Regimens highlighted in red contain an expensive drug that is not currently publicly funded for the regimen and treatment intent.
|
|
|
- Ralf Farmer
- 5 years ago
- Views:
Transcription
1 Palliative Breast Cancer Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for breast cancer used in the palliative setting. It is expected that the prescribing oncologist will select the regimen from the list of evidence-informed regimens that is most appropriate for their patient taking account of a variety of disease-specific and patient-related factors. Regimens highlighted in red contain an expensive drug that is not currently publicly funded for the regimen and treatment intent. Regimens highlighted in blue contain an expensive drug that is not currently publicly funded but where there is a universal compassionate access program in place. The administration of the drug supported by the systemic treatment funding model, but not the cost of drug. Breast Cancer AC DOXOrubicin 60 mg/m 2 IV day 1; Cyclophosphamide 600 mg/m 2 IV day 1. ANAS Anastrozole 1 mg PO daily CAPE Capecitabine mg/m 2 PO BID days Capecitabine mg/m 2 PO BID days 1 7. Q14 days CAPE+TRAS Capecitabine PO mg/m 2 BID days 1-14; CAPEDOCE Capecitabine 1000 mg/m 2 PO BID, days 1-14; DOCEtaxel 75 mg/m 2 IV day 1. CAPELAPA Capecitabine 1000 mg/m 2 PO BID days 1-14; Lapatinib 1250 mg PO daily. CAV Cyclophosphamide 1000 mg/m² IV day 1; DOXOrubicin 50 mg/m² IV day 1; vincristine 1.4 mg/m² IV day 1.
2 CISP(RT-W) CISplatin mg/m² IV day 1. Q7 days Concurrent with radiotherapy Breast Cancer CISPETOP(3D) CISplatin 25 mg/m 2 IV days 1-3; Etoposide 100 mg/m 2 IV days 1-3. CISPETOP(5D) CISplatin 20 mg/m² IV days 1-5; Etoposide 100 mg/m² IV days 1-5. CISPGEMC(W) CISplatin 30 mg/m 2 IV days 1, 8; Gemcitabine 750 mg/m 2 IV days 1, 8. CM(PO) Cyclophosphamide 50 mg PO daily; Methotrexate 2.5 mg PO BID days 1, 2. Q7 days CMF(PO) Cyclophosphamide 100 mg/m² PO days 1-14; Methotrexate 40 mg/m² IV days 1, 8; Fluorouracil 600 mg/m² IV days 1, 8. CMF(PO)+TRAS Cyclophosphamide 100 mg/m² PO days 1-14; Methotrexate 40 mg/m² IV days 1, 8; Fluorouracil 600 mg/m² IV days 1, 8. CRBP CARBOplatin AUC 6 IV day 1. Note: For use in triple negative or BRCA1/2 mutation-associated breast cancers CRBPETOP(5D) CARBOplatin AUC 5 IV days, 1. Etoposide 100 mg/m² IV days 1-5. CRBPGEMC CARBOplatin AUC 2 IV days 1, 8; Gemcitabine 800 mg/m2 IV days 1, 8. CRBPPACL CARBOplatin AUC 5-6 IV day 1; PACLitaxel 175 mg/m 2 IV day 1. DOCE DOCEtaxel 100 mg/m² IV day 1.
3 DOCE(W) DOCEtaxel mg/m 2 IV days 1, 8, 15. DOCE+PERT+TRAS DOCEtaxel mg/m 2 IV day 1; For cycle 1 only, trastuzumab and docetaxel may be given on day 2 DOCE+TRAS DOCEtaxel 100 mg/m 2 IV day 1; DOCEGEMC DOCEtaxel 75 mg/m² IV day 1; Gemcitabine 1000 mg/m² IV days 1, 8. DOXO DOXOrubicin 50 to 75 mg/m 2 IV day 1. DOXO(W) DOXOrubicin mg/m² IV days 1, 8, 15. Q21-28 days EPIR EPIrubicin mg/m 2 IV day 1. EPIR(W) EPIrubicin 25 mg/m² IV days 1, 8, 15. ERIB eribulin 1.4 mg/m² IV days 1, 8. ETOP(PO) Etoposide 50 mg PO days Alternative Dose and Schedule: Etoposide mg PO days EVEREXEM EXEM Everolimus 10 mg PO daily (5 mg may be considered for certain patients); Exemestane 25 mg PO daily Exemestane 25 mg PO daily FAC Fluorouracil 500 mg/m 2 IV day 1; DOXOrubicin 50 mg/m 2 IV day 1; Cyclophosphamide 500 mg/m 2 IV day 1. FEC50 Fluorouracil 500 mg/m² IV day 1; EPIrubicin 50 mg/m² IV day 1; Cyclophosphamide 500 mg/m² IV day 1.
4 GEMC Gemcitabine 1000 mg/m 2 IV days 1, 8. Gemcitabine 1000 mg/m 2 IV day 1, 8, 15. GOSE KADC LETR LPRL MEDR MEGE Goserelin 3.6 mg SC. Trastuzumab Emtansine 3.6 mg/kg IV Letrozole 2.5 mg PO daily Leuprolide 22.5 mg IM Q3 months Medroxyprogesterone 400 mg/day PO in divided doses Megestrol 160 mg PO daily NPAC nab-paclitaxel 260 mg/m 2 IV day 1. NPAC(W) nab-paclitaxel mg/ 2 IV weekly days 1, 8, 15. NPAC(W)+PERT+TRAS nab-paclitaxel mg/2 IV weekly days 1, 8 - Publicly funded under specific conditions, see PDRP (NDFP) eligibility forms For cycle 1 only, trastuzumab and nab-paclitaxel may be given on day 2 NPAC(W)+TRAS nab-paclitaxel 100 mg/m 2 weekly days 1, 8, 15. Alternative Trastuzumab schedule: Trastuzumab 4 mg/kg IV loading dose followed by 2 mg/kg IV days 1, 8, 15, 22. NPAC+PERT+TRAS nab-paclitaxel 260 mg/m2 IV day 1 - Publicly funded under specific conditions, see PDRP (NDFP) eligibility forms For cycle 1 only, trastuzumab and nab-paclitaxel may be given on day 2
5 NPAC+TRAS nab-paclitaxel 260 mg/m 2 IV day 1; PACL PACLitaxel 175 mg/m² IV day 1. PACL(W) PACLitaxel 80 mg/m² IV days 1, 8, 15. PACL(W)+PERT+TRAS PACLitaxel 80 mg/m 2 IV days 1, 8. (can be given on day 2 in cycle 1 only) Pertuzumab 840 mg IV loading dose followed by 420 mg IV day 1. For cycle 1 only, trastuzumab and paclitaxel may be given on day 2 PACL(W)+TRAS PACLitaxel 80 mg/m² IV days 1, 8, 15.. Alternative Trastuzumab schedule: Trastuzumab 4 mg/kg IV loading dose followed by 2 mg/kg IV days 1, 8, 15, 22. PACL+PERT+TRAS PACLitaxel 175 mg/m 2 IV day 1; For cycle 1 only, trastuzumab and paclitaxel may be given on day 2 PACL+TRAS PACLitaxel 175 mg/m² IV day 1; PERT+TRAS Pertuzumab 420 mg IV day 1. Trastuzumab 6 mg/kg IV. PDRP (NDFP) funding is contingent on the patient previously receiving chemotherapy. PMDR Pamidronate 90 mg IV day 1. TMXF TRAS Tamoxifen 20 mg PO daily Trastuzumab 8 mg/kg IV loading dose followed by 6 mg/kg IV PDRP (NDFP) funding is contingent on the patient previously receiving chemotherapy.
