Effects of coexpression of the LDL receptor and apoe on cholesterol metabolism and atherosclerosis in LDL receptor-deficient mice
|
|
|
- Adrian Fisher
- 5 years ago
- Views:
Transcription
1 Effects of coexpression of the LDL receptor and apoe on cholesterol metabolism and atherosclerosis in LDL receptor-deficient mice Masa-aki Kawashiri,* Yuzhen Zhang,* David Usher, Muredach Reilly,* Ellen Puré, and Daniel J. Rader 1, * Department of Medicine,* University of Pennsylvania School of Medicine, Philadelphia, PA, 19104; University of Delaware, Newark, DE 19716; and Wistar Institute, Philadelphia, PA and Ludwig Institute for Cancer Research, New York, NY Abstract The low density lipoprotein receptor (LDLR) plays a major role in regulation of plasma cholesterol levels as a ligand for apolipoprotein B-100 and apolipoprotein E (apoe). Consequently, LDLR-deficient mice fed a Westerntype diet develop significant hypercholesterolemia and atherosclerosis. ApoE not only mediates uptake of atherogenic lipoproteins via the LDLR and other cell-surface receptors, but also directly inhibits atherosclerosis. In this study, we examined the hypothesis that coexpression of the LDLR and apoe would have greater effects than either one alone on plasma cholesterol levels and the development of atherosclerosis in LDLR-deficient mice. LDLR-deficient mice fed a Western-type diet for 10 weeks were injected with recombinant adenoviral vectors encoding the genes for human LDLR, human apoe3, both LDLR and apoe3, or lacz (control). Plasma lipids were analyzed at several time points after vector injection. Six weeks after injection, mice were analyzed for extent of atherosclerosis by two independent methods. As expected, LDLR expression alone induced a significant reduction in plasma cholesterol due to reduced VLDL and LDL cholesterol levels, whereas overexpression of apoe alone did not reduce plasma cholesterol levels. When the LDLR and apoe were coexpressed in this model, the effects on plasma cholesterol levels were no greater than with expression of the LDLR alone. However, coexpression did result in a substantial increase in large apoerich HDL particles. In addition, although the combination of cholesterol reduction and apoe expression significantly reduced atherosclerosis, its effects were no greater than with expression of the LDLR or apoe alone. In summary, in this LDLR-deficient mouse model fed a Western-type diet, there was no evidence of an additive effect of expression of the LDLR and apoe on cholesterol reduction or atherosclerosis. Kawashiri, M-a., Y. Zhang, D. Usher, M. Reilly, E. Puré, and D. J. Rader. Effects of coexpression of the LDL receptor and apoe on cholesterol metabolism and atherosclerosis in LDL receptor-deficient mice. J. Lipid Res : Supplementary key words low density lipoprotein receptor lipoprotein metabolism gene transfer The low density lipoprotein receptor (LDLR) mediates the removal of LDL and remnant lipoproteins from circulation by binding to apolipoprotein B-100 (apob-100) and apolipoprotein E (apoe) and plays a major role in regulation of plasma cholesterol levels in humans (1). The LDLRdeficient mouse has modestly elevated levels of plasma cholesterol and, when fed a high fat, high cholesterol diet, develops much higher levels of cholesterol, xanthomas, and atherosclerosis (2). Liver-directed expression of human LDLR using an adenoviral vector in LDLR-deficient mice on a normal chow diet transiently reduced plasma cholesterol levels to normal (3). The effect of transient reduction of cholesterol on the development of atherosclerosis in the LDLR-deficient mouse has not been studied. ApoE is a 34-kD glycoprotein synthesized in variety of tissues, including liver and macrophages (4). As an integral surface component of lipoproteins, apoe mediates the uptake of chylomicron and VLDL remnants by acting as a ligand for LDLR (4), heparan sulfate proteoglycans (5), and the low density lipoprotein receptor related protein (LRP) (6). ApoE-deficient mice have high levels of remnant lipoproteins owing to defective clearance (7, 8). Liver-directed gene transfer of human apoe3 using adenoviral vectors decreased plasma cholesterol levels (9 11). Bone marrow transplantation of wild-type mouse bone marrow into apoe-deficient mice normalized the lipoprotein profile by providing a source of extrahepatic apoe (12, 13). Studies in apoe-deficient mice have established that approximately 1% of the normal plasma apoe con- Abbreviations: LDLR, low density lipoprotein receptor; apolipoprotein E, apoe; apob-100, apolipoprotein B-100; LRP, lipoprotein receptorrelated protein; HRP, horseradish peroxidase; DAB, diaminobenzidine tetrahydrochloride; FPLC, fast protein liquid chromatography. 1 To whom correspondence should be addressed at the University of Pennsylvania Medical Center, 654 Biomedical Research Building II/ III, 421 Curie Blvd., Philadelphia, PA rader@mail.med.upenn.edu Journal of Lipid Research Volume 42,
2 centration is adequate to normalize plasma cholesterol levels (14). The effects of overexpression of apoe on lipid levels in animal models other than apoe deficiency have also been studied. Overexpression of rat apoe in wild-type mice fed a high fat, high cholesterol diet reduced plasma cholesterol levels (15, 16). Overexpression of rat apoe in diabetic wild-type mice reduced plasma cholesterol and triglyceride levels (17). Overexpression of rat apoe in LDLR-deficient mice reduced VLDL cholesterol levels and accelerated VLDL clearance (18). Overexpression of human apoe3 in wild-type rabbits reduced VLDL cholesterol but increased LDL cholesterol levels (19). In fact, apoe-containing remnant lipoproteins have been shown to compete with LDL for binding to the LDL receptor (20). Expression of apoe also has significant effects on atherosclerosis. ApoE-deficient mice develop extensive atherosclerosis on a chow diet (7, 8, 21). Bone marrow transplantation of wild-type mouse bone marrow into apoe-deficient mice reduced progression of atherosclerosis (12, 13). Gene transfer and hepatic overexpression of human apoe3 in apoe-deficient mice induced marked regression of preexisting atherosclerosis (22, 23). In all of these experiments, apoe expression substantially reduced plasma cholesterol levels, making it difficult to determine the mechanism by which apoe reduced atherosclerosis. However, when WHHL rabbits were injected repeatedly with apoe for 8.5 months, there was no effect on plasma cholesterol levels but atherosclerosis was reduced (24). Gene transfer and hepatic overexpression of apoe inhibited the progression of early atherosclerotic lesions (25) and induced regression of advanced complex atherosclerotic lesions (26) in LDLR-deficient mice fed a Western-type diet despite having no significant effect on plasma cholesterol levels. Expression of apoe by the adrenals at plasma levels below the threshold for reducing plasma cholesterol levels still reduced atherosclerosis in apoe-deficient mice (27). Therefore, extravascular expression of apoe has direct effects on atherosclerosis independent of its ability to mediate hepatic uptake of atherogenic lipoproteins. In the current study, we tested the hypothesis that coexpression of the LDLR and apoe in LDLR-deficient mice fed a Western-type diet would have greater effects on cholesterol reduction and atherosclerosis than the expression of either gene alone. MATERIALS AND METHODS Mouse studies A first-generation recombinant adenovirus encoding the human LDLR cdna (28) was kindly provided by Dr. Karen Kozarsky. A second-generation recombinant adenovirus encoding the human apoe3 cdna (22, 25) was used for apoe3 gene transfer. As control adenoviruses, we used first- and second-generation vectors encoding beta galactosidase (AdlacZ and tsadlacz). A total of 40 LDLRdeficient male mice (back-crossed 10 times to C57BL/6 mice), 28 weeks old, were obtained from Jackson Labs (Bar Harbor, ME) and fed a Western-type diet (normal chow supplemented with 0.15% cholesterol and 20% butter fat) for 10 weeks before gene transfer. When the mice were 38 weeks of age, serum cholesterol was determined and the mice were divided into four groups such that the mean plasma cholesterol levels in each group were not different. The mice were injected intravenously with four different combinations of adenoviruses ( particles of each virus, particles total dose): 1) AdlacZ tsadlacz, 2) AdLDLR tsadlacz, 3) AdlacZ tsadapoe3, and 4) AdLDLR tsadapoe3). All the mice received the same total dose of virus. Each group contained 9 11 mice. Blood was obtained from the retroorbital plexus after a 4-h fast on days 0, 3, 7, 14, 21, 28, and 42 after adenovirus injection for analysis of transgene expression and lipids. Aliquots of plasma were stored at 20 C until quantification. All mice were killed 6 weeks after virus injection to quantify the extent of atherosclerotic lesions. Quantification of atherosclerotic lesions The extent of atherosclerosis was analyzed by two independent methods, en face analysis of the whole aorta and crosssectional analysis of the aortic root, as previously described (22, 29). Mice were anesthetized with xylozine/ketamine. After the aorta was gently perfused with ice-cold PBS via the left ventricle, the heart was severed just above the aortic root, cut at the base, then the upper half of the heart containing aortic root was embedded in OCT and frozen at 80 C. For the en face analysis, the rest of the aorta, from ascending aorta to common iliac artery, was removed and fixed in 10% formalin. After the adventitial and adipose tissue was removed, the aortic arches were cut open longitudinally, then stained with Sudan IV solution (0.5% Sudan IV, 35% ethanol, 50% acetone) for 8 min and destained with 80% ethanol for 5 min to eliminate background staining. Images were captured with a Leica MZ12 microscope and digitized, and Sudan IV stained lesion area was quantified using Image Pro Plus image analysis software (Media Cybernetics, Silver Spring, MD). All data capture and quantification was performed in a blinded fashion. Atherosclerosis was also quantified in the aortic root crosssections from the fresh-frozen OCT-embedded hearts (22, 29). Serial 8 M sections of the aortic root were cut and mounted on slides, then fixed in acetone, rehydrated in PBS containing 0.02% NaN3, and blocked with 1%BSA in PBS/NaN3. For detection of macrophages, sections were immunostained with monoclonal rat anti-murine MAC-1 antibody, monoclonal hamster anti-cd18, and monoclonal hamster anti-cd11c, followed by incubation with mouse anti-rat or goat anti-hamster IgG in the presence of 200- g/ml normal mouse IgG. Antibody reactivity was detected using HRP-conjugated biotin-streptavidin complexes and developed with diaminobenzidine tetrahydrchloride (DAB) as substrate. Images of immunostained aortic root sections, captured digitally with a video camera connected to a Leica microscope, were analyzed using computerized image analysis (Image Pro Plus, Media Cybernetics, Silver Spring, MD). Total lesion area in aortic root sections was measured by manually tracing entire lesions in 4 equally spaced aortic root sections per mouse. Macrophage lesion area was quantified in the same sections by determination of area that stained positively for macrophage markers. The percent of lesion occupied by macrophage-derived foam cells was calculated by dividing the macrophage lesion area by the total lesion area. The acquisition of images and analysis of lesions were performed in a blinded fashion. Analytical methods The plasma total cholesterol levels were measured in individual mice at each time point with an enzymatic assay on a Cobas Fara II (Roche Diagnostic System, Inc., Indianapolis, IN) with the use of Sigma reagents (Sigma, St. Louis, MO). Plasma humanspecific apoe levels were quantified in pooled plasma at each time point by enzyme-linked immunosorbent assay. Pooled plasma on days 0, 3, 7, and 14 from each group was subjected to fast protein 944 Journal of Lipid Research Volume 42, 2001
