SHANK3 Deficiency Impairs Heat Hyperalgesia and TRPV1 Signaling in Primary Sensory Neurons
|
|
|
- Cecil Nash
- 5 years ago
- Views:
Transcription
1 Article SHANK3 Deficiency Impairs Heat Hyperalgesia and TRPV1 Signaling in Primary Sensory Neurons Highlights d SHANK3 is expressed in primary sensory neurons and spinal cord central terminals d d d Shank3 haploinsufficiency in sensory neurons leads to impaired heat hyperalgesia SHANK3 in mouse and human DRG neurons regulates TRPV1 function SHANK3 interacts with TRPV1 and modulates presynaptic pain transmission Authors Qingjian Han, Yong Ho Kim, Xiaoming Wang,..., Yan Zhang, Yong-Hui Jiang, Ru-Rong Ji Correspondence yong-hui.jiang@dm.duke.edu (Y.-H.J.), ru-rong.ji@duke.edu (R.-R.J.) In Brief In this issue of Neuron, Han et al. describe how SHANK3, expressed by peripheral primary sensory neurons, regulates TRPV1 function and heat hyperalgesia after inflammation and nerve injury and therefore offer a mechanistic insight into pain dysregulation in autism. Han et al., 2016, Neuron 92, 1 15 December 21, 2016 ª 2016 Elsevier Inc.
2 Neuron Article SHANK3 Deficiency Impairs Heat Hyperalgesia and TRPV1 Signaling in Primary Sensory Neurons Qingjian Han, 1,4 Yong Ho Kim, 1,4 Xiaoming Wang, 2,4 Di Liu, 1 Zhi-Jun Zhang, 1 Alexandra L. Bey, 3 Mark Lay, 1 Wonseok Chang, 1 Temugin Berta, 1 Yan Zhang, 1 Yong-Hui Jiang, 2,3, * and Ru-Rong Ji 1,3,5, * 1 Department of Anesthesiology 2 Department of Pediatrics 3 Department of Neurobiology Duke University Medical Center, Durham, NC 27710, USA 4 Co-first author 5 Lead Contact *Correspondence: yong-hui.jiang@dm.duke.edu (Y.-H.J.), ru-rong.ji@duke.edu (R.-R.J.) SUMMARY Abnormal pain sensitivity is commonly associated with autism spectrum disorders (ASDs) and affects the life quality of ASD individuals. SHANK3 deficiency was implicated in ASD and pain dysregulation. Here, we report functional expression of SHANK3 in mouse dorsal root ganglion (DRG) sensory neurons and spinal cord presynaptic terminals. Homozygous and heterozygous Shank3 complete knockout (De4 22) results in impaired heat hyperalgesia in inflammatory and neuropathic pain. Specific deletion of Shank3 in Nav1.8-expressing sensory neurons also impairs heat hyperalgesia in homozygous and heterozygous mice. SHANK3 interacts with transient receptor potential subtype V1 (TRPV1) via Proline-rich region and regulates TRPV1 surface expression. Furthermore, capsaicininduced spontaneous pain, inward currents in DRG neurons, and synaptic currents in spinal cord neurons are all reduced after Shank3 haploinsufficiency. Finally, partial knockdown of SHANK3 expression in human DRG neurons abrogates TRPV1 function. Our findings reveal a peripheral mechanism of SHANK3, which may underlie pain deficits in SHANK3-related ASDs. INTRODUCTION Individuals with autism spectrum disorders (ASDs) show distinct neurobehavioral deficits, characterized by impairments in social interactions, deficits in communication, as well as repetitive behaviors (Jiang and Ehlers, 2013; Kaiser and Feng, 2015; Lord et al., 2000). ASDs are also characterized by wide sensory abnormalities related to touch, smell, taste, and pain (Allely, 2013; Klintwall et al., 2011). Environmental factors, including pesticides and fungicides have been linked to autism and neurodegeneration (Lord et al., 2000; Pearson et al., 2016). Pain is poorly assessed in ASD patients. They appear to have a higher tolerance to painful injuries such as bumps and cuts. As many as 70% of ASD patients show self-injurious behaviors, including head banging, skin picking, and self-biting (Furniss and Biswas, 2012). In sharp contrast, ASD children also display exaggerated behavioral responses to pain (Mandell et al., 2005; Nader et al., 2004; Rattaz et al., 2013). It was argued that ASD children have deficits in facial and behavioral expression of pain, which may lead caregivers to interpret this as pain insensitivity (Allely, 2013). This discrepancy results from our poor understanding of mechanisms underlying pain dysregulation in ASD. Interestingly, a recent study demonstrated that dysfunction of peripheral mechanosensory neurons contributes to tactile and behavioral deficits in mouse models of ASDs (Orefice et al., 2016). Numerous genes have been implicated in ASDs including those that regulate synaptic transmission (Jiang and Ehlers, 2013). SHANK/ProSAP family proteins act as synaptic scaffolding proteins at postsynaptic density (PSD) of excitatory glutamatergic synapses (Sheng and Kim, 2000). Human genetic evidence indicates that SHANK family genes are strong causative genes for idiopathic ASDs. SHANK3 is one of the best implicated genes in ASDs, and SHANK3 mutations were found in 2% of ASDs (Leblond et al., 2014; Moessner et al., 2007; Yi et al., 2016). Shank3 mutant mice exhibit dysregulation of glutamatergic synapses in their sizes, shapes, and functions, which is reminiscent of the phenotypes observed in mouse models of fragile X syndrome, Rett syndrome, and Angelman syndrome (Jiang and Ehlers, 2013; Wang et al., 2011, 2014a; Yoo et al., 2013). SHANK3 maps to the critical chromosome region of 22q13.3. Strikingly, clinical and genetic evaluation of 201 patients with Phelan- McDermid syndrome (PMS) (Phelan et al., 2001), also known as 22q13 deletion syndrome, which includes the deletion of the entire SHANK3 gene, demonstrates decreased pain sensitivity in 77% of patients (Sarasua et al., 2014). Different lines of Shank3 mutant mice, with deletions or mutations in different exons (exons 4 7, 9, 11, 13 16, and 21), have been reported. However, these lines of Shank3 mutant mice show variable molecular, synaptic, and behavioral phenotypes, in part due to different manipulations of Shank3 isoforms (Jiang and Ehlers, 2013). To develop a more relevant ASD model, we generated Shank3 complete deficient mice by deleting exons 4 Neuron 92, 1 15, December 21, 2016 ª 2016 Elsevier Inc. 1
3 to 22. This deletion causes the absence of the entire SHANK3 protein coding sequence (Wang et al., 2016). Shank3 De4 22 mouse has high construct validity because the deletion of Shank3 in mice closely mimics the SHANK3 defects found in most patients with ASDs. Notably, Shank3 De4 22 mice demonstrate robust behavioral phenotypes that recapitulate the major features in SHANK3-related ASD and PMS and highlight postsynaptic modulation of this gene in brain regions such as striatum (Wang et al., 2016). In this study, we investigated how SHANK3 regulates pain sensitivity in Shank3 De4 22 mice. Specifically, we examined the role of SHANK3 in dorsal root ganglion (DRG) primary sensory neurons of the peripheral nervous system. We also examined SHANK3 expression in presynaptic sites in spinal cord central terminals of DRG neurons. We have revealed a peripheral and presynaptic role of SHANK3 in pain transduction and transmission via regulating transient receptor potential ion channel subtype V1 (TRPV1). Our data have also demonstrated that haploinsufficiency of SHANK3 causes profound defects in TRPV1 function in mouse and human DRG neurons. RESULTS Heat Hyperalgesia, but Not Baseline Pain, Is Impaired in Shank3 De4 22 Mutant Mice To investigate a specific role of Shank3 in pain regulation, we tested baseline pain and inflammatory and neuropathic pain in Shank3 complete knockout mice, in which exons 4 to 22 were deleted (Figure 1A). In this study, we referred to De4 22 / mice as Shank3 / mice. We first compared the baseline thermal and mechanical sensitivity in homozygous (Shank3 / ), heterozygous (Shank3 +/ ), and wild-type (WT) mice. The hot plate test indicated that Shank3 / mice exhibited no change in withdrawal latency at different temperatures (50 C, 53 C, and 56 C), compared to Shank3 +/ and WT mice (Figure 1B). The von Frey test also showed no difference among three genotypes in paw withdrawal threshold to punctate mechanical stimuli (Figure 1C). Similarly, the cold sensitivity in cold plate and temperature preference test was unaltered in Shank3 +/ and Shank3 / mice (Figures 1D and S1A). Furthermore, the Hargreaves test showed intact response to radiant heat in Shank3 / mice (Figure 1E). Together, these data suggest that baseline pain sensitivity, i.e., normal pain perception and temperature sensation do not change in Shank3 global knockout (KO) mice. Next, we investigated whether chronic pain is altered in Shank3 mutant mice. Intraplantar injection of complete Freund s adjuvant (CFA) induced persistent inflammatory pain for >1 week, as characterized by heat hyperalgesia (Figure 1E). This heat hyperalgesia was significantly impaired in both Shank3 +/ mice and Shank3 / mice (F 2, 60 = , p < , two-way ANOVA; Figure 1E). There was also significant difference between homozygous and heterozygous mice (F 1, 40 = , p = , two-way ANOVA; Figure 1E). This result indicates that loss of one copy of Shank3 is sufficient to cause pain defects, which is reminiscent of autism patients with haploinsufficiency mutations of SHANK3. However, CFA-induced edema was normal in KO mice (Figure S1B), suggesting that inflammatory pain defect was not a result of impaired inflammation. Chronic constriction injury (CCI) also produced long-lasting heat hyperalgesia (>4 weeks; Figure 1F). This neuropathic pain symptom was reduced in both Shank3 / and Shank3 +/ mice (Figure 1F). Inflammatory and neuropathic pain is also characterized by mechanical allodynia, a reduction in the paw withdrawal threshold in the von Frey test. Shank3 +/ and Shank3 / mice displayed a mild, but significant reduction in mechanical allodynia after CFA (F 2, 72 = , p = , two-way ANOVA) and CCI (F 2, 90 = , p = , two-way ANOVA) (Figures S1C and S1D). In contrast, the acetone test showed that nerve injuryinduced cold allodynia was unaltered in Shank3 mutant mice (Figure S1E). Given a substantial reduction in heat hyperalgesia in Shank3 mutant mice, we postulated that heat transduction in sensory neurons could be impaired in these mice. The capsaicin receptor TRPV1 is expressed in C-fiber nociceptive neurons and upregulated in DRG neurons after inflammation and nerve injury (Ji et al., 2002; Obata et al., 2004). TRPV1 regulates heat transduction and is especially critical for the development of heat hyperalgesia (Caterina et al., 2000; Davis et al., 2000; Eskander et al., 2015). We tested capsaicin-induced pain in WT and Shank3 mutant mice. Intraplantar injection of capsaicin (10 mg) induced rapid (1 5 min) spontaneous pain (licking and flinching behavior) in WT mice, but this spontaneous pain was compromised in Shank3 / and Shank3 +/ mice (Figure 1G). Intraplantar capsaicin also resulted in neurogenic inflammation (paw edema), showing increased plasma extravasation in hindpaw tissue in the Evans Blue test (Figure 1H). The capsaicin-evoked plasma extravasation was reduced in Shank3 / and Shank3 +/ mice (Figure 1H). Interestingly, heterozygous and homozygous mice displayed similar defects in capsaicin responses (Figures 1G and 1H). Thus, Shank3 mutant mice have defects in both inflammation- and nerve injury-induced heat hyperalgesia due to compromised TRPV1 signaling. To determine if there is sensory neuron loss in mutant mice, we quantified the number of Nissl-stained neurons from every fifth DRG section of WT and Shank3 / mice, but did not see significant loss of DRG neurons in Shank3 / mice (1,756 ± 191 in WT DRGs versus 1,821.7 ± 181 in Shank3 / DRGs, p > 0.05; Figure S2A). The percentages of TRPV1 +, CGRP +, IB4 +, and NF200 + neurons in Shank3 / mice were unaltered (Figure S2A). Skin innervation of PGP9.5 + nerve fibers and spinal cord innervations of CGRP +, IB4 +, and NF200 + axonal terminals were also normal in KO mice (Figures S2B and S2C). Neither did we see neuronal loss in the spinal cord (Figure S2D). Together, we conclude that the deficit of heat hyperalgesia in Shank3 mutant mice is not due to cell loss and innervation deficits of DRG neurons. SHANK3 Is Expressed by Primary Sensory Neurons and their Spinal Cord Central Terminals We employed several different approaches to examine SHANK3 expression in DRG primary sensory neurons. RT-PCR analysis revealed the expression of all the Shank3 isoforms including Shank3a, Shank3b, Shank3c, Shank3d, and Shank3e in DRG, as well as spinal cord, cerebellum, and cortex tissues (Figure 2A). Western blotting showed SHANK3 protein expression in DRG and spinal cord of WT mice not Shank3 / mice, with lower 2 Neuron 92, 1 15, December 21, 2016
