HHS Public Access Author manuscript Clin Gastroenterol Hepatol. Author manuscript; available in PMC 2016 April 13.
|
|
|
- Mabel Rich
- 6 years ago
- Views:
Transcription
1 Relationship between changes in serum keratin 18 and changes in liver histology in children and adults with nonalcoholic fatty liver disease Raj Vuppalanchi 1, Ajay K. Jain 2, Ross Deppe 1, Katherine Yates 3, Megan Comerford 1, Howard C. Masuoka 1, Brent A. Neuschwander-Tetri 2, Rohit Loomba 4, Elizabeth M. Brunt 5, David E. Kleiner 6, Jean P. Molleston 7, Jeffrey B. Schwimmer 8, Joel E. Lavine 9, James Tonascia 3, and Naga Chalasani 1 1 Department of Medicine, Indiana University School of Medicine, Indianapolis, IN 2 Saint Louis University, St. Louis, MO 3 Bloomberg School of Public Health, The Johns Hopkins University, Baltimore, MD 4 University of California, San Diego, San Diego, CA 5 Washington University School of Medicine, St. Louis, MO 6 Laboratory of Pathology, National Cancer Institute, Bethesda, MD 7 Department of Medicine, Indiana University School of Medicine, Indianapolis, IN 8 Department of Pediatrics University of California, San Diego and Department of Gastroenterology, Rady Children's Hospital, San Diego. CA 9 Department of Pediatrics, Columbia University, New York, NY Abstract HHS Public Access Author manuscript Published in final edited form as: Clin Gastroenterol Hepatol December ; 12(12): e1-2. doi: /j.cgh Background & Aims Previous cross-sectional studies have shown that serum keratin 18 (K18) fragment levels predict liver histology in individuals with nonalcoholic fatty liver disease (NAFLD). We conducted a study to examine the relationship between changes in serum K18 levels and changes in liver histology in both adults and children with NAFLD. Author for Correspondence: Naga Chalasani, MD, FACG, David W. Crabb Professor& Director, Division of Gastroenterology and Hepatology, Indiana University School of Medicine, 1050 Wishard Boulevard, RG 4100, Indianapolis, IN 46202, Tel (317) , Fax (317) Disclosures: None relevant for this manuscript. Writing Assistance: None Author Contributions: Study concept and design: RV, BN, RL, NC Acquisition of data: RD, MC, EMB, JS, DEK Analysis and interpretation of data: KY, RV, NC Drafting of the manuscript: RV, AJ, NC Critical revision of the manuscript for important intellectual content: HM, BN, RL, JT, EMB, JS, JM, JL, NCDEK, NC Statistical analysis: KY, JT Obtained funding: NC Administrative, technical, or material support: NC Study supervision: NC
2 Vuppalanchi et al. Page 2 Methods Serum K18 levels were measured at baseline and at various time points in 231 adults and 152 children with NAFLD who participated in two randomized controlled trials. Liver biopsies from baseline and week 96 were reviewed centrally. Results There was greater decrease in serum K18 levels in adults with histological improvement at week 96 than those without histological improvement at week 16 (-193 ± 293 vs -139 ± 467 U/L, p<0.001), week 48 (-232 ± 360 vs -113 ± 425 U/L, p<0.001), and week 96 (-269 ± 368 vs -97 ± 400 U/L, p<0.001). There was greater decrease in serum K18 levels in children with histological improvement than those without histological improvement at week 48 (-197 ± 467 vs.-47 ± 350 U/L, p=0.005) and week 96 (-206 ± 432 vs. -2 ± 474 U/L, p<0.001). However, decrease in serum K18 was not better than decrease in ALT in identifying histological improvement in adults (AUROC, 0.71 [0.63, 0.80] vs 0.68 [0.61, 0.79], p=0.34) or children (0.72 [ ] vs [ ], p=0.42). Conclusion Decrease in serum K18 levels is strongly associated with improvement in liver histology in adults or children with NAFLD. However, K18 decrease did not perform better than ALT improvement in identifying histological changes in NAFLD. Keywords Serum K18; noninvasive biomarker; non-alcoholic steatohepatitis; PIVENS; TONIC Introduction Nonalcoholic fatty liver disease (NAFLD) is highly prevalent in the Western world and is rapidly evolving into a global problem due to the ongoing epidemic of obesity. 1-6 Histologically, it is characterized by a spectrum ranging from fatty liver (NAFL), a relatively benign condition, to nonalcoholic steatohepatitis (NASH). The latter condition may progress to cirrhosis, liver failure or hepatocellular cancer. 7-9 Currently, NASH is a histological diagnosis and requires a liver biopsy for the initial characterization and subsequent disease monitoring. 10, 11 Currently, liver histologic evaluation is the primary end point for therapeutic trials, and in fact, the American Association for the Study of Liver Disease (AASLD) recommends liver histology as the primary end point for phase 2b and phase 3 clinical trials. 11 Although liver biopsy is a routinely performed procedure, it is invasive, expensive, and associated with rare complications. Therefore, there is intense interest to identify noninvasive methods to predict liver histology in individuals with NAFLD. Several previous studies have shown that NASH is associated with increased apoptosis The cytoskeletal system of the hepatocytes contains intermediate filament proteins primarily made up of the keratins, K8 (previously called CK8) and K18 (previously called CK18) which are important for the integrity and mechanical stability of the hepatocytes. 15 Induction of apoptosis in liver disease results in early cleavage of K18 by caspases. These fragments are stable to proteolysis and are released into the circulation after the hepatocyte plasma membrane disrupts during the later stages of the apoptotic process. Several studies have reported that caspase-cleaved K18 fragment levels are significantly higher in serum or plasma of individuals with NASH, and correlate with steatosis, lobular inflammation and ballooning Therefore, serum or plasma K18 fragment levels offer great potential as a
3 Vuppalanchi et al. Page 3 noninvasive indicator of liver histology in individuals with NAFLD. An earlier crosssectional study by the NASH Clinical Research Network (NASH CRN) showed that plasma K18 fragment levels independently predicted NASH in adults with well-characterized NAFLD (area under the receiver operator curve, AUROC, 0.83; 95% CI: ). 20 A recent meta-analysis of several published cross-sectional studies revealed a pooled AUROC of 0.82 ( ), median sensitivity of 78% and median specificity of 87% for K18 fragment levels to predict NASH in adults with NAFLD. 21 However, a recent study consisting of 424 overweight/obese middle aged individuals with (n=300) and without NAFLD (n=124) showed plasma K18 has a high specificity for NAFLD (83%) and fibrosis (85%) but not for NASH (68%), and more importantly, its sensitivity was modest for NAFLD (63%), NASH (58%) and fibrosis (54%). 22 In a multicenter study of 201 children and adults with biopsy-proven NAFLD, plasma K18 levels were an excellent predictor of NASH with an AUROC of 0.93 (95% CI: ). 16 While many studies were conducted to examine the cross-sectional relationship between serum or plasma K18 levels and liver histology in individuals with NAFLD, there is limited data in terms of their value in monitoring changes in liver histology. Therefore, we conducted a study to examine the relationship between longitudinal changes in serum K18 levels and changes in liver histology in adults and children with well-characterized NAFLD who participated in recently published PIVENS and TONIC clinical trials. Patients and Methods The PIVENS and the TONIC clinical trials were conducted by the NASH CRN and their study design and results have been published previously Briefly, the PIVENS evaluated the efficacy of daily pioglitazone (30mg) or vitamin E (800 IU), vs. placebo in 247 nondiabetic, adult patients with histologically defined NASH. 25 Its primary end point was overall improvement in liver histology at week 96 compared to baseline. 25 The TONIC trial evaluated vitamin E (800 IU/day), metformin (1000 mg/day) or placebo for 96 weeks in a total of 173 patients (aged 8-17 years) with biopsy-confirmed NAFLD. 26 While sustained reduction in serum alanine aminotransferase (ALT) was the primary end point, overall improvement in liver histology at week 96 as compared to baseline was an important secondary end point that demonstrated improvement with vitamin E. 26 All participants provided an informed consent prior to their participation in these clinical trials which allowed for subsequent ancillary studies on archived biosamples. For both studies, baseline and 96-week liver biopsy specimens were formalin-fixed, paraffinembedded, and unstained slides were cut from tissue blocks and sent to the NASH CRN repository for preparation by a central laboratory and review by the NASH CRN Pathology Committee (10 hepatopathologists blinded to all clinical and group assignment data). Liver histology was scored according to the previously published NASH CRN histological scoring system. 27 In these studies, the overall improvement in liver histology was defined as a 1 point improvement in the hepatocellular ballooning score; no increase in the fibrosis score; and either a decrease in the NAFLD activity score (NAS) to a score 3 or a decrease in the NAS of 2 points, with at least a 1-point decrease in either the lobular inflammation or steatosis score. 25
4 Vuppalanchi et al. Page 4 Statistical Analysis Serum samples used in the current study were aliquots (0.5mL) from the original samples where blood from fasting subjects was collected into serum-separator tubes, allowed to clot for at least 30 minutes at room temperature, and centrifuged at 1,800 g for 15 minutes at 4 C. Aliquots of serum were immediately frozen at -70 C. Processing was completed within 2 hours, and samples were free of hemolysis. The serum caspase-cleaved K18 fragment levels were determined using the M30-Apoptosense ELISA, per the manufacturer's instructions (Peviva AB, Bromma, Sweden). The absorbance was determined at 450nm with the Vmax Kinetic Microplate reader by Molecular Devices M2 (Sunnyvale, CA). The standard curve and K18 values were determined using the Soft-max Pro software accompanying the microplate reader. For the remainder of the manuscript, serum K18 fragment levels are referred to as serum K18 levels. In separate analyses for adults with NASH (PIVENS trial) and children with NAFLD (TONIC trial), we compared trends in K18 from baseline through the period of treatment subdivided in three classifications: 1.) treatment group (placebo, vitamin E, and pioglitazone in the adult patients and placebo, vitamin E, and metformin in the children); 2.) patients with and without histological improvement; and 3.) patients whose NASH did or did not improve after 96 weeks of treatment. We calculated means, standard deviations, and 95% confidence limits for K18 levels and changes from baseline K18 levels at baseline and at 16 (for adults) or 24 (for children), 48, 72, and 96 weeks of treatment. P values (2 sided) for the differences in the K18 time trends by treatment group, histological improvement, and NASH resolution were derived from three separate multiple linear regression models for K18 change during treatment in relation to independent variables as follows: baseline K18 level, indicator variables for the classification group of interest, spline-type indicator variables for each time period, and interaction terms to allow the between group differences to vary with time; these regression models also included the use of generalized estimating equations (GEE) with robust variance estimation to account for within-patient correlations in repeated K18 measures. The resulting P values were derived from an F-test of the hypothesis that the time trends were equal across groups and time, i.e; the classification group term and all of the interaction terms were zero. The primary aim of the paper was to evaluate K18, separately for adults and children, as a biomarker for histological improvement and resolution of NASH over a 96 week period of treatment. We did this in three ways: 1.) multiple logistic regression models to derive the probability of overall histological improvement (and separately for specific components of improvement) and the probability of NASH resolution after 96 weeks as a function of changes in K18 after 96 weeks of treatment, controlling for baseline K18 levels and treatment group (specific to either the PIVENS or TONIC trials); 2.) comparison of the classification performance of K18 alone against ALT alone, and with the combination of the K18 and ALT with respect to discriminating power between histological improvement vs. not improved and NASH resolution vs. not resolved in both adults and children using cross-validated (jackknifed) areas-under the receiver-operating characteristic (AUROC) and P-values for differences in AUROC for K18 vs. ALT and for K18+ALT vs. ALT alone models 28 ; 3.) Secondary comparisons of the sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) were also made. The Stata 12 software (StatCorp) and SAS version 9.3 (SAS Institute Inc) were used for the statistical
