Supplementary Data Supplementary Table S1. IC 50 values of ipatasertib on cell viability in cell lines with
|
|
|
- Sydney Hubbard
- 6 years ago
- Views:
Transcription
1 Supplementary Data Supplementary Table S1. IC 50 values of ipatasertib on cell viability in cell lines with or without alterations in PTEN or PIK3CA. Cell Line Tissue Type Ipatasertib IC 50 (μmol/l) a PTEN b PIK3CA c 22rv1 Prostate 9.09 HETp.Q.546R AN3 CA Endometrial 0.63 N ASPC-1 Pancreatic 10 AU565 Breast 3.55 HET.p.L847F;HET.pK8 63I; HET.p.F872I BPH1 Prostate 10 BPH1025 Prostate 8.63 BT474 Breast 0.52 HET.p.K111N BT483 Breast 10 HET.p.E542K BT549 Breast 5.51 N;HOM.p.V 275fs*1 BxPC-3 Pancreatic 10 C33A Cervical 0.60 N CAL-120 Breast 10 CAL-51 Breast 0.36 N HET.p.E542K CAL-85-1 Breast 10 CAMA-1 Breast 0.26 P;HOM. p.d92h Caov-3 Ovarian 10 Capan-1 Pancreatic 10 Capan-2 Pancreatic 10 CFPAC-1 Pancreatic 10 HET.p.I391M Colo704 Ovarian 0.93 N DLD-1 Colon 10 HETp.E.545K;HET.p.D 549N DU145 Prostate 8.41 DU4475 Breast 10 ECC-1 Endometrial 1.05 N EFM-19 Breast 0.39 HOM.p.H1047L EFM-192A Breast 0.73 HET.pC420R EFO-21 Ovarian 0.51 N ES-2 Ovarian 10 EVSA-T Breast 0.07 N FU-OV-1 Ovarian 10 HCC-1143 Breast 10 HCC-1395 Breast 0.45 HCC-1419 Breast 10
2 Cell Line Tissue Type Ipatasertib IC 50 (μmol/l) a PTEN b PIK3CA c HCC-1569 Breast 0.88 HETDEL.26 HET.p.I391M 7fs*9 HCC-1954 Breast 1.01 HET.p.H1047R HCC-2218 Breast 4.44 HCC-38 Breast 10 N HET.p.W386L HCC-70 Breast 0.53 N HCC1428 Breast 10 HCT-116 Colon 10 HCT-15 Colon 10 HET.p.E545K;HET.p.D 549N HDQ-P1 Breast 10 HEC 1-B Endometrial 10 HEC-1A Endometrial 5.76 HPAC Pancreatic 10 HPAF-II Pancreatic 10 Hs 766T Pancreatic 10 HS578T Breast 2.22 HT-55 Colon 4.25 HT3 Cervical 10 Hup T3 Pancreatic 10 N HET.p.H556Q KLE Endometrial 10 KM12 Colon 2.63 N KP4 Pancreatic 10 KPL-1 Breast 1.95 HET.p.E545K LNCaP Prostate 0.11 N;HOM.p.K 6fs*4 LoVo Colon 3.36 MCF7 Breast 1.88 HET.p.E545K MDA-MB- Breast MDA-MB- Breast MDA-MB- 415 Breast 0.23 N;HOM.p.C 136Y;p.T27 7fs*13 MDA-MB- Breast 10 N HOM.p.I391M 436 MDA-MB- Breast 1.92 HET.p.E307 HET.p.H1047R 453 MDA-MB- 468 K Breast 10 N;HOM.c G>T HET.p.C769G;HET.p.T 435I
3 Cell Line Tissue Type Ipatasertib IC 50 (μmol/l) a PTEN b PIK3CA c MFM-223 Breast 0.42 HET.p.1047R;HET.p..E 176Q MiaPaCa2 Pancreatic 10 MS 751 Cervical 10 MT-3 Colon 1.66 HET.p.H1047R OV-90 Ovarian 10 Panc Pancreatic 10 Panc Pancreatic 7.38 Panc Pancreatic 7.22 Panc Pancreatic 10 Panc Pancreatic 10 Panc Pancreatic 10 N Panc-1 Pancreatic 10 PATU 8902 Pancreatic 10 N PATU Pancreatic 10 HET.p.I391M 8988T N PC-3 Prostate 1.37 N HET.p.N996H PC3M Prostate 1.45 N PC3M-LN4 Prostate N PC3MM2 Prostate 1.55 N PL45 Pancreatic 8.17 N PSN-1 Pancreatic 10 RKO Colon 10 HET.p.H1047R;HET.p.I 391M RL 95-2 Endometrial 0.54 N SiHa Cervical 10 SK-CO-1 Colon 10 SK-OV-3 Ovarian 2.46 HET.p.H1047R SK-UT-1B Endometrial 0.22 N SKBR3 Breast 0.81 SU Pancreatic 10 N SW 1990 Pancreatic 10 N T47D Breast 0.76 HET.p.H1047R TOV-112D Ovarian 10 TOV-21G Ovarian 0.65 N HET.p.H1047Y ZR-75-1 Breast 0.81 N;HOM.pL1 08R ZR Breast 10 HET.p.I391M
4 a Measured by the CellTiter-Glo Assay (Promega). b Negative protein (N) or known mutations in the gene are noted. c Known mutations are noted.
5 Supplementary Table S2. Percent tumor growth inhibition (%TGI) in xenograft models with or without alterations in PTEN or PIK3CA. Tumor Type Tumor Model Ipatasertib PIK3CA Max %TGI PTEN a mut/amp b Breast HCC H1047R Breast KPL4 135 H1047R Breast BT474M1 113 K111N Breast MCF7-neo/ Her2 65 E545K Breast MDA-MB Breast Fo5 88 Colon HM-7 89 H1047R Colon HCT H1047R Gastric HGC N GBM U87MG 164 N Melanoma A2058.x1 40 N Melanoma 537MEL 125 N NSCLC H520.x1 34 N amp NSCLC NCI-H460 7 E545K NSCLC NCI-H NSCLC NCI-H NSCLC EBC-1-11 Ovarian TOV-21G.x1 128 N H1047Y Ovarian IGROV N O1069W Ovarian SKOV3 40 H1047R Prostate 22RV1 68 Q546R Prostate LuCap amp Prostate LNCaP 188 N Prostate LuCap35V 97 Low Prostate LuCaP Low Prostate PC-3 93 N Prostate DU-145.x1 60 Prostate LuCap Amp = amplification, Max %TGI = maximum percent tumor growth inhibition, Mut = mutation, N = negative protein a Negative protein (N) or low protein expression for PTEN is noted. b Known mutations are noted.
6 Supplementary Table S3. PI3K/Akt pathway status in archival tumors. Archival tissue tumor samples were collected from the majority of patients and were assessed centrally for PTEN status by immunohistochemistry (IHC) and for PIK3CA and/or AKT mutations. PTEN expression was quantified by an H-score, ranging from complete loss compared to normal stroma (H-score 0) to normal expression (H-score 400). In addition to PIK3CA and AKT, mutations from other relevant signaling pathways were also examined, including BRAF, EGFR, ERBB2, FGFR3, FLT3, HRAS, JAK2, KIT, KRAS, MET, MYD88, NRAS, and RPPH1.
