Dopamine D1-Like Receptors Depress Excitatory Synaptic Transmissions in Striatal Neurons After Transient Forebrain Ischemia
|
|
|
- Theodore Kennedy
- 5 years ago
- Views:
Transcription
1 Dopamine D1-Like Receptors Depress Excitatory Synaptic Transmissions in Striatal Neurons After Transient Forebrain Ischemia Yuchun Zhang, PhD; Ping Deng, MD, PhD; Yiwen Ruan, MD, PhD; Zao C. Xu, MD, PhD Background and Purpose Spiny neurons in the neostriatum are highly vulnerable to ischemia. Despite an enormous body of research suggesting that dopamine is involved in ischemia-induced neuronal loss in the striatum, it remains unclear how dopamine interacts with the glutamatergic excitotoxicity that is widely accepted as a major cause of ischemic cell death. Our study was designed to investigate the effects of dopamine D1 receptor (D1R) activation on excitatory neurotransmission in postischemic striatal neurons. Methods We used the 4-vessel occlusion ischemia model and brain slice preparations. Whole-cell voltage-clamp recording was performed on striatal neurons to measure excitatory postsynaptic currents (EPSCs). Systemic administration of a D1R agonist after ischemia and hematoxylin/eosin staining were performed to evaluate the effects of D1R activation on ischemia-induced neuronal degeneration in the striatum. Results D1R activation depressed EPSCs in postischemic striatal neurons. The depression was attributable to inhibition of presynaptic release. An activator of camp-dependent protein kinase A (PKA) mimicked the depressive effects of D1R activation. Bath application of a PKA inhibitor blocked the depression of EPSCs, whereas intracellular postsynaptic application of the PKA inhibitor had no effect. The D1R agonist failed to reduce EPSC amplitude in the presence of an adenosine A1 receptor antagonist. Systemic administration of a D1R agonist after ischemia significantly attenuated ischemia-induced cell death in the striatum. Conclusions These results indicate that D1R activation presynaptically depresses excitatory synaptic transmission in striatal neurons after ischemia through activation of PKA and adenosine A1 receptors and thus demonstrate a novel mechanism of D1R-mediated protection against ischemia. (Stroke. 2008;39: ) Key Words: dopamine ischemia striatum excitatory postsynaptic currents adenosine The neostriatum, a brain region highly vulnerable to ischemia, is richly innervated by nigrostriatal dopaminergic projections in addition to the corticostriatal glutamatergic pathway. Microdialysis studies have demonstrated that ischemia induces an 500-fold increase in dopamine concentrations in the neostriatum. 1 Excessive dopamine is neurotoxic, as shown by the fact that depletion of dopaminergic neurons in the substantia nigra protects striatal neurons against ischemic insult. 2 The mechanisms underlying this neurotoxic effect have undergone extensive study. The molecular structure of dopamine makes it more likely to form reactive metabolites than other catecholamines, which consequently damage cellular components such as lipids, protein, and DNA. 3 Interestingly, subsequent studies from this laboratory have found that dopamine depletion does not bilaterally protect striatal neurons against ischemia. Postischemic cell death in the right striatum is alleviated by dopamine denervation in the right side, whereas no protection was observed in the left striatum after ipsilateral dopamine denervation. 4 Combined with the finding that dopamine receptors in the striatum change asymmetrically after unilateral dopamine depletion, 5 a hypothesis has been proposed that dopamine is also involved in postischemic neuronal injury through the dopamine receptor (DAR). Although both in vivo and in vitro studies have unanimously demonstrated involvement of the DAR in ischemic neuronal injury, the effects of DAR activation on ischemic cell death are highly controversial. For instance, systemic administration of a D1-like dopamine receptor (D1R) agonist confers neuroprotection against ischemia, 6 whereas other studies have suggested that blockade of D1Rs alleviates ischemic neuronal damage. 7 Likewise, the same is true for D2-like dopamine receptors (D2Rs). Both activation and inhibition of D2Rs have been shown to be protective by different research groups. 8,9 Our previous study has shown that excitatory synaptic transmission in striatal neurons is presynaptically enhanced after transient global ischemia, 10 and the enhancement of excitatory neurotransmission is thought to be associated with Received October 9, 2007; final revision received December 10, 2007; accepted January 3, From the Department of Anatomy and Cell Biology, Indiana University School of Medicine, Indianapolis. Correspondence to Zao C. Xu, MD, PhD, Department of Anatomy and Cell Biology, Indiana University School of Medicine, 635 Barnhill Dr, MS 507, Indianapolis, IN zxu@anatomy.iupui.edu 2008 American Heart Association, Inc. Stroke is available at DOI: /STROKEAHA
2 Zhang et al D1Rs Depress EPSCs in Spiny Neurons After Ischemia 2371 postischemic neuronal degeneration. Dopamine is able to modulate excitatory synaptic transmission in striatal neurons under control conditions and thus participates in motor learning and sensorimotor integration. 11 However, it is largely unknown how dopamine modulates excitatory neurotransmission in striatal neurons after ischemia or how it influences glutamatergic excitotoxicity. The present study was designed to elucidate these questions. Materials and Methods Transient Forebrain Ischemia Male Wistar rats (100 to 200 g; Charles River Laboratories, Wilmington, Mass) were subjected to transient forebrain ischemia via the 4-vessel occlusion method with modifications as described previously. 4,12 The animals were fasted overnight to provide uniform blood glucose levels. The animals were anesthetized with a mixture of 1% to 2% halothane in 33% O 2 /66% N 2. Bilateral vertebral arteries were electrocauterized. After craniotomy, a temperature probe was placed underneath the skull to maintain brain temperature at 37 C with a heating lamp via a feedback system. Silicone tubing was placed loosely around each common carotid artery to make bilateral common carotid arteries ready to be occluded. On the contralateral cranium, another hole was made to record the ischemic depolarization of striatal field potentials. The tip of the extracellular recording electrode was placed into the striatum with use of a stereotaxic instrument. The field potential was adjusted to zero before ischemia was instituted. During the occlusion procedure, bilateral common carotid arteries were clamped. Owing to the membrane depolarization induced by energy deprivation, the extracellular electrode detected the sustained field potential change, termed the ischemic depolarization (shift from 0 to 20 mv), within 2 to 3 minutes after occlusion. Occlusion was released when ischemic depolarization lasted for 20 minutes. The animals body temperature was maintained at 37 C with a temperature control system (VitaView 4.1, Mini Mitter Co, Bend, Ore). Experimental protocols were approved by the institutional animal care and use committees at the Indiana University School of Medicine. Brain Slice Preparation and Whole-Cell Voltage-Clamp Recording Brain slices were prepared from control and ischemic animals at different stages (3 and 9 hours) after reperfusion as described previously. 13 For whole-cell recording, patch electrodes were filled with an intracellular solution containing (in mmol/l): 43 CsCl, 92 CsMeSO 4, 5 TEA, 2 EGTA, 1 MgCl 2, 10 HEPES, and 4 ATP (Sigma, St. Louis, Mo). Neurobiotin (2%; Vector Laboratories, Burlingame, Calif) was included in the solution to allow verification of the identity of recorded neurons. The access resistance of the pipette was 10 to 15 M. Data were discarded if the access resistance varied by 20% during recording. Voltage-clamp recording was performed with an Axopatch 200 B amplifier (Axon Instruments, Foster City, Calif). Signals were filtered at 2 khz and digitized at a sampling rate of 5 khz with a data acquisition program (Axograph 4.9, Axon Instruments). Intrastriatal stimulation was delivered every 10 seconds to evoke postsynaptic responses. For the paired-pulse test, 2 stimuli with the same intensity were applied at an interval of 50 ms. The Axograph 4.9 program was used to perform the miniature excitatory postsynaptic current (mepsc) analysis. Data are presented as mean SEM. Paired student s t test was used for comparison of the percentage change in EPSC amplitude. Changes were considered significant at P In some experiments, neurobiotin was iontophoresed into the cell by passing depolarizing pulses after recording. The slice was then fixed in 4% paraformaldehyde overnight and incubated in 0.1% horseradish peroxidase conjugated avidin D (Vector Laboratories) in 0.01 mol/l phosphate-buffered saline with 0.5% Triton X-100 for 24 hours at room temperature. After detection of peroxidase activity with 3,3 -diaminobenzidine, slices containing labeled neurons were mounted on gelatin-coated slides and processed for light microscopy. Histologic Examination Histologic experiments were conducted to evaluate ischemic neuronal damage with or without D1R agonists. Twenty-four hours after ischemia, the rats were perfused transcardially with a mixture of 3.7% formaldehyde, 10% glacial acetic acid, and 80% methanol. Brain blocks containing neostriatum were dehydrated and embedded in paraffin. Serial coronal sections were cut into 10- m-thick slices selected from 3 interaural planes in the neostriatum, corresponding to 10.6, 8.7, and 8.08 mm. Four consecutive sections were taken from each plane. The sections were subsequently stained with hematoxylin/eosin. Two areas in the dorsal neostriatum (lateral and medial) were randomly selected in each hemisphere of a section by light microscopy ( 200) and digitized with an image analysis program (NIH Image 1.57). Dead cells show condensed nuclei and plasma membrane lysis, which can be readily separated from cells with normal morphology under a light microscope. Ischemic neuronal damage was evaluated by counting surviving neurons in each area. The experimenter conducting the counting was blinded to the experimental groups. For each rat, counting of surviving neurons was carried out in 48 microscopic fields ( m) in the dorsal part of the striatum in both hemispheres. Drug Application ( )-Bicuculline methiodide, ( )-2-amino-5-phosphonopentanoic acid, and 6-cyano-7-nitroquinoxaline-2,3-dione were purchased from Sigma. Drugs were applied via bath superfusion or recording pipette according to the experimental design. ( )-Bicuculline methiodide was used to block -aminobutyric acid A receptors at a concentration of 30 mol/l throughout the experiment. Sodium metabisulfite (Na 2 S 2 O 5,50 mol/l) was used to protect dopamine and dopaminergic agonists and antagonists from oxidation. For the histologic experiment, SKF38393 (5 mg/kg) was injected intraperitoneally immediately after ischemia. Results Activation of D1Rs Suppresses EPSCs in Spiny Neurons After Ischemia Whole-cell voltage-clamp recordings were performed on neurons in the dorsolateral striatum with medium-size somata in brain slices. Intrastriatal stimulation was delivered to evoke postsynaptic responses at a holding potential of 70 mv. After a stable whole-cell configuration was reached, we first determined the threshold of stimulus intensity. The threshold was defined as the stimulating current evoking the smallest detectable response from spiny neurons. Then a stimulation intensity of 2 times threshold was delivered to evoke EPSCs in the presence of the -aminobutyric acid A receptor antagonist ( )-bicuculline methiodide (30 mol/l). As shown in Figures 1A and 1B, the recorded neuron possessed a medium-size soma ( 15 m) with a large number of spines on its dendrites, indicating that the recorded neurons were medium spiny neurons. To examine the effects of D1R activation on EPSCs in postischemic spiny neurons, we applied the specific D1R agonist SKF38393 (10 mol/l) in the bath medium after a 5-minute baseline recording. SKF38393 reversibly suppressed EPSC amplitude at both 3 ( % of pre-skf38393 application, P 0.05, n 12) and 9 ( % of pre-skf38393 application, P 0.05, n 9; Figure 1) hours after ischemia. In contrast, EPSCs were potentiated by SKF38393 in control neurons ( % of pre-skf38393 application, P 0.05, n 6), which was consistent with a previous report. 14 The rising slope and decay time constant were not significantly altered by SKF38393 (data not shown). Also, SKF38393 did not change the holding current during recording.
