ScienceDirect. Study of degradation products at different MEA based capture pilot plants
|
|
|
- Arron Horton
- 5 years ago
- Views:
Transcription
1 Available online at ScienceDirect Energy Procedia 86 (2016 ) The 8th Trondheim Conference on CO 2 Capture, Transport and Storage Study of degradation products at different MEA based capture pilot plants D. Dux a, B. Schallert a * a E.ON Technologies GmbH, D Gelsenkirchen, Germany Abstract In the past, several research programs were undertaken at European Carbon Capture (CC) pilot plants. The results of these individual MEA based campaigns are brought together in order to better understand the solvent degradation at CC pilot plants. The data correlation analysis shows that nickel catalyzes significantly the formation of some degradation products (e.g. HEPO) at low concentrations of 2 mg/l. The iron impact was less clear than determined for nickel. All pilot plants show HEGly and HEPO as the main degradation products, followed by HEF. Within the group of carboxylic acids, formate is the major degradation product The The Authors. Authors. Published Published by Elsevier by Elsevier Ltd. This Ltd. is an open access article under the CC BY-NC-ND license ( Peer-review under responsibility of the Programme Chair of The 8th Trondheim Conference on CO2 Capture, Transport and Peer-review Storage. under responsibility of the Programme Chair of the 8th Trondheim Conference on CO 2 Capture, Transport and Storage Keywords: CO 2 capture; CC pilot plants; MEA; degradation products; HEGly-HEHEAA-HEPO system; metal catalysis 1. Introduction For post-combustion carbon capture, flue gas scrubbing with amine solvents is the most common option. 2- ethanolamine (MEA) has been widely used for long time for gas sweetening in refineries, and also for postcombustion capture from gas-fired plants. Due to the different composition of the flue gas from coal fired plants compared to e.g. gas turbines, the degradation occurring in the amine solvents has been found to be more complex. Much research has been undertaken on different amine solvents to find an efficient, degradation-resistant solvent with a low energy penalty. Despite all the different amines analysed, MEA remains the benchmark solvent for * Corresponding author. Tel.: ; fax: address: dinah.dux@eon.com The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license ( Peer-review under responsibility of the Programme Chair of the 8th Trondheim Conference on CO 2 Capture, Transport and Storage doi: /j.egypro
2 D. Dux and B. Schallert / Energy Procedia 86 ( 2016 ) carbon capture plants since it has been widely researched (Da Silva et al [1]). The EU funded many research projects in the past and also currently, to investigate the behaviour of MEA solvents under simulated (lab) conditions and in pilot plants. E.ON actively supported and co-funded these research projects. Additionally, EON has been involved in other industry funded carbon capture projects where MEA has been the benchmark solvent. During these projects, the degradation of the MEA solvent has been analysed at different intensities. Besides the degradation itself, corrosion was another aspect of interest in many campaigns as elevated Fe, Ni and Cr levels were observed in the amine solvent. The aim of this study is to compare the different pilot plant campaigns in order to identify any correlations within the solvent chemistry. This comparison is done anonymously as it is not our intention to make a comparison in respect to material selection or process technology of any individual plant. The focus lies rather on understanding, how the degradation compounds interact and how the degradation process could be interfered. Therefore, references [2] to [6] give an overview of the different research programs, from which data was taken for this study. Solvent reclaiming was done during some of these pilot plant campaigns, but usually towards the end of a campaign. Such data was not considered for evaluation, despite availability, as the solvent composition is changed by reclaiming. 2. Methods The chemical analyses of the different solvent samples were done within the individual CC pilot plant campaigns. The determination of the primary degradation products (mainly carboxylic acids) is typically done by Ion Chromatography (IC); laboratories proficient in this kind of technique and matrix were used throughout the different programs. All secondary degradation products (e.g. degradation products formed from other MEA-based degradation products) were determined by SINTEF, also within the individual research programs. SINTEF uses a combination of different analytical techniques, including GC-MS (Gas Chromatography Mass Spectrometry), LC- MS (Liquid Chromatography Mass Spectrometry) and IC-EX (Ion Chromatography with electrochemical detection) as described by Da Silva et al [1]. 3. Results 3.1. Solvent degradation Having a closer look to the degradation products of MEA, they can be divided into two groups: primary and secondary degradation products (Vevelstad et al [7]). Goff [8] described the initial oxidation of MEA to be a radical reaction, allowing for the development of different radical species, which further reacts with MEA or other degradation products. Figure 1 shows the typical primary degradation products observed in degraded MEA solvents. Comparing several pilot plant campaigns undertaken in the past years to optimize carbon capture at coal fired power plants it can be seen that formate is the dominant, primary oxidation product as shown in Figure 2. NH 3 Formic acid Glycolic acid MEA + O 2 Formaldehyde Acetaldehyde Acetic acid Oxalic acid Figure 1: Oxidative degradation products as described by da Silva et al [1]
3 264 D. Dux and B. Schallert / Energy Procedia 86 ( 2016 ) Figure 2: Primary degradation products (1 st DPs) for the different pilot plants compared in this study These carbonic acids are involved in further reactions with MEA forming the corresponding amide compound by dehydration (Figure 3). Other degradation products most likely will occur by further reactions between the different molecules present in solution. The formation of (2-Hydroxyethyl)-glycine (HEGly), for example, was described by Vevelstad [9] to be a two-step reaction between MEA, glyoxylic acid and formic acid (Figure 4). + - H 2 O R = H HEF R = CH 3 HEA R = CH 2 OH HHEA Figure 3: Reaction scheme between MEA and organic acids as described amongst others by da Silva et al [1] MEA + formic acid - H 2 O - CO 2 HEGly Figure 4: Formation mechanism of HEGly as suggested by Vevelstad [9] 3.2. HEF and formate The simplest reaction product is (2-Hydroxyethyl)formamide (HEF); formed by the reaction of formic acid with MEA. Lab studies found HEF to be one of the major degradation products formed during MEA degradation [1;7]. Even though it was already determined to be not the major degradation product at pilot plants (da Silva et al [1]), it was found in significant concentrations across all pilot plants compared in this study as shown in Figure 5.
4 D. Dux and B. Schallert / Energy Procedia 86 ( 2016 ) Figure 5: Secondary degradation products (2 nd DPs) as determined for the different pilot plant campaigns compared in this study While Vevelstad et al [10] reported that the formation of HEF is probably metal catalyzed; the pilot plant data did show varying dependencies on the typical dissolved heavy metals ions found in the solvent, e.g. iron, chromium and nickel. However, the reaction between MEA and formate to HEF is a simple condensation reaction. Figure 6 shows the correlation between HEF and formate concentration. All plants show a very similar correlation, accordingly, the amount of HEF present in the pilot plant campaigns depends mainly on the concentration of formate in the solvent, which varies for the different campaigns (Figure 7). The initial MEA oxidation rate of each plant is influenced by factors like oxygen availability in the solvent and radical formation; the latter one of course can be influenced by dissolved metals. Figure 8 shows the correlation of formate concentration with iron, chromium and nickel, respectively; of course, all three metals were present in the solvent at the same time. Some linear correlation with iron at low concentrations up to 1 mmol/l can be seen, but the magnitude of correlation differs between the plants. Chromium and nickel show only a strong correlation for one campaign at levels below 0.2 mmol/l; the effect in the
5 266 D. Dux and B. Schallert / Energy Procedia 86 ( 2016 ) other campaigns appears to be lower. The available data did not allow for quantitative evaluation of plant parameters like oxygen saturation, but a tendency towards higher formate formation in plants with higher oxygen concentrations in the flue gas, lower flue gas temperature and additional higher metal concentration in the solvent could be seen. Figure 6: Correlation of HEF with formate for the studied campaigns Figure 7: Formate formation for the studied CC pilot campaigns Figure 8: Formation of formate in correlation to iron, chromium and nickel in solution for the studied CC pilot plant campaigns The absolute equivalents of HEF and formate determined in the solvents were in a similar magnitude of about (HEF) to 1.0 (formate). Acetate is another simple carbonic acid, which follows a similar reaction scheme like formate by forming (2-Hydroxyethyl)acetamide (HEA). But in contrast to the HEF-formate system, the ratio between HEA to acetate is often stronger on the HEA side. In general, the analytical determination of acetate is more challenging compared to the other carbonic acids as the acetate signal is difficult to separate from glycolate within the commonly used ion chromatographic columns. Hence, the pilot plant data should be evaluated carefully. The correlation between HEA and acetate was as clear as found for the HEF-formate correlation for the studied pilot plants. Besides analytical uncertainty, this could also be due to competing reaction paths HEGly, HEHEAA and HEPO As widely known in the CCS community, N-(2-Hydroxyethlyl)glycine (HEGly) and N-(2- hydroxyethyl)piperazin-2-one (HEPO) are the major degradation products in MEA based CC pilot campaigns [1;7]. Da Silva et al suspected that the difference in HEGly to HEPO ratios of the different pilot plants is caused by differences in stripper operation [1]. In the following, the data of up to seven different pilot campaigns will be assessed to better understand the dependencies. The reaction from HEGly to HEPO was suggested by da Silva et al [1] and Strazisar [11] to be a two-step reaction, involving N-(2-Hydroxyethyl)-2-[(2-hydroxyethyl)amino]acetamide (HEHEAA) as interim product (Figure 9).
