Keratinocyte Stem Cells, Label-Retaining Cells and Possible Genome Protection Mechanisms
|
|
|
- Julia Ramsey
- 5 years ago
- Views:
Transcription
1 Keratinocyte Stem Cells, Label-Retaining Cells and Possible Genome Protection Mechanisms Christopher S. Potten EpiStem Ltd., Incubator Building, Manchester, UK Stem cells are the crucial cells upon which the entire tissue is dependent. Here we define and discuss what is meant by and known about keratinocyte stem cells. One way in which these cells have been studied is by their ability to retain radioactivity labelled thymidine for long periods of time (label retaining cells, LRCs). The underlying mechanism has been assumed in the past to be slow cycling but a more likely explanation is the selective segregation of old and new DNA strands (Cairns s hypothesis). Experiments in the small intestine indicate that the stem cells here are selectively sorting their DNA and becoming LRCs. A possible role for p53 in stem cell biology is presented. Key words: DNA strand segregation/epidermal proliferative unit/hair follicle bulge/keratinocyte stem cells/label retaining cells/p53 J Investig Dermatol Symp Proc 9: , 2004 Skin has evolved to be a tough, flexible, protective barrier. The outer layers of the epidermis, the cornified layers, are composed of hard, thin, plates of keratinous material, sometimes referred to as squames, which represent the remains of individual cells which are constantly being sloughed from the surface. Beneath the cornified layers are several suprabasal strata of metabolically active but reproductively dead epidermal cells, which are tightly bound to one another, the lower layer of which, the spinous layer, has an intricate interdigitating membrane with many desmosomes (see Fig 4 in Potten, 1994) that bind these cells to the lowermost layer, the basal layer, within which cell proliferation occurs. In the mouse, the basal layer is relatively flat, in the human epidermis the undulations may be greater, and the number of nucleated cell layers in the spinous and granular layers may be greater. Beneath the epidermis, there is a tough, elastic, dermis that contains many collagen and elastain fibers. The skin may also be covered by hair or fur that provides thermal and UV protection and beneath the dermis there may be an adipose layer providing thermal insulation. The skin provides an effective protection again UV damage, and minor and major wounds. The large constant cell loss from the surface is compensated for in steady state by cell division activity in the basal layer. A question that has interested cutaneous biologists for many years is whether all the cells in the basal layer possess an equal potential in terms of proliferative activity, or whether subclasses of proliferative cells can be identified. The skin possesses a powerful, regenerative potential if injury occurs, and an ancillary question has been whether all Abbreviations: EPU, epidermal proliferative unit; LRC, label-retaining cell Keynote presentation at the Montagna Symposium on the Biology of Skin, Snowmass, June the basal cells are equally efficient in their contribution to this regenerative wounding response. For many years, the basal layer was regarded as a homogeneous population with all cells being equipotential (Fig 1a); however, work in the early 1970s began to question this model and a more complex hierarchical, or lineage-based, concept became fairly rapidly accepted for the basal layer (Fig 1b). Here the basal layer was composed of a small number of self-maintaining stem cells that divided in an essentially asymmetric fashion, generating a daughter like themselves and a second daughter cell that entered a dividing transit population, a step that can be regarded as involving a differentiation event. In the dividing transit population, a series of cell divisions could occur before generating post-mitotic maturing cells that remained in the basal layer, until appropriate signals instigated the migration process from the basal layer to the suprabasal spinous layer. The number of generations in the dividing transit population is probably no more than three or four in mouse epidermis, but maybe somewhat greater in human epidermis where some cells of the last transit generation may be displaced into the first suprabasal layer. A fairly wide range of murine experiments provided evidence for this hierarchical organization. These ranged from classical cell kinetic experiments, such as the percent-labelled mitosis technique, analysis of clusters of tritiated thymidine-labelled cells with time, mathematical modelling studies, clonal regeneration following exposure to cytotoxic agents in vivo, clonal regeneration studies in vitro, studies of the retention of tritiated thymidine label in some basal cells when animals are labelled as babies and analyzed as adults (label-retention studies), considerations from chemical carcinogenesis experiments, spatial considerations and lineage tracking, studies into gap junctional communication and studies using transgenic models and, finally, concordance with other tissues, i.e., the acceptance of Copyright r 2004 by The Society for Investigative Dermatology, Inc. 183
2 184 POTTEN JID SYMPOSIUM PROCEEDINGS Figure 1 Possible cell lineage diagrams to consider for the proliferative organization of the basal layer of the epidermis. (a) Simple model where all basal cells are the same, dividing asymmetrically with random loss into the functional compartment. This model was generally assumed to apply to the basal layer of the epidermis in the 1950s and 1960s. (b) A short cell lineage with self-replacing stem cells and three dividing generations of transit cells. The stem cells now make up only a small proportion of the total proliferative compartment of the basal layer, that proportion being determined by the number of generations in the transit lineage. The stem cells (circles) divide essentially asymmetrically producing 1 further stem cell and the first of the dividing transit cells (squares). This can be regarded as a differentiation event. (c) A modification of lineage (b) where terminal differentiation may occur at any level in the dividing transit population. There are many variations of this theme. Model (b) or some modification of model (c) is believed to exist in mouse epidermal basal layer. (d) Is a slightly more complex version of model (b), where the differentiation event that distinguishes stem cells from dividing transit cells may be delayed at any point in the early stages of the dividing transit lineage, thus the differentiation event occurs at point x, y, or z. This model generates two classes of stem cells, the actual lineage ancestor stem cell and a category of potential stem cells that have yet to undergo the differentiation event that results in the production of a dividing transit cell. a unified scheme for cell replacement in all proliferating tissues. One of the earlier suggestions of this type of lineage organization arose out of studies into the spatial organization of murine and rodent epidermis, conducted by Christophers and colleagues, Mackenzie and colleagues, and Potten and colleagues, in the early 1970s (Mackenzie, 1969, 1970; Christophers, 1970, 1971a, b; Potten, 1974; Potten and Allen, 1975). These studies indicated that the cornified cells, the cells of the granular and spinous layers, were all arranged into discrete columns of cells, with their lateral junctions being coincident. These cells tended not to be circular piles of plates, but hexagonal in surface profile when viewed from above (see Fig 2c). The piles of hexagonal cells minimally overlapped with other hexagonal columns and the edges of the cells had a regular alternating interdigitation at the boundaries (see Fig 2b). These structural features implied a considerable organization in the basal layer, immediately beneath the columns, that insured a steady cell production (cell migration) that was coordinated from one column to the next. The implications were that each column represented the historical proliferative record of a small group of proliferative basal cells that lay beneath the column, and that these proliferative cells were a stable and permanent proliferative unit beneath each column. This led to the concept of the epidermal Figure 2 Images generated in the light or electron microscope relating to epidermal proliferative units (EPU). (a) A thin section (1 mm resin embedded mouse epidermis showing the basal layer, suprabasal cells, and columns of cornified cells). The boundaries of the columns are marked by arrows. (b) A higher power picture from the transmission electron microscope showing the columns of cornified cells with their minimal overlap and regular interdigitation. (c) A scanning electron microscope picture of the surface of the epidermis showing the hexagonal outlines and minimal overlap between adjacent columns. (d) Phase contrast image of a sheet of epidermis showing the outline of the column (EPU) with one cell labelled with tritiated thymidine with the silver grains in the autoradiograph showing up as light granules. Much of this work was a collaboration with Dr T Allen (Allen and Potten, 1974).
3 9 : 3 SEPTEMBER 2004 KERATINOCYTE STEM CELLS 185 Figure 3 Schematic representation of the epidermal proliferative unit (EPU), left-hand diagram showing a sectional profile at the top and a surface view through the columns to the basal layer beneath. The right-hand part of the diagram shows a cell lineage that can explain the cell replacement process. The position of cells in the lineage can be related to the EPU as seen in surface view by the color relationships. proliferative unit (EPU) (see Figs 2 and 3) (Potten, 1974, 1981). We can summarize the EPU in the mouse as consisting of about 10 basal cells with about 10 suprabasal cells arranged in a column, 3 4 of which were nucleated. About 1 cell per d enters the column and, hence, 1 cell per d leaves. The average cell cycle time in the mouse basal layer is about 100 h. Clonal regeneration studies following irradiation suggested less than 1 regenerative cell per EPU (Potten and Hendry, 1973), which taken together with cell kinetic and mathematical modelling studies, suggested one stem cell among the group of 10 in the basal layer, with a cell cycle time of about 200 h (Potten et al, 1984). This suggests the sort of lineage that is illustrated in Fig 3 or Fig 1b, with essentially three divisions in the dividing transit population but other arrangements such as that in Fig 1c are also possible. Various observations based on cell kinetic changes following injury, the position of label-retaining cells (LRC), morphology, and mathematical modelling, have suggested that the central region of the cluster of 10 basal cells is the most likely location for the single stem cell. Since the first description of the EPU, there has been considerable debate as to whether this concept has any application to the human. This is somewhat surprising since the epidermis in the majority of the body sites (abdomen, limbs, dorsum, etc.) are all characterized by a similar columnar organization in the cornified layers. The difficulty here is that the boundaries of the columns cannot be traced as distinctly to the basal layer as they can in the mouse; however, the existence of this level of organization in the upper strata implies a similar organizational structure lower down. The columnar organization and hexagonal surface view are often remarkably similar in human and mouse (Fig 4). Furthermore, b-galactosidase-transduced cells that were cultured and then transplanted back into skin as xenografts for humans or ordinary grafts for mouse, generated positively stained unitary structures, essentially indistinguishable for the two species and in both cases images that were highly consistent with the EPU concept (Mackenzie, 1997). One issue that needs to be defined is the question of what is meant by the term stem cells. A search through the literature quickly illustrates the fact that the concept of Figure 4 Comparison of human and rodent epidermal proliferative unit (EPU). Upper figures show evidence of columns of cornified cells in light and electron-microscope pictures. Middle panels show surface views of the epidermis stained with silver nitrate and the roughly hexagonal outline of cells. The lower panels show b- galactosidase stained units of proliferation in epidermis where cells have been transduced, cultured for a period of time as an organ culture and then grafted onto mice. The human image is from a xeonograft. I am grateful to Ian Mackenzie for these images (see Mackenzie, 1997) and they are reproduced with permission of the publishers.
