Influenza A virus infection predisposes hosts to secondary infection with different
|
|
|
- Cornelia Hill
- 6 years ago
- Views:
Transcription
1 IAI Accepted Manuscript Posted Online 19 September 2016 Infect. Immun. doi: /iai Copyright 2016, American Society for Microbiology. All Rights Reserved Influenza A virus infection predisposes hosts to secondary infection with different Streptococcus pneumoniae serotypes with similar outcome but serotype-specific manifestation Niharika Sharma-Chawla 1,2, Vicky Sender 3, Olivia Kershaw 4, Achim D. Gruber 4, Julia Volckmar 1, Birgitta Henriques-Normark 3,5, Sabine Stegemann-Koniszewski 1,2 and Dunja Bruder 1, Immune regulation group, Helmholtz Centre for Infection Research, Braunschweig, Germany 1 ; Infection immunology group, Institute of Medical Microbiology, Infection Prevention and Control, Otto-von-Guericke University, Magdeburg, Germany 2 ; Department of Microbiology, Tumor and Cell Biology; Karolinska Institutet, Stockholm, Sweden 3 ; Department of Veterinary Medicine, Institute of Veterinary Pathology, Free University Berlin, Germany 4 ; Department of Clinical Microbiology, Karolinska University Hospital, Stockholm, Sweden Running title: Bacterial strain-dependency in the IAV-S. pn. synergism Correspondence to Sabine Stegemann-Koniszewski (Sabine.Stegemann- Koniszewski@helmholtz-hzi.de) and Dunja Bruder (Dunja.Bruder@med.ovgu.de) 18 SSK and DB contributed equally to this work
2 21 Abstract Influenza A virus (IAV) and Streptococcus pneumoniae (S. pn.) are major causes of respiratory tract infections, particularly during co-infection. The synergism between these two pathogens is characterized by a complex network of dysregulated immune responses, some of which last until recovery post IAV infection. Despite the high serotype-diversity of S. pn. and the serotype-replacement observed since the introduction of conjugate vaccines, little is known about pneumococcal straindependency in the enhanced susceptibility to severe secondary S. pn. infection following IAV infection. Thus we studied how pre-infection with IAV alters host susceptibility to different S. pn. strains with varying degrees of invasiveness using a highly invasive serotype 4, an invasive serotype 7F and a carrier serotype 19F strain. A murine model of pneumococcal co-infection during the acute phase of IAV infection showed a significantly increased degree of pneumonia and mortality for all tested pneumococcal strains at otherwise sublethal doses. The incidence and kinetics of systemic dissemination however remained bacterial strain-dependent. Furthermore we observed strain-specific alterations in the pulmonary levels of alveolar macrophages, neutrophils and inflammatory mediators ultimately affecting immunopathology. During the recovery phase following IAV infection, bacterial growth in the lungs and systemic dissemination were enhanced in a strain-dependent manner. Altogether, this study shows that acute IAV infection predisposes the host to lethal S. pn. infection irrespective of the pneumococcal serotype, while the long lasting synergism between IAV and S. pn. is bacterial strain-dependent. These results hold implications for developing tailored therapeutic treatment regimens for dual infections during future IAV outbreaks. 2
3 44 Introduction Infection with secondary bacterial pathogens is attributed to be the major cause of excessive mortality during influenza A virus (IAV) outbreaks. This lethal synergism has been recognized as early as during the 1918/1919 IAV pandemic with an estimated global death toll of million (1, 2). Retrospective studies disclosed that 71% of the fatal cases during this pandemic were positive for Streptococcus pneumoniae (S. pn. or the pneumococcus) providing the first epidemiological evidence for viral-bacterial coinfections (2). A clear pre-disposition to bacterial disease was also evident in all of the succeeding influenza pandemics including the more recent 2009 H1N1 outbreak with a 10-55% higher incidence of hospitalizations and mortality due to bacterial pneumonia (3). Pneumococcal colonization is transient and asymptomatic in immunocompetent individuals and most commonly occurs in early childhood (4). At the same time however, pneumococci are able to cause a variety of diseases ranging from mild sinusitis and otitis media to more severe infections like sepsis and meningitis. Even though the introduction of the polyvalent pneumococcal conjugate vaccines (PCV) has reduced the incidence of childhood carriage and disease for the vaccine serotypes (3-5), 1.6 million people die from pneumococcal infections annually with the majority being children under the age of 5 (6). The development of pneumococcal disease depends on both bacterial factors such as the pneumococcal capsular type or serotype and the host innate immune response (7, 8). S. pn. expresses a plethora of virulence factors of which the encapsulating polysaccharide layer is the most important and best-studied so far (9, 10). The capsule confers anti-phagocytic and anti-opsonophagocytic properties that determine the invasive pneumococcal disease (IPD) potential of different serotypes (11). 3
4 IPD is defined as the recovery of S. pn. from a normally sterile site such as the blood or brain (7, 12, 13). To date, 97 distinct serotypes have been described based on unique chemical and immunogenic properties of their capsule (10, 14) and these serotypes can be divided into invasive as well as non-invasive/carrier serotypes (15, 16). Otherwise non-invasive serotypes are able to lethally infect immunocompromised patients reflecting the impact of host-immunity on the IPD potential (8, 17). Once pneumococci enter the airways, innate immune responses are initiated by lung resident alveolar macrophages (AM) (18) that release pro-inflammatory cytokines and chemokines to recruit pro-inflammatory cells such as polymorphonuclear cells (PMN) and mononuclear phagocytes into the lung parenchyma and alveoli to contain the infection (19). Several studies have illustrated a multifactorial nature of IAV-S. pn. co-pathogenesis with a plethora of underlying mechanisms (3, 20, 21). These include virus-mediated immune modulations such as aberrant inflammatory cell recruitment and function as well as increased cell death, often leading to changes in the anti-pneumococcal cytokine and chemokine responses (22, 23). Apart from the often devastating effect on anti-bacterial responses observed during acute influenza, some reports have demonstrated long-term immune defects. These include impaired neutrophil influx due to sustained desensitization of AM and the induction of an immune-suppressive state during recovery (3, 24, 25). However, the mechanisms underlying enhanced susceptibility to S. pn. following IAV infection are not fully understood and reports are at times contradictory. One major limitation of past studies is the use of singular S. pn. strains despite large differences in pathogenesis. Therefore it remains unclear if the identified mechanisms generally apply to strains of different pneumococcal serotypes. Importantly, blood cultures from living cases of the 1918 IAV pandemic revealed a higher prevalence of 4
5 less invasive serotypes in secondary pneumococcal infections than the common pathogenic serotypes found at that time (2, 26). This observation implicates a preference for otherwise colonizing strains to cause severe infections in IAV infected individuals and strongly supports the hypothesis that a pre-existing IAV infection influences the pathogenic effect of these strains. In this study we used an S. pn./iav co-infection mouse model to address host susceptibility to selected S. pn. strains with varying IPD potential during the acute and recovery phase of IAV. Our data show that acute IAV infection leads to equally fatal outcomes for all the tested S. pn. strains at otherwise sublethal doses. At the same time however, we detected S. pn. strain-specific changes in the underlying innate immune responses that presumably contributed to lung immunopathology. Following recovery from IAV infection, there were no significant effects on the survival following co-infection with any of the tested S. pn. strains. Nevertheless, pneumococcal growth in the airways and systemic dissemination were enhanced in a strain-specific manner late after IAV infection Materials and Methods Mice 7-8 weeks old C57BL/6JOlaHsd females were purchased from Harlan Winkelmann (Borchen, Germany) and Harlan Laboratories (Venray, Netherlands). Mice were housed in a specific pathogen free environment according to the guidelines of the regional animal care committees. All the experiments were approved and conducted in accordance to the guidelines set by the local animal welfare and ethics committees for the Helmholtz Centre for Infection Research (Niedersächsisches Landesamt für 5
6 Verbraucherschutz und Lebensmittelsicherheit) and the Karolinska Institute (KI; Stockholms Norra Djurförsöksetiska Nämnd) Bacterial and viral preparation For the S. pn. infections the serotype 4 strain TIGR4 (ATCC BAA-334 TM ), a serotype 19F strain (BHN100) and a 7F strain (BHN54) were used. All strains were obtained from the laboratory of B. Henriques-Normark (KI). The bacteria were grown to the mid-logarithmic growth phase in pre-warmed Todd-Hewitt Yeast (THY) medium (Todd-Hewitt broth (Sigma-Aldrich, Germany) supplemented with 1% yeast extract (Roth, Germany)) in a water bath at 37 C. Bacteria were harvested at an optical density of 0.35 to 0.50 at 620 nm (for T4 and 19F/7F respectively) by adding 10% v/v glycerol (Roth,Germany) and were frozen at -70 C. For animal infections, the frozen stocks were thawed, centrifuged at 6081xg at room temperature and washed once in 1 ml phosphate-buffered saline (PBS; Gibco, UK) before they were diluted to the desired concentration. The challenge dose was confirmed by plating 10-fold serial dilutions on blood agar plates (Columbia Agar with 5% sheep blood, BD Diagnostic Systems, Germany) that were incubated overnight at 37 C and 5% CO 2. At KI, the blood agar plates and THY medium were produced by the Karolinska Microbiology Laboratory (Solna, Sweden). For viral challenges, influenza A/PR/8/34 virus (H1N1; PR8) was produced in Madin-Darby canine kidney (MDCK) cells as described previously (27). The 50% tissue culture infectious dose (TCID 50 ) of the viral stock was determined by incubating 10-fold dilutions of virus stock solution on Madin-Darby canine kidney (MDCK) cells cultured in Dulbecco s Modified Eagle Medium (DMEM; Life Technologies, Germany) supplemented with % trypsin (Sigma-Aldrich, Germany) and 1% penicillin /streptomycin (ThermoFisher, USA). After 5 days, 0.5% chicken red blood cells 6
7 were added and the TCID 50 was calculated from the observed agglutination using the endpoint calculation by Reed and Muench (28). 138 Infection models Mice were weighed and anaesthetized through intraperitoneal administration of a mixture of ketamine and xylazine in PBS (100mg/kg ketamine, 10mg/kg xylazine). For bacterial challenges, mice were placed on their back on an intubation slope and the larynx was illuminated by an external cold-light source. A dose of 1x10 6 CFU S. pn. in 25µl PBS was instilled into the laryngopharynx for aspiration to the lower respiratory tract. For IAV challenges, anaesthetized mice were held upright with the head titled back slightly and a dose of 0.31TCID 50 in 25µl of PBS was administered dropwise to each nostril using a pipet. For all survival experiments following bacterial mono- or co-infection, mice were monitored three to six times per day and scored for the following parameters: body weight, movement, posture, pilo-erection, respiration, eye discharge, redness of the eye conjunctiva and response to stimulus. Moribund animals with severe symptoms of any one or a combination of the aforementioned parameters were euthanized and the infection was considered lethal. For all IAV mono-infections mice were monitored and weighed daily. The humane endpoint was set at 75% of the original body weight. 153 Assessment of the organ-wide bacterial burden To obtain nasopharyngeal lavage fluid, the trachea was exposed and the nasopharynx was flushed once with 1ml PBS from the trachea towards the nasal cavity. The lavage fluid was collected from the nostrils. For broncho-alveolar lavage fluid (BALF), the lungs were flushed once with 1ml PBS. For lung homogenates, the lungs were then aseptically excised and homogenized in 1ml PBS through a 100µm filter (Corning Inc., USA) using a syringe plunger. 5µl of 7
8 blood was collected from the tail vein and diluted in 45µl PBS for plating. Bacterial loads were determined by plating serial dilutions of the samples on blood agar plates. CFU were counted manually after incubating the plates for hours at 37 C with 5% CO Quantitative RT-PCR for viral load Lungs were perfused with 10ml PBS through the heart, excised and stored at -70 C in RNA later RM solution (Ambion, USA). RNA was extracted using the RNAeasy mini kit (Qiagen, Germany) according to the manufacturer s protocol. 1µg RNA was transcribed into cdna using the SuperScript III First-Strand Synthesis System (Invitrogen, USA). Purity and concentration of cdna was verified by performing a PCR for the housekeeping gene rps9. The quantitative RT-PCR performed to detect the absolute number of nucleoprotein (NP) copies in the samples was carried out on an ABI PRISM 7500 cycler (Applied Biosystems) using 35ng of cdna/sample. A standard curve was prepared using a reference plasmid standard with known numbers of NP copies/sample (3x10 1 3x10 9 ). The primers used were: rps9 5 CTGGACGAGGGCAAGATGAAGC and 3 TGACGTTGGCGGATGAGCACA; np 5 GAGGGGTGAGAATGGACGAAAAAC and 3 CAGGCAGGCAGGCAGGACTT (Eurofins MWG Operon, Germany). 175 Single cell preparation and staining for flow cytometry BALF was obtained as described above and the lungs were then perfused with PBS through the heart. The BALF was centrifuged at 4 C and 2000rpm in a table top centrifuge to pellet cells. The lungs were excised and manually minced on ice using scissors. Iscove s Modified Dulbecco s Medium (IMDM) with GlutaMAX-1 (Life Technologies, Germany) supplemented with 0.2mg/ml Collagenase D (Roche Diagnostics, Germany), 1mg/ml DNase (Sigma-Aldrich, Germany) and 5% fetal bovine serum (FBS Forte; Pan Biotech, 8
