Quo Vadis: Advanced Prostate Cancer Clinical Care and Clinical Research in the Era of Multiple Androgen Receptor-Directed Therapies
|
|
|
- Joan Washington
- 5 years ago
- Views:
Transcription
1 Review Article Quo Vadis: Advanced Prostate Cancer Clinical Care and Clinical Research in the Era of Multiple Androgen Receptor-Directed Therapies Won Kim, MD; and Charles J. Ryan, MD The novel androgen receptor-directed therapies abiraterone acetate and enzalutamide, having demonstrated improved survival in randomized phase 3 studies of men with metastatic castration-resistant prostate cancer, have ushered in a new era in the treatment of this disease. Additional novel androgen receptor-directed therapies, such as ARN-509 and orteronel, are in various phases of clinical trials and development. However, the emergence of therapeutic resistance and clinical disease progression is inevitable. Although advances in genomic technologies have led to unprecedented understanding of the biology of castration-resistant prostate cancer, efforts only now are underway to elucidate the mechanisms of resistance associated with abiraterone and enzalutamide. A tremendous challenge in the near future will be to determine the optimal sequence or combination of therapies to overcome resistance mechanisms. In this review, the current landscape of androgen receptor-directed therapies and future directions necessary to enhance their clinical efficacy for the maximal benefit of patients are discussed. Cancer 2015;121: VC 2014 American Cancer Society. KEYWORDS: metastatic castration-resistant prostate cancer, abiraterone acetate, enzalutamide, biomarkers. INTRODUCTION Prostate cancer is the second most commonly diagnosed malignancy in men and is responsible for >250,000 cancerrelated deaths worldwide. 1 Although surgery and radiotherapy offer curative potential for men with localized prostate cancer, men with advanced prostate cancer are burdened with an incurable disease. Androgen-deprivation therapy (ADT) is the standard of care for men with advanced prostate cancer and has been so for more than 70 years. 2 Unfortunately, virtually all men who receive ADT eventually experience progression to the lethal form of the disease: metastatic castrationresistant prostate cancer (mcrpc). Typically, death occurs 2 to 4 years after the onset of castration-resistance. Docetaxel received regulatory approval in 2004 as the first therapy to prolong overall survival in patients with mcrpc. 3 Since 2010, 5 additional agents cabazitaxel, 4 sipuleucel-t, 5 abiraterone acetate, 6,7 enzalutamide, 8,15 and radium have demonstrated profound survival impact on the lives of patients with mcrpc and have received regulatory approval (Table 1). Among these, the novel androgen receptor (AR)-targeted therapies abiraterone acetate and enzalutamide have the broadest clinical use. These therapies have provided new promise to patients with advanced prostate cancer; however, mcrpc remains incurable, causing great physical, emotional, and financial burdens. In this review, we examine the current landscape of AR-targeted therapies and how it may shape the future of clinical research and care of patients with advanced prostate cancer. Current Landscape of AR-Targeted Therapies Abiraterone acetate Cytochrome P450 17A1 (CYP17A1) is the rate-limiting enzyme in gonadal and extragonadal (ie, adrenal glands, prostate) androgen synthesis. Abiraterone acetate, the prodrug of abiraterone, is a potent CYP17A1 inhibitor that suppresses circulating serum androgens beyond what is possible through ADT alone (Fig. 1). In a phase 3 study of men with mcrpc postchemotherapy (COU-AA-301), treatment with the combination of abiraterone and prednisone improved survival compared with prednisone alone (14.8 months vs 10.9 months; hazard ratio [HR], 0.65; P<.001), leading to its initial regulatory approval in All secondary endpoints favored the patients who received abiraterone, including the time to prostate-specific antigen (PSA) progression (TPP) (10.2 months vs 6.6 months; P<.001), progression-free survival (PFS) (5.6 months vs 3.6 months; P<.001), and the PSA response rate (PSA decline 50%: 29% vs 6%; P<.001). In a Corresponding author: Charles J. Ryan, MD, Helen Diller Family Comprehensive Cancer Center, University of California-San Francisco, 1600 Divisadero Street, San Francisco, CA, 94115; Fax: (415) ; ryanc@medicine.ucsf.edu Helen Diller Family Comprehensive Cancer Center, University of California-San Francisco, San Francisco, California DOI: /cncr.28929, Received: February 25, 2014; Revised: June 20, 2014; Accepted: June 30, 2014, Published online September 18, 2014 in Wiley Online Library (wileyonlinelibrary.com) Cancer February 1,
2 Review Article TABLE 1. Phase 3 Studies Demonstrating Improved Survival in Patients With Metastatic Castrate-Resistant Prostate Cancer Regimens (Study Name) Reference Primary Endpoint Notes Docetaxel and prednisone vs mitoxantrone and prednisone (TAX-327) Tannock OS, 18.9 mo vs 16.5 mo (HR, 0.76; P 5.009) Sipuleucel-T vs placebo (IMPACT) Kantoff OS, 25.8 mo vs 21.7 mo (HR, 0.78; P 5.03) Cabazitaxel and prednisone vs mitoxantrone and prednisone (TROPIC) Abiraterone and prednisone vs placebo and prednisone (COU-AA-301) de Bono de Bono OS, 15.1 mo vs 12.7 mo (HR, 0.70; P<.0001) OS, 14.8 mo vs 10.9 mo (HR, 0.65; P<.001) Enzalutamide vs placebo (AFFIRM) Scher OS, 18.4 mo vs 13.6 mo (HR, 0.63; P<.001) Abiraterone and prednisone vs placebo and prednisone (COU-AA-302) Ryan OS, NYR vs mo (HR, 0.75; P 5.01); rpfs, 16.5 mo vs 8.3 mo (HR, 0.53; P<.001) Radium-223 vs placebo (ALSYMPCA) Parker OS, 14.0 mo vs 11.2 mo (HR, 0.70; P 5.002) Enzalutamide vs placebo (PREVAIL) Beer OS, 32.4 mo vs 30.2 mo (HR, 0.70; P<.0001); rpfs, NYR vs 3.9 mo (HR, 0.19; P<.0001) First phase 3 study to demonstrate a survival benefit in mcrpc Accrual between August 2003 and November 2007; asymptomatic or minimally symptomatic; chemotherapy-naive or postchemotherapy Accrual between January 2007 and October 2008; progressive disease after docetaxel Accrual between May 2008 and July 2009; progressive disease after docetaxel Accrual between September 2009 and November 2010; progressive disease after docetaxel; use of prednisone permitted but not required Accrual between April 2009 and June 2010; chemotherapy-naive; asymptomatic or minimally symptomatic; no prior ketoconazole (7 d); patients with visceral metastases were excluded Two or more bone metastases; no visceral metastases; postdocetaxel (or ineligible or unavailable); symptomatic disease Accrual between September 2010 and September 2012; chemotherapy-naive; use of prednisone permitted but not required; no prior abiraterone or ketoconazole; asymptomatic or minimally symptomatic; patients with visceral disease (including liver or lung metastases) were eligible Abbreviations: HR, hazard ratio; mcrpc, metastatic castration-resistant prostate cancer; NYR, not yet reached; OS, overall survival; rpfs, radiographic progression-free survival. second phase 3 study among chemotherapy-naive men with mcrpc (COU-AA-302) in which the coprimary endpoints were radiographic PFS (rpfs) and overall survival (OS), abiraterone again was superior to placebo (rpfs: HR, 0.43; 95% confidence interval [CI], ; P<.001; OS: HR, 0.75; 95% CI, ; P<.01). 7 Secondary endpoints, including risk of decline in Eastern Cooperative Oncology Group performance status, time to the initiation of cytotoxic chemotherapy, time to opiate use for cancer-related pain, and TPP, favored the abiraterone arm. Abiraterone requires the concomitant administration of prednisone to mitigate toxicities that result directly from the potent inhibition of CYP17A1 and the compensatory rise in adrenocorticotropic hormone, which leads to dramatically increased levels of adrenal steroid hormones upstream of CYP17A1 with potent mineralocorticoid activity. 10,11 Therefore, in addition to the side effects caused by mineralocorticoid excess, such as hypertension, hypokalemia, and edema, the potential toxicities associated with long-term prednisone use must be considered for patients who are under consideration for abiraterone treatment. Furthermore, recent investigations regarding ligand-receptor promiscuity of nuclear hormone receptors 12,13 and its potential role in therapeutic response and resistance raises further questions regarding steroid use. However, the clinical application of that work requires further clinical validation. Enzalutamide Enzalutamide is a novel antiandrogen that antagonizes the AR more potently than the previous generation of antiandrogens (eg, bicalutamide). Preclinical studies have 362 Cancer February 1, 2015
3 AR-Directed Therapies in Prostate CA/Kim and Ryan Figure 1. Androgen-receptor (AR)-directed therapies and mechanisms of action are illustrated, including novel androgen synthesis inhibitors and antiandrogens, and their mechanisms of action. Orteronel is a selective 17a-hydroxylase/C (17,20-lyase) inhibitor, but it loses its selectivity at high concentrations. Galeterone is a selective 17,20-lyase inhibitor and also acts as an AR antagonist and increases the rate of AR degradation. DOC indicates docetaxel; 17-OH, 17-hydroxy; CFG 920, a nonsteroidal cytochrome P inhibitor; VT 464, a nonsteroidal selective inhibitor of 17,20-lyase; ARN 909, an AR antagonist; ODM-201, an antiandrogen; AZD 3514, an oral drug that modulates AR signaling. demonstrated that enzalutamide binds to the AR with 8- fold greater affinity than bicalutamide and is active as an inhibitor in cells with AR amplification and cells in which bicalutamide has agonist activity. 14 Administered orally on a daily schedule, enzalutamide is generally well tolerated even in long-term use. In a randomized phase 3 study of 1199 men with mcrpc who had progressed after docetaxel chemotherapy (AFFIRM), enzalutamide demonstrated significantly improved OS compared with placebo (18.4 months vs 13.6 month; HR, 0.63; P<.001), leading to early unblinding of the study. 8 Enzalutamide was superior to placebo for all secondary endpoints, including PSA response rate (54% vs 2%; P<.001), soft tissue response rate (29% vs 4%; P<.001), and rpfs (8.3 months vs 2.9 months; HR, 0.40; P<.001). Enzalutamide received regulatory approval in 2013 in the postchemotherapy setting. A second phase 3 study of enzalutamide versus placebo in chemotherapy-naive patients with mcrpc (PRE- VAIL) focused on the coprimary endpoints of rpfs and OS. 15 Enzalutamide demonstrated a significant benefit in both endpoints, with a 30% reduction in the risk of death (HR, 0.70; P<.0001) and an 81% reduction in the risk of radiographic progression or death (HR, 0.19; P<.0001). The median OS was 32.4 months in the enzalutamide arm versus 30.2 months in the placebo arm. In the AFFIRM study, 5 of 800 patients who received enzalutamide had seizures, and there were no seizures in the control arm. In the PREVAIL study, 2 patients had seizures (1 patient in each study arm). Enzalutamide is known to cross the blood-brain barrier, and it is possible that it lowers the seizure threshold in some patients. Although rare, patients who have a history of seizures or other predisposition to seizures should not receive enzalutamide. Cancer February 1,