6 VINO Vinorelbine mg/m² IV days 1, 8, 15. Vinorelbine mg/m 2 days 1, 8. VINO+TRAS Vinorelbine mg/m² IV days 1, 8, 15. Trastuzumab 4 mg/kg IV loading dose followed by 2 mg/kg IV days 1, 8, 15, 22. Vinorelbine mg/m 2 days 1, 8. ZOLE Zoledronic Acid 4 mg IV day 1. DENO FLVS FLVSPALB LETRPALB Zoledronic acid 4 mg IV day 1. Q84 days Denosumab 120 mg SC not currently publicly funded for this regimen and intent Fulvestrant 500 mg IM on days 1, 15, 29 (loading dose) THEN Fulvestrant 500 mg IM not currently publicly funded for this regimen and intent Fulvestrant 500 mg IM days 1, 15, 29 (loading dose) not currently publicly funded for this regimen and intent THEN Fulvestrant 500 mg IM day 1; Palbociclib 125 mg PO days 1-21 not currently publicly funded for this regimen and intent. Letrozole 2.5 mg PO daily (continuously) not currently publicly funded for this regimen and intent; Palbociclib 125 mg PO days 1-21 not currently publicly funded for this regimen and intent. PGLDX Pegylated Liposomal Doxorubicin mg/m 2 IV day 1. Not publicly funded. Universal compassionate access program available Last Updated: September 2017
Appendix 2. Adjuvant Regimens. AC doxorubin 60 mg/m 2 every 3 weeks x 4 cycles Cyclophosphamide 600 mg/m 2
 Appendix 2 Adjuvant Regimens AC doxorubin 60 mg/m 2 every 3 weeks x 4 cycles Cyclophosphamide 600 mg/m 2 CMF IV cyclophosphamide 600 mg/m 2 days 1 & 8 every 4 weeks methotrexate 40 mg/m 2 for 6 cycles
Appendix 2 Adjuvant Regimens AC doxorubin 60 mg/m 2 every 3 weeks x 4 cycles Cyclophosphamide 600 mg/m 2 CMF IV cyclophosphamide 600 mg/m 2 days 1 & 8 every 4 weeks methotrexate 40 mg/m 2 for 6 cycles
COME HOME Innovative Oncology Business Solutions, Inc.
 Innovative Oncology Business Solutions, Inc. Breast Cancer Diagnostic/Therapeutic Pathway V11, April 2015 Required Structured Data Fields: ICD9 Code Stage Staging Components Performance Status Treatment
Innovative Oncology Business Solutions, Inc. Breast Cancer Diagnostic/Therapeutic Pathway V11, April 2015 Required Structured Data Fields: ICD9 Code Stage Staging Components Performance Status Treatment
Disease Site Sub-Disease Site Intent Regimen Code Regimen Details Status (as of October 17, 2017) Breast Not Applicable Adjuvant / Curative
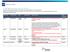 ST-QBP Regimen Request Status for 2017/18 Below are status of regimen requests submitted for funding considerations in FY 17/18 Q1 & Q2. Requests with status will be updated on the ST-QBP website and will
ST-QBP Regimen Request Status for 2017/18 Below are status of regimen requests submitted for funding considerations in FY 17/18 Q1 & Q2. Requests with status will be updated on the ST-QBP website and will
Disease Update: Metastatic Breast Cancer
 Disease Update: Metastatic Breast Cancer Aimee Faso, PharmD, BCOP, CPP Oncology Clinical Specialist, GI/Breast UNC Hospitals and Clinics August 2015 Objectives Identify treatment choices of metastatic
Disease Update: Metastatic Breast Cancer Aimee Faso, PharmD, BCOP, CPP Oncology Clinical Specialist, GI/Breast UNC Hospitals and Clinics August 2015 Objectives Identify treatment choices of metastatic
DOXOrubicin, Cyclophosphamide (AC 60/600) 21 day followed by weekly PACLitaxel (80) Therapy (AC-T) 261 CARBOplatin (AUC4-6) Monotherapy-21 days
 Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Treatment Options for Breast Cancer in Low- and Middle-Income Countries: Adjuvant and Metastatic Systemic Therapy
 Women s Empowerment Cancer Advocacy Network (WE CAN) Conference Bucharest, Romania October 2015 Treatment Options for Breast Cancer in Low- and Middle-Income Countries: Adjuvant and Metastatic Systemic
Women s Empowerment Cancer Advocacy Network (WE CAN) Conference Bucharest, Romania October 2015 Treatment Options for Breast Cancer in Low- and Middle-Income Countries: Adjuvant and Metastatic Systemic
FULCVR Some parts of regimen
 Disease Group APPROVED REGIMENS TO BE ADMINISTERED IN L4 SITE AC AC DOCE AC DOCE+TRAS AC AC (DD) AC (DD)+TRAS AC AC +TRAS AC +TRAS (RT W) CMF(PO) CMF(PO)+TRAS DOCETRAS CYCLDOCE CYCLDOCE+TRAS FEC100 FEC
Disease Group APPROVED REGIMENS TO BE ADMINISTERED IN L4 SITE AC AC DOCE AC DOCE+TRAS AC AC (DD) AC (DD)+TRAS AC AC +TRAS AC +TRAS (RT W) CMF(PO) CMF(PO)+TRAS DOCETRAS CYCLDOCE CYCLDOCE+TRAS FEC100 FEC
Triple Negative Breast Cancer: Part 2 A Medical Update
 Triple Negative Breast Cancer: Part 2 A Medical Update April 29, 2015 Tiffany A. Traina, MD Breast Medicine Service Memorial Sloan Kettering Cancer Center Weill Cornell Medical College Overview What is
Triple Negative Breast Cancer: Part 2 A Medical Update April 29, 2015 Tiffany A. Traina, MD Breast Medicine Service Memorial Sloan Kettering Cancer Center Weill Cornell Medical College Overview What is
Adjuvant Systemic Therapy in Early Stage Breast Cancer
 Adjuvant Systemic Therapy in Early Stage Breast Cancer Julie R. Gralow, M.D. Director, Breast Medical Oncology Jill Bennett Endowed Professor of Breast Cancer Professor, Global Health University of Washington
Adjuvant Systemic Therapy in Early Stage Breast Cancer Julie R. Gralow, M.D. Director, Breast Medical Oncology Jill Bennett Endowed Professor of Breast Cancer Professor, Global Health University of Washington
Protocol Number Tumour Group Protocol Name on NCCP website 22/02/ Lung Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy
 Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249
 Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
ST-QBP: Systemic Treatment Quality-Based Program (formerly STFM) DF: Drug Formulary GYNECOLOGICAL SKIN HEMATOLOGY ST- QBP
 Updates from October 17, 2017 Please note that the following are regimen updates applicable to webpage documents and/or Drug Formulary s regimen monographs, as indicated by checkmarks. : Systemic Treatment
Updates from October 17, 2017 Please note that the following are regimen updates applicable to webpage documents and/or Drug Formulary s regimen monographs, as indicated by checkmarks. : Systemic Treatment
Chapter. Contents Breast Cancer Adjuvant Epirubicin weekly. Docetaxel Copy No:
 Chapter 2: Breast Cancer Contents Chapter 2: Breast Cancer... 1 Breast Cancer... 2 Adjuvant...... 2 Epi-CMF... 2 FEC / docetaxel... 3 FEC100... 4 AC/EC/TC... 4 (neo) adjuvant... 5... 5 HER2 positive: TCarboH...
