Surgical Technique SUSTAIN -O. Radiolucent Spacer
|
|
|
- Oswin Wilson
- 5 years ago
- Views:
Transcription
1 Surgical Technique SUSTAIN -O Radiolucent Spacer
2 At Globus, we move with a sense of urgency to deliver innovations that improve the quality of life for patients with spinal disorders. We are inspired by the needs of these patients and also the needs of the surgeons and health care providers who treat them. This passion combined with Globus world class engineering transforms clinical insights into tangible spine care solutions. We are driven to provide the highest quality products to improve the techniques and outcomes of spine surgery so patients can resume their lives as quickly as possible. We extend our reach beyond our world class implants, instrumentation, and service by partnering with researchers and educators to advance the science and knowledge of spine care. The energy and enthusiasm each of us bring everyday to Globus is palpable. We are constantly in the pursuit of better patient care and understand that speed is critical because life cannot wait. 2
3 SUSTAIN -O Radiolucent Spacer SUSTAIN -O is an interbody fusion device made from radiolucent polymer (PEEK). The rounded corners, grip-style connection, and robust implant holder allow control during insertion and positioning. The anterior portion of the implant has a tapered leading edge for ease of insertion. The inferior and superior surfaces of the implant feature teeth to resist expulsion. To accomodate varying patient anatomy, SUSTain -O is offered in several height, width, and length configurations. SUSTAIN -O Radiolucent Spacer 1
4 SUSTAIN -O radiolucent spacer Directional teeth for expulsion resistance Radiographic markers confirm placement of implant Grip style connection for controlled implant positioning Tapered leading edge for easy insertion 2
5 Contents Implant Overview 4 Instrument Overview 5 Surgical Technique 1. Approach Creating Unilateral Access Endplate Preparation Distraction 11 Distraction Using Paddle Distractors, Trials or Scrapers Sizing 13 Sizing the Disc Space Using the Paddle Distractors and Scrapers Insertion 15 SUSTAIN -O Small Implant Set 16 SUSTAIN -O Implant Set 18 PRESERVE Posterior Unilateral Instrument Set 20 Posterior Disc Prep Instrument Set I 22 Posterior Disc Prep Instrument Set II 24 Additionally Available 26 Important Information 27 The Surgical Technique shown is for illustrative purposes only. The technique(s) actually employed in each case always depends on the medical judgment of the surgeon exercised before and during surgery as to the best mode of treatment for each patient. Additionally, as instruments may occasionally be updated, the instruments depicted in this Surgical Technique may not be exactly the same as the instruments currently available. Please consult with your sales representative or contact Globus directly for more information. SUSTAIN -O Radiolucent Spacer 3
6 implant Overview Features Tapered leading edge for easier insertion Rounded corners allow for rotation during insertion Grip-style connection for controlled implant positioning Teeth to resist explusion 1mm height increments for accurate fit 22mm, 26mm and 30mm lengths to accommodate patient anatomy 8mm, 10mm, and 12mm width options for an ideal fit 2mm (marker to edge) 8mm 8 13mm in 1mm increments, 15mm & 17mm 7 Lordosis Length 22mm 3mm (marker to edge) 10mm 7 Lordosis Length 26mm 3mm (marker to edge) 12mm 7 Lordosis Length 30mm 4
7 instrument Overview Distraction Instruments Scrapers Height Part No. 8mm mm mm mm mm mm mm mm Paddle Distractors Height Part No. 8mm mm mm mm mm mm mm mm SUSTAIN -O Radiolucent Spacer 5
8 Trials Trials, 8mm Wide x 22mm Long Trials, 10mm Wide x 22mm Long Height 22mm Height 22mm 8mm mm mm mm mm mm mm mm mm mm mm mm mm mm mm mm Trials, 10mm Wide x 26mm Long Trials, 10mm Wide x 30mm Long Height 26mm Height 30mm 8mm mm mm mm mm mm mm mm mm mm mm mm mm mm mm mm
9 Holder Instruments Implant Holder Holder, Straight Holder, Angled T-Handle SUSTAIN -O Radiolucent Spacer 7
10 MIS Instruments MIS Holder MIS Hex Driver, 2.5mm and 1.5mm MIS Handle Implant Jaw with Teeth, SUSTAIN -O Small Implant Jaw with Teeth, SUSTAIN -O 10mm Wide Implant Jaw with Teeth, SUSTAIN -O 12mm Wide Implant Jaw without Teeth, SUSTAIN -O Small Implant Jaw without Teeth, SUSTAIN -O 10mm Wide Implant Jaw without Teeth, SUSTAIN -O 12mm Wide
11 MIS Instruments (cont d) Slide Hammer, Quick Disconnect MIS Slap Hammer, Shaft 29mm MIS Holder MIS Handle (Assembled) MIS Holder MIS Handle MIS Slap Hammer, Shaft, 29mm Slide Hammer, Quick Disconnect (Assembled) SUSTAIN -O Radiolucent Spacer 9
12 sustain -o surgical technique Step 1 approach The patient is placed under anesthesia and positioned prone. Lateral C-arm fluoroscopy or other radiographic methods can be utilized throughout the surgery to ensure correct graft placement. The operative area is carefully cleaned and an incision is made at the appropriate fusion level(s). In addition to the described interbody fusion technique, supplemental posterior stabilization must be used at the appropriate level(s). Using an osteotome to create unilateral access Step 2 creating Unilateral Access Use an osteotome and a laminectomy punch to remove the inferior facet of the cranial vertebrae and the superior facet of the caudal vertebrae of the appropriate level(s). This creates a working unilateral access window to the disc. 10
13 Step 3 endplate Preparation Remove gross disc material with rongeurs or other suitable instruments. Insert the smallest Scraper into the disc space for further disc removal and endplate preparation, moving to larger Scrapers as needed. Careful disc removal and endplate preparation maximizes the potential for a successful fusion. Note: The anterior and lateral walls of the annulus should be preserved to provide peripheral support for the implant. Preparing endplates with scrapers Step 4 Distraction Distraction of the disc space aids in visualization as well as decompression and restoration of disc height. Distraction may be achieved using CREO, REVERE, or REVOLVE pedicle screws. Distraction using REVERE screws SUSTAIN -O Radiolucent Spacer 11
14 Distraction (cont d) Distraction Using Paddle Distractors, Trials or Scrapers To use the Paddle Distractors for distraction, begin with the smallest distractor and insert, using larger sizes until the desired distraction is achieved. Distraction of disc space using the Paddle Distractor Paddle Distractor in disc space To use the Scraper for distraction, begin with the smallest Scraper and insert, using larger sizes until the desired distraction is achieved. Distraction of disc space using the Scraper Scraper in disc space To use the Trial for distraction, begin with the smallest Trial and insert, using larger sizes until the desired distraction is achieved. Distraction of disc space using the Trial Trial in disc space Note: Use caution while using scrapers or Paddle Distractors for distraction to avoid damage to the endplates. 12
