MicroRNA-101 Regulates Multiple Developmental Programs to Constrain Excitation in Adult Neural Networks
|
|
|
- Theodora Cunningham
- 5 years ago
- Views:
Transcription
1 Article MicroRNA-101 Regulates Multiple Developmental Programs to Constrain Excitation in Adult Neural Networks Highlights d MiR-101 orchestrates early network development to limit excitation in the adult d d d Transient mir-101 blockade leads to hyper-excitability and memory deficits MiR-101 targets NKCC1 to facilitate the GABA switch and constrains dendrites Ank2 and Kif1a are targeted by mir-101 to limit formation of excitatory synapses Authors Giordano Lippi, Catarina C. Fernandes, Laura A. Ewell,..., Giuseppe Biagini, Gene W. Yeo, Darwin K. Berg Correspondence glippi@ucsd.edu (G.L.), dberg@ucsd.edu (D.K.B.) In Brief Lippi et al. discovered that microrna-101 regulates multiple genetic programs in parallel to orchestrate a major transition in early development, ensuring that adult neural circuits display appropriate excitability. MicroRNA-101 blockade induces hyper-excitability and memory deficits characteristic of numerous neurodevelopmental disorders. Lippi et al., 2016, Neuron 92, December 21, 2016 ª 2016 Elsevier Inc.
2 Neuron Article MicroRNA-101 Regulates Multiple Developmental Programs to Constrain Excitation in Adult Neural Networks Giordano Lippi, 1,5, * Catarina C. Fernandes, 1 Laura A. Ewell, 1 Danielle John, 1 Benedetto Romoli, 2 Giulia Curia, 2 Seth R. Taylor, 1 E. Paxon Frady, 1 Anne B. Jensen, 1 Jerry C. Liu, 1 Melanie M. Chaabane, 1 Cherine Belal, 1 Jason L. Nathanson, 3 Michele Zoli, 2 Jill K. Leutgeb, 1,4 Giuseppe Biagini, 2 Gene W. Yeo, 3 and Darwin K. Berg 1, * 1 Division of Biological Sciences, University of California, San Diego, La Jolla, CA 92093, USA 2 Department of Biomedical, Metabolic, and Neural Sciences, Center for Neuroscience and Neurotechnology, University of Modena and Reggio Emilia, Modena 41125, Italy 3 Institute for Genomic Medicine 4 Center for Neural Circuits and Behavior University of California, San Diego, La Jolla, CA 92093, USA 5 Lead Contact *Correspondence: glippi@ucsd.edu (G.L.), dberg@ucsd.edu (D.K.B.) SUMMARY A critical feature of neural networks is that they balance excitation and inhibition to prevent pathological dysfunction. How this is achieved is largely unknown, although deficits in the balance contribute to many neurological disorders. We show here that a microrna (mir-101) is a key orchestrator of this essential feature, shaping the developing network to constrain excitation in the adult. Transient early blockade of mir-101 induces long-lasting hyperexcitability and persistent memory deficits. Using target site blockers in vivo, we identify multiple developmental programs regulated in parallel by mir-101 to achieve balanced networks. Repression of one target, NKCC1, initiates the switch in g-aminobutyric acid (GABA) signaling, limits early spontaneous activity, and constrains dendritic growth. Kif1a and Ank2 are targeted to prevent excessive synapse formation. Simultaneous de-repression of these three targets completely phenocopies major dysfunctions produced by mir-101 blockade. Our results provide new mechanistic insight into brain development and suggest novel candidates for therapeutic intervention. INTRODUCTION Balanced excitation is a critical feature of properly functioning neural circuits. Aberrant activity is characteristic of numerous neurological disorders, including autism spectrum disorder (ASD), Rett syndrome, schizophrenia, and epilepsy (Belforte et al., 2010; Chao et al., 2010; Dzhala et al., 2005; Rubenstein and Merzenich, 2003; Yizhar et al., 2011; Zoghbi and Bear, 2012). In rodents, formative events in the first few weeks of post-natal life specify the ratio of excitatory and inhibitory input (E/I) and determine the excitability of neural circuits for the adult (Ben-Ari, 2002; Blankenship and Feller, 2010). During this time, the developing nervous system transitions from an initial period of rapid growth and exuberant synapse formation to a period during which circuits undergo refinement and consolidation (Blankenship and Feller, 2010; Kirkby et al., 2013). Disruptions in the timing or sequence of events comprising the growth phase and transition to consolidation can produce aberrant levels of excitation in the adult. How this complex process of network construction is guided to ensure proper excitation is largely unknown. MicroRNAs (mirnas) are strong candidates for coordinating complex developmental processes (Bian and Sun, 2011; Yoo et al., 2009). They are short non-coding RNAs that act as posttranscriptional regulators of gene expression (Bartel, 2004; Guo et al., 2010) by binding mrnas containing a mirna recognition element (MRE). A single mirna can target hundreds of different mrnas, orchestrating epigenetic regulation of large combinations of gene products and facilitating developmental switches (Makeyev et al., 2007; McNeill and Van Vactor, 2012). Little is known, however, about possible mirna involvement in post-natal brain development and long-term effects. Here we show that mir-101 regulates multiple post-natal developmental programs in parallel to constrain excitatory activity in the adult. Using target site blockers in vivo, we identify the molecular mechanisms used by mir-101 to produce balanced networks. It represses NKCC1 to initiate maturation of GABAergic signaling, to limit spontaneous synchronized activity, and to prevent excessive dendritic growth. A second developmental program involves repression of Kif1a and Ank2 to constrain excessive assembly of pre-synaptic components and reduce the density of glutamatergic synapses. Remarkably, simultaneous protection of all three targets recapitulates the core of the phenotype induced by mir-101 blockade. Neuron 92, , December 21, 2016 ª 2016 Elsevier Inc. 1337
3 RESULTS MiR-101 Is a Potential Master Regulator of Network Formation To identify mirnas orchestrating the complex series of events occurring during network formation, we performed small RNA sequencing of the mouse hippocampus on postnatal day 12 (P12). The hippocampus was chosen because of its known function and well defined circuitry; P12 was chosen because it is within a critical developmental window (Liu et al., 2006). The mirnas obtained were then ranked according to the following criteria. First, abundance, expressed as percentage of total counts. Highly abundant mirnas are more likely to have prominent roles. Second, upregulation during post-natal development. mirna levels rise when their function becomes necessary. Accordingly, we used qpcr to quantify the levels of top mirna candidates in the hippocampus from embryonic day 16 (E16) to adult. Third, enrichment of mirnas and mrnas in Argonaute (Ago) complexes (the effector of mirna function). We explored existing databases of Ago-miRNA-mRNA interactions that list the abundance of mirnas and their predicted mrna targets (Boudreau et al., 2014; Chi et al., 2009). mirnas were prioritized when they were enriched in Ago complexes and had predicted target transcripts (based on MRE) with known roles in neuronal development, and those targets were also abundant in the complexes. Of those, mir-101a and b stood out as the most promising. MiR-101a and b are highly expressed on P12 (Figure S1A, available online), increase 2- and 3-fold, respectively, during the relevant developmental window of E16 P12 (Figures S1B and S1C), represent some of the most abundant mirnas in Ago-miRNA-mRNA complexes in the cortex on P13, and have validated targets that are crucial for neuronal differentiation (Figure S1D). Notably, mir-101 is expressed not only in pyramidal neurons but also in interneurons (Figure S2A) and in non-neuronal cell types (Figures S2B and S2C). To assess the role of mir-101a and b in mediating early events in brain development, we performed a localized transient inhibition by injecting a fluorescein-tagged locked nucleic acid (LNA) antagonist for mir-101b (a-101.f) bilaterally into the dorsal hippocampus of P2 pups (Figure 1A). Sensor assays and qpcr experiments showed that the antagonist efficiently and selectively inhibited both mir-101a and b for up to 9 days compared with a control LNA (a-ctrl) and subsequently subsided (Figures S2D S2F and S3). Additional controls were performed to validate the antagonist and exclude that base composition or LNA length were responsible for the phenotype observed (Figures S2G S2I). The timing and transience of the inhibition were significant. It meant that any network defects persisting into adulthood must result from having interrupted early actions of mir-101 that normally have long-lasting effects. Although mir-101 is also highly expressed in the adult (Figures S1B and S1C), acute actions during adulthood would not have been compromised by application of a-101.f on P2. Similarly, injection on P2 would not perturb mir-101 actions during embryogenesis, e.g., effects on early cell proliferation and migration. Transient MiR-101 Inhibition in Early Life Produces Hyper-excitable Networks in the Adult To determine whether transitory inhibition of mir-101 during post-natal development creates long-lasting changes in circuit function, we tested the levels of excitability in multiple areas of the hippocampus with an array of techniques. First, we measured hippocampal single unit activity in freely behaving adult mice in an intact network long after mir-101 inhibition ended. During periods when animals were resting, pyramidal neurons had significantly elevated firing rates in a-101.f-treated animals compared with a-ctrl-treated controls (Figures 1B 1D and S4A S4C). This increase in baseline activity is consistent with hyper-excitable networks. Further evidence for increased excitation came from recordings of spontaneous excitatory postsynaptic currents (sepscs) in CA3 pyramidal neurons in acute hippocampal slices from young adults (P40). Both the frequency and amplitude of excitatory events were significantly increased by a-101.f compared with a-ctrl (Figures 1E 1G). Even more striking was the appearance of spontaneous high-frequency burst discharges that resembled spontaneous seizure-like events (SLEs) seen in half of the slices from a-101.f-treated animals (Figure 1E, bottom). To measure activity more broadly, we injected P2 pups with an adeno-associated virus encoding the calcium indicator GCaMP6f along with a-101.f and examined the dentate gyrus (DG), which is the main source of excitatory fibers to CA3 pyramidal neurons. Spontaneous calcium transients in acute P37 P40 slices were imaged using confocal microscopy (Figures 1H, 1I, S4D, and S4E). DG neurons from a-101.f-treated animals exhibited increased activity compared with a-ctrl (Figure 1J). Both the percentage of spontaneously active DG neurons and the frequency of calcium transients per active cell were increased (Figures 1K and 1L, bars labeled P2). In addition, neurons from a-101.f-treated animals occasionally showed prolonged bursts of excitatory activity (Figure 1J, bottom). Importantly, acute injections of a-101.f on P30, 8 10 days before calcium imaging, did not alter DG activity (Figures 1K and 1L, bars labeled P30). This is consistent with the earlier demonstration that P2 injection of a-101.f achieves only a transient blockade. Accordingly, mir-101 must act during post-natal development to subsequently determine excitability in the adult. Acute actions of mir-101 in the adult have other consequences (Lee et al., 2008; Vilardo et al., 2010). Because anomalous bursts of excitatory activity were detected in a-101.f-treated animals (Figures 1E and 1J), we asked whether the network presented major pathological features. Both the pentylentetrazol (PTZ) infusion test and Timm staining in the DG showed no difference between a-101.f-treated animals and littermate controls (Figures S4F S4H). Local field potential (LFP) video monitoring revealed that one a-101.f-treated animal of seven showed inter-ictal spikes and stage III seizures (data not shown) whereas all other animals did not. The maximal electroshock seizure (MES) test suggested that evoked seizures, although of similar severity, lasted significantly longer in a-101.f-treated animals than in a-ctrl controls (Figures S4I S4K). Together, the data indicate that, in a-101.f animals, the network is clearly prone to hyper-excitability but does not exhibit a full epileptic phenotype Neuron 92, , December 21, 2016
4 Figure 1. Transitory MiR-101 Inhibition Early in Development Induces Hyper-excitable Networks in the Young Adult (A) Schematic of the time course for mir-101 inhibition in vivo (green) and follow-up tests to assess consequences. (B and C) Single-unit recordings from the hippocampus in freely behaving mice (channels C1 C4) and scatterplots of relative waveform peak amplitudes (B) with mean waveforms plotted (C). (D) Mean spike rates for single units in a-101.fversus a-ctrl-treated mice. Median values, bold black line; inter-quartile range, box edges. (E) sepsc recordings in acute slices showing increased spontaneous excitatory activity in a-101.f-treated neurons (versus a-ctrl controls) on P40 (top two traces). Spontaneous high-frequency burst discharges were seen in half of the slices from a-101.f-treated animals (bottom trace). (F and G) Quantification of sepscs in CA3 reveals an increase in frequency (F) and amplitude (G) in a-101.ft-treated neurons. (H L) Confocal imaging of spontaneous calcium transients on P40. (H and I) Images of DG granule cells virally expressing GCaMP6f (top) and a scheme of the cells in the same field of view (bottom) color-coded for active (red) and inactive (white) cells for a-ctrl- (H) and a-101.f-treated animals (I) after injection on P2. Scale bars, 100 mm. (J) Representative calcium traces showing increases in event frequency (center) and prolonged transients (bottom) in a-101.f-treated animals compared with controls (top). DF, variation in fluorescence; F 0, baseline fluorescence. (K and L) Quantification of calcium activity showed an increase in the number of active cells (K) and in the frequency of events (L) in slices from P40 animals injected on P2 (P2 Injec.) but not when injected on P30 (P30 Injec.). Bar graphs (except D): mean ± SEM; Student s t test; Mann-Whitney U test (D). *p < 0.05, **p < 0.01, ***p < Transient Blockade of MiR-101 Early in Development Causes Memory Deficits in the Adult In many neurodevelopmental disorders, hyper-excitability is often accompanied by cognitive impairment. To determine whether the dysfunctions imposed by transitory mir-101 blockade correlate with long-lasting behavioral change, we tested young adult mice in a battery of tasks that probe hippocampal function. In the fear conditioning test, young adult mice that received a-101.f on P2 froze less in the context in which they received a foot shock (Figures 2A and 2B). This is consistent with the classical notion that formation of contextual memories is hippocampus-dependent (Curzon et al., 2009). Importantly, memory of the cue tone, which is, instead, amygdala-dependent, was not altered by mir-101 blockade. The spontaneous alternation assay requires a functionally intact dorsal hippocampus and is used to test spatial working memory in young rodents. Compared with controls, animals that received a-101.f completed fewer correct alternations, suggesting that they were less likely to remember the arm of the symmetrical Y maze that was last visited (Figures 2C 2E). Animals that received a-101.f also showed impairments in the object/place test, which measures the ability of the animals to remember the location of one of two identical objects (Figures 2F 2I). These data suggest that hippocampus-dependent contextual, working, and spatial memory were impaired in a-101.f-treated mice compared with controls. No significant differences were observed in the open field and the elevated plus maze tests, confirming that the observed memory effects were not a result of changes in anxiety levels or in general exploratory behavior (Figures 2J and 2K). Taken together, the results show that mir-101 function in the first 2 weeks of post-natal development is critical for subsequent neural circuit function. Transient loss of mir-101 regulation in the dorsal hippocampus has long-lasting consequences, leading to a hyper-excitable network and cognitive deficits. The profound effects of mir-101 blockade on network excitability in the adult emerge from disinhibiting mrna targets during early post-natal Neuron 92, , December 21,
