Fracture Fixation The following languages are included in this packet:
|
|
|
- Russell Bryan
- 6 years ago
- Views:
Transcription
1 Fracture Fixation The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk) For additional languages, visit our website Then click on the Prescribing Information option. For additional information and translations please contact the manufacturer or local distributor. M C 0086* P Wright Medical Technology, Inc. Wright Medical UK Ltd 1023 Cherry Road 3rd Avenue Memphis, TN Letchworth U.S.A. Herts, SG6 2JF. UK * the CE-Marking of Conformity is applied per catalog number and appears on the outer label, if applicable. October 2013 Printed in U.S.A indd 1
2 indd 2
3 Attention Operating Surgeon IMPORTANT MEDICAL INFORMATION WRIGHT MEDICAL Fracture Fixation ( ) EN Outline: I. General PRODUCT Information A. PATIENT SELECTION B. CONTRAINDICATIONS C. POTENTIAL COMPLICATIONS AND ADVERSE REACTIONS D. PRECAUTIONS E. HANDLING AND STERILIZATION F. STORAGE CONDITIONS II. Specific PRODUCT Information A. LOCON-T Distal Radius Plating System B. LOCON VLS Distal Radius Plating System C. EVOLVE Radial Head Plate System D. EVOLVE EPS System E. MICRONAIL Intramedullary Distal Radius System F. CHARLOTTE MTP Bone Fusion Plate indd 3
4 G. CHARLOTTE Multi-Use Compression Screw H. CHARLOTTE Compression Staple I. CHARLOTTE Quick Staple J. CHARLOTTE Snap-Off Screw K. CHARLOTTE Claw Plate L. CHARLOTTE 7.0 Multi-Use Compression Screw M. CHARLOTTE Carolina Jones Fracture System N. CHARLOTTE LisFranc Bone Screw O. CHARLOTTE LisFranc Plate DEFINITIONS Symbols and abbreviations may be used on the package label. The following table provides the definition of these symbols and abbreviations. Table 1. Definitions of Symbols and Abbreviations 4 Symbol g h D Definition Batch code Catalog number Do not re-use indd 4
5 Y i H l p N M P[]\ I K STERILE GAS J Caution, consult accompanying documents Consult operating instructions Use by Temperature limitation Keep dry Keep away from sunlight Date of manufacture Manufacturer Authorized EC Representative in the European Community Sterilized using ethylene oxide Sterilized using radiation Sterilized using gas plasma Sterilized using aseptic processing techniques For prescription use only indd 5
6 Abbreviation Ti Ti6Al4V CoCr SS UHMWPE Material Titanium Titanium Alloy Cobalt Chrome Alloy Stainless Steel Ultra High Molecular Weight Polyethylene 6 I. General PRODUCT Information Through the advancement of surgical fusion hardware, the surgeon has been provided a means of correcting deformity and reducing pain for many patients. While the implants used are largely successful in attaining these goals, it must be recognized that they are manufactured from metal, and that no implant can be expected to withstand the activity levels and loads as would normal, healthy bone after fusion occurs. Each patient must be evaluated by the surgeon to determine the risk/benefit relationship. In using fusion implants, the surgeon should be aware of the following: The correct selection and sizing of the implant is extremely important. Selection of the proper size, shape, and design of the implant increases the potential for success. The implants require careful seating and adequate bone support. In selecting patients for surgery, the following factors can be critical to the eventual success of the procedure: indd 6
7 1. Patient s occupation or activity. If the patient is involved in an occupation or activity which includes substantial lifting or muscle strain, the resultant forces can cause failure of the fixation, the device, or both. The implant will not restore function to the level expected with normal healthy bone, and the patient should not have unrealistic functional expectations. 2. Condition of senility, mental illness, or alcoholism. These conditions, among others, may cause the patient to ignore certain necessary limitations and precautions in the use of the implant, leading to failure or other complications. 3. Foreign body sensitivity. Where material sensitivity is suspected, appropriate tests should be made prior to material selection or implantation. A. PATIENT SELECTION Use of surgical fusion hardware requires consideration of the following general indications: Good condition of the patient Good neurovascular status Adequate skin coverage Possibility of a functional musculotendinous system Adequate bone stock to receive implant Availability of post-operative therapy Cooperative patient See Section II for specific product information indd 7
8 8 B. CONTRAINDICATIONS Infection Physiologically or psychologically inadequate patient Inadequate skin, bone, or neurovascular status Irreparable tendon system Possibility for conservative treatment Growing patients with open epiphyses Patients with high levels of activity C. POTENTIAL COMPLICATIONS AND ADVERSE REACTIONS In any surgical procedure, the potential for complications exists. The risks and complications with these implants include: Infection or painful, swollen or inflamed implant site Fracture of the implant Loosening or dislocation of the implant requiring revision surgery Bone resorption or over-production Allergic reaction(s) to implant material(s) Untoward histological responses possibly involving macrophages and/or fibroblasts Migration of particle wear debris possibly resulting in a bodily response Embolism See Section II for specific product information indd 8
9 D. PRECAUTIONS Following the instructions for use provided in product literature can minimize the potential for complications or adverse reactions with any implant. It is the responsibility of each surgeon using implants to consider the clinical and medical status of each patient and to be knowledgeable about all aspects of implant procedure and the potential complications that may occur. The benefits derived from implant surgery may not meet the patient s expectations or may deteriorate with time, necessitating revision surgery to replace the implant or to carry out alternative procedures. Revision surgeries with implants are common. The patient s mental status must also be considered. Willingness and/or ability to follow post-operative instructions may also impact the surgical outcome. Surgeons must balance many considerations to achieve the best result in individual patients. IF EXCESSIVE LOADING CANNOT BE PREVENTED, AN IMPLANT SHOULD NOT BE USED. The main goal of surgery with this implant is to establish bony fusion. Abnormal or excessive forces could lead to delayed union, non-union, or failure of the implant. Abnormal force loading and subsequent wear may be caused by: Uncorrected instability Improperly sized implant Inadequate soft tissue support Implant malposition Excessive motion indd 9
