Regenerative Product Portfolio. Trusted Clinical Solutions
|
|
|
- Cameron Bond
- 6 years ago
- Views:
Transcription
1 Regenerative Product Portfolio Trusted Clinical Solutions
2 Table Of Contents
3 3 Featured Products The Power Of Puros Allografts...5 Bone Grafts...6 Puros Allografts...6 Cortico-Cancellous Particulate...6 Cancellous Particulate...7 Cortical Particulate...7 Bone Block...8 Puros Putties...8 Puros DBM Putty...8 Puros DBM Putty With Chips...8 Xenografts...9 CopiOs Xenograft...9 Endobon Xenograft...9 Synthetic Bone Graft...10 NovaBone Dental Putty...10 Soft-Tissue Graft...11 Puros Dermis Allograft Tissue Matrix...11 Barrier Membranes...12 CopiOs Pericardium...12 Puros Pericardium...12 CopiOs Extend...13 Zimmer Socket Repair Membrane...13 Wound Care...14 Zimmer Collagen Plug, Tape and Patch...14 Procedure Kits...14 Sinus Crestal Approach Kit...15 Sinus Lateral Approach Kit...15 Screw Fixation Kit...16 Safescraper...16 Additional Regenerative Products Freeze Dried Bone Grafts...18 RegenerOss Particulate...18 DBM Putties...19 RegenerOss Putty Plus...19 RegenaVate DBM Products...20 Synthetic Bone Graft Substitutes...21 lngenios HA...21 lngenios ß-TCP...21 Barrier Membranes...22 BioMend & BioMend Extend...22 OsseoGuard & OsseoGuard Flex...22 Wound Care...23 RegenePro...23
4 Featured Products
5 The Power Of Puros Allografts 5 Clinicians around the globe have counted on the Puros family of allografts for hard- and soft-tissue augmentation procedures for years. The brand s renowned reputation is based on:* Consistent, clinically documented and predictable processing and configuration Allowing for creation of healthy, solid bone 1-3 Rapid, predictable turnover shown in human clinical studies 4-7 Natural, easy-to-use, terminally sterile options Quick hydration, five-year shelf life excluding RegenaVate and Puros Putties and storage at room temperature 8 excluding RegenaVate Frozen DBM. The Proprietary Tutoplast Process The proprietary Tutoplast process assures the highest standard of tissue quality with minimal risk of disease transmission. 8 That s why, for over 40 years, Tutoplast processed tissues have been used in more than five million procedures. 8 There Is No Comparison Zimmer Biomet Dental now offers the most comprehensive line of regenerative biologics available. This ever-expanding range of solutions provides the breadth and depth that clinicians need to complete regenerative procedures, while broadening the success of their practice. The Benefits Of The Multi-Step Tutoplast Process For Puros Particulate Bone Graft DELIPIDIZATION OSMOTIC TREATMENT OXIDATIVE TREATMENT LOW-DOSE G AMMA IRRADIATION SOLVENT DEHYDRATION The process preserves the valuable minerals in bone (minerals don t apply to soft tissues), collagen matrix and tissue integrity while inactivating pathogens and gently removing unwanted materials, such as cells, antigens and viruses 8 resulting in predictable, reliable and sterile allografts. * Claims referenced apply to Tutoplast processed products and exclude Puros DBM.
6 Bone Grafts Puros Allograft Particulates Puros Cortico-Cancellous Particulate Allograft Puros Cortico-Cancellous Particulate Allograft is an anatomic-based mix of 70% cortical and 30% cancellous bone particulate. Puros Cortico-Cancellous Particulate Allograft is used in procedures where space maintenance and faster remodeling 4-5 are desired.* This mixture combines the clinical advantages of both Puros Cortical and Puros Cancellous Particulate Allograft materials. Retains osseoconductive properties due to the preservation of the natural bone matrix collagen and mineral composition, trabecular pattern, and original porosity; 1-9 enabling the ingrowth of vascular and cellular connective tissue 7 Provides time-saving convenience by eliminating the need to mix various graft materials Both cortical and cancellous particulates come from a single donor Suggested Applications: Sinus lift/sinus floor elevation Ridge augmentation Puros Cortico-Cancellous Particles, 0.5 cc, μm Puros Cortico-Cancellous Particles, 1 cc, μm Puros Cortico-Cancellous Particles, 2 cc, μm Puros Cortico-Cancellous Particles, 0.5 cc, μm Puros Cortico-Cancellous Particles, 1 cc, μm Puros Cortico-Cancellous Particles, 2 cc, μm Shelf-life: Five (5) years * Cancellous bone has been shown to remodel faster than sintered bovine bone.
7 7 Puros Cancellous Particulate Allograft Puros Cancellous Particulate Allograft has a history of well-documented clinical results, is an easy-to-handle choice for predictable bone regeneration and acts as an osseoconductive scaffold for new bone formation. 1-9 In large-volume applications, prospective studies have documented faster bone regeneration at 6 months than grafts containing sintered bovine bone matrix 4,5 One study shows the use of tenting screws in combination with Puros Allograft resulted in an average 9.7 mm vertical augmentation in 4 to 5 months 10 In small-volume applications, regeneration of hard bone has been reported as early as 3 to 5 months 6,7,11 Retains osseoconductive properties due to the preservation of the natural bone matrix collagen and mineral composition, trabecular pattern, and original porosity; 1,9 enabling the ingrowth of vascular and cellular connective tissue 7 Shown Clinically Successful In: Regeneration of periodontal bone and furcation defects 1,9 Osseous defect regeneration 1,5-7,9,11 Regeneration of extraction sockets 6,7 and gaps around block grafts 6,7,11,12 Horizontal alveolar crest augmentation 6,7,11,12 and sinus augmentation 4, Puros Cancellous Particles, 0.5 cc, μm Puros Cancellous Particles, 1 cc, μm Puros Cancellous Particles, 2 cc, μm Puros Cancellous Particles, 0.5 cc, μm Puros Cancellous Particles, 1 cc, μm Puros Cancellous Particles, 2 cc, μm Shelf-life: Five (5) years Puros Cortical Particulate Allograft Puros Cortical Particulate Allograft is an easy way to naturally regenerate bone, with the particles having the density and strength of cortical autograft. It can be used alone or as a composite graft in space maintenance and volume enhancement procedures. 13 Without sacrificing ridge contour, cortical particles remodel into both a dense, lamellar structure as well as natural, viable bone with similar density to native bone 12 One study reported an average gain of 1.8 mm in bone thickness when used in a sandwich technique for the treatment of localized buccal dehiscence defects 14 One study found that by combining sandwich and mucogingival pouch flap techniques, there was a 1.5 to 3.5 mm gain in mean ridge thickness, and an 84% to 100% gain in mean ridge height 15 Shown Clinically Successful In: Puros Cortical Particles, 0.5 cc, μm Sinus augmentation 16, Puros Cortical Particles, 1 cc, μm Alveolar ridge augmentation 12,14, Puros Cortical Particles, 2 cc, μm Tent and sandwich grafting techniques Puros Cortical Particles, 0.5 cc, μm Puros Cortical Particles, 1 cc, μm Puros Cortical Particles, 2 cc, μm Shelf-life: Five (5) years
8 Bone Grafts Puros Block Allograft By eliminating the need to harvest an autogenous block graft, Puros Block Allografts may save time and help to reduce pain and can shorten the patient s rehabilitation period. A clinically documented solution for effectively restoring volume to severely resorbed ridges 2,3,18 Outcomes have been comparable to those generally reported for autogenous block grafting, but without the need for a second surgery to harvest bone Clinical reports have documented the ability to stabilize implants 5 to 6 months after grafting 2,3,18 Retains osseoconductive properties due to the preservation of the natural bone matrix collagen and mineral composition, trabecular pattern and original porosity 2, Puros Block Allograft, 10 mm Puros Block Allograft, 15 mm Shelf-life: Five (5) years Puros Demineralized Bone Matrix (DBM) Putty And Putty With Chips This moldable putty comprises 100% demineralized allograft and is sterilized using the proprietary Cancelle SP DBM Sterilization Process. This process sterilizes DBM Putty and Putty with Chips while inactivating or removing bacteria, viruses, fungi and spores, but preserves the biological integrity and natural collagen structure of bone. Puros DBM Putty with Chips has both cancellous and cortical mineralized chips for osseoconductivity as well as osseoinductive potential.* Pliable putty maintains its form and resists migration in a fluid environment Ready-to-use moldable formulation offers excellent handling and time-saving convenience Every donor lot is tested for osseoconductivity (OI) potential* and inflammatory response in an in vivo ectopic rat assay after sterilization 22 Puros DBM Putty and Putty with Chips are terminally sterilized to SAL 10-6 using low-temperature, low-dose gamma irradiation, which has shown an impact on the OI score results in an in vivo rat assay 23 * Findings from an in vivo rat assay are not necessarily predictive of human clinical results Puros DBM Putty, 0.5 cc Puros DBM Putty, 1 cc Puros DBM Putty, 2 cc Puros DBM Putty with Chips, 0.5 cc Puros DBM Putty with Chips, 1 cc Puros DBM Putty with Chips, 2 cc Shelf-life: One (1) year