3 liquid chromatography (FPLC) gel filtration (Pharmacia LKB Biotechnology) on two Superose 6 columns as previously described (11). Cholesterol concentrations in the fractions were determined with an enzymatic assay (Wako Pure Chemical Industries, Ltd.), and human-specific apoe levels in each fraction were quantified by the same method as above. The amount of cholesterol in each class was calculated using FPLC fractions #3 #10 for VLDL, #11 #24 for IDL/LDL, and #25 #40 for HDL. After separating the pooled plasma samples on FPLC, 6- l samples from three adjacent fractions were pooled and subjected to 3 20% gradient SDS-PAGE. Mouse apoa-i and mouse apob-48 and apob-100 were detected using rabbit anti-mouse apoa-i and apob (Biodesign International). Peroxidase-labeled goat anti-rabbit antibody ( Jackson Immuno Research) was used for detection. the level of the control mice by day 14. Mice injected with the apoe3 vector did not show a decrease in cholesterol relative to the control group, consistent with our previous report (25). Interestingly, coexpression of apoe3 with Statistical analysis Atherosclerotic lesion area data and lipids data on each day were subjected to ANOVA with the Kruskal-Wallis test, followed by the Dunn s multiple comparison test. Statistical significance for all comparisons was assigned at P Graphs and tables represent mean values SEM. RESULTS Effects on plasma lipid levels Male LDLR-deficient mice on a Western-type diet were injected with adenoviral vectors encoding the LDLR alone, apoe3 alone, LDLR plus apoe3, or control LacZ. Mean plasma levels of human apoe in mice coexpressing the LDLR (250 mg/dl) were 51% higher than in the mice injected with the apoe3 vector alone (166 mg/dl) on day 7, and 806% higher (LDLR apoe3; 154 mg/dl, apoe3 alone; 17 mg/dl) on day 14. Human apoe could be detected in both groups of mice injected with apoe vector throughout the experimental period but remained significantly higher in mice coexpressing the LDLR even after 6 weeks (LDLR apoe3; 22 mg/dl, apoe3 alone; 12 mg/dl). The effects on plasma cholesterol levels are shown in Table 1. Mice injected with control vector (AdlacZ) experienced transiently decreased levels of plasma cholesterol, which returned to baseline by day 14. Mice injected with the LDLR vector had a significant 75% decrease in cholesterol that was transient, similar to that previously reported in chow-fed mice (3), and cholesterol levels returned to TABLE 1. Changes in plasma total cholesterol after vector injection (mg/dl) Day 0 Day 3 Day 7 Day 14 Day 21 Day 28 Day 42 Control 1,946 1,442 1,331 1,453 1,497 1,572 1,909 (n 10) (373) (209) (217) (363) (290) (340) (318) LDLR 2, a 501 a 1,344 1,335 1,342 1,745 (n 9) (352) (278) (162) (299) (264) (240) (385) ApoE3 1,937 1,869 b 1,322 b 1,257 1,353 1,441 1,933 (n 10) (336) (234) (205) (142) (244) (287) (240) LDLR apoe3 1, a,c 628 a,c 1,120 1,342 1,616 1,939 (n 11) (308) (258) (102) (170) (301) (325) (423) Values are given as mean (SEM). a Significantly different from control, P b Significantly different from LDLR, P c Significantly different from apoe3, P Fig. 1. FPLC cholesterol profile. Pooled plasma samples were subjected to gel filtration using Superose 6 columns, and cholesterol level in each fraction was measured by using an enzymatic kit. A: Data from samples collected before injection of vectors. B: data from samples collected 3 days after injection of vectors. C: data from samples collected 7 days after injection of vectors. Squares, mice-injected AdlacZ/tsAdlacZ; diamonds, mice-injected AdLDLR; open triangles, mice-injected tsadapoe3; solid triangles, miceinjected tsadapoe3 with AdLDLR. Data are mean SE. Kawashiri et al. Effects of coexpression of LDLR and apoe 945
4 LDLR did not reduce cholesterol levels to any greater degree than expression of the LDLR alone. Thus, hepatic overexpression of apoe3 in LDLR-deficient mice had minimal effects on plasma cholesterol levels either alone or in coexpression with the LDLR. Effects on lipoprotein distribution To gain insight into the effects of apoe3 and LDLR expression on plasma lipoprotein, we separated lipoproteins by FPLC gel filtration. Prior to vector injection (Fig. 1A), there were no significant differences among the four groups. On day 3 after injection (Fig. 1B), expression of the LDLR alone markedly reduced the VLDL and IDL/ LDL fractions. In contrast, expression of apoe alone increased VLDL and IDL cholesterol compared with the control group. Coexpression of apoe3 with the LDLR resulted in a higher VLDL peak than expression of the LDLR alone and had no effect on IDL and LDL com- Fig. 2. Distribution of murine apoa-i (A) and murine apob (B) among lipoprotein classes. Six- l samples from three adjacent FPLC fractions were pooled and subjected to SDS-PAGE, followed by Western blotting analysis with the antibody against murine apoa-i and apob. C: Agarose electrophoresis of pooled FPLC fractions (VLDL #5 #7, LDL #16 #18, New peak #24 #26, and HDL #34 #36) of plasma obtained 3 days after injection from control and apoe LDLR vector-injected mice. The new cholesterol peak is noted to run in the alpha region. D: ApoE concentrations in FPLC fractions. Pooled plasma samples were subjected to gel filtration using Superose 6 columns, and humanspecific apoe levels were measured by ELISA. Squares: mice injected with control vector; open triangles, mice injected with apoe3 vector alone; filled triangles, mice injected with LDLR apoe3 vectors. 946 Journal of Lipid Research Volume 42, 2001
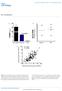
5 pared with expression of LDLR alone. In addition, coexpression of apoe3 and LDLR resulted in the appearance of a new peak (fractions #22 #33) between the LDL and HDL peaks. This peak is unlikely to be an artifact caused by the adenoviral vector itself because it is not seen in control, LDLR, or apoe vector-injected mice, but only in mice expressing both the LDLR and apoe. On day 7 after injection (Fig. 1C), expression of LDLR alone continued to result in substantially reduced VLDL, IDL, and LDL, whereas expression of apoe3 alone increased VLDL but modestly reduced LDL. The new cholesterol peak between LDL and HDL was still present on day 7 in the mice coexpressing the LDLR plus apoe. Western blotting for murine apoa-i (Fig. 2A) and apob (Fig. 2B) in the same FPLC fractions were performed to determine which class of lipoprotein contributed in the new cholesterol peak. The distribution of mouse apoa-i did not differ among the four groups around fractions #22 #33. Expression of the LDLR alone or apoe3 alone did not change apob distribution around fractions #22 #33. The peak between LDL and HDL was noted to contain substantial mouse apoa-i but little mouse apob, suggesting that it is a large HDL particle. We performed agarose electrophoresis on the FPLC-isolated fractions including this new peak and confirmed that it migrated in the alpha region, consistent with it being an HDL particle (Fig. 2C). The amount of human apoe in FPLC fractions was quantitated by ELISA (Fig. 2D). When apoe3 was expressed alone, human apoe was found mainly in VLDL peak and fractions #22 #30 representing large HDL. When apoe3 was coexpressed with the LDLR, less human apoe3 was found in VLDL and, interestingly, greater than 2-fold more apoe3 was found in large HDL compared with apoe3 expression alone. This suggests that the reduction in VLDL brought about by LDLR expression resulted in a shift of human apoe to apoe-rich large HDL particles. The amount of cholesterol in each lipoprotein fraction was calculated using the FPLC fraction cholesterol data. After control virus injection, both VLDL (Fig. 3A) and IDL/LDL cholesterol (Fig. 3B) decreased but HDL cholesterol (Fig. 3C) did not change. Expression of the LDLR alone decreased both the VLDL (90%) and IDL/LDL cholesterol (60%) levels, but did not change the HDL cholesterol level. Expression of apoe alone had little effect on the lipoprotein classes compared with controls. Interestingly, coexpression of apoe3 with LDLR decreased VLDL and IDL/LDL cholesterol similar to that of LDLR expression alone, but increased HDL cholesterol level to a greater extent than expression of either LDLR or apoe3 alone. Effects on atherosclerosis Atherosclerotic lesion area was quantified using two independent methods, en face analysis of the lipid-stained aorta and cross-sectional analysis of the aortic root. Both analytical methods produced similar results (Table 2). In the en face analysis, compared with mice injected with control vector, the mice expressing the LDLR alone had 43% reduced atherosclerosis (P 0.01), mice expressing apoe alone had 21% reduced atherosclerosis (P 0.19), TABLE 2. Atherosclerosis lesion area Fig. 3. Cholesterol changes in lipoprotein subclasses. Pooled plasma samples were subjected to gel filtration using Superose 6 columns, followed by cholesterol measurement in each fraction. After calculating the percentage of cholesterol recovered in the (A) VLDL, (B) IDL/LDL, and (C) HDL fractions, the actual VLDL-C, IDL/LDL-C, and HDL-C levels in plasma were calculated by multiplying each by the plasma cholesterol level. Squares: Mice-injected AdlacZ/tsAdlacZ; diamonds, mice-injected AdLDLR; open triangle, mice-injected tsadapoe3; solid triangle, mice-injected tsadapoe3 with AdLDLR. En Face Percent Lesion (%) Aortic Root Lesion Area ( m 10 3 ) Control (n 10) 8.9 (2.5) 373 (124) LDLR (n 9) 4.9 (1.6) a 208 (71) a ApoE3 (n 10) 6.6 (2.1) 273 (88) LDLR apoe3 (n 11) 5.5 (2.3) a 208 (66) a Values are given as mean (SEM). For both assays, the differences among the four groups were significant by ANOVA. a Significantly different from control (P 0.05) on post-test. Kawashiri et al. Effects of coexpression of LDLR and apoe 947