4 Review Article Considerations The postchemotherapy phase 3 studies of abiraterone and enzalutamide were placebo-controlled (placebo plus prednisone in the abiraterone study), because there were no standard-of-care treatment options known to prolong survival in this disease setting. However, it is fair to question whether placebo was the appropriate control for the prechemotherapy phase 3 trials of these drugs. The clinical benefit of docetaxel was known for years before those trials, and sipuleucel-t demonstrated its survival benefit in a phase 3 trial that was published before the enrollment period of the PREVAIL study (COU-AA-302 had completed enrollment before publication). That said, prostate cancer is a disease of older men, and many patients with mcrpc do not receive chemotherapy because of comorbidities, toxicities, or patient preferences. Sipuleucel-T is an option only for patients who have asymptomatic (or minimally symptomatic) mcrpc and does not impact the time to disease progression. Thus, abiraterone and enzalutamide represent effective and tolerable options for many patients, and their broad clinical use currently serves as evidence that the studies were conducted in a population in dire need. In addition, the phase 3 chemotherapy-naive studies used coprimary efficacy endpoints of rpfs and OS. rpfs was defined as freedom from death from any cause and freedom from progression of soft tissue or bone lesions. 7 The rationale for using rpfs as a coprimary endpoint stems from the finding that mcrpc is a disease with long median OS, 16 with multiple clinical events (eg, PSA progression, radiographic progression, decline in functional status, need for cytotoxic chemotherapy) that occur before death. Thus, the addition of rpfs to OS as a coprimary endpoint may provide a more comprehensive assessment benefit attributable an intervention. Both abiraterone and enzalutamide demonstrated significant improvement in rpfs. Enzalutamide also improved OS compared with placebo in this patient population; abiraterone demonstrated a strong trend toward OS improvement, but it fell short of statistical significance. Nevertheless, in the United States, the label for abiraterone was expanded to the chemotherapy-naive space based on these results; enzalutamide just recently received US FDA approval for its expanded indication after a priority review. AR-Directed Therapies in Development: Androgen Synthesis Inhibitors Orteronel (TAK-700) Orteronel, an imidazole derivative, is also an inhibitor of CYP17A1 although, unlike abiraterone, it is a nonsteroidal and reversible inhibitor. In addition, it is highly selective for 17,20-lyase (although, at higher concentrations, it does lose some selectivity and also inhibits 17a-hydroxylase). 17 A theoretical advantage of selective 17,20-lyase inhibition is that it may obviate the need for corticosteroid administration to mitigate the side effects associated with mineralocorticoid excess; the approved label for abiraterone acetate requires the daily coadministration of 10 mg prednisone. A phase 1/2 study in 96 patients with mcrcp demonstrated that orteronel (at various dose cohorts, including patients who did or did not receive prednisone) is well tolerated. The most common grade 3 adverse events were fatigue (9%) and diarrhea (3%). Orteronel also was clinically active, and 41% to 63% of patients (at various dose cohorts) achieved a PSA decline 50%. 18 It is noteworthy that the side-effect profile was similar to that of abiraterone acetate. Based on these data, 2 phase 3 studies of orteronel were initiated in patients with mcrpc (before and after docetaxel, mirroring the abiraterone and enzalutamide experiences). An interim analysis of the postchemotherapy study (ELM-PC 5) resulted in early termination for failing to satisfy the primary endpoint of improved OS. 19 It is worth noting that the median OS in patients who received orteronel was 17.0 months versus 15.2 months for patients who received placebo (HR, 0.88; 95% CI, ; P5.1898), comparing favorably to the abiraterone experience in similar patients. There were no safety concerns. 20 Similarly, results from the interim analysis of orteronel in patients with chemotherapy-naive mcrpc (ELM-PC 4) failed to demonstrate a significant improvement in OS, although it did produce a significant improvement in rpfs. 21 One of the reasons posited for the negative findings in those studies is that efficacious poststudy treatments became available during the study periods, as discussed further below. Currently, a randomized, phase 3, cooperative group study of orteronel with or without ADT in patients with metastatic, hormonesensitive prostate cancer is ongoing (National Clinical Trials [clinicaltrials.gov] identifier NCT ). Galeterone (TOK-001) Galeterone is a potent CYP17A1 inhibitor that, in preclinical studies, has demonstrated greater potency than abiraterone acetate. 22 In addition, galeterone has potent, direct ARantagonist activity as well as the effect of AR downregulation. Therefore, galeterone interrupts AR activity through multiple mechanisms. In a phase 1 study of 49 patients with mcrpc, galeterone was safe and well 364 Cancer February 1, 2015
5 AR-Directed Therapies in Prostate CA/Kim and Ryan tolerated, with grade 3 and 4 adverse events occurring at a frequency of 8% and 1%, respectively, and 11 patients (22%) had a PSA decline 50%. A phase 2 study of galeterone in patients with mcrpc is ongoing (NCT ). AR-Directed Therapies in Development: Antianderogens ARN-509 Like enzalutamide, ARN-509 is a potent AR antagonist that does not demonstrate significant AR-agonist activity, 23 particularly in the presence of amplified AR, which is common in the castration-resistant setting. 24 In prostate cancer cell lines, ARN-509 bound to AR with 5-fold to 10-fold greater affinity than bicalutamide; and, in murine xenograft models of mcrpc, it demonstrated greater antitumor activity than enzalutamide, with pharmacokinetic characteristics that potentially predicted a higher therapeutic index (maximal efficacy observed at lower steady-state plasma and brain levels compared with enzalutamide). In a phase 1 study of 39 patients with progressive mcrpc, ARN-509 was safe and well tolerated, with 4 grade 3 adverse events reported (3 which were considered unrelated to treatment; no seizures were reported). ARN-509 demonstrated clinical efficacy: 18 patients (60%) demonstrated a PSA decline 50%, and 5 of 10 evaluable patients who had measureable soft tissue lesions had a response of stable disease after >6 months. 25 Preliminary results from the phase 2 study, at the recommended ARN-509 dose of 240 mg daily, were reported in Forty-six chemotherapy-naive patients with mcrpc (25 treatment-naive, 21 postabiraterone therapy) were enrolled; and, at 12 weeks, 88% of the treatment-naive patients and 29% of the postabiraterone patients experienced a PSA response according to Prostate Cancer Clinical Trials Working Group (PCWG-2) criteria. 26 The phase 2 study also enrolled 47 patients with nonmetastatic CRPC, and 91% of those patients experienced a PSA response at 12 weeks. 27 Ongoing studies of ARN-509 include: a phase 3, placebo-controlled trial of ARN-509 in patients with nonmetastatic CRPC (NCT ); a phase 1b trial of ARN-509 in combination with abiraterone acetate and prednisone (NCT ); and a phase 2 randomized trial of ARN-509 in patients with biochemically relapsed, hormone-sensitive prostate cancer (NCT ). In the Pipeline There are multiple AR-directed therapies in early stages of clinical development. These include the following: 1. CFG-920, a nonsteroidal CYP17 inhibitor, currently in a phase 1/2 clinical trial (NCT ); 2. VT-464, a nonsteroidal selective inhibitor of 17,20- lyase, currently in a phase 1 trial (NCT ); 3. ODM-201, an antiandrogen that, in preclinical models, does not cross the brain-blood barrier, currently in a phase 2 clinical trial (NCT ); 4. AZD-3514, an oral drug that modulates AR signaling by 1) inhibition of AR nuclear translocation; and 2) down-regulation of AR levels 28 ; AZD-3514 is in a phase 1 clinical trial (NCT ); and 5. EZN-4176, an antisense oligonucleotide that binds to and degrades AR messenger RNA, leading to decreased AR expression; the phase 1 study was suspended, because dose escalation was limited by toxicities. 29 The profound impact demonstrated by abiraterone acetate and enzalutamide and the promising clinical activity of many AR-targeted drugs in development offer promise to the many patients burdened by advanced prostate cancer. However, to maximize the benefits of these therapies, further investigations are necessary on many fronts. Resistance to AR-Directed Therapies The oncogenic landscape of prostate cancer continues to be explored and charted through efforts fueled by advances in next-generation sequencing, leading to unprecedented understanding of the genetic basis of prostate cancer, providing investigators with a glimpse of the incredible molecular and clinical heterogeneity of the disease, and offering the possibility of precision medicine. However, in the era of targeted therapies, it is now necessary to take into account that the biology of prostate cancer that is resistant to abiraterone or enzalutamide (or both) is likely to be distinct from the disease that was previously termed simply castration-resistant prostate cancer (CRPC). Resistance to abiraterone and enzalutamide typically develops within 6 to 18 months of treatment initiation. Preclinical studies and increasing widespread clinical experience with these agents are elucidating various mechanisms of resistance associated with AR-targeted therapy, and similar resistance is expected for the AR-targeted therapies currently in development. Key potential resistance mechanisms against novel AR-directed therapies include further up-regulation of CYP17A1 activity and steroidogenesis 35,36 ; alterations in the AR itself, including AR mutations 37,38 and splice variants 35,39 that may promote ligand-independent activation of the AR signaling cascade; the promiscuous behavior of nuclear receptors, 13 including Cancer February 1,
6 TABLE 2. Proposed Disease States Within Castration-Resistant Prostate Cancer Ligand Dependent? AR Dependent? CRPC Mechanism Potential Clinical Scenario Yes Yes Increased androgen synthesis through overexpression of CYP17A1 or other steroidogenic enzymes No Yes AR mutation or splice variant allowing ligand-independent AR activation Yes No Ligand-mediated activation of alternative nuclear receptor Patient with disease progression after ketoconazole; experiences clinical response to more potent CYP17A1 inhibition with abiraterone acetate Patient has progression of disease after enzalutamide; mutational analysis reveals AR F876L mutation Patient experiencing disease progression after enzalutamide; analysis reveals induction of glucocorticoid receptor No No Neuroendocrine/small cell phenotype Patient progressing after multiple therapies; low/no PSA production; genomic analysis with MYC amplification or AURKA expression Abbreviations: AR, androgen receptor; AURKA, aurora kinase A; CRPC, castration-resistant prostate cancer; CYP17A1, cytochrome P450, family 17, subfamily A, polypeptide 1; FL, phenylalanine to leucine substitution at amino acid position 876 of the AR polypeptide; MYC, v-myc avian myelocytomatosis viral oncogene homolog; PSA, prostate-specific antigen. the recent finding that glucocorticoid receptor expression is induced in the setting of antiandrogen therapy 12 ; activation of non-ar pathways, such as the phosphoinositide 3- kinase (PI3K) and v-myc myelocytomatosis viral oncogene homolog (MYC) pathways 40,41 ; and phenotypic differentiation into a neuroendocrine or anaplastic phenotype. 42,43 Efforts are underway to understand mechanisms of resistance associated with the targeted treatment of advanced prostate cancer, and AR-directed therapies are a particular focus, including the 2 Stand Up 2 Cancer-Prostate Cancer Foundation Dream Team projects. 44,45 The promise of these efforts, which rely on multicenter expertise and collaboration, have generated great excitement. In addition, CRPC, which, over the past decade, has become the standard umbrella term to describe the disease state after progression on primary ADT, may no longer be adequate in this era of multiple therapeutic options. Terms that describe the disease state with regard to both the ligand and the receptor may be necessary to convey the disease biology (Table 2). For example, androgen-dependent, receptor-dependent CRPC may describe a cancer that has progressed after abiraterone but remains dependent on increased steroidogenesis; whereas androgen-independent, receptor-dependent CRPC may describe prostate cancer that has progressed through an AR variant that allows ligand-independent activation. 46 Sequential and Combination Therapy In light of the resistance mechanisms associated with ARdirected therapy, clinician investigators have initiated studies (Table 3) to answer 2 key questions: First, what is the optimal sequencing of therapy; and, second, what is the role of combination therapy? Sequencing The first phase 3 studies of both abiraterone acetate and enzalutamide were conducted in postchemotherapy patients and excluded prior receipt of the other agent. In the COU-AA-301 study, the PSA response rate was 29%, the median time to PSA progression was 10.2 months, and the median rpfs was 5.6 months. By comparison, in the COU-AA-302 study, the PSA response rate was 62%, the median time to PSA progression was 11.1 months, and the rpfs was 16.5 months. Similarly, in the AFFIRM study, the PSA response rate was 54%, the median time to PSA progression was 8.3 months, and the median rpfs was 8.3 months. In the PREVAIL study, the PSA response rate was 78%, the median time to PSA progression was 11.2 months, and the median rpfs was not yet reach (the lower bound of the 95% CI was 13.8 months). In addition, in retrospective analysis, the clinical activity of docetaxel after disease progression on abiraterone appeared to be less than expected. 47 Although any direct comparisons of these results are not conclusive, along with preclinical evidence pointing to the potential cross-resistance between AR-directed therapies and taxanes, 48 at least they raise the issue of sequencing these agents. Whether a clinical trial is appropriate to answer this question is up for debate. Based on the magnitude of benefit in the prechemotherapy setting, abiraterone has already been approved in the prechemotherapy space, and it is likely that enzalutamide will follow shortly. The early clinical use of 366 Cancer February 1, 2015