9 9 CopiOs Xenograft Particulates* Predictable Remodeling And Regeneration CopiOs Cancellous Particulate Xenografts are mineralized particulate cancellous bovine bone chips indicated for large and small bone defects 44,45 In small defects it has been reported into vital bone 44 During the remodeling process CopiOs Cancellous Particulate Xenografts act as an osseoconductive scaffold for new bone formation 44,46 Retains osseoconductive properties due to the preservation of the original bovine cancellous bone matrix collagen and mineral composition, trabecular pattern and original porosity 44,47 Biocompatable and well-tolerated by the host tissues in both animal and human studies 45,48 Alternative To Autogenous Bone CopiOs Cancellous Particulate Xenografts have been reported to be a viable alternative to autogenous bone grafts. 45,48 Tutoplast Process Sterilized and preserved using the proprietary Tutoplast process, CopiOs Cancellous Particulate Xenografts offers a high-quality option for successful bone regeneration. * Product only available in countries that honor the CE registration or have registration CopiOs Cancellous Particulate Xenograft, µm, 0.5 cc CopiOs Cancellous Particulate Xenograft, µm, 1 cc CopiOs Cancellous Particulate Xenograft, µm, 2 cc CopiOs Cancellous Particulate Xenograft, µm, 0.5 cc CopiOs Cancellous Particulate Xenograft, µm, 1 cc CopiOs Cancellous Particulate Xenograft, µm, 2 cc Shelf-life: Five (5) years Endobon Xenograft Granules Bovine-derived hydroxyapatite that has been fully deproteinated for safety An essentially non-resorbable material that is ideally suited for regeneration of defects when effective space maintenance is required Osseoconductive due to the interconnecting micro and macro pores for bony integration, which facilitate graft stability and vascular ingrowth Single-unit and value packs for sterility and value Endobon Xenograft Granules Are Indicated For Dental And/Or Oral Surgical Procedures, Such As: Filling defects after resection, cystectomy, apicoectomy or other defects in the alveolar ridge or wall Peri-implant defects Alveolar ridge augmentation, including aesthetic contouring defects Extraction socket grafting Sinus elevation ROX µm, 0.5 ml ROX µm, 1 ml ROX µm, 2 ml ROXLG µm, 2 ml ROXLG µm, 5 ml (5 1 ml each) ROXLG µm, 8 ml (8 1 ml each) Shelf-life: 18 months
10 Synthetic Bone Graft 10 NovaBone Dental Putty NovaBone Dental Putty is a next generation Calcium Phosphosilicate (CPS) Bone Graft substitute engineered for enhanced handling and uncompromised clinical performance. With a bimodal particle distribution of its active ingredient and using a Glycerin/PEG carrier, putty formulation allows for easier manipulation and demonstrates consistent resorption characteristics. Available in disposable uni-dose cartridges, the unique delivery system simplifies delivering the graft, thus facilitating minimally invasive techniques including hard-to-access defects such as gaps in immediate implant placement and crestal-approach sinus lifts. Cartridges are available in various sizes and are used in conjunction with NovaBone s cartridge dispenser. Each cartridge delivers 0.25 cc (Gray) to 0.5 cc (Blue) of putty. Putty is stable at room temperature, does not require refrigeration, has a four (4) year shelf-life, and appears radio-dense on radiographs. NovaBone Dental Putty Syringes - indicated for simple socket preservations and defects with easier access NovaBone Dental Putty Cartridges - indicated for minimally invasive surgeries including immediate implants and crestal sinus augmentation procedures Cartridge System EU cc Cartridges (Blue) 2 Pk EU cc Cartridges (Blue) 4 Pk EU cc Cartridges (Gray) 4 Pk EU4600 Cartridge Dispenser Shelf-life: Four (4) years Syringe Catalog Number EU1610 Description 0.5 cc Syringe 1 Pk
11 Soft-Tissue Grafts 11 Puros Dermis Allograft Tissue Matrix Ideal for aesthetic case requirements, Puros Dermis Allograft Tissue Matrix is a high-quality, natural, biocompatible matrix that is sterilized and preserved through the proprietary Tutoplast process to provide an easy-to-use, biocompatible, regenerative solution, for horizontal and vertical soft-tissue augmentation, 28,29 soft-tissue management and guided tissue regeneration procedures. Reduces morbidity and saves valuable chair time by eliminating the need to harvest an autogenous graft Provides an excellent healing environment and acts as a scaffold for the patient s own tissue to grow into and regenerate vital soft-tissue 28,29 Exhibits multi-directional strength 30 and exceptional adaptability to surface contours 49 Maintains space to allow for angiogenesis and tissue remodeling, and increases the volume of attached gingiva and connective tissue 28,29 Retains the natural collagen matrix, elastic content and mechanical properties of native dermis Easy-to-use with four convenient sizes and two different thicknesses can be cut to shape for specific surgical procedures Rehydrates in seconds, no refrigeration required Packaged sterile without residual antibiotics 8 Puros Dermis May Be Used In The Following: Both horizontal and vertical soft-tissue augmentation 28,29 Periodontal/peri-implant soft-tissue management Guided tissue regeneration procedures - Thin Puros Dermis Tissue Matrix, 10 x 10 mm, mm Puros Dermis Tissue Matrix, 10 x 20 mm, mm Puros Dermis Tissue Matrix, 10 x 40 mm, mm Puros Dermis Tissue Matrix, 20 x 40 mm, mm Shelf-life: Five (5) years - Thick Puros Dermis Tissue Matrix, 10 x 10 mm, mm Puros Dermis Tissue Matrix, 10 x 20 mm, mm Puros Dermis Tissue Matrix, 10 x 40 mm, mm Puros Dermis Tissue Matrix, 20 x 40 mm, mm Shelf-life: Five (5) years
12 Barrier Membranes 12 CopiOs Pericardium Membrane CopiOs Pericardium Membrane is made from bovine pericardium that provides a long-lasting, 32 conformable barrier strong 8 enough to meet most clinical needs and supple enough to adapt to challenging graft contours. Clinically demonstrated performance in guided bone regeneration procedures, 33,34 where ease of manipulation and adaptability to surface contours is essential Shown to provide a stable, long-lasting barrier during healing and integration of Puros Allografts, and staged or immediately placed implants 33,34 Supports an aesthetic soft-tissue response 33,34 through facilitation of cell attachment and proliferation and remodeling into vascularized, connective tissue 33,35 Retains the structure and composition of natural tissue due to the proprietary Tutoplast process, leading to optimal performance and handling 33, CopiOs Pericardium Membrane, 15 x 20 mm CopiOs Pericardium Membrane, 20 x 30 mm CopiOs Pericardium Membrane, 30 x 40 mm Shelf-life: Five (5) years Puros Pericardium Allograft Membrane Puros Pericardium Allograft Membrane provides a long-lasting 32 barrier when an optimum balance of strength and handling for graft containment are necessary. Retains the natural collagen matrix and mechanical properties of native pericardium due to the proprietary Tutoplast process Inhibits epithelial cell migration and maintains space for periodontal ligament and bone regeneration Puros Pericardium Membrane, 15 x 20 mm Puros Pericardium Membrane, 20 x 30 mm Puros Pericardium Membrane, 30 x 40 mm Shelf-life: Five (5) years
13 Barrier Membranes 13 Copios Extend Membrane CopiOs Extend Membrane is a long-lasting, resorbable collagen membrane designed to allow implant placement while providing ample time for regeneration. It conforms to the defect with enough structural integrity for space creation. CopiOs Extend Membrane lasts 6 to 9 months. Cell-Occlusive allows nutrients to permeate while occluding epithelial cells 43 Biocompatible highly purified, intact porcine dermis 43 Convenient handling conformable and easy to reposition in the defect Easy-to-Use supplied sterile and implantable dry or briefly hydrated 0190Z CopiOs Extend Membrane, 15 x 20 mm 0191Z CopiOs Extend Membrane, 20 x 30 mm 0192Z CopiOs Extend Membrane, 30 x 40 mm Shelf-life: Two (2) years Zimmer Socket Repair Membrane Zimmer Socket Repair Membrane is designed to assist wound healing in alveolar facial plate repair and residual ridge preservation following atraumatic, flapless single-root tooth extraction. Socket grafting can help to preserve bone volume for implant placement 36 The socket repair procedure is a flapless technique designed to preserve natural soft-tissue architecture and vascularity 36 Membrane is usually completely resorbed 26 to 38 weeks following surgery* 0154 Zimmer Socket Repair Membrane, 10 x 20 mm Shelf-life: Three (3) years * When not exposed, the resorption rate is 26 to 38 weeks; if left exposed, resorption time is shorter.
14 Wound Care 14 Zimmer Collagen Plug, Tape And Patch Highly porous, absorbable collagen wound dressings to help protect, heal and repair oral wounds. Protects Wound Bed adheres and provides coverage to oral wounds and sores Designed to Aid Healing porous, absorbable matrix supports delicate new tissue Versatile for Everyday Use three convenient shapes for common oral wounds and procedures Designed to resorb within a short timeframe Indicated for management of oral wounds and sores: - Denture sores - Oral ulcers (non-infected or viral) - Periodontal surgical wounds - Suture sites - Burns - Extraction sites - Surgical wounds - Traumatic wounds Zimmer Collagen Plug 10 mm x 20 mm Zimmer Collagen Tape 2.5 cm x 7.5 cm, 1.0 mm thick Zimmer Collagen Patch 2 cm x 4 cm, 3.0 mm thick 0100Z Zimmer Collagen Tape, 10 Pk 0101Z Zimmer Collagen Patch, 10 Pk 0102Z Zimmer Collagen Plug, 10 Pk Shelf-life: Three (3) years
15 Procedure Kits 15 Sinus Lift Solutions Sinus Crestal Approach Kit The Sinus Crestal Approach Kit is minimally invasive, designed for creating an osteotomy into the inferior cortical bone without tearing the Schneiderian membrane. S-reamer head has been designed with a special blade structure to leave a thin bone disk between membrane and reamer the reamer does not touch membrane directly Stoppers control the drilling depth of the S-reamer (10 pieces, 2 to 11 mm) and can also be mounted on the bone spreader and bone condenser S-reamer can be used for misaligned and septum cases Kit provides a complete set of tools for performing a crestal sinus lift SCAKIT Sinus Lift Kit, Crestal Approach Replacement parts also available. Sinus Lateral Approach Kit The Sinus Lateral Approach Kit provides the instruments and techniques for a sinus lift utilizing a lateral approach (modified Caldwell-Luc) procedure. Reamers designed to be used with conventional surgical motor handpiece at 2,000 rpm for quick entry into the sinus LS-reamer, used to create the lateral wall osteotomy, has been designed to help avoid arterial blood vessels Instruments provide method of opening the lateral wall near the crest Minimal flap and smaller window size compared to conventional techniques Designed to control drilling depth up to 3.5 mm without use of drill stops SLAKIT Sinus Lift Kit, Lateral Approach Replacement parts also available.
