Research Review PRODUCT REVIEW. About the Reviewer. Dr Jennifer Pilgrim MB ChB (Otago), FRACP. Atopic eczema
|
|
|
- Harold Logan
- 5 years ago
- Views:
Transcription
1 Research Review PRODUCT REVIEW Methylprednisolone aceponate 0.1% ointment/cream [ADVANTAN ] About the Reviewer Dr Jennifer Pilgrim MB ChB (Otago), FRACP A graduate of Otago University, Dr Jennifer Pilgrim undertook postgraduate training at Wadsworth VA-UCLA, California and St John s Hospital, London. She practices as a specialist dermatologist at Bowen Hospital, Wellington and also at Victoria University Student Health Service. Jennifer has extensive experience in a wide range of dermatological areas including paediatric and adolescent dermatology, minor surgery, tattoo removal, vulval disorders and some cosmetic procedures. About Research Review Research Review is an independent medical publishing organisation producing electronic publications in a wide variety of specialist areas. Product Reviews feature independent short summaries of major research affecting an individual medicine or technique. They include a background to the particular condition, a summary of the medicine and selected studies by a key New Zealand specialist with a comment on the relevance to New Zealand practice. Research Review publications are intended for New Zealand medical professionals. This review discusses the evidence in support of the use of methylprednisolone aceponate 0.1% ointment/cream [ADVANTAN ], a fourth-generation, non-halogenated topical corticosteroid approved for use in New Zealand for the management of atopic eczema. Methylprednisolone aceponate has the advantage of once-daily dosing, providing benefits in terms of patient compliance, and has an optimised efficacy/safety profile with minimal local or systemic adverse effects. This agent is suitable for use in adults and children and also for short-term use on the face. This review focuses largely on the use of methylprednisolone aceponate in children with atopic eczema. Methylprednisolone aceponate 0.1% ointment/cream is fully funded by Pharmac. Atopic eczema Atopic eczema (also known as atopic dermatitis) is a chronically relapsing, intensely pruritic inflammatory skin disease which favours flexural skin. 1 The condition typically develops in childhood, with approximately 80% of cases developing before the age of 5 years. 2 In the acute phase of the disease, erythema, vesicles, papules, crusts and weeping may be present, while in the chronic phase, lichenification and scaling of the skin are the predominant features. 3 Evidence suggests that approximately 60% of childhood eczema cases are free of symptoms by early adolescence, but symptoms may recur in adulthood, often as hand eczema. 1 Children with early-onset, severe atopic eczema associated with asthma and/or hay fever tend to have a worse prognosis. 1 One-third of community cases of atopic eczema are in adults. 1 Overall, approximately 80% of cases of atopic eczema in the community are mild. 1 Individuals with atopic eczema often have an inherited `atopic tendency, meaning that they may develop closely linked conditions such as asthma and rhinoconjunctivitis, although 20% of individuals with a clinically typical atopic distribution of eczema are not atopic. 1,4 Prevalence The overall prevalence of atopic eczema is 2-5%, with a prevalence of approximately 10-20% in children and young adults, thus making it the most common skin disease. 1,4-8 A 2001 NZ study reported an atopic eczema prevalence in children aged 6 7 years of 15% and a prevalence of 13% among those aged years. 6 The burden of atopic eczema Considerable physical and emotional morbidity may be present for the child with atopic eczema and his/her family, especially if the disease is poorly controlled. 1,9-12 In fact, an Australian study has shown that family stress related to the care of a child with moderate or severe atopic eczema is significantly greater than that for families of children with insulin-dependent diabetes mellitus. 11 There are many factors that contribute to reduced quality of life for sufferers and their families, including itching, soreness, psychological issues, physical issues, time involved in applying creams, frequent visits to the doctor, dietary issues and sleep loss due to the constant itch associated with the condition. 1,11,13 A NZ survey found that 14% of children and 16% of adolescents with atopic eczema had their sleep disturbed at least one night per week by their condition. 6 Another study revealed a mean of 1-2 hours of sleep lost each night for both sufferers and parents of those with moderate or severe forms of the condition. 11 It has also been shown that on average, individuals with severe atopic eczema take 5.3 days off school or work per year because of their disease and, while experiencing a flare, their concentration is significantly affected. 12 The economic costs associated with atopic eczema are high for both patient and community. 1 In Australia, it is estimated that the total cost of treating children with chronic eczema is $A316.7 million per year (this cost does not take into account treating children with recent-onset eczema). 7 Diagnosis The diagnosis of atopic eczema is a clinical one based on itching, redness and skin crease involvement, and takes into account the atopic stigmata of the condition. 1,4 The UK National Institute for Health and Clinical Excellence (NICE) 2007 guidelines recommend using the criteria outlined in Figure 1 for diagnosing atopic eczema in children. 14 The guidelines, which have taken into account all available severity tools (including the Eczema Area and Severity Index [EASI]), recommend that healthcare professionals adopt a holistic approach when assessing the severity of a child s atopic eczema (Table 1). 14,15 This assessment should guide treatment decisions. 1
2 Table 1: Assessing the severity of atopic eczema Child presents with rash Skin/physical severity Impact on quality of life and psychosocial wellbeing Clear Normal skin, no evidence of active atopic eczema No impact on quality of life Is it atopic eczema? Mild Moderate Areas of dry skin, infrequent itching (with or without small areas of redness) Areas of dry skin, frequent itching, redness (with or without excoriation and localised skin thickening) Little impact on everyday activities, sleep and psychosocial wellbeing Moderate impact on everyday activities and psychosocial wellbeing, frequently disturbed sleep Itchy skin condition and three or more of: Visible flexural eczema in children <18 months Personal history of flexural eczema aged <18 months Personal history of dry skin Personal history of asthma/allergic rhinitis History of atopic disease in first-degree relative Onset of signs and symptoms under the age of 2 years Severe Widespread areas of dry skin, incessant itching, redness (with or without excoriation, extensive skin thickening, bleeding, oozing, cracking and alteration of pigmentation) Severe limitation of everyday activities and psychosocial functioning, nightly loss of sleep YES NO (Adapted from UK NICE clinical guidelines 14 ) Treatment options As with other chronic conditions, the management plan for atopic eczema requires a multidisciplinary approach directed at longterm stabilisation, the prevention of flares and the avoidance of adverse effects. 4 Trigger factors such as irritants, contact allergens, food allergens and/or inhalant allergens need to be identified and managed, and treatment must be planned with a long-term perspective. Treating an exacerbation of atopic eczema involves efficient short-term control of acute symptoms and can be a therapeutic challenge. 4 Mild cases of atopic eczema may be managed by avoiding sources of irritation and using emollients, which should be continued even when the skin is clear. 14 In the UK, current first-line treatment of atopic eczema involves the use of emollients, topical corticosteroids and antihistamines. 1 Secondline treatments include ultraviolet light therapy (although this is not used in NZ for children) and allergen avoidance. 1 Thirdline treatments include systemic immunomodulatory agents (including azathioprine and cyclosporin A). 1 International guidelines recommend that a stepped approach to management be employed, with treatment tailored to the severity of the condition (see Figure 1) Treatment should be stepped up or down according to clinical response. Always use emollients, even when the skin is clear and add other treatments when required, with specialist advice where recommended (see page 6). Patients and their caregivers should be given advice on the quantities and frequency of treatments to be used and informed about recognising symptoms of bacterial and viral infection. A useful personal eczema management plan for patients with eczema is available from: Emollient therapy is crucial for maintaining skin hydration and improving skin barrier function, and is usually achieved by at least twice daily application of a moisturiser. 4 The use of emollients has been shown to significantly reduce the amount of topical corticosteroids used in infants with atopic eczema. 17 The jury seems to be out regarding the timing of the application of topical corticosteroids and emollients in conjunction with each other and, to date, no controlled trials have investigated this issue. Assess severity (see Table 1): Physical Psychological Social Discuss diagnosis and management options with child and parents/carers No active eczema (dry skin) Avoidance of irritants Identify and address specific trigger factors Mild eczema Mild-potency topical corticosteroids Seek alternative diagnosis Moderate eczema Moderatepotency topical corticosteroids Topical calcineurin inhibitors* Flares of eczema Step up therapy ladder Treat infection Review until condition settles Provide education for child, parents/carers Severe eczema Moderate/potent topical corticosteroids Topical calcineurin inhibitors* Bandages Phototherapy Systemic therapy (oral corticosteroids, non-steroidal immunosuppressants) Figure 1: Treatment algorithm for the diagnosis and treatment of paediatric atopic eczema. (Adapted from UK NICE clinical guidelines 14 ) * Pimecrolimus cream [Elidel ] is the only topical calcineurin inhibitor approved for use in NZ for atopic eczema. The agent is not funded in NZ. 2