6 Fig. 4. Effects of LDLR and apoe expression on macrophage content of atherosclerotic lesions in the aortic root. A: analysis of macrophage lesion area. * P 0.01, significantly different from control. B: percent of aortic root lesion occupied by macrophagederived foam cells. * P 0.05, significantly different from control. and mice coexpressing the LDLR and apoe had 38% reduced atherosclerosis (P 0.02). In the aortic root analysis, compared with controls, the mice expressing the LDLR alone had 44% reduced atherosclerosis (P 0.01), mice expressing apoe alone had 27% reduced atherosclerosis (P 0.16), and mice co-expressing the LDLR and apoe had 44% reduced atherosclerosis (P 0.007). By both methods of quantitation, coexpression of the LDLR and apoe did not reduce atherosclerosis to a greater extent than expression of the LDLR alone. We also determined the effects of LDLR and apoe expression on the composition of lesions by staining for macrophages and quantitating the lesion area (Fig. 4A) and the percent of the total lesion occupied by macrophage-derived foam cells (Fig. 4B). Expression of LDLR alone and apoe alone each significantly reduced the macrophage lesion area and percent lesion area. However, coexpression of the LDLR and apoe did not reduce macrophage lesion area to a greater extent than either one alone. DISCUSSION In these studies, we used somatic gene transfer to test the additive effects of coexpression of the LDLR and apoe compared with either one alone on cholesterol metabolism and atherosclerosis in LDLR-deficient mice fed a Western-type diet. Previous studies (3) had confirmed that expression of the LDLR using gene transfer in LDLR-deficient mice effectively reduced plasma cholesterol levels. On the other hand, gene transfer and hepatic overexpression of apoe alone in LDLR-deficient mice fed a Westerntype diet had little effect on plasma cholesterol levels (25). We hypothesized that hepatic gene transfer and coexpression of apoe and the LDLR in LDLR-deficient mice would result in a more substantial reduction in plasma cholesterol than either one alone. As expected, expression of the LDLR alone had a substantial effect in reducing plasma cholesterol levels in this mouse model. However, in contrast to our expectations, coexpression of apoe with the LDLR did not result in a greater reduction of plasma cholesterol than expression of the LDLR alone. However, coexpression did result in a substantial increase in large apoe-rich HDL particles compared with expression of apoe alone. This may be because when apoe was expressed alone, a substantial amount of apoe associated with VLDL and IDL particles; however, coexpression of the LDLR resulted in clearance of the majority of apob-containing lipoproteins, with the apoe then preferentially distributed to large HDL particles. As apoe in HDL is catabolized more slowly than apoe in apobcontaining lipoproteins (30, 31), this might also explain the seemingly paradoxical finding that human apoe levels were higher in mice coexpressing the LDLR than in those expressing apoe alone. The failure of apoe expression alone to reduce cholesterol levels in the absence of the LDLR is consistent with other published data. Plasma cholesterol levels in apoedeficient mice can be normalized by very low levels of apoe expression amounting to only about 1% of normal apoe levels (14). However, when apoe-deficient mice lacking the LDLR were transplanted with wild-type bone marrow (a source of macrophage-derived apoe) (32) or injected with an adenoviral vector expressing human apoe3 (33), there was no effect on plasma cholesterol levels. In fact, apoecontaining remnant lipoproteins can compete with LDL for uptake (20), and apoe inhibits lipolysis and catabolism of remnant lipoproteins (33). Finally, hepatic apoe promotes the hepatic secretion of VLDL triglycerides (34 37) and VLDL apob (38). Therefore any effect of apoe in promoting clearance of VLDL may be offset by its effect in promoting VLDL production. In the current study, LDLR expression alone effectively decreased both VLDL and IDL/LDL. ApoE3 expression alone increased VLDL and IDL, probably due to increased VLDL production and/or reduced VLDL lipolysis. Coexpression of apoe3 and LDLR decreased VLDL less effectively compared with expression of the LDLR alone, and had a similar effect on LDL compared with LDLR alone. Another goal of this study was to test the hypothesis that the effects of cholesterol reduction using LDLR gene transfer and apoe expression would be additive with regard to reduction in atherosclerosis. Abundant data in animals and humans indicate that long-term cholesterol reduction reduces atherosclerosis. A recombinant adeno-associated viral vector encoding the VLDL receptor reduced plasma cholesterol levels for up to 7 months and reduced atherosclerosis in LDLR-deficient mice fed a Western-type diet (39). ApoE has antiatherogenic effects that extend beyond 948 Journal of Lipid Research Volume 42, 2001
7 its ability to reduce plasma cholesterol levels. Macrophagederived apoe directly inhibits progression of atherosclerosis (12, 13, 40). Expression of apoe at levels that did not reduce plasma cholesterol levels reduced atherosclerosis in apoe-deficient mice (27). We previously showed that hepatic gene transfer of apoe in LDLR-deficient mice fed a Western-type diet reduced the progression of atherosclerosis despite having no effect on plasma cholesterol levels (25). Thus, cholesterol lowering alone and apoe expression in the absence of cholesterol reduction each have the ability individually to reduce progression of atherosclerosis in mice. In this study, even transient reduction in plasma cholesterol brought about by LDLR expression was associated with a significant reduction in atherosclerosis. However, our results indicate that in this experimental model there were not additive effects of cholesterol reduction and apoe expression in inhibiting the progression of atherosclerosis. However, these were short-term studies, and it is possible that longer term combined expression could have greater effects on lipoprotein metabolism and/or atherosclerosis. In summary, apoe3 and/or LDLR were expressed in the livers of LDLR-deficient mice fed a Western-type diet to test the additive effects on lipoprotein metabolism and the development of atherosclerosis. Overexpression of the LDLR alone reduced cholesterol levels transiently and had a significant effect on atherosclerosis, but the addition of apoe expression to LDLR expression did not reduce cholesterol levels or atherosclerosis to a greater extent than the LDLR alone. We are indebted to Dr. Karen F. Kozarsky for providing AdhLDLR and Dr. Kazuhisa Tsukamoto for making AdhapoE3; to Robert Hughes, Anna Lilluthan, Linda Morrell, and Anthony Secreto for excellent technical assistance; and to Drs. Jane Glick and Cyrille Maugeais for helpful discussions. This work was supported by grant HL57811 from the National Institutes of Health. D.J.R. is an Established Investigator of the American Heart Association. Manuscript received 13 September 2000 and in revised form 8 February REFERENCES 1. Brown, M. S., and J. L. Goldstein A receptor-mediated pathway for cholesterol homeostasis. Science. 232: Ishibashi, S., J. Goldstein, M. Brown, J. Herz, and D. Burns Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J. Clin. Invest. 93: Ishibashi, S., M. Brown, J. Goldstein, R. Gerard, R. Hammer, and J. Herz Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest. 92: Mahley, R. W Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 240: Mahley, R. W., and Z. S. Ji Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J. Lipid Res. 40: Rohlmann, A., M. Gotthardt, R. E. Hammer, and J. Herz Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remants. J. Clin. Invest. 101: Plump, A. S., J. D. Smith, T. Hayek, K. Aalto-Setälä, A. Walsh, J. G. Verstuyft, E. M. Rubin, and J. L. Breslow Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 71: Zhang, S., R. Reddick, J. Piedrahita, and N. Maeda Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 258: Stevenson, S., J. Marshall-Neff, B. Teng, C. Lee, S. Roy, and A. McClelland Phenotypic correction of hypercholesterolemia in apoe-deficient mice by adenovirus-mediated in vivo gene transfer. Arterio. Thromb. Vasc. Biol. 15: Kashyap, V. S., S. Santamarina-Fojo, D. R. Brown, C. L. Parrott, D. Applebaum-Bowden, S. Meyn, G. Talley, B. Paigen, N. Maeda, and H. B. Brewer, Jr Apolipoprotein E deficiency in mice: gene replacement and prevention of atherosclerosis using adenovirus vectors. J. Clin. Invest. 96: Tsukamoto, K., P. Smith, J. M. Glick, and D. J. Rader Liverdirected gene transfer and prolonged expression of three major human apoe isoforms in apoe-deficient mice. J. Clin. Invest. 100: Linton, M. F., J. B. Atkinson, and S. Fazio Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 267: Boisvert, W., J. Spangenberg, and L. Curtiss Treatment of severe hypercholesterolemia in apolipoprotein E-deficient mice by bone marrow transplantation. J. Clin. Invest. 96: Hasty, A. H., M. F. Linton, L. L. Swift, and S. Faxio Determination of the lower threshold of apolipoprotein E resulting in remnant lipoprotein clearance. J. Lipid Res. 40: Shimano, H., N. Yamada, M. Katsuki, M. Shimada, T. Gotoda, K. Harada, T. Murase, C. Fukazawa, F. Takaku, and Y. Yazaki Overexpression of apolipoprotein E in transgenic mice: marked reduction in plasma lipoproteins except high density lipoprotein and resistance against diet-induced hypercholesterolemia. Proc. Natl. Acad. Sci. USA 89: Shimano, H., N. Yamada, M. Katsuki, K. Yamamoto, T. Gotoda, K. Harada, M. Shimada, and Y. Yazaki Plasma lipoprotein metabolism in transgenic mice overexpressing apolipoprotein E. Accelerated clearance of lipoproteins containing apolipoprotein B. J. Clin. Invest. 90: Yamamoto, K., H. Shimano, M. Shimada, M. Kawamura, T. Gotoda, K. Harada, J. Ohsuga, Y. Yazaki, and N. Yamada Overexpression of apolipoprotein E prevents development of diabetic hyperlipidemia in transgenic mice. Diabetes. 44: Osuga, J., M. Yonemoto, N. Yamada, H. Shimano, H. Yagyu, K. Ohashi, K. Harada, T. Kamei, Y. Yazaki, and S. Ishibashi Cholesterol lowering in low density lipoprotein receptor knockout mice overexpressing apolipoprotein E. J. Clin. Invest. 102: Fan, J., S. J. Ji, Y. Huang, H. De Silva, D. Sanan, R. W. Mahley, T. L. Innerarity, and J. M. Taylor Increased expression of apolipoprotein E in transgenic rabbits results in reduced levels of very low density lipoproteins and an accumulation of low density lipoproteins in plasma. J. Clin. Invest. 101: Woollett, L. A., Y. Osono, J. Herz, and J. M. Dietschy Apolipoprotein E competitively inhibits receptor-dependent low density lipoprotein uptake by the liver but has no effect on cholesterol absorption or synthesis in the mouse. Proc. Natl. Acad. Sci. USA 92: Nakashima, Y., A. S. Plump, E. W. Raines, J. L. Breslow, and R. Ross ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. Vasc. Biol. 14: Tsukamoto, K., R. Tangirala, S. H. Chun, E. Puré, and D. J. Rader Rapid regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein E in apoe deficient mice. Arterioscler. Thromb. Vasc. Biol. 19: Desurmont, C., J. M. Caillaud, F. Emmanuel, P. Benoit, J. C. Fruchart, G. Castro, D. Branellec, J. M. Heard, and N. Duverger Complete atherosclerosis regression after human ApoE gene transfer in ApoE-deficient/nude mice. Arterioscler. Thromb. Vasc. Biol. 20: Yamada, N., I. Inoue, M. Kawamura, K. Harada, Y. Watanabe, H. Shimano, T. Gotoda, M. Shimada, K. Kohzaki, T. Tsukada, et al Apolipoprotein E prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbits. J. Clin. Invest. 89: Tsukamoto, K., R. Tangirala, S. Chun, D. Usher, E. Puré, and D. J. Rader Hepatic expression of apolipoprotein E inhibits pro- Kawashiri et al. Effects of coexpression of LDLR and apoe 949
