Westminster Hospital Medical School.) connection with the general problem of blood clotting, we have investigated. (Received June 18, 1935.
|
|
|
- Jasmine Simmons
- 5 years ago
- Views:
Transcription
1 173 6I2. II5.3 CHANGES IN THE COAGULABILITY OF THE BLOOD PRODUCED BY CITRIC ACID AND SOME OF ITS DECOMPOSITION PRODUCTS. BY DAVID DE SOUZA AND F. D. M. HOCKING. (From the John Burford Carlill Laboratories, Westminster Hospital Medical School.) (Received June 18, 1935.) FROM a survey of the literature relating to the production of hypercoagulability of the blood by the injection of citrates, it appears that the results have been obtained from experiments with sodium citrate. Neither citric acid nor any other of its compounds seems to have been much used. As a knowledge of the action of the citric acid molecule and its decomposition products might be of value not only per se but also in connection with the general problem of blood clotting, we have investigated the effects of the intramuscular injection of some of these substances on the alkali reserve and the coagulability of the blood. METHODS. Cats, anaesthetized by intraperitoneal injection of nembutal, 60 mg. per kg. body weight, were the subjects of the experiments. The substances to be tested were given by injection into the muscles. The methods of carrying out the experiments and the estimations have been described in detail in a previous paper [de Souza and Hocking, 1934]. RESULTS. In the first place it was necessary to find out whether the changes produced in the blood by intramuscular injection of sodium citrate were due entirely to the citric acid molecule or were influenced by the presence of sodium in it. For this purpose a series of experiments was carried out, in which citric acid was injected instead of sodium citrate. A single injection of citric acid, g. per kg. body weight, was given, that
2 174 D. DE SOUZA AND F. D. M. HOCKING. being the amount equivalent to sodium citrate g. per kg. body weight used in the previous investigation. A typical result is shown in Exp. 1. Exp. 1. Intramuscular injection of citric acid. Alkali reserve Coagulation time There was an increase in the alkali reserve from 56 to a maximum of 61 in 25 min., and a diminution in the coagulation time from 3X5 to a minimum of 2X8 in 15 min., with an indicated return towards the control in the former case and an almost complete return in the latter, 45 min. after the injection. The changes are much smaller than those with sodium citrate but are in the same direction. While the citric acid undergoes a certain amount of oxidation, it is evident that the presence of sodium in the citrate molecule leads to a greater increase in the alkali reserve, and this may account for the larger effect of the sodium citrate on coagulability. The action of ammonium chloride in diminishing the alkali reserve and increasing the coagulation time has already been described by us [1934]. Prof. Lovatt Evans suggested that it would be of interest to compare with this the action of ammonium citrate. This has been done in experiments on the same plan as the preceding. The amount of ammonium citrate injected was 0'118 g. per kg. body weight. Exp. 2 is an example. Bxp. 2. Intramuscular injection of ammonium citrate. Time after injection Alkali reserve Coagulation time * The alkali reserve increased from 52 to a maximum of 59 in 25 min. and remained practically at that level for the next 20 min. The coagulation time fell from 3x8 to a minimum of 2x5 in 15 min., returning to 3*3 in the next 10 min. and remaining there for the rest of the experiment. The result is like that from citric acid. The ammonia seems to have been removed from the citrate, perhaps to take part in the formation of urea, leaving the citric acid which has been oxidized to some extent.
3 CITRIC ACID AND BLOOD CLOTTING. Attention was directed next to some of the decomposition products of citric acid. At present no evidence is available as to which, if any, of those examined are formed from the breakdown of citric acid in the body. The action of acetone-dicarboxylic acid (CH2COOH.CO.CH2COOH) was first studied. Added to blood in a paraffined test-tube it converts the hemoglobin into acid haematin and acid hamatoporphyrin and causes the formation of a gel, which looks somewhat granular and does not undergo syneresis. Its sodium compound, formed by dissolving the acid in the calculated amount of NaOH solution, does not affect the haemoglobin and has been found to prevent the clotting of blood up to 48 hours. In the injection experiments the quantity of acetone-dicarboxylic acid put into the muscle was 0 07 g. per kg. body weight, that being the amount which would be obtained from g. of citric acid, equivalent to g. of sodium citrate taken as a standard. When the sodium compound was used it was freshly prepared from this amount of acetonedicarboxylic acid. Exp. 3. Exp. 3 shows the effects of the acid. Intramuscular injection of acetone-dicarboxylic acid. Alkali reserve Coagulation time 3.3 1F * The alkali reserve was not affected. The coagulation time diminished from 3.3 to a minimum of 1-8 in 5 min. then rose to 3 0 in the next 20 min. and remained near that value for the rest of the experiment. This kind of result was obtained in three experiments, but in one other the coagulation time increased after the injection from 3.3 to 4-5 in 15 min. and kept about that level for the next 20 min. It is notable that in this case the addition of the acid to the blood in vitro did not produce the usual granular-looking gel. Instead of this, the blood became thick and treacly and was still in this condition after 48 hours. Exp. 4 gives the type of result whenthe sodium compound was injected. Exp. 4. Intramuscular injection of sodium acetone-dicarboxylate. Alkali reserve Coagulation time
4 176 D. DE SOUZA AND F. D. M. HOCKING. Here there was an increase in the alkali reserve from 55 to 60 in 15 min., showing a tendency to rise slightly in the next 30 min. The coagulation time diminished in 5 mi. from 4*0 to 2-8 and rose again to 4*0 in the next 10 min., varying a little round 3-8 for the remaining 30 min. The lessening of the coagulation time seems always to occur in the first 5 min., with a return to the control value 10 min. later. As acetone is a product of the breakdown of acetone-dicarboxylic acid, a series of similar experiments was carried out with it. The amount injected was 0x125 g. per kg. body weight, about 4x5 times the quantity equivalent to g. of sodium citrate. There was no effect on the alkali reserve or on the coagulation time. The other decomposition product of citric acid examined was aconitic acid (CH2COOH.C.COOH:CHCOOH). The addition of this to blood in a paraffined test-tube leads to the formation of a granularlooking gel which resembles that due to acetone dicarboxylic acid and does not undergo syneresis. The haemoglobin is converted into acid hiematin. The sodium compound prevents coagulation and does not change the hiemoglobin. As the quantity of the acid corresponding to g. of sodium citrate is 0-08 g., that was the amount per kg. body weight injected into the muscles. Exp. 5 shows the result of an injection. Exp. 5. Intramuscular injection of aconitic acid. Time after injection Alkali reserve Coagulation time *8 There was practically no change in the alkali reserve. The coagulation time diminished from 3 0 to 2-3 in 5 min., then rose above the control level to 3-5 in the next 10 min., keeping about 3-8 for the rest of the time. In every experiment in which aconitic acid was injected the clotting time eventually exceeded the control. Sometimes it did so in the first 5 min., at others there was a previous diminution, as in Exp. 5. The sodium compound was freshly prepared from aconitic acid in the same way as sodium acetone-dicarboxylate from its acid. The results of its injection were very consistent and are seen in Exp. 6. Exp. 6. Intramuscular injection of sodium aconitate. Alkali reserve Coagulation time *5 2-5
5 CITRIC ACID AND BLOOD CLOTTING. 177 Time is expressed in minutes, the alkali reserve in c.c. of CO2 per The result is like that of injecting sodium citrate. The alkali reserve rose from 47 to 59 in 35 min., and the clotting time fell from 3-5 to 1-5 in 15 min., rising again to 2*5 in the next 10 min. DISCUSSION. In discussing the production of hypercoagulability of the blood by intramuscular injection of sodium citrate de Souza and Hocking [1934] pointed out that, while it might be due in part to the increase in the alkali reserve, there was some other factor besides this, possibly a small amount of citrate absorbed unchanged and circulating in the blood. The likelihood of its being a decomposition product of the citrate molecule was borne in mind and is strengthened by the experiments described here. Citric acid, as shown in Exp. 1, increases the alkali reserve and the coagulability of the blood, but not to the same extent as sodium citrate. The difference must be due to the presence of sodium in the citrate molecule, which thus supplies its own base for the formation of carbonate, whereas in the oxidation of citric acid to carbonate, base has to be obtained from the body. The greater rise in the alkali reserve accounts for the greater effect on coagulability. Ammonium citrate acts like citric acid (Exp. 2). As in the case of ammonium chloride, the ammonia is removed and perhaps goes to form urea, but here the alkali reserve is not diminished but increased, as the citric acid moiety is oxidized with the formation of carbonate. With regard to the decomposition products of citric acid no evidence has been found as to which are formed when citric acid is broken down in the body. Those studied here, acetone-dicarboxylic acid and aconitic acid, are known to be derived from citric acid when it is heated or acted upon by sulphuric acid. When injected into the muscles neither of them affects the alkali reserve. Acetone-dicarboxylic acid, however, may increase the coagulability of the blood as in Exp. 3, or in exceptional circumstances diminish it. Aconitic acid always diminishes the coagulability, sometimes after first increasing it, as in Exp. 5. The effects of the presence of sodium in the molecule are shown by the injection of the sodium compounds of the acids, which always cause a rise in the alkali reserve and an increase in the coagulability of the blood as in Exps. 4 and 6.
