Psychiatric Risk Factor ANK3/Ankyrin-G Nanodomains Regulate the Structure and Function of Glutamatergic Synapses
|
|
|
- Helen Horn
- 5 years ago
- Views:
Transcription
1 Neuron Article Psychiatric Risk Factor ANK3/Ankyrin-G Nanodomains Regulate the Structure and Function of Glutamatergic Synapses Katharine R. Smith, 1 Katherine J. Kopeikina, 1 Jessica M. Fawcett-Patel, 1 Katherine Leaderbrand, 2 Ruoqi Gao, 1 Britta Schürmann, 1 Kristoffer Myczek, 1 Jelena Radulovic, 2,3 Geoffrey T. Swanson, 3 and Peter Penzes 1,2, * 1 Department of Physiology 2 Department of Psychiatry and Behavioral Sciences 3 Department of Pharmacology Northwestern University Feinberg School of Medicine, Chicago, IL, USA *Correspondence: p-penzes@northwestern.edu SUMMARY Recent evidence implicates glutamatergic synapses as key pathogenic sites in psychiatric disorders. Common and rare variants in the ANK3 gene, encoding ankyrin-g, have been associated with bipolar disorder, schizophrenia, and autism. Here we demonstrate that ankyrin-g is integral to AMPAR-mediated synaptic transmission and maintenance of spine morphology. Using superresolution microscopy we find that ankyrin-g forms distinct nanodomain structures within the spine head and neck. At these sites, it modulates mushroom spine structure and function, probably as a perisynaptic scaffold and barrier within the spine neck. Neuronal activity promotes ankyrin-g accumulation in distinct spine subdomains, where it differentially regulates NMDA receptor-dependent plasticity. These data implicate subsynaptic nanodomains containing a major psychiatric risk molecule, ankyrin-g, as having location-specific functions and open directions for basic and translational investigation of psychiatric risk molecules. INTRODUCTION Synaptic plasticity is thought to underlie learning and memory, tuning of neural circuitry, and information storage in the brain. Two major postsynaptic processes are involved in plasticity of glutamatergic synapses: modifications in number of AMPA receptors (AMPARs) and alterations in size and shape of dendritic spines (Bosch and Hayashi, 2012). Spines are highly specialized and dynamic subcellular compartments that undergo structural plasticity (Sala and Segal, 2014). During one form of plasticity, long-term potentiation (LTP), spines become larger and more stable with an increased abundance of AMPARs, thereby strengthening synapse function (Makino and Malinow, 2009). The mechanisms by which this occurs have been extensively studied (Sala and Segal, 2014), though how this process may be disrupted in disease remains unclear. Spines harbor an array of scaffolding proteins (including PSD95), which are responsible for localization of membrane proteins such as AMPARs, enabling optimum positioning for synaptic transmission. Spines are also highly enriched in cytoskeletal elements including F-actin and b-spectrin (Blanpied et al., 2008; Cingolani and Goda, 2008). Recent studies using superresolution microscopy revealed that the organization of core synaptic components is more complex than previously appreciated; scaffolds and AMPARs are organized into subsynaptic domains that rearrange during plasticity (Kerr and Blanpied, 2012; MacGillavry et al., 2013; Nair et al., 2013). This phenomenon underscores the molecular complexity of the synapse, providing mechanistic insight into the workings of the PSD, and suggests how this function might go awry in pathogenic situations. Superresolution imaging therefore enables discovery of previously unanticipated architectural and functional features of synapses. Glutamatergic synapses and spine morphology have been implicated as key sites of pathogenesis in neuropsychiatric disorders including schizophrenia (SZ), autism spectrum disorders (ASDs), and intellectual disability (ID) (Penzes et al., 2011, 2013). Genetic studies support these findings by implicating genes that encode synaptic proteins in the etiology of these disorders (Gilman et al., 2011; Nurnberger et al., 2014; Purcell et al., 2014). Synaptic deficits and reduced plasticity have also been linked to bipolar disorder (BD) (Lin et al., 2012), although the synaptic biology that contributes to pathogenesis of BD remains elusive. BD and other neuropsychiatric disorders may share common genetic risk factors, the study of which might reveal shared pathogenic mechanisms. The human ANK3 gene is a leading BD risk gene also associated with SZ, ASD, and ID (Ferreira et al., 2008; Schulze et al., 2009). Rare pathogenic mutations in ANK3 were identified in patients with ID/ADHD (Iqbal et al., 2013) and ASD (Bi et al., 2012), making ANK3 a common genetic risk factor for neuropsychiatric diseases; however, the role that ANK3 may play in pathogenesis remains unknown. ANK3 encodes ankyrin-g, a scaffolding adaptor that links membrane proteins to the actin/b-spectrin cytoskeleton, organizing proteins into discrete domains at the plasma membrane Neuron 84, , October 22, 2014 ª2014 Elsevier Inc. 399
2 Neuron Ankyrin-G Nanodomains Regulate Dendritic Spines (Bennett and Healy, 2009). Multiple isoforms of ankyrin-g exist in neurons, sharing three conserved domains: an N-terminal membrane association domain, a spectrin-binding domain, and a C-terminal tail (Bennett and Healy, 2009). The 270/ 480 kda isoforms have well-documented roles at the axon initial segment (AIS) and nodes of Ranvier (NoR; Rasband, 2010). The role of the 190 kda isoform in neurons, however, is less well characterized. Some studies have suggested that ankyrin- G may also reside at synapses: giant isoforms are essential for stability of the presynapse at the Drosophila neuromuscular junction (Koch et al., 2008; Pielage et al., 2008) and proteomic analyses have detected ankyrin-g in rodent PSD preparations (Collins et al., 2006; Jordan et al., 2004; Nanavati et al., 2011). It remains unclear how synaptic ankyrin-g might impact spine organization or plasticity and contribute to disease pathogenesis. Here we demonstrate that ankyrin-g is integral to AMPARmediated synaptic transmission and maintenance of spine morphology. Using superresolution microscopy, we find ankyrin-g forms distinct nanodomain structures within the spine head and neck. At these sites, it modulates mushroom spine structure and function, probably as a perisynaptic scaffold and barrier within the spine neck. We show that neuronal activity promotes ankyrin-g accumulation in spine subdomains, where it contributes to NMDA receptor-dependent plasticity. These data implicate subsynaptic nanodomains of a major psychiatric risk molecule, ankyrin-g, as having location-specific functions and opens novel directions for basic and translational investigation of psychiatric risk molecules. RESULTS Ankyrin-G Knockdown Impairs AMPAR-Mediated Synaptic Transmission and Spine Maintenance Studies have suggested that ankyrin-g plays a role in neuronal function beyond its well-characterized actions at the AIS (Collins et al., 2006; Koch et al., 2008; Pielage et al., 2008). We hypothesized that ankyrin-g is involved in excitatory synaptic transmission. To test this, we utilized RNAi to knockdown ankyrin-g in mature cortical neuronal cultures. We tested four RNAi constructs in ankyrin-g-transfected HEK cells (Figure S1A available online) and used the most effective (RNAi 4) experimentally (Figures S1B and S1C). RNAi 4 led to significant reduction of ankyrin-g levels in the soma, AIS, and dendrites compared with control neurons (soma **p < 0.01, AIS and dendrite ****p < , two-way ANOVA, Figure S1C). To investigate synaptic function of ankyrin-g in neurons, we performed whole-cell electrophysiological recordings of miniature excitatory postsynaptic currents (mepscs) from control and knockdown neurons (Figure 1A). Ankyrin-G knockdown caused a significant 26% reduction in mepsc amplitude (*p = 0.015, t test, Figures 1B and 1C; Figure S1D) with no change in frequency (p = 0.621, t test, Figure 1D), suggesting that the effect of ankyrin-g knockdown is primarily postsynaptic. Since spine function and structure are linked, we tested whether ankyrin-g played a role in maintenance of spine morphology. Analysis of control or knockdown neurons imaged by confocal microscopy revealed that knockdown caused a significant 18% decrease in spine area (***p = , Figures 1E 1G; Figure S1E), contributed to by both a decrease in spine length (**p = ) and width (****p < , width:length: p = 0.412, t tests, Figure S1F). This effect on spine area was accompanied by an 18% decrease in spine density (*p = 0.030, Figure 1H); however, concurrent with no effect on mepsc frequency, there was no change in AMPAR cluster density as measured by confocal microscopy of knockdown neurons immunostained for GluA1 (Figure S1G). These experiments show that ankyrin-g is essential for maintaining proper AMPAR-mediated synaptic transmission and spine structure in cortical neurons. Ankyrin-G Is Present in Dendrites and Spines The effects of ankyrin-g knockdown on mepscs and spine structure led us to investigate ankyrin-g at synaptic sites. Confocal imaging of neurons immunostained for endogenous ankyrin-g revealed the expected localization in the AIS (open arrowheads, Figure 2A), but also a robust presence at the plasma membrane of the soma (arrows) and puncta throughout the dendritic tree (arrowheads). We examined ankyrin-g antibody specificity using two additional antibodies and found that all three had similar distributions (Figure S2A). Immunoreactivity from our ankyrin-g pab is highly reduced (72%) in ankyrin-g knockdown neurons (Figure S2B) and specifically recognizes both GFPtagged protein and bands of the correct molecular weight on western blots (Figure S2C). In GFP-expressing neurons, we observed ankyrin-g puncta in spines and along the dendritic shaft (Figure 2B). To confirm presence of ankyrin-g at synapses, we immunostained for ankyrin-g and either the presynaptic marker bassoon (Figure 2C) or postsynaptic scaffold PSD95 (Figure 2D); both markers exhibited substantial colocalization with ankyrin-g (Figure S2D). In cortical slices immunostained for ankyrin-g and bassoon, ankyrin-g also localized to the AIS and synaptic sites (Figure S2E). Our data thus indicate that endogenous ankyrin-g is present at synaptic sites in addition to the AIS. Ankyrin-G Forms Discrete Perisynaptic and Neck Nanodomains in Spines Recent studies indicate that molecules in spines are organized both spatially and functionally into subsynaptic domains (Chen and Sabatini, 2012; MacGillavry et al., 2011). We were therefore interested in the nanoscale organization of ankyrin-g at synapses with a view that this might reveal potential functions for this protein. Due to the limited resolution of confocal microscopy, we utilized a superresolution microscopy technique: structured illumination microscopy (SIM [Gustafsson, 2005]). To analyze postsynaptic organization, we imaged cortical neurons expressing GFP and immunostained for ankyrin-g (Figure 2E), which revealed that ankyrin-g is not contained within a single homogenous complex but is distributed into small domains throughout spine heads (Figure 2F). Surprisingly, we also found ankyrin-g localized to the spine neck (Figure 2F, open arrowheads), an understudied structure of the spine due to its size. Because the mean area of these domains was below the resolution of confocal microscopy, 131 ± 6 nm 2, we termed them nanodomains. To gain insight into the specific function of ankyrin-g, we analyzed the localization of ankyrin-g nanodomains relative to 400 Neuron 84, , October 22, 2014 ª2014 Elsevier Inc.
3 Neuron Ankyrin-G Nanodomains Regulate Dendritic Spines Figure 1. Ankyrin-G Knockdown Impairs Synaptic Transmission and Spine Maintenance (A) Representative AMPAR-mediated mepsc traces from control or ankyrin-g knockdown neurons. (B) Bar graph of mepsc amplitude (32.76 ± 3.68 pa to ± 1.21 pa, *p = 0.015, n = 15 cells), data are represented as mean ± SEM. (C) Cumulative probability graph of mepsc amplitudes (n = 15 cells). (D) Bar graph of mepsc frequency (p = 0.621, n = 15 cells; n.s., nonsignificant), data are represented as mean ± SEM. (E) Confocal images of control or ankyrin-g knockdown neurons. Scale bar = 5 mm. (F) Bar graph showing ankyrin-g RNAi causes a decrease in spine area compared with control (0.494 ± 0.16 mm 2 to ± mm 2, ***p = 0.003, n = cells), data are represented as mean ± SEM. (G) Cumulative probability graph of spine areas (n = cells). (H) Bar graph showing ankyrin-g RNAi causes a decrease in spine density compared with control (8.39 ± 0.43 to 6.80 ± 0.53 spines/10 mm,*p = 0.030, n = cells), data are represented as mean ± SEM. See also Figure S1. PSD95. We imaged GFP-expressing neurons immunostained for ankyrin-g and PSD95 (Figures 2G 2I and Figures S2F and S2G). Manders colocalization coefficients confirm that ankyrin-g overlaps with both PSD95 and bassoon to the same extent and is both pre- and postsynaptic in cortical neurons, in agreement with previous reports of ankyrin-g location at the presynapse (Figures S2F and S2G [Pielage et al., 2008]). Frequently, multiple ankyrin-g nanodomains were found adjacent to or directly overlapping with PSD95, with a mean distance from the center of an ankyrin-g nanodomain to the center of the PSD being ± mm. A linescan through the spine head shows that peaks of fluorescence for ankyrin-g and PSD95 do not overlap (Figure 2H) and suggest a perisynaptic location for ankyrin-g as confirmed by 3D reconstruction (Figure 2I, Figure S2I, and Movie S1). We categorized synapses into three groups to describe the relationship of ankyrin-g nanodomains to PSD95: (1) single nanodomain, (2) multiple nanodomains around the PSD, and (3) complete overlap of nanodomains (Figure S2H); 52% of spines fell into the second category, having two or more ankyrin-g nanodomains surrounding PSD95 (Figures 2J and 2K). Together, these SIM data led us to a model where ankyrin-g forms nanodomains in two main locations within the spine: in the spine head surrounding the PSD and within the spine neck (Figure 2L). Thus, ankyrin-g nanodomains are in a prominent position to potentially modulate glutamatergic synaptic transmission. Ankyrin-G Nanodomain Localization Associates with Spine Morphology To understand how differential localization of ankyrin-g nanodomains may correlate with spine morphology, we analyzed neurons stained for endogenous ankyrin-g. Our analysis of spine morphology showed that we could precisely measure spine geometry using SIM (Figures 3A 3D), producing values similar to other superresolution imaging studies (Tønnesen et al., 2014). Ankyrin-G nanodomains were present in 96% of spines and the number of nanodomains in the spine head directly correlated with the dimensions of the spine head (Figure 3F; Figures S3A and S3B). The mean ankyrin-g nanodomain area did not vary with spine head size (Figures 3E and 3G; Figures S3C and Neuron 84, , October 22, 2014 ª2014 Elsevier Inc. 401
4 Neuron Ankyrin-G Nanodomains Regulate Dendritic Spines Figure 2. Ankyrin-G Forms Nanodomains in the Heads and Necks of Spines (A) Confocal image of a neuron immunostained for ankyrin-g in the soma (arrows), axon (open arrowheads), and dendrites (closed arrowheads). Scale bar = 20 mm. (B) Dendrite from neuron expressing GFP showing punctate ankyrin-g staining in the dendritic shaft and spines (arrowheads). Scale bar = 5 mm. (legend continued on next page) 402 Neuron 84, , October 22, 2014 ª2014 Elsevier Inc.