7 AR-Directed Therapies in Prostate CA/Kim and Ryan TABLE 3. Current Clinical Investigations in Sequential and Combination Therapies for Advanced Prostate Cancer Sequencing studies Chemotherapy-naive mcrpc NCT : Concurrent vs sequential treatment with sipuleucel-t and enzalutamide in mcrpc Chemotherapy-naive or postchemotherapy mcrpc NCT : Concurrent vs sequential treatment with sipuleucel-t and abiraterone in men with mcrpc Combination studies AR therapies Chemotherapy-naive mcrpc NCT : A phase 3 study of enzalutamide with or without abiraterone in patients with mcrpc Chemotherapy-naive or postchemotherapy mcrpc NCT : A phase 1 study of ARN-509 in combination with abiraterone in mcrpc AR and non-ar therapies Chemotherapy-naive mcrpc NCT : A phase 1 study of enzalutamide and docetaxel in men with advanced prostate cancer NCT : Abiraterone acetate with or without dasatinib in treating patients with mcrpc NCT : A study of cabozantinib in combination with abiraterone in patients with bone-metastatic CRPC NCT : A phase 2 study of AMG386 and abiraterone in patients with mcrpc NCT : ARN509 plus everolimus in men with progressive metastatic castration-resistant prostate cancer after treatment with abiraterone acetate Chemotherapy-naive or postchemotherapy mcrpc NCT : A phase 1/2 study of HSP90 inhibitor AT13387 alone or in combination with abiraterone NCT : A phase 2 study of abiraterone with or without veliparib in treating patients with mcrpc NCT : BKM120 and abiraterone for patients with mcrpc Postchemotherapy mcrpc NCT : A phase 2 study of olaparib with abiraterone in treating mcrpc NCT : A phase 1/2 study of alisertib in combination with abiraterone and prednisone for patients with CRPC after progression on abiraterone NCT : Cabazitaxel and abiraterone in patients with mcrpc NCT : Tivozanib and enzalutamide in advanced prostate cancer Abbreviations: AR, androgen receptor; CRPC, castration-resistant prostate cancer; HSP90, heat-shock protein 90; mcrpc, metastatic castration-resistant prostate cancer; NCT, National Clinical Trials identifier. abiraterone (and even enzalutamide) by clinicians before chemotherapy may obviate this question. A more relevant question may be whether there is a role for sequencing the AR-directed therapies (ie, abiraterone followed by enzalutamide, or vice versa). Retrospective studies have demonstrated moderate clinical activity when 1 AR-directed therapy is followed by the other, with a median PFS of 3 to 4 months. However, there are some patients who do have significant clinical responses after progression on 1 AR-directed therapy, and prior response to abiraterone or enzalutamide is not predictive of a response to the other. Recently reported findings regarding potential mechanisms of resistance to ARtargeted therapies may shed some light. AR-V7 is a constitutively active AR splice variant that is present in patients with mcrpc. In a small study of 62 patients who were about to begin treatment with enzalutamide or abiraterone (31 patients in each arm), the AR-V7 variant was identified in 18 men (29%). 56 None of those 18 patients with the AR-V7 variant experienced a PSA response to either enzalutamide or abiraterone, whereas 27 of the 44 patients (62%) without the variant experienced a PSA response. It is noteworthy that 11 of the 12 AR-V7- positive patients (92%) who were about to begin enzalutamide had previously received abiraterone, and 2 of the 6 AR-V7-positive patients (33%) who were about to begin abiraterone had previously received enzalutamide. Another study evaluated AR amplification status using fluorescence in situ hybridization in 33 patients with mcrpc. 57 In that study, of 16 patients who were abiraterone-naive and enzalutamide-naive, 81% had AR amplification. In 11 patients who were resistant to abiraterone, 9% had AR amplification; and, in 6 patients who were resistant to enzalutamide, 66% had AR amplification. Although these were small studies, they suggest that the mechanisms of resistance to these AR-targeted therapies may indeed be distinct and that AR-V7 or AR amplification status may have a role as a biomarker in predicting response to abiraterone or enzalutamide. Furthermore, AR splice variant or amplification status may be able to determine whether sequencing AR-targeted therapies is a feasible strategy. For example, if the AR-V7 splice variant is identified in a patient who previously received abiraterone, then further treatment with enzalutamide may be futile; or a patient who previously received enzalutamide who retains AR amplification and is AR- Cancer February 1,
8 Review Article V7-negative may yet respond to abiraterone. These are conjectures based on preliminary results from small studies; clearly, prospectively analytical and clinical validation is necessary for any clinical application. However, these exciting findings provide a potential strategy by which clinicians may 1 day make treatment decisions. Combinations In general, combination treatments in advanced prostate cancer have not proven fruitful, although combination approaches with the novel agents discussed above have not yet been thoroughly evaluated. Docetaxel has been combined with calcitriol, 58 risedronate, 59 atrasentan, 60 zibotentan, 61 bevacizumab, 62 aflibercept, 63 and dasatinib 64 in large, randomized, placebo-controlled phase 3 studies, and all studies have been negative. However, it can be pointed out that, in every 1 of those trials, docetaxel was combined with a second agent that had not independently demonstrated survival benefit in patients with mcrpc. The current era, with multiple therapies available that have demonstrated survival benefit and promising therapies in clinical development (particularly ARdirected therapies), offers renewed promise. Rational combination therapies that address the various disease states in mcrpc have the potential to improve clinical outcomes for men with advanced prostate cancer. For example, in androgen-dependent, AR-dependent CRPC, it should be considered whether targeting both androgen synthesis and the AR concurrently can improve outcomes compared with sequential or monotherapy. A phase 3 clinical trial of enzalutamide with or without abiraterone (NCT ) will soon begin to enroll patients. Also, a phase 1 study of ARN-509 with abiraterone is ongoing (NCT ) and may lead to further investigation of this combination. Additional Considerations Another relevant question to be answered is whether the earlier use of AR-directed drugs like abiraterone and enzalutamide may prove to be the optimal use of these agents. A phase 2 trial of enzalutamide monotherapy in men with hormone therapy-naive prostate cancer produced impressive results: at 25 weeks, 93% of patients had a PSA decline 80%, for a median PSA decline of 99.6%. 65 Currently, there are ongoing, randomized phase 3 studies of ADT with or without abiraterone (NCT ) or orteronel (NCT ) in patients with newly diagnosed, metastatic, hormone-naive prostate cancer. Furthermore, studies of abiraterone in the neoadjuvant space have reported promising results, 66,67 and additional studies in both the neoadjuvant and adjuvant settings for high-risk prostate cancer are ongoing (NCT , abiraterone and luteinizing hormone-releasing hormone agonist with or without enzalutamide before surgery; NCT , enzalutamide in patients with high-risk prostate cancer after radical prostatectomy; NCT , enzalutamide, radiation, and hormone therapy for patients with intermediate-risk or high-risk prostate cancer). Looking forward, a fresh perspective on the process of therapy development is in order. Since 2010, 5 new agents have been approved, each or which has demonstrated improved OS in men with mcrpc. Given the paucity of therapeutic options available at the time, the improvement in OS depended largely on the efficacy of the drug in question. Abiraterone and enzalutamide in both the chemotherapy-naive studies and the postchemotherapy phase 3 studies were compared with placebo, as discussed above. Given the current standard of care, placebo will no longer be an acceptable control arm in clinical trials of patients with mcrpc. Appropriate control arms should be determined according to: 1) the patient population, ie, for asymptomatic or minimally symptomatic patients, an appropriate control arm may be sipuleucel-t or the AR-targeted therapies, whereas, in patients with symptomatic disease, chemotherapy may be more appropriate; and 2) the disease state, ie, (in the chemotherapy-naive space, abiraterone or enzalutamide would be reasonable choices, and sipuleucel-t also would be a reasonable choice for in asymptomatic patients; whereas, in the postdocetaxel space, cabazitaxel or radium-223 may be reasonable options. Practically speaking, because the AR-targeted therapies abiraterone and enzalutamide are in broadest clinical use, they may serve as an appropriate control arm for the majority of patient populations. In any case, the bar for improved OS will become more difficult to clear. Furthermore, the efficacy of sequential and subsequent therapies may preclude the further use of OS as a primary clinical trial endpoint, and surrogate and intermediate markers of clinical benefit in mcrpc are needed. This is illustrated in the recently reported results from the ELM-PC5 study of orteronel in postchemotherapy mcrpc and the ELM-PC4 study of orteronel in chemotherapy-naive patients, neither of which met their primary endpoints of efficacy. To further illustrate this point, in ELM-PC5, the median rpfs for the treatment versus placebo arms were 8.3 months and 5.7 months, respectively (HR, 0.76; P ), which is comparable to the median rpfs reported in the phase 3 COU-AA-301 and AFFIRM studies. In addition, the median OS for the placebo arm in the ELM-PC5 study was 368 Cancer February 1, 2015
9 AR-Directed Therapies in Prostate CA/Kim and Ryan longer than that for the placebo arm in the COU-AA-301 study, which may be an indication of a poststudy treatment effect on OS (the placebo arms of most contemporary trials have outperformed those of older trials, and the efficacy of poststudy treatment options available is a major reason). Furthermore, subgroup analyses demonstrated an OS benefit in the non-european and non-north American cohort, in which access to abiraterone was limited (and enzalutamide use was nonexistent). Thus, as novel therapies continue to advance through the clinical development pipeline, investigators must consider whether alternate endpoints (ie, surrogates) should be considered to define the true treatment effect of a drug. 68 For example, the label for abiraterone has been expanded based on the primary endpoint of rpfs. Other candidate surrogates include enumeration of circulating tumor cells, novel imaging biomarkers, and patient-reported outcomes Also, it is possible that these can be used in combination with known prognostic factors, such as serum lactate dehydrogenase and hemoglobin levels. 