Bioenergetic characterization of mouse podocytes
|
|
|
- Neil Anthony
- 5 years ago
- Views:
Transcription
1 Am J Physiol Cell Physiol 299: C464 C476, First published May 5, 2010; doi: /ajpcell Bioenergetic characterization of mouse podocytes Yoshifusa Abe, 1,2 Toru Sakairi, 1 Hiroshi Kajiyama, 3 Shashi Shrivastav, 1 Craig Beeson, 4 and Jeffrey B. Kopp 1 1 Kidney Disease Section, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland; 2 Department of Pediatrics, Showa University School of Medicine, Tokyo; and 3 Department of Rheumatology and Applied Immunology, Saitama Medical University, Saitama, Japan; and 4 Department of Pharmaceutical and Biomedical Science, Medical University of South Carolina, Charleston, South Carolina Submitted 29 December 2009; accepted in final form 4 May 2010 Abe Y, Sakairi T, Kajiyama H, Shrivastav S, Beeson C, Kopp JB. Bioenergetic characterization of mouse podocytes. Am J Physiol Cell Physiol 299: C464 C476, First published May 5, 2010; doi: /ajpcell Mitochondrial dysfunction contributes to podocyte injury, but normal podocyte bioenergetics have not been characterized. We measured oxygen consumption rates (OCR) and extracellular acidification rates (ECAR), using a transformed mouse podocyte cell line and the Seahorse Bioscience XF24 Extracellular Flux Analyzer. Basal OCR and ECAR were pmol/min and milli-ph units/min, respectively. The complex V inhibitor oligomycin reduced OCR to 45% of baseline rates, indicating that 55% of cellular oxygen consumption was coupled to ATP synthesis. Rotenone, a complex I inhibitor, reduced OCR to 25% of the baseline rates, suggesting that mitochondrial respiration accounted for 75% of the total cellular respiration. Thus 75% of mitochondrial respiration was coupled to ATP synthesis and 25% was accounted for by proton leak. Carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), which uncouples electron transport from ATP generation, increased OCR and ECAR to 360% and 840% of control levels. FCCP plus rotenone reduced ATP content by 60%, the glycolysis inhibitor 2-deoxyglucose reduced ATP by 35%, and 2-deoxyglucose in combination with FCCP or rotenone reduced ATP by 85%. The lactate dehydrogenase inhibitor oxamate and 2-deoxyglucose did not reduce ECAR, and 2-deoxyglucose had no effect on OCR, although 2-deoxyglucose reduced ATP content by 25%. Mitochondrial uncoupling induced by FCCP was associated with increased OCR with certain substrates, including lactate, glucose, pyruvate, and palmitate. Replication of these experiments in primary mouse podocytes yielded similar data. We conclude that mitochondria play the primary role in maintaining podocyte energy homeostasis, while glycolysis makes a lesser contribution. mitochondria; oxygen consumption rate; extracellular acidification rate PROGRESSIVE GLOMERULOSCLEROSIS of diverse etiologies, including focal segmental glomerulosclerosis (FSGS), diabetic nephropathy, IgA nephropathy, and lupus, is associated with injury and loss of podocytes. Multiple pathways of cellular injury probably contribute to podocyte depletion in these various disorders. A role for podocyte mitochondrial injury in FSGS is suggested by the fact that mutations in enzymes responsible for coenzyme Q generation [including para-hydroxybenzoate-polyprenyl transferase (COQ2) and decaprenyl diphosphate synthase subunit 2 (PDSS2)] and in the mitochondrial trna Leu(UUR) are associated with FSGS, as well as disorders in other tissues (4, 9, 14). Although mitochondrial function has been investigated in various cell types, a role of mitochondrial Address for reprint requests and other correspondence: J. Kopp, 10 Center Dr., NIH, Bethesda, MD ( jbkopp@nih.gov). C464 dysfunction as a contributor to podocyte injury in other glomerulopathies remains largely unexplored. Recently, the Seahorse Bioscience XF24 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA) has been used to monitor cell respiration together with extracellular ph as an indicator of anaerobic glycolysis (26). Therefore, we wanted to further characterize mitochondrial function in cultured podocytes with the Seahorse Bioscience XF24 Extracellular Flux Analyzer. In particular, we set out to develop estimates of the relative contributions of glycolysis and oxidative phosphorylation and to determine the preferred substrates for podocyte metabolism, using a transformed mouse podocyte cell line and primary cultures of mouse podocytes. METHODS Cell culture. The mouse podocyte cell line AI was established from glomeruli of transgenic mice bearing the podocin/rtta gene and the SV40 temperature-sensitive T antigen as described previously (10). For these studies, clone 1-1P4G5 was selected based on high expression of WT-1 and synaptopodin when cultured under nonpermissive conditions (37 C) for 5 days without medium change, suggesting some degree of differentiation (10). Cells were cultured in growth medium consisting of RPMI 1640, 10% fetal bovine serum, 100 U/ml of penicillin, and 100 g/ml streptomycin (obtained from GIBCO, Rockville, MD) under permissive conditions (33 C). Cells were trypsinized and transferred to the nonpermissive temperature of 37 C when cells reached 90% confluence. Primary glomerular cells were obtained from isolated glomeruli from 4 6 wk old FVB/N mice with the Dynabeads methods (11, 23). Briefly, purified glomeruli were placed in culture in RPMI 1640 medium supplemented with 10% fetal bovine serum. When colonies were established, the cells were harvested with trypsin and used at passage 3 or 4. For immunostaining, cells were fixed with 2% paraformaldehye and 4% sucrose, exposed to primary antibody (mouse monoclonal anti-wt-1 antibody, Upstate, Lake Placid, NY; mouse anti-nestin antibody, Millipore, Billerica, MA), exposed to fluorescent secondary antibody, and examined under fluorescent microscopy. Most cells in these primary glomerular cell cultures exhibited strong nuclear staining for WT-1 and for nestin within cytoplasmic filaments (data not shown); for simplicity, we refer to these cultures as primary podocytes, although by their nature these cells are not a homogenous population. Mice were cared for under a protocol that was approved in advance by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Animal Care and Use Committee, and all animal care conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Reagents. Sodium pyruvate, lactate, 2-deoxyglucose (2-DG), sodium oxamate, rotenone, carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), ouabain octahydrate, sodium palmitate, L-carnitine, and antimycin A were obtained from Sigma-Aldrich (St. Louis, MO), and oligomycin was obtained from Calbiochem (San Diego,
2 C465 CA). Concentrated stocks of 2-DG (1,000 mm) and oxamate (500 mm) were prepared in assay medium. Concentrated stocks of rotenone (1 mm) and oligomycin (1 mm) were prepared in DMSO. Concentrated stocks of FCCP (10 mm) and antimycin A (10 mm) were prepared in ethyl alcohol. Concentrated stocks of ouabain (50 mm) and L-carnitine (50 mm) were prepared in deionized water. Concentrated stocks of sodium palmitate (2 mm) were conjugated with 0.34 mm (2.267 g/dl) ultra-fatty acid-free bovine serum albumin (BSA). Ultra-fatty acid-free BSA was purchased from Roche Diagnostics (Indianapolis, IN). Measurements of oxygen consumption rate and extracellular acidification rate. A Seahorse Bioscience XF24-3 Extracellular Flux Analyzer was used to measure the rate change of dissolved O 2 and ph in medium immediately surrounding adherent cells cultured in a collagen I-coated XF24 V7 cell culture microplate (Seahorse Bioscience). Transformed mouse podocytes were seeded in XF24-well microplates at cells per well (area 0.32 cm 2 )in100 l of growth medium and then incubated at 37 C with 5% CO 2 overnight. The following day an additional 100 l of growth medium was added, and 2 days later medium was replaced. After incubation for a total of 5 days, growth medium was removed and replaced with 675 l of assay medium prewarmed to 37 C, composed of RPMI 1640 without bicarbonate containing (in mm) 10 KCl, 10 NaCl, 1 sodium pyruvate, and 3 lactate, and cultured at 37 C in room air. Primary mouse podocytes were seeded in XF24-well microplates at cells per well in 200 l of growth medium and then incubated at 37 C with 5% CO 2 overnight. The following day, growth medium was replaced with 675 l of assay