Name: Anna Dempniak Date: Wednesday 13 th 2018 Teacher: McGuckin Course code: SCH4UP. The Preparation of Esters
|
|
|
- Melvyn Snow
- 5 years ago
- Views:
Transcription
1 Name: Anna Dempniak Date: Wednesday 13 th 2018 Teacher: McGuckin Course code: SCH4UP The Preparation of Esters
2 Dempniak 2 Abstract The purpose of this lab was to observe the scents of six different esters. Esters were formed during the experiment by combining sulfuric acid, one of six alcohols, and one of four carboxylic acids in a test tube. The test tube was heated in a water bath on a hot plate to speed up the reaction, then mixed with distilled water and left to cool in ice water. This process was repeated six times with different alcohols and carboxylic acids resulting in six test tubes each containing a different type of ester. Each test tube was smelled and its scent was compared to the anticipated scent (based on the ester product of the predicted reaction). Test tubes three and six matched the predicted scent, while test tubes one and four kept some aspects of their predicted scent. Test tubes two and five held no trace of the predicted smell. Introduction Although most people are unaware of their existence, esters are one of the key pillars that make up life. Ester bonds can be found everywhere from the fat that makes up your leg to the phosphodiester backbone that forms DNA to the fragrant smell coming off an orange. (Pure Chemicals 2018) So what exactly is an ester? An ester is an organic compound containing at least two carbon chains and two oxygen atoms. The carbon chains must be connected to each other by one of the oxygen atoms, and one of the connecting carbon atoms must be double-bonded to the other oxygen atom.(bbc, 2014) Typically esters are created through a condensation reaction called esterfication. In esterfication, an organic compound containing a -OH group (an alcohol) is combined with an organic compound containing a -COOH group (a carboxylic acid). (BBC, 2014) The two hydrogen atoms from the two hydroxide groups in each compound pair with one of the oxygens from the hydroxide groups to form water, leaving the former alcohol and carboxylic acid connected by the remaining oxygen. One thing that esters are famous for is their unique odours and fragrances. Esters usually contain weak intermolecular forces which allows ester molecules to easily become gaseous and thus inhaled. (Dissenter 2014) Esters are responsible for the smells in many common fruits and plants, ranging from bananas to mint. (The Editors of Encyclopaedia Britannica, 2018) For example the ester Pentyl acetate smells like apples while Propyl acetace smells like pears. Esters include a whole range of easily available flavors: Methyl Salicylate smells minty, Ethyl butyrate has a pineapple scent, Isobutyl formate is described ethereal and slightly fruity, and Octyl acetate is the ester that gives oranges their
3 scent. (Pub Chem, 2018) Each combination of different carboxylic acid and alcohol results in a Dempniak 3 different ester with its own unique smell hence many different fragrances can be created using esters. Purpose The purpose of this lab was to observe the scent of six different esters by combing the necessary alcohol and carboxylic acid. Materials and Equipment 6 test tubes 1 mlmethanol pencil 1 ml ethanol Test tube rack 1 ml isobutyl alcohol Eyedropper 1 ml 2-methylpropanol (isobutyl alcohol) Scale 1 ml1-octanol 2 x 250 ml beakers 3 ml Glacial acetic acid 10mL graduated cylinder 1 ml Formic Acid Thermometer 1 ml Salicylic acid Safety googles 1 ml Butanoic acid Concentrated sulfuric acid 1mL 1-propanol 1-pentanol Procedure 1. Test tubes were labelled with the numbers one to six using pencil ml of tap water in a 250mL beaker was heated on hot plate to 60 C. 3. Alcohols were added to test tubes as follows:1 ml of 1-pentanol was added to test tube one, 1 ml of 1-propanol was added to test tube two, 1 ml of methanol was added to test tube three, 1 ml of ethanol was added to test tube four, 1 ml of isobutyl alcohol was added to test tube five, and 1 ml of 1-octanol was added to test tube six. 4. Four drops of sulphuric acid was added to each test tube. 5. One gram of salicyclic acid was weighed on scale. 6. Acids were added to test tubes as follows:1 ml of acetic acid was added to test tube one, two and six, 1 g of salicyclic acid was added to test tube three, 1 ml of butanoic acid was added to test tube four, and 1 ml of formic acid was added to test tube five.
4 7. Test tubes were heated in prepared beaker of hot water for fifteen minutes. 8. Test tubes were taken off water bath cooled in a beaker of cold water ml of water was added to each of the test tubes. 10. Test tubes were wafted and sniffed individually. The smell of each test tube was recorded. Results Odour of Test Tubes after Procedure Test tube Name of Ester Formed Predicted Odour Odour 1 Pentyl acetate Bananas/apples Slightly fruity with chemical smell Dempniak 4 2 Propyl acetate Pears Smells like nail polish remover 3 Methyl Salicylate Sweet, wintergreen Minty 4 Ethyl butyrate Pineapple Slightly fruity possibly apple smell with chemical smell 5 Isobutyl formate Ethereal, slightly fruity Smells like paint thinner 6 Octyl acetate Orange flavour Orange candy flavor Balanced Equations Test Tube 1 : Formation of Pentyl Acetate CH 3 COOH + C 5 H 12 O C 7 H 14 O 2 + H 2 O Test Tube 2 : Formation of Propyl Acetace CH 3 COOH + C 3 H 8 O C 5 H 10 O 2 + H 2 O Test Tube 3 : Formation of Methyl Salicylate C 7 H 6 O 3 + CH 3 OH C 8 H 8 O 3 + H 2 O Test Tube 4 : Formation of Ethyl Butyrate C 4 H 8 O 2 + C 2 H 6 O C 6 H 12 O 2 + H 2 O Test Tube 5 : Formation of Isobutyl formate CH 2 O 2 + C 4 H 10 O C 5 H 10 O 2 + H 2 O Test Tube 6 : Formation of Octyl acetate CH 3 COOH + C 8 H 18 O C 10 H 20 O 2 + H 2 O
5 Dempniak 5 Discussion Esters are a group of organic compounds noted for their wide range of odours and flavors. Since esters are created by mixing a carboxylic acid and an alcohol it is possible to synthesize a specific scent by combining the right carboxylic acid and alcohol. (The Editors of Encyclopaedia Britannica, 2018) This experiment attempted to create six different scents using four different carboxylic acids and six different alcohols. Pentyl acetate, which is known for its apple flavor, was formed using pentanol and acetic acid. Propyl acetate, known for a pear scent, was created from propanol and acetic acid. Likewise methyl salicylate (wintergreen scent) was formed from methanol and salicyclic acid, and ethyl butyrate (pineapple scent) was made from ethanol and butanoic acid. Octyl acetate (orange smell) was made from octanol and acetic acid. Finally isobutyl alcohol and formic acid were used to create isobutyl formate which was expected to have a fruity, ethereal smell. While the esters were relatively easy to synthesize in the laboratory, the majority of the esters formed did not give off the expected scent. Methyl salicyclate and Octyl acetate matched the predicted scent fairly closely. Pentyl acetate and ethyl burate smelled predominantly like chemicals but contained a slight fruity scent though it was hard to tell what fruit it was. Isobutyl formate and propyl acetate did not smell remotely ethereal or pear-like but smelled like paint thinner. The discrepancies between the predicted results and the observed results could be for a number of reasons. For one, throughout the entire experiment the fume-hood ran, ventilating the carboxylic acids. This spread a distinct chemical scent around the room which likely interfered with the smells of the esters even when they were taken outside to be tested. Secondly, during the experiment it was hard to come up with the correct words to describe smells and two people could describe the same smell somewhat differently. Compared to sight or sound, smell is a less objective sense. Anyone can tell if a chemical turns green, and it is easy to describe the visual difference. With smell however there is no set words to really describe smells except by comparing them to other scents or using vague words. For example isobutyl formate is described as an ethereal scent, ethereal being defined as delicate in a way that seems too perfect for this world (Merriam Webster, 2018). This description does not set down any clear guidelines as what someone considers delicate and perfect scent completely changes depending on the person. It is possible that although a particular scent may be deemed to have an apple flavor in this experiment, another experimenter might consider that flavor more of a pear scent. Finally, during the experiment no time was set aside for the experimenters nose to clear in between smelling test
6 Dempniak 6 tubes. When comparing flavors of cheese or wine usually there is some kind of palate cleanser in between samples so that the flavor of the last sample does not affect the flavor of the next. It is possible that since there was no time for the nose s palate to clear, the previous test tube s scent affected the next test tube s perceived scent. Although these sources of error could explain smaller discrepancies in scents theycannot account for the huge discrepancy between a fruity ethereal smell and the smell of paint thinner. Possibly the esters were not diluted enough and as a result the smells came across too strong. In order to determine for certain why the scents were so off the mark, further experimentation or research would be necessary. Despite the failure of this particular experiment to produce predictable and useful scents, esterfication has many uses in industry. Parabens, a type of ester that inhibits the growth of moulds and yeast, is used as a preservative in food and drugs.(pure Chemicals 2018) Manufacturers use esters with pleasant odours as ingredients in perfumes, as food flavourings that improve the smell and taste of processed food. Esters can be added to essential oils, cosmetics, and more to enhance their appeal to the consumer. (Pure Chemicals 2018) As its name suggests, esters are necessary to the manufacturing of polyester. Conclusion This experiment, although it did successfully form esters in all the test tubes, did not produce the expected smell in test tubes two and five. The reason for this discrepancy requires further experimentation or research before making any strong conclusions.