7 Supplementary Table S3. PI3K/Akt pathway status in archival tumors. Ipatasertib PTEN PIK3CA/AKT Other Tumor Type (mg) H-score Mutations Mutations Gastric 25 N/A N/A N/A Ovarian 25 0 MND MND Thyroid, Papillary MND MND Chondrosarcoma 50 N/A MND MND Colon PIK3CA H1047R KRAS G12A Head and Neck MND MND Breast 100 N/A PIK3CA R88Q MND Breast MND MND Colon MND MND Colon 200 N/A MND KRAS Q61L Colon PIK3CA H1047R MND Renal MND MND Cholangiocarcinoma MND MND Colon MND MND Colon MND KRAS G12A Breast 600 N/A N/A N/A Breast 600 N/A N/A N/A Breast MND MND Breast MND MND Breast MND NRAS Q61R Breast MND MND Breast PIK3CA E542K MND Breast MND MND Breast PIK3CA H1047R MND Breast MND MND Breast PIK3CA E545K MET N375S Breast MND MET 1010I Breast AKT1 E17K MND Chondrosarcoma 600 N/A MND MND Cholangiocarcinoma MND MND MND = mutation not detected, N/A = not available
8 Supplementary Table S3 (cont.) Tumor Type Ipatasertib (mg) PTEN H-score PIK3CA/AKT Mutations Other Mutations Colon MND KRAS G12D Colon MND KRAS G12V Colon MND MND Colon MND MND Endometrial 600 N/A N/A N/A Lung 600 N/A N/A N/A Neuroendocrine MND KRAS G12V Ovarian MND MND Pancreatic 600 N/A MND KRAS G12D Prostate 600 N/A N/A N/A Prostate 600 N/A N/A N/A Prostate 600 N/A N/A N/A Prostate 600 N/A N/A N/A Prostate MND MND Prostate MND MND Breast 800 N/A N/A N/A Colon MND MND Colon MND KRAS G12V Colon MND MND Colon MND MND NSCLC MND MND Unknown primary MND MND MND = mutation not detected, N/A = not available
9 Supplementary Table S4. Reasons for discontinuation of study Disease progression 25 mg (n = 3) Number (%) of Patients by Ipatasertib Daily Dose a 50 mg (n = 3) 100 mg (n = 3) 200 mg (n = 3) 400 mg (n = 3) 600 mg 800 mg (n = 30) b (n = 7) All Patients (n = 52) c 3 (100) 3 (100) 3 (100) 3 (100) 3 (100) 21 (70) 6 (86) 42 (81) Patient decision (20) 0 6 (12) Adverse event (3) 1 (14) 2 (4) Death (3) 0 1 (2) Physician decision (3) 0 1 (2) n = number of patients. a b c Patients are categorized by initial dose cohort assignment, regardless of subsequent dose modification. Thirty patients were enrolled in the 600 mg dosing cohort, but one enrolled patient was not treated with ipatasertib (GDC-0068); thus, 29 patients were treated. Fifty-two patients were enrolled in the study, but one enrolled patient was not treated with ipatasertib (GDC-0068); thus, 51 patients were treated.
10 Supplementary figure S1. Exposure plots confirm that exposures achieved in patients in the dose-escalation stage (right side) correlated with exposures in preclinical models at TGI 90 (left side). Preclinical in vivo efficacy data with ipatasertib was obtained in multiple xenograft models, including LNCaP prostate cancer (PTEN-null), U87MG glioma (PTEN-null), HGC-27 gastric cancer (PTEN-null), LuCaP141 prostate cancer (PIK3CA amplified), and TOV-21G-X1 ovarian cancer (PTEN-null, PIK3CA H1047R mutation, and KRAS G13C mutation). The dose of ipatasertib that produced 90% tumor growth inhibition (TGI 90 ) was determined in each xenograft model. The TGI 90 values for each cell line are graphed along the left of the panel, and the equivalent exposures obtained in patients are shown on the right.
11 Supplementary Figure S2. Relationship between exposure of ipatasertib to the most common ipatasertib-related AEs of fatigue, diarrhea, nausea, and rash. A, An analysis was performed to compare the dose (in mg along the horizontal axis) and exposure (at steady-state AUC in ng*hour/ml) of ipatasertib to the grades of the four most common AEs related to ipatasertib (fatigue/asthenia, diarrhea, nausea, and rash) for all treated patients. The grade of each AE is plotted as a color-coded circle: blue for Grade 1, yellow for Grade 2, red for Grade 3 and white for no AE. Overall, the severity of each of the four most common drug-related AEs is increased relative to the dose and exposures for ipatasertib. All of the Grade 3 AEs occur at ipatasertib doses > 600 mg with exposures above 2000 ng*hour/ml, and most of the Grade 2 AEs occur at similar high doses and exposures, except for Grade 2 fatigue which occurs at lower exposures. B, Time to onset in days (graphed on the horizontal axis) for the most common AEs related to ipatasertib (fatigue, nausea, diarrhea, and rash), ranked by severity from Grade 1 to Grade 2 along the vertical axis, was analyzed. All four AEs occurred early, during Cycle 1, although nausea and diarrhea tended to occur earlier, within the first week of dosing, whereas fatigue and rash tended to occur later at 7 to 14 days of dosing. Continued chronic dosing of ipatasertib beyond Cycle 1 did not lead to the further development of these most common AEs, as no diarrhea or rash occurred in patients after the first month of dosing, and only two patients developed fatigue or nausea after the first two months of dosing. C, Time to onset in days (graphed on the horizontal axis) for the worst grade for the most common AEs related to ipatasertib (ranked by severity of Grade 1, 2, or 3 for fatigue, diarrhea, nausea, and rash) was analyzed. Onset of the first Grade 1 to Grade 2 nausea or Grade 1 diarrhea both occurred on average within 7 days of dosing, whereas the higher grades of nausea and of diarrhea occurred within 14 days of dosing. The worst grade of fatigue (Grade 3) and of rash (Grade 2) also occurred later in the study, following 7 to 14 days. Chronic dosing of ipatasertib did not lead to the significant development of the higher grade AEs, as no Grade 3 nausea or Grade 2 rash is seen after the first cycle, and only 3 patients developed Grade 2 to Grade 3 fatigue or diarrhea after the first two cycles.
12 Supplementary figure 2A.
13 Supplementary figure 2B.
14 Supplementary figure 2C.
15 Supplementary Table S5. Common adverse events for ipatasertib in 10% of patients in any presented group MedDRA System Organ Class/ Preferred Term All AEs (n = 51) b All Patients Ipatasertib- Related AEs a (n = 51) b All AEs (n = 29) c 600 mg Cohort Ipatasertib- Related AEs a (n = 29) c Any Adverse Event, n (%) 51 (100) 47 (92.2) 29 (100) 29 (100) Diarrhea 37 (72.5) 35 (68.6) 29 (100) 29 (10%) Nausea 40 (78.4) 36 (70.6) 27 (93.1) 25 (86.2) Vomiting 30 (58.8) 26 (51) 23 (79.3) 20 (69) Asthenia 32 (62.7) 19 (37.3) 17 (58.6) 8 (27.6) Decreased appetite 22 (43.1) 12 (23.5) 10 (34.5) 4 (13.8) Hyperglycemia 18 (35.3) 17 (33.3) 9 (31) 8 (27.6) Dyspepsia 13 (25.5) 10 (19.6) 7 (24.1) 7 (24.1) Abdominal pain upper 8 (15.7) 6 (11.8) 6 (20.7) 5 (17.2) Back pain 11 (21.6) 0 6 (20.7) 0 Musculoskeletal pain 7 (13.7) 2 (3.9) 6 (20.7) 2 (6.9) Constipation 8 (15.7) 0 5 (17.2) 0 Headache 9 (17.6) 3 (5.9) 5 (17.2) 1 (3.4) Anemia 6 (11.8) 2 (3.9) 4 (13.8) 0 Cough 7 (13.7) 0 4 (13.8) 0 Dyspnea 5 (9.8) 1 (2) 4 (13.8) 1 (3.4) Pyrexia 9 (17.6) 0 4 (13.8) 0 Abdominal distension 4 (7.8) 2 (3.9) 3 (10.3) 2 (6.9) Abdominal pain 5 (9.8) 2 (3.9) 3 (10.3) 1 (3.4) Arthralgia 4 (7.8) 1 (2) 3 (10.3) 1 (3.4) Aspartate aminotransferase increased 3 (5.9) 1 (2) 3 (10.3) 1 (3.4) Dry skin 3 (5.9) 3 (5.9) 3 (10.3) 3 (10.3) Dysgeusia 7 (13.7) 6 (11.8) 3 (10.3) 2 (6.9)
16 Supplementary Table S5 (cont.) MedDRA System Organ Class/ Preferred Term All AEs (n = 51) b All Patients Ipatasertib- Related AEs a (n = 51) b All AEs (n = 29) c 600 mg Cohort Ipatasertib- Related AEs a (n = 29) c Hypomagnesemia 4 (7.8) 3 (5.9) 3 (10.3) 3 (10.3) Influenza like illness 3 (5.9) 0 3 (10.3) 0 Insomnia 4 (7.8) 0 3 (10.3) 0 Pain in extremity 5 (9.8) 0 3 (10.3) 0 Rash 7 (13.7) 6 (11.8) 3 (10.3) 3 (10.3) AE = adverse event; MedDRA = Medical Dictionary for Regulatory Activities; n = number of patients. Note: Patients are categorized by initial dose cohort assignment, regardless of subsequent dose modification. a b c Assessed by the investigator as at least in part attributable to ipatasertib. Fifty-two patients were enrolled in the study, but 1 enrolled patient was not treated with ipatasertib; thus, 51 patients were treated. Thirty patients were enrolled in the 600-mg dosing cohort, but 1 enrolled patient was not treated with ipatasertib; thus, 29 patients were treated.