3 Topical corticosteroids Mild atopic eczema may be managed sufficiently with emollients, but anti-inflammatory agents such as topical corticosteroids are required for moderate-to-severe symptoms resulting from acute flares and possibly for maintaining remission (when used intermittently). Topical corticosteroids, which are divided into four groups according to their strength (Class 1-4, see below), have been the gold standard in the treatment of atopic eczema for more than five decades. In treating atopic eczema the weakest possible steroid that will do the job should be used. However, at times it may be necessary to use a more potent corticosteroid to clear the skin. The NICE guidelines state that short treatment with a potent topical corticosteroid is as effective as a longer treatment with a mild preparation. 14 These agents are considered to be effective and safe when used correctly. 1 The following topical corticosteroids are available in NZ (some are available in combination with antibacterial or antifungal agents). Creams may be more suitable if the problem area is weeping, while ointments are recommended for drier areas. Lotions are more suitable for hairy areas. Continuous use for less than 1 month is considered short-term use, while use for greater than 3 months is considered long-term use. Class 1 (very potent): Clobetasol propionate Betamethasone dipropionate (in optimised vehicle) Class 2 (potent): Methylprednisolone aceponate Mometasone furoate Betamethasone valerate Betamethasone dipropionate Diflucortolone valerate Hydrocortisone 17-butyrate Class 3 (moderate): Clobetasone butyrate Triamcinolone acetonide Class 4 (mild): Hydrocortisone Therapeutic index Methylprednisolone aceponate has been awarded an excellent therapeutic rating by the German Society of Dermatology who have published a Therapeutic Index (TIX) for a number of topical corticosteroids. 18 The TIX describes the balance between the efficacy of a topical corticosteroid and its adverse effects (see Table 2). Table 2: The benefit-to-risk ratio as assessed by the Therapeutic Index (TIX) published by the German Society of Dermatology for a selection of Class 1 and 2 topical corticosteroids Topical corticosteroid Efficacy score Toxicity score TIX rating Methylprednisolone aceponate Mometasone furoate Clobetasol propionate Hydrocortisone butyrate Betamethasone valerate (Adapted from Ruzicka et al 2006) 18,19 Duration of topical corticosteroid use Mild atopic eczema may respond to low-potency topical corticosteroids within a few days, with symptoms clearing within 1-2 weeks. 20 Moderate disease may require more potent topical corticosteroids and these may need to be used for several weeks to clear symptoms. 20 Severe disease may only partially respond to potent topical corticosteroids after several months. 20 The NICE guidelines also suggest considering treating problem areas with topical corticosteroids for two consecutive days per week (often referred to as `weekend therapy ) in order to prevent flares in children experiencing 2-3 flares per month (this strategy should be reviewed for effectiveness within 3 to 6 months). 14 Treatment compliance issues Poor compliance with therapy is a major issue in treating patients with atopic eczema. Interestingly, the strongest predictor of treatment compliance is a good parent-doctor relationship. 15,21,22 Furthermore, a survey has shown that steroid phobia is a major contributor to poor treatment compliance and while the majority of patients receive topical corticosteroids to treat flares, 49% are concerned about using these agents. 12,23 Therefore, it is crucial that patients and their families are provided with simple, clear, unambiguous information on the management of this disease and are adequately educated regarding the risks and benefits of corticosteroid use. 7,15,24 Using topical corticosteroids that are dosed once daily as opposed to twice daily provides benefits in terms of patient compliance. About methylprednisolone aceponate Methylprednisolone aceponate 0.1% topical ointment or cream [Advantan ] is a fourth-generation, non-halogenated corticosteroid, available in NZ and approved by Medsafe for the management of atopic eczema in children and adults; specific indications include endogenous eczema, neurodermatitis and contact eczema. 25 The agent is fully funded by Pharmac and has a list price of NZ$4.95 per 15g tube. Clinicians may prescribe as many 15g tubes as deemed necessary for up to 3 months of treatment (see Prescribing Advantan section below for advice on determining the amount required). Efficacy data Methylprednisolone aceponate, dosed once daily, is considered to be a safe and effective acute and maintenance treatment choice for children (including infants) and adults with moderate-to-severe atopic eczema requiring fast and effective relief from their symptoms. 3,19,26-30 Its onset of activity is very rapid, with 50-80% of patients experiencing complete or distinct symptom improvement within 1 week of starting treatment and 90% experiencing such improvement within 3 weeks. 3 This is also true for patients with severe symptoms, who have been shown to experience fast and effective relief from reddening and itching. 3 Methylprednisolone aceponate compares favourably with other corticosteroids in its class and to tacrolimus. 3,19,26-30 The efficacy and tolerability of once daily methylprednisolone aceponate was shown to be equivalent to that of betamethasone valerate dosed twice daily in patients with various forms of eczema, with success rates of >85%. 19,31 Furthermore, a randomised, double-blinded comparative study comparing methylprednisolone aceponate 0.1% ointment once daily (n = 129) and the calcineurin inhibitor tacrolimus 0.03% ointment applied twice daily (n = 136) for 3 weeks in children and adolescents with severe to very severe flares of atopic eczema revealed a successful therapy rate in both groups of 67%. 26 Notably in that study, an approximate 70% reduction in itch was seen with methylprednisolone aceponate after 7 days and the agent was superior to tacrolimus for EASI, itch and sleep measures. Another study investigating the long-term management of moderate-to-severe atopic eczema in 221 individuals aged 12 years revealed that the agent applied twice weekly for 16 weeks along with an emollient effectively controlled the disease with a significantly reduced risk of relapse and no apparent adverse effects. 32 Safety data The overall incidence of adverse effects associated with methylprednisolone aceponate is approximately 5% and this agent exhibits a low incidence of systemic adverse effects. 3 When adverse effects do occur they are almost always mild-tomoderate in severity and do not usually result in the discontinuation of treatment. 3 The most commonly seen adverse effects are mild erythema, dryness and a sensation of burning, scaling and rash. 3,28 A number of studies have demonstrated that the incidence and severity of adverse effects with methylprednisolone aceponate are similar to those of less potent corticosteroids and significantly lower than those of other corticosteroids in the same class. 3 One such study was undertaken by Kecskés comparing methylprednisolone aceponate and mometasone furoate. 33 Their study revealed equal anti-inflammatory activity of the two agents and similar cortisol suppression, but significantly fewer local adverse effects with methylprednisolone aceponate than mometasone furoate. The favourable safety profile of methylprednisolone aceponate renders it a suitable agent for use in facial eczema, and postmarketing surveillance has confirmed the very good efficacy and tolerability of this agent when used for this indication. 34 The development of perioral dermatitis is not likely if use of the agent beyond clinical healing is avoided. 34 3
4 Contraindications Contraindications to the use of methylprednisolone aceponate include most viral diseases (e.g. varicella/ herpes zoster, vaccinia), and the presence of syphilitic or tuberculous processes and post-vaccination skin reactions in the area to be treated. 25 Pharmacological properties Methylprednisolone aceponate has a methyl group at C-6 and both of the alcohol residues attached to the five-membered ring (a propionate group at C-17 and an acetate group at C-21) are esterified. 3 The methyl group at C-6 is associated with a high intrinsic activity (drug-receptor binding), while the lipophilic diester grouping allows for good penetration into the stratum corneum. 29 The degree of penetration depends on the state of the skin and the conditions of application (open/occlusion). 25 Within the epidermis and dermis, methylprednisolone aceponate is rapidly metabolised by esterases to the active metabolite methylprednisolone-17-propionate, which binds 3-fold more strongly to glucocorticoid receptors than methylprednisolone aceponate. 3 Inflamed skin with its higher concentration of esterases, has been found to concentrate this active metabolite. 3 Within the skin, methylprednisolone-17-propionate is converted to methylprednisolone-21-propionate, which is hydrolysed and rendered relatively inactive. 3 Glucuronic acid rapidly inactivates any methylprednisolone-17-propionate entering the systemic circulation. 3 These metabolites are eliminated via the kidneys with a half-life of approximately 16 hours. 25 Unlike other potent corticosteroids, methylprednisolone aceponate does not have a halogen at C-9 and this feature allows for the high degree of dissociation between topical and systemic effects; nonhalogenated corticosteroids appear to preserve the function of the circadian rhythm of cortisol secretion even when used on large areas (40-60% of the skin surface). 25,29,30 Dosage and administration 25 One gram of cream or ointment contains 1mg (0.1%) methylprednisolone aceponate. The agent is for external topical use only. Generally, the formulation deemed most appropriate to the skin conditions is applied thinly once daily and should be used for no more than 12 weeks in adults and no more than 4 weeks in children. The cream formulation of Advantan has a high water content and a low fat content and is particularly suitable for the weeping stages of eczema. The ointment formulation of Advantan is suitable for skin that is dry. When being applied to the face, the agent should only be used for short periods and must not come into contact with the eyes. If signs of hypersensitivity or skin atrophy occur the agent should be discontinued. Use in children should be limited to the least amount required for therapeutic effect. Prolonged use in intertriginous areas and on flexures is undesirable. If fungal or bacterial infections are present, additional specific therapy is required. Bpac nz best practice recommendations point out that underuse of topical corticosteroids is far more common than overuse. 35 They recommend using `fingertip units (FTUs) as a measure for dosing, with one FTU being the amount of product that can be squeezed onto the top third of an adult finger and equating to approximately 0.5g. See Figure 2 for dosing using FTUs. ADULT (in FTUs) Face & neck Age 3 6 months 1 2 years 3 5 years 6 10 years Arm & hand Leg & foot Torso (front) Adult 2.5 Arm: 3.0 Hand: 1.0 Leg: 6.0 Foot: CHILD (in FTUs) 1.0 Face & neck Arm & Leg & Torso Back & hand foot (front) bottom Figure 2: Fingertip units (FTUs) for different areas of the body. One FTU is the amount of cream/ointment that can be squeezed onto the top third of an adult finger (approxiately 2.5cm). ADVANTAN s 15g tube dispenses approximately 0.5g per FTU How much should I prescribe? As a rough guide, if a 6-10 year-old child has the ointment or cream applied to their leg and foot once daily, they will require 4.5 FTUs per day (2.25g per day), equating to 15.75g of product per week. For this child, it would be appropriate to prescribe one tube of Advantan per week for up to 4 weeks. An adult patient would require 8 FTUs to cover their leg and foot once daily (4g per day) and would thus require 28g of product per week, or eight tubes of Advantan if a 4-week course is needed. These amounts are for one limb and so would need to be doubled for a bilateral presentation. Some patients requiring multiple tubes may be tempted to dispense them into a single container for ease of use. This should be discouraged as it has an unknown effect on the efficacy and safety of the product. Efficacy and safety of methylprednisolone aceponate 0.1% ointment/cream in major clinical trials Clinical experience with methylprednisolone aceponate (MPA) in eczema 36 Author: Fritsch P Summary: The efficacy of once or twice per day methylprednisolone aceponate (cream or ointment) was examined in six, multicentre, double-blind controlled trials in comparison with twice per day betamethasone valerate (cream) in a total of 1723 eczema patients (mean age ~45 years) over a period of up to 3 weeks. All regimens were found to be equally effective; >90% of patients were asymptomatic (erythema, vesiculation, weeping, crusting, scaling, lichenification) or greatly improved after 3 weeks. There were no differences between methylprednisolone aceponate and betamethasone valerate treatment, between cream and ointment formulations nor between once- and twice-daily application. Systemic side effects were not reported, while mild to moderate local complaints (itching, burning, erythema, dryness and scaling) were reported in 5.1 % of methylprednisolone aceponate recipients and 6.2% of betamethasone valerate recipients. Discontinuation for lack of efficacy occurred in 1.6% of methylprednisolone aceponate and 0.7% of betamethasone valerate recipients. Comment: There seems to be a lot of variables in this study, which makes it rather confusing. There is also no indication of the type of eczema treated, as acute vesicular eczema would be expected to respond differently from chronic lichenified eczema when using a cream rather than ointment formulation. It does support the safety and efficacy of methylprednisolone aceponate. It also confirms the current protocol of once daily use being appropriate. Effectiveness of topical methylprednisolone aceponate in eczema patients: results of the Swiss multicentre phase IV study 28 Author: Elsner P Summary: Use of methylprednisolone aceponate as a cream, ointment or fatty ointment was examined in a multicentre phase IV trial (average duration of treatment was 5 weeks) in 830 Swiss eczema patients ranging in age from 6 months to 97 years. Symptoms were cured or improved (investigator opinion) significantly in 87.4% of patients and with a response that was classified as fast or very fast onset in 79.4%; treatment discontinuation due to lack of efficacy occurred in 7.4% of patients. Patient tolerance was judged to be good or excellent in 92.3% of patients, although treatment was discontinued due to adverse events in 1.8% of patients. Comment: We do not have a fatty ointment formulation of methylprednisolone aceponate in NZ. The safe use of the product in very young infants is reassuring. It would be helpful to have information about which patients did not respond. Patients seem to have been included with types of eczema which would not generally be managed with methylprednisolone aceponate (e.g. acute contact dermatitis and seborrhoeic dermatitis). There is also no information about concomitant treatment with emollients. This would be helpful to elucidate treatment failures Back 4