7 Dempniak 7 References Admin. (2015, September 9). Esters: Its Chemical Nature, Properties and Uses. Retrieved June 14, 2018, from Pure Chemicals website: Carboxylic acids and esters. (2014). Retrieved June 14, 2018, from BBC website: _acids_esters/revision/5/ Clark, J. (2014). Introducing esters. Retrieved June 14, 2018, from Dissenter, J. (2014, February). Why do esters actually smell? Retrieved June 14, 2018, from The Editors of Encyclopaedia Britannica. (2018). Ester. Retrieved June 14, 2018, from Ethereal. (2018, June 15). Retrieved June 14, 2018, from Merriam Webster website: Isobutyl Formate. (n.d.). Retrieved June 14, 2018, from Pub Chem website:
CCMR Educational Programs
 CCMR Educational Programs Title: Date Created: July 21, 2006 Author(s): Appropriate Level: Abstract: Time Requirement: Frank La Gatta Esterfication Regents and Honors Chemistry An ester is produced when
CCMR Educational Programs Title: Date Created: July 21, 2006 Author(s): Appropriate Level: Abstract: Time Requirement: Frank La Gatta Esterfication Regents and Honors Chemistry An ester is produced when
The Preparation of Fragrant Esters
 The Objective Esters are the product of reaction of an organic (carboxylic acid) with an alcohol. Many esters are components of the essential oils of flowers and fruits. Several esters with pleasant fragrances
The Objective Esters are the product of reaction of an organic (carboxylic acid) with an alcohol. Many esters are components of the essential oils of flowers and fruits. Several esters with pleasant fragrances
12AL Experiment 8 (3 days): Synthesis of Isopentyl Acetate (aka: Banana Oil)
 12AL Experiment 8 (3 days): Synthesis of Isopentyl Acetate (aka: Banana Oil) Instructor Note: Day 1 (half of the class); Day 2 (other half); Day 3 (all students to finish up separation & purification);
12AL Experiment 8 (3 days): Synthesis of Isopentyl Acetate (aka: Banana Oil) Instructor Note: Day 1 (half of the class); Day 2 (other half); Day 3 (all students to finish up separation & purification);
TOPIC 5: ORGANIC CHEMISTRY APPENDICES
 TPI 5: RGANI HEMISTRY APPENDIES Appendix 5.1: Underwater Fireworks 3 Appendix 5.2: Preparation of Esters 6 Appendix 5.3: rganic Model-Building Presentation 9 Appendix 5.4: Esters: Flavours and Fragrances
TPI 5: RGANI HEMISTRY APPENDIES Appendix 5.1: Underwater Fireworks 3 Appendix 5.2: Preparation of Esters 6 Appendix 5.3: rganic Model-Building Presentation 9 Appendix 5.4: Esters: Flavours and Fragrances
A carboxylic acid is an organic compound that contains a carboxyl group, COOH
 1.6 Carboxylic Acids, Esters and Fats Carboxylic Acids A carboxylic acid is an organic compound that contains a carboxyl group, COOH These compounds are weak acids. Citrus fruits, crabapples, rhubarb,
1.6 Carboxylic Acids, Esters and Fats Carboxylic Acids A carboxylic acid is an organic compound that contains a carboxyl group, COOH These compounds are weak acids. Citrus fruits, crabapples, rhubarb,
Unit 2: Nature s Chemistry Topic 2 Consumer Products Summary Notes
 St Ninian s High School Chemistry Department National 5 Chemistry Unit 2: Nature s Chemistry Topic 2 Consumer Products Summary Notes Name Learning Outcomes After completing this topic you should be able
St Ninian s High School Chemistry Department National 5 Chemistry Unit 2: Nature s Chemistry Topic 2 Consumer Products Summary Notes Name Learning Outcomes After completing this topic you should be able
Ch14. Carboxylic Acids. Combining the hydroxyl and carbonyl functional groups. To make more powerful functional groups. version 1.