4 Figure 1. Shank3 Global Mutant Mice Exhibit Reductions in Heat Hyperalgesia after Inflammation and Nerve Injury and also in Capsaicin- Induced Pain (A) Schematic illustration of Shank3 complete knockout mice, in which exons 4 to 22 are deleted. De4 22 / mice = Shank3 / mice. (B D) Baseline pain sensitivity in homozygous (Shank3 / ), heterozygous (Shank3 +/ ), and WT (Shank3 +/+ ) mice. no significance, n.s.; one-way ANOVA; and n = 5 13 mice/group. (B) Hot plate test for heat sensitivity at 50 C, 53 C, and 56 C. (C) von Frey test for punctuate mechanical sensitivity. (D) Temperature preference test at 50 C/15 C and 21 C/5 C. (E and F) Hargreaves test for basal heat sensitivity before CFA injection (E) and CCI (F) and heat hyperalgesia after the CFA injection and CCI in three genotypes. Note that heat hyperalgesia is reduced in both Shank3 / and Shank3 +/ mice. # p < 0.05, one-way ANOVA; *p < 0.05, two-way ANOVA; and n = 5 7 mice / group. (G) Time course of intraplantar capsaicin (10 mg) induced spontaneous pain in three genotypes. # p < 0.05, one-way ANOVA; *p < 0.05, two-way ANOVA; and n = 5 9 mice/group. not significant, n.s. (H) Capsaicin-induced neurogenic inflammation, evaluated by plasma extravasation in ipsilateral paw in Evans Blue Dye (EBD) test 30 min after intraplantar capsaicin. The contralateral paw was included as control. # p < 0.05, one-way ANOVA and n = 5 6 mice/group. absorbance, Abs. All the data are mean ± SEM. Neuron 92, 1 15, December 21,
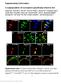
5 Please cite this article in press as: Han et al., SHANK3 Deficiency Impairs Heat Hyperalgesia and TRPV1 Signaling in Primary Sensory Neurons, Neuron Figure 2. Characterization of SHANK3 Expression in Mouse DRG and Spinal Cord (A) RT-PCR showing Shank3 isoforms in DRG, spinal cord (SC), cerebellum (CB), and cortex (CT). (B) Western blot showing SHANK3 expression in DRG, SC, CB, and CT. We loaded 50 or 5 mg proteins for DRG/SC and CB/CT samples, respectively. (C) IHC showing SHANK3 expression in DRG neurons of WT, but not KO mice. The scale bar represents 50 mm. (D) Double staining of SHANK3/Substance P (SP), SHANK3/IB4, and SHANK3/NF200 in DRG sections. The scale bars represent 50 mm. (E) Size distribution of SHANK3+ neurons and all neurons in DRG sections. A total of 1,896 neurons (including 1,245 SAHNK3+ neurons) from five mice were analyzed. (legend continued on next page) 4 Neuron 92, 1 15, December 21, 2016
6 expression levels compared with that of cerebellum and cortex (Figure 2B). We also examined the expression of different Shank3 isoforms in DRGs after CFA-induced inflammation and nerve injury (CCI), but did not find any upregulations of these isoforms, despite a moderate downregulation in Shank3 isoforms (Figure S1F). It is likely that pain-induced neuronal activity may cause the downregulation, because neuronal activity has been shown to downregulate Shank3 isoforms in cortical neurons (Wang et al., 2014b). Immunohistochemistry revealed that SHANK3 immunoreactivity (IR) is widely expressed in mouse DRG neurons, but SHANK3-IR in DRGs was lost in Shank3 / mice (Figure 2C). Double staining showed that SHANK3-IR was highly expressed in small-sized peptidergic (substance P + ) and non-peptidergic (IB4 + ) neurons, as well as in large-sized myelinated neurons (NF200 + ) in mouse DRG (Figure 2D). Cell size distribution analysis showed broad expression of SHANK3 in DRG neurons of different sizes, with predominant expression in small-sized neurons (<600 mm 2 ; Figure 2E). We also observed SHANK3-IR in nerve fibers in the hindpaw glabrous skin, due to axonal transport of SHANK3 from DRG cell bodies to peripheral terminals (Figure S3A). In situ hybridization also showed wide expression of Shank3 mrna in DRG neurons of WT mice, but not Shank3 / mice (Figure S3B). As in mouse DRG neurons, SHANK3 was widely expressed in rat DRG neurons too (Figures S3C and S3D). Dense SHANK3-IR was also found in the spinal cord dorsal horn, especially in the superficial layers (laminae I III) of WT mice, but not in KO mice (Figures 2F and S3E). As in DRG neurons, SHANK3-IR is present in both peptidergic (SP + ) and nonpeptidergic (IB4 + ) axonal terminals in the superficial dorsal horn (Figures 2F, S3E, and S3F). Interestingly, ablation of TRPV1-expressing C-fibers with systemic treatment of the neurotoxin resiniferatoxin (RTX) completely blocked the heat pain (Liu et al., 2010) and significantly reduced SHANK3-IR in the laminae I and IIo (Figures 2F and 2G). This result strongly suggests that SHANK3 in the superficial dorsal horn is originated, at least partly, from primary afferents. As expected, SHANK3-IR was also observed in many neurons in the deep dorsal horn (Figure S3E). Therefore, SHANK3 is expressed at both presynaptic sites (primary afferent terminals) and postsynaptic sites (neuronal somata) in the spinal cord dorsal horn. TRPV1 Currents in DRG Neurons Are Substantially Reduced in Shank3 Mutant Mice To determine the molecular and neural mechanism by which SHANK3 regulates pain, we employed patch-clamp recordings to assess TRPV1 function in dissociated small-diameter (<25 mm) DRG neurons of WT, Shank3 +/ and Shank3 / mice. Remarkably, inward currents induced by 100 nm capsaicin were substantially reduced in heterozygous mice and almost abolished in homozygous mice (Figures 3A and 3B). The percentage of DRG neurons responding to 100 nm capsaicin decreased from 55% (12/22) in WT mice to 30% (18/33 and 10/31) in Shank3 +/ and Shank3 / mice, respectively. Inward currents induced by a high dose of capsaicin (1 mm) were also drastically reduced in Shank3 +/ and Shank3 / mice (Figure 3B). In parallel, capsaicin-induced intracellular Ca 2+ increase was also reduced in Shank3-deficient neurons (Figures S4A and S4B). Interestingly, the TRPV1 defects in homozygous and heterozygous mice were comparable, and no statistic difference was found between these two genotypes (Figures 3A and 3B), indicating that haploinsufficiency of Shank3 is sufficient to disrupt TRPV1 function. Human genetics strongly suggests that SCN9A, the gene encoding sodium channel subunit Nav1.7, critically regulates pain sensitivity (Cox et al., 2006; Raouf et al., 2010). Interestingly, transient sodium currents, as well as Nav1.7-mediated sodium currents (isolated by Protoxin-II) (Sokolov et al., 2008) were normal in Shank3-deficient DRG neurons compared with WT neurons (Figure 3C). Neither did we find changes in neuronal excitability in Shank3 / mice: Shank3-deficient neurons and WT neurons fired action potentials at the same rate (Figure 3D). Thus, sodium channels such as Nav1.7 may not contribute to pain defects in Shank3 / mice. To rescue TRPV1 deficiency in Shank3-deficient neurons, we re-expressed Shank3 in these deficient mouse DRG neurons through electroporation of Shank3a construct. Notably, overexpressing Shank3-gfp in Shank3-deficient DRG neurons increased TRPV1 currents to the levels of WT neurons (Figure 3E). Thus, mouse Shank3 gene is critical for the function of TRPV1 in primary sensory neurons. Human SHANK3 mutation with a guanine insertion in exon 21 (InsG3680) is strongly associated with ASD (Durand et al., 2007). Overexpressing this SHANK3 mutant in Shank3-deficient DRG neurons failed to completely rescue TRPV1 currents (Figure 3E), despite higher expression of this mutant than WT Shank3 in DRG cultures after the transfection (Figure S4C). This result suggests an active role of SHANK3 mutation in regulating the TRPV1 function. Capsaicin-Induced Spinal Cord Synaptic Plasticity and Pain following Spinal Application Are Compromised in Shank3 Mutant Mice TRPV1 in central terminals of nociceptors regulates glutamate release from presynaptic terminals (Park et al., 2011). Next, we tested basal and capsaicin-evoked excitatory synaptic transmission in spinal cord slices of WT and Shank3 / mice, by recording miniature excitatory postsynaptic currents (mepscs) in lamina IIo neurons. These interneurons are predominantly excitatory, receive input from TRPV1-expressing primary afferents, and send output to lamina I projection neurons (Park et al., 2011; Todd, 2010). We found normal mepscs in Shank3-deficient lamina IIo neurons: both the frequency and (F) Double staining of SHANK3/IB4 in the superficial dorsal horn of control and RTX treated mouse. RTX (1 mg/kg body weight) was given daily for 3 days and mice were sacrificed 24 hr after the last injection. Box 1 and box 2 are enlarged in the upright images (box 1 and box 2 0 ) and further enlarged in the bottom images (box 1 and box 2 ). The scale bars represent 100 mm in top left images and 20 mm in other images. (G) Density of SHANK3-IR punctate in laminae I-IIo and IIi of the superficial dorsal horn of control and RTX treated mice. # p < 0.05; Student s t test; and n = 3 mice/ group. not significant, n.s. All of the data are mean ± SEM. Neuron 92, 1 15, December 21,
