CENTRAL REGULATION OF BLOOD PRESSURE IN HYPERTENSION
|
|
|
- Godfrey Ramsey
- 6 years ago
- Views:
Transcription
1 CENTRAL REGULATION OF BLOOD PRESSURE IN HYPERTENSION By JOO YUN JUN A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA
2 2012 Joo Yun Jun 2
3 To my parents and husband, for their unconditional love and support throughout the years 3
4 ACKNOWLEDGMENTS First of all, I would like to thank my mentor, Dr. Mohan Raizada, who has provided me wonderful training and enlightening experiences for my PhD. I have come to respect Mohan s brilliance as a scientist as well as his knowledge and understanding of things beyond. He also carries a great sense of humor with him, which has made my graduate study much enjoyable and valuable. I also appreciate the immense patience he has exercised with me through the years and the support he has provided as an advisor. He has been an amazing teacher, leader and motivator in my graduate years. Moreover, he taught me a lot about knowledge of life and it is always pleasure to have a chat with him. I would also like to acknowledge my committee members Dr. Sumners, Dr. Katovich, Dr. Waters and also lab members Dr. Zubcevic, Dr. Shan, Dr. Shi, Dr. Shenoy, Dr. Bradford, Jessica Marulanda, Fan Lin, Alisha Dunn, Sara Croft for offering their expertise, suggestions and support through my graduate study. Most importantly, I cannot express enough gratitude to my parents and my two sisters, who have supported me throughout these school years. My husband Arnold, for helping me finish all the work with his experiences. I could never have come this far without them. 4
5 TABLE OF CONTENTS page ACKNOWLEDGMENTS... 4 LIST OF FIGURES... 8 ABSTRACT CHAPTER 1 NEUROGENIC HYPERTENSION Central Regulation of Blood Pressure Oxidative Stress and Inflammation in Neurogenic Hypertension Endothelial Progenitor Cells and Vascular Dysfunction in Hypertension and Cardiovascular Diseases ROLE OF BRAIN MITOCHONDRIAL OXIDATIVE STRESS IN NEUROGENIC HYPERTENSION Increased Oxidative Stress in Neural Mechanisms of Hypertension Role of Brain Mitochondrial ROS in Neurogenic Hypertension Methods Animal Primary Neuronal Culture Measurement of ROS Production Telemetry Recordings of Arterial Pressure and Heart Rate Measurement of Full Spectral Analysis Implantation of Subcutaneous Osmotic Minipumps Intracerebroventricular mitotempo Infusion Immunohistochemistry Cardiac Pathology RNA Isolation and Real-Time PCR Results ICV Infusion of mitotempo Attenuates Ang II-induced Neurogenic Hypertension mitotempo Scavenges Ang II-induced Mitochondria ROS in Neuronal Cells in Primary Cultures Central Mitochondrial Superoxide Inhibition Influences Autonomic Nerve Activity in AngII-induced Neurogenic Hypertension ICV mitotempo Inhibits AngII-induced Microglia Activation in the PVN ICV mitotempo Inhibits Ang II-induced Increase in NADPH Oxidase mrna and Decrease in nnos mrna in the PVN ICV mitotempo Prevents Ang II-induced Cardiac Hypertrophy and Interstitial Fibrosis Discussion
6 3 BRAIN-MEDIATED DYSREGULATION OF THE BONE MARROW ACTIVITY IN NEUROGENIC HYPERTENSION Inflammation and Endothelial Progenitor Cells in Neurogenic Hypertension Methods Animal MNC Isolation from Blood and BM Isolation of EPCs from MNCs Direct Flow Cytometry (FACS) Analysis DiLDL and Lectin Staining Results Dysfunctional Endothelial Progenitor Cells in Chronic Ang II-induced Hypertension BM derived EPCs are dysfunctional in Ang II-induced hypertension Tube formation ability of cultured MNCs from Ang II infusion rats is diminished Ang II-induced Imbalance of EPCs and ICs BM-derived EPCs are reduced by chronic Ang II infusion Inflammatory cells are elevated in the BM and circulation by chronic Ang II infusion MitoTEMPO ICV Treatment Inhibits Elevated BM Inflammatory Cells and Reduced EPCs Discussion ROLE OF NDUFA10 IN NEUROGENIC HYPERTENSION Proteomic Approach of Hypertension Utilizing Spontaneous Hypertensive Rat Methods Animal Primary Neuronal Culture Western Blot Assay D Gel and Image Analysis Ndufa10 Overexpression Measurement of Mitochondrial and Cellular ROS Production Data and Statistical Analysis Results D Gel Analysis Revealed Differential Protein Expressions in PVN of WKY Rat and SHR Ndufa10 Expression is Increased in Cardiovascular Regulatory Regions of SHR Compared to WKY Ndufa10 Overexpression Induces Cellular and Mitochondrial Oxidative Stress in HEK 293 Cells Discussion CONCLUDING REMARKS AND FUTURE DIRECTIONS LIST OF REFERENCES
7 BIOGRAPHICAL SKETCH
8 LIST OF FIGURES Figure page 1-1 Central nervous system mediated circulatory regulation Hypothalamic and brain stem areas in cardiovascular regulatory brain regions Neuronal mechanisms of blood pressure regulation in the brain Animal experimental design The chemical structures of mitotempo and TEMPOL The representative software image of spectral analysis using Hey Presto Effects of mitotempo on Ang II-Induced Hypertension and heart rate Scavenging of AngII-induced superoxide in mitotempo treated neurons Effects of ICV mitotempo on autonomic nerve activity in Ang II-induced neurogenic hypertension Effects of ICV mitotempo on microglia activation and cytokine mrna in the PVN Effects of ICV mitotempo on NADPH oxidase and nnos mrna Effects of ICV mitotempo on cardiac hypertrophy and myocyte diameter Effects of ICV mitotempo on interstitial fibrosis of left ventricles DiLDL uptake and lectin binding of CD90 + /CD EPCs Dysfunctional BM EPCs by chronic Ang II infusion Decreased ability of tube formation by chronic Ang II infusion Reduced number of BM EPCs in Ang II induced hypertensive rats Elevated ICs and imbalance of EPC/IC in Ang II induced hypertension Effects of mitotempo ICV treatment on BM EPCs and ICs in Ang II induced hypertension Experimental design of 2D gel analysis Representative 2D-DIGE gel from PVN of SHR
9 4-3 The list of differentially expressed proteins from PVN of SHR compared to WKY Distribution of Ndufa10 in cardiovascular relevant brain regions of WKY and SHR Increased expression of Ndufa10 mrna and protein in SHR compared to WKY ncreased expression of Ndufa10 in cultured neurons from SHR compared to neurons from WKY Ndufa10 overexpression vector and its efficiency Increased cellular and mitochondrial oxidative stress by Ndufa10 overexpression AngII-AT 1 R mediated ROS production in neurons and glia cells Proposed hypothesis of activated microglia-neuron-astrocyte interaction in the CNS of hypertension Proposed regulatory mechanism of neural-microglial-bm connection in neurogenic hypertension
10 Abstract of Dissertation Presented to the Graduate School of the University of Florida in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy CENTRAL REGULATION OF BLOOD PRESSURE IN HYPERTENSION By Joo Yun Jun May 2012 Chair: Mohan K. Raizada Major: Medical Sciences Physiology and Pharmacology Hypertension is the underlying pathology in many cardiovascular diseases including stroke and renal failure. In past decades, neurogenic component of hypertension, defined as high blood pressure with elevated sympathetic drive, and angiotensin II (Ang II) mediated oxidative stress, have been suggested as major contributors in the pathogenesis of hypertension. We hypothesized that i. oxidative stress is primarily generated by mitochondria in the brain that contributes to Ang II induced neurogenic hypertension via the increase in sympathetic drive ii. iii. there is a neural-bone marrow (BM) connection that regulates endothelial progenitor cells (EPCs) and inflammatory cells (ICs) in hypertension an increased expression of Ndufa10, a subunit of mitochondrial electron transport chain in cardiovascular (CV) brain regions of the spontaneously hypertensive rat (SHR) is genetically linked to the development of neurogenic hypertension. First we observed beneficial effects of mitotempo, a scavenger of mitochondrial superoxide, on hypertension, autonomic function, BM-derived EPCs and ICs. Chronic Ang II infusion in Sprague Dawley (SD) rats resulted in an elevation of blood pressure and sympathetic vasomotor activity, decrease in spontaneous baroreceptor reflex gain, and increase in activated microglia in the PVN. Intracerebroventricular (ICV) 10
11 administration of mitotempo attenuated these changes. To further elucidate potential role of central nervous system on EPCs cardiovascular protective function, the numbers of circulating and BM EPCs compared to those of IC were determined. Ang IIinduced hypertension was associated with ~46% decrease in EPCs and ~250% increase in ICs, resulting in ~5 fold decrease of EPCs/ICs ratio in the BM. ICV mitotempo treatment normalized Ang II induced imbalance of EPC/IC ratio in the BM. Finally, 2D gel analysis and Western blot assay demonstrated a mitochondrial Ndufa10 is ~3 fold up-regulated in the PVN of SHR compared to WKY and that is associated with mitochondrial oxidative stress. In summary, these observations demonstrated that brain mitochondrial ROS play an important role in Ang II-induced hypertension that is associated with an imbalance in EPC/IC. Increased mitochondrial ROS may be a result of an increased expression of Ndufa10 in the brain. These data suggest that mitochondria ROS mediated imbalance in EPC/IC is responsible for the pathophysiology of hypertension. 11
12 CHAPTER 1 NEUROGENIC HYPERTENSION Central Regulation of Blood Pressure Hypertension is a chronic elevation of the blood pressure (BP) that is associated with cardiovascular (CV) diseases such as stroke, heart failure, and kidney disease. 1, 2 About % of cases are characterized as primary hypertension that has no clear medical cause, and there is no single factor underlying this multi-factorial disease. 3 In the past decades, significant progress has been made in the treatment of hypertension using inhibitors of rennin-angiotensin system including angiotensin-converting enzyme (ACE) inhibitors, Ang II receptor type 1 (AT 1 R) blockers, and diuretics and calcium channel blockers. 4-7 However, there have been extreme difficulties to manage hypertension in about 40% of hypertensive patients. These unresponsive patients frequently exhibit increased sympathetic outflow and dysfunctional baroreflex. 8, 9 Dysregulation of neural signal within the central nervous system (CNS) and autonomic nervous system (ANS) have been suggested as major contributors to the development of hypertension, and those are characterized as neurogenic components There are numerous studies linking the increased sympathetic outflow with elevation of BP in rat and human, suggesting that neurogenic hypertension is a cardiovascular disease with dysfunctional ANS. 8, 13, 14 The significance of sympathetic nervous system in the regulation of BP via the modulation of peripheral vascular tone and cardiac output is well established, however the brain involved mechanism in the pathophysiology of neurogenic hypertension associated with elevated sympathetic nerve activity (SNA) needs to be further investigated. 12
13 Autonomic nervous system is a powerful modulator of blood pressure, which is controlled by a network of brain nuclei mainly localized in the hypothalamus and brainstem (Figure 1-1). 15 These specialized nuclei include the paraventricular nucleus (PVN) adjacent to the third ventricle in hypothalamus, the subfornical organ (SFO) in the roof of the third ventricle, the organumvasculosum of the lamina terminalis (OVLT) in the forebrain, the nucleus of the solitary tract (NTS), and the rostral ventrolateral medulla (RVLM) in the brainstem (Figure 1-2 and 1-3). 16, 17 The nuclei are activated by not only peripheral circulating Ang II signal but also local activation of brain reninangiotensin system (RAS). 18,19 The RAS is an enzymatic cascade by which angiotensinogen is cleaved by renin and further cleaved by ACE to produce Ang II. RAS is well known systemic BP control mechanism 20, and the potent vasoconstrictive action of Ang II is a main contributor to the development of hypertension 21, 20, 22, 23 via the AT 1 R. 6, 24, 25 The brain expresses genes that encode all components of the RAS, 26 and the central actions of Ang II on BP regulation and fluid homeostasis are mediated via this receptor. 27, 28 Accordingly, high densities of this receptor within the brain are distributed in CV regulatory brain regions including the SFO, PVN, RVLM and the NTS. 29, 30, 31 Systemic infusion of Ang II increases AT 1 R mrna in the SFO leading high blood pressure, and pharmacological blockade of AT 1 R attenuates the pressor response to Ang II in the RVLM and PVN. 32, 33 AT 1 R expressing PVN neurons integrate peripheral Ang II signals from circumventricular organs such as SFO where blood brain barrier are incomplete (Figure 1-3). 34 Arginine vasopressin (AVP) is synthesized in the PVN and released from posterior pituitary (PP), which increase blood volume. These projections of SFO are innervated to PVN where its parvocellular neurons are transmitted to 13
14 sympathetic preganglionic motor neurons influencing sympathetic activity. 35 RVLM and NTS are in the brainstem area that provides a major input for maintaining blood pressure by regulating sympathetic nervous system as shown in Figure Increased RAS in the brainstem may also contribute to alterations in baroreflex function. Afferent baroreceptor input to the NTS manifests its action via directly mediating vagal control of heart rate, and involving a multisynaptic pathway with an excitatory projection from the NTS to the CVLM, a subsequent inhibitory projection to the RVLM, In the NTS, AT 1 R blockade facilitates baroreflex control of heart rate, and conversely, AT 1 R activation by peripheral Ang II depressed both sympathetic and vagal components of baroreflexinduced bradycardia, indicating that Ang II decreases baroreflex gain The greatest understanding of brain RAS dysfunction in hypertension has been achieved from studies of spontaneously hypertensive rat (SHR), a rat model of primary hypertension. In this animal model, the increase in brain angiotensinogen contributes to development of hypertension. 40, 41 Increased RAS components within the PVN, SFO, and NTS, as well as increased cellular levels of AT 1 R within the RVLM of SHR versus WKY animals 44 have all been documented. Furthermore, studies utilizing pharmacological blockers of the RAS have provided evidence of the brain RAS mediated centrally-induced hypertension. 45, 46 It was shown that the anti-hypertensive effect following the treatment of brain RAS blockers in hypertensive rats is mainly due to a decrease in sympathetic activity, 45, 47 suggesting that the mechanism by which increased brain RAS induces hypertension involves increased sympathetic vasomotor tone. In support of this, it has been shown that AT 1 Rs are associated with presympathetic vasomotor neurons in the RVLM, 48 and blockade of Ang II signal within 14
15 the RVLM decreased BP in the SHR but not in the WKY. 49 Thus, in contrast to normotensive rats, it appears that AT 1 R stimulation within the CV presympathetic brain areas contributes to the enhancement of sympathoexcitatory activity of the vasomotor neurons in hypertensive rats. This suggested that hypertension can be produced by up regulation of the brain RAS via a mechanism involving enhanced sympathetic outflow. However, the contribution of brain RAS to BP regulation in different areas or nuclei, and its precise mechanism in hypertension is still not fully understood. Additionally the effect of Ang II on the CV regulatory regions including PVN and RVLM neurons involves several potential mechanisms, for example an increase in reactive oxygen species (ROS), neuroinflammatory signals from microglia, and a decrease in the concentration 50, 51 of NO in the brain. Oxidative Stress and Inflammation in Neurogenic Hypertension Increased oxidative stress in the vasculature, the heart, the kidney, and the brain is associated with cardiovascular disease including hypertension The increased production of cellular oxidative stress by Ang II is well documented in the peripheral organs. Ang II activated NADPH oxidase (nicotinamide adenine dinucleotide phosphateoxidase) enzyme and subsequent production of cellular ROS were first investigated by Griendling et al Ang II infusion-induced hypertension shows increased vascular superoxide production 60 and adenoviral vector-mediated superoxide dismutase (SOD) in neuronal cells scavenged Ang II-induced increase in cellular superoxide. 61 Also, pharmacological blockade of the NADPH oxidase complex by apocynin (NADPH oxidase inhibitor) attenuates Ang II induced vascular superoxide production, and further prevents hypertension. 62, 63 Among the target organs of hypertensive vascular disease, the brain is most affected by oxidative stress. 64, 65 Although there have been many 15
16 studies regarding target organ damages in hypertension, relatively few studies have addressed the role of oxidative stress in the activation of the sympathetic nervous system. One of the critical roles of Ang II in the CNS is the activation of NADPH oxidase 66, 67 and the production of ROS resulting in the increase of central sympathetic outflow. Ang II stimulates cellular ROS production in cultured neurons isolated from the cardiovascular relevant brain areas, and this was prevented by the Ang II type 1 receptor (AT 1 ) antagonist losartan or superoxide dismutase mimetic TEMPOL 68, 69 treatment. In addition to NADPH oxidase-induced production of ROS in cytosol, mitochondria are another major source of ROS production in many cell types Mitochondria appear to be in the main loop of a vicious cycle of oxidative damage since mitochondria produce the majority of intra cellular oxidants providing main targets of redox signaling. 72, 75 This increases mitochondria-induced cellular oxidative stress and further induces oxidative damages in the organs. Therefore, the significance of increased oxidative stress in the CNS and that is involved with mitochondria induced ROS in the brain needs to be examined. Recent study from Nozoe et al has demonstrated that Ang II increases mitochondrial ROS production in the RVLM, leading to sympathetic activation. 76 Chan et al also demonstrated the role of mitochondrial electron transport chain complex in the RVLM of SHR and found that the impairment of mitochondrial ETC complexes contributes to chronic oxidative stress in the RVLM of SHR, leading to enhanced central sympathetic drive and hypertension. 77 Interestingly, studies have demonstrated that Ang II induced mitochondria-derived ROS production perpetuates NADPH oxidase-mediated production of ROS. This suggests a feed-forward activation 16
