Utilizing Seamless Adaptive Designs and Considering Multiplicity Adjustment for NASH Clinical Trials
|
|
|
- Adam Boyd
- 5 years ago
- Views:
Transcription
1 Utilizing Seamless Adaptive Designs and Considering Multiplicity Adjustment for NASH Clinical Trials Yeh-Fong Chen FDA/CDER/OB/DB3 Forum for Collaborative Research (Berkeley) 5/10/2017
2 Disclaimer This presentation reflects the views of the authors and should not be construed to represent the views or policies of the U.S. Food and Drug Administration. 2
3 Collaborators Feiran Jiao, Ph.D., FDA/CDER/OB/DB3 George Kordzakhia, Ph.D., FDA/CDER/OB/DB3 Min Min, Ph.D., FDA/CDER/OB/DB3 Lara Dimick, M.D., FDA/CDER/DGIEP 3
4 Clinical Trial Phases Objectives Phase 1: To test a new drug (or treatment), evaluate its safety, determine a safe dosage range, and identify side effects. Phase 2: To identify suitable dose(s) and further evaluate the safety of the study drug. Phase 3: To confirm the study drug s effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug to be used safely. Phase 4: To gather information on the drug's effect in various populations, including verifying and describing the clinical benefit and any side effects associated with long-term use after the drug has been approved and marketed. Source: Wikipedia 4
5 Conventional Phase 2 & Phase 3 5
6 Seamless Adaptive Designs 6
7 Clinical Trials in Pre-cirrhotic Non-alcoholic Steatohepatitis (NASH) NASH has been recognized as one of the leading causes of cirrhosis in adults and NASH-related cirrhosis is currently the second indication for liver transplants in the United States (Younossi et al., 2016). In a recently published study of 108 non-alcoholic fatty liver disease (NAFLD) patients who had serial biopsies, 47% of patients with NASH had a progression of fibrosis, and 18%, had spontaneous regression of fibrosis over a median follow-up period of 6.6 years (McPherson et al., 2015). Question: Can a clinical trial for treating NASH be conducted in less than 6.6 years? With innovative designs? 7
8 Four Types of Two-Stage Seamless Adaptive Designs Source: Chow SC and Lin M,
9 Analysis for Category II Adaptive Design 9
10 FDA Accelerated Approval Program Guidance for Industry-Expedited Programs for Serious Conditions Drugs & Biologics (FDA, 2014) states the following: FDA Guidance -Adaptive Designhttp:// uidances/ucm pdf.%20 10
11 Phase 3/4 Seamless Design for Accelerated Approval Phase 3 trial Placebo controlled Phase 4 trial Controlled for a Clinical Benefit Endpoint Results observed for a surrogate endpoint Full approval Submission of a marketing application IA* (SSR**) *Interim Analysis, ** Sample Size Re-estimation 11
12 Challenges with Accelerated Approval Trials Need to plan far in advance for the entire phase 3/4 trial with the Statistical Analysis Plan submitted prior to trial initiation Retention of patients in a placebo-controlled trial after marketing approval Placebo control is the best Potential for use of historical control, but lacking data for NASH at this time. Question: Can we consider other types of design? FDA Guidance for Industry E10 Choice of Control Group and Related Issues in Clinical Trials (FDA, 2001) 12
13 Primary Endpoint NASH Program Phase 3 (binary) Phase 4 (survival) Based on histological measurement Can be co-primary endpoints 1. Resolution of NASH without worsening of fibrosis 2. Reduction of fibrosis without worsening of NASH Time to all-cause mortality and liverrelated clinical outcomes, which may include but not limited to: Death (all cause) Model of end stage liver disease (MELD) score 15 Liver transplant Hospitalization 13
14 How to control overall Type I error rate? The overall alpha of 0.05 needs to be adjusted for the phase 3/4 trial Should we consider (α 3, α 4 )=(0.05, 0.05)? If only one single phase 3/4 trial is submitted for the marketing application, a much smaller alpha less than 0.05 may be considered (e.g., total p< ). FDA Guidance Providing Clinical Evidence of Effectiveness for Human Drug and Biological Products (FDA, 1998) 14
15 Proposed Phase 3/4 Seamless Adaptive Design for NASH Phase 3 Phase 4 R Drug Dose Placebo IA when p% of the population complete month 18 Efficacy based on histology: co-primary endpoints 1-Resolution of NASH without worsening of fibrosis 2-Reduction in fibrosis without worsening of NASH Composite endpoint for Phase 4 based on Progression to cirrhosis (histology) Overall mortality and liver clinical outcomes 15
16 Evaluation of Type I Error Controls In order to control the study-wise Type I error rate at 0.05, the following procedures are considered: 0.05 for both phases 3 and 4 (p 1 <0.05 & p 2 <0.05) 0.01 for phase 3 and 0.04 for phase 4 (p 1 <0.01 & p 2 <0.04) 0.01 for phase 3 (p 1 <0.01) 0.04 for phase 4 (p 2 <0.04) 0.01 for phase 3. If p 1 <0.01, then use 0.05, otherwise 0.04 for phase 4 (0.01/0.04/0.05) 16
17 Data Generation for Simulations Simulate two sets of repeated measurements by: y iik1 = α ik1 + B k1 + C j + ε iik1 z iik2 = α ik2 + B k2 + C j + ε iik2 i: treatment; j: subject; k: visit (k 1 = 5, k 2 = 10) α: treatment effect (fixed); B: visit effect (random); C: subject effect (random); ε: random error B MMM 0, V ; C~nnnnnn 0, σ c 2 ; ε nnnnnn 0, σ ε 2 Considering non-informative dropouts 17
18 Data Conversion 18
19 Example of Patient Profiles 19
20 n = 200/arm Simulation Settings Fixed effect parameter setup Replicate 10,000 times 20
21 Larger Biases With IA Conducted Earlier and More Dropouts 21
22 Type I Error Controlled But Conservative When C-statistic 22
23 Power Increases as C-statistic 23
24 Type I Error Rate When Dropout 24
25 Power When Dropout Rate 25
26 Case Example Two NASH trials are planned; one uses a phase 2/3/4 seamless design; the other one uses a phase 3/4 design. Question 1: What is a better approach to identifying safe and effective doses in phase 2 that can be further examined in phases 3 and 4? Consider interim efficacy or futility analysis? Question 2: Can the phase 4 data be combined from two separate trials and tested at α=0.05? No! 26
