Whisker growth induced by implantation of cultured vibrissa dermal papilla cells in the adult rat
|
|
|
- Brice Jared Jackson
- 5 years ago
- Views:
Transcription
1 /. Embryol. exp. Morph. 97, (1986) HI Printed in Great Britain The Company of Biologists Limited 1986 Whisker growth induced by implantation of cultured vibrissa dermal papilla cells in the adult rat KENNETH A. HORNE, COLIN A. B. JAHODA AND ROYF. OLIVER Department of Biological Sciences, University of Dundee, Dundee DD1 4HN, Scotland, UK SUMMARY Retention of the capacity to induce the growth of hair by cultured adult rat vibrissa dermal papilla cells has been investigated. Small pellets of serially cultured papilla cells were implanted into the bases of the exposed follicular epidermis of amputated adult rat vibrissa follicles. Amputated follicles that received no cell implants or implants of cultured dorsal skin fibroblasts were used as controls. Over 50 % of follicles implanted with cultured papilla cells in the passage range 1-3 grew hairs. In contrast none of the follicles that received late passage cells (range 6-15) produced hairs; and spontaneous regeneration of hair occurred in only 3 % of the control follicles. These results demonstrate that cultured papilla cells of early passage numbers retain their ability to induce hair growth. Histological examination confirmed that the implanted papilla cells interacted with follicular epidermis to organize the development of new, hairproducing bulbs, each containing a discrete dermal papilla. An important observation was that aggregative behaviour leading to papilla formation was only manifested by early passage papilla cell implants. This persisting embryonic characteristic appears to be an essential functional component of papilla cell activity which operates to regulate the profound morphogenetic changes that occur during the hair growth cycle. INTRODUCTION Mammalian hairs are formed by the differentiation and keratinization of cells produced in the germinative epidermal component at the base of the hair follicle. This epidermal matrix invests the dermal papilla, a discrete population of specialized fibroblasts. The importance of the dermal papilla in the growth of hair has been established in a series of experimental studies using the comparatively large rat vibrissa follicle, a model first used by Cohen (1961). Transplantation experiments have shown that freshly dissected papillae can induce hair growth when implanted into vibrissa follicles (Oliver, 1967; Ibrahim & Wright, 1977), and induce the development of new hair follicles when associated with ear or scrotal sac epidermis (Oliver, 1970). More recently it has been demonstrated that wounding of the dermal papilla in situ can lead to the production of longer hairs and extended growth cycles (Jahoda & Oliver, 1984b,c). Key words: hair growth, dermal papilla, cell culture, vibrissa, whisker, implantation, fibroblasts, rat.
2 112 K. A. HORNE, C. A. B. JAHODA AND R. F. OLIVER While postembryonic dermal papilla cells apparently remain mitotically quiescent (Pierard & de la Brassinne, 1975), it has now been shown that this intriguing population of cells can be grown in culture (Jahoda & Oliver, 1981, 1984a; Messenger, 1984). When compared with skin fibroblasts, cultured vibrissa papilla cells display unique morphological and behavioural characteristics and in particular tend to form discrete cell aggregates even after extended serial cultivation (Jahoda & Oliver, 1984a). This approach has opened up the possibility of analysing cultured papilla cells to determine their hair-growth-promoting characteristics. In this respect, it was important to establish whether the papilla cells also retained their ability to induce the growth of hair. It has been shown that permanent cessation of hair growth occurs following removal of more than the lower third of the vibrissa follicle (Oliver, 1966a,b, 1967). This finding has been exploited in the present work where retention of the functional capacity of cultured papilla cells was investigated by implanting serially cultured papilla cells into the bases of amputated follicles. The early fate of the implanted cells was followed by introducing papilla cells that had been labelled with [ 3 H]thymidine while growing in culture. As controls, follicles received implants of cultured skin fibroblasts. Here we amplify and present new findings to a preliminary account reported elsewhere (Jahoda, Horne & Oliver, 1984). MATERIALS AND METHODS Inbred hooded PVGC rats (colony, Dundee University) aged 3-6 months and of either sex were used to provide dermal papilla cells and skin fibroblasts for culture. Cell culture Dermal papilla cells Dermal papillae were dissected from the bulb regions of vibrissa follicles excised from the upper lips of freshly killed rats and stored in Eagle's minimal essential medium (MEM) as previously described (Jahoda & Oliver, 1981,1984a). Each papilla was cut into several pieces and the cut pieces of between 5 and 30 papillae were either grown on the glass coverslips of Cruickshank tissue culture chambers (Sterilin) and subcultured onto 35 mm plastic Petri dishes or grown directly in dishes at 37 C in an atmosphere of 5 % CO 2-95 % air. The culture medium consisted of Eagle's MEM containing 1 % L- glutamine (20min), 15% foetal calf serum (changed to 10% at passage 1), penicillin (SOi.u.mP 1 ) and streptomycin (SOjUgmP 1 ), all components from Gibco. The medium was changed every 3 or 4 days and when the cells started aggregating (Fig. 1) they were either prepared for implantation or subcultured. Cell dissociation was obtained using a solution of 0-25 % trypsin in phosphate-buffered saline EDTA (O^mgml" 1 ). A maximum dose of 2juCi of [ 3 H]thymidine (specific activity 45CimM~ 1 ; Radiochemical Centre, Amersham) was added over a 2 week period to passage 1 cells to provide labelled cells for implantation. Skin fibroblasts Skin fibroblasts were obtained from dorsal skin of rats of the same age range as those used to provide dermal papillae. Small pieces of dermis were dissected out and squashed under coverslips in 35 mm Petri dishes. When fibroblast outgrowth was established (Fig. 2), the coverslips and explants were removed. The same culture conditions and subculturing procedures used with papilla cells were employed.
3 Whisker growth induced by cultured dermal papilla cells 113 Fig. 1. Vibrissa dermal papilla cells exhibiting characteristic aggregative behaviour after 3 weeks in culture. xl55, phase contrast. Fig. 2. An equivalent culture of skin fibroblasts showing parallel cell alignment and complete coverage of the substrate. xl55, phase contrast. In vivo implantation Preparation of follicles Anaesthesia was induced by the intraperitoneal injection of Sagatal, ml 100g" 1 body weight. Since the major vibrissa follicles on the upper lip of the rat are arranged in a constant pattern of dorsoventrally and anteroposteriorly aligned rows, individual follicles can be surgically treated and subsequently identified. In this study all operations were performed on the three most posterior dorsoventral rows and the follicles were exposed as previously described (Oliver, 1966a; Jahoda & Oliver, 19846). The follicles selected for operation were identified and a record was made of the length of the vibrissae present in the follicles. Lengths of follicle were removed with a transverse cut, attempting to leave half or less of the upper region of the follicle in situ. The cut vibrissa shafts were plucked from the upper segments which were then available to receive cultured cell implants or to act as nonimplanted controls. Implantation of cultured cells Prior to implantation dermal papilla cells and fibroblast cultures were washed with phosphate-buffered saline (Oxoid Dulbecco 'A') and reincubated in medium containing 10 % rat serum. When the recipient follicles were prepared, the cells were harvested with a rubber policeman then divided into small pellets containing approximately cells. Using watchmaker's forceps a pellet was positioned within and around the opening of the follicular epidermis at the transected base of each follicle, essentially as described for the implantation of intact, freshly dissected dermal papillae (Oliver, 1967), and the lip stitched back in position. From 3-4 weeks postoperatively the follicle sites were examined under a stereoscopic microscope for evidence of fibre production, and the lengths of any hairs present were recorded.