6 D. Dux and B. Schallert / Energy Procedia 86 ( 2016 ) H + 2 O - H 2 O MEA HEGly HEHEAA Figure 9: Mechanism of HEPO formation [1;11] HEPO The first lab studies did not show HEGly and HEPO as important degradation products, only after adding metals and / or fly ash to the reaction vessels, formation of these compounds was observed [1;9]. Accordingly, the impact of typical metals present in pilot plant solvent was analyzed during the present study. Iron values were available for six cases, while chromium and nickel were only above detection limit for five of these six cases. Plotting HEGly, HEHEAA and HEPO against these metals was done for the available data in order to identify any correlations. However, one has to keep in mind that all metals are present within the solvents and that overlapping effects might occur. Figure 10: Correlation of HEGly, HEHEAA and HEPO with iron for the different pilot plants in this study Figure 11: Correlation of HEGly, HEHEAA and HEPO with chromium for the different pilot plants in this study Figure 12: Correlation of HEGly, HEHEAA and HEPO with nickel for the different pilot plants in this study
7 268 D. Dux and B. Schallert / Energy Procedia 86 ( 2016 ) A clear correlation between HEGly and any of the three metals could not be identified as the different trends drift apart quickly and other factors might be more important. HEHEAA shows some correlation with iron and nickel at very low concentrations. In case of iron, the campaigns show a correlation over a wider concentration range (up to 1 mmol/l) compared to nickel (up to 0.05 mmol/l), but the differences between the campaigns are stronger for iron than for nickel. However, less nickel data was available. The correlation with chromium suggests a stronger impact by other factors. A strong correlation was identified for HEPO formation. A strong linear increase in HEPO concentration was identified for a nickel concentration of up to 0.06 mmol/l. The data of five different pilot test campaigns align very well in this concentration range, indicating a dominant role of nickel in the formation of HEPO. The correlation to iron or chromium was less clear. Since the three metals appear in certain ratios, interferences between the individual correlations are possible, e.g. a slight positive correlation with the iron data can be caused by the good correlation with nickel, as nickel was present in the solvent as well. Sexton [12] used a mixture of chromium and nickel in his oxidative degradation experiments on MEA and found that the Cr/Ni mixture catalyzes the MEA oxidation to a greater extent than iron. However, at that time, degraded solvent samples were not analyzed for HEGly or HEPO, and about 90% of the raw peak area remained unidentified. It is clearly recommended to investigate further, if nickel solely or in combination with other metals catalyzes the degradation reaction. After a positive influence of nickel on the HEPO formation was identified, the correlation between HEGly, HEHEAA and HEPO was studied to better understand their dependencies within the reaction equilibrium; formation of HEPO in dependence on HEGly and HEHEAA, respectively is shown in Figure 13. Figure 13: HEPO concentration against HEGly and HEHEAA concentration for the different pilot plant campaigns in this study The correlation of HEPO against HEGly shows significant differences between the pilot plant campaigns. For one, the amount of HEPO formed per HEGly is not the same for the different campaigns. Additionally, it appears that there is a campaign specific limit on how much HEPO will be formed. The second step of the reaction appears to be a quick reaction as the HEPO values correlate well with the HEHEAA values of seven pilot plant campaigns. The concentration of HEPO is 7-10 times higher than the HEHEAA concentration, pointing to a strong equilibrium shift towards HEPO. Having this in mind, the first reaction step (HEGly to HEHEAA, Figure 9) is probably better characterized by plotting the sum of HEPO and HEHEAA against the HEGly concentration (Figure 14).
8 D. Dux and B. Schallert / Energy Procedia 86 ( 2016 ) Figure 14: Sum (HEPO and HEHEAA) concentration against HEGly concentration for the different pilot plant campaigns in this study Very different correlations were found for the seven different pilot campaigns. Some data show linear trends while others appear to follow a logarithmic function. But this could also be due to lower concentration ranges of the linear trending plants; with longer operation time a change might have been occurred. Additionally, the reaction from HEGly to HEHEAA is most likely impacted by plant specific factors like operation regime, plant design, solvent cycle time and alternate reactions. Based on this the formation of HEHEAA is the limiting step in HEPO formation. The amount of HEHEAA formed should possibly be manipulated by adjusting plant operation regime or plant design Magnitude of solvent degradation Figure 15 depicts the total accumulation of HEGly, HEHEAA and HEPO over time by plotting the sum of these compounds against operating hours. A linear trend was found for each pilot campaign. The typical mid-range formation rate of the system HEGly-HEHEAA-HEPO is between 0.06 mmol/(l*h) and 0.12 mmol/(l*h). The two pilot plant campaigns which are not falling into this range have a formation rate of 0.31 mmol/(l*h) and < 0.01 mmol/(l*h), respectively. The other secondary degradation products were found in significantly lower concentrations; HEF, for example, was determined in the range of 3 % to 20 % from the sum (HEGly, HEHEAA, HEPO) depending on the operating hours and pilot campaign. Figure 15: Sum (HEGly, HEHEAA, HEPO) concentration over time for the different pilot plant campaigns in this study Since the development over time is not linear for all compounds, Figure 16 shows the amount of secondary degradation products (2 nd DP) determined at around 600 operating hours at each CC pilot plant campaign. Of
9 270 D. Dux and B. Schallert / Energy Procedia 86 ( 2016 ) course, only compounds analyzed are shown, the absence of a compound does not necessarily mean that it was not present in the corresponding solvent of a campaign. The system HEGly-HEHEAA-HEPO dominated with midrange concentrations between 45 mmol/l and 70 mmol/l. The HEF concentration ranges from 0.3 mmol/l to 8.9 mmol/, with the majority falling into the range from 1.6 mmol/l to 4.8 mmol/l. the HEA concentration is mainly in the range from 0.1 mmol/l to 1.6 mmol/l, HEI concentrations are between 0.3 mmol/l to 2.9 mmol/l and OZD and HEEDA levels range from 0.1 mmol/l to 0.9 mmol/l. Figure 17 also shows the most typical primary degradation products (1 st DP) for the individual campaigns at around 600 operating hours. The formate concentration ranges from 3.4 mmol/l to 11.6 mmol/l, acetate concentration is between 0.1 mmol/l and 2.9 mmol/l, oxalate between 0.6 mmol/l and 1.6 mmol/l and glycolate between 0.5 mmol/l and 1.2 mmol/l. Strong variation is found across all campaigns and no mid-range section can be defined. Figure 16: Amount of secondary and primary degradation products at around 600 operating hours for the different pilot campaigns in this study The different behavior over time, meaning linear, exponential or other, causes a shift in concentration ratios with operation time. For a simplified comparison, the accumulated amount of degradation products was assumed to behave almost linearly due to the domination of the HEGly-HEHEAA-HEPO system. The accumulated amount of primary degradation products on the other hand shows a more exponential development. Hence, the ratio between secondary to primary degradation products will change with operating time. Accordingly, the solvent chemistry will change and alternate reactions might take place. TOC analysis of one plant indicated, that at the end of the campaign, a significant amount of unknown, organic degradation products was present in the solvent. Hence, the current, typical analytical scope does not cover the whole reaction chemistry. 4. Summary The correlation of the available, MEA based CC pilot plant campaign data as discussed above can be summarized as follows: The HEPO concentration shows a strong correlation with the nickel concentration in the solvent at low concentrations (< 0.04 mmol/l). The corresponding correlation between HEPO and iron was less significant, but some correlation at low concentrations might be given (< 1 mmol/l). HEHEAA formation is the limiting step in the equilibrium reaction of HEPO formation from HEGly. The HEF concentration correlates well with the formate concentration for all plants across all concentration ranges. The formate concentration shows some linear correlation with iron at iron levels below 1 mmol/l, which occurs to different extents for the different campaigns. A correlation between HEA and acetate could be seen, but showed more variation compared to HEF, which might be due to analytical issues. HEGly and HEPO are the most dominant degradation products and their sum together with HEHEAA shows a linear increase over time.
