1. Stowers Institute for Medical Research, Kansas City, MO 64110
|
|
|
- Howard Cole
- 5 years ago
- Views:
Transcription
1 Lipid droplets as fat storage organelles in Caenorhabditis elegans Ho Yi Mak 1, 2 1. Stowers Institute for Medical Research, Kansas City, MO Department of Molecular and Integrative Physiology, University of Kansas Medical Center, Kansas City, KS hym@stowers.org 1
2 Abstract Lipid droplets are evolutionarily conserved organelles where cellular fat storage and mobilization are exquisitely regulated. Recent studies have defined lipid droplets in C. elegans and explored how they are regulated by genetic and dietary factors. C. elegans offers unique opportunities to visualize lipid droplets at single cell resolution in live animals. The development of novel microscopy techniques and protein markers for lipid droplets will accelerate studies on how nutritional states and subcellular organization are linked in vivo. Together with powerful tools for genetic and biochemical analysis of metabolic pathways, alteration in lipid droplet abundance, size and distribution in C. elegans can be readily connected to whole animal energy homeostasis, behavior and life span. Therefore, further studies on lipid droplets in C. elegans promise to yield valuable insights that complement our knowledge gained from yeast, Drosophila and mammalian systems on cellular and organismal fat storage. 2
3 Introduction Lipid droplets are ubiquitous fat storage organelles that are conserved in yeast, C. elegans, Drosophila and mammals (1-3). They are also sites of regulated release of stored fat by lipases during cell growth and fasting (4). Therefore, lipid droplets are central to energy balance at cellular and organismal levels. Key structural and biochemical features of lipid droplets have been defined and it is generally accepted that neutral lipids such as triglycerides (TAG) and cholesterol esters (CE) are stored in the interior of the organelle that is delimited by a phospholipid monolayer (5-7). Current models suggest that fatty acid influx can be accommodated by neutral lipid synthesis and a concomitant change in lipid droplet size or number in a tissue specific manner (1, 8). For example, differentiation of mammalian white adipose cells are characterized by a decrease in lipid droplet number and an increase in lipid droplet size until a dominant unilocular lipid droplet remains (9, 10). The mechanisms that govern lipid droplet size and number in white adipose cells and other cell types are not fully understood. To study regulators of fat storage and lipid droplet size in metazoans, most studies have focused on the use of tissue culture cells and over-expressed lipid droplet associated proteins or vital dyes as markers. These studies yielded important insights into how conserved proteins and signaling pathways are coupled to control cellular fat storage. Nevertheless, extension of observations made in tissue culture cells into whole animal models is often time consuming and can lead to surprising results. In the last few years, C. elegans has emerged as an attractive model for studying fat storage in vivo (11, 12). Although C. elegans lack dedicated adipose tissues, its intestine serves as the main site for fat storage. Yolk lipoproteins are synthesized in the intestine, exported into the body cavity (pseudocoelomic space), and taken up by developing oocytes (13-15). This is remarkably similar to yolk deposition in chicken where yolk is synthesized in the liver and transported to the ovum via the bloodstream (16). Therefore, the C. elegans intestine may be viewed as a multi-functional organ that fulfills the roles of the liver and adipose tissues. Inspection of the sequenced genome of C. elegans and functional studies through analysis of genetic mutants revealed extensive conservation of genes involved in fatty acid synthesis, elongation, desaturation and degradation (17-23). Furthermore, fat storage in C. 3
4 elegans appears to be controlled by conserved insulin, TGF-β, serotonin, and mammalian target of rapamycin (mtor) signaling pathways (24-29). A number of reverse genetic techniques will accelerate functional dissection of signaling and protein interaction networks (30-32). The development of novel transgenic technologies has also enabled expression of native or heterologous proteins to near physiological levels (33). This is critical for structure-function analysis of specific proteins in vivo and the accurate targeting of fluorescent protein markers to lipid droplets and other sub-cellular organelles. The use of C. elegans offers a unique advantage of imaging lipid droplets in live animals at single cell resolution. Their intestinal cells support lipid droplet expansion to >10µm in diameter, similar to lipid droplets found in mammalian adipocytes (34). In contrary, adherent tissue culture cells have limited depth, which restricts spherical expansion of lipid droplets. Finally, C. elegans offers ample opportunities to connect alteration in cellular fat storage to whole animal physiology, behavior, and life-span (18, 35, 36). This review will focus on the discovery of lipid droplets in C. elegans, the methods to detect lipid droplets and the proteins that associate with lipid droplets in this model organism. Thorough understanding of these areas will no doubt provide answers to fundamental questions regarding how lipid droplets accommodate changes in the cellular demand and supply of fat in an evolutionarily conserved manner. Identification of lipid droplets in C. elegans In the absence of a specialized fat storage tissue, C. elegans store their fat primarily in the intestine. It is remarkable that the entire intestine is composed of only 20 cells, arranged in rings of two or four cells that enclose the lumen, which span almost the entire length of the animal (~1mm) (37, 38). Each intestinal cell is derived from a single embryonic blastomere: cell division ceases and organogenesis is complete before the hatching of a free-living animal (37, 38). Nevertheless, the intestinal cells increase in size that matches the growth of the animal as it progresses through 4 larval stages before maturing into a reproductive adult. One of the defining features of lipid droplets is the phospholipid monolayer that encloses their neutral lipid core, as demonstrated by transmission and freeze-fracture electron microscopy (5-7). This set lipid droplets apart from all other intracellular organelles that are bound by a 4
5 phospholipid bi-layer. Extensive ultra-structural studies have been performed in C. elegans, which revealed numerous vesicular compartments of similar size in the intestinal cells (37-39). These compartments may be differentiated by their electron densities. Two putative fat and lipoprotein storage compartments had been proposed: electron-lucent lipid droplets and electronopaque gut granules (37-40). Gut granules have also been detected using light microscopy and were subsequently defined as lysosome-related organelles (LROs) (41, 42). The appearance and relative abundance of these compartments is dependent on the developmental stages of the animal. However, membrane structures that surrounded these compartments were not studied in detail until recently. To positively identify lipid droplets using electron microscopy in C. elegans, Zhang et al took advantage of a mutant that appeared to undergo selective expansion of fat storage compartments (34) (Figure 1). The daf-22/thiolase gene encodes the terminal enzyme in a peroxisomal β-oxidation pathway. DAF-22/thiolase was originally identified as a key enzyme for processing the fatty acid moiety of the C. elegans dauer pheromone (43). Loss of DAF- 22/thiolase function in C. elegans specifically increases the level of triglycerides that are accommodated by expanded fat storage compartments in the intestinal cells (34). Since most other vesicular structures are <2µm in diameter, electron-lucent, grossly expanded compartments (> 10µm in diameter) could be readily identified by size. Indeed, it was found that such compartments were delimited by a phospholipid monolayer, thus confirming the existence of lipid droplets in C. elegans (34). Additional support came from the targeting of the adipose triglyceride lipase ortholog (ATGL-1) to the surface of expanded lipid droplets in daf-22 mutant animals (34). Since the initial report, lipid droplets have also been identified in wild-type animals by transmission electron microscopy (40). It was also determined that lipid droplets are distinct from lysosome related organelles (40), a compartment that was previously proposed to store fat in C. elegans (44). Furthermore, expanded lipid droplets have been reported in mutant animals that lack ACS-3/acyl-CoA synthetase (45). 5