6 178 D. DE SOUZA AND F. D. M. HOCKING. The interesting fact emerges that these two acids, obtained from the breakdown of citric acid, can alter the coagulability of the blood without affecting the alkali reserve. The further decomposition product, acetone, in the amount injected in these experiments, has no effect on either. SUMMARY. 1. Intramuscular injections of citric acid or ammonium citrate increase the alkali reserve and the coagulability of the blood. 2. Intramuscular injections of acetone-dicarboxylic acid or aconitic acid do not affect the alkali reserve, but may increase or diminish coagulability. 3. Intramuscular injections of sodium acetone-dicarboxylate or sodium aconitate increase the alkali reserve and the coagulability of the blood. 4. Intramuscular injections of acetone have no effect on alkali reserve or coagulability. REFERENCE. de Souza, D. and Hocking. F. D. M. (1934). J. Phy8iol. 83, 49.
establishing perfusion and of collecting and analysing the effluent fluid 1934]. Comparable increases in serum potassium were obtained when
![establishing perfusion and of collecting and analysing the effluent fluid 1934]. Comparable increases in serum potassium were obtained when establishing perfusion and of collecting and analysing the effluent fluid 1934]. Comparable increases in serum potassium were obtained when](/thumbs/95/123294922.jpg) 303 577.I74.5:612.I26 ACTION OF ADRENALINE ON THE SERUM POTASSIUM BY J. L. D'SILVA From the Department of Physiology, King's College, London (Received 24 March 1937) IN a previous communication it was
303 577.I74.5:612.I26 ACTION OF ADRENALINE ON THE SERUM POTASSIUM BY J. L. D'SILVA From the Department of Physiology, King's College, London (Received 24 March 1937) IN a previous communication it was
XXVI. STUDIES ON THE INTERACTION. OF AMINO-COMPOUNDS AND CARBOHYDRATES.
 XXVI. STUDIES ON THE INTERACTION. OF AMINO-COMPOUNDS AND CARBOHYDRATES. II. THE PREPARATION OF GLUCOSE UREIDE. BY ALEXANDER HYND. From the Department of Physiology, University of St Andrews. (Received
XXVI. STUDIES ON THE INTERACTION. OF AMINO-COMPOUNDS AND CARBOHYDRATES. II. THE PREPARATION OF GLUCOSE UREIDE. BY ALEXANDER HYND. From the Department of Physiology, University of St Andrews. (Received
clotting for a given time. This was found to be 57-1 mg. p.c.: Exp. 1 a. Ammon. ox. mg. p.c
 THE COAGULATION OF THE BLOOD. Part I. The Role of Calcium. BY H. W. C. VINES, M.B., Beit Memorial Fellow. IN all theories of coagulation the presence of calcium salts has been recognised as an essential
THE COAGULATION OF THE BLOOD. Part I. The Role of Calcium. BY H. W. C. VINES, M.B., Beit Memorial Fellow. IN all theories of coagulation the presence of calcium salts has been recognised as an essential
excreted, in spite of its constant presence in the blood. Similarly, a salt-free diet will rapidly cause the practical disappearance of chlorides
 THE REGULATION OF EXCRETION OF WATER BY THE KIDNEYS. I. By J. S. HALDANE, M.D., F.R.S. AND J. G. PRIESTLEY, B.M., Captain R.A.M.C., Beit Memorial Research Fellow. NUMEROUS observations tend to show that
THE REGULATION OF EXCRETION OF WATER BY THE KIDNEYS. I. By J. S. HALDANE, M.D., F.R.S. AND J. G. PRIESTLEY, B.M., Captain R.A.M.C., Beit Memorial Research Fellow. NUMEROUS observations tend to show that
15. Mixed fertilizers sources preparations- their compatibility advantages
 15. Mixed fertilizers sources preparations- their compatibility advantages Mixed fertilizers For over hundred years the mixed fertilizers are in use besides straight fertilizers. Many fertilizer mixtures
15. Mixed fertilizers sources preparations- their compatibility advantages Mixed fertilizers For over hundred years the mixed fertilizers are in use besides straight fertilizers. Many fertilizer mixtures
following experiments were designed to show the effects of changes in CO2 combining power in the blood of dogs after the administration of acid
 OBSERVATIONS ON THE FORMATION OF WHEALS V. THE EFFECTS OF VARIATION OF THE CO2 COMBINING POWER OF THE BLOOD ON HISTAMINE WHEALS By F. S. McCONNELL, W. K. WEAVER AND H. L. ALEXANDER (From the Department
OBSERVATIONS ON THE FORMATION OF WHEALS V. THE EFFECTS OF VARIATION OF THE CO2 COMBINING POWER OF THE BLOOD ON HISTAMINE WHEALS By F. S. McCONNELL, W. K. WEAVER AND H. L. ALEXANDER (From the Department
THE EFFECT OF ANTICOAGULANTS ON DETERMINA- TIONS OF INORGANIC PHOSPHATE AND PROTEIN IN PLASMA BY OLIVER HENRY GAEBLER
 THE EFFECT OF ANTICOAGULANTS ON DETERMINA TIONS OF INORGANIC PHOSPHATE AND PROTEIN IN PLASMA BY OLIVER HENRY GAEBLER (From the Department of Laboratories, Henry Ford Hospital, Detroit) (Received for publication,
THE EFFECT OF ANTICOAGULANTS ON DETERMINA TIONS OF INORGANIC PHOSPHATE AND PROTEIN IN PLASMA BY OLIVER HENRY GAEBLER (From the Department of Laboratories, Henry Ford Hospital, Detroit) (Received for publication,
THE EQUILIBRIUM BETWEEN ACTIVE NATIVE TRYPSIN AND INACTIVE DENATURED TRYPSIN
 Published Online: 20 January, 1934 Supp Info: http://doi.org/10.1085/jgp.17.3.393 Downloaded from jgp.rupress.org on November 8, 2018 THE EQUILIBRIUM BETWEEN ACTIVE NATIVE TRYPSIN AND INACTIVE DENATURED
Published Online: 20 January, 1934 Supp Info: http://doi.org/10.1085/jgp.17.3.393 Downloaded from jgp.rupress.org on November 8, 2018 THE EQUILIBRIUM BETWEEN ACTIVE NATIVE TRYPSIN AND INACTIVE DENATURED
Purity Tests for Modified Starches
 Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82 nd meeting 2016 Purity Tests for Modified Starches This monograph was also published in: Compendium
Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82 nd meeting 2016 Purity Tests for Modified Starches This monograph was also published in: Compendium
Separation of Plasma and Serum and Their Proteins from Whole Blood
 Separation of Plasma and Serum and Their Proteins from Whole Blood BCH 471 [Practical] BLOOD COMPOSITION Other names to blood cells Red blood cells (erythrocytes) White blood cells (leukocytes) Platelets
Separation of Plasma and Serum and Their Proteins from Whole Blood BCH 471 [Practical] BLOOD COMPOSITION Other names to blood cells Red blood cells (erythrocytes) White blood cells (leukocytes) Platelets
2. 2,4 Dinitro phenyl hydrazine (DNPH): I mm in 1N HCl. 5. Working standard: 1 in 20 dilution of the stock standard.
