Systematic Review Parker, Richard; Hodson, James; Rowe, Ian A C
|
|
|
- Meagan Miller
- 5 years ago
- Views:
Transcription
1 Systematic Review Parker, Richard; Hodson, James; Rowe, Ian A C DOI: /jgh Document Version Peer reviewed version Citation for published version (Harvard): Parker, R, Hodson, J & Rowe, IAC 2016, 'Systematic Review: Current Evidence in Non-Alcoholic Fatty Liver Disease Lacks Relevance to Patients with Advanced Fibrosis' Journal of Gastroenterology and Hepatology. Link to publication on Research at Birmingham portal Publisher Rights Statement: Checked for eligibility: 04/11/2016. This is the peer reviewed version of the following article:parker, R., Hodson, J., and Rowe, I. A. C. (2016) Systematic Review: Current Evidence in Non-Alcoholic Fatty Liver Disease Lacks Relevance to Patients with Advanced Fibrosis. Journal of Gastroenterology and Hepatology, which has been published in final form at /jgh This article may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Self-Archiving. General rights Unless a licence is specified above, all rights (including copyright and moral rights) in this document are retained by the authors and/or the copyright holders. The express permission of the copyright holder must be obtained for any use of this material other than for purposes permitted by law. Users may freely distribute the URL that is used to identify this publication. Users may download and/or print one copy of the publication from the University of Birmingham research portal for the purpose of private study or non-commercial research. User may use extracts from the document in line with the concept of fair dealing under the Copyright, Designs and Patents Act 1988 (?) Users may not further distribute the material nor use it for the purposes of commercial gain. Where a licence is displayed above, please note the terms and conditions of the licence govern your use of this document. When citing, please reference the published version. Take down policy While the University of Birmingham exercises care and attention in making items available there are rare occasions when an item has been uploaded in error or has been deemed to be commercially or otherwise sensitive. If you believe that this is the case for this document, please contact UBIRA@lists.bham.ac.uk providing details and we will remove access to the work immediately and investigate. Download date: 22. Apr. 2019
2 Systematic Review: Current Evidence in Non-Alcoholic Fatty Liver Disease Lacks Relevance to Patients with Advanced Fibrosis Richard Parker 1 James Hodson 2 Ian AC Rowe 3 1. Centre for Liver Research, University of Birmingham United Kingdom 2. Statistics Department, University Hospitals Birmingham NHS Foundation Trust, Birmingham UK 3. Leeds Institute for Data Analysis, University of Leeds, UK Corresponding author: Dr Richard Parker Centre for Liver Research 5 th Floor IBR University of Birmingham Birmingham B15 2TT United Kingdom richardparker@nhs.net (+44) (0) Disclosure This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as doi: /jgh.13625
3 None of the authors have any conflicts of interest to declare This work was supported by the European Association for the Study of the Liver (EASL) through a Sheila Sherlock Physician Scientist award to Dr Parker. There is no specific grant number attached to this award. Word count: Abstract: 238 Manuscript: 2588 Tables: 1 Figures: 4 Supplementary material: 5 tables, 4 figures Author contributions RP conceived the idea for this study, performed literature searches, data extraction, analysis and wrote the draft manuscript. JH assisted with data analysis and reviewed the draft manuscript. IAR conceived the idea for this study, data extraction and reviewed the draft manuscript. All authors approved the final manuscript. 2
4 Abstract Objectives Epidemiological data have shown that individuals with advanced fibrosis are at greatest risk of premature morbidity in NAFLD. Individuals included in clinical trials are often highly selected to remove confounding factors but selection can introduce bias and limit external validity. We examined the external validity of trials in non-alcoholic fatty liver disease (NAFLD) by examining characteristics of participants in observational studies (OS) and randomised controlled trials (RCT) in NAFLD. Design A systematic review was performed with structured literature searches for relevant OS and RCT using PubMed and Ovid Embase ( ). Identified studies were screened for inclusion by the authors and data extracted. Study populations were compared using t- tests to compare means and variances, in each case weighted by the size of individual studies. Dichotomous data were compared by Chi-squared test. Results In total 148 studies were included: 67 RCT and 81 OS including data from 44,860 individuals. Fifteen RCT participants differed from individuals in OS with regard to age, BMI, prevalence of DM, and gender (p<0.001 in each case). The most pronounced differences were seen between RCT participants and patients with advanced fibrosis. Comorbid conditions prevalent amongst individuals with NAFLD were frequent exclusion criteria in RCT. Conclusions The characteristics of participants in randomised controlled trials differ to those of the wider population of individuals with NAFLD. These differences may reduce the utility of trial data to individuals with NAFLD at greatest risk of death. Key words: Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis, Systematic review 3
5 Non-alcoholic fatty liver disease (NAFLD) is a spectrum of disease, ranging from simple hepatic steatosis through non-alcoholic steatohepatitis (NASH), to cirrhosis (1). NAFLD is common: hepatic steatosis is present in up to one-third of individuals (2) and NASH is seen in approximately 10% (3). Whilst the association of NAFLD per se with mortality is debated (4), large studies have shown that it is the subset of patients with advanced fibrosis who have an increased risk of liver-related morbidity as well as cardiovascular and neoplastic disease (5, 6). Given the high prevalence of NAFLD there is a need for effective therapies to prevent progression of disease and to treat established fibrosis. However, whilst many treatments have been trialed, few have shown categorical benefit in NASH (7). The external validity of a trial describes its relevance to a population outside of the trial s participants (8). Systematic analyses of trials in cardiology (9) and respiratory medicine (10, 11) have demonstrated have poor external validity, where commonly occurring medical conditions and age often exclude individuals from participation in trials (12). Poor external validity may promote ineffective treatments or, conversely, limit the acceptance of effective treatments (8). We used systematic review methods to assess the characteristics of participants included in randomised controlled trials (RCT) for treatment of NAFLD and the characteristics of individuals described in observational studies (OS) of NAFLD. This allowed comparison of the two groups and therefore an estimate of the external validity of RCTs performed in NAFLD to date. Methodology Literature search Literature searching was undertaken using three search strategies, to identify OS for NAFLD, OS reporting advanced fibrosis, and to identify randomised controlled trials in NAFLD. PubMed/MedLine and Ovid Embase were searched ( ), using the search terms: ((((randomised controlled trial) AND non alcoholic steatohepatitis) OR 4
6 NASH) OR NAFLD) OR non-alcoholic fatty liver disease) for RCT, and (((Prevalence[Title]) OR Natural history[title])) AND ((((non-alcoholic fatty liver disease) OR NASH) OR NAFLD) OR non-alcoholic steatohepatitis) and (fibrosis OR histology) AND (NASH OR nonalcoholic steatohepatitis) for OS. Results were limited to human studies and those published in English. The literature search was performed 1/7/2014 and updated on 11/8/2016. To examine grey literature for suitable studies the reference lists of included studies were searched for other suitable studies, and papers citing included studies were also reviewed. The title and abstract of papers found by the literature search were reviewed and unsuitable manuscripts or duplicate results excluded. Remaining papers were reviewed independently by two authors (RP, IAC) and disagreements resolved by consensus. This report is the only published account of this protocol. Inclusion and exclusion criteria Papers were included if they were full papers describing RCTs of interventions (lifestyle or pharmacological) in adult patients with any stage of NAFLD, or full papers reporting OS of prevalence or natural history of NAFLD in adults. Instances where the same cohort was described in more than one study were identified and included only once, with the study containing the most data included or the most recent report if descriptions were similar. Studies that had been published as abstracts, and those published in languages other than English were excluded. OS that only included particular groups, for example, studies reporting the prevalence of NAFLD amongst patients undergoing bariatric surgery, were also excluded. Data extraction Papers were reviewed and data extracted into a pre-prepared spreadsheet. Mean values for characteristics of trial participants in RCTs and individuals in OS were noted, specifically age, BMI, gender (% of male participants), prevalence of Diabetes mellitus (DM). For RCTs, type of intervention, primary outcome, secondary outcome and exclusion criteria were 5