5 Vuppalanchi et al. Page 5 Results Study Population analyses. P values of 0.05 or less were pre-specified as statistically significant; since these analyses involve both primary and secondary comparisons of many models, it was not feasible to use methods for correcting P values for multiplicity of comparison due to loss of statistical power. Where applicable, Spearman's rank correlation coefficient was used to estimate the correlations between serum K18 or ALT. K18 means (SD) were compared for baseline and end of trial histology features, separately for adults and children, and P values were derived from linear regression of the rank of the K18 value on the histological feature. Out of 243 adult patients with NASH who participated in the PIVENS trial, 231 serum samples from baseline, 186 samples from week 16, 189 samples from week 48 and 184 samples from week 96 were available for the current study. Out of 173 children and adolescents who participated in the TONIC trial, 152 serum samples from baseline, 125 samples from week 24, 128 samples from week 48 and 136 samples from week 96 were available for the current study. Longitudinal Changes in serum K18 levels PIVENS Trial At baseline, serum K18 levels among the 3 treatment groups were similar (placebo: 440 ± 350 U/L, vitamin E: 510 ± 350 U/L, and pioglitazone: 490 ± 410 U/L, p=0.50). Compared to placebo, serum K18 levels among individuals treated with vitamin E were reduced at week 16 (mean change from baseline -160 ± 300 vs. -50 ± 380 U/L, p=0.10), week 48 (-220 ± 390 vs. -10 ± 370 U/L, p=0.049), and week 96 (-200 ± 400 vs. 30 ± 400 U/L, p< 0.001) (Fig 1A). Similarly, compared to placebo, serum K18 levels among individuals treated with pioglitazone were reduced at week 16 (mean change from baseline -240 ± 390 vs. -50 ± 380 U/L, p=0.006), week 48 (-180 ± 370 vs. -10 ± 370 U/L, p = 0.003, and week 96 (-260 ± 400 vs. 30 ± 400 U/L, p<0.001) (Fig 1A). In a separate analysis, serum K18 levels were compared between adults with overall histological improvement (n=71) and without such histological improvement (n=113), irrespective of the treatment group. At baseline, mean serum K18 levels in the responder and non-responder groups were similar (478 ± 342 vs. 553 ± 342 U/L, p= 0.20). During the treatment period, compared to non-responders, individuals with overall histological improvement had a significantly greater decrease at week 16 (mean change from baseline -193 ± 293 vs ± 467 U/L, p<0.001), week 48 (-232 ± 360 vs ± 425 U/L, P<0.001), and week 96 (-269 ± 368 vs. -97 ± 400 U/L, P<0.001) (Fig 1B). TONIC Trial Serum K18 levels were similar among three groups at baseline (placebo: 456 ± 365 U/L, vitamin E: 474 ± 358 U/L and metformin: 495 ± 482 U/L, p=0.88). They decreased from baseline at weeks 48 and 96 with both vitamin E and metformin treatment; however, change in K18 at all visits was not statistically significantly associated with either treatment (Fig 2A). Compared to children without an overall histological improvement, those with overall histological improvement had a greater mean decrease in serum K18
6 Vuppalanchi et al. Page 6 levels at week 24 (-140 ± 376 vs. -19 ± 387 U/L, p= 0.02), week 48 (-197 ± 467 vs. -47 ± 350 U/L, p=0.005) and week 96 (-206 ± 432 vs. -2 ± 474 U/L, p<0.001) (Fig 2B). Relationship between change in serum K18 and changes in other histological features In adult participants of the PIVENS trial, there was a significant relationship between decrease in serum K18 levels and resolution of NASH and improvement in steatosis, inflammation, ballooning and NAS. Table 1 shows the relative odds of overall histological improvement, resolution of NASH and 1 point improvement in other histological parameters for every 50 U/L, 150 U/L, and 250 U/L decrease in serum K18 levels over 96 weeks. In general, there was a step-wise increase in the strength of relationship between improvement in all histological parameters (except for lobular inflammation or fibrosis) and the extent of decrease in serum K18 level (Table 1). For example, odds ratios for the relationship between overall improvement in histology and 50U/L, 150 U/L, and 250 U/L decrease in serum K18 levels were 1.18, 1.63, and 2.67 respectively. There was a statistically significant association between decrease in serum K18 levels and changes in fibrosis stage at p=0.05 (Table 1). In children and adolescents who participated in the TONIC trial, there was a significant relationship between decrease in serum K18 levels and resolution of NASH and improvement in steatosis, inflammation, ballooning, fibrosis stage and NAS (Table 2). Table 2 shows the relative odds of overall histological improvement, resolution of NASH and 1 point improvement in other histological parameters for every 50 U/L, 150 U/L, and 250 U/L decrease in serum K18 levels over 96 weeks. In general, there was a step-wise increase in the strength of relationship between improvement in all histological parameters and the level of decrease in serum K18 level (Table 2). For example, odds ratios for the relationship between overall improvement in histology and 50U/L, 150 U/L, and 250 U/L decrease in serum K18 levels were 1.27, 2.07, and 3.35 respectively. Decreases in serum K18 levels were significantly associated with improvement in fibrosis stage as well (p=0.02) (Table 2). Supplemental Tables 1 and 2 show sensitivity, specificity, positive predictive value and negative predictive values for various arbitrary thresholds for both overall histological improvement and resolution of NASH in PIVENS and TONIC clinical trials, respectively. For example, in the PIVENS trial, 50 U/L decrease in serum K18 levels had 71% sensitivity and 49% specificity in predicting overall histological improvement whereas 250 U/L decrease had 55% specificity and 57% specificity. A decrease in serum K18 level by 250 U/L had 44% sensitivity and 71% specificity for children in the TONIC trial. Relationship between change in ALT levels with serum K18 fragment levels and histological outcomes Serum K18 and ALT levels correlated strongly at baseline (r=0.51, p<0.001) and at week 96 (r=0.54, p<0.001) in adult participants of the PIVENS trial, and similarly, change from baseline at 96 weeks in serum K18 also strongly correlated with changes in ALT (r=0.63, p<0.001). The relative odds of overall histological improvement and resolution of NASH with every 10 U/L decrease in ALT levels over 96 weeks were 1.31 (95% CI: 1.11, 1.53, p=0.001) and 1.26 (95% CI: 1.08, 1.47, p=0.003), respectively.
7 Vuppalanchi et al. Page 7 Both cross-sectional and longitudinal correlations between serum K18 and ALT level were statistically significant in pediatric participants of the TONIC trial. The correlation between serum K18 and ALT level at baseline was r=0.70 (p<0.001) and at 96 weeks was r= 0.72 (p<0.001). Similarly in the TONIC trial, there was a strong correlation between change in ALT and change in serum K18 levels between baseline and week 96 (r=0.75, p<0.001). The relative odds of overall histological improvement and resolution of NASH with every 10 U/L decrease in ALT levels over 96 weeks were 1.28 (95% CI: 1.14, 1.42, p<0.001) and 1.37 (95% CI: 1.19, 1.58, p<0.001), respectively. Comparison of associations of change in K18 and change in ALT with change in liver histology Table 3 shows comparisons among change in K18, change in ALT and their combination as discriminators of changes in liver histology in the PIVENS trial. Compared to change in ALT, either change in serum K18 or their combination did not have significantly higher AUROCs for discriminating overall histological improvement, resolution of NASH, or 1 point decrease in steatosis, lobular inflammation, hepatocyte ballooning, or fibrosis stage (Table 3). Table 4 shows comparisons among change in K18, change in ALT and their combination as predictors of changes in liver histology in the TONIC trial. While the AUROCs for change in K18, change in ALT, or their combination to discriminate the overall histological improvement were not significantly different, change in ALT was significantly better than change in K18 to discriminate resolution of NASH (AUROC 0.84 (95% CI: ) vs (95% CI: ), p=0.005) (Table 4). However, compared to change in ALT, either change in serum K18 or their combination did not have significantly higher AUROCs for discriminating 1 point decrease in steatosis, lobular inflammation, hepatocyte ballooning, or fibrosis stage in the TONIC trial (Table 4). Relationship between early decrease in serum K18 and histological change at week 96 Using multiple linear regression, log change in serum K18 levels at week 16 (ΔK18 is change in log(k18 (U/L) at week 16) from log(baseline K18 (U/L)) was significantly associated with overall histological improvement (coefficient 0.33 (95% CI -0.59, -0.06), p=0.02) and resolution of NASH at week 96 (coefficient 0.25 (95% CI -0.48, -0.02), p=0.04) among the PIVENS participants,. However, there were no significant associations between ΔK18 at week 16 and change in steatosis, lobular inflammation, ballooning, or NAS. In the TONIC trial, log change in serum K18 levels at week 24 (ΔK18 is change in log(k18 U/L at week 24) from log(baseline)) was significantly associated with resolution of NASH at week 96 (coefficient 0.32 (95% CI -0.60, -0.05), p=0.02), but not with overall histological improvement or change in NAS or its individual components (data not shown). Cross-sectional relationship between serum K18 levels and liver histology Table 5 shows the cross-sectional relationship between serum K18 levels and various histological features at baseline and at 96 weeks for both adults and children (Tables 5). In general, there was a stronger relationship between serum K18 levels and various histological variables at 96 weeks, possibly because serum samples for K18 measurement were obtained on the same day or very close to the biopsy date. In the PIVENS trial, the median (IQR) duration between liver biopsy and serum K18 measurement was 49 (23, 95) days at baseline
8 Vuppalanchi et al. Page 8 Discussion whereas it was only 2 (1, 9) days at week 96. Similarly, in the TONIC trial, the median (IQR) duration between liver biopsy and serum K18 fragment measurement was 29 (14, 58) days at baseline whereas 2 (1, 2) days at week 96. In adults, at baseline, serum K18 levels were significantly associated with lobular inflammation (p<0.001), hepatocellular ballooning (p<0.001) and fibrosis stage (P<0.001), but not steatosis (p=0.86). At week 96, serum K18 levels were significantly associated with steatosis (p<0.001), lobular inflammation (p<0.001), hepatocellular ballooning (p<0.001), fibrosis stage (P<0.001) and presence of steatohepatitis (p<0.001). Serum K18 levels had significant correlation with NAS with a much stronger relationship at week 96 (r=0.52, p<0.001) than at baseline (r=0.32, p<0.001). In children, serum K18 levels at baseline were significantly associated with lobular inflammation (p<0.001) and steatohepatitis (p=0.04) but not with steatosis (p=0.75). There was a trend towards significance for hepatocellular ballooning (p=0.05) and fibrosis stage (p=0.1). At week 96, serum K18 levels were significantly associated with steatosis (p<0.001), inflammation (p<0.001), hepatocellular ballooning (p<0.001), fibrosis stage (p<0.001) and steatohepatitis (p<0.001). Serum K18 levels had a significant correlation with NAS at week 96 (r=0.62, p<0.001), but not at baseline (r=0.14, p=0.08). The main observations of this study are (a) decreases in serum K18 levels are significantly associated with improvement in liver histology in both adults and children with histologically confirmed NAFLD; (b) changes in serum K18 did not discriminate change in liver histology better than changes in ALT; also, a combination of change in serum K18 and change in ALT did not discriminate change in liver histology better than change in ALT alone; (c) serum K18 levels correlated significantly with serum ALT levels at baseline and at 96 weeks and importantly changes in serum K18 levels correlated significantly with changes in ALT; and (d) serum K18 levels exhibited the strongest cross-sectional relationship with liver histology in both adults and children with NAFLD when the duration between liver biopsy and serum K18 measurement was shorter suggesting that some features of NASH may fluctuate over time periods of months. There is great need to develop noninvasive tests to predict changes in liver histology in individuals with NAFLD, both for clinical practice and for clinical trials. Serum ALT is commonly used as a surrogate for assessing liver histology in a variety of liver diseases, but its utility in grading and staging liver histology in NAFLD is limited. 25, 26 Similarly, improvement in ALT is not a highly accurate predictor of improvement in liver histology either. This unmet need prompted us to investigate the utility of calculating changes in serum K18 levels as a surrogate marker for monitoring liver histology in individuals participating in clinical trials. However, this analysis of sample obtained in two large clinical trials indicates that measuring serum K18 offers no advantage over ALT, either alone or in combination with ALT, and appears to have limited clinical utility as a standalone noninvasive test.