16 Procedure Kits 16 Screw Fixation Kit The Screw Fixation System provides a compact solution for the temporary fixation and stabilization of bone transplants, suitable resorbable and non-resorbable bone replacement materials, and membranes for the alveolar ridge. Two color coded systems in 1.5 mmd MICRO (BLUE) and 2.0 mmd MINI (RED) offer concise and cost effective functional options. The color coding scheme for the two systems, the components and the screws, makes easy and rapid identification of the parts possible and simplifies parts matching. This modular storage system permits individual configuration and the open design ensures access during cleaning and sterilization. Fixation screws and mesh are manufactured from pure titanium or titanium alloy. They are biocompatible, corrosionproof and non-toxic in the biological environment. They allow imaging virtually free of artifacts. Safescraper TWIST Bone Collector Unique design provides 160 cutting area to effectively harvest up to 5 cc of cortical bone and facilitates access to difficult posterior regions Bone is contained in a sterile chamber Lateral opening system provides device stability for easy retrieval of harvested bone Secure & Simple The Screw Fixation System comes with power grip for secure and stable transfer to the surgical site. The screws are easily picked up and have a reliable connection to the driver. Compact The screw cartridges of the Screw Fixation System are more than simply storage containers. The cartridges make organization and sterilization of screws easy. Screw Fixation Kit Start-Up Kit Ordering Information Contents Assembled Start-Up Kit* Components Tray Z Screw Driver Handle Z Screw Driver Insert, Short Z Z Screw Driver Insert, Long Z Pilot Drill, Micro, 14 mml Z Pilot Block Drill, Micro Z * Fixation screws are sold separately. Screws are available in 1.5 mm and 2.0 mm diameters Disposable Cortical Bone Collector, 3 Pk Straight 3987 Disposable Cortical Bone Collector, 3 Pk Curved Shelf-life: Three (3) years
17 Additional Regenerative Products
18 Freeze Dried Bone Grafts 18 RegenerOss Allograft Broad selection of cadaveric-derived bone from a comprehensive tissue bank that conducts screening, recovery, autopsies, processing and packaging for all donor tissue. Aseptically processed for maximum regenerative properties without destruction of the biological properties of the tissue. All donor tissues receive multiple screening and cultures to ensure that the tissues are pathogen-free. RMCA205 Cancellous, Mineralized 0.5 cc µm RMCO205 Cortical, Mineralized 0.5 cc µm RMCA305 Cancellous, Mineralized 0.5 cc µm RMCO305 Cortical, Mineralized 0.5 cc µm RMCA505 Cancellous, Mineralized 0.5 cc µm RMCO505 Cortical, Mineralized 0.5 cc µm RMCA210 Cancellous, Mineralized 1 cc µm RMCO210 Cortical, Mineralized 1 cc µm RMCA310 Cancellous, Mineralized 1 cc µm RMCO310 Cortical, Mineralized 1 cc µm RMCA510 Cancellous, Mineralized 1 cc µm RMCO510 Cortical, Mineralized 1 cc µm RMCA220 Cancellous, Mineralized 2 cc µm RMCO220 Cortical, Mineralized 2 cc µm RMCA320 Cancellous, Mineralized 2 cc µm RMCO320 Cortical, Mineralized 2 cc µm RMCA520 Cancellous, Mineralized 2 cc µm RMCO520 Cortical, Mineralized 2 cc µm RDCA205 Cancellous, Partially Demineralized 0.5 cc µm RDCO205 Cortical, Partially Demineralized 0.5 cc µm RDCA305 Cancellous, Partially Demineralized 0.5 cc µm RDCO305 Cortical, Partially Demineralized 0.5 cc µm RDCA505 Cancellous, Partially Demineralized 0.5 cc µm RDCO505 Cortical, Partially Demineralized 0.5 cc µm RDCA210 Cancellous, Partially Demineralized 1 cc µm RDCO210 Cortical, Partially Demineralized 1 cc µm RDCA310 Cancellous, Partially Demineralized 1 cc µm RDCO310 Cortical, Partially Demineralized 1 cc µm RDCA510 Cancellous, Partially Demineralized 1 cc µm RDCO510 Cortical, Partially Demineralized 1 cc µm RDCA220 Cancellous, Partially Demineralized 2 cc µm RDCO220 Cortical, Partially Demineralized 2 cc µm RDCA320 Cancellous, Partially Demineralized 2 cc µm RDCO320 Cortical, Partially Demineralized 2 cc µm RDCA520 Cancellous, Partially Demineralized 2 cc µm RDCO520 Cortical, Partially Demineralized 2 cc µm Shelf-life: Two (2) - Five (5) years depending on lot
19 DBM Putties 19 RegenerOss Allograft Putty Plus Mineralized Plant-based carrier (derived from soybeans with no residual soy proteins) Provides graft containment Highly resistant to irrigation Available in 3 volumes: 0.5 cc, 1 cc and 2 cc (Four 0.5 cc Syringes) Contains 48% by weight DBM (28% Cortical and 20% cancellous chips) for handling and bone regeneration Moldable, non-toxic, lecithin carrier that is highly resistant to irrigation Osseoinductivity of every lot is validated by a cell proliferation assay Ergonomic design features a smaller diameter syringe with a curved tip to treat hard-to-reach defects ROAPM cc Syringe ROAPM10 1 cc Syringe ROAPM20 2 cc Syringe (Four 0.5 cc syringes) Value Pack Shelf-life: Two (2) years
20 DBM Putties 20 RegenaVate Formable DBM RegenaVate Formable DBM allograft contains demineralized bone matrix (DBM) and mineralized cortical cancellous bone chips in a porcine gelatin carrier. The product is available in two forms - Room Temperature (RT) and Frozen - to meet clinician preference. Induces bone formation and facilitates bone growth* The DBM is tested for osseoinductivity* in a scientifically-proven in vivo rat assay Mineralized chips provide for osseoconductivity Unique DBM provides handling flexibility Clinician can control product consistency: gel, paste or putty Room Temperature Z RegenaVate Formable DBM, RT, 1 cc Z RegenaVate Formable DBM, RT, 2 cc Shelf-life: Two (2) years Frozen Z RegenaVate Formable DBM Block, 1 cm x 1 cm x 0.5 cm, 0.5 cc Z RegenaVate Formable DBM Block, 1 cm x 2 cm x 0.5 cm, 1 cc Z RegenaVate Formable DBM Block, 1 cm x 4 cm x 0.5 cm, 2 cc Shelf-life: Two (2) years RegenaVate DBM Fill RegenaVate DBM Fill contains demineralized bone matrix (DBM) in a porcine gelatin carrier. The DBM is Flowable at 45 C and is a resilient solid at body temperature. Packaged in convenient syringes for ease of use The DBM is tested for osseoconductivity* in a scientifically-proven in vivo rat assay Clinician can control product consistency: gel, paste or putty Flowable DBM provides unique handling flexibility * These implants were evaluated in a human clinical study and were shown to induce bone formation. Each lot is tested using the athymic nude rat assay to verify osseoconductivity potential. Room Temperature Z RegenaVate DBM Fill, 0.5 cc Z RegenaVate DBM Fill, 1 cc Shelf-life: Two (2) years
21 Synthetic Bone Graft Substitutes 21 IngeniOs HA Synthetic Bone Particles Long-lasting IngeniOs HA Synthetic Bone Particles are 100% non-biologic particles are made of pure-phase hydroxyapatite (HA), a composition similar to HA found in naturally-occurring bone. Long-lasting osseoconductive support with negligible resorption over time to help provide long-term graft stability and maintenance of volume and aesthetic contours Up to 80% interconnected porosity allowing for vascularized bone formation, osseointegration and the natural remodeling process to occur within the graft framework 24 Radiopacity of material making it easy to identify on an X-ray Can be used as a bone graft extender to provide radiopacity or long-term volume preservation IngeniOs HA Synthetic Bone Particles, 0.25 cc, μm IngeniOs HA Synthetic Bone Particles, 0.5 cc, μm IngeniOs HA Synthetic Bone Particles, 1 cc, μm IngeniOs HA Synthetic Bone Particles, 2 cc, μm IngeniOs HA Synthetic Bone Particles, 0.5 cc, μm IngeniOs HA Synthetic Bone Particles, 1 cc, μm IngeniOs HA Synthetic Bone Particles, 2 cc, μm Shelf-life: Five (5) years IngeniOs ß-TCP Bioactive Synthetic Bone Particles Resorbable IngeniOs ß-TCP Bioactive Synthetic Bone Particles are 100% non-biologic particles are made of pure-phase beta tricalcium phosphate (ß-TCP) that is silicated, providing the potential for increased bioactivity. 24,25 Fully resorbable, non-biologic particles designed to resorb in balance with replacement by naturally-regenerating mineralized bone Up to 75% interconnected porosity designed to enable ingrowth of healthy bone tissue 24 Radiopacity of material making it easy to identify on an X-ray Can be used as a bone graft extender to extend volume or add radiopacity IngeniOs ß -TCP Bioactive Synthetic Bone Particles, 0.25 cc, μm IngeniOs ß -TCP Bioactive Synthetic Bone Particles, 0.5 cc, μm IngeniOs ß -TCP Bioactive Synthetic Bone Particles, 1 cc, μm IngeniOs ß -TCP Bioactive Synthetic Bone Particles, 2 cc, μm IngeniOs ß -TCP Bioactive Synthetic Bone Particles, 0.5 cc, μm IngeniOs ß -TCP Bioactive Synthetic Bone Particles, 1 cc, μm IngeniOs ß -TCP Bioactive Synthetic Bone Particles, 2 cc, μm Shelf-life: Five (5) years
22 Barrier Membranes 22 BioMend And BioMend Extend Resorbable Collagen Membranes Resorbable membranes that are rigid enough to create and maintain space. BioMend Membrane is resorbed in approximately eight (8) weeks. 30 BioMend Extend Membrane is resorbed in approximately 18 weeks. 30 Resorbable 30 eliminates second-stage surgery for membrane removal, reducing wound trauma and surgical chair time 30 Cell-Occlusive serves as barrier to prevent epithelial cell migration and allows passage of essential nutrients 30 Space-Maintaining provides rigid scaffold for tissue regeneration in GTR and GBR procedures 30 Excellent Handling tear resistant, suturable, pliable; easy to handle even when hydrated; conforms to defect morphology 38 BioMend Membrane 0103Z Resorbable Collagen Membrane 15 mm x 20 mm 0105Z Resorbable Collagen Membrane 20 mm x 30 mm 0107Z Resorbable Collagen Membrane 30 mm x 40 mm Shelf-life: Three (3) years BioMend Extend Membrane 0140Z Resorbable Collagen Membrane 15 mm x 20 mm 0141Z Resorbable Collagen Membrane 20 mm x 30 mm 0142Z Resorbable Collagen Membrane 30 mm x 40 mm Shelf-life: Three (3) years OsseoGuard And OsseoGuard Flex Resorbable Collagen Membranes OsseoGuard Resorbable collagen membranes made with highly purified, bovine-derived collagen sourced from safe sources Resorption profile of both membranes long enough to be well suited for GBR procedures Two different levels of drapability so that you can choose the membrane that best meets your needs OsseoGuard Membrane OG1520 Resorbable Collagen Membrane 15 mm x 20 mm OG2030 Resorbable Collagen Membrane 20 mm x 30 mm OG3040 Resorbable Collagen Membrane 30 mm x 40 mm Shelf-life: Three (3) years OsseoGuard Flex OsseoGuard Flex Membrane OGF1520 Resorbable Collagen Membrane 15 mm x 20 mm OGF2030 Resorbable Collagen Membrane 20 mm x 30 mm OGF3040 Resorbable Collagen Membrane 30 mm x 40 mm Shelf-life: Three (3) years