9 Germany) was freshly prepared for enzymatic digestion of the lung tissue. The minced lungs were suspended in 5ml of the digestion media for enzymatic digestion at 37 C for 45 minutes. The reaction was stopped by adding 5mM EDTA (working concentration) and the cell suspension was passed through a 100µm filter (Corning Inc., USA) followed by centrifugation at 420xg at 4 C. Erythrocyte lysis was performed and cells were resuspended for counting and staining. Single cell suspensions were incubated at room temperature in the dark in a mixture of the LIVE/DEAD fixable blue stain (ThermoFisher, USA) for dead cell exclusion and anti-mouse CD16/CD32 antibody (clone 93, purified; BioLegend, USA) for Fc-receptor blocking. Cell surface staining was then performed using antibodies specific for mouse F4/80 (clone BM8, APC; BioLegend, USA), CD11b (clone M1/70, pacific blue; BioLegend, USA) and Ly6G (clone 1A8, PE- Cy7; BioLegend, USA). Samples were acquired on a BD LSR II Fortessa using the FACS Diva (BD) software and analysis was performed using the FlowJo software (Tree Star, USA). Tissue-resident alveolar macrophages (AM; SSC high FSC high CD11b - F4/80 + autoflourescence + ), neutrophils (PMN; SSC high FSC high CD11b + Ly6G + F4/80 - ) and infiltrating mononuclear-phagocytes (IMP; CD11b + F4/80 -/low Ly6G - ) were identified through gating on the respective populations. 199 Histopathological analysis Lungs were fixed in 4% formalin and routinely embedded in paraffin. 5µm thick sections were cut, dewaxed and stained with hematoxylin and eosin. A blinded histological evaluation was performed by a veterinary pathologist certified by the European College of Veterinary Pathologists. The grade, extent and pattern of pneumonia were classified into broncho-interstitial pneumonia and broncho-pneumonia for IAV and S. pn. infection respectively. The scoring of lung 9
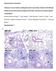
10 inflammation (grade 0 = not detected, grade 1 = minimal, grade 2 = mild; grade 3 = moderate and grade 4 = severe) was based on the number, kind (neutrophils, macrophages, lymphocytes) and location (interstitial, perivascular, intra-alveolar / - bronchial) of infiltrating cells. 209 Cytokine and chemokine detection For the detection of cytokines in lung homogenates, mouse ELISAMax kits (BioLegend, USA) were used according to the manufacturer s protocol. For the detection of chemokines in lung homogenates a mouse LEGENDplex TM pro-inflammatory chemokine kit (BioLegend, USA) was used according to the manufacturer s protocol. Samples were acquired on a BD LSR II Fortessa instrument and analyzed using the LEGENDplex TM v7.0 (Vigene Tech, USA) software. 215 Statistical analysis Graph Pad Prism 5.0 (Graph Pad software, USA) and RStudio (version , RStudio Inc., USA) were used to perform statistical analyses. The log-rank test was applied on the Kaplan Meier survival data. Otherwise, the oneway ANOVA with Bonferroni s multiple comparison test was used following logtransformation of the data to compare groups. The range of p values indicated is * p < 0.05; ** p < 0.01; *** p < Results IAV infection establishes a persisting and unresolved pneumonia even after complete viral clearance and recovery of the original body weight. Before establishing suited co-infection models, we characterized the underlying sublethal PR8/34 (H1N1) IAV mono-infection. Following intranasal infection, IAV infected mice started losing weight by day 4-5 post infection (pi) with a maximum weight loss of 10
11 % between days 7 and 9. By day 12 all mice recovered to 100% of the original body weight (Fig. 1a). High viral titers on day 7 and day 9 post IAV infection were consistent with the peak of weight loss (Fig. 1b) and viral clearance was observed by day 14 post IAV infection (Fig. 1b). Pulmonary histopathology was analyzed on days 7, 14 and 21 (Fig. 1c). Seven days post IAV infection inflammatory lesions were characterized by the accumulation of sloughed bronchial and alveolar epithelial cells and by an expansion of the alveolar septum, interstitium and bronchial lumen by neutrophils and numerous lymphocytes. At the later time-points, lesions were dominated by signs of advanced regeneration with severe hyperplasia of type II pneumocytes (Fig. 1c and supplementary Fig. S1b and c). Surprisingly, at the same time a wide-spread unresolved and partially active pneumonia was established by day 14 and day 21 post infection (Fig. 1c and supplementary Fig. S1a). Based on these findings, in our model the acute phase of IAV infection was represented by day 7 and the recovery phase persisted from day 14 until at least day 21 after IAV infection Acute IAV infection sensitizes the host to all tested S. pn. serotypes. To assess the effect of IAV infection on the susceptibility to different S. pn. serotypes we selected three strains with known differences in nasopharyngeal colonization and IPD potential. First, we characterized the infection with these strains alone as a reference for co-infection. We used the highly invasive strain TIGR4 (T4) of serotype 4 (27), an invasive strain of serotype 7F and a carrier strain of serotype 19F (29). According to previous reports, serotype 4 and serotype 7F harbor a high invasive disease potential while serotype 19F is less invasive (30-32). To determine the clinical nature of infection with these strains in vivo in our mouse model, naïve mice were infected with sub-lethal 11
12 doses of S. pn. T4, 19F or 7F. At 18hpi with T4, nasopharyngeal colonization and pneumonia were detected in 83% and 58% of the animals respectively (Fig. 2a). Furthermore, 21% of all the T4 infected animals showed bacteremia (Fig. 2a). In contrast, 100% of the 19F infected mice carried bacteria in the nasopharynx and 91% showed high bacterial loads in the lungs (Fig. 2a). Importantly, despite the high frequency of pneumonia, 19F infection did not cause bacteremia in any of the mice (Fig. 2a). Following infection with the 7F strain, 96% of the animals were colonized in the nasopharynx at 18hpi (Fig. 2a). Bacteria were detected in the lungs of 34% of the 7F infected animals at comparably low titers but not in the blood (Fig. 2a). Taken together, results from the mono-infections with the three selected S. pn. strains reflected clear differences in pathogenesis in our mouse model since only the S. pn. strain T4 caused IPD, while the S. pn. strains 19F and 7F were restricted to the respiratory tract at 18hpi. To gain insight into bacterial strain-dependent effects on susceptibility to S. pn. following IAV infection, co-infections with all three S. pn. strains were performed during the acute phase of IAV infection (day 7 post IAV infection) (Fig. 2b). The progression of the bacterial disease was assessed by the quantification of the bacterial load in the respiratory tract and blood at 18hpi as well as the approximate mortality rates over 96hpi. A non-significant tendency for increased bacterial loads in the nasopharynx was evident for all three pneumococcal strains when comparing mono-infection to coinfection at 18hpi (Table S1). At the same time, co-infection with all three bacterial strains resulted in exceptionally high and significantly elevated bacterial loads in the BALF and lung tissue compared to the respective S. pn. mono-infection (Fig. 2c and d). These results clearly demonstrated IAV-dependent and bacterial strain-independent pneumococcal outgrowth following co-infection during acute IAV infection. Nevertheless, 12
13 we observed a bacterial strain-dependent increase in the incidence of systemic disease following co-infection compared to the respective mono- infection (Fig. 2e). Only the strains previously classified as potentially invasive strains (T4 and 7F) demonstrated systemic dissemination at 18h post co-infection while 19F was fully restricted to the respiratory tract at this time point (Fig. 2e). Ultimately however, the underlying IAV infection led to significantly elevated mortality rates for all the tested S. pn. strains (79%, 75% and 63% following T4, 19F and 7F co-infection, respectively) without significant strain-specific differences (Fig. 2f). The overall high lethality observed following coinfection correlated well with the consistently high lung bacterial loads detected at 18hpi (Fig. 2d) and at the time point severely ill mice had to be euthanized according to the predefined endpoint criteria (data not shown). Interestingly, at the time of death 90% of the euthanized T4 co-infected animals showed bacteremia (median bacterial titer: 5.9x10 6 CFU/ml) (Fig. 2g and S2). In contrast, only 33% and 55% of the animals sacrificed following 19F and 7F co-infection showed bacteremia (Fig. 2g). Additionally, these bacteremic 19F and 7F co-infected mice yielded lower median bacterial titers than the bacteremic T4 co-infected mice (Fig. S2). Of note, survival studies for mono-infection with 19F and 7F using a 10-fold higher infection dose (1x10 7 CFU) than used for the coinfections still showed 100% survival without any bacteremia for all the mice (data not shown). Taken together, these results demonstrate acute IAV-infection to predispose the host to lethal secondary pneumococcal disease at low bacterial doses irrespective of the IPD potential of the S. pn. serotype. However, at the same time, the manifestation of disease was S. pn. strain-dependent regarding the incidence and kinetic of bacteremia. 13
14 The bacterial strain-dependent manifestation of secondary pneumococcal disease persists during the recovery phase of IAV infection. To assess whether long-term IAV-mediated enhanced susceptibility to pneumococcal disease occurs in a strain- and time-dependent manner, co-infections were performed during the recovery phase of IAV infection either on day 14 or day 21. Bacterial loads were determined 18 hours later (Fig. 2b). In general, for co-infections performed on day 14 post IAV infection the overall bacterial loads in BALF, lung and blood were reduced compared to co-infections performed on day 7 post IAV infection (Fig. 2c-e and 3a-c). Regarding the bacterial load in the nasopharynx, again there were no significant differences between co-infected and S. pn. mono-infected mice irrespective of the pneumococcal strain (Table S1). In contrast, delayed clearance was evident in the LRT for the two invasive strains T4 and 7F. Bacterial loads were higher in the BALF and lung tissue of the T4 and 7F co-infected mice compared to the respective mono-infections (significant for the 7F co-infected lungs, Fig. 3a and 3b). In line with the increased bacterial burden in the LRT, systemic dissemination occurred at a higher incidence in T4 and 7F co-infected than mono-infected mice (significant for T4, Fig. 3c). Of note, the 33% mortality of T4 co-infected mice (Fig. 3d) was accompanied by strong pneumonia and bacteremia in all mice with lethal infection (data not shown). Taken together, even though there was no significant increase in mortality in any of the groups co-infected 14 days following IAV infection (Fig. 3d), bacterial clearance was markedly and significantly delayed for two of the three strains (T4 and 7F). Therefore these results show enhanced susceptibility to IPD during recovery from IAV infection to depend on the pneumococcal strain. 14
15 When co-infections were performed 21 days following IAV infection there was still a trend, however not significant, towards delayed bacterial clearance from the LRT in all the co-infected groups (Table S2). However, at this time point post IAV infection, the pneumococcal infection remained restricted to the lungs for the strain 7F and the sporadic systemic dissemination observed only for the highly invasive strain T4 was not dependent on a previous IAV infection (Table S2). Taken together, the kinetic of enhanced long-term susceptibility to S. pn. was strain-dependent IAV-mediated changes in immune cell recruitment following co-infection are bacterial strain-dependent particularly during the acute phase of IAV infection. Of the different time points tested following IAV infection, the acute phase proved to be most detrimental to the host regarding susceptibility to secondary pneumococcal disease. The strong bacterial outgrowth in the LRT observed for all tested S. pn. strains pointed at defective innate immune responses. Therefore the recruitment of innate immune cells to the lung was assessed following co-infection with the three S. pn. strains on day 7 post IAV infection. Absolute numbers of i) alveolar macrophages (AM), ii) infiltrating mononuclear phagocytes (IMP), that are comprised of newly recruited monocytes, macrophages and dendritic cells, as well as iii) polymorphonuclear neutrophils (PMN) were determined in the lung. Strikingly, at 18h post co-infection the T4 and 19F co-infected groups showed significantly reduced numbers of AMs in the lung tissue compared to the S. pn. mono-infection (Fig. 4a). Of note, they were also marginally but not significantly reduced compared to the IAV mono-infection (Fig. 4a). In contrast, the number of IMPs was increased for all the co-infected groups compared to the respective S. pn. mono-infection at 18hpi (significantly for T4 and 7F co-infected 15
16 groups) (Fig. 4b). Importantly, the number of IMPs in all the co-infected groups remained comparable to the IAV mono-infection (Fig. 4b). Interestingly, PMN recruitment to the lung following bacterial mono-infection was significantly altered between the three different S. pn. strains. Here, the strongest recruitment of PMNs was detected following the 19F infection (Fig. 4c). Following co-infection, even though there were no significant changes between the co- and mono-infected groups for any of the tested pneumococcal strains, we observed a trend towards increased PMN numbers following co-infection for the strains T4 and 7F (Fig. 4c).However, there was a decrease following 19F coinfection when compared to the 19F mono-infection. Importantly, mean PMN numbers in all co-infected animals exceeded those present in the lung after IAV mono-infection. Of note, a similar pattern of differences was also observed for the BAL cells, except that the reduction of PMN in 19F co-infected mice was not observed (Fig. 4d, e and f). Importantly, the elevated PMN numbers observed for the invasive S. pn. strains T4 and 7F upon co-infection correlated well with the histopathological analysis performed 18h following co-infection (Fig. 5). Here, 80% and 100% of the T4 and 7F co-infected mice, respectively, showed the most severe grade of inflammation, while this was the case for only 57% of the 19F co-infected mice (Fig. 5a and b). However, the percentage of the lungs affected by bacterial broncho-pneumonia was comparable between all three strains post co-infection during acute IAV infection (Fig. 5c). Taken together, severe inflammatory lesions were observed for all the co-infected groups during the acute phase of IAV infection. Furthermore, the recruitment of innate immune cells to the lungs was determined following bacterial co-infection during the recovery phase of IAV infection. Of note, the number of AMs and IMPs detected in the lungs of co-infected mice were almost equal to 16