8 gression of atherosclerosis without reducing cholesterol levels in LDL receptor-deficient mice. Molecular Therapy. 1: Tangirala, R.K., D. Pratico, G. A. FitzGerald, S. Chun, K. Tsukamoto, C. Maugeais, D. C. Usher, E. Puré, and D. J. Rader Reduction of isoprostanes and regression of advanced atherosclerosis by apolipoprotein E. J. Biol. Chem. 276: Thorngate, F. E., L. L. Rudel, R. L. Walzem, and D. L. Williams Low levels of extrahepatic nonmacrophage ApoE inhibit atherosclerosis without correcting hypercholesterolemia in ApoEdeficient mice. Arterioscler. Thromb. Vasc. Biol. 20: Kozarsky, K. F., D. R. McKinley, L. L. Austin, S. E. Raper, L. D. Stratford-Perricaudet, and J. M. Wilson In vivo correction of low density lipoprotein receptor deficiency in the watanabe heritable hyperlipidemic rabbit with recombinant adenoviruses. J. Biol. Chem. 269: Tangirala, R. K., K. Tsukamoto, S. H. Chun, D. Usher, E. Puré, and D. J. Rader Regression of atherosclerosis induced by liverdirected gene transfer of apolipoprotein A-I in mice. Circulation. 100: Gregg, R. E., L. A. Zech, E. J. Schaefer, and H. B. Brewer, Jr Apolipoprotein E metabolism in normolipoproteinemic human subjects. J. Lipid Res. 25: Gregg, R. E., and H. B. Brewer, Jr In vivo metabolism of apolipoprotein E in humans. Meth. Enzy. 129: Linton, M. F., A. H. Hasty, V. R. Babaev, and S. Fazio Hepatic Apo E expression is required for remnant lipoprotein clearance in the absence of the low density lipoprotein receptor. J. Clin. Invest. 101: van Dijk, K. W., B. J. M. van Vlijmen, H. B. van t Hof, A. van der Zee, S. S. Fojo, T. J. Van Berkel, L. M. Havekes, and M. H. Hofker In LDL receptor-deficient mice, catabolism of remnant lipoproteins requires a high level of apoe but is inhibited by excess apoe. J. Lipid Res. 40: Mahley, R. W., and Y. Huang Apolipoprotein E: from atherosclerosis to Alzheimer s disease and beyond. Curr. Opin. Lipidol. 10: Tsukamoto, K., C. Maugeais, J. M. Glick, and D. J. Rader Markedly increased secretion of VLDL triglycerides induced by gene transfer of apolipoprotein E isoforms in apoe-deficient mice. J. Lipid Res. 41: Kuipers, F., M. C. Jong, Y. Lin, M. van Eck, R. Havinga, V. Blocks, H. J. Verkade, M. H. Hofker, H. Moshage, T. J. C. van Berkel, et al Impaired secretion of very low density lipoprotein-triglycerides by apolipoprotein E-deficient mouse hepatocytes. J. Clin. Invest. 100: Huang, Y., X. Q. Liu, S. C. J. Rall, J. M. Taylor, A. von Eckardstein, G. Assmann, and R. W. Mahley Overexpression and accumulation of apolipoprotein E as a cause of hypertriglyceridemia. J. Biol. Chem. 273: Maugeais, C., U. J. Tietge, K. Tsukamoto, J. M. Glick, and D. J. Rader Hepatic apolipoprotein E expression promotes very low density lipoprotein-apolipoprotein B production in vivo in mice. J. Lipid Res. 41: Chen, S. J., D. J. Rader, J. Tazelaar, M. Kawashiri, G. P. Gao, and J. M. Wilson Prolonged correction of hyperlipidemia in mice with familial hypercholesterolemia using an adeno-associated viral vector expressing VLDL receptor. Molecular Therapy. 2: Fazio, S., V. Babaev, A. Murray, A. Hasty, K. Carter, L. Gleaves, J. Atkinson, and M. Linton Increased atherosclerosis in mice reconstituted with apolipoprotein E null macrophages. Proc. Natl. Acad. Sci. USA. 94: Journal of Lipid Research Volume 42, 2001
Determination of the lower threshold of apolipoprotein E resulting in remnant lipoprotein clearance 1
 Determination of the lower threshold of apolipoprotein E resulting in remnant lipoprotein clearance 1 Alyssa H. Hasty,* MacRae F. Linton, 2,, Larry L. Swift,* and Sergio Fazio 2, *, Departments of Pathology,*
Determination of the lower threshold of apolipoprotein E resulting in remnant lipoprotein clearance 1 Alyssa H. Hasty,* MacRae F. Linton, 2,, Larry L. Swift,* and Sergio Fazio 2, *, Departments of Pathology,*
Hiroshi Hosoai,* Nancy R. Webb, Jane M. Glick, Uwe J. F. Tietge,* Matthew S. Purdom, Frederick C. de Beer, and Daniel J.
 Expression of serum amyloid A protein in the absence of the acute phase response does not reduce HDL cholesterol or apoa-i levels in human apoa-i transgenic mice Hiroshi Hosoai,* Nancy R. Webb, Jane M.
Expression of serum amyloid A protein in the absence of the acute phase response does not reduce HDL cholesterol or apoa-i levels in human apoa-i transgenic mice Hiroshi Hosoai,* Nancy R. Webb, Jane M.
Lipoprotein Clearance Mechanisms in LDL Receptor Deficient Apo-B48-only and Apo-B100-only Mice
 Lipoprotein Clearance Mechanisms in LDL Receptor Deficient Apo-B48-only and Apo-B100-only Mice Murielle M. Véniant,* Constance H. Zlot,* Rosemary L. Walzem, Vincenzo Pierotti,* Robert Driscoll,* David
Lipoprotein Clearance Mechanisms in LDL Receptor Deficient Apo-B48-only and Apo-B100-only Mice Murielle M. Véniant,* Constance H. Zlot,* Rosemary L. Walzem, Vincenzo Pierotti,* Robert Driscoll,* David
hypothesis in "knockout" mice lacking the low density lipoprotein
 Proc. Nati. Acad. Sci. USA Vol. 91, pp. 4431-4435, May 1994 Medical Sciences The two-receptor model of lipoprotein clearance: Tests of the hypothesis in "knockout" mice lacking the low density lipoprotein
Proc. Nati. Acad. Sci. USA Vol. 91, pp. 4431-4435, May 1994 Medical Sciences The two-receptor model of lipoprotein clearance: Tests of the hypothesis in "knockout" mice lacking the low density lipoprotein
review Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E
 review Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E Robert W. Mahley 1, *, and Zhong-Sheng Ji* Gladstone Institute of Cardiovascular
review Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E Robert W. Mahley 1, *, and Zhong-Sheng Ji* Gladstone Institute of Cardiovascular
Review Transgenic mouse models of lipoprotein metabolism
 Proc. Natl. Acad. Sci. USA Vol. 90, pp. 8314-8318, September 1993 Review Transgenic mouse models of lipoprotein metabolism and atherosclerosis (apolpoprotein/diet/colester) Jan L. Breslow Laboratory of
Proc. Natl. Acad. Sci. USA Vol. 90, pp. 8314-8318, September 1993 Review Transgenic mouse models of lipoprotein metabolism and atherosclerosis (apolpoprotein/diet/colester) Jan L. Breslow Laboratory of
Apoe Targeted Mutation Mice
 Apoe Targeted Mutation Mice Inactivation of the Apoe gene provides a mouse model for human atherogenesis and diverse applications in studies of lipid transport and metabolism Applications for the Apoe
Apoe Targeted Mutation Mice Inactivation of the Apoe gene provides a mouse model for human atherogenesis and diverse applications in studies of lipid transport and metabolism Applications for the Apoe
Severe Hypercholesterolemia, Hypertriglyceridemia, and Atherosclerosis in Mice Lacking Both Leptin and the Low Density Lipoprotein Receptor*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 276, No. 40, Issue of October 5, pp. 37402 37408, 2001 2001 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Severe Hypercholesterolemia,
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 276, No. 40, Issue of October 5, pp. 37402 37408, 2001 2001 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Severe Hypercholesterolemia,
Pathophysiology of Lipid Disorders
 Pathophysiology of Lipid Disorders Henry Ginsberg, M.D. Division of Preventive Medicine and Nutrition CHD in the United States CHD is the single largest killer of men and women 12 million have history
Pathophysiology of Lipid Disorders Henry Ginsberg, M.D. Division of Preventive Medicine and Nutrition CHD in the United States CHD is the single largest killer of men and women 12 million have history
Supplemental Material. Results
 Supplemental Material Results Fractionation of mouse plasma by high-resolution SEC. APOA1 eluted as a single major peak in fractions 16 of plasma (the apparent size of mature, lipidated HDL) when it was
Supplemental Material Results Fractionation of mouse plasma by high-resolution SEC. APOA1 eluted as a single major peak in fractions 16 of plasma (the apparent size of mature, lipidated HDL) when it was
Metabolism and Atherogenic Properties of LDL
 Metabolism and Atherogenic Properties of LDL Manfredi Rizzo, MD, PhD Associate Professor of Internal Medicine Faculty of Medicine, University of Palermo, Italy & Affiliate Associate Professor of Internal
Metabolism and Atherogenic Properties of LDL Manfredi Rizzo, MD, PhD Associate Professor of Internal Medicine Faculty of Medicine, University of Palermo, Italy & Affiliate Associate Professor of Internal
Inhibition of endothelial lipase causes increased HDL cholesterol levels in vivo
 Inhibition of endothelial lipase causes increased HDL cholesterol levels in vivo See the related Commentary beginning on page 318. Weijun Jin, John S. Millar, Uli Broedl, Jane M. Glick, and Daniel J. Rader
Inhibition of endothelial lipase causes increased HDL cholesterol levels in vivo See the related Commentary beginning on page 318. Weijun Jin, John S. Millar, Uli Broedl, Jane M. Glick, and Daniel J. Rader
Previous studies have provided evidence that atherosclerosis
 Effect of Macrophage-Derived Apolipoprotein E on Established Atherosclerosis in Apolipoprotein E Deficient Mice Weibin Shi, Xuping Wang, Nicholas J. Wang, William H. McBride, Aldons J. Lusis Abstract Apolipoprotein
Effect of Macrophage-Derived Apolipoprotein E on Established Atherosclerosis in Apolipoprotein E Deficient Mice Weibin Shi, Xuping Wang, Nicholas J. Wang, William H. McBride, Aldons J. Lusis Abstract Apolipoprotein
High density lipoprotein metabolism
 High density lipoprotein metabolism Lipoprotein classes and atherosclerosis Chylomicrons, VLDL, and their catabolic remnants Pro-atherogenic LDL HDL Anti-atherogenic Plasma lipid transport Liver VLDL FC
