DEFINING THE REFERENCE STANDARD FOR BIOMARKERS IN NASH: HISTOLOGY VERSUS CLINICAL OUTCOMES
|
|
|
- Laurel Wade
- 5 years ago
- Views:
Transcription
1 DEFINING THE REFERENCE STANDARD FOR BIOMARKERS IN NASH: HISTOLOGY VERSUS CLINICAL OUTCOMES Kathleen M Donohue MD, MSc Clinical Team Lead Liver & Inborn Errors Division of Gastroenterology & Inborn Errors Products FDA 1
2 Disclaimers Views expressed in this presentation are those of the speaker and do not necessarily represent an FDA official position I do not have any financial disclosures regarding pharmaceutical drug products 2
3 NASH reference standard? Accelerated Approval: Histology Resolution of steatohepatitis AND no worsening of liver fibrosis Improvement in liver fibrosis AND no worsening of steatohepatitis Both resolution of steatohepatitis and improvement in fibrosis Full Clinical Approval: Clinical Outcomes Progression to cirrhosis on histopathology Hepatic decompensation events MELD from 12 to > 15 Transplant All-cause mortality 3
4 Histology Standard N A S H DRUG HISTOLOGY CIRRHOSIS DECOMPENSATION TRANSPLANT MORTALITY Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease, IOM,
5 Histology Standard DRUG N A S H NEW BIOMARKER HISTOLOGY CIRRHOSIS DECOMPENSATION TRANSPLANT MORTALITY Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease, IOM,
6 Histology Standard N A S H NEW BIOMARKER Novel Pathway HISTOLOGY Fibrosis CIRRHOSIS DECOMPENSATION TRANSPLANT MORTALITY Inflammation DRUG Adapted from Fleming et al. Annals of Internal Medicine 1996 CGD Study Group NEJM
7 Histology Standard Novel Pathway N A S H NEW BIOMARKER HISTOLOGY Fibrosis CIRRHOSIS DECOMPENSATION TRANSPLANT MORTALITY Inflammation DRUG 7
8 Histology Standard DRUG Novel Pathway N A S H NEW BIOMARKER HISTOLOGY Fibrosis CIRRHOSIS DECOMPENSATION TRANSPLANT MORTALITY Inflammation 8
9 Benchmarking to histology DRUG Off Target Effects N A S H NEW BIOMARKER HISTOLOGY Fibrosis CIRRHOSIS DECOMPENSATION TRANSPLANT MORTALITY Inflammation 9
10 NASH reference standard? Accelerated Approval: Histology Resolution of steatohepatitis AND no worsening of liver fibrosis Improvement in liver fibrosis AND no worsening of steatohepatitis Both resolution of steatohepatitis and improvement in fibrosis Full Clinical Approval: Clinical Outcomes Progression to cirrhosis on histopathology Hepatic decompensation events MELD from 12 to > 15 Transplant All-cause mortality 10
11 Clinical outcome standard: Composite endpoint? 11
12 Composite Endpoints: Strengths statistical efficiency sample size, cost, time Avoids arbitrary choice among several important outcomes for same disease Kleist Applied Clinical Trials
13 Composite Endpoints: components Requirements clinically meaningful of similar importance to patients expected effects on each component are similar the clinically most important components should at least not be affected negatively Freemantle et al. JAMA 2003 Montori et. al BMJ
14 Composite Endpoints: Limitations Treatment effect often smallest on the most important outcome Inconsistent treatment effect on components e.g. chest pain, deaths Composite with nonfatal events (i.e. excluding death) is invalid censors worst outcome 14
15 Toward better biomarkers 15
16 FDA NASH Pipeline Pre-submission Meetings 8% 3% 8% 31% 32% 13% INDs Expedited Program Requests 6% initial Pediatric Study Plans 17% 36% Inter-Center Consultations 28% 10% 8% Biomarker Qualification Program EMA Collaborations
17 Reporting Biomarker Studies 17
18 QUADAS-2 18
19 QUADAS-2 Flow Diagram 19
20 Acknowledgements Slides from Erica Lyons & Stephanie Omokaro Lara Dimick-Santos Ruby Mehta Veronica Pei Suna Seo Frank Anania Roni Cherry-France Evangela Covert Anissa Davis-Williams Lisa Soule Dragos Roman Victor Crentsil Julie Beitz Mark Avigan John R. Senior 20
FDA Introductory Remarks Stephanie O. Omokaro, MD
 FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
FDA Regulatory Considerations for NASH Clinical Trial Endpoints Stephanie O. Omokaro, MD
 FDA Regulatory Considerations for NASH Clinical Trial Endpoints Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) U.S. Food and Drug Administration Center for Drug
FDA Regulatory Considerations for NASH Clinical Trial Endpoints Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) U.S. Food and Drug Administration Center for Drug
Utilizing Seamless Adaptive Designs and Considering Multiplicity Adjustment for NASH Clinical Trials
 Utilizing Seamless Adaptive Designs and Considering Multiplicity Adjustment for NASH Clinical Trials Yeh-Fong Chen FDA/CDER/OB/DB3 Forum for Collaborative Research (Berkeley) 5/10/2017 Disclaimer This
Utilizing Seamless Adaptive Designs and Considering Multiplicity Adjustment for NASH Clinical Trials Yeh-Fong Chen FDA/CDER/OB/DB3 Forum for Collaborative Research (Berkeley) 5/10/2017 Disclaimer This
Liver Forum Cirrhosis Working Group Arun J. Sanyal
 Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
NASH Regulatory Landscape. Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH
 NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
Approach to Clinical Trials in Drug Development : Eosinophilic Esophagitis (EoE) Outline. Outline
 Approach to Clinical Trials in Drug Development : Eosinophilic Esophagitis (EoE) Preeti Venkataraman, M.D. Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research
Approach to Clinical Trials in Drug Development : Eosinophilic Esophagitis (EoE) Preeti Venkataraman, M.D. Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research
Regulatory update from Europe:
 Regulatory update from Europe: Interim endpoints in phase 3 NASH trials Elmer Schabel MD The previously proposed/presented co-primary evaluation of two composite endpoints for the interim has been accepted:
Regulatory update from Europe: Interim endpoints in phase 3 NASH trials Elmer Schabel MD The previously proposed/presented co-primary evaluation of two composite endpoints for the interim has been accepted:
SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms
 SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms Claudia Filozof, MD PhD Executive Medical Director Covance Challenges in Clinical Development in NASH
SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms Claudia Filozof, MD PhD Executive Medical Director Covance Challenges in Clinical Development in NASH
Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH
 Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology
Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology
The EMA reflection paper on chronic liver disease and its implications for drug development in NASH
 The on chronic liver disease and its implications for drug development in NASH Content of the reflection paper and report from stakeholder meeting Elmer Schabel MD No conflict of interest. Content Overview:
The on chronic liver disease and its implications for drug development in NASH Content of the reflection paper and report from stakeholder meeting Elmer Schabel MD No conflict of interest. Content Overview:
ENCORE-PH Top-line Results
 ENCORE-PH Top-line Results Striving to improve human health December 5, 2018 NASDAQ CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements
ENCORE-PH Top-line Results Striving to improve human health December 5, 2018 NASDAQ CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements
Evercore ISI Presentation- Madrigal
 Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
Clinical Trial Endpoints from Mobile Technology: Regulatory Considerations September 29, 2016
 Clinical Trial Endpoints from Mobile Technology: Regulatory Considerations September 29, 2016 Elektra J. Papadopoulos, MD, MPH Clinical Outcome Assessments Staff Office of New Drugs Center for Drug Evaluation
Clinical Trial Endpoints from Mobile Technology: Regulatory Considerations September 29, 2016 Elektra J. Papadopoulos, MD, MPH Clinical Outcome Assessments Staff Office of New Drugs Center for Drug Evaluation
Performance Outcome Measures: A Regulatory Perspective
 Performance Outcome Measures: A Regulatory Perspective International Society for Pharmacoeconomics and Outcomes Research (ISPOR) May 18, 2015 Ashley F. Slagle, MS, PhD Study Endpoints and Labeling Development
Performance Outcome Measures: A Regulatory Perspective International Society for Pharmacoeconomics and Outcomes Research (ISPOR) May 18, 2015 Ashley F. Slagle, MS, PhD Study Endpoints and Labeling Development
September 30, Eric BASTINGS, MD. Acting Director Division of Neurology Products (DNP) Center for Drug Evaluation and Research (CDER)
 ISCTM Autumn Conference Options and methods to improve cognitive assessment in clinical trials of AD and its precursors September 30, 2013 Eric BASTINGS, MD Acting Director Division of Neurology Products
ISCTM Autumn Conference Options and methods to improve cognitive assessment in clinical trials of AD and its precursors September 30, 2013 Eric BASTINGS, MD Acting Director Division of Neurology Products
An Approach to Outcome Measure Development: A Regulatory Perspective
 An Approach to Outcome Measure Development: A Regulatory Perspective EMA Workshop on Alzheimer s disease November 24, 2014 Elektra J. Papadopoulos, MD, MPH Study Endpoints Team Study Endpoints and Labeling
An Approach to Outcome Measure Development: A Regulatory Perspective EMA Workshop on Alzheimer s disease November 24, 2014 Elektra J. Papadopoulos, MD, MPH Study Endpoints Team Study Endpoints and Labeling
MEETING PROSPECTUS. International Workshop on NASH Biomarkers
 International Workshop on NASH Biomarkers 2018 18-19 May 2018 Washington, D.C., USA MEETING PROSPECTUS www.expertmedicaleducation.com www.expertmedicalevents.com ADVANCING HEALTH GLOBALLY THROUGH INNOVATIVE
International Workshop on NASH Biomarkers 2018 18-19 May 2018 Washington, D.C., USA MEETING PROSPECTUS www.expertmedicaleducation.com www.expertmedicalevents.com ADVANCING HEALTH GLOBALLY THROUGH INNOVATIVE
GENFIT: New data to be presented at 2018 AASLD meeting, ahead of key results expected in 2018 and 2019
 GENFIT: New data to be presented at 2018 AASLD meeting, ahead of key results expected in 2018 and 2019 KOL event to be held ahead of expected data release with elafibranor in PBC by end of 2018 (Phase
GENFIT: New data to be presented at 2018 AASLD meeting, ahead of key results expected in 2018 and 2019 KOL event to be held ahead of expected data release with elafibranor in PBC by end of 2018 (Phase
Session III: Disease Definition WG Update
 Session III: Disease Definition WG Update Disease Definitions Working Group Presentation of WG Output: Manuscript Current Focus: NASH Resolution and Progression WG Output #1: Manuscript An evaluation of
Session III: Disease Definition WG Update Disease Definitions Working Group Presentation of WG Output: Manuscript Current Focus: NASH Resolution and Progression WG Output #1: Manuscript An evaluation of
Non-Alcoholic Fatty Liver Disease
 Non-Alcoholic Fatty Liver Disease None Disclosures Arslan Kahloon M.D Chief, Division of Gastroenterology and Hepatology University of Tennessee College of Medicine Chattanooga Objectives Understand the
Non-Alcoholic Fatty Liver Disease None Disclosures Arslan Kahloon M.D Chief, Division of Gastroenterology and Hepatology University of Tennessee College of Medicine Chattanooga Objectives Understand the
PMDA Considerations for Outcome Assessments
 PMDA Considerations for Outcome Assessments Keiju Motohashi Office of New Drug II Pharmaceuticals and Medical Devices Agency/ The University of Tokyo Hospital Disclaimer The views and opinions expressed
PMDA Considerations for Outcome Assessments Keiju Motohashi Office of New Drug II Pharmaceuticals and Medical Devices Agency/ The University of Tokyo Hospital Disclaimer The views and opinions expressed
Forward-looking Statements
 NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
Forward-looking Statements
 NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD. Pierre Bedossa. Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE
 PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD Pierre Bedossa Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE 1 PATHOLOGY OF NAFLD NAFLD: a chronic liver disease with a wide
PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD Pierre Bedossa Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE 1 PATHOLOGY OF NAFLD NAFLD: a chronic liver disease with a wide
Bariatric Surgery and Liver Transplantation
 Bariatric Surgery and Liver Transplantation Sammy Saab, MD, MPH, AGAF, FACG, FAASLD Professor of Medicine and Surgery Head, Outcomes Research in Hepatology David Geffen School of Medicine at UCLA Disclosures
Bariatric Surgery and Liver Transplantation Sammy Saab, MD, MPH, AGAF, FACG, FAASLD Professor of Medicine and Surgery Head, Outcomes Research in Hepatology David Geffen School of Medicine at UCLA Disclosures