6 Visualization of fat storage in C. elegans Attempts to visualize and quantitate fat storage long preceded the definitive identification of lipid droplets in C. elegans. Sudan Black has been widely adopted as a stain for intracellular fat since its first usage on bacteria (46). In C. elegans, animals that expressed a defective Insulin/IGF receptor ortholog DAF-2 showed more intense Sudan Black staining than wild-type animals (29). Subsequent biochemical measurement confirmed that loss of insulin signaling elevated triglycerides levels in C. elegans, validating Sudan Black staining as a method for visualization of fat storage (47). Sudan Black staining was most prominent in intestinal cells, thus giving rise to the notion that the intestine is the major fat storage organ in C. elegans. In addition, Sudan Black staining was observed in the hypodermis and gonad, suggesting that fat is exported from the intestine to other tissues. Although Sudan Black staining was widely employed in other studies (27, 48-50), the fixation protocol was not readily adaptable for large scale genetic and functional genomic screens. To overcome this, Nile Red and BODIPYconjugated fatty acid were used as alternatives for fat staining in live animals (47). Nile Red was first introduced as a fluorescent vital stain for intracellular lipid droplets in mammalian cells (51) while BODIPY-conjugated fatty acid had been used to monitor fatty acid uptake in mammalian cells (52). It was assumed that worms would ingest Nile Red or BODIPY-conjugated fatty acid from E. coli that had been grown in the presence of these dyes on agar plates. This was followed by absorption of the dyes through the intestinal epithelium and their accumulation in fat storage compartments in intestinal cells. The precise mechanisms of absorption and turn-over of these dyes are currently unknown. Nile Red staining was initially used to conduct a whole-genome RNAi screen, which resulted in the discovery of hundreds of gene inactivations that altered the intensity and/or pattern of staining within intestinal cells (47). Many genes with conserved function in fat metabolism were uncovered suggesting that Nile Red staining might serve as an indicator of fat storage in C. elegans. Nevertheless, recent evidence suggests that vital staining with Nile Red and BODIPY-conjugated fatty acid also illuminates lysosome related organelles and their accumulation in lipid droplets is highly dependent on genetic backgrounds (40, 44, 53-56). Therefore, future use of Nile Red as a vital dye for monitoring fat storage in C. elegans should always be validated with biochemical measurement of fat content. Further refinement may 6
7 include analysis of the emission spectrum of Nile Red which differs in lipid droplets versus lysosome related organelles (LROs) (40, 45). This is because Nile Red in a neutral lipid environment (i.e. lipid droplets) will emit yellow-gold fluorescence instead of red fluorescence (51). It is interesting to note that Nile Red, BODIPY or Oil-Red-O staining on fixed C. elegans samples appear to faithfully highlight lipid droplets and reflect biochemical measurements of fat content (40, 54-57). The continual demand for identification of fat storage compartments in live samples has led to the development and implementation of multiple label free lipid imaging techniques (58-60). The vibrations of C-H bond in lipids allow sensitive, label free detection of lipid droplets using Coherent Anti-Stokes Raman Scattering (CARS) microscopy in mammalian cells (59). This method was subsequently used to visualize fat storage compartments in live C. elegans (54, 61, 62). By exploiting vibrational characteristics of C=C double bonds, CARS microscopy also allowed the detection of changes in relative abundance of unsaturated fatty acids in mutant C. elegans that lacked specific fatty acid desaturases (62, 63). More recently, Stimulated Raman scattering (SRS) microscopy has been introduced (58). In comparison with CARS microscopy, SRS microscopy was reported to be more sensitive and less prone to background noise from structures unrelated to lipids. It was demonstrated in C. elegans that SRS microscopy could be used to visualize intracellular fat storage compartments and measure organismal fat content quantitatively (64). Using SRS microscopy, 272 gene inactivations by RNAi had been surveyed, which yielded 9 potentially new regulators of fat content in C. elegans (64). Although vesicular structures detected by CARS or SRS microscopy in C. elegans are likely to be lipid droplets as in mammalian cells, simultaneous imaging of fluorescent protein markers would be a logical step to determine if these label-free imaging techniques detect all lipid droplets. Lipid droplet associated proteins in C. elegans A large number of lipid droplet associated proteins have been identified from proteomics studies using mammalian and Drosophila cell and tissue extracts (65-68). In contrast, similar proteomics studies had not been carried out in C. elegans. This is in part because existing protocols for large-scale protein extraction and purification using frozen worm samples is 7
8 incompatible with lipid droplet preservation (Dawn Brasaemle; personal communication). Intriguingly, the Perilipin and CIDE families of proteins, which have been widely used as reference markers for lipid droplets in other organisms, are absent in C. elegans. Therefore, few C. elegans proteins have been reported to be genuinely associated with lipid droplets. A strategy forward will involve selecting conserved proteins from mammalian or Drosophila lipid droplet proteomes and interrogate their localization in C. elegans. It may be prudent to concentrate on proteins that have a clear role in fat metabolism or regulation of lipid droplet size or number. One such candidate is the Adipose Triglyceride Lipase ortholog, ATGL-1. The C. elegans ATGL-1 lipase is crucial for the balance of fat utilization and preservation in the developmentally arrested dauer larval stage (69). Long term (>1 month) survival of dauers is dependent on tight regulation of ATGL-1 activity by the AMP kinase (69). In Drosophila, the Adipose Triglyceride Lipase ortholog Brummer, when fused to green fluorescent protein (GFP), has been shown to localize to lipid droplet surface (70). Based on these results, it was satisfying to observe that the C. elegans ATGL-1::GFP fusion protein was also localized to the lipid droplet surface, both in wild-type and mutant animals with enlarged lipid droplets (40, 45). By coupling confocal microscopy with spectral analysis and linear un-mixing procedures, it was further demonstrated that ATGL-1::GFP marked lipid droplets are distinct from the auto-fluorescent lysosome related organelles (Figure 2) (40). Fat mobilization from lipid droplets is a highly regulated process that involves not only adipose triglyceride lipase, but its activator CGI58 and hormone sensitive lipase (4). Therefore, the C. elegans orthologs of CGI58 and hormone sensitive lipase are prime candidate markers for lipid droplets in future studies. The absence of Perilipin and CIDE families of proteins warrants further discussion. The Perilipin family members are found in mammals and invertebrates including Drosophila and Dictyostelium (71). However, no Perilipin orthologs have been identified through sequence homology in C. elegans and other nematode species. Since Dictyostelium is more primitive than nematodes, it is plausible that the Perilipin family was lost during evolution. Perilipin is known to play an important role in basal and hormone stimulated lipolysis through its association with the lipid droplet surface and its stimulatory activity toward adipose triglyceride lipase (ATGL) and hormone sensitive lipase (HSL) (72-77). The absence of Perilipin in C. elegans gives rise to an intriguing possibility that additional, Perilipin independent, regulatory mechanisms may exist for ATGL and HSL mediated lipolysis. Alternatively, proteins that share structural and 8
9 functional similarity with Perilipin may have greatly diverged in primary sequence that precludes their discovery through homology searches. In contrast to the Perilipins, the CIDE family proteins are found only in vertebrates (78). All three family members, CIDEA, CIDEB and CIDEC, have been shown to associate with lipid droplets and play important roles in regulating whole body fat storage and energy balance in mice and human (79-86). Ectopic over-expression of CIDE proteins modulates lipid droplet size (82, 83). One may speculate that the CIDE proteins have evolved in vertebrates to add an additional level of regulation for fat storage in lipid droplets. It is also plausible that divergent proteins substitute for CIDE protein functions in C. elegans and other invertebrates. Perspectives With the definitive identification of lipid droplets as fat storage organelles in C. elegans, now is the time to harness the forward and reverse genetic, biochemical and imaging tools available to study the cell biology of fat storage and mobilization in this well established model organism. Since the diet of C. elegans can be easily manipulated by feeding with different E. coli strains, analysis of lipid species and other metabolites will shed light on how dietary factors affect fat storage in lipid droplets (34, 56, 87, 88). Cell biological studies can also be coupled with powerful analytical methods that allow simultaneous monitoring of fatty acid absorption, elongation and de novo synthesis in C. elegans, which is yet to be employed in other metazoans (89). Although the intestinal cells are the major site of fat storage in C. elegans, with the advent of label free imaging techniques and fluorescent protein markers for lipid droplets, it will be of great interest to determine if the regulators of fat storage in intestinal cells function in a similar fashion in tissues such as the hypodermis and gonad. It is plausible that additional mechanisms may operate in these tissues to support rapid turn-over of fat for energy and reproduction. For example, small lipid droplets provide a larger surface area to volume ratio that favors lipolysis. The size of lipid droplets in the hypodermis and gonad of C. elegans may therefore be tightly regulated. The unique advantage of live animal imaging at single-cell resolution throughout the life cycle should see the use of C. elegans in addressing additional fundamental questions on lipid 9