17 Supplementary Table S6. All Grade 3 adverse events related to ipatasertib. Ipatasertib Dose (mg) a Adverse Event NCI CTCAE Grade SAE Onset Day Duration (Days) Action Taken with Ipatasertib 100 Hypercholesterolemia 3 No None 600 Diarrhea 3 No None 600 Diarrhea b 3 Yes 11 3 Dose reduced 600 Diarrhea b 3 No 34 8 Held 600 Diarrhea 3 No 11 3 Dose reduced 600 Diarrhea 3 No 15 5 Dose reduced 600 Asthenia 3 No Dose reduced 600 Toxic skin eruption 3 Yes 11 4 Held 600 Hyperglycemia 3 Yes 28 2 Held 600 Hypophosphatemia 3 No 35 3 None 800 Nausea c 3 No None 800 Asthenia c 3 No 21 8 Discontinued 800 Asthenia c 3 Yes 17 4 Dose reduced CTCAE = Common Terminology Criteria for Adverse Events v. 3.0; ID = identification; NCI = National Cancer Institute; QD = once daily; SAE = serious adverse event. a b c Patients are categorized by initial dose cohort assignment, regardless of subsequent dose modification. Patient experienced the same adverse event of diarrhea twice. Both the Grade 3 asthenia at 800 mg in one patient, and Grade 3 nausea in another patient qualified as dose-limiting toxicities (DLTs).
18 Supplementary Figure S3. Suppression of pgsk3β in platelet-rich plasma versus ipatasertib concentrations.
19 Supplementary figure S4. Pharmacodynamic (PD) dose-dependent elevations in glucose and insulin following treatment with ipatasertib. A, After 15 days of dosing, baseline glucose (white bars, in mg/dl) and maximum postdose glucose (gray bars) are shown for patients in Stage 1, ranked by increasing doses of ipatasertib (25 mg to 800 mg). Glucose values are generally below 200 mg/dl at ipatasertib doses < 600 mg. Beginning at 600 mg, however, higher glucoses can be observed and were more frequent at 800 mg. Elevations in glucose were transient and generally returned to baseline within 6 hours. B, After 15 days of dosing, baseline insulin (white bars, in mu/l) and maximum postdose insulin (gray) are shown for patients in Stage 1, ranked by increasing doses. Similar to the glucose elevations, insulin levels also increase beginning at 600 mg and increase further at 800 mg of ipatasertib. Elevations in insulin were transient and generally returned to baseline within 6 hours. C, As an representative example, glucose (red line, in mg/dl left side) and insulin (blue, in mu/l, right side) are shown with the exposures of ipatasertib (green) for a patient from Stage 1 treated with 600 mg of ipatasertib. Blood samples were checked on Day 1 and on Day 15 in Cycle 1. Glucose and insulin increased following ipatasertib, reaching a peak at 4 hours post-dose, but both values normalized within 6 hours. D, Changes in fasting pre-dose blood glucoses for all patients (at all dose levels) through Cycles 1 and 2 in Stage 1 are graphed as a percentage of the baseline values. The majority of patients show relatively stable glucose values, without significant elevations.
20 Supplementary figure S4A.
21 Supplementary figure S4B.
22 Supplementary figure S4C.
23 Supplementary figure S4D.
24 Supplementary Table S7. Hemoglobin A1C (HgbA1C) values for patients who had both pre-treatment and post-treatment values in Stages 1 and 2. Ipatasertib Dose (mg) Patient ID Day HbgA1C Ipatasertib Dose (mg) Patient ID Day HgbA1C
25 Supplementary Table S8. Summary data for patients with best radiographic stable disease (SD) by RECIST version 1.0 criteria. Stage Ipatasertib Dose (mg) Days on Study Best RECIST Response Cancer Diagnosis PTEN H-score a PIK3CA/AKT mutation a SD Ovarian 0 MND SD Chondrosarcoma NE NE SD Colorectal 100 PIK3CA H1047R SD Colorectal 150 MND 245 SD Chondrosarcoma NE NE SD Colorectal 100 MND 183 SD Colorectal 200 MND IR/SD b Prostate 300 MND 1072 IR/SD b Prostate 0 MND 85 IR/SD b Prostate NE NE 260 SD Breast 115 PIK3CA H1047R 55 SD Breast 0 MND 85 SD Breast 200 MND 229 SD Breast 300 AKT1 E17K 102 SD Breast NE NE SD Lung NE NE IR = incomplete response, mg = milligrams, MND = mutation not detected, NE = not evaluable, RECIST = Response Evaluation Criteria in Solid Tumors, SD = stable disease. a b PI3K/Akt pathway alteration was determined by central Genentech assessment. PTEN status was assessed by immunohistochemistry (H-score). PIK3CA or AKT1 mutations were assessed by RT-PCR analysis. Patients with metastatic castration-resistant prostate cancer who had no measurable target lesion at screening and had a best RECIST response of IR/SD.
Targeting the PI3K-Akt-mTOR pathway with GDC-0068, a novel selective ATP competitive Akt inhibitor
 Targeting the PI3K-Akt-mTOR pathway with GDC-0068, a novel selective ATP competitive Akt inhibitor Josep Tabernero, Andrés Cervantes, Cristina Saura, Desamparados Roda, Yibing Yan, Kui Lin, Sumati Murli,
Targeting the PI3K-Akt-mTOR pathway with GDC-0068, a novel selective ATP competitive Akt inhibitor Josep Tabernero, Andrés Cervantes, Cristina Saura, Desamparados Roda, Yibing Yan, Kui Lin, Sumati Murli,
America, Hershey, PA Australia, Melbourne, VIC Europe, Munich
 America, Hershey, PA Australia, Melbourne, VIC Europe, Munich info@vivopharm.com www.vivopharm.com Bladder MB-9-luc 2 Mouse urinary bladder carcinoma yes yes yes C57BL/6 2 Bone UMR-106 Rat osteosarcoma
America, Hershey, PA Australia, Melbourne, VIC Europe, Munich info@vivopharm.com www.vivopharm.com Bladder MB-9-luc 2 Mouse urinary bladder carcinoma yes yes yes C57BL/6 2 Bone UMR-106 Rat osteosarcoma
Supplementary Figure 1. a. b. Relative cell viability. Nature Genetics: doi: /ng SCR shyap1-1 shyap
 Supplementary Figure 1. a. b. p-value for depletion in vehicle (DMSO) 1e-05 1e-03 1e-01 1 0 1000 2000 3000 4000 5000 Genes log2 normalized shrna counts in T0 0 2 4 6 8 sh1 shluc 0 2 4 6 8 log2 normalized
Supplementary Figure 1. a. b. p-value for depletion in vehicle (DMSO) 1e-05 1e-03 1e-01 1 0 1000 2000 3000 4000 5000 Genes log2 normalized shrna counts in T0 0 2 4 6 8 sh1 shluc 0 2 4 6 8 log2 normalized
Supplementary Table 1: Summary of 33 Patients Treated with Abemaciclib in Dose Escalation
 Supplementary Table 1: Summary of 33 Patients Treated with Abemaciclib in Dose Escalation Cohort Dose and Frequency Age and Sex Tumor Type Number of Cycles Best Overall Response 4M Colorectal cancer -
Supplementary Table 1: Summary of 33 Patients Treated with Abemaciclib in Dose Escalation Cohort Dose and Frequency Age and Sex Tumor Type Number of Cycles Best Overall Response 4M Colorectal cancer -
Frequency(%) KRAS G12 KRAS G13 KRAS A146 KRAS Q61 KRAS K117N PIK3CA H1047 PIK3CA E545 PIK3CA E542K PIK3CA Q546. EGFR exon19 NFS-indel EGFR L858R
 Frequency(%) 1 a b ALK FS-indel ALK R1Q HRAS Q61R HRAS G13R IDH R17K IDH R14Q MET exon14 SS-indel KIT D8Y KIT L76P KIT exon11 NFS-indel SMAD4 R361 IDH1 R13 CTNNB1 S37 CTNNB1 S4 AKT1 E17K ERBB D769H ERBB
Frequency(%) 1 a b ALK FS-indel ALK R1Q HRAS Q61R HRAS G13R IDH R17K IDH R14Q MET exon14 SS-indel KIT D8Y KIT L76P KIT exon11 NFS-indel SMAD4 R361 IDH1 R13 CTNNB1 S37 CTNNB1 S4 AKT1 E17K ERBB D769H ERBB
Drug Niraparib Olaparib
 Dear NCCN Value Pathway Committee, We are making this submission to provide information that we believe is relevant for developing NCCN Categories of Preference for the use of PARP inhibitors in recurrent
Dear NCCN Value Pathway Committee, We are making this submission to provide information that we believe is relevant for developing NCCN Categories of Preference for the use of PARP inhibitors in recurrent
Nilotinib AEs (adverse events) in CML population:
 Nilotinib AEs (adverse events) in CML population: The percentages below were taken from a randomized trial of nilotinib 300mg BID in newly diagnosed Ph+ CML patients (N=279) taken from the Tasigna 2017
Nilotinib AEs (adverse events) in CML population: The percentages below were taken from a randomized trial of nilotinib 300mg BID in newly diagnosed Ph+ CML patients (N=279) taken from the Tasigna 2017
Supplemental text file, including:
 Supplemental text file, including: Supplemental Table S1: Kinase profile for XL147 Supplemental Table S2: Effects of XL147 on PI3K Pathway Signaling in MCF7 and PC-3 Cells Supplemental Table S3: XL147
Supplemental text file, including: Supplemental Table S1: Kinase profile for XL147 Supplemental Table S2: Effects of XL147 on PI3K Pathway Signaling in MCF7 and PC-3 Cells Supplemental Table S3: XL147
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/2/12/e1601756/dc1 Supplementary Materials for Syrosingopine sensitizes cancer cells to killing by metformin Don Benjamin, Marco Colombi, Sravanth K. Hindupur, Charles
advances.sciencemag.org/cgi/content/full/2/12/e1601756/dc1 Supplementary Materials for Syrosingopine sensitizes cancer cells to killing by metformin Don Benjamin, Marco Colombi, Sravanth K. Hindupur, Charles
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
Supplementary Materials for
 www.sciencetranslationalmedicine.org/cgi/content/full/2/43/43ra55/dc1 Supplementary Materials for Pathway-Based Identification of Biomarkers for Targeted Therapeutics: Personalized Oncology with PI3K Pathway
www.sciencetranslationalmedicine.org/cgi/content/full/2/43/43ra55/dc1 Supplementary Materials for Pathway-Based Identification of Biomarkers for Targeted Therapeutics: Personalized Oncology with PI3K Pathway
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Species Tumor Type Comment for in vivo work Lead Time for in vivo studies [weeks] MB-49-luc-2 Mouse urinary bladder carcinoma C57BL/6 2
![Species Tumor Type Comment for in vivo work Lead Time for in vivo studies [weeks] MB-49-luc-2 Mouse urinary bladder carcinoma C57BL/6 2 Species Tumor Type Comment for in vivo work Lead Time for in vivo studies [weeks] MB-49-luc-2 Mouse urinary bladder carcinoma C57BL/6 2](/thumbs/88/116309357.jpg) America, Hershey, PA Australia, Melbourne, VIC Europe, Munich info@vivopharm.com www.vivopharm.com Tissue Bladder Species Tumor Type Comment for in vivo work Lead Time for in vivo studies [weeks] MB-49-luc-
America, Hershey, PA Australia, Melbourne, VIC Europe, Munich info@vivopharm.com www.vivopharm.com Tissue Bladder Species Tumor Type Comment for in vivo work Lead Time for in vivo studies [weeks] MB-49-luc-
Study No Title : Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Human cell line list Production of Exosome Standards - Cell Lysates
 Human cell line list 2016 - Production of Exosome Standards - Cell Lysates 380 peripheral blood leukemia, pre-b cell 1301 blood leukemia, acute lymphoblastic, T cell 5637 bladder carcinoma 8305C thyroid
Human cell line list 2016 - Production of Exosome Standards - Cell Lysates 380 peripheral blood leukemia, pre-b cell 1301 blood leukemia, acute lymphoblastic, T cell 5637 bladder carcinoma 8305C thyroid
Supplementary Online Content
 Supplementary Online Content Jänne PA, van den Heuvel MM, Barlesi F, et al. Effect of selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRASmutant
Supplementary Online Content Jänne PA, van den Heuvel MM, Barlesi F, et al. Effect of selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRASmutant
Development of Rational Drug Combinations for Oncology Indications - An Industry Perspective
 Development of Rational Drug Combinations for Oncology Indications An Industry Perspective Stuart Lutzker, MDPhD Vice President Oncology Early Development Genentech Inc 1 Conventional Oncology Drug Development
Development of Rational Drug Combinations for Oncology Indications An Industry Perspective Stuart Lutzker, MDPhD Vice President Oncology Early Development Genentech Inc 1 Conventional Oncology Drug Development
Broad Spectrum, Targeted Approaches To Cancer Treatment
 SELECTIVELY KILLING CANCER CELLS Broad Spectrum, Targeted Approaches To Cancer Treatment Safe Harbor The presentation made during this meeting and other statements by ArQule may contain forward-looking
SELECTIVELY KILLING CANCER CELLS Broad Spectrum, Targeted Approaches To Cancer Treatment Safe Harbor The presentation made during this meeting and other statements by ArQule may contain forward-looking
Development of LOXO-101 in Adult and Pediatric Patients With Cancer
 Development of LOXO-101 in Adult and Pediatric Patients With Cancer Michael Craig Cox, PharmD, MHSc, BCOP Director, Clinical Development & Medical Affairs Loxo Oncology, Inc. 1 Thematic Convergence Underscores
Development of LOXO-101 in Adult and Pediatric Patients With Cancer Michael Craig Cox, PharmD, MHSc, BCOP Director, Clinical Development & Medical Affairs Loxo Oncology, Inc. 1 Thematic Convergence Underscores
ASCO Annual Meeting 2013, May 31 June , Chicago, IL
 Phase I Study of Safety and Pharmacokinetics of GDC-0917, an Antagonist of Inhibitor of Apoptosis Proteins in Patients with Refractory Solid Tumors or Lymphoma 1 A Tolcher, 1 K Papadopoulos, 1 A Patnaik,
Phase I Study of Safety and Pharmacokinetics of GDC-0917, an Antagonist of Inhibitor of Apoptosis Proteins in Patients with Refractory Solid Tumors or Lymphoma 1 A Tolcher, 1 K Papadopoulos, 1 A Patnaik,
Antitumor Activity of CUDC-305, a Novel Oral HSP90 Inhibitor, in Solid and Hematological Tumor Xenograft Models
 Antitumor Activity of CUDC-5, a Novel Oral HSP Inhibitor, in Solid and Hematological Tumor Xenograft Models Rudi Bao, MD/PhD April 1, 2 AACR 1th Annual Meeting 2 Experimental and Molecular Therapeutics
Antitumor Activity of CUDC-5, a Novel Oral HSP Inhibitor, in Solid and Hematological Tumor Xenograft Models Rudi Bao, MD/PhD April 1, 2 AACR 1th Annual Meeting 2 Experimental and Molecular Therapeutics
Sponsor / Company: Sanofi Drug substance(s): SAR (iniparib)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
Novel EGFR TKI Theliatinib: An Open Label, Dose Escalation Phase I Clinical Trial
 Novel EGFR TKI Theliatinib: An Open Label, Dose Escalation Phase I Clinical Trial 2014-309-00CH1 Presenter: Jifang Gong, Beijing Cancer Hospital Lin Shen 1, Li Zhang 2, Hongyun Zhao 2, Wenfeng Fang 2,
Novel EGFR TKI Theliatinib: An Open Label, Dose Escalation Phase I Clinical Trial 2014-309-00CH1 Presenter: Jifang Gong, Beijing Cancer Hospital Lin Shen 1, Li Zhang 2, Hongyun Zhao 2, Wenfeng Fang 2,
Figure S1 Time-dependent down-modulation of HER3 by EZN No Treatment. EZN-3920, 2 μm. Time, h
 Figure S1 Time-dependent down-modulation of HER3 by EZN-392 HE ER3 mrna A, %Contr rol 12 No Treatment EZN-392, 2 μm 1 8 6 4 2 2 8 24 Time, h Figure S2. Specific target down-modulation by HER3 (EZN-392)
Figure S1 Time-dependent down-modulation of HER3 by EZN-392 HE ER3 mrna A, %Contr rol 12 No Treatment EZN-392, 2 μm 1 8 6 4 2 2 8 24 Time, h Figure S2. Specific target down-modulation by HER3 (EZN-392)
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of
Supplementary Online Content
 Supplementary Online Content Powles T, O Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 openlabel
Supplementary Online Content Powles T, O Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 openlabel
Supplementary Information