7 recorded, as well as whether the trial reported a positive or negative finding. In the first instance, all OS were included in analysis of NAFLD as a whole, encompassing all stages of disease. Subsequent analyses were undertaken for biopsy-proven NASH and advanced fibrosis, including only OS that used and reported biopsy findings. For measurement of fibrosis in studies including biopsies, the Kleiner/Brunt classification was often used where F3 or F4 was taken to indicate advanced fibrosis, as is usual in the field. In studies that did not use the Kleiner/Brunt classification, advanced fibrosis was considered as bridging fibrosis or cirrhosis. The quality of included studies was assessed using the CONSORT guidelines for RCT (13) and the STROBE guidelines for observational studies (14). Data synthesis and analysis Many papers reported mean values for each arm of a trial but not an overall mean. Where this occurred, overall weighted means and variances were produced for the study as a whole, using the formulae and respectively. Once every study was represented by a single mean and variance, they were then split by study type (RCT or OS), and overall pooled means and variances calculated using the same approach. Comparisons were initially made between the study types with Kruskal-Wallis tests to produce an unweighted comparison of reported means or prevalences between the study types. As this does not take the size of studies into account, comparisons were then made using t-tests on the pooled means and variances. Variances were tested with F test, with Welch s correction used when they differed significantly by study type. Sub-group analyses were performed by stratifying data by geography, and by quality of RCTs. For the dichotomous outcomes (diabetes mellitus, gender), the overall rates were calculated by based on the total number of individuals with diabetes, or total number of 6
8 males in each type of study and calculating a percentage based on the total available data. This again removes the bias of larger studies dominating the data when only reported percentages were considered. Comparisons between study types were then performed using Chi-square tests. A p value of less than 0.05 was considered significant in all analyses. Prism v5.0 (Carlsbad, California USA) was used for statistical analysis. Results Literature search The search strategies resulted in a total of 1165 studies being identified. After removal of irrelevant studies and duplicated data, a total of 143 studies were included (figure 1). The quality of included studies, assessed with reference to the CONSORT and STROBE guidelines for RCT and OS respectively, showed most studies to be of high quality (supplementary figure 1). Characteristics of included studies In total eighty-one observational studies were included, including data on a total of 40,014 individuals with NAFLD. Twenty-four studies were population-based studies that described individuals with NAFLD in a general population, and fifty-two described characteristics of patients with NAFLD in secondary or tertiary centres. Seventy-three studies described all stages of NAFLD, including 34,147 individuals (supplementary table 1). Eighteen studies including 2,780 individuals described patients with biopsy-proven NASH (supplementary table 2) and 28 studies with 1938 individuals described characteristics of patients with advanced fibrosis (AF) (supplementary table 3). Sixty-seven RCTs were included. These studies included a total of 4,846 individuals (supplementary table 4). The methods used to diagnose NAFLD varied. Most population-based studies used imaging techniques, predominantly ultrasound, to define NAFLD whilst the majority of 7
9 secondary-care based studies used histology. NASH and AF were usually defined using the system proposed by Kleiner and Brunt (15), although some earlier studies used descriptive terms. In these cases, bridging fibrosis and/or cirrhosis were regard as advanced fibrosis. Several studies did not report the variables of interest, or did so in such a manner that we were unable to extract data for use in the present study. Available data are summarised in supplementary table 5. Comparison of Randomised Controlled Trials and Observational Studies in NAFLD Analysis of unadjusted study-reported means from included studies showed significant variance in age, BMI, prevalence of diabetes and distribution of gender across all study cohorts (figure 2). To compare age and BMI between types of study weighted means were calculated to reflect the relative size of each study and compared with student s t- test, and absolute prevalence of diabetes and male gender compared with Chi-squared test (table 1). RCT and OS showed statistically significant differences with respect to age (mean age RCT 50.0 years (SEM 0.09) vs. OS 49.4 years (SEM 0.06) student s t-test p<0.001) and BMI (32.1 kg/m 2 (0.05) vs (0.03), p<0.001)(table 1, figure 3). Prevalence of diabetes and gender were compared by Chi-squared test (table 1). RCT and OS showed statistically significant differences with regard to the prevalence of diabetes (8%, 337 of 4186 participants, vs. 24%, 5561 of individuals, p<0.001) and gender of participants (55% male, 2617 of 4733 participants, vs. 50%, 12,405 of 24,648 individuals, p<0.001) (table 1, figure 4). Subgroup analyses were performed to compare individuals with NASH or advanced fibrosis in OS to RCT participants. Age did not differ between RCT and individuals with biopsy-proven NASH (mean age RCT 50.0 years (0.09) vs (0.18), p=0.520) but did differ between RCT and individuals with advanced fibrosis (mean age 54.0 years (0.19), p<0.001) (table 1, figure 3). BMI differed significantly between RCT participants and 8
10 individuals with NASH (mean BMI RCT 32.1 (0.04) vs kg/m 2 (0.09) p=0.009), and also between RCT participants and individuals with advanced fibrosis (33.8 kg/m 2 (0.08), p<0.001) (table 1, figure 3). The prevalence of DM and gender also differed significantly between RCT participants and individuals with NASH or advanced fibrosis. In RCTs, 8%, (337 participants) had DM. In observational studies, 39% of individuals with NASH (829 of 2145 participants) and 45% of individuals with advanced fibrosis (782 of 1726 participants) had DM (Chi squared test p<0.001 in each case)(table 1, figure 4). In RCTs, 55% of participants were male compared to 47% of individuals with NASH (1170 of 2476 individuals) and 38% of individuals with advanced fibrosis (712 of 1869 individuals) (Chi squared test p<0.001 in each case) (table 1, figure 4). Analysis by geography Ethnicity is associated with marked phenotypic differences in NAFLD (16, 17). In view of this observational studies were stratified based on geographic location (supplementary figure 2). BMI and gender distribution showed significant differences by geographic location (Kruskal-Wallis test p< and <0.01 respectively). Accordingly, BMI and gender distribution in observational studies and RCTs were compared by geographical location. Significant differences remained between observational studies and RCTs (t-test of weighted means p<0.001). Again, these differences were most pronounced between RCTs and individuals with advanced fibrosis, with the exception of European data (supplementary figure 3). When considering results for gender, European data showed no differences between populations. In Asia and North America, RCTs contained significantly more men than were observed in epidemiological studies of advanced fibrosis (supplementary figure 4). 9
11 Exclusion criteria of RCT in NAFLD The exclusion criteria of all identified RCT were reviewed. Eighteen trials including 2008 participants (41% of all participants) excluded patients with cirrhosis. Participants with DM were excluded in 17 studies (1144 participants, 24% of total) and exclusion criteria based on medications to treat DM were reported in a further 22 studies (1643 participants, 34% of total). Thus, the presence of DM represented an absolute or relative exclusion criterion in 39 trials including 2,787 participants (58% of all RCT participants). Individuals using drugs to treat dyslipidaemia were excluded in 7 trials (375 participants, 8%). Discussion These data, derived from a robust systematic review, show that characteristics of individuals with NAFLD in observational studies differ from those included in RCT. These differences are statistically significant but are often small and may not be clinically significant. However, marked differences exist between RCT cohorts and individuals with advanced fibrosis who are more likely to progress to liver-related morbidity. This is compounded by the frequent exclusion of patients with cirrhosis or diabetes from trials. These differences may limit the application of RCT trial data to high-risk patients with NAFLD. There are important differences in susceptibility to insulin resitance and fatty liver btween indiviiduals of differing ethnicity (2, 16, 18). This was evident when studies were stratified by geography. Importantly, siginificant differences remained between OS and RCT populations. An explanation for the differences seen between OS and RCT may lie in the current paradigm that steatohepatitis is required for the development of liver fibrosis. Since this process takes many years to develop, RCTs in patients with NASH have often relied on histological criteria to recruit patients and assess efficacy. For instance the accepted endpoint of an <=2 point improvement in the NAFLD activity score (NAS) without 10