 Ch14 Carboxylic Acids Combining the hydroxyl and carbonyl functional groups. To make more powerful functional groups. version 1.0 Nick DeMello, PhD. 2007-2015 Ch14 Carboxylic Acids & Esters Carboxylic
Ch14 Carboxylic Acids Combining the hydroxyl and carbonyl functional groups. To make more powerful functional groups. version 1.0 Nick DeMello, PhD. 2007-2015 Ch14 Carboxylic Acids & Esters Carboxylic
22. The Fischer Esterification
 22. The Fischer Esterification A. Background Esters are an incredibly important functional group in organic chemistry. Esters are typically very pleasant smelling molecules and are therefore frequently
22. The Fischer Esterification A. Background Esters are an incredibly important functional group in organic chemistry. Esters are typically very pleasant smelling molecules and are therefore frequently
EXPERIMENT 8 (Organic Chemistry II) Carboxylic Acids Reactions and Derivatives
 EXPERIMENT 8 (rganic Chemistry II) Carboxylic Acids Reactions and Derivatives Pahlavan/Cherif Materials Medium test tubes (6) Test tube rack Beakers (50, 150, 400 ml) Ice Hot plate Graduated cylinders
EXPERIMENT 8 (rganic Chemistry II) Carboxylic Acids Reactions and Derivatives Pahlavan/Cherif Materials Medium test tubes (6) Test tube rack Beakers (50, 150, 400 ml) Ice Hot plate Graduated cylinders
Ch07. Carboxylic Acids. Combining the hydroxyl and carbonyl functional groups. To make organic acids. version 1.0
 Ch07 Carboxylic Acids Combining the hydroxyl and carbonyl functional groups. To make organic acids. version 1.0 Nick DeMello, PhD. 2007-2015 Important Dates This Wednesday: - Lab Checkout (you must check
Ch07 Carboxylic Acids Combining the hydroxyl and carbonyl functional groups. To make organic acids. version 1.0 Nick DeMello, PhD. 2007-2015 Important Dates This Wednesday: - Lab Checkout (you must check
Ex17. Analgesics, TLC Analysis. Analgesics. The Experiment. Part A. Carboxylic Acids. Part B. Willow Bark Esters & Esterification
 Ex17 Analgesics, TLC Analysis Analgesics Carboxylic Acids Structure Properties Willow Bark Esters & Esterification The Experiment Part A Use TLC to Separate Compounds Part B Determine Elution Solvents
Ex17 Analgesics, TLC Analysis Analgesics Carboxylic Acids Structure Properties Willow Bark Esters & Esterification The Experiment Part A Use TLC to Separate Compounds Part B Determine Elution Solvents
Prelab 6: Carboxylic Acids
 The Structure of Carboxylic Acids Prelab 6: Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group,
The Structure of Carboxylic Acids Prelab 6: Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group,
Preparation of Banana Oil
 Preparation of Banana Oil Introduction Many of the simple esters have pleasant fragrances which we find similar to that of fruits and flowers. These esters have been synthesized in laboratories and are
Preparation of Banana Oil Introduction Many of the simple esters have pleasant fragrances which we find similar to that of fruits and flowers. These esters have been synthesized in laboratories and are
Summary Consumer Products
 Summary Consumer Products National 4 Carbohydrates are naturally occurring compounds which contain the elements Carbon, Hydrogen and Oxygen, with the Hydrogen and Oxygen in the ratio of two to one. Plants
Summary Consumer Products National 4 Carbohydrates are naturally occurring compounds which contain the elements Carbon, Hydrogen and Oxygen, with the Hydrogen and Oxygen in the ratio of two to one. Plants
Carboxylic Acids, Esters and Acyl Chlorides
 R hemistry A 432 arboxylic Acids, Esters and Acyl hlorides arboxylic Acids, Esters and Acyl hlorides arboxylic acids contain the functional group, attached to an alkyl stem. They are widely found in nature,
R hemistry A 432 arboxylic Acids, Esters and Acyl hlorides arboxylic Acids, Esters and Acyl hlorides arboxylic acids contain the functional group, attached to an alkyl stem. They are widely found in nature,
Experiment 18: Esters
 1 Experiment 18: Esters group: Esters are derivatives of the carboxylic acids and contain the following functional R R' A pleasant, often fruity, odor is characteristic of some of the simpler esters. Ethyl
1 Experiment 18: Esters group: Esters are derivatives of the carboxylic acids and contain the following functional R R' A pleasant, often fruity, odor is characteristic of some of the simpler esters. Ethyl
Alcohols, Carboxylic Acids and Esters
 Alcohols, Carboxylic Acids and Esters Question Paper Level Subject Exam Board Unit Topic Difficulty Level Booklet GCSE Chemistry AQA C3 Alcohols, Carboxylic Acids and Esters Silver Level Question Paper
Alcohols, Carboxylic Acids and Esters Question Paper Level Subject Exam Board Unit Topic Difficulty Level Booklet GCSE Chemistry AQA C3 Alcohols, Carboxylic Acids and Esters Silver Level Question Paper
H O. rapidly reduces. They dissolve. because they can hydrogen bond to the water molecules.
 3.9 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
3.9 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
Nature s Chemistry Esters, Fats and Oils
 Cathkin High School CfE Higher Chemistry Nature s Chemistry Esters, Fats and Oils Page 1 of 18 No. Learning Outcome Understanding? 1 An ester can be identified from the name containing the -yl-oate endings.
Cathkin High School CfE Higher Chemistry Nature s Chemistry Esters, Fats and Oils Page 1 of 18 No. Learning Outcome Understanding? 1 An ester can be identified from the name containing the -yl-oate endings.
Esters An Introduction To Organic Chemistry Reactions
 We have made it easy for you to find a PDF Ebooks without any digging. And by having access to our ebooks online or by storing it on your computer, you have convenient answers with esters an introduction
We have made it easy for you to find a PDF Ebooks without any digging. And by having access to our ebooks online or by storing it on your computer, you have convenient answers with esters an introduction
The four levels of protein structure are: primary structure, secondary structure, tertiary structure, and quaternary structure.
 Proteins Proteins are organic complex nitrogenous compounds of high molecular weight, formed of C, H, O and N. They are formed of a number of amino acids linked together by peptide linkage [-CO-NH-]. Proteins
Proteins Proteins are organic complex nitrogenous compounds of high molecular weight, formed of C, H, O and N. They are formed of a number of amino acids linked together by peptide linkage [-CO-NH-]. Proteins
Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank.
 Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. 1. (3 points each) First set functional groups A. ether D. amine B.
Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. 1. (3 points each) First set functional groups A. ether D. amine B.
Organic Chemistry Part 2
 Organic Chemistry Part 2 Benzene Benzene is a special structure C 6 H 6 The carbon-carbon bonds aren t a single or double bond but something in-between Resonance bond CYCLIC HYDROCARBONS Carbon chains
Organic Chemistry Part 2 Benzene Benzene is a special structure C 6 H 6 The carbon-carbon bonds aren t a single or double bond but something in-between Resonance bond CYCLIC HYDROCARBONS Carbon chains
Calderglen High School CfE Higher Chemistry. Nature s Chemistry Esters, Fats and Oils. Page 1 of 11
 Calderglen High School CfE Higher Chemistry Nature s Chemistry Esters, Fats and Oils Page 1 of 11 No. Learning Outcome Understanding? 1 An ester can be identified from the name containing the -yl-oate
Calderglen High School CfE Higher Chemistry Nature s Chemistry Esters, Fats and Oils Page 1 of 11 No. Learning Outcome Understanding? 1 An ester can be identified from the name containing the -yl-oate
Carboxylic Acids and Esters
 arboxylic Acids and Esters N Goalby hemrevise.org - absorption IR Spectrum for arboxylic acids Butanoic acid 1 Solubility in Water The smaller carboxylic (up to 4) acids dissolve in water in all proportions
arboxylic Acids and Esters N Goalby hemrevise.org - absorption IR Spectrum for arboxylic acids Butanoic acid 1 Solubility in Water The smaller carboxylic (up to 4) acids dissolve in water in all proportions
Carbohydrates. Objectives. Background. Experiment 6
 1 of 6 3/15/2011 7:27 PM Experiment 6 Carbohydrates Objectives During this experiment you will look at some of the physical and chemical properties of carbohydrates. Many of the carbohydrates, especially
1 of 6 3/15/2011 7:27 PM Experiment 6 Carbohydrates Objectives During this experiment you will look at some of the physical and chemical properties of carbohydrates. Many of the carbohydrates, especially
Biomolecule: Carbohydrate
 Biomolecule: Carbohydrate This biomolecule is composed of three basic elements (carbon, hydrogen, and oxygen) in a 1:2:1 ratio. The most basic carbohydrates are simple sugars, or monosaccharides. Simple
Biomolecule: Carbohydrate This biomolecule is composed of three basic elements (carbon, hydrogen, and oxygen) in a 1:2:1 ratio. The most basic carbohydrates are simple sugars, or monosaccharides. Simple
Esters of various alkane acids (Item No.: P )
 Teacher's/Lecturer's Sheet Esters of various alkane acids (Item No.: P7173100) Curricular Relevance Area of Expertise: Chemie Education Level: Klasse 7-10 Topic: Organische Chemie Subtopic: Sauerstoffhaltige
Teacher's/Lecturer's Sheet Esters of various alkane acids (Item No.: P7173100) Curricular Relevance Area of Expertise: Chemie Education Level: Klasse 7-10 Topic: Organische Chemie Subtopic: Sauerstoffhaltige
Chemical Tests For Biologically Important Molecules Do not write on this document
 Chemical Tests For Biologically Important Molecules Do not write on this document Introduction The most common and important organic molecules found in living things fall into four classes: carbohydrates,
Chemical Tests For Biologically Important Molecules Do not write on this document Introduction The most common and important organic molecules found in living things fall into four classes: carbohydrates,
This topic will look at six important chemicals found inside your kitchen cupboard.