10 10 Uncorrected or recurrent deformity Patient misuse or overactivity Proper fixation at the time of surgery is critical to the success of the procedure. Bone stock must be adequate to support the device. Some preventative measures to consider to minimize the potential for complications: Follow guidelines for indications and contraindications provided above Identify prior pathology Stabilize collapse deformities Bone graft pre-existing cysts Use a properly sized implant Avoid K-wires and sutures through the implant Avoid flawing implant surfaces to minimize the potential for early fatigue failure. If complications develop, possible corrective procedures include: Implant removal Synovectomy Bone grafting of cysts Replacement of the implant Removal of the implant with fusion of the joint Over time, metallic implants may loosen, fracture, or cause pain after the bone fracture or osteotomy is healed. Removal of metallic implants is at the surgeon s discretion, and indd 10
11 the appropriateness of the selected procedure will be based on the surgeon s personal medical training and experience. It is imperative that adequate post-operative care and protection be provided by the surgeon. Recommendations Regarding Device Fragments 1. Use medical devices in accordance with their labeled indications and the manufacturer s instructions for use, especially during insertion and removal. 2. Inspect devices prior to use for damage during shipment or storage or any out-of-box defects that might increase the likelihood of fragmentation during a procedure. 3. Inspect devices immediately upon removal from the patient for any signs of breakage or fragmentation. 4. If the device is damaged, retain it to assist with the manufacturer s analysis of the event. 5. Carefully consider and discuss with the patient (if possible) the risks and benefits of retrieving vs. leaving the fragment in the patient. 6. Advise the patient of the nature and safety of unretrieved device fragments including the following information: a. The material composition of the fragment (if known); b. The size of the fragment (if known); c. The location of the fragment; d. The potential mechanisms for injury, e.g., migration, infection; e. Procedures or treatments that should be avoided such as MRI exams in the case of metallic fragments. This may help to reduce the possibility of a serious injury from the fragment indd 11
12 Clinical results depend on surgeon and technique, pre-operative and post-operative care, the implant, patient pathology and daily activity. It is important that surgeons obtain appropriate informed consent and discuss the potential for complications with each patient prior to surgery. This may include a review of alternative, non-implant procedures such as soft tissue reconstruction or arthrodesis. Concerning Magnetic Resonance Environments The devices described in this package insert have not been evaluated for safety and compatibility in the MR environment. The devices described in this package insert have not been tested for heating or migration in the MR environment. See Section II for specific product information. E. HANDLING AND STERILIZATION IMPLANTS The implants in this system are either provided sterile or non-sterile; the individual product s labeling will determine whether or not it is packaged sterile. Implants that are presented in instrument trays are provided non-sterile. Implants in sterile packaging should be inspected to ensure that the packaging has not been damaged or previously opened. The implants should be opened using aseptic OR technique; they should only be opened after the correct size has been determined. Implants provided non-sterile should be processed according to the recommended parameters for instruments (below). An implant should never be re-sterilized after contact with body tissues or fluids indd 12
13 Devices labeled for single-use only should never be reused. Reuse of these devices may potentially result in serious patient harm. Examples of hazards related to the reuse of these devices include, but are not limited to: significant degradation in device performance, cross-infection, and contamination INSTRUMENTS Surgical instruments (and non-sterile implants) should be cleaned and sterilized according to the following parameters: Cleaning & Disinfection Clean to remove gross contamination and disinfect to reduce the number of viable microorganisms. 1. Disassemble as per manufacturer instructions (if appropriate). 2. Rinse with cold tap water to remove gross contamination. 3. Bathe in an enzymatic detergent solution prepared per manufacturer directions for 5 minutes. 4. Scrub thoroughly with a soft brush and/or pipe cleaner; repeatedly flush any very narrow lumens with enzymatic detergent solution using a syringe. 5. Rinse with cold tap water for a minimum of one minute; use a syringe to repeatedly flush any very narrow lumens. 6. Bathe in a detergent solution prepared per manufacturer directions for 5 minutes. 7. Scrub thoroughly with a soft brush and/or pipe cleaner; repeatedly flush any very narrow lumens with detergent solution using a syringe indd 13
14 8. Rinse thoroughly / flush with deionized / reverse osmosis (RO/DI) water. 9. Sonicate for a minimum of 10 minutes in an enzymatic detergent solution prepared per manufacturer directions. 10. Rinse thoroughly / flush with RO/DI water. 11. Dry with a clean, soft, absorbent, disposable cloth. 12. Visually inspect for cleanliness. All visible surfaces, internal and external, should be visually inspected. If necessary re-clean until it is visibly clean. Note: Brushes (i.e. pipe cleaners) could be used for cleaning most lumens, however, the use of a syringe to flush narrow lumens with diameters less than or equal to inches is recommended. Sterilization 1. Double wrap the component in CSR wrap or a similar type non-woven medical grade wrapping material. 2. Autoclave according to the following parameters: 14 Steam Sterilization Cycle Type Parameter Minimum Set Point Exposure Temperature 270 F (132 C) Prevacuum 270 F (132 C) Exposure Time 4 minutes Dry Time 20 minutes indd 14
15 3. After sterilization, remove the component from its wrapping using accepted sterile technique with powder-free gloves. Ensure that implants are at room temperature prior to implantation. Avoid contact with hard objects that may cause damage. These recommendations are consistent with AAMI ST79 Table 5 guidelines and have been developed and tested using specific equipment. Due to variations in environment and equipment, it must be demonstrated that these recommendations produce sterility in your environment. If processing conditions, wrapping materials, or equipment changes occur, the effectiveness of the sterilization process must be demonstrated. For additional information see Wright s Cleaning and Handling of Wright Medical Instruments. F. STORAGE CONDITIONS All implants must be stored in a clean, dry environment and be protected from sunlight and extremes in temperature. II. Specific PRODUCT Information A. Locon-T Distal Radius Plating System The LOCON-T Distal Radial Plating System consists of right and left dorsal bone plates, volar T-plates, cancellous screws, cortical screws, buttress pins, and a dorsal plate extender. All components are manufactured from stainless steel indd 15
