Molecular pathology of paediatric central nervous system tumours
|
|
|
- Roderick Walker
- 5 years ago
- Views:
Transcription
1 Journal of Pathology J Pathol 2017; 241: Published online 10 November 2016 in Wiley Online Library (wileyonlinelibrary.com) DOI: /path.4813 INVITED REVIEW Molecular pathology of paediatric central nervous system tumours Jason CH Chiang and David W Ellison* Department of Pathology, St Jude Children s Research Hospital, Memphis, TN 38105, USA *Correspondence to: DW Ellison, MD, PhD, FRCPath, Department of Pathology, St Jude Children s Research Hospital, 262 Danny Thomas Place, Memphis, TN , USA. david.ellison@stjude.org Abstract Advances in our understanding of the biology of paediatric central nervous system (CNS) tumours have encouraged pathologists to use molecular markers alongside histopathological analysis for disease classification or prognostication and treatment stratification. In this article, we review molecular genetic alterations in paediatric CNS tumours, including those in low-grade and high-grade gliomas, ependymomas, and embryonal tumours. Some of these molecular changes with clinicopathological utility have been used for the first time in the most recent edition of the World Health Organization (WHO) classification of CNS tumours to define entities like ependymoma, RELA fusion-positive or diffuse midline glioma, H3 K27M-mutant. The classification of paediatric CNS tumours is entering a new era when histopathologists must work with molecular genetic data and their molecular pathology colleagues to provide an optimal diagnostic evaluation for their patients and clinical colleagues. Copyright 2016 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd. Keywords: nervous system; neoplasia; assay; fusion gene; histone mutation; chromothripsis Received 19 September 2016; Accepted 23 September 2016 No conflicts of interest were declared. Introduction Central nervous system (CNS) tumours are the leading cause of cancer-related death in children [1]. The overall average annual age-adjusted incidence rate is about 5.5 per While meningiomas and glioblastomas are more common in adults, the most common paediatric brain tumours are low-grade gliomas, such as pilocytic astrocytoma (15%), and embryonal tumours (12%), most of which are medulloblastomas. Embryonal tumours are the most common CNS tumour type in children aged 0 4 years. Supratentorial tumours account for about 25% of paediatric CNS tumours, followed by tumours arising in the cerebellum (20%), brainstem (12%), suprasellar region (8%), cranial nerves (7%), ventricles (6.4%), and spinal cord (4.3%). Until recently, the diagnosis of paediatric CNS tumours was based on histopathological features, and their classification generally followed that of adult tumours with similar appearances. However, advances in our understanding of tumour biology have provided opportunities to use molecular markers alongside histopathological analysis for disease classification, prognostication, and predicting therapy response. The pace of discovery has increased to such an extent, with the advent of high-throughput sequencing strategies, that molecular studies are becoming an essential part of the diagnostic workup. Furthermore, genomic discoveries have redefined relationships between tumours with similar and dissimilar histopathological features, and the distinction between adult and paediatric disease in related tumour types. Many molecular changes, from dominant-negative histone mutations and the hijacking of distal enhancer elements to new oncogenic gene fusion products and mutations, have been identified. Epigenetic dysregulation has also been revealed as a common theme across several tumour subtypes. The new 2016 World Health Organization (WHO) classification of tumours of the central nervous system will have a fundamental impact on clinical practice [2]; for the first time, molecular alterations are used in the classification of several entities. Major restructuring has occurred for adult diffuse gliomas, medulloblastomas, and other embryonal tumours in light of recent molecular discoveries. New entities defined by both histology and molecular signatures have emerged, including IDH-wild type and IDH-mutant glioblastomas, H3 K27M-mutant diffuse midline glioma, RELA fusion-positive ependymoma, WNT-activated and SHH-activated medulloblastomas, and C19MC-altered embryonal tumour with multilayered rosettes. These more homogeneous and narrowly defined entities are expected to facilitate patient stratification and precision therapy and to improve the design of clinical trials and experimental models. Paediatric gliomas and glioneuronal tumours About 55% of paediatric CNS tumours are gliomas [1]. Paediatric gliomas are heterogeneous, encompassing
2 160 JCH Chiang and DW Ellison low-grade tumours such as the cerebellar pilocytic astrocytoma, which is generally curable, WHO grade II diffuse gliomas, which resemble those that present in adults but are genetically and biologically distinct, and aggressive high-grade tumours such as the incurable diffuse pontine glioma, which would now be classified as diffuse midline glioma, H3 K27M-mutant [2]. Paediatric low-grade gliomas (LGGs) Paediatric LGGs are dominated by the pilocytic astrocytoma (PA; WHO grade I) and WHO grade II tumours, the diffuse astrocytoma (DA) or oligodendroglioma [2]. Two autosomal-dominant hereditary tumour syndromes, tuberous sclerosis complex (TSC) and neurofibromatosis type 1 (NF-1), are associated with the subependymal giant cell astrocytoma (SEGA) and optic pathway PA, respectively [3]. Unlike diffuse LGGs in adult patients, paediatric LGGs rarely harbour IDH1 or IDH2 mutations or undergo malignant transformation to higher-grade neoplasms [4]. Alterations of genes involved in the mitogen-activated protein kinase (MAPK) pathway are frequently found in paediatric LGGs, especially PAs [5,6], and BRAF alterations, particularly the KIAA1549 BRAF fusion duplication and the V600E mutation, are most common (Table 1) [7 10]. Other alterations that activate the MAPK pathway, such as mutations in NF1, RAF, RAS, and FGFR1, have also been identified in paediatric LGGs [5,11]. These molecular alterations are mutually exclusive, except in rare instances, as most paediatric LGGs and low-grade glioneuronal tumours harbour only one somatic genetic alteration that affects protein coding [5,12]. Patients with germline BRAF mutations and a glioma have not yet been reported. Pilocytic astrocytoma (PA) Approximately 85% of paediatric LGGs are PAs, arising most commonly in the cerebellum, but also in the diencephalon, optic pathways (optic nerve, chiasm, tracts, and radiation), and brainstem [1,13]. PAs presenting in the context of NF-1 arise most often in the optic pathways (66%) [14]. About 60 70% of sporadic PAs, especially those in the midline and cerebellum [15], but not in the cerebral hemispheres, harbour genomic rearrangement of BRAF. The most common fusion partner is KIAA1549 [7,10,16]. The KIAA1549-BRAF fusion variants usually occur in conjunction with a tandem duplication of the BRAF locus at 7q34, resulting in fusion products lacking the auto-inhibitory domain of BRAF. Other fusion partners such as MKRN1, CLCN6, GNAI1, FXR1, MACF1, FAM131B, and RNF130 have also been described [5,6,17]. The BRAF V600E mutation also occurs in about 9% of PAs, most commonly in diencephalic examples [18]. Other than BRAF alterations, alterations in FGFR1, KRAS, PTPN11, NTRK3, and NTRK2 have also been reported in sporadic PAs, but at much lower frequencies Table 1. Genetic alterations in paediatric low-grade gliomas Entities Mutations Structural alterations PA/PMA BRAF V600E KIAA1549-BRAF fusion (7q34 gain) FGFR1 (N546K, FGFR1 internal tandem duplication (ITD) K656E) NF1 DA BRAF V600E MYB/MYBL1 amplification or rearrangement IDH1/2 FGFR1 ITD or fusion H3 K27M O/OA FGFR1 FGFR1 ITD BRAF V600E MYB/MYBL1 rearrangement IDH1, IDH2 1p/19q co-deletion AG MYB/MYBL1 rearrangement QKI rearrangement PXA BRAF V600E 9p21.3 deletion (CDKN2A/B) TP53 DNET FGFR1 FGFR1 ITD BRAF V600E Chromosome 5 or 7 gain GG BRAF V600E Chromosome 5 and/or 7 gain KIAA1549-BRAF fusion DIA/DIG BRAF V600E 7q31 gain (MET ) Focal loss at 5q13.3, 21q22.11, and 10q21.3 PGNT SLC44A1-PRKCA fusion RGNT FGFR1 (N546K, K656E) PIC3CA DLGNT KIAA1549-BRAF fusion + 1p deletion Bold script = present in at least 50% of tumours. AG, angiocentric glioma; DA, diffuse astrocytoma; DIA, desmoplastic infantile astrocytoma; DIG, desmoplastic infantile ganglioglioma; DLGNT, diffuse leptomeningeal glioneuronal tumour; DNET, dysembryoplastic neuroepithelial tumour; GG, ganglioglioma, O, oligodendroglioma; OA, oligoastrocytoma; PA, pilocytic astrocytoma; PGNT, papillary glioneuronal tumour; PMA, pilomyxoid astrocytoma; PXA: pleomorphic xanthoastrocytoma; RGNT, rosette-forming glioneuronal tumour; SEGA, subependymal giant cell astrocytoma. (<5%), and they are usually in non-cerebellar PAs [5,6]. The second most common mutations in PAs are point mutations in FGFR1 s kinase domain, which activate the MAPK pathway. FGFR1-mutated PAs characteristically occur outside the cerebellum and in midline structures including the brainstem and third ventricle [19]. Internal tandem duplication of the tyrosine kinase domain of FGFR1 (FGFR1-ITD, FGFR1 TKDduplicated) is found rarely in PAs [5,6]. NF1 mutations are also rare in sporadic PAs [20]. The genetic alterations present in sporadic and NF1-asociated PAs converge on the RAS/RAF/MEK/MAPK, PI3K/AKT, and mtor signalling pathways to promote cell cycle progression and growth [21]. Pilomyxoid astrocytoma (PMA) PMA, a variant of PA, is recognized by its distinctive monophasic growth pattern and angiocentric arrangement in a myxoid matrix [22]. PMAs occur predominantly in infants and young children and involve the hypothalamic/chiasmatic region. The unfavourable location of the majority of PMAs often precludes complete surgical excision and these tumours have a relatively poor outcome compared with PAs [23]. The distinction between PMA and PA is not always clear-cut. The two tumours exist on a histopathological
3 Pediatric CNS tumors 161 spectrum, and PMAs often mature into PAs over time [24 28]. Many PMAs harbour a KIAA1549-BRAF fusion, further supporting their close relationship to PAs [24]. Paediatric low-grade diffuse gliomas Diffuse gliomas constitute less than 10% of paediatric LGGs [1]. Their infiltrative nature generally prevents a total surgical resection. Morphologically, they have an astrocytic, oligodendroglial, or mixed oligoastrocytic cytology and may be difficult to distinguish from PAs, especially in small biopsies. Most are diffuse astrocytomas (DAs), which occur primarily in the cerebral hemispheres and thalamus. The majority of paediatric DAs have alterations in MYB, MYBL1, FGFR1, orbraf [5,12,29]. MYB and MYBL1 alterations, which include episomal amplification and fusion with partner genes, such as PCDHGA1 and QKI, occur in approximately 25% of paediatric DAs [5,30]. The fusions result in loss of MYB s C-terminal negative regulatory domains. Partial duplication of MYBL1 generates a truncated protein, also without its regulatory domain [29]. Alterations in FGFR1, which are rare in paediatric DAs, include intragenic duplication of the tyrosine kinase domain (TKD) and fusion with TACC1 [5]. The intragenic TKD duplication induces FGFR1 autophosphorylation and activation of both MAPK and PI3K pathways. Approximately 25% of paediatric DAs harbour a BRAF V600E mutation [12,18]. Rare examples of paediatric low-grade DA have been shown to harbour an H3.3 K27M mutation, and this is not necessarily associated with a poor outcome [5], although H3 K27M-mutant diffuse midline glioma is generally regarded as high-grade disease [2]. Paediatric low-grade diffuse oligodendrogliomas lie on a morphological spectrum with dysembryoplastic neuroepithelial tumour (DNET) and share genetic alterations with this glioneuronal tumour, particularly FGFR1 mutations and TKD duplications [12]. As for DAs, diffuse oligodendrogliomas with IDH mutation and 1p/19q co-deletion (synchronous deletion of both chromosome 1p and chromosome 19q) are rare in the paediatric population, but can occur