 P & L. Johnson 2012 You may not realise it but everything is made of chemicals and therefore everything in your kitchen cupboard is a chemical. In fact some of the substances in your cupboard contain really
P & L. Johnson 2012 You may not realise it but everything is made of chemicals and therefore everything in your kitchen cupboard is a chemical. In fact some of the substances in your cupboard contain really
Lab 2. The Chemistry of Life
 Lab 2 Learning Objectives Compare and contrast organic and inorganic molecules Relate hydrogen bonding to macromolecules found in living things Compare and contrast the four major organic macromolecules:
Lab 2 Learning Objectives Compare and contrast organic and inorganic molecules Relate hydrogen bonding to macromolecules found in living things Compare and contrast the four major organic macromolecules:
CfE Higher Chemistry Homework. Unit 2: Natures Chemistry. The Chemistry of Cooking and Oxidation of Food. 1. Which of the following is an aldehyde?
 CfE Higher Chemistry Homework Unit 2: Natures Chemistry The Chemistry of Cooking and Oxidation of Food 1. Which of the following is an aldehyde? 2. Which is true of a compound with the following formula?
CfE Higher Chemistry Homework Unit 2: Natures Chemistry The Chemistry of Cooking and Oxidation of Food 1. Which of the following is an aldehyde? 2. Which is true of a compound with the following formula?
Carboxylic Acids and Esters
 24 Carboxylic Acids and Esters The sour tang in fruit juice comes from carboxylic acids. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and
24 Carboxylic Acids and Esters The sour tang in fruit juice comes from carboxylic acids. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and
LAB 4 Macromolecules
 LAB 4 Macromolecules Overview In addition to water and minerals, living things contain a variety of organic molecules. Most of the organic molecules in living organisms are of 4 basic types: carbohydrate,
LAB 4 Macromolecules Overview In addition to water and minerals, living things contain a variety of organic molecules. Most of the organic molecules in living organisms are of 4 basic types: carbohydrate,
Experiment Optional #2: The Synthesis of Aspirin
 Experiment Optional #2: The Synthesis of Aspirin The natural world provides us with many of the medications in common use today. Taxol is the common name of a medication used in treating certain cancers;
Experiment Optional #2: The Synthesis of Aspirin The natural world provides us with many of the medications in common use today. Taxol is the common name of a medication used in treating certain cancers;
A. Carboxylic acid functional groups contain the carboxyl structural feature. 1. Features of the carboxyl group
 Chapter 17 Carboxylic Acids and Their Derivatives Chem 306 Roper I. Overview A. Carboxylic acid functional groups contain the carboxyl structural feature. 1. Features of the carboxyl group 2. The reactivity
Chapter 17 Carboxylic Acids and Their Derivatives Chem 306 Roper I. Overview A. Carboxylic acid functional groups contain the carboxyl structural feature. 1. Features of the carboxyl group 2. The reactivity
6/9/2015. Unit 15: Organic Chemistry Lesson 15.2: Substituted Hydrocarbons & Functional Groups
 1-chloropropane 2-methylpropane 1-iodobutane Ethanoic Acid Unit 15: Organic Chemistry Lesson 15.2: Substituted Hydrocarbons & Functional Groups 43 It Ain t Just Hydrocarbons There are all sorts of organic
1-chloropropane 2-methylpropane 1-iodobutane Ethanoic Acid Unit 15: Organic Chemistry Lesson 15.2: Substituted Hydrocarbons & Functional Groups 43 It Ain t Just Hydrocarbons There are all sorts of organic
Oxidizing Alcohols. Questions. Prediction. Analysis. Safety Precautions. Materials. Conclusions. Procedure. 74 MHR Unit 1 Organic Chemistry
 xidizing Alcohols SKILL FUS Predicting Performing and recording Analyzing and interpreting Acidified potassium permanganate solution, KMn 4(aq), acts as an oxidizing agent when it comes in contact with
xidizing Alcohols SKILL FUS Predicting Performing and recording Analyzing and interpreting Acidified potassium permanganate solution, KMn 4(aq), acts as an oxidizing agent when it comes in contact with
Chapter 7-2 Hydrocarbons
 Chapter 7-1 Carbon C atom - atomic # is 6; it has 6 protons and therefore 6 electrons - is in group 14; it has 4 valence electrons - atomic mass is 12; it has 6 neutrons - shares electrons when forming
Chapter 7-1 Carbon C atom - atomic # is 6; it has 6 protons and therefore 6 electrons - is in group 14; it has 4 valence electrons - atomic mass is 12; it has 6 neutrons - shares electrons when forming
Carboxylic Acids and Their Derivatives
 arboxylic Acids and Their Derivatives Families ontaining the arbonyl Group Family Y Z Y Z aldehyde or ketone carboxylic acid or -- ester or -- acid halide or -F,-l,-Br,-I acid anhydride or amide or -N
arboxylic Acids and Their Derivatives Families ontaining the arbonyl Group Family Y Z Y Z aldehyde or ketone carboxylic acid or -- ester or -- acid halide or -F,-l,-Br,-I acid anhydride or amide or -N
For example, monosaccharides such as glucose are polar and soluble in water, whereas lipids are nonpolar and insoluble in water.