4 114 K. A. HORNE, C. A. B. JAHODA AND R. F. OLIVER Design of experiments 283 follicles in 45 rats were exposed to one of the four procedures employed. Details of the long-term experiments, in which follicles were biopsied from days after operation, are shown in Table 1. To study the early response of follicles to the procedures employed 30 follicles were used: 2 amputated, but nonimplanted, follicles (biopsied at 11 days), 6 follicles that had received cultured fibroblasts (biopsied at 5 or 7 days) and 22 follicles that were implanted with cultured papilla cells and biopsied from 1 h up to 11 days after operation. To determine the level of amputation, all of the operated follicles were fixed at biopsy for gross reconstruction with their removed lower portions which had been fixed after operation. In addition the exact level of cut was confirmed at histological examination of the biopsied follicles and, in 17 of the follicles that had received low passage number papilla cells, by histological examination of the removed lower portions. Histology and autoradiography Biopsied follicles and removed lower regions were fixed in formal saline, processed and embedded in paraffin wax before being serially sectioned at 8 jum parallel to the long axis of the follicle. Sections were stained in a combination of Weigert's haematoxylin, alcian blue and Curtis' Ponceau S. For autoradiography, slides with serial sections were taken to distilled water and dipped in Ilford Nuclear Emulsion Type K2. The sections were exposed for 14 days at 4 C and after developing and fixing were stained with haematoxylin and eosin. RESULTS Control experiments Nonimplanted controls An essential aspect of the study, that surgical removal of the lower third or more of the follicles would reliably ensure permanent cessation of hair growth, was clearly demonstrated. 87 of the 90 follicles in this control group did not produce hairs (Table 1). This was confirmed by histological examination of 40 of the biopsied follicles. With the exception of the three follicles that had produced hairs, there was no evidence of spontaneous regeneration of dermal papillae. The epidermal cells of the lower outer root sheaths had formed solid columns which terminated just above the level of the original cut (Fig. 3). Of the three follicles that had produced hairs two, biopsied at 49 and 162 days, had grown recognizable vibrissae following removal of their lower halves. In the third follicle, biopsied at 44 days and from which the lower two thirds had been removed, histological examination revealed the presence of two pelage-like follicles which were growing fine hairs, located just below the sebaceous gland. Apart from the size and anatomical similarity with pelage follicles (Fig. 14), the hairs had compartmentalized medullae in contrast with vibrissae which have an open, continuous medulla. Cultured fibroblast-implanted controls Of the 61 follicles that were implanted with cultured skin fibroblasts (35 receiving cells in the passage range 1-3 and 26 passage 4 or passage 5 cells), two produced hairs (Table 1). The 6 short-term follicles and 35 of the long-term
5 Whisker growth induced by cultured dermal papilla cells 115 follicles were examined histologically. Except for the two follicles that had produced hairs, these follicles were indistinguishable in appearance from nonimplanted controls, showing the presence of a thin layer of fibroblastic scar tissue over the base of the follicular epidermis (Fig. 4). One follicle, from which just the lower third had been excised and which was therefore within the level of expected spontaneous regeneration, had produced vibrissae. In the second follicle, where the lower two thirds had been removed, histology revealed a pelage-like follicle extending below the vibrissa sebaceous gland. Cultured papilla cell implants The results obtained from the 98 follicles that had received implants of cultured dermal papilla cells are shown in Table 1. None of the 42 follicles implanted with cells of high passage numbers (6-15) were observed to produce hairs. The follicles resembled control follicles, as shown in Figs 3 and 4, with no histological evidence of the presence of dermal papillae. In contrast 30 (53 %) of the 56 follicles that were implanted with cells in the passage range 1-3 were observed to have produced hairs either during routine observations (24 follicles) or at histology (6 follicles). Induced hair growth 27 of the 30 hair-producing follicles had grown vibrissae and a further three were found to be producing pelage-like hairs on histological examination. Vibrissae were first seen to emerge at 4 weeks after operation and the majority of those kept under extended observation produced hairs in the normal cyclic fashion. However, 5 follicles that had initially produced emergent vibrissae were revealed Procedure Nonimplanted controls Intact papilla implants Cultured fibroblast implants Cultured papilla cell implants Table 1. Summary of results of long-term study Biopsy time in days Passage number of cultured cells 1-3 4, ,15 Number of follicles Number growing vibrissae 2+1P P P 4+1P 0 0 P = number producing pelage-like hairs.
6 116 K. A. HORNE, C. A. B. JAHODA AND R. F. OLIVER on biopsy to have subsequently formed intrafollicular cysts containing coiled vibrissae (Fig. 13). Because of the varying biopsy times and the intermittent observations few definitive club or terminal hair lengths were obtained. However, hairs ranging in
7 Whisker growth induced by cultured dermal papilla cells 111 length from mm were observed growing from 14 of the follicles. This represented % of the expected hair length for the follicle positions. Analysis of the preimplantation level of follicle amputation revealed that 12 follicles had grown vibrissae after removal of between the lower third and lower half of the follicles and that 13 follicles had grown vibrissae after removal of more than the lower half (Fig. 5). Of the five follicles that had produced hairs after removal of the lower two thirds, two had grown vibrissae and three had developed as pelage-like follicles. Histological observations To determine the early fate of implanted papilla cells, 22 follicles were examined at intervals after operation. Shortly after implantation a variable number of papilla cells was seen located within the base of the outer root sheath where the cells appeared to be held in position by an underlying blood clot (Fig. 6). Papilla cells were also seen more diffusely spread within and outside the transected collagen capsule, sometimes associated with detached portions of the outer root sheath. Evidence that the implanted papilla cells formed the new papillae was provided by autoradiographic examination of follicles that had received papilla cells labelled with [ 3 H]thymidine. At 11 days labelled papilla cells were present within an indentation at the base of the otherwise now solid column of lower outer root sheath cells (Fig. 7). By 28 days, when whisker growth was occurring (Fig. 8), there was a new bulb at the site of papilla cell implantation, with an epidermal matrix containing active melanocytes organized around a discrete dermal papilla. Labelling of the dermal component of the follicle was strictly confined to cells within the body of the papilla and the papilla stalk (Fig. 9). Unlabelled cells were associated with the capillary network now established within the papilla. Examination of the 27 follicles that had produced vibrissae, and that were biopsied days after operation, showed that the majority of the follicles had lengthened and either projected below the level of the cut collagen capsule or Fig. 3. Nonimplanted control vibrissa follicle biopsied at 104 days after amputation of the lower one third of the follicle. The follicular epidermis (/<?) has formed a solid cord of cells which terminates above the transected rim of the collagen capsule (c). x30. Fig. 4. Detail of the base of a control follicle, biopsied at 35 days, from which the lower one third had been removed and which had received an implant of passage 2 skin fibroblasts. A thin layer of fibroblastic scar tissue has formed immediately below the follicular epidermis (fe) which displays the characteristic appearance of outer root sheath cells. X570. Fig. 5. Macrograph of two follicles (A and B) biopsied at 117 days after implantation of passage 2 papilla cells. The arrows indicate the original level of cut through the collagen capsule on the implanted upper segments. The removed lower portions are shown to the right. Follicle A, following a midfollicular amputation, was growing the curled whisker at left, which measured 11mm in length at the time of biopsy. The associated bulb can be seen as a pigmented area just below the cut capsule (see Fig. 10). Follicle B had the lower two thirds removed. An epithelial cyst projects below the cut capsule and histology revealed the presence of a large papilla and epidermal matrix high in the cyst wall and a coiled whisker within. xl3.
8 118 K. A. HORNE, C. A. B. JAHODA AND R. F. OLIVER 8
9 Whisker growth induced by cultured dermal papilla cells 119 showed lateral displacement of the bulb (Figs 10, 12, 13). Nineteen follicles showed the characteristic anatomy of vibrissa follicles with prominent and, generally, large dermal papillae. Seventeen of these follicles were in the midanagen phase of the hair growth cycle, one was in very early anagen (Fig. 11) and one was in telogen or the resting phase. Of particular interest was a follicle that had formed three separate bulbs at the base of the follicle (Fig. 12), each of which was producing its own whisker, 10 mm long when biopsied at 44 days. The remaining eight follicles, from which the lower half or more of the follicle had been removed, had formed epithelial cysts containing coiled vibrissa shafts. Five of these follicles had previously grown emergent hairs, but then hair growth continued below the skin surface. Two of these follicular cysts contained multiple bulbs. One contained two small bulbs (Fig. 13). In the second, four papilla/matrix associations had formed and a small, isolated clump of papilla cells was enclosed within the capsule. The three follicles that had produced pelage-like hairs were identified at histology (Fig. 14). As in the two similar follicles observed in control follicles, the lower two thirds of the follicles had been removed at operation. As with some of the control follicles, a further phenomenon revealed at histology was the presence, in five follicles, of sebaceous gland cells within the upper root sheath as apparent extensions of the sebaceous glands located within the collagen capsule. These sebaceous cells were seen in association with two of the pelage-like follicles (Fig. 14) and as additional histological features in three follicles growing vibrissae. Intact dermal papilla implants Three of the four follicles into which freshly dissected dermal papillae had been implanted produced generations of vibrissae ranging in length from mm. This represented % of the expected lengths for the follicle positions. Fig. 6. Upper region of a follicle biopsied 1 h after implantation of a pellet of cultured papilla cells in the base. The implant (/) is located within the proximal end of the follicular epidermis (fe) and a retaining blood clot (be) has formed at the site of amputation. x30. Fig. 7. Autoradiograph of the base of a follicle 11 days after implantation of papilla cells that had been labelled with [ 3 H]thymidine. Follicular epidermis (fe) is becoming organized around the labelled papilla cells (i) which have remained at the site of implantation. x400. Fig. 8. Follicle biopsied 28 days after implantation of passage 1 papilla cells labelled with [ 3 H]thymidine following removal of the lower half of the follicle. A new bulb with a discrete dermal papilla (dp) and investing epidermal matrix (em) has developed at the site of implantation. A pigmented whisker (w) was being produced. x30. Fig. 9. Autoradiograph showing detail of the bulb in Fig. 8. Labelling, not to be confused with pigment in the melanocytes (me) in the epidermal matrix (em) at right, is restricted to dermal cells within the papilla (dp) and its basal stalk. Unlabelled endothelial cells of the capillary network that has migrated into the papilla are also present. x550.