in older children and adolescents [5,12]. An important genetic distinction is emerging among diffuse low-grade gliomas at the older end of the paediatric age range, specifically between paediatric-type disease without IDH mutation and adult-type disease with IDH mutation. This distinction is associated with an important clinical difference. Paediatric-type disease generally has an indolent clinical course; these diffuse WHO grade II tumours rarely progress to grade III or grade IV tumours. In contrast, adult-type disease progresses in approximately 75% of cases [31]. Desmoplastic infantile astrocytoma (DIA) and ganglioglioma (DIGG) DIAs and DIGGs (WHO grade I) represent about 0.5% of paediatric CNS tumours [1]. They are histopathologically similar, a neoplastic neuronal element being the only distinction. Virtually all of these tumours occur in patients under the age of 2 years [32]. They are generally large cystic tumours arising in the parietal or frontal lobe. The molecular profiles of DIGGs and DIAs are essentially the same. Gain of 7q31 involving MET is found in more than 40% of DIAs/DIGGs. Focal recurrent copy number losses are found at 5q13.3, 21q22.11 and 10q21.3. Large chromosomal alterations are rare. About 10% of DIAs/DIGGs harbour a BRAF V600E mutation [33]. Diffuse leptomeningeal glioneuronal tumour (DLGNT) DLGNT is a new entity in the 2016 CNS WHO classification [2]. It commonly presents as diffuse leptomeningeal disease around the spinal cord in children or adolescents [34]. A parenchymal component is seen in about 80% of cases. The tumours contain oligodendrocyte-like cells with low levels of mitotic activity. About 25% of the tumours will show anaplastic progression. A ganglion cell component is found in approximately 15% of DLGNTs. KIAA1549-BRAF fusions and chromosome 1p deletion are seen in most tumours, with combined 19q deletion in about 20% [34,35]. Although this entity bears similarity to oligodendroglioma or some glioneuronal tumours, IDH and BRAF V600E mutations have never been demonstrated. Pleomorphic xanthoastrocytoma (PXA) PXA is a relatively rare (0.5 1%) tumour, typically affecting children and young adults [36]. Most PXAs are supratentorial (96%) with a predilection for the temporal lobe (46%). PXAs commonly arise in the superficial cerebral cortex and often extend into the leptomeninges [37]. Seizures are the presenting symptoms in 70% of patients. A BRAF V600E mutation occurs in about 65% of PXAs [18,33,38], especially those arising from the temporal lobe (90%). BRAF fusion duplication is not detected in PXAs. Homozygous deletion of 9p21.3, which contains CDKN2A/B, is seen in 60% of PXAs [39]. About 5% of PXAs harbour TP53 mutations [40]. PXAs may undergo malignant transformation, and anaplastic PXA has been added to the 2016 CNS WHO classification as a distinct (grade III) entity [2]. An anaplastic grading requires five or more mitoses per 10 high-power fields. Necrosis may be present, but the significance of necrosis in the absence of elevated mitotic activity is uncertain [41]. Anaplastic PXAs show a similar frequency of BRAF V600E mutation to grade II PXAs [18]. There is no correlation between TP53 mutation and the presence of anaplastic features [42]. Astroblastoma Astroblastoma is a rare tumour that develops in children and young adults as a well-circumscribed, peripheral hemispheric mass [43,44]. The tumour is characterized by astroblastomatous pseudorosettes, prominent
4 162 JCH Chiang and DW Ellison perivascular hyalinization, and pushing borders at the interface with adjacent brain. Astroblastoma is an entity in the 2016 WHO classification, without a designated grading [2]. About 70% of astroblastomas may harbour an MN1 alteration, including fusions with BEND2 or CXXC5 [45]. Gains of chromosomes 20q and 19 are observed in some tumours [43]. Subependymal giant cell astrocytoma (SEGA) SEGA is the most common CNS neoplasm in patients with tuberous sclerosis complex (TSC) and mutation of TSC1 or TSC2 [46]. SEGAs are typically located in the lateral ventricle, near the foramen of Monro along the subependymal region of the caudothalamic groove. Isolated SEGAs without other TSC-related lesions are likely due to somatic mosaicism or mutation of the TSC genes [47,48]. Glioneuronal tumours Glioneuronal tumours encompass heterogeneous tumours with a mixed glial and neuronal morphology. Ganglioglioma and dysembryoplastic neuroepithelial tumour (DNET) are relatively common among these tumours [1]. Ganglioglioma (GG) The histological hallmark of GGs is a combination of neoplastic ganglion cells and a glial component with features that can resemble the morphology of PA (most common) or a diffuse glioma (astrocytoma more commonly than oligodendroglioma). GGs are mostly supratentorial with a predilection (75%) for the temporal lobe [49]. Most patients (65%) present with a history of epilepsy [50]. A BRAF V600E mutation occurs in about 20 30% of GGs [18,33]. About 20% of GGs show gains of chromosome 5 or 7 (15% show gains of both) [51]. KIAA1549-BRAF fusions are very rare [52]. The glial component of GG may rarely (3 5%) undergo malignant transformation [49]. Anaplastic GGs commonly show substantial mitotic activity and, in some cases, endothelial proliferation and necrosis. About half of anaplastic GGs harbour a BRAF V600E mutation [18]. CDK4 amplification and loss of tumour suppressor genes such as CDKN2A/B and DMBT1 have been found to be present in the glial, but not the ganglion cell, component [53]. TP53 mutations in the malignant component have also been observed [54]. Dysembryoplastic neuroepithelial tumour (DNET) DNETs are low-grade (WHO grade I) tumours commonly associated with medically intractable seizures [55]. They are mostly supratentorial with a predilection for the temporal lobe. Several histological forms of DNET have been described [56 58]. Simple, complex, and diffuse DNETs and mixed DNET/GG show no difference in age of onset, associated seizure type, or outcome [57]. Most DNETs harbour an alteration in FGFR1, either a TKD duplication or a single nucleotide variation (SNV) [12,59]. BRAF V600E mutation is also seen in a minority of DNETs, more frequently in classic DNETs and in extratemporal locations [12,60]. Similar to GG and PA, 20% of DNETs show gains of chromosome 5 or 7 [51]. Paediatric high-grade diffuse gliomas (HGGs) Paediatric HGGs primarily encompass glioblastoma (GB) and its variants, which are WHO grade IV tumours, anaplastic astrocytoma (WHO grade III), and diffuse midline gliomas, including diffuse pontine glioma. Together, they comprise 15 20% of paediatric CNS tumours [1]. These tumours are characterized by brisk mitotic activity in grade III tumours and, additionally, microvascular proliferation and necrosis in grade IV tumours, similar to their adult counterparts. Although histologically similar, paediatric and adult HGGs can be distinguished by their clinical behaviours, locations, and genetic alterations (Table 2). Paediatric HGGs nearly always arise as primary malignant neoplasms and only rarely progress from tumours of lower grade [61,62]. The 2-year survival for paediatric HGGs ranges from 30% for tumours in the cerebral hemispheres down to less than 10% for diffuse pontine gliomas [63]. Similar to adult HGGs, paediatric HGGs commonly arise in the cerebral hemispheres [1]. However, high-grade pontine gliomas occur nearly always in children and account for nearly half of the paediatric HGGs. Midline HGGs arising from the thalamus (13%), cerebellum (5%), and spinal Table 2. Genetic alterations in paediatric high-grade gliomas Entities Mutations Structural alterations Anaplastic astrocytoma TP53 PDGFRA amplification/ rearrangement/ indels Glioblastoma TP53 CDKN2A deletion H3F3A (H3.3) G34R/V NTRK1-3 fusion genes PICK3CA/PIK3R1 BRAF V600E MET or EGFR amplification NF1 CDK4 or CDK6 amplification ATRX, DAXX MYCN amplification PDGFRA IDH1 FGFR1 Epithelioid glioblastomabraf V600E Diffuse midline glioma H3F3A (H3.3 K27M) HIST1H3A/B/C (H3.1) ACVR1 G328 Anaplastic PXA BRAF V600E 9p21.3 deletion (CDKN2A/B) TP53 Anaplastic GG BRAF V600E CDK4 amplification TP53 Loss of CDKN2A/B and DMBT1 Bold script = present in at least 50% of tumours. GG, ganglioglioma; PXA, pleomorphic xanthoastrocytoma.
5 Pediatric CNS tumors 163 cord (3%) are also much more common in the paediatric population. Compared with adult HGGs, paediatric HGGs carry significantly fewer genomic alterations, especially among tumours in infants less than 3 years of age [64,65]. Histone H3 K27M mutations are found in 80% of diffuse pontine gliomas [66]. They are also found in other midline HGGs arising in the thalamus, cerebellum or spinal cord. These were the first histone mutations to be identified in human cancer. About 75% of histone H3 mutations occur in H3F3A, encoding the H3.3 isoform, and 20 25% of mutations occur in HIST1H3B or rarely HIST1H3A/C, encoding H3.1 [65,67,68]. H3.3 and H3.1 mutations occur in a mutually exclusive manner. Activating somatic mutations in ACVR1, a type I receptor in the bone morphogenetic protein (BMP) signalling pathway, are identified in 25 30% of diffuse pontine gliomas. ACVR1 mutations almost always occur concurrently with a HIST1H3B K27M mutation in diffuse pontine gliomas that present at less than 5 years of age [69]. Hotspot G328 mutations are most common. While H3.1 K27M mutations are also found in thalamic HGGs, ACVR1 mutations have only been identified in diffuse pontine gliomas [65,67 69]. Approximately 30% of paediatric GBs harbour H3F3A mutations [70]. Histone H3.3, a replication-independent histone variant, is recruited to DNA via the ATRX DAXX heterodimer [70]. Approximately half of the paediatric GBs harbour TP53 mutations. Tumours with combined H3F3A, ATRX, and TP53 mutations frequently demonstrate alternative lengthening of telomeres (ALT). PPM1D, which is frequently overexpressed in medulloblastoma, has been found to be mutated recurrently in midline HGGs [71]. The mutations are gain-of-function changes that result in attenuated activity of p53 and of CHK2, a DNA damage response protein. Histone H3 K27 mutations are likely to be acting in a dominant fashion, as the mutant protein accounts for 5 15% of the total histone pool. The K27M mutation inhibits the polycomb repressive complex 2 (PRC2) [62], which leads to a global loss of lysine 27 trimethylation (H3K27me3) on wild-type histone H3 and derepression of PRC2 targets [72 75]. H3F3A G34R/V mutations are most commonly found in parietal, occipital, and temporal lobe HGGs from adolescents and young adults [66,70]. Mutations in ATRX and/or DAXX are identified in all tumours harbouring H3.3 G34 mutations. H3F3A G34-mutant HGGs, like the vast majority of paediatric HGGs, arise as primary malignancies, without evidence of a precursor lower-grade lesion [61]. Little is known about the function of the G34R/V mutations. H3F3A G34-mutant tumours show pronounced hypomethylation in subtelomeric regions [62]. The mutations reduce histone H3 lysine 36 trimethylation levels around histones carrying the mutation, but without the global dominant-negative effect of the K27M mutation [72]. Elevated MYCN levels have been observed in some tumours [76]. About 6 25% of paediatric hemispheric HGGs harbour a mutation in SETD2, an H3K36 methyltransferase, and show widespread H3K36me3 loss [65,77]. Frontal lobe HGGs in adolescents and young adults can harbour the IDH1 R132H mutation [62], the molecular hallmark of adult-type HGGs that progress from a grade II tumour. IDH1-mutant tumours account for less than 10% of paediatric HGGs [62]. H3F3A and IDH1 R132H mutations are mutually exclusive [70]. IDH-mutant tumours characteristically show global DNA hypermethylation known as glioma-cpg island methylator phenotype (G-CIMP), likely caused by the generation of R-2-hydroxyglutarate [78,79], whereas H3-mutant tumours show global hypomethylation signatures [62]. BRAF V600E mutations, NF1 mutations, and NTRK fusion genes are more commonly found in non-brainstem HGGs. About 10% of paediatric HGGs arising from the cerebral hemispheres harbour a BRAF V600E mutation [80]. BRAF V600E mutation has not been reported in diffuse pontine gliomas. BRAF fusion