 Supplementary Information Supplementary Figure 1. Effect of mir mimics and anti-mirs on DTPs a, Representative fluorescence microscopy images of GFP vector control or mir mimicexpressing parental and DTP
Supplementary Information Supplementary Figure 1. Effect of mir mimics and anti-mirs on DTPs a, Representative fluorescence microscopy images of GFP vector control or mir mimicexpressing parental and DTP
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:.38/nature83 Supplementary figure An Overview of Experimental Flow Hypothesis: The tumor microenvironment has a significant impact on cancer cell chemoresistance. Screen Coculture
SUPPLEMENTARY INFORMATION doi:.38/nature83 Supplementary figure An Overview of Experimental Flow Hypothesis: The tumor microenvironment has a significant impact on cancer cell chemoresistance. Screen Coculture
K-Ras signalling in NSCLC
 Targeting the Ras-Raf-Mek-Erk pathway Egbert F. Smit MD PhD Dept. Pulmonary Diseases Vrije Universiteit VU Medical Centre Amsterdam, The Netherlands K-Ras signalling in NSCLC Sun et al. Nature Rev. Cancer
Targeting the Ras-Raf-Mek-Erk pathway Egbert F. Smit MD PhD Dept. Pulmonary Diseases Vrije Universiteit VU Medical Centre Amsterdam, The Netherlands K-Ras signalling in NSCLC Sun et al. Nature Rev. Cancer
Immune checkpoint blockade in lung cancer
 Immune checkpoint blockade in lung cancer Raffaele Califano Department of Medical Oncology The Christie and University Hospital of South Manchester, Manchester, UK Outline Background Overview of the data
Immune checkpoint blockade in lung cancer Raffaele Califano Department of Medical Oncology The Christie and University Hospital of South Manchester, Manchester, UK Outline Background Overview of the data
CDK 4/6 Inhibitors: Efficacy and Side Effect Profile
 CDK 4/6 Inhibitors: Efficacy and Side Effect Profile Univ.-Prof. Dr. Christian F Singer, MPH Center for Breast Health, Medical University of Vienna Center for Familial Breast- and Ovarian Cancer, MUW Christian
CDK 4/6 Inhibitors: Efficacy and Side Effect Profile Univ.-Prof. Dr. Christian F Singer, MPH Center for Breast Health, Medical University of Vienna Center for Familial Breast- and Ovarian Cancer, MUW Christian
Supplementary Figure 1
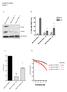 Supplementary Figure 1 A B HEC1A PTEN +/+ HEC1A PTEN -/- 16 HEC1A PTEN -/- 22 PTEN RAD51 % Cells with RAD51 foci 40 -IR +IR 30 20 10 * *! TUBULIN 0 HEC1A PTEN+/+ HEC1A PTEN-/- 16 HEC1A PTEN-/- 22 C D 1.0
Supplementary Figure 1 A B HEC1A PTEN +/+ HEC1A PTEN -/- 16 HEC1A PTEN -/- 22 PTEN RAD51 % Cells with RAD51 foci 40 -IR +IR 30 20 10 * *! TUBULIN 0 HEC1A PTEN+/+ HEC1A PTEN-/- 16 HEC1A PTEN-/- 22 C D 1.0
The AZ-Merck Collaboration
 The AZ-Merck Collaboration Institute of Medicine Washington, D.C. 10 Feb 2010 Pearl S. Huang, Ph.D. VP Oncology, Merck and Co. On behalf of the AZ/Merck Collaboration Team Combination Therapy is Standard
The AZ-Merck Collaboration Institute of Medicine Washington, D.C. 10 Feb 2010 Pearl S. Huang, Ph.D. VP Oncology, Merck and Co. On behalf of the AZ/Merck Collaboration Team Combination Therapy is Standard
Osimertinib Activity in Patients With Leptomeningeal Disease From Non-Small Cell Lung Cancer: Updated Results From the BLOOM Study
 Osimertinib Activity in Patients With Leptomeningeal Disease From Non-Small Cell Lung Cancer: Updated Results From the BLOOM Study Abstract 9002 Yang JC, Kim DW, Kim SW, Cho BC, Lee JS, Ye X, Yin X, Yang
Osimertinib Activity in Patients With Leptomeningeal Disease From Non-Small Cell Lung Cancer: Updated Results From the BLOOM Study Abstract 9002 Yang JC, Kim DW, Kim SW, Cho BC, Lee JS, Ye X, Yin X, Yang
NCCTG Status Report for Study N May 2010
 MARVEL: Marker Validation of Erlotinib in Lung Cancer - A Phase III Biomarker Validation Study of Second-Line Therapy in Patients With Advanced Non-Small Cell Lung Cancer (NSCLC) Randomized to Pemetrexed
MARVEL: Marker Validation of Erlotinib in Lung Cancer - A Phase III Biomarker Validation Study of Second-Line Therapy in Patients With Advanced Non-Small Cell Lung Cancer (NSCLC) Randomized to Pemetrexed
Supplementary Online Content
 Supplementary Online Content Kris MG, Johnson BE, Berry LD, et al. Using Multiplexed Assays of Oncogenic Drivers in Lung Cancers to Select Targeted Drugs. JAMA. doi:10.1001/jama.2014.3741 etable 1. Trials
Supplementary Online Content Kris MG, Johnson BE, Berry LD, et al. Using Multiplexed Assays of Oncogenic Drivers in Lung Cancers to Select Targeted Drugs. JAMA. doi:10.1001/jama.2014.3741 etable 1. Trials
Supplementary Information
 Supplementary Information - chimeric fusion transcript in human gastric cancer promotes tumorigenesis through activation of PI3K/AKT signaling Sun Mi Yun, Kwiyeom Yoon, Sunghoon Lee, Eunjeong Kim, Seong-Ho
Supplementary Information - chimeric fusion transcript in human gastric cancer promotes tumorigenesis through activation of PI3K/AKT signaling Sun Mi Yun, Kwiyeom Yoon, Sunghoon Lee, Eunjeong Kim, Seong-Ho
Nature Methods: doi: /nmeth Supplementary Figure 1
 Supplementary Figure 1 Finite-element analysis of cell cluster dynamics in different cluster trap architectures. (a) Cluster-Chip (b) Filter (c) A structure identical to the Cluster-Chip except that one
Supplementary Figure 1 Finite-element analysis of cell cluster dynamics in different cluster trap architectures. (a) Cluster-Chip (b) Filter (c) A structure identical to the Cluster-Chip except that one
LavaCell. Fluorescent Cell Stain. (version A) Catalog No
 LavaCell Fluorescent Cell Stain (version A) Catalog No. 15004 Active Motif North America 1914 Palomar Oaks Way, Suite 150 Carlsbad, California 92008, USA Toll free: 877 222 9543 Telephone: 760 431 1263
LavaCell Fluorescent Cell Stain (version A) Catalog No. 15004 Active Motif North America 1914 Palomar Oaks Way, Suite 150 Carlsbad, California 92008, USA Toll free: 877 222 9543 Telephone: 760 431 1263
Pembrolizumab for Patients With PD-L1 Positive Advanced Carcinoid or Pancreatic Neuroendocrine Tumors: Results From the KEYNOTE-028 Study
 Pembrolizumab for Patients With PD-L1 Positive Advanced Carcinoid or Pancreatic Neuroendocrine Tumors: Results From the KEYNOTE-28 Study Abstract 427O Mehnert JM, Bergsland E, O Neil BH, Santoro A, Schellens
Pembrolizumab for Patients With PD-L1 Positive Advanced Carcinoid or Pancreatic Neuroendocrine Tumors: Results From the KEYNOTE-28 Study Abstract 427O Mehnert JM, Bergsland E, O Neil BH, Santoro A, Schellens
Post-ASCO 2017 Cancer du sein Triple Négatif
 Post-ASCO 217 Cancer du sein Triple Négatif A.Ladjeroud, K.Bouzid Centre Pierre et Marie Curie- Alger Oran, 3 Septembre 217 Phase III Investigation of Neoadjuvant Carboplatin ± Veliparib in Combination
Post-ASCO 217 Cancer du sein Triple Négatif A.Ladjeroud, K.Bouzid Centre Pierre et Marie Curie- Alger Oran, 3 Septembre 217 Phase III Investigation of Neoadjuvant Carboplatin ± Veliparib in Combination
To assess safety profiles: significant laboratory changes and adverse events (AEs).
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
ALM301: Allosteric Isoform selective Akt inhibitor
 ALM301: Allosteric Isoform selective Akt inhibitor Background Akt1/2 selective inhibitors ALM301 Back-up compounds Akt2 selective inhibitors Approaches to Akt Inhibition Both ATP competitive and allosteric
ALM301: Allosteric Isoform selective Akt inhibitor Background Akt1/2 selective inhibitors ALM301 Back-up compounds Akt2 selective inhibitors Approaches to Akt Inhibition Both ATP competitive and allosteric
ARIAD Pharmaceuticals, Inc.
