Lipoprotein Clearance Mechanisms in LDL Receptor Deficient Apo-B48-only and Apo-B100-only Mice
|
|
|
- Brittney Day
- 5 years ago
- Views:
Transcription
1 Lipoprotein Clearance Mechanisms in LDL Receptor Deficient Apo-B48-only and Apo-B100-only Mice Murielle M. Véniant,* Constance H. Zlot,* Rosemary L. Walzem, Vincenzo Pierotti,* Robert Driscoll,* David Dichek,* Joachim Herz, and Stephen G. Young* *Gladstone Institute of Cardiovascular Disease, San Francisco, California ; Cardiovascular Research Institute and Department of Medicine, University of California, San Francisco, California 94143; Department of Molecular Biosciences, School of Veterinary Medicine, University of California, Davis, California 95616; and Department of Molecular Genetics, University of Texas Southwestern Medical School, Dallas, Texas Abstract The role of the low density lipoprotein receptor (LDLR) in the clearance of apo-b48 containing lipoproteins and the role of the LDLR-related protein (LRP) in the removal of apo-b100 containing lipoproteins have not been clearly defined. To address these issues, we characterized LDLRdeficient mice homozygous for an apo-b48-only allele, an apo-b100-only allele, or a wild-type apo-b allele (Ldlr / Apob 48/48, Ldlr / Apob 100/100, and Ldlr / Apob /, respectively). The plasma apo-b48 and LDL cholesterol levels were higher in Ldlr / Apob 48/48 mice than in Apob 48/48 mice, indicating that the LDL receptor plays a significant role in the removal of apo-b48 containing lipoproteins. To examine the role of the LRP in the clearance of apo-b100 containing lipoproteins, we blocked hepatic LRP function in Ldlr / Apob 100/100 mice by adenoviral-mediated expression of the receptor-associated protein (RAP). RAP expression did not change apo-b100 levels in Ldlr / Apob 100/100 mice. In contrast, RAP expression caused a striking increase in plasma apo-b48 levels in Apob 48/48 and Ldlr / Apob 48/48 mice. These data imply that LRP is important for the clearance of apo-b48 containing lipoproteins but plays no significant role in the clearance of apo-b100 containing lipoproteins. (J. Clin. Invest : ) Key words: low density lipoproteins LDL receptor related protein mouse apo-b radioimmunoassay LDL receptor deficiency adenovirus-mediated gene transfer Introduction Address correspondence to Murielle M. Véniant, Gladstone Institute of Cardiovascular Disease, P.O. Box , San Francisco, CA Phone: ; FAX: ; mveniant@gladstone.ucsf.edu Received for publication 1 June 1998 and accepted in revised form 27 August The Journal of Clinical Investigation Volume 102, Number 8, October 1998, The B apolipoproteins (apo-b100 and apo-b48) have been studied extensively because they play central roles in lipoprotein assembly, plasma lipid metabolism, and atherogenesis (1, 2). Apo-B100 is essential for the assembly of very low density lipoproteins (VLDL) 1 in the human liver and is virtually the only protein component of cholesterol-rich low density lipoproteins (LDL) (3). Apo-B48, a truncated apo-b containing the amino-terminal portion of apo-b100, is required for the assembly of chylomicrons in the intestine (2, 3). Apo-B48 is synthesized as a result of apo-b mrna editing, which changes codon 2153 (CAA, specifying Gln) to a translational stop codon (4). In some mammals, including mice and rats, but not humans, the liver expresses apo-b mrna editing activity and therefore synthesizes apo-b48 in addition to apo-b100 (5). In mice, approximately 70% of the hepatic apo-b transcripts code for apo-b48 (6). Apo-B100 containing LDL bind to and are taken up by the LDL receptor (LDLR), in both hepatic and extrahepatic tissues. When the LDLR is absent or defective, as in familial hypercholesterolemia, the plasma levels of apo-b100 and LDL cholesterol are markedly elevated (7). Apo-B48 lacks the portion of the apo-b molecule that interacts with the LDLR and therefore cannot directly mediate the uptake of lipoproteins by the LDLR (8). However, apo-b48 containing lipoproteins accommodate a large amount of apo-e (9), which is a ligand for both the LDLR and the LDLR-related protein (LRP) (10, 11). Several recent studies have suggested that the LRP plays a role in the removal of apo-b48 containing lipoproteins from the plasma (12, 13), but the relative importance of the LRP and the LDLR in the uptake of apo-b48 containing lipoproteins has never been defined precisely. In this study, we investigated the mechanisms for the uptake of apo-b48 and apo-b100 containing lipoproteins by the LDLR and by the LRP. Specifically, we sought to understand whether the LDLR has a significant role in the clearance of apo-b48 containing lipoproteins from the plasma. We also sought to determine whether the LRP, from the perspective of lipoprotein metabolism, was simply a receptor for the apo-b48 containing lipoproteins, or whether it also has a measurable effect on the metabolism of apo-b100 containing lipoproteins. To address these issues, we have characterized gene-targeted mice that synthesize exclusively apo-b48 ( apo-b48-only mice) or exclusively apo-b100 ( apo-b100-only mice), both in the presence and absence of LDLR expression and in the presence and absence of receptor-associated protein (RAP) expression. Our studies have shown that the LDLR does play a significant role in the clearance of apo-b48 containing lipoproteins, but that the LRP also has a major role. Interestingly, the LRP has no apparent role in the metabolism of apo-b Abbreviations used in this paper: Ad- -Gal, adenovirus containing the cdna for E. coli -galactosidase; Ad-RAP, adenovirus containing the cdna for rat receptor-associated protein; apo, apolipoprotein; FPLC, fast-performance liquid chromatography; HDL, high density lipoprotein; LDL, low density lipoprotein; LDLR, low density lipoprotein receptor; LRP, low density lipoprotein receptor-related protein; pfu, plaque-forming unit; RAP, receptor-associated protein; RIA, radioimmunoassay; VLDL, very low density lipoprotein. Mechanisms for the Clearance of Apo-B48 and Apo-B
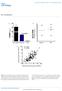
2 containing lipoproteins, even when LDLR activity is absent. Thus, from the perspective of lipoprotein metabolism, the LRP is an apo-b48 containing lipoprotein receptor. Methods Generation of LDLR-deficient mice that synthesize exclusively apo- B48 or apo-b100. Mice that synthesize exclusively apo-b48 (Apob 48/48 mice) or apo-b100 (Apob 100/100 mice) (14) were bred with LDLRdeficient mice (Ldlr / ) (15) to generate Ldlr / Apob 48/48 and Ldlr / Apob 100/100 mice. (Ldlr / Apob 48/48, Ldlr / Apob 100/100, Apoe / Apob 48/48, and Apoe / Apob 100/100 mice are currently available from The Jackson Laboratory [Bar Harbor, ME; We also generated a third group of mice, LDLR-deficient mice homozygous for a wild-type apo-b allele (Ldlr / Apob / mice). All three groups of mice had a similar genetic background ( 75% C57BL/6 and 25% 129/sv). For some experiments, we also used apo-e deficient mice with the targeted apo-b gene mutations (Apoe / Apob 48/48 and Apoe / Apob 100/100 mice), as well as apo-e deficient mice homozygous for a wild-type allele (Apoe / Apob / mice) (14, 16). The apo-e deficient mice also had a mixed genetic background ( 75% C57BL/6 and 25% 129/sv). All mice were weaned at 21 d, fed a chow diet, and housed in a full-barrier facility with a 12-h light/dark cycle. Plasma lipid and lipoprotein measurements. Total plasma cholesterol and triglyceride concentrations were measured on fresh plasma samples with colorimetric assays (16). Total cholesterol and triglyceride levels were measured at 5 mo of age, as well as immediately before and 5 d after intravenous injection of adenoviral preparations. The distribution of lipids within the plasma lipoprotein fractions was assessed by fast phase liquid chromatography (FPLC), using plasma pooled from 5 mice (14). Analysis of plasma lipoprotein sizes. Three lipoprotein fractions (VLDL [d g/ml], LDL [d g/ml], and HDL [d g/ml]) were prepared from plasma by ultracentrifugation (17). For these studies, plasma was pooled from 5 10 female mice of each genotype. VLDL diameters were determined by dynamic light scattering (17) and are reported as the mean SD (nm). LDL and HDL sizes were assessed by nondenaturing PAGE (17). Radioimmunoassays (RIAs) for mouse apo-b100. To measure the concentration of mouse apo-b100 in mouse plasma, we used two solid-phase, mab-based RIAs. The first was a competitive RIA that measured the ability of the apo-b100 in mouse plasma samples to compete with immobilized mouse LDL for binding to a 125 I-labeled mab against mouse apo-b100. (Mouse mabs specific for mouse apo- B100 were generated in Apob 48/48 mice that had been immunized with mouse apo-b100 [C.H. Zlot, L. Flynn, M.M. Véniant, E. Kim, M. Raabe, S.P.A. McCormick, P. Ambroziak, L.M. McEvoy, and S. Young, manuscript submitted for publication]. All mice are available from Jackson Labs.) Plasma apo-b100 levels in different groups of mice are presented as the percentage of the amount of apo-b100 in wild-type mice. Mouse apo-b100 concentrations were also measured with a direct-binding sandwich RIA (18, 19), using two different mouse apo-b100 specific mabs. Analysis of apo-b in the plasma by Western blotting. To compare the amounts of apo-b48 and apo-b100 in different plasma samples and to judge the relative amounts of apo-b48 and apo-b100 in mouse plasma, we performed Western blots of polyacrylamide/sds gel as previously described (16). The intensity of the bands was quantified with a GS300 transmittance/reflectance scanning densitometer and the GS-365 data system for densitometer (Hoefer Scientific Instruments, San Francisco, CA). Large-scale preparation of the recombinant adenovirus. A recombinant adenovirus containing the cdna for rat receptor-associated protein (Ad-RAP) has been described previously by Willnow and coworkers (12). As a control, we used an adenovirus containing the cdna for Escherichia coli -galactosidase (Ad- -Gal) (20). Recombinant adenoviruses were prepared on 293 cells as previously described (12). Plaque-forming units (pfu) were compared to viral particle numbers and were similar for both Ad-RAP and Ad- -Gal ( particles pfu). Injection of adenoviral preparations into mice. Mice were anesthetized with an intraperitoneal injection of avertin (0.02 mg/g of body weight). Blood samples ( l) were obtained from the retroorbital sinus of each mouse with heparinized collecting tubes. An external jugular vein was exposed, and Ad- -Gal or Ad-RAP ( particles diluted into 140 l of Tris-buffered saline) was injected intravenously with a 30-gauge needle. Statistical analysis. Mean lipid levels for each group of mice are reported, along with SEM. Differences in triglyceride levels, cholesterol levels, and apo-b100 levels were modeled by ANOVA with genotype being an intergroup factor. Differences in triglyceride and cholesterol levels measured before and after adenovirus injections were tested with a paired t test. Results Plasma lipids and lipoproteins in LDLR-deficient mice. We measured plasma lipid levels in each group of LDLR-deficient mice (Fig. 1). LDLR deficiency increased plasma cholesterol levels significantly, regardless of Apob genotype. However, the magnitude of this effect was greatest in the setting of the Apob 100 allele. The plasma cholesterol levels were lower in Figure 1. Bar graph comparing the plasma lipid levels from nine groups of mice. Mean plasma cholesterol and triglyceride levels were measured in chow-fed male mice after a 4-h fast. Error bars represent SEM. n represents the number of animals in each group. Statistical analyses were performed on both the cholesterol (a d) and triglyceride (e, f) measurements. Bars not sharing the same letter are significantly different (P 0.001) Véniant et al.
