Review Article The Controversial Clinicobiological Role of Breast Cancer Stem Cells
|
|
|
- Liliana Lawrence
- 6 years ago
- Views:
Transcription
1 Journal of Oncology Volume 2008, Article ID , 12 pages doi: /2008/ Review Article The Controversial Clinicobiological Role of Breast Cancer Stem Cells Claudia Casarsa, 1 Saro Oriana, 2 and Danila Coradini 1 1 Experimental Oncology Laboratory, Senology Center, Ambrosiana Clinic, Cesano Boscone, Milano, Italy 2 Surgery Department, Senology Center, Ambrosiana Clinic, Cesano Boscone, Milano, Italy Correspondence should be addressed to Danila Coradini, danila.coradini@yahoo.it Received 27 July 2008; Revised 5 December 2008; Accepted 23 December 2008 Recommended by Meenhard Herlyn Breast cancer remains a leading cause of morbidity and mortality in women mainly because of the propensity of primary breast tumors to metastasize. Growing experimental evidence suggests that cancer stem cells (CSCs) may contribute to tumor progression and metastasis spread. However, despite the tremendous clinical potential of such cells and their possible therapeutic management, the real nature of CSCs remains to be elucidated. Starting from what is currently known about normal mammary stem/progenitor cells, to better define the cell that originates a tumor or is responsible for metastatic spread, this review will discuss experimental evidence of breast cancer stem cells and speculate about the clinical importance and implications of their evaluation. Copyright 2008 Claudia Casarsa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. 1. Introduction Despite significant advances in diagnosis and clinical management, breast cancer remains a leading cause of morbidity and mortality in women [1], mainly owing to the propensity of primary breast tumors to metastasize to regional and distant sites such as lymph nodes, lung, liver, bone, and brain [2]. When the primary tumor is detected and removed before metastasis occurs, prognosis could be good and the chance of disease-free survival is high. However, if cancer cells have already begun to disseminate from the primary tumor and spread to other organs, current therapeutic strategies largely depend on the use of systemic cytotoxic drugs that frequently result in severe side effects on the patient and, in many cases, do not yield long-term success. This clinical scenario is further complicated by the fact that invasive breast cancers exhibit a wide range of morphological types, molecular profiles, and clinical behaviors. Not only there is a large variation in the nature of cell types between cancers, but even within a single tumor a significant heterogeneity in phenotype and genotype can be observed [3]. Based on growth patterns and cytological characteristics of the tumor cells, invasive breast cancers are categorized by the World Health Organization into 18 different histological subtypes, each of them associated with a diverse clinical behavior. In addition to morphology, invasive breast cancers can also be classified according to their proliferative potential (evaluated, e.g., by Ki67 expression) or the presence of such biological factors as hormone receptors (estrogen [ER] and progesterone [PgR]) or HER2/neu overexpression that are currently used in clinical practice to predict the prognosis and the response/resistance to cytotoxic and/or hormonal therapy [4 6]. Understanding the molecular causes of such a heterogeneity is therefore of paramount importance not only for the development of new therapeutic approaches, but also for a better knowledge of the biological bases of breast tumorigenesis and metastatic spread. To address these questions, over the past decade, scientists have used innovative technical strategies and approached new intriguing directions aimed to define the genetic and epigenetic profile of the single tumor and to better define the cell that originates a tumor or is responsible for metastatic spread. In particular, investigators focused their attention on the hypothesis that
2 2 Journal of Oncology breast cancer may be a stem cell disease, arising from tissue stem/progenitor cells or driven by cells with stem-cell properties [7]. 2. Isolation and Characterization of Mammary Stem/Progenitor Cells In the adult, cell loss, associated with the physiological tissue turnover, is compensated by the activity of specific cells, termed stem cells, which are so defined by their ability to self-renew and to generate the entire repertoire of the differentiated cells composing a given tissue. Since 1983, the existence of such stem cells has also been postulated in the mammary gland to explain the cellular dynamics underlying morphological changes throughout a woman s life, particularly during and after pregnancy [8]. In general, the identification and purification of normal stem cells are difficult tasks because of the paucity of stem cells in the tissue of origin and to the lack of stemnessspecific morphologic traits. Hence, animal models have been used, and the murine model, in particular, has supplied relevant data to improve our understanding of the cell biology of the mammary gland and to clarify the presence of stem/progenitor cell and differentiated cell compartments in the mammary gland [9, 10]. However, the information obtained from mice cannot be directly applied to humans (as in the case of murine exclusive cell surface markers) or may not be promptly translated to humans, as known in the hematopoietic field, where an opposite surface-antigen profile was observed in humans (CD34 + CD38 )withrespect to mice (CD34 CD38 + ). Several in vitro strategies have thus been developed to isolate and characterize human mammary stem/progenitor cells, based on the differential expression of some cell surface markers, the formation of mammospheres, and the use of fluorescent dyes Cell Surface Markers. The first experimental evidence of the existence of human mammary stem/progenitor cells was obtained by the in vitro isolation of multipotent epithelial cells, from normal human adult breast, according to their different expression of MUC-1 glycoprotein, CALLA/CD10, and epithelial-specific antigen (ESA). Using these markers, two epithelial-cell progenitor populations were distinguished, corresponding to the two components of normal mammary gland (myoepithelial cells, forming the basal layer of ducts, and epithelial cells, lining the lumen of ducts and forming the alveoli) [11 13]. Subsequently, Gudjonsson et al. [13], starting from the previous findings [14], provided in vivo evidence of the morphogenic potential of the MUC-1 /ESA + subpopulation, inoculating these cells subcutaneously in nude mice after pre-embedding them in a mixture of collagen gel and matrigel Mammosphere Formation. To identify a human mammary stem/progenitor-cell subpopulation, Dontu et al. [15] adopted a strategy similar to that employed for primary neural cells and based on the formation of floating spherical colonies used to define and measure stem cell-like behavior. In fact, contrary to the dogma that epithelial cell survival is anchorage-dependent, single cell suspensions of human mammary epithelial cells, obtained by mechanic/enzymatic dissociation, surprisingly survived in suspension and generated floating spherical colonies, termed nonadherent mammospheres [15]. The mammospheres contained numerous undifferentiated cells that, once isolated from the cluster, were able to generate new multilineage colonies, when cultured under differentiating conditions, and, in 3D culture, to reconstitute a functional mammary gland. However, the intrinsic dynamics of such cytospheres, as conventionally assayed, introduces several confounders, as reported in a recent paper by Singec et al. [16] who underlined the need to use more accurate conditions for assessing the clonality, number, and fate of stem cells. Although sphere formation may represent a useful culturing tool, it is not specific to stem cell characterization; any dividing cell from virtually any tissue will form floating cell clusters, when cultured in a serum-free medium and on a nonadherent substrate, owing to a predominant intercellular adhesiveness. Spontaneous sphere fusion may occur in normal as well as in neoplastic sphere cultures. Furthermore, in agreement with Singec et al. [16], we have observed that the mammospheres, supposedly rich in multilineage progenitors, have a very short life span (about 3-4 weeks), making it difficult to define the cells composing them as real mammary stem cells, which are longlived by definition. On account of all these criticisms, this experimental approach, based on the ability of the supposed mammary stem cells to generate clonal mammospheres under anchorage-independent culture conditions, has been defined a surrogate stem cell assay [17] Fluorescence Methods. To overcome the challenge represented by the limited availability of stemness markers and to take advantage of the ability of stem cells to extrude dyes, for example, Hoechst DNA-binding dye or rhodamine because the overexpression of some membrane transporter proteins, such as P-glycoproteins or breast cancer resistance proteins (BCRPs) [18, 19], fluorescent dyes have been used to identify and isolate by flow cytometry a small fraction of cells supposed to be stem progenitors [20]. This cell fraction, which amounts to around 0.2% of the total population, has been called side population (SP). However, several criticisms to such a sorting technique have been raised in the recent years principally concerning the toxicity of dyes used in the analysis [21] and the high assay variability associated with technical modifications required for each cell population under study; these limitations hamper the comparison of results obtained from different studies and affect cell selection for in vitro and in vivo growth experiments. In addition, recent findings from two teratocarcinoma cell lines indicate that Hoechst treatment, as performed during staining for SP analysis, can affect cell differentiation, suggesting other potential complications in the interpretation of data [22]. Recently, another approach, based on aldehyde dehydrogenase (ALDH) activity, has been proposed as a promising alternative to identify and characterize the human mammary stem/progenitor component in the mammary gland [23].
3 Journal of Oncology 3 CSC (a) Clonal evolution model (b) Cancer stem cell model Figure 1: Models of heterogeneity in solid cancer cells.(a)theclonal evolution model assumes that every cell in a tumor is potentially tumorinitiating. Progressionisgoverned by rarestochastic events operating in allcells. Cellswith mutations (yellow) that acquire growth advantage will dominate over all other cells in the tumor and will originate a new clone containing cells characterized by a different phenotype and having different proliferative potentials; in a clonogenicity or tumorigenicity assay, some of these cells (blue) would have a low probability of exhibiting this potential. (b) The cancer stem cell model states that a particular subset of tumor cells with stem cell-like properties, called cancer stem cell (CSC) (pink), drives tumor initiation, progression, and recurrence. CSCs are able to self-renew indefinitely and to differentiate, leading to the production of all cell types (blue) that make up the rest of the tumor. In clonogenic assays, CSCs have the potential to proliferate extensively and can form new tumors on transplantation. ALDH, a detoxifying enzyme responsible for the oxidation of intracellular aldehydes, is a putative candidate marker of stemness, since it is highly expressed in hematopoietic and neuronal stem/progenitor cells. Its presence can be evaluated by ALDEFLUOR kit: brightly fluorescent ALDH-expressing cells are easily detected by flow cytometry in the green fluorescence channel. Using such an approach, Ginestier et al. [23] showed that ALDH-positive cells formed mammospheres with high efficiency (10 ± 3.5%) when put into 96- well plates (1 cell/well), and displayed stem-like properties in terms of bilineage differentiation in vitro and outgrowth potential when inoculated in the mammary fat pad of humanized mice. However, even though the findings indicate that only ALDH-positive cells had phenotypic and functional characteristics of mammary stem cells, immunostaining of tissue sections using a monoclonal antibody against the first isoform of ALDH (ALDH1) did not detect any overlapping expression of several markers (e.g., CK5/6 and CK14), previouslyassociated withundifferentiated mammary epithelial cells, probably owing to the scarcity of this population. Analysis performed on mammosphere sections have shown that ALDH1-positive cells represented approximately 5% of the total cell population and expressed CK5/6 or CK14, supporting the hypothesis that ALDH1-positive cells represent the stem/progenitor population [13, 15]. Unfortunately, as highlighted by these inconclusive results, the efforts to purify adult stem cells from the human mammary gland have so far been hampered, on the one hand, by the lack of cell surface markers specific to undifferentiated or differentiated mammary cells and, on the other hand, by the lack of suitable in vivo assays for testing stem cell properties, with the consequence that human breast stem cells have not yet been extensively characterized. 3. Cancer Propagation Models To explain why not every cell within a tumor is capable of maintaining and/or reinitiating tumor growth, two models of heterogeneity in solid cancer have been proposed: the clonal evolution model (Figure 1(a)) and the cancer stem cell (CSC) model (Figure 1(b))[24 26]. The two main aspects of the clonal evolution model, first proposed by Nowell in 1976 [27], are (1) diversity within the tumor due to genetic instability and (2) selection of the cells with the most advantageous phenotype. In this respect, stem or differentiated cell characteristics (including selfrenewing capacity) are just simple phenotypes and, as such, can change. According to this model, any cancer cell can potentially become invasive and cause metastasis or become resistant to therapies and cause recurrence. The cancer stem cell (CSC) model (Figure 1(b))states that a particular subset of tumor cells with stem cell-like properties, called cancer stem cells, drives tumor initiation, progression, and recurrence. Since CSCs are widely believed to arise from normal stem or progenitor cells, the identification of stem cells in a tissue is of paramount importance to understand how a tumor arises. By definition, CSCs have the ability to self-renew indefinitely and to differentiate, which leads to the production of all cell types composing a tumor, both tumorigenic and nontumorigenic cells. But the latter lack the unlimited self-renewing capacity and the ability to reproduce the phenotypically diverse cell
4 4 Journal of Oncology Clonal succession creating a dominant clone CSC1 Mutation CSC2 Figure 2: Mixed model for the nature of sustained tumor growth. The tumor is originally driven by rare cells of one phenotype (CSC1, yellow), which may have stem/progenitor cell origin and give rise to the tumor bulk by producing terminally differentiated cells (blue). Subsequent mutations enhancing self-renewing capacity create a dominant subclone that is phenotypically different (CSC2, green) and more aggressive. If the CSC2 subclone does not display stem-like cell properties, such a subset will not be able to initiate tumors with a high frequency [30]. populations that make up the tumor bulk [28, 29]. Therefore, this model suggests that the presence of such rare tumorinitiating cells, in the heterogeneous mix of cells composing a tumor, is essential for neoplastic progression and metastatic spread [29, 30]. While the CSC model of carcinogenesis was described in the context of systemic malignancies as long ago as in the 1930s, only recently has it been extended to solid tumors, and during the last decade, several studies have provided evidence that CSCs may also exist in solid tumors including brain [31], lung [32], prostate [33], colon [34], liver [35], pancreas [36], and breast [37] carcinomas, as well as melanoma [38]. In breast tumorigenesis, the CSC model also seems to be supported by clinical observations indicating that, despite the fact that breast cancer patients may have hundreds or thousands of single disseminated cancer cells detectable in their bloodstream, only a very small percentage of cells progresses to form overt macroscopic metastases [39], and that metastatic tumors tend to reproduce a heterogeneity similar to the primary tumor [40].Since CSCs have been supposed to be responsible for the chemo- and radioresistance observed in several solid tumors [41, 42], the CSC model could explain such a finding with the ability of CSCs to escape cytotoxic drugs, via a high expression of specific drug transporter proteins, and to resist radiotherapy by increasing DNA repair activity. Unfortunately, the definitive proof of the cellular origin of CSCs they may arise either from normal resident stem cells within the tissue bearing the malignancy or from transformed progenitor cells that acquire the stem cell ability of self-renewal remains elusive and is a topic of intense debate as well as experimental investigation. In fact, if these cells arise from normal stem cells, then cancer cells could take advantage of the existing regulatory self-renewal pathways of stem cells. On the other hand, if these cells arise from mature, differentiated cells, multiple oncogenic mutations, affecting differentiation and self-renewal pathways are required for a cell to become tumorigenic and metastatic [43, 44]. It can be argued that mature cells have a very limited life span, and thus it is unlikely that all the necessary mutations could occur during the relatively short life of these cells. In contrast, the unlimited selfrenewing capacity of normal stem cells could enable them to accumulate the necessary mutations, despite the apparent paradox of the stem cell dogma, according to which a stem cell maintains its DNA constant through symmetric division [26, 45]. Thus, whereas the CSC model is highly hierarchical with a unique self-renewing cell type at the apex, the clonal evolution model attributes much of the intratumor variation to subclonal differences in mutational profile and implies that all cells, except the terminally differentiated ones, may have self-renewal capacity. Nevertheless, clonal evolution and CSC models share some aspects: in both, for instance, the tumor arises from a single cell that has acquired multiple mutations and has gained unlimited proliferative potential. This suggests that tumor heterogeneity could be explained by a new version of the clonal evolution model that incorporates some features of the CSC hypothesis [26]. A more intriguing model to explain the nature of sustained tumor growth is now emerging from the CSC model. According to this new model (Figure 2), tumors could originally be driven by rare CSCs (CSC1). Subsequent mutations, enhancing self-renewing capacity, could create a dominant, more aggressive subclone (CSC2), with a phenotypical aspect distinct from the original CSC. However, if the CSC2 subclone does not display stem-like properties, it should not be able to initiate tumors with a high frequency [46].