17 those in the S. pn. mono-infected groups at 18h following co-infection on day 14 post IAV infection (Fig. 6a and b). However, there were still trends for bacterial straindependent changes in the PMN numbers following co-infection (Fig. 6c). Here, the 19F co-infected group still showed marginally, though not significantly, lower cell numbers than the 19F mono-infected group whereas there was no difference in PMN numbers between the co-infected and mono-infected T4 groups and a trend for increased PMN numbers in the co-infected 7F group (Fig. 6c). By day 21 following IAV infection, AM and PMN numbers were unchanged between the co-infected and mono-infected groups for all bacterial strains (Fig. 6d). In contrast, the number of IMP remained marginally higher for the T4 co-infected group compared to the bacterial mono-infection (Fig. 6e). Altogether these data show that the recruitment of innate immune cells in response to S. pn. is substantially affected by a preceding IAV infection if co-infection occurs during the acute phase of the IAV infection. Most importantly, we identified both general, S. pn. strain-independent changes such as the increased presence of infiltrating cells following co-infection as well as strain-dependent changes such as altered AM and PMN numbers Changes in the cytokine and chemokine responses post co-infection during acute IAV infection are pneumococcal strain-dependent. The local inflammatory responses following infection are majorly orchestrated by a network of cytokines and chemokines. In order to obtain more insight into the pneumococcal strain-dependent inflammatory processes taking place following coinfection during acute IAV infection, we characterized and compared the lung inflammatory profile following S. pn. mono- and co-infection for the three strains. 17
18 Interleukin (IL)-6 and tumor necrosis factor (TNF)-α are multifunctional pro-inflammatory cytokines important for both local and systemic immune stimulation. For all three pneumococcal strains, co-infection during acute IAV infection led to a clear increase in the production of IL-6 compared to the respective bacterial mono-infection (Fig. 7a). Of note, this excess production of IL-6 observed following co-infection was significant only for the invasive strains T4 and 7F (Fig. 7a). Nevertheless we detected similarly high concentrations of IL-6 in all co-infected groups at 18hpi that demonstrated a strong inflammatory response irrespective of the co-infecting pneumococcal strain (Fig. 7a). Also for TNF-α, all the co-infected groups revealed elevated cytokine levels in the lung compared to the respective bacteria-only group (Fig. 7b). Of note, this increase was again strongest and significant only for co-infections with the strains T4 and 7F (Fig. 7b). Next, the lung protein concentrations of critical chemokines such as MCP-1 (monocyte chemoattractant protein-1), MIP-1 (macrophage inflammatory protein), KC (keratinocyte chemoattractant) and LIX (lipopolysaccharide-induced CXC chemokine), all of which are implicated in driving immunopathology in IAV infected patients was elucidated. For the bacterial mono-infection, we show that S. pn. strain 19F induced higher amounts of the neutrophil chemoattractants IL-6, KC and LIX compared to T4 and 7F (Fig. 7a, c and d). The least induction of IL-6, LIX and especially KC was detected after the 7F infection, which correlated well with the PMN counts observed in the lung following bacterial mono-infection (Fig. 7a, c and d). Following co-infection, KC levels were significantly elevated independent of the pneumococcal strain when compared to the respective bacterial mono-infection (Fig. 7c). Despite a trend for increased levels of LIX in the lungs of mice co-infected with T4 and 7F, there were no significant changes between coinfection and the bacterial mono-infection for any of the strains tested (Fig. 7d). In 18
19 contrast, both MCP-1, a potent monocytic chemoattractant, and MIP-1β, which is produced by macrophages to activate granulocytes, were significantly increased following co-infection compared to the respective bacterial mono-infection for all pneumococcal strains tested (Fig. 7e and f). Of note, in most cases, i.e. for TNF-α, KC, MCP-1and MIP-1β, the lowest levels were detected in the 19F co-infected group compared to the T4 and 7F co-infected groups (Fig 7b, c, e and f). In conclusion, when comparing between the mono- and co-infections, especially co-infections with T4 and 7F in contrast to co-infection with 19F showed a strongly synergistic rather than merely additive effect for nearly all the tested mediators. Furthermore, these data demonstrate that an underlying acute IAV infection indeed affects the local pro-inflammatory cytokine response towards S. pn. in a pneumococcal strain-dependent manner Discussion Bacterial co-infections during IAV infection remain a significant cause of hospitalizations and mortality world-wide. Moreover, as broadly used pneumococcal conjugate vaccines influence capsular switch and serotype distribution, understanding serotype-specific differences in secondary pneumococcal infection following IAV infection is of great importance. In order to elucidate pneumococcal strain-dependent effects in the synergism between IAV and S. pn., we determined the outcome and the host immune response following co-infection with three different pneumococcal serotypes. Our model differs from previous co-infection models as the three strains tested were exemplarily selected to represent strains of high, intermediate and low IPD potential. The data generated from this model shows clear strain-specific differences in the host immune 19
20 responses and implicates the use of different treatment strategies based on the coinfecting pneumococcal strain in the future. Interestingly, despite clear differences in the manifestation of mono-infection with the different strains, all the three strains caused similarly severe pneumonia and mortality following co-infection during acute IAV infection. This clearly demonstrated that acute IAV infection has devastating effects on anti-pneumococcal host defenses that are independent of the IPD potential of the co-infecting pneumococcal strain. Strikingly, even the carrier strain 19F caused severe disease and mortality following co-infection at a dose that was non-lethal in the absence of IAV infection in our model as well as in previously described studies (29, 33). IAV infection has been shown to also support the development of otitis media by 19F (34, 35). The strongest colonization was observed following 19F mono-infection compared to T4 and 7F however without any apparent disease symptoms. Importantly, these findings highlight the requirement of a fully competent innate immune system to confine this strain to its asymptomatic carrier state. In line with this, infections with 19F are most commonly found in children and immunecompromised patients (36). Furthermore, our findings are consistent with reports that bacterial strain-specific differences are surpassed and mortality does not correlate with the incidence of bacteremia during the acute phase of IAV infection (37, 38). In fact, mortality was proportional to the uncontrolled bacterial outgrowth in the LRT and not bacteremia as the co-infected animals that succumbed to the infection showed high variability in the extent of bacteremia. These results imply that one of the major causes for mortality was severe pneumonia. The consistently high bacterial loads detected in the lungs of mice co-infected with any of the tested S. pn. strains may further increase the cytolytic activity of bacterial virulence factors such as the pore-forming pneumolysin 20
21 (39), which is a potent pro-inflammatory signal. This in turn most likely explains the high levels of cytokines and chemokines in the lungs of all animals co-infected during acute IAV infection. In fact, computational modelling data have demonstrated that the robust local inflammatory responses induced by S. pn. were responsible for rapid mortality and IPD during active IAV co-infection (37). Next to the pneumococcal strain-independent effects mediated by IAV infection, we observed distinct changes in the inflammatory response mounted towards S. pn. coinfection that were dependent on the pneumococcal serotype. Depletion of AMs during the acute phase of IAV infection has previously been shown to contribute to severe secondary pneumococcal disease (40). Strikingly, in our model we did not observe a significant reduction of AM numbers in the lung during IAV mono-infection. However, coinfection during acute IAV infection led to a reduction in the mean AM counts that was particularly clear and also significant for the strains T4 and 19F. For co-infections with T4 this decrease in AM numbers was associated with an increase in the late-apoptotic or necrotic state of AMs (data not shown). Most likely, the loss in AMs in co-infection not only has detrimental effects on bacterial clearance but also on the resolution of inflammation as AM are the major effector cells to clear apoptotic PMN (41). Therefore, such a clear loss of AMs is likely to tip the balance towards a prolonged proinflammatory response culminating in immunopathology and morbidity (42, 43). For T4 co-infected mice, this scenario correlated well with the strong neutrophil influx and high levels of inflammatory mediators. In contrast, AM reduction was accompanied by a marginal decrease in lung neutrophil numbers following the 19F co-infection compared to the 19F mono-infection. Of note, also, the local concentrations of TNF-α and MIP-1β were marginally, but not significantly, lower for the 19F co-infected mice than for the T4 21
22 and 7F co-infected mice. This situation makes the AM decrease less consequential in the 19F anti-bacterial response when compared to the T4 co-infection. In our study, neutrophils emerged as the major cell type responsible for combating 19F infection, in line with the copious PMN influx observed during 19F mono-infection that was significantly elevated compared to T4 and 7F mono-infection. This is in accordance with a recent study that demonstrated complete abrogation of bacterial clearance in 19F precolonized animals upon neutrophil depletion (44). For co-infection with the strain 7F, cell numbers in the respiratory tract represented a more balanced state and the lethal disease outcome can most likely be attributed to local immunopathology, driven by the excessive inflammatory mediators and inflammatory monocytes observed in all 7F coinfected animals. For IAV infection alone, a strong TNF-α response has been shown to induce severe pathology (45) and thus neutralization protected against immunopathology mediated mortality (46, 47). Even though TNF-α neutralization failed to lower the disease severity following T4 co-infection (48), it still holds potential for a treatment strategy for the 7F co-infections and should be tested in the future. Altogether our study has identified several characteristics in the host response towards different pneumococcal strains following co-infection during acute IAV infection. Nevertheless, the outcome was equally devastating for all tested strains. Effective treatment of secondary pneumococcal infection following IAV infection has been suggested to combine antibiotic measures with immune modulators (49, 50). Therefore, future studies will be needed to exploit our findings regarding the serotype-specific inflammatory responses following co-infection for treatment regimens tailored to the coinfecting pneumococcal strain. 22
23 Importantly, we have extended our study of the S. pn. strain-dependency in host susceptibility and inflammatory responses following co-infection to the recovery phase of IAV infection. Histological analyses performed until 21 days post IAV infection revealed that pneumonia was established even after complete recovery of body weight and viral clearance. In fact, the few long-term follow up studies with small patient cohorts that survived acute H1N1 infection have revealed that structural abnormalities in the lung parenchyma can last up to a year after respiratory disease (51, 52). When co-infections were performed on day 14 following IAV infection, we indeed still observed impaired clearance of S. pn.. While survival was unaffected, we detected increased bacterial growth in the LRT and significantly increased incidence of systemic dissemination in a strain-dependent manner. In fact, IAV-induced impairment of AM sensitivity is proposed to persist until 100 days post IAV infection in mice (24). Furthermore, it has also been reported that a state of immunosuppression marked by the upregulation of IL-10 (25) is set up to promote repair and recovery post IAV infection, which may explain the marginally delayed bacterial clearance in all the co-infected animals until day 21 post IAV infection and the low levels of inflammation in the lungs compared to day 7 post IAV infection. In line with previous reports, our results show that IAV infection affects pneumococcal clearance even after elimination of the virus during recovery of the host. Importantly, we show a pneumococcal strain-dependency which implies that strains with higher IPD potential such as T4 to take advantage of the IAV pre-infected host for a longer time than colonizers such as 19F. Despite equally low mortality following coinfection with the different pneumococcal strains late following IAV infection in our model, we believe this finding holds implications e.g. for the surveillance of survivors of 23
