High flow nasal cannula therapy for respiratory support in children (Protocol)
|
|
|
- April Washington
- 5 years ago
- Views:
Transcription
1 High flow nasal cannula therapy for respiratory support in children (Protocol) Mayfield S, Jauncey-Cooke J, Hough JL, Schibler A, Gibbons K, Bogossian F This is a reprint of a Cochrane protocol, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2012, Issue 5
2 T A B L E O F C O N T E N T S HEADER ABSTRACT BACKGROUND OBJECTIVES METHODS ACKNOWLEDGEMENTS REFERENCES APPENDICES HISTORY CONTRIBUTIONS OF AUTHORS DECLARATIONS OF INTEREST i
3 [Intervention Protocol] High flow nasal cannula therapy for respiratory support in children Sara Mayfield 1,2, Jacqueline Jauncey-Cooke 1,2, Judith L Hough 3, Andreas Schibler 1,3, Kristen Gibbons 4, Fiona Bogossian 2 1 Paediatric Intensive Care Unit, Mater Children s Hospital, South Brisbane, Australia. 2 The University of Queensland, School of Nursing and Midwifery, Herston, Australia. 3 Mater Medical Research Institute, South Brisbane, Australia. 4 Clinical Research Support Unit, Mater Medical Research Institute, South Brisbane, Australia Contact address: Sara Mayfield, Sara.mayfield@mater.org.au. Editorial group: Cochrane Anaesthesia Group. Publication status and date: New, published in Issue 5, Citation: Mayfield S, Jauncey-Cooke J, Hough JL, Schibler A, Gibbons K, Bogossian F. High flow nasal cannula therapy for respiratory support in children. Cochrane Database of Systematic Reviews 2012, Issue 5. Art. No.: CD DOI: / CD A B S T R A C T This is the protocol for a review and there is no abstract. The objectives are as follows: We aim to determine if the use of HFNC is better than other forms of non-invasive therapy in paediatric patients who require respiratory support. B A C K G R O U N D Description of the condition Respiratory support is central to the care of critically ill children. Support may be needed due to underlying disease processes such as respiratory infections or pneumonia, neuromuscular disorders, cardiac conditions or failure, and other mechanisms such as upper airway obstruction, trauma and injury, or post-surgical interventions. Respiratory support can be delivered non-invasively in the form of oxygen therapy or continuous positive airway pressure (CPAP) or invasively via mechanical ventilation. Children with significant respiratory distress and hypoxaemia often require the latter. This may result in various forms of trauma to the lungs and airways, collectively known as ventilator induced lung injury (VILI) (Dahlem 2003; The ARDS Network 2000). While VILI is the major concern with intubation and mechanical ventilation, there are other effects on the body that need to be considered. The increased use of sedative drugs may lead to neuropathy or myopathy, which can increase recovery time. In turn there may be the need for cardiovascular support in the form of drug infusions to maintain blood pressure. All of these factors increase the costs of the care provided to the child. Non-invasive methods of ventilation are an ideal method of providing respiratory support without the need for intubation and may avoid some of the additional harm associated with positive pressure ventilation. Non-invasive ventilation can be as simple as oxygen therapy delivered via a face mask, nasal cannula or head box through to devices delivering CPAP via the face and mask interface or nasopharyngeal tubes, with pressure generated by a dedicated driver or water column (that is bubble CPAP) (Frey 2001; Frey 2003; Klein 1986). Those devices delivering CPAP can reduce the work of breathing and improve functional residual capacity, potentially avoiding intubation, reducing VILI, and preventing other possible causes of harm (Reid 1984; Thorsteinsson 2002). A disadvantage of this method of delivery is that it is cumber- 1
4 some and the masks and tubes used are poorly tolerated by young children and infants (Yong 2005). This can make the delivery of CPAP variable thereby resulting in ineffective ventilation. Having a system that can deliver CPAP and be comfortable and well tolerated by children is an important consideration in providing noninvasive respiratory support. Description of the intervention High flow nasal cannula (HFNC) therapy has recently been introduced to a range of patients from preterm infants to adults, addressing the need for a simple, effective method of providing respiratory support (Campbell 2006; McGinley 2009; McKiernan 2009; Shoemaker 2007). It has an advantage over simple oxygen therapy in that the gas mixture can be heated and humidified, thereby reducing damage to upper airway mucosa, and the concentration of inspired oxygen can also be titrated as required. This can prevent inflammatory reactions and the naso-pulmonary bronchoconstrictor reflex triggered by cold, dry air (Spentzas 2009). It has been shown that the delivery of nasal air at high flow rates may cause incidental delivery of CPAP (Dysart 2009; Spence 2007; Wilkinson 2008). The effects of this are yet to be fully understood. It may be that the high flow flushes the dead space of the nasopharyngeal cavity resulting in alveolar ventilation as a greater fraction of minute ventilation. It may also assist in the washout of carbon dioxide, which may then reduce apnoeas secondary to hypercapnia and improve ventilation (Dysart 2009). High flow rates may also provide an amount of positive pressure and thereby overcome upper airway obstruction, again improving ventilation (McGinley 2009). The amount of CPAP generated depends on the flow delivered relative to the size of the patient, the size of the nasal cannula used, and the potential leak around the nasal cannula (Kubicka 2008; Lampland 2009; Sreenan 2001). Three retrospective studies assessing HFNC therapy have demonstrated that overall ventilator days were significantly decreased after the introduction of this therapy when compared to retrospective historical control groups (McKiernan 2009; Schibler 2011; Shoemaker 2007). HFNC therapy has also been reported to be better tolerated by the patient than other forms of non-invasive ventilation (Roca 2010). This can reduce the need for sedation that is required to help tolerate more invasive or uncomfortable forms of respiratory support. Why it is important to do this review HFNC therapy is an emerging treatment option for the respiratory support of children, especially in the intensive care unit. To date, most of the findings have been from neonatal and adult studies, with little clinical experience reported in the paediatric population (McKiernan 2009). The Cochrane review on high flow nasal cannula from the Cochrane Neonatal Group concluded that there is insufficient evidence to determine HFNC effectiveness and that more research is needed (Wilkinson 2011). At present there is a protocol for the systematic review assessing HFNC effectiveness in the adult population, which is in progress with the Cochrane Anaesthesia Group (Corley in process). There is also a protocol with the Cochrane Acute Respiratory Infections Group assessing HFNC therapy for infants with bronchiolitis (Beggs 2012). This review differs in that it covers a wider age range and more diverse pathophysiologies. It is necessary to assess the use of this therapy amongst the paediatric population as there are potential risks associated with its use. Neonatal studies have described scalp emphysema and pneumocephalus as potential risks, along with nasal mucosal trauma and bleeding (Jasin 2008; Kopelman 2003). There is also concern that this therapy in the neonatal population provides unpredictable, high pressures that could damage the preterm lung (Lampland 2009). These risks need to be assessed in the paediatric population. HFNC has the potential to improve outcomes in critically ill children. It is readily applied and is not resource or cost intensive. Staff can easily be trained in the application of HFNC and in the care of children using this therapy. It may reduce the incidence of intubation in paediatrics and may reduce the length of intubation as HFNC holds potential as an adjunct between extubation and low flow nasal prong oxygen delivery. The potential also exists that children requiring this therapy may be cared for outside of the paediatric intensive care unit (PICU). O B J E C T I V E S We aim to determine if the use of HFNC is better than other forms of non-invasive therapy in paediatric patients who require respiratory support. M E T H O D S Criteria for considering studies for this review Types of studies We will include prospective, randomized controlled trials (RCTs) and quasi-randomized studies. Types of participants We will include paediatric participants aged from four weeks corrected age to 16 years requiring respiratory support. 2
5 Types of interventions For the purposes of this review, we will define high flow nasal oxygen as the delivery of heated, humidified oxygen or blended oxygen with air via a nasal cannula at flow rates of greater than 2 L/minute. HFNC will be compared with other means of noninvasive respiratory support, such as cot, hood or tent oxygen; low flow nasal cannula (flow rates equal to or less than 2 L/min); and continuous positive airway pressure (CPAP). Types of outcome measures Primary outcomes 1. Hospital mortality 2. Intubation rate 3. Treatment failure (defined by the use of additional respiratory support) Secondary outcomes 1. Duration of any form of respiratory support in hours (mechanical ventilation, non-invasive ventilation, high flow nasal cannula) 2. Length of stay in days in hospital 3. Clinical severity score 4. Length of PICU stay in days 5. Complications: air leaks (pneumothorax, pneumomediastinum, pneumopericardium or pulmonary interstitial emphysema (PIE)) reported either individually or as a composite outcome nasal trauma (defined as erythema or erosion of the nasal mucosa, nares or septum assessed by a blinded observer) nosocomial sepsis (defined as positive blood or cerebrospinal fluid (CSF) cultures) barotrauma gastrointestinal distention Additional outcomes measured in trials will be added as secondary outcomes following the literature search. Search methods for identification of studies SP (January 1980 to date); CINAHL via EBSCO Host (1982 to date); LILACS via the BIREME interface (1982 to date). We will search electronic databases of higher degree theses for relevant unpublished trials: Index to Theses (1950 to date), Australian Digital Theses Program (1997 to date) and Proquest Digital Dissertations (1980 to date). We will combine our MEDLINE search strategy with the Cochrane highly sensitive search strategy for