10 droplets. How is the interaction between lipid droplets and other subcellular organelles, such as the endoplasmic reticulum and mitochondria, maintained? What is the functional consequence of such interactions? Is the distribution and movement of lipid droplets regulated in different developmental stages and nutrient levels? Careful implementation of genetic, biochemical and microscopy techniques should yield insights that can be readily extended from C. elegans to mammals. 10
11 Acknowledgements I thank Ronald Cole for providing the image for Figure 2, Karen Reue and Meng Wang for comments and suggestions on the manuscript. Research on lipid droplets in our laboratory was supported by the Stowers Institute for Medical Research and in part by Research Grant 5-FY from the March of Dimes Foundation. 11
12 References 1. Farese RV, Jr. & Walther TC (2009) Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 139(5): Goodman JM (2009) Demonstrated and inferred metabolism associated with cytosolic lipid droplets. J Lipid Res 50(11): Martin S & Parton RG (2006) Lipid droplets: a unified view of a dynamic organelle. Nature reviews 7(5): Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, & Lass A (2009) Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res 50(1): Blanchette-Mackie EJ, et al. (1995) Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res 36(6): Robenek H, et al. (2009) Compartmentalization of proteins in lipid droplet biogenesis. Biochimica et biophysica acta 1791(6): Tauchi-Sato K, Ozeki S, Houjou T, Taguchi R, & Fujimoto T (2002) The surface of lipid droplets is a phospholipid monolayer with a unique Fatty Acid composition. The Journal of biological chemistry 277(46): Kuerschner L, Moessinger C, & Thiele C (2008) Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic 9(3): Hausman GJ & Richardson RL (1983) Cellular and vascular development in immature rat adipose tissue. J Lipid Res 24(5): Slavin BG (1979) Fine structural studies on white adipocyte differentiation. Anat Rec 195(1): Watts JL (2009) Fat synthesis and adiposity regulation in Caenorhabditis elegans. Trends Endocrinol Metab 20(2): Mullaney BC & Ashrafi K (2009) C. elegans fat storage and metabolic regulation. Biochimica et biophysica acta 1791(6): Grant B & Hirsh D (1999) Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Molecular biology of the cell 10(12): Hall DH, et al. (1999) Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev Biol 212(1): Kimble J & Sharrock WJ (1983) Tissue-specific synthesis of yolk proteins in Caenorhabditis elegans. Dev Biol 96(1):
13 16. Schneider WJ (1996) Vitellogenin receptors: oocyte-specific members of the low-density lipoprotein receptor supergene family. International review of cytology 166: Mak HY, Nelson LS, Basson M, Johnson CD, & Ruvkun G (2006) Polygenic control of Caenorhabditis elegans fat storage. Nat Genet 38: Kniazeva M, Crawford QT, Seiber M, Wang CY, & Han M (2004) Monomethyl branched-chain fatty acids play an essential role in Caenorhabditis elegans development. PLoS biology 2(9):E Kniazeva M, et al. (2003) Suppression of the ELO-2 FA elongation activity results in alterations of the fatty acid composition and multiple physiological defects, including abnormal ultradian rhythms, in Caenorhabditis elegans. Genetics 163(1): Walker AK, et al. (2010) Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes & development 24(13): Watts JL & Browse J (2002) Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America 99(9): Yang F, et al. (2006) An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature 442(7103): Van Gilst MR, Hadjivassiliou H, Jolly A, & Yamamoto KR (2005) Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS biology 3:e Greer ER, Perez CL, Van Gilst MR, Lee BH, & Ashrafi K (2008) Neural and molecular dissection of a C. elegans sensory circuit that regulates fat and feeding. Cell metabolism 8(2): Soukas AA, Kane EA, Carr CE, Melo JA, & Ruvkun G (2009) Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes & development 23(4): Srinivasan S, et al. (2008) Serotonin regulates C. elegans fat and feeding through independent molecular mechanisms. Cell metabolism 7(6): Sze JY, Victor M, Loer C, Shi Y, & Ruvkun G (2000) Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 403(6769): Jones KT, Greer ER, Pearce D, & Ashrafi K (2009) Rictor/TORC2 regulates Caenorhabditis elegans fat storage, body size, and development through sgk-1. PLoS biology 7(3):e Kimura KD, Tissenbaum HA, Liu Y, & Ruvkun G (1997) daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science (New York, N.Y 277:
14 30. Frokjaer-Jensen C, et al. (2010) Targeted gene deletions in C. elegans using transposon excision. Nat Methods 7(6): Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, & Ahringer J (2001) Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome biology 2(1):RESEARCH Robert V & Bessereau JL (2007) Targeted engineering of the Caenorhabditis elegans genome following Mos1-triggered chromosomal breaks. EMBO J 26(1): Frokjaer-Jensen C, et al. (2008) Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet 40(11): Zhang SO, et al. (2010) Genetic and dietary regulation of lipid droplet expansion in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America 107(10): Brock TJ, Browse J, & Watts JL (2006) Genetic regulation of unsaturated fatty acid composition in C. elegans. PLoS Genet 2(7):e Wang MC, O'Rourke EJ, & Ruvkun G (2008) Fat metabolism links germline stem cells and longevity in C. elegans. Science (New York, N.Y 322(5903): Leung B, Hermann GJ, & Priess JR (1999) Organogenesis of the Caenorhabditis elegans intestine. Dev Biol 216(1): Altun ZF & Hall DH (2009) Alimentary system, intestine. WormAtlas: 39. Albert PS & Riddle DL (1988) Mutants of Caenorhabditis elegans that form dauer-like larvae. Dev Biol 126(2): Zhang SO, Trimble R, Guo F, & Mak HY (2010) Lipid droplets as ubiquitous fat storage organelles in C. elegans. BMC Cell Biol 11: Clokey GV & Jacobson LA (1986) The autofluorescent "lipofuscin granules" in the intestinal cells of Caenorhabditis elegans are secondary lysosomes. Mechanisms of ageing and development 35(1): Hermann GJ, et al. (2005) Genetic analysis of lysosomal trafficking in Caenorhabditis elegans. Molecular biology of the cell 16(7): Butcher RA, et al. (2009) Biosynthesis of the Caenorhabditis elegans dauer pheromone. Proceedings of the National Academy of Sciences of the United States of America 106(6):
15 44. Schroeder LK, et al. (2007) Function of the Caenorhabditis elegans ABC transporter PGP-2 in the biogenesis of a lysosome-related fat storage organelle. Molecular biology of the cell 18(3): Mullaney BC, et al. (2010) Regulation of C. elegans fat uptake and storage by acyl-coa synthase-3 is dependent on NR5A family nuclear hormone receptor nhr-25. Cell metabolism 12(4): Burdon KL, Stokes JC, & Kimbrough CE (1942) Studies of the Common Aerobic Spore-forming Bacilli: I. Staining for Fat with Sudan Black B-safranin. Journal of bacteriology 43(6): Ashrafi K, et al. (2003) Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421: Ogg S, et al. (1997) The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389(6654): Ogg S & Ruvkun G (1998) The C. elegans PTEN homolog, DAF-18, acts in the insulin receptorlike metabolic signaling pathway. Mol Cell 2(6): McKay RM, McKay JP, Avery L, & Graff JM (2003) C elegans: a model for exploring the genetics of fat storage. Developmental cell 4(1): Greenspan P, Mayer EP, & Fowler SD (1985) Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol 100(3): Schaffer JE & Lodish HF (1994) Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell 79(3): Morck C, et al. (2009) Statins inhibit protein lipidation and induce the unfolded protein response in the non-sterol producing nematode Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America 106(43): Yen K, et al. (2010) A comparative study of fat storage quantitation in nematode Caenorhabditis elegans using label and label-free methods. PLoS One 5(9). 55. O'Rourke EJ, Soukas AA, Carr CE, & Ruvkun G (2009) C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell metabolism 10(5): Brooks KK, Liang B, & Watts JL (2009) The influence of bacterial diet on fat storage in C. elegans. PLoS One 4(10):e Klapper M, et al. (2011) Fluorescence-based fixative and vital staining of lipid droplets in Caenorhabditis elegans reveal fat stores using microscopy and flow cytometry approaches. J Lipid Res 52(6): Freudiger CW, et al. (2008) Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science (New York, N.Y 322(5909):
16 59. Nan X, Cheng JX, & Xie XS (2003) Vibrational imaging of lipid droplets in live fibroblast cells with coherent anti-stokes Raman scattering microscopy. J Lipid Res 44(11): Evans CL & Xie XS (2008) Coherent anti-stokes Raman scattering microscopy: chemical imaging for biology and medicine. Annu Rev Anal Chem (Palo Alto Calif) 1: Hellerer T, et al. (2007) Monitoring of lipid storage in Caenorhabditis elegans using coherent anti-stokes Raman scattering (CARS) microscopy. Proceedings of the National Academy of Sciences of the United States of America 104(37): Le TT, Duren HM, Slipchenko MN, Hu CD, & Cheng JX (2010) Label-free quantitative analysis of lipid metabolism in living Caenorhabditis elegans. J Lipid Res 51(3): Rinia HA, Burger KN, Bonn M, & Muller M (2008) Quantitative label-free imaging of lipid composition and packing of individual cellular lipid droplets using multiplex CARS microscopy. Biophysical journal 95(10): Wang MC, Min W, Freudiger CW, Ruvkun G, & Xie XS (2011) RNAi screening for fat regulatory genes with SRS microscopy. Nat Methods 8(2): Beller M, et al. (2006) Characterization of the Drosophila lipid droplet subproteome. Molecular & cellular proteomics : MCP 5(6): Brasaemle DL, Dolios G, Shapiro L, & Wang R (2004) Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. The Journal of biological chemistry 279(45): Cermelli S, Guo Y, Gross SP, & Welte MA (2006) The lipid-droplet proteome reveals that droplets are a protein-storage depot. Current biology : CB 16(18): Liu P, et al. (2004) Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. The Journal of biological chemistry 279(5): Narbonne P & Roy R (2009) Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature 457(7226): Gronke S, et al. (2005) Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell metabolism 1(5): Miura S, et al. (2002) Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. The Journal of biological chemistry 277(35): Greenberg AS, et al. (1991) Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. The Journal of biological chemistry 266(17):