3 Ldlr / Apob 48/48 mice (101 5 mg/dl) and higher in Ldlr / Apob 100/100 mice ( mg/dl) than in Ldlr / Apob / mice (159 8 mg/dl). The Ldlr / Apob 100/100 mice had significantly higher levels of -migrating lipoproteins and lower levels of pre- -migrating and -migrating lipoproteins than Ldlr / Apob 48/48 and Ldlr / Apob / mice (data not shown). Plasma triglyceride levels were significantly lower in the Ldlr / Apob 48/48 mice than in Ldlr / Apob / mice. The plasma cholesterol levels in Apoe / Apob 48/48 and Apoe / Apob / mice were much higher than in any of the groups of LDLR-deficient mice (Fig. 1). However, the total plasma cholesterol levels were nearly identical in the Apoe / Apob 100/100 and Ldlr / Apob 100/100 mice ( mg/dl). To assess the distribution of lipids within the plasma lipoproteins, we fractionated the plasma on an FPLC column (Fig. 2). The Ldlr / Apob 100/100 mice had higher LDL cholesterol levels and lower HDL cholesterol levels than Ldlr / Apob / mice. The LDL cholesterol levels were lower in Ldlr / Apob 48/48 than in Ldlr / Apob / mice. Interestingly, the height of the LDL cholesterol peak in the Ldlr / Apob 48/48 mice was virtually identical to that in Apob 100/100 mice, which had normal LDLR activity. However, it is important to note that both the total plasma cholesterol levels (Fig. 1) and the height of the LDL cholesterol peak (Fig. 2 A) were significantly higher in Ldlr / Apob 48/48 than in Apob 48/48 mice, indicating that LDLR deficiency raises LDL cholesterol levels even when apo-b100 synthesis is absent. In both Ldlr / Apob 100/100 and Ldlr / Apob / mice, as in human apo-b transgenic mice (6, 21, 22), a large fraction of the triglycerides was contained in the LDL fraction (Fig. 2 B). None of the LDLR-deficient mice had large amounts of cholesterol or triglycerides in the VLDL fraction. Indeed, the VLDL cholesterol and triglyceride peaks in the LDLR-deficient mice did not differ significantly from those in mice that Figure 2. Distribution of lipids within different lipoprotein fractions, as assessed by FPLC. A total of 150 l of mouse plasma (pooled from five male mice after a 4-h fast) was size-fractionated on an FPLC Superose 6 column, and the cholesterol and triglyceride contents of each fraction were measured with an enzymatic assay. A shows the distribution of cholesterol; B shows the distribution of triglycerides. Mechanisms for the Clearance of Apo-B48 and Apo-B
4 had normal levels of LDLR expression (Fig. 2). The relatively low levels of VLDL lipids is quite different from the situation in Apoe / Apob 100/100, Apoe / Apob /, or Apoe / Apob 48/48 mice. In the setting of apo-e deficiency, most of the lipids are within the VLDL, regardless of Apob genotype (14, 16). Analysis of lipoprotein particle sizes. There were significant differences in lipoprotein size in the different groups of mice, particularly with respect to VLDL size. The VLDL from Ldlr / Apob 100/100 mice were smaller than those from Ldlr / Apob / and Ldlr / Apob 48/48 mice (Table I). In the setting of apo-e deficiency, the results were quite different. The VLDL of Apoe / Apob / and Apoe / Apob 48/48 mice were smaller than those in Apoe / Apob 100/100 mice. The LDL were larger in Ldlr / Apob 100/100 mice than in Ldlr / Apob 48/48 (24.4 versus 23.0 nm). No differences in HDL size were observed. Apo-B levels in apo-e- and LDLR-deficient mice. To quantify the amount of apo-b100 in the plasma, we used mabbased RIAs (Fig. 3 A) as well as Western blot analysis (Fig. 3 B). Relative levels of apo-b48 in different mouse plasma samples were assessed by Western blots. Relative to Apob / mice, the plasma apo-b100 levels in Ldlr / Apob / and Ldlr / Apob 100/100 mice were increased by five- and eightfold, respectively (Fig. 3 A). LDLR deficiency resulted in a significant increase in the plasma levels of apo-b48, although the increase was much smaller than that caused by apo-e deficiency (Fig. 3 B). Densitometric analysis of Western blots revealed that the plasma apo-b48 levels in Ldlr / Apob 48/48 and Apoe / Apob 48/48 mice were increased by 2.5-fold and fivefold, respectively, compared with those in Apob 48/48 mice. The effect of apo-e deficiency on plasma apo-b100 levels was quite distinct from the changes caused by LDLR deficiency. Apo-B100 levels were 65% lower in Apoe / Apob / mice than in Apob / mice and were consistently higher in Apoe / Apob 100/100 than in Apob 100/100 mice. (Why apo-e deficiency produces a sharp ( 65%) decrease in mouse apo-b100 levels in the setting of the Apob allele but an increase ( 165%) in apo-b100 levels in the setting of the Apob 100 allele is not clear. We speculate that this difference may relate to the fact that the apo-b100 is produced by both the intestine and liver in the setting of the Apob 100 allele but apo-b100 is produced only by the liver in the setting of the Apob allele. In the setting of apo-e deficiency, it is possible that intestinal apo-b100 Table I. Mean VLDL Particle Diameters in Nine Different Genotypes of Mice Apob / Genotype Apob 48/48 Apob 100/100 Apoe / Apob / Apoe / Apob 48/48 Apoe / Apob 100/100 Ldlr / Apob / Ldlr / Apob 48/48 Ldlr / Apob 100/100 VLDL VLDL samples were prepared by ultracentrifugation from plasma pooled from five female mice from each group. The table shows population mean VLDL particle diameter (nm) SD. containing lipoproteins are cleared somewhat less efficiently by the LDLR than apo-b100 containing lipoproteins derived from the liver.) Metabolic effects of adenoviral-mediated overexpression of RAP in the liver. To assess the effect of the LRP on the metabolism of apo-b100 containing lipoproteins, we blocked hepatic LRP function in LDLR-deficient mice (both in Ldlr / Apob / and Ldlr / Apob 100/100 mice) and then determined whether the plasma apo-b100 levels were perturbed. To block LRP function, we used a recombinant adenovirus to overexpress RAP in the liver. We also defined the impact of RAP overexpression on plasma apo-b48 levels in both Ldlr / Apob / and Ldlr / Apob 48/48 mice. To be sure that RAP overexpression inhibited LRP function, we assessed the turnover of 125 I 2 -macroglobulin in wild-type and Ldlr / Apob / mice 5 d after the injection of Ad-RAP or Ad- -Gal. Ad- RAP, but not Ad- -Gal, dramatically retarded the clearance of 2 -macroglobulin in both groups of mice (data not shown). To assess the impact of RAP overexpression on apo-b metabolism, we measured plasma apo-b levels in Ldlr / Apob /, Ldlr / Apob 48/48, and Ldlr / Apob 100/100 mice before and 5 d after the injection of the adenoviral preparations. Of note, overexpression of RAP had no effect on plasma apo-b100 levels in Ldlr / Apob / or Ldlr / Apob 100/100 mice, as assessed by a mab-based RIA (Fig. 4 A) or by Western blot analysis (Fig. 4 B). These results in LDLR-deficient mice strongly suggest that blocking LRP function has little or no effect on the uptake and clearance of apo-b100 containing lipoproteins. The absence of a measurable effect of RAP overexpression on plasma apo-b100 levels stands in contrast to the effects on plasma apo-b48 levels. In both Ldlr / Apob / and Ldlr / Apob 48/48 mice, RAP overexpression caused a four- to fivefold increase in plasma apo-b48 levels (Fig. 4 B). The plasma lipids and the distribution of lipids within the lipoprotein fractions were also assessed 5 d after the injection of Ad- -Gal and Ad-RAP. Plasma lipid levels increased after the injection of both of the adenoviruses, although Ad-RAP had a much greater effect (Fig. 5, A and B). After the injection of Ad- -Gal, the plasma cholesterol and triglyceride levels increased by 30% and 100%, respectively, and the magnitude of the changes was similar in Ldlr / Apob /, Ldlr / Apob 48/48, and Ldlr / Apob 100/100 mice (Fig. 5, A and B). After injection of Ad-RAP, plasma cholesterol levels increased by 800% in the Ldlr / Apob 48/48 mice, 420% in the Ldlr / Apob / mice, and only 210% in the Ldlr / Apob 100/100 mice (Fig. 5 A). Triglyceride levels increased by 1500% in the Ldlr / Apob 48/48 mice, 659% in the Ldlr / Apob / mice, and 545% in the Ldlr / Apob 100/100 mice (Fig. 5 B). Although the percentage of changes in plasma lipid levels in Ldlr / Apob 48/48 mice and Ldlr / Apob 100/100 mice were quite different, it is important to remember that plasma apo-b levels increased by more than fourfold in the Ldlr / Apob 48/48 mice while they were unchanged in Ldlr / Apob 100/100 mice. Thus, relative to the number of apo-b containing lipoproteins in the plasma (see Fig. 4), the hyperlipidemic effect of RAP expression was likely no greater in Ldlr / Apob 48/48 mice than in Ldlr / Apob 100/100 mice. The fact that overexpression of RAP increased plasma lipid levels in the Ldlr / Apob 100/100 mice in the absence of any effect on plasma apo-b100 levels strongly suggested that RAP had the effect of increasing lipoprotein particle size. To gauge the size of lipoproteins in the plasma, we used FPLC fractionation studies to assess the distribution of lipids in the plasma 1562 Véniant et al.