4 186 POTTEN JID SYMPOSIUM PROCEEDINGS stem cells is remarkably context dependent. In 1990, a colleague and myself attempted to define what we meant by stem cells in the rapidly proliferating tissues of the body but basing our definition primarily on epithelial systems (Potten and Loeffler, 1990). The definition that we produced stated that stem cells are relatively undifferentiated, selfmaintaining proliferative cells, capable of many rounds of cell division, during which they produce a variety of progeny that may differentiate down various pathways. They are also capable of regenerating the tissue after injury when they usually adapt and vary the probability of self-maintenance. The definition includes the term undifferentiated, which again is a term subject to considerable misuse. We viewed differentiation as a permanent (not cyclical) relative change in the appearance or expression of some molecule in the cell that distinguishes it from other cells. The accumulation of more of this cellular product can be regarded as maturation. So a stem cell may be undifferentiated relative to other cells in the tissue but may, or may not, be differentiated relative to embryonic stem cells. Here we touch on the topic of stem cell plasticity. A subject of considerable current debate. This also leads to another area of potential confusion, concerning the ability of stem cells to differentiate. Stem cells can produce daughter cells that can differentiate down many different pathways. The best example here being the bone marrow stem cell, which can produce daughters that differentiate down many diverse pathways resulting in the stem cell acquiring the attribute of pluripotency. A strange concept, since the stem cell always remains as a stem cell by definition and it is some of its daughters that acquire the ability to express certain differentiation markers. This debate in terms of stem cell plasticity centers round the issue of whether the stem cells can be induced to generate daughters that make even wider ranges of differentiated progeny perhaps any, or all, of the possible differentiated cell types of the body, in which case the stem cells should be thought of as being totipotent like embryonic stem cells. We should perhaps think more along the lines of the experimentalist possessing a totipotent capability in terms of manipulation of stem cell progeny! The next step forward in our understanding of keratinocyte stem cells arose from observations by Bickenbach and colleagues, and Morris and colleagues, in the early and mid-1980s that administration of tritiated thymidine to baby mice resulted in the retention of some of this radioactive label in a few isolated cells in the epidermis of adults (Bickenbach, 1981; Mackenzie and Bickenbach, 1985; Morris et al, 1985). A wide range of subsequent studies suggested that these LRC were the slow cycling stem cells and various workers have subsequently attempted these approaches in other tissues. In the mouse, the studies involved a short series of repeated injections of tritiated thymidine into baby mice, with LRC being observed in the basal layer of the epidermis 3 12 wk later. As seen in sheets of epidermis, the LRC had a tendency to be located at the center of the EPU, where we believe the stem cells are located. They can be stimulated to divide by damage and they can also be shown to retain radiolabelled carcinogens for long periods of time (Morris et al, 1986) and, hence, are probably the carcinogen target cells. They can be shown to give rise to colonies in culture (Morris and Potten, 1994) and to be involved in the clonal regeneration processes following injury in vivo. They have subsequently been shown to share a number of other properties with stem cells (Li et al, 1998; Kaur and Li, 2000; Potten and Booth, 2002). LRC have subsequently been seen in various regions of the hair follicle, particularly the upper outer root sheath, and this led to another significant advance in skin stem cell studies, namely the identification of some highly efficient stem cells in a specialized region of the outer root sheath called the bulge (Cotsarelis et al, 1990; Morris and Potten, 1999). The term bulge has led to some confusion since some small follicles such as the underfur (zigzag) follicles in the mouse lack a definitive enlarged area. The term has been applied to vibrissae follicles where there is a clear swelling in the upper regions of the follicle well away from the lower permanent areas of the follicle. It is often used to define areas that contain LRC, sometimes many more LRC than would be predicted on the basis of stem cell considerations. Those in the field should, perhaps, consider defining precisely what the bulge area is, how it is identified and how far up, or down, the follicle it reaches. LRC can be seen in epidermis and in the hair follicle and for a variety of reasons it is believed that this approach provides a way of labelling keratinocyte stem cells; however, the underlying mechanisms involved here have not been extensively discussed or studied. The mechanism assumed by many to operate is that label incorporated into the DNA of stem cells in baby mice dilutes only very slowly because of the slow cell cycle time for these stem cells in the adult. But it is highly likely that in a wk-old mice which contains LRC, the stem cells will have been through at least ten divisions, probably significantly more because the cell cycle time in the juvenile phase of skin development will be much shorter. This effectively rules out slow cell cycle as being the explanation. There is commonly a somewhat puzzling use of the term quiescence for skin stem cells. If stem cells in epidermis or the outer root sheath are truly quiescent (i.e., non-cycling) then they cannot perform their function of producing cells for cell replacement. An alternative mechanism for the label retention was provided by a hypothesis put forward by Cairns in 1975, who suggested that stem cells would have evolved various protective mechanisms to conserve the genetic integrity of their DNA. DNA replication is probably one of the most hazardous events in the life of a cell. A simple and effective way of protecting stem cells against the risk of replicationinduced DNA errors is for the cell to have devised a mechanism to segregate the old (template) from the newly synthesized DNA strands at mitosis, retaining the old template strands in the stem cells which would thus be genetically pure, and discarding the newly synthesized strands with any replication induced errors to the daughter cell destined for differentiation and cell loss. Recent evidence in support of this hypothesis has been obtained for the stem cells in the small intestine, which will be discussed later in this manuscript (Potten et al, 2002). The next area to consider is the hair follicles. Hair follicles go through cyclic phases of active proliferation and hair production (the hair growth phase anagen) when the follicle is large with many cells. This is followed when there is a mature hair in the follicle, by a transition phase involving
5 9 : 3 SEPTEMBER 2004 regression of the follicle, apoptotic events and a change in morphology (the transition phase being called catagen). This is followed by a quiescent phase when there is no proliferative activity to be observed in the lower regions of the much smaller follicle (the telogen phase). The hair growth cycle in mice is highly synchronized for the first two cycles, with the growth phase lasting 21 d and cell cycle activity in the germinal region being very rapid (cell cycle times of about 12 h). In humans the growth phase can last for many months and the cycle time in the germinal region of the follicle is probably about 36 h. There are a large number of cells produced per d and with this level of cell production and length of growth, the anagen follicle must contain some cells at the origin of all the cell movement that maintain the cell production at least for the duration of anagen, i.e., some self-maintaining stem cells. In some species such as Angora rabbits, Merino sheep, and some dogs, the hair growth cycle can be considerably lengthened and in humans it may last as long as a year. It is somewhat surprising that the lineage organization in the growing follicle has not been extensively studied but a cell lineage of the sort seen in the epidermis and in all other rapidly replacing tissues, including the small intestine, which is structurally somewhat similar, is most likely to apply in the growing hair follicle. A major advance in our understanding of skin stem cell populations arose initially from early epidermal regeneration studies after various forms of injury (Albert et al, 1967a, b; Al Barwari and Potten, 1976; Morris and Argyris, 1983), followed by elegant work from 1990 to today, looking at hair follicle formation and the activity of LRC in the upper outer root sheath under a wide range of very elegant follicle manipulation studies (Cotsarelis et al, 1990; Kobayashi et al, 1993; Lavker et al, 1993; Rochat et al, 1994; Taylor et al, 2000; Oshima et al, 2001). These studies have shown that stem cells in the hair follicle bulge are remarkably potent reserve stem cells capable of forming hair follicles, sebaceous glands, and epidermis if those structures are injured; however, a question still remains, I believe, as to whether these bulge stem cells play any role in the normal hair growth cycles. So with these amazing advances which have occurred over the last few years involving some very precise and elegant experiments, we need to consider the stem cells in the epidermis, the resting and growing follicle, and the questions of how these are all inter-related during development, in steady state, during the hair growth cycles, and following injury. In 1969, we undertook a fairly detailed resin-embedded serial sectioning study during all the transition phases from telogen to anagen (Silver et al, 1969). Our interests were to identify the precursor cells (stem or melanoblast cells) for the anagen hair follicle melanocyte population. By tracing back from anagen 5 to telogen, it was possible to see a clear sequence of events from melanotic melanocytes through to dividing dendritic clear cells, back to individual clear cells in the follicle germ of many telogen follicles (see Fig 5). The conclusion from these experiments was that each telogen zigzag follicle germ contained 1, or at the maximum, 2 melanocyte stem cells (melanoblasts). These cells were also of interest to Bill Montana at that time (dendritic clear cells). The conclusions KERATINOCYTE STEM CELLS 187 that were drawn from our study were consistent with observations from a series of radiobiological studies where hair follicles were irradiated and the consequences analyzed for the follicular pigment cell precursor population Potten, 1968; Potten and Chase, 1970). Nishimura et al (2002) using clever modern molecular biology approaches recently identified melanocyte stem cells which fulfill stem cell criteria (Potten and Loeffler, 1990) using a lineage specific marker. Interestingly, they are located in the lowermost permanent portion throughout the hair cycle (see Fig 5). In zigzag hair follicles (80% of pelage hair follicles), the hair bulge area where the arrector pili muscle is attached corresponds to the lowermost permanent portion. In telogen, clear cells were previously found in the lowermost portion of the follicle, sometimes called the hair germ and this is located immediately above the dermal papilla (see Fig 7). Indeed, the process of stem cell activation and proliferation Nishimura et al showed is similar to the one described for clear cells in 1969 (Silver et al). Interestingly, Nishimura et al found additional melanoblasts below the bulge area (which they called the sub-bulge area) in the larger types of pelage follicles (guard or over hair follicles). This area often corresponds to the larger hair germ in the telogen (guard-hair follicles) (Nishimura, personal communication), which includes the cluster of cells found just below the attachment site of the arrector pili muscle in these larger follicles (Lavker et al, 1993). It is interesting that these recent elegant studies using a transgenic mouse system came to very similar conclusions (Nishimura et al, 2002) and indeed generated pictures very similar to those in our 1969 paper (see Fig 5). At the time of our studies into hair follicle melanocyte precursors, we regarded the region beneath a hair club in a telogen follicle as the follicle germ. This was the area where the melanoblasts were located. These stem cells were responsible, through an amplifying division scheme, for generating the clone of 30 or so melanocytes of the growing follicle (Potten, 1985). If the telogen follicle germ contains melanocyte stem cells it is not unlikely that it may also contain follicle bulb stem cells. There have been some recent helpful advances (Morris et al, 2004). Using mice having a Cre-recombinase construct with a keratin 15 promoter crossed with R26R reporter mice expressing Lac Z Morris et al described some lineage tracking of cells in the lower permanent region of the follicle (referred to as the bulge). The studies using isolated putative Lac Z or enhanced green fluorescent protein (EGFP) positive cells have shown that these lower follicle cells are capable of forming new anagen follicles, sebaceous glands, and epidermis when injected into scid mice; similar conclusions could be drawn from studies when Lac Z is permanently switched on in the mice. These studies clearly show the potency of these cells; however, questions remain about the number of putative stem cells (extent of Lac Z staining) in telogen follicles and the influence of other cell types in the cell extraction approaches used. Intuitively, one would expect a telogen follicle to contain only a few stem cells (say 4 6 in total) situated adjacent to the dermal papilla which on division form a cap over the