10 120 K. A. HORNE, C. A. B. JAHODA AND R. F. OLIVER
11 Whisker growth induced by cultured dermal papilla cells 121 DISCUSSION Fulfilment of experimental requirements An essential requirement of this study was to demonstrate the validity of using amputated vibrissa follicles to evaluate the inductive capacity of cultured dermal papilla cells. This was necessary because while permanent cessation of hair growth has been observed following removal of more than the lower third of vibrissa follicles, removal of less is associated with spontaneous papilla regeneration and renewed whisker growth (Oliver, 1966a,b, 1967; Ibrahim & Wright, 1982). The present results showed clearly that amputation of more than the lower third of vibrissa follicles reliably prevents further vibrissa growth. Only 3 % of the control follicles grew hairs (5 out of 151 follicles) and this included two follicles that produced fine, pelage-like hairs rather than vibrissae. This presence of hairs in the control follicles was found on histological examination subsequent to our earlier report (Jahoda et al. 1984). Induction of hair growth A major limitation of most culture systems is that the activities of cells grown in culture can rarely be related to their in situ function. Here we have demonstrated that cultured vibrissa cells of early passage numbers retain their functional capacity to induce the growth of hair. Over 50 % of follicles that were implanted with cultured papilla cells in the passage range 1-3 subsequently grew hairs (30 out of 56 follicles), 27 producing vibrissae and three pelage-like hairs. Histological studies of follicles biopsied at early intervals after implantation, in conjunction with the autoradiographic experiments, confirmed that the implanted papilla cells formed the new papillae responsible for vibrissa growth. The papilla cells interacted with follicular epidermis at the site of implantation to organize the development of new, hair-producing bulbs each containing a discrete dermal papilla. The majority of the follicles subsequently biopsied showed follicle lengthening and various stages of the growth cycle expected in follicles producing generations of hairs. Fig. 10. Section through follicle A in Fig. 5 showing the displaced bulb with its dermal papilla (dp) and epidermal matrix (em) projecting just below the cut capsule (c). x30. Fig. 11. Follicle biopsied 117 days after implantation of passage 2 papilla cells. This early anagen follicle is entering a new hair growth cycle with an epidermal matrix (em) developing around the papilla (dp). x30. Fig. 12. Follicle biopsied 44 days after implantation of passage 3 papilla cells. Three separate bulbs had formed (two of which can be seen in this section), each of which had produced a whisker 10 mm above the skin surface. x30. Fig. 13. Detail of base of a follicle biopsied 119 days after implantation of passage 2 papilla cells. Two bulbs, located within the cut edge of the capsule (c), were growing whiskers (w) within an intrafollicular cyst. x75. Fig. 14. Detail of the upper region of a follicle that had received a passage 3 papilla cell implant. This follicle had developed a hyperplastic sebaceous gland (sg) and a pelagelike follicle. The small bulb of this follicle (lower right) was producing a fine hair (h). X195.
12 122 K. A. HORNE, C. A. B. JAHODA AND R. F. OLIVER In all of these respects the cultured papilla cells behaved like freshly dissected papillae which previous work has shown induce whisker growth when implanted into similarly amputated vibrissa follicles (Oliver, 1967), a finding confirmed in this study by the three out of four follicles that produced vibrissae after implantation of intact papillae. A significant feature of the implanted papilla cells of early passage numbers was that they formed discrete cell masses which in turn formed associations with follicular epidermis. Presumably separate groupings of papilla cells occurred immediately after implantation in those follicles that produced multiple hairs. This aggregative property of papilla cells appears to be a fundamental characteristic related to their function. It is shown by the condensation of dermal cells to form papilla anlagen in the ontogenetic development of follicles (e.g. Wessells & Roessner, 1965); the tendency of adult papilla cells to form clumps in culture (Jahoda & Oliver, 1981, 1984a), and the fact that dermal papillae remain intact as discrete entities when transplanted into vibrissa follicles and a variety of ectopic sites, regardless of whether epidermal contact is made or not (Oliver, 1970; Young, 1977; Jahoda & Oliver, 1984c). In contrast to follicles receiving early passage number papilla cells, none of the 42 follicles that received late passage cells (range 6-15) produced hairs. Histological examination of the follicles revealed that the implanted papilla cells (in common with implanted fibroblasts) did not form recognizable cellular aggregates in situ. The implanted cells may have migrated from the implantation site, as has been demonstrated with follicle dermal sheath cells labelled with [ 3 H]thymidine (Horne, unpublished observations), and/or contributed to the fibroblastic layer at the follicle base. These observations suggest that loss of inductive potential by papilla cells after extended culture may be associated with loss of aggregative behaviour in vivo. The occasional development of pelage-like follicles (whether in the control or implanted groups) after removal of more than the lower two thirds of the follicle shows similarities to the reported regrowth of human axillary hair following amputation of the follicle up to the level of the sebaceous gland (Inaba, Anthony & McKinstry, 1979). A further regenerative phenomenon was the presence of sebaceous gland cells within the upper outer root sheath in some of the control and hair-growing follicles, a feature seen previously in a single amputated vibrissa follicle (Oliver, 1967). Our demonstration that cultured papilla cells can induce hair growth after reimplantation warrants examination of papilla cell properties in vitro even though, in monolayer cultures, the cells are unlikely to display all of the activities they exhibit in situ. We have now shown, for example, that human papilla cells from scalp and pubic follicles are targets of both androgen and glucocorticoid action (Murad etal. 1985). This approach may reveal the intrafollicular link between hormones and their effects on hair growth. By using clonal culture techniques it should also be possible to determine the number of divisions papilla cells will go through and still be competent to induce
13 Whisker growth induced by cultured dermal papilla cells 123 hair growth. While apparently postmitotic in situ (Pierard & de la Brassinne, 1975), the fact that papilla cells can divide in culture and yet subsequently perform normal functions demonstrates that they do not conform to the classical category of a postmitotic terminally differentiated cell type. The involvement of dermal cells other than papilla cells in follicle development and function has also to be considered. It has been shown, for example, that cells from newborn mouse skin form hair follicle structures when reassociated in transplantation chambers (Worst, MacKenzie & Fusenig, 1982). However, it remains unclear whether follicular dermal cells simply reassembled or whether all newborn dermal cells can become the dermal component of hair follicles. Our results indicate that adult skin fibroblasts are incapable of replacing dermal papilla cell function in the hair follicle. Papilla cells become distinct from surrounding mesenchyme as an apparently nondividing cell population early in follicle development (Wessells & Roessner, 1965). However, Oliver (1966a) and Ibrahim & Wright (1982) showed that cells from the follicular dermal sheath can form replacement dermal papillae after removal of the original papilla and up to one third of the lower follicle. Interestingly, dermal sheath cells from this regenerative region also display aggregative behaviour in culture while cells from the upper dermal sheath demonstrate normal fibroblast behaviour (Home, unpublished). Thus, as suggested by Oliver (1980), it may be appropriate to consider the lower follicle dermis, including the dermal papilla, as the crucial dermal element for hair production. Furthermore, our observations reinforce the idea (Jahoda & Oliver, 1984a) that cells of this dermal element are 'embryonic' in nature. This retention of embryonic properties into adulthood enables the papilla to regulate the profound morphogenetic changes that occur during the hair cycle; events which have been compared to those taking place during follicle development (e.g. Chase, 1965). Thus mesenchymal cell aggregative behaviour, which is a feature of skin appendage development, has been retained by dermal papilla cells as a property necessary for their morphogenetic role, and possibly also for maintaining follicle size (Jahoda & Oliver, 1984c). The important question now arises as to whether cultured papilla cells can induce hair follicle formation, an ability shown by intact adult papillae (Oliver, 1970). Experiments are now being performed to test this hypothesis. We are grateful to Mr G. Coghill for performing the autoradiography and Mr B. Pert for the photography. K.A.H. was supported by an MRC studentship. REFERENCES CHASE, H. B. (1965). Cycles and waves of hair growth. In Biology of the Skin and Hair Growth (ed. A. G. Lyne & B. F. Short), pp Sydney: Angus & Robertson. COHEN, J. (1961). The transplantation of individual rat and guinea-pig whisker papillae. /. Embryol. exp. Morph. 9, IBRAHIM, L. & WRIGHT, E. A. (1977). Inductive capacity of irradiated dermal papillae. Nature, Lond. 265,
14 124 K. A. HORNE, C. A. B. JAHODA AND R. F. OLIVER IBRAHIM, L. & WRIGHT, E. A. (1982). A quantitative study of hair growth using mouse and rat vibrissal follicles. /. Embryol. exp. Morph. 72, INABA, M., ANTHONY, J. & MCKINSTRY, C. (1979). Histologic study of the regeneration of axillary hair after removal with a subcutaneous tissue shaver. /. invest. Dermatol. 72, JAHODA, C. A. B., HORNE, K. A. & OLIVER, R. F. (1984). Induction of hair growth by implantation of cultured dermal papilla cells. Nature, Lond. 311, JAHODA, C. A. B. & OLIVER, R. F. (1981). The growth of vibrissa dermal papilla cells in vitro. Br. J. Dermatol. 105, JAHODA, C. A. B. & OLIVER, R. F. (1984a). Vibrissa dermal papilla cell aggregative behaviour in vivo and in vitro. J. Embryol. exp. Morph. 79, JAHODA, C. A. B. & OLIVER, R. F. (1984b). Changes in hair growth characteristics following the wounding of vibrissa follicles in the hooded rat. /. Embryol. exp. Morph. 83, JAHODA, C. A. B. & OLIVER, R. F. (1984C). Histological studies of the effects of wounding vibrissa follicles in the hooded rat. /. Embryol. exp. Morph. 83, MESSENGER, A. G. (1984). The culture of dermal papilla cells from human hair follicles. Br. J. Dermatol. 110, MURAD, S., HODGINS, M. B., JAHODA, C. A. B., OLIVER, R. F. & SIMPSON, N. B. (1985). Androgen receptors and metabolism in cultured dermal papilla cells from human hair follicles. Br. J. Dermatol. 113, 768. OLIVER, R. F. (1966a). Whisker growth after removal of the dermal papilla and lengths of the follicle in the hooded rat. /. Embryol. exp. Morph. 15, OLIVER, R. F. (1966&). Histological studies of whisker regeneration in the hooded rat. /. Embryol. exp. Morph. 16, OLIVER, R. F. (1967). The experimental induction of whisker growth in the hooded rat by implantation of dermal papillae. /. Embryol. exp. Morph. 18, OLIVER, R. F. (1970). The induction of hair follicle formation in the adult hooded rat by vibrissa dermal papillae. /. Embryol. exp. Morph. 23, OLIVER, R. F. (1980). Local interactions in mammalian hair growth. In The Skin of Vertebrates (ed. R. I. C. Spearman & P. A. Riley), pp London, New York: Academic Press. PIERARD, G. E. & DE LA BRASSINNE, M. (1975). Modulation of dermal cell activity during hair growth in the rat. /. cutan. Pathol. 2, WESSELS, N. K. & ROESSNER, K. D. (1965). Non-proliferation in dermal condensations of mouse vibrissae and pelage hair. DevlBiol. 12, WORST, P. K. M., MACKENZIE, I. C. & FUSENIG, N. E. (1982). Reformation of organised epidermal structure by transplantation of suspensions and cultures of epidermal and dermal cells. Cell Tissue Res. 225, YOUNG, R. D. (1977). Morphological and ultrastructural studies of the rat vibrissal follicle with special reference to the dermal papilla. Ph.D. Thesis, University of Dundee. (Accepted 20 May 1986)
Supplementary Information
 Supplementary Information Figure S1: Follicular melanocytes in the wound peripheral area migrate to the epidermis in response to wounding stimuli. Dorsal skin of Trp2-LacZ mice stained with X-gal and analyzed
Supplementary Information Figure S1: Follicular melanocytes in the wound peripheral area migrate to the epidermis in response to wounding stimuli. Dorsal skin of Trp2-LacZ mice stained with X-gal and analyzed
Supporting Information
 Supporting Information Plikus et al. 10.1073/pnas.1215935110 SI Text Movies S1, S2, S3, and S4 are time-lapse recordings from individually cultured Period2 Luc vibrissa follicles show that circadian cycles
Supporting Information Plikus et al. 10.1073/pnas.1215935110 SI Text Movies S1, S2, S3, and S4 are time-lapse recordings from individually cultured Period2 Luc vibrissa follicles show that circadian cycles
THE INDUCTION OF HAIR FOLLICLES BY EMBRYONIC DERMAL PAPILLAE*
 THE JouR AL OF INVESTIGATIVE DERMATOLOGY Copyright 1970 by The Williams & Wilkins Co. Vol. 55, No. 6 Printed in U.S.A. THE INDUCTION OF HAIR FOLLICLES BY EMBRYONIC DERMAL PAPILLAE* EDWARD J. KOLLAR, PH.D.