7 Figure 3. Capsaicin Responses in DRG and Spinal Cord Neurons Are Reduced in Shank3 +/ and Shank3 / Mice (A) Traces of 100 nm capsaicin-induced inward currents in small-sized DRG neurons from WT, Shank3 +/, and Shank3 / mice. (B) Amplitude of capsaicin (100 nm and 1 mm) currents in DRG neurons from three genotypes. # p < 0.05 versus WT control; not significant, n.s.; one-way ANOVA; and n = neurons/group. (C) Traces of transient sodium currents and Nav1.7-mediated sodium currents in small-sized DRG neurons of WT and KO mice. The Nav1.7-mediated sodium currents were isolated by 10 nm Pro-toxin II. The quantification of the currents is shown on the right. not significant, n.s. n = neurons/group. (D) Traces of action potentials in small-sized DRG neurons of WT and KO mice. The quantification of firing frequency of action potentials is shown on the right. n = neurons/group. (E) Deficiency of TRPV1 currents in DRG neurons of Shank3 / mice is rescued by re-expression of WT Shank3 (Shank3-Res), but not mutant Shank3 (Shank3- INS) inshank3-deficient neurons. The quantification of TRPV1 currents is shown on the right. # p < 0.05 versus Shank3 / control; not significant, n.s.; one-way ANOVA; and n = 6 9 neurons/group. (F) Traces of mepscs in lamina IIo neurons of spinal cord slices of WT and Shank3 / mice and the effects of capsaicin (100 nm and 1 mm). (G) Frequency of mepscs in lamina IIo neurons of spinal cord slices of WT and Shank3 / mice. # p < 0.05; not significant, n.s.; two-way ANOVA; and n = 6 neurons/group. (H) Spontaneous pain induced by intrathecal capsaicin (500 ng, i.t.) is reduced in Shank3 +/ and Shank3 / mice. # p < 0.05; one-way ANOVA; and n = 5 10 mice/ group. All the data are mean ± SEM. amplitude of mepsc were comparable in WT and Shank3 / mice (Figures 3F, 3G, S4D, and S4E). Capsaicin produced dose-dependent increases in mepsc frequency, but not mepsc amplitude in WT mice; but these mepsc frequency increases were substantially reduced after Shank3 deficiency (Figures 3F and 3G). Thus, SHANK3 is also required for TRPV1-mediated synaptic plasticity (mepsc frequency increase) via presynaptic modulation. Given an important role of TRPM8 in cold sensation (Bautista et al., 2007), we tested the function of TRPM8 in DRG and spinal 6 Neuron 92, 1 15, December 21, 2016
8 cord neurons in WT and Shank3 / mice. Menthol induced comparable increases in intracellular Ca 2+ in DRG neurons and mepsc in spinal lamina IIo neurons of WT and Shank3 / mice (Figures S4F S4H), suggesting an intact TRPM8 signaling after Shank3 deficiency. TRPC4 is expressed in DRG neurons and regulated in neuropathic pain (Staaf et al., 2009). Gadolinium chloride (GdCl 3 ), an agonist of TRPC4, induced comparable inward currents in DRG neurons and mepsc frequency increases in spinal cord neurons in WT and Shank3 KO mice (Figures S4I S4K), suggesting that TRPC4 function does not require SHANK3. Spinal capsaicin injection via intrathecal (i.t.) route induces robust spontaneous pain via central TRPV1 at primary afferent terminals (Park et al., 2011). This spontaneous pain was reduced in both Shank3 +/ and Shank3 / mice (Figure 3H). Capsaicin also elicited phosphorylation of extracellular signal-regulated kinase (perk) in superficial dorsal horn neurons, an essential step for central sensitization in persistent pain (Ji et al., 2003). The capsaicin-induced perk was abolished in Shank3 / mice (Figure S5). Thus, Shank3 is also important for central TRPV1 signaling in the spinal cord. Interaction of SHANK3 with TRPV1 in Native DRG Neurons and Heterologous ND7/23 Cells Our behavioral and electrophysiological data support a functional interaction of SHANK3 and TRPV1. We further examined biochemical interactions between these two important proteins. Double staining revealed co-localization of TRPV1 with SHANK3 in mouse DRG neurons (Figure 4A). TRPV1 is also co-localized with SHANK3 in spinal axonal terminals in the superficial dorsal horn (Figure 4B). The co-immunoprecipitation (coip) experiment showed possible interaction of SHANK3 and TRPV1 in mouse DRG (Figure 4C). Surface biotinylation analysis revealed a reduction of TRPV1 surface expression in Shank3-deficient DRG neurons (Figure 4D). We further tested SHANK3/TRPV1 interaction in heterologous ND7/23 cells, which are hybrid cells of neuroblastoma and DRG neurons (Dunn et al., 1991). The coip experiment validated the SHANK3/TRPV1 interaction in ND7/23 cells expressing SHANK3 and TRPV1 (Figures 4E and 4F). Surface biotinylation analysis also showed increased surface expression of TRPV1 in ND7/23 cells over-expressing Shank3 (Figure 4G). These results suggest that SHANK3 controls the function TRPV1 by modulating its trafficking and surface expression. SHANK3a consists of multiple domains for protein-protein interactions: ankyrin repeats (ANK), SH3 domain, PSD-95/Dlg/ ZO-1 (PDZ) domain, Proline-rich region, and sterile alpha motif (SAM) domain (Figure 5A) (Jiang and Ehlers, 2013; Sheng and Kim, 2000). To define how SHANK3 interacts with TRPV1, we constructed four expression vectors: SHANK3a, PDZ , Proline-rich 891 1,299, and Homer-SAM 1,302 1,730 and overexpressed them with TRPV1 constructor in ND7/23 cells. We found the region 891 1,299 of Proline-rich domain, but not PDZ and Homer-SAM 1,302 1,730 domains was sufficient for the SHANK3/ TRPV1 interaction (Figure 5B). Competing experiments further showed that the Proline-rich 891 1,299 domain and SHANK3a competed for the interaction with TRPV1 in ND7/23 cells (Figure 5C). Transfection of WT mouse DRG neurons with the Proline-rich 891 1,299 region of Shank3a drastically reduced TRPV1 currents (Figures 5D and 5E), suggesting a role of this SHANK3 region in regulating TRPV1 function in native DRG neurons. It is noteworthy that human SHANK3 mutation InsG3680 resulted in a truncated SHANK3 ending at the site 1,227 of the Proline-rich 891 1,299 region (Durand et al., 2007), and this mutation also affected TRPV1 function in DRG neurons (Figure 3E). Inflammatory Pain, Heat Pain, and TRPV1 Signaling Are Impaired after Sensory Neuron-Specific Shank3 Deletion To confirm a specific role of peripheral SHANK3 in pain regulation, we generated Shank3 conditional KO (CKO) mice by crossing Shank3-floxed mice with Na V 1.8-Cre mice (Figure 6A), leading to specific deletion of Shank3 in nociceptive neurons as well as some low-threshold A-fiber neurons (Agarwal et al., 2004; Shields et al., 2012). We then compared baseline pain and inflammatory pain in three genotypes: littermate control mice (Shank3 f/f ), heterozygous mice (Na V 1.8-cre;Shank3 f/+ ), and homozygous mice (Na V 1.8-cre;Shank3 f/f ) (Figure 6B). We found a profound reduction (80%) of SHANK3-IR in the superficial dorsal horn (laminae I III) in Na V 1.8-cre;Shank3 f/f mice (Figures 6C and 6D), further supporting that SHANK3-IR in the superficial dorsal horn is largely originated from primary sensory neurons. Heterozygous CKO mice also showed a 60% reduction in SHANK3-IR in laminae I III (Figure 6D). Remarkably, baseline heat sensitivity in hot plate and Hargreaves tests was impaired in homozygous mice showing increased withdrawal latency (Figures 6E 6G). However, temperature preference tests showed no difference among three genotypes in mild test temperatures (21 C/21 C and 15 C/25 C; Figure S6A). We also failed to see changes in body temperature (rectal temperature) in Shank3 conditional mutant mice and Trpv1 null mice (Figure S6B). Deficit in baseline heat sensitivity did not occur in heterozygous mutant mice (Figures 6E and 6F). CFA-induced heat hyperalgesia was reduced in both homozygous and heterozygous CKO mice (Figure 6G). The von Frey test showed comparable baseline mechanical sensitivity in three genotypes, but CFA-induced mechanical allodynia was slightly decreased in homozygous and heterozygous mice (Figure S6C). Capsaicininduced spontaneous pain was substantially reduced in homozygous and heterozygous CKO mice following intraplantar or intrathecal injection (Figures 6H and 6I). It is noteworthy that SHANK3-IR in deep dorsal horn neurons (laminae IV and V) was unaltered (Figure S6D). Collectively, these data from Shank3 CKO mice strongly suggest that deletion of Shank3 in sensory neurons can recapitulate all pain defects in Shank3 global KO mice. Moreover, Shank3 homozygous, but not heterozygous, CKO mice showed heat deficit, reminiscent of Trpv1 KO mice (Caterina et al., 2000). SHANK3 Expression in Human DRG Neurons Regulates TRPV1 Signaling To evaluate the translational potential of this study, we further tested if SHANK3 would regulate TRPV1 function in human DRG neurons obtained from non-diseased donors. Immunohistochemistry and size frequency analysis showed very broad expression of SHANK3 in all types of human DRG neurons (Figures 7A and 7B). We also observed a high-level of co-localization of SHANK3 and TRPV1 in human DRG neurons (Figure 7C). Neuron 92, 1 15, December 21,
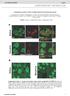
9 Please cite this article in press as: Han et al., SHANK3 Deficiency Impairs Heat Hyperalgesia and TRPV1 Signaling in Primary Sensory Neurons, Neuron Figure 4. Characterization of SHANK3 and TRPV1 Interaction in Mouse DRG Neurons and ND7/23 Cells (A) Double staining showing co-localization of SHANK3 and TRPV1 in DRG neurons: red channel and green channel are from single focal plane of a DRG section, and the merged image is the orthogonal view (XY, XZ, and YZ) of z stack. The scale bar represents 50 mm. (B) Double labeling of SHANK3 and TRPV1 in axonal terminals in superficial spinal dorsal horn. The small box in the top image is enlarged in three separate boxes with single and merged images. The scale bars represent 50 mm (top) and 5 mm (bottom). (C) CoIP showing SHANK3/TRPV1 interaction in DRGs. DRG lysate was immunoprecipitated with TRPV1 antibody, and immunoblotted with SHANK3 or TRPV1 antibody as indicated. This experiment was repeated three times. (D) Surface biotinylation showing decreased surface expression of TRPV1 in cultured DRG neurons of Shank3 / mice. The quantification of surface and lysate TRPV1 expression in WT and Shank3 KO mice is shown on the right. #p < 0.05; Student s t test; and n = 4 cultures/group. (E and F) CoIP analysis in ND7/23 cells. The lysate of ND7/23 cells overexpressing SHANK3-GFP and TRPV1 was immunoprecipitated with GFP antibody (E) or TRPV1 antibody (F) and then immunoblotted with anti-shank3 or anti-trpv1 antibody. Each of the experiments was repeated three times. (G) Surface biotinylation showing increased surface expression of TRPV1 after Shank3 overexpression in ND7/23 cells. The quantification of surface and lysate expression of TRPV1 in ND7/23 cells is shown on the right. #p < 0.05; Student s t test; and n = 3 cultures/group. transferrin receptor, TfR. All the data are mean ± SEM. Co-localization of SHANK3/TRPV1 was further observed in axons in human spinal nerve (Figure 7C), suggesting active axonal transport of both proteins. To define a critical role of SHANK3 in regulating human TRPV1 function, we developed an effective method to knock down SHANK3 expression in 8 Neuron 92, 1 15, December 21, 2016 dissociated human DRG neurons using small interfering RNA (sirna). Figure 7D shows that siglo-labeled sirna could be uptaken by all human DRG neurons in primary cultures. Treatment of human DRG cultures with selective SHANK3 sirna (100 nm) lead to a partial reduction in SHANK3-IR (Figure 7E), which can mimic haploinsufficiency of SHANK3 in human. Patch clamp-recordings in siglo-labeled small-diameter human DRG neurons (<50 mm) showed that capsaicin-induced inward currents were substantially suppressed (85% and 79% inhibition at 0.1 and 1 mm capsaicin, respectively) by SHANK3 sirna, despite a partial (50%) knockdown of SHANK3 expression (Figures 7E 7G). By contrast, the SHANK3 sirna treatment did not change TRPV1 mrna levels in human DRG cultures (Figure S7), arguing against the possibility of regulating TRPV1 expression by SHANK3. Competing experiments by overexpression of the Proline-rich891 1,299 region further suggested an
