Esterase Inhibitors Prevent Lysosomal Enzyme Redistribution in Two Noninvasive Models of Experimental Pancreatitis
|
|
|
- Blanche Flowers
- 5 years ago
- Views:
Transcription
1 GASTROENTEROLOGY 1989;96:853-9 Esterase nhibitors Prevent Lysosomal Enzyme Redistribution in Two Noninvasive Models of Experimental Pancreatitis G. OHSHO, A. K. SALUA, U. LEL, A. SENGUPTA, and M.1. STEER Department of Surgery and Division of Gastroenterology, Harvard Digestive Diseases Center and Charles A. Dana Research Laboratories, Beth srael Hospital and Harvard Medical School, Boston, Massachusetts Earlier studies have indicated that lysosomal enzymes such as cathepsin B become redistributed within pancreatic acinar cells during the early stages of both diet- and secretagogue-induced acute pancreatitis. As a result, cathepsin B and digestive zymogens became colocalized within large cytoplasmic vacuoles. As cathepsin B can activate trypsinogen, this co localization could result in intracellular digestive enzyme activation. The present study investigates the protective effects of gabexate mesilate (FOY) and camostate (FOY 35) on both ofthese noninvasive models of experimental pancreatitis. These esterase inhibitors prevented the hyperamylasemia, pancreatic edema, and acinar cell vacuolization that characterize secretagogue-induced pancreatitis and the hyperamylasemia and mortality that characterize diet-induced pancreatitis. n addition, FOY and FOY 35 were found to significantly decrease the subcellular redistribution of cathepsin B that occurs in both models. These findings indicate that enzyme activity sensitive to inhibition by FOY and FOY 35 may be critical to the redistribution phenomenon that characterizes both of these models of pancreatitis. t is generally believed that intra pancreatic digestive enzyme activation, as well as injury to the gland by activated proteolytic and lipolytic enzymes, is an important early event in the pathogenesis of acute pancreatitis. Until recently, it was assumed that digestive enzyme activation occurred within the pancreatic ductal space or in the duodenum. Recent studies, however, have suggested that digestive enzyme activation might occur within acinar cells. This conclusion was based on the results of studies using the secretagogue- and diet-induced models of experimental acute pancreatitis that indicated that, early in the evolution of those forms of the disease, digestive enzymes and lysosomal hydrolases become colocalized within cytoplasmic vacuoles (1-4). As the lysosomal enzyme cathepsin B is known to be capable of activating trypsinogen (5) and trypsin can activate the remaining digestive enzyme zymogens, the observed colocalization could result in intravacuolar digestive enzyme activation. n the present communication we report the results of studies that evaluated the effects of the potent new low molecular weight enzyme inhibitors gabexate mesilate (FOY) and camostate (FOY 35) on (a) edematous pancreatitis induced by infusing rats with a dose of the cholecystokinin-pancreozymin analogue caerulein in excess of that which causes maximal rates of digestive enzyme secretion from the pancreas and (b) necrotizing pancreatitis induced by feeding young female mice a cholinedeficient ethionine-supplemented (CDE) diet. n accord with reports by others (6), we have found that FOY protects against the interstitial pancreatic edema and hyperamylasemia that characterize the secretagogue-induced model of pancreatitis. We have also noted that FOY 35 reduces the mortality rate and degree of hyperamylasemia that follow ingestion of the CDE diet. These observations are consistent with the previous report that FOY has a similar effect on this model (7). We have also found thatredistribution of the lysosomal enzyme cathepsin B from the lysosome- and mitochondria-enriched Abbreviation used in this paper: ede. choline-deficient ethionine supplemented by the American Gastroenterological Association /89/$3.5
2 854 OHSHO ET AL. GASTROENTEROLOGY Vol. 96, No.3 subcellular fraction to the zymogen granule-enriched subcellular fraction is also reduced by these enzyme inhibitors. This latter observation suggests that activated enzymes may play an important role in the redistribution phenomenon, which is the earliest event that has been noted to characterize these models of acute pancreatitis. Materials and Methods Male Wi star rats (9-12 g) from Charles River Breeding Laboratories, Wilmington, Mass. were used for the secretagogue-induced pancreatitis experiments. A PE-5 cannula was placed in the jugular vein and its patency was maintained by continuous infusion of a solution containing NaCl (15 mm) and heparin (3 Ulml) at a rate of.2 mllh as described earlier (8). The animals were housed in shoe-box cages and allowed h to recover from the effects of anesthesia. Animals were subsequently divided into the following three groups: (a) control-infused only with the heparin-saline solution at a rate of.58 mllh for 3.5 h; (b) caerulein-infused with the heparin-saline solution as above (.58 mllh, 3.5 h) but with caerulein added to the infusate such that each animal received 5 JLg/kg h; (c) FaY plus caerulein-identical to caerulein but FaY infused for 2 h before and throughout the 3.5 h of caerulein infusion. The dose of FaY administered was 5 mg/kg. h. Female CD-l mice (1-14 g) from Charles River Breeding Laboratories were used for the diet-induced pancreatitis experiments. After an initial 24-h period of fasting, they were fed g/mouse of either regular laboratory diet (control group) or of the CDE diet containing.5% ethionine (U.S. Biochemical Corp., Cleveland, Ohio). When tested, FaY 35 was added to the diets and administered over 24 h such that a total dose of 6 mg/kg was consumed. After completion of the 24-h period during which animals were fed one of the diets with or without FaY 35, all mice were again fasted for 24 h. They were then either killed for use in biochemical experiments or given regular laboratory diet ad libitum for the following 3 days to allow for determination of mortality rates. Gabexate mesilate (FaY) and camostate (FaY 35) were the kind gifts of Ono Pharmaceuticals, Osaka, Japan. Other reagents were obtained from previously reported sources. n Vitro Amylase Secretion Dispersed rat and mouse pancreatic acllll were prepared by collagenase (Cooper Diagnostics, Freehold, N.J.) digestion and gentle shearing as previously described (9). Acini were suspended in HE PES-Ringer buffer (ph 7.4) containing (mm) NaC (115), KC (5), MgS4 (), Na 2 HP 4 (1), HEPES (1), CaCl 2 (1.26), and glucose (15). n addition, the buffer contained Eagle's basal amino acids, bovine serum albumin (.1%), and soybean trypsin inhibitor (.1 %, Cooper Diagnostics) and was saturated by bubbling with O 2, The acini were pre incubated in HEPES Ringer buffer (37 C) with or without FaY (1-4 M) for 3 min and then suspended in the same buffer containing varying concentrations of secretagogues. Amylase secretion was measured over the ensuing 3 min and expressed as the percentage of total amylase content. Net stimulated amylase secretion was calculated by subtracting the percentage of amylase secreted in the absence from that in the presence of the secretagogue. Subcellular Fractionation At selected times, rats and mice were killed by cervical dislocation, portions of the pancreas were quickly removed and trimmed of fat, and the pancreas was homogenized with a glass-teflon homogenizer in buffer (ph 6.5) containing 5 mm MOPS, 1 mm MgS4, and 25 mm sucrose. Unbroken cells and debris were removed by low-speed centrifugation (15 g. 15 min, 4 C) and the resulting supernatant was used for subcellular fractionation as described by Tartakoff and Jamieson (1) with minor modifications (8). The low-speed supernatant was considered to be the entire sample for later calculations. That supernatant was centrifuged (13 g. 15 min. 4 C) and the resulting zymogen granule-rich pellet was harvested. The remaining supernatant was centrifuged (12, g, 12 min. 4 C) to obtain a pellet that was rich in lysosomes and mitochondria. The remaining supernatant was centrifuged (15. g. 6 min, 4 C) to obtain a microsome-rich pellet and a supernatant whose contents were considered "soluble." The purity of each of these subcellular fractions was evaluated using marker enzymes with findings identical to those previously reported from this laboratory (8). Other portions of the pancreas were homogenized in phosphate-buffered saline containing.5% Triton X- using a Brinkman polytron (Brinkman nstruments, nc.. Westbury, N.Y.) and used to quantitate total enzyme content of the pancreas. Assays Amylase activity in serum and in subcellular fractions from the pancreas was measured using soluble starch as the substrate according to the method of Bernfield (11). Cathepsin B activity in pancreas fractions was measured fluorimetrically using the substrate CBZ-arginyl-arginine,B-naphthylamide (Bacham Bioscience nc.; Philadelphia, Pa.) as described by McDonald and Ellis with minor modifications (3.12). Deoxyribonucleic acid was measured according to the method of Labarca and Paigen (13) using calf thymus deoxyribonucleic acid (DNA) as the standard. The development of pancreatic edema was quantitated by comparing the pancreas weight obtained immediately after killing the animals ("wet weight") to that of the same sample after incubation at 15 C for 48 h ("dry weight"). Microscopy Samples of rat pancreatic tissue were fixed by immersion for 12 h in a mixture containing 95% ethanolsaturated picric acid/formalin/acetic acid (8: 15: 5. voll vollvol). After paraffin embedding, sectioning. and stain-
3 March 1989 ESTERASES N PANCREATTS 855 ing with hematoxylin-eosin, the sections were examined by a blinded observer and changes were graded on a -4 + scale. Mouse pancreatic fragments were fixed by immersion for 12 h in Zenker's fluid followed by overnight washing in running water. They were immersed in 15% sucrose-enriched phosphate-buffered saline and cryosectioned at 6 p.m. The sections were treated with Lugol's iodine-thiosulfate, stained with hematoxylin-eosin, and examined, as were rat samples, by a blinded observer. A 25 3 E 2 E "- " D D 2 15 " " " 1 E E D 5 D B Analysis of Data The results reported in this communication represent the mean ± SEM for n determinations. Differences between groups were evaluated using Student's t-test, with significant differences defined as those associated with a probability value of <.5. Mortality data were analyzed using the technique of Kaplan-Meier and evaluated using the. method with Yates' correction (14). Results Effects of FOY and FOY 35 on Serum Amylase and Morphologic Changes n agreement with previous reports from this (3,8) and other laboratories (15), infusion of a supramaximally stimulating dose of caerulein (5 JLg/kg. h) for 3.5 h was found to cause an increase in serum amylase levels (Figure la) and the appearance of pancreatic edema. The latter could be objectively quantitated by measuring the pancreatic dry to wet weight ratio, which was found to be 28.3% ± 1.6% in control samples and 13.1% ±.6% in samples taken from supramaximally stimulated rats. Other histologic changes that were noted included acinar cell vacuolization and the appearance, within the pancreatic parenchyma, of an inflammatory cell infiltrate (Table 1). When FOY (5 mg/kg. h) was infused before as well as with caerulein, the caerulein-induced increase in serum amylase was markedly attenuated (Figure 1). n addition, the dry to wet weight ratio of the pancreas was increased to 24.7% ±.2%, indicating that pancreatic edema had been prevented by FOY. These effects of FOY were all highly significant (p <.1). n addition, neither an inflammatory cell infiltrate nor evidence of acinar cell vacuolization was noted in animals given FOY (Table 1). Administration of the CDE diet was also found to cause hyperamylasemia (Figure B) and acinar cell vacuolization. n addition, ingestion of the CDE diet was associated with a 6% mortality rate (Figure 2). Similar observations have been reported previously from this laboratory (16). Addition of FOY 35 to the CDE diet resulted in a reduction in the degree of hyperamylasemia (Figure Control CER FOY Control CDE FOY35 +CER +CDE Figure 1. Effects of FOY and FOY 35 on hyperamylasemia of pancreatitis. A. Rats were infused with heparin-saline ("control"), heparin-saline plus 5 ltg/kg. h caerulein ("CER"), or heparin-saline plus caerulein plus 5 mg/ kg h FOY ("FOY + CER"), and serum amylase activity was measured as described in the text. Results represent mean and vertical bars the SEM value of duplicate measurements, each made using 7-12 different animals in each group. B. Mice were fed regular laboratory diet ("control") the CDE diet ("CDE"), or the CDE diet combined with FOY 35 ("FOY 35 + CDE") and serum amylase activity was measured as described in the text. Results represent mean and vertical bars the SEM value of duplicate measurements, each made using 9-15 different animals in each group. * p <.1 when caerulein or CDE alone were compared with control. * * p <.1 when FOY + caerulein or FOY 35 + CDE were compared with caerulein or CDE alone. B) and in the mortality rate (Figure 2). Others have noted that FOY has a similar effect when tested using the CDE diet-induced model of pancreatitis (7). The degree of acinar cell vacuolization was not altered by administration of FOY 35 with the CDE diet. Effect of FOY on Caerulein Stimulation of n Vitro Amylase Secretion Caerulein caused a dose-dependent stimulation of amylase secretion from dispersed rat pancre- Table 1. Effect of Caerulein and FOY on Microscopic Changes nduced by Supramaximal Stimulation with Caerulein nflammatory Acinar cell nfusion group n c ell infiltrate vacuolization Control 8 Caerulein Caerulein + FOY 5 Rats were infused with heparin-saline ("control"), heparin-saline plus caerulein ("caerulein"), or heparin-saline plus FOY plus caerulein ("caerulein + FOY "), for 3.5 h as described in the text. n addition, the animals given FOY received that agent for 2 h before caerulein infusion. There were n rats in each group. Histologic changes were blindly graded on a scale from (no change) to 4+ (maximum change).