17 73. Miyoshi H, et al. (2007) Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. The Journal of biological chemistry 282(2): Miyoshi H, et al. (2006) Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. The Journal of biological chemistry 281(23): Sztalryd C, et al. (2003) Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol 161(6): Subramanian V, et al. (2004) Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. The Journal of biological chemistry 279(40): Yamaguchi T, Omatsu N, Matsushita S, & Osumi T (2004) CGI-58 interacts with perilipin and is localized to lipid droplets. Possible involvement of CGI-58 mislocalization in Chanarin-Dorfman syndrome. The Journal of biological chemistry 279(29): Wu C, Zhang Y, Sun Z, & Li P (2008) Molecular evolution of Cide family proteins: novel domain formation in early vertebrates and the subsequent divergence. BMC evolutionary biology 8: Rubio-Cabezas O, et al. (2009) Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO molecular medicine 1(5): Keller P, et al. (2008) Fat-specific protein 27 regulates storage of triacylglycerol. The Journal of biological chemistry 283(21): Li JZ, et al. (2007) Cideb regulates diet-induced obesity, liver steatosis, and insulin sensitivity by controlling lipogenesis and fatty acid oxidation. Diabetes 56(10): Puri V, et al. (2007) Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. The Journal of biological chemistry 282(47): Puri V, et al. (2008) Cidea is associated with lipid droplets and insulin sensitivity in humans. Proceedings of the National Academy of Sciences of the United States of America 105(22): Zhou Z, et al. (2003) Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat Genet 35(1): Nishino N, et al. (2008) FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. The Journal of clinical investigation 118(8): Toh SY, et al. (2008) Up-regulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS One 3(8):e
18 87. Geier FM, Want EJ, Leroi AM, & Bundy JG (2011) Cross-platform comparison of Caenorhabditis elegans tissue extraction strategies for comprehensive metabolome coverage. Anal Chem 83(10): Pungaliya C, et al. (2009) A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America 106(19): Perez CL & Van Gilst MR (2008) A 13C isotope labeling strategy reveals the influence of insulin signaling on lipogenesis in C. elegans. Cell metabolism 8(3):
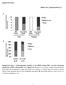
19 Figure legends Figure 1. Lipid droplets in C. elegans intestinal cells. (A) A wild-type larval stage L4 animal. The first intestinal segment is boxed in red. Scale bar, 100µm. (B-C) Electron micrographs showing cross-sections of the first intestinal segment in wild-type (B) and daf-22(-) (C) mutant animals. Red asterisks indicate enlarged lipid droplets. Scale bar, 5µm. (D) Electron micrograph showing a phospholipid monolayer (red arrowhead) that surrounds an expanded lipid droplet (boxed area in inset) in contrast to phospholipid bilayers (red arrows) of nearby organelles. The lumen of the intestine is at the bottom of the inset. (B-D) Reprint from Zhang et al, Proc Natl Acad Sci U S A.;107: Figure 2. ATGL-1::GFP as a marker for lipid droplets in C. elegans intestinal cells. Confocal microscopy and linear un-mixing distinguishes lipid droplets that are marked by ATGL-1::GFP fusion protein (in green) and auto-fluorescent lysosome related organelles (LROs) (in red). Scale bar, 10µm. 19
20 Figure 1 Mak A wild type C * * * wild type D daf-22(-) daf-22(-) 1µm 50nm B
21 Figure 2 Mak autofluorescence ATGL-1::GFP
Regulation of Lipid Homeostasis: Lipid Droplets
 Regulation of Lipid Homeostasis: Lipid Droplets Bernd Helms Article Brand Recter Hendrik Mertens 1 The Basics I: FAs The Basics II: FA Activation 2 Basics III: TG-FA Interplay. Why? Adipocytes 3 Foam cells
Regulation of Lipid Homeostasis: Lipid Droplets Bernd Helms Article Brand Recter Hendrik Mertens 1 The Basics I: FAs The Basics II: FA Activation 2 Basics III: TG-FA Interplay. Why? Adipocytes 3 Foam cells
Master class Biomolecular Sciences Molecular Cell Biology.
 Master class Biomolecular Sciences Molecular Cell Biology. 04-09-08: Paul van Bergen en Henegouwen. Clathrin-indep Endocytosis 11-09-08: Willem Stoorvogel. Endocytosis and MHC classii 18-09-08: X-track
Master class Biomolecular Sciences Molecular Cell Biology. 04-09-08: Paul van Bergen en Henegouwen. Clathrin-indep Endocytosis 11-09-08: Willem Stoorvogel. Endocytosis and MHC classii 18-09-08: X-track
Qualifying Fat Storage Phenotypes Using Gas Chromatography in Caenorhabditis elegans
 University of Colorado, Boulder CU Scholar Undergraduate Honors Theses Honors Program Spring 2014 Qualifying Fat Storage Phenotypes Using Gas Chromatography in Caenorhabditis elegans Sabrina Fechtner University
University of Colorado, Boulder CU Scholar Undergraduate Honors Theses Honors Program Spring 2014 Qualifying Fat Storage Phenotypes Using Gas Chromatography in Caenorhabditis elegans Sabrina Fechtner University
Chapter 1 The Lipid Droplet: a Dynamic Organelle, not only Involved in the Storage and Turnover of Lipids
 Chapter 1 The Lipid Droplet: a Dynamic Organelle, not only Involved in the Storage and Turnover of Lipids Sven-Olof Olofsson, Pontus Boström, Jens Lagerstedt, Linda Andersson, Martin Adiels, Jeanna Perman,
Chapter 1 The Lipid Droplet: a Dynamic Organelle, not only Involved in the Storage and Turnover of Lipids Sven-Olof Olofsson, Pontus Boström, Jens Lagerstedt, Linda Andersson, Martin Adiels, Jeanna Perman,
Fat synthesis and adiposity regulation in Caenorhabditis elegans
 Review Fat synthesis and adiposity regulation in Caenorhabditis elegans Jennifer L. Watts School of Molecular Biosciences, Washington State University, Pullman, WA 99164, USA Understanding the regulation
Review Fat synthesis and adiposity regulation in Caenorhabditis elegans Jennifer L. Watts School of Molecular Biosciences, Washington State University, Pullman, WA 99164, USA Understanding the regulation
Specific polyunsaturated fatty acids modulate lipid delivery and oocyte development in C. elegans revealed by molecular-selective label-free imaging
 Specific polyunsaturated fatty acids modulate lipid delivery and oocyte development in C. elegans revealed by molecular-selective label-free imaging Wei-Wen Chen 1,2,3,+, Yung-Hsiang Yi 4,5,+, Cheng-Hao
Specific polyunsaturated fatty acids modulate lipid delivery and oocyte development in C. elegans revealed by molecular-selective label-free imaging Wei-Wen Chen 1,2,3,+, Yung-Hsiang Yi 4,5,+, Cheng-Hao
2013 John Wiley & Sons, Inc. All rights reserved. PROTEIN SORTING. Lecture 10 BIOL 266/ Biology Department Concordia University. Dr. S.
 PROTEIN SORTING Lecture 10 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University Introduction Membranes divide the cytoplasm of eukaryotic cells into distinct compartments. The endomembrane
PROTEIN SORTING Lecture 10 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University Introduction Membranes divide the cytoplasm of eukaryotic cells into distinct compartments. The endomembrane
Introduction. Biochemistry: It is the chemistry of living things (matters).
 Introduction Biochemistry: It is the chemistry of living things (matters). Biochemistry provides fundamental understanding of the molecular basis for the function and malfunction of living things. Biochemistry
Introduction Biochemistry: It is the chemistry of living things (matters). Biochemistry provides fundamental understanding of the molecular basis for the function and malfunction of living things. Biochemistry
Problem Set #5 4/3/ Spring 02
 Question 1 Chloroplasts contain six compartments outer membrane, intermembrane space, inner membrane, stroma, thylakoid membrane, and thylakoid lumen each of which is populated by specific sets of proteins.
Question 1 Chloroplasts contain six compartments outer membrane, intermembrane space, inner membrane, stroma, thylakoid membrane, and thylakoid lumen each of which is populated by specific sets of proteins.
Lysosomes, Peroxisomes and Centrioles. Hüseyin Çağsın
 Lysosomes, Peroxisomes and Centrioles Hüseyin Çağsın Lysosomes Outline Endosomes Molecule transport to the lysosomes Endocytosis Exocytosis Autophagy Vacuoles Peroxisomes Centrioles Lysosomes Lysosomes
Lysosomes, Peroxisomes and Centrioles Hüseyin Çağsın Lysosomes Outline Endosomes Molecule transport to the lysosomes Endocytosis Exocytosis Autophagy Vacuoles Peroxisomes Centrioles Lysosomes Lysosomes
Cell Cell
 Go to cellsalive.com. Select Interactive Cell Models: Plant and Animal. Fill in the information on Plant and Animal Organelles, then Click on Start the Animation Select Plant or Animal Cell below the box.
Go to cellsalive.com. Select Interactive Cell Models: Plant and Animal. Fill in the information on Plant and Animal Organelles, then Click on Start the Animation Select Plant or Animal Cell below the box.
