SJO SILICONE IMPLANTS The following languages are included in this packet:
|
|
|
- Peregrine Williamson
- 6 years ago
- Views:
Transcription
1 SJO SILICONE IMPLANTS The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk) For additional languages, visit our website Then click on the Prescribing Information button. For additional information and translations please contact the manufacturer or local distributor. M C 0086* P Wright Medical Technology, Inc. Wright Medical UK Ltd 1023 Cherry Road 3rd Avenue Memphis, TN Letchworth U.S.A. Herts, SG6 2JF UK * The CE-Marking of Conformity is applied per catalog number and appears on the outer label, if applicable. October 2013 Printed in U.S.A.
2 Attention Operating Surgeon IMPORTANT MEDICAL INFORMATION SMALL JOINT ORTHOPEDIC (SJO) (SILICONE IMPLANTS) ( ) OUTLINE: I. DEFINITIONS II. GENERAL PRODUCT INFORMATION A. PATIENT SELECTION B. CONTRAINDICATIONS C. POTENTIAL COMPLICATIONS AND ADVERSE REACTIONS D. PRECAUTIONS E. HANDLING AND STERILIZATION F. STORAGE CONDITIONS EN III. SPECIFIC PRODUCT INFORMATION A. SWANSON RADIAL HEAD IMPLANT B. SWANSON HAMMERTOE IMPLANT C. SWANSON FINGER JOINT IMPLANT AND GROMMET D. SWANSON FLEXIBLE HINGE TOE IMPLANT AND GROMMET E. SWANSON WRIST JOINT IMPLANT F. SWANSON TRAPEZIUM IMPLANT/TIE-IN TRAPEZIUM G. SWANSON TENDON SPACER H. SWANSON BONE PLUG (CEMENT RESTRICTOR) I. SWANSON INCISION DRAIN 1
3 I. DEFINITIONS Symbols and abbreviations may be used on the package label. The following table provides the definition of these symbols and abbreviations. Table 1. Definitions of Symbols and Abbreviations Symbol g h D Y i H l p N Definition Batch code Catalog number Do not re-use Caution, consult accompanying documents Consult operating instructions Use by Temperature limitation Keep dry Keep away from sunlight Date of manufacture 2
4 M P[]\ I K Manufacturer Authorized EC Representative in the European Community Sterilized using ethylene oxide Sterilized using radiation STERILE GAS Sterilized using gas plasma J Sterilized using aseptic processing techniques For prescription use only Abbreviation Ti Ti6Al4V CoCr SS UHMWPE Material Titanium Titanium Alloy Cobalt Chrome Alloy Stainless Steel Ultra High Molecular Weight Polyethylene 3
5 4 II. GENERAL PRODUCT INFORMATION Through the advancement of partial and total joint replacement, the surgeon has been provided a means of restoring mobility, correcting deformity, and reducing pain for many patients. While the prostheses used are largely successful in attaining these goals, it must be recognized that they are manufactured from silicone, metal, and ceramic materials, and that any joint replacement system cannot be expected to withstand the activity levels and loads as would normal, healthy bone. In addition, the system will not be as strong, reliable, or durable as a natural human joint. In using joint prostheses, the surgeon should be aware of the following: A. All products are manufactured from silicone elastomer, EXCEPT the finger, toe, and wrist grommets, which are manufactured from titanium. B. The judgement by a surgeon to implant silicone elastomer implants is a risk/benefit decision which must take into account the patient s needs and desire in addition to the surgeon s knowledge of expected results and complications as well as therapeutic alternatives. Wright Medical Technology, Inc., can provide a bibliography of articles on the use and complications of silicone elastomer implants to any physician. Please write or call Wright Medical Technology, Inc. C. The correct selection and sizing of the prosthesis is extremely important. Selection of the proper size, shape, and design of the prosthesis increases the potential for success in joint replacement. Joint prostheses require careful seating and adequate bone support. D. Wright Medical Technology, Inc., does not recommend a particular surgical technique when using the implant. Proper surgical procedures and techniques are necessarily the responsibility of the medical professional. Each surgeon must evaluate the appropriateness of the surgical technique used based on personal medical training and experience.
6 E. Reshaping of the implant should be avoided because it can compromise or destroy the structural integrity and the functionality of the implant. F. For all implants, a sizing set is available for proper size determination during surgery (supplied nonsterile and not suitable for implantation) G. In selecting patients for joint replacements, the following factors can be critical to the eventual success of the procedure: 1. Patient s occupation or activity. If the patient is involved in an occupation or activity which includes substantial lifting or muscle strain, the resultant forces can cause failure of the fixation, the device, or both. The prosthesis will not restore function to the level expected with normal healthy bone, and the patient should not have unrealistic functional expectations. 2. Condition of senility, mental illness, or alcoholism. These conditions, among others, may cause the patient to ignore certain necessary limitations and precautions in the use of the prosthesis, leading to failure or other complications. 3. Foreign body sensitivity. Where material sensitivity is suspected, appropriate tests should be made prior to material selection or implantation. A. PATIENT SELECTION Any joint implant arthroplasty requires consideration of the following general indications: Good condition of the patient Good neurovascular status Adequate skin coverage Possibility of a functional musculotendinous system 5
7 Adequate bone stock to receive implant Availability of post-operative therapy Cooperative patient See Section II for specific product information. B. CONTRAINDICATIONS Infection Physiologically or psychologically inadequate patient Inadequate skin, bone, or neurovascular status Irreparable tendon system Possibility for conservative treatment Growing patients with open epiphyses Patients with high levels of activity See Section II for specific product information. C. POTENTIAL COMPLICATIONS AND ADVERSE REACTIONS In any surgical procedure, the potential for complications exists. The risks and complications with silicone implants include: Infection or painful, swollen or inflamed implant site Fracture of the implant Loosening or dislocation of the prosthesis requiring revision surgery 6
8 Bone restoration or over-production Allergic reaction(s) to prosthesis material(s) Untoward histological responses possibly involving macrophages and/or fibroblasts Migration of particle wear debris possibly resulting in a bodily response Embolism Some degree of particle formation is inevitable with all implants including those made of silicone elastomer. The amount will vary with factors such as patient activity, joint stability or instability post-implantation, implant position and the amount of soft tissue support. The patient s biological response to these particles is variable, but can include local synovitis and bone lysis in contiguous bones. See Section II for specific product information. D. PRECAUTIONS Following the instructions for use provided in product literature can minimize the potential for complications or adverse reactions with any implant. It is the responsibility of each surgeon using implants to consider the clinical and medical status of each patient and to be knowledgeable about all aspects of implant procedure and the potential complications that may occur. The benefits derived from implant surgery may not meet the patient s expectations or may deteriorate with time, necessitating revision surgery to replace the implant or to carry out alternative procedures. Revision surgeries with implants are common. The patient s mental status must also be considered. Willingness and/or ability to follow post-operative instructions may also impact the surgical outcome. Surgeons must balance many considerations to achieve the best result in individual patients. 7
