Chapter 20 Lipids. Organic and Biochem
|
|
|
- Derick Welch
- 5 years ago
- Views:
Transcription
1 Chapter 20 Lipids rganic and Biochem
2 20.1 Introduction Found in living organisms Insoluble in water but Soluble in non-polar substances Example of Lipid Solvent: diethyl ether Polar groups in lipids are Smaller than the non-polar groups Two most Common Roles Energy Storage Cell Membrane bi-layer
3 1 gram of fat = 9 kcal 1 gram of carbohydrate = 4 kcal Also act as Chemical Messengers Four groups o Fats and Waxes o Complex Lipids o Steroids o Arachidonic acid derivatives
4 20.2 Structure of Triglycerides Triesters of _Glycerol and long-chain carboxylic acids called fatty acids Animal fats and vegetable oils
5 The carboxylic acids have the following in common: Almost all Unbranched carbon chain Even number of carbons ther than some double bonds and CH they have no other functional groups
6 Two groups of fatty acids Saturated fatty acids o Have only single bonds o Solids at Room Temp. Unsaturated fatty acids o Have at least 1 double bond o Cis isomers dominate o Liquids at Room Temp.
7 Saturated Refers to the Chains Not Really the Hydrogens VanderWaal forces weakly pack Structure Double bonds interrupt VanderWaal forces: Interrupt packing
8 Fatty acid nomenclature o Common names dominate o Example: o Stearic Acid : (18:0) o leic Acid : (18:1) o Linoleic Acid : (18:2) o Linolenic Acid : (18:3) o Table 20.1 (pg 472) # of Carbon s in Chain # of Double bonds in Chain
9
10 Triglycerides Glycerol typically is esterified by three different carboxylic acids Example: Palmitate (16:0) lcate (18:1) Stearate (18:0)
11 20.3 Properties of Fats Animal fats: SLIDS Plant or fish: LIQUIDS ILS Solid fats Saturated fats Vegetable oils Unsaturated fats Table 20.2 (pg 474)
12
13 Hydrogenation Add H 2 across the double bond of an unsaturated fat Liquid becomes a solid Modern Butter/Lard Substitutes
14 Saponification Glycerides can be reacted with a base to form sodium salts (soap) How to Make Soap Fight Club?
15 Box: 20C Soaps and Detergents Surfactants Hydrophobic Hydrophilic Demo water, oil, soap suspensions Surfactant Video
16 20.4 Complex Lipids Components of cell membranes Two types: o Phospholipids o Glycolipids
17 Phospholipids Contain alcohol with: _1-2 Fatty acids and Phospho complex o Glycerophospholipids: Structural Alcohol is glycerol o Sphingolipids: Sphingosine instead of Glycerol
18 Glycolipids Sphingosine with a carbohydrate and Fatty Acid
19 20.5 Membranes Formed by complex lipids Unsaturated fatty acids are important components Purpose is to separate cells from the external environment and to provide transport for nutrients and waste products
20 Lipid bilayers Two rows of complex lipid molecules arranged tail to tail Hydrophobic tales point toward each other leaving the Hydrophilic heads projecting to the inner and outer surfaces of the membrane IMPRTANT substances must be able to transport or cross the membrane
21
22
23 Cell Membrane/Lipid Animations tutorial
24 20.6 Glycerophospholipids Also called phosphoglycerides Structure is similar to simple fats Primary component of Cell Membrane Alcohol is glycerol 2 H groups esterified by fatty acids 3 rd H esterified by a phosphate group which is esterified to another alcohol group (Choline) Three important examples Phosphatidylcholines (Lecithin) Cephalins phosphatidylethanolamine & phosphatidylserine Phosphatidyl inositols (PI)
25 Phosphatidylcholines Common Name: Lecithin (Page 479) Draw the Molecule and ID the Polar/Non-Polar portions H 2 C C Stearic acid HC C Linolenic acid H 2 C H 2 C C P - Stearic acid CH 2 CH 2 N + Choline - HC C Linolenic acid H 3 C N + CH 2 CH 2 P
26 Cephalins Similar to Lecithin ethanolamine and/or serine substituted for choline Page 480: H 2 C C Stearic acid - HC C Linolenic acid + H 3 N CH 2 CH 2 P - H 2 C C Stearic acid C HC CH 2 - P HC C Linolenic acid NH 3 +
27 Phosphatidyl inositols (PI) Important to biological membranes as signaling molecules Page 480 H 2 C C Stearic acid HC C Linolenic acid H 2 C P R H H H H H H H H H H H PI (1 phosphate group) Can have more phosphate groups attached (PIP 2 )
28 20.7 Sphingolipids Coating of nerve axons ( myelin ) Alcohol is sphingosine NH 2 group connected to fatty acid with an Amide bond H group esterified by phosphorylcholine Ceramide : Fatty acid/sphingosine combination
29 Glycerol Lipids vs. Sphingosine Lipids How many bonding sites are found on Glycerol? Look up Sphingosine on page 480. How many bonding sites are found on Sphingosine? H 3 C CH CH H HC CH 2 H NH 2
30 Draw a Sphingolipid (Sphingomyelin)that has a Fatty acid bonded to Sphingosine s #1 bonding site and a phosphate-choline complex bonded to bonding site #2 (look at page 481 for help) ID the Polar/Non-Polar portions of the molecule Does this molecule look similar to a Glycerophospholipid?
31 Multiple Sclerosis: Nerve axons are protected by myelin sheath MS results in the gradual degradation of the myelin sheath. Nerve axons short circuit Symptoms: Lack of coordination Muscle weariness Loss of vision
32 20.8 Glycolipids Contain a carbohydrate ne important Example: Cerbrosides H H H H H H HN H H H H 1 Fatty acid (only example with a 24 C) Brain Lipid
33 20.9 Steroids 3 rd class of lipids Contain the following ring structure: Label parts (Page 483) There may be double bonds and other components
34 Cholesterol Most abundant steroid in the body Serves as a membrane component in red blood cells and myelinated nerve cells Serves as a raw material for _ther Steroids_
35 Cholesterol Exists in: Free Form: H Esterified with fatty acid Form: R
36 Cholesterol is gained 2 ways Ingested in diet Manufactured in Liver Cholesterol is transported throughout the body via Lipoproteins Read Page 487.
37 HDL: High Density Lipoprotein 50% Protein content - 18% Cholesterol Important for transfer of cholesterol to Liver Good Cholesterol LDL: Low Density Lipoprotein 25% Protein content - 45% Cholesterol Important for cholesterol metabolism (carries cholesterol to cells) Coated Pits LDL Receptor sites Bad Cholesterol if a lack of LDL receptor sites exist.