5 Figure 3. Assessment of apo-b48 and apo-b100 levels in the plasma of mice with different genotypes. (A) Quantification of apo-b100 levels in Apob /, Apoe / Apob /, Ldlr / Apob /, Apob 100/100, Apoe / Apob 100/100, and Ldlr / Apob 100/100 mice, as judged by apo-b100 specific competitive and sandwich RIAs. (B) Western blot analysis of apo- B48 and apo-b100 levels in the plasma of Apob /, Apoe / Apob /, and Ldlr / Apob / mice (top panel); Apob 48/48, Apoe / Apob 48/48, Ldlr / Apob 48/48 mice (middle panel); and Apob 100/100, Apoe / Apob 100/100, and Ldlr / Apob 100/100 mice (bottom panel). Plasma samples were size-fractionated on a 4% polyacrylamide/sds gel, and Western blots were performed with a rabbit antiserum specific for mouse apo- B100. As quantified by scanning densitometer, the apo-b48 levels in Ldlr / Apob 48/48 mice were increased by % compared with those in Apob 48/48 mice. Apo-E deficiency resulted in a striking increase in apo-b48 levels; apo-b48 levels in Apoe / Apob 48/48 mice were increased by %, compared with those in Apob 48/48 mice. Scanning densitometer evaluation of the apo-b100 bands were in agreement with the quantification performed by RIA. 5 d after Ad-RAP administration. There was a striking increase in VLDL cholesterol in the Ldlr / Apob 48/48, Ldlr / Apob /, and Ldlr / Apob 100/100 mice after Ad-RAP injection (Fig. 6, C and D; compare with the baseline distribution of lipids in Fig. 2). After the injection of Ad- -Gal, there were increased lipids in the VLDL fraction (Fig. 6, A and B), although the magnitude of this effect was less than that observed after Ad-RAP. In addition to the FPLC fractionation studies, we also assessed VLDL particle diameter in Ldlr / Apob 48/48 and Ldlr / Mechanisms for the Clearance of Apo-B48 and Apo-B
6 Figure 4. Comparisons of plasma apo-b levels before and 5 d after intravenous injection of either Ad- -Gal or Ad-RAP. (A) Comparison of apo-b100 levels in Ldlr / Apob 100/100 before and after injection of Ad- -Gal or Ad-RAP as determined by a mab-based sandwich RIA. Data are expressed as cpm bound in the RIA when 0.5 l of plasma was added to the well. Identical findings were noted when 2.5 l of plasma was added to each well. (B) Western blot analysis of plasma apo-b levels in Ldlr / Apob /, Ldlr / Apob 48/48, and Ldlr / Apob 100/100 mice, both before and after injection of either Ad- -Gal (left panel) or Ad-RAP (right panel). As judged by densitometry, overexpression of RAP had no effect on plasma apo-b100 levels in Ldlr / Apob / ( arbitrary units [AU] before injection of Ad-RAP; AU after injection of Ad-RAP) or Ldlr / Apob 100/100 mice ( AU before and AU after injection of Ad-RAP). In Ldlr / Apob / mice, apo- B48 levels were AU before and AU after injection; in Ldlr / Apob 48/48 mice, apo-b48 levels were AU before and AU after injection. Apob 100/100 mice after Ad-RAP injection. The mean VLDL diameter increased by 36% to 81 nm in Ldlr / Apob 48/48 mice and by 34% to 50 nm in the Ldlr / Apob 100/100 mice (baseline sizes are shown in Table I). Thus, the percentage increase in VLDL diameter with Ad-RAP was similar for apo-b48 and apo-b100 containing lipoproteins. Apo-B48 and apo-b100 levels after expression of RAP in wild-type and Apob 48/48 mice. In addition to assessing the effects of RAP in LDLR-deficient mice, we determined its effect on apo-b100 and apo-b48 levels in wild-type mice (Fig. 7). In those mice, we suspected that RAP overexpression would result in increased plasma apo-b100 levels, since high levels of RAP expression interfere with the binding of lipoproteins to the LDLR, at least to a small extent (23, 24). This suspicion was confirmed. In wild-type mice, RAP overexpression increased plasma apo-b100 levels twofold (Fig. 7). The effects of RAP overexpression on the apo-b48 levels in wild-type mice were more intriguing because they shed light on the issue of whether the clearance of apo-b48 containing lipoproteins normally depends largely or entirely on the LDLR or depends on both the LDLR and the LRP. If the clearance of apo-b48 containing lipoproteins in wild-type mice were entirely or largely due to the LDLR, one would expect to observe an twofold elevation in plasma apo-b48 levels, similar in magnitude to the increase in plasma apo-b100 levels. This was not the case; RAP overexpression in wild-type mice increased apo-b48 levels sixfold. Finding a much greater increase in plasma apo-b48 levels strongly suggests that the LRP has a substantial role in the clearance of apo-b48 containing lipoproteins, even in the setting of normal levels of LDLR expression. Consistent with these results, we observed a sevenfold increase in plasma apo- B48 levels in Apob 48/48 mice after the injection of Ad-RAP. Discussion The role of the LDLR in removing apo-b100 containing lipoproteins from the plasma is well established (25), and recent studies have implicated the LRP in the clearance of apo-b48 containing lipoproteins in animals that are deficient in the LDL receptor (12, 13, 26). In the current study, we sought to define whether the LRP can play a role in removing apo- B100 containing lipoproteins from the plasma and whether the LDLR has a significant role in the clearance of apo-b48 containing lipoproteins. To examine these issues, we used adenoviral-mediated overexpression of RAP to block LRP function in LDLR-deficient mice that synthesized exclusively apo-b48 or apo-b100. The use of apo-b48-only and apo Véniant et al.
7 Figure 5. Total plasma (A) cholesterol and (B) triglyceride levels measured both before and 5 d after intravenous injection of Ad- -Gal or Ad- RAP into Ldlr Apob, Ldlr Apob 48/48, and Ldlr Apob 100/100 mice. Error bars represent SEM. n indicates the number of mice in each group. B100-only mice allowed us to examine lipoprotein metabolism in a less complex setting, one where the normal competition between apo-b48 and apo-b100 containing lipoproteins for metabolic processing and uptake (27) is nonexistent. Our experiments described here have clarified the roles of both the LRP and the LDLR by showing that the LRP has little if any capacity to remove apo-b100 containing lipoproteins from the plasma and that the LDLR plays a significant role in the clearance of apo-b48 containing lipoproteins. If the LRP played a significant role in removing apo-b100 containing lipoproteins from the plasma, we reasoned that blocking LRP function with RAP would cause significantly increased plasma levels of the apo-b100 containing lipoproteins. Our experiments demonstrated that this did not occur. As judged by Western blots as well as mab-based RIAs, RAP overexpression did not affect the plasma levels of apo-b100 in Ldlr / Apob 100/100 or Ldlr / Apob / mice. This finding is especially noteworthy, given the fact that the apo-b containing lipoproteins in the RAP-expressing mice were large and buoyant. In contrast, RAP overexpression increased apo-b48 levels fivefold in both Ldlr / Apob 48/48 and Ldlr / Apob / mice. The striking effects on plasma apo-b48 levels, along with the absence of an effect on apo-b100 levels, indicate that the LRP is, from the perspective of lipoprotein metabolism, a receptor only for the apo-b48 containing lipoproteins. The uptake of apo-b containing remnant lipoproteins by the LRP is mediated by apo-e (28). Interestingly, the amount of apo-e on circulating remnant lipoproteins may be insufficient for uptake by the LRP; rather, these lipoproteins appear to require a supplemental dose of apo-e before uptake occurs (29). The laboratory of Linton and Fazio reported data suggesting that the apo-e supplement must be provided by hepatocytes (30). They found that apo-e rich remnant lipoproteins were readily taken up (as a result of the LRP) by hepatocytes that were deficient in the LDLR but not by hepatocytes that lacked both the LDLR and apo-e. These experiments suggested that the uptake of apo-e containing remnant lipoproteins by the LRP is largely or completely dependent on the ability of hepatocytes to synthesize and secrete apo-e (30). These data led to an intriguing hypothesis: that the apo-e se- Mechanisms for the Clearance of Apo-B48 and Apo-B
8 Figure 6. Distribution of plasma lipids 5 d after injection of Ad- -Gal or Ad-RAP. A total of 150 l of plasma (pooled from five mice in each group) was size-fractionated on an FPLC column. A and B show lipid distributions after Ad- -Gal injection; C and D show lipid distributions after Ad-RAP injection. Cholesterol distributions are shown in A and C; triglyceride distributions are shown in B and D. As illustrated by these FPLC profiles, Ad-RAP had a more potent hyperlipidemic effect than Ad- -Gal. In addition, Ad-RAP had a more potent HDLlowering effect than Ad- -Gal. creted by hepatocytes initially binds to the LRP, and the bound apo-e subsequently attaches to remnant lipoproteins. Remnant lipoproteins supplemented with apo-e in this fashion would then be internalized by the LRP. Alternatively, the Figure 7. Western blot analysis of plasma apo-b levels in wild-type and Apob 48/48 mice, both before and after injection of Ad-RAP. In wild-type mice, apo-b100 levels increased approximately twofold after injection of Ad-RAP ( AU before and AU after injection). In wild-type mice, apo-b48 levels increased sixfold after injection of Ad-RAP ( AU before and AU, after injection); in Apob 48/48 mice, apo-b48 levels increased sevenfold after injection of Ad-RAP ( AU before and AU, after injection). apo-e secreted by hepatocytes into the space of Disse may be captured by proteoglycans and then facilitate the LRP-mediated uptake of lipoproteins (31). However, regardless of how apo-e supplementation occurs, our data indicate that the LRP is active only in the uptake of apo-b48 containing lipoproteins. We suggest that the presence of the carboxyl terminus of apo-b100 (amino acids ) on the surface of the lipoprotein prevents the lipoprotein particle from binding a sufficient dose of supplemental apo-e, thereby preventing its uptake via the LRP. The studies of Willnow et al. (12), Ishibashi et al. (11, 32), and Rohlmann et al. (13) highlighted the role of the LRP in the uptake of apo-b48 containing lipoproteins in LDLR-deficient mice. However, the issue of whether the LDLR plays a significant role in the uptake of apo-b48 containing lipoproteins has never been addressed definitively in in vivo models. Our studies with Ldlr / Apob 48/48 and Apob 48/48 mice provide clear insights into this issue. The Ldlr / Apob 48/48 mice did not have severe hypercholesterolemia, but they had two- to threefold higher plasma apo-b48 levels, higher total cholesterol levels, and much higher LDL cholesterol levels than the Apob 48/48 mice. These observations indicate that the LDLR normally plays a significant role in the clearance of apo-b48 containing lipoproteins. The fact that the LDLR has an easily detectable role in the clearance of apo-b48 containing lipoproteins raises the question of whether the LDLR, under normal circumstances, clears most or all of the apo-b48 containing lipoproteins from the plasma. Our data would argue that it does not; rather, we believe that the LRP has a key role in the clearance of apo-b48 containing lipoproteins, even with physiologically normal levels of LDLR expression. First, in the setting of homozygosity for the Apob 48 allele, we documented that apo-e deficiency caused a far greater increase in plasma apo-b48 levels than LDLR deficiency, suggesting that a significant fraction of 1566 Véniant et al.