5 Reduction of relapses of atopic dermatitis with methylprednisolone aceponate cream twice weekly in addition to maintenance treatment with emollient: a multicentre, randomized, double-blind, controlled study 32 Authors: Peserico A et al Summary: The long-term efficacy (16 weeks) of methylprednisolone aceponate 0.1% cream twice weekly, in addition to an emollient (Advabase ), was examined in a multicentre, randomised, double-blind, controlled study of 249 patients after stabilisation of an acute severe or very severe flare of atopic dermatitis with methylprednisolone aceponate alone. Time to relapse (primary endpoint) of atopic dermatitis was longer in the methylprednisolone aceponate group than in the emollient only group, and the probability of being relapse free at 16 weeks was 87.1% in the methylprednisolone aceponate group versus 65.8% in those receiving emollient only. Methylprednisolone aceponate twice weekly induced a 3.5-fold lower risk of relapse than emollient alone (hazard ratio 3.5, 95% CI ; p < ). Methylprednisolone aceponate was also superior to emollient for all other efficacy endpoints (relapse rate, disease status, patient s assessment of intensity of itch, the EASI, Investigator s Global Assessment score, affected body surface area, Dermatology Life Quality Index (DLQI) and children s DLQI, patient s and investigator s global assessment of response and patient s assessment of sleep quality). Both treatments were well tolerated, with the frequency of adverse events with the emollient alone being higher than with the study drug (24% vs 15%); none of the adverse events reported during the maintenance phase were considered to be due to the study drug, and no serious adverse events were reported. Comment: The use of maintenance therapy to prolong remissions is not well documented. Generally, once the eczema is under control topical steroids are discontinued and only reinstated when a flare occurs. Given the high safety profile and patient tolerance of the medication, this is a useful management suggestion. It is a bit surprising that the rate of adverse effects is higher with the emollient base (Advabase ) as this must be the base of the active medication. Effectiveness and tolerability of methylprednisolone aceponate (Advantan ) in the treatment of eczematous disorders of the face 34 Authors: Ruzicka T and Zaumseil RP Summary: The tolerability of methylprednisolone aceponate cream and/or ointment once daily for a maximum of 4 weeks was examined in a multicentre, post-marketing surveillance study in 575 patients (aged 3 months to 87 years) with eczematous dermatitis of the face (primarily neurodermatitis [46%] and contact eczema [25%]). Methylprednisolone aceponate was generally well tolerated, no skin infections or atrophy were reported and very good tolerability was reported by 86% of patients and 89% of doctors. Efficacy of methylprednisolone aceponate, based on doctor s assessment at the end of therapy, was asymptomatic in 66.3% of patients and distinct improvement in 32.9%. Comment: I am not certain what a diagnosis of neurodermatitis implies and whether cream and ointment are being used in the same patient. The tolerability and efficacy of the product indicates its usefulness in facial dermatoses. Methylprednisolone aceponate (MPA) - use and clinical experience in children 27 Author: Rampini E Summary: In order to assess the clinical efficacy and tolerability of 0.1% methylprenisolone aceponate cream and ointment once or twice daily for a maximum of 21 days, two double-blind, multicentre clinical studies were performed in children aged 14 years suffering from atopic dermatitis and compared with treatment with 0.25% prednicarbate. An additional trial examined the systemic safety (endogenous cortisol production after 7 days application over a large skin area [mean 12.% of skin surface]) of 0.1% methylprenisolone aceponate to 0.1% hydrocortisone 17-butyrate. There was no significant difference in response rates between methylprenisolone aceponate (once daily 96.3%; twice daily 97.4%) and prednicarbate (once daily 98.1%; twice daily 100%) in the efficacy trials (n = 108 and 78), while the cortisol levels were unchanged after 7 days exposure to either methylprenisolone aceponate (14.22 vs µg/dl) or 0.1% hydrocortisone 17-butyrate (11.70 vs µg/dl). In the efficacy trials, a total of 36 children aged 3 years were treated with methylprenisolone aceponate or prednicarbate. The response rate in both groups was 100%. Comment: Prednicarbate is not available in NZ. The response rates comparing once daily with twice daily use confirm the NZ guidelines on once daily use regime. Being able to reassure parents that there is no evidence of systemic absorption after 21 days (and therefore any systemic side effects) will go a long way in improving compliance. More prolonged use data would be interesting, but with the high response rate, use is generally for short periods only. Methylprednisolone aceponate in eczema and other inflammatory skin disorders a clinical update 19 Author: Ruzicka T Comment: This is a useful overview of methylprednisolone aceponate with regards dosing regime, safety (both locally and systemically), tolerability and efficacy. It confirms the use of once-daily treatment being efficacious in a wide range of age groups and this, in conjunction with the rapid onset of improvement, increases compliance. The safety profile in terms of local tolerability and systemic effects are well documented. Studies on the suppression of the hypothalamic-pituitary axis show minimal effects, giving it a reassuring advantage in long-term use in children. This review also documents its safety and efficacy in a wide range of facial dermatoses, but the benefits of its use for the scalp is not relevant in NZ where we have no access to the milk formulation or the alcohol solution. Take-home messages Methylprednisolone aceponate is a useful agent in the management of atopic eczema in paediatric and adult populations Methylprednisolone aceponate has a high efficacy to safety ratio compared with topical steroids of similar potency The increased compliance with once daily dosing, as opposed to twice daily, is also significant (time taken to apply products repeatedly is acknowledged by patients as a reason for treatment failure) Patients are often reluctant to use ointment-based products as they are sticky, harder to apply (so more time consuming) and leave greasy residue on clothing and bedding Ointments are often superior in terms of hydration and efficacy Methylprednisolone aceponate ointment has the property of an ointment, but the cosmetic acceptability and ease of application of a cream 5
6 Questions frequently asked by GPs When should I refer to a specialist dermatologist? Immediate (same day) referral to a specialist dermatologist should be sought if eczema herpeticum is suspected. Urgent referral (within 3 weeks) should be undertaken if atopic eczema is severe or failing to respond to 2 weeks of optimal topical therapy. Specialist referral is also recommended for paediatric patients in whom the diagnosis is uncertain, the eczema is severe with frequent flares and/or is not responding to appropriate treatment, facial atopic eczema is present and not responding to appropriate topical therapy, there is severe psychosocial impact, the family needs guidance and support for management of the child s eczema, and for patients where employment is at risk. Patients should also be referred to a specialist if oral steroids are required on a regular basis (more than two courses yearly) or if large amounts of topical steroids are being required and the patient is not compliant with ancillary measures (medicated baths, emollients). When should tar or other additives be added? Adding tar to steroid creams increases the efficacy of the steroid without having to resort to using a stronger product. This is particularly helpful in locations such as the genital area where strong steroids are contraindicated. Tar is also useful in lichenified eczema or difficultto-treat areas such as the hands and feet. Tar is a messy product and patients need to be made aware of this. Salicylic acid is useful as a descaling agent in hyperkeratotic eczema and menthol can be a useful addition to act as a counterirritant - some patients find the slight sting of menthol preferable to itch. Mixing additives to steroid creams will incur a cost, which can be considerable. Generally, the use of additives is better left to specialist dermatologists with experience individualising creams for very specific purposes and patients. When should oral antihistamines be prescribed? Oral antihistamines can be invaluable in managing itchy patients. Eczema has been described as an itch that rashes. Scratching exacerbates the eczema, making the condition more likely to become chronic as well as leading to infection. Nocturnal scratching is a significant problem as it disturbs sleep for the patient as well as other household members. Non-sedating antihistamines during the day are helpful, but probably the sedating antihistamines at night are the most beneficial. The sedative effect aids deeper sleep, so scratching is less likely to occur. Many patients state that they start to itch before the rash appears and if they could only stop the scratching they would not have any rash. Should allergy tests be undertaken and which ones? Patients always hope there is an easy answer to finding a single cause of their eczema and fail to understand that it is usually a multifactorial condition. They pin their hopes on finding an explanation in a single dietary or environmental allergen and they do need to understand that this is rarely the case. Some children, in particular, are put on very odd exclusion diets by their well-intentioned parents. I think it can help to arrange screening IgE and RAST tests which may find dietary allergens to be avoided, but may also reassure parents that their children can be allowed to eat a wide variety of foods without making their diets too restrictive. There is also not a complete correlation between the results of the tests and what happens in real life when the person actually consumes the food. There are limited numbers of foods which can be tested and tests are not uniformly available in NZ. I think IgE and RAST tests are indicated when there is a strong