 -1 Estimation of Alanine Transaminase (ALT) (Mohun and Cook, 1957) Reagents I. Buffered substrate: [100 mm phosphate buffer, 200mM DL-alanine; 2 mm 2-oxo glutarate.}- Dissolved 1.5 g di potassium hydrogen
-1 Estimation of Alanine Transaminase (ALT) (Mohun and Cook, 1957) Reagents I. Buffered substrate: [100 mm phosphate buffer, 200mM DL-alanine; 2 mm 2-oxo glutarate.}- Dissolved 1.5 g di potassium hydrogen
Ready reckoners for phosphorus fertilizers
 Ready reckoners for phosphorus s (by Dr. Jagdev Sharma, Senior Scientist (Soil Science) and Dr. A. K. Upadhyay, Senior Scientist (Soil Science) These ready reckoners have been prepared to help farmers/
Ready reckoners for phosphorus s (by Dr. Jagdev Sharma, Senior Scientist (Soil Science) and Dr. A. K. Upadhyay, Senior Scientist (Soil Science) These ready reckoners have been prepared to help farmers/
(ethanol) suggests that it is similar to the diuresis following ingestion of water.
 435 J. Physiol. (I946) I04, 435-442 6I2.464.I THE EFFECT OF ETHYL ALCOHOL AND SOME OTHER DIURETICS ON CHLORIDE EXCRETION IN MAN BY M. GRACE EGGLETON AND ISABEL G. SMITH, From the Physiology Department,
435 J. Physiol. (I946) I04, 435-442 6I2.464.I THE EFFECT OF ETHYL ALCOHOL AND SOME OTHER DIURETICS ON CHLORIDE EXCRETION IN MAN BY M. GRACE EGGLETON AND ISABEL G. SMITH, From the Physiology Department,
Nutrients. Chapter 25 Nutrition, Metabolism, Temperature Regulation
 Chapter 25 Nutrition, Metabolism, Temperature Regulation 25-1 Nutrients Chemicals used by body to produce energy, provide building blocks or function in other chemical reactions Classes Carbohydrates,
Chapter 25 Nutrition, Metabolism, Temperature Regulation 25-1 Nutrients Chemicals used by body to produce energy, provide building blocks or function in other chemical reactions Classes Carbohydrates,
NOTICE. Ref. No. BCKV-RNARC (Comp 4)/quotation 1/14-15 Date:
 Ref. No. BCKV-RNARC (Comp 4)/quotation 1/14-15 Date: 24.02.2015 NOTICE The following item with specification listed below is required for the research on Increasing use efficiency of nitrogen fertilizers.
Ref. No. BCKV-RNARC (Comp 4)/quotation 1/14-15 Date: 24.02.2015 NOTICE The following item with specification listed below is required for the research on Increasing use efficiency of nitrogen fertilizers.
SOME OBSERVATIONS UPON SODIUM ALGINATE. By 0. M. SOLANDT. From the Physiological Laboratory, Cambridge.
 582.6 SOME OBSERVATIONS UPON SODIUM ALGINATE. By 0. M. SOLANDT. From the Physiological Laboratory, Cambridge. (Received for publication 13th December 1940.) ALGINIC acid was discovered by Stanford in 1883
582.6 SOME OBSERVATIONS UPON SODIUM ALGINATE. By 0. M. SOLANDT. From the Physiological Laboratory, Cambridge. (Received for publication 13th December 1940.) ALGINIC acid was discovered by Stanford in 1883
FulvicForce offers an ideal replacement for. creatine. FulvicForce boosts immune system and fights. inflammation. FulvicForce is effective in weight
 FulvicForce fulvic acid in Sport and Training Research confirms that fulvic acid possesses truly remarkable biological, nutritional and remedial properties. It is these same properties, which make FulvicForce
FulvicForce fulvic acid in Sport and Training Research confirms that fulvic acid possesses truly remarkable biological, nutritional and remedial properties. It is these same properties, which make FulvicForce
estimates were made of the normal rate of increase in plasma urea over periods in skin and in plasma, hypertonic sodium chloride solution was
 482 J. Physiol. (I95I) II5, 482-487 THE STTE OF BODY WTER IN THE CT BY M. GRCE EGGLETON From the Department of Physiology, University College, London (Received 5 July 1951) In the course of an investigation
482 J. Physiol. (I95I) II5, 482-487 THE STTE OF BODY WTER IN THE CT BY M. GRCE EGGLETON From the Department of Physiology, University College, London (Received 5 July 1951) In the course of an investigation
possibility of a secretion of adrenaline from the suprarenal glands resulting
 355 J Physiol. (I942) IOI, 355-36I 6i2.014.465:577 I74.5 THE EFFECT OF ANAESTHESIA ON THE ADRENALINE CONTENT OF THE SUPRARENAL GLANDS BY P. C. ELMES AND A. A. JEFFERSON From the Department of Pharmacology,
355 J Physiol. (I942) IOI, 355-36I 6i2.014.465:577 I74.5 THE EFFECT OF ANAESTHESIA ON THE ADRENALINE CONTENT OF THE SUPRARENAL GLANDS BY P. C. ELMES AND A. A. JEFFERSON From the Department of Pharmacology,
(From Washington Square College, New York University.)
 6I2.III.22 THE MEASUREMENT OF RED CELL VOLUME. II. Alterations in cell volume in solutions of various tonicities. BY ERIC PONDER AND GEORGE SASLOW. (From Washington Square College, New York University.)
6I2.III.22 THE MEASUREMENT OF RED CELL VOLUME. II. Alterations in cell volume in solutions of various tonicities. BY ERIC PONDER AND GEORGE SASLOW. (From Washington Square College, New York University.)
necessity for an investigation into possible different types of urine acidity. In
 456 J. Physiol. (I947) io6, 456-465 6I2.46i SOME FACTORS AFFECTING THE ACIDITY OF URINE IN MAN BY M. GRACE EGGLETON From the Department of Physiology, University College, London (Received 22 February 1947)
456 J. Physiol. (I947) io6, 456-465 6I2.46i SOME FACTORS AFFECTING THE ACIDITY OF URINE IN MAN BY M. GRACE EGGLETON From the Department of Physiology, University College, London (Received 22 February 1947)
longitudinal sinus. A decrease in blood flow was observed when the pressure
 362 J. Physiol. (I942) IOI, 362-368 6I2.I44:6I2.824 THE EFFECT OF VARIATIONS IN THE SU.BARACHNOID PRESSURE ON THE VENOUS PRESSURE IN THE SUPERIOR LONGITUDINAL SINUS AND IN THE TORCULAR OF THE DOG BY T.
362 J. Physiol. (I942) IOI, 362-368 6I2.I44:6I2.824 THE EFFECT OF VARIATIONS IN THE SU.BARACHNOID PRESSURE ON THE VENOUS PRESSURE IN THE SUPERIOR LONGITUDINAL SINUS AND IN THE TORCULAR OF THE DOG BY T.
completenutrition I R E L A N D The Role of Fulvic Acid in Sport and Exercise By Peter Gouge BSc (Hons) Nutrition, RNutr
 The Role of Fulvic Acid in Sport and Exercise By Peter Gouge BSc (Hons) Nutrition, RNutr Copyright 2009 Anaerobic Exercise (Absence of Oxygen) Aerobic Exercise (Presence of Oxygen) To discuss the positive
The Role of Fulvic Acid in Sport and Exercise By Peter Gouge BSc (Hons) Nutrition, RNutr Copyright 2009 Anaerobic Exercise (Absence of Oxygen) Aerobic Exercise (Presence of Oxygen) To discuss the positive
CHAPTER 3: MATERIALS AND METHODS
 CHAPTER 3: MATERIALS AND METHODS Materials and Methods. The from different husks, fruits, and vegetables peels were estimated quantitatively by the following volumetric procedure s such as Bertrand s,
CHAPTER 3: MATERIALS AND METHODS Materials and Methods. The from different husks, fruits, and vegetables peels were estimated quantitatively by the following volumetric procedure s such as Bertrand s,
Feedstuffs Analysis G-22-1 PROTEIN
 Feedstuffs Analysis G-22-1 PROTEIN PRINCIPLE SCOPE Many modifications of the Kjeldahl method have been accepted for the estimation of protein in organic materials. It comprises sample oxidation and conversion
Feedstuffs Analysis G-22-1 PROTEIN PRINCIPLE SCOPE Many modifications of the Kjeldahl method have been accepted for the estimation of protein in organic materials. It comprises sample oxidation and conversion
AQA B3.3 Homeostasis LEVEL 1
 AQA B3.3 Homeostasis LEVEL 1 176 minutes 176 marks Page 1 of 48 ## (a) The table shows the compounds and ions dissolved in a student s urine. Compound or ion Percentage of total urea 60 negative ions 25
AQA B3.3 Homeostasis LEVEL 1 176 minutes 176 marks Page 1 of 48 ## (a) The table shows the compounds and ions dissolved in a student s urine. Compound or ion Percentage of total urea 60 negative ions 25
conductivity after its precipitation indicated that salts had been held freezing point or conductivity than the precipitation of the same
 THE EFFECT ON THE MOLECULAR CONCENTRATION AND ELECTRICAL CONDUCTIVITY OF MUSCLE EXTRACTS OF REMOVAL OF THE PROTEIDS. BY G. N. STEWART, Western Reserve University, Cleveland, U.S.A. (Preliminary Note.)