23 References 23 1 Tsao YP, Neiva R, Al-Shammari K, Oh TJ, Wang HL. Effects of a mineralized human cancellous bone allograft in regeneration of mandibular Class II furcation defects. J Periodontol. 2006;77: Keith JD Jr, Petrungaro P, Leonetti JA, Elwell CW Jr, Zeren KJ, Caputo C, et al. Clinical and histologic evaluation of a mineralized block allograft: results from the developmental period ( ). Int J Periodontics Restorative Dent. 2006;26: Leonetti JA, Koup R. Localized maxillary ridge augmentation with a block allograft for dental implant placement: case reports. Implant Dent. 2003;12: Froum SJ, Wallace SS, Elian N, Cho SC, Tarnow DP. Comparison of mineralized cancellous bone allograft (Puros) and anorganic bovine bone matrix (Bio-Oss) for sinus augmentation: histomorphometry at 26 to 32 weeks after grafting. Int J Periodontics Restorative Dent. 2006;26: Noumbissi SS, Lozada JL, Boyne PJ, Rohrer MD, Clem D, Kim JS, Prasad H. Clinical, histologic, and histomorphometric evaluation of mineralized solvent-dehydrated bone allograft (Puros) in human maxillary sinus grafts. J Oral Implantol. 2005;31: Block MS, Finger I, Lytle R. Human mineralized bone in extraction sites before implant placement. Preliminary results. J Amer Dent Assoc. 2002;133: Minichetti JC, D Amore JC, Hong AYJ, Cleveland DB. Human histologic analysis of mineralized bone allograft (Puros) placement before implant surgery. J Oral Implantol. 2004;30: Data on file with RTI Surgical, Inc. 9 Davi E, Aslan M, Simsek G, Yilmaz AB. The effects of bone chips dehydrated with surgical solvent on healing bone defects. JIMR. 2002;30: Le B, Rohrer MD, Prassad HS. Screw tent-pole grafting technique for reconstruction of large vertical alveolar ridge defects using human mineralized allograft for implant site preparation. J Oral Maxillofac Surg. 68: , Block MS, Degen M. Horizontal ridge augmentation using human mineralized particulate bone: preliminary results. J Oral Maxillofac Surg. 2004;62(Suppl 2): Le B, Burstein J, Sedghizadeh PP. Cortical tenting grafting technique in the severely atrophic alveolar ridge for implant site development. Implant Dent. 2008;17: Wang HL, Boyapati L. PASS principles for predictable bone regeneration. Implant Dent. 2006;15: Park SH, Wang HL. Management of localized buccal dehiscence defect with allografts and acellular dermal matrix. Int J Periodontics Restorative Dent. 2006;26: Park SH, Wang HL. Mucogingival pouch flap for sandwich bone augmentation: technique and rationale. Implant Dent. 2005;14: Schlegel KA, Schultze-Mosgau S, Wiltfang J, Neukam FW, Rupprecht S, Thorwarth M. Changes in mineralization of free autogenous bone grafts used for sinus floor elevation. Clin Oral Implants Res. 2006;17: Rubio de Rezende ML, Nasciemento de Melo LG, Hamata MM, Monteiro-Amado F. Particulate inlay nasal graft with immediate dental implant placement in a patient with repaired alveolar cleft; case report. Implant Dent. 2008;17: Keith JD Jr, Salama MA. Ridge preservation and augmentation using regenerative materials to enhance implant predictability and esthetics. Compend Contin Educ Dent. 2007;28: Schwartz-Arad D, Levin L, Sigal L. Surgical success of intraoral autogenous block onlay bone grafting for alveolar ridge augmentation. Implant Dent. 2005;14: Levin L, Nitzan D, Schwartz-Arad D. Success of dental implants placed in intraoral block bone grafts. J Periodontol. 2007;78: von Arx T, Buser D. Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: a clinical study with 42 patients. Clin Oral Implants Res. 2006;17: Urist MR. Bone: Formation by autoinduction. Science. 1965;150: Effect of terminal gamma sterilization on osteoinductivity. White paper available from RTI Surgical, Inc. 24 Data on file with curasan AG. 25 Pietak AM, Reid JW, Stott MJ, Sayer M. Silicon substitution in the calcium phosphate bioceramics. Biomaterial. 28 (2008) C. Knabe, P. Ducheyne. Chapter 6 Cellular response to bioactive ceramics, In: Handbook of Bioceramics and their Applications. Ed: Prof. Dr. Tadashi Kokubo, Woodhead Publishing Inc., Cambridge, UK, 2008, p Greenspan DC, Hernandez R, Faleris J. Histology of surgically implanted Tutoplast processed dermis; RTI Surgical, Inc. 28 Petrungaro P. Correction of iatrogenic gingival recession in the esthetic zone. Inside Dentistry. 2007;11: Petrungaro P. Acellular dermal matrix tissue grafts. Inside Dentistry. 2010;6: Li ST, Chen HC, Lee NS, Ringshia R, Yuen D. A Comparative Study of Zimmer BioMend and BioMend Extend Membranes Made at Two Different Manufacturing Facilities. Zimmer Dental White Paper: Wang HL, Carrol WJ. Guided bone regeneration using bone grafts and collagen membranes. Implant Dent. 2001;32(7): Rothamel D, Schwarz F, Sager M, Herten M, Sculean A, Becker J. Biodegradation of differently cross-linked collagen membranes: an experimental study in the rat. Clin Oral Implants Res. 2005;16(3): Kistler S, Bayer G, Kistler F, Am Lech L. Experience with the biological Tutodent membrane in implant practice. Implantologie Zeitung Journal. 2004;8(7): Simsek B, Simsek S. Evaluation of success rates of immediate and delayed implants after tooth extraction. Chinese Medical Journal. 2003;116(8): Steigmann M. Pericardium membrane and xenograft particulate grafting materials for horizontal alveolar ridge defects. Implant Dent. 2006;15: Sclar AG. Ridge preservation for optimum esthetics and function: the Bio-Col technique. Postgraduate Dentistry. 1999;6: Elian N, Cho SC, Froum S, Smith RB, Tarnow DP. A simplified socket classification and repair technique. Pract Proced Aesthet Dent. 2007;19:99-104, quiz Data on file with Collagen Matrix, Inc. 39 Yuen D, Junchaya C, Zuclich G, Ulreich JB, Lin H, Li S. A resorbable, reconstituted, type I collagen membrane for guided tissue regeneration and soft tissue augmentation. Society for Biomaterials, Sixth World Biomaterials Congress Transactions, p. 1288, Bunyaratavei P, Wang HL Collagen membranes: a review. J Periodoritol. 2001;7 2: Wallace SS, Mazor Z, Froum SJ, Cho SC, Tamow DP. Schneiderian membrane perforation rate during sinus elevation using piezosurgery: clinical results of 100 consecutive cases. IntJ Periodontics Restorative Dent. 2007;27: Wang HL, Shotwell JL, ltose T, Neiva RF. Multidisciplinary treatment approach for enhancement of implant esthetics. lmplant Dent. 2005;14: Data on file with Collagen Matrix, Inc. 44 Tudor C. Srour S, Thorwarth M, Wehrhan F, Stockmann P, Neukam FW et al. Bone regeneration in osseous defects application of particulated human bovine materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105: Instructions for Use. 46 Trentz OA, Hoerstrup SP, Sun LK, Bestmann L, Platz A, Trentz OL. Osteoblasts response to allogenic and xenogenic solvent dehydrated cancellous bone in vitro. Biomaterials. 2003;24: Tadic D, Epple M. A thorough physicochemical investigation of 14 calcium phosphate based bone substitution materials in comparison to natural bone. Biomaterials. 2004;25: Ploger M, Wolf HK, Schau I, von der Haar A. Rekonstrucktion and Augmentation mittels eines kortikospongiösen Tutodent CS Blocks. BDIZ Konkret. 2005;2: Onur R, Singla A. Solvent-dehydrated cadaveric dermis: a new allograft for pubovaginal sling surgery. J Urol. 2005:12:
24 Contact us at or visit zimmerbiometdental.com Zimmer Biomet Dental Global Headquarters 4555 Riverside Drive Palm Beach Gardens, FL Tel: Fax: Unless otherwise indicated, as referenced herein, all trademarks are the property of Zimmer Biomet; and all products are manufactured by one or more of the dental subsidiaries of Zimmer Biomet Holdings, Inc., and distributed and marketed by Zimmer Biomet Dental (and, in the case of distribution and marketing, its authorized marketing partners). Cancelle SP is a registered trademark of RTI Surgical, Inc. Tutoplast is a registered trademark of Tutogen Medical GmbH. Safescraper is a trademark of C.G.M. S.P.A. NovaBone is a registered trademark of NovaBone Products, LLC. BioMend, BioMend Extend, CopiOs, CopiOs Extend, OsseoGuard, OsseoGuard Flex, and Socket Repair Membranes are manufactured by Collagen Matrix, Inc. IngeniOs products are manufactured by Curasan AG. Safescraper is manufactured by META Advanced Medical Technology. NovaBone Dental Putty is manufactured by NovaBone Products, LLC. Puros is manufactured by RTI Surgical, Inc. CopiOs Xenograft and CopiOs Pericardium are manufactured by Tutogen Medical GmbH. RegenaVate is manufactured by RTI Surgical, Inc. RegenePro is manufactured by Syntacoll GmbH. RegenerOss Allograft Putty Plus is manufactured by Interpore Cross International with tissue provided by LifeLink Tissue Bank. (AATB Certified). Screw Fixation Kits are manufactured by Medicon e.g. Endobon is manufactured by Biomet France, Sarl. RegenerOss Allograft Particulate products are distributed by Zimmer Biomet Dental and processed by Community Tissue Services. Prior to August, 2016, distinct lots of RegenerOss Allograft Particulate products were processed by University of Miami Tissue Bank (UMTB). Orders may be fulfilled with product originating from either or both tissue establishment until depletion of UMTB stock. For additional product information, please refer to the individual product labeling or instructions for use. Product clearance and availability may be limited to certain countries/regions. This material is intended for clinicians only and does not comprise medical advice or recommendations. This material may not be copied or reprinted without the express written consent of Zimmer Biomet. ZB0011 REV A 11/ Zimmer Biomet. All rights reserved.
Puros Cancellous Particulate Allograft & Puros Block Allograft
 Puros Cancellous Particulate Allograft & Puros Block Allograft Puros Cancellous Particulate Allograft The Natural Choice For Healthy 1 Bone Growth. 1. Proven, Predictable Regeneration Acts as an osteoconductive
Puros Cancellous Particulate Allograft & Puros Block Allograft Puros Cancellous Particulate Allograft The Natural Choice For Healthy 1 Bone Growth. 1. Proven, Predictable Regeneration Acts as an osteoconductive
Puros Cancellous Particulate Allograft & Puros Block Allograft
 Puros Cancellous Particulate Allograft & Puros Block Allograft Puros Cancellous Particulate Allograft The Natural Choice For Healthy 1 Bone Growth. 1. Proven, Predictable Regeneration Acts as an osteoconductive
Puros Cancellous Particulate Allograft & Puros Block Allograft Puros Cancellous Particulate Allograft The Natural Choice For Healthy 1 Bone Growth. 1. Proven, Predictable Regeneration Acts as an osteoconductive
REGENERATION BY DESIGN
 REGENERATION BY DESIGN ALLOGRAFT, XENOGRAFT, PUTT Y & BIOACTIVE GLASS FOR BONE REGENERATION INSTRUMENTS FOR COLLECTING BONE BARRIER MEMBRANES FOR GUIDED REGENERATION GUIDED IMPLANT TREATMENT Wide Selection
REGENERATION BY DESIGN ALLOGRAFT, XENOGRAFT, PUTT Y & BIOACTIVE GLASS FOR BONE REGENERATION INSTRUMENTS FOR COLLECTING BONE BARRIER MEMBRANES FOR GUIDED REGENERATION GUIDED IMPLANT TREATMENT Wide Selection
An ever-expanding range of trusted clinical solutions
 Regenerative ortfolio An ever-expanding range of trusted clinical solutions TABLE OF CONTENTS Introduction 4 uros Allografts and the Tutoplast Tissue Sterilization rocess 6 Hard Tissue Augmentation 10
Regenerative ortfolio An ever-expanding range of trusted clinical solutions TABLE OF CONTENTS Introduction 4 uros Allografts and the Tutoplast Tissue Sterilization rocess 6 Hard Tissue Augmentation 10
More than bone regeneration. A total solution.
 More than bone regeneration. A total solution. More than a dental implant company. A total solution. When it comes to treatment options, your patients want positive results both functionally and esthetically.
More than bone regeneration. A total solution. More than a dental implant company. A total solution. When it comes to treatment options, your patients want positive results both functionally and esthetically.
Versatile grafting Solutions
 Versatile grafting Solutions A Canadian company serving Canadian dentists since 1997 Here s why your colleagues are calling us for their bone regeneration needs Founded in 1997 Citagenix has been providing
Versatile grafting Solutions A Canadian company serving Canadian dentists since 1997 Here s why your colleagues are calling us for their bone regeneration needs Founded in 1997 Citagenix has been providing
BONE AUGMENTATION AND GRAFTING
 1 A Computer-Guided Bone Block Harvesting Procedure: A Proof-of-Principle Case Report and Technical Notes Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review
1 A Computer-Guided Bone Block Harvesting Procedure: A Proof-of-Principle Case Report and Technical Notes Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review
Innovative Range of Regenerative Solutions
 TM Innovative Range of Regenerative Solutions MIS Implant Technologies Ltd. All rights reserved. Optimal volumes and quality of hard and soft tissue are required to satisfy the goals of oral rehabilitation
TM Innovative Range of Regenerative Solutions MIS Implant Technologies Ltd. All rights reserved. Optimal volumes and quality of hard and soft tissue are required to satisfy the goals of oral rehabilitation
A WIDE RANGE OF REGENERATIVE SOLUTIONS
 A WIDE RANGE OF REGENERATIVE SOLUTIONS INDICATIONS: 1/ SOCKET AND RIDGE PRESERVATION 2/ FILLING OF EXTRACTION SOCKETS Biomaterials offers portfolio of regenerative materials for implantology, aimed at
A WIDE RANGE OF REGENERATIVE SOLUTIONS INDICATIONS: 1/ SOCKET AND RIDGE PRESERVATION 2/ FILLING OF EXTRACTION SOCKETS Biomaterials offers portfolio of regenerative materials for implantology, aimed at
Biomaterials Line. MIS Corporation. All Rights Reserved.