suggestion of a dietary trigger or when the patient is not responding and continues to have severe disease despite optimal management and compliance. Prick testing can be useful in patients who seem to react in certain environments or in a seasonal pattern. I sometimes do these in families contemplating acquiring a cat or dog when there is an atopic child - it is easier to get goldfish in the first place than part with the family cat if it turns out to be an eczema trigger. Routine prick tests only measure response to a few allergens. More extensive testing is available in some centres, but this may only be possible privately and is costly. Patch testing is indicated if it seems likely there is a contact allergen, but this is of little benefit in the majority of atopic individuals. Patch tests can be very expensive and may not be available with public funding. When should alternative therapy such as immunosuppressants be considered? The decision to start alternative therapies such as immunosuppressants (including cyclosporin, methotrexate, azathioprine) should not be taken lightly. These are all drugs associated with potential significant side effects, but they can be highly effective. It should be emphasised that these are only part of a management plan and do not replace the need for emollients and other topical therapies. They are almost never first-line therapy. With increasing awareness of these products there can be pressure from some patients who view them as an easy option. Patients who may benefit from these medications should be referred to specialists familiar with their use. These types of drugs should probably only be initiated by specialist dermatologists to ensure they are used appropriately and adequately monitored. References 1. Hoare C et al. Systematic review of treatments for atopic eczema. Health Technol Assess. 2000;4(37): Williams HC and Wüthrich B. The natural history of atopic dermatitis. Williams HC, editor. Atopic dermatitis. Cambridge: Cambridge University Press; Luger TA. Balancing efficacy and safety in the management of atopic dermatitis: the role of methylprednisolone aceponate. J Eur Acad Dermatol Venereol. 2011;25(3): Darsow U et al. For the European Task Force on Atopic Dermatitis/EADV Eczema Task Force. ETFAD/EADV eczema task force 2009 position paper on diagnosis and treatment of atopic dermatitis. J Eur Acad Dermatol Venereol. 2010;24(3): Williams HC. Is the prevalence of atopic dermatitis increasing? Clin Exp Dermatol. 1992;17: Asher MI et al. The burden of symptoms of asthma, allergic rhinoconjunctivitis and atopic eczema in children and adolescents in six New Zealand centres: ISAAC Phase One. N Z Med J. 114(1128): Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics 2003;21(2): Simpson CR et al. Trends in the epidemiology and prescribing of medication for eczema in England. J R Soc Med. 2009;102(3): Alvarenga TM and Caldeira AP. Quality of life in pediatric patients with atopic dermatitis. J Pediatr (Rio J). 2009;85(5): Carroll CL et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22(3): Su JC et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76: Zuberbier T et al. Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol. 2006;118(1): Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract Aug;60(8): National Institute for Health and Clinical Excellence. Atopic eczema in children: management of atopic eczema in children from birth up to the age of 12 years. London: NICE, Available from: (Accessed August 2012) 15. Lewis-Jones S et al. Management of atopic eczema in children aged up to 12 years: summary of NICE guidance. BMJ. 2007;335: Akdis CA et al. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report. Allergy 2006;61(8): Grimalt R et al. The steroid-sparing effect of an emollient therapy in infants with atopic dermatitis: a randomized controlled study. Dermatology 2007;214(1): Luger TA. Topical dermatotherapy with glucocorticoids therapeutic index. Guidelines of the German Dermatology Society. AWMF Ruzicka T. Methylprednisolone aceponate in eczema and other inflammatory skin disorders a clinical update. Int J Clin Pract. 2006;60(1): DermNet NZ. Treatment of atopic dermatitis. Online resource available from: (Accessed Aug 2012) 21. Ohya et al. Psychosocial factors and adherence to treatment advice in childhood atopic dermatitis. J Invest Dermatol 2001;117: Santer M et al. Experiences of carers managing childhood eczema and their views on its treatment: a qualitative study. Br J Gen Pract. 2012;62(597):e Fischer G. Compliance problems in paediatric atopic eczema. Australas J Dermatol. 1996;37 Suppl 1:S Ricci G. Three years of Italian experience of an educational program for parents of young children affected by atopic dermatitis: improving knowledge produces lower anxiety levels in parents of children with atopic dermatitis. Pediatr Dermatol. 2009;26(1): Medsafe. New Zealand Medicines and Medical Devices Safety Authority. ADVANTAN Data Sheet. Available from: (Accessed Aug 2012) 26. Bieber T et al. Efficacy and safety of methylprednisolone aceponate ointment 0.1% compared to tacrolimus 0.03% in children and adolescents with an acute flare of severe atopic dermatitis. Allergy 2007;62(2): Rampini E. Methylprednisolone aceponate (MPA) - use and clinical experience in children. J Dermatol Treat. 1992;3(Suppl 2): Elsner P. Effectiveness of topical methylprednisolone aceponate in eczema patients: results of the Swiss multicentre phase IV study. Anktuelle Dermatologie 1995;21(5): Zaumseil RP et al. Methylprednisolone aceponate (MPA) a new therapeutic for eczema: a pharmacological overview. J Derm Treat. 1992;3 Suppl 2: Blume-Peytavi U and Wahn U. Optimizing the treatment of atopic dermatitis in children: a review of the benefit/ risk ratio of methylprednisolone aceponate. J Eur Acad Dermatol Venereol. 2011;25(5): Albrecht G. Clinical comparison of methylprednisolone aceponate and prednicarbate in chronic eczema. J Eur Acad Dermatol Venereol. 1994;3(Suppl 1):S42 S Peserico A. Reduction of relapses of atopic dermatitis with methylprednisolone aceponate cream twice weekly in addition to maintenance treatment with emollient: a multicentre, randomized, double-blind, controlled study. Br J Dermatol. 2008;158(4): Kecskés A et al. Comparison of the local and systemic side effects of methylprednisolone aceponate and mometasone furoate applied as ointments with equal anti-inflammatory activity. J Am Acad Dermatol. 1993;29: Ruzicka T and Zaumseil RP. Effectiveness and tolerability of methylprednisolone aceponate (Advantan ) in the treatment of eczematous disorders of the face. H-G Z Hautkr 2002;77(4): bpacnz Topical corticosteroid treatment for skin conditions. Reviewer Amanda Oakley. BPJ Issue 23 September Available from: (Accessed Aug 2012) 36. Fritsch P. Clinical experience with methylprednisolone aceponate (MPA) in eczema. J Derm Treat. 1992;3(2):17-19 This publication has been created with an educational grant from CSL Biotherapies NZ Limited. The content is entirely independent and based on published studies and the author s opinions. It may not reflect the views of CSL Biotherapies. Please consult the full Data Sheet at before prescribing. Treatment decisions based on these data are the full responsibility of the prescribing physician RESEARCH REVIEW 6
Pharmacologic Treatment of Atopic Dermatitis
 J KMA Pharmacotherapeutics Pharmacologic Treatment of Atopic Dermatitis Chun Wook Park, MD Department of Dermatology, Hallym University College of Medicine E mail : dermap@paran.com J Korean Med Assoc
J KMA Pharmacotherapeutics Pharmacologic Treatment of Atopic Dermatitis Chun Wook Park, MD Department of Dermatology, Hallym University College of Medicine E mail : dermap@paran.com J Korean Med Assoc
15 minute eczema consultation
 THERAPY WORKSHOP 15 minute eczema consultation History Current treatments Examination Treatment Plan Written action plan Soap substitute/bath oil Antiseptic baths Emollients Topical steroids Other treatments
THERAPY WORKSHOP 15 minute eczema consultation History Current treatments Examination Treatment Plan Written action plan Soap substitute/bath oil Antiseptic baths Emollients Topical steroids Other treatments
Children s Hospital Of Wisconsin
 Children s Hospital Of Wisconsin Co-Management Guidelines To support collaborative care, we have developed guidelines for our community providers to utilize when referring to, and managing patients with,
Children s Hospital Of Wisconsin Co-Management Guidelines To support collaborative care, we have developed guidelines for our community providers to utilize when referring to, and managing patients with,
RELEVANT DISCLOSURES ATOPIC DERMATITIS / ECZEMA MANAGING ECZEMA IN INFANTS AND CHILDREN
 RELEVANT DISCLOSURES MANAGING ECZEMA IN INFANTS AND CHILDREN Advisory board member - MEDA (Elidel), Speaking honoraria Bayer (Advantan) Advisory board, consultant, speaker: Pfizer, Abbvie, Janssen, Elli
RELEVANT DISCLOSURES MANAGING ECZEMA IN INFANTS AND CHILDREN Advisory board member - MEDA (Elidel), Speaking honoraria Bayer (Advantan) Advisory board, consultant, speaker: Pfizer, Abbvie, Janssen, Elli
TCIs are only available on prescription and are usually started by a dermatology specialist.
 (TCIs) What are topical calcineurin inhibitors? Topical calcineurin inhibitors are treatments that alter the immune system and have been developed for controlling eczema. There are two types available:
(TCIs) What are topical calcineurin inhibitors? Topical calcineurin inhibitors are treatments that alter the immune system and have been developed for controlling eczema. There are two types available:
過敏病科中心. Allergy Centre. Eczema. Allergy Centre 過敏病科中心. Allergy Centre. For enquiries and appointments, please contact us at:
 Allergy Centre 過敏病科中心 Eczema For enquiries and appointments, please contact us at: Allergy Centre 9/F, Li Shu Pui Block Hong Kong Sanatorium & Hospital 2 Village Road, Happy Valley, Hong Kong Tel: 2835
Allergy Centre 過敏病科中心 Eczema For enquiries and appointments, please contact us at: Allergy Centre 9/F, Li Shu Pui Block Hong Kong Sanatorium & Hospital 2 Village Road, Happy Valley, Hong Kong Tel: 2835
Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57
 Atopic eczema in under 12s: diagnosis and management Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Atopic eczema in under 12s: diagnosis and management Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Atopic Eczema with detail on how to apply wet wraps
 Atopic Eczema with detail on how to apply wet wraps Dr Carol Hlela Consultant Dermatologist Head of Unit, Department of Dermatology, Paediatrics Red Cross Children s Hospital, UCT Red Cross War Memorial
Atopic Eczema with detail on how to apply wet wraps Dr Carol Hlela Consultant Dermatologist Head of Unit, Department of Dermatology, Paediatrics Red Cross Children s Hospital, UCT Red Cross War Memorial
Medication Policy Manual. Topic: Dupixent, dupilumab Date of Origin: March 10, Committee Approval: March 10, 2017 Next Review Date: May 2018
 Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru493 Topic: Dupixent, dupilumab Date of Origin: March 10, 2017 Committee Approval: March 10, 2017
Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru493 Topic: Dupixent, dupilumab Date of Origin: March 10, 2017 Committee Approval: March 10, 2017
New Medicine Report. Pimecrolimus. RED- Hospital only Date of Last Revision 6 th March 2003
 New Medicine Report Document Status Pimecrolimus Reviewed by Suffolk D&T RED- Hospital only Date of Last Revision 6 th March 2003 Approved Name Pimecrolimus Trade Name Elidel Manufacturer Novartis Legal
New Medicine Report Document Status Pimecrolimus Reviewed by Suffolk D&T RED- Hospital only Date of Last Revision 6 th March 2003 Approved Name Pimecrolimus Trade Name Elidel Manufacturer Novartis Legal
Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57
 Atopic eczema in under 12s: diagnosis and management Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57 NICE 2007. All rights reserved. Contents Introduction... 4 Child-centred care...