Chapter 2: Breast Cancer Contents Chapter 2: Breast Cancer... 1 Breast Cancer... 2 Adjuvant...... 2 Epi-CMF... 2 FEC / docetaxel... 3 FEC100... 4 AC/EC/TC... 4 (neo) adjuvant... 5... 5 HER2 positive: TCarboH...
Breast Cancer Clinical Pathway Committee Development Meeting
 Breast Cancer Clinical Pathway Committee Development Meeting Agenda Start Time Topic 8:0 am 8:0 am Welcome, Introductions, and Objectives for the Session 8:0 am 8: am Value-based Care in Breast Cancer
Breast Cancer Clinical Pathway Committee Development Meeting Agenda Start Time Topic 8:0 am 8:0 am Welcome, Introductions, and Objectives for the Session 8:0 am 8: am Value-based Care in Breast Cancer
Myeloma. Alternative Schedule: Bortezomib 1.3 mg/m² SC days 1, 4, 8, 11. Q21 days
 Palliative Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for myeloma used in the palliative setting. It is expected that the prescribing oncologist will select
Palliative Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for myeloma used in the palliative setting. It is expected that the prescribing oncologist will select
Therapy Side Effects
 Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Therapy Side Effects Therapy Side Effects Versions 2004 2011: Albert / Bischoff / Costa / Friedrichs / Göhring / Jackisch/
Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Therapy Side Effects Therapy Side Effects Versions 2004 2011: Albert / Bischoff / Costa / Friedrichs / Göhring / Jackisch/
StrandAdvantage Tissue-Specific Cancer Genomic Tests. Empowering Crucial First-Line Therapy Decisions for Your Patient
 StrandAdvantage Tissue-Specific Cancer Genomic Tests Empowering Crucial First-Line Therapy Decisions for Your Patient Harness the power of precision medicine with StrandAdvantage Precision medicine in
StrandAdvantage Tissue-Specific Cancer Genomic Tests Empowering Crucial First-Line Therapy Decisions for Your Patient Harness the power of precision medicine with StrandAdvantage Precision medicine in
It is a malignancy originating from breast tissue
 59 Breast cancer 1 It is a malignancy originating from breast tissue including both early stages which are potentially curable, and metastatic breast cancer (MBC) which is usually incurable. Most breast
59 Breast cancer 1 It is a malignancy originating from breast tissue including both early stages which are potentially curable, and metastatic breast cancer (MBC) which is usually incurable. Most breast
亞東紀念醫院 Breast Cancer 化學治療處方集
 亞東紀念醫院 Breast Cancer 化學治療處方集 2008-08 制定 最近修改日期 :2015-01 CMF Breast cancer 化學治療處方參考集 Adjuvant Classic CMF Cyclophosphamide 100mg/m2 PO qd; D1-D14 Methotrexate 40mg/m 2 in N/S 100 ml IV drip 30 mins; D1,
亞東紀念醫院 Breast Cancer 化學治療處方集 2008-08 制定 最近修改日期 :2015-01 CMF Breast cancer 化學治療處方參考集 Adjuvant Classic CMF Cyclophosphamide 100mg/m2 PO qd; D1-D14 Methotrexate 40mg/m 2 in N/S 100 ml IV drip 30 mins; D1,
Clinical Management Guideline for Breast Cancer
 Initial Evaluation Clinical Stage Pre-Treatment Evaluation Treatment and pathological stage Adjuvant Treatment Less than 4 positive lymph nodes ER Positive HER2 Negative (see page 2 & 3 ) Primary Diagnosis:
Initial Evaluation Clinical Stage Pre-Treatment Evaluation Treatment and pathological stage Adjuvant Treatment Less than 4 positive lymph nodes ER Positive HER2 Negative (see page 2 & 3 ) Primary Diagnosis:
Adjuvant/Curative/Neo-adjuvant High Grade and Burkitt s Lymphoma Regimens. High Grade Lymphoma
 Adjuvant/Curative/Neo-adjuvant High Grade and Burkitt s Lymphoma Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for high grade and Burkitt s lymphoma used in the
Adjuvant/Curative/Neo-adjuvant High Grade and Burkitt s Lymphoma Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for high grade and Burkitt s lymphoma used in the
Haematology, Oncology and Palliative Care Directorate.
 Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Verzenio) Reference Number: CP.PHAR.355 Effective Date: 10.24.17 Last Review Date: 02.19 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this
Clinical Policy: (Verzenio) Reference Number: CP.PHAR.355 Effective Date: 10.24.17 Last Review Date: 02.19 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this
Breast Cancer Breast Managed Clinical Network
 Initial Evaluation Clinical Stage Pre-Treatment Evaluation Treatment and pathological stage Less than 4 positive lymph nodes Adjuvant Treatment ER Positive HER2 Negative (see page 2 & 3 ) HER2 Positive
Initial Evaluation Clinical Stage Pre-Treatment Evaluation Treatment and pathological stage Less than 4 positive lymph nodes Adjuvant Treatment ER Positive HER2 Negative (see page 2 & 3 ) HER2 Positive
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Herceptin) Reference Number: ERX.SPA.42 Effective Date: 07.01.16 Last Review Date: 05/17 Line of Business: Commercial [Prescription Drug Plan] Revision Log See Important Reminder at the
Clinical Policy: (Herceptin) Reference Number: ERX.SPA.42 Effective Date: 07.01.16 Last Review Date: 05/17 Line of Business: Commercial [Prescription Drug Plan] Revision Log See Important Reminder at the
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Proposed Health Technology Appraisal Everolimus in combination with exemestane for the treatment of advanced or metastatic HER2 negative, oestrogen
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Proposed Health Technology Appraisal Everolimus in combination with exemestane for the treatment of advanced or metastatic HER2 negative, oestrogen
Palliative Low Grade Lymphoma & Hairy Cell Leukemia Regimens. Low Grade Lymphoma
 Palliative Low Grade Lymphoma & Hairy Cell Leukemia Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for low grade lymphoma and Hairy Cell leukemia used in the palliative
Palliative Low Grade Lymphoma & Hairy Cell Leukemia Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for low grade lymphoma and Hairy Cell leukemia used in the palliative
Metastatic Breast Cancer What is new? Subtypes and variation?
