Membrane Transport II (Osmosis) Linda S. Costanzo, Ph.D.
|
|
|
- Magdalene Johnson
- 6 years ago
- Views:
Transcription
1 Membrane Transport II (Osmosis) Linda S. Costanzo, Ph.D. OBJECTIVES: 1. Be able to define and calculate osmolarity 2. Describe osmosis across a semipermeable membrane and the volume changes that will occur as a result. 3. Be able to calculate osmotic pressure and understand the factors that contribute to it. 4. Understand the role of reflection coefficient in determining effective osmotic pressure. 5. Understand how a difference in effective osmotic pressure drives osmotic water flow. 6. Compare osmosis to diffusion of water. Suggested Reading: Physiology, edited by: R.M. Berne and M. N. Levy, Mosby, 6 th Ed. pp Physiology, L.S. Costanzo, Saunders, pp Most water flow across cell membranes occurs by osmosis, not by diffusion. Osmosis is the topic of today's lecture, but first we will clarify the concepts of osmolarity and osmotic pressure. I. OSMOLARITY Osmolarity is the concentration of osmotically active particles in a single solution. To calculate osmolarity, one must know the concentration of the solute and whether that solute dissociates in solution. For example, urea does not dissociate in solution; one mole of urea is one mole in solution. However, in solution NaCl dissociates into two particles (or nearly two particles) and CaCl 2 dissociates into three particles (or nearly three). The symbol "g" gives the number of particles in solution for a given solute. "g" also takes into account whether there is complete dissociation or not; for example, "g" for NaCl is not 2.0, but is slightly less than 2.0 (e.g., 1.85) because in solution some of the Na + and Cl - interact with each other. Thus, g for urea is 1.0; g for NaCl is slightly less than 2.0 as explained; g for CaCl 2 is slightly less than 3.0. The units of "g" are osmoles/mole. The units of osmolarity are osmoles/l or mosmoles/l. Osmolarity = g x C
2 If two solutions have the same calculated osmolarity, they are called isosmotic. If two solutions have different calculated osmolarities, the solution with the higher osmolarity is hyperosmotic and the solution with the lower osmolarity is hyposmotic. Osmolarity is calculated for one solution. There is no membrane separating one solution from another and no water flow (that's osmotic pressure and osmosis... coming up!). II. OSMOTIC PRESSURE AND OSMOSIS A. Example of osmosis Osmosis is the flow of water across a semipermeable membrane due to a difference in solute concentration. This difference in solute concentration creates an osmotic pressure difference and the osmotic pressure difference is the driving force for water flow. The figure below illustrates the concept of osmosis. In both A and B, there is a semipermeable membrane separating two solutions, one containing solute (the circles), the other containing only water. The membrane is impermeable to the solute, but permeable to water. In A, the two solutions are open to the atmosphere. The solute in Solution 1 creates an osmotic pressure, which produces a reduction in hydrostatic pressure in Solution 1 (the higher the osmotic pressure, the lower the hydrostatic pressure). As a result, there is a hydrostatic pressure gradient established between Solution 2 and Solution 1, with the greater hydrostatic pressure in Solution 2. Water flows from 2 to 1, driven by this hydrostatic pressure gradient. With time, the volume in 1 increases and the volume in 2 decreases. In B, the two solutions are closed and pressure is applied with a piston to stop the flow of water from 2 to 1. The pressure required to stop water flow is the osmotic pressure of Solution 1. This pressure can be measured experimentally.
3 B. Calculating osmotic pressure The osmotic pressure (B) of Solution 1 in the above example is determined by two factors: the concentration of osmotically active particles in the solution and whether the membrane is impermeable to the solute (i.e., whether the solute remains in Solution 1). Effective osmotic pressure is calculated by the van't Hoff equation, which converts solute concentration into pressure, taking into account whether the solute remains in the original compartment: B = g C F RT where B is osmotic pressure (or effective osmotic pressure), g is number of solute particles per mole in solution, C is concentration of solute, RT is gas constant x absolute temperature, and F is a new term called reflection coefficient. C. Reflection coefficient The reflection coefficient (F) is a dimensionless (no units) number that describes the ease with which the solute crosses the membrane. (Yes, reflection coefficient is related to permeability, in case you were wondering.) Values for reflection coefficient vary between 1.0 and zero as follows:
4 F = 1.0 means the membrane is impermeable to the solute and the solute stays in the original compartment. Referring to the figure above, solute would remain in Solution 1. In this case, the solute exerts its full osmotic effect, the osmotic pressure is maximal, and water flow is maximal. Examples of solutes with F = 1.0 are proteins, which are impermeable across cell membranes and capillaries. F = 0 means that the membrane is freely permeable to the solute and the solute can diffuse down its concentration gradient from the original compartment. Recalling diffusion for a moment, this means that eventually, the solute concentration will be the same in both compartments. In this case, there will be no effective osmotic pressure and no water flow. Notice that, in the van't Hoff equation, when F = 0 the effective osmotic pressure also equals zero. An example of a solute with F = 0 (or close to 0) is urea. F = between 0 and 1.0. Most solutes fall in between they are neither completely impermeable nor freely permeable. Thus, effective osmotic pressure and water flow fall somewhere between their maximal possible values and zero. Notice in the van't Hoff equation, when F is between 0 and 1.0, that effective osmotic pressure is less than maximal, but greater than zero. There are many examples of solutes with F between 0 and 1: mannitol, glucose, NaCl, KCl, CaCl 2. In general, the larger the solute, the higher the value of F. D. Osmotic pressure difference drives water flow For osmosis to occur (water flow due to an osmotic pressure difference), what we really care about is the effective osmotic pressure difference between two
5 solutions separated by a membrane. In practice, if you're given two solutions, calculate the effective osmotic pressure of each one (units of pressure in mm Hg or atm), then take the difference to determine the driving force for water flow. Follow these rules to determine the direction of water flow: If the two solutions have the same effective osmotic pressure, they are isotonic and no water flows between them. If the two solutions have different effective osmotic pressures, the one with the higher pressure is called hypertonic and the one with the lower pressure is called hypotonic. Very important: water flows from the hypotonic solution into the hypertonic solution, from lower effective osmotic pressure to higher effective osmotic pressure. Consider the following example, based on the figure below that illustrates the difference between osmolarity and osmotic pressure and the importance of the reflection coeffi g = 1 for all solutes F albumin = 1.0 F globulin = 1.0 F urea = 0 In A, the two solutions are isosmotic, since their calculated osmolarities are equal. They also are isotonic because their calculated effective osmotic pressures are equal (same F for albumin and globulin). In B, the two solutions are also isosmotic, but they are not isotonic: the urea solution is hypotonic and the
6 albumin solution is hypertonic; thus water would flow from right to left, from hypotonic to hypertonic. III. OSMOSIS VS DIFFUSION OF WATER Osmosis of water should be distinguished from diffusion of water (with osmosis being much more important across biological membranes). Diffusion of water obeys Fick's Law of diffusion and depends on the concentration difference of water and the surface area for diffusion. Surface area for diffusion is proportional to the area of water-filled pores in the membrane (r 2 ). Osmosis of water depends on an osmotic pressure difference (see above). Water flow due to a pressure difference (rather than a concentration difference) is based on Poiseuille's law which states that flow is proportional to r 4 of the water-filled pores. Thus, osmosis of water is much faster than diffusion of water because of its fourth power relationship with pore radius! Flow = B r 4 )P 8 0 )x III. PRACTICE PROBLEMS 1. Red blood cells are placed in 150 mm NaCl and no change in their cell volume is observed. It can be concluded that: A. 150 mm NaCl is hypertonic B. 150 mm NaCl is hypotonic C. ICF has an effective osmotic pressure of 300 mosm. D. NaCl acts as if it were an undissociated molecule E. NaCl has a higher permeability than water in red blood cells. 2. Red blood cells are placed in 300 mm solution of a nonelectrolyte with a reflection coefficient of 0.4 and the cells swell. It may be concluded that: A. a 300 mm solution of this substance is isotonic. B. the solution has approximately the effective osmotic pressure of 60 mm NaCl.
