HHS Public Access Author manuscript Nat Chem Biol. Author manuscript; available in PMC 2011 October 01.
|
|
|
- Clifton Copeland
- 6 years ago
- Views:
Transcription
1 A whole organism screen identifies novel regulators of fat storage George A. Lemieux 1, Jason Liu 2, Nasima Mayer 3, Roland J. Bainton 3, Kaveh Ashrafi 2,*, and Zena Werb 1,* 1 Department of Anatomy, University of California, San Francisco CA , USA 2 Department of Physiology, University of California, San Francisco CA , USA 3 Department of Anesthesia, University of California, San Francisco CA , USA Abstract HHS Public Access Author manuscript Published in final edited form as: Nat Chem Biol April ; 7(4): doi: /nchembio.534. The regulation of energy homeostasis integrates diverse biological processes ranging from behavior to metabolism and is linked fundamentally to numerous disease states. To identify new molecules that can bypass homeostatic compensatory mechanisms of energy balance in intact animals, we screened for small molecule modulators of C. elegans fat content. We report on several molecules that modulate fat storage without obvious deleterious effects on feeding, growth, and reproduction. A subset of these compounds also altered fat storage in mammalian and insect cell culture. We found that one of the newly identified compounds exerts its effects in C. elegans through a pathway that requires novel functions of an AMP-activated kinase catalytic subunit and a transcription factor previously unassociated with fat regulation. Thus, our strategy identifies small molecules that are effective within the context of intact animals and reveals relationships between new pathways that operate across phyla to influence energy homeostasis. INTRODUCTION In metazoans, energy homeostasis is regulated by cellular and organism-wide networks that control behavior and the intertwined physiologies of nutrient uptake, transport, lipid synthesis, storage and utilization [1]. Excessive fat accumulation and aberrant metabolism underlie susceptibility to numerous pathologies ranging from type II diabetes to certain forms of cancer [2]. Pharmacological agents that modulate energy balance in the context of whole organisms could significantly facilitate the understanding of and treatment of metabolic disorders. However, the homeostatic nature of energy regulatory networks and the sophisticated chemo-protective barriers of intact organisms render many compounds that Users may view, print, copy, download and text and data- mine the content in such documents, for the purposes of academic research, subject always to the full Conditions of use: Correspondence and requests for materials should be addressed to K.A. (kaveh.ashrafi@ucsf.edu) or Z.W. (zena.werb@ucsf.edu). * These authors contributed equally to this work. Author Contributions. G.A.L., K.A. and Z.W. conceived the study design. G.A.L., J.L., and N.M. performed the experiments. G.A.L., K.A., R.B. and Z.W. analyzed the data. G.A.L., K.A. and Z.W. wrote the paper. All the authors read, revised and approved the manuscript. Competing Interests. The authors declare no competing interests
2 Lemieux et al. Page 2 RESULTS show excellent in vitro or in cell-based efficacy ineffective when administered to whole animals [3, 4]. One approach to this challenge is to conduct screens on whole animals, rather than pre-determined targets, to identify compounds that elicit desired responses in intact animals [5]. C. elegans offers the possibility for relatively cost-effective whole-animal screening [6 8]. Additionally, the speed and ease with which C. elegans can be genetically manipulated provides a currently unparalleled experimental path for elucidating mechanisms that underlie a compound s actions [9]. For instance, many fundamental components of neural signaling cascades were first discovered by screening for mutants that were either resistant or hypersensitive to application of various neurotransmitters to whole nematodes [10, 11]. This approach is not restricted to identifying a compound s binding target, but importantly, can reveal combinations of pathways that mediate the physiological consequences of compound activity. Since the maintenance of energy homeostasis is central to life, the underlying molecular mechanisms of its regulation are highly conserved across phylogeny. As in mammals, fat in C. elegans is derived from conversion of absorbed nutrients and other internal resources through de novo synthesis as well as the direct uptake of dietary fatty acids [12]. A majority of C. elegans extractable fatty acids is derived from dietary uptake of palmitic acid and shorter chain fatty acids [13]. In addition, many of the known mammalian metabolic regulatory pathways operate similarly in C. elegans [12, 14]. These include fat and sugar uptake, transport, synthesis and degradation mechanisms, their transcriptional regulators such as SREBP (sterol response element binding protein) [15], cellular fuel gauge mechanisms such as TOR (target of rapamycin)[16], and neuroendocrine regulators such as insulin [17]. Moreover, in C. elegans as in mammals, serotonin, dopamine and neuropeptide Y-like signaling pathways regulate food-related behaviors [18 20]. Thus, while the mammalian regulatory pathways involving energy homeostasis are more sophisticated than that of nematodes, the core mechanisms are largely conserved. To generate small molecule tools for probing the complex mechanisms of energy homeostasis, we developed a forward chemical screen for novel compounds that alter fat accumulation in C. elegans. Here, we report on several molecules that either elevate or depress fat storage. By combining compound treatment with systematic gene inactivation, we found that the fat lowering activity of one of the newly identified compounds is ultimately dependent on AMP-activated kinase (AMPK) as well as a transcription factor with no previously identified role in fat metabolism. These results demonstrate the utility of our approach in identifying compounds that exert their effects through evolutionarily conserved pathways as well as revealing new fat regulatory modules. A whole organism screen for modulators of lipid metabolism We chose as the basis for our assay the solvatochromatic vital dye Nile Red that has been used extensively in multiple experimental systems to identify and characterize metabolic pathways involved in fat metabolism [15, 21 24]. Nile Red staining of C. elegans intestinal
3 Lemieux et al. Page 3 lipid-containing organelles is reproducible, exhibits a large dynamic range, and is sensitive to numerous genetic and environmental manipulations expected to alter fat content [21]. In addition, it allows for the characterization of metabolic regulatory pathways that are impervious to analysis with less sensitive fixed staining or biochemical extraction methods [22]. To validate our approach, we tested whether known pharmacological modifiers of mammalian lipid metabolism altered C. elegans Nile Red staining. Animals treated with 1 mm 5-aminoimidazole-4-carboxyamide ribonucleoside (1, AICAR), an agonist of AMPactivated kinase (AMPK) [25], exhibited decreased staining, as would be predicted when AMPK is activated. The patterns and extents of fat reduction by AICAR were comparable to that seen upon treatment with 100 µm Fluoxetine (2), a serotonin re-uptake inhibitor (Fig. 1a, b) [18]. These experiments indicate that C. elegans fat storage can be modulated at the level of primary signal transduction (AICAR) and neurotransmitter release (Fluoxetine) by pharmacological agents known to modulate mammalian fat. We next screened previously uncharacterized small molecules for those that altered Nile Red staining. Developmentally synchronized nematodes were cultured in 384-well plates with individual compounds (5 µm average concentration), E. coli (food) and Nile Red for two days; then the content of each well was imaged by automated fluorescence microscopy (Fig. 1c). Nematodes were scored for growth and Nile Red fluorescence levels. We found that of 3200 compounds of a commercially available diversity library whose compounds physical-chemical properties were similar to most orally available drugs, 1% lowered and 0.4% elevated Nile Red staining more than two-fold relative to control treated animals. An additional 1.2% of compounds caused lethality or inhibited development. While the hit rate of this screen is high relative to in vitro high-throughput screens, it is comparable to other whole organism phenotypic screens [8]. Of the identified compounds, we further characterized 10 that caused highly reproducible Nile Red phenotypes without obvious developmental abnormalities. Compounds H17 (3), F21 (4), F17 (5), A15 (6), A13 (7), I13 (8), C5 (9) and F14(10) (Fig. 2a) reduced Nile Red staining, while H6 (11) and E8 (12) (Fig. 2b) caused increased staining. Highlighting the dynamic range of Nile Red, exposure of animals to 10 µm H17 reduced staining by >20- fold, while that of E8 elevated staining by >4-fold relative to control treated animals (Fig. 2c). Compounds that increased or decreased Nile Red staining similarly affected staining of the animals by BODIPY-conjugated fatty acids (Supplementary Fig. S1a, b), a structurally unrelated vital stain for fat. In addition, we measured the bulk ratio of triglycerides to protein extractable from nematodes that were treated with each of the compounds that reduced Nile Red staining. We found that compounds H17, F21, F17, A13 and I13 significantly decreased this ratio in a manner that correlated with their activity for reducing Nile Red staining (Supplementary Fig. S1c). No significant changes in total fat content were detected for compounds A15, C5 and F14, which may reflect the sensitivity differences or compartment/tissue specificity of the vital dye assays compared to the whole animal extraction assay. In the 10 compounds, eight different core scaffolds are represented, reflecting the structurally diverse library from which they were derived. The 4-hydroxy-2-oxo quinoline carboxamides F17 and F21 are structural analogs and induced similar fat phenotypes (Fig.