3 2372 Stroke August 2008 We also included the specific D1R antagonist SCH23390 (1 mol/l) in the extracellular medium and found that subsequent application of SKF38393 failed to depress EPSC amplitude ( % of pre-skf38393 application, P 0.05, n 5). Alternatively, we applied dopamine (50 mol/l) after preincubation with sulpiride (10 mol/l), a D2R antagonist. Dopamine decreased the EPSC amplitude by % (P 0.05, n 5; Figure 1D) in the presence of sulpiride. These results suggest that SKF38393 suppresses excitatory synaptic transmission in spiny neurons after ischemia by activating D1Rs. Figure 1. D1R activation depressed EPSCs in postischemic spiny neurons. A, Photograph of a stained spiny neuron filled intracellularly with neurobiotin after recording. B, Spiny dendrites inside box in A viewed at higher magnification. C, SKF38393 depressed EPSCs in spiny neurons 3 and 9 hours after ischemia, although it enhanced EPSCs in control neurons. Data were averaged from peak EPSC amplitudes during the last 3 minutes of each phase. D, Dopamine depressed EPSCs in the presence of sulpiride, but SKF38393 had no effect on EPSC amplitude after pretreatment with SCH Upper and lower edges of the box indicate the 75th and 25th percentiles of the data set, respectively. The line in the box indicates the median of the data. Two whiskers indicate the minimum and maximum data values, unless outliers (gray circles) are present, in which case the whiskers extend to a maximum of 1.5 times the interquartile range. E, Time course of the percentage change in EPSC amplitude in neurons after ischemia. The black bold line denotes the phase of drug application. F, Sample traces taken from times denoted by the numbers in E. A Presynaptic Mechanism Is Responsible for the Depressive Effects of D1Rs We recorded mepscs without adding tetrodotoxin, which has no effect on spontaneous EPSCs in postischemic spiny neurons. 10 We found that mepsc frequency decreased by % (P 0.05, n 10; Figures 2A and 2B) in the presence of SKF38393 while mepsc amplitude was unchanged under the same conditions ( % of pre- SKF38393 application, P 0.05, n 10; Figures 2A and 2B). We also examined the effects of D1R activation on pairedpulse ratio. Figures 2C and 2D show that the magnitude of the paired-pulse ratio was increased in 6 of 6 cells tested, with an average % increase (P 0.05). These results suggest that a presynaptic, but not a postsynaptic, mechanism is responsible for the depressive effects of D1R activation on excitatory inputs of postischemic spiny neurons. The Depressive Effect of D1R Activation Is Dependent on PKA D1R activation triggers a G protein mediated signal cascade that stimulates adenylyl cyclase and subsequently, an activator of camp-dependent protein kinase A (PKA). We therefore tested whether this pathway was involved in the SKF38393-induced EPSC depression. 8-Br-cAMP (100 mol/l), a membrane-permeable PKA activator, reversibly reduced the evoked EPSC to % of baseline (P 0.05, n 7; Figures 3A and 3C), an effect comparable to that of SKF Preincubating the slices with 10 mol/l Rp-cAMP, a membrane-permeable PKA inhibitor, blocked the depressive effects of SKF38393 on EPSC amplitude ( % of pre-skf38393 application, P 0.05, n 6; Figures 3B and 3C). H89 (5 mol/l), a structurally distinct
4 Zhang et al D1Rs Depress EPSCs in Spiny Neurons After Ischemia 2373 Figure 2. A presynaptic mechanism was responsible for the D1R-induced depression of EPSCs in postischemic spiny neurons. A, Sample traces of mepscs recorded from postischemic spiny neurons before, during, and after SKF38393 application. B, Box plot showing the group data of SKF38393-induced changes in mepsc frequency and amplitude. C, Sample traces of paired-pulse stimulation before and during SKF38393 application. D, Paired-pulse ratio changes in individual neuron before and during SKF38393 application. PKA inhibitor, had a comparable lack of effect in blocking the SKF38393-induced EPSC depression ( % of pre-skf38393 application, P 0.05, n 5; Figure 3C). Electron microscopy study has shown that presynaptic D1Rs are very rare under normal conditions, 15 although the expression pattern of D1Rs after ischemia is unclear. Our data, however, suggest that D1R activation presynaptically depresses excitatory neurotransmission in spiny neurons after ischemia. To further examine the locus of D1R effects, we intracellularly applied PKI 6-22 (20 mol/l), a PKA inhibitor, to postsynaptic spiny neurons through a recording pipette. Unlike bath-applied PKA inhibitors, SKF38393 (10 mol/l) still depressed the EPSCs to % of baseline (P 0.05, n 6; Figure 4) in the presence of intracellular PKI 6-22, an extent comparable to that of SKF38393 Figure 4. Inactivation of PKA in postsynaptic neurons did not block the D1R depression of EPSCs in postischemic spiny neurons. A1, Intracellularly applied PKI 6-22 did not block the SKF38393-induced EPSC depression in a sample neuron. A2, Representative traces taken from the time indicated by numbers in A1. B, Intracellularly applied 8-Br-cAMP did not occlude the SKF38393-induced EPSC depression in a sample neuron. C, Group data showing the percentage change in EPSC amplitude by the drugs indicated in each bar. alone. To confirm that PKI 6-22 intracellularly applied through a recording pipette can inhibit PKA in postsynaptic neurons; we performed another experiment to serve as a control. Activation of D1R is known to potentiate EPSCs by PKA under control condition. 16 We tested the effect of SKF38393 on EPSC amplitude in control spiny neurons with an internal solution containing PKI 6-22 and found that SKF38393 failed to potentiate EPSCs ( % of pre- SKF38393 application, P 0.05, n 4), suggesting that intrapipette PKI 6-22 was functional. If D1R activation depresses EPSCs after ischemia without the involvement of PKA in postsynaptic neurons, then preactivation of PKA in postsynaptic neurons should not occlude the SKF38393 effect on EPSC depression. We tested our prediction by intracellular application of 8-Br-cAMP (100 mol/l) and found that SKF38393 still depressed EPSCs in the presence of intracellular 8-Br-cAMP ( % of pre-skf38393 application, P 0.05, n 5; Figures 4B and 4C). Figure 3. PKA mediated D1R depression of EPSCs in postischemic spiny neurons. A, 8-Br-cAMP reduced the amplitude of EPSCs in a sample neuron. B, SKF38393 had no effect on EPSC amplitude with Rp-cAMP in the bath medium in a sample neuron. C, Group data showing the percentage change in EPSC amplitude by the drugs indicated in each bar. H89 was applied in a manner similar to Rp-cAMP.
