Manufacturing Methods of Suppositories
|
|
|
- Bennett Jeffry Scott
- 5 years ago
- Views:
Transcription
1 Manufacturing Methods of Suppositories 1- Hand rolling method:classification of Suppositories Based on molding suppositories with fingers after the formation of a plastic mass In mortar add:- 1- A plastic-like mass is prepared by triturating grated cocoa butter & active ingredients in a mortar. 2- Pestle should be used to force the mass together against the sides of the mortar. To form plastic mass (It may be warmed very gently temp below 30 o C. 4- Cohesive mass is rolled into cylinder. 5- The total mass is weighed to record the total weight. (the mass is divided into the No. of the dose.) 6- The mass will quickly solidify & lose it plasticity) 7- The cone & pointed cylinder are the shapes that easily formed by hand rolling procedure. 8- Wrapped & packed. 2-Compression method Based on forcing suppository Base & medicaments into special compression mold. 3-Fusion method 1-Based on melting the suppository base (vehicle) & then disperse or dissolve the drug in the melted base. 2- pour the mixture into mold. Allow it to congeal. 3- remove from mold. Quality Control Tests QUALITY CONTROL procedures listed in the US Pharmacopeia (USP30-NF25) for manufactured suppositories include identification, assay, and, in some cases, NOTES NOTES Pharmaceutics III Page 1
2 water content, residual solvent, dissolution, and content uniformity 1- Appearance of suppository, include odor, color, surface condition & shape. 2- Content uniformity : to ensure that each individual supp. contains the labeled content 3-Weight variation: should be within 5% 4- Melting point/melting Zone: Macro-melting point test :- is a measure of the time needed for the entire suppository to melt when immersed in a constant-temp. at 37 C water bath. Micro-melting point:- is the melting range measured in capillary tubes for the base only. 5-Breaking test (fragility test) Is the test that carried out to measure the brittleness or fragility of suppository. Erweka hardness tester-is used to determine the weight at which the suppository collapse. 6- Dissolution rate The in-vitro release pattern is measured. The volume surrounding suppository is constant & the drug content is measured at different intervals The time versus drug concentration can be obtained. Pharmaceutics III Page 2
3 7- Liquefaction time Liquefaction testing provides information on the behavior of a suppository when subjected to a maximum temperature of 37 C. The test commonly used is Krowczynski s method, which measures the time required for a suppository to liquefy under pressures similar to those found in the rectum in the presence of water at 37 C. In general, liquefaction should take no longer than about 30 minutes. 8- Melting and solidification time There is a relationship between melting and solidification that is important to characterize. The release of the active ingredient from the vehicle is related to the melting point of the vehicle and the solubility of the drug in the vehicle. Suppositories undergo three changes in phase during their life. First, they are melted and then solidified; upon administration, they are again melted. An understanding of these factors and their relationships is critical for evaluating the bioavailability of the final suppository formulation. The higher the melting point, the later the drug effects appear. If too high, the drug effect does not appear. Various methods are available to measure it, including Shukoff s method, the European Pharmacopoeia also describes a procedure. Pharmaceutics III Page 3
4 Sterile Products Sterile Pharmaceutical Products Parenterals Ophthalmic preparations Radiopharmaceutical preparations They are sterile products intended for administration by injection under or through the skin or directly into body fluids, tissue or organs Sterile- free from any contaminating microorganism Small Volume Parenterals or Large Volume Parenterals Physiology:- Located outside the alimentary canal. Disadvantage Pain and discomfort Incorrect drug dose ( impossible to overcome the error Restricted to hospital or specialized personnel ROUTE OF PARENTERAL ADMINISTRATION In pharmacology and toxicology, a route of administration means the path by which a drug, fluid, poison or other substance is brought into contact with the body. Parenteral by injection or infusion 1- Subcutaneous injections NOTES Pharmaceutics III Page 4
5 Injecting a fluid or a solid pellet into the layer of loose connective and adipose tissue directly underlying two outer layers ( epidermis and dermis) of the skin. injections are used to administer vaccines and medications, A pellet may be injected to deliver longlasting doses of medication Common drugs for this route are vaccines, insulin, and epinepherine Drugs by this route have a slow onset of action than an IM or IV Following administration, the site of injection, the body temperature, age, degree of massage at the injection site are factors affecting drug distribution. NOTES Pharmaceutics III Page 5
6 The injection of a substance directly into a relaxed muscle. It is used for particular forms of medication that are administered in small amounts. Intramuscular injections are often given in the: Deltoid muscle in the shoulder (for rapid absorption of drug) astus lateralis muscle of the thigh entrogluteal and dorsogluteal muscles in the buttock Deltoid muscle :- thick, flat triangular muscle responsible for the round shape of the shoulder. Vastus lateralis muscle NOTES Pharmaceutics III Page 6
7 Ventrogluteal muscles Dorsogluteal muscles IM injections are preferred to be isotonic Absorption is relatively rapid compared to SC. Pharmaceutics III Page 7
8 For SC or IM injection there is a delay in the systemic effects of the drug (the drug first has to pass through the epithelial cells & the capillaries walls before entering into the blood. This occurs by passive diffusion. 3- Intravenous injections - Volume varies from less 1 ml- 500 ml. Giving of liquid substances directly into a vein into blood for a faster effect. The word intravenous simply means "within a vein". IV rapidly increases the concentration of the drug in the blood plasma, but the concentration soon falls due to reversible transfer of the drug from plasma into body tissue (Process of drug distribution). That followed by irreversible excretion and metabolism Pharmaceutics III Page 8
9 IV products are usually aqueous or hydroalcoholic solutions O/W emulsion with controlled particle size can be used Volume less than 10 ml is termed intravenous BOLUS Advantage 100 % bioavailability Rapid response Plasma concentration after 4 mins Constant blood level may be obtained Used for the administration of irritant or caustic drugs due to: Rapid dilution by the circulating blood Insensitivity of venous wall to pain Disadvantage Sudden excessive drug concentration at a target organ (drug shock) Once the dose is taken, anti-doting may be impossible Thrombosis is possible if extreme sites are used for injection E.g ankle, wrist or when the drug is potentially irritant Pharmaceutics III Page 9
10 IV infusion Term IV infusion is used for volume more than 10 ml. IV infusions administers a large volume of fluid at a slow rate and ensures that the drug enters the general circulation at a constant rate. The drug plasma rises soon after the start of infusion and achieves a steady state when rate of drug addition equals the rate of drug loss. Upon stopping infusion, elimination of the drug from the body by metabolism and /or excretion generally follow first order (proportional to the drug concentration) The term also available for Large volume parenteral (LVP) range ml. IV admixture IV combination for LVP prior or during administration (as a unit product) is known as IV admixture. Used to provide continuous or prolonged drug effect Specialized Routes Intra-arterial : direct injection into an artery (due to the fast flow of the blood in the artery, the drug will rapidly disperse throughout the blood system or to target the drug to a specific organ or tissue that is served by the artery) Intra-spinal: into spinal canal (aq. Solution injected in a volume less than 20 ml into a particular area of the spinal column) Intra-thecal: direct into cerebral spinal fluid Intra- articular: injection into a joint (aq. Solution or suspension) Intra cardial : direct injection into the heart ( aqueous solution applied in emergency) Intra-pleural: inject into the pleural cavity or a lung Pharmaceutics III Page 10
11 SPECIALIZED LVP AND STERILE SOLUTIONS Hyperalimentation: The administration of Nutrients and Vitamins by intravenous feeding, especially to individuals unable to take in food through the alimentary tract It is a medical procedure used for individuals who cannot get nutrients from food. This is done mainly due to impaired gastrointestinal (GI) conditions such as severe malabsorption, progressed eating disorders etc. Dialysis is a process for removing waste and excess water from the blood, and is used primarily as an artificial replacement for lost kidney function in people with renal failure. A- Peritoneal dialysis solution Sterile solutions injected (and continuously withdrawn) into the abdominal cavity for bathing the peritoneum for minutes Used: to rapid removal of drugs or chemical toxicity To counteract acute renal failure (toxic or metabolites diffuse into circulating fluids & removed) In peritoneal dialysis, a sterile solution containing glucose (called dialysate) is run through a tube into the peritoneal cavity, the abdominal body cavity around the intestine, where the peritoneal membrane acts as a partially permeable membrane. The peritoneal membrane or peritoneum is a layer of tissue containing blood vessels that lines and surrounds the peritoneal, or abdominal, cavity and the internal abdominal organs (stomach, Pharmaceutics III Page 11