5 Journal of Oncology 5 CSC Progression CSC Mutation mcsc Invasion Blood vessels Metastasis Figure 3: Metastatic evolution. Genetic and epigenetic mechanisms may cause the generation of a self-renewing metastatic cancer stem cell subclone (mcsc, yellow), phenotypically different from the CSC that is driving tumorigenesis (pink). Through a series of invasive processes, mcsc enters blood vessels and colonizes distant organs according to the seed and soil hypothesis. Blue cells represent bulk tumor cells [47]. Although metastasis is the predominant cause of lethality in breast cancer patients, metastatic spread is a highly inefficient process, since very few cells successfully colonize distant sites. A possible explanation of this inefficiency is provided by the CSC model, according to which genetic and epigenetic mechanisms may generate, within the primary tumor, a self-renewing metastatic cancer stem cell (mcsc) characterized by an immunophenotype different from the CSC that is driving tumorigenesis (Figure 3). This is suggested by the observation that in metastatic sites, some cell subpopulations with self-renewing features display acellsurfacemarkerprofiledifferent from the CSC that originated the primary tumor [47]. Through a series of invasive processes, this new mcsc subclone could enter blood vessels and colonize distant organs according to the seed and soil hypothesis. 4. Isolation and Characterization of Breast Cancer Stem Cells On account of accumulating evidence and according to the CSC model that assumes a tissue stem/progenitor cell as the origin of a tumor, some methods adopted to isolate and cultivate normal mammary stem/progenitor cells have also been applied to breast cancer tissue. However, there may be biases, inherent in the techniques applied, that can affect experiment reproducibility. In fact, some technical approaches, including the strong enzymatic digestion of breast tissue necessary to disaggregate the connective tissue surrounding the mammary gland, can cause damage to cells or loss of some particular surface markers, hampering the identification of unique markers for the putative CSCs (authors unpublished data). Furthermore, since cell
6 6 Journal of Oncology recovery from solid tissue is usually low (rarely exceeding 10%), the samples obtained may not be representative of the original lesion, owing to the rare presence of CSCs in the tumor mass, actual CSCs may be lost, while other cells may be mistakenly identified as CSCs [26]. The first experimental clues about the existence of putative CSCs came from the observation that, even when using immortalized cancer cell lines, large numbers of cells (in the range ) must be injected into experimental animals to initiate a tumor and, in spite of that, only a very small proportion of these cells will go on to form metastases [48]. Additional experimental studies showed that while early steps in hematogenous metastasis (intravasation, survival, arrest, and extravasation) can be remarkably efficient, with over 80% of cells successfully completing the metastatic process at this point, only a small subset of these cells (i.e., about 2%, depending on the experimental model) can initiate growth as micrometastases and that an even smaller subset (i.e., about 0.02%, depending on the experimental model) is able to persist and grow into macroscopic tumors. This suggests that the initial growth of micrometastases represents the critical decision-making stage Surface Markers. After the identification of stemness specific markers in hematopoietic tumors, considerable progress has also been made in the elucidation of the biological properties of breast cancer stem cells. Al-Hajj et al. [37] first demonstrated the presence of a cell subpopulation displaying stem cell properties, characterized by the cell surface marker profile CD44 + /CD24 low /lin, in solid tissues and in pleural effusions of patients with advanced-stage metastatic breast cancer. This phenotype displayed a 10- to 50-fold increase in the ability to form tumors in NOD/SCID mice over unfractionated tumor cells. In addition, the authors demonstrated that cells with such a specific cell surface antigen profile could successfully and efficiently grow as tumor xenografts in immunodeficient mice; the highest capacity to form tumors was observed after injection of 200 cells with the ESA + /CD44 + /CD24 low /lin phenotype. However, the heterogeneous expression patterns of ESA, CD44, or CD24, observed by FACS analysis in secondary lesions, support the hypothesis that the CD44 + /CD24 low /lin profile could be the marker of the putative breast CSC phenotype, since it recapitulates the heterogeneous complexity of the tumors from which it has been isolated. Such a hypothesis could be corroborated by a study in which Ince et al. [49] observed the presence of two different populations of mammary epithelial cells in the tumor of a single patient. Only one population was myoepithelial-like and was able to give rise to tumors with heterogeneous histology and to be tumorigenic when injected into mammary fat pads of immunodeficient mice Fluorescence Methods. Similar to normal breast stem cells, CSCs have also been investigated according to their expression of aldehyde dehydrogenase (ALDH) activity. Ginestier et al. [23] analyzed the tumorigenicity of ALDEFLUOR-positive populations isolated from two metastatic and two primary invasive ductal carcinomas (three triple negative tumors ER-negative, PR-negative, and HER2-negative and one ER-positive, PR-positive, and HER2-negative tumor) that were transplanted into the humanized cleared fat pad of immunodeficient mice, immediately after surgery with no previous cultivation. To test tumorigenicity, cell sorting was performed at early passages in animals, in order to minimize the variability introduced by the xenotransplant model. ALDEFLUOR-positive cells represented 3% to 10% of the total cell population, and 500 positive cells were able to generate a tumor in as few as 40 days. Significantly, the concomitant presence of the ALDEFLUOR-positive phenotype and of the previously described breast CSC phenotype (CD44 + /CD24 /low )was observed in a small cell fraction of the three triple-negative tumors (range %), whereas tumor cells generated from one metastatic tumor (pleural effusion) showed a high percentage of overlapping cell fraction (1.16%) that gave rise to outgrowth from as few as 20 cells. Conversely, ALDEFLUOR-negative cells, though bearing the CD44 + /CD24 /low phenotype, were not tumorigenic, even when cells/fat pad were implanted. This suggests that the concomitant presence of ALDEFLUOR-positive and CD44 + /CD24 /low phenotypes may characterize progenitor cells with proliferative potential. When assessing the potential use of ALDH1 to detect malignant mammary stem/progenitor cells in situ on breast cancer tissue sections, Ginestier et al. [23] found that ALDH1 expression correlated with the histoclinical parameters, suggesting the use of this marker as a powerful predictor of poor clinical outcome. So far, therefore, the ALDEFLUOR assay, overcoming the limited availability of CSC-specific surface markers, seems to represent the pivotal tool for the isolation of cell populations with high tumor-initiating capability or cell populations with stem-like properties in normal tissue, allowing the identification of stem/progenitor cells involved in normal mammary development and may be in tumor transformation. As regards the use of Hoechst to detect the side population and to identify CSCs, thus bypassing the lack of universally accepted surface-antigen markers, several limitations are emerging in addition to the technical criticisms described for the methods of normal mammary stem cell isolation; they include the low cell recovery from tumor tissue that does not reflect the entire cohort of cancer cells, and the toxicity of the dye that precludes its use for functional CSC assays in vitro and in vivo [50] Self-Renewal Pathways. Since another trait shared by normal stem cells and CSCs is the ability to self-renew, the deregulation of key pathways involved in such a pivotal cellular function has been presumed to be implicated in breast carcinogenesis and more thoroughly investigated. Experimental evidence indicates that carcinogenesis in the mammary gland, and in other solid organs, might result in the transformation of stem and/or progenitor cells because of the deregulation of self-renewal pathways, including Notch, Wnt, Hedgehog, and the transcription factor Bmi1 [51] and suggests that the targeting of self-renewal pathways might provide a specific approach to eradicate CSCs [52]. However,
7 Journal of Oncology 7 in developing and testing compounds against putative CSCs, several uncertainties must be faced and elucidated, first of all the possible instability of CSCs that could hamper specific cell targeting. 5. Limits to the Xenograft Approach There are several limitations to the use of xenograft assays as proof of stemness. The most relevant is that tumor growth and stem cell phenotype are not only determined by intrinsic characteristics of tumor cells but are also influenced by the microenvironment in which cells grow. In this respect, the heterogeneity found in animal experimental models does not prove that normal or malignant stem cells undergo asymmetric division but just reflects a change in cell surface antigen expression induced by environmental conditions. Simply injecting tumor cells into mice without measuring the time of latency required to form a palpable tumor mass may induce to draw wrong conclusions about their absolute tumorigenic potential, which may be influenced by environmental conditions. Another important limitation to CSC investigation is the efficiency of thein vivo model used. It is well known that xenograft is less efficient than syngeneic transplant because of the presence of animal growth factors that could interact with their equivalent human receptors and provide confounding stimuli for the transplanted human stem/progenitor cells. In a recent paper, Kelly et al. [53], challenging the CSC hypothesis, proposed that xenograft may select a dominant clone capable of surviving and maintaining tumor outgrowth in a foreign environment, and stressed the importance of performing a tumorigenic assay using cells sorted from the patient s tumor to avoid cell variability and the selection of a dominant cell subpopulation, after several serial passages in animals, whereas the majority of cells die owing to the lack of appropriate supporting factors. As regards the mammary gland, in order to study human breast carcinogenesis, it is central to establish a model system that more accurately recapitulates normal breast epithelial development in rodents. For cancer cells, such a system should also correlate with the clinical behavior of the source tumor in patients. However, the inability of human breast epithelial cells to colonize mouse mammary fat pads represents a constant problem. The importance of both species- and tissue-specific influences has been highlighted in the studies by Kuperwasser et al. [54], which indicated that, although outgrowths can be generated in the murine humanized mammary fat pads, the repopulating frequency by normal breast stem cells remains relatively low. This suggests that the expression of some markers of the inoculated cells could be influenced by circulating or locally produced animal-specific factors. For example, estrogen has been found to profoundly affect the growth of ER-negative breast cancer cells because circulating mouse estrogens led to recruitment of bone marrow-derived stromal cells and promoted the growth of tumors in virgin mice [55]. However, despite these limitations, xenograft models still represent an essential tool for in vivo carcinogenesis studies. Meanwhile, the great challenge in stem cell investigation will be the standardization of an orthotopic model in which the whole tumor bulk could arise from a single definitively characterized human breast CSC. 6. Issues Concerning Established Breast Cancer Cell Lines Despite the intriguing results so far obtained, the use of established breast cancer cell lines as experimental models to collect data regarding CSCs is not without pitfalls, the main one being the attempt to apply stem-cell concepts to breast cell lines. Established cell lines, in fact, are cultivated in artificial conditions (depending on the experimental model) for many generations, with the risk that the unavoidable selection induced by the serum media blurs the distinction between tumorigenic and nontumorigenic clones. For example, in vitro culture conditions, such as growth with or without serum medium, could contribute to the functional differences found between non-cscs and CSCs, including a diverse proliferative activity. It is unlikely that the so-called cancer stem cells derived from established cell lines are the stem cells that make up a tumor. It is more likely that the stem cell component indicated as responsible for maintaining the line is in reality only a subpopulation of cells having a high-proliferative rate and being able to form clonal aggregates in the presence of additional techniques, for example, retroviral marking [16]. Therefore, extending the CSC concept to cell lines could be misleading as is the case with the results reported by Sheridan et al. [56]. In a series of established breast cancer cell lines (MDA-MB-231, TMD-436, Hs578T, SUM1315, HBL-100, and MDA-MB-468), the authors observed a high percentage (>30%) of CD44 + /CD24 /low cells which were highly efficient in initiating a tumor in experimental settings, and concluded that those cell lines were composed mainly of cancer stem cells. However, since the CD44 + /CD24 /low phenotype has been shown to be insufficient to confer stem-like properties [23], such an enrichment in CD44 + /CD24 /low phenotype in established cell lines could be hypothesized to be a purification marker without functional implications. Therefore, it is crucial to demonstrate that such a subpopulation, contained in established breast cancer cell lines and displaying a putative stem-like phenotype, has the real functional characteristics of CSCs: self-renewal and differentiation capability. Awaiting these confirmations as well as more standardized protocols for the isolation and expansion of CSCs from tissue, the established breast cancer cell line model still remains a useful experimental tool to test drugs, radioresistance, and antibodies against the surface markers associated with the putative stem-like phenotype. 7. Microenvironment and Stem Cell Niche Despite the extensive use of the in vivo model, in which human tumor cells are injected into immunodeficient mice, significant challenges are pending in experimental settings with CSCs, mostly related to the biological and technical