10 D. Dux and B. Schallert / Energy Procedia 86 ( 2016 ) Conclusions The comparison of the different CC pilot campaigns leads to following conclusions: Longer operation time for pilot campaigns is required as degradation product distribution will change with time. The impact on solvent behavior needs to be determined. Reliable, analytical data is required to investigate and understand dependencies. Hence the analytical scope of pilot campaigns should not neglect periodic sampling of the solvent. At this point, it is not clear, if the HEGly formation is catalyzed in any form or if the reaction depends on other factors. However, since HEGly consumes MEA by further reactions, it needs to be limited within the solvent to reduce the overall MEA loss. The initial oxidation to carbonic acids should be minimized in order to reduce amide formation, since this is another pathway of MEA consumption. Nickel and potentially iron play an important role in some of the degradation reactions. The correlations have shown that a limitation to mmol/l ( mg/l) nickel and 0.1 mmol/l (~ 6 mg/l) iron within the solvent is preferable. Further recommended studies: Impact of other metals on solvent degradation. Potential candidates could be molybdenum (as it occurs as part of stainless steel corrosion) and metals commonly found in fly ash (e.g. vanadium and manganese). More research into the initial MEA oxidation mechanism is required. And more importantly, how to control this initial degradation in a pilot plant (on site variation of oxygen saturation and / or cycle time, if possible). Comparison of the campaigns from different CC pilot plants provided a valuable insight into the chemistry, but plant design and operational conditions need to be considered to complete the data assessment. References [1] Da Silva et al, E., Lepaumier, H., Grimstvedt, A., Vevelstad, S.J., Einbu, A., Vernstad, K., Svendsen, H.F.,Zahlsen, K., Understanding 2- ethanolamine degradation in postcombustion CO 2 capture, I&EC Res., 51 (2012) [2] Aker Clean Carbon, results from ACC MTU test campaign MEA at Longannet, May-Nov 2009 [3] CESAR CO 2 enhanced separation and recovery; SEVENTH FRAMEWORK PROGRAMME THEME 5 Energy; Energy : Advanced separation techniques; Collaborative Project GA No [4] DONG long term MEA campaign; results provided within research program [5] SOLVIT: Results from Heilbronn test plant MEA reference campaign, March May 2012 [6] OCTAVIUS: Optmisation of CO 2 capture technology allowing verification and implementation at utility scale; SEVENTH FRAMEWORK PROGRAMME THEME ENERGY &6.2.1; Optimising the integration of CO2 capture into power plants; Collaborative Project GA No [7] Vevelstad, S. J.; Grimstvedt, A.; Elnan, J.; da Silva et al, E.F.; Sevendsen, H. F.; Oxidative degradation of 2-ethanolamine; the effect of oxygen concentration and temperature on product formation; International Journal of Greenhouse Gas Control, 18 (2013), [8] Goff, G. S.; Rochelle, G. T.; Monoethanolamine degradation: O 2 mass transfer effects under CO 2 capture conditions; Ind. Eng. Chem. Res., 43 (2004), [9] Vevelstad, S. J.; Grimstvedt, A.; Knuutila, H.; da Silva et al, E.F.; Svendsen H. F.; Influence of experimental setup on amine degradation, International Journal of Greenhouse Gas Control, 28 (2014), [10] Vevelstad, S. J.; Grimstvedt, A.; Knuutila, H.; Svendsen H. F.; Thermal degradation on already oxidatively degraded solutions; Energy Procedia, 37 (2013), [11] Strazisar, B.R., Anderson, R.R., White, C.M.; Degradation pathways for monoethanolamine in a CO 2 capture facility; Energy & Fuels, 17 (2003), [12] Sexton, A. J.; Rochelle, G. T.; Catalysts and Inhibitors for MEA Oxidation; Energy Procedia, 1 (2009),
A study of thermal degradation of different amines and their resulting degradation products
 1 A study of thermal degradation of different amines and their resulting degradation products Ingvild Eide-Haugmo a, Hélène Lepaumier a, Aslak Einbu b, Kai Vernstad b, Eirik Falck da Silva b, Hallvard
1 A study of thermal degradation of different amines and their resulting degradation products Ingvild Eide-Haugmo a, Hélène Lepaumier a, Aslak Einbu b, Kai Vernstad b, Eirik Falck da Silva b, Hallvard
Degradation of amines in CO 2 Capture
 Degradation of amines in CO 2 Capture Gary T. Rochelle, Stephanie Freeman, Alex Voice, Fred Closmann Luminant Carbon Management Program The University of Texas at Austin Presented at TCCS-6 June, 2011
Degradation of amines in CO 2 Capture Gary T. Rochelle, Stephanie Freeman, Alex Voice, Fred Closmann Luminant Carbon Management Program The University of Texas at Austin Presented at TCCS-6 June, 2011
BIO-CHEMICALS FROM CONVERSION OF BIO-ETHANOL USING VARIOUS SINGLE OXIDES
 BIO-CHEMICALS FROM CONVERSION OF BIO-ETHANOL USING VARIOUS SINGLE OXIDES Nattapron Siribanluehan a, Sirirat Jitkarnka a a The Petroleum and Petrochemical College, Chulalongkorn University, Bangkok, Thailand
BIO-CHEMICALS FROM CONVERSION OF BIO-ETHANOL USING VARIOUS SINGLE OXIDES Nattapron Siribanluehan a, Sirirat Jitkarnka a a The Petroleum and Petrochemical College, Chulalongkorn University, Bangkok, Thailand
COMPARISON OF THE PHASE BEHAVIOUR OF HIGH MOLECULAR MASS ALCOHOLS IN VARIOUS SUPERCRITICAL FLUIDS
 COMPARISON OF THE PHASE BEHAVIOUR OF HIGH MOLECULAR MASS ALCOHOLS IN VARIOUS SUPERCRITICAL FLUIDS C.E. Schwarz, J.H. Knoetze* Department of Process Engineering, University of Stellenbosch, Private Bag
COMPARISON OF THE PHASE BEHAVIOUR OF HIGH MOLECULAR MASS ALCOHOLS IN VARIOUS SUPERCRITICAL FLUIDS C.E. Schwarz, J.H. Knoetze* Department of Process Engineering, University of Stellenbosch, Private Bag
Structure and activity relationships for amine based CO2 absorbents-i Singh, P.; Niederer, J. P. M.; Versteeg, Geert
 University of Groningen Structure and activity relationships for amine based CO2 absorbents-i Singh, P.; Niederer, J. P. M.; Versteeg, Geert Published in: International Journal of Greenhouse Gas Control
University of Groningen Structure and activity relationships for amine based CO2 absorbents-i Singh, P.; Niederer, J. P. M.; Versteeg, Geert Published in: International Journal of Greenhouse Gas Control
Method Development and Validation for Nutraceuticals
 White Paper Method Development and Validation for Nutraceuticals Maud Silvent Technical Specialist David Neville Technical Specialist Method Development and Validation for Nutraceuticals Introduction Nutraceutical
White Paper Method Development and Validation for Nutraceuticals Maud Silvent Technical Specialist David Neville Technical Specialist Method Development and Validation for Nutraceuticals Introduction Nutraceutical
Applicant Name: GD Sigelei Electroinc Tech Co., Ltd B7 Building, No.1 District, Xicheng Science and Technology Park, Hengli Town, Dongguan, China
 Date: Jan.17, 2018 Applicant Name: GD Sigelei Electroinc Tech Co., Ltd Applicant Add.: B7 Building, No.1 District, Xicheng Science and Technology Park, Hengli Town, Dongguan, China Test sample(s) was(were)
Date: Jan.17, 2018 Applicant Name: GD Sigelei Electroinc Tech Co., Ltd Applicant Add.: B7 Building, No.1 District, Xicheng Science and Technology Park, Hengli Town, Dongguan, China Test sample(s) was(were)
USER SPECIFICATIONS FOR QUINTOLUBRIC 888 Series DESCRIPTION OF THE MOST IMPORTANT PROPERTIES AND THE POSSIBLE VARIATIONS AND TOLERANCES
 USER SPECIFICATIONS FOR QUINTOLUBRIC 888 Series OVERVIEW The QUINTOLUBRIC 888 Series of fluids are based on a synthetic organic ester, which is the main base component, plus a number of selected additives
USER SPECIFICATIONS FOR QUINTOLUBRIC 888 Series OVERVIEW The QUINTOLUBRIC 888 Series of fluids are based on a synthetic organic ester, which is the main base component, plus a number of selected additives
Chemicals Based on Ethylene
 Chemicals Based on Ethylene Ethylene is sometimes known as the king of petrochemicals because more commercial chemicals are produced from ethylene than from any other intermediate. This unique position
Chemicals Based on Ethylene Ethylene is sometimes known as the king of petrochemicals because more commercial chemicals are produced from ethylene than from any other intermediate. This unique position
22. The Fischer Esterification
 22. The Fischer Esterification A. Background Esters are an incredibly important functional group in organic chemistry. Esters are typically very pleasant smelling molecules and are therefore frequently
22. The Fischer Esterification A. Background Esters are an incredibly important functional group in organic chemistry. Esters are typically very pleasant smelling molecules and are therefore frequently