12 Spleen, liver, and intestines). Diffusion and osmosis drive waste products and excess fluid through the peritoneum into the dialysate until the dialysate approaches equilibrium with the body's fluids. Then the dialysate is drained, discarded, and replaced with fresh dialysate. This exchange is repeated 4-5 times per day; automatic systems can run more frequent exchange cycles overnight. Peritoneal dialysis is less efficient than hemodialysis, but because it is carried out for a longer period of time the net effect in terms of removal of waste products and of salt and water are similar to hemodialysis. Peritoneal dialysis is carried out at home by the patient, often without help. This frees patients from the routine of having to go to a dialysis clinic on a fixed schedule multiple times per week. Peritoneal dialysis can be performed with little to no specialized equipment (other than bags of fresh dialysate). Ex: glucose solution containing the same ionic contents of the extra-cellular fluids Pharmaceutics III Page 12
13 B- Haemodialysis solution In hemodialysis, the patient's blood is pumped through the blood compartment of a dialyzer, exposing it to a partially permeable membrane. The dialyzer is composed of thousands of tiny synthetic hollow fibers. The fiber wall acts as the semipermeable membrane. Blood flows through the fibers, dialysis solution flows around the outside of the fibers, and water and wastes move between these two solutions. The cleansed blood is then returned via the circuit back to the body. Ultrafiltration occurs by increasing the hydrostatic pressure across the dialyzer membrane. This usually is done by applying a negative pressure to the dialysate compartment of the dialyzer. This pressure gradient causes water and dissolved solutes to move from blood to dialysate, and allows the removal of several litres of excess fluid during a typical 4-hour treatment. Pharmaceutics III Page 13
14 Is an electrolytes solution simulating body fluid and it is used for artificial kidney apparatus C- Irrigation solutions Used to irrigate and clean body cavities wounds. They must be sterile, pyrogen free Normal saline may be used as irrigative solution If designed (labeled) irrigative should not used as parenteral Injection Dosages Small volume parenterals - are sterile and Pyrogen free. - are packed in volume up to 100 ml. as:- Single- dose ampoules (glass thin walled containers made of type I borosilicate glass or may be plastic) Pharmaceutics III Page 14
15 Multiple- dose vials:- thick-walled glass & the container is sealed with rubber closure Prefilled syringes:- is aseptically filled into sterile syringes. The packed solution has a high level of sterility assurance & does not contain an antimicrobial preservatives. The product is available for immediate use. (use is limited because. It is expensive) Pharmaceutics III Page 15
16 ADDITIVES Substances added to the product to:- Provide efficacy Safe Elegant products Maintain pharmaceutical stability Ensure sterility Aid in parenteral administration Antioxidants Role of antioxidants An antioxidant is a molecule that inhibits the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. In turn, these radicals can start a chain reaction. When the chain reaction occurs in a cell, it can cause damage or death to the cell. Antioxidants terminate these chain reactions by removing free radical intermediates, and inhibit other oxidation reactions. They do this by being oxidized themselves, so antioxidants are often reducing agents such as this, ascorbic acid, or polyphenols. To prevent degradation active ingredients and maintain product stability during shelflife. Pharmaceutics III Page 16
17 Stability of drugs that possess the low oxidation potential are susceptible to oxidative degradation. This can be prevented by applying different techniques:- The common antioxidant parenteral are: Sodium Bi-sulfite, Sodium meta- bisulfite Ascorbic acid Mechanisms of Action for aqueous They will preferentially oxidized instead of the drug or block oxidative chain reaction Displace the air by inert gas bubbled to solution prior to filling and sealing in the final products. Eg. N 2 and CO 2 Addition of free radical scavengers to block the oxidative chain reaction Eg. Vit. E and Vit. C Pharmaceutics III Page 17
18 Antimicrobial (preservatives): have a dual role A- In multiple dose: act as bacteriostatic 1- (inhibit the growth of any microbes accidentally introduced due to repeated doses 2- protect the product from minor contamination during formulation process Required for all multiple-dose products Not used for LVP (toxic effect) Added to the most unit-dose solutions that are terminally sterilized (Terminal sterilization is the process of sterilizing the final packaged product ) Preservative Should be compatible with the drug & excipients or injection components e.g containers & closures Effective in non-toxic concentration Effective after formulation and near the expiration date Antimicrobial test should be evaluate the antimicrobial activity of preservative in the final packaged product Buffers Added to resist ph changes during storage Requirements for ideal buffer Have adequate buffer capacity to maintain the ph of the product at stable value Ideal ph of parenteral product is ph 7.4 At ph above 9 lead to necrosis If below ph 3 pain in tissues Doesn't cause drug degradation Pharmaceutics III Page 18
19 E.g. Citrate buffer increase the degradation of Vit. B 12 Doesn't affect the isotonicity and stability of the product. ph of many official preparations is adjusted due to 1- To increase the stability of injection 2- minimize pain, irritation, necrosis 3- To provide unsuitable conditions for the growth of microorganisms 4- To enhance physiological activity, ph of product should be kept as physiologically possible with acceptance stability 5- control the drug solubility (as desired) 6- provide unsatisfactory conditions for growth of micro-organism (M.O cant resist low or high ph) 7- The ph of the product can be influenced by product degradation, container, stopper, diffusion of gases The product, is buffered to obtain maxm. solubility and resist change in ph. Buffers for parenteral use either weak acid and its salts or weak bases and its salts Sequestering agents (chelating agents) Used to complex or inactivate e.g metals copper, iron and zinc Metal contamination related to: raw materials, solvent e.g water Rubber stoppers and containers equipment ex. Of chelating agents: 1- Ethylenediamine tetra acetic acid (EDTA) 2- salts of citric and tartaric acid there activity increase in presence of reducing agents Pharmaceutics III Page 19
20 Inert gases Usually, Applied to enhance the integrity of oxygensensitive medications (increase stability) to replace oxygen the main cause of decomposition Ex: nitrogen and CO 2 For best results Use boiling water to remove air Purging the container with inert gases prior to filling Use glass-seal ampoules provide a barrier for gas transmission Use of butyl rubber stock better resistance to gas permeation than other rubbers Solubilizing agents and surfactants Solubilizing agents To increase drug solubility e.g lecithin, PEG, glycerol, ethyl alcohol (steroids, fatsoluble vit.) Surfactants SAA to increase dispersion of colloidal particles, increase powder wettability and prevent crystal growth for suspension & provide acceptable syringability. E.g Lecithin- Obtained from egg yolk, or soya beans sources. Osmotic pressure and tonicity adjustment agents Osmosis = passage of solvent molecules through semipermiable membrane from dilute to concentrated soln until equilibrium Osmotic pressure = force or pressure responsible for this process Iso-osmotic (isosmotic ) solutions = have the same osmotic pressure Pharmaceutics III Page 20
21 Iso-tonic solutions = solutions have the same osmotic pressure as body fluids (electrolytes concentrations) Hyper tonic solutions = solutions have higher O.P. than body fluids. Disadv. 1- allow water to move outwards from RBCs through their semipermeable membrane lead to shrink of cells (reversible mechanism) 2- Drain fluids extensively from body tissue and organs Ex: hypertonic solution used for: * NaCl solution used for abortion or emesis by local action * Wrong administration of large volume hypertonic normal saline may cause hemorrhage encephalopathy (drainage of brain fluids) and renal failure (kidney necrosis, and to pregnant females abortion. Hypo-tonic solutions = solutions have lower O.P. than body fluids. Disadv. 1- allow water to move into RBCs through their semipermeable membrane lead to swell rapidly and brust (haemolysis) of cells (irreversible mechanism) 2- not recommended in parenterals (can be adjusted by NacL, KCl, glucose, mannitol) Osmolarity of plasma = 306 milliosomole mosmol /liter Pharmaceutics III Page 21
22 Parenterals deviate from this value cause: Hemolysis of RBCs, Tissue irritation Pain on injection Shrinking of RBCs Large volume parenteral should be isotonic Small volume S.C, I.M, I.V may be hypertonic Large volume parenteral should be isotonic (have osmolarity ranging from mosmol/L) ex: glucose 5% infusion (= 280 mosmol/l) 0.9% Sod. Chloride (308 mosmol/l) Pharmaceutics III Page 22
23 Vehicle Aqueous Non aqueous Should be pharmacologically inert, non toxic, compatible with blood, maintain solubility of the drug Physically and chemically stable Unaffected by the ph changes. Not interfere with the therapeutic activity of injection Water Advantage Ideal for most injections Well tolerated Safest Easiest to administer Help to provide uniform dose More accurately measured Easy visual inspection for particulate contamination, chemical (ppt), color change Disadvantage May accelerate drug hydrolysis resulting in an inert or toxic products (powder for reconstitution) Not suitable for poorly soluble drugs Injection of distilled water may cause a rise in body temp. (pyrogenic = fever producing) Water free from this reaction = apyrogenic Pyrogen It is Lipid in nature and may be produced by many organisms, yeast, gram ve & gram +ve bacteria, fungi & viruses (non volatile) Non- microbial Pyrogens are :- some steroids & plasma components as they produce a pyrogenic response if injected Endotoxins isolated from the outer membrane of gram ve bacteria are composed of lipids that also produce Pharmaceutics III Page 23
24 significant physiological changes when injected (pyrogenic effect) They are fever-producing substances due to their toxic effect The most potent are associated with gram negative bacteria Source of pyrogen: solvent, medicament, additives, apparatus, containers Water is the greatest source of pyrogen in parenterals The contamination of LVP with pyrogen is serious, owing to the large volumes that are administered to seriously ill patients Types of water in pharmacy are 1- Purified water, USP is free of dissolved or solid impurities. is intended for use in the preparation of aqueous dosage forms, except those intended for parenteral administration (Injections) Should be freshly boiled and cooled immediately before use. Pharmaceutics III Page 24