9 IF EXCESSIVE LOADING CANNOT BE PREVENTED, AN IMPLANT SHOULD NOT BE USED. One of the goals of implant surgery is to minimize production of wear particles. It can never be eliminated because of all the moving parts, e.g., implants that articulate against bone, wear to some degree. In an implant arthroplasty, clinically significant wear can result from normal biomechanical forces. Abnormal or excessive force will further increase clinically significant wear. Abnormal force loading and subsequent wear may be caused by: Uncorrected instability Improperly sized implant Inadequate soft tissue support Implant malposition Excessive motion Uncorrected or recurrent deformity Patient misuse or overactivity Intra-operative fixation Some preventative measures to consider to minimize the potential for complications: Follow guidelines for indications and contraindications provided above Identify prior pathology Stabilize collapse deformities Bone graft pre-existing cysts 8
10 Use a properly sized implant Avoid K-wires and sutures through the implant If complications develop, possible corrective procedures include: Implant removal Synovectomy Bone grafting of cysts Replacement of the implant Removal of the implant with fusion of the joint Clinical results depend on surgeon and technique, pre-operative and post-operative care, the implant, patient pathology and daily activity. It is important that surgeons obtain appropriate informed consent and discuss the potential for complications with each patient prior to surgery. This may include a review of alternative, non-implant procedures such as soft tissue reconstruction or arthrodesis. Recommendations Regarding Device Fragments Use medical devices in accordance with their labeled indications and Wright Medical Technology s instructions for use, especially during insertion and removal. Inspect devices prior to use for damage during shipment or storage or any out-of-box defects that might increase the likelihood of fragmentation during a procedure. Inspect devices immediately upon removal from the patient for any signs of breakage or fragmentation. If the device is damaged, retain it to assist with Wright Medical Technology s analysis of the event. 9
11 Carefully consider and discuss with the patient (if possible) the risks and benefits of retrieving vs. leaving the fragment in the patient. Advise the patient of the nature and safety of unretrieved device fragments including the following information: a. The material composition of the fragment (if known); b. The size of the fragment (if known); c. The location of the fragment; d. The potential mechanisms for injury, e.g., migration, infection; e. Procedures or treatments that should be avoided such as MRI exams in the case of metallic fragments. This may help to reduce the possibility of a serious injury from the fragment. Concerning Magnetic Resonance Environments The devices described in this package insert have not been evaluated for safety and compatibility in the MR environment. The devices described in this package insert have not been tested for heating or migration in the MR environment. See Section II for specific product information. E. HANDLING AND STERILIZATION IMPLANTS The implants described in this package insert are either provided sterile or non-sterile as indicated on the individual product s label. Implants that are presented in instrument trays are provided non-sterile. 10
12 Implants in sterile packaging should be inspected to ensure that the packaging has not been damaged or previously opened. If the inner package integrity has been compromised, contact the manufacturer for further instructions. The implants should be opened using aseptic OR technique; they should only be opened after the correct size has been determined. This product is for single use only. An implant should never be re-sterilized after contact with body tissues or fluids. Devices labeled for single-use only should never be reused. Reuse of these devices may potentially result in serious patient harm. Examples of hazards related to the reuse of these devices include, but are not limited to: significant degradation in device performance, cross-infection, and contamination. Implants provided non-sterile should be processed according to the recommended parameters for instruments (below). INSTRUMENTS Surgical instruments (and non-sterile implants) should be cleaned and sterilized according to the following parameters: Cleaning 1. Disassemble all components as per manufacturer instructions (if appropriate). 2. Rinse with cold tap water to remove gross contamination. 3. Bathe in an enzymatic detergent solution prepared per manufacturer directions for 5 minutes. 4. Scrub thoroughly with a soft brush and/or pipe cleaner; repeatedly flush any very narrow lumens with enzymatic detergent solution using a syringe. 11
13 5. Rinse with cold tap water for a minimum of one minute; use a syringe to repeatedly flush any very narrow lumens. 6. Bathe in a detergent solution prepared per manufacturer directions for 5 minutes. 7. Scrub thoroughly with a soft brush and/or pipe cleaner; repeatedly flush any very narrow lumens with detergent solution using a syringe. 8. Rinse thoroughly/flush with deionized/reverse osmosis (RO/DI) water. 9. Sonicate for a minimum of 10 minutes in an enzymatic detergent solution prepared per manufacturer directions. 10. Rinse thoroughly/flush with RO/DI water. 11. Dry with a clean, soft, absorbent, disposable cloth. 12. Visually inspect for cleanliness. All visible surfaces, internal and external, should be visually inspected. If necessary re-clean until it is visibly clean. Note: Brushes (i.e. pipe cleaners) could be used for cleaning most lumens, however, the use of a syringe to flush narrow lumens with diameters less than or equal to inches is recommended. Sterilization The minimum recommended steam sterilization conditions for Wright reusable instruments (and non-sterile implants) are as follows: 1. Double wrap the component in an FDA-cleared CSR wrap or similar type nonwoven medical grade wrapping material. 2. Autoclave according to the following parameters: 12