THE EFFECT ON THE MOLECULAR CONCENTRATION AND ELECTRICAL CONDUCTIVITY OF MUSCLE EXTRACTS OF REMOVAL OF THE PROTEIDS. BY G. N. STEWART, Western Reserve University, Cleveland, U.S.A. (Preliminary Note.)
University College, London.)
 6I2.I2I:547.472*3 LACTIC ACID FORMATION AND REMOVAL WITH CHANGE OF BLOOD REACTION. BY M. GRACE EGGLETON1 AND C. LOVATT EVANS. (From the Department of Physiology and Biochemistry, University College, London.)
6I2.I2I:547.472*3 LACTIC ACID FORMATION AND REMOVAL WITH CHANGE OF BLOOD REACTION. BY M. GRACE EGGLETON1 AND C. LOVATT EVANS. (From the Department of Physiology and Biochemistry, University College, London.)
CRYSTALLINE PEPSIN V. ISOLATION OF CRYSTALLINE PEPSIN FROM BOVINE GASTRIC JUICE BY JOHN H. NORTHROP
 CRYSTALLINE PEPSIN V. ISOLATION OF CRYSTALLINE PEPSIN FROM BOVINE GASTRIC JUICE BY JOHN H. NORTHROP (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J.) (Accepted
CRYSTALLINE PEPSIN V. ISOLATION OF CRYSTALLINE PEPSIN FROM BOVINE GASTRIC JUICE BY JOHN H. NORTHROP (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J.) (Accepted
A modification of Fiske and Subbarow's method for determination of phosphocreatine 1,
 A modification of Fiske and Subbarow's method for determination of phosphocreatine 1, By N.-O. Abdon (Lund) and Erik Jacobsen (Copenhagen). (From the Pharmacological Department, University of Lund, Sweden.)
A modification of Fiske and Subbarow's method for determination of phosphocreatine 1, By N.-O. Abdon (Lund) and Erik Jacobsen (Copenhagen). (From the Pharmacological Department, University of Lund, Sweden.)
6I2.744.I5: e3. sufficiently high'. There exists in such cases a certain concentration of the. by direct analysis.
 194 THE DIFFUSION OF ACTATE INTO AND FROM MUSCE. BY S. C. DEVADATTA. 6I2.744.I5:547.472e3 (From the Department of Physiology, Edinburgh University.) CERTAIN constituents of the voluntary muscles of the
194 THE DIFFUSION OF ACTATE INTO AND FROM MUSCE. BY S. C. DEVADATTA. 6I2.744.I5:547.472e3 (From the Department of Physiology, Edinburgh University.) CERTAIN constituents of the voluntary muscles of the
ON TEA TANNIN ISOLATED FROM GREEN TEA.
 70 [Vol. 6 ON TEA TANNIN ISOLATED FROM GREEN TEA. By MICHIYO TSUJIMIIRA. (Received September 8th., 1930). The author(1) has recently isolated Tea catechin from green tea and pro posed the following formula
70 [Vol. 6 ON TEA TANNIN ISOLATED FROM GREEN TEA. By MICHIYO TSUJIMIIRA. (Received September 8th., 1930). The author(1) has recently isolated Tea catechin from green tea and pro posed the following formula
points raised, and the following is an account of what I have done under touched, but my work has fallen under two main heads:
 NOTES ON CREATININE. BY P. C. COLLS, late Assistant Demonstrator in Physiology, King's College, London. (From the Physiological Laboratory, King's College, London.) ABOUT two years ago, a lengthy correspondence
NOTES ON CREATININE. BY P. C. COLLS, late Assistant Demonstrator in Physiology, King's College, London. (From the Physiological Laboratory, King's College, London.) ABOUT two years ago, a lengthy correspondence
INTRODUCTION. IN a previous paper(l) we have been able to show that adrenaline may
 REVERSAL OF THE ACTION OF ADRENALINE. BY B. A. McSWINEY AND G. L. BROWN. (From the Department of Physiology, University of Manchester.) INTRODUCTION. IN a previous paper(l) we have been able to show that
REVERSAL OF THE ACTION OF ADRENALINE. BY B. A. McSWINEY AND G. L. BROWN. (From the Department of Physiology, University of Manchester.) INTRODUCTION. IN a previous paper(l) we have been able to show that
CHEMISTRY OF LIFE 30 JANUARY 2013
 CHEMISTRY OF LIFE 30 JANUARY 2013 Lesson Description In this lesson, we will: Investigate the structure and function of molecules that are essential for life. Key Concepts Terminology A molecule is any
CHEMISTRY OF LIFE 30 JANUARY 2013 Lesson Description In this lesson, we will: Investigate the structure and function of molecules that are essential for life. Key Concepts Terminology A molecule is any
April 08, biology 2201 ch 11.3 excretion.notebook. Biology The Excretory System. Apr 13 9:14 PM EXCRETORY SYSTEM.
 Biology 2201 11.3 The Excretory System EXCRETORY SYSTEM 1 Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid base concentrations and metabolite concentrations
Biology 2201 11.3 The Excretory System EXCRETORY SYSTEM 1 Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid base concentrations and metabolite concentrations
for Medical Research. (Received May 10th, 1922.)
 XLV. NOTE ON URINARY TIDES AND EXCRETORY RHYTHM. BY JAMES ARGYLL CAMPBELL AND THOMAS ARTHUR WEBSTER. From the Department of Applied Physiology, National Institute for Medical Research. (Received May 10th,
XLV. NOTE ON URINARY TIDES AND EXCRETORY RHYTHM. BY JAMES ARGYLL CAMPBELL AND THOMAS ARTHUR WEBSTER. From the Department of Applied Physiology, National Institute for Medical Research. (Received May 10th,
University of Manchester.)
 6I2.744.2:547.292-II5 THE LACTIC ACID METABOLISM OF FROG'S MUSCLE POISONED WITH IODOACETIC ACID. I. The lactic acid metabolism of anaerobic iodoacetate muscle. II. The lactic acid metabolism of aerobic
6I2.744.2:547.292-II5 THE LACTIC ACID METABOLISM OF FROG'S MUSCLE POISONED WITH IODOACETIC ACID. I. The lactic acid metabolism of anaerobic iodoacetate muscle. II. The lactic acid metabolism of aerobic
INTERMEDIATE 1 1 Food and Diet. These elements are present in compounds - not as free elements.