16 INDICATIONS Use of the LOCON-T Distal Radial Plating System is intended to be used for fixation of unstable distal radial fractures in which closed reduction is not suitable: Joint destruction and/or subluxation visible on x-ray; Failed fracture fixation with or without bone graft; Osteotomy and repair of distal radius malunion with or without bone graft; Displaced or non-displaced fracture which may or may not involve angulation or fragmentation of bone; Dorsal plates are indicated for use with comminuted articular fractures, shearing fractures of the articular surface, severely comminuted extra-articular fractures, and fractures in which reduction has been lost following fixation with percutaneous pins with or without an external fixator; T-plates are indicated for use with volar articular shearing fractures. CONTRAINDICATIONS The LOCON-T Distal Radial Plating System is contraindicated for the following: In patients with probable history of infection or current infection Overt infection Skeletally immature patients indd 16
17 B. LOCON VLS Distal Radius Plating System The Locon VLS Distal Radius Plating System consists of right and left volar bone plates, cancellous screws, and cortical screws. All components are manufactured from stainless steel. INDICATIONS Use of the Locon VLS Distal Radius Plating System is intended to be used for fixation of unstable distal radial fractures in which closed reduction is not suitable: Joint destruction and/or subluxation visible on x-ray; Failed fracture fixation with or without bone graft; Osteotomy and repair of distal radius malunion with or without bone graft; Displaced or non-displaced fracture which may or may not involve angulation or fragmentation of bone; Volar plates are indicated for use with comminuted articular fractures, shearing fractures of the articular surface, severely comminuted extra-articular fractures, and fractures in which reduction has been lost following fixation with percutaneous pins with or without an external fixator; Locking Volar plates are indicated for use with volar articular shearing fractures indd 17
18 CONTRAINDICATIONS The Locon VLS Distal Radius Plating System is contraindicated for the following: In patients with probable history of infection or current infection Overt infection Skeletally immature patients C. EVOLVE Radial Head Plate System The EVOLVE Radial Head Plate System consists of plates, cancellous screws, and locking screws. All components are manufactured from stainless steel. INDICATIONS Use of the EVOLVE Radial Head Plate System is intended to be used for fixation of unstable radial fractures in which closed reduction is not suitable. CONTRAINDICATIONS The EVOLVE Radial Plate System is contraindicated for the following: In patients with probable history of infection or current infection Overt infection Skeletally immature patients indd 18
19 D. EVOLVE EPS System The EVOLVE EPS System consists of a variety of pre-contoured plate geometries. The plates feature compression slots and locking screw holes. The associated screws are available in a range of lengths. All components are manufactured from stainless steel. INDICATIONS The EVOLVE EPS System is intended for fixation of fractures, osteotomies and nonunions of the olecranon, humerus, radius and ulna. CONTRAINDICATIONS The EVOLVE EPS System is contraindicated for the following: In patients with probable history of infection or current infection Overt infection Skeletally immature patients E. MICRONAIL Intramedullary Distal Radius System The MICRONAIL Intramedullary Distal Radius System consists of Distal Radius Implants, Cortical Bone Screws, and Buttress Screws. All components are manufactured from Titanium indd 19
20 INDICATIONS The MICRONAIL Intramedullary Distal Radius System is intended to be used for the fixation of unstable distal radius fractures in which closed reduction is not suitable: Joint destruction and/or subluxation visible on x-ray; Failed fracture fixation with or without bone graft; Osteotomy and repair of distal radius malunion with or without bone graft; Displaced or non-displaced fracture which may or may not involve angulation or fragmentation of bone; Comminuted articular fractures, shearing fractures of the articular surface, severely comminuted extra-articular fractures, and fractures in which reduction has been lost following fixation with percutaneous pins with or without an external fixator. CONTRAINDICATIONS The MICRONAIL Intramedullary Distal Radius System is contraindicated for the following: In patients with probable history of infection or current infection Overt infection Skeletally immature patients indd 20
21 F. CHARLOTTE MTP Bone Fusion Plate The CHARLOTTE MTP Bone Fusion Plate System consists of plates in left and right configurations and screws. All screws and plates are manufactured from stainless steel. INDICATIONS The CHARLOTTE MTP Bone Fusion Plate System is intended to help increase the rate of bony union, and to maintain the position of the toe during fusion. Once the joint has fused, the plate is secondary in the transmission of gait forces. Indications for Use: Fractures, osteotomies or arthrodesis of the first metatarsal-phalangeal joint Deformity due to hallux valgus Deformity due to arthritis in the first metatarsal-phalangeal joint Loss of motion- hallux rigidus Pain associated with osteoarthritis or rheumatoid arthritis in the first metatarsalphalangeal joint Revision procedures where other treatments or devices have failed; and Chronic instability in the first metatarsal-phalangeal joint indd 21
22 G. CHARLOTTE Multi-Use Compression Screw The CHARLOTTE Multi-Use Compression Screw is offered in various diameters and lengths. It is offered in short and long thread lengths and has self drilling and self tapping features on both distal and proximal threads. All screws are manufactured from stainless steel. INDICATIONS The CHARLOTTE Multi-Use Compression Screw is indicated for fixation of bone fractures or for bone reconstruction. Examples include: Mono or Bi-Cortical osteotomies in the foot or hand Distal or Proximal metatarsal or metacarpal osteotomies Weil osteotomy Fusion of the first metatarsophalangeal joint and interphalangeal joint Fixation of osteotomies for Hallux Valgus treatment (such as Scarf, Chevron, etc.) Akin type osteotomy Arthrodesis base first metatarsal cuneiform joint to reposition and stabilize metatarsus varus primus Calcaneus/ cuboid arthrodesis Talar/ navicular arthrodesis indd 22