Very-Long Chain Fatty Acid Biosynthesis
 Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
7.06 Cell Biology EXAM #3 April 24, 2003
 7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
Supplemental Figures
 Supplemental Figures Supplemental Figure 1. Fasting-dependent regulation of the SREBP ortholog SBP-1 and lipid homeostasis mediated by the SIRT1 ortholog SIR-2.1 in C. elegans. (A) Wild-type or sir-2.1(lof)
Supplemental Figures Supplemental Figure 1. Fasting-dependent regulation of the SREBP ortholog SBP-1 and lipid homeostasis mediated by the SIRT1 ortholog SIR-2.1 in C. elegans. (A) Wild-type or sir-2.1(lof)
doi: /j.bbrc
 doi: 10.1016/j.bbrc.2009.11.006 Fatty Acid Metabolism is Involved in Stress Resistance Mechanisms of Caenorhabditis elegans Makoto Horikawa a, b, Kazuichi Sakamoto a, 1 a Graduate School of Life and Environmental
doi: 10.1016/j.bbrc.2009.11.006 Fatty Acid Metabolism is Involved in Stress Resistance Mechanisms of Caenorhabditis elegans Makoto Horikawa a, b, Kazuichi Sakamoto a, 1 a Graduate School of Life and Environmental
Intracellular Compartments and Protein Sorting
 Intracellular Compartments and Protein Sorting Intracellular Compartments A eukaryotic cell is elaborately subdivided into functionally distinct, membrane-enclosed compartments. Each compartment, or organelle,
Intracellular Compartments and Protein Sorting Intracellular Compartments A eukaryotic cell is elaborately subdivided into functionally distinct, membrane-enclosed compartments. Each compartment, or organelle,
FATTY ACID METABOLISM IN CAENORHABDITIS ELEGANS: CHARACTERIZATION OF THE DELTA 9 FATTY ACID DESATURASES AND IDENTIFICATION OF A KEY REGULATOR, NHR-80
 FATTY ACID METABOLISM IN CAENORHABDITIS ELEGANS: CHARACTERIZATION OF THE DELTA 9 FATTY ACID DESATURASES AND IDENTIFICATION OF A KEY REGULATOR, NHR-80 By TRISHA JANE BROCK A dissertation submitted in partial
FATTY ACID METABOLISM IN CAENORHABDITIS ELEGANS: CHARACTERIZATION OF THE DELTA 9 FATTY ACID DESATURASES AND IDENTIFICATION OF A KEY REGULATOR, NHR-80 By TRISHA JANE BROCK A dissertation submitted in partial
CHM333 LECTURE 34: 11/30 12/2/09 FALL 2009 Professor Christine Hrycyna
 Lipid Metabolism β-oxidation FA Acetyl-CoA Triacylglycerols (TAGs) and glycogen are the two major forms of stored energy in vertebrates Glycogen can supply ATP for muscle contraction for less than an hour
Lipid Metabolism β-oxidation FA Acetyl-CoA Triacylglycerols (TAGs) and glycogen are the two major forms of stored energy in vertebrates Glycogen can supply ATP for muscle contraction for less than an hour
The Cell Organelles. Eukaryotic cell. The plasma membrane separates the cell from the environment. Plasma membrane: a cell s boundary
 Eukaryotic cell The Cell Organelles Enclosed by plasma membrane Subdivided into membrane bound compartments - organelles One of the organelles is membrane bound nucleus Cytoplasm contains supporting matrix
Eukaryotic cell The Cell Organelles Enclosed by plasma membrane Subdivided into membrane bound compartments - organelles One of the organelles is membrane bound nucleus Cytoplasm contains supporting matrix
In The Name Of God. In The Name Of. EMRI Modeling Group
 In The Name Of God In The Name Of God EMRI Modeling Group Cells work together in functionally related groups called tissues Types of tissues: Epithelial lining and covering Connective support Muscle movement
In The Name Of God In The Name Of God EMRI Modeling Group Cells work together in functionally related groups called tissues Types of tissues: Epithelial lining and covering Connective support Muscle movement
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM. Triacylglycerol and Fatty Acid Metabolism
 ANSC/NUTR 618 LIPIDS & LIPID METABOLISM II. Triacylglycerol synthesis A. Overall pathway Glycerol-3-phosphate + 3 Fatty acyl-coa à Triacylglycerol + 3 CoASH B. Enzymes 1. Acyl-CoA synthase 2. Glycerol-phosphate
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM II. Triacylglycerol synthesis A. Overall pathway Glycerol-3-phosphate + 3 Fatty acyl-coa à Triacylglycerol + 3 CoASH B. Enzymes 1. Acyl-CoA synthase 2. Glycerol-phosphate
Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins
 Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins - Cholesterol: It is a sterol which is found in all eukaryotic cells and contains an oxygen (as a hydroxyl group OH) on Carbon number
Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins - Cholesterol: It is a sterol which is found in all eukaryotic cells and contains an oxygen (as a hydroxyl group OH) on Carbon number
Molecular Cell Biology Problem Drill 16: Intracellular Compartment and Protein Sorting
 Molecular Cell Biology Problem Drill 16: Intracellular Compartment and Protein Sorting Question No. 1 of 10 Question 1. Which of the following statements about the nucleus is correct? Question #01 A. The
Molecular Cell Biology Problem Drill 16: Intracellular Compartment and Protein Sorting Question No. 1 of 10 Question 1. Which of the following statements about the nucleus is correct? Question #01 A. The
Thursday, October 16 th
 Thursday, October 16 th Good morning. Those of you needing to take the Enzymes and Energy Quiz will start very soon. Students who took the quiz Wednesday: Please QUIETLY work on the chapter 6 reading guide.
Thursday, October 16 th Good morning. Those of you needing to take the Enzymes and Energy Quiz will start very soon. Students who took the quiz Wednesday: Please QUIETLY work on the chapter 6 reading guide.
number Done by Corrected by Doctor Faisal Al-Khatibe
 number 24 Done by Mohammed tarabieh Corrected by Doctor Faisal Al-Khatibe 1 P a g e *Please look over the previous sheet about fatty acid synthesis **Oxidation(degradation) of fatty acids, occurs in the
number 24 Done by Mohammed tarabieh Corrected by Doctor Faisal Al-Khatibe 1 P a g e *Please look over the previous sheet about fatty acid synthesis **Oxidation(degradation) of fatty acids, occurs in the
Cellular control of cholesterol. Peter Takizawa Department of Cell Biology
 Cellular control of cholesterol Peter Takizawa Department of Cell Biology Brief overview of cholesterol s biological role Regulation of cholesterol synthesis Dietary and cellular uptake of cholesterol
Cellular control of cholesterol Peter Takizawa Department of Cell Biology Brief overview of cholesterol s biological role Regulation of cholesterol synthesis Dietary and cellular uptake of cholesterol
Companion to Biosynthesis of Ketones & Cholesterols, Regulation of Lipid Metabolism Lecture Notes
 Companion to Biosynthesis of Ketones & Cholesterols, Regulation of Lipid Metabolism Lecture Notes The major site of acetoacetate and 3-hydorxybutyrate production is in the liver. 3-hydorxybutyrate is the
Companion to Biosynthesis of Ketones & Cholesterols, Regulation of Lipid Metabolism Lecture Notes The major site of acetoacetate and 3-hydorxybutyrate production is in the liver. 3-hydorxybutyrate is the
Summary and concluding remarks
 Summary and concluding remarks This thesis is focused on the role and interaction of different cholesterol and phospholipid transporters. Cholesterol homeostasis is accomplished via a tightly regulated
Summary and concluding remarks This thesis is focused on the role and interaction of different cholesterol and phospholipid transporters. Cholesterol homeostasis is accomplished via a tightly regulated
Lipids. Lipids: a Diverse group of chemicals. Storage Lipids: derivatives of fatty acids. 11/21/10
 1 Lipids Lehninger 3 rd ed. Chapter 11 (For biosynthesis see Chapter 21) 2 Lipids: a Diverse group of chemicals Insolubility in water. Fats and oils: energy stores. Phospholipids and sterols: structural
1 Lipids Lehninger 3 rd ed. Chapter 11 (For biosynthesis see Chapter 21) 2 Lipids: a Diverse group of chemicals Insolubility in water. Fats and oils: energy stores. Phospholipids and sterols: structural
Cell morphology. Cell organelles structure and function. Chapter 1: UNIT 1. Dr. Charushila Rukadikar
 UNIT 1 Cell morphology Cell organelles structure and function Chapter 1: Dr. Charushila Rukadikar Assistant Professor Department Of Physiology ZMCH, Dahod Physiology The science that is concerned with
UNIT 1 Cell morphology Cell organelles structure and function Chapter 1: Dr. Charushila Rukadikar Assistant Professor Department Of Physiology ZMCH, Dahod Physiology The science that is concerned with
BME NEUROSCIENCE PRINCIPLES OF NEURAL SCIENCE 1 ST SEMESTER GRADUATE COURSE HYOUNG F. KIM
 BME NEUROSCIENCE PRINCIPLES OF NEURAL SCIENCE 1 ST SEMESTER GRADUATE COURSE HYOUNG F. KIM BASIC CONCEPT OF CELL BIOLOGY CELL & NEURON What are the differences? BASIC CONCEPT OF CELL 1. What are the cells
BME NEUROSCIENCE PRINCIPLES OF NEURAL SCIENCE 1 ST SEMESTER GRADUATE COURSE HYOUNG F. KIM BASIC CONCEPT OF CELL BIOLOGY CELL & NEURON What are the differences? BASIC CONCEPT OF CELL 1. What are the cells
Alternative splicing. Biosciences 741: Genomics Fall, 2013 Week 6
 Alternative splicing Biosciences 741: Genomics Fall, 2013 Week 6 Function(s) of RNA splicing Splicing of introns must be completed before nuclear RNAs can be exported to the cytoplasm. This led to early
Alternative splicing Biosciences 741: Genomics Fall, 2013 Week 6 Function(s) of RNA splicing Splicing of introns must be completed before nuclear RNAs can be exported to the cytoplasm. This led to early
HHS Public Access Author manuscript Nat Chem Biol. Author manuscript; available in PMC 2011 October 01.