4 856 OHSHO ET AL. GASTROENTEROLOGY Vol. 96, No.3 l _._ i 6 Jl O t r - - r J Days Figure 2. Effects of FOY 35 on mortality rate of CDE dietinduced pancreatitis. Mice were fed the CDE diet alone (-"-, n = 32) or the CDE diet plus FOY 35 (e- --e, n = 32) for 24 h as described in the text. The statistical significance of changes was evaluated by comparing the mortality rates. * p <.1. and ** p <.1. atic acini with maximal rates of net amylase secretion (1.2% ± 1.4% per 3 min) observed in the presence of 1-1 M caerulein. Higher doses of the secretagogue resulted in lower rates of amylase secretion (17). Addition of FOY (1-4 M) to a suspension of dispersed acini did not alter the response of the acini to caerulein, i.e., in the presence of FOY, maximal caerulein-stimulated net amylase secretion (1.9% ±.5% per 3 min) was still observed when the caerulein concentration was 1-1 M and lower rates of secretion were noted when higher concentrations of the secretagogue were present. n vitro caerulein-stimulated amylase secretion in mouse pancreatic acini was 7.3% ±.2% of total content per 3 min. n acini taken from mice fed the CDE diet, in vitro caerulein-stimulated amylase secretion was reduced to only 1.6% ±.4% of total content per 3 min. This phenomenon has been reported previously (9). A similar reduction in caerulein-stimulated amylase secretion was observed when acini taken from mice fed the CDE diet with FOY 35 were evaluated (net stimulated amylase secretion = 1.2% ±.3% of total content per 3 min). Effects of FOY and FOY 35 on Subcellular Distribution of Amylase The distribution of amylase in the various subcellular fractions of pancreas obtained from control animals was similar to that previously reported (8) (Figures 3A and 3B). Although most of the amylase activity was recovered from particulate fractions (zymogen granules, endoplasmic reticulum) a significant amount was present in the soluble fraction. This soluble amylase activity presumably results from organelle disruption during tissue processing. Alternatively, some or all of it may represent enzymes already discharged into the ductal space at the time the animals were killed. Supramaximal 6 A -:J' 5 ll ' 5 ll KP 12KP 15KP 15KS B 1.3KP 12KP 15KS 15KP Figure 3. Effect of FOY and FOY 35 on subcellular distribution of amylase during pancreatitis. A. Results from rats infused with or without caerulein with or without FOY. B. Results from mice fed the CDE diet with or without FOY 35. Open bars depict control animals, solid bars represent those given only caerulein or the CDE diet, and cross-hatched bars indicate the animals given either caerulein with FOY or the CDE diet with,foy 35. The subcellular fractions are the 13 g x 15 min pellet (1.3 KP), the 12, g x 12 min pellet (12 KP), the 15, g x 6 min pellet (15 KP), and the 15, g x 6 min supernatant (15 KS). Results represent mean and vertical bars the SEM values for 5-12 different animals and separate subcellular fractionations in each group. * p <.1 when caerulein or CDE alone was compared with control group. ** p <.1 when FOY + caerulein was compared with caerulein alone. Unless indicated, such comparisons revealed p values >.5. stimulation with caerulein resulted in an increase in the amount of amylase recovered from the soluble fraction, a change that had previously been reported and interpreted to indicate that supramaximal stimulation causes amylase to localize in fragile organelles that are subject to disruption during tissue processing (8). A corresponding decrease in the amylase activity present in the particulate fractions was also noted. Administration of FOY at the time of caerulein infusion prevented the alterations in amylase distribution induced by caerulein infusion (Figure 3A). Thus, in samples obtained from animals infused with caerulein plus FOY, the amylase con-
5 March 1989 ESTERASES N PANCREATTS 857 tent of the soluble fraction was similar to that noted in control animals not given a supramaximal dose of caerulein and significantly lower than that observed after caerulein infusion without FOY (p <.1). Administration of the CDE diet to mice also resulted in a significant increase in the amylase activity of the soluble fraction (Figure 3B). FOY 35, however, did not alter this redistribution of amylase activity. The CDE diet caused the digestive enzyme content of the pancreas to rise from a control value of 1.33 ±.35 D/JLg DNA to a level of 3.56 ±.3 U/JLg DNA (p <.1)' as enzyme synthesis continues while enzyme secretion is reduced (18,19). Administration of FOY 35 did not reduce the elevation in pancreatic amylase content that occurred after the ingestion of the CDE diet. After ingestion of the CDE diet containing FOY 35, the amylase content of the pancreas was found to be 3.27 ±.29 U/JLg DNA. Effects of FOY on Subcellular Distribution of Cathepsin B The cathepsin B content of the pancreas, expressed as units per microgram of DNA, was not altered by either supramaximal stimulation with caerulein on administration of the CDE diet, and was unaffected by either FOY or FOY 35 (not shown). Subcellular fractionation of samples obtained from control animals revealed that cathepsin B activity was recovered, primarily, in the 12, g pellet (Figures 4A and 4B) identified earlier (3) as being enriched in mitochondria and lysosomes. A significant portion of the cathepsin B activity was also present in the 13 g pellet. This observation has been interpreted to indicate the presence of heavy lysosomes. We have previously reported that supramaximal stimulation with caerulein causes a redistribution of cathepsin B. n subcellular fractionation experiments such as this, the redistribution of cathepsin B causes an increased amount to be recovered in the 13 g pellet, whereas that in the 12, g pellet declines (3). The redistribution of cathepsin B caused by caerulein infusion was found to be markedly reduced by infusion of FOY (p <.1) (Figure 4A). The CDE diet also caused the cathepsin B activity to increase in the 13 g pellet and to decrease in the 12, g pellet (Figure 4B). Administration of FOY 35 with the CDE diet partially, but significantly (p <.1), reduced this redistribution of cathepsin B (Figure 4B). Discussion FOY and FOY 35 are synthetic guanidino acid esters that inhibit a number of esterases including trypsin, phospholipase A z, kallikrein, plasmin, 5 A,..." -' 4 g l- LL. 3...,!1i!,.z. 2 1 nil 7 6 B 1 Lo KP 12KP 15KP 15KS 1.3KP 12KP 15KP 15KS Figure 4. Effect of FOY and FOY 35 on subcellular distribution of cathepsin B. Experimental groups and subcellular fractions are as described in Figure 3. A. Results for rats given caerulein with or without FOY. B. Results for mice fed the CDE diet with or without FOY 35. n panel A: * p <.1 when caerulein was compared with control; * * p <.1 when FOY + caerulein was compared with caerulein alone. n panel B: * p <.1 when CDE was compared with control; * * p <.1 when FOY + CDE was compared with CDE alone. Unless otherwise indicated, such comparisons revealed p values >.5. thrombin, and the C 1R and C 1 esterases (2-22). FOY 35, in contrast to FOY, is absorbed from the gastrointestinal tract, and circulating levels of the active esterase inhibitor can be detected after oral administration. The relatively low molecular weight of FOY and FOY 35 (417 and 495 daltons, respectively) has suggested that they might gain entry into the pancreas from the vascular space and has encouraged studies evaluating their use in the therapy of acute pancreatitis. n this communication, we have confirmed several recent reports that have indicated that these agents can exert a protective effect against secretagogue- (6,23) as well as diet- (7) induced pancreatitis. FOY was found to prevent the hyperamylasemia, pancreatic edema, inflammatory cell infiltrate, and acinar cell vacuolization that follow supramaximal stimulation with caerulein (Figure la, Table 1). Similarly, FOY 35 was noted to reduce the hyperamylasemia and mortality rate that occur after administration of the CDE diet (Fig-