17 mechanism of cellular ROS production between NADHP oxidase and mitochondria. 78 It is tempting to suggest that NADPH oxidase-derived ROS first trigger Ca 2+ accumulation within mitochondria and this subsequently induce mitochondrial superoxide generation, which further activates NADPH oxidase and mitochondria. However, additional studies need to establish a more direct link between AngII-NADPH oxidase-mitochondria in neuronal redox signaling and the pathophysiology of neurogenic hypertension. Both human and animal studies have provided strong evidence that increase in inflammatory modulators and overall inflammatory status are critical in cardiovascular diseases and hypertension. 79 The levels of plasma inflammatory cytokines and other markers of inflammation are increased with the progression of hypertension, and suppressing immune response produces beneficial effects. 80 Studies have demonstrated that circulating levels of TNF-α, IL6, and adhesion molecules such as P- selectin are increased in patients with primary hypertension. 81, 82 Inhibitors of the reninangiotensin system, which are effective antihypertensive treatment in some patients, are shown to decrease C-reactive protein, IL6 and TNF-α levels and inflammation. 83 In animal studies, T cells play an important role in Ang II-induced inflammation and vascular dysfunction in hypertension. 84, 85 The SHR also exhibits increased levels of activated monocytes and that is associated with high blood pressure. 86 Ang II promotes leukocyte-endothelial interaction contributing to vascular inflammation, along with increases in the expression of cytokines IL-6, IL-1β and TNFα. 87 Although the participation of peripheral cytokines and proinflammatory modulators in hypertension has been established, there is no clear evidence available for their involvement in neuroinflammation in neurogenic hypertension. Cytokines modulate neuronal activity 17
18 and receptors for cytokines are found in different cell types in the brain. 88 Recent study from Cardinale et al demonstrated that bilateral NF-kB blockade in the PVN using NKkB decoy oligodeoxynucleotide and serine mutated inhibitory kb (AdIkB) reduced proinflammatory cytokines such as TNF-α, IL-1β, IL-6, MCP-1 and ROS production in Ang II-induced hypertension. 89 In addition, nnos mrna level was increased by bilateral NF-kB inhibition, resulting in increase in NO bioavailability and decrease in plasma NE levels. 89 NO is a well-known sympathoinhibitory neurotransmitter, and the expression of nnos and plasma NE are indirect indicator of neuronal activity and sympathetic outflow. Another study provided the evidence that TNF-α increase Ca 2+ influx into hippocampal pyramidal neurons 90 and IL-1β has similar effect on neuronal activity that increase Ca 2+ current via phosphorylation of N-methyl-D-aspartic acid receptor. 91 These findings indicate that central cytokines levels are associated with sympathetic drive and hypertension. Inflammatory cytokines are polypeptides and peripherally produced cytokines are not able to cross BBB. However, there are multiple ways of influencing CNS to modulate cardiovascular system when cytokines are transported from plasma. As discussed above, brain has a specific area called circumventricular organ (SFO, OVLT, AP), in which the BBB is not present. Variety of signals including AngII and cytokines are transmitted through these areas. Also, CNS produce cytokines locally by activated monocytes, macrophages and activated mictoglia. 88, 92 mrnas and proteins of TNFα, IL-6 and IL-1 have been identified in different region of brain including hippocampus and brainstem. 93 It seems that both peripheral cytokines and centrally generated cytokines contribute to the neuroinflammatory activation in neurogenic hypertension. The inflammatory process in the CV regulatory regions of the brain is 18
19 associated with the modulation of autonomic nervous system, and microglia is one of the important cell types for triggering neuroinflammation in the brain. Microglia, as local microphages in the brain are activated and increased in response to brain injury, and observed in the patients with neurodegenerative disease including Alzheimer and Parkinson s disease. 94, 95 Activated microglia produce inflammatory cytokines such as NF-kB, IL-6, TNF-α within the brain 96 and these cytokines are the important stimulators of sympathetic activation. Shi et al has demonstrated that chronic Ang II infusion induced hypertension which is associated with an increase in the activated microglia in the PVN. 97 Recent study from Miyoshi et al also provided the evidence indicating that neurons and microglia express AT 1 R, that are primarily responsible for Ang II-induced TGF-β production in the CNS. 98 Systemic administration of centrally acting AT 1 R blocker ameliorates these responses. 99 However the involvement of microglia activation in peripheral inflammatory process including increase in bone marrow (BM)-derived inflammatory cells in neurogenic hypertension is not yet clear. Endothelial Progenitor Cells and Vascular Dysfunction in Hypertension and Cardiovascular Diseases Accumulating studies have shown that improvement of the ability of endothelial repair function with BM stem cells is important in hypertension-induced vascular pathophysiology. 100, 101 Endothelial progenitor cells (EPCs) are BM derived endothelial stem cells and released into the circulation to maintain vascular homeostasis. 102 In order to replace damaged endothelium, EPCs are mobilized to the site of injury and involved in the repair process. However, dysfunctional and reduced number of EPCs are unable to perform blood vessel regeneration in damaged vasculature in hypertension. Decreased function and number of EPCs are correlated in patients with 19
20 hypertension, obesity, chronic kidney disease, and immune diseases. 103, 104 Patients with diabetes or pulmonary hypertension also showed dysfunctional EPCs are associated with endothelial dysfunction. 105, 106 Animal studies have demonstrated that dysfunctional EPCs are responsible for the increase in EPC ROS, NADPH oxidase and the decrease in NO availability. 106 Endtmann et al have demonstrated that Ang II decreases EPC numbers and function by increasing oxidative stress and apoptosis and this effect is blocked by AT 1 antagonist, demonstrating Ang II is an important signaling trigger. 105 As discussed above, Ang II-induced central production of neuroinflammatory process including PVN microglia activation, which directly or indirectly raises cytokines, chemokines, ROS etc., stimulates neuronal activity. Considering that BM is efficiently innervated by cholinergic nerve fibers from the sympathetic nervous system, altered sympathetic drive induced by Ang II from the brain to the BM may impair EPCs function and increases peripheral inflammatory cells from BM to the circulation resulting in a compromised vascular repair mechanism in hypertension. Based on the available evidence, it is tempting to suggest that peripheral inflammatory signals including circulating Ang II propagates across the BBB into the CV relevant brain regions and mediate sympathetic activation participating further generation of inflammatory modulators in the periphery such as BM. Therefore, CNS may play a key role in the regulation of endothelial dysfunction and inflammation in hypertension. This suggests the plausible evidence of the existence of a functional neural-inflammatory-bm communication that is responsible for the pathophysiology of neurogenic hypertension. 20
21 Figure 1-1. Central nervous system mediated circulatory regulation.the figure illustrates simplified schematics of neural control of circulation. Peripheral circulating Ang II signal activates hypothalamic CV regulatory regions and signals transmits to brain stem regions. Activated aortic and carotid afferent bodies also send a neuronal signal to the brainstem, which send an output signal to the effector organs (heart, arteries, veins, adrenal gland and bone marrow) via both the sympathetic and parasympathetic efferents, thereby regulating cardiac output (by regulating rate and force of heart contractions) and peripheral resistance (by regulating the contractility of the arteries and veins, and adrenaline synthesis). The preganglionic neurones mostly signal vie acetylcholine (Ach) while the postganglionic neuronal transmitter is mainly noradrenaline (NA). 21
22 Figure 1-2. Hypothalamic and brain stem areas in cardiovascular regulatory brain regions. Saggital diagram of the rat brain indicatesthe cardiovascular regulatory brain regions with high densities of AT 1 receptors. Circumventrical organs such as SFO that lack a BBB and exposed to influences of the peripheral renin-angiotensin system. The area in the boxes highlights the specific nuclei within the hypothalamus and medulla oblongata involved in cardiovascular homeostasis. SFO, OVLT, PVN in the blue dotted box, NTS, RVLM, CVLM-in the red dotted box. 22
23 Figure 1-3. Neuronal mechanisms of blood pressure regulation in the brain. Arginine vasopressin (AVP), posterior pituitary (PP), Intermediolateral cell column (IML).Arginine vasopressin (AVP) is synthesized in the PVN and released from posterior pituitary (PP) that results in increase of blood volume. Increase in baroreceptor afferent activity is transmitted to NTS to inhibit efferent sympathetic nerve activity. Increase in oxidative stress in these areas is associated with increased neuronal activity resulting in high blood pressure. 23
24 CHAPTER 2 ROLE OF BRAIN MITOCHONDRIAL OXIDATIVE STRESS IN NEUROGENIC HYPERTENSION Increased Oxidative Stress in Neural Mechanisms of Hypertension Accumulating evidence indicates that increased oxidative stress in the vasculature, the heart, the kidney, and the brain is associated with cardiovascular disease including hypertension Indeed, excessive production of reactive oxygen species (ROS) in the brain nuclei not only is an important signaling trigger but also plays a crucial role in the regulation of sympathetic nerve activity. 55, 108, 109 Circulating Ang II has been shown to increase cellular superoxide production in the brain and that is mediated by NADPH oxidase. 69, 78 NADHP oxidase is multisubunit membrane-bound enzyme complex and Ang II is the trigger of NADPH oxidase activation. Ang II-induced stimulation of ROS production may compromise sympathoinhibitory mechanisms in the CNS, thereby contributing to chronic increase in blood pressure. Importantly, it has been reported that elevated ROS production in the key CV regulatory nuclei such as the PVN and the RVLM leads to increase in sympathetic activity and BP. 109 The basal level of O 2 - and H 2 O 2 in the RVLM that generates and maintains sympathetic vasomotor tone, is elevated in animal models of hypertension. 110 For example, a study by Kimura et al showed that overexpression of inducible nitric oxide synthase in the RVLM produced hypertension by increasing ROS levels in normotensive WKY rats. 111 Davisson s group demonstrated that increased O 2 - In the forebrain of mice is associated with cardiac dysfunction in myocardiac infarction. 112 Our previous studies have demonstrated that soluble epoxide-mediated BP regulation in the SHR is mediated by NADPH oxidase-derived generation in forebrain nuclei. 69 Taken together these 24
25 observations suggest that accumulating ROS and NO availability in the CV brain regions play a key role in the development of neurogenic hypertension. Role of Brain Mitochondrial ROS in Neurogenic Hypertension Although Ang II-activated NADPH oxidase is known to be the major source of ROS production in hypertension, 66, 67 the contribution of mitochondrial superoxide generation by Ang II in these regions of the brain is relatively unclear in neurogenic hypertension. Mitochondria are another major source of cellular superoxide generation and it has been evidenced that mitochondrial ROS is involved in the pathogenesis of cardiovascular, metabolic and neurodegenerative diseases. 113, 114 Recently, Chan et al investigated the role of mitochondrial electron transport chain in the brain and dysfunctional electron transport chain in the RVLM of SHR results in oxidative stress by inhibiting chain complex activity III or I. 77 Recent study by Dikalova et al also demonstrated that mitochondrial superoxide is important for the development of hypertension by using mitochondria targeting antioxidant mitotempo. 115 Systemic infusion of mitotempo in Ang II induced hypertensive mice improved endothelial function and NADPH oxidase activity. 115 However, the contribution of the brain mitochondrial ROS in the regulation of sympathetic activation and hypertension cannot be deduced from these studies since mitotempo, like TEMPOL is likely to cross the BBB (Figure 2-2). Given that targeting mitochondria for intracellular ROS and excessive oxidative stress may be significantly effective in the regulation of blood pressure, it is possible that mitochondrial-derived oxidative stress in the brain alters neural redox state, and initiates development of neurogenic hypertension. However, potential mechanisms by which brain mitochondrial oxidative stress alters autonomic nervous system and 25
26 cardiovascular parameters in neurogenic hypertension have not been tested yet. Hence, we hypothesized that mitochondrial ROS in the brain is responsible for dysfunctional neural signals in pathophysiology of hypertension. We investigated the effects of central administration of mitochondria targeted antioxidant, mitotempo on autonomic function, cardiac hypertrophy, and brain microglia activation including cytokine release in Ang II-induced neurogenic hypertension. Methods Animal Adult male Sprague-Dawley (SD) rats aged 6 to 7 weeks were purchased from Charles River Laboratories (Wilmington, MA). Rats were individually housed in a temperature-controlled room (22 C to 23 C) with a 12:12-hour light-dark cycle. Rat chow (Harlan Tekland) and water were provided by Animal Care Services. All experimental procedures were approved by the University of Florida Institute Animal Care and Use Committee. Primary Neuronal Culture Neuronal cells in primary culture from the brainstem and hypothalamus of one day-old SD were established. Brains were isolated from neonatal rats and hypothalamic and brainstem areas were dissected, and trypsinized (Worthington Biochemical, cat# 3667) for 15 min at 37 C to dissociate individual neurons. Cells were then plated in poly-l-lysine (Sigma, cat# P-1274) pre-coated 6 or 12 well culture dishes. After 48 hours, cells were treated with anti-mitotic agent, arabinoside (Cytosine 1-B-D, cat# C- 1768)for 2 days and media was replaced with DMEM +10% FBS. Cultures were established for days prior to use in the experiments. Previous studies have shown that the culture contain <95% neuronal cells and remaining being astrocytes
27 Measurement of ROS Production Eleven to thirteen days of cultured neurons were treated with AngII (500nM), or co-treatment with mitotempo (2 and 5nM, Enzolifescience, ALX ) for 4 hours. Cellular superoxide was measured by DHE (dihydroethidium, Invitrogen) fluorescent staining and mitochondrial superoxide was measured by MitoSOX Red staining (Invitrogen). DHE (1nM) was added to neurons for 30 min at 37 C and cells were washed with PBS three times. For the detection of mitochondrial ROS, neurons were incubated with 5uM of mitosox red dye for 10 min at 37 C and washed with PBS three times. Additionally, mitotracker green (Invitrogen) staining was used for the mitochondrial subcelluar location of MitoSOX. Cells were fixed with 4% PFA to be examined. Images were obtained from Zeiss Axioplan 2 Fluorescent Microscope. Telemetry Recordings of Arterial Pressure and Heart Rate Male SD rats (6 to 7 weeks old) were anesthetized with a mixture of O 2 (1 L/min) and isoflurane (2% to 4%) during the surgery. A radiotransmitter (TA11PAC40, Data Sciences International) was implanted to record arterial pressure and heart rate from the abdominal aorta. About ~1 inch long incision was made in the middle of abdominal skin and aorta was exposed and isolated carefully. The catheter from the telemetry transducer was inserted using curved needle into the vessel toward the heart and sealed with 3M vetbond. Transducer was sutured (3-0 nylon, non-absorbable) with inner skin to secure its position under the skin. Second suture was placed to close up the outer skin and additional surgical clips were used. To minimize post-operational pain, bolus injection of buprenorphine (0.03 mg/kg SC) was administered after the surgery. Rats were allowed to recover for 7 to 10 days before baseline telemetric measurements 27
28 were taken. Telemetry recording was performed every 3-4 days. A full spectral analysis was performed on the blood pressure signal. Measurement of Full Spectral Analysis The variance of blood pressure, heart rate and pulse interval variance was calculated from the dark period (12:00am-5:00am) data collected for 10 min every hour. Pulse intervals were calculated in miliseconds (ms) by inversion of heart rate values. The spectral analysis was performed using the software Hey Presto. The low frequency component of the power spectrum (LF(SBP)) indicating sympathetic drive included the power from 0.04 to 0.15 Hz, and the high frequency component the power spectrum (HF(PI)) indicating parasympathetic drive included the power from 0.15 Hz to 0.25 Hz. The power of each band was calculated as integral of power spectral density under the curve in the frequency range. LF(SBP) and HF(PI) were computed in absolute units in mmhg 2 and in normalized units expressed as a percentage of the total power. sbrg(pi) was computed in unites of ms/mmhg indicating spontaneous barareflex gain. Figure 2-3 shows the representative image of the software. Implantation of Subcutaneous Osmotic Minipumps Rats were further assigned to subgroups (n=5-8) to receive Ang II (200 ng/kg/minute), 0.9% saline or mitotempo (Enzo Life Science,100 or 170 ng/kg/min) delivered via an osmotic minipump (No. 2004, ALZET).Pumps were prepared and filled with drugs day before the implantation and incubated at 37 C for overnight. A small incision was made between the scapulae and pumps were placed subcutaneously. Skin was clipped and pumps were secured. Each pump lasts for 4 weeks from the day of drug preparation and animals were euthanized before day 28 (Figure 2-1). 28
29 Intracerebroventricular mitotempo Infusion Ten to fourteen days after implantation of telemetry transducers, rats were subjected to second surgery of intracerebroventricular (ICV) cannulae implantation. As shown in figure 2-1 experimental design, either the ICV or SC infusion of mitotempo started on day 0. In brief, rats were anesthetized with a 4% isoflurane/o 2 mixture, and the head was positioned in a Kopf stereotaxic apparatus (Harvard apparatus) using the earplugs. Incision was made and the bregma was exposed. Surgical drill (Complete bone micro drill system, , Harvard apparatus) was used to make a hole to place a cannula inside to the brain. An infusion cannula (Brain infusion kit 1 3-5mm, ALZET) was implanted into the left cerebroventricle (1.3 mm caudal to bregma, 1.5 mm lateral to the midline, and 4.5 mm ventral to the dura). Small amount of dental cement was added to secure the cannular for the period of experiments. A four-week osmotic minipump was connected to the infusion cannula via the catheter tube to deliver mitotempo (Enzo Life Science, 100 or 170 ng/kg/min). Immunohistochemistry Brains were post-fixed with 10% PFA for one hour and placed into 30% sucrose for 2-3 days until they are ready. OCT embedded brains were frozen prior to sectioning and cut into 10~20 µm coronal sections including PVN. Sections were then incubated with 0.1% triton X-100 followed by serum incubation for one hour. Primary anti-iba-1 antibody (Waco, cat# ), a specific marker for microglia was diluted into 1:500 and incubated for 3 hours at room temperature. After washing three times with PBS, anti-rabbit IgG (1:200, VEGTOR, cat# BA-1000) antibody was used as a secondary antibody that is conjugated with 3,3'-diaminobenzidine (DAB). Activated microglia were 29