9 apo-b48 lipoproteins is cleared from the plasma via a second receptor that recognizes apo-e (i.e., the LRP). Second, in wildtype mice, adenoviral-mediated overexpression of RAP produced a far greater increase in apo-b48 levels than in apo- B100 levels. Both observations strongly suggest that the LRP plays a fundamental role in the clearance of apo-b48 containing lipoproteins, even with normal LDLR expression, and that it is not simply a back-up for the LDLR. A primary role for the LRP in lipoprotein uptake is also supported by the recent studies of Rohlmann et al. (13). They inactivated the LRP gene in the liver and documented a twofold increase in hepatic LDLR expression. That observation suggests that the LRP is normally involved in lipoprotein uptake and that eliminating LRP-mediated uptake results in an upregulation of LDLR expression. Our measurements of VLDL size provided intriguing insights into the metabolism of apo-b48 and apo-b100. The mean VLDL particle diameter was 40% smaller in Apoe / Apob 48/48 mice than in Apob 48/48 or Apoe / Apob 100/100 mice. Similarly, the mean VLDL particle diameter was 40% smaller in Ldlr / Apob 100/100 mice than in Apoe / Apob 100/100 mice. These reductions correspond to more than a 2.5-fold reduction in particle volume. What is the likely explanation for these findings? We believe that VLDL are small in Apoe / Apob 48/48 mice because defective clearance by the LDLR and LRP prolongs their circulation time, leading to a more extensive hydrolysis of core lipids. Similarly, we suspect that the small size of VLDL in Ldlr / Apob 100/100 mice is due to absent LDLR uptake and virtually nonexistent LRP uptake, leading to more complete removal of core lipids. To our knowledge, these data are the first to raise the possibility that the efficiency of receptor-mediated uptake could have striking influences on the size of circulating VLDL. In the prior studies by Willnow and coworkers (12) and in our current studies, adenoviral expression of RAP in the setting of LDLR deficiency resulted in turbid plasma and grossly elevated plasma cholesterol and triglyceride levels. The hyperlipidemia observed after Ad-RAP injection was much more severe than that in mice lacking LRP gene expression in the liver (13). Our experiments indicate that the Ad-RAP hyperlipidemia can be resolved into two components. In the case of Ldlr / Apob 48/48 and Ldlr / Apob / mice, one component results from a profound accumulation of apo-b48 containing lipoproteins. However, the Ad-RAP hyperlipidemia also involves a second component, inasmuch as the Ldlr / Apob 100/100 mice developed hypercholesterolemia and hypertriglyceridemia without an increase in apo-b100 levels. The second component leads to the appearance of larger and more buoyant lipoproteins in the plasma, perhaps as a result of altered lipolytic processing of lipoproteins. At least in part, the larger lipoproteins could relate to the viral infection itself. In our experiments, and in those of Tsukamoto (33), the injection of Ad- -Gal led to a shift toward larger lipoproteins (more VLDL on the FPLC fractionation studies), although the magnitude of this effect was less than that observed with Ad-RAP. In their original description of the LDLR knockout mice, Ishibashi et al. (15) noted that the hypercholesterolemia was only mild to moderate, in contrast to the severe hypercholesterolemia that occurs in LDLR-deficient humans. They reasoned that the mild phenotype in the mouse probably relates to the ability of mice to synthesize apo-b48 containing lipoproteins in the liver. In our studies, we have demonstrated that their reasoning was correct: the Ldlr / Apob 100/100 mice had much higher LDL cholesterol levels than the Ldlr / Apob / mice. Another difference between the Ldlr / Apob 100/100 and Ldlr / Apob / mice also deserves underscoring. The mild hypercholesterolemia in Ldlr / Apob / mice prevents them from developing significant atherosclerosis when fed a low-fat, chow diet (34). Although we have not yet performed systematic studies of atherosclerosis in the three groups of LDLRdeficient mice described in this paper, we have noted very severe atherosclerosis throughout the arterial tree in a few Ldlr / Apob 100/100 mice that were maintained on a low-fat chow diet for 10 mo (M. Véniant and S. Young, unpublished observations). Moreover, we have noted severe atherosclerosis in a large number of chow-fed Ldlr / Apobec1 / mice, which also synthesize exclusively apo-b100 (35). We suspect that the Ldlr / Apob 100/100 and Ldlr / Apobec1 / mice will be very attractive as experimental models for atherosclerosis studies. Like most humans with atherosclerosis, these animals have high plasma levels of apo-b100 containing, cholesterolrich LDL. Acknowledgments We thank S. Ordway and G. Howard for reviewing the manuscript and J. Caroll and N. Shea for preparing the illustrations. This work was supported by National Institutes of Health grant HL47660 (to S.G. Young). References 1. Havel, R.J., and J.P. Kane Introduction: structure and metabolism of plasma lipoproteins. In The Metabolic and Molecular Bases of Inherited Disease, 7th ed., Vol. 2. C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, editors. McGraw-Hill, New York Young, S.G Recent progress in understanding apolipoprotein B. Circulation. 82: Kane, J.P Apolipoprotein B: structural and metabolic heterogeneity. Annu. Rev. Physiol. 45: Davidson, N.O., S. Anant, and A.J. MacGinnitie Apolipoprotein B messenger RNA editing: insights into the molecular regulation of post-transcriptional cytidine deamination. Curr. Opin. Lipidol. 6: Greeve, J., I. Altkemper, J.-H. Dieterich, H. Greten, and E. Windler Apolipoprotein B mrna editing in 12 different mammalian species: hepatic expression is reflected in low concentrations of apob-containing plasma lipoproteins. J. Lipid Res. 34: Purcell-Huynh, D.A., R.V. Farese, Jr., D.F. Johnson, L.M. Flynn, V. Pierotti, D.L. Newland, M.F. Linton, D.A. Sanan, and S.G. Young Transgenic mice expressing high levels of human apolipoprotein B develop severe atherosclerotic lesions in response to a high-fat diet. J. Clin. Invest. 95: Goldstein, J.L., and M.S. Brown Familial hypercholesterolemia. In The Metabolic Basis of Inherited Disease, 6th ed., C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, editors. McGraw-Hill, New York Marcel, Y.L., T.L. Innerarity, C. Spilman, R.W. Mahley, A.A. Protter, and R.W. Milne Mapping of human apolipoprotein B antigenic determinants. Arteriosclerosis. 7: Milne, R.W., P.K. Weech, L. Blanchette, J. Davignon, P. Alaupovic, and Y.L. Marcel Isolation and characterization of apolipoprotein B-48 and B-100 very low density lipoproteins from type III hyperlipoproteinemic subjects. J. Clin. Invest. 73: Mahley, R.W Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 240: Ishibashi, S., J. Herz, N. Maeda, J.L. Goldstein, and M.S. Brown The two-receptor model of lipoprotein clearance: tests of the hypothesis in knockout mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc. Natl. Acad. Sci. USA. 91: Willnow, T.E., Z. Sheng, S. Ishibashi, and J. Herz Inhibition of hepatic chylomicron remnant uptake by gene transfer of a receptor antagonist. Science. 264: Rohlmann, A., M. Gotthardt, R.E. Hammer, and J. Herz Inducible inactivation of hepatic LRP gene by Cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J. Clin. Invest. 101: Mechanisms for the Clearance of Apo-B48 and Apo-B
10 14. Farese, R.V., Jr., M.M. Véniant, C.M. Cham, L.M. Flynn, V. Pierotti, J.F. Loring, M. Traber, S. Ruland, R.S. Stokowski, D. Huszar, et al Phenotypic analysis of mice expressing exclusively apolipoprotein B48 or apolipoprotein B100. Proc. Natl. Acad. Sci. USA. 93: Ishibashi, S., M.S. Brown, J.L. Goldstein, R.D. Gerard, R.E. Hammer, and J. Herz Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest. 92: Véniant, M.M., V. Pierotti, D. Newland, C.M. Cham, D.A. Sanan, R.L. Walzem, and S.G. Young Susceptibility to atherosclerosis in mice expressing exclusively apolipoprotein B48 or apolipoprotein B100. J. Clin. Invest. 100: Walzem, R.L., P.A. Davis, and R.J. Hansen Overfeeding increases very low density lipoprotein diameter and causes the appearance of a unique lipoprotein particle in association with failed yolk deposition. J. Lipid Res. 35: Kim, E., C.M. Cham, M.M. Véniant, P. Ambroziak, and S.G. Young Dual mechanisms for the low plasma levels of truncated apolipoprotein B proteins in familial hypobetalipoproteinemia. Analysis of a new mouse model with a nonsense mutation in the Apob gene. J. Clin. Invest. 101: Raabe, M., L.M. Flynn, C.F. Zlot, J.S. Wong, M. Véniant, R.L. Hamilton, and S.G. Young Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc. Natl. Acad. Sci. USA. 95: Dong, G., A.H. Schulick, M.B. DeYoung, and D.A. Dichek Identification of a cis-acting sequence in the human plasminogen activator inhibitor type-1 gene that mediates transforming growth factor- 1 responsiveness in endothelium in vivo. J. Biol. Chem. 271: Linton, M.F., R.V. Farese, Jr., G. Chiesa, D.S. Grass, P. Chin, R.E. Hammer, H.H. Hobbs, and S.G. Young Transgenic mice expressing high plasma concentrations of human apolipoprotein B100 and lipoprotein(a). J. Clin. Invest. 92: Kim, E., and S.G. Young Genetically modified mice for the study of apolipoprotein B. J. Lipid Res. 39: Mokuno, H., S. Brady, L. Kotite, J. Herz, and R.J. Havel Effect of the 39-kDa receptor-associated protein on the hepatic uptake and endocytosis of chylomicron remnants and low density lipoproteins in the rat. J. Biol. Chem. 269: Bu, G Receptor-associated protein: a specialized chaperone and antagonist for members of the LDL receptor gene family. Curr. Opin. Lipidol. 9: Goldstein, J.L., M.S. Brown, R.G.W. Anderson, D.W. Russell, and W.J. Schneider Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu. Rev. Cell Biol. 1: Willnow, T.E., S.A. Armstrong, R.E. Hammer, and J. Herz Functional expression of low density lipoprotein receptor-related protein is controlled by receptor-associated protein in vivo. Proc. Natl. Acad. Sci. USA. 92: Schneeman, B.O., L. Kotite, K.M. Todd, and R.J. Havel Relationships between the responses of triglyceride-rich lipoproteins in blood plasma containing apolipoproteins B-48 and B-100 to a fat-containing meal in normolipidemic humans. Proc. Natl. Acad. Sci. USA. 90: Beisiegel, U., W. Weber, G. Ihrke, J. Herz, and K.K. Stanley The LDL-receptor-related protein, LRP, is an apolipoprotein E-binding protein. Nature. 341: Kowal, R.C., J. Herz, J.L. Goldstein, V. Esser, and M.S. Brown Low density lipoprotein receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein E-enriched lipoproteins. Proc. Natl. Acad. Sci. USA. 86: Linton, M.F., A.H. Hasty, V.R. Babaev, and S. Fazio Hepatic apo E expression is required for remnant lipoprotein clearance in the absence of the low density lipoprotein receptor. J. Clin. Invest. 101: Mahley, R.W Heparan sulfate proteoglycan/low density lipoprotein receptor-related protein pathway involved in type III hyperlipoproteinemia and Alzheimer s disease. Isr. J. Med. Sci. 32: Ishibashi, S., S. Perrey, Z. Chen, J.-I. Osuga, M. Shimada, K. Ohashi, K. Harada, Y. Yazaki, and N. Yamada Role of the low density lipoprotein (LDL) receptor pathway in the metabolism of chylomicron remnants. A quantitative study in knockout mice lacking the LDL receptor, apolipoprotein E, or both. J. Biol. Chem. 271: Tsukamoto, K., P. Smith, J.M. Glick, and D.J. Rader Liverdirected gene transfer and prolonged expression of three major human apoe isoforms in apoe-deficient mice. J. Clin. Invest. 100: Ishibashi, S., J.L. Goldstein, M.S. Brown, J. Herz, and D.K. Burns Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor negative mice. J. Clin. Invest. 93: Powell-Braxton, L., M. Véniant, R.D. Latvala, K.-I. Hirano, W.B. Won, J. Ross, N. Dybdal, C.H. Zlot, S.G. Young, and N.O. Davidson A mouse model of human familial hypercholesterolemia: markedly elevated low density lipoprotein cholesterol levels and severe atherosclerosis on a low-fat, chow diet. Nat. Med. 4: Véniant et al.
review Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E
 review Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E Robert W. Mahley 1, *, and Zhong-Sheng Ji* Gladstone Institute of Cardiovascular
review Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E Robert W. Mahley 1, *, and Zhong-Sheng Ji* Gladstone Institute of Cardiovascular
hypothesis in "knockout" mice lacking the low density lipoprotein
 Proc. Nati. Acad. Sci. USA Vol. 91, pp. 4431-4435, May 1994 Medical Sciences The two-receptor model of lipoprotein clearance: Tests of the hypothesis in "knockout" mice lacking the low density lipoprotein
Proc. Nati. Acad. Sci. USA Vol. 91, pp. 4431-4435, May 1994 Medical Sciences The two-receptor model of lipoprotein clearance: Tests of the hypothesis in "knockout" mice lacking the low density lipoprotein
行政院國家科學委員會補助專題研究計畫成果報告
 NSC892314B002270 898 1 907 31 9010 23 1 Molecular Study of Type III Hyperlipoproteinemia in Taiwan β β ε E Abstract β Type III hyperlipoproteinemia (type III HLP; familial dysbetalipoproteinemia ) is a
NSC892314B002270 898 1 907 31 9010 23 1 Molecular Study of Type III Hyperlipoproteinemia in Taiwan β β ε E Abstract β Type III hyperlipoproteinemia (type III HLP; familial dysbetalipoproteinemia ) is a
review Genetically modified mice for the study of apolipoprotein B Edward Kim 1 and Stephen G. Young
 review Genetically modified mice for the study of apolipoprotein B Edward Kim 1 and Stephen G. Young Gladstone Institute of Cardiovascular Disease, San Francisco, CA 94141-9100, and Cardiovascular Research
review Genetically modified mice for the study of apolipoprotein B Edward Kim 1 and Stephen G. Young Gladstone Institute of Cardiovascular Disease, San Francisco, CA 94141-9100, and Cardiovascular Research
lipoprotein receptor (low density lipoprotein-related protein/receptor-associated protein/cholesteryl esters)
 Proc. Natl. Acad. Sci. USA Vol. 92, pp. 4611-4615, May 1995 Medical Sciences Initial hepatic removal of chylomicron remnants is unaffected but endocytosis is delayed in mice lacking the low density lipoprotein
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 4611-4615, May 1995 Medical Sciences Initial hepatic removal of chylomicron remnants is unaffected but endocytosis is delayed in mice lacking the low density lipoprotein
The bridging-function of hepatic lipase reduces both apo-b48-and apo-b100- containing lipoproteins in LDL receptor deficient apo-b48-only and apo-
 The bridging-function of hepatic lipase reduces both apo-b48-and apo-b100- containing lipoproteins in LDL receptor deficient apo-b48-only and apo- B100-only mice. Helén L. Dichek 1, Kun Qian, and Nalini
The bridging-function of hepatic lipase reduces both apo-b48-and apo-b100- containing lipoproteins in LDL receptor deficient apo-b48-only and apo- B100-only mice. Helén L. Dichek 1, Kun Qian, and Nalini
Effects of coexpression of the LDL receptor and apoe on cholesterol metabolism and atherosclerosis in LDL receptor-deficient mice
 Effects of coexpression of the LDL receptor and apoe on cholesterol metabolism and atherosclerosis in LDL receptor-deficient mice Masa-aki Kawashiri,* Yuzhen Zhang,* David Usher, Muredach Reilly,* Ellen
Effects of coexpression of the LDL receptor and apoe on cholesterol metabolism and atherosclerosis in LDL receptor-deficient mice Masa-aki Kawashiri,* Yuzhen Zhang,* David Usher, Muredach Reilly,* Ellen
Behind LDL: The Metabolism of ApoB, the Essential Apolipoprotein in LDL and VLDL
 Behind LDL: The Metabolism of ApoB, the Essential Apolipoprotein in LDL and VLDL Sung-Joon Lee, PhD Division of Food Science Institute of Biomedical Science and Safety Korea University Composition of Lipoproteins:
Behind LDL: The Metabolism of ApoB, the Essential Apolipoprotein in LDL and VLDL Sung-Joon Lee, PhD Division of Food Science Institute of Biomedical Science and Safety Korea University Composition of Lipoproteins:
Lipoproteins Metabolism
 Lipoproteins Metabolism LEARNING OBJECTIVES By the end of this Lecture, the student should be able to describe: What are Lipoproteins? Describe Lipoprotein Particles. Composition of Lipoproteins. The chemical
Lipoproteins Metabolism LEARNING OBJECTIVES By the end of this Lecture, the student should be able to describe: What are Lipoproteins? Describe Lipoprotein Particles. Composition of Lipoproteins. The chemical
Summary and concluding remarks
 Summary and concluding remarks This thesis is focused on the role and interaction of different cholesterol and phospholipid transporters. Cholesterol homeostasis is accomplished via a tightly regulated
Summary and concluding remarks This thesis is focused on the role and interaction of different cholesterol and phospholipid transporters. Cholesterol homeostasis is accomplished via a tightly regulated
Pathophysiology of Lipid Disorders
 Pathophysiology of Lipid Disorders Henry Ginsberg, M.D. Division of Preventive Medicine and Nutrition CHD in the United States CHD is the single largest killer of men and women 12 million have history
Pathophysiology of Lipid Disorders Henry Ginsberg, M.D. Division of Preventive Medicine and Nutrition CHD in the United States CHD is the single largest killer of men and women 12 million have history
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism
 ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism I. Chylomicrons (exogenous pathway) A. 83% triacylglycerol, 2% protein, 8% cholesterol plus cholesterol esters, 7% phospholipid (esp. phosphatidylcholine)
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism I. Chylomicrons (exogenous pathway) A. 83% triacylglycerol, 2% protein, 8% cholesterol plus cholesterol esters, 7% phospholipid (esp. phosphatidylcholine)
Metabolism and Atherogenic Properties of LDL
 Metabolism and Atherogenic Properties of LDL Manfredi Rizzo, MD, PhD Associate Professor of Internal Medicine Faculty of Medicine, University of Palermo, Italy & Affiliate Associate Professor of Internal
Metabolism and Atherogenic Properties of LDL Manfredi Rizzo, MD, PhD Associate Professor of Internal Medicine Faculty of Medicine, University of Palermo, Italy & Affiliate Associate Professor of Internal
A Deficiency of Microsomal Triglyceride Transfer Protein Reduces Apolipoprotein B Secretion*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 275, No. 11, Issue of March 17, pp. 7515 7520, 2000 2000 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. A Deficiency of
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 275, No. 11, Issue of March 17, pp. 7515 7520, 2000 2000 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. A Deficiency of
The risk of developing atherosclerosis and coronary heart
 Increased LDL Cholesterol and Atherosclerosis in LDL Receptor Deficient Mice With Attenuated Expression of Scavenger Receptor B1 Dennis Huszar, Mariet Lee Varban, Franz Rinninger, Roslyn Feeley, Takeshi
Increased LDL Cholesterol and Atherosclerosis in LDL Receptor Deficient Mice With Attenuated Expression of Scavenger Receptor B1 Dennis Huszar, Mariet Lee Varban, Franz Rinninger, Roslyn Feeley, Takeshi
Lipid Metabolism in Familial Hypercholesterolemia
 Lipid Metabolism in Familial Hypercholesterolemia Khalid Al-Rasadi, BSc, MD, FRCPC Head of Biochemistry Department, SQU Head of Lipid and LDL-Apheresis Unit, SQUH President of Oman society of Lipid & Atherosclerosis
Lipid Metabolism in Familial Hypercholesterolemia Khalid Al-Rasadi, BSc, MD, FRCPC Head of Biochemistry Department, SQU Head of Lipid and LDL-Apheresis Unit, SQUH President of Oman society of Lipid & Atherosclerosis
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM The LDL Receptor, LDL Uptake, and the Free Cholesterol Pool
 ANSC/NUTR 618 LIPIDS & LIPID METABOLISM The, LDL Uptake, and the Free Cholesterol Pool I. Michael Brown and Joseph Goldstein A. Studied families with familial hypercholesterolemia. B. Defined the relationship
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM The, LDL Uptake, and the Free Cholesterol Pool I. Michael Brown and Joseph Goldstein A. Studied families with familial hypercholesterolemia. B. Defined the relationship
 DOI: 10.1038/ncb2210 b. ICAM1 ng ml -1 P = 0.0001 Small RNA (15-30nts) ng ml -1 Cell Lysate Exosome HDL Plasma HDL Normal Human HDL mirnas R = 0.45 P < 0.0001 Normal Human Exosome mirnas Figure S1. Characterization
DOI: 10.1038/ncb2210 b. ICAM1 ng ml -1 P = 0.0001 Small RNA (15-30nts) ng ml -1 Cell Lysate Exosome HDL Plasma HDL Normal Human HDL mirnas R = 0.45 P < 0.0001 Normal Human Exosome mirnas Figure S1. Characterization
Plasma lipoproteins & atherosclerosis by. Prof.Dr. Maha M. Sallam
 Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
The regulation of the production of apob from liver is
 Normal Production Rate of Apolipoprotein B in LDL Receptor Deficient Mice John S. Millar, Cyrille Maugeais, Ilia V. Fuki, Daniel J. Rader Abstract The low density lipoprotein (LDL) receptor is well known
Normal Production Rate of Apolipoprotein B in LDL Receptor Deficient Mice John S. Millar, Cyrille Maugeais, Ilia V. Fuki, Daniel J. Rader Abstract The low density lipoprotein (LDL) receptor is well known
Hepatic uptake of chylomicron remnants
 Hepatic uptake of chylomicron remnants Allen D. Cooper Research Institute, Palo Alto Medical Foundation, 860 Bryant Street, Palo Alto, CA 94301, and Department of Medicine, Stanford University, Stanford,
Hepatic uptake of chylomicron remnants Allen D. Cooper Research Institute, Palo Alto Medical Foundation, 860 Bryant Street, Palo Alto, CA 94301, and Department of Medicine, Stanford University, Stanford,
Lipoprotein receptors in the liver. Control signals for plasma cholesterol traffic.
 Lipoprotein receptors in the liver. Control signals for plasma cholesterol traffic. M S Brown, J L Goldstein J Clin Invest. 1983;72(3):743-747. https://doi.org/10.1172/jci111044. Research Article Find
Lipoprotein receptors in the liver. Control signals for plasma cholesterol traffic. M S Brown, J L Goldstein J Clin Invest. 1983;72(3):743-747. https://doi.org/10.1172/jci111044. Research Article Find
Plasma cholesterol has been established as a predictor of
 Dietary Fat Induced Alterations in Atherosclerosis Are Abolished by ACAT2-Deficiency in ApoB100 Only, LDLr / Mice Thomas A. Bell III, Kathryn Kelley, Martha D. Wilson, Janet K. Sawyer, Lawrence L. Rudel
Dietary Fat Induced Alterations in Atherosclerosis Are Abolished by ACAT2-Deficiency in ApoB100 Only, LDLr / Mice Thomas A. Bell III, Kathryn Kelley, Martha D. Wilson, Janet K. Sawyer, Lawrence L. Rudel
Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry
 Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry Learning Objectives 1. Define lipoproteins and explain the rationale of their formation in blood. 2. List different
Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry Learning Objectives 1. Define lipoproteins and explain the rationale of their formation in blood. 2. List different
Role of apolipoprotein B-containing lipoproteins in the development of atherosclerosis Jan Borén MD, PhD
 Role of apolipoprotein B-containing lipoproteins in the development of atherosclerosis Jan Borén MD, PhD Our laboratory focuses on the role of apolipoprotein (apo) B- containing lipoproteins in normal
Role of apolipoprotein B-containing lipoproteins in the development of atherosclerosis Jan Borén MD, PhD Our laboratory focuses on the role of apolipoprotein (apo) B- containing lipoproteins in normal
Fig. 1 Family pedigree of Patient 1 (upper) and Patient 2 (lower).
 Fig. 1 Family pedigree of Patient 1 (upper) and Patient 2 (lower). Fig. 2 Determination of cholesteryl ester net transfer rate from HDL to VLDL and LDL. Table 1 Serum lipids and apoprotein levels in the
Fig. 1 Family pedigree of Patient 1 (upper) and Patient 2 (lower). Fig. 2 Determination of cholesteryl ester net transfer rate from HDL to VLDL and LDL. Table 1 Serum lipids and apoprotein levels in the
Severe Hypercholesterolemia, Hypertriglyceridemia, and Atherosclerosis in Mice Lacking Both Leptin and the Low Density Lipoprotein Receptor*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 276, No. 40, Issue of October 5, pp. 37402 37408, 2001 2001 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Severe Hypercholesterolemia,
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 276, No. 40, Issue of October 5, pp. 37402 37408, 2001 2001 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Severe Hypercholesterolemia,
Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice.
 Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
High density lipoprotein metabolism
 High density lipoprotein metabolism Lipoprotein classes and atherosclerosis Chylomicrons, VLDL, and their catabolic remnants Pro-atherogenic LDL HDL Anti-atherogenic Plasma lipid transport Liver VLDL FC
High density lipoprotein metabolism Lipoprotein classes and atherosclerosis Chylomicrons, VLDL, and their catabolic remnants Pro-atherogenic LDL HDL Anti-atherogenic Plasma lipid transport Liver VLDL FC
Type III Hyperlipoproteinemia and Spontaneous Atherosclerosis in Mice Resulting from Gene Replacement of Mouse Apoe with Human APOE *2
 Type III Hyperlipoproteinemia and Spontaneous Atherosclerosis in Mice Resulting from Gene Replacement of Mouse Apoe with Human APOE *2 Patrick M. Sullivan,* Hafid Mezdour,* Steven H. Quarfordt, and Nobuyo
Type III Hyperlipoproteinemia and Spontaneous Atherosclerosis in Mice Resulting from Gene Replacement of Mouse Apoe with Human APOE *2 Patrick M. Sullivan,* Hafid Mezdour,* Steven H. Quarfordt, and Nobuyo
Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice
 Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice Atherosclerosis, 2007 Chiba T, Shinozaki S, Nakazawa T, et al. Present by Sudaporn Pummoung Apolipoprotein E (apoe( apoe)
Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice Atherosclerosis, 2007 Chiba T, Shinozaki S, Nakazawa T, et al. Present by Sudaporn Pummoung Apolipoprotein E (apoe( apoe)
Determination of the lower threshold of apolipoprotein E resulting in remnant lipoprotein clearance 1
 Determination of the lower threshold of apolipoprotein E resulting in remnant lipoprotein clearance 1 Alyssa H. Hasty,* MacRae F. Linton, 2,, Larry L. Swift,* and Sergio Fazio 2, *, Departments of Pathology,*
Determination of the lower threshold of apolipoprotein E resulting in remnant lipoprotein clearance 1 Alyssa H. Hasty,* MacRae F. Linton, 2,, Larry L. Swift,* and Sergio Fazio 2, *, Departments of Pathology,*
Chapter VIII: Dr. Sameh Sarray Hlaoui
 Chapter VIII: Dr. Sameh Sarray Hlaoui Lipoproteins a Lipids are insoluble in plasma. In order to be transported they are combined with specific proteins to form lipoproteins: Clusters of proteins and lipids.