38 20.10 Steroid Hormones Cholesterol is the raw material for the synthesis of steroid hormones Fig (pg 488) Progesterone is the primary steroid hormone that all others come from 2 major groups of Steroid Hormones Adrenocorticoids Sex Hormones
39 H 3 C C H 3 C H Cholesterol Progesterone H H C H 2 CH C 3 H H H C H 2 CH C H Testosterone Cortisol Aldosterone Adrenocorticoid Hormones
40 Adrenocorticoids Products of adrenal glands Two groups: Mineralocorticoids Regulate the concentration of ions (Na + and K + ) Aldosterone: controls re-absorption of ions Glucocorticoids Regulate glucose and glycogen levels in body Cortisol and Cortisone also act as anti-inflammatories Synthetic corticoid: Prednisolone - used to treat inflammatory diseases (arthritis, asthma, crohn s)
41 H C H 2 C H H C H 2 C H H Cortisol Cortisone
42 Sex Hormones Testosterone Most important male sex hormone Not only produced by males Balance of Testosterone to progesterone/estradiol determines sexual differentiation Synthesized from cholesterol Estradiol Synthesized from Testosterone With progesterone regulates menstrual cycle
43 Conversion of steroids: Testosterone Estradiol Loss of H & Keto-enol tautomerism Draw Testosterone & Estradiol H H H Testosterone Estradiol
44 20.11 Bile Salts Products of the oxidation of cholesterol Produced in the LIVER Stored in the Gall Bladder Powerful detergents Disperse dietary lipids in the small intestine Forms an emulsion with lipids Removes excess cholesterol Form clumps_ Functions: _Emulsify lipids for digestion _neutralize stomach acid
45 Show Figure pg 493 H 3 C H - NH H 3 C H NH H H H H S If too much cholesterol is present with the bile salts in the gall bladder Gallstones can form.
46 Fourth Group: Arachiodonic Acid Lipids Prostaglandins Mediate hormonal actions Cause inflammation and swelling Thomboxanes Causes blood to clot Leukotrienes Responsible for muscle contractions (Non-conscious muscles especially) Mediates hormone action Lungs? Increased levels cause Asthma All come from the precursor Arachiodonic Acid
47 Aspirin inhibits an enzyme in the conversion reactions of Arachiodonic acid. Aspirin lowers levels of the first 2 lipids Anti-inflammatory Thins blood
15.1 Lipids 15.2 Fatty Acids. Copyright 2009 by Pearson Education, Inc.
 Chapter 15 Lipids 15.1 Lipids 15.2 Fatty Acids Copyright 2009 by Pearson Education, Inc. 1 Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but
Chapter 15 Lipids 15.1 Lipids 15.2 Fatty Acids Copyright 2009 by Pearson Education, Inc. 1 Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but
Functions of Lipids. - Storage Fats are long term energy (9 kcal/g) while carbohydrates are quick energy (4 kcal/g).
 Chapter 8: Lipids Functions of Lipids - Storage Fats are long term energy (9 kcal/g) while carbohydrates are quick energy (4 kcal/g). - Membrane Components Lipid barriers keep water out. - Messengers Hormones
Chapter 8: Lipids Functions of Lipids - Storage Fats are long term energy (9 kcal/g) while carbohydrates are quick energy (4 kcal/g). - Membrane Components Lipid barriers keep water out. - Messengers Hormones
Lecture 3 6/28/10. Membrane Lipids. Importance of Membranes. Categories of Lipids. Lipids: Chapter 20 Sections 4-7. ! Membranes are important in
 Lecture 3 Lipids: Chapter 20 Sections 4-7! The most polar lipids are found in the membranes of cells and organelles! Why?! These lipids are amphipathic! Membranes are complex and have many components Membrane
Lecture 3 Lipids: Chapter 20 Sections 4-7! The most polar lipids are found in the membranes of cells and organelles! Why?! These lipids are amphipathic! Membranes are complex and have many components Membrane
Classification, functions and structure
 Classification, functions and structure Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine and Pharmacy "Nicolae Testemitanu" Lipids
Classification, functions and structure Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine and Pharmacy "Nicolae Testemitanu" Lipids
Biological role of lipids
 Lipids Lipids Organic compounds present in living organisms, insoluble in water but able to be extracted by organic solvents such as: chloroform, acetone, benzene. Extraction = the action of taking out
Lipids Lipids Organic compounds present in living organisms, insoluble in water but able to be extracted by organic solvents such as: chloroform, acetone, benzene. Extraction = the action of taking out
Lipids. Lipids: Defined. ClassificaCon by FuncCon 10/19/09. Chapter 21
 Lipids Chapter 21 Lipids: Defined They are a family of substances that are insoluble in water, but soluble in non polar solvents and solvents of low polarity ClassificaCon by FuncCon Energy storage Lipids
Lipids Chapter 21 Lipids: Defined They are a family of substances that are insoluble in water, but soluble in non polar solvents and solvents of low polarity ClassificaCon by FuncCon Energy storage Lipids
Lipids Definition. Definition: Water insoluble No common structure (though generally large R groups)
 Lipids Definition Definition: Water insoluble No common structure (though generally large R groups) Water Solubility (Hydrophilic) What makes molecules water soluble (hydrophilic)? Like dissolves like
Lipids Definition Definition: Water insoluble No common structure (though generally large R groups) Water Solubility (Hydrophilic) What makes molecules water soluble (hydrophilic)? Like dissolves like
Definition: Water insoluble No common structure (though generally large R groups)
 Lipids Definition Definition: Water insoluble No common structure (though generally large R groups) Water Solubility (Hydrophilic) What makes molecules water soluble (hydrophilic)? Like dissolves like
Lipids Definition Definition: Water insoluble No common structure (though generally large R groups) Water Solubility (Hydrophilic) What makes molecules water soluble (hydrophilic)? Like dissolves like
Lipids fatty, oily, or waxy hydrophobic organic compounds.