30 visualized. To obtain the images of PVN sections, an Olympus BX41 microscope was used. Cardiac Pathology Hearts were collected from the rats at the end of the experiment, and processed for cardiac morphology and histological examination, as described previously. 118 Briefly, left ventricles were first separated and rinsed with PBS to remove residual blood before weighing and fixing with 10% paraformaldehyde. Later ventricles were embedded in paraffin and cross-sectioned into 4µm. Sections were stained with either hematoxylineosin for the myocyte diameter measurement or with pico-sirius red dye for interstitial fibrosis measurement. Twenty-five to thirty images were taken from each section using Olympus BX41 microscope and analyzed with the image J software from NIH. RNA Isolation and Real-Time PCR To analyze mrna levels from the PVN, hypothalamic tissues including PVN were dissected. Coronal segments were first sliced according to Paxinos and Watson (The Rat Brain: In Stereotaxic Coordinates) and small blocks of each area were excised (2.0mm wide and high). Total RNA was prepared using RNeasy kit (Qiagen) according to the manufacturer s instruction. About 200 to 300ng of purified RNA were reverse transcribed using high-capacity cdna reverse transcription kit (Bio-Rad Laboratories). Quantitative Real-Time PCR was performed with specific primers and probes of IL1β (Rn _m1), TNF-α (Rn _m1), and CD11b (Rn _m1), NADPH oxidase (Rn _m1; p22-phox, Rn _m1; Gp9-1phox), nnos (Rn _m1; NOS2) by using PRISM 7000 sequence detection system (Applied 30
31 Biosystem). Data were normalized to 18s ribosomal RNA (Hs _s1) and GAPDH (9Rn _m1). Results ICV Infusion of mitotempo Attenuates Ang II-induced Neurogenic Hypertension Despite recent studies demonstrated the role of oxidative stress and inflammation in Ang II-induced hypertension, the role of brain mitochondrial ROS associated with pathophysiology of neurogenic hypertension has not been examined. To test whether scavenging of mitochondrial ROS in the brain can prevent Ang II-mediated development of hypertension, Ang II-infused SD rats were co-treated with mitotempo either subcutaneously or intracerebroventricularly for 4 weeks. Two different doses of mitotempo (100ng/kg/min and 170ng/kg/min) were administrated based on previous study. 119 Over the experimental period, chronic subcutaneous (SC) infusion of Ang II (200ng/kg/min) increases mean arterial pressure (control: 98±2mmHg; n=8, Ang II: 177±6 mmhg; n = 8). Although SC mitotempo did not prevent the Ang II-induced increase in MAP, intracerebroventricular (ICV) infusion of mitotempo significantly attenuated the increase of MAP. A dose of 100ng/kg/min caused ~30mmHg decrease (Ang II: 177±6 mmhg; n = 8 vs. 146±12 mmhg; n=6) while the dose of 170ng/kg/min resulted in ~60mmHg decrease in MAP (Ang II: 177±6 mmhg; n = 8 vs. 112±13 mmhg; n=8) (Figure 2-4A and B). ICV mitotempo alone had no effect on MAP (control: 98±2mmHg; n=8, mitotempo alone: 104ng/kg/min; n=4). Heart rate in control group has decreased as the rats aged, however they did not show significance changes between the mitotempo treatment groups (Figure 2-4C). 31
32 mitotempo Scavenges Ang II-induced Mitochondria ROS in Neuronal Cells in Primary Cultures To further verify mitotempo s superoxide scavenging function in neurons, Ang IItreated (500nM) primary neuronal cultures were co-treated with mitotempo (2 or 5nM) for 4 hours followed by staining with mitosox (a mitochondrial specific superoxide detector). Mitochondrial-localized actions of mitotempo were confirmed by co-staining with a mitochondrial specific fluorescence dye (i.e., MitoTracker Green). Figure 2-5B shows Ang II increased mitochondrial superoxide oxidized by mitosox was completely diminished in the neuron. Additionally, levels of total cellular ROS as detected by DHE (dihydroetidium, excitation 490 nm/emission 585 nm) staining were normalized by mitotempo treatment (Figure 2-5A). This suggests that mitotempo specifically decrease not only mitochondrial ROS but also total cellular ROS in Ang II-treated neurons. The neuronal cultures that is used in the experiment contains >90% of neurons, however still has <10% of glia and astrocytes. The possibility of gila and astrocytes induced mitochondrial oxidative stress stained by mitosox cannot be ruled out. Central Mitochondrial Superoxide Inhibition Influences Autonomic Nerve Activity in AngII-induced Neurogenic Hypertension Full spectral analysis was performed based on the data obtained from 24 hours of telemetry recording to investigate whether mitochondrial ROS influences autonomic functions (Figure 2-3). Spectral analysis using telemetry data is non-invasive classical methods of quantification of the cardiovascular variability, and the variance of systolic blood pressure and heart beat intervals that provide an insight into autonomic control of the circulation in hypertensive subjects. 120 The LF component of systolic blood pressure power spectrum, LF(SBP) is considered as a marker of oscillations of the sympathetic 32
33 activity addressed to resistant arteries, and HF(PI), the high frequency pulse interval variability indicates a marker of cardiac parasympathetic drive. Also, sbrg (PI) is considered spontaneous cardiac baroreceptor reflex gain. LF (SBP) and sbrg (PI) indicate ~6 fold increase in sympathetic vasomotor drive [( LF (SBP) control: ±0.2 ms 2 /mmhg 2, AngII: ±0.3 ms 2 /mmhg 2 ] and ~3 fold decrease in cardiac spontaneous baroreflex gain [( sbrg (PI) control: ±0.1 ms/mmhg, AngII: ±0.06 ms/mmhg)], respectively after 4 weeks of Ang II-infusion (Figure 2-6). However, ICV mitotempo could normalize these changes to the control level [100ng/kg/min, 170ng/kg/min: ( LF (SBP) ±0.7, ±0.3 ms 2 /mmhg 2, ( sbrg (PI): -0.07±0.04, ±0.1 ms/mmhg]. Cardiac parasympathetic drive measured by HF (PI) did not show significant changes in any of these groups (Figure 2-6C), yet the ratio of LF to HF, which is an indication of vasovagal balance, was ~2.5 fold elevated by Ang II (Figure 2-6D). ICV mitotempo treatment was able to attenuate Ang II-mediated change in the vasovagal balance (Figure 2-6D). ICV mitotempo Inhibits AngII-induced Microglia Activation in the PVN Shi et al have shown that chronic Ang II infusion results in microglial activation and increases mrna levels of various inflammatory genes in the PVN. 121 To test if mitochondrial ROS inhibition would influence microglial activation, we determined the effects of ICV mitotempo treatment on mrna levels cytokines and CD11b, a marker of activated microglia in Ang II-induced hypertensive rats. Consist with previous findings Ang II increased Iba1 positive cells by ~85% and CD11b expressing cells (i.e., activated microglia) by ~1.6 fold in the PVN.ICV mitotempo significantly reduced the number of Iba1 expressing microglia and the level of CD11b mrna in the PVN to the control levels (Figure 2-7A and B). In addition, Ang II increased mrna levels of IL1β and TNFα in the 33
34 PVN as ~1.6 folds and~2.3 folds, respectively, while ICV mitotempo completely abolished the increase of IL1β and TNFα transcript levels (Figure 2-7C-E). ICV mitotempo Inhibits Ang II-induced Increase in NADPH Oxidase mrna and Decrease in nnos mr N A in the PVN Although Ang II-induced increase in ROS production in the brain is mediated by NADPH oxidase, the involvement of mitochondria in the activity of NADPH oxidase in neuronal ROS production is not yet tested. mrnas were prepared from PVN and Realtim PCR was performed to determine the levels of NADPH oxidase subunit p22 phox, gp91 and nnos mrna. Figure 2-8 shows Ang II infusion significantly increases p22 mrna by ~1.9 fold and decreases nnos mrna by ~2 fold in the PVN. ICV mitotempo treatment prevents these changes and normalized to the control levels. These data suggests that mitocondria ROS inversely affect NADHP oxidase mrna as well as nnos mrna, resulting in the imbalance of cellular redox state, and excessive oxidative stress. ICV mitotempo Prevents Ang II-induced Cardiac Hypertrophy and Interstitial Fibrosis At the end of the experiments (27days after telemetry recording), rats were euthanized and hearts were collected to determine the effects of ICV mitotempo treatment on Ang II-induced cardiac pathology. Chronic Ang II infusion resulted in an increase in the heart weight/body weight ratio (control: 3.0±0.2, AngII: 4.15±0.1) and cardiac myocyte diameter (control: 12.48±0.9µm, AngII: 15.8±1.2µm), two indicators of cardiac hypertrophy. However, the change of heart/body weight ratio and hypertrophy by Ang II infusion was normalized with the mitotempo ICV treatment (100ng/kg/min: 3.4±0.8, 170ng/kg/min: 3.5±0.3) (Figure 2-9A). Also, ICV mitotempo treatment 34
35 prevented increase in myocyte diameter (13.1±1.1µm) (Figure 2-9B).Interestingly, SC infusion of mitotempo that did not affect MAP, had no beneficial effects on cardiac hypertrophy (14.8±0.8µm). To determine cardiac interstitial fibrosis, left ventricles were stained with pico-sirius Red dye and examined with Olympus BX41 microscope. Consistently, interstitial fibrotic area is significantly increased with Ang II infusion (control: 4±0.5, Ang II: 15±1.1 area %) but inhibited with mitotempo ICV treatment (ICV mitotempo: 6±0.8, SC mitotempo: 16±0.9) (Figure 2-10A). Figure 2-10B shows quantification graph from Image J software (NIH). Discussion The most significant finding of this study is that increase in brain mitochondrial ROS is responsible for the changes in autonomic function and microglial activation in Ang II-induced neurogenic hypertension. Oxidative stress in the CV relevant regions in the brain has been associated with the pathogenesis of the hypertension and observed in experimental rat model. Studies have shown that SOD mimetic, TEMPOL or adenoviral-mediated deliver of SOD gene in the SFO or RVLM decrease blood pressure and prevented hypertension. 56, 122, 123 It is well established that Ang II increases NADPH oxidase mediated oxidative stress in the brain and that is associated with neurogenic hypertension. 61, 124, 125 NADPH oxidase is the enzyme complex, mainly activated by Ang II. Although NADPH oxidase mediated ROS production is well documented, the involvement of mitochondria, as another source of ROS production in the brain is not completely understood. Antioxidant treatment such as vitamin E in clinical trials has not been very successful in hypertensive patients, 126 which might be explained by the failure of 35
36 targeting ROS in the subcellular level such as mitochondria. Recent study demonstrated the protective effects of mitochondrial targeting antioxidant on different animal models of hypertension. 77, 127, 128 The mitochondrial targeting antioxidant, mitotempo infusion improved endothelial function by preventing loss of endothelial nitric oxide in Ang II or DOCA salt-induced hypertension. 119 Also, the possibility of NADPH oxidase activation by mitochondrial ROS has been suggested. 119 As shown in figure 2-8A, in fact, Ang IImediated increase in NADPH oxidase mrna was blocked by ICV mitotempo treatment in the PVN. It is possible to suggest that mitochondria superoxide production can be triggered by NADPH oxidase activation through Ca 2+ accumulation within the mitochondria, and mitochondrial ROS would regulate NADPH oxidase vice versa. Therefore, it is tempting to propose that targeting mitochondria to inhibit cellular ROS can be more effective in hypertensive disease. In this study, we used ICV administration of mitotempo to examine the central role of mitochondrial ROS and to demonstrate its beneficial effects on neurogenic hypertension and brain microglia. ICV infusion of mitotempo prevents hypertension via the reduced microglia activation and sympathetic outflow. However, comparable dose of SC infusion of mitotempo did not prevent hypertension. This observation is in contrast to the previous observation of Dikalova et al who determined an attenuation of hypertension by SC administration of mitotempo in mice. 119 This discrepancy in the results may likely be due to an increased accessibility of mitotempo in the brains of mice, as a result of the previously reported altered permeability of the blood brain barrier in mice subjected to twice the concentration of Ang II compared to the one used in the rats in the present study. 129 This is particularly relevant in view of evidence that 36
37 autonomic regions of the brain are highly vascularized. 87 However, differences in other humoral responses and metabolic processes between the two species cannot be ruled out at this point. Finally, our findings establish that Ang II-induced mitochondrial ROS in the brain triggers neuronal activity that results in increased sympathetic drive, decreased baroreceptor reflex gain, PVN microglia activation, and cardiac hypertrophy. Additionally increased cytokines in the PVN is a marker of proinflammatory signal in the CNS that is associated with increased sympathetic drive. Thus, we speculate that central production of mitochondrial ROS is an important signaling mechanism, mediating autonomic function in pathophysiology of Ang II-induced neurogenic hypertension. 37
38 Figure 2-1. Animal experimental design. Radiotelemetry was implanted on day -10 and subcutaneous Ang II with eigher ICV or subcutaneous mitotempo are administrated at day 0. Blood pressure and heart rate were monitored twice a week for 24 hours until the end of expreriment. Brains and hearts were collected for further histology and bone marrow cells were isolated to enrich EPCs and ICs. 38
39 Figure 2-2. The chemical structures of mitotempo and TEMPOL. An antioxidant TEMPOL is a superoxide dismutase mimetic and mitotempo is mitochondria targeting antioxidant. Their structure is similar but conjugation of a lipophilic triphenylphosphonium cation to the structure of TEMPOL allows targeting of an antioxidant to the mitochondria. TEMPOL is known to cross BBB and based on the structural similarity mitotempo has a great chance to pass BBB. 39
40 Figure 2-3. The representative software image of spectral analysis using Hey Presto. Spectral analysis is non-invasive classical methods of quantification of the cardiovascular variability, and the variance of systolic blood pressure and heart beat intervals that provide an insight into autonomic controls. The variance of blood pressure, heart rate and pulse interval was calculated from the telemetry data collected for each 10 min every hour until 24 hours. Pulse intervals were calculated in miliseconds (ms) by inversion of heart rate values. 40
41 Figure 2-4. Effects of mitotempo on Ang II-Induced Hypertension and heart rate. A, ICV mitotempo significantly attenuated MAP in Ang II-induced hypertension in a dose dependent manner (100 and 170ng/kg/min). *P<0.05, **P<0.01 vs control. B, SC mitotempo did not attenuate MAP. C, Heart rate did not show differences between mitotempo treatment groups. Bar graph is mean±sem. **P<0.01 vs control. 41
42 Figure 2-5. Scavenging of AngII-induced superoxide in mitotempo treated neurons.a, Cellular superoxide stained by dihydroethidium. Relative fluorescence units (RFU s) were detected by microplate reader. *P<0.05 vs control, #P<0.05 vs Ang II. B, Representative images of neurons. mitosox red staining is used to detect mitochondrial superoxide and mitotracker green is used for mitochondrial localization. 42
43 Figure 2-6. Effects of ICV mitotempo on autonomic nerve activity in Ang II-induced neurogenic hypertension. A, LF(SBP): Sympathetic vasomotor drive. B, HF(PI): Cardiac parasympathetic drive. C, sbrg(pi): Cardiac spontaneous baroreflex gain. D, LF/HF: Vasovagal balance. *P<0.05 vs control, #P<0.05 vs Ang II. 43
44 Figure 2-7. Effects of ICV mitotempo on microglia activation and cytokine mrna in the PVN. A, Ang II-induced Iba-1 positive activated microglia within PVN were reduced to control level by ICV mitotempo treatment. B, Quantification of the number of Iba-1 positive microglia in the PVN. C, mrna of CD11b, a marker of activated microglia. D, mrna of IL1β. E, mrna of TNFα. mitotempo alone group was not included in the data since we did not see any significant changes in MAP and HR. *P<0.05 vs control, #P<0.05 vs Ang II. 44
45 Figure 2-8. Effects of ICV mitotempo on NADPH oxidase and nnos mrna. A, p22 phox and gp99 phox mrna.b, nnos mrna. mitotempo treatment normalized p22phox and nnos mrna levels. *P<0.05, vs control, #P<0.05 vs Ang II. 45
46 Figure 2-9. Effects of ICV mitotempo on cardiac hypertrophy and myocyte diameter. A, The Ratio of heart weight to body weight and body weight did not change between groups. B, Cardiac myocyte diameter measured from H&E stained left ventricle section. There were no significant changes in their body weight between groups. *P<0.05, **P<0.01 vs control, #P<0.05 vs Ang II. C, Representative pictures of hearts from each group. 46
47 Figure Effects of ICV mitotempo on interstitial fibrosis of left ventricles. A, Representative left ventricle sections of sirius red staining positive fibrotic areas. B, Quantification graph generated from Image J software.icv mitotempo treatment prevented the increase in interstitial fibrotic areas in left ventricles induced by Ang II. *P<0.05 vs control, #P<0.05 vs Ang II. 47
48 CHAPTER 3 BRAIN-MEDIATED DYSREGULATION OF THE BONE MARROW ACTIVITY IN NEUROGENIC HYPERTENSION Inflammation and Endothelial Progenitor Cells in Neurogenic Hypertension It is well established that hypertension is associated with increase in inflammatory modulators in the circulation as well as in the central nervous system Chronic inhibition of inflammatory markers prevents ischemic-induced vascular pathology in type II diabetic mice, 133 and increased inflammation in the circumventricular organs and brainstem is associated with increase in the sympathetic drive. 134 Vascular inflammation in the brainstem of the SHR, the rat model of neurogenic hypertension was increased with elevated inflammatory cytokines such as IL-1β, IL-6, and TNF-α. 132 The treatment of Ang II type 1 receptor (AT 1 R) blocker with access to the brain showed antihypertensive effects and inhibited cerebrovascular inflammation including reduced 135, 136 macrophage infiltration and decreased cytokine expressions such as TNF-a, IL1b. These data and Felder s work 137 allow us to propose that vascular inflammation in cardiovascular disease including hypertension may be CNS regulated, and inflammatory process in the CV regulatory regions of the brain is associated with modulation of the autonomic function. In particular, there is supportive evidence that the vascular inflammation and brain cytokines play an important role in the pathogenesis of hypertension. 138 Furthermore, expression of inflammatory cytokines is increased in the cardiovascular relevant brain regions of various animal model of hypertension. 132, 139 For example adeno-associated viral mediated IL-10 overexpression in the PVN attenuates Ang II-induced hypertension, 121 and IL-6 microinjection in the NTS attenuates the barorceptor reflex gain function in rats. 140 In addition, ICV infusion of IL1α, a 48