 7. Biomaterials Line MIS Corporation. All Rights Reserved. 6. MIS s Quality System complies with international quality standards: ISO 13485: 2003 - Quality Management System for Medical Devices, ISO 9001:
7. Biomaterials Line MIS Corporation. All Rights Reserved. 6. MIS s Quality System complies with international quality standards: ISO 13485: 2003 - Quality Management System for Medical Devices, ISO 9001:
Case Study. Case # 1 Author: Dr. Suheil Boutros (USA) 2013 Zimmer Dental, Inc. All rights reserved. 6557, Rev. 03/13.
 Placement of a Zimmer Trabecular Metal Dental Implant with Simultaneous Ridge Augmentation and Immediate Non-Functional Loading Following Tooth Extraction and Orthodontic Treatment for Implant Site Development
Placement of a Zimmer Trabecular Metal Dental Implant with Simultaneous Ridge Augmentation and Immediate Non-Functional Loading Following Tooth Extraction and Orthodontic Treatment for Implant Site Development
Bringing you Geistlich biocompatibility with improved application and handling benefits. Your combination for success
 Bringing you Geistlich biocompatibility with improved application and handling benefits Your combination for success Geistlich Combi-Kit Collagen: Combining ease and predictablility Geistlich Combi-Kit
Bringing you Geistlich biocompatibility with improved application and handling benefits Your combination for success Geistlich Combi-Kit Collagen: Combining ease and predictablility Geistlich Combi-Kit
FDBA(Freeze Dried Bone Allograft) SureOss Cortical Bone - Powder / Chip. OsteOss Cortical with Cancellous Bone - Powder / Chip
 PERIODONTAL Bone & Skin Allograft Products FDBA(Freeze Dried Bone Allograft) SureOss - Powder / Chip OsteOss Cortical with - Powder / Chip DFDBA(Demineralized Freeze Dried Bone Allograft) SureOss -D Demineralized
PERIODONTAL Bone & Skin Allograft Products FDBA(Freeze Dried Bone Allograft) SureOss - Powder / Chip OsteOss Cortical with - Powder / Chip DFDBA(Demineralized Freeze Dried Bone Allograft) SureOss -D Demineralized
BioVin Collagen Membrane
 BioVin Collagen Membrane Resorbable cross-linked collagen membrane BioVin Bovine Bone Bovine Bone Substitute OToss Synthetic Bone Synthetic Resorbable Biphasic Calcium Phosphate OToss Synthetic Bone Inject
BioVin Collagen Membrane Resorbable cross-linked collagen membrane BioVin Bovine Bone Bovine Bone Substitute OToss Synthetic Bone Synthetic Resorbable Biphasic Calcium Phosphate OToss Synthetic Bone Inject
The Original remains unique.
 The Original remains unique. Geistlich leading regeneration 2A, 2B Geistlich is the world leader in regenerative dentistry. We transform natural biomaterials into safe and reliable treatment methods that
The Original remains unique. Geistlich leading regeneration 2A, 2B Geistlich is the world leader in regenerative dentistry. We transform natural biomaterials into safe and reliable treatment methods that
botiss biomaterials bone & tissue regeneration collacone max Innovative composite matrix socket preservation form-fitting resorbable composite
 bone & tissue regeneration botiss biomaterials socket preservation Innovative composite matrix form-fitting resorbable composite 1 Socket preservation safeguarding your sockets collacone.. max flexbone*
bone & tissue regeneration botiss biomaterials socket preservation Innovative composite matrix form-fitting resorbable composite 1 Socket preservation safeguarding your sockets collacone.. max flexbone*
botiss dental bone & tissue regeneration biomaterials collacone max Innovative composite matrix socket preservation form-fitting resorbable composite
 dental bone & tissue regeneration botiss biomaterials socket preservation Innovative composite matrix form-fitting resorbable composite 1 botiss regeneration system maxresorb flexbone* collacone.. max
dental bone & tissue regeneration botiss biomaterials socket preservation Innovative composite matrix form-fitting resorbable composite 1 botiss regeneration system maxresorb flexbone* collacone.. max
Cerasorb M DENTAL. O:\Zulassung\Cerasorb Dental Kanada 2013\Texte\Cerasorb M Dental final IFU docx
 Cerasorb M DENTAL Resorbable, pure-phase beta-tricalcium phosphate matrix with interconnecting porosity for bone regeneration for use in dental and maxillofacial surgery DESCRIPTION: Cerasorb M DENTAL
Cerasorb M DENTAL Resorbable, pure-phase beta-tricalcium phosphate matrix with interconnecting porosity for bone regeneration for use in dental and maxillofacial surgery DESCRIPTION: Cerasorb M DENTAL
REGENERATIONTIME. A Case Report by. Ridge Augmentation and Delayed Implant Placement on an Upper Lateral Incisor
 A Case Report by Dr. Daniele Cardaropoli Ridge Augmentation and Delayed Implant Placement on an Upper Lateral Incisor The Situation An adult female patient presented with an endodontic/prosthetic failure
A Case Report by Dr. Daniele Cardaropoli Ridge Augmentation and Delayed Implant Placement on an Upper Lateral Incisor The Situation An adult female patient presented with an endodontic/prosthetic failure
Guided Tissue and Bone Regeneration
 Guided Tissue and Bone Regeneration One toolbox for your needs! Tefguide 9000 742 401 12x24 mm pc 1 Osgide 9000 701 520 15 x 20 mm pc 1 Osbone 9000 800 255 250-1000 μm 0.25 cc 5 Product Specifications
Guided Tissue and Bone Regeneration One toolbox for your needs! Tefguide 9000 742 401 12x24 mm pc 1 Osgide 9000 701 520 15 x 20 mm pc 1 Osbone 9000 800 255 250-1000 μm 0.25 cc 5 Product Specifications
Citagenix Catalogue 2017 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES
 Citagenix Catalogue 2017 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES 100% Allograft Hydratable Inductive Matrix NEW Create your customized osteoinductive graft by combining
Citagenix Catalogue 2017 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES 100% Allograft Hydratable Inductive Matrix NEW Create your customized osteoinductive graft by combining
Scientific & Clinical Evidence Jason membrane
 Scientific & Clinical Evidence Jason membrane Pericardium GBR/GTR Membrane Facts - CE since 2009 - so far no serious clinical complication or objection - approx. 250.000 successful clinical treatments
Scientific & Clinical Evidence Jason membrane Pericardium GBR/GTR Membrane Facts - CE since 2009 - so far no serious clinical complication or objection - approx. 250.000 successful clinical treatments
THE NEW STANDARD OF EXCELLENCE IN BIOMATERIALS. Collagenated heterologous cortico-cancellous bone mix + TSV Gel GTO I N S P I R E D B Y N A T U R E
 GTO THE NEW STANDARD OF EXCELLENCE IN BIOMATERIALS Collagenated heterologous cortico-cancellous bone mix + TSV Gel R E G E N E R A T I O N S C I E N C E I N S P I R E D B Y N A T U R E A unique biotechnology
GTO THE NEW STANDARD OF EXCELLENCE IN BIOMATERIALS Collagenated heterologous cortico-cancellous bone mix + TSV Gel R E G E N E R A T I O N S C I E N C E I N S P I R E D B Y N A T U R E A unique biotechnology
Symbios Xenograft Granules Porcine Bone Graft Material
 Symbios Xenograft Granules Porcine Bone Graft Material 32671122-USX-1607_Symbios Highlight Brochure.indd 1 2016-09-27 12:12 Symbios Xenograft Granules Porcine bone graft material Symbios Xenograft Granules
Symbios Xenograft Granules Porcine Bone Graft Material 32671122-USX-1607_Symbios Highlight Brochure.indd 1 2016-09-27 12:12 Symbios Xenograft Granules Porcine bone graft material Symbios Xenograft Granules
Procedure Manual and Catalog
 Procedure Manual and Catalog TM Why SynthoGraft? SynthoGraft offers a unique structure which provides stability, while its micro-porosity allows for rapid vascularization and subsequent resorption. Although
Procedure Manual and Catalog TM Why SynthoGraft? SynthoGraft offers a unique structure which provides stability, while its micro-porosity allows for rapid vascularization and subsequent resorption. Although
DRIVING THE FUTURE. About HOSPITAL INNOVATIONS. Strong commitment to healthcare innovation
 idente Catalogue About HOSPITAL INNOVATIONS Since 2008, Hospital Innovations has been supplying a growing range of specialist products for use in orthopaedic and corrective surgery; with an emphasis on
idente Catalogue About HOSPITAL INNOVATIONS Since 2008, Hospital Innovations has been supplying a growing range of specialist products for use in orthopaedic and corrective surgery; with an emphasis on
BEGO BIOMATERIALS When the result counts
 BEGO BIOMATERIALS When the result counts Partners in Progress INTRO Challenge what exists get the right answers We practise systematic thinking with a passion and we are never satisfied with the status
BEGO BIOMATERIALS When the result counts Partners in Progress INTRO Challenge what exists get the right answers We practise systematic thinking with a passion and we are never satisfied with the status
Bone augmentation with biomaterials
 Patient information dental bone & tissue regeneration botiss biomaterials Bone augmentation with biomaterials established safe natural X100 Implantation stability is crucial for success Atrophy of the
Patient information dental bone & tissue regeneration botiss biomaterials Bone augmentation with biomaterials established safe natural X100 Implantation stability is crucial for success Atrophy of the
REASONS TO USE R.T.R.