 Lipids Lipids Lipids fatty, oily, or waxy hydrophobic organic compounds. u long hydrocarbon chain u composed of CHO Diverse group u fats u oils u waxes u steroids Do not form polymers u big molecules made
Lipids Lipids Lipids fatty, oily, or waxy hydrophobic organic compounds. u long hydrocarbon chain u composed of CHO Diverse group u fats u oils u waxes u steroids Do not form polymers u big molecules made
BIOB111_CHBIO - Tutorial activity for Session 12
 BIOB111_CHBIO - Tutorial activity for Session 12 General topic for week 6 Session 12 Lipids Useful Links: 1. Animations on Cholesterol (its synthesis, lifestyle factors, LDL) http://www.wiley.com/college/boyer/0470003790/animations/cholesterol/cholesterol.htm
BIOB111_CHBIO - Tutorial activity for Session 12 General topic for week 6 Session 12 Lipids Useful Links: 1. Animations on Cholesterol (its synthesis, lifestyle factors, LDL) http://www.wiley.com/college/boyer/0470003790/animations/cholesterol/cholesterol.htm
CHAPTER 28 LIPIDS SOLUTIONS TO REVIEW QUESTIONS
 28 09/16/2013 17:44:40 Page 415 APTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents
28 09/16/2013 17:44:40 Page 415 APTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents
CHAPTER 28 LIPIDS SOLUTIONS TO REVIEW QUESTIONS
 HAPTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents and their insolubility
HAPTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents and their insolubility
Seminar 6 Theoretical part
 Seminar 6 Theoretical part Lipids are a heterogeneous group of naturally occurring organic compounds, classified together on the basis of their common solubility properties. Lipids are insoluble in water,
Seminar 6 Theoretical part Lipids are a heterogeneous group of naturally occurring organic compounds, classified together on the basis of their common solubility properties. Lipids are insoluble in water,
Chemistry 1506: Allied Health Chemistry 2. Section 8: Lipids. Biochemical Esters and Hydrocarbons. Outline
 hemistry 1506 Dr. unter s lass Section 8 Notes - Page 1/21 hemistry 1506: Allied ealth hemistry 2 Section 8: Lipids Biochemical Esters and ydrocarbons utline SETIN 8.1 INTRDUTIN...2 SETIN SETIN SETIN 8.2
hemistry 1506 Dr. unter s lass Section 8 Notes - Page 1/21 hemistry 1506: Allied ealth hemistry 2 Section 8: Lipids Biochemical Esters and ydrocarbons utline SETIN 8.1 INTRDUTIN...2 SETIN SETIN SETIN 8.2
Chapter 19 Lecture Outline
 Chapter 19 Lecture utline Prepared by Andrea D. Leonard University of Louisiana at Lafayette Lipids! Introduction to Lipids! Lipids are biomolecules that are soluble in organic solvents and insoluble in
Chapter 19 Lecture utline Prepared by Andrea D. Leonard University of Louisiana at Lafayette Lipids! Introduction to Lipids! Lipids are biomolecules that are soluble in organic solvents and insoluble in
I. Structure and Properties of Lipids
 I. Structure and Properties of Lipids Lipids: A diverse group of compounds characterized by their low solubility in water and a high solubility in organic solvents such as chloroform and methanol. Nonpolar
I. Structure and Properties of Lipids Lipids: A diverse group of compounds characterized by their low solubility in water and a high solubility in organic solvents such as chloroform and methanol. Nonpolar
Chemistry 506: Allied Health Chemistry 2. Chapter 17: Lipids. Biochemical Esters and Hydrocarbons
 hemistry 506 Dr. unter s lass hapter 17. hemistry 506: Allied ealth hemistry 2 1 hapter 17: Lipids Biochemical Esters and ydrocarbons Introduction to General, rganic & Biochemistry, 5 th Edition by Bettelheim
hemistry 506 Dr. unter s lass hapter 17. hemistry 506: Allied ealth hemistry 2 1 hapter 17: Lipids Biochemical Esters and ydrocarbons Introduction to General, rganic & Biochemistry, 5 th Edition by Bettelheim
Lipids. Polar bears have a large reserve of lipids.
 Chapter 28 Lipids Polar bears have a large reserve of lipids. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and Susan Arena Course 28.1 Lipids:
Chapter 28 Lipids Polar bears have a large reserve of lipids. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and Susan Arena Course 28.1 Lipids:
Introduction to the Study of Lipids
 Introduction to the Study of Lipids Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General
Introduction to the Study of Lipids Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General
CH 3. Lipids CHAPTER SUMMARY
 H 3 C H 3 C 15 H 3 C H Views of Cholesterol APTER SUMMARY 15.1 The Nature of can best be defined as biomolecules which are soluble to a great extent in solvents. In contrast to carbohydrates, proteins
H 3 C H 3 C 15 H 3 C H Views of Cholesterol APTER SUMMARY 15.1 The Nature of can best be defined as biomolecules which are soluble to a great extent in solvents. In contrast to carbohydrates, proteins
Lipids and Membranes
 Lipids Lipids are hydrophobic or amphiphilic insoluble in water soluble in organic solvents soluble in lipids Lipids are used as energy storage molecules structural components of membranes protective molecules
Lipids Lipids are hydrophobic or amphiphilic insoluble in water soluble in organic solvents soluble in lipids Lipids are used as energy storage molecules structural components of membranes protective molecules
Chapter 11: Lipids. Voet & Voet: Pages
 Chapter 11: Lipids Voet & Voet: Pages 380-394 Slide 1 Lipids Lipids are distinguished by their high solubility in non polar solvents and low solubility in H2O Diverse group of compounds including Fats,
Chapter 11: Lipids Voet & Voet: Pages 380-394 Slide 1 Lipids Lipids are distinguished by their high solubility in non polar solvents and low solubility in H2O Diverse group of compounds including Fats,
Factors to Consider in the Study of Biomolecules
 Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General structure and functional groups
Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General structure and functional groups
Chemistry B11 Chapters 15 Lipids
 Chapters 15 ipids ipids: are family of biomolecules that have the common property of being soluble in organic solvents but not in water. Role of lipids: they have three important roles in nature: 1. They
Chapters 15 ipids ipids: are family of biomolecules that have the common property of being soluble in organic solvents but not in water. Role of lipids: they have three important roles in nature: 1. They
Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson
 Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson Link download full: http://testbankair.com/download/test-bank-forlehninger-principles-of-biochemistry-5th-edition-by-nelson/ Chapter
Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson Link download full: http://testbankair.com/download/test-bank-forlehninger-principles-of-biochemistry-5th-edition-by-nelson/ Chapter
Lipids and Membranes
 Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Lipids and Membranes I. overview Lipids are related
Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Lipids and Membranes I. overview Lipids are related
Organic molecules highly hydrophobic and water insoluble.