9 Vuppalanchi et al. Page 9 Although there has been much enthusiasm for investigating plasma/serum K18 as a noninvasive biomarker in NAFLD (3 meta-analyses already published), 21, 29, 30 there are many important knowledge gaps regarding our understanding of serum/plasma K18 behavior in various physiological and pathological states. For example, scant data exists regarding serum/plasma K18 levels in the control population and thus it is currently not possible to establish a normal range for this analyte. Our review of the literature produced 5 datasets consisting of 600 person reference population where K18 fragment (M30 antigen) levels were measured by Peviva's M30 Apoptosense ELISA; the median serum/plasma K18 levels ranged between U/L with 75 th percentile values ranging between U/L (Supplemental Table 1). 9-11, 22, 31 However, most subjects in these studies are largely Caucasian and their anthropometric and clinical characteristics were not described in detail. Furthermore, other characteristics of circulating K18 (e.g. day-to-day variability, effect of exercise, fasting, meals, inter-current illness, co-morbidities and medications such as statins) have not been investigated. Some aspects of our study deserve further discussion. By using samples and data from 400 children and adults with well characterized NAFLD whose liver histology at baseline and at 96 weeks was centrally reviewed in a blinded fashion by a group of expert hepatopathologists, this study represents a substantive investigation of longitudinal changes in K18 levels as a surrogate marker for monitoring liver histology in NAFLD. A potential limitation is that our study was conducted on stored biosamples from previously completed clinical trials. The effect of sample storage on K18 levels has not been examined and for this study, the sample collection and storage were conducted in a rigorous fashion according to a pre-established standard operating procedure. In this study, we only measured K18 fragments (M30 antigen) but not total K18 (uncleaved K18, M65 antigen) and thus our study cannot shed light on the utility of changes in total K18 as a surrogate for liver histology monitoring. However, in five cross-sectional studies reported to date with M65 antigen in individuals with NAFLD there is no indication that it is more useful than M30 antigen in predicting NASH. 30 In summary, we comprehensively investigated the relationship between changes in serum K18 levels and changes in liver histology in adults and children with well characterized NAFLD who underwent paired liver biopsies at least 96 weeks apart. There was a strong relationship between change in serum K18 levels and important histological outcomes, but their AUROCs were mostly under 0.85 indicating limited clinical utility. Moreover, changes in serum K18 levels did not perform better than changes in ALT in predicting changes in liver histology in NAFLD. Supplementary Material Acknowledgments Refer to Web version on PubMed Central for supplementary material. Grant Support: Administrative supplements awarded under the American Recovery and Reinvestment Act of 2009 (ARRA) for 3K24DK S1 to Dr. Chalasani.
10 Vuppalanchi et al. Page 10 References 1. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011; 34: [PubMed: ] 2. Bellentani S, Scaglioni F, Marino M, et al. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010; 28: [PubMed: ] 3. Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004; 40: [PubMed: ] 4. Gupta R, Bhangoo A, Matthews NA, et al. The prevalence of non-alcoholic fatty liver disease and metabolic syndrome in obese children. J Pediatr Endocrinol Metab. 2011; 24: [PubMed: ] 5. Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005; 288:E [PubMed: ] 6. Farrell GC, Wong VW, Chitturi S. NAFLD in Asia--as common and important as in the West. Nat Rev Gastroenterol Hepatol. 2013; 10: [PubMed: ] 7. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012; 107: [PubMed: ] 8. Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999; 116: [PubMed: ] 9. Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010; 51: [PubMed: ] 10. Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010; 52: [PubMed: ] 11. Sanyal AJ, Brunt EM, Kleiner DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011; 54: [PubMed: ] 12. Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004; 39: [PubMed: ] 13. Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003; 125: [PubMed: ] 14. Canbay A, Feldstein AE, Higuchi H, et al. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003; 38: [PubMed: ] 15. Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008; 129: [PubMed: ] 16. Feldstein AE, Alkhouri N, De Vito R, et al. Serum cytokeratin-18 fragment levels are useful biomarkers for nonalcoholic steatohepatitis in children. Am J Gastroenterol. 2013; 108: [PubMed: ] 17. Yilmaz Y, Dolar E, Ulukaya E, et al. Elevated serum levels of caspase-cleaved cytokeratin 18 (CK18-Asp396) in patients with nonalcoholic steatohepatitis and chronic hepatitis. C Med Sci Monit. 2009; 15:CR [PubMed: ] 18. Tsutsui M, Tanaka N, Kawakubo M, et al. Serum fragmented cytokeratin 18 levels reflect the histologic activity score of nonalcoholic fatty liver disease more accurately than serum alanine aminotransferase levels. J Clin Gastroenterol. 2010; 44: [PubMed: ] 19. Shen J, Chan HL, Wong GL, et al. Non-invasive diagnosis of non-alcoholic steatohepatitis by combined serum biomarkers. J Hepatol. 2012; 56: [PubMed: ] 20. Feldstein AE, Wieckowska A, Lopez AR, et al. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009; 50: [PubMed: ]
11 Vuppalanchi et al. Page 11 Abbreviations ALT 21. Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011; 43: [PubMed: ] 22. Cusi K, Chang Z, Harrison S, et al. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2014; 60: [PubMed: ] 23. Chalasani NP, Sanyal AJ, Kowdley KV, et al. Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemp Clin Trials. 2009; 30: [PubMed: ] 24. Lavine JE, Schwimmer JB, Molleston JP, et al. Treatment of nonalcoholic fatty liver disease in children: TONIC trial design. Contemp Clin Trials. 2010; 31: [PubMed: ] 25. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010; 362: [PubMed: ] 26. Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011; 305: [PubMed: ] 27. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005; 41: [PubMed: ] 28. Cui J. Estimating AIDS incidence and jack-knife variance from a continuous delay distribution and incomplete data. Stat Med. 1999; 18: [PubMed: ] 29. Chen J, Zhu Y, Zheng Q, et al. Serum cytokeratin-18 in the diagnosis of non-alcoholic steatohepatitis: A meta-analysis. Hepatol Res Kwok R, Tse YK, Wong GL, et al. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease - the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2014; 39: [PubMed: ] 31. Peviva. M30 Apoptosense ELISA, REF 10010, Instructions for Use Page Yagmur E, Trautwein C, Leers MP, et al. Elevated apoptosis-associated cytokeratin 18 fragments (CK18Asp386) in serum of patients with chronic liver diseases indicate hepatic and biliary inflammation. Clin Biochem. 2007; 40: [PubMed: ] 33. Deppe RB, Comerford M, Masuoka HC, et al. Serum CK18 but Not HMGB1 Levels Are Elevated in Adults With Primary Sclerosing Cholangitis. Gastroenterology. 2013; 144:S967 S Cortez-Pinto, Hea. Plasma cytokeratin 18 in a healthy population: How does it correlate with metabolic factors and presence of steatosis. Submitted to EASL AUROC K18 Keratin 18 NAS NAFLD NASH NASH CRN PIVENS TONIC Alanine aminotransferase Area Under the Receiver Operator Characteristic NAFLD Activity Score Nonalcoholic fatty liver disease Nonalcoholic steatohepatitis Nonalcoholic Steatohepatitis Clinical Research Network Pioglitazone versus vitamin E versus placebo for the treatment of nondiabetic patients with non-alcoholic steatohepatitis Treatment of NAFLD in Children
12 Vuppalanchi et al. Page 12 Figure 1. Changes in serum K18 levels in the PIVENS trial Panel A: Changes in serum K18 levels in three treatment groups (placebo, pioglitazone and vitamin E) during the PIVENS trial. Panel B: Changes in serum K18 levels in adults who achieved histological improvement as compared to those who did not achieve histological improvement in the PIVENS trial. Histological improvement was defined based on specified change in NAS and no progression of fibrosis as described in the text. Panel C: Changes in serum K18 levels in adults with and without resolution of NASH in the PIVENS trial.
13 Vuppalanchi et al. Page 13 Figure 2. Changes serum K18 fragment levels during the TONIC trial Panel A: Changes in serum K18 levels in three treatment groups (placebo, metformin and vitamin E) during the TONIC trial. Panel B: Changes in serum K18 levels in adults who achieved histological improvement as compared to those who did not achieve histological improvement in the TONIC trial. Histological improvement was defined based on change in NAS and no progression of fibrosis as described in the text. Panel C: Changes in serum K18 levels in adults with and without resolution of NASH in the TONIC trial.
14 Vuppalanchi et al. Page 14 Table 1 Association between change in serum K18 and change in liver histology in adults who participated in the PIVENS trial Relative Odds (95% CI) of improved histology per decrease in K18 over 96 weeks (N=174) * Histological Change over 96 weeks 50 U/L decrease 150 U/L decrease 250 U/L decrease P AUROC (95% CI) Overall histological improvement 1.18 (1.08, 1.29) 1.63 (1.24, 2.14) 2.67 (1.44, 3.57) < (0.63, 0.80) Resolution of NASH 1.14 (1.05, 1.25) 1.50 (1.16, 1.94) 1.97 (1.280, 3.02) (0.56, 0.73) 1 point improvement: Steatosis grade 1.20 (1.11, 1.30) 1.73 (1.35, 2.21) 2.49 (1.65, 3.76) < (0.67, 0.81) Lobular inflammation 1.04 (0.98, 1.11) 1.13 (0.95, 1.36) 1.23 (0.92, 1.67) (0.53, 0.70) Hepatocellular ballooning 1.16 (1.08, 1.26) 1.57 (1.32, 2.10) 2.13 (1.44, 3.16) < (0.58, 0.75) NAFLD activity score 1.18 (1.10, 1.28) 1.66 (1.20, 1.64) 2.33 (1.58, 3.44) < (0.69, 0.83) Fibrosis stage 1.07 (1.00, 1.15) 1.22 (1.00, 1.50) 1.41 (1.00, 1.97) (0.45, 0.62) * Patients with paired K18 scores (baseline and 96 weeks) and paired histology were included in analyses. Number in analyses=174 participants; for histological improvement model, number=141. Overall histological improvement response defined as 1 or more points in the ballooning score; no increase in the fibrosis score; and either a decrease in the NAS to a score of 3 points or less, or a decrease in the NAS of at least 2 points, with a 1 point decrease in either the lobular inflammation or the steatosis score. P determined from logistic regression of change in histological feature on change in K18 as a continuous variable after controlling for baseline K18 level and the treatment group. Cross-validated area under the receiver operating characteristic (AUROC) curves using a jackknife procedure were used.