medium. To investigate coupling efficiency (defined as the reciprocal relationship between rates of oxidative phosphorylation and glycolysis, adjusted to meet cellular energy needs) and spare respiratory capacity, five different assay media were employed: RPMI 1640 without bicarbonate and glucose, RPMI 1640 without bicarbonate and glucose but containing 3 mm lactate, RPMI 1640 without bicarbonate containing 11 mm glucose, RPMI 1640 without bicarbonate and glucose but containing 10 mm pyruvate, and RPMI 1640 without bicarbonate containing 11 mm glucose and 10 mm pyruvate. All of these media included 2 mm L-glutamine (Table 1). To assess -oxidation in podocytes, RPMI 1640 containing 11 mm glucose and 0.5 mm carnitine was employed as an assay medium, and sodium palmitate was administered at the final concentration of 200 M. Measurements of oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were performed after equilibration in assay medium for 1 h. Briefly, the Seahorse analyzer uses a cartridge with 24 optical fluorescent O 2 and ph sensors that are embedded in a sterile disposable cartridge, 1 for each well. Before each rate measurement, the plungers mix assay media in each well for 8 min to allow the oxygen partial pressure to reach equilibrium. For measurements of rates, the plungers gently descend into the wells, forming a chamber that entraps the cells in an 7- l volume. Measurements of O 2 concentration and ph are periodically made over 4 min, and OCR and ECAR are obtained from the slopes of concentration change in these parameters vs. time. After the rate measurements, the plungers ascend and the plate is gently agitated to reequilibrate the medium. OCR is reported in the unit of picomoles per minute and ECAR is reported in milli-ph units (mph) per minute. Baseline rates are measured four times. One or more testing chemicals are preloaded in the reagent delivery chambers of the sensor cartridge and then pneumatically injected into the wells to reach the desired final working concentration. After 2 min of mixing, postexposure OCR and ECAR measurements are made four to six times. The averages of four baseline rates and up to six test rates were used for data analysis. ATP quantification. Podocytes were seeded in collagen I-coated 96-well microplates (Discovery Labware, Becton Dickinson, Franklin Lakes, NJ) at cells per well (0.32 cm 2 )in100 l of growth medium. Cells were incubated at 37 C overnight, with addition of another 100 l of growth medium on the next day and replacement of medium 2 days later. After incubation for 5 days, assays were initiated by removing the growth medium from each well and replacing it with 100 l of assay medium. Cells were exposed to vehicle or compound for 45 min in the 37 C incubator before the ATP assay started. The quantity of ATP present in the test cells in each well was measured by CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI). The ATP assay was performed according to the manufacturer s instruction. Luminescence intensity from each well was measured with a FLUOstar Optima plate reader (BMG Labtech, Offenberg, Germany). Calcein acetoxymethyl ester cell viability assay. Cell viability after 30-min exposure to compounds or vehicle was determined with the calcein acetoxymethyl ester (AM) assay. Podocytes were seeded in collagen I-coated 96-well black microplates (Discovery Labware, Becton Dickinson) at cells per well (0.32 cm 2 )in100 l of growth medium. Cells were incubated at 37 C overnight, with addition of another 100 l of growth medium on the next day and replacement of medium 2 days later. After incubation for 5 days, assays were initiated by removing the growth medium from each well and replacing it with 100 l of assay medium. Cells were exposed to vehicle or compound for 30 min at 37 C. Calcein AM (Invitrogen, Carlsbad, CA) staining solution was prepared by diluting to 1 M in Hanks balanced salt solution (HBSS) immediately before use. The assay was performed by first removing the assay medium containing the compounds and then washing each well with 200 l of HBSS; 100 lof1 M calcein AM staining solution was then added to each well. After incubation at 37 C for 30 min, the fluorescence intensity of each well was measured with a FLUOstar Optima plate reader (BMG Labtech). Statistical analysis. Statistical analysis was performed with Prism (GraphPad, San Diego, CA). Data are presented as means SD, unless stated otherwise. For OCR and ECAR measurements over time, the area under the curve was generated by an algorithm executed by the Seahorse device and these values were compared between experimental and control groups. Analyses included Student t-test and ANOVA. A P value of 0.05 was considered significant. RESULTS Baseline bioenergetics. We first investigated respiration in transformed mouse podocytes, assessed as OCR, and glycolytic lactic acid production, assessed as ECAR. Basal cellular OCR and ECAR were found to be pmol/min per cells and mph/min per cells Table 1. Assay media used to investigate coupling efficiency and spare respiratory capacity Standard Assay Medium Control Medium Glucose Medium Pyruvate Medium Glucose Pyruvate Medium Lactate Medium Glucose, mm Pyruvate, mm Lactate, mm 3 3 NaCl, mm 10 KCl, mm 10 Glutamine, mm
3 C466 (initial cell count), respectively (Fig. 1A). We wanted to investigate whether the bioenergetic profile was similar in primary podocyte cultures to what we found in transformed podocytes. Basal cellular OCR and ECAR were found to be pmol/min per cells and mph/min per cells (initial cell count), respectively (Fig. 1A; each data point represents mean SD, n 20). Contribution of ATP turnover, proton leak, and nonmitochondrial respiration to total cellular respiration. We next carried out titration studies for oligomycin (which blocks the mitochondrial complex V, where the electron chain is coupled to ATP synthesis) and rotenone (which blocks complex I, thereby eliminating mitochondrial respiration) and assessed OCR, ECAR, and ATP generation. In the presence of increasing doses of oligomycin and rotenone for 45 min, OCR was reduced, while ECAR was simultaneously increased (Fig. 2, A D), indicating that the cells shifted mitochondrial respiration to glycolysis. In contrast to cancer cells, however, in podocytes the shift to glycolysis was unable to completely fulfill cellular energy demand, and consequently ATP charge fell (Fig. 2, E and F). Mitochondrial function comprises coupled and uncoupled respiration. Coupled respiration generates ATP, while uncoupled respiration involves the futile cycle of proton pumping and proton leak back across the inner mitochondrial membrane. Using maximally effective doses of oligomycin and rotenone, we found that oligomycin reduced OCR to 47% of Fig. 1. Baseline energetics and intracellular ATP levels. A: basal oxygen consumption rate (OCR, left y-axis) and basal extracellular acidification rate (ECAR, right y-axis) in transformed podocytes and primary podocytes. OCR was significantly higher in primary podocytes (P , unpaired t-test), while ECAR was similar in the 2 cell types. Each data point represents mean SD (n 20) and is representative of 11 independent experiments in transformed podocytes and 3 independent experiments in primary podocytes. mph, milli-ph units. B: intracellular ATP level was measured in response to mitochondrial uncoupler carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP, 3 M), glycolysis inhibitor 2-deoxyglucose (2-DG, 100 mm), and complex I inhibitor rotenone (1 M) treatment for 45 min, individually or in combination as shown, in cultured mouse podocytes. ATP level is expressed as % of control, which was defined as the baseline value in cells exposed only to vehicle. Each data point represents mean SD (n 4). Control vs. 2-DG, FCCP, 2-DG vs. FCCP, and 2-DG FCCP rotenone, all ***P vs. vehicle control, by ANOVA. This is representative of 2 independent experiments. Viability of the treated cells remained similar to control, as shown by calcein AM stain (top). Each data point represents mean SD (n 4; all P 0.05 vs. vehicle control, by ANOVA). C: administering 2-DG before FCCP and rotenone injection had no additional effect on OCR and ECAR, but administering 2-DG after FCCP and rotenone injection decreased ECAR. Each data point represents mean SE (n 5), and results are representative of 2 independent experiments.