4 Lemieux et al. Page 4 2a, c). In contrast, the 2-aryl substituted quinoline 4-carboxamides F14 and H6 share a similar scaffold but induced moderately low fat and high fat phenotypes, respectively. The effects of compounds F21, F17, A13, H17, F14 and E8 were dose-dependent and saturable at levels that were compound-dependent (F21, F17, A13: Fig. 2d; H17, F14, E8: Supplementary Fig. S1d), with EC 50 s between µm. These data are consistent with the hypothesis that the compounds induce fat reduction or elevation through specific, saturable interactions with the fat regulatory machinery and not as the result of non-specific toxicological responses to xenobiotics. To ascertain further that the newly identified compounds alter fat metabolism in the nematode, we examined their effects on transcriptional expression of fat-7, a C. elegans ortholog of mammalian stearoyl-coa desaturase-1 (SCD-1), which catalyzes the conversion of saturated stearoyl-coa into mono-unsaturated oleoyl-coa [26]. These Δ 9 desaturases regulate the ratio of unsaturated to saturated fats and are transcriptionally sensitive to the metabolic state of the cell, being induced during periods of anabolic activity and repressed during periods of fat utilization (i.e., starvation) [23, 27, 28]. We therefore expected that many small molecules that affect fat storage should in turn alter expression of this enzyme. Transgenic C. elegans carrying a fat-7::gfp reporter express FAT-7:GFP in intestinal cells, where it is most strongly visible in the first anterior pair of cells (Fig. 2e, top panel). Nematodes expressing FAT-7:GFP were cultured with 10 µm of each of the compounds shown in Fig. 2a and b or DMSO as a control. Induced changes in Nile Red intensity generally paralleled the extent of induced change in fat-7::gfp expression. Nematodes cultured on Nile Red-reducing compounds H17, A13, A15, F17, F21, and C5 exhibited reduced levels of fat-7 expression (Fig. 2e, f), while the Nile Red-increasing compound E8 caused increased expression (Fig. 2e, f). Taken together, these findings indicate that the screening strategy succeeded in identifying compounds that alter C. elegans fat levels. Properties of newly identified fat regulatory compounds To investigate whether the observed changes in fat could be attributed to non-specific deleterious consequences of compound treatment, we examined growth rates, fecundity, and feeding rates of compound treated animals. In all 10 cases, compound treatments induced only very slight developmental delays with no correlation between severity of the fat phenotype and growth rate (Supplementary Fig. S2a). None of the compounds significantly altered fecundity of animals (Supplementary Fig. S2b). Compounds that induced a low fat phenotype caused either normal (H17, F21, F14, I13 and C5) or elevated (F17, A15 and A13) feeding rates (Supplementary Fig. S2c). The two compounds that increased fat storage (H6, E8) did not alter feeding rates. Taken together, these results suggest that the observed fat phenotypes are not merely due to general sickness or altered feeding behavior. Our results echo recent genetic studies in C. elegans [18], in that fat content is not merely a passive consequence of feeding behavior. The excess feeding rate of animals with reduced fat levels may be indicative of compensatory mechanisms. For an initial assessment of whether the newly identified fat regulatory compounds may have utility in the study of fat metabolism other than in C. elegans, we examined consequences of treatment of the mouse 3T3-L1 cell culture model of adipogenesis [29] and
5 Lemieux et al. Page 5 the macrophage-like Drosophila Schneider S2 cells [30] with each of the 10 compounds. Upon induction of adipogenesis, 3T3-L1 cells developed a rounded morphology and contained numerous intracellular lipid droplets (Fig. 3a). Concomitant treatment of preadipocyte 3T3-L1 cells with the adipogenic cocktail and each of A13, I13 or F21 compounds that reduce fat in C. elegans resulted in a dose-dependent reduction in lipid droplet formation (Fig. 3a, b). While lipid droplets in 3T3-L1 cells are derived from de novo fatty acid synthesis, S2 cells store lipids in droplets after uptake of lipoprotein complexes [30]. Of the 10 compounds tested, only A15 induced a significant alteration in the appearance of fat depots of these cells. A15 treated cells exhibited abnormally large lipid droplets (Fig. 3c, d), a phenotype similar to that observed by RNAi of genes involved in phospholipid biosynthesis [30]. Thus, the C. elegans Nile Red screen identified compounds that also modulate fat storage in mammalian and insect cell-based assays of fat metabolism. The finding that only four of the ten compounds showed activity in 3T3-L1 and S2 cells may mean that fat effects of some of the compounds are C. elegans specific. However, it is also likely that each cell-based assay only represents a relatively limited metabolic space compared to a whole organism system. For example, some compounds may modulate fat storage in C. elegans through cell non-autonomous processes that originate in the nervous system of the nematode. To begin to understand how these compounds modulate fat storage we returned to C. elegans to examine the genetic requirements for the compounds activities. Genetic analyses of the mechanisms of fat modulation We treated C. elegans with a variety of genetically altered backgrounds with each of the ten compounds. We first asked whether the Nile Red-increasing E8 and H6 could retain their effects in animals deficient in SREBP, a transcription factor that regulates expression of lipid synthesis genes in mammals and C. elegans [31, 32]. RNAi mediated depletion of sbp-1 dramatically reduced Nile Red staining [21] (Supplementary Fig. 3a, b) that was unaffected by concomitant E8 or H6 treatment suggesting that Nile Red increasing effects of these compounds cannot bypass sbp-1 function. We next queried the effects of compound treatment in animals deficient in fat metabolism due to loss of fatty acyl-coa synthase genes, in nutrient sensing due to loss of either rictor/torc2 or AMPK signaling pathways, in bbs-7 and tub-1, C. elegans orthologs of human obesity genes [33, 34], or in insulin signaling due to loss of daf-16, encoding a FOXO-like transcription factor that is required for many of the phenotypes caused by reduction of insulin signaling [35]. In all cases, animals were susceptible to fat reduction by each of the Nile Red reducing compounds (H17, F21, F17, I13, A13, A15, C5, and F14; Supplementary Fig. 3c, d, data not shown). The only exception was that animals deficient in aak-1, which encodes a catalytic subunit of AMPK, were selectively resistant to the Nile Red-reducing effects of F17 (Fig. 4a, b). aak-1 selectively mediates the effects of F17 AMPK is an evolutionarily conserved hetero-trimeric serine/threonine protein kinase complex that negatively regulates energy consuming pathways such as fat synthesis and positively regulates pathways of energy production such as fatty acid β-oxidation and glycolysis [36]. Like humans, C. elegans has two different genes (aak-1, aak-2) that encode different catalytic α-subunits. Control treated aak-1 mutants exhibited Nile Red staining that
6 Lemieux et al. Page 6 was similar to wild-type animals (Fig. 4a top row); thus the resistance of aak-1 mutants to F17 induced fat reduction was not simply a consequence of excess basal staining (Fig. 4a middle row, b). Moreover, aak-1 mutants remained susceptible to the effects of F21, despite structural similarity of this compound to F17 (Fig. 2a), suggesting that distinct mechanisms underlie the observed effects of these newly discovered compounds. While the extended lifespan of insulin signaling deficient animals [37] and excess fat content of hibernating C. elegans [38] requires aak-2, the reduced fat phenotype of F17 or F21 (Fig. 4a, b, Supplementary Fig. S4a) did not depend on the presence of this AMPK catalytic subunit. Mice deficient in SCD-1 exhibit increased fatty acid oxidation in the liver that is correlated with increased activation of AMPK [39]. Consistent with this finding, RNAi mediated inactivation of fat-7 in C. elegans results in reduced fat storage [23]. Given our earlier observation that treatment of C. elegans with F17 decreased fat-7 expression (Fig. 2e, f), we investigated whether the F17-aak-1 mediated fat reduction required fat-7. We subjected both wild-type and aak-1 mutants to either fat-7 RNAi or vector control and then treated them with F17 or vehicle control. Wild-type animals treated with fat-7 RNAi exhibited decreased Nile Red staining that was decreased even further with F17 treatment (Supplementary Fig. S4b). While mutants in aak-1 partially abrogated the effects of F17, exposure to fat-7 RNAi induced a similar percentage change in fat reduction whether animals were treated with vehicle control or with F17 (Supplementary Fig. S4b). The additive, independent effects of these interactions indicate that the loss of FAT-7:GFP expression upon treatment with F17 is likely a consequence of the fat reduction rather than the mechanism by which F17 reduces fat storage. The existence of a discreet function for aak-1 has until now remained largely uncharacterized. With several known direct and indirect agonists of AMPK complexes already known, we examined the effects of these molecules on wild-type and aak-1 animals to discern whether any of these known agents exhibit F17 s selective dependence on aak-1. The riboside AICAR is converted in cells to an AMP mimetic (ZMP) and is thought to activate AMPK directly by binding to the AMP-binding allosteric regulatory site [40]. C. elegans treated with 1 mm AICAR have reduced Nile Red staining (Fig. 1). However, aak-1 mutants fail to suppress the fat-reducing effects of AICAR indicating that, unlike F17, AICAR reduces fat in the nematode through a mechanism independent of aak-1 (Supplementary Fig. S4c). Another class of AMPK activating agents, the biguanides (i.e., metformin, phenformin, buformin) are thought to act indirectly on AMPK by elevating the AMP:ATP ratio through interference with mitochondrial electron transport [41]. Treating animals with 7.5 mm phenformin (13) resulted in a developmental delay that was not suppressed in aak-1 mutants (Supplementary Fig. S4d). In addition, we also treated C. elegans with the direct AMPK activator A [42]. However this β1-complex selective agonist did not affect Nile Red staining at concentrations up to 300 µm (data not shown). Thus, F17 possesses the unique ability to modulate C. elegans fat levels potently through a pathway that utilizes an AMPK complex whose function until now has been undefined.
7 Lemieux et al. Page 7 Structural requirements for fat reduction through aak-1 To understand the structure-activity relationship for the 4-hydroxy 2-oxo-quinoline scaffold found in F17 and F21 better, we examined analogs with different 4-hydroxy quinoline substituents (R1, R2: Table 1). Compounds bearing 6-membered and larger ring systems such as pyridyl and pyrimidyl in place of the thiazolyl substituent at R2 did not exhibit low fat phenotypes (Table 1, compounds F (14),-9110 (15),-8895 (16),-4816 (17),-4038 (18), (19)). However, other 5-membered thiazol substituted compounds reduced levels of fat storage relative to control treated animals (Table 1, compounds F (20), (21), (22), (23), (24)) Compound F that was substituted with a 4- phenyl group on the thiazol ring (R2) and an N-allyl group on the quinoline ring (R1) had the most potent fat reducing effect and, similar to F17, required presence of aak-1. The presence of the large-branched alkyl side chain at the R1 position of the quinoline ring was not absolutely required for aak-1 mediated fat reduction since F had an activity profile comparable to F17. While most conservative substitutions around the thiazol ring system were tolerated in terms of targeting the aak-1 pathway, the fat-reducing effects of compound F that has a 5-ethyl substituted thiadiazol ring system at R2 were unaffected by loss of aak-1. These findings echo the preceding epistasis data (Fig. 4) demonstrating that the low fat phenotypes exhibited by F17 and F21-treated animals are due to the interaction of these structurally similar compounds with distinct pathways. Additionally, we found that more potent modulators of the AMPK pathway can be identified through modification of the core scaffold of F17. F17 s fat phenotype also depends on a transcription factor Although loss of aak-1 abrogated the fat reducing effects of F17, the requirement for aak-1 was only partial. To gain further insights into the fat reducing effects of F17, we screened through 975 clones representing approximately 70% of the genes that are predicted to encode kinases and transcription factors in the C. elegans genome. Among these, we found that RNAi-mediated inactivation of K08F8.2, which encodes a transcription factor that contains a basic leucine-zipper (bzip) DNA binding domain [43], blocked approximately one third of F17 s Nile Red reducing phenotype (Fig. 5. Supplementary Fig. S5). While aak-1 suppressed 60 65% of the fat reducing effects of F17, RNAi inactivation of K08F8.2 in the aak-1 mutant background resulted in virtually complete abrogation of F17 s fat reducing effects (Fig. 5). Although not definitive proof, this additive suppressive interaction suggests that K08F8.2 acts in parallel to aak-1 to mediate F17 s reduced fat phenotype, rather than as a pathway component of AAK-1 signaling. Like aak-1 mutants, K08F8.2 RNAi failed to suppress the fat reducing effects of F21 (Fig. 5), further indicating that F17 and F21 modulate fat metabolism through distinct mechanisms. F17 activates AMPK in human liver-derived cells To assess whether F17 affects the AMPK pathway in organisms other than C. elegans, we treated HepG2 human hepatocarcinoma cells with this compound. We used this cell line because it has been used frequently to study AMPK and its activating compounds and because liver cells are important sites of fat metabolism. Fixation and staining of this cell line with a neutral lipid specific stain revealed the presence of numerous lipid droplets
8 Lemieux et al. Page 8 DISCUSSION contained within the cytoplasm of each cell (Fig. 6a). Treatment with F17 (25 µm) markedly reduced the number of lipid droplets in each cell (Fig. 6a, Supplementary Fig. S6a). To assess the effects of F17 on AMPK signaling directly, we treated cells with either F17, AICAR as a positive control, F17 with AMPK inhibitor compound C [44], F21 or DMSO as a vehicle control. AMPK inhibits fat synthesis by phosphorylation and inactivation of acetyl coenzyme A carboxylase (ACC) [45], which catalyzes the rate-limiting step in de novo fatty acid synthesis. Treatment with either AICAR or F17, but not F21, induced phosphorylation of ACC. F17-induced ACC phosphorylation was inhibited by simultaneous treatment with compound C (Fig. 6b, Supplementary Fig. S6b). We also observed that phosphorylation of AMPK-α, which is characteristic of its activation, was induced by AICAR and F17 treatment, but not by F21 ((Fig. 6b, Supplementary Fig. S6b). These results indicate that F17 acts as an agonist of AMPK signaling in human cells at much lower concentrations than AICAR, the widely used direct activator of AMPK. The concentration at which F17 activates the AMPK pathway in mammalian cells was also similar to that of a recently reported agonist A (25), a compound discovered through screening ~700,000 small molecules in an in vitro assay directly targeting purified AMPK [42]. Thus, these findings indicate that screening in C. elegans can yield compounds that act on pathways of therapeutic interest across phyla. Discovery of fat regulatory compounds in whole organisms Just as small molecules have helped drive understanding of many biological pathways at the cellular level, there is a need for pharmacological tools to interrogate complex processes of whole organism physiology. Fish, amphibians and nematodes [5 8] have proven to be compatible with the demands of systematic forward chemical screening in intact, multicellular animals. Although our C. elegans fat screen is amenable to very large scale screening, our findings indicate that even a small screen is sufficient to identify diverse, novel compounds that modulate fat content, a physiologically relevant phenotype that is the outcome of an array of integrated pathways. Here, we have reported on numerous novel small molecules that modulate intestinal labeling of C. elegans with vital dyes that stain lipid-containing compartments, modulate total triglyceride content and affect the expression of a key lipogenesis gene, whose expression is correlated with triglyceride storage in nematodes and mammals. In addition, we found that several of these molecules also modulate fat accumulation in both mammalian and Drosophila cell culture. These findings indicate that C. elegans vital Nile Red staining provides an effective strategy for identifying small molecules that perturb fat storage in C. elegans and mammalian systems. Our results stand in contrast to several recent publications that have implied that vital dye staining is non-predictive of changes in global fat metabolism in C. elegans [46 48]. One explanation for this discrepancy is that biochemical measurements of bulk triglycerides extracted from populations of whole animals do not exhibit the same sensitivity and/or compartment specificity afforded by vital dye-based imaging.