24 severe IAV infections during outbreaks of pneumococcal strains with moderate to high IPD potential. Altogether, the results of our study strongly highlight the need to identify the serotype infecting the patient before administering standard treatment regimens such as e.g. the increasingly used corticosteroids (45, 50, 53). Following co-infection during the acute phase of IAV infection the invasive strains T4 and 7F caused stronger pro-inflammatory responses compared to the 19F strain. Therefore immunosuppression along with antiviral and antibacterial treatment may work better for these strains. In case of coinfection with colonizing strains such as the 19F, a strong bacteriostatic antibiotic along with antivirals may be more potent in preventing mortality. Certainly, these hypotheses will need to be tested in future studies, for which we believe our results provide a valuable first basis. Furthermore, our results imply the imperative need to strongly consider the pneumococcal serotype when studying the mechanisms underlying severe IAV-S. pn. co-infections in animal models
25 References 1. Taubenberger JK, Morens DM Influenza: the mother of all pandemics. Emerg Infect Dis 12: Chien YW, Klugman KP, Morens DM Bacterial pathogens and death during the 1918 influenza pandemic. N Engl J Med 361: McCullers JA The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol 12: Khan MN, Pichichero ME The host immune dynamics of pneumococcal colonization: implications for novel vaccine development. Hum Vaccin Immunother 10: van den Boogaard FE, van Gisbergen KP, Vernooy JH, Medema JP, Roelofs JJ, van Zoelen MA, Endeman H, Biesma DH, Boon L, van 't Veer C, de Vos AF, van der Poll T Granzyme A impairs host defense during Streptococcus pneumoniae pneumonia. Am J Physiol Lung Cell Mol Physiol doi: /ajplung :ajplung Anonymous Pneumococcal conjugate vaccine for childhood immunization--who position paper. Wkly Epidemiol Rec 82: Chiavolini D, Pozzi G, Ricci S Animal Models of Streptococcus pneumoniae Disease. Clinical Microbiology Reviews 21: Weinberger DM, Harboe ZB, Sanders EAM, Ndiritu M, Klugman KP, Rückinger S, Dagan R, Adegbola R, Cutts F, Johnson HL, O'Brien KL, Scott JA, Lipsitch M Association of Serotype with Risk of Death Due to Pneumococcal Pneumonia: A Meta-Analysis. Clinical Infectious Diseases 51: de Vos AF, Dessing MC, Lammers AJ, de Porto AP, Florquin S, de Boer OJ, de Beer R, Terpstra S, Bootsma HJ, Hermans PW, van 't Veer C, van der Poll T The polysaccharide capsule of Streptococcus pneumonia partially impedes MyD88-mediated immunity during pneumonia in mice. PLoS One 10:e Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin Microbiol Rev 28: Weinberger DM, Trzcinski K, Lu YJ, Bogaert D, Brandes A, Galagan J, Anderson PW, Malley R, Lipsitch M Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog 5:e Wartha F, Beiter K, Albiger B, Fernebro J, Zychlinsky A, Normark S, Henriques-Normark B Capsule and D-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell Microbiol 9: Camberlein E, Cohen JM, Jose R, Hyams CJ, Callard R, Chimalapati S, Yuste J, Edwards LA, Marshall H, van Rooijen N, Noursadeghi M, Brown JS Importance of bacterial replication and alveolar macrophage-independent clearance mechanisms during early lung infection with Streptococcus pneumoniae. Infect Immun 83: Rodgers GL, Klugman KP The future of pneumococcal disease prevention. Vaccine 29 Suppl 3:C Sandgren A, Albiger B, Orihuela CJ, Tuomanen E, Normark S, Henriques-Normark B Virulence in mice of pneumococcal clonal types with known invasive disease potential in humans. J Infect Dis 192: Blomberg C, Dagerhamn J, Dahlberg S, Browall S, Fernebro J, Albiger B, Morfeldt E, Normark S, Henriques-Normark B Pattern of accessory regions and invasive disease potential in Streptococcus pneumoniae. J Infect Dis 199:
26 Luján M, Gallego M, Belmonte Y, Fontanals D, Vallès J, Lisboa T, Rello J Influence of pneumococcal serotype group on outcome in adults with bacteraemic pneumonia. European Respiratory Journal 36: Sun K, Gan Y, Metzger DW Analysis of murine genetic predisposition to pneumococcal infection reveals a critical role of alveolar macrophages in maintaining the sterility of the lower respiratory tract. Infect Immun 79: Haste LV, Hulland K, Bolton S, Yesilkaya H, McKechnie K, Andrew PW Development and Characterisation of a Long Term Murine Model of Streptococcus pneumoniae Infection of the Lower Airways. Infect Immun doi: /iai Robinson KM, Kolls JK, Alcorn JF The immunology of influenza virus-associated bacterial pneumonia. Curr Opin Immunol 34C: Hussell T, Cavanagh MM The innate immune rheostat: influence on lung inflammatory disease and secondary bacterial pneumonia. Biochem Soc Trans 37: Ballinger MN, Standiford TJ Postinfluenza bacterial pneumonia: host defenses gone awry. J Interferon Cytokine Res 30: Chertow DS, Memoli MJ Bacterial coinfection in influenza: a grand rounds review. JAMA 309: Didierlaurent A, Goulding J, Patel S, Snelgrove R, Low L, Bebien M, Lawrence T, van Rijt LS, Lambrecht BN, Sirard JC, Hussell T Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med 205: van der Sluijs KF, van Elden LJ, Nijhuis M, Schuurman R, Pater JM, Florquin S, Goldman M, Jansen HM, Lutter R, van der Poll T IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol 172: Klugman KP, Chien YW, Madhi SA Pneumococcal pneumonia and influenza: a deadly combination. Vaccine 27 Suppl 3:C9-C Stegemann S, Dahlberg S, Kröger A, Gereke M, Bruder D, Henriques-Normark B, Gunzer M Increased Susceptibility for Superinfection with <italic>streptococcus pneumoniae</italic> during Influenza Virus Infection Is Not Caused by TLR7-Mediated Lymphopenia. PLoS ONE 4:e Reed LJ, Muench H A simple method of estimating fifty per cent endpoints Am J Hyg 27: McCullers JA, McAuley JL, Browall S, Iverson AR, Boyd KL, Henriques Normark B Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J Infect Dis 202: Brueggemann AB, Griffiths DT, Meats E, Peto T, Crook DW, Spratt BG Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J Infect Dis 187: Sandgren A, Sjostrom K, Olsson-Liljequist B, Christensson B, Samuelsson A, Kronvall G, Henriques Normark B Effect of clonal and serotype-specific properties on the invasive capacity of Streptococcus pneumoniae. J Infect Dis 189: Sjostrom K, Spindler C, Ortqvist A, Kalin M, Sandgren A, Kuhlmann-Berenzon S, Henriques- Normark B Clonal and capsular types decide whether pneumococci will act as a primary or opportunistic pathogen. Clin Infect Dis 42: Blevins LK, Wren JT, Holbrook BC, Hayward SL, Swords WE, Parks GD, Alexander-Miller MA Coinfection with Streptococcus pneumoniae negatively modulates the size and composition of the ongoing influenza-specific CD8(+) T cell response. J Immunol 193:
27 Short KR, Reading PC, Brown LE, Pedersen J, Gilbertson B, Job ER, Edenborough KM, Habets MN, Zomer A, Hermans PW, Diavatopoulos DA, Wijburg OL Influenza-induced inflammation drives pneumococcal otitis media. Infect Immun 81: Wren JT, Blevins LK, Pang B, King LB, Perez AC, Murrah KA, Reimche JL, Alexander-Miller MA, Swords WE Influenza A virus alters pneumococcal nasal colonization and middle ear infection independently of phase variation. Infect Immun 82: Ahn JG, Choi SY, Kim DS, Kim KH Changes in pneumococcal nasopharyngeal colonization among children with respiratory tract infections before and after use of the two new extendedvalency pneumococcal conjugated vaccines. Infect Dis (Lond) 47: Smith MW, Schmidt JE, Rehg JE, Orihuela CJ, McCullers JA Induction of pro- and antiinflammatory molecules in a mouse model of pneumococcal pneumonia after influenza. Comp Med 57: Mina MJ, Klugman KP The role of influenza in the severity and transmission of respiratory bacterial disease. Lancet Respir Med 2: Harvey RM, Hughes CE, Paton AW, Trappetti C, Tweten RK, Paton JC The impact of pneumolysin on the macrophage response to Streptococcus pneumoniae is strain-dependent. PLoS One 9:e Ghoneim HE, Thomas PG, McCullers JA Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J Immunol 191: Knapp S, Leemans JC, Florquin S, Branger J, Maris NA, Pater J, van Rooijen N, van der Poll T Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med 167: Schneider C, Nobs SP, Heer AK, Kurrer M, Klinke G, van Rooijen N, Vogel J, Kopf M Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog 10:e Purnama C, Ng SL, Tetlak P, Setiagani YA, Kandasamy M, Baalasubramanian S, Karjalainen K, Ruedl C Transient ablation of alveolar macrophages leads to massive pathology of influenza infection without affecting cellular adaptive immunity. Eur J Immunol 44: Wilson R, Cohen JM, Jose RJ, de Vogel C, Baxendale H, Brown JS Protection against Streptococcus pneumoniae lung infection after nasopharyngeal colonization requires both humoral and cellular immune responses. Mucosal Immunol 8: La Gruta NL, Kedzierska K, Stambas J, Doherty PC A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol 85: Peper RL, Van Campen H Tumor necrosis factor as a mediator of inflammation in influenza A viral pneumonia. Microb Pathog 19: Hussell T, Pennycook A, Openshaw PJ Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur J Immunol 31: Ellis GT, Davidson S, Crotta S, Branzk N, Papayannopoulos V, Wack A TRAIL+ monocytes and monocyte-related cells cause lung damage and thereby increase susceptibility to influenza- Streptococcus pneumoniae coinfection. EMBO Rep 16: Karlstrom A, Heston SM, Boyd KL, Tuomanen EI, McCullers JA Toll-like receptor 2 mediates fatal immunopathology in mice during treatment of secondary pneumococcal pneumonia following influenza. J Infect Dis 204: Ghoneim HE, McCullers JA Adjunctive corticosteroid therapy improves lung immunopathology and survival during severe secondary pneumococcal pneumonia in mice. J Infect Dis 209: Edgeworth D, Brohan J, O'Neill S, Maher M, Breen D, Murphy D Pulmonary sequelae of severe H1N1 infection treated with high frequency oscillatory ventilation. Ir Med J 106:
28 Luyt C-E, Combes A, Becquemin M-H, Beigelman-Aubry C, Hatem S, Brun A-L, Zraik N, Carrat F, Grenier PA, Richard J-CM, Mercat A, Brochard L, Brun-Buisson C, Chastre J LOng-term outcomes of pandemic 2009 influenza a(h1n1)-associated severe ards. Chest 142: Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, Lim WS Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev 3:CD
29 Funding This study was supported by the International Research Training Group 1273 (IRTG1273) funded by the German Research Foundation (DFG), the Swedish Research Council, Knut and Alice Wallenberg foundation, the Swedish foundation for strategic research (SSF) and by ALF grants from Stockholm city council. DB was supported by the President s Initiative and Networking Fund of the Helmholtz Association of German Research Centers (HGF) under contract number W2/W3-029 and by a grant from the German Research Foundation (SFB854) Acknowledgments We thank Silvia Prettin, Tatjana Hirsch and Nicole Peters for their help in sample collection and Julia D. Boehme, Marcus Gereke, Andreas Jeron and Priya Sakthivel for their helpful suggestions during experimental planning Author contribution Experimental planning, execution and data analysis: NSC, SSK, VS, JV; Mouse histology: OK, AG; Project consumables/animals and analysis tools: DB, BHN; Manuscript writing: NSC, SSK, DB; Manuscript proof-reading: all; Project design and supervision: SSK, BHN, DB Competing financial interests No conflict of financial interest declared by the authors
30 753 Figure legends Figure 1: Persisting unresolved pneumonia post IAV infection. WT C57BL/6J mice were intra-nasally infected with 0.31TCID 50 of the A/PR8/34 H1N1 strain of IAV or treated with PBS as control on day 0. (a) Changes in body-weight are represented as percentage relative to the starting weight. Data are shown as mean ± SEM. (b) Absolute quantification of viral nucleoprotein (NP) copy numbers in the lungs of PBS-treated and IAV-infected mice at the indicated number of days post IAV infection. Lines indicate the median. Statistical analysis was performed using the one-way ANOVA with Bonferroni s multiple comparison test with the asterisk * indicating significant differences between the groups, *P<0.05; **P<0.01; ***P< Data are compiled of at least two independent experiments with 3-5 mice in each group. (c) Representative example (one out of n=3) of histopathological changes in the lungs of IAV infected or PBS treated mice at 7, 14 and 21 days post infection analyzed following H and E staining. Lungs of PBS treated mice were unchanged. For day 7 after IAV infection the arrowheads indicate an attenuation of the bronchial epithelium and the neutrophils and macrophages within the alveoli are encircled; for day 14 after IAV infection the arrowheads indicate type II hyperplasia and the neutrophils and macrophages in the bronchi (indicating bronchitis) are encircled; for day 21 after IAV infection the arrowheads indicate type II pneumocyte hyperplasia with increased numbers of lymphocytes in the surrounding interstitium (encircled) Figure 2: Enhanced susceptibility to secondary S. pn. infection during the acute phase of IAV infection. Groups of 5-6 WT C57BL/6J mice were oro-pharyngeally infected with 1x10 6 CFU of S. pn. strain T4, 19F or 7F. (a) Incidence of colonization, 30
31 pneumonia and bacteremia at 18hpi with S. pn. strains T4, 19F or 7F according to the bacterial burden detected in the nasopharynx, post-lavage lungs and blood for all bacterial mono-infections performed in the study. Data are shown as mean ± SEM. (b) Schematic diagram for co-infections with S. pn. strains T4, 19F or 7F post IAV infection. Mice were infected with 1x10 6 CFU of S. pn. strains T4, 19F or 7F on day 7 post infection with 0.31TCID 50 of IAV or PBS treatment. (c - e) Bacterial burden in the (c) BALF, (d) post-lavage lungs and (e) blood at 18h after secondary infection with T4, 19F or 7F. Lines indicate the median. Statistical analysis was performed using the one-way ANOVA with Bonferroni s multiple comparison test with asterisks * indicating significant differences, *P<0.05; **P<0.01; ***P< (f) Survival rates of mice infected with S. pn. T4, 19F or 7F on day 7 post IAV infection or PBS treatment. The asterisk * indicates significant differences assessed by the Log-rank test on the Kaplan-Meier survival data for the co-infected groups compared to the S. pn. only groups, *P<0.05; **P<0.01. (g) Incidence of colonization, pneumonia and bacteremia in the co-infected and single S. pn. infected mice with lethal infection according to the bacterial burden detected in the nasopharynx, whole lung and blood respectively Figure 3: Strain-specific alterations in the course of secondary pneumococcal disease during recovery from IAV infection. Mice were infected with 1x10 6 CFU of S. pn. strain T4, 19F or 7F on day 14 post infection with 0.31TCID 50 of IAV or PBS treatment. (a - c) Bacterial burden in the (a) BALF, (b) post-lavage lungs and (c) blood at 18h post-secondary infection with T4, 19F or 7F. Lines indicate the median. Statistical analysis was performed using the one-way ANOVA with Bonferroni s multiple comparison test with the asterisk * indicative of significant differences between the co- 31