duplications commonly found in PAs are not seen in HGGs. Approximately 50% of epithelioid GBs, a newly recognized variant in the 2016 CNS WHO classification [2], harbour a BRAF V600E mutation [81,82]. Epithelioid GBs have a predilection for children and younger adults, typically presenting as sharply demarcated superficial cerebral or diencephalic masses. Such cases may have an associated low-grade precursor, often but not invariably showing features of PXA [83]. Sporadic NF1 mutations are seen in 5 25% of paediatric HGGs, more commonly in HGGs arising from the cerebral hemispheres [65,67 70]. Activating fusions of the NTRK family of neurotrophin receptors (NTRK1 3) are seen in about 5% of all paediatric HGGs, with a high frequency (about 50%) observed in children younger than 3 years of age [5,6,65]. FGFR1 activating mutations are found predominantly in thalamic HGGs [68]. Mutations in TP53, PDGFRA, PIK3CA, and PIK3R1 are found in HGGs from all locations. TP53 mutations are found in more than half of paediatric HGGs, including diffuse pontine gliomas and other midline HGGs [65,67 70]. PDGFRA alterations, including amplification, rearrangement, indels, and mutations, are the most common RTK alterations and are found in about 30% of paediatric HGGs [65,84 88]. Mutations in the downstream PI3-kinase catalytic (PIK3CA) or regulatory (PIK3R1) subunits occur in 10 25% of paediatric HGGs. CDKN2A homozygous deletion is observed in about 25% of non-brainstem HGGs [86]. In diffuse pontine gliomas, focal amplifications of CDK4 or CDK6 are observed [85,88]. Interestingly, gene expression analyses have demonstrated close similarity between paediatric and adult HGGs. Three major groups of paediatric HGGs correlate with the Proneural, Proliferative, and Mesenchymal groups identified in adult HGGs [84,86,87]. Another classification divides both adult and paediatric GBs into six molecular groups: IDH, K27, G34, RTK I (PDGFRA), Mesenchymal, and RTK II (classic) [62]. The IDH group is characterized by tumours with IDH1
6 164 JCH Chiang and DW Ellison or IDH2 mutations, mostly from young adults. K27 and G34 are the only groups associated with H3F3A mutations and include the youngest patients. Tumours in the K27 subgroup have a midline location (midbrain, brainstem, spinal cord), whereas G34 tumours most commonly arise in the cerebral white matter. The RTK I group, which shows a patient age range similar to that of the IDH group (median age 36 years old), is associated with PDGFRA amplification. The RTK II, or classic, group is enriched with tumours harbouring alterations typically present in adult primary GBs. The RTK II (classic) group shows significant overlap with Verhaak s Classic group, and the RTK I (PDGFRA), IDH, and K27 groups show significant overlap with Verhaak s Proneural group [89]. An additional group with a GB morphology but a high frequency of BRAF V600E mutation and a distinct methylation signature, which resembles that of PXA, has a more favourable outcome than GBs and is termed PXA-like [90]. The IDH, PXA-like, and G34 groups are associated with a longer overall survival than expected for GBs, whereas the K27, RTK, and Mesenchymal groups are associated with the shortest survival [62,90]. The presence of amplified oncogenes, such as EGFR, PDGFR, and MYCN, is associated with a poor outcome across groups. Ependymomas Ependymomas are the second most common malignant brain tumour in childhood [91], usually arising in the posterior fossa of young children in the first decade of life. Several recent studies demonstrated distinct molecular groups of ependymoma [92 97], each characterized by distinct clinical, anatomic, and genetic features. Tumours from the three principal CNS anatomic sites (supratentorial region, posterior fossa, spinal cord) can be distinguished by their gene expression profiles [98]. Two distinct molecular subtypes of posterior fossa ependymomas have been identified [93]. The more common group A posterior fossa ependymomas, 48% of ependymomas from all sites [99], show minimal genomic alterations, occur predominantly in infants, and demonstrate activation of cancer-related signalling pathways, including those associated with VEGF, PDGFR, integrin, and MAPK. Group A tumours have elevated methylation across CpG islands, which is associated with epigenetic silencing of differentiation genes [96]. Group B posterior fossa ependymomas are less frequent than group A tumours and occur in adolescents and young adults. They generally display chromosome-wide ploidy changes. The mutation rates in both group A and group B are low, and no recurrent mutations have so far been found in either group A or group B posterior fossa ependymomas [96]. Approximately 70% of supratentorial ependymomas harbour C11orf95 RELA fusions within chromosome 11q, as a result of chromothripsis, a catastrophic chromosome shattering with erroneous DNA repair that results in the shuffling of DNA segments across the affected chromosome(s) [97,100]. C11orf95 RELA fusion transcripts are never found in posterior fossa tumours, once more highlighting the distinction between ependymomas at different CNS locations. C11orf95 RELA fusions lead to activation of the NF-κB signalling pathway [97,101]. RELA fusion-positive ependymoma is a newly recognized entity in the 2016 CNS WHO classification [2]. MAMLD1 YAP1 or less commonly FAM118B YAP1 fusions are seen in RELA fusion-negative supratentorial ependymomas [97,102]. YAP1 fusion-positive tumours are more common in young children and show no evidence of chromothripsis. Subependymomas in all three CNS locations show predominantly balanced genomes and are much more common in adults [102]. The existence of nine molecular groups based on DNA methylation profiling supratentorial RELA, supratentorial YAP1, supratentorial subependymoma, posterior fossa group A, posterior fossa group B, posterior fossa subependymoma, spinal cord classic ependymoma, spinal cord myxopapillary ependymoma, and spinal cord subependymoma has been demonstrated [102]. The outcome of ependymoma in children, especially in infants, is poor; almost half die as a result of their disease. Supratentorial RELA fusion-positive and posterior fossa group A ependymomas together make up two-thirds of all cases and are associated with poor overall survival when compared with the other groups. However, these findings have yet to be confirmed in cohorts of patients treated on clinical trials. Embryonal tumours Embryonal tumours (Table 3), including medulloblastoma, atypical teratoid/rhabdoid tumour (AT/RT), and CNS primitive neuroectodermal tumours (CNS- PNETs), are the most prevalent paediatric malignant CNS tumours, together comprising 15 20% of all CNS tumours in children younger than 14 years of age [1]. These tumours are very rare in adults. Medulloblastoma Medulloblastoma is the most common childhood malignant CNS tumour [1]. By using a combination of therapies including surgical resection, risk-adjusted irradiation, and adjuvant chemotherapy, about 70% of paediatric medulloblastomas can be cured, although debilitating long-term sequelae are seen in some patients [103]. Medulloblastoma is a clinically and molecularly heterogeneous disease [ ]. Three histologically defined variants are recognized alongside the classic tumour: desmoplastic/nodular (D/N), medulloblastoma with extensive nodularity (MBEN), and large cell/anaplastic (LC/A) [2]. Four molecular groups have been identified through transcriptome profiling: WNT (10%), SHH (30%), group 3 (15%),
7 Pediatric CNS tumors 165 Table 3. Genetic alterations in embryonal tumours Entities Defining molecular features Mutations Structural alterations MB, WNT-activated Activation of WNT pathway CTNNB1 Monosomy 6 DDX3X Chromatin remodelling genes TP53 MB, SHH-activated and TP53-wildtype Activation of SHH pathway PTCH1 9q loss SMO 10q loss SUFU TERT promoter MB, SHH-activated and TP53-mutant Activation of SHH pathway (TP53) MYCN amplification GLI2 amplification 17p loss Tetraploidy MB, group 3 Elevated expression of MYC SMARCA4 Isochromosome 17q Chromatin remodelling genes Enhancer hijacking (GFI1/GF1B) Genes of TGF-β pathway MYC amplification MB, group 4 Chromatin remodelling genes Isochromosome 17q Enhancer hijacking (GFI1/GF1B) MYCN amplification AT/RT Loss of INI1 or BRG1 SMARCB1 22q deletion SMARCA4 Monosomy 22 ETMR Chromosome 19q13.42 (Chromosome 19q13.42 amplification, fusion) microrna cluster amplification CNS NB-FOXR2 FOXR2 overexpression FOXR2 fusion products Chromosome 1q gain Loss of 16q Chromosome 8 gain CNS EFT-CIC Up-regulation of ETS CIC fusion or deletion transcription factor family CNS HGNET-MN1 MN1 rearrangement Loss of 16q Chromosome 8 gain CNS HGNET-BCOR BCOR alterations Bold script = present in at least 50% of tumours. AT/RT, atypical teratoid/rhabdoid tumour; ETMR, embryonal tumour with multilayered rosettes; CNS EFT-CIC, CNS Ewing sarcoma family tumour with CIC alteration; CNS HGNET-BCOR, CNS high-grade neuroepithelial tumour with BCOR alteration; CNS HGNET-MN1, CNS high-grade neuroepithelial tumour with MN1 alteration; CNS NB-FOXR2, CNS neuroblastoma with FOXR2 activation; MB, medulloblastoma. and group 4 (45%) [ ]. The 2016 WHO classification recognizes five genetically defined types of medulloblastoma: WNT-activated, SHH-activated and TP53-mutant, SHH-activated and TP53-wild type, and non-wnt/non-shh incorporating group 3 and group 4 tumours [2]. The WNT group, the least common and the most homogeneous group, is characterized by activation of the WNT signalling pathway [105]. CTNNB1 mutations are identified in approximately 90% of tumours [108]. Nuclear accumulation of β-catenin is routinely used as a biomarker for WNT pathway activation, often in conjunction with the detection of monosomy 6, which is found in 80 85% of WNT medulloblastomas [107,109]. Mutations in DDX3X, an RNA helicase, are detected in 50% of the WNT tumours [108,110,111]. Mutations in chromatin remodelling genes, including SMARCA4 (25%), MLL2 (12.5%), CREBBP (6%), TRAPP (3%), and MED13 (3%), are also found in about half of the tumours. TP53 mutations are present in 15% of WNT tumours and are not associated with the poor prognosis associated with TP53-mutant SHH tumours [111,112]. Indeed, WNT-activated medulloblastomas are associated with an excellent outcome; patients without high-risk clinical factors, such as metastatic disease, nearly all survive following treatments with standard therapies [104,107]. In an attempt to reduce the long-term adverse CNS effects of irradiation, WNT medulloblastoma has become the focus of trials in which it is regarded as low-risk disease and suitable for reduced adjuvant therapy. The SHH molecular group is the most heterogeneous, and all histological variants occur in this group. D/N tumours and MBENs are all classified as SHH-activated and account for just over half of SHH tumours [107,113], and LC/A tumours occur in this group almost as frequently as they do in group 3 [107]. Clinically, SHH medulloblastomas present most frequently in infants and adults; throughout most of childhood, they are relatively uncommon. Across three age-related categories: infant (0 4 years), children (4 17 years), and adult (>17 years), SHH medulloblastomas show striking clinicopathological and genetic diversity [114]. MBENs fall into the infant category, while classic and LC/A tumours are most frequent in childhood. Adult tumours mostly have a D/N morphology. Genetic alterations are unequally spread across the categories [114]. SUFU mutations tend to occur in the