7 C. this substance will remain in the extracellular fluid compartment. D. the solution is isosmotic with 60 mm NaCl. E. the effective osmotic pressure of this solution is 150 mosm. 3. Solutions 1 and 2 contain different concentrations of the same solute. After a brief time, the volume of Solution 1 decreases, and the volume of Solution 2 increases. Which of the following statements explains or describes these initial volume changes? A. Initially, the effective osmotic pressure of Solution 1 is greater than the effective osmotic pressure of Solution 2. B. The observed volume changes would have been greater if the reflection coefficient of the solute is 1.0 rather than 0.5. C. The observed volume changes would have been greater if the solute concentration of Solution 1 was doubled. D. The reflection coefficient of the solute is zero. 4. Which of the following pairs of solutions is both isosmotic and isotonic. Assume that NaCl is completely dissociated (i.e., g = 2) and the following values for σ. Protein 1.0 Sucrose 0.6 NaCl 0.5 Urea 0 Water 0 A. 100 mm protein; 100 mm sucrose B. 100 mm sucrose; 100 mm NaCl C. 100 mm urea; 50 mm NaCl D. 100 mm urea; water E. None of the above ANSWERS
8 1. C. (Since no volume change occurred, 150 mm NaCl must be isotonic with respect to the ICF. That is it must be in osmotic equilibrium with it. Since the osmotic pressure of the NaCl is approximately 2 x 150 = 300 mosm, this must also be the osmotic pressure of the ICF.) 2. B. (Since the substance is a nonelectrolyte its osmotic pressure is 1 mosmole/mmole x 300 mm = 300 mosm. However, its effective osmotic pressure with respect to red blood cell membranes is: σ x 300 = 0.4 x 300 = 120 mosm. That is it has the same effect as a substance with σ = 1, but at an osmotic pressure of 120 mosm. Such as substance would be NaCl at 60 mm, since it would also have an osmotic pressure of 2 x 60 = 120 mosm. This is a hypotonic solution. That is why a 300 mosm solution of this substance causes the cells to swell. The solution is clearly not isosmotic with respect to 60 mm NaCl. The latter has far fewer dissolved particles than the solution in question.) 3. B. Since water flowed into Solution 2 (its volume increased), the initial effective osmotic pressure of Solution 2 must have been greater than that of Solution 1. The higher the reflection coefficient, the greater the effective osmotic pressure difference and the greater the water flow. If the solute concentration of Solution was doubled, the initial effective osmotic pressure difference would have been decreased. If the reflection coefficient was zero, there would have been no water flow. 4. E.
Cellular Physiology. Body Fluids: 1) Water: (universal solvent) Body water varies based on of age, sex, mass, and body composition
 Membrane Physiology Body Fluids: 1) Water: (universal solvent) Body water varies based on of age, sex, mass, and body composition H 2 O ~ 73% body weight Low body fat; Low bone mass H 2 O ( ) ~ 60% body
Membrane Physiology Body Fluids: 1) Water: (universal solvent) Body water varies based on of age, sex, mass, and body composition H 2 O ~ 73% body weight Low body fat; Low bone mass H 2 O ( ) ~ 60% body
Lab 4: Osmosis and Diffusion
 Page 4.1 Lab 4: Osmosis and Diffusion Cells need to obtain water and other particles from the fluids that surround them. Water and other particles also move out of cells. Osmosis (for water) and diffusion
Page 4.1 Lab 4: Osmosis and Diffusion Cells need to obtain water and other particles from the fluids that surround them. Water and other particles also move out of cells. Osmosis (for water) and diffusion
Membrane Dynamics. The body is mostly water. Figure 5.1a Body Fluid Compartments. Figure 5.1c Body Fluid Compartments 2016 Pearson Education, Inc.
 Figure 5.1a Body Fluid Compartments Membrane Dynamics The body is mostly water Figure 5.1c Body Fluid Compartments 1 Osmotic Equilibrium Chemical Disequilibrium ØWater moves freely between the ECF and
Figure 5.1a Body Fluid Compartments Membrane Dynamics The body is mostly water Figure 5.1c Body Fluid Compartments 1 Osmotic Equilibrium Chemical Disequilibrium ØWater moves freely between the ECF and
There are mainly two types of transport :
 There are mainly two types of transport : # Type one: Passive diffusion 1- which does not require additional energy and occurs down the concentration gradient (high low concentration) " Down Hill" (^_^
There are mainly two types of transport : # Type one: Passive diffusion 1- which does not require additional energy and occurs down the concentration gradient (high low concentration) " Down Hill" (^_^
Ch 3 Membrane Transports
 Ch 3 Membrane Transports what's so dynamic about cell membranes? living things get nutrients and energy from the envrionment this is true of the entire organism and each cell this requires transport in/out
Ch 3 Membrane Transports what's so dynamic about cell membranes? living things get nutrients and energy from the envrionment this is true of the entire organism and each cell this requires transport in/out
LAB 4: OSMOSIS AND DIFFUSION
 Page 4.1 LAB 4: OSMOSIS AND DIFFUSION Cells need to obtain water and other particles from the fluids that surround them. Water and other particles also move out of cells. Osmosis (for water) and diffusion
Page 4.1 LAB 4: OSMOSIS AND DIFFUSION Cells need to obtain water and other particles from the fluids that surround them. Water and other particles also move out of cells. Osmosis (for water) and diffusion
The table indicates how changing the variable listed alone will alter diffusion rate.
 Rate of Diffusion (flux) Concentration gradient substance x surface area of membrane x lipid solubility = Distance (thickness of membrane) x molecular weight Table 3-1: Factors Influencing the Rate of
Rate of Diffusion (flux) Concentration gradient substance x surface area of membrane x lipid solubility = Distance (thickness of membrane) x molecular weight Table 3-1: Factors Influencing the Rate of
Consider the structure of the plasma membrane (fig. 8.6)- phospholipid bilayer with peripheral and integral proteins.