5 Figure 2. Transient MiR-101 Blockade Early in Development Produces Memory Impairment in the Adult (A) Fear conditioning test pairing shock with context and tone cue. (B) Animals receiving a-101.f spend less time freezing when re-exposed to the context where they received the shock, indicating impaired association between context and shock. Exposure to a different cage (Altered) induced little freezing in either group. (C) Alternation test involving a symmetrical Y maze with examples of correct and incorrect alternations. (D and E) Animals treated with a-101.f performed fewer correct alternations than controls while traveling the same distance (E), suggesting impaired spatial working memory. (F) Object/place test comparing time spent at the new versus old object position. (G I) Animals with a-101.f spent less time at the new object position (G and H) while traveling the same distance (I), suggesting impaired spatial memory. The discrimination index (H) suggests that the animals have a modest bias toward the location of the object that was not moved. (J) Open field tests showed that a-101.f treatment does not alter the distance traveled by the mice (left), time spent in the central zone of the arena (center), or number of transitions from the periphery to the center of the arena (right). The mir-101 antagonist does not, therefore, alter exploratory behavior or novelty-induced anxiety levels. (K) Elevated plus maze test. No significant difference was detected between the groups in time spent in the open arms (left, index of anxiety) or total distance traveled (right). Mean ± SEM, Student s t test, Welch s test (H); *p < 0.05, **p < life. Identifying such targets will help to understand the developmental programs that converge to establish a balanced network. MiR-101 Targets NKCC1 to Facilitate the GABA Switch, a Critical Event in Development To identify the mechanisms by which mir-101 affects the assembly of balanced circuits, we tested individual candidate mrna targets. We compiled a list of potential mir-101 targets, combining TARGETSCAN predicted interactions with existing high-throughput sequencing of RNA isolated by cross-linking immunoprecipitation (HITS-CLIP) databases from both the mouse cortex on P13 (Chi et al., 2009) and the human cortex (Boudreau et al., 2014). We selected 17 putative mir-101 targets most frequently associated in vivo with the Ago-miRNA-mRNA complexes. We then used qpcr to determine which candidates decreased in the hippocampus between P7 and P11 (low P11- to-p7 ratio), as expected in response to the mir-101 increase, and, conversely, which increased in response to mir-101 blockade by a-101.f, as expected for a de-repressed target. A final selection criterion was based on the encoded protein being relevant for neural development. Seven top candidate mrnas emerged (Table S1). Most promising was the chloride importer NKCC1 because it declines during the second week of post-natal life in rodents and, along with an increase in the chloride exporter KCC2, is responsible for maturation of GABAergic signaling, rendering it inhibitory (Ben-Ari, 2002; Blankenship and Feller, 2010; Rivera et al., 1999). Disruption of the GABA switch during development has profound consequences for network excitability and E/I (Cancedda et al., 2007; Chen and Kriegstein, 2015; Deidda et al., 2015; Dzhala et al., 2005; He et al., 2014; Liu et al., 2006). What causes NKCC1 to decline, however, has long been the focus of investigation but remains unknown. Because the NKCC1 mrna contains a predicted mir-101 MRE, we tested the hypothesis that mir-101 drives maturation of GABAergic signaling by repressing NKCC1 and that disrupting the timing of the GABA switch causes the hyper-excitability observed in a-101.f-treated animals Neuron 92, , December 21, 2016
6 Figure 3. MiR-101 Shapes Network Development in Part by Targeting NKCC1 (A) HEK cells expressing a luciferase construct encoding the 3 0 UTR of NKCC1 showed increased luciferase activity (normalized for Renilla activity) when co-transfected with an a-101 construct. (B) Injection of a-101.f on P2 increased NKCC1 mrna levels in vivo (left, normalized for Hprt1) but did not change KCC2 levels (right), as quantified by qpcr on P8. (C) Western blot analysis confirmed increased NKCC1 levels both in cytosolic protein and membrane/organelle fractions from P8 acute hippocampal slices of animals treated with a-101.f on P2 (four to five slices from the dorsal hippocampus from animals). Quantification of band intensity on western blots (legend continued on next page) Neuron 92, , December 21,
7 To determine whether mir-101 is critical for the decline in NKCC1 during development, we first employed cell culture. Cells expressing a luciferase construct containing the NKCC1 3 0 UTR showed increased luciferase activity when treated with a-101 (a-101.f without a fluorescent tag) compared with a-ctrl-treated cells (Figure 3A). Further, a-101.f increased both NKCC1 messenger and protein levels in vivo, indicating that endogenous mir-101 directly targets the NKCC1 3 0 UTR to degrade the mrna (Figures 3B and 3C). In contrast, KCC2 levels were not changed by mir-101 blockade (Figure 3B). Sensor assays using wild-type and mutated sequences helped confirm the MRE in the NKCC1 3 0 UTR that mir-101 binds to repress its translation (Figures 3D 3F). Experiments in cell culture confirmed the effect of preventing mir-101 regulation of NKCC1. Patch-clamp recording from primary neuron cultures transfected with a-101 indicated that many more neurons displayed a depolarized reversal potential for GABA (E GABA ), as seen in responses to pico-spritzed application of GABA, at a time when the gradient should have matured to make GABA inhibitory (Figure S5A). Consistent with this, neurons transfected with a-101 showed a much greater likelihood of displaying a depolarizing response to GABA, as monitored with a calcium-dependent fluor, than those receiving a-ctrl (Figures 3G, 3H, and S5B). To assess the effects of mir-101 on E GABA under conditions where the neuronal connections were more similar to those in vivo, we compared the GABA responses of neurons in acute slices from a-101.f-treated with control mice on P8. Consistent with cell culture results (Figure S5A), a-101.f-treated neurons retained a more depolarized value for E GABA (Figures 3I 3K). To determine whether mir-101 regulation of NKCC1 mrna alone was responsible for the delayed maturation of E GABA, we employed an LNA target site blocker (TSB; Figures S5C and S5D). This is a novel and powerful strategy that allows the manipulation in vivo of a single mirna-mrna interaction without affecting the basal levels of mrna targets. With this tool, we established a mechanistic link between the regulation of an individual mrna and specific aspects of the phenotype induced by mirna blockade. The NKCC1-TSB prevents binding of mir-101 to the mir-101 MRE in the 3 0 UTR of NKCC1, thereby freeing the transcript from mir-101 inhibitory regulation. All other mir-101 targets would still be subject to mir-101 repression; any phenotype observed would be exclusively due to NKCC1 de-repression. Injection of the NKCC1-TSB did indeed result in a higher level of the NKCC1 transcript (Figure S5D) and a more depolarized E GABA, phenocopying the effect of a-101.f on the chloride gradient (Figures 3I 3K). The results clearly demonstrate that mir-101 directly suppresses NKCC1 levels and, thereby, promotes timely maturation of the chloride gradient to render GABA inhibitory. Maturation of the chloride gradient is an essential feature of early post-natal development whose initiation was not previously understood. A delay in this maturation, as in the case of mir-101 blockade, would be expected to have major consequences for the subsequent development of neural networks (Liu et al., 2006). Protection of NKCC1 Alone Is Not Sufficient to Recapitulate a-101.f-induced Network Dysfunction When the hippocampal circuit is being established, large spontaneous synchronized events (SEs) are the major form of network activity (Ben-Ari, 2002; Blankenship and Feller, 2010; Bonifazi et al., 2009). SEs are generated by the concomitant action of depolarizing GABA and glutamate and are thought to drive synapse formation and help establish properly balanced local circuits (Cancedda et al., 2007; Deidda et al., 2015; Kirkby et al., 2013). Using calcium imaging, we tested whether the extended period of depolarized E GABA induced by mir-101 blockade affected network dynamics. Acute hippocampal slices from a-101.f-treated P8 mice showed increased calcium transients relative to controls (Figures 4A 4C). SEs occurred more frequently and included larger cell ensembles (Figures 4D and 4E). Asynchronous events also occurred more frequently (Figure 4F). In addition, we observed other signs of hyper-excitability, as in the case of double SEs (i.e., two events occurring at a very short inter-event interval; Figure 4G) and saw large synchronous bursts (Figure S5E). Consistent with a role for GABAergic excitation in promoting SEs (Blankenship and Feller, 2010; Bonifazi et al., 2009), disinhibition of NKCC1 alone (NKCC1-TSB), which prolongs the period of depolarizing GABA, was sufficient to replicate the effect of a-101.f on the frequency of SEs (Figure 4D). Importantly, NKCC1-TSB failed to replicate other aspects of the a-101.f phenotype, such as increases in the total number of events (normalized first for b-actin and then for a-ctrl fraction 1) for a-101.f yielded F1 = 1.65, F2 = 1.95, F3 = 0.15, and F4 = 0.03, whereas, for a-ctrl, it yielded F1 = 1.00, F2 = 1.35, F3 = 0.02, and F4 = (D F) Sensor technology was used to demonstrate in vivo that the NKCC1 mir-101 MRE affects protein expression levels. (D) Top: scheme of the lentiviral construct showing a portion of the NKCC1 3 0 UTR cloned downstream of red fluorescent protein excited at 670nm (RFP 670 ). Bottom: sequence of the NKCC1 wild-type and the mutated mir-101 MREs. (E) Images of the CA1 area from animals that received the wild-type (top) or the mutated NKCC1 sensor (bottom). (F) Quantification of RFP 670 labeling, normalized to GFP, shows that the NKCC1 wild-type 3 0 UTR reduces RFP 670 fluorescence compared with a control sensor. The reduction is rescued when the mir-101 MRE is mutated. (G and H) Loading hippocampal neurons in culture on days in vitro (DIV) 8 or 10 with fluorescent acetoxymethyl ester (Fluo-4AM) and challenging 1 hr later with GABA (in the presence of glutamate receptor blockers) revealed more responding cells when the cultures had been transfected with a-101. (Responding cells were defined as those having DF/F 0 R 50% in response to GABA and are expressed as the percentage of all viable cells, as defined by those responding to 1 M KCl at the end of the recording). Images of loaded hippocampal neurons (G) and quantification of GABA responses (H). (I K) Antagonizing mir-101 (a-101.f) or protecting NKCC1 from mir-101 inhibition (NKCC1-TSB) delays E GABA maturation in vivo. (I) Gramicidin-perforated patch-clamp recordings of GABA-mediated currents from CA3 pyramidal neurons in P7 P8 hippocampal slices obtained at the indicated clamp potentials. (J and K) Linear fit of current/voltage (I-V) plots used to estimate E GABA (K) in CA3 neurons receiving a-ctrl, a-101.f, or NKCC1-TSB. Scale bars, 100 pa, 200 ms. Bar graphs: mean ± SEM, Student s t test, one-way ANOVA with Tukey s multiple comparison test (F and K); *p < 0.05, **p < Neuron 92, , December 21, 2016
8 Figure 4. NKCC1-TSB Recapitulates Some, but Not All, Aspects of the a-101.f Phenotype Shown is confocal imaging of spontaneous calcium transients in the CA3 region of acute P8 hippocampal slices. (A) Images of CA3 from a-ctrl- (left, top), a-101.f-treated (center, top), and NKCC1-TSB (right, top) mice showing cells participating in synchronous events (bottom, coded red), firing asynchronously (blue) or being inactive (showing only KCl-induced depolarization, white). Scale bars, 100 mm. (B) Raster plots of activity in movies of a-ctrl (left), a-101.f (center), and NKCC1-TSB (right) with principal component analyses of population activity shown below to identify synchronous events (red dots). (C G) MiR-101 inhibition increased the total number of events (C), including synchronous events (D), percentage of cells participating in synchronous events (E), incidence of asynchronous events (F), and frequency of double events (G, as shown in B, center, red boxes). Of these, de-repression of NKCC1 alone via NKCC1- TSB recapitulated only the effects on synchronous events (D). Partial knockdown of NKCC1 through an sirna (si-nkcc1) co-injected with a-101.f completely rescued the entire phenotype induced by the absence of mir-101, indicating that NKCC1 expression is necessary, although not sufficient, for all aspects of the phenotype. Bar graphs: mean ± SEM, one-way ANOVA with Tukey s multiple comparison test; **p < 0.01, ***p < 0.001, ****p < Neuron 92, , December 21,
9 (Figure 4C), the proportion of cells synchronized (Figure 4E), and the numbers of asynchronous events and double events (Figures 4F and 4G). Partial knockdown of NKCC1 with a small interfering RNA (sirna) in the presence of a-101.f (Figure S5D), however, rescued the entire phenotype induced by a-101.f (Figures 4C 4G); this indicates that overexpression of NKCC1 is essential, although not sufficient, to generate all aspects of the a-101.f phenotype observed here. Taken together, the data indicate that NKCC1 repression by mir-101 is necessary to facilitate the GABA switch and to limit the frequency of SEs. Other mir-101 targets, however, must also be repressed to prevent excessive network activity. MiR-101 Represses Multiple Programs in Parallel to Constrain Spontaneous Network Activity To further elucidate the regulatory mechanisms by which mir-101 limits spontaneous activity, we examined six more candidates from the prioritized list (Table S1). We reasoned that multiple mrna targets would have to be released simultaneously if we were to phenocopy all aspects of mir-101 blockade. Accordingly, we designed TSBs for each mrna target and distributed them into three groups (G1, G2, and G3; Figure 5A), configured to maximize synergistic effects of the protected mrnas while avoiding TSB combinations vulnerable to heteroduplex formation (Figure S6). All groups also contained NKCC1-TSB to ensure that the NKCC1 component of the phenotype was included as a baseline. To assess their unique contributions, the groups were individually injected on P2, and their effects on spontaneous activity were monitored on P8 via calcium imaging. Remarkably, each group recapitulated unique aspects of the a-101.f phenotype not achieved by NKCC1-TSB alone (Figures 5B 5E and S7). The presynaptic G1 mimicked the increase in total activity primarily by increasing asynchronous events (Figures 5B and 5C). This is consistent with reports that Kif1a overexpression induces the formation of presynaptic boutons (Kondo et al., 2012) and that Ank2 stabilizes synapses (Bulat et al., 2014; Pielage et al., 2008). The glia G2 instead recapitulated the increase in the size of cell ensembles recruited in each synchronous event (Figure 5D). The finding is consistent with the role of Abca1 in meeting the high lipid demand of rapidly expanding membranes during dendritic growth and synaptogenesis (Table S1). The neuronal excitability G3 uniquely accounted for the large number of double events (Figure 5E). This may reflect the regulation of extrasynaptic glutamate by system xct, thought to promote neuronal excitability in early circuits (Table S1). The selective effects of the groups on mrna levels were confirmed by qpcr (Figure S7). Because G1 phenocopied most aspects of a-101.f-induced changes in network activity, we further validated both Ank2 and Kif1a regulation by mir-101 (Figures S7 and S8). First, mir-101 inhibition was shown to increase protein levels (Figures S7G and S7H). Second, sensor assays were used to identify the mir-101 MREs for each and demonstrate specificity of the TSBs (Figure S8). Last, simultaneous knockdown of Ank2 and Kif1a (Figures 5F and 5G) was shown to rescue the increases in asynchronous and total calcium events (Figures 5I and 5J) induced by a-101.f but not the increase in SEs (Figure 5H). The results indicate a specific role for the two presynaptic proteins in determining network activity. An important feature of mir-101 revealed by a-101.f blockade is that actions during early post-natal life have lasting effects into adulthood. We hypothesized that the additional effects induced by G1, over and above that of NKCC1-TSB alone, could be sufficient to recapitulate the long-lasting effects on excitability seen with a-101.f. Indeed, calcium imaging in acute hippocampal slices prepared on P40 showed that G1, but not NKCC1-TSB, fully mimicked a-101.f in producing increased levels of activity and increased numbers of active cells in the DG (Figures 5K 5M), hallmarks of a hyper-excitable network. The results indicate that mir-101 prevents excessive activity and that its actions early in development have lasting effects in the adult. It achieves this by repressing multiple developmental programs in parallel that control separate aspects of circuit formation, acting in different neuronal compartments and even in different cell types. Remarkably, protecting three core mrna targets (NKCC1, Kif1a, and Ank2) from mir-101 regulation recapitulates the long-term hyper-excitability induced by a-101.f. MiR-101 Targets Ank2 and Kif1a Complement NKCC1 in Constraining Synaptic Input Having identified three core mrna targets that mir-101 regulates to produce balanced networks, we then probed how they affect connectivity at the single-cell level. SEs and early spontaneous activity are known to guide synapse formation and network construction (Kirkby et al., 2013). Given that mir-101 blockade early in development increases spontaneous activity levels, we asked whether it affected synaptic input in a way likely to alter E/I. Recording from CA3 pyramidal neurons in P11 hippocampal acute slices revealed a clear increase in sepsc frequency for animals receiving a-101.f on P2 compared with controls receiving a-ctrl (Figures 6A and 6B). No change was seen in the spontaneous inhibitory postsynaptic current (sipsc) frequency (Figure 6C) or in mean amplitude (Figures S9A and S9B). Importantly, the ratio of sepscs/sipscs was greatly increased, indicating an imbalance in E/I (Figure 6D). This suggests that the a-101.f-induced increase in early spontaneous network activity may have caused excessive synaptogenesis favoring excitatory input. To test this hypothesis, we recorded miniature excitatory and inhibitory postsynaptic currents (mepscs and mipscs, respectively) from CA3 pyramidal neurons in acute P11 hippocampal slices. Because mepscs and mipscs represent the spontaneous release of individual synaptic vesicles in the absence of action potentials, their frequencies are often used to assess relative numbers of synaptic contacts (although other factors can sometimes also affect spontaneous release). Indeed, a-101.f increased the frequency of both mepscs and mipscs, consistent with increases in the numbers of both glutamatergic and GABAergic synapses (Figures 6E 6G, S9C, and S9D). No changes in mean amplitude were recorded (Figures S9E S9H). Importantly, the increase in mepsc frequency was proportionately greater, thereby increasing the ratio of mepsc/mipsc frequencies in a-101.f-treated animals (Figure 6H) and affecting E/I Neuron 92, , December 21, 2016