 INTERMEDIATE 1 1 Food and Diet FOOD AND DIET The main elements present in the human body are: Hydrogen Oxygen Nitrogen Carbon These elements are present in compounds - not as free elements. Unlike plants,
INTERMEDIATE 1 1 Food and Diet FOOD AND DIET The main elements present in the human body are: Hydrogen Oxygen Nitrogen Carbon These elements are present in compounds - not as free elements. Unlike plants,
Aim: To study the effect of ph on the action of salivary amylase. NCERT
 Exercise 28 Aim: To study the effect of ph on the action of salivary amylase. Principle: Optimal activity for most of the enzymes is generally observed between ph 5.0 and 9.0. However, a few enzymes, e.g.,
Exercise 28 Aim: To study the effect of ph on the action of salivary amylase. Principle: Optimal activity for most of the enzymes is generally observed between ph 5.0 and 9.0. However, a few enzymes, e.g.,
belonging to the pseudoglobulins, forming a heat-stable, dialysable vasoconstrictor (Received 2 April 1942)
 284 J. Physiol. (I942) IOI, 284-288 6I2.462.1:6I2.I46 PREPARATION AND SOME PROPERTIES OF HYPERTENSIN (ANGIOTONIN) BY P. EDMAN, U. S. VON EULER, E. JORPES AND 0. T. SJOSTRAND From the Physiology Department
284 J. Physiol. (I942) IOI, 284-288 6I2.462.1:6I2.I46 PREPARATION AND SOME PROPERTIES OF HYPERTENSIN (ANGIOTONIN) BY P. EDMAN, U. S. VON EULER, E. JORPES AND 0. T. SJOSTRAND From the Physiology Department
blue, buffer excretion by titrating back to ph 3*7 with.1 N hydrochloric
 CALCIUM CHLORIDE ACIDOSIS. BY J. B. S. HALDANE, R. HILL, AND J. M. LUCK. (From the Biochemical Laboratory, Cambridge.) GYORGY(1) has shown that calcium chloride, when administered to babies, causes an
CALCIUM CHLORIDE ACIDOSIS. BY J. B. S. HALDANE, R. HILL, AND J. M. LUCK. (From the Biochemical Laboratory, Cambridge.) GYORGY(1) has shown that calcium chloride, when administered to babies, causes an
Experimental. Schmidt, in his experiments, boiled his solutions
 PROTECTION OF TRYPSIN FROM DESTRUCTION BY HEAT. BY D. IL DE SOUZA. (From the Institute of Physiology, University College, London.) E. W. SCHMIDT' has recently claimed: that trypsin in the presence of peptone,
PROTECTION OF TRYPSIN FROM DESTRUCTION BY HEAT. BY D. IL DE SOUZA. (From the Institute of Physiology, University College, London.) E. W. SCHMIDT' has recently claimed: that trypsin in the presence of peptone,
Cellular Respiration Notes. Biology - Mrs. Kaye
 Cellular Respiration Notes Biology - Mrs. Kaye Energy Transfer In cellular respiration, chemical energy is converted into usable energy which is converted into heat energy. ATP and ADP ATP acts as an energy
Cellular Respiration Notes Biology - Mrs. Kaye Energy Transfer In cellular respiration, chemical energy is converted into usable energy which is converted into heat energy. ATP and ADP ATP acts as an energy
THE EFFECT OF EXTRACTS OF SUPRARENAL CORTEX ON THE BLOOD CALCIUM
 35 THE EFFECT OF EXTRACTS OF SUPRARENAL CORTEX ON THE BLOOD CALCIUM BY L. MIRVISH AND L. P. BOSMAN. (From the Department of Biochemistry, University of Cape Town.) (Received 12th February 1929.) INTRODUCTION.
35 THE EFFECT OF EXTRACTS OF SUPRARENAL CORTEX ON THE BLOOD CALCIUM BY L. MIRVISH AND L. P. BOSMAN. (From the Department of Biochemistry, University of Cape Town.) (Received 12th February 1929.) INTRODUCTION.
(A) Urea cycle (B) TCA cycle (C) Glycolysis (D) Pyruvate oxidation (E) Respiratory chain
 Biochemistry - Problem Drill 15: Citric Acid Cycle No. 1 of 10 1. Which of the following statements is not a metabolic pathway involved in carbohydrate catabolism and ATP production. (A) Urea cycle (B)
Biochemistry - Problem Drill 15: Citric Acid Cycle No. 1 of 10 1. Which of the following statements is not a metabolic pathway involved in carbohydrate catabolism and ATP production. (A) Urea cycle (B)
Metabolism of echitamine and plumbagin in rats
 J. Biosci., Vol. 3, Number 4, December 1981, pp. 395-400. Printed in India. Metabolism of echitamine and plumbagin in rats B. CHANDRASEKARAN and B. NAGARAJAN Microbiology Division, Cancer Institute, Madras
J. Biosci., Vol. 3, Number 4, December 1981, pp. 395-400. Printed in India. Metabolism of echitamine and plumbagin in rats B. CHANDRASEKARAN and B. NAGARAJAN Microbiology Division, Cancer Institute, Madras
Cellular Respiration Guided Notes
 Respiration After you hear word 'respiration', you may now think about breathing. During breathing, the is entered with each inhale and is released with each exhale. You may have noticed that breathing
Respiration After you hear word 'respiration', you may now think about breathing. During breathing, the is entered with each inhale and is released with each exhale. You may have noticed that breathing
THE EFFECT OF DENATURATION ON THE VISCOSITY OF PROTEIN SYSTEMS BY M. L. ANSON A~D A. E. MIRSKY. (Accepted for publication, December 2, 1931)
 THE EFFECT OF DENATURATION ON THE VISCOSITY OF PROTEIN SYSTEMS BY M. L. ANSON A~D A. E. MIRSKY (From tke Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. Y., and the ttospital
THE EFFECT OF DENATURATION ON THE VISCOSITY OF PROTEIN SYSTEMS BY M. L. ANSON A~D A. E. MIRSKY (From tke Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. Y., and the ttospital
Examination of Chemicals in Trap Cases. (Phenolphthalein)
 Introduction Examination of Chemicals in Trap Cases (Phenolphthalein) Although a number of different techniques using different chemicals such as fluorescent dyes, starch powder, phenolphthalein powders
Introduction Examination of Chemicals in Trap Cases (Phenolphthalein) Although a number of different techniques using different chemicals such as fluorescent dyes, starch powder, phenolphthalein powders
might be due to a direct action on the thyroid, like that of the thiouracil
 288 J. Physiol. (1953) I20, 288-297 COMPARISON OF THE EFFECTS OF THIOURACIL, THY- ROXINE AND CORTISONE ON THE THYROID FUNCTION OF RABBITS BY N. B. MYANT* From the Department of Clinical Research, University
288 J. Physiol. (1953) I20, 288-297 COMPARISON OF THE EFFECTS OF THIOURACIL, THY- ROXINE AND CORTISONE ON THE THYROID FUNCTION OF RABBITS BY N. B. MYANT* From the Department of Clinical Research, University
substance or substances the glycogen of the heart is derived. The
 612.173: 612.396.112 THE SOURCE OF THE HEART GLYCOGEN. By J. YULE BOGUE, C. LOVATT EVANS, and R. A. GREGORY.' From the Department of Physiology, Biochemistry, and Pharmacology, University College, London.
612.173: 612.396.112 THE SOURCE OF THE HEART GLYCOGEN. By J. YULE BOGUE, C. LOVATT EVANS, and R. A. GREGORY.' From the Department of Physiology, Biochemistry, and Pharmacology, University College, London.