 Topic 8: MEMBRANE TRANSPORT (lectures 11-12) OBJECTIVES: 1. Have a basic appreciation of the chemical characteristics of substances that impact their ability to travel across plasma membranes. 2. Know
Topic 8: MEMBRANE TRANSPORT (lectures 11-12) OBJECTIVES: 1. Have a basic appreciation of the chemical characteristics of substances that impact their ability to travel across plasma membranes. 2. Know
 Taking care of business Go to this page and enter room SJ123: http://tinyurl.com/physclicker Take 2 minutes to complete this survey: http://tinyurl.com/physdis Online quiz this weekend: Released Thursday
Taking care of business Go to this page and enter room SJ123: http://tinyurl.com/physclicker Take 2 minutes to complete this survey: http://tinyurl.com/physdis Online quiz this weekend: Released Thursday
Terminology. Terminology. Terminology. Molarity number of moles of solute / Liter of solution. a) Terminology b) Body Fluid Compartments
 Integrative Sciences: Biological Systems A Fall 2011 Body Fluids Compartments, Renal Clearance and Renal Excretion of Drugs Monday, November 21, 2011 Lisa M. Harrison-Bernard, Ph.D. Department of Physiology;
Integrative Sciences: Biological Systems A Fall 2011 Body Fluids Compartments, Renal Clearance and Renal Excretion of Drugs Monday, November 21, 2011 Lisa M. Harrison-Bernard, Ph.D. Department of Physiology;
Physiology of the body fluids, Homeostasis
 Physiology of the body fluids, Homeostasis Tamas Banyasz The Body as an open system 1. Open system: The body exchanges material and energy with its environment 2. Homeostasis: The process through which
Physiology of the body fluids, Homeostasis Tamas Banyasz The Body as an open system 1. Open system: The body exchanges material and energy with its environment 2. Homeostasis: The process through which
WATER AND SOLUTE MOVEMENT THROUGH RED BLOOD CELLS
 WATER AND SOLUTE MOVEMENT THROUGH RED BLOOD CELLS Purpose This exercise is designed to demonstrate the properties of cellular membranes and the movement of water and solutes across them. In this lab, you
WATER AND SOLUTE MOVEMENT THROUGH RED BLOOD CELLS Purpose This exercise is designed to demonstrate the properties of cellular membranes and the movement of water and solutes across them. In this lab, you
Transport Across the Cell Membrane 11/5/07
 11/5/07 "The difference between the internal and external chemical composition of a cell represents a degree of order, that can be maintained only by a barrier to free movement into and out of the cell.
11/5/07 "The difference between the internal and external chemical composition of a cell represents a degree of order, that can be maintained only by a barrier to free movement into and out of the cell.
Lujain Al_Adayleh. Amani Nofal. Mohammad khatatbeh
 4 Lujain Al_Adayleh Amani Nofal Mohammad khatatbeh Recap : Filtration: the movement according to the differences of pressure. **note: not all particles have the same solubility through plasma membrane.
4 Lujain Al_Adayleh Amani Nofal Mohammad khatatbeh Recap : Filtration: the movement according to the differences of pressure. **note: not all particles have the same solubility through plasma membrane.
Cellular Transport Notes
 Cellular Transport Notes About Cell Membranes All cells have a cell membrane Functions: a. Controls what enters and exits the cell to maintain an internal balance called homeostasis b. Provides protection
Cellular Transport Notes About Cell Membranes All cells have a cell membrane Functions: a. Controls what enters and exits the cell to maintain an internal balance called homeostasis b. Provides protection
Chapter 4 Cell Membrane Transport
 Chapter 4 Cell Membrane Transport Plasma Membrane Review o Functions Separate ICF / ECF Allow exchange of materials between ICF / ECF such as obtaining O2 and nutrients and getting rid of waste products
Chapter 4 Cell Membrane Transport Plasma Membrane Review o Functions Separate ICF / ECF Allow exchange of materials between ICF / ECF such as obtaining O2 and nutrients and getting rid of waste products
Cell Processes: Osmosis
 www.mathbench.umd.edu./modules-au Osmosis May 2015 page 1 Cell Processes: Osmosis URL: http://mathbench.org.au/cellular-processes/osmosis/ Learning Outcomes After completing this module you should be able
www.mathbench.umd.edu./modules-au Osmosis May 2015 page 1 Cell Processes: Osmosis URL: http://mathbench.org.au/cellular-processes/osmosis/ Learning Outcomes After completing this module you should be able
A Closer Look at Cell Membranes. Chapter 5 Part 2
 A Closer Look at Cell Membranes Chapter 5 Part 2 5.5 Membrane Trafficking By processes of endocytosis and exocytosis, vesicles help cells take in and expel particles that are too big for transport proteins,
A Closer Look at Cell Membranes Chapter 5 Part 2 5.5 Membrane Trafficking By processes of endocytosis and exocytosis, vesicles help cells take in and expel particles that are too big for transport proteins,
Physiology. Cases and Problems FOURTH EDITION
 Physiology Cases and Problems FOURTH EDITION Physiology Cases and Problems FOURTH EDITION Linda S. Costanzo, Ph.D. Professor of Physiology and Biophysics Medical College of Virginia Virginia Commonwealth
Physiology Cases and Problems FOURTH EDITION Physiology Cases and Problems FOURTH EDITION Linda S. Costanzo, Ph.D. Professor of Physiology and Biophysics Medical College of Virginia Virginia Commonwealth
Biology. Membranes.