12 worsening of fibrosis (19), specifically excludes patients with cirrhosis since there is no way to evaluate whether fibrosis has worsened in this group. Whilst RCT are necessarily different to real life clinical practice, use of this endpoint skews trial populations away from the groups at greatest risk of liver related morbidity and mortality, and towards younger patients with earlier disease. This questions the value of current surrogate endpoints in NAFLD trials and raises important issues regarding the definition of such outcome measures in early phase studies for patients with NASH. In other liver diseases, such as hepatitis B virus infection, when liver disease is treated in patients with cirrhosis there is evidence of a reduction in fibrosis progression (20) and a concurrent reduction in the risk of liver related events. IT is important to discover whether this can also be achieved in patients with NASH and advanced fibrosis. An additional cause for the differences in observational cohorts and trial participants is the stringent exclusion criteria applied in RCT. In particular, diabetes per se or use of medications for diabetes is a frequent cause of exclusion of patients from trials, while nearly a quarter of patients (24%) have DM in observational studies of NAFLD and nearly half of patients (45%) with advanced fibrosis have DM. In some trials, for example trials of metformin or thiazolidinediones, limitations on diabetic patients or diabetic medications may be justified but the applicability of these findings to patients with advanced fibrosis is then limited. The design of trials that exclude patients with both diabetes and cirrhosis is thus a major barrier to external validity since many of those patients at the greatest risk of liver related death are not represented in these studies. Recent notable trials in NAFLD have suggested that studies are bcoming more inclusive. For example, the recent trial of Elafibranor included around reported that over 30% of participants had diabetes (21). The American Association for the Study of Liver Disease (AASLD) published a consensus statement regarding trial design in NAFLD (22). This was added to in 2015 by a report of a meeting between representatives of the AASLD the US Food and Drug Administration (FDA) (19). These documents provide guidance that aims to achieve greater consistency in 11
13 trial design and to define outcomes relevant to patients. They do not provide recommendations on measures that might strengthen external validity per se but do recommend that trials in NAFLD should target specific groups, in particular those at risk of progression to cirrhosis, those with cirrhosis, and post-transplant patients. Advice from regulatory bodies regarding measures to improve of review external validity of trials is either lacking or non-binding. These findings are similar to studies in other disease areas. In cardiovascular disease advanced patient age and the presence of co-morbidity were identified as important factors limiting external validity (9). The issue of co-morbidity is also important in patients with NAFLD. The association of NAFLD with the metabolic syndrome raises the risk of coexisting cardiovascular disease, prior extra-hepatic cancer, and other obesity related complications (23) that may also impact on recruitment into clinical trials. It is important that these co-morbidities are considered in future trial design. It is likely that inclusion of patients with significant co-morbidities would increase the risk of competing mortality such that in large scale, long duration licensing studies the number of patients required for sufficient statistical power would also be increased. Thus there is a short-term disincentive to include such patients in registration studies. In the long-term however it is preferable that physicians and patients understand the likely benefits and harms of treatment and this can only be achieved through the inclusion of such patients in licensing studies of novel therapeutics for patients with NASH. This study has several limitations. It is limited by missing data, which also reflects data being presented in a manner that was not suitable for analysis in this study. Nevertheless, the comprehensive systematic review and consequent large numbers studies included goes some way to limiting the impact of these missing data. There is also a risk of bias in the selection of patients for inclusion in observational studies of NASH and advanced fibrosis, through selection for biopsy, which may only be performed in patients who appear at high risk to clinicians. This is also the case in observational studies based in 12
14 secondary care where a degree of selection bias may be present. The larger community based studies using non-invasive methods to diagnose NAFLD are less at risk of these biases. In conclusion, we have identified differences in the characteristics of patients identified in observational studies of NASH, particularly those with advanced fibrosis, and those patients enrolled in RCTs of new therapeutic approaches. These findings, whilst partially explained by the therapies that have been trialed and by the choice of surrogate endpoint, highlight a risk that future studies will have limited external validity. References 1. Chalasani N, Younossi Z, Lavine JE et al. The Diagnosis and Management of Non- Alcoholic Fatty Liver Disease: Practice Guideline By the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7): Browning JD, Szczepaniak LS, Dobbins R et al. Prevalence of Hepatic Steatosis in an Urban Population in the United States: Impact of Ethnicity. Hepatology. 2004;40(6): Williams CD, Stengel J, Asike MI et al. Prevalence of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis Among a Largely Middle-Aged Population Utilizing Ultrasound and Liver Biopsy: A Prospective Study. Gastroenterology. 2011;140(1): Lazo M, Hernaez R, Bonekamp S et al. Non-Alcoholic Fatty Liver Disease and Mortality Among Us Adults: Prospective Cohort Study. Bmj. 2011; Adams LA, Lymp JF, St Sauver J et al. The Natural History of Nonalcoholic Fatty Liver Disease: A Population-Based Cohort Study. Gastroenterology. 2005;129(1): Ekstedt M, Hagström H, Nasr P et al. Fibrosis Stage is the Strongest Predictor for Disease specific Mortality in Nafld After Up to 33 Years of Follow up. Hepatology. 2015;61 (5): Musso G, Gambino R, Cassader M et al. A Meta-Analysis of Randomized Trials for the Treatment of Nonalcoholic Fatty Liver Disease. Hepatology. 2010;52(1): Rothwell PM. External Validity of Randomised Controlled Trials: to Whom Do the Results of This Trial Apply?. The Lancet. 2005;365(9453): Steg PG, López-Sendón J, De Sa EL et al. External Validity of Clinical Trials in Acute Myocardial Infarction. Archives of internal medicine. 2007;167(1): Travers J, Marsh S, Williams M et al. External Validity of Randomised Controlled Trials in Asthma: To Whom Do the Results of the Trials Apply? Thorax. 13
15 2007;62(3): Travers J, Marsh S, Caldwell B et al. External Validity of Randomized Controlled Trials in Copd. Respiratory medicine. 2007;101(6): Van Spall HGC, Toren A, Kiss A et al. Eligibility Criteria of Randomized Controlled Trials Published in High-Impact General Medical Journals: A Systematic Sampling Review. JAMA. 2007;297(11): Schulz KF, Altman DG, Moher D. Consort 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMC medicine. 2010;8(1): Von Elm E, Altmans DG, Egger M et al. The Strengthening the Reporting of Observational Studies in Epidemiology (Strobe) Statement: Guidelines for Reporting Observational Studies. International Journal of Surgery. 2014;12: Kleiner DE, Brunt EM, Van Natta M et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology. 2005;41(6): Mohanty SR, Troy TN, Huo D et al. Influence of Ethnicity on Histological Differences in Non-Alcoholic Fatty Liver Disease. Journal of Hepatology. 2009;50: Bambha K, Belt P, Abraham M et al. Ethnicity and Nonalcoholic Fatty Liver Disease. Hepatology. 2012;55: Raji A, Seely EW, Arky RA et al. Body Fat Distribution and Insulin Resistance in Healthy Asian Indians and Caucasians. The Journal of Clinical Endocrinology & Metabolism. 2001;86(11): Sanyal AJ, Friedman SL, Mccullough AJ et al. Challenges and Opportunities in Drug and Biomarker Development for Nonalcoholic Steatohepatitis: Findings and Recommendations From an American Association for the Study of Liver Diseases - U.s. Food and Drug Administration Joint Workshop. Hepatology. 2015;61 (4): Marcellin P, Gane E, Buti M et al. Regression of Cirrhosis During Treatment With Tenofovir Disoproxil Fumarate for Chronic Hepatitis B: A 5-Year Open-Label Follow- Up Study. The Lancet. 2013;381(9865): Ratziu V, Harrison SA, Francque S et al. Elafibranor, an Agonist of the Peroxisome Proliferator∠Activated Receptor∠α and∠Î, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150(5): e Sanyal AJ, Brunt EM, Kleiner DE et al. Endpoints and Clinical Trial Design for Nonalcoholic Steatohepatitis. Hepatology. 2011;54(1): Armstrong MJ, Adams LA, Canbay A et al. Extrahepatic Complications of Nonalcoholic Fatty Liver Disease. Hepatology. 2014;59(3):
16 Weighted means of age and BMI Observational studies RCT cohorts All NAFLD NASH Adv. fibrosis Mean SEM Mean SE M Difference between means p t-test Mean SEM Difference between means p t-test Mean SEM Difference between means p t-test Age (years) < < BMI (kg/m 2 ) < < Calculated prevalence of diabetes, distribution of gender Observational studies RCT cohorts All NAFLD NASH Advanced fibrosis Prevalence of Diabetes (%) Male gender (%) prevalence prevalence p Chi-squared prevalence p Chi-squared prevalence p Chi-squared 8 24 < < < < < <0.001 Table 1: Characteristics of RCT and observational study cohorts. p-value refers to difference of observational studies to RCT cohort. 15
17 Figure Legends Figure 1. Flow chart of literature search outcomes.
18 Figure 2: Distribution of variables between types of study: A age, B BMI, where each point represents the mean value from an individual trial. C prevalence of diabetes and D male gender, each data point represents prevalence within an individual study. Lines at median and interquartile range. *p<0.05, **p<0.001, ***p<0.001 by Kruskal-Wallis test.
19 Figure 3: Age and BMI of participants in observational studies and RCTs. Data are shown as weighted mean, horizontal lines represent standard deviation. Dashed line at RCT mean. ***p<0.001 by student s t-test
20 Figure 4: Prevalence of diabetes and distribution of gender in observational studies and RCTs. ***p<0.001 by Chi-squared test
Where a licence is displayed above, please note the terms and conditions of the licence govern your use of this document.