 Biology 4A Laboratory Biologically Important Molecules Objectives Perform tests to detect the presence of carbohydrates, lipids, proteins, and nucleic acids Recognize the importance of a control in a biochemical
Biology 4A Laboratory Biologically Important Molecules Objectives Perform tests to detect the presence of carbohydrates, lipids, proteins, and nucleic acids Recognize the importance of a control in a biochemical
ENZYME ACTIVITY. Introduction
 ENZYME ACTIVITY This activity is an alternative to the titration proposed for Enzyme Catalysis (AP Bio Lab #2, Biology Lab Manual). There are numerous alternative lab activities that measure the rate of
ENZYME ACTIVITY This activity is an alternative to the titration proposed for Enzyme Catalysis (AP Bio Lab #2, Biology Lab Manual). There are numerous alternative lab activities that measure the rate of
Carbohydrates Chemical Composition and Identification
 Carbohydrates Chemical Composition and Identification Introduction: Today, scientists use a combination of biology and chemistry for their understanding of life and life processes. Thus, an understanding
Carbohydrates Chemical Composition and Identification Introduction: Today, scientists use a combination of biology and chemistry for their understanding of life and life processes. Thus, an understanding
CHAPTER4 ANSWERS. Multiple Choice Questions. Short Answer Questions. 1. (b) 2. (d) 3. (a) 4. (c) 5. (c) 6. (b) 7. (a) 8. (b)
 CHAPTER4 ANSWERS Multiple Choice Questions 1. (b) 2. (d) 3. (a) 4. (c) 5. (c) 6. (b) 7. (a) 8. (b) 9. (a) 10. (d) 11. (a) 12. (d) 13. (b) 14. (a) 15. (c) 16. (c) 17. (c) 18. (d) 19. (c) 20. (a) 21. (b)
CHAPTER4 ANSWERS Multiple Choice Questions 1. (b) 2. (d) 3. (a) 4. (c) 5. (c) 6. (b) 7. (a) 8. (b) 9. (a) 10. (d) 11. (a) 12. (d) 13. (b) 14. (a) 15. (c) 16. (c) 17. (c) 18. (d) 19. (c) 20. (a) 21. (b)
Properties of Alcohols and Phenols Experiment #3
 Properties of Alcohols and Phenols Experiment #3 Objectives: To observe the solubility of alcohols relative to their chemical structure, to perform chemical tests to distinguish primary, secondary and
Properties of Alcohols and Phenols Experiment #3 Objectives: To observe the solubility of alcohols relative to their chemical structure, to perform chemical tests to distinguish primary, secondary and
Esters. What intermolecular forces do you think esters have? δ + CH 3
 Esters What intermolecular forces do you think esters have? ow will these intermolecular forces affect their: Melting and boiling points compared to alkanes Solubility in water δ 3 δ + 3 Dipole dipole
Esters What intermolecular forces do you think esters have? ow will these intermolecular forces affect their: Melting and boiling points compared to alkanes Solubility in water δ 3 δ + 3 Dipole dipole
3016 Oxidation of ricinoleic acid (from castor oil) with KMnO 4 to azelaic acid
 6 Oxidation of ricinoleic acid (from castor oil) with KMnO 4 to azelaic acid CH -(CH ) OH (CH ) -COOH KMnO 4 /KOH HOOC-(CH ) -COOH C H 4 O (.) KMnO 4 KOH (.) (6.) C H 6 O 4 (.) Classification Reaction
6 Oxidation of ricinoleic acid (from castor oil) with KMnO 4 to azelaic acid CH -(CH ) OH (CH ) -COOH KMnO 4 /KOH HOOC-(CH ) -COOH C H 4 O (.) KMnO 4 KOH (.) (6.) C H 6 O 4 (.) Classification Reaction
CH 3 CH 2 CH 2 CH 2 OH
 1 The alcohols form a homologous series. The first member is methanol and the fourth is butanol. 3 O methanol 3 2 2 2 O butanol (a) Give two general characteristics of a homologous series. (ii) alculate
1 The alcohols form a homologous series. The first member is methanol and the fourth is butanol. 3 O methanol 3 2 2 2 O butanol (a) Give two general characteristics of a homologous series. (ii) alculate
MiSP ENZYME ACTION Teacher Guide, L1 - L3. Introduction
 MiSP ENZYME ACTION Teacher Guide, L1 - L3 Introduction The subject of this unit, enzymes, is typically a high school topic and is studied in depth in Advanced Placement Biology. Even so, it can be successfully
MiSP ENZYME ACTION Teacher Guide, L1 - L3 Introduction The subject of this unit, enzymes, is typically a high school topic and is studied in depth in Advanced Placement Biology. Even so, it can be successfully
Preparation and Comparison of Soaps Minneapolis Community and Tech. College C1152 Principles of Chemistry II v.5.10
 Preparation and Comparison of Soaps Minneapolis Community and Tech. College C1152 Principles of Chemistry II v.5.10 Name Introduction Vegetable oil, like all animal and vegetable fats, are made up of a
Preparation and Comparison of Soaps Minneapolis Community and Tech. College C1152 Principles of Chemistry II v.5.10 Name Introduction Vegetable oil, like all animal and vegetable fats, are made up of a
(LM pages 91 98) Time Estimate for Entire Lab: 2.5 to 3.0 hours. Special Requirements
 Laboratory 7 Chemical Aspects of Digestion (LM pages 91 98) Time Estimate for Entire Lab: 2.5 to 3.0 hours Special Requirements Incubation. Students should start these sections at the beginning of the
Laboratory 7 Chemical Aspects of Digestion (LM pages 91 98) Time Estimate for Entire Lab: 2.5 to 3.0 hours Special Requirements Incubation. Students should start these sections at the beginning of the
Topic 4.5 COMPOUNDS CONTAINING THE CARBONYL GROUP. Aldehydes and Ketones Carboxylic Acids and their Salts Esters Acyl Chlorides and Acid Anhydrides
 Topic 4.5 MPUNDS NTAINING TE ARBNYL GRUP Aldehydes and Ketones arboxylic Acids and their Salts Esters Acyl hlorides and Acid Anhydrides ALDEYDES AND KETNES 1. Introduction Aldehydes and ketones are collectively
Topic 4.5 MPUNDS NTAINING TE ARBNYL GRUP Aldehydes and Ketones arboxylic Acids and their Salts Esters Acyl hlorides and Acid Anhydrides ALDEYDES AND KETNES 1. Introduction Aldehydes and ketones are collectively
Reading 3.2 Why do different food molecules provide different amounts of energy?
 Reading 3.2 Why do different food molecules provide different amounts of energy? Getting Started The following four items have carbohydrate, protein, and fat molecules. Honey has simple sugars. Rice has
Reading 3.2 Why do different food molecules provide different amounts of energy? Getting Started The following four items have carbohydrate, protein, and fat molecules. Honey has simple sugars. Rice has
BIOLOGY 1101 LAB 1: OSMOSIS & DIFFUSION. READING: Please read pages & in your text prior to lab.