10 Figure 5. Prolin-Rich Domain Is Essential for the SHANK3 and TRPV1 Interaction in Heterologous Cells and DRG Neurons (A) Diagram of full length SHANK3a and SHANK3a mutant proteins. (B and C) CoIP showing the interaction between TRPV1 and the Proline-rich (Pro) domain of SHANK3 in ND7/23 cells. Each of the experiments was repeated three times. (B) Interaction of TRPV1 with SHANK3 mutants. SHANK3or its mutantsweretransientlyco-expressed together with TRPV1, and cell lysate was immunoprecipitated with GFP antibody and then immunoblotted with GFP or TRPV1 antibody as indicated. (C) Proline-rich region competes the interaction between SHANK3 and TRPV1. SHANK3 mutants were co-transfected with full length SHANK3 and TRPV1, and cell lysate was immunoprecipitated with TRPV1 antibody, and then immunoblotted with GFP, TRPV1, or SHANK3 antibody. (D and E) Competition experiment showing that transfection of WT mouse DRG neurons with Proline-rich 891 1,299 region of SHANK3a profoundly inhibits TRPV1 currents. (D) Traces of TRPV1 currents in DRG neurons. (E) Amplitudes of inward currents. # p < 0.01 versus WT control; unpaired t test; and n = 8 10 neurons/ group. All the data are mean ± SEM. active role of this SHANK3 region in TRPV1 function regulation in human primary sensory neurons (Figures 7H and 7I). Therefore, SHANK3 haploinsufficiency also causes a profound impairment of TRPV1 function in human DRG neurons. DISCUSSION Using Shank3 complete knockout mice with deletion of exons 4 to 22 (De4 22 Shank3 / ), we have made several interesting findings in this study. First, SHANK3 is broadly expressed in cell bodies and central terminals of DRG primary sensory neurons. Second, same as in human SHANK3-related disorders, Shank3 haploinsufficiency in mice causes marked deficits in inflammatory pain and neuropathic pain with predominant reductions in heat hyperalgesia. Third, Shank3 CKO mice with Shank3 deficiency in Nav1.8-expressing sensory neurons can recapitulate all the pain defects in global knockout mice and display additional deficits in baseline heat sensitivity. Fourth, SHANK3 regulates TRPV1 function in both primary sensory neurons and presynaptic terminals and is also essential for capsaicin-evoked pain at both peripheral and central sites. Fifth, SHANK3 interacts with TRPV1 via the Proline-rich region of SHANK3 and regulates the trafficking and surface expression of TRPV1. Finally, SHANK3 is expressed in human DRG neurons and SHANK3 knockdown dramatically disrupts TRPV1 currents in human sensory neurons. Peripheral Modulation of Pain and Heat Sensation by SHANK3 via Interaction with TRPV1 To the best of our knowledge, this is the first study to demonstrate a peripheral role of SHANK3. We found broad SHANK3 expression in primary sensory neurons of both mouse and human DRGs. All the major Shank3 isoforms, including Shank3a, Shank3b, Shank3c, Shank3d, and Shank3e and full-length SHANK3 protein expressed in mouse DRGs. Importantly, specific deletion of Shank3 in Nav1.8-expressing sensory neurons recapitulated all the pain deficits in Shank3 global KO mice, including severe defect in heat hyperalgesia and mild defect in mechanical allodynia. Thus, peripheral SHANK3 plays an essential role in driving heightened pain sensitivity after inflammation and nerve injury. It is noteworthy that Shank3 deletion does not cause neuronal loss in DRG and spinal cord. Neither does this deletion affect peripheral and central innervations of DRG neurons. Thus, pain defects in Shank3 mutant mice are not caused by developmental defects in sensory neurons and their projections. TRPV1 is one of the most investigated transduction molecules in pain research (Caterina and Julius, 2001; Patapoutian et al., 2009). Little is known about scaffold proteins for TRPV1, although the A-kinase anchoring protein (AKAP) regulates PKA phosphorylation of TRPV1 and hyperalgesia (Jeske et al., 2008). Our findings demonstrated that SHANK3 is a scaffold protein for TRPV1 and critically regulates TRPV1 function in mouse and human primary sensory neurons. First, SHANK3 is co-localized with TRPV1 in sensory neurons and nerve axons of mouse and human DRGs. Second, capsaicin-induced inward currents are dramatically reduced in mouse DRG neurons after Shank3 haploinsufficiency. Strikingly, sirna-mediated partial knockdown of SHANK3 resulted in a substantial reduction in TRPV1 currents in human DRG neurons. Third, Shank3 re-expression in Shank3-deficint DRG neurons rescued TRPV1 currents. Fourth, capsaicin-induced spontaneous pain and inflammationand nerve injury-induced heat hyperalgesia are markedly reduced in Shank3 global and conditional mutant mice. Fifth, Neuron 92, 1 15, December 21,
11 Figure 6. Shank3 in Sensory Neurons Is Required for Heat Transduction, CFA-Induced Heat Hyperalgesia, and Capsaicin-Evoked Pain (A) Schematic illustration of Shank3 conditional knockout mice with Shank3 deletion in Nav1.8-expressing sensory neurons. (B) Genotyping of Shank3 conditional knockout mice for Shank3 f/f and Nav1.8-Cre genotypes. (C) Double staining of SHANK3 and IB4 on spinal cord dorsal horn from Shank3 f/f and Nav1.8-Cre;Shank3 f/f mice. The scale bar represents 100 mm. (D) Quantification of SHANK3-IR in laminae I III and laminae IV-V of spinal cord from Shank3 f/f, Nav1.8-Cre;Shank3 f/+, and Nav1.8-Cre;Shank3 f/f mice. # p < 0.05; one-way ANOVA; and n = 5 mice/group. (E) Hot plate test for heat sensitivity in three genotypes, # p < 0.05, one-way ANOVA; *p < 0.05, two-way ANOVA; and n = 5 10 mice/group. not significant, n.s. (F) Temperature preference test at 50 C/15 C and 21 C/5 C in three genotypes, # p < 0.05, one-way ANOVA and n = 6 mice/group. (G) CFA-induced heat hyperalgesia in three genotypes. # p < 0.05, one-way ANOVA; *p < 0.05, two-way ANOVA; and n = 5 10 mice/group. not significant, n.s. (H and I) Spontaneous pain induced by intraplantar capsaicin (10 mg, i.pl., H) or intrathecal capsaicin (500 ng, i.t., I) in three genotypes. # p < 0.05, one-way ANOVA; *p < 0.05, two-way ANOVA; and n = 5 10 mice/group. not significant, n.s. All the data are expressed as mean ± SEM. capsaicin-induced neurogenic inflammation, but not CFAinduced edema is abrogated in Shank3 mutant mice. The coip experiment further demonstrated possible physical interaction of SHANK3 and TRPV1 in both native DRG neurons and heterologous ND7/23 cells. As a scaffold protein, SHANK3 appears to control TRPV1 function in sensory neurons via regulating the surface expression of TRPV1. SHANK3 is known to interact with NMDAR and mglur5 via PDZ and Homer binding motif, respectively (Sheng and Kim, 2000; Tu et al., 1999). Of great interest, SHANK3 interacts with TRPV1 via the Proline-rich region of 10 Neuron 92, 1 15, December 21, 2016
12 Figure 7. SHANK3 Regulates TRPV1 Function in Human DRG Neurons (A) SHANK3-IR in human DRG neurons. The enlarged SHANK3 immunostaining is shown in the box of left image (upright). The Nissl staining is shown in the same box (low right). The scale bars represent 500 mm (left) and 200 mm (right). (B) Size distribution of SHANK3-IR neurons and all neurons in human DRG sections. A total of 2,199 neurons from three human DRGs were analyzed. (C) Double labeling of SHANK3 with TRPV1 in human DRG and spinal nerve. The scale bars represent 200 mm. (D) Immunocytochemistry showing knockdown of SHANK3 expression by SHANK3 sirna treatment (100 nm, 48 hr) in cultured human DRG neurons. The scale bar represents 200 mm. (E) Intensity of SHANK3-IR neurons after SHANK3 sirna and control (con) sirna treatment. # p < 0.05; Student s t test; and n = neurons/group. (F and G) Inhibition of TRPV1 currents in human DRG neurons following SHANK3 sirna treatment (100 nm, 48 hr). (F) Typical traces of capsaicin-induced inward currents in cultured human DRG neurons. (G) Quantification of capsaicin-induced inward currents in human DRG neurons. # p < 0.05 versus control sirna; unpaired t test; and n = neurons/group. (H and I) Proline-rich 891 1,299 domain of SHANK3a is required for TRPV1 function in human DRG neurons. (H) Typical traces of capsaicin-induced inward currents in cultured human DRG neurons. (I) Quantification of capsaicin-induced inward currents in human DRG neurons. # p < 0.05 versus control; unpaired t test; and n = 6 7 neurons/group. All the data are expressed as mean ± SEM. SHANK3. This region is essential for the SHANK3 binding to TRPV1 and also important for regulating TRPV1 function in mouse and human DRG neurons. Consistently, an ASD-associated human mutation (InsG3680), which generates a truncated protein ending of SHANK3 in this region (Durand et al., 2007), also disrupted the TRPV1 function in DRG neurons. Future studies are warranted to determine the specific sequences on both proteins that are responsible for the SHANK3/TRPV1 interaction. Our results also show that neuronal excitability did not change after Shank3 deficiency in DRG neurons, suggesting that SHANK3 specifically regulates the transduction, but not the conduction of pain in primary sensory neurons. In addition to capsaicin-induced pain and injury-induced heat hyperalgesia, basal heat sensitivity in all the tests was impaired in homozygous CKO mice, but not global knockout mice. This discrepancy suggests that peripheral and central SHANK3 may regulate heat Neuron 92, 1 15, December 21,