27 Considering Two Doses in Phase 3/4 We compared four procedures (1) Sequential: (α 3, α 4 )=(0.05/2, 0.05/2) for each dose (2) Bonferroni: (α 3, α 4 )=(0.01/2, 0.04/2) with a recycled alpha (3) Bonferoni 3 + Hochberg 4 If winning on both doses, the phase 4 uses Hochberg 0.05 If winning on only one dose, the phase 4 uses Hochberg If failing on both doses, the phase 4 uses Hochberg 0.04 (4) Hochberg 3&4 If winning on both doses, the phase 4 uses Hochberg 0.05 If winning on only one dose, the phase 4 uses Hochberg 0.04 If failing on both doses, the phase 4 uses Hochberg
28 Type I errors are controlled 28
29 Bonf 3 + Hoch 4 and Hochberg 3&4 are equally powerful 29
30 Hochberg is more powerful at phase 3 30
31 SED (Chen et al., 2014) for Handling Placebo Dropouts at Phase 4 For NASH trials, the placebo lead-in could be replaced by Vitamin E non-responders. 31
32 External Control in Phase 4 To address the issues (e.g., ethical concern, extensive dropouts) associated with the inclusion of a long term placebo arm To eliminate biases with external controls and to maintain the integrity matching methods (e.g., propensity scores) to optimize comparability of similar participating subjects Survival analyses to adjust for important baseline covariates May need to consider a conservative method for handling intermittent missing data in the external control arm 32
33 Take-Home Messages A two-stage seamless adaptive design can speed up the drug development process. We studied several procedures that aimed to control the overall Type I error rate. The sequential testing procedure is conservative but not powerful. When the surrogate and clinical endpoints are highly correlated, the studied procedures appear to be too conservative and thus more research is needed to enhance trial efficiency. To avoid bias due to potential extensive dropouts from placebo patients in phase 4, different designs including adding an external control can be considered, but the Statistical Analysis Plan needs to be specified prospectively. 33
34 References Chen YF, Zhang X., Tamura R, Chen C-M. (2014). A sequential enriched design for target patient population in psychiatric clinical trials, Statistics in Medicine, 33(17), Chow, S. C., & Lin, M. (2015). Analysis of Two-Stage Adaptive Seamless Trial Design. Pharmaceutica Analytica Acta, 6, 341. Hermansen, S. W. (2008). Evaluating predictive models: computing and interpreting the c statistic. In Data Mining and Predictive Modeling, SAS Global Forum (pp ). McPherson, S., Hardy, T., Henderson, E., Burt, A. D., Day, C. P., & Anstee, Q. M. (2015). Evidence of NAFLD progression from steatosis to fibrosingsteatohepatitis using paired biopsies: implications for prognosis and clinical management. Journal of hepatology, 62(5), Younossi, Z. M., Koenig, A. B., Abdelatif, D., Fazel, Y., Henry, L., & Wymer, M. (2016). Global epidemiology of nonalcoholic fatty liver disease meta analytic assessment of prevalence, incidence and outcomes. Hepatology, 24(1),
35 References (Continued) US Food and Drug Administration. (1998). Guidance for industry: Providing Clinical Evidence of Effectiveness for Human Drug and Biological Products. Silver Spring: US Food and Drug Administration. Retrieved September, 2, 2016 from tion/guidances/ucm pdf US Food and Drug Administration. (2001). Guidance for industry: E10 Choice of Control Group and Related Issues in Clinical Trials. Silver Spring: US Food and Drug Administration. Retrieved September, 2, 2016 from uidances/ucm pdf US Food and Drug Administration. (2014). Guidance for industry: Expedited programs for serious conditions drugs and biologics. Silver Spring: US Food and Drug Administration. Retrieved September, 2, 2016 from uidances/ucm pdf 35
36
SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms
 SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms Claudia Filozof, MD PhD Executive Medical Director Covance Challenges in Clinical Development in NASH
SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms Claudia Filozof, MD PhD Executive Medical Director Covance Challenges in Clinical Development in NASH
FDA Introductory Remarks Stephanie O. Omokaro, MD
 FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
NASH Regulatory Landscape. Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH
 NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
DEFINING THE REFERENCE STANDARD FOR BIOMARKERS IN NASH: HISTOLOGY VERSUS CLINICAL OUTCOMES
 DEFINING THE REFERENCE STANDARD FOR BIOMARKERS IN NASH: HISTOLOGY VERSUS CLINICAL OUTCOMES Kathleen M Donohue MD, MSc Clinical Team Lead Liver & Inborn Errors Division of Gastroenterology & Inborn Errors
DEFINING THE REFERENCE STANDARD FOR BIOMARKERS IN NASH: HISTOLOGY VERSUS CLINICAL OUTCOMES Kathleen M Donohue MD, MSc Clinical Team Lead Liver & Inborn Errors Division of Gastroenterology & Inborn Errors
Disease Burden of Non Alcoholic Fatty Liver Disease (NAFLD)
 Disease Burden of Non Alcoholic Fatty Liver Disease (NAFLD) Dr. H. Razavi May 31, 2017 First European NASH NAFLD Summit Disclosure: This work with funded by a multi-sponsored research grant from Intercept,
Disease Burden of Non Alcoholic Fatty Liver Disease (NAFLD) Dr. H. Razavi May 31, 2017 First European NASH NAFLD Summit Disclosure: This work with funded by a multi-sponsored research grant from Intercept,
PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD. Pierre Bedossa. Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE
 PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD Pierre Bedossa Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE 1 PATHOLOGY OF NAFLD NAFLD: a chronic liver disease with a wide
PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD Pierre Bedossa Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE 1 PATHOLOGY OF NAFLD NAFLD: a chronic liver disease with a wide
The EMA reflection paper on chronic liver disease and its implications for drug development in NASH
 The on chronic liver disease and its implications for drug development in NASH Content of the reflection paper and report from stakeholder meeting Elmer Schabel MD No conflict of interest. Content Overview:
The on chronic liver disease and its implications for drug development in NASH Content of the reflection paper and report from stakeholder meeting Elmer Schabel MD No conflict of interest. Content Overview:
FDA Regulatory Considerations for NASH Clinical Trial Endpoints Stephanie O. Omokaro, MD
 FDA Regulatory Considerations for NASH Clinical Trial Endpoints Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) U.S. Food and Drug Administration Center for Drug
FDA Regulatory Considerations for NASH Clinical Trial Endpoints Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) U.S. Food and Drug Administration Center for Drug
Volixibat for non-alcoholic steatohepatitis (NASH)
 NIHR Innovation Observatory Evidence Briefing: October 2017 Volixibat for non-alcoholic steatohepatitis (NASH) NIHRIO (HSRIC) ID: 10240 NICE ID: 9524 LAY SUMMARY Non-alcoholic steatohepatitis (NASH) is
NIHR Innovation Observatory Evidence Briefing: October 2017 Volixibat for non-alcoholic steatohepatitis (NASH) NIHRIO (HSRIC) ID: 10240 NICE ID: 9524 LAY SUMMARY Non-alcoholic steatohepatitis (NASH) is
Regulatory update from Europe:
 Regulatory update from Europe: Interim endpoints in phase 3 NASH trials Elmer Schabel MD The previously proposed/presented co-primary evaluation of two composite endpoints for the interim has been accepted:
Regulatory update from Europe: Interim endpoints in phase 3 NASH trials Elmer Schabel MD The previously proposed/presented co-primary evaluation of two composite endpoints for the interim has been accepted:
MEETING PROSPECTUS. International Workshop on NASH Biomarkers
 International Workshop on NASH Biomarkers 2018 18-19 May 2018 Washington, D.C., USA MEETING PROSPECTUS www.expertmedicaleducation.com www.expertmedicalevents.com ADVANCING HEALTH GLOBALLY THROUGH INNOVATIVE
International Workshop on NASH Biomarkers 2018 18-19 May 2018 Washington, D.C., USA MEETING PROSPECTUS www.expertmedicaleducation.com www.expertmedicalevents.com ADVANCING HEALTH GLOBALLY THROUGH INNOVATIVE
Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH
 Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology
Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology
Liver biopsy as the gold standard for diagnosis. Pierre BEDOSSA
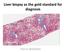 Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
SAF score and mortality in NAFLD after up to 41 years of follow-up
 SAF score and mortality in NAFLD after up to 41 years of follow-up Hannes Hagström, Patrik Nasr, Mattias Ekstedt, Stergios Kechagias, Per Stal, Pierre Bedossa and Rolf Hultcrantz Journal Article N.B.:
SAF score and mortality in NAFLD after up to 41 years of follow-up Hannes Hagström, Patrik Nasr, Mattias Ekstedt, Stergios Kechagias, Per Stal, Pierre Bedossa and Rolf Hultcrantz Journal Article N.B.:
Using Statistical Principles to Implement FDA Guidance on Cardiovascular Risk Assessment for Diabetes Drugs
 Using Statistical Principles to Implement FDA Guidance on Cardiovascular Risk Assessment for Diabetes Drugs David Manner, Brenda Crowe and Linda Shurzinske BASS XVI November 9-13, 2009 Acknowledgements
Using Statistical Principles to Implement FDA Guidance on Cardiovascular Risk Assessment for Diabetes Drugs David Manner, Brenda Crowe and Linda Shurzinske BASS XVI November 9-13, 2009 Acknowledgements
GR-MD-02 for Indication of NASH Cirrhosis: NASH-CX Clinical Trial Results
 GR-MD-02 for Indication of NASH Cirrhosis: NASH-C Clinical Trial Results Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 1 2018 2017 Galectin
GR-MD-02 for Indication of NASH Cirrhosis: NASH-C Clinical Trial Results Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 1 2018 2017 Galectin
Possibilities and risks associated with directly moving from Phase I to Phase III: Adaptive designs and Co
 Possibilities and risks associated with directly moving from Phase I to Phase III: Adaptive designs and Co Dominik Heinzmann, PhD Global Development Team Leader Breast/Gyn Cancer Associate Director Biostatistics,
Possibilities and risks associated with directly moving from Phase I to Phase III: Adaptive designs and Co Dominik Heinzmann, PhD Global Development Team Leader Breast/Gyn Cancer Associate Director Biostatistics,
Non-Alcoholic Fatty Liver Diseasean underestimated epidemic
 Non-Alcoholic Fatty Liver Diseasean underestimated epidemic Amir Shlomai MD,PhD Head, Department of Medicine D The Liver Institute Rabin Medical Center, Beilinson Hospital The IASLD semi-annual meeting-
Non-Alcoholic Fatty Liver Diseasean underestimated epidemic Amir Shlomai MD,PhD Head, Department of Medicine D The Liver Institute Rabin Medical Center, Beilinson Hospital The IASLD semi-annual meeting-
Clinical Trials & Endpoints in NASH Cirrhosis
 Clinical Trials & Endpoints in NASH Cirrhosis April 25, 2018 Peter G. Traber, MD CEO & CMO, Galectin Therapeutics 2018 Galectin Therapeutics NASDAQ: GALT For more information, see galectintherapeutics.com
Clinical Trials & Endpoints in NASH Cirrhosis April 25, 2018 Peter G. Traber, MD CEO & CMO, Galectin Therapeutics 2018 Galectin Therapeutics NASDAQ: GALT For more information, see galectintherapeutics.com
Accelerating Innovation in Statistical Design
 Implementing a National Cancer Clinical Trials System for the 21 st Century, Workshop #2 Session #5: Accelerating Innovation Through Effective Partnerships Accelerating Innovation in Statistical Design
Implementing a National Cancer Clinical Trials System for the 21 st Century, Workshop #2 Session #5: Accelerating Innovation Through Effective Partnerships Accelerating Innovation in Statistical Design
Evercore ISI Presentation- Madrigal
 Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH?
 WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH? Helena Cortez-Pinto Laboratório de Nutrição, FML, Serviço de Gastrenterologia, Hospital St Maria, Lisboa, Portugal EASL Governing Board:
WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH? Helena Cortez-Pinto Laboratório de Nutrição, FML, Serviço de Gastrenterologia, Hospital St Maria, Lisboa, Portugal EASL Governing Board:
Liver Pathology in the 0bese
 Liver Pathology in the 0bese Rob Goldin Centre for Pathology, Imperial College r.goldin@imperial.ac.uk Ludwig et al. Non-alcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease.
Liver Pathology in the 0bese Rob Goldin Centre for Pathology, Imperial College r.goldin@imperial.ac.uk Ludwig et al. Non-alcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease.
Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical
 Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical Disclosure Naim Alkhouri, MD discloses the following relationships with commercial companies: Membership in the Speakers Bureau for Alexion
Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical Disclosure Naim Alkhouri, MD discloses the following relationships with commercial companies: Membership in the Speakers Bureau for Alexion
Normal ALT for men 30 IU/L 36% US males abnormal. Abnl ALT. Assess alcohol use/meds. Recheck in 6-8 weeks. still pos
 Fatty liver disease Its not just for big boys anymore Ken Flora, MD, FAASLD, FACG, AGAF No disclosures Common situation Normal ALT for men 30 IU/L 36% US males abnormal Normal ALT for women 20 IU/L 28%
Fatty liver disease Its not just for big boys anymore Ken Flora, MD, FAASLD, FACG, AGAF No disclosures Common situation Normal ALT for men 30 IU/L 36% US males abnormal Normal ALT for women 20 IU/L 28%
Statistical Challenges for Novel Technologies. Roseann M. White, MA Director of Pragmatic Clinical Trial Statistics Duke Clinical Research Institute
 Statistical Challenges for Novel Technologies Roseann M. White, MA Director of Pragmatic Clinical Trial Statistics Duke Clinical Research Institute ISCTM 2016 Autumn Conference, Philadelphia, PA 27 September
Statistical Challenges for Novel Technologies Roseann M. White, MA Director of Pragmatic Clinical Trial Statistics Duke Clinical Research Institute ISCTM 2016 Autumn Conference, Philadelphia, PA 27 September
Clinical trial design issues and options for the study of rare diseases
 Clinical trial design issues and options for the study of rare diseases November 19, 2018 Jeffrey Krischer, PhD Rare Diseases Clinical Research Network Rare Diseases Clinical Research Network (RDCRN) is
Clinical trial design issues and options for the study of rare diseases November 19, 2018 Jeffrey Krischer, PhD Rare Diseases Clinical Research Network Rare Diseases Clinical Research Network (RDCRN) is
Accepted Manuscript. Nonalcoholic Fatty Liver Disease and Mortality. Norbert Stefan, MD
 Accepted Manuscript Nonalcoholic Fatty Liver Disease and Mortality Norbert Stefan, MD PII: S1542-3565(18)30161-7 DOI: 10.1016/j.cgh.2018.02.016 Reference: YJCGH 55704 To appear in: Clinical Gastroenterology
Accepted Manuscript Nonalcoholic Fatty Liver Disease and Mortality Norbert Stefan, MD PII: S1542-3565(18)30161-7 DOI: 10.1016/j.cgh.2018.02.016 Reference: YJCGH 55704 To appear in: Clinical Gastroenterology
Accelerating Phase II-III Oncology Drug Development Through the Use of Adaptive Designs
 Accelerating Phase II-III Oncology Drug Development Through the Use of Adaptive Designs - Jonathan R. Smith, Ph.D. June 15th, 2004, DIA Annual Meeting, Washington Outline of Presentation Oncology Background
Accelerating Phase II-III Oncology Drug Development Through the Use of Adaptive Designs - Jonathan R. Smith, Ph.D. June 15th, 2004, DIA Annual Meeting, Washington Outline of Presentation Oncology Background
Forward-looking Statements
 NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
NON-ALCOHOLIC FATTY LIVER DISEASE:
 NON-ALCOHOLIC FATTY LIVER DISEASE: ROLE OF THE PRIMARY PROVIDER Archita P. Desai, MD Assistant Professor of Medicine University of Arizona 25 th Annual Southwestern Conference on Medicine Outline Pathophysiology
NON-ALCOHOLIC FATTY LIVER DISEASE: ROLE OF THE PRIMARY PROVIDER Archita P. Desai, MD Assistant Professor of Medicine University of Arizona 25 th Annual Southwestern Conference on Medicine Outline Pathophysiology
XII Michelangelo Foundation Seminar
 XII Michelangelo Foundation Seminar Paradigm shift? The Food and Drug Administration collaborative project P. Cortazar, Silver Spring, USA FDA Perspective: Moving from Adjuvant to Neoadjuvant Trials in
XII Michelangelo Foundation Seminar Paradigm shift? The Food and Drug Administration collaborative project P. Cortazar, Silver Spring, USA FDA Perspective: Moving from Adjuvant to Neoadjuvant Trials in
NONALCOHOLIC FATTY LIVER DISEASE. Non-Alcoholic Fatty Liver Disease (NAFLD) Primary NAFLD. April 13, 2012
 NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Primary NAFLD Simple Steatosis Fatty hepatocytes Intracellular fat
NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Primary NAFLD Simple Steatosis Fatty hepatocytes Intracellular fat
EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease.
 Commentary. EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease. Christopher D. Byrne 1,2, Giovanni Targher 3 1 Nutrition and Metabolism, Faculty of Medicine,
Commentary. EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease. Christopher D. Byrne 1,2, Giovanni Targher 3 1 Nutrition and Metabolism, Faculty of Medicine,
DIAGNOSIS OF NASH LIVER BIOPSY. PHC
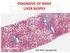 DIAGNOSIS OF NASH LIVER BIOPSY PHC 2018 - www.aphc.info 1 THE NATURAL HISTORY OF NAFLD STEATOSIS (NAFL) STEATOHEPATITIS (NASH) FIBROSIS CIRRHOSIS HCC UNDER THE LENS : THE 3 HISTOLOGICAL COMPONENTS OF NAFLD
DIAGNOSIS OF NASH LIVER BIOPSY PHC 2018 - www.aphc.info 1 THE NATURAL HISTORY OF NAFLD STEATOSIS (NAFL) STEATOHEPATITIS (NASH) FIBROSIS CIRRHOSIS HCC UNDER THE LENS : THE 3 HISTOLOGICAL COMPONENTS OF NAFLD
Practical Statistical Reasoning in Clinical Trials
 Seminar Series to Health Scientists on Statistical Concepts 2011-2012 Practical Statistical Reasoning in Clinical Trials Paul Wakim, PhD Center for the National Institute on Drug Abuse 10 January 2012
Seminar Series to Health Scientists on Statistical Concepts 2011-2012 Practical Statistical Reasoning in Clinical Trials Paul Wakim, PhD Center for the National Institute on Drug Abuse 10 January 2012
AAIM: GI Workshop Follow Up to Case Studies. Non-alcoholic Fatty Liver Disease Ulcerative Colitis Crohn s Disease
 AAIM: GI Workshop Follow Up to Case Studies Non-alcoholic Fatty Liver Disease Ulcerative Colitis Crohn s Disease Daniel Zimmerman, MD VP and Medical Director, RGA Global October 2015 Non-alcoholic Fatty
AAIM: GI Workshop Follow Up to Case Studies Non-alcoholic Fatty Liver Disease Ulcerative Colitis Crohn s Disease Daniel Zimmerman, MD VP and Medical Director, RGA Global October 2015 Non-alcoholic Fatty
Non-alcoholic fatty liver disease: time to take note and manage. Philip Newsome Professor of Hepatology & Director of Centre for Liver Research
 Non-alcoholic fatty liver disease: time to take note and manage Philip Newsome Professor of Hepatology & Director of Centre for Liver Research Disclosures Consultancy, Co-ordinating Investigator roles
Non-alcoholic fatty liver disease: time to take note and manage Philip Newsome Professor of Hepatology & Director of Centre for Liver Research Disclosures Consultancy, Co-ordinating Investigator roles
Forward-looking Statements
 Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding
Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding
Update on Non-Alcoholic Fatty Liver Disease. Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI
 Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) NON-ALCOHOLIC STEATOHEPATITIS (NASH) ADDRESSING A GROWING SILENT EPIDEMIC
 NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
EVALUATION REPORT
 International Workshop on NASH Biomarkers 2017 5-6 May 2017 Washington, D.C., EVALUATION REPORT www.expertmedicalevents.com/nashbiomarkers EXECUTIVE SUMMARY INTRODUCTION The 2 nd International Workshop
International Workshop on NASH Biomarkers 2017 5-6 May 2017 Washington, D.C., EVALUATION REPORT www.expertmedicalevents.com/nashbiomarkers EXECUTIVE SUMMARY INTRODUCTION The 2 nd International Workshop
EMA Workshop on Multiplicity Issues in Clinical Trials 16 November 2012, EMA, London, UK
 EMA Workshop on Multiplicity Issues in Clinical Trials 16 November 2012, EMA, London, UK (http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/events/2012/06/event_detai l_000589.jsp). Summary
EMA Workshop on Multiplicity Issues in Clinical Trials 16 November 2012, EMA, London, UK (http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/events/2012/06/event_detai l_000589.jsp). Summary
Bariatric Surgery and Liver Transplantation
 Bariatric Surgery and Liver Transplantation Sammy Saab, MD, MPH, AGAF, FACG, FAASLD Professor of Medicine and Surgery Head, Outcomes Research in Hepatology David Geffen School of Medicine at UCLA Disclosures
Bariatric Surgery and Liver Transplantation Sammy Saab, MD, MPH, AGAF, FACG, FAASLD Professor of Medicine and Surgery Head, Outcomes Research in Hepatology David Geffen School of Medicine at UCLA Disclosures
Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry
 Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
PEDIATRIC FOIE GRAS: NON-ALCOHOLIC FATTY LIVER DISEASE
 PEDIATRIC FOIE GRAS: NON-ALCOHOLIC FATTY LIVER DISEASE Updates on New insights into NAFLD and NASH pathophysiology New AASLD/AGA/ACG guidelines for NAFLD and NASH, as pertains to pediatrics Evidence-based
PEDIATRIC FOIE GRAS: NON-ALCOHOLIC FATTY LIVER DISEASE Updates on New insights into NAFLD and NASH pathophysiology New AASLD/AGA/ACG guidelines for NAFLD and NASH, as pertains to pediatrics Evidence-based
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) NON-ALCOHOLIC STEATOHEPATITIS (NASH) ADDRESSING A GROWING SILENT EPIDEMIC
 NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
Designing a Bayesian randomised controlled trial in osteosarcoma. How to incorporate historical data?
 Designing a Bayesian randomised controlled trial in osteosarcoma: How to incorporate historical data? C. Brard, L.V Hampson, M-C Le Deley, G. Le Teuff SCT - Montreal, May 18th 2016 Brard et al. Designing
Designing a Bayesian randomised controlled trial in osteosarcoma: How to incorporate historical data? C. Brard, L.V Hampson, M-C Le Deley, G. Le Teuff SCT - Montreal, May 18th 2016 Brard et al. Designing
ADDING VALUE AND EXPERTISE FOR SUCCESSFUL MARKET ACCESS. Per Sørensen, Lundbeck A/S
 ADDING VALUE AND EXPERTISE FOR SUCCESSFUL MARKET ACCESS Per Sørensen, Lundbeck A/S Market Access - price & reimbursement Situation today within psychiatric and neurological diseases Increased requirements
ADDING VALUE AND EXPERTISE FOR SUCCESSFUL MARKET ACCESS Per Sørensen, Lundbeck A/S Market Access - price & reimbursement Situation today within psychiatric and neurological diseases Increased requirements
AVOIDING BIAS AND RANDOM ERROR IN DATA ANALYSIS
 AVOIDING BIAS AND RANDOM ERROR IN DATA ANALYSIS Susan S. Ellenberg, Ph.D. Perelman School of Medicine University of Pennsylvania FDA Clinical Investigator Course Silver Spring, MD November 14, 2018 OVERVIEW
AVOIDING BIAS AND RANDOM ERROR IN DATA ANALYSIS Susan S. Ellenberg, Ph.D. Perelman School of Medicine University of Pennsylvania FDA Clinical Investigator Course Silver Spring, MD November 14, 2018 OVERVIEW
Disclosure. Objectives. Smash the Nash: A practical approach to fatty liver disease
 Smash the Nash: A practical approach to fatty liver disease Bruce D. Askey, MS, ANP-BC Associate Lecturer North Andover, MA Adult Nurse Practitioner Dept. of Hepatology/Gastroenterology Guthrie Clinic
Smash the Nash: A practical approach to fatty liver disease Bruce D. Askey, MS, ANP-BC Associate Lecturer North Andover, MA Adult Nurse Practitioner Dept. of Hepatology/Gastroenterology Guthrie Clinic
Bio Predictive. FibroTest Scientific Publications. Ratziu 2016 FibroMax NASH. Friedman 2016 FibroTest NASH EASL Key Publications for 2017
 EASL 2017 Scientific Publications Ratziu 2016 NASH Friedman 2016 NASH SteatoTest and sensitive markers of improvement in NASH trial using Elafibranor Elafibranor, an agonist of the peroxisome proliferator
EASL 2017 Scientific Publications Ratziu 2016 NASH Friedman 2016 NASH SteatoTest and sensitive markers of improvement in NASH trial using Elafibranor Elafibranor, an agonist of the peroxisome proliferator
Therapeutic Approaches to Cirrhotic versus Pre-Cirrhotic NASH
 www.alacrita.com Therapeutic Approaches to Cirrhotic versus Pre-Cirrhotic NASH 2nd Annual NASH Summit Europe October 23-24, 2018 Frankfort, Germany Peter G. Traber, MD Partner, Alacrita Consulting Alacrita
www.alacrita.com Therapeutic Approaches to Cirrhotic versus Pre-Cirrhotic NASH 2nd Annual NASH Summit Europe October 23-24, 2018 Frankfort, Germany Peter G. Traber, MD Partner, Alacrita Consulting Alacrita
UMHS-PUHSC JOINT INSTITUTE
 Role of Visceral Adiposity in the Pathogenesis of Non-Alcoholic Fatty Liver Disease in Lean versus Obese Patients: A Comparative Study between Patients at UMHS versus PUHSC Lai WEI and Anna LOK W Zhang,
Role of Visceral Adiposity in the Pathogenesis of Non-Alcoholic Fatty Liver Disease in Lean versus Obese Patients: A Comparative Study between Patients at UMHS versus PUHSC Lai WEI and Anna LOK W Zhang,
NAFLD/NASH. Definitions. Pathology NASH. Vicki Shah PA-C, MMS Rush University Hepatology
 NAFLD/NASH Vicki Shah PA-C, MMS Rush University Hepatology Definitions NAFLD Evidence of hepatic steatosis by histology (5%) or imaging No causes for secondary fat accumulation EtOH, Drugs, hereditary
NAFLD/NASH Vicki Shah PA-C, MMS Rush University Hepatology Definitions NAFLD Evidence of hepatic steatosis by histology (5%) or imaging No causes for secondary fat accumulation EtOH, Drugs, hereditary
Personalised medicine with state-of-the-art MR image analysis
 Personalised medicine with state-of-the-art MR image analysis Biomarkers as tests Diagnostic pathways in chronic disease Heart failure EKG BNP Echo/cardiac MRI COPD CXR Pulmonary function tests Chest CT
Personalised medicine with state-of-the-art MR image analysis Biomarkers as tests Diagnostic pathways in chronic disease Heart failure EKG BNP Echo/cardiac MRI COPD CXR Pulmonary function tests Chest CT
What are the regulatory issues. prevention trials?