Chapter VIII: Dr. Sameh Sarray Hlaoui Lipoproteins a Lipids are insoluble in plasma. In order to be transported they are combined with specific proteins to form lipoproteins: Clusters of proteins and lipids.
Human LDL Receptor / LDLR ELISA Pair Set
 Human LDL Receptor / LDLR ELISA Pair Set Catalog Number : SEK10231 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in
Human LDL Receptor / LDLR ELISA Pair Set Catalog Number : SEK10231 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in
Cholesterol metabolism. Function Biosynthesis Transport in the organism Hypercholesterolemia
 Cholesterol metabolism Function Biosynthesis Transport in the organism Hypercholesterolemia - component of all cell membranes - precursor of bile acids steroid hormones vitamin D Cholesterol Sources: dietary
Cholesterol metabolism Function Biosynthesis Transport in the organism Hypercholesterolemia - component of all cell membranes - precursor of bile acids steroid hormones vitamin D Cholesterol Sources: dietary
Hiroshi Hosoai,* Nancy R. Webb, Jane M. Glick, Uwe J. F. Tietge,* Matthew S. Purdom, Frederick C. de Beer, and Daniel J.
 Expression of serum amyloid A protein in the absence of the acute phase response does not reduce HDL cholesterol or apoa-i levels in human apoa-i transgenic mice Hiroshi Hosoai,* Nancy R. Webb, Jane M.
Expression of serum amyloid A protein in the absence of the acute phase response does not reduce HDL cholesterol or apoa-i levels in human apoa-i transgenic mice Hiroshi Hosoai,* Nancy R. Webb, Jane M.
2.5% of all deaths globally each year. 7th leading cause of death by % of people with diabetes live in low and middle income countries
 Lipid Disorders in Diabetes (Diabetic Dyslipidemia) Khosrow Adeli PhD, FCACB, DABCC Head and Professor, Clinical Biochemistry, The Hospital for Sick Children, University it of Toronto Diabetes A Global
Lipid Disorders in Diabetes (Diabetic Dyslipidemia) Khosrow Adeli PhD, FCACB, DABCC Head and Professor, Clinical Biochemistry, The Hospital for Sick Children, University it of Toronto Diabetes A Global
Lipid Metabolism Prof. Dr. rer physiol. Dr.h.c. Ulrike Beisiegel
 Lipid Metabolism Department of Biochemistry and Molecular Biology II Medical Center Hamburg-ppendorf 1 Lipids. visceral fat. nutritional lipids 0 1.5 3 4.5 9 h. serum lipids. lipid accumulation in the
Lipid Metabolism Department of Biochemistry and Molecular Biology II Medical Center Hamburg-ppendorf 1 Lipids. visceral fat. nutritional lipids 0 1.5 3 4.5 9 h. serum lipids. lipid accumulation in the
Development of an RNA Interference Therapeutic Targeting Angiopoietin-Like Protein 3 for Treatment of Hyperlipidemia
 Development of an RNA Interference Therapeutic Targeting Angiopoietin-Like Protein 3 for Treatment of Hyperlipidemia November 12 th, 2018 So C. Wong Arrowhead Pharmaceuticals Inc. Disclosures All authors
Development of an RNA Interference Therapeutic Targeting Angiopoietin-Like Protein 3 for Treatment of Hyperlipidemia November 12 th, 2018 So C. Wong Arrowhead Pharmaceuticals Inc. Disclosures All authors
Apo E structure determines VLDL clearance and atherosclerosis risk in mice
 Apo E structure determines VLDL clearance and atherosclerosis risk in mice Christopher Knouff, 1 Myron E. Hinsdale, 1 Hafid Mezdour, 1 Michael K. Altenburg, 1 Masahiko Watanabe, 1 Steven H. Quarfordt,
Apo E structure determines VLDL clearance and atherosclerosis risk in mice Christopher Knouff, 1 Myron E. Hinsdale, 1 Hafid Mezdour, 1 Michael K. Altenburg, 1 Masahiko Watanabe, 1 Steven H. Quarfordt,
is degraded slowly and it accumulates to massive levels in B48 (11), a unique form ofapo-b that is present in chylomicrons
 Proc. Natl Acad. Sci. USA Vol. 79, pp. 3623-3627, June 1982 Medical Sciences Hepatic uptake of chylomicron remnants in WHHL rabbits: A mechanism genetically distinct from the low density lipoprotein receptor
Proc. Natl Acad. Sci. USA Vol. 79, pp. 3623-3627, June 1982 Medical Sciences Hepatic uptake of chylomicron remnants in WHHL rabbits: A mechanism genetically distinct from the low density lipoprotein receptor
APOB (Human) ELISA Kit
 APOB (Human) ELISA Kit Catalog Number KA4330 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
APOB (Human) ELISA Kit Catalog Number KA4330 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Hypertriglyceridemia. Ara Metjian, M.D. Resident s Report 20 December 2002
 Hypertriglyceridemia Ara Metjian, M.D. Resident s Report 20 December 2002 Review of Lipids Chylomicrons (CM): Dietary lipids absorbed through the GI tract are assembled intracellularly into CM. Very Low
Hypertriglyceridemia Ara Metjian, M.D. Resident s Report 20 December 2002 Review of Lipids Chylomicrons (CM): Dietary lipids absorbed through the GI tract are assembled intracellularly into CM. Very Low
Characterization of apolipoprotein E7 (Glu 244 Lys, Glu 245 Lys), a mutant apolipoprotein E associated with hyperlipidemia and atherosclerosis
 Characterization of apolipoprotein E7 (Glu 244 Lys, Glu 245 Lys), a mutant apolipoprotein E associated with hyperlipidemia and atherosclerosis Taku Yamamura, 1 Li-Ming Dong, 2 and Akira Yamamoto Department
Characterization of apolipoprotein E7 (Glu 244 Lys, Glu 245 Lys), a mutant apolipoprotein E associated with hyperlipidemia and atherosclerosis Taku Yamamura, 1 Li-Ming Dong, 2 and Akira Yamamoto Department
Apolipoprotein E (apoe) plays a central role in the
 Atherosclerosis and Lipoproteins Harmful Effects of Increased LDLR Expression in Mice With Human APOE*4 But Not APOE*3 Sudi I. Malloy,* Michael K. Altenburg,* Christopher Knouff,* Lorraine Lanningham-Foster,
Atherosclerosis and Lipoproteins Harmful Effects of Increased LDLR Expression in Mice With Human APOE*4 But Not APOE*3 Sudi I. Malloy,* Michael K. Altenburg,* Christopher Knouff,* Lorraine Lanningham-Foster,
Metabolism of lipoproteins containing apolipoprotein B in hepatic lipase-deficient mice
 Metabolism of lipoproteins containing apolipoprotein B in hepatic lipase-deficient mice Shiqiang Qiu,* Nathalie Bergeron,* Leila Kotite,* Ronald M. Krauss, Andre Bensadoun, and Richard J. Havel 1, * Cardiovascular
Metabolism of lipoproteins containing apolipoprotein B in hepatic lipase-deficient mice Shiqiang Qiu,* Nathalie Bergeron,* Leila Kotite,* Ronald M. Krauss, Andre Bensadoun, and Richard J. Havel 1, * Cardiovascular
N-3 Fatty Acids Non-HDL-Cand LDL-C Thomas Dayspring MD, FACP
 Omega or N-3 Fatty Acids (FA) significantly reduce TG synthesis and significantly deplete the TG content of VLDL particles indicated by significantly reduced V. FA are the substrate for TG synthesis. N3-FA
Omega or N-3 Fatty Acids (FA) significantly reduce TG synthesis and significantly deplete the TG content of VLDL particles indicated by significantly reduced V. FA are the substrate for TG synthesis. N3-FA
Hepatic Remnant Lipoprotein Clearance by Heparan Sulfate Proteoglycans and Low-Density Lipoprotein Receptors Depend on Dietary Conditions in Mice
 Hepatic Remnant Lipoprotein Clearance by Heparan Sulfate Proteoglycans and Low-Density Lipoprotein Receptors Depend on Dietary Conditions in Mice Erin M. Foley,* Philip L.S.M. Gordts,* Kristin I. Stanford,*
Hepatic Remnant Lipoprotein Clearance by Heparan Sulfate Proteoglycans and Low-Density Lipoprotein Receptors Depend on Dietary Conditions in Mice Erin M. Foley,* Philip L.S.M. Gordts,* Kristin I. Stanford,*
Apolipoprotein E (apoe) and the low-density lipoprotein
 Human LDL Receptor Enhances Sequestration of ApoE4 and VLDL Remnants on the Surface of Hepatocytes but Not Their Internalization in Mice Michael Altenburg, Jose Arbones-Mainar, Lance Johnson, Jennifer
Human LDL Receptor Enhances Sequestration of ApoE4 and VLDL Remnants on the Surface of Hepatocytes but Not Their Internalization in Mice Michael Altenburg, Jose Arbones-Mainar, Lance Johnson, Jennifer
Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved
 1 Supplemental Figure Legends Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved PCSK9 concentrations. 4 Plasma mature and furin-cleaved PCSK9s were measured by a sandwich
1 Supplemental Figure Legends Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved PCSK9 concentrations. 4 Plasma mature and furin-cleaved PCSK9s were measured by a sandwich
Ezetimibe: a selective inhibitor of cholesterol absorption
 European Heart Journal Supplements (2001) 3 (Supplement E), E6 E10 Ezetimibe: a selective inhibitor of cholesterol absorption Dipartimento di Scienze Farmacologiche, Universita degli Studi di Milano, Milano,
European Heart Journal Supplements (2001) 3 (Supplement E), E6 E10 Ezetimibe: a selective inhibitor of cholesterol absorption Dipartimento di Scienze Farmacologiche, Universita degli Studi di Milano, Milano,
Ezetimibe: a selective inhibitor of cholesterol absorption
 European Heart Journal Supplements (2001) 3 (Supplement E), E6 E10 Ezetimibe: a selective inhibitor of cholesterol absorption Dipartimento di Scienze Farmacologiche, Universita degli Studi di Milano, Milano,
European Heart Journal Supplements (2001) 3 (Supplement E), E6 E10 Ezetimibe: a selective inhibitor of cholesterol absorption Dipartimento di Scienze Farmacologiche, Universita degli Studi di Milano, Milano,
METHODS. Proc. Natl. Acad. Sci. USA Vol. 95, pp , December 1998 Medical Sciences. Contributed by Richard J. Havel, October 30, 1998
 Proc. Natl. Acad. Sci. USA Vol. 95, pp. 15647 15652, December 1998 Medical Sciences Lamellar lipoproteins uniquely contribute to hyperlipidemia in mice doubly deficient in apolipoprotein E and hepatic
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 15647 15652, December 1998 Medical Sciences Lamellar lipoproteins uniquely contribute to hyperlipidemia in mice doubly deficient in apolipoprotein E and hepatic
ApoVLDL of the Watanabe Heritable Hyperlipidemic rabbit and the cholesterol-fed rabbit
 ApoVLDL of the Watanabe Heritable Hyperlipidemic rabbit and the cholesterol-fed rabbit Takanobu Wakasugi, Hiroshi Mabuchi, Yasuyuki Sakai, Takeshi Sakai, Akira Yoshimura, Akira Watanabe, Junji Koizumi,
ApoVLDL of the Watanabe Heritable Hyperlipidemic rabbit and the cholesterol-fed rabbit Takanobu Wakasugi, Hiroshi Mabuchi, Yasuyuki Sakai, Takeshi Sakai, Akira Yoshimura, Akira Watanabe, Junji Koizumi,
Inhibition of endothelial lipase causes increased HDL cholesterol levels in vivo
 Inhibition of endothelial lipase causes increased HDL cholesterol levels in vivo See the related Commentary beginning on page 318. Weijun Jin, John S. Millar, Uli Broedl, Jane M. Glick, and Daniel J. Rader
Inhibition of endothelial lipase causes increased HDL cholesterol levels in vivo See the related Commentary beginning on page 318. Weijun Jin, John S. Millar, Uli Broedl, Jane M. Glick, and Daniel J. Rader
2.5. AMPK activity
 Supplement Fig. A 3 B phos-ampk 2.5 * Control AICAR AMPK AMPK activity (Absorbance at 45 nm) 2.5.5 Control AICAR Supplement Fig. Effects of AICAR on AMPK activation in macrophages. J774. macrophages were
Supplement Fig. A 3 B phos-ampk 2.5 * Control AICAR AMPK AMPK activity (Absorbance at 45 nm) 2.5.5 Control AICAR Supplement Fig. Effects of AICAR on AMPK activation in macrophages. J774. macrophages were
Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins
 Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins - Cholesterol: It is a sterol which is found in all eukaryotic cells and contains an oxygen (as a hydroxyl group OH) on Carbon number
Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins - Cholesterol: It is a sterol which is found in all eukaryotic cells and contains an oxygen (as a hydroxyl group OH) on Carbon number
Lipids, Lipoproteins and Cardiovascular Risk: Getting the Most out of New and Old Biomarkers. New and Old Biomarkers. Disclosures
 Lipids, Lipoproteins and Cardiovascular Risk: Getting the Most out of New and Old Biomarkers William Cromwell, MD, FAHA, FNLA Diplomate, American Board of Clinical Lipidology Chief Lipoprotein and Metabolic
Lipids, Lipoproteins and Cardiovascular Risk: Getting the Most out of New and Old Biomarkers William Cromwell, MD, FAHA, FNLA Diplomate, American Board of Clinical Lipidology Chief Lipoprotein and Metabolic
Abstract. Introduction
 In the Absence of the Low Density Lipoprotein Receptor, Human Apolipoprotein C1 Overexpression in Transgenic Mice Inhibits the Hepatic Uptake of Very Low Density Lipoproteins via a Receptor-associated
In the Absence of the Low Density Lipoprotein Receptor, Human Apolipoprotein C1 Overexpression in Transgenic Mice Inhibits the Hepatic Uptake of Very Low Density Lipoproteins via a Receptor-associated
LDL Uptake Cell-Based Assay Kit
 LDL Uptake Cell-Based Assay Kit Catalog Number KA1327 100 assays Version: 07 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
LDL Uptake Cell-Based Assay Kit Catalog Number KA1327 100 assays Version: 07 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
The carboxyl terminus in apolipoprotein E2 and the seven amino acid repeat in apolipoprotein E-Leiden: role in receptor-binding activity
 The carboxyl terminus in apolipoprotein E2 and the seven amino acid repeat in apolipoprotein E-Leiden: role in receptor-binding activity Li-Ming Dong,* Thomas L. Innerarity,*, Kay S. Arnold,* Yvonne M.