identifying randomized controlled trials (RCTs) as suggested in the Cochrane handbook for Systematic Reviews of Interventions (Higgins 2011). We will adopt the MEDLINE search strategy (see Appendix 1) for searching in all other databases. For ongoing trials, we will search the Meta Register of Controlled Trials ( and the National Research Register ( Searching other resources We will handsearch citations from included studies. We will not exclude studies on the basis of language. We will contact authors known in the field to determine if unpublished work is available. Data collection and analysis Selection of studies Six authors ( SM, JJC, FB, JH, AS, KG) will undertake the review. We will use the search strategy described to obtain titles and abstracts of studies that may be relevant to the review. Two authors (SM and JJC) will independently perform this screening. We will discard studies that are not applicable, and the reason for each trial that is excluded will be documented. We will resolve disagreements by consulting with a third author (FB), who will decide on inclusion or not. We will compile a list of eligible trials, with a unique identifier, on a form for eligible trials contained within the data extraction form (see Appendix 2). Electronic searches We will obtain all relevant studies irrespective of language or publication status (published, unpublished, in press, and in progress) using the following methods. We will apply no limits in terms of language or year of publication. We will search the current issue of the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library); MED- LINE via Ovid SP (January 1966 to date); EMBASE via Ovid Data extraction and management We will adapt the standardized Cochrane Anasthesia Review Group (CARG) data extraction form (Appendix 2) to capture relevant data specific to this review. We (SM and JJC) will use this form independently to extract and collect data from the relevant studies. We (SM and JJC) will resolve any discrepancies in the data extracted by discussion. 3
6 Assessment of risk of bias in included studies Two authors (SM and JJC) will independently assess the methodological quality of the eligible trials. Any disagreements will be resolved by a third author (FB). We will include a risk of bias table as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The judgements low risk, high risk and unclear risk will be used in the table to determine bias. SM will enter the data into the Review manager Software (RevMan 5.1) with verification of data entry conducted independently. The following domains will be assessed. 1.Random sequence generation. 2. Allocation concealment. 3. Blinding of participants and personnel, and outcome assessors. 4. Intention-to-treat analysis. We will use pre-defined criteria for treatment failure (switching to an alternative respiratory support modality) and intubation. Measures of treatment effect We will summarize trials that meet the inclusion criteria in tables to allow for comparison of characteristics and quality. Excluded studies will be tabulated with the reason for exclusion documented. For dichotomous outcome data, such as mortality, we will use risk ratio (RR) to determine effect. It will be displayed on a table as number with event and number without event. For continuous data we will collect the mean and standard deviation and display it on a table. If different scales are used to measure continuous data across trials we will calculate the standardized mean difference (SMD) to determine treatment effect. We will analyse outcomes from comparable trials with 95% confidence intervals (CI) to estimate each trial s treatment effect. We will compare the results graphically using forest plots, with risk ratio (RR) as the point estimate for dichotomous outcomes and mean difference (MD) as the point estimate for continuous outcomes. Dealing with missing data We will contact the corresponding author of the study to source missing data. If the corresponding author does not respond, or if it is not possible to find them, then we will include the trial in question in the review but we will analyse the effects of its inclusion or exclusion on the overall results as part of the sensitivity analysis. Assessment of heterogeneity We will analyse heterogeneity using the Chi 2 test on N-1 degrees of freedom, with an alpha of 0.1 used for statistical significance, and with the I 2 statistic (Higgins 2011). I 2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity, respectively. We will set an I 2 threshold of greater than 50% to indicate a substantial variation across trials due to heterogeneity. We will use a fixed-effect model if we find insignificant heterogeneity between trials. We will use a random-effects model if significant heterogeneity exists between trials (Higgins 2011). We will test for homogeneity between trials for each outcome using the Cochran s Q statistic, with P less than or equal to We will assess the clinical diversity (clinical heterogeneity) and methodological diversity (risk of bias assessment) of the included studies. We will undertake subgroup analysis to examine possible clinical variability when the I 2 statistic is less than 50% but heterogeneity remains statistically significant. We will analyse outcome data from trial populations rather than individuals in order to explain possible sources of variability ( Higgins 2011). Assessment of reporting biases We will assess publication bias or small study effects by preparing a funnel plot. We will test for funnel plot asymmetry if there are greater than 10 studies included in the meta-analysis. Data synthesis We will review the summary tables of included trials to identify clinical heterogeneity amongst trials. If there are two or more randomized trials with comparable populations undergoing similar interventions, we will do a meta-analysis with a random-effects model using RevMan 5.1. Subgroup analysis and investigation of heterogeneity We will perform subgroup analysis to explore possible sources of heterogeneity (for example participants, interventions). Heterogeneity among participants could be related to age, corrected gestational age and underlying pathophysiological condition. Heterogeneity in treatment could be related to the amount of flow delivered in relation to body weight. We will explore the impact of differing flow rates with a subgroup analysis. We will tabulate and assess adverse effects with descriptive techniques as they are likely to be different for the various subgroups. Where possible, we will calculate the RR with 95% CI for each adverse effect. We will use a narrative summary of findings when there is clear evidence of heterogeneity amongst trials (Sutton 2008). We will examine differences in populations based on the following. 1. Age (corrected). 2. Pathophysiology, as follows: type 1 respiratory failure; type 2 respiratory failure; parenchymal lung disease; neuromuscular disorders; respiratory drive; airway obstruction; preterm birth. 4
7 Sensitivity analysis We will perform a sensitivity analysis, exploring the causes of heterogeneity and the robustness of results if there is an adequate number of studies. We will perform sensitivity analysis of trials with low-risk of bias versus high-risk of bias. We will compare random-effects model and fixed-effect model estimates for each outcome variable. (SoF) table using the GRADE software. The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers, within a study, the risk of bias (methodologic quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias (Guyatt 2008). Summary of findings We will use the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence associated with specific outcomes: mortality, intubation, failure of treatment or escalation to non-invasive ventilation, length of PICU stay, length of time on any form of respiratory support, oxygenation and respiratory assessment tools in our review and construct a summary of findings A C K N O W L E D G E M E N T S We would like to thank Mathew Zacharis (content editor), Cathal Walsh (Statistical editor), Dominic Wilkinson, Oliver Karam, Mark Davies (peer reviewers) for their help and editorial advice during the preparation of this protocol for the systematic review. R E F E R E N C E S Additional references Beggs 2012 Beggs, Wong ZH, Kaul S, Ogden KJ, Walters JAE. Highflow nasal cannula therapy for infants with bronchiolitis. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: / CD009609] Campbell 2006 Campbell DM, Shah PS, Shah V, Kelly EN. Nasal continuous positive airway pressure from high flow cannula versus Infant Flow for preterm infants. Journal of Perinatology 2006;26: Corley in process Corley A, Rickard CM, Aitken LM, Johnston A, Barnett A, Fraser JF. High flow nasal cannula for respiratory support in adult intensive care patients. Registered title: Dahlem 2003 Dahlem P, Van Aalderen WM, Hamaker ME, Dijkfraaf MG, Bos AP. Incidence and short-term outcome of acute lung injury in mechanically ventilated children. European Respiratory Journal 2003;22(6): [PUBMED: ] Dysart 2009 Dysart K, Miller TL, Wolfson MR, Shaffer TH. Reserach in high flow therapy: mechanisms of action. Respiratory Medicine 2009;103: Frey 2001 Frey B, McQuillan PJ, Shann F, Freezer N. Nasopharyngeal oxygen therapy produces positive end-expiratory pressure in infants. European Journal of Pediatrics 2001;160: Frey 2003 Frey B, Shann F. Oxygen administration in infants. Archives of Disease in Childhood: Fetal and Neonatal Edition 2003;88 (2):84 8. [MEDLINE: ] Guyatt 2008 Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ. What is quality of evidence and why is it important to clinicians. BMJ 2008;336: [MEDLINE: ] Higgins 2011 Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version [updated March 2011]. The Cochrane Collaboration, Available from Jasin 2008 Jasin LR, Kern S, Thopmson S, Walter C, Rone JM, Yohannan MD. Subcutaneous scalp emphysema, pneumoorbitis and pneumocephalus in a neonate on high humidity high flow nasal cannula. Journal of Perinatology 2008;28: [DOI: /jp ] Klein 1986 Klein M, Reynolds LG. Relief of sleep-related oropharyngeal airway obstruction by continuous insufflation of the pharynx. Lancet 1986;1: Kopelman 2003 Kopelman AE, Holbert D. Use of oxygen cannulas in extremely low birthweight infants is associated with mucosal trauma and bleeding, and possibly with coagulase-negative staphylococcal sepsis. Journal of Perinatology 2003;23:94 7. Kubicka 2008 Kubicka ZJ, Limauro J, Darnell RA. Heated humidified high-flow nasal cannula therapy: yet another way to deliver continuous positive airway pressure?. Pediatrics 2008;121:
8 Lampland 2009 Lampland AL, Plumm B, Meyers PA, Worwa CT, Mammel MC. Observational study of humidified high flow nasal cannula compared with nasal continuous positive airway pressure. Journal of Pediatrics 2009;154: McGinley 2009 McGinley B, Halbower A, Schwartz AR, Smith PL, Patil SP, Schneider H. Effect of a high-flow open nasal cannula system on obstructive sleep apnoea in children. Pediatrics 2009;124: McKiernan 2009 McKiernan C, Chua LC, Vistanier PF, Allen H. High flow nasal cannulae therapy in infants with bronchiolitis. Journal of Pediatrics 2009;156: Reid 1984 Reid LM. Lung growth in health and disease. British Journal of Diseases of the Chest 1984;78: RevMan 5.1 The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, Roca 2010 Roca O, Riera J, Torres F, Masclans J. High-flow oxygen therapy in acute respiratory failure. Respiratory Care 2010; 55(4): Schibler 2011 Schibler A, Pham TMT, Dunster KR, Foster K, Barlow A, Gibbons K, Hough JL. Reduced intubation rates for infants after introduction of high flow nasal prong oxygen delivery. Intensive Care Medicine 2011;3: [DOI: / s ] Shoemaker 2007 Shoemaker MT, Pierce MR, Yoder BA, DiGeronimo RJ. High flow nasal cannula versus nasal CPAP for neonatal respiratory disease: a retrospective study. Journal of Perinatology 2007;27: Spence 2007 Spence KL, Murphy D, Kilian C, McGonigle R, Kilani RA. High flow nasal cannula as a device to provide continuous positive airway pressure in infants. Journal of Perinatology 2007;27: Spentzas 2009 Spentzas T, Minarik M, Patters AB, Vinson B, Stidham G. Children with respiratory distress treated with high-flow nasal cannula. Journal of Intensive Care Medicine 2009;24 (5): Sreenan 2001 Sreenan C, Lemke RP, Hudson-Mason A, Osiovich H. High-flow nasal cannulae in the management of apnoea of prematurity: a comparison with conventional nasal continuous positive airway pressure. Pediatrics 2001;107: Sutton 2008 Sutton AH, HIggins JP. Recent developments in meta-analysis. Statistics in Medicine 2008;27: [PUBMED: ] The ARDS Network 2000 The ARDS Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The New England Journal of Medicine 2000;342: [PUBMED: ] Thorsteinsson 2002 Thorsteinsson A, Werner O, Jonmaker C, Larsson A. Airway closure in anaesthetized infants and children: the influence of inspiratory pressures and volumes. Acta Anaesthesiologica Scandinavica 2002;46: Wilkinson 2008 Wilkinson DJ, Anderson CC, Smith K, Holberton J. Pharyngeal pressure with high-flow nasal cannulae in premature infants. Journal of Perinatology 2008;28:42 7. Wilkinson 2011 Wilkinson D, Andersen C, O Donnell CPF, De Paoli AG. High flow nasal cannula for respiratory support in preterm infants. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: / CD pub2] Yong 2005 Yong SC, Chen SJ, Boo NY. Incidence of nasal trauma associated with nasal prong versus nasal mask during continuous positive airway pressure treatment in very low birthweight infants: a randomised control study. Archives of Diseases in Childhood. Fetal and Neonatal Edition 2005;90: F Indicates the major publication for the study 6
9 A P P E N D I C E S Appendix 1. Search strategy for MEDLINE (Ovid SP) 1. (exp Oxygen Inhalation Therapy/ and intubation rates*.af.) or (high flow adj3 (nasal or prong or cannula)).mp. or (nasal adj3 oxygen).mp. 2. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh and 2 Appendix 2. Data extraction form Review title or ID Study ID (surname of first author and year first full report of study was published e.g. Smith 2001) Report IDs of other reports of this study (e.g. duplicate publications, follow-up studies) 1. General Information 7
10 Date form completed (dd/mm/yyyy) Name/ID of person extracting data Report title (title of paper/ abstract/ report that data are extracted from) Report ID (ID for this paper/ abstract/ report) Reference details Report author contact details Publication type (e.g. full report, abstract, letter) Study funding sources (including role of funders) Possible conflicts of interest (for study authors) 2. Study Eligibility Study Characteristics Type of study Eligibility criteria (Insert eligibility criteria for each characteristic as defined in the Protocol) Randomized Controlled Trial Controlled Clinical Trial (quasi-randomized trial) Yes No Unclear Location in text 8
11 Participants Paediatric patients aged from 4 weeks corrected age to 16 years Types of intervention High flow nasal Oxygen(heated/ humidified, flow >2lt/kg/min) Comparator: non invasive respiratory support such as cot/tent/ hood, low flow oxygen or CPAP Types of outcome measures INCLUDE Hospital mortality; intubation rate; treatment failure. Secondary: duration of any form of respiratory support; length of hospital stay; clinical severity score; length of PICU stay; complications- air leak, nasal trauma, nosocomial sepsis, barotrauma, gastrointestinal distention EXCLUDE Reason for exclusion DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW 3. Methods Descriptions as stated in report/paper Location in text 9
12 Aim of study Design (e.g. parallel, crossover, cluster) Unit of allocation (by individuals, cluster/ groups or body parts) Start date End date Total study duration Ethical approval needed/ obtained for study Yes No Unclear 4. Risk of Bias assessment See Chapter 8 of the Cochrane Handbook (Higgins 2011) Domain Risk of bias Support for judgement Location in text Random sequence generation (selection bias) Allocation concealment (selection bias) Low risk High risk Unclear 10
13 Blinding of participants and personnel (performance bias) (if required) Outcome group: All/ Outcome group: Blinding of outcome assessment (detection bias) (if required) Outcome group: All/ Outcome group: Incomplete outcome data (attrition bias) Selective outcome reporting? (reporting bias) Other bias 5. Participants Provide overall data and, if available, comparative data for each intervention or comparison group. Description as stated in report/paper Location in text Total no. randomized (or total pop. at start of study for NRCTs) 11
14 Clusters (if applicable, no., type, no. people per cluster) Withdrawals and exclusions (if not provided below by outcome) Age Sex Severity of illness Co-morbidities Other treatment received (additional to study intervention) Subgroups measured Subgroups reported 6. Intervention groups Copy and paste table for each intervention and comparison group Intervention Group 1 Description as stated in report/paper Location in text Group name HFNC 12
15 No. randomized to group (specify whether no. people or clusters) Description (include sufficient detail for replication, e.g. content, dose, components) Duration of treatment period Timing (e.g. frequency, duration of each episode) Delivery (e.g. mechanism, medium, intensity, fidelity) Co-interventions Comparison Group 1 Description as stated in report/paper Location in text Group name Invasive Ventilation No. randomized to group (specify whether no. people or clusters) Description (include sufficient detail for replication, e.g. content, dose, components) Duration of treatment period 13
16 Timing (e.g. frequency, duration of each episode) Delivery (e.g. mechanism, medium, intensity, fidelity) Co-interventions Comparison Group 2 Description as stated in report/paper Location in text Group name Non-Invasive Ventilation No. randomized to group (specify whether no. people or clusters) Description (include sufficient detail for replication, e.g. content, dose, components) Duration of treatment period Timing (e.g. frequency, duration of each episode) Delivery (e.g. mechanism, medium, intensity, fidelity) Co-interventions 14
17 7. Outcomes Copy and paste table for each outcome. Outcome 1 Description as stated in report/paper Location in text Outcome name Hospital Mortality Time points measured Time points reported Outcome definition (with diagnostic criteria if relevant) Person measuring/reporting Unit of measurement (if relevant) Scales: upper and lower limits (indicate whether high or low score is good) Is outcome/tool validated? Yes No Unclear 15
18 Imputation of missing data (e.g. assumptions made for ITT analysis) Assumed risk estimate (e.g. baseline or population risk noted in Background) Power Outcome 2 Description as stated in report/paper Location in text Outcome name Intubation Rate Time points measured Time points reported Outcome definition (with diagnostic criteria if relevant) Person measuring/reporting Unit of measurement (if relevant) Scales: upper and lower limits (indicate whether high or low score is good) Is outcome/tool validated? Yes No Unclear 16
19 Imputation of missing data (e.g. assumptions made for ITT analysis) Assumed risk estimate (e.g. baseline or population risk noted in Background) Power Outcome 3 Description as stated in report/paper Location in text Outcome name Treatment Faiulre- escalation to other form of respiratory support Time points measured Time points reported Outcome definition (with diagnostic criteria if relevant) Person measuring/reporting Unit of measurement (if relevant) Scales: upper and lower limits (indicate whether high or low score is good) Is outcome/tool validated? Yes No Unclear 17
20 Imputation of missing data (e.g. assumptions made for ITT analysis) Assumed risk estimate (e.g. baseline or population risk noted in Background) Power Outcome 4 Description as stated in report/paper Location in text Outcome name Duration of respiratory support Time points measured Time points reported Outcome definition (with diagnostic criteria if relevant) Person measuring/reporting Unit of measurement (if relevant) Scales: upper and lower limits (indicate whether high or low score is good) Is outcome/tool validated? Yes No Unclear 18
21 Imputation of missing data (e.g. assumptions made for ITT analysis) Assumed risk estimate (e.g. baseline or population risk noted in Background) Power Outcome 5 Description as stated in report/paper Location in text Outcome name Complications- air leak, nasal trauma, nosocomial sepsis, barotrauma, gastrointestinal distention Time points measured Time points reported Outcome definition (with diagnostic criteria if relevant) Person measuring/reporting Unit of measurement (if relevant) Scales: upper and lower limits (indicate whether high or low score is good) Is outcome/tool validated? Yes No Unclear 19
22 Imputation of missing data (e.g. assumptions made for ITT analysis) Assumed risk estimate (e.g. baseline or population risk noted in Background) Power Outcome 6 Description as stated in report/paper Location in text Outcome name Length of Hospital stay Time points measured Time points reported Outcome definition (with diagnostic criteria if relevant) Person measuring/reporting Unit of measurement (if relevant) Scales: upper and lower limits (indicate whether high or low score is good) Is outcome/tool validated? Yes No Unclear 20