 Metastatic Breast Cancer What is new? Subtypes and variation? Anne Blaes, MD, MS University of Minnesota, Division of Hematology/Oncology Director, Adult Cancer Survivor Program Current estimates for metastatic
Metastatic Breast Cancer What is new? Subtypes and variation? Anne Blaes, MD, MS University of Minnesota, Division of Hematology/Oncology Director, Adult Cancer Survivor Program Current estimates for metastatic
BREAST CANCER (RECURRENT OR METASTATIC) TREATMENT REGIMENS (Part 1 of 5)
 BREAST CANCER (RECURRENT METASTATIC) TREATMENT S (Part 1 of 5) Clinical Trials: The NCCN recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection,
BREAST CANCER (RECURRENT METASTATIC) TREATMENT S (Part 1 of 5) Clinical Trials: The NCCN recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection,
Chemotherapy With or Without Targeted Drugs* in Metastatic Breast Cancer
 Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Chemotherapy With or Without Targeted Drugs* in Metastatic Breast Cancer * Substances without published evidence based on at
Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Chemotherapy With or Without Targeted Drugs* in Metastatic Breast Cancer * Substances without published evidence based on at
METASTATIC BREAST CANCER: CONTROLLING THE HERD WHEN THE HORSES ARE OUT OF THE BARN
 METASTATIC BREAST CANCER: CONTROLLING THE HERD WHEN THE HORSES ARE OUT OF THE BARN V A L L E R I E G O R D O N B S C, M D, F R C P C M E D I C A L O N C O L O G I S T A S S I S T A N T P R O F E S S O
METASTATIC BREAST CANCER: CONTROLLING THE HERD WHEN THE HORSES ARE OUT OF THE BARN V A L L E R I E G O R D O N B S C, M D, F R C P C M E D I C A L O N C O L O G I S T A S S I S T A N T P R O F E S S O
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Verzenio) Reference Number: CP.PHAR.355 Effective Date: 10.24.17 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this
Clinical Policy: (Verzenio) Reference Number: CP.PHAR.355 Effective Date: 10.24.17 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced
 ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
RECENT ADVANCES BREAST CANCER THERAPY. Bhanu Priya B, 2 Basavanna P L, 1 Postgraduate, 2 Professor Department of Pharmacology, MMC&RI, Mysore
 RECENT ADVANCES BREAST CANCER THERAPY 1 Bhanu Priya B, 2 Basavanna P L, 1 Postgraduate, 2 Professor Department of Pharmacology, MMC&RI, Mysore Brief overview Introduction Etiology Pathogenesis Symptoms
RECENT ADVANCES BREAST CANCER THERAPY 1 Bhanu Priya B, 2 Basavanna P L, 1 Postgraduate, 2 Professor Department of Pharmacology, MMC&RI, Mysore Brief overview Introduction Etiology Pathogenesis Symptoms
Guidelines for the Use of Anti-Emetics with Chemotherapy
 Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Working Formulary January 2013 Oncology Chemotherapy Regimens
 Working Formulary January 2013 Oncology Chemotherapy Regimens In the currently changing commissioning landscape, this document is intended to represent the up to date list of non clinical trial chemotherapy
Working Formulary January 2013 Oncology Chemotherapy Regimens In the currently changing commissioning landscape, this document is intended to represent the up to date list of non clinical trial chemotherapy
Objectives: Describe poly-adp-ribose polymerase (PARP) inhibitors mechanism of action.
 1 2 3 Role of PARP Inhibitors in Metastatic Breast Cancer Catie Chatowsky, PharmD PGY1 Pharmacy Resident Disclosure: I have nothing to disclose. Objectives: Describe poly-adp-ribose polymerase (PARP) inhibitors
1 2 3 Role of PARP Inhibitors in Metastatic Breast Cancer Catie Chatowsky, PharmD PGY1 Pharmacy Resident Disclosure: I have nothing to disclose. Objectives: Describe poly-adp-ribose polymerase (PARP) inhibitors
Medical Therapies in Ovarian Cancer The Arabic Perspectives. Mezghani Bassem -Tunisia
 Tunisian Health System: Social Welfare with a Public insurance for all citizens including Indigent persons. (± Additional private insurance) Choice: Public Hospital/Private Clinics (Indigents Public H)
Tunisian Health System: Social Welfare with a Public insurance for all citizens including Indigent persons. (± Additional private insurance) Choice: Public Hospital/Private Clinics (Indigents Public H)
LUNG. Tumour Group: Regimen name / acronym. Place in therapy. Induction chemotherapy. Regimen name / acronym. Place in therapy
 Tumour Group: LUNG Non-small cell lung cancer Adjuvant Vinorelbine PO* / Cisplatin 1 x x First line Vinorelbine IV / Cisplatin x First line Vinorelbine IV / Carboplatin x cisplatin Vinorelbine PO* / Cisplatin
Tumour Group: LUNG Non-small cell lung cancer Adjuvant Vinorelbine PO* / Cisplatin 1 x x First line Vinorelbine IV / Cisplatin x First line Vinorelbine IV / Carboplatin x cisplatin Vinorelbine PO* / Cisplatin
Beyond the Guidelines: Clinical Investigators Provide Their Perspectives on Current Strategies and Ongoing Research in the Management of Breast Cancer
 Beyond the Guidelines: Clinical Investigators Provide Their Perspectives on Current Strategies and Ongoing Research in the Management of Breast Cancer Wednesday, December 11, 2013 7:30 PM 9:30 PM San Antonio,
Beyond the Guidelines: Clinical Investigators Provide Their Perspectives on Current Strategies and Ongoing Research in the Management of Breast Cancer Wednesday, December 11, 2013 7:30 PM 9:30 PM San Antonio,
Breast Cancer. Metastatic NCCN GUIDELINES FOR PATIENTS Available online at NCCN.org/patients. Please complete. our online survey at
 NCCN GUIDELINES FOR PATIENTS 2018 Please complete our online survey at NCCN.org/patients/survey Breast Cancer Metastatic Presented with support from: Available online at NCCN.org/patients Ü Metastatic
NCCN GUIDELINES FOR PATIENTS 2018 Please complete our online survey at NCCN.org/patients/survey Breast Cancer Metastatic Presented with support from: Available online at NCCN.org/patients Ü Metastatic
Horizon Scanning Centre November Vinflunine (Javlor) monotherapy for advanced breast cancer SUMMARY NIHR HSC ID: 7887
 Horizon Scanning Centre November 2012 Vinflunine (Javlor) monotherapy for advanced breast cancer SUMMARY NIHR HSC ID: 7887 This briefing is based on information available at the time of research and a