15 Step 5 Sizing Assemble the desired Trial onto the T-Handle. Insert the smallest Trial into the disc space, using gentle impaction if needed. Determine which Trial and corresponding graft best fits the prepared disc space. A secure fit is desirable in order to maintain disc height and stabilize the segment, and can be confirmed using fluoroscopy and tactile feel. Sizing the disc space using the Trial SUSTAIN -O Radiolucent Spacer 13
16 Sizing (cont d) Sizing the Disc Space Using the Paddle Distractors and Scrapers Alternately, Paddle Distractors and Scrapers may be used to size the disc space, inserting horizontally, and rotating to determine the appropriate height. Note: Use caution while using Scrapers or Paddle Distractors for sizing to avoid damage to the endplates. Sizing the disc space using Paddle Distractor Sizing disc space using Scraper 14
17 Step 6 insertion Select an appropriately sized spacer and fill the chamber with autogenous bone graft material. Insert into the intervertebral space using the Holder. To seat the spacer, gently impact the Holder until the spacer is in the desired position. The spacer should be recessed into the disc space. Supplemental autogenous bone graft material should be placed around the spacer. Compression may be necessary to help restore sagittal alignment and resist posterior migration. Supplemental fixation using CREO, REVERE or REVOLVE pedicle screws is required. Final implant position SUSTAIN -O Radiolucent Spacer 15
18 16 SUSTAIN -O SMALL IMPLANT SET
19 SUSTAIN -O Small Implant Set SUSTAIN Radiolucent Spacer Oblique, Small, 8x22 Length Part No. Qty 8mm mm mm mm mm mm mm mm mm mm SUSTAIN Radiolucent Spacer Oblique, Small, 10x22 Length Part No. Qty 8mm mm mm mm mm mm mm mm mm mm SUSTAIN Radiolucent Oblique, Small, Module Assembly Items highlighted in gray are additionally available. SUSTAIN -O Radiolucent Spacer 17
20 18 SUSTAIN -O IMPLANT SET
21 SUSTAIN -O Implant Set SUSTAIN Radiolucent Spacer Oblique, 10x26 Length Part No. Qty 8mm mm mm mm mm mm mm mm mm mm SUSTAIN Radiolucent Spacer Oblique, 12x26 Length Part No. Qty 8mm mm mm mm mm mm mm mm mm mm SUSTAIN Radiolucent Spacer Oblique, 10x30 Length Part No. Qty 8mm mm mm mm mm mm mm mm mm mm SUSTAIN Radiolucent Spacer Oblique, 12x30 Length Part No. Qty 8mm mm mm mm mm mm mm mm mm mm SUSTAIN Radiolucent Oblique, Implant Module Items highlighted in gray are additionally available. SUSTAIN -O Radiolucent Spacer 19
22 PRESERVE INSTRUMENT SET
23 PRESERVE Posterior Unilateral Instrument Set Instruments Qty Instruments Qty Implant Holder T-Handle Paddle Distractor, 15mm Paddle Distractor, 17mm Holder, Straight Slide Hammer, Quick Disconnect Holder, Angled Trial Shaft, 8x22mm wide, 8mm, Trial, SUSTAIN -R Oblique, 26mm length, 8mm 1 SUSTAIN Oblique, Small Trial, SUSTAIN -R Oblique, 26mm length, 9mm Trial, SUSTAIN -R Oblique, 26mm length, 10mm Trial, SUSTAIN -R Oblique, 26mm length, 11mm Trial Shaft, 8x22mm, 9mm, SUSTAIN Oblique, Small Trial Shaft, 8x22mm, 10mm, SUSTAIN Oblique, Small Trial, SUSTAIN -R Oblique, 26mm length, 12mm Trial Shaft, 8x22mm wide, 11mm, Trial, SUSTAIN -R Oblique, 26mm length, 13mm 1 SUSTAIN Oblique, Small Trial, SUSTAIN -R Oblique, 26mm length, 15mm Trial Shaft, 8x22mm wide, 12mm, Trial, SUSTAIN -R Oblique, 26mm length, 17mm 1 SUSTAIN Oblique, Small Trial, SUSTAIN -R Oblique, 30mm length, 8mm Trial, SUSTAIN -R Oblique, 30mm length, 9mm Trial, SUSTAIN -R Oblique, 30mm length, 10mm Trial Shaft, 8x22mm wide, 13mm, SUSTAIN Oblique, Small Trial Shaft, 8x22mm wide, 15mm, SUSTAIN Oblique, Small Trial, SUSTAIN -R Oblique, 30mm length, 11mm Trial Shaft, 8x22mm wide, 17mm, Trial, SUSTAIN -R Oblique, 30mm length, 12mm 1 SUSTAIN Oblique, Small Trial, SUSTAIN -R Oblique, 30mm length, 13mm Trial Shaft, 10x22mm wide, 8mm, Trial, SUSTAIN -R Oblique, 30mm length, 15mm Trial, SUSTAIN -R Oblique, 30mm length, 17mm Scraper, Oblique, 8mm Scraper, Oblique, 9mm 1 SUSTAIN Oblique, Small Trial Shaft, 10x22mm wide, 9mm, SUSTAIN Oblique, Small Trial Shaft, 10x22mm wide, 10mm, SUSTAIN Oblique, Small Scraper, Oblique, 10mm Trial Shaft, 10x22mm wide, 11mm, Scraper, Oblique, 11mm 1 SUSTAIN Oblique, Small Scraper, Oblique, 12mm Scraper, Oblique, 13mm Scraper, Oblique, 15mm Scraper, Oblique, 17mm Paddle Distractor, 8mm Trial Shaft, 10x22mm wide, 12mm, SUSTAIN Oblique, Small Trial Shaft, 10x22mm wide, 13mm, SUSTAIN Oblique, Small Trial Shaft, 10x22mm wide, 15mm, SUSTAIN Oblique, Small Paddle Distractor, 9mm Trial Shaft, 10x22mm wide, 17mm, Paddle Distractor, 10mm 1 SUSTAIN Oblique, Small Paddle Distractor, 11mm PRESERVE Posterior Unilateral Instruments Paddle Distractor, 12mm Paddle Distractor, 13mm 1 SUSTAIN -O Radiolucent Spacer 21
24 POSTERIOR DISC PREP INSTRUMENTS I SET
25 Posterior Disc Prep Instruments I Set Instruments Qty Push Rod Assembly, Bone Funnel Nerve Retractor, 5mm, Suction Nerve Retractor, Corner Nerve Root Retractor, Fine, 5mm Nerve Root Retractor, Medium, 10mm Bone Funnel Bone Funnel - Tube Disc Rongeur, 250x2mm, Straight Disc Rongeur, 250x2mm, Up Biting Disc Rongeur, 250x4mm, Straight Disc Rongeur, 250x6mm, Straight Disc Rongeur, 250x4mm, Up Biting Disc Rongeur, 250x4mm, Down Biting Kerrison, 250x3mm, Straight Kerrison, 250x5mm, Straight Lamina Spreader, Hinged Graphic Case SUSTAIN -O Radiolucent Spacer 23
26 POSTERIOR DISC PREP INSTRUMENTS II SET
27 Posterior Disc Prep Instruments II Set Instruments Qty Bone Curette, 6.5x9.5mm, Straight Bone Curette, 6.5x9.5mm, Right Bone Curette, 6.5x9.5mm, Left Bone Curette, 6.5x9.5mm, Up Pushing Bone Curette, 6.5x9.5mm, Down Pushing Rake, 8mm, Straight Rake, 8mm, Angled Bone Curette, 5.0x7.5mm, Straight Bone Curette, 5.0x7.5mm, Up Pushing Bone Curette, 5.0x7.5mm, Down Pushing Bone Curette, 8.0x11.5mm, Straight Bone Curette, 8.0x11.5mm, Right Bone Curette, 8.0x11.5mm, Left Bone Curette, 5.0x10mm, Axial Osteotome, 7mm Rasp, 8x20mm, Knurled, Straight Rasp, 8x20mm, Knurled, Angled Ring Curette, 6mm, Straight Ring Curette, 6mm, Angled Right Ring Curette, 6mm, Angled Left Graphic Case II SUSTAIN -O Radiolucent Spacer 25