 UNIT 5. LIPIDS OUTLINE 5.1. Introduction. 5.2. Fatty acids. 5.3. Eicosanoids. 5.4. Triacylglycerols = Triglycerides. 5.5. Waxes. 5.6. Membrane lipids: glycerophospholipids and sphingolipids. 5.7. Isoprenoids
UNIT 5. LIPIDS OUTLINE 5.1. Introduction. 5.2. Fatty acids. 5.3. Eicosanoids. 5.4. Triacylglycerols = Triglycerides. 5.5. Waxes. 5.6. Membrane lipids: glycerophospholipids and sphingolipids. 5.7. Isoprenoids
Organism. Organ and organ systems. Tissue. Cells. Carbohydrates Proteins Lipids Nucleic Acids
 hemistry of Life rganism rgan and organ systems Tissue ells arbohydrates Proteins Lipids Nucleic Acids Hydrocarbons, Alcohols, Phenols, Ethers, Thiols, Aldehydes, Ketones, arboxylic Acids, Esters, Amines,
hemistry of Life rganism rgan and organ systems Tissue ells arbohydrates Proteins Lipids Nucleic Acids Hydrocarbons, Alcohols, Phenols, Ethers, Thiols, Aldehydes, Ketones, arboxylic Acids, Esters, Amines,
GUTS Lecture Syllabus for Lipid Structure and Nomenclature
 GUTS Lecture Syllabus for Lipid Structure and Nomenclature For Questions or Assistance contact: Dr. Gwen Sancar, gsancar@ad.unc.edu Learning bjectives After completing the GUTS lecture and associated self-
GUTS Lecture Syllabus for Lipid Structure and Nomenclature For Questions or Assistance contact: Dr. Gwen Sancar, gsancar@ad.unc.edu Learning bjectives After completing the GUTS lecture and associated self-
Topic 3: Molecular Biology
 Topic 3: Molecular Biology 3.2 Carbohydrates and Lipids Essen=al Understanding: Carbon, hydrogen and oxygen are used to supply and store energy. Carbohydrates CARBOHYDRATES CHO sugars Primarily consist
Topic 3: Molecular Biology 3.2 Carbohydrates and Lipids Essen=al Understanding: Carbon, hydrogen and oxygen are used to supply and store energy. Carbohydrates CARBOHYDRATES CHO sugars Primarily consist
Lipids and Classification:
 Lipids and Classification: Lipids: Biological lipids are a chemically diverse group of organic compounds which are insoluble or only poorly soluble in water. They are readily soluble in non-polar solvents
Lipids and Classification: Lipids: Biological lipids are a chemically diverse group of organic compounds which are insoluble or only poorly soluble in water. They are readily soluble in non-polar solvents
By: Dr Hadi Mozafari 1
 Biological lipids are a chemically diverse group of compounds, the common and defining feature of which is their insolubility in water. By: Dr Hadi Mozafari 1 Fats and oils are the principal stored forms
Biological lipids are a chemically diverse group of compounds, the common and defining feature of which is their insolubility in water. By: Dr Hadi Mozafari 1 Fats and oils are the principal stored forms
Carboxylic acids is а compound whose characteristic functional group is the carboxyl group -COOH, example:
 Carboxylic acids LECTURE 3 Carboxylic acids is а compound whose characteristic functional group is the carboxyl group -COOH, example: Lipids: classification, structure and biological role. By/Arshed Abd
Carboxylic acids LECTURE 3 Carboxylic acids is а compound whose characteristic functional group is the carboxyl group -COOH, example: Lipids: classification, structure and biological role. By/Arshed Abd
Dr. Nafith Abu Tarboush
 4 Dr. Nafith Abu Tarboush June 24 th 2013 Ahmad Moayd 1 Definition and general properties refer to slide no. 2 Lipids: macromolecules made from Alcohol and Fatty acid bonded by ester linkage. Amphipathic
4 Dr. Nafith Abu Tarboush June 24 th 2013 Ahmad Moayd 1 Definition and general properties refer to slide no. 2 Lipids: macromolecules made from Alcohol and Fatty acid bonded by ester linkage. Amphipathic
Chemistry 1120 Exam 2 Study Guide
 Chemistry 1120 Exam 2 Study Guide Chapter 6 6.1 Know amines are derivatives of ammonia, which is not an amine. Classify amines as primary, secondary or tertiary. Master Tutor Section 6.1 Review Section
Chemistry 1120 Exam 2 Study Guide Chapter 6 6.1 Know amines are derivatives of ammonia, which is not an amine. Classify amines as primary, secondary or tertiary. Master Tutor Section 6.1 Review Section
Chapter 28: Lipids In a slightly different order than in your textbook Fatty Acids
 common name hapter 28: Lipids In a slightly different order than in your textbook Fatty Acids shorthand notations #of : # of = #of, location of = line structure comments butyric acid 4:0 in butterfat lauric
common name hapter 28: Lipids In a slightly different order than in your textbook Fatty Acids shorthand notations #of : # of = #of, location of = line structure comments butyric acid 4:0 in butterfat lauric
MCQS ON LIPIDS. Dr. RUCHIKA YADU
 MCQS ON LIPIDS Dr. RUCHIKA YADU Q1. THE FATS AND OILS ARE RESPECTIVELY RICH IN a) Unsaturated fatty acids b) Saturated fatty acids c) Saturated and unsaturated fatty acids d) None of these Q2. ESSENTIAL
MCQS ON LIPIDS Dr. RUCHIKA YADU Q1. THE FATS AND OILS ARE RESPECTIVELY RICH IN a) Unsaturated fatty acids b) Saturated fatty acids c) Saturated and unsaturated fatty acids d) None of these Q2. ESSENTIAL
Lipids. Lipids. Jiří Jonák and Lenka Fialová Institute of Medical Biochemistry, 1st Medical Faculty of the Charles University, Prague
 Lipids Jiří Jonák and Lenka Fialová Institute of Medical Biochemistry, 1st Medical Faculty of the Charles University, Prague Lipids 1. General introduction 2. Nomenclature of fatty acids 3. Degradation
Lipids Jiří Jonák and Lenka Fialová Institute of Medical Biochemistry, 1st Medical Faculty of the Charles University, Prague Lipids 1. General introduction 2. Nomenclature of fatty acids 3. Degradation
Nafith Abu Tarboush DDS, MSc, PhD
 Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush Lipids (cholesterol, cholesterol esters, phospholipids & triacylglycerols) combined with proteins (apolipoprotein) in
Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush Lipids (cholesterol, cholesterol esters, phospholipids & triacylglycerols) combined with proteins (apolipoprotein) in
Lipids do not like water! (aka: hydrophobic) Generally insoluble
 Lipids Lipids Lipids do not like water! (aka: hydrophobic) Generally insoluble Lipids They act like this because of their molecular structure (non-polar) Lipids are made mostly of C and H atoms, with O
Lipids Lipids Lipids do not like water! (aka: hydrophobic) Generally insoluble Lipids They act like this because of their molecular structure (non-polar) Lipids are made mostly of C and H atoms, with O
Chapter 8. Functions of Lipids. Structural Nature of Lipids. BCH 4053 Spring 2001 Chapter 8 Lecture Notes. Slide 1. Slide 2.