9 Lemieux et al. Page 9 Use of small molecules to uncover physiological functions Perspectives METHODS Materials One of the compounds identified by the primary Nile Red screen, F17, reduced fat content in C. elegans through pathways involving the AMPK-α1 catalytic subunit. Similar to the human and mouse genomes, the C. elegans genome encodes two α, two β, and five γ subunits that may interact to form different AMPK complexes with discrete activities [36]. In mammals, AMPK complexes exhibit varying tissue expression patterns and even different subcellular localizations [49]. In addition, individual complexes respond to different stimuli to mediate varied functions from distinct peripheral energy consumption pathways to feeding behaviors in the central nervous system [36]. In C. elegans, the function of the catalytic subunit encoded by aak-1 is dispensable for both the insulin-dependent lifespan extension phenotype mediated by aak-2 [37] and in the maintenance of an alternative hibernative state that also requires aak-2 [38]. As aak-1 mutants exhibit no fat storage phenotype of their own, it was only through F17-treatment that we identified a function for aak-1 in modulating fat storage in C. elegans. Similarly, we found that F17 s fat reducing effects depend on K08F8.2 a transcription factor containing a bzip DNA binding motif [43]. As in aak-1, inactivation of K08F8.2 has not been previously implicated in fat regulation, highlighting the utility of small molecules in uncovering physiological roles for various pathways. While the direct binding target(s) of F17 remains to be elucidated, these studies highlight the power of combining pharmacology with genetics to identify in vivo pathways through which a compound exerts its effects. Finally, in addition to causing fat reduction, F17 treatment caused feeding increase, however, the molecular mechanisms through which F17 elicits the observed feeding effects remain to be elucidated. An advantage of whole animal phenotypic screening is that it does not depend on any preconceived notions as to which pathways should be targeted for generating desired outcomes. Counter to the prevailing paradigm for generation of therapeutics through targetbased screening, many of the staples of modern medicine emerged serendipitously when unanticipated effects of particular compounds were noted in whole animals [50]. It remains to be seen whether any newly identified C. elegans fat regulatory compounds will exert similar effects in other intact species. It is, however, noteworthy that the chemical structures of most enzymatic cofactors, metabolic intermediates, neurotransmitters, and lipid-based signaling intermediates are absolutely conserved from nematodes to humans. Given that known mammalian fat regulatory compounds exert similar effects in C. elegans and at least one of the newly discovered compounds, F17, activates AMPK signaling in C. elegans and mammalian cell lines, it is possible that many of the newly identified compounds will be portable to other systems. The diverse compound screening library was purchased from SPECS via the Small Molecule Development Center at UCSF. Compounds H17, F21, F17, A13, I13, A15, C5, F14, H6 and E8 for follow-up studies were repurchased from SPECS. Compounds F17, F21, F , F , F , F , F , F , F , F ,
10 Lemieux et al. Page 10 Nematode strains Compound screening Quantitative imaging F , and F were purchased from Chembridge. BODIPY labeled fatty acid (BODIPY-FA: 4,4-difluoro-5-octyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoic acid) and lipidtox neutral lipid stain were from Invitrogen. AMPK agonist AICAR was from Toronto Research Chemicals, Inc. AMPK agonist A was from Tocris Bioscience. The AMPK antagonist compound C was from EMD4Biosciences. Except where noted, all other chemicals were from Sigma-Aldrich. All antibodies were from Cell Signaling Technologies. All cell culture media were purchased from Invitrogen. Cell lines and heat inactivated fetal bovine serum (FBS) were obtained from the UCSF cell culture facility. Wild-type: N2 (Bristol), aak-1 (tm1944) III, aak-2 (ok524) X, tub-1 (nr2044) II, osm-12 (n1606) III, lpo-6 (mg360) II, daf-16 (MgDf47) I, mod-1(ok103) V, fat-7::gfp: lin-15 (n765) X; waex15[fat-7::gfp]. (See Supplemental Methods for a detailed description) Compounds from a SPECS diversity library assembled by the Small Molecule Discovery Center (QB3/UCSF) were screened by culturing synchronized L1 animals with E. coli OP-50, 100 nm Nile Red and the compound library at an average concentration of 5 µm in 384-well plates. After 2 days of culture at 20 C, brightfield and red fluorescence images were acquired using an automated microscope. Phenotypes for growth, and red-fluorescence staining patterns were scored if more than 75% of animals in a well exhibited the phenotype. All image data intended for quantitative comparison were acquired at the same subsaturating exposure time. Fluorescence images of 7 10 nematodes were used to quantify lipid storage using ImageJ ( The total integrated fluorescence intensity from the two most anterior intestinal cells in images was quantified. Alternatively, to measure the integrated intensity of intestinal lipid droplets, independent of intestinal haze, a 2-dimensional spot-enhancing Gaussian filter was used to create a binary mask that identified the fluorescent lipid droplets in the first two anterior pairs of intestinal cells from images of nematodes. The mask was applied to the original image and the sum of the unmasked pixel intensities was evaluated. Pharyngeal pumping assay Developmentally synchronized L1 animals were added to 24 well plates (approximately 25 nematodes per well) containing NGM-agar seeded with E.coli OP-50 and either experimental compound ( µm), 5-hydroxytryptamine (5 mm) or DMSO. Plates were incubated for hr at 20 C. The number of pharyngeal contractions in a 10 s period was recorded using an electronic counter. The pumping rate reported is the average rate of young adult animals per condition. C. elegans growth and egg-laying assays, cell culture assays, western blotting and RNAi treatments, are described in Supplementary Methods.