23 Imputation of missing data (e.g. assumptions made for ITT analysis) Assumed risk estimate (e.g. baseline or population risk noted in Background) Power Outcome 7 Description as stated in report/paper Location in text Outcome name Clinical severity score Time points measured Time points reported Outcome definition (with diagnostic criteria if relevant) Person measuring/reporting Unit of measurement (if relevant) Scales: upper and lower limits (indicate whether high or low score is good) Is outcome/tool validated? Yes No Unclear 21
24 Imputation of missing data (e.g. assumptions made for ITT analysis) Assumed risk estimate (e.g. baseline or population risk noted in Background) Power Outcome 8- secondary outcome Description as stated in report/paper Location in text Outcome name Length of PICU stay Time points measured Time points reported Outcome definition (with diagnostic criteria if relevant) Person measuring/reporting Unit of measurement (if relevant) Scales: upper and lower limits (indicate whether high or low score is good) Is outcome/tool validated? Yes No Unclear 22
25 Imputation of missing data (e.g. assumptions made for ITT analysis) Assumed risk estimate (e.g. baseline or population risk noted in Background) Power 8. Results Copy and paste the appropriate table for each outcome, including additional tables for each time point and subgroup as required. Dichotomous outcome Outcome HFNC Invasive Ventilation Non Invasive Ventilation Details No. event with No. without event No. event with No. without event No. event with No. without event Intubation Failure Treatment of Hospital Mortality Complications Continuous outcome 23
26 Outcome Unit of Measurement HFNC Group Invasive Ventilation Group Non Invasive Group Details n M SD n M SD n M SD Length of PICU stay Clinical Severity Score Duration of Respiratory support LOS- Hospital 9. Applicability Have important populations been excluded from the study? (consider disadvantaged populations, and possible differences in the intervention effect) Is the intervention likely to be aimed at disadvantaged groups? (e.g..lower socioeconomic groups) Does the study directly address the review question? (any issues of partial or indirect applicability) Yes No Unclear Yes No Unclear Yes No Unclear 24
27 10.Other information Description as stated in report/paper Location in text Key conclusions of study authors References to other relevant studies Correspondence required for further study information (from whom, what and when) H I S T O R Y Protocol first published: Issue 5, 2012 C O N T R I B U T I O N S O F A U T H O R S Conceiving the review: Sara Mayfield (SM) Co-ordinating the review: SM Undertaking manual searches: SM Screening search results: SM and Jacqui Jauncey-Cooke (JJC) Organizing retrieval of papers: SM Screening retrieved papers against inclusion criteria: SM and JJC Appraising quality of papers: SM, JJC and Judith Hough (JH) Abstracting data from papers: SM and JJC Writing to authors of papers for additional information: SM Providing additional data about papers: SM Obtaining and screening data on unpublished studies: SM Data management for the review: SM, JJC and Fiona Bogossian (FB) Entering data into Review Manager (RevMan 5.1): Sara Mayfield RevMan statistical data: SM and Kristen Gibbons (KG) Other statistical analysis not using RevMan: KG 25
28 Interpretation of data: SM, JJC, FB, JH and Andreas Schibler (AS) Statistical inferences: SM, JJC, FB and KG Writing the review: SM Securing funding for the review: n/a Performing previous work that was the foundation of the present study: Guarantor for the review (one author): FB Person responsible for reading and checking review before submission: JJC, FB, AS and JH D E C L A R A T I O N S O F I N T E R E S T Sara Mayfield and Andreas Schibler are working on studies with HFNC in the paediatric intensive care unit where they work. To date the trials are observational in nature and not in line with RCTs, however this may change in the future. However, any trial that may be included from these authors will be acknowledged and assessed by an independent person (JH, FB or JJC). Andreas Schibler: Fisher and Paykel have supported my research by funding one project and supplying equipment in another. Neither projects are published as yet, but may be eligible for inclusion in this review. If any of the studies in the review are deemed to hold a conflict of interest with me, then they will be assessed by an independent person (JH, FB, SM or JJC). Jacqueline Jauncey-Cooke: none known. Judith L Hough: none known: Kristen Gibbons: none known. Fiona Bogossian: none known. 26
ROLE OF PRESSURE IN HIGH FLOW THERAPY
 ROLE OF PRESSURE IN HIGH FLOW THERAPY Thomas L. Miller, PhD, MEd Director, Clinical Research and Education Vapotherm, Inc. Research Assistant Professor of Pediatrics Jefferson Medical College This information
ROLE OF PRESSURE IN HIGH FLOW THERAPY Thomas L. Miller, PhD, MEd Director, Clinical Research and Education Vapotherm, Inc. Research Assistant Professor of Pediatrics Jefferson Medical College This information
Nottingham Children s Hospital
 High Flow Nasal Cannula Therapy Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Guide line for the use of HFNCT (High Flow Nasal Cannula Therapy) Contact Name
High Flow Nasal Cannula Therapy Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Guide line for the use of HFNCT (High Flow Nasal Cannula Therapy) Contact Name
heated humidified high-flow nasal cannula therapy in children F A Hutchings, 1 T N Hilliard, 1 P J Davis 2 Review
 1 Department of Paediatric Respiratory Medicine, Bristol Royal Hospital for Children, Bristol, UK 2 Department of Paediatric Intensive Care, Bristol Royal Hospital for Children, Bristol, UK Correspondence
1 Department of Paediatric Respiratory Medicine, Bristol Royal Hospital for Children, Bristol, UK 2 Department of Paediatric Intensive Care, Bristol Royal Hospital for Children, Bristol, UK Correspondence
Cochrane Pregnancy and Childbirth Group Methodological Guidelines
 Cochrane Pregnancy and Childbirth Group Methodological Guidelines [Prepared by Simon Gates: July 2009, updated July 2012] These guidelines are intended to aid quality and consistency across the reviews
Cochrane Pregnancy and Childbirth Group Methodological Guidelines [Prepared by Simon Gates: July 2009, updated July 2012] These guidelines are intended to aid quality and consistency across the reviews
High-flow nasal cannula use in a paediatric intensive care unit over 3 years
 High-flow nasal cannula use in a paediatric intensive care unit over 3 years Tracey I Wraight and Subodh S Ganu Respiratory illness and/or distress is the commonest reason for non-elective paediatric intensive
High-flow nasal cannula use in a paediatric intensive care unit over 3 years Tracey I Wraight and Subodh S Ganu Respiratory illness and/or distress is the commonest reason for non-elective paediatric intensive
5/3/2012. Goals and Objectives HFNC. High-Flow Oxygen Therapy: Real Benefit or Just a Fad?
 High-Flow Oxygen Therapy: Real Benefit or Just a Fad? Timothy R. Myers MBA, RRT-NPS Director, Women s & Children s Respiratory Care & Procedural Services and Pediatric Heart Center Rainbow Babies & Children
High-Flow Oxygen Therapy: Real Benefit or Just a Fad? Timothy R. Myers MBA, RRT-NPS Director, Women s & Children s Respiratory Care & Procedural Services and Pediatric Heart Center Rainbow Babies & Children
Children & Young People s Directorate Paediatric-Neonatal Guidelines Checklist & Version Control Sheet
 Children & Young People s Directorate Paediatric-Neonatal Guidelines Checklist & Version Control Sheet 1. Name of Guideline / Policy/ Procedure 2. Purpose of Procedure/ Guidelines/ Protocol Guideline for
Children & Young People s Directorate Paediatric-Neonatal Guidelines Checklist & Version Control Sheet 1. Name of Guideline / Policy/ Procedure 2. Purpose of Procedure/ Guidelines/ Protocol Guideline for
Clinical Guideline: Heated Humidified High Flow Nasal Cannula (HHHFNC) Guideline
 EOE Neonatal ODN Clinical Guideline: Heated Humidified High Flow Nasal Cannula (HHHFNC) Guideline Authors: Dr Eliana Panayiotou and Dr Bharat Vakharia For use in: EoE Neonatal Units Guidance specific to
EOE Neonatal ODN Clinical Guideline: Heated Humidified High Flow Nasal Cannula (HHHFNC) Guideline Authors: Dr Eliana Panayiotou and Dr Bharat Vakharia For use in: EoE Neonatal Units Guidance specific to
OmniOx (HFT 500) Jan Prepared by MEKICS. OmniOx-HFT500 Jan ver1.1 1
 OmniOx (HFT 500) Jan. 2013. Prepared by MEKICS OmniOx-HFT500 Jan. 2013 ver1.1 1 0. Index 1. Introduce of OmniOx (HTF500) 2. Specification (HFT, CPAP+) 3. The World Best Features 2 OmniOx-HFT500 Jan. 2013
OmniOx (HFT 500) Jan. 2013. Prepared by MEKICS OmniOx-HFT500 Jan. 2013 ver1.1 1 0. Index 1. Introduce of OmniOx (HTF500) 2. Specification (HFT, CPAP+) 3. The World Best Features 2 OmniOx-HFT500 Jan. 2013
Critical Appraisal Practicum. Fabio Di Bello Medical Implementation Manager
 Critical Appraisal Practicum Fabio Di Bello Medical Implementation Manager fdibello@ebsco.com What we ll talk about today: DynaMed process for appraising randomized trials and writing evidence summaries
Critical Appraisal Practicum Fabio Di Bello Medical Implementation Manager fdibello@ebsco.com What we ll talk about today: DynaMed process for appraising randomized trials and writing evidence summaries
Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE
 Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE Indications for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) administration, the patient should be: Spontaneously
Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE Indications for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) administration, the patient should be: Spontaneously
KAMIT CAN, AYSE BERNA ANIL, MURAT ANIL, NESLIHAN ZENGIN, ALKAN BAL, YUKSEL BICILIOGLU, GAMZE GOKALP, FATIH DURAK AND GULBERAT INCE
 R E S E A R C H P A P E R Impact of High-flow Nasal Cannula Therapy in Quality Improvement and Clinical Outcomes in a Non-invasive Ventilation Device-free Pediatric Intensive Care Unit FULVA KAMIT CAN,
R E S E A R C H P A P E R Impact of High-flow Nasal Cannula Therapy in Quality Improvement and Clinical Outcomes in a Non-invasive Ventilation Device-free Pediatric Intensive Care Unit FULVA KAMIT CAN,
Systematic reviews and meta-analyses of observational studies (MOOSE): Checklist.