14 Steam Sterilization Cycle Type Parameter Set Point Exposure Temperature 270 F (132 C) Prevacuum Exposure Time 4 minutes 270 F (132 C) Dry Time 20 minutes 3. After sterilization, remove the component from its wrapping using accepted sterile technique with powder-free gloves. Ensure that implants are at room temperature prior to implantation. Avoid contact with hard objects that may cause damage. These recommendations are consistent with AAMI ST79 Table 5 guidelines and have been developed and tested using specific equipment. Due to variations in environment and equipment, it must be demonstrated that these recommendations produce sterility in your environment. If processing conditions, wrapping materials, or equipment changes occur, the effectiveness of the sterilization process must be demonstrated. For additional information see Wright s Cleaning and Handling of Wright Medical Instruments. F. STORAGE CONDITIONS All implants must be stored in a clean, dry environment and be protected from sunlight and extremes in temperature. 13
15 III. SPECIFIC PRODUCT INFORMATION A. SWANSON RADIAL HEAD IMPLANT DESCRIPTION Available in Standard and Extra Long Sizes, the Swanson Radial Head Implant is a pliable, one-piece intramedullary-stemmed cuffed implant designed to help preserve the joint space and relationships of the radio-humeral and proximal radio-ulnar joints following radial head resection for rheumatoid, degenerative, or traumatic arthritis. It has also been used as a primary replacement following radial head resection for fractures. It is designed specifically for radiohumeral arthroplasty. INDICATIONS Replacement of the radial head for rheumatoid, degenerative, or post-traumatic disabilities presenting pain, crepitation, and decreased motion at the radio-humeral and/or proximal radio-ulnar joint with: joint destruction and/or subluxation visible on X-ray and/or resistance to conservative treatment Primary replacement after fracture of the radial head Symptomatic sequelae after radial head resection NOTE: The use of extra long radial head implant is indicated when there is a lack of bone stock at the radial neck and the distance between the capitellum and the proximal radius is too long for the conventional style implant. This situation can be seen when there has been a comminuted radial head fracture 14
16 including the neck or following an overzealous bone removal in radial head excision procedures. Evidence of joint narrowing secondary to radio-humeral joint synovitis is not a contraindication to radial head implant replacement combined with elbow synovectomy. CONTRAINDICATIONS Growing children with open epiphyses Dislocation of radius on ulna that would allow a radio-humeral articulation B. SWANSON HAMMERTOE (WEIL DESIGN) IMPLANT DESCRIPTION The Swanson Hammertoe Implant is a flexible double stemmed implant specifically designed for the proximal interphalangeal joint of the lateral toes. It is used as an adjunct to resection arthroplasty in cases of moderate to severe hammertoe deformities of toes 2 through 5. The implant is symmetrical; therefore, there are no proximal/distal nor lateral/ medial designations. INDICATIONS Semi-rigid or rigid hammertoe deformity associated with degenerative arthritis Semi-rigid or rigid hammertoe deformity associated with rheumatoid arthritis Revision of a failed arthroplasty or arthrodesis 15
17 C. SWANSON FINGER JOINT IMPLANT AND GROMMET DESCRIPTION The Swanson Finger Joint Implant is a flexible, intramedullary-stemmed, one-piece implant developed for reconstruction of finger joints to help restore function to hands disabled by rheumatoid, degenerative or post-traumatic arthritis. The midsection of the load-distributing flexible hinge implant has been designed to flex easily while, at the same time, maintaining vertical stability: it acts as both a spacer and a flexible hinge. The Swanson Finger Joint Grommet II is a thin, titanium shield designed for use with the Swanson Finger Joint Implant in severe rheumatoid arthritis patients where cutting or abrasion of the flexible implant from contact with thin, sharp bone edges could occur, or in patients who have high activity levels. It is contoured to conform to the shape of the stem and midsection of the flexible implant and is fabricated from unalloyed titanium for surgical application. The distal grommet is used on the distal stem and the proximal grommet on the proximal stem. Sizes 3-9 packages of the Swanson Finger Implant contains a pair of matching proximal and distal grommets. INDICATIONS Metacarpophalangeal Joint Rheumatoid or post-traumatic disabilities with: Fixed or stiff MP joints X-ray evidence of joint destruction or subluxation Ulnar drift, noncorrectable by surgery, of soft tissues alone Contracted intrinsic and extrinsic musculature and ligament system Associated stiff interphalangeal joints 16
18 Proximal Interphalangeal Joint Rheumatoid, degenerative or post-traumatic disabilities with: Destroyed or joints subluxed Stiffened joints in which a joint tissue release alone would be inadequate PRECAUTIONS Fitting of the grommet requires a precise press-fit. It must be accurately centered; otherwise it may impinge the cortex in the intramedullary canal on one side and could cause bone resorption. Unless the shoulders of the grommet can be fitted into metaphyseal bone, rotation of the grommet could occur. In certain cases of severe metacarpophalangeal joint dislocation, additional bone must be removed to obtain joint reduction and the implant may have to be used without the grommet. D. SWANSON FLEXIBLE HINGE TOE IMPLANT AND GROMMET (STANDARD AND SHORT STEM) DESCRIPTION The Swanson Flexible Hinge Toe Implant is a double stemmed flexible hinge implant designed to restore function to the metatarsophalangeal joints disabled by rheumatoid, degenerative, or post-traumatic arthritis. In the first metatarsophalangeal joint, the implant is used in cases of moderate to severe hallux valgus deformity secondary to rheumatoid arthritis, or to senile degenerative arthritis and in cases of bony destruction on both sides of the joint. In the lateral metatarsophalangeal joints, the implant is used in cases of dislocation and extension contracture of the metatarsophalangeal joint, in cases of bone destruction of one or both joint surfaces as in rheumatoid arthritis, and in cases of dislocation resulting from resection of the base of one or both joint surfaces as in rheumatoid arthritis. 17
19 The Swanson Flexible Hinge Toe Joint Grommet is a thin titanium shield designed for use with the Swanson Flexible Hinge Toe Implant in rheumatoid patients where cutting or abrasion of the flexible implant from contact with thin, sharp bone edges can occur, or in patients who have high activity levels. It is contoured to conform to the shape of the midsection of the flexible implant and is fabricated from unalloyed titanium for surgical application. The distal grommet is used on the distal stem and the proximal grommet is used on the proximal stem to protect the implant from biomechanical shearing forces of sharp bone edges during joint motion. INDICATIONS In cases of rheumatoid arthritis presenting a moderate to severe hallux valgus deformity, lateral toe involvement, radiographic evidence of erosion, cyst formation and narrowing of the first metatarsophalangeal joint and contractual deformities. In cases of severe senile hallux valgus deformity. NOTE: Care must be taken to preserve part of the head to prevent shift of the weight bearing to the second toe. In cases of moderate to severe hallux valgus deformity secondary to degenerative or post-traumatic arthritis. For revision of previous procedures when there is evidence of bony destruction involving both sides of the joint and for revision of failed single stem arthroplasty. In cases of rheumatoid arthritis of the lateral toes presenting moderate to severe deformity and radiographic evidence of erosion, cyst formation, and narrowing of the metatarsophalangeal joint. 18