 BCH 4053 Spring 2001 Chapter 8 Lecture Notes 1 Chapter 8 Lipids 2 Functions of Lipids Energy Storage Thermal Insulation Structural Components of Membranes Protective Coatings of Plants and Insects Hormonal
BCH 4053 Spring 2001 Chapter 8 Lecture Notes 1 Chapter 8 Lipids 2 Functions of Lipids Energy Storage Thermal Insulation Structural Components of Membranes Protective Coatings of Plants and Insects Hormonal
TEST BANK FOR LEHNINGER PRINCIPLES OF BIOCHEMISTRY 6TH EDITION BY NELSON
 Link full download: https://testbankservice.com/download/testbank-for-lehninger-principles-of-biochemistry-6th-edition-bynelson TEST BANK FOR LEHNINGER PRINCIPLES OF BIOCHEMISTRY 6TH EDITION BY NELSON
Link full download: https://testbankservice.com/download/testbank-for-lehninger-principles-of-biochemistry-6th-edition-bynelson TEST BANK FOR LEHNINGER PRINCIPLES OF BIOCHEMISTRY 6TH EDITION BY NELSON
Phospholipids Metabolism
 Chapter VI: Phospholipids Metabolism Dr. Sameh Sarray Hlaoui Phospholipids Features: Amphipatic: - Hydrophobic head: fatty acids - Hydropholic head: P group+ alcohol Composed of alcohol attached by a phosphodiester
Chapter VI: Phospholipids Metabolism Dr. Sameh Sarray Hlaoui Phospholipids Features: Amphipatic: - Hydrophobic head: fatty acids - Hydropholic head: P group+ alcohol Composed of alcohol attached by a phosphodiester
Experiment 12 Lipids. Structures of Common Fatty Acids Name Number of carbons
 Experiment 12 Lipids Lipids are a class of biological molecules that are insoluble in water and soluble in nonpolar solvents. There are many different categories of lipids and each category has different
Experiment 12 Lipids Lipids are a class of biological molecules that are insoluble in water and soluble in nonpolar solvents. There are many different categories of lipids and each category has different
2013 W. H. Freeman and Company. 10 Lipids
 2013 W. H. Freeman and Company 10 Lipids CHAPTER 10 Lipids Key topics: Biological roles of lipids Structure and properties of storage lipids Structure and properties of membrane lipids Structure and properties
2013 W. H. Freeman and Company 10 Lipids CHAPTER 10 Lipids Key topics: Biological roles of lipids Structure and properties of storage lipids Structure and properties of membrane lipids Structure and properties
Chemistry 1050 Exam 3 Study Guide
 Chapter 12 Chemistry 1050 Exam 3 Study Guide 12.1 a) Identify alkenes, alkynes and aromatics as unsaturated hydrocarbons. Determine the number of hydrogen atoms needed to complete an alkene structure.
Chapter 12 Chemistry 1050 Exam 3 Study Guide 12.1 a) Identify alkenes, alkynes and aromatics as unsaturated hydrocarbons. Determine the number of hydrogen atoms needed to complete an alkene structure.
CHY2026: General Biochemistry. Unit 3: Lipid Chemistry
 CHY2026: General Biochemistry Unit 3: Lipid Chemistry Lipids The are heterogeneous and are related to fatty acids They include fats, oils and waxes Fatty acids have the general formula R-COOH where R =
CHY2026: General Biochemistry Unit 3: Lipid Chemistry Lipids The are heterogeneous and are related to fatty acids They include fats, oils and waxes Fatty acids have the general formula R-COOH where R =
Lipids are used to store and excess energy from extra carbohydrates in animals
 Lipids Lipids are a major source of energy used by cells, however lipids are more difficult for your body to break down. They produce nearly twice the amount of energy than proteins or carbohydrates. Lipids
Lipids Lipids are a major source of energy used by cells, however lipids are more difficult for your body to break down. They produce nearly twice the amount of energy than proteins or carbohydrates. Lipids
Chapter 26 Biochemistry 5th edition. phospholipids. Sphingolipids. Cholesterol. db=books&itool=toolbar
 http://www.ncbi.nlm.nih.gov/sites/entrez? db=books&itool=toolbar 1 The surface of a soap bubble is a bilayer formed by detergent molecules 2 Chapter 26 Biochemistry 5th edition phospholipids Sphingolipids
http://www.ncbi.nlm.nih.gov/sites/entrez? db=books&itool=toolbar 1 The surface of a soap bubble is a bilayer formed by detergent molecules 2 Chapter 26 Biochemistry 5th edition phospholipids Sphingolipids
Chemistry 107 Exam 3 Study Guide
 Chapter 7 Chemistry 107 Exam 3 Study Guide 7.1 Recognize the aldehyde, ketone and hydroxyl (-OH) functional groups found in carbohydrates. Differentiate between mono-, di-, and polysaccharides. Master
Chapter 7 Chemistry 107 Exam 3 Study Guide 7.1 Recognize the aldehyde, ketone and hydroxyl (-OH) functional groups found in carbohydrates. Differentiate between mono-, di-, and polysaccharides. Master
General Biochemistry-1 BCH 202
 General Biochemistry-1 BCH 202 1 I would like to acknowledge Dr. Farid Ataya for his valuable input & help in this course. 2 Outline Lipids Definition, function, fatty acids, classification: simple lipids:
General Biochemistry-1 BCH 202 1 I would like to acknowledge Dr. Farid Ataya for his valuable input & help in this course. 2 Outline Lipids Definition, function, fatty acids, classification: simple lipids:
3.1.3 Lipids. Source: AQA Spec
 alevelbiology.co.uk SPECIFICATION Triglycerides and phospholipids are two groups of lipid. Triglycerides are formed by the condensation of one molecule of glycerol and three molecules of fatty acid. A
alevelbiology.co.uk SPECIFICATION Triglycerides and phospholipids are two groups of lipid. Triglycerides are formed by the condensation of one molecule of glycerol and three molecules of fatty acid. A
Chapter 2 The Chemistry of Life Part 2
 Chapter 2 The Chemistry of Life Part 2 Carbohydrates are Polymers of Monosaccharides Three different ways to represent a monosaccharide Carbohydrates Carbohydrates are sugars and starches and provide
Chapter 2 The Chemistry of Life Part 2 Carbohydrates are Polymers of Monosaccharides Three different ways to represent a monosaccharide Carbohydrates Carbohydrates are sugars and starches and provide
Lesson 2. Biological Molecules. Introduction to Life Processes - SCI 102 1
 Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
BIOLOGY 111. CHAPTER 2: The Chemistry of Life Biological Molecules
 BIOLOGY 111 CHAPTER 2: The Chemistry of Life Biological Molecules The Chemistry of Life : Learning Outcomes 2.4) Describe the significance of carbon in forming the basis of the four classes of biological
BIOLOGY 111 CHAPTER 2: The Chemistry of Life Biological Molecules The Chemistry of Life : Learning Outcomes 2.4) Describe the significance of carbon in forming the basis of the four classes of biological
2.3 Carbon-Based Molecules CARBON BASED MOLECULES
 CARBON BASED MOLECULES KEY CONCEPTS Carbon-based molecules are the foundation of life. Lipids are one class of organic molecules. This group includes fats, oils, waxes, and steroids. Lipids are made of
CARBON BASED MOLECULES KEY CONCEPTS Carbon-based molecules are the foundation of life. Lipids are one class of organic molecules. This group includes fats, oils, waxes, and steroids. Lipids are made of
Chapter Sections: 3.1 Carbon s Place in the Living World 3.2 Functional Groups 3.3 Carbohydrates 3.4 Lipids 3.5 Proteins 3.