 3 REASONS TO USE R.T.R. AFTER EACH EXTRACTION Fully resorbable ß-TCP material RTR 3raisons 120x280.indd 1 16/06/15 10:52 1AVOID SPONTANEOUS RIDGE RESORPTION After tooth extraction, spontaneous healing
3 REASONS TO USE R.T.R. AFTER EACH EXTRACTION Fully resorbable ß-TCP material RTR 3raisons 120x280.indd 1 16/06/15 10:52 1AVOID SPONTANEOUS RIDGE RESORPTION After tooth extraction, spontaneous healing
Alpha-Bio's GRAFT complete portfolio bone substitutes resorbable membranes best combination of quality & value with efficient, easy-to-use products
 Alpha-Bio Tec, a world leader in advanced implantology solutions, is proud to present its growing biomaterials product line: Alpha-Bio's GRAFT, which has taken a leading position in the global dental biomaterials
Alpha-Bio Tec, a world leader in advanced implantology solutions, is proud to present its growing biomaterials product line: Alpha-Bio's GRAFT, which has taken a leading position in the global dental biomaterials
Inion BioRestore. Bone Graft Substitute. Product Overview
 Inion BioRestore Bone Graft Substitute Product Overview Inion BioRestore Introduction Inion BioRestore is a synthetic bone graft substitute, which remodels into bone and is easy to use. Inion BioRestore
Inion BioRestore Bone Graft Substitute Product Overview Inion BioRestore Introduction Inion BioRestore is a synthetic bone graft substitute, which remodels into bone and is easy to use. Inion BioRestore
The Essential Choice. With the Benefits of Biologic Predictability
 The Essential Choice With the Benefits of Biologic Predictability Documented, Reliable, Experienced is the essential choice for your daily regenerative needs. Throughout our long history and dedication
The Essential Choice With the Benefits of Biologic Predictability Documented, Reliable, Experienced is the essential choice for your daily regenerative needs. Throughout our long history and dedication
The Essential Choice. With the Benefits of Biologic Predictability
 The Essential Choice With the Benefits of Biologic Predictability Documented, Reliable, Experienced is the essential choice for your daily regenerative needs. Throughout our long history and dedication
The Essential Choice With the Benefits of Biologic Predictability Documented, Reliable, Experienced is the essential choice for your daily regenerative needs. Throughout our long history and dedication
Product Catalog. Dental Bone & Tissue Regeneration
 Product Catalog Dental Bone & Tissue Regeneration Bone graft regeneration Guided bone substitutes regeneration Osteogenesis Controlled resorption rate Angiogenesis Long term volume stability Remodeling
Product Catalog Dental Bone & Tissue Regeneration Bone graft regeneration Guided bone substitutes regeneration Osteogenesis Controlled resorption rate Angiogenesis Long term volume stability Remodeling
international biologics product catalog
 international biologics product catalog 99.2% average implant success rate 1 BioHorizons is committed to developing evidence-based and scientifically-proven products. This commitment started with the launch
international biologics product catalog 99.2% average implant success rate 1 BioHorizons is committed to developing evidence-based and scientifically-proven products. This commitment started with the launch
RPC. Surgical Solutions USA Regenerative Product Catalog
 RPC Surgical Solutions USA Regenerative Product Catalog RESORBABLE COLLAGEN MEMBRANES (BOVINE) CYTOPLAST RTM Collagen Resorption time of 26-38 weeks RTM1520 $185 15mm x 20mm Membranes per box: 2 RTM2030
RPC Surgical Solutions USA Regenerative Product Catalog RESORBABLE COLLAGEN MEMBRANES (BOVINE) CYTOPLAST RTM Collagen Resorption time of 26-38 weeks RTM1520 $185 15mm x 20mm Membranes per box: 2 RTM2030
Surgical Solutions USA Regenerative Product Catalog USA
 RPC Surgical Solutions Regenerative Product Catalog RESORBABLE COLLAGEN MEMBRANES (BOVINE) CYTOPLAST RTM Collagen Resorption time of 26-38 weeks RTM1520 $185 15mm x 20mm Membranes per box: 2 RTM2030 $230
RPC Surgical Solutions Regenerative Product Catalog RESORBABLE COLLAGEN MEMBRANES (BOVINE) CYTOPLAST RTM Collagen Resorption time of 26-38 weeks RTM1520 $185 15mm x 20mm Membranes per box: 2 RTM2030 $230
HOSPITAL INNOVATIONS
 idente Catalogue About HOSPITAL INNOVATIONS Since 2008, Hospital Innovations has been supplying a growing range of specialist products for use in orthopaedic and corrective surgery; with an emphasis on
idente Catalogue About HOSPITAL INNOVATIONS Since 2008, Hospital Innovations has been supplying a growing range of specialist products for use in orthopaedic and corrective surgery; with an emphasis on
Don t Let Life Pass You By Because Of Oral Bone Loss
 Don t Let Life Pass You By Because Of Oral Bone Loss Ask For Dental Implant Solutions From BIOMET 3i Scan With Your Smartphone! In order to scan QR codes, your mobile device must have a QR code reader
Don t Let Life Pass You By Because Of Oral Bone Loss Ask For Dental Implant Solutions From BIOMET 3i Scan With Your Smartphone! In order to scan QR codes, your mobile device must have a QR code reader
DEMINERALIZED BONE MATRIX
 DEMINERALIZED BONE MATRIX DEMINERALIZED BONE MATRIX GRAFT OVERVIEW BioAdapt is a pre-formed demineralized bone matrix (DBM) comprised of 00 percent donated human musculoskeletal tissue. BioAdapt DBM provides
DEMINERALIZED BONE MATRIX DEMINERALIZED BONE MATRIX GRAFT OVERVIEW BioAdapt is a pre-formed demineralized bone matrix (DBM) comprised of 00 percent donated human musculoskeletal tissue. BioAdapt DBM provides
Case Report. RapidSorb Rapid Resorbable Fixation System. Ridge augmentation in a one-step surgical protocol.
 Case Report RapidSorb Rapid Resorbable Fixation System. Ridge augmentation in a one-step surgical protocol. RapidSorb Rapid Resorbable Fixation System. Ridge augmentation in a one-step surgical protocol.
Case Report RapidSorb Rapid Resorbable Fixation System. Ridge augmentation in a one-step surgical protocol. RapidSorb Rapid Resorbable Fixation System. Ridge augmentation in a one-step surgical protocol.
Bone & Membrane. Catalogue Biologic Regenerative Products ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES
 Bone & Membrane Biologic Regenerative Products Catalogue 2016 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES PTFE Surgical Sutures Bone & Membrane Biologic Regenerative Products
Bone & Membrane Biologic Regenerative Products Catalogue 2016 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES PTFE Surgical Sutures Bone & Membrane Biologic Regenerative Products
Pre op Failed endodontic treatment with sinus involvement.
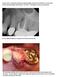 Case #1 of 10 consecutive extraction sockets grafted with Socket Graft Putty, covered with Socket Seal and sealed with Periacryl. I D # HEU This patient is a 66 year old female. Pre op Failed endodontic
Case #1 of 10 consecutive extraction sockets grafted with Socket Graft Putty, covered with Socket Seal and sealed with Periacryl. I D # HEU This patient is a 66 year old female. Pre op Failed endodontic
We Want to Keep You Smiling. Bone Regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide
 We Want to Keep You Smiling Bone Regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide Strong Bone for a Healthier Smile Strong and healthy teeth provide a feeling of well-being, self-confidence and
We Want to Keep You Smiling Bone Regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide Strong Bone for a Healthier Smile Strong and healthy teeth provide a feeling of well-being, self-confidence and
A wide variety of membrane options
 A wide variety of membrane options BOVINE Bovine collagen membrane Neomem is a resorbable collagen membrane derived from highly purified type I collagen fibres from bovine Achilles tendon. The macromolecular
A wide variety of membrane options BOVINE Bovine collagen membrane Neomem is a resorbable collagen membrane derived from highly purified type I collagen fibres from bovine Achilles tendon. The macromolecular
We want to keep you smiling. Bone regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide
 We want to keep you smiling Bone regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide Strong bone for a healthier smile Strong and healthy teeth provide a feeling of well-being, self-confidence and
We want to keep you smiling Bone regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide Strong bone for a healthier smile Strong and healthy teeth provide a feeling of well-being, self-confidence and
Cytoflex Barrier Membrane Clinical Evaluation
 Cytoflex Barrier Membrane Clinical Evaluation Historical Background Guided tissue regeneration is a well established concept in the repair of oral bone defects. The exclusion of soft tissue epithelial
Cytoflex Barrier Membrane Clinical Evaluation Historical Background Guided tissue regeneration is a well established concept in the repair of oral bone defects. The exclusion of soft tissue epithelial
Dental Implants: A Predictable Solution for Tooth Loss. Reena Talwar, DDS PhD FRCD(C) Oral & Maxillofacial Surgeon Associate Clinical Professor
 Dental Implants: A Predictable Solution for Tooth Loss Reena Talwar, DDS PhD FRCD(C) Oral & Maxillofacial Surgeon Associate Clinical Professor What are Dental Implants? Titanium posts used to replace missing
Dental Implants: A Predictable Solution for Tooth Loss Reena Talwar, DDS PhD FRCD(C) Oral & Maxillofacial Surgeon Associate Clinical Professor What are Dental Implants? Titanium posts used to replace missing
Sinus Lift. A Key Step Toward Restoring Your Smile
 Sinus Lift A Key Step Toward Restoring Your Smile Sinus Lift A Key Step Towards Restoring Your Smile What Can I Expect From A Sinus Lift Procedure? There are different approaches to doing a sinus lift
Sinus Lift A Key Step Toward Restoring Your Smile Sinus Lift A Key Step Towards Restoring Your Smile What Can I Expect From A Sinus Lift Procedure? There are different approaches to doing a sinus lift
Symbios. Product Catalog
 Symbios Product Catalog Solutions for all your regenerative needs The comprehensive portfolio for hard and soft tissue regeneration provides ease of use and safety for dental clinicians and their patients.
Symbios Product Catalog Solutions for all your regenerative needs The comprehensive portfolio for hard and soft tissue regeneration provides ease of use and safety for dental clinicians and their patients.
chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute.
 chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute. Osteoconductive Resorbable Synthetic chronos Bone Void Filler chronos granules and preforms are synthetic, porous, osteoconductive,
chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute. Osteoconductive Resorbable Synthetic chronos Bone Void Filler chronos granules and preforms are synthetic, porous, osteoconductive,
SPINE BIOMATERIALS Crop Bleed
 A comprehensive guide to biomaterials offered by Posterolateral DBX PUTTY DBX STRIP DBX MIX DBX INJECT chronos Strip chronos Granules chronos Preforms PROCURE CHOICE Marrow Aspiration Kit Bone Marrow Aspiration
A comprehensive guide to biomaterials offered by Posterolateral DBX PUTTY DBX STRIP DBX MIX DBX INJECT chronos Strip chronos Granules chronos Preforms PROCURE CHOICE Marrow Aspiration Kit Bone Marrow Aspiration
( ) 2009;28(2):89-94
 ( ) 2009;28(2):89-94 Osseointegration is important in the functional aspect, however, esthetics is also important, especially in the maxillary anterior region. An adequate surgical technique is necessary
( ) 2009;28(2):89-94 Osseointegration is important in the functional aspect, however, esthetics is also important, especially in the maxillary anterior region. An adequate surgical technique is necessary
RELIABLE WHEN IT COUNTS. The unique collagenase-resistant membrane protects bone graft and supports treatment success even when exposed 4
 RELIABLE WHEN IT COUNTS 1 RELIABLE WHEN IT COUNTS RESISTANT TO EXPOSURE The unique collagenase-resistant membrane protects bone graft and supports treatment success even when exposed 4 RELIABLE BARRIER
RELIABLE WHEN IT COUNTS 1 RELIABLE WHEN IT COUNTS RESISTANT TO EXPOSURE The unique collagenase-resistant membrane protects bone graft and supports treatment success even when exposed 4 RELIABLE BARRIER
Alveolar Ridge Augmentation with Titanium Mesh and Particulate Allograft A Case Report
 Alveolar Ridge Augmentation with Titanium Mesh and Particulate Allograft A Case Report Dr. Pratibha Borasi, Dr. Praneeta Kamble Department of Periodontics, Nair Hospital Dental College, Mumbai, Maharashtra,
Alveolar Ridge Augmentation with Titanium Mesh and Particulate Allograft A Case Report Dr. Pratibha Borasi, Dr. Praneeta Kamble Department of Periodontics, Nair Hospital Dental College, Mumbai, Maharashtra,
Bone augmentation with maxgraft
 Patient information bone & tissue regeneration botiss biomaterials Bone augmentation with maxgraft established safe X100 natural Implantation stability is crucial for success Atrophy of the jaw bone loss
Patient information bone & tissue regeneration botiss biomaterials Bone augmentation with maxgraft established safe X100 natural Implantation stability is crucial for success Atrophy of the jaw bone loss
Management of a complex case
 2 Soft- and hard-tissue reconstruction of a severely deficient site prior to implant placement: a case report Management of a complex case Younes Khosroshahy, DDS, MFDS RCS (Eng), Dip Imp Dent RCSEd, Blue
2 Soft- and hard-tissue reconstruction of a severely deficient site prior to implant placement: a case report Management of a complex case Younes Khosroshahy, DDS, MFDS RCS (Eng), Dip Imp Dent RCSEd, Blue
Extraction with Immediate Implant Placement and Ridge Preservation in the Posterior
 Extraction with Immediate Implant Placement and Ridge Preservation in the Posterior by Timothy F. Kosinski, DDS, MAGD The following case presentation illustrates the diagnosis, planning and treatment for
Extraction with Immediate Implant Placement and Ridge Preservation in the Posterior by Timothy F. Kosinski, DDS, MAGD The following case presentation illustrates the diagnosis, planning and treatment for
BONE GRAFTING. Product Catalog
 BONE GRAFTING Product Catalog PRODUCT LINE Titanium Mesh Surgitime Titanium Titanium Foil Surgitime Titanium Seal Polytetraflouroethylene Membrane Surgitime PTFE Bone Screws Fixation Set We innovate to
BONE GRAFTING Product Catalog PRODUCT LINE Titanium Mesh Surgitime Titanium Titanium Foil Surgitime Titanium Seal Polytetraflouroethylene Membrane Surgitime PTFE Bone Screws Fixation Set We innovate to
Regeneration Bone Grafting & Soft Tissue Management
 Regeneration Bone Grafting & Soft Tissue Management Human Histology OSTEON II 26.5 months Regeneration Products information Table of contents Bone Graft Material OSTEON II OSTEON OSTEON II Collagen OSTEON
Regeneration Bone Grafting & Soft Tissue Management Human Histology OSTEON II 26.5 months Regeneration Products information Table of contents Bone Graft Material OSTEON II OSTEON OSTEON II Collagen OSTEON
Redefining Regeneration
 Redefining Regeneration Taking Volume to the MAX One Product, One Treatment, Real VOLUME Buccal Bone Loss Socket Preservation Lateral / Vertical Augmentation Grafting Material Scaffold Barrier (4-6 months)
Redefining Regeneration Taking Volume to the MAX One Product, One Treatment, Real VOLUME Buccal Bone Loss Socket Preservation Lateral / Vertical Augmentation Grafting Material Scaffold Barrier (4-6 months)
Socket Treatment. Procedure Guide
 Socket Treatment Procedure Guide www.implantdirect.com 888.649.6425 Extraction Healing and Ridge Resorption The Why Working with patients to educate them on the benefits of bone maintenance post extraction
Socket Treatment Procedure Guide www.implantdirect.com 888.649.6425 Extraction Healing and Ridge Resorption The Why Working with patients to educate them on the benefits of bone maintenance post extraction
CONFORM FlEX. Demineralized cancellous bone
 CONFORM FlEX Demineralized cancellous bone TABlE OF CONTENTS introduction 2 FEATURES AND BENEFiTS 4 PROCESSiNg AND PACKAgiNg information 5 MUSCUlOSKElETAl TRANSPlANT FOUNDATiON (MTF) 6 ORDERiNg information
CONFORM FlEX Demineralized cancellous bone TABlE OF CONTENTS introduction 2 FEATURES AND BENEFiTS 4 PROCESSiNg AND PACKAgiNg information 5 MUSCUlOSKElETAl TRANSPlANT FOUNDATiON (MTF) 6 ORDERiNg information
Crestal Sinus Augmentation: A Simplified Approach to Implant Placement in the Posterior Maxilla
 IJOICR 10.5005/jp-journals-10012-1036 RECENT TECHNICAL ADVANCES Crestal Sinus Augmentation: A Simplified Approach to Implant Placement in the Posterior Maxilla Crestal Sinus Augmentation: A Simplified
IJOICR 10.5005/jp-journals-10012-1036 RECENT TECHNICAL ADVANCES Crestal Sinus Augmentation: A Simplified Approach to Implant Placement in the Posterior Maxilla Crestal Sinus Augmentation: A Simplified
Product Information. MIS Corporation. All Rights Reserved.
 Product Information MIS Corporation. All Rights Reserved. MIS Warranty: MIS exercises great care and effort in maintaining the superior quality of its products. All MIS products are guaranteed to be free
Product Information MIS Corporation. All Rights Reserved. MIS Warranty: MIS exercises great care and effort in maintaining the superior quality of its products. All MIS products are guaranteed to be free
BIOMATERIAL COLLECTION
 BIOMATERIAL COLLECTION www.implantdirect.com Innovation. Quality. Service. Value. INDEX GRAFTING PAGE Allograft Bone Grafting DirectGen 1-2 Bone Grafting DirectGen Putty 3 Bone Grafting DirectGen FLEX
BIOMATERIAL COLLECTION www.implantdirect.com Innovation. Quality. Service. Value. INDEX GRAFTING PAGE Allograft Bone Grafting DirectGen 1-2 Bone Grafting DirectGen Putty 3 Bone Grafting DirectGen FLEX
Regeneration Bone Grafting & Soft Tissue Management
 Regeneration Bone Grafting & Soft Tissue Management 1 Regeneration Bone Grafting & Soft Tissue Management Table of contents Bone Graft Material OSTEON II OSTEON OSTEON II Collagen OSTEON Collagen 5 6
Regeneration Bone Grafting & Soft Tissue Management 1 Regeneration Bone Grafting & Soft Tissue Management Table of contents Bone Graft Material OSTEON II OSTEON OSTEON II Collagen OSTEON Collagen 5 6
Rehabilitating a Compromised Site for Restoring Form, Function and Esthetics- A Case Report
 Research & Reviews: Journal of Dental Sciences Rehabilitating a Compromised Site for Restoring Form, Function and Esthetics- A Case Report Priyanka Prakash* Division of Periodontology, Department of Dental
Research & Reviews: Journal of Dental Sciences Rehabilitating a Compromised Site for Restoring Form, Function and Esthetics- A Case Report Priyanka Prakash* Division of Periodontology, Department of Dental
Evaluation of different grafting materials in three-wall intra-bony defects around dental implants in beagle dogs
 Current Applied Physics 5 (2005) 507 511 www.elsevier.com/locate/cap Evaluation of different grafting materials in three-wall intra-bony defects around dental implants in beagle dogs Ui-Won Jung a, Hee-Il
Current Applied Physics 5 (2005) 507 511 www.elsevier.com/locate/cap Evaluation of different grafting materials in three-wall intra-bony defects around dental implants in beagle dogs Ui-Won Jung a, Hee-Il
Thick vs. Thin Gingival Biotypes: A Key Determinant in Treatment Planning for Dental Implants
 r s Thick vs. Thin Gingival Biotypes: A Key Determinant in Treatment Planning for Dental Implants richard t. kao, dds, phd; mark c. fagan, ms, dds; and gregory j. conte, ms, dmd abstract During the treatment
r s Thick vs. Thin Gingival Biotypes: A Key Determinant in Treatment Planning for Dental Implants richard t. kao, dds, phd; mark c. fagan, ms, dds; and gregory j. conte, ms, dmd abstract During the treatment
Clinical Case Reports using Cytoplast GTR Barrier Membranes
 Clinical Case Reports using Cytoplast GTR Barrier Membranes Barry K. Bartee, DDS, MD The Cytoplast Technique: Extraction Site Grafting Without Primary Closure 1. 1. Preoperative view. To maximize the result
Clinical Case Reports using Cytoplast GTR Barrier Membranes Barry K. Bartee, DDS, MD The Cytoplast Technique: Extraction Site Grafting Without Primary Closure 1. 1. Preoperative view. To maximize the result
Bone Grafting for Socket Preservation
 Bone Grafting for Socket Preservation Dr. Karl R. Koerner Normal extraction facial bone loss. Excessive force. Commonly the thickness of facial bone. Hussain, A. et al. Ridge preservation comparing a nonresorbable
Bone Grafting for Socket Preservation Dr. Karl R. Koerner Normal extraction facial bone loss. Excessive force. Commonly the thickness of facial bone. Hussain, A. et al. Ridge preservation comparing a nonresorbable
Clinical cases by Dr. Fernando Rojas-Vizcaya. botiss. dental bone & tissue regeneration. biomaterials. strictly biologic
 Clinical cases by Dr. Fernando Rojas-Vizcaya dental bone & tissue regeneration botiss biomaterials strictly biologic botiss BTR system: BONE biologic potential bovine block & granules: pure bone mineral
Clinical cases by Dr. Fernando Rojas-Vizcaya dental bone & tissue regeneration botiss biomaterials strictly biologic botiss BTR system: BONE biologic potential bovine block & granules: pure bone mineral
Table of Contents. Training kit-introduction 3. How to Activiate 4-5. Expert Tip 6-7. Indications Tips 8-9. Sinus Lift 10-11
 Training Kit Table of Contents Training kit-introduction 3 How to Activiate 4-5 Expert Tip 6-7 Indications Tips 8-9 Sinus Lift 10-11 Augma Biomaterials 3 Augma Biomaterials invites you to join the circle
Training Kit Table of Contents Training kit-introduction 3 How to Activiate 4-5 Expert Tip 6-7 Indications Tips 8-9 Sinus Lift 10-11 Augma Biomaterials 3 Augma Biomaterials invites you to join the circle
ADVANCED BONE GRAFT SYSTEM OVERVIEW
 ADVANCED BONE GRAFT SYSTEM OVERVIEW NANOSS BIOACTIVE ADVANCED BONE GRAFT Table Of Contents INTRODUCTION System Overview... 1 NANOSS BIOACTIVE COMPONENTS Comparison of nanoss Bioactive and Human Bone...
ADVANCED BONE GRAFT SYSTEM OVERVIEW NANOSS BIOACTIVE ADVANCED BONE GRAFT Table Of Contents INTRODUCTION System Overview... 1 NANOSS BIOACTIVE COMPONENTS Comparison of nanoss Bioactive and Human Bone...