 1 Biology Membranes 2015 10 28 www.njctl.org 2 Vocabulary active transport carrier protein channel protein concentration gradient diffusion enzymatic activity facilitated diffusion fluid mosaic hypertonic
1 Biology Membranes 2015 10 28 www.njctl.org 2 Vocabulary active transport carrier protein channel protein concentration gradient diffusion enzymatic activity facilitated diffusion fluid mosaic hypertonic
5.6 Diffusion, Membranes, and Metabolism
 5.6 Diffusion, Membranes, and Metabolism Concentration of a substance Number of atoms or molecules in a given volume Concentration gradient of a substance A difference in concentration between two regions
5.6 Diffusion, Membranes, and Metabolism Concentration of a substance Number of atoms or molecules in a given volume Concentration gradient of a substance A difference in concentration between two regions
Transport across the cell membrane
 Transport across the cell membrane Learning objectives Body compartments ECF and ICF Constituents Lipid Bilayer: Barrier to water and water-soluble substances ions glucose H 2 O urea CO 2 O 2 N 2 halothane
Transport across the cell membrane Learning objectives Body compartments ECF and ICF Constituents Lipid Bilayer: Barrier to water and water-soluble substances ions glucose H 2 O urea CO 2 O 2 N 2 halothane
Passive Cellular Transport. Unit 2 Lesson 4
 Unit 2 Lesson 4 Students will be able to: Define passive transport Enumerate the three types of passive transport Described each type of passive transport: osmosis, diffusion, and facilitated diffusion
Unit 2 Lesson 4 Students will be able to: Define passive transport Enumerate the three types of passive transport Described each type of passive transport: osmosis, diffusion, and facilitated diffusion
Interactions Between Cells and the Extracellular Environment
 Chapter 6 Interactions Between Cells and the Extracellular Environment Et Extracellular lll environment Includes all parts of the body outside of cells Cells receive nourishment Cells release waste Cells
Chapter 6 Interactions Between Cells and the Extracellular Environment Et Extracellular lll environment Includes all parts of the body outside of cells Cells receive nourishment Cells release waste Cells
[S] [S] Hypertonic [H O] [H 2 O] g. Osmosis is the diffusion of water through membranes! 15. Osmosis. Concentrated sugar solution
![[S] [S] Hypertonic [H O] [H 2 O] g. Osmosis is the diffusion of water through membranes! 15. Osmosis. Concentrated sugar solution [S] [S] Hypertonic [H O] [H 2 O] g. Osmosis is the diffusion of water through membranes! 15. Osmosis. Concentrated sugar solution](/thumbs/88/115710711.jpg) Concentrated sugar solution Sugar molecules (Water molecules not shown) 100ml 100ml Hypertonic [S] g [H2 Hypotonic [H O] 2 O] [H 2 O] g Semipermeable Dilute sugar solution (100ml) Time 125ml Osmosis 75ml
Concentrated sugar solution Sugar molecules (Water molecules not shown) 100ml 100ml Hypertonic [S] g [H2 Hypotonic [H O] 2 O] [H 2 O] g Semipermeable Dilute sugar solution (100ml) Time 125ml Osmosis 75ml
BASIC MEDICAL SCIENCE OF THE RENAL AND URINARY SYSTEMS
 Ch01M3428.qxd 12/5/06 6:47 M age 1 BASIC MEDICAL SCIENCE OF THE RENAL AND URINARY SYSTEMS Basic principles 3 Organization of the kidneys 13 Renal function 39 The kidneys in disease 65 The lower urinary
Ch01M3428.qxd 12/5/06 6:47 M age 1 BASIC MEDICAL SCIENCE OF THE RENAL AND URINARY SYSTEMS Basic principles 3 Organization of the kidneys 13 Renal function 39 The kidneys in disease 65 The lower urinary
Unit 3: Cellular Processes. 1. SEPARTION & PROTECTION: the contents of the cell from the. 2. TRANSPORT: the transport of in and out of the cell
 Unit 3: Cellular Processes Name: Aim #14 Cell Membrane: How does the cell membrane function to maintain homeostasis? Date: _ I. The Cell Membrane: What is it? Also known as A thin structure that acts as
Unit 3: Cellular Processes Name: Aim #14 Cell Membrane: How does the cell membrane function to maintain homeostasis? Date: _ I. The Cell Membrane: What is it? Also known as A thin structure that acts as
Cell Membrane (Transport) Notes
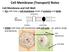 Cell Membrane (Transport) Notes Cell Membrane and Cell Wall: ALL cells have a cell membrane made of proteins and lipids protein channel Cell Membrane Layer 1 Layer 2 lipid bilayer protein pump SOME cells
Cell Membrane (Transport) Notes Cell Membrane and Cell Wall: ALL cells have a cell membrane made of proteins and lipids protein channel Cell Membrane Layer 1 Layer 2 lipid bilayer protein pump SOME cells
LAB 04 Diffusion and Osmosis
 LAB 04 Diffusion and Osmosis Objectives: Describe the physical mechanisms of diffusion and osmosis. Understand the relationship between surface area and rate of diffusion. Describe how molar concentration
LAB 04 Diffusion and Osmosis Objectives: Describe the physical mechanisms of diffusion and osmosis. Understand the relationship between surface area and rate of diffusion. Describe how molar concentration
Body Fluid Compartments and Cell Membranes Linda S. Costanzo, Ph.D.
 Body Fluid Compartments and Cell Membranes Linda S. Costanzo, Ph.D. OBJECTIVES: 1. Describe the major and minor body fluid compartments. 2. Compare the total cation and anion concentrations in meq/l in
Body Fluid Compartments and Cell Membranes Linda S. Costanzo, Ph.D. OBJECTIVES: 1. Describe the major and minor body fluid compartments. 2. Compare the total cation and anion concentrations in meq/l in
FLEXIBLE, SELECTIVELY PERMEABLE boundary that helps control what enters and leaves the cell. Composed of: a. Two layers of PHOSPHOLIPIDS molecules
 FLEXIBLE, SELECTIVELY PERMEABLE boundary that helps control what enters and leaves the cell. Composed of: a. Two layers of PHOSPHOLIPIDS molecules arranged with POLAR HEADS facing outside and NON-POLAR
FLEXIBLE, SELECTIVELY PERMEABLE boundary that helps control what enters and leaves the cell. Composed of: a. Two layers of PHOSPHOLIPIDS molecules arranged with POLAR HEADS facing outside and NON-POLAR
Contents. Module A Cells and Cell Processes. Module B Continuity and Unity Of Life. Introduction to Keystone Finish Line Biology...