NCUT National Centre for Upgrading Technology
 NCUT National Centre for Upgrading Technology a Canada Alberta alliance for bitumen and heavy oil research Comparison of the Reactivity of Naphthenic Acids in Athabasca bitumen and San Joaquin Valley Parviz
NCUT National Centre for Upgrading Technology a Canada Alberta alliance for bitumen and heavy oil research Comparison of the Reactivity of Naphthenic Acids in Athabasca bitumen and San Joaquin Valley Parviz
Refinery Wastewater Operations: Challenges Created from the Processing of Opportunity Crudes
 Refinery Wastewater Operations: Challenges Created from the Processing of Opportunity Crudes Roy Hernandez-Mena 1* and David R. Marrs, P.E. 2 1 Baker Hughes, Inc. 12645 W. Airport Blvd., Sugar Land, TX
Refinery Wastewater Operations: Challenges Created from the Processing of Opportunity Crudes Roy Hernandez-Mena 1* and David R. Marrs, P.E. 2 1 Baker Hughes, Inc. 12645 W. Airport Blvd., Sugar Land, TX
A New Method for the Early Detection of Edible Oil Oxidation
 WHITE PAPER Early Detection of Edible Oil Oxidation A New Method for the Early Detection of Edible Oil Oxidation Edible oils are used in a wide range of culinary applications. Oils containing unsaturated
WHITE PAPER Early Detection of Edible Oil Oxidation A New Method for the Early Detection of Edible Oil Oxidation Edible oils are used in a wide range of culinary applications. Oils containing unsaturated
Association des Amidonneries de Céréales de l Union Européenne
 aac Association des Amidonneries de Céréales de l Union Européenne SUMMARY REPORT Eco-profile of starch and related products Based on a report by Dr Ian Boustead January 2001 1 Introduction The concept
aac Association des Amidonneries de Céréales de l Union Européenne SUMMARY REPORT Eco-profile of starch and related products Based on a report by Dr Ian Boustead January 2001 1 Introduction The concept
LC Columns - Exceed the limit. A premium inert range of LC columns delivering optimal peak shape. ProteCol -P PEEK lined
 ProteCol LC Columns - Exceed the limit A premium inert range of LC columns delivering optimal peak shape. ProteCol -G Glass lined ProteCol -P PEEK lined B C A D E F A Column end cap B PEEK frit housing
ProteCol LC Columns - Exceed the limit A premium inert range of LC columns delivering optimal peak shape. ProteCol -G Glass lined ProteCol -P PEEK lined B C A D E F A Column end cap B PEEK frit housing
Thermal Stability of Oleic Acid and Ethyl Linoleate
 Chapter 3.1 Thermal Stability of leic Acid and Ethyl Linoleate The first part of this work consisted of studying the thermal stability of oleic acid, which was initially a candidate as a starting material
Chapter 3.1 Thermal Stability of leic Acid and Ethyl Linoleate The first part of this work consisted of studying the thermal stability of oleic acid, which was initially a candidate as a starting material
Experiences with Zinc Injection at Obrigheim Nuclear Power Plant. Ingolf BRIESEN, Obrigheim NPP, Germany
 Experiences with Zinc Injection at Obrigheim Nuclear Power Plant Ingolf BRIESEN, Obrigheim NPP, Germany 1. Introduction/Background Obrigheim Nuclear Power Plant (KWO) went on-line in October 1968. The
Experiences with Zinc Injection at Obrigheim Nuclear Power Plant Ingolf BRIESEN, Obrigheim NPP, Germany 1. Introduction/Background Obrigheim Nuclear Power Plant (KWO) went on-line in October 1968. The
Contribution of Drinking Water to Dietary Requirements of Essential Metals
 Contribution of Drinking Water to Dietary Requirements of Essential Metals Michelle Deveau Water Quality Science Division Health Canada May 7, 2008 Workshop on Health Risk Assessment of Essential Metals
Contribution of Drinking Water to Dietary Requirements of Essential Metals Michelle Deveau Water Quality Science Division Health Canada May 7, 2008 Workshop on Health Risk Assessment of Essential Metals
An Investigative Study of Reactions Involving Glucosinolates and Isothiocyanates
 An Investigative Study of Reactions Involving Glucosinolates and Isothiocyanates Alzea Chrisel H. Alea 1, Diane Elaine T. Co 2 and Marissa G Noel 3* 1,2,3Chemistry Department, De La Salle University, 2401
An Investigative Study of Reactions Involving Glucosinolates and Isothiocyanates Alzea Chrisel H. Alea 1, Diane Elaine T. Co 2 and Marissa G Noel 3* 1,2,3Chemistry Department, De La Salle University, 2401
DET REPORT NO. 69 JUNE 2015
 1.) THINKING BEYOND THE NPD BOX - INEXPENSIVE CONVERSION OF NPD EQUIPMENT TO MULTIPLE MODES OF SELECTIVE GC DETECTION. 2.) GC-CCID DIFFERENTIATION BETWEEN SATURATE VS. UNSATURATE AND MONO-UNSATURATE VS.
1.) THINKING BEYOND THE NPD BOX - INEXPENSIVE CONVERSION OF NPD EQUIPMENT TO MULTIPLE MODES OF SELECTIVE GC DETECTION. 2.) GC-CCID DIFFERENTIATION BETWEEN SATURATE VS. UNSATURATE AND MONO-UNSATURATE VS.
Supporting Information
 Supporting Information Combined EC-NMR and In Situ FTIR Spectroscopic Studies of Glycerol Electrooxidation on Pt/C, PtRu/C, and PtRh/C Long Huang, Jia-Yu Sun, Shuo-Hui Cao, Mei Zhan, Zu-Rong Ni, Hui-Jun
Supporting Information Combined EC-NMR and In Situ FTIR Spectroscopic Studies of Glycerol Electrooxidation on Pt/C, PtRu/C, and PtRh/C Long Huang, Jia-Yu Sun, Shuo-Hui Cao, Mei Zhan, Zu-Rong Ni, Hui-Jun
Local Structure Analysis of de-nox Catalyst Prepared by the Glycothermal Method
 Local Structure Analysis of de-nox Catalyst Prepared by the Glycothermal Method K. Kagawa a, H. Deguchi a, M. Horiuchi b, M. Takahashi c, S. Iwamoto c, and M. Inoue c a Kansai Electric Power Co., INC.
Local Structure Analysis of de-nox Catalyst Prepared by the Glycothermal Method K. Kagawa a, H. Deguchi a, M. Horiuchi b, M. Takahashi c, S. Iwamoto c, and M. Inoue c a Kansai Electric Power Co., INC.
Prelab 6: Carboxylic Acids
 The Structure of Carboxylic Acids Prelab 6: Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group,
The Structure of Carboxylic Acids Prelab 6: Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group,
Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n
 Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n Acid Derivatives and their Names - Acid Halides have a Cl or Br instead of OH. Replace ic acid with yl halide, such as propionyl chloride (a common
Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n Acid Derivatives and their Names - Acid Halides have a Cl or Br instead of OH. Replace ic acid with yl halide, such as propionyl chloride (a common
Differentiation Between Nutritional and Carcinogenic Selenium via Speciation Analyses
 Presented at the AOAC Conference, September 29, 2015 Differentiation Between Nutritional and Carcinogenic Selenium via Speciation Analyses Russell Gerads Business Development Director Brooks Applied Labs
Presented at the AOAC Conference, September 29, 2015 Differentiation Between Nutritional and Carcinogenic Selenium via Speciation Analyses Russell Gerads Business Development Director Brooks Applied Labs
Analytical Method for 2, 4, 5-T (Targeted to Agricultural, Animal and Fishery Products)
 Analytical Method for 2, 4, 5-T (Targeted to Agricultural, Animal and Fishery Products) The target compound to be determined is 2, 4, 5-T. 1. Instrument Liquid Chromatograph-tandem mass spectrometer (LC-MS/MS)
Analytical Method for 2, 4, 5-T (Targeted to Agricultural, Animal and Fishery Products) The target compound to be determined is 2, 4, 5-T. 1. Instrument Liquid Chromatograph-tandem mass spectrometer (LC-MS/MS)
OCR A GCSE Chemistry. Topic 6: Global challenges. Organic chemistry. Notes.
 OCR A GCSE Chemistry Topic 6: Global challenges Organic chemistry Notes C6.2a recognise functional groups and identify members of the same homologous series Prefixes (beginning of the name) o remember
OCR A GCSE Chemistry Topic 6: Global challenges Organic chemistry Notes C6.2a recognise functional groups and identify members of the same homologous series Prefixes (beginning of the name) o remember
Key Words: Brassica oleraceae, glucosinolate, liquid chromatography mass spectrometry, FNH-I-003
 IDENTIFICATION OF MAJOR GLUCOSINOLATES IN BROCCOLI (Brassica oleracea var. italica) BY LIQUID CHROMATOGRAPHY MASS SPECTROMETRY (LC-MS) AND DETERMINATION OF ANTICANCER PROPERTIES OF BROCCOLI EXTRACTS Carlos
IDENTIFICATION OF MAJOR GLUCOSINOLATES IN BROCCOLI (Brassica oleracea var. italica) BY LIQUID CHROMATOGRAPHY MASS SPECTROMETRY (LC-MS) AND DETERMINATION OF ANTICANCER PROPERTIES OF BROCCOLI EXTRACTS Carlos
Risk Assessment of NIAS in Food Contact Adhesives
 Dr. Monika Tönnießen and Dr. Matthias Frischmann September 2017 Risk Assessment of IAS in Food ontact Adhesives Globally relevant content 2/8 Risk Assessment of IAS in Food ontact Adhesives What are IAS?