8 8 Journal of Oncology complexities associated with identifying, quantifying, and longitudinally monitoring CSCs in a complex in vivo environment. In particular, as already mentioned, xenograft may fail to reveal possible tumor growth-sustaining cells because the heterologous microenvironment precludes essential interactions with support cells. As recently shown in an elegant study by Naumov et al. [57], many established human tumor cell lines, which had previously been described as nontumorigenic (including the breast cancer cell line MDA-MB-436), were potentially tumorigenic but dormant. In fact, when these cells were injected into animals and allowed to grow for a much longer period of time than a normal tumor growth assay (up to 12 months), spontaneous tumors eventually began to develop after an initial dormancy period and a switch to an angiogenic phenotype. Therefore, this study provides a conceptual framework to approach the problem that a supportive microenvironment is required for tumor outgrowth in CSCs-involving studies. In adults, normal stem cells reside in a physiologically unique microenvironment called stem cell niche. This niche, mainly composed of fibroblasts and myoepithelial cells, provides a physical anchoring site for stem cells via adhesion molecules linking the stem cells to the extracellular matrix. Support cells act as a hub in orientating dividing stem cells to hold one daughter cell in the niche, while the other one exits the niche and undergoes transit amplification followed by differentiation [58, 59]. Under normal physiological conditions, the niche provides fine control over cell proliferation, typically balancing proliferation and apoptosis through paracrine factors, so that the stem cell population remains undifferentiated and maintains a constant size [60, 61]. The effectors mediating heterotypic cell interactions within the niche include a number of soluble factors and cell surface receptors. Interestingly, some of these molecules, including Wnt, Notch, TGF-β, bone morphogenetic proteins (BMPs), and others [62 65], are known to be involved in tumor development, and emerging data support the idea that a cancer stem cell niche could also exist, and that interactions with such a tumor niche may sustain a self-renewing population of tumor cells [66]. Alterations affecting stromal cells,such as local modifications of tissue homeostasis induced by chronic inflammation, have been shown to promote formation of epithelial tumors, and very recent papers have described the generation of pluripotent stem cells from fibroblasts. This evidence supports the possibility that cancer may arise from just a few mutations in resident tissue stem/progenitor cells or even differentiated cells leading to a stem-like phenotype [67, 68]. Indeed, if signaling pathways are dysregulated, the niche may be converted into a microenvironment that favors uncontrolled proliferation and expansion of an altered stem cell population. Similarly, a cancer stem cell could be hypothesized to remain dormant in a metastatic site until activated by abnormal signaling from the microenvironment [66]. Currently, evidence of an anatomically and/or physiologically specialized environment that constitutes a true CSC niche is scarce, and the identity of a stem cell niche within the mouse mammary gland has not been defined. Conversely, a putative stem cell niche which gives rise to at least three lineage-restricted cell types outside the stem cell zone has been identified in the adult human breast [28]. The most likely location of the niche in the mature gland could be the ducts, where in situ analysis identified a narrow region of quiescent cells that were stained for putative stem cell markers including chondroitin sulphate, K6a, CK15, and SSEA-4 [69]. However, the data published so far do not exclude the possible existence of other niches or models of niche. In particular, the model of the estrogendriven stem cell niche consists of three different cell types: the ER-positive sensor cells, the EGFR-positive stromal cells, and the ER-negative stem cells. All of them could remain quiescent until they are switched on by estrogens. In response to estrogens, the ER-positive sensor cells synthesize and secrete amphiregulin that activates EGFR-positive stromal cells which in turn activate ER-negative stem cells [70], although the identity of the stromal factors interacting with the epithelial components of the stem cell niche remains to be revealed. In addition, the similarities between stem cell niches in different tissues remain poorly understood, in particular whether tissue-specific stem cells can be regulated by stem cell niches in other organs or whether vice versa ectopic mesenchymal stem cells may colonize a breast niche and influence its behavior. Studies by Hochedlinger et al. [71] support the notion that a malignant genome can be reprogrammed to exhibit a normal-like phenotype when transferred into a new biological context, whereas Blanpain et al. [72] have reported that epithelial stem cells are able to generate their own microenvironment. Tumor cells also have the well-established ability to interact with their surrounding environment and to influence it profoundly; examples are neoangiogenesis, recruitment of immune cells, and modification of tissue architecture. All these findings have important and provocative implications for understanding metastatic growth in secondary sites. 8. CSCs and Metastasis Since the majority of breast cancer deaths occur as a result of metastatic disease rather than from the effects of the primary tumor, one of the biggest challenges is the identification, as early as possible, of patients harboring metastatic cells. In fact, the persistence of disease at a low or undetectable level (the so-called minimal residual disease ) is a common feature of breast cancer as supported by autoptic findings [73] as well as by the accumulating evidence that breast cancer patients, even with no indication of metastatic spread by current clinical parameters, have individual tumor cells in their blood [74, 75]. Several studies have shown that detection of isolated tumor cells in the bone marrow is an independent prognostic factor. However, even though approximately 30% of breast cancer patients may have micrometastatic disease in their bone marrow at the onset, only 30 50% of them will go on to develop clinically evident metastases within 5 years [76, 77]. The presence of such cells in the bone marrow is particularly interesting since bone represents one of the most common sites for breast cancer metastasis, together
9 Journal of Oncology 9 with regional lymph nodes, lung, liver, and brain, all of which may represent putative nichesfor disseminating tumor cells according to the hypothesis that cancer cells can arrest and grow in favorite metastatic sites. This seed and soil theory, first proposed in 1889 by Paget [78], predicts that a cancer cell (the seed ) can survive in and colonize only secondary sites (the soil ) that produce growth factors capable of significantly influencing cell behavior [79, 80], and has largely withstood the test of time [81]. In the case of breast cancer, disseminated carcinoma cells are detectable in the bone marrow [82], and recent findings indicate that most cancer cells found in the bone marrow have a breast CSC phenotype [83]. However, recent experimental observations demonstrated a direct involvement of the bone marrow-derived cells in the development of human epithelial tumors, suggesting that CSCs may originate from bone marrowderived cells [84]. Furthermore, a very recent paper from Mylona et al. [85] indicated that, in clinical breast cancer tissues, the CD44 + /CD24 /low phenotype (i.e., the phenotype experimentally associated with stemness) had no significant correlation with clinical outcome. This is in agreement with a previous paper by Abraham et al. [86] that found no correlation between CD44 + /CD24 /low tumor cell prevalence and tumor progression, in terms of event-free and overall survival. Conversely and quite surprisingly, the prevalence of CD44 /CD24 + tumor cells was found to exert an unfavorable impact on patients relapse-free and overall survival. This suggests that, although CD44 + /CD24 /low breast tumor cells may be highly efficient in initiating tumors in animal experimental models, in patients, these cells could be associated with the development of distant metastasis, particularly bone metastases, rather than with clinical outcome. Therefore, CD44 + /CD24 /low tumor cells could be a subclass of tumorigenic cells characterized by a great metastatic potential, maybe due to the role of CD44 as a homing receptor for distant tissue compartments. 9. Clinical Implications of CSC Paradigm and Future Directions The hypothesis that only CSCs are capable of reinitiating growth to form metastases in distant sites has fundamental clinical implications in terms of both prognosis and therapy since it provides an explanation of the limits to many current breast cancer treatments [87, 88]. In fact, the main goal of the current therapeutic strategies is represented by the gold standard of tumor shrinkage. However, if a tumor is maintained by a small subpopulation of CSCs that is constitutively resistant to therapeutic agents, tumor shrinkage results in the selective killing of the more differentiated, nontumorigenic cells that make up the bulk of the tumor, while leaving cancer stem cells viable and able to continue to maintain and/or reinitiate tumor growth on a metastatic site. Thus, current therapies fail to account for potential molecular and proliferative differences in the various subpopulations of tumor cells, and may be ineffective on the more aggressive and dangerous subgroup that constituted by CSCs. Since CSCs may constitute metastasis precursor cells, as suggested by the detection of disseminated tumor cells with a breast CSC phenotype in the bone marrow of breast cancer patients [83], it is of paramount importance to develop reliable diagnostic tools through the identification of additional and more specific markers for CSCs or even for niche cells, although this could be a difficult task due to the complexity of niche composition [89]. Difficult but not impossible, since recently Calabrese et al. [90] were able to visualize brain CSCs surviving in a vascular niche that secretes factors which promote their long-term growth and self-renewal. In addition, innovative technologies, using sensitive imaging techniques, have recently permitted real-time monitoring of CSC presence and viability as well as analysis of the angiogenic switch [91]. However, although this imaging technique can be extremely valuable in order to achieve a greater understanding of the biology of CSCs and their relationship with the stromal compartment, it cannot be used, at least at present, for intravital monitoring of such elusive cells in patients. Conversely, more clinically relevant imaging techniques, such as high-resolution magnetic resonance imaging (MRI) [92] and three-dimensional highfrequency ultrasound [93], are currently being developed for the study of CSCs in preclinical models. These techniques look promising for future clinical applications to determine prognosis, monitor therapeutic efficacy, and possibly affect therapies. In addition to its impact on diagnosis and prognosis, the CSC hypothesis also has significant implications for the therapy of breast cancer. Since current therapies do not target the tumor-initiating cells effectively, as implied by the large number of patients who relapse after adjuvant chemotherapeutic and/or hormonal treatment [94], there is a need for therapeutic agents specifically directed against CSCs. These specific agents could be added to conventional cytotoxic drugs, designed to kill actively dividing cells, and be able to eradicate the metastatic disease. Thus, they would turn cancer, if detected at early stage, into a curable disease limited to the primary organ. Although we still know too little about the molecular features distinguishing CSCs from the bulk of tumor cells to develop a smart drug, the significant advances in the field indicate that CSCs could soon represent a really useful target. 10. Conclusions Recent findings in breast biology have provided support for the CSC hypothesis, but researchers still face many challenges. First, attention should be paid to the accuracy of experimental methods for the isolation and propagation of CSCs derived from clinical samples, with a particular emphasis on cell culture environment (substrate, atmosphere, and medium) that has a critical role in standardizing the culture conditions for breast cancer progression studies [49]. Secondly, more accurate techniques should be used for the sphere formation assay, so as to determine a selfrenewing capability sufficient to classify a cancer cell as a cancer stem cell, and to avoid conflicting results obtained by different groups. Moreover, it is necessary to pursue the
10 10 Journal of Oncology clinical demonstration that CSCs can be used as a prognostic indicator of disease progression and to identify the mechanisms by which CSCs escape conventional therapies in order to develop new specific therapeutic approaches. Finally, there is the need to find the definitive evidence of the existence of CSCs and to identify the stromarelated factors that influence the development and spread of CSCs. Since CSCs have not yet been fully defined, their existence in breast cancer cannot conclusively be proven, and competitive hypotheses, which may explain some of the puzzling features of certain tumor cell populations, should be taken into account. The most exciting of these competitive hypotheses implies the reversible epithelial-to-mesenchymal transition, the developmental process in which epithelial cells acquire the migratory properties of mesenchymal cells. As shown in a very recent paper [95], the induction of the epithelial-mesenchymal transition could stimulate breast cells to adopt characteristics of stem cells. This suggests that CSCs are not distinct entities but rather tumor cells that transiently acquire stem cell-like properties as a consequence of an epithelial-mesenchymal transition. Undoubtedly, such a link between epithelial-mesenchymal transition and stem cell phenotype further fuels the debated question about the existence of CSCs and holds a number of interesting implications for the biology of epithelial cells, including the possibility that the stem cells of certain epithelial organs such as mammary glands may acquire many of the attributes of the mesenchymal cell state that confer them an increased tumorigenic potential. The advent of new technologies, including gene expression profiling and proteomics, and the ability to apply them tosmallnumbersofcellswillprobablyhelptosolvesuch open questions. References [1] A. Jemal, R. Siegel, E. Ward, et al., Cancer statistics, 2006, CA: A Cancer Journal for Clinicians, vol. 56, no. 2, pp , [2] A. F. Chambers, A. C. Groom, and I. C. MacDonald, Dissemination and growth of cancer cells in metastatic sites, Nature Reviews Cancer, vol. 2, no. 8, pp , [3] F. A. Tavassoli, P. Devilee, and World Health Organization Classification of Tumors, Pathology and Genetics of Tumours of the Breast and Female Genital Organs, IARC Press, Lyon, France, [4] M. Duffy, Estrogen receptors: role in breast cancer, Critical Reviews in Clinical Laboratory Sciences, vol. 43, no. 4, pp , [5] R. Schiff,S.A.Massarweh,J.Shou,etal., Advancedconcepts in estrogen receptor biology and breast cancer endocrine resistance: implicated role of growth factor signaling and estrogen receptor coregulators, Cancer Chemotherapy and Pharmacology, vol. 56, supplement 1, pp , [6] J. S. Ross and J. A. Fletcher, The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy, Stem Cells, vol. 16, no. 6, pp , [7] L. E. Ailles and I. L. Weissman, Cancer stem cells in solid tumors, Current Opinion in Biotechnology, vol. 18, no. 5, pp , [8] J. Taylor-Papadimitriou, E. B. Lane, and S. E. Chang, Cell lineages and interactions in neoplastic expression in the human breast, in Understanding Breast Cancer: Clinical and Laboratory Concepts, M. A. Rich, J. C. Hager, and P. Furmanski, Eds., pp , Marcel Dekker, New York, NY, USA, [9] M. Shackleton, F. Vaillant, K. J. Simpson, et al., Generation of a functional mammary gland from a single stem cell, Nature, vol. 439, no. 7072, pp , [10] J. Regan and M. Smalley, Prospective isolation and functional analysis of stem and differentiated cells from the mouse mammary gland, Stem Cell Reviews, vol. 3, no. 2, pp , [11] J. Stingl, C. J. Eaves, U. Kuusk, and J. T. Emerman, Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast, Differentiation, vol. 63, no. 4, pp , [12] P. S. Rudland, R. Barraclough, D. G. Fernig, and J. A. Smith, Mammary stem cells in normal development and cancer, in Stem Cell, C. S. Potten, Ed., pp , Accademic Press, San Diego, Calif, USA, [13] T. Gudjonsson, R. Villadsen, H. L. Nielsen, L. Rønnov-Jessen, M. J. Bissell, and O. W. Petersen, Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties, Genes & Development,vol.16,no.6,pp , [14] J. Yang, R. C. Guzman, N. Popnikolov, et al., Phenotypic characterization of collagen gel embedded primary human breast epithelial cells in athymic nude mice, Cancer Letters, vol. 81, no. 2, pp , [15] G. Dontu, W. M. Abdallah, J. M. Foley, et al., In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells, Genes & Development, vol. 17, no. 10, pp , [16] I. Singec, R. Knoth, R. P. Meyer, et al., Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology, Nature Methods, vol. 3, no. 10, pp , [17] J. Stingl and C. Caldas, Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis, Nature Reviews Cancer, vol. 7, no. 10, pp , [18] L. A. Doyle, W. Yang, L. V. Abruzzo, et al., A multidrug resistance transporter from human MCF-7 breast cancer cells, Proceedings of the National Academy of Sciences of the United States of America, vol. 95, no. 26, pp , [19] M. Kim, H. Turnquist, J. Jackson, et al., The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst and is overexpressed in hematopoietic stem cells, Clinical Cancer Research, vol. 8, no. 1, pp , [20] A. J. Alvi, H. Clayton, C. Joshi, et al., Functional and molecular characterisation of mammary side population cells, Breast Cancer Research, vol. 5, no. 1, pp. R1 R8, [21] R. P. Hill, Identifying cancer stem cells in solid tumors: case not proven, Cancer Research, vol. 66, no. 4, pp , [22] D. Adamski, J.-F. Mayol, N. Platet, F. Berger, F. Hérodin, and D. Wion, Effects of Hoechst on C2C12 and PC12 cell differentiation, FEBS Letters, vol. 581, no. 16, pp , [23] C. Ginestier, M. H. Hur, E. Charafe-Jauffret, et al., ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome, Cell Stem Cell, vol. 1, no. 5, pp , 2007.