20 PRECAUTIONS Fitting of the grommet requires a precise press fit. It must be accurately centered; otherwise it may impinge the cortex in the intramedullary canal on one side and could cause bone resorption. Unless the shoulders of the grommet can be fitted into metaphyseal bone, rotation of the grommet could occur. In certain cases of severe metatarsophalangeal joint dislocation, additional bone must be removed to obtain joint reduction and the implant may have to be used without the grommet. E. SWANSON WRIST JOINT IMPLANT AND GROMMET DESCRIPTION The Swanson Wrist Joint Implant is a one-piece, intramedullary stemmed implant fabricated from silicone elastomer. It is designed for use in implant resection arthroplasty of the radiocarpal joint. The Swanson Wrist Joint Grommet is a thin, titanium shield designed to modify the Swanson Wrist Joint Implant in selected cases. It is contoured to conform to the shape of the mid-section of the flexible implant and is fabricated from unalloyed titanium for surgical application. To protect the implant from the shearing forces of sharp bone edges, the distal grommet is normally used on the dorsal surface and the proximal grommet on the palmar surface. Use of the grommet-modified implant is indicated in patients where cutting or abrasion of the flexible implant from contact with resected bone is likely to occur. Each package of the Swanson Wrist Joint Grommet contains matching proximal and distal pairs. 19
21 INDICATIONS Arthritic or traumatic disability resulting in: instability of the wrist due to subluxation or dislocation of the radiocarpal joint severe deviation of the wrist causing musculotendinous imbalance of the digits stiffness or fusion of the wrist in a non-functional position stiffness of the wrist where movement is a requirement for hand function F. SWANSON TRAPEZIUM IMPLANT/TIE-IN TRAPEZIUM DESCRIPTION The Swanson Trapezium Implant/TIE-IN Trapezium is a flexible, one-piece, intramedullary stemmed implant developed to help restore function to thumbs disabled by degenerative arthritis or post-traumatic arthritis. It is designed to replace the trapezium bone in an attempt to preserve the anatomical relationships of the basal joints of the thumb after resection arthroplasty by acting as a space filler. INDICATIONS Degenerative or post-traumatic arthritis (e.g., following an old Bennett fracture) Disabilities of the thumb basal joints with localized bony changes Localized pain and palpable crepitation during circumduction movement with axial compression of involved thumb ( grind test ) NOTE: Dr. Swanson has described a grind test which may be used in localizing the cause of the patient s complaint. To perform this test, the patient s thumb is held securely in the examiner s right hand, and the base of the patient s thumb 20
22 is held in the examiner left thumb and index finger. If the test is positive, passive circumduction of the thumb while axial compression is applied will produce pain, crepitation and subluxation localized at the carpometacarpal joint. Decreased motion, decreased pinch and decreased grip strength X-ray evidence of arthritic changes of the trapeziometacarpal, trapezioscaphoid, trapeziotrapezoid, and trapezium second metacarpal joints, singly or in combination Associated unstable, stiff or painful distal joints of thumb or swan-neck deformity CONTRAINDICATIONS Severe displacement, resorption or involvement of contiguous carpal bones G. SWANSON TENDON SPACER DESCRIPTION The Swanson Tendon Spacer is a temporary passive spacer used to facilitate two-stage reconstruction of flexor and extensor tendons of the hand. INDICATIONS Reconstruction of flexor or extensor tendons of the fingers, thumb, and wrist Scarred or adherent tendons following trauma or failed primary repair Absence of tendon sheath Scarred or adherent non-functional tendon pulleys Ruptured tendon 21
23 H. SWANSON BONE PLUG (CEMENT RESTRICTOR) DESCRIPTION The Swanson Bone Plug is a flexible silicone elastomer plug designed to fit snugly into the intramedullary canal to restrict migration of poly (methyl) methacrylate bone cement. It can be used in total large joint replacement arthroplasty involving the hip, knee, shoulder or elbow joints which requires poly (methyl) methacrylate cement fixation of an intramedullary stem(s). INDICATIONS Any total large joint replacement arthroplasty, which requires poly (methyl) methacrylate cement fixation of an intramedullary stem. I. SWANSON INCISION DRAIN DESCRIPTION The Swanson Incision Drain is a thin, flat, flexible silicone elastomer drain designed for post-operative drainage of blood and fluids from incisions such as those involved with arthroplasty of the small joints of the upper and lower extremities. INDICATIONS Drainage of smaller incisions, which do not require suction drainage, or as an adjunct to suction drainage in medium sized incisions. The drains may be useful in cases where decreased scar formation and tissue reaction are important, such as in hand and foot surgery or plastic surgery. 22
24 PRECAUTIONS While the technique of insertion of the Swanson Incision Drain may vary among physicians, there are several important points to observe: Upon insertion, the drain should be wrinkled or twisted to form a path for drainage of fluids. When removing drain from package, immerse directly in sterile saline. The initial dressing should be moist so that it acts as a wick to draw the fluids out of the wound. Handling of the drain should be done with blunt instruments to avoid surface trauma or contamination with foreign bodies. Rinse the drain thoroughly with sterile saline solution before insertion. The Swanson Hammertoe (Weil Design) Implant was designed by Lowell Scott Weil, D.P.M., F.A.C.F.S., Chicago, Illinois All other Swanson Implants were designed by Alfred B. Swanson, M.D., F.A.C.S., Grand Rapids, Michigan Trademarks and Registered Trademarks are owned or licensed by Wright Medical Technology, Inc. 23
The following languages are included in this packet:
 DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO MIS FOREFOOT SCREW
 DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM
 EN CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM 150871-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
EN CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM 150871-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
BIOFOAM BONE WEDGE
 BIOFOAM BONE WEDGE 150837-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
BIOFOAM BONE WEDGE 150837-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
MICA SCREWS For additional information and translations please contact the manufacturer or local distributor.
 MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE
 ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EXTERNAL FIXATION SYSTEMS The following languages are included in this packet:
 EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
Metallic Internal Fixation Devices The following languages are included in this packet:
 Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM
 METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
DARCO HEADED CANNULATED SCREWS
 DARCO HEADED CANNULATED SCREWS 151677-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO HEADED CANNULATED SCREWS 151677-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
STABILIZATION AND FRACTURE FIXATION
 STABILIZATION AND FRACTURE FIXATION 150838-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
STABILIZATION AND FRACTURE FIXATION 150838-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
METAL HEMI SYSTEM
 METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM The following languages are included in this packet:
 ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
The following languages are included in this packet:
 OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
SWANSON. Flexible Hinge Toe Implant SURGIC AL TECHNIQUE
 SWANSON Flexible Hinge Toe Implant SURGIC AL TECHNIQUE SWANSON flexible HINGE TOE IMPLANT with SWANSON Flexible Hinge Grommet surgical technique presented by ALFRED B. SWANSON, MD, FACS, GRAND RAPIDS,
SWANSON Flexible Hinge Toe Implant SURGIC AL TECHNIQUE SWANSON flexible HINGE TOE IMPLANT with SWANSON Flexible Hinge Grommet surgical technique presented by ALFRED B. SWANSON, MD, FACS, GRAND RAPIDS,
ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM
 ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM 150884-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM 150884-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
Fracture Fixation The following languages are included in this packet:
 Fracture Fixation 150846-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
Fracture Fixation 150846-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
SWANSON. Hammertoe Implant SURGICAL TECHNIQUE
 Hammertoe Implant SURGICAL TECHNIQUE hammertoe IMPLANT surgical technique presented by LOWELL SCOTT WEIL, DPM HANDLING AND STERILIZATION This product has been sterilized and should be considered sterile
Hammertoe Implant SURGICAL TECHNIQUE hammertoe IMPLANT surgical technique presented by LOWELL SCOTT WEIL, DPM HANDLING AND STERILIZATION This product has been sterilized and should be considered sterile
SALVATION EXTERNAL FIXATION SYSTEM
 SALVATION EXTERNAL FIXATION SYSTEM 151660-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
SALVATION EXTERNAL FIXATION SYSTEM 151660-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE
 CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
TOTAL ELBOW SYSTEM The following languages are included in this packet:
 TOTAL ELBOW SYSTEM 115298-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch) Türkçe
TOTAL ELBOW SYSTEM 115298-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch) Türkçe
SALVATION EXTERNAL FIXATION SYSTEM
 SALVATION EXTERNAL FIXATION SYSTEM 151660-2 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
SALVATION EXTERNAL FIXATION SYSTEM 151660-2 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
CANNULATED SCREW SYSTEM The following languages are included in this packet:
 CANNULATED SCREW SYSTEM 137183-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -Chinese (sch) Türkçe
CANNULATED SCREW SYSTEM 137183-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -Chinese (sch) Türkçe
BIOARCH SUBTALAR IMPLANT SYSTEM
 BIOARCH SUBTALAR IMPLANT SYSTEM 137308-4 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
BIOARCH SUBTALAR IMPLANT SYSTEM 137308-4 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
BIOFOAM ANKLE SPACER BLOCK
 BIOFOAM ANKLE SPACER BLOCK 145782-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
BIOFOAM ANKLE SPACER BLOCK 145782-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
INVISION TOTAL ANKLE REVISION SYSTEM The following languages are included in this packet:
 INVISION TOTAL ANKLE REVISION SYSTEM 151678-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 -
INVISION TOTAL ANKLE REVISION SYSTEM 151678-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 -
TOTAL ANKLE SYSTEMS
 TOTAL ANKLE SYSTEMS 150864-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
TOTAL ANKLE SYSTEMS 150864-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
OSKAR Radial Head Prosthesis
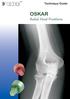 Technique Guide OSKAR Radial Head Prosthesis DESCRIPTION The OSKAR Radial Head Implant is a pliable, onepiece intramedullary-stemmed cuffed implant designed to help preserve the joint space and relationships
Technique Guide OSKAR Radial Head Prosthesis DESCRIPTION The OSKAR Radial Head Implant is a pliable, onepiece intramedullary-stemmed cuffed implant designed to help preserve the joint space and relationships
Silicone Finger Implant
 Silicone Finger Implant Manufactured from high tear resistant implant grade silicone Surgical Technique Eleven, evenly scaled sizes for comprehensive anatomical fit Simple, precise instrumentation Designed
Silicone Finger Implant Manufactured from high tear resistant implant grade silicone Surgical Technique Eleven, evenly scaled sizes for comprehensive anatomical fit Simple, precise instrumentation Designed
MICROPORT KNEE SYSTEMS The following languages are included in this packet:
 EN English (en) MICROPORT KNEE SYSTEMS 151251-0 The following languages are included in this packet: For additional languages, visit our website ortho.microport.com Then click on the Prescribing Information
EN English (en) MICROPORT KNEE SYSTEMS 151251-0 The following languages are included in this packet: For additional languages, visit our website ortho.microport.com Then click on the Prescribing Information
Integra. Silicone Toe System SURGICAL TECHNIQUE
 Silicone Toe System SURGICAL TECHNIQUE Silicone Toe System Table of Contents Indications...2 Contraindications...2 Warnings...2 Precautions...2 Adverse Events...2 Flexible Great Toe (FGT)...3 Surgical
Silicone Toe System SURGICAL TECHNIQUE Silicone Toe System Table of Contents Indications...2 Contraindications...2 Warnings...2 Precautions...2 Adverse Events...2 Flexible Great Toe (FGT)...3 Surgical
Silicone PIP, MCP & MCP-X (PreFlex)
 Silicone PIP, MCP & MCP-X (PreFlex) Finger Joint Arthroplasty Operative Technique Silicone PIP Silicone MCP Silicone PreFlex (MCP-X) Stryker Disclaimer This publication sets forth detailed recommended
Silicone PIP, MCP & MCP-X (PreFlex) Finger Joint Arthroplasty Operative Technique Silicone PIP Silicone MCP Silicone PreFlex (MCP-X) Stryker Disclaimer This publication sets forth detailed recommended
Integra. Silicone PIP Implant SURGICAL TECHNIQUE
 Integra Silicone PIP Implant SURGICAL TECHNIQUE Table of Contents Indications For Use...2 Contraindications...2 Warnings and Precautions...2 Surgical Technique Preoperative Assessment... 3 Step 1: Initial
Integra Silicone PIP Implant SURGICAL TECHNIQUE Table of Contents Indications For Use...2 Contraindications...2 Warnings and Precautions...2 Surgical Technique Preoperative Assessment... 3 Step 1: Initial
SWANSON BASAL THUMB IMPLANT SYSTEM. surgical technique presented by ALFRED B. SWANSON, MD, FACS, GRAND RAPIDS, MICHIGAN.