Horizon Scanning Centre November 2012 Vinflunine (Javlor) monotherapy for advanced breast cancer SUMMARY NIHR HSC ID: 7887 This briefing is based on information available at the time of research and a
Adjuvant Chemotherapy + Trastuzumab
 Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Adjuvant Chemotherapy + Trastuzumab (Optimal Drugs / Dosage / Trastuzumab) Adjuvant Chemotherapy (Optimal Drugs / Optimal Dosage
Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Adjuvant Chemotherapy + Trastuzumab (Optimal Drugs / Dosage / Trastuzumab) Adjuvant Chemotherapy (Optimal Drugs / Optimal Dosage
MASCC Guidelines for Antiemetic control: An update
 MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
Updates From San Antonio Breast Cancer Symposium 2017
 Updates From San Antonio Breast Cancer Symposium 2017 Rob Coleman University of Sheffield Presentation Outline New Insights into adjuvant endocrine treatment Duration of treatment Perioperative therapy
Updates From San Antonio Breast Cancer Symposium 2017 Rob Coleman University of Sheffield Presentation Outline New Insights into adjuvant endocrine treatment Duration of treatment Perioperative therapy
For Health Professionals Who Care For Cancer Patients
 April 2018 Volume 21, No. 4 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice New Programs: Palbociclib for Metastatic Breast Cancer, Adjuvant Capecitabine for Breast
April 2018 Volume 21, No. 4 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice New Programs: Palbociclib for Metastatic Breast Cancer, Adjuvant Capecitabine for Breast
Form 2023 R2.0: Ovarian Cancer Pre-HSCT Data
 Key Fields Sequence Number Date Received: - - CIBMTR Center Number: CIBMTR Recipient ID: Today's Date: - - Date of HSCT for which this form is being completed: - - HSCT type: (check all that apply) Autologous
Key Fields Sequence Number Date Received: - - CIBMTR Center Number: CIBMTR Recipient ID: Today's Date: - - Date of HSCT for which this form is being completed: - - HSCT type: (check all that apply) Autologous
TRANSPARENCY COMMITTEE OPINION. 15 February 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 February 2006 Taxotere 20 mg, concentrate and solvent for solution for infusion B/1 vial of Taxotere and 1 vial
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 February 2006 Taxotere 20 mg, concentrate and solvent for solution for infusion B/1 vial of Taxotere and 1 vial
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting
 West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
Treatment of Metastatic Breast Cancer. Prof RCCoombes Imperial College London
 Treatment of Metastatic Breast Cancer Prof RCCoombes Imperial College London Metastatic Breast Cancer: General Guidelines Specialized oncology nurses (if possible specialized breast nurses) should be part
Treatment of Metastatic Breast Cancer Prof RCCoombes Imperial College London Metastatic Breast Cancer: General Guidelines Specialized oncology nurses (if possible specialized breast nurses) should be part
癌症診療指引175 Chemotherapy regimens for primary or adjuvant therapy
 癌症診療指引175 Chemotherapy regimens for primary or adjuvant therapy Preferred regimens for HER2-positive disease AC followed by T chemotherapy with Trastuzumab Doxorubicin 60 1 Q3W 1 Cyclophosphamide 600 1
癌症診療指引175 Chemotherapy regimens for primary or adjuvant therapy Preferred regimens for HER2-positive disease AC followed by T chemotherapy with Trastuzumab Doxorubicin 60 1 Q3W 1 Cyclophosphamide 600 1
Oncological Treatment of Breast Cancer
 Oncological Treatment of Breast Cancer Pathway of Care Core Network Team Publication date October 2017 Expected review date October 2019 Version number 22 Version status Final Table of Contents 1.0 ONCOLOGY
Oncological Treatment of Breast Cancer Pathway of Care Core Network Team Publication date October 2017 Expected review date October 2019 Version number 22 Version status Final Table of Contents 1.0 ONCOLOGY
AGO e. V. in der DGGG e.v. sowie in der DKG e.v.
 AGO e. V. in der DGGG e.v. sowie in der DKG e.v. Guidelines Breast Version 2015.1 www.ago-online.de Disease-Free and Overall Survival in Metastatic Breast Cancer AGO e. V. in der DGGG e.v. sowie in der
AGO e. V. in der DGGG e.v. sowie in der DKG e.v. Guidelines Breast Version 2015.1 www.ago-online.de Disease-Free and Overall Survival in Metastatic Breast Cancer AGO e. V. in der DGGG e.v. sowie in der
Etudes cliniques Service d Oncologie - Radiothérapie
 Etudes cliniques Service d Oncologie - Radiothérapie Juillet 2016 SEIN-NEO ADJUVANT LORELEI -GO28888-1319 BCG : A phase II randomized, double-blind, parallel cohort study of neoadjuvant letrozole + GDC-0032
Etudes cliniques Service d Oncologie - Radiothérapie Juillet 2016 SEIN-NEO ADJUVANT LORELEI -GO28888-1319 BCG : A phase II randomized, double-blind, parallel cohort study of neoadjuvant letrozole + GDC-0032
Evolving Paradigms in HER2+ MBC: Strategies for Individualizing Therapy with Available Agents
 Evolving Paradigms in HER2+ MBC: Strategies for Individualizing Therapy with Available Agents Kimberly L. Blackwell MD Professor Department of Medicine and Radiation Oncology Duke University Medical Center
Evolving Paradigms in HER2+ MBC: Strategies for Individualizing Therapy with Available Agents Kimberly L. Blackwell MD Professor Department of Medicine and Radiation Oncology Duke University Medical Center
Advances in Breast Cancer Therapeutics in the Adjuvant and Metastatic Settings. Eve Rodler, MD University of California at Davis October 2016
 Advances in Breast Cancer Therapeutics in the Adjuvant and Metastatic Settings Eve Rodler, MD University of California at Davis October 2016 17th Annual Advances in Oncology September 30-October 1, 2016
Advances in Breast Cancer Therapeutics in the Adjuvant and Metastatic Settings Eve Rodler, MD University of California at Davis October 2016 17th Annual Advances in Oncology September 30-October 1, 2016
A) DIAGNOSIS & WORKUP
 Metastatic Breast Cancer Updated September 2015 by Dr. Ko (Medical Oncologist, Abbotsford Cancer Centre) Reviewed by Dr. Eitan Amir (Staff Medical Oncologist, University of Toronto) DISCLAIMER: The following
Metastatic Breast Cancer Updated September 2015 by Dr. Ko (Medical Oncologist, Abbotsford Cancer Centre) Reviewed by Dr. Eitan Amir (Staff Medical Oncologist, University of Toronto) DISCLAIMER: The following