25 2- Water for injection, USP: Used for parenteral solutions which are to be sterilized after their preparation. It is obtained by sterilizing pyrogen-free distilled water immediately after its collection. 3- Sterile water for injection, USP: water for injection which has been sterilized and packaged in single dose containers not greater than 1 liter size. it must be pyrogen-free not contain an antimicrobial agent or other added substance. This water is intended to be used as a solvent, vehicle, and diluent for alreadysterilized packaged injectable medications. The one-liter bottles not administered intravenously because they have no tonicity. used for reconstitution of multiple dose medication e.g antibiotics. In use, the water is aseptically added to the vial of medication to prepare the desired injection. 4- Bacteriostatic water for injection: sterile water for injection containing one or more suitable antimicrobial agents (to increase flexibility to multiple dose). The container label must state the name and proportion of the antimicrobial agent. Sterile vehicle in the preparation of small volumes of injectable preparations. Bacteriostatic agent gives the flexibility for multiple-dose vials. The preservative will destroy the contaminant microorganism Parenterals administered in large volumes is restricted due to excessive toxic amounts of antimicrobial agents that may be injected along with the medication. Generally, If volumes of greater than 5 ml of solvent are required, sterile water for injection rather than bacteriostatic water for injection is preferred. Pharmaceutics III Page 25
26 Sodium chloride injection, USP It is a sterile isotonic solution of sodium chloride (155 m Eq/L= ionic content of plasma) in water for injection. It contains no antimicrobial agents. used as a sterile vehicle in preparing solutions or suspensions of drugs for parenteral administration. Sodium chloride injection is frequently used as a catheter or IV infusion Ringer s injection, USP A sterile soln of NaCl, KCl, CaCl 2 (conc. similar to body fluids). Used as vehicle for drug or electrolytes replenisher Lactated ringer s injection, USP Lactated ringer s injection, USPA sterile soln of NaCl, KCl, CaCl 2 + sod. lactate Used as vehicle for drug or electrolytes replenisher Non- aqueous solutions If the drug is unstable in aqueous systems it may be necessary to use an alternative (nonaqueous solvent). To: 1- Increase drug stability 2-Improve solubility of poorly soluble drugs A-Water miscible non aqueous Ethyl alcohol (low conc to increase solubility) e.g dioxin and phenytoin injection BP Propylene glycol (PG) (non toxic, rapidly metabolized and excreted) Glycerol and polyethylene glycol (PEG) B-Water immiscible solvent Mainly used to dissolve drugs that are insoluble in water. Eg: steroids, hormones, vitamins Fixed oils of vegetable origin. 1- Almond Oil, (glycerides of oleic acid) used as a solvent for oily phenol injections, Pharmaceutics III Page 26
27 2- Olive oil, sesame oil, maize oil, cottonseed oil, Soya oil and castor oil are all suitable for parenteral use. N.B = mineral oils (paraffins) not used for parenteral (not metabolized) Dosage forms for parenteral use A- solution Drug+ preservative + water solution sterile microfilteration 0.22 µm filter for heat sensitive preparation For thermostable: terminally autoclavesterilized after filling (assure sterility and package) Parenteral Suspension Ready-to-use injection Reconstituted prior to administer May contain 0.5-5% solid (reach 30% with antibiotics) Particle size less than 5 µm Should have good syringeability and injectability Bacteriostatic sodium chloride injection, USP It is a sterile isotonic solution of sodium chloride (0.9%) in water for injection. It contains one or more antimicrobial agents. Not packed in container greater than 30 ml Should be labeled Not for use in new born (toxicity) Syringeability = handling characteristics of suspension while withdrawing it from container to a syringe + its clogging and foaming tendency Injectability = suspension characteristics during injection (required pressure for injection Thixotropy = Preparation that is solid in absence of shearing force become fluid if shaken- Pharmaceutics III Page 27
28 - the original structure is resumed after few mins. at rest Advantage Suitable for insoluble drugs Increased stability Possible depot action Disadvantage Difficult formulation and manufacturing Patient discomfort Difficult dose uniformity Needs more additives 1-Surfactant: needed for wetting hydrophobic drug and prevent crystal growth e.g : lecithin, polysorbate --tween 80, 60 2-Suspending agent: to control sedimentation rate ( viscosity ): gelatin, povidone, CMC To hold suspended particles enough time for accurate dose Prevent reformation of particle clumps 3- ph adjustment : citric acid, sod. citrate 4- Antioxidant: ascorbic acid, sod. Bi sulfite, or metabisulfite, sod. Formaldhyde, thiourea 5-Preservative: should be tested to determine the most effective conc. Preparation steps of Parenteral suspension Sterilization and milling of active ingredients Sterilization of vehicle Or Dissolve all soluble components and sterilize them by autoclave or sterile filtration Add previously sterilized powder to the sterile soln aseptically Aseptic wetting and dispersion of active ingredients Aseptic milling of bulk suspension Aseptic filling of the bulk suspension in suitable containers Pharmaceutics III Page 28
29 Methods for preparation of sterile drug powder Sterile re-crystalization Dissolve drug--- sterile by filtration (0.022 um) Sterile anti-solvent --- then added to drug solution to allow drug to crystallize in situ then filter aseptically Spray drying Spray the drug soln. into dry chamber to come in contact with hot sterile gas (air) Rapid evaporation leaving sterile uniform drug powder Adv. Suitable for heat sensitive drug powder Uniform particle size Low particulate contamination Low price and rapid process Lyophilization Separation of solid substance from solution by freezing. (Evaporate the solvent by sublimation) Adv. Suitable for heat and oxygen sensitive drug More rapid solubility (rapid re-constituation) Reduced contamination Disadv. Expensive Difficult to form crystalline powder Low solvent content Parenteral Emulsion Suitable for IV administration --- Dispersed droplets p.s = um --- Stablized by surfactants (lecithin, tween 80, gelatin, serum albumin and CMC) Application A source of calories and essential fatty acids Pharmaceutics III Page 29
30 Vehicle for drugs showing more stability, safety and efficacy in emulsion form. Ex: dexamethazone palmitate parenteral emulsion 5-6 active >>> its solution form Diazepam emulsion showed lower toxicity than solution. Physostigmine salicylate emulsion more stable than its soluton Disadv. of parenteral emulsion 1- Unstable product, difficult sterilization of the product (heat influence stability & impossible filtration) 2- rapid growth of M.O (not contain preservative) 3-difficult manufacturing steps under aseptic condition. 4- Unstable system upon storage (increase globule size--- cause thrombosis if injected IV) 5- Long term emulsion therapy may lead to overloading syndrome (fever, anemia, hepato-spleenomegaly. Dry powder Unstable drugs presented in form of dry pd for reconstitution prior to administration with vehicle. If solution will be the final dosage form should labeled for injection or sterile If suspension sterile for suspension Ex: sterile ampicillin trihydrate for suspension Containers and package of parenterals - Integral part of the product (bec. Stability, potency, safety) Should reject any component of packaging which is not safe, incompatible or affect stability) Pharmaceutics III Page 30
31 1- Glass containers: - multi-dose (vial) Single dose (Ampoule) Large volume parenteral L P (now used in plastic forms) Role of containers and closures that are used for packing of parenterals 1- maintain the sterility of the packed product 2- Withstand sterilization process 3- Allow withdrawal of the contents Materials of parenteral containers A- Glass 1- transparent and chemically inert, 2- excellent compatibility 3- Good product presentation, 4- good product identification colored glass used to resist deterioration of the product by light Carbon& sulfur, iron & manganese (Amber) Compd of cadmium and sulfur (yellow) Cobalt oxide or cupric oxide (blue) Iron oxide, manganese dioxide & chromium dioxide (Green) Types of glass 1- Type I Neural (neutral or borosilicate) 2- Type II (Soda glass with a surface treatment) 3- Type III (Soda glass of limited alkalinity) 4- NP glass ( Soda glass for non parenteral use) Glass bottle are normally made of glass type II Plastics containers 1- Poly vinyl chloride P C collapsible bags are used to package most infusion fluids. Pharmaceutics III Page 31
32 They are designed with port for the attachment of administration set and an additive port for addition of small-volume parenteral fluids. Resistant to impact Flexible and collapse during fluid administration & do not require an air inlet system. 2- Semi- rigid polyethylene containers :- These containers are intended for single use. Rubber Closure 1- saturated elastomers Copolymer of poly-isobutylene and polyisoprene Ethylene propylene rubber Ethylene propylene diene rubber Silicone rubber 2- Unsaturated elastomers Poly-isoprene Poly butadiene Styrene butadiene Poly-chloroprene Compatibility with the preparation Leaching form stopper is the most common leading to turbidity or precipitation Reaction with preparation Sorption of preservative or other ingredients Methods of evaluation: Microscopic examination of particulate matter Evaluation of solution by ultraviolet Infrared, thin layer chromatography,. Pharmaceutics III Page 32
Parenteral products-definition
 Parenteral products-definition Parenteral preparations are sterile, pyrogen-free liquids (solutions, emulsions, or suspensions) or solid dosage forms packaged in either single-dose or multidose containers.
Parenteral products-definition Parenteral preparations are sterile, pyrogen-free liquids (solutions, emulsions, or suspensions) or solid dosage forms packaged in either single-dose or multidose containers.