 Obstructive sleep apnoea and retinopathy in patients with type 2 diabetes Altaf, Quratul-ain; Dodson, Paul; Ali, Asad; Raymond, Neil T; Wharton, Helen; Hampshire- Bancroft, Rachel; Shah, Mirriam; Shepherd,
Obstructive sleep apnoea and retinopathy in patients with type 2 diabetes Altaf, Quratul-ain; Dodson, Paul; Ali, Asad; Raymond, Neil T; Wharton, Helen; Hampshire- Bancroft, Rachel; Shah, Mirriam; Shepherd,
Screening for ovarian cancer Kehoe, Sean
 Screening for ovarian cancer Kehoe, Sean DOI: 10.1016/j.maturitas.2015.05.009 License: Creative Commons: Attribution-NonCommercial-NoDerivs (CC BY-NC-ND) Document Version Peer reviewed version Citation
Screening for ovarian cancer Kehoe, Sean DOI: 10.1016/j.maturitas.2015.05.009 License: Creative Commons: Attribution-NonCommercial-NoDerivs (CC BY-NC-ND) Document Version Peer reviewed version Citation
NONALCOHOLIC FATTY LIVER DISEASE. Non-Alcoholic Fatty Liver Disease (NAFLD) Primary NAFLD. April 13, 2012
 NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Primary NAFLD Simple Steatosis Fatty hepatocytes Intracellular fat
NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Primary NAFLD Simple Steatosis Fatty hepatocytes Intracellular fat
The EARNEST study : interarm blood pressure differences should also be recorded Moody, William; Ferro, Charles; Townend, Jonathan
 The EARNEST study : interarm blood pressure differences should also be recorded Moody, William; Ferro, Charles; Townend, Jonathan DOI: 10.1016/j.ahj.2014.05.006 License: None: All rights reserved Document
The EARNEST study : interarm blood pressure differences should also be recorded Moody, William; Ferro, Charles; Townend, Jonathan DOI: 10.1016/j.ahj.2014.05.006 License: None: All rights reserved Document
A different view on immunity Dempsey, Claire; Jones, Nicholas
 A different view on immunity Dempsey, Claire; Jones, Nicholas DOI: 10.1097/TP.0000000000000738 License: None: All rights reserved Document Version Peer reviewed version Citation for published version (Harvard):
A different view on immunity Dempsey, Claire; Jones, Nicholas DOI: 10.1097/TP.0000000000000738 License: None: All rights reserved Document Version Peer reviewed version Citation for published version (Harvard):
Update on Non-Alcoholic Fatty Liver Disease. Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI
 Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
CDHNF & NASPGHAN A Partnership for Research and Education for Children s Digestive and Nutritional Health
 CDHNF & NASPGHAN A Partnership for Research and Education for Children s Digestive and Nutritional Health Obesity and NAFLD Definitions: Nonalcoholic steatohepatitis (NASH) and nonalcoholic fatty liver
CDHNF & NASPGHAN A Partnership for Research and Education for Children s Digestive and Nutritional Health Obesity and NAFLD Definitions: Nonalcoholic steatohepatitis (NASH) and nonalcoholic fatty liver
The role of non-invasivemethods in evaluating liver fibrosis of patients with non-alcoholic steatohepatitis
 The role of non-invasivemethods in evaluating liver fibrosis of patients with non-alcoholic steatohepatitis Objectives: Liver biopsy is the gold standard for diagnosing the extent of fibrosis in NAFLD/NASH;
The role of non-invasivemethods in evaluating liver fibrosis of patients with non-alcoholic steatohepatitis Objectives: Liver biopsy is the gold standard for diagnosing the extent of fibrosis in NAFLD/NASH;
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery
NICE guideline Published: 6 July 2016 nice.org.uk/guidance/ng49
 Non-alcoholic fatty liver disease (NAFLD): assessment and management NICE guideline Published: 6 July 20 nice.org.uk/guidance/ng49 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Non-alcoholic fatty liver disease (NAFLD): assessment and management NICE guideline Published: 6 July 20 nice.org.uk/guidance/ng49 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
MY APPROACH to the use of NOACs for stroke prevention in patients with atrial fibrillation Lip, Gregory
 MY APPROACH to the use of NOACs for stroke prevention in patients with atrial fibrillation Lip, Gregory DOI: 10.1016/j.tcm.2014.05.012 License: Other (please specify with Rights Statement) Document Version
MY APPROACH to the use of NOACs for stroke prevention in patients with atrial fibrillation Lip, Gregory DOI: 10.1016/j.tcm.2014.05.012 License: Other (please specify with Rights Statement) Document Version
The use of random projections for the analysis of mass spectrometry imaging data Palmer, Andrew; Bunch, Josephine; Styles, Iain
 The use of random projections for the analysis of mass spectrometry imaging data Palmer, Andrew; Bunch, Josephine; Styles, Iain DOI: 10.1007/s13361-014-1024-7 Citation for published version (Harvard):
The use of random projections for the analysis of mass spectrometry imaging data Palmer, Andrew; Bunch, Josephine; Styles, Iain DOI: 10.1007/s13361-014-1024-7 Citation for published version (Harvard):
Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical
 Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical Disclosure Naim Alkhouri, MD discloses the following relationships with commercial companies: Membership in the Speakers Bureau for Alexion
Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical Disclosure Naim Alkhouri, MD discloses the following relationships with commercial companies: Membership in the Speakers Bureau for Alexion
FDA Introductory Remarks Stephanie O. Omokaro, MD
 FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014
 Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
SAF score and mortality in NAFLD after up to 41 years of follow-up
 SAF score and mortality in NAFLD after up to 41 years of follow-up Hannes Hagström, Patrik Nasr, Mattias Ekstedt, Stergios Kechagias, Per Stal, Pierre Bedossa and Rolf Hultcrantz Journal Article N.B.:
SAF score and mortality in NAFLD after up to 41 years of follow-up Hannes Hagström, Patrik Nasr, Mattias Ekstedt, Stergios Kechagias, Per Stal, Pierre Bedossa and Rolf Hultcrantz Journal Article N.B.:
Non-Alcoholic Fatty Liver Diseasean underestimated epidemic
 Non-Alcoholic Fatty Liver Diseasean underestimated epidemic Amir Shlomai MD,PhD Head, Department of Medicine D The Liver Institute Rabin Medical Center, Beilinson Hospital The IASLD semi-annual meeting-
Non-Alcoholic Fatty Liver Diseasean underestimated epidemic Amir Shlomai MD,PhD Head, Department of Medicine D The Liver Institute Rabin Medical Center, Beilinson Hospital The IASLD semi-annual meeting-
AAIM: GI Workshop Follow Up to Case Studies. Non-alcoholic Fatty Liver Disease Ulcerative Colitis Crohn s Disease
 AAIM: GI Workshop Follow Up to Case Studies Non-alcoholic Fatty Liver Disease Ulcerative Colitis Crohn s Disease Daniel Zimmerman, MD VP and Medical Director, RGA Global October 2015 Non-alcoholic Fatty
AAIM: GI Workshop Follow Up to Case Studies Non-alcoholic Fatty Liver Disease Ulcerative Colitis Crohn s Disease Daniel Zimmerman, MD VP and Medical Director, RGA Global October 2015 Non-alcoholic Fatty
UMHS-PUHSC JOINT INSTITUTE
 Role of Visceral Adiposity in the Pathogenesis of Non-Alcoholic Fatty Liver Disease in Lean versus Obese Patients: A Comparative Study between Patients at UMHS versus PUHSC Lai WEI and Anna LOK W Zhang,
Role of Visceral Adiposity in the Pathogenesis of Non-Alcoholic Fatty Liver Disease in Lean versus Obese Patients: A Comparative Study between Patients at UMHS versus PUHSC Lai WEI and Anna LOK W Zhang,
WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH?
 WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH? Helena Cortez-Pinto Laboratório de Nutrição, FML, Serviço de Gastrenterologia, Hospital St Maria, Lisboa, Portugal EASL Governing Board:
WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH? Helena Cortez-Pinto Laboratório de Nutrição, FML, Serviço de Gastrenterologia, Hospital St Maria, Lisboa, Portugal EASL Governing Board:
EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease.