13 sensitivity in a different, even opposite way. A deficit in peripheral heat transduction could be masked by enhanced central processing of heat signaling in global KO mice. It is conceivable that loss of TRPV1 function in Shank3-deficient sensory neurons is responsible for this altered heat sensitivity in CKO mice. SHANK3 may also regulate additional thermal sensors or signaling molecules in the PNS and CNS. Notably, TRPV1 primarily regulates heat hyperalgesia after tissue injury, but only has a partial role in heat-induced nociceptive responses (Caterina et al., 2000; Davis et al., 2000). Apart from TRPV1, several thermal sensors have been implicated in extreme heat (TRPV2) and painful heat such as TRPM3 and calcium-activated chloride channel anoctamin 1 (ANO1) (Caterina et al., 1999; Cho et al., 2012; Vriens et al., 2011). By contrast, cold sensitivity and TRPM8 function do not change in Shank3 KO and CKO mice. Presynaptic Modulation of Pain by SHANK3 Almost all SHANK3-related studies focus on postsynaptic mechanisms in the brain. Strikingly, we saw prominent SHANK3 expression in central terminals in the superficial dorsal horn. These SHANK3-expressing axonal terminals are presynaptic components of the first-order nociceptive synapses that co-express TRPV1 (Todd, 2010). The presynaptic expression of SHANK3 is also supported by the evidence that SHANK3-IR is dramatically reduced in the superficial dorsal horn after C-fiber ablation and Shank3 deletion in primary sensory neurons. As a functional measurement of presynaptic modulation, TRPV1- medicated mepsc frequency increase in spinal lamina IIo neurons is substantially reduced in Shank3 mutant mice. Spinal TRPV1 was also implicated in the generation of mechanical allodynia (Eskander et al., 2015; Kim et al., 2012). Consistently, mechanical allodynia after CFA and CCI was partially reduced in Shank3 mutant mice. However, defect in heat hyperalgesia is the primary phenotype in these mice. Heat hyperalgesia can be regulated by both peripheral and central TRPV1. Inflammation not only induces heat hyperalgesia, but also increases TRPV1 expression in DRG neurons and axonal transport of TRPV1 to skin nerve terminals (Ji et al., 2002). SHANK3 is also present in axonal terminals and presynaptic components of hippocampal neurons (Halbedl et al., 2016), but the function of this presynaptic SHANK3 was not investigated in this study. Remarkably, deletion of the autism gene Mecp2 in DRG mechanosensory neurons results in loss of presynaptic inhibition in the spinal cord, tactile hypersensitivity, and behavioral phenotypes of ASD (Orefice et al., 2016). It remains to be investigated if spinal inhibitory synaptic transmission is also altered after SHANK3 deficiency. Conclusions Abnormal sensory integration is commonly associated with ASD patients and significantly affects the life quality of the individuals. Pain in ASD patients is difficult to assess in part due to their deficits in communication. It is generally believed that ASDs are a group of developmental brain disorders due to dysregulation of excitatory and inhibitory synaptic transmission in the cortex (Jiang and Ehlers, 2013; Tabuchi et al., 2007). However, peripheral and presynaptic mechanisms of ASDs have begun to be revealed (Orefice et al., 2016). It is conceivable that different autism genes in sensory neurons may regulate different sensory modalities, such as pain and touch by Shank3 and Mecp2, respectively. Remarkably, SHANK3 deletion in PMS results in high pain threshold in 77% of patients (p = ) and high pain tolerance is becoming more common with increased age (89%, p = ). PMS patients also exhibit significant self-injury behaviors such as biting (46%, p = ) and hair pulling (41%, p = ), but insignificant touch hypersensitivity (46%, p = 0.699) (Sarasua et al., 2014). Using the newly generated Shank3 complete knockout mice with deletion of exons 4 to 22 (Wang et al., 2016), we have demonstrated that like human SHANK3 deficiency, haploinsufficiency of Shank3 in mice leads to substantial impairment in TRPV1 function and heat hyperalgesia, and these deficits can be reproduced by Shank3 deletion in mouse and human sensory neurons. Our data strongly suggest a peripheral mechanism of ASDs by which SHANK3 expression in primary sensory DRG neurons controls the surface expression and function of TRPV1 to regulate heat pain transduction and heat hyperalgesia associated inflammatory and neuropathic pain. Our results also revealed a presynaptic mechanism of SHANK3 by which SHANK3 expression in superficial spinal presynaptic terminals controls TRPV1-mediated neurotransmitter release in the spinal cord pain circuit under injury conditions. Thus, our findings have offered a mechanistic insight into pain deficits in ASDs. On the other hand, targeting peripheral SHANK3 may also help alleviate chronic pain in non-asd patients. Given an important role of SHANK3 in postsynaptic modulation of glutamatergic synaptic transmission and well-demonstrated role of spinal cord postsynaptic plasticity in driving central sensitization and chronic pain (Ji et al., 2003), SHANK3 could also contribute to enhanced pain states after inflammation and nerve injury via modulating postsynaptic plasticity in spinal cord dorsal horn neurons. Therefore, SHANK3 modulates inflammatory and neuropathic pain via peripheral, presynaptic, and postsynaptic mechanisms. However, defects in heat transduction and heat hyperalgesia in Shank3 mutant mice could be primarily mediated by peripheral SHANK3 via its interaction with TRPV1. EXPERIMENTAL PROCEDURES Reagents We purchased capsaicin, CFA, menthol, and gadolinium chloride from Sigma- Aldrich, sirna from OriGene, resiniferatoxin from LC Laboratories, and ProTx- II from Peptide Institute, Inc. Animals Shank3 complete knockout mice (exons 4 to 22 deletion, De4 22 /, C57BL/6 background RRID: MGI: ) and Shank3 flox/fllox mice were generated at Duke University Medical Center (Wang et al., 2016). Nav1.8-cre; Shank3 flox/flox mice were generated by mating Nav1.8-cre mice with Shank3 flox/flox mice (RRID: MGI: ). Adult male C57BL/6 mice (8 12 weeks) were used for behavioral and biochemical studies. Young C57BL/6 mice (5 8 weeks) were used for electrophysiological studies in spinal cord slices. All the animal procedures were approved by the Institutional Animal Care & Use Committee (IACUC) of Duke University. See Supplemental Experimental Procedures for details. Animal Models of Pain and Intrathecal Injection To produce inflammatory pain, diluted CFA (20 ml) was injected into the plantar surface of a hindpaw. To produce CCI in mice, three loose silk ligatures were tied around the sciatic nerve. Capsaicin was injected via intraplantar 12 Neuron 92, 1 15, December 21, 2016
14 (3 or 10 mg) or intrathecal route (0.5 mg) to induce spontaneous pain. For intrathecal injection, spinal cord puncture was made with a 30G needle. See Supplemental Experimental Procedures for details. Behavioral Testing Capsaicin-induced spontaneous pain was assessed by counting the time (s) spent on flicking, biting, and flinching for the first 5 min after the injection. Mechanical sensitivity was tested with von Frey hairs and thermal sensitivity was tested using Hargreaves apparatus. A two temperature choice test was used to assess temperature preference. Cold allodynia was assessed by acetone test. See Supplemental Experimental Procedures for details. Whole-Cell Patch-Clamp Recordings in Dissociated Mouse DRG Neurons DRGs were aseptically removed from mice and digested with collagenase and dispase-ii for 120 min. Cells were placed on glass coverslips coated with poly- D-lysine and grown in a neurobasal defined medium for 24 hr before experiments. Whole-cell voltage- and current-clamp recordings were performed at 28 C to measure capsaicin-induced currents, transient sodium currents, and action potentials, respectively. See Supplemental Experimental Procedures for details. Whole-Cell Patch-Clamp Recordings in Dissociated Human DRG Neurons Non-diseased human DRGs were obtained from the National Disease Research Interchange (NDRI). Postmortem L3 L5 DRGs were dissected from six donors and delivered in ice-cold culture medium. Human DRG cultures were prepared as previously reported (Xu et al., 2015). DRGs were digested for 120 min with collagenase Type II and dispase II. DRG neurons were plated on poly-d-lysine-coated glass coverslips and grown in neurobasal medium. Whole-cell patch-clamp recordings in small (<50 mm) human DRG neurons were conducted at room temperature. See Supplemental Experimental Procedures for details. Spinal Cord Slice Preparation and Patch-Clamp Recordings The L3 L5 lumbar spinal cord segment was removed from mice under urethane anesthesia ( g/kg, i.p.) and kept in preoxygenated ice-cold artificial cerebrospinal fluid (acsf) solution. Transverse slices ( mm) were cut on a vibrating microslicer. The whole-cell patch-clamp recordings were made from lamina IIo neurons in voltage clamp mode to record spontaneous EPSCs (sepscs) and mepscs. See Supplemental Experimental Procedures for details. Immunohistochemistry in Rodent and Human Tissues Animals were perfused through the ascending aorta with 4% paraformaldehyde. Spinal cord, skin DRG, and nerve sections were cut in a cryostat. The sections were blocked with 2% goat or horse serum and then incubated overnight at 4 C with primary antibodies followed by Cy3-, Cy5, or FITC-conjugated secondary antibodies. Human DRGs were fixed in 4% paraformaldehyde for 24 hr and processed for immunofluorescence. See Supplemental Experimental Procedures for details. Cell Culture and Transfection ND7/23 cells were cultured in high glucose DMEM supplemented with 10% fetal bovine serum. Transfection was performed with Lipofectamine 2000 Reagent. For electroporation, freshly disassociated mouse or human DRG neurons were transfected with 4D-Nucleofector System (Lonza). For sirna transfection, 100 pmol sirna was mixed with 5 pmol siglo control and 1.5 ml TransIT-2020 Transfection Reagent (Mirus). See Supplemental Experimental Procedures for details. Immunoprecipitation Transfected ND7/23 cells or cultured primary DRG neurons were lysed in icecold immunoprecipitation buffer. The lysate was immunoprecipitated with 0.5 mg of a rabbit anti-gfp antibody or goat anti-trpv1 antibody and then incubated with protein G-Sepharose beads (Roche). See Supplemental Experimental Procedures for details. Surface Protein Biotinylation Plasma membrane protein expression was detected after protein biotinylation. Solubilized lysates were pulled down on Streptavidin Agarose Resin overnight on rotor at 4 C. The pellet was then incubated in SDS-PAGE loading buffer and sample buffer for western blot analysis. See Supplemental Experimental Procedures for details. Western Blot The samples were separated on an SDS-PAGE gel, transferred, and probed with primary antibodies against SHANK3 (rabbit, 1:5,000, provided by Paul Worley from Johns Hopkins Medical School). See Supplemental Experimental Procedures for details. RT-PCR Tissues were rapidly isolated in RNase free conditions. Total RNAs were extracted using RNeasy Plus Mini Kit (QIAGEN). RNAs (0.5 1 mg) were reverse-transcribed using the SuperScript III RT (Invitrogen). See Supplemental Experimental Procedures for details. Statistical Analyses All data were expressed as mean ± SEM. Biochemical, electrophysiological, and behavioral data were analyzed using Student s t test (two groups) and one-way or two-way ANOVA followed by post hoc Bonferroni test. The criterion for statistical significance was p < See Supplemental Experimental Procedures for details. SUPPLEMENTAL INFORMATION Supplemental Information includes Supplemental Experimental Procedures and seven figures and can be found with this article online at org/ /j.neuron AUTHOR CONTRIBUTIONS Q.H., Y.H.K., and X.W. designed the experiments; Q.H. performed behavioral, immunohistochemical, in situ hybridization, and coip experiments; Y.H.K. conducted patch-clamp recordings in mouse and human neurons; X.W. generated Shank3 (De4 22) KO mice and Shank3 floxed mice and conducted PCR and western blot studies; D.L. conducted patch-clamp recordings in mouse DRG and spinal cord neurons; Z.-J.Z. did histochemical experiments; A.L.B. did genotyping; M.L. did in situ hybridization; W.C. conducted recordings in mouse DRG neurons; T.B. and Y.Z. performed immunostaining; R.-R.J. and Y.-H.J. conceived and supervised the project; and R.-R.J., Q.H., and Y.-H.J. wrote the paper. ACKNOWLEDGMENTS This project was supported in part by NIH RO1 grants NS87988, DE17794, and DE22743 to R.-R.J. and MH and MH to Y.-H.J. Received: April 14, 2016 Revised: September 4, 2016 Accepted: October 27, 2016 Published: December 1, 2016 REFERENCES Agarwal, N., Offermanns, S., and Kuner, R. (2004). Conditional gene deletion in primary nociceptive neurons of trigeminal ganglia and dorsal root ganglia. Genesis 38, Allely, C.S. (2013). Pain sensitivity and observer perception of pain in individuals with autistic spectrum disorder. Sci. World J. 2013, Bautista, D.M., Siemens, J., Glazer, J.M., Tsuruda, P.R., Basbaum, A.I., Stucky, C.L., Jordt, S.E., and Julius, D. (2007). The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, Neuron 92, 1 15, December 21,