11 Journal of Oncology 11 [24] T.Reya,S.J.Morrison,M.F.Clarke,andI.L.Weissman, Stem cells, cancer, and cancer stem cells, Nature, vol. 414, no. 6859, pp , [25] R. Bjerkvig, B. B. Tysnes, K. S. Aboody, J. Najbauer, and A. J. A. Terzis, The origin of the cancer stem cell: current controversies and new insights, Nature Reviews Cancer, vol. 5, no. 11, pp , [26] L. L. Campbell and K. Polyak, Breast tumor heterogeneity: cancer stem cells or clonal evolution? Cell Cycle, vol. 6, no. 19, pp , [27] P. C. Nowell, The clonal evolution of tumor cell populations, Science, vol. 194, no. 4260, pp , [28] R. Villadsen, A. J. Fridriksdottir, L. Rønnov-Jessen, et al., Evidence for a stem cell hierarchy in the adult human breast, Journal of Cell Biology, vol. 177, no. 1, pp , [29] M. S. Wicha, S. Liu, and G. Dontu, Cancer stem cells: an old idea a paradigm shift, Cancer Research, vol. 66, no. 4, pp , [30] R. Pardal, M. F. Clarke, and S. J. Morrison, Applying the principles of stem-cell biology to cancer, Nature Reviews Cancer, vol. 3, no. 12, pp , [31] S. K. Singh, I. D. Clarke, M. Terasaki, et al., Identification of a cancer stem cell in human brain tumors, Cancer Research, vol. 63, no. 18, pp , [32] C. D. Peacock and D. N. Watkins, Cancer stem cells and the ontogeny of lung cancer, Journal of Clinical Oncology, vol. 26, no. 17, pp , [33] N. J. Maitland and A. T. Collins, Prostate cancer stem cells: anewtargetfortherapy, Journal of Clinical Oncology, vol. 26, no. 17, pp , [34] L. Ricci-Vitiani, D. G. Lombardi, E. Pilozzi, et al., Identification and expansion of human colon-cancer-initiating cells, Nature, vol. 445, no. 7123, pp , [35] S. Sell and H. L. Leffert, Liver cancer stem cells, Journal of Clinical Oncology, vol. 26, no. 17, pp , [36] C. J. Lee, J. Dosch, and D. M. Simeone, Pancreatic cancer stem cells, Journal of Clinical Oncology, vol. 26, no. 17, pp , [37] M. Al-Hajj, M. S. Wicha, A. Benito-Hernandez, S. J. Morrison, and M. F. Clarke, Prospective identification of tumorigenic breast cancer cells, Proceedings of the National Academy of Sciences of the United States of America, vol. 100, no. 7, pp , [38] M. R. Kamstrup, R. Gniadecki, and G. L. Skovgaard, Putative cancer stem cells in cutaneous malignancies, Experimental Dermatology, vol. 16, no. 4, pp , [39] L. Weiss, Metastatic inefficiency, Advances in Cancer Research, vol. 54, pp , [40] T. Brabletz, A. Jung, S. Spaderna, F. Hlubek, and T. Kirchner, Migrating cancer stem cells an integrated concept of malignant tumour progression, Nature Reviews Cancer, vol. 5, no. 9, pp , [41] P. Ø. Sakariassen, H. Immervoll, and M. Chekenya, Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies, Neoplasia, vol. 9, no. 11, pp , [42] M. Baumann, M. Krause, and R. Hill, Exploring the role of cancer stem cells in radioresistance, Nature Reviews Cancer, vol. 8, no. 7, pp , [43] M. Al-Hajj and M. F. Clarke, Self-renewal and solid tumor stem cells, Oncogene, vol. 23, no. 43, pp , [44] D. Hanahan and R. A. Weinberg, The hallmarks of cancer, Cell, vol. 100, no. 1, pp , [45] J. Marx, Cancer research. Mutant stem cells may seed cancer, Science, vol. 301, no. 5638, pp , [46] J. M. Adams and A. Strasser, Is tumor growth sustained by rare cancer stem cells or dominant clones? Cancer Research, vol. 68, no. 11, pp , [47] J. E. Visvader and G. J. Lindeman, Cancer stem cells in solid tumours: accumulating evidence and unresolved questions, Nature Reviews Cancer, vol. 8, no. 10, pp , [48] D. R. Welch, Technical considerations for studying cancer metastasis in vivo, Clinical and Experimental Metastasis, vol. 15, no. 3, pp , [49] T. A. Ince, A. L. Richardson, G. W. Bell, et al., Transformation of different human breast epithelial cell types leads to distinct tumor phenotypes, Cancer Cell, vol. 12, no. 2, pp , [50] A. Hadnagy, L. Gaboury, R. Beaulieu, and D. Balicki, SP analysis may be used to identify cancer stem cell populations, Experimental Cell Research, vol. 312, no. 19, pp , [51] S. Liu, G. Dontu, and M. S. Wicha, Mammary stem cells, self-renewal pathways, and carcinogenesis, Breast Cancer Research, vol. 7, no. 3, pp , [52] M. Kubo, M. Nakamura, A. Tasaki, et al., Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer, Cancer Research, vol. 64, no. 17, pp , [53] P. N. Kelly, A. Dakic, J. M. Adams, S. L. Nutt, and A. Strasser, Tumor growth need not be driven by rare cancer stem cells, Science, vol. 317, no. 5836, p. 337, [54] C. Kuperwasser, T. Chavarria, M. Wu, et al., Reconstruction of functionally normal and malignant human breast tissues in mice, Proceedings of the National Academy of Sciences of the United States of America, vol. 101, no. 14, pp , [55] P. B. Gupta, D. Proia, O. Cingoz, et al., Systemic stromal effects of estrogen promote the growth of estrogen receptornegative cancers, Cancer Research, vol. 67, no. 5, pp , [56] C. Sheridan, H. Kishimoto, R. K. Fuchs, et al., CD44 + /CD24 -breast cancer cells exhibit enhanced invase properties: an early step necessary for metastasis, Breast Cancer Research, vol. 8, no. 5, article R59, pp. 1 13, [57] G. N. Naumov, E. Bender, D. Zurakowski, et al., A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype, Journal of the National Cancer Institute, vol. 98, no. 5, pp , [58] L. Li and T. Xie, Stem cell niche: structure and function, Annual Review of Cell and Developmental Biology, vol. 21, pp , [59] A. Spradling, D. Drummond-Barbosa, and T. Kai, Stem cells find their niche, Nature, vol. 414, no. 6859, pp , [60] T. Tumbar, G. Guasch, V. Greco, et al., Defining the epithelial stem cell niche in skin, Science, vol. 303, no. 5656, pp , [61] J. Zhang, C. Niu, L. Ye, et al., Identification of the haematopoietic stem cell niche and control of the niche size, Nature, vol. 425, no. 6960, pp , [62]G.Turashvili,J.Bouchal,G.Burkadze,andZ.Kolar, Wnt signalling pathway in mammary gland development and carcinogenesis, Pathobiology, vol. 73, no. 5, pp , 2006.