5 Neuron Ankyrin-G Nanodomains Regulate Dendritic Spines Figure 3. Ankyrin-G Nanodomain Localization Associates with Spine Morphology, Based on SIM Imaging (A D) Frequency distributions of spine geometry: head area (A), head width (B), neck width (C), and neck length (D). (E) Frequency distribution of ankyrin-g nanodomain area. (F) Correlation plot of number of ankyrin-g nanodomains versus spine head area, n = 122 spines, 9 cells. (G) Correlation plot of nanodomain area versus area of spine head (n.s.). (H) Frequency histogram of PSD95 puncta area. (I) Bar graph showing mean nanodomain and PSD95 area, ****p = , data are represented as mean ± SEM. (J) Correlation plot of number of nanodomains versus area of PSD. (K) Correlation plot of nanodomain area versus area of PSD (n.s.). (L) Bar graph of spine head, nanodomain, and PSD95 areas in spines with ankyrin-g in the head and neck or head only. Head area: ± mm 2 to ± mm 2, **p = ; ankyrin-g nanodomain area: ± mm 2 to ± mm 2, p = 0.174; PSD95 area: ± mm 2 to ± mm 2, p = 0.350, n = 122 spines, 9 cells. Data are represented as mean ± SEM. See also Figure S3. S3D), suggesting that they do not multimerize. PSD95 clusters had a mean area of ± mm 2 (Figures 3H and 3I), and this area correlated with the number of nanodomains in the spine head, rather than their mean area (Figures 3J and 3K). In addition to its presence in the spine head, 74% of spines had ankyrin-g nanodomains in the neck (Figure S3E), and 99% of these spines also had nanodomains in the head, correlating the presence of ankyrin-g in the neck to its presence in the head (Figure S3F). (C and D) Confocal images of neurons stained for ankyrin-g and bassoon (C) and PSD95 (D). Arrowheads, colocalization. Scale bar = 5 mm. (E) SIM z stack maximum projection of GFP-expressing neuron (left) immunostained for ankyrin-g (right, dendrite outlined in yellow). Scale bar = 5 mm. (F) Ratiometric SIM images of spines expressing GFP and immunostained for ankyrin-g from image in (E). Arrowheads, spine head nanodomains; open arrowheads, spine neck nanodomains. Scale bar = 0.2 mm. (G) SIM image of GFP-expressing neuron immunostained for ankyrin-g (red) and PSD95 (blue). Scale bar = 2 mm. Box indicates spine in (H) and (I). (H) Linescan through spine head from boxed spine in (G). (I) 3D reconstruction of boxed spine in (G). Scale bar = 0.5 mm. See also Movie S1. (J) SIM images of PSDs from GFP-expressing neurons immunostained for ankyrin-g and PSD95. Scale bar = mm. (K) Quantification of percentage of PSDs with each distribution. (L) Schematic diagram of observed distributions of ankyrin-g nanodomains within spines. See also Figure S2. Neuron 84, , October 22, 2014 ª2014 Elsevier Inc. 403
6 Neuron Ankyrin-G Nanodomains Regulate Dendritic Spines Figure 4. A Short Isoform of Ankyrin-G Regulates Spine Maintenance (A) Schematic of the functional domains of 190 kda and 270 kda ankyrin-g isoforms. Note the serine-rich (Ser-rich) domain and tail insert are only present in the 270 kda isoform. (B) SDS-PAGE and western blots of synaptoneurosomes probed with antibodies to ankyrin-g, PSD95, and spectrin. Lysate, whole-cell cortical lysate; syn, synaptoneurosomes. (C) Confocal images of neurons expressing GFPAnkG190 or GFPAnkG270. Scale bar = 20 mm. Open arrowheads, AIS. Inset, zoom of dendrite; scale bar = 5 mm. (D) Bar graph showing dendrite:axon intensity ratio of GFPAnkG190 or GFPAnkG270 (**p = , n = 8 10 cells), data are represented as mean ± SEM. (E) Bar graph showing reduced intensity of GFPAnkG270 in spines compared with GFPAnkG190 (***p = , n = cells), data are represented as mean ± SEM. (F) Confocal images of neurons expressing mcherry alone, +GFPAnkG190, or +GFPAnkG270. Scale bar = 5 mm. (legend continued on next page) 404 Neuron 84, , October 22, 2014 ª2014 Elsevier Inc.
7 Neuron Ankyrin-G Nanodomains Regulate Dendritic Spines The presence of ankyrin-g in the spine neck in addition to the head directly correlated with the spine head area, as spines with neck nanodomains had significantly larger head areas compared with spines where ankyrin-g was absent from the neck, with no effect on the area of ankyrin-g or PSD95 clusters (Figure 3L). Together, these data suggest a distinct role for ankyrin-g at synapses that differs from classical scaffolding proteins such as PSD95; ankyrin-g is found in discrete nanodomains that are located both perisynaptically and in the spine neck. Moreover, the number of nanodomains in the spine head or associated with the PSD is correlated with both spine head size and PSD size, with the additional presence of ankyrin-g in the neck associated with larger spine heads. A Short Form of Ankyrin-G Is Localized to Spines There are multiple isoforms of ankyrin-g in the brain, with two of the most prevalent being the 190 and 270 kda isoforms (Figure 4A [Bennett and Healy, 2009]). The 270 kda isoform is restricted to axons due to the presence of a serine-rich domain and a long, C-terminal tail (Zhang and Bennett, 1998); however, the shorter 190 kda isoform lacks these domains. Given our data showing endogenous ankyrin-g at the PSD and the prevalence of the 190 kda isoform in the rat frontal cortex (Figure S4A), we hypothesized that the short isoform might be localized to spines. Consistent with this idea, synaptoneurosome preparations from rat cortex revealed significant enrichment of the 190 kda isoform of ankyrin-g in synaptic lysate (Figure 4B). We expressed GFP-tagged 190 kda and 270 kda ankyrin-g in neurons and used confocal imaging to determine their localization (Figure 4C). Expression of GFPAnkG270 was restricted to the axon and soma with low expression in dendrites. In comparison, GFPAnkG190 was expressed in the AIS, soma, throughout the dendritic tree, and in spines (Figure 4C, inset). Quantification of ankyrin-g abundance in axonal and dendritic compartments showed equal intensity of GFPAnkG190 in both compartments, compared with GFPAnkG270, which was far more localized to the axon (**p = ; Figure 4D). Quantification of the fluorescence intensity of both isoforms in spines revealed 60% less spine localization of GFPAnkG270 compared with GFPAnkG190 and a reduced spine:shaft ratio (***p < ; Figures 4E and S4B), suggesting that the 190 kda isoform is localized at synaptic sites in cortical neurons. Ankyrin-G Isoforms Differentially Regulate Spine Morphology The differential distribution of ankyrin-g isoforms suggests that they may have distinct effects on spine morphology. We expressed GFPAnkG190 and GFPAnkG270 isoforms with mcherry in neurons and imaged them with confocal microscopy (Figure 4F). Overexpression of GFPAnkG190 caused a 24% increase in spine area compared with control (**p < , one-way ANOVA; Figure 4G), contributed by both an increase in spine length and head width and no effect on spine density (Figure S4C), suggesting that at this mature time point, ankyrin-g190 is primarily modulating the maintenance of existing spines. Conversely, overexpression of GFPAnkG270 caused a 22% decrease in spine area (*p = ; Figures 4F and 4G). To confirm a specific role for 190 kda ankyrin-g in spine maintenance and growth, we coexpressed ankyrin-g RNAi with either RNAi-resistant AnkG190 or AnkG270 constructs and assessed the ability of each to rescue the ankyrin-g knockdown phenotype. Confocal imaging revealed that RNAi-resistant constructs had the expected expression patterns (Figures S4D and S4E). RNAi-resistant GFPAnkG190 completely rescued the effects of RNAi knockdown; however, coexpression of the GFPAnkG270 RNAi-resistant construct augmented the effects of RNAi knockdown (similar to its overexpression phenotype), causing a 24% decrease in spine area (Figures 4H and 4I and Figure S4F). This supports our hypothesis that ankyrin-g190 is primarily responsible for the effects we observe on spine morphology. 190 kda Ankyrin-G Subspine Localization Impacts Spine Morphology We then utilized SIM to precisely localize GFPAnkG190 in spines. We imaged neurons expressing mcherry and GFPAnkG190 and found enrichment of the 190 kda isoform in spine heads and necks (Figure 5A). Linescans through the dendritic shaft and individual spines illustrate enrichment of GFPAnkG190 within the spine head (arrowheads) and peaks of fluorescence in the spine neck (open arrowheads). Within single spine heads, there are often multiple peaks of GFPAnkG190 fluorescence (Figure 5A, zoom), highlighted by linescans across the spine head (Figures 5B and 5C). SIM imaging of neurons expressing GFPAnkG190 and immunostained for PSD95 showed that GFPAnkG190 surrounds the central PSD rather than completely overlapping with it (Figure S5A), supporting a perisynaptic function for AnkG190 in the spine head. Given the correlations between endogenous ankyrin-g localization in the head and neck and spine geometry we hypothesized that AnkG190 overexpression in these spine regions may impact spine morphology. We analyzed neurons overexpressing GFPAnkG190 and found that the presence of GFPAnkG190 anywhere in the spine causes enlargement of head and neck width in mushroom spines compared to spines without GFPAnkG190 or spines from neurons expressing mcherry alone (**p = , **p = ; Figures 5D and 5E). This effect was specific to mushroom-type spines and not thin spines (Figures S5B and S5C), although the overexpression of GFPAnkG190 caused no changes in the abundance (G) Bar graph of spine area showing overexpression of GFPAnkG190 causes increased spine area compared with control, whereas overexpression of GFPAnkG270 causes a decreased spine area (0.485 ± mm 2 to ± mm 2 [AnkG190] or to ± mm 2 [AnkG270], ****p = , **p < 0.01, *p < 0.05, n = cells), data are represented as mean ± SEM. (H) Confocal images of cortical neurons transfected with control RNAi, ankyrin-g RNAi, ankyrin-g RNAi + 190rescue, or ankyrin-g RNAi + 270rescue. Scale bar = 5 mm. (I) Bar graphs showing decreased spine area in knockdown neurons compared with control, rescued by 190 kda but not 270 kda expression (***p = , **p < 0.01, *p < 0.05, n = cells), data are represented as mean ± SEM. See also Figure S4. Neuron 84, , October 22, 2014 ª2014 Elsevier Inc. 405
8 Neuron Ankyrin-G Nanodomains Regulate Dendritic Spines Figure kda Ankyrin-G Modulates Spine Head and Neck Width, Based on SIM Imaging (A) SIM image of neuron expressing GFPAnkG190. Scale bar = 2 mm. Box, spine in zoom; white lines, linescans in (B) and (C). Scale bar = 1 mm. (B and C) Linescans of ankyrin-g intensity from shaft through neck and head (B) or across spine head (C). (D and E) Bar graphs of mushroom spine head (D) and neck (E) widths on overexpression of or absence of GFPAnkG190 compared with mcherry control, (**p = , **p = , n = 131 spines), data are represented as mean ± SEM. (F) Bar graphs of spine head area and neck width on overexpression or absence of GFPAnkG190 in the head only, split by spine type (head area: ± mm 2 to ± mm 2, **p = , neck width: p = 0.367), data are represented as mean ± SEM. (G) Bar graphs of spine head area and neck width on overexpression or absence of GFPAnkG190 in the head and neck, split by spine type (head area: ± mm 2 to ± mm 2, **p < , neck width: ± mm to ± mm, ****p < ), data are represented as mean ± SEM. (H) Schematic diagram showing GFPAnkG190 overexpression in the head only causes increased spine head area (F). However, additional overexpression in the neck increases spine head enlargement and neck thickening (G). See also Figure S5. of mushroom spines (Figure S5D). We then analyzed the effects of the presence of GFPAnkG190 overexpression in specific regions on spine geometry. Overexpression of GFPAnkG190 in the head significantly increased head area by 59% in mushroom spines only (**p = , Mann-Whitney U; Figure 5F), with no effect on neck width. In spines where GFPAnkG190 was overexpressed in the neck in addition to the head, there was a larger 70% increase in head area and 23% increase in neck width (**p < , ****p < , Mann-Whitney U; Figure 5G), with no effect on thin spines. Therefore, the overexpression of GFPAnkG190 in mushroom but not thin spines causes changes in morphology, dependent on the location of GFPAnkG190; overexpression in the head only causes head enlargement, whereas additional overexpression in the neck causes further head enlargement and neck thickening (Figure 5H). b-spectrin Binding Is Essential for 190 kda Ankyrin-G Localization and Spine Morphology Ankyrin-G is known to be an essential link between the plasma membrane and the cytoskeleton (Bennett and Healy, 2009). We used STRING network analysis to identify candidate proteins that may act downstream of ankyrin-g to mediate the effects we observe on spines (Figure 6A). This revealed multiple membrane proteins but only one candidate likely to link ankyrin-g to the cytoskeleton, b-spectrin (SPTBN4). A binding partner and modulator of the actin cytoskeleton, b-spectrin has been shown to be present in spines (Ursitti et al., 2001). We hypothesized that the interaction between ankyrin-g and b-spectrin is required for ankyrin-g s function. We created GFPAnkGAAA that contains a mutation rendering it unable to bind b-spectrin (Figure 6B [Kizhatil et al., 2007]). This mutant displayed significantly reduced localization to spines compared with wild-type GFPAnkG190 (Figure 6C). Linescans of individual spines (Figure 6C, zooms) showed that enrichment of GFPAnkG190 in spine heads (*p < 0.05, **p < 0.01, two-way ANOVA; Figure 6D) is abolished in neurons expressing the GFPAnkGAAA (Figure 6E; *p % 0.05, **p < 0.01, ***p < 0.001, two-way ANOVA). Indeed, the mutant had a diffuse distribution with significantly higher expression in the dendritic 406 Neuron 84, , October 22, 2014 ª2014 Elsevier Inc.