23 H. CHARLOTTE Compression Staple The CHARLOTTE Compression Staple is offered in several sizes with barbs to prevent back out and a diamond shape slot compression feature. All staples are manufactured from stainless steel. INDICATIONS The CHARLOTTE Compression Staple is intended to be used for fixation such as: LisFranc arthrodesis, mono or bi-cortical osteotomies in the forefoot, first metatarsophalangeal arthrodesis, Akin osteotomy, midfoot and hindfoot arthrodeses or osteotomies, fixation of osteotomies for hallux valgus treatment (Scarf and Chevron), and arthrodesis of the metatarsocuneiform joint to reposition and stabilize metatarsus primus varus. I. charlotte Quick Staple The CHARLOTTE Quick Staple features barbs to prevent back out. All staples are manufactured from stainless steel. INDICATIONS The CHARLOTTE Quick Staple is intended to be used for wedge osteotomy of the first phalanx (Akin osteotomy), in the treatment of hallux-valgus in order to correct a remaining valgus or pronation of the first ray, and external rotation, and wind-swept toes indd 23
24 J. CHARLOTTE Snap-Off Screw The CHARLOTTE Snap-Off Screw is offered in various diameters and lengths. All screws are manufactured from titanium. INDICATIONS The CHARLOTTE Snap-Off Screw is indicated for fixation of bone fractures or for bone reconstruction. Examples include: Fixation of Small Bone Fragments Weil osteotomy Mono-cortical fixation Osteotomies and fractures fixation in the foot and hand 24 K. charlotte Claw Plate The CHARLOTTE CLAW Plate consists of plates and locking-screws in various lengths. All plates and screws are manufactured from stainless steel. INDICATIONS The CHARLOTTE CLAW Plate is intended to be used for fixation such as: LisFranc arthrodesis, mono or bi-cortical osteotomies in the forefoot, first metatarsophalangeal arthrodesis, Akin osteotomy, midfoot and hindfoot arthrodeses or osteotomies, fixation indd 24
25 of osteotomies for hallux valgus treatment (Scarf and Chevron), and arthrodesis of the metatarsocuneiform joint to reposition and stabilize metatarsus primus varus. L. charlotte 7.0 mm Multi-Use Compression Screw The CHARLOTTE 7.0 mm Multi-Use Compression Screw is a self drilling screw offered in various lengths and distal thread lengths. Washers are offered for oblique and straight screw placement. All screws and washers are manufactured from stainless steel. INDICATIONS The CHARLOTTE 7.0 mm Multi-Use Compression Screw is indicated for fixation of bone fractures or for bone reconstruction. Examples include: Fixation of bone fragments, in long bones or small bones fractures Fracture management in the foot or hand Arthrodesis in hand, foot or ankle surgery Mono- or Bi-cortical osteotomies in the foot or hand or in long bones Hindfoot arthrodesis M. charlotte Carolina Jones Fracture System The CHARLOTTE Carolina Jones Fracture Screw is offered in various diameters and lengths. All screws are manufactured from stainless steel indd 25
26 INDICATIONS The CHARLOTTE Carolina Jones Fracture System is indicated for fixation of bone fractures or for bone reconstruction of the 5th Metatarsal. Examples include: Fixation of malunions and nonunions Acute fractures Avulsion fractures Repetitive stress fractures Jones Fractures Malleolar Fractures Talus Fractures Greater Tuberosity Fractures N. charlotte LisFranc Bone Screw The CHARLOTTE LisFranc Bone Screw is offered in various diameters and lengths. All screws are manufactured from stainless steel. INDICATIONS The CHARLOTTE LisFranc Bone Screw is intended to be used for fixation such as: LisFranc arthrodesis, first metatarsophalangeal arthrodesis, midfoot and hindfoot arthrodeses or osteotomies, fixation of osteotomies for hallux valgus treatment (Scarf indd 26
27 and Chevron), and arthrodesis of the metatarsocuneiform joint to reposition and stabilize metatarsus primus varus. O. charlotte LisFranc Plate The CHARLOTTE LisFranc Plate consists of plates, non-locking screws, and locking screws. All components are manufactured from stainless steel. INDICATIONS The CHARLOTTE LisFranc Plate is intended to be used for fixation such as: LisFranc arthrodesis, mono or bi-cortical osteotomies in the forefoot, first metatarsophalangeal arthrodesis, Akin osteotomy, midfoot and hindfoot arthrodeses or osteotomies, fixation of osteotomies for hallux valgus treatment (Scarf and Chevron), and arthrodesis of the metatarsocuneiform joint to reposition and stabilize metatarsus primus varus. This implant should only be used with a CHARLOTTE plate and screw system. Combination with other implants or instruments is not permissible. Trademarks and Registered Trademarks are owned by Wright Medical Technology, Inc indd 27
DARCO MIS FOREFOOT SCREW
 DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM
 EN CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM 150871-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
EN CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM 150871-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
The following languages are included in this packet:
 DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
MICA SCREWS For additional information and translations please contact the manufacturer or local distributor.
 MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE
 ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
BIOFOAM BONE WEDGE
 BIOFOAM BONE WEDGE 150837-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
BIOFOAM BONE WEDGE 150837-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
Metallic Internal Fixation Devices The following languages are included in this packet:
 Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
EXTERNAL FIXATION SYSTEMS The following languages are included in this packet:
 EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
STABILIZATION AND FRACTURE FIXATION
 STABILIZATION AND FRACTURE FIXATION 150838-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
STABILIZATION AND FRACTURE FIXATION 150838-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
DARCO HEADED CANNULATED SCREWS
 DARCO HEADED CANNULATED SCREWS 151677-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO HEADED CANNULATED SCREWS 151677-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM
 METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM The following languages are included in this packet:
 ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
METAL HEMI SYSTEM
 METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM
 ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM 150884-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM 150884-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
SALVATION EXTERNAL FIXATION SYSTEM
 SALVATION EXTERNAL FIXATION SYSTEM 151660-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
SALVATION EXTERNAL FIXATION SYSTEM 151660-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
SALVATION EXTERNAL FIXATION SYSTEM
 SALVATION EXTERNAL FIXATION SYSTEM 151660-2 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
SALVATION EXTERNAL FIXATION SYSTEM 151660-2 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
The following languages are included in this packet:
 OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
CANNULATED SCREW SYSTEM The following languages are included in this packet:
 CANNULATED SCREW SYSTEM 137183-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -Chinese (sch) Türkçe
CANNULATED SCREW SYSTEM 137183-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -Chinese (sch) Türkçe