THE JouR AL OF INVESTIGATIVE DERMATOLOGY Copyright 1970 by The Williams & Wilkins Co. Vol. 55, No. 6 Printed in U.S.A. THE INDUCTION OF HAIR FOLLICLES BY EMBRYONIC DERMAL PAPILLAE* EDWARD J. KOLLAR, PH.D.
This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur.
 Background Knowledge Functions of normal skin Background Knowledge This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur. Learning
Background Knowledge Functions of normal skin Background Knowledge This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur. Learning
Lab 7: Integumentary System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS
 Lab 7: Integumentary System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS Pre-Lab Activity 1: 1. a. epidermis b. dermis c. hypodermis d. adipose tissue e. hair f. sebaceous gland g. sweat gland 2. a Pre-Lab
Lab 7: Integumentary System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS Pre-Lab Activity 1: 1. a. epidermis b. dermis c. hypodermis d. adipose tissue e. hair f. sebaceous gland g. sweat gland 2. a Pre-Lab
The site of action of the ichthyosis locus (ic) in the mouse, as determined by dermal-epidermal recombinations
 /. Embryol. exp. Morph. Vol. 32, 3, pp. 715-721, 1974 715 Printed in Great Britain The site of action of the ichthyosis locus (ic) in the mouse, as determined by dermal-epidermal recombinations BY MARGARET
/. Embryol. exp. Morph. Vol. 32, 3, pp. 715-721, 1974 715 Printed in Great Britain The site of action of the ichthyosis locus (ic) in the mouse, as determined by dermal-epidermal recombinations BY MARGARET
Chapter 4 Opener Pearson Education, Inc.
 Chapter 4 Opener Introduction The integumentary system is composed of: Skin Hair Nails Sweat glands Oil glands Mammary glands The skin is the most visible organ of the body Clinicians can tell a lot about
Chapter 4 Opener Introduction The integumentary system is composed of: Skin Hair Nails Sweat glands Oil glands Mammary glands The skin is the most visible organ of the body Clinicians can tell a lot about
in alopeeia areata are not entirely defined. Sabouraud (1) made the fundamental observation
 MORPHOLOGIC CHANGES IN PILOSEBACEOUS UNITS AND ANAGEN HAIRS IN ALOPECIA AREATA* The normal growth of scalp hair is a result of a continuous, rapid proliferation of tissue by the matrix of the hair bulb
MORPHOLOGIC CHANGES IN PILOSEBACEOUS UNITS AND ANAGEN HAIRS IN ALOPECIA AREATA* The normal growth of scalp hair is a result of a continuous, rapid proliferation of tissue by the matrix of the hair bulb
Morphogenesis by dissociated immature rat testicular cells in primary culture
 /. Einhryol. exp. Morph. Vol. 44, pp. 297-302, 1978 297 Printed in Great Britain (p Company of Biologists Limited 1978 SHORT PAPER Morphogenesis by dissociated immature rat testicular cells in primary
/. Einhryol. exp. Morph. Vol. 44, pp. 297-302, 1978 297 Printed in Great Britain (p Company of Biologists Limited 1978 SHORT PAPER Morphogenesis by dissociated immature rat testicular cells in primary
Integumentary System. Integumentary System
 1. General aspects a. The integumentary system consists of several organs major organ of the system is the skin other organs are relatively small and they can be considered as specialized structures of
1. General aspects a. The integumentary system consists of several organs major organ of the system is the skin other organs are relatively small and they can be considered as specialized structures of
Trichofolliculoma of the Guinea Pig 1,2
 Trichofolliculoma of the Guinea Pig 1,2 Raymond D. Ediger, Garrett S. Dill, Jr., and Robert M. Kovatch, Aerobiology and Evaluation Laboratories and Medical Sciences Laboratories, Fort Detrick, Frederick,
Trichofolliculoma of the Guinea Pig 1,2 Raymond D. Ediger, Garrett S. Dill, Jr., and Robert M. Kovatch, Aerobiology and Evaluation Laboratories and Medical Sciences Laboratories, Fort Detrick, Frederick,
Human Anatomy & Physiology
 PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 5 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 5 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
Overview of the Integumentary System. Lab #7. Layers of the epidermis are known as strata. Organization of the Epidermis: Layers of the Epidermis
 Overview of the Integumentary System Lab #7 Integumentary System Organization of the Epidermis: Layers of the epidermis are known as strata Figure 5 2 Layers of the Epidermis Top: Free surface of skin
Overview of the Integumentary System Lab #7 Integumentary System Organization of the Epidermis: Layers of the epidermis are known as strata Figure 5 2 Layers of the Epidermis Top: Free surface of skin
Lesson 3: The Human Integumentary and Fascial Systems
 Basic Human Anatomy Lesson 3: The Human Integumentary and Fascial Systems Welcome to Lesson 3 of the Basic Human Anatomy Course. Today, we ll be studying the Human Integumentary and Fascial Systems. I
Basic Human Anatomy Lesson 3: The Human Integumentary and Fascial Systems Welcome to Lesson 3 of the Basic Human Anatomy Course. Today, we ll be studying the Human Integumentary and Fascial Systems. I
Ex. 7: Integumentary
 Collin County Community College BIOL. 2401 Ex. 7: Integumentary. Skin or Integument Consists of three major regions Epidermis outermost superficial region Dermis middle region Hypodermis (superficial fascia)
Collin County Community College BIOL. 2401 Ex. 7: Integumentary. Skin or Integument Consists of three major regions Epidermis outermost superficial region Dermis middle region Hypodermis (superficial fascia)
Introduction. Skin and Body Membranes. Cutaneous Membranes Skin 9/14/2017. Classification of Body Membranes. Classification of Body Membranes
 Introduction Skin and Body Membranes Body membranes Cover surfaces Line body cavities Form protective and lubricating sheets around organs Classified in 5 categories Epithelial membranes 3 types- cutaneous,
Introduction Skin and Body Membranes Body membranes Cover surfaces Line body cavities Form protective and lubricating sheets around organs Classified in 5 categories Epithelial membranes 3 types- cutaneous,
Further studies on the melanophores of periodic albino mutant of Xenopus laevis
 J. Embryol. exp. Morph. 91, 65-78 (1986) 65 Printed in Great Britain The Company of Biologists Limited 1986 Further studies on the melanophores of periodic albino mutant of Xenopus laevis T. FUKUZAWA AND
J. Embryol. exp. Morph. 91, 65-78 (1986) 65 Printed in Great Britain The Company of Biologists Limited 1986 Further studies on the melanophores of periodic albino mutant of Xenopus laevis T. FUKUZAWA AND
INTEGUMENTARY 1-Epidermis, 2-Dermis, Structure of thick and thin skin I- Epidermis . Stratum basale
 INTEGUMENTARY The skin (integument, cutis ) and its derivatives constitute the integumentary system. It form the external covering of the body and is the largest organ of the body. The skin consists of
INTEGUMENTARY The skin (integument, cutis ) and its derivatives constitute the integumentary system. It form the external covering of the body and is the largest organ of the body. The skin consists of
INTEGUMENTARY SYSTEM CHAPTER 4
 INTEGUMENTARY SYSTEM CHAPTER 4 FUNCTIONS Waterproofs Protein called keratin Protection 1 st line of defense against pathogens, chemicals & abrasions Insulation Regulates heat loss by controlling blood
INTEGUMENTARY SYSTEM CHAPTER 4 FUNCTIONS Waterproofs Protein called keratin Protection 1 st line of defense against pathogens, chemicals & abrasions Insulation Regulates heat loss by controlling blood
Ch 4. Skin and Body Membranes
 Ch 4 Skin and Body Membranes TITLE HISTOLOGY SLIDES & NOTES ESSENTIAL QUESTION What tissues compose the integumentary system? Stratified Squamous Epithelium Stratified = several layers; Squamous = shape
Ch 4 Skin and Body Membranes TITLE HISTOLOGY SLIDES & NOTES ESSENTIAL QUESTION What tissues compose the integumentary system? Stratified Squamous Epithelium Stratified = several layers; Squamous = shape
Integumentary System
 Chapter 5 Integumentary System 5-1 Skin: composed of dermis and epidermis Dermis. Gives structural strength. C.T. with many fibers, fibroblasts, macrophages. Some adipocytes and blood vessels. Contains
Chapter 5 Integumentary System 5-1 Skin: composed of dermis and epidermis Dermis. Gives structural strength. C.T. with many fibers, fibroblasts, macrophages. Some adipocytes and blood vessels. Contains
SKIN HISTOLOGY the microscopic anatomy of the Integument. Mikrogeo. com
 SKIN HISTOLOGY the microscopic anatomy of the Integument Mikrogeo. com Hair follicles, sweat glands, sebaceous glands (even teeth) are products of the epidermis,embryologically speaking ectododerm, that
SKIN HISTOLOGY the microscopic anatomy of the Integument Mikrogeo. com Hair follicles, sweat glands, sebaceous glands (even teeth) are products of the epidermis,embryologically speaking ectododerm, that
Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs
 Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs Classification of Body Membranes Epithelial membranes Cutaneous
Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs Classification of Body Membranes Epithelial membranes Cutaneous
LESSON ASSIGNMENT. The Human Integumentary and Fascial Systems. After completing this lesson, you should be able to:
 LESSON ASSIGNMENT LESSON 3 The Human Integumentary and Fascial Systems. TEXT ASSIGNMENT Paragraphs 3-1 through 3-14. LESSON OBJECTIVES After completing this lesson, you should be able to: 3-1. Define integumentary
LESSON ASSIGNMENT LESSON 3 The Human Integumentary and Fascial Systems. TEXT ASSIGNMENT Paragraphs 3-1 through 3-14. LESSON OBJECTIVES After completing this lesson, you should be able to: 3-1. Define integumentary
The Integumentary System
 The Integumentary System Integument is skin Skin and its appendages make up the integumentary system A fatty layer (hypodermis) lies deep to it Two distinct regions Epidermis Dermis PHL 212 1 Function
The Integumentary System Integument is skin Skin and its appendages make up the integumentary system A fatty layer (hypodermis) lies deep to it Two distinct regions Epidermis Dermis PHL 212 1 Function
Histopathology: skin pathology
 Histopathology: skin pathology These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information
Histopathology: skin pathology These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information
B. Incorrect! The ectoderm does not produce the dermis. C. Incorrect! The dermis is derived from the mesoderm.
 Human Anatomy - Problem Drill 04: The Integumentary System Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as 1. From the inner cell
Human Anatomy - Problem Drill 04: The Integumentary System Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as 1. From the inner cell
B. Connective tissue membranes lubricate & cushion 1. made of areolar tissue 2. synovial line fibrous joint capsules & secrete fluid
 I. Body Membranes A. Epithelial membranes cover & line 1. epithelial sheet over underlying connective tissue 2. 3 types a. Cutaneous = skin b. Mucous = lines cavities open to exterior Skin and Body Membranes
I. Body Membranes A. Epithelial membranes cover & line 1. epithelial sheet over underlying connective tissue 2. 3 types a. Cutaneous = skin b. Mucous = lines cavities open to exterior Skin and Body Membranes
ELECTRON MICROSCOPY OF A SMALL PIGMENTED CUTANEOUS LESION*
 ELECTRON MICROSCOPY OF A SMALL PIGMENTED CUTANEOUS LESION* The description of the lesion in the title of this rcport is intentionally non-committal. Diagnosed clinically as a lentigo, it was removed as
ELECTRON MICROSCOPY OF A SMALL PIGMENTED CUTANEOUS LESION* The description of the lesion in the title of this rcport is intentionally non-committal. Diagnosed clinically as a lentigo, it was removed as
ABCD rule. apocrine glands. arrector pili. ceruminous glands. contact dermatitis
 ABCD rule assessing moles: asymmetric, broder irregularity, color, diameter (larger than 6mm) apocrine glands arrector pili sweat glands in the pubic and underarm areas that secrete thicker sweat, that
ABCD rule assessing moles: asymmetric, broder irregularity, color, diameter (larger than 6mm) apocrine glands arrector pili sweat glands in the pubic and underarm areas that secrete thicker sweat, that
Anatomy Ch 6: Integumentary System
 Anatomy Ch 6: Integumentary System Introduction: A. Organs are body structures composed of two or more different tissues. B. The skin and its accessory organs make up the integumentary system. Types of
Anatomy Ch 6: Integumentary System Introduction: A. Organs are body structures composed of two or more different tissues. B. The skin and its accessory organs make up the integumentary system. Types of
Chapter 5. Integumentary System 5-1
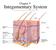 Chapter 5 Integumentary System 5-1 Structures that are part of the integument Skin Hair Nails Glands Overview of Functions Protection Sensation Temperature regulation Vitamin D production Excretion Immunity
Chapter 5 Integumentary System 5-1 Structures that are part of the integument Skin Hair Nails Glands Overview of Functions Protection Sensation Temperature regulation Vitamin D production Excretion Immunity
TRANSFER OF PREMELANOSOMES INTO THE KERATINIZING CELLS OF ALBINO HAIR FOLLICLE
 TRANSFER OF PREMELANOSOMES INTO THE KERATINIZING CELLS OF ALBINO HAIR FOLLICLE PAUL F. PARAKKAL. From the Department of Dermatology, Boston University School of Medicine, Boston, Massachusetts 02118 INTRODUCTION
TRANSFER OF PREMELANOSOMES INTO THE KERATINIZING CELLS OF ALBINO HAIR FOLLICLE PAUL F. PARAKKAL. From the Department of Dermatology, Boston University School of Medicine, Boston, Massachusetts 02118 INTRODUCTION
Skin and Body Membranes
 4 Skin and Body Membranes PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Skin and Body Membranes
4 Skin and Body Membranes PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Skin and Body Membranes
(From the Arnold Biological Laboratory, Brown University, Providence, Rhode Island) Materials and Methods
 HISTOLOGY AND CYTOCHEMISTRY OF HUMAN SKIN XI. THF. DISTRIBUTION OF /~-GLucURONIDASE* BY WILLIAM MONTAGNA, PH.D. (From the Arnold Biological Laboratory, Brown University, Providence, Rhode Island) PLATES
HISTOLOGY AND CYTOCHEMISTRY OF HUMAN SKIN XI. THF. DISTRIBUTION OF /~-GLucURONIDASE* BY WILLIAM MONTAGNA, PH.D. (From the Arnold Biological Laboratory, Brown University, Providence, Rhode Island) PLATES
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Skin and Body Membranes
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Skin and Body Membranes 4 Body Membranes Function of body membranes Cover body surfaces Line body cavities
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Skin and Body Membranes 4 Body Membranes Function of body membranes Cover body surfaces Line body cavities
Study Guide for Bio 101 Lecture Exam 3
 Study Guide for Bio 101 Lecture Exam 3 Please note that this study guide is a listing of objectives that you are required to master for this course. However, items mentioned in class or in laboratory as
Study Guide for Bio 101 Lecture Exam 3 Please note that this study guide is a listing of objectives that you are required to master for this course. However, items mentioned in class or in laboratory as
Skin (Integumentary System) Wheater, Chap. 9
 Skin (Integumentary System) Wheater, Chap. 9 Skin (Integument) Consists of skin and associated derivatives Largest organ of body (21 ft 2 ; 9 lbs.; has 11 miles of blood vessels) Functions: Protection
Skin (Integumentary System) Wheater, Chap. 9 Skin (Integument) Consists of skin and associated derivatives Largest organ of body (21 ft 2 ; 9 lbs.; has 11 miles of blood vessels) Functions: Protection
The Integumentary System : Embryology & Genetic Bases. Purnomo Soeharso Department of Medical Biology FMUI
 The Integumentary System : Embryology & Genetic Bases Purnomo Soeharso Department of Medical Biology FMUI Tissue organization of the skin (integumentum) : - Epidermis stratified epithelium on the outer
The Integumentary System : Embryology & Genetic Bases Purnomo Soeharso Department of Medical Biology FMUI Tissue organization of the skin (integumentum) : - Epidermis stratified epithelium on the outer
Anatomy and Physiology Homework: Chapters 3-4
 Anatomy and Physiology Homework: Chapters 3-4 CHAPTER 3: Cells and Tissues 1. The smallest unit of living tissue is called a. All living organisms are composed of these basic units where all life processes
Anatomy and Physiology Homework: Chapters 3-4 CHAPTER 3: Cells and Tissues 1. The smallest unit of living tissue is called a. All living organisms are composed of these basic units where all life processes
Chapter 6: Skin and the Integumentary System
 Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 10 th ed. Chapter 6: Skin and the Integumentary System Chapter 6: Skin and the Integumentary System I. Skin and Its Tissues A. Introduction
Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 10 th ed. Chapter 6: Skin and the Integumentary System Chapter 6: Skin and the Integumentary System I. Skin and Its Tissues A. Introduction
FIBROSING ALOPECIA IN A PATTERN DISTRIBUTION IN TWO BROTHERS WITH PILI MULTIGEMINI
 FIBROSING ALOPECIA IN A PATTERN DISTRIBUTION IN TWO BROTHERS WITH PILI MULTIGEMINI B D S B S M Department of Dermatology and Venereology, ed al a lty, ed al n er ty o a Summary. presence of several hairs
FIBROSING ALOPECIA IN A PATTERN DISTRIBUTION IN TWO BROTHERS WITH PILI MULTIGEMINI B D S B S M Department of Dermatology and Venereology, ed al a lty, ed al n er ty o a Summary. presence of several hairs
Chapter 6: Integumentary System
 Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 12 th ed. Chapter 6: Skin and the Integumentary System Chapter 6: Integumentary System I. Introduction 1. The skin is composed of of tissues.
Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 12 th ed. Chapter 6: Skin and the Integumentary System Chapter 6: Integumentary System I. Introduction 1. The skin is composed of of tissues.
Study the Effect of Illumination Time Parameter of Diode Laser on Wound Healing Process
 The 1 st Regional Conference of Eng. Sci. NUCEJ Spatial ISSUE vol.11, No.2, 2008 pp 536-543 Study the Effect of Illumination Time Parameter of Diode Laser on Wound Healing Process Assist. Prof. Dr. Munqith
The 1 st Regional Conference of Eng. Sci. NUCEJ Spatial ISSUE vol.11, No.2, 2008 pp 536-543 Study the Effect of Illumination Time Parameter of Diode Laser on Wound Healing Process Assist. Prof. Dr. Munqith
Levels of Organization
 Levels of Organization Oklahoma Laws Violators can be fined, arrested or jailed for making ugly faces at a dog. Females are forbidden from doing their own hair without being licensed by the state. Dogs
Levels of Organization Oklahoma Laws Violators can be fined, arrested or jailed for making ugly faces at a dog. Females are forbidden from doing their own hair without being licensed by the state. Dogs
Experimental Neoplastic Formation in Embryonic Chick Brains
 Experimental Neoplastic Formation in Embryonic Chick Brains by BENGT KALLEN 1 From the Tornblad Institute of Comparative Embryology, Lund WITH TWO PLATES IN mammalian teratology, a malformation consisting
Experimental Neoplastic Formation in Embryonic Chick Brains by BENGT KALLEN 1 From the Tornblad Institute of Comparative Embryology, Lund WITH TWO PLATES IN mammalian teratology, a malformation consisting
LIST OF ORGANS FOR HISTOPATHOLOGICAL ANALYSIS:!! Neural!!!!!!Respiratory:! Brain : Cerebrum,!!! Lungs and trachea! Olfactory, Cerebellum!!!!Other:!
 LIST OF ORGANS FOR HISTOPATHOLOGICAL ANALYSIS:!! Neural!!!!!!Respiratory:! Brain : Cerebrum,!!! Lungs and trachea! Olfactory, Cerebellum!!!!Other:! Spinal cord and peripheral nerves! Eyes, Inner ear, nasal
LIST OF ORGANS FOR HISTOPATHOLOGICAL ANALYSIS:!! Neural!!!!!!Respiratory:! Brain : Cerebrum,!!! Lungs and trachea! Olfactory, Cerebellum!!!!Other:! Spinal cord and peripheral nerves! Eyes, Inner ear, nasal
The Integumentary System: An Overview
 The Integumentary System: An Overview Functions: Protective covering Helps regulate body temperature Retards water loss from deeper tissues Houses sensory receptors Synthesizes biochemicals Excretes small
The Integumentary System: An Overview Functions: Protective covering Helps regulate body temperature Retards water loss from deeper tissues Houses sensory receptors Synthesizes biochemicals Excretes small
Cochlear anatomy, function and pathology III. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology III Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (3) on the cochlear lateral wall and Reissner s membrane:
Cochlear anatomy, function and pathology III Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (3) on the cochlear lateral wall and Reissner s membrane:
Chapter 5 The Integumentary System. Copyright 2009, John Wiley & Sons, Inc. 1
 Chapter 5 The Integumentary System Copyright 2009, John Wiley & Sons, Inc. 1 Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails, and
Chapter 5 The Integumentary System Copyright 2009, John Wiley & Sons, Inc. 1 Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails, and
Skin and Body Membranes
 Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 4 Skin and Body Membranes Slides 4.1 4.32 Lecture Slides in PowerPoint by Jerry L. Cook Skin and Body Membranes Function
Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 4 Skin and Body Membranes Slides 4.1 4.32 Lecture Slides in PowerPoint by Jerry L. Cook Skin and Body Membranes Function
Hole s Essentials of Human Anatomy & Physiology
 Hole s Essentials of Human Anatomy & Physiology David Shier Jackie Butler Ricki Lewis Created by Dr. Melissa Eisenhauer Head Athletic Trainer/Assistant Professor Trevecca Nazarene University Chapter 6
Hole s Essentials of Human Anatomy & Physiology David Shier Jackie Butler Ricki Lewis Created by Dr. Melissa Eisenhauer Head Athletic Trainer/Assistant Professor Trevecca Nazarene University Chapter 6
Unit 4 - The Skin and Body Membranes 1
 Unit 4 - The Skin and Body Membranes 1 I. Unit 4: Skin and Body Membranes A. Body Membranes 1. Function of body membranes a) Cover body surfaces b) Line body cavities c) Form protective sheets around organs
Unit 4 - The Skin and Body Membranes 1 I. Unit 4: Skin and Body Membranes A. Body Membranes 1. Function of body membranes a) Cover body surfaces b) Line body cavities c) Form protective sheets around organs
Observations on the Pathology of Lesions Associated with Stephanofilaria dinniki Round, 1964 from the Black Rhinoceros (Diceros bicornis)
 Journal of Helminthology, ~ol. XXXVIII, Nos. 1/2, 1964, pp. 171-174. Observations on the Pathology of Lesions Associated with Stephanofilaria dinniki Round, 1964 from the Black Rhinoceros (Diceros bicornis)
Journal of Helminthology, ~ol. XXXVIII, Nos. 1/2, 1964, pp. 171-174. Observations on the Pathology of Lesions Associated with Stephanofilaria dinniki Round, 1964 from the Black Rhinoceros (Diceros bicornis)
The Effect of Cortisone on Cell Proliferation and Migration in Peripheral Nerves undergoing Wallerian degeneration
 The Effect of Cortisone on Cell Proliferation and Migration in Peripheral Nerves undergoing Wallerian by G. A. THOMAS 1 From the Department of Anatomy, Guy's Hospital Medical School, London INTRODUCTION
The Effect of Cortisone on Cell Proliferation and Migration in Peripheral Nerves undergoing Wallerian by G. A. THOMAS 1 From the Department of Anatomy, Guy's Hospital Medical School, London INTRODUCTION
INTEGUMENTARY SYSTEM PART I: FUNCTIONS & EPIDERMIS
 INTEGUMENTARY SYSTEM PART I: FUNCTIONS & EPIDERMIS Integumentary System Cutaneous membrane Epidermis (5-layers) made up of epithelial tissue only Dermis (2-layers) contains connective tissue, vessels,
INTEGUMENTARY SYSTEM PART I: FUNCTIONS & EPIDERMIS Integumentary System Cutaneous membrane Epidermis (5-layers) made up of epithelial tissue only Dermis (2-layers) contains connective tissue, vessels,
Integumentary System and Body Membranes
 Integumentary System and Body Membranes The Skin and its appendages hair, nails, and skin glands Anatomy/Physiology NHS http://www.lab.anhb.uwa.edu.au/mb140/corepages/integumentary/integum.htm I. System
Integumentary System and Body Membranes The Skin and its appendages hair, nails, and skin glands Anatomy/Physiology NHS http://www.lab.anhb.uwa.edu.au/mb140/corepages/integumentary/integum.htm I. System
Integumentary System
 Integumentary System Overview Functions 1. Protection 2. Excretion of wastes 3. Maintenance of T b 4. Synthesis of vitamin D 3 5. Storage of lipids 6. Detection of sensory stimuli Epidermis Tissue types
Integumentary System Overview Functions 1. Protection 2. Excretion of wastes 3. Maintenance of T b 4. Synthesis of vitamin D 3 5. Storage of lipids 6. Detection of sensory stimuli Epidermis Tissue types
Histology of Integumentary System
 Histology of Integumentary System CONTENT / Topics Integumentary System Skin, thick - Major Layers Epidermis Dermis Hair Skin, hairy and Hair Follicle Sebaceous Glands Sebaceous Gland Sweat Glands Merocrine
Histology of Integumentary System CONTENT / Topics Integumentary System Skin, thick - Major Layers Epidermis Dermis Hair Skin, hairy and Hair Follicle Sebaceous Glands Sebaceous Gland Sweat Glands Merocrine
Ch. 4: Skin and Body Membranes
 Ch. 4: Skin and Body Membranes I. Body Membranes A. Function of body membranes 1. Cover body surfaces 2. Line body cavities 3. Form protective sheets around organs II. Classification of Body Membranes
Ch. 4: Skin and Body Membranes I. Body Membranes A. Function of body membranes 1. Cover body surfaces 2. Line body cavities 3. Form protective sheets around organs II. Classification of Body Membranes
2/5/2019. Organ System: Skin or Integumentary System. Hypodermis (or superficial fascia) Integumentary System - Learn and Understand
 Integumentary System - Learn and Understand Skin is an organ comprised of all four tissues Each layer of the skin contributes to one or more of its numerous functions Skin is both strong and flexible Keratinization
Integumentary System - Learn and Understand Skin is an organ comprised of all four tissues Each layer of the skin contributes to one or more of its numerous functions Skin is both strong and flexible Keratinization