10 Figure 5. Additional mrna Targets Must Be Protected to Reproduce the a.101.f Phenotype (A) Grouping (left) of TSBs (right, numbers in brackets indicate the MREs for each mrna) according to the general theme of the target (center). (B E) Calcium imaging on P8 indicated that G1 uniquely recapitulates the increase in total activity (B) primarily because of more asynchronous events (C), G2 the increase in cells participating in SEs (D), and G3 the increase in double events (E). (F J) Simultaneous knockdown of Ank2 and Kif1a rescues only certain aspects of the a-101.f phenotype. qpcr shows the effect of two sirnas injected on P2 against Ank2 (si-ank2, F) and Kif1a (si-kif1a, G) levels on P8. Co-injection of sirnas for Ank2 and Kif1a rescues the increase in frequency of total (I) and asynchronous (J) events but not of synchronous events (H) induced by a-101.f. (K M) Calcium imaging on P40 revealed that network abnormalities persist in young adults receiving G1, but not NKCC1-TSB, on P2. Representative calcium traces (K). Quantification of calcium event frequency (L) and percentage of active cells (M). Bar graphs: mean ± SEM, one-way ANOVA with Tukey s multiple comparison test (B J), Kruskal Wallis with Dunn s multiple comparison test (L and M); *p < 0.05, **p < 0.01, ***p < Neuron 92, , December 21,
11 Figure 6. MiR-101 Targets NKCC1, Kif1a, and Ank2 to Restrain Synaptic Events and Balance E/I (A) Recording sepscs in P11 acute hippocampal slices from a-ctrl and a-101.f-treated mice. (B D) The a-101.f treatment increased the frequency of sepscs (B) but not sipscs (C), and, therefore, increased the ratio of sepscs/ sipscs (D). (E) Traces of mepscs (at 80 mv) and mipscs (at 0 mv) from a-ctrl- (top, left), a-101.f-treated (bottom, left), NKCC1-TSB-treated (top, right), and G1-treated (bottom, right) animals. (F H) Both mepsc (F) and mipsc (G) frequencies were increased (see also Figure S9D), although mepscs proportionately more so by a-101.f, causing an increased mepsc/mipsc (H). G1 treatment better replicated a-101.f than NKCC1- TSB (F and H), indicating the additive effects of multiple pathways. Bar graphs: mean ± SEM, one-way ANOVA with Tukey s multiple comparison test (B D), Kruskal Wallis with Dunn s multiple comparison test (F H); *p < 0.05, **p < 0.01, ***p < To identify the mechanisms by which specific mir-101 targets regulate synaptogenesis and determine E/I, we employed TSBs. We compared NKCC1-TSB, which completely recapitulates the increase in SEs induced by a-101.f, with G1, which phenocopies more closely the overall phenotype of a-101.f, including longlasting effects. G1 was more effective than NKCC1-TSB alone in elevating mepsc frequency on P11 (Figures 6E and 6F), whereas both yielded mipsc frequencies equivalent to that of a-101.f (Figure 6G). As a result, G1 elevated the ratio of mepsc/mipsc frequencies, whereas NKCC1-TSB appeared to be less effective (Figure 6H). The additional contributions of G1 were also apparent in the kinds of synaptic events seen. CA3 pyramidal neurons receive three major excitatory inputs that can be distinguished by analyzing the shape of individual synaptic events. mepscs with an amplitude greater than 30 pa almost exclusively originate from mossy fiber (MF) input (Henze et al., 1997). Compared with controls, a-101.f-treated animals showed an increase in the frequency of MF events (Figure S9I). G1-treated animals fully recapitulated this increase, whereas NKCC1-TSB-treated animals were not different from controls (Figure S9I). This suggests that both a-101.f and G1 induce an increase in the density of MF synapses (number per unit dendrite length), whereas NKCC1-TSB does not. Other synaptic input to CA3 pyramidal neurons comes from commissural association fibers (A/Cs) and the perforant path where the mepsc time to peak (TTP) is delayed because of den- dritic filtering (Perez-Rosello et al., 2011). Examining mepscs smaller than 30 pa (to exclude MF synaptic events) yielded a slower TTP for NKCC1-TSB on average than for G1 (Figure S9J). This is consistent with proportionately more of the synaptic contacts being located farther away in the dendritic tree as a result of NKCC1-TSB treatment compared with G1. NKCC1 and Kif1a/Ank2 Play Complementary Roles in Promoting Connectivity Premature maturation of the GABA switch or selective local removal of depolarizing GABAergic input during development greatly reduces dendritic complexity (Cancedda et al., 2007; Chen and Kriegstein, 2015). This, together with the observation above that NKCC1-TSB increased the relative proportion of distal mepscs seen in CA3 pyramidal neurons without increasing MF events, suggested the hypothesis that release of NKCC1 from mir-101 regulation may preferentially enhance dendritic arbor complexity, whereas release of Ank2 and Kif1a may have a complementary effect in more directly promoting synaptic density. To visualize changes in dendritic morphology, we co-injected the inhibitors on P2, along with a viral construct encoding EGFP, and then imaged CA1 and CA3 pyramidal proximal dendrites on P8 (Figure 7A). NKCC1-TSB alone fully matched the increases in length of primary dendritic branches produced by a-101.f in CA1 (Figures 7B and 7C) and both primary and secondary branches in CA3, whereas the branch number was unchanged (Figures S9K S9M). G1 also recapitulated the a-101.f effect (data not shown), as expected, because it contains NKCC1-TSB. Previous work has shown that overexpression of Kif1a increases the number of presynaptic boutons (Kondo et al., 2012), whereas Ank Neuron 92, , December 21, 2016
12 Figure 7. MiR-101 Repression of NKCC1 Restrains Dendritic Development, but Additional Targeting of Ank2 and Kif1a Is Necessary to Limit Formation of Presynaptic Puncta and Spinogenesis (A) Sparse labeling of hippocampal pyramidal neurons with rabies virus expressing EGFP (top; scale bar, 50 mm) and characterization of dendritic morphology on P8 with NeuronJ (bottom). (B and C) NKCC1-TSB recapitulates the increase in average dendritic length of CA1 primary (B) and CA3 primary and secondary dendrites (C) induced by mir-101. (D) Presynaptic VGlut1 immunostaining in the stratum radiatum on P15 where CA3 A/Cs are prevalent. Scale bar, 50 mm. (E and F) G1, but not NKCC1-TSB, recapitulates the increase induced by a-101.f. Scale bar, 5 mm. Representative images (E) of the area in CA3 used for the analysis (corresponding to the white box in D). Quantification of pre-synaptic puncta (F). (G) Confocal imaging was used to analyze dendritic protrusions on primary proximal dendrites on P11. Scale bar, 5 mm. (H and I) The increases in total protrusion density (H) and mushroom spine density (I) induced by a-101.f are phenocopied by G1 but not by NKCC1-TSB. Bar graphs: mean ± SEM, one-way ANOVA with Tukey s multiple comparison test; *p < 0.05, **p < 0.01, ***p < controls synapse stability (Bulat et al., 2014). To test whether these actions could explain mechanistically the difference between NKCC1-TSB and G1, we first immunostained for vesicular glutamate transporter (VGluT1) as a presynaptic marker of glutamatergic synapses. G1, but not NKCC1-TSB, was able to phenocopy, on P15, the increased numbers of puncta for VGluT1 seen in the stratum radiatum, where A/Cs are prevalent (Figures 7D 7F). The increase in VGluT1 presynaptic puncta induced by a-101.f injected on P2 was maintained on P30, a time when synapse formation and refinement are mostly completed (a-101.f had 1.6-fold more puncta than a-ctrl, Student s t test, p < 0.01). Further, quantification on P11 of dendritic protrusions also revealed clear differences on the postsynaptic side. G1, but not NKCC1-TSB, mimicked the increased density of dendritic Neuron 92, , December 21,
13 protrusions both in the CA1 and CA3 seen with a-101.f (Figures 7G 7I). Notably, G1 also reproduced most closely the increase in mushroom spines, the type thought likely to represent mature glutamatergic synapses (Chen and Sabatini, 2012). These results at the single-cell level elucidate the mechanistic details of how mir-101 curtails the development of excitatory input to generate a balanced network in the adult. Suppression of NKCC1 by mir-101 limits spontaneous activity and the activity-dependent genetic programs that promote dendritic growth. Repression of additional components (Kif1a/Ank2) is needed to constrain the formation of presynaptic boutons and the stabilization of synapses. Inhibition of these two developmental programs in parallel allows pyramidal neurons to receive appropriate excitatory input and avoid pathological levels of activity. Further, the fact that both CA1 and CA3 pyramidal cells are affected similarly by manipulation of mir-101 and its targets suggests that these are core mechanisms. DISCUSSION Creating robust neural networks with balanced E/I is essential for proper brain function, as evidenced by the many crippling neurological disorders reflecting deficiencies in this process. mirnas are attractive candidates for coordinating the many pathways that must act in parallel during network formation to achieve a proper final balance. We show here that mir-101 performs such a role, inhibiting NKCC1, together with Ank2, Kif1a, and other mrna targets, at a critical time during post-natal development to achieve optimal network excitability in the adult. Other mirnas have previously been shown to regulate various aspects of neuronal development and morphology (Bian and Sun, 2011; Lippi et al., 2011; McNeill and Van Vactor, 2012; Schratt et al., 2006), but the present work represents a first in terms of identifying a single player orchestrating a comprehensive effect on neural circuit properties to maintain network stability long after the actual instruction. MiR-101 Regulation of a Critical Phase in Network Development to Achieve a Balanced State It has long been known that early post-natal neural development in rodents includes a period of exuberant growth that subsequently transitions into a phase of pruning and consolidation. This transition is when the fundamental properties of neural networks are established, including the general number and strength of synapses. Errors in the growth phase or in the timing of the transition have long-term consequences for network function (Cancedda et al., 2007; Chen and Kriegstein, 2015; Deidda et al., 2015; Dzhala et al., 2005; He et al., 2014; Liu et al., 2006). The elevation of mir-101 levels that occurs at this time suggested that it played a role; specific blockade of mir-101 by injection of a-101.f on P2 confirmed it. The blockade produced profound changes in network excitability and behavior that persisted well into adulthood. Importantly, these effects were driven exclusively by mir-101 events in early post-natal life because the blockade achieved by a-101.f expired within 9 days of injection, as evidenced by qpcr of both the mir and its targets. Further, blockade of mir-101 in the adult did not replicate any of these effects, indicating that its expression in the adult must serve other purposes (Lee et al., 2008; Vilardo et al., 2010). The results permit the unambiguous conclusion that early mir-101 action has long-lasting effects. This outcome is quite different from those reported previously for mirna actions in the nervous system, such as those of mir-128, which appears to act in the adult to regulate ion channels and ERK2 signaling pathways (Tan et al., 2013). The fact that high levels of mir-101 have also been found in the cortex at the end of the second week of post-natal life (Chi et al., 2009) suggests a global role in the developing brain that merits further investigation. Coordination of Distinct Programs by MiR-101 for a Common Purpose The ability of mir-101 to coordinate the development of a major brain feature (i.e., balanced networks) by regulating key independent programs in parallel is well illustrated by its actions on pyramidal neurons in the hippocampus. During development, the neurons elaborate extensive dendritic branches and receive large numbers of synaptic contacts. This development depends in part on local GABAergic signaling initially being depolarizing. Selective silencing of that GABAergic input (Chen and Kriegstein, 2015) or accelerated maturation of the chloride gradient to render GABA inhibitory impairs dendritic arborization (Cancedda et al., 2007). Conversely, TSB protection of NKCC1 against mir-101 regulation prolongs the period of depolarizing GABA and increases the frequency of SEs, triggering activitydependent gene programs that induce the neurons to extend longer dendritic branches. The additional protection of Ank2 and Kif1a obtained by G1, however, is required to increase the density of synapses formed on the branches, as seen immunohistochemically. Previous studies have suggested roles for Ank2 and Kif1a in synapse formation or stabilization (Bulat et al., 2014; Koch et al., 2008; Kondo et al., 2012; Pielage et al., 2008; Stephan et al., 2015; Yonekawa et al., 1998). Consistent with this, G1, which protects Ank2 and Kif1a along with NKCC1, increased both the density of VGluT1-containing presynaptic puncta and the density of dendritic protrusions, whereas NKCC1-TSB affected only dendritic arborization. The overall outcome is that G1, but not NKCC1-TSB alone, causes an imbalance in E/I and produces long-lasting hyper-excitability. Notably, G2 and G3 contain TSBs protecting yet other mir-101 targets responsible for unique aspects of the changes in spontaneous network activity induced by a-101.f. An interesting question for the future is whether G2 and G3 may recapitulate pathological aspects of a-101.f that were not detected in G1-treated adults, such as the SLE and excitatory bursts. Perhaps surprisingly, mir-101 does not appear to regulate KCC2, although a developmental increase in KCC2 during the second week of post-natal life normally complements the decrease in NKCC1 to execute maturation of the chloride gradient (Ben-Ari, 2002; Blankenship and Feller, 2010; Rivera et al., 1999). MiR-101 does not, for example, target any components of the RE1-silencing transcription factor (REST) complex, a repressor that inhibits KCC2 early in development (Karadsheh and Delpire, 2001), before transcription factors potently induce its expression (Medina et al., 2014). Upstream events are most 1348 Neuron 92, , December 21, 2016