12 12 Journal of Oncology [63] G. Dontu, K. W. Jackson, E. McNicholas, M. J. Kawamura, W. M. Abdallah, and M. S. Wicha, Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells, Breast Cancer Research, vol. 6, no. 6, pp. R605 R615, [64] S. Liu, G. Dontu, I. D. Mantle, et al., Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells, Cancer Research, vol. 66, no. 12, pp , [65] B. Bierie and H. L. Moses, TGF-β and cancer, Cytokine & Growth Factor Reviews, vol. 17, no. 1-2, pp , [66] L. Li and W. B. Neaves, Normal stem cells and cancer stem cells: the niche matters, Cancer Research, vol. 66, no. 9, pp , [67] K. Takahashi, K. Tanabe, M. Ohnuki, et al., Induction of pluripotent stem cells from adult human fibroblasts by defined factors, Cell, vol. 131, no. 5, pp , [68] M. Wernig, A. Meissner, R. Foreman, et al., In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state, Nature, vol. 448, no. 7151, pp , [69] M. A. LaBarge, O. W. Petersen, and M. J. Bissell, Of microenvironments and mammary stem cells, Stem Cell Reviews, vol. 3, no. 2, pp , [70] C. Brisken and S. Duss, Stem cells and the stem cell niche in the breast: an integrated hormonal and developmental perspective, Stem Cell Reviews, vol. 3, no. 2, pp , [71] K. Hochedlinger, R. Blelloch, C. Brennan, et al., Reprogramming of a melanoma genome by nuclear transplantation, Genes & Development, vol. 18, no. 15, pp , [72] C. Blanpain, W. E. Lowry, A. Geoghegan, L. Polak, and E. Fuchs, Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche, Cell, vol. 118, no. 5, pp , [73] J. Folkman and R. Kalluri, Cancer without disease, Nature, vol. 427, no. 6977, p. 787, [74] S. Meng, D. Tripathy, E. P. Frenkel, et al., Circulating tumor cells in patients with breast cancer dormancy, Clinical Cancer Research, vol. 10, no. 24, pp , [75] A. Ring, I. E. Smith, and M. Dowsett, Circulating tumour cells in breast cancer, Lancet Oncology, vol. 5, no. 2, pp , [76] G. Wiedswang, E. Borgen, R. Kåresen, et al., Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer, Journal of Clinical Oncology, vol. 21, no. 18, pp , [77] G. Gebauer, T. Fehm, E. Merkle, E. P. Beck, N. Lang, and W. Jäger, Epithelial cells in bone marrow of breast cancer patients at time of primary surgery: clinical outcome during long-term follow-up, Journal of Clinical Oncology, vol. 19, no. 16, pp , [78] S. Paget, The distribution of secondary growths in cancer of the breast, The Lancet, vol. 133, no. 3421, pp , [79] J. A. Aguirre-Ghiso, L. Ossowski, and S. K. Rosenbaum, Green fluorescent protein tagging of extracellular signalregulated kinase and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth, Cancer Research, vol. 64, no. 20, pp , [80] J. Folkman, Role of angiogenesis in tumor growth and metastasis, Seminars in Oncology, vol. 29, no. 6, supplement 16, pp , [81] I. J. Fidler, Seed and soil revisited: contribution of the organ microenvironment to cancer metastasis, Surgical Oncology Clinics of North America, vol. 10, no. 2, pp , [82]P.A.Phadke,R.R.Mercer,J.F.Harms,etal., Kinetics of metastatic breast cancer cell trafficking in bone, Clinical Cancer Research, vol. 12, no. 5, pp , [83] M. Balic, H. Lin, L. Young, et al., Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype, Clinical Cancer Research, vol. 12, no. 19, pp , [84] C. R. Cogle, N. D. Theise, D. Fu, et al., Bone marrow contributes to epithelial cancers in mice and humans as developmental mimicry, Stem Cells, vol. 25, no. 8, pp , [85] E. Mylona, I. Giannopoulou, E. Fasomytakis, et al., The clinicopathologic and prognostic significance of CD44 + /CD24 /low and CD44 /CD24 + tumor cells in invasive breast carcinomas, Human Pathology, vol. 39, no. 7, pp , [86] B. K. Abraham, P. Fritz, M. McClellan, P. Hauptvogel, M. Athelogou, and H. Brauch, Prevalence of CD44 + /CD24 /low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis, Clinical Cancer Research, vol. 11, no. 3, pp , [87] M. Al-Hajj, M. W. Becker, M. Wicha, I. Weissman, and M. F. Clarke, Therapeutic implications of cancer stem cells, Current Opinion in Genetics and Development, vol.14,no.1, pp , [88] M. Dean, Cancer stem cells: redefining the paradigm of cancer treatment strategies, Molecular Interventions, vol. 6, no. 3, pp , [89] J. B. Sneddon and Z. Werb, Location, location, location: the cancer stem cell niche, Cell Stem Cell, vol. 1, no. 6, pp , [90] C. Calabrese, H. Poppleton, M. Kocak, et al., A perivascular niche for brain tumor stem cells, Cancer Cell, vol.11,no.1, pp , [91] L. S. Hart and W. S. El-Deiry, Invincible, but not invisible: imaging approaches toward in vivo detection of cancer stem cells, Journal of Clinical Oncology, vol. 26, no. 17, pp , [92] C. Heyn, J. A. Ronald, L. T. Mackenzie, et al., In vivo magnetic resonance imaging of single cells in mouse brain with optical validation, Magnetic Resonance in Medicine, vol. 55, no. 1, pp , [93] K. C. Graham, L. A. Wirtzfeld, L. T. MacKenzie, et al., Three-dimensional high-frequency ultrasound imaging for longitudinal evaluation of liver metastases in preclinical models, Cancer Research, vol. 65, no. 12, pp , [94] Early Breast Cancer Trialists Collaborative Group (EBCTCG), Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials, The Lancet, vol. 365, no. 9472, pp , [95] S. A. Mani, W. Guo, M.-J. Liao, et al., The epithelialmesenchymal transition generates cells with properties of stem cells, Cell, vol. 133, no. 4, pp , 2008.
13 MEDIATORS of INFLAMMATION The Scientific World Journal Gastroenterology Research and Practice Journal of Diabetes Research International Journal of Journal of Endocrinology Immunology Research Disease Markers Submit your manuscripts at BioMed Research International PPAR Research Journal of Obesity Journal of Ophthalmology Evidence-Based Complementary and Alternative Medicine Stem Cells International Journal of Oncology Parkinson s Disease Computational and Mathematical Methods in Medicine AIDS Behavioural Neurology Research and Treatment Oxidative Medicine and Cellular Longevity
Characteristics of Cancer Stem Cells (CSCs)
 GENReports: Market & Tech Analysis Characteristics of Cancer Stem Cells (CSCs) > Enal Razvi, Ph.D. Biotechnology Analyst, Managing Director Select Biosciences, Inc. enal@selectbio.us! Topic,IntroducEon,and,Scope!
GENReports: Market & Tech Analysis Characteristics of Cancer Stem Cells (CSCs) > Enal Razvi, Ph.D. Biotechnology Analyst, Managing Director Select Biosciences, Inc. enal@selectbio.us! Topic,IntroducEon,and,Scope!
Contents 1 The Windows of Susceptibility to Breast Cancer 2 The So Called Pre-Neoplastic Lesions and Carcinoma In Situ
 Contents 1 The Windows of Susceptibility to Breast Cancer... 1 1.1 Introduction... 1 1.2 Risk Factor and Etiological Agents... 2 1.3 The Concept of the Windows of Susceptibility to Carcinogenesis... 5
Contents 1 The Windows of Susceptibility to Breast Cancer... 1 1.1 Introduction... 1 1.2 Risk Factor and Etiological Agents... 2 1.3 The Concept of the Windows of Susceptibility to Carcinogenesis... 5
TUMOR INITIATING CELLS: THE STEM CELL THEORY OF CANCER
 Pr John DE VOS Département d Ingénierie Cellulaire et Tissulaire INSERM 1183 - IRMB Hôpital St Eloi - CHU de Montpellier john.devos@inserm.fr @_jdevos_ TUMOR INITIATING CELLS: THE STEM CELL THEORY OF CANCER
Pr John DE VOS Département d Ingénierie Cellulaire et Tissulaire INSERM 1183 - IRMB Hôpital St Eloi - CHU de Montpellier john.devos@inserm.fr @_jdevos_ TUMOR INITIATING CELLS: THE STEM CELL THEORY OF CANCER
Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features
 Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features Loretta Gammaitoni, Lidia Giraudo, Valeria Leuci, et al. Clin Cancer Res
Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features Loretta Gammaitoni, Lidia Giraudo, Valeria Leuci, et al. Clin Cancer Res
Basement membrane in lobule.
 Bahram Memar, MD Basement membrane in lobule. Normal lobule-luteal phase Normal lobule-follicular phase Lactating breast Greater than 95% are adenocarcinomas in situ carcinomas and invasive carcinomas.
Bahram Memar, MD Basement membrane in lobule. Normal lobule-luteal phase Normal lobule-follicular phase Lactating breast Greater than 95% are adenocarcinomas in situ carcinomas and invasive carcinomas.
Hematopoiesis. - Process of generation of mature blood cells. - Daily turnover of blood cells (70 kg human)
 Hematopoiesis - Process of generation of mature blood cells - Daily turnover of blood cells (70 kg human) 1,000,000,000,000 total cells 200,000,000,000 red blood cells 70,000,000,000 neutrophils Hematopoiesis
Hematopoiesis - Process of generation of mature blood cells - Daily turnover of blood cells (70 kg human) 1,000,000,000,000 total cells 200,000,000,000 red blood cells 70,000,000,000 neutrophils Hematopoiesis
A NOVEL CANCER STEM CELL (CSC) DRUG DISCOVERY PLATFORM. Collaborative Opportunity
 A NOVEL CANCER STEM CELL (CSC) DRUG DISCOVERY PLATFORM Collaborative Opportunity March 2014 PURPOSE Cancer Research UK funded Investigators have established in vitro and in vivo models of CSCs for use
A NOVEL CANCER STEM CELL (CSC) DRUG DISCOVERY PLATFORM Collaborative Opportunity March 2014 PURPOSE Cancer Research UK funded Investigators have established in vitro and in vivo models of CSCs for use
- is a common disease - 1 person in 3 can expect to contract cancer at some stage in their life -1 person in 5 can expect to die from it
 MBB157 Dr D Mangnall The Molecular Basis of Disease CANCER Lecture 1 One of the simpler (and better) definitions of cancer comes from the American Cancer Society, who define cancer as; 'Cancer is a group
MBB157 Dr D Mangnall The Molecular Basis of Disease CANCER Lecture 1 One of the simpler (and better) definitions of cancer comes from the American Cancer Society, who define cancer as; 'Cancer is a group
Research Article Stromal Expression of CD10 in Invasive Breast Carcinoma and Its Correlation with ER, PR, HER2-neu, and Ki67
 SAGE-Hindawi Access to Research International Breast Cancer Volume 20, Article ID 47957, 4 pages doi:0.406/20/47957 Research Article Stromal Expression of CD0 in Invasive Breast Carcinoma and Its Correlation
SAGE-Hindawi Access to Research International Breast Cancer Volume 20, Article ID 47957, 4 pages doi:0.406/20/47957 Research Article Stromal Expression of CD0 in Invasive Breast Carcinoma and Its Correlation
Neoplasia part I. Dr. Mohsen Dashti. Clinical Medicine & Pathology nd Lecture
 Neoplasia part I By Dr. Mohsen Dashti Clinical Medicine & Pathology 316 2 nd Lecture Lecture outline Review of structure & function. Basic definitions. Classification of neoplasms. Morphologic features.
Neoplasia part I By Dr. Mohsen Dashti Clinical Medicine & Pathology 316 2 nd Lecture Lecture outline Review of structure & function. Basic definitions. Classification of neoplasms. Morphologic features.
Cancer Stem Cells & Glioblastoma
 Cancer Stem Cells & Glioblastoma JP Hugnot «Brain plasticity, Neural stem cells and Glial tumors» INSERM U1051-UM2 Institut des Neurosciences de Montpellier Montpellier 1-Stem cells and Brain Stem Cells
Cancer Stem Cells & Glioblastoma JP Hugnot «Brain plasticity, Neural stem cells and Glial tumors» INSERM U1051-UM2 Institut des Neurosciences de Montpellier Montpellier 1-Stem cells and Brain Stem Cells
Predictive Assays in Radiation Therapy
 Outline Predictive Assays in Radiation Therapy Radiation Biology Introduction Early predictive assays Recent trends in predictive assays Examples for specific tumors Summary Lecture 4-23-2014 Introduction
Outline Predictive Assays in Radiation Therapy Radiation Biology Introduction Early predictive assays Recent trends in predictive assays Examples for specific tumors Summary Lecture 4-23-2014 Introduction
Breast cancer as a systemic disease: a view of metastasis
 Review Click here for more articles from the symposium doi: 10.1111/joim.12084 Breast cancer as a systemic disease: a view of metastasis A. J. Redig 1 & S. S. McAllister 1,2,3 From the 1 Division of Hematology,
Review Click here for more articles from the symposium doi: 10.1111/joim.12084 Breast cancer as a systemic disease: a view of metastasis A. J. Redig 1 & S. S. McAllister 1,2,3 From the 1 Division of Hematology,
Differential Connexin Function Enhances Self-Renewal in Glioblastoma
 CSU ID: 2558414 Name: Sonal Patel Differential Connexin Function Enhances Self-Renewal in Glioblastoma Cancer is one of the most common disease in the US with more than a million people affected every
CSU ID: 2558414 Name: Sonal Patel Differential Connexin Function Enhances Self-Renewal in Glioblastoma Cancer is one of the most common disease in the US with more than a million people affected every
BY Mrs. K.SHAILAJA., M. PHARM., LECTURER DEPT OF PHARMACY PRACTICE, SRM COLLEGE OF PHARMACY
 BY Mrs. K.SHAILAJA., M. PHARM., LECTURER DEPT OF PHARMACY PRACTICE, SRM COLLEGE OF PHARMACY Cancer is a group of more than 100 different diseases that are characterized by uncontrolled cellular growth,
BY Mrs. K.SHAILAJA., M. PHARM., LECTURER DEPT OF PHARMACY PRACTICE, SRM COLLEGE OF PHARMACY Cancer is a group of more than 100 different diseases that are characterized by uncontrolled cellular growth,
September 20, Submitted electronically to: Cc: To Whom It May Concern:
 History Study (NOT-HL-12-147), p. 1 September 20, 2012 Re: Request for Information (RFI): Building a National Resource to Study Myelodysplastic Syndromes (MDS) The MDS Cohort Natural History Study (NOT-HL-12-147).
History Study (NOT-HL-12-147), p. 1 September 20, 2012 Re: Request for Information (RFI): Building a National Resource to Study Myelodysplastic Syndromes (MDS) The MDS Cohort Natural History Study (NOT-HL-12-147).