新竹馬偕紀念醫院癌症中心 乳癌化學治療藥物處方
 新竹馬偕紀念醫院癌症中心 乳癌化學治療藥物處方 文件修訂記錄 修正次數 修正日期 修正版別 修 改 內 容 1 2011.04.07 1.0 初次訂定 2 2013.05.08 2.0 修訂 3 2013.04.30 3.0 修訂 :Triple-Negative Breast Cancer 處方 新增 :Neoadjuvant-p7~8 4 2014.04.29 4.0 修訂 :FEC + Trastuzumab
新竹馬偕紀念醫院癌症中心 乳癌化學治療藥物處方 文件修訂記錄 修正次數 修正日期 修正版別 修 改 內 容 1 2011.04.07 1.0 初次訂定 2 2013.05.08 2.0 修訂 3 2013.04.30 3.0 修訂 :Triple-Negative Breast Cancer 處方 新增 :Neoadjuvant-p7~8 4 2014.04.29 4.0 修訂 :FEC + Trastuzumab
Breast cancer. Prof Arlene Chan Medical Oncologist Director Breast Clinical Trials Unit, Mount Hospital Vice-Chair Breast Cancer Research Centre - WA
 Breast cancer rof Arlene Chan Medical Oncologist Director Breast Clinical Trials Unit, Mount Hospital Vice-Chair Breast Cancer Research Centre - WA Breast cancer Incidence of Breast Cancer by Stage at
Breast cancer rof Arlene Chan Medical Oncologist Director Breast Clinical Trials Unit, Mount Hospital Vice-Chair Breast Cancer Research Centre - WA Breast cancer Incidence of Breast Cancer by Stage at
Reviewed by Dr. Eitan Amir (Staff Medical Oncologist, University of Toronto)
 Metastatic Breast Cancer Updated June 2017 by Dr. Veitch (Tom Baker Cancer Centre) Reviewed by Dr. Eitan Amir (Staff Medical Oncologist, University of Toronto) DISCLAIMER: The following are study notes
Metastatic Breast Cancer Updated June 2017 by Dr. Veitch (Tom Baker Cancer Centre) Reviewed by Dr. Eitan Amir (Staff Medical Oncologist, University of Toronto) DISCLAIMER: The following are study notes
Immunoconjugates in Both the Adjuvant and Metastatic Setting
 Immunoconjugates in Both the Adjuvant and Metastatic Setting Mark Pegram, M.D. Director, Stanford Breast Oncology Program Co-Director, Molecular Therapeutics Program Trastuzumab Treatment of Breast Tumor
Immunoconjugates in Both the Adjuvant and Metastatic Setting Mark Pegram, M.D. Director, Stanford Breast Oncology Program Co-Director, Molecular Therapeutics Program Trastuzumab Treatment of Breast Tumor
Perjeta (pertuzumab)
 Perjeta (pertuzumab) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/201809/16/2018 POLICY A. INDICATIONS The indications
Perjeta (pertuzumab) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/201809/16/2018 POLICY A. INDICATIONS The indications
ASCO 2017 BREAST CANCER HIGHLIGHTS
 Post-ASCO 24 th June 2017, Dolce La Hulpe, Belgium ASCO 2017 BREAST CANCER HIGHLIGHTS Martine J. Piccart-Gebhart, MD, PhD Jules Bordet Institute, Brussels, Belgium Université Libre de Bruxelles Breast
Post-ASCO 24 th June 2017, Dolce La Hulpe, Belgium ASCO 2017 BREAST CANCER HIGHLIGHTS Martine J. Piccart-Gebhart, MD, PhD Jules Bordet Institute, Brussels, Belgium Université Libre de Bruxelles Breast
Effectively managing patients receiving oral anticancer therapies to treat MBC: from a BCN perspective. Gillian Kruss
 Effectively managing patients receiving oral anticancer therapies to treat MBC: from a BCN perspective Gillian Kruss New drug developments / new challenges More effective treatments (prolong O.S / PFS)
Effectively managing patients receiving oral anticancer therapies to treat MBC: from a BCN perspective Gillian Kruss New drug developments / new challenges More effective treatments (prolong O.S / PFS)
Expanding Therapeutic Strategies for HER2-Positive Metastatic Breast Cancer
 Expanding Therapeutic Strategies for HER2-Positive Metastatic Breast Cancer Sara A. Hurvitz, MD, FACP Associate Professor of Medicine University of California Los Angeles Los Angeles, California Trastuzumab
Expanding Therapeutic Strategies for HER2-Positive Metastatic Breast Cancer Sara A. Hurvitz, MD, FACP Associate Professor of Medicine University of California Los Angeles Los Angeles, California Trastuzumab
ASCO and San Antonio Updates
 ASCO and San Antonio Updates 30 th Annual Miami Breast Cancer Conference March 7-10, 2013 Debu Tripathy, MD Professor of Medicine University of Southern California Norris Comprehensive Cancer Center Breakthroughs
ASCO and San Antonio Updates 30 th Annual Miami Breast Cancer Conference March 7-10, 2013 Debu Tripathy, MD Professor of Medicine University of Southern California Norris Comprehensive Cancer Center Breakthroughs
Highlights. Padova,
 Highlights P Pronzato Padova, 17.11.2012 Last 12 Months Main Meetings SABCS 2011 (San Antonio) EBCC 8 2012 (Wien) ASCO 2012 (Chicago) ESMO/ECCO 2012 (Wien) The Medical Oncology Job Risk Manager Strategy
Highlights P Pronzato Padova, 17.11.2012 Last 12 Months Main Meetings SABCS 2011 (San Antonio) EBCC 8 2012 (Wien) ASCO 2012 (Chicago) ESMO/ECCO 2012 (Wien) The Medical Oncology Job Risk Manager Strategy
CANCER PREVENTION AND ACCESS TO MEDICINES. Gracemarie Bricalli ESMO Head of International Affairs
 CANCER PREVENTION AND ACCESS TO MEDICINES Gracemarie Bricalli ESMO Head of International Affairs ESMO s 2020 Vision: Access to cancer care ESMO s 2020 vision statement recognises that progress in the management
CANCER PREVENTION AND ACCESS TO MEDICINES Gracemarie Bricalli ESMO Head of International Affairs ESMO s 2020 Vision: Access to cancer care ESMO s 2020 vision statement recognises that progress in the management
Breast Cancer Clinical Trials in Georgia
 www.georgiacore.org www.georgiatrials.org Breast Clinical Trials in Georgia Breast in Georgia: The Facts As the leading cause of cancer incidence among Georgia females, breast cancer accounts for 32 percent
www.georgiacore.org www.georgiatrials.org Breast Clinical Trials in Georgia Breast in Georgia: The Facts As the leading cause of cancer incidence among Georgia females, breast cancer accounts for 32 percent
National Horizon Scanning Centre. Bevacizumab (Avastin) in combination with non-taxanes for metastatic breast cancer - first line therapy
 Bevacizumab (Avastin) in combination with non-taxanes for metastatic breast cancer - first line therapy December 2007 This technology summary is based on information available at the time of research and
Bevacizumab (Avastin) in combination with non-taxanes for metastatic breast cancer - first line therapy December 2007 This technology summary is based on information available at the time of research and
THE ESMO INTERNATIONAL ANTINEOPLASTIC MEDICINES SURVEY: HOW AVAILABLE ARE THE WHO ESSENTIAL CANCER MEDICINES?