14 likely responsible, coordinating the repression of NKCC1 via mir-101 while releasing expression of KCC2 through other mechanisms. The results clearly demonstrate that mir-101 regulates multiple developmental programs at a critical early stage to achieve the goal of a stable, balanced network in the adult. It does this by constraining the growth phase and facilitating the transition to consolidation, thereby limiting the development of excitatory input. Importantly, blockade of these mir-101 actions does not simply delay development; instead, it causes persistent deficits that apparently cannot be fully compensated even in adulthood. In addition to the targets identified here, mir-101 almost certainly regulates many other mrnas simultaneously to produce the global effect of controlling E/I and stabilizing network function. The candidate approach utilized in this work, in particular the use of TSBs, should also be effective in parsing out additional contributions of other targets repressed by mir-101. Pathological Consequences and Behavioral Relevance Previous studies have linked mir-101 to various neurological disorders because of its regulation of specific targets, as in the case of Atxn1 in spinocerebellar ataxia type I (Lee et al., 2008) and the amyloid precursor protein in Alzheimer s disease (Vilardo et al., 2010). In contrast, our findings define a much broader role for mir-101 in neural development, coordinating multiple pathways in early post-natal life to achieve long-lasting network stability. Disruption of mir-101 action causes hyper-excitability and cognitive impairments. These pathological outcomes reflect features common to many neurodevelopmental disorders (Belforte et al., 2010; Chao et al., 2010; Rubenstein and Merzenich, 2003; Yizhar et al., 2011; Zoghbi and Bear, 2012) in which mirna dysfunctions have often been reported (Im and Kenny, 2012; Wu et al., 2016). Interestingly, several of the mir-101 targets identified here have been linked to specific features of neurodevelopmental disorders. NKCC1 and the maturation of the chloride gradient have been linked to seizure susceptibility (Dzhala et al., 2005). Kif1a mutations in humans cause cognitive impairment, atrophy, neuropathy, and epilepsy (Lee et al., 2015). Both Slc7a11 and Ank2 belong to modules of ASD risk genes that have a key role in neural development and in establishing connectivity of glutamatergic neurons; they are themselves regulated by the fragile X mental retardation protein (FMRP; Parikshak et al., 2013; Voineagu et al., 2011). Notably, mir- 101 levels are altered in patients with ASD (Mundalil Vasu et al., 2014) and schizophrenia (Beveridge et al., 2010). MiR- 101 has also been linked to fragile X syndrome (Zongaro et al., 2013) because of its ability to regulate FMRP, and it has been connected to Rett syndrome because it is responsive to the transcriptional regulator Mecp2, which, in mutated form, can cause Rett syndrome (Wu et al., 2010). These reports indicate the biomedical relevance of the regulatory cascades controlled by mir-101. Taken together, these findings suggest that mir-101 regulates a highly interconnected gene network that controls the phase of initial growth in neural nets, the strength of excitatory input within them, and its balance with inhibition. Our work provides new insight into brain development and reveals potential targets for therapeutic intervention to compensate or reverse multiple disease states. EXPERIMENTAL PROCEDURES Animals Animals were housed under 12/12 hr light/dark cycle (7:00 a.m. 7:00 p.m.) at 23 C±1 C with free access to food and water. All experimental procedures at UCSD were performed as approved by the Institutional Animal Care and Use Committee and according to the NIH Guidelines for the Care and Use of Laboratory Animals. Behavioral and in vivo experiments at UNIMORE were conducted in accordance with the European Community Council Directive (86/609/EEC) of November 24, 1986, and approved by the ethics committee. RNA Sequencing and qpcr mirna analysis was performed as described previously (Zisoulis et al., 2010), and sequencing was based on Illumina s Small RNA Digital Gene Expression v1.5 protocol. Semiquantitative PCR was used to measure mirna levels and their mrna targets in RNA from dorsal hippocampus or hippocampal slices as described previously (Lippi et al., 2011) using the mirneasy mini kit (QIAGEN). Both here and below, see the Supplemental Experimental Procedures for details. Behavior Animals received LNAs or viral constructs on P2 and, after reaching 8 weeks, were subjected to a battery of behavioral tests using standard procedures as described. Electrical Recordings In vivo single-unit recording was performed with implanted microdrive recording devices using electrode assemblies with four tetrodes. Single units were isolated, and firing rates were determined for periods when animals were quietly resting (characterized by the absence of a hippocampal theta rhythm). Histological analysis of serial sections was used to confirm electrode positions in the cornu Ammonis (CA) cell layers. Patch-clamp recording from neurons in acute slices was performed using standard techniques. To determine E GABA, perforated patch-clamp recordings were obtained with gramicidin in the patch electrode. Calcium Imaging Calcium imaging was performed on neurons in acute slices on P8 after loading with calcium fluor. Imaging on P40 was performed using virally encoded GCaMP6 co-injected on P2 with LNA antagonists. A custom-made MATLAB pipeline was used for analysis. SUPPLEMENTAL INFORMATION Supplemental Information includes Supplemental Experimental Procedures, nine figures, and one table and can be found with this article online at dx.doi.org/ /j.neuron AUTHOR CONTRIBUTIONS G.L., A.B.J., E.P.F., S.R.T., J.C.L., and M.M.C. performed calcium imaging experiments. C.C.F. and D.J. performed electrophysiological experiments. J.L.N. and G.W.Y. performed small RNA sequencing. L.A.E., J.K.L., and G.L. performed in vivo recordings. B.R., G.C., G.B., and M.Z. performed behavioral tests and MES tests. E.P.F. wrote the code for calcium imaging analysis. C.B. performed western blot analysis. G.L. performed all remaining experiments. G.L. and D.K.B. wrote the paper. All authors performed data analysis and reviewed and commented on versions of the manuscript. ACKNOWLEDGMENTS We thank E. Terenziani, H. Andrews, E. Ng, I. Lai, and H. Zhang for help with calcium imaging analysis; X. Wang for LNA injections; M. Lippi for helping Neuron 92, , December 21,
15 with the analysis code; L. Manca, A. Tombesi, and A. Vilella for technical assistance with in situ hybridization experiments; V. Jayaraman and K. Svoboda from the GENIE Project for providing the AAV GCaMP6f; and C. Barbato and F. Ruberti for reagents. We thank N.C. Spitzer, M. Scanziani, S. Leutgeb, W.B. Kristan, and D. Dulcis for reviewing versions of the manuscript. This work was supported by grants from the NIH (2R01NS and 1R21NS087342), TRDRP (22XT-0016, 21FT-0027, and 25IP-0019), and UNIMORE FAR2014 (to M.Z.). A.B.J. was supported by the Lundbeck Foundation. Received: April 25, 2016 Revised: October 7, 2016 Accepted: November 3, 2016 Published: December 8, 2016 REFERENCES Bartel, D.P. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, Belforte, J.E., Zsiros, V., Sklar, E.R., Jiang, Z., Yu, G., Li, Y., Quinlan, E.M., and Nakazawa, K. (2010). Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat. Neurosci. 13, Ben-Ari, Y. (2002). Excitatory actions of gaba during development: the nature of the nurture. Nat. Rev. Neurosci. 3, Beveridge, N.J., Gardiner, E., Carroll, A.P., Tooney, P.A., and Cairns, M.J. (2010). Schizophrenia is associated with an increase in cortical microrna biogenesis. Mol. Psychiatry 15, Bian, S., and Sun, T. (2011). Functions of noncoding RNAs in neural development and neurological diseases. Mol. Neurobiol. 44, Blankenship, A.G., and Feller, M.B. (2010). Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat. Rev. Neurosci. 11, Bonifazi, P., Goldin, M., Picardo, M.A., Jorquera, I., Cattani, A., Bianconi, G., Represa, A., Ben-Ari, Y., and Cossart, R. (2009). GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science 326, Boudreau, R.L., Jiang, P., Gilmore, B.L., Spengler, R.M., Tirabassi, R., Nelson, J.A., Ross, C.A., Xing, Y., and Davidson, B.L. (2014). Transcriptome-wide discovery of microrna binding sites in human brain. Neuron 81, Bulat, V., Rast, M., and Pielage, J. (2014). Presynaptic CK2 promotes synapse organization and stability by targeting Ankyrin2. J. Cell Biol. 204, Cancedda, L., Fiumelli, H., Chen, K., and Poo, M.M. (2007). Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J. Neurosci. 27, Chao, H.T., Chen, H., Samaco, R.C., Xue, M., Chahrour, M., Yoo, J., Neul, J.L., Gong, S., Lu, H.C., Heintz, N., et al. (2010). Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 468, Chen, J., and Kriegstein, A.R. (2015). A GABAergic projection from the zona incerta to cortex promotes cortical neuron development. Science 350, Chen, Y., and Sabatini, B.L. (2012). Signaling in dendritic spines and spine microdomains. Curr. Opin. Neurobiol. 22, Chi, S.W., Zang, J.B., Mele, A., and Darnell, R.B. (2009). Argonaute HITS-CLIP decodes microrna-mrna interaction maps. Nature 460, Curzon, P., Rustay, N.R., and Browman, K.E. (2009). Cued and contextual fear conditioning for rodents. In Methods of Behavior Analysis in Neuroscience, Second Edition, J.J. Buccafusco, ed. (CRC Press/Taylor & Francis). Deidda, G., Allegra, M., Cerri, C., Naskar, S., Bony, G., Zunino, G., Bozzi, Y., Caleo, M., and Cancedda, L. (2015). Early depolarizing GABA controls critical-period plasticity in the rat visual cortex. Nat. Neurosci. 18, Dzhala, V.I., Talos, D.M., Sdrulla, D.A., Brumback, A.C., Mathews, G.C., Benke, T.A., Delpire, E., Jensen, F.E., and Staley, K.J. (2005). NKCC1 transporter facilitates seizures in the developing brain. Nat. Med. 11, Guo, H., Ingolia, N.T., Weissman, J.S., and Bartel, D.P. (2010). Mammalian micrornas predominantly act to decrease target mrna levels. Nature 466, He, Q., Nomura, T., Xu, J., and Contractor, A. (2014). The developmental switch in GABA polarity is delayed in fragile X mice. J. Neurosci. 34, Henze, D.A., Card, J.P., Barrionuevo, G., and Ben-Ari, Y. (1997). Large amplitude miniature excitatory postsynaptic currents in hippocampal CA3 pyramidal neurons are of mossy fiber origin. J. Neurophysiol. 77, Im, H.I., and Kenny, P.J. (2012). MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 35, Karadsheh, M.F., and Delpire, E. (2001). Neuronal restrictive silencing element is found in the KCC2 gene: molecular basis for KCC2-specific expression in neurons. J. Neurophysiol. 85, Kirkby, L.A., Sack, G.S., Firl, A., and Feller, M.B. (2013). A role for correlated spontaneous activity in the assembly of neural circuits. Neuron 80, Koch, I., Schwarz, H., Beuchle, D., Goellner, B., Langegger, M., and Aberle, H. (2008). Drosophila ankyrin 2 is required for synaptic stability. Neuron 58, Kondo, M., Takei, Y., and Hirokawa, N. (2012). Motor protein KIF1A is essential for hippocampal synaptogenesis and learning enhancement in an enriched environment. Neuron 73, Lee, Y., Samaco, R.C., Gatchel, J.R., Thaller, C., Orr, H.T., and Zoghbi, H.Y. (2008). mir-19, mir-101 and mir-130 co-regulate ATXN1 levels to potentially modulate SCA1 pathogenesis. Nat. Neurosci. 11, Lee, J.R., Srour, M., Kim, D., Hamdan, F.F., Lim, S.H., Brunel-Guitton, C., Décarie, J.C., Rossignol, E., Mitchell, G.A., Schreiber, A., et al. (2015). De novo mutations in the motor domain of KIF1A cause cognitive impairment, spastic paraparesis, axonal neuropathy, and cerebellar atrophy. Hum. Mutat. 36, Lippi, G., Steinert, J.R., Marczylo, E.L., D Oro, S., Fiore, R., Forsythe, I.D., Schratt, G., Zoli, M., Nicotera, P., and Young, K.W. (2011). Targeting of the Arpc3 actin nucleation factor by mir-29a/b regulates dendritic spine morphology. J. Cell Biol. 194, Liu, Z., Neff, R.A., and Berg, D.K. (2006). Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science 314, Makeyev, E.V., Zhang, J., Carrasco, M.A., and Maniatis, T. (2007). The microrna mir-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mrna splicing. Mol. Cell 27, McNeill, E., and Van Vactor, D. (2012). MicroRNAs shape the neuronal landscape. Neuron 75, Medina, I., Friedel, P., Rivera, C., Kahle, K.T., Kourdougli, N., Uvarov, P., and Pellegrino, C. (2014). Current view on the functional regulation of the neuronal K(+)-Cl(-) cotransporter KCC2. Front. Cell. Neurosci. 8, 27. Mundalil Vasu, M., Anitha, A., Thanseem, I., Suzuki, K., Yamada, K., Takahashi, T., Wakuda, T., Iwata, K., Tsujii, M., Sugiyama, T., and Mori, N. (2014). Serum microrna profiles in children with autism. Mol. Autism 5, 40. Parikshak, N.N., Luo, R., Zhang, A., Won, H., Lowe, J.K., Chandran, V., Horvath, S., and Geschwind, D.H. (2013). Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell 155, Perez-Rosello, T., Baker, J.L., Ferrante, M., Iyengar, S., Ascoli, G.A., and Barrionuevo, G. (2011). Passive and active shaping of unitary responses from associational/commissural and perforant path synapses in hippocampal CA3 pyramidal cells. J. Comput. Neurosci. 31, Pielage, J., Cheng, L., Fetter, R.D., Carlton, P.M., Sedat, J.W., and Davis, G.W. (2008). A presynaptic giant ankyrin stabilizes the NMJ through regulation of presynaptic microtubules and transsynaptic cell adhesion. Neuron 58, Rivera, C., Voipio, J., Payne, J.A., Ruusuvuori, E., Lahtinen, H., Lamsa, K., Pirvola, U., Saarma, M., and Kaila, K. (1999). The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, Neuron 92, , December 21, 2016
16 Rubenstein, J.L., and Merzenich, M.M. (2003). Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2, Schratt, G.M., Tuebing, F., Nigh, E.A., Kane, C.G., Sabatini, M.E., Kiebler, M., and Greenberg, M.E. (2006). A brain-specific microrna regulates dendritic spine development. Nature 439, Stephan, R., Goellner, B., Moreno, E., Frank, C.A., Hugenschmidt, T., Genoud, C., Aberle, H., and Pielage, J. (2015). Hierarchical microtubule organization controls axon caliber and transport and determines synaptic structure and stability. Dev. Cell 33, Tan, C.L., Plotkin, J.L., Venø, M.T., von Schimmelmann, M., Feinberg, P., Mann, S., Handler, A., Kjems, J., Surmeier, D.J., O Carroll, D., et al. (2013). MicroRNA-128 governs neuronal excitability and motor behavior in mice. Science 342, Vilardo, E., Barbato, C., Ciotti, M., Cogoni, C., and Ruberti, F. (2010). MicroRNA-101 regulates amyloid precursor protein expression in hippocampal neurons. J. Biol. Chem. 285, Voineagu, I., Wang, X., Johnston, P., Lowe, J.K., Tian, Y., Horvath, S., Mill, J., Cantor, R.M., Blencowe, B.J., and Geschwind, D.H. (2011). Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474, Wu, H., Tao, J., Chen, P.J., Shahab, A., Ge, W., Hart, R.P., Ruan, X., Ruan, Y., and Sun, Y.E. (2010). Genome-wide analysis reveals methyl-cpg-binding protein 2-dependent regulation of micrornas in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA 107, Wu, Y.E., Parikshak, N.N., Belgard, T.G., and Geschwind, D.H. (2016). Genome-wide, integrative analysis implicates microrna dysregulation in autism spectrum disorder. Nat. Neurosci. 19, Yizhar, O., Fenno, L.E., Prigge, M., Schneider, F., Davidson, T.J., O Shea, D.J., Sohal, V.S., Goshen, I., Finkelstein, J., Paz, J.T., et al. (2011). Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, Yonekawa, Y., Harada, A., Okada, Y., Funakoshi, T., Kanai, Y., Takei, Y., Terada, S., Noda, T., and Hirokawa, N. (1998). Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J. Cell Biol. 141, Yoo, A.S., Staahl, B.T., Chen, L., and Crabtree, G.R. (2009). MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature 460, Zisoulis, D.G., Lovci, M.T., Wilbert, M.L., Hutt, K.R., Liang, T.Y., Pasquinelli, A.E., and Yeo, G.W. (2010). Comprehensive discovery of endogenous Argonaute binding sites in Caenorhabditis elegans. Nat. Struct. Mol. Biol. 17, Zoghbi, H.Y., and Bear, M.F. (2012). Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb. Perspect. Biol. 4, a Zongaro, S., Hukema, R., D Antoni, S., Davidovic, L., Barbry, P., Catania, M.V., Willemsen, R., Mari, B., and Bardoni, B. (2013). The 3 0 UTR of FMR1 mrna is a target of mir-101, mir-129-5p and mir-221: implications for the molecular pathology of FXTAS at the synapse. Hum. Mol. Genet. 22, Neuron 92, , December 21,
17 Neuron, Volume 92 Supplemental Information MicroRNA-101 Regulates Multiple Developmental Programs to Constrain Excitation in Adult Neural Networks Giordano Lippi, Catarina C. Fernandes, Laura A. Ewell, Danielle John, Benedetto Romoli, Giulia Curia, Seth R. Taylor, E. Paxon Frady, Anne B. Jensen, Jerry C. Liu, Melanie M. Chaabane, Cherine Belal, Jason L. Nathanson, Michele Zoli, Jill K. Leutgeb, Giuseppe Biagini, Gene W. Yeo, and Darwin K. Berg
18 SUPPLEMENTAL INFORMATION SUPPLEMENTAL FIGURES Figure S1. Features identifying mir-101 as a top candidate for mediating a major transition in early brain development. Related to Figure 1. (A) Pie chart showing the proportion of total sequenced reads obtained from small RNA deep sequencing (Illumina) for the indicated mirs in P12 hippocampus (data averaged for 6 wild-type
19 2 animals). (B and C) Twelve of the most expressed mirs were chosen and their developmental regulation tested. (Excluded at this stage were mirs such as mir-9 and the let-7 family that are known to be highly expressed during embryonic life and thought to affect stem cell renewal and early differentiation; Bian et al., 2011). The bar graphs represent the abundance of each mir at P3, P12, and adult (> P40), normalized first for housekeeping gene snorna-412 and then for the amount present at embryonic day (E) 16, as assessed by qpcr analysis. Four candidates showed increased expression levels during the relevant developmental window (E16-P12): mir-127, mir-101a and b, and mir-218. Of these four, only mir-101 was reported to be highly enriched in Ago-miR-mRNA complexes as measured by HITS-CLIP experiments in the cortex at P13 (Chi et al., 2009), suggesting that mir-101 is preferentially loaded in the RISC complex that mediates post-transcriptional repression. The mir-101a and b isoforms differ by only one nucleotide (Figure S2H) and have identical seed sequence. (D) Putative targets of mir- 101 derived from the HITS-CLIP database (Chi et al., 2009) were functionally annotated through DAVID and ordered by ascending false discovery rate (FDR). Also listed is the % of all mir-101 targets for that biological theme, and fold-enrichment compared to the entire mouse transcriptome (see Supplemental Experimental Procedures). MiR-101a and b are relatively abundant and enriched in the Ago complex (consistent with Chi et al., 2009; Eacker et al., 2011; Zongaro et al., 2013), increase substantially during the relevant time window (consistent with Zongaro et al., 2013), and have potential targets highly relevant for neuronal development, making them top candidates for mediating the transition.