6 188 POTTEN JID SYMPOSIUM PROCEEDINGS Figure 5 Hair follicle melanocyte precursor cells (stem cells) recognized in (upper panels) thin resin cut sections, stained with alkaline toluidine blue and in (lower panels) Dct-lac Z transgenic mice (lac Z reporter under the control of enzyme dopachrome tautomerase promoter). The similarity between the images generated and the conclusions drawn from these two studies performed 33 y apart, is remarkable. Both show the presence of melanocyte stem cells in the lowermost permanent portion, which corresponds to the telogen hair germ in most pelage follicles. Reproduced with permission from Silver et al (1969) and with the kind permission of Nishimura et al (2002). developing anagen dermal papilla. Only a few of these would be expected to be visible in sections. Any more than this would imply some redundancy and raise questions about the function of the additional cells, whether they divide and if so where their daughters are placed, and most importantly, where do all the positive cells end up in the anagen follicle and what happens to them at the next catagen. There is a difficulty in discriminating between blue staining of cells and the functional role of each cell in three dimensions and over time in a dynamic system like telogen anagen hair follicle. One of the major observations in the Morris paper was the identification of gene expression profiles for these putative stem cells which, among other things, provides a means of identifying and manipulating these stem cells. The keratinocyte stem cell populations may be related in the way that is illustrated in Figs 6 and 7. Diagrams similar to Fig 7 have been published before (Lavker and Sun, 2000). What, in my view, remains uncertain is the amplification divisions between the hair follicle bulge reserve stem cells and the steady-state stem cells in epidermis and any stem cells in the growing hair follicle. It has been proposed by some that at each new hair growth cycle, stem cells or stem cell progeny migrate from the bulge region to the follicle germ, to initiate a new hair growth cycle, a complicated concept that involves difficult cell migratory processes, complex regulatory mechanisms, and possible stem divisional activity in a tissue that has evolved to be tough with extensive cell-to-cell adhesion. This clearly can happen if the follicle is injured but it seems unnecessarily complicated to evoke such a mechanism for the steady-state unperturbed series of hair growth cycles when the anagen precursor stem cell could reside in the telogen germ region. But I do note that in some recent publications the bulge concept has been expanded to incorporate all the permanent lower regions of the follicle, to include what was previously regarded as the telogen germ. (See also Morris et al, 2004.) So, there is an issue of how the bulge is defined. Is it a region of the upper outer root sheath beneath the sebaceous gland or is it the entire permanent region of the follicle that includes the follicle germ. If the latter, then the role of the upper outer root sheath cells in hair cycles becomes less clear. The different stem cell locations suggested in Fig 7 imply specific local environments (niches) for the stem cells. In the EPU, this may be the adjacent, more immature dividing transit cells, with some ill-defined influence of the underlying dermal tissue. For the bulge, there is clearly also the possibility of a niche environment being supplied by the local keratinocytes (of uncertain lineage status) while, in the telogen follicle, a niche will be provided by the neighboring quiescent keratinocytes, together with an important and known influence emanating from the dermal papilla. In the
7 9 : 3 SEPTEMBER 2004 KERATINOCYTE STEM CELLS 189 Figure 6 The possible inter-relationships between cell lineages in the epidermis (epidermal proliferative unit (EPU)), a presumed cell lineage within the anagen hair follicle and a possible interrelationship between these and the important reserve stem cells that occur in the upper outer root sheath or bulge region of the hair follicle. Some of the markers that have been associated with these cells are shown on the lower right. growing follicle, the nature of a niche is less clear since the location of the stem cells is less clear. But it is also likely to involve neighboring immature transit cells and a dermal papilla component. What can we learn from the stem cell organization in other tissues? A tissue with some similarity to the epidermis is the dorsal surface of the tongue. In mice this has been studied in some detail to define a tongue proliferative unit which is directly analogous to the EPU. Here the architecture is much more complex but the organization and lineage structure is essentially the same (Hume and Potten, 1976). The crypts in the small intestine are another tissue organized in a very similar fashion with cell lineages and lineage ancestor stem cells. Similar lineages have been described for the glandular elements of the stomach, the crypts in the large intestine and although the structural relationships between the lineages and the tissue are ill defined, similar lineage organizations are clearly present during spermatogenesis and bone marrow hematopoiesis. The thing that differs mainly in these tissues is the number of generations of cells that are seen in the dividing transit compartment. The number of generations determines the degree of amplification in cell production numbers each time a stem cell divides and it also inversely determines the frequency with which stem cells can be found in the proliferating regions of the tissue. These range from about 10% of the proliferating cells in some epithelia to a fraction of 1% in the bone marrow or testis. One complication that needs to be considered in relation to stem cell populations is the point of commitment between the stem cell compartment and the dividing transit compartment. In Fig 1a c, this is shown at the point of division of the stem cell which, as a consequence, is an asymmetric division; the two daughters differing at the point of division. Figure 7 The spatial relationship of the stem cell compartments as seen in Fig 6, represented diagrammatically for telogen and anagen hair follicles. (Modified from Potten and Booth, 2002.)
8 190 POTTEN JID SYMPOSIUM PROCEEDINGS But it is possible that this commitment may be delayed one or a few divisions down the cell lineage as illustrated in Fig 1d. This provides an hierarchy, or age structure, within a stem cell compartment and a version of this scheme is believed to occur in the bone marrow lineage and in the small intestinal crypt. Is this what we have in the skin? (see Fig 6). It is interesting to note that in Fig 1d the cells within the stem cell compartment are now dividing in an essentially symmetric fashion. There have been some impressive advances recently, stemming from the work of Kaur and colleagues, and Morris and colleagues, in the identification and characterization of stem cells in epidermis and hair follicle (Morris et al, 1991; Li et al, 1998; Kaur and Li, 2000; Tani et al, 2000). From these and other studies, it is possible to draw up a list of features that are associated with keratinocyte stem cells and these are shown in Table I. These recent advances are important in that they allow the stem cells to be identified, potentially isolated and, as a consequence, characterized better, leading to the possibility of manipulation and expansion of these cells in culture. The small intestinal epithelium is a model system that may provide some insight into the mechanisms underlying LRC, some confirmation of their stem cell status, and an indication of one way in which cancer induction is minimized in the epidermis. The small intestinal epithelium is a rapidly proliferating tissue with a high degree of structural Table I. Properties of some keratinocyte stem cells Have a high colony-forming ability in vitro. Holoclones Located in interfollicular epidermis, specific locations in a rete ridge configuration, in the bulb or matrix of growing hair follicles. In the germ of telogen follicles, and in the bulge or upper outer root sheath Fixed cells (strong adherence or anchorage) Long-term proliferative capability Low proportion of cells in S þ G2/M (long cell cycle) (quiescent?) Present in total keratinocyte population at 4% 8 þ %. Early lineage cells express a 2 b 1 integrins Strong adhesion to basal lamina extracellular matrix, type IV collagen, or fibronectin Express keratin 19, keratin 15, CD34 Express integrin a 6 strongly but weak expression of CD71 a bright (a 6 CD71 dim ). Retain 3 HTdR label when treated as babies (LRC) Small cells with a high nuclear to cytoplasmic ratio The LRC can form colonies in vitro are predominantly a bright 6 CD71 dim divide when skin is injured tend to be at center of EPU retain radiolabelled skin carcinogens a A cell surface proliferation marker recognized by monoclonal antibody 10G7, which turns out to be transferrin receptor. Information taken from Potten and Booth (2002) but see also Braun et al (2003), Trempus et al (2003), Morris et al (2004). LRC, label-retaining cell; EPU, epidermal proliferative unit. polarity. Cell function is achieved by differentiated cells on finger-like projections into the lumen of the intestine, the villi, whereas cell proliferation to counterbalance cell death and loss from the villus is achieved by cell division activity in small closed proliferative units at the base of the villi, called crypts. Each crypt is a flask-shaped structure with about 250 cells in total, these being arranged as a series of annuli of about 16 cells. The bulk of the cells divide with an average intermitotic time of 12 h in the mouse, the same as the average cell cycle time in a growing hair follicle, which has a similar degree of polarity. It is now widely accepted that cell proliferation in the crypt is achieved within a series of about 5 cell lineages, each lineage with its own selfmaintaining lineage ancestor stem cell. There are about 6 generations in the cell lineage in the small intestine, possibly 7 or 8 in the large intestine where similar numbers of lineages and, hence, lineage ancestor stem cells are believed to exist. In the absence of stem cell specific markers (although some are now in the process of being developed), the small intestinal epithelium represents a unique and valuable cell biological model system, since the position of a cell in the cell lineage can be directly related to its topographical position within the crypt. A wide variety of experiments have indicated that the lineage ancestor cells are to be found in the annulus of 16 cells positioned about 4 or 5 cell positions from the base of the crypt immediately above a functional differentiated component called the Paneth cells. The lineage that best explains the cell replacement process is the one illustrated in Fig 1c with the commitment to differentiation that separates stem cells from non-stem cells occurring not at the level of the lineage ancestor cell divisions, but 1 or 2 generations down the lineage. The cells at the stem cell location in the small intestinal crypts of mouse appear to have a cell cycle time twice as long as the majority of the crypt cells, i.e., they pass through a cycle once a day and, hence, in the lifespan of the laboratory mouse these cells may divide a thousand times. Alternatively, more complex models of the hierarchy can be envisaged with master stem cells dividing very rarely, producing progeny that divide rapidly before undergoing differentiation into the dividing transit compartment although there is no experimental evidence to support such a model. Once a cell leaves the proliferative compartment in the crypt, it has a life expectancy of about 3 4 d before being shed as a worn-out cell from the tip of the villus (see Fig 8 and Potten et al, 1997; Potten, 1998; Marshman et al, 2002). The small intestine of both mouse and man has a greater mass than in the large intestine by factors of between 3 and 4, the stem cells are proliferating 1.5 times faster in the small intestine, and there are two to three times more total stem cells in the small intestine than the large intestine and three to four times more total stem cell divisions in a lifespan. All of these numbers would suggest that the stem cells in the small intestine should have a significantly higher risk of incurring the sequence of genetic changes that lead to cancer than the stem cells in the large intestine. But the cancer incidence data clearly show that the small intestine has about 70 times less cancer than the large intestine, which implies that the stem cells in the small bowel are very efficiently protected against the genetic risks associated with cancer development.