Dr. Monika Tönnießen and Dr. Matthias Frischmann September 2017 Risk Assessment of IAS in Food ontact Adhesives Globally relevant content 2/8 Risk Assessment of IAS in Food ontact Adhesives What are IAS?
Acetaldehyde Production from Ethanol over Ni-Based Catalysts
 Chiang Mai J. Sci. 2008; 35(1) KC-019 171 Chiang Mai J. Sci. 2008; 35(1) : 171-177 www.science.cmu.ac.th/journal-science/josci.html Contributed Paper Acetaldehyde Production from Ethanol over Ni-Based
Chiang Mai J. Sci. 2008; 35(1) KC-019 171 Chiang Mai J. Sci. 2008; 35(1) : 171-177 www.science.cmu.ac.th/journal-science/josci.html Contributed Paper Acetaldehyde Production from Ethanol over Ni-Based
THE RELATIONSHIP BETWEEN TWO METHODS FOR EVALUATING FIVE-CARBON SUGARS IN EUCALYPTUS EXTRACTION LIQUOR
 THE RELATIONSHIP BETWEEN TWO METHODS FOR EVALUATING FIVE-CARBON SUGARS IN EUCALYPTUS EXTRACTION LIQUOR Congcong Chi, a,b* Zeng Zhang, a Weiwei Ge, a and Hasan Jameel b Alkaline pre-extraction and hydrothermal
THE RELATIONSHIP BETWEEN TWO METHODS FOR EVALUATING FIVE-CARBON SUGARS IN EUCALYPTUS EXTRACTION LIQUOR Congcong Chi, a,b* Zeng Zhang, a Weiwei Ge, a and Hasan Jameel b Alkaline pre-extraction and hydrothermal
Carboxylic Acids and their Derivatives I
 2302272 Org Chem II Part I Lecture 5 Carboxylic Acids and their Derivatives I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 20 in Organic Chemistry,
2302272 Org Chem II Part I Lecture 5 Carboxylic Acids and their Derivatives I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 20 in Organic Chemistry,
National 5 Unit Two : Nature s Chemistry
 National 5 Unit Two : Nature s Chemistry Fuels A fuel is a chemical which burns, giving off energy. Combustion is a reaction of a substance with oxygen giving off energy. The test for oxygen is it relights
National 5 Unit Two : Nature s Chemistry Fuels A fuel is a chemical which burns, giving off energy. Combustion is a reaction of a substance with oxygen giving off energy. The test for oxygen is it relights
Available online at ScienceDirect. Energy Procedia 78 (2015 )
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 78 (2015 ) 2772 2777 6th International Building Physics Conference, IBPC 2015 Evaluation of sensation of dryness in airway under
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 78 (2015 ) 2772 2777 6th International Building Physics Conference, IBPC 2015 Evaluation of sensation of dryness in airway under
5124 SCIENCE (PHYSICS AND CHEMISTRY)
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS GCE Ordinary Level www.xtremepapers.com MARK SCHEME for the October/November 2010 question paper for the guidance of teachers 5124 SCIENCE (PHYSICS AND
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS GCE Ordinary Level www.xtremepapers.com MARK SCHEME for the October/November 2010 question paper for the guidance of teachers 5124 SCIENCE (PHYSICS AND
Analyte Proficiency From All Labs # Analytes: 26 Sample # Statistical Summary # Labs Reporting: 82 Urea Issue Date : 06/30/2016
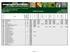 Analyte Proficiency From All Labs # Analytes: 26 Sample # 160511 Statistical Summary # Labs Reporting: 82 Urea Issue Date : 06/30/2016 Analyte Code Analyte # Tests Submitted # Tests in Robust Calculations
Analyte Proficiency From All Labs # Analytes: 26 Sample # 160511 Statistical Summary # Labs Reporting: 82 Urea Issue Date : 06/30/2016 Analyte Code Analyte # Tests Submitted # Tests in Robust Calculations
Summary p. 1 What Are Dietary Reference Intakes? p. 2 Approach for Setting Dietary Reference Intakes p. 7 Nutrient Functions and the Indicators Used
 Summary p. 1 What Are Dietary Reference Intakes? p. 2 Approach for Setting Dietary Reference Intakes p. 7 Nutrient Functions and the Indicators Used to Estimate Requirements p. 10 Criteria and Proposed
Summary p. 1 What Are Dietary Reference Intakes? p. 2 Approach for Setting Dietary Reference Intakes p. 7 Nutrient Functions and the Indicators Used to Estimate Requirements p. 10 Criteria and Proposed
Smoke Analytes Sub-Group (SMA) 2017 Report
 Smoke Analytes Sub-Group (SMA) 2017 Report Smoke Science and Product Technology Study Groups Kitzbühel, October 11, 2017 SMA SG Sub-Group name change In January 2017, SG changed its name to Smoke Analytes
Smoke Analytes Sub-Group (SMA) 2017 Report Smoke Science and Product Technology Study Groups Kitzbühel, October 11, 2017 SMA SG Sub-Group name change In January 2017, SG changed its name to Smoke Analytes
Application Note # FTMS-46 solarix XR: Analysis of Complex Mixtures
 Application Note # FTMS-46 solarix XR: Analysis of Complex Mixtures Introduction Natural organic matter (NOM) as well as crude oil and crude oil fractions are very complex mixtures of organic compounds
Application Note # FTMS-46 solarix XR: Analysis of Complex Mixtures Introduction Natural organic matter (NOM) as well as crude oil and crude oil fractions are very complex mixtures of organic compounds
Syringe Pump Application Note AN27. Figure 1: Phase diagram of water showing vapor-liquid relationship for subcritical water
 Measurement of Aqueous Solubility of Compounds at High Temperature Using a Dynamic Flow Apparatus and a Teledyne Isco Syringe Pump Jerry W. King & Keerthi Srinivas, University of Arkansas, Dept. of Chemical
Measurement of Aqueous Solubility of Compounds at High Temperature Using a Dynamic Flow Apparatus and a Teledyne Isco Syringe Pump Jerry W. King & Keerthi Srinivas, University of Arkansas, Dept. of Chemical
Studies on Thermal Oxidation Stability of Aviation Lubricating Oils
 MATEC Web of Conferences 114, 000 (017) DOI: 10.1051/ matecconf/017114000 MAE 017 Studies on Thermal Oxidation Stability of Aviation Lubricating Oils Nan Wu 1,, Zhi-Min Zong 1,a, Yi-Wei Fei and Jun Ma
MATEC Web of Conferences 114, 000 (017) DOI: 10.1051/ matecconf/017114000 MAE 017 Studies on Thermal Oxidation Stability of Aviation Lubricating Oils Nan Wu 1,, Zhi-Min Zong 1,a, Yi-Wei Fei and Jun Ma
WHAT ARE FERTILIZERS
 FERTILIZER INDUSTRY WHAT ARE FERTILIZERS FERTILIZERS ARE COMPOUNDS GIVEN TO PLANTS WITH THE INTENTION OF PROMOTING GROWTH; THEY ARE USUALLY APPLIED EITHER VIA THE SOIL, FOR UPTAKE BY PLANT ROOTS, OR BY
FERTILIZER INDUSTRY WHAT ARE FERTILIZERS FERTILIZERS ARE COMPOUNDS GIVEN TO PLANTS WITH THE INTENTION OF PROMOTING GROWTH; THEY ARE USUALLY APPLIED EITHER VIA THE SOIL, FOR UPTAKE BY PLANT ROOTS, OR BY
ESTERS, MULTIPURPOSE GROUP V - BASE FLUIDS, PROPERTIES AND APPLICATIONS
 F. Bongardt Esters, multipurpose group V... Frank Bongardt ISSN 0350-350X GOMABN 55, 2, 101-108 Professional paper ESTERS, MULTIPURPOSE GROUP V - BASE FLUIDS, PROPERTIES AND APPLICATIONS Abstract Lube
F. Bongardt Esters, multipurpose group V... Frank Bongardt ISSN 0350-350X GOMABN 55, 2, 101-108 Professional paper ESTERS, MULTIPURPOSE GROUP V - BASE FLUIDS, PROPERTIES AND APPLICATIONS Abstract Lube
Additives for polyolefines: chemistry involved and innovative effects
 Additives for polyolefines: chemistry involved and innovative effects Abstract Because many synthetic resins suffer from photo degradation, the investigation of ways to inhibit or at least retard this