 A whole organism screen identifies novel regulators of fat storage George A. Lemieux 1, Jason Liu 2, Nasima Mayer 3, Roland J. Bainton 3, Kaveh Ashrafi 2,*, and Zena Werb 1,* 1 Department of Anatomy, University
A whole organism screen identifies novel regulators of fat storage George A. Lemieux 1, Jason Liu 2, Nasima Mayer 3, Roland J. Bainton 3, Kaveh Ashrafi 2,*, and Zena Werb 1,* 1 Department of Anatomy, University
Fatty Acid Degradation. Catabolism Overview. TAG and FA 11/11/2015. Chapter 27, Stryer Short Course. Lipids as a fuel source diet Beta oxidation
 Fatty Acid Degradation Chapter 27, Stryer Short Course Catabolism verview Lipids as a fuel source diet Beta oxidation saturated Unsaturated dd chain Ketone bodies as fuel Physiology High energy More reduced
Fatty Acid Degradation Chapter 27, Stryer Short Course Catabolism verview Lipids as a fuel source diet Beta oxidation saturated Unsaturated dd chain Ketone bodies as fuel Physiology High energy More reduced
Lipids: Fats, Oils & Waxes: AP Biology
 Lipids: Fats, Oils & Waxes: Lipids long term energy storage concentrated energy *9 Cal/gram Lipids: Triglycerides Lipids are composed of C, H, O u long hydrocarbon chains (H-C) Family groups u fats u phospholipids
Lipids: Fats, Oils & Waxes: Lipids long term energy storage concentrated energy *9 Cal/gram Lipids: Triglycerides Lipids are composed of C, H, O u long hydrocarbon chains (H-C) Family groups u fats u phospholipids
Integration Of Metabolism
 Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
LIPID METABOLISM
 LIPID METABOLISM LIPOGENESIS LIPOGENESIS LIPOGENESIS FATTY ACID SYNTHESIS DE NOVO FFA in the blood come from :- (a) Dietary fat (b) Dietary carbohydrate/protein in excess of need FA TAG Site of synthesis:-
LIPID METABOLISM LIPOGENESIS LIPOGENESIS LIPOGENESIS FATTY ACID SYNTHESIS DE NOVO FFA in the blood come from :- (a) Dietary fat (b) Dietary carbohydrate/protein in excess of need FA TAG Site of synthesis:-
Zool 3200: Cell Biology Exam 4 Part I 2/3/15
 Name: Trask Zool 3200: Cell Biology Exam 4 Part I 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Name: Trask Zool 3200: Cell Biology Exam 4 Part I 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Zool 3200: Cell Biology Exam 4 Part I 2/3/15
 Name: Key Trask Zool 3200: Cell Biology Exam 4 Part I 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Name: Key Trask Zool 3200: Cell Biology Exam 4 Part I 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Lehninger 5 th ed. Chapter 17
 Lehninger 5 th ed. Chapter 17 December 26, 2010 Prof. Shimon Schuldiner Email: Shimon.Schuldiner@huji.ac.il Phone: 6585992 CHAPTER 17 Fatty Acid Catabolism Key topics: How fats are digested in animals
Lehninger 5 th ed. Chapter 17 December 26, 2010 Prof. Shimon Schuldiner Email: Shimon.Schuldiner@huji.ac.il Phone: 6585992 CHAPTER 17 Fatty Acid Catabolism Key topics: How fats are digested in animals
Homework Hanson section MCB Course, Fall 2014
 Homework Hanson section MCB Course, Fall 2014 (1) Antitrypsin, which inhibits certain proteases, is normally secreted into the bloodstream by liver cells. Antitrypsin is absent from the bloodstream of
Homework Hanson section MCB Course, Fall 2014 (1) Antitrypsin, which inhibits certain proteases, is normally secreted into the bloodstream by liver cells. Antitrypsin is absent from the bloodstream of
BIOL 4374/BCHS 4313 Cell Biology Exam #1 February 13, 2001
 BIOL 4374/BCHS 4313 Cell Biology Exam #1 February 13, 2001 SS# Name This exam is worth a total of 100 points. The number of points each question is worth is shown in parentheses. Good luck! 1. (2) The
BIOL 4374/BCHS 4313 Cell Biology Exam #1 February 13, 2001 SS# Name This exam is worth a total of 100 points. The number of points each question is worth is shown in parentheses. Good luck! 1. (2) The
BCM 221 LECTURES OJEMEKELE O.
 BCM 221 LECTURES BY OJEMEKELE O. OUTLINE INTRODUCTION TO LIPID CHEMISTRY STORAGE OF ENERGY IN ADIPOCYTES MOBILIZATION OF ENERGY STORES IN ADIPOCYTES KETONE BODIES AND KETOSIS PYRUVATE DEHYDROGENASE COMPLEX
BCM 221 LECTURES BY OJEMEKELE O. OUTLINE INTRODUCTION TO LIPID CHEMISTRY STORAGE OF ENERGY IN ADIPOCYTES MOBILIZATION OF ENERGY STORES IN ADIPOCYTES KETONE BODIES AND KETOSIS PYRUVATE DEHYDROGENASE COMPLEX
The Cell. Copyright 2003 Pearson Education, Inc. publishing as Benjamin Cummings
 The Cell Cell Theory The cell is the basic structural and functional unit of life The organism activity depends on individual and collective activity of cells Biochemical activities of cells are dictated
The Cell Cell Theory The cell is the basic structural and functional unit of life The organism activity depends on individual and collective activity of cells Biochemical activities of cells are dictated
Zool 3200: Cell Biology Exam 4 Part II 2/3/15
 Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
ab SREBP-2 Translocation Assay Kit (Cell-Based)
 ab133114 SREBP-2 Translocation Assay Kit (Cell-Based) Instructions for Use For analysis of translocation of SREBP-2 into nuclei. This product is for research use only and is not intended for diagnostic
ab133114 SREBP-2 Translocation Assay Kit (Cell-Based) Instructions for Use For analysis of translocation of SREBP-2 into nuclei. This product is for research use only and is not intended for diagnostic
BIOLOGICAL CHEMISTRY Prof. J.H.P. Bayley, Dr. R.M. Adlington and Dr. L. Smith Trinity Term First Year. Lecture 2 Hagan Bayley
 BIOLOGICAL CHEMISTRY Prof. J.H.P. Bayley, Dr. R.M. Adlington and Dr. L. Smith Trinity Term 2007 - First Year Lecture 2 Hagan Bayley Introduction to the macromolecules of life and cell structures. Introduction
BIOLOGICAL CHEMISTRY Prof. J.H.P. Bayley, Dr. R.M. Adlington and Dr. L. Smith Trinity Term 2007 - First Year Lecture 2 Hagan Bayley Introduction to the macromolecules of life and cell structures. Introduction
Chapt. 10 Cell Biology and Biochemistry. The cell: Student Learning Outcomes: Describe basic features of typical human cell
 Chapt. 10 Cell Biology and Biochemistry Cell Chapt. 10 Cell Biology and Biochemistry The cell: Lipid bilayer membrane Student Learning Outcomes: Describe basic features of typical human cell Integral transport
Chapt. 10 Cell Biology and Biochemistry Cell Chapt. 10 Cell Biology and Biochemistry The cell: Lipid bilayer membrane Student Learning Outcomes: Describe basic features of typical human cell Integral transport
MCB130 Midterm. GSI s Name:
 1. Peroxisomes are small, membrane-enclosed organelles that function in the degradation of fatty acids and in the degradation of H 2 O 2. Peroxisomes are not part of the secretory pathway and peroxisomal
1. Peroxisomes are small, membrane-enclosed organelles that function in the degradation of fatty acids and in the degradation of H 2 O 2. Peroxisomes are not part of the secretory pathway and peroxisomal
THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS
 Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Cell Structure and Function
 Household pin w/ bactera Cell Structure and Function Chapter 4 Same bacteria on pinhead Fig. 4-1c, p.50 Review: Ionic Bonds Na has 11p and 10e making it (+) Cl has 18e and 17 p making it (-) The attraction
Household pin w/ bactera Cell Structure and Function Chapter 4 Same bacteria on pinhead Fig. 4-1c, p.50 Review: Ionic Bonds Na has 11p and 10e making it (+) Cl has 18e and 17 p making it (-) The attraction
Very-Long Chain Fatty Acid Biosynthesis
 Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Chapter 1 Membrane Structure and Function
 Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
MILK BIOSYNTHESIS PART 3: FAT
 MILK BIOSYNTHESIS PART 3: FAT KEY ENZYMES (FROM ALL BIOSYNTHESIS LECTURES) FDPase = fructose diphosphatase Citrate lyase Isocitrate dehydrogenase Fatty acid synthetase Acetyl CoA carboxylase Fatty acyl
MILK BIOSYNTHESIS PART 3: FAT KEY ENZYMES (FROM ALL BIOSYNTHESIS LECTURES) FDPase = fructose diphosphatase Citrate lyase Isocitrate dehydrogenase Fatty acid synthetase Acetyl CoA carboxylase Fatty acyl
Cell Structure & Function. Source:
 Cell Structure & Function Source: http://koning.ecsu.ctstateu.edu/cell/cell.html Definition of Cell A cell is the smallest unit that is capable of performing life functions. http://web.jjay.cuny.edu/~acarpi/nsc/images/cell.gif
Cell Structure & Function Source: http://koning.ecsu.ctstateu.edu/cell/cell.html Definition of Cell A cell is the smallest unit that is capable of performing life functions. http://web.jjay.cuny.edu/~acarpi/nsc/images/cell.gif