 BASAL THUB IPLANT SYSTE surgical technique presented by ALFRED B. SWANSON, D, FACS, GRAND RAPIDS, ICHIGAN. BASAL THUB IPLANT SWANSON basal thumb IPLANT as described by Alfred B. Swanson, D general PRECAUTIONS
BASAL THUB IPLANT SYSTE surgical technique presented by ALFRED B. SWANSON, D, FACS, GRAND RAPIDS, ICHIGAN. BASAL THUB IPLANT SWANSON basal thumb IPLANT as described by Alfred B. Swanson, D general PRECAUTIONS
SWANSON. Flexible Hinge Toe Implant with SWANSON Flexible Hinge Grommet
 Flexible Hinge Toe Implant with SWANSON Flexible Hinge Grommet flexible HINGE TOE IMPLANT with SWANSON Flexible Hinge Grommet surgical technique presented by ALFRED B. SWANSON, MD, FACS, GRAND RAPIDS,
Flexible Hinge Toe Implant with SWANSON Flexible Hinge Grommet flexible HINGE TOE IMPLANT with SWANSON Flexible Hinge Grommet surgical technique presented by ALFRED B. SWANSON, MD, FACS, GRAND RAPIDS,
Ascension. Silicone MCP surgical technique. surgical technique Ascension Silicone MCP
 Ascension Silicone MCP surgical technique WW 2 Introduction This manual describes the sequence of techniques and instruments used to implant the Ascension Silicone MCP (FIGURE 1A). Successful use of this
Ascension Silicone MCP surgical technique WW 2 Introduction This manual describes the sequence of techniques and instruments used to implant the Ascension Silicone MCP (FIGURE 1A). Successful use of this
ORTHOFLEX. Silicone Hammertoe Implant SURGICAL TECHNIQUE
 ORTHOFLEX Silicone Hammertoe Implant SURGICAL TECHNIQUE Contents Chapter 1 4 Product Information 4 Device Description Chapter 2 4 Intended Use 5 Indications 5 Contraindications Chapter 3 6 Surgical Technique
ORTHOFLEX Silicone Hammertoe Implant SURGICAL TECHNIQUE Contents Chapter 1 4 Product Information 4 Device Description Chapter 2 4 Intended Use 5 Indications 5 Contraindications Chapter 3 6 Surgical Technique
Lesser MPJ Hemi Implant
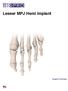 Lesser MPJ Hemi Implant Surgical Technique Contents Product The BioPro Lesser MPJ Hemi Implant is a simple, durable, metallic hemiarthroplasty resurfacing prosthesis for the treatment of arthritis, Freiberg
Lesser MPJ Hemi Implant Surgical Technique Contents Product The BioPro Lesser MPJ Hemi Implant is a simple, durable, metallic hemiarthroplasty resurfacing prosthesis for the treatment of arthritis, Freiberg
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
WristMotion Wrist Hemiarthroplasty System Instructions for Use
 WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Bipolar Radial Head System
 Bipolar Radial Head System Katalyst Surgical Technique DESCRIPTION The Katalyst Telescoping Bipolar Radial Head implant restores the support and bearing surface of the radial head in the face of fracture,
Bipolar Radial Head System Katalyst Surgical Technique DESCRIPTION The Katalyst Telescoping Bipolar Radial Head implant restores the support and bearing surface of the radial head in the face of fracture,
FUSEFORCE. Hand Fixation System SURGICAL TECHNIQUE
 FUSEFORCE Hand Fixation System SURGICAL TECHNIQUE Contents Chapter 1 3 Chapter 2 4 Appendix A 7 Intended Use Surgical Technique Ordering Information Indication Sizing Reference Proper surgical procedures
FUSEFORCE Hand Fixation System SURGICAL TECHNIQUE Contents Chapter 1 3 Chapter 2 4 Appendix A 7 Intended Use Surgical Technique Ordering Information Indication Sizing Reference Proper surgical procedures
Integra. PyroSphere System SURGICAL TECHNIQUE
 Integra PyroSphere System SURGICAL TECHNIQUE Table of Contents Indications For Use...02 Contraindications...02 Warnings...03 Precautions...03 Patient Positioning...03 Surgical Technique... 04 Step 1 Initial
Integra PyroSphere System SURGICAL TECHNIQUE Table of Contents Indications For Use...02 Contraindications...02 Warnings...03 Precautions...03 Patient Positioning...03 Surgical Technique... 04 Step 1 Initial
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE. This Package Insert is for product distributed in the US only.
 TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
REPIPHYSIS LIMB SALVAGE SYSTEM
 REPIPHYSIS LIMB SALVAGE SYSTEM 150800-0 For additional information please contact the manufacturer or local distributor. M MicroPort Orthopedics Inc. 5677 Airline Rd Arlington, TN 38002 U.S.A. i Y October
REPIPHYSIS LIMB SALVAGE SYSTEM 150800-0 For additional information please contact the manufacturer or local distributor. M MicroPort Orthopedics Inc. 5677 Airline Rd Arlington, TN 38002 U.S.A. i Y October
The Rheumatoid Hand Deformities & Management. Dr. Anirudh Sharma Resident Department of Orthopedics
 + The Rheumatoid Hand Deformities & Management Dr. Anirudh Sharma Resident Department of Orthopedics + Why is Rheumatoid Arthritis important? + RA is a very debilitating disease median life expectancy
+ The Rheumatoid Hand Deformities & Management Dr. Anirudh Sharma Resident Department of Orthopedics + Why is Rheumatoid Arthritis important? + RA is a very debilitating disease median life expectancy
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Integra. Katalyst Bipolar Radial Head System SURGICAL TECHNIQUE
 Integra Katalyst Bipolar Radial Head System SURGICAL TECHNIQUE Surgical Technique As the manufacturer of this device, Integra does not practice medicine and does not recommend this or any other surgical
Integra Katalyst Bipolar Radial Head System SURGICAL TECHNIQUE Surgical Technique As the manufacturer of this device, Integra does not practice medicine and does not recommend this or any other surgical
Technique Guide Wrist Hemiarthroplasty System
 Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
Precautionary Statement ( )
 Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Integra. Thumb Joint Replacement PATIENT INFORMATION. with PyroCarbon implants
 Integra Thumb Joint Replacement with PyroCarbon implants PATIENT INFORMATION Anatomy of the Hand The hand is composed of many different bones, muscles and ligaments that allow for a large amount of movement
Integra Thumb Joint Replacement with PyroCarbon implants PATIENT INFORMATION Anatomy of the Hand The hand is composed of many different bones, muscles and ligaments that allow for a large amount of movement
SURGICAL TECHNIQUE GUIDE
 DANGER indicates an imminently hazardous situation which, if not avoided, will result in death or serious injury. WARNING indicates a potentially hazardous situation which, if not avoided, could result
DANGER indicates an imminently hazardous situation which, if not avoided, will result in death or serious injury. WARNING indicates a potentially hazardous situation which, if not avoided, could result
Duraloc CONSTRAINED LINER
 SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
INBONE II. Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT TIBIAL REAMING SPECIFICATION
 INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT TIBIAL REAMING SPECIFICATION INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT Tibial Reaming Specification Contents Chapter 1 4 Product
INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT TIBIAL REAMING SPECIFICATION INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT Tibial Reaming Specification Contents Chapter 1 4 Product
Implantable K-wire SURGIC A L T ECHNIQUE
 Implantable K-wire SURGIC A L T ECHNIQUE Contents Chapter 1 4 Product Information 4 Device Description 4 Indications 4 Contraindications Chapter 2 5 Surgical Technique 5 Hammertoe Correction 6 MTP Joint
Implantable K-wire SURGIC A L T ECHNIQUE Contents Chapter 1 4 Product Information 4 Device Description 4 Indications 4 Contraindications Chapter 2 5 Surgical Technique 5 Hammertoe Correction 6 MTP Joint
Ascension. MCP surgical technique. surgical technique Ascension MCP PyroCarbon Total Joint
 Ascension MCP surgical technique PyroCarbon Total Joint WW 1.0 Table of Contents 2.0 Introduction............................ 2 3.0 Ascension MCP Implants................ 2 4.0 Instrumentation for MCP
Ascension MCP surgical technique PyroCarbon Total Joint WW 1.0 Table of Contents 2.0 Introduction............................ 2 3.0 Ascension MCP Implants................ 2 4.0 Instrumentation for MCP
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
TriboFit Hip System. Issue 3 - August 2013 English
 Important Information: Please read prior to use in a clinical setting. The surgeon should be familiar with the operative technique and all the information in this insert. Description: The TriboFit Hip
Important Information: Please read prior to use in a clinical setting. The surgeon should be familiar with the operative technique and all the information in this insert. Description: The TriboFit Hip
Metacarpophalangeal Joint Implant Arthroplasty REHABILITATION PROTOCOL
 Andrew McNamara, MD The Orthopaedic and Fracture Clinic 1431 Premier Drive Mankato, MN 56001 507-386-6600 Metacarpophalangeal Joint Implant Arthroplasty REHABILITATION PROTOCOL Patient Name: Date: Diagnosis:
Andrew McNamara, MD The Orthopaedic and Fracture Clinic 1431 Premier Drive Mankato, MN 56001 507-386-6600 Metacarpophalangeal Joint Implant Arthroplasty REHABILITATION PROTOCOL Patient Name: Date: Diagnosis:
Document No Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE
 Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
Integra. Modular Radial Head System SURGICAL TECHNIQUE
 Integra Modular Radial Head System SURGICAL TECHNIQUE Table of Contents System Overview...2 Indications and Contraindications... 3 Modular Radial Head Implant Technique...4 Component Dimensions...8 Implant
Integra Modular Radial Head System SURGICAL TECHNIQUE Table of Contents System Overview...2 Indications and Contraindications... 3 Modular Radial Head Implant Technique...4 Component Dimensions...8 Implant
Progeny Hip Stem. Surgical Protocol and Product Specifications
 Progeny Hip Stem Surgical Protocol and Product Specifications Progeny Hip Stem Introduction With emphasis on maximum stability and ease of use, the StelKast ProgenyTM Hip System provides the surgeon with
Progeny Hip Stem Surgical Protocol and Product Specifications Progeny Hip Stem Introduction With emphasis on maximum stability and ease of use, the StelKast ProgenyTM Hip System provides the surgeon with
Instructions for use for Adler Cemented Total Hip Replacement Prosthesis
 0434 Page 1 of 5 Instructions use Important Inmation on Adler Cemented Femoral Hip Stems and Modular Heads For use by an Accredited Orthopaedic Surgeon only DEVICE DESCRIPTION General Inmation The advancement
0434 Page 1 of 5 Instructions use Important Inmation on Adler Cemented Femoral Hip Stems and Modular Heads For use by an Accredited Orthopaedic Surgeon only DEVICE DESCRIPTION General Inmation The advancement
8 Recovering From HAND FRACTURE SURGERY
 8 Recovering From HAND FRACTURE SURGERY Hand fractures are caused by trauma and result in breaking (fracturing) the phalanges or metacarpals. Surgery involves achieving acceptable alignment and providing
8 Recovering From HAND FRACTURE SURGERY Hand fractures are caused by trauma and result in breaking (fracturing) the phalanges or metacarpals. Surgery involves achieving acceptable alignment and providing
Doc. No.: IU/8 for. Rev. No.: 07 ResTOR Prosthesis
 0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Modular Ulnar Head surgical technique. Transforming Extremities
 First Choice Modular Ulnar Head surgical technique Transforming Extremities instrumentation Head and Collar Trials Assembly Pad Starter Awl Trial Extractor Osteotomy Guide Stem Trials Implant Impactor
First Choice Modular Ulnar Head surgical technique Transforming Extremities instrumentation Head and Collar Trials Assembly Pad Starter Awl Trial Extractor Osteotomy Guide Stem Trials Implant Impactor
Important notice: the device(s) can be prescribed and implanted only by a doctor legally authorized to perform this type of surgery.
 rev.10 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH - EVOLIS/GMK KNEE PROSTHESIS - INSTRUCTIONS FOR USE Important notice: the device(s) can be prescribed
rev.10 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH - EVOLIS/GMK KNEE PROSTHESIS - INSTRUCTIONS FOR USE Important notice: the device(s) can be prescribed
Integra. Modular Radial Head System SURGICAL TECHNIQUE
 Integra Modular Radial Head System SURGICAL TECHNIQUE Table of Contents System Overview...2 Indications and Contraindications... 3 Modular Radial Head Implant Technique...4 Component Dimensions...8 Implant
Integra Modular Radial Head System SURGICAL TECHNIQUE Table of Contents System Overview...2 Indications and Contraindications... 3 Modular Radial Head Implant Technique...4 Component Dimensions...8 Implant
Salto Talaris Total Ankle Prosthesis
 Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
INTELLIGENT SPINAL SYSTEM
 INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
Reprocessing Guide. Autoclavable Arthroscope and Hardware Sterilization Tray
 Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
SWANSON. Flexible Finger Joint Implant SURGIC AL TECHNIQUE
 SWANSON Flexible Finger Joint Implant SURGIC AL TECHNIQUE Contents Preface 4 Chapter 1 5 5 6 7 7 7 8 Chapter 2 9 9 15 15 16 19 19 Appendix A 20 Appendix B 22 Design Surgeon Introduction Device Description
SWANSON Flexible Finger Joint Implant SURGIC AL TECHNIQUE Contents Preface 4 Chapter 1 5 5 6 7 7 7 8 Chapter 2 9 9 15 15 16 19 19 Appendix A 20 Appendix B 22 Design Surgeon Introduction Device Description
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
STERILE IMPLANT PRODUCT INSERT PATIENT-FITTED TEMPOROMANDIBULAR JOINT RECONSTRUCTION PROSTHESIS SYSTEM
 STERILE IMPLANT PRODUCT INSERT PATIENT-FITTED TEMPOROMANDIBULAR JOINT RECONSTRUCTION PROSTHESIS SYSTEM CAUTION United States Federal Law restricts this device to sale by or on the order of a physician.