The carboxyl terminus in apolipoprotein E2 and the seven amino acid repeat in apolipoprotein E-Leiden: role in receptor-binding activity Li-Ming Dong,* Thomas L. Innerarity,*, Kay S. Arnold,* Yvonne M.
 Glossary For TheFatNurse s For All Ages Series Apolipoprotein B (APOB or ApoB) are the primary apolipoproteins of chylomicrons and low-density lipoproteins (LDL - known commonly by the misnomer "bad cholesterol"
Glossary For TheFatNurse s For All Ages Series Apolipoprotein B (APOB or ApoB) are the primary apolipoproteins of chylomicrons and low-density lipoproteins (LDL - known commonly by the misnomer "bad cholesterol"
Supplementary Figure 1: Additional metabolic parameters of obesity mouse models and controls. (a) Body weight, (b) blood glucose and (c) insulin
 Supplementary Figure 1: Additional metabolic parameters of obesity mouse models and controls. (a) Body weight, (b) blood glucose and (c) insulin resistance index of homeostatic model assessment (HOMA IR)
Supplementary Figure 1: Additional metabolic parameters of obesity mouse models and controls. (a) Body weight, (b) blood glucose and (c) insulin resistance index of homeostatic model assessment (HOMA IR)
Deficiency of low density lipoprotein receptors in liver and adrenal gland of the WHHL rabbit, an animal model of
 Proc. Natl. Acad. Sci. USA Vol. 78, No. 4, pp. 2268-2272, April 1981 Biochemistry Deficiency of low density lipoprotein receptors in liver and adrenal gland of the WHHL rabbit, an animal model of familial
Proc. Natl. Acad. Sci. USA Vol. 78, No. 4, pp. 2268-2272, April 1981 Biochemistry Deficiency of low density lipoprotein receptors in liver and adrenal gland of the WHHL rabbit, an animal model of familial
LIPIDS OF BLOODSTREAM
 HEC No. 1428 ISSN No. 1681-5491 CARDIOLOGY MEDICAL CHANNEL Vol. 15, No. 2 APRIL - JUNE 2009 EDITORIAL LIPIDS OF BLOODSTREAM 1. ZAFAR ALAM MAHMOOD 2. SYED WASEEMUDDIN AHMED 3. MOHAMMAD SUALEH 4. SAAD BIN
HEC No. 1428 ISSN No. 1681-5491 CARDIOLOGY MEDICAL CHANNEL Vol. 15, No. 2 APRIL - JUNE 2009 EDITORIAL LIPIDS OF BLOODSTREAM 1. ZAFAR ALAM MAHMOOD 2. SYED WASEEMUDDIN AHMED 3. MOHAMMAD SUALEH 4. SAAD BIN
Byung Hong Chung 1, * and Nassrin Dashti
 Lipolytic remnants of human VLDL produced in vitro: effect of HDL levels in the lipolysis mixtures on the apocs to apoe ratio and metabolic properties of VLDL core remnants Byung Hong Chung 1, * and Nassrin
Lipolytic remnants of human VLDL produced in vitro: effect of HDL levels in the lipolysis mixtures on the apocs to apoe ratio and metabolic properties of VLDL core remnants Byung Hong Chung 1, * and Nassrin
Supplemental Material. Results
 Supplemental Material Results Fractionation of mouse plasma by high-resolution SEC. APOA1 eluted as a single major peak in fractions 16 of plasma (the apparent size of mature, lipidated HDL) when it was
Supplemental Material Results Fractionation of mouse plasma by high-resolution SEC. APOA1 eluted as a single major peak in fractions 16 of plasma (the apparent size of mature, lipidated HDL) when it was
 Glossary For TheFatNurse s For All Ages Series Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Apolipoprotein
Glossary For TheFatNurse s For All Ages Series Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Apolipoprotein
Fig. S1. REGN1500 reduces plasma levels of cholesterol, TG and NEFA in WT and Ldlr -/- mice. (A) WT
 Figure Legends for Supplementary Figures. Fig. S1. REGN15 reduces plasma levels of cholesterol, TG and NEF in WT and Ldlr -/- mice. () WT and Ldlr -/- mice were injected with control IgG or REGN15 (1 mg/kg)
Figure Legends for Supplementary Figures. Fig. S1. REGN15 reduces plasma levels of cholesterol, TG and NEF in WT and Ldlr -/- mice. () WT and Ldlr -/- mice were injected with control IgG or REGN15 (1 mg/kg)
Cardiovascular Controversies: Emerging Therapies for Lowering Cardiovascular Risk
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
LDL Uptake Cell-Based Assay Kit
 LDL Uptake Cell-Based Assay Kit Item No. 10011125 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
LDL Uptake Cell-Based Assay Kit Item No. 10011125 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Lipid metabolism in familial hypercholesterolemia
 Lipid metabolism in familial hypercholesterolemia Khalid Al-Rasadi, BSc, MD, FRCPC Head of Biochemistry Department, SQU Head of Lipid and LDL-Apheresis Unit, SQUH President of Oman society of Lipid & Atherosclerosis
Lipid metabolism in familial hypercholesterolemia Khalid Al-Rasadi, BSc, MD, FRCPC Head of Biochemistry Department, SQU Head of Lipid and LDL-Apheresis Unit, SQUH President of Oman society of Lipid & Atherosclerosis
 Acetyl CoA HMG CoA Mevalonate (C6) Dimethylallyl Pyrophosphate isopentenyl Pyrophosphate (C5) Geranyl Pyrophosphate (C10) FarnesylPyrophosphate (C15) Squalene (C30) Lanosterol (C30) 7 Dehydrocholesterol
Acetyl CoA HMG CoA Mevalonate (C6) Dimethylallyl Pyrophosphate isopentenyl Pyrophosphate (C5) Geranyl Pyrophosphate (C10) FarnesylPyrophosphate (C15) Squalene (C30) Lanosterol (C30) 7 Dehydrocholesterol
The New Gold Standard for Lipoprotein Analysis. Advanced Testing for Cardiovascular Risk
 The New Gold Standard for Lipoprotein Analysis Advanced Testing for Cardiovascular Risk Evolution of Lipoprotein Testing The Lipid Panel Total Cholesterol = VLDL + LDL + HDL Evolution of Lipoprotein Testing
The New Gold Standard for Lipoprotein Analysis Advanced Testing for Cardiovascular Risk Evolution of Lipoprotein Testing The Lipid Panel Total Cholesterol = VLDL + LDL + HDL Evolution of Lipoprotein Testing
Nature Genetics: doi: /ng.3561
 Supplementary Figure 1 Pedigrees of families with APOB p.gln725* mutation and APOB p.gly1829glufs8 mutation (a,b) Pedigrees of families with APOB p.gln725* mutation. (c) Pedigree of family with APOB p.gly1829glufs8
Supplementary Figure 1 Pedigrees of families with APOB p.gln725* mutation and APOB p.gly1829glufs8 mutation (a,b) Pedigrees of families with APOB p.gln725* mutation. (c) Pedigree of family with APOB p.gly1829glufs8
Separation of HDL Particles by Immunoprecipitation
 Sun Diagnostics, LLC Separation of HDL Particles by Immunoprecipitation Rae-Anne Nguyen and John H. Contois 13 Introduction We all know that HDL cholesterol concentration is inversely associated with coronary
Sun Diagnostics, LLC Separation of HDL Particles by Immunoprecipitation Rae-Anne Nguyen and John H. Contois 13 Introduction We all know that HDL cholesterol concentration is inversely associated with coronary
1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones?
 1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones? 3How are dietary lipids transported? 4How lipids synthesized in the liver are transported? 5 Lipoprotien
1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones? 3How are dietary lipids transported? 4How lipids synthesized in the liver are transported? 5 Lipoprotien
PLASMA LIPOPROTEINS AND LIPIDS DETERMINATION OF PLASMA CHOLESTEROL AND TRIGLICERIDE LEVEL
 PLASMA LIPOPROTEINS AND LIPIDS DETERMINATION OF PLASMA CHOLESTEROL AND TRIGLICERIDE LEVEL Lipids are characterized by low polarity and limited solubility in water. Their plasma concentration is about 500-600
PLASMA LIPOPROTEINS AND LIPIDS DETERMINATION OF PLASMA CHOLESTEROL AND TRIGLICERIDE LEVEL Lipids are characterized by low polarity and limited solubility in water. Their plasma concentration is about 500-600
Hepatic secretion of apob-100 is impaired in hypobetalipoproteinemic mice with an apob-38.9-specifying allele
 Hepatic secretion of apob-100 is impaired in hypobetalipoproteinemic mice with an apob-38.9-specifying allele Zhouji Chen, 1, * Robin L. Fitzgerald,* Gang Li, 2, * Nicholas O. Davidson, and Gustav Schonfeld
Hepatic secretion of apob-100 is impaired in hypobetalipoproteinemic mice with an apob-38.9-specifying allele Zhouji Chen, 1, * Robin L. Fitzgerald,* Gang Li, 2, * Nicholas O. Davidson, and Gustav Schonfeld
LDL receptor related protein mediates cellsurface clustering and hepatic sequestration of chylomicron remnants in LDLR-deficient mice
 LDL receptor related protein mediates cellsurface clustering and hepatic sequestration of chylomicron remnants in LDLR-deficient mice Kenneth C.-W. Yu,, Wei Chen, Allen D. Cooper J Clin Invest. 2001;107(11):1387-1394.