8 166 JCH Chiang and DW Ellison infant category, while SMO mutations occur mostly in adults (30% of the tumours). MYCN and GLI2 amplification are detected mainly in the childhood group. PTCH1 alterations are common across the age groups (42% infant, 36% childhood, and 54% adult SHH tumours). The high prevalence of PTCH1 and SMO mutations in adult SHH medulloblastomas predicts responsiveness to SMO inhibitors, whereas the presence of downstream MYCN amplification or GLI2 amplification/mutations predicts lack of response. TERT promoter mutations (C228T and C250T) occur in 38% of SHH medulloblastomas and are present in 80% of adult SHH tumours [115,116]. Mutations in chromatin remodelling genes, including MLL2 (12%), BCOR (3%), and LBD1 (3%), occur in 21% of the tumours [110]. Mutations in DDX3X are seen in 11% of SHH tumours [108,110,111]. SHH tumours frequently showed loss of the whole of chromosome arm 9q [111]. Gorlin syndrome (GS; naevoid basal cell carcinoma syndrome) caused by de novo (60% of cases) or inherited germline PTCH1 mutations is an autosomal-dominant disorder characterized by developmental defects and various forms of cancer including medulloblastoma [117]. About 5% of patients develop D/N medulloblastoma during infancy [118]. The outcome for patients with GS and D/N medulloblastoma is mostly favourable following conventional therapy [119]. However, the presence of PTEN or GNAS alterations may be associated with a relatively poor prognosis [120]. Highlighting the heterogeneity of SHH medulloblastoma is the contrast between the outcomes of two tumour types in different categories. MBENs in the infant category are associated with an excellent outcome, whereas SHH-activated LC/A tumours with MYCN amplification or a TP53 mutation are very aggressive, nearly always presenting with metastatic disease [104,105,107,113,114]. SHH-activated, TP53-mutant medulloblastoma is a genetically defined entity in the 2016 WHO classification [2]. TP53 mutations occur in 13% of SHH tumours, and many of these are germline mutations (Li Fraumeni syndrome) [112,114]. SHH medulloblastomas with germline TP53 mutations frequently harbour amplifications in MYCN (42%) and/or GLI2 (30%) [109]. Childhood SHH tumours with TP53 mutations have an appalling outcome, especially in the presence of MYCN or GLI2 amplification [105]. SHH tumours with TP53 mutations commonly have tetraploidy [111]. Most SHH medulloblastomas with a TP53 mutation have an LC/A morphology and are high-risk tumours [2]. Non-WNT/non-SHH group 3 and group 4 medulloblastomas can be separated on gene expression or DNA methylation profiling, but overlap to some extent [109]. Both groups show a male preponderance. Isochromosome 17q occurs in both groups, though it is more common in group 4 tumours (80%) than in group 3 (26%) [109]. Both groups demonstrate recurrent structural alterations, including deletions, duplications, inversions, and complex rearrangements. Structural alterations that aberrantly induce the proto-oncogenes GFI1 or GF1B through highly active enhancer elements (enhancer hijacking) [121] occur in 41% of group 3 and 10% of group 4 tumours [122]. GFI1 and GFI1B are activated in a mutually exclusive manner. Mutations in the chromatin remodelling KDM gene family (KDM1A, KDM3A, KDM4C, KDM5A, KDM5B, KDM6A, and KDM7A) and gain of EZH2 occur only in group 3 and group 4 medulloblastomas [110]. Patients with group 3 medulloblastoma have the worst outcome of the four molecular groups [105,123]. These tumours are more common in boys, often show an LC/A morphology, and nearly 50% are metastatic at the time of diagnosis [105]. They are characterized by MYC overexpression and in 17% of cases they harbour MYC amplification [123]. Group 3 tumours with metastasis, isochromosome 17q, or MYC amplification carry a dismal prognosis, but group 3 tumours with classic histology are standard-risk tumours [2]. Copy number changes that target genes in the TGF-β signalling pathway occur in 20% of group 3 tumours [109,124], and PVT1 alterations are present in 12% of the tumours [109]. Mutations in chromatin remodelling genes including SMARCA4 (11%), KDM gene family members (8%), MLL2 (4%), GPS2 (3%), MLL3 (1%), CREBBP (1%), and CHD7 (1%) are detected in 28.5% of group 3 tumours [108,110,111]. Group 4 is the most common molecular group with a strong gender bias (three times more male than female patients) [105]. Nearly all tumours show classic histology. About 10% harbour SNCAIP tandem duplications, with OTX2 amplification in 5.5% and CDK6 amplification in 5% of tumours [109]. Mutations in chromatin remodelling genes, including KDMA6A (13%), other KDM family members (4%), MLL3 (3%), CHD7 (3%), and ZMYM3 (3%), are seen across approximately 30% of cases [108,110,111]. Copy number changes that target genes in the NF-κB signalling pathway occur in group 4 tumours [109]. MYCN amplification seen in 5% of group 4 tumours is associated with a poor outcome [105]. Atypical teratoid/rhabdoid tumour (AT/RT) AT/RTs are highly malignant tumours typically occurring before 5 years of age, with a male predominance (male to female ratio: :1) and an estimated prevalence of 1 2% among paediatric CNS tumours [1, ]. A significant proportion of AT/RTs arise in children younger than 2 years. They are the most common malignant CNS tumour of children below 1 year of age. AT/RTs arise in supratentorial or posterior fossa locations with an approximately equal frequency, but rarely arise in the spinal cord. Infratentorial tumours located in the cerebellar hemispheres, cerebellopontine angle, or brainstem are more frequent in the first 2 years of life. AT/RTs are characterized by loss of INI1/SMARCB1/ BAF47 expression due to either germline (25 35%) or to somatic SMARCB1 mutations and/or deletions on chromosome 22q [ ]. INI1 is a core
9 Pediatric CNS tumors 167 member of the adenosine triphosphate (ATP)-dependent SWI/SNF chromatin-remodelling complex. AT/RTs have an extremely low mutation rate, with INI1 alterations the sole recurrent event [134]. Loss of another SWI/SNF chromatin-remodelling complex member, BRG1/SMARCA4, due to SMARCA4 mutation is seen in rare AT/RTs [135]. Loss of nuclear INI1 or BRG1 immunoreactivity is routinely used for the diagnosis of these tumours. If an embryonal tumour has histological features and a polyimmunophenotype to suggest a diagnosis of AT/RT, but expresses both INI1 and BRG1, then a descriptive diagnosis of CNS embryonal tumour with rhabdoid features is available in the 2016 WHO classification [2]. The diagnosis of AT/RT itself requires immunohistochemical confirmation of the characteristic molecular deficit. One recent study has identified three distinct molecular groups of AT/RT (TYR, SHH, and MYC), which are associated with differences in demographics, tumour location, and types of INI1 or epigenetic alteration [136]. AT/RT-TYR tumours are mostly (77%) infratentorial with large INI1 deletions and overexpression of melanosomal genes, such as MITF, TYR (tyrosinase) or DCT. Monosomy 22 is seen in nearly 80% of the tumours. Tyrosinase is highly expressed in almost every case in this subgroup, but not in the other two subgroups, and may therefore serve as a specific marker. More than 80% of AT/RT-TYR tumours occur in patients younger than 1 year of age. The AT/RT-SHH group encompasses both supratentorial (55%) and infratentorial (45%) tumours and harbours focal INI1 or BRG1 alterations and overexpression of SHH pathway genes, including MYCN and GLI2. NOTCH signalling is also active in this group. Genome-wide hypermethylation is seen in both ATRT-TYR and ATRT-SHH subgroups. AT/RT-MYC tumours occur in patients older than 6 years of age and are mostly supratentorial (61%) with focal INI1 deletions and overexpression of MYC and the HOX gene cluster. C19MC-altered embryonal tumour with multilayered rosettes (ETMR) ETMR occurs in young children and is associated with a poor prognosis; nearly all patients die within 1 2 years of diagnosis [137,138]. ETMR encompasses three histopathological patterns, including embryonal tumour with abundant neuropil and true rosettes (ETANTR), ependymoblastoma, and medulloepithelioma [90]. Genome-wide DNA methylation and copy number analyses have demonstrated biological overlap between these three histological entities [139]. ETMR is characterized by amplification of a locus at chromosome 19q13.42 that contains a microrna cluster (C19MC) [140]. A fusion product containing C19MC and a nearby gene TTYH1 has also been identified in ETMR, which likely amplifies the expression of C19MC using the promoter of TTYH1 [141]. Up-regulation of DNMT3B DNA methyltransferase in ETMR may explain its distinctive epigenetic pattern [139]. Interphase fluorescence in situ hybridization (ifish) for C19MC is now routinely used for the diagnosis of this group of aggressive embryonal tumours, a group now defined by its common cytogenetic alteration and designated embryonal tumour with multilayered rosettes, C19MC-altered in the new WHO classification. In the absence of C19MC amplification, a tumour with the typical histological features of an ETMR or ependymoblastoma (5%) should be diagnosed as embryonal tumour with multilayered rosettes, NOS. However, up to one quarter of embryonal tumours with the typical features of a medulloepithelioma lack C19MC amplification [138], so medulloepithelioma remains as a diagnostic entity in the WHO classification. Newly identified CNS embryonal tumour entities Using integrated genomic analyses, principally genome-wide DNA methylation profiling, a recent study has identified four new groups of high-grade CNS tumours [45]. The four new entities are CNS neuroblastoma with FOXR2 activation (CNS NB-FOXR2), CNS Ewing sarcoma family tumour with CIC alteration (CNS EFT-CIC), CNS high-grade neuroepithelial tumour with MN1 alteration (CNS HGNET-MN1), and CNS high-grade neuroepithelial tumour with BCOR alteration (CNS HGNET-BCOR). The CNS NB-FOXR2 group encompasses tumours characterized in most cases by an embryonal cytology, including the morphologies classified as CNS neuroblastoma and CNS ganglioneuroblastoma. Tumours in this group express OLIG2 and synaptophysin. Gain of chromosome arm 1q is associated with the CNS NB-FOXR2 entity (98%), with 50% showing loss of 16q and 30% gain of chromosome 8. Genomic fusion events lead to increased FOXR2 expression in these tumours. The CNS EFT-CIC group also contains a high frequency of tumours with an embryonal cytology, but there is more morphological variability among these tumours. Fusion events or deletion involving CIC are associated with up-regulation of the ETS transcription factor family, including ETV1, ETV4, ETV5, FLI1, and ETS1 in these tumours. The CNS HGNET-MN1 group includes a high proportion of tumours with the morphology ascribed to astroblastomas. These tumours particularly affect female patients and present at an older age and have a better outcome than many CNS embryonal tumours. Genomic rearrangement fuses MN1 with partner genes that include CXXC5 and BEND2. About 30% of the tumours show loss of chromosome 16q, and 16% show gain of chromosome 8. The CNS HGNET-BCOR group shows a variety of CNS tumour histologies and only in rare instances exhibits an embryonal morphology. Most tumours display balanced copy number profiles and BCOR alterations, including in-frame deletion/internal tandem duplications and frameshift mutations, and 79% of tumours demonstrate nuclear β-catenin immunoreactivity. The full clinicopathological profiles of these four tumour groups have yet to be fully elucidated.