High density lipoprotein metabolism Lipoprotein classes and atherosclerosis Chylomicrons, VLDL, and their catabolic remnants Pro-atherogenic LDL HDL Anti-atherogenic Plasma lipid transport Liver VLDL FC
Summary and concluding remarks
 Summary and concluding remarks This thesis is focused on the role and interaction of different cholesterol and phospholipid transporters. Cholesterol homeostasis is accomplished via a tightly regulated
Summary and concluding remarks This thesis is focused on the role and interaction of different cholesterol and phospholipid transporters. Cholesterol homeostasis is accomplished via a tightly regulated
Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice
 Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice Atherosclerosis, 2007 Chiba T, Shinozaki S, Nakazawa T, et al. Present by Sudaporn Pummoung Apolipoprotein E (apoe( apoe)
Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice Atherosclerosis, 2007 Chiba T, Shinozaki S, Nakazawa T, et al. Present by Sudaporn Pummoung Apolipoprotein E (apoe( apoe)
Human LDL Receptor / LDLR ELISA Pair Set
 Human LDL Receptor / LDLR ELISA Pair Set Catalog Number : SEK10231 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in
Human LDL Receptor / LDLR ELISA Pair Set Catalog Number : SEK10231 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in
Effect of atorvastatin on plasma apoe metabolism in patients with combined hyperlipidemia
 Effect of atorvastatin on plasma apoe metabolism in patients with combined hyperlipidemia Jeffrey S. Cohn, 1, * Michel Tremblay,* Rami Batal,* Hélène Jacques,* Lyne Veilleux,* Claudia Rodriguez,* P. Hugh
Effect of atorvastatin on plasma apoe metabolism in patients with combined hyperlipidemia Jeffrey S. Cohn, 1, * Michel Tremblay,* Rami Batal,* Hélène Jacques,* Lyne Veilleux,* Claudia Rodriguez,* P. Hugh
Apolipoprotein E (apoe) and the low-density lipoprotein
 Human LDL Receptor Enhances Sequestration of ApoE4 and VLDL Remnants on the Surface of Hepatocytes but Not Their Internalization in Mice Michael Altenburg, Jose Arbones-Mainar, Lance Johnson, Jennifer
Human LDL Receptor Enhances Sequestration of ApoE4 and VLDL Remnants on the Surface of Hepatocytes but Not Their Internalization in Mice Michael Altenburg, Jose Arbones-Mainar, Lance Johnson, Jennifer
The risk of developing atherosclerosis and coronary heart
 Increased LDL Cholesterol and Atherosclerosis in LDL Receptor Deficient Mice With Attenuated Expression of Scavenger Receptor B1 Dennis Huszar, Mariet Lee Varban, Franz Rinninger, Roslyn Feeley, Takeshi
Increased LDL Cholesterol and Atherosclerosis in LDL Receptor Deficient Mice With Attenuated Expression of Scavenger Receptor B1 Dennis Huszar, Mariet Lee Varban, Franz Rinninger, Roslyn Feeley, Takeshi
Plasma concentrations of HDL cholesterol (HDL-C) and
 Dose-Dependent Acceleration of High-Density Lipoprotein Catabolism by Endothelial Lipase Cyrille Maugeais, PhD; Uwe J.F. Tietge, MD; Uli C. Broedl, MD; Dawn Marchadier, MS; William Cain, PhD; Mary G. McCoy,
Dose-Dependent Acceleration of High-Density Lipoprotein Catabolism by Endothelial Lipase Cyrille Maugeais, PhD; Uwe J.F. Tietge, MD; Uli C. Broedl, MD; Dawn Marchadier, MS; William Cain, PhD; Mary G. McCoy,
2.5% of all deaths globally each year. 7th leading cause of death by % of people with diabetes live in low and middle income countries
 Lipid Disorders in Diabetes (Diabetic Dyslipidemia) Khosrow Adeli PhD, FCACB, DABCC Head and Professor, Clinical Biochemistry, The Hospital for Sick Children, University it of Toronto Diabetes A Global
Lipid Disorders in Diabetes (Diabetic Dyslipidemia) Khosrow Adeli PhD, FCACB, DABCC Head and Professor, Clinical Biochemistry, The Hospital for Sick Children, University it of Toronto Diabetes A Global
Hepatic lipase deficiency decreases the selective uptake of HDL-cholesteryl esters in vivo
 Hepatic lipase deficiency decreases the selective uptake of HDL-cholesteryl esters in vivo Gilles Lambert, 1, * Marcelo J. A. Amar,* Pascal Martin,* Jamila Fruchart-Najib, Bernhard Föger,* Robert D. Shamburek,*
Hepatic lipase deficiency decreases the selective uptake of HDL-cholesteryl esters in vivo Gilles Lambert, 1, * Marcelo J. A. Amar,* Pascal Martin,* Jamila Fruchart-Najib, Bernhard Föger,* Robert D. Shamburek,*
Role of apolipoprotein B-containing lipoproteins in the development of atherosclerosis Jan Borén MD, PhD
 Role of apolipoprotein B-containing lipoproteins in the development of atherosclerosis Jan Borén MD, PhD Our laboratory focuses on the role of apolipoprotein (apo) B- containing lipoproteins in normal
Role of apolipoprotein B-containing lipoproteins in the development of atherosclerosis Jan Borén MD, PhD Our laboratory focuses on the role of apolipoprotein (apo) B- containing lipoproteins in normal
Type III Hyperlipoproteinemia and Spontaneous Atherosclerosis in Mice Resulting from Gene Replacement of Mouse Apoe with Human APOE *2
 Type III Hyperlipoproteinemia and Spontaneous Atherosclerosis in Mice Resulting from Gene Replacement of Mouse Apoe with Human APOE *2 Patrick M. Sullivan,* Hafid Mezdour,* Steven H. Quarfordt, and Nobuyo
Type III Hyperlipoproteinemia and Spontaneous Atherosclerosis in Mice Resulting from Gene Replacement of Mouse Apoe with Human APOE *2 Patrick M. Sullivan,* Hafid Mezdour,* Steven H. Quarfordt, and Nobuyo
Apolipoprotein C-III deficiency accelerates triglyceride hydrolysis by lipoprotein lipase in wild-type and apoe knockout mice
 Apolipoprotein C-III deficiency accelerates triglyceride hydrolysis by lipoprotein lipase in wild-type and apoe knockout mice Miek C. Jong, 1, * Patrick C. N. Rensen, 1, Vivian E. H. Dahlmans,* Hans van
Apolipoprotein C-III deficiency accelerates triglyceride hydrolysis by lipoprotein lipase in wild-type and apoe knockout mice Miek C. Jong, 1, * Patrick C. N. Rensen, 1, Vivian E. H. Dahlmans,* Hans van
The mouse has become the pre-eminent species for the
 Brief Reviews Diet and Murine Atherosclerosis Godfrey S. Getz, Catherine A. Reardon Abstract Lipid-enriched diets are often used to induce or accelerate the rate of atherosclerotic lesion development in
Brief Reviews Diet and Murine Atherosclerosis Godfrey S. Getz, Catherine A. Reardon Abstract Lipid-enriched diets are often used to induce or accelerate the rate of atherosclerotic lesion development in
2.5. AMPK activity
 Supplement Fig. A 3 B phos-ampk 2.5 * Control AICAR AMPK AMPK activity (Absorbance at 45 nm) 2.5.5 Control AICAR Supplement Fig. Effects of AICAR on AMPK activation in macrophages. J774. macrophages were
Supplement Fig. A 3 B phos-ampk 2.5 * Control AICAR AMPK AMPK activity (Absorbance at 45 nm) 2.5.5 Control AICAR Supplement Fig. Effects of AICAR on AMPK activation in macrophages. J774. macrophages were
Behind LDL: The Metabolism of ApoB, the Essential Apolipoprotein in LDL and VLDL
 Behind LDL: The Metabolism of ApoB, the Essential Apolipoprotein in LDL and VLDL Sung-Joon Lee, PhD Division of Food Science Institute of Biomedical Science and Safety Korea University Composition of Lipoproteins:
Behind LDL: The Metabolism of ApoB, the Essential Apolipoprotein in LDL and VLDL Sung-Joon Lee, PhD Division of Food Science Institute of Biomedical Science and Safety Korea University Composition of Lipoproteins:
LDL Uptake Cell-Based Assay Kit
 LDL Uptake Cell-Based Assay Kit Catalog Number KA1327 100 assays Version: 07 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
LDL Uptake Cell-Based Assay Kit Catalog Number KA1327 100 assays Version: 07 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
The regulation of the production of apob from liver is
 Normal Production Rate of Apolipoprotein B in LDL Receptor Deficient Mice John S. Millar, Cyrille Maugeais, Ilia V. Fuki, Daniel J. Rader Abstract The low density lipoprotein (LDL) receptor is well known
Normal Production Rate of Apolipoprotein B in LDL Receptor Deficient Mice John S. Millar, Cyrille Maugeais, Ilia V. Fuki, Daniel J. Rader Abstract The low density lipoprotein (LDL) receptor is well known
Bone marrow-derived HL mitigates bone marrow-derived CETP-mediated decreases in HDL in mice globally deficient in HL and the LDLr
 Bone marrow-derived HL mitigates bone marrow-derived CETP-mediated decreases in HDL in mice globally deficient in HL and the Neil J. Hime, 1,2 Audrey S. Black, David J. Bonnet, and Linda K. Curtiss Department
Bone marrow-derived HL mitigates bone marrow-derived CETP-mediated decreases in HDL in mice globally deficient in HL and the Neil J. Hime, 1,2 Audrey S. Black, David J. Bonnet, and Linda K. Curtiss Department
Chylomicron remnant uptake in the livers of mice expressing human apolipoproteins E3, E2 (Arg158 Cys), and E3-Leiden
 Chylomicron remnant uptake in the livers of mice expressing human apolipoproteins E3, E2 (Arg158 Cys), and E3-Leiden Sung-Joon Lee,*, Itamar Grosskopf,*,, ** Sungshin Y. Choi, and Allen D. Cooper 1, *,
Chylomicron remnant uptake in the livers of mice expressing human apolipoproteins E3, E2 (Arg158 Cys), and E3-Leiden Sung-Joon Lee,*, Itamar Grosskopf,*,, ** Sungshin Y. Choi, and Allen D. Cooper 1, *,
Review Gene therapy for lipid disorders Masa-aki Kawashiri and Daniel J Rader
 Review Gene therapy for lipid disorders Masa-aki Kawashiri and Daniel J Rader University of Pennsylvania, Philadelphia, Pennsylvania, USA Received: 18 September 2000 Accepted: 24 September 2000 Published:
Review Gene therapy for lipid disorders Masa-aki Kawashiri and Daniel J Rader University of Pennsylvania, Philadelphia, Pennsylvania, USA Received: 18 September 2000 Accepted: 24 September 2000 Published:
Mice Expressing Dysfunctional Apolipoprotein E
 Type IIl Hyperlipoproteinemic Phenotype in Transgenic Mice Expressing Dysfunctional Apolipoprotein E Sergio Fazio, Ya-Li Lee, Zhong-Sheng Ji, and Stanley C. Rail, Jr. Gladstone Institute ofcardiovascular
Type IIl Hyperlipoproteinemic Phenotype in Transgenic Mice Expressing Dysfunctional Apolipoprotein E Sergio Fazio, Ya-Li Lee, Zhong-Sheng Ji, and Stanley C. Rail, Jr. Gladstone Institute ofcardiovascular
APOB (Human) ELISA Kit
 APOB (Human) ELISA Kit Catalog Number KA4330 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
APOB (Human) ELISA Kit Catalog Number KA4330 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
In vivo contribution of LCAT to apolipoprotein B lipoprotein cholesteryl esters in LDL receptor and apolipoprotein E knockout mice
 In vivo contribution of LCAT to apolipoprotein B lipoprotein cholesteryl esters in LDL receptor and apolipoprotein E knockout mice James W. Furbee, Jr.,* Omar Francone, and John S. Parks 1, * Department
In vivo contribution of LCAT to apolipoprotein B lipoprotein cholesteryl esters in LDL receptor and apolipoprotein E knockout mice James W. Furbee, Jr.,* Omar Francone, and John S. Parks 1, * Department
YENHONG ZHU*, STEFANO BELLOSTA, CLAUS LANGER, FRANCO BERNINI, ROBERT ROBERT W. MAHLEY, GERD ASSMANN*,, AND ARNOLD VON ECKARDSTEIN* E.