The Integumentary System
 The Integumentary System Skin (Integument) Consists of two major regions 1. Epidermis superficial region 2. Dermis middle region Hypodermis (superficial fascia) deepest region Subcutaneous layer deep to
The Integumentary System Skin (Integument) Consists of two major regions 1. Epidermis superficial region 2. Dermis middle region Hypodermis (superficial fascia) deepest region Subcutaneous layer deep to
Supporting Information
 Supporting Information CD200 Expressing Human Basal Cell Carcinoma Cells Initiate Tumor Growth Chantal S Colmont 1, Antisar BenKetah 1, Simon H Reed 2, Nga Voong 3, William Telford 3, Manabu Ohyama 4,
Supporting Information CD200 Expressing Human Basal Cell Carcinoma Cells Initiate Tumor Growth Chantal S Colmont 1, Antisar BenKetah 1, Simon H Reed 2, Nga Voong 3, William Telford 3, Manabu Ohyama 4,
How to decipher a pathology report for alopecia
 How to decipher a pathology report for alopecia DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY Lynne J. Goldberg, MD S063-Hair Disorders Made Easier DISCLOSURES I do not have any relationships with industry
How to decipher a pathology report for alopecia DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY Lynne J. Goldberg, MD S063-Hair Disorders Made Easier DISCLOSURES I do not have any relationships with industry
Mesenchymal control over elongating and branching morphogenesis in salivary gland development
 J. Embryol. exp. Morph. Vol. 66, pp. 9-, 98 9 Printed in Great Britain Company of Biologists Limited 98 Mesenchymal control over elongating and branching morphogenesis in salivary gland development ByHIROYUKI
J. Embryol. exp. Morph. Vol. 66, pp. 9-, 98 9 Printed in Great Britain Company of Biologists Limited 98 Mesenchymal control over elongating and branching morphogenesis in salivary gland development ByHIROYUKI
Male pattern hair loss: current understanding
 Review Male pattern hair loss: current understanding David A. Whiting, MD Introduction The most common form of human hair loss is androgenic alopecia (AGA). It affects at least 50% of men by the age of
Review Male pattern hair loss: current understanding David A. Whiting, MD Introduction The most common form of human hair loss is androgenic alopecia (AGA). It affects at least 50% of men by the age of
CHAPTER 5 INTEGUMENTARY
 CHAPTER 5 INTEGUMENTARY skin under the skin other stuff cutaneous layer hypodermis (subcutaneous) accessory structures Cutaneous layer = skin epithelial layers = connective tissue layer = dermis Subcutaneous
CHAPTER 5 INTEGUMENTARY skin under the skin other stuff cutaneous layer hypodermis (subcutaneous) accessory structures Cutaneous layer = skin epithelial layers = connective tissue layer = dermis Subcutaneous
IN the skin of the mouse we find that the dermal papilla of growing hair
 (240 Cyclic Changes in Polysaccharides of the Papilla of the Hair Follicle By WILLIAM MONTAGNA, HERMAN B. CHASE, JANET D. MALONE, AND HELEN P. MELARAGNO (From Brown University, Providence, Rhode Island,
(240 Cyclic Changes in Polysaccharides of the Papilla of the Hair Follicle By WILLIAM MONTAGNA, HERMAN B. CHASE, JANET D. MALONE, AND HELEN P. MELARAGNO (From Brown University, Providence, Rhode Island,
(From Arnold Biological Laboratory, Brown University, ProVence) P~s 3 To 5. Materials and Methods
 Published Online: 25 January, 1955 Supp Info: http://doi.org/10.1083/jcb.1.1.13 Downloaded from jcb.rupress.org on June 21, 2018 HISTOLOGY AND CYTOCHEMISTRY OF HUMAN SKIN IX. THE Dm~mUT~ON Ot NoN-SP~.c~Ic
Published Online: 25 January, 1955 Supp Info: http://doi.org/10.1083/jcb.1.1.13 Downloaded from jcb.rupress.org on June 21, 2018 HISTOLOGY AND CYTOCHEMISTRY OF HUMAN SKIN IX. THE Dm~mUT~ON Ot NoN-SP~.c~Ic
The Integumentary System
 The Integumentary System The Integumentary System Integument is skin Skin and its appendages make up the integumentary system (See if you can name some appendages) A fatty layer (hypodermis) lies deep
The Integumentary System The Integumentary System Integument is skin Skin and its appendages make up the integumentary system (See if you can name some appendages) A fatty layer (hypodermis) lies deep
Tips on getting the most from your alopecia pathology reports. D irector, H a ir C linic, Boston Medical C e n ter
 Tips on getting the most from your alopecia pathology reports Lynne J. Goldberg, MD J a g Bhawan Professor o f Dermatology a n d Pathology & Laboratory Medicine Boston U n iversity School of Medicine D
Tips on getting the most from your alopecia pathology reports Lynne J. Goldberg, MD J a g Bhawan Professor o f Dermatology a n d Pathology & Laboratory Medicine Boston U n iversity School of Medicine D
SKIN. 3. How is the skin structured around the finger joints to allow for flexible movement of the fingers?
 SKIN Objectives for Exam #1: 1. List various skin structures and describe their functions. 2. Describe skin responses to increases and decreases in body temperature. 3. Provide examples of various skin
SKIN Objectives for Exam #1: 1. List various skin structures and describe their functions. 2. Describe skin responses to increases and decreases in body temperature. 3. Provide examples of various skin
Describe the functions of the vertebrate integumentary system. Discuss the structure of the skin and how it relates to function.
 Chapter 5 Describe the functions of the vertebrate integumentary system. Discuss the structure of the skin and how it relates to function. Explain the basis for different skin colors. Describe the structure
Chapter 5 Describe the functions of the vertebrate integumentary system. Discuss the structure of the skin and how it relates to function. Explain the basis for different skin colors. Describe the structure
Integumentary System. 2/20/02 S. Davenport 1
 Integumentary System 2/20/02 S. Davenport 1 Functions of Skin Protection Temperature regulation Sensation Excretion Vitamin D production 2/20/02 S. Davenport 2 Protection A Barrier Three types of barriers:
Integumentary System 2/20/02 S. Davenport 1 Functions of Skin Protection Temperature regulation Sensation Excretion Vitamin D production 2/20/02 S. Davenport 2 Protection A Barrier Three types of barriers:
The Integumentary System: ANATOMY Includes: - Skin (integument) MEMBRANES. PHYSIOLOGY (functions) Protection. EPITHELIAL (cont.
 Did you know. Membranes & The Integumentary System The skin is the largest organ of the human body. It has a surface area of about 25 square-feet! You shed about 1.5 pounds of skin particles each year.
Did you know. Membranes & The Integumentary System The skin is the largest organ of the human body. It has a surface area of about 25 square-feet! You shed about 1.5 pounds of skin particles each year.
ALL PHOTOS ARE IDENTIFIED IN THE LOWER RIGHT CORNER WITH THE MAGNIFICATION POWER THAT THE PHOTO WAS TAKEN WITH. SCAN - THIS IS A VERY LOW POWER IMAGE
 ALL PHOTOS ARE IDENTIFIED IN THE LOWER RIGHT CORNER WITH THE MAGNIFICATION POWER THAT THE PHOTO WAS TAKEN WITH. SCAN - THIS IS A VERY LOW POWER IMAGE THAT WE USE WHEN A SAMPLE IS SO BIG THAT YOU CAN T
ALL PHOTOS ARE IDENTIFIED IN THE LOWER RIGHT CORNER WITH THE MAGNIFICATION POWER THAT THE PHOTO WAS TAKEN WITH. SCAN - THIS IS A VERY LOW POWER IMAGE THAT WE USE WHEN A SAMPLE IS SO BIG THAT YOU CAN T
11/8/2012. Chapter 6 Part 1 Objectives: Skin = Integument = Cutaneous Membrane. The Structure of Skin. Epidermis
 Chapter 6 Part 1 Objectives: Define organ, and associate the skin as an organ of the integumentary system. List the general functions of the skin. Describe the structure of the layers of the skin. Summarize
Chapter 6 Part 1 Objectives: Define organ, and associate the skin as an organ of the integumentary system. List the general functions of the skin. Describe the structure of the layers of the skin. Summarize
Hair. 5 B Notes. Hair 10/1/14. The Integumentary System
 Hair 5 B Notes The Integumentary System Aler=ng the body to presence of insects on the skin Guarding the scalp against physical trauma, heat loss, and sunlight En=re surface except palms, soles, lips,
Hair 5 B Notes The Integumentary System Aler=ng the body to presence of insects on the skin Guarding the scalp against physical trauma, heat loss, and sunlight En=re surface except palms, soles, lips,
EXPERIMENTAL THERMAL BURNS I. A study of the immediate and delayed histopathological changes of the skin.