10 168 JCH Chiang and DW Ellison Choroid plexus tumours Choroid plexus tumours, including choroid plexus papilloma (CPP, WHO grade I), atypical choroid plexus papilloma (acpp, WHO grade II), and choroid plexus carcinoma (CPC, WHO grade III), are rare intraventricular neoplasms predominantly occurring in children under 2 years of age [126]. No significant differences are observed when the molecular alterations of CPPs and acpps are compared [142]; hyperdiploidy is common in both. Genomic, transcriptomic, and DNA methylation profiling has demonstrated clear segregation of CPCs from CPPs and acpps [142]. Germline alterations of TP53 (Li Fraumeni syndrome) predispose to CPC [143,144]. Somatic TP53 mutations are observed in 60% of CPCs, and increased copies of mutated TP53 are associated with a worse outcome [142]. Deletion of PTEN has also been implicated in CPC [ ]. CPCs are characterized by complex chromosomal alterations associated with patient age and prognosis [147]. Gains on chromosomes 1, 12, and 20; losses of chromosomes 5, 18, and 22; and uniparental disomy (UPD) are common [142,146]. Chromosome 17 is most frequently affected by UPD. About 90% of CPCs exhibiting UPD of chromosome 17 harbour TP53 mutations. Chromosomal losses of 9, 19p, and 22q (hypodiploid CPC) are significantly more frequent in children younger than 3 years old, whereas gains of chromosomes 7, 8q, 14q, 19, and 21q (hyperdiploid CPCs) prevail in older patients [142,147]. TP53 mutations are more common in hypodiploid CPCs (90%) than in hyperdiploid CPCs (50%) [142]. Loss of 12q is associated with a shorter survival [147]. TAF12, NFYC, and RAD54L have been identified as potential oncogenes in CPC [148]. Germ cell tumours Worldwide, intracranial germ cell tumours account for 3 4% of paediatric brain tumours [149]; in Japan, however, their incidence is significantly higher, at over 15%. Pure germinomas are most common, and generally have a good prognosis. KIT mutations are the most common alterations in intracranial germ cell tumours and are more often observed in pure germinomas (25% of cases) than in mixed tumours [150,151]. KRAS and NRAS mutations are also identified. KIT, KRAS, and NRAS mutations are mutually exclusive and together account for about 45% of cases. Recurrent focal amplifications affecting MTOR, CBL, and AKT1 are also found, indicating deregulation of the KIT/RAS and AKT/mTOR pathways in over 50% of intracranial germ cell tumours. Germline variants in JMJD1C are enriched in Japanese patients and may provide a potential explanation for the greater incidence of intracranial germ cell tumours in this ethnic group. JMJD1C is an H3K9 demethylase essential for maintenance of male germ cells [152]. Craniopharyngiomas Craniopharyngiomas account for 3 5% of paediatric brain tumours and two histological subtypes are recognized: adamantinomatous and papillary [2]. The adamantinomatous variant predominates in children, but can arise throughout adulthood. Almost all adamantinomatous craniopharyngiomas harbour an activating mutation in CTNNB1 [153]. Papillary craniopharyngiomas are typically an adult disease and are characterized by BRAF V6000E mutation [154]. Summary The 2016 WHO classification of CNS tumours represents a pivotal moment when molecular alterations have been integrated alongside histopathological features for the first time. Molecular genetic studies have revealed signature molecular alterations: histone H3 mutations for some paediatric high-grade gliomas, RELA fusions for supratentorial ependymomas, loss of INI1/BRG1 expression for AT/RTs, C19MC copy number alteration for ETMRs, and clinically important molecular groups of medulloblastoma. These will be supplemented by others by the time of the next edition and fundamentally alter the perspective of the diagnostic process linking histopathological and molecular testing for the optimum evaluation of paediatric CNS tumours. References 1. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in Neuro Oncol 2014; 16(suppl 4): iv Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016; 131: Listernick R, Ferner RE, Liu GT, et al. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol 2007; 61: Buccoliero AM, Castiglione F, Degl Innocenti DR, et al. IDH1 mutation in pediatric gliomas: has it a diagnostic and prognostic value? Fetal Pediatr Pathol 2012; 31: Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nature Genet 2013; 45: Jones DT, Hutter B, Jager N, et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nature Genet 2013; 45: Pfister S, Janzarik WG, Remke M, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest 2008; 118: Bar EE, Lin A, Tihan T, et al. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol 2008; 67: Sievert AJ, Jackson EM, Gai X, et al. Duplication of 7q34 in pediatric low-grade astrocytomas detected by high-density single-nucleotide polymorphism-based genotype arrays results in a novel BRAF fusion gene. Brain Pathol 2009; 19:
Neuropathology Evening Session: Case 3
 Neuropathology Evening Session: Case 3 Christine E. Fuller, MD Cincinnati Children s Hospital Medical Center Disclosure of Relevant Financial Relationships USCAP requires that all faculty in a position
Neuropathology Evening Session: Case 3 Christine E. Fuller, MD Cincinnati Children s Hospital Medical Center Disclosure of Relevant Financial Relationships USCAP requires that all faculty in a position
2017 Diagnostic Slide Session Case 3
 2017 Diagnostic Slide Session Case 3 Andrew Gao, MD Lili-Naz Hazrati, MD, PhD Cynthia Hawkins, MD, PhD Hospital for Sick Children and University of Toronto, Toronto, Canada Disclosures: none Clinical History
2017 Diagnostic Slide Session Case 3 Andrew Gao, MD Lili-Naz Hazrati, MD, PhD Cynthia Hawkins, MD, PhD Hospital for Sick Children and University of Toronto, Toronto, Canada Disclosures: none Clinical History
Gliomas in the 2016 WHO Classification of CNS Tumors
 Gliomas in the 2016 WHO Classification of CNS Tumors Hindi N Al-Hindi, MD, FCAP Consultant Neuropathologist and Head Section of Anatomic Pathology Department of Pathology and Laboratory Medicine King Faisal
Gliomas in the 2016 WHO Classification of CNS Tumors Hindi N Al-Hindi, MD, FCAP Consultant Neuropathologist and Head Section of Anatomic Pathology Department of Pathology and Laboratory Medicine King Faisal
Childhood Brain and Spinal Cord Tumors Treatment Overview (PDQ )
 1 di 8 04/03/2017 07.31 NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute
1 di 8 04/03/2017 07.31 NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute
Genomic analysis of childhood High grade glial (HGG) brain tumors
 Genomic analysis of childhood High grade glial (HGG) brain tumors Linda D Cooley Children s Mercy, Kansas City The Children s Mercy Hospital, 2017 Genomic analysis of childhood High grade glial (HGG) brain
Genomic analysis of childhood High grade glial (HGG) brain tumors Linda D Cooley Children s Mercy, Kansas City The Children s Mercy Hospital, 2017 Genomic analysis of childhood High grade glial (HGG) brain
CNS pathology Third year medical students. Dr Heyam Awad 2018 Lecture 12: CNS tumours 2/3
 CNS pathology Third year medical students Dr Heyam Awad 2018 Lecture 12: CNS tumours 2/3 Pilocytic astrocytoma Relatively benign ( WHO grade 1) Occurs in children and young adults Mostly: in the cerebellum
CNS pathology Third year medical students Dr Heyam Awad 2018 Lecture 12: CNS tumours 2/3 Pilocytic astrocytoma Relatively benign ( WHO grade 1) Occurs in children and young adults Mostly: in the cerebellum
CNS TUMORS. D r. Ali Eltayb ( U. of Omdurman. I ). M. Path (U. of Alexandria)
 CNS TUMORS D r. Ali Eltayb ( U. of Omdurman. I ). M. Path (U. of Alexandria) CNS TUMORS The annual incidence of intracranial tumors of the CNS ISmore than intraspinal tumors May be Primary or Secondary
CNS TUMORS D r. Ali Eltayb ( U. of Omdurman. I ). M. Path (U. of Alexandria) CNS TUMORS The annual incidence of intracranial tumors of the CNS ISmore than intraspinal tumors May be Primary or Secondary
Tumors of the Nervous System
 Tumors of the Nervous System Peter Canoll MD. PhD. What I want to cover What are the most common types of brain tumors? Who gets them? How do they present? What do they look like? How do they behave? 1
Tumors of the Nervous System Peter Canoll MD. PhD. What I want to cover What are the most common types of brain tumors? Who gets them? How do they present? What do they look like? How do they behave? 1
Classification of Diffuse Gliomas: Progress, Pearls and Pitfalls. Rob Macaulay Neuropathologist, MCC October 21, 2017
 Classification of Diffuse Gliomas: Progress, Pearls and Pitfalls Rob Macaulay Neuropathologist, MCC October 21, 2017 Objectives Explain why the designation high grade glioma is preferable to GBM for intraoperative
Classification of Diffuse Gliomas: Progress, Pearls and Pitfalls Rob Macaulay Neuropathologist, MCC October 21, 2017 Objectives Explain why the designation high grade glioma is preferable to GBM for intraoperative
WHO 2016 CNS Tumor Classification Update. DISCLOSURES (Arie Perry, MD) PATTERN RECOGNITION. Arie Perry, M.D. Director, Neuropathology
 WHO 2016 CNS Tumor Classification Update Arie Perry, M.D. Director, Neuropathology DISCLOSURES (Arie Perry, MD) I have no financial relationships to disclose. - and - I will not discuss off label use or
WHO 2016 CNS Tumor Classification Update Arie Perry, M.D. Director, Neuropathology DISCLOSURES (Arie Perry, MD) I have no financial relationships to disclose. - and - I will not discuss off label use or
Pr D.Figarella-Branger Service d Anatomie Pathologique et de Neuropathologie, La Timone, Marseille UMR 911 Inserm, Université d Aix-Marseille
 Novelties in the WHO 2016 classification of brain tumours Pr D.Figarella-Branger Service d Anatomie Pathologique et de Neuropathologie, La Timone, Marseille UMR 911 Inserm, Université d Aix-Marseille The
Novelties in the WHO 2016 classification of brain tumours Pr D.Figarella-Branger Service d Anatomie Pathologique et de Neuropathologie, La Timone, Marseille UMR 911 Inserm, Université d Aix-Marseille The
Peter Canoll MD. PhD.
 Tumors of the Nervous System Peter Canoll MD. PhD. What I want to cover What are the most common types of brain tumors? Who gets them? How do they ypresent? What do they look like? How do they behave?
Tumors of the Nervous System Peter Canoll MD. PhD. What I want to cover What are the most common types of brain tumors? Who gets them? How do they ypresent? What do they look like? How do they behave?
Case 1. Maysa Al-Hussaini MD FRCPath
 Case 1 Maysa Al-Hussaini MD FRCPath MAYSA King AL-HUSSAINI Hussein Cancer MD Center MRCPATH KING HUSSEIN Amman CANCER Jordan CENTER Clinical history 4 year old boy History of frontal headache, sleepiness.
Case 1 Maysa Al-Hussaini MD FRCPath MAYSA King AL-HUSSAINI Hussein Cancer MD Center MRCPATH KING HUSSEIN Amman CANCER Jordan CENTER Clinical history 4 year old boy History of frontal headache, sleepiness.
Tumours of the Central Nervous System (CNS) Molecular Information Reporting Guide
 Sponsored by Tumours of the Central Nervous System (CNS) Molecular Information Reporting Guide Family/Last name Given name(s) Date of birth DD MM YYYY Patient identifiers Date of request Accession/Laboratory
Sponsored by Tumours of the Central Nervous System (CNS) Molecular Information Reporting Guide Family/Last name Given name(s) Date of birth DD MM YYYY Patient identifiers Date of request Accession/Laboratory
WHO 2016 CNS TUMOR CLASSIFICATION UPDATE. Arie Perry, M.D. Director, Neuropathology
 WHO 2016 CNS TUMOR CLASSIFICATION UPDATE Arie Perry, M.D. Director, Neuropathology DISCLOSURES (Arie Perry, MD) I have no financial relationships to disclose. - and - I will not discuss off label use or
WHO 2016 CNS TUMOR CLASSIFICATION UPDATE Arie Perry, M.D. Director, Neuropathology DISCLOSURES (Arie Perry, MD) I have no financial relationships to disclose. - and - I will not discuss off label use or
Diagnostic application of SNParrays to brain cancers
 Diagnostic application of SNParrays to brain cancers Adriana Olar 4/17/2018 No disclosures 55 yo M, focal motor seizure T2 T1-post C DIAGNOSIS BRAIN, LEFT FRONTAL LOBE, BIOPSY: - DIFFUSE GLIOMA, OLIGODENDROGLIAL
Diagnostic application of SNParrays to brain cancers Adriana Olar 4/17/2018 No disclosures 55 yo M, focal motor seizure T2 T1-post C DIAGNOSIS BRAIN, LEFT FRONTAL LOBE, BIOPSY: - DIFFUSE GLIOMA, OLIGODENDROGLIAL
SYSTEMIC MANAGEMENT OF PEDIATRIC PRIMARY BRAIN TUMORS
 SYSTEMIC MANAGEMENT OF PEDIATRIC PRIMARY BRAIN TUMORS María E. Echevarría, MD Assistant Professor University of Puerto Rico Medical Sciences Campus DISCLOSURES No disclosures INTRODUCTION Pediatric CNS
SYSTEMIC MANAGEMENT OF PEDIATRIC PRIMARY BRAIN TUMORS María E. Echevarría, MD Assistant Professor University of Puerto Rico Medical Sciences Campus DISCLOSURES No disclosures INTRODUCTION Pediatric CNS
Pediatric CNS Tumors. Disclosures. Acknowledgements. Introduction. Introduction. Posterior Fossa Tumors. Whitney Finke, MD
 Pediatric CNS Tumors Disclosures Whitney Finke, MD Neuroradiology Fellow PGY-6 University of Utah Health Sciences Center Salt Lake City, Utah None Acknowledgements Introduction Nicholas A. Koontz, MD Luke
Pediatric CNS Tumors Disclosures Whitney Finke, MD Neuroradiology Fellow PGY-6 University of Utah Health Sciences Center Salt Lake City, Utah None Acknowledgements Introduction Nicholas A. Koontz, MD Luke
General: Brain tumors are lesions that have mass effect distorting the normal tissue and often result in increased intracranial pressure.