9 Neuron Ankyrin-G Nanodomains Regulate Dendritic Spines Figure 6. b-spectrin Binding Is Crucial for Ankyrin-G Synaptic Targeting and Function (A) STRING analysis of ANK3 interactors. Red rectangle highlights ANK3-SPTBN4 (b-spectrin) interaction. Line colors represent types of evidence: pink, experimental; green, text mining; blue, databases; purple, homology. (B) Schematic showing the functional domains of 190 kda isoform of ankyrin-g and the 999 DAR/ AAA mutation that disrupts ankyrin-g binding to b-spectrin. (C) Confocal images of neurons expressing mcherry alone, +GFPAnkG190, or +GFPAnkGAAA. Scale bar = 5 mm. Zoom of spines with example linescan; arrowheads, AnkG in head or shaft. Scale bar = 1 mm. (D F) Linescan analysis of GFPAnkG190 and GFPAnkGAAA in spines cotransfected with mcherry, *p < 0.05, **p < 0.01, ***p < 0.001, n = 7 12 cells, data are represented as mean ± SEM. (G) Spine:shaft ratios of GFPAnkG fluorescence intensity show a decrease in ratio for GFPAnkGAAA compared to GFPAnkG190 (**p = , n = 6 cells), data are represented as mean ± SEM. (H) Bar graph of effects of GFPAnkG190 and GFPAnkGAAA overexpression on spine area (**p < 0.01, *p < 0.01, n = cells), data are represented as mean ± SEM. (I) Bar graph of spine areas in the presence or absence of GFPAnkG190 or GFPAnkGAAA (***p < 0.001, n = cells), data are represented as mean ± SEM. (J) Bar graph of percentage of spine enlargement in the presence or absence of GFPAnkG190/AAA (*p = , n = cells), data are represented as mean ± SEM. (K) Confocal images of neurons expressing GFP, GFPAnkG190, or GFPAnkGAAA and immunostained for GluA1. Scale bar = 5 mm. (L) Bar graph of GluA1 cluster area quantification (0.216 ± to ± 0.009, *p = 0.03, n = cells), data are represented as mean ± SEM. See also Figure S6. shaft and collection at the base of spines (*p % 0.05, **p < 0.01, ***p < 0.001, two-way ANOVA; Figure 6F). The spine to shaft ratio of GFPAnkGAAA was reduced significantly by 36% (Figure 6G) and more than 60% of spines had a spine to shaft ratio of 0.5 or less (Figure S6A), indicative of enrichment in the dendritic shaft rather than in the spine head. GFPAnkGAAA expression caused a decrease in spine area, contrary to the increase seen with GFPAnkG190 overexpression (**p < , *p < 0.001, p < 0.05, one-way ANOVA; Figure 6H). The interaction between ankyrin-g and b-spectrin therefore is critical for targeting ankyrin-g to spines in cortical neurons. We also asked whether the interaction between ankyrin-g and b-spectrin was important for spine enlargement. We analyzed the area of spines imaged by confocal microscopy and categorized by the presence or absence of GFPAnkG190 or GFPAnkGAAA. Spines containing GFPAnkG190 were 47% larger than spines without (***p < 0.001, one-way ANOVA; Figure 6I) but there was no difference between the areas of spines containing GFPAnkGAAA compared with spines without (p = n.s., one-way ANOVA). Spines with GFPAnkG190 demonstrated 32% enlargement compared to spines containing GFPAnkGAAA, which only showed 20% Neuron 84, , October 22, 2014 ª2014 Elsevier Inc. 407
10 Neuron Ankyrin-G Nanodomains Regulate Dendritic Spines Figure 7. Ankyrin-G Confines AMPARs to Spines (A) Confocal images of control or knockdown neurons immunostained for GluA1 (red). Arrowheads, GluA1 in spines. Scale bar = 5 mm. (B and C) Bar graphs showing quantification of GluA1 spine content (B) and cluster area (C) (from ± mm 2 to ± mm 2, *p = 0.011, **p = , n = cells), data are represented as mean ± SEM. (legend continued on next page) 408 Neuron 84, , October 22, 2014 ª2014 Elsevier Inc.
11 Neuron Ankyrin-G Nanodomains Regulate Dendritic Spines enlargement (*p = , t test; Figure 6J). Likewise, in SIM analysis, GFPAnkGAAA overexpression abolished the effects of GFPAnkG190 overexpression on mushroom spines (Figure 5G). Neither head area nor neck width was increased, even with GFPAnkGAAA in both the head and neck (Figure S6C). Even when GFPAnkGAAA is targeted to spines, its ability to increase spine size is compromised, showing that the ability of ankyrin-g to bind b-spectrin is not only important for ankyrin-g targeting but also for mediating changes in morphology. We then tested the impact of 190 kda ankyrin-g on AMPAR clusters by analyzing GluA1 cluster area in neurons transfected with GFPAnkG190 or GFPAnkGAAA. We found a 17% increase of cluster area with expression of GFPAnkG190, which was abolished with GFPAnkGAAA (*p = 0.031, one-way ANOVA; Figures 6K and 6L), further pointing to the necessity of the ankyrin-gb-spectrin interaction for correct spine morphology and AMPAR clustering. Ankyrin-G Regulates AMPARs in Dendritic Spines Our electrophysiological experiments show that ankyrin-g knockdown reduces mepsc amplitude (Figures 1A 1C), suggesting reduction in synaptic AMPARs. We were able to rescue this phenotype by coexpression of RNAi-resistant AnkG190 (Figures S7A S7C), suggesting that synaptic ankyrin-g may play a role in AMPAR regulation in spines. Confocal imaging of neurons costained for ankyrin-g and GluA1 revealed that 60% of ankyrin-g puncta colocalized with GluA1 puncta (Figures S7D and S7E). To test whether ankyrin-g modulates GluA1 clustering in spines, we immunostained ankyrin-g RNAi-transfected neurons for GluA1, followed by confocal imaging (Figure 7A). Ankyrin-G knockdown generated a 38% reduction in GluA1 intensity in the spine and a 22% reduction in GluA1 cluster area (intensity: *p = , area: *p = 0.045, t test, Figures 7B and 7C), pointing to a mechanism where ankyrin-g knockdown reduces GluA1 levels in spines and accounts for the reduced mepsc amplitude. To understand the mechanism underlying regulation of AMPAR by ankyrin-g, we used SIM to examine subsynaptic localization with GluA1. Ankyrin-G nanodomains were partially overlapping with and around GluA1 clusters (Figure 7D and Figure S7F). Indeed, the mean distance between the center of GluA1 and ankyrin-g was ± mm, very similar to that of PSD95 and ankyrin-g, supporting a perisynaptic localization for ankyrin-g. This partial colocalization suggests that ankyrin-g may interact with AMPARs. To test this in vivo, we performed coimmunoprecipitation experiments from rat cortical lysate, showing that ankyrin-g interacts in a complex with GluA1 and b-spectrin (Figures 7E and 7F). A significant fraction of ankyrin-g in spines did not colocalize with GluA1, suggesting other potential mechanisms of regulation. GluA1 cluster size was not correlated with ankyrin-g nanodomain area or number in the spine head (Figures 7G and 7H). However, in spines where ankyrin-g was present in the neck in addition to the head, the mean GluA1 cluster area was significantly increased by 38% (*p = 0.033, t test, Figure 7I), whereas the PSD95 area was independent of the presence of ankyrin-g in the neck (p = 0.350) and solely dependent on ankyrin-g in the head. To assess the effects of ankyrin-g presence in the neck in addition to the head on GluA1 clusters, we imaged neurons overexpressing GFPAnkG190 and immunostained for GluA1 (Figure 7J). Overexpression of GFPAnkG190 in the head and neck caused a significant increase in GluA1 cluster area, whereas overexpression in the head alone did not have significant effects (p = 0.004, one-way ANOVA; Figures 7J and 7K). If ankyrin-g contributes to scaffolding or retention of AMPARs in spines, we would expect alterations in AMPAR surface dynamics upon manipulation of ankyrin-g expression. We performed fluorescence recovery after photobleaching (FRAP) of GluA1-SEP clusters to measure AMPAR mobility in neurons overexpressing AnkG190. Overexpression of AnkG190 reduced the amount of GluA1-SEP fluorescence recovery in spines compared with control neurons (Figures 7L and 7M), and calculation of the total mobile fraction of GluA1-SEP in each condition revealed a 39% decrease in GluA1-SEP mobility in AnkG190- overexpressing neurons compared with control (p = 0.072, t test; Figure 7N). This suggests ankyrin-g overexpression in spines increases AMPAR stability. Ankyrin-G Contributes to the Regulation of Chemical LTP-Induced Spine Plasticity Due to the effects that manipulation of ankyrin-g expression had on spine morphology and function, we hypothesized that ankyrin-g might play a role in activity-dependent spine plasticity. We used a well-characterized chemical LTP (cltp) protocol shown to cause rapid activation of NMDA receptors, causing increased spine area and density (Chung et al., 2004; Liao (D) SIM images of a spine from a GFP-expressing neuron immunostained for ankyrin-g (red) and GluA1 (blue). Scale bar = 0.2 mm. Closed arrowheads, spine head nanodomains; open arrowheads, neck nanodomains. White in bottom left panel, colocalization highlighter. Last two panels show ratiometric images of ankyrin-g and GluA1 localization, respectively. (E and F) Western blots of coimmunoprecipitation experiments of ankyrin-g with GluA1 and spectrin from rat cortex. migg, control mouse IgG; IP, immunoprecipitation. (G and H) Correlation plots of nanodomain area (G) and number in the spine head (H) versus GluA1 puncta area. (I) Bar graph showing GluA1 and PSD95 puncta area in spines with ankyrin-g in the head only or in the head and neck, *p = 0.033, data are represented as mean ± SEM. (J) SIM image of spine from GFPAnkG190-expressing neuron immunostained for GluA1. Scale bar = 0.2 mm. (K) Bar graph showing GluA1 puncta area in spines with GFPAnkG190 overexpressed in the head only or in the head and neck (*p = 0.04, n = 75 spines), data are represented as mean ± SEM. (L) Representative time lapse of GluA1-SEP fluorescence recovery in control or AnkG190 overexpressing neurons in FRAP experiment. Scale bar = 1 mm. (M) Quantification of GluA1-SEP fluorescence cluster intensity over time. Data are fitted with single exponentials (colored lines); data are represented as mean ± SEM. (N) Bar graph of mobile fraction in control and AnkG190-overexpressing neurons (60.95% ± 5.0% to 37.26% ± 4.3%, **p = , n = 10 cells), data are represented as mean ± SEM. See also Figure S7. Neuron 84, , October 22, 2014 ª2014 Elsevier Inc. 409
12 Neuron Ankyrin-G Nanodomains Regulate Dendritic Spines (legend on next page) 410 Neuron 84, , October 22, 2014 ª2014 Elsevier Inc.