 ARIAD Pharmaceuticals, Inc. June 8, 2016 David Sachs Non-small cell lung cancer 1 ARIAD clinical trial patient Some of the statements in this presentation constitute forward looking statements under the
ARIAD Pharmaceuticals, Inc. June 8, 2016 David Sachs Non-small cell lung cancer 1 ARIAD clinical trial patient Some of the statements in this presentation constitute forward looking statements under the
Phase 1b KEYNOTE-200 (STORM):
 Phase 1b KEYNOTE-2 (STORM): A study of an intravenously delivered oncolytic virus, Coxsackievirus A21 in combination with pembrolizumab in advanced cancer patients Hardev S. Pandha 1, Kevin J. Harrington
Phase 1b KEYNOTE-2 (STORM): A study of an intravenously delivered oncolytic virus, Coxsackievirus A21 in combination with pembrolizumab in advanced cancer patients Hardev S. Pandha 1, Kevin J. Harrington
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK
Cabozantinib (Cometriq )
 Cabozantinib (Cometriq ) Workshop dose escalation EMA 4/5 Dec 2014 Frans Opdam, internist-clinical pharmacologist Clinical assessor, Dutch Medicines Agency Phase 3 Study XL184-301 (EXAM): Design Cabozantinib
Cabozantinib (Cometriq ) Workshop dose escalation EMA 4/5 Dec 2014 Frans Opdam, internist-clinical pharmacologist Clinical assessor, Dutch Medicines Agency Phase 3 Study XL184-301 (EXAM): Design Cabozantinib
HansaBioMed Catalog 2014 for Exosome Research. X. Human Cell Lines...
 HansaBioMed Catalog 2014 for Exosome Research X..... HansaBioMed Catalog 2014 HUMAN CELL LINES 380 leukemia, pre-b cell 1301 leukemia, acute lymphoblastic, T cell suspension, morphology round, small cells
HansaBioMed Catalog 2014 for Exosome Research X..... HansaBioMed Catalog 2014 HUMAN CELL LINES 380 leukemia, pre-b cell 1301 leukemia, acute lymphoblastic, T cell suspension, morphology round, small cells
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
ArQule CorporateUpdate
 ArQule April 2015 1 ArQule CorporateUpdate Safe Harbor This presentation and other statements by ArQule may contain forwardlooking statements within the meaning of the Private Securities Litigation Reform
ArQule April 2015 1 ArQule CorporateUpdate Safe Harbor This presentation and other statements by ArQule may contain forwardlooking statements within the meaning of the Private Securities Litigation Reform
2016 Year-End Results and Conference Call. March 14, 2017
 2016 Year-End Results and Conference Call March 14, 2017 Forward Looking Statement This communication contains "forward-looking" statements within the meaning of the Private Securities Litigation Reform
2016 Year-End Results and Conference Call March 14, 2017 Forward Looking Statement This communication contains "forward-looking" statements within the meaning of the Private Securities Litigation Reform
Drafting a Coverage Authorization Request Letter
 Drafting a Coverage Authorization Request Letter The following information is presented for informational purposes only and is not intended to provide reimbursement or legal advice. Laws, regulations,
Drafting a Coverage Authorization Request Letter The following information is presented for informational purposes only and is not intended to provide reimbursement or legal advice. Laws, regulations,
BR is an established treatment regimen for CLL in the front-line and R/R settings
 Idelalisib plus bendamustine and rituximab (BR) is superior to BR alone in patients with relapsed/refractory CLL: Results of a phase III randomized double-blind placebo-controlled study Andrew D. Zelenetz,
Idelalisib plus bendamustine and rituximab (BR) is superior to BR alone in patients with relapsed/refractory CLL: Results of a phase III randomized double-blind placebo-controlled study Andrew D. Zelenetz,
A PHASE 1 STUDY OF TRC105 (ANTI- ADVANCED SOLID TUMORS
 ASCO 2011 Abstract Number: 3073 A PHASE 1 STUDY OF TRC105 (ANTI- CD105 ANTIBODY) IN PATIENTS WITH ADVANCED SOLID TUMORS J. W. Goldman, M. S. Gordon, H. Hurwitz, R. Pili, D. S. Mendelson, B. J. Adams, D.
ASCO 2011 Abstract Number: 3073 A PHASE 1 STUDY OF TRC105 (ANTI- CD105 ANTIBODY) IN PATIENTS WITH ADVANCED SOLID TUMORS J. W. Goldman, M. S. Gordon, H. Hurwitz, R. Pili, D. S. Mendelson, B. J. Adams, D.
Figure S1. ERBB3 mrna levels are elevated in Luminal A breast cancers harboring ERBB3
 Supplemental Figure Legends. Figure S1. ERBB3 mrna levels are elevated in Luminal A breast cancers harboring ERBB3 ErbB3 gene copy number gain. Supplemental Figure S1. ERBB3 mrna levels are elevated in
Supplemental Figure Legends. Figure S1. ERBB3 mrna levels are elevated in Luminal A breast cancers harboring ERBB3 ErbB3 gene copy number gain. Supplemental Figure S1. ERBB3 mrna levels are elevated in
Genetic Alteration Panels
 TM Genetic Alteration Panels GENETIC ALTERATION PANELS Table of Contents AKT Genetic Alteration Panel (ATCC No. TCP-1029 )...1 BRAF Genetic Alteration Panel (ATCC No. TCP-1032 )...5 EGFR Genetic Alteration
TM Genetic Alteration Panels GENETIC ALTERATION PANELS Table of Contents AKT Genetic Alteration Panel (ATCC No. TCP-1029 )...1 BRAF Genetic Alteration Panel (ATCC No. TCP-1032 )...5 EGFR Genetic Alteration
New Targeted Agents Demonstrate Greater Efficacy and Tolerability in the Treatment of HER2-positive Breast Cancer
 New Evidence reports on presentations given at ASCO 2012 New Targeted Agents Demonstrate Greater Efficacy and Tolerability in the Treatment of HER2-positive Breast Cancer Presentations at ASCO 2012 Breast
New Evidence reports on presentations given at ASCO 2012 New Targeted Agents Demonstrate Greater Efficacy and Tolerability in the Treatment of HER2-positive Breast Cancer Presentations at ASCO 2012 Breast
RXDX-101 & RXDX-102. Justin Gainor, MD February 20 th, 2014
 RXDX-101 & RXDX-102 Justin Gainor, MD February 20 th, 2014 Background Chromosomal fusions are important oncogenic drivers in NSCLC - ALK Rearrangements (4-6%) - ROS1 Rearrangements (1-2%) - RET Rearrangements
RXDX-101 & RXDX-102 Justin Gainor, MD February 20 th, 2014 Background Chromosomal fusions are important oncogenic drivers in NSCLC - ALK Rearrangements (4-6%) - ROS1 Rearrangements (1-2%) - RET Rearrangements
DCC-2618, a novel pan-kit and PDGFR
 DCC-2618, a novel pan-kit and PDGFRα Kinase switch control inhibitor demonstrates encouraging activity in patients (pts) with Gastrointestinal Stromal Tumors (GIST) N. Somaiah, A. Razak, M. Gordon, F.
DCC-2618, a novel pan-kit and PDGFRα Kinase switch control inhibitor demonstrates encouraging activity in patients (pts) with Gastrointestinal Stromal Tumors (GIST) N. Somaiah, A. Razak, M. Gordon, F.