71 The incorporation of surrogate biomarkers into current clinical trials and practice will require a greater understanding of the molecular and biologic characteristics of advanced prostate cancer, demonstration of analytic and clinical validation (as well as clinical utility) of candidate surrogates, 72 and, finally, integration into prospective clinical trials. Conclusion The success of the novel AR-directed therapies abiraterone acetate and enzalutamide has ushered in a new, promising era in the treatment of advanced prostate cancer. Capitalizing on the finding that retained AR activity is the hallmark of CRPC, additional AR-directed therapies are in development. However, not all patients derive clinical benefit from these drugs, and all patients who initially benefit inevitably acquire resistance and experience disease progression. The ligand and receptor states in CRPC are variable, leading to different resistance mechanisms; how AR-directed therapies further alter disease biology is under active investigation. Key future directions include optimizing the sequence and combination of novel therapeutics as well as validating surrogate endpoints to foster the ongoing development of these drugs efforts that will profoundly impact the lives of men with prostate cancer. FUNDING SUPPORT No specific funding was disclosed. CONFLICT OF INTEREST DISCLOSURES Dr. Ryan reports honoraria from Janssen Biotech. REFERENCES 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61: Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen, and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1: Tannock IF, de Wit R, Berry WR, et al; TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351: de Bono JS, Oudard S, Ozquroglu M, et al; TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376: Kantoff PW, Higano CS, Shore ND, et al; IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363: de Bono JS, Logothetis CJ, Molina A, et al; COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364: Ryan CJ, Smith MR, de Bono JS, et al; COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368: Scher HI, Fizazi K, Saad F, et al; AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367: Parker C, Nilsson S, Heinrich D, et al; ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369: Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castrationresistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26: Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28: Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155: Richards J, Lin AC, Hay CW, et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res. 2012;72: Tran C, Ouk S, Clegg NJ, et al. Development of a secondgeneration antiandrogen for treatment of advanced prostate cancer. Science. 2009;324: Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J med 2014;371: Halabi S, Vogelzang NJ, Ou SS, Owzar K, Archer L, Small EJ. Progression-free survival as a predictor of overall survival in men with castrate-resistant prostate cancer. J Clin Oncol. 2009;27: Kaku T, Hitaka T, Ojida A, et al. Discovery of orteronel (TAK- 700), a naphthylmethylimidazole derivative, as a highly selective 17,20-lyase inhibitor with potential utility in the treatment of prostate cancer. Bioorg Med Chem. 2011;19: Agus DB, Stadler WM, Shevrin DH, et al. Safety, efficacy, and pharmacodynamics of the investigational agent TAK-700 in metastatic castration-resistant prostate cancer (mcrpc): updated data from a phase I/II study [abstract]. J Clin Oncol. 2011;29(suppl). Abstract Dreicer R, Jones R, Oudard S, et al. Results from a phase 3, randomized, double-blind, multicenter, placebo-controlled trial of orteronel (TAK-700) plus prednisone in patients with metastatic castration-resistant prostate cancer (mcrpc) that has progressed during or following decetaxel-based therapy (ELM-PC 5 trial) [abstract]. J Clin Oncol. 2014;32(suppl 4). Abstract Takeda Pharmaceutical Company. Takeda announces unblinding of phase 3 study of orteronel in patients with metastatic castrationresistant prostate cancer that progressed post-chemotherapy based on Cancer February 1,
10 Review Article interim analysis [press release]. Osaka, Japan: Takeda Pharmaceutical Company; de Wit R, Fizazi K, Jinga V, et al. Phase 3, randomized, placebocontrolled trial of orteronel (TAK-700) plus prednisone in patients (pts) with chemotherapy-naive metastatic castration-resistant prostate cancer (mcrpc) (ELM-PC 4 trial). J Clin Oncol. 2014;32(5 suppl). Abstract Vasaitis T, Belosay A, Schayowitz A, et al. Androgen receptor inactivation contributes to antitumor efficacy of 17a-hydroxylase/17,20- lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta- 5,16-diene in prostate cancer. Mol Cancer Ther. 2008;7: Clegg NJ, Wongvipat J, Joseph JD, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72: Friedlander TW, Roy R, Tomlins SA, et al. Common structural and epigenetic changes in the genome of castration-resistant prostate cancer. Cancer Res. 2012;72: Rathkopf DE, Morris MJ, Fox JJ, et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2013;31: Rathkopf DE, Emmanuel SA, Shore ND, et al. ARN-509 in men with metastatic castration-resistant prostate cancer (mcrpc) [abstract]. J Clin Oncol. 2013;31(suppl 6). Abstract Smith MR, Antonarakis ES, Ryan CJ, et al. ARN-509 in men with high-risk nonmetastatic castration-resistant prostate cancer (mcrpc) [abstract]. J Clin Oncol. 2013;31(suppl 6). Abstract Loddick SA, Ross SJ, Thomason AG, et al. AZD3514: a small molecule that modulates androgen receptor signaling and function in vitro and in vivo. Mol Cancer Ther. 2013;12: Bianchini D, Omlin A, Pezaro C, et al. First-in-human phase I study of EZN-4176, a locked nucleic acid antisense oligonucleotide to exon 4 of the androgen receptor mrna in patients with castration-resistant prostate cancer. BrJCancer.2013;109: Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18: Barbieri CE, Baca SC, Lawrence MS, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44: GrassoCS,WuYM,RobinsonDR,etal.Themutationallandscapeof lethal castration-resistant prostate cancer. Nature. 2012;487: Baca SC, Prandi D, Lawrence MS, et al. Punctuated evolution of prostate cancer genomes. Cell. 2013;153: Berger MF, Lawrence MS, Demichelis F, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470: Mostaghel EA, Maarck BT, Plymate SR, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17: Cai C, Chen S, Ng P, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71: Joseph JD, Lu N, Qian J, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3: Korpal M, Korn JM, Gao X, et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discov. 2013;3: Nyquist MD, Li Y, Hwang TH, et al. TALEN-engineered AR gene rearrangements reveal endocrine uncoupling of androgen receptor in prostate cancer. Proc Natl Acad Sci U S A. 2013;110: Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTENdeficient prostate cancer. Cancer Cell. 2011;19: Gao L, Schwartzman J, Gibbs A, et al. Androgen receptor promotes ligand-independent prostate cancer progression through c-myc upregulation [serial online]. PLoS One. 2013;8:e Mosquera JM, Beltran H, Park K, et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia. 2013;15: Terry S, Maille P, Baaddi H, et al. Cross modulation between the androgen receptor axis and protocadherin-pc in mediating neuroendocrine transdifferentiation and therapeutic resistance of prostate cancer. Neoplasia. 2013;15: Stand Up 2 Cancer-Prostate Cancer Foundation Dream Team. Precision Therapy of Advanced Prostate Cancer. Available at: www. aacr.org/funding/pages/sutc-dream-team.aspx?itemid=2. Accessed September 11, Stand Up 2 Cancer-Prostate Cancer Foundation Dream Team. Targeting Adaptive Pathways in Metastatic Treatment-Resistant Prostate Cancer. Available at: aspx?itemid=3. Accessed September 11, Nelson PS. Molecular states underlying androgen receptor activation: a framework for therapeutics targeting androgen signaling in prostate cancer. J Clin Oncol. 2012;30: Mezynski J, Pezaro C, Bianchini D, et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann Oncol. 2012;23: van Soest RJ, van Royen ME, de Morree ES, et al. Cross-resistance between taxanes and new hormonal agents abiraterone and enzalutamide may affect drug sequence choices in metastatic castrationresistant prostate cancer. Eur J Cancer. 2013;49: Loriot Y, Bianchini D, Ileana E, et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann Oncol. 2013;24: Noonan KL, North S, Bitting RL, Armstrong AJ, Ellard SL, Chi KN. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24: Schrader AJ, Boegemann M, Ohlmann CH, et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol. 2014;65: Thomsen FB, Roder MA, Rathenborg P, Brasso K, Borre M, Iversen P. Enzalutamide treatment in patients with metastatic castrationresistant prostate cancer progressing after chemotherapy and abiraterone acetate. Scand J Urol. 2014;48: Badrising S, van der Noort V, van Oort IM, et al. Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatment. Cancer. 2014;120: Schmid SC, Geith A, Boker A, et al. Enzalutamide after docetaxel and abiraterone therapy in metastatic castration-resistant prostate cancer. Adv Ther. 2014;31: Bianchini D, Lorente D, Rodriguez-Vida A, et al. Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abiraterone. Eur J Cancer. 2014;50: Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Enlg J Med 2014;371: Small EJ, Youngren J, Thomas G, et al. Androgen receptor (AR) amplification in patients with metastatic castration-resistant prostate cancer (mcrpc) refractory to therapy with abiraterone or enzalutamide: preliminary results from the SU2C/PCF/AACR West Coast Prostate Cancer Dream Team (WCDT) [abstract]. J Clin Oncol. 2014;32(5 suppl). Abstract Scher HI, Jia X, Chi K, et al. Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J Clin Oncol. 2011;29: Meulenbeld HJ, van Werkhoven ED, Coenen JL, et al. Randomised phase II/III study of docetaxel with or without risedronate in patients with metastatic castration resistant prostate cancer (CRPC), the Netherlands Prostate Study (NePro). Eur J Cancer. 2012;48: Quinn DI, Tangen CM, Hussain M, et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castrationresistant prostate cancer (SWOG S0421): a randomised phase 3 trial. Lancet Oncol. 2013;14: Cancer February 1, 2015
Sequencing Strategies in Metastatic Castration Resistant Prostate Cancer (MCRPC)
 Sequencing Strategies in Metastatic Castration Resistant Prostate Cancer (MCRPC) Amit Bahl Consultant Oncologist Bristol Cancer Institute Clinical Director Spire Specialist Care Centre UK Disclosures Advisory
Sequencing Strategies in Metastatic Castration Resistant Prostate Cancer (MCRPC) Amit Bahl Consultant Oncologist Bristol Cancer Institute Clinical Director Spire Specialist Care Centre UK Disclosures Advisory
Second line hormone therapies. Dr Lisa Pickering Consultant Medical Oncologist ESMO Preceptorship Singapore 2017
 Second line hormone therapies Dr Lisa Pickering Consultant Medical Oncologist ESMO Preceptorship Singapore 2017 Disclosures Institutional Research Support/P.I. Employee Consultant Major Stockholder Speakers
Second line hormone therapies Dr Lisa Pickering Consultant Medical Oncologist ESMO Preceptorship Singapore 2017 Disclosures Institutional Research Support/P.I. Employee Consultant Major Stockholder Speakers
Novel treatment for castration-resistant prostate cancer
 Novel treatment for castration-resistant prostate cancer Cora N. Sternberg, MD, FACP Chair, Department of Medical Oncology San Camillo and Forlanini Hospitals Rome, Italy Treatment options for patients
Novel treatment for castration-resistant prostate cancer Cora N. Sternberg, MD, FACP Chair, Department of Medical Oncology San Camillo and Forlanini Hospitals Rome, Italy Treatment options for patients
SESSIONE PLATINUM SERIES (Best Papers Poster o Abstract on Prostate Cancer) In Oncologia
 SESSIONE PLATINUM SERIES (Best Papers Poster o Abstract on Prostate Cancer) In Oncologia Divisione di Oncologia Medica Unità Tumori Genitourinari SESSIONE PLATINUM SERIES (Best Papers Poster o Abstract
SESSIONE PLATINUM SERIES (Best Papers Poster o Abstract on Prostate Cancer) In Oncologia Divisione di Oncologia Medica Unità Tumori Genitourinari SESSIONE PLATINUM SERIES (Best Papers Poster o Abstract
Secondary Hormonal therapies in mcrpc
 Secondary Hormonal therapies in mcrpc Ravindran Kanesvaran Consultant,Division of Medical Oncology National Cancer Centre Singapore 1 Disclosures Research Support/P.I. Sanofi Consultant Major Stockholder
Secondary Hormonal therapies in mcrpc Ravindran Kanesvaran Consultant,Division of Medical Oncology National Cancer Centre Singapore 1 Disclosures Research Support/P.I. Sanofi Consultant Major Stockholder
2014 Treatment Paradigms in mcrpc Docetaxel in hormone sensitive PC
 Ronald de Wit Erasmus MC Cancer Institute The Netherlands 2014 Treatment Paradigms in mcrpc Docetaxel in hormone sensitive PC Disclosures Sanofi ; research grant support, consultancy and speaker fees Astellas;
Ronald de Wit Erasmus MC Cancer Institute The Netherlands 2014 Treatment Paradigms in mcrpc Docetaxel in hormone sensitive PC Disclosures Sanofi ; research grant support, consultancy and speaker fees Astellas;
Session 4 Chemotherapy for castration refractory prostate cancer First and second- line chemotherapy
 Session 4 Chemotherapy for castration refractory prostate cancer First and second- line chemotherapy October- 2015 ESMO 2004 October- 2015 Fyraftensmøde 2 2010 October- 2015 Fyraftensmøde 3 SWOG 9916 OS
Session 4 Chemotherapy for castration refractory prostate cancer First and second- line chemotherapy October- 2015 ESMO 2004 October- 2015 Fyraftensmøde 2 2010 October- 2015 Fyraftensmøde 3 SWOG 9916 OS
Until 2004, CRPC was consistently a rapidly lethal disease.