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AD Award Number: W81XWH-04-1-0618 TITLE: Are Breast Tumor Stem Cells Responsible for Metastasis and Angiogenesis PRINCIPAL INVESTIGATOR: Quintin Pan, Ph.D. CONTRACTING ORGANIZATION: University of Michigan
AD Award Number: W81XWH-04-1-0618 TITLE: Are Breast Tumor Stem Cells Responsible for Metastasis and Angiogenesis PRINCIPAL INVESTIGATOR: Quintin Pan, Ph.D. CONTRACTING ORGANIZATION: University of Michigan
Maram Abdaljaleel, MD Dermatopathologist and Neuropathologist University of Jordan, School of Medicine
 Maram Abdaljaleel, MD Dermatopathologist and Neuropathologist University of Jordan, School of Medicine The most common non-skin malignancy of women 2 nd most common cause of cancer deaths in women, following
Maram Abdaljaleel, MD Dermatopathologist and Neuropathologist University of Jordan, School of Medicine The most common non-skin malignancy of women 2 nd most common cause of cancer deaths in women, following
Metastasis progression
 Metastasis progression Mieloma multiplo Linear Progression Cancer cells disseminate through the organism after acquiring metastatic features inside the primary cancer Parallel progression Cancer cells
Metastasis progression Mieloma multiplo Linear Progression Cancer cells disseminate through the organism after acquiring metastatic features inside the primary cancer Parallel progression Cancer cells
Extended Mammosphere Culture of Human Breast Cancer Cells
 Extended Mammosphere Culture of Human Breast Cancer Cells Application Note The PromoCell 3D Tumorsphere Medium XF The PromoCell 3D Tumorsphere Medium XF has been designed to meet your requirements for
Extended Mammosphere Culture of Human Breast Cancer Cells Application Note The PromoCell 3D Tumorsphere Medium XF The PromoCell 3D Tumorsphere Medium XF has been designed to meet your requirements for
Beverly A. Teicher, PhD DCTD/NCI. The content reflects my professional opinions, not an NCI policy statement.
 Beverly A. Teicher, PhD DCTD/NCI The content reflects my professional opinions, not an NCI policy statement. Outline 1. Transplantable Syngeneic Tumors 2. Human Tumor Xenografts 3. Disseminated Disease
Beverly A. Teicher, PhD DCTD/NCI The content reflects my professional opinions, not an NCI policy statement. Outline 1. Transplantable Syngeneic Tumors 2. Human Tumor Xenografts 3. Disseminated Disease
DOCTORAL THESIS SUMMARY
 UNIVERSITY OF MEDICINE AND PHARMACY CRAIOVA FACULTY OF MEDICINE DOCTORAL THESIS SUMMARY CLINICO-IMAGING STUDY OF INVASIVE DUCTAL BREAST CARCINOMAS CORRELATED TO HORMONAL RECEPTORS AND HER2/NEU ONCOPROTEIN
UNIVERSITY OF MEDICINE AND PHARMACY CRAIOVA FACULTY OF MEDICINE DOCTORAL THESIS SUMMARY CLINICO-IMAGING STUDY OF INVASIVE DUCTAL BREAST CARCINOMAS CORRELATED TO HORMONAL RECEPTORS AND HER2/NEU ONCOPROTEIN
Cancer stem cells old concepts, new insights
 Review (2008) 15, 947 958 & 2008 Nature Publishing Group All rights reserved 1350-9047/08 $30.00 www.nature.com/cdd Cancer stem cells old concepts, new insights L Vermeulen 1, MR Sprick 1, K Kemper 1,
Review (2008) 15, 947 958 & 2008 Nature Publishing Group All rights reserved 1350-9047/08 $30.00 www.nature.com/cdd Cancer stem cells old concepts, new insights L Vermeulen 1, MR Sprick 1, K Kemper 1,
Contemporary Classification of Breast Cancer
 Contemporary Classification of Breast Cancer Laura C. Collins, M.D. Vice Chair of Anatomic Pathology Professor of Pathology Beth Israel Deaconess Medical Center and Harvard Medical School Boston, MA Outline
Contemporary Classification of Breast Cancer Laura C. Collins, M.D. Vice Chair of Anatomic Pathology Professor of Pathology Beth Israel Deaconess Medical Center and Harvard Medical School Boston, MA Outline
Stem Cells. Induced Stem Cells
 Induced Stem Cells Stem Cells Mouse and human somatic cells can either be reprogrammed to a pluripotent state or converted to another lineage with a combination of transcription factors suggesting that
Induced Stem Cells Stem Cells Mouse and human somatic cells can either be reprogrammed to a pluripotent state or converted to another lineage with a combination of transcription factors suggesting that
let-7 Regulates Self Renewal and Tumorigenicity of Breast Cancer Cells
 let-7 Regulates Self Renewal and Tumorigenicity of Breast Cancer Cells Fengyan Yu, 1,2 Herui Yao, 1 Pengcheng Zhu, 2 Xiaoqin Zhang, 1 Qiuhui Pan, 1 Chang Gong, 1 Yijun Huang, 3 Xiaoqu Hu, 1 Fengxi Su,
let-7 Regulates Self Renewal and Tumorigenicity of Breast Cancer Cells Fengyan Yu, 1,2 Herui Yao, 1 Pengcheng Zhu, 2 Xiaoqin Zhang, 1 Qiuhui Pan, 1 Chang Gong, 1 Yijun Huang, 3 Xiaoqu Hu, 1 Fengxi Su,
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AD Award Number: W81XWH-09-1-0129 TITLE: Targeting Ovarian Carcinoma Stem Cells PRINCIPAL INVESTIGATOR: Richard Jones, M.D. CONTRACTING ORGANIZATION: Johns Hopkins University Baltimore, MD 21205 REPORT
AD Award Number: W81XWH-09-1-0129 TITLE: Targeting Ovarian Carcinoma Stem Cells PRINCIPAL INVESTIGATOR: Richard Jones, M.D. CONTRACTING ORGANIZATION: Johns Hopkins University Baltimore, MD 21205 REPORT
Supplementary Figure 1. Identification of tumorous sphere-forming CSCs and CAF feeder cells. The LEAP (Laser-Enabled Analysis and Processing)
 Supplementary Figure 1. Identification of tumorous sphere-forming CSCs and CAF feeder cells. The LEAP (Laser-Enabled Analysis and Processing) platform with laser manipulation to efficiently purify lung
Supplementary Figure 1. Identification of tumorous sphere-forming CSCs and CAF feeder cells. The LEAP (Laser-Enabled Analysis and Processing) platform with laser manipulation to efficiently purify lung
CRIPTO-1 A POSSIBLE NEW BIOMARKER IN GLIOBLASTOMA MULTIFORME PIA OLESEN, MD, PHD STUDENT
 CRIPTO-1 A POSSIBLE NEW BIOMARKER IN GLIOBLASTOMA MULTIFORME PIA OLESEN, MD, PHD STUDENT Glioblastoma WHO Grade IV Glioma Heterogenic Undiffenrentiated phenotype 50% of all Gliomas Around 600 patients
CRIPTO-1 A POSSIBLE NEW BIOMARKER IN GLIOBLASTOMA MULTIFORME PIA OLESEN, MD, PHD STUDENT Glioblastoma WHO Grade IV Glioma Heterogenic Undiffenrentiated phenotype 50% of all Gliomas Around 600 patients
Can we prevent metastasis?
 Can we prevent metastasis? A research example to translate from the bench to the bedside Diane Palmieri, Ph.D. Women s Cancers Section Laboratory of Molecular Pharmacology CCR, NCI Some Basic Truths Most
Can we prevent metastasis? A research example to translate from the bench to the bedside Diane Palmieri, Ph.D. Women s Cancers Section Laboratory of Molecular Pharmacology CCR, NCI Some Basic Truths Most
CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow
 White Paper September 2016 CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow Lily C. Trajman, PhD Introduction: Hematopoietic Stem Cells (HSCs)
White Paper September 2016 CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow Lily C. Trajman, PhD Introduction: Hematopoietic Stem Cells (HSCs)
Oncolytic Virotherapy: Targeting Cancer Stem Cells
 Oncolytic Virotherapy: Targeting Cancer Stem Cells Cancer Stem Cells (CSCs) or Cancer Initiating Cells (CICs) A consensus of five defining criteria has been established to affirm the existence of CICs:
Oncolytic Virotherapy: Targeting Cancer Stem Cells Cancer Stem Cells (CSCs) or Cancer Initiating Cells (CICs) A consensus of five defining criteria has been established to affirm the existence of CICs:
Lecture 1: Carcinogenesis
 Lecture 1: Carcinogenesis Anti-cancer (oncology agents): These are perhaps the most dangerous of drugs, other than the narcotic analgesics. This is due to their toxicities. Killing or inhibiting cancer
Lecture 1: Carcinogenesis Anti-cancer (oncology agents): These are perhaps the most dangerous of drugs, other than the narcotic analgesics. This is due to their toxicities. Killing or inhibiting cancer
World Congress on Breast Cancer
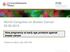 World Congress on Breast Cancer 05.08.2015 How pregnancy at early age protects against breast cancer Fabienne Meier-Abt, MD PhD Background: Early age pregnancy protects against breast cancer. MacMahon,
World Congress on Breast Cancer 05.08.2015 How pregnancy at early age protects against breast cancer Fabienne Meier-Abt, MD PhD Background: Early age pregnancy protects against breast cancer. MacMahon,
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/22278 holds various files of this Leiden University dissertation. Author: Cunha Carvalho de Miranda, Noel Filipe da Title: Mismatch repair and MUTYH deficient
Cover Page The handle http://hdl.handle.net/1887/22278 holds various files of this Leiden University dissertation. Author: Cunha Carvalho de Miranda, Noel Filipe da Title: Mismatch repair and MUTYH deficient
Breast cancer stem cells: implications for therapy of breast cancer
 Breast cancer stem cells: implications for therapy of breast cancer Author Morrison, Brian, Schmidt, Chris, Lakhani, Sunil, Reynolds, Brent, Lopez, Alejandro Published 2008 Journal Title Breast Cancer
Breast cancer stem cells: implications for therapy of breast cancer Author Morrison, Brian, Schmidt, Chris, Lakhani, Sunil, Reynolds, Brent, Lopez, Alejandro Published 2008 Journal Title Breast Cancer
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. Confirmation of Dnmt1 conditional knockout out mice. a, Representative images of sorted stem (Lin - CD49f high CD24 + ), luminal (Lin - CD49f low CD24 + )
Supplementary Figures Supplementary Figure 1. Confirmation of Dnmt1 conditional knockout out mice. a, Representative images of sorted stem (Lin - CD49f high CD24 + ), luminal (Lin - CD49f low CD24 + )
Type of file: PDF Size of file: 0 KB Title of file for HTML: Supplementary Information Description: Supplementary Figures
 Type of file: PDF Size of file: 0 KB Title of file for HTML: Supplementary Information Description: Supplementary Figures Supplementary Figure 1 mir-128-3p is highly expressed in chemoresistant, metastatic
Type of file: PDF Size of file: 0 KB Title of file for HTML: Supplementary Information Description: Supplementary Figures Supplementary Figure 1 mir-128-3p is highly expressed in chemoresistant, metastatic
Cancer Biology Course. Invasion and Metastasis
 Cancer Biology Course Invasion and Metastasis 2016 Lu-Hai Wang NHRI Cancer metastasis Major problem: main reason for killing cancer patients, without it cancer can be cured or controlled. Challenging questions:
Cancer Biology Course Invasion and Metastasis 2016 Lu-Hai Wang NHRI Cancer metastasis Major problem: main reason for killing cancer patients, without it cancer can be cured or controlled. Challenging questions:
Neoplasia literally means "new growth.
 NEOPLASIA Neoplasia literally means "new growth. A neoplasm, defined as "an abnormal mass of tissue the growth of which exceeds and is uncoordinated with that of the normal tissues and persists in the
NEOPLASIA Neoplasia literally means "new growth. A neoplasm, defined as "an abnormal mass of tissue the growth of which exceeds and is uncoordinated with that of the normal tissues and persists in the
Triple Negative Breast Cancer
 Triple Negative Breast Cancer Prof. Dr. Pornchai O-charoenrat Division of Head-Neck & Breast Surgery Department of Surgery Faculty of Medicine Siriraj Hospital Breast Cancer Classification Traditional
Triple Negative Breast Cancer Prof. Dr. Pornchai O-charoenrat Division of Head-Neck & Breast Surgery Department of Surgery Faculty of Medicine Siriraj Hospital Breast Cancer Classification Traditional
Recent advances in breast cancers
 Recent advances in breast cancers Breast cancer is a hetrogenous disease due to distinct genetic alterations. Similar morphological subtypes show variation in clinical behaviour especially in response
Recent advances in breast cancers Breast cancer is a hetrogenous disease due to distinct genetic alterations. Similar morphological subtypes show variation in clinical behaviour especially in response
Aberrant cell Growth. Younas Masih New Life College of Nursing Karachi. 3/4/2016 Younas Masih ( NLCON)
 Aberrant cell Growth Younas Masih New Life College of Nursing Karachi 1 Objectives By the end of this session the learners will be able to, Define the characteristics of the normal cell Describe the characteristics
Aberrant cell Growth Younas Masih New Life College of Nursing Karachi 1 Objectives By the end of this session the learners will be able to, Define the characteristics of the normal cell Describe the characteristics
Identifying Cancer Stem Cells in Solid Tumors: Case Not Proven
 Identifying Cancer Stem Cells in Solid Tumors: Case Not Proven Richard P. Hill Ontario Cancer Institute, Princess Margaret Hospital, University Health Network and Department of Medical Biophysics, University
Identifying Cancer Stem Cells in Solid Tumors: Case Not Proven Richard P. Hill Ontario Cancer Institute, Princess Margaret Hospital, University Health Network and Department of Medical Biophysics, University
number Done by Corrected by Doctor Maha Shomaf
 number 19 Done by Waseem Abo-Obeida Corrected by Abdullah Zreiqat Doctor Maha Shomaf Carcinogenesis: the molecular basis of cancer. Non-lethal genetic damage lies at the heart of carcinogenesis and leads
number 19 Done by Waseem Abo-Obeida Corrected by Abdullah Zreiqat Doctor Maha Shomaf Carcinogenesis: the molecular basis of cancer. Non-lethal genetic damage lies at the heart of carcinogenesis and leads
Claudin-4 Expression in Triple Negative Breast Cancer: Correlation with Androgen Receptors and Ki-67 Expression
 Claudin-4 Expression in Triple Negative Breast Cancer: Correlation with Androgen Receptors and Ki-67 Expression Mona A. Abd-Elazeem, Marwa A. Abd- Elazeem Pathology department, Faculty of Medicine, Tanta
Claudin-4 Expression in Triple Negative Breast Cancer: Correlation with Androgen Receptors and Ki-67 Expression Mona A. Abd-Elazeem, Marwa A. Abd- Elazeem Pathology department, Faculty of Medicine, Tanta
Introduction. Cancer Biology. Tumor-suppressor genes. Proto-oncogenes. DNA stability genes. Mechanisms of carcinogenesis.