4 C467 Fig. 2. Dose titration of oligomycin and rotenone. Shown are OCR, ECAR, and ATP levels for transformed podocytes. F 1F 0 ATP synthase inhibitor oligomycin and complex I inhibitor rotenone suppressed respiration (A, B) and stimulated glycolysis (C, D). Each data point represents mean SD (n 4), and results are representative of 2 independent experiments. With oligomycin and with rotenone, ATP levels fell, indicating that increased glycolysis was insufficient to compensate for reduced respiration and thus to meet cellular energy demand (E, F). ATP level is expressed as % of control, which was the value in cells exposed only to vehicle. Each data point represents mean SD (n 4), and results are representative of 2 independent experiments (**P 0.01 vs. vehicle control, by ANOVA). baseline rates, indicating that 53% of cellular oxygen consumption was related to ATP synthesis. Rotenone reduced OCR to 23% of the baseline rates, suggesting that mitochondrial respiration accounted for 77% of the total cellular respiration. Thus in transformed podocytes 69% (53%/77%) of mitochondrial respiration was coupled to ATP synthesis, and 31% of mitochondrial respiration was accounted for by proton leak (Fig. 3). The rotenone-resistant rate reflects the nonmitochondrial respiration rate, which includes substrate oxidation and cell surface oxygen consumption (7). With regard to primary podocytes, oligomycin and rotenone reduced OCR to 41% and 23%, respectively. These results indicate that 59% of cellular oxygen consumption was related to ATP synthesis and mitochondrial respiration accounted for 77% of the total cellular respiration. Thus in primary podocytes 77% (59%/77%) of mitochondrial respiration was
5 C468 Fig. 3. Contribution of mitochondrial respiration to cellular ATP synthesis. Mitochondrial respiration was calculated from OCR under basal conditions and after the addition of oligomycin (1 M) and rotenone (1 M). Each data point represents mean SD of the last 3 of 6 rates for transformed podocytes (n 10 replicates, left) and primary podocytes (n 6 replicates, right). Experiment was performed 3 times with transformed podocytes and once with primary podocytes. These data indicate that in both transformed and primary podocytes mitochondrial respiration accounts for 77% of OCR and ATP generation accounts for 53% and 59% of OCR in transformed podocytes and primary podocytes, respectively. coupled to ATP synthesis, and 23% of mitochondrial respiration was accounted for by proton leak (Fig. 3). To evaluate whether the effect of oligomycin to inhibit OCR was via inhibition of Na -K -transporting ATPase, titration study was performed with ouabain, a specific inhibitor of Na -K -transporting ATPase. Ouabain (from 0.03 to 5 mm) had no effect on OCR (data not shown). These findings suggest that the effect of oligomycin is not due to inhibition of the plasma membrane Na -K -ATPase and that OCR is strongly coupled to ATP usage. Contribution of mitochondrial respiration and of glycolysis to ATP charge. We characterized the relative contribution of glycolysis and mitochondrial function to ATP charge in transformed podocytes. We measured cellular ATP concentration in cells exposed for 45 min to eight conditions: vehicle, the glycolysis inhibitor 2-DG, the ionophore and mitochondrial uncoupler FCCP, the mitochondrial complex I inhibitor rotenone, FCCP plus rotenone, 2-DG plus FCCP, 2-DG plus rotenone, and 2-DG plus FCCP plus rotenone (Fig. 1B). 2-DG, FCCP, and rotenone treatment each reduced ATP content by 20 35%. Of those three treatments, 2-DG was the most effective at reducing ATP (35% reduction). FCCP plus rotenone reduced ATP content by 60%, and 2-DG in combination with FCCP or rotenone reduced ATP by 85%. Finally, the combination of 2-DG, FCCP, and rotenone essentially eliminated ATP production. Thus 2-DG decreased ATP charge more than FCCP or rotenone did, and 2-DG plus FCCP (as well as 2-DG plus rotenone) decreased ATP charge more than FCCP plus rotenone did. These data indicate that ATP generation shifts from oxidative phosphorylation to glycolysis when mitochondrial function is impaired (Fig. 1B). Although 1 mm pyruvate was included in the assay medium, administering 2-DG before FCCP injection had no additional effect on OCR and ECAR but administering 2-DG after FCCP injection decreased ECAR (Fig. 1C). These data indicate that FCCP can activate glycolysis but transformed podocytes do not depend on anaerobic glycolysis to generate ATP. Furthermore, oxidative phosphorylation cannot be activated by 2-DG, and ATP charge is not fully maintained after inhibition of either glycolysis or mitochondrial function. Maximal mitochondrial respiratory capacity and cellular response to suppression of mitochondrial respiration. We examined the effect on OCR, ECAR, and intracellular ATP levels of uncoupling respiration from oxidative phosphorylation. In the presence of increasing doses of FCCP, OCR and ECAR were both increased to 360% and 840% of control levels (Fig. 4). Compared with blocking by the combination of 2-DG, rotenone, and FCCP (Fig. 1B), intracellular ATP content was lowered by 70% in the presence of FCCP alone compared with untreated controls (Fig. 4). These data suggest that podocytes, faced with partial suppression of mitochondrial function, fail to upregulate glycolysis sufficiently to sustain cellular ATP levels. After a maximal response was reached, higher FCCP concentrations ( 5 M) were noted to be toxic to podocytes, manifested as decreased OCR (data not shown). We extended these findings to primary mouse podocytes, in which FCCP increased both OCR and ECAR, as was observed in transformed podocytes (Fig. 4, B and D). Coupling efficiency and spare respiratory capacity. To assess the optimal energy substrate for podocytes, we investigated coupling efficiency and spare respiratory capacity. Coupling efficiency is assessed by the administration of oligomycin, and then spare respiratory capacity is assessed by the administration of FCCP. Pyruvate and pyruvate plus glucose increased the OCR in each phase (Table 2). In addition to increasing OCR, it is apparent that exogenous pyruvate is additive in enhancing spare respiratory capacity in the presence of FCCP in both transformed podocytes and primary podocytes (Fig. 5A). Interestingly, 3 mm lactate could also enhance spare respiratory capacity, and did so to a greater extent even than glucose in primary podocytes. Furthermore, FCCP with 2 mm glutamine could increase OCR especially in primary podocytes. Although calculated ATP turnover was not altered, after FCCP, proton leak (expressed as % of basal rates) and mitochondrial respiration (also expressed as % of basal rates) decreased in the presence of lactate, glucose, pyruvate, and glucose plus pyruvate (Fig. 5B). To investigate the mechanisms for this reduced mitochondrial respiration and reduced proton leak, rotenone was added in the presence and absence of FCCP, using glucose plus pyruvate medium (Fig. 5C). In the absence of FCCP, rotenone completely inhibited OCR, but when FCCP was added first and rotenone added later, rotenone was not able to completely inhibit OCR. By contrast, when the complex III inhibitor antimycin was used in place of rotenone, OCR was completely blocked whether FCCP was present or absent (Fig. 5C). In this experiment, mitochondrial respiration and proton leak were lowest in the rotenone group in the presence of FCCP (Fig. 5D).