AP Biology Cells: Chapters 4 & 5
 AP Biology Cells: Chapters 4 & 5 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The was the first unifying principle of biology. a. spontaneous generation
AP Biology Cells: Chapters 4 & 5 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The was the first unifying principle of biology. a. spontaneous generation
UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following are synthesized along various sites of the endoplasmic reticulum
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following are synthesized along various sites of the endoplasmic reticulum
A comparative study of fat storage quantitation in nematode Caenorhabditis elegans using label and label-free methods
 University of Massachusetts Medical School escholarship@umms Program in Gene Function and Expression Publications and Presentations Molecular, Cell and Cancer Biology 9-24-2010 A comparative study of fat
University of Massachusetts Medical School escholarship@umms Program in Gene Function and Expression Publications and Presentations Molecular, Cell and Cancer Biology 9-24-2010 A comparative study of fat
CELLS. Cells. Basic unit of life (except virus)
 Basic unit of life (except virus) CELLS Prokaryotic, w/o nucleus, bacteria Eukaryotic, w/ nucleus Various cell types specialized for particular function. Differentiation. Over 200 human cell types 56%
Basic unit of life (except virus) CELLS Prokaryotic, w/o nucleus, bacteria Eukaryotic, w/ nucleus Various cell types specialized for particular function. Differentiation. Over 200 human cell types 56%
Introduction to metabolic regulation. Prof K Syed Department of Biochemistry & Microbiology University of Zululand Room no. 247
 Introduction to metabolic regulation Prof K Syed Department of Biochemistry & Microbiology University of Zululand Room no. 247 SyedK@unizulu.ac.za Topics Metabolism Metabolism: Categories Important metabolic
Introduction to metabolic regulation Prof K Syed Department of Biochemistry & Microbiology University of Zululand Room no. 247 SyedK@unizulu.ac.za Topics Metabolism Metabolism: Categories Important metabolic
Lipids digestion and absorption, Biochemistry II
 Lipids digestion and absorption, blood plasma lipids, lipoproteins Biochemistry II Lecture 1 2008 (J.S.) Triacylglycerols (as well as free fatty acids and both free and esterified cholesterol) are very
Lipids digestion and absorption, blood plasma lipids, lipoproteins Biochemistry II Lecture 1 2008 (J.S.) Triacylglycerols (as well as free fatty acids and both free and esterified cholesterol) are very
Biosynthesis of Fatty Acids. By Dr.QUTAIBA A. QASIM
 Biosynthesis of Fatty Acids By Dr.QUTAIBA A. QASIM Fatty Acids Definition Fatty acids are comprised of hydrocarbon chains terminating with carboxylic acid groups. Fatty acids and their associated derivatives
Biosynthesis of Fatty Acids By Dr.QUTAIBA A. QASIM Fatty Acids Definition Fatty acids are comprised of hydrocarbon chains terminating with carboxylic acid groups. Fatty acids and their associated derivatives
Lysosomes. Gr: lysis solution, soma body. Membrane bounded vesicles. Usually round ovoid or irregular electron dense bodies m.
 Lysosomes Gr: lysis solution, soma body Membrane bounded vesicles Usually round ovoid or irregular electron dense bodies 0.05 0.5 m. Lysosomes No. varies from a few to several hundred per cell, in different
Lysosomes Gr: lysis solution, soma body Membrane bounded vesicles Usually round ovoid or irregular electron dense bodies 0.05 0.5 m. Lysosomes No. varies from a few to several hundred per cell, in different
Cellular compartments
 Cellular compartments 1. Cellular compartments and their function 2. Evolution of cellular compartments 3. How to make a 3D model of cellular compartment 4. Cell organelles in the fluorescent microscope
Cellular compartments 1. Cellular compartments and their function 2. Evolution of cellular compartments 3. How to make a 3D model of cellular compartment 4. Cell organelles in the fluorescent microscope
Cholesterol and its transport. Alice Skoumalová
 Cholesterol and its transport Alice Skoumalová 27 carbons Cholesterol - structure Cholesterol importance A stabilizing component of cell membranes A precursor of bile salts A precursor of steroid hormones
Cholesterol and its transport Alice Skoumalová 27 carbons Cholesterol - structure Cholesterol importance A stabilizing component of cell membranes A precursor of bile salts A precursor of steroid hormones
Module C CHEMISTRY & CELL BIOLOGY REVIEW
 Module C CHEMISTRY & CELL BIOLOGY REVIEW Note: This module is provided for A&P courses that do not have a prerequisite class which includes chemistry and cell biology. Content covered by required prerequisite
Module C CHEMISTRY & CELL BIOLOGY REVIEW Note: This module is provided for A&P courses that do not have a prerequisite class which includes chemistry and cell biology. Content covered by required prerequisite
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10643 Supplementary Table 1. Identification of hecw-1 coding polymorphisms at amino acid positions 322 and 325 in 162 strains of C. elegans. WWW.NATURE.COM/NATURE 1 Supplementary Figure
doi:10.1038/nature10643 Supplementary Table 1. Identification of hecw-1 coding polymorphisms at amino acid positions 322 and 325 in 162 strains of C. elegans. WWW.NATURE.COM/NATURE 1 Supplementary Figure
Practice Exam 2 MCBII
 1. Which feature is true for signal sequences and for stop transfer transmembrane domains (4 pts)? A. They are both 20 hydrophobic amino acids long. B. They are both found at the N-terminus of the protein.
1. Which feature is true for signal sequences and for stop transfer transmembrane domains (4 pts)? A. They are both 20 hydrophobic amino acids long. B. They are both found at the N-terminus of the protein.
BBSG 501 Section 4 Metabolic Fuels, Energy and Order Fall 2003 Semester
 BBSG 501 Section 4 Metabolic Fuels, Energy and Order Fall 2003 Semester Section Director: Dave Ford, Ph.D. Office: MS 141: ext. 8129: e-mail: fordda@slu.edu Lecturers: Michael Moxley, Ph.D. Office: MS
BBSG 501 Section 4 Metabolic Fuels, Energy and Order Fall 2003 Semester Section Director: Dave Ford, Ph.D. Office: MS 141: ext. 8129: e-mail: fordda@slu.edu Lecturers: Michael Moxley, Ph.D. Office: MS
Cholesterol metabolism. Function Biosynthesis Transport in the organism Hypercholesterolemia
 Cholesterol metabolism Function Biosynthesis Transport in the organism Hypercholesterolemia - component of all cell membranes - precursor of bile acids steroid hormones vitamin D Cholesterol Sources: dietary
Cholesterol metabolism Function Biosynthesis Transport in the organism Hypercholesterolemia - component of all cell membranes - precursor of bile acids steroid hormones vitamin D Cholesterol Sources: dietary
Don t Freak Out. Test on cell organelle on Friday!
 Cell Structure 1 Don t Freak Out Test on cell organelle on Friday! This test should be a buffer test and help raise your overall test score. All information will come from this week! 2 Cells Provide Compartments
Cell Structure 1 Don t Freak Out Test on cell organelle on Friday! This test should be a buffer test and help raise your overall test score. All information will come from this week! 2 Cells Provide Compartments
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): Overall, this is an interesting study. The authors used a custom microfluidic device to deliver precise concentrations of bacterial food to worms
Reviewers' comments: Reviewer #1 (Remarks to the Author): Overall, this is an interesting study. The authors used a custom microfluidic device to deliver precise concentrations of bacterial food to worms
Novel Effects of Mibefradil, An Anti-Cancer Drug, on White Adipocytes
 Georgia State University ScholarWorks @ Georgia State University Biology Theses Department of Biology 8-8-2017 Novel Effects of Mibefradil, An Anti-Cancer Drug, on White Adipocytes Sonia Thompson Follow
Georgia State University ScholarWorks @ Georgia State University Biology Theses Department of Biology 8-8-2017 Novel Effects of Mibefradil, An Anti-Cancer Drug, on White Adipocytes Sonia Thompson Follow
Protein Trafficking in the Secretory and Endocytic Pathways
 Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
The endoplasmic reticulum is a network of folded membranes that form channels through the cytoplasm and sacs called cisternae.