Supplemental Methods LC-MS Assay for NAD Concentration
 Supplemental Methods LC-MS Assay for NAD Concentration The concentration of NAD in cells was determined by a non-validated liquid chromatography tandem mass spectrometry (LC-MS/MS) assay using 13 C 5 -NAD
Supplemental Methods LC-MS Assay for NAD Concentration The concentration of NAD in cells was determined by a non-validated liquid chromatography tandem mass spectrometry (LC-MS/MS) assay using 13 C 5 -NAD
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
Phase 1 study of AG-881, an inhibitor of mutant IDH1/IDH2, in patients with advanced IDH mutant solid tumors, including glioma
 2002 Phase 1 study of AG-881, an inhibitor of mutant IDH1/IDH2, in patients with advanced IDH mutant solid tumors, including glioma Ingo K Mellinghoff 1, Marta Penas-Prado 2, Katherine B Peters 3, Timothy
2002 Phase 1 study of AG-881, an inhibitor of mutant IDH1/IDH2, in patients with advanced IDH mutant solid tumors, including glioma Ingo K Mellinghoff 1, Marta Penas-Prado 2, Katherine B Peters 3, Timothy
ArQule Jefferies Global Healthcare Conference June 2015
 ArQule Jefferies Global Healthcare Conference June 2015 1 ArQule Corporate Update Safe Harbor This presentation and other statements by ArQule may contain forward-looking statements within the meaning
ArQule Jefferies Global Healthcare Conference June 2015 1 ArQule Corporate Update Safe Harbor This presentation and other statements by ArQule may contain forward-looking statements within the meaning
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Phase 1 Trial of ALN-VSP in Cancers Involving the Liver. Annual Meeting of the Controlled Release Society August 2, 2011
 Phase 1 Trial of ALN-VSP in Cancers Involving the Liver Annual Meeting of the Controlled Release Society August 2, 2011 Agenda ALN-VSP: Background Phase 1 Trial Study Design Safety Data Tumor Response
Phase 1 Trial of ALN-VSP in Cancers Involving the Liver Annual Meeting of the Controlled Release Society August 2, 2011 Agenda ALN-VSP: Background Phase 1 Trial Study Design Safety Data Tumor Response
Efficacy of larotrectinib in adolescents and young adults with TRK fusion cancer
 Efficacy of larotrectinib in adolescents and young adults with TRK fusion cancer Soledad Gallego, 1 Valentina Boni, 2 Ulrik Lassen, 3 Anna Farago, 4 Wafik El-Deiry, 5 David Hong, 6 Blanca López-Ibor, 2
Efficacy of larotrectinib in adolescents and young adults with TRK fusion cancer Soledad Gallego, 1 Valentina Boni, 2 Ulrik Lassen, 3 Anna Farago, 4 Wafik El-Deiry, 5 David Hong, 6 Blanca López-Ibor, 2
INNOVAZIONI TERAPEUTICHE IN ONCOLOGIA MEDICA CAGLIARI GIUGNO 2005 Policlinico Universitario - Cagliari LAPATINIB
 INNOVAZIONI TERAPEUTICHE IN ONCOLOGIA MEDICA CAGLIARI 23-24 GIUGNO 2005 Policlinico Universitario - Cagliari LAPATINIB Elena Massa CATTEDRA DI ONCOLOGIA MEDICA UNIVERSITA DEGLI STUDI DI CAGLIARI ErbB Inhibition
INNOVAZIONI TERAPEUTICHE IN ONCOLOGIA MEDICA CAGLIARI 23-24 GIUGNO 2005 Policlinico Universitario - Cagliari LAPATINIB Elena Massa CATTEDRA DI ONCOLOGIA MEDICA UNIVERSITA DEGLI STUDI DI CAGLIARI ErbB Inhibition
Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma (BCC): 24-month update of the pivotal ERIVANCE BCC study
 Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma (BCC): 24-month update of the pivotal ERIVANCE BCC study A Sekulic, 1 MR Migden, 2 AE Oro, 3 L Dirix, 4 K Lewis,
Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma (BCC): 24-month update of the pivotal ERIVANCE BCC study A Sekulic, 1 MR Migden, 2 AE Oro, 3 L Dirix, 4 K Lewis,
Sponsor Novartis. Generic Drug Name Pasireotide. Therapeutic Area of Trial Cushing s disease. Protocol Number CSOM230B2208E1
 Sponsor Novartis Generic Drug Name Pasireotide Therapeutic Area of Trial Cushing s disease Protocol Number CSOM230B2208E1 Title Extension to a multicenter, open-label study to assess the safety and efficacy
Sponsor Novartis Generic Drug Name Pasireotide Therapeutic Area of Trial Cushing s disease Protocol Number CSOM230B2208E1 Title Extension to a multicenter, open-label study to assess the safety and efficacy
INSTRUCTIONS: 1. Use codetable on page 1 for modifications / termination reasons
 Radiation Therapy Oncology Group Phase III Head & Neck Cancer Treatment Summary Form AMENDED DATA YES INSTRUCTIONS: 1 Use codetable on page 1 for modifications / termination reasons SUMMARY OF SYSTEMIC
Radiation Therapy Oncology Group Phase III Head & Neck Cancer Treatment Summary Form AMENDED DATA YES INSTRUCTIONS: 1 Use codetable on page 1 for modifications / termination reasons SUMMARY OF SYSTEMIC
Next Generation Sequencing in Clinical Practice: Impact on Therapeutic Decision Making
 Next Generation Sequencing in Clinical Practice: Impact on Therapeutic Decision Making November 20, 2014 Capturing Value in Next Generation Sequencing Symposium Douglas Johnson MD, MSCI Vanderbilt-Ingram
Next Generation Sequencing in Clinical Practice: Impact on Therapeutic Decision Making November 20, 2014 Capturing Value in Next Generation Sequencing Symposium Douglas Johnson MD, MSCI Vanderbilt-Ingram
Discovery And Development Of A Monoclonal Antibody Against A Novel Target For The Treatment Of Colorectal Cancer
 1 Discovery And Development Of A Monoclonal Antibody Against A Novel Target For The Treatment Of Colorectal Cancer Philip M. Arlen, MD 16th Drug Discovery Summit and 3rd Discovery Chemistry & Drug Design
1 Discovery And Development Of A Monoclonal Antibody Against A Novel Target For The Treatment Of Colorectal Cancer Philip M. Arlen, MD 16th Drug Discovery Summit and 3rd Discovery Chemistry & Drug Design
DAVAOncology, LP...facilitating successful drug development
 DAVAOncology, L Tyrosine Receptor Kinase I3K p85 p110 TEN TORC1 RAS I2 I3 Efficacy of targeted therapy is challenged by the complexity of tumor biology, including cross talk and redundancies between divergent
DAVAOncology, L Tyrosine Receptor Kinase I3K p85 p110 TEN TORC1 RAS I2 I3 Efficacy of targeted therapy is challenged by the complexity of tumor biology, including cross talk and redundancies between divergent
Constan'ne S Tam Victorian Comprehensive Cancer Center Melbourne, Australia
 Constan'ne S Tam Victorian Comprehensive Cancer Center Melbourne, Australia BGB-3111: Kinase Selec.vity Rela.ve to Ibru.nib Equipotent against BTK compared to ibru.nib Higher selec.vity vs EGFR, ITK, JAK3,
Constan'ne S Tam Victorian Comprehensive Cancer Center Melbourne, Australia BGB-3111: Kinase Selec.vity Rela.ve to Ibru.nib Equipotent against BTK compared to ibru.nib Higher selec.vity vs EGFR, ITK, JAK3,
Lung Cancer Case. Since the patient was symptomatic, a targeted panel was sent. ALK FISH returned in 2 days and was positive.