6 C469 Fig. 4. Dose titration of FCCP. The mitochondrial uncoupler FCCP increased OCR (A, B), due to increased aerobic metabolism, and increased ECAR (C, D), due to increased glycolysis consequent to decreased mitochondrial ATP generation, with a net effect of decreased cellular ATP levels (E). ATP level is expressed as % of control, which was the value in cells exposed only to vehicle. The effect on primary cells was similar to that on transformed podocytes. Each data point represents mean SD (n 4), and results are representative of 2 and 3 independent experiments in B and D and in A, C, and E, respectively. *P 0.05, **P 0.01 vs. vehicle control, by ANOVA. We next investigated the effects of various glycolytic substrates, using the substrates listed in Table 1. After addition of oligomycin there was a modest numerical increase in podocyte ECAR with glucose medium (reflecting increased glycolysis as compensation for inhibited mitochondrial ATP synthesis) but little change with pyruvate medium and with glucose plus pyruvate medium (Fig. 5E). After the addition of FCCP, podocyte ECAR increased with all three media; the peak
7 C470 Table 2. Oxygen consumption rate of podocytes incubated in presence of various metabolic substrates Basal Oligomycin FCCP Rotenone Transformed podocytes Control Lactate Glucose Pyruvate Glucose pyruvate Primary cells Control Lactate Glucose Pyruvate Glucose pyruvate Data (in pmol/min) are means SD oxygen consumption rates (OCR), with 4 replicates. Podocytes were cultured in control medium containing 2 mm L-glutamine or this medium supplemented with 3 mm lactate, mm glucose, 10 mm pyruvate, or mm glucose 10 mm pyruvate. Values for basal OCR, postoligomycin OCR, and postrotenone OCR were obtained 45 min, 79 min, and 147 min, respectively, after the start of OCR measurement. The value for post-carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) OCR was taken at the peak value following FCCP injection. ECAR was highest in glucose plus pyruvate medium, next highest in glucose medium, and third highest in pyruvate medium. By contrast, podocyte ECAR showed no increase following addition of oligomycin and addition of FCCP in control medium (containing only glutamine), consistent with the lack of glycolytic substrate in this medium (data not shown). These data suggest that podocytes utilize glucose and to a lesser extent pyruvate in the tricarboxylic acid (TCA) cycle, leading to CO 2 production and extracellular acidification. Cellular responses to suppression of glycolysis. As podocytes appeared to have limited glycolytic reserve, we next examined glycolytic rates in greater detail. The contribution of lactic acid production from glycolysis to ECAR was assessed with two inhibitors. Oxamate inhibits lactate dehydrogenase, which converts pyruvate to lactate during the last step of glycolysis. 2-DG is a glucose analog and inhibits hexokinase, the first enzyme in the glycolysis pathway, which converts glucose to glucose-6-phosphate. Neither inhibitor affected OCR (Fig. 6, A and B), but, interestingly, both agents increased ECAR (Fig. 6, C and D). ATP synthesis was only modestly affected by oxamate, as might be expected since glycolysis is intact (Fig. 6E). By contrast, ATP synthesis dropped by 25% with high concentrations of 2-DG. These data indicate that mitochondrial function, while the dominant contributor to the energy budget, cannot fully compensate for impaired glycolysis (Fig. 6F). Alternative mitochondrial substrates: fatty acid oxidation. We examined the ability of transformed podocytes to utilize fatty acids as a metabolic fuel source in the presence of a maximally effective dose of the uncoupling agent FCCP. Palmitate with a BSA carrier significantly increased OCR and ATP charge more than basal medium containing BSA (Fig. 7), while ECAR was increased to a similar extent under both conditions (data not shown). These data suggest that transformed podocytes can use palmitate as a fuel source. However, these findings were not seen in primary podocytes (data not shown). DISCUSSION We have studied the bioenergetic profile of cultured mouse podocytes, including both transformed cells and primary cells. We measured OCR and ECAR, using a Seahorse extracellular flux analyzer, and cellular ATP levels in response to various inhibitors of mitochondrial function and of glycolysis and in response to provision of various energy substrates. Previous studies using a similar technique to characterize cellular bioenergetics have studied cancer lines, isolated synaptosomes, and myocytes (3, 21, 22, 26). To our knowledge, this is the first study using transformed cell lines and primary cells derived from the mouse kidney. Our principal findings are as follows. First, mitochondrial respiration accounts for 77% of cellular respiration and 75% of this coupled to ATP synthesis. Second, inhibition of glycolysis or of oxidative phosphorylation each reduced ATP levels, suggesting that podocytes have limited ability to increase oxidative phosphorylation or glycolysis to preserve energy homeostasis under these conditions. Third, cultured podocytes utilize a range of substrates, including glucose, pyruvate, palmitate, and lactate. Podocytes differ from previously studied cancer cells in two ways: podocytes do not depend on glycolysis for generation of ATP, and inhibition of glycolysis does not lead to an increase in oxidative phosphorylation, leading to a fall in cellular ATP content. In the human non-small cell carcinoma cell lines H460 and A549, Wu et al. (26) found that there was an effective compensatory upregulation of glycolysis following the administration of oligomycin to block oxidative phosphorylation, and this response was able to sustain ATP level. By contrast, we have shown that podocytes lack this ability to increase glycolysis when mitochondrial function is blocked (Table 3). This finding has important implications for podocyte biology, underscoring the importance of the mitochondria to podocyte homeostasis. Cultured podocytes demonstrated additional bioenergetic differences from cancer cells. In cultured human non-small cell carcinoma cell, oxamate reduced ECAR by 80% and 2-DG reduced ECAR and increased OCR (26). The oxamate-sensitive portion of ECAR reflects the glycolysis rate, while the oxamate-insensitive ECAR is due to nonglycolytic acidification, mostly acidification by CO 2.CO 2 is the end product of the TCA cycle in mitochondria, and after generation of carbonic acid this contributes to extracellular acidification. Somewhat surprisingly, in podocytes, oxamate had little effect on OCR or
8 C471 Fig. 5. Coupling efficiency and spare respiratory capacity with various energy substrates. A: podocytes were exposed to 1 M oligomycin, 3 M FCCP, and 1 M rotenone. Each data point represents mean SE (n 4), and results are representative of 4 and 3 independent experiments in transformed podocytes and primary podocytes, respectively. Viability of the treated cells remained similar to control by calcein AM stain (data not shown). B: baseline OCR was defined as the average of 4 points of OCR before addition of oligomycin. ATP turnover, proton leak, and mitochondrial respiration were calculated from data obtained (average of 4 points of OCR after injection of oligomycin and average of last 2 points before addition of rotenone) with different energy substrates and were normalized to baseline OCR. C: podocytes were exposed to 1 M oligomycin, 3 M FCCP, 1 M antimycin A, or 1 M rotenone in glucose pyruvate assay medium. Each data point represents mean SE (n 5), and results are representative of 3 independent experiments in transformed podocytes. Viability of the treated cells remained similar to control by calcein AM stain (data not shown). D: baseline OCR was defined as the average of 4 points of OCR before addition of oligomycin. ATP turnover, proton leak, and mitochondrial respiration were calculated from data obtained (average of 4 points of OCR after injection of oligomycin and average of last 2 points before addition of antimycin A or rotenone) and were normalized to baseline OCR. E: ECAR was measured in parallel with respiration. Results are normalized to ECAR in glucose medium before addition of oligomycin. Each data point represents mean SE (n 4), and results are representative of 4 independent experiments in transformed podocytes.