28 Additionally Available Part No. Descriptions Trial, SUSTAIN -R Oblique 26mm length, 14mm Trial, SUSTAIN -R Oblique, 26mm length, 16mm Trial, SUSTAIN -R Oblique 30mm length, 14mm Trial, SUSTAIN -R Oblique, 30mm length, 16mm Scraper, Oblique, 7mm Scraper, Oblique, 14mm Scraper, Oblique, 16mm Paddle Distractor, 7mm Paddle Distractor, 14mm Paddle Distractor, 16mm MIS Holder MIS Hex Driver, 2.5mm and 1.5mm MIS Handle Implant Jaw, with Teeth, Sustain -O Small Implant Jaw, without Teeth, SUSTAIN -O small Implant Jaw, with Teeth, Sustain -O, 10mm Wide Implant Jaw, without Teeth, SUSTAIN -O 10mm Wide Implant Jaw, with Teeth, Sustain -O, 12mm Wide Implant Jaw, without Teeth, SUSTAIN -O 12mm Wide MIS Slap Hammer, Shaft, 29mm Slide Hammer, Quick Disconnect Trial Shaft, 8mm wide,14mm, SUSTAIN Oblique, Small Trial Shaft, 8mm wide, 16mm, SUSTAIN Oblique, Small Trial Shaft, 10mm wide,14mm, SUSTAIN Oblique, Small Trial Shaft, 10mm wide, 16mm, SUSTAIN Oblique, Small 26
29 IMPORTANT INFORMATION ON THE sustain and sustain -r spacers DESCRIPTION SUSTAIN and SUSTAIN Radiolucent (SUSTAIN -R) Spacers are devices that can be used as intervertebral fusion devices or as vertebral body replacement devices. These spacers are available in different shapes and heights to accommodate various surgical approaches and anatomical needs. Protrusions on the superior and inferior surfaces of each device grip the endplates of the adjacent vertebrae to resist expulsion. Each spacer has an axial hole to allow grafting material to be packed inside the spacer. These spacers are used to provide structural stability in skeletally mature individuals following discectomy, corpectomy, or vertebrectomy (including partial). Lumbar spacers may be inserted using a posterior, transforaminal, anterior, anterolateral, or lateral lumbar approach. Cervical spacers are inserted using an anterior cervical approach. The SUSTAIN Spacers are made from commercially pure titanium or titanium alloy as specified in ASTM F67, F136, and F1295. The SUSTAIN R Spacers are made from radiolucent PEEK polymer with titanium alloy or tantalum markers as specified in ASTM F136, F560, F1295, and F2026. INDICATIONS When used as lumbar intervertebral body fusion devices, SUSTAIN and SUSTAIN Radiolucent (SUSTAIN R) Spacers are intended for use in patients with degenerative disc disease (DDD) at one or two contiguous levels of the lumbosacral spine (L2-S1). DDD is defined as discogenic back pain with degeneration of the disc confirmed by history and radiographic studies. These patients should be skeletally mature and have had at least six (6) months of non-operative treatment. In addition, these patients may have up to Grade 1 spondylolisthesis or retrolisthesis at the involved level(s). The SUSTAIN and SUSTAIN -R Spacers are to be filled with autogenous bone graft material. These devices are intended to be used with supplemental fixation, such as the REVERE, REVOLVE or BEACON Stabilization Systems. When used as cervical intervertebral body fusion devices, the SUSTAIN and SUSTAIN -R Spacers are intended for use in skeletally mature patients with degenerative disc disease (DDD) of the cervical spine (C2-T1) at one level. DDD is defined as discogenic pain with degeneration of the disc confirmed by history and radiographic studies. These patients should be skeletally mature and have had at least six (6) weeks of non-operative treatment. The SUSTAIN and SUSTAIN -R Spacers are to be filled with autogenous bone graft material. These devices are intended to be used with supplemental fixation, such as the ASSURE, PROVIDENCE or XTEND Anterior Cervical Plate Systems. When used as vertebral body replacement devices, SUSTAIN and SUSTAIN -R Spacers are intended for use in the thoracolumbar spine (T1-L5) to replace a collapsed, damaged or unstable vertebral body due to tumor or trauma (i.e., fracture). The spacers are intended to be used with supplemental spinal fixation systems that have been labeled for use in the thoracic and/or lumbar spine (i.e., posterior pedicle screw and rod systems, anterior plate systems and anterior screw and rod systems). The interior of the spacer can be packed with bone grafting material. SUSTAIN and SUSTAIN -R Spacers are designed to provide anterior spinal column support even in the absence of fusion for a prolonged period. WARNINGS One of the potential risks identified with this system is death. Other potential risks which may require additional surgery, include: device component fracture, loss of fixation, non-union, fracture of the vertebrae, neurological injury, and vascular or visceral injury. Interbody fusion devices for the treatment of degenerative conditions are designed to withstand both full load bearing and the loads associated with long-term use which could result from the presence of non-union or delayed union. Certain degenerative diseases or underlying physiological conditions such as diabetes, rheumatoid arthritis, or osteoporosis may alter the healing process, thereby increasing the risk of implant breakage or spinal fracture. These warnings do not include all adverse effects which could occur with surgery in general, but are important considerations particular to orthopedic implants. General surgical risks should be explained to the patient prior to surgery. Use this device as supplied and in accordance with the handling and use information provided below. PRECAUTIONS The implantation of these devices should be performed only by experienced spinal surgeons with specific training in the use of this system because this is a technically demanding procedure presenting a risk of serious injury to the patient. Preoperative planning and patient anatomy should be considered when selecting implant size. Surgical implants must never be reused. An explanted implant must never be reimplanted. Even though the device appears undamaged, it may have small defects and internal stress patterns which could lead to breakage. Adequately instruct the patient. Mental or physical impairment which compromises or prevents a patient s ability to comply with necessary limitations or precautions may place that patient at a particular risk during postoperative rehabilitation. For optimal implant performance, the surgeon should consider the levels of implantation, patient weight, patient activity level, other patient conditions, etc. which may impact the performance of the system. SUSTAIN and SUSTAIN -R Spacers have not been evaluated for safety and compatibility in the MR environment. SUSTAIN and SUSTAIN -R Spacers have not been tested for heating or migration in the MR environment. CONTRAINDICATIONS Use of SUSTAIN and SUSTAIN -R Spacer(s) is contraindicated in patients with the following conditions: 1. Active systemic infection, infection localized to the site of the proposed implantation, or when the patient has demonstrated allergy or foreign body sensitivity to any of the implant materials. 2. Prior fusion at the level(s) to be treated. 3. Severe osteoporosis may prevent adequate fixation and thus preclude the use of this or any other orthopedic implant. 