 What are the regulatory issues that impact endpoints for prevention trials? Ann T. Farrell, M.D. Deputy Division Director Division of Drug Oncology Products Office of New Drugs Center for Drug Evaluation
What are the regulatory issues that impact endpoints for prevention trials? Ann T. Farrell, M.D. Deputy Division Director Division of Drug Oncology Products Office of New Drugs Center for Drug Evaluation
Defining the gold standard in biomarker validation for NASH
 Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
Corporate Presentation
 Corporate Presentation February 2, 2017 NASDAQ: GALT www.galectintherapeutics.com 2017 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains, in addition to historical information,
Corporate Presentation February 2, 2017 NASDAQ: GALT www.galectintherapeutics.com 2017 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains, in addition to historical information,
Diseases to Watch. Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-alcoholic
 Diseases to Watch Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-alcoholic steatohepatitis (NASH) - Prevalence and Symptoms - Risk Factors and Potential treatments - Target identification for NASH Robert
Diseases to Watch Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-alcoholic steatohepatitis (NASH) - Prevalence and Symptoms - Risk Factors and Potential treatments - Target identification for NASH Robert
Case #1. Digital Slides 11/6/ year old woman presented with abnormal liver function tests. Liver Biopsy to r/o autoimmune hepatitis
 45 year old woman presented with abnormal liver function tests Liver Biopsy to r/o autoimmune hepatitis Further down. ANA 1: 160; ASMA 1:80 ANA 1: 160; ASMA 1:80 IgG = 14.5 g/l (upper normal range: 16)
45 year old woman presented with abnormal liver function tests Liver Biopsy to r/o autoimmune hepatitis Further down. ANA 1: 160; ASMA 1:80 ANA 1: 160; ASMA 1:80 IgG = 14.5 g/l (upper normal range: 16)
Update on Nonalcoholic Fatty Liver Disease. Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic
 Update on Nonalcoholic Fatty Liver Disease Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic Outline Defining the phenotypes of nonalcoholic fatty liver disease NAFLD Diagnostics
Update on Nonalcoholic Fatty Liver Disease Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic Outline Defining the phenotypes of nonalcoholic fatty liver disease NAFLD Diagnostics
«STEATOSI EPATICA ED EPATOPATIE METABOLICHE» Ester Vanni Division of Gastroenterology University of Turin
 «STEATOSI EPATICA ED EPATOPATIE METABOLICHE» Ester Vanni Division of Gastroenterology University of Turin OUTLINE NAFLD overview NAFLD and menarche NAFLD and pregnancy NAFLD and menopause Other metabolic
«STEATOSI EPATICA ED EPATOPATIE METABOLICHE» Ester Vanni Division of Gastroenterology University of Turin OUTLINE NAFLD overview NAFLD and menarche NAFLD and pregnancy NAFLD and menopause Other metabolic
EVALUATION OF ABNORMAL LIVER TESTS
 EVALUATION OF ABNORMAL LIVER TESTS MIA MANABAT DO PGY6 MOA 119 TH ANNUAL SPRING SCIENTIFIC CONVENTION MAY 19, 2018 EVALUATION OF ABNORMAL LIVER TESTS Review of liver enzymes vs liver function tests Clinical
EVALUATION OF ABNORMAL LIVER TESTS MIA MANABAT DO PGY6 MOA 119 TH ANNUAL SPRING SCIENTIFIC CONVENTION MAY 19, 2018 EVALUATION OF ABNORMAL LIVER TESTS Review of liver enzymes vs liver function tests Clinical
INVESTOR PRESENTATION. November 16 th, 2015
 INVESTOR PRESENTATION November 16 th, 2015 1 Disclaimer Important information and forward looking statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE SINCE APRIL
INVESTOR PRESENTATION November 16 th, 2015 1 Disclaimer Important information and forward looking statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE SINCE APRIL
Ocaliva (obeticholic acid tablets)
 Ocaliva (obeticholic acid tablets) Policy Number: 5.01.619 Last Review: 11/2018 Origination: 11/2016 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Ocaliva (obeticholic acid tablets) Policy Number: 5.01.619 Last Review: 11/2018 Origination: 11/2016 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Statistics for Clinical Trials: Basics of Phase III Trial Design
 Statistics for Clinical Trials: Basics of Phase III Trial Design Gary M. Clark, Ph.D. Vice President Biostatistics & Data Management Array BioPharma Inc. Boulder, Colorado USA NCIC Clinical Trials Group
Statistics for Clinical Trials: Basics of Phase III Trial Design Gary M. Clark, Ph.D. Vice President Biostatistics & Data Management Array BioPharma Inc. Boulder, Colorado USA NCIC Clinical Trials Group
CORPORATE PRESENTATION
 CORPORATE PRESENTATION NASDAQ: GALT www.galectintherapeutics.com 1 Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within the meaning
CORPORATE PRESENTATION NASDAQ: GALT www.galectintherapeutics.com 1 Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within the meaning
Improving Access to Quality Medical Care Webinar Series
 Improving Access to Quality Medical Care Webinar Series Presented by The Arizona Telemedicine Program and the Southwest Telehealth Resource Center 2015 UA Board of Regents Welcome AZ, UT, CO, NM & NV FLEX
Improving Access to Quality Medical Care Webinar Series Presented by The Arizona Telemedicine Program and the Southwest Telehealth Resource Center 2015 UA Board of Regents Welcome AZ, UT, CO, NM & NV FLEX
Challenges in the Diagnosis of Steatohepatitis
 The Bugaboos of Fatty Liver Disease: Ballooning and Fibrosis Hans Popper Hepatopathology Society Companion Meeting San Antonio, Tx March, 2017 David Kleiner, M.D., Ph.D. NCI/Laboratory of Pathology Challenges
The Bugaboos of Fatty Liver Disease: Ballooning and Fibrosis Hans Popper Hepatopathology Society Companion Meeting San Antonio, Tx March, 2017 David Kleiner, M.D., Ph.D. NCI/Laboratory of Pathology Challenges
Liver Forum Cirrhosis Working Group Arun J. Sanyal
 Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
P values From Statistical Design to Analyses to Publication in the Age of Multiplicity
 P values From Statistical Design to Analyses to Publication in the Age of Multiplicity Ralph B. D Agostino, Sr. PhD Boston University Statistics in Medicine New England Journal of Medicine March 2, 2017
P values From Statistical Design to Analyses to Publication in the Age of Multiplicity Ralph B. D Agostino, Sr. PhD Boston University Statistics in Medicine New England Journal of Medicine March 2, 2017
M30 Apoptosense ELISA. A biomarker assay for detection and screening of NASH
 M30 Apoptosense ELISA A biomarker assay for detection and screening of NASH NASH A Global Disease In the Western countries, Non-Alcoholic Fatty Liver Disease (NAFLD) is the most common liver disease, strongly
M30 Apoptosense ELISA A biomarker assay for detection and screening of NASH NASH A Global Disease In the Western countries, Non-Alcoholic Fatty Liver Disease (NAFLD) is the most common liver disease, strongly
Fatty Liver Disease A growing epidemic
 Fatty Liver Disease A growing epidemic Updates in GIM for Primary Care Don C. Rockey March 9 th, 2018 Disclosures 2018 Research Funding (all to MUSC) NIH/NIDDK Actelion Pharmaceuticals Gilead Sciences
Fatty Liver Disease A growing epidemic Updates in GIM for Primary Care Don C. Rockey March 9 th, 2018 Disclosures 2018 Research Funding (all to MUSC) NIH/NIDDK Actelion Pharmaceuticals Gilead Sciences
An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications
 REVIEW An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications Naim Alkhouri, M.D.,*, and Andrea Scott, B.S.* Nonalcoholic fatty liver disease (NAFLD)
REVIEW An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications Naim Alkhouri, M.D.,*, and Andrea Scott, B.S.* Nonalcoholic fatty liver disease (NAFLD)
Big Data Training for Translational Omics Research. Session 1, Day 3, Liu. Case Study #2. PLOS Genetics DOI: /journal.pgen.