11 Lemieux et al. Page 11 Supplementary Material Acknowledgements References Refer to Web version on PubMed Central for supplementary material. We are grateful to Dr. David Lum for his help and advice with the initial C. elegans experiments. We are thankful to Dr. Brendan Mullaney and Dr. Katherine Cunningham for helpful discussions and comments on the manuscript. We thank Dr. Kurt Thorn and the Nikon Imaging Center for use of the Nikon 6D automated epifluorescence microscope and help with imaging as well as the Small Molecule Discovery Center at the California Institute for Quantitative Biomedical Research at UCSF for providing the small molecule library. This work was supported by grants from the National Cancer Institute and National Institute of Environmental Health Sciences (ES012801, ES and CA056721) to Z.W., the National Institute of Diabetes and Digestive and Kidney Diseases (DK070149) to K.A., the National Institute of General Medicine (GM081863) to R.B. and a Byers Award to K.A. and R.B. 1. Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001; 104: [PubMed: ] 2. Kopelman PG. Obesity as a medical problem. Nature. 2000; 404: [PubMed: ] 3. Knight ZA, Shokat KM. Chemical genetics: where genetics and pharmacology meet. Cell. 2007; 128: [PubMed: ] 4. Stelling J, et al. Robustness of cellular functions. Cell. 2004; 118: [PubMed: ] 5. Wheeler GN, Brandli AW. Simple vertebrate models for chemical genetics and drug discovery screens: Lessons from zebrafish and Xenopus. Dev Dyn. 2009; 238: [PubMed: ] 6. Moy TI, et al. High-throughput screen for novel antimicrobials using a whole animal infection model. ACS Chem Biol. 2009; 4: [PubMed: ] 7. Petrascheck M, Ye X, Buck LB. An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature. 2007; 450: [PubMed: ] 8. Kwok TC, et al. A small-molecule screen in C. elegans yields a new calcium channel antagonist. Nature. 2006; 441: [PubMed: ] 9. Jones AK, Buckingham SD, Sattelle DB. Chemistry-to-gene screens in Caenorhabditis elegans. Nat Rev Drug Discov. 2005; 4: [PubMed: ] 10. Schafer WR, Sanchez BM, Kenyon CJ. Genes affecting sensitivity to serotonin in Caenorhabditis elegans. Genetics. 1996; 143: [PubMed: ] 11. Weinshenker D, Garriga G, Thomas JH. Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J Neurosci Oct; 15(10): , (1995). [PubMed: ] 12. Watts JL. Fat synthesis and adiposity regulation in Caenorhabditis elegans. Trends Endocrinol Metab. 2009; 20: [PubMed: ] 13. Perez CL, Van Gilst MR. A 13C isotope labeling strategy reveals the influence of insulin signaling on lipogenesis in C. elegans. Cell Metab. 2008; 8: [PubMed: ] 14. Jones KT, Ashrafi K. Caenorhabditis elegans as an emerging model for studying the basic biology of obesity. Dis Model Mech. 2009; 2: [PubMed: ] 15. McKay RM, et al. C elegans: a model for exploring the genetics of fat storage. Dev Cell. 2003; 4: [PubMed: ] 16. Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004; 131: [PubMed: ] 17. Kimura KD, et al. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997; 277: [PubMed: ]
12 Lemieux et al. Page Srinivasan S, et al. Serotonin regulates C. elegans fat and feeding through independent molecular mechanisms. Cell Metab. 2008; 7: [PubMed: ] 19. de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998; 94: [PubMed: ] 20. Suo S, Culotti JG, Van Tol HH. Dopamine counteracts octopamine signalling in a neural circuit mediating food response in C. elegans. EMBO J. 2009; 28: [PubMed: ] 21. Ashrafi K, et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003; 421: [PubMed: ] 22. Greenspan P, Mayer EP, Fowler SD. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985; 100: [PubMed: ] 23. Van Gilst MR, et al. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol. 2005; 3:e53. [PubMed: ] 24. Jones KS, et al. A high throughput live transparent animal bioassay to identify non-toxic small molecules or genes that regulate vertebrate fat metabolism for obesity drug development. Nutr Metab (Lond). 2008; 5:23. [PubMed: ] 25. Sullivan JE, et al. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994; 353: [PubMed: ] 26. Watts JL, Browse J. A palmitoyl-coa-specific delta9 fatty acid desaturase from Caenorhabditis elegans. Biochem Biophys Res Commun. 2000; 272: [PubMed: ] 27. Van Gilst MR, Hadjivassiliou H, Yamamoto KR. A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc Natl Acad Sci U S A. 2005; 102: [PubMed: ] 28. Yang F, et al. An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature. 2006; 442: [PubMed: ] 29. MacDougald OA, Lane MD. Transcriptional regulation of gene expression during adipocyte differentiation. Annu Rev Biochem. 1995; 64: [PubMed: ] 30. Guo Y, et al. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008; 453: [PubMed: ] 31. Horton JD, et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci U S A. 2003; 100: [PubMed: ] 32. Kniazeva M, et al. Monomethyl branched-chain fatty acids play an essential role in Caenorhabditis elegans development. PLoS Biol. 2004; 2:E257. [PubMed: ] 33. Shiri-Sverdlov R, et al. Identification of TUB as a novel candidate gene influencing body weight in humans. Diabetes. 2006; 55: [PubMed: ] 34. Goldstone AP, Beales PL. Genetic obesity syndromes. Front Horm Res. 2008; 36: [PubMed: ] 35. Ogg S, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997; 389: [PubMed: ] 36. Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009; 89: [PubMed: ] 37. Apfeld J, et al. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004; 18: [PubMed: ] 38. Narbonne P, Roy R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature. 2009; 457: [PubMed: ] 39. Dobrzyn P, et al. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci U S A Apr 27; 101(17): Epub 2004 Apr 19. [PubMed: ] 40. Corton JM, et al. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995; 229: [PubMed: ]
13 Lemieux et al. Page Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000; 348(Pt 3): [PubMed: ] 42. Cool B, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006; 3: [PubMed: ] 43. Wang X, Jia H, Chamberlin HM. The bzip proteins CES-2 and ATF-2 alter the timing of transcription for a cell-specific target gene in C. elegans. Dev Biol. 2006; 289: [PubMed: ] 44. Zhou G, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001; 108: [PubMed: ] 45. Davies SP, Sim AT, Hardie DG. Location and function of three sites phosphorylated on rat acetyl- CoA carboxylase by the AMP-activated protein kinase. Eur J Biochem. 1990; 187: [PubMed: ] 46. O'Rourke EJ, et al. C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metab. 2009; 10: [PubMed: ] 47. Brooks KK, Liang B, Watts JL. The influence of bacterial diet on fat storage in C. elegans. PLoS One. 2009; 4:e7545. [PubMed: ] 48. Morck C, et al. Statins inhibit protein lipidation and induce the unfolded protein response in the non-sterol producing nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2009; 106: [PubMed: ] 49. Kodiha M, et al. Localization of AMP kinase is regulated by stress, cell density, and signaling through the MEK-->ERK1/2 pathway. Am J Physiol Cell Physiol. 2007; 293:C1427 C1436. [PubMed: ] 50. MacRae CA, Peterson RT. Zebrafish-based small molecule discovery. Chem Biol. 2003; 10: [PubMed: ]
14 Lemieux et al. Page 14 Figure 1. Pharmacological modulation of Nile Red staining in C. elegans (a) Young adult animals were cultured from first larval stage of development with Nile Red and either 1 mm AICAR, 100 µm Fluoxetine or 0.1% DMSO as a control. A representative image for each case is shown. Animals are orientated anterior (left) to posterior (right). The anterior half of each intestine is shown for Fluoxetine and AICAR treated animals. Scale bar = 40 µm (b) The integrated fluorescence intensity of the two anterior-most intestinal cells was measured for 5 7 animals. Error bars represent the standard error of the mean. (c) An outline of screen used to identify new small molecule modulators of fat metabolism.
15 Lemieux et al. Page 15 Figure 2. Fat metabolism in C. elegans is modulated by diverse compounds (a,b) Structures of fat-reducing (a) and fat-increasing (b) compounds and associated Nile Red phenotypes. All images were acquired from C. elegans that were treated with 10 µm compound or 0.1% DMSO as a vehicle control. Scale bar, 20 µm. Because of the large dynamic range of the effects of compounds, shown images for the series of fat reducing compounds were acquired at different exposure intensities than those of fat increasing compounds to facilitate visualization. (c) Quantification of the integrated intensity of Nile Red-stained lipid droplets in the anterior-most 3-pairs of intestinal cells of 10 animals per
16 Lemieux et al. Page 16 condition cultured as in a and b. Images used for quantification were all acquired with the same exposure time to allow direct comparison between all conditions. ** p < 0.01: twotailed t-test. (d) The integrated intensities of the anterior intestinal cells of 7 10 animals per serial dilution of compound. Error bars: ± s.d. (e) An overlay of green fluorescence and bright field images (top panel) showing fat-7::gfp expression in the first anterior pair of intestinal cells. Scale bar, 20 µm. Green fluorescence images of the first pair of intestinal cells from transgenic animals cultured with either 0.1% DMSO or with 10 µm compound. E8 and adjacent control images were acquired at different exposure time to facilitate visualization. (f) The mean fluorescence intensities of the first anterior intestinal cells of were quantified. n = 5 7 animals per condition *** p < 0.05: two-tailed t-test.
17 Lemieux et al. Page 17 Figure 3. Compounds that lower Nile Red staining in C. elegans modulate lipid accumulation in mammalian and insect cell culture models of adipogenesis and lipid uptake (a) NIH 3T3-L1 pre-adipocytes were differentiated in the presence of either DMSO or 20 µm of each compound, then fixed, stained with Oil Red O and counterstained with hematoxylin. Scale bar = 40 µm. (b) NIH 3T3-L1 cells were differentiated in the presence of different concentrations of compound then fixed and stained with Oil Red O. The amount of bound dye was extracted and quantified by measuring absorbance at 520 nm. (c) Drosophila Schneider S2 cells were cultured overnight in medium supplemented with oleate-albumin complexes. The cells were then fixed and stained with green LipidTOX neutral lipid stain to visualize lipid droplets then counter-stained with DAPI. Confocal microscopic images are overlaid. Example cells in each inset are enlarged to illustrate the droplet size phenotype. Scale bars are 10 µm. (d) The maximum size of lipid droplets in each cell was quantified for 3 5 independent image fields for more than 20 cells. Error bars represent the standard error of the mean.
18 Lemieux et al. Page 18 Figure 4. The fat and feeding phenotypes induced by F17 but not F21 are due to AMPK (a) Nile red staining of wild-type (top row), aak-1 mutant animals (middle row) and aak-2 mutant nematodes (bottom row) cultured with 0.1% DMSO (Control, left column), 10 µm F17 (middle column) and 10 µm F21 (right column). Scale bar = 20 µm (b) The integrated fluorescence intensity of the anterior intestine was quantified for wild-type, aak-1 and aak-2 mutant nematodes cultured with 0.1% DMSO (black), 10 µm F17 (light gray) and 10 µm F21 (dark gray) animals were quantified for each condition. Error bars represent the standard error of the mean.
19 Lemieux et al. Page 19 Figure 5. Loss of K08F8.2 partially suppresses the F17 low fat phenotype First larval stage wild-type and aak-1 mutant C. elegans were cultured on HT115 E.coli expressing either a control double stranded RNA or double stranded RNA targeting K08F8.2 along with Nile Red. After 2 days of culture at 20 C the medium was supplemented with either 10 µm F17, 10 µm F21 or 0.1% DMSO as a vehicle control. After an additional 24 hr of culture, the integrated fluorescence intensity of the first two intestinal cells of gravid adults was quantified. For each genetic background, fluorescence intensities from compound-treated animals were normalized to DMSO-treated animals of the same genetic background to correct for modest fat increasing effects of K08F8.2 RNAi. Error bars represent the standard error of the mean of 5 7 individual measurements. * p < 0.02: twotailed t-test.
20 Lemieux et al. Page 20 Figure 6. F17 activates the AMPK pathway and reduces the number of lipid droplets in HepG2 hepatocarcinoma cells (a) HepG2 cells were treated with F17 (25 µm) or 0.25% DMSO as a control. The cells were fixed and stained with LipidTOX neutral lipid stain (red) and counterstained with DAPI (blue). Scale bars represent 10 µm. (b) HepG2 cells were treated with either 2 mm AICAR or AICAR vehicle control (0.2% DMSO) 30 µm F17, F17 vehicle control (0.3% DMSO), simultaneously with 30 µm F17 and 20 µm Compound C, or with 30 µm F21. Western blots of extracts for anti-phospho ACC (pacc), anti-acc total protein (ACC), anti-phospho
21 Lemieux et al. Page 21 AMPKα (pampkα) or anti-ampkα (AMPKα) are shown. Full length blots are shown in Supplementary Fig. S7.