 Systematic reviews and meta-analyses of observational studies (MOOSE): Checklist. MOOSE Checklist Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease:
Systematic reviews and meta-analyses of observational studies (MOOSE): Checklist. MOOSE Checklist Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease:
Nasal versus oral intubation for mechanical ventilation of newborn infants (Review)
 Nasal versus oral intubation for mechanical ventilation of newborn infants (Review) Spence K, Barr P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published
Nasal versus oral intubation for mechanical ventilation of newborn infants (Review) Spence K, Barr P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published
CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) DEFINITION
 CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) DEFINITION Method of maintaining low pressure distension of lungs during inspiration and expiration when infant breathing spontaneously Benefits Improves oxygenation
CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) DEFINITION Method of maintaining low pressure distension of lungs during inspiration and expiration when infant breathing spontaneously Benefits Improves oxygenation
Breathing exercises for chronic obstructive pulmonary disease (Protocol)
 Breathing exercises for chronic obstructive pulmonary disease (Protocol) Holland AE, Hill C, McDonald CF This is a reprint of a Cochrane protocol, prepared and maintained by The Cochrane Collaboration
Breathing exercises for chronic obstructive pulmonary disease (Protocol) Holland AE, Hill C, McDonald CF This is a reprint of a Cochrane protocol, prepared and maintained by The Cochrane Collaboration
Acute Paediatric Respiratory Pathway
 Guideline for the use of high Flow Nasal Cannula Oxygen Therapy (Optiflow or Airvo) in Children with Bronchiolitis or an acute respiratory illness Introduction: High flow nasal cannula (HFNC) oxygen enables
Guideline for the use of high Flow Nasal Cannula Oxygen Therapy (Optiflow or Airvo) in Children with Bronchiolitis or an acute respiratory illness Introduction: High flow nasal cannula (HFNC) oxygen enables
NON INVASIVE LIFE SAVERS. Non Invasive Ventilation (NIV)
 Table 1. NIV: Mechanisms Of Action Decreases work of breathing Increases functional residual capacity Recruits collapsed alveoli Improves respiratory gas exchange Reverses hypoventilation Maintains upper
Table 1. NIV: Mechanisms Of Action Decreases work of breathing Increases functional residual capacity Recruits collapsed alveoli Improves respiratory gas exchange Reverses hypoventilation Maintains upper
Controlled Trials. Spyros Kitsiou, PhD
 Assessing Risk of Bias in Randomized Controlled Trials Spyros Kitsiou, PhD Assistant Professor Department of Biomedical and Health Information Sciences College of Applied Health Sciences University of
Assessing Risk of Bias in Randomized Controlled Trials Spyros Kitsiou, PhD Assistant Professor Department of Biomedical and Health Information Sciences College of Applied Health Sciences University of
Cochrane Breast Cancer Group
 Cochrane Breast Cancer Group Version and date: V3.2, September 2013 Intervention Cochrane Protocol checklist for authors This checklist is designed to help you (the authors) complete your Cochrane Protocol.
Cochrane Breast Cancer Group Version and date: V3.2, September 2013 Intervention Cochrane Protocol checklist for authors This checklist is designed to help you (the authors) complete your Cochrane Protocol.
MINDFULNESS-BASED INTERVENTIONS IN EPILEPSY
 03 March 2016; v.1 MINDFULNESS-BASED INTERVENTIONS IN EPILEPSY AIM This review aimed to evaluate the effectiveness of mindfulness as a therapeutic intervention for people with epilepsy. METHODS Criteria
03 March 2016; v.1 MINDFULNESS-BASED INTERVENTIONS IN EPILEPSY AIM This review aimed to evaluate the effectiveness of mindfulness as a therapeutic intervention for people with epilepsy. METHODS Criteria
PROSPERO International prospective register of systematic reviews
 PROSPERO International prospective register of systematic reviews High-dose chemotherapy followed by autologous haematopoietic cell transplantation for children, adolescents and young adults with first
PROSPERO International prospective register of systematic reviews High-dose chemotherapy followed by autologous haematopoietic cell transplantation for children, adolescents and young adults with first
Downloaded from:
 Arnup, SJ; Forbes, AB; Kahan, BC; Morgan, KE; McKenzie, JE (2016) The quality of reporting in cluster randomised crossover trials: proposal for reporting items and an assessment of reporting quality. Trials,
Arnup, SJ; Forbes, AB; Kahan, BC; Morgan, KE; McKenzie, JE (2016) The quality of reporting in cluster randomised crossover trials: proposal for reporting items and an assessment of reporting quality. Trials,
PROSPERO International prospective register of systematic reviews
 PROSPERO International prospective register of systematic reviews Closed reduction methods for acute anterior shoulder dislocation [Cochrane Protocol] Kanthan Theivendran, Raj Thakrar, Subodh Deshmukh,
PROSPERO International prospective register of systematic reviews Closed reduction methods for acute anterior shoulder dislocation [Cochrane Protocol] Kanthan Theivendran, Raj Thakrar, Subodh Deshmukh,
Kugelman A, Riskin A, Said W, Shoris I, Mor F, Bader D.
 Heated, Humidified High-Flow Nasal Cannula (HHHFNC) vs. Nasal Intermittent Positive Pressure Ventilation (NIPPV) for the Primary Treatment of RDS, A Randomized, Controlled, Prospective, Pilot Study Kugelman
Heated, Humidified High-Flow Nasal Cannula (HHHFNC) vs. Nasal Intermittent Positive Pressure Ventilation (NIPPV) for the Primary Treatment of RDS, A Randomized, Controlled, Prospective, Pilot Study Kugelman
ORIGINAL ARTICLE. DD Woodhead, DK Lambert, JM Clark and RD Christensen. Intermountain Healthcare, McKay-Dee Hospital, Ogden, UT, USA
 ORIGINAL ARTICLE Comparing two methods of delivering high-flow gas therapy by nasal cannula following endotracheal extubation: a prospective, randomized, masked, crossover trial DD Woodhead, DK Lambert,
ORIGINAL ARTICLE Comparing two methods of delivering high-flow gas therapy by nasal cannula following endotracheal extubation: a prospective, randomized, masked, crossover trial DD Woodhead, DK Lambert,
Standards for the reporting of new Cochrane Intervention Reviews
 Methodological Expectations of Cochrane Intervention Reviews (MECIR) Standards for the reporting of new Cochrane Intervention Reviews 24 September 2012 Preface The standards below summarize proposed attributes
Methodological Expectations of Cochrane Intervention Reviews (MECIR) Standards for the reporting of new Cochrane Intervention Reviews 24 September 2012 Preface The standards below summarize proposed attributes
Meta-analyses: analyses:
 Meta-analyses: analyses: how do they help, and when can they not? Lee Hooper Senior Lecturer in research synthesis & nutrition l.hooper@uea.ac.uk 01603 591268 Aims Systematic Reviews Discuss the scientific
Meta-analyses: analyses: how do they help, and when can they not? Lee Hooper Senior Lecturer in research synthesis & nutrition l.hooper@uea.ac.uk 01603 591268 Aims Systematic Reviews Discuss the scientific
Advantages and disadvantages of different nasal CPAP systems in newborns
 Intensive Care Med (2004) 30:926 930 DOI 10.1007/s00134-004-2267-8 N E O N A T A L A N D P A E D I A T R I C I N T E N S I V E C A R E V. Buettiker M. I. Hug O. Baenziger C. Meyer B. Frey Advantages and
Intensive Care Med (2004) 30:926 930 DOI 10.1007/s00134-004-2267-8 N E O N A T A L A N D P A E D I A T R I C I N T E N S I V E C A R E V. Buettiker M. I. Hug O. Baenziger C. Meyer B. Frey Advantages and
Modalities and Complications Associated With the Use of High-Flow Nasal Cannula: Experience in a Pediatric ICU
 Modalities and Complications Associated With the Use of High-Flow Nasal Cannula: Experience in a Pediatric ICU Florent Baudin MD MSc, Sebastien Gagnon, Benjamin Crulli MD, François Proulx MD, Philippe
Modalities and Complications Associated With the Use of High-Flow Nasal Cannula: Experience in a Pediatric ICU Florent Baudin MD MSc, Sebastien Gagnon, Benjamin Crulli MD, François Proulx MD, Philippe
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library)
 A systematic review of smoking cessation and relapse prevention interventions in parents of babies admitted to a neonatal unit (after delivery) Divya Nelson, Sarah Gentry, Caitlin Notley, Henry White,
A systematic review of smoking cessation and relapse prevention interventions in parents of babies admitted to a neonatal unit (after delivery) Divya Nelson, Sarah Gentry, Caitlin Notley, Henry White,
Effect of peak inspiratory pressure on the development. of postoperative pulmonary complications.
 Effect of peak inspiratory pressure on the development of postoperative pulmonary complications in mechanically ventilated adult surgical patients: a systematic review protocol Chelsa Wamsley Donald Missel
Effect of peak inspiratory pressure on the development of postoperative pulmonary complications in mechanically ventilated adult surgical patients: a systematic review protocol Chelsa Wamsley Donald Missel
School of Dentistry. What is a systematic review?
 School of Dentistry What is a systematic review? Screen Shot 2012-12-12 at 09.38.42 Where do I find the best evidence? The Literature Information overload 2 million articles published a year 20,000 biomedical
School of Dentistry What is a systematic review? Screen Shot 2012-12-12 at 09.38.42 Where do I find the best evidence? The Literature Information overload 2 million articles published a year 20,000 biomedical
LRI Children s Hospital
 LRI Children s Hospital Humidified High Flow Nasal Cannula (HHFNC) Oxygen Therapy Staff relevant to: Medical and Nursing staff Team approval date: 5.01.18 Version: 1 Revision due: January 2020 Written
LRI Children s Hospital Humidified High Flow Nasal Cannula (HHFNC) Oxygen Therapy Staff relevant to: Medical and Nursing staff Team approval date: 5.01.18 Version: 1 Revision due: January 2020 Written
Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants(review)
 Cochrane Database of Systematic Reviews Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants (Review) SubramaniamP,HoJJ,DavisPG Subramaniam
Cochrane Database of Systematic Reviews Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants (Review) SubramaniamP,HoJJ,DavisPG Subramaniam
Newborn Life Support. NLS guidance.