32 infected and S. pn. only groups, *P<0.05;**P<0.01;***P< (d) Survival rates of mice co-infected on day 14 post IAV infection or infected with S. pn. only Figure 4: Strain-dependent changes in the innate immune cell composition of the lungs of mice co-infected during the acute phase of IAV infection. Mice were infected with 1x10 6 CFU of S. pn. strain T4, 19F or 7F or treated with PBS on day 7 post infection with 0.31TCID 50 of IAV or PBS treatment. Absolute numbers of (a) alveolar macrophages (#AM), (b) infiltrating mononuclear phagocytes (#IMP) and (c) polymorphonuclear cells (#PMN) in post-lavage lungs at 18h after co-infection. Absolute numbers of (d) alveolar macrophages (#AM), (e) infiltrating mononuclear phagocytes (#IMP) and (f) polymorphonuclear cells (#PMN) in BALF 18h after co-infection. Data are shown as Tukey box plots with lines indicating the median. Data are compiled of n>5 from at least two independent experiments with 2-3 WT C57BL/6J mice per group. Statistical analysis was performed using the one-way ANOVA with Bonferroni s multiple comparison test with the asterisk * indicative of significant differences, *P<0.05;**P< Figure 5: Histopathological changes in the lung tissue of S. pn. mono-infected mice and mice co-infected during the acute phase of IAV infection. Mice were infected with 1x10 6 CFU of S. pn. strain T4, 19F or 7F on day 7 post infection with 0.31TCID 50 of IAV or PBS treatment. (a) Representative example (one out of n=3-7) of the histopathological changes examined by H and E staining of the lungs of co-infected and S. pn. mono-infected mice at 18h post infection with T4, 19F or 7F. Arrowheads indicate selected regions of broncho-pneumonia. (b) Histopathological scores for 32
33 inflammation observed in the lungs of co-infected and S. pn. mono-infected mice. Acute inflammation was characterized as perivascular and interstitial immune cell infiltration. (c) Percentage of the lung affected by broncho-pneumonia in the co-infected and S. pn. mono-infected mice. Histological analysis was performed twice with 2-4 mice per group. Lines indicate the median Figure 6: Inflammatory cell profiles in the lungs of mice S. pn. mono-infected or co-infected during the recovery phase of IAV infection. Mice were infected with 1x10 6 CFU of S. pn. strain T4, 19F or 7F or treated with PBS on day 14 post infection with 0.31TCID 50 of IAV or PBS treatment. Absolute numbers of (a) alveolar macrophages (#AM), (b) infiltrating mononuclear phagocytes (#IMP) and (c) polymorphonuclear cells (#PMN) in post-lavage lungs 18h after the bacterial infection or PBS treatment. Mice were infected with 1x10 6 CFU of S. pn. strain T4, 19F or 7F or treated with PBS on day 21 post infection with 0.31TCID 50 of IAV or PBS treatment. Absolute numbers of (d) alveolar macrophages (#AM), (e) infiltrating cells (#IMP) and (f) polymorphonuclear cells (#PMN) in post-lavage lungs 18h after the bacterial infection or PBS treatment. All data are shown as Tukey box plots with lines indicating the median. Data are compiled of at least two independent experiments with 3-4 WT mice per group Figure 7: Pro-inflammatory cytokine and chemokine profiles in the lungs of mice S. pn. mono-infected or co-infected during the acute phase of IAV infection. Mice were infected with 1x10 6 CFU of S. pn. strain T4, 19F or 7F or treated with PBS on day 7 post infection with 0.31TCID 50 of IAV or PBS treatment. Protein concentrations of (a) IL-6 and (b) TNF-α in the homogenates of post-lavage lungs at 18h after the secondary 33
34 bacterial infection or PBS treatment. Data are shown as mean ± SEM of the number of mice/group indicated in parentheses and are compiled from two independent infection experiments with 1-4 mice per group. Protein concentrations of (c) KC, (d) LIX, (e) MCP- 1 and (f) MIP-1β in the homogenates of post-lavage lungs at 18h after the secondary bacterial infection or PBS treatment. Data are shown as mean ± SEM of the number of mice/group indicated in parentheses and are compiled from two independent infections with 2-5 mice per group. Statistical analysis was performed using the one-way ANOVA with Bonferroni s multiple comparison post-test. Asterisks * indicate significant differences, *P<0.05;**P<0.01;***P<
35
36
37
38
39
Influenza A virus infection predisposes hosts to secondary infection with different
 Supplementary information Influenza A virus infection predisposes hosts to secondary infection with different Streptococcus pneumoniae serotypes with similar outcome but serotype-specific manifestation.
Supplementary information Influenza A virus infection predisposes hosts to secondary infection with different Streptococcus pneumoniae serotypes with similar outcome but serotype-specific manifestation.
Supplementary Figures
 Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Supporting Information
 Supporting Information Valkenburg et al. 10.1073/pnas.1403684111 SI Materials and Methods ELISA and Microneutralization. Sera were treated with Receptor Destroying Enzyme II (RDE II, Accurate) before ELISA
Supporting Information Valkenburg et al. 10.1073/pnas.1403684111 SI Materials and Methods ELISA and Microneutralization. Sera were treated with Receptor Destroying Enzyme II (RDE II, Accurate) before ELISA
Supporting Information
 Supporting Information Aldridge et al. 10.1073/pnas.0900655106 Fig. S1. Flow diagram of sublethal (a) and lethal (b) influenza virus infections. (a) Infection of lung epithelial cells by influenza virus
Supporting Information Aldridge et al. 10.1073/pnas.0900655106 Fig. S1. Flow diagram of sublethal (a) and lethal (b) influenza virus infections. (a) Infection of lung epithelial cells by influenza virus
Streptococcus pneumonia
 Streptococcus pneumonia The pneumococci (S. pneumoniae) are gram-positive diplococci. Often lancet shaped or arranged in chains, possessing a capsule of polysaccharide that permits typing with specific
Streptococcus pneumonia The pneumococci (S. pneumoniae) are gram-positive diplococci. Often lancet shaped or arranged in chains, possessing a capsule of polysaccharide that permits typing with specific
In vitro bactericidal assay Fig. S8 Gentamicin protection assay Phagocytosis assay
 In vitro bactericidal assay Mouse bone marrow was isolated from the femur and the tibia. Cells were suspended in phosphate buffered saline containing.5% BSA and 2 mm EDTA and filtered through a cell strainer.
In vitro bactericidal assay Mouse bone marrow was isolated from the femur and the tibia. Cells were suspended in phosphate buffered saline containing.5% BSA and 2 mm EDTA and filtered through a cell strainer.
Abstract: I. A ims Aim 1:
 Abstract: Previous work from our laboratory demonstrated that obese mice have alterations in antiviral cytokine gene expression when infected with the influenza virus. Since these cytokines play a major
Abstract: Previous work from our laboratory demonstrated that obese mice have alterations in antiviral cytokine gene expression when infected with the influenza virus. Since these cytokines play a major
Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD-
 Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Pneumococcal vaccines. Safety & Efficacy. Prof. Rajesh Kumar, MD PGIMER School of Public Health Chandigarh
 Pneumococcal vaccines Safety & Efficacy Prof. Rajesh Kumar, MD PGIMER School of Public Health Chandigarh Disclosure Slide X X I DO NOT have any significant or other financial relationships with industry
Pneumococcal vaccines Safety & Efficacy Prof. Rajesh Kumar, MD PGIMER School of Public Health Chandigarh Disclosure Slide X X I DO NOT have any significant or other financial relationships with industry
Thesis for doctoral degree (Ph.D.) 2007 Molecular epidemiology of pneumococcal carriage and invasive disease Karin Sjöström.
 Thesis for doctoral degree (Ph.D.) 2007 Thesis for doctoral degree (Ph.D.) 2007 Molecular epidemiology of pneumococcal carriage and invasive disease Karin Sjöström Molecular Epidemiology of Pneumococcal
Thesis for doctoral degree (Ph.D.) 2007 Thesis for doctoral degree (Ph.D.) 2007 Molecular epidemiology of pneumococcal carriage and invasive disease Karin Sjöström Molecular Epidemiology of Pneumococcal
Supporting Information
 Supporting Information Desnues et al. 10.1073/pnas.1314121111 SI Materials and Methods Mice. Toll-like receptor (TLR)8 / and TLR9 / mice were generated as described previously (1, 2). TLR9 / mice were
Supporting Information Desnues et al. 10.1073/pnas.1314121111 SI Materials and Methods Mice. Toll-like receptor (TLR)8 / and TLR9 / mice were generated as described previously (1, 2). TLR9 / mice were
COPD lungs show an attached stratified mucus layer that separate. bacteria from the epithelial cells resembling the protective colonic
 COPD lungs show an attached stratified mucus layer that separate bacteria from the epithelial cells resembling the protective colonic mucus SUPPLEMENTARY TABLES AND FIGURES Tables S1 S8, page 1 and separate
COPD lungs show an attached stratified mucus layer that separate bacteria from the epithelial cells resembling the protective colonic mucus SUPPLEMENTARY TABLES AND FIGURES Tables S1 S8, page 1 and separate
ABIMMUNE Repurposing disused antibiotics with immune modulators as antimicrobial strategy for respiratory tract infections
 ABIMMUNE Repurposing disused antibiotics with immune modulators as antimicrobial strategy for respiratory tract infections Jean-Claude Sirard Christophe Carnoy Fiordiligie Casilag Delphine Cayet The partners
ABIMMUNE Repurposing disused antibiotics with immune modulators as antimicrobial strategy for respiratory tract infections Jean-Claude Sirard Christophe Carnoy Fiordiligie Casilag Delphine Cayet The partners
Increased susceptibility for superinfection with Streptococcus pneumoniae during influenza virus infection is not caused by TLR7-mediated lymphopenia.
 Increased susceptibility for superinfection with Streptococcus pneumoniae during influenza virus infection is not caused by TLR7-mediated lymphopenia. Item Type Article Authors Stegemann, Sabine; Dahlberg,
Increased susceptibility for superinfection with Streptococcus pneumoniae during influenza virus infection is not caused by TLR7-mediated lymphopenia. Item Type Article Authors Stegemann, Sabine; Dahlberg,
Medical Virology Immunology. Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University
 Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
PNEUMONIA : PROMISE FULFILLED? Regina Berba MD FPSMID
 PNEUMONIA : PROMISE FULFILLED? Regina Berba MD FPSMID Objectives of Lecture Know the quality of current evidence based guidelines on immunization Appreciate the performance of pneumonia vaccines in terns
PNEUMONIA : PROMISE FULFILLED? Regina Berba MD FPSMID Objectives of Lecture Know the quality of current evidence based guidelines on immunization Appreciate the performance of pneumonia vaccines in terns
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland Approved for public release; distribution unlimited
 AD (Leave blank) Award Number: W81XWH-07-1-0345 TITLE: Second-Generation Therapeutic DNA Lymphoma Vaccines PRINCIPAL INVESTIGATOR: Larry W. Kwak, M.D., Ph.D. CONTRACTING ORGANIZATION: University of Texas
AD (Leave blank) Award Number: W81XWH-07-1-0345 TITLE: Second-Generation Therapeutic DNA Lymphoma Vaccines PRINCIPAL INVESTIGATOR: Larry W. Kwak, M.D., Ph.D. CONTRACTING ORGANIZATION: University of Texas
Online Data Supplement. Induction of Pulmonary Granuloma Formation by Propionibacterium acnes is regulated by. MyD88 and Nox2
 Online Data Supplement Induction of Pulmonary Granuloma Formation by Propionibacterium acnes is regulated by MyD88 and Nox2 Jessica L. Werner *, Sylvia G. Escolero *, Jeff T. Hewlett *, Tim N. Mak *3,
Online Data Supplement Induction of Pulmonary Granuloma Formation by Propionibacterium acnes is regulated by MyD88 and Nox2 Jessica L. Werner *, Sylvia G. Escolero *, Jeff T. Hewlett *, Tim N. Mak *3,
SUPPLEMENTARY INFORMATION. CXCR4 inhibitors could benefit to HER2 but not to Triple-Negative. breast cancer patients
 SUPPLEMENTARY INFORMATION CXCR4 inhibitors could benefit to HER2 but not to Triple-Negative breast cancer patients Lefort S. 1,2, Thuleau A. 3, Kieffer Y. 1,2, Sirven P. 1,2, Bieche I. 4, Marangoni E.
SUPPLEMENTARY INFORMATION CXCR4 inhibitors could benefit to HER2 but not to Triple-Negative breast cancer patients Lefort S. 1,2, Thuleau A. 3, Kieffer Y. 1,2, Sirven P. 1,2, Bieche I. 4, Marangoni E.
Understanding mortality from pandemic and seasonal influenza
 Understanding mortality from pandemic and seasonal influenza Jonathan A. McCullers Associate Member Department of Infectious Diseases St. Jude Children s Research Hospital H1 H2 H3 H4 H5 H6 H7 H8 H9 H10
Understanding mortality from pandemic and seasonal influenza Jonathan A. McCullers Associate Member Department of Infectious Diseases St. Jude Children s Research Hospital H1 H2 H3 H4 H5 H6 H7 H8 H9 H10
Suppl Video: Tumor cells (green) and monocytes (white) are seeded on a confluent endothelial
 Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Interferon γ regulates idiopathic pneumonia syndrome, a. Th17 + CD4 + T-cell-mediated GvH disease
 Interferon γ regulates idiopathic pneumonia syndrome, a Th17 + CD4 + T-cell-mediated GvH disease Nora Mauermann, Julia Burian, Christophe von Garnier, Stefan Dirnhofer, Davide Germano, Christine Schuett,
Interferon γ regulates idiopathic pneumonia syndrome, a Th17 + CD4 + T-cell-mediated GvH disease Nora Mauermann, Julia Burian, Christophe von Garnier, Stefan Dirnhofer, Davide Germano, Christine Schuett,
Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and
 Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and Thy1 in NH cells derived from the lungs of naïve mice.
Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and Thy1 in NH cells derived from the lungs of naïve mice.
INTRABULBAR INOCULATION OF JAPANESE ENCEPHALITIS VIRUS TO MICE
 THE KURUME MEDICAL JOURNAL Vol. 15, No. 1, 1968 INTRABULBAR INOCULATION OF JAPANESE ENCEPHALITIS VIRUS TO MICE TOSHINORI TSUCHIYA Department of Microbiology, and Department of Ophthalmology, Kurume University
THE KURUME MEDICAL JOURNAL Vol. 15, No. 1, 1968 INTRABULBAR INOCULATION OF JAPANESE ENCEPHALITIS VIRUS TO MICE TOSHINORI TSUCHIYA Department of Microbiology, and Department of Ophthalmology, Kurume University
10.00 PBS OVA OVA+isotype antibody 8.00 OVA+anti-HMGB1. PBS Methatroline (mg/ml)
 RESEARCH ARTICLE Penh (100% of PBS) 1 PBS 8.00 +anti-hmgb1 6.00 4.00 p=0.054 Cellular & Molecular Immunology advance online publication, PBS 3.12 6.25 Methatroline (mg/ml) Neutrophil isolation and culture
RESEARCH ARTICLE Penh (100% of PBS) 1 PBS 8.00 +anti-hmgb1 6.00 4.00 p=0.054 Cellular & Molecular Immunology advance online publication, PBS 3.12 6.25 Methatroline (mg/ml) Neutrophil isolation and culture
Benefits of the pneumococcal immunisation programme in children in the United Kingdom
 Benefits of the pneumococcal immunisation programme in children in the United Kingdom 2006-2014 Professor Mary P E Slack mpeslack@gmail.com March 2015 Disclosure of interest The presenter has received
Benefits of the pneumococcal immunisation programme in children in the United Kingdom 2006-2014 Professor Mary P E Slack mpeslack@gmail.com March 2015 Disclosure of interest The presenter has received
SUPPLEMENTARY METHODS
 SUPPLEMENTARY METHODS Histological analysis. Colonic tissues were collected from 5 parts of the middle colon on day 7 after the start of DSS treatment, and then were cut into segments, fixed with 4% paraformaldehyde,
SUPPLEMENTARY METHODS Histological analysis. Colonic tissues were collected from 5 parts of the middle colon on day 7 after the start of DSS treatment, and then were cut into segments, fixed with 4% paraformaldehyde,
Malik Sallam. Ola AL-juneidi. Ammar Ramadan. 0 P a g e
 1 Malik Sallam Ola AL-juneidi Ammar Ramadan 0 P a g e Today's lecture will be about viral upper respiratory tract infections. Those include: common cold, sinusitis, otitis, etc. Infections in the upper
1 Malik Sallam Ola AL-juneidi Ammar Ramadan 0 P a g e Today's lecture will be about viral upper respiratory tract infections. Those include: common cold, sinusitis, otitis, etc. Infections in the upper
MATERIALS AND METHODS. Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All
 MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
Haemophilus influenzae
 Haemophilus influenzae type b Severe bacterial infection, particularly among infants During late 19th century believed to cause influenza Immunology and microbiology clarified in 1930s Haemophilus influenzae
Haemophilus influenzae type b Severe bacterial infection, particularly among infants During late 19th century believed to cause influenza Immunology and microbiology clarified in 1930s Haemophilus influenzae
Primary Adult Naïve CD4+ CD45RA+ Cells. Prepared by: David Randolph at University of Alabama, Birmingham
 Primary Adult Naïve CD4+ CD45RA+ Cells Prepared by: David Randolph (drdrdr@uab.edu) at University of Alabama, Birmingham Goal: To obtain large numbers of highly pure primary CD4+ CD45RO- CD25- cells from
Primary Adult Naïve CD4+ CD45RA+ Cells Prepared by: David Randolph (drdrdr@uab.edu) at University of Alabama, Birmingham Goal: To obtain large numbers of highly pure primary CD4+ CD45RO- CD25- cells from
Supplementary Materials for
 immunology.sciencemag.org/cgi/content/full/2/16/eaan6049/dc1 Supplementary Materials for Enzymatic synthesis of core 2 O-glycans governs the tissue-trafficking potential of memory CD8 + T cells Jossef
immunology.sciencemag.org/cgi/content/full/2/16/eaan6049/dc1 Supplementary Materials for Enzymatic synthesis of core 2 O-glycans governs the tissue-trafficking potential of memory CD8 + T cells Jossef
Biological Consulting Services
 Biological Consulting Services of North Florida/ Inc. May 13, 2009 Aphex BioCleanse Systems, Inc. Dear Sirs, We have completed antimicrobial efficacy study on the supplied Multi-Purpose Solution. The testing
Biological Consulting Services of North Florida/ Inc. May 13, 2009 Aphex BioCleanse Systems, Inc. Dear Sirs, We have completed antimicrobial efficacy study on the supplied Multi-Purpose Solution. The testing
Supplemental Information. Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection. Cell Host & Microbe, Volume 15
 Cell Host & Microbe, Volume 15 Supplemental Information Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection Arya Khosravi, Alberto Yáñez, Jeremy G. Price, Andrew Chow, Miriam Merad, Helen
Cell Host & Microbe, Volume 15 Supplemental Information Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection Arya Khosravi, Alberto Yáñez, Jeremy G. Price, Andrew Chow, Miriam Merad, Helen
Exercise Alters Stress and Inflammatory Response in Lungs of Mice
 Exercise Alters Stress and Inflammatory Response in Lungs of Mice Joseph Sevcik1, Shibani Naik2, Marian L Kohut1,2 1Department of Kinesiology, Iowa State University, Ames, IA 2Immunobiology Department,
Exercise Alters Stress and Inflammatory Response in Lungs of Mice Joseph Sevcik1, Shibani Naik2, Marian L Kohut1,2 1Department of Kinesiology, Iowa State University, Ames, IA 2Immunobiology Department,
HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates
 HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates Department of Microbiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA
HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates Department of Microbiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA
Innate immunity. Abul K. Abbas University of California San Francisco. FOCiS
 1 Innate immunity Abul K. Abbas University of California San Francisco FOCiS 2 Lecture outline Components of innate immunity Recognition of microbes and dead cells Toll Like Receptors NOD Like Receptors/Inflammasome
1 Innate immunity Abul K. Abbas University of California San Francisco FOCiS 2 Lecture outline Components of innate immunity Recognition of microbes and dead cells Toll Like Receptors NOD Like Receptors/Inflammasome
Quantifying Bordetella-induced Neutrophil Infiltration Using a Myeloperoxidase Assay
 Quantifying Bordetella-induced Neutrophil Infiltration Using a Myeloperoxidase Assay Sandra M. Fuentes, Research Scholar Faculty Mentor: Dr. Eric Harvill The Pennsylvania State University Abstract Bacteria
Quantifying Bordetella-induced Neutrophil Infiltration Using a Myeloperoxidase Assay Sandra M. Fuentes, Research Scholar Faculty Mentor: Dr. Eric Harvill The Pennsylvania State University Abstract Bacteria
Effects of PCV7 and PCV13 on invasive pneumococcal disease and carriage in Stockholm, Sweden
 ORIGINAL ARTICLE RESPIRATORY INFECTIONS Effects of PCV7 and PCV13 on invasive pneumococcal disease and carriage in Stockholm, Sweden Ilias Galanis 1,, Ann Lindstrand 1,2,, Jessica Darenberg 1,, Sarah Browall
ORIGINAL ARTICLE RESPIRATORY INFECTIONS Effects of PCV7 and PCV13 on invasive pneumococcal disease and carriage in Stockholm, Sweden Ilias Galanis 1,, Ann Lindstrand 1,2,, Jessica Darenberg 1,, Sarah Browall
What is the immune system? Types of Immunity. Pasteur and rabies vaccine. Historical Role of smallpox. Recognition Response
 Recognition Response Effector memory What is the immune system? Types of Immunity Innate Adaptive Anergy: : no response Harmful response: Autoimmunity Historical Role of smallpox Pasteur and rabies vaccine
Recognition Response Effector memory What is the immune system? Types of Immunity Innate Adaptive Anergy: : no response Harmful response: Autoimmunity Historical Role of smallpox Pasteur and rabies vaccine
ADI_Res_Bull_2013_Pneumococcal_Vaccine_Tests
 ADI_Res_Bull_2013_Pneumococcal_Vaccine_Tests Most non-vaccinated humans and some animals have a natural exposure to non-virulent strains Streptococcus pneumonia, and therefore contain high levels of antibodies
ADI_Res_Bull_2013_Pneumococcal_Vaccine_Tests Most non-vaccinated humans and some animals have a natural exposure to non-virulent strains Streptococcus pneumonia, and therefore contain high levels of antibodies
Pneumococcal vaccines
 Pneumococcal vaccines Marco Aurélio Sáfadi, MD, PhD FCM da Santa Casa de São Paulo Challenges in establishing the baseline burden of disease, before implementing a vaccination program S. pneumoniae disease
Pneumococcal vaccines Marco Aurélio Sáfadi, MD, PhD FCM da Santa Casa de São Paulo Challenges in establishing the baseline burden of disease, before implementing a vaccination program S. pneumoniae disease
EXPERIMENTAL SALMONELLOSIS
 EXPERIMENTAL SALMONELLOSIS INTRACELLULAR GROWTH OF Salmonella enteritidis INGESTED IN MONONUCLEAR PHAGOCYTES OF MICE, AND CELLULAR BASIS OF IMMUNITY SUSUMU MITSUHASHI, ICHIEI SATO, AND TOKUMITSU TANAKA
EXPERIMENTAL SALMONELLOSIS INTRACELLULAR GROWTH OF Salmonella enteritidis INGESTED IN MONONUCLEAR PHAGOCYTES OF MICE, AND CELLULAR BASIS OF IMMUNITY SUSUMU MITSUHASHI, ICHIEI SATO, AND TOKUMITSU TANAKA
In vitro human regulatory T cell expansion
 - 1 - Human CD4 + CD25 + regulatory T cell isolation, Workflow in vitro expansion and analysis In vitro human regulatory T cell expansion Introduction Regulatory T (Treg) cells are a subpopulation of T
- 1 - Human CD4 + CD25 + regulatory T cell isolation, Workflow in vitro expansion and analysis In vitro human regulatory T cell expansion Introduction Regulatory T (Treg) cells are a subpopulation of T
The Annexin V Apoptosis Assay
 The Annexin V Apoptosis Assay Development of the Annexin V Apoptosis Assay: 1990 Andree at al. found that a protein, Vascular Anticoagulant α, bound to phospholipid bilayers in a calcium dependent manner.
The Annexin V Apoptosis Assay Development of the Annexin V Apoptosis Assay: 1990 Andree at al. found that a protein, Vascular Anticoagulant α, bound to phospholipid bilayers in a calcium dependent manner.
Prevention and treatment of swine-origin influenza virus with interferon: an in vivo and ex vivo study
 RESEARCH FUND FOR THE CONTROL OF INFECTIOUS DISEASES Prevention and treatment of swine-origin influenza virus with interferon: an in vivo and ex vivo study JM Nicholls *, RWY Chan, E Fish K e y M e s s
RESEARCH FUND FOR THE CONTROL OF INFECTIOUS DISEASES Prevention and treatment of swine-origin influenza virus with interferon: an in vivo and ex vivo study JM Nicholls *, RWY Chan, E Fish K e y M e s s
Clinical manifestations of invasive pneumococcal disease by vaccine and non-vaccine types
 ORIGINAL ARTICLE PULMONARY INFECTIONS Clinical manifestations of invasive pneumococcal disease by vaccine and non-vaccine types Sarah Browall 1,12, Erik Backhaus 2,12, Pontus Naucler 1,3, Ilias Galanis
ORIGINAL ARTICLE PULMONARY INFECTIONS Clinical manifestations of invasive pneumococcal disease by vaccine and non-vaccine types Sarah Browall 1,12, Erik Backhaus 2,12, Pontus Naucler 1,3, Ilias Galanis
Cells and viruses. Human isolates (A/Kawasaki/173/01 [H1N1], A/Yokohama/2057/03 [H3N2],
![Cells and viruses. Human isolates (A/Kawasaki/173/01 [H1N1], A/Yokohama/2057/03 [H3N2], Cells and viruses. Human isolates (A/Kawasaki/173/01 [H1N1], A/Yokohama/2057/03 [H3N2],](/thumbs/86/93801604.jpg) Supplementary information Methods Cells and viruses. Human isolates (A/Kawasaki/173/01 [H1N1], A/Yokohama/2057/03 [H3N2], and A/Hong Kong/213/03 [H5N1]) were grown in Madin-Darby canine kidney (MDCK) cells
Supplementary information Methods Cells and viruses. Human isolates (A/Kawasaki/173/01 [H1N1], A/Yokohama/2057/03 [H3N2], and A/Hong Kong/213/03 [H5N1]) were grown in Madin-Darby canine kidney (MDCK) cells
Alberta Health and Wellness Public Health Notifiable Disease Management Guidelines August Pneumococcal Disease, Invasive (IPD)
 August 2011 Pneumococcal Disease, Invasive (IPD) Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) Case Definition August
August 2011 Pneumococcal Disease, Invasive (IPD) Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) Case Definition August
pplementary Figur Supplementary Figure 1. a.
 pplementary Figur Supplementary Figure 1. a. Quantification by RT-qPCR of YFV-17D and YFV-17D pol- (+) RNA in the supernatant of cultured Huh7.5 cells following viral RNA electroporation of respective
pplementary Figur Supplementary Figure 1. a. Quantification by RT-qPCR of YFV-17D and YFV-17D pol- (+) RNA in the supernatant of cultured Huh7.5 cells following viral RNA electroporation of respective
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Supplementary Figure 1. Antibiotic partially rescues mice from sepsis. (ab) BALB/c mice under CLP were treated with antibiotic or PBS.