 1 Lecture Objectives Know the histologic features of the most common tumors of the CNS. Know the differences in behavior of the different tumor types. Be aware of the treatment modalities in the various
1 Lecture Objectives Know the histologic features of the most common tumors of the CNS. Know the differences in behavior of the different tumor types. Be aware of the treatment modalities in the various
The new WHO 2016 classification of brain tumors what neurosurgeons need to know
 DOI 10.1007/s00701-016-3062-3 REVIEW ARTICLE - BRAIN TUMORS The new WHO 2016 classification of brain tumors what neurosurgeons need to know Rouzbeh Banan 1 & Christian Hartmann 1 Received: 8 July 2016
DOI 10.1007/s00701-016-3062-3 REVIEW ARTICLE - BRAIN TUMORS The new WHO 2016 classification of brain tumors what neurosurgeons need to know Rouzbeh Banan 1 & Christian Hartmann 1 Received: 8 July 2016
Pediatric Brain Tumors: Innovative Genomic Information Is Transforming the Diagnostic and Clinical Landscape
 VOLUME 33 NUMBER 27 SEPTEMBER 20 2015 JOURNAL OF CLINICAL ONCOLOGY R E V I E W A R T I C L E Pediatric Brain Tumors: Innovative Genomic Information Is Transforming the Diagnostic and Clinical Landscape
VOLUME 33 NUMBER 27 SEPTEMBER 20 2015 JOURNAL OF CLINICAL ONCOLOGY R E V I E W A R T I C L E Pediatric Brain Tumors: Innovative Genomic Information Is Transforming the Diagnostic and Clinical Landscape
Applications of molecular neuro-oncology - a review of diffuse glioma integrated diagnosis and emerging molecular entities
 Wood et al. Diagnostic Pathology (2019) 14:29 https://doi.org/10.1186/s13000-019-0802-8 REVIEW Applications of molecular neuro-oncology - a review of diffuse glioma integrated diagnosis and emerging molecular
Wood et al. Diagnostic Pathology (2019) 14:29 https://doi.org/10.1186/s13000-019-0802-8 REVIEW Applications of molecular neuro-oncology - a review of diffuse glioma integrated diagnosis and emerging molecular
Karl Kashofer, Phd Institut für Pathologie Medizinische Universität Graz
 Expanding on WHO guideline compliant molecular testing of central nervous system tumors by low density whole genome sequencing. Karl Kashofer, Phd Institut für Pathologie Medizinische Universität Graz
Expanding on WHO guideline compliant molecular testing of central nervous system tumors by low density whole genome sequencing. Karl Kashofer, Phd Institut für Pathologie Medizinische Universität Graz
Supra- and infratentorial brain tumors from childhood to maternity
 Supra- and infratentorial brain tumors from childhood to maternity What to expect? I am going to show you the characteristic imaging findings of following tumors: Thierry A.G.M. Huisman, MD, FICIS, EQNR
Supra- and infratentorial brain tumors from childhood to maternity What to expect? I am going to show you the characteristic imaging findings of following tumors: Thierry A.G.M. Huisman, MD, FICIS, EQNR
Site Specific Coding Rules MALIGNANT CENTRAL NERVOUS SYSTEM TUMORS
 Multiple Primary and Histology Site Specific Coding Rules MALIGNANT CENTRAL NERVOUS SYSTEM TUMORS 1 Prerequisites 2 Completion of Multiple Primary and Histology General Coding Rules 3 There are many ways
Multiple Primary and Histology Site Specific Coding Rules MALIGNANT CENTRAL NERVOUS SYSTEM TUMORS 1 Prerequisites 2 Completion of Multiple Primary and Histology General Coding Rules 3 There are many ways
Histopathological Study and Categorisation of Brain Tumors
 Histopathological Study and Categorisation of Brain Tumors Ruchira Wadhwa 1*, Purvi Patel 2, Hansa Goswami 3 1 Third Year Resident, 2 Assistant Professor, 3 Professor and Head, Department of Pathology,
Histopathological Study and Categorisation of Brain Tumors Ruchira Wadhwa 1*, Purvi Patel 2, Hansa Goswami 3 1 Third Year Resident, 2 Assistant Professor, 3 Professor and Head, Department of Pathology,
Supplemental Information. Molecular, Pathological, Radiological, and Immune. Profiling of Non-brainstem Pediatric High-Grade
 Cancer Cell, Volume 33 Supplemental Information Molecular, Pathological, Radiological, and Immune Profiling of Non-brainstem Pediatric High-Grade Glioma from the HERBY Phase II Randomized Trial Alan Mackay,
Cancer Cell, Volume 33 Supplemental Information Molecular, Pathological, Radiological, and Immune Profiling of Non-brainstem Pediatric High-Grade Glioma from the HERBY Phase II Randomized Trial Alan Mackay,
I have no conflicts of interest in relation to this presentation. Vogel FS & Burger PC 3/28/2016
 IF THIS IS NOT GLIOBLASTOMA, THEN WHAT IS IT? Murat Gokden, MD Department of Pathology/Neuropathology University of Arkansas for Medical Sciences Little Rock, AR mgokden@uams.edu I have no conflicts of
IF THIS IS NOT GLIOBLASTOMA, THEN WHAT IS IT? Murat Gokden, MD Department of Pathology/Neuropathology University of Arkansas for Medical Sciences Little Rock, AR mgokden@uams.edu I have no conflicts of
Clinical significance of genetic analysis in glioblastoma treatment
 Clinical significance of genetic analysis in glioblastoma treatment Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan Koji Yoshimoto Can we get prognostic
Clinical significance of genetic analysis in glioblastoma treatment Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan Koji Yoshimoto Can we get prognostic
Clinically Useful Next Generation Sequencing and Molecular Testing in Gliomas MacLean P. Nasrallah, MD PhD
 Clinically Useful Next Generation Sequencing and Molecular Testing in Gliomas MacLean P. Nasrallah, MD PhD Neuropathology Fellow Division of Neuropathology Center for Personalized Diagnosis (CPD) Glial
Clinically Useful Next Generation Sequencing and Molecular Testing in Gliomas MacLean P. Nasrallah, MD PhD Neuropathology Fellow Division of Neuropathology Center for Personalized Diagnosis (CPD) Glial
Review: Diagnostic, prognostic and predictive relevance of molecular markers in gliomas
 Review: Diagnostic, prognostic and predictive relevance of molecular markers in gliomas Sebastian Brandner MD FRCPath 1 and Andreas von Deimling 2 MD 1) Division of Neuropathology, The National Hospital
Review: Diagnostic, prognostic and predictive relevance of molecular markers in gliomas Sebastian Brandner MD FRCPath 1 and Andreas von Deimling 2 MD 1) Division of Neuropathology, The National Hospital
Cynthia Hawkins. Division of Pathology, Labatt Brain Tumour Research Centre, The Hospital for Sick Children, University of Toronto, Canada
 Cynthia Hawkins Division of Pathology, Labatt Brain Tumour Research Centre, The Hospital for Sick Children, University of Toronto, Canada To apply a practical diagnostic approach to pediatric high grade
Cynthia Hawkins Division of Pathology, Labatt Brain Tumour Research Centre, The Hospital for Sick Children, University of Toronto, Canada To apply a practical diagnostic approach to pediatric high grade
Updates on the CNS classifica3on of brain tumors
 27 th Annual Mee-ng of the IAP-AD "New Horizons in Surgical Pathology Conrad Dubai, UAE Friday, 27 November 2015 Updates on the CNS classifica3on of brain tumors Eyas M Ha=ab, MD Department of Pathology
27 th Annual Mee-ng of the IAP-AD "New Horizons in Surgical Pathology Conrad Dubai, UAE Friday, 27 November 2015 Updates on the CNS classifica3on of brain tumors Eyas M Ha=ab, MD Department of Pathology
Tumors of the Central Nervous System
 Tumors of the Central Nervous System 1 Financial Disclosures I have NO SIGNIFICANT FINANCIAL, GENERAL, OR OBLIGATION INTERESTS TO REPORT Introduction General: Brain tumors are lesions that have mass effect
Tumors of the Central Nervous System 1 Financial Disclosures I have NO SIGNIFICANT FINANCIAL, GENERAL, OR OBLIGATION INTERESTS TO REPORT Introduction General: Brain tumors are lesions that have mass effect
Pleomorphic Xanthoastrocytoma
 Pleomorphic Xanthoastrocytoma Christine E. Fuller Keywords Pleomorphic xanthoastrocytoma; Pleomorphic xanthoastrocytoma with anaplastic features 2.1 OVERVIEW Pleomorphic xanthoastrocytoma (PXA) is an uncommon
Pleomorphic Xanthoastrocytoma Christine E. Fuller Keywords Pleomorphic xanthoastrocytoma; Pleomorphic xanthoastrocytoma with anaplastic features 2.1 OVERVIEW Pleomorphic xanthoastrocytoma (PXA) is an uncommon
Genomic Methods in Cancer Epigenetic Dysregulation
 Genomic Methods in Cancer Epigenetic Dysregulation Clara, Lyon 2018 Jacek Majewski, Associate Professor Department of Human Genetics, McGill University Montreal, Canada A few words about my lab Genomics
Genomic Methods in Cancer Epigenetic Dysregulation Clara, Lyon 2018 Jacek Majewski, Associate Professor Department of Human Genetics, McGill University Montreal, Canada A few words about my lab Genomics
MOLECULAR DIAGNOSTICS OF GLIOMAS
 MOLECULAR DIAGNOSTICS OF GLIOMAS Arie Perry, M.D. Director, Neuropathology Division DIFFUSE GLIOMAS Cell types Astrocytomas (A) Oligodendrogliomas (O) Mixed oligoastrocytoma (MOA) Three WHO grades: II,
MOLECULAR DIAGNOSTICS OF GLIOMAS Arie Perry, M.D. Director, Neuropathology Division DIFFUSE GLIOMAS Cell types Astrocytomas (A) Oligodendrogliomas (O) Mixed oligoastrocytoma (MOA) Three WHO grades: II,
SUPPLEMENTARY INFORMATION
 VOLUME: 1 ARTICLE NUMBER: 0027 In the format provided by the authors and unedited. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy
VOLUME: 1 ARTICLE NUMBER: 0027 In the format provided by the authors and unedited. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy
Molecular Testing of Brain Tumor
 Journal of Pathology and Translational Medicine 2017; 51: 205-223 REVIEW Molecular Testing of Brain Tumor Sung-Hye Park 1,2 Jaekyung Won 1 Seong-Ik Kim 1 Yujin Lee 1 Chul-Kee Park 3 Seung-Ki Kim 3 Seung-Hong
Journal of Pathology and Translational Medicine 2017; 51: 205-223 REVIEW Molecular Testing of Brain Tumor Sung-Hye Park 1,2 Jaekyung Won 1 Seong-Ik Kim 1 Yujin Lee 1 Chul-Kee Park 3 Seung-Ki Kim 3 Seung-Hong
The mutations that drive cancer. Paul Edwards. Department of Pathology and Cancer Research UK Cambridge Institute, University of Cambridge
 The mutations that drive cancer Paul Edwards Department of Pathology and Cancer Research UK Cambridge Institute, University of Cambridge Previously on Cancer... hereditary predisposition Normal Cell Slightly
The mutations that drive cancer Paul Edwards Department of Pathology and Cancer Research UK Cambridge Institute, University of Cambridge Previously on Cancer... hereditary predisposition Normal Cell Slightly
Brain Tumors. Medulloblastoma. Pilocytic astrocytoma: Ahmed Koriesh, MD. Pathological finding
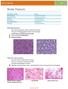 NeuroPathology Page 8 Brain Tumors Pathological finding Pseudorosette Rosenthal fibers Rosettes Wet Keratin Psammoma bodies Fried egg Tumor Ependymoma, SEGA Pilocytic astrocytoma Medulloblastoma Craniopharyngioma
NeuroPathology Page 8 Brain Tumors Pathological finding Pseudorosette Rosenthal fibers Rosettes Wet Keratin Psammoma bodies Fried egg Tumor Ependymoma, SEGA Pilocytic astrocytoma Medulloblastoma Craniopharyngioma
2018 Diagnostic Slide Session Case #8
 2018 Diagnostic Slide Session Case #8 Angela N. Viaene, MacLean P. Nasrallah, and Zissimos Mourelatos Hospital of the University of Pennsylvania AANP June 9, 2018 Disclosures: none Clinical History Healthy,
2018 Diagnostic Slide Session Case #8 Angela N. Viaene, MacLean P. Nasrallah, and Zissimos Mourelatos Hospital of the University of Pennsylvania AANP June 9, 2018 Disclosures: none Clinical History Healthy,
Morphological features and genetic alterations
 Morphological features and genetic alterations Tutor : Audrey Rousseau Caget Lise: Université d Angers Iorio Vittoria: Seconda Università degli studi di Napoli Manaila Roxana: Iuliu Hatieganu University
Morphological features and genetic alterations Tutor : Audrey Rousseau Caget Lise: Université d Angers Iorio Vittoria: Seconda Università degli studi di Napoli Manaila Roxana: Iuliu Hatieganu University
Pediatric Brain Tumors: Updates in Treatment and Care
 Pediatric Brain Tumors: Updates in Treatment and Care Writer Classroom Rishi R. Lulla, MD MS Objectives Introduce the common pediatric brain tumors Discuss current treatment strategies for pediatric brain
Pediatric Brain Tumors: Updates in Treatment and Care Writer Classroom Rishi R. Lulla, MD MS Objectives Introduce the common pediatric brain tumors Discuss current treatment strategies for pediatric brain
Development of Carcinoma Pathways
 The Construction of Genetic Pathway to Colorectal Cancer Moriah Wright, MD Clinical Fellow in Colorectal Surgery Creighton University School of Medicine Management of Colon and Diseases February 23, 2019
The Construction of Genetic Pathway to Colorectal Cancer Moriah Wright, MD Clinical Fellow in Colorectal Surgery Creighton University School of Medicine Management of Colon and Diseases February 23, 2019
Astroblastoma: Radiologic-Pathologic Correlation and Distinction from Ependymoma
 AJNR Am J Neuroradiol 23:243 247, February 2002 Case Report Astroblastoma: Radiologic-Pathologic Correlation and Distinction from Ependymoma John D. Port, Daniel J. Brat, Peter C. Burger, and Martin G.
AJNR Am J Neuroradiol 23:243 247, February 2002 Case Report Astroblastoma: Radiologic-Pathologic Correlation and Distinction from Ependymoma John D. Port, Daniel J. Brat, Peter C. Burger, and Martin G.