Additives for polyolefines: chemistry involved and innovative effects Abstract Because many synthetic resins suffer from photo degradation, the investigation of ways to inhibit or at least retard this
FATTY ACID PROFILING BY GAS CHROMATOGRAPHY FOR THE SHERLOCK MIS
 FATTY ACID PROFILING BY GAS CHROMATOGRAPHY FOR THE SHERLOCK MIS Traditional gas chromatography of complex mixtures of compounds requires precision on the part of the chromatography equipment and considerable
FATTY ACID PROFILING BY GAS CHROMATOGRAPHY FOR THE SHERLOCK MIS Traditional gas chromatography of complex mixtures of compounds requires precision on the part of the chromatography equipment and considerable
Greg Patterson C.C.A. President A&L Canada Laboratories
 Welcome Greg Patterson C.C.A. President A&L Canada Laboratories Compost Quality Consumer of the Future Population dynamics - more and more people involved in use of compost Better educated on what is expected
Welcome Greg Patterson C.C.A. President A&L Canada Laboratories Compost Quality Consumer of the Future Population dynamics - more and more people involved in use of compost Better educated on what is expected
Analyses no.: Telephone: Fax: Telephone: Fax:
 Analysis order for biogas Analyses no.: Date of receipt: Sampling vessel: Contractor = Invoice recipient Copy of the test report for: LUFA Customer no. (if available) LUFA Customer no. (if available) Name,
Analysis order for biogas Analyses no.: Date of receipt: Sampling vessel: Contractor = Invoice recipient Copy of the test report for: LUFA Customer no. (if available) LUFA Customer no. (if available) Name,
CYCLOSERINE Proposal for revision of The International Pharmacopoeia (August 2012)
 August 2012 RESTRICTED CYCLOSERINE Proposal for revision of The International Pharmacopoeia (August 2012) Draft for comment This document was provided by a quality control expert. Should you have any comments
August 2012 RESTRICTED CYCLOSERINE Proposal for revision of The International Pharmacopoeia (August 2012) Draft for comment This document was provided by a quality control expert. Should you have any comments
Fig.1. Denatonium benzoate (DB) chemical structure
 ILIADE code 280 CLEN Method Determination of Denatonium Benzoate in Alcoholic Products by HPLC-UV 1 Scope The purpose of this method is verification of fulfilment of the legislative requirements on denatured
ILIADE code 280 CLEN Method Determination of Denatonium Benzoate in Alcoholic Products by HPLC-UV 1 Scope The purpose of this method is verification of fulfilment of the legislative requirements on denatured
Colloidal metal oxide nanocrystal catalysis by sustained chemically driven ligand displacement
 Colloidal metal oxide nanocrystal catalysis by sustained chemically driven ligand displacement Jonathan De Roo 1,2,3 *, Isabel Van Driessche 1, José C. Martins 2 and Zeger Hens 3,4 * 1 Sol-gel Center for
Colloidal metal oxide nanocrystal catalysis by sustained chemically driven ligand displacement Jonathan De Roo 1,2,3 *, Isabel Van Driessche 1, José C. Martins 2 and Zeger Hens 3,4 * 1 Sol-gel Center for
Factors to Consider in the Study of Biomolecules
 Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General structure and functional groups
Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General structure and functional groups
Mass Spectrometry Made Simple for Clinical Diagnostic Labs
 Mass Spectrometry Made Simple for Clinical Diagnostic Labs The SCIEX Topaz System with FDA-Cleared Topaz Vitamin D 200M Assay Kit Mass Spectrometry Made Simple SCIEX Topaz System for Clinical Diagnostics
Mass Spectrometry Made Simple for Clinical Diagnostic Labs The SCIEX Topaz System with FDA-Cleared Topaz Vitamin D 200M Assay Kit Mass Spectrometry Made Simple SCIEX Topaz System for Clinical Diagnostics
Student Handout. This experiment allows you to explore the properties of chiral molecules. You have
 Student Handout This experiment allows you to explore the properties of chiral molecules. You have learned that some compounds exist as enantiomers non-identical mirror images, such as your left and right
Student Handout This experiment allows you to explore the properties of chiral molecules. You have learned that some compounds exist as enantiomers non-identical mirror images, such as your left and right
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Safety Assessment of Hypobromous Acid (220 ppm as Br 2 ) Used as a Beef Carcass Wash
 Safety Assessment of Hypobromous Acid (220 ppm as Br 2 ) Used as a Beef Carcass Wash Prepared for: Enviro Tech Chemical Services Modesto, CA Prepared by: Duncan Turnbull, DPhil, DABT Senior Science Advisor
Safety Assessment of Hypobromous Acid (220 ppm as Br 2 ) Used as a Beef Carcass Wash Prepared for: Enviro Tech Chemical Services Modesto, CA Prepared by: Duncan Turnbull, DPhil, DABT Senior Science Advisor
Simultaneous quantification of cellular lipids and carotenoids inside Chlorella vulgaris using Raman spectrometry
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 61 (2014 ) 829 833 The 6 th International Conference on Applied Energy ICAE2014 Simultaneous quantification of cellular lipids and
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 61 (2014 ) 829 833 The 6 th International Conference on Applied Energy ICAE2014 Simultaneous quantification of cellular lipids and
Analytical methods and laboratory choice for food composition, an introduction
 Analytical methods and laboratory choice for food composition, an introduction Paul Hulshof, Global challenges Research Fund Foodomp workshop, Pretoria, 5-9 Feb, 2018 General Reasons for analyzing food
Analytical methods and laboratory choice for food composition, an introduction Paul Hulshof, Global challenges Research Fund Foodomp workshop, Pretoria, 5-9 Feb, 2018 General Reasons for analyzing food
MEA Oxidation in CO 2 Capture Inhibitor Screening with Hot-gas FTIR
 MEA xidation in C 2 Capture Inhibitor Screening with Hot-gas FTIR Alexander Voice Gary T. Rochelle 17.May.2011 Luminant Carbon Management Program University of Texas, Dept. Chem. Eng. Amine xidation in
MEA xidation in C 2 Capture Inhibitor Screening with Hot-gas FTIR Alexander Voice Gary T. Rochelle 17.May.2011 Luminant Carbon Management Program University of Texas, Dept. Chem. Eng. Amine xidation in
OXIDATION AND ANTIOXIDANT PROTECTION IN RAW MATERIALS AND FEEDS
 EVALUATION OF OXIDATION AND ANTIOXIDANT PROTECTION IN RAW MATERIALS AND FEEDS Sergi Carné and Javier Estévez Technical Department, Industrial Técnica Pecuaria, S.A. (ITPSA); scarne@itpsa.com 1 LIPID OXIDATION
EVALUATION OF OXIDATION AND ANTIOXIDANT PROTECTION IN RAW MATERIALS AND FEEDS Sergi Carné and Javier Estévez Technical Department, Industrial Técnica Pecuaria, S.A. (ITPSA); scarne@itpsa.com 1 LIPID OXIDATION
ETHYLENE GLYCOL. Table 1.1 Physical properties of Ethylene glycol
 ETHYLENE GLYCOL Introduction [1]: Glycols are dihydric alcohols having an aliphatic carbon chain. They have the general chemical formula C n H 2n (OH) 2. is the simplest and the most important of the glycols.
ETHYLENE GLYCOL Introduction [1]: Glycols are dihydric alcohols having an aliphatic carbon chain. They have the general chemical formula C n H 2n (OH) 2. is the simplest and the most important of the glycols.