9 9 : 3 SEPTEMBER 2004 KERATINOCYTE STEM CELLS 191 Figure 8 The small intestinal proliferative units. The left-hand picture shows a scanning electron micrograph showing the villus covered with epithelial cells and a single crypt embedded in the fracture zone at the base of the villus. The right-hand image shows a section cut through the small intestine showing that it is a simple columnar epithelium 1 cell thick. The center panel shows an abbreviated diagrammatic representation of the cell lineage that is now believed to explain the cell replacement process. The figure illustrates how the position of a cell in the cell lineage can be precisely related to topographical position of cells on the long axis of the crypt. cp ¼ cell position. In 1975, Cairns proposed that a very simple but totally effective way of protecting a stem cell against DNA replication-induced error would be to have evolved a mechanism (completely undefined or studied) for sorting the template strands of DNA which would be error-free from the newly synthesized strands of DNA that might contain replication-induced errors. The template strands would be conserved within the cell destined to remain as a stem cell, while the newly synthesized strands with potential errors would be discarded to the daughter cell destined for differentiation and loss from the tissue which, in the murine small intestine, would occur 5 7 d after birth from a stem cell (the villus transit time plus the transit time through the cell lineages). If this hypothesis is valid it should be possible to label the template strands at times when stem cells are making new stem cells and, hence, new template strands, ideally at the penultimate divisions before the stem cells assume their steady-state asymmetric division mode. Stem cells make more stem cells in two situations, one is during tissue development in the postnatal animal and the second is following cytotoxic insult such as radiation where stem cells are killed. Figure 9 shows that LRC can be observed in both these situations and that for the developmental scenario the optimum time for labelling is about 6 wk of age, after which LRC can be observed at 11 wk which represents a time interval during which the stem cells would be predicted to have undergone between 20 and 40 cell divisions. Also shown in Fig 9 is the appearance of these LRC and the fact that they appear with the highest frequency at cell positions 4 5 (the stem cell location). The post-irradiation regeneration situation tends to generate higher yields of LRC but they still occur with the highest frequency at the stem cell position (Potten et al, 2002). These LRC can be seen to undergo mitosis. Subsequent bromodeoxyuridine labelling (a series of BrdU injections) to a 11-wk mouse after generating LRC by labelling with tritiated thymidine at 6 wk or, a series of bromodeoxyuridine injections on day 8 after a dose of radiation when LRC were generated by tritiated thymidine administration over days 1 4, can result in labelling all the LRC with bromodeoxyuridine. This shows that all the LRC at the stem cell position are cycling. This allowed a more definitive experiment to be undertaken to address the issue of DNA strand segregation. In these experiments, which have been conducted more than once for both the juvenile labelling and for the post-irradiation regeneration labelling, LRC (tritiated thymidine) have subsequently had their newly synthesized strands labelled with BrdU. Immediately after the BrdU labelling, all of the LRC at the stem cell position are doubly labelled. Over a period of 2 d (two divisions since there is a 24 h cell cycle) the doubly labelled cells become singly labelled with tritiated thymidine which persists in the LRC for the duration of the experiment (up to about 10 d) (see Fig 10). These experiments strongly suggest, since the two labels segregate differently, that selective strand segregation as described by the Cairns s hypothesis must be operating. This, of course, has fairly major implications in several areas of cancer biology, cancer genetics and ageing (telomere shortening). p53 is a tumor suppressor gene that can be regarded as the guardian of the genome (Lane, 1992) for adult stem cells and for embryos. Wild-type p53 protein keeps cells normal and the gene is mutated in many tumors. It can be shown to play an important role in deleting genetically abnormal embryos and cells in embryos (Norimura et al, 1996). It is implicated in the initiation of apoptosis or in cell cycle arrest and repair processes. It may be implicated in the permanent cell cycle arrest (premature differentiation) post-irradiation. Recent work using a fibroblast cell line, where p53 has been deleted but can be switched on by a zinc responsive promoter, has shown, using time-lapse studies, that when p53 is switched on in these otherwise p53 null cells, an asymmetric cell division mode is assumed with one daughter remaining proliferatively active and the other undergoing a differentiation event. It was subsequently shown that in these asymmetrically dividing cells, the DNA strands were asymmetrically distributed (using bromodeoxyuridine
10 192 POTTEN JID SYMPOSIUM PROCEEDINGS Figure 9 The results of experiments designed to generate label-retaining cells (LRC) in the stem cell compartment of the crypts of the small intestine of the mouse. Upper left panel shows the frequency of LRC when tritiated thymidine is administered over a period of time to different aged mice. The maximum yield is obtained when animals of about 6 wk are exposed to tritiated thymidine with the crypts being subsequently analyzed at 11 wk. An alternative strategy for generating LRC is to label with tritiated thymidine over a period of 4 d after a dose of radiation designed to kill stem cells and induce stem cell regeneration. This protocol generally produces higher yields of LRC as shown in the upper right-hand panel. The LRC at the stem cell position are shown by the three images lower left. Modified from Potten et al (2002). labelling and DNA quantification on cesium chloride gradients). There was also an asymmetric distribution of the bromodexoyuridine label in binucleate cells generated by cytoclasium D (Merok et al, 2002). What these studies suggest is that p53 may be involved in the asymmetric divisions that occur in stem cells and in the selective DNA strand segregation process that we have just described. The interesting feature illustrated by the small intestine is that some of these processes regulated by p53 differ in the stem cell and the dividing transit compartment. p53-dependent apoptosis appears to be exclusively a property associated with stem cells, whereas cell cycle arrest (G2 in these cells) is something exclusively associated with the dividing transit cells (see Potten, 2004). Asymmetric divisions and selective strands segregation may also be an exclusive property of the stem cell compartment and one regulated in some way by the p53 gene, see Fig 11. A secondary corollary to the Cairns hypothesis was that the stem cells would have to have a prohibition of sister chromatid exchange phenomenon since this would mix the strands. Such a prohibition may result in compromising excision repair processes, since some of the enzymes are common to the two processes and, as a consequence, the stem cells selectively segregating their DNA might be predicted to be extremely radiosensitive. Apoptosis is easily recognized in small intestinal crypts both at the level of the transmission electron microscope and also in good-quality hematoxylin and eosin paraffin sections. In the mid-1970 s (Potten, 1977) we instigated a program of work to quantitate the time course, the cell positional distribution and the dose response for apoptosis in the small intestinal crypts of the mouse. These experiments have been expanded and reviewed in several publications (Potten, 1996, 1995, 2004; Potten et al, 1997). The results can be summarized as follows: 1. Following a dose of radiation, apoptotic cells can be observed in the crypt within a period of 1 2 h, with maximal values occurring at between 3 and 6 h, after which there is a steady return to control levels. 2. When the yield of apoptosis following a dose of radiation is studied on a cell positional basis, it is clear that the
11 9 : 3 SEPTEMBER 2004 KERATINOCYTE STEM CELLS 193 Figure 10 The results of an experiment where label retaining cells (LRC) (tritiated thymidine) were subsequently relabelled with bromodeoxyuridine (BrdU) to generate 3 HTdR labelled template strands and BrdU-labelled newly synthesized strands. The segregation of the two labels was then studied over a period of up to 10 d as described in experiments where label retention was generated in two different ways described in Fig 9. Initially, the LRC are all doubly labelled, showing that all the LRC are passing through the cell cycle. The 3 HTdR label persists through the course of the experiment (10 d equivalent to 10 cell cycles), while the bromodeoxyuridine disappears from the LRC over a period of 2 3 d (equivalent to 2 3 cell cycles). These experiments provide convincing evidence that the rare stem cells located at cell position 4 in the crypt, selectively segregate old and new strands at mitosis as suggested by Cairns (1975). Reproduced with permission from Potten et al (2002). cells that die at 3 6 h post-irradiation occur with the highest probability at cell positions 4 5. It is not the rapidly proliferating cells that die via apoptosis, but a small cohort that are located at the 4th 5th position in the crypt, i.e., at the stem cell position. The majority of the rapidly dividing cells do not enter apoptosis particularly at early times, but appear to undergo a permanent cell cycle arrest (a process we refer to as premature differentiation, Paulus et al, 1992). 3. The dose response curve shows that these apoptosis susceptible cells at cell position 4 5 are exquisitely radiosensitive. Increases in the number of apoptoses per crypt above the spontaneous levels can be detected after doses as low as Gy. The dose response saturates at around Gy at which point about 5 cells at the stem cell location die per crypt. This apoptotic response is independent of dose rate and linear energy transfer, suggesting a lack of an ability to repair the damage (Hendry et al, 1982). This radiation-induced apoptosis is totally p53 dependent (absent in p53 null mice, Merritt et al, 1994) and appears to be restricted to about 5 cells at the stem cell position. This suggests that these cells have an extremely rapid and efficient damage detection mechanism, which results in the activation of an altruistic cell suicide thus removing the damage. This of course is an efficient way of deleting any random errors that might occur in the template strands conserved in the stem cells. Thus, the small intestinal crypts are protected by essentially three mechanisms. Firstly, selective retention of template strands in the stem cells eliminating the risk of replication- Figure 11 A hypothetical role for p53 in regulating altruistic apoptosis in stem cells while at the same time triggering, via p21, cell cycle arrest and repair processes in dividing transit cells and potential stem cells. There are some indications with cell culture systems that p53 may also be involved in regulating asymmetric division processes and selective strand segregation both of which are properties associated exclusively with the stem cell compartment. Reproduced with permission from Potten et al (2003).
The concept of stem cells with their dependent cell
 Keratinocyte Stem Cells: a Commentary 1 Christopher S. Potten*²³ and Catherine Booth³ *Epithelial Biology Department, Paterson Institute for Cancer Research, Christie Hospital NHS Trust, Manchester, U.K.;
Keratinocyte Stem Cells: a Commentary 1 Christopher S. Potten*²³ and Catherine Booth³ *Epithelial Biology Department, Paterson Institute for Cancer Research, Christie Hospital NHS Trust, Manchester, U.K.;
Keratinocyte stem cells: targets for cutaneous carcinogens
 Keratinocyte stem cells: targets for cutaneous carcinogens Rebecca J. Morris J Clin Invest. 2000;106(1):3-8. https://doi.org/10.1172/jci10508. Perspective A skin cancer seen in the clinic is in reality
Keratinocyte stem cells: targets for cutaneous carcinogens Rebecca J. Morris J Clin Invest. 2000;106(1):3-8. https://doi.org/10.1172/jci10508. Perspective A skin cancer seen in the clinic is in reality
The Beauty of the Skin
 The Beauty of the Skin Rose-Anne Romano, Ph.D Assistant Professor Department of Oral Biology School of Dental Medicine State University of New York at Buffalo The Big Question How do approximately 50 trillion
The Beauty of the Skin Rose-Anne Romano, Ph.D Assistant Professor Department of Oral Biology School of Dental Medicine State University of New York at Buffalo The Big Question How do approximately 50 trillion
Skin (Integumentary System) Wheater, Chap. 9
 Skin (Integumentary System) Wheater, Chap. 9 Skin (Integument) Consists of skin and associated derivatives Largest organ of body (21 ft 2 ; 9 lbs.; has 11 miles of blood vessels) Functions: Protection
Skin (Integumentary System) Wheater, Chap. 9 Skin (Integument) Consists of skin and associated derivatives Largest organ of body (21 ft 2 ; 9 lbs.; has 11 miles of blood vessels) Functions: Protection
Chapter 4 Opener Pearson Education, Inc.