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
THE BIOCHEMICAL JOURNAL, ERRATUMi. Vol. XXVII, p. 1753, line 22 for 1 cc. M/45 phosphate read 1 cc. M/15 phosphate
 THE BIOCHEMICAL JOURNAL, 1933 ERRATUMi Vol. XXVII, p. 1753, line 22 for 1 cc. M/45 phosphate read 1 cc. M/15 phosphate CCXXXVI. OXIDATION OF FATTY ACIDS IN THE LIVER'. BY JUDA HIRSCH QUASTEL AND ARNOLD
THE BIOCHEMICAL JOURNAL, 1933 ERRATUMi Vol. XXVII, p. 1753, line 22 for 1 cc. M/45 phosphate read 1 cc. M/15 phosphate CCXXXVI. OXIDATION OF FATTY ACIDS IN THE LIVER'. BY JUDA HIRSCH QUASTEL AND ARNOLD
administration of adrenaline or in cases of increased perfusion pressure. approximately the same within fairly wide variations of the systemic
 6I2. I72. I THE DISTRIBUTION OF THE BLOOD IN THE CORONARY BLOOD VESSELS. BY G. V. ANREP, A. BLALOCK AND M. HAMMOUDA. (From the Physiological Laboratory, Cambridge.) As a result of experiments on perfused
6I2. I72. I THE DISTRIBUTION OF THE BLOOD IN THE CORONARY BLOOD VESSELS. BY G. V. ANREP, A. BLALOCK AND M. HAMMOUDA. (From the Physiological Laboratory, Cambridge.) As a result of experiments on perfused
Soil organic matter composition, decomposition, mineralization and immobilization
 Soil organic matter composition, decomposition, mineralization and immobilization SOIL ORGANIC MATTER Substances containing carbon are organic matter. Soil organic matter consists of decomposing plant
Soil organic matter composition, decomposition, mineralization and immobilization SOIL ORGANIC MATTER Substances containing carbon are organic matter. Soil organic matter consists of decomposing plant
Furthermore, added choline may exert relatively little effect when. naturally occurring lipotropic factors are present in appreciable amounts
 343 6I2.352.2:547.922 THE EFFECTS OF CHOLESTEROL AND CHOLINE ON LIVER FAT BY C. H. BEST AND JESSIE H. RIDOUT (From the School of Hygiene, University of Toronto) (Received January 27, 1936) THE results
343 6I2.352.2:547.922 THE EFFECTS OF CHOLESTEROL AND CHOLINE ON LIVER FAT BY C. H. BEST AND JESSIE H. RIDOUT (From the School of Hygiene, University of Toronto) (Received January 27, 1936) THE results
THIONYL IODIDE. Part II. Rate of Decomposition and Spectroscopic Studies BY M. R. ASWATHANARAYANA RAO. Introduction
 THIONYL IODIDE Part II. Rate of Decomposition and Spectroscopic Studies BY M. R. ASWATHANARAYANA RAO (Department of Chemistry, University of Mysore Central College, Bangalore) Received February 6, 194
THIONYL IODIDE Part II. Rate of Decomposition and Spectroscopic Studies BY M. R. ASWATHANARAYANA RAO (Department of Chemistry, University of Mysore Central College, Bangalore) Received February 6, 194
HAGEDORN AND JENSEN TO THE DETER- REDUCING SUGARS. MINATION OF LARGER QUANTITIES OF XIV. AN APPLICATION OF THE METHOD OF
 XIV. AN APPLICATION OF THE METHOD OF HAGEDORN AND JENSEN TO THE DETER- MINATION OF LARGER QUANTITIES OF REDUCING SUGARS. By CHARLES SAMUEL HANES (Junior Scholar of the Exhibition of 1851). From the Botany
XIV. AN APPLICATION OF THE METHOD OF HAGEDORN AND JENSEN TO THE DETER- MINATION OF LARGER QUANTITIES OF REDUCING SUGARS. By CHARLES SAMUEL HANES (Junior Scholar of the Exhibition of 1851). From the Botany
Exercise 15-B PHYSIOLOGICAL CHARACTERISTICS OF BACTERIA CONTINUED: AMINO ACID DECARBOXYLATION, CITRATE UTILIZATION, COAGULASE & CAMP TESTS
 Exercise 15-B PHYSIOLOGICAL CHARACTERISTICS OF BACTERIA CONTINUED: AMINO ACID DECARBOXYLATION, CITRATE UTILIZATION, COAGULASE & CAMP TESTS Decarboxylation of Amino Acids and Amine Production The decarboxylation
Exercise 15-B PHYSIOLOGICAL CHARACTERISTICS OF BACTERIA CONTINUED: AMINO ACID DECARBOXYLATION, CITRATE UTILIZATION, COAGULASE & CAMP TESTS Decarboxylation of Amino Acids and Amine Production The decarboxylation
GRADE 11 NOVEMBER 2013 LIFE SCIENCES P1 MEMORANDUM
 NATIONAL SENIOR CERTIFICATE GRADE 11 NOVEMBER 2013 LIFE SCIENCES P1 MEMORANDUM MARKS: 150 This memorandum consists of 7 pages. 2 LIFE SCIENCES P1 (NOVEMBER 2013) SECTION A QUESTION 1 1.1 1.1.1 A 1.2 1.2.1
NATIONAL SENIOR CERTIFICATE GRADE 11 NOVEMBER 2013 LIFE SCIENCES P1 MEMORANDUM MARKS: 150 This memorandum consists of 7 pages. 2 LIFE SCIENCES P1 (NOVEMBER 2013) SECTION A QUESTION 1 1.1 1.1.1 A 1.2 1.2.1
There are enzymes in biological washing powders. Biological washing powder has to be used at temperatures below 45 C.
 There are enzymes in biological washing powders. Biological washing powder has to be used at temperatures below 45 C. The enzymes in biological washing powders do not work on the stains on clothes at temperatures
There are enzymes in biological washing powders. Biological washing powder has to be used at temperatures below 45 C. The enzymes in biological washing powders do not work on the stains on clothes at temperatures
The molecule that serves as the major source of readily available body fuel is: a. fat. b. glucose. c. acetyl CoA. d. cellulose.
 The molecule that serves as the major source of readily available body fuel is: a. fat. b. glucose. c. acetyl CoA. d. cellulose. Dietary fats are important because: a. they keep blood pressure normal.
The molecule that serves as the major source of readily available body fuel is: a. fat. b. glucose. c. acetyl CoA. d. cellulose. Dietary fats are important because: a. they keep blood pressure normal.
Food acidity FIRST LAB
 Food acidity FIRST LAB objective To determine total acidity of milk, juice, vinegar and oil acid value Food acidity Food acids are usually organic acids, with citric, malic, lactic, tartaric, and acetic
Food acidity FIRST LAB objective To determine total acidity of milk, juice, vinegar and oil acid value Food acidity Food acids are usually organic acids, with citric, malic, lactic, tartaric, and acetic
EFFECTS OF ANTICOAGULANTS ON THE ph. (Studies on the blood ph estimated by the glass electrode method. II)
 The Journal of Biochemistry, vol. 22, No. 2. EFFECTS OF ANTICOAGULANTS ON THE ph OF THE BLOOD. (Studies on the blood ph estimated by the glass electrode method. II) BY HISATO YOSHIMURA (From the First
The Journal of Biochemistry, vol. 22, No. 2. EFFECTS OF ANTICOAGULANTS ON THE ph OF THE BLOOD. (Studies on the blood ph estimated by the glass electrode method. II) BY HISATO YOSHIMURA (From the First
Cycling of Organic and Inorganic Matter
 Cycling of Matter Build your Own Notes: Use these topics as guidelines to create your own notes from the page given udy Notes/Questions Cycling of Organic and Inorganic Matter Organic matter always contains
Cycling of Matter Build your Own Notes: Use these topics as guidelines to create your own notes from the page given udy Notes/Questions Cycling of Organic and Inorganic Matter Organic matter always contains
HEMOTOLOGY. B. Helps stabilize body temperature -heats up and cools down slowly which moderates body temp
 I. Body H 2 O = HEMOTOLOGY A. Variable quantities 1. sweating and urination ( ) decreases H 2 O 2. drinking H 2 O increases B. Water is found in two compartments 1. contains 2/3 of all water in your body
I. Body H 2 O = HEMOTOLOGY A. Variable quantities 1. sweating and urination ( ) decreases H 2 O 2. drinking H 2 O increases B. Water is found in two compartments 1. contains 2/3 of all water in your body
PURIFICATION OF PROTHROMBIN AND THROMBIN : CHEMICAL PROPERTIES OF PURIFIED PREPARATIONS*
 PURIFICATION OF PROTHROMBIN AND THROMBIN : CHEMICAL PROPERTIES OF PURIFIED PREPARATIONS* BY WALTER H. SEEGERS (Prom the Department of Pathology, State University of Zowa, Iowa City) (Received for publication,
PURIFICATION OF PROTHROMBIN AND THROMBIN : CHEMICAL PROPERTIES OF PURIFIED PREPARATIONS* BY WALTER H. SEEGERS (Prom the Department of Pathology, State University of Zowa, Iowa City) (Received for publication,
Fate of Dietary Protein
 Fate of Dietary Protein Dietary protein Stomach: l, pepsin Denatured and partially hydrolyzed protein (large polypeptides) small intestine: proteases Amino acids and dipeptides intestinal lining: proteases