13 Neuron Ankyrin-G Nanodomains Regulate Dendritic Spines et al., 2001; Xie et al., 2007). We performed cltp with control or knockdown neurons, imaged them by confocal microscopy, and analyzed spine area and density (Figures 8A 8C). We observed significant increases in spine size (27%) and density (25%) (area: **p = , density: *p = 0.023, t tests; Figures 8B and 8C), although in ankyrin-g knockdown neurons, there were no such increases after cltp treatment (area: p = 0.710, density: p = 0.663, t tests; Figures 8B and 8C), suggesting that ankyrin- G knockdown occludes the structural changes induced by cltp. Ankyrin-G Accumulates in Synapses during cltp We next asked how ankyrin-g might be involved in spine enlargement during cltp. We costained GFP-transfected neurons for ankyrin-g and imaged them by confocal microscopy (Figure 8D). Ankyrin-G accumulated in spines after cltp treatment, characterized by an increase in the ankyrin-g spine-toshaft ratio (**p = , Mann-Whitney U; Figure 8E). We used SIM to assess changes in distribution of ankyrin-g nanodomains after cltp (Figure 8F). We again saw increases in spine head area (24%), width (16%), and length (12%) (area: **p = , width: *p = 0.018, length: *p = 0.047, Mann-Whitney U; Figure 8G; Figures S8A S8C), which corroborates recent superresolution studies (Tønnesen et al., 2014). The number of nanodomains in the spine head increased significantly after cltp treatment (Figure 8H), even when normalized to spine head area (Figure S8D), suggesting that the increase in ankyrin-g nanodomain number is not solely due to increased spine head size but that ankyrin-g is enriched in the spine head after cltp. Correlations between spine head area and ankyrin-g nanodomain number were similar for both control and cltp conditions (Figures S8E and S8F). Spine head enlargement after cltp correlated significantly with the number of ankyrin-g nanodomains in the spine head, with no enlargement in spines lacking ankyrin-g (Figure 8I), underlining the potential importance of ankyrin-g in this process. Spine neck width increased by 20% after cltp treatment (***p < , Mann-Whitney U, Figure 8J), with no change in spine neck length (p = 0.303, Mann-Whitney U, Figure S8G). Interestingly, spines with ankyrin-g in their neck in addition to their head did not undergo cltp-dependent spine head enlargement (Figure 8K), potentially due to the fact that this subset of spines already has large head areas that might not be able to expand further. Presence of ankyrin-g in the spine neck had no impact on the effect of cltp on spine neck width (Figure 8L), supporting a role for ankyrin-g in regulating spine neck width in basal and active conditions. SIM imaging of neurons immunostained for ankyrin-g and GluA1 showed a significant increase in overlap between ankyrin-g nanoclusters and GluA1 (from 8% to 23%, ***p = , Figures 8M and 8N), suggesting that ankyrin-g moves to overlap more fully with AMPARs during cltp. Increased Association of Ankyrin-G with the Plasma Membrane and Cytoskeleton In Vivo To determine whether AnkG190 translocates in vivo in response to a physiological learning paradigm, we analyzed subcellular fractions from the hippocampus of mice subjected to fear conditioning, a well-characterized model of learning and memory. AnkG190 was enriched in the membrane (84%) and cytoskeletal fractions (60%) from trained compared with naive mice but not in the cytosolic fraction (cytoskeleton: *p = 0.045, membrane: **p = , cytosol: p = 0.488, t tests, Figures 8O 8Q). These data suggest that ankyrin-g has greater association with both the cell membrane and cytoskeleton following a learning task in mice. DISCUSSION ANK3 (ankyrin-g) is emerging as a shared risk factor for multiple psychiatric disorders including SZ, ASD, ID, and BD Figure 8. Ankyrin-G in Dendritic Spines Is Required for LTP (A) Confocal images of control or knockdown neurons treated with control or chemical LTP (cltp) protocol. Scale bar = 5 mm. (B and C) Bar graphs of spine areas (B) and spine density (C) in control or cltp conditions, in control, or knockdown neurons (**p = , *p = 0.023, n = cells), data are represented as mean ± SEM. (D) Confocal images of control and cltp treated cortical neurons expressing GFP and costained with ankyrin-g (red). Arrowheads, ankyrin-g puncta. Scale bar = 5 mm. (E) Quantification of spine:shaft ratio of ankyrin-g after treatment with cltp protocol (**p < 0.01, n = cells), data are represented as mean ± SEM. (F) SIM images of spines from GFP-expressing cortical neurons stained for ankyrin-g (red) in control or cltp conditions. Scale bar = 0.25 mm. (G) Bar graph showing increased spine head area in cltp versus control, based on SIM images (0.530 ± mm 2 to ± mm 2, **p = , n = spines), data are represented as mean ± SEM. (H) Bar graph showing increased nanodomain number in spine heads after cltp, based on SIM imaging, ***p < , n = spines, data are represented as mean ± SEM. (I) Graph of correlation between change in spine head area of cltp spines compared with mean control spine area and number of ankyrin-g nanodomains in the spine head, as determined by immunostaining for ankyrin-g subsequent to cltp. ***p < , Spearman correlation = (J) Bar graph showing increased spine neck width after cltp treatment compared with control, based on SIM images (from ± mm to ± mm, ***p < , n = spine necks), data are represented as mean ± SEM. (K) Bar graph of spine head area after cltp treatment in spines with/without ankyrin-g in their necks, based on SIM images (*p = 0.037, n = spines), data are represented as mean ± SEM. (L) Bar graph of spine neck width after cltp treatment in spines with/without ankyrin-g in their necks, based on SIM images (**p = 0.009, **p = 0.007, n = spines), data are represented as mean ± SEM. (M) SIM images of control or cltp treated neurons immunostained for ankyrin-g (red) and GluA1 (green). Scale bar = 2 mm. (N) Bar graph showing increased overlap of ankyrin-g nanodomains with GluA1 clusters after cltp (***p = , n = 5 cells), data are represented as mean ± SEM. (O Q) Analysis of ankyrin-g distribution in hippocampal cytoskeletal (P), membrane (Q), and cytosolic (R) subcellular fractions from naive or fear-conditioned mice. Western blots: upper blots are probed with ankyrin-g and lower blots are probed with actin (*p = 0.045, **p = , n = 5 mice), data are represented as mean ± SEM. See also Figure S8. Neuron 84, , October 22, 2014 ª2014 Elsevier Inc. 411
14 Neuron Ankyrin-G Nanodomains Regulate Dendritic Spines (Bi et al., 2012; Ferreira et al., 2008; Iqbal et al., 2013; Schulze et al., 2009). These disorders are unified by glutamatergic synapse pathology, yet little is known about potential functions of ankyrin-g in spines. Roles for ankyrin-g at other types of synapses have begun to emerge: organizing GABAergic synapses at the AIS (Huang et al., 2010) and stabilizing presynaptic structure at the Drosophila NMJ (Pielage et al., 2008). Our data support a model (Figures S8H S8J) whereby ankyrin-g functions at glutamatergic synapses and thereby may contribute to disease pathogenesis. Superresolution microscopy identified ankyrin-g nanodomains localized perisynaptically in the spine head and also in the spine neck. These nanodomains contribute to AMPAR-mediated synaptic transmission by controlling AMPAR cluster stability, spine head size, neck width, and cltp-dependent spine enlargement. Furthermore, stimuli that induce plasticity or learning in vivo induce accumulation of ankyrin-g to synapses, the cytoskeleton, and membranes, thus supporting a role for ankyrin-g in synaptic plasticity and a mechanism by which the glutamatergic synaptic pathology may be mediated in neuropsychiatric disorders. Superresolution Imaging Identifies Subsynaptic Ankyrin-G Nanodomains Confocal imaging indicated the presence of ankyrin-g in spines and its colocalization with glutamatergic synaptic markers; however, the resolution of this technique does not reveal subsynaptic detail. The advent of superresolution microscopy has facilitated imaging beyond the diffraction limit of light, providing unprecedented insight into subcellular architecture (Tønnesen and Nägerl, 2013). Several recent studies have used these techniques to reveal that AMPARs, PSD95, actin, and CaMKII are localized to subsynaptic domains (Frost et al., 2010; Lu et al., 2014; MacGillavry et al., 2013; Nair et al., 2013). Superresolution approaches have not yet been used to investigate synaptic function of newly identified psychiatric risk genes. Our measurements of spine geometry derived from SIM images are consistent with other reports using superresolution microscopy (Tønnesen et al., 2014), and we observe very similar changes in spine neck width during LTP, underlining the accuracy of our imaging technique. Ankyrin-G forms nanodomains of 130 nm in diameter, three times smaller than the PSD itself, and organizes perisynaptically and in the spine neck. This is in contrast to classical PSD scaffolds that are large heterogeneous platforms (MacGillavry et al., 2011). This difference is highlighted by the observation that ankyrin-g nanodomain number but not area correlates with spine head size, suggesting that they are discrete entities and do not multimerize. Ankyrin-G nanodomains were not located directly within the PSD, but surrounding the PSD, or within the spine neck away from the synapse. PALM imaging has recently identified a similar localization for CamKII, a key protein involved in synaptic plasticity (Lu et al., 2014). Our SIM localization data suggest a function for ankyrin-g that differs from a classical PSD scaffolding protein. Ankyrin-G Nanodomain Function in Spines At the AIS, ankyrin-g is known to mediate recruitment and organization of molecules, but how ankyrin-g may regulate the structure and function of glutamatergic synapses is unknown. We show that b-spectrin is in a complex with ankyrin-g and AMPARs. b-spectrin is a component of the submembranous cytoskeleton, previously reported to interact with ankyrin-g (Bennett and Healy, 2009), is abundant in dendrites and spines (Ursitti et al., 2001), and has been shown to regulate spine morphology by bundling actin filaments (Nestor et al., 2011). By introducing point mutations known to abolish ankyrin-g s interaction with b-spectrin, we prevented targeting of ankyrin- G to spines and caused a reduction in spine size. This mutation also abolishes the effect of AnkG190 overexpression on spine head area, neck width, and GluA1 cluster area, pointing to b-spectrin being a major mediator of ankyrin-g function in spines. This is similar to interactions at the Drosophila NMJ, where b-spectrin recruits ankyrin2 and is responsible for the structure and organization of the synapse (Pielage et al., 2006). These results indicate that interaction with the cytoskeleton, through b-spectrin, is important for both localization of ankyrin- G to spines and its function at synapses. At the nanoscale, ankyrin-g showed only partial overlap with PSD95 with a mean distance of 378 nm between the center of PSD95 puncta and the surrounding ankyrin-g nanodomains. This perisynaptic position of ankyrin-g corresponds with the pool of F-actin that resides 300 nm from the PSD center (Frost et al., 2010), placing ankyrin-g in an ideal position to modulate this pool of actin. Indeed, the morphology of the PSD is dynamically regulated by the actin cytoskeleton that surrounds it (Blanpied et al., 2008) and this can have direct effects on clustering of AMPARs and efficacy of synaptic transmission (MacGillavry et al., 2013). We found that spine head and PSD size correlated with the number of ankyrin-g puncta in the spine head, suggesting that ankyrin-g may link to the actin cytoskeleton and be important for modulation of the PSD. Ankyrin-G nanodomains also partially overlap with GluA1 puncta in spines, showing perisynaptic localization based on SIM. Coimmunoprecipitation experiments indicate that ankyrin-g, AMPARs, and b-spectrin participate in a common complex, supporting our model in which ankyrin-g links a perisynaptic pool of AMPARs to the b-spectrin-actin cytoskeleton, therefore stabilizing these receptors. Our FRAP data showed that overexpression of AnkG190 caused a significant reduction in AMPAR mobility in spines, further supporting the function of ankyrin-g as an additional synaptic scaffold. The interaction between ankyrin-g and GluA1 is likely to be indirect and could be mediated by multiple proteins, such as the accessory protein stargazin, which mediates the interaction between AMPARs and PSD95 (Bats et al., 2007) and the b-spectrin-actin cytoskeleton adaptor protein 4.1N, which regulates the exocytosis of GluA1-AMPARS (Lin et al., 2009). It is also important to emphasize that ankyrin-g may be mediating these effects by altering the actin cytoskeleton, which could cause changes in receptor scaffolding, trafficking, and other related indirect mechanisms. In addition to their perisynaptic localization, ankyrin-g nanodomains were present in the neck of 74% of spines analyzed. Localization in spine neck is particularly intriguing, as it has been reported for few other proteins including DARPP-32 (Blom et al., 2013), septin 7 (Tada et al., 2007), and synaptopodin 412 Neuron 84, , October 22, 2014 ª2014 Elsevier Inc.