22 Lemieux et al. Page 22 Table 1 Nile Red phenotypes of different 4-hydroxy-2-oxo-quinoline carboxamides. Compound R 1 R 2 Nile Red (% of Control) 1 aak-1 suppressed F17 14 yes F methyl 28 yes F yes F yes F21 7 no F yes F no F nd 2 F nd F nd F nd F nd F nd 1 Integrated intensity of wild-type C. elegans treated with compound expressed as a percentage of DMSO-treated control. 2 Not Determined (nd)
ab Adipogenesis Assay Kit (Cell-Based)
 ab133102 Adipogenesis Assay Kit (Cell-Based) Instructions for Use For the study of induction and inhibition of adipogenesis in adherent cells. This product is for research use only and is not intended
ab133102 Adipogenesis Assay Kit (Cell-Based) Instructions for Use For the study of induction and inhibition of adipogenesis in adherent cells. This product is for research use only and is not intended
Supplemental Data. Article. Serotonin Regulates C. elegans Fat and Feeding. through Independent Molecular Mechanisms
 Cell Metabolism, Volume 7 Supplemental Data Article Serotonin Regulates C. elegans Fat and Feeding through Independent Molecular Mechanisms Supriya Srinivasan, Leila Sadegh, Ida C. Elle, Anne G.L. Christensen,
Cell Metabolism, Volume 7 Supplemental Data Article Serotonin Regulates C. elegans Fat and Feeding through Independent Molecular Mechanisms Supriya Srinivasan, Leila Sadegh, Ida C. Elle, Anne G.L. Christensen,
BIOL212 Biochemistry of Disease. Metabolic Disorders - Obesity
 BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
Role of fatty acids in the development of insulin resistance and type 2 diabetes mellitus
 Emerging Science Role of fatty acids in the development of insulin resistance and type 2 diabetes mellitus George Wolf Insulin resistance is defined as the reduced responsiveness to normal circulating
Emerging Science Role of fatty acids in the development of insulin resistance and type 2 diabetes mellitus George Wolf Insulin resistance is defined as the reduced responsiveness to normal circulating
ab SREBP-2 Translocation Assay Kit (Cell-Based)
 ab133114 SREBP-2 Translocation Assay Kit (Cell-Based) Instructions for Use For analysis of translocation of SREBP-2 into nuclei. This product is for research use only and is not intended for diagnostic
ab133114 SREBP-2 Translocation Assay Kit (Cell-Based) Instructions for Use For analysis of translocation of SREBP-2 into nuclei. This product is for research use only and is not intended for diagnostic
Integration Of Metabolism
 Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Fat synthesis and adiposity regulation in Caenorhabditis elegans
 Review Fat synthesis and adiposity regulation in Caenorhabditis elegans Jennifer L. Watts School of Molecular Biosciences, Washington State University, Pullman, WA 99164, USA Understanding the regulation
Review Fat synthesis and adiposity regulation in Caenorhabditis elegans Jennifer L. Watts School of Molecular Biosciences, Washington State University, Pullman, WA 99164, USA Understanding the regulation
Lehninger 5 th ed. Chapter 17
 Lehninger 5 th ed. Chapter 17 December 26, 2010 Prof. Shimon Schuldiner Email: Shimon.Schuldiner@huji.ac.il Phone: 6585992 CHAPTER 17 Fatty Acid Catabolism Key topics: How fats are digested in animals
Lehninger 5 th ed. Chapter 17 December 26, 2010 Prof. Shimon Schuldiner Email: Shimon.Schuldiner@huji.ac.il Phone: 6585992 CHAPTER 17 Fatty Acid Catabolism Key topics: How fats are digested in animals
Qualifying Fat Storage Phenotypes Using Gas Chromatography in Caenorhabditis elegans
 University of Colorado, Boulder CU Scholar Undergraduate Honors Theses Honors Program Spring 2014 Qualifying Fat Storage Phenotypes Using Gas Chromatography in Caenorhabditis elegans Sabrina Fechtner University
University of Colorado, Boulder CU Scholar Undergraduate Honors Theses Honors Program Spring 2014 Qualifying Fat Storage Phenotypes Using Gas Chromatography in Caenorhabditis elegans Sabrina Fechtner University
Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original
 Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original phenotypic screening was n=40. For specific tests, the
Supplementary Fig. 1 eif6 +/- mice show a reduction in white adipose tissue, blood lipids and normal glycogen synthesis. The cohort of the original phenotypic screening was n=40. For specific tests, the
Fig. S1. Dose-response effects of acute administration of the β3 adrenoceptor agonists CL316243, BRL37344, ICI215,001, ZD7114, ZD2079 and CGP12177 at
 Fig. S1. Dose-response effects of acute administration of the β3 adrenoceptor agonists CL316243, BRL37344, ICI215,001, ZD7114, ZD2079 and CGP12177 at doses of 0.1, 0.5 and 1 mg/kg on cumulative food intake
Fig. S1. Dose-response effects of acute administration of the β3 adrenoceptor agonists CL316243, BRL37344, ICI215,001, ZD7114, ZD2079 and CGP12177 at doses of 0.1, 0.5 and 1 mg/kg on cumulative food intake
Metabolism of cardiac muscle. Dr. Mamoun Ahram Cardiovascular system, 2013
 Metabolism of cardiac muscle Dr. Mamoun Ahram Cardiovascular system, 2013 References This lecture Mark s Basic Medical Biochemistry, 4 th ed., p. 890-891 Hand-out Why is this topic important? Heart failure
Metabolism of cardiac muscle Dr. Mamoun Ahram Cardiovascular system, 2013 References This lecture Mark s Basic Medical Biochemistry, 4 th ed., p. 890-891 Hand-out Why is this topic important? Heart failure
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach
![klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach](/thumbs/91/104639484.jpg) DOI: 10.1038/ncb1891 A. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach embryos let hatch overnight transfer to RNAi plates; incubate 5 days at 15 C RNAi food L1 worms adult worms
DOI: 10.1038/ncb1891 A. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach embryos let hatch overnight transfer to RNAi plates; incubate 5 days at 15 C RNAi food L1 worms adult worms
UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
Biochemistry: A Short Course
 Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 28 Fatty Acid Synthesis 2013 W. H. Freeman and Company Chapter 28 Outline 1. The first stage of fatty acid synthesis is transfer
Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 28 Fatty Acid Synthesis 2013 W. H. Freeman and Company Chapter 28 Outline 1. The first stage of fatty acid synthesis is transfer
CHM333 LECTURE 34: 11/30 12/2/09 FALL 2009 Professor Christine Hrycyna
 Lipid Metabolism β-oxidation FA Acetyl-CoA Triacylglycerols (TAGs) and glycogen are the two major forms of stored energy in vertebrates Glycogen can supply ATP for muscle contraction for less than an hour
Lipid Metabolism β-oxidation FA Acetyl-CoA Triacylglycerols (TAGs) and glycogen are the two major forms of stored energy in vertebrates Glycogen can supply ATP for muscle contraction for less than an hour
Project Summary. Funded by The Beef Checkoff. Regulation of Marbling Development in Beef Cattle by Specific Fatty Acids
 Project Summary Regulation of Marbling Development in Beef Cattle by Specific Fatty Acids Principal Investigators: S. Smith 1, B. Johnson 2 and M. Doumit 3 Texas A&M University 1, Texas Tech University
Project Summary Regulation of Marbling Development in Beef Cattle by Specific Fatty Acids Principal Investigators: S. Smith 1, B. Johnson 2 and M. Doumit 3 Texas A&M University 1, Texas Tech University
LIPID METABOLISM
 LIPID METABOLISM LIPOGENESIS LIPOGENESIS LIPOGENESIS FATTY ACID SYNTHESIS DE NOVO FFA in the blood come from :- (a) Dietary fat (b) Dietary carbohydrate/protein in excess of need FA TAG Site of synthesis:-
LIPID METABOLISM LIPOGENESIS LIPOGENESIS LIPOGENESIS FATTY ACID SYNTHESIS DE NOVO FFA in the blood come from :- (a) Dietary fat (b) Dietary carbohydrate/protein in excess of need FA TAG Site of synthesis:-
number Done by Corrected by Doctor Faisal Al-Khatibe
 number 24 Done by Mohammed tarabieh Corrected by Doctor Faisal Al-Khatibe 1 P a g e *Please look over the previous sheet about fatty acid synthesis **Oxidation(degradation) of fatty acids, occurs in the
number 24 Done by Mohammed tarabieh Corrected by Doctor Faisal Al-Khatibe 1 P a g e *Please look over the previous sheet about fatty acid synthesis **Oxidation(degradation) of fatty acids, occurs in the
ab Hepatic Lipid Accumulation/ Steatosis Assay Kit
 ab133131 Hepatic Lipid Accumulation/ Steatosis Assay Kit Instructions for Use For evaluating steatosis risk of drug candidates using Oil Red O to stain neutral lipids in hepatocytes. This product is for
ab133131 Hepatic Lipid Accumulation/ Steatosis Assay Kit Instructions for Use For evaluating steatosis risk of drug candidates using Oil Red O to stain neutral lipids in hepatocytes. This product is for
THE GLUCOSE-FATTY ACID-KETONE BODY CYCLE Role of ketone bodies as respiratory substrates and metabolic signals
 Br. J. Anaesth. (1981), 53, 131 THE GLUCOSE-FATTY ACID-KETONE BODY CYCLE Role of ketone bodies as respiratory substrates and metabolic signals J. C. STANLEY In this paper, the glucose-fatty acid cycle
Br. J. Anaesth. (1981), 53, 131 THE GLUCOSE-FATTY ACID-KETONE BODY CYCLE Role of ketone bodies as respiratory substrates and metabolic signals J. C. STANLEY In this paper, the glucose-fatty acid cycle
GPR120 *** * * Liver BAT iwat ewat mwat Ileum Colon. UCP1 mrna ***
 a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
Companion to Biosynthesis of Ketones & Cholesterols, Regulation of Lipid Metabolism Lecture Notes
 Companion to Biosynthesis of Ketones & Cholesterols, Regulation of Lipid Metabolism Lecture Notes The major site of acetoacetate and 3-hydorxybutyrate production is in the liver. 3-hydorxybutyrate is the
Companion to Biosynthesis of Ketones & Cholesterols, Regulation of Lipid Metabolism Lecture Notes The major site of acetoacetate and 3-hydorxybutyrate production is in the liver. 3-hydorxybutyrate is the
Supplementary materials
 Supplementary materials Chemical library from ChemBridge 50,240 structurally diverse small molecule compounds dissolved in DMSO Hits Controls: No virus added μ Primary screening at 20 g/ml of compounds
Supplementary materials Chemical library from ChemBridge 50,240 structurally diverse small molecule compounds dissolved in DMSO Hits Controls: No virus added μ Primary screening at 20 g/ml of compounds
FAD FADH2. glycerol-3- phosphate. dehydrogenase. This DHAP is metabolically no different from that produced in glycolysis.
 1 Lipid Metabolism: ow that we are aware of the types of lipids in our bodies, it is important to see how we make them or break them. We will start our discussion with triacylglyceride degradation, and
1 Lipid Metabolism: ow that we are aware of the types of lipids in our bodies, it is important to see how we make them or break them. We will start our discussion with triacylglyceride degradation, and
ANSC/NUTR 618 Lipids & Lipid Metabolism
 I. Overall concepts A. Definitions ANC/NUTR 618 Lipids & Lipid Metabolism 1. De novo synthesis = synthesis from non-fatty acid precursors a. Carbohydrate precursors (glucose, lactate, and pyruvate) b.