5 2374 Stroke August 2008 Figure 5. D1R-mediated depression of EPSCs was dependent on A1Rs. A, 1,3- Dipropyl-8-cyclopentylxanthine blocked the SKF38393-induced EPSC depression in a sample neuron. B, 1,3-Dipropyl-8- cyclopentylxanthine blocked the depressive effects of 8-Br-cAMP in a sample neuron. C, Cyclopentyladenosine occluded the effects of SKF38393 in a sample neuron. D, AM251 had no effect on SKF38393-induced EPSC depression in a sample neuron. E, Group data showing the percentage change in EPSC amplitude by the drugs indicated in each bar. Activation of A1Rs Is Required for D1R-Mediated Depression of EPSCs Presynaptic depression could be either homosynaptic or heterosynaptic. Adenosine A1 receptors (A1Rs) are known to inhibit synaptic glutamate release and are reported to act downstream of D1R activation. 17 To test whether this was true in our case, we preincubated brain slices with the A1R antagonist 1,3-dipropyl-8-cyclopentylxanthine (0.5 mol/l) and found that both SKF38393 and 8-Br-cAMP failed to depress EPSCs in postischemic spiny neurons ( % for SKF38393, P 0.05, n 6; Figures 5A and 5E; % for 8-BrcAMP, P 0.05, n 5; Figures 5B and 5E). Pretreatment with cyclopentyladenosine (0.1 mol/l), an A1R agonist, also occluded SKF38393-induced EPSC depression ( % of pre-skf38393 application, P 0.05, n 5; Figures 5C and 5E), suggesting that A1R activation mediated the effects of D1Rs. We also tested the effects of AM251 (1 mol/l), an endocannabinoid CB1 receptor antagonist, because CB1 receptors have been shown to induce presynaptic depression. As shown in Figure 5, AM251 did not block the effects of SKF38393 ( % of pre-skf38393 application, P 0.05, n 5; Figures 5D and 5E). Systemic Administration of a D1R Agonist Attenuated Ischemic Neuronal Injury To test whether depression of EPSCs can protect striatal neurons against ischemia, we intraperitoneally injected SKF38393 (5 mg/kg) immediately after ischemia, and histologic slides were prepared to evaluate ischemic damage. It is worthwhile to point out that previous studies regarding the effects of a D1R agonist on ischemic injury are controversial. A substantial source of the debate is the variation in brain temperature after ischemia, 6,7 because it is well known that hypothermia can dramatically alleviate ischemic neuronal injury. To reduce the variation in brain temperature and make the results more consistent, we maintained the animals body temperature at 37 C after ischemia until they were euthanized. Survival of striatal neurons was assessed 24 hours after termination of ischemia. Normal striatal neurons are characterized by a round soma and clear intact nuclei by hematoxylin/eosin analyses (Figure 6B), whereas dying neurons appear shrunken with pyknotic nuclei (Figure 6A). In animals that received saline injection after ischemia, the number of surviving neurons in each image was (n 6, see Methods), which was consistent with our previous study in the same ischemia model. 4 The number of surviving neurons in
6 Zhang et al D1Rs Depress EPSCs in Spiny Neurons After Ischemia 2375 Figure 6. In vivo administration of a D1R agonist after ischemia alleviated striatal neuronal injury. A and B, Representative images of hematoxylin/eosin staining in saline- and SKF38393-treated groups, respectively. Bar 50 m. C, Group data showing that the number of surviving neuron was increased by SKF38393 injection. D, A schematic drawing shows that activation of D1Rs on neighboring cells (green) induces the formation of adenosine, which subsequently inhibits synaptic glutamate release from presynaptic terminals (red) through A1R activation in postischemic striatum. Depression of EPSCs in striatal neurons (yellow) might alleviate ischemic injury. animals that received SKF38393 injection after ischemia showed a dramatic increase, to (n 10, P 0.05; Figure 6C). Discussion Our results have demonstrated that D1R activation depresses excitatory neurotransmission in postischemic striatal neurons through activation of PKA and adenosine A1Rs, and such depression might contribute to D1R-induced neuroprotection (Figure 6D). Excitatory synaptic transmission has received much attention in ischemic cell death because of its important role in excitotoxicity. It is therefore reasonable to consider that facilitation of synaptic glutamate release would potentiate synaptic activity and neuronal injury after ischemia. This idea is supported by both in vivo and in vitro studies. BAPTA-AM protects cultured neurons against oxygenglucose deprivation (OGD) by inhibiting glutamate release rather than buffering calcium in postsynaptic cells. 18 Our previous studies also showed that synaptic glutamate release is facilitated in striatal spiny neurons after transient global ischemia, 10 whereas it is inhibited in ischemia-resistant cholinergic interneurons. 19 It is thought that the differential changes in synaptic glutamate release of these 2 types of neurons are associated with their distinct sensitivity to ischemia. In this regard, modulation of excitatory synaptic transmission may be a factor that influences ischemic outcome. Changes in synaptic strength, eg, long-term potentiation, were observed in striatal spiny neurons after OGD and have been proposed as a putative mechanism of selective cell death in the striatum because it was not observed in other ischemia-resistant striatal neurons. 20 Study of brain slices subjected to OGD have demonstrated that ischemic long-term potentiation in spiny neurons is amplified by endogenous activation of dopamine D1Rs. 21 Excitatory postsynaptic potentials failed to increase after OGD in the presence of a D1R antagonist or in D1R -/- mice, 21 demonstrating an endogenous role for D1Rs in ischemia-induced synaptic plasticity. However, our studies, based on an in vivo ischemia model, found that ischemia-induced enhancement of excitatory neurotransmission 10 can be suppressed by exogenous activation of D1Rs. The result is not surprising, because our study revealed an exogenous effect of D1Rs on postischemic synaptic activity. Furthermore, a previous study from this laboratory revealed a similar discrepancy. For example, excitatory neurotransmission in cholinergic interneuron is presynaptically depressed after in vivo ischemia, 19 but it does not show significant changes after in vitro OGD. 20 We speculate that the difference may result from the distinct experimental conditions, eg, the ischemia model and different stages after insult. It is well known that D1R activation potentiates EPSCs in normal striatal neurons through PKA phosphorylating postsynaptic -amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. 16 However, we demonstrated an alternative pathway of D1R signaling in postischemic striatal neurons, ie, D1R- PKA-A1R-EPSC (Figure 6D). It is interesting to explore why D1Rs activate a different pathway after ischemia. It has been known that a similar mechanism underlies D1R depression of EPSCs in normal nucleus accumbens neurons, a process that is N-methyl-D-aspartate receptor dependent. 22 Indeed, calcium influx through N-methyl-D-aspartate receptors mediates multiple dopamine effects in the striatum. 23 On the basis of these previous studies, we speculate that other postischemic changes in striatal neurons, eg, elevated calcium concentrations, promote D1Rinduced adenosine formation and thus depress EPSCs. It should also be pointed out that our recordings were performed in brain slices taken from rats subjected to in vivo ischemia, and there was no ischemic insult during recording. Thus, any observed difference in D1R effects between control and ischemic conditions can be attributed to the actions of ischemia that occurred in vivo. Dopamine exacerbates ischemic cell death, as indicated by the evidence that depletion of dopamineregic neurons in the