Fate of Dietary Protein Dietary protein Stomach: l, pepsin Denatured and partially hydrolyzed protein (large polypeptides) small intestine: proteases Amino acids and dipeptides intestinal lining: proteases
Amino Acid Metabolism
 Amino Acid Metabolism Fate of Dietary Protein Dietary protein Stomach: l, pepsin Denatured and partially hydrolyzed protein (large polypeptides) small intestine: proteases Amino acids and dipeptides intestinal
Amino Acid Metabolism Fate of Dietary Protein Dietary protein Stomach: l, pepsin Denatured and partially hydrolyzed protein (large polypeptides) small intestine: proteases Amino acids and dipeptides intestinal
UNDERSTANDING YOUR WATER PROFILE PRESENTED BY POULTRY PARTNERS AND AHPD
 UNDERSTANDING YOUR WATER PROFILE PRESENTED BY POULTRY PARTNERS AND AHPD WHY DOES IT MATTER? Water intake for commercial poultry breeds is 1.5-2x greater than feed intake Commercial birds drink more now
UNDERSTANDING YOUR WATER PROFILE PRESENTED BY POULTRY PARTNERS AND AHPD WHY DOES IT MATTER? Water intake for commercial poultry breeds is 1.5-2x greater than feed intake Commercial birds drink more now
6I I:6I hypophysectomy. This diminution of diabetes is shown particularly as. hypophysectomized or totally decerebrated [Houssay and
 6I2.466.6I:6I2.492.5 KETOSIS IN THE PANCREATIC AND PHLORRHIZIN DIABETES OF HYPOPHYSECTOMIZED DOGS. BY CIRO T. RIETTI. (Institute of Physiology, Faculty of Medicine, Buenos Ayres.) IN the hypophysectomized
6I2.466.6I:6I2.492.5 KETOSIS IN THE PANCREATIC AND PHLORRHIZIN DIABETES OF HYPOPHYSECTOMIZED DOGS. BY CIRO T. RIETTI. (Institute of Physiology, Faculty of Medicine, Buenos Ayres.) IN the hypophysectomized
M1. (a) (concentration high) in the hepatic portal vein is blood with glucose absorbed from the intestine 1
 M. (a) (concentration high) in the hepatic portal vein is blood with glucose absorbed from the intestine concentration is lower in the hepatic vein because insulin (has caused) glucose to be converted
M. (a) (concentration high) in the hepatic portal vein is blood with glucose absorbed from the intestine concentration is lower in the hepatic vein because insulin (has caused) glucose to be converted
: /18
 612.461.23: 616-001.17/18 SOME OBSERVATIONS ON THE COMPARATIVE EFFECTS OF COLD AND BURNS ON PROTEIN METABOLISM IN RATS. By G. H. LATHE 1 and R. A. PETERS. From the Department of Biochemistry, Oxford. (Received
612.461.23: 616-001.17/18 SOME OBSERVATIONS ON THE COMPARATIVE EFFECTS OF COLD AND BURNS ON PROTEIN METABOLISM IN RATS. By G. H. LATHE 1 and R. A. PETERS. From the Department of Biochemistry, Oxford. (Received
WHY DO WE NEED AN EXCRETORY SYSTEM? Function: To eliminate waste To maintain water and salt balance To maintain blood pressure
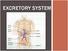 EXCRETORY SYSTEM WHY DO WE NEED AN EXCRETORY SYSTEM? Function: To eliminate waste To maintain water and salt balance To maintain blood pressure These wastes include: Carbon dioxide Mostly through breathing
EXCRETORY SYSTEM WHY DO WE NEED AN EXCRETORY SYSTEM? Function: To eliminate waste To maintain water and salt balance To maintain blood pressure These wastes include: Carbon dioxide Mostly through breathing
Barsoum & Gaddum [1935a], working on dogs, found that the histamine. obtained a similar effect by severely restricting the arterial blood supply to
![Barsoum & Gaddum [1935a], working on dogs, found that the histamine. obtained a similar effect by severely restricting the arterial blood supply to Barsoum & Gaddum [1935a], working on dogs, found that the histamine. obtained a similar effect by severely restricting the arterial blood supply to](/thumbs/89/99230787.jpg) 297 J. Physiol. (I944) I03, 297-305 547*78iT5:6I6-005.2 LIBERATION OF HISTAMINE DURING REACTIVE HYPERAEMIA AND MUSCLE CONTRACTION IN MAN BY G. V. ANREP, G. S. BARSOUM, S. SALAMA AND Z. SOUIDAN From the
297 J. Physiol. (I944) I03, 297-305 547*78iT5:6I6-005.2 LIBERATION OF HISTAMINE DURING REACTIVE HYPERAEMIA AND MUSCLE CONTRACTION IN MAN BY G. V. ANREP, G. S. BARSOUM, S. SALAMA AND Z. SOUIDAN From the
4.1 Cycling of Matter Date: Cycling of Organic and Inorganic Matter. Build your Own Notes:
 4.1 Cycling of Matter Date: Build your Own Notes: Use these topics as guidelines to create your own notes for 4.1 from pages 83 84 Study Notes/Questions Cycling of Organic and Inorganic Matter Matter is
4.1 Cycling of Matter Date: Build your Own Notes: Use these topics as guidelines to create your own notes for 4.1 from pages 83 84 Study Notes/Questions Cycling of Organic and Inorganic Matter Matter is
PYRROLE AS A CATALYST FOR CERTAIN BIOLOGICAL OXIDATIONS
 PYRROLE AS A CATALYST FOR CERTAIN BIOLOGICAL OXIDATIONS BY FREDERICK BERNHEIM AND MARY L. C. BERNHEIM* (From the Departments of Physiology and Biochemistry, Duke University School of Medicine, Durham)
PYRROLE AS A CATALYST FOR CERTAIN BIOLOGICAL OXIDATIONS BY FREDERICK BERNHEIM AND MARY L. C. BERNHEIM* (From the Departments of Physiology and Biochemistry, Duke University School of Medicine, Durham)
BIOB111 - Tutorial activity for Session 25
 BIOB111 - Tutorial activity for Session 25 General topics for week 14 Session 25 The metabolism of proteins Students are asked to draw the concept map showing all details of protein metabolism 1 Instructions:
BIOB111 - Tutorial activity for Session 25 General topics for week 14 Session 25 The metabolism of proteins Students are asked to draw the concept map showing all details of protein metabolism 1 Instructions:
PHYSIOLOGICAL DISTURBANCES DURING EXPERIMENTAL
 PHYSIOLOGICAL DISTURBANCES DURING EXPERIMENTAL DIPHTHERITIC INTOXICATION. III. RESPIRATORY QUOTIENTS AND METABOLIC RATE 1 By HERMAN YANNET AND WALTER GOLDFARB (From the Department of Pediatrics and Physiology,
PHYSIOLOGICAL DISTURBANCES DURING EXPERIMENTAL DIPHTHERITIC INTOXICATION. III. RESPIRATORY QUOTIENTS AND METABOLIC RATE 1 By HERMAN YANNET AND WALTER GOLDFARB (From the Department of Pediatrics and Physiology,
Connections of Carbohydrate, Protein, and Lipid Metabolic Pathways
 OpenStax-CNX module: m44441 1 Connections of Carbohydrate, Protein, and Lipid Metabolic Pathways OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
OpenStax-CNX module: m44441 1 Connections of Carbohydrate, Protein, and Lipid Metabolic Pathways OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
CURVE OF SUGAR EXCRETION IN SEVERE DIABETES.
 CURVE OF SUGAR EXCRETION IN SEVERE DIABETES. BY HANNAH FELSHER. (From the Otho S. A. Sprague Memorial Institute Laboratory oj Clinical Research, Rush Medical College, Chicago.) (Received for publication,
CURVE OF SUGAR EXCRETION IN SEVERE DIABETES. BY HANNAH FELSHER. (From the Otho S. A. Sprague Memorial Institute Laboratory oj Clinical Research, Rush Medical College, Chicago.) (Received for publication,
Properties of Oxidized Cassava Starch as Influenced by Oxidant Concentration and Reaction Time
 Properties of Oxidized Cassava Starch as Influenced by Oxidant Concentration and Reaction Time P-STARCH-26 Kunruedee Sangseethong 1 and Klanarong Sriroth 2,3 1 Cassava and Starch Technology Research Unit,
Properties of Oxidized Cassava Starch as Influenced by Oxidant Concentration and Reaction Time P-STARCH-26 Kunruedee Sangseethong 1 and Klanarong Sriroth 2,3 1 Cassava and Starch Technology Research Unit,
Using the Organic Acids Test Part 5 Dr. Jeff Moss
 Using organic acids to resolve chief complaints and improve quality of life in chronically ill patients Part V Jeffrey Moss, DDS, CNS, DACBN jeffmoss@mossnutrition.com 413-530-08580858 (cell) 1 Summer
Using organic acids to resolve chief complaints and improve quality of life in chronically ill patients Part V Jeffrey Moss, DDS, CNS, DACBN jeffmoss@mossnutrition.com 413-530-08580858 (cell) 1 Summer
SAINSBURY, M.D., M.R.C.P.