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE
 CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
CHARLOTTE. 3.0 and 4.3 Multi-Use Compression Screws SURGIC A L T ECHNIQUE
 CHARLOTTE 3.0 and 4.3 Multi-Use Compression Screws SURGIC A L T ECHNIQUE CHARLOTTE 3.0 and 4.3 Multi-Use Compression Screws SURGICAL TECHNIQUE Surgical Technique as described by: Robert Anderson, MD Bruce
CHARLOTTE 3.0 and 4.3 Multi-Use Compression Screws SURGIC A L T ECHNIQUE CHARLOTTE 3.0 and 4.3 Multi-Use Compression Screws SURGICAL TECHNIQUE Surgical Technique as described by: Robert Anderson, MD Bruce
CHARLOTTE. Compression Staple SURGIC A L T ECHNIQUE
 CHARLOTTE Compression Staple SURGIC A L T ECHNIQUE CHARLOTTE Compression Staple SURGICAL TECHNIQUE Surgical Technique as described by: Robert Anderson, MD Bruce Cohen, MD W. Hodges Davis, MD Contents Chapter
CHARLOTTE Compression Staple SURGIC A L T ECHNIQUE CHARLOTTE Compression Staple SURGICAL TECHNIQUE Surgical Technique as described by: Robert Anderson, MD Bruce Cohen, MD W. Hodges Davis, MD Contents Chapter
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
BIOARCH SUBTALAR IMPLANT SYSTEM
 BIOARCH SUBTALAR IMPLANT SYSTEM 137308-4 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
BIOARCH SUBTALAR IMPLANT SYSTEM 137308-4 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
CHARLOTTE Lisfranc. Recontruction System SURGICAL TECHNIQUE
 CHARLOTTE Lisfranc Recontruction System SURGICAL TECHNIQUE Contents Preface 3 Chapter 1 4 Chapter 2 4 4 4 4 4 Chapter 3 5 5 5 6 6 6 7 7 8 Chapter 4 8 8 9 10 Appendix 10 Introduction Indications and Contraindications
CHARLOTTE Lisfranc Recontruction System SURGICAL TECHNIQUE Contents Preface 3 Chapter 1 4 Chapter 2 4 4 4 4 4 Chapter 3 5 5 5 6 6 6 7 7 8 Chapter 4 8 8 9 10 Appendix 10 Introduction Indications and Contraindications
INVISION TOTAL ANKLE REVISION SYSTEM The following languages are included in this packet:
 INVISION TOTAL ANKLE REVISION SYSTEM 151678-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 -
INVISION TOTAL ANKLE REVISION SYSTEM 151678-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 -
BIOFOAM ANKLE SPACER BLOCK
 BIOFOAM ANKLE SPACER BLOCK 145782-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
BIOFOAM ANKLE SPACER BLOCK 145782-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
TOTAL ELBOW SYSTEM The following languages are included in this packet:
 TOTAL ELBOW SYSTEM 115298-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch) Türkçe
TOTAL ELBOW SYSTEM 115298-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch) Türkçe
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
SJO SILICONE IMPLANTS The following languages are included in this packet:
 SJO SILICONE IMPLANTS 150823-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
SJO SILICONE IMPLANTS 150823-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
TOTAL ANKLE SYSTEMS
 TOTAL ANKLE SYSTEMS 150864-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
TOTAL ANKLE SYSTEMS 150864-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
Technique Guide Lapidus Arthrodesis System
 Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
MICA. Minimally Invasive Foot Surgery CHEVRON OSTEOTOMY SURGIC AL TECHNIQUE
 MICA Minimally Invasive Foot Surgery CHEVRON OSTEOTOMY SURGIC AL TECHNIQUE Contents Chapter 1 4 Introduction Chapter 2 5 Indications and Warnings Chapter 3 6 Patient Positioning and Set Up Chapter 4 7
MICA Minimally Invasive Foot Surgery CHEVRON OSTEOTOMY SURGIC AL TECHNIQUE Contents Chapter 1 4 Introduction Chapter 2 5 Indications and Warnings Chapter 3 6 Patient Positioning and Set Up Chapter 4 7
SpeedTip CCS 2.2, 3.0
 PRODUCT INFORMATION SpeedTip CCS 2.2, 3.0 Cannulated Compression Screws APTUS 2 SpeedTip CCS 2.2, 3.0 Cannulated Compression Screws SpeedTip CCS * 2.2, 3.0 Cannulated Compression Screws A new generation
PRODUCT INFORMATION SpeedTip CCS 2.2, 3.0 Cannulated Compression Screws APTUS 2 SpeedTip CCS 2.2, 3.0 Cannulated Compression Screws SpeedTip CCS * 2.2, 3.0 Cannulated Compression Screws A new generation
Integra. ADVANSYS Plating System SURGICAL TECHNIQUE
 Integra ADVANSYS Plating System SURGICAL TECHNIQUE Table of Contents Dorsal Lisfranc Plate Indications...2 Contraindications...2 Description...2 Surgical Technique...3 Surgical Site Preparation...3 Step
Integra ADVANSYS Plating System SURGICAL TECHNIQUE Table of Contents Dorsal Lisfranc Plate Indications...2 Contraindications...2 Description...2 Surgical Technique...3 Surgical Site Preparation...3 Step
FUSEFORCE. Hand Fixation System SURGICAL TECHNIQUE
 FUSEFORCE Hand Fixation System SURGICAL TECHNIQUE Contents Chapter 1 3 Chapter 2 4 Appendix A 7 Intended Use Surgical Technique Ordering Information Indication Sizing Reference Proper surgical procedures
FUSEFORCE Hand Fixation System SURGICAL TECHNIQUE Contents Chapter 1 3 Chapter 2 4 Appendix A 7 Intended Use Surgical Technique Ordering Information Indication Sizing Reference Proper surgical procedures
MICROPORT KNEE SYSTEMS The following languages are included in this packet:
 EN English (en) MICROPORT KNEE SYSTEMS 151251-0 The following languages are included in this packet: For additional languages, visit our website ortho.microport.com Then click on the Prescribing Information
EN English (en) MICROPORT KNEE SYSTEMS 151251-0 The following languages are included in this packet: For additional languages, visit our website ortho.microport.com Then click on the Prescribing Information
ORTHOLOC 3Di. Foot Reconstruction System SURGIC AL TECHNIQUE
 ORTHOLOC 3Di Foot Reconstruction System S C R E W TA R G E T I N G G U I D E SURGIC AL TECHNIQUE SURGEON DESIGN TEAM The ORTHOLOC 3Di Foot Reconstruction System was developed in conjuction with: ORTHOLOC
ORTHOLOC 3Di Foot Reconstruction System S C R E W TA R G E T I N G G U I D E SURGIC AL TECHNIQUE SURGEON DESIGN TEAM The ORTHOLOC 3Di Foot Reconstruction System was developed in conjuction with: ORTHOLOC
Implantable K-wire SURGIC A L T ECHNIQUE
 Implantable K-wire SURGIC A L T ECHNIQUE Contents Chapter 1 4 Product Information 4 Device Description 4 Indications 4 Contraindications Chapter 2 5 Surgical Technique 5 Hammertoe Correction 6 MTP Joint
Implantable K-wire SURGIC A L T ECHNIQUE Contents Chapter 1 4 Product Information 4 Device Description 4 Indications 4 Contraindications Chapter 2 5 Surgical Technique 5 Hammertoe Correction 6 MTP Joint
ORTHOLOC CROSSCHECK TECHNOLOGY OVERVIEW FOR HOSPITAL VALUE ANALYSIS COMMITTEE
 ORTHOLOC CROSSCHECK TECHNOLOGY OVERVIEW FOR HOSPITAL VALUE ANALYSIS COMMITTEE Indications for Use The ORTHOLOC 3Di Foot Reconstruction System is intended for use in stabilization of fresh fractures, revision
ORTHOLOC CROSSCHECK TECHNOLOGY OVERVIEW FOR HOSPITAL VALUE ANALYSIS COMMITTEE Indications for Use The ORTHOLOC 3Di Foot Reconstruction System is intended for use in stabilization of fresh fractures, revision
Variable Angle LCP Forefoot/Midfoot System 2.4/2.7. Procedure specific plates for osteotomies, arthrodeses and fractures of the foot.