 Commentary. EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease. Christopher D. Byrne 1,2, Giovanni Targher 3 1 Nutrition and Metabolism, Faculty of Medicine,
Commentary. EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease. Christopher D. Byrne 1,2, Giovanni Targher 3 1 Nutrition and Metabolism, Faculty of Medicine,
Standards for the reporting of new Cochrane Intervention Reviews
 Methodological Expectations of Cochrane Intervention Reviews (MECIR) Standards for the reporting of new Cochrane Intervention Reviews 24 September 2012 Preface The standards below summarize proposed attributes
Methodological Expectations of Cochrane Intervention Reviews (MECIR) Standards for the reporting of new Cochrane Intervention Reviews 24 September 2012 Preface The standards below summarize proposed attributes
Non-Alcoholic Fatty Liver Disease
 Non-Alcoholic Fatty Liver Disease None Disclosures Arslan Kahloon M.D Chief, Division of Gastroenterology and Hepatology University of Tennessee College of Medicine Chattanooga Objectives Understand the
Non-Alcoholic Fatty Liver Disease None Disclosures Arslan Kahloon M.D Chief, Division of Gastroenterology and Hepatology University of Tennessee College of Medicine Chattanooga Objectives Understand the
PEDIATRIC FOIE GRAS: NON-ALCOHOLIC FATTY LIVER DISEASE
 PEDIATRIC FOIE GRAS: NON-ALCOHOLIC FATTY LIVER DISEASE Updates on New insights into NAFLD and NASH pathophysiology New AASLD/AGA/ACG guidelines for NAFLD and NASH, as pertains to pediatrics Evidence-based
PEDIATRIC FOIE GRAS: NON-ALCOHOLIC FATTY LIVER DISEASE Updates on New insights into NAFLD and NASH pathophysiology New AASLD/AGA/ACG guidelines for NAFLD and NASH, as pertains to pediatrics Evidence-based
Evercore ISI Presentation- Madrigal
 Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
Normal ALT for men 30 IU/L 36% US males abnormal. Abnl ALT. Assess alcohol use/meds. Recheck in 6-8 weeks. still pos
 Fatty liver disease Its not just for big boys anymore Ken Flora, MD, FAASLD, FACG, AGAF No disclosures Common situation Normal ALT for men 30 IU/L 36% US males abnormal Normal ALT for women 20 IU/L 28%
Fatty liver disease Its not just for big boys anymore Ken Flora, MD, FAASLD, FACG, AGAF No disclosures Common situation Normal ALT for men 30 IU/L 36% US males abnormal Normal ALT for women 20 IU/L 28%
Diseases to Watch. Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-alcoholic
 Diseases to Watch Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-alcoholic steatohepatitis (NASH) - Prevalence and Symptoms - Risk Factors and Potential treatments - Target identification for NASH Robert
Diseases to Watch Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-alcoholic steatohepatitis (NASH) - Prevalence and Symptoms - Risk Factors and Potential treatments - Target identification for NASH Robert
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) NON-ALCOHOLIC STEATOHEPATITIS (NASH) ADDRESSING A GROWING SILENT EPIDEMIC
 NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
Where a licence is displayed above, please note the terms and conditions of the licence govern your use of this document.
 Self-injurious, aggressive and destructive behaviour in young children with a moderate to profound intellectual disability Handley, Louise; Adams, Dawn; Simkiss, Douglas; Oliver, Christopher DOI: 10.1016/j.paed.2013.04.004
Self-injurious, aggressive and destructive behaviour in young children with a moderate to profound intellectual disability Handley, Louise; Adams, Dawn; Simkiss, Douglas; Oliver, Christopher DOI: 10.1016/j.paed.2013.04.004
Liver Pathology in the 0bese
 Liver Pathology in the 0bese Rob Goldin Centre for Pathology, Imperial College r.goldin@imperial.ac.uk Ludwig et al. Non-alcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease.
Liver Pathology in the 0bese Rob Goldin Centre for Pathology, Imperial College r.goldin@imperial.ac.uk Ludwig et al. Non-alcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease.
National Horizon Scanning Centre. Enhanced Liver Fibrosis Test (ELF) for evaluating liver fibrosis. June 2008
 Enhanced Liver Fibrosis Test (ELF) for evaluating liver fibrosis June 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Enhanced Liver Fibrosis Test (ELF) for evaluating liver fibrosis June 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Challenges in the Diagnosis of Steatohepatitis
 The Bugaboos of Fatty Liver Disease: Ballooning and Fibrosis Hans Popper Hepatopathology Society Companion Meeting San Antonio, Tx March, 2017 David Kleiner, M.D., Ph.D. NCI/Laboratory of Pathology Challenges
The Bugaboos of Fatty Liver Disease: Ballooning and Fibrosis Hans Popper Hepatopathology Society Companion Meeting San Antonio, Tx March, 2017 David Kleiner, M.D., Ph.D. NCI/Laboratory of Pathology Challenges
PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD. Pierre Bedossa. Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE
 PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD Pierre Bedossa Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE 1 PATHOLOGY OF NAFLD NAFLD: a chronic liver disease with a wide
PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD Pierre Bedossa Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE 1 PATHOLOGY OF NAFLD NAFLD: a chronic liver disease with a wide
Non-Alcoholic Fatty Liver Disease (NAFLD)
 HEPATO-PANCREATO-BILIARY STOMACH CANCER PROGRAM Non-Alcoholic Fatty Liver Disease (NAFLD) Steatosis and Non-Alcoholic Steatohepatitis (NASH) Management Recommendations UCSF Fresno Department of Surgery
HEPATO-PANCREATO-BILIARY STOMACH CANCER PROGRAM Non-Alcoholic Fatty Liver Disease (NAFLD) Steatosis and Non-Alcoholic Steatohepatitis (NASH) Management Recommendations UCSF Fresno Department of Surgery
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) NON-ALCOHOLIC STEATOHEPATITIS (NASH) ADDRESSING A GROWING SILENT EPIDEMIC
 NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
«STEATOSI EPATICA ED EPATOPATIE METABOLICHE» Ester Vanni Division of Gastroenterology University of Turin
 «STEATOSI EPATICA ED EPATOPATIE METABOLICHE» Ester Vanni Division of Gastroenterology University of Turin OUTLINE NAFLD overview NAFLD and menarche NAFLD and pregnancy NAFLD and menopause Other metabolic
«STEATOSI EPATICA ED EPATOPATIE METABOLICHE» Ester Vanni Division of Gastroenterology University of Turin OUTLINE NAFLD overview NAFLD and menarche NAFLD and pregnancy NAFLD and menopause Other metabolic
Results. NeuRA Worldwide incidence April 2016
 Introduction The incidence of schizophrenia refers to how many new cases there are per population in a specified time period. It is different from prevalence, which refers to how many existing cases there
Introduction The incidence of schizophrenia refers to how many new cases there are per population in a specified time period. It is different from prevalence, which refers to how many existing cases there
CONSORT 2010 checklist of information to include when reporting a randomised trial*
 CONSORT 2010 checklist of information to include when reporting a randomised trial* Section/Topic Title and abstract Introduction Background and objectives Item No Checklist item 1a Identification as a
CONSORT 2010 checklist of information to include when reporting a randomised trial* Section/Topic Title and abstract Introduction Background and objectives Item No Checklist item 1a Identification as a
Systematic Reviews. Simon Gates 8 March 2007
 Systematic Reviews Simon Gates 8 March 2007 Contents Reviewing of research Why we need reviews Traditional narrative reviews Systematic reviews Components of systematic reviews Conclusions Key reference
Systematic Reviews Simon Gates 8 March 2007 Contents Reviewing of research Why we need reviews Traditional narrative reviews Systematic reviews Components of systematic reviews Conclusions Key reference
Update on Clinical Management
 Non-Alcoholic Fatty Liver Disease Update on Clinical Management Lisa J. Yoo, D.O. Gastroenterologist Regional Gastroenterology INTRODUCTION Non-alcoholic fatty liver disease (NAFLD) is characterized by
Non-Alcoholic Fatty Liver Disease Update on Clinical Management Lisa J. Yoo, D.O. Gastroenterologist Regional Gastroenterology INTRODUCTION Non-alcoholic fatty liver disease (NAFLD) is characterized by
The QUOROM Statement: revised recommendations for improving the quality of reports of systematic reviews
 The QUOROM Statement: revised recommendations for improving the quality of reports of systematic reviews David Moher 1, Alessandro Liberati 2, Douglas G Altman 3, Jennifer Tetzlaff 1 for the QUOROM Group
The QUOROM Statement: revised recommendations for improving the quality of reports of systematic reviews David Moher 1, Alessandro Liberati 2, Douglas G Altman 3, Jennifer Tetzlaff 1 for the QUOROM Group
Nonalcoholic fatty liver disease (NAFLD) is the most common
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2008;6:1249 1254 Cytokeratin 18 Fragment Levels as a Noninvasive Biomarker for Nonalcoholic Steatohepatitis in Bariatric Surgery Patients DIMA L. DIAB,* LISA YERIAN,
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2008;6:1249 1254 Cytokeratin 18 Fragment Levels as a Noninvasive Biomarker for Nonalcoholic Steatohepatitis in Bariatric Surgery Patients DIMA L. DIAB,* LISA YERIAN,
National Horizon Scanning Centre. Transient elastography (FibroScan) for evaluating liver fibrosis. April 2008
 Transient elastography (FibroScan) for evaluating liver fibrosis April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not
Transient elastography (FibroScan) for evaluating liver fibrosis April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not
An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications
 REVIEW An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications Naim Alkhouri, M.D.,*, and Andrea Scott, B.S.* Nonalcoholic fatty liver disease (NAFLD)
REVIEW An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications Naim Alkhouri, M.D.,*, and Andrea Scott, B.S.* Nonalcoholic fatty liver disease (NAFLD)
Systematic Reviews and Meta- Analysis in Kidney Transplantation
 Systematic Reviews and Meta- Analysis in Kidney Transplantation Greg Knoll MD MSc Associate Professor of Medicine Medical Director, Kidney Transplantation University of Ottawa and The Ottawa Hospital KRESCENT
Systematic Reviews and Meta- Analysis in Kidney Transplantation Greg Knoll MD MSc Associate Professor of Medicine Medical Director, Kidney Transplantation University of Ottawa and The Ottawa Hospital KRESCENT
Liver biopsy as the gold standard for diagnosis. Pierre BEDOSSA
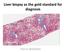 Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
Where a licence is displayed above, please note the terms and conditions of the licence govern your use of this document.