49 proinflammatory cytokine increase sympathetic nerve activity resulting in high BP. In previous study, shi et al have demonstrated that microglia in the PVN are activated in response to chronic Ang II infusion and produce a variety of inflammatory mediators, including cytokines. 121 Microglia produces neurotoxic responses through production of cytokines and ROS. This damages the BBB integrity resulting in the infiltration of inflammatory cells in the brain. 141 It is reported that activated microglia cells are increased in the central nervous system from the patients with neurodegenerative disease, such as Alzheimer and Parkinson s disease. 94, 95 However, the involvement of brain in peripheral BM-derived inflammatory signaling and the regulation of endothelial regenerationin hypertension has not been studied extensively. EPCs are BM derived stem cells that are important components in the endothelial repair process after vascular injury in cardiovascular disease. It is well established that endothelial dysfunction is a relatively early event in hypertension-induced vascular pathogenesis, therefore preventing endothelial damage from high blood pressure could be a critical therapeutic strategy for hypertension. Since various cardiovascular disorders are found to be associated with loss of intact vasculature and vascular inflammation, well-functioning EPCs might not only be important for maintaining intact vasculature in healthy individuals, but also for preventing various cardiovascular disorders including diabetes and hypertension. There are reports that dysfunctional EPCs induce oxidative stress and inflammatory cytokines that could result in vasoconstriction, inflammation, and vascular fibrosis. 106, 142, 143 Moreover, the number of circulating EPCs are decreased, and the ability of their functions are impaired in both , 144 experimental animal models and human patients of cardiovascular disease. 49
50 Considering the dysfunctional sympathetic and parasympathetic drive in neurogenic hypertension, it is tempting to suggest that altered sympathetic drive to BM may contribute to EPC impairment. This contention is supported by recent experiment in diabetes with retinopathy, a pathophysiology that is also associated with increase in proinflammatory cytokines and microglia cells in the eye. 145 A reduction in the number of nerve terminal ending in the BM and impaired sympathetic drive has been associated with diabetic retinopathy. 146 This study was designed to investigate the hypothesis that there is central regulation of BM EPCs and a functional balance between inflammatory cells (ICs) and EPCs in neurogenic hypertension. W e observed the effects of Ang II infusion on the numbers and functions of BM-derived EPCs and the ratio of EPCs/ICs in Ang II-induced rat model of hypertension. Additionally, mitochondrial targeting superoxide scavenger, mitotempo ICV treatment induced antihypertensive model (descried in Chapter 2) was utilized to investigate the functional connection of BM and brain. Methods Animal Adult male Sprague-Dawley (SD) rats aged 6 to 7 weeks were purchased from Charles River Laboratories (Wilmington, MA). Rats were individually housed in a temperature-controlled room (22 C to 23 C) with a 12:12-hour light-dark cycle. Rat chow (Harlan Tekland) and water were provided by Animal Care Services. All experimental procedures were approved by the University of Florida Institute Animal Care and Use Committee. 50
51 MNC Isolation from Blood and BM Rats were subjected to anesthesia with isoflurane and about 10 ml of blood was drawn from abdominal vein. Collected blood was diluted with PBS+2%FBS+1mM EDTA followed by the addition of 10ml of Ficoll-Paque (GE Healthcare, Cat# ).Yellow buffy coat was obtained after centrifugation at 1200rpm for 25min and was transferred to a new tube to isolate mononuclear cell (MNC) pallet. To remove residual red blood cells in the pellet, ammonium chloride (STEM CELL technology, Cat# 07850) was added and incubated for 10min on ice. Cells were then centrifuged twice after washing with PBS+2% FBS+1mM EDTA to remove residual ammonium chloride. White MNC pellet was obtained after second centrifugation at 1200rpm. For BM MNCs, intact femur and tibia were isolated from rats and rinsed with PBS+2% FBS+1mM EDTA buffer followed by cleaning and removing muscle and fat. The tips of the bones were cut to flush out bone marrow cells using syringes into 50ml tube. MNC pellets were obtained by spinning down at 1200rpm for 15mins at room temperature. Ammonium chloride was added to remove RBCs for 10 min on ice same as blood MNC isolation followed by 2 times washing with PBS+2% FBS+1mM EDTA. Isolation of EPCs from MNCs MNCs were prepared in the 5ml tubes at a concentration of cells/ml in PBS+2% FBS+1mM EDTA. 50ul of EasySep negative selection cocktail (STEM CELL technology) is added to the cells and incubated for 10 min at room temperature followed by incubation with 50ul of EasySep Magnatic Nanoparticles (STEM CELL technology). Tubes were placed into the EasySep Magnet for 5min at room temperature for the negative selection. The EasySep Magnet generates a high-gradient magnetic field in the interior cavity that is strong enough to separate cells labeled with EasySep 51
52 Magnetic Particles. The magnetically labeled cells remain bound inside the tube, held by the magnetic field when unlabeled cells within the magnet are transferred to the new tube placed outside. This step was repeated for three times. After centrifugation for 10min at 1200rpm, cell pellets were re-suspended in 100ul of PBS+2% FBS+1mM EDTA at a concentration of cells or fewer with 5ul of mouse serum for positive selection (STEM CELL technology). CD90 + antibody cocktail and Magnetic nanoparticles from the positive selection kit (STEM CELL technology) were added to the cell suspension. Tubes containing cell mixture were placed in the magnet for 5min incubation. The magnetically labeled cells remained in the tube, held by magnetic field of the EaseSep Magnets while the rest of unlabeled cells were removed. This step was repeated for three times to isolate less contaminated CD90 + cells. Cells were plated in 96well plate in Stem Span media (STEM CELL technology Cat# 09650) and functional assay. Direct Flow Cytometry (FACS) Analysis To profile the level of inflammation MNCs from BM and blood were prepared in a concentration of x 10 6 cells/100ul in 2%FBS, 1mM EDTA and 1xPBS mixture media. Antibodies were from AbD Serotec (Alex647 conjugated CD4/5/8/3/68, RPE conjugated CD25, FITC conjugated CD45, Percp-cy5.5 conjugated CD90) as recommended by the company. Cells are incubated with antibodies for 45 minute at 4 o C. Individual antibodies were prepared in each cell suspension as control. After twice of washing, cells were fixed with 2% paraformaldehyde for later analysis. All samples were read on LSR-II (BD Biosystems) in University of Florida Interdisciplinary Center for 52
53 Biotechnology Research (ICBR) and data were analyzed withfacs Diva software, version DiLDL and Lectin Staining CD90 + cells are examined for DiLDL (1,1-dioctadecyl-3,3,3,3- tetramethylindocarbocyanine low density lipoprotein) uptake and lectin binding to confirm its EPC characteristics. First, isolated cells are incubated with DiLDL (final con. 10 µg/ml, Invitrogen) at 37 C for 1 h followed by twice of washing with PBS. Then cells were fixed in 2% paraformaldehyde for 10 min and counterstained with 20 µg/ml FITClabelled lectin from Ulexeuropaeus (L9006, Sigma) for 1 h at 37 C in the dark. After washing cells with PBS, double-positive Dil LDL/Lectin cells were observed from Olympus BX41 fluorescence microscope. Results Dysfunctional Endothelial Progenitor Cells in Chronic Ang II-induced Hypertension BM derived EPCs are dysfunctional in AngII-induced hypertension CD90 + (Thy-1 + ) and CD4 - /CD5 - /CD8 - markers were used to isolate EPCs. They are known to be human CD34+ cells in rats. 102, 147 In order to separate CD90 + /CD4 - /CD5 - /CD8 - cells from mononuclear cells (MNCs), magnetic beads with antibodies were utilized. These cells stained positive for LDL uptake and Lectin binding (Figure 3-1). Double positive staining confirmed the characteristics of EPCs. Second, functional assays were performed to determine if CD90 + /CD4 - /CD5 - /CD8 - cells demonstrate EPCs characteristics, and the effects of chronic Ang II infusion on BM derived EPCs. Cells were plated onto 96 well for 24 hours and the abilities of proliferation and migration toward SDF-1 were measured by fluorescence and luminescence, respectively. Both 53
54 proliferation and migration abilities are significantly reduced after 6 (~70%) and 12 weeks (~40%) of Ang II infusion compared to control, but not after 4 weeks infusion (Figure 3-2A and B).However, Ang 1-7 pretreatment with EPCs for 24 hour did not improve these abilities toward SDF (Figure 3-2C and D). Tube formation ability of cultured MNCs from Ang II infusion rats is diminished Isolated MNCs were plated in fibronectin pre-coated 6-well plates and maintained with endothelial basal medium for 3 weeks until they differentiate into endothelial cells. Cells were then transferred to 96-well Matrigel matrix plate (BD BioCoat TM Angiogenesis System Endothelial Cell Tube Formation, Cat #: ) at 2.5~3x10 4 cells/ml and incubated for 12 hours at 37, 5% CO 2. Then cells were monitored under microscope (bright field) every 2-3 hours to identify the ability of tube formation (Figure 3-3A and B). The length of tubes and the number of branches from each cell were measured. Cells continued to form tubes until 10 hours after plating. Analysis with Image J demonstrated that the length of tubes and the number of branches were significantly reduced in cells from Ang II infusion rats compared to cells from control rats (Figure 3-3C and D). Ang II-induced Imbalance of EPCs and ICs BM-derived EPCs are reduced by chronic Ang II infusion To investigate the effects of Ang II on the EPC numbers, BM-derived MNCs were isolated from femur and tibia of control rats and Ang II infused rats (4, 6 and 12 weeks infusion). BM EPC numbers were measured from MNCs sorted by FACS analysis. CD90 + /CD cells were counted as EPCs. Increased mean arterial pressure confirmed hypertension induction by Ang II infusion in SD rats (4-12 weeks infusion: mmHg of MAP) (Figure 3-4A). As discussed above, the number of EPCs was significantly reduced by 50% from 4 weeks to 12 weeks of Ang II infusion (4 week: 54
55 73±4, 6 week: 36±5, 12 week: 38±8 % of control) (Figure 3-4B), when blood pressure increased. Blood EPCs showed similar trend but were not significantly reduced in neither of the Ang II infusion rats. Inflammatory cells are elevated in the BM and circulation by chronic Ang II infusion To further investigate the involvement of inflammatory cells in chronic Ang II infusion model of hypertension, BM-derived MNCs were isolated to profile the changes in the number of inflammatory cells (ICs). Inflammatory cells such as CD4 + /8 + (T lymphocytes), CD4 + /8 + /25 + (T regulatory cells), CD45 + /3 + (T lymphocytes), and CD68 + (macrophages) were measured by FACS analysis to compare the changes of its numbers from MNCs. Chronic Ang II infusion resulted in ~250% increases in BMderived ICs and similar trend in blood ICs (Figure 3-6B). As a result, there were significant decreases in the EPCs/ICs ratios (control: 1±0.2, Ang II: EPCs/CD4 + /8 + (0.75±0.06 to 0.2±0.02), EPCs/CD4 + /8 + /25 + (0.6±0.08 to 0.23±0.01), EPC/CD45 + /3 + (0.79±0.1 to 0.21±0.03), EPC/CD68 + (0.62±0.08 to 0.3±0.05) in the BM and blood by 4 to 12 weeks of Ang II infusion (Figure 3-6). MitoTEMPO ICV Treatment Inhibits Elevated BM Inflammatory Cells and Reduced EPCs The effect of ICV mitotempo on BM EPCs and ICs was studied to determine if central alteration of hypertension correct the imbalance in EPC/IC. Ang II-induced decrease in the number of BM EPCs was completely restored by ICV mitotempo treatment (Ang II: 52±4%, ICV mitotempo: 88±6%, SC mitotempo: 49±8% of control) (Figure. 3-6A). In addition, BM IC levels were normalized by ICV mitotempo treatment (Figure. 3-6B). As a result, there was a ~5 fold decrease in the EPCs/ICs ratio in the BM and ICV, but not SC mitotempo normalized this (Figure. 3-6C).These results 55
56 demonstrate the existence of functional neural-bm interaction in Ang II-induced neurogenic hypertension. Discussion This study demonstrates that Ang II-induced hypertension that exhibits neurogenic component is associated with a decrease in the number of EPCs, the function of EPCs and EPCs/ICs ratio. This may compromise the ability of EPCs to repair vasculature and contribute to the establishment of hypertension-linked pathophysiology. This significant finding suggests that there is a neural-bm connection that regulates inflammatory status and helps to maintain healthy endothelium in normal animals. Furthermore, brain mitochondrial oxidative stress plays an important role in the regulation of the neural-bm communication. It is shown that dysfunctional EPCs are associated with human metabolic and cardiovascular diseases, increase in NADPH oxidase activity and decrease in NO production in hypertension. 105, 148, 149 Recent study from Jarajapu et al described that blockade of NADPH oxidase restores vasoreparative function in diabetic EPCs. 150 The protective role of EPCs and its regulatory mechanism in neurogenic hypertension has become attractive in past years. Although damaged endothelium and imbalanced vascular homeostasis in cardiovascular disease are associated with dysfunctional EPCs, it has not been evidenced that there is a central regulation of EPCs. Present study suggests that Ang II-induced dysfunctional EPCs and the role of central mitochondrial ROS in the regulation of EPCs and ICs in hypertension. We demonstrated that there is a dysfunctional regulatory link between brain and BM in Ang II-induced hypertension and brain mitochondrial ROS plays an important role in neural- BM connection. As shown in Figure 3-4A, chronic Ang II infusion reduced BM EPC number in 4, 6, and 12 weeks of infusion in a time dependent manner. The functional 56
57 ability of migration and proliferation toward SDF was also decreased in 6 and 12 weeks infusion of Ang II (Figure 3-2A and B).These detrimental effects of Ang II on EPCs from the hypertensive rats could be induced by either vasoconstrictive signals from circulating Ang II levels or brain-mediated sympathetic drive or both. Further experiments will be required to elucidate more precise mechanisms regarding this matter. The possibility of direct Ang II signals into BM sympathetic nerve endings causing EPCs to dysfunctional cannot be ruled out from this study. Increased vascular inflammation in hypertension and cardiovascular disease are well established based on both clinical and animal studies. 104, 105 Harrison s group was among the first to demonstrate the role of T cells and adaptive immune system in the pathogenesis of hypertension. 85 In the present study, Ang II infusion increased BM inflammatory cells (ICs) and reduced EPCs/ICs ratio accordingly. mitotempo, mitochondria targeting antioxidant ICV treatment significantly restored the Ang IIinduced changes of EPCs and ICs in the BM, demonstrating the functional connection of neural-bm interaction. As discussed above, increased circulating Ang II may directly affects the increase in BM ICs resulting in vascular inflammation. However, the novelty of the present study is that inhibition of the brain ROS is able to attenuate changes in BM activity and inflammatory status, which supports a brain-bm communication. The functional balance of inflammatory cells and EPCs needs to be maintained in normal vascular physiology. However, in hypertensive disease increased sympathetic drive and oxidative stress within the brain may increase BM ICs and decrease EPCs resulting in impaired vascular pathophysiology. Mitochondrial ROS contributes to increase in sympathetic activation and neuroinflammation by activating microglia in the 57
58 PVN. This concept is supported by the following evidence. First, Ang II hypertension increases activated microglia and proinflammatory cytokines in the PVN 97 (and the study in chapter 2). Second, interruption of microglia activation by either minocycline 97 or by mitotempo attenuates hypertension, and third, hypertension-induced imbalance in BM activity is prevented by central mitotempo treatment, indicating the existence of regulatory link between the autonomic brain regulatory regions and the BM. Finally, these data demonstrate that dysfunctional neural-bm communicatory pathway may be the critical mechanism in pathophysiology of Ang II-induced neurogenic hypertension. 58
59 Figure 3-1. DiLDL uptake and lectin binding of CD90 + /CD EPCs. Isolated EPCs from BM were stained with lectin (green, excitation wavelength 477 nm) or DiLDL (red, excitation wavelength 543 nm). Double positive cells appearing yellow in the merge were identified as EPCs. 59
60 Figure 3-2. Dysfunctional BM EPCs by chronic Ang II infusion. A, Migration assay toward SDF. B, Prolifertation assay toward SDF. C, Ang 1-7 pretreatment before migration assay. D, Ang 1-7 pretreatment before proliferation assay.*p<0.05, #P<0.01 vs control. n=
61 Figure 3-3. Decreased ability of tube formation by chronic Ang II infusion. A and B, Representative images of tube formation from control rat and Ang II infusion rat. C, The length of tubes from each group were analyzed using Image J. D, The number of branches were counted and quantificated by graph. *P<0.05 vs control. n=
62 Figure 3-4. Reduced number of BM EPCs in Ang II induced hypertensive rats. A, Tail cuff measured mean arterial pressure (MAP). Systemic Ang II infusion elevates MAP. B, Decreased EPC numbers by Ang II infusion. *P<0.05, #P<0.01 vs control. n=
63 Figure 3-5. Elevated ICs and imbalance of EPC/IC in Ang II induced hypertension. CD4 + /8 + (T lymphocytes), CD4 + /8 + /25 + (T regulatory cells), CD45 + /3 + (T lymphocytes), CD68 + (macrophages) *P<0.05, #P<0.01 vs control. n=
64 Figure 3-6. Effects of mitotempo ICV treatment on BM EPCs and ICs in Ang II induced hypertension. A, ICV mitotempo normalized the decreased number of EPCs (CD4 - /5 - /8 - /90 + ) by Ang II infusion to control level. B, ICV mitotempo normalized increases in BM derived inflammatory cells. C, The ratio of EPCs to ICs. *P<0.05 vs control, #P<0.05 vs Ang II by 1-way ANOVA followed by Bonferoni. n=7=8. 64