 Variable Angle LCP Forefoot/Midfoot System 2.4/2.7. Procedure specific plates for osteotomies, arthrodeses and fractures of the foot. Compression technology Variable angle locking technology Anatomic and
Variable Angle LCP Forefoot/Midfoot System 2.4/2.7. Procedure specific plates for osteotomies, arthrodeses and fractures of the foot. Compression technology Variable angle locking technology Anatomic and
Integra Total Foot System 2
 Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
SWANSON. Hammertoe Implant SURGICAL TECHNIQUE
 Hammertoe Implant SURGICAL TECHNIQUE hammertoe IMPLANT surgical technique presented by LOWELL SCOTT WEIL, DPM HANDLING AND STERILIZATION This product has been sterilized and should be considered sterile
Hammertoe Implant SURGICAL TECHNIQUE hammertoe IMPLANT surgical technique presented by LOWELL SCOTT WEIL, DPM HANDLING AND STERILIZATION This product has been sterilized and should be considered sterile
Precautionary Statement ( )
 Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Ratcheting Compression Plating System. Surgical Technique
 Ratcheting Compression Plating System Surgical Technique Acumed is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches
Ratcheting Compression Plating System Surgical Technique Acumed is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches
Telya. Varisation staple. Surgical Technique. Irish Distributer: O'Mara Medical Tel
 Surgical Technique Indications The varisation is used to stabilize an osteotomy of the great toe proximal phalanx (Akin osteotomy), in order to correct : Hallux valgus interphalangeus, Phalangeal pronation,
Surgical Technique Indications The varisation is used to stabilize an osteotomy of the great toe proximal phalanx (Akin osteotomy), in order to correct : Hallux valgus interphalangeus, Phalangeal pronation,
Page 1 of 7 Doc. No.: IU/15 Rev. No.: 05 Rev. Date:03MAY2018. Instructions for use for ATLAS Tibial Fracture(TF) Nail
 Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
Integra. surgical technique. Advansys Midfoot Plating System. eng. D.L.P. Dorsal Lisfranc Plate. M.L.P. Medial Lisfranc Plate
 eng Integra Advansys Midfoot Plating System surgical technique D.L.P. Dorsal Lisfranc Plate M.L.P. Medial Lisfranc Plate Table of Contents Advansys Medial Lisfranc Plate (DLP)... 4 Indications... 4 Contraindications...
eng Integra Advansys Midfoot Plating System surgical technique D.L.P. Dorsal Lisfranc Plate M.L.P. Medial Lisfranc Plate Table of Contents Advansys Medial Lisfranc Plate (DLP)... 4 Indications... 4 Contraindications...
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Technique Guide Hammertoe Correction System
 Technique Guide Hammertoe Correction System TM The ToeMATE Hammertoe Correction System is an easy to implant bone screw system intended for the correction of hammertoe deformity. It is provided in a complete
Technique Guide Hammertoe Correction System TM The ToeMATE Hammertoe Correction System is an easy to implant bone screw system intended for the correction of hammertoe deformity. It is provided in a complete
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
DRACO FOOT & ANKLE SYSTEM PRODUCT INFORMATION
 DRACO FOOT & ANKLE SYSTEM PRODUCT INFORMATION 2 DRACO FOOT & ANKLE SYSTEM www.hnmtotal.com DRACO Foot & Ankle System CONTENTS MetaFuse L Plate 3 MetaFuse BLP Plate 4 MetaFuse Midfoot/Lisfranc Plate 5 MetaFuse
DRACO FOOT & ANKLE SYSTEM PRODUCT INFORMATION 2 DRACO FOOT & ANKLE SYSTEM www.hnmtotal.com DRACO Foot & Ankle System CONTENTS MetaFuse L Plate 3 MetaFuse BLP Plate 4 MetaFuse Midfoot/Lisfranc Plate 5 MetaFuse
Use of the 20 Memory Staple in Osteotomies of Fusions of the Forefoot
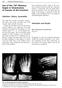 168 Forefoot Reconstruction Use of the 20 Memory Staple in Osteotomies of Fusions of the Forefoot Definition, History, Generalities This staple first provides a permanent compression both in the prongs
168 Forefoot Reconstruction Use of the 20 Memory Staple in Osteotomies of Fusions of the Forefoot Definition, History, Generalities This staple first provides a permanent compression both in the prongs
LOWER EXTREMITIES SINGLE-USE INSTRUMENTATION SURGICAL IMPLANTATION TECHNIQUE. First Metatarsal Phalangeal Joint FOOT THE DIFFERENCE IS MOVING.
 LOWER EXTREMITIES SINGLE-USE INSTRUMENTATION SURGICAL IMPLANTATION TECHNIQUE First Metatarsal Phalangeal Joint FOOT THE DIFFERENCE IS MOVING. CARTIVA SYNTHETIC CARTILAGE IMPLANT SURGICAL IMPLANTATION TECHNIQUE
LOWER EXTREMITIES SINGLE-USE INSTRUMENTATION SURGICAL IMPLANTATION TECHNIQUE First Metatarsal Phalangeal Joint FOOT THE DIFFERENCE IS MOVING. CARTIVA SYNTHETIC CARTILAGE IMPLANT SURGICAL IMPLANTATION TECHNIQUE
Important Information on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only
 0434 Page 1 of 6 Instructions use Important Inmation on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: Atlas Hip Fracture (HF) Nail is an intramedullary interlocking
0434 Page 1 of 6 Instructions use Important Inmation on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: Atlas Hip Fracture (HF) Nail is an intramedullary interlocking
PL ATING SYSTEM. Pre-contoured implants Versatile fixation system Hexalobe screw recess design Locked fixation
 I N N O VA T I O N ME A NS M OT I O N FOOTMOTION PL ATING SYSTEM Pre-contoured implants Versatile fixation system Hexalobe screw recess design Locked fixation FOOTMOTION PLATING SYSTEM Indications: The
I N N O VA T I O N ME A NS M OT I O N FOOTMOTION PL ATING SYSTEM Pre-contoured implants Versatile fixation system Hexalobe screw recess design Locked fixation FOOTMOTION PLATING SYSTEM Indications: The
Compression Screw 3.2 Bioabsorbable. Product information
 Compression Screw 3.2 Bioabsorbable Product information Caution The current product description is not sufficient for the immediate application of instruments and implants. Instructional training by an
Compression Screw 3.2 Bioabsorbable Product information Caution The current product description is not sufficient for the immediate application of instruments and implants. Instructional training by an
WristMotion Wrist Hemiarthroplasty System Instructions for Use
 WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
SALVATION 3Di. Plating System SURGICAL TECHNIQUE
 SALVATION 3Di Plating System SURGICAL TECHNIQUE Contents Chapter 1 4 Introduction Chapter 2 5 Intended use 5 Indications 5 Contraindications Chapter 3 6 Device Description Chapter 4 7 Preoperative Planning
SALVATION 3Di Plating System SURGICAL TECHNIQUE Contents Chapter 1 4 Introduction Chapter 2 5 Intended use 5 Indications 5 Contraindications Chapter 3 6 Device Description Chapter 4 7 Preoperative Planning
Technique Guide. 6.5 mm Midfoot Fusion Bolt. For intramedullary fixation of the medial column of the foot.