Corporate Update. NASDAQ: GALT April 9, 2018
 Corporate Update April 9, 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within
Corporate Update April 9, 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) NON-ALCOHOLIC STEATOHEPATITIS (NASH) ADDRESSING A GROWING SILENT EPIDEMIC
 NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical
 Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical Disclosure Naim Alkhouri, MD discloses the following relationships with commercial companies: Membership in the Speakers Bureau for Alexion
Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical Disclosure Naim Alkhouri, MD discloses the following relationships with commercial companies: Membership in the Speakers Bureau for Alexion
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) NON-ALCOHOLIC STEATOHEPATITIS (NASH) ADDRESSING A GROWING SILENT EPIDEMIC
 NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
EVALUATION REPORT
 International Workshop on NASH Biomarkers 2017 5-6 May 2017 Washington, D.C., EVALUATION REPORT www.expertmedicalevents.com/nashbiomarkers EXECUTIVE SUMMARY INTRODUCTION The 2 nd International Workshop
International Workshop on NASH Biomarkers 2017 5-6 May 2017 Washington, D.C., EVALUATION REPORT www.expertmedicalevents.com/nashbiomarkers EXECUTIVE SUMMARY INTRODUCTION The 2 nd International Workshop
Defining the gold standard in biomarker validation for NASH
 Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
Regulatory Updates Veronica Miller, PhD
 Regulatory Updates Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH PARIS_NASH_2018 2 PARIS NASH 2018 Disclosures Dr. Veronica Miller is an employee of the Forum for Collaborative
Regulatory Updates Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH PARIS_NASH_2018 2 PARIS NASH 2018 Disclosures Dr. Veronica Miller is an employee of the Forum for Collaborative
Life After SVR for Cirrhotic HCV
 Life After SVR for Cirrhotic HCV KIM NEWNHAM MN, NP CIRRHOSIS CARE CLINIC UNIVERSITY OF ALBERTA Objectives To review the benefits of HCV clearance in cirrhotic patients To review some of the emerging data
Life After SVR for Cirrhotic HCV KIM NEWNHAM MN, NP CIRRHOSIS CARE CLINIC UNIVERSITY OF ALBERTA Objectives To review the benefits of HCV clearance in cirrhotic patients To review some of the emerging data
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014
 Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
Therapeutic Approaches to Cirrhotic versus Pre-Cirrhotic NASH
 www.alacrita.com Therapeutic Approaches to Cirrhotic versus Pre-Cirrhotic NASH 2nd Annual NASH Summit Europe October 23-24, 2018 Frankfort, Germany Peter G. Traber, MD Partner, Alacrita Consulting Alacrita
www.alacrita.com Therapeutic Approaches to Cirrhotic versus Pre-Cirrhotic NASH 2nd Annual NASH Summit Europe October 23-24, 2018 Frankfort, Germany Peter G. Traber, MD Partner, Alacrita Consulting Alacrita
Update on Non-Alcoholic Fatty Liver Disease. Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI
 Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
Non-alcoholic fatty liver disease: time to take note and manage. Philip Newsome Professor of Hepatology & Director of Centre for Liver Research
 Non-alcoholic fatty liver disease: time to take note and manage Philip Newsome Professor of Hepatology & Director of Centre for Liver Research Disclosures Consultancy, Co-ordinating Investigator roles
Non-alcoholic fatty liver disease: time to take note and manage Philip Newsome Professor of Hepatology & Director of Centre for Liver Research Disclosures Consultancy, Co-ordinating Investigator roles
GR-MD-02 for Indication of NASH Cirrhosis: NASH-CX Clinical Trial Results
 GR-MD-02 for Indication of NASH Cirrhosis: NASH-C Clinical Trial Results Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 1 2018 2017 Galectin
GR-MD-02 for Indication of NASH Cirrhosis: NASH-C Clinical Trial Results Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 1 2018 2017 Galectin
The future of liver transplantation for viral hepatitis
 The future of liver transplantation for viral hepatitis François Durand Hepatology & Liver Intensive Care Hospital Beaujon, Clichy University Paris Diderot France Liver transplantation in France 2013:
The future of liver transplantation for viral hepatitis François Durand Hepatology & Liver Intensive Care Hospital Beaujon, Clichy University Paris Diderot France Liver transplantation in France 2013:
Clinical Trials & Endpoints in NASH Cirrhosis
 Clinical Trials & Endpoints in NASH Cirrhosis April 25, 2018 Peter G. Traber, MD CEO & CMO, Galectin Therapeutics 2018 Galectin Therapeutics NASDAQ: GALT For more information, see galectintherapeutics.com
Clinical Trials & Endpoints in NASH Cirrhosis April 25, 2018 Peter G. Traber, MD CEO & CMO, Galectin Therapeutics 2018 Galectin Therapeutics NASDAQ: GALT For more information, see galectintherapeutics.com
HABP/VABP Patient Outcomes from Previously Conducted Trials
 HABP/VABP Patient Outcomes from Previously Conducted Trials Daniel Rubin, Ph.D. Food and Drug Administration Disclaimer: Slides represent views of the presenter and not necessarily of the FDA 1 Outline
HABP/VABP Patient Outcomes from Previously Conducted Trials Daniel Rubin, Ph.D. Food and Drug Administration Disclaimer: Slides represent views of the presenter and not necessarily of the FDA 1 Outline
Personalised medicine with state-of-the-art MR image analysis
 Personalised medicine with state-of-the-art MR image analysis Biomarkers as tests Diagnostic pathways in chronic disease Heart failure EKG BNP Echo/cardiac MRI COPD CXR Pulmonary function tests Chest CT
Personalised medicine with state-of-the-art MR image analysis Biomarkers as tests Diagnostic pathways in chronic disease Heart failure EKG BNP Echo/cardiac MRI COPD CXR Pulmonary function tests Chest CT
Discrimination and Reclassification in Statistics and Study Design AACC/ASN 30 th Beckman Conference
 Discrimination and Reclassification in Statistics and Study Design AACC/ASN 30 th Beckman Conference Michael J. Pencina, PhD Duke Clinical Research Institute Duke University Department of Biostatistics
Discrimination and Reclassification in Statistics and Study Design AACC/ASN 30 th Beckman Conference Michael J. Pencina, PhD Duke Clinical Research Institute Duke University Department of Biostatistics
WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH?
 WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH? Helena Cortez-Pinto Laboratório de Nutrição, FML, Serviço de Gastrenterologia, Hospital St Maria, Lisboa, Portugal EASL Governing Board:
WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH? Helena Cortez-Pinto Laboratório de Nutrição, FML, Serviço de Gastrenterologia, Hospital St Maria, Lisboa, Portugal EASL Governing Board:
Accelerating Drug Development through Collaboration
 Accelerating Drug Development through Collaboration October 7, 2015 Veronica Miller, PhD UC Berkeley School of Public Health Forum for Collaborative HIV Research Washington DC www.hivforum.org 1 Forum
Accelerating Drug Development through Collaboration October 7, 2015 Veronica Miller, PhD UC Berkeley School of Public Health Forum for Collaborative HIV Research Washington DC www.hivforum.org 1 Forum
Forward-looking Statements
 Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding
Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding
OA Biomarkers: What is required for validation and qualification? Part I. Evaluation Frameworks
 OA Biomarkers: What is required for validation and qualification? Part I. Evaluation Frameworks Michael C. Nevitt, PhD Dept of Epidemiology and Biostatistics University of California, San Francisco OAI
OA Biomarkers: What is required for validation and qualification? Part I. Evaluation Frameworks Michael C. Nevitt, PhD Dept of Epidemiology and Biostatistics University of California, San Francisco OAI
Improving the Lives of Patients with Liver Diseases
 Improving the Lives of Patients with Liver Diseases Corporate Presentation March 2019 Safe Harbor Statement This presentation contains "forward-looking" statements that involve risks, uncertainties and
Improving the Lives of Patients with Liver Diseases Corporate Presentation March 2019 Safe Harbor Statement This presentation contains "forward-looking" statements that involve risks, uncertainties and
Non-Alcoholic Fatty Liver Diseasean underestimated epidemic
 Non-Alcoholic Fatty Liver Diseasean underestimated epidemic Amir Shlomai MD,PhD Head, Department of Medicine D The Liver Institute Rabin Medical Center, Beilinson Hospital The IASLD semi-annual meeting-
Non-Alcoholic Fatty Liver Diseasean underestimated epidemic Amir Shlomai MD,PhD Head, Department of Medicine D The Liver Institute Rabin Medical Center, Beilinson Hospital The IASLD semi-annual meeting-
Quantitative Assessment of the Liver: Breath Tests. M. Shadab Siddiqui, M.D. Virginia Commonwealth University
 Quantitative Assessment of the Liver: Breath Tests M. Shadab Siddiqui, M.D. Virginia Commonwealth University Objectives Principles of breath tests Breath tests in NAFLD Potential applications of breath
Quantitative Assessment of the Liver: Breath Tests M. Shadab Siddiqui, M.D. Virginia Commonwealth University Objectives Principles of breath tests Breath tests in NAFLD Potential applications of breath
EASL International Liver Congress Paris, France 14 April 2018
 NGM282 Improves Fibrosis and NASH-Related Histology in 12 Weeks in Patients With Biopsy-Confirmed NASH, Which is Preceded By Significant Decreases in Hepatic Steatosis, Liver Transaminases and Fibrosis
NGM282 Improves Fibrosis and NASH-Related Histology in 12 Weeks in Patients With Biopsy-Confirmed NASH, Which is Preceded By Significant Decreases in Hepatic Steatosis, Liver Transaminases and Fibrosis
PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective
 PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective Davy Chiodin, VP - Regulatory Science, QA and Compliance, Acerta Pharma (A Member of the AstraZeneca
PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective Davy Chiodin, VP - Regulatory Science, QA and Compliance, Acerta Pharma (A Member of the AstraZeneca
Collaborative Approaches for Developing Kidney Safety Biomarkers
 Collaborative Approaches for Developing Kidney Safety Biomarkers John Michael Sauer, PhD Executive Director of the Predictive Safety Testing Consortium (PSTC) C-Path: A Public-Private Partnership Act as
Collaborative Approaches for Developing Kidney Safety Biomarkers John Michael Sauer, PhD Executive Director of the Predictive Safety Testing Consortium (PSTC) C-Path: A Public-Private Partnership Act as
Organ Allocation in Pennsylvania: Current concepts and future directions
 Organ Allocation in Pennsylvania: Current concepts and future directions David Goldberg, MD, MSCE Assistant Professor of Medicine and Epidemiology Medical Director of Living Donor Liver Transplantation
Organ Allocation in Pennsylvania: Current concepts and future directions David Goldberg, MD, MSCE Assistant Professor of Medicine and Epidemiology Medical Director of Living Donor Liver Transplantation