I. Overall concepts A. Definitions ANC/NUTR 618 Lipids & Lipid Metabolism 1. De novo synthesis = synthesis from non-fatty acid precursors a. Carbohydrate precursors (glucose, lactate, and pyruvate) b.
doi: /j.bbrc
 doi: 10.1016/j.bbrc.2009.11.006 Fatty Acid Metabolism is Involved in Stress Resistance Mechanisms of Caenorhabditis elegans Makoto Horikawa a, b, Kazuichi Sakamoto a, 1 a Graduate School of Life and Environmental
doi: 10.1016/j.bbrc.2009.11.006 Fatty Acid Metabolism is Involved in Stress Resistance Mechanisms of Caenorhabditis elegans Makoto Horikawa a, b, Kazuichi Sakamoto a, 1 a Graduate School of Life and Environmental
BALANCING THE SCALES USING A NOVEL CELLULAR ENERGY SENSOR
 The West London Medical Journal 2010 Vol 2 No 4 pp 29-35 BALANCING THE SCALES USING A NOVEL CELLULAR ENERGY SENSOR Sairah Akbar The topic of obesity is rarely out of the public eye with an increasingly
The West London Medical Journal 2010 Vol 2 No 4 pp 29-35 BALANCING THE SCALES USING A NOVEL CELLULAR ENERGY SENSOR Sairah Akbar The topic of obesity is rarely out of the public eye with an increasingly
SUPPLEMENTARY INFORMATION
 doi: 1.138/nature7221 Brown fat selective genes 12 1 Control Q-RT-PCR (% of Control) 8 6 4 2 Ntrk3 Cox7a1 Cox8b Cox5b ATPase b2 ATPase f1a1 Sirt3 ERRα Elovl3/Cig3 PPARα Zic1 Supplementary Figure S1. stimulates
doi: 1.138/nature7221 Brown fat selective genes 12 1 Control Q-RT-PCR (% of Control) 8 6 4 2 Ntrk3 Cox7a1 Cox8b Cox5b ATPase b2 ATPase f1a1 Sirt3 ERRα Elovl3/Cig3 PPARα Zic1 Supplementary Figure S1. stimulates
Oxidation of Long Chain Fatty Acids
 Oxidation of Long Chain Fatty Acids Dr NC Bird Oxidation of long chain fatty acids is the primary source of energy supply in man and animals. Hibernating animals utilise fat stores to maintain body heat,
Oxidation of Long Chain Fatty Acids Dr NC Bird Oxidation of long chain fatty acids is the primary source of energy supply in man and animals. Hibernating animals utilise fat stores to maintain body heat,
AMPK Phosphorylation Assay Kit
 AMPK Phosphorylation Assay Kit Catalog Number KA3789 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
AMPK Phosphorylation Assay Kit Catalog Number KA3789 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Mangifera indica activates key metabolic master switch enzymes The innovation for well-aging
 Mangifera indica activates key metabolic master switch enzymes The innovation for well-aging Dr. S. Buchwald-Werner 6. May 2015 Vitafoods Europe Conference Healthy Aging Session Consumer demand for healthy-aging
Mangifera indica activates key metabolic master switch enzymes The innovation for well-aging Dr. S. Buchwald-Werner 6. May 2015 Vitafoods Europe Conference Healthy Aging Session Consumer demand for healthy-aging
Energy metabolism - the overview
 Energy metabolism - the overview Josef Fontana EC - 40 Overview of the lecture Important terms of the energy metabolism The overview of the energy metabolism The main pathways of the energy metabolism
Energy metabolism - the overview Josef Fontana EC - 40 Overview of the lecture Important terms of the energy metabolism The overview of the energy metabolism The main pathways of the energy metabolism
AMPK. Tomáš Kučera.
 AMPK (AMP- ACTIVATED PROTEIN KINASE ) Tomáš Kučera tomas.kucera@lfmotol.cuni.cz Department of Medical Chemistry and Clinical Biochemistry 2nd Faculty of Medicine, Charles University in Prague and Motol
AMPK (AMP- ACTIVATED PROTEIN KINASE ) Tomáš Kučera tomas.kucera@lfmotol.cuni.cz Department of Medical Chemistry and Clinical Biochemistry 2nd Faculty of Medicine, Charles University in Prague and Motol
Supplemental Figures
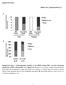 Supplemental Figures Supplemental Figure 1. Fasting-dependent regulation of the SREBP ortholog SBP-1 and lipid homeostasis mediated by the SIRT1 ortholog SIR-2.1 in C. elegans. (A) Wild-type or sir-2.1(lof)
Supplemental Figures Supplemental Figure 1. Fasting-dependent regulation of the SREBP ortholog SBP-1 and lipid homeostasis mediated by the SIRT1 ortholog SIR-2.1 in C. elegans. (A) Wild-type or sir-2.1(lof)
Regulation of Lipid Homeostasis: Lipid Droplets
 Regulation of Lipid Homeostasis: Lipid Droplets Bernd Helms Article Brand Recter Hendrik Mertens 1 The Basics I: FAs The Basics II: FA Activation 2 Basics III: TG-FA Interplay. Why? Adipocytes 3 Foam cells
Regulation of Lipid Homeostasis: Lipid Droplets Bernd Helms Article Brand Recter Hendrik Mertens 1 The Basics I: FAs The Basics II: FA Activation 2 Basics III: TG-FA Interplay. Why? Adipocytes 3 Foam cells
BBSG 501 Section 4 Metabolic Fuels, Energy and Order Fall 2003 Semester
 BBSG 501 Section 4 Metabolic Fuels, Energy and Order Fall 2003 Semester Section Director: Dave Ford, Ph.D. Office: MS 141: ext. 8129: e-mail: fordda@slu.edu Lecturers: Michael Moxley, Ph.D. Office: MS
BBSG 501 Section 4 Metabolic Fuels, Energy and Order Fall 2003 Semester Section Director: Dave Ford, Ph.D. Office: MS 141: ext. 8129: e-mail: fordda@slu.edu Lecturers: Michael Moxley, Ph.D. Office: MS
Introduction to metabolic regulation. Prof K Syed Department of Biochemistry & Microbiology University of Zululand Room no. 247
 Introduction to metabolic regulation Prof K Syed Department of Biochemistry & Microbiology University of Zululand Room no. 247 SyedK@unizulu.ac.za Topics Metabolism Metabolism: Categories Important metabolic
Introduction to metabolic regulation Prof K Syed Department of Biochemistry & Microbiology University of Zululand Room no. 247 SyedK@unizulu.ac.za Topics Metabolism Metabolism: Categories Important metabolic
BCM 221 LECTURES OJEMEKELE O.
 BCM 221 LECTURES BY OJEMEKELE O. OUTLINE INTRODUCTION TO LIPID CHEMISTRY STORAGE OF ENERGY IN ADIPOCYTES MOBILIZATION OF ENERGY STORES IN ADIPOCYTES KETONE BODIES AND KETOSIS PYRUVATE DEHYDROGENASE COMPLEX
BCM 221 LECTURES BY OJEMEKELE O. OUTLINE INTRODUCTION TO LIPID CHEMISTRY STORAGE OF ENERGY IN ADIPOCYTES MOBILIZATION OF ENERGY STORES IN ADIPOCYTES KETONE BODIES AND KETOSIS PYRUVATE DEHYDROGENASE COMPLEX
Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells.
 Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells. a. Schematic of the V-ATPase proton pump macro-complex structure. The V1 complex is composed of
Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells. a. Schematic of the V-ATPase proton pump macro-complex structure. The V1 complex is composed of
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Roles of Lipids. principal form of stored energy major constituents of cell membranes vitamins messengers intra and extracellular
 Roles of Lipids principal form of stored energy major constituents of cell membranes vitamins messengers intra and extracellular = Oxidation of fatty acids Central energy-yielding pathway in animals. O
Roles of Lipids principal form of stored energy major constituents of cell membranes vitamins messengers intra and extracellular = Oxidation of fatty acids Central energy-yielding pathway in animals. O
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
SUPPLEMENTARY FIGURE S1: nlp-22 is expressed in the RIA interneurons and is secreted. (a) An animal expressing both the RIA specific reporter
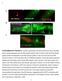 1 SUPPLEMENTARY FIGURE S1: nlp-22 is expressed in the RIA interneurons and is secreted. (a) An animal expressing both the RIA specific reporter Pglr-3:mCherry (red) and Pnlp-22:gfp (green) shows co-localization
1 SUPPLEMENTARY FIGURE S1: nlp-22 is expressed in the RIA interneurons and is secreted. (a) An animal expressing both the RIA specific reporter Pglr-3:mCherry (red) and Pnlp-22:gfp (green) shows co-localization
Cell Cell
 Go to cellsalive.com. Select Interactive Cell Models: Plant and Animal. Fill in the information on Plant and Animal Organelles, then Click on Start the Animation Select Plant or Animal Cell below the box.
Go to cellsalive.com. Select Interactive Cell Models: Plant and Animal. Fill in the information on Plant and Animal Organelles, then Click on Start the Animation Select Plant or Animal Cell below the box.
UNIT 1: Introduction to metabolic regulation
 UNIT 1: Introduction to metabolic regulation Prof K Syed Department of Biochemistry & Microbiology University of Zululand Room no. 247 SyedK@unizulu.ac.za Topics Metabolism Metabolism: Categories Important
UNIT 1: Introduction to metabolic regulation Prof K Syed Department of Biochemistry & Microbiology University of Zululand Room no. 247 SyedK@unizulu.ac.za Topics Metabolism Metabolism: Categories Important
Mamofillin New aesthetic perspective
 New aesthetic perspective info@ White adipose tissue (WAT) White adipose tissue (WAT) is the prevalent type in human adults functioning as the major storage site for the lipids absorbed from daily intake
New aesthetic perspective info@ White adipose tissue (WAT) White adipose tissue (WAT) is the prevalent type in human adults functioning as the major storage site for the lipids absorbed from daily intake
Biosynthesis of Fatty Acids. By Dr.QUTAIBA A. QASIM
 Biosynthesis of Fatty Acids By Dr.QUTAIBA A. QASIM Fatty Acids Definition Fatty acids are comprised of hydrocarbon chains terminating with carboxylic acid groups. Fatty acids and their associated derivatives
Biosynthesis of Fatty Acids By Dr.QUTAIBA A. QASIM Fatty Acids Definition Fatty acids are comprised of hydrocarbon chains terminating with carboxylic acid groups. Fatty acids and their associated derivatives
Metabolic integration and Regulation
 Metabolic integration and Regulation 109700: Graduate Biochemistry Trimester 2/2016 Assistant Prof. Dr. Panida Khunkaewla kpanida@sut.ac.th School of Chemistry Suranaree University of Technology 1 Overview
Metabolic integration and Regulation 109700: Graduate Biochemistry Trimester 2/2016 Assistant Prof. Dr. Panida Khunkaewla kpanida@sut.ac.th School of Chemistry Suranaree University of Technology 1 Overview
Part-4. Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death
 Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
H 2 S: Synthesis and functions
 H 2 S: Synthesis and functions 1 Signaling gas molecules: O 2, NO and CO Then, H 2 S - Fourth singling gas molecule after O 2, NO and CO 2 Nothing Rotten About Hydrogen Sulfide s Medical Promise Science
H 2 S: Synthesis and functions 1 Signaling gas molecules: O 2, NO and CO Then, H 2 S - Fourth singling gas molecule after O 2, NO and CO 2 Nothing Rotten About Hydrogen Sulfide s Medical Promise Science
What systems are involved in homeostatic regulation (give an example)?