 BIOLOGY 1101 LAB 1: OSMOSIS & DIFFUSION READING: Please read pages 27-31 & 83-86 in your text prior to lab. INTRODUCTION: All living things depend on water. A water molecule is made up of an oxygen atom
BIOLOGY 1101 LAB 1: OSMOSIS & DIFFUSION READING: Please read pages 27-31 & 83-86 in your text prior to lab. INTRODUCTION: All living things depend on water. A water molecule is made up of an oxygen atom
Fuels. 1. Combustion is an example of an exothermic reaction which will give out energy, endothermic reactions are the opposite
 Fuels 1. ombustion is an example of an exothermic reaction which will give out energy, endothermic reactions are the opposite overed ( ) ow well can you do this? 2. The energy given out by a fuel can be
Fuels 1. ombustion is an example of an exothermic reaction which will give out energy, endothermic reactions are the opposite overed ( ) ow well can you do this? 2. The energy given out by a fuel can be
12BL Experiment 2: Extraction & Saponification of Trimyristin from Nutmeg
 12BL Experiment 2: Extraction & Saponification of Trimyristin from Nutmeg Safety: Proper lab goggles/glasses must be worn (even over prescription glasses). Heating of organic solvents releases irritating
12BL Experiment 2: Extraction & Saponification of Trimyristin from Nutmeg Safety: Proper lab goggles/glasses must be worn (even over prescription glasses). Heating of organic solvents releases irritating
Do Now: Sort the following into the order of life from smallest to largest:
 Do Now: Sort the following into the order of life from smallest to largest: organ, molecule, atom, organelle, cell, organ system, tissue, organism Correct Order: atom, molecule, organelle, cell, tissue,
Do Now: Sort the following into the order of life from smallest to largest: organ, molecule, atom, organelle, cell, organ system, tissue, organism Correct Order: atom, molecule, organelle, cell, tissue,
R O R' Acid anhydride. Acid halide. Carboxylic acid. Ester O O O O. Nitrile Acyl phosphate Thioester. Amide
 Chapter 10. Carboxylic Acids and Derivatives Carboxylic acid X Acid halide ' Acid anhydride Ester ' P N 2 C N S' Amide Nitrile Acyl phosphate Thioester The common structural feature of all these compounds
Chapter 10. Carboxylic Acids and Derivatives Carboxylic acid X Acid halide ' Acid anhydride Ester ' P N 2 C N S' Amide Nitrile Acyl phosphate Thioester The common structural feature of all these compounds
Alcohols and Ethers. Alcohols
 Alcohols and Ethers A patient does not experience pain during surgery when given a general anesthetic. The earliest anesthetics, used during the Civil War, belonged to a class of chemical compounds called
Alcohols and Ethers A patient does not experience pain during surgery when given a general anesthetic. The earliest anesthetics, used during the Civil War, belonged to a class of chemical compounds called
CfE Higher Chemistry. Unit 2: Natures Chemistry. Esters, Fats and Oils, Soaps
 CfE igher Chemistry Unit 2: Natures Chemistry Esters, Fats and ils, Soaps 27/02/2018 Lesson Starter: Name / Draw these molecules a) b) c) d) e) 2-methylpentan-1-ol f) Pentanoic acid 27/02/2018 Esters 27/02/2018
CfE igher Chemistry Unit 2: Natures Chemistry Esters, Fats and ils, Soaps 27/02/2018 Lesson Starter: Name / Draw these molecules a) b) c) d) e) 2-methylpentan-1-ol f) Pentanoic acid 27/02/2018 Esters 27/02/2018
You Are What You Eat
 An Investigation of Macromolecules Student Materials Introduction....2 Pre-Lab Questions.5 Lab Protocol..6 Post-Lab Questions and Analysis 9 Last updated: September 26 th, 2017 1 Introduction When deciding
An Investigation of Macromolecules Student Materials Introduction....2 Pre-Lab Questions.5 Lab Protocol..6 Post-Lab Questions and Analysis 9 Last updated: September 26 th, 2017 1 Introduction When deciding
Identification of Organic Compounds Lab
 Identification of Organic Compounds Lab Introduction All organic compounds contain the element carbon (C). Organic compounds usually also contain oxygen (O) or hydrogen (H) or both. They may also contain
Identification of Organic Compounds Lab Introduction All organic compounds contain the element carbon (C). Organic compounds usually also contain oxygen (O) or hydrogen (H) or both. They may also contain
Biology 20 Laboratory Life s Macromolecules OBJECTIVE INTRODUCTION
 Biology 20 Laboratory Life s Macromolecules OBJECTIVE To observe and record reactions between three classes of macromolecules in the presence of simple chemical indictors. To be able to distinguish positive
Biology 20 Laboratory Life s Macromolecules OBJECTIVE To observe and record reactions between three classes of macromolecules in the presence of simple chemical indictors. To be able to distinguish positive
Organic Chemistry. AQA Chemistry topic 7
 rganic hemistry AQA hemistry topic 7 7.1 arbon ompounds as fuels and feedstock rude il rude oil is a finite resource found in rocks. It s the remains of an ancient biomass consisting mainly of plankton
rganic hemistry AQA hemistry topic 7 7.1 arbon ompounds as fuels and feedstock rude il rude oil is a finite resource found in rocks. It s the remains of an ancient biomass consisting mainly of plankton
cyclobutane Benzene Ring phenyl
 ow many carbons and hydrogens in the following? More rganic Today eview hydrocarbons Functional Groups Condensation eaction Biopolymers A. 6 C, 14 B. 6 C, 15 C. 6 C, 16 3 1 2 D. 7 C, 15 3 1 1 3 E. 7 C,
ow many carbons and hydrogens in the following? More rganic Today eview hydrocarbons Functional Groups Condensation eaction Biopolymers A. 6 C, 14 B. 6 C, 15 C. 6 C, 16 3 1 2 D. 7 C, 15 3 1 1 3 E. 7 C,
Who Killed the Flowers? Teacher Information
 Who Killed the Flowers? Teacher Information Summary: Mrs. Powell, the science teacher, suspects that someone killed some flowers in the school s greenhouse by urinating on them. In this science lab, students
Who Killed the Flowers? Teacher Information Summary: Mrs. Powell, the science teacher, suspects that someone killed some flowers in the school s greenhouse by urinating on them. In this science lab, students
Lesmahagow High School
 Lesmahagow High School Higher Chemistry Alcohols and Esters - Past Paper Homework Questions . Carbohydrates are an essential part of our diet. (a) Why are carbohydrates an important part of our diet? (b)
Lesmahagow High School Higher Chemistry Alcohols and Esters - Past Paper Homework Questions . Carbohydrates are an essential part of our diet. (a) Why are carbohydrates an important part of our diet? (b)
OCR A GCSE Chemistry. Topic 6: Global challenges. Organic chemistry. Notes.
 OCR A GCSE Chemistry Topic 6: Global challenges Organic chemistry Notes C6.2a recognise functional groups and identify members of the same homologous series Prefixes (beginning of the name) o remember
OCR A GCSE Chemistry Topic 6: Global challenges Organic chemistry Notes C6.2a recognise functional groups and identify members of the same homologous series Prefixes (beginning of the name) o remember
STUDENT ACTIVITY GUIDE
 Page 1/5 ACTIVITY OBJECTIVE STUDENT ACTIVITY GUIDE WHAT AFFECTS YEAST GROWTH? Taken from IFT Experiments in Food Science Series In this activity, you will (1) determine what factors affect the growth of
Page 1/5 ACTIVITY OBJECTIVE STUDENT ACTIVITY GUIDE WHAT AFFECTS YEAST GROWTH? Taken from IFT Experiments in Food Science Series In this activity, you will (1) determine what factors affect the growth of
Investigating the Oxidative Rancidity of Polyunsaturated Oils
 1 Investigating the Oxidative Rancidity of Polyunsaturated Oils Research Question How do different storage temperatures (-20.0, 4.0, 25.0, 40.0, 60.0 ºC) of sunflower oil affect the relative concentration
1 Investigating the Oxidative Rancidity of Polyunsaturated Oils Research Question How do different storage temperatures (-20.0, 4.0, 25.0, 40.0, 60.0 ºC) of sunflower oil affect the relative concentration
Macromolecules. Small molecules that join together to form one large polymer molecules.