9 C472 Fig. 6. Dose titration of oxamate and 2-DG. Oxamate and 2- DG did not affect OCR (A, B), but each agent increased ECAR (C, D). Oxamate had only a modest effect on cellular ATP levels, while maximal doses of 2-DG (100 mm) decreased cellular ATP levels by 25% (E, F); this indicates that podocytes are largely dependent on oxidative phosphorylation rather than glycolysis. ATP level is expressed as % of control, which was the baseline value in cells exposed only to assay medium, *P 0.05, **P 0.01 vs. vehicle control, by ANOVA. cellular ATP, suggesting that, unlike what is seen in many tumors and cell lines, podocytes primarily oxidize their glycolytic pyruvate, and thus it appears that little lactic acid is produced from glycolysis, while oxamate increased ECAR. This increase in ECAR may be due to increased amounts of pyruvate being metabolized to CO 2 within mitochondria, ultimately generating protons, rather than being converted to lactate, which generates only one proton per molecule compared with three protons generated per molecule of pyruvate that is metabolized to CO 2. On the other hand, 2-DG, which blocks glycolysis, reduced cellular ATP and was not associated with an increase in OCR. This indicates that when glycolysis is inhibited podocytes do not increase mitochondrial activity to make up the energy deficit. These findings suggest that cul-
10 C473 Fig. 7. Palmitate as an energy substrate. A: sodium palmitate (200 M) conjugated with ultra-fatty acid-free bovine serum albumin (BSA) was administered by injection into the assay medium at the time point shown. Oligomycin (1 M) and FCCP (20 M) were injected at subsequent time points as shown. OCR was significantly higher with palmitate compared with the control medium (P for area under the curve, by ANOVA). Each data point represents mean SE (n 5), and results are representative of 2 independent experiments. Further, ATP charge was significantly higher with palmitate as an energy source after FCCP administration compared with control (P 0.001, unpaired t-test), as shown in B. Each data point represents mean SD (n 4), and results are representative of 2 independent experiments. tured podocytes have limited ability to increase mitochondrial function when the need arises. In the present study, exogenous lactate increased OCR to the same degree as glucose. Movement of lactate occurs via two types of transporters selective for monocarboxylates, the monocarboxylate transporters (MCTs) and sodium-coupled monocarboxylate transporters (SMCTs) (5, 6, 13, 27). The MCT family now comprises 14 members, of which 4 (MCT1 Table 3. Comparison of mitochondrial bioenergetic profile Present Study Wu et al. (2007) Human Non-Small Cell Carcinoma Cell Lines Transformed podocytes Primary podocytes H460 A549 Basal OCR, nmol min cells Basal ECAR, mph min cells Mitochondrial respiration, % ATP turnover, % Proton leak, % Fraction of baseline OCR, % Oligomycin Rotenone Oxamate Deoxyglucose Uncoupler* Fraction of baseline ECAR, % Oligomycin Rotenone Oxamate Deoxyglucose FCCP Fraction of baseline ATP content, % Oligomycin Rotenone Oxamate Deoxyglucose Uncoupler* Values from present study are given for mean SD maximum change, relevant to control conditions; denotes estimates from previously published data by Wu et al. (26). ECAR, extracellular acidification rate; mph, milli-ph units. *Uncoupling agent employed: FCCP for present study and 2,4-dinitrophenol for Wu et al.
11 C474 MCT4) have been demonstrated experimentally to catalyze the proton-linked transport of metabolically important monocarboxylates. SMCTs comprise SMCT1 (SLC5A8) and SMCT2 (SLC5A12). MCT1, MCT2, SMCT1, and SMCT2 are expressed in tubular cells. To our knowledge, there are no reports that both of these are expressed in the podocytes. In the present study we confirmed mrna expression of MCT1 in both transformed podocytes and primary podocytes (data not shown). Further investigation will be required to investigate how podocytes take up exogenous lactate and to define the importance of this pathway. Furthermore, FCCP plus glutamine increased OCR, especially in primary podocytes. In the present study, assay media were added 1 h before the measurement. One possible explanation is that endogenous glycogen or glutamine is mobilized to provide a substrate for glycolysis (17). Nonmitochondrial oxygen consumption is composed chiefly of cell surface oxygen consumption, peroxisomal oxygen consumption, and substrate oxidation. In this study, nonmitochondrial respiration was 23% in transformed podocytes and primary podocytes. Herst and Berridge (7) studied 19 cancer cell lines and found wide variability (1% to 96%) in the extent to which mitochondrial respiration accounts for cellular oxygen consumption, and the extent of cell surface oxygen consumption varied among those cell lines, ranging from 1% to 80%. Thus, while there is much variability in the rates of nonmitochondrial oxygen consumption among cell types, the results from transformed podocytes and primary podocytes are quite similar to each other. Proton leak dissipates 20% of the energy derived from mitochondrial function in cells and tissues derived from many animal species (1). The physiological function of proton leak may include heat production and prevention of oxidative stress caused by reactive oxygen species (ROS) (1). Indeed, we found that proton leak accounts for the loss of 31% and 23% of total energy generated in transformed and primary podocytes, respectively. Rotenone, a complex I inhibitor, suppresses mitochondrial respiration. In the presence of lactate, glucose, pyruvate, and glucose plus pyruvate, the fraction of OCR that was inhibited by rotenone fell compared with the fraction of OCR that was inhibited by rotenone in basal medium. The reasons for this decrease in mitochondrial respiration when these energy substrates are present are uncertain. It may be that these metabolic substrates stimulate glycolysis and thereby reduce mitochondrial respiration; such coupling has been shown previously (3). Since all these measurements were made after the administration of the uncoupler FCCP, it is also possible that these findings in some way reflect an artifact. For example, FCCP uncoupling might alter complex I function or might affect or the uptake of rotenone. We are not aware of other investigations of rotenone-inhibitable mitochondrial respiration in other cell types in the presence of the range of substrates we used, and so we cannot determine whether these findings extend to other cell types. Finally, the increase in OCR that we noted with certain substrates before oligomycin administration could be due to an increase of nonmitochondrial respiration including substrate oxidation and/or cell surface oxygen consumption. Choi et al. (3) studied synaptosomes isolated from mouse cerebral cortex and showed that exogenous glucose and pyruvate were additive in enhancing spare respiratory capacity following FCCP (as we found in podocytes) and that pyruvate increased the proton leak conductance estimated from oligomycin-insensitive respiration (as we found in podocytes). Since proton leak rate is highly dependent upon the mitochondrial membrane potential (18) and pyruvate causes further hyperpolarization of the intrasynaptosomal mitochondria even in the presence of glucose (12), these authors suggested that the increased proton leak might be accounted for by this increase in mitochondrial membrane potential and further that pyruvate selectively energized a population of synaptosomes that had impaired glucose utilization (3). These authors did not use rotenone to measure mitochondrial respiration. Our data, derived from podocytes rather than isolated synaptosomes, indicate that proton leak is actually decreased by glucose, by pyruvate, and especially by a combination of these substrates. Exposure of cells to elevated glucose concentrations induces mitochondrial hyperpolarization and generation of mitochondrial superoxide, which is coupled with an accelerated electron flux across the renal mitochondria (16, 24). Proton leak has been shown to decrease ROS generation, whereas ROS have been shown to induce proton leak. This suggests the existence of a feedback loop between proton leak and ROS (2). Although high ROS generation is expected in the presence of elevated glucose concentrations, the calculated proton leak in podocytes was lowest in glucose plus pyruvate medium. Thus our data cannot be readily explained by a link between ROS and proton leak. At this time, we do not have a hypothesis to explain these findings in podocytes. Fig. 8. Electron and energy flow in mitochondria. Arrows represent the flow of electrons through the respiratory complexes. The generation of mitochondrial membrane potential is either used to generate ATP or dissipated as a proton leak. ROS, reactive oxygen species. Figure is modified from Brookes (2).