20 3 Figure S2. MiR-101 is highly expressed in multiple cell-types and is efficiently blocked by a-101.f. Related to Figure 1. (A) In situ hybridization shows that mir-101 is expressed in hippocampus, both in the pyramidal layer of CA1 (1) and in the hilus (2) where interneurons, highlighted by the white arrows, are prevalent. Nissl staining (left
21 4 panels) shows cell nuclei and ribosomal RNA associated with the rough endoplasmic reticulum. (3-4) Expression of mir-124, a brain enriched mir, was used for comparison. Scale bar = 20 µm. (B and C) Tables showing mir-101a and b levels in different neuronal subtypes and non-neuronal brain cells. Relative abundance is expressed on a 1-5 scale (n =rare, nnnnn = very abundant). Expression levels are extrapolated from a cell-type-based analysis (He et al., 2012) performed in (B) mouse brain (mirap) or (C) rat primary cultures (Jovicic et al. 2013). Both mir-101a and b are expressed at medium-to-high levels in all cell-types. (D to F) Sensor technology was used to demonstrate that mir-101 is present in vivo at high levels and can be blocked by a-101.f. (D) Top, scheme of the lentiviral construct encoding the mir-101 sensor (SEN.101). The sensor has six exactly complementary mir-101b MREs cloned downstream from RFP 670. Bottom, sequence of one mir-101 MRE. (E) Images of the CA1 area from animals that received the SEN.101, together with either a-ctrl (top) or a-101.f (bottom). (F) Quantification of RFP 670 labeling, normalized for GFP, shows that the six mir-101 MREs strongly reduce RFP 670 fluorescence normally (in the presence of a-ctrl) and that it is partially rescued by a-101.f. In contrast, RFP 670 in the control sensor (SEN.Ctrl) is not reduced by expression in the cells and is unaffected by a-101.f. (G to I) Additional validation of the LNAs used. We tested two additional scrambled control oligos, one equivalent in length to the mir-101 inhibitor a-scr.101 and the other equivalent in length to the TSB for NKCC1, a-scr.nkcc1, with the same overall nucleotide composition. These were tested at P8 on spontaneous calcium activity compared to the original a-ctrl and confirmed as having no effect on total calcium events (G), frequency of SEs (H), or percentage of synchronized cells (I). Bar graphs: mean ± s.e.m. One-way ANOVA. **p<0.01; ***p<0.001.
22 5
23 6 Figure S3. Validation of mir-101 inhibition by a-101.f. Related to Figure 1. (A) High-resolution picture of a- 101.F distribution in the dorsal hippocampus (CA1, CA3, and dentate gyrus (DG)), as reflected by the 5 6-FAM (a single isomer derivative of fluorescein) green tag, of a P8 animal that was injected with a-101.f at P2. The image shows that the antagonist entered the cells and accumulated in the cytoplasm during the 6-day period. Individual regions (boxes 1 and 2, lower panel) are magnified (boxes 1 and 2, upper panel). Also shown are cell nuclei (DRAQ5 staining, a far-red DNA stain, top right panel), astrocytes (GFAP immunostaining, bottom left), and the merge of all channels (bottom right). The level of a-101.f was high in the pyramidal cell layer, in astrocytes along the dorsal edge of the stratum lacunosum moleculare (box 2), and in other cell-types (most likely interneurons, glia, and endothelial cells) scattered along all strata. This is consistent with the chemistry of in vivo-ready LNAs that are not cell-type specific. Scale bar: 100 µm. (B) Illustration showing the complementarity of a-101.f with mir-101b (vertical red bars indicating the complementary bases) which is essential for the inhibition. The efficacy of the mir- 101 inhibition is best monitored by measuring the levels of known mir-101 mrna targets such as App and Atxn1 (Vilardo et al., 2010; Lee et al., 2008). qpcr confirmed increased levels of App (C) and Atxn1 (D) at P7 in a-101.ftreated animals versus a-ctrl-treated animals. The inhibition subsided by P13. (E) High-resolution picture of a-101.f distribution in a P15 dorsal hippocampus, as reflected by the 5 6-FAM green tag (left) combined with DRAQ5 staining for cell nuclei and GFAP staining for astrocytes (right), after injection of a-101.f at P2. Traces of the antagonist are detected only along the needle track by this time (white dotted lines). Magnification of the indicated CA3 area (box 1 from top panel) shows clusters of extracellular a-101.f deposits and few green cell bodies (insert 1, bottom left panels). An image in the same area, but 80 µm more caudally, shows no green fluorescence though it has DRAQ5 and GFAP (insert 1, bottom right panels). Scale bar: 100 µm. (F and G) Direct qpcr of mir levels indicates that a-101.f effectively decreased mir-101a (F) and mir-101b (G) levels in the dorsal hippocampus at P7 in vivo. The effect is diminished at P11 and completely subsides at P15, confirming the fluorescence results above, and indicating that injection of a-101.f at P2 inhibits mir-101 transiently. (H) Illustration showing the sequence similarity (indicated by vertical red lines) among mir-101a, mir-101b, mir-144, and mir-132, particularly in the seed sequence (bases 2-8). As a result, mir-144 and mir-132 represent good candidates for assessing off-target effects of the mir-101 antagonist. Evidence for the specificity of a-101.f comes from the finding that it had no detectable off-target effects on the levels of mir-144 (I) or mir-132 (J) measured by qpcr, and no effect on the
24 7 levels of two known mir-132 targets, namely p250gap (Wayman et al., 2008) (K) and Mecp2 (Klein et al., 2007) (L), tested by qpcr. Bar graphs: mean ± s.e.m. Student s t-test, *p<0.05; **p<0.01.
25 8 Figure S4. Hyper-excitable network activity in a-101.f-treated young adult mice. Related to Figure 1. (A) Action potential (spike) raster of principal cells simultaneously recorded from an a-ctrl mouse. Each row represents
26 9 the spiking activity of a single cell, with tick marks indicating spikes. The concurrent behavioral state of the animal was determined by spectral analysis of the simultaneously recorded local field potential (LFP) trace (bottom). Periods with high theta-to-delta ratios (>2) were considered theta states and were removed from analysis (cyan). Periods with low theta-to-delta ratios (<2) were considered moments when the animal was quietly resting and were included in analysis (red). Spike rates reported in the main text were calculated by counting the number of spikes occurring during non-theta states, divided by seconds spent in non-theta states. (B) Recording from an a-101.f mouse, as in (A). (C) Coronal histological section stained with cresyl violet showing typical placement of tetrode in the principal cell layer of CA1 (tetrode track highlighted with red circle). Scale bars (C and D): 200 µm. (D) Representative image of P40 hippocampus after co-injection of virally encoded GCaMP6f and a-101.f at P2. Regions expressing the green GCaMP6f fluor received the mir-101 inhibitor a-101.f as well, though the latter is no longer detectable at this age. The white rectangle indicates the DG area used for analysis. (E) Average length of calcium events revealed by GCaMP6f was not changed by the a-101.f treatment at P2 (1 frame = 328 ms) or by acute injection of a-101.f at P30. (F to I) The pathological activity of a-101.f-treated mice does not represent an epileptic phenotype. (F) PTZ-induced seizure susceptibility in adult animals treated at P2 with a-101.f is not different from a-ctrl-treated controls. (G and H) Mossy fiber sprouting, a hallmark of temporal lobe epilepsy, was not observed in cohorts of a-ctrl- or a-101.f- treated animals. Example sections of Timm staining with zinccontaining fibers stained black reveal low levels of dark granules in the inner molecular layer of the dentate gyrus in both control (G, top) and a-101.f-treated animals (G, bottom). On average there was no difference in mossy fiber sprouting between controls and a-101.f-treated animals (H). (I to K) Analysis of seizures induced by MES test showed that a-101.f-treated animals had seizures with similar severity score (I) but significantly longer duration (J and K) than a-ctrl-treated mice, indicating a network prone to pathological activity. Representative ECoG traces of seizures induced by MES test in a-ctrl- (J, top) and a-101.f-treated (J, bottom) mice. Epidural electrodes were implanted in frontal cortex (fc). Black boxes indicate duration of electrical stimulus; red dots indicate artifacts induced by the experimenter positioning the ear electrodes. Bar graphs in (E to I) represent mean ± s.e.m.; in (K) are shown the median value (bold black line, a-ctrl = 3, a-101.f = 9.5) and inter-quartile range (box edges). Student s t- test, except K, where Mann-Whitney U test was used; *p<0.05.
27 10 Figure S5. Effects of a-101.f and NKCC1-TSB. Related to Figures 3, 4. (A) Blockade of mir-101 extends the depolarizing period of GABA responses. Right, graph of mean amplitudes of hippocampal neuron responses in culture to picospritzer-applied GABA, plotted as a function of membrane potential (as done for neurons in slices in
28 11 Figure 3F-3H). Left, the extrapolated E GABA values suggests that blockade of mir-101 delays the maturation of the chloride gradient, as predicted by the effects on NKCC1. (B) Traces of typical calcium transients induced by adding GABA to neurons in culture at DIV8. Grey, a neuron that responded to GABA; red, one that did not. Addition of 1M KCl was used to induce depolarization and visualize viable neurons. A dotted horizontal line represents an example of the threshold used to classify responders and non-responders. (C) Diagram showing mir-101b targeting of NKCC1 (top) and NKCC1-TSB protection (bottom). Top, mir-101b is incorporated in the RISC complex and through base-pair recognition (inset box) binds the MRE (highlighted in yellow) on the NKCC1 mrna to induce repression (red arrow). Bottom, the NKCC1-TSB binds exclusively to the mir-101 MRE on the NKCC1 mrna and protects it from mir-101 repression. All other mir-101 targets (X mrna) are still repressed. The use of TSBs offers a critical advantage over manipulations used previously (e.g. over-expression of a microrna-resistant version of the target, or direct sirna against the target) because it does not interfere with the basal level of the mrna. This is particularly important when the dosage of the targeted mrna has fundamental developmental implications. (D) Injection of a-101.f at P2 increased NKCC1 mrna levels at P8 as quantified by qpcr and normalized for the housekeeping gene Hprt1. NKCC1-TSB injected at P2 was at least as effective as a-101.f in increasing NKCC1 mrna levels at P8. Regulation of NKCC1 by mir-101 in vivo had been suggested by previous work in humans (Boudreau et al., 2014). Two sirnas against NKCC1 were also tested. While si-nkcc1 induced a significant down-regulation, si-nkcc1.2 had no effect. Because sirnas are GapmeRs (Exiqon) and are processed by different intracellular machinery, the effect of both sirnas on NKCC1 levels was compared to a control sirna (si-ctrl). (E) Selective effect of a-101.f on event frequencies. To visualize neuronal activity at P8, we loaded acute hippocampal slices with the calcium indicator Fluo-4AM and imaged calcium transients with a confocal microscope. A raster plot of population activity (upper) and principal component analysis (bottom) are shown, as in Figure 4B. A burst of spontaneous calcium transients from an a-101.f-treated mouse is indicated in the lower trace (red box). Bar graphs: mean ± s.e.m; Student s t-test. *p<0.05; **p<0.01, ***p<0.001.