 1 Supplementary Figure 1. Antibiotic partially rescues mice from sepsis. (ab) BALB/c mice under CLP were treated with antibiotic or PBS. (a) Survival curves. WT Sham (n=5), WT CLP or WT CLP antibiotic
1 Supplementary Figure 1. Antibiotic partially rescues mice from sepsis. (ab) BALB/c mice under CLP were treated with antibiotic or PBS. (a) Survival curves. WT Sham (n=5), WT CLP or WT CLP antibiotic
Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice
 Research article Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice Arash Shahangian, 1,2 Edward K. Chow, 3 Xiaoli Tian, 4 Jason R. Kang, 1 Amir Ghaffari, 1,2 Su Y. Liu, 1,2 John
Research article Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice Arash Shahangian, 1,2 Edward K. Chow, 3 Xiaoli Tian, 4 Jason R. Kang, 1 Amir Ghaffari, 1,2 Su Y. Liu, 1,2 John
Pneumococcal Disease and Pneumococcal Vaccines
 Pneumococcal Disease and Epidemiology and Prevention of - Preventable Diseases Note to presenters: Images of vaccine-preventable diseases are available from the Immunization Action Coalition website at
Pneumococcal Disease and Epidemiology and Prevention of - Preventable Diseases Note to presenters: Images of vaccine-preventable diseases are available from the Immunization Action Coalition website at
Bacterial diseases caused by Streptoccus pneumoniae in children
 Bacterial diseases caused by Streptoccus pneumoniae in children Bactermia 85% Bacterial pneumonia 66% Bacterial meningitis 50% Otitis media 40% Paranasal sinusitis 40% 0% 10% 20% 30% 40% 50% 60% 70% 80%
Bacterial diseases caused by Streptoccus pneumoniae in children Bactermia 85% Bacterial pneumonia 66% Bacterial meningitis 50% Otitis media 40% Paranasal sinusitis 40% 0% 10% 20% 30% 40% 50% 60% 70% 80%
Ex vivo Human Antigen-specific T Cell Proliferation and Degranulation Willemijn Hobo 1, Wieger Norde 1 and Harry Dolstra 2*
 Ex vivo Human Antigen-specific T Cell Proliferation and Degranulation Willemijn Hobo 1, Wieger Norde 1 and Harry Dolstra 2* 1 Department of Laboratory Medicine - Laboratory of Hematology, Radboud University
Ex vivo Human Antigen-specific T Cell Proliferation and Degranulation Willemijn Hobo 1, Wieger Norde 1 and Harry Dolstra 2* 1 Department of Laboratory Medicine - Laboratory of Hematology, Radboud University
Splenectomy Vaccine Protocol PIDPIC
 Splenectomy Vaccine Protocol PIDPIC 6.24.14 Rationale Spleen clears encapsulated bacteria and infected erythrocytes Serves as one of the largest lymphoid tissues where B cells are educated against encapsulated
Splenectomy Vaccine Protocol PIDPIC 6.24.14 Rationale Spleen clears encapsulated bacteria and infected erythrocytes Serves as one of the largest lymphoid tissues where B cells are educated against encapsulated
Appendix A: Disease-Specific Chapters
 Ministry of Health and Long-Term Care Infectious Diseases Protocol Appendix A: Disease-Specific Chapters Chapter: Haemophilus influenzae disease, all types, invasive Effective: May 2018 Haemophilus influenzae,
Ministry of Health and Long-Term Care Infectious Diseases Protocol Appendix A: Disease-Specific Chapters Chapter: Haemophilus influenzae disease, all types, invasive Effective: May 2018 Haemophilus influenzae,
Evaluation of Antibacterial Effect of Odor Eliminating Compounds
 Evaluation of Antibacterial Effect of Odor Eliminating Compounds Yuan Zeng, Bingyu Li, Anwar Kalalah, Sang-Jin Suh, and S.S. Ditchkoff Summary Antibiotic activity of ten commercially available odor eliminating
Evaluation of Antibacterial Effect of Odor Eliminating Compounds Yuan Zeng, Bingyu Li, Anwar Kalalah, Sang-Jin Suh, and S.S. Ditchkoff Summary Antibiotic activity of ten commercially available odor eliminating
In vitro human regulatory T cell expansion
 - 1 - Human CD4 + CD25 + CD127 dim/- regulatory T cell Workflow isolation, in vitro expansion and analysis In vitro human regulatory T cell expansion Introduction Regulatory T (Treg) cells are a subpopulation
- 1 - Human CD4 + CD25 + CD127 dim/- regulatory T cell Workflow isolation, in vitro expansion and analysis In vitro human regulatory T cell expansion Introduction Regulatory T (Treg) cells are a subpopulation
Evaluation of directed and random motility in microslides Assessment of leukocyte adhesion in flow chambers
 Evaluation of directed and random motility in microslides Motility experiments in IBIDI microslides, image acquisition and processing were performed as described. PMN, which ended up in an angle < 180
Evaluation of directed and random motility in microslides Motility experiments in IBIDI microslides, image acquisition and processing were performed as described. PMN, which ended up in an angle < 180
Re: SCN-Lifequest: Bird flu study in mice, H1 N1/MAE-01
 ST&T DEPARTMENTS: PRODUCT DEVELOPMENT & CLINICAL RESEARCH MS. SIMONE DERAYEH PHARMACOLOGY/TOXICOLOGY DR. ALDRICH DR. SNODGRASS GENERAL MEDICINE, MEDICAL CONSULTATION DR. ALDRICH DR. SNODGRASS INTERNATIONAL
ST&T DEPARTMENTS: PRODUCT DEVELOPMENT & CLINICAL RESEARCH MS. SIMONE DERAYEH PHARMACOLOGY/TOXICOLOGY DR. ALDRICH DR. SNODGRASS GENERAL MEDICINE, MEDICAL CONSULTATION DR. ALDRICH DR. SNODGRASS INTERNATIONAL
Heat-killed Lactobacillus casei
 Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection Yu-Jin Jung, Young-Tae Lee,
Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection Yu-Jin Jung, Young-Tae Lee,
Streptococcus pneumoniae
 Streptococcus pneumoniae EPIDEMIOLOGY AND PREVENTION 2^4. November 2007 Prof. Dr. Kathrin Mühlemann Klinik und Poliklinik für Infektionskrankheiten Universität Bern, INSELSPITAL S. pneumoniae: epidemiology
Streptococcus pneumoniae EPIDEMIOLOGY AND PREVENTION 2^4. November 2007 Prof. Dr. Kathrin Mühlemann Klinik und Poliklinik für Infektionskrankheiten Universität Bern, INSELSPITAL S. pneumoniae: epidemiology
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): In the manuscript Rational Combination of CXCL11-Expressing Oncolytic Virus and PD-L1 Blockade Works Synergistically to Enhance Therapeutic Efficacy
Reviewers' comments: Reviewer #1 (Remarks to the Author): In the manuscript Rational Combination of CXCL11-Expressing Oncolytic Virus and PD-L1 Blockade Works Synergistically to Enhance Therapeutic Efficacy
Live Attenuated Influenza Virus Increases Pneumococcal Translocation and Persistence Within the Middle Ear
 J Infect Dis. 2015 Jul 15; 212(2): 195 201. Published online 2014 Dec 11. doi: 10.1093/infdis/jiu804 PMCID: PMC4654764 PMID: 25505300 Live Attenuated Influenza Virus Increases Pneumococcal Translocation
J Infect Dis. 2015 Jul 15; 212(2): 195 201. Published online 2014 Dec 11. doi: 10.1093/infdis/jiu804 PMCID: PMC4654764 PMID: 25505300 Live Attenuated Influenza Virus Increases Pneumococcal Translocation
The Involvement of IL-17A in the Murine Response to Sub-Lethal Inhalational Infection with Francisella tularensis
 The Involvement of IL-17A in the Murine Response to Sub-Lethal Inhalational Infection with Francisella tularensis Gal Markel 1, Erez Bar-Haim 1, Eran Zahavy 2, Hila Cohen 1, Ofer Cohen 1, Avigdor Shafferman
The Involvement of IL-17A in the Murine Response to Sub-Lethal Inhalational Infection with Francisella tularensis Gal Markel 1, Erez Bar-Haim 1, Eran Zahavy 2, Hila Cohen 1, Ofer Cohen 1, Avigdor Shafferman
however, and the present communication is concerned with some of
 THE AGGLUTINATION OF HUMAN ERYTHROCYTES MODIFIED BY TREATMENT WITH NEWCASTLE DISEASE AND INFLUENZA VIRUS' ALFRED L. FLORMAN' Pediatric Service and Division of Bacteriology, The Mount Sinai Hospital, New
THE AGGLUTINATION OF HUMAN ERYTHROCYTES MODIFIED BY TREATMENT WITH NEWCASTLE DISEASE AND INFLUENZA VIRUS' ALFRED L. FLORMAN' Pediatric Service and Division of Bacteriology, The Mount Sinai Hospital, New
HISTO-PHYSIOLOGY HISTO-PHYSIOLOGY HISTO-PHYSIOLOGY. 09-Mar-15. Dr. Muhammad Tariq Javed. RESPIRATORY SYSTEM Lec-1
 RESPIRATORY SYSTEM Lec-1 Dr. Muhammad Tariq Javed Professor Department of Pathology, University of Agriculture, Faisalabad. Email: mtjaved@uaf.edu.pk Web: http://www.geocities.ws/mtjaved 1 2 Conducting
RESPIRATORY SYSTEM Lec-1 Dr. Muhammad Tariq Javed Professor Department of Pathology, University of Agriculture, Faisalabad. Email: mtjaved@uaf.edu.pk Web: http://www.geocities.ws/mtjaved 1 2 Conducting
Pearson r = P (one-tailed) = n = 9
 8F4-Specific Lysis, % 1 UPN1 UPN3 8 UPN7 6 Pearson r =.69 UPN2 UPN5 P (one-tailed) =.192 4 UPN8 n = 9 2 UPN9 UPN4 UPN6 5 1 15 2 25 8 8F4, % Max MFI Supplementary Figure S1. AML samples UPN1-UPN9 show variable
8F4-Specific Lysis, % 1 UPN1 UPN3 8 UPN7 6 Pearson r =.69 UPN2 UPN5 P (one-tailed) =.192 4 UPN8 n = 9 2 UPN9 UPN4 UPN6 5 1 15 2 25 8 8F4, % Max MFI Supplementary Figure S1. AML samples UPN1-UPN9 show variable
Respiratory syncytial virus (RSV) and respiratory bacteria which comes first?
 Respiratory syncytial virus (RSV) and respiratory bacteria which comes first? Prof. David Greenberg MD The Pediatric Infectious Disease Unit Soroka University Medical Center The Faculty of Health Sciences,
Respiratory syncytial virus (RSV) and respiratory bacteria which comes first? Prof. David Greenberg MD The Pediatric Infectious Disease Unit Soroka University Medical Center The Faculty of Health Sciences,
IFNg. IFNg IL-5 IL-13 IL-17 IL-22. LTi NCR+ ILC3. IL-17 IL-22 IFNg
 Group 1 ILC T-Bet Eomes Nkp46 NK1.1 NK cells IFNg T-Bet ILC1 IFNg low RORgt Group 2 ILC RORa CD127 ILC2 IL-5 IL-13 Group 3 ILC RORc CD127 AhR T-Bet AhR LTi c-kit; CD4+/- NCR+ ILC3 c-kit; Nkp46 IL-17 IL-22
Group 1 ILC T-Bet Eomes Nkp46 NK1.1 NK cells IFNg T-Bet ILC1 IFNg low RORgt Group 2 ILC RORa CD127 ILC2 IL-5 IL-13 Group 3 ILC RORc CD127 AhR T-Bet AhR LTi c-kit; CD4+/- NCR+ ILC3 c-kit; Nkp46 IL-17 IL-22
Participation of CD11c Leukocytes in Methicillin-Resistant Staphylococcus aureus Clearance from the Lung
 INFECTION AND IMMUNITY, May 2011, p. 1898 1904 Vol. 79, No. 5 0019-9567/11/$12.00 doi:10.1128/iai.01299-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Participation of CD11c
INFECTION AND IMMUNITY, May 2011, p. 1898 1904 Vol. 79, No. 5 0019-9567/11/$12.00 doi:10.1128/iai.01299-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Participation of CD11c
Title: Successive influenza virus infection and Streptococcus pneumoniae stimulation alter human dendritic cell function
 Author's response to reviews Title: Successive influenza virus infection and Streptococcus pneumoniae stimulation alter human dendritic cell function Authors: Yuet Wu (zacharywu03@yahoo.com.hk) Huawei
Author's response to reviews Title: Successive influenza virus infection and Streptococcus pneumoniae stimulation alter human dendritic cell function Authors: Yuet Wu (zacharywu03@yahoo.com.hk) Huawei
Zheng, BJ; Du, LY; Zhao, GY; Lin, YP; Sui, HY; Chan, C; Ma, S; Guan, Y; Yuen, KY. Citation Hong Kong Medical Journal, 2008, v. 14 suppl. 4, p.