12 We were somewhat surprised to see that rotenone and antimycin had slightly different quantitative effects on OCR inhibition. Antimycin, a complex III inhibitor, completely inhibited OCR both in the presence and in the absence of FCCP. It is known that the combination of carbonyl cyanide m-chlorophenylhydrazone with either oligomycin or antimycin gives rise to the elevated levels of ROS in both HeLa cells and HeEB1 cells, while each compound alone has a much smaller effect (8); thus the combination of two drugs could increase mitochondrial membrane potential. However, if the generation of mitochondrial membrane potential is inhibited by FCCP before antimycin, a part of the flow of energy can generate proton leak, since proton leak is mediated by electron flow via complexes I, III, and IV (Fig. 8) (2). If the generation of mitochondrial membrane potential is inhibited by FCCP before rotenone, the flow of energy or electrons cannot generate proton leak. Otherwise, since it is reported that high glucose exposure leads to reduced complex III activity in rat renal proximal cells (16), the same amount of antimycin could inhibit OCR more effectively than rotenone did. Mitochondrial uncoupling induced by FCCP was associated with increased OCR with some but not all substrates. In transformed podocytes these substrates included lactate, glucose, pyruvate, and palmitate, while in primary podocytes these substrates included lactate, glucose, and pyruvate. These data are in general similar to those of other cell types, in which uncoupling leads to increase OCR with pyruvate and fatty acid (octanoic acid) (3, 26). To the extent that the response to mitochondrial uncoupling can provide a valid model of cellular response to increased energy demands, these findings may shed light on the utilization of various substrates under conditions of cell stress. Furthermore, it is known that mitochondrial uncoupling leads to stimulation of fatty acid -oxidation in several studies with other cell types (19, 20, 25). With regard to fatty acid utilization in podocytes, Mayrhofer et al. (15) showed that mouse podocytes express CD36, which is a key cell surface molecule that promotes the uptake of long-chain fatty acids, and that disrupted fatty acid metabolism in concert with an impaired antioxidant defense mechanism may play a role in puromycin aminonucleoside nephrosis in rat podocytes. Our study has certain limitations. First, much of our data derived from podocyte cell lines, which have the advantage of providing a stable cell phenotype over time. It is possible that transformation alters cellular bioenergetics. To address this possibility, we confirmed key aspects of our work in primary mouse podocytes. Second, the timescale of our experimental manipulations was necessarily fairly short, on the order of 3 h, and so the effect of particular interventions over a period of days or weeks could not be assessed. Third, these data may or may not reflect the bioenergetic profile of podocytes in vivo, where these cells receive cues from other cells and a specialized extracellular matrix and are bathed in a highly complex plasma milieu, with lower oxygen tension than is typical in cell culture. In summary, we have developed an initial bioenergetic profile of cultured mouse podocytes and conclude that mitochondria play the primary role in maintaining energy homeostasis, while glycolysis makes a lesser contribution. Podocytes can use a diverse set of substrates, including glucose, lactate, pyruvate and, in transformed podocytes but not primary podocytes, palmitate. ACKNOWLEDGMENTS We are grateful to Hideko Takahashi and Huiyan Lu for technical assistance with animal care, to Dr. Hisashi Hasumi and Dr. Masaya Baba for valuable advice, and to Dr. Kevin Bittman and Dr. Min Wu, both of Seahorse Bioscience, for valuable advice in the design and interpretation of particular experiments. We thank Dr. Michael Sack for critical review of the manuscript. GRANTS This study was supported by the NIDDK Intramural Research Program, under project ZO1-DK DISCLOSURES No conflicts of interest, financial or otherwise, are declared by the author(s). REFERENCES C Brand MD. The efficiency and plasticity of mitochondrial energy transduction. Biochem Soc Trans 33: , Brookes PS. Mitochondrial H leak and ROS generation: an odd couple. Free Radic Biol Med 38: 12 23, Choi SW, Gerencser AA, Nicholls DG. Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J Neurochem 109: , Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, Caridi G, Piemonte F, Montini G, Ghiggeri GM, Murer L, Barisoni L, Pastore A, Muda AO, Valente ML, Bertini E, Emma F. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol 18: , Ganapathy V, Thangaraju M, Gopal E, Martin PM, Itagaki S, Miyauchi S, Prasad PD. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J 10: , Halestrap AP, Meredith D. The SLC16 gene family from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Arch 447: , Herst PM, Berridge MV. Cell surface oxygen consumption: a major contributor to cellular oxygen consumption in glycolytic cancer cell lines. Biochim Biophys Acta 1767: , Hoffmann S, Spitkovsky D, Radicella JP, Epe B, Wiesner RJ. Reactive oxygen species derived from the mitochondrial respiratory chain are not responsible for the basal levels of oxidative base modifications observed in nuclear DNA of mammalian cells. Free Radic Biol Med 36: , Hotta O, Inoue CN, Miyabayashi S, Furuta T, Takeuchi A, Taguma Y. Clinical and pathologic features of focal segmental glomerulosclerosis with mitochondrial trnaleu(uur) gene mutation. Kidney Int 59: , Kajiyama H, Titus S, Austin CP, Chiotos K, Matsumoto T, Sakairi T, Kopp JB. Tetracycline-inducible gene expression in conditionally immortalized mouse podocytes. Am J Nephrol 29: , Katsuya K, Yaoita E, Yoshida Y, Yamamoto Y, Yamamoto T. An improved method for primary culture of rat podocytes. Kidney Int 69: , Kauppinen RA, Nicholls DG. Synaptosomal bioenergetics. The role of glycolysis, pyruvate oxidation and responses to hypoglycaemia. Eur J Biochem 158: , Li H, Myeroff L, Smiraglia D, Romero MF, Pretlow TP, Kasturi L, Lutterbaugh J, Rerko RM, Casey G, Issa JP, Willis J, Willson JK, Plass C, Markowitz SD. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc Natl Acad Sci USA 100: , Lopez LC, Schuelke M, Quinzii CM, Kanki T, Rodenburg RJ, Naini A, Dimauro S, Hirano M. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am J Hum Genet 79: , Mayrhofer C, Krieger S, Huttary N, Chang MW, Grillari J, Allmaier G, Kerjaschki D. Alterations in fatty acid utilization and an impaired antioxidant defense mechanism are early events in podocyte injury: a proteomic analysis. Am J Pathol 174: , Munusamy S, MacMillan-Crow LA. Mitochondrial superoxide plays a crucial role in the development of mitochondrial dysfunction during high glucose exposure in rat renal proximal tubular cells. Free Radic Biol Med 46: , 2009.