 Using the CHA2DS2-VASc score for stroke prevention in atrial fibrillation Nielsen, Peter Brønnum; Skjøth, Flemming; Rasmussen, Lars Hvilsted; Larsen, Torben Bjerregaard; Lip, Gregory DOI: 10.1016/j.cjca.2015.01.034
Using the CHA2DS2-VASc score for stroke prevention in atrial fibrillation Nielsen, Peter Brønnum; Skjøth, Flemming; Rasmussen, Lars Hvilsted; Larsen, Torben Bjerregaard; Lip, Gregory DOI: 10.1016/j.cjca.2015.01.034
Non-alcoholic fatty liver disease: time to take note and manage. Philip Newsome Professor of Hepatology & Director of Centre for Liver Research
 Non-alcoholic fatty liver disease: time to take note and manage Philip Newsome Professor of Hepatology & Director of Centre for Liver Research Disclosures Consultancy, Co-ordinating Investigator roles
Non-alcoholic fatty liver disease: time to take note and manage Philip Newsome Professor of Hepatology & Director of Centre for Liver Research Disclosures Consultancy, Co-ordinating Investigator roles
Zhengtao Liu 1,2,3*, Shuping Que 4*, Lin Zhou 1,2,3 Author affiliation:
 Dose-response Relationship of Serum Uric Acid with Metabolic Syndrome and Non-alcoholic Fatty Liver Disease Incidence: AMeta-analysis of Prospective Studies Zhengtao Liu 1,2,3*, Shuping Que 4*, Lin Zhou
Dose-response Relationship of Serum Uric Acid with Metabolic Syndrome and Non-alcoholic Fatty Liver Disease Incidence: AMeta-analysis of Prospective Studies Zhengtao Liu 1,2,3*, Shuping Que 4*, Lin Zhou
SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms
 SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms Claudia Filozof, MD PhD Executive Medical Director Covance Challenges in Clinical Development in NASH
SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms Claudia Filozof, MD PhD Executive Medical Director Covance Challenges in Clinical Development in NASH
NAFLD: evidence-based management. Curso de residentes AEEH Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain
 NAFLD: evidence-based management Curso de residentes AEEH 2017 Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain Clinical case - 55 yo female - Sent for incidental steatosis at abdominal
NAFLD: evidence-based management Curso de residentes AEEH 2017 Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain Clinical case - 55 yo female - Sent for incidental steatosis at abdominal
NONALCOHOLIC FATTY LIVER DISEASE
 NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD, MSc Hepatology and Liver Transplantation University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Terminology Pathogenesis
NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD, MSc Hepatology and Liver Transplantation University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Terminology Pathogenesis
Paris Hepatology Conference PROGNOSIS OF NASH
 Paris Hepatology Conference PROGNOSIS OF NASH Palais des Congrès Monday 15th January 2018 14:45-15:00 PHC 2018 - www.aphc.info Driven to care Disclosures Advisory committees Speaking and teaching Research
Paris Hepatology Conference PROGNOSIS OF NASH Palais des Congrès Monday 15th January 2018 14:45-15:00 PHC 2018 - www.aphc.info Driven to care Disclosures Advisory committees Speaking and teaching Research
PREVALENCE OF NAFLD & NASH
 - - PREVALENCE OF & USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology 2011; 140:124-31) Dallas Heart Study Prevalence Numbers (Browning et al., Hepatology 2004;40:1387-95)
- - PREVALENCE OF & USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology 2011; 140:124-31) Dallas Heart Study Prevalence Numbers (Browning et al., Hepatology 2004;40:1387-95)
NAFLD: US GUIDELINES. US Guidelines for NAFLD
 NAFLD: US GUIDELINES Arun J Sanyal M.D. Charles Caravati Professor of Medicine Virginia Commonwealth University School of Medicine US Guidelines for NAFLD Represents consensus amongst AGA, AASLD and ACG
NAFLD: US GUIDELINES Arun J Sanyal M.D. Charles Caravati Professor of Medicine Virginia Commonwealth University School of Medicine US Guidelines for NAFLD Represents consensus amongst AGA, AASLD and ACG
LIVER PATHOLOGY. Thursday 28 th November 2013
 LIVER PATHOLOGY Thursday 28 th November 2013 Liver biopsy assessment of steatosis Amar Paul Dhillon Royal Free Hospital RIBA, London Thursday 28th November 2013 NAFLD didn t exist before 2001 and liver
LIVER PATHOLOGY Thursday 28 th November 2013 Liver biopsy assessment of steatosis Amar Paul Dhillon Royal Free Hospital RIBA, London Thursday 28th November 2013 NAFLD didn t exist before 2001 and liver
General. Regulatory Alert. Recommendations. Guideline Title. Bibliographic Source(s) Guideline Status. FDA Warning/Regulatory Alert
 General Guideline Title Non-alcoholic fatty liver disease (NAFLD): assessment and management. Bibliographic Source(s) National Guideline Centre. Non-alcoholic fatty liver disease (NAFLD): assessment and
General Guideline Title Non-alcoholic fatty liver disease (NAFLD): assessment and management. Bibliographic Source(s) National Guideline Centre. Non-alcoholic fatty liver disease (NAFLD): assessment and
NAFLD & NASH. Naga Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of GI and Hepatology
 NAFLD & NASH Naga Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of GI and Hepatology Indiana University School of Medicine ACG Midwest Regional Course,
NAFLD & NASH Naga Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of GI and Hepatology Indiana University School of Medicine ACG Midwest Regional Course,
Cochrane Breast Cancer Group
 Cochrane Breast Cancer Group Version and date: V3.2, September 2013 Intervention Cochrane Protocol checklist for authors This checklist is designed to help you (the authors) complete your Cochrane Protocol.
Cochrane Breast Cancer Group Version and date: V3.2, September 2013 Intervention Cochrane Protocol checklist for authors This checklist is designed to help you (the authors) complete your Cochrane Protocol.
Early life determinants of Non-Alcoholic Fatty Liver Disease and NASH DR JULIANA MUIVA-GITOBU KENYA PAEDIATRIC ASSOCIATION CONFERENCE APRIL 2016.
 Early life determinants of Non-Alcoholic Fatty Liver Disease and NASH DR JULIANA MUIVA-GITOBU KENYA PAEDIATRIC ASSOCIATION CONFERENCE APRIL 2016. Outline Definition NAFLD and NASH Magnitude of the problem
Early life determinants of Non-Alcoholic Fatty Liver Disease and NASH DR JULIANA MUIVA-GITOBU KENYA PAEDIATRIC ASSOCIATION CONFERENCE APRIL 2016. Outline Definition NAFLD and NASH Magnitude of the problem
What is NAFLD?.NASH? Presenter Disclosure Information. Learning Objectives. Case 1: Rob. Questions Pertinent to Rob
 Presenter Disclosure Information 5 6pm Nonalcoholic Fatty Liver Disease (NAFLD): Another Obesity-Related Epidemic SPEAKER Elliot Tapper, MD The following relationships exist related to this presentation:
Presenter Disclosure Information 5 6pm Nonalcoholic Fatty Liver Disease (NAFLD): Another Obesity-Related Epidemic SPEAKER Elliot Tapper, MD The following relationships exist related to this presentation:
Laboratory analysis of the obese child recommendations and discussion. MacKenzi Hillard May 4, 2011
 Laboratory analysis of the obese child recommendations and discussion MacKenzi Hillard May 4, 2011 aka: What to do with Fasting Labs The Obesity Epidemic The prevalence of obesity in adolescents has tripled
Laboratory analysis of the obese child recommendations and discussion MacKenzi Hillard May 4, 2011 aka: What to do with Fasting Labs The Obesity Epidemic The prevalence of obesity in adolescents has tripled
WORLDWIDE EPIDEMIOLOGY OF NASH
 WORLDWIDE EPIDEMIOLOGY OF NASH Stefano Bellentani, M.D., Ph.D. Chief of Gastroenterology and Hepatology Service Clinica Santa Chiara Locarno Switzerland & Fondazione Italiana Fegato (FIF), Bassovizza (Trieste),
WORLDWIDE EPIDEMIOLOGY OF NASH Stefano Bellentani, M.D., Ph.D. Chief of Gastroenterology and Hepatology Service Clinica Santa Chiara Locarno Switzerland & Fondazione Italiana Fegato (FIF), Bassovizza (Trieste),
FATTY LIVER DISEASE (NAFLD) (NASH) A GROWING
 NON ALCOHOLIC FATTY LIVER DISEASE () & NON ALCOHOLIC S T E ATO H E PAT I T I S () ADDRESSING A GROWING SILENT EPIDEMIC Prevalence of & USA Prevalence in Middle Age Patients San Antonio, Texas (Williams
NON ALCOHOLIC FATTY LIVER DISEASE () & NON ALCOHOLIC S T E ATO H E PAT I T I S () ADDRESSING A GROWING SILENT EPIDEMIC Prevalence of & USA Prevalence in Middle Age Patients San Antonio, Texas (Williams
Update on Nonalcoholic Fatty Liver Disease. Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic
 Update on Nonalcoholic Fatty Liver Disease Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic Outline Defining the phenotypes of nonalcoholic fatty liver disease NAFLD Diagnostics
Update on Nonalcoholic Fatty Liver Disease Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic Outline Defining the phenotypes of nonalcoholic fatty liver disease NAFLD Diagnostics
Defining the gold standard in biomarker validation for NASH
 Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
Fatty liver disease: What do we know?