 Contents Introduction to Keystone Finish Line Biology...5 Module A Cells and Cell Processes Unit 1 Basic Biological Principles...7 Lesson 1 Unifying Characteristics of Life BIO.A.1.1.1, BIO.A.1.2.1...8
Contents Introduction to Keystone Finish Line Biology...5 Module A Cells and Cell Processes Unit 1 Basic Biological Principles...7 Lesson 1 Unifying Characteristics of Life BIO.A.1.1.1, BIO.A.1.2.1...8
Diffusion, osmosis, transport mechanisms 43
 Diffusion, osmosis, transport mechanisms 43 DIFFUSION, OSMOSIS AND TRANSPORT MECHANISMS The cell membrane is a biological membrane that separates the interior of all cells from the outside environment
Diffusion, osmosis, transport mechanisms 43 DIFFUSION, OSMOSIS AND TRANSPORT MECHANISMS The cell membrane is a biological membrane that separates the interior of all cells from the outside environment
Principles of Fluid Balance
 Principles of Fluid Balance I. The Cellular Environment: Fluids and Electrolytes A. Water 1. Total body water (TBW) = 60% of total body weight 2. Fluid Compartments in the Body a. Intracellular Compartment
Principles of Fluid Balance I. The Cellular Environment: Fluids and Electrolytes A. Water 1. Total body water (TBW) = 60% of total body weight 2. Fluid Compartments in the Body a. Intracellular Compartment
CHAPTER. Movement Across Plasma Membrane. Chapter 6 Outline. Diffusion Osmosis. Membrane Potential Cell Signaling
 CHAPTER 6 Interaction Between Cells and the Extracellular Environment Chapter 6 Outline Extracellular Environment Diffusion Osmosis Carrier-Mediated Carrier Mediated Transport Membrane Potential Cell Signaling
CHAPTER 6 Interaction Between Cells and the Extracellular Environment Chapter 6 Outline Extracellular Environment Diffusion Osmosis Carrier-Mediated Carrier Mediated Transport Membrane Potential Cell Signaling
Passive Transport. Does not expend cellular energy for the movement to take place. Ex-rolling down a hill
 Passive Transport Fluid Mosaic Model Passive Transport Does not expend cellular energy for the movement to take place Ex-rolling down a hill Parts of a Solution Solute: what gets dissolved Solvent: What
Passive Transport Fluid Mosaic Model Passive Transport Does not expend cellular energy for the movement to take place Ex-rolling down a hill Parts of a Solution Solute: what gets dissolved Solvent: What
Body Fluid Compartments
 Yıldırım Beyazıt University Faculty of Medicine Department of Physiology Body Fluid Compartments Dr. Sinan Canan Body fluid balance 1 Body fluid compartments 2 Water distribution Tissue % Water Blood 83,0
Yıldırım Beyazıt University Faculty of Medicine Department of Physiology Body Fluid Compartments Dr. Sinan Canan Body fluid balance 1 Body fluid compartments 2 Water distribution Tissue % Water Blood 83,0
Dr. Mohamed S. Daoud Biochemistry Department College of Science, KSU. Dr. Mohamed Saad Daoud
 Dr. Mohamed S. Daoud Biochemistry Department College of Science, KSU 1 Course symbol: BCH 472 Course Title: Biochemistry of biological fluids Credit hours: 3(2+1) 2 Clinical Biochemistry is one of the
Dr. Mohamed S. Daoud Biochemistry Department College of Science, KSU 1 Course symbol: BCH 472 Course Title: Biochemistry of biological fluids Credit hours: 3(2+1) 2 Clinical Biochemistry is one of the
Membrane Transport. Anatomy 36 Unit 1
 Membrane Transport Anatomy 36 Unit 1 Membrane Transport Cell membranes are selectively permeable Some solutes can freely diffuse across the membrane Some solutes have to be selectively moved across the
Membrane Transport Anatomy 36 Unit 1 Membrane Transport Cell membranes are selectively permeable Some solutes can freely diffuse across the membrane Some solutes have to be selectively moved across the
Transport through membranes
 Transport through membranes Membrane transport refers to solute and solvent transfer across both cell membranes, epithelial and capillary membranes. Biological membranes are composed of phospholipids stabilised
Transport through membranes Membrane transport refers to solute and solvent transfer across both cell membranes, epithelial and capillary membranes. Biological membranes are composed of phospholipids stabilised
What is the function of the cell membrane?
 What is the function of the cell membrane? 1. DIFFUSION: The movement of molecules from an area of high concentration to an area of lower concentration. Why do molecules move from high concentration to
What is the function of the cell membrane? 1. DIFFUSION: The movement of molecules from an area of high concentration to an area of lower concentration. Why do molecules move from high concentration to
David Huang! AP Biology! Oct. 4,2013! AP Biology Osmosis Laboratory Analysis! Introduction:!! There are several different methods for the
 David Huang AP Biology Oct. 4,2013 AP Biology Osmosis Laboratory Analysis Introduction: There are several different methods for the transportation of molecules across the phospholipid bilayer. These transportation
David Huang AP Biology Oct. 4,2013 AP Biology Osmosis Laboratory Analysis Introduction: There are several different methods for the transportation of molecules across the phospholipid bilayer. These transportation
Outline. Membrane Structure and Function. Membrane Models Fluid-Mosaic. Chapter 5
 Membrane Structure and Function Chapter 5 Membrane Models Fluid-Mosaic Outline Plasma Membrane Structure and Function Protein Functions Plasma Membrane Permeability! Diffusion! Osmosis! Transport Via Carrier
Membrane Structure and Function Chapter 5 Membrane Models Fluid-Mosaic Outline Plasma Membrane Structure and Function Protein Functions Plasma Membrane Permeability! Diffusion! Osmosis! Transport Via Carrier
Membrane transport. Small molecules. pumps. Large molecules
 Cell Membrane and Transport Review Sheet Transport of nutrients, ions, and excretory substances from one side to the other is a major function of the cell membrane. A number of different means have been
Cell Membrane and Transport Review Sheet Transport of nutrients, ions, and excretory substances from one side to the other is a major function of the cell membrane. A number of different means have been
CH 7.2 & 7.4 Biology
 CH 7.2 & 7.4 Biology LABEL THE MEMBRANE Phospholipids Cholesterol Peripheral proteins Integral proteins Cytoskeleton Cytoplasm Extracellular fluid Most of the membrane A phospholipid bi-layer makes up
CH 7.2 & 7.4 Biology LABEL THE MEMBRANE Phospholipids Cholesterol Peripheral proteins Integral proteins Cytoskeleton Cytoplasm Extracellular fluid Most of the membrane A phospholipid bi-layer makes up
Review: Cellular Transport
 Review: Cellular Transport OSMOSIS 1. Label the pictures below ( isotonic, hypertonic, or hypotonic). The dots represent solutes. A. B. C. 2. means there is a GREATER concentration of solute molecules
Review: Cellular Transport OSMOSIS 1. Label the pictures below ( isotonic, hypertonic, or hypotonic). The dots represent solutes. A. B. C. 2. means there is a GREATER concentration of solute molecules
Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are
 Fluid, Electrolyte, and Acid-Base Balance Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60%
Fluid, Electrolyte, and Acid-Base Balance Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60%
Lesson Overview. 7.3 Cell Transport
 7.3 THINK ABOUT IT When thinking about how cells move materials in and out, it can be helpful to think of a cell as a nation. The boundaries of a nation are its borders, and nearly every country tries
7.3 THINK ABOUT IT When thinking about how cells move materials in and out, it can be helpful to think of a cell as a nation. The boundaries of a nation are its borders, and nearly every country tries
INVESTIGATION : Determining Osmolarity of Plant Tissue
 INVESTIGATION : Determining Osmolarity of Plant Tissue AP Biology This lab investigation has two main components. In the first component, you will learn about the osmolarity of plant tissues and the property
INVESTIGATION : Determining Osmolarity of Plant Tissue AP Biology This lab investigation has two main components. In the first component, you will learn about the osmolarity of plant tissues and the property
Body Water ANS 215 Physiology and Anatomy of Domesticated Animals
 Body Water ANS 215 Physiology and Anatomy of Domesticated Animals I. Body Water A. Water is the most abundant constituent comprising 60% of total body weight. 1. Solvent for many chemicals of the body
Body Water ANS 215 Physiology and Anatomy of Domesticated Animals I. Body Water A. Water is the most abundant constituent comprising 60% of total body weight. 1. Solvent for many chemicals of the body
Razi Kittaneh & Tamer Barakat. Bayan Abusheikha. Faisal Mohammed
 3 Razi Kittaneh & Tamer Barakat Bayan Abusheikha Faisal Mohammed Transport and Osmolality In the last lecture we briefly talked about Transport, there are 2 types of transport: 1) Passive Transport 2)
3 Razi Kittaneh & Tamer Barakat Bayan Abusheikha Faisal Mohammed Transport and Osmolality In the last lecture we briefly talked about Transport, there are 2 types of transport: 1) Passive Transport 2)
Describe the Fluid Mosaic Model of membrane structure.
 Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membranebound organelles. In this topic, we will examine the structure and
Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membranebound organelles. In this topic, we will examine the structure and
Passive Transport: Practice Problems PAP BIOLOGY
 Passive Transport: Practice Problems PAP BIOLOGY #1 Draw a diagram where the cell has low concentration of salt molecules and the environment it is in has a high concentration of salt molecules in a water
Passive Transport: Practice Problems PAP BIOLOGY #1 Draw a diagram where the cell has low concentration of salt molecules and the environment it is in has a high concentration of salt molecules in a water
Ch. 5 Homeostasis & Cell Transport
 Ch. 5 Homeostasis & Cell Transport 5.1 Homeostasis & Permeability Homeostasis ability of cell to maintain balance needed for life To maintain balance: cells must transport needed materials into cells &
Ch. 5 Homeostasis & Cell Transport 5.1 Homeostasis & Permeability Homeostasis ability of cell to maintain balance needed for life To maintain balance: cells must transport needed materials into cells &
μ i = chemical potential of species i C i = concentration of species I
 BIOE 459/559: Cell Engineering Membrane Permeability eferences: Water Movement Through ipid Bilayers, Pores and Plasma Membranes. Theory and eality, Alan Finkelstein, 1987 Membrane Permeability, 100 Years
BIOE 459/559: Cell Engineering Membrane Permeability eferences: Water Movement Through ipid Bilayers, Pores and Plasma Membranes. Theory and eality, Alan Finkelstein, 1987 Membrane Permeability, 100 Years
PROBLEM SET 7.1 FLUID VOLUMES, GLOMERULAR FILTRATION AND CLEARANCE
 PROBLEM SET 7.1 FLUID VOLUMES, GLOMERULAR FILTRATION AND CLEARANCE ANSWER KEY 1. The time course of decay of plasma [inulin] shown in Fig. 6.1.1 can be simultaneously used to determine the ECF volume and
PROBLEM SET 7.1 FLUID VOLUMES, GLOMERULAR FILTRATION AND CLEARANCE ANSWER KEY 1. The time course of decay of plasma [inulin] shown in Fig. 6.1.1 can be simultaneously used to determine the ECF volume and
The Plasma Membrane - Gateway to the Cell
 The Plasma Membrane - Gateway to the Cell 1 Photograph of a Cell Membrane 2 Cell Membrane The cell membrane is flexible and allows a unicellular organism to move 3 Homeostasis Balanced internal condition
The Plasma Membrane - Gateway to the Cell 1 Photograph of a Cell Membrane 2 Cell Membrane The cell membrane is flexible and allows a unicellular organism to move 3 Homeostasis Balanced internal condition
Cellular Transport Notes
 Cellular Transport Notes About Cell Membranes 1.All cells have a cell membrane a.controls what enters and exits the cell to maintain an internal balance called homeostasis b.provides protection and support
Cellular Transport Notes About Cell Membranes 1.All cells have a cell membrane a.controls what enters and exits the cell to maintain an internal balance called homeostasis b.provides protection and support
Membrane Transport. Biol219 Lecture 9 Fall 2016
 Membrane Transport Permeability - the ability of a substance to pass through a membrane Cell membranes are selectively permeable Permeability is determined by A. the phospholipid bilayer and B. transport
Membrane Transport Permeability - the ability of a substance to pass through a membrane Cell membranes are selectively permeable Permeability is determined by A. the phospholipid bilayer and B. transport
BIPN100 Human Physiology 1 Bill Kristan, Ph.D.
 1. Dr K s office hours: In office Tuesdays 2:30 3:30 PM (In office: 3122A Pacific Hall) Group Friday 11 noon (MeeAng room: 3502 Pacific Hall) 2. Dr K s problem solving session: not this week BIPN100 Human
1. Dr K s office hours: In office Tuesdays 2:30 3:30 PM (In office: 3122A Pacific Hall) Group Friday 11 noon (MeeAng room: 3502 Pacific Hall) 2. Dr K s problem solving session: not this week BIPN100 Human
Lecture Overview. Cell Membrane. Marieb s Human Anatomy and Physiology. Chapter 3 Cell Membranes Movement Across the Cell Membrane Lecture 7
 Marieb s Human Anatomy and Physiology Marieb Hoehn Chapter 3 Cell Membranes Movement Across the Cell Membrane Lecture 7 1 The cell membrane Lecture Overview Osmotic pressure and tonicity Movement of substances
Marieb s Human Anatomy and Physiology Marieb Hoehn Chapter 3 Cell Membranes Movement Across the Cell Membrane Lecture 7 1 The cell membrane Lecture Overview Osmotic pressure and tonicity Movement of substances
FIGURE A. The phosphate end of the molecule is polar (charged) and hydrophilic (attracted to water).
 PLASMA MEMBRANE 1. The plasma membrane is the outermost part of a cell. 2. The main component of the plasma membrane is phospholipids. FIGURE 2.18 A. The phosphate end of the molecule is polar (charged)
PLASMA MEMBRANE 1. The plasma membrane is the outermost part of a cell. 2. The main component of the plasma membrane is phospholipids. FIGURE 2.18 A. The phosphate end of the molecule is polar (charged)
URINARY SYSTEM. Primary functions. Major organs & structures
 URINARY SYSTEM Primary functions Excretion of metabolic wastes Regulation of water and ion balances Regulation of blood pressure Vitamin D activation Regulation of rbc s (erythropoietin) Gluconeogenesis
URINARY SYSTEM Primary functions Excretion of metabolic wastes Regulation of water and ion balances Regulation of blood pressure Vitamin D activation Regulation of rbc s (erythropoietin) Gluconeogenesis
Section 4: Cellular Transport. Cellular transport moves substances within the cell and moves substances into and out of the cell.
 Section 4: Cellular transport moves substances within the cell and moves substances into and out of the cell. Essential Questions What are the processes of diffusion, facilitated diffusion, and active
Section 4: Cellular transport moves substances within the cell and moves substances into and out of the cell. Essential Questions What are the processes of diffusion, facilitated diffusion, and active
BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes
 BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes The cell membrane is the gateway into the cell, and must allow needed things such as nutrients into the cell without letting them escape.
BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes The cell membrane is the gateway into the cell, and must allow needed things such as nutrients into the cell without letting them escape.
9/20/2016 CHAPTER 7 LECTURE NOTES. Section Objectives. Explain how a cell s plasma membrane functions.