 Until 2004, CRPC was consistently a rapidly lethal disease. the entry in systemic disease is declared on a an isolated PSA recurrence after local treatment so!!! The management of CRPC and MCRPC is different
Until 2004, CRPC was consistently a rapidly lethal disease. the entry in systemic disease is declared on a an isolated PSA recurrence after local treatment so!!! The management of CRPC and MCRPC is different
Evolution or revolution in the treatment of prostate cancer
 Evolution or revolution in the treatment of prostate cancer de Johann Sebastian de Bono, MB, ChB, FRCP, MSc, PhD Professor of Experimental Cancer Medicine Department of Medicine/ Drug Development Unit
Evolution or revolution in the treatment of prostate cancer de Johann Sebastian de Bono, MB, ChB, FRCP, MSc, PhD Professor of Experimental Cancer Medicine Department of Medicine/ Drug Development Unit
Anti-Androgen Therapies for Prostate Cancer: A Focused Review
 Anti-Androgen Therapies for Prostate Cancer: A Focused Review Nischala Ammannagari, MD, and Saby George, MD, FACP Abstract Among men in the United States, prostate cancer is the most common malignancy
Anti-Androgen Therapies for Prostate Cancer: A Focused Review Nischala Ammannagari, MD, and Saby George, MD, FACP Abstract Among men in the United States, prostate cancer is the most common malignancy
Castrate resistant prostate cancer: the future of anti-androgens.
 Castrate resistant prostate cancer: the future of anti-androgens. Dmitri Pchejetski 1,2*, Heba Alshaker 3, Justin Stebbing 3,4* 1. Department of Medicine, Imperial College, London, UK 2. School of Medicine,
Castrate resistant prostate cancer: the future of anti-androgens. Dmitri Pchejetski 1,2*, Heba Alshaker 3, Justin Stebbing 3,4* 1. Department of Medicine, Imperial College, London, UK 2. School of Medicine,
Androgens and prostate cancer: insights from abiraterone acetate and other novel agents
 Androgens and prostate cancer: insights from abiraterone acetate and other novel agents Ian Davis Ludwig Institute for Cancer Research Austin Health, Melbourne, Australia Supported in part by an Australian
Androgens and prostate cancer: insights from abiraterone acetate and other novel agents Ian Davis Ludwig Institute for Cancer Research Austin Health, Melbourne, Australia Supported in part by an Australian
Lower Baseline PSA Predicts Greater Benefit From Sipuleucel-T
 Lower Baseline PSA Predicts Greater Benefit From Sipuleucel-T Schelhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, Kantoff PW. Lower baseline prostate-specific antigen is associated with a greater
Lower Baseline PSA Predicts Greater Benefit From Sipuleucel-T Schelhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, Kantoff PW. Lower baseline prostate-specific antigen is associated with a greater
Index Patients 3& 4. Guideline Statements 10/11/2014. Enzalutamide Reduced the Risk of Death
 //4 Prolonged Radiographic Progression-Free Survival Reduced the Risk of Death Overall ITT Population Estimated median rpfs, months (9% CI): : NYR (.8 NYR); placebo:.9 (.7.4) rpfs (%) ( Enza 9 8 7 4 8
//4 Prolonged Radiographic Progression-Free Survival Reduced the Risk of Death Overall ITT Population Estimated median rpfs, months (9% CI): : NYR (.8 NYR); placebo:.9 (.7.4) rpfs (%) ( Enza 9 8 7 4 8
Treatment sequencing in metastatic castrate resistant prostate cancer
 (2014) 16, 426 431 2014 AJA, SIMM & SJTU. All rights reserved 1008 682X www.asiaandro.com; www.ajandrology.com Prostate Cancer Open Access REVIEW Treatment sequencing in metastatic castrate resistant prostate
(2014) 16, 426 431 2014 AJA, SIMM & SJTU. All rights reserved 1008 682X www.asiaandro.com; www.ajandrology.com Prostate Cancer Open Access REVIEW Treatment sequencing in metastatic castrate resistant prostate
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Abiraterone for the treatment of metastatic castration-resistant prostate cancer that has progressed on or after a docetaxel-based chemotherapy regimen Disease
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Abiraterone for the treatment of metastatic castration-resistant prostate cancer that has progressed on or after a docetaxel-based chemotherapy regimen Disease
Management of Incurable Prostate Cancer in 2014
 Management of Incurable Prostate Cancer in 2014 Julie N. Graff, MD, MCR Portland VA Medical Center Assistant Professor of Medicine Knight Cancer Institute, OHSU 2014: Cancer Estimates Stage at Diagnosis
Management of Incurable Prostate Cancer in 2014 Julie N. Graff, MD, MCR Portland VA Medical Center Assistant Professor of Medicine Knight Cancer Institute, OHSU 2014: Cancer Estimates Stage at Diagnosis
New Treatment Modalities and Clinical Trials for HRPC 계명의대 김천일
 New Treatment Modalities and Clinical Trials for HRPC 계명의대 김천일 Castrate-Resistant Prostate Cancer (CRPC) Current standard therapy Androgen receptor (AR) in CRPC New systemic therapies Hormonal therapy
New Treatment Modalities and Clinical Trials for HRPC 계명의대 김천일 Castrate-Resistant Prostate Cancer (CRPC) Current standard therapy Androgen receptor (AR) in CRPC New systemic therapies Hormonal therapy
*For reprints and all correspondence: Nobuaki Matsubara, Kashiwanoha, Kashiwa, Chiba , Japan.
 Japanese Journal of Clinical Oncology, 2015, 45(8) 774 779 doi: 10.1093/jjco/hyv070 Advance Access Publication Date: 15 May 2015 Original Article Original Article A multicenter retrospective analysis of
Japanese Journal of Clinical Oncology, 2015, 45(8) 774 779 doi: 10.1093/jjco/hyv070 Advance Access Publication Date: 15 May 2015 Original Article Original Article A multicenter retrospective analysis of
Management of castrate resistant disease: after first line hormone therapy fails
 Management of castrate resistant disease: after first line hormone therapy fails Rob Jones Consultant in Medical Oncology Beatson Cancer Centre Glasgow Relevant Disclosure I have received research support
Management of castrate resistant disease: after first line hormone therapy fails Rob Jones Consultant in Medical Oncology Beatson Cancer Centre Glasgow Relevant Disclosure I have received research support
New Treatment Options for Prostate Cancer
 New Treatment Options for Prostate Cancer Moderator: Jeremy P. Goldberg, President, JPG Healthcare LLC Panelists: Philip Kantoff, MD, Director, Lank Center for Genitourinary Oncology, Dana- Farber Cancer
New Treatment Options for Prostate Cancer Moderator: Jeremy P. Goldberg, President, JPG Healthcare LLC Panelists: Philip Kantoff, MD, Director, Lank Center for Genitourinary Oncology, Dana- Farber Cancer
Management of castrate resistant disease; after first line hormone therapy fails
 Management of castrate resistant disease; after first line hormone therapy fails Dr. Syed A Hussain Clinical Senior Lecturer and Consultant in Medical Oncology University of Liverpool and Clatterbridge
Management of castrate resistant disease; after first line hormone therapy fails Dr. Syed A Hussain Clinical Senior Lecturer and Consultant in Medical Oncology University of Liverpool and Clatterbridge
When exogenous testosterone therapy is. adverse responses can be induced.
 Theoretical tips It has been reasoned that discontinuation of ADT in nonorchiectomized patients may have detrimental effect on patients with CRPC as discontinuation of ADT can result in renewed release
Theoretical tips It has been reasoned that discontinuation of ADT in nonorchiectomized patients may have detrimental effect on patients with CRPC as discontinuation of ADT can result in renewed release
Group Sequential Design: Uses and Abuses
 Group Sequential Design: Uses and Abuses Susan Halabi Department of Biostatistics and Bioinformatics, Duke University October 23, 2015 susan.halabi@duke.edu What Does Interim Data Say? 2 Group Sequential
Group Sequential Design: Uses and Abuses Susan Halabi Department of Biostatistics and Bioinformatics, Duke University October 23, 2015 susan.halabi@duke.edu What Does Interim Data Say? 2 Group Sequential
Have we optimized the use of Androgen Receptor pathway targeted drugs in Castrate-Resistant Prostate Cancer?
 Have we optimized the use of Androgen Receptor pathway targeted drugs in Castrate-Resistant Prostate Cancer? Karim Fizazi, MD, PhD Institut Gustave Roussy Villejuif, France Disclosure Participation to
Have we optimized the use of Androgen Receptor pathway targeted drugs in Castrate-Resistant Prostate Cancer? Karim Fizazi, MD, PhD Institut Gustave Roussy Villejuif, France Disclosure Participation to
Advanced Prostate Cancer
 Advanced Prostate Cancer SAMO Masterclass 4 th March 2016 Aurelius Omlin Conflicts of interest Advisory Rolle: Astra Zeneca, Astellas, Bayer, Janssen, Pfizer, Sanofi Aventis Research support: TEVA, Janssen
Advanced Prostate Cancer SAMO Masterclass 4 th March 2016 Aurelius Omlin Conflicts of interest Advisory Rolle: Astra Zeneca, Astellas, Bayer, Janssen, Pfizer, Sanofi Aventis Research support: TEVA, Janssen
Philip Kantoff, MD Dana-Farber Cancer Institute
 CHEMOTHERAPY FOR MCRPC Philip Kantoff, MD Dana-Farber Cancer Institute Harvard Medical School 1 Disclosure of Financial Relationships With Any Commercial Interest Name Nature of Financial Commercial Interests
CHEMOTHERAPY FOR MCRPC Philip Kantoff, MD Dana-Farber Cancer Institute Harvard Medical School 1 Disclosure of Financial Relationships With Any Commercial Interest Name Nature of Financial Commercial Interests
Strategic decisions for systemic treatment. metastatic castration resistant prostate cancer (mcrpc)
 Strategic decisions for systemic treatment metastatic castration resistant prostate cancer (mcrpc) SAMO Luzern 14.09.2012 Richard Cathomas Onkologie Kantonsspital Graubünden richard.cathomas@ksgr.ch mcrpc
Strategic decisions for systemic treatment metastatic castration resistant prostate cancer (mcrpc) SAMO Luzern 14.09.2012 Richard Cathomas Onkologie Kantonsspital Graubünden richard.cathomas@ksgr.ch mcrpc
 www.drpaulmainwaring.com Figure 1 Androgen action Harris W P et al. (2009) Nat Clin Pract Urol doi:10.1038/ncpuro1296 Figure 2 Mechanisms of castration resistance in prostate cancer Harris W P et al. (2009)
www.drpaulmainwaring.com Figure 1 Androgen action Harris W P et al. (2009) Nat Clin Pract Urol doi:10.1038/ncpuro1296 Figure 2 Mechanisms of castration resistance in prostate cancer Harris W P et al. (2009)
Sequencing treatment for metastatic prostate cancer
 11 Sequencing treatment for metastatic prostate cancer SOPHIE MERRICK, STYLIANI GERMANOU, ROGER KIRBY AND SIMON CHOWDHURY In the past 10 years there have been significant advances in the understanding
11 Sequencing treatment for metastatic prostate cancer SOPHIE MERRICK, STYLIANI GERMANOU, ROGER KIRBY AND SIMON CHOWDHURY In the past 10 years there have been significant advances in the understanding
Circulating tumor cells as biomarker for hormonal treatment in breast and prostate cancer. Michal Mego
 National Cancer Institute, Slovakia Translational Research Unit Circulating tumor cells as biomarker for hormonal treatment in breast and prostate cancer Michal Mego 2 nd Department of Oncology, Faculty
National Cancer Institute, Slovakia Translational Research Unit Circulating tumor cells as biomarker for hormonal treatment in breast and prostate cancer Michal Mego 2 nd Department of Oncology, Faculty
PROSTATE CANCER HORMONE THERAPY AND BEYOND. Przemyslaw Twardowski MD Professor of Oncology Department of Urologic Oncology John Wayne Cancer Institute
 PROSTATE CANCER HORMONE THERAPY AND BEYOND Przemyslaw Twardowski MD Professor of Oncology Department of Urologic Oncology John Wayne Cancer Institute Disclosures I am a Consultant for Bayer and Sanofi-Aventis
PROSTATE CANCER HORMONE THERAPY AND BEYOND Przemyslaw Twardowski MD Professor of Oncology Department of Urologic Oncology John Wayne Cancer Institute Disclosures I am a Consultant for Bayer and Sanofi-Aventis
Management of castration resistant prostate cancer after first line hormonal therapy fails
 Management of castration resistant prostate cancer after first line hormonal therapy fails Simon Crabb Senior Lecturer in Medical Oncology University of Southampton WHAT ARE THE AIMS OF TREATMENT? Cure?