 EXPERIMENTAL THERMAL BURNS I A study of the immediate and delayed histopathological changes of the skin. RJ Brennan, M.D. and B. Rovatti M.D. The purpose of this study was to determine the progressive
EXPERIMENTAL THERMAL BURNS I A study of the immediate and delayed histopathological changes of the skin. RJ Brennan, M.D. and B. Rovatti M.D. The purpose of this study was to determine the progressive
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 5 The Integumentary System Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails,
Principles of Anatomy and Physiology 14 th Edition CHAPTER 5 The Integumentary System Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails,
Supplementary Figure 1 The ability to regenerate an ear hole is discontinuous with wound healing. Ear-hole closure at D85 for each sex within each
 Supplementary Figure 1 The ability to regenerate an ear hole is discontinuous with wound healing. Ear-hole closure at D85 for each sex within each species observed. Data show a binary response to a 4 mm
Supplementary Figure 1 The ability to regenerate an ear hole is discontinuous with wound healing. Ear-hole closure at D85 for each sex within each species observed. Data show a binary response to a 4 mm
Prelab #4 BLOOD; BONE MARROW; RESPIRATORY; INTEGUEMENT Page 1
 Prelab #4 BLOOD; BONE MARROW; RESPIRATORY; INTEGUEMENT Page 1 Blood Slide 101 This a classic slide of blood cells using a Wright stain. Inspect red blood cells and their appearance. Note the approximate
Prelab #4 BLOOD; BONE MARROW; RESPIRATORY; INTEGUEMENT Page 1 Blood Slide 101 This a classic slide of blood cells using a Wright stain. Inspect red blood cells and their appearance. Note the approximate
The Integumentary System
 PowerPoint Lecture Slides prepared by Meg Flemming Austin Community College C H A P T E R 5 The Integumentary System Chapter 5 Learning Outcomes 5-1 5-2 5-3 5-4 5-5 Describe the main structural features
PowerPoint Lecture Slides prepared by Meg Flemming Austin Community College C H A P T E R 5 The Integumentary System Chapter 5 Learning Outcomes 5-1 5-2 5-3 5-4 5-5 Describe the main structural features
Integumentary System. Packet #12
 Integumentary System Packet #12 Introduction Skin/Integument Skin, considered an organ, is the major component of the integumentary system. The integumentary system is also composed of other accessory
Integumentary System Packet #12 Introduction Skin/Integument Skin, considered an organ, is the major component of the integumentary system. The integumentary system is also composed of other accessory
Identification of the spermatogenic stages in living seminiferous tubules of man
 Identification of the spermatogenic stages in living seminiferous tubules of man V. Nikkanen, K.-O. S\l=o"\derstr\l=o"\m and M. Parvinen Department of Obstetrics and Gynecology, Turku University Central
Identification of the spermatogenic stages in living seminiferous tubules of man V. Nikkanen, K.-O. S\l=o"\derstr\l=o"\m and M. Parvinen Department of Obstetrics and Gynecology, Turku University Central
Histopathology of Melanoma
 THE YALE JOURNAL OF BIOLOGY AND MEDICINE 48, 409-416 (1975) Histopathology of Melanoma G. J. WALKER SMITH Department ofpathology, Yale University School ofmedicine, 333 Cedar Street, New Haven, Connecticut
THE YALE JOURNAL OF BIOLOGY AND MEDICINE 48, 409-416 (1975) Histopathology of Melanoma G. J. WALKER SMITH Department ofpathology, Yale University School ofmedicine, 333 Cedar Street, New Haven, Connecticut
The Beauty of the Skin
 The Beauty of the Skin Rose-Anne Romano, Ph.D Assistant Professor Department of Oral Biology School of Dental Medicine State University of New York at Buffalo The Big Question How do approximately 50 trillion
The Beauty of the Skin Rose-Anne Romano, Ph.D Assistant Professor Department of Oral Biology School of Dental Medicine State University of New York at Buffalo The Big Question How do approximately 50 trillion
Mast cells in leprosy
 Original article: Mast cells in leprosy *Dr. Navdeep Kaur, 1 Dr. S.S. Hiremath, 2 Dr. Suresh K.K. 3 1Post Graduate, 2 Professor, 3 Professor, Department of Pathology, JJM Medical College, Davangere, Karnataka,
Original article: Mast cells in leprosy *Dr. Navdeep Kaur, 1 Dr. S.S. Hiremath, 2 Dr. Suresh K.K. 3 1Post Graduate, 2 Professor, 3 Professor, Department of Pathology, JJM Medical College, Davangere, Karnataka,
Melanoma-Back to Basics I Thought I Knew Ya! Paul K. Shitabata, M.D. Dermatopathologist APMG
 Melanoma-Back to Basics I Thought I Knew Ya! Paul K. Shitabata, M.D. Dermatopathologist APMG At tumor board, a surgeon insists that all level II melanomas are invasive since they have broken through the
Melanoma-Back to Basics I Thought I Knew Ya! Paul K. Shitabata, M.D. Dermatopathologist APMG At tumor board, a surgeon insists that all level II melanomas are invasive since they have broken through the
7/10/18. Introduction. Integumentary System. Physiology. Anatomy. Structure of the Skin. Epidermis
 Introduction Integumentary System Chapter 22 Skin is largest and heaviest organ of body (7% of body weight) Houses receptors for touch, heat, cold, movement, and vibration No other body system is more
Introduction Integumentary System Chapter 22 Skin is largest and heaviest organ of body (7% of body weight) Houses receptors for touch, heat, cold, movement, and vibration No other body system is more
The Integumentary System
 The Integumentary System The Integumentary System Integument is skin Skin and its appendages make up the integumentary system A fatty layer (hypodermis) lies deep to it Two distinct regions Epidermis Dermis
The Integumentary System The Integumentary System Integument is skin Skin and its appendages make up the integumentary system A fatty layer (hypodermis) lies deep to it Two distinct regions Epidermis Dermis
Skin. Kristine Krafts, M.D.
 Skin Kristine Krafts, M.D. Skin Lecture Objectives Describe the functions of skin. Describe the structure, location and function of the cell types found in epidermis: keratinocytes, melanocytes, Langerhans
Skin Kristine Krafts, M.D. Skin Lecture Objectives Describe the functions of skin. Describe the structure, location and function of the cell types found in epidermis: keratinocytes, melanocytes, Langerhans
Chapter 6: Skin & the. 6.1 Skin and its Tissues 6.2 Accessory Organs of the Skin 6.3 Regulation of Body Temperature 6.4 Healing of Wounds
 Skin & the Integumentary System 6.1-6.2 September 10, 2012 Chapter 6: Skin & the Integumentary System 6.1 Skin and its Tissues 6.2 Accessory Organs of the Skin 6.3 Regulation of Body Temperature 6.4 Healing
Skin & the Integumentary System 6.1-6.2 September 10, 2012 Chapter 6: Skin & the Integumentary System 6.1 Skin and its Tissues 6.2 Accessory Organs of the Skin 6.3 Regulation of Body Temperature 6.4 Healing
Dr Narmeen S. Ahmad. Lab 1
 Dr Narmeen S. Ahmad Lab 1 1 Tissues are groups of cells with a common structure (form) and function (job). There are (4) types of tissue: 1. Epithelial 2. Connective 3. Muscle 4. Nervous 2 Epithelial cells
Dr Narmeen S. Ahmad Lab 1 1 Tissues are groups of cells with a common structure (form) and function (job). There are (4) types of tissue: 1. Epithelial 2. Connective 3. Muscle 4. Nervous 2 Epithelial cells
Case Report A Rare Cutaneous Adnexal Tumor: Malignant Proliferating Trichilemmal Tumor
 Case Reports in Medicine Volume 2015, Article ID 742920, 4 pages http://dx.doi.org/10.1155/2015/742920 Case Report A Rare Cutaneous Adnexal Tumor: Malignant Proliferating Trichilemmal Tumor Omer Alici,
Case Reports in Medicine Volume 2015, Article ID 742920, 4 pages http://dx.doi.org/10.1155/2015/742920 Case Report A Rare Cutaneous Adnexal Tumor: Malignant Proliferating Trichilemmal Tumor Omer Alici,
Effect of glucose on beta cell proliferation and population size in organ culture of foetal and neonatal rat pancreases
 J. Embryol. exp. Morph. 74, 303-312 (1983) 3Q3 Printed in Great Britain The Company of Biologists Limited 1983 Effect of glucose on beta cell proliferation and population size in organ culture of foetal
J. Embryol. exp. Morph. 74, 303-312 (1983) 3Q3 Printed in Great Britain The Company of Biologists Limited 1983 Effect of glucose on beta cell proliferation and population size in organ culture of foetal
The Cardiovascular System (Part II)
 The Cardiovascular System (Part II) 1 Aortic arch derivatives Aortic arches 2 Pharyngeal and aortic arches 4 th week 3 (1st, 2nd pairs disappeared) 6 th week (37 days) 4 8th week: transformed into the
The Cardiovascular System (Part II) 1 Aortic arch derivatives Aortic arches 2 Pharyngeal and aortic arches 4 th week 3 (1st, 2nd pairs disappeared) 6 th week (37 days) 4 8th week: transformed into the
21 Skin and it s Accessories
 21 Skin and it s Accessories 21-001 Skin 1 2 Sweat glands Adipose tissue 3 Retinaculum cutis 21-01. Skin of the palm 1. General view. Human, H-E stain, x 3.3. Opening of sweat gland duct Stratum corneum
21 Skin and it s Accessories 21-001 Skin 1 2 Sweat glands Adipose tissue 3 Retinaculum cutis 21-01. Skin of the palm 1. General view. Human, H-E stain, x 3.3. Opening of sweat gland duct Stratum corneum