Atopic eczema in under 12s: diagnosis and management Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57 NICE 2007. All rights reserved. Contents Introduction... 4 Child-centred care...
Comparative efficacy of topical mometasone furoate 0.1% cream vs topical tacrolimus 0.03% ointment in the treatment of atopic dermatitis
 Original Article Comparative efficacy of topical mometasone furoate 0.1% cream vs topical tacrolimus 0.03% ointment in the treatment of atopic dermatitis Md Alauddin Khan *, Lubna Khondker **, Dilshad
Original Article Comparative efficacy of topical mometasone furoate 0.1% cream vs topical tacrolimus 0.03% ointment in the treatment of atopic dermatitis Md Alauddin Khan *, Lubna Khondker **, Dilshad
What s Topical About Topicals?
 What s Topical About Topicals? Megha M. Tollefson, MD Associate Professor of Dermatology and Pediatrics July 29, 2017 2015 MFMER 3513105-1 Disclosures None 2015 MFMER 3513105-2 Outline Topical steroids
What s Topical About Topicals? Megha M. Tollefson, MD Associate Professor of Dermatology and Pediatrics July 29, 2017 2015 MFMER 3513105-1 Disclosures None 2015 MFMER 3513105-2 Outline Topical steroids
Prescribing Information
 Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
ATOPIC ECZEMA. What are the aims of this leaflet?
 ATOPIC ECZEMA What are the aims of this leaflet? This leaflet has been written to help you understand more about atopic eczema. It tells you what it is, what causes it, what can be done about it, and where
ATOPIC ECZEMA What are the aims of this leaflet? This leaflet has been written to help you understand more about atopic eczema. It tells you what it is, what causes it, what can be done about it, and where
Steroid use in managing your child s Atopic Eczema
 Steroid use in managing your child s Atopic Eczema Clinical Nurse Specialist for Paediatric Dermatology (01284) 713575 Step up step down approach: Addressograph Severe Call your General Practitioner (GP)
Steroid use in managing your child s Atopic Eczema Clinical Nurse Specialist for Paediatric Dermatology (01284) 713575 Step up step down approach: Addressograph Severe Call your General Practitioner (GP)
TOPCORT Cream/Ointment (Mometasone furoate 0.1%)
 Published on: 10 Jul 2014 TOPCORT Cream/Ointment (Mometasone furoate 0.1%) Composition TOPCORT Cream Mometasone Furoate, IP... 0.1% w/w In a cream base... q.s. TOPCORT Ointment Mometasone Furoate, IP...
Published on: 10 Jul 2014 TOPCORT Cream/Ointment (Mometasone furoate 0.1%) Composition TOPCORT Cream Mometasone Furoate, IP... 0.1% w/w In a cream base... q.s. TOPCORT Ointment Mometasone Furoate, IP...
Diagnosis and Management of Common and Infective Skin Diseases in Children at primary care level
 Diagnosis and Management of Common and Infective Skin Diseases in Children at primary care level Dr Ng Su Yuen Paediatrician and Paediatric Dermatologist Hospital Pulau Pinang Outline Common inflammatory
Diagnosis and Management of Common and Infective Skin Diseases in Children at primary care level Dr Ng Su Yuen Paediatrician and Paediatric Dermatologist Hospital Pulau Pinang Outline Common inflammatory
Dermatitis (inflammatory skin condition) Nonallergic. dermatitis. Non-atopic eczema (non- IgE mediated)
 Atopic Eczema Dermatitis (inflammatory skin condition) Allergic dermatitis -eczema Nonallergic dermatitis Atopic eczema (IgE mediated) Non-atopic eczema (non- IgE mediated) Pathophysiology of Eczema Allergy
Atopic Eczema Dermatitis (inflammatory skin condition) Allergic dermatitis -eczema Nonallergic dermatitis Atopic eczema (IgE mediated) Non-atopic eczema (non- IgE mediated) Pathophysiology of Eczema Allergy
LRI Children s Hospital
 Atopic Eczema Care LRI Children s Hospital Staff relevant to: Clinical staff working within the UHL Children s Hospital. Team approval date: May 2017 Version: V 4 Revision due: May 2020 Written by: K.
Atopic Eczema Care LRI Children s Hospital Staff relevant to: Clinical staff working within the UHL Children s Hospital. Team approval date: May 2017 Version: V 4 Revision due: May 2020 Written by: K.
Core Safety Profile. AT/H/PSUR/0013/002 Date of FAR:
 ore afety Profile Active substance: Methylprednisolon aceponate Pharmaceutical form(s)/strength: ream / 0.1 % Fatty ointment / 0.1 % utaneous emulsion / 0.1 % intment / 0.1 % utaneous solution / 0.1 %
ore afety Profile Active substance: Methylprednisolon aceponate Pharmaceutical form(s)/strength: ream / 0.1 % Fatty ointment / 0.1 % utaneous emulsion / 0.1 % intment / 0.1 % utaneous solution / 0.1 %
Allergy Medications. Antihistamines. are very safe. Although usually taken as tablets, they may be prescribed as a liquid or syrup for young children
 The treatments prescribed for allergy control the symptoms and reactions; they do not cure the condition. However, using treatments as prescribed can show a huge change in a patient s health, mood and
The treatments prescribed for allergy control the symptoms and reactions; they do not cure the condition. However, using treatments as prescribed can show a huge change in a patient s health, mood and
The safety and effectiveness of Dupixent in pediatric patients have not been established (1).
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.30 Subject: Dupixent Page: 1 of 6 Last Review Date: September 15, 2017 Dupixent Description Dupixent
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.30 Subject: Dupixent Page: 1 of 6 Last Review Date: September 15, 2017 Dupixent Description Dupixent
Managing and Minimizing Flare-ups in Atopic Dermatitis
 Managing and Minimizing Flare-ups in Atopic Dermatitis Importance of the skin barrier & how commonly used drugs are impacting it Dr. Benjamin Barankin, MD FRCPC Medical Director & Founder of Toronto Dermatology
Managing and Minimizing Flare-ups in Atopic Dermatitis Importance of the skin barrier & how commonly used drugs are impacting it Dr. Benjamin Barankin, MD FRCPC Medical Director & Founder of Toronto Dermatology
The Itch That Rashes. Sarah D. Cipriano, MD, MPH, MS Resident, Dermatology University of Utah
 The Itch That Rashes Sarah D. Cipriano, MD, MPH, MS Resident, Dermatology University of Utah 1 Conflict of Interest No conflict of interest Will discuss off label use of medications 2 3 Most likely diagnosis?
The Itch That Rashes Sarah D. Cipriano, MD, MPH, MS Resident, Dermatology University of Utah 1 Conflict of Interest No conflict of interest Will discuss off label use of medications 2 3 Most likely diagnosis?
ECZEMA SOCIETY OF CANADA ATOPIC DERMATITIS: PATIENT INSIGHTS REPORT MILD-TO-MODERATE DISEASE
 ECZEMA SOCIETY OF CANADA ATOPIC DERMATITIS: PATIENT INSIGHTS REPORT MILD-TO-MODERATE DISEASE 1 OVERVIEW OF SURVEY RESULTS INTRODUCTION PATIENT INSIGHTS BY THE NUMBERS: Adult Atopic Dermatitis (AD) In the
ECZEMA SOCIETY OF CANADA ATOPIC DERMATITIS: PATIENT INSIGHTS REPORT MILD-TO-MODERATE DISEASE 1 OVERVIEW OF SURVEY RESULTS INTRODUCTION PATIENT INSIGHTS BY THE NUMBERS: Adult Atopic Dermatitis (AD) In the
Prescribing Information. Taro-Clobetasol. Taro-Clobetasol
 Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
Eczema & Dermatitis Clinical features: Histopathological features: Classification:
 Eczema & Dermatitis Eczema is an inflammatory reactive pattern of skin to many and different stimuli characterized by itching, redness, scaling and clustered papulovesicles. Eczema and dermatitis are synonymous
Eczema & Dermatitis Eczema is an inflammatory reactive pattern of skin to many and different stimuli characterized by itching, redness, scaling and clustered papulovesicles. Eczema and dermatitis are synonymous
KEY MESSAGES. Psoriasis patients are more prone to cardiovascular diseases, stroke, lymphoma and non-melanoma skin cancers, and increased mortality.
 KEY MESSAGES Psoriasis is a genetically determined, systemic immune-mediated chronic inflammatory disease that affects primarily the skin and joints. Psoriasis Vulgaris is characterised by well-demarcated
KEY MESSAGES Psoriasis is a genetically determined, systemic immune-mediated chronic inflammatory disease that affects primarily the skin and joints. Psoriasis Vulgaris is characterised by well-demarcated
Technology appraisal guidance Published: 25 August 2004 nice.org.uk/guidance/ta82
 Tacrolimus and pimecrolimus for atopic eczema Technology appraisal guidance Published: 25 August 2004 nice.org.uk/guidance/ta82 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Tacrolimus and pimecrolimus for atopic eczema Technology appraisal guidance Published: 25 August 2004 nice.org.uk/guidance/ta82 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate).
 DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
DATA SHEET. Betamethasone dipropionate equivalent to betamethasone 0.5mg/g (0.05% w/w).
 DATA SHEET 1. DIPROSONE DIPROSONE (0.05% w/w) cream DIPROSONE (0.05% w/w) ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Betamethasone dipropionate equivalent to betamethasone 0.5mg/g (0.05% w/w).
DATA SHEET 1. DIPROSONE DIPROSONE (0.05% w/w) cream DIPROSONE (0.05% w/w) ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Betamethasone dipropionate equivalent to betamethasone 0.5mg/g (0.05% w/w).