A subpopulation of nociceptors specifically linked to itch
 Supplementary Information A subpopulation of nociceptors specifically linked to itch Liang Han 1, Chao Ma 3,4, Qin Liu 1,2, Hao-Jui Weng 1,2, Yiyuan Cui 5, Zongxiang Tang 1,2, Yushin Kim 1, Hong Nie 4,
Supplementary Information A subpopulation of nociceptors specifically linked to itch Liang Han 1, Chao Ma 3,4, Qin Liu 1,2, Hao-Jui Weng 1,2, Yiyuan Cui 5, Zongxiang Tang 1,2, Yushin Kim 1, Hong Nie 4,
Modulation of TRP channels by resolvins in mouse and human
 July 9, 2015, Ion Channel Retreat, Vancouver Ion Channel and Pain Targets Modulation of TRP channels by resolvins in mouse and human Ru-Rong Ji, PhD Pain Research Division Department of Anesthesiology
July 9, 2015, Ion Channel Retreat, Vancouver Ion Channel and Pain Targets Modulation of TRP channels by resolvins in mouse and human Ru-Rong Ji, PhD Pain Research Division Department of Anesthesiology
Sensory coding and somatosensory system
 Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
Charlie Taylor, PhD CpTaylor Consulting Chelsea, MI, USA
 Contribution of Calcium Channel α 2 δ Binding Sites to the Pharmacology of Gabapentin and Pregabalin Charlie Taylor, PhD CpTaylor Consulting Chelsea, MI, USA Disclosure Information Charlie Taylor, PhD
Contribution of Calcium Channel α 2 δ Binding Sites to the Pharmacology of Gabapentin and Pregabalin Charlie Taylor, PhD CpTaylor Consulting Chelsea, MI, USA Disclosure Information Charlie Taylor, PhD
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Introduction to some interesting research questions: Molecular biology of the primary afferent nociceptor
 Introduction to some interesting research questions: Molecular biology of the primary afferent nociceptor NOCICEPTORS ARE NOT IDENTICAL PEPTIDE SubP/CGRP Trk A NON-PEPTIDE IB4 P2X 3 c-ret Snider and McMahon
Introduction to some interesting research questions: Molecular biology of the primary afferent nociceptor NOCICEPTORS ARE NOT IDENTICAL PEPTIDE SubP/CGRP Trk A NON-PEPTIDE IB4 P2X 3 c-ret Snider and McMahon
SUPPLEMENTARY INFORMATION. The Calcium-activated Chloride Channel Anoctamin 1 acts as a Heat. Sensor in Nociceptive Neurons
 SUPPLEMENTARY INFORMATION The Calcium-activated Chloride Channel Anoctamin 1 acts as a Heat Sensor in Nociceptive Neurons Hawon Cho, Young Duk Yang, Jesun Lee, Byeongjoon Lee, Tahnbee Kim Yongwoo Jang,
SUPPLEMENTARY INFORMATION The Calcium-activated Chloride Channel Anoctamin 1 acts as a Heat Sensor in Nociceptive Neurons Hawon Cho, Young Duk Yang, Jesun Lee, Byeongjoon Lee, Tahnbee Kim Yongwoo Jang,
Supplementary Table 1. List of primers used in this study
 Supplementary Table 1. List of primers used in this study Gene Forward primer Reverse primer Rat Met 5 -aggtcgcttcatgcaggt-3 5 -tccggagacacaggatgg-3 Rat Runx1 5 -cctccttgaaccactccact-3 5 -ctggatctgcctggcatc-3
Supplementary Table 1. List of primers used in this study Gene Forward primer Reverse primer Rat Met 5 -aggtcgcttcatgcaggt-3 5 -tccggagacacaggatgg-3 Rat Runx1 5 -cctccttgaaccactccact-3 5 -ctggatctgcctggcatc-3
Potential for delta opioid receptor agonists as analgesics in chronic pain therapy
 Potential for delta opioid receptor agonists as analgesics in chronic pain therapy David Kendall & Bengt von Mentzer; PharmNovo AB/UK Alex Conibear & Eamonn Kelly, University of Bristol Junaid Asghar,
Potential for delta opioid receptor agonists as analgesics in chronic pain therapy David Kendall & Bengt von Mentzer; PharmNovo AB/UK Alex Conibear & Eamonn Kelly, University of Bristol Junaid Asghar,
Special Issue on Pain and Itch
 Special Issue on Pain and Itch Title: Recent Progress in Understanding the Mechanisms of Pain and Itch Guest Editor of the Special Issue: Ru-Rong Ji, PhD Chronic pain is a major health problem world-wide.
Special Issue on Pain and Itch Title: Recent Progress in Understanding the Mechanisms of Pain and Itch Guest Editor of the Special Issue: Ru-Rong Ji, PhD Chronic pain is a major health problem world-wide.
TMC9 as a novel mechanosensitive ion channel
 TMC9 as a novel mechanosensitive ion channel Mechanical forces play numerous roles in physiology. When an object contacts our skin, it exerts a force that is encoded as touch or pain depending on its intensity.
TMC9 as a novel mechanosensitive ion channel Mechanical forces play numerous roles in physiology. When an object contacts our skin, it exerts a force that is encoded as touch or pain depending on its intensity.
Seizure: the clinical manifestation of an abnormal and excessive excitation and synchronization of a population of cortical
 Are There Sharing Mechanisms of Epilepsy, Migraine and Neuropathic Pain? Chin-Wei Huang, MD, PhD Department of Neurology, NCKUH Basic mechanisms underlying seizures and epilepsy Seizure: the clinical manifestation
Are There Sharing Mechanisms of Epilepsy, Migraine and Neuropathic Pain? Chin-Wei Huang, MD, PhD Department of Neurology, NCKUH Basic mechanisms underlying seizures and epilepsy Seizure: the clinical manifestation
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
Supporting Information
 ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were
 Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Pharmacology of Pain Transmission and Modulation
 Pharmacology of Pain Transmission and Modulation 2 Jürg Schliessbach and Konrad Maurer Nociceptive Nerve Fibers Pain is transmitted to the central nervous system via thinly myelinated Aδ and unmyelinated
Pharmacology of Pain Transmission and Modulation 2 Jürg Schliessbach and Konrad Maurer Nociceptive Nerve Fibers Pain is transmitted to the central nervous system via thinly myelinated Aδ and unmyelinated
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Behavioural effects of ketamine in non-stressed and stressed mice. Naive C57BL/6 adult male mice (n=10/group) were given a single dose of saline vehicle or ketamine (3.0 mg/kg,
Supplementary Figure 1. Behavioural effects of ketamine in non-stressed and stressed mice. Naive C57BL/6 adult male mice (n=10/group) were given a single dose of saline vehicle or ketamine (3.0 mg/kg,
Intracellular signalling cascades associated with TRP channels
 Gerald Thiel Intracellular signalling cascades associated with TRP channels Current state of investigations and potential applications Saarland University, Germany Campus Saarbrücken Department of Medical
Gerald Thiel Intracellular signalling cascades associated with TRP channels Current state of investigations and potential applications Saarland University, Germany Campus Saarbrücken Department of Medical
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature06994 A phosphatase cascade by which rewarding stimuli control nucleosomal response A. Stipanovich*, E. Valjent*, M. Matamales*, A. Nishi, J.H. Ahn, M. Maroteaux, J. Bertran-Gonzalez,
doi: 10.1038/nature06994 A phosphatase cascade by which rewarding stimuli control nucleosomal response A. Stipanovich*, E. Valjent*, M. Matamales*, A. Nishi, J.H. Ahn, M. Maroteaux, J. Bertran-Gonzalez,
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Atlas representations of the midcingulate (MCC) region targeted in this study compared against the anterior cingulate (ACC) region commonly reported. Coronal sections are shown on
Supplementary Figure 1 Atlas representations of the midcingulate (MCC) region targeted in this study compared against the anterior cingulate (ACC) region commonly reported. Coronal sections are shown on
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5
 The Journal of Clinical Investigation CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5 Bao-Chun Jiang, 1 De-Li Cao, 1 Xin Zhang, 1 Zhi-Jun Zhang, 1,2 Li-Na He, 1 Chun-Hua Li, 1
The Journal of Clinical Investigation CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5 Bao-Chun Jiang, 1 De-Li Cao, 1 Xin Zhang, 1 Zhi-Jun Zhang, 1,2 Li-Na He, 1 Chun-Hua Li, 1
When cells are already maximally potentiated LTP is occluded.
 When cells are already maximally potentiated LTP is occluded. Stein, V et al., (2003) J Neurosci, 23:5503-6606. Also found in Rat Barrel Cortex Ehrlich & Malinow (2004) J. Neurosci. 24:916-927 Over-expression
When cells are already maximally potentiated LTP is occluded. Stein, V et al., (2003) J Neurosci, 23:5503-6606. Also found in Rat Barrel Cortex Ehrlich & Malinow (2004) J. Neurosci. 24:916-927 Over-expression
211MDS Pain theories
 211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
Social deficits in Shank3-deficient mouse models of autism are rescued by histone deacetylase (HDAC) inhibition
 SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41593-018-0110-8 In the format provided by the authors and unedited. Social deficits in Shank3-deficient mouse models of autism are rescued by
SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41593-018-0110-8 In the format provided by the authors and unedited. Social deficits in Shank3-deficient mouse models of autism are rescued by
Serotonergic Control of the Developing Cerebellum M. Oostland
 Serotonergic Control of the Developing Cerebellum M. Oostland Summary Brain development is a precise and crucial process, dependent on many factors. The neurotransmitter serotonin is one of the factors
Serotonergic Control of the Developing Cerebellum M. Oostland Summary Brain development is a precise and crucial process, dependent on many factors. The neurotransmitter serotonin is one of the factors
The Nervous System: Neural Tissue Pearson Education, Inc.
 13 The Nervous System: Neural Tissue Introduction Nervous System Characteristics Controls and adjust the activity of the body Provides swift but brief responses The nervous system includes: Central Nervous
13 The Nervous System: Neural Tissue Introduction Nervous System Characteristics Controls and adjust the activity of the body Provides swift but brief responses The nervous system includes: Central Nervous
LESSON 3.3 WORKBOOK. Why does applying pressure relieve pain?