 THE ACTION OF SALTS UPON HEAT COAGULATION. BY SYDNEY RINGER, M.D., F.R.S., AND HARRINGTON SAINSBURY, M.D., M.R.C.P. IN a previous paper on the influence of certain salts upon the act of clotting' a fluid
THE ACTION OF SALTS UPON HEAT COAGULATION. BY SYDNEY RINGER, M.D., F.R.S., AND HARRINGTON SAINSBURY, M.D., M.R.C.P. IN a previous paper on the influence of certain salts upon the act of clotting' a fluid
Higher Biology. Unit 2: Metabolism and Survival Topic 2: Respiration. Page 1 of 25
 Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
Topic 4: Enzymes and metabolism
 Topic 4: Enzymes and metabolism 1. An is a living molecule produced by glands to digest food in the alimentary canal. living molecule produced by cells to synthesise complex molecules from simpler ones.
Topic 4: Enzymes and metabolism 1. An is a living molecule produced by glands to digest food in the alimentary canal. living molecule produced by cells to synthesise complex molecules from simpler ones.
found it difficult to express all the fluid from the loop. 32-2
 487 J. Physiol. (I940) 98, 487-49I 6i2.364:615.782.57 THE ABSORPTION OF WATER FROM THE COLON OF THE RAT UNDER URETHANE ANAESTHESIA By B. L. ANDREW, J. N. DAVIDSON AND R. C. GARRY From the Physiology Department,
487 J. Physiol. (I940) 98, 487-49I 6i2.364:615.782.57 THE ABSORPTION OF WATER FROM THE COLON OF THE RAT UNDER URETHANE ANAESTHESIA By B. L. ANDREW, J. N. DAVIDSON AND R. C. GARRY From the Physiology Department,
Cell Biology Sub-Topic (1.6) Respiration
 Cell Biology Sub-Topic (1.6) Respiration On completion of this subtopic I will be able to state that: Glucose is a source of energy in the cell. The chemical energy stored in glucose is released by a series
Cell Biology Sub-Topic (1.6) Respiration On completion of this subtopic I will be able to state that: Glucose is a source of energy in the cell. The chemical energy stored in glucose is released by a series
during the 8 days prior to the first intraperitoneal
 EXPERIMENTAL HYPERTONICITY: ALTERATIONS IN THE DISTRIBUTION OF BODY WATER, AND THE CAUSE OF DEATH 1 By ALEXANDER W. WINKLER, J. RUSSELL ELKINTON,2 JAMES HOPPER, JR.,8 AND HEBBEL E. HOFF (From the Department
EXPERIMENTAL HYPERTONICITY: ALTERATIONS IN THE DISTRIBUTION OF BODY WATER, AND THE CAUSE OF DEATH 1 By ALEXANDER W. WINKLER, J. RUSSELL ELKINTON,2 JAMES HOPPER, JR.,8 AND HEBBEL E. HOFF (From the Department
3.12 Polymers. As with esters, the OH from the carboxylic acid is lost with the H from the alcohol.
 3.12 s Condensation polymerisation: This is the joining of 2 monomers while eliminating a small molecule - H 2 O or HCl The functional group on one monomer joins with a different functional group in another
3.12 s Condensation polymerisation: This is the joining of 2 monomers while eliminating a small molecule - H 2 O or HCl The functional group on one monomer joins with a different functional group in another
Introduction to Microbiology BIOL 220, Summer Session 1, 1996 Exam # 2
 Name I. Multiple Choice (1 point each) Introduction to Microbiology BIOL 220, Summer Session 1, 1996 Exam # 2 D 1. Which transport process requires energy? A. Osmosis C. Diffusion B. Facilitated diffusion
Name I. Multiple Choice (1 point each) Introduction to Microbiology BIOL 220, Summer Session 1, 1996 Exam # 2 D 1. Which transport process requires energy? A. Osmosis C. Diffusion B. Facilitated diffusion
Unit Seven Blood and Immunity
 Unit Seven Blood and Immunity I. Introduction A. Definition Blood is a sticky fluid that is heavier and thicker than water. Blood is a type of, whose cells and suspended in a liquid intercellular material.
Unit Seven Blood and Immunity I. Introduction A. Definition Blood is a sticky fluid that is heavier and thicker than water. Blood is a type of, whose cells and suspended in a liquid intercellular material.
Transfer of food energy to chemical energy. Includes anabolic and catabolic reactions. The cell is the metabolic processing center
 Metabolism There are a lot of diagrams here. DO NOT, I repeat, DO NOT get overly anxious or excited about them. We will go through them again slowly!! Read the slides, read the book, DO NOT TAKE NOTES.
Metabolism There are a lot of diagrams here. DO NOT, I repeat, DO NOT get overly anxious or excited about them. We will go through them again slowly!! Read the slides, read the book, DO NOT TAKE NOTES.
Medicine, Cambridge, England, and Wuppertal, B.A.O.R.
 182 J. Physiol. (I948) I07, i82-i86 6I2.46I.62 PHOSPHATE CLEARANCES IN INFANTS AND ADULTS BY R. F. A. DEAN AND R. A. McCANCE From the Medical Research Council, Department. of Experimental Medicine, Cambridge,
182 J. Physiol. (I948) I07, i82-i86 6I2.46I.62 PHOSPHATE CLEARANCES IN INFANTS AND ADULTS BY R. F. A. DEAN AND R. A. McCANCE From the Medical Research Council, Department. of Experimental Medicine, Cambridge,
The Cardiovascular System home study course
 The Cardiovascular System home study course harmony house holistic therapy treatment centre and training academy www.harmony-house.org 1 Copyright 2010 by Mark and Katy Rogers All rights reserved. No part
The Cardiovascular System home study course harmony house holistic therapy treatment centre and training academy www.harmony-house.org 1 Copyright 2010 by Mark and Katy Rogers All rights reserved. No part
ENZYME CONCENTRATIONS AND ENZYME ACTIVITY: PLANNING SHEET
 Activity 2.11 Student Sheet ENZYME CONCENTRATIONS AND ENZYME ACTIVITY: PLANNING SHEET To investigate how enzyme concentration can affect the initial rate of reaction. Wear eye protection, lab coats and
Activity 2.11 Student Sheet ENZYME CONCENTRATIONS AND ENZYME ACTIVITY: PLANNING SHEET To investigate how enzyme concentration can affect the initial rate of reaction. Wear eye protection, lab coats and
Citation Acta medica Nagasakiensia. 1965, 9(
 NAOSITE: Nagasaki University's Ac Title Comparative Biochemistry of Hemoglo Author(s) Muta, Ikuo Citation Acta medica Nagasakiensia. 1965, 9( Issue Date 1965-03-25 URL http://hdl.handle.net/10069/15492
NAOSITE: Nagasaki University's Ac Title Comparative Biochemistry of Hemoglo Author(s) Muta, Ikuo Citation Acta medica Nagasakiensia. 1965, 9( Issue Date 1965-03-25 URL http://hdl.handle.net/10069/15492
Cellular Respiration
 Cellular Respiration 1. To perform cell work, cells require energy. a. A cell does three main kinds of work: i. Mechanical work, such as the beating of cilia, contraction of muscle cells, and movement
Cellular Respiration 1. To perform cell work, cells require energy. a. A cell does three main kinds of work: i. Mechanical work, such as the beating of cilia, contraction of muscle cells, and movement
technique by Hemingway [1931] makes it possible to
![technique by Hemingway [1931] makes it possible to technique by Hemingway [1931] makes it possible to](/thumbs/90/101795941.jpg) 6I2.464 THE ACTION OF CYANIDE ON THE ISOLATED MAMMALIAN KIDNEY. BY L. E. BAYLISS' AND E. LUNDSGAARD2 (Copenhagen). (From the Department of Physiology and Biochemistry, University College, London.) THE
6I2.464 THE ACTION OF CYANIDE ON THE ISOLATED MAMMALIAN KIDNEY. BY L. E. BAYLISS' AND E. LUNDSGAARD2 (Copenhagen). (From the Department of Physiology and Biochemistry, University College, London.) THE