Application Note. Introduction
 Simultaneously Measuring Oxidation of Exogenous and Endogenous Fatty Acids Using the XF Palmitate-BSA FAO Substrate with the Agilent Seahorse XF Cell Mito Stress Test Application Note Introduction The
Simultaneously Measuring Oxidation of Exogenous and Endogenous Fatty Acids Using the XF Palmitate-BSA FAO Substrate with the Agilent Seahorse XF Cell Mito Stress Test Application Note Introduction The
Yoshifusa Abe, 1,2 Toru Sakairi, 3 Craig Beeson, 4 and Jeffrey B. Kopp 2 1
 Am J Physiol Renal Physiol 35: F1477 F149, 13. First published September 18, 13; doi:1.1152/ajprenal.182.13. TGF- 1 stimulates mitochondrial oxidative phosphorylation and generation of reactive oxygen
Am J Physiol Renal Physiol 35: F1477 F149, 13. First published September 18, 13; doi:1.1152/ajprenal.182.13. TGF- 1 stimulates mitochondrial oxidative phosphorylation and generation of reactive oxygen
Rapid Analysis of Glycolytic and Oxidative Substrate Flux of Cancer Cells in a Microplate
 Rapid Analysis of Glycolytic and Oxidative Substrate Flux of Cancer Cells in a Microplate Lisa S. Pike Winer, Min Wu* Seahorse Bioscience Inc., North Billerica, Massachusetts, United States of America
Rapid Analysis of Glycolytic and Oxidative Substrate Flux of Cancer Cells in a Microplate Lisa S. Pike Winer, Min Wu* Seahorse Bioscience Inc., North Billerica, Massachusetts, United States of America
Identifying Metabolic Phenotype Switches in Cancer Cells Using the Agilent Seahorse XF Analyzer in an Hypoxic Environment
 Identifying Metabolic Phenotype Switches in Cancer Cells Using the Agilent Seahorse XF Analyzer in an Hypoxic Environment Application Note Introduction Decreased oxygen levels, or hypoxia, and hypoxic-mediated
Identifying Metabolic Phenotype Switches in Cancer Cells Using the Agilent Seahorse XF Analyzer in an Hypoxic Environment Application Note Introduction Decreased oxygen levels, or hypoxia, and hypoxic-mediated
Performing Bioenergetic Analysis: Seahorse XFe96 Analyzer
 icell Cardiomyocytes 2 Application Protocol Performing Bioenergetic Analysis: Seahorse XFe96 Analyzer Introduction The myocardium is the most metabolically active tissue in the body and is highly sensitive
icell Cardiomyocytes 2 Application Protocol Performing Bioenergetic Analysis: Seahorse XFe96 Analyzer Introduction The myocardium is the most metabolically active tissue in the body and is highly sensitive
Quantifying Cellular ATP Production Rate Using Agilent Seahorse XF Technology
 White Paper Quantifying Cellular ATP Production Rate Using Agilent Seahorse XF Technology Authors Natalia Romero George Rogers Andy Neilson Brian P. Dranka Agilent Technologies, Inc., Lexington, MA, USA
White Paper Quantifying Cellular ATP Production Rate Using Agilent Seahorse XF Technology Authors Natalia Romero George Rogers Andy Neilson Brian P. Dranka Agilent Technologies, Inc., Lexington, MA, USA
Electron Transport and Oxidative. Phosphorylation
 Electron Transport and Oxidative Phosphorylation Electron-transport chain electron- Definition: The set of proteins and small molecules involved in the orderly sequence of transfer to oxygen within the
Electron Transport and Oxidative Phosphorylation Electron-transport chain electron- Definition: The set of proteins and small molecules involved in the orderly sequence of transfer to oxygen within the
Cell Metabolism Assays. for. Immunology. Research
 the immunity-metabolism link Cell Metabolism Assays for Immunology Research GOLD STANDARD ASSAYS FOR MEASURING METABOLIC RePROGRAMMING Metabolic Signatures Cell activation, amplification, and effector
the immunity-metabolism link Cell Metabolism Assays for Immunology Research GOLD STANDARD ASSAYS FOR MEASURING METABOLIC RePROGRAMMING Metabolic Signatures Cell activation, amplification, and effector
Medical Biochemistry and Molecular Biology department
 Medical Biochemistry and Molecular Biology department Cardiac Fuels [Sources of energy for the Cardiac muscle] Intended learning outcomes of the lecture: By the end of this lecture you would be able to:-
Medical Biochemistry and Molecular Biology department Cardiac Fuels [Sources of energy for the Cardiac muscle] Intended learning outcomes of the lecture: By the end of this lecture you would be able to:-
Nafith Abu Tarboush DDS, MSc, PhD
 Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush OMM: permeable to small molecules (MW
Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush OMM: permeable to small molecules (MW
Agilent Technologies: Seahorse XF Introduction
 Agilent Technologies: Seahorse XF Introduction Tools for investigating cell metabolism diagnostic procedures. 1 The Engines Cells Generate Energy via Two Metabolic Pathways Glycolysis [ H + ] Respiration
Agilent Technologies: Seahorse XF Introduction Tools for investigating cell metabolism diagnostic procedures. 1 The Engines Cells Generate Energy via Two Metabolic Pathways Glycolysis [ H + ] Respiration
MitoXpress FAO Assay. For the measurement of Fatty Acid Oxidation
 MitoXpress FAO Assay For the measurement of Fatty Acid Oxidation Companion Kit to be used in combination with Luxcel s MitoXpress Xtra - Oxygen Consumption Assay ILLUMINATING DISCOVERY TABLE OF CONTENTS
MitoXpress FAO Assay For the measurement of Fatty Acid Oxidation Companion Kit to be used in combination with Luxcel s MitoXpress Xtra - Oxygen Consumption Assay ILLUMINATING DISCOVERY TABLE OF CONTENTS
ab Glycolysis Stress Test Companion Assay
 Version 1 Last updated 12 July 2017 ab222945 Glycolysis Stress Test Companion Assay For the characterization of glycolytic stress in live cells when used in combination with Glycolysis Assay [Extracellular
Version 1 Last updated 12 July 2017 ab222945 Glycolysis Stress Test Companion Assay For the characterization of glycolytic stress in live cells when used in combination with Glycolysis Assay [Extracellular
Cell Metabolism Assays. for OMICS. Research
 Gene-Protein-metabolism links Cell Metabolism Assays for OMICS Research STANDARD Parameters of Functional METABOLIsm Genomics Cells use gene expression to synthesize proteins and other products that are
Gene-Protein-metabolism links Cell Metabolism Assays for OMICS Research STANDARD Parameters of Functional METABOLIsm Genomics Cells use gene expression to synthesize proteins and other products that are
Vocabulary. Chapter 20: Electron Transport and Oxidative Phosphorylation
 Vocabulary ATP Synthase: the enzyme responsible for production of ATP in mitochondria Chemiosmotic Coupling: the mechanism for coupling electron transport to oxidative phosphorylation; it requires a proton
Vocabulary ATP Synthase: the enzyme responsible for production of ATP in mitochondria Chemiosmotic Coupling: the mechanism for coupling electron transport to oxidative phosphorylation; it requires a proton
Chapter 9 Notes. Cellular Respiration and Fermentation
 Chapter 9 Notes Cellular Respiration and Fermentation Objectives Distinguish between fermentation and anaerobic respiration. Name the three stages of cellular respiration and state the region of the cell
Chapter 9 Notes Cellular Respiration and Fermentation Objectives Distinguish between fermentation and anaerobic respiration. Name the three stages of cellular respiration and state the region of the cell
Chapter 14 - Electron Transport and Oxidative Phosphorylation
 Chapter 14 - Electron Transport and Oxidative Phosphorylation The cheetah, whose capacity for aerobic metabolism makes it one of the fastest animals Prentice Hall c2002 Chapter 14 1 14.4 Oxidative Phosphorylation
Chapter 14 - Electron Transport and Oxidative Phosphorylation The cheetah, whose capacity for aerobic metabolism makes it one of the fastest animals Prentice Hall c2002 Chapter 14 1 14.4 Oxidative Phosphorylation
Ryan McGarrigle, Ph.D.