15 Vuppalanchi et al. Page 15 Table 2 Association between change in serum K18 and change in liver histology in children who participated in the TONIC trial Relative Odds (95% CI) of improved histology per decrease in K18 over 96 weeks (N=120) * Histological Change over 96 weeks 50 U/L decrease 150 U/L decrease 250 U/L decrease P AUROC (95% CI) Overall histological improvement 1.27 (1.12, 1.45) 2.07 (1.41, 3.03) 3.35 (1.78, 6.33) < (0.63, 0.81) Resolution of NASH 1.17 (1.06, 1.29) 1.59 (1.18, 2.14) 2.16 (1.31, 3.56) (0.58, 0.79) 1 point improvement: Steatosis grade 1.13 (1.05, 1.22) 1.44 (1.16, 1.80) 1.85 (1.285, 2.66) (0.54, 0.74) Lobular inflammation 1.09 (1.02, 1.16) 1.28 (1.06, 1.56) 1.52 (1.10, 2.10) (0.46, 0.67) Hepatocellular ballooning 1.09 (1.01, 1.17) 1.29 (1.04, 1.60) 1.53 (1.07, 2.19) (0.54, 0.74) NAFLD activity score 1.24 (1.13, 1.36) 1.89 (1.43, 2.49) 2.89 (1.82, 4.59) < (0.64, 0.84) Fibrosis stage 1.09 (1.02, 1.17) 1.30 (1.05, 1.61) 1.55 (1.09, 2.21) (0.42, 0.63) * Patients with paired K-18 scores (baseline and 96 weeks) and paired histology were included in analyses. Number in analyses=120 participants; for NASH resolution models, number=99. Overall histological improvement response defined as a decrease in the NAS of at least 2 points and no increase in the fibrosis score P-value was determined from logistic regression of change in histological feature on change in K18 as a continuous variable after controlling for baseline K18 level and the treatment group. Cross-validated area under the receiver operating characteristic (AUROC) curves using a jackknife procedure were used.
16 Vuppalanchi et al. Page 16 Table 3 Comparison between changes in serum K18 and serum ALT in identifying changes in liver histology in adult participants of the PIVENS trial AUROC (95% C.I.) P-value Histological Change over 96 weeks Change in K18 Change in ALT Changes in K18 + ALT K18 vs ALT K18+ ALT vs ALT Overall histological improvement 0.71 (0.63, 0.80) 0.68 (0.59, 0.76) 0.70 (0.61, 0.79) Resolution of NASH 0.64 (0.56, 0.73) 0.67 (0.59, 0.75) 0.68 (0.59, 0.76) point improvement: Steatosis grade 0.74 (0.67, 0.81) 0.76 (0.69, 0.84) 0.77 (0.70, 0.84) Lobular inflammation 0.62 (0.53, 0.70) 0.65 (0.56, 0.73) 0.64 (0.56, 0.73) Hepatocellular ballooning 0.67 (0.58, 0.75) 0.60 (0.51, 0.69) 0.65 (0.57, 0.73) NAFLD activity score 0.76 (0.69, 0.83) 0.76 (0.69, 0.86) 0.77 (0.70, 0.84) Fibrosis stage 0.53 (0.45, 0.62) 0.61 (0.52, 0.70) 0.57 (0.49, 0.66) * Participants with paired K18 scores (baseline and 96 weeks), paired ALT measures and paired histology were included in analyses (N=174). Overall histological improvement response defined as 1 or more points in the ballooning score; no increase in the fibrosis score; and either a decrease in the NAS to a score of 3 points or less, or a decrease in the NAS of at least 2 points, with a 1 point decrease in either the lobular inflammation or the steatosis score. Cross-validated (jackknifed) areas-under the receiver-operating characteristic (AUROC) and P-values for differences in AUROC for K18 vs. ALT and for K18+ALT vs. ALT alone models are presented.
17 Vuppalanchi et al. Page 17 Table 4 Comparison between changes in serum K18 and serum ALT in identifying changes in liver histology in pediatric participants of the TONIC trial AUROC (95% C.I.) P-value Histological Change over 96 weeks Change in K18 Change in ALT Changes in K18 + ALT K18 vs ALT K18+ ALT vs ALT Overall histological improvement 0.72 (0.63, 0.81) 0.79 (0.70, 0.87) 0.79 (0.71, 0.87) Resolution of NASH 0.69 (0.58, 0.79) 0.84 (0.76, 0.93) 0.83 (0.75, 0.92) Improvement of at least 1 point: Steatosis grade 0.64 (0.54, 0.74) 0.72 (0.63, 0.81) 0.71 (0.61, 0.80) Lobular inflammation 0.57 (0.46, 0.67) 0.58 (0.48, 0.69) 0.55 (0.44, 0.66) Hepatocellular ballooning 0.64 (0.54, 0.74) 0.68 (0.59, 0.78) 0.66 (0.56, 0.76) NAFLD activity score 0.74 (0.64, 0.84) 0.81 (0.73, 0.89) 0.80 (0.72, 0.89) Fibrosis stage 0.52 (0.42, 0.62) 0.54 (0.43, 0.64) 0.50 (0.39, 0.60) * Participants with paired K-18 scores (baseline and 96 weeks), paired ALT measures and paired histology were included in analyses (N=117). Overall histological improvement response defined as no increase in the fibrosis score; and a decrease in the NAS of at least 2 points. Cross-validated (jackknifed) areas-under the receiver-operating characteristic (AUROC) and P-values for differences in AUROC for K18 vs. ALT and for K18+ALT vs. ALT alone models are presented.
18 Vuppalanchi et al. Page 18 Steatosis Table 5 Cross-sectional relationship between serum K18 level and histological features of NAFLD at baseline and end of trial K18 levels at baseline (U/L, mean±sd) P * K18 levels at end of trial (U/L, mean±sd) P * PIVENS <33% 497 ± ± 270 < % 467 ± ± 319 Lobular Inflammation < 2 foci 395 ± 337 < ± 253 < foci 529 ± ± 365 Ballooning None to few 344 ± 208 < ± 205 <0.001 Many 628 ± ± 342 Fibrosis None 261 ± ± 190 <0.001 Mild 461 ± 352 < ± 262 Moderate 545 ± ± 313 Bridging/Cirrhosis 575 ± ± 389 Steatohepatitis None 238 ± 160 < ±173 <0.001 Borderline 362 ± ± 141 Definite 539 ± ± 340 Steatosis TONIC <33% 519 ± ± 249 < % 458 ± ± 366 Lobular Inflammation < 2 foci 361 ± ± 212 < foci 570 ± ± 397 Ballooning None to few 452 ± ± 245 <0.001 Many 550 ± ± 393 Fibrosis None 489 ± ± 223 <0.001 Mild 373 ± ± 347
19 Vuppalanchi et al. Page 19 K18 levels at baseline (U/L, mean±sd) P * K18 levels at end of trial (U/L, mean±sd) P * Moderate 458 ± ± 400 Bridging 800 ± ± 335 Steatohepatitis <0.001 None 384± ± 219 Borderline 433± ± 241 Definite 557± ± 356 * No. of participants: PIVENS (N=231 at baseline, N=182 at 96 weeks); TONIC (N=152 at baseline, N=134 at 96 weeks); P (2-sided) determined from linear regression of the rank of the K18 value on the histological feature Although all PIVENS participants had steatohepatitis at entry based on local pathologist's assessment, 25 individuals were deemed not to have sufficient evidence of steatohepatitis upon central review. 4 PIVENS and no TONIC participants were diagnosed with cirrhosis at baseline; and 9 PIVENS and no TONIC participants diagnosed with cirrhosis at end of trial (96 weeks).
M30 Apoptosense ELISA. A biomarker assay for detection and screening of NASH
 M30 Apoptosense ELISA A biomarker assay for detection and screening of NASH NASH A Global Disease In the Western countries, Non-Alcoholic Fatty Liver Disease (NAFLD) is the most common liver disease, strongly
M30 Apoptosense ELISA A biomarker assay for detection and screening of NASH NASH A Global Disease In the Western countries, Non-Alcoholic Fatty Liver Disease (NAFLD) is the most common liver disease, strongly
Nonalcoholic fatty liver disease (NAFLD) is the most common
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2008;6:1249 1254 Cytokeratin 18 Fragment Levels as a Noninvasive Biomarker for Nonalcoholic Steatohepatitis in Bariatric Surgery Patients DIMA L. DIAB,* LISA YERIAN,
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2008;6:1249 1254 Cytokeratin 18 Fragment Levels as a Noninvasive Biomarker for Nonalcoholic Steatohepatitis in Bariatric Surgery Patients DIMA L. DIAB,* LISA YERIAN,
Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical
 Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical Disclosure Naim Alkhouri, MD discloses the following relationships with commercial companies: Membership in the Speakers Bureau for Alexion
Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical Disclosure Naim Alkhouri, MD discloses the following relationships with commercial companies: Membership in the Speakers Bureau for Alexion
CDHNF & NASPGHAN A Partnership for Research and Education for Children s Digestive and Nutritional Health
 CDHNF & NASPGHAN A Partnership for Research and Education for Children s Digestive and Nutritional Health Obesity and NAFLD Definitions: Nonalcoholic steatohepatitis (NASH) and nonalcoholic fatty liver
CDHNF & NASPGHAN A Partnership for Research and Education for Children s Digestive and Nutritional Health Obesity and NAFLD Definitions: Nonalcoholic steatohepatitis (NASH) and nonalcoholic fatty liver
Update on Non-Alcoholic Fatty Liver Disease. Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI
 Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
PEDIATRIC FOIE GRAS: NON-ALCOHOLIC FATTY LIVER DISEASE
 PEDIATRIC FOIE GRAS: NON-ALCOHOLIC FATTY LIVER DISEASE Updates on New insights into NAFLD and NASH pathophysiology New AASLD/AGA/ACG guidelines for NAFLD and NASH, as pertains to pediatrics Evidence-based
PEDIATRIC FOIE GRAS: NON-ALCOHOLIC FATTY LIVER DISEASE Updates on New insights into NAFLD and NASH pathophysiology New AASLD/AGA/ACG guidelines for NAFLD and NASH, as pertains to pediatrics Evidence-based
In Search of New Biomarkers for Nonalcoholic Fatty Liver Disease
 REVIEW In Search of New Biomarkers for Nonalcoholic Fatty Liver Disease Ting-Ting Chan, M.R.C.P., and Vincent Wai-Sun Wong, M.D. Nonalcoholic fatty liver disease (NAFLD) affects 15% to 40% of the general
REVIEW In Search of New Biomarkers for Nonalcoholic Fatty Liver Disease Ting-Ting Chan, M.R.C.P., and Vincent Wai-Sun Wong, M.D. Nonalcoholic fatty liver disease (NAFLD) affects 15% to 40% of the general
NONALCOHOLIC FATTY LIVER DISEASE. Non-Alcoholic Fatty Liver Disease (NAFLD) Primary NAFLD. April 13, 2012
 NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Primary NAFLD Simple Steatosis Fatty hepatocytes Intracellular fat
NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Primary NAFLD Simple Steatosis Fatty hepatocytes Intracellular fat
NON-ALCOHOLIC FATTY LIVER DISEASE:
 NON-ALCOHOLIC FATTY LIVER DISEASE: ROLE OF THE PRIMARY PROVIDER Archita P. Desai, MD Assistant Professor of Medicine University of Arizona 25 th Annual Southwestern Conference on Medicine Outline Pathophysiology
NON-ALCOHOLIC FATTY LIVER DISEASE: ROLE OF THE PRIMARY PROVIDER Archita P. Desai, MD Assistant Professor of Medicine University of Arizona 25 th Annual Southwestern Conference on Medicine Outline Pathophysiology
Challenges in the Diagnosis of Steatohepatitis
 The Bugaboos of Fatty Liver Disease: Ballooning and Fibrosis Hans Popper Hepatopathology Society Companion Meeting San Antonio, Tx March, 2017 David Kleiner, M.D., Ph.D. NCI/Laboratory of Pathology Challenges