Product Guide for LudgerSep TM R1 HPLC Column for DMB labelled Sialic Acid Analysis
 Product Guide for LudgerSep TM R1 HPLC Column for DMB labelled Sialic Acid Analysis Product # LS-R1-4.6x150 Ludger Document # LS-R1-DMB-Guide-v5.1 Ludger Ltd Culham Science Centre Oxford OX14 3EB United
Product Guide for LudgerSep TM R1 HPLC Column for DMB labelled Sialic Acid Analysis Product # LS-R1-4.6x150 Ludger Document # LS-R1-DMB-Guide-v5.1 Ludger Ltd Culham Science Centre Oxford OX14 3EB United
Product Guide for LudgerSep TM ur2 UHPLC Column for DMB Sialic Acid Analysis
 Product Guide for LudgerSep TM ur2 UHPLC Column for DMB Sialic Acid Analysis Product # LS-UR2-2.1x100 Ludger Document # LS-uR2-DMB-Guide-v2.1 Ludger Ltd Culham Science Centre Oxford OX14 3EB United Kingdom
Product Guide for LudgerSep TM ur2 UHPLC Column for DMB Sialic Acid Analysis Product # LS-UR2-2.1x100 Ludger Document # LS-uR2-DMB-Guide-v2.1 Ludger Ltd Culham Science Centre Oxford OX14 3EB United Kingdom
Biomolecules. Unit 3
 Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Savesta Herbals is engaged in manufacturing
 Savesta Herbals is engaged in manufacturing standardized herbal extracts that synergistically combine the ancient wisdom of Ayurveda with the modern production and QC methods. Our state-of- the- art production
Savesta Herbals is engaged in manufacturing standardized herbal extracts that synergistically combine the ancient wisdom of Ayurveda with the modern production and QC methods. Our state-of- the- art production
PHOTOCATALYTIC DECONTAMINATION OF CHLORANTRANILIPROLE RESIDUES IN WATER USING ZnO NANOPARTICLES. DR. A. RAMESH, Ph.D, D.Sc.,
 PHOTOCATALYTIC DECONTAMINATION OF CHLORANTRANILIPROLE RESIDUES IN WATER USING ZnO NANOPARTICLES DR. A. RAMESH, Ph.D, D.Sc., raamesh_a@yahoo.co.in 1 OBJECTIVES Determination of persistence and photolysis
PHOTOCATALYTIC DECONTAMINATION OF CHLORANTRANILIPROLE RESIDUES IN WATER USING ZnO NANOPARTICLES DR. A. RAMESH, Ph.D, D.Sc., raamesh_a@yahoo.co.in 1 OBJECTIVES Determination of persistence and photolysis
Part I. Boolean modelling exercises
 Part I. Boolean modelling exercises. Glucose repression of Icl in yeast In yeast Saccharomyces cerevisiae, expression of enzyme Icl (isocitrate lyase-, involved in the gluconeogenesis pathway) is important
Part I. Boolean modelling exercises. Glucose repression of Icl in yeast In yeast Saccharomyces cerevisiae, expression of enzyme Icl (isocitrate lyase-, involved in the gluconeogenesis pathway) is important
Supplementary Information
 Supplementary Information Levulinic esters from the acid-catalysed reactions of sugar and alcohol as part of bio-refinery Xun Hu and Chun-Zhu Li* Fuels and Energy Technology Institute, Curtin University
Supplementary Information Levulinic esters from the acid-catalysed reactions of sugar and alcohol as part of bio-refinery Xun Hu and Chun-Zhu Li* Fuels and Energy Technology Institute, Curtin University
August Determination of Pindone in Agricultural Products by LC-MS/MS
 August 2011 237 LC-MS/MS 23 2 22 Determination of Pindone in Agricultural Products by LC-MS/MS Shizuka S6>ID, Satoru N:BDID and Rieko M6IHJ96 National Institute of Health Sciences: 1 18 1 Kamiyoga, Setagaya-ku,
August 2011 237 LC-MS/MS 23 2 22 Determination of Pindone in Agricultural Products by LC-MS/MS Shizuka S6>ID, Satoru N:BDID and Rieko M6IHJ96 National Institute of Health Sciences: 1 18 1 Kamiyoga, Setagaya-ku,
A pillar[2]arene[3]hydroquinone which can self-assemble to a molecular zipper in the solid state
![A pillar[2]arene[3]hydroquinone which can self-assemble to a molecular zipper in the solid state A pillar[2]arene[3]hydroquinone which can self-assemble to a molecular zipper in the solid state](/thumbs/86/93673402.jpg) A pillar[2]arene[3]hydroquinone which can self-assemble to a molecular zipper in the solid state Mingguang Pan, Min Xue* Department of Chemistry, Zhejiang University, Hangzhou 310027, P. R. China Fax:
A pillar[2]arene[3]hydroquinone which can self-assemble to a molecular zipper in the solid state Mingguang Pan, Min Xue* Department of Chemistry, Zhejiang University, Hangzhou 310027, P. R. China Fax:
Organic Chemistry. AQA Chemistry topic 7
 rganic hemistry AQA hemistry topic 7 7.1 arbon ompounds as fuels and feedstock rude il rude oil is a finite resource found in rocks. It s the remains of an ancient biomass consisting mainly of plankton
rganic hemistry AQA hemistry topic 7 7.1 arbon ompounds as fuels and feedstock rude il rude oil is a finite resource found in rocks. It s the remains of an ancient biomass consisting mainly of plankton
EXPERIMENT 13 QUALITATIVE ANALYSIS
 PURPOSE: 1. To separate and identify each cation in a mixture of Ag +, Cu 2+, Zn 2+, and Ca 2+ cations. 2. To identify the cations present in three individually assigned unknown mixtures that may contain
PURPOSE: 1. To separate and identify each cation in a mixture of Ag +, Cu 2+, Zn 2+, and Ca 2+ cations. 2. To identify the cations present in three individually assigned unknown mixtures that may contain
69. On the Mechanism o f Thiamine Action, II.
 302 [Vol. 27, 69. On the Mechanism o f Thiamine Action, II. Department By Shunzi MIZUHARA, Ryohei TAMURA, and Hidetaka ARATA. of Biological Chemistry, Okayama University Medical School. {Comm. by T. SHIMIZU,
302 [Vol. 27, 69. On the Mechanism o f Thiamine Action, II. Department By Shunzi MIZUHARA, Ryohei TAMURA, and Hidetaka ARATA. of Biological Chemistry, Okayama University Medical School. {Comm. by T. SHIMIZU,
Lesmahagow High School
 Lesmahagow High School Higher Chemistry Alcohols and Esters - Past Paper Homework Questions . Carbohydrates are an essential part of our diet. (a) Why are carbohydrates an important part of our diet? (b)
Lesmahagow High School Higher Chemistry Alcohols and Esters - Past Paper Homework Questions . Carbohydrates are an essential part of our diet. (a) Why are carbohydrates an important part of our diet? (b)
Available online at ScienceDirect. Energy Procedia 72 (2015 )
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 72 (2015 ) 270 277 International Scientific Conference Environmental and Climate Technologies CONECT 2014 How to assess involvement
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 72 (2015 ) 270 277 International Scientific Conference Environmental and Climate Technologies CONECT 2014 How to assess involvement
CONTINUOUS ESTERIFICATION IN SUPERCRITICAL CARBON DIOXIDE
 CONTINUOUS ESTERIFICATION IN SUPERCRITICAL CARBON DIOXIDE Hassan S. Ghaziaskar* and Ali Daneshfar Department of Chemistry, Isfahan University of Technology Isfahan, 84154, I.R. Iran. Email: ghazi@cc.iut.ac.ir
CONTINUOUS ESTERIFICATION IN SUPERCRITICAL CARBON DIOXIDE Hassan S. Ghaziaskar* and Ali Daneshfar Department of Chemistry, Isfahan University of Technology Isfahan, 84154, I.R. Iran. Email: ghazi@cc.iut.ac.ir
Carboxylic Acids and Carboxylic Acid Deriva3ves. Nucleophilic Acyl Subs0tu0on (Addi0on- Elimina0on)
 Carboxylic Acids and Carboxylic Acid Deriva3ves Nucleophilic Acyl Subs0tu0on (Addi0on- Elimina0on) 1 Carboxylic Compounds Acyl group bonded to X, an electronega3ve atom or leaving group Includes: X = halide
Carboxylic Acids and Carboxylic Acid Deriva3ves Nucleophilic Acyl Subs0tu0on (Addi0on- Elimina0on) 1 Carboxylic Compounds Acyl group bonded to X, an electronega3ve atom or leaving group Includes: X = halide
Simple Method (IS-MRM) to Monitor Lysophospholipids and Phospholipids During LC-MS Method Development via In-Source CID
 Simple Method (IS-MRM) to Monitor Lysophospholipids and Phospholipids During LC-MS Method Development via In-Source CID James Little, Eastman Chemical Company, Kingsport, TN Overview Phospholipids and
Simple Method (IS-MRM) to Monitor Lysophospholipids and Phospholipids During LC-MS Method Development via In-Source CID James Little, Eastman Chemical Company, Kingsport, TN Overview Phospholipids and
Rec Alkaline Presentation. Jarmo Pudas, Development Director
 Rec Alkaline Presentation Jarmo Pudas, Development Director Alkaline battery ingredients All types of batteries contain electrolysis main element allowing current to flow through the specific mixture.
Rec Alkaline Presentation Jarmo Pudas, Development Director Alkaline battery ingredients All types of batteries contain electrolysis main element allowing current to flow through the specific mixture.
New generation of phosphate-esters for MWF: balancing performance, labeling and economics.