Biomarkers in Neuro-Ophthalmic Tumors
 Biomarkers in Neuro-Ophthalmic Tumors Fausto J. Rodríguez MD Department of Pathology Johns Hopkins University School of Medicine Disclosure of Relevant Financial Relationships Disclosure of Relevant Financial
Biomarkers in Neuro-Ophthalmic Tumors Fausto J. Rodríguez MD Department of Pathology Johns Hopkins University School of Medicine Disclosure of Relevant Financial Relationships Disclosure of Relevant Financial
The 2016 WHO classification of central nervous system tumors: what neurologists need to know
 REVIEW C URRENT OPINION The 2016 WHO classification of central nervous system tumors: what neurologists need to know John C. DeWitt a,, Andreas Mock a,b,c,, and David N. Louis a Purpose of review The 2016
REVIEW C URRENT OPINION The 2016 WHO classification of central nervous system tumors: what neurologists need to know John C. DeWitt a,, Andreas Mock a,b,c,, and David N. Louis a Purpose of review The 2016
Enterprise Interest None
 Enterprise Interest None Heterogeneous chromosomal profiles in a unique series of DIPG in children and young adults European Congress of Pathology Amsterdam, 6 th September 2017 Charlotte Dufour, Romain
Enterprise Interest None Heterogeneous chromosomal profiles in a unique series of DIPG in children and young adults European Congress of Pathology Amsterdam, 6 th September 2017 Charlotte Dufour, Romain
Pathologic Analysis of CNS Surgical Specimens
 2015 Kenneth M. Earle Memorial Neuropathology Review Pathologic Analysis of CNS Surgical Specimens Peter C. Burger, MD Interdisciplinary Quality Control Familiarity with entities Use of diagnostic algorithm
2015 Kenneth M. Earle Memorial Neuropathology Review Pathologic Analysis of CNS Surgical Specimens Peter C. Burger, MD Interdisciplinary Quality Control Familiarity with entities Use of diagnostic algorithm
H3F3A K27M Mutation in Pediatric CNS Tumors. A Marker for Diffuse High-Grade Astrocytomas
 Anatomic Pathology / H3.3 Mutations in Pediatric Diffuse High-Grade Astrocytomas H3F3A K27M Mutation in Pediatric CNS Tumors A Marker for Diffuse High-Grade Astrocytomas Gerrit H. Gielen, MD, 1 Marco Gessi,
Anatomic Pathology / H3.3 Mutations in Pediatric Diffuse High-Grade Astrocytomas H3F3A K27M Mutation in Pediatric CNS Tumors A Marker for Diffuse High-Grade Astrocytomas Gerrit H. Gielen, MD, 1 Marco Gessi,
Disclaimers. Molecular pathology of brain tumors. Some aspects only. Some details are inevitably personal opinions
 Molecular pathology of brain tumors Disclaimers Some aspects only H.K. Ng The Chinese University of Hong Kong Some details are inevitably personal opinions Free ppt : http://www.acp.cuhk.edu.hk/hkng Why
Molecular pathology of brain tumors Disclaimers Some aspects only H.K. Ng The Chinese University of Hong Kong Some details are inevitably personal opinions Free ppt : http://www.acp.cuhk.edu.hk/hkng Why
BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma
 BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma by Matthew R. Mistry A thesis submitted in conformity with the requirements for the degree
BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma by Matthew R. Mistry A thesis submitted in conformity with the requirements for the degree
Molecular Genetics of Paediatric Tumours. Gino Somers MBBS, BMedSci, PhD, FRCPA Pathologist-in-Chief Hospital for Sick Children, Toronto, ON, CANADA
 Molecular Genetics of Paediatric Tumours Gino Somers MBBS, BMedSci, PhD, FRCPA Pathologist-in-Chief Hospital for Sick Children, Toronto, ON, CANADA Financial Disclosure NanoString - conference costs for
Molecular Genetics of Paediatric Tumours Gino Somers MBBS, BMedSci, PhD, FRCPA Pathologist-in-Chief Hospital for Sick Children, Toronto, ON, CANADA Financial Disclosure NanoString - conference costs for
Neuronal and Mixed Neuronal- Glial and Embryonal Tumors
 Neuronal and Mixed Neuronal- Glial and Embryonal Tumors Alexander R. Judkins, MD, FRCP (Edin) Pathologist-in-Chief Children s Hospital Los Angeles Associate Professor (Clinical Scholar) & Vice Chair Department
Neuronal and Mixed Neuronal- Glial and Embryonal Tumors Alexander R. Judkins, MD, FRCP (Edin) Pathologist-in-Chief Children s Hospital Los Angeles Associate Professor (Clinical Scholar) & Vice Chair Department
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES CENTRAL NERVOUS SYSTEM MEDULLOBLASTOMA AND PNET CNS Site Group Medulloblastoma and PNET Author: Dr. Norm Laperriere 1. INTRODUCTION 3 2. PREVENTION
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES CENTRAL NERVOUS SYSTEM MEDULLOBLASTOMA AND PNET CNS Site Group Medulloblastoma and PNET Author: Dr. Norm Laperriere 1. INTRODUCTION 3 2. PREVENTION
Examining large groups of cancer patients to identify ways of predicting which therapies cancers might respond to.
 Stratified Medicine Examining large groups of cancer patients to identify ways of predicting which therapies cancers might respond to. Looking in detail at cancer cells and their genetic make up. Permit
Stratified Medicine Examining large groups of cancer patients to identify ways of predicting which therapies cancers might respond to. Looking in detail at cancer cells and their genetic make up. Permit
Five Most Common Problems in Surgical Neuropathology
 Five Most Common Problems in Surgical Neuropathology If the brain were so simple that we could understand it, we would be so simple that we couldn t Emerson Pugh What is your greatest difficulty in neuropathology?
Five Most Common Problems in Surgical Neuropathology If the brain were so simple that we could understand it, we would be so simple that we couldn t Emerson Pugh What is your greatest difficulty in neuropathology?
Brain Tumors in Children
 Brain Tumors in Children Michael A. Grotzer University Children s Hospital of Zurich, Switzerland Incidence of Childhood Cancer CNS Tumors Acute lymphoblastic Leukemia Neuroblastoma Non-Hodgkin Lymphoma
Brain Tumors in Children Michael A. Grotzer University Children s Hospital of Zurich, Switzerland Incidence of Childhood Cancer CNS Tumors Acute lymphoblastic Leukemia Neuroblastoma Non-Hodgkin Lymphoma
Precision medicine: How to exploit the growing knowledge on the evolving genomes of cells to improve cancer prevention and therapy.
 Precision medicine: How to exploit the growing knowledge on the evolving genomes of cells to improve cancer prevention and therapy Joe Costello, PhD Department of Neurological Surgery A more accurate and
Precision medicine: How to exploit the growing knowledge on the evolving genomes of cells to improve cancer prevention and therapy Joe Costello, PhD Department of Neurological Surgery A more accurate and
Supplementary Information
 Rise in Glioblastoma Multiforme incidence in England 1995 2015 suggests an adverse environmental or lifestyle factor Alasdair Philips, Denis L Henshaw, Graham Lamburn, Michael J O Carroll Supplementary
Rise in Glioblastoma Multiforme incidence in England 1995 2015 suggests an adverse environmental or lifestyle factor Alasdair Philips, Denis L Henshaw, Graham Lamburn, Michael J O Carroll Supplementary
Genomic Analysis in the Practice of Surgical Neuropathology. The Emory Experience
 Genomic Analysis in the Practice of Surgical Neuropathology The Emory Experience Stewart G. Neill, MD; Debra F. Saxe, PhD; Michael R. Rossi, PhD; Matthew J. Schniederjan, MD; Daniel J. Brat, MD, PhD The
Genomic Analysis in the Practice of Surgical Neuropathology The Emory Experience Stewart G. Neill, MD; Debra F. Saxe, PhD; Michael R. Rossi, PhD; Matthew J. Schniederjan, MD; Daniel J. Brat, MD, PhD The
Effective January 1, 2018 ICD O 3 codes, behaviors and terms are site specific
 Effective January 1, 2018 codes, behaviors and terms are site specific /N 8551/3 Acinar adenocarcinoma (C34. _) Lung primaries diagnosed prior to 1/1/2018 use code 8550/3 For prostate (all years) see 8140/3
Effective January 1, 2018 codes, behaviors and terms are site specific /N 8551/3 Acinar adenocarcinoma (C34. _) Lung primaries diagnosed prior to 1/1/2018 use code 8550/3 For prostate (all years) see 8140/3
SPECIAL SLIDE SEMINAR CASE 3
 SPECIAL SLIDE SEMINAR CASE 3 Tihana Džombeta, MD Leo Pažanin, MD, PhD Department of Pathology, School of Medicine, University of Zagreb Department of Pathology, Clinical Hospital Centre Sestre milosrdnice
SPECIAL SLIDE SEMINAR CASE 3 Tihana Džombeta, MD Leo Pažanin, MD, PhD Department of Pathology, School of Medicine, University of Zagreb Department of Pathology, Clinical Hospital Centre Sestre milosrdnice
Brain tumors are, as a group, the most common
 See the corresponding editorial in this issue, pp 133 134. J Neurosurg Pediatrics 8:000 000, 8:135 148, 2011 Multidisciplinary management of childhood brain tumors: a review of outcomes, recent advances,
See the corresponding editorial in this issue, pp 133 134. J Neurosurg Pediatrics 8:000 000, 8:135 148, 2011 Multidisciplinary management of childhood brain tumors: a review of outcomes, recent advances,
Childhood Brain and Spinal Cord Tumors Treatment Overview (PDQ )
 1 di 14 27/11/2016 17.42 NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute
1 di 14 27/11/2016 17.42 NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute
Anaplastic Pilocytic Astrocytoma: The fusion of good and bad
 Anaplastic Pilocytic Astrocytoma: The fusion of good and bad Alexandrina Nikova 1, Charalampos-Chrysovalantis Chytoudis-Peroudis 2, Penelope Korkolopoulou 3 and Dimitrios Kanakis 4 Abstract 5 Pilocytic
Anaplastic Pilocytic Astrocytoma: The fusion of good and bad Alexandrina Nikova 1, Charalampos-Chrysovalantis Chytoudis-Peroudis 2, Penelope Korkolopoulou 3 and Dimitrios Kanakis 4 Abstract 5 Pilocytic
Protocol Abstract and Schema
 Protocol Abstract and Schema A Phase I Trial of p28 (NSC745104), a Non-HDM2 mediated peptide inhibitor of p53 ubiquitination in pediatric patients with recurrent or progressive CNS tumors Description and
Protocol Abstract and Schema A Phase I Trial of p28 (NSC745104), a Non-HDM2 mediated peptide inhibitor of p53 ubiquitination in pediatric patients with recurrent or progressive CNS tumors Description and
2018 ICD-O-3 Updates in Table Format with Annotation for Reference
 Status Histology Description (this may be preferred term or a synonym) Report Comments New term 8010 3 Urachal carcinoma (C65.9, C66.9, C67._, C68._) New term 8013 3 Combined large cell neuroendocrine
Status Histology Description (this may be preferred term or a synonym) Report Comments New term 8010 3 Urachal carcinoma (C65.9, C66.9, C67._, C68._) New term 8013 3 Combined large cell neuroendocrine
Effective January 1, 2018 ICD O 3 codes, behaviors and terms are site specific
 Effective January 1, 2018 codes, behaviors and terms are site specific Status /N 8010/3 Urachal carcinoma (C65.9, C66.9, C67. _, C68._) 8013/3 Combined large cell neuroendocrine carcinoma (C34. _, C37.9)
Effective January 1, 2018 codes, behaviors and terms are site specific Status /N 8010/3 Urachal carcinoma (C65.9, C66.9, C67. _, C68._) 8013/3 Combined large cell neuroendocrine carcinoma (C34. _, C37.9)
The genetic landscape of ganglioglioma
 Pekmezci et al. Acta Neuropathologica Communications (2018) 6:47 https://doi.org/10.1186/s40478-018-0551-z RESEARCH The genetic landscape of ganglioglioma Open Access Melike Pekmezci 1, Javier E. Villanueva-Meyer
Pekmezci et al. Acta Neuropathologica Communications (2018) 6:47 https://doi.org/10.1186/s40478-018-0551-z RESEARCH The genetic landscape of ganglioglioma Open Access Melike Pekmezci 1, Javier E. Villanueva-Meyer
USCAP Pediatrics Evening Subspecialty Conference 2015
 USCAP Pediatrics Evening Subspecialty Conference 2015 Sunday 22 March 2015 Alexander Lazar MD/PhD Department of Pathology S Section of Bone Soft TIssue Pathology Sarcoma Research Center The Case Patient
USCAP Pediatrics Evening Subspecialty Conference 2015 Sunday 22 March 2015 Alexander Lazar MD/PhD Department of Pathology S Section of Bone Soft TIssue Pathology Sarcoma Research Center The Case Patient
The New WHO Classification and the Role of Integrated Molecular Profiling in the Diagnosis of Malignant Gliomas
 The New WHO Classification and the Role of Integrated Molecular Profiling in the Diagnosis of Malignant Gliomas Stefan Prokop, MD Neuropathology Fellow Hospital of the University of Pennsylvania Background
The New WHO Classification and the Role of Integrated Molecular Profiling in the Diagnosis of Malignant Gliomas Stefan Prokop, MD Neuropathology Fellow Hospital of the University of Pennsylvania Background
Nature Genetics: doi: /ng.2995
 Supplementary Figure 1 Kaplan-Meier survival curves of patients with brainstem tumors. (a) Comparison of patients with PPM1D mutation versus wild-type PPM1D. (b) Comparison of patients with PPM1D mutation
Supplementary Figure 1 Kaplan-Meier survival curves of patients with brainstem tumors. (a) Comparison of patients with PPM1D mutation versus wild-type PPM1D. (b) Comparison of patients with PPM1D mutation
Embryonal tumor with multilayered rosettes, C19MC-altered: Report of an extremely rare malignant pediatric central nervous system neoplasm
 745208SCO0010.1177/2050313X17745208SAGE Open Medical Case ReportsTariq et al. case-report2017 Case Report SAGE Open Medical Case Reports Embryonal tumor with multilayered rosettes, C19MC-altered: Report
745208SCO0010.1177/2050313X17745208SAGE Open Medical Case ReportsTariq et al. case-report2017 Case Report SAGE Open Medical Case Reports Embryonal tumor with multilayered rosettes, C19MC-altered: Report
SALSA MLPA probemix P175-A3 Tumour Gain Lot A3-0714: As compared to the previous version A2 (lot A2-0411), nine probes have a small change in length.