 Chapter Sections: 3.1 Carbon s Place in the Living World 3.2 Functional Groups 3.3 Carbohydrates 3.4 Lipids 3.5 Proteins 3.6 Nucleic Acids Student Goals: By the end of this lecture series, students should
Chapter Sections: 3.1 Carbon s Place in the Living World 3.2 Functional Groups 3.3 Carbohydrates 3.4 Lipids 3.5 Proteins 3.6 Nucleic Acids Student Goals: By the end of this lecture series, students should
Lipids. OpenStax College
 OpenStax-CNX module: m44401 1 Lipids OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you will be able
OpenStax-CNX module: m44401 1 Lipids OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you will be able
The main biological functions of the many varied types of lipids include: energy storage protection insulation regulation of physiological processes
 Big Idea In the biological sciences, a dehydration synthesis (condensation reaction) is typically defined as a chemical reaction that involves the loss of water from the reacting molecules. This reaction
Big Idea In the biological sciences, a dehydration synthesis (condensation reaction) is typically defined as a chemical reaction that involves the loss of water from the reacting molecules. This reaction
Organic Molecules. 8/27/2004 Mr. Davenport 1
 Organic Molecules 8/27/2004 Mr. Davenport 1 Carbohydrates Commonly called sugars and starches Consist of C, H, O with H:O ration 2:1 Usually classified as to sugar units Monosaccharide are single sugar
Organic Molecules 8/27/2004 Mr. Davenport 1 Carbohydrates Commonly called sugars and starches Consist of C, H, O with H:O ration 2:1 Usually classified as to sugar units Monosaccharide are single sugar
Lipid Chemistry. Presented By. Ayman Elsamanoudy Salwa Abo El-khair
 Lipid Chemistry Presented By Ayman Elsamanoudy Salwa Abo El-khair 4 Objectives: 1. By the end of this chapter the student should be able to: define lipids. describe the biological importance of lipids.
Lipid Chemistry Presented By Ayman Elsamanoudy Salwa Abo El-khair 4 Objectives: 1. By the end of this chapter the student should be able to: define lipids. describe the biological importance of lipids.
Ahmad O. Olimat. Abdallah Al-Qawasmeh. Dr.Mamoun
 10 Ahmad O. Olimat Abdallah Al-Qawasmeh Mohammed Yousef Dr.Mamoun A QUICK RECAP Eicosanoids They are derived from Arachidonic acid, a fatty acid that contains 20 carbon atoms and four double bonds. They
10 Ahmad O. Olimat Abdallah Al-Qawasmeh Mohammed Yousef Dr.Mamoun A QUICK RECAP Eicosanoids They are derived from Arachidonic acid, a fatty acid that contains 20 carbon atoms and four double bonds. They
FAT. Dr. Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology
 FAT Dr. Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology OBJECTIVES LECTURE By the end of this lecture, student can: Define what is lipid/fat
FAT Dr. Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology OBJECTIVES LECTURE By the end of this lecture, student can: Define what is lipid/fat
Chemistry Chapter 21
 Chemistry 2100 Chapter 21 Lipids Fa3y Acids CH oleic acid (mp 4 C) CH stearic acid (mp 70 C) Triacylglycerols Fatty Acids! The fatty acid components of triglycerides have certain things in common: 1.
Chemistry 2100 Chapter 21 Lipids Fa3y Acids CH oleic acid (mp 4 C) CH stearic acid (mp 70 C) Triacylglycerols Fatty Acids! The fatty acid components of triglycerides have certain things in common: 1.
Biomolecules. Unit 3
 Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Sphingolipids. Sphingolipids are an additional type of membrane lipids, after glycerophospholipids, galactolipids and sulfolipids
 Lipids 2 Steven E. Massey, Ph.D. Assistant Professor of Bioinformatics Department of Biology and Environmental Sciences University of Puerto Rico Río Piedras Office & Lab: Bioinformatics Lab NCN#343B Tel:
Lipids 2 Steven E. Massey, Ph.D. Assistant Professor of Bioinformatics Department of Biology and Environmental Sciences University of Puerto Rico Río Piedras Office & Lab: Bioinformatics Lab NCN#343B Tel:
Classification of Lipids
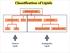 Classification of Lipids Neutral Lipids Amphipathic Lipids Amphipathic Lipids Most cell-membrane lipids are one of two main classes of amphipathic hydrolyzable lipids. Glycerophospholipids (phosphoglycerides):
Classification of Lipids Neutral Lipids Amphipathic Lipids Amphipathic Lipids Most cell-membrane lipids are one of two main classes of amphipathic hydrolyzable lipids. Glycerophospholipids (phosphoglycerides):
LIPIDS II: TRIACYLGLYCEROLS:
 LIPIDS II: TRIACYLGLYCEROLS: How are they broken down? o Hydrolyzed into 3 fatty acids and 1 glycerol o Physiologically in body: Enzyme called a LIPASE present in adipocytes and intestines o Saponification
LIPIDS II: TRIACYLGLYCEROLS: How are they broken down? o Hydrolyzed into 3 fatty acids and 1 glycerol o Physiologically in body: Enzyme called a LIPASE present in adipocytes and intestines o Saponification
Chem 431A-L24-F 07 admin: Last time: We finished Chapt 7, started Chapt 10 FA s and TG s FA=fatty acid, TG=triglycerides or triacylglycerols
 Chem 431A-L24-F'07 page 1 of 5 Chem 431A-L24-F 07 admin: Last time: We finished Chapt 7, started Chapt 10 FA s and TG s FA=fatty acid, TG=triglycerides or triacylglycerols (0) REVIEW: FA s are very reduced
Chem 431A-L24-F'07 page 1 of 5 Chem 431A-L24-F 07 admin: Last time: We finished Chapt 7, started Chapt 10 FA s and TG s FA=fatty acid, TG=triglycerides or triacylglycerols (0) REVIEW: FA s are very reduced
LIPIDS OF PHYSIOLOGICAL SIGNIFICANCE
 LIPIDS OF PHYSIOLOGICAL SIGNIFICANCE Original slides. Important. 436 Notes 438 notes Extra information رابط التعديل: https://docs.google.com/document/d/1wvdec1atp7j- ZKWOUSukSLsEcosjZ0AqV4z2VcH2TA0/edit?usp=sharing
LIPIDS OF PHYSIOLOGICAL SIGNIFICANCE Original slides. Important. 436 Notes 438 notes Extra information رابط التعديل: https://docs.google.com/document/d/1wvdec1atp7j- ZKWOUSukSLsEcosjZ0AqV4z2VcH2TA0/edit?usp=sharing
Fats, Cholesterol, and Hormones
 Fats, Cholesterol, and Hormones 1 Types of Fats Lipids biological origin sparingly soluble in water Main classes of lipids Fatty Acids long hydrocarbon chains with a carboxylic acid on one end Triacylglycerols