The Bugaboos of Fatty Liver Disease: Ballooning and Fibrosis Hans Popper Hepatopathology Society Companion Meeting San Antonio, Tx March, 2017 David Kleiner, M.D., Ph.D. NCI/Laboratory of Pathology Challenges
Nonalcoholic Fatty Liver Disease: Definitions, Risk Factors, and Workup
 REVIEW REVIEW Nonalcoholic Fatty Liver Disease: Definitions, Risk Factors, and Workup Puneet Puri, M.B.B.S., M.D. and Arun J. Sanyal, M.B.B.S., M.D. Nonalcoholic fatty liver disease (NAFLD) is defined
REVIEW REVIEW Nonalcoholic Fatty Liver Disease: Definitions, Risk Factors, and Workup Puneet Puri, M.B.B.S., M.D. and Arun J. Sanyal, M.B.B.S., M.D. Nonalcoholic fatty liver disease (NAFLD) is defined
What is NAFLD?.NASH? Presenter Disclosure Information. Learning Objectives. Case 1: Rob. Questions Pertinent to Rob
 Presenter Disclosure Information 5 6pm Nonalcoholic Fatty Liver Disease (NAFLD): Another Obesity-Related Epidemic SPEAKER Elliot Tapper, MD The following relationships exist related to this presentation:
Presenter Disclosure Information 5 6pm Nonalcoholic Fatty Liver Disease (NAFLD): Another Obesity-Related Epidemic SPEAKER Elliot Tapper, MD The following relationships exist related to this presentation:
Normal ALT for men 30 IU/L 36% US males abnormal. Abnl ALT. Assess alcohol use/meds. Recheck in 6-8 weeks. still pos
 Fatty liver disease Its not just for big boys anymore Ken Flora, MD, FAASLD, FACG, AGAF No disclosures Common situation Normal ALT for men 30 IU/L 36% US males abnormal Normal ALT for women 20 IU/L 28%
Fatty liver disease Its not just for big boys anymore Ken Flora, MD, FAASLD, FACG, AGAF No disclosures Common situation Normal ALT for men 30 IU/L 36% US males abnormal Normal ALT for women 20 IU/L 28%
The role of non-invasivemethods in evaluating liver fibrosis of patients with non-alcoholic steatohepatitis
 The role of non-invasivemethods in evaluating liver fibrosis of patients with non-alcoholic steatohepatitis Objectives: Liver biopsy is the gold standard for diagnosing the extent of fibrosis in NAFLD/NASH;
The role of non-invasivemethods in evaluating liver fibrosis of patients with non-alcoholic steatohepatitis Objectives: Liver biopsy is the gold standard for diagnosing the extent of fibrosis in NAFLD/NASH;
NONALCOHOLIC FATTY LIVER DISEASE
 NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD, MSc Hepatology and Liver Transplantation University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Terminology Pathogenesis
NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD, MSc Hepatology and Liver Transplantation University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Terminology Pathogenesis
NAFLD & NASH. Naga Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of GI and Hepatology
 NAFLD & NASH Naga Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of GI and Hepatology Indiana University School of Medicine ACG Midwest Regional Course,
NAFLD & NASH Naga Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of GI and Hepatology Indiana University School of Medicine ACG Midwest Regional Course,
Non-Alcoholic Fatty Liver Disease (NAFLD)
 HEPATO-PANCREATO-BILIARY STOMACH CANCER PROGRAM Non-Alcoholic Fatty Liver Disease (NAFLD) Steatosis and Non-Alcoholic Steatohepatitis (NASH) Management Recommendations UCSF Fresno Department of Surgery
HEPATO-PANCREATO-BILIARY STOMACH CANCER PROGRAM Non-Alcoholic Fatty Liver Disease (NAFLD) Steatosis and Non-Alcoholic Steatohepatitis (NASH) Management Recommendations UCSF Fresno Department of Surgery
Postmenopausal women are at an increased risk
 AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 64, NO. 1, 2016 A Longer Duration of Estrogen Deficiency Increases Fibrosis Risk Among Postmenopausal Women With Nonalcoholic Fatty
AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 64, NO. 1, 2016 A Longer Duration of Estrogen Deficiency Increases Fibrosis Risk Among Postmenopausal Women With Nonalcoholic Fatty
WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH?
 WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH? Helena Cortez-Pinto Laboratório de Nutrição, FML, Serviço de Gastrenterologia, Hospital St Maria, Lisboa, Portugal EASL Governing Board:
WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH? Helena Cortez-Pinto Laboratório de Nutrição, FML, Serviço de Gastrenterologia, Hospital St Maria, Lisboa, Portugal EASL Governing Board:
Non-Alcoholic Fatty Liver Diseasean underestimated epidemic
 Non-Alcoholic Fatty Liver Diseasean underestimated epidemic Amir Shlomai MD,PhD Head, Department of Medicine D The Liver Institute Rabin Medical Center, Beilinson Hospital The IASLD semi-annual meeting-
Non-Alcoholic Fatty Liver Diseasean underestimated epidemic Amir Shlomai MD,PhD Head, Department of Medicine D The Liver Institute Rabin Medical Center, Beilinson Hospital The IASLD semi-annual meeting-
EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease.
 Commentary. EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease. Christopher D. Byrne 1,2, Giovanni Targher 3 1 Nutrition and Metabolism, Faculty of Medicine,
Commentary. EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease. Christopher D. Byrne 1,2, Giovanni Targher 3 1 Nutrition and Metabolism, Faculty of Medicine,
2 nd International Workshop on NASH Biomarkers, Washington DC, May 5-6, 2017
 Hepatic Proton Density Fat Fraction Correlates With Histologic Measures of Steatosis and Is Responsive to Changes in Those Measures in a Multi-center Nonalcoholic Steatohepatitis Clinical Trial Michael
Hepatic Proton Density Fat Fraction Correlates With Histologic Measures of Steatosis and Is Responsive to Changes in Those Measures in a Multi-center Nonalcoholic Steatohepatitis Clinical Trial Michael
Non-Alcoholic Steatohepatitis (NASH): What the Gastroenterologist Should Know
 Non-Alcoholic Steatohepatitis (NASH): What the Gastroenterologist Should Know Naga P. Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of GI and Hepatology
Non-Alcoholic Steatohepatitis (NASH): What the Gastroenterologist Should Know Naga P. Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of GI and Hepatology
NAFLD: US GUIDELINES. US Guidelines for NAFLD
 NAFLD: US GUIDELINES Arun J Sanyal M.D. Charles Caravati Professor of Medicine Virginia Commonwealth University School of Medicine US Guidelines for NAFLD Represents consensus amongst AGA, AASLD and ACG
NAFLD: US GUIDELINES Arun J Sanyal M.D. Charles Caravati Professor of Medicine Virginia Commonwealth University School of Medicine US Guidelines for NAFLD Represents consensus amongst AGA, AASLD and ACG
Evercore ISI Presentation- Madrigal
 Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
Update on Clinical Management
 Non-Alcoholic Fatty Liver Disease Update on Clinical Management Lisa J. Yoo, D.O. Gastroenterologist Regional Gastroenterology INTRODUCTION Non-alcoholic fatty liver disease (NAFLD) is characterized by
Non-Alcoholic Fatty Liver Disease Update on Clinical Management Lisa J. Yoo, D.O. Gastroenterologist Regional Gastroenterology INTRODUCTION Non-alcoholic fatty liver disease (NAFLD) is characterized by
Non-Alcoholic Fatty Liver Disease
 Non-Alcoholic Fatty Liver Disease None Disclosures Arslan Kahloon M.D Chief, Division of Gastroenterology and Hepatology University of Tennessee College of Medicine Chattanooga Objectives Understand the
Non-Alcoholic Fatty Liver Disease None Disclosures Arslan Kahloon M.D Chief, Division of Gastroenterology and Hepatology University of Tennessee College of Medicine Chattanooga Objectives Understand the
Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease
 1 Liver Unit, Newcastle Upon Tyne Hospitals NHS Trust, Freeman Hospital, Newcastle upon Tyne, UK 2 Institute of Cellular Medicine, Faculty of Medical Sciences, Newcastle University, Newcastle Upon Tyne,
1 Liver Unit, Newcastle Upon Tyne Hospitals NHS Trust, Freeman Hospital, Newcastle upon Tyne, UK 2 Institute of Cellular Medicine, Faculty of Medical Sciences, Newcastle University, Newcastle Upon Tyne,
AAIM: GI Workshop Follow Up to Case Studies. Non-alcoholic Fatty Liver Disease Ulcerative Colitis Crohn s Disease
 AAIM: GI Workshop Follow Up to Case Studies Non-alcoholic Fatty Liver Disease Ulcerative Colitis Crohn s Disease Daniel Zimmerman, MD VP and Medical Director, RGA Global October 2015 Non-alcoholic Fatty
AAIM: GI Workshop Follow Up to Case Studies Non-alcoholic Fatty Liver Disease Ulcerative Colitis Crohn s Disease Daniel Zimmerman, MD VP and Medical Director, RGA Global October 2015 Non-alcoholic Fatty
Obesity, metabolic syndrome (MetS), and type 2 diabetes
 Noninvasive Diagnosis of NASH and Liver Fibrosis Within the Spectrum of NAFLD Naim Alkhouri, MD, and Arthur J. McCullough, MD Dr. Alkhouri and Dr. McCullough are affiliated with the Department of Gastroenterology
Noninvasive Diagnosis of NASH and Liver Fibrosis Within the Spectrum of NAFLD Naim Alkhouri, MD, and Arthur J. McCullough, MD Dr. Alkhouri and Dr. McCullough are affiliated with the Department of Gastroenterology
CASE REPORT. Abstract. Introduction
 CASE REPORT A Customized Online Nutrition Guidance System Is Effective for Treating Patients with Nonalcoholic Fatty Liver Disease by Supporting Continuity of Diet Therapy at Home: A Pilot Study Tomonori
CASE REPORT A Customized Online Nutrition Guidance System Is Effective for Treating Patients with Nonalcoholic Fatty Liver Disease by Supporting Continuity of Diet Therapy at Home: A Pilot Study Tomonori
Lavine, MD, PhD; 4 Anna Mae Diehl, MD; 5 Elizabeth M. Brunt, MD; 6 Kenneth Cusi, MD; 7 Michael Charlton, MD; 8 Arun J. Sanyal, MD
 Page 1 of 59 The Diagnosis and Management of Non-alcoholic Fatty Liver Disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and
Page 1 of 59 The Diagnosis and Management of Non-alcoholic Fatty Liver Disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and
Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts NAS score for NASH diagnosis
 Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts NAS score for NASH diagnosis Alina M. Allen, Meng Yin, Sudhakar K. Venkatesh, Taofic Mounajjed, Todd A. Kellogg,
Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts NAS score for NASH diagnosis Alina M. Allen, Meng Yin, Sudhakar K. Venkatesh, Taofic Mounajjed, Todd A. Kellogg,
IS LIVER BIOPSY NECESSARY IN PATIENTS WITH NONALCOHOLIC FATTY LIVER DISEASE?