 Postsynaptic potentials small changes in voltage (membrane potential) due to the binding of neurotransmitter. Receptor-gated ion channels ion channels that open or close in response to the binding of a
Postsynaptic potentials small changes in voltage (membrane potential) due to the binding of neurotransmitter. Receptor-gated ion channels ion channels that open or close in response to the binding of a
TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice
 Research article TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice Tong Liu, 1 Temugin Berta, 1 Zhen-Zhong Xu, 1 Chul-Kyu Park, 1 Ling Zhang, 1 Ning
Research article TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice Tong Liu, 1 Temugin Berta, 1 Zhen-Zhong Xu, 1 Chul-Kyu Park, 1 Ling Zhang, 1 Ning
Central Terminal Sensitization of TRPV1 by Descending Serotonergic Facilitation Modulates Chronic Pain
 Article Central Terminal Sensitization of TRPV1 by Descending Serotonergic Facilitation Modulates Chronic Pain Yu Shin Kim, 1,7 Yuxia Chu, 2,7 Liang Han, 1 Man Li, 2,3 Zhe Li, 1 Pamela Colleen LaVinka,
Article Central Terminal Sensitization of TRPV1 by Descending Serotonergic Facilitation Modulates Chronic Pain Yu Shin Kim, 1,7 Yuxia Chu, 2,7 Liang Han, 1 Man Li, 2,3 Zhe Li, 1 Pamela Colleen LaVinka,
Presynaptic NMDA receptor control of spontaneous and evoked activity By: Sally Si Ying Li Supervisor: Jesper Sjöström
 Presynaptic NMDA receptor control of spontaneous and evoked activity By: Sally Si Ying Li Supervisor: Jesper Sjöström NMDA receptors are traditionally known to function as post-synaptic coincidence detectors.
Presynaptic NMDA receptor control of spontaneous and evoked activity By: Sally Si Ying Li Supervisor: Jesper Sjöström NMDA receptors are traditionally known to function as post-synaptic coincidence detectors.
Activity Dependent Changes At the Developing Neuromuscular Junction
 Activity Dependent Changes At the Developing Neuromuscular Junction (slides 16, 17 and 18 have been slightly modified for clarity) MCP Lecture 2-3 9.013/7.68 04 Neuromuscular Junction Development 1. Muscle
Activity Dependent Changes At the Developing Neuromuscular Junction (slides 16, 17 and 18 have been slightly modified for clarity) MCP Lecture 2-3 9.013/7.68 04 Neuromuscular Junction Development 1. Muscle
Pain Pathways. Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH
 Pain Pathways Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH Objective To give you a simplistic and basic concepts of pain pathways to help understand the complex issue of pain Pain
Pain Pathways Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH Objective To give you a simplistic and basic concepts of pain pathways to help understand the complex issue of pain Pain
Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity
 Manuscript EMBO-2010-76298 Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity Yuki Fujita, Shota Endo, Toshiyuki Takai and Toshihide Yamashita Corresponding author: Toshihide
Manuscript EMBO-2010-76298 Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity Yuki Fujita, Shota Endo, Toshiyuki Takai and Toshihide Yamashita Corresponding author: Toshihide
Repeated intra-articular injections of acidic saline produce long-lasting joint pain and. widespread hyperalgesia
 Repeated intra-articular injections of acidic saline produce long-lasting joint pain and widespread hyperalgesia N. Sugimura 1, M. Ikeuchi 1 *, M. Izumi 1, T. Kawano 2, K. Aso 1, T. Kato 1, T. Ushida 3,
Repeated intra-articular injections of acidic saline produce long-lasting joint pain and widespread hyperalgesia N. Sugimura 1, M. Ikeuchi 1 *, M. Izumi 1, T. Kawano 2, K. Aso 1, T. Kato 1, T. Ushida 3,
Part 11: Mechanisms of Learning
 Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Lecture 22: A little Neurobiology
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
Risk Factors That Predispose Patients to Orofacial Pain
 Risk Factors That Predispose Patients to Orofacial Pain Paul Durham, PhD Distinguished Professor of Cell Biology Director Center for Biomedical & Life Sciences Missouri State University Cluster Headache
Risk Factors That Predispose Patients to Orofacial Pain Paul Durham, PhD Distinguished Professor of Cell Biology Director Center for Biomedical & Life Sciences Missouri State University Cluster Headache
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Pathophysiology of Pain
 Pathophysiology of Pain Wound Inflammatory response Chemical mediators Activity in Pain Path PAIN http://neuroscience.uth.tmc.edu/s2/chapter08.html Chris Cohan, Ph.D. Dept. of Pathology/Anat Sci University
Pathophysiology of Pain Wound Inflammatory response Chemical mediators Activity in Pain Path PAIN http://neuroscience.uth.tmc.edu/s2/chapter08.html Chris Cohan, Ph.D. Dept. of Pathology/Anat Sci University
Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +.
![Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +. Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +.](/thumbs/90/102196023.jpg) Research Interests Advanced cognitive learning is encoded in distributed circuits that span multiple forebrain areas. Further, synaptic plasticity and neural network theories hypothesize that essential
Research Interests Advanced cognitive learning is encoded in distributed circuits that span multiple forebrain areas. Further, synaptic plasticity and neural network theories hypothesize that essential
Analgesia Mediated by the TRPM8 Cold Receptor in Chronic Neuropathic Pain
 Current Biology 16, 1591 1605, August 22, 2006 ª2006 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2006.07.061 Analgesia Mediated by the TRPM8 Cold Receptor in Chronic Neuropathic Pain Article Clare
Current Biology 16, 1591 1605, August 22, 2006 ª2006 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2006.07.061 Analgesia Mediated by the TRPM8 Cold Receptor in Chronic Neuropathic Pain Article Clare
CHAPTER 10 THE SOMATOSENSORY SYSTEM
 CHAPTER 10 THE SOMATOSENSORY SYSTEM 10.1. SOMATOSENSORY MODALITIES "Somatosensory" is really a catch-all term to designate senses other than vision, hearing, balance, taste and smell. Receptors that could
CHAPTER 10 THE SOMATOSENSORY SYSTEM 10.1. SOMATOSENSORY MODALITIES "Somatosensory" is really a catch-all term to designate senses other than vision, hearing, balance, taste and smell. Receptors that could
How Nicotinic Signaling Shapes Neural Networks
 How Nicotinic Signaling Shapes Neural Networks Darwin K. Berg Division of Biological Sciences University of California, San Diego Nicotinic Cholinergic Signaling Uses the transmitter ACh to activate cation-selective
How Nicotinic Signaling Shapes Neural Networks Darwin K. Berg Division of Biological Sciences University of California, San Diego Nicotinic Cholinergic Signaling Uses the transmitter ACh to activate cation-selective
(a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable
 Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Diabetic Complications Consortium
 Diabetic Complications Consortium Application Title: Cathepsin S inhibition and diabetic neuropathy Principal Investigator: Nigel A Calcutt 1. Project Accomplishments: We investigated the efficacy of cathepsin
Diabetic Complications Consortium Application Title: Cathepsin S inhibition and diabetic neuropathy Principal Investigator: Nigel A Calcutt 1. Project Accomplishments: We investigated the efficacy of cathepsin
PAIN MANAGEMENT in the CANINE PATIENT
 PAIN MANAGEMENT in the CANINE PATIENT Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physio), CAFCI, CCRT Part 1: Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physio), CAFCI, CCRT 1 Pain is the most common reason
PAIN MANAGEMENT in the CANINE PATIENT Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physio), CAFCI, CCRT Part 1: Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physio), CAFCI, CCRT 1 Pain is the most common reason
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1. SybII and Ceb are sorted to distinct vesicle populations in astrocytes. Nature Neuroscience: doi: /nn.
 Supplementary Figure 1 SybII and Ceb are sorted to distinct vesicle populations in astrocytes. (a) Exemplary images for cultured astrocytes co-immunolabeled with SybII and Ceb antibodies. SybII accumulates
Supplementary Figure 1 SybII and Ceb are sorted to distinct vesicle populations in astrocytes. (a) Exemplary images for cultured astrocytes co-immunolabeled with SybII and Ceb antibodies. SybII accumulates
The Egyptian Journal of Hospital Medicine (January 2018) Vol. 70 (12), Page
 The Egyptian Journal of Hospital Medicine (January 2018) Vol. 70 (12), Page 2172-2177 Blockage of HCN Channels with ZD7288 Attenuates Mechanical Hypersensitivity in Rats Model of Diabetic Neuropathy Hussain
The Egyptian Journal of Hospital Medicine (January 2018) Vol. 70 (12), Page 2172-2177 Blockage of HCN Channels with ZD7288 Attenuates Mechanical Hypersensitivity in Rats Model of Diabetic Neuropathy Hussain
ANATOMY AND PHYSIOLOGY OF NEURONS. AP Biology Chapter 48
 ANATOMY AND PHYSIOLOGY OF NEURONS AP Biology Chapter 48 Objectives Describe the different types of neurons Describe the structure and function of dendrites, axons, a synapse, types of ion channels, and
ANATOMY AND PHYSIOLOGY OF NEURONS AP Biology Chapter 48 Objectives Describe the different types of neurons Describe the structure and function of dendrites, axons, a synapse, types of ion channels, and
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Introduction to Neurobiology
 Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
LESSON 3.3 WORKBOOK. Why does applying pressure relieve pain? Workbook. Postsynaptic potentials
 Depolarize to decrease the resting membrane potential. Decreasing membrane potential means that the membrane potential is becoming more positive. Excitatory postsynaptic potentials (EPSP) graded postsynaptic
Depolarize to decrease the resting membrane potential. Decreasing membrane potential means that the membrane potential is becoming more positive. Excitatory postsynaptic potentials (EPSP) graded postsynaptic
Chapter 37&38. Nervous Systems. EQ: How do animals sense and respond to the world around them?