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS (Diabetes Mellitus Part 1): An Overview
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS (Diabetes Mellitus Part 1): An Overview
Enzyme-coupled Receptors. Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors
 Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
23.1 Lipid Metabolism in Animals. Chapter 23. Micelles Lipid Metabolism in. Animals. Overview of Digestion Lipid Metabolism in
 Denniston Topping Caret Copyright! The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chapter 23 Fatty Acid Metabolism Triglycerides (Tgl) are emulsified into fat droplets
Denniston Topping Caret Copyright! The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chapter 23 Fatty Acid Metabolism Triglycerides (Tgl) are emulsified into fat droplets
Master class Biomolecular Sciences Molecular Cell Biology.
 Master class Biomolecular Sciences Molecular Cell Biology. 04-09-08: Paul van Bergen en Henegouwen. Clathrin-indep Endocytosis 11-09-08: Willem Stoorvogel. Endocytosis and MHC classii 18-09-08: X-track
Master class Biomolecular Sciences Molecular Cell Biology. 04-09-08: Paul van Bergen en Henegouwen. Clathrin-indep Endocytosis 11-09-08: Willem Stoorvogel. Endocytosis and MHC classii 18-09-08: X-track
Fatty acid breakdown
 Fatty acids contain a long hydrocarbon chain and a terminal carboxylate group. Most contain between 14 and 24 carbon atoms. The chains may be saturated or contain double bonds. The complete oxidation of
Fatty acids contain a long hydrocarbon chain and a terminal carboxylate group. Most contain between 14 and 24 carbon atoms. The chains may be saturated or contain double bonds. The complete oxidation of
ANSC/NUTR 601 GENERAL ANIMAL NUTRITION Stearoyl-CoA desaturase, VLDL metabolism, and obesity
 ANSC/NUTR 601 GENERAL ANIMAL NUTRITION Stearoyl-CoA desaturase, VLDL metabolism, and obesity I. Stearoyl coenzyme A desaturase and obesity in rodents A. Stearoyl coenzyme A desaturase (SCD) is the 9 desaturase.
ANSC/NUTR 601 GENERAL ANIMAL NUTRITION Stearoyl-CoA desaturase, VLDL metabolism, and obesity I. Stearoyl coenzyme A desaturase and obesity in rodents A. Stearoyl coenzyme A desaturase (SCD) is the 9 desaturase.
Supplementary Table 1. Primer Sequences Used for Quantitative Real-Time PCR
 Supplementary Table 1. Primer Sequences Used for Quantitative Real-Time PCR Gene Forward Primer (5-3 ) Reverse Primer (5-3 ) cadl CTTGGGGGCGCGTCT CTGTTCTTTTGTGCCGTTTCG cyl-coenzyme Dehydrogenase, very
Supplementary Table 1. Primer Sequences Used for Quantitative Real-Time PCR Gene Forward Primer (5-3 ) Reverse Primer (5-3 ) cadl CTTGGGGGCGCGTCT CTGTTCTTTTGTGCCGTTTCG cyl-coenzyme Dehydrogenase, very
In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic
 Glycolysis 1 In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic glycolysis. If this pyruvate is converted instead
Glycolysis 1 In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic glycolysis. If this pyruvate is converted instead
Free Fatty Acid Assay Kit (Fluorometric)
 Product Manual Free Fatty Acid Assay Kit (Fluorometric) Catalog Number STA-619 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Triglycerides (TAG) are a type of lipid
Product Manual Free Fatty Acid Assay Kit (Fluorometric) Catalog Number STA-619 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Triglycerides (TAG) are a type of lipid
1. Stowers Institute for Medical Research, Kansas City, MO 64110
 Lipid droplets as fat storage organelles in Caenorhabditis elegans Ho Yi Mak 1, 2 1. Stowers Institute for Medical Research, Kansas City, MO 64110 2. Department of Molecular and Integrative Physiology,
Lipid droplets as fat storage organelles in Caenorhabditis elegans Ho Yi Mak 1, 2 1. Stowers Institute for Medical Research, Kansas City, MO 64110 2. Department of Molecular and Integrative Physiology,
Homeostatic Control Systems
 Homeostatic Control Systems In order to maintain homeostasis, control system must be able to Detect deviations from normal in the internal environment that need to be held within narrow limits Integrate
Homeostatic Control Systems In order to maintain homeostasis, control system must be able to Detect deviations from normal in the internal environment that need to be held within narrow limits Integrate
Jonathan Kindberg BNF Molecular Biology Through Discovery. Research Proposal
 Jonathan Kindberg BNF0 491 Molecular Biology Through Discovery Research Proposal The effect of a single nucleotide polymorphism in the NPY protein and ethanol consumption in mice (08 December 2012) I.
Jonathan Kindberg BNF0 491 Molecular Biology Through Discovery Research Proposal The effect of a single nucleotide polymorphism in the NPY protein and ethanol consumption in mice (08 December 2012) I.
REGULATION OF ENZYME ACTIVITY. Medical Biochemistry, Lecture 25
 REGULATION OF ENZYME ACTIVITY Medical Biochemistry, Lecture 25 Lecture 25, Outline General properties of enzyme regulation Regulation of enzyme concentrations Allosteric enzymes and feedback inhibition
REGULATION OF ENZYME ACTIVITY Medical Biochemistry, Lecture 25 Lecture 25, Outline General properties of enzyme regulation Regulation of enzyme concentrations Allosteric enzymes and feedback inhibition
Acetyl-CoA or BC acyl-coa. Int1-ACP. FabH. FabF. Anti-FabF Platensimycin ACP. Fak
 Initiation Acetyl-CoA AccBCDA FabZ Int-ACP Elongation [N+] FabG Int1-ACP Acetyl-CoA or BC acyl-coa FabH FabD CoA Malonyl-ACP Malonyl CoA ACP Anti-FabI Triclosan AFN-15 FabI acyl-acp PlsX PlsY Pls C Lipoic
Initiation Acetyl-CoA AccBCDA FabZ Int-ACP Elongation [N+] FabG Int1-ACP Acetyl-CoA or BC acyl-coa FabH FabD CoA Malonyl-ACP Malonyl CoA ACP Anti-FabI Triclosan AFN-15 FabI acyl-acp PlsX PlsY Pls C Lipoic
nature methods Organelle-specific, rapid induction of molecular activities and membrane tethering
 nature methods Organelle-specific, rapid induction of molecular activities and membrane tethering Toru Komatsu, Igor Kukelyansky, J Michael McCaffery, Tasuku Ueno, Lidenys C Varela & Takanari Inoue Supplementary
nature methods Organelle-specific, rapid induction of molecular activities and membrane tethering Toru Komatsu, Igor Kukelyansky, J Michael McCaffery, Tasuku Ueno, Lidenys C Varela & Takanari Inoue Supplementary
Multiple choice: Circle the best answer on this exam. There are 12 multiple choice questions, each question is worth 3 points.
 CHEM 4420 Exam 4 Spring 2015 Dr. Stone Page 1 of 6 Name Use complete sentences when requested. There are 120 possible points on this exam. Therefore there are 20 bonus points. Multiple choice: Circle the
CHEM 4420 Exam 4 Spring 2015 Dr. Stone Page 1 of 6 Name Use complete sentences when requested. There are 120 possible points on this exam. Therefore there are 20 bonus points. Multiple choice: Circle the
AMPK. Tomáš Kuc era. Ústav lékar ské chemie a klinické biochemie 2. lékar ská fakulta, Univerzita Karlova v Praze
 AMPK (AMP- ACTIVATED PROTEIN KINASE ) Tomáš Kuc era Ústav lékar ské chemie a klinické biochemie 2. lékar ská fakulta, Univerzita Karlova v Praze 2013 AMPK AMP-ACTIVATED PROTEIN KINASE present in all eukaryotic
AMPK (AMP- ACTIVATED PROTEIN KINASE ) Tomáš Kuc era Ústav lékar ské chemie a klinické biochemie 2. lékar ská fakulta, Univerzita Karlova v Praze 2013 AMPK AMP-ACTIVATED PROTEIN KINASE present in all eukaryotic
Fatty Acid Degradation. Catabolism Overview. TAG and FA 11/11/2015. Chapter 27, Stryer Short Course. Lipids as a fuel source diet Beta oxidation
 Fatty Acid Degradation Chapter 27, Stryer Short Course Catabolism verview Lipids as a fuel source diet Beta oxidation saturated Unsaturated dd chain Ketone bodies as fuel Physiology High energy More reduced
Fatty Acid Degradation Chapter 27, Stryer Short Course Catabolism verview Lipids as a fuel source diet Beta oxidation saturated Unsaturated dd chain Ketone bodies as fuel Physiology High energy More reduced
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
LDL Uptake Cell-Based Assay Kit
 LDL Uptake Cell-Based Assay Kit Catalog Number KA1327 100 assays Version: 07 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
LDL Uptake Cell-Based Assay Kit Catalog Number KA1327 100 assays Version: 07 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
REGULATORY MECHANISMS OF TRANSCRIPTION FACTOR FUNCTION
 Transcription Regulation And Gene Expression in Eukaryotes Cycle G2 (lecture 13709) RG Clerc 04.04.2012 REGULATORY MECHANISMS OF TRANSCRIPTION FACTOR FUNCTION Protein synthesized Protein phosphorylated
Transcription Regulation And Gene Expression in Eukaryotes Cycle G2 (lecture 13709) RG Clerc 04.04.2012 REGULATORY MECHANISMS OF TRANSCRIPTION FACTOR FUNCTION Protein synthesized Protein phosphorylated
Regulation of Metabolism
 Regulation of Metabolism Pratt and Cornely Chapter 19 Regulation by Compartmentalization Form of reciprocal regulation Degradation vs biosynthesis Requires transporters 1 Specialization of organs Fuel
Regulation of Metabolism Pratt and Cornely Chapter 19 Regulation by Compartmentalization Form of reciprocal regulation Degradation vs biosynthesis Requires transporters 1 Specialization of organs Fuel
Using the Organic Acids Test Part 5 Dr. Jeff Moss
 Using organic acids to resolve chief complaints and improve quality of life in chronically ill patients Part V Jeffrey Moss, DDS, CNS, DACBN jeffmoss@mossnutrition.com 413-530-08580858 (cell) 1 Summer
Using organic acids to resolve chief complaints and improve quality of life in chronically ill patients Part V Jeffrey Moss, DDS, CNS, DACBN jeffmoss@mossnutrition.com 413-530-08580858 (cell) 1 Summer
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10643 Supplementary Table 1. Identification of hecw-1 coding polymorphisms at amino acid positions 322 and 325 in 162 strains of C. elegans. WWW.NATURE.COM/NATURE 1 Supplementary Figure
doi:10.1038/nature10643 Supplementary Table 1. Identification of hecw-1 coding polymorphisms at amino acid positions 322 and 325 in 162 strains of C. elegans. WWW.NATURE.COM/NATURE 1 Supplementary Figure