STERILE IMPLANT PRODUCT INSERT PATIENT-FITTED TEMPOROMANDIBULAR JOINT RECONSTRUCTION PROSTHESIS SYSTEM CAUTION United States Federal Law restricts this device to sale by or on the order of a physician.
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
Hip Resurfacing System
 Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
Integra. Spider and Mini Spider Limited Wrist Fusion System SURGICAL TECHNIQUE
 Integra Spider and Mini Spider Limited Wrist Fusion System SURGICAL TECHNIQUE Table of contents Description... 02 Indications... 02 Contraindications... 02 Surgical Technique... 03 Spider Introduction-Four
Integra Spider and Mini Spider Limited Wrist Fusion System SURGICAL TECHNIQUE Table of contents Description... 02 Indications... 02 Contraindications... 02 Surgical Technique... 03 Spider Introduction-Four
SURGICAL TECHNIQUE CHI. Cannulated Hemi Implants 1 ST MPJ ARTHROPLASTY
 CHI Cannulated Hemi Implants 1 ST MPJ ARTHROPLASTY SURGICAL TECHNIQUE CHI Cannulated Hemi Implants Design Rationale Pain of the great toe joint often leads to altered lifestyles. The goal of MPJ implant
CHI Cannulated Hemi Implants 1 ST MPJ ARTHROPLASTY SURGICAL TECHNIQUE CHI Cannulated Hemi Implants Design Rationale Pain of the great toe joint often leads to altered lifestyles. The goal of MPJ implant
CableFIX Xpress Carpometacarpal Fixation System. Operative technique
 CableFIX Xpress Carpometacarpal Fixation System Operative technique CableFIX Xpress Carpometacarpal Fixation System CableFIX Xpress Carpometacarpal Fixation System Contents 1. Indications and contraindications...
CableFIX Xpress Carpometacarpal Fixation System Operative technique CableFIX Xpress Carpometacarpal Fixation System CableFIX Xpress Carpometacarpal Fixation System Contents 1. Indications and contraindications...
Rehabilitation after Total Elbow Arthroplasty
 Rehabilitation after Total Elbow Arthroplasty Total Elbow Atrthroplasty Total elbow arthroplasty (TEA) Replacement of the ulnohumeral articulation with a prosthetic device. Goal of TEA is to provide pain
Rehabilitation after Total Elbow Arthroplasty Total Elbow Atrthroplasty Total elbow arthroplasty (TEA) Replacement of the ulnohumeral articulation with a prosthetic device. Goal of TEA is to provide pain
For use by an Accredited Orthopaedic Surgeon only
 Instructions use Page 1 of 6 For use by an Accredited Orthopaedic Surgeon only 1. DEVICE DESCRIPTION: The advancement of partial and total hip replacement has provided surgeons with the means of restoring
Instructions use Page 1 of 6 For use by an Accredited Orthopaedic Surgeon only 1. DEVICE DESCRIPTION: The advancement of partial and total hip replacement has provided surgeons with the means of restoring
Foot & Ankle. Smart Toe II. Intramedullary Implant. Operative Technique. Foot & Ankle
 Foot & Ankle Smart Toe II Intramedullary Implant Operative Technique Foot & Ankle Smart Toe This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments.
Foot & Ankle Smart Toe II Intramedullary Implant Operative Technique Foot & Ankle Smart Toe This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments.
LOWER EXTREMITIES SINGLE-USE INSTRUMENTATION SURGICAL IMPLANTATION TECHNIQUE. First Metatarsal Phalangeal Joint FOOT THE DIFFERENCE IS MOVING.
 LOWER EXTREMITIES SINGLE-USE INSTRUMENTATION SURGICAL IMPLANTATION TECHNIQUE First Metatarsal Phalangeal Joint FOOT THE DIFFERENCE IS MOVING. CARTIVA SYNTHETIC CARTILAGE IMPLANT SURGICAL IMPLANTATION TECHNIQUE
LOWER EXTREMITIES SINGLE-USE INSTRUMENTATION SURGICAL IMPLANTATION TECHNIQUE First Metatarsal Phalangeal Joint FOOT THE DIFFERENCE IS MOVING. CARTIVA SYNTHETIC CARTILAGE IMPLANT SURGICAL IMPLANTATION TECHNIQUE
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician.
 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH Mpact 3D Metal Implants and Augments 3D Metal INSTRUCTION FOR USE Important notice: the device(s) can
CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH Mpact 3D Metal Implants and Augments 3D Metal INSTRUCTION FOR USE Important notice: the device(s) can
Ceramic Femoral Heads and Acetabular Cup Liners
 Important Information: Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution: Federal (U.S.A) law restricts this device to sale by or on the
Important Information: Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution: Federal (U.S.A) law restricts this device to sale by or on the
ORTHOSPHERE Spherical Interpositional Implant
 ORTHOSPHERE Spherical Interpositional Implant ORTHOSPHERE spherical interpositional implant surgical technique presented by J. H. CALANDRUCCIO, MD M. T. JOBE, MD Proper surgical procedures and techniques
ORTHOSPHERE Spherical Interpositional Implant ORTHOSPHERE spherical interpositional implant surgical technique presented by J. H. CALANDRUCCIO, MD M. T. JOBE, MD Proper surgical procedures and techniques
Integra. Silicone MCP Joint Prosthesis SURGICAL TECHNIQUE
 Integra Silicone MCP Joint Prosthesis SURGICAL TECHNIQUE Table of Contents Introduction Indications For Use...2 Contraindications...2 Warnings and Precautions... 2 System Overview... 3 Surgical Technique
Integra Silicone MCP Joint Prosthesis SURGICAL TECHNIQUE Table of Contents Introduction Indications For Use...2 Contraindications...2 Warnings and Precautions... 2 System Overview... 3 Surgical Technique
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