 Kelly Harvey, ANNP NWNODN, previously Wythenshawe Hospital has shared this presentation with the understanding that it is for personal use following your attendance at the 8th Annual Senior Neonatal Nursing
Kelly Harvey, ANNP NWNODN, previously Wythenshawe Hospital has shared this presentation with the understanding that it is for personal use following your attendance at the 8th Annual Senior Neonatal Nursing
High-flow nasal cannula therapy for infants with bronchiolitis (Review)
 High-flow nasal cannula therapy for infants with bronchiolitis (Review) Beggs S, Wong ZH, Kaul S, Ogden KJ, Walters JAE This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration
High-flow nasal cannula therapy for infants with bronchiolitis (Review) Beggs S, Wong ZH, Kaul S, Ogden KJ, Walters JAE This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration
Cochrane Bone, Joint & Muscle Trauma Group How To Write A Protocol
 A p r i l 2 0 0 8 Cochrane Bone, Joint & Muscle Trauma Group How To Write A Protocol This booklet was originally produced by the Cochrane Renal Group to make the whole process of preparing a protocol as
A p r i l 2 0 0 8 Cochrane Bone, Joint & Muscle Trauma Group How To Write A Protocol This booklet was originally produced by the Cochrane Renal Group to make the whole process of preparing a protocol as
Agomelatine versus placebo: A meta-analysis of published and unpublished trials
 Agomelatine versus placebo: A meta-analysis of published and unpublished trials (Protocol for a systematic review, Ulm, January 17, 2011) Markus Kösters, Andrea Cipriani, Giuseppe Guaiana, Thomas Becker
Agomelatine versus placebo: A meta-analysis of published and unpublished trials (Protocol for a systematic review, Ulm, January 17, 2011) Markus Kösters, Andrea Cipriani, Giuseppe Guaiana, Thomas Becker
Title Neonatal and Paediatric High-Flow Nasal Cannula Oxygen Therapy Guideline. Department Paediatrics / Neonates Date Issued
 Document Control Title Author Neonatal and Paediatric High-Flow Nasal Cannula Oxygen Therapy Guideline Author s job title Directorate Medical Version Department Paediatrics / Neonates Date Issued Status
Document Control Title Author Neonatal and Paediatric High-Flow Nasal Cannula Oxygen Therapy Guideline Author s job title Directorate Medical Version Department Paediatrics / Neonates Date Issued Status
Robert M. Jacobson, M.D. Department of Pediatric and Adolescent Medicine Mayo Clinic, Rochester, Minnesota
 How to Conduct a Systematic Review: A Workshop 24 th Annual Primary Care Research Methods & Statistics Conference, San Antonio, Texas Saturday, December 3, 2011 Robert M. Jacobson, M.D. Department of Pediatric
How to Conduct a Systematic Review: A Workshop 24 th Annual Primary Care Research Methods & Statistics Conference, San Antonio, Texas Saturday, December 3, 2011 Robert M. Jacobson, M.D. Department of Pediatric
[population] or for TREATMENT: [Intervention or intervention contrast] for [health problem] in [population] Review information
![[population] or for TREATMENT: [Intervention or intervention contrast] for [health problem] in [population] Review information [population] or for TREATMENT: [Intervention or intervention contrast] for [health problem] in [population] Review information](/thumbs/89/100111423.jpg) Model Review (Version 1.0): for PREVENTION: [Intervention] for prevention of [health... Page 1 of 28 Model Review (Version 1.0): for PREVENTION: [Intervention] for prevention of [health problem] in [population]
Model Review (Version 1.0): for PREVENTION: [Intervention] for prevention of [health... Page 1 of 28 Model Review (Version 1.0): for PREVENTION: [Intervention] for prevention of [health problem] in [population]
PRESSURES DELIVERED BY NASAL HIGH FLOW THERAPY DURING
 PRESSURES DELIVERED BY NASAL HIGH FLOW THERAPY DURING ALL PHASES OF THE RESPIRATORY CYCLE Rachael L. Parke, RN, MHSc (Hons) Cardiothoracic and Vascular Intensive Care Unit Auckland City Hospital Private
PRESSURES DELIVERED BY NASAL HIGH FLOW THERAPY DURING ALL PHASES OF THE RESPIRATORY CYCLE Rachael L. Parke, RN, MHSc (Hons) Cardiothoracic and Vascular Intensive Care Unit Auckland City Hospital Private
Screening for prostate cancer (Review)
 Ilic D, Neuberger MM, Djulbegovic M, Dahm P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2013, Issue 1 http://www.thecochranelibrary.com
Ilic D, Neuberger MM, Djulbegovic M, Dahm P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2013, Issue 1 http://www.thecochranelibrary.com
Systems differ in their ability to deliver optimal humidification
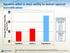 Average Absolute Humidity (mg H 2 O/L) Systems differ in their ability to deliver optimal humidification 45 Flows Tested 40 35 30 Optiflow Airvo 2 Vapotherm Vapotherm 5 L/min 10L/min 20L/min 30L/min 40L/min
Average Absolute Humidity (mg H 2 O/L) Systems differ in their ability to deliver optimal humidification 45 Flows Tested 40 35 30 Optiflow Airvo 2 Vapotherm Vapotherm 5 L/min 10L/min 20L/min 30L/min 40L/min
Appendix Document A1: Search strategy for Medline (1960 November 2015)
 Appendices: Appendix Document A1: Search strategy for Medline (1960 November 2015) Appendix Table A1: Detailed Risk of Bias Table Appendix Figure A1: Funnel plot Appendix Figure A2: Sensitivity analysis
Appendices: Appendix Document A1: Search strategy for Medline (1960 November 2015) Appendix Table A1: Detailed Risk of Bias Table Appendix Figure A1: Funnel plot Appendix Figure A2: Sensitivity analysis
Instrument for the assessment of systematic reviews and meta-analysis
 Appendix II Annex II Instruments for the assessment of evidence As detailed in the main body of the methodological appendix (Appendix II, "Description of the methodology utilised for the collection, assessment
Appendix II Annex II Instruments for the assessment of evidence As detailed in the main body of the methodological appendix (Appendix II, "Description of the methodology utilised for the collection, assessment
This is a pre-copyedited, author-produced PDF of an article accepted for publication in Journal of Neonatal Nursing following peer review.
 This is a pre-copyedited, author-produced PDF of an article accepted for publication in Journal of Neonatal Nursing following peer review. The version of record [Journal of Neonatal Nursing (February 2013)
This is a pre-copyedited, author-produced PDF of an article accepted for publication in Journal of Neonatal Nursing following peer review. The version of record [Journal of Neonatal Nursing (February 2013)
Workshop: Cochrane Rehabilitation 05th May Trusted evidence. Informed decisions. Better health.
 Workshop: Cochrane Rehabilitation 05th May 2018 Trusted evidence. Informed decisions. Better health. Disclosure I have no conflicts of interest with anything in this presentation How to read a systematic
Workshop: Cochrane Rehabilitation 05th May 2018 Trusted evidence. Informed decisions. Better health. Disclosure I have no conflicts of interest with anything in this presentation How to read a systematic
Web appendix: Supplementary data
 Web appendix: Supplementary data Azad MA, Coneys JG, Kozyrskyj AL, Field CJ, Ramsey CD, Becker AB, Friesen C, Abou-Setta AM, Zarychanski R. Probiotic supplementation during pregnancy or infancy for the
Web appendix: Supplementary data Azad MA, Coneys JG, Kozyrskyj AL, Field CJ, Ramsey CD, Becker AB, Friesen C, Abou-Setta AM, Zarychanski R. Probiotic supplementation during pregnancy or infancy for the
Guidelines and Best Practices for High Flow Nasal Cannula (HFNC) Pediatric Pocket Guide
 Guidelines Best Practices for High Flow Nasal Cannula (HFNC) Pediatric Pocket Guide Patient Selection Diagnoses Patient presents with one or more of the following signs or symptoms of respiratory distress:
Guidelines Best Practices for High Flow Nasal Cannula (HFNC) Pediatric Pocket Guide Patient Selection Diagnoses Patient presents with one or more of the following signs or symptoms of respiratory distress:
Web Appendix 1: Literature search strategy. BTS Acute Hypercapnic Respiratory Failure (AHRF) write-up. Sources to be searched for the guidelines;
 Web Appendix 1: Literature search strategy BTS Acute Hypercapnic Respiratory Failure (AHRF) write-up Sources to be searched for the guidelines; Cochrane Database of Systematic Reviews (CDSR) Database of
Web Appendix 1: Literature search strategy BTS Acute Hypercapnic Respiratory Failure (AHRF) write-up Sources to be searched for the guidelines; Cochrane Database of Systematic Reviews (CDSR) Database of
Meta Analysis. David R Urbach MD MSc Outcomes Research Course December 4, 2014
 Meta Analysis David R Urbach MD MSc Outcomes Research Course December 4, 2014 Overview Definitions Identifying studies Appraising studies Quantitative synthesis Presentation of results Examining heterogeneity
Meta Analysis David R Urbach MD MSc Outcomes Research Course December 4, 2014 Overview Definitions Identifying studies Appraising studies Quantitative synthesis Presentation of results Examining heterogeneity
Randomised controlled trial of weaning strategies for preterm infants on nasal continuous positive airway pressure
 Tang et al. BMC Pediatrics (2015) 15:147 DOI 10.1186/s12887-015-0462-0 RESEARCH ARTICLE Open Access Randomised controlled trial of weaning strategies for preterm infants on nasal continuous positive airway
Tang et al. BMC Pediatrics (2015) 15:147 DOI 10.1186/s12887-015-0462-0 RESEARCH ARTICLE Open Access Randomised controlled trial of weaning strategies for preterm infants on nasal continuous positive airway
High-Flow Nasal Cannula Utilization in Pediatric Critical Care
 High-Flow Nasal Cannula Utilization in Pediatric Critical Care Kristen D Coletti MD, Dayanand N Bagdure MBBS, Linda K Walker MD, Kenneth E Remy MD MHSc, and Jason W Custer MD BACKGROUND: High-flow nasal
High-Flow Nasal Cannula Utilization in Pediatric Critical Care Kristen D Coletti MD, Dayanand N Bagdure MBBS, Linda K Walker MD, Kenneth E Remy MD MHSc, and Jason W Custer MD BACKGROUND: High-flow nasal
Non Invasive Ventilation In Preterm Infants. Manuel Sanchez Luna Hospital General Universitario Gregorio Marañón Complutense University Madrid
 Non Invasive Ventilation In Preterm Infants Manuel Sanchez Luna Hospital General Universitario Gregorio Marañón Complutense University Madrid Summary Noninvasive ventilation begings in the delivery room
Non Invasive Ventilation In Preterm Infants Manuel Sanchez Luna Hospital General Universitario Gregorio Marañón Complutense University Madrid Summary Noninvasive ventilation begings in the delivery room
High Flow Nasal Cannula Oxygen HFNC. Dr I S Kalla Department of Pulmonology University of the Witwatersrand
 786 High Flow Nasal Cannula Oxygen HFNC Dr I S Kalla Department of Pulmonology University of the Witwatersrand Disclaimer I was a scep@c un@l I used it Now I am a firm believer HFNC The Fisher and Paykel
786 High Flow Nasal Cannula Oxygen HFNC Dr I S Kalla Department of Pulmonology University of the Witwatersrand Disclaimer I was a scep@c un@l I used it Now I am a firm believer HFNC The Fisher and Paykel
Problem solving therapy
 Introduction People with severe mental illnesses such as schizophrenia may show impairments in problem-solving ability. Remediation interventions such as problem solving skills training can help people
Introduction People with severe mental illnesses such as schizophrenia may show impairments in problem-solving ability. Remediation interventions such as problem solving skills training can help people
Outcomes assessed in the review
 The effectiveness of mechanical compression devices in attaining hemostasis after removal of a femoral sheath following femoral artery cannulation for cardiac interventional procedures Jones T Authors'
The effectiveness of mechanical compression devices in attaining hemostasis after removal of a femoral sheath following femoral artery cannulation for cardiac interventional procedures Jones T Authors'
Results. NeuRA Motor dysfunction April 2016
 Introduction Subtle deviations in various developmental trajectories during childhood and adolescence may foreshadow the later development of schizophrenia. Studies exploring these deviations (antecedents)
Introduction Subtle deviations in various developmental trajectories during childhood and adolescence may foreshadow the later development of schizophrenia. Studies exploring these deviations (antecedents)
Evidence Summary For the Ghana Essential Medicines Committee
 Evidence Summary For the Ghana Essential Medicines Committee Title: Caffeine Citrate for treating apnoea in preterm infants Formulation: 1. Injection: 20 mg/ml (equivalent to 10 mg caffeine base/ml) 2.