Hepatology for the Nonhepatologist
 Hepatology for the Nonhepatologist Kenneth E. Sherman, MD, PhD Gould Professor of Medicine Director, Division of Digestive Diseases University of Cincinnati College of Medicine Cincinnati, Ohio Learning
Hepatology for the Nonhepatologist Kenneth E. Sherman, MD, PhD Gould Professor of Medicine Director, Division of Digestive Diseases University of Cincinnati College of Medicine Cincinnati, Ohio Learning
DIAGNOSIS OF NASH LIVER BIOPSY. PHC
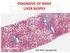 DIAGNOSIS OF NASH LIVER BIOPSY PHC 2018 - www.aphc.info 1 THE NATURAL HISTORY OF NAFLD STEATOSIS (NAFL) STEATOHEPATITIS (NASH) FIBROSIS CIRRHOSIS HCC UNDER THE LENS : THE 3 HISTOLOGICAL COMPONENTS OF NAFLD
DIAGNOSIS OF NASH LIVER BIOPSY PHC 2018 - www.aphc.info 1 THE NATURAL HISTORY OF NAFLD STEATOSIS (NAFL) STEATOHEPATITIS (NASH) FIBROSIS CIRRHOSIS HCC UNDER THE LENS : THE 3 HISTOLOGICAL COMPONENTS OF NAFLD
The Impact of HBV Therapy on Fibrosis and Cirrhosis
 The Impact of HBV Therapy on Fibrosis and Cirrhosis Jordan J. Feld, MD, MPH Associate Professor of Medicine University of Toronto Hepatologist Toronto Centre for Liver Disease Sandra Rotman Centre for
The Impact of HBV Therapy on Fibrosis and Cirrhosis Jordan J. Feld, MD, MPH Associate Professor of Medicine University of Toronto Hepatologist Toronto Centre for Liver Disease Sandra Rotman Centre for
The Chronic Liver Disease Foundation (CLDF) and the International Coalition of Hepatology Education Providers (IC-HEP) present:
 The Chronic Liver Disease Foundation (CLDF) and the International Coalition of Hepatology Education Providers (IC-HEP) present: Certified by: Provided by: Endorsed by: Hepatic Encephalopathy Hepatic Encephalopathy:
The Chronic Liver Disease Foundation (CLDF) and the International Coalition of Hepatology Education Providers (IC-HEP) present: Certified by: Provided by: Endorsed by: Hepatic Encephalopathy Hepatic Encephalopathy:
INVESTOR PRESENTATION
 SEPTEMBER 2018 INVESTOR PRESENTATION September 2018 I. PIPELINE II. INTRODUCTION TO NASH III. GENFIT ASSETS IN NASH IV. BEYOND NASH V. MOVING FORWARD VI. CORPORATE HIGHLIGHTS 1 Disclaimer Important Information
SEPTEMBER 2018 INVESTOR PRESENTATION September 2018 I. PIPELINE II. INTRODUCTION TO NASH III. GENFIT ASSETS IN NASH IV. BEYOND NASH V. MOVING FORWARD VI. CORPORATE HIGHLIGHTS 1 Disclaimer Important Information
EVALUATION OF ABNORMAL LIVER TESTS
 EVALUATION OF ABNORMAL LIVER TESTS MIA MANABAT DO PGY6 MOA 119 TH ANNUAL SPRING SCIENTIFIC CONVENTION MAY 19, 2018 EVALUATION OF ABNORMAL LIVER TESTS Review of liver enzymes vs liver function tests Clinical
EVALUATION OF ABNORMAL LIVER TESTS MIA MANABAT DO PGY6 MOA 119 TH ANNUAL SPRING SCIENTIFIC CONVENTION MAY 19, 2018 EVALUATION OF ABNORMAL LIVER TESTS Review of liver enzymes vs liver function tests Clinical
EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease.
 Commentary. EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease. Christopher D. Byrne 1,2, Giovanni Targher 3 1 Nutrition and Metabolism, Faculty of Medicine,
Commentary. EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease. Christopher D. Byrne 1,2, Giovanni Targher 3 1 Nutrition and Metabolism, Faculty of Medicine,
Corporate Presentation
 Developing Innovative Therapies for Patients Suffering from Life-threatening Diseases Corporate Presentation NasdaqCM: LJPC July 2014 Forward-Looking Statements These slides contain "forward-looking" statements
Developing Innovative Therapies for Patients Suffering from Life-threatening Diseases Corporate Presentation NasdaqCM: LJPC July 2014 Forward-Looking Statements These slides contain "forward-looking" statements
The Effect of Antiviral Therapy on Liver Fibrosis in CHC. Jidong Jia Beijing Friendship Hospital, Capital Medical University
 The Effect of Antiviral Therapy on Liver Fibrosis in CHC Jidong Jia Beijing Friendship Hospital, Capital Medical University 2016-5-29 1 Disclosure Consultation for Abbvie, BMS, Gilead, MSD, Novartis and
The Effect of Antiviral Therapy on Liver Fibrosis in CHC Jidong Jia Beijing Friendship Hospital, Capital Medical University 2016-5-29 1 Disclosure Consultation for Abbvie, BMS, Gilead, MSD, Novartis and
Jan vp24.i. GENFIT Overview. January 2014
 Jan 2014. vp24.i GENFIT Overview January 2014 1 Disclaimer Forward Looking Statement GENFIT IS A PUBLIC COMPANY LISTED ON NYSE EURONEXT (ALTERNEXT PARIS) STOCK EXCHANGE SINCE 2006. THIS DOCUMENT DOES NOT
Jan 2014. vp24.i GENFIT Overview January 2014 1 Disclaimer Forward Looking Statement GENFIT IS A PUBLIC COMPANY LISTED ON NYSE EURONEXT (ALTERNEXT PARIS) STOCK EXCHANGE SINCE 2006. THIS DOCUMENT DOES NOT