29 12 Figure S6. Example of a mir-101 recognition element and corresponding TSB. Related to Figures 4, 5. Potential targets of mir-101 were selected from an existing database derived from HITS-CLIP in the cortex at P13 (Chi et al., 2009). (A) The panel was assembled combining elements from USCS browser and Starbase. Top, scheme of mouse chromosome 1 (mm8 assembly). The red rectangle indicates the area carrying the Kif1a gene. Below the chromosome is shown a segment of the Kif1a gene sequence. The Kif1a gene is transcribed from the (-) strand as indicated by the purple bar with white arrows pointing left. The sequence including the mir-101 MRE is highly conserved (Vertebrate Conservation) and drops substantially at the end of the MRE (right part of graph). Immediately under the conservation profile and aligned to the (-) strand are shown clusters of HITS-CLIP tags used to build the Ago-miRNA ternary map shown below (Chi et al., 2009). The map was derived comparing the HITS- CLIP tags with the top predicted mirs sites. In this case the ternary map contains MREs for mir-101 and mir-708. The sequence for Kif1a-TSB2 is shown at the bottom. Kif1a-TSB2 was designed to prevent binding of mir-101 to the mir-101 recognition element in the 3 -UTR of Kif1a, thereby freeing the transcript from mir-101 inhibition. All
30 13 other mir-101 targets would still be subjected to mir-101 regulation. To obtain specificity and reduce the risk of heteroduplex formation, the TSB was designed to overlap both MREs. MiR-708 is several orders of magnitude less abundant than mir-101 in the hippocampus at P12, making it unlikely to be a critical regulator. To confirm this, we designed a TSB specific for the mir-708 MRE (Kif1a-708-TSB) that would not bind to the mir-101 MRE. Injection of Kif1a-708-TSB at P2 in the dorsal hippocampus did not change the levels of Kif1a mrna measured with qpcr (a-ctrl = 1 ± 0.14; a-101.f = 0.88 ± 0.06; n = 3 animals). (B) Example of bioinformatics analysis performed during TSB design with the proprietary Exiqon tool ( In green is the hybridization score. TSBs were designed and grouped to avoid scores >40.
31 14 Figure S7. MiR-101 represses multiple mrna targets to regulate spontaneous network activity. Related to Figure 5. (A) Raster plot of activity in a movie for Group 1 target-site blockers (G1, Presynaptic ), which contains TSBs for Ank2 and Kif1a, along with NKCC1-TSB. Each row represents an individual cell, and each black horizontal line the duration of the calcium transients determined by how long ΔF/F 0 is greater than threshold. High asynchronous activity is apparent between SEs. This is consistent with reports that Kif1a overexpression induces presynaptic boutons (Kondo et al., 2012) and that Ank2 stabilizes synapses (Bulat et al., 2014; Pielage at al., 2008).
32 15 (B) qpcr validated that G1 protected Ank2 (left) and Kif1a (right), normalized for control (a-ctrl). In contrast, neither G2 nor G3 had an effect on either Ank2 or Kif1a. (C) Raster plot for G2 ( Glial ), which contains TSBs for Abca1 and Ndrg2, along with NKCC1-TSB. The fraction of cells participating in each event is high. This is consistent with the role of Abca1 in meeting the high lipid demand of rapidly expanding membranes during dendritic growth and synaptogenesis (Karasinska et al., 2009). (D) qpcr showed significantly increased mrna levels for Ndrg2 (left) and Abca1 (right), while G1 and G3 had no effect. (E) Raster plot for G3 ( Neuronal Excitability ), which contains TSBs for Pmca2 and Slc7a11, along with NKCC1-TSB. A high frequency of double events is seen. This may reflect the regulation of extrasynaptic glutamate by system xct, which is thought to promote neuronal excitability in early circuits (Blankeship et al., 2010; De Bundel et al., 2011). (F) qpcr documented an increase in mrna levels for Pmca2 (left) and Slc7a11 (right), while G1 and G2 had no effect on these parameters. (G and H) Ank2 and Kif1a protein levels increase after mir-101 blockade. (G) Images of Ank2 immunostaining in CA3 at P8 (left). Quantification of the integrated density of the Ank2 signal (right). (H) Immunostaining analysis for Kif1a, as done for Ank2. Bar graphs: mean ± s.e.m. Student s t-test, *p<0.05; **p<0.01; ***p<0.001.
33 16
34 17 Figure S8. MiR-101 represses Ank2 and Kif1a; the TSBs are specific. Related to Figure 5. (A and B) Sensor technology was used to demonstrate in vivo that Ank2 and Kif1a are targets of mir-101. (A) Top, scheme of the lentiviral construct encoding the Ank2 sensor. Bottom, sequence of the Ank2 mir-101 MREs cloned downstream the RFP 670. (B) Quantification of RFP 670 to GFP intensity shows that mutation of the mir-101 MREs in both the Ank2 and Kif1a sensors induces an increase in RFP 670 protein levels, as it would be expected after losing mir-101 repression. Interestingly, while the Kif1a wild-type mir-101 MREs induced a significant reduction in RFP 670 compared to the control sensor, the Ank2 wild-type mir-101 MREs did not. This is likely due to a positive regulator of translation contained in the Ank2 3 -UTR. (C to F) Specificity of the TSBs. Example of possible offtarget interactions between TSBs and non-specific mrnas (C). The levels of all off-target mrnas that showed high to moderate complementarity to the TSBs contained in G1 were tested via qpcr (D). No difference was seen at the mrna level (E and F). Sensor technology was used to confirm at the protein level that TSBs had no off-target effects. (E) Scheme of the viral construct used for this experiment, with the off-target recognition elements from the indicated mrnas. (F) Co-injection of G1 at P2 did not alter RFP 670 expression at P7. Bar graphs: mean ± s.e.m. Student s t-test except (B) One-way ANOVA. *p<0.05; **p<0.01.
35 18 Figure S9. Effects of a-101.f vs. NKCC1-TSB and G1 on event frequencies and dendritic branches. Related to Figures 6, 7. Patch-clamp recording of synaptic events in CA3 pyramidal neurons in acute hippocampal slices
How Nicotinic Signaling Shapes Neural Networks
 How Nicotinic Signaling Shapes Neural Networks Darwin K. Berg Division of Biological Sciences University of California, San Diego Nicotinic Cholinergic Signaling Uses the transmitter ACh to activate cation-selective
How Nicotinic Signaling Shapes Neural Networks Darwin K. Berg Division of Biological Sciences University of California, San Diego Nicotinic Cholinergic Signaling Uses the transmitter ACh to activate cation-selective
Correlated network activity in the developing hippocampus: role in synaptogenesis
 Enrico Cherubini Correlated network activity in the developing hippocampus: role in synaptogenesis SPACE PHYSICS and BIOLOGY Dubna, December 19-23, 2010 The construction of the brain relies on genetic
Enrico Cherubini Correlated network activity in the developing hippocampus: role in synaptogenesis SPACE PHYSICS and BIOLOGY Dubna, December 19-23, 2010 The construction of the brain relies on genetic
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Chapter 2. Investigation into mir-346 Regulation of the nachr α5 Subunit
 15 Chapter 2 Investigation into mir-346 Regulation of the nachr α5 Subunit MicroRNA s (mirnas) are small (< 25 base pairs), single stranded, non-coding RNAs that regulate gene expression at the post transcriptional
15 Chapter 2 Investigation into mir-346 Regulation of the nachr α5 Subunit MicroRNA s (mirnas) are small (< 25 base pairs), single stranded, non-coding RNAs that regulate gene expression at the post transcriptional
Serotonergic Control of the Developing Cerebellum M. Oostland
 Serotonergic Control of the Developing Cerebellum M. Oostland Summary Brain development is a precise and crucial process, dependent on many factors. The neurotransmitter serotonin is one of the factors
Serotonergic Control of the Developing Cerebellum M. Oostland Summary Brain development is a precise and crucial process, dependent on many factors. The neurotransmitter serotonin is one of the factors
When cells are already maximally potentiated LTP is occluded.
 When cells are already maximally potentiated LTP is occluded. Stein, V et al., (2003) J Neurosci, 23:5503-6606. Also found in Rat Barrel Cortex Ehrlich & Malinow (2004) J. Neurosci. 24:916-927 Over-expression
When cells are already maximally potentiated LTP is occluded. Stein, V et al., (2003) J Neurosci, 23:5503-6606. Also found in Rat Barrel Cortex Ehrlich & Malinow (2004) J. Neurosci. 24:916-927 Over-expression
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References
 File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
Zhu et al, page 1. Supplementary Figures
 Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
SUPPLEMENTARY INFORMATION. Supplementary Figure 1
 SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
Is Intrinsic Hyperexcitability in CA3 the Culprit for Seizures in Rett Syndrome?
 Current Literature In Basic Science Is Intrinsic Hyperexcitability in CA3 the Culprit for Seizures in Rett Syndrome? Network Hyperexcitability in Hippocampal Slices From Mecp2 Mutant Mice Revealed by Voltage-Sensitive
Current Literature In Basic Science Is Intrinsic Hyperexcitability in CA3 the Culprit for Seizures in Rett Syndrome? Network Hyperexcitability in Hippocampal Slices From Mecp2 Mutant Mice Revealed by Voltage-Sensitive
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Bidirectional optogenetic modulation of the tonic activity of CEA PKCδ + neurons in vitro. a, Top, Cell-attached voltage recording illustrating the blue light-induced increase in
Supplementary Figure 1 Bidirectional optogenetic modulation of the tonic activity of CEA PKCδ + neurons in vitro. a, Top, Cell-attached voltage recording illustrating the blue light-induced increase in
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
Dep. Control Time (min)
 aa Control Dep. RP 1s 1 mv 2s 1 mv b % potentiation of IPSP 2 15 1 5 Dep. * 1 2 3 4 Time (min) Supplementary Figure 1. Rebound potentiation of IPSPs in PCs. a, IPSPs recorded with a K + gluconate pipette
aa Control Dep. RP 1s 1 mv 2s 1 mv b % potentiation of IPSP 2 15 1 5 Dep. * 1 2 3 4 Time (min) Supplementary Figure 1. Rebound potentiation of IPSPs in PCs. a, IPSPs recorded with a K + gluconate pipette
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) 108 (b-c) (d) (e) (f) (g)
 Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) In four mice, cre-dependent expression of the hyperpolarizing opsin Arch in pyramidal cells
Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) In four mice, cre-dependent expression of the hyperpolarizing opsin Arch in pyramidal cells
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
Supplementary Figure 1. mir124 does not change neuron morphology and synaptic
 Supplementary Figure 1. mir124 does not change neuron morphology and synaptic density. Hippocampal neurons were transfected with mir124 (containing DsRed) or DsRed as a control. 2 d after transfection,
Supplementary Figure 1. mir124 does not change neuron morphology and synaptic density. Hippocampal neurons were transfected with mir124 (containing DsRed) or DsRed as a control. 2 d after transfection,
Nature Neuroscience: doi: /nn Supplementary Figure 1. Trial structure for go/no-go behavior
 Supplementary Figure 1 Trial structure for go/no-go behavior a, Overall timeline of experiments. Day 1: A1 mapping, injection of AAV1-SYN-GCAMP6s, cranial window and headpost implantation. Water restriction
Supplementary Figure 1 Trial structure for go/no-go behavior a, Overall timeline of experiments. Day 1: A1 mapping, injection of AAV1-SYN-GCAMP6s, cranial window and headpost implantation. Water restriction
Excitatory/Inhibitory Synaptic Imbalance Leads to Hippocampal Hyperexcitability in Mouse Models of Tuberous Sclerosis
 Article Excitatory/Inhibitory Synaptic Imbalance Leads to Hippocampal Hyperexcitability in Mouse Models of Tuberous Sclerosis Helen S. Bateup, Caroline A. Johnson, Cassandra L. Denefrio, Jessica L. Saulnier,
Article Excitatory/Inhibitory Synaptic Imbalance Leads to Hippocampal Hyperexcitability in Mouse Models of Tuberous Sclerosis Helen S. Bateup, Caroline A. Johnson, Cassandra L. Denefrio, Jessica L. Saulnier,
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
Cholinergic Activation of M2 Receptors Leads to Context- Dependent Modulation of Feedforward Inhibition in the Visual Thalamus
 Cholinergic Activation of M2 Receptors Leads to Context- Dependent Modulation of Feedforward Inhibition in the Visual Thalamus Miklos Antal., Claudio Acuna-Goycolea., R. Todd Pressler, Dawn M. Blitz, Wade
Cholinergic Activation of M2 Receptors Leads to Context- Dependent Modulation of Feedforward Inhibition in the Visual Thalamus Miklos Antal., Claudio Acuna-Goycolea., R. Todd Pressler, Dawn M. Blitz, Wade
Memory Systems II How Stored: Engram and LTP. Reading: BCP Chapter 25
 Memory Systems II How Stored: Engram and LTP Reading: BCP Chapter 25 Memory Systems Learning is the acquisition of new knowledge or skills. Memory is the retention of learned information. Many different
Memory Systems II How Stored: Engram and LTP Reading: BCP Chapter 25 Memory Systems Learning is the acquisition of new knowledge or skills. Memory is the retention of learned information. Many different
Synaptic Integration
 Synaptic Integration 3 rd January, 2017 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Excitatory Synaptic Actions Excitatory Synaptic Action
Synaptic Integration 3 rd January, 2017 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Excitatory Synaptic Actions Excitatory Synaptic Action
Supplementary Figure 1. Basic properties of compound EPSPs at
 Supplementary Figure 1. Basic properties of compound EPSPs at hippocampal CA3 CA3 cell synapses. (a) EPSPs were evoked by extracellular stimulation of the recurrent collaterals and pharmacologically isolated
Supplementary Figure 1. Basic properties of compound EPSPs at hippocampal CA3 CA3 cell synapses. (a) EPSPs were evoked by extracellular stimulation of the recurrent collaterals and pharmacologically isolated
TNS Journal Club: Interneurons of the Hippocampus, Freund and Buzsaki
 TNS Journal Club: Interneurons of the Hippocampus, Freund and Buzsaki Rich Turner (turner@gatsby.ucl.ac.uk) Gatsby Unit, 22/04/2005 Rich T. Introduction Interneuron def = GABAergic non-principal cell Usually
TNS Journal Club: Interneurons of the Hippocampus, Freund and Buzsaki Rich Turner (turner@gatsby.ucl.ac.uk) Gatsby Unit, 22/04/2005 Rich T. Introduction Interneuron def = GABAergic non-principal cell Usually
Suppl. Information Supplementary Figure 1. Strategy/latency analysis of individual mice during maze learning. a,
 Goal-oriented searching mediated by ventral hippocampus early in trial-and-error learning Ruediger, S, Spirig, D., Donato, F., Caroni, P. Suppl. Information Supplementary Figure 1. Strategy/latency analysis
Goal-oriented searching mediated by ventral hippocampus early in trial-and-error learning Ruediger, S, Spirig, D., Donato, F., Caroni, P. Suppl. Information Supplementary Figure 1. Strategy/latency analysis
Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons
 Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons Peter Osseward, Uri Magaram Department of Neuroscience University of California, San Diego La Jolla, CA 92092 possewar@ucsd.edu
Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons Peter Osseward, Uri Magaram Department of Neuroscience University of California, San Diego La Jolla, CA 92092 possewar@ucsd.edu
Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC
 1 2 1 3 Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC 4 5 6 7 (a) Reconstructions of LII/III GIN-cells with somato-dendritic compartments in orange and axonal arborizations
1 2 1 3 Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC 4 5 6 7 (a) Reconstructions of LII/III GIN-cells with somato-dendritic compartments in orange and axonal arborizations
Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex
 Supplementary Information Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex Luc Gentet, Yves Kremer, Hiroki Taniguchi, Josh Huang, Jochen Staiger and Carl
Supplementary Information Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex Luc Gentet, Yves Kremer, Hiroki Taniguchi, Josh Huang, Jochen Staiger and Carl
Supplemental Information. A Visual-Cue-Dependent Memory Circuit. for Place Navigation
 Neuron, Volume 99 Supplemental Information A Visual-Cue-Dependent Memory Circuit for Place Navigation Han Qin, Ling Fu, Bo Hu, Xiang Liao, Jian Lu, Wenjing He, Shanshan Liang, Kuan Zhang, Ruijie Li, Jiwei
Neuron, Volume 99 Supplemental Information A Visual-Cue-Dependent Memory Circuit for Place Navigation Han Qin, Ling Fu, Bo Hu, Xiang Liao, Jian Lu, Wenjing He, Shanshan Liang, Kuan Zhang, Ruijie Li, Jiwei
Chapter 6 subtitles postsynaptic integration
 CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 6 subtitles postsynaptic integration INTRODUCTION (1:56) This sixth and final chapter deals with the summation of presynaptic currents. Glutamate and
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 6 subtitles postsynaptic integration INTRODUCTION (1:56) This sixth and final chapter deals with the summation of presynaptic currents. Glutamate and
Supplementary Figure 1. GABA depolarizes the majority of immature neurons in the
 Supplementary Figure 1. GABA depolarizes the majority of immature neurons in the upper cortical layers at P3 4 in vivo. (a b) Cell-attached current-clamp recordings illustrate responses to puff-applied
Supplementary Figure 1. GABA depolarizes the majority of immature neurons in the upper cortical layers at P3 4 in vivo. (a b) Cell-attached current-clamp recordings illustrate responses to puff-applied
Nature Methods: doi: /nmeth Supplementary Figure 1. Activity in turtle dorsal cortex is sparse.