15 Neuron Ankyrin-G Nanodomains Regulate Dendritic Spines (Deller et al., 2000). At the AIS, ankyrin-g acts as a barrier to limit the lateral mobility of ion channels and protein diffusion (Rasband, 2010). The additional presence of ankyrin-g in the spine neck correlated with increased size of AMPAR clusters in the spine head, but not the size of the PSD, again suggesting a role for modulating the abundance of dynamic, diffusive proteins, rather than the relatively static PSD. Ankyrin-G may act as a barrier in the spine neck, limiting the mobility of GluA1 out of the spine, promoting the retention of AMPARs in the spine head and thereby maintaining synaptic function. This proposed mechanism is supported by experimental data and mathematical models that indicate that the spine neck plays an essential role in limiting diffusion (Ashby et al., 2006; Kusters et al., 2013; Simon et al., 2014). Ankyrin-G Regulates Spine Neck Width Due to previous resolution limitations, proteins that affect neck morphology have not been extensively studied. Our GFPAnkG190 overexpression experiments show that ankyrin- G can alter spine neck width in addition to head size; when overexpressed in the spine head, only spine head area increased; however, when overexpressed in the spine head and neck concurrently, head area increased even more and was accompanied by neck thickening. Spine neck changes have recently been shown to be a critical parameter of spine plasticity (Tønnesen et al., 2014), and it is emerging that spine necks are essential for myriad spine functions, including modulating AMPAR diffusion (Ashby et al., 2006; Kusters et al., 2013; Simon et al., 2014), compartmentalization of signaling (Grunditz et al., 2008; Murakoshi et al., 2011; Noguchi et al., 2005), and regulating diffusion across the neck during activity (Bloodgood and Sabatini, 2005). The presence of the leading psychiatric risk gene ankyrin-g within the spine neck places it at a critical crossroads for influencing synaptic functions. Ankyrin-G Contributes to cltp-dependent Plasticity in Spines Knockdown of ankyrin-g impairs cltp-dependent enlargement of spines, suggesting that ankyrin-g contributes to structural changes during cltp. Neuronal activity caused accumulation of ankyrin-g in spines and SIM revealed that this was due to an increase in number, but not size, of ankyrin-g nanoclusters, indicating protein enrichment. Considering our data showing ankyrin-g overexpression in spines causes increased spine area, we propose that cltp-dependent accumulation of ankyrin-g in spines is likely to play a key role in spine enlargement during synaptic plasticity. During this early stage of cltp, rapid polymerization of actin and activation of CamKII occurs with specific proteins being trafficked into the spine, including AMPARs and actin remodeling proteins (Sala and Segal, 2014). This, combined with the location of ankyrin-g nanodomains at regulatory sites for plasticity (Cingolani and Goda, 2008), suggests that ankyrin-g may play a role in the modulation of cltp. SIM showed that colocalization of ankyrin-g nanoclusters with GluA1 also increases after cltp, indicative of increased participation in multiprotein complexes that may stabilize AMPARs at synapses. If ankyrin-g is an additional scaffold in the spine, it might be involved in localization and support of multiple membrane proteins including ion channels and NMDARs, which are essential for cltp induction and expression. Further work will be required to dissect the exact role of ankyrin-g in this process. In support of our findings in cultured neurons, analysis of hippocampal lysates from mice that had undergone a learning task demonstrated significantly more ankyrin-g in the membrane and cytoskeletal fractions in trained mice. These data show that a physiological learning paradigm can cause relocation of a pool of ankyrin-g, supporting the validity of our cellular findings and pointing to a mechanism involving ankyrin-g in learning and memory. This is also supported by recent data showing ankyrin-g knockdown in Drosophila causes shortterm memory defects (Iqbal et al., 2013) and suggests that roles of ankyrin-g in learning and memory are worthy of further investigation. Role for Ankyrin-G 190 kda Isoform in Synapse Maintenance and Plasticity The longer isoforms of ankyrin-g have well-defined functions at the AIS and NoR (Rasband, 2010); however, our data indicate a role for 190 kda ankyrin-g in the maintenance and function of mature cortical synapses. This isoform has been identified in PSDs (Nanavati et al., 2011), and our synaptoneurosome preparations corroborate an enrichment of this isoform at the synapse. GFPAnkG190 was enriched at postsynaptic sites, compared with GFPAnkG270, which was restricted to the AIS and SIM revealed that GFPAnkG190 overexpression yielded morphological changes in mushroom, but not thin spines. We show that knockdown of ankyrin-g in mature neurons caused reduced mepsc amplitude and spine area. However, these effects on spines were rescued by RNAi-resistant AnkG190 but not AnkG270. Together, our overexpression and rescue experiments and the enrichment of the 190 kda ankyrin-g isoform in spines suggest that this isoform mediates morphological effects on dendritic spines. Ankyrin-G and Synaptic Pathology in Psychiatric Disorders Though linked to SZ, ASD, and ID, ANK3 is most strongly linked to BD where glutamatergic synaptic pathology has been less extensively investigated. A previous study reported that 190 kda ankyrin-g becomes specifically enriched in the PSD upon treatment with lithium, a common drug used to BD (Nanavati et al., 2011). We show that cltp increases ankyrin-g in spines, in a similar manner to lithium treatment. Lithium has been shown to enhance LTP (Voytovych et al., 2012), suggesting that ankyrin-g in dendritic spines may be at the convergence between plasticity and therapeutic pathways relevant for BD. Our findings show that Ankyrin-G forms nanostructures within synapses, both in the spine neck and head. While spine size and density have been investigated, other parameters such as spine neck length and width could be relevant for pathogenesis as in an animal model of Rett syndrome (Belichenko et al., 2009). This stresses the importance of examining disease-relevant molecules at higher resolution to provide clues as to their specialized functions and role in pathogenesis. Human neuropathological studies using superresolution microscopy would provide invaluable insight into pathogenesis. Our study suggests Neuron 84, , October 22, 2014 ª2014 Elsevier Inc. 413
16 Neuron Ankyrin-G Nanodomains Regulate Dendritic Spines that understanding how subsynaptic phenotypes are modulated by molecular pathways, and how these phenotypes (including nanoscale localization of molecules) are affected in psychiatric disorders, may allow for more accurate mapping of diseaserelevant genes and pathways onto specific elements of subcellular architecture. EXPERIMENTAL PROCEDURES Cell Culture Dissociated cultures of primary cortical neurons were prepared from embryonic day 18 Sprague-Dawley rat embryos as described previously (Srivastava et al., 2012). Immunocytochemistry and Confocal Microscopy Neurons were fixed, stained, imaged, and quantified as previously described (Srivastava et al., 2012). Structured Illumination Microscopy and Analysis Multichannel structured illumination microscopy (SIM) images were acquired using a Nikon Structured Illumination superresolution microscope using a NA objective and reconstructed using Nikon Elements software. Single plane or z stack (z = 0.2 mm) images were processed and analyzed using Nikon Elements software, MetaMorph, and ImageJ. Single-spine analyses were carried out on 100 spines across five neurons per condition. Electrophysiology Cultured cortical neurons were recorded in whole-cell configuration 3 4 days posttransfection (24 25 days in vitro) as described previously (Srivastava et al., 2012). SUPPLEMENTAL INFORMATION Supplemental Information includes Supplemental Experimental Procedures, eight figures, and one movie and can be found with this article online at AUTHOR CONTRIBUTIONS K.R.S. performed and analyzed confocal imaging, electrophysiology, and some SIM experiments, led the project, and wrote the paper. K.J.K. performed SIM imaging, analysis, and wrote the paper. J.F.P. performed molecular biology. R.G., B.S., and K.M. performed biochemistry. K.L. and J.R. performed behavioral and fractionation experiments. G.T.S. supervised electrophysiology and advised on the project. P.P. supervised the project and wrote the paper. ACKNOWLEDGMENTS This work was supported by R01MH071316, R01MH to P.P., R01NS to G.T.S., R01MH to J.R., and a Marie Curie Outgoing Postdoctoral Fellowship (302281) to K.R.S. We are grateful to Bryan Copits and Claire Vernon for assistance with electrophysiology, Tristan Hedrick for help with brain slicing, Vann Bennett for the goat ankyrin-g Ab, and Karen Zito, Jubao Duan, and members of the P.P. lab for helpful discussions. We thank NU Nikon Cell Imaging Facility for use of the N-SIM and spinning-disc confocal and Teng Leong Chew, Constadina Arvanitis, and Joshua Rappoport for assistance with imaging and analysis. All experiments involving animals were performed according to the Institutional Animal Care and Use Committee of NU. Accepted: October 2, 2014 Published: October 22, 2014 REFERENCES Ashby, M.C., Maier, S.R., Nishimune, A., and Henley, J.M. (2006). Lateral diffusion drives constitutive exchange of AMPA receptors at dendritic spines and is regulated by spine morphology. J. Neurosci. 26, Bats, C., Groc, L., and Choquet, D. (2007). The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron 53, Belichenko, P.V., Wright, E.E., Belichenko, N.P., Masliah, E., Li, H.H., Mobley, W.C., and Francke, U. (2009). Widespread changes in dendritic and axonal morphology in Mecp2-mutant mouse models of Rett syndrome: evidence for disruption of neuronal networks. J. Comp. Neurol. 514, Bennett, V., and Healy, J. (2009). Membrane domains based on ankyrin and spectrin associated with cell-cell interactions. Cold Spring Harb. Perspect. Biol. 1, a Bi, C., Wu, J., Jiang, T., Liu, Q., Cai, W., Yu, P., Cai, T., Zhao, M., Jiang, Y.H., and Sun, Z.S. (2012). Mutations of ANK3 identified by exome sequencing are associated with autism susceptibility. Hum. Mutat. 33, Blanpied, T.A., Kerr, J.M., and Ehlers, M.D. (2008). Structural plasticity with preserved topology in the postsynaptic protein network. Proc. Natl. Acad. Sci. USA 105, Blom, H., Rönnlund, D., Scott, L., Westin, L., Widengren, J., Aperia, A., and Brismar, H. (2013). Spatial distribution of DARPP-32 in dendritic spines. PLoS ONE 8, e Bloodgood, B.L., and Sabatini, B.L. (2005). Neuronal activity regulates diffusion across the neck of dendritic spines. Science 310, Bosch, M., and Hayashi, Y. (2012). Structural plasticity of dendritic spines. Curr. Opin. Neurobiol. 22, Chen, Y., and Sabatini, B.L. (2012). Signaling in dendritic spines and spine microdomains. Curr. Opin. Neurobiol. 22, Chung, H.J., Huang, Y.H., Lau, L.F., and Huganir, R.L. (2004). Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J. Neurosci. 24, Cingolani, L.A., and Goda, Y. (2008). Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci. 9, Collins, M.O., Husi, H., Yu, L., Brandon, J.M., Anderson, C.N., Blackstock, W.P., Choudhary, J.S., and Grant, S.G. (2006). Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J. Neurochem. 97 (Suppl 1 ), Deller, T., Merten, T., Roth, S.U., Mundel, P., and Frotscher, M. (2000). Actin-associated protein synaptopodin in the rat hippocampal formation: localization in the spine neck and close association with the spine apparatus of principal neurons. J. Comp. Neurol. 418, Ferreira, M.A., O Donovan, M.C., Meng, Y.A., Jones, I.R., Ruderfer, D.M., Jones, L., Fan, J., Kirov, G., Perlis, R.H., Green, E.K., et al.; Wellcome Trust Case Control Consortium (2008). Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat. Genet. 40, Frost, N.A., Shroff, H., Kong, H., Betzig, E., and Blanpied, T.A. (2010). Singlemolecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron 67, Gilman, S.R., Iossifov, I., Levy, D., Ronemus, M., Wigler, M., and Vitkup, D. (2011). Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron 70, Grunditz, A., Holbro, N., Tian, L., Zuo, Y., and Oertner, T.G. (2008). Spine neck plasticity controls postsynaptic calcium signals through electrical compartmentalization. J. Neurosci. 28, Gustafsson, M.G. (2005). Nonlinear structured-illumination microscopy: wide-field fluorescence imaging with theoretically unlimited resolution. Proc. Natl. Acad. Sci. USA 102, Huang, B., Babcock, H., and Zhuang, X. (2010). Breaking the diffraction barrier: super-resolution imaging of cells. Cell 143, Neuron 84, , October 22, 2014 ª2014 Elsevier Inc.
17 Neuron Ankyrin-G Nanodomains Regulate Dendritic Spines Iqbal, Z., Vandeweyer, G., van der Voet, M., Waryah, A.M., Zahoor, M.Y., Besseling, J.A., Roca, L.T., Vulto-van Silfhout, A.T., Nijhof, B., Kramer, J.M., et al. (2013). Homozygous and heterozygous disruptions of ANK3: at the crossroads of neurodevelopmental and psychiatric disorders. Hum. Mol. Genet. 22, Jordan, B.A., Fernholz, B.D., Boussac, M., Xu, C., Grigorean, G., Ziff, E.B., and Neubert, T.A. (2004). Identification and verification of novel rodent postsynaptic density proteins. Mol. Cell. Proteomics 3, Kerr, J.M., and Blanpied, T.A. (2012). Subsynaptic AMPA receptor distribution is acutely regulated by actin-driven reorganization of the postsynaptic density. J. Neurosci. 32, Kizhatil, K., Yoon, W., Mohler, P.J., Davis, L.H., Hoffman, J.A., and Bennett, V. (2007). Ankyrin-G and beta2-spectrin collaborate in biogenesis of lateral membrane of human bronchial epithelial cells. J. Biol. Chem. 282, Koch, I., Schwarz, H., Beuchle, D., Goellner, B., Langegger, M., and Aberle, H. (2008). Drosophila ankyrin 2 is required for synaptic stability. Neuron 58, Kusters, R., Kapitein, L.C., Hoogenraad, C.C., and Storm, C. (2013). Shapeinduced asymmetric diffusion in dendritic spines allows efficient synaptic AMPA receptor trapping. Biophys. J. 105, Liao, D., Scannevin, R.H., and Huganir, R. (2001). Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J. Neurosci. 21, Lin, D.T., Makino, Y., Sharma, K., Hayashi, T., Neve, R., Takamiya, K., and Huganir, R.L. (2009). Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat. Neurosci. 12, Lin, C.Y., Sawa, A., and Jaaro-Peled, H. (2012). Better understanding of mechanisms of schizophrenia and bipolar disorder: from human gene expression profiles to mouse models. Neurobiol. Dis. 45, Lu, H.E., MacGillavry, H.D., Frost, N.A., and Blanpied, T.A. (2014). Multiple spatial and kinetic subpopulations of CaMKII in spines and dendrites as resolved by single-molecule tracking PALM. J. Neurosci. 34, MacGillavry, H.D., Kerr, J.M., and Blanpied, T.A. (2011). Lateral organization of the postsynaptic density. Mol. Cell. Neurosci. 48, MacGillavry, H.D., Song, Y., Raghavachari, S., and Blanpied, T.A. (2013). Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron 78, Makino, H., and Malinow, R. (2009). AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron 64, Murakoshi, H., Wang, H., and Yasuda, R. (2011). Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature 472, Nair, D., Hosy, E., Petersen, J.D., Constals, A., Giannone, G., Choquet, D., and Sibarita, J.B. (2013). Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. J. Neurosci. 33, Nanavati, D., Austin, D.R., Catapano, L.A., Luckenbaugh, D.A., Dosemeci, A., Manji, H.K., Chen, G., and Markey, S.P. (2011). The effects of chronic treatment with mood stabilizers on the rat hippocampal post-synaptic density proteome. J. Neurochem. 119, Nestor, M.W., Cai, X., Stone, M.R., Bloch, R.J., and Thompson, S.M. (2011). The actin binding domain of bi-spectrin regulates the morphological and functional dynamics of dendritic spines. PLoS ONE 6, e Noguchi, J., Matsuzaki, M., Ellis-Davies, G.C., and Kasai, H. (2005). Spineneck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron 46, Nurnberger, J.I., Jr., Koller, D.L., Jung, J., Edenberg, H.J., Foroud, T., Guella, I., Vawter, M.P., and Kelsoe, J.R.; Psychiatric Genomics Consortium Bipolar Group (2014). Identification of pathways for bipolar disorder: a meta-analysis. JAMA Psychiatry 71, Penzes, P., Cahill, M.E., Jones, K.A., VanLeeuwen, J.E., and Woolfrey, K.M. (2011). Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 14, Penzes, P., Buonanno, A., Passafaro, M., Sala, C., and Sweet, R.A. (2013). Developmental vulnerability of synapses and circuits associated with neuropsychiatric disorders. J. Neurochem. 126, Pielage, J., Fetter, R.D., and Davis, G.W. (2006). A postsynaptic spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J. Cell Biol. 175, Pielage, J., Cheng, L., Fetter, R.D., Carlton, P.M., Sedat, J.W., and Davis, G.W. (2008). A presynaptic giant ankyrin stabilizes the NMJ through regulation of presynaptic microtubules and transsynaptic cell adhesion. Neuron 58, Purcell, S.M., Moran, J.L., Fromer, M., Ruderfer, D., Solovieff, N., Roussos, P., O Dushlaine, C., Chambert, K., Bergen, S.E., Kähler, A., et al. (2014). A polygenic burden of rare disruptive mutations in schizophrenia. Nature 506, Rasband, M.N. (2010). The axon initial segment and the maintenance of neuronal polarity. Nat. Rev. Neurosci. 11, Sala, C., and Segal, M. (2014). Dendritic spines: the locus of structural and functional plasticity. Physiol. Rev. 94, Schulze, T.G., Detera-Wadleigh, S.D., Akula, N., Gupta, A., Kassem, L., Steele, J., Pearl, J., Strohmaier, J., Breuer, R., Schwarz, M., et al.; NIMH Genetics Initiative Bipolar Disorder Consortium (2009). Two variants in Ankyrin 3 (ANK3) are independent genetic risk factors for bipolar disorder. Mol. Psychiatry 14, Simon, C.M., Hepburn, I., Chen, W., and De Schutter, E. (2014). The role of dendritic spine morphology in the compartmentalization and delivery of surface receptors. J. Comput. Neurosci. 36, Srivastava, D.P., Copits, B.A., Xie, Z., Huda, R., Jones, K.A., Mukherji, S., Cahill, M.E., VanLeeuwen, J.E., Woolfrey, K.M., Rafalovich, I., et al. (2012). Afadin is required for maintenance of dendritic structure and excitatory tone. J. Biol. Chem. 287, Tada, T., Simonetta, A., Batterton, M., Kinoshita, M., Edbauer, D., and Sheng, M. (2007). Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr. Biol. 17, Tønnesen, J., and Nägerl, U.V. (2013). Superresolution imaging for neuroscience. Exp. Neurol. 242, Tønnesen, J., Katona, G., Rózsa, B., and Nägerl, U.V. (2014). Spine neck plasticity regulates compartmentalization of synapses. Nat. Neurosci. 17, Ursitti, J.A., Martin, L., Resneck, W.G., Chaney, T., Zielke, C., Alger, B.E., and Bloch, R.J. (2001). Spectrins in developing rat hippocampal cells. Brain Res. Dev. Brain Res. 129, Voytovych, H., Kriváneková, L., and Ziemann, U. (2012). Lithium: a switch from LTD- to LTP-like plasticity in human cortex. Neuropharmacology 63, Xie, Z., Srivastava, D.P., Photowala, H., Kai, L., Cahill, M.E., Woolfrey, K.M., Shum, C.Y., Surmeier, D.J., and Penzes, P. (2007). Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron 56, Zhang, X., and Bennett, V. (1998). Restriction of 480/270-kD ankyrin G to axon proximal segments requires multiple ankyrin G-specific domains. J. Cell Biol. 142, Neuron 84, , October 22, 2014 ª2014 Elsevier Inc. 415
18 Neuron, Volume 84 Supplemental Information Psychiatric Risk Factor ANK3/Ankyrin-G Nanodomains Regulate the Structure and Function of Glutamatergic Synapses Katharine R. Smith, Katherine J. Kopeikina, Jessica M. Fawcett-Patel, Katherine Leaderbrand, Ruoqi Gao, Britta Schürmann, Kristoffer Myczek, Jelena Radulovic, Geoffrey T. Swanson, and Peter Penzes
19
20
21
22
23
24
25
Authors: K. L. Arendt, M. Royo, M. Fernández-Monreal, S. Knafo, C. N. Petrok, J.