Nash with cirrhosis icd 10
 Free, official coding info for 2018 ICD-10-CM K75.81 - includes detailed crosswalks, DRG grouping and more. Free, official coding info for 2018 ICD-10-CM K74.69 - includes detailed crosswalks, DRG grouping
Free, official coding info for 2018 ICD-10-CM K75.81 - includes detailed crosswalks, DRG grouping and more. Free, official coding info for 2018 ICD-10-CM K74.69 - includes detailed crosswalks, DRG grouping
INVESTOR PRESENTATION. November 16 th, 2015
 INVESTOR PRESENTATION November 16 th, 2015 1 Disclaimer Important information and forward looking statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE SINCE APRIL
INVESTOR PRESENTATION November 16 th, 2015 1 Disclaimer Important information and forward looking statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE SINCE APRIL
Panel 1 Discussion. Capitalizing on the Totality of Evidence to Streamline Approvals for Supplemental Indications. #FriendsAM17
 Panel 1 Discussion Capitalizing on the Totality of Evidence to Streamline Approvals for Supplemental Indications #FriendsAM17 Panel 1 Participants Moderator: George Demetri, Dana-Farber Cancer Institute,
Panel 1 Discussion Capitalizing on the Totality of Evidence to Streamline Approvals for Supplemental Indications #FriendsAM17 Panel 1 Participants Moderator: George Demetri, Dana-Farber Cancer Institute,
A pathologist, a radiologist and a hepatologist walked into a bar
 A pathologist, a radiologist and a hepatologist walked into a bar Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology Saint Louis
A pathologist, a radiologist and a hepatologist walked into a bar Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology Saint Louis
Overview of PSC Making the Diagnosis
 Overview of PSC Making the Diagnosis Tamar Taddei, MD Assistant Professor of Medicine Yale University School of Medicine Overview Definition Epidemiology Diagnosis Modes of presentation Associated diseases
Overview of PSC Making the Diagnosis Tamar Taddei, MD Assistant Professor of Medicine Yale University School of Medicine Overview Definition Epidemiology Diagnosis Modes of presentation Associated diseases
Bariatric Surgery in NASH Results, indications and contra-indications
 Bariatric Surgery in NASH Results, indications and contra-indications Guillaume Lassailly CHRU de Lille Conflit of interest No conflict of interest related to this lecture Contract with: Novartis Gilead
Bariatric Surgery in NASH Results, indications and contra-indications Guillaume Lassailly CHRU de Lille Conflit of interest No conflict of interest related to this lecture Contract with: Novartis Gilead
Hepatitis C wi w t i h Ju J dy y W y W a y t a t t
 Hepatitis C with Judy Wyatt Hepatitis C and the histopathologist Pre-2006 biopsy based treatment of moderate-severe chronic hepatitis Now biopsy for: Watchful waiting, to confirm mild disease? Cirrhosis
Hepatitis C with Judy Wyatt Hepatitis C and the histopathologist Pre-2006 biopsy based treatment of moderate-severe chronic hepatitis Now biopsy for: Watchful waiting, to confirm mild disease? Cirrhosis
A COMPANY INSPIRED BY REGENERATION
 OUR FOCUS A COMPANY INSPIRED BY REGENERATION MEET ALLY, a teacher, mother and motorcycle enthusiast with primary biliary cholangitis*(pbc), a rare and potentially fatal non-viral liver disease. OUR FOCUS
OUR FOCUS A COMPANY INSPIRED BY REGENERATION MEET ALLY, a teacher, mother and motorcycle enthusiast with primary biliary cholangitis*(pbc), a rare and potentially fatal non-viral liver disease. OUR FOCUS
1 ST EUROPEAN. NASH Workshop BARCELONA, SPAIN 8-9 MARCH 2019 REQUEST FOR SUPPORT.
 1 ST EUROPEAN NASH Workshop BARCELONA, SPAIN 8-9 MARCH 2019 REQUEST FOR SUPPORT NEEDS ASSESSMENT NEEDS ASSESSMENT Non-alcoholic fatty liver disease (NAFLD) is an emerging liver disease affecting 25 % of
1 ST EUROPEAN NASH Workshop BARCELONA, SPAIN 8-9 MARCH 2019 REQUEST FOR SUPPORT NEEDS ASSESSMENT NEEDS ASSESSMENT Non-alcoholic fatty liver disease (NAFLD) is an emerging liver disease affecting 25 % of
NONALCOHOLIC FATTY LIVER DISEASE. Non-Alcoholic Fatty Liver Disease (NAFLD) Primary NAFLD. April 13, 2012
 NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Primary NAFLD Simple Steatosis Fatty hepatocytes Intracellular fat
NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Primary NAFLD Simple Steatosis Fatty hepatocytes Intracellular fat
DENOMINATOR: All patients aged 18 years and older with a diagnosis of chronic hepatitis C cirrhosis
 Quality ID #401: Hepatitis C: Screening for Hepatocellular Carcinoma (HCC) in Patients with Cirrhosis National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Preventive Care