65 CHAPTER 4 ROLE OF NDUFA10 IN NEUROGENIC HYPERTENSION Proteomic Approach of Hypertension Utilizing Spontaneous Hypertensive Rat Hypertension is a primary cause in many cardiovascular disease, including stroke, diabetes, ischemic heart disease, and renal failure. The onset of hypertension is also a product of a natural aging process, however its severity and timing is usually associated with certain lifestyle relating factors such as diet, exercise level, and body weight. From the past decades it is hypothesized that certain gene expression profiles are altered in hypertensive patients compared to healthy individuals, 151 and some of which may be inheritable. 152 However, whether this gene expression alteration is pro-hypertensive or simply a secondary effect of high blood pressure remains unclear. In the majority of cases there is no single factor underlying the onset of hypertension, and such cases are considered to be primary hypertension. Primary hypertension is a complex polygenic trait with underlying genetic components, and many of them are still unknown. This contributes to 90-95% of all hypertensive cases. 153, 154 In past decades, the central roles of sympathetic/parasympathetic drive in the development of hypertension have been emphasized. Treatments with drugs blocking the renin-angiotensin system (e.g. angiotensin-converting enzyme inhibitors, angiotensin receptor type 1 blockers) are significantly effective in hypertensive patients, however in a considerable number of patients they fail to control high blood pressure These pharmacotherapy resistant cases have been indicated to exhibit neurogenic component with increased sympathetic drive and altered neural cardiovascular mechanism. Thus, understanding of the cellular and molecular mechanisms of neural control of cardiovascular function is of extreme important in order to establish more effective treatment strategies. 65
66 Autonomic nervous system is a powerful modulator of blood pressure regulation, which is controlled by a complicated network of brain nuclei mainly localized in the hypothalamus and brainstem. 15 These specialized nuclei include the paraventricular nucleus (PVN) adjacent to the third ventricle in hypothalamus, the subfornical organ (SFO) in the roof of the third ventricle, the organum vasculosum of the lamina terminalis (OVLT) in the forebrain, the nucleus of the solitary tract (NTS), and the rostral ventrolateral medulla (RVLM) in the brainstem. 16, 17 Circumventricular organs such as SFO and OVLT are BBB incompletes areas within the brain, which allow circulating Ang II signals from periphery to activate these nuclei and to transmit to PVN and RVLM resulting in stimulation of sympathetic drive. Collectively, the evidence led us to hypothesize that altered expression of key regulatory gene/associated gene(s)in CV relevant regions is linked to neurogenic hypertension. We decided to investigate this hypothesis by the use of proteomic profiling of the PVN from hypertensive rats. SHR, an animal model of human primary hypertension and age matched WKY rats were utilized. The rational for detecting the PVN of the brain for protein profiling was that it is a key cardiovascular regulatory nuclei located in the hypothalamus, transmitting signals from circumventricular organ to brainstem such as RVLM and NTS. Methods Animal Spontaneously hypertensive rats (SHRs) aged 12 weeks and age matched WKY rats were purchased from Charles River Laboratories (Wilmington, MA). Rats were individually housed in a temperature-controlled room (22 C to 23 C) with a 12:12-hour light-dark cycle. Rat chow (Harlan Tekland) and water were provided by Animal Care 66
67 Services. All experimental procedures were approved by the University of Florida Institute Animal Care and Use Committee. Primary Neuronal Culture Neuronal cells in primary culture from the brainstem and hypothalamus of one day-old SD were established. Brains were isolated from neonatal rats and hypothalamic and brain stem areas were dissected into separate dishes. Tissues were trypsinized for 15 min at 37 C to dissociate individual neurons. Cells are then plated in poly-l-lysine pre-coated 6 or 12 well culture dishes. DMEM with 10% FBS were used as cultural media and neurons are maintained for days prior to use in the experiments. Antimitotic agent, arabinoside-c was treated 3 days after plating culture and changed media 3 days later to increase the purity of the neuronal culture. Western Blot Assay PVN proteins were extracted from 3 WKY control rats and SHR experimental rats at 20 weeks in age. Protein concentration was measured in the supernatant with the Bradford assay kit (BioRad), using BSA (bovine serum albumin) as standard protein. Fifteen to twenty micrograms of proteins were run on a 12% SDS-PAGE, and the proteins were transferred onto a nitrocellulose membrane. After1 hour blocking with 5% milk in Tris-buffered saline-tween 20, the membrane was probed with the rabbit polyclonal antibody against Ndufa10 (1µg/ml in 3% milk/tris-buffered saline-tween 20, XW -7882, ProSci) overnight at 4ºC. The membrane was washed 3 times for 10 minutes in Tris-buffered saline-tween 20 and incubated with anti-chicken IgG-horseradish peroxidase-conjugated secondary antibody (1:5000) for 1 hour at room temperature. After final washes, the membrane was incubated with chemiluminescent agent for 1 67
68 minute and then exposed to film to visualize protein bands. β-actin was used as a loading control. 2D Gel and Image Analysis The experimental scheme is described in Figure 4-1. PVN proteins were extracted and prepared same way as Western blot assay. The first dimension was performed in an EttanIPGphor (GE Healthcare) using DryStrips ph 3 11 NL, 24 cm (GE Healthcare). Samples were loaded on the acid end of the strip with loading device. The isoelectrofocusing conditions were those recommended by the manufacturer for the type of strip used. The SDS-PAGE (12%) was developed in an EttanDALTsix(GE Healthcare) at 257C and 1 W/gel for 12 h. Proteins were visualized by staining with SYPRO Ruby Protein Gel Stain. The image analysis was performed with DeCyder v.6.5 software (GE Healthcare). The differently labeled protein spots in the same gel were detected with the Differential In-gel Analysis (DIA) mode of the software using its proprietary algorithms. Spot images from 3 different gels were matched with the Biological Variation Analysis (BVA) mode. The normalized ratios of each protein spot from each gel of all 3 replicates were standardized according to internal standard. Proteins were selected when the average fold difference of SHR/WKY was greater than 1.5 or lower than -1.5, and when the p-value of Student T-test of was less than Ndufa10 Overexpression For Ndufa10 overexpression in neuronal cells, AAV-plasmid vector inserted with ndufa10 cdna were produced. Full-length cdnas cloned in the pexpress-1 mammalian expression vector are commercially available from Open Biosystem. First, ndufa10 cdnas was cloned to AAV plasmid vector ptr-uf22, which is regulated by the 381-bp CMV immediate early gene enhancer, 1352-bp chicken β-actin promoter and the - 68
69 exon1-intron1 woodchuck post-transcriptional regulatory element (WPRE). The plasmids were amplified and purified by using CompactPrep Plasmid Midi Kit (Qiagen 12843) and then transfected into human HEK 293 cells using standard procedures 159. ptr-uf22-ndufa10 and GFP control vectors were transfected to neuronal HEK 293 cells for 24 and 48 hours. mrna were collected at various time points (24 h, 48 h) to measure Ndufa10 expression. Efficiency will be checked by GFP expression under fluorescence microscope. Measurement of Mitochondrial and Cellular ROS Production Eleven to thirteen days neuronal cultures were treated with Ang II (500nM), or overexpressed with ptr-uf22-ndufa10. Cellular superoxide production was measured by DHE (dihydroethidium, Invitrogen) fluorescent staining and mitochondrial superoxide were measured by MitoSOX Red staining (Invitrogen). DHE (1nM) was added to neurons for 30 min at 37 C and cells were washed with PBS three times. For the detection of mitochondrial ROS, neurons were incubated with 5uM of mitosox red dye for 10 min at 37 C and washed with PBS three times. DAPI was used as nucleus counter staining before cells were fixed. Images were obtained from Zeiss Axioplan 2 Fluorescent Visualization Microscope. Data and Statistical Analysis Data were expressed as mean±sem. 2-way ANOVAs or 1-way ANOVAs, and the Bonferroni post-test was used to allow multiple comparisons of cardiovascular variables across time and between different groups. Paired/unpaired Student t-test was used for further comparisons between 2 groups where applicable, with P<0.05 considered significant. 69
70 Results 2D Gel Analysis Revealed Differential Protein Expressions in PVN of WKY Rat and SHR First, in collaboration with Dr. Sally Yuan, we compared 2D gel profiles of the PVN protein from WKY and SHR carried out at the ICBR Core Facility. A representative protein gel image is shown in Figure 4-2. PVN proteins were extracted f rom 3 W KY control rats (MAP= mmhg) and 3 SHR experimental rats (MAP= mmhg) at 20 weeks in age. At least 1600 spots were obtained in each gel but only 1000 spots were matched in all three gels (Figure 4-3). The image analysis was performed with DeCyder v.6.5 software (GE Healthcare). The differently labeled protein spots in the same gel were detected with the Differential In-gel Analysis (DIA) mode of the software using its proprietary algorithms. Spot images from 3 different gels were matched with the Biological Variation Analysis (BVA) mode. The normalized ratios of each protein spot from each gel of all 3 replicates were standardized by the internal control. The standardized and averaged ratio between differentially expressed proteins was calculated. BVA was also used for statistical analysis. Proteins were selected when the average fold difference of SHR/WKY was greater than 1.5 or lower than -1.5, and when the p-value of Student T-test was less than Figure 4-3 shows the list of proteins that has differently expressed between WKY rat and SHR PVN. Glutathione transferase omega-1, Glutathione S-transferase Mu-1, and Nucleoside diphosphoate kinase A were shown to increase in the SHR by ~3.22, ~3.89, and ~5.57 fold, respectively compared to WKY. Lamin-B1 protein, in contrast was decreased by ~4.15 fold in the SHR. We selected NADH dehydrogenase 1 alpha subcomplex 10 encoded by Ndufa10 since it showed the highest expression difference between the two strains. This protein showed 70
71 in a two different spots with different molecular weights. The ratio of SHR/WKY was 6.17-fold increased in the spot 720 whereas it was 5.75-fold decreased in the spot 753 (Figure 4-3). We hypothesized that post-translational modification is processed in the SHR responsible for the precursor of two different sizes of Ndufa10 proteins. However, further experiments would be required to confirm this. Ndufa10 Expression is Increased in Cardiovascular Regulatory Regions of SHR Compared to WKY Western blot analysis was carried out to verify the result of the 2D analysis with an anti-ndufa10 antibody (Prosci XW-7882). Figure 4-4 shows a comparison of Ndufa10 protein levels in different CV relevant brain regions in WKY rats and SHR. Ndufa10 was present in all CV relevant brain regions tested: PVN, RVLM, OVLT, SFO and NTS. In addition, there were significant increases in Ndufa10 protein levels in the SHR compared to WKY rats (PVN: ~2, RVLM: ~1.2, NTS: ~1.3, SFO: ~1.5, OVLT: ~3 fold) (Figure 4-5). Ndufa10 from SHR showed greater molecular weight compared to one f rom W KY. There is a study by Meng et al also showing that Ndufa10 expressed in left ventricular of SHR had greater molecular weight than in left ventricular of WKY rats suggesting that Ndufa10 might be involved in development of cardiac hypertrophy. 160 The exact mechanism regarding Ndufa10 post-transcriptional modification needs to be further examined. Neuronal cells cultured from hypothalamic brain of prehypertensive SHR were used to determine if Ndufa10 is genetically linked to hypertension. In the SHR, mrna and protein levels of Ndufa10 were 1.3 fold and 3.5 fold increased respectively compared to WKY neurons (Figure 4-6). 71
72 Ndufa10 Overexpression Induces Cellular and Mitochondrial Oxidative Stress in HEK 293 Cells We determined if Ndufa10 is involved with cellular oxidative stress with the use of a human cell line HEK293. Full-length cdnas of ndufa10 was cloned in the pexpress-1 mammalian expression vector. ndufa10 cdnas was first cloned to AAV plasmid vector ptr-uf22, which is regulated by the 381-bp CMV immediate early gene enhancer, 1352-bp chicken β-actin promoter and the -exon1-intron1 woodchuck posttranscriptional regulatory element (WPRE) as shown in Figure 4-7A. The plasmids were amplified and transfected into human HEK 293 cells using standard procedures. mrnas were collected at various time points (24 h, 48 h) to measure Ndufa10 transcription. Ndufa10 mrna was 2~2.2 fold higher after 24-48hours of transfection with Ndufa10 plasmid compared with GFP plasmid (Figure 4-7B). Cellular superoxide was measured by DHE red fluorescence and mitochondrial superoxide was measured by mitosox red staining. Cellular ROS was ~2 fold increased with ndufa10 overexpression (Figure 4-8A). Additionally, the amount of H 2 O 2 produced by Ndufa10 overexpression was ~2 fold increased after 48 hours of transfection as shown in Figure 4-8B. Mito SOX staining confirmed the increase in oxidative stress is generated from mitochondria (Figure 4-8C). Discussion The purpose of the present study was to determine if there are hypertension-linked protein(s) are present in the PVN of the SHR. These proteomic studies have revealed that NADH dehydrogenase (ubiquinone) 1 alpha subcomplex 10 (Ndufa10) is significantly up-regulated in the PVN and other CV regulatory regions of the SHR. In addition, overexpression of ndufa10 significantly induced cellular and mitochondrial 72
73 ROS generation in HEK 293 cells. Our observation of increased cellular oxidative stress by Ndufa10 overexpression is supported by Jin X et al who suggested that Ndufa10 expression in the left ventricular of SHR heart is involved in the increased production of ROS leading to hypertrophic heart. 160 It is tempting to suggest that abnormally regulated Ndufa10 expression may cause excessive ROS production in the CV regulatory regions, leading to high blood pressure and neurogenic hypertension. Ndufa10 is one of the 45 subunits in electron transport chain complex I and has dehydrogenase activity. Recent study from Olsson et al found that Ndufa10 is one of decreased genes involved in oxidative phosphorylation in human pancreatic islets from patients with type 2- diabetes. 161 Another study demonstrated that Ndufa10 mutations disrupted the start codon and amino acid substitution cause complex 1 deficiency in a patient with Leith disease. 162 However, its involvement of cellular signaling pathway in neuronal activity and sympathetic drive in neurogenic hypertension is not yet tested. It is possible that differentially regulated Ndufa10 would induce the mitochondria electron transport chain malfunction that leads electrons to leak out of the transport chain leading to excessive ROS production and neural firing in the brain. It is well established that cellular ROS are important for normal signaling pathway in the heart, the kidney and the brain. 74, 163, 164 Chronic increases of ROS production 109, especially in CNS are strongly implicated in development of hypertension. Circulating Ang II induced hypertension also involves an increase in intracellular superoxide levels in neurons. 56 Ang II-induced stimulation of ROS production may stimulate sympathetic nerve activity in the CNS, thereby contributing to chronic increase in blood pressure. Cellular oxidative stress mediated by NADHP oxidase, an enzyme 73
74 complex that is mainly activated by Ang II is well documented. However, as an important source of cellular ROS production, mitochondrial mediated oxidative stress in hypertension has not been tested. The leakage of electrons from electron transport chain complexes results in the cellular accumulation of ROS including superoxide anion and hydrogen peroxide. Mitochondrial ROS is produced when the enzymatic activities of electron transport chain complex I are reduced and dysfunctional, resulting in the accumulation of electrons, which facilitates the direct transfer of electrons to molecular oxygen. 77, 168 However, it has been suggested the possibility of NADPH oxidase activation by mitochondrial ROS. 119 It is possible that mitochondria superoxide production can be first triggered by NADPH oxidase activation through Ca 2+ accumulation within the mitochondria, and subsequently produced mitochondrial ROS could inversely activate NADPH oxidase. Studies have shown that mitochondria-derived ROS in CNS mediated elevated sympathetic nerve activity in hypertension and that is induced by Ang II infusion. 169 A recent Study by Chan et al also showed that impaired mitochondrial activity increased ROS production in the RVLM, and the administration of coenzyme Q10, which is an anti-oxidative enzyme, restored electron transport chain function and attenuated hypertension. 77 In a view of this, Ndufa10 as the one of the electron transport chain complex subunit may be the key regulator of mitochondrial and cellular ROS generation in CNS and the contributor to neural mechanism of neurogenic hypertension. Altered expression of Ndufa10 in pre-hypertensive stage and its effects on mitochondrial ROS production in the brain of SHR suggests that there might be a genetic correlation between Ndufa10 and development of neurogenic hypertension. However the exact 74
75 mechanisms in which cellular signaling involving the protein expression regulation are not fully identified. 75
76 Figure 4-1. Experimental design of 2D gel analysis. PVN proteins are isolated from the brain of SHR and WKY rats and two-dimentional difference gel electrophoresis (2D-DIGE) was performed to compare protein expression level between SHR and WKY rats. Additionaly, protein and mrna were prepared from both tissue and primary neuronal culture for Westen blot analysis and real-time PCR. 76
77 Figure 4-2. Representative 2D-DIGE gel from PVN of SHR. Each boxed spot shows significantly up or down regulated protein in SHR compared to WKY.PVN proteins were extracted from 3 WKY rats and SHR rats at 20 weeks in age. At least 1600 spots were obtained in each gel but only 1000 spots were matched in all three gels. n=3. 77
78 Figure 4-3. The list of differentially expressed proteins from PVN of SHR compared to WKY.The image analysis was performed with DeCyder v.6.5 software (GE Healthcare). The differently labeled protein spots in the same gel were detected with the Differential In-gel Analysis (DIA) mode of the software using its proprietary algorithms. Spot images from 3 different gels were matched with the Biological Variation Analysis (BVA) mode. The normalized ratios of each protein spot from each gel of all 3 replicates were standardized by internal control. The standardized and averaged ratio between differentially expressed proteins was calculated. BVA was also used for statistical analysis. Proteins were selected when the average fold difference of SHR/WKY was greater than 1.5 or lower than -1.5, and when the p-value of Student T-test of was less than n=3. 78
79 Figure 4-4. Distribution of Ndufa10 in cardiovascular relevant brain regions of WKY and SHR. A, Ndufa10 protein expression from different CV regulatory regions f rom W KY. B, Ndufa10 protein expression from different CV regulatory regions from SHR. C and D, Quantification of the bands density. n=
80 Figure 4-5. Increased expression of Ndufa10 mrna and protein in SHR compared to WKY. Ndufa10 expression is upregulated in the PVN, RVLM, OVLT, SFO and NTS of SHR.There were significant increases in Ndufa10 protein levels in the SHR compared to WKY rats (PVN: ~2, RVLM: ~1.2, NTS: ~1.3, SFO: ~1.5, OVLT: ~3 fold). *P<0.05 vs WKY. n=