4. Conditions that may place excessive stresses on bone and implants, such as severe obesity or degenerative diseases, are relative contraindications. The decision whether to use these devices in such conditions must be made by the physician taking into account the risks versus the benefits to the patient. 5. Patients whose activity, mental capacity, mental illness, alcoholism, drug abuse, occupation, or lifestyle may interfere with their ability to follow postoperative restrictions and who may place undue stresses on the implant during bony healing and may be at a higher risk of implant failure. 6. Any condition not described in the indications for use. COMPLICATIONS AND POSSIBLE ADVERSE EVENTS Prior to surgery, patients should be made aware of the following possible adverse effects in addition to the potential need for additional surgery to correct these effects: Loosening, bending or breakage of components Displacement/migration of device components Tissue sensitivity to implant material Potential for skin breakdown and/or wound complications Non-union or delayed union or mal-union Infection Nerve damage, including loss of neurological function (sensory and/or motor), paralysis, dysesthesia, hyperesthesia, paresthesia, radiculopathy, reflex deficit, cauda equina syndrome Dural tears, cerebral spinal fluid leakage Fracture of vertebrae Foreign body reaction (allergic) to components or debris Vascular or visceral injury SUSTAIN -O Radiolucent Spacer 27
30 IMPORTANT INFORMATION ON THE sustain and sustain -r spacers (cont d) Change in spinal curvature, loss of correction, height and/or reduction Urinary retention or loss of bladder control or other types of disorders of the urogenital system Ileus, gastritis, bowel obstruction or other types of gastrointestinal system compromise Reproductive system compromise including impotence, sterility, loss of consortium and sexual dysfunction. Pain or discomfort Bursitis Decrease in bone density due to stress shielding Loss of bone or fracture of bone above or below the level of surgery Bone graft donor site pain, fracture, and/or delayed wound healing Restriction of activities Lack of effective treatment of symptoms for which surgery was intended Need for additional surgical intervention Death PACKAGING SUSTAIN implants may be supplied pre-packaged and sterile, using gamma irradiation. The integrity of the sterile packaging should be checked to ensure that sterility of the contents is not compromised. Packaging should be carefully checked for completeness and all components should be carefully checked to ensure that there is no damage prior to use. Damaged packages or products should not be used, and should be returned to Globus Medical. During surgery, after the correct size has been determined, remove the implants from the packaging using aseptic technique. The instrument sets are provided nonsterile and are steam sterilized prior to use, as described in the STERILIZATION section below. Following use or exposure to soil, instruments must be cleaned, as described in the CLEANING section below. HANDLING All instruments and implants should be treated with care. Improper use or handling may lead to damage and/or possible malfunction. Instruments should be checked to ensure that they are in working order prior to surgery. All instruments should be inspected prior to use to ensure that there is no unacceptable deterioration such as corrosion, discoloration, pitting, cracked seals, etc. Non-working or damaged instruments should not be used, and should be returned to Globus Medical. CLEANING All instruments that can be disassembled must be disassembled for cleaning. All handles must be detached. Instruments may be reassembled following sterilization. The instruments should be cleaned using neutral cleaners before sterilization and introduction into a sterile surgical field or (if applicable) return of the product to Globus Medical. Cleaning and disinfecting of instruments can be performed with aldehydefree solvents at higher temperatures. Cleaning and decontamination must include the use of neutral cleaners followed by a deionized water rinse. Note: certain cleaning solutions such as those containing formalin, glutaraldehyde, bleach and/or other alkaline cleaners may damage some devices, particularly instruments; these solutions should not be used. The following cleaning methods should be observed when cleaning instruments after use or exposure to soil, and prior to sterilization: 1. Immediately following use, ensure that the instruments are wiped down to remove all visible soil and kept from drying by submerging or covering with a wet towel. 2. Disassemble all instruments that can be disassembled. 3. Rinse the instruments under running tap water to remove all visible soil. Flush the lumens a minimum of 3 times, until the lumens flush clean. 4. repare Enzol (or a similar enzymatic detergent) per manufacturer s recommendations. 5. Immerse the instruments in the detergent and allow them to soak for a minimum of 2 minutes. 6. Use a soft bristled brush to thoroughly clean the instruments. Use a pipe cleaner for any lumens. Pay close attention to hard to reach areas. 7. Using a sterile syringe, draw up the enzymatic detergent solution. Flush any lumens and hard to reach areas until no soil is seen exiting the area. 8. Remove the instruments from the detergent and rinse them in running warm tap water. 9. Prepare Enzol (or a similar enzymatic detergent) per manufacturer s recommendations in an ultrasonic cleaner. 10. Completely immerse the instruments in the ultrasonic cleaner and ensure detergent is in lumens by flushing the lumens. Sonicate for a minimum of 3 minutes. 11. Remove the instruments from the detergent and rinse them in running deionized water or reverse osmosis water for a minimum of 2 minutes. 