 Fatty liver disease: What do we know? Prof. Dr. Claus Niederau Katholische Kliniken Oberhausen ggmbh St. Josef-Hospital Academic Teaching Hospital University of Duisburg-Essen NAFLD Non-Alcoholic Fatty
Fatty liver disease: What do we know? Prof. Dr. Claus Niederau Katholische Kliniken Oberhausen ggmbh St. Josef-Hospital Academic Teaching Hospital University of Duisburg-Essen NAFLD Non-Alcoholic Fatty
Nonalcoholic Fatty Liver Disease: Definitions, Risk Factors, and Workup
 REVIEW REVIEW Nonalcoholic Fatty Liver Disease: Definitions, Risk Factors, and Workup Puneet Puri, M.B.B.S., M.D. and Arun J. Sanyal, M.B.B.S., M.D. Nonalcoholic fatty liver disease (NAFLD) is defined
REVIEW REVIEW Nonalcoholic Fatty Liver Disease: Definitions, Risk Factors, and Workup Puneet Puri, M.B.B.S., M.D. and Arun J. Sanyal, M.B.B.S., M.D. Nonalcoholic fatty liver disease (NAFLD) is defined
Downloaded from:
 Arnup, SJ; Forbes, AB; Kahan, BC; Morgan, KE; McKenzie, JE (2016) The quality of reporting in cluster randomised crossover trials: proposal for reporting items and an assessment of reporting quality. Trials,
Arnup, SJ; Forbes, AB; Kahan, BC; Morgan, KE; McKenzie, JE (2016) The quality of reporting in cluster randomised crossover trials: proposal for reporting items and an assessment of reporting quality. Trials,
The diagnosis of Chronic Pancreatitis
 The diagnosis of Chronic Pancreatitis 1. Background The diagnosis of chronic pancreatitis (CP) is challenging. Chronic pancreatitis is a disease process consisting of: fibrosis of the pancreas (potentially
The diagnosis of Chronic Pancreatitis 1. Background The diagnosis of chronic pancreatitis (CP) is challenging. Chronic pancreatitis is a disease process consisting of: fibrosis of the pancreas (potentially
Lipid and Bile Acids as NAFLD- Related Biomarkers
 Lipid and Bile Acids as NAFLD- Related Biomarkers Puneet Puri, MBBS, MD Division of Gastroenterology, Hepatology and Nutrition Virginia Commonwealth University, Richmond, VA 1st International Workshop
Lipid and Bile Acids as NAFLD- Related Biomarkers Puneet Puri, MBBS, MD Division of Gastroenterology, Hepatology and Nutrition Virginia Commonwealth University, Richmond, VA 1st International Workshop
Metabolically healthy obesity and NAFLD
 News & Views Metabolically healthy obesity and NAFLD Giovanni Targher and Christopher D. Byrne Giovanni Targher is at the Section of Endocrinology, Diabetes and Metabolism, Department of Medicine, University
News & Views Metabolically healthy obesity and NAFLD Giovanni Targher and Christopher D. Byrne Giovanni Targher is at the Section of Endocrinology, Diabetes and Metabolism, Department of Medicine, University
American Journal of Oral Medicine and Radiology
 American Journal of Oral Medicine and Radiology e - ISSN - XXXX-XXXX ISSN - 2394-7721 Journal homepage: www.mcmed.us/journal/ajomr PREVALENCE OF NONALCOHOLIC FATTY LIVER DISEASE AMONG TYPE 2 DIABETIC POPULATION
American Journal of Oral Medicine and Radiology e - ISSN - XXXX-XXXX ISSN - 2394-7721 Journal homepage: www.mcmed.us/journal/ajomr PREVALENCE OF NONALCOHOLIC FATTY LIVER DISEASE AMONG TYPE 2 DIABETIC POPULATION
Systematic reviews and meta-analyses of observational studies (MOOSE): Checklist.
 Systematic reviews and meta-analyses of observational studies (MOOSE): Checklist. MOOSE Checklist Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease:
Systematic reviews and meta-analyses of observational studies (MOOSE): Checklist. MOOSE Checklist Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease:
NAFLD & NASH: Russian perspective
 NAFLD & NASH: Russian perspective Vasily Isakov, MD, PhD Professor, Chief, Department Gastroenterology & Hepatology, Federal Research Center of nutrition, biotechnology and food safety Disclosures Received
NAFLD & NASH: Russian perspective Vasily Isakov, MD, PhD Professor, Chief, Department Gastroenterology & Hepatology, Federal Research Center of nutrition, biotechnology and food safety Disclosures Received
Predictors of Severity of Alcohol Withdrawal in Hospitalized Patients
 Elmer Original Article ress Predictors of Severity of Alcohol Withdrawal in Hospitalized Patients Radhames Ramos a, Thierry Mallet b, Anthony DiVittis c b, d, e, Ronny Cohen Abstract Background: Alcohol
Elmer Original Article ress Predictors of Severity of Alcohol Withdrawal in Hospitalized Patients Radhames Ramos a, Thierry Mallet b, Anthony DiVittis c b, d, e, Ronny Cohen Abstract Background: Alcohol
Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry
 Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Utilizing Seamless Adaptive Designs and Considering Multiplicity Adjustment for NASH Clinical Trials
 Utilizing Seamless Adaptive Designs and Considering Multiplicity Adjustment for NASH Clinical Trials Yeh-Fong Chen FDA/CDER/OB/DB3 Forum for Collaborative Research (Berkeley) 5/10/2017 Disclaimer This
Utilizing Seamless Adaptive Designs and Considering Multiplicity Adjustment for NASH Clinical Trials Yeh-Fong Chen FDA/CDER/OB/DB3 Forum for Collaborative Research (Berkeley) 5/10/2017 Disclaimer This
Results. NeuRA Treatments for internalised stigma December 2017
 Introduction Internalised stigma occurs within an individual, such that a person s attitude may reinforce a negative self-perception of mental disorders, resulting in reduced sense of selfworth, anticipation
Introduction Internalised stigma occurs within an individual, such that a person s attitude may reinforce a negative self-perception of mental disorders, resulting in reduced sense of selfworth, anticipation
Continuous update of the WCRF-AICR report on diet and cancer. Protocol: Breast Cancer. Prepared by: Imperial College Team
 Continuous update of the WCRF-AICR report on diet and cancer Protocol: Breast Cancer Prepared by: Imperial College Team The current protocol for the continuous update should ensure consistency of approach
Continuous update of the WCRF-AICR report on diet and cancer Protocol: Breast Cancer Prepared by: Imperial College Team The current protocol for the continuous update should ensure consistency of approach
Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts NAS score for NASH diagnosis
 Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts NAS score for NASH diagnosis Alina M. Allen, Meng Yin, Sudhakar K. Venkatesh, Taofic Mounajjed, Todd A. Kellogg,
Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts NAS score for NASH diagnosis Alina M. Allen, Meng Yin, Sudhakar K. Venkatesh, Taofic Mounajjed, Todd A. Kellogg,
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library)
 A systematic review of smoking cessation and relapse prevention interventions in parents of babies admitted to a neonatal unit (after delivery) Divya Nelson, Sarah Gentry, Caitlin Notley, Henry White,
A systematic review of smoking cessation and relapse prevention interventions in parents of babies admitted to a neonatal unit (after delivery) Divya Nelson, Sarah Gentry, Caitlin Notley, Henry White,
Disclosure. Objectives. Smash the Nash: A practical approach to fatty liver disease
 Smash the Nash: A practical approach to fatty liver disease Bruce D. Askey, MS, ANP-BC Associate Lecturer North Andover, MA Adult Nurse Practitioner Dept. of Hepatology/Gastroenterology Guthrie Clinic
Smash the Nash: A practical approach to fatty liver disease Bruce D. Askey, MS, ANP-BC Associate Lecturer North Andover, MA Adult Nurse Practitioner Dept. of Hepatology/Gastroenterology Guthrie Clinic
Non alcoholic fatty liver disease and atherosclerosis Raul Santos, MD
 Non alcoholic fatty liver disease and atherosclerosis Raul Santos, MD Sao Paulo Medical School Hospital Sao Paulo, Brazil Disclosure Honoraria received for consult and/or speaker : Astra Zeneca, Amgen,