 Endoplasmic reticulum (ER) The endoplasmic reticulum is a network of folded membranes that form channels through the cytoplasm and sacs called cisternae. Cisternae serve as channels for the transport of
Endoplasmic reticulum (ER) The endoplasmic reticulum is a network of folded membranes that form channels through the cytoplasm and sacs called cisternae. Cisternae serve as channels for the transport of
UNIT 1: Introduction to metabolic regulation
 UNIT 1: Introduction to metabolic regulation Prof K Syed Department of Biochemistry & Microbiology University of Zululand Room no. 247 SyedK@unizulu.ac.za Topics Metabolism Metabolism: Categories Important
UNIT 1: Introduction to metabolic regulation Prof K Syed Department of Biochemistry & Microbiology University of Zululand Room no. 247 SyedK@unizulu.ac.za Topics Metabolism Metabolism: Categories Important
Supplemental Data. Article. Serotonin Regulates C. elegans Fat and Feeding. through Independent Molecular Mechanisms
 Cell Metabolism, Volume 7 Supplemental Data Article Serotonin Regulates C. elegans Fat and Feeding through Independent Molecular Mechanisms Supriya Srinivasan, Leila Sadegh, Ida C. Elle, Anne G.L. Christensen,
Cell Metabolism, Volume 7 Supplemental Data Article Serotonin Regulates C. elegans Fat and Feeding through Independent Molecular Mechanisms Supriya Srinivasan, Leila Sadegh, Ida C. Elle, Anne G.L. Christensen,
 Glossary For TheFatNurse s For All Ages Series Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Apolipoprotein
Glossary For TheFatNurse s For All Ages Series Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Apolipoprotein
Endoplasmic Reticulum
 Endoplasmic Reticulum What s ER? How is ER? Why is ER? definition description functions Nissl s bodies neurons Berg s bodies hepatocytes Organelle structure histocytochemical evidences Ergastoplasm pancreatic
Endoplasmic Reticulum What s ER? How is ER? Why is ER? definition description functions Nissl s bodies neurons Berg s bodies hepatocytes Organelle structure histocytochemical evidences Ergastoplasm pancreatic
Cell Quality Control. Peter Takizawa Department of Cell Biology
 Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Synthesis and elongation of fatty acids
 Synthesis and elongation of fatty acids A molecular caliper mechanism for determining very long-chain fatty acid length Vladimir Denic and Jonathan S. Weissman (2007) Cell 130, 663-677 February 28, 2008
Synthesis and elongation of fatty acids A molecular caliper mechanism for determining very long-chain fatty acid length Vladimir Denic and Jonathan S. Weissman (2007) Cell 130, 663-677 February 28, 2008
/searchlist/6850.html Tour of the Cell 1
 http://www.studiodaily.com/main /searchlist/6850.html Tour of the Cell 1 2011-2012 Cytology: science/study of cells To view cells: Light microscopy resolving power: measure of clarity Electron microscopy
http://www.studiodaily.com/main /searchlist/6850.html Tour of the Cell 1 2011-2012 Cytology: science/study of cells To view cells: Light microscopy resolving power: measure of clarity Electron microscopy
Structure & Function of Cells
 Anatomy & Physiology 101-805 Unit 4 Structure & Function of Cells Paul Anderson 2011 Anatomy of a Generalised Cell Attached or bound ribosomes Cilia Cytosol Centriole Mitochondrion Rough endoplasmic reticulum
Anatomy & Physiology 101-805 Unit 4 Structure & Function of Cells Paul Anderson 2011 Anatomy of a Generalised Cell Attached or bound ribosomes Cilia Cytosol Centriole Mitochondrion Rough endoplasmic reticulum
Lipid Metabolism. Remember fats?? Triacylglycerols - major form of energy storage in animals
 Remember fats?? Triacylglycerols - major form of energy storage in animals Your energy reserves: ~0.5% carbs (glycogen + glucose) ~15% protein (muscle, last resort) ~85% fat Why use fat for energy? 1 gram
Remember fats?? Triacylglycerols - major form of energy storage in animals Your energy reserves: ~0.5% carbs (glycogen + glucose) ~15% protein (muscle, last resort) ~85% fat Why use fat for energy? 1 gram
cholesterol structure Cholesterol FAQs Cholesterol promotes the liquid-ordered phase of membranes Friday, October 15, 2010
 cholesterol structure most plasma cholesterol is in the esterified form (not found in cells or membranes) cholesterol functions in all membranes (drives formation of lipid microdomains) cholesterol is
cholesterol structure most plasma cholesterol is in the esterified form (not found in cells or membranes) cholesterol functions in all membranes (drives formation of lipid microdomains) cholesterol is
Lecture 36. Key Concepts. Overview of lipid metabolism. Reactions of fatty acid oxidation. Energy yield from fatty acid oxidation
 Lecture 36 Lipid Metabolism 1 Fatty Acid Oxidation Ketone Bodies Key Concepts Overview of lipid metabolism Reactions of fatty acid oxidation Energy yield from fatty acid oxidation Formation of ketone bodies
Lecture 36 Lipid Metabolism 1 Fatty Acid Oxidation Ketone Bodies Key Concepts Overview of lipid metabolism Reactions of fatty acid oxidation Energy yield from fatty acid oxidation Formation of ketone bodies
Lecture 15 The Lipid Bilayer: A Dynamic Self- Assembled Structure of Multiple Lipid Classes
 Lecture 15 The Lipid Bilayer: A Dynamic Self- Assembled Structure of Multiple Lipid Classes LIPIDS-Biological molecules with low solubility in water and high solubility in non-polar solvents -Lipids form
Lecture 15 The Lipid Bilayer: A Dynamic Self- Assembled Structure of Multiple Lipid Classes LIPIDS-Biological molecules with low solubility in water and high solubility in non-polar solvents -Lipids form
Gut Reaction. Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD
 Gut Reaction Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD Ley, R. et al (2005) PNAS vol. 102 no. 31 Bacterial diversity in the distal gut (ceca) of C57BL6 mice. (A) Phylogenetic tree of
Gut Reaction Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD Ley, R. et al (2005) PNAS vol. 102 no. 31 Bacterial diversity in the distal gut (ceca) of C57BL6 mice. (A) Phylogenetic tree of
Intracellular vesicular traffic. B. Balen
 Intracellular vesicular traffic B. Balen Three types of transport in eukaryotic cells Figure 12-6 Molecular Biology of the Cell ( Garland Science 2008) Endoplasmic reticulum in all eucaryotic cells Endoplasmic
Intracellular vesicular traffic B. Balen Three types of transport in eukaryotic cells Figure 12-6 Molecular Biology of the Cell ( Garland Science 2008) Endoplasmic reticulum in all eucaryotic cells Endoplasmic
Bio10 Cell Structure SRJC
 3.) Cell Structure and Function Structure of Cell Membranes Fluid mosaic model Mixed composition: Phospholipid bilayer Glycolipids Sterols Proteins Fluid Mosaic Model Phospholipids are not packed tightly
3.) Cell Structure and Function Structure of Cell Membranes Fluid mosaic model Mixed composition: Phospholipid bilayer Glycolipids Sterols Proteins Fluid Mosaic Model Phospholipids are not packed tightly
BIOLOGY 111. CHAPTER 3: The Cell: The Fundamental Unit of Life
 BIOLOGY 111 CHAPTER 3: The Cell: The Fundamental Unit of Life The Cell: The Fundamental Unit of Life Learning Outcomes 3.1 Explain the similarities and differences between prokaryotic and eukaryotic cells
BIOLOGY 111 CHAPTER 3: The Cell: The Fundamental Unit of Life The Cell: The Fundamental Unit of Life Learning Outcomes 3.1 Explain the similarities and differences between prokaryotic and eukaryotic cells
endomembrane system internal membranes origins transport of proteins chapter 15 endomembrane system
 endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
Programmed Cell Death (apoptosis)
 Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
The Cell and Cellular transport
 Cell theory (1838): The Cell 1. All organisms are composed of one or more cells, and the life processes of metabolism and heredity occur within these cells. 2. Cells are the smallest living things, the
Cell theory (1838): The Cell 1. All organisms are composed of one or more cells, and the life processes of metabolism and heredity occur within these cells. 2. Cells are the smallest living things, the
Lipid droplet biogenesis: a novel process of self-digestion as a strategy of survival to stress
 Lipid droplet biogenesis: a novel process of self-digestion as a strategy of survival to stress Memoria del trabajo experimental para optar al grado de doctor, correspondiente al Programa de Doctorado
Lipid droplet biogenesis: a novel process of self-digestion as a strategy of survival to stress Memoria del trabajo experimental para optar al grado de doctor, correspondiente al Programa de Doctorado
MBB317. Dr D MANGNALL OBESITY. Lecture 2
 MBB317 Dr D MANGNALL OBESITY Lecture 2 When the structure of the insulin receptor was first discovered it was assumed that the active beta subunit tyrosine kinase would phosphorylate some intracellular
MBB317 Dr D MANGNALL OBESITY Lecture 2 When the structure of the insulin receptor was first discovered it was assumed that the active beta subunit tyrosine kinase would phosphorylate some intracellular