 Chapter 4 Opener Introduction The integumentary system is composed of: Skin Hair Nails Sweat glands Oil glands Mammary glands The skin is the most visible organ of the body Clinicians can tell a lot about
Chapter 4 Opener Introduction The integumentary system is composed of: Skin Hair Nails Sweat glands Oil glands Mammary glands The skin is the most visible organ of the body Clinicians can tell a lot about
Ch 4. Skin and Body Membranes
 Ch 4 Skin and Body Membranes TITLE HISTOLOGY SLIDES & NOTES ESSENTIAL QUESTION What tissues compose the integumentary system? Stratified Squamous Epithelium Stratified = several layers; Squamous = shape
Ch 4 Skin and Body Membranes TITLE HISTOLOGY SLIDES & NOTES ESSENTIAL QUESTION What tissues compose the integumentary system? Stratified Squamous Epithelium Stratified = several layers; Squamous = shape
Supporting Information
 Supporting Information Plikus et al. 10.1073/pnas.1215935110 SI Text Movies S1, S2, S3, and S4 are time-lapse recordings from individually cultured Period2 Luc vibrissa follicles show that circadian cycles
Supporting Information Plikus et al. 10.1073/pnas.1215935110 SI Text Movies S1, S2, S3, and S4 are time-lapse recordings from individually cultured Period2 Luc vibrissa follicles show that circadian cycles
ANAT3231: lectures overview
 ANAT3231: lectures overview Stem Cell Biology Stem Cell Technology Resources: http://php.med.unsw.edu.au/cell biology/ Essential Cell Biology 3 rd edition Alberts Dr Annemiek Beverdam School of Medical
ANAT3231: lectures overview Stem Cell Biology Stem Cell Technology Resources: http://php.med.unsw.edu.au/cell biology/ Essential Cell Biology 3 rd edition Alberts Dr Annemiek Beverdam School of Medical
Integumentary System. Integumentary System
 1. General aspects a. The integumentary system consists of several organs major organ of the system is the skin other organs are relatively small and they can be considered as specialized structures of
1. General aspects a. The integumentary system consists of several organs major organ of the system is the skin other organs are relatively small and they can be considered as specialized structures of
7/10/18. Introduction. Integumentary System. Physiology. Anatomy. Structure of the Skin. Epidermis
 Introduction Integumentary System Chapter 22 Skin is largest and heaviest organ of body (7% of body weight) Houses receptors for touch, heat, cold, movement, and vibration No other body system is more
Introduction Integumentary System Chapter 22 Skin is largest and heaviest organ of body (7% of body weight) Houses receptors for touch, heat, cold, movement, and vibration No other body system is more
ANAT3231: lectures overview
 ANAT3231: lectures overview Stem Cell Biology Stem Cell Technology Resources: http://php.med.unsw.edu.au/cell biology/ Essential Cell Biology 3 rd edition Alberts Dr Annemiek Beverdam School of Medical
ANAT3231: lectures overview Stem Cell Biology Stem Cell Technology Resources: http://php.med.unsw.edu.au/cell biology/ Essential Cell Biology 3 rd edition Alberts Dr Annemiek Beverdam School of Medical
Lab 7: Integumentary System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS
 Lab 7: Integumentary System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS Pre-Lab Activity 1: 1. a. epidermis b. dermis c. hypodermis d. adipose tissue e. hair f. sebaceous gland g. sweat gland 2. a Pre-Lab
Lab 7: Integumentary System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS Pre-Lab Activity 1: 1. a. epidermis b. dermis c. hypodermis d. adipose tissue e. hair f. sebaceous gland g. sweat gland 2. a Pre-Lab
SKIN. 3. How is the skin structured around the finger joints to allow for flexible movement of the fingers?
 SKIN Objectives for Exam #1: 1. List various skin structures and describe their functions. 2. Describe skin responses to increases and decreases in body temperature. 3. Provide examples of various skin
SKIN Objectives for Exam #1: 1. List various skin structures and describe their functions. 2. Describe skin responses to increases and decreases in body temperature. 3. Provide examples of various skin
The Integumentary System: An Overview
 The Integumentary System: An Overview Functions: Protective covering Helps regulate body temperature Retards water loss from deeper tissues Houses sensory receptors Synthesizes biochemicals Excretes small
The Integumentary System: An Overview Functions: Protective covering Helps regulate body temperature Retards water loss from deeper tissues Houses sensory receptors Synthesizes biochemicals Excretes small
Integumentary System
 Integumentary System Overview Functions 1. Protection 2. Excretion of wastes 3. Maintenance of T b 4. Synthesis of vitamin D 3 5. Storage of lipids 6. Detection of sensory stimuli Epidermis Tissue types
Integumentary System Overview Functions 1. Protection 2. Excretion of wastes 3. Maintenance of T b 4. Synthesis of vitamin D 3 5. Storage of lipids 6. Detection of sensory stimuli Epidermis Tissue types
2/5/2019. Organ System: Skin or Integumentary System. Hypodermis (or superficial fascia) Integumentary System - Learn and Understand
 Integumentary System - Learn and Understand Skin is an organ comprised of all four tissues Each layer of the skin contributes to one or more of its numerous functions Skin is both strong and flexible Keratinization
Integumentary System - Learn and Understand Skin is an organ comprised of all four tissues Each layer of the skin contributes to one or more of its numerous functions Skin is both strong and flexible Keratinization
Epidermal Label-Retaining Cells: Background and Recent Applications
 Epidermal Label-Retaining Cells: Background and Recent Applications KristinM.BraunandFionaM.Watt Keratinocyte Laboratory, Cancer Research UK London Research Institute, London, UK Epidermal label-retaining
Epidermal Label-Retaining Cells: Background and Recent Applications KristinM.BraunandFionaM.Watt Keratinocyte Laboratory, Cancer Research UK London Research Institute, London, UK Epidermal label-retaining
Cell Growth and Division
 Name Class Date 10 Cell Growth and Division Big idea Growth, Development, and Reproduction Q: How does a cell produce a new cell? WHAT I KNOW WHAT I LEARNED 10.1 Why do cells divide? 10.2 How do cells
Name Class Date 10 Cell Growth and Division Big idea Growth, Development, and Reproduction Q: How does a cell produce a new cell? WHAT I KNOW WHAT I LEARNED 10.1 Why do cells divide? 10.2 How do cells
B. Incorrect! The ectoderm does not produce the dermis. C. Incorrect! The dermis is derived from the mesoderm.
 Human Anatomy - Problem Drill 04: The Integumentary System Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as 1. From the inner cell
Human Anatomy - Problem Drill 04: The Integumentary System Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as 1. From the inner cell
****************************************************************************************************** INTEGUMENTARY SYSTEM
 BIOLOGY 211: HUMAN ANATOMY & PHYSIOLOGY ****************************************************************************************************** INTEGUMENTARY SYSTEM ******************************************************************************************************
BIOLOGY 211: HUMAN ANATOMY & PHYSIOLOGY ****************************************************************************************************** INTEGUMENTARY SYSTEM ******************************************************************************************************
Skin. Kristine Krafts, M.D.
 Skin Kristine Krafts, M.D. Skin Lecture Objectives Describe the functions of skin. Describe the structure, location and function of the cell types found in epidermis: keratinocytes, melanocytes, Langerhans
Skin Kristine Krafts, M.D. Skin Lecture Objectives Describe the functions of skin. Describe the structure, location and function of the cell types found in epidermis: keratinocytes, melanocytes, Langerhans
Integumentary System (Script) Slide 1: Integumentary System. Slide 2: An overview of the integumentary system
 Integumentary System (Script) Slide 1: Integumentary System Slide 2: An overview of the integumentary system Skin is the body s largest and heaviest organ making up 15% of body weight. Most skin is 1 to
Integumentary System (Script) Slide 1: Integumentary System Slide 2: An overview of the integumentary system Skin is the body s largest and heaviest organ making up 15% of body weight. Most skin is 1 to
INTEGUMENTARY SYSTEM CHAPTER 4
 INTEGUMENTARY SYSTEM CHAPTER 4 FUNCTIONS Waterproofs Protein called keratin Protection 1 st line of defense against pathogens, chemicals & abrasions Insulation Regulates heat loss by controlling blood
INTEGUMENTARY SYSTEM CHAPTER 4 FUNCTIONS Waterproofs Protein called keratin Protection 1 st line of defense against pathogens, chemicals & abrasions Insulation Regulates heat loss by controlling blood
Cancer Cells. It would take another 20 years and a revolution in the techniques of biological research to answer these questions.
 Cancer Cells Cancer, then, is a disease in which a single normal body cell undergoes a genetic transformation into a cancer cell. This cell and its descendants, proliferating across many years, produce
Cancer Cells Cancer, then, is a disease in which a single normal body cell undergoes a genetic transformation into a cancer cell. This cell and its descendants, proliferating across many years, produce
Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs
 Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs Classification of Body Membranes Epithelial membranes Cutaneous
Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs Classification of Body Membranes Epithelial membranes Cutaneous
Unit 4 - The Skin and Body Membranes 1
 Unit 4 - The Skin and Body Membranes 1 I. Unit 4: Skin and Body Membranes A. Body Membranes 1. Function of body membranes a) Cover body surfaces b) Line body cavities c) Form protective sheets around organs
Unit 4 - The Skin and Body Membranes 1 I. Unit 4: Skin and Body Membranes A. Body Membranes 1. Function of body membranes a) Cover body surfaces b) Line body cavities c) Form protective sheets around organs
INTEGUMENTARY 1-Epidermis, 2-Dermis, Structure of thick and thin skin I- Epidermis . Stratum basale
 INTEGUMENTARY The skin (integument, cutis ) and its derivatives constitute the integumentary system. It form the external covering of the body and is the largest organ of the body. The skin consists of
INTEGUMENTARY The skin (integument, cutis ) and its derivatives constitute the integumentary system. It form the external covering of the body and is the largest organ of the body. The skin consists of
KEY CONCEPT Cells have distinct phases of growth, reproduction, and normal functions.