 Chapter 37&38 Nervous Systems EQ: How do animals sense and respond to the world around them? The Nervous System Function? sense the internal and external environment, coordinate actions, transmit response
Chapter 37&38 Nervous Systems EQ: How do animals sense and respond to the world around them? The Nervous System Function? sense the internal and external environment, coordinate actions, transmit response
TGF-β Signaling Regulates Neuronal C1q Expression and Developmental Synaptic Refinement
 Supplementary Information Title: TGF-β Signaling Regulates Neuronal C1q Expression and Developmental Synaptic Refinement Authors: Allison R. Bialas and Beth Stevens Supplemental Figure 1. In vitro characterization
Supplementary Information Title: TGF-β Signaling Regulates Neuronal C1q Expression and Developmental Synaptic Refinement Authors: Allison R. Bialas and Beth Stevens Supplemental Figure 1. In vitro characterization
Module H NERVOUS SYSTEM
 Module H NERVOUS SYSTEM Topic from General functions of the nervous system Organization of the nervous system from both anatomical & functional perspectives Gross & microscopic anatomy of nervous tissue
Module H NERVOUS SYSTEM Topic from General functions of the nervous system Organization of the nervous system from both anatomical & functional perspectives Gross & microscopic anatomy of nervous tissue
Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007)
 Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007) Introduction Adrian s work on sensory coding Spinal cord and dorsal root ganglia Four somatic sense modalities Touch Mechanoreceptors
Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007) Introduction Adrian s work on sensory coding Spinal cord and dorsal root ganglia Four somatic sense modalities Touch Mechanoreceptors
Receptors and Neurotransmitters: It Sounds Greek to Me. Agenda. What We Know About Pain 9/7/2012
 Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
MOLECULAR AND CELLULAR NEUROSCIENCE
 MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
Nervous System Review
 Nervous System Review Name: Block: 1. Which processes are involved in the movement of molecule Y from point X to point Z? A. exocytosis and diffusion B. endocytosis and diffusion C. exocytosis and facilitated
Nervous System Review Name: Block: 1. Which processes are involved in the movement of molecule Y from point X to point Z? A. exocytosis and diffusion B. endocytosis and diffusion C. exocytosis and facilitated
Chapter Nervous Systems
 The Nervous System Chapter Nervous Systems Which animals have nervous systems? (Which do not) What are the basic components of a NS? What kind of fish performs brain operations? What differentiates one
The Nervous System Chapter Nervous Systems Which animals have nervous systems? (Which do not) What are the basic components of a NS? What kind of fish performs brain operations? What differentiates one
Supplemental information Acid-sensing ion channel 1a contributes to hippocampal LTP inducibility through multiple mechanisms
 Supplemental information Acid-sensing ion channel 1a contributes to hippocampal LTP inducibility through multiple mechanisms Ming-Gang Liu, Hu-Song Li, Wei-Guang Li, Yan-Jiao Wu, Shi-Ning Deng, Chen Huang,
Supplemental information Acid-sensing ion channel 1a contributes to hippocampal LTP inducibility through multiple mechanisms Ming-Gang Liu, Hu-Song Li, Wei-Guang Li, Yan-Jiao Wu, Shi-Ning Deng, Chen Huang,
Resolving TRPV1- and TNF- -Mediated Spinal Cord Synaptic Plasticity and Inflammatory Pain with Neuroprotectin D1
 15072 The Journal of Neuroscience, October 19, 2011 31(42):15072 15085 Neurobiology of Disease Resolving TRPV1- and TNF- -Mediated Spinal Cord Synaptic Plasticity and Inflammatory Pain with Neuroprotectin
15072 The Journal of Neuroscience, October 19, 2011 31(42):15072 15085 Neurobiology of Disease Resolving TRPV1- and TNF- -Mediated Spinal Cord Synaptic Plasticity and Inflammatory Pain with Neuroprotectin
What Cell Make Up the Brain and Spinal Cord
 What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Applied Neuroscience. Conclusion of Science Honors Program Spring 2017
 Applied Neuroscience Conclusion of Science Honors Program Spring 2017 Review Circle whichever is greater, A or B. If A = B, circle both: I. A. permeability of a neuronal membrane to Na + during the rise
Applied Neuroscience Conclusion of Science Honors Program Spring 2017 Review Circle whichever is greater, A or B. If A = B, circle both: I. A. permeability of a neuronal membrane to Na + during the rise
Ube3a is required for experience-dependent maturation of the neocortex
 Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
The Pain Pathway. dorsal root ganglion. primary afferent nociceptor. TRP: Transient Receptor Potential
 Presented by Issel Anne L. Lim 1 st Year PhD Candidate Biomedical Engineering Johns Hopkins University 580.427/580.633 Ca Signals in Biological Systems Outline The Pain Pathway TRP: Transient Receptor
Presented by Issel Anne L. Lim 1 st Year PhD Candidate Biomedical Engineering Johns Hopkins University 580.427/580.633 Ca Signals in Biological Systems Outline The Pain Pathway TRP: Transient Receptor
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
SYNAPTIC COMMUNICATION
 BASICS OF NEUROBIOLOGY SYNAPTIC COMMUNICATION ZSOLT LIPOSITS 1 NERVE ENDINGS II. Interneuronal communication 2 INTERNEURONAL COMMUNICATION I. ELECTRONIC SYNAPSE GAP JUNCTION II. CHEMICAL SYNAPSE SYNAPSES
BASICS OF NEUROBIOLOGY SYNAPTIC COMMUNICATION ZSOLT LIPOSITS 1 NERVE ENDINGS II. Interneuronal communication 2 INTERNEURONAL COMMUNICATION I. ELECTRONIC SYNAPSE GAP JUNCTION II. CHEMICAL SYNAPSE SYNAPSES
Supplemental Figure 1. Western blot analysis indicated that MIF was detected in the fractions of
 Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Synaptic plasticityhippocampus. Neur 8790 Topics in Neuroscience: Neuroplasticity. Outline. Synaptic plasticity hypothesis
 Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Psychophysical laws. Legge di Fechner: I=K*log(S/S 0 )
 Psychophysical laws Legge di Weber: ΔS=K*S Legge di Fechner: I=K*log(S/S 0 ) Sensory receptors Vision Smell Taste Touch Thermal senses Pain Hearing Balance Proprioception Sensory receptors Table 21-1 Classification
Psychophysical laws Legge di Weber: ΔS=K*S Legge di Fechner: I=K*log(S/S 0 ) Sensory receptors Vision Smell Taste Touch Thermal senses Pain Hearing Balance Proprioception Sensory receptors Table 21-1 Classification
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11905 WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 Quantification of integrated chorda tympani nerve responses. Quantification
SUPPLEMENTARY INFORMATION doi:10.1038/nature11905 WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 Quantification of integrated chorda tympani nerve responses. Quantification
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Lesson 14. The Nervous System. Introduction to Life Processes - SCI 102 1
 Lesson 14 The Nervous System Introduction to Life Processes - SCI 102 1 Structures and Functions of Nerve Cells The nervous system has two principal cell types: Neurons (nerve cells) Glia The functions
Lesson 14 The Nervous System Introduction to Life Processes - SCI 102 1 Structures and Functions of Nerve Cells The nervous system has two principal cell types: Neurons (nerve cells) Glia The functions
Nature Neuroscience: doi: /nn Supplementary Figure 1. Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons
 Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
Electron micrograph of phosphotungstanic acid-stained exosomes derived from murine
 1 SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Physical properties of murine DC-derived exosomes. a, Electron micrograph of phosphotungstanic acid-stained exosomes derived from
1 SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Physical properties of murine DC-derived exosomes. a, Electron micrograph of phosphotungstanic acid-stained exosomes derived from
Reversing the Effects of Fragile X Syndrome
 CLINICAL IMPLICATIONS OF BASIC RESEARCH Paul J. Lombroso, M.D., Marilee P. Ogren, Ph.D. Assistant Editors Reversing the Effects of Fragile X Syndrome MARILEE P. OGREN, PH.D., AND PAUL J. LOMBROSO, M.D.
CLINICAL IMPLICATIONS OF BASIC RESEARCH Paul J. Lombroso, M.D., Marilee P. Ogren, Ph.D. Assistant Editors Reversing the Effects of Fragile X Syndrome MARILEE P. OGREN, PH.D., AND PAUL J. LOMBROSO, M.D.
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Ch. 47 Somatic Sensations: Tactile and Position Senses (Reading Homework) - Somatic senses: three types (1) Mechanoreceptive somatic senses: tactile
 Ch. 47 Somatic Sensations: Tactile and Position Senses (Reading Homework) - Somatic senses: three types (1) Mechanoreceptive somatic senses: tactile and position sensations (2) Thermoreceptive senses:
Ch. 47 Somatic Sensations: Tactile and Position Senses (Reading Homework) - Somatic senses: three types (1) Mechanoreceptive somatic senses: tactile and position sensations (2) Thermoreceptive senses:
Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture
 Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture Nanna Goldman *, Michael Chen *, Takumi Fujita *, Qiwu Xu, Weiguo Peng, Wei Liu, Tina K. Jensen, Yong Pei, Fushun Wang, Xiaoning
Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture Nanna Goldman *, Michael Chen *, Takumi Fujita *, Qiwu Xu, Weiguo Peng, Wei Liu, Tina K. Jensen, Yong Pei, Fushun Wang, Xiaoning
Nature Neuroscience: doi: /nn Supplementary Figure 1. Iliopsoas and quadratus lumborum motor neurons in the L2 spinal segment.
 Supplementary Figure 1 Iliopsoas and quadratus lumborum motor neurons in the L2 spinal segment. (A) IL and QL motor neurons were labeled after CTb-488 (green) muscle injections at birth. At P4, the L2
Supplementary Figure 1 Iliopsoas and quadratus lumborum motor neurons in the L2 spinal segment. (A) IL and QL motor neurons were labeled after CTb-488 (green) muscle injections at birth. At P4, the L2
Coding of Sensory Information
 Coding of Sensory Information 22 November, 2016 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Sensory Systems Mediate Four Attributes of
Coding of Sensory Information 22 November, 2016 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Sensory Systems Mediate Four Attributes of
SUPPLEMENTARY INFORMATION. Supplementary Figure 1
 SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
EE 791 Lecture 2 Jan 19, 2015
 EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
Glycine-gated ion channels Converging mechanism and therapeutic potentials
 Glycine-gated ion channels Converging mechanism and therapeutic potentials Li Zhang Laboratory for Integrative Neuroscience, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health,
Glycine-gated ion channels Converging mechanism and therapeutic potentials Li Zhang Laboratory for Integrative Neuroscience, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health,
293T cells were transfected with indicated expression vectors and the whole-cell extracts were subjected
 SUPPLEMENTARY INFORMATION Supplementary Figure 1. Formation of a complex between Slo1 and CRL4A CRBN E3 ligase. (a) HEK 293T cells were transfected with indicated expression vectors and the whole-cell
SUPPLEMENTARY INFORMATION Supplementary Figure 1. Formation of a complex between Slo1 and CRL4A CRBN E3 ligase. (a) HEK 293T cells were transfected with indicated expression vectors and the whole-cell
Chapter 17. Nervous System Nervous systems receive sensory input, interpret it, and send out appropriate commands. !
 Chapter 17 Sensory receptor Sensory input Integration Nervous System Motor output Brain and spinal cord Effector cells Peripheral nervous system (PNS) Central nervous system (CNS) 28.1 Nervous systems
Chapter 17 Sensory receptor Sensory input Integration Nervous System Motor output Brain and spinal cord Effector cells Peripheral nervous system (PNS) Central nervous system (CNS) 28.1 Nervous systems
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress REDUCING THE PAIN FACTOR AN UPDATE ON PERI-OPERATIVE ANALGESIA Sandra Forysth, BVSc DipACVA Institute of Veterinary,
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress REDUCING THE PAIN FACTOR AN UPDATE ON PERI-OPERATIVE ANALGESIA Sandra Forysth, BVSc DipACVA Institute of Veterinary,
Nervous System. 2. Receives information from the environment from CNS to organs and glands. 1. Relays messages, processes info, analyzes data
 Nervous System 1. Relays messages, processes info, analyzes data 2. Receives information from the environment from CNS to organs and glands 3. Transmits impulses from CNS to muscles and glands 4. Transmits
Nervous System 1. Relays messages, processes info, analyzes data 2. Receives information from the environment from CNS to organs and glands 3. Transmits impulses from CNS to muscles and glands 4. Transmits
Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission
 Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission Xiaona Du, 1 Han Hao, 1 Yuehui Yang, 1 Sha Huang, 1 Caixue Wang, 1 Sylvain Gigout, 2 Rosmaliza Ramli, 2,3 Xinmeng
Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission Xiaona Du, 1 Han Hao, 1 Yuehui Yang, 1 Sha Huang, 1 Caixue Wang, 1 Sylvain Gigout, 2 Rosmaliza Ramli, 2,3 Xinmeng
Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent neurons innervating the dura
 Washington University School of Medicine Digital Commons@Becker Open Access Publications 212 Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent
Washington University School of Medicine Digital Commons@Becker Open Access Publications 212 Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent
Medical Neuroscience Tutorial
 Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