 Title Studies of SARS virus vaccines Author(s) Zheng, BJ; Du, LY; Zhao, GY; Lin, YP; Sui, HY; Chan, C; Ma, S; Guan, Y; Yuen, KY Citation Hong Kong Medical Journal, 2008, v. 14 suppl. 4, p. 39-43 Issued
Title Studies of SARS virus vaccines Author(s) Zheng, BJ; Du, LY; Zhao, GY; Lin, YP; Sui, HY; Chan, C; Ma, S; Guan, Y; Yuen, KY Citation Hong Kong Medical Journal, 2008, v. 14 suppl. 4, p. 39-43 Issued
Pneumococcal Vaccine Effectiveness. Steven Black, MD Center for Global Health Cincinnati Children s s Hospital Cincinnati, Ohio USA
 Pneumococcal Vaccine Effectiveness Steven Black, MD Center for Global Health Cincinnati Children s s Hospital Cincinnati, Ohio USA Overview Possible effectiveness outcomes for pneumococcal vaccines Pre-licensure
Pneumococcal Vaccine Effectiveness Steven Black, MD Center for Global Health Cincinnati Children s s Hospital Cincinnati, Ohio USA Overview Possible effectiveness outcomes for pneumococcal vaccines Pre-licensure
Blocking Interhost Transmission of Influenza Virus by Vaccination in the Guinea Pig Model
 JOURNAL OF VIROLOGY, Apr. 2009, p. 2803 2818 Vol. 83, No. 7 0022-538X/09/$08.00 0 doi:10.1128/jvi.02424-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Blocking Interhost Transmission
JOURNAL OF VIROLOGY, Apr. 2009, p. 2803 2818 Vol. 83, No. 7 0022-538X/09/$08.00 0 doi:10.1128/jvi.02424-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Blocking Interhost Transmission
Supplementary Figure 1. Immune profiles of untreated and PD-1 blockade resistant EGFR and Kras mouse lung tumors (a) Total lung weight of untreated
 1 Supplementary Figure 1. Immune profiles of untreated and PD-1 blockade resistant EGFR and Kras mouse lung tumors (a) Total lung weight of untreated (U) EGFR TL mice (n=7), Kras mice (n=7), PD-1 blockade
1 Supplementary Figure 1. Immune profiles of untreated and PD-1 blockade resistant EGFR and Kras mouse lung tumors (a) Total lung weight of untreated (U) EGFR TL mice (n=7), Kras mice (n=7), PD-1 blockade
WHO biosafety risk assessment and guidelines for the production and quality control of human influenza pandemic vaccines: Update
 WHO biosafety risk assessment and guidelines for the production and quality control of human influenza pandemic vaccines: Update 23 July 2009 Introduction This document updates guidance 1 from the World
WHO biosafety risk assessment and guidelines for the production and quality control of human influenza pandemic vaccines: Update 23 July 2009 Introduction This document updates guidance 1 from the World
FIG S1 Replication rates of S. suis strain 10, 10Δsly, and 10cpsΔEF on mono- and virus pre-infected porcine PCLS.
 A strain 10 10cps EF B strain 10 H1N1 + strain 10 10cps EF H1N1 + 10cps EF 10 8 10 sly 10 7 H3N2 + strain 10 H3N2 + 10cps EF CFU/ml media 10 7 10 6 10 5 10 4 CFU/ml media 10 6 10 5 10 4 10 3 0 2 4 8 12
A strain 10 10cps EF B strain 10 H1N1 + strain 10 10cps EF H1N1 + 10cps EF 10 8 10 sly 10 7 H3N2 + strain 10 H3N2 + 10cps EF CFU/ml media 10 7 10 6 10 5 10 4 CFU/ml media 10 6 10 5 10 4 10 3 0 2 4 8 12
ADCC Assay Protocol Vikram Srivastava 1, Zheng Yang 1, Ivan Fan Ngai Hung 2, Jianqing Xu 3, Bojian Zheng 3 and Mei- Yun Zhang 3*
 ADCC Assay Protocol Vikram Srivastava 1, Zheng Yang 1, Ivan Fan Ngai Hung 2, Jianqing Xu 3, Bojian Zheng 3 and Mei- Yun Zhang 3* 1 Department of Microbiology, Li Ka Shing Faculty of Medicine, University
ADCC Assay Protocol Vikram Srivastava 1, Zheng Yang 1, Ivan Fan Ngai Hung 2, Jianqing Xu 3, Bojian Zheng 3 and Mei- Yun Zhang 3* 1 Department of Microbiology, Li Ka Shing Faculty of Medicine, University
Pneumococcal Vaccine in Children: current situation
 Pneumococcal Vaccine in Children: current situation LAU Yu Lung Chair Professor of Paediatrics Doris Zimmern Professor in Community Child Health LKS Faculty of Medicine, The University of Hong Kong Chairman
Pneumococcal Vaccine in Children: current situation LAU Yu Lung Chair Professor of Paediatrics Doris Zimmern Professor in Community Child Health LKS Faculty of Medicine, The University of Hong Kong Chairman
Influenza Infection Leads to Increased Susceptibility to Subsequent Bacterial Superinfection by Impairing NK Cell Responses in the Lung
 This information is current as of July 19, 2018. Supplementary Material References Subscription Permissions Email Alerts Influenza Infection Leads to Increased Susceptibility to Subsequent Bacterial Superinfection
This information is current as of July 19, 2018. Supplementary Material References Subscription Permissions Email Alerts Influenza Infection Leads to Increased Susceptibility to Subsequent Bacterial Superinfection
SUPPLEMENTAL INFORMATIONS
 1 SUPPLEMENTAL INFORMATIONS Figure S1 Cumulative ZIKV production by testis explants over a 9 day-culture period. Viral titer values presented in Figure 1B (viral release over a 3 day-culture period measured
1 SUPPLEMENTAL INFORMATIONS Figure S1 Cumulative ZIKV production by testis explants over a 9 day-culture period. Viral titer values presented in Figure 1B (viral release over a 3 day-culture period measured
2. Innate immunity 2013
 1 Innate Immune Responses 3 Innate immunity Abul K. Abbas University of California San Francisco The initial responses to: 1. Microbes: essential early mechanisms to prevent, control, or eliminate infection;
1 Innate Immune Responses 3 Innate immunity Abul K. Abbas University of California San Francisco The initial responses to: 1. Microbes: essential early mechanisms to prevent, control, or eliminate infection;
Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538
 Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538 Background: TIGIT is a co-inhibitory receptor that is highly expressed in Natural Killer (NK) cells, activated CD4+, CD8+ and regulatory
Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538 Background: TIGIT is a co-inhibitory receptor that is highly expressed in Natural Killer (NK) cells, activated CD4+, CD8+ and regulatory
Organic dust-induced interleukin-12 production activates T- and natural killer cells
 Eur Respir J 22; 2: 686 69 DOI:.1183/931936.2.222 Printed in UK all rights reserved Copyright #ERS Journals Ltd 22 European Respiratory Journal ISSN 93-1936 Organic dust-induced interleukin-12 production
Eur Respir J 22; 2: 686 69 DOI:.1183/931936.2.222 Printed in UK all rights reserved Copyright #ERS Journals Ltd 22 European Respiratory Journal ISSN 93-1936 Organic dust-induced interleukin-12 production
Update of WHO biosafety risk assessment and guidelines for the production and quality control of human influenza pandemic vaccines
 Update of WHO biosafety risk assessment and guidelines for the production and quality control of human influenza pandemic vaccines 28 May 2009 Introduction This document updates WHO guidance 1 to national
Update of WHO biosafety risk assessment and guidelines for the production and quality control of human influenza pandemic vaccines 28 May 2009 Introduction This document updates WHO guidance 1 to national
Phosphate buffered saline (PBS) for washing the cells TE buffer (nuclease-free) ph 7.5 for use with the PrimePCR Reverse Transcription Control Assay
 Catalog # Description 172-5080 SingleShot Cell Lysis Kit, 100 x 50 µl reactions 172-5081 SingleShot Cell Lysis Kit, 500 x 50 µl reactions For research purposes only. Introduction The SingleShot Cell Lysis
Catalog # Description 172-5080 SingleShot Cell Lysis Kit, 100 x 50 µl reactions 172-5081 SingleShot Cell Lysis Kit, 500 x 50 µl reactions For research purposes only. Introduction The SingleShot Cell Lysis
Clinical Infectious Diseases Advance Access published April 11, What is the real role of respiratory viruses in severe community-acquired
 Clinical Infectious Diseases Advance Access published April 11, 2014 1 What is the real role of respiratory viruses in severe community-acquired pneumonia? Olli Ruuskanen 1 and Asko Järvinen 2 1 Department
Clinical Infectious Diseases Advance Access published April 11, 2014 1 What is the real role of respiratory viruses in severe community-acquired pneumonia? Olli Ruuskanen 1 and Asko Järvinen 2 1 Department
La risposta immune all infezione da virus ebola. Chiara Agrati, PhD
 La risposta immune all infezione da virus ebola Chiara Agrati, PhD Pathogenetic mechanisms This virus infection is able to: - disable the immune system, preventing an effective protective immune response
La risposta immune all infezione da virus ebola Chiara Agrati, PhD Pathogenetic mechanisms This virus infection is able to: - disable the immune system, preventing an effective protective immune response
Streptococcus pneumoniae CDC
 Streptococcus pneumoniae CDC Pneumococcal Disease Infection caused by the bacteria, Streptococcus pneumoniae» otitis media 20 million office visits (28-55% Strep)» pneumonia 175,000 cases annually» meningitis
Streptococcus pneumoniae CDC Pneumococcal Disease Infection caused by the bacteria, Streptococcus pneumoniae» otitis media 20 million office visits (28-55% Strep)» pneumonia 175,000 cases annually» meningitis
Supporting Information
 Supporting Information Yen et al. 10.1073/pnas.1111000108 SI Materials and Methods Cells. Madin Darby canine kidney (MDCK) cells and human embryonic kidney 293T cells were obtained from the American Type
Supporting Information Yen et al. 10.1073/pnas.1111000108 SI Materials and Methods Cells. Madin Darby canine kidney (MDCK) cells and human embryonic kidney 293T cells were obtained from the American Type
Rapid antigen-specific T cell enrichment (Rapid ARTE)
 Direct ex vivo characterization of human antigen-specific CD154+CD4+ T cell Rapid antigen-specific T cell enrichment (Rapid ARTE) Introduction Workflow Antigen (ag)-specific T cells play a central role
Direct ex vivo characterization of human antigen-specific CD154+CD4+ T cell Rapid antigen-specific T cell enrichment (Rapid ARTE) Introduction Workflow Antigen (ag)-specific T cells play a central role
Role of cyclooxygenase-2 in H5N1 viral pathogenesis and the potential use of its inhibitors
 Title Role of cyclooxygenase-2 in HN viral pathogenesis and the potential use of its inhibitors Author(s) Lee, MY; Cheung, CY; Peiris, JSM Citation Hong Kong Medical Journal, 2, v. 9 n. Suppl. 4, p. 29-
Title Role of cyclooxygenase-2 in HN viral pathogenesis and the potential use of its inhibitors Author(s) Lee, MY; Cheung, CY; Peiris, JSM Citation Hong Kong Medical Journal, 2, v. 9 n. Suppl. 4, p. 29-
Kinetics of Coinfection with Influenza A Virus and Streptococcus pneumoniae
 Kinetics of Coinfection with Influenza A Virus and Streptococcus pneumoniae Amber M. Smith 1 *, Frederick R. Adler 2, Ruy M. Ribeiro 3,4, Ryan N. Gutenkunst 5, Julie L. McAuley 6, Jonathan A. McCullers
Kinetics of Coinfection with Influenza A Virus and Streptococcus pneumoniae Amber M. Smith 1 *, Frederick R. Adler 2, Ruy M. Ribeiro 3,4, Ryan N. Gutenkunst 5, Julie L. McAuley 6, Jonathan A. McCullers
Investigating respiratory disease
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Investigating respiratory disease Author : David Gibson Categories : Vets Date : August 3, 2009 David Gibson explores diagnostic
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Investigating respiratory disease Author : David Gibson Categories : Vets Date : August 3, 2009 David Gibson explores diagnostic
"Non-invasive Pneumococcal Diseases dynamics & the Host Age" Roman Prymula Ministry of Health, the Czech Republic
 "Non-invasive Pneumococcal Diseases dynamics & the Host Age" Roman Prymula Ministry of Health, the Czech Republic Disease Caused by S. pneumoniae S. pneumoniae Eustachian Tube Nasopharynx Pharynx Nasal
"Non-invasive Pneumococcal Diseases dynamics & the Host Age" Roman Prymula Ministry of Health, the Czech Republic Disease Caused by S. pneumoniae S. pneumoniae Eustachian Tube Nasopharynx Pharynx Nasal
Nature Immunology: doi: /ni Supplementary Figure 1. Production of cytokines and chemokines after vaginal HSV-2 infection.
 Supplementary Figure 1 Production of cytokines and chemokines after vaginal HSV-2 infection. C57BL/6 mice were (a) treated intravaginally with 20 µl of PBS or infected with 6.7x10 4 pfu of HSV-2 in the
Supplementary Figure 1 Production of cytokines and chemokines after vaginal HSV-2 infection. C57BL/6 mice were (a) treated intravaginally with 20 µl of PBS or infected with 6.7x10 4 pfu of HSV-2 in the