 THE ESMO INTERNATIONAL ANTINEOPLASTIC MEDICINES SURVEY: HOW AVAILABLE ARE THE WHO ESSENTIAL CANCER MEDICINES? Alexandru ENIU, MD, PhD Chair, ESMO Global Policy Committee Department of Breast Tumors Cancer
THE ESMO INTERNATIONAL ANTINEOPLASTIC MEDICINES SURVEY: HOW AVAILABLE ARE THE WHO ESSENTIAL CANCER MEDICINES? Alexandru ENIU, MD, PhD Chair, ESMO Global Policy Committee Department of Breast Tumors Cancer
Systemic therapy: HER-2 update. Hans Wildiers Multidisciplinair Borst Centrum/Algemene medische oncologie UZ Leuven
 Systemic therapy: HER-2 update Hans Wildiers Multidisciplinair Borst Centrum/Algemene medische oncologie UZ Leuven New drugs Strategic issues Specific anti-her2 drugs Lapa$nib /Nera$nib Baselga & Swain,
Systemic therapy: HER-2 update Hans Wildiers Multidisciplinair Borst Centrum/Algemene medische oncologie UZ Leuven New drugs Strategic issues Specific anti-her2 drugs Lapa$nib /Nera$nib Baselga & Swain,
Neo-adjuvant and adjuvant treatment for HER-2+ breast cancer
 Neo-adjuvant and adjuvant treatment for HER-2+ breast cancer Angelo Di Leo «Sandro Pitigliani» Medical Oncology Unit Hospital of Prato Istituto Toscano Tumori Prato, Italy NOAH: Phase III, Open-Label Trial
Neo-adjuvant and adjuvant treatment for HER-2+ breast cancer Angelo Di Leo «Sandro Pitigliani» Medical Oncology Unit Hospital of Prato Istituto Toscano Tumori Prato, Italy NOAH: Phase III, Open-Label Trial
COST CONSIDERATIONS Union for International Cancer Control 2014 Review of Cancer Medicines on the WHO List of Essential Medicines!!!!!!!!!
 UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
Recent advances in the management of metastatic breast cancer in older adults
 Recent advances in the management of metastatic breast cancer in older adults Laura Biganzoli Medical Oncology Dept New Hospital of Prato Istituto Toscano Tumori Italy Important recent advances in the
Recent advances in the management of metastatic breast cancer in older adults Laura Biganzoli Medical Oncology Dept New Hospital of Prato Istituto Toscano Tumori Italy Important recent advances in the
GSK Medicine: Study Number: Title: Rationale: Study Period: Objectives: Indication: Study Investigators/Centers: Research Methods: Data Source
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY CLASSIFICATION
 GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
Recent Update in Management of Breast Cancer: Medical Oncology. Jin Hee Ahn, M.D., PhD. 23-April-2015
 2015 GBCC & 4 th IBCS 1/37 Recent Update in Management of Breast Cancer: Medical Oncology Jin Hee Ahn, M.D., PhD. 23-April-2015 Department of Oncology, Asan Medical Center, UUCM, Seoul, Korea 2/37 3/37
2015 GBCC & 4 th IBCS 1/37 Recent Update in Management of Breast Cancer: Medical Oncology Jin Hee Ahn, M.D., PhD. 23-April-2015 Department of Oncology, Asan Medical Center, UUCM, Seoul, Korea 2/37 3/37
Chemotherapy as Primary or Adjuvant Therapy (HER2-POSTIVE)
 乳癌抗癌藥物治療指引 Chemotherapy as Primary or Adjuvant Therapy (HER2-POSTIVE) PREFERRED REGIMENS AC followed by Paclitaxel with Trastuzumab Doxorubicin 60 1 Q3W 4 1 Trastuzumab 4 2 mg/kg 1 QW 12 Paclitaxel 80
乳癌抗癌藥物治療指引 Chemotherapy as Primary or Adjuvant Therapy (HER2-POSTIVE) PREFERRED REGIMENS AC followed by Paclitaxel with Trastuzumab Doxorubicin 60 1 Q3W 4 1 Trastuzumab 4 2 mg/kg 1 QW 12 Paclitaxel 80
Breast Cancer RESULT INTERPRETATION THERAPRINT OVERVIEW
 Breast Cancer PAGE 1 OF 7 Patient: DOB: Patient #: Gender: Customer Ref.: SPECIMEN Requisition: Collection Date: Date Received: Report Date: Specimen Site: Tumor Origin: Unspecified Breast PHYSICIAN Ordering
Breast Cancer PAGE 1 OF 7 Patient: DOB: Patient #: Gender: Customer Ref.: SPECIMEN Requisition: Collection Date: Date Received: Report Date: Specimen Site: Tumor Origin: Unspecified Breast PHYSICIAN Ordering
For Health Professionals Who Care For Cancer Patients
 March 2018 Volume 21, No. 3 For Health Professionals Who Care For Cancer s Inside This Issue: Editor s Choice New Programs: Nivolumab for Squamous Cell Cancer of the Head and Neck, Ibrutinib for Mantle-Cell
March 2018 Volume 21, No. 3 For Health Professionals Who Care For Cancer s Inside This Issue: Editor s Choice New Programs: Nivolumab for Squamous Cell Cancer of the Head and Neck, Ibrutinib for Mantle-Cell
Clinical Management Guideline for Small Cell Lung Cancer
 Diagnosis and Staging: Key Points 1. Ensure a CT scan that is
Diagnosis and Staging: Key Points 1. Ensure a CT scan that is
Supplementary Online Content
 Supplementary Online Content Rugo HS, Klein P, Melin SA, et al. Association between use of a scalp cooling device and alopecia after chemotherapy for breast cancer. JAMA. doi:10.1001/jama.2016.21038 Additional
Supplementary Online Content Rugo HS, Klein P, Melin SA, et al. Association between use of a scalp cooling device and alopecia after chemotherapy for breast cancer. JAMA. doi:10.1001/jama.2016.21038 Additional
Clinical Research on PARP Inhibitors and Triple-Negative Breast Cancer (TNBC)
 Clinical Research on PARP Inhibitors and Triple-Negative Breast Cancer (TNBC) Eric P Winer, MD Disclosures for Eric P Winer, MD No real or apparent conflicts of interest to disclose Key Topics: PARP and
Clinical Research on PARP Inhibitors and Triple-Negative Breast Cancer (TNBC) Eric P Winer, MD Disclosures for Eric P Winer, MD No real or apparent conflicts of interest to disclose Key Topics: PARP and
Sustained benefits for women with HER2-positive early breast cancer JORGE MADRID BIG GOCCHI PROTOCOLO HERA
 Sustained benefits for women with HER2-positive early breast cancer JORGE MADRID BIG GOCCHI PROTOCOLO HERA The fascinating history of Herceptin 1981 1985 1987 1990 1992 1998 2000 2005 2006 2008 2011 Murine
Sustained benefits for women with HER2-positive early breast cancer JORGE MADRID BIG GOCCHI PROTOCOLO HERA The fascinating history of Herceptin 1981 1985 1987 1990 1992 1998 2000 2005 2006 2008 2011 Murine
Customizing Therapeutic Strategies in the Management of Metastatic Breast Cancer