 5.1 The Cell Cycle KEY CONCEPT Cells have distinct phases of growth, reproduction, and normal functions. Objective: Cells have distinct phases of growth, reproduction and normal functions. APK: Why do
5.1 The Cell Cycle KEY CONCEPT Cells have distinct phases of growth, reproduction, and normal functions. Objective: Cells have distinct phases of growth, reproduction and normal functions. APK: Why do
Supplementary Information
 Supplementary Information Figure S1: Follicular melanocytes in the wound peripheral area migrate to the epidermis in response to wounding stimuli. Dorsal skin of Trp2-LacZ mice stained with X-gal and analyzed
Supplementary Information Figure S1: Follicular melanocytes in the wound peripheral area migrate to the epidermis in response to wounding stimuli. Dorsal skin of Trp2-LacZ mice stained with X-gal and analyzed
Chapter 5. Integumentary System 5-1
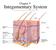 Chapter 5 Integumentary System 5-1 Structures that are part of the integument Skin Hair Nails Glands Overview of Functions Protection Sensation Temperature regulation Vitamin D production Excretion Immunity
Chapter 5 Integumentary System 5-1 Structures that are part of the integument Skin Hair Nails Glands Overview of Functions Protection Sensation Temperature regulation Vitamin D production Excretion Immunity
Epithelial stem cells in the skin: definition, markers, localization and functions
 Exp Dermatol 1999: 8: 80 88 Copyright C Munksgaard 1999 Printed in Denmark All rights reserved Controversies in Experimental Dermatology Section Editor: Ralf Paus, Berlin ISSN 0906-6705 Epithelial stem
Exp Dermatol 1999: 8: 80 88 Copyright C Munksgaard 1999 Printed in Denmark All rights reserved Controversies in Experimental Dermatology Section Editor: Ralf Paus, Berlin ISSN 0906-6705 Epithelial stem
Chapter 5: Integumentary System
 Chapter 5: Integumentary System I. Overview of the Integumentary System A. List the five major functions of the integumentary system: 1. 2. 3. 4. 5. Il. Skin A. Epidermis 1. The epidermis consists of 2.
Chapter 5: Integumentary System I. Overview of the Integumentary System A. List the five major functions of the integumentary system: 1. 2. 3. 4. 5. Il. Skin A. Epidermis 1. The epidermis consists of 2.
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Skin and Body Membranes
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Skin and Body Membranes 4 Body Membranes Function of body membranes Cover body surfaces Line body cavities
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Skin and Body Membranes 4 Body Membranes Function of body membranes Cover body surfaces Line body cavities
Prelab #4 BLOOD; BONE MARROW; RESPIRATORY; INTEGUEMENT Page 1
 Prelab #4 BLOOD; BONE MARROW; RESPIRATORY; INTEGUEMENT Page 1 Blood Slide 101 This a classic slide of blood cells using a Wright stain. Inspect red blood cells and their appearance. Note the approximate
Prelab #4 BLOOD; BONE MARROW; RESPIRATORY; INTEGUEMENT Page 1 Blood Slide 101 This a classic slide of blood cells using a Wright stain. Inspect red blood cells and their appearance. Note the approximate
DEBRIDEMENT: ANATOMY and PHYSIOLOGY. Professor Donald G. MacLellan Executive Director Health Education & Management Innovations
 DEBRIDEMENT: ANATOMY and PHYSIOLOGY Professor Donald G. MacLellan Executive Director Health Education & Management Innovations ANATOMY and PHYSIOLOGY Epidermal Layers ECM Structure Dermis Structure Skin
DEBRIDEMENT: ANATOMY and PHYSIOLOGY Professor Donald G. MacLellan Executive Director Health Education & Management Innovations ANATOMY and PHYSIOLOGY Epidermal Layers ECM Structure Dermis Structure Skin
Introduction. Skin and Body Membranes. Cutaneous Membranes Skin 9/14/2017. Classification of Body Membranes. Classification of Body Membranes
 Introduction Skin and Body Membranes Body membranes Cover surfaces Line body cavities Form protective and lubricating sheets around organs Classified in 5 categories Epithelial membranes 3 types- cutaneous,
Introduction Skin and Body Membranes Body membranes Cover surfaces Line body cavities Form protective and lubricating sheets around organs Classified in 5 categories Epithelial membranes 3 types- cutaneous,
THE sebaceous glands of the rabbit consist of clusters of about ten cells
 79 On the Relationship between Mammary, Sweat, and Sebaceous Glands By D. B. CARLISLE (From the Department of Zoology and Comparative Anatomy, Oxford, and the Plymouth Laboratory of the Marine Biological
79 On the Relationship between Mammary, Sweat, and Sebaceous Glands By D. B. CARLISLE (From the Department of Zoology and Comparative Anatomy, Oxford, and the Plymouth Laboratory of the Marine Biological
Anatomy and Physiology Homework: Chapters 3-4
 Anatomy and Physiology Homework: Chapters 3-4 CHAPTER 3: Cells and Tissues 1. The smallest unit of living tissue is called a. All living organisms are composed of these basic units where all life processes
Anatomy and Physiology Homework: Chapters 3-4 CHAPTER 3: Cells and Tissues 1. The smallest unit of living tissue is called a. All living organisms are composed of these basic units where all life processes
Fig. 1. Simple columnar epithelial cells lining the small intestine.
 Mitosis Prelab Reading Fig. 1. Simple columnar epithelial cells lining the small intestine. The tall cells pictured in Fig. 1 form the lining of the small intestine in humans and other animals. These cells
Mitosis Prelab Reading Fig. 1. Simple columnar epithelial cells lining the small intestine. The tall cells pictured in Fig. 1 form the lining of the small intestine in humans and other animals. These cells
The Integumentary System
 The Integumentary System Integument is skin Skin and its appendages make up the integumentary system A fatty layer (hypodermis) lies deep to it Two distinct regions Epidermis Dermis PHL 212 1 Function
The Integumentary System Integument is skin Skin and its appendages make up the integumentary system A fatty layer (hypodermis) lies deep to it Two distinct regions Epidermis Dermis PHL 212 1 Function
4 Skin and Body Membranes Study Guide
 Name: SKIN AND BODY MEMBRANES: 4 Skin and Body Membranes Study Guide Period: Body membranes, which cover body surfaces, line its cavities, and form protective sheets around organs, fall into two major
Name: SKIN AND BODY MEMBRANES: 4 Skin and Body Membranes Study Guide Period: Body membranes, which cover body surfaces, line its cavities, and form protective sheets around organs, fall into two major
Mitosis in Onion Root Tip Cells
 Mitosis in Onion Root Tip Cells A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules, or chromosomes.
Mitosis in Onion Root Tip Cells A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules, or chromosomes.
Ch. 4: Skin and Body Membranes
 Ch. 4: Skin and Body Membranes I. Body Membranes A. Function of body membranes 1. Cover body surfaces 2. Line body cavities 3. Form protective sheets around organs II. Classification of Body Membranes
Ch. 4: Skin and Body Membranes I. Body Membranes A. Function of body membranes 1. Cover body surfaces 2. Line body cavities 3. Form protective sheets around organs II. Classification of Body Membranes
Mitosis in Onion Root Tip Cells
 Mitosis in Onion Root Tip Cells A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules, or chromosomes.
Mitosis in Onion Root Tip Cells A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules, or chromosomes.
Anatomy Ch 6: Integumentary System
 Anatomy Ch 6: Integumentary System Introduction: A. Organs are body structures composed of two or more different tissues. B. The skin and its accessory organs make up the integumentary system. Types of
Anatomy Ch 6: Integumentary System Introduction: A. Organs are body structures composed of two or more different tissues. B. The skin and its accessory organs make up the integumentary system. Types of
Keywords: Daughter Cells Asexual Reproduction Sexual Reproduction Chromosomes Chromatin Homologous Chromosomes Diploid
 Name: CP Biology Unit 6: Cell Growth and Development Students will be able to: 6.1 Understand and explain the different aspects of the eukaryotic cell cycle. Explain how cell size is related to cell division
Name: CP Biology Unit 6: Cell Growth and Development Students will be able to: 6.1 Understand and explain the different aspects of the eukaryotic cell cycle. Explain how cell size is related to cell division
Keywords: Daughter Cells Asexual Reproduction Sexual Reproduction Chromosomes Chromatin Homologous Chromosomes Diploid
 Name: CP Biology Unit 5: Cell Growth and Development Students will be able to: 5.1 Understand and explain the different aspects of the eukaryotic cell cycle. Explain how cell size is related to cell division
Name: CP Biology Unit 5: Cell Growth and Development Students will be able to: 5.1 Understand and explain the different aspects of the eukaryotic cell cycle. Explain how cell size is related to cell division
Chapter 5 The Integumentary System. Copyright 2009, John Wiley & Sons, Inc. 1
 Chapter 5 The Integumentary System Copyright 2009, John Wiley & Sons, Inc. 1 Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails, and
Chapter 5 The Integumentary System Copyright 2009, John Wiley & Sons, Inc. 1 Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails, and
Integument. Squamous Cell Carcinoma. Melanoma. Largest organ 30% of all clinical diagnoses 1/3 of all tumors
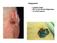 Squamous Cell Carcinoma Integument Largest organ 30% of all clinical diagnoses 1/3 of all tumors Melanoma Epidermis Stratified, squamous keratinized epithelium Derived from ectoderm Appendages hair follicles
Squamous Cell Carcinoma Integument Largest organ 30% of all clinical diagnoses 1/3 of all tumors Melanoma Epidermis Stratified, squamous keratinized epithelium Derived from ectoderm Appendages hair follicles
Human Anatomy & Physiology
 PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 5 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 5 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
Study Guide for Bio 101 Lecture Exam 3
 Study Guide for Bio 101 Lecture Exam 3 Please note that this study guide is a listing of objectives that you are required to master for this course. However, items mentioned in class or in laboratory as
Study Guide for Bio 101 Lecture Exam 3 Please note that this study guide is a listing of objectives that you are required to master for this course. However, items mentioned in class or in laboratory as
Intrinsic Patterns of Behavior of Epithelial Stem Cells
 Intrinsic Patterns of Behavior of Epithelial Stem Cells Debbie Tudor, Matthew Locke, Eleri Owen-Jones, and Ian C. Mackenzie University of Wales College of Medicine, Heath Park, Cardiff, UK The early concepts
Intrinsic Patterns of Behavior of Epithelial Stem Cells Debbie Tudor, Matthew Locke, Eleri Owen-Jones, and Ian C. Mackenzie University of Wales College of Medicine, Heath Park, Cardiff, UK The early concepts
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 4 The Tissue Level of Organization Introduction The purpose of this chapter is to: Learn about the various types of tissues and their origins
Principles of Anatomy and Physiology 14 th Edition CHAPTER 4 The Tissue Level of Organization Introduction The purpose of this chapter is to: Learn about the various types of tissues and their origins
BIO 130 Anatomy and Physiology Spring, 2016 Exam 3 Name: Course ID Number. Section 1 Answer questions 1 40 on the scan sheet.