Evidence Summary For the Ghana Essential Medicines Committee Title: Caffeine Citrate for treating apnoea in preterm infants Formulation: 1. Injection: 20 mg/ml (equivalent to 10 mg caffeine base/ml) 2.
Ireland CJ, Chapman TM, Mathew SF, Herbison GP, Zacharias M
 Cochrane Database of Systematic Reviews Continuous positive airway pressure(cpap) during the postoperative period for prevention of postoperative morbidity and mortality following major abdominal surgery
Cochrane Database of Systematic Reviews Continuous positive airway pressure(cpap) during the postoperative period for prevention of postoperative morbidity and mortality following major abdominal surgery
NIV - BI-LEVEL POSITIVE AIRWAY PRESSURE (BIPAP)
 Introduction NIV - BI-LEVEL POSITIVE AIRWAY PRESSURE (BIPAP) Noninvasive ventilation (NIV) is a method of delivering oxygen by positive pressure mask that allows for the prevention or postponement of invasive
Introduction NIV - BI-LEVEL POSITIVE AIRWAY PRESSURE (BIPAP) Noninvasive ventilation (NIV) is a method of delivering oxygen by positive pressure mask that allows for the prevention or postponement of invasive
Systematic Reviews and Meta- Analysis in Kidney Transplantation
 Systematic Reviews and Meta- Analysis in Kidney Transplantation Greg Knoll MD MSc Associate Professor of Medicine Medical Director, Kidney Transplantation University of Ottawa and The Ottawa Hospital KRESCENT
Systematic Reviews and Meta- Analysis in Kidney Transplantation Greg Knoll MD MSc Associate Professor of Medicine Medical Director, Kidney Transplantation University of Ottawa and The Ottawa Hospital KRESCENT
PROSPERO International prospective register of systematic reviews
 PROSPERO International prospective register of systematic reviews Effectiveness of progressive muscle relaxation training for adults diagnosed with schizophrenia: a systematic review protocol Carlos Melo-Dias,
PROSPERO International prospective register of systematic reviews Effectiveness of progressive muscle relaxation training for adults diagnosed with schizophrenia: a systematic review protocol Carlos Melo-Dias,
Results. NeuRA Hypnosis June 2016
 Introduction may be experienced as an altered state of consciousness or as a state of relaxation. There is no agreed framework for administering hypnosis, but the procedure often involves induction (such
Introduction may be experienced as an altered state of consciousness or as a state of relaxation. There is no agreed framework for administering hypnosis, but the procedure often involves induction (such
CPAP failure in preterm infants: incidence, predictors and consequences
 CPAP failure in preterm infants: incidence, predictors and consequences SUPPLEMENTAL TEXT METHODS Study setting The Royal Hobart Hospital has an 11-bed combined Neonatal and Paediatric Intensive Care Unit
CPAP failure in preterm infants: incidence, predictors and consequences SUPPLEMENTAL TEXT METHODS Study setting The Royal Hobart Hospital has an 11-bed combined Neonatal and Paediatric Intensive Care Unit
Systematic Review & Course outline. Lecture (20%) Class discussion & tutorial (30%)
 Systematic Review & Meta-analysisanalysis Ammarin Thakkinstian, Ph.D. Section for Clinical Epidemiology and Biostatistics Faculty of Medicine, Ramathibodi Hospital Tel: 02-201-1269, 02-201-1762 Fax: 02-2011284
Systematic Review & Meta-analysisanalysis Ammarin Thakkinstian, Ph.D. Section for Clinical Epidemiology and Biostatistics Faculty of Medicine, Ramathibodi Hospital Tel: 02-201-1269, 02-201-1762 Fax: 02-2011284
High-Flow Nasal Cannulae in Very Preterm Infants after Extubation
 original article High-Flow Nasal Cannulae in Very Preterm Infants after Extubation Brett J. Manley, M.B., B.S., Louise S. Owen, M.D., Lex W. Doyle, M.D., Chad C. Andersen, M.B., B.S., David W. Cartwright,
original article High-Flow Nasal Cannulae in Very Preterm Infants after Extubation Brett J. Manley, M.B., B.S., Louise S. Owen, M.D., Lex W. Doyle, M.D., Chad C. Andersen, M.B., B.S., David W. Cartwright,
Alcohol interventions in secondary and further education
 National Institute for Health and Care Excellence Guideline version (Draft for Consultation) Alcohol interventions in secondary and further education NICE guideline: methods NICE guideline Methods
National Institute for Health and Care Excellence Guideline version (Draft for Consultation) Alcohol interventions in secondary and further education NICE guideline: methods NICE guideline Methods
Systematic review with multiple treatment comparison metaanalysis. on interventions for hepatic encephalopathy
 Systematic review with multiple treatment comparison metaanalysis on interventions for hepatic encephalopathy Hepatic encephalopathy (HE) is a reversible neuropsychiatric syndrome associated with severe
Systematic review with multiple treatment comparison metaanalysis on interventions for hepatic encephalopathy Hepatic encephalopathy (HE) is a reversible neuropsychiatric syndrome associated with severe
Learning from Systematic Review and Meta analysis
 Learning from Systematic Review and Meta analysis Efficacy and Safety of Antiscabietic Agents: A Systematic Review and Network Meta analysis of Randomized Controlled Trials KUNLAWAT THADANIPON, MD 4 TH
Learning from Systematic Review and Meta analysis Efficacy and Safety of Antiscabietic Agents: A Systematic Review and Network Meta analysis of Randomized Controlled Trials KUNLAWAT THADANIPON, MD 4 TH
PROSPERO International prospective register of systematic reviews
 PROSPERO International prospective register of systematic reviews Drug-eluting balloon angioplasty versus non-stenting balloon angioplasty for peripheral arterial disease of the lower limbs [Cochrane Protocol]
PROSPERO International prospective register of systematic reviews Drug-eluting balloon angioplasty versus non-stenting balloon angioplasty for peripheral arterial disease of the lower limbs [Cochrane Protocol]
Data extraction. Specific interventions included in the review Dressings and topical agents in relation to wound healing.
 Systematic reviews of wound care management: (2) dressings and topical agents used in the healing of chronic wounds Bradley M, Cullum N, Nelson E A, Petticrew M, Sheldon T, Torgerson D Authors' objectives
Systematic reviews of wound care management: (2) dressings and topical agents used in the healing of chronic wounds Bradley M, Cullum N, Nelson E A, Petticrew M, Sheldon T, Torgerson D Authors' objectives
Effective Health Care Program
 Comparative Effectiveness Review Number 68 Effective Health Care Program Noninvasive Positive-Pressure Ventilation (NPPV) for Acute Respiratory Failure Executive Summary Background Acute respiratory failure
Comparative Effectiveness Review Number 68 Effective Health Care Program Noninvasive Positive-Pressure Ventilation (NPPV) for Acute Respiratory Failure Executive Summary Background Acute respiratory failure
Results. NeuRA Treatments for internalised stigma December 2017
 Introduction Internalised stigma occurs within an individual, such that a person s attitude may reinforce a negative self-perception of mental disorders, resulting in reduced sense of selfworth, anticipation
Introduction Internalised stigma occurs within an individual, such that a person s attitude may reinforce a negative self-perception of mental disorders, resulting in reduced sense of selfworth, anticipation
Checklist for Randomized Controlled Trials. The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews
 The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews Checklist for Randomized Controlled Trials http://joannabriggs.org/research/critical-appraisal-tools.html www.joannabriggs.org
The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews Checklist for Randomized Controlled Trials http://joannabriggs.org/research/critical-appraisal-tools.html www.joannabriggs.org
The QUOROM Statement: revised recommendations for improving the quality of reports of systematic reviews
 The QUOROM Statement: revised recommendations for improving the quality of reports of systematic reviews David Moher 1, Alessandro Liberati 2, Douglas G Altman 3, Jennifer Tetzlaff 1 for the QUOROM Group
The QUOROM Statement: revised recommendations for improving the quality of reports of systematic reviews David Moher 1, Alessandro Liberati 2, Douglas G Altman 3, Jennifer Tetzlaff 1 for the QUOROM Group
Respiratory muscle training for cervical spinal cord injury (Review)
 Respiratory muscle training for cervical spinal cord injury (Review) Berlowitz D, Tamplin J This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in
Respiratory muscle training for cervical spinal cord injury (Review) Berlowitz D, Tamplin J This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in
*Ensure CRS is calculated using room air saturation. RN/RT to: - Contact physician
 TEXAS CHILDREN S HOSPITAL EVIDENCE-BASED OUTCOMES CENTER High Flow Nasal Cannula (HFNC) Therapy: Initiation and Escalation for Respiratory Distress Evidence-Informed Pathway Target Group See TCH Bronchiolitis
TEXAS CHILDREN S HOSPITAL EVIDENCE-BASED OUTCOMES CENTER High Flow Nasal Cannula (HFNC) Therapy: Initiation and Escalation for Respiratory Distress Evidence-Informed Pathway Target Group See TCH Bronchiolitis
Feng-Yi Lai, RN, MSN, Instructor Department of Nursing, Shu-Zen College of Medicine and Management, Asphodel Yang, RN, PhD, Associate Professor
 Feng-Yi Lai, RN, MSN, Instructor Department of Nursing, Shu-Zen College of Medicine and Management, Asphodel Yang, RN, PhD, Associate Professor Department of Nursing, Central Taiwan University of Science
Feng-Yi Lai, RN, MSN, Instructor Department of Nursing, Shu-Zen College of Medicine and Management, Asphodel Yang, RN, PhD, Associate Professor Department of Nursing, Central Taiwan University of Science
Von Reuss and CPAP, Disclosures CPAP. Noninvasive respiratory therapieswhy bother? Noninvasive respiratory therapies- types
 Noninvasive respiratory therapiesby a nose? NEO- The Conference for Neonatology February 21, 2014 Disclosures I have no relevant financial relationships to disclose or conflicts of interest to release.