 Customizing Therapeutic Strategies in the Management of Metastatic Breast Cancer Vandana G Abramson, MD Assistant Professor of Medicine Division of Hematology/Oncology Vanderbilt University Breast Cancer
Customizing Therapeutic Strategies in the Management of Metastatic Breast Cancer Vandana G Abramson, MD Assistant Professor of Medicine Division of Hematology/Oncology Vanderbilt University Breast Cancer
非臨床試験 臨床の立場から 京都大学医学部附属病院戸井雅和
 資料 2 2 非臨床試験 臨床の立場から 京都大学医学部附属病院戸井雅和 1 Preclinical studies Therapeutic Window: Efficacy/Toxicity Disease Specificity Subtype Specificity Combination: Concurrent/Sequential Therapeutic situation: Response/
資料 2 2 非臨床試験 臨床の立場から 京都大学医学部附属病院戸井雅和 1 Preclinical studies Therapeutic Window: Efficacy/Toxicity Disease Specificity Subtype Specificity Combination: Concurrent/Sequential Therapeutic situation: Response/
CLINICAL TRIALS ACC. Jul 2016
 CLINICAL TRIALS ACC Jul 2016 Glioblastoma BRAIN A071102 A Phase II/III Randomized Trial of Veliparib or Placebo in Combination With Temozolomide in Newly Diagnosed Glioblastoma With MGMT Promoter Hypermethylation
CLINICAL TRIALS ACC Jul 2016 Glioblastoma BRAIN A071102 A Phase II/III Randomized Trial of Veliparib or Placebo in Combination With Temozolomide in Newly Diagnosed Glioblastoma With MGMT Promoter Hypermethylation
CCC Chemotherapy Protocols V9.0
 CCC Chemotherapy Protocols V9.0 General observations 3 Breast cancer 5 Gastrointestinal cancer Oesophagus 17 Gastric 19 Pancreas 22 Cholangiocarcinoma 24 Hepatocellular carcinoma 25 Neuroendocrine tumours
CCC Chemotherapy Protocols V9.0 General observations 3 Breast cancer 5 Gastrointestinal cancer Oesophagus 17 Gastric 19 Pancreas 22 Cholangiocarcinoma 24 Hepatocellular carcinoma 25 Neuroendocrine tumours
MEDICAL ONCOLOGY NEWS IN BREAST CANCER 2014
 MEDICAL ONCOLOGY NEWS IN BREAST CANCER 2014 Dr Thomas Yau Clinical Assistant Professor MBBS(HK), MRCP (UK), FHKCP (Med Onc), FHKAM( Medicine), FRCP(London) Queen Mary Hospital The University of Hong Kong
MEDICAL ONCOLOGY NEWS IN BREAST CANCER 2014 Dr Thomas Yau Clinical Assistant Professor MBBS(HK), MRCP (UK), FHKCP (Med Onc), FHKAM( Medicine), FRCP(London) Queen Mary Hospital The University of Hong Kong
Locally Advanced Breast Cancer: Systemic and Local Therapy
 Locally Advanced Breast Cancer: Systemic and Local Therapy Joseph A. Sparano, MD Professor of Medicine & Women s Health Albert Einstein College of Medicine Associate Chairman, Department of Oncology Montefiore
Locally Advanced Breast Cancer: Systemic and Local Therapy Joseph A. Sparano, MD Professor of Medicine & Women s Health Albert Einstein College of Medicine Associate Chairman, Department of Oncology Montefiore
Positive HER-2 tumor. How to incorporate the new drugs into neoadjuvance
 Oncology Department Vall d Hebron University Hospital Barcelona. Spain Positive HER-2 tumor. How to incorporate the new drugs into neoadjuvance Javier Cortés June/2013 MD Anderson experience Buzdar et
Oncology Department Vall d Hebron University Hospital Barcelona. Spain Positive HER-2 tumor. How to incorporate the new drugs into neoadjuvance Javier Cortés June/2013 MD Anderson experience Buzdar et
Supplementary Online Content
 Supplementary Online Content Spring LM, Gupta A, Reynolds KL, et al. Neoadjuvant endocrine therapy for estrogen receptor positive breast cancer: a systematic review and meta-analysis. JAMA Oncol. Published
Supplementary Online Content Spring LM, Gupta A, Reynolds KL, et al. Neoadjuvant endocrine therapy for estrogen receptor positive breast cancer: a systematic review and meta-analysis. JAMA Oncol. Published
Emetogenicity level 1. Emetogenicity level 2
 Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
Locally Advanced Breast Cancer: Systemic and Local Therapy
 Locally Advanced Breast Cancer: Systemic and Local Therapy Joseph A. Sparano, MD Professor of Medicine & Women s Health Albert Einstein College of Medicine Associate Chairman, Department of Oncology Montefiore
Locally Advanced Breast Cancer: Systemic and Local Therapy Joseph A. Sparano, MD Professor of Medicine & Women s Health Albert Einstein College of Medicine Associate Chairman, Department of Oncology Montefiore
1 The Cancer Programs Regulation (AR 242/98) is amended by this Regulation.
 Alberta Regulation 18/2005 Cancer Programs Act AMENDMENT REGULATION Filed: February 22, 2005 For information only: Made by the Minister of Health and Wellness (M.O. 9/2005) on February 17, 2005 pursuant
Alberta Regulation 18/2005 Cancer Programs Act AMENDMENT REGULATION Filed: February 22, 2005 For information only: Made by the Minister of Health and Wellness (M.O. 9/2005) on February 17, 2005 pursuant
Latest News in Breast Cancer Research
 Latest News in Breast Cancer Research Highlights from the 2011 San Antonio Breast Cancer Symposium www.cancercare.org This special edition of the CancerCare Connect booklet series highlights cutting-edge
Latest News in Breast Cancer Research Highlights from the 2011 San Antonio Breast Cancer Symposium www.cancercare.org This special edition of the CancerCare Connect booklet series highlights cutting-edge
DR LUIS MANSO UNIDAD TUMORES DE MAMA Y GINECOLÓGICOS HOSPITAL 12 DE OCTUBRE MADRID
 DR LUIS MANSO UNIDAD TUMORES DE MAMA Y GINECOLÓGICOS HOSPITAL 12 DE OCTUBRE MADRID RESUMEN DE ARTICULOS THERESA BOLERO 3 NOAH UP-DATE GEPAR SIXTO RADIOTHERAPY EBCTCG CTCs MISCELANEAS Lancet Oncol 2014;
DR LUIS MANSO UNIDAD TUMORES DE MAMA Y GINECOLÓGICOS HOSPITAL 12 DE OCTUBRE MADRID RESUMEN DE ARTICULOS THERESA BOLERO 3 NOAH UP-DATE GEPAR SIXTO RADIOTHERAPY EBCTCG CTCs MISCELANEAS Lancet Oncol 2014;
COME HOME Innovative Oncology Business Solutions, Inc.
 COME HOME Thyroid Cancer pathway development worksheet, v9 April 13, 2015 Required Structured Data: Stage Staging Components Staging Date Histology Quality Measure(s): Staging (clinical or pathologic)
COME HOME Thyroid Cancer pathway development worksheet, v9 April 13, 2015 Required Structured Data: Stage Staging Components Staging Date Histology Quality Measure(s): Staging (clinical or pathologic)