 BIO 130 Anatomy and Physiology Spring, 2016 Exam 3 Name: Course ID Number Section 1 Answer questions 1 40 on the scan sheet. 1. Which of the following is NOT a characteristic of epithelial tissue? a. It
BIO 130 Anatomy and Physiology Spring, 2016 Exam 3 Name: Course ID Number Section 1 Answer questions 1 40 on the scan sheet. 1. Which of the following is NOT a characteristic of epithelial tissue? a. It
Mitosis and the Cell Cycle
 Mitosis and the Cell Cycle Chapter 12 The Cell Cycle: Cell Growth & Cell Division Where it all began You started as a cell smaller than a period at the end of a sentence Getting from there to here Cell
Mitosis and the Cell Cycle Chapter 12 The Cell Cycle: Cell Growth & Cell Division Where it all began You started as a cell smaller than a period at the end of a sentence Getting from there to here Cell
SUPPLEMENTARY INFORMATION
 b 350 300 250 200 150 100 50 0 E0 E10 E50 E0 E10 E50 E0 E10 E50 E0 E10 E50 Number of organoids per well 350 300 250 200 150 100 50 0 R0 R50 R100 R500 1st 2nd 3rd Noggin 100 ng/ml Noggin 10 ng/ml Noggin
b 350 300 250 200 150 100 50 0 E0 E10 E50 E0 E10 E50 E0 E10 E50 E0 E10 E50 Number of organoids per well 350 300 250 200 150 100 50 0 R0 R50 R100 R500 1st 2nd 3rd Noggin 100 ng/ml Noggin 10 ng/ml Noggin
Dr Narmeen S. Ahmad. Lab 1
 Dr Narmeen S. Ahmad Lab 1 1 Tissues are groups of cells with a common structure (form) and function (job). There are (4) types of tissue: 1. Epithelial 2. Connective 3. Muscle 4. Nervous 2 Epithelial cells
Dr Narmeen S. Ahmad Lab 1 1 Tissues are groups of cells with a common structure (form) and function (job). There are (4) types of tissue: 1. Epithelial 2. Connective 3. Muscle 4. Nervous 2 Epithelial cells
...if the bulge does not support interfollicular epidermis, how is this compartment maintained?
 Perspectives S t e m c e l l s o p i n i o n Epidermal homeostasis: do committed progenitors work while stem cells sleep? Philip Jones and Benjamin D. Simons Abstract Tracking the fate of cells in murine
Perspectives S t e m c e l l s o p i n i o n Epidermal homeostasis: do committed progenitors work while stem cells sleep? Philip Jones and Benjamin D. Simons Abstract Tracking the fate of cells in murine
Cytological and Histological Study of Adult and Neonate Epidermis in Thick and Thin Skin of Various Anatomical Sites
 Available online on www.ijpqa.com International Journal of Pharmaceutical Quality Assurance 218; 9(2); 174-179 doi: 1.25258/ijpqa.v9i2.13642 ISSN 975 956 Research Article Cytological and Histological Study
Available online on www.ijpqa.com International Journal of Pharmaceutical Quality Assurance 218; 9(2); 174-179 doi: 1.25258/ijpqa.v9i2.13642 ISSN 975 956 Research Article Cytological and Histological Study
You might be interested in reading an excerpt from Dimensions
 Take notes (minimum 1 page) on the following reading. Then review using the animation (link at bottom) and answer the quiz questions at the bottom of the webpage. To get credit for this assignment you
Take notes (minimum 1 page) on the following reading. Then review using the animation (link at bottom) and answer the quiz questions at the bottom of the webpage. To get credit for this assignment you
Hole s Essentials of Human Anatomy & Physiology
 Hole s Essentials of Human Anatomy & Physiology David Shier Jackie Butler Ricki Lewis Created by Dr. Melissa Eisenhauer Head Athletic Trainer/Assistant Professor Trevecca Nazarene University Chapter 6
Hole s Essentials of Human Anatomy & Physiology David Shier Jackie Butler Ricki Lewis Created by Dr. Melissa Eisenhauer Head Athletic Trainer/Assistant Professor Trevecca Nazarene University Chapter 6
Skin. Lecture #14. Ref:
 Skin Lecture #14 Ref: http://www.ccunix.ccu.edu.tw/~chenmsl/tea/skin_910721.htm Structure of Skin 1. Epidermis 2. Dermis 3. Subcutis 4. Hair follicle 5. Sebaceous gland 6. Sweat gland Skin Largest human
Skin Lecture #14 Ref: http://www.ccunix.ccu.edu.tw/~chenmsl/tea/skin_910721.htm Structure of Skin 1. Epidermis 2. Dermis 3. Subcutis 4. Hair follicle 5. Sebaceous gland 6. Sweat gland Skin Largest human
Ex. 7: Integumentary
 Collin County Community College BIOL. 2401 Ex. 7: Integumentary. Skin or Integument Consists of three major regions Epidermis outermost superficial region Dermis middle region Hypodermis (superficial fascia)
Collin County Community College BIOL. 2401 Ex. 7: Integumentary. Skin or Integument Consists of three major regions Epidermis outermost superficial region Dermis middle region Hypodermis (superficial fascia)
This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur.
 Background Knowledge Functions of normal skin Background Knowledge This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur. Learning
Background Knowledge Functions of normal skin Background Knowledge This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur. Learning
INTEGUMENTARY SYSTEM PART I: FUNCTIONS & EPIDERMIS
 INTEGUMENTARY SYSTEM PART I: FUNCTIONS & EPIDERMIS Integumentary System Cutaneous membrane Epidermis (5-layers) made up of epithelial tissue only Dermis (2-layers) contains connective tissue, vessels,
INTEGUMENTARY SYSTEM PART I: FUNCTIONS & EPIDERMIS Integumentary System Cutaneous membrane Epidermis (5-layers) made up of epithelial tissue only Dermis (2-layers) contains connective tissue, vessels,
Chapter 05. Lecture Outline. See separate PowerPoint slides for all figures and tables pre-inserted into PowerPoint without notes.
 Chapter 05 Lecture Outline See separate PowerPoint slides for all figures and tables pre-inserted into PowerPoint without notes. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Chapter 05 Lecture Outline See separate PowerPoint slides for all figures and tables pre-inserted into PowerPoint without notes. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
The Integumentary System : Embryology & Genetic Bases. Purnomo Soeharso Department of Medical Biology FMUI
 The Integumentary System : Embryology & Genetic Bases Purnomo Soeharso Department of Medical Biology FMUI Tissue organization of the skin (integumentum) : - Epidermis stratified epithelium on the outer
The Integumentary System : Embryology & Genetic Bases Purnomo Soeharso Department of Medical Biology FMUI Tissue organization of the skin (integumentum) : - Epidermis stratified epithelium on the outer
Unit I Problem 9 Histology: Basic Tissues of The Body
 Unit I Problem 9 Histology: Basic Tissues of The Body - What is the difference between cytology and histology? Cytology: it is the study of the structure and functions of cells and their contents. Histology:
Unit I Problem 9 Histology: Basic Tissues of The Body - What is the difference between cytology and histology? Cytology: it is the study of the structure and functions of cells and their contents. Histology:
Unit 4 The Integumentary System
 Unit 4 The Integumentary System I. Classification of Body Membranes A. Epithelial Membranes (3) 1. Cutaneous Membrane > Stratified Squamous > Sits on Dense Connective Tissue > Skin: Epidermis & Dermis
Unit 4 The Integumentary System I. Classification of Body Membranes A. Epithelial Membranes (3) 1. Cutaneous Membrane > Stratified Squamous > Sits on Dense Connective Tissue > Skin: Epidermis & Dermis
Chapter 5 The Integumentary System. Copyright 2009, John Wiley & Sons, Inc. 1
 Chapter 5 The Integumentary System Copyright 2009, John Wiley & Sons, Inc. 1 Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails, and
Chapter 5 The Integumentary System Copyright 2009, John Wiley & Sons, Inc. 1 Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails, and
CELL CYCLE INTRODUCTION PART I ANIMAL CELL CYCLE INTERPHASE EVOLUTION/HEREDITY UNIT. Activity #3
 AP BIOLOGY EVOLUTION/HEREDITY UNIT Unit 1 Part 3 Chapter 12 Activity #3 INTRODUCTION CELL CYCLE NAME DATE PERIOD The nuclei in cells of eukaryotic organisms contain chromosomes with clusters of genes,
AP BIOLOGY EVOLUTION/HEREDITY UNIT Unit 1 Part 3 Chapter 12 Activity #3 INTRODUCTION CELL CYCLE NAME DATE PERIOD The nuclei in cells of eukaryotic organisms contain chromosomes with clusters of genes,
Stem Cells. Induced Stem Cells
 Induced Stem Cells Stem Cells Mouse and human somatic cells can either be reprogrammed to a pluripotent state or converted to another lineage with a combination of transcription factors suggesting that
Induced Stem Cells Stem Cells Mouse and human somatic cells can either be reprogrammed to a pluripotent state or converted to another lineage with a combination of transcription factors suggesting that
Student Objectives. 7. Describe the structure of nails.
 Student Objectives When you have completed the exercises in this chapter, you will have accomplished the following objectives: The Skin 1. Name the tissue types composing the epidermis and dermis. List
Student Objectives When you have completed the exercises in this chapter, you will have accomplished the following objectives: The Skin 1. Name the tissue types composing the epidermis and dermis. List
Integumentary System
 Chapter 5 Integumentary System 5-1 Skin: composed of dermis and epidermis Dermis. Gives structural strength. C.T. with many fibers, fibroblasts, macrophages. Some adipocytes and blood vessels. Contains
Chapter 5 Integumentary System 5-1 Skin: composed of dermis and epidermis Dermis. Gives structural strength. C.T. with many fibers, fibroblasts, macrophages. Some adipocytes and blood vessels. Contains
Fish Skin Grafts Promote Superior Cell Ingrowth Compared to Amnion Allografts, Human Cadaver Skin and Mammalian Extracellular Matrix (ECM)
 Fish Skin Grafts Promote Superior Cell Ingrowth Compared to Amnion Allografts, Human Cadaver Skin and Mammalian Extracellular Matrix (ECM) Christopher L. Winters, DPM American Health Network Indianapolis,
Fish Skin Grafts Promote Superior Cell Ingrowth Compared to Amnion Allografts, Human Cadaver Skin and Mammalian Extracellular Matrix (ECM) Christopher L. Winters, DPM American Health Network Indianapolis,
Cell Death & Renewal (part 2)
 17 Cell Death & Renewal (part 2) Programmed Cell Death A major signaling pathway that promotes cell survival is initiated by the enzyme PI 3-kinase, which phosphorylates PIP2 to form PIP3, which activates
17 Cell Death & Renewal (part 2) Programmed Cell Death A major signaling pathway that promotes cell survival is initiated by the enzyme PI 3-kinase, which phosphorylates PIP2 to form PIP3, which activates
Ch 5: Integumentary System
 Ch 5: Integumentary System You gotta have skin; All you really need is skin. Skin's the thing, that if you've got it outside, It helps keep your insides in. Alan Sherman (1924-1973) Developed by John Gallagher,
Ch 5: Integumentary System You gotta have skin; All you really need is skin. Skin's the thing, that if you've got it outside, It helps keep your insides in. Alan Sherman (1924-1973) Developed by John Gallagher,
Biology: Cell Division and Cancer
 Cancer Cells [Photo by Dr. Cecil Fox via Wikimedia Commons] Biology: Cell Division and Cancer High School 9-12 Corin Malone Auburn Riverside High School, Auburn School District Materials adapted from Not
Cancer Cells [Photo by Dr. Cecil Fox via Wikimedia Commons] Biology: Cell Division and Cancer High School 9-12 Corin Malone Auburn Riverside High School, Auburn School District Materials adapted from Not
Histology of Integumentary System
 Histology of Integumentary System CONTENT / Topics Integumentary System Skin, thick - Major Layers Epidermis Dermis Hair Skin, hairy and Hair Follicle Sebaceous Glands Sebaceous Gland Sweat Glands Merocrine
Histology of Integumentary System CONTENT / Topics Integumentary System Skin, thick - Major Layers Epidermis Dermis Hair Skin, hairy and Hair Follicle Sebaceous Glands Sebaceous Gland Sweat Glands Merocrine
Stem cells in gastrointestinal epithelium: numbers, characteristics and death
 Stem cells in gastrointestinal epithelium: numbers, characteristics and death Christopher S. Potten Epithelial Biology, Paterson Institute for Cancer Research, Christie Hospital NHS Trust, Manchester M20
Stem cells in gastrointestinal epithelium: numbers, characteristics and death Christopher S. Potten Epithelial Biology, Paterson Institute for Cancer Research, Christie Hospital NHS Trust, Manchester M20
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 5 The Integumentary System Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails,
Principles of Anatomy and Physiology 14 th Edition CHAPTER 5 The Integumentary System Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails,
Histology = the study of tissues. Tissue = a complex of cells that have a common function
 { EPITHELIAL TISSUE Histology = the study of tissues Tissue = a complex of cells that have a common function The Four Primary Tissue Types: Epithelium (epithelial tissue) covers body surfaces, lines body
{ EPITHELIAL TISSUE Histology = the study of tissues Tissue = a complex of cells that have a common function The Four Primary Tissue Types: Epithelium (epithelial tissue) covers body surfaces, lines body
Levels of Organization
 Levels of Organization Oklahoma Laws Violators can be fined, arrested or jailed for making ugly faces at a dog. Females are forbidden from doing their own hair without being licensed by the state. Dogs
Levels of Organization Oklahoma Laws Violators can be fined, arrested or jailed for making ugly faces at a dog. Females are forbidden from doing their own hair without being licensed by the state. Dogs
Skin is a multilayered organ that covers and protects the body.
 Section 1: Skin is a multilayered organ that covers and protects the body. K What I Know W What I Want to Find Out L What I Learned Essential Questions What are the four tissue types that are found in
Section 1: Skin is a multilayered organ that covers and protects the body. K What I Know W What I Want to Find Out L What I Learned Essential Questions What are the four tissue types that are found in
Skin The Integumentary System
 Skin The Integumentary System THINK about IT What s the largest organ in your body? No, it is not your ears or stomach, or even your lungs or heart. By far the largest human organ is the skin. If that
Skin The Integumentary System THINK about IT What s the largest organ in your body? No, it is not your ears or stomach, or even your lungs or heart. By far the largest human organ is the skin. If that
Histopathology: skin pathology
 Histopathology: skin pathology These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information
Histopathology: skin pathology These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information
5.1. KEY CONCEPT Cells have distinct phases of growth, reproduction, and normal functions. 68 Reinforcement Unit 2 Resource Book
 5.1 THE CELL CYCLE KEY CONCEPT Cells have distinct phases of growth, reproduction, and normal functions. Cells have a regular pattern of growth, DNA duplication, and division that is called the cell cycle.
5.1 THE CELL CYCLE KEY CONCEPT Cells have distinct phases of growth, reproduction, and normal functions. Cells have a regular pattern of growth, DNA duplication, and division that is called the cell cycle.
Chapter 6: Integumentary System
 Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 12 th ed. Chapter 6: Skin and the Integumentary System Chapter 6: Integumentary System I. Introduction 1. The skin is composed of of tissues.
Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 12 th ed. Chapter 6: Skin and the Integumentary System Chapter 6: Integumentary System I. Introduction 1. The skin is composed of of tissues.
SKIN HISTOLOGY the microscopic anatomy of the Integument. Mikrogeo. com
 SKIN HISTOLOGY the microscopic anatomy of the Integument Mikrogeo. com Hair follicles, sweat glands, sebaceous glands (even teeth) are products of the epidermis,embryologically speaking ectododerm, that
SKIN HISTOLOGY the microscopic anatomy of the Integument Mikrogeo. com Hair follicles, sweat glands, sebaceous glands (even teeth) are products of the epidermis,embryologically speaking ectododerm, that
(From The Rockefeller Institute) Materials and Methods. Observations with the Electron Microscope
 ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF THE PAPILLOMA VIRUS IN THE SKIN OF THE RABBIT* BY ROBERT S. STONE,~ M.D., RICHARD E. SHOPE, M.D., DAN H. MOORE, P,~.D. (From The Rockefeller Institute) PLATES
ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF THE PAPILLOMA VIRUS IN THE SKIN OF THE RABBIT* BY ROBERT S. STONE,~ M.D., RICHARD E. SHOPE, M.D., DAN H. MOORE, P,~.D. (From The Rockefeller Institute) PLATES
Integumentary System and Body Membranes
 Integumentary System and Body Membranes The Skin and its appendages hair, nails, and skin glands Anatomy/Physiology NHS http://www.lab.anhb.uwa.edu.au/mb140/corepages/integumentary/integum.htm I. System
Integumentary System and Body Membranes The Skin and its appendages hair, nails, and skin glands Anatomy/Physiology NHS http://www.lab.anhb.uwa.edu.au/mb140/corepages/integumentary/integum.htm I. System
The Integementary System. The Skin & Its Parts
 The Integementary System The Skin & Its Parts General Structure 2. Accessory structures: hair, nails, exocrine glands 1. Cutaneous membrane: various layers Major Functions 1. Protection 2. Temperature
The Integementary System The Skin & Its Parts General Structure 2. Accessory structures: hair, nails, exocrine glands 1. Cutaneous membrane: various layers Major Functions 1. Protection 2. Temperature
Lecture Overview. Chapter 4 Epithelial Tissues Lecture 9. Introduction to Tissues. Epithelial Tissues. Glandular Epithelium
 Visual Anatomy & Physiology First Edition Martini & Ober Chapter 4 Lecture 9 Lecture Overview Introduction to Tissues Location General characteristics Functions Classification Glandular Epithelium 2 Where
Visual Anatomy & Physiology First Edition Martini & Ober Chapter 4 Lecture 9 Lecture Overview Introduction to Tissues Location General characteristics Functions Classification Glandular Epithelium 2 Where
Cell Birth and Death. Chapter Three
 Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Anatomy and Physiology I Student Outline The Integumentary System. Integumentary System. Page 1
 Anatomy and Physiology I Student Outline The Integumentary System Integumentary System Page 1 Have a very clear understanding of the each particular tissue and their unique functions in each layer of the
Anatomy and Physiology I Student Outline The Integumentary System Integumentary System Page 1 Have a very clear understanding of the each particular tissue and their unique functions in each layer of the
Cell Division Questions. Mitosis and Meiosis
 Cell Division Questions Mitosis and Meiosis 1 10 Do not write outside the box 5 Figure 3 shows a pair of chromosomes at the start of meiosis. The letters represent alleles. Figure 3 E E e e F F f f 5 (a)
Cell Division Questions Mitosis and Meiosis 1 10 Do not write outside the box 5 Figure 3 shows a pair of chromosomes at the start of meiosis. The letters represent alleles. Figure 3 E E e e F F f f 5 (a)
Keratinocyte Stem Cell Assays: An Evolving Science
 Keratinocyte Stem Cell Assays: An Evolving Science Pritinder Kaur, Amy Li, Richard Redvers, and Ivan Bertoncellow Epithelial Stem Cell Biology Laboratory; whaemopoiesis Laboratory, Peter MacCallum Cancer
Keratinocyte Stem Cell Assays: An Evolving Science Pritinder Kaur, Amy Li, Richard Redvers, and Ivan Bertoncellow Epithelial Stem Cell Biology Laboratory; whaemopoiesis Laboratory, Peter MacCallum Cancer
ALL PHOTOS ARE IDENTIFIED IN THE LOWER RIGHT CORNER WITH THE MAGNIFICATION POWER THAT THE PHOTO WAS TAKEN WITH. SCAN - THIS IS A VERY LOW POWER IMAGE
 ALL PHOTOS ARE IDENTIFIED IN THE LOWER RIGHT CORNER WITH THE MAGNIFICATION POWER THAT THE PHOTO WAS TAKEN WITH. SCAN - THIS IS A VERY LOW POWER IMAGE THAT WE USE WHEN A SAMPLE IS SO BIG THAT YOU CAN T
ALL PHOTOS ARE IDENTIFIED IN THE LOWER RIGHT CORNER WITH THE MAGNIFICATION POWER THAT THE PHOTO WAS TAKEN WITH. SCAN - THIS IS A VERY LOW POWER IMAGE THAT WE USE WHEN A SAMPLE IS SO BIG THAT YOU CAN T
CELL CYCLE INTRODUCTION PART I ANIMAL CELL CYCLE INTERPHASE
 CELL CYCLE INTRODUCTION The nuclei in cells of eukaryotic organisms contain chromosomes with clusters of genes, discrete units of hereditary information consisting of double-stranded DNA. Structural proteins
CELL CYCLE INTRODUCTION The nuclei in cells of eukaryotic organisms contain chromosomes with clusters of genes, discrete units of hereditary information consisting of double-stranded DNA. Structural proteins
LESSON ASSIGNMENT. The Human Integumentary and Fascial Systems. After completing this lesson, you should be able to:
 LESSON ASSIGNMENT LESSON 3 The Human Integumentary and Fascial Systems. TEXT ASSIGNMENT Paragraphs 3-1 through 3-14. LESSON OBJECTIVES After completing this lesson, you should be able to: 3-1. Define integumentary
LESSON ASSIGNMENT LESSON 3 The Human Integumentary and Fascial Systems. TEXT ASSIGNMENT Paragraphs 3-1 through 3-14. LESSON OBJECTIVES After completing this lesson, you should be able to: 3-1. Define integumentary