 CHAPTER 7 LECTURE NOTES Kennedy biol. 1ab Section Objectives Explain how a cell s plasma membrane functions. Relate the function of the plasma membrane to the fluid mosaic model. All living cells must
CHAPTER 7 LECTURE NOTES Kennedy biol. 1ab Section Objectives Explain how a cell s plasma membrane functions. Relate the function of the plasma membrane to the fluid mosaic model. All living cells must
How Cells Work. Chapter 4
 How Cells Work Chapter 4 Energy Laws Energy is the capacity to do work The total amount of energy in the universe is constant-energy can t be created or destroyed..only transferred! Energy is flowing from
How Cells Work Chapter 4 Energy Laws Energy is the capacity to do work The total amount of energy in the universe is constant-energy can t be created or destroyed..only transferred! Energy is flowing from
CELL BOUNDARIES. Cells create boundaries through: Cell Membranes made of the phospholipid bilayer Cell Walls made of cellulose in plants
 CELL BOUNDARIES CELL BOUNDARIES Cells create boundaries through: Cell Membranes made of the phospholipid bilayer Cell Walls made of cellulose in plants TYPES OF MEMBRANES Some substances = too large or
CELL BOUNDARIES CELL BOUNDARIES Cells create boundaries through: Cell Membranes made of the phospholipid bilayer Cell Walls made of cellulose in plants TYPES OF MEMBRANES Some substances = too large or
Osmoregulation and Osmotic Balance
 OpenStax-CNX module: m44808 1 Osmoregulation and Osmotic Balance OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this
OpenStax-CNX module: m44808 1 Osmoregulation and Osmotic Balance OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this
Biology. Slide 1 / 74. Slide 2 / 74. Slide 3 / 74. Membranes. Vocabulary
 Slide 1 / 74 Slide 2 / 74 iology Membranes 2015-10-28 www.njctl.org Vocabulary Slide 3 / 74 active transport carrier protein channel protein concentration gradient diffusion enzymatic activity facilitated
Slide 1 / 74 Slide 2 / 74 iology Membranes 2015-10-28 www.njctl.org Vocabulary Slide 3 / 74 active transport carrier protein channel protein concentration gradient diffusion enzymatic activity facilitated
Phospholipids. Extracellular fluid. Polar hydrophilic heads. Nonpolar hydrophobic tails. Polar hydrophilic heads. Intracellular fluid (cytosol)
 Module 2C Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membrane- bound organelles. In this module, we will examine the
Module 2C Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membrane- bound organelles. In this module, we will examine the
Microcirculation and Edema. Faisal I. Mohammed MD, PhD.
 Microcirculation and Edema Faisal I. Mohammed MD, PhD. Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchang in capillaries. Outline what
Microcirculation and Edema Faisal I. Mohammed MD, PhD. Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchang in capillaries. Outline what
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Basic mechanisms of Kidney function
 Excretion Basic mechanisms of Kidney function Urine formation in Amphibians Urine formation in Mammals Urine formation in Insects Nitrogen balance Kidneys The most fundamental function of kidneys) is to
Excretion Basic mechanisms of Kidney function Urine formation in Amphibians Urine formation in Mammals Urine formation in Insects Nitrogen balance Kidneys The most fundamental function of kidneys) is to
Electrolytes Solution
 Electrolytes Solution Substances that are not dissociated in solution are called nonelectrolytes, and those with varying degrees of dissociation are called electrolytes. Urea and dextrose are examples
Electrolytes Solution Substances that are not dissociated in solution are called nonelectrolytes, and those with varying degrees of dissociation are called electrolytes. Urea and dextrose are examples
Maintained by plasma membrane controlling what enters & leaves the cell
 CELL TRANSPORT AND HOMEOSTASIS Homeostasis Balanced internal condition of cells Also called equilibrium Maintained by plasma membrane controlling what enters & leaves the cell Functions of Plasma Membrane
CELL TRANSPORT AND HOMEOSTASIS Homeostasis Balanced internal condition of cells Also called equilibrium Maintained by plasma membrane controlling what enters & leaves the cell Functions of Plasma Membrane
Gateway to the Cell 11/1/2012. The cell membrane is flexible and allows a unicellular organism to move FLUID MOSAIC MODEL
 Gateway to the Cell The cell membrane is flexible and allows a unicellular organism to move Isolates the cell, yet allows communication with its surroundings fluid mosaics = proteins (and everything else)
Gateway to the Cell The cell membrane is flexible and allows a unicellular organism to move Isolates the cell, yet allows communication with its surroundings fluid mosaics = proteins (and everything else)
Equilibrium when two areas have the same concentration or are filled evenly
 Aim: How does the cell membrane function to maintain homeostasis? Do Now: Describe what homeostasis is. Homework: Vocab: Homeostasis, equilibrium, concentration gradient, diffusion, carrier protein, osmosis,
Aim: How does the cell membrane function to maintain homeostasis? Do Now: Describe what homeostasis is. Homework: Vocab: Homeostasis, equilibrium, concentration gradient, diffusion, carrier protein, osmosis,
DIFFUSON AND OSMOSIS INTRODUCTION diffusion concentration gradient. net osmosis water potential active transport
 DIFFUSON AND OSMOSIS NAME DATE INTRODUCTION The life of a cell is dependent on efficiently moving material into and out of the cell across the cell membrane. Raw materials such as oxygen and sugars needed
DIFFUSON AND OSMOSIS NAME DATE INTRODUCTION The life of a cell is dependent on efficiently moving material into and out of the cell across the cell membrane. Raw materials such as oxygen and sugars needed
Ch. 7 Diffusion, Osmosis, and Movement across a Membrane
 Ch. 7 Diffusion, Osmosis, and Movement across a Membrane Diffusion Spontaneous movement of particles from an area of high concentration to an area of low concentration Does not require energy (exergonic)
Ch. 7 Diffusion, Osmosis, and Movement across a Membrane Diffusion Spontaneous movement of particles from an area of high concentration to an area of low concentration Does not require energy (exergonic)
Ch3: Cellular Transport Review KEY
 Ch3: Cellular Transport Review KEY OSMOSIS Label the pictures below ( isotonic, hypertonic, or hypotonic environments) hypotonic hypertonic isotonic hypertonic means there is a GREATER concentration of
Ch3: Cellular Transport Review KEY OSMOSIS Label the pictures below ( isotonic, hypertonic, or hypotonic environments) hypotonic hypertonic isotonic hypertonic means there is a GREATER concentration of
BIOL 305L Spring 2019 Laboratory Six
 Please print Full name clearly: BIOL 305L Spring 2019 Laboratory Six Osmosis in potato and carrot samples Introduction Osmosis is the movement of water molecules through a selectively permeable membrane
Please print Full name clearly: BIOL 305L Spring 2019 Laboratory Six Osmosis in potato and carrot samples Introduction Osmosis is the movement of water molecules through a selectively permeable membrane
PHYSIOLOGY 2017: OPTO 5344 Lecture 1. Transport across the cell membrane Constanzo 1. I. Introduction
 PHYSIOLOGY 2017: OPTO 5344 Lecture 1. Transport across the cell membrane Constanzo 1 I. Introduction Fig. 1 Composition and size of cells and organelles (Ganong, 21 st edition) Water - 70-85% of cell mass
PHYSIOLOGY 2017: OPTO 5344 Lecture 1. Transport across the cell membrane Constanzo 1 I. Introduction Fig. 1 Composition and size of cells and organelles (Ganong, 21 st edition) Water - 70-85% of cell mass
BIOL 347L Laboratory Three
 Introduction BIOL 347L Laboratory Three Osmosis in potato and carrot samples Osmosis is the movement of water molecules through a selectively permeable membrane into a region of higher solute concentration,
Introduction BIOL 347L Laboratory Three Osmosis in potato and carrot samples Osmosis is the movement of water molecules through a selectively permeable membrane into a region of higher solute concentration,
Equilibrium is a condition of balance. Changes in temperature, pressure or concentration can cause a shift in the equilibrium.