Quality ID #401: Hepatitis C: Screening for Hepatocellular Carcinoma (HCC) in Patients with Cirrhosis National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Preventive Care
Challenges in the Diagnosis of Steatohepatitis
 The Bugaboos of Fatty Liver Disease: Ballooning and Fibrosis Hans Popper Hepatopathology Society Companion Meeting San Antonio, Tx March, 2017 David Kleiner, M.D., Ph.D. NCI/Laboratory of Pathology Challenges
The Bugaboos of Fatty Liver Disease: Ballooning and Fibrosis Hans Popper Hepatopathology Society Companion Meeting San Antonio, Tx March, 2017 David Kleiner, M.D., Ph.D. NCI/Laboratory of Pathology Challenges
NONALCOHOLIC FATTY LIVER DISEASE
 NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD, MSc Hepatology and Liver Transplantation University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Terminology Pathogenesis
NONALCOHOLIC FATTY LIVER DISEASE Kiran Bambha, MD, MSc Hepatology and Liver Transplantation University of Colorado Denver April 13, 2012 Non-Alcoholic Fatty Liver Disease (NAFLD) Terminology Pathogenesis
Treatment of Hepatitis C Recurrence after Liver Transplantation. Maria Carlota Londoño Liver Unit Hospital Clínic Barcelona
 Treatment of Hepatitis C Recurrence after Liver Transplantation Maria Carlota Londoño Liver Unit Hospital Clínic Barcelona Agenda 1. Introduction 2. Treatment options for hepatitis C recurrence after transplantation
Treatment of Hepatitis C Recurrence after Liver Transplantation Maria Carlota Londoño Liver Unit Hospital Clínic Barcelona Agenda 1. Introduction 2. Treatment options for hepatitis C recurrence after transplantation
Consensus AASLD-EASL HBV Treatment Endpoint and HBV Cure Definition
 Consensus AASLD-EASL HBV Treatment Endpoint and HBV Cure Definition Anna S. Lok, MD, DSc Alice Lohrman Andrews Professor in Hepatology Director of Clinical Hepatology Assistant Dean for Clinical Research
Consensus AASLD-EASL HBV Treatment Endpoint and HBV Cure Definition Anna S. Lok, MD, DSc Alice Lohrman Andrews Professor in Hepatology Director of Clinical Hepatology Assistant Dean for Clinical Research
Liver Pathology and the Clinician in 2015: At the Crossroads. Thomas D. Schiano, M.D. Mount Sinai Medical Center New York, New York
 Liver Pathology and the Clinician in 2015: At the Crossroads Thomas D. Schiano, M.D. Mount Sinai Medical Center New York, New York DISCLOSURES Consultant for: Salix Merck Gilead BMS Synageva Research funding:
Liver Pathology and the Clinician in 2015: At the Crossroads Thomas D. Schiano, M.D. Mount Sinai Medical Center New York, New York DISCLOSURES Consultant for: Salix Merck Gilead BMS Synageva Research funding:
Case #1. Digital Slides 11/6/ year old woman presented with abnormal liver function tests. Liver Biopsy to r/o autoimmune hepatitis
 45 year old woman presented with abnormal liver function tests Liver Biopsy to r/o autoimmune hepatitis Further down. ANA 1: 160; ASMA 1:80 ANA 1: 160; ASMA 1:80 IgG = 14.5 g/l (upper normal range: 16)
45 year old woman presented with abnormal liver function tests Liver Biopsy to r/o autoimmune hepatitis Further down. ANA 1: 160; ASMA 1:80 ANA 1: 160; ASMA 1:80 IgG = 14.5 g/l (upper normal range: 16)
Liver Transplantation: The End of the Road in Chronic Hepatitis C Infection
 University of Massachusetts Medical School escholarship@umms UMass Center for Clinical and Translational Science Research Retreat 2012 UMass Center for Clinical and Translational Science Research Retreat
University of Massachusetts Medical School escholarship@umms UMass Center for Clinical and Translational Science Research Retreat 2012 UMass Center for Clinical and Translational Science Research Retreat
Liver Forum 7: Summary of Proceedings Thursday, October 19, 2017 Washington D.C.
 Liver Forum 7: Summary of Proceedings Thursday, October 19, 2017 Washington D.C. Facilitating Collaborative Research in Drug Development and Health Policy 1608 Rhode Island Avenue NW, Suite 212, Washington
Liver Forum 7: Summary of Proceedings Thursday, October 19, 2017 Washington D.C. Facilitating Collaborative Research in Drug Development and Health Policy 1608 Rhode Island Avenue NW, Suite 212, Washington
INVESTOR PRESENTATION
 2017.04.24 INVESTOR PRESENTATION APRIL 2017 1 Disclaimer Important Information and Forward Looking Statements THIS PRESENTATION HAS BEEN PREPARED BY GENFIT AND IS FOR INFORMATION PURPOSES ONLY. CERTAIN
2017.04.24 INVESTOR PRESENTATION APRIL 2017 1 Disclaimer Important Information and Forward Looking Statements THIS PRESENTATION HAS BEEN PREPARED BY GENFIT AND IS FOR INFORMATION PURPOSES ONLY. CERTAIN
Disclosures. The Typical Therapeutic Pyramid $$$ The NAFLD Umbrella. The Big Question: What are the treatment options for NASH?