Krüppel-like family of transcription factors: an emerging new frontier in fat biology
 Review 622 International Journal of Biological Sciences 2009; 5(6):622-636 Ivyspring International Publisher. All rights reserved Krüppel-like family of transcription factors: an emerging new frontier
Review 622 International Journal of Biological Sciences 2009; 5(6):622-636 Ivyspring International Publisher. All rights reserved Krüppel-like family of transcription factors: an emerging new frontier
Lipid Metabolism. Catabolism Overview
 Lipid Metabolism Pratt & Cornely, Chapter 17 Catabolism Overview Lipids as a fuel source from diet Beta oxidation Mechanism ATP production Ketone bodies as fuel 1 High energy More reduced Little water
Lipid Metabolism Pratt & Cornely, Chapter 17 Catabolism Overview Lipids as a fuel source from diet Beta oxidation Mechanism ATP production Ketone bodies as fuel 1 High energy More reduced Little water
Bio 401 Sp2014. Multiple Choice (Circle the letter corresponding to the correct answer)
 MIDTERM EXAM KEY (60 pts) You should have 5 pages. Please put your name on every page. You will have 70 minutes to complete the exam. You may begin working as soon as you receive the exam. NOTE: the RED
MIDTERM EXAM KEY (60 pts) You should have 5 pages. Please put your name on every page. You will have 70 minutes to complete the exam. You may begin working as soon as you receive the exam. NOTE: the RED
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 25 Metabolism and Nutrition Metabolic Reactions Metabolism refers to all of the chemical reactions taking place in the body. Reactions that break
Principles of Anatomy and Physiology 14 th Edition CHAPTER 25 Metabolism and Nutrition Metabolic Reactions Metabolism refers to all of the chemical reactions taking place in the body. Reactions that break
cholesterol structure Cholesterol FAQs Cholesterol promotes the liquid-ordered phase of membranes Friday, October 15, 2010
 cholesterol structure most plasma cholesterol is in the esterified form (not found in cells or membranes) cholesterol functions in all membranes (drives formation of lipid microdomains) cholesterol is
cholesterol structure most plasma cholesterol is in the esterified form (not found in cells or membranes) cholesterol functions in all membranes (drives formation of lipid microdomains) cholesterol is
Rescue of mutant rhodopsin traffic by metformin-induced AMPK activation accelerates photoreceptor degeneration Athanasiou et al
 Supplementary Material Rescue of mutant rhodopsin traffic by metformin-induced AMPK activation accelerates photoreceptor degeneration Athanasiou et al Supplementary Figure 1. AICAR improves P23H rod opsin
Supplementary Material Rescue of mutant rhodopsin traffic by metformin-induced AMPK activation accelerates photoreceptor degeneration Athanasiou et al Supplementary Figure 1. AICAR improves P23H rod opsin
Molecular Cell Biology Problem Drill 16: Intracellular Compartment and Protein Sorting
 Molecular Cell Biology Problem Drill 16: Intracellular Compartment and Protein Sorting Question No. 1 of 10 Question 1. Which of the following statements about the nucleus is correct? Question #01 A. The
Molecular Cell Biology Problem Drill 16: Intracellular Compartment and Protein Sorting Question No. 1 of 10 Question 1. Which of the following statements about the nucleus is correct? Question #01 A. The
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): Overall, this is an interesting study. The authors used a custom microfluidic device to deliver precise concentrations of bacterial food to worms
Reviewers' comments: Reviewer #1 (Remarks to the Author): Overall, this is an interesting study. The authors used a custom microfluidic device to deliver precise concentrations of bacterial food to worms
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Behavioural effects of ketamine in non-stressed and stressed mice. Naive C57BL/6 adult male mice (n=10/group) were given a single dose of saline vehicle or ketamine (3.0 mg/kg,
Supplementary Figure 1. Behavioural effects of ketamine in non-stressed and stressed mice. Naive C57BL/6 adult male mice (n=10/group) were given a single dose of saline vehicle or ketamine (3.0 mg/kg,
Specific polyunsaturated fatty acids modulate lipid delivery and oocyte development in C. elegans revealed by molecular-selective label-free imaging
 Specific polyunsaturated fatty acids modulate lipid delivery and oocyte development in C. elegans revealed by molecular-selective label-free imaging Wei-Wen Chen 1,2,3,+, Yung-Hsiang Yi 4,5,+, Cheng-Hao
Specific polyunsaturated fatty acids modulate lipid delivery and oocyte development in C. elegans revealed by molecular-selective label-free imaging Wei-Wen Chen 1,2,3,+, Yung-Hsiang Yi 4,5,+, Cheng-Hao
Gene Polymorphisms and Carbohydrate Diets. James M. Ntambi Ph.D
 Gene Polymorphisms and Carbohydrate Diets James M. Ntambi Ph.D Fatty Acids that Flux into Tissue Lipids are from Dietary Sources or are Made De novo from Glucose or Fructose Glucose Fructose Acetyl-CoA
Gene Polymorphisms and Carbohydrate Diets James M. Ntambi Ph.D Fatty Acids that Flux into Tissue Lipids are from Dietary Sources or are Made De novo from Glucose or Fructose Glucose Fructose Acetyl-CoA
he micrornas of Caenorhabditis elegans (Lim et al. Genes & Development 2003)
 MicroRNAs: Genomics, Biogenesis, Mechanism, and Function (D. Bartel Cell 2004) he micrornas of Caenorhabditis elegans (Lim et al. Genes & Development 2003) Vertebrate MicroRNA Genes (Lim et al. Science
MicroRNAs: Genomics, Biogenesis, Mechanism, and Function (D. Bartel Cell 2004) he micrornas of Caenorhabditis elegans (Lim et al. Genes & Development 2003) Vertebrate MicroRNA Genes (Lim et al. Science
BIOLOGY 311C - Brand Spring 2010
 BIOLOGY 311C - Brand Spring 2010 NAME (printed very legibly) KEY UT-EID EXAMINATION III Before beginning, check to be sure that this exam contains 8 pages (including front and back) numbered consecutively,
BIOLOGY 311C - Brand Spring 2010 NAME (printed very legibly) KEY UT-EID EXAMINATION III Before beginning, check to be sure that this exam contains 8 pages (including front and back) numbered consecutively,
Generating Spontaneous Copy Number Variants (CNVs) Jennifer Freeman Assistant Professor of Toxicology School of Health Sciences Purdue University
 Role of Chemical lexposure in Generating Spontaneous Copy Number Variants (CNVs) Jennifer Freeman Assistant Professor of Toxicology School of Health Sciences Purdue University CNV Discovery Reference Genetic
Role of Chemical lexposure in Generating Spontaneous Copy Number Variants (CNVs) Jennifer Freeman Assistant Professor of Toxicology School of Health Sciences Purdue University CNV Discovery Reference Genetic
Lecture #27 Lecturer A. N. Koval
 Lecture #27 Lecturer A. N. Koval Hormones Transduce Signals to Affect Homeostatic Mechanisms Koval A. (C), 2011 2 Lipophilic hormones Classifying hormones into hydrophilic and lipophilic molecules indicates
Lecture #27 Lecturer A. N. Koval Hormones Transduce Signals to Affect Homeostatic Mechanisms Koval A. (C), 2011 2 Lipophilic hormones Classifying hormones into hydrophilic and lipophilic molecules indicates
Membrane Lipids & Cholesterol Metabolism
 Membrane Lipids & Cholesterol Metabolism Learning Objectives 1. How Are Acylglycerols and Compound Lipids Produced? 2. The synthesis of Sphingolipids from Ceramide 3. Diseases due to Disruption of Lipid
Membrane Lipids & Cholesterol Metabolism Learning Objectives 1. How Are Acylglycerols and Compound Lipids Produced? 2. The synthesis of Sphingolipids from Ceramide 3. Diseases due to Disruption of Lipid
Fatty acids synthesis
 Fatty acids synthesis The synthesis start from Acetyl COA the first step requires ATP + reducing power NADPH! even though the oxidation and synthesis are different pathways but from chemical part of view
Fatty acids synthesis The synthesis start from Acetyl COA the first step requires ATP + reducing power NADPH! even though the oxidation and synthesis are different pathways but from chemical part of view
Lipids. Lipids: a Diverse group of chemicals. Storage Lipids: derivatives of fatty acids. 11/21/10
 1 Lipids Lehninger 3 rd ed. Chapter 11 (For biosynthesis see Chapter 21) 2 Lipids: a Diverse group of chemicals Insolubility in water. Fats and oils: energy stores. Phospholipids and sterols: structural
1 Lipids Lehninger 3 rd ed. Chapter 11 (For biosynthesis see Chapter 21) 2 Lipids: a Diverse group of chemicals Insolubility in water. Fats and oils: energy stores. Phospholipids and sterols: structural
Glucose. Glucose. Insulin Action. Introduction to Hormonal Regulation of Fuel Metabolism
 Glucose Introduction to Hormonal Regulation of Fuel Metabolism Fasting level 3.5-5 mmol (1 mmol = 18 mg/dl) Postprandial 6-10 mmol Amount of glucose in circulation is dependent on: Absorption from the
Glucose Introduction to Hormonal Regulation of Fuel Metabolism Fasting level 3.5-5 mmol (1 mmol = 18 mg/dl) Postprandial 6-10 mmol Amount of glucose in circulation is dependent on: Absorption from the
THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS
 Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Synthesis and elongation of fatty acids
 Synthesis and elongation of fatty acids A molecular caliper mechanism for determining very long-chain fatty acid length Vladimir Denic and Jonathan S. Weissman (2007) Cell 130, 663-677 February 28, 2008
Synthesis and elongation of fatty acids A molecular caliper mechanism for determining very long-chain fatty acid length Vladimir Denic and Jonathan S. Weissman (2007) Cell 130, 663-677 February 28, 2008
LDL Uptake Flow Cytometry Assay Kit
 LDL Uptake Flow Cytometry Assay Kit Item No. 601470 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
LDL Uptake Flow Cytometry Assay Kit Item No. 601470 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION

 Supplementary Figure 1 a OD c. 1. 1... Time [min] 1 p
Supplementary Figure 1 a OD c. 1. 1... Time [min] 1 p