7 2376 Stroke August 2008 substantia nigra alleviates neuronal degeneration after ischemia. 2 It has been shown that dopamine-mediated toxicity might also involve glutamate. This was deduced initially from studies in which dopamine denervation attenuated glutamateinduced striatal lesions. 24 Elevated levels of dopamine inhibit glutamate uptake, perhaps via the action of dopamine-derived reactive oxygen species. 25 This could cause an elevation of synaptic glutamate levels, which leads to overactivation of glutamate receptors and cell death. Aside from the ability of dopamine to produce reactive oxygen species, dopamine can also be involved in ischemic injury through receptor-dependent mechanisms. Dopamine can protect cultured striatal neurons against glutamate-induced cell death through D1R-mediated increases in intracellular camp. 26 Studies with in vivo ischemia model have shown comparable results. 6 In conclusion, our study not only confirmed previous reports that dopamine is involved in glutamate excitotoxicity but also, more important, demonstrated that a subtype of dopamine receptor, D1Rs, can inhibit synaptic glutamate release after ischemia and might underlie the neuroprotective mechanisms of D1R activation. Our study described a correlation between EPSC depression and alleviated cell death by D1R activation. Obviously, correlation does not mean causation. To test for functional causality, we need to prevent the D1R-induced EPSC depression in vivo and then examine ischemic injury. Unfortunately, such experiments are not feasible owing to technical restrictions. It is extremely difficult, if not impossible, to selectively manipulate synaptic receptors without interfering with extrasynaptic receptors in intact brains. However, as advances in genetic manipulation and molecular biology techniques continue, we believe that our concern could be resolved in the near future. Acknowledgments We are grateful to Dr Zhigang Lei, Department of Anatomy and Cell Biology, Indiana University School of Medicine, for the exploratory work involved in this project. We thank Dr Edwin Richard for proofreading the manuscript. Sources of Funding This work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke grant R01 NS38053 (Z.C.X.) and by an American Heart Association fellowship ( Z, Y.Z.; Z, P.D.). None. Disclosures References 1. Globus MY, Busto R, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of ischemia on the in vivo release of striatal dopamine, glutamate, and -aminobutyric acid studied by intracerebral microdialysis. J Neurochem. 1988;51: Globus MY, Ginsberg MD, Dietrich WD, Busto R, Scheinberg P. Substantia nigra lesion protects against ischemic damage in the striatum. Neurosci Lett. 1987;80: Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14: Ren Y, Li X, Xu ZC. Asymmetrical protection of neostriatal neurons against transient forebrain ischemia by unilateral dopamine depletion. Exp Neurol. 1997;146: Xu ZC, Ling G, Sahr RN, Neal-Beliveau BS. Asymmetrical changes of dopamine receptors in the striatum after unilateral dopamine depletion. Brain Res. 2005;1038: Armentero MT, Fancellu R, Nappi G, Blandini F. Dopamine receptor agonists mediate neuroprotection in malonate-induced striatal lesion in the rat. Exp Neurol. 2002;178: Yamamoto Y, Tanaka T, Shibata S, Watanabe S. Involvement of D1 dopamine receptor mechanism in ischemia-induced impairment of CA1 presynaptic fiber spikes in rat hippocampal slices. Brain Res. 1994;665: Okada Y, Sakai H, Kohiki E, Suga E, Yanagisawa Y, Tanaka K, Hadano S, Osuga H, Ikeda JE. A dopamine D4 receptor antagonist attenuates ischemia-induced neuronal cell damage via upregulation of neuronal apoptosis inhibitory protein. J Cereb Blood Flow Metab. 2005;25: Zou S, Li L, Pei L, Vukusic B, Van Tol HH, Lee FJ, Wan Q, Liu F. Protein-protein coupling/uncoupling enables dopamine D2 receptor regulation of AMPA receptor-mediated excitotoxicity. J Neurosci. 2005;25: Zhang Y, Deng P, Li Y, Xu ZC. Enhancement of excitatory synaptic transmission in spiny neurons after transient forebrain ischemia. J Neurophysiol. 2006;95: Reynolds JN, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 2002;15: Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10: Deng P, Pang ZP, Zhang Y, Xu ZC. Increase of delayed rectifier potassium currents in large aspiny neurons in the neostriatum following transient forebrain ischemia. Neuroscience. 2005;131: Umemiya M, Raymond LA. Dopaminergic modulation of excitatory postsynaptic currents in rat neostriatal neurons. J Neurophysiol. 1997;78: Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15: Price CJ, Kim P, Raymond LA. D1 dopamine receptor-induced cyclic AMP-dependent protein kinase phosphorylation and potentiation of striatal glutamate receptors. J Neurochem. 1999;73: Ding L, Perkel DJ, Farries MA. Presynaptic depression of glutamatergic synaptic transmission by D1-like dopamine receptor activation in the avian basal ganglia. J Neurosci. 2003;23: Abdel-Hamid KM, Tymianski M. Mechanisms and effects of intracellular calcium buffering on neuronal survival in organotypic hippocampal cultures exposed to anoxia/aglycemia or to excitotoxins. J Neurosci. 1997;17: Pang ZP, Deng P, Ruan YW, Xu ZC. Depression of fast excitatory synaptic transmission in large aspiny neurons of the neostriatum after transient forebrain ischemia. J Neurosci. 2002;22: Calabresi P, Saulle E, Centonze D, Pisani A, Marfia GA, Bernardi G. Post-ischaemic long-term synaptic potentiation in the striatum: a putative mechanism for cell type-specific vulnerability. Brain. 2002;125: Saulle E, Centonze D, Martin AB, Moratalla R, Bernardi G, Calabresi P. Endogenous dopamine amplifies ischemic long-term potentiation via D1 receptors. Stroke. 2002;33: Harvey J, Lacey MG. A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J Neurosci. 1997;17: Konradi C, Leveque JC, Hyman SE. Amphetamine and dopamineinduced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci. 1996;16: Filloux F, Wamsley JK. Dopaminergic modulation of excitotoxicity in rat striatum: evidence from nigrostriatal lesions. Synapse. 1991;8: Berman SB, Hastings TG. Inhibition of glutamate transport in synaptosomes by dopamine oxidation and reactive oxygen species. J Neurochem. 1997;69: Amano T, Ujihara H, Matsubayashi H, Sasa M, Yokota T, Tamura Y, Akaike A. Dopamine-induced protection of striatal neurons against kainate receptor-mediated glutamate cytotoxicity in vitro. Brain Res. 1994; 655:61 69.
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
Supporting Information
 ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
Enhanced excitatory synaptic transmission in spiny neurons of rat striatum after unilateral dopamine denervation
 Neuroscience Letters 308 (2001) 201±205 www.elsevier.com/locate/neulet Enhanced excitatory synaptic transmission in spiny neurons of rat striatum after unilateral dopamine denervation Zhiping Pang, Guang
Neuroscience Letters 308 (2001) 201±205 www.elsevier.com/locate/neulet Enhanced excitatory synaptic transmission in spiny neurons of rat striatum after unilateral dopamine denervation Zhiping Pang, Guang
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Neurons of the Bed Nucleus of the Stria Terminalis (BNST)
 Neurons of the Bed Nucleus of the Stria Terminalis (BNST) Electrophysiological Properties and Their Response to Serotonin DONALD G. RAINNIE a Harvard Medical School and Department of Psychiatry, Brockton
Neurons of the Bed Nucleus of the Stria Terminalis (BNST) Electrophysiological Properties and Their Response to Serotonin DONALD G. RAINNIE a Harvard Medical School and Department of Psychiatry, Brockton
Basal Ganglia Anatomy, Physiology, and Function. NS201c
 Basal Ganglia Anatomy, Physiology, and Function NS201c Human Basal Ganglia Anatomy Basal Ganglia Circuits: The Classical Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate
Basal Ganglia Anatomy, Physiology, and Function NS201c Human Basal Ganglia Anatomy Basal Ganglia Circuits: The Classical Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate
Astrocyte signaling controls spike timing-dependent depression at neocortical synapses
 Supplementary Information Astrocyte signaling controls spike timing-dependent depression at neocortical synapses Rogier Min and Thomas Nevian Department of Physiology, University of Berne, Bern, Switzerland
Supplementary Information Astrocyte signaling controls spike timing-dependent depression at neocortical synapses Rogier Min and Thomas Nevian Department of Physiology, University of Berne, Bern, Switzerland
SUPPLEMENTARY INFORMATION. Supplementary Figure 1
 SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
Ube3a is required for experience-dependent maturation of the neocortex
 Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
MOLECULAR AND CELLULAR NEUROSCIENCE
 MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
Part 11: Mechanisms of Learning
 Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Chapter 2: Cellular Mechanisms and Cognition
 Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Is action potential threshold lowest in the axon?