 Session 1, Day 3, Liu Case Study #2 PLOS Genetics DOI:10.1371/journal.pgen.1005910 Enantiomer Mirror image Methadone Methadone Kreek, 1973, 1976 Methadone Maintenance Therapy Long-term use of Methadone
Session 1, Day 3, Liu Case Study #2 PLOS Genetics DOI:10.1371/journal.pgen.1005910 Enantiomer Mirror image Methadone Methadone Kreek, 1973, 1976 Methadone Maintenance Therapy Long-term use of Methadone
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Supplementary Figure 1. Recursive partitioning using PFS data in patients with advanced NSCLC with non-squamous histology treated in the placebo pemetrexed arm of LUME-Lung 2. (A)
SUPPLEMENTARY MATERIAL Supplementary Figure 1. Recursive partitioning using PFS data in patients with advanced NSCLC with non-squamous histology treated in the placebo pemetrexed arm of LUME-Lung 2. (A)
NAFLD: evidence-based management. Curso de residentes AEEH Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain
 NAFLD: evidence-based management Curso de residentes AEEH 2017 Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain Clinical case - 55 yo female - Sent for incidental steatosis at abdominal
NAFLD: evidence-based management Curso de residentes AEEH 2017 Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain Clinical case - 55 yo female - Sent for incidental steatosis at abdominal
Research in Hepatology What is hot and what is not!
 Research in Hepatology What is hot and what is not! Anna SF Lok, MD University of Michigan Ann Arbor, MI, USA Please consider the environment before printing this PowerPoint Outline How to define hot?
Research in Hepatology What is hot and what is not! Anna SF Lok, MD University of Michigan Ann Arbor, MI, USA Please consider the environment before printing this PowerPoint Outline How to define hot?
Investigator Initiated Study Proposal Form
 Please submit completed form to IISReview@KCI1.com Date Submitted Name & Title Institution Address Phone Number Email Address Principal Investigator / Institution YES NO Multi Center Study Acelity Product(s)
Please submit completed form to IISReview@KCI1.com Date Submitted Name & Title Institution Address Phone Number Email Address Principal Investigator / Institution YES NO Multi Center Study Acelity Product(s)
Preface: Nonalcoholic Fatty Liver Disease: An Expanding Health Care Epidemic
 NASH and NAFLD Preface: Nonalcoholic Fatty Liver Disease: An Expanding Health Care Epidemic David E. Bernstein xiii Clinical and Economic Burden of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis
NASH and NAFLD Preface: Nonalcoholic Fatty Liver Disease: An Expanding Health Care Epidemic David E. Bernstein xiii Clinical and Economic Burden of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis
NAFLD 2017 Identifying and managing disease while waiting for a cure
 NAFLD 2017 Identifying and managing disease while waiting for a cure A. Sidney Barritt IV, MD, MSCR Associate Professor of Medicine UNC Liver Center High Impact Hepatology 4 November 2017 Disclosures I
NAFLD 2017 Identifying and managing disease while waiting for a cure A. Sidney Barritt IV, MD, MSCR Associate Professor of Medicine UNC Liver Center High Impact Hepatology 4 November 2017 Disclosures I
Methodological aspects of non-inferiority and equivalence trials
 Methodological aspects of non-inferiority and equivalence trials Department of Clinical Epidemiology Leiden University Medical Center Capita Selecta 09-12-2014 1 Why RCTs Early 1900: Evidence based on
Methodological aspects of non-inferiority and equivalence trials Department of Clinical Epidemiology Leiden University Medical Center Capita Selecta 09-12-2014 1 Why RCTs Early 1900: Evidence based on
Regulatory Considerations for Seamless Oncology Drug Development Expansion Cohorts
 Regulatory Considerations for Seamless Oncology Drug Development Expansion Cohorts National Cancer Policy Forum - The Drug Development Paradigm in Oncology: Managing Benefit and Risk in Seamless Cancer
Regulatory Considerations for Seamless Oncology Drug Development Expansion Cohorts National Cancer Policy Forum - The Drug Development Paradigm in Oncology: Managing Benefit and Risk in Seamless Cancer