Regeneration Bone Grafting & Soft Tissue Management
 Regeneration Bone Grafting & Soft Tissue Management OSTEON TM II Table of Contents Bone Graft Material OSTEON TM II Collagen 04 OSTEON TM Collagen 06 OSTEON TM II 08 OSTEON TM 12 ORTHOPEDIC OSTEON TM 14
Regeneration Bone Grafting & Soft Tissue Management OSTEON TM II Table of Contents Bone Graft Material OSTEON TM II Collagen 04 OSTEON TM Collagen 06 OSTEON TM II 08 OSTEON TM 12 ORTHOPEDIC OSTEON TM 14
B U I L D S T R O N G B O N E F A S T
 B U I L D S T R O N G B O N E F A S T Putty MIS Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
B U I L D S T R O N G B O N E F A S T Putty MIS Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
Consensus Report Tissue augmentation and esthetics (Working Group 3)
 B. Klinge Thomas F. Flemmig Consensus Report Tissue augmentation and esthetics (Working Group 3) Members of working group: Matteo Chiapasco Jan-Eirik Ellingsen Ronald Jung Friedrich Neukam Isabella Rocchietta
B. Klinge Thomas F. Flemmig Consensus Report Tissue augmentation and esthetics (Working Group 3) Members of working group: Matteo Chiapasco Jan-Eirik Ellingsen Ronald Jung Friedrich Neukam Isabella Rocchietta
SMARTBRANE SIMPLE RELIABLE PURE NEW! Resorbable Pericardium Membrane MORE ECONOMIC. THE SMALLEST MEMBRANE 10 x 10 mm. 10 x10
 SMARTBRANE Resorbable Pericardium Membrane 10 x10 NEW! THE SMALLEST MEMBRANE 10 x 10 mm SIMPLE RELIABLE PURE MORE ECONOMIC SIMPLE Optimized handling properties ensuring straight-forward application The
SMARTBRANE Resorbable Pericardium Membrane 10 x10 NEW! THE SMALLEST MEMBRANE 10 x 10 mm SIMPLE RELIABLE PURE MORE ECONOMIC SIMPLE Optimized handling properties ensuring straight-forward application The
PERIODONTAL Bone & Skin Allograft Products
 PERIODONTAL Bone & Skin Allograft Products SureOss Family SureOss Cortical Bone - / SureOss Plus Cortical Bone with CA - / SureOss Collagen Cortical Bone with Collagen - Sponge Block SureOss Paste Cortical
PERIODONTAL Bone & Skin Allograft Products SureOss Family SureOss Cortical Bone - / SureOss Plus Cortical Bone with CA - / SureOss Collagen Cortical Bone with Collagen - Sponge Block SureOss Paste Cortical
BIOMATERIAL COLLECTION
 BIOMATERIAL COLLECTION www.implantdirect.com Innovation. Quality. Service. Value. INDEX GRAFTING PAGE Xenograft Bone Grafting DirectOss 1 Allograft Bone Grafting DirectGen 2-3 Bone Grafting DirectGen Putty
BIOMATERIAL COLLECTION www.implantdirect.com Innovation. Quality. Service. Value. INDEX GRAFTING PAGE Xenograft Bone Grafting DirectOss 1 Allograft Bone Grafting DirectGen 2-3 Bone Grafting DirectGen Putty
Soft-Tissue Success with the. Clinical Success. Proven Matrix. with the Proven. Geistlich Mucograft Geistlich Mucograft Seal
 Soft-Tissue Success with the Clinical Success Proven Matrix with the Proven Bone Geistlich Mucograft Substitute Seal The Softer Side of Geistlich Innovation Decades of collagen expertise leads naturally
Soft-Tissue Success with the Clinical Success Proven Matrix with the Proven Bone Geistlich Mucograft Substitute Seal The Softer Side of Geistlich Innovation Decades of collagen expertise leads naturally
Socket preservation in the daily practice: A clinical case report
 Clinical Socket preservation in the daily practice: A clinical case report Rabih Abi Nader 1 and Carine Tabarani 2 Abstract Soft tissue contour depends on the underlying bone anatomy. Following tooth extraction,
Clinical Socket preservation in the daily practice: A clinical case report Rabih Abi Nader 1 and Carine Tabarani 2 Abstract Soft tissue contour depends on the underlying bone anatomy. Following tooth extraction,
botiss maxgraft bonering botiss maxgraft bonebuilder Because one option is not enough. PROCESSED ALLOGENIC BONERING
 Product Information Allograft Biomaterials@Straumann Because one option is not enough. botiss maxgraft bonering PROCESSED ALLOGENIC BONERING botiss maxgraft bonebuilder CUSTOMIZED ALLOGENIC BONE BLOCK
Product Information Allograft Biomaterials@Straumann Because one option is not enough. botiss maxgraft bonering PROCESSED ALLOGENIC BONERING botiss maxgraft bonebuilder CUSTOMIZED ALLOGENIC BONE BLOCK
Resorbable bilayer synthetic membrane Biomimetic tissue-engineered matrix for GBR and GTR
 Resorbable bilayer synthetic membrane Biomimetic tissue-engineered matrix for GBR and GTR Patented Jet-Spraying technology: Full barrier effect during 4 weeks, and complete resorption in 6 months PATENTED
Resorbable bilayer synthetic membrane Biomimetic tissue-engineered matrix for GBR and GTR Patented Jet-Spraying technology: Full barrier effect during 4 weeks, and complete resorption in 6 months PATENTED
Periimplant Regeneration Fenestration
 Indication Sheet PIR Periimplant Regeneration Fenestration Treatment concept of Dr. Jean-Pierre Gardella (surgeon) and Dr. Christian Richelme (prosthodontist), Marseille, France > Filling of a peri-implant
Indication Sheet PIR Periimplant Regeneration Fenestration Treatment concept of Dr. Jean-Pierre Gardella (surgeon) and Dr. Christian Richelme (prosthodontist), Marseille, France > Filling of a peri-implant
P105 Predictable Bone Grafting for Site Preparation for Implants and Prosthetics Workshop JAMES GRISDALE, DDS THURSDAY, FEBRUARY 26
 P105 Predictable Bone Grafting for Site Preparation for Implants and Prosthetics Workshop JAMES GRISDALE, DDS THURSDAY, FEBRUARY 26 Please complete the speaker evaluation form in the Midwinter Meeting
P105 Predictable Bone Grafting for Site Preparation for Implants and Prosthetics Workshop JAMES GRISDALE, DDS THURSDAY, FEBRUARY 26 Please complete the speaker evaluation form in the Midwinter Meeting
Tooth out what's next?
 Tooth out what's next Content >> Treatment Options >> Clinical Cases >> Product Information >> Product related FAQs >> Why Ridge Preservation > Minimise invasion: Bone volume preservation > Minimise invasion:
Tooth out what's next Content >> Treatment Options >> Clinical Cases >> Product Information >> Product related FAQs >> Why Ridge Preservation > Minimise invasion: Bone volume preservation > Minimise invasion:
SCA Kit Sinus Crestal Approach Kit
 SCA Kit Sinus Crestal Approach Kit Introduction 3S of SCA Kit SCA Kit Composition Component(I-drill) Component(S-Reamer) Component(Stopper) Component(Depth Gauge) Component(Bone carrier) Component(Bone
SCA Kit Sinus Crestal Approach Kit Introduction 3S of SCA Kit SCA Kit Composition Component(I-drill) Component(S-Reamer) Component(Stopper) Component(Depth Gauge) Component(Bone carrier) Component(Bone
Limited bone availability makes implant placement challenging
 Bone Grafting: Essential Indications and Techniques in Implant Dentistry Limited bone availability makes implant placement challenging and sometimes unpredictable. Candidates for implant therapy must have
Bone Grafting: Essential Indications and Techniques in Implant Dentistry Limited bone availability makes implant placement challenging and sometimes unpredictable. Candidates for implant therapy must have
Case reports by Dr. Roland Török IMPLANT INSTITUTE TÖRÖK. botiss. dental bone & tissue regeneration. biomaterials.
 Case reports by Dr. Roland Török dental bone & tissue regeneration Dr. Med. STOM. roland török IMPLANT INSTITUTE TÖRÖK botiss biomaterials strictly biologic botiss BTR system: BONE bovine block & granules:
Case reports by Dr. Roland Török dental bone & tissue regeneration Dr. Med. STOM. roland török IMPLANT INSTITUTE TÖRÖK botiss biomaterials strictly biologic botiss BTR system: BONE bovine block & granules:
Product Information. When one option is not enough.
 Product Information Biomaterials@Straumann When one option is not enough. Content BONE SUBSTITUTES Straumann AlloGraft granules Processed human allograft 6 MEMBRANES Straumann Membrane Plus Collagen membrane
Product Information Biomaterials@Straumann When one option is not enough. Content BONE SUBSTITUTES Straumann AlloGraft granules Processed human allograft 6 MEMBRANES Straumann Membrane Plus Collagen membrane
The Use of DynaMatrix Extracellular Membrane for Gingival Augmentation: A Case Series Dr. Stephen Saroff, DDS
 The Use of DynaMatrix Extracellular Membrane for Gingival Augmentation: A Case Series Dr. Stephen Saroff, DDS LOCALIZED RECESSION ON TOOTH #25 DUE TO BONE RECESSION (PRE OP) Introduction Tissue grafting
The Use of DynaMatrix Extracellular Membrane for Gingival Augmentation: A Case Series Dr. Stephen Saroff, DDS LOCALIZED RECESSION ON TOOTH #25 DUE TO BONE RECESSION (PRE OP) Introduction Tissue grafting
Laboratory Solutions. Optimization By Design
 Laboratory Solutions Optimization By Design Digital Dentistry Solutions Business Optimization With BellaTek Digital Dentistry Solutions Optimize the solutions you give to your customers with BellaTek premium
Laboratory Solutions Optimization By Design Digital Dentistry Solutions Business Optimization With BellaTek Digital Dentistry Solutions Optimize the solutions you give to your customers with BellaTek premium
MANAGEMENT OF ATROPHIC ANTERIOR MAXILLA USING RIDGE SPLIT TECHNIQUE, IMMEDIATE IMPLANTATION AND TEMPORIZATION
 Case Report International Journal of Dental and Health Sciences Volume 02, Issue 06 MANAGEMENT OF ATROPHIC ANTERIOR MAXILLA USING RIDGE SPLIT TECHNIQUE, IMMEDIATE IMPLANTATION AND TEMPORIZATION Rakshith
Case Report International Journal of Dental and Health Sciences Volume 02, Issue 06 MANAGEMENT OF ATROPHIC ANTERIOR MAXILLA USING RIDGE SPLIT TECHNIQUE, IMMEDIATE IMPLANTATION AND TEMPORIZATION Rakshith
Surgery All at Once : Socket preservation and immediate placement of an implant in an infected site in the anterior region a Case Report
 Surgery All at Once : Socket preservation and immediate placement of an implant in an infected site in the anterior region a Case Report W.P. van der Schoor*, A.R.M. van der Schoor Tooth extraction followed
Surgery All at Once : Socket preservation and immediate placement of an implant in an infected site in the anterior region a Case Report W.P. van der Schoor*, A.R.M. van der Schoor Tooth extraction followed
Periimplant Regeneration Fenestration
 Indication Sheet PIR-1 Periimplant Regeneration Fenestration Treatment concept of Dr. Jean-Pierre Gardella (surgeon) and Dr. Christian Richelme (prosthodontist), Marseille, France > Filling of a peri-implant
Indication Sheet PIR-1 Periimplant Regeneration Fenestration Treatment concept of Dr. Jean-Pierre Gardella (surgeon) and Dr. Christian Richelme (prosthodontist), Marseille, France > Filling of a peri-implant
Step-by-Step Step-by-Step. Internal Hex. Implant System MAKE IT SIMLE
 4. 7. Step-by-Step Cemented Step-by-Step Bridge BONDBONE Using Abutments Internal Hex. Implant System MAKE IT SIMLE MIS Corporation. All Rights Reserved. Published by MIS, which reserves the right to ameliorate
4. 7. Step-by-Step Cemented Step-by-Step Bridge BONDBONE Using Abutments Internal Hex. Implant System MAKE IT SIMLE MIS Corporation. All Rights Reserved. Published by MIS, which reserves the right to ameliorate
Multi-Modality Anterior Extraction Site Grafting Increased Predictability for Aesthetics Michael Tischler, DDS
 Page 1 of 5 Issue Date: March 2003, Posted On: 8/1/2005 Multi-Modality Anterior Extraction Site Grafting Increased Predictability for Aesthetics Michael Tischler, DDS The extraction of teeth creates a
Page 1 of 5 Issue Date: March 2003, Posted On: 8/1/2005 Multi-Modality Anterior Extraction Site Grafting Increased Predictability for Aesthetics Michael Tischler, DDS The extraction of teeth creates a