PARENTERAL PREPARATIONS
 PARENTERAL PREPARATIONS INTRODUCTION Parenteral (Gk, para enteron, beside the intestine) dosage forms differ from all other drug dosage forms, because they are injected directly into body tissue through
PARENTERAL PREPARATIONS INTRODUCTION Parenteral (Gk, para enteron, beside the intestine) dosage forms differ from all other drug dosage forms, because they are injected directly into body tissue through
Parenteral Products. By: Howida Kamal, Ph.D
 Parenteral Products By: Howida Kamal, Ph.D Dosage forms Route of administration Enteral INTO Parenteral ONTO Topical Dosage forms Physical form Solid Liquid Semi-solid Powder Granules Tablets Capsules
Parenteral Products By: Howida Kamal, Ph.D Dosage forms Route of administration Enteral INTO Parenteral ONTO Topical Dosage forms Physical form Solid Liquid Semi-solid Powder Granules Tablets Capsules
COLLEGE OF PHARMACY STERILE PRODUCTS PHT 434. Dr. Mohammad Javed Ansari Ph.D Contact info:
 COLLEGE OF PHARMACY STERILE PRODUCTS PHT 434 Dr. Mohammad Javed Ansari Ph.D Contact info: javedpharma@gmail.com OBJECTIVES OF THE LECTURE At the end of this lecture, you will be aware of: What are factors
COLLEGE OF PHARMACY STERILE PRODUCTS PHT 434 Dr. Mohammad Javed Ansari Ph.D Contact info: javedpharma@gmail.com OBJECTIVES OF THE LECTURE At the end of this lecture, you will be aware of: What are factors
DRAFT PROPOSAL FOR REVISION OF GENERAL MONOGRAPHS: PARENTERAL PREPARATIONS. (July 2012) Draft for comment
 1 2 3 4 5 DRAFT PROPOSAL FOR REVISION OF July 2012 RESTRICTED GENERAL MONOGRAPHS: PARENTERAL PREPARATIONS (July 2012) Draft for comment This document was provided by a quality control expert. Should you
1 2 3 4 5 DRAFT PROPOSAL FOR REVISION OF July 2012 RESTRICTED GENERAL MONOGRAPHS: PARENTERAL PREPARATIONS (July 2012) Draft for comment This document was provided by a quality control expert. Should you
Dr.N.Damodharan Professor and head Department of pharmaceutics SRM college of pharmacy
 Dr.N.Damodharan Professor and head Department of pharmaceutics SRM college of pharmacy Suppositories are solid dosage forms intended for insertion into body orifices where they melt, soften, or dissolve
Dr.N.Damodharan Professor and head Department of pharmaceutics SRM college of pharmacy Suppositories are solid dosage forms intended for insertion into body orifices where they melt, soften, or dissolve
1 INJECTIONS INTRODUCTION
 1 INJECTIONS INTRODUCTION Parenteral articles are preparations intended for injection through the skin or other external boundary tissue, rather than through the alimentary canal, so that the active substances
1 INJECTIONS INTRODUCTION Parenteral articles are preparations intended for injection through the skin or other external boundary tissue, rather than through the alimentary canal, so that the active substances
Industrial Pharmacy (3) Solutions as a dosage form. DR.Saad.M.YACOUB
 Industrial Pharmacy (3) Solutions as a dosage form DR.Saad.M.YACOUB Solutions: definition A solution is a homogenous one-phase system consisting of two or more components. The solvent, or mixture of solvents,
Industrial Pharmacy (3) Solutions as a dosage form DR.Saad.M.YACOUB Solutions: definition A solution is a homogenous one-phase system consisting of two or more components. The solvent, or mixture of solvents,
Suppository Chapter Content
 10 min SUPPOSITORY Suppository Chapter Content 1. Suppositories and Factors Affecting Drug Absorption 2. Ideal Suppository and Different Types of Bases 3. Methods of Suppository Manufacturing Suppository
10 min SUPPOSITORY Suppository Chapter Content 1. Suppositories and Factors Affecting Drug Absorption 2. Ideal Suppository and Different Types of Bases 3. Methods of Suppository Manufacturing Suppository
Emulsions. Purpose of emulsions and of emulsification:
 Pharmacist Ghada Hamid Emulsions Emulsion is a dispersion in which the dispersed phase is composed of small globules of a liquid distributed throughout a vehicle in which it is immiscible. The dispersed
Pharmacist Ghada Hamid Emulsions Emulsion is a dispersion in which the dispersed phase is composed of small globules of a liquid distributed throughout a vehicle in which it is immiscible. The dispersed
BRIEFING 1 INJECTIONS INTRODUCTION
 BRIEFING 1 Injections, USP 28 page 2201. The United States Pharmacopeia is the coordinating pharmacopeia in the efforts toward international harmonization of the specifications provided in this general
BRIEFING 1 Injections, USP 28 page 2201. The United States Pharmacopeia is the coordinating pharmacopeia in the efforts toward international harmonization of the specifications provided in this general
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5
 Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
ERT 430 PHARMACEUTICAL PROCESS ENGINEERING PARENTERAL FORMULATION
 ERT 430 PHARMACEUTICAL PROCESS ENGINEERING PARENTERAL FORMULATION OUTLINE Introduction Parenteral Product Requirement Parenteral Product Formulation: Solution Emulsion Suspension Evaluation of Parenteral
ERT 430 PHARMACEUTICAL PROCESS ENGINEERING PARENTERAL FORMULATION OUTLINE Introduction Parenteral Product Requirement Parenteral Product Formulation: Solution Emulsion Suspension Evaluation of Parenteral
LIQUID PREPARATIONS FOR ORAL USE. Final text for addition to The International Pharmacopoeia (November 2007)
 November 2007 LIQUID PREPARATIONS FOR ORAL USE Final text for addition to The International Pharmacopoeia (November 2007) This monograph was adopted at the Forty-second WHO Expert Committee on Specifications
November 2007 LIQUID PREPARATIONS FOR ORAL USE Final text for addition to The International Pharmacopoeia (November 2007) This monograph was adopted at the Forty-second WHO Expert Committee on Specifications
NANO 243/CENG 207 Course Use Only
 L6. Drug Administration & Transport by Fluid Motion April 19, 2018 Part I: Drug Administration Routes of Drug Administration Topical: local effect, substance is applied directly where its action is desired.
L6. Drug Administration & Transport by Fluid Motion April 19, 2018 Part I: Drug Administration Routes of Drug Administration Topical: local effect, substance is applied directly where its action is desired.
LECTURE 10. PACKAGING TECHNOLOGY
 LECTURE 10. PACKAGING TECHNOLOGY The increasing demand for fresh and quality packaged food, consumer convenience and manufacturers concern for longer shelf life of the food products is driving the market
LECTURE 10. PACKAGING TECHNOLOGY The increasing demand for fresh and quality packaged food, consumer convenience and manufacturers concern for longer shelf life of the food products is driving the market
University of Sulaimani School of Pharmacy Dept. of Pharmaceutics Third level - Second semester
 University of Sulaimani School of Pharmacy Dept. of Pharmaceutics Third level - Second semester 5/21/2017 Pharmaceutical Compounding, Dr. rer. nat. Rebaz Ali 1 Outlines Why rectal or vaginal route? Suppository
University of Sulaimani School of Pharmacy Dept. of Pharmaceutics Third level - Second semester 5/21/2017 Pharmaceutical Compounding, Dr. rer. nat. Rebaz Ali 1 Outlines Why rectal or vaginal route? Suppository
BIOL 347L Laboratory Three
 Introduction BIOL 347L Laboratory Three Osmosis in potato and carrot samples Osmosis is the movement of water molecules through a selectively permeable membrane into a region of higher solute concentration,
Introduction BIOL 347L Laboratory Three Osmosis in potato and carrot samples Osmosis is the movement of water molecules through a selectively permeable membrane into a region of higher solute concentration,
REFERENCES OVERVIEW ADVANTAGES AEROSOLS. Aerosols. and Foams
 COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING This material has been reproduced and communicated to you by or on behalf of Monash University pursuant to Part VB of the Copyright Act 1968
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING This material has been reproduced and communicated to you by or on behalf of Monash University pursuant to Part VB of the Copyright Act 1968
1 INJECTIONS INTRODUCTION
 1 INJECTIONS INTRODUCTION Parenteral articles are preparations intended for injection through the skin or other external boundary tissue, rather than through the alimentary canal, so that the active substances
1 INJECTIONS INTRODUCTION Parenteral articles are preparations intended for injection through the skin or other external boundary tissue, rather than through the alimentary canal, so that the active substances
Pharmaceutics I صيدالنيات 1. Unit 2 Route of Drug Administration
 Pharmaceutics I صيدالنيات 1 Unit 2 Route of Drug Administration 1 Routs of Drug administration The possible routes of drug entry into the body may be divided into two classes: Parenteral Rout Enteral Rout
Pharmaceutics I صيدالنيات 1 Unit 2 Route of Drug Administration 1 Routs of Drug administration The possible routes of drug entry into the body may be divided into two classes: Parenteral Rout Enteral Rout
6. SR, XR, CR, and TR are examples of formulations. A) oral B) modified release C) repeat action D) soft gels
 1. Which is a parenteral route of administration? A) oral B) vaginal C) sublingual D) rectal 2. A local effect occurs A) at the site of administration. B) throughout the body. C) only after intravenous
1. Which is a parenteral route of administration? A) oral B) vaginal C) sublingual D) rectal 2. A local effect occurs A) at the site of administration. B) throughout the body. C) only after intravenous
13. SUPPOSITORY Suppository Bases. The active substance is prepared in a suitable bases. An ideal suppository bases should carry:
 13. SUPPOSITORY They are solid single-dose preparations whose shapes, volumes and consistencies are suitable for rectal administration. There are also such vaginal preparations in the treatment of local
13. SUPPOSITORY They are solid single-dose preparations whose shapes, volumes and consistencies are suitable for rectal administration. There are also such vaginal preparations in the treatment of local
EH1008 Biomolecules. Inorganic & Organic Chemistry. Water. Lecture 2: Inorganic and organic chemistry.