1 ST EUROPEAN. NASH Workshop BARCELONA, SPAIN 8-9 MARCH 2019 REQUEST FOR SUPPORT.
 1 ST EUROPEAN NASH Workshop BARCELONA, SPAIN 8-9 MARCH 2019 REQUEST FOR SUPPORT NEEDS ASSESSMENT NEEDS ASSESSMENT Non-alcoholic fatty liver disease (NAFLD) is an emerging liver disease affecting 25 % of
1 ST EUROPEAN NASH Workshop BARCELONA, SPAIN 8-9 MARCH 2019 REQUEST FOR SUPPORT NEEDS ASSESSMENT NEEDS ASSESSMENT Non-alcoholic fatty liver disease (NAFLD) is an emerging liver disease affecting 25 % of
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Abetalipoproteinemia NASH and, 537 Acquired errors of metabolism NASH and, 536 537 Amiodarone steatohepatitis due to, 527 Anticonvulsant mood
Note: Page numbers of article titles are in boldface type. A Abetalipoproteinemia NASH and, 537 Acquired errors of metabolism NASH and, 536 537 Amiodarone steatohepatitis due to, 527 Anticonvulsant mood
Statistical considerations in indirect comparisons and network meta-analysis
 Statistical considerations in indirect comparisons and network meta-analysis Said Business School, Oxford, UK March 18-19, 2013 Cochrane Comparing Multiple Interventions Methods Group Oxford Training event,
Statistical considerations in indirect comparisons and network meta-analysis Said Business School, Oxford, UK March 18-19, 2013 Cochrane Comparing Multiple Interventions Methods Group Oxford Training event,
Steatotic liver disease
 Steatotic liver disease Fatty liver disease Prof. Dr. ANNE HOORENS Non-Neoplastic Liver Pathology December 8th 2018 Working Group of Digestive Pathology Belgian Society of Pathology OUTLINE NAFLD = Non-Alcoholic
Steatotic liver disease Fatty liver disease Prof. Dr. ANNE HOORENS Non-Neoplastic Liver Pathology December 8th 2018 Working Group of Digestive Pathology Belgian Society of Pathology OUTLINE NAFLD = Non-Alcoholic
Non-alcoholic fatty liver disease and associated risk factors among hemodialysis patients
 Non-alcoholic fatty liver disease and associated risk factors among hemodialysis patients Sima Golmohamadi Hamid Reza Omrani Nadia Asadi Lotfollah Asgari Zahra Fathi Department of Internal Medicine, School
Non-alcoholic fatty liver disease and associated risk factors among hemodialysis patients Sima Golmohamadi Hamid Reza Omrani Nadia Asadi Lotfollah Asgari Zahra Fathi Department of Internal Medicine, School
Estimands in oncology
 Estimands in oncology Steven Teerenstra 1,2 1 Radboud university Nijmegen medical center, NL 2 Statistical assessor at Medicines Evaluation Board (MEB), NL Disclaimer 2 Views expressed my own, based on
Estimands in oncology Steven Teerenstra 1,2 1 Radboud university Nijmegen medical center, NL 2 Statistical assessor at Medicines Evaluation Board (MEB), NL Disclaimer 2 Views expressed my own, based on
Innovative Analytic Approaches in the Context of a Global Pediatric IBD Drug Development
 Innovative Analytic Approaches in the Context of a Global Pediatric IBD Drug Development Margaret Gamalo-Siebers, PhD Global Statistical Sciences, Eli Lilly & Co. Pediatric Inflammatory Bowel Disease (IBD)
Innovative Analytic Approaches in the Context of a Global Pediatric IBD Drug Development Margaret Gamalo-Siebers, PhD Global Statistical Sciences, Eli Lilly & Co. Pediatric Inflammatory Bowel Disease (IBD)
Zhengtao Liu 1,2,3*, Shuping Que 4*, Lin Zhou 1,2,3 Author affiliation:
 Dose-response Relationship of Serum Uric Acid with Metabolic Syndrome and Non-alcoholic Fatty Liver Disease Incidence: AMeta-analysis of Prospective Studies Zhengtao Liu 1,2,3*, Shuping Que 4*, Lin Zhou
Dose-response Relationship of Serum Uric Acid with Metabolic Syndrome and Non-alcoholic Fatty Liver Disease Incidence: AMeta-analysis of Prospective Studies Zhengtao Liu 1,2,3*, Shuping Que 4*, Lin Zhou
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014
 Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
Preliminary clinical experience with Shear Wave Dispersion Imaging for liver viscosity
 Preliminary clinical experience with Shear Wave Dispersion Imaging for liver viscosity Dr. Katsutoshi Sugimoto Department of Gastroenterology and Hepatology, Tokyo Medical University, Japan Introduction
Preliminary clinical experience with Shear Wave Dispersion Imaging for liver viscosity Dr. Katsutoshi Sugimoto Department of Gastroenterology and Hepatology, Tokyo Medical University, Japan Introduction
Obesity, Inflammation and Liver Cancer
 Obesity, Inflammation and Liver Cancer Richard Moreau, M.D., 1 INSERM U773, Centre de Recherche Biomédicale Bichat-Beaujon CRB3, 2 Université Denis Diderot Paris 7, 3 Service d Hépatologie, Hôpital Beaujon,
Obesity, Inflammation and Liver Cancer Richard Moreau, M.D., 1 INSERM U773, Centre de Recherche Biomédicale Bichat-Beaujon CRB3, 2 Université Denis Diderot Paris 7, 3 Service d Hépatologie, Hôpital Beaujon,
NAFLD & NASH. Naga Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of GI and Hepatology
 NAFLD & NASH Naga Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of GI and Hepatology Indiana University School of Medicine ACG Midwest Regional Course,
NAFLD & NASH Naga Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of GI and Hepatology Indiana University School of Medicine ACG Midwest Regional Course,
Improving the Lives of Patients with Liver Diseases
 Improving the Lives of Patients with Liver Diseases Corporate Presentation March 2019 Safe Harbor Statement This presentation contains "forward-looking" statements that involve risks, uncertainties and
Improving the Lives of Patients with Liver Diseases Corporate Presentation March 2019 Safe Harbor Statement This presentation contains "forward-looking" statements that involve risks, uncertainties and
Nash with cirrhosis icd 10
 Free, official coding info for 2018 ICD-10-CM K75.81 - includes detailed crosswalks, DRG grouping and more. Free, official coding info for 2018 ICD-10-CM K74.69 - includes detailed crosswalks, DRG grouping
Free, official coding info for 2018 ICD-10-CM K75.81 - includes detailed crosswalks, DRG grouping and more. Free, official coding info for 2018 ICD-10-CM K74.69 - includes detailed crosswalks, DRG grouping
Non-Alcoholic Fatty Liver Disease
 Non-Alcoholic Fatty Liver Disease None Disclosures Arslan Kahloon M.D Chief, Division of Gastroenterology and Hepatology University of Tennessee College of Medicine Chattanooga Objectives Understand the
Non-Alcoholic Fatty Liver Disease None Disclosures Arslan Kahloon M.D Chief, Division of Gastroenterology and Hepatology University of Tennessee College of Medicine Chattanooga Objectives Understand the
Company Overview. September 2018 NASDAQ: MDGL
 Company Overview September 2018 NASDAQ: MDGL 1 Forward Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our future financial or business
Company Overview September 2018 NASDAQ: MDGL 1 Forward Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our future financial or business