 Proc. Natl. Acad. Sci. USA Vol. 95, pp. 7585 7590, June 1998 Medical Sciences Low-dose expression of a human apolipoprotein E transgene in macrophages restores cholesterol efflux capacity of apolipoprotein
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 7585 7590, June 1998 Medical Sciences Low-dose expression of a human apolipoprotein E transgene in macrophages restores cholesterol efflux capacity of apolipoprotein
Pathway of biogenesis of apolipoprotein E-containing HDL in vivo with the participation of ABCA1 and LCAT
 www.biochemj.org Biochem. J. (2007) 403, 359 367 (Printed in Great Britain) doi:10.1042/bj20061048 359 Pathway of biogenesis of apolipoprotein E-containing HDL in vivo with the participation of ABCA1 and
www.biochemj.org Biochem. J. (2007) 403, 359 367 (Printed in Great Britain) doi:10.1042/bj20061048 359 Pathway of biogenesis of apolipoprotein E-containing HDL in vivo with the participation of ABCA1 and
Hepatic lipase expression in macrophages contributes to atherosclerosis in apoe-deficient and LCAT-transgenic mice
 Hepatic lipase expression in macrophages contributes to atherosclerosis in apoe-deficient and LCAT-transgenic mice Zengxuan Nong, 1 Herminia González-Navarro, 1 Marcelo Amar, 1 Lita Freeman, 1 Catherine
Hepatic lipase expression in macrophages contributes to atherosclerosis in apoe-deficient and LCAT-transgenic mice Zengxuan Nong, 1 Herminia González-Navarro, 1 Marcelo Amar, 1 Lita Freeman, 1 Catherine
Atherosclerosis in perlecan heterozygous mice
 Atherosclerosis in perlecan heterozygous mice Reeba K. Vikramadithyan,* Yuko Kako,* Guangping Chen,* Yunying Hu,* Eri Arikawa-Hirasawa, Yoshihiko Yamada, and Ira J. Goldberg 1, * Department of Medicine,*
Atherosclerosis in perlecan heterozygous mice Reeba K. Vikramadithyan,* Yuko Kako,* Guangping Chen,* Yunying Hu,* Eri Arikawa-Hirasawa, Yoshihiko Yamada, and Ira J. Goldberg 1, * Department of Medicine,*
Reverse Transport of Cholesterol Is the Reason for Resistance to Development of Atherosclerosis in Prague Hereditary Hypercholesterolemic (PHHC) Rat
 Physiol. Res. 63: 591-596, 2014 Reverse Transport of Cholesterol Is the Reason for Resistance to Development of Atherosclerosis in Prague Hereditary Hypercholesterolemic (PHHC) Rat M. SCHMIEDTOVA 1, M.
Physiol. Res. 63: 591-596, 2014 Reverse Transport of Cholesterol Is the Reason for Resistance to Development of Atherosclerosis in Prague Hereditary Hypercholesterolemic (PHHC) Rat M. SCHMIEDTOVA 1, M.
Lipid/Lipoprotein Structure and Metabolism (Overview)
 Lipid/Lipoprotein Structure and Metabolism (Overview) Philip Barter President, International Atherosclerosis Society Centre for Vascular Research University of New South Wales Sydney, Australia Disclosures
Lipid/Lipoprotein Structure and Metabolism (Overview) Philip Barter President, International Atherosclerosis Society Centre for Vascular Research University of New South Wales Sydney, Australia Disclosures
Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved
 1 Supplemental Figure Legends Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved PCSK9 concentrations. 4 Plasma mature and furin-cleaved PCSK9s were measured by a sandwich
1 Supplemental Figure Legends Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved PCSK9 concentrations. 4 Plasma mature and furin-cleaved PCSK9s were measured by a sandwich
Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice.
 Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
Apo E structure determines VLDL clearance and atherosclerosis risk in mice
 Apo E structure determines VLDL clearance and atherosclerosis risk in mice Christopher Knouff, 1 Myron E. Hinsdale, 1 Hafid Mezdour, 1 Michael K. Altenburg, 1 Masahiko Watanabe, 1 Steven H. Quarfordt,
Apo E structure determines VLDL clearance and atherosclerosis risk in mice Christopher Knouff, 1 Myron E. Hinsdale, 1 Hafid Mezdour, 1 Michael K. Altenburg, 1 Masahiko Watanabe, 1 Steven H. Quarfordt,
Hepatic Remnant Lipoprotein Clearance by Heparan Sulfate Proteoglycans and Low-Density Lipoprotein Receptors Depend on Dietary Conditions in Mice
 Hepatic Remnant Lipoprotein Clearance by Heparan Sulfate Proteoglycans and Low-Density Lipoprotein Receptors Depend on Dietary Conditions in Mice Erin M. Foley,* Philip L.S.M. Gordts,* Kristin I. Stanford,*
Hepatic Remnant Lipoprotein Clearance by Heparan Sulfate Proteoglycans and Low-Density Lipoprotein Receptors Depend on Dietary Conditions in Mice Erin M. Foley,* Philip L.S.M. Gordts,* Kristin I. Stanford,*
Human LDL ELISA Kit. Innovative Research, Inc.
 Human LDL ELISA Kit Catalog No: IRKTAH2582 Lot No: SAMPLE INTRODUCTION Human low-density lipoprotein (LDL) transports cholesterol from the liver to tissues where it is incorporated into cell membranes.
Human LDL ELISA Kit Catalog No: IRKTAH2582 Lot No: SAMPLE INTRODUCTION Human low-density lipoprotein (LDL) transports cholesterol from the liver to tissues where it is incorporated into cell membranes.
Nature Genetics: doi: /ng.3561
 Supplementary Figure 1 Pedigrees of families with APOB p.gln725* mutation and APOB p.gly1829glufs8 mutation (a,b) Pedigrees of families with APOB p.gln725* mutation. (c) Pedigree of family with APOB p.gly1829glufs8
Supplementary Figure 1 Pedigrees of families with APOB p.gln725* mutation and APOB p.gly1829glufs8 mutation (a,b) Pedigrees of families with APOB p.gln725* mutation. (c) Pedigree of family with APOB p.gly1829glufs8
Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry
 Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry Learning Objectives 1. Define lipoproteins and explain the rationale of their formation in blood. 2. List different
Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry Learning Objectives 1. Define lipoproteins and explain the rationale of their formation in blood. 2. List different
Journal of the American College of Cardiology Vol. 50, No. 24, by the American College of Cardiology Foundation ISSN /07/$32.