 Technique Guide 6.5 mm Midfoot Fusion Bolt. For intramedullary fixation of the medial column of the foot. Table of Contents Introduction 6.5 mm Midfoot Fusion Bolt 2 AO Principles 4 Indications 5 Surgical
Technique Guide 6.5 mm Midfoot Fusion Bolt. For intramedullary fixation of the medial column of the foot. Table of Contents Introduction 6.5 mm Midfoot Fusion Bolt 2 AO Principles 4 Indications 5 Surgical
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
ORTHOLOC 3Di. Foot Reconstruction System Midfoot Fusion Plate SURGICAL TECHNIQUE
 ORTHOLOC 3Di Foot Reconstruction System Midfoot Fusion Plate SURGICAL TECHNIQUE ORTHOLOC Foot Reconstruction System Midfoot Fusion Plate SURGICAL TECHNIQUE Surgeon Design Team The ORTHOLOC 3Di Foot Reconstruction
ORTHOLOC 3Di Foot Reconstruction System Midfoot Fusion Plate SURGICAL TECHNIQUE ORTHOLOC Foot Reconstruction System Midfoot Fusion Plate SURGICAL TECHNIQUE Surgeon Design Team The ORTHOLOC 3Di Foot Reconstruction
SURGICAL TECHNIQUE. Compression screw FOREFOOT SOLUTIONS
 SURGICAL TECHNIQUE Compression screw FOREFOOT SOLUTIONS SCARF OSTEOTOMY The Scarf osteotomy consists of a horizontal cut and two transversal cuts of the first metatarsal, allowing for a broad range of
SURGICAL TECHNIQUE Compression screw FOREFOOT SOLUTIONS SCARF OSTEOTOMY The Scarf osteotomy consists of a horizontal cut and two transversal cuts of the first metatarsal, allowing for a broad range of
SURGICAL TECHNIQUE GUIDE
 DANGER indicates an imminently hazardous situation which, if not avoided, will result in death or serious injury. WARNING indicates a potentially hazardous situation which, if not avoided, could result
DANGER indicates an imminently hazardous situation which, if not avoided, will result in death or serious injury. WARNING indicates a potentially hazardous situation which, if not avoided, could result
Technique Guide Wrist Hemiarthroplasty System
 Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
Knee spanning solutions
 Knee spanning solutions System features Indications Intended to be used on adults or pediatric patients as required for fracture fixation (open or closed); post-traumatic joint contracture which has resulted
Knee spanning solutions System features Indications Intended to be used on adults or pediatric patients as required for fracture fixation (open or closed); post-traumatic joint contracture which has resulted
The Flower Medial Column Fusion Plate
 The Flower Medial Column Fusion Plate PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION
The Flower Medial Column Fusion Plate PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION
MetaFix Ludloff Plate
 Merete MetaFix Ludloff Plate Low Profile Locking Bone Plate System Surgical Technique and Ordering Information - Content - Content 1. Description.................................................. 3 2.
Merete MetaFix Ludloff Plate Low Profile Locking Bone Plate System Surgical Technique and Ordering Information - Content - Content 1. Description.................................................. 3 2.
SWANSON. Flexible Hinge Toe Implant SURGIC AL TECHNIQUE
 SWANSON Flexible Hinge Toe Implant SURGIC AL TECHNIQUE SWANSON flexible HINGE TOE IMPLANT with SWANSON Flexible Hinge Grommet surgical technique presented by ALFRED B. SWANSON, MD, FACS, GRAND RAPIDS,
SWANSON Flexible Hinge Toe Implant SURGIC AL TECHNIQUE SWANSON flexible HINGE TOE IMPLANT with SWANSON Flexible Hinge Grommet surgical technique presented by ALFRED B. SWANSON, MD, FACS, GRAND RAPIDS,
ALLOPURE. Bicortical Allograft Wedges Specific for Evans and Cotton Osteotomies SURGICAL TECHNIQUE
 ALLOPURE Bicortical Allograft Wedges Specific for Evans and Cotton Osteotomies SURGICAL TECHNIQUE Contents Chapter 1 4 Chapter 2 5 5 5 Chapter 3 6 6 7 8 8 Appendix A 9 Introduction Intended Use Indications
ALLOPURE Bicortical Allograft Wedges Specific for Evans and Cotton Osteotomies SURGICAL TECHNIQUE Contents Chapter 1 4 Chapter 2 5 5 5 Chapter 3 6 6 7 8 8 Appendix A 9 Introduction Intended Use Indications
AcUMEDr. AcUTRAKr. Headless Compression Screw
 AcUMEDr AcUTRAKr Headless Compression Screw AcUTRAKr HEADLESS COMPRESSION SCREW Since 1988 Acumed has been designing innovative solutions for the demanding situations facing orthopedic surgeons, hospitals
AcUMEDr AcUTRAKr Headless Compression Screw AcUTRAKr HEADLESS COMPRESSION SCREW Since 1988 Acumed has been designing innovative solutions for the demanding situations facing orthopedic surgeons, hospitals
DARCO. Bow 2 Plate SURGIC AL TECHNIQUE
 DARCO Bow 2 Plate SURGIC AL TECHNIQUE Contents 2 Preface 3 Chapter 1 4 Chapter 2 5 6 7 8 9 Appendix 10 10 11 Intended Use Indications/Contraindications Design Rationale Preoperative Planning Surgical Technique
DARCO Bow 2 Plate SURGIC AL TECHNIQUE Contents 2 Preface 3 Chapter 1 4 Chapter 2 5 6 7 8 9 Appendix 10 10 11 Intended Use Indications/Contraindications Design Rationale Preoperative Planning Surgical Technique
ADVANSYS Midfoot plating system
 T.T.C. Tibio-Talo-Calcaneus plate TTC ENGLISH ADVANSYS Midfoot plating system Surgical technique Or t h o pa e d i c s LOWER EXTREMITY T.T.C. (DORSAL LISFRANC PLATE) Introduction Indications The Newdeal
T.T.C. Tibio-Talo-Calcaneus plate TTC ENGLISH ADVANSYS Midfoot plating system Surgical technique Or t h o pa e d i c s LOWER EXTREMITY T.T.C. (DORSAL LISFRANC PLATE) Introduction Indications The Newdeal
IMPORTANT MEDICAL INFORMATION Advanced Orthopaedic Solutions INTRAMEDULLARY NAILS Warnings and Precautions (SINGLE USE ONLY)
 IMPORTANT MEDICAL INFORMATION Advanced Orthopaedic Solutions INTRAMEDULLARY NAILS Warnings and Precautions (SINGLE USE ONLY) IMPORTANT NOTE Intramedullary nails provide an alternative to open reduction
IMPORTANT MEDICAL INFORMATION Advanced Orthopaedic Solutions INTRAMEDULLARY NAILS Warnings and Precautions (SINGLE USE ONLY) IMPORTANT NOTE Intramedullary nails provide an alternative to open reduction