LDL receptor related protein mediates cellsurface clustering and hepatic sequestration of chylomicron remnants in LDLR-deficient mice Kenneth C.-W. Yu,, Wei Chen, Allen D. Cooper J Clin Invest. 2001;107(11):1387-1394.
Hypertriglyceridemia: Why, When, and How to Treat. Gregory Cohn, MD, FNLA, FASPC
 Hypertriglyceridemia: Why, When, and How to Treat Gregory Cohn, MD, FNLA, FASPC DISCLOSURES Consultant to Akcea Therapeutics (in the past 12 months). OUTLINE I. Lipoproteins II. Non-HDL-C III. Causes and
Hypertriglyceridemia: Why, When, and How to Treat Gregory Cohn, MD, FNLA, FASPC DISCLOSURES Consultant to Akcea Therapeutics (in the past 12 months). OUTLINE I. Lipoproteins II. Non-HDL-C III. Causes and
Measurement of fasting serum apob-48 levels in normolipidemic and hyperlipidemic subjects by ELISA 1
 Measurement of fasting serum apob-48 levels in normolipidemic and hyperlipidemic subjects by ELISA 1 Naohiko Sakai, 2,3, * Yoshiaki Uchida, 2, Koji Ohashi,* Toshiyuki Hibuse,* Yasuhiko Saika,* Yoshiaki
Measurement of fasting serum apob-48 levels in normolipidemic and hyperlipidemic subjects by ELISA 1 Naohiko Sakai, 2,3, * Yoshiaki Uchida, 2, Koji Ohashi,* Toshiyuki Hibuse,* Yasuhiko Saika,* Yoshiaki
Relative roles of LDLr and LRP in the metabolism of chylomicron remnants in genetically manipulated mice
 Relative roles of LDLr and LRP in the metabolism of chylomicron remnants in genetically manipulated mice Ian J. Martins, 1, * Eugene Hone,* Caroline Chi,* Ulrich Seydel,* Ralph N. Martins, and Trevor G.
Relative roles of LDLr and LRP in the metabolism of chylomicron remnants in genetically manipulated mice Ian J. Martins, 1, * Eugene Hone,* Caroline Chi,* Ulrich Seydel,* Ralph N. Martins, and Trevor G.
Bone marrow-derived HL mitigates bone marrow-derived CETP-mediated decreases in HDL in mice globally deficient in HL and the LDLr
 Bone marrow-derived HL mitigates bone marrow-derived CETP-mediated decreases in HDL in mice globally deficient in HL and the Neil J. Hime, 1,2 Audrey S. Black, David J. Bonnet, and Linda K. Curtiss Department
Bone marrow-derived HL mitigates bone marrow-derived CETP-mediated decreases in HDL in mice globally deficient in HL and the Neil J. Hime, 1,2 Audrey S. Black, David J. Bonnet, and Linda K. Curtiss Department
Walter B. Bayubay CLS (ASCP), AMT, MA Ed, CPI
 Walter B. Bayubay CLS (ASCP), AMT, MA Ed, CPI Biochemical Analysis (Lipid Panel) Analyte Total Cholesterol Reference Range Patient A < 200 241 LDL-C /= 40 38 Triglycerides
Walter B. Bayubay CLS (ASCP), AMT, MA Ed, CPI Biochemical Analysis (Lipid Panel) Analyte Total Cholesterol Reference Range Patient A < 200 241 LDL-C /= 40 38 Triglycerides
Juxtapid. Juxtapid (lomitapide) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Juxtapid Page: 1 of 6 Last Review Date: September 20, 2018 Juxtapid Description Juxtapid (lomitapide)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Juxtapid Page: 1 of 6 Last Review Date: September 20, 2018 Juxtapid Description Juxtapid (lomitapide)
Is it really that simple? Alyssa Hasty, PhD Associate Professor Molecular Physiology and Biophysics
 Alyssa Hasty, PhD Associate Professor Molecular Physiology and Biophysics Why we care about hepatic lipogenesis Control of lipid synthesis What can go wrong in humans Animal models dlto study lipoprotein
Alyssa Hasty, PhD Associate Professor Molecular Physiology and Biophysics Why we care about hepatic lipogenesis Control of lipid synthesis What can go wrong in humans Animal models dlto study lipoprotein
Mechanisms Underlying the Atherosclerosis Risk Associated with Apolipoprotein E Isoforms in Humans. Michael Kelly Altenburg
 Mechanisms Underlying the Atherosclerosis Risk Associated with Apolipoprotein E Isoforms in Humans Michael Kelly Altenburg A dissertation submitted to the faculty of the University of North Carolina at
Mechanisms Underlying the Atherosclerosis Risk Associated with Apolipoprotein E Isoforms in Humans Michael Kelly Altenburg A dissertation submitted to the faculty of the University of North Carolina at
Lipoprotein Formation, Structure and Metabolism: Cholesterol Balance and the Regulation of Plasma Lipid Levels
 Lipoprotein Formation, Structure and Metabolism: Balance and the Regulation of Plasma Lipid Levels David E. Cohen, MD, PhD Director of Hepatology, Gastroenterology Division, Brigham and Women s Hospital
Lipoprotein Formation, Structure and Metabolism: Balance and the Regulation of Plasma Lipid Levels David E. Cohen, MD, PhD Director of Hepatology, Gastroenterology Division, Brigham and Women s Hospital
Lipids digestion and absorption, Biochemistry II
 Lipids digestion and absorption, blood plasma lipids, lipoproteins Biochemistry II Lecture 1 2008 (J.S.) Triacylglycerols (as well as free fatty acids and both free and esterified cholesterol) are very
Lipids digestion and absorption, blood plasma lipids, lipoproteins Biochemistry II Lecture 1 2008 (J.S.) Triacylglycerols (as well as free fatty acids and both free and esterified cholesterol) are very
Novel Reduction of PCSK9 Expression: Mechanistic Insights into the Anti-Atherosclerotic & Hypolipidemic Effects of Heat Shock Protein 27
 Novel Reduction of PCSK9 Expression: Mechanistic Insights into the Anti-Atherosclerotic & Hypolipidemic Effects of Heat Shock Protein 27 Ed O Brien, Jean-Claude Bakala-N Goma, Chunhua Shi Cumming School
Novel Reduction of PCSK9 Expression: Mechanistic Insights into the Anti-Atherosclerotic & Hypolipidemic Effects of Heat Shock Protein 27 Ed O Brien, Jean-Claude Bakala-N Goma, Chunhua Shi Cumming School
ATP III (Adult Treatment Panel III) CLASSIFICATION C IN ADULTS
 LABORATORY AND RISK FACTORS OF ATHEROSCLEROSIS S R. Mohammadi Biochemist (Ph.D.) Faculty member of Medical Faculty RISK FACTORS FOR CHD Clinical Risk Factors Laboratory Risk Factors MAJOR CLINICAL RISK
LABORATORY AND RISK FACTORS OF ATHEROSCLEROSIS S R. Mohammadi Biochemist (Ph.D.) Faculty member of Medical Faculty RISK FACTORS FOR CHD Clinical Risk Factors Laboratory Risk Factors MAJOR CLINICAL RISK
Lipid/Lipoprotein Structure and Metabolism (Overview)
 Lipid/Lipoprotein Structure and Metabolism (Overview) Philip Barter President, International Atherosclerosis Society Centre for Vascular Research University of New South Wales Sydney, Australia Disclosures
Lipid/Lipoprotein Structure and Metabolism (Overview) Philip Barter President, International Atherosclerosis Society Centre for Vascular Research University of New South Wales Sydney, Australia Disclosures
1) DNA unzips - hydrogen bonds between base pairs are broken by special enzymes.
 Biology 12 Cell Cycle To divide, a cell must complete several important tasks: it must grow, during which it performs protein synthesis (G1 phase) replicate its genetic material /DNA (S phase), and physically
Biology 12 Cell Cycle To divide, a cell must complete several important tasks: it must grow, during which it performs protein synthesis (G1 phase) replicate its genetic material /DNA (S phase), and physically
Suppl. Table 1: CV of pooled lipoprotein fractions analysed by ESI-MS/MS
 Supplement VLDL LDL HDL PC 3.3 1.77 1.3 LPC 4.82 2.5.35 SM 3.1 4.6 1.92 CER 2.17 6.3 4.15 PE 3.18 1.93 2.79 PE-pl 13.18 1.9 2.32 CE 2.9.65.4 FC.36 3.5 2.54 Suppl. Table 1: CV of pooled lipoprotein fractions
Supplement VLDL LDL HDL PC 3.3 1.77 1.3 LPC 4.82 2.5.35 SM 3.1 4.6 1.92 CER 2.17 6.3 4.15 PE 3.18 1.93 2.79 PE-pl 13.18 1.9 2.32 CE 2.9.65.4 FC.36 3.5 2.54 Suppl. Table 1: CV of pooled lipoprotein fractions
Chapter (5) Etiology of Low HDL- Cholesterol
 Chapter (5) Etiology of Low HDL- Cholesterol The aim of this chapter is to summarize the different etiological factors mainly the role of life-style and different disease conditions contributing to the
Chapter (5) Etiology of Low HDL- Cholesterol The aim of this chapter is to summarize the different etiological factors mainly the role of life-style and different disease conditions contributing to the
Hydrophobic Surfactant Treatment Prevents Atherosclerosis in the Rabbit
 Hydrophobic Surfactant Treatment Prevents Atherosclerosis in the Rabbit RAPID PUBLICATIONS J. B. RODGERS, E. C. KYRIAKIDES, B. KAPUSCINSKA, S. K. PENG, and W. J. BOCHENEK, Department of Medicine, Albany
Hydrophobic Surfactant Treatment Prevents Atherosclerosis in the Rabbit RAPID PUBLICATIONS J. B. RODGERS, E. C. KYRIAKIDES, B. KAPUSCINSKA, S. K. PENG, and W. J. BOCHENEK, Department of Medicine, Albany
Transgenic rabbits as models for atherosclerosis research
 review Transgenic rabbits as models for atherosclerosis research Margaret E. Brousseau 1 and Jeffrey M. Hoeg 2 Lipid Metabolism Laboratory, JM-USDA/HNRCA at Tufts University, Boston, MA 02111 and Molecular
review Transgenic rabbits as models for atherosclerosis research Margaret E. Brousseau 1 and Jeffrey M. Hoeg 2 Lipid Metabolism Laboratory, JM-USDA/HNRCA at Tufts University, Boston, MA 02111 and Molecular
Hepatic lipase expression in macrophages contributes to atherosclerosis in apoe-deficient and LCAT-transgenic mice
 Hepatic lipase expression in macrophages contributes to atherosclerosis in apoe-deficient and LCAT-transgenic mice Zengxuan Nong, 1 Herminia González-Navarro, 1 Marcelo Amar, 1 Lita Freeman, 1 Catherine
Hepatic lipase expression in macrophages contributes to atherosclerosis in apoe-deficient and LCAT-transgenic mice Zengxuan Nong, 1 Herminia González-Navarro, 1 Marcelo Amar, 1 Lita Freeman, 1 Catherine
Central role of apociii
 University of Copenhagen & Copenhagen University Hospital Central role of apociii Anne Tybjærg-Hansen MD DMSc Copenhagen University Hospital and Faculty of Health and Medical Sciences, University of Copenhagen,
University of Copenhagen & Copenhagen University Hospital Central role of apociii Anne Tybjærg-Hansen MD DMSc Copenhagen University Hospital and Faculty of Health and Medical Sciences, University of Copenhagen,