 Journal of the American College of Cardiology Vol. 50, No. 24, 2007 2007 by the American College of Cardiology Foundation ISSN 0735-1097/07/$32.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2007.07.081
Journal of the American College of Cardiology Vol. 50, No. 24, 2007 2007 by the American College of Cardiology Foundation ISSN 0735-1097/07/$32.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2007.07.081
Abstract. Introduction
 Substitution of the Carboxyl-terminal Domain of apo AI with apo AII Sequences Restores the Potential of HDL to Reduce the Progression of Atherosclerosis in apo E Knockout Mice Paul Holvoet, Sophie Danloy,
Substitution of the Carboxyl-terminal Domain of apo AI with apo AII Sequences Restores the Potential of HDL to Reduce the Progression of Atherosclerosis in apo E Knockout Mice Paul Holvoet, Sophie Danloy,
行政院國家科學委員會補助專題研究計畫成果報告
 NSC892314B002270 898 1 907 31 9010 23 1 Molecular Study of Type III Hyperlipoproteinemia in Taiwan β β ε E Abstract β Type III hyperlipoproteinemia (type III HLP; familial dysbetalipoproteinemia ) is a
NSC892314B002270 898 1 907 31 9010 23 1 Molecular Study of Type III Hyperlipoproteinemia in Taiwan β β ε E Abstract β Type III hyperlipoproteinemia (type III HLP; familial dysbetalipoproteinemia ) is a
The bridging-function of hepatic lipase reduces both apo-b48-and apo-b100- containing lipoproteins in LDL receptor deficient apo-b48-only and apo-
 The bridging-function of hepatic lipase reduces both apo-b48-and apo-b100- containing lipoproteins in LDL receptor deficient apo-b48-only and apo- B100-only mice. Helén L. Dichek 1, Kun Qian, and Nalini
The bridging-function of hepatic lipase reduces both apo-b48-and apo-b100- containing lipoproteins in LDL receptor deficient apo-b48-only and apo- B100-only mice. Helén L. Dichek 1, Kun Qian, and Nalini
Apolipoprotein E (apoe) plays a central role in the
 Atherosclerosis and Lipoproteins Harmful Effects of Increased LDLR Expression in Mice With Human APOE*4 But Not APOE*3 Sudi I. Malloy,* Michael K. Altenburg,* Christopher Knouff,* Lorraine Lanningham-Foster,
Atherosclerosis and Lipoproteins Harmful Effects of Increased LDLR Expression in Mice With Human APOE*4 But Not APOE*3 Sudi I. Malloy,* Michael K. Altenburg,* Christopher Knouff,* Lorraine Lanningham-Foster,
lipoprotein receptor (low density lipoprotein-related protein/receptor-associated protein/cholesteryl esters)
 Proc. Natl. Acad. Sci. USA Vol. 92, pp. 4611-4615, May 1995 Medical Sciences Initial hepatic removal of chylomicron remnants is unaffected but endocytosis is delayed in mice lacking the low density lipoprotein
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 4611-4615, May 1995 Medical Sciences Initial hepatic removal of chylomicron remnants is unaffected but endocytosis is delayed in mice lacking the low density lipoprotein
Investigations on the mechanism of hypercholesterolemia observed in copper deficiency in rats
 J. Biosci., Vol. 12, Number 2, June 1987, pp. 137 142. Printed in India. Investigations on the mechanism of hypercholesterolemia observed in copper deficiency in rats P. VALSALA and P. A. KURUP Department
J. Biosci., Vol. 12, Number 2, June 1987, pp. 137 142. Printed in India. Investigations on the mechanism of hypercholesterolemia observed in copper deficiency in rats P. VALSALA and P. A. KURUP Department
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism
 ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism I. Chylomicrons (exogenous pathway) A. 83% triacylglycerol, 2% protein, 8% cholesterol plus cholesterol esters, 7% phospholipid (esp. phosphatidylcholine)
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism I. Chylomicrons (exogenous pathway) A. 83% triacylglycerol, 2% protein, 8% cholesterol plus cholesterol esters, 7% phospholipid (esp. phosphatidylcholine)
Mechanisms of obesity and related pathologies: Role of apolipoprotein E in the development of obesity
 MINIREVIEW Mechanisms of obesity and related pathologies: Role of apolipoprotein E in the development of obesity Kyriakos E. Kypreos 1, Iordanes Karagiannides 2, Elisavet H. Fotiadou 1, Eleni A. Karavia
MINIREVIEW Mechanisms of obesity and related pathologies: Role of apolipoprotein E in the development of obesity Kyriakos E. Kypreos 1, Iordanes Karagiannides 2, Elisavet H. Fotiadou 1, Eleni A. Karavia
Basic Science Reports
 Basic Science Reports Long-Term Stable Expression of Human Apolipoprotein A-I Mediated by Helper-Dependent Adenovirus Gene Transfer Inhibits Atherosclerosis Progression and Remodels Atherosclerotic Plaques
Basic Science Reports Long-Term Stable Expression of Human Apolipoprotein A-I Mediated by Helper-Dependent Adenovirus Gene Transfer Inhibits Atherosclerosis Progression and Remodels Atherosclerotic Plaques
Apolipoprotein E Deficiency in Mice: Gene Replacement and Prevention of Atherosclerosis Using Adenovirus Vectors
 Apolipoprotein E Deficiency in Mice: Gene Replacement and Prevention of Atherosclerosis Using Adenovirus Vectors Vikram S. Kashyap, Silvia Santamarina-Fojo, David R. Brown, Catherine L. Parrott, Deborah
Apolipoprotein E Deficiency in Mice: Gene Replacement and Prevention of Atherosclerosis Using Adenovirus Vectors Vikram S. Kashyap, Silvia Santamarina-Fojo, David R. Brown, Catherine L. Parrott, Deborah
Susceptibility to Diet-Induced Atherosclerosis in Transgenic Mice Expressing a Dysfunctional Human Apolipoprotein E(Arg 112,Cysl42)
 1873 Susceptibility to Diet-Induced Atherosclerosis in Transgenic Mice Expressing a Dysfunctional Human Apolipoprotein E(Arg 112,Cysl42) Sergio Fazio, David A. Sanan, Ya-Li Lee, Zhong-Sheng Ji, Robert
1873 Susceptibility to Diet-Induced Atherosclerosis in Transgenic Mice Expressing a Dysfunctional Human Apolipoprotein E(Arg 112,Cysl42) Sergio Fazio, David A. Sanan, Ya-Li Lee, Zhong-Sheng Ji, Robert
LDL Uptake Cell-Based Assay Kit
 LDL Uptake Cell-Based Assay Kit Item No. 10011125 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
LDL Uptake Cell-Based Assay Kit Item No. 10011125 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Brief Review. Godfrey S. Getz, Catherine A. Reardon
 Brief Review Animal Models of Atherosclerosis Godfrey S. Getz, Catherine A. Reardon Abstract Atherosclerosis is a chronic inflammatory disorder that is the underlying cause of most cardiovascular disease.
Brief Review Animal Models of Atherosclerosis Godfrey S. Getz, Catherine A. Reardon Abstract Atherosclerosis is a chronic inflammatory disorder that is the underlying cause of most cardiovascular disease.
Reduction in Serum Lecithin:Cholesterol Acyltransferase Activity Prior to the Occurrence of Ketosis and Milk Fever in Cows
 FULL PAPER Biochemistry Reduction in Serum Lecithin:Cholesterol Acyltransferase Activity Prior to the Occurrence of Ketosis and Milk Fever in Cows Hisami NAKAGAWA-UETA 1) and Norio KATOH 2) * 1) Ishikawa
FULL PAPER Biochemistry Reduction in Serum Lecithin:Cholesterol Acyltransferase Activity Prior to the Occurrence of Ketosis and Milk Fever in Cows Hisami NAKAGAWA-UETA 1) and Norio KATOH 2) * 1) Ishikawa
Transgenic rabbits as models for atherosclerosis research
 review Transgenic rabbits as models for atherosclerosis research Margaret E. Brousseau 1 and Jeffrey M. Hoeg 2 Lipid Metabolism Laboratory, JM-USDA/HNRCA at Tufts University, Boston, MA 02111 and Molecular
review Transgenic rabbits as models for atherosclerosis research Margaret E. Brousseau 1 and Jeffrey M. Hoeg 2 Lipid Metabolism Laboratory, JM-USDA/HNRCA at Tufts University, Boston, MA 02111 and Molecular
1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones?
 1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones? 3How are dietary lipids transported? 4How lipids synthesized in the liver are transported? 5 Lipoprotien
1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones? 3How are dietary lipids transported? 4How lipids synthesized in the liver are transported? 5 Lipoprotien
review Genetically modified mice for the study of apolipoprotein B Edward Kim 1 and Stephen G. Young
 review Genetically modified mice for the study of apolipoprotein B Edward Kim 1 and Stephen G. Young Gladstone Institute of Cardiovascular Disease, San Francisco, CA 94141-9100, and Cardiovascular Research
review Genetically modified mice for the study of apolipoprotein B Edward Kim 1 and Stephen G. Young Gladstone Institute of Cardiovascular Disease, San Francisco, CA 94141-9100, and Cardiovascular Research
Separation of HDL Particles by Immunoprecipitation
 Sun Diagnostics, LLC Separation of HDL Particles by Immunoprecipitation Rae-Anne Nguyen and John H. Contois 13 Introduction We all know that HDL cholesterol concentration is inversely associated with coronary
Sun Diagnostics, LLC Separation of HDL Particles by Immunoprecipitation Rae-Anne Nguyen and John H. Contois 13 Introduction We all know that HDL cholesterol concentration is inversely associated with coronary
Endothelial lipase (EL) is a member of the triglyceride
 Upregulation of Macrophage Endothelial Lipase by Toll-Like Receptors 4 and 3 Modulates Macrophage Interleukin-10 and -12 Production Xun Wang, Weijun Jin, Daniel J. Rader Abstract Limited data suggest that
Upregulation of Macrophage Endothelial Lipase by Toll-Like Receptors 4 and 3 Modulates Macrophage Interleukin-10 and -12 Production Xun Wang, Weijun Jin, Daniel J. Rader Abstract Limited data suggest that
Characterization of apolipoprotein E7 (Glu 244 Lys, Glu 245 Lys), a mutant apolipoprotein E associated with hyperlipidemia and atherosclerosis
 Characterization of apolipoprotein E7 (Glu 244 Lys, Glu 245 Lys), a mutant apolipoprotein E associated with hyperlipidemia and atherosclerosis Taku Yamamura, 1 Li-Ming Dong, 2 and Akira Yamamoto Department
Characterization of apolipoprotein E7 (Glu 244 Lys, Glu 245 Lys), a mutant apolipoprotein E associated with hyperlipidemia and atherosclerosis Taku Yamamura, 1 Li-Ming Dong, 2 and Akira Yamamoto Department
Taylor Yohe. Project Advisor: Dr. Martha A. Belury. Department of Human Nutrition at the Ohio State University
 Atherosclerosis Development and the Inflammatory Response of Hepatocytes to Sesame Oil Supplementation Taylor Yohe Project Advisor: Dr. Martha A. Belury Department of Human Nutrition at the Ohio State
Atherosclerosis Development and the Inflammatory Response of Hepatocytes to Sesame Oil Supplementation Taylor Yohe Project Advisor: Dr. Martha A. Belury Department of Human Nutrition at the Ohio State
Plasma cholesterol has been established as a predictor of
 Dietary Fat Induced Alterations in Atherosclerosis Are Abolished by ACAT2-Deficiency in ApoB100 Only, LDLr / Mice Thomas A. Bell III, Kathryn Kelley, Martha D. Wilson, Janet K. Sawyer, Lawrence L. Rudel
Dietary Fat Induced Alterations in Atherosclerosis Are Abolished by ACAT2-Deficiency in ApoB100 Only, LDLr / Mice Thomas A. Bell III, Kathryn Kelley, Martha D. Wilson, Janet K. Sawyer, Lawrence L. Rudel
Storage of human plasma samples leads to alterations in the lipoprotein distribution of apoc-iii and apoe
 methods Storage of human plasma samples leads to alterations in the lipoprotein distribution of apoc-iii and apoe Jeffrey S. Cohn, 1 Claudia Rodriguez, Hélène Jacques, Michel Tremblay, and Jean Davignon
methods Storage of human plasma samples leads to alterations in the lipoprotein distribution of apoc-iii and apoe Jeffrey S. Cohn, 1 Claudia Rodriguez, Hélène Jacques, Michel Tremblay, and Jean Davignon
Plasma lipoproteins & atherosclerosis by. Prof.Dr. Maha M. Sallam
 Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
Hepatic uptake of chylomicron remnants
 Hepatic uptake of chylomicron remnants Allen D. Cooper Research Institute, Palo Alto Medical Foundation, 860 Bryant Street, Palo Alto, CA 94301, and Department of Medicine, Stanford University, Stanford,
Hepatic uptake of chylomicron remnants Allen D. Cooper Research Institute, Palo Alto Medical Foundation, 860 Bryant Street, Palo Alto, CA 94301, and Department of Medicine, Stanford University, Stanford,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
Lipoprotein receptors in the liver. Control signals for plasma cholesterol traffic.