81 Figure 4-6. Increased expression of Ndufa10 in cultured neurons from SHR compared to neurons from WKY.In the SHR, mrna and protein levels of Ndufa10 was ~1.3 fold and ~3.5 fold increased respectively compared to WKY neurons. *P<0.05 vs WKY by t-test. n=
82 Figure 4-7. Ndufa10 overexpression vector and its efficiency. A, AAV-plasmid vector inserted with ndufa10 cdna or hgfp.ndufa10 cdnas was first cloned to AAV plasmid vector ptr-uf22, which is regulated by the 381-bp CMV enhancer, chicken β-actin promoter and the -exon1-intron1 woodchuck posttranscriptional regulatory element (WPRE). The plasmids were amplified and transfected into human HEK 293 cells. B, ptr-uf22-ndufa10 and GFP vectors were transfected to HEK 293 cells. Ndufa10 mrna is fold increased in ndufa10 vector transfected cells after 24 and 48 hours. *P<0.05 vs vector control. n=
83 Figure 4-8. Increased cellular and mitochondrial oxidative stress by Ndufa10 overexpression. A, Cellular superoxide is measured by DHE fluorescence (dihydroetidium, excitation 490 nm/emission 585 nm). In the presence of Ndufa10 vector transfection for 24 hours, DHE is ~2 fold increased. B, The production of hydrogen peroxide is increased after 48 hours of Ndufa10 overexpression. C, Mitochondrial specific fluorescenc dye MitoSox staining comfirmed that Ndufa10 overexpression-induced ROS is from mitochondria. Images were taked from Zeiss Axioplan 2 Fluorescent microscope. *P<0.05. n=5. 83
84 CHAPTER 5 CONCLUDING REMARKS AND FUTURE DIRECTIONS We propose the following hypothesis based on our data and available evidence from the literature. First, neurogenic hypertension is associated with increased oxidative stress in the brain and mitochondrial ROS are the major contributor for the pathophysiology of neurogenic hypertension. Second, dysfunctional EPCs and elevated levels of ICs, and the imbalance of EPC/IC ratio are regulated by central nervous system, and there exist a neural-bm communication in hypertension. Third, circulating Ang II activates neuron and microglia that generate cellular and mitochondrial ROS, stimulating central proinflammatory cytokines, especially in the PVN. These modulators further increase neuronal activity in CV regulatory regions resulting in elevated sympathetic nerve activity and neurogenic hypertension. Oxidative stress in the brain plays an important role in the sympathetic nerve activation. NADPH oxidase is a well-accepted major player of cellular ROS production. 63, 170 However, the role of mitochondria-derived ROS and its contribution to the cellular ROS in neuron and microglia within CNS has not been tested. Figure 5-1 describes NADPH oxidase and mitochondria-mediated ROS production in neurons and glia cells. Ang II signal stimulates AT 1 R, which activates NADPH oxidase subunits such as gp91phox and Nox1/2, subsequently leading to superoxide production. Recent findings indicate that NADPH oxidase-derived cellular ROS triggers Ca 2+ accumulation within the mitochondria resulting in mitochondrial ROS generation. 76,171 They also suggested that mitochondria-derived ROS mediate neuronal activity and sympathetic activation. 76,171 In the present study we have demonstrated that ICV treatment with mitotempo completely abolish Ang II-induced development of neurogenic 84
85 hypertension, and mitochondrial protein Ndufa10 expression is increased in the brain of SHR. We propose that mitochondrial ROS triggered by Ang II or altered expression of Ndufa10 may contribute to increase in NADPH oxidase activity and cellular ROS within CNS, resulting in neuronal sympathoexcitation (Figure 5-1). However, the mechanism by which mitochondrial ROS and NADPH oxidase-induced ROS are linked in feedforward fashion remains to be investigated in neurogenic hypertension. Previous study has evidenced the importance of activated microglia in Ang IIinduced neurogenic hypertension and increase in the number of Iba1 positive microglia in the PVN. 97 Activated microglia in the brain known to mediate neurotoxicity by producing pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α in neurodegenerative disorders, aging, and diabetic retinopathy. 145, As shown in our data, chronic Ang II-infusion activates PVN microglia and increase cytokine mrna levels that is attenuated by mitotempo treatment. These suggest that oxidative stress plays an import role in microglia activation and cytokine release. Microglia and astrocyte express AT 1 R, which mediate Ang II signals and produce cytokines. 175, 176 In Figure 5-2, we propose that neurons are activated by these cytokines and produce mitochondrial and cellular ROS that is further activate microglia. The possibility of microglia generated ROS affecting neuron and microglia itself cannot be ruled out. Stimulation of proinflammatory cytokines and other mediators within the brain induces a neuroinflammatory response that is associated with increase in peripheral inflammatory response in cardiovascular diseases such as hypertension. Under normal conditions, healthy endothelial cells contribute to maintaining blood vessel integrity and vascular tone along with smooth muscle cells. EPCs participate in the repair and 85
86 regeneration of endothelial cell injury induced during the course of normal stress and environmental insult to maintain vascular homeostasis. 177 We propose that an imbalance in EPC/IC ratio and associated EPC dysfunction compromise their repair capacity leading to vascular pathophysiology, the hallmark of chronic hypertension. Thus, we believe that changes in BM or circulating EPC number and function may serve as a biomarker for cardiovascular endothelial dysfunction. According to the Department of Human Health and Services Report in 2009, a reduction in EPC numbers and decreased functional ability are currently being used to predict potential cardiovascular diseases in clinical studies. Patients with ischemic cardiomyopathy exhibit increased number of EPCs in early disease stages but decreased in the latter stages of congestive heart failure. 178 Also diabetes, chronic kidney disease, pulmonary hypertension and aging showed decreased number of EPCs associated with endothelial dysfunction. 102, 103, 105,100, 179 To increase the number and function of endogenous EPCs or to infuse exogenous healthy EPCs could be the pharmacological or genetic targets for cardiovascular disease treatments. It is well established that damaged endothelium and imbalance in vascular homeostasis are associated with decreased number and dysfunctional EPCs in hypertension. However, there has been no evidence if there is a central regulation of EPCs in hypertension. Endothelial dysfunction is an early event in high blood pressureinduced vascular pathophysiology and that is associated with increased sympathetic outflow. It is tempting to suggest that there is a neural regulatory mechanism for EPC mobilization by sympathetic stimulation to BM. Our results from mitotempo ICV administration demonstrated the possible regulatory mechanisms of central-bm 86
87 communication in neurogenic hypertension. mitotempo ICV infusion prevents Ang IIinduced decrease in BM EPCs and increase in BM ICs. Figure 5-3 summarizes the hypothesis of regulatory mechanism of neural-microglial-bm connectionin neurogenic hypertension. There is no clear evidence in the literature to support the involvement of CNS in the regulation of EPCs and ICs in cardiovascular disorder such as hypertension so far. Therefore, the exact mechanism in which CNS regulates EPCs and ICs by the innervating nerves to BM via sympathetic outflow is uncertain. Additionally the identification and characterization of EPC are still controversial. There are number of different surface markers of EPCs that are being used in the number of studies. It is required to establish a specific cell surface marker to unambiguously distinguish EPCs from closely related other cells. Utilizing different neurogenic hypertensive model such as SHR needs to be required to confirm neural-bm communicatory connection. Moreover, targeting specific CV regulatory area such as PVN in the brain to stimulate sympathetic nervous system-bm innervations pathway would be interesting. Lastly, the involvement of renin-angiotensin system including AT 1 R, Ang 1-7, ACE and ACE2 in the regulation of brain-bm regulatory mechanism cannot be ruled out. This study provides evidence in support of neural-microglial-bm regulation of cardiovascular system. It raises important issues regarding neuroinflammatory signals in other CV regulatory regions including NTS and RVLM, and BM-neural retrograde signal transduction with the involvement of microglia. Additional examination would be necessary to provide further proof of concept for this novel neural-peripheral axis. 87
88 Figure 5-1. AngII-AT 1 R mediated ROS production in neurons and glia cells. NADPH oxidase is activated by AT 1 R, producing cellular ROS. In addition, mitochondrial ROS Ca 2+ accumulation is triggered by AT 1 R and NADHP oxidase, which produce superoxide. Increased expression of Ndufa10 results in mitochondrial ROS production, leading to increase in Ang II-independent cellular ROS production. Phospholipase C (PLC), Phosphokinase C (PKC), cellular superoxide dismutase (SOD1), SOD mimetic (TEMPOL), mitochondrial suporoxide dismutase (SOD2), mitotempo (mitochondrial targeting antioxidant). 88
89 Figure 5-2. Proposed hypothesis of activated microglia-neuron-astrocyte interaction in the CNS of hypertension.at 1 Rs are present in microglia, neuron and astrocyte. Microglia are activated by Ang II signal, oxidative stress and microglia activator factors released from nearby damaged neuron such as MMP3 and neuromelanin. Cytokines are released from activated microglia and they stimulate microglia as autocrine signaling. Neuronal activity is increased by cytokines from microglia and astrocyte and produce ROS, resulting in increase in sympathetic drive. 89
90 Figure 5-3. Proposed regulatory mechanism of neural-microglial-bm connection in neurogenic hypertension.at 1 Rin circumventricular organs (SFO, OVLT) are activated by increased circulating Ang II, and signals are transmitted to the PVN neuron and microglia. Increased neuronal mitochondrial ROS mediates microglial activation and neuronal activity, resulting in increase in sympathetic drive from brain to BM. An imbalance in EPCs/ICs is due to the decrease in EPCs and increase in ICs associated with blood pressure and cardiovascular pathophysiology. Peripheral Ang II also directly affects BM EPCs and ICs. (Modified from Zubcevic et al 2011) 90
Blood Pressure Fox Chapter 14 part 2
 Vert Phys PCB3743 Blood Pressure Fox Chapter 14 part 2 T. Houpt, Ph.D. 1 Cardiac Output and Blood Pressure How to Measure Blood Pressure Contribution of vascular resistance to blood pressure Cardiovascular
Vert Phys PCB3743 Blood Pressure Fox Chapter 14 part 2 T. Houpt, Ph.D. 1 Cardiac Output and Blood Pressure How to Measure Blood Pressure Contribution of vascular resistance to blood pressure Cardiovascular
Blood pressure. Formation of the blood pressure: Blood pressure. Formation of the blood pressure 5/1/12
 Blood pressure Blood pressure Dr Badri Paudel www.badripaudel.com Ø Blood pressure means the force exerted by the blood against the vessel wall Ø ( or the force exerted by the blood against any unit area
Blood pressure Blood pressure Dr Badri Paudel www.badripaudel.com Ø Blood pressure means the force exerted by the blood against the vessel wall Ø ( or the force exerted by the blood against any unit area
Therefore MAP=CO x TPR = HR x SV x TPR
 Regulation of MAP Flow = pressure gradient resistance CO = MAP TPR Therefore MAP=CO x TPR = HR x SV x TPR TPR is the total peripheral resistance: this is the combined resistance of all blood vessels (remember
Regulation of MAP Flow = pressure gradient resistance CO = MAP TPR Therefore MAP=CO x TPR = HR x SV x TPR TPR is the total peripheral resistance: this is the combined resistance of all blood vessels (remember
Monday, 17 April 2017 BODY FLUID HOMEOSTASIS
 Monday, 17 April 2017 BODY FLUID HOMEOSTASIS Phenomenon: shipwrecked sailor on raft in ocean ("water, water everywhere but not a drop to drink") Why are the sailors thirsty? (What stimulated thirst?) Why
Monday, 17 April 2017 BODY FLUID HOMEOSTASIS Phenomenon: shipwrecked sailor on raft in ocean ("water, water everywhere but not a drop to drink") Why are the sailors thirsty? (What stimulated thirst?) Why
BIPN100 F15 Human Physiol I (Kristan) Lecture 14 Cardiovascular control mechanisms p. 1
 BIPN100 F15 Human Physiol I (Kristan) Lecture 14 Cardiovascular control mechanisms p. 1 Terms you should understand: hemorrhage, intrinsic and extrinsic mechanisms, anoxia, myocardial contractility, residual
BIPN100 F15 Human Physiol I (Kristan) Lecture 14 Cardiovascular control mechanisms p. 1 Terms you should understand: hemorrhage, intrinsic and extrinsic mechanisms, anoxia, myocardial contractility, residual
Autonomic Nervous System
 Autonomic Nervous System Keri Muma Bio 6 Organization of the Nervous System Efferent Division Somatic Nervous System Voluntary control Effector = skeletal muscles Muscles must be excited by a motor neuron
Autonomic Nervous System Keri Muma Bio 6 Organization of the Nervous System Efferent Division Somatic Nervous System Voluntary control Effector = skeletal muscles Muscles must be excited by a motor neuron
Veins. VENOUS RETURN = PRELOAD = End Diastolic Volume= Blood returning to heart per cardiac cycle (EDV) or per minute (Venous Return)
 Veins Venous system transports blood back to heart (VENOUS RETURN) Capillaries drain into venules Venules converge to form small veins that exit organs Smaller veins merge to form larger vessels Veins
Veins Venous system transports blood back to heart (VENOUS RETURN) Capillaries drain into venules Venules converge to form small veins that exit organs Smaller veins merge to form larger vessels Veins
Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D.
 Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D. OBJECTIVES: 1. Describe the Central Nervous System Ischemic Response. 2. Describe chemical sensitivities of arterial and cardiopulmonary chemoreceptors,
Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D. OBJECTIVES: 1. Describe the Central Nervous System Ischemic Response. 2. Describe chemical sensitivities of arterial and cardiopulmonary chemoreceptors,
Cardiovascular System B L O O D V E S S E L S 2
 Cardiovascular System B L O O D V E S S E L S 2 Blood Pressure Main factors influencing blood pressure: Cardiac output (CO) Peripheral resistance (PR) Blood volume Peripheral resistance is a major factor
Cardiovascular System B L O O D V E S S E L S 2 Blood Pressure Main factors influencing blood pressure: Cardiac output (CO) Peripheral resistance (PR) Blood volume Peripheral resistance is a major factor
Cardiovascular Physiology IV.
 Cardiovascular Physiology IV. 48. Short-term control mechanisms of arterial blood pressure. 49. Long-term control of arterial blood pressure. Ferenc Domoki, November 14 2017. Challenges/expectations Blood
Cardiovascular Physiology IV. 48. Short-term control mechanisms of arterial blood pressure. 49. Long-term control of arterial blood pressure. Ferenc Domoki, November 14 2017. Challenges/expectations Blood
Blood Pressure Regulation. Faisal I. Mohammed, MD,PhD
 Blood Pressure Regulation Faisal I. Mohammed, MD,PhD 1 Objectives Outline the short term and long term regulators of BP Know how baroreceptors and chemoreceptors work Know function of the atrial reflex.
Blood Pressure Regulation Faisal I. Mohammed, MD,PhD 1 Objectives Outline the short term and long term regulators of BP Know how baroreceptors and chemoreceptors work Know function of the atrial reflex.
Properties of Pressure
 OBJECTIVES Overview Relationship between pressure and flow Understand the differences between series and parallel circuits Cardiac output and its distribution Cardiac function Control of blood pressure
OBJECTIVES Overview Relationship between pressure and flow Understand the differences between series and parallel circuits Cardiac output and its distribution Cardiac function Control of blood pressure
Blood Pressure Regulation. Slides 9-12 Mean Arterial Pressure (MAP) = 1/3 systolic pressure + 2/3 diastolic pressure
 Sheet physiology(18) Sunday 24-November Blood Pressure Regulation Slides 9-12 Mean Arterial Pressure (MAP) = 1/3 systolic pressure + 2/3 diastolic pressure MAP= Diastolic Pressure+1/3 Pulse Pressure CO=MAP/TPR
Sheet physiology(18) Sunday 24-November Blood Pressure Regulation Slides 9-12 Mean Arterial Pressure (MAP) = 1/3 systolic pressure + 2/3 diastolic pressure MAP= Diastolic Pressure+1/3 Pulse Pressure CO=MAP/TPR
Lab Period: Name: Physiology Chapter 14 Blood Flow and Blood Pressure, Plus Fun Review Study Guide
 Lab Period: Name: Physiology Chapter 14 Blood Flow and Blood Pressure, Plus Fun Review Study Guide Main Idea: The function of the circulatory system is to maintain adequate blood flow to all tissues. Clinical
Lab Period: Name: Physiology Chapter 14 Blood Flow and Blood Pressure, Plus Fun Review Study Guide Main Idea: The function of the circulatory system is to maintain adequate blood flow to all tissues. Clinical
Cardiovascular system: Blood vessels, blood flow. Latha Rajendra Kumar, MD
 Cardiovascular system: Blood vessels, blood flow Latha Rajendra Kumar, MD Outline 1- Physical laws governing blood flow and blood pressure 2- Overview of vasculature 3- Arteries 4. Capillaries and venules
Cardiovascular system: Blood vessels, blood flow Latha Rajendra Kumar, MD Outline 1- Physical laws governing blood flow and blood pressure 2- Overview of vasculature 3- Arteries 4. Capillaries and venules
Ch 9. The Autonomic Nervous System
 Ch 9 The Autonomic Nervous System SLOs Review the organization of the ANS Describe how neural regulation of smooth and cardiac muscles differs from that of skeletal muscles Describe the structure and innervation
Ch 9 The Autonomic Nervous System SLOs Review the organization of the ANS Describe how neural regulation of smooth and cardiac muscles differs from that of skeletal muscles Describe the structure and innervation
Introduction. Acute sodium overload produces renal tubulointerstitial inflammation in normal rats
 Acute sodium overload produces renal tubulointerstitial inflammation in normal rats MI Roson, et al. Kidney International (2006) Introduction Present by Kanya Bunnan and Wiraporn paebua Tubular sodium
Acute sodium overload produces renal tubulointerstitial inflammation in normal rats MI Roson, et al. Kidney International (2006) Introduction Present by Kanya Bunnan and Wiraporn paebua Tubular sodium
REGULATION OF CARDIOVASCULAR SYSTEM
 REGULATION OF CARDIOVASCULAR SYSTEM Jonas Addae Medical Sciences, UWI REGULATION OF CARDIOVASCULAR SYSTEM Intrinsic Coupling of cardiac and vascular functions - Autoregulation of vessel diameter Extrinsic
REGULATION OF CARDIOVASCULAR SYSTEM Jonas Addae Medical Sciences, UWI REGULATION OF CARDIOVASCULAR SYSTEM Intrinsic Coupling of cardiac and vascular functions - Autoregulation of vessel diameter Extrinsic
CASE 13. What neural and humoral pathways regulate arterial pressure? What are two effects of angiotensin II?