 New generation of phosphate-esters for MWF: balancing performance, labeling and economics. Claude-Emmanuel Hédoire 1), 1) Solvay Novecare, Aubervilliers, France 1 Introduction Phosphate-esters are well
New generation of phosphate-esters for MWF: balancing performance, labeling and economics. Claude-Emmanuel Hédoire 1), 1) Solvay Novecare, Aubervilliers, France 1 Introduction Phosphate-esters are well
4 Types of Organic Polar Reactions
 Objective 12 Apply Reactivity Principles to Electrophilic Addition Reactions 1: Alkenes Identify structural features (pi bond) and electrophiles Use curved arrows to predict product 4 Types of Organic
Objective 12 Apply Reactivity Principles to Electrophilic Addition Reactions 1: Alkenes Identify structural features (pi bond) and electrophiles Use curved arrows to predict product 4 Types of Organic
Analysis of HMF by HPLC
 COST Action 927 Training School Building Skills on the Analysis of Thermal Process Contaminants in Foods 22-26 October 2007, Ankara Analysis of HMF by HPLC Vural Gökmen O O OH Background O COOH O R 2 Carbonyl
COST Action 927 Training School Building Skills on the Analysis of Thermal Process Contaminants in Foods 22-26 October 2007, Ankara Analysis of HMF by HPLC Vural Gökmen O O OH Background O COOH O R 2 Carbonyl
Automated Purification and Analytical Reinjection of a Small Molecule Drug, Probenecid, on a Gilson LC/MS Dual Function System
 Automated Purification and Analytical Reinjection of a Small Molecule Drug, Probenecid, on a Gilson LC/MS Dual Function System Keywords Introduction Application Note PHA0413 High Pressure Liquid Chromatography
Automated Purification and Analytical Reinjection of a Small Molecule Drug, Probenecid, on a Gilson LC/MS Dual Function System Keywords Introduction Application Note PHA0413 High Pressure Liquid Chromatography
Thermal Degradation Studies of Electronic Cigarette Liquids Part 2: Development of a Model Reaction System Used to Study α-dicarbonyl Formation
 Thermal Degradation Studies of Electronic Cigarette Liquids Part 2: Development of a Model Reaction System Used to Study α-dicarbonyl Formation Melvin, M.S.; Avery, K.C.; Ballentine, R.M.; Gardner, W.P.;
Thermal Degradation Studies of Electronic Cigarette Liquids Part 2: Development of a Model Reaction System Used to Study α-dicarbonyl Formation Melvin, M.S.; Avery, K.C.; Ballentine, R.M.; Gardner, W.P.;
PROPOSAL FOR REVISION OF MONOGRAPH PUBLISHED IN The International Pharmacopoeia: REVISION OF ph test ABACAVIR ORAL SOLUTION (JULY 2012)
 July 2012 RESTRICTED PROPOSAL FOR REVISION OF MONOGRAPH PUBLISHED IN The International Pharmacopoeia: REVISION OF ph test ABACAVIR ORAL SOLUTION (JULY 2012) PROPOSED REVISION FOR COMMENT The background
July 2012 RESTRICTED PROPOSAL FOR REVISION OF MONOGRAPH PUBLISHED IN The International Pharmacopoeia: REVISION OF ph test ABACAVIR ORAL SOLUTION (JULY 2012) PROPOSED REVISION FOR COMMENT The background
ABACAVIR SULFATE Proposal for revision of The International Pharmacopoeia (August 2012)
 August 2012 RESTRICTED ABACAVIR SULFATE Proposal for revision of The International Pharmacopoeia (August 2012) Draft for comment This document was provided by a quality control expert. Should you have
August 2012 RESTRICTED ABACAVIR SULFATE Proposal for revision of The International Pharmacopoeia (August 2012) Draft for comment This document was provided by a quality control expert. Should you have
Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond
 Biology 12 - Biochemistry Practice Exam KEY Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Biology 12 - Biochemistry Practice Exam KEY Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Chapter Three (Biochemistry)
 Chapter Three (Biochemistry) 1 SECTION ONE: CARBON COMPOUNDS CARBON BONDING All compounds can be classified in two broad categories: organic compounds and inorganic compounds. Organic compounds are made
Chapter Three (Biochemistry) 1 SECTION ONE: CARBON COMPOUNDS CARBON BONDING All compounds can be classified in two broad categories: organic compounds and inorganic compounds. Organic compounds are made
CORESTA RECOMMENDED METHOD NÄ 9
 CORESTA RECOMMENDED METHOD NÄ 9 DETERMINATION OF NICOTINE IN CIGARETTE FILTERS BY GAS CHROMATOGRAPHIC ANALYSIS (April 2009) 0. INTRODUCTION In 2001 the CORESTA Routine Analytical Chemistry Sub-Group was
CORESTA RECOMMENDED METHOD NÄ 9 DETERMINATION OF NICOTINE IN CIGARETTE FILTERS BY GAS CHROMATOGRAPHIC ANALYSIS (April 2009) 0. INTRODUCTION In 2001 the CORESTA Routine Analytical Chemistry Sub-Group was
Organic Chemistry Diversity of Carbon Compounds
 Organic Chemistry Diversity of Carbon Compounds Hydrocarbons The Alkanes The Alkenes The Alkynes Naming Hydrocarbons Cyclic Hydrocarbons Alkyl Groups Aromatic Hydrocarbons Naming Complex Hydrocarbons Chemical
Organic Chemistry Diversity of Carbon Compounds Hydrocarbons The Alkanes The Alkenes The Alkynes Naming Hydrocarbons Cyclic Hydrocarbons Alkyl Groups Aromatic Hydrocarbons Naming Complex Hydrocarbons Chemical
Bottom Ash Data Week 38
 Bottom Ash Data 2018 Week 38 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on October 4, 2018. The data represents bottom ash composite results for
Bottom Ash Data 2018 Week 38 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on October 4, 2018. The data represents bottom ash composite results for
New Annex 15 Updated Requirements & Approach to Validation. Presented by Ashley Isbel 10 th August 2015
 New Annex 15 Updated Requirements & Approach to Validation Presented by Ashley Isbel 10 th August 2015 EU GMP Guide Annex 15 Qualification & Validation 2001 Current version of Annex 15 published Nov 2012
New Annex 15 Updated Requirements & Approach to Validation Presented by Ashley Isbel 10 th August 2015 EU GMP Guide Annex 15 Qualification & Validation 2001 Current version of Annex 15 published Nov 2012
Key Advantages of Comprehensive Cannabis Analysis
 Comprehensive Cannabis Analysis: Pesticides, Aflatoxins, Terpenes, and High Linear Dynamic Range Potency from One Extract Using One Column and One Solvent System Robert Di Lorenzo 1, Diana Tran 2, KC Hyland
Comprehensive Cannabis Analysis: Pesticides, Aflatoxins, Terpenes, and High Linear Dynamic Range Potency from One Extract Using One Column and One Solvent System Robert Di Lorenzo 1, Diana Tran 2, KC Hyland
Product Safety Assessment Propoxylated/Ethoxylated Ethylenediamine Polyol
 Product Safety Assessment Propoxylated/Ethoxylated Ethylenediamine Polyol Select a Topic: Names Product Overview Manufacture of Product Product Description Product Uses Exposure Potential Health Information
Product Safety Assessment Propoxylated/Ethoxylated Ethylenediamine Polyol Select a Topic: Names Product Overview Manufacture of Product Product Description Product Uses Exposure Potential Health Information
8 Micronutrients Overview & Dietary Reference Intakes (DRIs)
 8 Micronutrients Overview & Dietary Reference Intakes (DRIs) Micronutrients consist of vitamins and minerals. In this chapter, an overview of vitamins and minerals will be presented followed by a description
8 Micronutrients Overview & Dietary Reference Intakes (DRIs) Micronutrients consist of vitamins and minerals. In this chapter, an overview of vitamins and minerals will be presented followed by a description
Carbohydrates. Objectives. Background. Experiment 6
 1 of 6 3/15/2011 7:27 PM Experiment 6 Carbohydrates Objectives During this experiment you will look at some of the physical and chemical properties of carbohydrates. Many of the carbohydrates, especially
1 of 6 3/15/2011 7:27 PM Experiment 6 Carbohydrates Objectives During this experiment you will look at some of the physical and chemical properties of carbohydrates. Many of the carbohydrates, especially
Acrylamide in Foods: An Important International Issue
 Acrylamide in Foods: An Important International Issue 2013 Fera-JIFSAN Annual Symposium FDA, Harvey W. Wiley Bldg Auditorium College Park, MD June 12-13, 2013 Acrylamide in Foods Acrylamide - industrial
Acrylamide in Foods: An Important International Issue 2013 Fera-JIFSAN Annual Symposium FDA, Harvey W. Wiley Bldg Auditorium College Park, MD June 12-13, 2013 Acrylamide in Foods Acrylamide - industrial
Introduction to Biochemistry
 Life is Organized in Increasing Levels of Complexity Introduction to Biochemistry atom simple molecule What is the chemical makeup of living things? macromolecule organ organ system organism organelle
Life is Organized in Increasing Levels of Complexity Introduction to Biochemistry atom simple molecule What is the chemical makeup of living things? macromolecule organ organ system organism organelle
STEFES GMBH D Hamburg, Wendenstr. 21b Tel +49(0) Fax +49(0)
 Sanovita Produktions- und Vertriebs GmbH D-78532 Tuttlingen, Bahnhofstrasse 71 Telefon: +49 (0) 7461 9335-0 Telefax: +49 (0) 7461 9335-44 info@sanovita-gmbh.de www.sanovita-gmbh.de STEFES GMBH D- 20097
Sanovita Produktions- und Vertriebs GmbH D-78532 Tuttlingen, Bahnhofstrasse 71 Telefon: +49 (0) 7461 9335-0 Telefax: +49 (0) 7461 9335-44 info@sanovita-gmbh.de www.sanovita-gmbh.de STEFES GMBH D- 20097