 Macromolecules Polymerisation: Polymerisation is the joining of small molecules (monomers), into chains of a very large molecule (polymer). The monomers can be as atoms, simple molecules of ethen as in
Macromolecules Polymerisation: Polymerisation is the joining of small molecules (monomers), into chains of a very large molecule (polymer). The monomers can be as atoms, simple molecules of ethen as in
Chemistry of food and FOOD GRAINS Build a simple calorimeter. Regina Zibuck
 Chemistry of food and FOOD GRAINS Build a simple calorimeter Regina Zibuck rzibuck@wayne.edu Build and use a calorimeter (15 %) include calibration data in the 5 sheets. Written test (85 %) (Students are
Chemistry of food and FOOD GRAINS Build a simple calorimeter Regina Zibuck rzibuck@wayne.edu Build and use a calorimeter (15 %) include calibration data in the 5 sheets. Written test (85 %) (Students are
Organic Chemistry. Chapter 23. Hill, Petrucci, McCreary & Perry 4 th. Ed. Alkane to Substituent Group methane CH 4 methyl CH 3
 hapter 23 rganic hemistry ill, Petrucci, Mcreary & Perry 4 th Ed. Alkane to Substituent Group methane 4 methyl 3 ethane 3 3 ethyl 3 2 propane 3 2 3 propyl 3 2 2 isopropyl ( 3 ) 2 or 3 3 butyl 3 2 2 2 butane
hapter 23 rganic hemistry ill, Petrucci, Mcreary & Perry 4 th Ed. Alkane to Substituent Group methane 4 methyl 3 ethane 3 3 ethyl 3 2 propane 3 2 3 propyl 3 2 2 isopropyl ( 3 ) 2 or 3 3 butyl 3 2 2 2 butane
National 5 Unit Two : Nature s Chemistry
 National 5 Unit Two : Nature s Chemistry Fuels A fuel is a chemical which burns, giving off energy. Combustion is a reaction of a substance with oxygen giving off energy. The test for oxygen is it relights
National 5 Unit Two : Nature s Chemistry Fuels A fuel is a chemical which burns, giving off energy. Combustion is a reaction of a substance with oxygen giving off energy. The test for oxygen is it relights
DO NOW: What is a catalyst?
 AGENDA ABSENT Block Nov 5 th /6 th -Week 13 TOPIC: Enzymes OBJ : 1-4 DO NOW: What is a catalyst? Science of Life EXT: Enzyme Lab DUE DATE: 11-10 DW: -----------------------------------------------------------------------------------------------------------------
AGENDA ABSENT Block Nov 5 th /6 th -Week 13 TOPIC: Enzymes OBJ : 1-4 DO NOW: What is a catalyst? Science of Life EXT: Enzyme Lab DUE DATE: 11-10 DW: -----------------------------------------------------------------------------------------------------------------
Chemistry Chapter 19
 hemistry 2100 hapter 19 arboxyl Derivatives In this chapter, we study three classes of compounds derived from carboxylic acids; anhydrides, esters, and amides. Each is related to a carboxyl group by loss
hemistry 2100 hapter 19 arboxyl Derivatives In this chapter, we study three classes of compounds derived from carboxylic acids; anhydrides, esters, and amides. Each is related to a carboxyl group by loss
large molecules small molecules fuels carbon
 4 10 5 12 6 14 4 2 6 3 8 methane ethane propane butane, pentane & hexane n 2n+2 Saturated hydrocarbons Insoluble in water n 2n Unsaturated hydrocarbons Insoluble in water Burns in plentiful 2 with a clean
4 10 5 12 6 14 4 2 6 3 8 methane ethane propane butane, pentane & hexane n 2n+2 Saturated hydrocarbons Insoluble in water n 2n Unsaturated hydrocarbons Insoluble in water Burns in plentiful 2 with a clean
Naming Organic Halide Organic Halide: is a compound that contains one or more halogen atoms (F, Cl, Br, I) as part of its molecular structure.
 Naming Organic Halide Organic Halide: is a compound that contains one or more halogen atoms (F, Cl, Br, I) as part of its molecular structure. Organic halides have many important uses including: fire retardation,
Naming Organic Halide Organic Halide: is a compound that contains one or more halogen atoms (F, Cl, Br, I) as part of its molecular structure. Organic halides have many important uses including: fire retardation,
Chapter 4 - Carbon Compounds
 Chapter 4 - Carbon Compounds Carbon compounds organic compounds are tied up with living organisms. So much so, that as we have seen, the presence of methane might be considered an indicator of life. Methane
Chapter 4 - Carbon Compounds Carbon compounds organic compounds are tied up with living organisms. So much so, that as we have seen, the presence of methane might be considered an indicator of life. Methane
Firrhill High School CfE Higher Chemistry
 Firrhill High School CfE Higher Chemistry Unit 2 Homework Natures Chemistry 1 1. Write the general formula for the alkanols. Alcohols, Carboxylic Acids and Esters 2. Draw the structural formulae for each
Firrhill High School CfE Higher Chemistry Unit 2 Homework Natures Chemistry 1 1. Write the general formula for the alkanols. Alcohols, Carboxylic Acids and Esters 2. Draw the structural formulae for each
WHAT IS A LIPID? OBJECTIVE The objective of this worksheet is to understand the structure and function of lipids
 WHAT IS A LIPID? OBJECTIVE The objective of this worksheet is to understand the structure and function of lipids PART A: Understanding Lipids Lipids are more commonly known as fats and include triglycerides,
WHAT IS A LIPID? OBJECTIVE The objective of this worksheet is to understand the structure and function of lipids PART A: Understanding Lipids Lipids are more commonly known as fats and include triglycerides,
Guided Inquiry Skills Lab. Additional Lab 1 Making Models of Macromolecules. Problem. Introduction. Skills Focus. Materials.