12. Dry instruments using a clean soft cloth and filtered pressurized air. 13. Visually inspect each instrument for visible soil. If visible soil is present, then repeat cleaning process starting with Step 3. CONTACTIN FORMATION Globus Medical may be contacted at GLOBUS1 ( ). A surgical technique manual may be obtained by contacting Globus Medical. STERILIZATION SUSTAIN implants are available sterile or nonsterile. The instruments used with these devices are only available nonsterile. Sterile SUSTAIN implants are sterilized by gamma radiation, validated to ensure a Sterility Assurance Level (SAL) of Sterile implants are packaged in a heat sealed, double foil pouch. The expiration date is provided in the package label. Implants are considered sterile unless the packaging has been opened or damaged. Nonsterile SUSTAIN implants and instruments have been validated to ensure an SAL of The use of an FDAcleared wrap is recommended, per the Association for the Advancement of Medical Instrumentation (AAMI) ST79, Comprehensive Guide to Steam Sterilization and Sterility Assurance in Health Care Facilities. It is the end user s responsibility to use only sterilizers and accessories (such as sterilization wraps, sterilization pouches, chemical indicators, biological indicators, and sterilization cassettes) that have been cleared by the FDA for the selected sterilization cycle specifications (time and temperature). When using a rigid sterilization container, the following must be taken into consideration for proper sterilization of Globus devices and loaded graphic cases: Recommended sterilization parameters are listed in the table below. Only FDA-cleared rigid sterilization containers for use with pre-vacuum steam sterilization may be used. When selecting a rigid sterilization container, it must have a minimum filter area of 176 in 2 total, or a minimum of four (4) 7.5in diameter filters. No more than one (1) loaded graphic case or its contents can be placed directly into a rigid sterilization container. Stand-alone modules/racks or single devices must be placed, without stacking, in a container basket to ensure optimal ventilation. The rigid sterilization container manufacturer s instructions for use are to be followed; if questions arise, contact the manufacturer of the specific container for guidance. Refer to AAMI ST79 for additional information concerning the use of rigid sterilization containers. For implants and instruments provided NONSTERILE, sterilization is recommended (wrapped or containerized) as follows: Method Cycle Type Temperature Exposure Time Drying Time Steam Pre-vacuum 132º C 4 Minutes 30 Minutes (270º F) These parameters are validated to sterilize only this device. If other products are added to the sterilizer, the recommended parameters are not valid and new cycle parameters must be established by the user. The sterilizer must be properly installed, maintained, and calibrated. Ongoing testing must be performed to confirm inactivation of all forms of viable microorganisms. CAUTION: Federal (USA) Law Restricts this Device to Sale by or on the order of a Physician. REV J 28
31 Notes SUSTAIN -O Radiolucent Spacer 29
32 Globus Medical Valley Forge Business Center 2560 General Armistead Avenue Audubon, PA Customer Service: Phone GLOBUS1 (or ) Fax GLOBUS3 (or ) 2014 Globus Medical. All rights reserved. Patents pending. Life moves us is a registered trademark of Globus Medical. ASSURE, BEACON, CREO, PRESERVE, PROVIDENCE, REVERE, REVOLVE, SUSTAIN and XTEND are registered trademarks of Globus Medical. Please refer to package insert for description, indications, contraindications, warnings, precautions and other important information. P: RMS UK Limited 28 Trinity Road, Nailsea, Somerset, BS48 4NU England GMTGD
Posterior Lumbar Interbody Fusion System
 Px Posterior Lumbar Interbody Fusion System Px PEEK INTERBODY FUSION SYSTEM INDICATIONS FOR USE The Innovasis Px PEEK IBF System is an intervertebral body fusion device for use in patients with degenerative
Px Posterior Lumbar Interbody Fusion System Px PEEK INTERBODY FUSION SYSTEM INDICATIONS FOR USE The Innovasis Px PEEK IBF System is an intervertebral body fusion device for use in patients with degenerative
C-THRU Anterior Spinal System
 C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
nvp Posterior Lumbar Interbody Fusion System
 nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System
 nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
product overview Implant heights range from 8mm-20mm in 2mm increments, with two lordocic angle options of 6 and 12.
 ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
nvt Transforaminal Lumbar Interbody Fusion System
 nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
TABLE OF CONTENTS. ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2. Implant Specifications 3. Instrument Features 4
 Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
CROSS -FUSE P E E K V B R / I B F SYST E M
 S U R G I C A L T E C H N I Q U E CROSS -FUSE P E E K V B R / I B F SYST E M S U R G I C A L S Y S T E M O V E R V I E W 2 CROSS-FUSE P E E K V B R / I B F S Y S T E M S U R G I C A L T E C H N I Q U E
S U R G I C A L T E C H N I Q U E CROSS -FUSE P E E K V B R / I B F SYST E M S U R G I C A L S Y S T E M O V E R V I E W 2 CROSS-FUSE P E E K V B R / I B F S Y S T E M S U R G I C A L T E C H N I Q U E
Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF)
 Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF) Preoperative Planning Preoperative planning is necessary for the correct selection of lumbar interbody fusion devices.
Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF) Preoperative Planning Preoperative planning is necessary for the correct selection of lumbar interbody fusion devices.
Alamo T Transforaminal Lumbar Interbody System Surgical Technique
 Transforaminal Lumbar Interbody System Surgical Technique Table of Contents Indications and Device Description.............. 1 Alamo T Implant Features and Instruments...........2 Surgical Technique......................
Transforaminal Lumbar Interbody System Surgical Technique Table of Contents Indications and Device Description.............. 1 Alamo T Implant Features and Instruments...........2 Surgical Technique......................
TABLE OF CONTENTS. Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview. Implants 3. Instruments 5. Surgical Technique 10
 Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
Surgical Technique. Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion
 Surgical Technique Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 5 Disc Exposure 5 Disc and
Surgical Technique Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 5 Disc Exposure 5 Disc and
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
OPERATIVE TECHNIQUE. CONSTRUX Mini PTC. Mini PTC Spacer System
 OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
Surgical Technique. Apache Anterior Lumbar Interbody Fusion
 Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
Alamo C. Cervical Interbody System Surgical Technique. An Alliance Partners Company
 Cervical Interbody System Surgical Technique Table of Contents Indications for Use................................1 Device Description............................... 1 Alamo C Instruments..............................
Cervical Interbody System Surgical Technique Table of Contents Indications for Use................................1 Device Description............................... 1 Alamo C Instruments..............................
EXACTECH SPINE. Operative Technique. Cervical Spacer System. Surgeon focused. Patient driven. TM
 EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
PILLAR AL. Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) PEEK Spacer System OPERATIVE TECHNIQUE
 PILLAR AL PEEK Spacer System Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) OPERATIVE TECHNIQUE Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE TECHNIQUE 3 OPERATIVE
PILLAR AL PEEK Spacer System Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) OPERATIVE TECHNIQUE Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE TECHNIQUE 3 OPERATIVE
SURGICAL TECHNIQUE MANUAL. InterFuse T
 1 CONTENTS InterFuse T Product Description 3 Indications for Use 3 X-Ray Marker Locations 4 Product Specifications 4 Instrument Set 5 Step 1 Preoperative Planning 8 Patient Positioning 8 Step 2 Disc Removal
1 CONTENTS InterFuse T Product Description 3 Indications for Use 3 X-Ray Marker Locations 4 Product Specifications 4 Instrument Set 5 Step 1 Preoperative Planning 8 Patient Positioning 8 Step 2 Disc Removal
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use
 Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. Oblique Lumbar Interbody Fusion
 SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. ACDF Anterior Cervical Discectomy and Fusion
 SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
Patient Information MIS LLIF. Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine...2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine...2 General Conditions of the Spine....4 What is Spondylolisthesis....5
VTI INTERFUSE T SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. 1/20
 VTI INTERFUSE T SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. 1/20 CONTENTS InterFuse T Product Description Indications for Use X-Ray Marker Locations Product Specifications Instrument Set 3 4 5 STEP
VTI INTERFUSE T SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. 1/20 CONTENTS InterFuse T Product Description Indications for Use X-Ray Marker Locations Product Specifications Instrument Set 3 4 5 STEP
Cervical Spacer System surgical technique
 Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
HawkeyeTM Peek. surgical technique
 HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
Solitaire Anterior Spinal System
 Surgical Technique Solitaire Anterior Spinal System Independent Stabilization for the Anterior Column Available in Titanium and Contents Introduction... Page 1 Design Features... Page 2 Instruments...
Surgical Technique Solitaire Anterior Spinal System Independent Stabilization for the Anterior Column Available in Titanium and Contents Introduction... Page 1 Design Features... Page 2 Instruments...
Zeus Intervertebral Body Fusion Devices PACKAGE INSERT
 LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
Spartan S 3 Facet Screw System PACKAGE INSERT
 LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
Surgical Technique Manual
 InterFuse S Interbody Fusion System Surgical Technique Manual VERTEBRAL TECHNOLOGIES MS 4043-02 Rev. O Product Overview Introduction The VTI InterFuse S implant is an interbody fusion device that combines
InterFuse S Interbody Fusion System Surgical Technique Manual VERTEBRAL TECHNOLOGIES MS 4043-02 Rev. O Product Overview Introduction The VTI InterFuse S implant is an interbody fusion device that combines
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System
 X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
SURGICAL TECHNIQUE GUIDE TRESTLE. Anterior Cervical Plating System
 SURGICAL TECHNIQUE GUIDE TRESTLE Anterior Cervical Plating System 2 SURGICAL TECHNIQUE GUIDE SURGICAL TECHNIQUE GUIDE System Features Large window enables visualization of graft site and end plates Screw
SURGICAL TECHNIQUE GUIDE TRESTLE Anterior Cervical Plating System 2 SURGICAL TECHNIQUE GUIDE SURGICAL TECHNIQUE GUIDE System Features Large window enables visualization of graft site and end plates Screw
X-spine Systems, Inc. Spider Cervical Plating System
 X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
EFSPINE CERVICAL COMBINED SET DISC PROTHESIS ORGANIZER BOX
 EFSPINE CERVICAL COMBINED SET INSTRUMENTS CERVICAL CAGE & DISC PROTHESIS ORGANIZER BOX Cervical Thoracic Thoraco - Lumbar Sacral EFSPINE CERVICAL COMBINED SET CERVICAL IMPLANTS INTRODUCTION Cervical Disc
EFSPINE CERVICAL COMBINED SET INSTRUMENTS CERVICAL CAGE & DISC PROTHESIS ORGANIZER BOX Cervical Thoracic Thoraco - Lumbar Sacral EFSPINE CERVICAL COMBINED SET CERVICAL IMPLANTS INTRODUCTION Cervical Disc
T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system.
 T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system. One instrument, one technique Accommodates both open and MIS approaches A PEEK implant that works with patient anatomy Streamlined
T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system. One instrument, one technique Accommodates both open and MIS approaches A PEEK implant that works with patient anatomy Streamlined
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System
 X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
Gallery Laminoplasty Spine System
 Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Apache Cervical Interbody Fusion Device. Surgical Technique. Page of 13. LC-005 Rev F
 LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
VTI INTERFUSE S SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK.
 VTI INTERFUSE S SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. CONTENTS InterFuse S Product Description Indications for Use X-Ray Marker Locations and Product Specifications Instrument Set 3 4 5-7 STEP
VTI INTERFUSE S SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. CONTENTS InterFuse S Product Description Indications for Use X-Ray Marker Locations and Product Specifications Instrument Set 3 4 5-7 STEP
Amendia Interbody Fusion Devices PACKAGE INSERT
 LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
Zimmer Anterior Buttress Plate System. Surgical Technique
 Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
ACP1 CERVICAL PLATE SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE II.
 I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
Interbody fusion cage for the transforaminal approach. Travios. Surgical Technique
 Interbody fusion cage for the transforaminal approach Travios Surgical Technique Image intensifier control This description alone does not provide sufficient background for direct use of DePuy Synthes
Interbody fusion cage for the transforaminal approach Travios Surgical Technique Image intensifier control This description alone does not provide sufficient background for direct use of DePuy Synthes
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
Surgical Technique. TraXis. Precision. Transforaminal Lumbar Interbody Fusion Spacer. The Art & Science of Spine Surgery
 Surgical Technique Precision TraXis Transforaminal Lumbar Interbody Fusion Spacer The Art & Science of Spine Surgery Table of Contents Introduction 3 Indications 4 Key Instruments 5 Surgical Technique
Surgical Technique Precision TraXis Transforaminal Lumbar Interbody Fusion Spacer The Art & Science of Spine Surgery Table of Contents Introduction 3 Indications 4 Key Instruments 5 Surgical Technique
Thoracolumbar Solutions. Zyston Curve. Interbody Spacer System. Surgical Technique Guide
 Thoracolumbar Solutions Zyston Curve Interbody Spacer System Surgical Technique Guide 2 Zyston Curve Interbody Spacer System Surgical Technique Guide The Zyston Curve Interbody System is designed to optimize
Thoracolumbar Solutions Zyston Curve Interbody Spacer System Surgical Technique Guide 2 Zyston Curve Interbody Spacer System Surgical Technique Guide The Zyston Curve Interbody System is designed to optimize
Table of Contents.
 surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
Zavation Z-Link Cervical
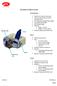 Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE
 OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
2. Active systemic infection or infection localized to the site of the proposed implantation is contraindications to implantation.
 OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
Imola Lateral IBF System Surgical Technique
 Imola Lateral IBF System Surgical Technique IMOLA CIRCUIT TABLE OF CONTENTS Design Rationale Instructions for Use Surgical Technique 1. Table Mounting 2. Surgical Planning & Targeting 3. Access and Preparation
Imola Lateral IBF System Surgical Technique IMOLA CIRCUIT TABLE OF CONTENTS Design Rationale Instructions for Use Surgical Technique 1. Table Mounting 2. Surgical Planning & Targeting 3. Access and Preparation
Battalion TM Universal Spacer System
 Battalion TM Universal Spacer System GENERAL INFORMATION: The Battalion Universal Spacer System (Battalion System) is an intervertebral body fusion device with implants of various lengths, widths, heights,
Battalion TM Universal Spacer System GENERAL INFORMATION: The Battalion Universal Spacer System (Battalion System) is an intervertebral body fusion device with implants of various lengths, widths, heights,
X-spine Systems, Inc. Certex Spinal Fixation System
 X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
Patient Information MIS LLIF. Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine....2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine....2 General Conditions of the Spine....4 What is Spondylolisthesis....5
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
L-VARLOCK. Posterior Lumbar Cage with adjustable lordosis. S urgical T echnique
 L-VARLOCK Posterior Lumbar Cage with adjustable lordosis S urgical T echnique Introduction Designed and manufactured by KISCO International, L-VARLOCK cages are made of titanium alloy Ti 6AI 4V (standards
L-VARLOCK Posterior Lumbar Cage with adjustable lordosis S urgical T echnique Introduction Designed and manufactured by KISCO International, L-VARLOCK cages are made of titanium alloy Ti 6AI 4V (standards
EIT TLIF Cage. For Natural Bone Ingrowth with EIT Cellular Titanium
 EIT TLIF Cage For Natural Bone Ingrowth with EIT Cellular Titanium EIT TLIF Cage Surgical Technique EIT Cellular Titanium provides active fusion area» ~ 80% porosity» ~ 650 µm diamond pore size» open interconnected
EIT TLIF Cage For Natural Bone Ingrowth with EIT Cellular Titanium EIT TLIF Cage Surgical Technique EIT Cellular Titanium provides active fusion area» ~ 80% porosity» ~ 650 µm diamond pore size» open interconnected
Advantage ALIF. Keith Shevlin Managing Director
 Advantage ALIF Unit 10, 9-11 Myrtle Street, Crows Nest NSW 2065 Keith Shevlin Managing Director keithshevlin@precisionsurgical.com.au Advantage ALIF Introduction & Indications for Use 1 Surgical Technique
Advantage ALIF Unit 10, 9-11 Myrtle Street, Crows Nest NSW 2065 Keith Shevlin Managing Director keithshevlin@precisionsurgical.com.au Advantage ALIF Introduction & Indications for Use 1 Surgical Technique
Patient Information MIS TLIF. Transforaminal Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS TLIF Transforaminal Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques MIS TLIF Table of Contents Anatomy of Spine..............................................
Patient Information MIS TLIF Transforaminal Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques MIS TLIF Table of Contents Anatomy of Spine..............................................
Patient Information ACDF. Anterior Cervical Discectomy and Fusion
 Patient Information ACDF Anterior Cervical Discectomy and Fusion Table of Contents Anatomy of the Spine...2-3 General Conditions of the Cervical Spine...4 5 What is an ACDF?...6 How is an ACDF performed?...7
Patient Information ACDF Anterior Cervical Discectomy and Fusion Table of Contents Anatomy of the Spine...2-3 General Conditions of the Cervical Spine...4 5 What is an ACDF?...6 How is an ACDF performed?...7
InFix. Anterior Lumbar Device. Surgical Technique Guide
 InFix Anterior Lumbar Device Surgical Technique Guide 2 InFix Anterior Lumbar Device Surgical Technique Guide InFix Anterior Lumbar System s modular design is intended to restore lordosis, disc height
InFix Anterior Lumbar Device Surgical Technique Guide 2 InFix Anterior Lumbar Device Surgical Technique Guide InFix Anterior Lumbar System s modular design is intended to restore lordosis, disc height
BAK/C Cervical Anterior Interbody Fusion System
 Surgical Technique BAK/C Cervical Anterior Interbody Fusion System The Comfortable Choice for Cervical Fusion BAK/C Cervical Surgical Technique 1 The BAK/C Cervical Fusion System is an alternative to conventional
Surgical Technique BAK/C Cervical Anterior Interbody Fusion System The Comfortable Choice for Cervical Fusion BAK/C Cervical Surgical Technique 1 The BAK/C Cervical Fusion System is an alternative to conventional
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental fixation is used.
 Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Cervical Screw System
 Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
The Fusion of. Strength, Design. Performance. and
 The Fusion of Strength, Design and Performance Interbody Fusion Platform Interbody Fusion Platform The DePuy Spine Interbody Fusion platform is designed to provide an open architecture implant in a load-sharing
The Fusion of Strength, Design and Performance Interbody Fusion Platform Interbody Fusion Platform The DePuy Spine Interbody Fusion platform is designed to provide an open architecture implant in a load-sharing
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
A U X I L I A R Y C O N N E C T O R S Surgical Technique
 A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
The Omega LIF System devices are made from Titanium alloy Ti6Al4V ELI, ASTM F136.
 Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
Patient Information MIS TLIF. Transforaminal Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS TLIF Transforaminal Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques MIS TLIF Table of Contents Anatomy of Spine...2 General Conditions of the Spine...4 6 MIS-TLIF
Patient Information MIS TLIF Transforaminal Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques MIS TLIF Table of Contents Anatomy of Spine...2 General Conditions of the Spine...4 6 MIS-TLIF
Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine.
 Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation
Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation
Transforaminal Lumbar Interbody Fusion Cage (TLIF)
 Transforaminal Lumbar Interbody Fusion age (TLIF) 990100010 DOULE ENGINE MEDIL MTERIL O., LTD. No. 218 Houxiang Road, Haicang District, Xiamen 361022, P.R.hina Tel: +86 592 6087101 Fax: +86 592 6587078
Transforaminal Lumbar Interbody Fusion age (TLIF) 990100010 DOULE ENGINE MEDIL MTERIL O., LTD. No. 218 Houxiang Road, Haicang District, Xiamen 361022, P.R.hina Tel: +86 592 6087101 Fax: +86 592 6587078
ACP. Anterior Cervical Plate System SURGICAL TECHNIQUE
 ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
GIZA Surgical Technique
 GIZA Surgical Technique Vertebral Body Replacement System Manufactured by Titanium alloy material provides mechanical integrity during insertion and distraction, x-ray visibility, and biocompatibility*
GIZA Surgical Technique Vertebral Body Replacement System Manufactured by Titanium alloy material provides mechanical integrity during insertion and distraction, x-ray visibility, and biocompatibility*
100 Interpace Parkway Parsippany, NJ
 100 Interpace Parkway Parsippany, NJ 07054 www.biometspine.com 800-526-2579 All trademarks are the property of Biomet, Inc. or one of its subsidiaries, unless otherwise indicated. Rx Only. 2009 EBI, LLC.