Fats, Cholesterol, and Hormones 1 Types of Fats Lipids biological origin sparingly soluble in water Main classes of lipids Fatty Acids long hydrocarbon chains with a carboxylic acid on one end Triacylglycerols
Essential Components of Food
 Essential Components of Food The elements of life living things are mostly (98%) made of 6 elements: C carbon H hydrogen O oxygen P phosphorus N nitrogen S sulphur -each element makes a specific number
Essential Components of Food The elements of life living things are mostly (98%) made of 6 elements: C carbon H hydrogen O oxygen P phosphorus N nitrogen S sulphur -each element makes a specific number
Dr. Nafith Abu Tarboush
 5 Dr. Nafith Abu Tarboush June 25 th 2013 Mohammad Abu Dosh Sheet 5.. Lipids ( Dr. Nafith ) : Classification of fatty acids : - they are classified depending on the existence of double bonds to : 1) Saturated
5 Dr. Nafith Abu Tarboush June 25 th 2013 Mohammad Abu Dosh Sheet 5.. Lipids ( Dr. Nafith ) : Classification of fatty acids : - they are classified depending on the existence of double bonds to : 1) Saturated
BCH 3000 PRINCIPLES OF BIOCHEMISTRY
 BCH 3000 PRINCIPLES OF BIOCHEMISTRY (Semester 2-2012/13) 1 LIPID Learning outcome (Objectives) Function and distribution. Characteristics of fatty acids-structure and chemical properties. Saturated and
BCH 3000 PRINCIPLES OF BIOCHEMISTRY (Semester 2-2012/13) 1 LIPID Learning outcome (Objectives) Function and distribution. Characteristics of fatty acids-structure and chemical properties. Saturated and
26.1 Acetyl Coenzyme A
 Chapter 26 Lipids Lipids Lipids are naturally occurring substances grouped together on the basis of a common property they they are more soluble in nonpolar solvents than in water. Some of the most important
Chapter 26 Lipids Lipids Lipids are naturally occurring substances grouped together on the basis of a common property they they are more soluble in nonpolar solvents than in water. Some of the most important
PHOSPHOLIPIDS METABOLISM. BY Dr. Walid Said Zaki Dr. Marwa Ali LECTURER OF BIOCHEMISTRY AND MOLECULAR BIOLOGY
 PHOSPHOLIPIDS METABOLISM BY Dr. Walid Said Zaki Dr. Marwa Ali LECTURER OF BIOCHEMISTRY AND MOLECULAR BIOLOGY 1. State the definition and classification of Phospholipids. 2. Describe the general structure
PHOSPHOLIPIDS METABOLISM BY Dr. Walid Said Zaki Dr. Marwa Ali LECTURER OF BIOCHEMISTRY AND MOLECULAR BIOLOGY 1. State the definition and classification of Phospholipids. 2. Describe the general structure
Lipids Types, Food Sources, Functions
 Lipids Types, Food Sources, Functions What Are Lipids? Lipids Diverse group of molecules that are insoluble in water Fats The lipid content of diets and foods 1 Lipids in Body Cells and Tissues Types of
Lipids Types, Food Sources, Functions What Are Lipids? Lipids Diverse group of molecules that are insoluble in water Fats The lipid content of diets and foods 1 Lipids in Body Cells and Tissues Types of
Chem 5 PAL Worksheet Lipids Smith text Chapter 15
 Chem 5 PAL Worksheet Lipids Smith text Chapter 15 Principle: Fatty acids are carboxylic acids with long (usually > 14) carbon chains which can be saturated (no carbon-carbon double bonds) are unsaturated
Chem 5 PAL Worksheet Lipids Smith text Chapter 15 Principle: Fatty acids are carboxylic acids with long (usually > 14) carbon chains which can be saturated (no carbon-carbon double bonds) are unsaturated
Nebal Al - Gallab. Shatha Al - Jabri. Mamoon Ahram
 10 Nebal Al - Gallab Shatha Al - Jabri Mamoon Ahram Note: the doctor showed extra examples, they were in the slides, you can refer to them... Naming of Fatty Acids - 1 st Method ( IUPAC system ) We start
10 Nebal Al - Gallab Shatha Al - Jabri Mamoon Ahram Note: the doctor showed extra examples, they were in the slides, you can refer to them... Naming of Fatty Acids - 1 st Method ( IUPAC system ) We start
Dietary fat supplies essential body tissue needs, both as an energy fuel and a structural material.
 Chapter 3 Fats Chapter 3 Lesson 3.1 Key Concepts Dietary fat supplies essential body tissue needs, both as an energy fuel and a structural material. Foods from animal and plant sources supply distinct
Chapter 3 Fats Chapter 3 Lesson 3.1 Key Concepts Dietary fat supplies essential body tissue needs, both as an energy fuel and a structural material. Foods from animal and plant sources supply distinct
Lipids. Lipids: a Diverse group of chemicals. Storage Lipids: derivatives of fatty acids. 11/21/10
 1 Lipids Lehninger 3 rd ed. Chapter 11 (For biosynthesis see Chapter 21) 2 Lipids: a Diverse group of chemicals Insolubility in water. Fats and oils: energy stores. Phospholipids and sterols: structural
1 Lipids Lehninger 3 rd ed. Chapter 11 (For biosynthesis see Chapter 21) 2 Lipids: a Diverse group of chemicals Insolubility in water. Fats and oils: energy stores. Phospholipids and sterols: structural
Biomolecules Lecture Carbohydrates Lipids Proteins Nucleic Acids
 www.aiouinfo.com Biomolecules Lecture Carbohydrates Lipids Proteins Nucleic Acids Carbohydrates Monosaccharides 4 Examples: 1. Glucose 2. Fructose 3. Ribose 4. Deoxyribose Glucose Structure Function of
www.aiouinfo.com Biomolecules Lecture Carbohydrates Lipids Proteins Nucleic Acids Carbohydrates Monosaccharides 4 Examples: 1. Glucose 2. Fructose 3. Ribose 4. Deoxyribose Glucose Structure Function of
Lipids. Types of lipids. Fatty acid structure. Lipid functions. Fatty acid structure
 Lipids Types of lipids lassification of Lipids Fatty Acids Glycerides Nonglycerol Lipids omplex Lipids Structure of Biological Membranes Fatty Acids Saturated Unsaturated omplex Lipids Lipoproteins Glycolipids
Lipids Types of lipids lassification of Lipids Fatty Acids Glycerides Nonglycerol Lipids omplex Lipids Structure of Biological Membranes Fatty Acids Saturated Unsaturated omplex Lipids Lipoproteins Glycolipids
Dr. Nafith Abu Tarboush
 6 Dr. Nafith Abu Tarboush June 26 th 2013 Noor Salem 1 Not corrected Review Lipids are composed of two main connected parts : o Alcohol Sphingosine Glycerol o Fatty Acids (3 in number) Saturated Long Chain
6 Dr. Nafith Abu Tarboush June 26 th 2013 Noor Salem 1 Not corrected Review Lipids are composed of two main connected parts : o Alcohol Sphingosine Glycerol o Fatty Acids (3 in number) Saturated Long Chain
CHEM 3331 Fundamentals of Biochemistry. Organic and Biochemistry for Today Spencer L. Seager / Michael R. Slabaugh. Lipids