 Cancer Biology Chapter 18 Eric J. Hall., Amato Giaccia, Radiobiology for the Radiologist Introduction Tissue homeostasis depends on the regulated cell division and self-elimination (programmed cell death)
Cancer Biology Chapter 18 Eric J. Hall., Amato Giaccia, Radiobiology for the Radiologist Introduction Tissue homeostasis depends on the regulated cell division and self-elimination (programmed cell death)
Chapter 7 Conclusions
 VII-1 Chapter 7 Conclusions VII-2 The development of cell-based therapies ranging from well-established practices such as bone marrow transplant to next-generation strategies such as adoptive T-cell therapy
VII-1 Chapter 7 Conclusions VII-2 The development of cell-based therapies ranging from well-established practices such as bone marrow transplant to next-generation strategies such as adoptive T-cell therapy
BIO 302: MARCH 4 & 6, 2014
 BIO 302: MARCH 4 & 6, 2014 WEEK 8 LECTURE 2: CANCER AS A COMPLEX ADAPTIVE SYSTEM Dr. George Poste Chief Scientist, Complex Adaptive Systems Initiative and Del E. Webb Chair in Health Innovation Arizona
BIO 302: MARCH 4 & 6, 2014 WEEK 8 LECTURE 2: CANCER AS A COMPLEX ADAPTIVE SYSTEM Dr. George Poste Chief Scientist, Complex Adaptive Systems Initiative and Del E. Webb Chair in Health Innovation Arizona
CELL BIOLOGY - CLUTCH CH CANCER.
 !! www.clutchprep.com CONCEPT: OVERVIEW OF CANCER Cancer is a disease which is primarily caused from misregulated cell division, which form There are two types of tumors - Benign tumors remain confined
!! www.clutchprep.com CONCEPT: OVERVIEW OF CANCER Cancer is a disease which is primarily caused from misregulated cell division, which form There are two types of tumors - Benign tumors remain confined
Overview of Cancer. Mylene Freires Advanced Nurse Practitioner, Haematology
 Overview of Cancer Mylene Freires Advanced Nurse Practitioner, Haematology Aim of the Presentation Review basic concepts of cancer Gain some understanding of the socio-economic impact of cancer Order of
Overview of Cancer Mylene Freires Advanced Nurse Practitioner, Haematology Aim of the Presentation Review basic concepts of cancer Gain some understanding of the socio-economic impact of cancer Order of
Question 1 A. ER-, PR-, HER+ B. ER+, PR+, HER2- C. ER-, PR+, HER2- D. ER-, PR-, HER2- E. ER-, PR+, HER2+
 Triple Negative Breast Cancer Laura C. Collins, M.D. Department of Pathology Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA Question 1 The tumor depicted on the next slide
Triple Negative Breast Cancer Laura C. Collins, M.D. Department of Pathology Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA Question 1 The tumor depicted on the next slide
Convergent and Divergent Mechanisms in Aging and Cancer
 Convergent and Divergent Mechanisms in Aging and Cancer Mariana S. De Lorenzo, PhD Department of Cell Biology & Molecular Medicine delorems@umdnj.edu LEARNING OBJECTIVES 1. To identify convergent and divergent
Convergent and Divergent Mechanisms in Aging and Cancer Mariana S. De Lorenzo, PhD Department of Cell Biology & Molecular Medicine delorems@umdnj.edu LEARNING OBJECTIVES 1. To identify convergent and divergent
Supplementary Figures
 Supplementary Figures Supplementary Figure 1 DOT1L regulates the expression of epithelial and mesenchymal markers. (a) The expression levels and cellular localizations of EMT markers were confirmed by
Supplementary Figures Supplementary Figure 1 DOT1L regulates the expression of epithelial and mesenchymal markers. (a) The expression levels and cellular localizations of EMT markers were confirmed by
Haematopoietic stem cells
 Haematopoietic stem cells Neil P. Rodrigues, DPhil NIH Centre for Biomedical Research Excellence in Stem Cell Biology Boston University School of Medicine neil.rodrigues@imm.ox.ac.uk Haematopoiesis: An
Haematopoietic stem cells Neil P. Rodrigues, DPhil NIH Centre for Biomedical Research Excellence in Stem Cell Biology Boston University School of Medicine neil.rodrigues@imm.ox.ac.uk Haematopoiesis: An
The Avatar System TM Yields Biologically Relevant Results
 Application Note The Avatar System TM Yields Biologically Relevant Results Liquid biopsies stand to revolutionize the cancer field, enabling early detection and noninvasive monitoring of tumors. In the
Application Note The Avatar System TM Yields Biologically Relevant Results Liquid biopsies stand to revolutionize the cancer field, enabling early detection and noninvasive monitoring of tumors. In the
Animal Tissue Culture SQG 3242 Biology of Cultured Cells. Dr. Siti Pauliena Mohd Bohari
 Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
Biochemistry of Cancer and Tumor Markers
 Biochemistry of Cancer and Tumor Markers The term cancer applies to a group of diseases in which cells grow abnormally and form a malignant tumor. It is a long term multistage genetic process. The first
Biochemistry of Cancer and Tumor Markers The term cancer applies to a group of diseases in which cells grow abnormally and form a malignant tumor. It is a long term multistage genetic process. The first
Molecular Characterization of Breast Cancer: The Clinical Significance
 Molecular Characterization of : The Clinical Significance Shahla Masood, M.D. Professor and Chair Department of Pathology and Laboratory Medicine University of Florida College of Medicine-Jacksonville
Molecular Characterization of : The Clinical Significance Shahla Masood, M.D. Professor and Chair Department of Pathology and Laboratory Medicine University of Florida College of Medicine-Jacksonville
Breast Cancer. Most common cancer among women in the US. 2nd leading cause of death in women. Mortality rates though have declined
 Breast Cancer Most common cancer among women in the US 2nd leading cause of death in women Mortality rates though have declined 1 in 8 women will develop breast cancer Breast Cancer Breast cancer increases
Breast Cancer Most common cancer among women in the US 2nd leading cause of death in women Mortality rates though have declined 1 in 8 women will develop breast cancer Breast Cancer Breast cancer increases
Disclosure of Relevant Financial Relationships. Breast Pathology Evening Specialty Conference Case #4. Clinical Case: Pathologic Features
 Breast Pathology Evening Specialty Conference Case #4 K.P. Siziopikou, MD, PhD Professor of Pathology Director of Breast Pathology and Breast Pathology Fellowship Program Northwestern University Feinberg
Breast Pathology Evening Specialty Conference Case #4 K.P. Siziopikou, MD, PhD Professor of Pathology Director of Breast Pathology and Breast Pathology Fellowship Program Northwestern University Feinberg
Breast Cancer. Excess Estrogen Exposure. Alcohol use + Pytoestrogens? Abortion. Infertility treatment?
 Breast Cancer Breast Cancer Excess Estrogen Exposure Nulliparity or late pregnancy + Early menarche + Late menopause + Cystic ovarian disease + External estrogens exposure + Breast Cancer Excess Estrogen
Breast Cancer Breast Cancer Excess Estrogen Exposure Nulliparity or late pregnancy + Early menarche + Late menopause + Cystic ovarian disease + External estrogens exposure + Breast Cancer Excess Estrogen
Breast Cancer. Saima Saeed MD
 Breast Cancer Saima Saeed MD Breast Cancer Most common cancer among women in the US 2nd leading cause of death in women 1 in 8 women will develop breast cancer Incidence/mortality rates have declined Breast
Breast Cancer Saima Saeed MD Breast Cancer Most common cancer among women in the US 2nd leading cause of death in women 1 in 8 women will develop breast cancer Incidence/mortality rates have declined Breast
BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES. Overview and Mechanism of Action Dr.
 BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES Overview and Mechanism of Action Dr. Leah Klapper, CSO 88 BL-8040: Novel CXCR4 Antagonist For Hematological Cancers Indications:
BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES Overview and Mechanism of Action Dr. Leah Klapper, CSO 88 BL-8040: Novel CXCR4 Antagonist For Hematological Cancers Indications:
Enterprise Interest Nothing to declare
 Enterprise Interest Nothing to declare Update of mixed tumours of the GI tract, the pancreas and the liver Introduction to the concept of mixed tumours and clinical implication Jean-Yves SCOAZEC Surgical
Enterprise Interest Nothing to declare Update of mixed tumours of the GI tract, the pancreas and the liver Introduction to the concept of mixed tumours and clinical implication Jean-Yves SCOAZEC Surgical
Supplementary Materials. for Garmy-Susini, et al, Integrin 4 1 signaling is required for lymphangiogenesis and tumor metastasis
 Supplementary Materials for Garmy-Susini, et al, Integrin 4 1 signaling is required for lymphangiogenesis and tumor metastasis 1 Supplementary Figure Legends Supplementary Figure 1: Integrin expression
Supplementary Materials for Garmy-Susini, et al, Integrin 4 1 signaling is required for lymphangiogenesis and tumor metastasis 1 Supplementary Figure Legends Supplementary Figure 1: Integrin expression
5K ALDEFLUOR-positive/ CXCR1-negative. 5K ALDEFLUOR-positive/ CXCR1-positive BAAA BAAA CXCR1-APC BAAA BAAA CXCR1-APC
 A +DEAB -DEAB K ALDEFLUOR-positive/ CXCR-negative BAAA BAAA CXCR-APC B +DEAB -DEAB K ALDEFLUOR-positive/ CXCR-positive BAAA BAAA CXCR-APC C Supplemental Figure. Tumorigenicity of the ALDEFLUOR-positive/CXCR-positive
A +DEAB -DEAB K ALDEFLUOR-positive/ CXCR-negative BAAA BAAA CXCR-APC B +DEAB -DEAB K ALDEFLUOR-positive/ CXCR-positive BAAA BAAA CXCR-APC C Supplemental Figure. Tumorigenicity of the ALDEFLUOR-positive/CXCR-positive
mirna Dr. S Hosseini-Asl
 mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
Quantification of early stage lesions for loss of p53 should be shown in the main figures.