Non alcoholic fatty liver disease and atherosclerosis Raul Santos, MD Sao Paulo Medical School Hospital Sao Paulo, Brazil Disclosure Honoraria received for consult and/or speaker : Astra Zeneca, Amgen,
NAFLD/NASH. Definitions. Pathology NASH. Vicki Shah PA-C, MMS Rush University Hepatology
 NAFLD/NASH Vicki Shah PA-C, MMS Rush University Hepatology Definitions NAFLD Evidence of hepatic steatosis by histology (5%) or imaging No causes for secondary fat accumulation EtOH, Drugs, hereditary
NAFLD/NASH Vicki Shah PA-C, MMS Rush University Hepatology Definitions NAFLD Evidence of hepatic steatosis by histology (5%) or imaging No causes for secondary fat accumulation EtOH, Drugs, hereditary
Template 1 for summarising studies addressing prognostic questions
 Template 1 for summarising studies addressing prognostic questions Instructions to fill the table: When no element can be added under one or more heading, include the mention: O Not applicable when an
Template 1 for summarising studies addressing prognostic questions Instructions to fill the table: When no element can be added under one or more heading, include the mention: O Not applicable when an
First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st NAFLD/NASH : an expanding burden on liver health
 First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st 2017 NAFLD/NASH : an expanding burden on liver health Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière,
First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st 2017 NAFLD/NASH : an expanding burden on liver health Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière,
NASH Regulatory Landscape. Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH
 NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
Fatty Liver Disease A growing epidemic
 Fatty Liver Disease A growing epidemic Updates in GIM for Primary Care Don C. Rockey March 9 th, 2018 Disclosures 2018 Research Funding (all to MUSC) NIH/NIDDK Actelion Pharmaceuticals Gilead Sciences
Fatty Liver Disease A growing epidemic Updates in GIM for Primary Care Don C. Rockey March 9 th, 2018 Disclosures 2018 Research Funding (all to MUSC) NIH/NIDDK Actelion Pharmaceuticals Gilead Sciences
Internal Medicine Grand Rounds University of Texas Southwestern Medical Center October 5, 2018
 Internal Medicine Grand Rounds University of Texas Southwestern Medical Center October 5, 2018 Nonalcoholic Fatty Liver Disease (NAFLD) Turns 38-What Have We Learned? Jay D. Horton, M.D. This is to acknowledge
Internal Medicine Grand Rounds University of Texas Southwestern Medical Center October 5, 2018 Nonalcoholic Fatty Liver Disease (NAFLD) Turns 38-What Have We Learned? Jay D. Horton, M.D. This is to acknowledge
NON-ALCOHOLIC STEATOHEPATITIS AND NON-ALCOHOLIC FATTY LIVER DISEASES
 NON-ALCOHOLIC STEATOHEPATITIS AND NON-ALCOHOLIC FATTY LIVER DISEASES Preface Zobair M. Younossi xiii Epidemiology and Natural History of NAFLD and NASH 1 Janus P. Ong and Zobair M. Younossi Understanding
NON-ALCOHOLIC STEATOHEPATITIS AND NON-ALCOHOLIC FATTY LIVER DISEASES Preface Zobair M. Younossi xiii Epidemiology and Natural History of NAFLD and NASH 1 Janus P. Ong and Zobair M. Younossi Understanding
Bariatric Surgery and Liver Transplantation
 Bariatric Surgery and Liver Transplantation Sammy Saab, MD, MPH, AGAF, FACG, FAASLD Professor of Medicine and Surgery Head, Outcomes Research in Hepatology David Geffen School of Medicine at UCLA Disclosures
Bariatric Surgery and Liver Transplantation Sammy Saab, MD, MPH, AGAF, FACG, FAASLD Professor of Medicine and Surgery Head, Outcomes Research in Hepatology David Geffen School of Medicine at UCLA Disclosures
Patients With NASH and Cryptogenic Cirrhosis Are Less Likely Than Those With Hepatitis C to Receive Liver Transplants
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2011;9:700 704 Patients With NASH and Cryptogenic Cirrhosis Are Less Likely Than Those With Hepatitis C to Receive Liver Transplants JACQUELINE G. O LEARY, CARMEN
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2011;9:700 704 Patients With NASH and Cryptogenic Cirrhosis Are Less Likely Than Those With Hepatitis C to Receive Liver Transplants JACQUELINE G. O LEARY, CARMEN
Citation Characteristics of Research Published in Emergency Medicine Versus Other Scientific Journals
 ORIGINAL CONTRIBUTION Citation Characteristics of Research Published in Emergency Medicine Versus Other Scientific From the Division of Emergency Medicine, University of California, San Francisco, CA *
ORIGINAL CONTRIBUTION Citation Characteristics of Research Published in Emergency Medicine Versus Other Scientific From the Division of Emergency Medicine, University of California, San Francisco, CA *
The EMA reflection paper on chronic liver disease and its implications for drug development in NASH
 The on chronic liver disease and its implications for drug development in NASH Content of the reflection paper and report from stakeholder meeting Elmer Schabel MD No conflict of interest. Content Overview:
The on chronic liver disease and its implications for drug development in NASH Content of the reflection paper and report from stakeholder meeting Elmer Schabel MD No conflict of interest. Content Overview:
M30 Apoptosense ELISA. A biomarker assay for detection and screening of NASH
 M30 Apoptosense ELISA A biomarker assay for detection and screening of NASH NASH A Global Disease In the Western countries, Non-Alcoholic Fatty Liver Disease (NAFLD) is the most common liver disease, strongly
M30 Apoptosense ELISA A biomarker assay for detection and screening of NASH NASH A Global Disease In the Western countries, Non-Alcoholic Fatty Liver Disease (NAFLD) is the most common liver disease, strongly
Beyond the liver in patients with non-alcoholic fatty liver disease (NAFLD) cause for concern?
 Editorial Beyond the liver in patients with non-alcoholic fatty liver disease (NAFLD) cause for concern? Matthew J. Armstrong 1,2, Geoffrey Haydon 1, Wing-Kin Syn 3,4 1 Liver Unit, Queen Elizabeth University
Editorial Beyond the liver in patients with non-alcoholic fatty liver disease (NAFLD) cause for concern? Matthew J. Armstrong 1,2, Geoffrey Haydon 1, Wing-Kin Syn 3,4 1 Liver Unit, Queen Elizabeth University
Consensus Statement on Ethnopharmacological Field Studies ConSEPTFS (Draft version)
 Consensus Statement on Ethnopharmacological Field Studies ConSEPTFS (Draft version) Prepared by Michael Heinrich (UCL School of Pharmacy, London, UK), Andreas Lardos (Zürich, CH); Marco Leonti (Univ. Cagliari,
Consensus Statement on Ethnopharmacological Field Studies ConSEPTFS (Draft version) Prepared by Michael Heinrich (UCL School of Pharmacy, London, UK), Andreas Lardos (Zürich, CH); Marco Leonti (Univ. Cagliari,
AASLD Immune tolerant phase HBV NAFLD diagnostic HCC
 AASLD 2016 Immune tolerant phase HBV NAFLD diagnostic HCC Immune tolerant 3 Modified from Chan HLY and Wong VWS. Hepatitis B. In Zakim and Boyers s Hepatology 2012 2015 AMERICAN ASSOCIATION FOR THE S1T6UDY
AASLD 2016 Immune tolerant phase HBV NAFLD diagnostic HCC Immune tolerant 3 Modified from Chan HLY and Wong VWS. Hepatitis B. In Zakim and Boyers s Hepatology 2012 2015 AMERICAN ASSOCIATION FOR THE S1T6UDY
Prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus patients in a tertiary care hospital of Bihar
 Original Research Article Prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus patients in a tertiary care hospital of Bihar Naresh Kumar 1, Jyoti Kumar Dinkar 2*, Chandrakishore
Original Research Article Prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus patients in a tertiary care hospital of Bihar Naresh Kumar 1, Jyoti Kumar Dinkar 2*, Chandrakishore