 CASE 13 A 57-year-old man with long-standing diabetes mellitus and newly diagnosed hypertension presents to his primary care physician for follow-up. The patient has been trying to alter his dietary habits
CASE 13 A 57-year-old man with long-standing diabetes mellitus and newly diagnosed hypertension presents to his primary care physician for follow-up. The patient has been trying to alter his dietary habits
Blood Pressure Regulation -1
 CVS Physiology Lecture 18 Blood Pressure Regulation -1 Please study the previous sheet before studying this one, even if the first part in this sheet is revision. In the previous lecture we were talking
CVS Physiology Lecture 18 Blood Pressure Regulation -1 Please study the previous sheet before studying this one, even if the first part in this sheet is revision. In the previous lecture we were talking
Nervous System. Master controlling and communicating system of the body. Secrete chemicals called neurotransmitters
 Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Nervous Systems: Diversity & Functional Organization
 Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Blood Pressure Regulation 2. Faisal I. Mohammed, MD,PhD
 Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
Blood Pressure Regulation 2. Faisal I. Mohammed, MD,PhD
 Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
CNS Effects of Aldosterone :
 CNS Effects of Aldosterone : Critical Roles in salt-sensitive sensitive hypertension and CHF. Frans HH Leenen MD, PhD, FRCPC, FAHA 2 Renin Angiotensin Aldosterone System Circulatory RAAS Tissue RAAS: -
CNS Effects of Aldosterone : Critical Roles in salt-sensitive sensitive hypertension and CHF. Frans HH Leenen MD, PhD, FRCPC, FAHA 2 Renin Angiotensin Aldosterone System Circulatory RAAS Tissue RAAS: -
SUPPLEMENTAL DATA. Lumen area ( m 2 )
 Elastin Lumen area ( m 2 ) Media to lumen ratio (x1) H.E. Medium thickness ( m) Medium area ( m 2 ) SUPPLEMENTAL DATA A (Bmal1 flox/flox ) (SM-Bmal1 -/- ) B 1 8 8 6 6 4 4 2 2 1µm 5 8 4 6 3 2 4 1 2 Supplemental
Elastin Lumen area ( m 2 ) Media to lumen ratio (x1) H.E. Medium thickness ( m) Medium area ( m 2 ) SUPPLEMENTAL DATA A (Bmal1 flox/flox ) (SM-Bmal1 -/- ) B 1 8 8 6 6 4 4 2 2 1µm 5 8 4 6 3 2 4 1 2 Supplemental
Brain-derived neurotrophic factor modulates angiotensin signaling in the hypothalamus to increase blood pressure in rats
 Am J Physiol Heart Circ Physiol 38: H612 H622, 215. First published January 9, 215; doi:1.1152/ajpheart.776.214. Brain-derived neurotrophic factor modulates angiotensin signaling in the hypothalamus to
Am J Physiol Heart Circ Physiol 38: H612 H622, 215. First published January 9, 215; doi:1.1152/ajpheart.776.214. Brain-derived neurotrophic factor modulates angiotensin signaling in the hypothalamus to
T. Laitinen Departments of Physiology and Clinical Physiology, University of Kuopio and Kuopio University Hospital, Kuopio, Finland
 AUTONOMOUS NEURAL REGULATION T. Laitinen Departments of Physiology and Clinical Physiology, University of Kuopio and Kuopio University Hospital, Kuopio, Finland Keywords: Autonomic nervous system, sympathetic
AUTONOMOUS NEURAL REGULATION T. Laitinen Departments of Physiology and Clinical Physiology, University of Kuopio and Kuopio University Hospital, Kuopio, Finland Keywords: Autonomic nervous system, sympathetic
Autonomic Nervous System
 Autonomic Nervous System Autonomic nervous system organization Sympathetic Nervous System division of the autonomic nervous system that arouses the body, mobilizing its energy in stressful situations
Autonomic Nervous System Autonomic nervous system organization Sympathetic Nervous System division of the autonomic nervous system that arouses the body, mobilizing its energy in stressful situations
NROSCI/BIOSC 1070 and MSNBIO 2070 September 25, 2017 Cardiovascular 5 Control of Vascular Resistance
 NROSCI/BIOSC 1070 and MSNBIO 2070 September 25, 2017 Cardiovascular 5 Control of Vascular Resistance Baroreceptors and Baroreceptor Reflexes In order to maintain stable blood pressure, it is necessary
NROSCI/BIOSC 1070 and MSNBIO 2070 September 25, 2017 Cardiovascular 5 Control of Vascular Resistance Baroreceptors and Baroreceptor Reflexes In order to maintain stable blood pressure, it is necessary
Autonomic Nervous System Dr. Ali Ebneshahidi
 Autonomic Nervous System Dr. Ali Ebneshahidi Nervous System Divisions of the nervous system The human nervous system consists of the central nervous System (CNS) and the Peripheral Nervous System (PNS).
Autonomic Nervous System Dr. Ali Ebneshahidi Nervous System Divisions of the nervous system The human nervous system consists of the central nervous System (CNS) and the Peripheral Nervous System (PNS).
Autonomic Nervous System
 Autonomic Nervous System 6 th March, 2015 & 19 th March, 2015 Touqeer Ahmed Ph.D. Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Autonomic Nervous System Role
Autonomic Nervous System 6 th March, 2015 & 19 th March, 2015 Touqeer Ahmed Ph.D. Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Autonomic Nervous System Role
Autonomic Nervous System
 Autonomic Nervous System Touqeer Ahmed PhD 3 rd March, 2017 Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Nervous System Divisions The peripheral nervous system
Autonomic Nervous System Touqeer Ahmed PhD 3 rd March, 2017 Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Nervous System Divisions The peripheral nervous system
Systems Neuroscience November 21, 2017 The autonomic nervous system
 Systems Neuroscience November 21, 2017 The autonomic nervous system Daniel C. Kiper kiper@ini.phys.ethz.ch http: www.ini.unizh.ch/~kiper/system_neurosci.html How is the organization of the autonomic nervous
Systems Neuroscience November 21, 2017 The autonomic nervous system Daniel C. Kiper kiper@ini.phys.ethz.ch http: www.ini.unizh.ch/~kiper/system_neurosci.html How is the organization of the autonomic nervous
Cardiac Output 1 Fox Chapter 14 part 1
 Vert Phys PCB3743 Cardiac Output 1 Fox Chapter 14 part 1 T. Houpt, Ph.D. Regulation of Heart & Blood Pressure Keep Blood Pressure constant if too low, not enough blood (oxygen, glucose) reaches tissues
Vert Phys PCB3743 Cardiac Output 1 Fox Chapter 14 part 1 T. Houpt, Ph.D. Regulation of Heart & Blood Pressure Keep Blood Pressure constant if too low, not enough blood (oxygen, glucose) reaches tissues
The Autonomic Nervous System Outline of class lecture for Physiology
 The Autonomic Nervous System Outline of class lecture for Physiology 1 After studying the endocrine system you should be able to: 1. Describe the organization of the nervous system. 2. Compare and contrast
The Autonomic Nervous System Outline of class lecture for Physiology 1 After studying the endocrine system you should be able to: 1. Describe the organization of the nervous system. 2. Compare and contrast
CHAPTER 15 LECTURE OUTLINE
 CHAPTER 15 LECTURE OUTLINE I. INTRODUCTION A. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. B. Operation of the ANS to maintain homeostasis,
CHAPTER 15 LECTURE OUTLINE I. INTRODUCTION A. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. B. Operation of the ANS to maintain homeostasis,
Neuropsychiatry Block
 Neuropsychiatry Block Physiology of the Autonomic Nervous System By Laiche Djouhri, PhD Dept. of Physiology Email: ldjouhri@ksu.edu.sa Ext:71044 References The Autonomic Nervous System and the Adrenal
Neuropsychiatry Block Physiology of the Autonomic Nervous System By Laiche Djouhri, PhD Dept. of Physiology Email: ldjouhri@ksu.edu.sa Ext:71044 References The Autonomic Nervous System and the Adrenal
1. Antihypertensive agents 2. Vasodilators & treatment of angina 3. Drugs used in heart failure 4. Drugs used in arrhythmias
 1. Antihypertensive agents 2. Vasodilators & treatment of angina 3. Drugs used in heart failure 4. Drugs used in arrhythmias Only need to know drugs discussed in class At the end of this section you should
1. Antihypertensive agents 2. Vasodilators & treatment of angina 3. Drugs used in heart failure 4. Drugs used in arrhythmias Only need to know drugs discussed in class At the end of this section you should
Structure and organization of blood vessels
 The cardiovascular system Structure of the heart The cardiac cycle Structure and organization of blood vessels What is the cardiovascular system? The heart is a double pump heart arteries arterioles veins
The cardiovascular system Structure of the heart The cardiac cycle Structure and organization of blood vessels What is the cardiovascular system? The heart is a double pump heart arteries arterioles veins
Circulation. Blood Pressure and Antihypertensive Medications. Venous Return. Arterial flow. Regulation of Cardiac Output.
 Circulation Blood Pressure and Antihypertensive Medications Two systems Pulmonary (low pressure) Systemic (high pressure) Aorta 120 mmhg Large arteries 110 mmhg Arterioles 40 mmhg Arteriolar capillaries
Circulation Blood Pressure and Antihypertensive Medications Two systems Pulmonary (low pressure) Systemic (high pressure) Aorta 120 mmhg Large arteries 110 mmhg Arterioles 40 mmhg Arteriolar capillaries
Chapter 16. APR Enhanced Lecture Slides
 Chapter 16 APR Enhanced Lecture Slides See separate PowerPoint slides for all figures and tables pre-inserted into PowerPoint without notes and animations. Copyright The McGraw-Hill Companies, Inc. Permission
Chapter 16 APR Enhanced Lecture Slides See separate PowerPoint slides for all figures and tables pre-inserted into PowerPoint without notes and animations. Copyright The McGraw-Hill Companies, Inc. Permission
Blood Pressure Regulation Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
 Blood Pressure Regulation Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction There are two basic mechanisms for regulating
Blood Pressure Regulation Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction There are two basic mechanisms for regulating
General organization of central and peripheral components of the nervous system
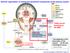 General organization of central and peripheral components of the nervous system Today we are focusing on the ANS Part of ANS?? Life depends on the innervation of the viscera... all the rest is biological
General organization of central and peripheral components of the nervous system Today we are focusing on the ANS Part of ANS?? Life depends on the innervation of the viscera... all the rest is biological
Special Lecture 11/08/2013. Hypertension Dr. HN Mayrovitz
 Special Lecture 11/08/2013 Hypertension Dr. HN Mayrovitz Arterial Blood Pressure (ABP) Major Factors Summarized Sympathetic Hormones Arteriole MAP ~ Q x TPR + f (V / C) SV x HR Renal SBP Hypertension =
Special Lecture 11/08/2013 Hypertension Dr. HN Mayrovitz Arterial Blood Pressure (ABP) Major Factors Summarized Sympathetic Hormones Arteriole MAP ~ Q x TPR + f (V / C) SV x HR Renal SBP Hypertension =
Cardiovascular Protection and the RAS
 Cardiovascular Protection and the RAS Katalin Kauser, MD, PhD, DSc Senior Associate Director, Boehringer Ingelheim Pharmaceutical Inc. Micardis Product Pipeline Scientific Support Ridgefield, CT, USA Cardiovascular
Cardiovascular Protection and the RAS Katalin Kauser, MD, PhD, DSc Senior Associate Director, Boehringer Ingelheim Pharmaceutical Inc. Micardis Product Pipeline Scientific Support Ridgefield, CT, USA Cardiovascular
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 15 The Autonomic Nervous System Comparison of Somatic and Autonomic Nervous Systems The somatic nervous system includes both sensory and motor
Principles of Anatomy and Physiology 14 th Edition CHAPTER 15 The Autonomic Nervous System Comparison of Somatic and Autonomic Nervous Systems The somatic nervous system includes both sensory and motor
number Done by Corrected by Doctor
 number 13 Done by Tamara Wahbeh Corrected by Doctor Omar Shaheen In this sheet the following concepts will be covered: 1. Divisions of the nervous system 2. Anatomy of the ANS. 3. ANS innervations. 4.
number 13 Done by Tamara Wahbeh Corrected by Doctor Omar Shaheen In this sheet the following concepts will be covered: 1. Divisions of the nervous system 2. Anatomy of the ANS. 3. ANS innervations. 4.
Presenter: Tom Mulvey
 Slides are from Level 3 Biology Course Content Day, 7 th November 2012 Presenter: Tom Mulvey Teachers are free to use these for teaching purposes with appropriate acknowledgement Blood Pressure Ways of
Slides are from Level 3 Biology Course Content Day, 7 th November 2012 Presenter: Tom Mulvey Teachers are free to use these for teaching purposes with appropriate acknowledgement Blood Pressure Ways of
Inhibition of TNF in the Brain Reverses Alterations in RAS Components and Attenuates Angiotensin II-Induced Hypertension
 Inhibition of TNF in the Brain Reverses Alterations in RAS Components and Attenuates Angiotensin II-Induced Hypertension Srinivas Sriramula 1,2, Jeffrey P. Cardinale 1, Joseph Francis 1 * 1 Comparative
Inhibition of TNF in the Brain Reverses Alterations in RAS Components and Attenuates Angiotensin II-Induced Hypertension Srinivas Sriramula 1,2, Jeffrey P. Cardinale 1, Joseph Francis 1 * 1 Comparative
Neuroimmunology. Innervation of lymphoid organs. Neurotransmitters. Neuroendocrine hormones. Cytokines. Autoimmunity
 Neuroimmunology Innervation of lymphoid organs Neurotransmitters Neuroendocrine hormones Cytokines Autoimmunity CNS has two ways of contacting and regulating structures in the periphery Autonomic
Neuroimmunology Innervation of lymphoid organs Neurotransmitters Neuroendocrine hormones Cytokines Autoimmunity CNS has two ways of contacting and regulating structures in the periphery Autonomic
Chapter 14 The Autonomic Nervous System Chapter Outline
 Chapter 14 The Autonomic Nervous System Chapter Outline Module 14.1 Overview of the Autonomic Nervous System (Figures 14.1 14.3) A. The autonomic nervous system (ANS) is the involuntary arm of the peripheral
Chapter 14 The Autonomic Nervous System Chapter Outline Module 14.1 Overview of the Autonomic Nervous System (Figures 14.1 14.3) A. The autonomic nervous system (ANS) is the involuntary arm of the peripheral
The Autonomic Nervous System
 The Autonomic Nervous System Responsible for control of visceral effectors and visceral reflexes: smooth muscle, glands, the heart. e.g. blood pressure, cardiac output, plasma glucose The autonomic system
The Autonomic Nervous System Responsible for control of visceral effectors and visceral reflexes: smooth muscle, glands, the heart. e.g. blood pressure, cardiac output, plasma glucose The autonomic system
Physiology of Circulation
 Physiology of Circulation Dr. Ali Ebneshahidi Blood vessels Arteries: Blood vessels that carry blood away from the heart to the lungs and tissues. Arterioles are small arteries that deliver blood to the
Physiology of Circulation Dr. Ali Ebneshahidi Blood vessels Arteries: Blood vessels that carry blood away from the heart to the lungs and tissues. Arterioles are small arteries that deliver blood to the
Heart. Large lymphatic vessels Lymph node. Lymphatic. system Arteriovenous anastomosis. (exchange vessels)
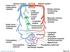 Venous system Large veins (capacitance vessels) Small veins (capacitance vessels) Postcapillary venule Thoroughfare channel Heart Large lymphatic vessels Lymph node Lymphatic system Arteriovenous anastomosis
Venous system Large veins (capacitance vessels) Small veins (capacitance vessels) Postcapillary venule Thoroughfare channel Heart Large lymphatic vessels Lymph node Lymphatic system Arteriovenous anastomosis
Physiology Unit 3 CARDIOVASCULAR PHYSIOLOGY: THE VASCULAR SYSTEM
 Physiology Unit 3 CARDIOVASCULAR PHYSIOLOGY: THE VASCULAR SYSTEM In Physiology Today Hemodynamics F = ΔP/R Blood flow (F) High to low pressure Rate = L/min Pressure (P) Hydrostatic pressure Pressure exerted
Physiology Unit 3 CARDIOVASCULAR PHYSIOLOGY: THE VASCULAR SYSTEM In Physiology Today Hemodynamics F = ΔP/R Blood flow (F) High to low pressure Rate = L/min Pressure (P) Hydrostatic pressure Pressure exerted
Impact factor: Reporter:4A1H0019 Chen Zi Hao 4A1H0023 Huang Wan ting 4A1H0039 Sue Yi Zhu 4A1H0070 Lin Guan cheng 4A1H0077 Chen Bo xuan
 Curcumin Protects Neonatal Rat Cardiomyocytes against High Glucose-Induced Apoptosis via PI3K/Akt Signalling Pathway Wei Yu,1,2 Wenliang Zha,1 Zhiqiang Ke,1 Qing Min,2 Cairong Li,1 Huirong Sun,3 and Chao
Curcumin Protects Neonatal Rat Cardiomyocytes against High Glucose-Induced Apoptosis via PI3K/Akt Signalling Pathway Wei Yu,1,2 Wenliang Zha,1 Zhiqiang Ke,1 Qing Min,2 Cairong Li,1 Huirong Sun,3 and Chao
Cardiovascular System. Blood Vessel anatomy Physiology & regulation
 Cardiovascular System Blood Vessel anatomy Physiology & regulation Path of blood flow Aorta Arteries Arterioles Capillaries Venules Veins Vena cava Vessel anatomy: 3 layers Tunica externa (adventitia):
Cardiovascular System Blood Vessel anatomy Physiology & regulation Path of blood flow Aorta Arteries Arterioles Capillaries Venules Veins Vena cava Vessel anatomy: 3 layers Tunica externa (adventitia):
Blood pressure control Contin. Reflex Mechanisms. Dr. Hiwa Shafiq
 Blood pressure control Contin. Reflex Mechanisms Dr. Hiwa Shafiq 17-12-2018 A. Baroreceptor reflexes Baroreceptors (stretch receptors) located in the walls of several large systemic arteries( specially
Blood pressure control Contin. Reflex Mechanisms Dr. Hiwa Shafiq 17-12-2018 A. Baroreceptor reflexes Baroreceptors (stretch receptors) located in the walls of several large systemic arteries( specially
Introduction to Autonomic
 Part 2 Autonomic Pharmacology 3 Introduction to Autonomic Pharmacology FUNCTIONS OF THE AUTONOMIC NERVOUS SYSTEM The autonomic nervous system (Figure 3 1) is composed of the sympathetic and parasympathetic
Part 2 Autonomic Pharmacology 3 Introduction to Autonomic Pharmacology FUNCTIONS OF THE AUTONOMIC NERVOUS SYSTEM The autonomic nervous system (Figure 3 1) is composed of the sympathetic and parasympathetic
BIOL241 - Lecture 12a
 Cranial Nerves, source: training.seer.cancer.gov Nervous System Overview BIOL241 - Lecture 12a 1 Topics Divisions of the NS: CNS and PNS Structure and types of neurons Synapses Structure and function of
Cranial Nerves, source: training.seer.cancer.gov Nervous System Overview BIOL241 - Lecture 12a 1 Topics Divisions of the NS: CNS and PNS Structure and types of neurons Synapses Structure and function of
Cigarette Smoke Exposure and HIV-Related Neurologic Disease Progression Basic Mechanisms and Clinical Consequences
 Cigarette Smoke Exposure and HIV-Related Neurologic Disease Progression Basic Mechanisms and Clinical Consequences Walter Royal, III, MD Professor of Neurology University of Maryland School of Medicine
Cigarette Smoke Exposure and HIV-Related Neurologic Disease Progression Basic Mechanisms and Clinical Consequences Walter Royal, III, MD Professor of Neurology University of Maryland School of Medicine
Adipose Tissue as an Endocrine Organ. Abdel Moniem Ibrahim, MD Professor of Physiology Cairo University
 Adipose Tissue as an Endocrine Organ Abdel Moniem Ibrahim, MD Professor of Physiology Cairo University Functions of Adipose Tissue Adipose tissue expresses and secretes a variety of bioactive peptides,
Adipose Tissue as an Endocrine Organ Abdel Moniem Ibrahim, MD Professor of Physiology Cairo University Functions of Adipose Tissue Adipose tissue expresses and secretes a variety of bioactive peptides,
Autonomic nervous system
 Autonomic nervous system Key notes Autonomic: an independent system that runs on its own The ANS is a visceral and involuntary sensory and motor system The visceral motor fibers in the autonomic nerves
Autonomic nervous system Key notes Autonomic: an independent system that runs on its own The ANS is a visceral and involuntary sensory and motor system The visceral motor fibers in the autonomic nerves
Cardiovascular regulation. Neural and hormonal Regulation Dr Badri Paudel GMC. 1-Local factors (autoregulation) Control of Blood Flow
 Neural and hormonal Regulation Dr Badri Paudel GMC 1 Cardiovascular regulation Fa c t o r s i nvo l v e d i n t h e regulation of cardiovascular function include: 1- Autoregulation 2- Neural regulation
Neural and hormonal Regulation Dr Badri Paudel GMC 1 Cardiovascular regulation Fa c t o r s i nvo l v e d i n t h e regulation of cardiovascular function include: 1- Autoregulation 2- Neural regulation
Autonomic Division of NS
 Autonomic Division of NS Compare and contrast the structures of the sympathetic and the parasympathetic divisions, including functions and neurotransmitters. Show the levels of integration in the ANS,
Autonomic Division of NS Compare and contrast the structures of the sympathetic and the parasympathetic divisions, including functions and neurotransmitters. Show the levels of integration in the ANS,
3/10/2009 VESSELS PHYSIOLOGY D.HAMMOUDI.MD. Palpated Pulse. Figure 19.11
 VESSELS PHYSIOLOGY D.HAMMOUDI.MD Palpated Pulse Figure 19.11 1 shows the common sites where the pulse is felt. 1. Temporal artery at the temple above and to the outer side of the eye 2. External maxillary
VESSELS PHYSIOLOGY D.HAMMOUDI.MD Palpated Pulse Figure 19.11 1 shows the common sites where the pulse is felt. 1. Temporal artery at the temple above and to the outer side of the eye 2. External maxillary
Otto Loewi (88) German pharmacologist. Page 1. Sir Henry Hallett Dale
 Regulation of Cardiovascular functions Goals: Maintain normal blood pressure (heart pump + vascular resistance + blood volume) Adaptation of blood pressure to special circumstances (redistribution) Maintains
Regulation of Cardiovascular functions Goals: Maintain normal blood pressure (heart pump + vascular resistance + blood volume) Adaptation of blood pressure to special circumstances (redistribution) Maintains
Human Anatomy. Autonomic Nervous System
 Human Anatomy Autonomic Nervous System 1 Autonomic Nervous System ANS complex system of nerves controls involuntary actions. Works with the somatic nervous system (SNS) regulates body organs maintains
Human Anatomy Autonomic Nervous System 1 Autonomic Nervous System ANS complex system of nerves controls involuntary actions. Works with the somatic nervous system (SNS) regulates body organs maintains
Blood Pressure. a change in any of these could cause a corresponding change in blood pressure
 Blood Pressure measured as mmhg Main factors affecting blood pressure: 1. cardiac output 2. peripheral resistance 3. blood volume a change in any of these could cause a corresponding change in blood pressure
Blood Pressure measured as mmhg Main factors affecting blood pressure: 1. cardiac output 2. peripheral resistance 3. blood volume a change in any of these could cause a corresponding change in blood pressure
The Nervous System: Neural Tissue Pearson Education, Inc.