 Disclosures I have the following relationships to disclose: Ethicon Endo Surgery Inc. Galectin Therapeutics Synageva Biopharma Raptor Pharmaceuticals I will be discussing off label use of medications in
Disclosures I have the following relationships to disclose: Ethicon Endo Surgery Inc. Galectin Therapeutics Synageva Biopharma Raptor Pharmaceuticals I will be discussing off label use of medications in
Update on Nonalcoholic Fatty Liver Disease. Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic
 Update on Nonalcoholic Fatty Liver Disease Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic Outline Defining the phenotypes of nonalcoholic fatty liver disease NAFLD Diagnostics
Update on Nonalcoholic Fatty Liver Disease Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic Outline Defining the phenotypes of nonalcoholic fatty liver disease NAFLD Diagnostics
M30 Apoptosense ELISA. A biomarker assay for detection and screening of NASH
 M30 Apoptosense ELISA A biomarker assay for detection and screening of NASH NASH A Global Disease In the Western countries, Non-Alcoholic Fatty Liver Disease (NAFLD) is the most common liver disease, strongly
M30 Apoptosense ELISA A biomarker assay for detection and screening of NASH NASH A Global Disease In the Western countries, Non-Alcoholic Fatty Liver Disease (NAFLD) is the most common liver disease, strongly
Q4 Report Webcast February 7, 2019 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO
 Q4 Report 2018 Webcast February 7, 2019 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation has been prepared by Calliditas Therapeutics AB
Q4 Report 2018 Webcast February 7, 2019 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation has been prepared by Calliditas Therapeutics AB
An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications
 REVIEW An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications Naim Alkhouri, M.D.,*, and Andrea Scott, B.S.* Nonalcoholic fatty liver disease (NAFLD)
REVIEW An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications Naim Alkhouri, M.D.,*, and Andrea Scott, B.S.* Nonalcoholic fatty liver disease (NAFLD)
Advances in Understanding Hepatic Fibrosis and Chronic Liver Disease
 Advances in Understanding Hepatic Fibrosis and Chronic Liver Disease Scott Friedman, M.D. Fishberg Professor of Medicine Dean for Therapeutic Discovery Chief, Division of Liver Diseases Icahn School of
Advances in Understanding Hepatic Fibrosis and Chronic Liver Disease Scott Friedman, M.D. Fishberg Professor of Medicine Dean for Therapeutic Discovery Chief, Division of Liver Diseases Icahn School of
Hepatopulmonary Syndrome: An Update
 Hepatopulmonary Syndrome: An Update Michael J. Krowka MD Professor of Medicine Division of Pulmonary and Critical Care Division of Gastroenterology and Hepatology Mayo Clinic Falk Liver Week October 11,
Hepatopulmonary Syndrome: An Update Michael J. Krowka MD Professor of Medicine Division of Pulmonary and Critical Care Division of Gastroenterology and Hepatology Mayo Clinic Falk Liver Week October 11,
PROs for Drug Development. Melanie Blank, MD
 PROs for Drug Development in Chronic Kidney Disease Melanie Blank, MD Disclaimer The views expressed here represent my opinions and do not necessarily represent the views of the FDA. Overview Stagnation
PROs for Drug Development in Chronic Kidney Disease Melanie Blank, MD Disclaimer The views expressed here represent my opinions and do not necessarily represent the views of the FDA. Overview Stagnation
tenofovir disoproxil (as fumarate), 245mg, film-coated tablet (Viread ) SMC No. (720/11) Gilead Sciences Ltd
 tenofovir disoproxil (as fumarate), 245mg, film-coated tablet (Viread ) SMC No. (720/11) Gilead Sciences Ltd 05 August 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
tenofovir disoproxil (as fumarate), 245mg, film-coated tablet (Viread ) SMC No. (720/11) Gilead Sciences Ltd 05 August 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
NONALCOHOLIC STEATOHEPATITIS (NASH) - OPPORTUNITY ANALYSIS AND FORECASTS TO EVENT-DRIVEN UPDATE
 REFERENCE CODE GDHC034POA PUBLICAT ION DATE MARCH 2014 NONALCOHOLIC STEATOHEPATITIS (NASH) - - EVENT-DRIVEN UPDATE Executive Summary NASH: Key Metrics in Six Major Pharmaceutical Markets 2012 Epidemiology
REFERENCE CODE GDHC034POA PUBLICAT ION DATE MARCH 2014 NONALCOHOLIC STEATOHEPATITIS (NASH) - - EVENT-DRIVEN UPDATE Executive Summary NASH: Key Metrics in Six Major Pharmaceutical Markets 2012 Epidemiology
Hepatocellular Carcinoma: Epidemiology and Screening
 Hepatocellular Carcinoma: Epidemiology and Screening W. Ray Kim, MD Professor and Chief Gastroenterology and Hepatology Stanford University School of Medicine Case A 67 year old Filipino-American woman
Hepatocellular Carcinoma: Epidemiology and Screening W. Ray Kim, MD Professor and Chief Gastroenterology and Hepatology Stanford University School of Medicine Case A 67 year old Filipino-American woman
HBV Forum 2 April 18 th 2017 Hilton Amsterdam.
 HBV Forum 2 April 18 th 2017 Hilton Amsterdam www.forumresearch.org Detection, Assessment and Management of DILI During Drug Development for HBV: The IQ DILI Initiative. Arie Regev, MD Global Patient Safety
HBV Forum 2 April 18 th 2017 Hilton Amsterdam www.forumresearch.org Detection, Assessment and Management of DILI During Drug Development for HBV: The IQ DILI Initiative. Arie Regev, MD Global Patient Safety
Assessment of sofosbuvir (Sovaldi )
 Assessment of sofosbuvir (Sovaldi ) Summary of the national assessments of sofosbuvir (Sovaldi ) for the treatment of Hepatitis C December 18, 2014 Health Care Institute, Diemen, NL Lead Partner WP5 EUnetHTA
Assessment of sofosbuvir (Sovaldi ) Summary of the national assessments of sofosbuvir (Sovaldi ) for the treatment of Hepatitis C December 18, 2014 Health Care Institute, Diemen, NL Lead Partner WP5 EUnetHTA
Obesity, Inflammation and Liver Cancer
 Obesity, Inflammation and Liver Cancer Richard Moreau, M.D., 1 INSERM U773, Centre de Recherche Biomédicale Bichat-Beaujon CRB3, 2 Université Denis Diderot Paris 7, 3 Service d Hépatologie, Hôpital Beaujon,
Obesity, Inflammation and Liver Cancer Richard Moreau, M.D., 1 INSERM U773, Centre de Recherche Biomédicale Bichat-Beaujon CRB3, 2 Université Denis Diderot Paris 7, 3 Service d Hépatologie, Hôpital Beaujon,
End Stage Liver Disease & Disease Specific Indications for Liver Transplant. Susan Kang, RN, MSN, ANP-BC
 End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP-BC Introduction (https://www.srtr.org) What does the liver do? STORAGE METABOLIC DETOXIFICATION SYNTHETIC
End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP-BC Introduction (https://www.srtr.org) What does the liver do? STORAGE METABOLIC DETOXIFICATION SYNTHETIC
End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP BC
 End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP BC Introduction (https://www.srtr.org) 1 What does the liver do? STORAGE METABOLIC DETOXIFICATION SYNTHETIC
End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP BC Introduction (https://www.srtr.org) 1 What does the liver do? STORAGE METABOLIC DETOXIFICATION SYNTHETIC