 Supplementary information to: Is action potential threshold lowest in the axon? Maarten H. P. Kole & Greg J. Stuart Supplementary Fig. 1 Analysis of action potential (AP) threshold criteria. (a) Example
Supplementary information to: Is action potential threshold lowest in the axon? Maarten H. P. Kole & Greg J. Stuart Supplementary Fig. 1 Analysis of action potential (AP) threshold criteria. (a) Example
The control of spiking by synaptic input in striatal and pallidal neurons
 The control of spiking by synaptic input in striatal and pallidal neurons Dieter Jaeger Department of Biology, Emory University, Atlanta, GA 30322 Key words: Abstract: rat, slice, whole cell, dynamic current
The control of spiking by synaptic input in striatal and pallidal neurons Dieter Jaeger Department of Biology, Emory University, Atlanta, GA 30322 Key words: Abstract: rat, slice, whole cell, dynamic current
Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons
 11488 Journal of Physiology (2001), 532.3, pp.731 748 731 Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons Chiung-Chun Huang, Shiow-Win
11488 Journal of Physiology (2001), 532.3, pp.731 748 731 Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons Chiung-Chun Huang, Shiow-Win
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/317/5841/183/dc1 Supporting Online Material for Astrocytes Potentiate Transmitter Release at Single Hippocampal Synapses Gertrudis Perea and Alfonso Araque* *To whom
www.sciencemag.org/cgi/content/full/317/5841/183/dc1 Supporting Online Material for Astrocytes Potentiate Transmitter Release at Single Hippocampal Synapses Gertrudis Perea and Alfonso Araque* *To whom
Synaptic Plasticity and the NMDA Receptor
 Synaptic Plasticity and the NMDA Receptor Lecture 4.2 David S. Touretzky November, 2015 Long Term Synaptic Plasticity Long Term Potentiation (LTP) Reversal of LTP Long Term Depression (LTD) Reversal of
Synaptic Plasticity and the NMDA Receptor Lecture 4.2 David S. Touretzky November, 2015 Long Term Synaptic Plasticity Long Term Potentiation (LTP) Reversal of LTP Long Term Depression (LTD) Reversal of
Supplementary Information
 Supplementary Information D-Serine regulates cerebellar LTD and motor coordination through the 2 glutamate receptor Wataru Kakegawa, Yurika Miyoshi, Kenji Hamase, Shinji Matsuda, Keiko Matsuda, Kazuhisa
Supplementary Information D-Serine regulates cerebellar LTD and motor coordination through the 2 glutamate receptor Wataru Kakegawa, Yurika Miyoshi, Kenji Hamase, Shinji Matsuda, Keiko Matsuda, Kazuhisa
MOLECULAR BIOLOGY OF DRUG ADDICTION. Sylvane Desrivières, SGDP Centre
 1 MOLECULAR BIOLOGY OF DRUG ADDICTION Sylvane Desrivières, SGDP Centre Reward 2 Humans, as well as other organisms engage in behaviours that are rewarding The pleasurable feelings provide positive reinforcement
1 MOLECULAR BIOLOGY OF DRUG ADDICTION Sylvane Desrivières, SGDP Centre Reward 2 Humans, as well as other organisms engage in behaviours that are rewarding The pleasurable feelings provide positive reinforcement
Decreased Frequency But Not Amplitude of Quantal Synaptic Responses Associated with Expression of Corticostriatal Long-Term Depression
 The Journal of Neuroscience, November 1, 1997, 17(21):8613 8620 Decreased Frequency But Not Amplitude of Quantal Synaptic Responses Associated with Expression of Corticostriatal Long-Term Depression Sukwoo
The Journal of Neuroscience, November 1, 1997, 17(21):8613 8620 Decreased Frequency But Not Amplitude of Quantal Synaptic Responses Associated with Expression of Corticostriatal Long-Term Depression Sukwoo
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM
 STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
Synaptic Integration
 Synaptic Integration 3 rd January, 2017 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Excitatory Synaptic Actions Excitatory Synaptic Action
Synaptic Integration 3 rd January, 2017 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Excitatory Synaptic Actions Excitatory Synaptic Action
Neuroscience 201A (2016) - Problems in Synaptic Physiology
 Question 1: The record below in A shows an EPSC recorded from a cerebellar granule cell following stimulation (at the gap in the record) of a mossy fiber input. These responses are, then, evoked by stimulation.
Question 1: The record below in A shows an EPSC recorded from a cerebellar granule cell following stimulation (at the gap in the record) of a mossy fiber input. These responses are, then, evoked by stimulation.
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/312/5779/1533/dc1 Supporting Online Material for Long-Term Potentiation of Neuron-Glia Synapses Mediated by Ca 2+ - Permeable AMPA Receptors Woo-Ping Ge, Xiu-Juan Yang,
www.sciencemag.org/cgi/content/full/312/5779/1533/dc1 Supporting Online Material for Long-Term Potentiation of Neuron-Glia Synapses Mediated by Ca 2+ - Permeable AMPA Receptors Woo-Ping Ge, Xiu-Juan Yang,
Adenosine A 2A Receptors Are Essential for Long-Term Potentiation of NMDA-EPSCs at Hippocampal Mossy Fiber Synapses
 Article Adenosine A 2A Receptors Are Essential for Long-Term Potentiation of NMDA-EPSCs at Hippocampal Mossy Fiber Synapses Nelson Rebola, 1,3 Rafael Lujan, 2 Rodrigo A. Cunha, 1 and Christophe Mulle 3,
Article Adenosine A 2A Receptors Are Essential for Long-Term Potentiation of NMDA-EPSCs at Hippocampal Mossy Fiber Synapses Nelson Rebola, 1,3 Rafael Lujan, 2 Rodrigo A. Cunha, 1 and Christophe Mulle 3,
Chapter 3 subtitles Action potentials
 CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
Synaptic Transmission: Ionic and Metabotropic
 Synaptic Transmission: Ionic and Metabotropic D. Purves et al. Neuroscience (Sinauer Assoc.) Chapters 5, 6, 7. C. Koch. Biophysics of Computation (Oxford) Chapter 4. J.G. Nicholls et al. From Neuron to
Synaptic Transmission: Ionic and Metabotropic D. Purves et al. Neuroscience (Sinauer Assoc.) Chapters 5, 6, 7. C. Koch. Biophysics of Computation (Oxford) Chapter 4. J.G. Nicholls et al. From Neuron to
Supplementary Figure 1. Basic properties of compound EPSPs at
 Supplementary Figure 1. Basic properties of compound EPSPs at hippocampal CA3 CA3 cell synapses. (a) EPSPs were evoked by extracellular stimulation of the recurrent collaterals and pharmacologically isolated
Supplementary Figure 1. Basic properties of compound EPSPs at hippocampal CA3 CA3 cell synapses. (a) EPSPs were evoked by extracellular stimulation of the recurrent collaterals and pharmacologically isolated
Synaptic plasticityhippocampus. Neur 8790 Topics in Neuroscience: Neuroplasticity. Outline. Synaptic plasticity hypothesis
 Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Cellular Neurobiology / BIPN 140
 SECOND MIDTERM EXAMINATION Fall, 2015 GENERAL INSTRUCTIONS 1. Please write your name on ALL 6 pages. 2. Please answer each question IN THE SPACE ALLOTTED. 1) /10 pts 2) /10 pts 3) /15 pts 4) /15 pts 5)
SECOND MIDTERM EXAMINATION Fall, 2015 GENERAL INSTRUCTIONS 1. Please write your name on ALL 6 pages. 2. Please answer each question IN THE SPACE ALLOTTED. 1) /10 pts 2) /10 pts 3) /15 pts 4) /15 pts 5)
Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels. Vahri Beaumont and Robert S. Zucker
 Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels Vahri Beaumont and Robert S. Zucker Background I h channels discovered in 1976 (Noma A. and Irisawa H.) Voltage-gated
Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels Vahri Beaumont and Robert S. Zucker Background I h channels discovered in 1976 (Noma A. and Irisawa H.) Voltage-gated
BIPN140 Lecture 8: Synaptic Transmission II
 BIPN140 Lecture 8: Synaptic Transmission II 1. Postsynaptic Receptors: Metabotropic & Ionotropic 2. Postsynaptic Responses (Postsynaptic Potentials, PSPs) 3. Neurotransmitters Su (FA16) Chemical Synapse:
BIPN140 Lecture 8: Synaptic Transmission II 1. Postsynaptic Receptors: Metabotropic & Ionotropic 2. Postsynaptic Responses (Postsynaptic Potentials, PSPs) 3. Neurotransmitters Su (FA16) Chemical Synapse:
The wufless-ness of glutamate!
 The wufless-ness of glutamate! EXCITOTOXINS are substances, usually acidic amino acids, that react with specialized receptors in the brain in such a way as to lead to destruction of certain types of neurons.
The wufless-ness of glutamate! EXCITOTOXINS are substances, usually acidic amino acids, that react with specialized receptors in the brain in such a way as to lead to destruction of certain types of neurons.
The action potential travels down both branches because each branch is a typical axon with voltage dependent Na + and K+ channels.
 BIO 360 - MIDTERM FALL 2018 This is an open book, open notes exam. PLEASE WRITE YOUR NAME ON EACH SHEET. Read each question carefully and answer as well as you can. Point values are shown at the beginning
BIO 360 - MIDTERM FALL 2018 This is an open book, open notes exam. PLEASE WRITE YOUR NAME ON EACH SHEET. Read each question carefully and answer as well as you can. Point values are shown at the beginning
NS219: Basal Ganglia Anatomy
 NS219: Basal Ganglia Anatomy Human basal ganglia anatomy Analagous rodent basal ganglia nuclei Basal ganglia circuits: the classical model of direct and indirect pathways + Glutamate + - GABA - Gross anatomy
NS219: Basal Ganglia Anatomy Human basal ganglia anatomy Analagous rodent basal ganglia nuclei Basal ganglia circuits: the classical model of direct and indirect pathways + Glutamate + - GABA - Gross anatomy
Neurons, Synapses, and Signaling
 Neurons, Synapses, and Signaling The Neuron is the functional unit of the nervous system. Neurons are composed of a cell body, which contains the nucleus and organelles; Dendrites which are extensions
Neurons, Synapses, and Signaling The Neuron is the functional unit of the nervous system. Neurons are composed of a cell body, which contains the nucleus and organelles; Dendrites which are extensions
Supplementary Figure 1. SybII and Ceb are sorted to distinct vesicle populations in astrocytes. Nature Neuroscience: doi: /nn.