Beacon Hospital Annual Study Morning Treatment of Atopic Dermatitis (Eczema) in Primary Care
 Beacon Hospital Annual Study Morning Treatment of Atopic Dermatitis (Eczema) in Primary Care Alan D. Irvine MD DSc Lecture overview What causes AD How to get optimise results in primary care Eczema and
Beacon Hospital Annual Study Morning Treatment of Atopic Dermatitis (Eczema) in Primary Care Alan D. Irvine MD DSc Lecture overview What causes AD How to get optimise results in primary care Eczema and
DIPROSONE OV Cream and Ointment do not contain preservatives, parabens or lanolin.
 PRODUCT INFORMATION DIPROSONE OV (OPTIMISED VEHICLE) CREAM AND OINTMENT NAME OF THE MEDICINE DIPROSONE OV Cream and Ointment (Betamethasone dipropionate) Chemistry Abstracts Service (CAS) registry number:
PRODUCT INFORMATION DIPROSONE OV (OPTIMISED VEHICLE) CREAM AND OINTMENT NAME OF THE MEDICINE DIPROSONE OV Cream and Ointment (Betamethasone dipropionate) Chemistry Abstracts Service (CAS) registry number:
Assessing the Current Treatment of Atopic Dermatitis: Unmet Needs
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
An Everyday Guide to Eczema
 An Everyday Guide to Eczema By Dr. Kristel Polder, Board-Certified Dermatologist Developed in Partnership with Who is affected by eczema? 32 million people in the US 1 in 5 children 1 in 12 adults *www.eczema.org
An Everyday Guide to Eczema By Dr. Kristel Polder, Board-Certified Dermatologist Developed in Partnership with Who is affected by eczema? 32 million people in the US 1 in 5 children 1 in 12 adults *www.eczema.org
Management of eczema in infants and children Assoc Prof David Orchard Director, Department of Dermatology Royal Children s Hospital
 Atopic dermatitis definition Management of eczema in infants and children Assoc Prof David Orchard Director, Department of Dermatology Royal Children s Hospital Atopic dermatitis is long lasting (chronic)
Atopic dermatitis definition Management of eczema in infants and children Assoc Prof David Orchard Director, Department of Dermatology Royal Children s Hospital Atopic dermatitis is long lasting (chronic)
RECLASSIFICATION SUBMISSION. EUMOVATE Eczema and Dermatitis Cream. (Clobetasone butyrate 0.05%) From Prescription Only Medicine
 RECLASSIFICATION SUBMISSION EUMOVATE Eczema and Dermatitis Cream (Clobetasone butyrate 0.05%) From Prescription Only Medicine To Pharmacist Only Medicine NOVEMBER 2000 MEETING PART A 1. International non-proprietary
RECLASSIFICATION SUBMISSION EUMOVATE Eczema and Dermatitis Cream (Clobetasone butyrate 0.05%) From Prescription Only Medicine To Pharmacist Only Medicine NOVEMBER 2000 MEETING PART A 1. International non-proprietary
Professor Rohan Ameratunga Clinical Immunologist and Allergist Auckland
 Professor Rohan Ameratunga Clinical Immunologist and Allergist Auckland 16:30-17:25 WS #170: Eczema Management 17:35-18:30 WS #182: Eczema Management (Repeated) Managing ECZEMA A/Prof Rohan Ameratunga
Professor Rohan Ameratunga Clinical Immunologist and Allergist Auckland 16:30-17:25 WS #170: Eczema Management 17:35-18:30 WS #182: Eczema Management (Repeated) Managing ECZEMA A/Prof Rohan Ameratunga
New Zealand Data Sheet
 New Zealand Data Sheet 1. PRODUCT NAME Resolve Plus 1.0 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Resolve Plus 1.0% Cream (1% w/w hydrocortisone, 2% w/w miconazole) Excipient(s) with known effect Resolve
New Zealand Data Sheet 1. PRODUCT NAME Resolve Plus 1.0 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Resolve Plus 1.0% Cream (1% w/w hydrocortisone, 2% w/w miconazole) Excipient(s) with known effect Resolve
Contact Dermatitis In Atopic Patients
 Contact Dermatitis In Atopic Patients Jenny Murase, MD Palo Alto Foundation Medical Group Director of Patch Testing University of California, San Francisco Associate Clinical Professor Disclosures Consultant
Contact Dermatitis In Atopic Patients Jenny Murase, MD Palo Alto Foundation Medical Group Director of Patch Testing University of California, San Francisco Associate Clinical Professor Disclosures Consultant
Recommended management of eczema in older patients
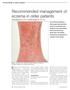 Recommended management of eczema in older patients Victoria Sherman MA, MRCP and Daniel Creamer BSc, MD, FRCP Our series Prescribing in older people gives practical advice for successful management of
Recommended management of eczema in older patients Victoria Sherman MA, MRCP and Daniel Creamer BSc, MD, FRCP Our series Prescribing in older people gives practical advice for successful management of
Eczema. By:- Dr. Naif Al-Shahrani Salman bin Abdazziz University
 Eczema By:- Dr. Naif Al-Shahrani Salman bin Abdazziz University Dermatitis= Eczema =Spongiosis Eczema Atopic Seborrheic Contact Allergic Irritant Nummular Asteatotic Stasis Neurodermatitis/Lichen Simplex
Eczema By:- Dr. Naif Al-Shahrani Salman bin Abdazziz University Dermatitis= Eczema =Spongiosis Eczema Atopic Seborrheic Contact Allergic Irritant Nummular Asteatotic Stasis Neurodermatitis/Lichen Simplex
What is atopic dermatitis?
 What is atopic dermatitis? Complex inflammatory skin disorder intense pruritus cutaneous hyperreactivity immune dysregulation Chronic with exacerbations and remissions Affects all ages, but more common
What is atopic dermatitis? Complex inflammatory skin disorder intense pruritus cutaneous hyperreactivity immune dysregulation Chronic with exacerbations and remissions Affects all ages, but more common
Atopic dermatitis/ eczema (a condition that makes skin red and itchy):
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Atopic dermatitis/ eczema (a condition that makes skin red and itchy): Atopic dermatitis (AD) is a common skin condition with
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Atopic dermatitis/ eczema (a condition that makes skin red and itchy): Atopic dermatitis (AD) is a common skin condition with
1 g cream or ointment contains 1 mg (0.1%) methylprednisolone aceponate.
 ADVANTAN Methylprednisolone aceponate 0.1% ointment/cream Presentation 1 g cream or ointment contains 1 mg (0.1%) methylprednisolone aceponate. Uses Actions After topical application, ADVANTAN suppresses
ADVANTAN Methylprednisolone aceponate 0.1% ointment/cream Presentation 1 g cream or ointment contains 1 mg (0.1%) methylprednisolone aceponate. Uses Actions After topical application, ADVANTAN suppresses
CENTENE PHARMACY AND THERAPEUTICS NEW DRUG REVIEW 3Q17 July August
 BRAND NAME Dupixent GENERIC NAME dupilumab MANUFACTURER Regeneron DATE OF APPROVAL March 28, 2017 PRODUCT LAUNCH DATE First week of April 2017 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review
BRAND NAME Dupixent GENERIC NAME dupilumab MANUFACTURER Regeneron DATE OF APPROVAL March 28, 2017 PRODUCT LAUNCH DATE First week of April 2017 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review
Eucrisa. Eucrisa (crisaborole) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.25 Subject: Eucrisa Page: 1 of 6 Last Review Date: September 15, 2017 Eucrisa Description Eucrisa
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.25 Subject: Eucrisa Page: 1 of 6 Last Review Date: September 15, 2017 Eucrisa Description Eucrisa
Update on emollients
 Update on emollients Amal Mhanna, MD Pediatric Dermatologist Clemenceau Medical Center Disclosure: I was a member of an advisory board y for J&J and received honoraria. Emollients and moisturizers are
Update on emollients Amal Mhanna, MD Pediatric Dermatologist Clemenceau Medical Center Disclosure: I was a member of an advisory board y for J&J and received honoraria. Emollients and moisturizers are
Constitutional eczema
 Patient information Constitutional eczema What is constitutional eczema? Constitutional eczema, also called atopic eczema, is a form of eczema that mainly occurs in childhood. Eczema usually starts before
Patient information Constitutional eczema What is constitutional eczema? Constitutional eczema, also called atopic eczema, is a form of eczema that mainly occurs in childhood. Eczema usually starts before
Contact Allergy Testing (Patch Testing) Information for parents and carers of children up to 12 years of age
 Contact Allergy Testing (Patch Testing) Information for parents and carers of children up to 12 years of age Dermatology Department The aim of this leaflet is to give you information about contact allergy
Contact Allergy Testing (Patch Testing) Information for parents and carers of children up to 12 years of age Dermatology Department The aim of this leaflet is to give you information about contact allergy
PRODUCT INFORMATION ELEUPHRAT CREAM, OINTMENT AND LOTION. Betamethasone dipropionate equivalent to betamethasone 0.5 mg/g (0.
 PRODUCT INFORMATION ELEUPHRAT CREAM, OINTMENT AND LOTION NAME OF THE MEDICINE Betamethasone dipropionate equivalent to betamethasone 0.5 mg/g (0.05% w/w) Chemical Structure: Betamethasone dipropionate
PRODUCT INFORMATION ELEUPHRAT CREAM, OINTMENT AND LOTION NAME OF THE MEDICINE Betamethasone dipropionate equivalent to betamethasone 0.5 mg/g (0.05% w/w) Chemical Structure: Betamethasone dipropionate
Atopic dermatitis: tacrolimus vs. topical corticosteroid use
 Atopic dermatitis: tacrolimus vs. topical corticosteroid use Langa Y, BPharm Van der Merwe E, BPharm, MSc (Pharmacology) Correspondence to: Elsabe van der Merwe, e-mail: elsabev@medikredit.co.za Keywords:
Atopic dermatitis: tacrolimus vs. topical corticosteroid use Langa Y, BPharm Van der Merwe E, BPharm, MSc (Pharmacology) Correspondence to: Elsabe van der Merwe, e-mail: elsabev@medikredit.co.za Keywords:
Dermatology Literary Review
 Emollients and ageing skin: optimising effectiveness and safety of Nursing, Vol. 25, No.11, pages 596-598. 6 Article outlines the causes of dryness in ageing skin and concludes that emollients are the
Emollients and ageing skin: optimising effectiveness and safety of Nursing, Vol. 25, No.11, pages 596-598. 6 Article outlines the causes of dryness in ageing skin and concludes that emollients are the
Dupixent (dupilumab)
 Dupixent (dupilumab) Line(s) of Business: HMO; PPO; QUEST Integration Effective Date: TBD POLICY A. INDICATIONS The indications below including FDA-approved indications and compendial uses are considered
Dupixent (dupilumab) Line(s) of Business: HMO; PPO; QUEST Integration Effective Date: TBD POLICY A. INDICATIONS The indications below including FDA-approved indications and compendial uses are considered
Skin disorders. Seborrhoeic dermatitis Search date April 2010 Luigi Naldi ...