Management of castration resistant prostate cancer after first line hormonal therapy fails Simon Crabb Senior Lecturer in Medical Oncology University of Southampton WHAT ARE THE AIMS OF TREATMENT? Cure?
ESMO SUMMIT MIDDLE EAST 2018
 ESMO SUMMIT MIDDLE EAST 2018 14 Years of progress in Prostate Cancer Standards of Care and new targets Name Ronald de Wit 6-7 April 2018, Dubai, UAE CONFLICT OF INTEREST DISCLOSURE Sub-title Sanofi Roche
ESMO SUMMIT MIDDLE EAST 2018 14 Years of progress in Prostate Cancer Standards of Care and new targets Name Ronald de Wit 6-7 April 2018, Dubai, UAE CONFLICT OF INTEREST DISCLOSURE Sub-title Sanofi Roche
8/31/ ) Intermittent androgen deprivation in androgen-sensitive PCa. 1) Alpharadin (Ra223) in CRPC with bone metastases
 Bruce J. Roth, M.D. Clinical Trials: Medivation, Oncogenix 1) Alpharadin (Ra223) in CRPC with bone metastases 2) Enzalutamide (MDV-31) in CRPC and prior docetaxel 3) Abiraterone in chemo-naïve CRPC 4)
Bruce J. Roth, M.D. Clinical Trials: Medivation, Oncogenix 1) Alpharadin (Ra223) in CRPC with bone metastases 2) Enzalutamide (MDV-31) in CRPC and prior docetaxel 3) Abiraterone in chemo-naïve CRPC 4)
Hormonal Manipulations in CRPC. NW Clarke Professor of Urological Oncology Manchester UK
 Hormonal Manipulations in CRPC NW Clarke Professor of Urological Oncology Manchester UK Standard Treatment of CRPC Pre 2004 (and in 2013?) PSA progression 99m Tc BS negative CT scan large lymph node component
Hormonal Manipulations in CRPC NW Clarke Professor of Urological Oncology Manchester UK Standard Treatment of CRPC Pre 2004 (and in 2013?) PSA progression 99m Tc BS negative CT scan large lymph node component
Published on The YODA Project (
 Principal Investigator First Name: David Last Name: Lorente Degree: MD Primary Affiliation: Medical Oncology Service, Hospital Provincial de Castellón E-mail: lorente.davest@gmail.com Phone number: +34
Principal Investigator First Name: David Last Name: Lorente Degree: MD Primary Affiliation: Medical Oncology Service, Hospital Provincial de Castellón E-mail: lorente.davest@gmail.com Phone number: +34
Perspective on endocrine and chemotherapy agents. Cora N. Sternberg Department of Medical Oncology San Camillo & Forlanini Hospitals Rome, Italy
 Perspective on endocrine and chemotherapy agents Cora N. Sternberg Department of Medical Oncology San Camillo & Forlanini Hospitals Rome, Italy Disclosures Dr. Sternberg has received research funding for
Perspective on endocrine and chemotherapy agents Cora N. Sternberg Department of Medical Oncology San Camillo & Forlanini Hospitals Rome, Italy Disclosures Dr. Sternberg has received research funding for
Management of Prostate Cancer
 Management of Prostate Cancer An ESMO Perspective Alan Horwich Conflicts of Interest Disclosure Alan Horwich I have no personal conflicts of interest relating to prostate cancer. European Incidence and
Management of Prostate Cancer An ESMO Perspective Alan Horwich Conflicts of Interest Disclosure Alan Horwich I have no personal conflicts of interest relating to prostate cancer. European Incidence and
Joelle Hamilton, M.D.
 Joelle Hamilton, M.D. www.urologycentersalabama.com Case Presentation: CRPC, Rising PSA 70 yo healthy, fit, active man post RALP 8 years prior with rising PSA Rising PSA from 0.02 nadir to 3.4 thus ADT
Joelle Hamilton, M.D. www.urologycentersalabama.com Case Presentation: CRPC, Rising PSA 70 yo healthy, fit, active man post RALP 8 years prior with rising PSA Rising PSA from 0.02 nadir to 3.4 thus ADT
Advanced Prostate Cancer. Searching for Optimal Therapy Sequence and Assessing Emerging Treatment Options
 Advanced Prostate Cancer Searching for Optimal Therapy Sequence and Assessing Emerging Treatment Options Disclaimer This slide deck in its original and unaltered format is for educational purposes and
Advanced Prostate Cancer Searching for Optimal Therapy Sequence and Assessing Emerging Treatment Options Disclaimer This slide deck in its original and unaltered format is for educational purposes and
Advanced Prostate Cancer. November Jose W. Avitia, M.D
 Advanced Prostate Cancer November 4 2017 Jose W. Avitia, M.D In 2017 161,000 new cases of prostate cancer diagnosed in US, mostly with elevated PSA 5-10% will present with metastatic disease In 2017: 26,000
Advanced Prostate Cancer November 4 2017 Jose W. Avitia, M.D In 2017 161,000 new cases of prostate cancer diagnosed in US, mostly with elevated PSA 5-10% will present with metastatic disease In 2017: 26,000
A Forward Look at Options for. In Prostate Cancer
 A Forward Look at Options for Prostate Cancer Charles J Ryan, MD Associate Professor of Medicine Helen Diller Family Comprehensive Cancer Center University of California, San Francisco UC 1 SF UC SF Castration
A Forward Look at Options for Prostate Cancer Charles J Ryan, MD Associate Professor of Medicine Helen Diller Family Comprehensive Cancer Center University of California, San Francisco UC 1 SF UC SF Castration
Apalutamide in the treatment of castrate-resistant prostate cancer: evidence from clinical trials
 811450TAU0010.1177/1756287218811450Therapeutic Advances in UrologyVS Koshkin and EJ Small review-article2018 Therapeutic Advances in Urology Review Apalutamide in the treatment of castrate-resistant prostate
811450TAU0010.1177/1756287218811450Therapeutic Advances in UrologyVS Koshkin and EJ Small review-article2018 Therapeutic Advances in Urology Review Apalutamide in the treatment of castrate-resistant prostate
What will change for men with advanced prostate cancer in the next 24 months? ESO Observatory: Perspective on endocrine and chemotherapy agents
 Perspective on endocrine and chemotherapy agents Cora N. Sternberg Department of Medical Oncology San Camillo & Forlanini Hospitals Rome, Italy Disclosures Dr.Sternberg has received research funding for
Perspective on endocrine and chemotherapy agents Cora N. Sternberg Department of Medical Oncology San Camillo & Forlanini Hospitals Rome, Italy Disclosures Dr.Sternberg has received research funding for
Michiel H.F. Poorthuis*, Robin W.M. Vernooij*, R. Jeroen A. van Moorselaar and Theo M. de Reijke
 First-line non-cytotoxic therapy in chemotherapynaive patients with metastatic castration-resistant prostate cancer: a systematic review of 10 randomised clinical trials Michiel H.F. Poorthuis*, Robin
First-line non-cytotoxic therapy in chemotherapynaive patients with metastatic castration-resistant prostate cancer: a systematic review of 10 randomised clinical trials Michiel H.F. Poorthuis*, Robin
SUPPLEMENTARY APPENDIX. COU-AA-301 enrolled men with pathologically confirmed mcrpc who had received previous
 SUPPLEMENTARY APPENDIX Methods Subjects COUAA30 enrolled men with pathologically confirmed mcrpc who had received previous treatment with docetaxel chemotherapy and had documented PSA progression according
SUPPLEMENTARY APPENDIX Methods Subjects COUAA30 enrolled men with pathologically confirmed mcrpc who had received previous treatment with docetaxel chemotherapy and had documented PSA progression according
SOGUG meeting New drugs after docetaxel chemotherapy in patient with mcrpc
 SOGUG meeting New drugs after docetaxel chemotherapy in patient with mcrpc Stéphane OUDARD, MD, PhD Head of the Oncology department Georges Pompidou Hospital, Paris France University Rene Descartes, Paris
SOGUG meeting New drugs after docetaxel chemotherapy in patient with mcrpc Stéphane OUDARD, MD, PhD Head of the Oncology department Georges Pompidou Hospital, Paris France University Rene Descartes, Paris
ASCO 2012 Genitourinary tumors
 ASCO 2012 Genitourinary tumors Post ASCO Bern 14-06-2012 Dr. med. Richard Cathomas leitender Arzt Onkologie, KSGR, Chur Renal cell cancer Changes in first line treatment? Prostate cancer 3 positive phase
ASCO 2012 Genitourinary tumors Post ASCO Bern 14-06-2012 Dr. med. Richard Cathomas leitender Arzt Onkologie, KSGR, Chur Renal cell cancer Changes in first line treatment? Prostate cancer 3 positive phase
METASTATIC PROSTATE CANCER MANAGEMENT K I R U B E L T E F E R A M. D. T R I H E A LT H C A N C E R I N S T I T U T E 0 1 / 3 1 /
 METASTATIC PROSTATE CANCER MANAGEMENT K I R U B E L T E F E R A M. D. T R I H E A LT H C A N C E R I N S T I T U T E 0 1 / 3 1 / 2 0 1 8 Prostate Cancer- Statistics Most common cancer in men after a skin
METASTATIC PROSTATE CANCER MANAGEMENT K I R U B E L T E F E R A M. D. T R I H E A LT H C A N C E R I N S T I T U T E 0 1 / 3 1 / 2 0 1 8 Prostate Cancer- Statistics Most common cancer in men after a skin
Prostate Cancer Management: From Early Chemical Recurrence to HRPC (excluding Immunotherapy).
 Thanks to: The Medical Educator Consortium Luis Raez, MD, Florida International University 15th ed. Prostate Cancer Management: From Early Chemical Recurrence to HRPC (excluding Immunotherapy). Mayer Fishman,
Thanks to: The Medical Educator Consortium Luis Raez, MD, Florida International University 15th ed. Prostate Cancer Management: From Early Chemical Recurrence to HRPC (excluding Immunotherapy). Mayer Fishman,
When exogenous testosterone therapy is. adverse responses can be induced.
 Theoretical tips It has been reasoned that discontinuation of ADT in non orchiectomized patients may have detrimental effect on patients with CRPC as discontinuation of ADT can result in renewed release
Theoretical tips It has been reasoned that discontinuation of ADT in non orchiectomized patients may have detrimental effect on patients with CRPC as discontinuation of ADT can result in renewed release
mcrpc in 2016 How to decide the optimal treatment? N. Mottet
 mcrpc in 2016 How to decide the optimal treatment? N. Mottet Disclosures Conflict of interest Chairman EAU PCa guidelines..... Therefore I'm 100% biased Castrate-resistant prostate cancer (CRPC) Definition
mcrpc in 2016 How to decide the optimal treatment? N. Mottet Disclosures Conflict of interest Chairman EAU PCa guidelines..... Therefore I'm 100% biased Castrate-resistant prostate cancer (CRPC) Definition
Please consider the following information on ZYTIGA (abiraterone acetate). ZYTIGA - Compendia Communication - NCCN LATITUDE and STAMPEDE June 2017
 Page 1 of 2 Janssen Scientific Affairs, LLC 1125 Trenton-Harbourton Road PO Box 200 Titusville, NJ 08560 800.526.7736 tel 609.730.3138 fax June 08, 2017 Joan McClure 275 Commerce Drive #300 Fort Washington,
Page 1 of 2 Janssen Scientific Affairs, LLC 1125 Trenton-Harbourton Road PO Box 200 Titusville, NJ 08560 800.526.7736 tel 609.730.3138 fax June 08, 2017 Joan McClure 275 Commerce Drive #300 Fort Washington,
Incorporating New Agents into the Treatment Paradigm for Prostate Cancer
 Incorporating New Agents into the Treatment Paradigm for Prostate Cancer Dr. Celestia S. Higano FACP, Professor, Medicine and Urology, Uni. of Washington Member, Fred Hutchinson Cancer Research Center
Incorporating New Agents into the Treatment Paradigm for Prostate Cancer Dr. Celestia S. Higano FACP, Professor, Medicine and Urology, Uni. of Washington Member, Fred Hutchinson Cancer Research Center
Updates in Prostate Cancer Treatment 2018
 Updates in Prostate Cancer Treatment 2018 Mountain States Cancer Conference Elaine T. Lam, MD November 3, 2018 Learning Objectives Understand the difference between hormone sensitive and castration resistant
Updates in Prostate Cancer Treatment 2018 Mountain States Cancer Conference Elaine T. Lam, MD November 3, 2018 Learning Objectives Understand the difference between hormone sensitive and castration resistant
Rationale Design of Combination Therapy in Prostate Cancer: Targeting the AR and PI3K pathways
 Rationale Design of Combination Therapy in Prostate Cancer: Targeting the AR and PI3K pathways Brett S Carver, MD Assistant Attending, Department of Surgery/Urology Prostate Cancer Therapeutics: A Changed
Rationale Design of Combination Therapy in Prostate Cancer: Targeting the AR and PI3K pathways Brett S Carver, MD Assistant Attending, Department of Surgery/Urology Prostate Cancer Therapeutics: A Changed
Management Options in Advanced Prostate Cancer: What is the Role for Sipuleucel-T?