 SUPPLEMENTARY INFORMATION Title: PIP 3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane Authors: K. L. Arendt, M. Royo, M. Fernández-Monreal, S. Knafo, C.
SUPPLEMENTARY INFORMATION Title: PIP 3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane Authors: K. L. Arendt, M. Royo, M. Fernández-Monreal, S. Knafo, C.
SUPPLEMENTARY FIGURE LEGENDS
 SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
Supplementary Figure 1. mir124 does not change neuron morphology and synaptic
 Supplementary Figure 1. mir124 does not change neuron morphology and synaptic density. Hippocampal neurons were transfected with mir124 (containing DsRed) or DsRed as a control. 2 d after transfection,
Supplementary Figure 1. mir124 does not change neuron morphology and synaptic density. Hippocampal neurons were transfected with mir124 (containing DsRed) or DsRed as a control. 2 d after transfection,
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Endocytic Trafficking and Recycling Maintain apoolofmobilesurfaceampareceptors Required for Synaptic Potentiation
 Article Endocytic Trafficking and Recycling Maintain apoolofmobilesurfaceampareceptors Required for Synaptic Potentiation Enrica Maria Petrini, 1,2 Jiuyi Lu, 3 Laurent Cognet, 4 Brahim Lounis, 4 Michael
Article Endocytic Trafficking and Recycling Maintain apoolofmobilesurfaceampareceptors Required for Synaptic Potentiation Enrica Maria Petrini, 1,2 Jiuyi Lu, 3 Laurent Cognet, 4 Brahim Lounis, 4 Michael
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Supplemental Materials Molecular Biology of the Cell
 Supplemental Materials Molecular Biology of the Cell Garcia-Alvarez et al. Supplementary Figure Legends Figure S1.Expression and RNAi-mediated silencing of STIM1 in hippocampal neurons (DIV, days in vitro).
Supplemental Materials Molecular Biology of the Cell Garcia-Alvarez et al. Supplementary Figure Legends Figure S1.Expression and RNAi-mediated silencing of STIM1 in hippocampal neurons (DIV, days in vitro).
Wenqin Hu, Cuiping Tian, Tun Li, Mingpo Yang, Han Hou & Yousheng Shu
 Distinct contributions of Na v 1.6 and Na v 1.2 in action potential initiation and backpropagation Wenqin Hu, Cuiping Tian, Tun Li, Mingpo Yang, Han Hou & Yousheng Shu Supplementary figure and legend Supplementary
Distinct contributions of Na v 1.6 and Na v 1.2 in action potential initiation and backpropagation Wenqin Hu, Cuiping Tian, Tun Li, Mingpo Yang, Han Hou & Yousheng Shu Supplementary figure and legend Supplementary
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/7/308/ra4/dc1 Supplementary Materials for Antipsychotics Activate mtorc1-dependent Translation to Enhance Neuronal Morphological Complexity Heather Bowling, Guoan
www.sciencesignaling.org/cgi/content/full/7/308/ra4/dc1 Supplementary Materials for Antipsychotics Activate mtorc1-dependent Translation to Enhance Neuronal Morphological Complexity Heather Bowling, Guoan
Nature Neuroscience: doi: /nn Supplementary Figure 1. ACx plasticity is required for fear conditioning.
 Supplementary Figure 1 ACx plasticity is required for fear conditioning. (a) Freezing time of conditioned and control mice before CS presentation and during CS presentation in a new context. Student s
Supplementary Figure 1 ACx plasticity is required for fear conditioning. (a) Freezing time of conditioned and control mice before CS presentation and during CS presentation in a new context. Student s
Supplementary Figure 1. Microglia do not show signs of classical immune activation following MD a-b. Images showing immunoreactivity for MHCII (a)
 1 Supplementary Figure 1. Microglia do not show signs of classical immune activation following MD a-b. Images showing immunoreactivity for MHCII (a) and CD45 (b) in fixed sections of binocular visual cortex
1 Supplementary Figure 1. Microglia do not show signs of classical immune activation following MD a-b. Images showing immunoreactivity for MHCII (a) and CD45 (b) in fixed sections of binocular visual cortex
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Palmitoylation regulates glutamate receptor distributions in postsynaptic densities through control of PSD95 conformation and orientation
 Palmitoylation regulates glutamate receptor distributions in postsynaptic densities through control of PSD95 conformation and orientation Okunola Jeyifous a,b,1, Eric I. Lin a,b,1, Xiaobing Chen b,c, Sarah
Palmitoylation regulates glutamate receptor distributions in postsynaptic densities through control of PSD95 conformation and orientation Okunola Jeyifous a,b,1, Eric I. Lin a,b,1, Xiaobing Chen b,c, Sarah
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References
 File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
Ionotropic glutamate receptors (iglurs)
 Ionotropic glutamate receptors (iglurs) GluA1 GluA2 GluA3 GluA4 GluN1 GluN2A GluN2B GluN2C GluN2D GluN3A GluN3B GluK1 GluK2 GluK3 GluK4 GluK5 The general architecture of receptor subunits Unique properties
Ionotropic glutamate receptors (iglurs) GluA1 GluA2 GluA3 GluA4 GluN1 GluN2A GluN2B GluN2C GluN2D GluN3A GluN3B GluK1 GluK2 GluK3 GluK4 GluK5 The general architecture of receptor subunits Unique properties
Schwarz et al. Activity-Dependent Ubiquitination of GluA1 Mediates a Distinct AMPAR Endocytosis
 Schwarz et al Activity-Dependent Ubiquitination of GluA1 Mediates a Distinct AMPAR Endocytosis and Sorting Pathway Supplemental Data Supplemental Fie 1: AMPARs undergo activity-mediated ubiquitination
Schwarz et al Activity-Dependent Ubiquitination of GluA1 Mediates a Distinct AMPAR Endocytosis and Sorting Pathway Supplemental Data Supplemental Fie 1: AMPARs undergo activity-mediated ubiquitination
Postsynaptic scaffold proteins in health and disease Dr. Jonathan Hanley
 Postsynaptic Scaffold Proteins in Health and Disease 1 School of Biochemistry University of Bristol, UK Talk outline Introduction to synapses, their plasticity and molecular organization Focus on excitatory
Postsynaptic Scaffold Proteins in Health and Disease 1 School of Biochemistry University of Bristol, UK Talk outline Introduction to synapses, their plasticity and molecular organization Focus on excitatory
The Nogo Receptor Family Restricts Synapse Number in the Developing Hippocampus
 Article The Nogo Receptor Family Restricts Synapse Number in the Developing Hippocampus Zachary P. Wills,, Caleigh Mandel-Brehm, Alan R. Mardinly, Alejandra E. McCord, Roman J. Giger, 3,4 and Michael E.
Article The Nogo Receptor Family Restricts Synapse Number in the Developing Hippocampus Zachary P. Wills,, Caleigh Mandel-Brehm, Alan R. Mardinly, Alejandra E. McCord, Roman J. Giger, 3,4 and Michael E.
Supporting Information
 ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
Serotonergic Control of the Developing Cerebellum M. Oostland
 Serotonergic Control of the Developing Cerebellum M. Oostland Summary Brain development is a precise and crucial process, dependent on many factors. The neurotransmitter serotonin is one of the factors
Serotonergic Control of the Developing Cerebellum M. Oostland Summary Brain development is a precise and crucial process, dependent on many factors. The neurotransmitter serotonin is one of the factors
Synaptic plasticityhippocampus. Neur 8790 Topics in Neuroscience: Neuroplasticity. Outline. Synaptic plasticity hypothesis
 Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Supplementary Figure 1. SybII and Ceb are sorted to distinct vesicle populations in astrocytes. Nature Neuroscience: doi: /nn.
 Supplementary Figure 1 SybII and Ceb are sorted to distinct vesicle populations in astrocytes. (a) Exemplary images for cultured astrocytes co-immunolabeled with SybII and Ceb antibodies. SybII accumulates
Supplementary Figure 1 SybII and Ceb are sorted to distinct vesicle populations in astrocytes. (a) Exemplary images for cultured astrocytes co-immunolabeled with SybII and Ceb antibodies. SybII accumulates
Structural basis for the role of inhibition in facilitating adult brain plasticity
 Structural basis for the role of inhibition in facilitating adult brain plasticity Jerry L. Chen, Walter C. Lin, Jae Won Cha, Peter T. So, Yoshiyuki Kubota & Elly Nedivi SUPPLEMENTARY FIGURES 1-6 a b M
Structural basis for the role of inhibition in facilitating adult brain plasticity Jerry L. Chen, Walter C. Lin, Jae Won Cha, Peter T. So, Yoshiyuki Kubota & Elly Nedivi SUPPLEMENTARY FIGURES 1-6 a b M
Memory Systems II How Stored: Engram and LTP. Reading: BCP Chapter 25
 Memory Systems II How Stored: Engram and LTP Reading: BCP Chapter 25 Memory Systems Learning is the acquisition of new knowledge or skills. Memory is the retention of learned information. Many different
Memory Systems II How Stored: Engram and LTP Reading: BCP Chapter 25 Memory Systems Learning is the acquisition of new knowledge or skills. Memory is the retention of learned information. Many different
SUPPLEMENTARY INFORMATION
 Figure S1. Loss of Ena/VASP proteins inhibits filopodia and neuritogenesis. (a) Bar graph of filopodia number per stage 1 control and mmvvee (Mena/ VASP/EVL-null) neurons at 40hrs in culture. Loss of all
Figure S1. Loss of Ena/VASP proteins inhibits filopodia and neuritogenesis. (a) Bar graph of filopodia number per stage 1 control and mmvvee (Mena/ VASP/EVL-null) neurons at 40hrs in culture. Loss of all
pt305-camkii stabilizes a learning-induced increase in AMPA receptors for ongoing memory consolidation after classical conditioning
 Received 6 Nov 213 Accepted 25 Apr 214 Published 3 May 214 pt35- stabilizes a learning-induced increase in AMPA receptors for ongoing memory consolidation after classical conditioning Souvik Naskar 1,,
Received 6 Nov 213 Accepted 25 Apr 214 Published 3 May 214 pt35- stabilizes a learning-induced increase in AMPA receptors for ongoing memory consolidation after classical conditioning Souvik Naskar 1,,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
Zhu et al, page 1. Supplementary Figures
 Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
BIPN140 Lecture 12: Synaptic Plasticity (II)
 BIPN140 Lecture 12: Synaptic Plasticity (II) 1. Early v.s. Late LTP 2. Long-Term Depression 3. Molecular Mechanisms of Long-Term Depression: NMDA-R dependent 4. Molecular Mechanisms of Long-Term Depression:
BIPN140 Lecture 12: Synaptic Plasticity (II) 1. Early v.s. Late LTP 2. Long-Term Depression 3. Molecular Mechanisms of Long-Term Depression: NMDA-R dependent 4. Molecular Mechanisms of Long-Term Depression:
Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC
 1 2 1 3 Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC 4 5 6 7 (a) Reconstructions of LII/III GIN-cells with somato-dendritic compartments in orange and axonal arborizations
1 2 1 3 Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC 4 5 6 7 (a) Reconstructions of LII/III GIN-cells with somato-dendritic compartments in orange and axonal arborizations
The Neuregulin-1 Receptor ErbB4 Controls Glutamatergic Synapse Maturation and Plasticity
 Article The Neuregulin-1 Receptor ErbB4 Controls Glutamatergic Synapse Maturation and Plasticity Bo Li, 1 Ran-Sook Woo, 2 Lin Mei, 2 and Roberto Malinow 1, * 1 Cold Spring Harbor Laboratory, Cold Spring
Article The Neuregulin-1 Receptor ErbB4 Controls Glutamatergic Synapse Maturation and Plasticity Bo Li, 1 Ran-Sook Woo, 2 Lin Mei, 2 and Roberto Malinow 1, * 1 Cold Spring Harbor Laboratory, Cold Spring
Disrupting GluA2-GAPDH Interaction Affects Axon and Dendrite Development
 Disrupting GluA2-GAPDH Interaction Affects Axon and Dendrite Development 1 Frankie Hang Fung Lee, 1 Ping Su, 1 Yu Feng Xie, 1 Kyle Ethan Wang, 2 Qi Wan and 1,3 Fang Liu 1 Campbell Research Institute, Centre
Disrupting GluA2-GAPDH Interaction Affects Axon and Dendrite Development 1 Frankie Hang Fung Lee, 1 Ping Su, 1 Yu Feng Xie, 1 Kyle Ethan Wang, 2 Qi Wan and 1,3 Fang Liu 1 Campbell Research Institute, Centre
Supplementary Figure 1. SDS-FRL localization of CB 1 in the distal CA3 area of the rat hippocampus. (a-d) Axon terminals (t) in stratum pyramidale
 Supplementary Figure 1. SDS-FRL localization of CB 1 in the distal CA3 area of the rat hippocampus. (a-d) Axon terminals (t) in stratum pyramidale (b) show stronger immunolabeling for CB 1 than those in
Supplementary Figure 1. SDS-FRL localization of CB 1 in the distal CA3 area of the rat hippocampus. (a-d) Axon terminals (t) in stratum pyramidale (b) show stronger immunolabeling for CB 1 than those in
Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +.
![Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +. Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +.](/thumbs/90/102196023.jpg) Research Interests Advanced cognitive learning is encoded in distributed circuits that span multiple forebrain areas. Further, synaptic plasticity and neural network theories hypothesize that essential
Research Interests Advanced cognitive learning is encoded in distributed circuits that span multiple forebrain areas. Further, synaptic plasticity and neural network theories hypothesize that essential
Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission
 Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission Jesica Raingo, Mikhail Khvotchev, Pei Liu, Frederic Darios, Ying C. Li, Denise
Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission Jesica Raingo, Mikhail Khvotchev, Pei Liu, Frederic Darios, Ying C. Li, Denise
Glial-Derived Prodegenerative Signaling in the Drosophila Neuromuscular System
 Article in the Drosophila Neuromuscular System Lani C. Keller, 1 Ling Cheng, 1 Cody J. Locke, 1 Martin Müller, 1 Richard D. Fetter, 1,2 and Graeme W. Davis 1, * 1 Department of Biochemistry and Biophysics,
Article in the Drosophila Neuromuscular System Lani C. Keller, 1 Ling Cheng, 1 Cody J. Locke, 1 Martin Müller, 1 Richard D. Fetter, 1,2 and Graeme W. Davis 1, * 1 Department of Biochemistry and Biophysics,
Supplemental information Acid-sensing ion channel 1a contributes to hippocampal LTP inducibility through multiple mechanisms
 Supplemental information Acid-sensing ion channel 1a contributes to hippocampal LTP inducibility through multiple mechanisms Ming-Gang Liu, Hu-Song Li, Wei-Guang Li, Yan-Jiao Wu, Shi-Ning Deng, Chen Huang,
Supplemental information Acid-sensing ion channel 1a contributes to hippocampal LTP inducibility through multiple mechanisms Ming-Gang Liu, Hu-Song Li, Wei-Guang Li, Yan-Jiao Wu, Shi-Ning Deng, Chen Huang,
1) Drop off in the Bi 150 box outside Baxter 331 or to the head TA (jcolas).
 Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
How Nicotinic Signaling Shapes Neural Networks
 How Nicotinic Signaling Shapes Neural Networks Darwin K. Berg Division of Biological Sciences University of California, San Diego Nicotinic Cholinergic Signaling Uses the transmitter ACh to activate cation-selective
How Nicotinic Signaling Shapes Neural Networks Darwin K. Berg Division of Biological Sciences University of California, San Diego Nicotinic Cholinergic Signaling Uses the transmitter ACh to activate cation-selective
SUPPLEMENTARY INFORMATION
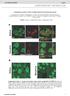 doi: 10.1038/nature06994 A phosphatase cascade by which rewarding stimuli control nucleosomal response A. Stipanovich*, E. Valjent*, M. Matamales*, A. Nishi, J.H. Ahn, M. Maroteaux, J. Bertran-Gonzalez,
doi: 10.1038/nature06994 A phosphatase cascade by which rewarding stimuli control nucleosomal response A. Stipanovich*, E. Valjent*, M. Matamales*, A. Nishi, J.H. Ahn, M. Maroteaux, J. Bertran-Gonzalez,
Neuronal plasma membrane
 ORGANELLES ORGANELLES Neuronal plasma membrane The neuronal plasma membrane contains several local domains with unique properties Presynaptic terminal Endoplasmic Reticulum In neurons the Nissl bodies
ORGANELLES ORGANELLES Neuronal plasma membrane The neuronal plasma membrane contains several local domains with unique properties Presynaptic terminal Endoplasmic Reticulum In neurons the Nissl bodies
Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) 108 (b-c) (d) (e) (f) (g)
 Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) In four mice, cre-dependent expression of the hyperpolarizing opsin Arch in pyramidal cells
Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) In four mice, cre-dependent expression of the hyperpolarizing opsin Arch in pyramidal cells
Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory
 (2011) 16, 156 170 & 2011 Macmillan Publishers Limited All rights reserved 1359-4184/11 www.nature.com/mp ORIGINAL ARTICLE Mechanisms for acute stress-induced enhancement of glutamatergic transmission
(2011) 16, 156 170 & 2011 Macmillan Publishers Limited All rights reserved 1359-4184/11 www.nature.com/mp ORIGINAL ARTICLE Mechanisms for acute stress-induced enhancement of glutamatergic transmission
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Bidirectional optogenetic modulation of the tonic activity of CEA PKCδ + neurons in vitro. a, Top, Cell-attached voltage recording illustrating the blue light-induced increase in
Supplementary Figure 1 Bidirectional optogenetic modulation of the tonic activity of CEA PKCδ + neurons in vitro. a, Top, Cell-attached voltage recording illustrating the blue light-induced increase in
Nature Neuroscience: doi: /nn Supplementary Figure 1. Distribution of starter cells for RV-mediated retrograde tracing.
 Supplementary Figure 1 Distribution of starter cells for RV-mediated retrograde tracing. Parcellation of cortical areas is based on Allen Mouse Brain Atlas and drawn to scale. Thick white curves, outlines
Supplementary Figure 1 Distribution of starter cells for RV-mediated retrograde tracing. Parcellation of cortical areas is based on Allen Mouse Brain Atlas and drawn to scale. Thick white curves, outlines
293T cells were transfected with indicated expression vectors and the whole-cell extracts were subjected
 SUPPLEMENTARY INFORMATION Supplementary Figure 1. Formation of a complex between Slo1 and CRL4A CRBN E3 ligase. (a) HEK 293T cells were transfected with indicated expression vectors and the whole-cell
SUPPLEMENTARY INFORMATION Supplementary Figure 1. Formation of a complex between Slo1 and CRL4A CRBN E3 ligase. (a) HEK 293T cells were transfected with indicated expression vectors and the whole-cell
Nature Immunology: doi: /ni.3631
 Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Ube3a is required for experience-dependent maturation of the neocortex
 Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
SUPPLEMENTARY INFORMATION. Supplementary Figure 1
 SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
Nature Methods: doi: /nmeth Supplementary Figure 1. Activity in turtle dorsal cortex is sparse.
 Supplementary Figure 1 Activity in turtle dorsal cortex is sparse. a. Probability distribution of firing rates across the population (notice log scale) in our data. The range of firing rates is wide but
Supplementary Figure 1 Activity in turtle dorsal cortex is sparse. a. Probability distribution of firing rates across the population (notice log scale) in our data. The range of firing rates is wide but
Axon initial segment position changes CA1 pyramidal neuron excitability
 Axon initial segment position changes CA1 pyramidal neuron excitability Cristina Nigro and Jason Pipkin UCSD Neurosciences Graduate Program Abstract The axon initial segment (AIS) is the portion of the
Axon initial segment position changes CA1 pyramidal neuron excitability Cristina Nigro and Jason Pipkin UCSD Neurosciences Graduate Program Abstract The axon initial segment (AIS) is the portion of the
Nature Neuroscience: doi: /nn Supplementary Figure 1. Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites.
 Supplementary Figure 1 Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites. Staining with fluorescence antibodies to detect GFP (Green), β-galactosidase (magenta/white). (a, b) Class
Supplementary Figure 1 Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites. Staining with fluorescence antibodies to detect GFP (Green), β-galactosidase (magenta/white). (a, b) Class
BISP194: MOLECULAR MECHANISM OF SYNAPTIC PLASTICITY Spring Quarter
 BISP194: MOLECULAR MECHANISM OF SYNAPTIC PLASTICITY Spring Quarter 2011 Instructor: Class Website: Gentry N. Patrick (gpatrick@ucsd.edu) http://www.biology.ucsd.edu/classes/bisp194.sp11 Class Meetings:
BISP194: MOLECULAR MECHANISM OF SYNAPTIC PLASTICITY Spring Quarter 2011 Instructor: Class Website: Gentry N. Patrick (gpatrick@ucsd.edu) http://www.biology.ucsd.edu/classes/bisp194.sp11 Class Meetings:
Figure S1. (A) SDS-PAGE separation of GST-fusion proteins purified from E.coli BL21 strain is shown. An equal amount of GST-tag control, LRRK2 LRR
 Figure S1. (A) SDS-PAGE separation of GST-fusion proteins purified from E.coli BL21 strain is shown. An equal amount of GST-tag control, LRRK2 LRR and LRRK2 WD40 GST fusion proteins (5 µg) were loaded
Figure S1. (A) SDS-PAGE separation of GST-fusion proteins purified from E.coli BL21 strain is shown. An equal amount of GST-tag control, LRRK2 LRR and LRRK2 WD40 GST fusion proteins (5 µg) were loaded
SUPPLEMENTARY INFORMATION
 DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
Nature Neuroscience: doi: /nn Supplementary Figure 1. MADM labeling of thalamic clones.
 Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Dynamic mechanisms of neuroligin-dependent presynaptic terminal assembly in living cortical neurons
 Bury and Sabo Neural Development 2014, 9:13 RESEARCH ARTICLE Open Access Dynamic mechanisms of neuroligin-dependent presynaptic terminal assembly in living cortical neurons Luke AD Bury 1 and Shasta L
Bury and Sabo Neural Development 2014, 9:13 RESEARCH ARTICLE Open Access Dynamic mechanisms of neuroligin-dependent presynaptic terminal assembly in living cortical neurons Luke AD Bury 1 and Shasta L
Supplementary Figure 1. Basic properties of compound EPSPs at
 Supplementary Figure 1. Basic properties of compound EPSPs at hippocampal CA3 CA3 cell synapses. (a) EPSPs were evoked by extracellular stimulation of the recurrent collaterals and pharmacologically isolated
Supplementary Figure 1. Basic properties of compound EPSPs at hippocampal CA3 CA3 cell synapses. (a) EPSPs were evoked by extracellular stimulation of the recurrent collaterals and pharmacologically isolated
Supplementary Figure 1: Validation of labeling specificity of immature OSNs and presynaptic terminals. (A) (B) (C) (D) (E)
 Supplementary Figure 1: Validation of labeling specificity of immature OSNs and presynaptic terminals. (A) Confocal images of septal olfactory epithelium of an adult Gγ8-sypGFP-tdTom mouse showing colocalization
Supplementary Figure 1: Validation of labeling specificity of immature OSNs and presynaptic terminals. (A) Confocal images of septal olfactory epithelium of an adult Gγ8-sypGFP-tdTom mouse showing colocalization
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
Neuronal plasma membrane
 ORGANELLES ORGANELLES Neuronal plasma membrane The neuronal plasma membrane contains several local domains with unique properties Presynaptic terminal Endoplasmic Reticulum In neurons the Nissl bodies
ORGANELLES ORGANELLES Neuronal plasma membrane The neuronal plasma membrane contains several local domains with unique properties Presynaptic terminal Endoplasmic Reticulum In neurons the Nissl bodies
Lecture 7: Roles for MAGUKS in Activity-dependent Synaptogenesis MCP
 Lecture 7: Roles for MAGUKS in Activity-dependent Synaptogenesis MCP 9.013 04 Po st -S yn ap (P ti SD c ) De ns it y PSD site en face.25 µm From: Kennedy (2000) Science MEMBRANE ASSOCIATED GUANYLATE KINASES
Lecture 7: Roles for MAGUKS in Activity-dependent Synaptogenesis MCP 9.013 04 Po st -S yn ap (P ti SD c ) De ns it y PSD site en face.25 µm From: Kennedy (2000) Science MEMBRANE ASSOCIATED GUANYLATE KINASES
Synaptic Plasticity and Memory
 Synaptic Plasticity and Memory Properties and synaptic mechanisms underlying the induction of long-term potentiation (LTP) The role of calcium/calmodulin-dependent kinase II (CamKII) in the induction,
Synaptic Plasticity and Memory Properties and synaptic mechanisms underlying the induction of long-term potentiation (LTP) The role of calcium/calmodulin-dependent kinase II (CamKII) in the induction,
Chapter 3. Expression of α5-megfp in Mouse Cortical Neurons. on the β subunit. Signal sequences in the M3-M4 loop of β nachrs bind protein factors to
 22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
Plasticity of Cerebral Cortex in Development
 Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Potential Treatment and Current Research in Phelan-McDermid Syndrome. 11/16/2016 Frambu Center for Rare Disorders
 Potential Treatment and Current Research in Phelan-McDermid Syndrome 11/16/2016 Frambu Center for Rare Disorders Genetics is Complicated! Deletion 22q13: Therapies Under Investigation Intranasal insulin
Potential Treatment and Current Research in Phelan-McDermid Syndrome 11/16/2016 Frambu Center for Rare Disorders Genetics is Complicated! Deletion 22q13: Therapies Under Investigation Intranasal insulin
Dep. Control Time (min)
 aa Control Dep. RP 1s 1 mv 2s 1 mv b % potentiation of IPSP 2 15 1 5 Dep. * 1 2 3 4 Time (min) Supplementary Figure 1. Rebound potentiation of IPSPs in PCs. a, IPSPs recorded with a K + gluconate pipette
aa Control Dep. RP 1s 1 mv 2s 1 mv b % potentiation of IPSP 2 15 1 5 Dep. * 1 2 3 4 Time (min) Supplementary Figure 1. Rebound potentiation of IPSPs in PCs. a, IPSPs recorded with a K + gluconate pipette
Classification of Synapses Using Spatial Protein Data
 Classification of Synapses Using Spatial Protein Data Jenny Chen and Micol Marchetti-Bowick CS229 Final Project December 11, 2009 1 MOTIVATION Many human neurological and cognitive disorders are caused
Classification of Synapses Using Spatial Protein Data Jenny Chen and Micol Marchetti-Bowick CS229 Final Project December 11, 2009 1 MOTIVATION Many human neurological and cognitive disorders are caused
Chapter 2. Investigation into mir-346 Regulation of the nachr α5 Subunit
 15 Chapter 2 Investigation into mir-346 Regulation of the nachr α5 Subunit MicroRNA s (mirnas) are small (< 25 base pairs), single stranded, non-coding RNAs that regulate gene expression at the post transcriptional
15 Chapter 2 Investigation into mir-346 Regulation of the nachr α5 Subunit MicroRNA s (mirnas) are small (< 25 base pairs), single stranded, non-coding RNAs that regulate gene expression at the post transcriptional
F-actin VWF Vinculin. F-actin. Vinculin VWF
 a F-actin VWF Vinculin b F-actin VWF Vinculin Supplementary Fig. 1. WPBs in HUVECs are located along stress fibers and at focal adhesions. (a) Immunofluorescence images of f-actin (cyan), VWF (yellow),
a F-actin VWF Vinculin b F-actin VWF Vinculin Supplementary Fig. 1. WPBs in HUVECs are located along stress fibers and at focal adhesions. (a) Immunofluorescence images of f-actin (cyan), VWF (yellow),
Sustained CPEB-Dependent Local Protein Synthesis Is Required to Stabilize Synaptic Growth for Persistence of Long-Term Facilitation in Aplysia
 Article Sustained CPEB-Dependent Local Protein Synthesis Is Required to Stabilize Synaptic Growth for Persistence of Long-Term Facilitation in Aplysia Maria Concetta Miniaci, 2,4 Joung-Hun Kim, 2,5 Sathyanarayanan
Article Sustained CPEB-Dependent Local Protein Synthesis Is Required to Stabilize Synaptic Growth for Persistence of Long-Term Facilitation in Aplysia Maria Concetta Miniaci, 2,4 Joung-Hun Kim, 2,5 Sathyanarayanan
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Project report October 2012 March 2013 CRF fellow: Principal Investigator: Project title:
 Project report October 2012 March 2013 CRF fellow: Gennaro Napolitano Principal Investigator: Sergio Daniel Catz Project title: Small molecule regulators of vesicular trafficking to enhance lysosomal exocytosis
Project report October 2012 March 2013 CRF fellow: Gennaro Napolitano Principal Investigator: Sergio Daniel Catz Project title: Small molecule regulators of vesicular trafficking to enhance lysosomal exocytosis
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
Neuronal Differentiation: CNS Synapse Formation
 Neuronal Differentiation: CNS Synapse Formation Comparison of the NMJ and Central Synapses Sanes and Lichtman 2001 Comparison of the NMJ and Central Synapses (1) Each neuron in the CNS is innervated by
Neuronal Differentiation: CNS Synapse Formation Comparison of the NMJ and Central Synapses Sanes and Lichtman 2001 Comparison of the NMJ and Central Synapses (1) Each neuron in the CNS is innervated by
Dynamic Partitioning of a GPI-Anchored Protein in Glycosphingolipid-Rich Microdomains Imaged by Single-Quantum Dot Tracking
 Additional data for Dynamic Partitioning of a GPI-Anchored Protein in Glycosphingolipid-Rich Microdomains Imaged by Single-Quantum Dot Tracking Fabien Pinaud 1,3, Xavier Michalet 1,3, Gopal Iyer 1, Emmanuel
Additional data for Dynamic Partitioning of a GPI-Anchored Protein in Glycosphingolipid-Rich Microdomains Imaged by Single-Quantum Dot Tracking Fabien Pinaud 1,3, Xavier Michalet 1,3, Gopal Iyer 1, Emmanuel
NMDAR-dependent Argonaute 2 phosphorylation regulates mirna activity and dendritic spine plasticity
 Article NMDAR-dependent Argonaute 2 phosphorylation regulates mirna activity and dendritic spine plasticity Dipen Rajgor 1, Thomas M Sanderson 2, Mascia Amici 2, Graham L Collingridge 2,3,4 & Jonathan
Article NMDAR-dependent Argonaute 2 phosphorylation regulates mirna activity and dendritic spine plasticity Dipen Rajgor 1, Thomas M Sanderson 2, Mascia Amici 2, Graham L Collingridge 2,3,4 & Jonathan
Neurotrophin receptor p75 NTR mediates Huntington s disease associated synaptic and memory dysfunction
 The Journal of Clinical Investigation Neurotrophin receptor p75 NTR mediates Huntington s disease associated synaptic and memory dysfunction Verónica Brito, 1,2,3 Albert Giralt, 1,2,3 Lilian Enriquez-Barreto,
The Journal of Clinical Investigation Neurotrophin receptor p75 NTR mediates Huntington s disease associated synaptic and memory dysfunction Verónica Brito, 1,2,3 Albert Giralt, 1,2,3 Lilian Enriquez-Barreto,
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures
 Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
The Neurobiology of Learning and Memory
 The Neurobiology of Learning and Memory JERRY W. RUDY University of Colorado, Boulder Sinauer Associates, Inc. Publishers Sunderland, Massachusetts 01375 Table of Contents CHAPTER 1 Introduction: Fundamental
The Neurobiology of Learning and Memory JERRY W. RUDY University of Colorado, Boulder Sinauer Associates, Inc. Publishers Sunderland, Massachusetts 01375 Table of Contents CHAPTER 1 Introduction: Fundamental
Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells.
 Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells. a. Schematic of the V-ATPase proton pump macro-complex structure. The V1 complex is composed of
Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells. a. Schematic of the V-ATPase proton pump macro-complex structure. The V1 complex is composed of
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1 SNARE Probes for FRET/2pFLIM Analysis Used in the Present Study. mturquoise (mtq) and Venus (Ven) are in blue and yellow, respectively. The soluble N-ethylmaleimide-sensitive
Supplementary Figure 1 Supplementary Figure 1 SNARE Probes for FRET/2pFLIM Analysis Used in the Present Study. mturquoise (mtq) and Venus (Ven) are in blue and yellow, respectively. The soluble N-ethylmaleimide-sensitive
effects on organ development. a-f, Eye and wing discs with clones of ε j2b10 show no
 Supplementary Figure 1. Loss of function clones of 14-3-3 or 14-3-3 show no significant effects on organ development. a-f, Eye and wing discs with clones of 14-3-3ε j2b10 show no obvious defects in Elav
Supplementary Figure 1. Loss of function clones of 14-3-3 or 14-3-3 show no significant effects on organ development. a-f, Eye and wing discs with clones of 14-3-3ε j2b10 show no obvious defects in Elav
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
Supplementary Figure S1
 Supplementary Figure S1 Supplementary Figure S1. PARP localization patterns using GFP-PARP and PARP-specific antibody libraries GFP-PARP localization in non-fixed (A) and formaldehyde fixed (B) GFP-PARPx
Supplementary Figure S1 Supplementary Figure S1. PARP localization patterns using GFP-PARP and PARP-specific antibody libraries GFP-PARP localization in non-fixed (A) and formaldehyde fixed (B) GFP-PARPx
Expanded View Figures
 PEX13 functions in selective autophagy Ming Y Lee et al Expanded View Figures Figure EV1. PEX13 is required for Sindbis virophagy. A, B Quantification of mcherry-capsid puncta per cell (A) and GFP-LC3
PEX13 functions in selective autophagy Ming Y Lee et al Expanded View Figures Figure EV1. PEX13 is required for Sindbis virophagy. A, B Quantification of mcherry-capsid puncta per cell (A) and GFP-LC3
Supplementary Figure 1
 Supplementary Figure 1 Miniature microdrive, spike sorting and sleep stage detection. a, A movable recording probe with 8-tetrodes (32-channels). It weighs ~1g. b, A mouse implanted with 8 tetrodes in
Supplementary Figure 1 Miniature microdrive, spike sorting and sleep stage detection. a, A movable recording probe with 8-tetrodes (32-channels). It weighs ~1g. b, A mouse implanted with 8 tetrodes in
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses Yichen Shi 1,2, Peter Kirwan 1,2, James Smith 1,2, Hugh P.C. Robinson 3 and Frederick
SUPPLEMENTARY INFORMATION Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses Yichen Shi 1,2, Peter Kirwan 1,2, James Smith 1,2, Hugh P.C. Robinson 3 and Frederick
1) Drop off in the Bi 150 box outside Baxter 331 or to the head TA (jcolas).
 Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Nature Neuroscience doi: /nn Supplementary Figure 1. Characterization of viral injections.
 Supplementary Figure 1 Characterization of viral injections. (a) Dorsal view of a mouse brain (dashed white outline) after receiving a large, unilateral thalamic injection (~100 nl); demonstrating that
Supplementary Figure 1 Characterization of viral injections. (a) Dorsal view of a mouse brain (dashed white outline) after receiving a large, unilateral thalamic injection (~100 nl); demonstrating that
Supplementary Information
 Supplementary Information Title Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis Authors Shin H. Kang, Ying Li, Masahiro Fukaya, Ileana Lorenzini,
Supplementary Information Title Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis Authors Shin H. Kang, Ying Li, Masahiro Fukaya, Ileana Lorenzini,
Lecture: Introduction to nervous system development
 Lecture: Introduction to nervous system development Prof. Ilan Davis, Department of Biochemistry. Wellcome Senior Research Fellow Senior Research Fellow, Jesus College ilan.davis@bioch.ox.ac.uk http://www.ilandavis.com
Lecture: Introduction to nervous system development Prof. Ilan Davis, Department of Biochemistry. Wellcome Senior Research Fellow Senior Research Fellow, Jesus College ilan.davis@bioch.ox.ac.uk http://www.ilandavis.com
Supplementary information. The Light Intermediate Chain 2 Subpopulation of Dynein Regulates Mitotic. Spindle Orientation
 Supplementary information The Light Intermediate Chain 2 Subpopulation of Dynein Regulates Mitotic Spindle Orientation Running title: Dynein LICs distribute mitotic functions. Sagar Mahale a, d, *, Megha
Supplementary information The Light Intermediate Chain 2 Subpopulation of Dynein Regulates Mitotic Spindle Orientation Running title: Dynein LICs distribute mitotic functions. Sagar Mahale a, d, *, Megha
LDLR-related protein 10 (LRP10) regulates amyloid precursor protein (APP) trafficking and processing: evidence for a role in Alzheimer s disease
 Brodeur et al. Molecular Neurodegeneration 2012, 7:31 RESEARCH ARTICLE Open Access LDLR-related protein 10 (LRP10) regulates amyloid precursor protein (APP) trafficking and processing: evidence for a role
Brodeur et al. Molecular Neurodegeneration 2012, 7:31 RESEARCH ARTICLE Open Access LDLR-related protein 10 (LRP10) regulates amyloid precursor protein (APP) trafficking and processing: evidence for a role
Multiple Autism-Linked Genes Mediate Synapse Elimination via Proteasomal Degradation of a Synaptic Scaffold PSD-95
 Multiple Autism-Linked Genes Mediate Synapse Elimination via Proteasomal Degradation of a Synaptic Scaffold PSD-95 Nien-Pei Tsai, 1,4 Julia R. Wilkerson, 1,4 Weirui Guo, 1 Marina A. Maksimova, 1 George
Multiple Autism-Linked Genes Mediate Synapse Elimination via Proteasomal Degradation of a Synaptic Scaffold PSD-95 Nien-Pei Tsai, 1,4 Julia R. Wilkerson, 1,4 Weirui Guo, 1 Marina A. Maksimova, 1 George
Supplementary Information
 1 Supplementary Information A role for primary cilia in glutamatergic synaptic integration of adult-orn neurons Natsuko Kumamoto 1,4,5, Yan Gu 1,4, Jia Wang 1,4, Stephen Janoschka 1,2, Ken-Ichi Takemaru
1 Supplementary Information A role for primary cilia in glutamatergic synaptic integration of adult-orn neurons Natsuko Kumamoto 1,4,5, Yan Gu 1,4, Jia Wang 1,4, Stephen Janoschka 1,2, Ken-Ichi Takemaru