 Supplementary Figure 1 SybII and Ceb are sorted to distinct vesicle populations in astrocytes. (a) Exemplary images for cultured astrocytes co-immunolabeled with SybII and Ceb antibodies. SybII accumulates
Supplementary Figure 1 SybII and Ceb are sorted to distinct vesicle populations in astrocytes. (a) Exemplary images for cultured astrocytes co-immunolabeled with SybII and Ceb antibodies. SybII accumulates
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Introduction to Neurobiology
 Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
Dopamine in Ube3a m-/p+ mice. Online Supplemental Material
 Online Supplemental Material S1 Supplemental Figure 1. Schematic of rate-dependent intracranial self-stimulation (ICSS) (A) Mice implanted with monopolar stimulating electrodes to the medial forebrain
Online Supplemental Material S1 Supplemental Figure 1. Schematic of rate-dependent intracranial self-stimulation (ICSS) (A) Mice implanted with monopolar stimulating electrodes to the medial forebrain
Dania Ahmad. Tamer Barakat + Dania Ahmad. Faisal I. Mohammed
 16 Dania Ahmad Tamer Barakat + Dania Ahmad Faisal I. Mohammed Revision: What are the basic types of neurons? sensory (afferent), motor (efferent) and interneuron (equaled association neurons). We classified
16 Dania Ahmad Tamer Barakat + Dania Ahmad Faisal I. Mohammed Revision: What are the basic types of neurons? sensory (afferent), motor (efferent) and interneuron (equaled association neurons). We classified
Endogenous Dopamine Amplifies Ischemic Long-Term Potentiation via D1 Receptors
 Endogenous Dopamine Amplifies Ischemic Long-Term Potentiation via D1 Receptors Emilia Saulle, MD; Diego Centonze, MD; Ana B. Martín, PhD; Rosario Moratalla, PhD; Giorgio Bernardi, MD; Paolo Calabresi,
Endogenous Dopamine Amplifies Ischemic Long-Term Potentiation via D1 Receptors Emilia Saulle, MD; Diego Centonze, MD; Ana B. Martín, PhD; Rosario Moratalla, PhD; Giorgio Bernardi, MD; Paolo Calabresi,
NS200: In vitro electrophysiology section September 11th, 2013
 NS200: In vitro electrophysiology section September 11th, 2013 Quynh Anh Nguyen, 4 th Year Nicoll Lab quynhanh.nguyen@ucsf.edu N276 Genentech Hall, Mission Bay Outline Part I: Theory Review of circuit
NS200: In vitro electrophysiology section September 11th, 2013 Quynh Anh Nguyen, 4 th Year Nicoll Lab quynhanh.nguyen@ucsf.edu N276 Genentech Hall, Mission Bay Outline Part I: Theory Review of circuit
CASE 49. What type of memory is available for conscious retrieval? Which part of the brain stores semantic (factual) memories?
 CASE 49 A 43-year-old woman is brought to her primary care physician by her family because of concerns about her forgetfulness. The patient has a history of Down syndrome but no other medical problems.
CASE 49 A 43-year-old woman is brought to her primary care physician by her family because of concerns about her forgetfulness. The patient has a history of Down syndrome but no other medical problems.
Cellular mechanisms of information transfer: neuronal and synaptic plasticity
 Cellular mechanisms of information transfer: neuronal and synaptic plasticity Ivan Pavlov (UCL Institute of Neurology, UK) Anton Chizhov (Ioffe Physical Technical Institute) Pavel Zykin (St.-Petersburg
Cellular mechanisms of information transfer: neuronal and synaptic plasticity Ivan Pavlov (UCL Institute of Neurology, UK) Anton Chizhov (Ioffe Physical Technical Institute) Pavel Zykin (St.-Petersburg
Ultrastructural Contributions to Desensitization at the Cerebellar Mossy Fiber to Granule Cell Synapse
 Ultrastructural Contributions to Desensitization at the Cerebellar Mossy Fiber to Granule Cell Synapse Matthew A.Xu-Friedman and Wade G. Regehr Department of Neurobiology, Harvard Medical School, Boston,
Ultrastructural Contributions to Desensitization at the Cerebellar Mossy Fiber to Granule Cell Synapse Matthew A.Xu-Friedman and Wade G. Regehr Department of Neurobiology, Harvard Medical School, Boston,
Lecture 22: A little Neurobiology
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
Neuroscience: Exploring the Brain, 3e. Chapter 4: The action potential
 Neuroscience: Exploring the Brain, 3e Chapter 4: The action potential Introduction Action Potential in the Nervous System Conveys information over long distances Action potential Initiated in the axon
Neuroscience: Exploring the Brain, 3e Chapter 4: The action potential Introduction Action Potential in the Nervous System Conveys information over long distances Action potential Initiated in the axon
Activity Dependent Changes At the Developing Neuromuscular Junction
 Activity Dependent Changes At the Developing Neuromuscular Junction (slides 16, 17 and 18 have been slightly modified for clarity) MCP Lecture 2-3 9.013/7.68 04 Neuromuscular Junction Development 1. Muscle
Activity Dependent Changes At the Developing Neuromuscular Junction (slides 16, 17 and 18 have been slightly modified for clarity) MCP Lecture 2-3 9.013/7.68 04 Neuromuscular Junction Development 1. Muscle
Chapter 5 subtitles GABAergic synaptic transmission
 CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 5 subtitles GABAergic synaptic transmission INTRODUCTION (2:57) In this fifth chapter, you will learn how the binding of the GABA neurotransmitter to
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 5 subtitles GABAergic synaptic transmission INTRODUCTION (2:57) In this fifth chapter, you will learn how the binding of the GABA neurotransmitter to
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References
 File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC
 1 2 1 3 Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC 4 5 6 7 (a) Reconstructions of LII/III GIN-cells with somato-dendritic compartments in orange and axonal arborizations
1 2 1 3 Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC 4 5 6 7 (a) Reconstructions of LII/III GIN-cells with somato-dendritic compartments in orange and axonal arborizations
Section: Chapter 5: Multiple Choice. 1. The structure of synapses is best viewed with a(n):
 Section: Chapter 5: Multiple Choice 1. The structure of synapses is best viewed with a(n): p.155 electron microscope. light microscope. confocal microscope. nissle-stained microscopic procedure. 2. Electron
Section: Chapter 5: Multiple Choice 1. The structure of synapses is best viewed with a(n): p.155 electron microscope. light microscope. confocal microscope. nissle-stained microscopic procedure. 2. Electron
Chapter 6 subtitles postsynaptic integration
 CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 6 subtitles postsynaptic integration INTRODUCTION (1:56) This sixth and final chapter deals with the summation of presynaptic currents. Glutamate and
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 6 subtitles postsynaptic integration INTRODUCTION (1:56) This sixth and final chapter deals with the summation of presynaptic currents. Glutamate and
Excitatory synaptic transmission in the central nervous system. Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus
 Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus M. Frerking*, C. C. H. Petersen*, and R. A. Nicoll* Departments of *Cellular and Molecular Pharmacology and Physiology,
Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus M. Frerking*, C. C. H. Petersen*, and R. A. Nicoll* Departments of *Cellular and Molecular Pharmacology and Physiology,
DOPAMINE DA NEURON ANATOMY AND PHYSIOLOGY
 9 DOPAMINE ANTHONY A. GRACE Studies into the regulation of the dopamine (DA) system and its postsynaptic actions are often stymied by the myriad of actions that this neurotransmitter can produce. Thus,
9 DOPAMINE ANTHONY A. GRACE Studies into the regulation of the dopamine (DA) system and its postsynaptic actions are often stymied by the myriad of actions that this neurotransmitter can produce. Thus,
Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817.
 Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817. Four (4) hallmark clinical signs: 1) Tremor: (Note -
Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817. Four (4) hallmark clinical signs: 1) Tremor: (Note -
Axon initial segment position changes CA1 pyramidal neuron excitability
 Axon initial segment position changes CA1 pyramidal neuron excitability Cristina Nigro and Jason Pipkin UCSD Neurosciences Graduate Program Abstract The axon initial segment (AIS) is the portion of the
Axon initial segment position changes CA1 pyramidal neuron excitability Cristina Nigro and Jason Pipkin UCSD Neurosciences Graduate Program Abstract The axon initial segment (AIS) is the portion of the
Cholinergic Activation of M2 Receptors Leads to Context- Dependent Modulation of Feedforward Inhibition in the Visual Thalamus
 Cholinergic Activation of M2 Receptors Leads to Context- Dependent Modulation of Feedforward Inhibition in the Visual Thalamus Miklos Antal., Claudio Acuna-Goycolea., R. Todd Pressler, Dawn M. Blitz, Wade
Cholinergic Activation of M2 Receptors Leads to Context- Dependent Modulation of Feedforward Inhibition in the Visual Thalamus Miklos Antal., Claudio Acuna-Goycolea., R. Todd Pressler, Dawn M. Blitz, Wade
Vizcarra-Chacón et al. BMC Neuroscience 2013, 14:60
 Vizcarra-Chacón et al. BMC Neuroscience 2013, 14:60 RESEARCH ARTICLE Open Access Contribution of different classes of glutamate receptors in the corticostriatal polysynaptic responses from striatal direct
Vizcarra-Chacón et al. BMC Neuroscience 2013, 14:60 RESEARCH ARTICLE Open Access Contribution of different classes of glutamate receptors in the corticostriatal polysynaptic responses from striatal direct
Alterations in Synaptic Strength Preceding Axon Withdrawal
 Alterations in Synaptic Strength Preceding Axon Withdrawal H. Colman, J. Nabekura, J.W. Lichtman presented by Ana Fiallos Synaptic Transmission at the Neuromuscular Junction Motor neurons with cell bodies
Alterations in Synaptic Strength Preceding Axon Withdrawal H. Colman, J. Nabekura, J.W. Lichtman presented by Ana Fiallos Synaptic Transmission at the Neuromuscular Junction Motor neurons with cell bodies
Supplementary Information
 Hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K + channels Meena S. George, L.F. Abbott, Steven A. Siegelbaum Supplementary Information Part 1: Supplementary Figures
Hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K + channels Meena S. George, L.F. Abbott, Steven A. Siegelbaum Supplementary Information Part 1: Supplementary Figures
Muscarinic Receptors Differentially Modulate the Persistent Potassium Current in Striatal Spiny Projection Neurons
 RAPID COMMUNICATION Muscarinic Receptors Differentially Modulate the Persistent Potassium Current in Striatal Spiny Projection Neurons LISA A. GABEL AND ERIC S. NISENBAUM Department of Psychology, University
RAPID COMMUNICATION Muscarinic Receptors Differentially Modulate the Persistent Potassium Current in Striatal Spiny Projection Neurons LISA A. GABEL AND ERIC S. NISENBAUM Department of Psychology, University
Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids
 Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids Solange P Brown 1 3,Stephan D Brenowitz 1,3 & Wade G Regehr 1 Many types of neurons can release
Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids Solange P Brown 1 3,Stephan D Brenowitz 1,3 & Wade G Regehr 1 Many types of neurons can release
Astrocytes gate Hebbian synaptic plasticity in the striatum
 Received 13 Dec 215 Accepted 4 Nov 216 Published 2 Dec 216 Astrocytes gate Hebbian synaptic plasticity in the striatum Silvana Valtcheva 1,2 & Laurent Venance 1,2 DOI: 1.138/ncomms13845 OPEN Astrocytes,
Received 13 Dec 215 Accepted 4 Nov 216 Published 2 Dec 216 Astrocytes gate Hebbian synaptic plasticity in the striatum Silvana Valtcheva 1,2 & Laurent Venance 1,2 DOI: 1.138/ncomms13845 OPEN Astrocytes,
Presynaptic Depression of Glutamatergic Synaptic Transmission by D1-Like Dopamine Receptor Activation in the Avian Basal Ganglia
 6086 The Journal of Neuroscience, July 9, 2003 23(14):6086 6095 Behavioral/Systems/Cognitive Presynaptic Depression of Glutamatergic Synaptic Transmission by D1-Like Dopamine Receptor Activation in the
6086 The Journal of Neuroscience, July 9, 2003 23(14):6086 6095 Behavioral/Systems/Cognitive Presynaptic Depression of Glutamatergic Synaptic Transmission by D1-Like Dopamine Receptor Activation in the
Ionotropic glutamate receptors (iglurs)
 Ionotropic glutamate receptors (iglurs) GluA1 GluA2 GluA3 GluA4 GluN1 GluN2A GluN2B GluN2C GluN2D GluN3A GluN3B GluK1 GluK2 GluK3 GluK4 GluK5 The general architecture of receptor subunits Unique properties
Ionotropic glutamate receptors (iglurs) GluA1 GluA2 GluA3 GluA4 GluN1 GluN2A GluN2B GluN2C GluN2D GluN3A GluN3B GluK1 GluK2 GluK3 GluK4 GluK5 The general architecture of receptor subunits Unique properties
Nature Methods: doi: /nmeth Supplementary Figure 1. Activity in turtle dorsal cortex is sparse.
 Supplementary Figure 1 Activity in turtle dorsal cortex is sparse. a. Probability distribution of firing rates across the population (notice log scale) in our data. The range of firing rates is wide but
Supplementary Figure 1 Activity in turtle dorsal cortex is sparse. a. Probability distribution of firing rates across the population (notice log scale) in our data. The range of firing rates is wide but
Prolonged Synaptic Integration in Perirhinal Cortical Neurons
 RAPID COMMUNICATION Prolonged Synaptic Integration in Perirhinal Cortical Neurons JOHN M. BEGGS, 1 JAMES R. MOYER, JR., 1 JOHN P. MCGANN, 2 AND THOMAS H. BROWN 1 3 1 Department of Psychology, 2 Interdepartmental
RAPID COMMUNICATION Prolonged Synaptic Integration in Perirhinal Cortical Neurons JOHN M. BEGGS, 1 JAMES R. MOYER, JR., 1 JOHN P. MCGANN, 2 AND THOMAS H. BROWN 1 3 1 Department of Psychology, 2 Interdepartmental
SUPPLEMENTARY INFORMATION
 doi: 1.138/nature6416 Supplementary Notes Spine Ca 2+ signals produced by glutamate uncaging We imaged uncaging-evoked [Ca 2+ ] transients in neurons loaded with a green Ca 2+ - sensitive indicator (G;
doi: 1.138/nature6416 Supplementary Notes Spine Ca 2+ signals produced by glutamate uncaging We imaged uncaging-evoked [Ca 2+ ] transients in neurons loaded with a green Ca 2+ - sensitive indicator (G;
Lack of GPR88 enhances medium spiny neuron activity and alters. motor- and cue- dependent behaviors
 Lack of GPR88 enhances medium spiny neuron activity and alters motor- and cue- dependent behaviors Albert Quintana, Elisenda Sanz, Wengang Wang, Granville P. Storey, Ali D. Güler Matthew J. Wanat, Bryan
Lack of GPR88 enhances medium spiny neuron activity and alters motor- and cue- dependent behaviors Albert Quintana, Elisenda Sanz, Wengang Wang, Granville P. Storey, Ali D. Güler Matthew J. Wanat, Bryan
ASYMMETRICAL CHANGES OF EXCITATORY SYNAPTIC TRANSMISSION IN DOPAMINE-DENERVATED STRIATUM AFTER TRANSIENT FOREBRAIN ISCHEMIA
 www.neuroscience-ibro.com Neuroscience Vol. 114, No. 2, pp. 317^326, 2002 ß 2002 IBRO. Published by ElsevierScience Ltd All rights reserved. Printed in Great Britain PII: S 0 3 0 6-4 5 2 2( 0 2) 0 0 3
www.neuroscience-ibro.com Neuroscience Vol. 114, No. 2, pp. 317^326, 2002 ß 2002 IBRO. Published by ElsevierScience Ltd All rights reserved. Printed in Great Britain PII: S 0 3 0 6-4 5 2 2( 0 2) 0 0 3
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed.,
 Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by B.-W. Ku,
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by B.-W. Ku,
Basal Ganglia. Introduction. Basal Ganglia at a Glance. Role of the BG
 Basal Ganglia Shepherd (2004) Chapter 9 Charles J. Wilson Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Introduction A set of nuclei in the forebrain and midbrain area in mammals, birds, and reptiles.
Basal Ganglia Shepherd (2004) Chapter 9 Charles J. Wilson Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Introduction A set of nuclei in the forebrain and midbrain area in mammals, birds, and reptiles.
Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit
 Br. J. Pharmac. (1971), 41, 331-338. Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit D. D. CHRIST AND S. NISHI Neurophysiology Laboratory, Department of Pharmacology,
Br. J. Pharmac. (1971), 41, 331-338. Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit D. D. CHRIST AND S. NISHI Neurophysiology Laboratory, Department of Pharmacology,
Synaptic Plasticity and Memory
 Synaptic Plasticity and Memory Properties and synaptic mechanisms underlying the induction of long-term potentiation (LTP) The role of calcium/calmodulin-dependent kinase II (CamKII) in the induction,
Synaptic Plasticity and Memory Properties and synaptic mechanisms underlying the induction of long-term potentiation (LTP) The role of calcium/calmodulin-dependent kinase II (CamKII) in the induction,
Fundamentals of the Nervous System and Nervous Tissue: Part C
 PowerPoint Lecture Slides prepared by Janice Meeking, Mount Royal College C H A P T E R 11 Fundamentals of the Nervous System and Nervous Tissue: Part C Warm Up What is a neurotransmitter? What is the
PowerPoint Lecture Slides prepared by Janice Meeking, Mount Royal College C H A P T E R 11 Fundamentals of the Nervous System and Nervous Tissue: Part C Warm Up What is a neurotransmitter? What is the
Thursday, January 22, Nerve impulse
 Nerve impulse Transmembrane Potential caused by ions moving through cell membrane at different rates Two main ions of concern Na + - Sodium K + - potassium Cell membrane not freely permeable therefore
Nerve impulse Transmembrane Potential caused by ions moving through cell membrane at different rates Two main ions of concern Na + - Sodium K + - potassium Cell membrane not freely permeable therefore
Supplementary Figure 1. ACE robotic platform. A. Overview of the rig setup showing major hardware components of ACE (Automatic single Cell
 2 Supplementary Figure 1. ACE robotic platform. A. Overview of the rig setup showing major hardware components of ACE (Automatic single Cell Experimenter) including the MultiClamp 700B, Digidata 1440A,
2 Supplementary Figure 1. ACE robotic platform. A. Overview of the rig setup showing major hardware components of ACE (Automatic single Cell Experimenter) including the MultiClamp 700B, Digidata 1440A,
Ivy/Neurogliaform Interneurons Coordinate Activity in the Neurogenic Niche
 Ivy/Neurogliaform Interneurons Coordinate Activity in the Neurogenic Niche Sean J. Markwardt, Cristina V. Dieni, Jacques I. Wadiche & Linda Overstreet-Wadiche Supplementary Methods. Animals We used hemizygous
Ivy/Neurogliaform Interneurons Coordinate Activity in the Neurogenic Niche Sean J. Markwardt, Cristina V. Dieni, Jacques I. Wadiche & Linda Overstreet-Wadiche Supplementary Methods. Animals We used hemizygous
5-Nervous system II: Physiology of Neurons
 5-Nervous system II: Physiology of Neurons AXON ION GRADIENTS ACTION POTENTIAL (axon conduction) GRADED POTENTIAL (cell-cell communication at synapse) SYNAPSE STRUCTURE & FUNCTION NEURAL INTEGRATION CNS
5-Nervous system II: Physiology of Neurons AXON ION GRADIENTS ACTION POTENTIAL (axon conduction) GRADED POTENTIAL (cell-cell communication at synapse) SYNAPSE STRUCTURE & FUNCTION NEURAL INTEGRATION CNS
Basal Ganglia General Info
 Basal Ganglia General Info Neural clusters in peripheral nervous system are ganglia. In the central nervous system, they are called nuclei. Should be called Basal Nuclei but usually called Basal Ganglia.
Basal Ganglia General Info Neural clusters in peripheral nervous system are ganglia. In the central nervous system, they are called nuclei. Should be called Basal Nuclei but usually called Basal Ganglia.
10.1: Introduction. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial cells) Dendrites.
 10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
Summarized by B.-W. Ku, E. S. Lee, and B.-T. Zhang Biointelligence Laboratory, Seoul National University.
 Chapter 2. The Cellular l and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 3 rd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2008. Summarized by B.-W. Ku,
Chapter 2. The Cellular l and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 3 rd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2008. Summarized by B.-W. Ku,
previously shown (10), however, this manipulation by itself does not reliably result in the development of a large
 Proc. Nati. Acad. Sci. USA Vol. 85, pp. 9346-9350, December 1988 Neurobiology Long-term potentiation differentially affects two components of synaptic responses in hippocampus (plasticity/n-methyl-d-aspartate/d-2-amino-5-phosphonovglerate/facilitation)
Proc. Nati. Acad. Sci. USA Vol. 85, pp. 9346-9350, December 1988 Neurobiology Long-term potentiation differentially affects two components of synaptic responses in hippocampus (plasticity/n-methyl-d-aspartate/d-2-amino-5-phosphonovglerate/facilitation)
Chapter 2. The Cellular and Molecular Basis of Cognition
 Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga,, R. B. Ivry,, and G. R. Mangun,, Norton, 2002. Summarized by B.-W. Ku,
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga,, R. B. Ivry,, and G. R. Mangun,, Norton, 2002. Summarized by B.-W. Ku,
Bidirectional NMDA receptor plasticity controls CA3 output and heterosynaptic metaplasticity
 Bidirectional NMDA receptor plasticity controls CA output and heterosynaptic metaplasticity David L. Hunt, Nagore Puente, Pedro Grandes, Pablo E. Castillo a NMDAR EPSC (pa) - - -8-6 -4 - st 5 nd 5 b NMDAR
Bidirectional NMDA receptor plasticity controls CA output and heterosynaptic metaplasticity David L. Hunt, Nagore Puente, Pedro Grandes, Pablo E. Castillo a NMDAR EPSC (pa) - - -8-6 -4 - st 5 nd 5 b NMDAR
Intrinsic and Synaptic Properties of Neurons in an Avian Thalamic Nucleus During Song Learning
 J Neurophysiol 88: 1903 1914, 2002; 10.1152/jn.01043.2001. Intrinsic and Synaptic Properties of Neurons in an Avian Thalamic Nucleus During Song Learning MINMIN LUO AND DAVID J. PERKEL Department of Neuroscience,
J Neurophysiol 88: 1903 1914, 2002; 10.1152/jn.01043.2001. Intrinsic and Synaptic Properties of Neurons in an Avian Thalamic Nucleus During Song Learning MINMIN LUO AND DAVID J. PERKEL Department of Neuroscience,
DIFFERENTIAL CHANGES OF SYNAPTIC TRANSMISSION IN SPINY NEURONS OF RAT NEOSTRIATUM FOLLOWING TRANSIENT FOREBRAIN ISCHEMIA
 www.elsevier.com/locate/neuroscience Neuroscience Vol. 105, No. 1, pp. 139^152, 2001 ß 2001 IBRO. Published by Elsevier Science Ltd Printed in Great Britain. All rights reserved PII: S 0 3 0 6-4 5 2 2(
www.elsevier.com/locate/neuroscience Neuroscience Vol. 105, No. 1, pp. 139^152, 2001 ß 2001 IBRO. Published by Elsevier Science Ltd Printed in Great Britain. All rights reserved PII: S 0 3 0 6-4 5 2 2(
Anatomy of the basal ganglia. Dana Cohen Gonda Brain Research Center, room 410
 Anatomy of the basal ganglia Dana Cohen Gonda Brain Research Center, room 410 danacoh@gmail.com The basal ganglia The nuclei form a small minority of the brain s neuronal population. Little is known about
Anatomy of the basal ganglia Dana Cohen Gonda Brain Research Center, room 410 danacoh@gmail.com The basal ganglia The nuclei form a small minority of the brain s neuronal population. Little is known about
When cells are already maximally potentiated LTP is occluded.
 When cells are already maximally potentiated LTP is occluded. Stein, V et al., (2003) J Neurosci, 23:5503-6606. Also found in Rat Barrel Cortex Ehrlich & Malinow (2004) J. Neurosci. 24:916-927 Over-expression
When cells are already maximally potentiated LTP is occluded. Stein, V et al., (2003) J Neurosci, 23:5503-6606. Also found in Rat Barrel Cortex Ehrlich & Malinow (2004) J. Neurosci. 24:916-927 Over-expression
Cogs 107b Systems Neuroscience lec9_ neuromodulators and drugs of abuse principle of the week: functional anatomy
 Cogs 107b Systems Neuroscience www.dnitz.com lec9_02042010 neuromodulators and drugs of abuse principle of the week: functional anatomy Professor Nitz circa 1986 neurotransmitters: mediating information
Cogs 107b Systems Neuroscience www.dnitz.com lec9_02042010 neuromodulators and drugs of abuse principle of the week: functional anatomy Professor Nitz circa 1986 neurotransmitters: mediating information
Presynaptic NMDA receptor control of spontaneous and evoked activity By: Sally Si Ying Li Supervisor: Jesper Sjöström
 Presynaptic NMDA receptor control of spontaneous and evoked activity By: Sally Si Ying Li Supervisor: Jesper Sjöström NMDA receptors are traditionally known to function as post-synaptic coincidence detectors.
Presynaptic NMDA receptor control of spontaneous and evoked activity By: Sally Si Ying Li Supervisor: Jesper Sjöström NMDA receptors are traditionally known to function as post-synaptic coincidence detectors.
Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons
 Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons Peter Osseward, Uri Magaram Department of Neuroscience University of California, San Diego La Jolla, CA 92092 possewar@ucsd.edu
Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons Peter Osseward, Uri Magaram Department of Neuroscience University of California, San Diego La Jolla, CA 92092 possewar@ucsd.edu
Nature Neuroscience: doi: /nn Supplementary Figure 1. Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons
 Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
Notes are online at The Neuron
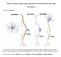 Notes are online at http://cogsci.ucsd.edu/~clovett/neuronotescogs17.pdf A. What is a neuron? The Neuron 1. A neuron is a type of cell that receives and transmits information in the Central Nervous System
Notes are online at http://cogsci.ucsd.edu/~clovett/neuronotescogs17.pdf A. What is a neuron? The Neuron 1. A neuron is a type of cell that receives and transmits information in the Central Nervous System
1) Drop off in the Bi 150 box outside Baxter 331 or to the head TA (jcolas).
 Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
photometry on the extruded cytoplasm.
 Answers To Midterm 2011 Question 1. a) Isoproterenol. Used to dissect presynaptic and postsynaptic components of sympathetic modulation of neuromuscular junction (Orbelli effect). Specifically activates
Answers To Midterm 2011 Question 1. a) Isoproterenol. Used to dissect presynaptic and postsynaptic components of sympathetic modulation of neuromuscular junction (Orbelli effect). Specifically activates