 13 The Nervous System: Neural Tissue Introduction Nervous System Characteristics Controls and adjust the activity of the body Provides swift but brief responses The nervous system includes: Central Nervous
13 The Nervous System: Neural Tissue Introduction Nervous System Characteristics Controls and adjust the activity of the body Provides swift but brief responses The nervous system includes: Central Nervous
Chapters 9 & 10. Cardiorespiratory System. Cardiovascular Adjustments to Exercise. Cardiovascular Adjustments to Exercise. Nervous System Components
 Cardiorespiratory System Chapters 9 & 10 Cardiorespiratory Control Pulmonary ventilation Gas exchange Left heart Arterial system Tissues Right heart Lungs Pulmonary ventilation Cardiovascular Regulation-
Cardiorespiratory System Chapters 9 & 10 Cardiorespiratory Control Pulmonary ventilation Gas exchange Left heart Arterial system Tissues Right heart Lungs Pulmonary ventilation Cardiovascular Regulation-
Composed by Natalia Leonidovna Svintsitskaya, Associate professor of the Chair of Human Anatomy, Candidate of Medicine
 Theoretical background to the study of the autonomic nervous system. Sympathetic and parasympathetic divisions of the autonomic nervous system. Features of the structure, function Composed by Natalia Leonidovna
Theoretical background to the study of the autonomic nervous system. Sympathetic and parasympathetic divisions of the autonomic nervous system. Features of the structure, function Composed by Natalia Leonidovna
In the name of GOD. Animal models of cardiovascular diseases: myocardial infarction & hypertension
 In the name of GOD Animal models of cardiovascular diseases: myocardial infarction & hypertension 44 Presentation outline: Cardiovascular diseases Acute myocardial infarction Animal models for myocardial
In the name of GOD Animal models of cardiovascular diseases: myocardial infarction & hypertension 44 Presentation outline: Cardiovascular diseases Acute myocardial infarction Animal models for myocardial
Integrated Cardiopulmonary Pharmacology Third Edition
 Integrated Cardiopulmonary Pharmacology Third Edition Chapter 3 Pharmacology of the Autonomic Nervous System Multimedia Directory Slide 19 Slide 37 Slide 38 Slide 39 Slide 40 Slide 41 Slide 42 Slide 43
Integrated Cardiopulmonary Pharmacology Third Edition Chapter 3 Pharmacology of the Autonomic Nervous System Multimedia Directory Slide 19 Slide 37 Slide 38 Slide 39 Slide 40 Slide 41 Slide 42 Slide 43
Autonomic nervous system
 15 Autonomic nervous system The role of nervous system ANTICIPATION Cortex Potential input Potential output Cortex Integration Sensor Input Output Effector REGULATION Feedback regulation http://www.slideshare.net/drpsdeb/presentations
15 Autonomic nervous system The role of nervous system ANTICIPATION Cortex Potential input Potential output Cortex Integration Sensor Input Output Effector REGULATION Feedback regulation http://www.slideshare.net/drpsdeb/presentations
Hypothalamic TLR2 triggers sickness behavior via a microglia-neuronal axis
 Hypothalamic TLR triggers sickness behavior via a microglia-neuronal axis Sungho Jin, *, Jae Geun Kim,, *, Jeong Woo Park, Marco Koch,, Tamas L. Horvath and Byung Ju Lee Department of Biological Sciences,
Hypothalamic TLR triggers sickness behavior via a microglia-neuronal axis Sungho Jin, *, Jae Geun Kim,, *, Jeong Woo Park, Marco Koch,, Tamas L. Horvath and Byung Ju Lee Department of Biological Sciences,
Biology 218 Human Anatomy
 Chapter 20 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Introduction (p. 632) 1. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. 2.
Chapter 20 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Introduction (p. 632) 1. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. 2.
Outline Urinary System. Urinary System and Excretion. Urine. Urinary System. I. Function II. Organs of the urinary system
 Outline Urinary System Urinary System and Excretion Bio105 Chapter 16 Renal will be on the Final only. I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of
Outline Urinary System Urinary System and Excretion Bio105 Chapter 16 Renal will be on the Final only. I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of
B-cell. Astrocyte SCI SCI. T-cell
 RF #2015 P-01 PI: Azizul Haque, PhD Grant Title: Targeting Enolase in Spinal Cord Injury 12-month Technical Progress Report Progress Report (First Six Months): Enolase is one of the most abundantly expressed
RF #2015 P-01 PI: Azizul Haque, PhD Grant Title: Targeting Enolase in Spinal Cord Injury 12-month Technical Progress Report Progress Report (First Six Months): Enolase is one of the most abundantly expressed
Chapter 14 Blood Vessels, Blood Flow and Pressure Exam Study Questions
 Chapter 14 Blood Vessels, Blood Flow and Pressure Exam Study Questions 14.1 Physical Law Governing Blood Flow and Blood Pressure 1. How do you calculate flow rate? 2. What is the driving force of blood
Chapter 14 Blood Vessels, Blood Flow and Pressure Exam Study Questions 14.1 Physical Law Governing Blood Flow and Blood Pressure 1. How do you calculate flow rate? 2. What is the driving force of blood
Lujain Hamdan. Ayman Musleh & Yahya Salem. Mohammed khatatbeh
 12 Lujain Hamdan Ayman Musleh & Yahya Salem Mohammed khatatbeh the last lecture, we have studied the differences between the two divisions of the ANS: sympathetic and parasympathetic pathways which work
12 Lujain Hamdan Ayman Musleh & Yahya Salem Mohammed khatatbeh the last lecture, we have studied the differences between the two divisions of the ANS: sympathetic and parasympathetic pathways which work
2.4 Autonomic Nervous System
 2.4 Autonomic Nervous System The ANS regulates visceral activities normally outside the realm of consciousness and voluntary control: Circulation. Digestion. Sweating. Pupillary size. The ANS consists
2.4 Autonomic Nervous System The ANS regulates visceral activities normally outside the realm of consciousness and voluntary control: Circulation. Digestion. Sweating. Pupillary size. The ANS consists
Nox-Dependent Mechanisms of Cardiomyocyte Dysfunction in a Model of Pressure Overload
 Nox-Dependent Mechanisms of Cardiomyocyte Dysfunction in a Model of Pressure Overload Giovanna Frazziano, PhD Vascular Medicine Institute Department of Pharmacology and Chemical Biology University of Pittsburgh
Nox-Dependent Mechanisms of Cardiomyocyte Dysfunction in a Model of Pressure Overload Giovanna Frazziano, PhD Vascular Medicine Institute Department of Pharmacology and Chemical Biology University of Pittsburgh
Autonomic Nervous System. Introduction
 Autonomic Nervous System Introduction 1 The nervous system is divided into: 1- the central nervous system (CNS; the brain and spinal cord) 2- the peripheral nervous system (PNS; neuronal tissues outside
Autonomic Nervous System Introduction 1 The nervous system is divided into: 1- the central nervous system (CNS; the brain and spinal cord) 2- the peripheral nervous system (PNS; neuronal tissues outside
Analysis of AVP functions via V1a and V1b receptors with knockout mice. Akito Tanoue
 Analysis of AVP functions via V1a and V1b receptors with knockout mice Akito Tanoue Department of Pharmacology, National Research Institute for Child Health and Development Arginine-Vasopressin (AVP) is
Analysis of AVP functions via V1a and V1b receptors with knockout mice Akito Tanoue Department of Pharmacology, National Research Institute for Child Health and Development Arginine-Vasopressin (AVP) is
BIOH111. o Cell Module o Tissue Module o Skeletal system o Muscle system o Nervous system o Endocrine system o Integumentary system
 BIOH111 o Cell Module o Tissue Module o Skeletal system o Muscle system o Nervous system o Endocrine system o Integumentary system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
BIOH111 o Cell Module o Tissue Module o Skeletal system o Muscle system o Nervous system o Endocrine system o Integumentary system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
Physiology of Circulation. Dr. Hiwa Shafiq 16/12/2018
 Physiology of Circulation Dr. Hiwa Shafiq 16/12/2018 Overview of the circulation The function of the circulation is to: 1. transport nutrients to the body tissues 2. transport waste products away 3. conduct
Physiology of Circulation Dr. Hiwa Shafiq 16/12/2018 Overview of the circulation The function of the circulation is to: 1. transport nutrients to the body tissues 2. transport waste products away 3. conduct
FLASH CARDS. Kalat s Book Chapter 10 Alphabetical
 FLASH CARDS www.biologicalpsych.com Kalat s Book Chapter 10 Alphabetical AgRP AgRP Agouti-related peptide; synthesized in hypothalamus. Acts as an appetite stimulator. Also decreases metabolism. aldosterone
FLASH CARDS www.biologicalpsych.com Kalat s Book Chapter 10 Alphabetical AgRP AgRP Agouti-related peptide; synthesized in hypothalamus. Acts as an appetite stimulator. Also decreases metabolism. aldosterone
InteractionofInflammatoryResponseandSympatheticNervousSystemintheCentralRegulationofChronicHeartFailureinRats
 : I Surgeries and Cardiovascular System Volume 16 Issue 4 Version 1.0 Year 2016 Type: Double Blind Peer Reviewed International Research Journal Publisher: Global Journals Inc. (USA) Online ISSN: 2249-4618
: I Surgeries and Cardiovascular System Volume 16 Issue 4 Version 1.0 Year 2016 Type: Double Blind Peer Reviewed International Research Journal Publisher: Global Journals Inc. (USA) Online ISSN: 2249-4618
MOLECULAR AND CELLULAR NEUROSCIENCE
 MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
Dania Ahmad. Tamer Barakat + Dania Ahmad. Faisal I. Mohammed
 16 Dania Ahmad Tamer Barakat + Dania Ahmad Faisal I. Mohammed Revision: What are the basic types of neurons? sensory (afferent), motor (efferent) and interneuron (equaled association neurons). We classified
16 Dania Ahmad Tamer Barakat + Dania Ahmad Faisal I. Mohammed Revision: What are the basic types of neurons? sensory (afferent), motor (efferent) and interneuron (equaled association neurons). We classified
Biology 218 Human Anatomy
 Chapter 17 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Overview of the Nervous System (p. 537) 1. The nervous system and the endocrine system are the body s major control and integrating centers.
Chapter 17 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Overview of the Nervous System (p. 537) 1. The nervous system and the endocrine system are the body s major control and integrating centers.
The Autonomic Nervous
 Autonomic Nervous System The Autonomic Nervous Assess Prof. Fawzia Al-Rouq System Department of Physiology College of Medicine King Saud University LECTUR (1) Functional Anatomy & Physiology of Autonomic
Autonomic Nervous System The Autonomic Nervous Assess Prof. Fawzia Al-Rouq System Department of Physiology College of Medicine King Saud University LECTUR (1) Functional Anatomy & Physiology of Autonomic
Autonomic regulation of islet hormone secretion
 Autonomic regulation of islet hormone secretion Implications for health and disease Billy & Bree Paper 1: Autonomic regulation of islet hormone secretion : Implications for health and disease By Team BBB
Autonomic regulation of islet hormone secretion Implications for health and disease Billy & Bree Paper 1: Autonomic regulation of islet hormone secretion : Implications for health and disease By Team BBB
Physiology lecture 15 Hemodynamic
 Physiology lecture 15 Hemodynamic Dispensability (D) : proportional change in volume per unit change in pressure D = V/ P*V It is proportional (divided by the original volume). Compliance (C) : total change
Physiology lecture 15 Hemodynamic Dispensability (D) : proportional change in volume per unit change in pressure D = V/ P*V It is proportional (divided by the original volume). Compliance (C) : total change
Autonomic Nervous System. Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry
 Autonomic Nervous System Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Peripheral Nervous System A. Sensory Somatic Nervous System B. Autonomic Nervous System 1. Sympathetic Nervous
Autonomic Nervous System Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Peripheral Nervous System A. Sensory Somatic Nervous System B. Autonomic Nervous System 1. Sympathetic Nervous
Chapter 9. Nervous System
 Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Supplementary Fig. 1. Identification of acetylation of K68 of SOD2
 Supplementary Fig. 1. Identification of acetylation of K68 of SOD2 A B H. sapiens 54 KHHAAYVNNLNVTEEKYQEALAK 75 M. musculus 54 KHHAAYVNNLNATEEKYHEALAK 75 X. laevis 55 KHHATYVNNLNITEEKYAEALAK 77 D. rerio
Supplementary Fig. 1. Identification of acetylation of K68 of SOD2 A B H. sapiens 54 KHHAAYVNNLNVTEEKYQEALAK 75 M. musculus 54 KHHAAYVNNLNATEEKYHEALAK 75 X. laevis 55 KHHATYVNNLNITEEKYAEALAK 77 D. rerio
(D) (E) (F) 6. The extrasystolic beat would produce (A) increased pulse pressure because contractility. is increased. increased
 Review Test 1. A 53-year-old woman is found, by arteriography, to have 5% narrowing of her left renal artery. What is the expected change in blood flow through the stenotic artery? Decrease to 1 2 Decrease
Review Test 1. A 53-year-old woman is found, by arteriography, to have 5% narrowing of her left renal artery. What is the expected change in blood flow through the stenotic artery? Decrease to 1 2 Decrease