 hapter 8 Lipids HEM 3331 Fundamentals of Biochemistry hapter 8 Lipids rganic and Biochemistry for Today Spencer L. Seager / Michael R. Slabaugh Mr. Kevin A. Boudreaux Angelo State University www.angelo.edu/faculty/kboudrea
hapter 8 Lipids HEM 3331 Fundamentals of Biochemistry hapter 8 Lipids rganic and Biochemistry for Today Spencer L. Seager / Michael R. Slabaugh Mr. Kevin A. Boudreaux Angelo State University www.angelo.edu/faculty/kboudrea
Biomolecules: lipids
 Biomolecules: lipids Organic biomolecules: lipids Organic amphiphilic compounds insoluble in water Easily extracted from animal and vegetal cells using apolar solvents Fundamental to build cell's shape
Biomolecules: lipids Organic biomolecules: lipids Organic amphiphilic compounds insoluble in water Easily extracted from animal and vegetal cells using apolar solvents Fundamental to build cell's shape
CELL MEMBRANES (MAS)
 CELL MEMBRANES (MAS) 1 CELL MEMBRANE area of the cell immediately surrounding the cytoplasm the most conserved structure in living cells. Every living thing on this planet has some type of membrane 2 Anatomy
CELL MEMBRANES (MAS) 1 CELL MEMBRANE area of the cell immediately surrounding the cytoplasm the most conserved structure in living cells. Every living thing on this planet has some type of membrane 2 Anatomy
History. Aron first proposed that fat may be essential for normal growth Tested on animals-vitamins A,D,E added. Fat deficiency severely affected
 Chapter 5 LIPIDS History 1918 Aron first proposed that fat may be essential for normal growth Tested on animals-vitamins A,D,E added Fat deficiency severely affected Bone growth Reproduction Called Vitamin
Chapter 5 LIPIDS History 1918 Aron first proposed that fat may be essential for normal growth Tested on animals-vitamins A,D,E added Fat deficiency severely affected Bone growth Reproduction Called Vitamin
Lipids, Biological Membranes and Cellular Transport. 阮雪芬 May/9/2004
 Lipids, Biological Membranes and Cellular Transport 阮雪芬 May/9/2004 Outline Introduction Fatty Acids Triacylglycerols Polar lipids Steroids and other lipids Biological membranes Membrane transport Examples
Lipids, Biological Membranes and Cellular Transport 阮雪芬 May/9/2004 Outline Introduction Fatty Acids Triacylglycerols Polar lipids Steroids and other lipids Biological membranes Membrane transport Examples
Biology. Chapter 3. Molecules of Life. Concepts and Applications 9e Starr Evers Starr
 Biology Concepts and Applications 9e Starr Evers Starr Chapter 3 Molecules of Life 2015 3.1 What Are the Molecules of Life? The molecules of life contain a high proportion of carbon atoms: Complex carbohydrates
Biology Concepts and Applications 9e Starr Evers Starr Chapter 3 Molecules of Life 2015 3.1 What Are the Molecules of Life? The molecules of life contain a high proportion of carbon atoms: Complex carbohydrates
Some Interesting Nutritional Biochemistry of Sugars
 Some Interesting Nutritional Biochemistry of Sugars 1 The Fructose Paradox: Sweet Poison Very sweet sugar Cheap to produce (high fructose corn syrup) Low Glycemic Index.but, it s a nutritional nightmare!
Some Interesting Nutritional Biochemistry of Sugars 1 The Fructose Paradox: Sweet Poison Very sweet sugar Cheap to produce (high fructose corn syrup) Low Glycemic Index.but, it s a nutritional nightmare!
OBJECTIVE. Lipids are largely hydrocarbon derivatives and thus represent
 Paper 4. Biomolecules and their interactions Module 20: Saturated and unsaturated fatty acids, Nomenclature of fatty acids and Essential and non-essential fatty acids OBJECTIVE The main aim of this module
Paper 4. Biomolecules and their interactions Module 20: Saturated and unsaturated fatty acids, Nomenclature of fatty acids and Essential and non-essential fatty acids OBJECTIVE The main aim of this module
Chapter 3- Organic Molecules
 Chapter 3- Organic Molecules CHNOPS Six of the most abundant elements of life (make up 95% of the weight of all living things)! What are they used for? Structures, enzymes, energy, hormones, DNA How do
Chapter 3- Organic Molecules CHNOPS Six of the most abundant elements of life (make up 95% of the weight of all living things)! What are they used for? Structures, enzymes, energy, hormones, DNA How do
Lipids: Fats, Oils & Waxes: AP Biology
 Lipids: Fats, Oils & Waxes: Lipids long term energy storage concentrated energy *9 Cal/gram Lipids: Triglycerides Lipids are composed of C, H, O u long hydrocarbon chains (H-C) Family groups u fats u phospholipids
Lipids: Fats, Oils & Waxes: Lipids long term energy storage concentrated energy *9 Cal/gram Lipids: Triglycerides Lipids are composed of C, H, O u long hydrocarbon chains (H-C) Family groups u fats u phospholipids
How to maximize fat energy? Swine. Poultry. Shrimp. Technical brochure about the molecular structure and mode of action of lysolecithins
 Introduction «Lecithin and lysolecithin «Normal fat digestion «Mode of action lysolecithins «Conclusions «Swine Poultry Fish How to maximize fat energy? Shrimp Technical brochure about the molecular structure
Introduction «Lecithin and lysolecithin «Normal fat digestion «Mode of action lysolecithins «Conclusions «Swine Poultry Fish How to maximize fat energy? Shrimp Technical brochure about the molecular structure
Lipids and Biological Membranes
 Lipids and Biological Membranes Lipids: Found in all living organisms Especially important as components of biological membranes Defined functionally, not structurally, as compounds that are totally or
Lipids and Biological Membranes Lipids: Found in all living organisms Especially important as components of biological membranes Defined functionally, not structurally, as compounds that are totally or
What is the intermolecular force present in these molecules? A) London B) dipole-dipole C) hydrogen bonding D) ion-dipole E) None. D.
 REVIEW SHEET CHP 7, FRST AND DEAL 1. (7.1) Types of Attractive Forces (Intermolecular forces (IMF)). IMF s are attractive forces between molecules due to electrostatic attraction. Therefore a molecule
REVIEW SHEET CHP 7, FRST AND DEAL 1. (7.1) Types of Attractive Forces (Intermolecular forces (IMF)). IMF s are attractive forces between molecules due to electrostatic attraction. Therefore a molecule