 Additional Lab 1 Making Models of Macromolecules Guided Inquiry Skills Lab Problem How do monomers join together to form polymers? Introduction A small number of elements make up most of the mass of your
Additional Lab 1 Making Models of Macromolecules Guided Inquiry Skills Lab Problem How do monomers join together to form polymers? Introduction A small number of elements make up most of the mass of your
Enzyme Action: Testing Catalase Activity
 Enzyme Action: Testing Catalase Activity Pennsylvania Science Standards: S11.A.1.1.4 S11.A.1.3.1 S11.A.2.2.2.1 S11.A.2.2.2.2 Keystone Eligible Content Bio.B.4.1.1, Bio.B.4.1.2, and Bio.B.4.2.5 Introduction
Enzyme Action: Testing Catalase Activity Pennsylvania Science Standards: S11.A.1.1.4 S11.A.1.3.1 S11.A.2.2.2.1 S11.A.2.2.2.2 Keystone Eligible Content Bio.B.4.1.1, Bio.B.4.1.2, and Bio.B.4.2.5 Introduction
Name: Per. Date: / 71 points MACROMOLECULE LAB: Testing for the Presence of Macromolecules
 Name: Per. Date: / 71 points MACROMOLECULE LAB: Testing for the Presence of Macromolecules Introduction: There are four broad classes of macromolecules that can be found in living systems. Each type of
Name: Per. Date: / 71 points MACROMOLECULE LAB: Testing for the Presence of Macromolecules Introduction: There are four broad classes of macromolecules that can be found in living systems. Each type of
Lect 2- Organic Chem Biomolecules student copy
 3 pts ec Lect 2- Organic Chem Biomolecules student copy printing Other organic compounds Take a cheeseburger... hamburger, covered with American (yellow) cheese on a hamburger bun... yummy! Now, if you
3 pts ec Lect 2- Organic Chem Biomolecules student copy printing Other organic compounds Take a cheeseburger... hamburger, covered with American (yellow) cheese on a hamburger bun... yummy! Now, if you
THERMALLY OXIDIZED SOYA BEAN OIL interacted with MONO- and DIGLYCERIDES of FATTY ACIDS
 THERMALLY OXIDIZED SOYA BEAN OIL interacted with MONO- and DIGLYCERIDES of FATTY ACIDS Prepared at the 39th JECFA (1992), published in FNP 52 Add 1 (1992). Metals and arsenic specifications revised at
THERMALLY OXIDIZED SOYA BEAN OIL interacted with MONO- and DIGLYCERIDES of FATTY ACIDS Prepared at the 39th JECFA (1992), published in FNP 52 Add 1 (1992). Metals and arsenic specifications revised at
Burping Yeast: An Investigation of Cellular Respiration
 Burping Yeast: An Investigation of Cellular Respiration Student Materials Introduction... 2 Lab Protocol... 4 Data Collection Worksheet... 6 Pre-Lab Questions... 7 Post-Lab Questions and Analysis... 8
Burping Yeast: An Investigation of Cellular Respiration Student Materials Introduction... 2 Lab Protocol... 4 Data Collection Worksheet... 6 Pre-Lab Questions... 7 Post-Lab Questions and Analysis... 8
Organic. Carbon Chemistry
 Today Organic Carbon Chemistry Organic You know more than you think already What you will need Lewis dot, VSEPR VB, hybrid orbitals, MO electronegativity intermolecular forces Two hurdles we will deal
Today Organic Carbon Chemistry Organic You know more than you think already What you will need Lewis dot, VSEPR VB, hybrid orbitals, MO electronegativity intermolecular forces Two hurdles we will deal
4. CARBON AND ITS COMPOUND
 . ARBN AND ITS MPUND rganic hemistry- The study of carbon compounds. rganic ompounds- The compounds of carbon (except the oxides of carbon, carbonates, hydro carbonates and carbides) are called organic
. ARBN AND ITS MPUND rganic hemistry- The study of carbon compounds. rganic ompounds- The compounds of carbon (except the oxides of carbon, carbonates, hydro carbonates and carbides) are called organic
Problem Based Practical Activities Problem 10: Patient prognosis
 Learn Chemistry Problem Based Practical Activities Developed by Dr Catherine Smith, RSC School Teacher Fellow at the University of Leicester 2011-2012 This resource was produced as part of the National
Learn Chemistry Problem Based Practical Activities Developed by Dr Catherine Smith, RSC School Teacher Fellow at the University of Leicester 2011-2012 This resource was produced as part of the National
B07 Alcohols, Corboxylic Acids & Esters.notebook. November 19, Alcohols
 Alcohols There is more to alcohol than just beverages. In fact, most alcohols are poisonous. THis is what makes them effective as disinfectants and cleaners. 1 What makes an alcohol and alcohol? If a hydrocarbon
Alcohols There is more to alcohol than just beverages. In fact, most alcohols are poisonous. THis is what makes them effective as disinfectants and cleaners. 1 What makes an alcohol and alcohol? If a hydrocarbon
CH [2] (ii) Give the structural formula of another hydrocarbon which is isomeric with the above.
![CH [2] (ii) Give the structural formula of another hydrocarbon which is isomeric with the above. CH [2] (ii) Give the structural formula of another hydrocarbon which is isomeric with the above.](/thumbs/75/72821632.jpg) 1 The alkenes are unsaturated hydrocarbons. They form a homologous series, the members of which have the same chemical properties. They undergo addition reactions and are easily oxidised. (a) The following
1 The alkenes are unsaturated hydrocarbons. They form a homologous series, the members of which have the same chemical properties. They undergo addition reactions and are easily oxidised. (a) The following
CLASS SET. Modeling Life s Important Compounds. AP Biology
 Modeling Life s Important Compounds AP Biology CLASS SET OBJECTIVES: Upon completion of this activity, you will be able to: Explain the connection between the sequence and the subcomponents of a biological
Modeling Life s Important Compounds AP Biology CLASS SET OBJECTIVES: Upon completion of this activity, you will be able to: Explain the connection between the sequence and the subcomponents of a biological
Properties of Alcohols and Phenols Experiment #3
 Properties of Alcohols and Phenols Experiment #3 bjectives: (A) To observe the solubility of alcohols relative to their chemical structure and (B) chemical tests will be performed to distinguish primary,
Properties of Alcohols and Phenols Experiment #3 bjectives: (A) To observe the solubility of alcohols relative to their chemical structure and (B) chemical tests will be performed to distinguish primary,
Enzyme Action: Testing Catalase Activity
 Enzyme Action: Testing Catalase Activity LabQuest 6A Many organisms can decompose hydrogen peroxide (H 2 O 2 ) enzymatically. Enzymes are globular proteins, responsible for most of the chemical activities
Enzyme Action: Testing Catalase Activity LabQuest 6A Many organisms can decompose hydrogen peroxide (H 2 O 2 ) enzymatically. Enzymes are globular proteins, responsible for most of the chemical activities
Core Lab #3 Investigating Diabetes Mellitus
 Name: Introduction Diabetes is a malfunction of one of the major homeostatic mechanisms in the body the endocrine system. Two hormones, insulin and glucagon control the level of sugar in the blood. The
Name: Introduction Diabetes is a malfunction of one of the major homeostatic mechanisms in the body the endocrine system. Two hormones, insulin and glucagon control the level of sugar in the blood. The
THERMALLY OXIDIZED SOYA BEAN OIL
 THERMALLY OXIDIZED SOYA BEAN OIL Prepared at the 39th JECFA (1992), published in FNP 52 Add 1 (1992). Metals and arsenic specifications revised at the 55th JECFA (2000). An ADI of 0-3 mg/kg bw was established
THERMALLY OXIDIZED SOYA BEAN OIL Prepared at the 39th JECFA (1992), published in FNP 52 Add 1 (1992). Metals and arsenic specifications revised at the 55th JECFA (2000). An ADI of 0-3 mg/kg bw was established
Salicylic acid. Hi I m Molly Cool. Extracted from the bark of the willow tree and used to ease aches and pains and reduce fever -
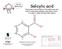 Salicylic acid Extracted from the bark of the willow tree and used to ease aches and pains and reduce fever - it is used to make the pain-killer aspirin and makeitmolecular designed by 7 C Carbon (black)
Salicylic acid Extracted from the bark of the willow tree and used to ease aches and pains and reduce fever - it is used to make the pain-killer aspirin and makeitmolecular designed by 7 C Carbon (black)
Experiment 9 Amino Acids and Proteins
 Experiment 9 Amino Acids and Proteins Proteins are very important biological molecules, with many possible functions. Enzymes are proteins that catalyze biological reactions. There are transport proteins
Experiment 9 Amino Acids and Proteins Proteins are very important biological molecules, with many possible functions. Enzymes are proteins that catalyze biological reactions. There are transport proteins
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1)
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 7. CARBOXYLIC ACIDS AND THEIR
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 7. CARBOXYLIC ACIDS AND THEIR