6 858 OHSHO ET AL. GASTROENTEROLOGY Vol. 96, No.3 ures 1B and 2). These observations indicate that enzyme activity subject to FOY inhibition plays an important role in the evolution of both of these models of pancreatitis but, by themselves, these findings do not further define the events that are modulated by the esterase inhibitors. The ability of FOY to reduce the severity of secretagogue-induced pancreatitis might be explained by interference by FOY with either the binding or subsequent acinar cell stimulation by caeruleini.e., that FOY might act by preventing secretagogueinduced supramaximal stimulation. This possibility was tested by measuring in vitro caerulein-stimulated amylase secretion from acini in the presence and absence of 1-4 M FOY. That concentration of FOY was chosen after preliminary calculations indicated that infusion of 5 mg/kg. h of FOY in 1-g rats for 3 h would achieve a FOY level in blood :51-4 M. The observation that 1-4 M FOY does not interfere with the stimulation of amylase release induced by low concentrations of caerulein and does not prevent the inhibition of secretion that follows exposure to high concentrations of the secretagogue indicates that FOY does not interfere with binding or the subsequent stimulus-secretion coupling events that follow the binding of caerulein to surface membrane receptors. We have previously shown that the CDE diet interferes with stimulus-secretion coupling at a step subsequent to hormone-receptor binding and before membrane phosphoinositide hydrolysis by phospholipase C (9). As a result, acini taken from mice fed the CDE diet are unresponsive to in vitro stimulation by secretagogues such as caerulein. We considered the possibility that FOY 35 might protect against CDE diet-induced pancreatitis by preventing the development of this defect in stimulus-secretion coupling. To test this possibility, acini taken from mice given the CDE diet with or without FOY 35 were prepared and in vitro caerulein stimulation of amylase secretion was evaluated. FOY 35 did not prevent the reduction in caerulein-stimulated amylase secretion that occurred after administration of the CDE diet. This observation indicates that FOY 35 does not protect against CDE diet-induced pancreatitis by preventing the defect in stimulus-secretion coupling caused by the ethionine-containing diet. Both the secretagogue and the diet-induced models of experimental pancreatitis are characterized by the formation of large cytoplasmic vacuoles that contain both digestive enzyme zymogens and lysosomal hydrolases. n the diet model, these vacuoles arise by crinophagy (i.e., fusion between zymogen granules and lysosomes), whereas in the secretagogue model, the vacuoles appear to develop as a result of both crinophagy and a defect in the normal sorting mechanisms that segregate lysosomal hydrolases from digestive zymogens during intracellular transport (1-4). n both models, colocalization of lysosomal hydrolases and digestive zymogens in large cytoplasmic vacuoles has been noted to precede the appearance of cell injury. As the lysosomal hydrolase cathepsin B can activate trypsinogen and trypsin can activate the other zymogens, this colocalization phenomenon might result in intracellular digestive enzyme activation and be an important event in the evolution of these forms of pancreatitis. n our previously reported studies, colocalization of digestive enzymes and lysosomal hydrolases within the large vacuoles was demonstrated using the morphologic techniques of immunolocalization and enzyme cytochemistry (1-4). n the secretagogue-induced model, subcellular fractionation studies were also performed (3). They indicated that a redistribution of cathepsin B from the lysosomeand mitochondria-enriched fraction to the more dense zymogen granule-enriched fraction corresponded to the morphologically demonstrated colocalization. n this communication, we report the results of similar subcellular fractionation studies, performed after administration of the CDE diet. Once again, redistribution of cathepsin B to the zymogen granule-enriched fraction was noted. The most intriguing observation reported in this communication is the finding that FOY and FOY 35 prevent the phenomenon of cathepsin B redistribution that characterizes both of these models of pancreatitis. Thus, FOY administration markedly reduces the increase in cathepsin B noted in the 13 g pellet and the decrease in the 12, g pellet after subcellular fractionation of samples taken from rats subject to supramaximal stimulation with caerulein (Figure 4A) and from mice fed the CDE diet (Figure 4B). As a result of these observations, we conclude that the redistribution phenomenon (and presumably the co localization of digestive zymogens and lysosomal hydrolases) is dependent on esterase activity, which is susceptible to inhibition by FOY and FOY 35. Further identification and characterization of that enzyme activity may shed important light on the pathophysiology of acute pancreatitis. Candidate enzymes should include the phospholipases as well as the proteases that can be inhibited by FOY. Administration of FOY was found to prevent both cytoplasmic vacuole formation (Table 1) and the increase in soluble amylase activity that is observed when the pancreas is fractionated after supramaximal stimulation with caerulein (Figure 3A). t is likely that these two effects are interrelated as the increase in soluble amylase activity probably results from rupture of the large cytoplasmic vacuoles that
7 March 1989 ESTERASES N PANCREATTS 859 have been noted to become increasingly fragile during supramaximal stimulation with caerulein (3). On the other hand, FOY 35 does not prevent vacuole formation (not shown) or alter the increase in soluble amylase activity in fractions taken from mice fed the CDE diet (Figure 3B). As both FOY and FOY 35 reduce cathepsin B redistribution, an explanation for the differing effect of FOY and FOY 35 on vacuole formation and on the distribution of amylase activity is not readily apparent from these studies. These observations, however, might suggest that vacuole formation is not the consequence of cathepsin B redistribution. Further, these findings may indicate that vacuole formation either precedes or occurs independently of cathepsin B redistribution, and that ii the secretagogue model, but not in the diet model, of pancreatitis, vacuole formation involves the action of esterases that are subject to inhibition by FOY. Further clarification of these issues must await the results of future studies. References 1. Koike H, Steer ML, Meldolesi J. Pancreatic effects of ethionine: blockade of exocytosis and appearance of crinophagy and autophagy precede cellular necrosis. Am J Physiol 1982;242:G Watanabe O. Baccino FM. Steer ML. Meldolesi J. Effects of supramaximal caerulein stimulation on the ultrastructure of rat pancreatic acinar cell: early morphological changes during the development of experimental pancreatitis. Am J Physiol 1984;246:G Saluja A. Hashimoto S. Saluja M. Powers RE. Meldolesi J. Steer ML. Subcellular redistribution of lysosomal enzymes during caerulein-induced pancreatitis. Am J Physiol 1987; 253:G Saito. Hashimoto S. Saluja A. Steer ML. Meldolesi J. ntracellular transport of pancreatic zymogens during caerulein supramaximal stimulation. Am J Physio1987;253:G Greenbaum LA. Hirshkowitz A. Endogenous cathepsin activates trypsinogen in extracts of dog pancreas. Proc Soc Exp Bio Med 1961;17: Wisner JR. Renner G. Grendell JH. Niederau C. Ferrell LD. Gabexate mesilate (FOY) protects against ceruletide-induced acute pancreatitis in the rat. Pancreas 1987;2: Niedereau C. Liddle RA. Ferrell LD. Grendell JH. Beneficial effects of cholecystokinin-receptor blockade and inhibition of proteolytic enzyme activity in experimental acute hemorrhagic pancreatitis in mice. Evidence for cholecystokinin as a major factor in development of acute pancreatitis. J Clin nvest 1986;78: Saluja A. Saito. Saluja M. et al. n-vivo rat pancreatic acinar cell function during supramaximal stimulation with caerulein. Am J Physio1985;249:G Powers RE. Saluja AK. Houlihan MJ, Steer ML. Diminished agonist-stimulated inositol trisphosphate generation block stimulus-secretion coupling in mouse pancreatic acini during diet-induced experimental pancreatitis. J Clin nvest 1986;77: Tartakoff A. Jamieson JE. Fractionation of guinea pig pancreas. Methods Enzymo1974;31: Bernfield P. Amylases X and B. n: Colowick SP. Kaplan ND. eds. Methods in enzymology. Volume 1. New York: Academic. 1955: McDonald JK. Ellis S. On the substrate specificity of cathepsin B, and B2 including a new flu orogenic substrate for cathepsin B,. Life Sci 1975;17: Labarca C. Paigen K. A simple. rapid and sensitive DNA assay procedure. Anal Biochem 198;12: Colten T. Statistics in medicine. Boston: Little. Brown. 1974: Lampel M. Kern H. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch [Aj1977;373: Manabe T. Steer ML. Experimental acute pancreatitis in mice: protective effects of glucagon. Gastroenterology 1979;76: Williams JA. Korc MA. Dormer RL. Action of secretagogues on a new preparation of functionally intact isolated pancreatic acini. Am J Physio1978;235:E Lombardi B. Estes LW. Longnecker DS. Acute hemorrhagic pancreatitis (massive necrosis) with fat necrosis induced in mice by DL-ethionine fed with a choline deficient diet. Am J Patho1975;79: Gilliland L. Steer ML. Effects of ethionine on digestive enzyme synthesis and discharge by mouse pancreas. Am J Physio198;239:G Muramatsu M. Fujii S. nhibitory effects of w-guanidino esters on trypsin. plasmin. plasma kallikrein and thrombin. Biochim Biophys Acta 1972;268: Tamura Y. Hirado M. Okamura Y. Minato Y. Fujii S. Synthetic inhibitors of trypsin. plasmin. kallikrein. thrombin. C,r and C, esterase. Biochim Biophys Acta 1977;484: Freise J, Magerstedt p. Schmid K. nhibition of phospholipase A2 by gabexate mesilate. camostate and aprotinin. Enzyme 1983;3: Okegawa T. Fujita T. Yamamoto Y. et al. Effects of FOY on experimental acute pancreatitis. Gendai ryo (Current Medicine) 1974;6:1-15. Received March Accepted September Address requests for reprints to: Michael L. Steer. M.D. Department of Surgery. Beth srael Hospital. 33 Brookline Avenue. Boston. Massachusetts This study was supported by National nstitutes of Health grants AM and AM G. Ohshio was supported by funds from the Uehara Memorial Foundation.