Flower Medium Headless & Cannulated Screws
 Flower Medium Headless & Cannulated Screws PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION
Flower Medium Headless & Cannulated Screws PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION
RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON
 ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
DYNAMIC, TRANSVERSE COMPRESSION. Low Profile, Anatomic Design, Type II Anodized. Mechanical Compression Designed to Stimulate the Fusion Process
 CoLink_XP_ST_080218.pdf 1 8/2/18 8:36 AM SURGICAL TECHNIQUE CoLink XP Plates DYNAMIC, TRANSVERSE COMPRESSION C M Y CM MY CY CMY K MTP Std. Plate MTP Revision Plate Lapidus Standard +1mm and +2mm Y Plate
CoLink_XP_ST_080218.pdf 1 8/2/18 8:36 AM SURGICAL TECHNIQUE CoLink XP Plates DYNAMIC, TRANSVERSE COMPRESSION C M Y CM MY CY CMY K MTP Std. Plate MTP Revision Plate Lapidus Standard +1mm and +2mm Y Plate
Hallux Valgus Deformity OPERATIVE TECHNIQUE
 Hallux Valgus Deformity OPERATIVE TECHNIQUE Table of Contents 1 ORDERING INFORMATION 3 OPERATIVE TECHNIQUE 9 INDICATIONS FOR USE The surgical technique shown is for illustrative purposes only. The technique(s)
Hallux Valgus Deformity OPERATIVE TECHNIQUE Table of Contents 1 ORDERING INFORMATION 3 OPERATIVE TECHNIQUE 9 INDICATIONS FOR USE The surgical technique shown is for illustrative purposes only. The technique(s)
CHARLOTTE. Snap-Off Screws SURGICAL TECHNIQUE
 CHARLOTTE Snap-Off Screws SURGICAL TECHNIQUE CHARLOTTE snap-off screws surgical technique SURGICAL ADVISORS ROBERT ANDERSON, MD BRUCE COHEN, MD W. HODGES DAVIS, MD Proper surgical procedures and techniques
CHARLOTTE Snap-Off Screws SURGICAL TECHNIQUE CHARLOTTE snap-off screws surgical technique SURGICAL ADVISORS ROBERT ANDERSON, MD BRUCE COHEN, MD W. HODGES DAVIS, MD Proper surgical procedures and techniques
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
TTA Wedge System INSTRUCTIONS FOR USE
 TTA Wedge System INSTRUCTIONS FOR USE 1 INSTRUCTIONS FOR USE The OssAbility TTA Wedge System consists of the following products: Wedge Implants Osteotomy Guide Advancement Levers Osteotomy Planning Overlay
TTA Wedge System INSTRUCTIONS FOR USE 1 INSTRUCTIONS FOR USE The OssAbility TTA Wedge System consists of the following products: Wedge Implants Osteotomy Guide Advancement Levers Osteotomy Planning Overlay
Surgical Technique. Cannulated Angled Blade Plate 3.5 and 4.5, 90
 Surgical Technique Cannulated Angled Blade Plate 3.5 and 4.5, 90 Cannulated Angled Blade Plate 3.5 and 4.5, 90 Table of contents Indications/Contraindications 2 Implants 3 Surgical technique 5 Implant
Surgical Technique Cannulated Angled Blade Plate 3.5 and 4.5, 90 Cannulated Angled Blade Plate 3.5 and 4.5, 90 Table of contents Indications/Contraindications 2 Implants 3 Surgical technique 5 Implant
Flower Opening Wedge Plate
 Flower Opening Wedge Plate PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION T-PLATE, OBLIQUE
Flower Opening Wedge Plate PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION T-PLATE, OBLIQUE
Zimmer Small Fragment Universal Locking System. Surgical Technique
 Zimmer Small Fragment Universal Locking System Surgical Technique Zimmer Small Fragment Universal Locking System 1 Zimmer Small Fragment Universal Locking System Surgical Technique Table of Contents Introduction
Zimmer Small Fragment Universal Locking System Surgical Technique Zimmer Small Fragment Universal Locking System 1 Zimmer Small Fragment Universal Locking System Surgical Technique Table of Contents Introduction
Indications: ATLAS HF Nail is indicated for fractures of the femur including: Contraindications:
 Page 1 of 6 Important Inmation on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: Atlas Hip Fracture (HF) Nail is an intramedullary interlocking nail with
Page 1 of 6 Important Inmation on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: Atlas Hip Fracture (HF) Nail is an intramedullary interlocking nail with
The Flower Forefoot PROCEDURE GUIDE.
 The Flower Forefoot PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION T-PLATE, OBLIQUE MTP
The Flower Forefoot PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION T-PLATE, OBLIQUE MTP
CHARLOTTE. 7.0 Multi-Use Compression Screw System SURGICAL TECHNIQUE
 CHARLOTTE 7.0 Multi-Use Compression Screw System SURGICAL TECHNIQUE Contents Chapter 1 1 Chapter 2 2 2 3 4 Appendix A 5 6 Appendix B 7 8 Preoperative Planning Surgical Technique Subtalar Arthrodesis Guide
CHARLOTTE 7.0 Multi-Use Compression Screw System SURGICAL TECHNIQUE Contents Chapter 1 1 Chapter 2 2 2 3 4 Appendix A 5 6 Appendix B 7 8 Preoperative Planning Surgical Technique Subtalar Arthrodesis Guide
Integra. Silicone PIP Implant SURGICAL TECHNIQUE
 Integra Silicone PIP Implant SURGICAL TECHNIQUE Table of Contents Indications For Use...2 Contraindications...2 Warnings and Precautions...2 Surgical Technique Preoperative Assessment... 3 Step 1: Initial
Integra Silicone PIP Implant SURGICAL TECHNIQUE Table of Contents Indications For Use...2 Contraindications...2 Warnings and Precautions...2 Surgical Technique Preoperative Assessment... 3 Step 1: Initial
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
Instructions for use for Bone Plates, Bone Screws, Intramedullary Nails, Pins and Wires
 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
6.5 mm midfoot fusion bolt
 6.5 mm midfoot fusion bolt For intramedullary fixation of the medial column of the foot SurgIcal technique Table of Contents Introduction 6.5 mm Midfoot Fusion Bolt 2 AO Principles 4 Indications 5 Surgical
6.5 mm midfoot fusion bolt For intramedullary fixation of the medial column of the foot SurgIcal technique Table of Contents Introduction 6.5 mm Midfoot Fusion Bolt 2 AO Principles 4 Indications 5 Surgical