 Supplementary Figure 1 Activity in turtle dorsal cortex is sparse. a. Probability distribution of firing rates across the population (notice log scale) in our data. The range of firing rates is wide but
Supplementary Figure 1 Activity in turtle dorsal cortex is sparse. a. Probability distribution of firing rates across the population (notice log scale) in our data. The range of firing rates is wide but
Axon initial segment position changes CA1 pyramidal neuron excitability
 Axon initial segment position changes CA1 pyramidal neuron excitability Cristina Nigro and Jason Pipkin UCSD Neurosciences Graduate Program Abstract The axon initial segment (AIS) is the portion of the
Axon initial segment position changes CA1 pyramidal neuron excitability Cristina Nigro and Jason Pipkin UCSD Neurosciences Graduate Program Abstract The axon initial segment (AIS) is the portion of the
Structural basis for the role of inhibition in facilitating adult brain plasticity
 Structural basis for the role of inhibition in facilitating adult brain plasticity Jerry L. Chen, Walter C. Lin, Jae Won Cha, Peter T. So, Yoshiyuki Kubota & Elly Nedivi SUPPLEMENTARY FIGURES 1-6 a b M
Structural basis for the role of inhibition in facilitating adult brain plasticity Jerry L. Chen, Walter C. Lin, Jae Won Cha, Peter T. So, Yoshiyuki Kubota & Elly Nedivi SUPPLEMENTARY FIGURES 1-6 a b M
Synaptic plasticityhippocampus. Neur 8790 Topics in Neuroscience: Neuroplasticity. Outline. Synaptic plasticity hypothesis
 Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Tuning properties of individual circuit components and stimulus-specificity of experience-driven changes.
 Supplementary Figure 1 Tuning properties of individual circuit components and stimulus-specificity of experience-driven changes. (a) Left, circuit schematic with the imaged component (L2/3 excitatory neurons)
Supplementary Figure 1 Tuning properties of individual circuit components and stimulus-specificity of experience-driven changes. (a) Left, circuit schematic with the imaged component (L2/3 excitatory neurons)
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Behavioural effects of ketamine in non-stressed and stressed mice. Naive C57BL/6 adult male mice (n=10/group) were given a single dose of saline vehicle or ketamine (3.0 mg/kg,
Supplementary Figure 1. Behavioural effects of ketamine in non-stressed and stressed mice. Naive C57BL/6 adult male mice (n=10/group) were given a single dose of saline vehicle or ketamine (3.0 mg/kg,
Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission
 Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission Jesica Raingo, Mikhail Khvotchev, Pei Liu, Frederic Darios, Ying C. Li, Denise
Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission Jesica Raingo, Mikhail Khvotchev, Pei Liu, Frederic Darios, Ying C. Li, Denise
Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane
 Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane potential recorded from POMC neurons following treatment with
Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane potential recorded from POMC neurons following treatment with
Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +.
![Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +. Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +.](/thumbs/90/102196023.jpg) Research Interests Advanced cognitive learning is encoded in distributed circuits that span multiple forebrain areas. Further, synaptic plasticity and neural network theories hypothesize that essential
Research Interests Advanced cognitive learning is encoded in distributed circuits that span multiple forebrain areas. Further, synaptic plasticity and neural network theories hypothesize that essential
Supplementary Figure 1. SybII and Ceb are sorted to distinct vesicle populations in astrocytes. Nature Neuroscience: doi: /nn.
 Supplementary Figure 1 SybII and Ceb are sorted to distinct vesicle populations in astrocytes. (a) Exemplary images for cultured astrocytes co-immunolabeled with SybII and Ceb antibodies. SybII accumulates
Supplementary Figure 1 SybII and Ceb are sorted to distinct vesicle populations in astrocytes. (a) Exemplary images for cultured astrocytes co-immunolabeled with SybII and Ceb antibodies. SybII accumulates
Lisa M. Giocomo & Michael E. Hasselmo
 Mol Neurobiol (2007) 36:184 200 DOI 10.1007/s12035-007-0032-z Neuromodulation by Glutamate and Acetylcholine can Change Circuit Dynamics by Regulating the Relative Influence of Afferent Input and Excitatory
Mol Neurobiol (2007) 36:184 200 DOI 10.1007/s12035-007-0032-z Neuromodulation by Glutamate and Acetylcholine can Change Circuit Dynamics by Regulating the Relative Influence of Afferent Input and Excitatory
Acetylcholine again! - thought to be involved in learning and memory - thought to be involved dementia (Alzheimer's disease)
 Free recall and recognition in a network model of the hippocampus: simulating effects of scopolamine on human memory function Michael E. Hasselmo * and Bradley P. Wyble Acetylcholine again! - thought to
Free recall and recognition in a network model of the hippocampus: simulating effects of scopolamine on human memory function Michael E. Hasselmo * and Bradley P. Wyble Acetylcholine again! - thought to
Functional Development of Neuronal Networks in Culture -An in vitro Assay System of Developing Brain for Endocrine Disruptors
 Functional Development of Neuronal Networks in Culture -An in vitro Assay System of Developing Brain for Endocrine Disruptors Masahiro Kawahara and Yoichiro Kuroda Tokyo Metropolitan Institute for Neuroscience
Functional Development of Neuronal Networks in Culture -An in vitro Assay System of Developing Brain for Endocrine Disruptors Masahiro Kawahara and Yoichiro Kuroda Tokyo Metropolitan Institute for Neuroscience
Supplementary Information
 1 Supplementary Information A role for primary cilia in glutamatergic synaptic integration of adult-orn neurons Natsuko Kumamoto 1,4,5, Yan Gu 1,4, Jia Wang 1,4, Stephen Janoschka 1,2, Ken-Ichi Takemaru
1 Supplementary Information A role for primary cilia in glutamatergic synaptic integration of adult-orn neurons Natsuko Kumamoto 1,4,5, Yan Gu 1,4, Jia Wang 1,4, Stephen Janoschka 1,2, Ken-Ichi Takemaru
Comparison of open chromatin regions between dentate granule cells and other tissues and neural cell types.
 Supplementary Figure 1 Comparison of open chromatin regions between dentate granule cells and other tissues and neural cell types. (a) Pearson correlation heatmap among open chromatin profiles of different
Supplementary Figure 1 Comparison of open chromatin regions between dentate granule cells and other tissues and neural cell types. (a) Pearson correlation heatmap among open chromatin profiles of different
Supplementary Table I Blood pressure and heart rate measurements pre- and post-stroke
 SUPPLEMENTARY INFORMATION doi:10.1038/nature09511 Supplementary Table I Blood pressure and heart rate measurements pre- and post-stroke Pre Post 7-days Systolic Diastolic BPM Systolic Diastolic BPM Systolic
SUPPLEMENTARY INFORMATION doi:10.1038/nature09511 Supplementary Table I Blood pressure and heart rate measurements pre- and post-stroke Pre Post 7-days Systolic Diastolic BPM Systolic Diastolic BPM Systolic
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Atlas representations of the midcingulate (MCC) region targeted in this study compared against the anterior cingulate (ACC) region commonly reported. Coronal sections are shown on
Supplementary Figure 1 Atlas representations of the midcingulate (MCC) region targeted in this study compared against the anterior cingulate (ACC) region commonly reported. Coronal sections are shown on
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10776 Supplementary Information 1: Influence of inhibition among blns on STDP of KC-bLN synapses (simulations and schematics). Unconstrained STDP drives network activity to saturation
doi:10.1038/nature10776 Supplementary Information 1: Influence of inhibition among blns on STDP of KC-bLN synapses (simulations and schematics). Unconstrained STDP drives network activity to saturation
Supplementary Figure 1. SDS-FRL localization of CB 1 in the distal CA3 area of the rat hippocampus. (a-d) Axon terminals (t) in stratum pyramidale
 Supplementary Figure 1. SDS-FRL localization of CB 1 in the distal CA3 area of the rat hippocampus. (a-d) Axon terminals (t) in stratum pyramidale (b) show stronger immunolabeling for CB 1 than those in
Supplementary Figure 1. SDS-FRL localization of CB 1 in the distal CA3 area of the rat hippocampus. (a-d) Axon terminals (t) in stratum pyramidale (b) show stronger immunolabeling for CB 1 than those in
Part 11: Mechanisms of Learning
 Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Relative expression of K IR2.1 transcript to enos was reduced 29-fold in capillaries from knockout animals. Relative expression of K IR2.1 transcript to enos was reduced 29-fold
Supplementary Figure 1 Relative expression of K IR2.1 transcript to enos was reduced 29-fold in capillaries from knockout animals. Relative expression of K IR2.1 transcript to enos was reduced 29-fold
Nature Medicine: doi: /nm.4084
 Supplementary Figure 1: Sample IEDs. (a) Sample hippocampal IEDs from different kindled rats (scale bar = 200 µv, 100 ms). (b) Sample temporal lobe IEDs from different subjects with epilepsy (scale bar
Supplementary Figure 1: Sample IEDs. (a) Sample hippocampal IEDs from different kindled rats (scale bar = 200 µv, 100 ms). (b) Sample temporal lobe IEDs from different subjects with epilepsy (scale bar
Modeling of Hippocampal Behavior
 Modeling of Hippocampal Behavior Diana Ponce-Morado, Venmathi Gunasekaran and Varsha Vijayan Abstract The hippocampus is identified as an important structure in the cerebral cortex of mammals for forming
Modeling of Hippocampal Behavior Diana Ponce-Morado, Venmathi Gunasekaran and Varsha Vijayan Abstract The hippocampus is identified as an important structure in the cerebral cortex of mammals for forming
Wnt Signaling Mediates Experience-Related Regulation of Synapse Numbers and Mossy Fiber Connectivities in the Adult Hippocampus
 Article Wnt Signaling Mediates Experience-Related Regulation of Synapse Numbers and Mossy Fiber Connectivities in the Adult Hippocampus Nadine Gogolla, 1,2 Ivan Galimberti, 1 Yuichi Deguchi, 1 and Pico
Article Wnt Signaling Mediates Experience-Related Regulation of Synapse Numbers and Mossy Fiber Connectivities in the Adult Hippocampus Nadine Gogolla, 1,2 Ivan Galimberti, 1 Yuichi Deguchi, 1 and Pico
Marianna Szemes 1, Rachel L Davies 2, Claire LP Garden 3 and Maria M Usowicz 4*
 Szemes et al. Molecular Brain 2013, 6:33 RESEARCH Open Access Weaker control of the electrical properties of cerebellar granule cells by tonically active GABA A receptors in the Ts65Dn mouse model of Down
Szemes et al. Molecular Brain 2013, 6:33 RESEARCH Open Access Weaker control of the electrical properties of cerebellar granule cells by tonically active GABA A receptors in the Ts65Dn mouse model of Down
Embryonic MGE Cells as a Treatment for Epilepsy December 1, 2012
 Embryonic MGE Cells as a Treatment for Epilepsy December 1, 2012 Scott C. Baraban, PhD University of California, San Francisco American Epilepsy Society Annual Meeting Disclosure Name of Commercial Interest
Embryonic MGE Cells as a Treatment for Epilepsy December 1, 2012 Scott C. Baraban, PhD University of California, San Francisco American Epilepsy Society Annual Meeting Disclosure Name of Commercial Interest
Synaptotagmin-7-Mediated Asynchronous Release Boosts High-Fidelity Synchronous Transmission at a Central Synapse
 Article Synaptotagmin-7-Mediated Asynchronous Release Boosts High-Fidelity Synchronous Transmission at a Central Synapse Highlights d Syt7 KO does not alter fast release or short-term plasticity of calyx
Article Synaptotagmin-7-Mediated Asynchronous Release Boosts High-Fidelity Synchronous Transmission at a Central Synapse Highlights d Syt7 KO does not alter fast release or short-term plasticity of calyx
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature21682 Supplementary Table 1 Summary of statistical results from analyses. Mean quantity ± s.e.m. Test Days P-value Deg. Error deg. Total deg. χ 2 152 ± 14 active cells per day per mouse
doi:10.1038/nature21682 Supplementary Table 1 Summary of statistical results from analyses. Mean quantity ± s.e.m. Test Days P-value Deg. Error deg. Total deg. χ 2 152 ± 14 active cells per day per mouse
TITLE: Altered Astrocyte-Neuron Interactions and Epileptogenesis in Tuberous Sclerosis Complex Disorder
 AD Award Number: W81XW -12-1-0196 TITLE: Altered Astrocyte-Neuron Interactions and Epileptogenesis in Tuberous Sclerosis Complex Disorder PRINCIPAL INVESTIGATOR: Dr. David Sulzer, Ph.D CONTRACTING ORGANIZATION:
AD Award Number: W81XW -12-1-0196 TITLE: Altered Astrocyte-Neuron Interactions and Epileptogenesis in Tuberous Sclerosis Complex Disorder PRINCIPAL INVESTIGATOR: Dr. David Sulzer, Ph.D CONTRACTING ORGANIZATION:
NNZ-2566 in Rett Syndrome and Autism Spectrum Disorders Role and Update
 NNZ-2566 in Rett Syndrome and Autism Spectrum Disorders Role and Update 1 Overview The natural growth factor IGF-1 is broken down in the body to IGF-1[1-3] NNZ-2566 is an analogue of IGF-1[1-3] developed
NNZ-2566 in Rett Syndrome and Autism Spectrum Disorders Role and Update 1 Overview The natural growth factor IGF-1 is broken down in the body to IGF-1[1-3] NNZ-2566 is an analogue of IGF-1[1-3] developed
Astrocyte signaling controls spike timing-dependent depression at neocortical synapses
 Supplementary Information Astrocyte signaling controls spike timing-dependent depression at neocortical synapses Rogier Min and Thomas Nevian Department of Physiology, University of Berne, Bern, Switzerland
Supplementary Information Astrocyte signaling controls spike timing-dependent depression at neocortical synapses Rogier Min and Thomas Nevian Department of Physiology, University of Berne, Bern, Switzerland
Sleep-Wake Cycle I Brain Rhythms. Reading: BCP Chapter 19
 Sleep-Wake Cycle I Brain Rhythms Reading: BCP Chapter 19 Brain Rhythms and Sleep Earth has a rhythmic environment. For example, day and night cycle back and forth, tides ebb and flow and temperature varies
Sleep-Wake Cycle I Brain Rhythms Reading: BCP Chapter 19 Brain Rhythms and Sleep Earth has a rhythmic environment. For example, day and night cycle back and forth, tides ebb and flow and temperature varies
mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons
 Supplemental Material mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons Antoine de Chevigny, Nathalie Coré, Philipp Follert, Marion Gaudin, Pascal
Supplemental Material mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons Antoine de Chevigny, Nathalie Coré, Philipp Follert, Marion Gaudin, Pascal
Nature Neuroscience: doi: /nn Supplementary Figure 1. ACx plasticity is required for fear conditioning.