Pathobiology of Experimental Acute Pancreatitis
 Pathobiology of Experimental Acute Pancreatitis The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Steer, M. L. 1992. Pathobiology
Pathobiology of Experimental Acute Pancreatitis The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Steer, M. L. 1992. Pathobiology
Zymogen and Lysosomal Enzyme Colocalization
 Pancreatic Duct Obstruction in Rabbits Causes Digestive Zymogen and Lysosomal Enzyme Colocalization Ashok Saluja,* Manju Saluja,* Antonello Villa,* Ubaldo Leli,* Peter Rutledge,* Jacopo Meldolesi,$ and
Pancreatic Duct Obstruction in Rabbits Causes Digestive Zymogen and Lysosomal Enzyme Colocalization Ashok Saluja,* Manju Saluja,* Antonello Villa,* Ubaldo Leli,* Peter Rutledge,* Jacopo Meldolesi,$ and
A Cholecystokinin-releasing Factor Mediates Ethanol-induced Stimulation of Rat Pancreatic Secretion
 A Cholecystokinin-releasing Factor Mediates Ethanol-induced Stimulation of Rat Pancreatic Secretion A.K. Saluja, L. Lu, Y. Yamaguchi, B. Hofbauer, M. Rünzi, R. Dawra, M. Bhatia, and M.L. Steer Department
A Cholecystokinin-releasing Factor Mediates Ethanol-induced Stimulation of Rat Pancreatic Secretion A.K. Saluja, L. Lu, Y. Yamaguchi, B. Hofbauer, M. Rünzi, R. Dawra, M. Bhatia, and M.L. Steer Department
Experimental Acute Pancreatitis in Mice
 GASTROENTEROLOGY 76:529-534, 1979 Experimental Acute Pancreatitis in Mice Protective Effects of Glucagon T ADAO MANABE, M.D., and MICHAEL L. STEER, M.D. Department of Surgery, Beth Israel Hospital, and
GASTROENTEROLOGY 76:529-534, 1979 Experimental Acute Pancreatitis in Mice Protective Effects of Glucagon T ADAO MANABE, M.D., and MICHAEL L. STEER, M.D. Department of Surgery, Beth Israel Hospital, and
Lysosomal Enzymes and Pancreatitis
 620 EDITORIALS GASTROENTEROLOGY Vol. 109, No. 2 MA. &adenosyi-l-methionine attenuates alcohol-induced liver injury in the baboon. Hepatology 1990;11:165-172. 31. Lieber CS, Robins S J, Leo MA. Hepatic
620 EDITORIALS GASTROENTEROLOGY Vol. 109, No. 2 MA. &adenosyi-l-methionine attenuates alcohol-induced liver injury in the baboon. Hepatology 1990;11:165-172. 31. Lieber CS, Robins S J, Leo MA. Hepatic
EFFECT OF HORMONES ON PANCREATIC MACROMOLECULAR TRANSPORT
 GASTROENTEROLOGY 68: 1536-1542, 1975 Copyright 1975 by The Williams & Wilkins Co. Vol. 68, No.6 Printed in U.S.A. EFFECT OF HORMONES ON PANCREATIC MACROMOLECULAR TRANSPORT MANJIT SiNGH, M.D., F.R.C.P.
GASTROENTEROLOGY 68: 1536-1542, 1975 Copyright 1975 by The Williams & Wilkins Co. Vol. 68, No.6 Printed in U.S.A. EFFECT OF HORMONES ON PANCREATIC MACROMOLECULAR TRANSPORT MANJIT SiNGH, M.D., F.R.C.P.
COLLOID DROPLET FORMATION IN DOG THYROID IN VITRO
 COLLOID DROPLET FORMATION IN DOG THYROID IN VITRO Induction by Dibutyryl Cyclic-AMP I. PASTAN and S. HI. WOLLMAN. Froml the National Institute of Arthritis and Metabolic Diseases and the National Cancer
COLLOID DROPLET FORMATION IN DOG THYROID IN VITRO Induction by Dibutyryl Cyclic-AMP I. PASTAN and S. HI. WOLLMAN. Froml the National Institute of Arthritis and Metabolic Diseases and the National Cancer
THE DEPENDENCE OF EXOCRINE PANCREATIC SECRETION ON INSULIN IN SHEEP
 Quarterly Journal of Experimental Physiology (1984) 69, 35-39 3 5 Printed in Great Britain THE DEPENDENCE OF EXOCRINE PANCREATIC SECRETION ON INSULIN IN SHEEP STEFAN PIERZYNOWSKI AND W. BAREJ The Institute
Quarterly Journal of Experimental Physiology (1984) 69, 35-39 3 5 Printed in Great Britain THE DEPENDENCE OF EXOCRINE PANCREATIC SECRETION ON INSULIN IN SHEEP STEFAN PIERZYNOWSKI AND W. BAREJ The Institute
and Its Inhibitor Human-Polymorphonuclear-Leucocyte Neutral Protease Studies with Fluorescein-Labelled Polymeric Collagen Fibrils as a Substrate
 Eur. J. Biochem. 67, 165169 (1976) HumanPolymorphonuclearLeucocyte Neutral Protease and Its Inhibitor Studies with FluoresceinLabelled Polymeric Collagen Fibrils as a Substrate Frank S. STEVEN, David W.
Eur. J. Biochem. 67, 165169 (1976) HumanPolymorphonuclearLeucocyte Neutral Protease and Its Inhibitor Studies with FluoresceinLabelled Polymeric Collagen Fibrils as a Substrate Frank S. STEVEN, David W.
CAUSE-EFFECT RELATIONSHIPS BETWEEN ZYMOGEN ACTIVATION AND OTHER EARLY EVENTS IN SECRETAGOGUE-INDUCED ACUTE
 Page 1 of 38 Articles in PresS. Am J Physiol Gastrointest Liver Physiol (March 1, 2007). doi:10.1152/ajpgi.00543.2006 Van Acker, 1 CAUSE-EFFECT RELATIONSHIPS BETWEEN ZYMOGEN ACTIVATION AND OTHER EARLY
Page 1 of 38 Articles in PresS. Am J Physiol Gastrointest Liver Physiol (March 1, 2007). doi:10.1152/ajpgi.00543.2006 Van Acker, 1 CAUSE-EFFECT RELATIONSHIPS BETWEEN ZYMOGEN ACTIVATION AND OTHER EARLY
TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
 Journal of Supramolecular Structure 4:441 (401)-447 (407) (1976) TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
Journal of Supramolecular Structure 4:441 (401)-447 (407) (1976) TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
Experiments were carried out then with the object of producing complete disappearance of the A
 Relation of Glucagon to A Cells of the Pancreas*. (22339) SERGIO A. BENCOSME AND J. FREI. (Introduced by J.S.L. Browne Departament of pathology, Queen`s University, Kingston, Ontario, Canada. In spite
Relation of Glucagon to A Cells of the Pancreas*. (22339) SERGIO A. BENCOSME AND J. FREI. (Introduced by J.S.L. Browne Departament of pathology, Queen`s University, Kingston, Ontario, Canada. In spite
Synopsis. Received March 2, adrenaline. Mosinger and Kujalova (1964) reported that adrenaline-induced lipolysis
 Studies on Reduction of Lipolysis in Adipose Tissue on Freezing and Thawing YASUSHI SAITO1, NoBUO MATSUOKA1, AKIRA KUMAGAI1, HIROMICHI OKUDA2, AND SETSURO FUJII3 Chiba University, Chiba 280, Japan, 2Department
Studies on Reduction of Lipolysis in Adipose Tissue on Freezing and Thawing YASUSHI SAITO1, NoBUO MATSUOKA1, AKIRA KUMAGAI1, HIROMICHI OKUDA2, AND SETSURO FUJII3 Chiba University, Chiba 280, Japan, 2Department
Iodide transport in isolated cells of mouse submaxillary gland
 J. Biosci., Vol. 10, Number 3, September 1986, pp. 303 309. Printed in India. Iodide transport in isolated cells of mouse submaxillary gland R. K. BANERJEE*, A. K. BOSE, T. K. CHAKRABORTY, P. K. DE and
J. Biosci., Vol. 10, Number 3, September 1986, pp. 303 309. Printed in India. Iodide transport in isolated cells of mouse submaxillary gland R. K. BANERJEE*, A. K. BOSE, T. K. CHAKRABORTY, P. K. DE and
PEPSIN SECRETION DURING DAMAGE BY ETHANOL AND SALICYLIC ACID
 GASTROENTEROLOGY Copyriht 1972 by The Williams & Wilkins Co. Vol. 62. No. 3 Printed in U.S. A. PEPSIN SECRETION DURING DAMAGE BY ETHANOL AND SALICYLIC ACID LEONARD R. JOHNSON, PH.D. Department of Physiology
GASTROENTEROLOGY Copyriht 1972 by The Williams & Wilkins Co. Vol. 62. No. 3 Printed in U.S. A. PEPSIN SECRETION DURING DAMAGE BY ETHANOL AND SALICYLIC ACID LEONARD R. JOHNSON, PH.D. Department of Physiology
Caerulein-Induced Acute Necrotizing Pancreatitis in Mice: Protective Effects of Proglumide, Benzotript, and Secretin
 GASTRONTROLOGY 198;88:119-4 Caerulein-Induced Acute Necrotizing Pancreatitis in Mice: Protective ffects of Proglumide, Benzotript, and Secretin CLAUS NIDRAU, LINDA D. FRRLL, and JAMS H. GRNDLL Department
GASTRONTROLOGY 198;88:119-4 Caerulein-Induced Acute Necrotizing Pancreatitis in Mice: Protective ffects of Proglumide, Benzotript, and Secretin CLAUS NIDRAU, LINDA D. FRRLL, and JAMS H. GRNDLL Department
hydrolyzes ATP to exchange 3 Na + in for 2 K + out generate the transcellular Na + and K + gradients provides the electrochemical gradient
 Regulation of pancreatic excretory function by ion channels Viktoria Venglovecz 2015 Morphology of the pancreas Composition of pancreatic juice 1 2 liters of pancreatic juice per day acini secrete isotonic,
Regulation of pancreatic excretory function by ion channels Viktoria Venglovecz 2015 Morphology of the pancreas Composition of pancreatic juice 1 2 liters of pancreatic juice per day acini secrete isotonic,
INCREASE IN ACCUMULATION OF L-DOPA (3,4-DIHYDROXY PHENYLALANINE) IN BRAIN SLICES BY ALCOHOL
 INCREASE IN ACCUMULATION OF L-DOPA (3,4-DIHYDROXY PHENYLALANINE) IN BRAIN SLICES BY ALCOHOL KENICHI KANIIKE* AND HIROSHI YOSHIDA Department of Pharmacology, Faculty of Medicine, Osaka University, Osaka
INCREASE IN ACCUMULATION OF L-DOPA (3,4-DIHYDROXY PHENYLALANINE) IN BRAIN SLICES BY ALCOHOL KENICHI KANIIKE* AND HIROSHI YOSHIDA Department of Pharmacology, Faculty of Medicine, Osaka University, Osaka
lysosomes Ingested materials Defective cell components Degrades macromolecules of all types:
 lysosomes Digests Ingested materials Defective cell components Degrades macromolecules of all types: Proteins Nucleic acids Carbohydrates Lipids Single membrane bound vesicle, contains up to 50 digestive
lysosomes Digests Ingested materials Defective cell components Degrades macromolecules of all types: Proteins Nucleic acids Carbohydrates Lipids Single membrane bound vesicle, contains up to 50 digestive
FOCUS SubCell. For the Enrichment of Subcellular Fractions. (Cat. # ) think proteins! think G-Biosciences
 169PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS SubCell For the Enrichment of Subcellular Fractions (Cat. # 786 260) think
169PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS SubCell For the Enrichment of Subcellular Fractions (Cat. # 786 260) think
The gallbladder. Bile secretion:
 The gallbladder is a thin walled green muscular sac on the inferior surface of the liver. The gallbladder stores bile that is not immediately needed for digestion and concentrates it. When the muscular
The gallbladder is a thin walled green muscular sac on the inferior surface of the liver. The gallbladder stores bile that is not immediately needed for digestion and concentrates it. When the muscular
PRODUCT INFORMATION & MANUAL
 PRODUCT INFORMATION & MANUAL Mitochondrial Extraction Kit NBP2-29448 Research use only. Not for diagnostic or therapeutic procedures www.novusbio.com P: 303.760.1950 P: 888.506.6887 F: 303.730.1966 technical@novusbio.com
PRODUCT INFORMATION & MANUAL Mitochondrial Extraction Kit NBP2-29448 Research use only. Not for diagnostic or therapeutic procedures www.novusbio.com P: 303.760.1950 P: 888.506.6887 F: 303.730.1966 technical@novusbio.com
ASSAY OF SPHINGOMYELINASE ACTIVITY
 ASSAY OF SPHINGOMYELINASE ACTIVITY Protocol for Protein Extraction Stock Solution 1. Leupeptin/hydrochloride (FW 463.0,
ASSAY OF SPHINGOMYELINASE ACTIVITY Protocol for Protein Extraction Stock Solution 1. Leupeptin/hydrochloride (FW 463.0,
EFFECTS OF EXOGENOUSLY ADDED SHORT-CHAIN FATTY ACIDS ON PANCREATIC EXOCRINE SECRETION IN DOMESTIC RABBIT
 EFFECTS OF EXOGENOUSLY ADDED SHORT-CHAIN FATTY ACIDS ON PANCREATIC EXOCRINE SECRETION IN DOMESTIC RABBIT DOJANA N 1., POP A 2., PAPUC C 3. 1 Department of Animal Physiology, Faculty of Veterinary Medicine,
EFFECTS OF EXOGENOUSLY ADDED SHORT-CHAIN FATTY ACIDS ON PANCREATIC EXOCRINE SECRETION IN DOMESTIC RABBIT DOJANA N 1., POP A 2., PAPUC C 3. 1 Department of Animal Physiology, Faculty of Veterinary Medicine,
DIGESTIVE SYSTEM II ACCESSORY DIGESTIVE ORGANS
 DIGESTIVE SYSTEM II ACCESSORY DIGESTIVE ORGANS Dr. Larry Johnson Texas A& M University Objectives Distinguish between the parotid and submandibular salivary glands. Understand and identify the structural
DIGESTIVE SYSTEM II ACCESSORY DIGESTIVE ORGANS Dr. Larry Johnson Texas A& M University Objectives Distinguish between the parotid and submandibular salivary glands. Understand and identify the structural
Therapeutic Regimens in Acute Experimental Hemorrhagic Pancreatitis
 GASTROENTEROLOGY 1988;95:1648-57 Therapeutic Regimens in Acute Experimental Hemorrhagic Pancreatitis Effects of Hydration, Oxygenation, Peritoneal Lavage, and a Potent Protease Inhibitor CLAUS NIEDERAU,
GASTROENTEROLOGY 1988;95:1648-57 Therapeutic Regimens in Acute Experimental Hemorrhagic Pancreatitis Effects of Hydration, Oxygenation, Peritoneal Lavage, and a Potent Protease Inhibitor CLAUS NIEDERAU,
Study of the main chemical components of Ganoderma lucidum
 Study of the main chemical components of Ganoderma lucidum Yasuo Komota et al Tokyo Medical and Dental University [Purpose] As part of the means for exerting quality control on Ganoderma lucidum 50% ethanol
Study of the main chemical components of Ganoderma lucidum Yasuo Komota et al Tokyo Medical and Dental University [Purpose] As part of the means for exerting quality control on Ganoderma lucidum 50% ethanol
(Received 6 August 1979)
 J. Phyoiol. (1980), 303, pp. 33-41 33 With 2 text-figurew Printed in Great Britain PARALLEL SECRETION OF ENZYMES BY THE RABBIT PANCREAS BY E. L. GILLILAND* AND G. GLAZER From the Academic Surgical Unit,
J. Phyoiol. (1980), 303, pp. 33-41 33 With 2 text-figurew Printed in Great Britain PARALLEL SECRETION OF ENZYMES BY THE RABBIT PANCREAS BY E. L. GILLILAND* AND G. GLAZER From the Academic Surgical Unit,
Human Saliva as a Convenient Source of Ribonuclease. By S. BRADBURY
 Human Saliva as a Convenient Source of Ribonuclease 323 By S. BRADBURY (From the Cytological Laboratory, Department of Zoology, University Museum, Oxford) SUMMARY Saliva, heated to 80 C for 10 minutes
Human Saliva as a Convenient Source of Ribonuclease 323 By S. BRADBURY (From the Cytological Laboratory, Department of Zoology, University Museum, Oxford) SUMMARY Saliva, heated to 80 C for 10 minutes
Study of the main chemical components of Ganoderma lucidum
 Study of the main chemical components of Ganoderma lucidum Yasuo Komota et al Tokyo Medical and Dental University [Purpose] As part of the means for exerting quality control on Ganoderma lucidum 50% ethanol
Study of the main chemical components of Ganoderma lucidum Yasuo Komota et al Tokyo Medical and Dental University [Purpose] As part of the means for exerting quality control on Ganoderma lucidum 50% ethanol
Thursday, October 16 th
 Thursday, October 16 th Good morning. Those of you needing to take the Enzymes and Energy Quiz will start very soon. Students who took the quiz Wednesday: Please QUIETLY work on the chapter 6 reading guide.
Thursday, October 16 th Good morning. Those of you needing to take the Enzymes and Energy Quiz will start very soon. Students who took the quiz Wednesday: Please QUIETLY work on the chapter 6 reading guide.
Supporting Information
 Supporting Information Gerasimenko et al..73/pnas.39 SI Materials and Methods Reagents used in this study include Fluo-4/Fura- (Invitrogen), thapsigargin (albiochem), collagenase (Worthington), palmitoleic
Supporting Information Gerasimenko et al..73/pnas.39 SI Materials and Methods Reagents used in this study include Fluo-4/Fura- (Invitrogen), thapsigargin (albiochem), collagenase (Worthington), palmitoleic
TEMPORARY INHIBITION OF TRYPSIN*
 TEMPORARY INHIBITION OF TRYPSIN* BY M. LASKOWSKI AND FENG CHI WU (From the Department oj Biochemistry, Marquette University School of Medicine, Milwaukee, Wisconsin) (Received for publication, April 30,
TEMPORARY INHIBITION OF TRYPSIN* BY M. LASKOWSKI AND FENG CHI WU (From the Department oj Biochemistry, Marquette University School of Medicine, Milwaukee, Wisconsin) (Received for publication, April 30,
Instructions for Use. APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests
 3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
Pancreas composed of 2 parts: 1- exocrine gland 2- endocrine gland
 pancreas Pancreas composed of 2 parts: 1- exocrine gland 2- endocrine gland Acute pancreatitis Inflammation of the pancreas associated with acinar cell injury Clinical features: 1-abdominal pain cardinal
pancreas Pancreas composed of 2 parts: 1- exocrine gland 2- endocrine gland Acute pancreatitis Inflammation of the pancreas associated with acinar cell injury Clinical features: 1-abdominal pain cardinal
Cooke, Nahrwold and Grossman, 1967]. In the present experiments, attempts. Wales, 2033, Australia.
![Cooke, Nahrwold and Grossman, 1967]. In the present experiments, attempts. Wales, 2033, Australia. Cooke, Nahrwold and Grossman, 1967]. In the present experiments, attempts. Wales, 2033, Australia.](/thumbs/96/128693653.jpg) Quarterly Journal of Experimental Phyeiology (1973) 58, 335-343 BASAL AND POSTPRANDIAL PANCREATIC SECRETION IN RATS. By H. M. SiHw and T. J. HEATH. From the School of Physiology and Pharmacology, The University
Quarterly Journal of Experimental Phyeiology (1973) 58, 335-343 BASAL AND POSTPRANDIAL PANCREATIC SECRETION IN RATS. By H. M. SiHw and T. J. HEATH. From the School of Physiology and Pharmacology, The University
Broad Spectrum Protease Inhibitor Cocktail
 Broad Spectrum Protease Inhibitor Cocktail Catalog number: AR1182 Boster s Broad Spectrum Protease Inhibitor Cocktail is a complex of various protease inhibitors, which has been tested for inhibiting proteases
Broad Spectrum Protease Inhibitor Cocktail Catalog number: AR1182 Boster s Broad Spectrum Protease Inhibitor Cocktail is a complex of various protease inhibitors, which has been tested for inhibiting proteases
Day Date Title Instructor 5 th Ed 6 th Ed. Protein digestion and AA absorption
 Day Date Title Instructor 5 th Ed 6 th Ed 1 Tuesday 18 April 2017 Protein digestion and AA absorption D S Jairajpuri 250 256 250 256 2 Wednesday 19 April 2017 Removal of nitrogen and urea cycle D S Jairajpuri
Day Date Title Instructor 5 th Ed 6 th Ed 1 Tuesday 18 April 2017 Protein digestion and AA absorption D S Jairajpuri 250 256 250 256 2 Wednesday 19 April 2017 Removal of nitrogen and urea cycle D S Jairajpuri
A small, membrane-bound compartment capable of performing all the basic functions of life
 AP Biology The Cell The Cell Cell: A small, membrane-bound compartment capable of performing all the basic functions of life Discovery of Cells: - 17 th century - A Dutch clothing dealer named Antonie
AP Biology The Cell The Cell Cell: A small, membrane-bound compartment capable of performing all the basic functions of life Discovery of Cells: - 17 th century - A Dutch clothing dealer named Antonie
Standard 2 Exam Biology. 2. This macromolecule is responsible for short term energy storage and structural support in plants
 1. This macromolecule is responsible for structural support, movement, enzymatic activity, cell communication, and is made of amino acids. a. Lipids b. Carbohydrates c. Proteins d. Nucleic Acids e. ATP
1. This macromolecule is responsible for structural support, movement, enzymatic activity, cell communication, and is made of amino acids. a. Lipids b. Carbohydrates c. Proteins d. Nucleic Acids e. ATP
interstitium at pressures below the maximum secretory pressure of the pancreas. The ink
 Gut, 1970, 11, 69-73 Effect of pressure on the integrity of the duct-acinar system of the pancreas R. C. PIROLA1 AND A. E. DAVIS From the Department of Medicine, Prince Henry Hospital, Sydney, Australia
Gut, 1970, 11, 69-73 Effect of pressure on the integrity of the duct-acinar system of the pancreas R. C. PIROLA1 AND A. E. DAVIS From the Department of Medicine, Prince Henry Hospital, Sydney, Australia
VAMP8 is the v-snare that mediates basolateral exocytosis in a mouse model of alcoholic pancreatitis
 Research article VAMP8 is the v-snare that mediates basolateral exocytosis in a mouse model of alcoholic pancreatitis Laura I. Cosen-Binker, 1 Marcelo G. Binker, 1 Cheng-Chun Wang, 2 Wanjin Hong, 2 and
Research article VAMP8 is the v-snare that mediates basolateral exocytosis in a mouse model of alcoholic pancreatitis Laura I. Cosen-Binker, 1 Marcelo G. Binker, 1 Cheng-Chun Wang, 2 Wanjin Hong, 2 and
150 mm HCO How Does the Pancreas Do It? Clues from Computer Modelling of the Duct Cell
 JOP. J. Pancreas (Online) 2001; 2(4 Suppl):198202. 150 mm How Does the Pancreas Do It? Clues from Computer Modelling of the Duct Cell Yoshiro Sohma 1, Michael A Gray 2, Yusuke Imai 1, Barry E Argent 2
JOP. J. Pancreas (Online) 2001; 2(4 Suppl):198202. 150 mm How Does the Pancreas Do It? Clues from Computer Modelling of the Duct Cell Yoshiro Sohma 1, Michael A Gray 2, Yusuke Imai 1, Barry E Argent 2
PREVIOUS work has shown that ingestion
 192 B. C. DILWORTH, C. D. SCHULTZ AND E. J. DAY Summit, Pennsylvania, for their cooperative efforts and grant-in-aid in support of this work. REFERENCES Dilworth, B. C, C. D. Schultz and E. J. Day, 1970.
192 B. C. DILWORTH, C. D. SCHULTZ AND E. J. DAY Summit, Pennsylvania, for their cooperative efforts and grant-in-aid in support of this work. REFERENCES Dilworth, B. C, C. D. Schultz and E. J. Day, 1970.
Ultrastructural Study of Human Natural Killer CNK) Cell*)
 Hiroshima Journal of Medical Sciences Vol. 31, No. 1, March, 1982 HJIM 31-6 31 Ultrastructural Study of Human Natural Killer CNK) Cell*) Yoshinori KAWAGUCHI, Eishi KITTAKA, Yoshito TANAKA, Takeo TANAKA
Hiroshima Journal of Medical Sciences Vol. 31, No. 1, March, 1982 HJIM 31-6 31 Ultrastructural Study of Human Natural Killer CNK) Cell*) Yoshinori KAWAGUCHI, Eishi KITTAKA, Yoshito TANAKA, Takeo TANAKA
Week 3 The Pancreas: Pancreatic ph buffering:
 Week 3 The Pancreas: A gland with both endocrine (secretion of substances into the bloodstream) & exocrine (secretion of substances to the outside of the body or another surface within the body) functions
Week 3 The Pancreas: A gland with both endocrine (secretion of substances into the bloodstream) & exocrine (secretion of substances to the outside of the body or another surface within the body) functions
FOR OPTIMAL GUT HEALTH KEMIN.COM/GUTHEALTH
 FOR OPTIMAL GUT HEALTH KEMIN.COM/GUTHEALTH ALETA A SOURCE OF 1,3-BETA GLUCANS Aleta is highly bioavailable, offering a concentration greater than 5% of 1,3-beta glucans. Aleta provides a consistent response
FOR OPTIMAL GUT HEALTH KEMIN.COM/GUTHEALTH ALETA A SOURCE OF 1,3-BETA GLUCANS Aleta is highly bioavailable, offering a concentration greater than 5% of 1,3-beta glucans. Aleta provides a consistent response
An Exploratory Study on the Development of an Animal Model of Acute Pancreatitis Following Nicotine Exposure
 TOBACCO INDUCED DISEASES Vol. 1, No. 3: 213-217 (2003) PTID Society An Exploratory Study on the Development of an Animal Model of Acute Pancreatitis Following Nicotine Exposure Chowdhury P Department of
TOBACCO INDUCED DISEASES Vol. 1, No. 3: 213-217 (2003) PTID Society An Exploratory Study on the Development of an Animal Model of Acute Pancreatitis Following Nicotine Exposure Chowdhury P Department of
PREPARED BY P.DHARANI PRASAD II YEAR B.PHARM II SEM SUB:PATHOPHYSIOLOGY
 CELL INJURY UNIT I PREPARED BY P.DHARANI PRASAD II YEAR B.PHARM II SEM SUB:PATHOPHYSIOLOGY DETECTION OF CELLULAR CHANGES AFTER INJURY BY: LIGHT MICROSCOPY OR GROSS EXAMINATION DETECT CHANGES HOURS TO DAYS
CELL INJURY UNIT I PREPARED BY P.DHARANI PRASAD II YEAR B.PHARM II SEM SUB:PATHOPHYSIOLOGY DETECTION OF CELLULAR CHANGES AFTER INJURY BY: LIGHT MICROSCOPY OR GROSS EXAMINATION DETECT CHANGES HOURS TO DAYS
Effect of infusions of phosphatides upon the atherosclerotic aorta in situ and as an ocular aortic implant*
 Volume 1 Number 4 Effect of infusions of phosphatides upon the atherosclerotic aorta in situ and as an ocular aortic implant* SANFORD. BYERS and MEYER FRIEDMAK Harold Brunn Institute, Mount Zion Hospital
Volume 1 Number 4 Effect of infusions of phosphatides upon the atherosclerotic aorta in situ and as an ocular aortic implant* SANFORD. BYERS and MEYER FRIEDMAK Harold Brunn Institute, Mount Zion Hospital
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
satisfactorily as a means of altering experimentally the ph of the upper
 THE REACTION QF HUMAN DUODENAL CONTENTS TO ACID AND ALKALINE MEAT MIXTURES By STACY R. METTIER (From I1e Thorndike Memorial Laboratory, Boston City Hospital, and the Department of Medicine, Harvard Medical
THE REACTION QF HUMAN DUODENAL CONTENTS TO ACID AND ALKALINE MEAT MIXTURES By STACY R. METTIER (From I1e Thorndike Memorial Laboratory, Boston City Hospital, and the Department of Medicine, Harvard Medical
Chapter 1 Plasma membranes
 1 of 5 TEXTBOOK ANSWERS Chapter 1 Plasma membranes Recap 1.1 1 The plasma membrane: keeps internal contents of the cell confined to one area keeps out foreign molecules that damage or destroy the cell
1 of 5 TEXTBOOK ANSWERS Chapter 1 Plasma membranes Recap 1.1 1 The plasma membrane: keeps internal contents of the cell confined to one area keeps out foreign molecules that damage or destroy the cell
1-It is to prevent back flow of fecal content from colon into small intestine.
 Function of the ileocecal valve: 1-It is to prevent back flow of fecal content from colon into small intestine. 2-The wall of the ileum for several centimeters preceding valve has a thickened muscular
Function of the ileocecal valve: 1-It is to prevent back flow of fecal content from colon into small intestine. 2-The wall of the ileum for several centimeters preceding valve has a thickened muscular
REGULATION OF ENZYME ACTIVITY. Medical Biochemistry, Lecture 25
 REGULATION OF ENZYME ACTIVITY Medical Biochemistry, Lecture 25 Lecture 25, Outline General properties of enzyme regulation Regulation of enzyme concentrations Allosteric enzymes and feedback inhibition
REGULATION OF ENZYME ACTIVITY Medical Biochemistry, Lecture 25 Lecture 25, Outline General properties of enzyme regulation Regulation of enzyme concentrations Allosteric enzymes and feedback inhibition
LAB 3: Biomolecules and Digestion
 Page 3.1 LAB 3: Biomolecules and Digestion Food taken into our bodies must first be broken down by mechanical and chemical digestion before it can be absorbed and used as an energy source. The chemical
Page 3.1 LAB 3: Biomolecules and Digestion Food taken into our bodies must first be broken down by mechanical and chemical digestion before it can be absorbed and used as an energy source. The chemical
Silver-Impregnation of the Golgi Complex in Epididymal Epithelial Cells of Mice
 CELL STRUCTURE AND FUNCTION 8, 339-346 (1984) C by Japan Society for Cell Biology Silver-Impregnation of the Golgi Complex in Epididymal Epithelial Cells of Mice Ikuo Yamaoka, Sumie Katsuta and Yoshimi
CELL STRUCTURE AND FUNCTION 8, 339-346 (1984) C by Japan Society for Cell Biology Silver-Impregnation of the Golgi Complex in Epididymal Epithelial Cells of Mice Ikuo Yamaoka, Sumie Katsuta and Yoshimi
Correction. (originally cited in print as reference #219; now cited as references 195 and 222)
 GASTROENTEROLOGY 2007;133:1056 Correction Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology 2007;132:1127-1151. The published version of this review article,
GASTROENTEROLOGY 2007;133:1056 Correction Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology 2007;132:1127-1151. The published version of this review article,
SensoLyte pnpp Alkaline Phosphatase Assay Kit *Colorimetric*
 SensoLyte pnpp Alkaline Phosphatase Assay Kit *Colorimetric* Catalog # 72146 Kit Size 500 Assays (96-well plate) Optimized Performance: This kit is optimized to detect alkaline phosphatase activity Enhanced
SensoLyte pnpp Alkaline Phosphatase Assay Kit *Colorimetric* Catalog # 72146 Kit Size 500 Assays (96-well plate) Optimized Performance: This kit is optimized to detect alkaline phosphatase activity Enhanced
Pancreas Fox Chapter 18 part 2 (also Chapter 19.3 & 19.4)
 Vert Phys PCB3743 Pancreas Fox Chapter 18 part 2 (also Chapter 19.3 & 19.4) T. Houpt, Ph.D. Anatomy of Digestive System Peristalsis Stomach and Acid Secretion Liver and Bile Secretion Pancreas and pancreatic
Vert Phys PCB3743 Pancreas Fox Chapter 18 part 2 (also Chapter 19.3 & 19.4) T. Houpt, Ph.D. Anatomy of Digestive System Peristalsis Stomach and Acid Secretion Liver and Bile Secretion Pancreas and pancreatic
Cell Injury MECHANISMS OF CELL INJURY
 Cell Injury MECHANISMS OF CELL INJURY The cellular response to injurious stimuli depends on the following factors: Type of injury, Its duration, and Its severity. Thus, low doses of toxins or a brief duration
Cell Injury MECHANISMS OF CELL INJURY The cellular response to injurious stimuli depends on the following factors: Type of injury, Its duration, and Its severity. Thus, low doses of toxins or a brief duration
SUPPLEMENTARY INFORMATION In format provided by JAATTELA (NOVEMBER 2005)
 Box S1: Methods for studying lysosomal function and integrity Volume and distribution of the acidic compartment. Acridine orange is a metachromatic fluorochrome and a weak base that accumulates in the
Box S1: Methods for studying lysosomal function and integrity Volume and distribution of the acidic compartment. Acridine orange is a metachromatic fluorochrome and a weak base that accumulates in the
1. Arrows A, B, and C in the diagram below represent the processes necessary to make the energy stored in food available for muscle activity.
 1. Arrows A, B, and C in the diagram below represent the processes necessary to make the energy stored in food available for muscle activity. The correct sequence of processes represented by A, B, and
1. Arrows A, B, and C in the diagram below represent the processes necessary to make the energy stored in food available for muscle activity. The correct sequence of processes represented by A, B, and
year 7 REVISION Spring Assessment - Biology
 year 7 REVISION Spring Assessment - Biology year 7 REVISION Microscope and Cells THE MICROSCOPE Used to view things too small for the naked eye. Consists of lenses of different magnification to make things
year 7 REVISION Spring Assessment - Biology year 7 REVISION Microscope and Cells THE MICROSCOPE Used to view things too small for the naked eye. Consists of lenses of different magnification to make things
Effect of 6-Aminonicotinamide on the activity of hexokinase and lactate dehydrogenase isoenzymes in regions of the rat brain
 J. Biosci., Vol. 6, Number 3, September 1984, pp. 331-336. Printed in India. Effect of 6-Aminonicotinamide on the activity of hexokinase and lactate dehydrogenase isoenzymes in regions of the rat brain
J. Biosci., Vol. 6, Number 3, September 1984, pp. 331-336. Printed in India. Effect of 6-Aminonicotinamide on the activity of hexokinase and lactate dehydrogenase isoenzymes in regions of the rat brain
Digestive Lecture Test Questions Set 4
 Digestive Lecture Test Questions Set 4 1. Which of the following is not associated directly with the small intestine: a. villi b. circular folds c. microvilli d. haustrae e. secretin 2. The largest (longest)
Digestive Lecture Test Questions Set 4 1. Which of the following is not associated directly with the small intestine: a. villi b. circular folds c. microvilli d. haustrae e. secretin 2. The largest (longest)
Acute Hemorrhagic Pancreatic Necrosis in Mice
 Acute Hemorrhagic Pancreatic Necrosis in Mice Induction in Male Mice Treated With Estradiol K. N. RAO, PhD, P. K. EAGON, PhD, K. OKAMURA, MD, D. H. VAN THIEL, MD, J. S. GAVALER, BS, R. H. KELLY, PhD, and
Acute Hemorrhagic Pancreatic Necrosis in Mice Induction in Male Mice Treated With Estradiol K. N. RAO, PhD, P. K. EAGON, PhD, K. OKAMURA, MD, D. H. VAN THIEL, MD, J. S. GAVALER, BS, R. H. KELLY, PhD, and
Phospholipid/Calcium-Dependent Protein Kinase
 J. Clin. Biochem. Nutr., 2, 171-177, 1987 Phospholipid/Calcium-Dependent Protein Kinase Activity in Human Cortisol-Hypersecreting Adrenocortical Adenoma Tatsuo ISHIZUKA,1,* Hiroshi MURASE,1 Midori YASUE,1
J. Clin. Biochem. Nutr., 2, 171-177, 1987 Phospholipid/Calcium-Dependent Protein Kinase Activity in Human Cortisol-Hypersecreting Adrenocortical Adenoma Tatsuo ISHIZUKA,1,* Hiroshi MURASE,1 Midori YASUE,1
Manual (Second edition)
 Reagent for RNA Extraction ISOGENⅡ Manual (Second edition) Code No. 311-07361 Code No. 317-07363 NIPPON GENE CO., LTD. Table of contents I Product description 1 II Product content 1 III Storage 1 IV Precautions
Reagent for RNA Extraction ISOGENⅡ Manual (Second edition) Code No. 311-07361 Code No. 317-07363 NIPPON GENE CO., LTD. Table of contents I Product description 1 II Product content 1 III Storage 1 IV Precautions
Effect of phospholipase-d on rat kidney mitochondria*
 J. Biosci., Vol. 1, Number 1, March 1979, pp. 75 82. Printed in India. Effect of phospholipase-d on rat kidney mitochondria* S. N. A. ZAIDI, A. C. SHIPSTONE and N. K. GARG Division of Biochemistry, Central
J. Biosci., Vol. 1, Number 1, March 1979, pp. 75 82. Printed in India. Effect of phospholipase-d on rat kidney mitochondria* S. N. A. ZAIDI, A. C. SHIPSTONE and N. K. GARG Division of Biochemistry, Central
All organisms must obtain and process essential nutrients (food) *** Exception: Venus Fly Traps undergo photosynthesis but needs source of nitrogen
 All organisms must obtain and process essential nutrients (food) AUTOTROPHS self feeder makes their own food eg. Plants do not require a digestive tract *** Exception: Venus Fly Traps undergo photosynthesis
All organisms must obtain and process essential nutrients (food) AUTOTROPHS self feeder makes their own food eg. Plants do not require a digestive tract *** Exception: Venus Fly Traps undergo photosynthesis
ab CytoPainter Golgi/ER Staining Kit
 ab139485 CytoPainter Golgi/ER Staining Kit Instructions for Use Designed to detect Golgi bodies and endoplasmic reticulum by microscopy This product is for research use only and is not intended for diagnostic
ab139485 CytoPainter Golgi/ER Staining Kit Instructions for Use Designed to detect Golgi bodies and endoplasmic reticulum by microscopy This product is for research use only and is not intended for diagnostic
1) Four main feeding mechanisms of animals a) Suspension feeders i) (1) Humpback whales b) Substrate feeders i)
 1 AP Biology March 2008 Digestion Chapter 41 Homeostatic mechanisms manage an animal s energy budget. 1) Four main feeding mechanisms of animals Suspension feeders (1) Humpback whales Substrate feeders
1 AP Biology March 2008 Digestion Chapter 41 Homeostatic mechanisms manage an animal s energy budget. 1) Four main feeding mechanisms of animals Suspension feeders (1) Humpback whales Substrate feeders
Effect of Muscular Exercise on Adrenaline and Noradrenaline Secretion of the Adrenal Gland in the Dog
 Tohoku J. exp. Med., 1966, 88, 361-366 Effect of Muscular Exercise on Adrenaline and Noradrenaline Secretion of the Adrenal Gland in the Dog Sennosuke Ohukuzi Deparment of Physiology (Prof. T. Suzuki),
Tohoku J. exp. Med., 1966, 88, 361-366 Effect of Muscular Exercise on Adrenaline and Noradrenaline Secretion of the Adrenal Gland in the Dog Sennosuke Ohukuzi Deparment of Physiology (Prof. T. Suzuki),
4. Lysosomes, Smooth Endoplasmic Reticulum, Mitochondria, and Inclusions
 4. Lysosomes, Smooth Endoplasmic Reticulum, Mitochondria, and Inclusions Undergraduate Graduate Histology Lecture Series Larry Johnson, Professor Veterinary Integrative Biosciences Texas A&M University
4. Lysosomes, Smooth Endoplasmic Reticulum, Mitochondria, and Inclusions Undergraduate Graduate Histology Lecture Series Larry Johnson, Professor Veterinary Integrative Biosciences Texas A&M University
Chapter 15 Gastrointestinal System
 Chapter 15 Gastrointestinal System Dr. LL Wang E-mail: wanglinlin@zju.edu.cn Rm 608, Block B, Research Building, School of Medicine, Zijingang Campus Pancreatic Secretion The exocrine cells in the pancreas
Chapter 15 Gastrointestinal System Dr. LL Wang E-mail: wanglinlin@zju.edu.cn Rm 608, Block B, Research Building, School of Medicine, Zijingang Campus Pancreatic Secretion The exocrine cells in the pancreas
Mansoura university Faculty of medicine Histology and cell Biology Department Curriculum Content And Logbook
 Mansoura university Faculty of medicine Histology and cell Biology Department Curriculum Content And Logbook For the 1 st year Medical Student s In Histology and cell Biology Mansoura university Faculty
Mansoura university Faculty of medicine Histology and cell Biology Department Curriculum Content And Logbook For the 1 st year Medical Student s In Histology and cell Biology Mansoura university Faculty
Proteases in germinating finger millet (Eleusine coracana) seeds
 Biosci., Vol. 5, Number 3, September 1983, pp. 219 224. Printed in India. Proteases in germinating finger millet (Eleusine coracana) seeds Introduction U. VIDYAVATHI, B. SHIVARAJ and T. N. PATTABIRAMAN
Biosci., Vol. 5, Number 3, September 1983, pp. 219 224. Printed in India. Proteases in germinating finger millet (Eleusine coracana) seeds Introduction U. VIDYAVATHI, B. SHIVARAJ and T. N. PATTABIRAMAN
Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples:
 Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
High resolution structural evidence suggests the Sarcoplasmic Reticulum forms microdomains with Acidic Stores (lyososomes) in the heart.
 High resolution structural evidence suggests the Sarcoplasmic Reticulum forms microdomains with Acidic Stores (lyososomes) in the heart. Daniel Aston, Rebecca A. Capel, Kerrie L. Ford, Helen C. Christian,
High resolution structural evidence suggests the Sarcoplasmic Reticulum forms microdomains with Acidic Stores (lyososomes) in the heart. Daniel Aston, Rebecca A. Capel, Kerrie L. Ford, Helen C. Christian,
Substrate Specificity and Salt Inhibition of Five Proteinases Isolated from the Pyloric Caeca and Stomach of Sardine
 Agric. Biol. Chem., 46 (6), 1565~1569, 1982 1565 Substrate Specificity and Salt Inhibition of Five Proteinases Isolated from the Pyloric Caeca and Stomach of Sardine Minoru Noda, Thanh Vo Van, Isao Kusakabe
Agric. Biol. Chem., 46 (6), 1565~1569, 1982 1565 Substrate Specificity and Salt Inhibition of Five Proteinases Isolated from the Pyloric Caeca and Stomach of Sardine Minoru Noda, Thanh Vo Van, Isao Kusakabe
Chapter 26 The Digestive System
 Chapter 26 The Digestive System Digestive System Gastroenterology is the study of the stomach and intestine. Digestion Catabolism Absorption Anabolism The actions of the digestive system are controlled
Chapter 26 The Digestive System Digestive System Gastroenterology is the study of the stomach and intestine. Digestion Catabolism Absorption Anabolism The actions of the digestive system are controlled
Biol110L-Cell Biology Lab Spring Quarter 2012 Module 1-4 Friday April 13, 2012 (Start promptly; work fast; the protocols take ~4 h)
 Biol110L-Cell Biology Lab Spring Quarter 2012 Module 1-4 Friday April 13, 2012 (Start promptly; work fast; the protocols take ~4 h) A. Microscopic Examination of the Plasma Membrane and Its Properties
Biol110L-Cell Biology Lab Spring Quarter 2012 Module 1-4 Friday April 13, 2012 (Start promptly; work fast; the protocols take ~4 h) A. Microscopic Examination of the Plasma Membrane and Its Properties
marked secretion ofcatecholamines and a subsequent inhibition ofsecretion although the basal secretion shows an initial rise.
 J. Physiol. (1969), 2, pp. 797-85 797 With 7 text-ftgurem Printed in Great Britain SODIUM IONS AND THE SECRETION OF CATECHOLAMINES By P. BANKS, ROSEMARY BIGGINS, R. BISHOP, B. CHRISTIAN AND N. CURRIE From
J. Physiol. (1969), 2, pp. 797-85 797 With 7 text-ftgurem Printed in Great Britain SODIUM IONS AND THE SECRETION OF CATECHOLAMINES By P. BANKS, ROSEMARY BIGGINS, R. BISHOP, B. CHRISTIAN AND N. CURRIE From
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
Macromolcules, Enzymes, & Cells Intro
 Name: Date: 1. The distortion (change in shape) of enzyme molecules which occurs at high temperatures is known as 5. A characteristic shared by all enzymes, hormones, and antibodies is that their function
Name: Date: 1. The distortion (change in shape) of enzyme molecules which occurs at high temperatures is known as 5. A characteristic shared by all enzymes, hormones, and antibodies is that their function
Procaspase-3. Cleaved caspase-3. actin. Cytochrome C (10 M) Z-VAD-fmk. Procaspase-3. Cleaved caspase-3. actin. Z-VAD-fmk
 A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
Do Now Makeups. 4. In which organelle would water and dissolved materials be stored? A. 1 B. 2 C. 3 D. 5. A. mitochondria B.
 Do Now Makeups Name: Date: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell? A. mitochondria B. centrosomes
Do Now Makeups Name: Date: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell? A. mitochondria B. centrosomes
THE ACTIVATION OF PROTEOLYSIS IN THE ACROSOME REACTION OF GUINEA-PIG SPERM
 J. Cell Sci. 32, 153-164 (1978) 153 Printed in Great Britain Company of Biologists Limited lgys THE ACTIVATION OF PROTEOLYSIS IN THE ACROSOME REACTION OF GUINEA-PIG SPERM D. P. L. GREEN Physiological Laboratory,
J. Cell Sci. 32, 153-164 (1978) 153 Printed in Great Britain Company of Biologists Limited lgys THE ACTIVATION OF PROTEOLYSIS IN THE ACROSOME REACTION OF GUINEA-PIG SPERM D. P. L. GREEN Physiological Laboratory,
Stimulation of Active K + Transport by Anti-L Antibodies in Trypsin-Treated Low Potassium Sheep Erythrocytes
 LETTER TO THE EDITOR Stimulation of Active K + Transport by Anti-L Antibodies in Trypsin-Treated Low Potassium Sheep Erythrocytes Dear Sir: In this letter we attempt to resolve a discrepancy on the effect
LETTER TO THE EDITOR Stimulation of Active K + Transport by Anti-L Antibodies in Trypsin-Treated Low Potassium Sheep Erythrocytes Dear Sir: In this letter we attempt to resolve a discrepancy on the effect
Effects of Insulin and Glucagon on Elasticity of Lipid Bilayers Modified by Rat Liver Plasma Membrane Fragments
 Gen. Physiol. Biophys. (1988), 7, 537 542 537 Short communication Effects of Insulin and Glucagon on Elasticity of Lipid Bilayers Modified by Rat Liver Plasma Membrane Fragments J. KAVEČANSKÝ 1, T. HIANIK
Gen. Physiol. Biophys. (1988), 7, 537 542 537 Short communication Effects of Insulin and Glucagon on Elasticity of Lipid Bilayers Modified by Rat Liver Plasma Membrane Fragments J. KAVEČANSKÝ 1, T. HIANIK
6. The diagram below represents an interaction between parts of an organism.
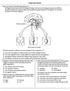 Endocrine Review 1. Base your answer to the following question on the diagram below and on your knowledge of biology. Each arrow in the diagram represents a different hormone released by the pituitary
Endocrine Review 1. Base your answer to the following question on the diagram below and on your knowledge of biology. Each arrow in the diagram represents a different hormone released by the pituitary
Cell Structure. Present in animal cell. Present in plant cell. Organelle. Function. strength, resist pressure created when water enters
 Cell Structure Though eukaryotic cells contain many organelles, it is important to know which are in plant cells, which are in animal cells and what their functions are. Organelle Present in plant cell
Cell Structure Though eukaryotic cells contain many organelles, it is important to know which are in plant cells, which are in animal cells and what their functions are. Organelle Present in plant cell
Separation of Plasma and Serum and Their Proteins from Whole Blood
 Separation of Plasma and Serum and Their Proteins from Whole Blood BCH 471 [Practical] BLOOD COMPOSITION Other names to blood cells Red blood cells (erythrocytes) White blood cells (leukocytes) Platelets
Separation of Plasma and Serum and Their Proteins from Whole Blood BCH 471 [Practical] BLOOD COMPOSITION Other names to blood cells Red blood cells (erythrocytes) White blood cells (leukocytes) Platelets
OF FATTY LIVERS. XLII. PROTEIN AND THE DIETARY PRODUCTION. On a number of occasions however use of this diet has failed to produce in our
 XLII. PROTEIN AND THE DIETARY PRODUCTION OF FATTY LIVERS. BY HAROLD JOHN CHANNON AND HARRY WILKINSON. From the Department of Biochemistry, The University, Liverpool. (Received December 20th, 1934.) THE
XLII. PROTEIN AND THE DIETARY PRODUCTION OF FATTY LIVERS. BY HAROLD JOHN CHANNON AND HARRY WILKINSON. From the Department of Biochemistry, The University, Liverpool. (Received December 20th, 1934.) THE
Relaxation responses of aortic rings from salt-loaded high calcium fed rats to potassium chloride, calcium chloride and magnesium sulphate
 Pathophysiology 4 (1998) 275 280 Relaxation responses of aortic rings from salt-loaded high calcium fed rats to potassium chloride, calcium chloride and magnesium sulphate B.J. Adegunloye, O.A. Sofola
Pathophysiology 4 (1998) 275 280 Relaxation responses of aortic rings from salt-loaded high calcium fed rats to potassium chloride, calcium chloride and magnesium sulphate B.J. Adegunloye, O.A. Sofola
PHYSIOLOGY OF THE DIGESTIVE SYSTEM
 Student Name CHAPTER 26 PHYSIOLOGY OF THE DIGESTIVE SYSTEM D igestion is the process of breaking down complex nutrients into simpler units suitable for absorption. It involves two major processes: mechanical
Student Name CHAPTER 26 PHYSIOLOGY OF THE DIGESTIVE SYSTEM D igestion is the process of breaking down complex nutrients into simpler units suitable for absorption. It involves two major processes: mechanical
ENZYME FORMATION IN LYSOZYME LYSATE OF BACILUS SUBTILIS
 The Journal of Biochemistry, Vol. 44, No. 12, 1957 ENZYME FORMATION IN LYSOZYME LYSATE OF BACILUS SUBTILIS It was already reported that the whole lysate obtained by the treat ment of Bacillus subtilis
The Journal of Biochemistry, Vol. 44, No. 12, 1957 ENZYME FORMATION IN LYSOZYME LYSATE OF BACILUS SUBTILIS It was already reported that the whole lysate obtained by the treat ment of Bacillus subtilis
Biochemistry Name: Practice Questions
 Name: Practice Questions 1. Carbohydrate molecules A and B come in contact with the cell membrane of the same cell. Molecule A passes through the membrane readily, but molecule B does not. It is most likely
Name: Practice Questions 1. Carbohydrate molecules A and B come in contact with the cell membrane of the same cell. Molecule A passes through the membrane readily, but molecule B does not. It is most likely
Using a technique by which it is possible to study gastro-intestinal absorption
 531 J. Physiol. (I956) I34, 53I-537 THE ABSORPTION OF GLUCOSE BY THE INTACT RAT BY P. C. REYNELL AND G. H. SPRAY From the Nuffield Department of Clinical Medicine, University of Oxford (Received 30 May
531 J. Physiol. (I956) I34, 53I-537 THE ABSORPTION OF GLUCOSE BY THE INTACT RAT BY P. C. REYNELL AND G. H. SPRAY From the Nuffield Department of Clinical Medicine, University of Oxford (Received 30 May