 EH1008 Biomolecules Lecture 2: Inorganic and organic chemistry limian.zheng@ucc.ie 1 Inorganic & Organic Chemistry Inorganic Chemistry: generally, substances that do not contain carbon Inorganic molecules:
EH1008 Biomolecules Lecture 2: Inorganic and organic chemistry limian.zheng@ucc.ie 1 Inorganic & Organic Chemistry Inorganic Chemistry: generally, substances that do not contain carbon Inorganic molecules:
PRESCRIBING INFORMATION. Dextrose Injection USP. (Concentrated Dextrose for Intravenous Administration) 50% (500 mg/ml) Fluid and Nutrient Replenisher
 PRESCRIBING INFORMATION Dextrose Injection USP (Concentrated Dextrose for Intravenous Administration) 50% (500 mg/ml) Fluid and Nutrient Replenisher Pfizer Canada Inc. 17300 Trans-Canada Highway Kirkland,
PRESCRIBING INFORMATION Dextrose Injection USP (Concentrated Dextrose for Intravenous Administration) 50% (500 mg/ml) Fluid and Nutrient Replenisher Pfizer Canada Inc. 17300 Trans-Canada Highway Kirkland,
Dr.N Damodharan Professor and Head. Department of Pharmaceutics SRM College of Pharmacy
 Dr.N Damodharan Professor and Head Department of Pharmaceutics SRM College of Pharmacy 1 Definition: - Medicines prepared according to the formulae of Galen. - A medicinal preparation composed mainly of
Dr.N Damodharan Professor and Head Department of Pharmaceutics SRM College of Pharmacy 1 Definition: - Medicines prepared according to the formulae of Galen. - A medicinal preparation composed mainly of
BIOL 305L Spring 2019 Laboratory Six
 Please print Full name clearly: BIOL 305L Spring 2019 Laboratory Six Osmosis in potato and carrot samples Introduction Osmosis is the movement of water molecules through a selectively permeable membrane
Please print Full name clearly: BIOL 305L Spring 2019 Laboratory Six Osmosis in potato and carrot samples Introduction Osmosis is the movement of water molecules through a selectively permeable membrane
Functional Excipients for Suppository Applications
 Functional Excipients for Suppository Applications Skin Delivery Platform 2016 1 The Skin Delivery Platform Major areas of activity are in 4 pillars PLATFORM : SKIN DELIVERY Dermal Drug Delivery Mildness
Functional Excipients for Suppository Applications Skin Delivery Platform 2016 1 The Skin Delivery Platform Major areas of activity are in 4 pillars PLATFORM : SKIN DELIVERY Dermal Drug Delivery Mildness
Petrolatum. Stage 4, Revision 1. Petrolatum is a purified semi solid mixture of hydrocarbons obtained from petroleum.
 1 001-1208PDG.pdf Petrolatum Stage 4, Revision 1 Definition Petrolatum is a purified semi solid mixture of hydrocarbons obtained from petroleum. It may contain a suitable antioxidant. Description and Solubility
1 001-1208PDG.pdf Petrolatum Stage 4, Revision 1 Definition Petrolatum is a purified semi solid mixture of hydrocarbons obtained from petroleum. It may contain a suitable antioxidant. Description and Solubility
1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small
 Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
Transport. Slide 1 of 47. Copyright Pearson Prentice Hall
 & Transport 1 of 47 Learning Targets TN Standard CLE 3216.1.3 Explain how materials move into and out of cells. CLE 3216.1.5 Investigate how proteins regulate the internal environment of a cell through
& Transport 1 of 47 Learning Targets TN Standard CLE 3216.1.3 Explain how materials move into and out of cells. CLE 3216.1.5 Investigate how proteins regulate the internal environment of a cell through
Purposes of suppositories
 Suppositories Definition Are conical or ovoid, solid prep. For insertion into the body cavity where they melt,dissolve or disperse and exert a local or a systemic effect. Their basis is fat,a wax or glycerol-gelatin
Suppositories Definition Are conical or ovoid, solid prep. For insertion into the body cavity where they melt,dissolve or disperse and exert a local or a systemic effect. Their basis is fat,a wax or glycerol-gelatin
Chapter 12. Excretion and the Interaction of Systems
 Chapter 12 Excretion and the Interaction of Systems 1 2 Goals for This Chapter 1. Identify the main structures and functions of the human excretory system 2. Explain the function of the nephron 3. Describe
Chapter 12 Excretion and the Interaction of Systems 1 2 Goals for This Chapter 1. Identify the main structures and functions of the human excretory system 2. Explain the function of the nephron 3. Describe
DIFFUSON AND OSMOSIS INTRODUCTION diffusion concentration gradient. net osmosis water potential active transport
 DIFFUSON AND OSMOSIS NAME DATE INTRODUCTION The life of a cell is dependent on efficiently moving material into and out of the cell across the cell membrane. Raw materials such as oxygen and sugars needed
DIFFUSON AND OSMOSIS NAME DATE INTRODUCTION The life of a cell is dependent on efficiently moving material into and out of the cell across the cell membrane. Raw materials such as oxygen and sugars needed
Slide 2 of 47. Copyright Pearson Prentice Hall. End Show
 2 of 47 7-3 Cell Boundaries All cells are surrounded by a thin, flexible barrier known as the cell membrane. Many cells also produce a strong supporting layer around the membrane known as a cell wall.
2 of 47 7-3 Cell Boundaries All cells are surrounded by a thin, flexible barrier known as the cell membrane. Many cells also produce a strong supporting layer around the membrane known as a cell wall.
MONOGRAPHS (USP) Saccharin Sodium
 Vol. 31(4) [July Aug. 2005] HARMONIZATION 1225 MONOGRAPHS (USP) BRIEFING Saccharin Sodium, USP 28 page 1745 and page 612 of PF 31(2) [Mar. Apr. 2005]. The United States Pharmacopeia is the coordinating
Vol. 31(4) [July Aug. 2005] HARMONIZATION 1225 MONOGRAPHS (USP) BRIEFING Saccharin Sodium, USP 28 page 1745 and page 612 of PF 31(2) [Mar. Apr. 2005]. The United States Pharmacopeia is the coordinating
Topical Preparations
 Topical Preparations One of the functions of the skin is to protect the internal body components against the external environment and thus to control the passage of chemicals into and out of the body.
Topical Preparations One of the functions of the skin is to protect the internal body components against the external environment and thus to control the passage of chemicals into and out of the body.
6.02 Uniformity of Dosage Units
 6.02 Uniformity of Dosage Units Change 1. Content Uniformity, 3. Criteria and Table 6.02-2 as follows: 1. Content Uniformity Select not less than 30 units, and proceed as follows for the dosage form designated.
6.02 Uniformity of Dosage Units Change 1. Content Uniformity, 3. Criteria and Table 6.02-2 as follows: 1. Content Uniformity Select not less than 30 units, and proceed as follows for the dosage form designated.
» Monohydrate Citric Acid contains one molecule of water of hydration. It contains not less than 99.5 percent and not more than 100.
 BRIEFING Citric Acid, Monohydrate. The European Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the compendial standards for the Citric Acid, Monohydrate monograph,
BRIEFING Citric Acid, Monohydrate. The European Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the compendial standards for the Citric Acid, Monohydrate monograph,
BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes
 BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes The cell membrane is the gateway into the cell, and must allow needed things such as nutrients into the cell without letting them escape.
BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes The cell membrane is the gateway into the cell, and must allow needed things such as nutrients into the cell without letting them escape.
NORMOSOL -R MULTIPLE ELECTROLYTES INJECTION TYPE 1, USP For Replacing Acute Losses of Extracellular Fluid Flexible Plastic Container
 NORMOSOL -R MULTIPLE ELECTROLYTES INJECTION TYPE 1, USP For Replacing Acute Losses of Extracellular Fluid Flexible Plastic Container R x only DESCRIPTION Normosol-R is a sterile, nonpyrogenic isotonic
NORMOSOL -R MULTIPLE ELECTROLYTES INJECTION TYPE 1, USP For Replacing Acute Losses of Extracellular Fluid Flexible Plastic Container R x only DESCRIPTION Normosol-R is a sterile, nonpyrogenic isotonic
Lab #6: Cellular Transport Mechanisms Lab
 Lab #6: Cellular Transport Mechanisms Lab OVERVIEW One of the major functions of the plasma membrane is to regulate the movement of substances into and out of the cell. This process is essential in maintaining
Lab #6: Cellular Transport Mechanisms Lab OVERVIEW One of the major functions of the plasma membrane is to regulate the movement of substances into and out of the cell. This process is essential in maintaining
Physical Pharmacy. Interfacial phenomena. Khalid T Maaroof MSc. Pharmaceutical sciences School of pharmacy Pharmaceutics department
 Physical Pharmacy Interfacial phenomena Khalid T Maaroof MSc. Pharmaceutical sciences School of pharmacy Pharmaceutics department 1 Introduction The boundary between two phases is generally described as
Physical Pharmacy Interfacial phenomena Khalid T Maaroof MSc. Pharmaceutical sciences School of pharmacy Pharmaceutics department 1 Introduction The boundary between two phases is generally described as
APPENDIX 1 SUMMARY OF PRODUCT CHARACTERISTICS
 SmPC Country: Lebanon Date of approval : 17/08/2011 Procedure : National APPENDIX 1 SUMMARY OF PRODUCT CHARACTERISTICS 1- NAME OF THE MEDICINAL PRODUCT DECAN, concentrate for solution for infusion 2- QUALITATIVE
SmPC Country: Lebanon Date of approval : 17/08/2011 Procedure : National APPENDIX 1 SUMMARY OF PRODUCT CHARACTERISTICS 1- NAME OF THE MEDICINAL PRODUCT DECAN, concentrate for solution for infusion 2- QUALITATIVE
Pharmaceutical Preparation For Internal Use
 Pharmaceutical Preparation For Internal Use 1. Solid Preparations (Tablet, Capsule, Pill) 2. Liquid Preparations (Aqua, Syrup, Elixir, Extract, Liquor, Emulsion, Mixture, Infusion, Decoction). 3. Powder
Pharmaceutical Preparation For Internal Use 1. Solid Preparations (Tablet, Capsule, Pill) 2. Liquid Preparations (Aqua, Syrup, Elixir, Extract, Liquor, Emulsion, Mixture, Infusion, Decoction). 3. Powder
Unit 3 : Homeostasis and Cell Transport
 Unit 3 : Homeostasis and Cell Transport A. Maintaining Homeostasis 1. Homeostasis process of regulating and maintaining a constant internal env. 2. Need to maintain homeostasis of water levels, glucose
Unit 3 : Homeostasis and Cell Transport A. Maintaining Homeostasis 1. Homeostasis process of regulating and maintaining a constant internal env. 2. Need to maintain homeostasis of water levels, glucose
Know your Ingredients: Triclosan, SD Alcohol 40, Propylene Glycol, Propylene Glycol, Tetrasodium EDTA, D&C Green 5, Sodium Stearate, and water.
 Urinary System There are four eliminative channels comprised of organs which help keep our bodies free from toxins, the intestinal, respiratory, urinary and integumentary systems are all responsible for
Urinary System There are four eliminative channels comprised of organs which help keep our bodies free from toxins, the intestinal, respiratory, urinary and integumentary systems are all responsible for
The Mechanics of the Dental Anesthetic Cartridge
 The Mechanics of the Dental Anesthetic Cartridge Sponsored by Pierrel, makers of Orabloc (Articaine hydrochloridre 40mg/ml and epinephrine Injection) Abstract Local anesthesia is the fundamental part of
The Mechanics of the Dental Anesthetic Cartridge Sponsored by Pierrel, makers of Orabloc (Articaine hydrochloridre 40mg/ml and epinephrine Injection) Abstract Local anesthesia is the fundamental part of
Contents. Introduction
 Introduction Contents Classification of Monophasic liquid Formulation Consideration Manufacturing Consideration Recent advance in monophasic liquid Recent approach in monophasic liquid formulation Conclusion
Introduction Contents Classification of Monophasic liquid Formulation Consideration Manufacturing Consideration Recent advance in monophasic liquid Recent approach in monophasic liquid formulation Conclusion
Excretion and Water Balance
 Excretion and Water Balance In the body, water is found in three areas, or compartments: Plasma, the liquid portion of the blood without the blood cells, makes up about 7 percent of body fluid. The intercellular
Excretion and Water Balance In the body, water is found in three areas, or compartments: Plasma, the liquid portion of the blood without the blood cells, makes up about 7 percent of body fluid. The intercellular
Paper No.: 13 Paper Title: Food Additives Module 2. Functional Classification of Food Additives
 Paper No.: 13 Paper Title: Food Additives Module 2. Functional Classification of Food Additives 2.1 Introduction According to the Food Protection Committee of the Food and Nutrition Board, food additives
Paper No.: 13 Paper Title: Food Additives Module 2. Functional Classification of Food Additives 2.1 Introduction According to the Food Protection Committee of the Food and Nutrition Board, food additives
FORMULATION CHOICE. How and why they are chosen. Dr Andy Fowles On behalf of ECPA Specification Expert Group
 FORMULATION CHOICE How and why they are chosen Dr Andy Fowles On behalf of ECPA Specification Expert Group Topics Why formulate? How to identify formulation options Drivers Principle formulation type overview
FORMULATION CHOICE How and why they are chosen Dr Andy Fowles On behalf of ECPA Specification Expert Group Topics Why formulate? How to identify formulation options Drivers Principle formulation type overview
SOFT GELATIN CAPSULE. Dr. Nawal Ayash
 SOFT GELATIN CAPSULE Dr. Nawal Ayash 1 Soft gelatin capsule DEFINITION:- soft Gelatin capsules are one piece, hermetically sealed, soft gelatin shells containing a liquid, a suspension, or a semisolid.
SOFT GELATIN CAPSULE Dr. Nawal Ayash 1 Soft gelatin capsule DEFINITION:- soft Gelatin capsules are one piece, hermetically sealed, soft gelatin shells containing a liquid, a suspension, or a semisolid.
METOLOSE: CONTENTS PAGE
 METOLOSE: CONTENTS PAGE 2 Preface What is Metolose Substitution types Specifications 1) Available grades & viscosity 2) Nomenclature 3) Packaging Characteristics of Metolose Properties of Metolose 1) Powder
METOLOSE: CONTENTS PAGE 2 Preface What is Metolose Substitution types Specifications 1) Available grades & viscosity 2) Nomenclature 3) Packaging Characteristics of Metolose Properties of Metolose 1) Powder
BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes
 BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes The cell membrane is the gateway into the cell, and must allow needed things such as nutrients into the cell without letting them escape.
BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes The cell membrane is the gateway into the cell, and must allow needed things such as nutrients into the cell without letting them escape.
Chapter 5: Analysis of water content, total solids & water activity
 Chapter 5: Analysis of water content, total solids & water activity 1 Water is an essential constituent of many foods. It may occur as an intracellular or extracellular component in vegetable and animal
Chapter 5: Analysis of water content, total solids & water activity 1 Water is an essential constituent of many foods. It may occur as an intracellular or extracellular component in vegetable and animal
BRIEFING. Nonharmonized attributes: Identification, Heavy metals, Characters, Labeling, Bacterial endotoxins, Sterility, Storage.
 BRIEFING Citric Acid, Anhydrous, page 872 of PF 28(3) [May June 2002]. The European Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the compendial standards for the
BRIEFING Citric Acid, Anhydrous, page 872 of PF 28(3) [May June 2002]. The European Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the compendial standards for the
Innovations in Design: NIA-West. Missy Lowery, MSc Head of Integrated Marketing Capsugel, now a Lonza company 11/13/2017
 Innovations in Design: NIA-West Missy Lowery, MSc Head of Integrated Marketing Capsugel, now a Lonza company 11/13/2017 1 Innovations in Design: How Do You Stand Out? If all other competitors are the same
Innovations in Design: NIA-West Missy Lowery, MSc Head of Integrated Marketing Capsugel, now a Lonza company 11/13/2017 1 Innovations in Design: How Do You Stand Out? If all other competitors are the same
FIGURE A. The phosphate end of the molecule is polar (charged) and hydrophilic (attracted to water).
 PLASMA MEMBRANE 1. The plasma membrane is the outermost part of a cell. 2. The main component of the plasma membrane is phospholipids. FIGURE 2.18 A. The phosphate end of the molecule is polar (charged)
PLASMA MEMBRANE 1. The plasma membrane is the outermost part of a cell. 2. The main component of the plasma membrane is phospholipids. FIGURE 2.18 A. The phosphate end of the molecule is polar (charged)
Body Water ANS 215 Physiology and Anatomy of Domesticated Animals
 Body Water ANS 215 Physiology and Anatomy of Domesticated Animals I. Body Water A. Water is the most abundant constituent comprising 60% of total body weight. 1. Solvent for many chemicals of the body
Body Water ANS 215 Physiology and Anatomy of Domesticated Animals I. Body Water A. Water is the most abundant constituent comprising 60% of total body weight. 1. Solvent for many chemicals of the body
Unit 1 Matter & Energy for Life
 Unit 1 Matter & Energy for Life Chapter 2 Interaction of Cell Structure Biology 2201 Primary Membrane Function: Homeostasis Conditions in the cell must remain more or less constant under many different
Unit 1 Matter & Energy for Life Chapter 2 Interaction of Cell Structure Biology 2201 Primary Membrane Function: Homeostasis Conditions in the cell must remain more or less constant under many different
Antioxidants. An antioxidant is a molecule that inhibits the oxidation of other molecules.
 1 2 3 4 Antioxidants An agents which inhibit oxidation and are commonly used to prevent rancidity of oils and fats or deterioration of other materials through oxidative process. Ascorbic acid, Butylated
1 2 3 4 Antioxidants An agents which inhibit oxidation and are commonly used to prevent rancidity of oils and fats or deterioration of other materials through oxidative process. Ascorbic acid, Butylated
1.14. Passive Transport
 Passive Transport 1.14 Simple Diffusion Cell s are selectively permeable only certain substances are able to pass through them. As mentioned in section 1.2, cell s are largely composed of a phospholipid
Passive Transport 1.14 Simple Diffusion Cell s are selectively permeable only certain substances are able to pass through them. As mentioned in section 1.2, cell s are largely composed of a phospholipid
Suppositories. Suppositories: 08/10/2016
 Suppositories Suppositories: Suppositories are solid dosage forms intended for insertion into body orifices where they melt, soften, or dissolve and exert localized or systemic effects. A suppositories
Suppositories Suppositories: Suppositories are solid dosage forms intended for insertion into body orifices where they melt, soften, or dissolve and exert localized or systemic effects. A suppositories
July 2010 Abridged version, MAIL PO Box Seattle, WA USA. STREET 2201 Westlake Avenue, Suite 200 Seattle, WA USA
 A HealthTech Report Stability data on chlorhexidine formulations: PATH summary July 2010 Abridged version, 2012 MAIL PO Box 900922 Seattle, WA 98109 USA STREET 2201 Westlake Avenue, Suite 200 Seattle,
A HealthTech Report Stability data on chlorhexidine formulations: PATH summary July 2010 Abridged version, 2012 MAIL PO Box 900922 Seattle, WA 98109 USA STREET 2201 Westlake Avenue, Suite 200 Seattle,
UNIT 2 DIABETES REVIEW
 UNIT 2 DIABETES REVIEW Pancreas is unable to make insulin. Therefore, glucose cannot get into the cells for energy. Insulin is made, but cell receptors do not work at getting recognizing that insulin.
UNIT 2 DIABETES REVIEW Pancreas is unable to make insulin. Therefore, glucose cannot get into the cells for energy. Insulin is made, but cell receptors do not work at getting recognizing that insulin.
Fats and oils. Three fatty acids combine with one glycerol to form a triglyceride Fat found in foods is made up of triglycerides Fat
 Fats and oils Lipids is a general term for both fats and oils Fats are lipids that are solid at room temperature while oils are lipids that are liquid at room temperature Fats and oils are made up of carbon,
Fats and oils Lipids is a general term for both fats and oils Fats are lipids that are solid at room temperature while oils are lipids that are liquid at room temperature Fats and oils are made up of carbon,
Each cell has its own border, which separates the cell from its surroundings and also determines what comes in and what goes out.
 7.3 Cell Transport Wednesday, December 26, 2012 10:02 AM Vocabulary: Diffusion: process in which cells become specialized in structure and function Facilitated diffusion: process of diffusion in which
7.3 Cell Transport Wednesday, December 26, 2012 10:02 AM Vocabulary: Diffusion: process in which cells become specialized in structure and function Facilitated diffusion: process of diffusion in which
LAB 4: OSMOSIS AND DIFFUSION
 Page 4.1 LAB 4: OSMOSIS AND DIFFUSION Cells need to obtain water and other particles from the fluids that surround them. Water and other particles also move out of cells. Osmosis (for water) and diffusion
Page 4.1 LAB 4: OSMOSIS AND DIFFUSION Cells need to obtain water and other particles from the fluids that surround them. Water and other particles also move out of cells. Osmosis (for water) and diffusion
Diffusion & Osmosis - Exercise 4
 Diffusion & Osmosis - Exercise 4 Objectives -Define: Solvent, Solute, and Solution -Define: Diffusion, Selectively permeable membrane, Osmosis, and Dialysis -Understand rule of thumb: Concentration will
Diffusion & Osmosis - Exercise 4 Objectives -Define: Solvent, Solute, and Solution -Define: Diffusion, Selectively permeable membrane, Osmosis, and Dialysis -Understand rule of thumb: Concentration will
Diffusion, osmosis, transport mechanisms 43
 Diffusion, osmosis, transport mechanisms 43 DIFFUSION, OSMOSIS AND TRANSPORT MECHANISMS The cell membrane is a biological membrane that separates the interior of all cells from the outside environment
Diffusion, osmosis, transport mechanisms 43 DIFFUSION, OSMOSIS AND TRANSPORT MECHANISMS The cell membrane is a biological membrane that separates the interior of all cells from the outside environment
Body Fluids and Fluid Compartments
 Body Fluids and Fluid Compartments Bởi: OpenStaxCollege The chemical reactions of life take place in aqueous solutions. The dissolved substances in a solution are called solutes. In the human body, solutes
Body Fluids and Fluid Compartments Bởi: OpenStaxCollege The chemical reactions of life take place in aqueous solutions. The dissolved substances in a solution are called solutes. In the human body, solutes
Plasma Membrane Function
 Plasma Membrane Function Cells have to maintain homeostasis, they do this by controlling what moves across their membranes Structure Double Layer of phospholipids Head (polar) hydrophiliclikes water -
Plasma Membrane Function Cells have to maintain homeostasis, they do this by controlling what moves across their membranes Structure Double Layer of phospholipids Head (polar) hydrophiliclikes water -
DRY SYRUPS SWAPNA.M. Ist semester DEPARTMENT OF PHARMACEUTICS UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES KAKATIYA UNIVERSITY, WARANGAL SEMINAR BY
 DRY SYRUPS SEMINAR BY SWAPNA.M M.PHARMACY Ist semester DEPARTMENT OF PHARMACEUTICS UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES KAKATIYA UNIVERSITY, WARANGAL CONTENTS DEFINITION CHARACTERISTICS OF SUSPENSIONS
DRY SYRUPS SEMINAR BY SWAPNA.M M.PHARMACY Ist semester DEPARTMENT OF PHARMACEUTICS UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES KAKATIYA UNIVERSITY, WARANGAL CONTENTS DEFINITION CHARACTERISTICS OF SUSPENSIONS
Enhanced delivery methods for greater efficacy
 On-Line Formulation Training - Anywhere In The World - Enhanced delivery methods for greater efficacy Belinda Carli Director, Institute of Personal Care Science Image showing absorbance in the outer stratum
On-Line Formulation Training - Anywhere In The World - Enhanced delivery methods for greater efficacy Belinda Carli Director, Institute of Personal Care Science Image showing absorbance in the outer stratum
ONLINE HEMODIALYSIS TRAINING SESSION 1
 ONLINE HEMODIALYSIS TRAINING SESSION 1 This document is a supplement to the Online Training. Do not reproduce. Copyright Dialysis4Career. All Rights Reserved. The Renal System - A highly sophisticated
ONLINE HEMODIALYSIS TRAINING SESSION 1 This document is a supplement to the Online Training. Do not reproduce. Copyright Dialysis4Career. All Rights Reserved. The Renal System - A highly sophisticated
Excretion (IGCSE Biology Syllabus )
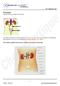 Excretion (IGCSE Biology Syllabus 2016-2018) Structure of the Kidney Excretion is the removal from organisms of toxic materials, the waste products of metabolism and substances in excess of requirements
Excretion (IGCSE Biology Syllabus 2016-2018) Structure of the Kidney Excretion is the removal from organisms of toxic materials, the waste products of metabolism and substances in excess of requirements
BIOCHEMISTRY OF BLOOD
 BCH 471 BIOCHEMISTRY OF BLOOD Amal Alamri Experiment 1 Separation of Plasma and Serum from Whole Blood Whole Blood It is living tissue that circulates through the heart, arteries, veins, and capillaries
BCH 471 BIOCHEMISTRY OF BLOOD Amal Alamri Experiment 1 Separation of Plasma and Serum from Whole Blood Whole Blood It is living tissue that circulates through the heart, arteries, veins, and capillaries
INTERMEDIATE 1 1 Food and Diet. These elements are present in compounds - not as free elements.
 INTERMEDIATE 1 1 Food and Diet FOOD AND DIET The main elements present in the human body are: Hydrogen Oxygen Nitrogen Carbon These elements are present in compounds - not as free elements. Unlike plants,
INTERMEDIATE 1 1 Food and Diet FOOD AND DIET The main elements present in the human body are: Hydrogen Oxygen Nitrogen Carbon These elements are present in compounds - not as free elements. Unlike plants,
EXCRETION QUESTIONS. Use the following information to answer the next two questions.
 EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
UNIVERSITY OF MEDICAL SCIENCES, ONDO DEPARTMENT OF PHYSIOLOGY PHS 211 TRANSPORT MECHANISM LECTURER: MR A.O. AKINOLA
 UNIVERSITY OF MEDICAL SCIENCES, ONDO DEPARTMENT OF PHYSIOLOGY PHS 211 TRANSPORT MECHANISM LECTURER: MR A.O. AKINOLA OUTLINE Introduction Basic mechanisms Passive transport Active transport INTRODUCTION
UNIVERSITY OF MEDICAL SCIENCES, ONDO DEPARTMENT OF PHYSIOLOGY PHS 211 TRANSPORT MECHANISM LECTURER: MR A.O. AKINOLA OUTLINE Introduction Basic mechanisms Passive transport Active transport INTRODUCTION
Practical 3 Extemporaneous Preparations Solutions. Name: Group: Date:
 Practical 3 Extemporaneous Preparations Solutions Name: Group: Date: FILTRATION Unwanted particulate matter may be removed from solutions by the process of filtration. Filtration may be either fine or
Practical 3 Extemporaneous Preparations Solutions Name: Group: Date: FILTRATION Unwanted particulate matter may be removed from solutions by the process of filtration. Filtration may be either fine or
Inorganic pharmaceutical chemistry. Replacement Therapy Lec 2
 Inorganic pharmaceutical chemistry Replacement Therapy Lec 2 Replacement Therapy The basic objective of replacement therapy is to restore the volume and composition of the body fluids to normal one. Volume
Inorganic pharmaceutical chemistry Replacement Therapy Lec 2 Replacement Therapy The basic objective of replacement therapy is to restore the volume and composition of the body fluids to normal one. Volume
MONOGRAPHS (NF) Pharmacopeial Forum 616 HARMONIZATION Vol. 31(2) [Mar. Apr. 2005]
![MONOGRAPHS (NF) Pharmacopeial Forum 616 HARMONIZATION Vol. 31(2) [Mar. Apr. 2005] MONOGRAPHS (NF) Pharmacopeial Forum 616 HARMONIZATION Vol. 31(2) [Mar. Apr. 2005]](/thumbs/86/93540561.jpg) 616 HARMONIZATION Vol. 31(2) [Mar. Apr. 2005] the recorder. The substances are eluted in the following order: o-toluenesulfonamide, p-toluenesulfonamide, and caffeine. The test is not valid unless the
616 HARMONIZATION Vol. 31(2) [Mar. Apr. 2005] the recorder. The substances are eluted in the following order: o-toluenesulfonamide, p-toluenesulfonamide, and caffeine. The test is not valid unless the
The Plasma Membrane - Gateway to the Cell
 The Plasma Membrane - Gateway to the Cell 1 Photograph of a Cell Membrane 2 Cell Membrane The cell membrane is flexible and allows a unicellular organism to move 3 Homeostasis Balanced internal condition
The Plasma Membrane - Gateway to the Cell 1 Photograph of a Cell Membrane 2 Cell Membrane The cell membrane is flexible and allows a unicellular organism to move 3 Homeostasis Balanced internal condition
30.1 Organization of the Human Body
 30.1 Organization of the Human Body Organization of the Body The levels of organization in the body include cells, tissues, organs, and organ systems. At each level of organization, these parts of the
30.1 Organization of the Human Body Organization of the Body The levels of organization in the body include cells, tissues, organs, and organ systems. At each level of organization, these parts of the
Normosol -R and 5% Dextrose Injection MULTIPLE ELECTROLYTES AND 5% DEXTROSE INJECTION TYPE 1, USP For Replacing Acute Losses of Extracellular Fluid
 Normosol -R and 5% Dextrose Injection MULTIPLE ELECTROLYTES AND 5% DEXTROSE INJECTION TYPE 1, USP For Replacing Acute Losses of Extracellular Fluid R x only Flexible Plastic Container DESCRIPTION Normosol-R
Normosol -R and 5% Dextrose Injection MULTIPLE ELECTROLYTES AND 5% DEXTROSE INJECTION TYPE 1, USP For Replacing Acute Losses of Extracellular Fluid R x only Flexible Plastic Container DESCRIPTION Normosol-R
Written Response #1: True/False
 Written Response #1: True/False 1. Osmosis means to absorb something. 2. Cells are able to excrete waste. 3. Cells obtain energy by gaining nutrition from food. 4. Plants use sunlight for food. 5. Plants
Written Response #1: True/False 1. Osmosis means to absorb something. 2. Cells are able to excrete waste. 3. Cells obtain energy by gaining nutrition from food. 4. Plants use sunlight for food. 5. Plants
Experiment 2: Melting Points and the Identification of an Unknown and Cholesterol from Human Gallstones
 1 Experiment 2: Melting Points and the Identification of an Unknown and Cholesterol from Human Gallstones Part 1. Melting Points and the Identification of an Unknown Read pp 211-220, Chapter 14, (especially
1 Experiment 2: Melting Points and the Identification of an Unknown and Cholesterol from Human Gallstones Part 1. Melting Points and the Identification of an Unknown Read pp 211-220, Chapter 14, (especially
Dairy Update. finnesota EXTENSION SERVICE UNIVERSITY OF MINNESOTA ANIMAL SCIENCE EXTENSION. Issue 112 November, 1992
 , finnesota EXTENSION SERVICE UNIVERSITY OF MINNESOTA ANIMAL SCIENCE EXTENSION Department of Animal Science 101 Haecker Hall 1364 Eckles Avenue St. Paul, Minnesota 55108 (612) 624 4995 FAX: (612) 625 1283
, finnesota EXTENSION SERVICE UNIVERSITY OF MINNESOTA ANIMAL SCIENCE EXTENSION Department of Animal Science 101 Haecker Hall 1364 Eckles Avenue St. Paul, Minnesota 55108 (612) 624 4995 FAX: (612) 625 1283
Stability of micronutrients in PN what is known and what can be claimed? Prof. Mike.Allwood Pharmacy Consultant
 Stability of micronutrients in PN what is known and what can be claimed? Prof. Mike.Allwood Pharmacy Consultant Pharmaceutical interactions 1. Physical incompatibility = precipitation Caused by: Certain
Stability of micronutrients in PN what is known and what can be claimed? Prof. Mike.Allwood Pharmacy Consultant Pharmaceutical interactions 1. Physical incompatibility = precipitation Caused by: Certain
Reference ID:
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PRISMASOL and PHOXILLUM safely and effectively. See full prescribing information for PRISMASOL and
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PRISMASOL and PHOXILLUM safely and effectively. See full prescribing information for PRISMASOL and
Lesson Overview. 7.3 Cell Transport
 7.3 THINK ABOUT IT When thinking about how cells move materials in and out, it can be helpful to think of a cell as a nation. The boundaries of a nation are its borders, and nearly every country tries
7.3 THINK ABOUT IT When thinking about how cells move materials in and out, it can be helpful to think of a cell as a nation. The boundaries of a nation are its borders, and nearly every country tries
Pectins. Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82 nd meeting 2016
 Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82 nd meeting 2016 Pectins This monograph was also published in: Compendium of Food Additive Specifications.
Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82 nd meeting 2016 Pectins This monograph was also published in: Compendium of Food Additive Specifications.
PHARMACEUTICS I صيدالنيات 1 UNIT 1 INTRODUCTION
 PHARMACEUTICS I صيدالنيات 1 UNIT 1 INTRODUCTION 1 PHARMACEUTICS Pharmaceutics is the science of dosage form design. The general area of study concerned with the formulation, manufacture, stability, and
PHARMACEUTICS I صيدالنيات 1 UNIT 1 INTRODUCTION 1 PHARMACEUTICS Pharmaceutics is the science of dosage form design. The general area of study concerned with the formulation, manufacture, stability, and
LAB.2. Tablet Production Methods
 LAB.2 Tablet Production Methods Dry methods Direct compression Dry granulation Wet methods Wet granulation Regardless whether tablets are made by direct compression or granulation, the first step, milling
LAB.2 Tablet Production Methods Dry methods Direct compression Dry granulation Wet methods Wet granulation Regardless whether tablets are made by direct compression or granulation, the first step, milling
*Sections or subsections omitted from the full prescribing information are not 6 ADVERSE REACTIONS
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PRISMASOL and PHOXILLUM safely and effectively. See full prescribing information for PRISMASOL and
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PRISMASOL and PHOXILLUM safely and effectively. See full prescribing information for PRISMASOL and
Lab 4: Osmosis and Diffusion
 Page 4.1 Lab 4: Osmosis and Diffusion Cells need to obtain water and other particles from the fluids that surround them. Water and other particles also move out of cells. Osmosis (for water) and diffusion
Page 4.1 Lab 4: Osmosis and Diffusion Cells need to obtain water and other particles from the fluids that surround them. Water and other particles also move out of cells. Osmosis (for water) and diffusion
Mixed solvent systems;; spirits, and elixirs
 Mixed solvent systems;; spirits, and elixirs By Dr. Mohammed Sattar 2016/2017 1 Outlines Ø Elixirs Ø Applications Ø Formulation Ø Spirits Ø Applications Ø Formulation 2 1 Non aqueous systems it may be
Mixed solvent systems;; spirits, and elixirs By Dr. Mohammed Sattar 2016/2017 1 Outlines Ø Elixirs Ø Applications Ø Formulation Ø Spirits Ø Applications Ø Formulation 2 1 Non aqueous systems it may be
Chapter MEMBRANE TRANSPORT
 Chapter 3 I MEMBRANE TRANSPORT The cell membrane, or plasma membrane, is the outermost layer of the cell. It completely surrounds the protoplasm or living portion of the cell, separating the cell s interior
Chapter 3 I MEMBRANE TRANSPORT The cell membrane, or plasma membrane, is the outermost layer of the cell. It completely surrounds the protoplasm or living portion of the cell, separating the cell s interior