 Seborrhoeic Search date April 21 Luigi Naldi.................................................. ABSTRACT INTRODUCTION: Seborrhoeic affects at least 1% of the population. Malassezia (Pityrosporum) ovale
Seborrhoeic Search date April 21 Luigi Naldi.................................................. ABSTRACT INTRODUCTION: Seborrhoeic affects at least 1% of the population. Malassezia (Pityrosporum) ovale
Atopic dermatitis Tacrolimus vs topical corticosteroid use
 Atopic dermatitis Tacrolimus vs topical corticosteroid use Yolande Langa, BPharm Elsabé van der Merwe, BPharm; MSc (Pharmacology) Abstract Atopic dermatitis (AD), the dermatologic manifestation of the
Atopic dermatitis Tacrolimus vs topical corticosteroid use Yolande Langa, BPharm Elsabé van der Merwe, BPharm; MSc (Pharmacology) Abstract Atopic dermatitis (AD), the dermatologic manifestation of the
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Technology Appraisals and Guidance Information Services Static List Review (SLR) Title and TA publication number of static topic: Final decision: TA82;
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Technology Appraisals and Guidance Information Services Static List Review (SLR) Title and TA publication number of static topic: Final decision: TA82;
METHYLPREDNISOLONE ACEPONATE (MPA)
 RE AFETY PRFILE ETHYLPREDNILNE AEPNATE (PA) 4.2 Posology and method of administration PA is to be applied thinly once daily to the affected areas and rubbed in lightly. PA cream/ointment/fatty ointment:
RE AFETY PRFILE ETHYLPREDNILNE AEPNATE (PA) 4.2 Posology and method of administration PA is to be applied thinly once daily to the affected areas and rubbed in lightly. PA cream/ointment/fatty ointment:
Topical Immunomodulator Step Therapy Program
 Topical Immunomodulator Step Therapy Program Policy Number: 5.01.557 Last Review: 8/2017 Origination: 7/2013 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) BCBSKC will provide
Topical Immunomodulator Step Therapy Program Policy Number: 5.01.557 Last Review: 8/2017 Origination: 7/2013 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) BCBSKC will provide
CHAPTER 1. Eczema Basics
 CHAPTER 1 Eczema Basics Definition Eczema is an inflammatory skin condition, characterised by ichtyosis (dry skin), erythema (redness), excoriation (interruption of the skin), scratching lesions, lichenification
CHAPTER 1 Eczema Basics Definition Eczema is an inflammatory skin condition, characterised by ichtyosis (dry skin), erythema (redness), excoriation (interruption of the skin), scratching lesions, lichenification
ATOPIC DERMATITIS: A BLUEPRINT FOR SUCCESS. Sierra Wolter MD, FAAD Pediatric Dermatology University of Arizona, College of Medicine
 ATOPIC DERMATITIS: A BLUEPRINT FOR SUCCESS Sierra Wolter MD, FAAD Pediatric Dermatology University of Arizona, College of Medicine THE PLAN Is it atopic dermatitis? What is atopic dermatitis? Guidelines
ATOPIC DERMATITIS: A BLUEPRINT FOR SUCCESS Sierra Wolter MD, FAAD Pediatric Dermatology University of Arizona, College of Medicine THE PLAN Is it atopic dermatitis? What is atopic dermatitis? Guidelines
Eucrisa. Eucrisa (crisaborole) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Eucrisa Page: 1 of 7 Last Review Date: June 22, 2018 Eucrisa Description Eucrisa (crisaborole)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Eucrisa Page: 1 of 7 Last Review Date: June 22, 2018 Eucrisa Description Eucrisa (crisaborole)
ECZEMA. Introduction. Differential diagnoses. Starship Children s Health Clinical Guideline
 Introduction Differential Diagnoses Indications for Admission Inpatient Treatment Flow Chart for Inpatient Care Outpatient Treatment References See also Auckland Regional Clinical Pathways for Eczema (with
Introduction Differential Diagnoses Indications for Admission Inpatient Treatment Flow Chart for Inpatient Care Outpatient Treatment References See also Auckland Regional Clinical Pathways for Eczema (with
Learning Circle: Jan 26, 2011 Childhood Eczema
 Learning Circle: Jan 26, 2011 Childhood Eczema Wingfield Rehmus, MD MPH BC Children s Hospital Clinical Assistant Professor, UBC Department of Paediatrics Associate Member, UBC Department of Dermatology
Learning Circle: Jan 26, 2011 Childhood Eczema Wingfield Rehmus, MD MPH BC Children s Hospital Clinical Assistant Professor, UBC Department of Paediatrics Associate Member, UBC Department of Dermatology
Topical Doxepin Prior Authorization with Quantity Limit Program Summary
 Topical Doxepin Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-3 Agent(s) Indication(s) Dosage & Administration Doxepin 5% cream Prudoxin (doxepin) cream 5% KS_PS_Topical_Doxepin_PAQL_ProgSum_AR1018
Topical Doxepin Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-3 Agent(s) Indication(s) Dosage & Administration Doxepin 5% cream Prudoxin (doxepin) cream 5% KS_PS_Topical_Doxepin_PAQL_ProgSum_AR1018
Teledermatology Paediatric eczema. Dr Carolyn Charman Consultant Dermatologist Royal Devon and Exeter Hospital
 Teledermatology Paediatric eczema Dr Carolyn Charman Consultant Dermatologist Royal Devon and Exeter Hospital NHS e-referral teledermatology Rapid access to diagnosis / management advice from local integrated
Teledermatology Paediatric eczema Dr Carolyn Charman Consultant Dermatologist Royal Devon and Exeter Hospital NHS e-referral teledermatology Rapid access to diagnosis / management advice from local integrated
The role of the practice nurse in managing psoriasis in primary care
 The role of the practice nurse in managing psoriasis in primary care Item type Authors Publisher Journal Article Buckley, David Nursing in General Practice Nursing in general practice Downloaded 16-Sep-2016
The role of the practice nurse in managing psoriasis in primary care Item type Authors Publisher Journal Article Buckley, David Nursing in General Practice Nursing in general practice Downloaded 16-Sep-2016
Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Atopic Dermatitis (AD)
 Diagnosis: ATOPIC DERMATITIS (AD) Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Atopic Dermatitis (AD) PATIENT ADVICE: Unfortunately, there is no cure for atopic dermatitis, so
Diagnosis: ATOPIC DERMATITIS (AD) Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Atopic Dermatitis (AD) PATIENT ADVICE: Unfortunately, there is no cure for atopic dermatitis, so
Lead team presentation
 Lead team presentation Dupilumab for treating adults with moderate to severe atopic dermatitis [ID1048] 1 st Appraisal Committee meeting Committee B Chair: Amanda Adler Lead team: Diar Fattah, Danielle
Lead team presentation Dupilumab for treating adults with moderate to severe atopic dermatitis [ID1048] 1 st Appraisal Committee meeting Committee B Chair: Amanda Adler Lead team: Diar Fattah, Danielle
8-year surveillance 2016 Atopic eczema in under 12s (2007) NICE guideline CG57
 8-year 2016 Atopic eczema in under 12s (2007) NICE guideline CG57 Appendix A: decision matrix year Diagnosis 57 01 What criteria should be used to diagnose atopic eczema in children and how do they vary
8-year 2016 Atopic eczema in under 12s (2007) NICE guideline CG57 Appendix A: decision matrix year Diagnosis 57 01 What criteria should be used to diagnose atopic eczema in children and how do they vary
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2116-3 Program Prior Authorization/Medical Necessity Medications Dupixent (dupilumab) P&T Approval Date 1/2017, 5/2017, 7/2017
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2116-3 Program Prior Authorization/Medical Necessity Medications Dupixent (dupilumab) P&T Approval Date 1/2017, 5/2017, 7/2017
Running head: ATOPIC DERMATITIS, ITS IMPACT AND TREATMENT
 Atopic Dermatitis, Its Impact and Treatment Running head: ATOPIC DERMATITIS, ITS IMPACT AND TREATMENT The Impact of Treatment on Quality of Life of Children with Atopic Dermatitis and their Families Consuela
Atopic Dermatitis, Its Impact and Treatment Running head: ATOPIC DERMATITIS, ITS IMPACT AND TREATMENT The Impact of Treatment on Quality of Life of Children with Atopic Dermatitis and their Families Consuela
For topical use only. Not for oral, ophthalmic, or intravaginal use.
 DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
1g cream or ointment contains 1 mg methylprednisolone aceponate.
 CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
Vulval dermatoses. Dr Fiona Lewis, Consultant Dermatologist St John s Institute of Dermatology, London & Heatherwood & Wexham Park Hospital, Slough
 Vulval dermatoses Dr Fiona Lewis, Consultant Dermatologist St John s Institute of Dermatology, London & Heatherwood & Wexham Park Hospital, Slough Pigmentation Vulvodynia Ulcers Genetic Pruritus VULVAL
Vulval dermatoses Dr Fiona Lewis, Consultant Dermatologist St John s Institute of Dermatology, London & Heatherwood & Wexham Park Hospital, Slough Pigmentation Vulvodynia Ulcers Genetic Pruritus VULVAL
Is it allergy? Debbie Shipley
 Is it allergy? Debbie Shipley Topics Food Allergy and Eczema Hand Eczema and Patch Testing Urticaria Tackling Allergy Gell and Coombs classification Skin conditions with possible allergic component Allergy
Is it allergy? Debbie Shipley Topics Food Allergy and Eczema Hand Eczema and Patch Testing Urticaria Tackling Allergy Gell and Coombs classification Skin conditions with possible allergic component Allergy
Using Your ESP* in Pharmacy: How to Improve Treatment Adherence and Patient Outcomes in Psoriasis (*Expanded Scope of Practice)
 Using Your ESP* in Pharmacy: How to Improve Treatment Adherence and Patient Outcomes in Psoriasis (*Expanded Scope of Practice) Patient Case Study in Psoriasis Patient Case Study in Psoriasis William Smith,
Using Your ESP* in Pharmacy: How to Improve Treatment Adherence and Patient Outcomes in Psoriasis (*Expanded Scope of Practice) Patient Case Study in Psoriasis Patient Case Study in Psoriasis William Smith,
Dermatology elective for yr. 5. Natta Rajatanavin, MD. Div. of dermatology Dep. Of Medicine, Ramathibodi Hospital Mahidol University 23 rd Feb 2015
 Dermatology elective for yr. 5 Natta Rajatanavin, MD. Div. of dermatology Dep. Of Medicine, Ramathibodi Hospital Mahidol University 23 rd Feb 2015 How to diagnosis and manage eczema and psoriasis. Objectives
Dermatology elective for yr. 5 Natta Rajatanavin, MD. Div. of dermatology Dep. Of Medicine, Ramathibodi Hospital Mahidol University 23 rd Feb 2015 How to diagnosis and manage eczema and psoriasis. Objectives
Lichen Sclerosus. Exceptional healthcare, personally delivered
 Lichen Sclerosus Exceptional healthcare, personally delivered Lichen Sclerosus (LS) is an itchy skin condition usually affecting genital skin, but it can occur elsewhere. It affects women more often than
Lichen Sclerosus Exceptional healthcare, personally delivered Lichen Sclerosus (LS) is an itchy skin condition usually affecting genital skin, but it can occur elsewhere. It affects women more often than
NEW ZEALAND DATA SHEET 1 LOCOID 2 QUALITATIVE AND QUANTITATIVE COMPOSTION 3 PHARMACEUTICAL FORM 4 CLINICAL PARTICULARS
 NEW ZEALAND DATA SHEET 1 LOCOID Lipocream Ointment Topical Emulsion (Locoid Crelo ) Scalp Lotion hydrocortisone butyrate 2 QUALITATIVE AND QUANTITATIVE COMPOSTION Each formulation contains active ingredient
NEW ZEALAND DATA SHEET 1 LOCOID Lipocream Ointment Topical Emulsion (Locoid Crelo ) Scalp Lotion hydrocortisone butyrate 2 QUALITATIVE AND QUANTITATIVE COMPOSTION Each formulation contains active ingredient
(minutes for web publishing)
 Dermatology Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 20 October 2017 (minutes for web publishing) Dermatology Subcommittee minutes are published in accordance
Dermatology Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 20 October 2017 (minutes for web publishing) Dermatology Subcommittee minutes are published in accordance
UPDATES IN ATOPIC DERMATITIS
 UPDATES IN ATOPIC DERMATITIS Amanda Hess, MMS, PA-C President-Elect, AAPA-AAI Arizona Asthma and Allergy Institute, Scottsdale, AZ LEARNING OBJECTIVES Discuss epidemiology, risk factors, and causes of
UPDATES IN ATOPIC DERMATITIS Amanda Hess, MMS, PA-C President-Elect, AAPA-AAI Arizona Asthma and Allergy Institute, Scottsdale, AZ LEARNING OBJECTIVES Discuss epidemiology, risk factors, and causes of
NOVASONE CREAM, OINTMENT AND LOTION PRODUCT INFORMATION
 NAME OF THE MEDICINE Mometasone furoate 0.1% (1 mg/g) Chemical structure: NOVASONE CREAM, OINTMENT AND LOTION PRODUCT INFORMATION Mometasone furoate is 9,21-dichloro-11ß,17-dihydroxy-16 -methylpregna-1,4-diene-
NAME OF THE MEDICINE Mometasone furoate 0.1% (1 mg/g) Chemical structure: NOVASONE CREAM, OINTMENT AND LOTION PRODUCT INFORMATION Mometasone furoate is 9,21-dichloro-11ß,17-dihydroxy-16 -methylpregna-1,4-diene-
AUSTRALIAN PRODUCT INFORMATION RESOLVE PLUS 1.0 (MICONAZOLE NITRATE, HYDROCORTISONE) CREAM
 AUSTRALIAN PRODUCT INFORMATION RESOLVE PLUS 1.0 (MICONAZOLE NITRATE, HYDROCORTISONE) CREAM 1 NAME OF THE MEDICINE Miconazole nitrate, Hydrocortisone. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Miconazole
AUSTRALIAN PRODUCT INFORMATION RESOLVE PLUS 1.0 (MICONAZOLE NITRATE, HYDROCORTISONE) CREAM 1 NAME OF THE MEDICINE Miconazole nitrate, Hydrocortisone. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Miconazole
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ovixan 1 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram cream contains 1 mg mometasone furoate Excipients with known
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ovixan 1 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram cream contains 1 mg mometasone furoate Excipients with known
PRESCRIBING INFORMATION. Cream 0.025% Topical Corticosteroid
 PRESCRIBING INFORMATION Pr PROPADERM (beclomethasone dipropionate) Cream 0.025% Topical Corticosteroid Valeant Canada LP 2150 St-Elzear Blvd. West, Laval, Quebec, Canada H7L 4A8 Date of preparation: October
PRESCRIBING INFORMATION Pr PROPADERM (beclomethasone dipropionate) Cream 0.025% Topical Corticosteroid Valeant Canada LP 2150 St-Elzear Blvd. West, Laval, Quebec, Canada H7L 4A8 Date of preparation: October
Novan Announces Promising Clinical Results with SB414
 Novan Announces Promising Clinical Results with SB414 In the recently completed Phase 1b trial for atopic dermatitis, clinical efficacy measures were highly correlated with critical and disease-relevant
Novan Announces Promising Clinical Results with SB414 In the recently completed Phase 1b trial for atopic dermatitis, clinical efficacy measures were highly correlated with critical and disease-relevant
SUMMARY OF THE PRODUCT CHARACTERISTICS
 SUMMARY OF THE PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Zoviduo 50 mg/g and 10 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 gram cream contains 50 mg aciclovir and 10 mg hydrocortisone.
SUMMARY OF THE PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Zoviduo 50 mg/g and 10 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 gram cream contains 50 mg aciclovir and 10 mg hydrocortisone.
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Psoriasis: the management of psoriasis 1.1 Short title Psoriasis 2 The remit The Department of Health has asked NICE: 'to produce
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Psoriasis: the management of psoriasis 1.1 Short title Psoriasis 2 The remit The Department of Health has asked NICE: 'to produce
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME KENACOMB ear drops NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g contains the following active ingredients: 0.025% gramicidin, 0.25% neomycin, 100,000 units nystatin,
1. PRODUCT NAME KENACOMB ear drops NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g contains the following active ingredients: 0.025% gramicidin, 0.25% neomycin, 100,000 units nystatin,
ATOPIC DERMATITIS IN CHILDREN. Simple choice questions
 ATOPIC DERMATITIS IN CHILDREN Simple choice questions 1. Which is the principal cause of atopic dermatitisdevelopment in little age children? A. Psychoemotional factors. B. Habitual antigens. C. Food allergy.
ATOPIC DERMATITIS IN CHILDREN Simple choice questions 1. Which is the principal cause of atopic dermatitisdevelopment in little age children? A. Psychoemotional factors. B. Habitual antigens. C. Food allergy.
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Advantan cream 0.1% w/w Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g Advantan cream contains 1 mg (0.1%) methylprednisolone aceponate.
1 NAME OF THE MEDICINAL PRODUCT Advantan cream 0.1% w/w Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g Advantan cream contains 1 mg (0.1%) methylprednisolone aceponate.
Atopic Dermatitis: Therapeutic Challenges
 Atopic Dermatitis: Therapeutic Challenges PDA August 14, 2009 Jon Hanifin OHSU, Portland Dominant Concepts in Atopic Dermatitis Allergy / Immunology Era: 1915-2006 The Epidermal Era: 2006---- Barrier dysfunction
Atopic Dermatitis: Therapeutic Challenges PDA August 14, 2009 Jon Hanifin OHSU, Portland Dominant Concepts in Atopic Dermatitis Allergy / Immunology Era: 1915-2006 The Epidermal Era: 2006---- Barrier dysfunction
Food allergies and eczema
 Department of Dermatology Food allergies and eczema Information for parents and carers Eczema, also known as atopic eczema or atopic dermatitis, is a skin condition that causes inflammation and irritation.
Department of Dermatology Food allergies and eczema Information for parents and carers Eczema, also known as atopic eczema or atopic dermatitis, is a skin condition that causes inflammation and irritation.
PNW EPC Drug Effectiveness Review Project Summary Report Atopic Dermatitis New Drug Evaluation: Dupilumab
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Elements for a Public Summary
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Psoriasis (excluding widespread plaque psoriasis) Psoriasis is a common chronic skin disorder. Estimates of the prevalence (proportion
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Psoriasis (excluding widespread plaque psoriasis) Psoriasis is a common chronic skin disorder. Estimates of the prevalence (proportion
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Haelan Tape Fludroxycortide 4 micrograms per square centimetre Tape 2. QUALITATIVE AND QUANTITATIVE COMPOSITION The tape is impregnated
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Haelan Tape Fludroxycortide 4 micrograms per square centimetre Tape 2. QUALITATIVE AND QUANTITATIVE COMPOSITION The tape is impregnated
Psoriasis: Causes, Symptoms, And Treatment
 Psoriasis: Causes, Symptoms, And Treatment We all know that a healthy immune system is good. But, do you know that an overactive immune system can cause certain conditions like Psoriasis? Read on to find
Psoriasis: Causes, Symptoms, And Treatment We all know that a healthy immune system is good. But, do you know that an overactive immune system can cause certain conditions like Psoriasis? Read on to find
Volume 2; Number 2 February 2008
 Volume 2; Number 2 February 2008 CONTENTS Page 2 Page 3 Page 3 Page 4 Page 6 New Drug Assessment: Rufinamide (Inovelon) New Drug Assessment: Zoledronic acid infusion (Aclasta) New Drug Assessment: Mesalazine
Volume 2; Number 2 February 2008 CONTENTS Page 2 Page 3 Page 3 Page 4 Page 6 New Drug Assessment: Rufinamide (Inovelon) New Drug Assessment: Zoledronic acid infusion (Aclasta) New Drug Assessment: Mesalazine