 SALSA MLPA probemix P175-A3 Tumour Gain Lot A3-0714: As compared to the previous version A2 (lot A2-0411), nine probes have a small change in length. This SALSA probemix is for basic research only! This
SALSA MLPA probemix P175-A3 Tumour Gain Lot A3-0714: As compared to the previous version A2 (lot A2-0411), nine probes have a small change in length. This SALSA probemix is for basic research only! This
A clinical perspective on neuropathology and molecular genetics in brain tumors
 A clinical perspective on neuropathology and molecular genetics in brain tumors M.J. van den Bent Erasmus MC Cancer Institute Rotterdam, the Netherlands Disclosures Member speakersbureau: MSD Consultancy:
A clinical perspective on neuropathology and molecular genetics in brain tumors M.J. van den Bent Erasmus MC Cancer Institute Rotterdam, the Netherlands Disclosures Member speakersbureau: MSD Consultancy:
What yield in the last decade about Molecular Diagnostics in Neuro
 What yield in the last decade about Molecular Diagnostics in Neuro Oncology? Raphael Salles S.Medeiros Neuropathologist at HC FMUSP Clinical Research Project Manager at Oncology department at Hospital
What yield in the last decade about Molecular Diagnostics in Neuro Oncology? Raphael Salles S.Medeiros Neuropathologist at HC FMUSP Clinical Research Project Manager at Oncology department at Hospital
Brain tumors: tumor types
 Brain tumors: tumor types Tumor types There are more than 120 types of brain tumors. Today, most medical institutions use the World Health Organization (WHO) classification system to identify brain tumors.
Brain tumors: tumor types Tumor types There are more than 120 types of brain tumors. Today, most medical institutions use the World Health Organization (WHO) classification system to identify brain tumors.
MALIGNANT GLIOMAS: TREATMENT AND CHALLENGES
 MALIGNANT GLIOMAS: TREATMENT AND CHALLENGES DISCLOSURE No conflicts of interest to disclose Patricia Bruns APRN, CNS Givens Brain Tumor Center Abbott Northwestern Hospital October 12, 2018 OBJECTIVES THEN
MALIGNANT GLIOMAS: TREATMENT AND CHALLENGES DISCLOSURE No conflicts of interest to disclose Patricia Bruns APRN, CNS Givens Brain Tumor Center Abbott Northwestern Hospital October 12, 2018 OBJECTIVES THEN
Chapter 1 Introduction
 Chapter 1 Introduction Men think epilepsy divine, merely because they do not understand it. But if they called everything divine which they do not understand, why, there would be no end to divine things.
Chapter 1 Introduction Men think epilepsy divine, merely because they do not understand it. But if they called everything divine which they do not understand, why, there would be no end to divine things.
Q&A. Fabulous Prizes. Collecting Cancer Data:CNS 2/7/12. NAACCR Webinar Series Collecting Cancer Data Central Nervous System
 Collecting Cancer Data Central Nervous System NAACCR 2012 2013 Webinar Series 2/7/2013 Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants
Collecting Cancer Data Central Nervous System NAACCR 2012 2013 Webinar Series 2/7/2013 Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants
R1601 Essential Immunohistochemical and Molecular Markers for General CNS Glial Tumors
 October 22, 2018 12:00-1:00 PM Background The World Health Organization Classification of tumors of the Central Nervous System has recently been revised. There is now greater emphasis on molecular phenotype
October 22, 2018 12:00-1:00 PM Background The World Health Organization Classification of tumors of the Central Nervous System has recently been revised. There is now greater emphasis on molecular phenotype
Key Words: Brain tumor, BRAF, Glioneuronal, MAPK, Pilocytic astrocytoma.
 J Neuropathol Exp Neurol Copyright Ó 2012 by the American Association of Neuropathologists, Inc. Vol. 71, No. 1 January 2012 pp. 66Y72 ORIGINAL ARTICLE BRAF Alterations in Primary Glial and Glioneuronal
J Neuropathol Exp Neurol Copyright Ó 2012 by the American Association of Neuropathologists, Inc. Vol. 71, No. 1 January 2012 pp. 66Y72 ORIGINAL ARTICLE BRAF Alterations in Primary Glial and Glioneuronal
Advances in Brain Tumor Research: Leveraging BIG data for BIG discoveries
 Advances in Brain Tumor Research: Leveraging BIG data for BIG discoveries Jill Barnholtz-Sloan, PhD Associate Professor & Associate Director for Bioinformatics and Translational Informatics jsb42@case.edu
Advances in Brain Tumor Research: Leveraging BIG data for BIG discoveries Jill Barnholtz-Sloan, PhD Associate Professor & Associate Director for Bioinformatics and Translational Informatics jsb42@case.edu
Advances In Orbital Neuropathology
 Advances In Orbital Neuropathology Charles G. Eberhart, MD PhD Associate Professor of Pathology, Ophthalmology and Oncology Johns Hopkins University School of Medicine Overview Non-neoplastic lesions Microphthalmos/pseudoglioma
Advances In Orbital Neuropathology Charles G. Eberhart, MD PhD Associate Professor of Pathology, Ophthalmology and Oncology Johns Hopkins University School of Medicine Overview Non-neoplastic lesions Microphthalmos/pseudoglioma
The Cancer Genome Atlas Research Network* abstract
 The new england journal of medicine established in 1812 June 25, 2015 vol. 372 no. 26 Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas The Cancer Genome Atlas Research Network*
The new england journal of medicine established in 1812 June 25, 2015 vol. 372 no. 26 Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas The Cancer Genome Atlas Research Network*
SYLLABUS SWI/SNF-dependent tumors
 SYLLABUS SWI/SNF-dependent tumors F Le Loarer, MD, PhD Centre Leon Berard, Department of Pathology, Lyon, FRANCE Since the seminal description in 1999 of recurrent inactivation of SMARCB1- which encodes
SYLLABUS SWI/SNF-dependent tumors F Le Loarer, MD, PhD Centre Leon Berard, Department of Pathology, Lyon, FRANCE Since the seminal description in 1999 of recurrent inactivation of SMARCB1- which encodes
Tumor suppressor genes D R. S H O S S E I N I - A S L
 Tumor suppressor genes 1 D R. S H O S S E I N I - A S L What is a Tumor Suppressor Gene? 2 A tumor suppressor gene is a type of cancer gene that is created by loss-of function mutations. In contrast to
Tumor suppressor genes 1 D R. S H O S S E I N I - A S L What is a Tumor Suppressor Gene? 2 A tumor suppressor gene is a type of cancer gene that is created by loss-of function mutations. In contrast to
Difficult Diagnoses and Controversial Entities in Neoplastic Lung
 Difficult Diagnoses and Controversial Entities in Neoplastic Lung Lynette M. Sholl, M.D. Associate Pathologist, Brigham and Women s Hospital Chief, Pulmonary Pathology Service Associate Professor, Harvard
Difficult Diagnoses and Controversial Entities in Neoplastic Lung Lynette M. Sholl, M.D. Associate Pathologist, Brigham and Women s Hospital Chief, Pulmonary Pathology Service Associate Professor, Harvard
TUMORS of nervous system
 TUMORS of nervous system By: Shifaa Alqa qa Done By : Ola Hijjawi CNS tumors : The annual incidence of CNS tumors ranges from 10 to 17 per 100,000 persons for intracranial tumors and 1 to 2 per 100,000
TUMORS of nervous system By: Shifaa Alqa qa Done By : Ola Hijjawi CNS tumors : The annual incidence of CNS tumors ranges from 10 to 17 per 100,000 persons for intracranial tumors and 1 to 2 per 100,000
Case Presentation: USCAP Jason T. Huse, MD, PhD Assistant Member Department of Pathology Memorial Sloan Kettering Cancer Center
 Case Presentation: USCAP 2016 Jason T. Huse, MD, PhD Assistant Member Department of Pathology Memorial Sloan Kettering Cancer Center Case History 53 year old female with a long standing history of migraines
Case Presentation: USCAP 2016 Jason T. Huse, MD, PhD Assistant Member Department of Pathology Memorial Sloan Kettering Cancer Center Case History 53 year old female with a long standing history of migraines
Cancer. The fundamental defect is. unregulated cell division. Properties of Cancerous Cells. Causes of Cancer. Altered growth and proliferation
 Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Phase II Pediatric Study With Dabrafenib in Combination With Trametinib in Patients With HGG and LGG
 Find Studies About Studies Submit Studies Resources About Site Phase II Pediatric Study With Dabrafenib in Combination With Trametinib in Patients With HGG and LGG The safety and scientific validity of
Find Studies About Studies Submit Studies Resources About Site Phase II Pediatric Study With Dabrafenib in Combination With Trametinib in Patients With HGG and LGG The safety and scientific validity of
Overview of Brain Tumors ICRO 2015 SRMS IMS, Bareilly Prof Kamal Sahni
 Overview of Brain Tumors ICRO 2015 SRMS IMS, Bareilly Prof Kamal Sahni 1 Overview of Brain Tumors Incidence, prevalence & mortality Metastatic vs. Primary CNS tumors =10:1 World wide incidence of Primary
Overview of Brain Tumors ICRO 2015 SRMS IMS, Bareilly Prof Kamal Sahni 1 Overview of Brain Tumors Incidence, prevalence & mortality Metastatic vs. Primary CNS tumors =10:1 World wide incidence of Primary
Predictive biomarker profiling of > 1,900 sarcomas: Identification of potential novel treatment modalities
 Predictive biomarker profiling of > 1,900 sarcomas: Identification of potential novel treatment modalities Sujana Movva 1, Wenhsiang Wen 2, Wangjuh Chen 2, Sherri Z. Millis 2, Margaret von Mehren 1, Zoran
Predictive biomarker profiling of > 1,900 sarcomas: Identification of potential novel treatment modalities Sujana Movva 1, Wenhsiang Wen 2, Wangjuh Chen 2, Sherri Z. Millis 2, Margaret von Mehren 1, Zoran
In 1988 Dumas-Duport et al. first used
 Copyright 2009, Barrow Neurological Institute Dysembryoplastic Neuroepithelial Tumor: A Review Mark Garrett, MD Jennifer Eschbacher, MD Peter Nakaji, MD Most DNETs are benign, low-grade lesions. However,
Copyright 2009, Barrow Neurological Institute Dysembryoplastic Neuroepithelial Tumor: A Review Mark Garrett, MD Jennifer Eschbacher, MD Peter Nakaji, MD Most DNETs are benign, low-grade lesions. However,
Cancer. The fundamental defect is. unregulated cell division. Properties of Cancerous Cells. Causes of Cancer. Altered growth and proliferation
 Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
CHAPTER 11 Tumors Originating in the Brain Medulloblastomas, PNETs and Ependymomas
 Tumors Originating in the Brain Medulloblastomas, PNETs and Ependymomas Foolishly, I waited 7 months before I joined this (or any) group. By that time, my son had radiation, chemo, and a recurrence of
Tumors Originating in the Brain Medulloblastomas, PNETs and Ependymomas Foolishly, I waited 7 months before I joined this (or any) group. By that time, my son had radiation, chemo, and a recurrence of
Molecular Markers in Acute Leukemia. Dr Muhd Zanapiah Zakaria Hospital Ampang
 Molecular Markers in Acute Leukemia Dr Muhd Zanapiah Zakaria Hospital Ampang Molecular Markers Useful at diagnosis Classify groups and prognosis Development of more specific therapies Application of risk-adjusted
Molecular Markers in Acute Leukemia Dr Muhd Zanapiah Zakaria Hospital Ampang Molecular Markers Useful at diagnosis Classify groups and prognosis Development of more specific therapies Application of risk-adjusted