 Copy into Note Packet and Return to Teacher Cells and Their Environment Section 1: Passive Transport Objectives Relate concentration gradients, diffusion, and equilibrium. Predict the direction of water
Copy into Note Packet and Return to Teacher Cells and Their Environment Section 1: Passive Transport Objectives Relate concentration gradients, diffusion, and equilibrium. Predict the direction of water
Transport of Solutes and Water
 Transport of Solutes and Water Across cell membranes 1. Simple and Facilitated diffusion. 2. Active transport. 3. Osmosis. Simple diffusion Simple diffusion - the red particles are moving from an area
Transport of Solutes and Water Across cell membranes 1. Simple and Facilitated diffusion. 2. Active transport. 3. Osmosis. Simple diffusion Simple diffusion - the red particles are moving from an area
Cell Membrane-Structure and Function
 Cell Membrane-Structure and Function BIO 250 Living things are composed of cells and cell products (extracellular) Cells are the basic unit of structure They are the basic unit of function They vary in
Cell Membrane-Structure and Function BIO 250 Living things are composed of cells and cell products (extracellular) Cells are the basic unit of structure They are the basic unit of function They vary in
Cell Boundaries Section 7-3
 Cell Boundaries Section 7-3 The most important parts of a cell are its borders, which separate the cell from its surroundings. The cell membrane is a thin, flexible barrier that surrounds all cells. The
Cell Boundaries Section 7-3 The most important parts of a cell are its borders, which separate the cell from its surroundings. The cell membrane is a thin, flexible barrier that surrounds all cells. The
Diffusion, Osmosis and Active Transport
 Diffusion, Osmosis and Active Transport Part A: Diffusion A living cell interacts constantly with the environmental medium that surrounds it. The plasma membrane surrounding a cell is a living, selectively
Diffusion, Osmosis and Active Transport Part A: Diffusion A living cell interacts constantly with the environmental medium that surrounds it. The plasma membrane surrounding a cell is a living, selectively
Each cell has its own border, which separates the cell from its surroundings and also determines what comes in and what goes out.
 7.3 Cell Transport Wednesday, December 26, 2012 10:02 AM Vocabulary: Diffusion: process in which cells become specialized in structure and function Facilitated diffusion: process of diffusion in which
7.3 Cell Transport Wednesday, December 26, 2012 10:02 AM Vocabulary: Diffusion: process in which cells become specialized in structure and function Facilitated diffusion: process of diffusion in which
The Transport of Materials Across Cell Membranes
 The Transport of Materials Across Cell Membranes EK 2.B.1.b. LO 2.10 The Plasma Membrane 2 EK 2.B.1.b. LO 2.10 The Plasma Membrane The cell membrane is said to be semi permeable or selectively permeable
The Transport of Materials Across Cell Membranes EK 2.B.1.b. LO 2.10 The Plasma Membrane 2 EK 2.B.1.b. LO 2.10 The Plasma Membrane The cell membrane is said to be semi permeable or selectively permeable
EXERCISE Transport Mechanisms in the Body
 EXERCISE Transport Mechanisms in the Body 2 OBJECTIVES After completing these activities, you should be able to: Understand the differences between passive and active processes of transport Define diffusion,
EXERCISE Transport Mechanisms in the Body 2 OBJECTIVES After completing these activities, you should be able to: Understand the differences between passive and active processes of transport Define diffusion,
Slide 2 of 47. Copyright Pearson Prentice Hall. End Show
 2 of 47 7-3 Cell Boundaries All cells are surrounded by a thin, flexible barrier known as the cell membrane. Many cells also produce a strong supporting layer around the membrane known as a cell wall.
2 of 47 7-3 Cell Boundaries All cells are surrounded by a thin, flexible barrier known as the cell membrane. Many cells also produce a strong supporting layer around the membrane known as a cell wall.
The Plasma Membrane - Gateway to the Cell
 The Plasma Membrane - Gateway to the Cell 1 Photograph of a Cell Membrane 2 Cell Membrane The cell membrane is flexible and allows a unicellular organism to move 3 Homeostasis Balanced internal condition
The Plasma Membrane - Gateway to the Cell 1 Photograph of a Cell Membrane 2 Cell Membrane The cell membrane is flexible and allows a unicellular organism to move 3 Homeostasis Balanced internal condition
Renal Physiology. April, J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine.
 Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
1. How many fatty acid molecules combine with a glycerol to form a phospholipid molecule? A. 1 B. 2 C. 3 D. 4
 Topic 3: Movement of substances across cell membrane 1. How many fatty acid molecules combine with a glycerol to form a phospholipid molecule? A. 1 B. 2 C. 3 D. 4 Directions: Questions 2 and 3 refer to
Topic 3: Movement of substances across cell membrane 1. How many fatty acid molecules combine with a glycerol to form a phospholipid molecule? A. 1 B. 2 C. 3 D. 4 Directions: Questions 2 and 3 refer to
Measuring Osmotic Potential
 Measuring Osmotic Potential INTRODUCTION All cells require essential materials to ensure their survival. Chemical, physical, and biological processes are used to move these materials inside of cells. Similar
Measuring Osmotic Potential INTRODUCTION All cells require essential materials to ensure their survival. Chemical, physical, and biological processes are used to move these materials inside of cells. Similar
II. Active Transport (move molecules against conc. gradient - cell must expend energy) (uses carrier proteins)
 Chapter 5 - Homeostasis and Transport I. Passive Transport (no energy from cell required) A. Diffusion 1. movement of molecules from an area of higher concentration to an area of lower concentration a.
Chapter 5 - Homeostasis and Transport I. Passive Transport (no energy from cell required) A. Diffusion 1. movement of molecules from an area of higher concentration to an area of lower concentration a.
BIOLOGY - CLUTCH CH.44 - OSMOREGULATION AND EXCRETION.
 !! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
!! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
PAPER 12: MEMBRANE BIOPHYSICS. Module 18: Osmosis (pressure, Osmotic equilibrium, Donnan equilibrium, flow of water & solute), Electroosmosis
 PAPER 12: MEMBRANE BIOPHYSICS Module 18: Osmosis (pressure, Osmotic equilibrium, Donnan equilibrium, flow of water & solute), Electroosmosis Introduction: Think about a soap bubble, which consists of a
PAPER 12: MEMBRANE BIOPHYSICS Module 18: Osmosis (pressure, Osmotic equilibrium, Donnan equilibrium, flow of water & solute), Electroosmosis Introduction: Think about a soap bubble, which consists of a