 Clinical Medicine Insights: Oncology Consise Review Open Access Full open access to this and thousands of other papers at http://www.la-press.com. Management Options in Advanced Prostate Cancer: What is
Clinical Medicine Insights: Oncology Consise Review Open Access Full open access to this and thousands of other papers at http://www.la-press.com. Management Options in Advanced Prostate Cancer: What is
SUMMARY. 3. Emerging understanding of mechanisms of resistance to current treatments
 SUMMARY 1. Discuss the active agents in prostate cancer currently available in Australia 2. Celebrate the growing role for Prostate Medical Oncologists in Multi Disc Teams active treaments overall survival
SUMMARY 1. Discuss the active agents in prostate cancer currently available in Australia 2. Celebrate the growing role for Prostate Medical Oncologists in Multi Disc Teams active treaments overall survival
Prostate Cancer 2009 MDV Anti-Angiogenesis. Anti-androgen Radiotherapy Surgery Androgen Deprivation Therapy. Docetaxel/Epothilone
 Prostate Cancer 2009 Anti-Angiogenesis MDV 3100 Anti-androgen Radiotherapy Surgery Androgen Deprivation Therapy Docetaxel/Epothilone Abiraterone DC therapy Bisphosphonates Denosumab Secondary Hormonal
Prostate Cancer 2009 Anti-Angiogenesis MDV 3100 Anti-androgen Radiotherapy Surgery Androgen Deprivation Therapy Docetaxel/Epothilone Abiraterone DC therapy Bisphosphonates Denosumab Secondary Hormonal
Prostate cancer update: Dr Robert Huddart Cancer Clinic London
 Prostate cancer update: 2013 Dr Robert Huddart Cancer Clinic London Recent developments Improved imaging New radiotherapy technologies Radiotherapy for advanced disease Intermittent hormone therapy New
Prostate cancer update: 2013 Dr Robert Huddart Cancer Clinic London Recent developments Improved imaging New radiotherapy technologies Radiotherapy for advanced disease Intermittent hormone therapy New
Developmental Therapeutics for Genitourinary Malignancies
 Developmental Therapeutics for Genitourinary Malignancies Russell Szmulewitz, MD April 2018 Disclosure Information 23 rd Annual Developmental Therapeutics Symposium Name of Speaker I have the following
Developmental Therapeutics for Genitourinary Malignancies Russell Szmulewitz, MD April 2018 Disclosure Information 23 rd Annual Developmental Therapeutics Symposium Name of Speaker I have the following
GU Guidelines Update Meeting: M0 Castrate Resistant Prostate Cancer. Dr. Simon Yu Nov 18, 2017
 GU Guidelines Update Meeting: M0 Castrate Resistant Prostate Cancer Dr. Simon Yu Nov 18, 2017 Faculty/Presenter Disclosure Faculty: Dr. Simon Yu Relationships with commercial interests: Grants/Research
GU Guidelines Update Meeting: M0 Castrate Resistant Prostate Cancer Dr. Simon Yu Nov 18, 2017 Faculty/Presenter Disclosure Faculty: Dr. Simon Yu Relationships with commercial interests: Grants/Research
ORIGINAL PAPER. Gianpaolo Perletti 1,2, Elena Monti 1, Emanuela Marras 1, Anne Cleves 3, Vittorio Magri 4, Alberto Trinchieri 5, Paul S.
 ORIGINAL PAPER DOI: 10.4081/aiua.2015.2.121 Efficacy and safety of second-line agents for treatment of metastatic castration-resistant prostate cancer progressing after docetaxel. A systematic review and
ORIGINAL PAPER DOI: 10.4081/aiua.2015.2.121 Efficacy and safety of second-line agents for treatment of metastatic castration-resistant prostate cancer progressing after docetaxel. A systematic review and
SIMPOSIO. Radioterapia stereotassica e nuovi farmaci nel tumore e della prostata metastatico
 SIMPOSIO Radioterapia stereotassica e nuovi farmaci nel tumore e della prostata metastatico Definition of Oligometastatic PCa 1-3 synchronous metastases (bone and/or lymph nodes) 2-5 synchronous metastases
SIMPOSIO Radioterapia stereotassica e nuovi farmaci nel tumore e della prostata metastatico Definition of Oligometastatic PCa 1-3 synchronous metastases (bone and/or lymph nodes) 2-5 synchronous metastases
Summary... 2 GENITOURINARY TUMOURS - PROSTATE... 3
 ESMO 2016 Congress 7-11 October, 2016 Copenhagen, Denmark Table of Contents Summary... 2 GENITOURINARY TUMOURS - PROSTATE... 3 Custirsen provides no additional survival benefit to cabazitaxel/prednisone
ESMO 2016 Congress 7-11 October, 2016 Copenhagen, Denmark Table of Contents Summary... 2 GENITOURINARY TUMOURS - PROSTATE... 3 Custirsen provides no additional survival benefit to cabazitaxel/prednisone
Recent Progress in Management of Advanced Prostate Cancer
 Review Article [1] April 15, 2005 By Philip W. Kantoff, MD [2] Androgen-deprivation therapy, usually with combined androgen blockade, is standard initial treatment for advanced prostate cancer. With failure
Review Article [1] April 15, 2005 By Philip W. Kantoff, MD [2] Androgen-deprivation therapy, usually with combined androgen blockade, is standard initial treatment for advanced prostate cancer. With failure
Advanced Prostate Cancer
 Advanced Prostate Cancer January 13, 2017 Sindu Kanjeekal MD FRCPC Medical Oncology and Hematology Regional Systemic Quality Lead Erie St Clair Adjunct Professor Schulich School of Medicine and University
Advanced Prostate Cancer January 13, 2017 Sindu Kanjeekal MD FRCPC Medical Oncology and Hematology Regional Systemic Quality Lead Erie St Clair Adjunct Professor Schulich School of Medicine and University
Policy. not covered Sipuleucel-T. Considerations Sipuleucel-T. Description Sipuleucel-T. be medically. Sipuleucel-T. covered Q2043.
 Cellular Immunotherapy forr Prostate Cancer Policy Number: 8.01.53 Origination: 11/2010 Last Review: 11/2014 Next Review: 11/2015 Policy BCBSKC will provide coverage for cellular immunotherapy for prostate
Cellular Immunotherapy forr Prostate Cancer Policy Number: 8.01.53 Origination: 11/2010 Last Review: 11/2014 Next Review: 11/2015 Policy BCBSKC will provide coverage for cellular immunotherapy for prostate
January Abiraterone pre-docetaxel for patients with asymptomatic or minimally symptomatic metastatic castration resistant prostate cancer
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Abiraterone pre-docetaxel for asymptomatic/minimally symptomatic metastatic castration resistant prostate cancer Abiraterone pre-docetaxel for patients with asymptomatic
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Abiraterone pre-docetaxel for asymptomatic/minimally symptomatic metastatic castration resistant prostate cancer Abiraterone pre-docetaxel for patients with asymptomatic
Patients Living Longer: The Promise of Newer Therapies
 Patients Living Longer: The Promise of Newer Therapies David M. Nanus, MD! Chief, Division of Hematology and Medical Oncology! Weill Cornell Medicine! New York Presbyterian Hospital!! Demographics 180,890
Patients Living Longer: The Promise of Newer Therapies David M. Nanus, MD! Chief, Division of Hematology and Medical Oncology! Weill Cornell Medicine! New York Presbyterian Hospital!! Demographics 180,890
Prostate Cancer: Vision of the Future By: H.R.Jalalian
 1 H. R. Jalalian Hematologist&Oncologist Baqiyatallah University of Medical Sciences 2 State of the art: vision on the future Diagnosis Surgery Radiotherapy Medical Oncology 3 Early Detection PSA sensitivity
1 H. R. Jalalian Hematologist&Oncologist Baqiyatallah University of Medical Sciences 2 State of the art: vision on the future Diagnosis Surgery Radiotherapy Medical Oncology 3 Early Detection PSA sensitivity
Androgen synthesis inhibitors in the treatment of castration resistant prostate cancer
 (2014) 16, 387 400 2014 AJA, SIMM & SJTU. All rights reserved 1008-682X www.asiaandro.com; www.ajandrology.com Prostate Cancer Open Access INVITED REVIEW Androgen synthesis inhibitors in the treatment
(2014) 16, 387 400 2014 AJA, SIMM & SJTU. All rights reserved 1008-682X www.asiaandro.com; www.ajandrology.com Prostate Cancer Open Access INVITED REVIEW Androgen synthesis inhibitors in the treatment
This article is authors accepted manuscripts in Asian Journal of Andrology.
 This article is authors accepted manuscripts in Asian Journal of Andrology. OnlineFirst Author s accepted Manuscripts are PDF versions of manuscripts that have been peer reviewed and accepted for publication,
This article is authors accepted manuscripts in Asian Journal of Andrology. OnlineFirst Author s accepted Manuscripts are PDF versions of manuscripts that have been peer reviewed and accepted for publication,
Management of castrate resistant disease: after first line hormone therapy fails
 Management of castrate resistant disease: after first line hormone therapy fails Rob Jones Consultant in Medical Oncology Beatson Cancer Centre Glasgow Rhona McMenemin Consultant in Clinical Oncology The
Management of castrate resistant disease: after first line hormone therapy fails Rob Jones Consultant in Medical Oncology Beatson Cancer Centre Glasgow Rhona McMenemin Consultant in Clinical Oncology The
Mapping the Complexity of Androgen Signaling In Prostate Cancer Progression Eleni Efstathiou MD PhD
 Mapping the Complexity of Androgen Signaling In Prostate Cancer Progression Eleni Efstathiou MD PhD The University of Athens Medical School Dept of Clinical Therapeutics Prostate Cancer Evolution Chemotherapy
Mapping the Complexity of Androgen Signaling In Prostate Cancer Progression Eleni Efstathiou MD PhD The University of Athens Medical School Dept of Clinical Therapeutics Prostate Cancer Evolution Chemotherapy
American Urological Association (AUA) Guideline
 1 Approved by the AUA Board of Directors May 2018 Authors disclosure of potential conflicts of interest and author/staff contributions appear at the end of the article. 2018 by the American Urological
1 Approved by the AUA Board of Directors May 2018 Authors disclosure of potential conflicts of interest and author/staff contributions appear at the end of the article. 2018 by the American Urological
Management of mcrpc: Hormonal therapy and treatment sequence for CRPC
 Management of mcrpc: Hormonal therapy and treatment sequence for CRPC Professor Bertrand Tombal, MD, PhD Cliniques universitaires Saint-Luc Université catholique de Louvain Brussels, Belgium Credentials
Management of mcrpc: Hormonal therapy and treatment sequence for CRPC Professor Bertrand Tombal, MD, PhD Cliniques universitaires Saint-Luc Université catholique de Louvain Brussels, Belgium Credentials
SYSTEMIC THERAPIES FOR CRPC: Chemotherapy and Radium-223
 SYSTEMIC THERAPIES FOR CRPC: Chemotherapy and Radium-223 ELENA CASTRO Spanish National Cancer Research Centre Prostate Preceptorship. Lugano 4-5 October 2018 Disclosures Participation in advisory boards:
SYSTEMIC THERAPIES FOR CRPC: Chemotherapy and Radium-223 ELENA CASTRO Spanish National Cancer Research Centre Prostate Preceptorship. Lugano 4-5 October 2018 Disclosures Participation in advisory boards:
UPDATE ON RECENT CUTTING-EDGE TRIALS: TREATMENTS NOW AVAILABLE FOR NEWLY DIAGNOSED mhspc PATIENTS
 UPDATE ON RECENT CUTTING-EDGE TRIALS: TREATMENTS NOW AVAILABLE FOR NEWLY DIAGNOSED mhspc PATIENTS Dr. Neal Shore, Carolina Urologic Research Centre, USA Assoc. Prof. Neeraj Agarwal, Huntsman Cancer Institute,
UPDATE ON RECENT CUTTING-EDGE TRIALS: TREATMENTS NOW AVAILABLE FOR NEWLY DIAGNOSED mhspc PATIENTS Dr. Neal Shore, Carolina Urologic Research Centre, USA Assoc. Prof. Neeraj Agarwal, Huntsman Cancer Institute,
Principal Investigator. General Information. Conflict of Interest Published on The YODA Project (http://yoda.yale.edu)
 Principal Investigator First Name: Antonio Last Name: Finelli Degree: MD, MSc, FRCSC Primary Affiliation: Princess Margaret Cancer Centre E-mail: antonio.finelli@uhn.ca Phone number: 416-946-4501 x2851
Principal Investigator First Name: Antonio Last Name: Finelli Degree: MD, MSc, FRCSC Primary Affiliation: Princess Margaret Cancer Centre E-mail: antonio.finelli@uhn.ca Phone number: 416-946-4501 x2851
Lessons from in-vivo models of castration-resistant prostate cancer
 REVIEW C URRENT OPINION Lessons from in-vivo models of castration-resistant prostate cancer Dong Lin a,b, Peter W. Gout b, and Yuzhuo Wang a,b,c Purpose of review Although the treatment of castration-resistant
REVIEW C URRENT OPINION Lessons from in-vivo models of castration-resistant prostate cancer Dong Lin a,b, Peter W. Gout b, and Yuzhuo Wang a,b,c Purpose of review Although the treatment of castration-resistant
NCCN Guidelines for Prostate Cancer V Web teleconference 06/17/16 and 06/30/17
 Guideline Page and Request PROS-1 Submission from Myriad Genetic Laboratories, Inc. Request addition of recommendation for genetic risk assessment/testing to the Initial Clinical Assessment algorithm for
Guideline Page and Request PROS-1 Submission from Myriad Genetic Laboratories, Inc. Request addition of recommendation for genetic risk assessment/testing to the Initial Clinical Assessment algorithm for
Chemohormonal Therapy For Prostate Cancer. What is old, is new again!
 Chemohormonal Therapy For Prostate Cancer What is old, is new again! Mount Tremblant January 20, 2017 Kala S. Sridhar MD, MSc, FRCPC Medical Oncologist, Princess Margaret Hospital Head, GU Medical Oncology
Chemohormonal Therapy For Prostate Cancer What is old, is new again! Mount Tremblant January 20, 2017 Kala S. Sridhar MD, MSc, FRCPC Medical Oncologist, Princess Margaret Hospital Head, GU Medical Oncology
Urological Science xxx (2015) 1e5. Contents lists available at ScienceDirect. Urological Science. journal homepage:
 Urological Science xxx (2015) 1e5 Contents lists available at ScienceDirect Urological Science journal homepage: www.urol-sci.com Original article The efficacy of abiraterone acetate in treating Taiwanese
Urological Science xxx (2015) 1e5 Contents lists available at ScienceDirect Urological Science journal homepage: www.urol-sci.com Original article The efficacy of abiraterone acetate in treating Taiwanese
Have we optimized the use of Androgen Receptor pathway targeted drugs in Castrate-Resistant Prostate Cancer?
 Have we optimized the use of Androgen Receptor pathway targeted drugs in Castrate-Resistant Prostate Cancer? Karim Fizazi, MD, PhD Institut Gustave Roussy Villejuif, France Disclosure Participation to
Have we optimized the use of Androgen Receptor pathway targeted drugs in Castrate-Resistant Prostate Cancer? Karim Fizazi, MD, PhD Institut Gustave Roussy Villejuif, France Disclosure Participation to
Francesco Massari Oncologia Medica Azienda Ospedaliero Universitaria di Bologna Policlinico S. Orsola-Malpighi
 Focus sulla malattia metastatica ormonosensibile (mhspc) ADT e Chemioterapia: quando e a chi? Francesco Massari Oncologia Medica Azienda Ospedaliero Universitaria di Bologna Policlinico S. Orsola-Malpighi
Focus sulla malattia metastatica ormonosensibile (mhspc) ADT e Chemioterapia: quando e a chi? Francesco Massari Oncologia Medica Azienda Ospedaliero Universitaria di Bologna Policlinico S. Orsola-Malpighi
POSITIVE CHMP OPINION FOR XTANDI (ENZALUTAMIDE) IN ADVANCED PROSTATE CANCER 1
 POSITIVE CHMP OPINION FOR XTANDI (ENZALUTAMIDE) IN ADVANCED PROSTATE CANCER 1 Enzalutamide recommended for approval in the European Union (EU) for the treatment of adult men with metastatic castration-resistant
POSITIVE CHMP OPINION FOR XTANDI (ENZALUTAMIDE) IN ADVANCED PROSTATE CANCER 1 Enzalutamide recommended for approval in the European Union (EU) for the treatment of adult men with metastatic castration-resistant
original research Abstract Introduction
 original research A prognostic model for stratifying clinical outcomes in chemotherapy-naive metastatic castration-resistant prostate cancer patients treated with abiraterone acetate Daniel Joseph Khalaf,
original research A prognostic model for stratifying clinical outcomes in chemotherapy-naive metastatic castration-resistant prostate cancer patients treated with abiraterone acetate Daniel Joseph Khalaf,
Challenges in the sequencing of therapies for the management of metastatic castrationresistant
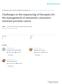 See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/261569460 Challenges in the sequencing of therapies for the management of metastatic castrationresistant
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/261569460 Challenges in the sequencing of therapies for the management of metastatic castrationresistant
PROSTATE CANCER Importance of Molecular Characteristics in Support of Therapeutic Decisions
 PROSTATE CANCER Importance of Molecular Characteristics in Support of Therapeutic Decisions Outline Prognostic and diagnostic value of pathologic and molecular alterations in prostate cancer Current status
PROSTATE CANCER Importance of Molecular Characteristics in Support of Therapeutic Decisions Outline Prognostic and diagnostic value of pathologic and molecular alterations in prostate cancer Current status
HHS Public Access Author manuscript Prostate Cancer Prostatic Dis. Author manuscript; available in PMC 2015 December 01.
 Prospective Evaluation of Low-Dose Ketoconazole Plus Hydrocortisone (HC) in Docetaxel Pre-treated Castration- Resistant Prostate Cancer (CRPC) Patients Ernest N. Lo, M.D. 1, Laurel A. Beckett, Ph.D. 1,
Prospective Evaluation of Low-Dose Ketoconazole Plus Hydrocortisone (HC) in Docetaxel Pre-treated Castration- Resistant Prostate Cancer (CRPC) Patients Ernest N. Lo, M.D. 1, Laurel A. Beckett, Ph.D. 1,
Prognostic Model Predicting Metastatic Castration-Resistant Prostate Cancer Survival in Men Treated With Second-Line Chemotherapy
 DOI:10.1093/jnci/djt280 Advance Access publication October 17, 2013 The Author 2013. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
DOI:10.1093/jnci/djt280 Advance Access publication October 17, 2013 The Author 2013. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
The Role of Immunotherapy in Prostate Cancer: What s Trending?
 The Role of Immunotherapy in Prostate Cancer: What s Trending? Douglas G. McNeel, MD, PhD University of Wisconsin Carbone Cancer Center Madison, Wisconsin Prostate cancer rationale for immune therapies
The Role of Immunotherapy in Prostate Cancer: What s Trending? Douglas G. McNeel, MD, PhD University of Wisconsin Carbone Cancer Center Madison, Wisconsin Prostate cancer rationale for immune therapies
Bridging the Gap From Knowledge to Practice in Castration-Resistant Prostate Cancer A CME Activity
 Overview Bridging the Gap From Knowledge to Practice in Castration-Resistant Prostate Cancer A CME Activity The selection of therapy for patients with castration-resistant prostate cancer (CRPC) remains
Overview Bridging the Gap From Knowledge to Practice in Castration-Resistant Prostate Cancer A CME Activity The selection of therapy for patients with castration-resistant prostate cancer (CRPC) remains
A SPECIAL MEETING REVIEW EDITION. Special Reporting on: PLUS Meeting Abstract Summaries. With Expert Commentary by:
 August 2013 Volume 11, Issue 8, Supplement 11 A SPECIAL MEETING REVIEW EDITION Highlights in Advanced Prostate Cancer From the 2013 American Urological Association Annual Meeting and the 2013 American
August 2013 Volume 11, Issue 8, Supplement 11 A SPECIAL MEETING REVIEW EDITION Highlights in Advanced Prostate Cancer From the 2013 American Urological Association Annual Meeting and the 2013 American
Saad et al [12] Metastatic CRPC. Bhoopalam et al [14] M0 PCa on ADT <1 yr vs >1 yr ADT
![Saad et al [12] Metastatic CRPC. Bhoopalam et al [14] M0 PCa on ADT <1 yr vs >1 yr ADT Saad et al [12] Metastatic CRPC. Bhoopalam et al [14] M0 PCa on ADT <1 yr vs >1 yr ADT](/thumbs/71/65630457.jpg) Evolution of Treatment Options for Patients with and Bone Metastases Trials of Treatments for Castration-Resistant Prostrate Cancer Mentioned in This Review Bisphosphonates (Zometa) 4 mg IV 8 mg IV ( to
Evolution of Treatment Options for Patients with and Bone Metastases Trials of Treatments for Castration-Resistant Prostrate Cancer Mentioned in This Review Bisphosphonates (Zometa) 4 mg IV 8 mg IV ( to
CME Baseline Curriculum Report
 CME Baseline Curriculum Report October 14, 2010 For CME Activity: Managing Advanced Prostate Cancer in the Community Setting: A Case-based Curriculum Supported by an independent educational grant from
CME Baseline Curriculum Report October 14, 2010 For CME Activity: Managing Advanced Prostate Cancer in the Community Setting: A Case-based Curriculum Supported by an independent educational grant from
Targeting the Androgen Receptor in Castration Resistant Prostate Cancer. Raoul S. Concepcion, MD,FACS IPCU January 2017
 Targeting the Androgen Receptor in Castration Resistant Prostate Cancer Raoul S. Concepcion, MD,FACS IPCU January 2017 Consultant: Myriad, CUSP, Tolmar, Integra Cloud, Cellay Speakers Bureau: Dendreon,
Targeting the Androgen Receptor in Castration Resistant Prostate Cancer Raoul S. Concepcion, MD,FACS IPCU January 2017 Consultant: Myriad, CUSP, Tolmar, Integra Cloud, Cellay Speakers Bureau: Dendreon,
Con$nuing Care for Your Pa$ents with Metasta$c CRPC
 27 th Annual InternaAonal Prostate Cancer Symposium Update January 26, 2017 Con$nuing Care for Your Pa$ents with Metasta$c CRPC Michael S. Cookson, MD, MMHC Professor and Chair Department of Urology University
27 th Annual InternaAonal Prostate Cancer Symposium Update January 26, 2017 Con$nuing Care for Your Pa$ents with Metasta$c CRPC Michael S. Cookson, MD, MMHC Professor and Chair Department of Urology University