Noninvasive respiratory therapiesby a nose? NEO- The Conference for Neonatology February 21, 2014 Disclosures I have no relevant financial relationships to disclose or conflicts of interest to release.
Strategies to increase the uptake of the influenza vaccine by healthcare workers: A summary of the evidence
 Strategies to increase the uptake of the influenza vaccine by healthcare workers: A summary of the evidence This evidence summary document has been prepared for the National Collaborating Centres for Public
Strategies to increase the uptake of the influenza vaccine by healthcare workers: A summary of the evidence This evidence summary document has been prepared for the National Collaborating Centres for Public
Early Rehabilitation in the ICU: Do We Still Need Chest Physiotherapy?
 Early Rehabilitation in the ICU: Do We Still Need Chest Physiotherapy? Michelle Kho, PT, PhD Assistant Professor, School of Rehabilitation Science, McMaster University Adjunct Assistant Professor, Department
Early Rehabilitation in the ICU: Do We Still Need Chest Physiotherapy? Michelle Kho, PT, PhD Assistant Professor, School of Rehabilitation Science, McMaster University Adjunct Assistant Professor, Department
A Systematic Review and Meta-Analysis of Pre-Transfusion Hemoglobin Thresholds for Allogeneic Red Blood Cell Transfusions
 A Systematic Review and Meta-Analysis of Pre-Transfusion Hemoglobin Thresholds for Allogeneic Red Blood Cell Transfusions Authors: Lesley J.J. Soril 1,2, MSc; Laura E. Leggett 1,2, MSc; Joseph Ahn, MSc
A Systematic Review and Meta-Analysis of Pre-Transfusion Hemoglobin Thresholds for Allogeneic Red Blood Cell Transfusions Authors: Lesley J.J. Soril 1,2, MSc; Laura E. Leggett 1,2, MSc; Joseph Ahn, MSc
AEROSURF Phase 2 Program Update Investor Conference Call
 AEROSURF Phase 2 Program Update Investor Conference Call November 12, 2015 Forward Looking Statement To the extent that statements in this presentation are not strictly historical, including statements
AEROSURF Phase 2 Program Update Investor Conference Call November 12, 2015 Forward Looking Statement To the extent that statements in this presentation are not strictly historical, including statements
Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials(review)
 Cochrane Database of Systematic Reviews Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials (Review) Anglemyer A, Horvath HT, Bero L Anglemyer
Cochrane Database of Systematic Reviews Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials (Review) Anglemyer A, Horvath HT, Bero L Anglemyer
Quality Design Limitations Inconsistency Indirectness Imprecision studies. Other. considerations Heliox Control (95% Absolute
 Question 7: In the child with asthma exacerbation in the ED, should heliox driven albuterol vs. oxygen or room air driven albuterol be used to prevent hospitalization, decrease time in the ED or improve
Question 7: In the child with asthma exacerbation in the ED, should heliox driven albuterol vs. oxygen or room air driven albuterol be used to prevent hospitalization, decrease time in the ED or improve
Template for MECIR (Review)
 Template for MECIR (Review) This guidance document contains information regarding the Cochrane Collaboration's mandatory MECIR Conduct and Reporting Standards and editorial suggestions specific to PaPaS,
Template for MECIR (Review) This guidance document contains information regarding the Cochrane Collaboration's mandatory MECIR Conduct and Reporting Standards and editorial suggestions specific to PaPaS,
Checklist for Randomized Controlled Trials. The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews
 The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews Checklist for Randomized Controlled Trials http://joannabriggs.org/research/critical-appraisal-tools.html www.joannabriggs.org
The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews Checklist for Randomized Controlled Trials http://joannabriggs.org/research/critical-appraisal-tools.html www.joannabriggs.org
Nonsteroidal anti-inflammatory drugs and perioperative bleeding in paediatric tonsillectomy (Review)
 Nonsteroidal anti-inflammatory drugs and perioperative bleeding in paediatric tonsillectomy (Review) Lewis SR, Nicholson A, Cardwell ME, Siviter G, Smith AF This is a reprint of a Cochrane review, prepared
Nonsteroidal anti-inflammatory drugs and perioperative bleeding in paediatric tonsillectomy (Review) Lewis SR, Nicholson A, Cardwell ME, Siviter G, Smith AF This is a reprint of a Cochrane review, prepared
PROSPERO International prospective register of systematic reviews
 PROSPERO International prospective register of systematic reviews Effectiveness of collaborative care in patients with combined physical disorders and depression or anxiety disorder: a systematic review
PROSPERO International prospective register of systematic reviews Effectiveness of collaborative care in patients with combined physical disorders and depression or anxiety disorder: a systematic review
Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease(review)
 Cochrane Database of Systematic Reviews Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease(review) Puhan MA, Gimeno-Santos E, Cates CJ, Troosters T Puhan MA, Gimeno-Santos
Cochrane Database of Systematic Reviews Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease(review) Puhan MA, Gimeno-Santos E, Cates CJ, Troosters T Puhan MA, Gimeno-Santos
9/15/2017. Disclosures. Heated High Flow Nasal Cannula: Hot Air or Optimal Noninvasive Support? Objectives. Aerogen Pharma
 Heated High Flow Nasal Cannula: Hot Air or Optimal Noninvasive Support? Rob DiBlasi RRT-NPS, FAARC Program Manager Research/QI, Respiratory Therapy Principle Investigator, Seattle Children s Research Institute
Heated High Flow Nasal Cannula: Hot Air or Optimal Noninvasive Support? Rob DiBlasi RRT-NPS, FAARC Program Manager Research/QI, Respiratory Therapy Principle Investigator, Seattle Children s Research Institute
Traumatic brain injury
 Introduction It is well established that traumatic brain injury increases the risk for a wide range of neuropsychiatric disturbances, however there is little consensus on whether it is a risk factor for
Introduction It is well established that traumatic brain injury increases the risk for a wide range of neuropsychiatric disturbances, however there is little consensus on whether it is a risk factor for
Critical appraisal: Systematic Review & Meta-analysis
 Critical appraisal: Systematic Review & Meta-analysis Atiporn Ingsathit MD.PhD. Section for Clinical Epidemiology and biostatistics Faculty of Medicine Ramathibodi Hospital Mahidol University What is a
Critical appraisal: Systematic Review & Meta-analysis Atiporn Ingsathit MD.PhD. Section for Clinical Epidemiology and biostatistics Faculty of Medicine Ramathibodi Hospital Mahidol University What is a
SECTION 1: INCLUSION, EXCLUSION & RANDOMISATION INFORMATION
 SECTION 1: INCLUSION, EXCLUSION & RANDOMISATION INFORMATION DEMOGRAPHIC INFORMATION Given name Family name Date of birth Consent date Gender Female Male Date of surgery INCLUSION & EXCLUSION CRITERIA YES
SECTION 1: INCLUSION, EXCLUSION & RANDOMISATION INFORMATION DEMOGRAPHIC INFORMATION Given name Family name Date of birth Consent date Gender Female Male Date of surgery INCLUSION & EXCLUSION CRITERIA YES
Introduction to systematic reviews/metaanalysis
 Introduction to systematic reviews/metaanalysis Hania Szajewska The Medical University of Warsaw Department of Paediatrics hania@ipgate.pl Do I needknowledgeon systematicreviews? Bastian H, Glasziou P,
Introduction to systematic reviews/metaanalysis Hania Szajewska The Medical University of Warsaw Department of Paediatrics hania@ipgate.pl Do I needknowledgeon systematicreviews? Bastian H, Glasziou P,
GE Healthcare. Non Invasive Ventilation (NIV) For the Engström Ventilator. Relief, Relax, Recovery
 GE Healthcare Non Invasive Ventilation (NIV) For the Engström Ventilator Relief, Relax, Recovery COPD is currently the fourth leading cause of death in the world, and further increases in the prevalence
GE Healthcare Non Invasive Ventilation (NIV) For the Engström Ventilator Relief, Relax, Recovery COPD is currently the fourth leading cause of death in the world, and further increases in the prevalence
Distraction techniques
 Introduction are a form of coping skills enhancement, taught during cognitive behavioural therapy. These techniques are used to distract and draw attention away from the auditory symptoms of schizophrenia,
Introduction are a form of coping skills enhancement, taught during cognitive behavioural therapy. These techniques are used to distract and draw attention away from the auditory symptoms of schizophrenia,