 Lung Cancer Case Jonathan Riess, M.D. M.S. Assistant Professor of Medicine University of California Davis School of Medicine UC Davis Comprehensive Cancer Center 63 year-old woman, never smoker, presents
Lung Cancer Case Jonathan Riess, M.D. M.S. Assistant Professor of Medicine University of California Davis School of Medicine UC Davis Comprehensive Cancer Center 63 year-old woman, never smoker, presents
Diagnostic test Suggested website label Description Hospitals available
 Diagnostic test Suggested website label Description Hospitals available Abbott Molecular Inc, PATHVYSION HER-2 DNA Probe Kit (FISH) PathVysion kit A diagnostic tool used to determine whether a particular
Diagnostic test Suggested website label Description Hospitals available Abbott Molecular Inc, PATHVYSION HER-2 DNA Probe Kit (FISH) PathVysion kit A diagnostic tool used to determine whether a particular
Supplementary Material
 Supplementary Material Original article in Gastric Cancer A novel splice variant of XIAP-associated factor 1 (XAF1) is expressed in peripheral blood containing gastric cancer-derived circulating tumor
Supplementary Material Original article in Gastric Cancer A novel splice variant of XIAP-associated factor 1 (XAF1) is expressed in peripheral blood containing gastric cancer-derived circulating tumor
Supplementary Online Content
 Supplementary Online Content Chacón MR, Enrico DH, Burton J, Waisberg FD, Videla VM. Incidence of placebo adverse events in randomized clinical trials of targeted and immunotherapy cancer drugs in the
Supplementary Online Content Chacón MR, Enrico DH, Burton J, Waisberg FD, Videla VM. Incidence of placebo adverse events in randomized clinical trials of targeted and immunotherapy cancer drugs in the
Juyoun Jin, D.V.M., Ph.D. Institute for Refractory Cancer Research, Samsung Medical Center
 Juyoun Jin, D.V.M., Ph.D. Institute for Refractory Cancer Research, Samsung Medical Center Overview of Anticancer Drug Development Discovery Non-clinical development Clinical Trial Target Identification
Juyoun Jin, D.V.M., Ph.D. Institute for Refractory Cancer Research, Samsung Medical Center Overview of Anticancer Drug Development Discovery Non-clinical development Clinical Trial Target Identification
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
REWRITING CANCER TREATMENT THROUGH EPIGENETIC MEDICINES
 REWRITING CANCER TREATMENT THROUGH EPIGENETIC MEDICINES May 18, 2017 Molecularly Defined Solid Tumor Program Update FORWARD-LOOKING STATEMENTS Any statements in this press release about future expectations,
REWRITING CANCER TREATMENT THROUGH EPIGENETIC MEDICINES May 18, 2017 Molecularly Defined Solid Tumor Program Update FORWARD-LOOKING STATEMENTS Any statements in this press release about future expectations,
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Emerging Therapies for HCV: Highlights from AASLD 2012 (Part 2)
 Emerging Therapies for HCV: Highlights from AASLD 2012 (Part 2) Goals for Hepatitis C Therapy Compared to PegIFN α/rbv, new treatment regimens for chronic hepatitis C should offer: Improved efficacy Efficacy
Emerging Therapies for HCV: Highlights from AASLD 2012 (Part 2) Goals for Hepatitis C Therapy Compared to PegIFN α/rbv, new treatment regimens for chronic hepatitis C should offer: Improved efficacy Efficacy
Nature Medicine: doi: /nm.4078
 Supplementary Figure 1. Cetuximab induces ER stress response in DiFi cells. (a) Scheme of SILAC proteome. (b) MS-base read out of SILAC experiment. The histogram of log 2 -transformed normalized H/L ratios
Supplementary Figure 1. Cetuximab induces ER stress response in DiFi cells. (a) Scheme of SILAC proteome. (b) MS-base read out of SILAC experiment. The histogram of log 2 -transformed normalized H/L ratios
I. Diagnosis of the cancer type in CUP
 Latest Research: USA I. Diagnosis of the cancer type in CUP II. Outcomes of site-specific therapy of the cancer type in CUP a. Prospective clinical trial b. Retrospective clinical trials 1 Latest Research:
Latest Research: USA I. Diagnosis of the cancer type in CUP II. Outcomes of site-specific therapy of the cancer type in CUP a. Prospective clinical trial b. Retrospective clinical trials 1 Latest Research:
GSK Medicine: Study Number: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Part A Part B Part C
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Supplementary Information Titles Journal: Nature Medicine
 Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
KEYTRUDA is also indicated in combination with pemetrexed and platinum chemotherapy for the
 FDA-Approved Indication for KEYTRUDA (pembrolizumab) in Combination With Carboplatin and Either Paclitaxel or Nab-paclitaxel for the Firstline Treatment of Patients With Metastatic Squamous Non Small Cell
FDA-Approved Indication for KEYTRUDA (pembrolizumab) in Combination With Carboplatin and Either Paclitaxel or Nab-paclitaxel for the Firstline Treatment of Patients With Metastatic Squamous Non Small Cell
ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015. ClinicalTrials.gov ID: NCT
 ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015 ClinicalTrials.gov ID: NCT01378962 Study Identification Unique Protocol ID: ML25514 Brief Title: A Study
ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015 ClinicalTrials.gov ID: NCT01378962 Study Identification Unique Protocol ID: ML25514 Brief Title: A Study
Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
 Antitumor Activity and Safety of FPA144, an ADCCenhanced, FGFR2b Isoform-Selective Monoclonal Antibody, in Patients with FGFR2b + Gastric Cancer and Advanced Solid Tumors Jeeyun Lee 1, Johanna Bendell
Antitumor Activity and Safety of FPA144, an ADCCenhanced, FGFR2b Isoform-Selective Monoclonal Antibody, in Patients with FGFR2b + Gastric Cancer and Advanced Solid Tumors Jeeyun Lee 1, Johanna Bendell
Initial Results from an Open-label, Doseescalation Phase I Study of the Oral BRAF Inhibitor LGX818 in BRAF V600 mutant Advanced Melanoma
 Initial Results from an Open-label, Doseescalation Phase I Study of the Oral BRAF Inhibitor LGX818 in BRAF V600 mutant Advanced Melanoma Reinhard Dummer, 1 Caroline Robert, 2 Marta Nyakas, 3 Grant McArthur,
Initial Results from an Open-label, Doseescalation Phase I Study of the Oral BRAF Inhibitor LGX818 in BRAF V600 mutant Advanced Melanoma Reinhard Dummer, 1 Caroline Robert, 2 Marta Nyakas, 3 Grant McArthur,
Predictive biomarker profiling of > 1,900 sarcomas: Identification of potential novel treatment modalities
 Predictive biomarker profiling of > 1,900 sarcomas: Identification of potential novel treatment modalities Sujana Movva 1, Wenhsiang Wen 2, Wangjuh Chen 2, Sherri Z. Millis 2, Margaret von Mehren 1, Zoran
Predictive biomarker profiling of > 1,900 sarcomas: Identification of potential novel treatment modalities Sujana Movva 1, Wenhsiang Wen 2, Wangjuh Chen 2, Sherri Z. Millis 2, Margaret von Mehren 1, Zoran
PRODUCT INFORMATION 1 ABOUT THIS GUIDE DOSAGE FORM AND STRENGTH STORAGE AND HANDLING
 DOSING GUIDE INDICATION ONIVYDE (irinotecan liposome injection) is indicated, in combination with fluorouracil (5-FU) and leucovorin (LV), for the treatment of patients with metastatic adenocarcinoma of
DOSING GUIDE INDICATION ONIVYDE (irinotecan liposome injection) is indicated, in combination with fluorouracil (5-FU) and leucovorin (LV), for the treatment of patients with metastatic adenocarcinoma of
PBI-05204, an inhibitor of Akt, FGF-2, NF-κb and p70s6k
 PBI-05204, an inhibitor of Akt, FGF-2, NF-κb and p70s6k S. Bidyasar, R. Kurzrock, G. S. Falchook, A. Naing, J. J. Wheler, J. Durand, P. Yang, M. J. Johansen, R. A. Newman, R. Khan, D. Hong MD Anderson
PBI-05204, an inhibitor of Akt, FGF-2, NF-κb and p70s6k S. Bidyasar, R. Kurzrock, G. S. Falchook, A. Naing, J. J. Wheler, J. Durand, P. Yang, M. J. Johansen, R. A. Newman, R. Khan, D. Hong MD Anderson
List of Available TMAs in the PRN
 TMA RPCI_BrainCa01 RPCI_BrCa03 RPCI_BrCa04 RPCI_BrCa05 RPCI_BrCa0 RPCI_BrCa07 RPCI_BrCa08 RPCI_BrCa15 RPCI_BrCa1 RPCI_BrCa17 RPCI_BrCa18 RPCI_BrCa19 RPCI_BrCa20 RPCI_BrCa21 RPCI_BrCa24 RPCI_BrCa25 RPCI_BrCa2
TMA RPCI_BrainCa01 RPCI_BrCa03 RPCI_BrCa04 RPCI_BrCa05 RPCI_BrCa0 RPCI_BrCa07 RPCI_BrCa08 RPCI_BrCa15 RPCI_BrCa1 RPCI_BrCa17 RPCI_BrCa18 RPCI_BrCa19 RPCI_BrCa20 RPCI_BrCa21 RPCI_BrCa24 RPCI_BrCa25 RPCI_BrCa2
Approval of pembrolizumab (MSI- H/dMMR) and considerations for site-agnostic development of drugs in oncology
 Approval of pembrolizumab (MSI- H/dMMR) and considerations for site-agnostic development of drugs in oncology Steven Lemery, MD, MHS Associate Director, DOP2 Traditional development paradigm Based on tumor
Approval of pembrolizumab (MSI- H/dMMR) and considerations for site-agnostic development of drugs in oncology Steven Lemery, MD, MHS Associate Director, DOP2 Traditional development paradigm Based on tumor
Plasma-Seq conducted with blood from male individuals without cancer.
 Supplementary Figures Supplementary Figure 1 Plasma-Seq conducted with blood from male individuals without cancer. Copy number patterns established from plasma samples of male individuals without cancer
Supplementary Figures Supplementary Figure 1 Plasma-Seq conducted with blood from male individuals without cancer. Copy number patterns established from plasma samples of male individuals without cancer
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine
MEDICAL POLICY. SUBJECT: MOLECULAR PANEL TESTING OF CANCERS TO IDENTIFY TARGETED THERAPIES (Excluding NSCLC and CRC) EFFECTIVE DATE: 12/21/17
 MEDICAL POLICY SUBJECT: MOLECULAR PANEL TESTING OF PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: MOLECULAR PANEL TESTING OF PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