 Reviewer #1 (Remarks to the Author): Expert in prostate cancer The manuscript "Clonal dynamics following p53 loss of heterozygosity in Kras-driven cancers" uses a number of novel genetically engineered
Reviewer #1 (Remarks to the Author): Expert in prostate cancer The manuscript "Clonal dynamics following p53 loss of heterozygosity in Kras-driven cancers" uses a number of novel genetically engineered
Mario Giuliano Trieste Novembre 2015
 Mario Giuliano Trieste 20-21 Novembre 2015 Metastatic Cascade Main Actors A small fraction of cells detaching from primary tumors end up forming metastatic lesions. 1 0 Tumor Circulating Tumor Cells (CTCs)
Mario Giuliano Trieste 20-21 Novembre 2015 Metastatic Cascade Main Actors A small fraction of cells detaching from primary tumors end up forming metastatic lesions. 1 0 Tumor Circulating Tumor Cells (CTCs)
BREAST PATHOLOGY. Fibrocystic Changes
 BREAST PATHOLOGY Lesions of the breast are very common, and they present as palpable, sometimes painful, nodules or masses. Most of these lesions are benign. Breast cancer is the 2 nd most common cause
BREAST PATHOLOGY Lesions of the breast are very common, and they present as palpable, sometimes painful, nodules or masses. Most of these lesions are benign. Breast cancer is the 2 nd most common cause
Biochemistry of Carcinogenesis. Lecture # 35 Alexander N. Koval
 Biochemistry of Carcinogenesis Lecture # 35 Alexander N. Koval What is Cancer? The term "cancer" refers to a group of diseases in which cells grow and spread unrestrained throughout the body. It is difficult
Biochemistry of Carcinogenesis Lecture # 35 Alexander N. Koval What is Cancer? The term "cancer" refers to a group of diseases in which cells grow and spread unrestrained throughout the body. It is difficult
Evaluation the Correlation between Ki67 and 5 Years Disease Free Survival of Breast Cancer Patients
 BIOSCIENCES BIOTECHNOLOGY RESEARCH ASIA, December 2015. Vol. 12(3), 2221-2225 Evaluation the Correlation between Ki67 and 5 Years Disease Free Survival of Breast Cancer Patients S.M. Hosseini¹, H. Shahbaziyan
BIOSCIENCES BIOTECHNOLOGY RESEARCH ASIA, December 2015. Vol. 12(3), 2221-2225 Evaluation the Correlation between Ki67 and 5 Years Disease Free Survival of Breast Cancer Patients S.M. Hosseini¹, H. Shahbaziyan
TITLE: Notch in Pathological Angiogenesis and Lymphangiogenesis
 Award Number: W81XWH-10-1-0304 TITLE: Notch in Pathological Angiogenesis and Lymphangiogenesis PRINCIPAL INVESTIGATOR: Minji Kim CONTRACTING ORGANIZATION: Columbia University New York, NY 10032 REPORT
Award Number: W81XWH-10-1-0304 TITLE: Notch in Pathological Angiogenesis and Lymphangiogenesis PRINCIPAL INVESTIGATOR: Minji Kim CONTRACTING ORGANIZATION: Columbia University New York, NY 10032 REPORT
Challenges for 3D Cancer Cell Culture On Basement Membrane. AACR, 2009 Presented by Hynda K. Kleinman, PhD
 Management of Disease Through DNA Repair Challenges for 3D Cancer Cell Culture On Basement Membrane AACR, 2009 Presented by Hynda K. Kleinman, PhD ams biotechnology (europe) ltd info@amsbio.com www.amsbio.com
Management of Disease Through DNA Repair Challenges for 3D Cancer Cell Culture On Basement Membrane AACR, 2009 Presented by Hynda K. Kleinman, PhD ams biotechnology (europe) ltd info@amsbio.com www.amsbio.com
T Cell Development. Xuefang Cao, MD, PhD. November 3, 2015
 T Cell Development Xuefang Cao, MD, PhD November 3, 2015 Thymocytes in the cortex of the thymus Early thymocytes development Positive and negative selection Lineage commitment Exit from the thymus and
T Cell Development Xuefang Cao, MD, PhD November 3, 2015 Thymocytes in the cortex of the thymus Early thymocytes development Positive and negative selection Lineage commitment Exit from the thymus and
A holistic approach to targeting breast cancer part II: Micronutrient synergy. Presented by: Dr. Neha Shanker DRRI
 A holistic approach to targeting breast cancer part II: Micronutrient synergy Presented by: Dr. Neha Shanker DRRI Overview of the previous webinar In the last presentation we talked about: Increase in
A holistic approach to targeting breast cancer part II: Micronutrient synergy Presented by: Dr. Neha Shanker DRRI Overview of the previous webinar In the last presentation we talked about: Increase in
Material and Methods. Flow Cytometry Analyses:
 Material and Methods Flow Cytometry Analyses: Immunostaining of breast cancer cells for HER2 was performed by incubating cells with anti- HER2/neu APC (Biosciences, Cat# 340554), anti-her2/neu PE (Biosciences,
Material and Methods Flow Cytometry Analyses: Immunostaining of breast cancer cells for HER2 was performed by incubating cells with anti- HER2/neu APC (Biosciences, Cat# 340554), anti-her2/neu PE (Biosciences,
DOWNLOAD OR READ : UNDERSTANDING BREAST CANCER CELL BIOLOGY AND THERAPY A VISUAL APPROACH PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : UNDERSTANDING BREAST CANCER CELL BIOLOGY AND THERAPY A VISUAL APPROACH PDF EBOOK EPUB MOBI Page 1 Page 2 understanding breast cancer cell biology and therapy a visual approach understanding
DOWNLOAD OR READ : UNDERSTANDING BREAST CANCER CELL BIOLOGY AND THERAPY A VISUAL APPROACH PDF EBOOK EPUB MOBI Page 1 Page 2 understanding breast cancer cell biology and therapy a visual approach understanding
CONTRACTING ORGANIZATION: The Walter & Eliza Hall Institute Melbourne 3050 Australia
 AD Award Number: W81XWH-05-1-0506 TITLE: Breast Stem Cell Markers and Tumor Stem Cells in BRCA1, BRCA2 and Non- BRCA 1/2 Women PRINCIPAL INVESTIGATOR: Geoffrey J Lindeman, Ph.D. Jane E Visvade, Ph.D. Joseph
AD Award Number: W81XWH-05-1-0506 TITLE: Breast Stem Cell Markers and Tumor Stem Cells in BRCA1, BRCA2 and Non- BRCA 1/2 Women PRINCIPAL INVESTIGATOR: Geoffrey J Lindeman, Ph.D. Jane E Visvade, Ph.D. Joseph
Does EMT Contribute to Radiation Resistance in Human Breast Cancer?
 AD Award Number: W81XWH-10-1-0592 TITLE: Does EMT Contribute to Radiation Resistance in Human Breast Cancer? PRINCIPAL INVESTIGATOR: Anupama Munshi, Ph.D CONTRACTING ORGANIZATION: University of Oklahoma
AD Award Number: W81XWH-10-1-0592 TITLE: Does EMT Contribute to Radiation Resistance in Human Breast Cancer? PRINCIPAL INVESTIGATOR: Anupama Munshi, Ph.D CONTRACTING ORGANIZATION: University of Oklahoma
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3021 Supplementary figure 1 Characterisation of TIMPless fibroblasts. a) Relative gene expression of TIMPs1-4 by real time quantitative PCR (RT-qPCR) in WT or ΔTimp fibroblasts (mean ±
DOI: 10.1038/ncb3021 Supplementary figure 1 Characterisation of TIMPless fibroblasts. a) Relative gene expression of TIMPs1-4 by real time quantitative PCR (RT-qPCR) in WT or ΔTimp fibroblasts (mean ±
stem cell products Basement Membrane Matrix Products Rat Mesenchymal Stem Cell Growth and Differentiation Products
 stem cell products Basement Membrane Matrix Products Rat Mesenchymal Stem Cell Growth and Differentiation Products Stem Cell Qualified Extracellular Matrix Proteins Stem cell research requires the finest
stem cell products Basement Membrane Matrix Products Rat Mesenchymal Stem Cell Growth and Differentiation Products Stem Cell Qualified Extracellular Matrix Proteins Stem cell research requires the finest
UCLA Nutrition Noteworthy
 UCLA Nutrition Noteworthy Title Effects of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Breast Cancer Permalink https://escholarship.org/uc/item/12g6398b Journal Nutrition Noteworthy, 2(1) ISSN 1556-1895
UCLA Nutrition Noteworthy Title Effects of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Breast Cancer Permalink https://escholarship.org/uc/item/12g6398b Journal Nutrition Noteworthy, 2(1) ISSN 1556-1895
Neoplasia 18 lecture 8. Dr Heyam Awad MD, FRCPath
 Neoplasia 18 lecture 8 Dr Heyam Awad MD, FRCPath ILOS 1. understand the angiogenic switch in tumors and factors that stimulate and inhibit angiogenesis. 2. list the steps important for tumor metastasis
Neoplasia 18 lecture 8 Dr Heyam Awad MD, FRCPath ILOS 1. understand the angiogenic switch in tumors and factors that stimulate and inhibit angiogenesis. 2. list the steps important for tumor metastasis
What is Cancer? Petra Ketterl, MD Medical Oncology and Functional Medicine
 What is Cancer? Petra Ketterl, MD Medical Oncology and Functional Medicine What is Cancer? Layman s terms: cancer starts when cells grow out of control (in any place in the body) and crowd out normal cells
What is Cancer? Petra Ketterl, MD Medical Oncology and Functional Medicine What is Cancer? Layman s terms: cancer starts when cells grow out of control (in any place in the body) and crowd out normal cells
The Hallmarks of Cancer
 The Hallmarks of Cancer Theresa L. Hodin, Ph.D. Clinical Research Services Theresa.Hodin@RoswellPark.org Hippocrates Cancer surgery, circa 1689 Cancer Surgery Today 1971: Nixon declares War on Cancer
The Hallmarks of Cancer Theresa L. Hodin, Ph.D. Clinical Research Services Theresa.Hodin@RoswellPark.org Hippocrates Cancer surgery, circa 1689 Cancer Surgery Today 1971: Nixon declares War on Cancer
Understanding and Optimizing Treatment of Triple Negative Breast Cancer
 Understanding and Optimizing Treatment of Triple Negative Breast Cancer Edith Peterson Mitchell, MD, FACP Clinical Professor of Medicine and Medical Oncology Program Leader, Gastrointestinal Oncology Department
Understanding and Optimizing Treatment of Triple Negative Breast Cancer Edith Peterson Mitchell, MD, FACP Clinical Professor of Medicine and Medical Oncology Program Leader, Gastrointestinal Oncology Department
REVIEWS BREAST CANCER METASTASIS: MARKERS AND MODELS. Britta Weigelt*, Johannes L. Peterse and Laura J. van t Veer*
 BREAST CANCER METASTASIS: MARKERS AND MODELS Britta Weigelt*, Johannes L. Peterse and Laura J. van t Veer* Abstract Breast cancer starts as a local disease, but it can metastasize to the lymph nodes and
BREAST CANCER METASTASIS: MARKERS AND MODELS Britta Weigelt*, Johannes L. Peterse and Laura J. van t Veer* Abstract Breast cancer starts as a local disease, but it can metastasize to the lymph nodes and
Breast cancer stem cells: treatment resistance and therapeutic opportunities
 Breast cancer stem cells: treatment resistance and therapeutic opportunities Author Al-Ejeh, Fares, E.Smart, Chanel, Morrison, Brian, Chenevix-Trench, Georgia, Lopez, Alejandro, R.Lakhani, Sunil, P.Brown,
Breast cancer stem cells: treatment resistance and therapeutic opportunities Author Al-Ejeh, Fares, E.Smart, Chanel, Morrison, Brian, Chenevix-Trench, Georgia, Lopez, Alejandro, R.Lakhani, Sunil, P.Brown,
Outline of the presentation
 Outline of the presentation Breast cancer subtypes and classification Clinical need in estrogen-positive (ER+) metastatic breast cancer (mbc) Sulforaphane and SFX-01: the preclinical evidence STEM Phase
Outline of the presentation Breast cancer subtypes and classification Clinical need in estrogen-positive (ER+) metastatic breast cancer (mbc) Sulforaphane and SFX-01: the preclinical evidence STEM Phase
609G: Concepts of Cancer Genetics and Treatments (3 credits)
 Master of Chemical and Life Sciences Program College of Computer, Mathematical, and Natural Sciences 609G: Concepts of Cancer Genetics and Treatments (3 credits) Text books: Principles of Cancer Genetics,
Master of Chemical and Life Sciences Program College of Computer, Mathematical, and Natural Sciences 609G: Concepts of Cancer Genetics and Treatments (3 credits) Text books: Principles of Cancer Genetics,
SIBLINGs, cancer's multifunctional weapons
 SIBLINGs, cancer's multifunctional weapons 6/18/08 Akeila Bellahcène and Vincent Castronovo of the Metastasis Research laboratory of the University of Liège are among the first researchers to have discovered
SIBLINGs, cancer's multifunctional weapons 6/18/08 Akeila Bellahcène and Vincent Castronovo of the Metastasis Research laboratory of the University of Liège are among the first researchers to have discovered
Clinical Oncology - Science in focus - Editorial. Understanding oestrogen receptor function in breast cancer, and its interaction with the
 Clinical Oncology - Science in focus - Editorial TITLE: Understanding oestrogen receptor function in breast cancer, and its interaction with the progesterone receptor. New preclinical findings and their
Clinical Oncology - Science in focus - Editorial TITLE: Understanding oestrogen receptor function in breast cancer, and its interaction with the progesterone receptor. New preclinical findings and their
Overview of the core ideas in cancer research
 Overview of the core ideas in cancer research Paul Edwards Cancer Research UK Cambridge Institute and Department of Pathology, University of Cambridge This lecture Overview of the ideas that provide the
Overview of the core ideas in cancer research Paul Edwards Cancer Research UK Cambridge Institute and Department of Pathology, University of Cambridge This lecture Overview of the ideas that provide the
OMICS Journals are welcoming Submissions
 OMICS Journals are welcoming Submissions OMICS International welcomes submissions that are original and technically so as to serve both the developing world and developed countries in the best possible
OMICS Journals are welcoming Submissions OMICS International welcomes submissions that are original and technically so as to serve both the developing world and developed countries in the best possible
Skin squamous cell carcinoma propagating cells increase with tumour progression and invasiveness
 Manuscript EMBO-2012-83706 Skin squamous cell carcinoma propagating cells increase with tumour progression and invasiveness Gaëlle Lapouge, Benjamin Beck, Dany Nassar, Christine Dubois, Sophie Dekoninck
Manuscript EMBO-2012-83706 Skin squamous cell carcinoma propagating cells increase with tumour progression and invasiveness Gaëlle Lapouge, Benjamin Beck, Dany Nassar, Christine Dubois, Sophie Dekoninck
BIT 120. Copy of Cancer/HIV Lecture
 BIT 120 Copy of Cancer/HIV Lecture Cancer DEFINITION Any abnormal growth of cells that has malignant potential i.e.. Leukemia Uncontrolled mitosis in WBC Genetic disease caused by an accumulation of mutations
BIT 120 Copy of Cancer/HIV Lecture Cancer DEFINITION Any abnormal growth of cells that has malignant potential i.e.. Leukemia Uncontrolled mitosis in WBC Genetic disease caused by an accumulation of mutations
Chapter 10. Summary, conclusions and future perspectives
 Chapter 10 Summary, conclusions and future perspectives 10.1 SUMMARY In this thesis, a new tumor imaging tracer in nuclear medicine is studied. This 123 tracer, L-3-[ I]Iodo-alpha-methyl-tyrosine (IMT),
Chapter 10 Summary, conclusions and future perspectives 10.1 SUMMARY In this thesis, a new tumor imaging tracer in nuclear medicine is studied. This 123 tracer, L-3-[ I]Iodo-alpha-methyl-tyrosine (IMT),