 Rev. Med. Chir. Soc. Med. Nat., Iaşi 2016 vol. 120, no. 3 INTERNAL MEDICINE - PEDIATRICS UPDATES IS LIVER BIOPSY NECESSARY IN PATIENTS WITH NONALCOHOLIC FATTY LIVER DISEASE? R. S. Gavril 1, Laura Mihalache
Rev. Med. Chir. Soc. Med. Nat., Iaşi 2016 vol. 120, no. 3 INTERNAL MEDICINE - PEDIATRICS UPDATES IS LIVER BIOPSY NECESSARY IN PATIENTS WITH NONALCOHOLIC FATTY LIVER DISEASE? R. S. Gavril 1, Laura Mihalache
Original article J Bas Res Med Sci 2014; 1(1):50-55.
 The effect of pioglitazone and metformin on non-alcoholic fatty liver: A double blind clinical trial study Kourosh Sayehmiri 1, 2, Khairollah Asadollahi 1, 2*, Mariam Yaghubi 1, Ghobad Abangah 3, Hassan
The effect of pioglitazone and metformin on non-alcoholic fatty liver: A double blind clinical trial study Kourosh Sayehmiri 1, 2, Khairollah Asadollahi 1, 2*, Mariam Yaghubi 1, Ghobad Abangah 3, Hassan
Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH
 Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology
Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology
Steatotic liver disease
 Steatotic liver disease Fatty liver disease Prof. Dr. ANNE HOORENS Non-Neoplastic Liver Pathology December 8th 2018 Working Group of Digestive Pathology Belgian Society of Pathology OUTLINE NAFLD = Non-Alcoholic
Steatotic liver disease Fatty liver disease Prof. Dr. ANNE HOORENS Non-Neoplastic Liver Pathology December 8th 2018 Working Group of Digestive Pathology Belgian Society of Pathology OUTLINE NAFLD = Non-Alcoholic
Non-alcoholic fatty liver disease: time to take note and manage. Philip Newsome Professor of Hepatology & Director of Centre for Liver Research
 Non-alcoholic fatty liver disease: time to take note and manage Philip Newsome Professor of Hepatology & Director of Centre for Liver Research Disclosures Consultancy, Co-ordinating Investigator roles
Non-alcoholic fatty liver disease: time to take note and manage Philip Newsome Professor of Hepatology & Director of Centre for Liver Research Disclosures Consultancy, Co-ordinating Investigator roles
Liver Pathology in the 0bese
 Liver Pathology in the 0bese Rob Goldin Centre for Pathology, Imperial College r.goldin@imperial.ac.uk Ludwig et al. Non-alcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease.
Liver Pathology in the 0bese Rob Goldin Centre for Pathology, Imperial College r.goldin@imperial.ac.uk Ludwig et al. Non-alcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease.
The role of ARFI and APRI in diagnosis of liver fibrosis on patients with common chronic liver diseases
 RESEARCH ARTICLE The role of ARFI and APRI in diagnosis of liver fibrosis on patients with common chronic liver diseases Objective: This study aimed to investigate the value of liver fibrosis assessment
RESEARCH ARTICLE The role of ARFI and APRI in diagnosis of liver fibrosis on patients with common chronic liver diseases Objective: This study aimed to investigate the value of liver fibrosis assessment
Improving Access to Quality Medical Care Webinar Series
 Improving Access to Quality Medical Care Webinar Series Presented by The Arizona Telemedicine Program and the Southwest Telehealth Resource Center 2015 UA Board of Regents Welcome AZ, UT, CO, NM & NV FLEX
Improving Access to Quality Medical Care Webinar Series Presented by The Arizona Telemedicine Program and the Southwest Telehealth Resource Center 2015 UA Board of Regents Welcome AZ, UT, CO, NM & NV FLEX
Lipid and Bile Acids as NAFLD- Related Biomarkers
 Lipid and Bile Acids as NAFLD- Related Biomarkers Puneet Puri, MBBS, MD Division of Gastroenterology, Hepatology and Nutrition Virginia Commonwealth University, Richmond, VA 1st International Workshop
Lipid and Bile Acids as NAFLD- Related Biomarkers Puneet Puri, MBBS, MD Division of Gastroenterology, Hepatology and Nutrition Virginia Commonwealth University, Richmond, VA 1st International Workshop
PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD. Pierre Bedossa. Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE
 PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD Pierre Bedossa Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE 1 PATHOLOGY OF NAFLD NAFLD: a chronic liver disease with a wide
PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD Pierre Bedossa Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE 1 PATHOLOGY OF NAFLD NAFLD: a chronic liver disease with a wide
FDA Introductory Remarks Stephanie O. Omokaro, MD
 FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
Transient elastography in chronic liver diseases of other etiologies
 4 Post Meeting A.I.S.F. Unmet Clinical Needs in Hepatology: New and upcoming diagnostic tools" Transient elastography in chronic liver diseases of other etiologies Dr. Vincenza Calvaruso Gastroenterologia
4 Post Meeting A.I.S.F. Unmet Clinical Needs in Hepatology: New and upcoming diagnostic tools" Transient elastography in chronic liver diseases of other etiologies Dr. Vincenza Calvaruso Gastroenterologia
NAFLD/NASH. Definitions. Pathology NASH. Vicki Shah PA-C, MMS Rush University Hepatology
 NAFLD/NASH Vicki Shah PA-C, MMS Rush University Hepatology Definitions NAFLD Evidence of hepatic steatosis by histology (5%) or imaging No causes for secondary fat accumulation EtOH, Drugs, hereditary
NAFLD/NASH Vicki Shah PA-C, MMS Rush University Hepatology Definitions NAFLD Evidence of hepatic steatosis by histology (5%) or imaging No causes for secondary fat accumulation EtOH, Drugs, hereditary
SAF score and mortality in NAFLD after up to 41 years of follow-up
 SAF score and mortality in NAFLD after up to 41 years of follow-up Hannes Hagström, Patrik Nasr, Mattias Ekstedt, Stergios Kechagias, Per Stal, Pierre Bedossa and Rolf Hultcrantz Journal Article N.B.:
SAF score and mortality in NAFLD after up to 41 years of follow-up Hannes Hagström, Patrik Nasr, Mattias Ekstedt, Stergios Kechagias, Per Stal, Pierre Bedossa and Rolf Hultcrantz Journal Article N.B.:
Preface: Nonalcoholic Fatty Liver Disease: An Expanding Health Care Epidemic
 NASH and NAFLD Preface: Nonalcoholic Fatty Liver Disease: An Expanding Health Care Epidemic David E. Bernstein xiii Clinical and Economic Burden of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis
NASH and NAFLD Preface: Nonalcoholic Fatty Liver Disease: An Expanding Health Care Epidemic David E. Bernstein xiii Clinical and Economic Burden of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis
Update on Nonalcoholic Fatty Liver Disease. Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic
 Update on Nonalcoholic Fatty Liver Disease Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic Outline Defining the phenotypes of nonalcoholic fatty liver disease NAFLD Diagnostics
Update on Nonalcoholic Fatty Liver Disease Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic Outline Defining the phenotypes of nonalcoholic fatty liver disease NAFLD Diagnostics
HEP DART 2017, Kona, Hawaii
 HEP DART 2017, Kona, Hawaii Rong Yu 1, Ke Xu 1, Jing Li 1, Tong Sun 1, Shengjiang Zhang 2, Jinhua Shao 2, Jin Sun 2, Qiong He 3, Jianwen Luo 3, Cheng Wang 4, Yudong Wang 4, Jing Chen 4, Vanessa Wu 4, George
HEP DART 2017, Kona, Hawaii Rong Yu 1, Ke Xu 1, Jing Li 1, Tong Sun 1, Shengjiang Zhang 2, Jinhua Shao 2, Jin Sun 2, Qiong He 3, Jianwen Luo 3, Cheng Wang 4, Yudong Wang 4, Jing Chen 4, Vanessa Wu 4, George
NAFLD/NASH in Sub- Saharan Africa
 NAFLD/NASH in Sub- Saharan Africa Corné Kruger Gastroenterologist Durbanville Mediclinic Cape Town Liver Interest group meeting: Cape Town 2015 Introduction NAFLD is the most common liver disease disease
NAFLD/NASH in Sub- Saharan Africa Corné Kruger Gastroenterologist Durbanville Mediclinic Cape Town Liver Interest group meeting: Cape Town 2015 Introduction NAFLD is the most common liver disease disease
Patients With Diabetes and Chronic Liver Disease Are at Increased Risk for Overall Mortality: A Population Study From the United States
 Patients With Diabetes and Chronic Liver Disease Are at Increased Risk for Overall Mortality: A Population Study From the United States Maria Stepanova, 1,2 Stephen Clement, 3 Robert Wong, 4 Sammy Saab,
Patients With Diabetes and Chronic Liver Disease Are at Increased Risk for Overall Mortality: A Population Study From the United States Maria Stepanova, 1,2 Stephen Clement, 3 Robert Wong, 4 Sammy Saab,
Serum fragmented cytokeratin 18 levels reflect the histological activity. score of nonalcoholic fatty liver disease more accurately than serum
 Serum fragmented cytokeratin 18 levels reflect the histological activity score of nonalcoholic fatty liver disease more accurately than serum alanine aminotransferase levels Masaru Tsutsui, BE, * Naoki
Serum fragmented cytokeratin 18 levels reflect the histological activity score of nonalcoholic fatty liver disease more accurately than serum alanine aminotransferase levels Masaru Tsutsui, BE, * Naoki
Serum Levels of CK18 M30 and Leptin Are Useful Predictors of Steatohepatitis and Fibrosis in Paediatric NAFLD
 ORIGINAL ARTICLE: HEPATOLOGY AND NUTRITION Serum Levels of CK18 M30 and Leptin Are Useful Predictors of Steatohepatitis and Fibrosis in Paediatric NAFLD Emer Fitzpatrick, y Ragai R. Mitry, y Alberto Quaglia,
ORIGINAL ARTICLE: HEPATOLOGY AND NUTRITION Serum Levels of CK18 M30 and Leptin Are Useful Predictors of Steatohepatitis and Fibrosis in Paediatric NAFLD Emer Fitzpatrick, y Ragai R. Mitry, y Alberto Quaglia,
Liver biopsy as the gold standard for diagnosis. Pierre BEDOSSA
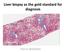 Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
LIVER, PANCREAS, AND BILIARY TRACT
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2012;10:1028 1033 LIVER, PANCREAS, AND BILIARY TRACT Prevalence and Indicators of Portal Hypertension in Patients With Nonalcoholic Fatty Liver Disease FLAVIA D.
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2012;10:1028 1033 LIVER, PANCREAS, AND BILIARY TRACT Prevalence and Indicators of Portal Hypertension in Patients With Nonalcoholic Fatty Liver Disease FLAVIA D.
An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications
 REVIEW An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications Naim Alkhouri, M.D.,*, and Andrea Scott, B.S.* Nonalcoholic fatty liver disease (NAFLD)
REVIEW An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications Naim Alkhouri, M.D.,*, and Andrea Scott, B.S.* Nonalcoholic fatty liver disease (NAFLD)
Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease
 Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease David E. Kleiner, 1 Elizabeth M. Brunt, 2 Mark Van Natta, 3 Cynthia Behling, 4 Melissa J. Contos, 5 Oscar W.
Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease David E. Kleiner, 1 Elizabeth M. Brunt, 2 Mark Van Natta, 3 Cynthia Behling, 4 Melissa J. Contos, 5 Oscar W.
LIVER PATHOLOGY. Thursday 28 th November 2013
 LIVER PATHOLOGY Thursday 28 th November 2013 Liver biopsy assessment of steatosis Amar Paul Dhillon Royal Free Hospital RIBA, London Thursday 28th November 2013 NAFLD didn t exist before 2001 and liver
LIVER PATHOLOGY Thursday 28 th November 2013 Liver biopsy assessment of steatosis Amar Paul Dhillon Royal Free Hospital RIBA, London Thursday 28th November 2013 NAFLD didn t exist before 2001 and liver
Internal Medicine Grand Rounds University of Texas Southwestern Medical Center October 5, 2018
 Internal Medicine Grand Rounds University of Texas Southwestern Medical Center October 5, 2018 Nonalcoholic Fatty Liver Disease (NAFLD) Turns 38-What Have We Learned? Jay D. Horton, M.D. This is to acknowledge
Internal Medicine Grand Rounds University of Texas Southwestern Medical Center October 5, 2018 Nonalcoholic Fatty Liver Disease (NAFLD) Turns 38-What Have We Learned? Jay D. Horton, M.D. This is to acknowledge
Fatty Liver Disease A growing epidemic
 Fatty Liver Disease A growing epidemic Updates in GIM for Primary Care Don C. Rockey March 9 th, 2018 Disclosures 2018 Research Funding (all to MUSC) NIH/NIDDK Actelion Pharmaceuticals Gilead Sciences
Fatty Liver Disease A growing epidemic Updates in GIM for Primary Care Don C. Rockey March 9 th, 2018 Disclosures 2018 Research Funding (all to MUSC) NIH/NIDDK Actelion Pharmaceuticals Gilead Sciences
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) NON-ALCOHOLIC STEATOHEPATITIS (NASH) ADDRESSING A GROWING SILENT EPIDEMIC
 NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
Repeating measurements by transient elastography in nonalcoholic fatty liver disease patients with high liver stiffness
 bs_bs_banner doi:10.1111/jgh.14311 HEPATOLOGY Repeating measurements by transient elastography in nonalcoholic fatty liver disease patients with high liver stiffness Jeremy Chak-Lun Chow,* Grace Lai-Hung
bs_bs_banner doi:10.1111/jgh.14311 HEPATOLOGY Repeating measurements by transient elastography in nonalcoholic fatty liver disease patients with high liver stiffness Jeremy Chak-Lun Chow,* Grace Lai-Hung
Bariatric Surgery and Liver Transplantation
 Bariatric Surgery and Liver Transplantation Sammy Saab, MD, MPH, AGAF, FACG, FAASLD Professor of Medicine and Surgery Head, Outcomes Research in Hepatology David Geffen School of Medicine at UCLA Disclosures
Bariatric Surgery and Liver Transplantation Sammy Saab, MD, MPH, AGAF, FACG, FAASLD Professor of Medicine and Surgery Head, Outcomes Research in Hepatology David Geffen School of Medicine at UCLA Disclosures
Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD
 Diabetes Care 1 Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD Jessica Bazick, 1 Michele Donithan, 2 Brent A. Neuschwander-Tetri,
Diabetes Care 1 Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD Jessica Bazick, 1 Michele Donithan, 2 Brent A. Neuschwander-Tetri,
Prevalence of Non-alcoholic Fatty Liver Disease in the Russian Federation: the Open, Multicenter, Prospective Study, DIREG 1
 American Journal of Clinical Medicine Research, 2015, Vol. 3, No. 2, 31-36 Available online at http://pubs.sciepub.com/ajcmr/3/2/3 Science and Education Publishing DOI:10.12691/ajcmr-3-2-3 Prevalence of
American Journal of Clinical Medicine Research, 2015, Vol. 3, No. 2, 31-36 Available online at http://pubs.sciepub.com/ajcmr/3/2/3 Science and Education Publishing DOI:10.12691/ajcmr-3-2-3 Prevalence of
NON-ALCOHOLIC STEATOHEPATITIS AND NON-ALCOHOLIC FATTY LIVER DISEASES
 NON-ALCOHOLIC STEATOHEPATITIS AND NON-ALCOHOLIC FATTY LIVER DISEASES Preface Zobair M. Younossi xiii Epidemiology and Natural History of NAFLD and NASH 1 Janus P. Ong and Zobair M. Younossi Understanding
NON-ALCOHOLIC STEATOHEPATITIS AND NON-ALCOHOLIC FATTY LIVER DISEASES Preface Zobair M. Younossi xiii Epidemiology and Natural History of NAFLD and NASH 1 Janus P. Ong and Zobair M. Younossi Understanding
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) NON-ALCOHOLIC STEATOHEPATITIS (NASH) ADDRESSING A GROWING SILENT EPIDEMIC
 NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
The Absence of Obstructive Sleep Apnea May Protect against Non- Alcoholic Fatty Liver in Patients Undergoing Bariatric Surgery
 The Absence of Obstructive Sleep Apnea May Protect against Non- Alcoholic Fatty Liver in Patients Undergoing Bariatric Surgery The Harvard community has made this article openly available. Please share
The Absence of Obstructive Sleep Apnea May Protect against Non- Alcoholic Fatty Liver in Patients Undergoing Bariatric Surgery The Harvard community has made this article openly available. Please share
PROGRESSION TO FIBROSIS IN PATIENTS WITH NONALCOHOLIC FATTY LIVER DISEASE (NAFLD) THE VALUE OF NONINVASIVE MARKERS
 Supplement 1/2015, 4 th ISAA PROGRESSION TO FIBROSIS IN PATIENTS WITH NONALCOHOLIC FATTY LIVER DISEASE (NAFLD) THE VALUE OF NONINVASIVE MARKERS Denisa DOBRIN 1, Tiberiu Ioan NANEA 2, Cristian Mihai POMOHACI
Supplement 1/2015, 4 th ISAA PROGRESSION TO FIBROSIS IN PATIENTS WITH NONALCOHOLIC FATTY LIVER DISEASE (NAFLD) THE VALUE OF NONINVASIVE MARKERS Denisa DOBRIN 1, Tiberiu Ioan NANEA 2, Cristian Mihai POMOHACI
Fatty liver disease: What do we know?
 Fatty liver disease: What do we know? Prof. Dr. Claus Niederau Katholische Kliniken Oberhausen ggmbh St. Josef-Hospital Academic Teaching Hospital University of Duisburg-Essen NAFLD Non-Alcoholic Fatty
Fatty liver disease: What do we know? Prof. Dr. Claus Niederau Katholische Kliniken Oberhausen ggmbh St. Josef-Hospital Academic Teaching Hospital University of Duisburg-Essen NAFLD Non-Alcoholic Fatty
New Discriminant Method for Identifying the Aggressive Disease Phenotype of Non-alcoholic Fatty Liver Disease
 ORIGINAL ARTICLE New Discriminant Method for Identifying the Aggressive Disease Phenotype of Non-alcoholic Fatty Liver Disease Yusuke Kawamura 1,2, Kenji Ikeda 1,2, Yasuji Arase 1,2, Shunichiro Fujiyama
ORIGINAL ARTICLE New Discriminant Method for Identifying the Aggressive Disease Phenotype of Non-alcoholic Fatty Liver Disease Yusuke Kawamura 1,2, Kenji Ikeda 1,2, Yasuji Arase 1,2, Shunichiro Fujiyama
NAFLD: evidence-based management. Curso de residentes AEEH Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain
 NAFLD: evidence-based management Curso de residentes AEEH 2017 Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain Clinical case - 55 yo female - Sent for incidental steatosis at abdominal
NAFLD: evidence-based management Curso de residentes AEEH 2017 Salvador Augustin, MD Liver Unit Vall d Hebron Hospital Barcelona, Spain Clinical case - 55 yo female - Sent for incidental steatosis at abdominal
NASH UPDATE ON DIAGNOSTICS AND THERAPY. Arun J Sanyal MBBS, MD Virginia Commonwealth University School of Medicine
 NASH UPDATE ON DIAGNOSTICS AND THERAPY Arun J Sanyal MBBS, MD Virginia Commonwealth University School of Medicine Conflicts of interest Salaried employee: of VCU Member of Board: McGuire VA Research Institute,
NASH UPDATE ON DIAGNOSTICS AND THERAPY Arun J Sanyal MBBS, MD Virginia Commonwealth University School of Medicine Conflicts of interest Salaried employee: of VCU Member of Board: McGuire VA Research Institute,
Laboratory analysis of the obese child recommendations and discussion. MacKenzi Hillard May 4, 2011
 Laboratory analysis of the obese child recommendations and discussion MacKenzi Hillard May 4, 2011 aka: What to do with Fasting Labs The Obesity Epidemic The prevalence of obesity in adolescents has tripled
Laboratory analysis of the obese child recommendations and discussion MacKenzi Hillard May 4, 2011 aka: What to do with Fasting Labs The Obesity Epidemic The prevalence of obesity in adolescents has tripled
Early life determinants of Non-Alcoholic Fatty Liver Disease and NASH DR JULIANA MUIVA-GITOBU KENYA PAEDIATRIC ASSOCIATION CONFERENCE APRIL 2016.
 Early life determinants of Non-Alcoholic Fatty Liver Disease and NASH DR JULIANA MUIVA-GITOBU KENYA PAEDIATRIC ASSOCIATION CONFERENCE APRIL 2016. Outline Definition NAFLD and NASH Magnitude of the problem
Early life determinants of Non-Alcoholic Fatty Liver Disease and NASH DR JULIANA MUIVA-GITOBU KENYA PAEDIATRIC ASSOCIATION CONFERENCE APRIL 2016. Outline Definition NAFLD and NASH Magnitude of the problem
Treatment of NASH: What Helps Beyond Weight Loss?