 Supplementary Figure 1 ACx plasticity is required for fear conditioning. (a) Freezing time of conditioned and control mice before CS presentation and during CS presentation in a new context. Student s
Supplementary Figure 1 ACx plasticity is required for fear conditioning. (a) Freezing time of conditioned and control mice before CS presentation and during CS presentation in a new context. Student s
Chapter 5 subtitles GABAergic synaptic transmission
 CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 5 subtitles GABAergic synaptic transmission INTRODUCTION (2:57) In this fifth chapter, you will learn how the binding of the GABA neurotransmitter to
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 5 subtitles GABAergic synaptic transmission INTRODUCTION (2:57) In this fifth chapter, you will learn how the binding of the GABA neurotransmitter to
Supplementary Information
 Supplementary Information D-Serine regulates cerebellar LTD and motor coordination through the 2 glutamate receptor Wataru Kakegawa, Yurika Miyoshi, Kenji Hamase, Shinji Matsuda, Keiko Matsuda, Kazuhisa
Supplementary Information D-Serine regulates cerebellar LTD and motor coordination through the 2 glutamate receptor Wataru Kakegawa, Yurika Miyoshi, Kenji Hamase, Shinji Matsuda, Keiko Matsuda, Kazuhisa
NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3
 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES Neurons communicate with other neurons or target cells at synapses. Chemical synapse: a very narrow
NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES Neurons communicate with other neurons or target cells at synapses. Chemical synapse: a very narrow
Supplementary Figure 1: Kv7 currents in neonatal CA1 neurons measured with the classic M- current voltage-clamp protocol.
 Supplementary Figures 1-11 Supplementary Figure 1: Kv7 currents in neonatal CA1 neurons measured with the classic M- current voltage-clamp protocol. (a), Voltage-clamp recordings from CA1 pyramidal neurons
Supplementary Figures 1-11 Supplementary Figure 1: Kv7 currents in neonatal CA1 neurons measured with the classic M- current voltage-clamp protocol. (a), Voltage-clamp recordings from CA1 pyramidal neurons
Supplementary Figure 1. ACE robotic platform. A. Overview of the rig setup showing major hardware components of ACE (Automatic single Cell
 2 Supplementary Figure 1. ACE robotic platform. A. Overview of the rig setup showing major hardware components of ACE (Automatic single Cell Experimenter) including the MultiClamp 700B, Digidata 1440A,
2 Supplementary Figure 1. ACE robotic platform. A. Overview of the rig setup showing major hardware components of ACE (Automatic single Cell Experimenter) including the MultiClamp 700B, Digidata 1440A,
Physiology of synapses and receptors
 Physiology of synapses and receptors Dr Syed Shahid Habib Professor & Consultant Clinical Neurophysiology Dept. of Physiology College of Medicine & KKUH King Saud University REMEMBER These handouts will
Physiology of synapses and receptors Dr Syed Shahid Habib Professor & Consultant Clinical Neurophysiology Dept. of Physiology College of Medicine & KKUH King Saud University REMEMBER These handouts will
The Neurobiology of Learning and Memory
 The Neurobiology of Learning and Memory JERRY W. RUDY University of Colorado, Boulder Sinauer Associates, Inc. Publishers Sunderland, Massachusetts 01375 Table of Contents CHAPTER 1 Introduction: Fundamental
The Neurobiology of Learning and Memory JERRY W. RUDY University of Colorado, Boulder Sinauer Associates, Inc. Publishers Sunderland, Massachusetts 01375 Table of Contents CHAPTER 1 Introduction: Fundamental
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supporting Information
 ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
Reversing the Effects of Fragile X Syndrome
 CLINICAL IMPLICATIONS OF BASIC RESEARCH Paul J. Lombroso, M.D., Marilee P. Ogren, Ph.D. Assistant Editors Reversing the Effects of Fragile X Syndrome MARILEE P. OGREN, PH.D., AND PAUL J. LOMBROSO, M.D.
CLINICAL IMPLICATIONS OF BASIC RESEARCH Paul J. Lombroso, M.D., Marilee P. Ogren, Ph.D. Assistant Editors Reversing the Effects of Fragile X Syndrome MARILEE P. OGREN, PH.D., AND PAUL J. LOMBROSO, M.D.
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates
 Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
The Role of Mitral Cells in State Dependent Olfactory Responses. Trygve Bakken & Gunnar Poplawski
 The Role of Mitral Cells in State Dependent Olfactory Responses Trygve akken & Gunnar Poplawski GGN 260 Neurodynamics Winter 2008 bstract Many behavioral studies have shown a reduced responsiveness to
The Role of Mitral Cells in State Dependent Olfactory Responses Trygve akken & Gunnar Poplawski GGN 260 Neurodynamics Winter 2008 bstract Many behavioral studies have shown a reduced responsiveness to
Authors: K. L. Arendt, M. Royo, M. Fernández-Monreal, S. Knafo, C. N. Petrok, J.
 SUPPLEMENTARY INFORMATION Title: PIP 3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane Authors: K. L. Arendt, M. Royo, M. Fernández-Monreal, S. Knafo, C.
SUPPLEMENTARY INFORMATION Title: PIP 3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane Authors: K. L. Arendt, M. Royo, M. Fernández-Monreal, S. Knafo, C.
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature05772 SUPPLEMENTARY INFORMATION Supplemental figure 1. Enrichment facilitates learning. a. Images showing a home cage and a cage used for environmental enrichment (EE). For EE up to
doi: 10.1038/nature05772 SUPPLEMENTARY INFORMATION Supplemental figure 1. Enrichment facilitates learning. a. Images showing a home cage and a cage used for environmental enrichment (EE). For EE up to
Assessing mouse behaviors: Modeling pediatric traumatic brain injury
 Assessing mouse behaviors: Modeling pediatric traumatic brain injury Bridgette Semple Ph.D. Postdoctoral Fellow, Noble Laboratory Department of Neurological Surgery, UCSF Pediatric Traumatic Brain Injury
Assessing mouse behaviors: Modeling pediatric traumatic brain injury Bridgette Semple Ph.D. Postdoctoral Fellow, Noble Laboratory Department of Neurological Surgery, UCSF Pediatric Traumatic Brain Injury
SYNAPTIC COMMUNICATION
 BASICS OF NEUROBIOLOGY SYNAPTIC COMMUNICATION ZSOLT LIPOSITS 1 NERVE ENDINGS II. Interneuronal communication 2 INTERNEURONAL COMMUNICATION I. ELECTRONIC SYNAPSE GAP JUNCTION II. CHEMICAL SYNAPSE SYNAPSES
BASICS OF NEUROBIOLOGY SYNAPTIC COMMUNICATION ZSOLT LIPOSITS 1 NERVE ENDINGS II. Interneuronal communication 2 INTERNEURONAL COMMUNICATION I. ELECTRONIC SYNAPSE GAP JUNCTION II. CHEMICAL SYNAPSE SYNAPSES
What effect would an AChE inhibitor have at the neuromuscular junction?
 CASE 4 A 32-year-old woman presents to her primary care physician s office with difficulty chewing food. She states that when she eats certain foods that require a significant amount of chewing (meat),
CASE 4 A 32-year-old woman presents to her primary care physician s office with difficulty chewing food. She states that when she eats certain foods that require a significant amount of chewing (meat),
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1. Differential expression of mirnas from the pri-mir-17-92a locus.
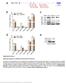 Supplementary Figure 1 Differential expression of mirnas from the pri-mir-17-92a locus. (a) The mir-17-92a expression unit in the third intron of the host mir-17hg transcript. (b,c) Impact of knockdown
Supplementary Figure 1 Differential expression of mirnas from the pri-mir-17-92a locus. (a) The mir-17-92a expression unit in the third intron of the host mir-17hg transcript. (b,c) Impact of knockdown
Nature Neuroscience: doi: /nn Supplementary Figure 1. Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons
 Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
serotonin in learning and plasticity
 serotonin in learning and plasticity pt.1 immediate action L P H N NRX N N R X N CDH RhoA/ROCK RAC1 DAG [Ca2+] camp GIRK2 P11 Gq CASK PICK1 VELI MINT-1 CaMK Ca2+ channel AC Gi mglur7 mglur5 Glutamate NMDAR
serotonin in learning and plasticity pt.1 immediate action L P H N NRX N N R X N CDH RhoA/ROCK RAC1 DAG [Ca2+] camp GIRK2 P11 Gq CASK PICK1 VELI MINT-1 CaMK Ca2+ channel AC Gi mglur7 mglur5 Glutamate NMDAR
1) Drop off in the Bi 150 box outside Baxter 331 or to the head TA (jcolas).
 Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
Cellular Neurobiology / BIPN 140
 SECOND MIDTERM EXAMINATION Fall, 2015 GENERAL INSTRUCTIONS 1. Please write your name on ALL 6 pages. 2. Please answer each question IN THE SPACE ALLOTTED. 1) /10 pts 2) /10 pts 3) /15 pts 4) /15 pts 5)
SECOND MIDTERM EXAMINATION Fall, 2015 GENERAL INSTRUCTIONS 1. Please write your name on ALL 6 pages. 2. Please answer each question IN THE SPACE ALLOTTED. 1) /10 pts 2) /10 pts 3) /15 pts 4) /15 pts 5)
Supplementary Figure 1 hlrrk2 promotes CAP dependent protein translation.
 ` Supplementary Figure 1 hlrrk2 promotes CAP dependent protein translation. (a) Overexpression of hlrrk2 in HeLa cells enhances total protein synthesis in [35S] methionine/cysteine incorporation assays.
` Supplementary Figure 1 hlrrk2 promotes CAP dependent protein translation. (a) Overexpression of hlrrk2 in HeLa cells enhances total protein synthesis in [35S] methionine/cysteine incorporation assays.
Resonant synchronization of heterogeneous inhibitory networks
 Cerebellar oscillations: Anesthetized rats Transgenic animals Recurrent model Review of literature: γ Network resonance Life simulations Resonance frequency Conclusion Resonant synchronization of heterogeneous
Cerebellar oscillations: Anesthetized rats Transgenic animals Recurrent model Review of literature: γ Network resonance Life simulations Resonance frequency Conclusion Resonant synchronization of heterogeneous
Light-evoked hyperpolarization and silencing of neurons by conjugated polymers
 Light-evoked hyperpolarization and silencing of neurons by conjugated polymers Paul Feyen 1,, Elisabetta Colombo 1,2,, Duco Endeman 1, Mattia Nova 1, Lucia Laudato 2, Nicola Martino 2,3, Maria Rosa Antognazza
Light-evoked hyperpolarization and silencing of neurons by conjugated polymers Paul Feyen 1,, Elisabetta Colombo 1,2,, Duco Endeman 1, Mattia Nova 1, Lucia Laudato 2, Nicola Martino 2,3, Maria Rosa Antognazza
MCB MIDTERM EXAM #1 MONDAY MARCH 3, 2008 ANSWER KEY
 MCB 160 - MIDTERM EXAM #1 MONDAY MARCH 3, 2008 ANSWER KEY Name ID# Instructions: -Only tests written in pen will be regarded -Please submit a written request indicating where and why you deserve more points
MCB 160 - MIDTERM EXAM #1 MONDAY MARCH 3, 2008 ANSWER KEY Name ID# Instructions: -Only tests written in pen will be regarded -Please submit a written request indicating where and why you deserve more points
Psychiatric Risk Factor ANK3/Ankyrin-G Nanodomains Regulate the Structure and Function of Glutamatergic Synapses
 Neuron Article Psychiatric Risk Factor ANK3/Ankyrin-G Nanodomains Regulate the Structure and Function of Glutamatergic Synapses Katharine R. Smith, 1 Katherine J. Kopeikina, 1 Jessica M. Fawcett-Patel,
Neuron Article Psychiatric Risk Factor ANK3/Ankyrin-G Nanodomains Regulate the Structure and Function of Glutamatergic Synapses Katharine R. Smith, 1 Katherine J. Kopeikina, 1 Jessica M. Fawcett-Patel,
Alterations in Synaptic Strength Preceding Axon Withdrawal
 Alterations in Synaptic Strength Preceding Axon Withdrawal H. Colman, J. Nabekura, J.W. Lichtman presented by Ana Fiallos Synaptic Transmission at the Neuromuscular Junction Motor neurons with cell bodies
Alterations in Synaptic Strength Preceding Axon Withdrawal H. Colman, J. Nabekura, J.W. Lichtman presented by Ana Fiallos Synaptic Transmission at the Neuromuscular Junction Motor neurons with cell bodies
Outline. Outline. Background Literature and Slides 10/23/2013. The Schizophrenia Research Forum online: Molecules, Neural Networks and Behavior
 Background Literature and Slides Molecules, Neural Networks and Behavior Roberto Fernández Galán, PhD Assistant Professor of Neurosciences Mt. Sinai Health Care Foundation Scholar Alfred P. Sloan Research
Background Literature and Slides Molecules, Neural Networks and Behavior Roberto Fernández Galán, PhD Assistant Professor of Neurosciences Mt. Sinai Health Care Foundation Scholar Alfred P. Sloan Research
Sample Lab Report 1 from 1. Measuring and Manipulating Passive Membrane Properties
 Sample Lab Report 1 from http://www.bio365l.net 1 Abstract Measuring and Manipulating Passive Membrane Properties Biological membranes exhibit the properties of capacitance and resistance, which allow
Sample Lab Report 1 from http://www.bio365l.net 1 Abstract Measuring and Manipulating Passive Membrane Properties Biological membranes exhibit the properties of capacitance and resistance, which allow
Synaptic Plasticity and the NMDA Receptor
 Synaptic Plasticity and the NMDA Receptor Lecture 4.2 David S. Touretzky November, 2015 Long Term Synaptic Plasticity Long Term Potentiation (LTP) Reversal of LTP Long Term Depression (LTD) Reversal of
Synaptic Plasticity and the NMDA Receptor Lecture 4.2 David S. Touretzky November, 2015 Long Term Synaptic Plasticity Long Term Potentiation (LTP) Reversal of LTP Long Term Depression (LTD) Reversal of
Ube3a is required for experience-dependent maturation of the neocortex
 Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
TITLE: Altered Astrocyte-Neuron Interactions and Epileptogenesis in Tuberous Sclerosis Complex Disorder
 AWARD NUMBER: W81XWH-12-1-0196 TITLE: Altered Astrocyte-Neuron Interactions and Epileptogenesis in Tuberous Sclerosis Complex Disorder PRINCIPAL INVESTIGATOR: Dr. David Sulzer, Ph.D. CONTRACTING ORGANIZATION:
AWARD NUMBER: W81XWH-12-1-0196 TITLE: Altered Astrocyte-Neuron Interactions and Epileptogenesis in Tuberous Sclerosis Complex Disorder PRINCIPAL INVESTIGATOR: Dr. David Sulzer, Ph.D. CONTRACTING ORGANIZATION:
