U5 snrna Interacts with Exon Sequences at 5 and 3 Splice Sites
|
|
|
- Robert Warner
- 5 years ago
- Views:
Transcription
1 Cell, Vol. 68, , February 21, 1992, Copyright by Cell Press U5 snrna Interacts with Exon Sequences at 5 and 3 Splice Sites A. J. Newman and C. Norman MRC Laboratory of Molecular Biology Hills Road Cambridge CB2 2QH England Summary U5 snrna is an essential pre-mrna splicing factor whose function remains enigmatic. Specific mutations in a conserved single-stranded loop sequence in yeast U5 snrna can activate cleavage of Gl *A mutant premrnas at aberrant 5 splice sites and facilitate processing of dead-end lariat intermediates to mrna. Activation of aberrant 5 cleavage sites involves base pairing between US snrna and nucleotides upstream of the cleavage site. Processing of dead-end lariat intermediates to mrna correlates with base pairing between U5 and the first two bases in exon 2. The loop sequence in U5 snrna may therefore be intimately involved in the transesterification reactions at S and 3 splice sites. This pattern of interactions is strikingly reminiscent of exon recognition events in group II self-splicing introns and is consistent with the notion that U5 snrna may be related to a specific functional domain from a group II-like self-splicing ancestral intron. Introduction Splicing of mrna precursors occurs in a complex, dynamic structure called the spliceosome. This particle is formed on the pre-mrna by the ordered interaction of a set of snrnp particles containing five snrnas: Ul, U2, U4AJ6, and U5 (for recent reviews see Steitz et al., 1988; Ruby and Abelson, 1991; Lamond, 1991; Guthrie, 1991). Within this complex, introns are removed via a two-step transesterification process. First, 5 splice site cleavage involves nucleophilic attack by the 2 -OH of a specific adenosine residue near the 3 end of the intron. This gives the 5 exon and an intron-exon 2 lariat in which the S end of the intron is covalently attached via a 5,2 phosphodiester linkage to the adenosine to form a branch. Second, these intermediates are resolved via nucleophilic attack of the 3 -OH of the 5 exon at the 3 splice site to give the mrna and lariat intron products (reviewed by Sharp, 1987). Group II autocatalytic introns follow an identical two-step transesterification pathway, also yielding lariat RNA molecules as intermediates and products (Van der Veen et al., 1986; Peebles et al., 1986; Schmelzer and Schweyen, 1986). In these self-splicing introns, catalysis and specificity are achieved by formation of characteristic and highly conserved secondary and tertiary structures that establish the appropriate architecture for the transesterification reactions (Michel and Jacquier, 1987; Jacquier and Michel, 1987; reviewed by Michel et al., 1989). In contrast to the highly structured nature of group II introns, nuclear pre-mrna introns apparently have only short, conserved sequences at the splice junctions (and to a variable degree at the branchpoint) to establish specificity. Nevertheless, theclosemechanisticsimilarity between spliceosomal splicing and group II autocatalytic splicing argues that the recognition and catalytic functions of spliceosomes could be substantially RNA based. Indeed, it has often been suggested that these two types of splicing could have a common ancestry (Cech, 1986; Jacquier, 1990; Jacquier and Michel, 1990). According to this view, transacting spliceosome components would have inherited the functions of recognition and catalysis, which are intrinsic properties of self-splicing introns. The precise functions of snrna and protein splicing factors are currently under intensive study. It is well established that Ul and U2 snrnps play essential roles early in spliceosome assembly (Ruby and Abelson, 1988; Lamond et al., 1989; Barabino et al., 1989, 1990; Seraphin and Rosbash, 1989). Ul is involved in 5 splice site recognition and U2 in branchpoint recognition. Both of these interactions involve base pairing between the snrna and intron sequences (Zhuang and Weiner, 1986,1989; Parker et al., 1987; Wu and Manley, 1989; Seraphin et al., 1988; Siliciano and Guthrie, 1988). The functions of U4, U5, and U6 snrnas and a large number of other characterized splicing factors are not yet understood (reviewed by Ruby and Abelson, 1991; Guthrie, 1991). These factors include several proteins that have homologies with ATP-dependent RNA helicases (Dalbadie-McFarland and Abelson, 1990; Jamieson et al., 1991; Strauss and Guthrie, 1991). U4/U6 and U5 apparently associate to form a tri-snrnp particle (Cheng and Abelson, 1987; Konarska and Sharp, 1987; Lossky et al., 1987; Pinto and Steitz, 1989) before entering the spliceosome(lammet al., 1991;Seraphinetal., 1991). USsnRNP is a large particle with many protein components (Bach et al., 1989), including one polypeptide of >200 kd originally identified in yeast as the product of the PRPB gene (Lossky et al., 1987; Whittaker et al., 1990). This protein is highly conserved phylogenetically, implying that it has some important function (Anderson et al., 1989; Pinto and Steitz, 1989; Garcia-Blanc0 et al., 1990). The role of U5 snrnp in splicing remains enigmatic. Some evidence suggests that U5 snrnp or a UBassociated protein may interact with intron 3 splice sites (Chabot et al., 1985; Gerke and Steitz, 1986; Tazi et al., 1986), while evidence from yeast suggests that U5 is involved somehow in 5 splice site cleavage (Newman and Norman, 1991). U5 snrna has a highly conserved single-stranded loop sequence that includes the invariant nine nucleotide motif GCCUUUUAC (Patterson and Guthrie, 1987; Guthrie and Patterson, 1988). In U5 snrnp this loop is susceptible to chemical modification (Black and Pinto, 1989) and can base pair with other nucleic acids (Lamm et al., 1991). Specific mutations in this loop can activate aberrant processing pathways in spliceosomes assembled with pre-
2 Cell 744 mrna harboring mutations of the first nucleotide (Gl) of the intron. These aberrant pathways involve either activation of new 5 cleavage sites in the vicinity of the authentic site, or onward processing of dead-end lariat intermediates derived from cleavage at the authentic 5 splice site (Newman and Norman, 1991). This paper presents a more detailed analysis of the effects of mutations in the yeast U5 snrna loop sequence on 5 and 3 splice site utilization in vivo. The results reveal that the conserved U5 snrna loop sequence interacts with exon sequences at both S and 3 splice sites and may therefore play an important role in the transesterification reactions themselves. The U5 snrna-exon interactions are strikingly reminiscent of events in group I I autocatalytic splicing where base pairing between exon sequences and a single-stranded loop (the intron D3 loop) plays a crucial role in splice site specification. This finding underscores the mechanistic similarity between group II and spliceosomal splicing. Results The aim of the experiments described below is to identify the molecular basis of the activation by U5 snrna loop mutations of aberrant processing pathways and thereby clarify the role of U5 snrnp in pre-mrna splicing. It has been shown that mutations in the U5 loop motif GCCUUU- UAC can activate at least three aberrant processing pathways for Gl-A mutant pre-mrnas: (1) cleavage at an aberrant 5 splice site 12 bases 5 of the authentic site, activated by loop mutation GCCUUCUAC; (2) cleavage at an aberrant 5 splice site four bases 3 of the authentic site; and (3) onward processing of dead-end lariat intermediates to authentic mrna. Pathways 2 and 3 were simultaneously activated by the loop sequence GCGGUAUCC, which has four base changes relative to the wild-type sequence (Newman and Norman, 1991). In principle, the activation of aberrant mrna production using the 5 splice sites at -12 and +4 (pathways 1 and 2) could result from the effects of U5 snrna mutations on processing of intermediates to mrna rather than on 5 splice site cleavage. However, both of these splice sites are 5 of a G residue, which experiments elsewhere have shown is compatible with efficient processing of intermediates to mrna (Seraphin and Rosbash, 1990). Moreover, analysis of the lariat intermediates produced from Gl-+A mutant pre-mrna showed directly that processing of the -12 and +4 intermediates to mrna is not rate limiting (Newman and Norman, 1991). These considerations argue emphatically that production of these aberrant m RNAs is activated via alterat ions in 5 splice site cleavage specificity. The experimental approach used here is similar to that previously adopted to look at dominant effects of U5 snrna mutations on processing of Gl-+A 5 splice site mutant pre-mrnas. Haploid strains of yeast were made in which reporter pre-mrnas carrying splice site mutations are transcribed from expression cassettes transplaced at the chromosomal URA3 locus. RNA processing is monitored by primer extension or activity assays. All the strains used here have a chromosomal copy of the snr7 gene, which encodes wild-type U5 snrna. Dominant effectsof U5 snrna mutationson splicing of the reporter construct pre-mrnas are assayed by introducing an additional copy of the snr7 gene, encoding U5 with one or more base substitutions in the conserved loop motif, on a low copy number centromeric shuttle vector. Dead-End Lariat Processing and Altered Specificity of 5 Splice Site Cleavage To investigate the molecular basis for activation of cleavage at the splice site at +4 and processing of dead-end lariat intermediates, a number of variants of the original U5 snrna mutant GCGGUAUCC were made by oligonucleotide-directed mutagenesis (Kunkel, 1985). These U5 snrna loop sequencevariants were introduced on low copy number centromere-based plasmid vectors into a strain that produces Gl-A mutant pre-mrna from a transplaced expression cassette. Poly(A) RNA was isolated from these transformants and analyzed by primer extension using reverse transcriptase and an end-labeled oligonucleotide primer specific for exon 2 of the expressioncassette. Eightvariantswereexamined, togetherwith the wild-type snr7 gene, for their effects on processing of Gl-+A mutant pre-mrna (Figure 1, left panel). The two aberrant processing pathways can be cleanly separated. Loop sequences that have U mutated to A at position 6 in the motif activate cleavage at the aberrant 5 splice site at +4 (lanes 3, 4, 5, and 8). Loop sequences that have CU mutated to GG at positions 3-4 in the motif (lanes 2,3, and 6-10) activate authentic mrna production from dead-end lariats. Authentic mrna is barely visible in lane 8 but is readily detectable by 6-galactosidase assay using an analogous CYH2lacZ expression cassette (data not shown). Variable levels of authentic mrna are produced, since the efficiency of this pathway is affected by mutations elsewhere in the U5 snrna loop. The molecular events underlying this pathway are discussed below. Requirements for Cleavage of the 5 Splice Site at +4 To explore the requirements for cleavage at +4 in greater detail, a search was carried out for dominant suppressor U5 snrna mutationsthat activate cleavage at this5 splice site. A frame shift was introduced into the Gl+A IacZ fusion reporter construct by a single base deletion in exon 2 so that mrnas arising from splicing at the +4 cleavage site retain the natural reading frame and can therefore produce 6-galactosidase. A strain expressing this premrna was challenged by transformation with a library of mutant U5 snrna genes on a low copy number vector. In this library the identity of bases 2-8 in the loop motif was randomized by site-directed mutagenesis (Newman and Norman, 1991). Transformants that expressed 6-galactosidase were identified by color test using a chromogenic substrate, and the plasmid-borne snr7 gene was isolated and sequenced in each case. Seven different loop sequences were present. Some were isolated independently several times in this screen. A common feature of these
3 Interaction of U5 snrna with Exon Sequences 745 Figure 1. Cleavage at the Aberrant 5 Splice Site at +4 and Onward Processing of Dead-End vvvvvvvvvv vvvvvvvv~o Lariat Intermediates Can Be Separated Geneti- U-lVVVVV~4: 4aovvvva~ =J % caky xwaa4=3wa132 3waaa3wa33 33D www3wwwww 3www~wwwww Strains of yeast that express Gl-A 5 splice vwwvwwwwww owwowwwwww vvvvvvvvvv site mutant CYH2 pre-mrnas (ieft panel) or wwwwwwwwww wwwwwwwwww AG-AA 3 splice site mutant pre-mrnas (riaht panel) were transformed with a set of lowc~py number centromeric shuttle plasmids carrying snr7genesencodingu5snrnaswith theloop motif mutations indicated. Poly(A)+ RNA was isolated and analyzed by primer extension using an oligonucleotide primer specific for CYHP gene exon 2 sequences ([90/4043]; see Experimental Procedures). In each panel, lane 1 is the wild-type U5 gene (loop sequence GCCUU- UUAC). Lane 3 is the loop sequence GCGGU- AUCC (4 base changes from the wild type) that activates both cleavage at +4 and onward processing of lariat intermediates (see text), and the other lanes show variants of this mutant loop sequence. The expression cassette promoter has one major and two adjacent minor transcription start sites 10 nucleotides apart; only the most prominent (and most 53 of the Gl>A [5 splice site] AG>M [3 Splice sita] resulting mrnas is marked by the arrows at the left of the figure. The internal standard is derivedfromendogenouscyh2mrnas(multiple 5 ends). Pre-mRNAs are not shown. The central lanes contain end-labeled markers (psr322 cut with Mspl). mrna [+4] mrna l-121 w Internal standard b ivs.e2 lariat ) intermediate.:. s.!:: :i ii:li:. :y,.i :.I_; : 4 mrna [authentic] L GCCUQJUAC +* 3 INTRON CCUUGAUGUAU&ACUCUGCACUGGAGACACGA EXONl 5 4 * GCCUU&JAC 4 = GCCUUSUAC x311 Gl>A pre-mrna Figure 2. U-A Mutation at Position 6 in the U5 snrna Loop Motif Activates Cleavage at the Aberrant 5 Splice Site at +4 A strain of yeast that expresses Gl-A mutant pre-mrna from an expression cassette transplaced at the MA3 locus was transformed with low copy number Centromeric plasmids carrying various snr7 genes as indicated. Poly(A)+ RNA was isolated from these strains and analyzed by primer extension using a primer (190/4043); see Experimental Procedures) specific for CYHZ gene exon 2 sequences, The wild-type loop sequence (GCCUUUUAC) is in lane 1. Lanes 2-4 show the effects of single base changes at loop position 6. Lanes 5-l 1 are the loop sequences isolated from a loop mutation library by demanding cleavage at the aberrant 5 splice site at +4 (see text), Pre-mRNAs are not shown, The cartoon on the right shows the sequence surrounding the 5 splice site in CYH2 pre-mrna and (below the substrate sequence) the loop mutations and cleavage sites that give rise to +4 and -12 mrnas (see also Figure 3 and its legend).
4 Cdl 746 seven loop sequences is that they have an A at loop position 6, and all seven loops activate cleavage at +4 as expected (Figure 2). Indeed, while several loops have additional mutations, the single base change U6 to A in the loop is sufficient to activate this aberrant pathway (Figure 2, lane 3). Model for 5 Splice Site Cleavage In earlier work (Newman and Norman, 1991) it was found that the single base change U6 to C in the loop motif activated cleavage at an aberrant Ysplice site at -12 in exon 1 of the same Gl-A mutant reporter construct (Figure 2, lane 2). The radically different effects of the U6-A and U6-C mutations suggested a model (Figure 3) whose main feature is a direct base-pairing interaction between the U5 snrna loop and bases in the pre-mrna immediately upstream of the cleavage site. The loop sequence is known from biochemical data to be accessible in the snrnp so that it can base pair with other nucleic acids (Lamm et al., 1991). The base changes at position 6 in the snrna loop allow the formation of a new base pair with a nucleotide in the pre-mrna near the cleavage site that is activated. An important feature of the model is that the aberrant cleavage site in each case lies between the two nucleotides that are opposite positions 3 and 4 of the U5 snrna loop. Activation of a Minor 5 Cleavage Site at -11 The model outlined above is readily testable. Previous work revealed a third minor aberrant 5 cleavage site in G 1 -A pre-mrna. The wild-type splicing machinery cuts inefficiently at the sequence GlUCACGU 11 bases upstream of the authentic 5 splice site (Newman and Norman, 1991). This site is 5 to a U, so, as expected, molecules that have been cut here accumulate as dead-end lariat intermediates (Seraphin and Rosbash, 1990). If the model shown in Figure 3 is correct, the clear prediction is that cleavage at the aberrant site at -11 should be activated by a single base change U to C at position 5 in the l GCCUQJUAC I* 3 INTRON~CCUUGAUGUAU&~ACUCUGCACUGGAGACACGA~EXO~J~ 5 4 * 4 * GCCUUAUAC GCCUUQJAC 6 I+:1 c-121 Figure 3. Model for Activation of Aberrant 5 Cleavage Sites Involving Direct Interaction between the U5 snrna Loop Motif and Gl-A Mutant Pre-mRNA The substrate sequence is shown 5 +3 right to left, with the Gl-A 5 splice site mutation underlined. Below the pre-mrna sequence are shown the U5 snrna loop mutations U6-A and U6-C, which activate cleavage of the pre-mrna at the sites at +4 and -12. In each case a new base pair is formed by the U5 snrna loop mutation. The position of the aberrant cleavage is conserved with respect to the loop sequence; the cut always occurs opposite the phosphodiester bond between loop nucleotides 3 and 4. Above the pre-mrna sequence is a prediction: cleavage at the aberrant site at -11 should be activated by a U-C mutation at loop position 5 (see text). U5 snrna loop. The experiment is shown in Figure 4, where Gl-A mutant pre-mrna is challenged with all three single base changes at loop positions 5 and 6 in U5 snrna. A primer specific for intron sequences was used to assay the steady-state levels of lariat splicing intermediates. The striking result is that the single base change U to C at loop position 5 strongly and specifically activates cleavage at the -11 site, just as predicted from the model in Figure 3. Activation of Cleavage at the 5 Splice Site at -12 Further evidence in support of this model is shown in Figure 5. This experiment was designed to explore the requirements for cleavage at the aberrant site at -12 in exon 1. Nucleotides -14A and -15G in the substrate, predicted by the model to base pair with U5 snrna, were altered to give four new variant pre-mrnas. Each of these substrates still has the original Gl-A mutation. These premrnas were challenged with U5 snrna genes carrying each of the three single base mutations at loop positions 5 and 6. Each of the substrate mutations abolishes cleavage at -12, which, in the context of the wild-type exon 1 sequence, occurs efficiently in the presence of the mutation U to C at loop position 6 (Figure 5, lane 2 in each panel). Instead, cleavage at -12 is now activated (with variable efficiency) by other U5 snrna loop mutations. Thus, for example, cleavage of -14A+C is activated by mutation of U to G at loop position 5; -15G-U is activated by mutation of U to A at position 6; -15G-C is activated by mutation of U to G at position 6. It is interesting that for -15G-U, where cleavage at -12 is activated by mutation of U6 to A, the -12 site outcompetes the site at +4 that would normally be efficiently cut. These data indicate that nucleotides 5 and 6 in the U5 snrna loop motif can base pair with the nucleotides at positions 2 and 3 upstream of the cleavage site in the substrate. Taken together these results strongly support the model shown in Figure 3. The main features of the model are that the aberrant 5 splice site cleavage reactions are related to a direct RNA-RNA interaction between the loop sequence of U5 snrna and pre-mrna sequences just upstream of each cleavage site. Crucially, in each case the precise site of cleavage is af a conserved position with respect to the U5 snrna loop sequence: it is always between the two bases that lie opposite nucleotides 3 and 4 in the loop. U5 snrna and Processing of Dead-End Lariat Intermediates The interaction seen here between the U5 snrna loop and the 5 cleavage site has a striking parallel in an intramolecular recognition event that plays a major role in determining the 5 splice site in group II autocatalytic introns. In group II splicing, an intron sequence (EBSl) displayed on a single-stranded loop (the D3 loop) base pairs with a complementary target sequence (IBSl) just upstream of the 5 splice site (Jacquier and Michel, 1987; Michel and Jacquier, 1987; Figure 6A). It has recently been demonstrated that the EBSl-IBSl helix plays a critical role in determining the cleavage site; cleavage always occurs
5 Interaction of U5 snrna with Exon Sequences 747 L GCCUQJUAC t* 3 INTRON CCUUGAUGUAUgGAcUCUGCACLkGAGACACGA t * 4 l GCCUUAUAC GCCUUQJAC EXONl 5 Pre-mRNA a exon2 Branch IVS.E2 IVS.EP [-111 [AUTHENTIC] Gl>A pre-mrna Figure 4. Cutting at the Aberrant 5 Cleavage Site at -11 Is Activated by a U-C Mutation at Position 5 in the U5 snrna Loop A strain that expresses GI-A mutant pre-mrna was transformed with low copy number centromeric plasmids carrying snr7 genes encoding U5 snrna as follows: lane 1, wild-type U5 snrna; lanes 2-7, six single base changes at loop positions 5 and 6. Poly(A)+ RNA was isolated and the steady-state levels of dead-end lariat intermediates were assayed by primer extension using a primer ([90/4103]; see Experimental Procedures) specific for sequences near the 5 end of the CYH2 intron (see the cartoon at the left). Each lariat intermediate gives a doublet of primer extension products. at a position that is conserved with respect to the EBSl sequence (Jacquier and Jacquesson-Breuleux, 1991). The similarity between the behavior of U5 snrna and group II intron EBSl is clear, and provides an important clue to understanding the other type of aberrant processing pathway activated by specific U5 snrna mutations: the onward processing of dead-end lariat intermediates from Gl-+A pre-mrnas to give authentic mrna. The clue is that in many group II introns the D3 loop can also pair with bases at the 5 end of exon 2. This interaction, which is mediated by D3 loop sequences immediately 5 to EBSl, contributes to 3 splice site selection and activation in group II introns (Jacquier and Jacquesson- Breuleux, 1991; Figure 6A). The results of the experiment shown in Figure 1 (left panel) suggested that two adjacent base changes CU to GG at positions 3-4 of the U5 snrna loop motif were necessary to see onward processing of dead-end lariat intermediates from Gl+A pre-mrna. By analogy with the D3 loop-exon 2 interaction in group II introns alluded to above, a simple hypothesis would be that the U5 snrna loop is in a position to base pair with exon 2 and thus influence 3 splice site cleavage and lariat intermediate processing. The sequence of the 5 end of exon 2 in the pre-mrnas used in this work is AGlCCGG, where AG is the conserved dinucleotide at the 3 end of the intron. This information leads to the specific prediction that there might be a direct interaction between bases 3-4of the U5 snrna loop (GG in the loop mutation concerned) and the first two bases in exon 2 (which are CC) and that this interaction could somehow activate utilization of the authentic 3 splice site. One prediction of this hypothesis is that appropriate U5 loop mutations might have similar effects on processing of other dead-end intermediates: for example, those that arise from processing of pre-mrnas with 3 splice site mutations, where onward processing is blocked because of defective recognition or activation of the mutant 3 splice site. To explore this question, reporter constructs were made with a single base change AG to AA at the B terminal G of the intron. This mutation allows accurate and efficient 5 splice site cleavage but interferes strongly with 3 splice site cleavage, so that dead-end lariat intermediates accumulate (Newman and Norman, 1991; Figure 1, right panel). AG-AA mutant pre-mrna was then challenged with the same set of U5 snrna loop mutations that was
6 Cell 748 L GCCUGUUAC t*,, 3 INTRON CCUUGAUGUAU~GACUCUGCACUGGAGACACGA EXONl 5 4 * 4 * GCCUU&JAC GCCUUCUAC mrna [+4] bw*- mrna [-121 w Internal standard b Gl>A Gl>A Gl >A Gl A Gl>A - 14A>G 14A>C 15G>lJ 15G>C Figure 5. Base Pairing between the U5 snrna Loop and the Pre-mRNA Activates Cleavage at the Aberrant 5 Splice Site at -12 Single base changes A[-1 41 to G or C and G[-151 to U or C were introduced into a Gl -A mutant CYHP reporter construct. The A[-141 and G[-151 positions in the pre-mrna are indicated diagramatically at the top of the figure, Strains expressing each of these pre-mrnas ([A-E]) were then transformed with low copy number centromeric plasmids carrying snr7 (U5 snrna) genes with the loop sequences shown, Lane 1, wild-type GCCUUUUAC; lane 2. U6-C; lane 3, U6+A; lane 4, U6+G; lane 5, U5-C; lane 6, U5-A; lane 7, U5-G. Poly(A) RNA was isolated from each of these strains and assayed by primer extension using an oligonucleotide specific for exon 2 ([90/4043]; see Experimental Procedures). The mrna products derived from cleavage at the +4 and -12 sites are indicated. Only the major product from each site is marked (the promoter has major and minor transcription start sites 10 bases apart). Pre-mRNAs and lariat intermediates are not shown. surveyed previously for effects on Gl-A mutant premrna processing (Figure 1, compare right and left panels). The same subset of these U5 loop mutations produces authentic mrna from both Gl-A and AG-AA lariat intermediates. These loop sequences all have CU mutated to GG at positions 3-4. As with the Gi+A substrate, the level of authentic mrna produced is affected by mutations elsewhere in the loop motif. The molecular basis for this is not yet clear. It is obvious from this experiment, however, that activation of the aberrant Vcleavage site at +4 only occurs with the Gl-A mutant pre-mrna, consistent with earlier results (Newman and Norman, 1991). We have taken advantage of this production of authentic mrna from AC-AA 3 splice site mutant pre-mrna to screen U5 loop mutation libraries for loop mutations that activate dead-end lariat intermediate processing. These screens are free of potential complications arising from aberrant 5 splice site cleavages. Base Pairing between U5 snrna and Exon 2 One clear prediction of the model for U5 snrna loop-exon 2 interactions is that the loop sequence demanded for onward processing of dead-end intermediates will vary if the sequence at the 5 end of exon 2 is altered artificially. Therefore, a number of AG-AA mutant CYHPlacZ reporter constructs were made with changes in the first three nucleotides of exon 2. Libraries of U5 snrna genes with randomized loop sequences on low copy number shuttle vectors were introduced into strains carrying these new reporter constructs. Clones that tested positive for f3-galactosidase expression were colony purified, and the episomal snr7 gene was isolated and sequenced. Table 1 shows the results from some of these screening experiments using a library randomized at loop positions 2-4. For most of these substrates only a single loop sequence emerged from the screen that was capable of producing authentic IacZ fusion mrna, and for each substrate this single sequence was isolated repeatedly from the library.
7 Interaction of U5 snrna with Exon Sequences 749 A I UUU U : A G c u c UA CC UA UA GC GC UA,&AU GA C C A C A UA G UUG CG AU 5 3 Figure 6. Comparison of Predicted Secondary Structures of a Generalized Group II Self-Splicing lntron and the Major Stem-Loop in Yeast U5 snrna (A) Secondary structure of a generalized group II self-splicing intron (adapted from Michel et al. [1989]). Features of interest relevant to the results presented here are displayed on the D3 single-stranded loop sequence. These are EBSl (exon-binding site l), which is complementary to and base pairs with IBSl (intron-binding site l), in exon 1 adjacent to the 5 splice site. Also, in the D3 loop, upstream of EBSl, is a base that is complementary to the first base of exon 2 (both marked plus and indicated by a large arrowhead). The Sand 3 splice sites are indicated by small arrowheads marked 5 and 3. (B) Secondary structure of the major stem-loop in yeast U5 snrna (Patterson and Guthrie, 1987). The terminal loop sequence GCCUUUUAC is identical in all organisms examined so far. In this paper loop motif bases 5 and 6 are shown to be capable of base pairing with nucleotides upstream of the 5 splice site. Also, loop motif bases 4 and 3 can base pair with nucleotides 1 and 2 of exon 2. Furthermore, the loop sequence isolated varies according to the identity of the first two nucleotides of exon 2 in the reporter pre-mrna, so that the U5 loop and exon 2 sequences remain complementary (allowing a GU base pair in one case). These results clearly implicate nucleotides 3 and 4 of the U5 snrna loop motif in a base-pairing interaction with bases 2 and 1 of exon 2. The results do not address the possibility of base pairing involving position 2 of the U5 loop motif; this base always emerged as a C, as it is in the wild-type loop sequence. Screening of a library of U5 snrna genes that was randomized at loop motif residues 2-8 also gave loop sequences that could base pair with the first two bases in exon 2 (data not shown). The processing of some of these variant exon 2 premrnas has been examined at the RNA level, and two examples are shown in Figure 7. The results emphasize the specificity of the U5 loop-exon 2 interaction that activates onward processing of lariat intermediates. Substrates with different exon 2 sequences (AAICCGG and AAICGCG) were challenged with a series of U5 loop mutations, including those (Figure 7, lanes 6-9) isolated in screens using reporter constructs with these exon 2 sequences. There is a striking pattern of activity in this assay, in that authentic mrna is produced only when bases 3 and 4 in the U5 loop motif are complementary to bases 2 and 1 in exon 2. These results clearly indicate that the U5 snrna loop must at some point in the splicing pathway lie in close proximity with exon 2 sequences at the 3 splice site, so that these two sequences can interact directly. Discussion The results presented here suggest that the conserved single-stranded loop sequence in U5 snrna must be in close proximity to exon sequences at the 5 splice site prior to or during the first transesterification reaction of pre-mrna splicing. When normal definition of the 5 cleav age site is perturbed by a mutation in the conserved Gl
8 Cell 750 Table 1 U5 snrna Loop Sequences isolated by Screening Loop Mutation Libraries for Processing of AG-AA (3 splice Site) Mutant Pre-mRNAs Substrate Sequence ~ Intron-AA.CCGG-Exon 2 AA CGCG AA.UCGG AA.CGGG U5 snrna Loop Isolated 34 GCGGUUUAC GCCGUUUAC GCGGUUUAC GCGAUUUAC GCCGUUUAC Exon 2 mutationswere introduced into an AG-AA 3 splice site mutant IacZ fusion reporter construct as indicated (left column), and these cassettes were transplaced at the URA3 locus. A low copy number centromeric library of snr7 (U5 snrna) genes randomized at loop motif positions 2-4 was then Introduced Into these strains. Clones that were colony purified, and the centromeric plasmid was isolated by recovery in Escherrchia coli. The snr7 gene was sequenced, and the loop motif recovered is shown in the righthand column. Not all the variant exon 2 sequences that we have tested gave clear results, in some instances there was insufficient discrimination between posrtrves and background 0.galactosidase actrvity (data not shown) position in the intron, the U5 snrna loop sequence can play a decisive role in determining the 5 cleavage site. Definition of the cleavage site in these circumstances can be simply explained by base pairing between the U5 snrna loop and nucleotides upstream of the cleavage site. The position of cleavage is apparently conserved with respect to the U5 snrna loop, so that the location of USexon 1 base pairing determines the cleavage site. Specific US snrna loop mutations can also activate processing of dead-end lariat intermediates to mrna. Activation of this aberrant processing pathway correlates with formationof base pairs between U5snRNAand nucleotides at the 5 end of exon 2. This suggests that the U5 snpna loop sequence must also be in close proximity to the 3 cleavage site prior to or during the second transesterification reaction. Interactions between U5 snrna and Substrate Sequences The interaction between U5 snrna and exon 1 is reminiscent of a base-pairing scheme in group II autocatalytic mrna [authentic] b 1 mrna [authentic] Internal standard t Internal standard ivs.e2 lariat F intermediate ivs. E2 lariat intermediate AA. CCGG AA.CGCG Figure 7. Evidence for Interaction between the U5 snrna Loop Motif (Nucleotides 4 and 3) and Exon 2 Nucleotides 1 and 2 Strains that express 3 splice site mutant (AG-AA) CYHP pre-mrna were transformed with low copy number plasmids carrying various snr7 genes (encoding U5 snrna) as follows: lane 1, wild-type U5 snrna; lanes 2-9, loop motif mutations as indicated. Left panel. substrate 3 splice sate sequence is AAKCGG. Right panel: substrate 3 splice site sequence is AA/CGCG. For these substrates processing of dead-end lariat intermediates to mrna is activated by U5 snrna loop mutations that establrsh complementamy between U5 and the first 2 bases of exon 2 in the substrate. Pre-mRNAs are not shown. The manor species that migrates more slowly than the IVS.E2 primer extension product has not been characterized, but may indicate use of a cryptic branchpoint. The central lanes and the lane at the extreme left are markers made by end-labeling a psr322/mspl digest.
9 Interaction of U5 snrna with Exon Sequences 751 introns that makes an important contribution to 5 splice site recognition. In group II introns this base pairing involves two short complementary sequences that lie at the 3 end of exon 1 (the intron-binding site IBSl) and in a single-stranded loop in the intron (the exon-binding site EBSl). EBSl is displayed on the D3 stem-loop (Jacquier and Michel, 1987; Michel and Jacquier, 1987; Figure 6). Furthermore, the site of 5 splice site cleavage is fixed relative to the EBSl sequence (Jacquier and Jacquesson- Breuleux, 1991). There is a striking parallel here, then, between the U5 snrna loop and the group II self-splicing intron D3 loop in the way these elements interact with exon 1 sequences and contribute to 5 splice site specificity. This similarity between the U5 snrna loop and the group II intron D3 loop extends also to the potential for interaction of these loop sequences with nucleotides in exon 2 immediately downstream of the 3 splice site. In group II self-splicing introns there is frequently complementarity between D3 loop sequences 5 of EBSl and bases at the extreme 5 end of exon 2 (Michel et al., 1989; Figure 6). Base pairing between these sequences can affect 3 splice site selection and cleavage (Jacquier and Jacquesson-Breuleux, 1991). The results presented in this paper show that base pairs can form between nucleotides in the U5 snrna loop and the first two bases in exon 2 in the substrate. This interaction in some way activates 3 splice site cleavage so that dead-end lariat intermediates are processed onward to give authentic mrna. In contrast to the situation in group II introns, however, where the interactions between the D3 loop sequence and exon sequences put both 5 and 3 cleavage sites opposite the same position on the D3 loop, in the U&mediated aberrant processing pathways described here the 5 and 3 cleavages appear to occur one base out of register with respect to the U5 loop sequence. That is, 5 splice site cleavage always occurs between the two nucleotides opposite loop motif positions 3 and 4, whereas 3 cleavage is apparently between the two bases opposite loop positions4 and 5. The significance of this is not currently clear. In group II autocatalytic splicing one of the functions of EBSl-IBSl helix formation is thought to be tethering of the exon 1 intermediate prior to 3 splice site cleavage (Michel et al., 1989). Perhaps in the spliceosome this is achieved by some other interaction, and the relative positions of the exon 1 3 Of-i and U5 loop sequence can or must alter before 3 splice site cleavage occurs. Currently, the identity of factors involved in the recognition of the conserved intron sequences at the 3 end of introns remains uncertain. Specification of the 3 splice site apparently focuses on uridine-rich sequences and conserved intron nucleotides immediately upstream of the 3 cleavage site (Patterson and Guthrie, 1991). In the experiments reported here, interactions between U5 snrna and exon 2 can profoundly affect the rate of the 3 splice site transesterification for dead-end lariat intermediates. There has been no sign of any activation of cryptic or aberrant 3 cleavage sites, so at present it remains uncertain whether U5 snrna ordinarily plays any part in specifying the actual site of 3 splice site cleavage. Functions of U5 snrna in Pre-mRNA Splicing To what extent do the aberrant processing pathways activated by U5 loop mutations reflect the normal course of events? In other words, do the usual functions of U5 snrna involve direct interactions with exon sequences at 5 and 3 splice sites in wild-type pre-mrna? Certainly this is the most straightforward model for U5 snrnp function, although we cannot exclude the formal possibility that the U5 snrna mutations studied here have elicited new phenotypes unrelated to the normal function of U5 snrnp. Ultimately, biochemical experiments may be necessary to settle this issue, but there is compelling circumstantial evidence that the functions in which U5 is implicated by the experiments reported here fit neatly into the normal pathway of events. It is well-established that Ul snrna interacts with 5 splice sites by base pairing, and while this is an essential early event in spliceosome assembly, it is certainly not the only event that determines the 5 splice site. For example, mutation of the highly conserved G5 nucleotide in yeast introns causes the activation of aberrant cleavage sites nearby (Parker and Guthrie, 1985; Jacquier et al., 1985) and these aberrant events are not suppressed by the appropriatecompensating base changes in Ul snrna(siliciano and Guthrie, 1988; Seraphin et al., 1988). Also, mutation of the first base of introns (Gl) allows cleavage at the authentic 5 splice site to give exon 1 and lariat intronexon 2 intermediates, but further processing is blocked (Newman et al., 1985; Parker and Guthrie, 1985; Aebi et al., 1986, 1987). Here again, this defect is not rescued by the compensating base change in Ul snrna (Siliciano and Guthrie, 1988). These findings suggest that 5 splice site sequences may be under surveillance by other factors after their initial recognition by Ul. In any case the aberrant processing events activated by U5 mutations show a conventional dependence on Ul snrna. Substrates with two adjacent base changes at the 5 splice site (GU+AC) accumulate only pre-mrna, with virtually no 5 cleavages even in the presence of appropriate U5 snrna loop mutations. US-mediated aberrant cleavages (as well as cleavage at the authentic site) can be reactivated by introduction of an appropriate suppressor Ul snrna gene carrying two compensating base changes (data not shown). This implies that both aberrant and authentic cleavages are dependent on interaction of Ul snrna at the normal 5 splice site at some stage in the splicing pathway. Another point arguing that U8mediated aberrant cleavages are closely related to the normal pathway is that the subsequent onward processing of intermediates is subject to the usual constraints with respect to the identity of the base downstream of the 5 cleavage site. This is the base that becomes linked 2,5 at the intron branchpoint. For the cleavages at -12 and +4, which are 5 of a G residue, the lariat intermediates are efficiently processed onward to mrnas. In contrast, the cleavage at -11 is 5 of a U residue and gives rise to dead-end lariat intermediates. We speculate that correct positioning of U5 at the 5 splice site could ordinarily be achieved by some interaction (which may or may not directly involve Ul) that is critically
10 Cell 752 dependent on intron nucleotide Gl. In pre-mrnas with Gl -+A or C mutations, precise positioning of U5 becomes uncoupled from the authentic 5 splice site and can be directed by the U5 loop: pre-mrna base pairing to nearby aberrant cleavage sites. Ui and U5 could be present simultaneously at the 5 splice site, or, alternatively, Ul may leave before U.5 appears. Evolutionary Considerations Given that group II self splicing and spliceosomal splicing share the same reaction mechanism, there has been speculation that these two processes are evolutionarily related (Cech, 1986; Michel et al., 1989; Jacquier, 1990). With the exception of the U2 snrna:branchpoint and group II domain VI helices (where in each case the A that attacks the 5 splice site is thought to be bulged out of the helix), it has hitherto been difficult to see any correspondence between functional domains in group II introns and their hypothetical spliceosomal counterparts. In the case of the group II D3 stem-loop and U5 snrna, however, there are clear structural and functional similarities. Could lj5 have been derived from the D3 stem-loop of a group II-like ancestor intron? Clearly the rigid exon sequence specificity of the EBSl element of a group II-like intron D3 loop would need to be lost or suppressed if D3 loop functions were transferred to a separate snrnp particle capable of acting in trans on any intron in any premrna, regardless of the upstream exon sequence. Possibly, in this hypothetical transition from an autocatalytic group II-like ancestor to a spliceosome in which the domains of the self-splicing intron have been transformed into discrete trans-acting factors it may have been beneficial or essential to preserve certain aspects of the original reaction scheme. One such aspect could be the formation of a base-paired helix at the 5 cleavage site, which is also common to group I autocatalytic introns (reviewed by Cech, 1990). The central nine bases of the U5 snrna loop are GCCU- UUUAC in all organisms analyzed so far (Guthrie and Patterson, 1988). If the U5 snrna loop does not normally display significant exon sequence specificity-this having been supplanted by recognition of conserved intron signals by factors such as Ul -why then is this sequence so rigidly COrtSSNSd? One partial explanation is that this loop motif could be suitably promiscuous in its base-pairing behavior. It has recently been shown that U can base pair with G or C in addition to its usual Watson-Crick partner A. An RNA helix incorporating these unusual base pairs proves to be highly regular and undistorted (Holbrook et al., 1991). Perhaps the U-rich U5 snrna loop sequence GCCUUUUAC is compatible with direct interaction between U5 and nucleotides upstream of 5 splice sites, irrespective of the exon sequence. Protein splicing factors could of course play a role in maintaining or modulating this indiscriminate behavior. Apparently, US-mediated sequence-specific effects on 5 splice site cleavage only become manifest when mutations in the first base of the intron cause a severe defect in the rate and fidelity of an earlier 5 splice site recognition step. This earlier event would normally play an instructive role in determining the location of the US-exon 1 interaction. In any case, while it looks as if there is still much to discover about splice site recognition, the results presented here suggest that the U5 snrna loop sequence is intimately involved in the transesterification reactions at both 5 and 3 splice sites. The underlying similarities between the group II D3 loop and U5 snrna that have become apparent from this set of experiments beg the question: Are there other instances among the spliceosomal snrnas of structures or functions that betray an autocatalytic ancestry? Splice site selection and reactivity in group II introns are determined by multiple RNA-based interactions. There may be further clues here to the functions of the spliceosomal snrnas. Experimental Procedures DNA Manipulations Mutagenesis of the yeast U5 snrna gene (snr7) was via standard methods (Kunkel, 1985), using the following oligonucleotide primers U5 snrna loop GCCUUUUAC: Loop 2-S-N: [89/1997], dcggatggttctggnnnnnnncaagaac- CATGTT. Loop 2-4-N: [91/1573], datggttctggtaaannncaagaaccatgt. Loop CS-UGA: [91/1574], datggttctggtaaaahgcaagaacca- TGT. Loop M-C: [91/582], datggttctggtaagaggcaagaaccatg. Loop US-GA: [91/1334], datggttctggtaayaggcaagmccatg. Loop GCGGUAUCC single base variants were made using the following oligonucleotides. [90/2654], dgatggttctgggatacggcaagaacca. [90/2655], dgatggttctgggataacgcaagaacca. [90/2656], dgatggltctgggaaaccgcaagaacca. [90/2660], dgatggtlctgggacaccgcaagaacca. [90/2661], dgatggttctggtataccgcaagaacca. The primer for snr7 gene sequencing was ( , daacgccctc- CTTACTCATTG. The basic structure of the CYHPlacZ and ADHCYH2 expression cassettes has been described (Newman and Norman, 1991). CYH2lacZ expression cassettes embedded in URA3 in Bluescript KS+ (positive strand packaged) were mutagenized with the following oligonucleotides. Exon 2 frameshift i-l]: [90/4029], dtctacccttaccgctgta- CAAAAA. 3 Splice site AGAAAIAC: [90/906], dccttaccggktgtacaaaa Exon 2 Cl-T: [91/1575], dctacccttaccgattgtacaaaaaa. Exon 2 Cl-G: [91/1576], dctacccttaccgcttgtacaaaaaa. Exon 2 Cl-A: , dctacccttaccgtttgtacaaaaaa. Exon 2 CCGG-CGCG: [91/2103], dctacccttacgcgttgtacaa- AAAA. Exon 2 CCGG-CACG: [91/2104], dctacccttacgtgttgtacaa- AAAA. Exon 2 C2-AGT: [91/2660], dctacccttacchgttgtacaaaaaa. ADHCYHP expression cassettes (AG-AA) embedded in URA3 in Bluescript KS+ (negative strand packaged) were mutagenized wtth the following oligonucleotides. Exon 2 CP-AGT: [91/2754], dttttttgtacaacdggtaagggta- GAAT. Exon 2 CCGG-CGCG: [91/2406], dttttttgtacaacgcgtaagg- GTAGAAT. Exon2CCGG-CACG: [91/2407],dTTTTTTGTACAACACGTAAGGG- TAGAAT. ADHCYH2 expression cassettes (Gl-A) embedded in URA3 in BS (positive strand packaged) were mutagenized with the following oligonucleotides.
11 Interaction of U5 snrna with Exon Sequences 753 Exon 1 A[-14]-CG: [91/1132], dtgagacgtgaccsctgtgcttt- CTAG. Exon 1 G[-15]-TC: [91 I1 1331, dtgagacgtgacctrtgtgcttt- CTAG. Ambiguity code is as follows: R = AG; S = CG; D = AGT; H = ACT; K = GT; Y = CT; N = TCGA. Yeast Manipulations Transplacements, 8-galactosidase assays, and RNA preparations were as previously described (Newman and Norman, 1991). RNA Analysis All RNA analysis was performed using poly(a) RNA isolated by chromatography on oligo(dt)-cellulose. Primers for reverse transcription were [90/4043], dggggtgctttctgtgcttaccgattct (for exon 2 in ADHCYHZ), and [90/4103], dccatgatatacacacgacatatt- GGTTGCAC (intron primer for mapping 5 cleavage sites in CYH2 intron-exon 2 lariat intermediates). Acknowledgments We are grateful to Wes Sundquist, Kiyoshi Nagai, Jon Karn, Ellen Gottlieb, Mair Churchill, Nick Dibb, Victoria Smith, and Mike Gait for constructive criticism of the manuscript. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 USC Section 1734 solely to indicate this fact. Received November 8, 1991; revised December 3, 1991 References Aebi, M., Hornig, H., Padgett, R. A., Reiser, J., and Weissmann, C. (1986). Sequence requirements for splicing of higher eukaryotic nuclear pre-mrna. Cell 47, Aebi, M., Hornig. H., and Weissmann, C. (1987). 5 Cleavage site in eukaryotic pre-mrna splicing is determined by the overall 5 splice region, not by the conserved 5 GU. Cell 50, Anderson, G. J., Bach, M.. Luhrmann, R., and Beggs, J. D. (1969). Conservation between yeast and man of a protein associated with U5 small nuclear ribonucleoprotein. Nature 342, , Bach, M., Winkelmann, G., and Luhrmann, R. (1989). 20s small nuclear ribonucleoprotein U5 shows a surprisingly complex protein composition. Proc. Natl. Acad. Sci. USA 86, Barabino, S. M. L., Sproat, B. S., Ryder, U.. Blencowe, B. J., and Lamond, A. I. (1989). Mapping U2 snrnp-pre-mrna interactions using biotinylated oligonucleotides made of 2 -OMe RNA. EMBO J. 8, Barabino, S. M. L., Blencowe, B. J., Ryder, U., Sproat, B. S., and Lamond, A. I. (1990). Targeted snrnp depletion reveals an additional role for mammalian Ul snrnp in spliceosome assembly. Cell 63, Black, D. L., and Pinto, A. L. (1989). U5 small nuclear ribonucleoprotein: RNA structure analysis and ATP-dependent interaction with U4/ U6. Mol. Cell. Biol. 9, Cech, T. R. (1986). The generality of self-splicing RNA: relationship to nuclear mrna splicing. Cell 44, Cech, T. R. (1990). Self-splicing of group I introns. Annu. Rev. Biothem. 59, Chabot, B., Black, D. L., LeMaster, D. M., and Steitz, J. A. (1985). The 3 splice site of pre-messenger RNA is recognised by a small nuclear ribonucleoprotein. Science 230, Cheng, S. C., and Abelson, J. (1987). Spliceosome assembly in yeast. Genes Dev Dalbadie-McFarland, G.. and Abelson, J. (1990). PRPS: a helicase-like protein required for mrna splicing in yeast. Proc. Natl. Acad. Sci. USA 87, Garcia-Blanco, M. A., Anderson, G. J., Beggs, J. D., and Sharp, P. A. (1990). A mammalian protein of 220 kda binds pre-mrnas in the spliceosome: a potential homologue of the yeast PRP8 protein. Proc. Natl. Acad. Sci. USA 87, Gerke, V., and Steitz, J. A. (1986). A protein associated with small nuclear ribonucleoprotein particles recognizes the 3 splice site of premessenger RNA. Cell 47, Guthrie, C. (1991). Messenger RNA splicing in yeast: clues to why the spliceosome is a ribonucleoprotein. Science 253, 157-l 63. Guthrie, C., and Patterson, 6. (1988). Spliceosomal snrnas. Annu. Rev. Genet. 22, Holbrook, S. R., Cheong, C., Tinoco, I., and Kim, S. (1991). Crystal structure of an RNA double helix incorporating a track of non-watson- Crick base pairs. Nature 353, Jacquier, A. (1990). Self-splicing groupll and nuclear pre-mrna introns: how similar are they? Trends Biochem. Sci. 75, Jacquier, A., and Jacquesson-Breuleux, N. (1991). Splice site selection and the role of the lariat in agroup II intron. J. Mol. Biol. 219, Jacquier, A., and Michel, F. (1987). Multiple exon-binding sites in class II self-splicing introns. Cell Jacquier, A., and Michel, F. (1990). Base-pairing interactions involving the 5 and 3 -terminal nucleotides of group II self-splicing introns. J. Mol. Biol. 273, Jacquier, A., Rodriguez, J. R., and Rosbash, M. (1985). Aquantitative analysis of the effects of 5 junction and TACTAAC box mutants and mutant combinations on yeast mrna splicing. Cell 43, Jamieson, D. J., Rahe, B., Pringle, J., and Beggs, J. D. (1991). A suppressor of a yeast splicing mutation (prp8-1) encodes a putative ATP-dependent RNA helicase. Nature 349, Konarska, M. M., and Sharp, P. A. (1987). Interactions between small nuclear ribonucleoprotein particles in formation of spliceosomes. Cell 49, Kunkel, T. A. (1985). Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc. Natl. Acad. Sci. USA82, Lamm, G. M.. Blencowe, B. J., Sproat, B. S., Iribarren, A. M., Ryder, U., and Lamond,A. I. (1991). AntisenseprobescontainingP-aminoadenosine allow efficient depletion of U5 snrnp from HeLa splicing extracts Nucl. Acids Res. 79, Lamond, A. I. (1991). Nuclear RNA processing. Curr. Opin. Cell Biol. 3, Lamond, A. I., Sproat, B., Ryder, U., and Hamm, J. (1989). Probing the structure and function of U2 snrnp with antisense oligonucleotides made of 2 -OMe RNA. Cell 58, Lossky, M., Anderson, G. J.. Jackson, S. P., and Beggs, J. (1987). Identification of a yeast snrnp protein and detection of snrnp-snrnp interactions. Cell 57, Michel, F., and Jacquier, A. (1987). Long-range intron-exon and intronintron pairings involved in self-splicing of class II catalytic introns. Cold Spring Harbor Symp. &ant. Biol. 52, Michel, F., Umesono, K., and Ozeki, H. (1989). Comparative and functional anatomy of group II catalytic introns. Gene 82, Newman, A., and Norman, C. (1991). Mutations in yeast U5 snrna alter the specificity of 5 splice-site cleavage. Cell 65, II Newman, A. J., Lin. R.. Cheng, S., and Abelson, J. (1985). Molecular consequences of specific intron mutations on yeast mrna splicing in vivo and in vitro. Cell 42, Parker, R., and Guthrie, C. (1985). A point mutation in the conserved hexanucleotide at a yeast 5 splice junction uncouples recognition, cleavage, and ligation. Cell 47, Parker, R., Siliciano, P. G., and Guthrie, C. (1987). Recognition of the TACTAAC box during mrna splicing in yeast involves base pairing to the UP-like snrna. Cell 49, Patterson, B., and Guthrie, C. (1987). An essential yeast snrna with a U&like domain is required for splicing in viva. Cell 49, Patterson, B., and Guthrie, C. (1991). A U-rich tract enhances usage of an alternative 3 splice site in yeast. Cell 64, Peebles, C. L., Perlman, C. S., Mecklenburg, K. L., Petrillo, M. L.,
1. Identify and characterize interesting phenomena! 2. Characterization should stimulate some questions/models! 3. Combine biochemistry and genetics
 1. Identify and characterize interesting phenomena! 2. Characterization should stimulate some questions/models! 3. Combine biochemistry and genetics to gain mechanistic insight! 4. Return to step 2, as
1. Identify and characterize interesting phenomena! 2. Characterization should stimulate some questions/models! 3. Combine biochemistry and genetics to gain mechanistic insight! 4. Return to step 2, as
Figure mouse globin mrna PRECURSOR RNA hybridized to cloned gene (genomic). mouse globin MATURE mrna hybridized to cloned gene (genomic).
 Splicing Figure 14.3 mouse globin mrna PRECURSOR RNA hybridized to cloned gene (genomic). mouse globin MATURE mrna hybridized to cloned gene (genomic). mrna Splicing rrna and trna are also sometimes spliced;
Splicing Figure 14.3 mouse globin mrna PRECURSOR RNA hybridized to cloned gene (genomic). mouse globin MATURE mrna hybridized to cloned gene (genomic). mrna Splicing rrna and trna are also sometimes spliced;
RECAP (1)! In eukaryotes, large primary transcripts are processed to smaller, mature mrnas.! What was first evidence for this precursorproduct
 RECAP (1) In eukaryotes, large primary transcripts are processed to smaller, mature mrnas. What was first evidence for this precursorproduct relationship? DNA Observation: Nuclear RNA pool consists of
RECAP (1) In eukaryotes, large primary transcripts are processed to smaller, mature mrnas. What was first evidence for this precursorproduct relationship? DNA Observation: Nuclear RNA pool consists of
the basis of the disease is diverse (see ref. 16 and references within). One class of thalassemic lesions is single-base substitutions
 Proc. Natl. Acad. Sci. USA Vol. 87, pp. 6253-6257, August 1990 Biochemistry Mechanism for cryptic splice site activation during pre-mrna splicing (thalassemia/ul small nuclear ribonucleoprotein particle/5'
Proc. Natl. Acad. Sci. USA Vol. 87, pp. 6253-6257, August 1990 Biochemistry Mechanism for cryptic splice site activation during pre-mrna splicing (thalassemia/ul small nuclear ribonucleoprotein particle/5'
Spliceosome Pathway. is required for a stable interaction between U2 snrnp and
 MOLECULAR AND CELLULAR BIOLOGY, Sept. 1988, p. 3755-3760 Vol. 8, No. 9 0270-7306/88/093755-06$02.00/0 Copyright 1988, American Society for Microbiology Early Commitment of Yeast Pre-mRNA to the Spliceosome
MOLECULAR AND CELLULAR BIOLOGY, Sept. 1988, p. 3755-3760 Vol. 8, No. 9 0270-7306/88/093755-06$02.00/0 Copyright 1988, American Society for Microbiology Early Commitment of Yeast Pre-mRNA to the Spliceosome
Processing of RNA II Biochemistry 302. February 13, 2006
 Processing of RNA II Biochemistry 302 February 13, 2006 Precursor mrna: introns and exons Intron: Transcribed RNA sequence removed from precursor RNA during the process of maturation (for class II genes:
Processing of RNA II Biochemistry 302 February 13, 2006 Precursor mrna: introns and exons Intron: Transcribed RNA sequence removed from precursor RNA during the process of maturation (for class II genes:
Processing of RNA II Biochemistry 302. February 14, 2005 Bob Kelm
 Processing of RNA II Biochemistry 302 February 14, 2005 Bob Kelm What s an intron? Transcribed sequence removed during the process of mrna maturation (non proteincoding sequence) Discovered by P. Sharp
Processing of RNA II Biochemistry 302 February 14, 2005 Bob Kelm What s an intron? Transcribed sequence removed during the process of mrna maturation (non proteincoding sequence) Discovered by P. Sharp
Processing of RNA II Biochemistry 302. February 18, 2004 Bob Kelm
 Processing of RNA II Biochemistry 302 February 18, 2004 Bob Kelm What s an intron? Transcribed sequence removed during the process of mrna maturation Discovered by P. Sharp and R. Roberts in late 1970s
Processing of RNA II Biochemistry 302 February 18, 2004 Bob Kelm What s an intron? Transcribed sequence removed during the process of mrna maturation Discovered by P. Sharp and R. Roberts in late 1970s
UNDERSTANDING THE ROLE OF ATP HYDROLYSIS IN THE SPLICEOSOME
 UNDERSTANDING THE ROLE OF ATP HYDROLYSIS IN THE SPLICEOSOME RNA Splicing Lecture 2, Biological Regulatory Mechanisms, H. Madhani Dept. of Biochemistry and Biophysics MAJOR MESSAGES Eight essential ATPases
UNDERSTANDING THE ROLE OF ATP HYDROLYSIS IN THE SPLICEOSOME RNA Splicing Lecture 2, Biological Regulatory Mechanisms, H. Madhani Dept. of Biochemistry and Biophysics MAJOR MESSAGES Eight essential ATPases
RECAP (1)! In eukaryotes, large primary transcripts are processed to smaller, mature mrnas.! What was first evidence for this precursorproduct
 RECAP (1) In eukaryotes, large primary transcripts are processed to smaller, mature mrnas. What was first evidence for this precursorproduct relationship? DNA Observation: Nuclear RNA pool consists of
RECAP (1) In eukaryotes, large primary transcripts are processed to smaller, mature mrnas. What was first evidence for this precursorproduct relationship? DNA Observation: Nuclear RNA pool consists of
Evidence that U5 snrnp recognizes the 3 splice site for catalytic step II in mammals
 The EMBO Journal Vol.16 No.15 pp.4746 4759, 1997 Evidence that U5 snrnp recognizes the 3 splice site for catalytic step II in mammals Maria Dolores Chiara 1, Leon Palandjian, sites in pre-mrna very near
The EMBO Journal Vol.16 No.15 pp.4746 4759, 1997 Evidence that U5 snrnp recognizes the 3 splice site for catalytic step II in mammals Maria Dolores Chiara 1, Leon Palandjian, sites in pre-mrna very near
particles at the 3' splice site
 Proc. Nati. Acad. Sci. USA Vol. 89, pp. 1725-1729, March 1992 Biochemistry The 35-kDa mammalian splicing factor SC35 mediates specific interactions between Ul and U2 small nuclear ribonucleoprotein particles
Proc. Nati. Acad. Sci. USA Vol. 89, pp. 1725-1729, March 1992 Biochemistry The 35-kDa mammalian splicing factor SC35 mediates specific interactions between Ul and U2 small nuclear ribonucleoprotein particles
U2 toggles iteratively between the stem IIa and stem IIc conformations to promote pre-mrna splicing
 U2 toggles iteratively between the stem IIa and stem IIc conformations to promote pre-mrna splicing Angela K. Hilliker, 1 Melissa A. Mefford, 2 and Jonathan P. Staley 1,3 1 Department of Molecular Genetics
U2 toggles iteratively between the stem IIa and stem IIc conformations to promote pre-mrna splicing Angela K. Hilliker, 1 Melissa A. Mefford, 2 and Jonathan P. Staley 1,3 1 Department of Molecular Genetics
The conserved central domain of yeast U6 snrna: Importance of U2-U6 helix I a in spliceosome assembly
 RNA (2002), 8:997 1010+ Cambridge University Press+ Printed in the USA+ Copyright 2002 RNA Society+ DOI: 10+1017+S1355838202025013 The conserved central domain of yeast U6 snrna: Importance of U2-U6 helix
RNA (2002), 8:997 1010+ Cambridge University Press+ Printed in the USA+ Copyright 2002 RNA Society+ DOI: 10+1017+S1355838202025013 The conserved central domain of yeast U6 snrna: Importance of U2-U6 helix
REGULATED SPLICING AND THE UNSOLVED MYSTERY OF SPLICEOSOME MUTATIONS IN CANCER
 REGULATED SPLICING AND THE UNSOLVED MYSTERY OF SPLICEOSOME MUTATIONS IN CANCER RNA Splicing Lecture 3, Biological Regulatory Mechanisms, H. Madhani Dept. of Biochemistry and Biophysics MAJOR MESSAGES Splice
REGULATED SPLICING AND THE UNSOLVED MYSTERY OF SPLICEOSOME MUTATIONS IN CANCER RNA Splicing Lecture 3, Biological Regulatory Mechanisms, H. Madhani Dept. of Biochemistry and Biophysics MAJOR MESSAGES Splice
The nuclear pre-mrna introns of eukaryotes are removed by
 U4 small nuclear RNA can function in both the major and minor spliceosomes Girish C. Shukla and Richard A. Padgett* Department of Molecular Biology, Lerner Research Institute, Cleveland Clinic Foundation,
U4 small nuclear RNA can function in both the major and minor spliceosomes Girish C. Shukla and Richard A. Padgett* Department of Molecular Biology, Lerner Research Institute, Cleveland Clinic Foundation,
Splicing function of mammalian U6 small nuclear RNA: Conserved
 Proc. Nati. Acad. Sci. SA Vol. 91, pp. 903-907, February 1994 Biochemistry Splicing function of mammalian 6 small nuclear RNA: Conserved positions in central domain and helix I are essential during the
Proc. Nati. Acad. Sci. SA Vol. 91, pp. 903-907, February 1994 Biochemistry Splicing function of mammalian 6 small nuclear RNA: Conserved positions in central domain and helix I are essential during the
Mechanism of splicing
 Outline of Splicing Mechanism of splicing Em visualization of precursor-spliced mrna in an R loop Kinetics of in vitro splicing Analysis of the splice lariat Lariat Branch Site Splice site sequence requirements
Outline of Splicing Mechanism of splicing Em visualization of precursor-spliced mrna in an R loop Kinetics of in vitro splicing Analysis of the splice lariat Lariat Branch Site Splice site sequence requirements
Alan Weiner BIOCHEM 530 Friday, MEB 248 October 23, 2015 RNA structure, the ribosome, structure-based drug design
 Alan Weiner BIOCHEM 530 Friday, MEB 248 October 23, 2015 RNA structure, the ribosome, structure-based drug design Crick's "Central Dogma"? DNA makes RNA makes protein Crick's "Central Dogma"? DNA makes
Alan Weiner BIOCHEM 530 Friday, MEB 248 October 23, 2015 RNA structure, the ribosome, structure-based drug design Crick's "Central Dogma"? DNA makes RNA makes protein Crick's "Central Dogma"? DNA makes
Received 26 January 1996/Returned for modification 28 February 1996/Accepted 15 March 1996
 MOLECULAR AND CELLULAR BIOLOGY, June 1996, p. 3012 3022 Vol. 16, No. 6 0270-7306/96/$04.00 0 Copyright 1996, American Society for Microbiology Base Pairing at the 5 Splice Site with U1 Small Nuclear RNA
MOLECULAR AND CELLULAR BIOLOGY, June 1996, p. 3012 3022 Vol. 16, No. 6 0270-7306/96/$04.00 0 Copyright 1996, American Society for Microbiology Base Pairing at the 5 Splice Site with U1 Small Nuclear RNA
L I F E S C I E N C E S
 1a L I F E S C I E N C E S 5 -UUA AUA UUC GAA AGC UGC AUC GAA AAC UGU GAA UCA-3 5 -TTA ATA TTC GAA AGC TGC ATC GAA AAC TGT GAA TCA-3 3 -AAT TAT AAG CTT TCG ACG TAG CTT TTG ACA CTT AGT-5 NOVEMBER 2, 2006
1a L I F E S C I E N C E S 5 -UUA AUA UUC GAA AGC UGC AUC GAA AAC UGU GAA UCA-3 5 -TTA ATA TTC GAA AGC TGC ATC GAA AAC TGT GAA TCA-3 3 -AAT TAT AAG CTT TCG ACG TAG CTT TTG ACA CTT AGT-5 NOVEMBER 2, 2006
GENOME-WIDE COMPUTATIONAL ANALYSIS OF SMALL NUCLEAR RNA GENES OF ORYZA SATIVA (INDICA AND JAPONICA)
 GENOME-WIDE COMPUTATIONAL ANALYSIS OF SMALL NUCLEAR RNA GENES OF ORYZA SATIVA (INDICA AND JAPONICA) M.SHASHIKANTH, A.SNEHALATHARANI, SK. MUBARAK AND K.ULAGANATHAN Center for Plant Molecular Biology, Osmania
GENOME-WIDE COMPUTATIONAL ANALYSIS OF SMALL NUCLEAR RNA GENES OF ORYZA SATIVA (INDICA AND JAPONICA) M.SHASHIKANTH, A.SNEHALATHARANI, SK. MUBARAK AND K.ULAGANATHAN Center for Plant Molecular Biology, Osmania
RNA Processing in Eukaryotes *
 OpenStax-CNX module: m44532 1 RNA Processing in Eukaryotes * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you
OpenStax-CNX module: m44532 1 RNA Processing in Eukaryotes * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you
Eukaryotic mrna is covalently processed in three ways prior to export from the nucleus:
 RNA Processing Eukaryotic mrna is covalently processed in three ways prior to export from the nucleus: Transcripts are capped at their 5 end with a methylated guanosine nucleotide. Introns are removed
RNA Processing Eukaryotic mrna is covalently processed in three ways prior to export from the nucleus: Transcripts are capped at their 5 end with a methylated guanosine nucleotide. Introns are removed
Pre-mRNA has introns The splicing complex recognizes semiconserved sequences
 Adding a 5 cap Lecture 4 mrna splicing and protein synthesis Another day in the life of a gene. Pre-mRNA has introns The splicing complex recognizes semiconserved sequences Introns are removed by a process
Adding a 5 cap Lecture 4 mrna splicing and protein synthesis Another day in the life of a gene. Pre-mRNA has introns The splicing complex recognizes semiconserved sequences Introns are removed by a process
Association of U6 snrna with the 5'-splice site region of pre-mrna in the spliceosome
 Association of U6 snrna with the 5'-splice site region of pre-mrna in the spliceosome Hitoshi Sawa and Yoshiro Shimura^ Department of Biophysics, Faculty of Science, Kyoto University, Kyoto 606, Japan
Association of U6 snrna with the 5'-splice site region of pre-mrna in the spliceosome Hitoshi Sawa and Yoshiro Shimura^ Department of Biophysics, Faculty of Science, Kyoto University, Kyoto 606, Japan
The organization of 3' splice-site sequences in mammalian introns
 The organization of 3' splice-site sequences in mammalian introns Robin Reed Department of Cellular and Molecular Physiology, Harvard Medical School, Boston, Massachusetts 02115 USA A model pre-mrna substrate
The organization of 3' splice-site sequences in mammalian introns Robin Reed Department of Cellular and Molecular Physiology, Harvard Medical School, Boston, Massachusetts 02115 USA A model pre-mrna substrate
The U1 snrnp Base Pairs with the 5 Splice Site within a Penta-snRNP Complex
 MOLECULAR AND CELLULAR BIOLOGY, May 2003, p. 3442 3455 Vol. 23, No. 10 0270-7306/03/$08.00 0 DOI: 10.1128/MCB.23.10.3442 3455.2003 Copyright 2003, American Society for Microbiology. All Rights Reserved.
MOLECULAR AND CELLULAR BIOLOGY, May 2003, p. 3442 3455 Vol. 23, No. 10 0270-7306/03/$08.00 0 DOI: 10.1128/MCB.23.10.3442 3455.2003 Copyright 2003, American Society for Microbiology. All Rights Reserved.
The role of the mammalian branchpoint sequence in pre-mrna splicing
 The role of the mammalian branchpoint sequence in pre-mrna splicing Robin Reed and Tom Maniatis Department of Biochemistry and Molecular Biology, Harvard University, Cambridge, Massachusetts 02138 USA
The role of the mammalian branchpoint sequence in pre-mrna splicing Robin Reed and Tom Maniatis Department of Biochemistry and Molecular Biology, Harvard University, Cambridge, Massachusetts 02138 USA
Polyomaviridae. Spring
 Polyomaviridae Spring 2002 331 Antibody Prevalence for BK & JC Viruses Spring 2002 332 Polyoma Viruses General characteristics Papovaviridae: PA - papilloma; PO - polyoma; VA - vacuolating agent a. 45nm
Polyomaviridae Spring 2002 331 Antibody Prevalence for BK & JC Viruses Spring 2002 332 Polyoma Viruses General characteristics Papovaviridae: PA - papilloma; PO - polyoma; VA - vacuolating agent a. 45nm
integral components of snrnps (26-30), whereas PRP19 is not tightly associated with any of the five snrnas required
 Proc. Natl. Acad. Sci. USA Vol. 90, pp. 10821-10825, November 1993 Biochemistry Yeast precursor mrna processing protein PRP19 associates with the spliceosome concomitant with or just after dissociation
Proc. Natl. Acad. Sci. USA Vol. 90, pp. 10821-10825, November 1993 Biochemistry Yeast precursor mrna processing protein PRP19 associates with the spliceosome concomitant with or just after dissociation
Role of small nuclear RNAs in eukaryotic gene expression
 The Authors Journal compilation 2013 Biochemical Society Essays Biochem. (2013) 54, 79 90: doi: 10.1042/BSE0540079 Role of small nuclear RNAs in eukaryotic gene expression 6 Saba Valadkhan 1 and Lalith
The Authors Journal compilation 2013 Biochemical Society Essays Biochem. (2013) 54, 79 90: doi: 10.1042/BSE0540079 Role of small nuclear RNAs in eukaryotic gene expression 6 Saba Valadkhan 1 and Lalith
The 59 and 39 domains of yeast U6 snrna: Lsm proteins facilitate binding of Prp24 protein to the U6 telestem region
 RNA (2002), 8:1011 1033+ Cambridge University Press+ Printed in the USA+ Copyright 2002 RNA Society+ DOI: 10+1017+S1355838202026092 The 59 and 39 domains of yeast U6 snrna: Lsm proteins facilitate binding
RNA (2002), 8:1011 1033+ Cambridge University Press+ Printed in the USA+ Copyright 2002 RNA Society+ DOI: 10+1017+S1355838202026092 The 59 and 39 domains of yeast U6 snrna: Lsm proteins facilitate binding
Role of the snrnas in spliceosomal active site
 RNA Biology ISSN: 1547-6286 (Print) 1555-8584 (Online) Journal homepage: http://www.tandfonline.com/loi/krnb20 Role of the snrnas in spliceosomal active site Saba Valadkhan To cite this article: Saba Valadkhan
RNA Biology ISSN: 1547-6286 (Print) 1555-8584 (Online) Journal homepage: http://www.tandfonline.com/loi/krnb20 Role of the snrnas in spliceosomal active site Saba Valadkhan To cite this article: Saba Valadkhan
REGULATED AND NONCANONICAL SPLICING
 REGULATED AND NONCANONICAL SPLICING RNA Processing Lecture 3, Biological Regulatory Mechanisms, Hiten Madhani Dept. of Biochemistry and Biophysics MAJOR MESSAGES Splice site consensus sequences do have
REGULATED AND NONCANONICAL SPLICING RNA Processing Lecture 3, Biological Regulatory Mechanisms, Hiten Madhani Dept. of Biochemistry and Biophysics MAJOR MESSAGES Splice site consensus sequences do have
MCB Chapter 11. Topic E. Splicing mechanism Nuclear Transport Alternative control modes. Reading :
 MCB Chapter 11 Topic E Splicing mechanism Nuclear Transport Alternative control modes Reading : 419-449 Topic E Michal Linial 14 Jan 2004 1 Self-splicing group I introns were the first examples of catalytic
MCB Chapter 11 Topic E Splicing mechanism Nuclear Transport Alternative control modes Reading : 419-449 Topic E Michal Linial 14 Jan 2004 1 Self-splicing group I introns were the first examples of catalytic
Mammalian pre-mrna branch site selection by U2 snrnp involves base pairing
 Mammalian pre-mrna branch site selection by U2 snrnp involves base pairing Jian Wu and James L. Manley Department of Biological Sciences, Columbia University, New York, New York 10027 USA SV40 early pre-mrna
Mammalian pre-mrna branch site selection by U2 snrnp involves base pairing Jian Wu and James L. Manley Department of Biological Sciences, Columbia University, New York, New York 10027 USA SV40 early pre-mrna
Practice Problems 8. a) What do we define as a beneficial or advantageous mutation to the virus? Why?
 Life Sciences 1a Practice Problems 8 1. You have two strains of HIV one is a wild type strain of HIV and the second has acquired a mutation in the gene encoding the protease. This mutation has a dual effect
Life Sciences 1a Practice Problems 8 1. You have two strains of HIV one is a wild type strain of HIV and the second has acquired a mutation in the gene encoding the protease. This mutation has a dual effect
Determinants of Plant U12-Dependent Intron Splicing Efficiency
 This article is published in The Plant Cell Online, The Plant Cell Preview Section, which publishes manuscripts accepted for publication after they have been edited and the authors have corrected proofs,
This article is published in The Plant Cell Online, The Plant Cell Preview Section, which publishes manuscripts accepted for publication after they have been edited and the authors have corrected proofs,
Genetics. Instructor: Dr. Jihad Abdallah Transcription of DNA
 Genetics Instructor: Dr. Jihad Abdallah Transcription of DNA 1 3.4 A 2 Expression of Genetic information DNA Double stranded In the nucleus Transcription mrna Single stranded Translation In the cytoplasm
Genetics Instructor: Dr. Jihad Abdallah Transcription of DNA 1 3.4 A 2 Expression of Genetic information DNA Double stranded In the nucleus Transcription mrna Single stranded Translation In the cytoplasm
Pyrimidine Tracts between the 5 Splice Site and Branch Point Facilitate Splicing and Recognition of a Small Drosophila Intron
 MOLECULAR AND CELLULAR BIOLOGY, May 1997, p. 2774 2780 Vol. 17, No. 5 0270-7306/97/$04.00 0 Copyright 1997, American Society for Microbiology Pyrimidine Tracts between the 5 Splice Site and Branch Point
MOLECULAR AND CELLULAR BIOLOGY, May 1997, p. 2774 2780 Vol. 17, No. 5 0270-7306/97/$04.00 0 Copyright 1997, American Society for Microbiology Pyrimidine Tracts between the 5 Splice Site and Branch Point
8 Suppression Analysis
 Genetic Techniques for Biological Research Corinne A. Michels Copyright q 2002 John Wiley & Sons, Ltd ISBNs: 0-471-89921-6 (Hardback); 0-470-84662-3 (Electronic) 8 Suppression Analysis OVERVIEW Suppression
Genetic Techniques for Biological Research Corinne A. Michels Copyright q 2002 John Wiley & Sons, Ltd ISBNs: 0-471-89921-6 (Hardback); 0-470-84662-3 (Electronic) 8 Suppression Analysis OVERVIEW Suppression
An ATP-independent complex commits pre-mrna to the mammaiian spliceosome assembly pathway
 An ATP-independent complex commits pre-mrna to the mammaiian spliceosome assembly pathway Susan Michaud and Robin Reed Department of Cellular and Molecular Physiology and Program in Cellular and Developmental
An ATP-independent complex commits pre-mrna to the mammaiian spliceosome assembly pathway Susan Michaud and Robin Reed Department of Cellular and Molecular Physiology and Program in Cellular and Developmental
Molecular Biology (BIOL 4320) Exam #2 May 3, 2004
 Molecular Biology (BIOL 4320) Exam #2 May 3, 2004 Name SS# This exam is worth a total of 100 points. The number of points each question is worth is shown in parentheses after the question number. Good
Molecular Biology (BIOL 4320) Exam #2 May 3, 2004 Name SS# This exam is worth a total of 100 points. The number of points each question is worth is shown in parentheses after the question number. Good
Chapter 10 - Post-transcriptional Gene Control
 Chapter 10 - Post-transcriptional Gene Control Chapter 10 - Post-transcriptional Gene Control 10.1 Processing of Eukaryotic Pre-mRNA 10.2 Regulation of Pre-mRNA Processing 10.3 Transport of mrna Across
Chapter 10 - Post-transcriptional Gene Control Chapter 10 - Post-transcriptional Gene Control 10.1 Processing of Eukaryotic Pre-mRNA 10.2 Regulation of Pre-mRNA Processing 10.3 Transport of mrna Across
Frank Rigo and Harold G. Martinson*
 MOLECULAR AND CELLULAR BIOLOGY, Jan. 2008, p. 849 862 Vol. 28, No. 2 0270-7306/08/$08.00 0 doi:10.1128/mcb.01410-07 Copyright 2008, American Society for Microbiology. All Rights Reserved. Functional Coupling
MOLECULAR AND CELLULAR BIOLOGY, Jan. 2008, p. 849 862 Vol. 28, No. 2 0270-7306/08/$08.00 0 doi:10.1128/mcb.01410-07 Copyright 2008, American Society for Microbiology. All Rights Reserved. Functional Coupling
LS1a Fall 2014 Problem Set #4 Due Monday 11/3 at 6 pm in the drop boxes on the Science Center 2 nd Floor
 LS1a Fall 2014 Problem Set #4 Due Monday 11/3 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
LS1a Fall 2014 Problem Set #4 Due Monday 11/3 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
Mechanisms of alternative splicing regulation
 Mechanisms of alternative splicing regulation The number of mechanisms that are known to be involved in splicing regulation approximates the number of splicing decisions that have been analyzed in detail.
Mechanisms of alternative splicing regulation The number of mechanisms that are known to be involved in splicing regulation approximates the number of splicing decisions that have been analyzed in detail.
ZHIHONG JIANG, 1 JOCELYN COTE, 1 JENNIFER M. KWON, 2 ALISON M. GOATE, 3
 MOLECULAR AND CELLULAR BIOLOGY, June 2000, p. 4036 4048 Vol. 20, No. 11 0270-7306/00/$04.00 0 Copyright 2000, American Society for Microbiology. All Rights Reserved. Aberrant Splicing of tau Pre-mRNA Caused
MOLECULAR AND CELLULAR BIOLOGY, June 2000, p. 4036 4048 Vol. 20, No. 11 0270-7306/00/$04.00 0 Copyright 2000, American Society for Microbiology. All Rights Reserved. Aberrant Splicing of tau Pre-mRNA Caused
Life Sciences 1A Midterm Exam 2. November 13, 2006
 Name: TF: Section Time Life Sciences 1A Midterm Exam 2 November 13, 2006 Please write legibly in the space provided below each question. You may not use calculators on this exam. We prefer that you use
Name: TF: Section Time Life Sciences 1A Midterm Exam 2 November 13, 2006 Please write legibly in the space provided below each question. You may not use calculators on this exam. We prefer that you use
Exon Mutations Uncouple 5 Splice Site Selection from Ul snrna Pairing
 Cell, Vol. 63, 619-629, November 2, 1990, Copyright 0 1990 by Cell press Exon Mutations Uncouple 5 Splice Site Selection from Ul snrna Pairing l Bertrand S6raphin t and Michael Rosbash Howard Hughes Medical
Cell, Vol. 63, 619-629, November 2, 1990, Copyright 0 1990 by Cell press Exon Mutations Uncouple 5 Splice Site Selection from Ul snrna Pairing l Bertrand S6raphin t and Michael Rosbash Howard Hughes Medical
the essential splicing factor PSF bind stably to premrna prior to catalytic step 11 of the splicing
 The EMBO Journal vol.13 no.14 pp.3356-3367, 1994 A novel set of spliceosome-associated proteins and the essential splicing factor PSF bind stably to premrna prior to catalytic step 11 of the splicing reaction
The EMBO Journal vol.13 no.14 pp.3356-3367, 1994 A novel set of spliceosome-associated proteins and the essential splicing factor PSF bind stably to premrna prior to catalytic step 11 of the splicing reaction
A Novel Type of Splicing Enhancer Regulating Adenovirus Pre-mRNA Splicing
 A Novel Type of Splicing Enhancer Regulating Adenovirus Pre-mRNA Splicing Oliver Mühlemann, Bai-Gong Yue, Svend Petersen-Mahrt and Göran Akusjärvi Mol. Cell. Biol. 2000, 20(7):2317. DOI: 10.1128/MCB.20.7.2317-2325.2000.
A Novel Type of Splicing Enhancer Regulating Adenovirus Pre-mRNA Splicing Oliver Mühlemann, Bai-Gong Yue, Svend Petersen-Mahrt and Göran Akusjärvi Mol. Cell. Biol. 2000, 20(7):2317. DOI: 10.1128/MCB.20.7.2317-2325.2000.
V23 Regular vs. alternative splicing
 Regular splicing V23 Regular vs. alternative splicing mechanistic steps recognition of splice sites Alternative splicing different mechanisms how frequent is alternative splicing? Effect of alternative
Regular splicing V23 Regular vs. alternative splicing mechanistic steps recognition of splice sites Alternative splicing different mechanisms how frequent is alternative splicing? Effect of alternative
Materials and Methods , The two-hybrid principle.
 The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
Introduction retroposon
 17.1 - Introduction A retrovirus is an RNA virus able to convert its sequence into DNA by reverse transcription A retroposon (retrotransposon) is a transposon that mobilizes via an RNA form; the DNA element
17.1 - Introduction A retrovirus is an RNA virus able to convert its sequence into DNA by reverse transcription A retroposon (retrotransposon) is a transposon that mobilizes via an RNA form; the DNA element
Genetics and Genomics in Medicine Chapter 6 Questions
 Genetics and Genomics in Medicine Chapter 6 Questions Multiple Choice Questions Question 6.1 With respect to the interconversion between open and condensed chromatin shown below: Which of the directions
Genetics and Genomics in Medicine Chapter 6 Questions Multiple Choice Questions Question 6.1 With respect to the interconversion between open and condensed chromatin shown below: Which of the directions
Alternative RNA processing: Two examples of complex eukaryotic transcription units and the effect of mutations on expression of the encoded proteins.
 Alternative RNA processing: Two examples of complex eukaryotic transcription units and the effect of mutations on expression of the encoded proteins. The RNA transcribed from a complex transcription unit
Alternative RNA processing: Two examples of complex eukaryotic transcription units and the effect of mutations on expression of the encoded proteins. The RNA transcribed from a complex transcription unit
Variant Classification. Author: Mike Thiesen, Golden Helix, Inc.
 Variant Classification Author: Mike Thiesen, Golden Helix, Inc. Overview Sequencing pipelines are able to identify rare variants not found in catalogs such as dbsnp. As a result, variants in these datasets
Variant Classification Author: Mike Thiesen, Golden Helix, Inc. Overview Sequencing pipelines are able to identify rare variants not found in catalogs such as dbsnp. As a result, variants in these datasets
MECHANISMS OF ALTERNATIVE PRE-MESSENGER RNA SPLICING
 Annu. Rev. Biochem. 2003. 72:291 336 doi: 10.1146/annurev.biochem.72.121801.161720 Copyright 2003 by Annual Reviews. All rights reserved First published online as a Review in Advance on February 27, 2003
Annu. Rev. Biochem. 2003. 72:291 336 doi: 10.1146/annurev.biochem.72.121801.161720 Copyright 2003 by Annual Reviews. All rights reserved First published online as a Review in Advance on February 27, 2003
1. Investigate the structure of the trna Synthase in complex with a trna molecule. (pdb ID 1ASY).
 Problem Set 11 (Due Nov 25 th ) 1. Investigate the structure of the trna Synthase in complex with a trna molecule. (pdb ID 1ASY). a. Why don t trna molecules contain a 5 triphosphate like other RNA molecules
Problem Set 11 (Due Nov 25 th ) 1. Investigate the structure of the trna Synthase in complex with a trna molecule. (pdb ID 1ASY). a. Why don t trna molecules contain a 5 triphosphate like other RNA molecules
Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression. Chromatin Array of nuc
 Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression Chromatin Array of nuc 1 Transcriptional control in Eukaryotes: Chromatin undergoes structural changes
Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression Chromatin Array of nuc 1 Transcriptional control in Eukaryotes: Chromatin undergoes structural changes
In vitro reconstitution of snrnps: a reconstituted U4/U6 snrnp participates in splicing complex formation
 In vitro reconstitution of snrnps: a reconstituted U4/U6 snrnp participates in splicing complex formation Claudio W' Pikielny, Albrecht Bindereif, 1 and Michael R. Green Department of Biochemistry and
In vitro reconstitution of snrnps: a reconstituted U4/U6 snrnp participates in splicing complex formation Claudio W' Pikielny, Albrecht Bindereif, 1 and Michael R. Green Department of Biochemistry and
MODULE 3: TRANSCRIPTION PART II
 MODULE 3: TRANSCRIPTION PART II Lesson Plan: Title S. CATHERINE SILVER KEY, CHIYEDZA SMALL Transcription Part II: What happens to the initial (premrna) transcript made by RNA pol II? Objectives Explain
MODULE 3: TRANSCRIPTION PART II Lesson Plan: Title S. CATHERINE SILVER KEY, CHIYEDZA SMALL Transcription Part II: What happens to the initial (premrna) transcript made by RNA pol II? Objectives Explain
Interaktionen von RNAs und Proteinen
 Sonja Prohaska Computational EvoDevo Universitaet Leipzig May 5, 2017 RNA-protein binding vs. DNA-protein binding Is binding of proteins to RNA the same as binding of proteins to DNA? Will the same rules
Sonja Prohaska Computational EvoDevo Universitaet Leipzig May 5, 2017 RNA-protein binding vs. DNA-protein binding Is binding of proteins to RNA the same as binding of proteins to DNA? Will the same rules
Islamic University Faculty of Medicine
 Islamic University Faculty of Medicine 2012 2013 2 RNA is a modular structure built from a combination of secondary and tertiary structural motifs. RNA chains fold into unique 3 D structures, which act
Islamic University Faculty of Medicine 2012 2013 2 RNA is a modular structure built from a combination of secondary and tertiary structural motifs. RNA chains fold into unique 3 D structures, which act
7.012 Quiz 3 Answers
 MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
Human Genome: Mapping, Sequencing Techniques, Diseases
 Human Genome: Mapping, Sequencing Techniques, Diseases Lecture 4 BINF 7580 Fall 2005 1 Let us review what we talked about at the previous lecture. Please,... 2 The central dogma states that the transfer
Human Genome: Mapping, Sequencing Techniques, Diseases Lecture 4 BINF 7580 Fall 2005 1 Let us review what we talked about at the previous lecture. Please,... 2 The central dogma states that the transfer
TRANSCRIPTION. DNA à mrna
 TRANSCRIPTION DNA à mrna Central Dogma Animation DNA: The Secret of Life (from PBS) http://www.youtube.com/watch? v=41_ne5ms2ls&list=pl2b2bd56e908da696&index=3 Transcription http://highered.mcgraw-hill.com/sites/0072507470/student_view0/
TRANSCRIPTION DNA à mrna Central Dogma Animation DNA: The Secret of Life (from PBS) http://www.youtube.com/watch? v=41_ne5ms2ls&list=pl2b2bd56e908da696&index=3 Transcription http://highered.mcgraw-hill.com/sites/0072507470/student_view0/
Computational Identification and Prediction of Tissue-Specific Alternative Splicing in H. Sapiens. Eric Van Nostrand CS229 Final Project
 Computational Identification and Prediction of Tissue-Specific Alternative Splicing in H. Sapiens. Eric Van Nostrand CS229 Final Project Introduction RNA splicing is a critical step in eukaryotic gene
Computational Identification and Prediction of Tissue-Specific Alternative Splicing in H. Sapiens. Eric Van Nostrand CS229 Final Project Introduction RNA splicing is a critical step in eukaryotic gene
Introduction to Cancer Biology
 Introduction to Cancer Biology Robin Hesketh Multiple choice questions (choose the one correct answer from the five choices) Which ONE of the following is a tumour suppressor? a. AKT b. APC c. BCL2 d.
Introduction to Cancer Biology Robin Hesketh Multiple choice questions (choose the one correct answer from the five choices) Which ONE of the following is a tumour suppressor? a. AKT b. APC c. BCL2 d.
Pseudouridines in U2 snrna stimulate the ATPase activity of Prp5 during spliceosome assembly
 Article Pseudouridines in U2 snrna stimulate the ATPase activity of Prp5 during spliceosome assembly Guowei Wu 1,, Hironori Adachi 1, Junhui Ge 2, David Stephenson 1, Charles C Query 3 & Yi-Tao Yu 1,*
Article Pseudouridines in U2 snrna stimulate the ATPase activity of Prp5 during spliceosome assembly Guowei Wu 1,, Hironori Adachi 1, Junhui Ge 2, David Stephenson 1, Charles C Query 3 & Yi-Tao Yu 1,*
Bio 111 Study Guide Chapter 17 From Gene to Protein
 Bio 111 Study Guide Chapter 17 From Gene to Protein BEFORE CLASS: Reading: Read the introduction on p. 333, skip the beginning of Concept 17.1 from p. 334 to the bottom of the first column on p. 336, and
Bio 111 Study Guide Chapter 17 From Gene to Protein BEFORE CLASS: Reading: Read the introduction on p. 333, skip the beginning of Concept 17.1 from p. 334 to the bottom of the first column on p. 336, and
SPLICING SILENCING BY THE CUGBP2 SPLICING FACTOR: MECHANISM OF ACTION AND COMBINATORIAL CODE FOR SPLICING SILENCING WITH IMPLICATIONS FOR
 SPLICING SILENCING BY THE CUGBP2 SPLICING FACTOR: MECHANISM OF ACTION AND COMBINATORIAL CODE FOR SPLICING SILENCING WITH IMPLICATIONS FOR AUTOREGULATION by Jill A. Dembowski Bachelor of Science, Clarion
SPLICING SILENCING BY THE CUGBP2 SPLICING FACTOR: MECHANISM OF ACTION AND COMBINATORIAL CODE FOR SPLICING SILENCING WITH IMPLICATIONS FOR AUTOREGULATION by Jill A. Dembowski Bachelor of Science, Clarion
Studying Alternative Splicing
 Studying Alternative Splicing Meelis Kull PhD student in the University of Tartu supervisor: Jaak Vilo CS Theory Days Rõuge 27 Overview Alternative splicing Its biological function Studying splicing Technology
Studying Alternative Splicing Meelis Kull PhD student in the University of Tartu supervisor: Jaak Vilo CS Theory Days Rõuge 27 Overview Alternative splicing Its biological function Studying splicing Technology
Hands-On Ten The BRCA1 Gene and Protein
 Hands-On Ten The BRCA1 Gene and Protein Objective: To review transcription, translation, reading frames, mutations, and reading files from GenBank, and to review some of the bioinformatics tools, such
Hands-On Ten The BRCA1 Gene and Protein Objective: To review transcription, translation, reading frames, mutations, and reading files from GenBank, and to review some of the bioinformatics tools, such
TRANSLATION: 3 Stages to translation, can you guess what they are?
 TRANSLATION: Translation: is the process by which a ribosome interprets a genetic message on mrna to place amino acids in a specific sequence in order to synthesize polypeptide. 3 Stages to translation,
TRANSLATION: Translation: is the process by which a ribosome interprets a genetic message on mrna to place amino acids in a specific sequence in order to synthesize polypeptide. 3 Stages to translation,
Multifactorial Interplay Controls the Splicing Profile of Alu-Derived Exons
 MOLECULAR AND CELLULAR BIOLOGY, May 2008, p. 3513 3525 Vol. 28, No. 10 0270-7306/08/$08.00 0 doi:10.1128/mcb.02279-07 Copyright 2008, American Society for Microbiology. All Rights Reserved. Multifactorial
MOLECULAR AND CELLULAR BIOLOGY, May 2008, p. 3513 3525 Vol. 28, No. 10 0270-7306/08/$08.00 0 doi:10.1128/mcb.02279-07 Copyright 2008, American Society for Microbiology. All Rights Reserved. Multifactorial
mirna Dr. S Hosseini-Asl
 mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
SUPPLEMENTAL DATA RESULTS
 SUPPLEMENTAL DATA RESULTS Ded1-mediated ribosomal scanning is less leaky than scanning promoted by eifs 4A/4B/4F. The efficiency of leaky scanning in the presence of Ded1 or of eifs 4A/4B/4F was investigated
SUPPLEMENTAL DATA RESULTS Ded1-mediated ribosomal scanning is less leaky than scanning promoted by eifs 4A/4B/4F. The efficiency of leaky scanning in the presence of Ded1 or of eifs 4A/4B/4F was investigated
reads observed in trnas from the analysis of RNAs carrying a 5 -OH ends isolated from cells induced to express
 Supplementary Figure 1. VapC-mt4 cleaves trna Ala2 in E. coli. Histograms representing the fold change in reads observed in trnas from the analysis of RNAs carrying a 5 -OH ends isolated from cells induced
Supplementary Figure 1. VapC-mt4 cleaves trna Ala2 in E. coli. Histograms representing the fold change in reads observed in trnas from the analysis of RNAs carrying a 5 -OH ends isolated from cells induced
INTRODUCTION. Splicing of eukaryotic messenger RNA precursors (pre-mrna) is a complex process that. Nucleic Acids Research ABSTRACT
 Volume 17 Number 16 1989 Voue1 eerhoiouetcetree ubr1 99NcecAis egaaino ladu nnsrvas ifrnilitrcin fsma Oligonucleoticle-targeted degradation of Ul and U2 snrnas reveals differential interactions of sinian
Volume 17 Number 16 1989 Voue1 eerhoiouetcetree ubr1 99NcecAis egaaino ladu nnsrvas ifrnilitrcin fsma Oligonucleoticle-targeted degradation of Ul and U2 snrnas reveals differential interactions of sinian
The 5 0 EndofU2snRNAIsinCloseProximity to U1 and Functional Sites of the Pre-mRNA in Early Spliceosomal Complexes
 Article The 5 0 EndofU2snRNAIsinCloseProximity to U1 and Functional Sites of the Pre-mRNA in Early Spliceosomal Complexes Gizem Dönmez, 1 Klaus Hartmuth, 1 Berthold Kastner, 1 Cindy L. Will, 1 and Reinhard
Article The 5 0 EndofU2snRNAIsinCloseProximity to U1 and Functional Sites of the Pre-mRNA in Early Spliceosomal Complexes Gizem Dönmez, 1 Klaus Hartmuth, 1 Berthold Kastner, 1 Cindy L. Will, 1 and Reinhard
The Biology and Genetics of Cells and Organisms The Biology of Cancer
 The Biology and Genetics of Cells and Organisms The Biology of Cancer Mendel and Genetics How many distinct genes are present in the genomes of mammals? - 21,000 for human. - Genetic information is carried
The Biology and Genetics of Cells and Organisms The Biology of Cancer Mendel and Genetics How many distinct genes are present in the genomes of mammals? - 21,000 for human. - Genetic information is carried
Alternative splicing. Biosciences 741: Genomics Fall, 2013 Week 6
 Alternative splicing Biosciences 741: Genomics Fall, 2013 Week 6 Function(s) of RNA splicing Splicing of introns must be completed before nuclear RNAs can be exported to the cytoplasm. This led to early
Alternative splicing Biosciences 741: Genomics Fall, 2013 Week 6 Function(s) of RNA splicing Splicing of introns must be completed before nuclear RNAs can be exported to the cytoplasm. This led to early
Computational Biology I LSM5191
 Computational Biology I LSM5191 Aylwin Ng, D.Phil Lecture Notes: Transcriptome: Molecular Biology of Gene Expression II TRANSLATION RIBOSOMES: protein synthesizing machines Translation takes place on defined
Computational Biology I LSM5191 Aylwin Ng, D.Phil Lecture Notes: Transcriptome: Molecular Biology of Gene Expression II TRANSLATION RIBOSOMES: protein synthesizing machines Translation takes place on defined
Identification of cis-acting Intron and Exon Regions in Influenza
 MOLECULAR AND CELLULAR BIOLOGY, Mar. 1992, p. 962-970 0270-7306/92/030962-09$02.00/0 Copyright ) 1992, American Society for Microbiology Vol. 12, No. 3 Identification of cis-acting Intron and Exon Regions
MOLECULAR AND CELLULAR BIOLOGY, Mar. 1992, p. 962-970 0270-7306/92/030962-09$02.00/0 Copyright ) 1992, American Society for Microbiology Vol. 12, No. 3 Identification of cis-acting Intron and Exon Regions
Lecture 2: Virology. I. Background
 Lecture 2: Virology I. Background A. Properties 1. Simple biological systems a. Aggregates of nucleic acids and protein 2. Non-living a. Cannot reproduce or carry out metabolic activities outside of a
Lecture 2: Virology I. Background A. Properties 1. Simple biological systems a. Aggregates of nucleic acids and protein 2. Non-living a. Cannot reproduce or carry out metabolic activities outside of a
Novel RNAs along the Pathway of Gene Expression. (or, The Expanding Universe of Small RNAs)
 Novel RNAs along the Pathway of Gene Expression (or, The Expanding Universe of Small RNAs) Central Dogma DNA RNA Protein replication transcription translation Central Dogma DNA RNA Spliced RNA Protein
Novel RNAs along the Pathway of Gene Expression (or, The Expanding Universe of Small RNAs) Central Dogma DNA RNA Protein replication transcription translation Central Dogma DNA RNA Spliced RNA Protein
Alternative Splicing and Genomic Stability
 Alternative Splicing and Genomic Stability Kevin Cahill cahill@unm.edu http://dna.phys.unm.edu/ Abstract In a cell that uses alternative splicing, the total length of all the exons is far less than in
Alternative Splicing and Genomic Stability Kevin Cahill cahill@unm.edu http://dna.phys.unm.edu/ Abstract In a cell that uses alternative splicing, the total length of all the exons is far less than in
Generation of antibody diversity October 18, Ram Savan
 Generation of antibody diversity October 18, 2016 Ram Savan savanram@uw.edu 441 Lecture #10 Slide 1 of 30 Three lectures on antigen receptors Part 1 : Structural features of the BCR and TCR Janeway Chapter
Generation of antibody diversity October 18, 2016 Ram Savan savanram@uw.edu 441 Lecture #10 Slide 1 of 30 Three lectures on antigen receptors Part 1 : Structural features of the BCR and TCR Janeway Chapter
Yeast U1 snrnp pre-mrna complex formation without U1snRNA pre-mrna base pairing
 RNA (2001), 7:133 142+ Cambridge University Press+ Printed in the USA+ Copyright 2001 RNA Society+ Yeast U1 snrnp pre-mrna complex formation without U1snRNA pre-mrna base pairing HANSEN DU and MICHAEL
RNA (2001), 7:133 142+ Cambridge University Press+ Printed in the USA+ Copyright 2001 RNA Society+ Yeast U1 snrnp pre-mrna complex formation without U1snRNA pre-mrna base pairing HANSEN DU and MICHAEL
V24 Regular vs. alternative splicing
 Regular splicing V24 Regular vs. alternative splicing mechanistic steps recognition of splice sites Alternative splicing different mechanisms how frequent is alternative splicing? Effect of alternative
Regular splicing V24 Regular vs. alternative splicing mechanistic steps recognition of splice sites Alternative splicing different mechanisms how frequent is alternative splicing? Effect of alternative
SR Proteins. The SR Protein Family. Advanced article. Brenton R Graveley, University of Connecticut Health Center, Farmington, Connecticut, USA
 s s Brenton R Graveley, University of Connecticut Health Center, Farmington, Connecticut, USA Klemens J Hertel, University of California Irvine, Irvine, California, USA Members of the serine/arginine-rich
s s Brenton R Graveley, University of Connecticut Health Center, Farmington, Connecticut, USA Klemens J Hertel, University of California Irvine, Irvine, California, USA Members of the serine/arginine-rich
Section Chapter 14. Go to Section:
 Section 12-3 Chapter 14 Go to Section: Content Objectives Write these Down! I will be able to identify: The origin of genetic differences among organisms. The possible kinds of different mutations. The
Section 12-3 Chapter 14 Go to Section: Content Objectives Write these Down! I will be able to identify: The origin of genetic differences among organisms. The possible kinds of different mutations. The
L I F E S C I E N C E S
 1a L I F E S C I E N C E S 5 -UUA AUA UUC GAA AGC UGC AUC GAA AAC UGU GAA UCA-3 5 -TTA ATA TTC GAA AGC TGC ATC GAA AAC TGT GAA TCA-3 3 -AAT TAT AAG CTT TCG ACG TAG CTT TTG ACA CTT AGT-5 NOVEMBER 2, 2006
1a L I F E S C I E N C E S 5 -UUA AUA UUC GAA AGC UGC AUC GAA AAC UGU GAA UCA-3 5 -TTA ATA TTC GAA AGC TGC ATC GAA AAC TGT GAA TCA-3 3 -AAT TAT AAG CTT TCG ACG TAG CTT TTG ACA CTT AGT-5 NOVEMBER 2, 2006
Comparative analysis detects dependencies among the 5 splice-site positions
 Comparative analysis detects dependencies among the 5 splice-site positions IDO CARMEL, SAAR TAL, IDA VIG, and GIL AST Department of Human Genetics and Molecular Medicine, Sackler Faculty of Medicine,
Comparative analysis detects dependencies among the 5 splice-site positions IDO CARMEL, SAAR TAL, IDA VIG, and GIL AST Department of Human Genetics and Molecular Medicine, Sackler Faculty of Medicine,
1) DNA unzips - hydrogen bonds between base pairs are broken by special enzymes.
 Biology 12 Cell Cycle To divide, a cell must complete several important tasks: it must grow, during which it performs protein synthesis (G1 phase) replicate its genetic material /DNA (S phase), and physically
Biology 12 Cell Cycle To divide, a cell must complete several important tasks: it must grow, during which it performs protein synthesis (G1 phase) replicate its genetic material /DNA (S phase), and physically
SUPPLEMENTARY INFORMATION
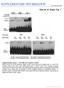 doi:10.1038/nature10495 WWW.NATURE.COM/NATURE 1 2 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 3 4 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 5 6 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 7 8 WWW.NATURE.COM/NATURE
doi:10.1038/nature10495 WWW.NATURE.COM/NATURE 1 2 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 3 4 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 5 6 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 7 8 WWW.NATURE.COM/NATURE
Investigating the mechanism of alternative splicing regulation of the RNA-binding proteins T-STAR and Sam68
 Investigating the mechanism of alternative splicing regulation of the RNA-binding proteins T-STAR and Sam68 Marina Danilenko Institute of Genetic Medicine A thesis submitted for the degree of Doctor of
Investigating the mechanism of alternative splicing regulation of the RNA-binding proteins T-STAR and Sam68 Marina Danilenko Institute of Genetic Medicine A thesis submitted for the degree of Doctor of