 THE RED SECTION 821 see related editorial on page x Treatment of NASH: What Helps Beyond Weight Loss? Bubu A. Banini, MD, PhD1 and Arun J. Sanyal, MBBS, MD1 Am J Gastroenterol 2017; 112:821 824; doi: 10.1038/ajg.2017.83;
THE RED SECTION 821 see related editorial on page x Treatment of NASH: What Helps Beyond Weight Loss? Bubu A. Banini, MD, PhD1 and Arun J. Sanyal, MBBS, MD1 Am J Gastroenterol 2017; 112:821 824; doi: 10.1038/ajg.2017.83;
The effect of aerobic exercise on serum level of liver enzymes and liver echogenicity in patients with non-alcoholic fatty liver disease
 Gastroenterology and Hepatology From Bed to Bench. 2013 RIGLD, Research Institute for Gastroenterology and Liver Diseases ORIGINAL ARTICLE The effect of aerobic exercise on serum level of liver enzymes
Gastroenterology and Hepatology From Bed to Bench. 2013 RIGLD, Research Institute for Gastroenterology and Liver Diseases ORIGINAL ARTICLE The effect of aerobic exercise on serum level of liver enzymes
A pathologist, a radiologist and a hepatologist walked into a bar
 A pathologist, a radiologist and a hepatologist walked into a bar Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology Saint Louis
A pathologist, a radiologist and a hepatologist walked into a bar Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology Saint Louis
Ocaliva (obeticholic acid tablets)
 Ocaliva (obeticholic acid tablets) Policy Number: 5.01.619 Last Review: 11/2018 Origination: 11/2016 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Ocaliva (obeticholic acid tablets) Policy Number: 5.01.619 Last Review: 11/2018 Origination: 11/2016 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
PREVALENCE OF NAFLD & NASH
 - - PREVALENCE OF & USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology 2011; 140:124-31) Dallas Heart Study Prevalence Numbers (Browning et al., Hepatology 2004;40:1387-95)
- - PREVALENCE OF & USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology 2011; 140:124-31) Dallas Heart Study Prevalence Numbers (Browning et al., Hepatology 2004;40:1387-95)
At Least 1 in 5 Patients in Your Practice Have Fatty Liver
 At Least 1 in 5 Patients in Your Practice Have Fatty Liver What Can You Tell Your Patients Magnus McLeod MD FRCPC Assistant Professor Dalhousie University 30-NOV-2017 NAFLD Non-Alcoholic Fatty Liver Disease
At Least 1 in 5 Patients in Your Practice Have Fatty Liver What Can You Tell Your Patients Magnus McLeod MD FRCPC Assistant Professor Dalhousie University 30-NOV-2017 NAFLD Non-Alcoholic Fatty Liver Disease
NAFLD: ACG Southern Regional Course Nashville, TN. Speaking - None
 NAFLD: 2015 Naga Chalasani, MD, FACG David W. Crabb Professor of Medicine Director, Division of GI and Hepatology Indiana University School of Medicine ACG Southern Regional Course Nashville, TN Disclosures
NAFLD: 2015 Naga Chalasani, MD, FACG David W. Crabb Professor of Medicine Director, Division of GI and Hepatology Indiana University School of Medicine ACG Southern Regional Course Nashville, TN Disclosures
Patients With NASH and Cryptogenic Cirrhosis Are Less Likely Than Those With Hepatitis C to Receive Liver Transplants
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2011;9:700 704 Patients With NASH and Cryptogenic Cirrhosis Are Less Likely Than Those With Hepatitis C to Receive Liver Transplants JACQUELINE G. O LEARY, CARMEN
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2011;9:700 704 Patients With NASH and Cryptogenic Cirrhosis Are Less Likely Than Those With Hepatitis C to Receive Liver Transplants JACQUELINE G. O LEARY, CARMEN
Liver fibrosis, inflammation, and steatosis are
 Prospective Biopsy-Controlled Evaluation of Cell Death Biomarkers for Prediction of Liver Fibrosis and Nonalcoholic Steatohepatitis Diana Joka, 1 Kristin Wahl, 1 Sarah Moeller, 1 Jerome Schlue, 2 Bernhard
Prospective Biopsy-Controlled Evaluation of Cell Death Biomarkers for Prediction of Liver Fibrosis and Nonalcoholic Steatohepatitis Diana Joka, 1 Kristin Wahl, 1 Sarah Moeller, 1 Jerome Schlue, 2 Bernhard
Screening cardiac patients for advanced liver disease
 HKASLD 30 th ASM and International Symposium on Hepatology 2017 Screening cardiac patients for advanced liver disease 5 Nov 2017 Dr. Lau Yue Leung Joulen Pamela Youde Nethersole Eastern Hospital NAFLD
HKASLD 30 th ASM and International Symposium on Hepatology 2017 Screening cardiac patients for advanced liver disease 5 Nov 2017 Dr. Lau Yue Leung Joulen Pamela Youde Nethersole Eastern Hospital NAFLD
NAFLD & NASH: Russian perspective
 NAFLD & NASH: Russian perspective Vasily Isakov, MD, PhD Professor, Chief, Department Gastroenterology & Hepatology, Federal Research Center of nutrition, biotechnology and food safety Disclosures Received
NAFLD & NASH: Russian perspective Vasily Isakov, MD, PhD Professor, Chief, Department Gastroenterology & Hepatology, Federal Research Center of nutrition, biotechnology and food safety Disclosures Received
Fatty Liver Disease. Mark Thursz. Imperial College
 Fatty Liver Disease Mark Thursz Imperial College Non-Alcoholic Fatty Liver Disease UK adult obesity (BMI>30) 1980: 6% [M], 8% [F]. 1997: 17% [M], 20% [F]. By 2004, 23.6% of men and 23.8% of women were
Fatty Liver Disease Mark Thursz Imperial College Non-Alcoholic Fatty Liver Disease UK adult obesity (BMI>30) 1980: 6% [M], 8% [F]. 1997: 17% [M], 20% [F]. By 2004, 23.6% of men and 23.8% of women were
Effect of Vitamin E or Metformin for Treatment of Nonalcoholic Fatty Liver Disease in Children and Adolescents JAMA. 2011;305(16):
 ORIGINAL CONTRIBUTION Effect of or for Treatment of Nonalcoholic Fatty Liver Disease in Children and Adolescents The TONIC Randomized Controlled Trial Joel E. Lavine, MD, PhD Jeffrey B. Schwimmer, MD Mark
ORIGINAL CONTRIBUTION Effect of or for Treatment of Nonalcoholic Fatty Liver Disease in Children and Adolescents The TONIC Randomized Controlled Trial Joel E. Lavine, MD, PhD Jeffrey B. Schwimmer, MD Mark
ALT and aspartate aminotransferase (AST) levels were measured using the α-ketoglutarate reaction (Roche,
 Supplemental Methods Analytical determinations ALT and aspartate aminotransferase (AST) levels were measured using the α-ketoglutarate reaction (Roche, Basel, Switzerland). Glucose, triglyceride, total
Supplemental Methods Analytical determinations ALT and aspartate aminotransferase (AST) levels were measured using the α-ketoglutarate reaction (Roche, Basel, Switzerland). Glucose, triglyceride, total
A Modification of the Brunt System for Scoring Liver Histology of Patients with Non-Alcoholic Fatty Liver Disease
 Arch Iran Med 2010; 13 (1): 38 44 Original Article A Modification of the Brunt System for Scoring Liver Histology of Patients with Non-Alcoholic Fatty Liver Disease Shahin Merat MD*, Farzaneh Khadem-Sameni
Arch Iran Med 2010; 13 (1): 38 44 Original Article A Modification of the Brunt System for Scoring Liver Histology of Patients with Non-Alcoholic Fatty Liver Disease Shahin Merat MD*, Farzaneh Khadem-Sameni
The Skinny On Non Alcoholic Fatty Liver Disease
 The Skinny On Non Alcoholic Fatty Liver Disease UCSF Advances in Internal Medicine Monika Sarkar, MD, MAS UCSF Division of GI/Hepatology June 24th, 2015 Non Alcoholic Fatty Liver Disease: Outline Pathogenesis
The Skinny On Non Alcoholic Fatty Liver Disease UCSF Advances in Internal Medicine Monika Sarkar, MD, MAS UCSF Division of GI/Hepatology June 24th, 2015 Non Alcoholic Fatty Liver Disease: Outline Pathogenesis
AASLD Immune tolerant phase HBV NAFLD diagnostic HCC
 AASLD 2016 Immune tolerant phase HBV NAFLD diagnostic HCC Immune tolerant 3 Modified from Chan HLY and Wong VWS. Hepatitis B. In Zakim and Boyers s Hepatology 2012 2015 AMERICAN ASSOCIATION FOR THE S1T6UDY
AASLD 2016 Immune tolerant phase HBV NAFLD diagnostic HCC Immune tolerant 3 Modified from Chan HLY and Wong VWS. Hepatitis B. In Zakim and Boyers s Hepatology 2012 2015 AMERICAN ASSOCIATION FOR THE S1T6UDY
Non-invasive diagnostic biomarkers
 Non-invasive diagnostic biomarkers Liver Forum, November 12 th, 2015 Rohit Loomba, MD, MHSc Professor of Medicine (with tenure) Director, NAFLD Research Center, Division of Gastroenterology, Department
Non-invasive diagnostic biomarkers Liver Forum, November 12 th, 2015 Rohit Loomba, MD, MHSc Professor of Medicine (with tenure) Director, NAFLD Research Center, Division of Gastroenterology, Department
FATTY LIVER DISEASE (NAFLD) (NASH) A GROWING
 NON ALCOHOLIC FATTY LIVER DISEASE () & NON ALCOHOLIC S T E ATO H E PAT I T I S () ADDRESSING A GROWING SILENT EPIDEMIC Prevalence of & USA Prevalence in Middle Age Patients San Antonio, Texas (Williams
NON ALCOHOLIC FATTY LIVER DISEASE () & NON ALCOHOLIC S T E ATO H E PAT I T I S () ADDRESSING A GROWING SILENT EPIDEMIC Prevalence of & USA Prevalence in Middle Age Patients San Antonio, Texas (Williams
Case #1. Digital Slides 11/6/ year old woman presented with abnormal liver function tests. Liver Biopsy to r/o autoimmune hepatitis
 45 year old woman presented with abnormal liver function tests Liver Biopsy to r/o autoimmune hepatitis Further down. ANA 1: 160; ASMA 1:80 ANA 1: 160; ASMA 1:80 IgG = 14.5 g/l (upper normal range: 16)
45 year old woman presented with abnormal liver function tests Liver Biopsy to r/o autoimmune hepatitis Further down. ANA 1: 160; ASMA 1:80 ANA 1: 160; ASMA 1:80 IgG = 14.5 g/l (upper normal range: 16)
tage Percent Total & over Total & over Men Women Men Women
 Paul Angulo, MD, FACG, AGAF Professor of Medicine, Section Chief of Hepatology Division i i of Digestive i Diseases and Nutrition i University of Kentucky Medical Center Lexington, KY Paul Angulo, MD University
Paul Angulo, MD, FACG, AGAF Professor of Medicine, Section Chief of Hepatology Division i i of Digestive i Diseases and Nutrition i University of Kentucky Medical Center Lexington, KY Paul Angulo, MD University
Nonalcoholic fatty liver disease (NAFLD) and nonalcoholic. Noninvasive Assessment of Nonalcoholic Fatty Liver Disease in Obese or Overweight Patients
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2012;10:1162 1168 Noninvasive Assessment of Nonalcoholic Fatty Liver Disease in Obese or Overweight Patients SVEN M. A. FRANCQUE,* AN VERRIJKEN, ILSE MERTENS, GUY
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2012;10:1162 1168 Noninvasive Assessment of Nonalcoholic Fatty Liver Disease in Obese or Overweight Patients SVEN M. A. FRANCQUE,* AN VERRIJKEN, ILSE MERTENS, GUY
Viral hepatitis and Hepatocellular Carcinoma
 Viral hepatitis and Hepatocellular Carcinoma Hashem B. El-Serag, MD, MPH Dan L. Duncan Professor of Medicine Chief, Gastroenterology and Hepatology Houston VA & Baylor College of Medicine Houston, TX Outline
Viral hepatitis and Hepatocellular Carcinoma Hashem B. El-Serag, MD, MPH Dan L. Duncan Professor of Medicine Chief, Gastroenterology and Hepatology Houston VA & Baylor College of Medicine Houston, TX Outline
«STEATOSI EPATICA ED EPATOPATIE METABOLICHE» Ester Vanni Division of Gastroenterology University of Turin
 «STEATOSI EPATICA ED EPATOPATIE METABOLICHE» Ester Vanni Division of Gastroenterology University of Turin OUTLINE NAFLD overview NAFLD and menarche NAFLD and pregnancy NAFLD and menopause Other metabolic
«STEATOSI EPATICA ED EPATOPATIE METABOLICHE» Ester Vanni Division of Gastroenterology University of Turin OUTLINE NAFLD overview NAFLD and menarche NAFLD and pregnancy NAFLD and menopause Other metabolic