100 Interpace Parkway Parsippany, NJ 07054 www.biometspine.com 800-526-2579 All trademarks are the property of Biomet, Inc. or one of its subsidiaries, unless otherwise indicated. Rx Only. 2009 EBI, LLC.
ATEC Porous Ti System
 ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
OPERATIVE TECHNIQUE COVER IMAGE OPTIONAL (DETAIL) IMAGE SKYHAWK. lateral interbody fusion system lateral plate system
 OPERATIVE TECHNIQUE COVER IMAGE OPTIONAL (DETAIL) IMAGE SKYHAWK lateral interbody fusion system lateral plate system TABLE OF CONTENTS Introduction 1 Pre-Operative Technique 2 Operative Technique 3 Part
OPERATIVE TECHNIQUE COVER IMAGE OPTIONAL (DETAIL) IMAGE SKYHAWK lateral interbody fusion system lateral plate system TABLE OF CONTENTS Introduction 1 Pre-Operative Technique 2 Operative Technique 3 Part
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
X-spine Systems, Inc. Fixcet Spinal Facet Screw System
 X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
Y o u r Id e a s En g i n e e r e d t o Li f e
 ISSYS LP Spinal Fixation System Surgical Guide Y o u r Id e a s En g i n e e r e d t o Li f e In t r o d u c t i o n ISSYS LP Sp i n a l Fixation System The foundation of the ISSYS LP Spinal Fixation System
ISSYS LP Spinal Fixation System Surgical Guide Y o u r Id e a s En g i n e e r e d t o Li f e In t r o d u c t i o n ISSYS LP Sp i n a l Fixation System The foundation of the ISSYS LP Spinal Fixation System
XRL A modular expandable radiolucent vertebral body replacement system
 XRL A modular expandable radiolucent vertebral body replacement system This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE Table of Contents Introduction XRL 2 AO Spine Principles
XRL A modular expandable radiolucent vertebral body replacement system This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE Table of Contents Introduction XRL 2 AO Spine Principles
OPERATIVE TECHNIQUE PTC PEEK FORZA. spacer system
 OPERATIVE TECHNIQUE PTC PEEK FORZA spacer system TABLE OF CONTENTS Introduction 1 Operative Technique 2 Instruments 12 FORZA PEEK Part Numbers 20 FORZA PTC Part Numbers 22 Modular Implant Inserter 24 Disassembly
OPERATIVE TECHNIQUE PTC PEEK FORZA spacer system TABLE OF CONTENTS Introduction 1 Operative Technique 2 Instruments 12 FORZA PEEK Part Numbers 20 FORZA PTC Part Numbers 22 Modular Implant Inserter 24 Disassembly
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
TraXis TLIF Interbody System
 TraXis TLIF Interbody System Surgical Technique Solutions by the people of Zimmer Spine. zimmerspine.com A TLIF that s ahead of the curve. From the people of Zimmer Spine. Minimally Invasive Surgery (MIS)
TraXis TLIF Interbody System Surgical Technique Solutions by the people of Zimmer Spine. zimmerspine.com A TLIF that s ahead of the curve. From the people of Zimmer Spine. Minimally Invasive Surgery (MIS)
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
OPERATIVE TECHNIQUE. anterior cervical plating system
 OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
Synex System TECHNIQUE GUIDE. An expandable vertebral body replacement device
 Synex System TECHNIQUE GUIDE An expandable vertebral body replacement device Original Instruments and Implants of the Association for the Study of Internal Fixation AO ASIF Synex System Overview The Synex
Synex System TECHNIQUE GUIDE An expandable vertebral body replacement device Original Instruments and Implants of the Association for the Study of Internal Fixation AO ASIF Synex System Overview The Synex
Module: #15 Lumbar Spine Fusion. Author(s): Jenni Buckley, PhD. Date Created: March 27 th, Last Updated:
 Module: #15 Lumbar Spine Fusion Author(s): Jenni Buckley, PhD Date Created: March 27 th, 2011 Last Updated: Summary: Students will perform a single level lumbar spine fusion to treat lumbar spinal stenosis.
Module: #15 Lumbar Spine Fusion Author(s): Jenni Buckley, PhD Date Created: March 27 th, 2011 Last Updated: Summary: Students will perform a single level lumbar spine fusion to treat lumbar spinal stenosis.
INTELLIGENT SPINAL SYSTEM
 INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
Silverstone Interbody System Surgical Technique
 Silverstone Interbody System Surgical Technique TABLE OF CONTENTS Design Rationale Instructions for Use Surgical Technique 1. Disc Space Preparation 2. Distraction 3. Implant Selection 4. Implant Placement
Silverstone Interbody System Surgical Technique TABLE OF CONTENTS Design Rationale Instructions for Use Surgical Technique 1. Disc Space Preparation 2. Distraction 3. Implant Selection 4. Implant Placement
VTI INTERLINK PEDICLE SCREW SYSTEM
 VTI INTERLINK PEDICLE SCREW SYSTEM SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. DEVICE DESCRIPTION The VTI InterLink Pedicle Screw System is comprised of polyaxial pedicle screws in various diameters
VTI INTERLINK PEDICLE SCREW SYSTEM SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. DEVICE DESCRIPTION The VTI InterLink Pedicle Screw System is comprised of polyaxial pedicle screws in various diameters
T.L.I.F. Surgical Technique. Featuring the T.L.I.F. SG Instruments, VG2 PLIF Allograft, and the MONARCH Spine System.
 Surgical Technique T.L.I.F. Transforaminal Lumbar Interbody Fusion Featuring the T.L.I.F. SG Instruments, VG2 PLIF Allograft, and the MONARCH Spine System. CONSULTING SURGEON Todd Albert, M.D. Rothman
Surgical Technique T.L.I.F. Transforaminal Lumbar Interbody Fusion Featuring the T.L.I.F. SG Instruments, VG2 PLIF Allograft, and the MONARCH Spine System. CONSULTING SURGEON Todd Albert, M.D. Rothman