 Lipoprotein receptors in the liver. Control signals for plasma cholesterol traffic. M S Brown, J L Goldstein J Clin Invest. 1983;72(3):743-747. https://doi.org/10.1172/jci111044. Research Article Find
Lipoprotein receptors in the liver. Control signals for plasma cholesterol traffic. M S Brown, J L Goldstein J Clin Invest. 1983;72(3):743-747. https://doi.org/10.1172/jci111044. Research Article Find
Hypertriglyceridemia. Ara Metjian, M.D. Resident s Report 20 December 2002
 Hypertriglyceridemia Ara Metjian, M.D. Resident s Report 20 December 2002 Review of Lipids Chylomicrons (CM): Dietary lipids absorbed through the GI tract are assembled intracellularly into CM. Very Low
Hypertriglyceridemia Ara Metjian, M.D. Resident s Report 20 December 2002 Review of Lipids Chylomicrons (CM): Dietary lipids absorbed through the GI tract are assembled intracellularly into CM. Very Low
Abstract. Introduction
 In the Absence of the Low Density Lipoprotein Receptor, Human Apolipoprotein C1 Overexpression in Transgenic Mice Inhibits the Hepatic Uptake of Very Low Density Lipoproteins via a Receptor-associated
In the Absence of the Low Density Lipoprotein Receptor, Human Apolipoprotein C1 Overexpression in Transgenic Mice Inhibits the Hepatic Uptake of Very Low Density Lipoproteins via a Receptor-associated
Novel Reduction of PCSK9 Expression: Mechanistic Insights into the Anti-Atherosclerotic & Hypolipidemic Effects of Heat Shock Protein 27
 Novel Reduction of PCSK9 Expression: Mechanistic Insights into the Anti-Atherosclerotic & Hypolipidemic Effects of Heat Shock Protein 27 Ed O Brien, Jean-Claude Bakala-N Goma, Chunhua Shi Cumming School
Novel Reduction of PCSK9 Expression: Mechanistic Insights into the Anti-Atherosclerotic & Hypolipidemic Effects of Heat Shock Protein 27 Ed O Brien, Jean-Claude Bakala-N Goma, Chunhua Shi Cumming School
 DOI: 10.1038/ncb2210 b. ICAM1 ng ml -1 P = 0.0001 Small RNA (15-30nts) ng ml -1 Cell Lysate Exosome HDL Plasma HDL Normal Human HDL mirnas R = 0.45 P < 0.0001 Normal Human Exosome mirnas Figure S1. Characterization
DOI: 10.1038/ncb2210 b. ICAM1 ng ml -1 P = 0.0001 Small RNA (15-30nts) ng ml -1 Cell Lysate Exosome HDL Plasma HDL Normal Human HDL mirnas R = 0.45 P < 0.0001 Normal Human Exosome mirnas Figure S1. Characterization
Treatment of Atherosclerosis in 2007
 Treatment of Atherosclerosis in 2007 Szilard Voros, M.D. Medical Director Cardiovascular MR and CT Piedmont Hospital, Piedmont Hospital Our Paradigm Genotype Phenotype Environment Atherosclerotic Disease
Treatment of Atherosclerosis in 2007 Szilard Voros, M.D. Medical Director Cardiovascular MR and CT Piedmont Hospital, Piedmont Hospital Our Paradigm Genotype Phenotype Environment Atherosclerotic Disease
Increased levels of low density lipoprotein (LDL) and cholesterolenriched
 Compared with saturated fatty acids, dietary monounsaturated fatty acids and carbohydrates increase atherosclerosis and VLDL cholesterol levels in LDL receptor-deficient, but not apolipoprotein E-deficient,
Compared with saturated fatty acids, dietary monounsaturated fatty acids and carbohydrates increase atherosclerosis and VLDL cholesterol levels in LDL receptor-deficient, but not apolipoprotein E-deficient,
Cholesterol metabolism. Function Biosynthesis Transport in the organism Hypercholesterolemia
 Cholesterol metabolism Function Biosynthesis Transport in the organism Hypercholesterolemia - component of all cell membranes - precursor of bile acids steroid hormones vitamin D Cholesterol Sources: dietary
Cholesterol metabolism Function Biosynthesis Transport in the organism Hypercholesterolemia - component of all cell membranes - precursor of bile acids steroid hormones vitamin D Cholesterol Sources: dietary
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): Bempedoic acid (ETC-1002) is an ATP-citrate lyase (ACL) inhibitor that in clinical studies has been shown to decrease plasma LDL cholesterol apparently
Reviewers' comments: Reviewer #1 (Remarks to the Author): Bempedoic acid (ETC-1002) is an ATP-citrate lyase (ACL) inhibitor that in clinical studies has been shown to decrease plasma LDL cholesterol apparently
ApoA-I deficiency causes both hypertriglyceridemia and. and increased atherosclerosis in human apob transgenic
 ApoA-I deficiency causes both hypertriglyceridemia and increased atherosclerosis in human apob transgenic mice Emanuel Voyiaziakis,* Ira J. Goldberg,* Andrew S. Plump, Edward M. Rubin, Jan L. Breslow,
ApoA-I deficiency causes both hypertriglyceridemia and increased atherosclerosis in human apob transgenic mice Emanuel Voyiaziakis,* Ira J. Goldberg,* Andrew S. Plump, Edward M. Rubin, Jan L. Breslow,
Lipid Metabolism Prof. Dr. rer physiol. Dr.h.c. Ulrike Beisiegel
 Lipid Metabolism Department of Biochemistry and Molecular Biology II Medical Center Hamburg-ppendorf 1 Lipids. visceral fat. nutritional lipids 0 1.5 3 4.5 9 h. serum lipids. lipid accumulation in the
Lipid Metabolism Department of Biochemistry and Molecular Biology II Medical Center Hamburg-ppendorf 1 Lipids. visceral fat. nutritional lipids 0 1.5 3 4.5 9 h. serum lipids. lipid accumulation in the
Lipid Metabolism in Familial Hypercholesterolemia
 Lipid Metabolism in Familial Hypercholesterolemia Khalid Al-Rasadi, BSc, MD, FRCPC Head of Biochemistry Department, SQU Head of Lipid and LDL-Apheresis Unit, SQUH President of Oman society of Lipid & Atherosclerosis
Lipid Metabolism in Familial Hypercholesterolemia Khalid Al-Rasadi, BSc, MD, FRCPC Head of Biochemistry Department, SQU Head of Lipid and LDL-Apheresis Unit, SQUH President of Oman society of Lipid & Atherosclerosis
Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins
 Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins - Cholesterol: It is a sterol which is found in all eukaryotic cells and contains an oxygen (as a hydroxyl group OH) on Carbon number
Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins - Cholesterol: It is a sterol which is found in all eukaryotic cells and contains an oxygen (as a hydroxyl group OH) on Carbon number
Paradoxical Enhancement of Atherosclerosis by Probucol Treatment in Apolipoprotein E deficient Mice
 Paradoxical Enhancement of Atherosclerosis by Probucol Treatment in Apolipoprotein E deficient Mice Sunny H. Zhang,* Robert L. Reddick,* Elena Avdievich,* L. Kester Surles,* Robert G. Jones,* John B. Reynolds,*
Paradoxical Enhancement of Atherosclerosis by Probucol Treatment in Apolipoprotein E deficient Mice Sunny H. Zhang,* Robert L. Reddick,* Elena Avdievich,* L. Kester Surles,* Robert G. Jones,* John B. Reynolds,*
Downloaded from by on November 8, 2018 The metabolic syndrome is the clustering of insulin
 Combined Adipocyte-Macrophage Fatty Acid Binding Protein Deficiency Improves Metabolism, Atherosclerosis, and Survival in Apolipoprotein E Deficient Mice Jeffrey B. Boord, MD*; Kazuhisa Maeda, MD, PhD*;
Combined Adipocyte-Macrophage Fatty Acid Binding Protein Deficiency Improves Metabolism, Atherosclerosis, and Survival in Apolipoprotein E Deficient Mice Jeffrey B. Boord, MD*; Kazuhisa Maeda, MD, PhD*;
rapid communication Journal of Lipid Research Volume 39,
 rapid communication Human apolipoprotein A-II is a pro-atherogenic molecule when it is expressed in transgenic mice at a level similar to that in humans: evidence of a potentially relevant species-specific
rapid communication Human apolipoprotein A-II is a pro-atherogenic molecule when it is expressed in transgenic mice at a level similar to that in humans: evidence of a potentially relevant species-specific
Chapter VIII: Dr. Sameh Sarray Hlaoui
 Chapter VIII: Dr. Sameh Sarray Hlaoui Lipoproteins a Lipids are insoluble in plasma. In order to be transported they are combined with specific proteins to form lipoproteins: Clusters of proteins and lipids.
Chapter VIII: Dr. Sameh Sarray Hlaoui Lipoproteins a Lipids are insoluble in plasma. In order to be transported they are combined with specific proteins to form lipoproteins: Clusters of proteins and lipids.
Niacin Metabolism: Effects on Cholesterol
 Niacin Metabolism: Effects on Cholesterol By Julianne R. Edwards For Dr. William R. Proulx, PhD, RD Associate Professor of Nutrition and Dietetics In partial fulfillments for the requirements of NUTR342
Niacin Metabolism: Effects on Cholesterol By Julianne R. Edwards For Dr. William R. Proulx, PhD, RD Associate Professor of Nutrition and Dietetics In partial fulfillments for the requirements of NUTR342
Metabolism of lipoproteins containing apolipoprotein B in hepatic lipase-deficient mice
 Metabolism of lipoproteins containing apolipoprotein B in hepatic lipase-deficient mice Shiqiang Qiu,* Nathalie Bergeron,* Leila Kotite,* Ronald M. Krauss, Andre Bensadoun, and Richard J. Havel 1, * Cardiovascular
Metabolism of lipoproteins containing apolipoprotein B in hepatic lipase-deficient mice Shiqiang Qiu,* Nathalie Bergeron,* Leila Kotite,* Ronald M. Krauss, Andre Bensadoun, and Richard J. Havel 1, * Cardiovascular
Hypertriglyceridemia, Inflammation, & Pregnancy
 Hypertriglyceridemia, Inflammation, & Pregnancy Richard L. Nemiroff, MD, FACOG, NLA Professor, Clinical Gynecology Perelman School of Medicine University of Pennsylvania Philadelphia, PA Disclosure of
Hypertriglyceridemia, Inflammation, & Pregnancy Richard L. Nemiroff, MD, FACOG, NLA Professor, Clinical Gynecology Perelman School of Medicine University of Pennsylvania Philadelphia, PA Disclosure of
Massive xanthomatosis and altered composition of atherosclerotic lesions in hyperlipidemic mice lacking acyl CoA:cholesterol acyltransferase 1
 Massive xanthomatosis and altered composition of atherosclerotic lesions in hyperlipidemic mice lacking acyl CoA:cholesterol acyltransferase 1 Michel Accad, 1,2,3 Steven J. Smith, 1,3 Dale L. Newland,
Massive xanthomatosis and altered composition of atherosclerotic lesions in hyperlipidemic mice lacking acyl CoA:cholesterol acyltransferase 1 Michel Accad, 1,2,3 Steven J. Smith, 1,3 Dale L. Newland,
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM The LDL Receptor, LDL Uptake, and the Free Cholesterol Pool
 ANSC/NUTR 618 LIPIDS & LIPID METABOLISM The, LDL Uptake, and the Free Cholesterol Pool I. Michael Brown and Joseph Goldstein A. Studied families with familial hypercholesterolemia. B. Defined the relationship
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM The, LDL Uptake, and the Free Cholesterol Pool I. Michael Brown and Joseph Goldstein A. Studied families with familial hypercholesterolemia. B. Defined the relationship