 Cardiomyocyte function characterised through combined analysis of metabolic flux, beat rate and cellular oxygenation and its application in in vitro ischemia reperfusion modelling Ryan McGarrigle, Ph.D.
Cardiomyocyte function characterised through combined analysis of metabolic flux, beat rate and cellular oxygenation and its application in in vitro ischemia reperfusion modelling Ryan McGarrigle, Ph.D.
Islet Assay Using the Agilent Seahorse XF24 Islet Capture Microplate
 Islet Assay Using the Agilent Seahorse XF24 Islet Capture Microplate Technical Overview Introduction Agilent Seahorse XF Analyzers are most commonly used with an adherent monolayer of cells attached to
Islet Assay Using the Agilent Seahorse XF24 Islet Capture Microplate Technical Overview Introduction Agilent Seahorse XF Analyzers are most commonly used with an adherent monolayer of cells attached to
) one consumes in breathing is converted to:, which of the following would be found in the oxidized state?
 MCB 102: Pantea s Sxn Chapter 19 Problem Set Answer Key 1) Page: 690 Ans: E Almost all of the oxygen (O 2 ) one consumes in breathing is converted to: A) acetyl-coa. B) carbon dioxide (CO 2 ). C) carbon
MCB 102: Pantea s Sxn Chapter 19 Problem Set Answer Key 1) Page: 690 Ans: E Almost all of the oxygen (O 2 ) one consumes in breathing is converted to: A) acetyl-coa. B) carbon dioxide (CO 2 ). C) carbon
ab Fatty Acid Oxidation Assay
 Version 3 Last updated 22 June 2017 ab217602 Fatty Acid Oxidation Assay For the convenient measurement of Fatty Acid Oxidation (FAO) in live cells when used in combination with Extracellular Oxygen Consumption
Version 3 Last updated 22 June 2017 ab217602 Fatty Acid Oxidation Assay For the convenient measurement of Fatty Acid Oxidation (FAO) in live cells when used in combination with Extracellular Oxygen Consumption
Mitochondria and ATP Synthesis
 Mitochondria and ATP Synthesis Mitochondria and ATP Synthesis 1. Mitochondria are sites of ATP synthesis in cells. 2. ATP is used to do work; i.e. ATP is an energy source. 3. ATP hydrolysis releases energy
Mitochondria and ATP Synthesis Mitochondria and ATP Synthesis 1. Mitochondria are sites of ATP synthesis in cells. 2. ATP is used to do work; i.e. ATP is an energy source. 3. ATP hydrolysis releases energy
Respiration. Respiration. How Cells Harvest Energy. Chapter 7
 How Cells Harvest Energy Chapter 7 Respiration Organisms can be classified based on how they obtain energy: autotrophs: are able to produce their own organic molecules through photosynthesis heterotrophs:
How Cells Harvest Energy Chapter 7 Respiration Organisms can be classified based on how they obtain energy: autotrophs: are able to produce their own organic molecules through photosynthesis heterotrophs:
Citric Acid Cycle and Oxidative Phosphorylation
 Citric Acid Cycle and Oxidative Phosphorylation Page by: OpenStax Summary The Citric Acid Cycle In eukaryotic cells, the pyruvate molecules produced at the end of glycolysis are transported into mitochondria,
Citric Acid Cycle and Oxidative Phosphorylation Page by: OpenStax Summary The Citric Acid Cycle In eukaryotic cells, the pyruvate molecules produced at the end of glycolysis are transported into mitochondria,
A) Choose the correct answer: 1) Reduction of a substance can mostly occur in the living cells by:
 Code: 1 1) Reduction of a substance can mostly occur in the living cells by: (a) Addition of oxygen (b) Removal of electrons (c) Addition of electrons (d) Addition of hydrogen 2) Starting with succinate
Code: 1 1) Reduction of a substance can mostly occur in the living cells by: (a) Addition of oxygen (b) Removal of electrons (c) Addition of electrons (d) Addition of hydrogen 2) Starting with succinate
XF Data Normalization by the Agilent Seahorse XF Imaging and Normalization System
 Application Note Normalization XF Data Normalization by the Agilent Seahorse XF Imaging and Normalization System Authors Yoonseok Kam Kellie Chadwick Ned Jastromb Brian P. Dranka Agilent Technologies,
Application Note Normalization XF Data Normalization by the Agilent Seahorse XF Imaging and Normalization System Authors Yoonseok Kam Kellie Chadwick Ned Jastromb Brian P. Dranka Agilent Technologies,
Oxidative Phosphorylation
 Electron Transport Chain (overview) The NADH and FADH 2, formed during glycolysis, β- oxidation and the TCA cycle, give up their electrons to reduce molecular O 2 to H 2 O. Electron transfer occurs through
Electron Transport Chain (overview) The NADH and FADH 2, formed during glycolysis, β- oxidation and the TCA cycle, give up their electrons to reduce molecular O 2 to H 2 O. Electron transfer occurs through
Metabolism of Carbohydrates Inhibitors of Electron Transport Chain
 Paper : 04 Module : 19 Principal Investigator Paper Coordinator Content Reviewer Content Writer Dr.S.K.Khare,Professor IIT Delhi. Dr. Ramesh Kothari,Professor UGC-CAS Department of Biosciences Saurashtra
Paper : 04 Module : 19 Principal Investigator Paper Coordinator Content Reviewer Content Writer Dr.S.K.Khare,Professor IIT Delhi. Dr. Ramesh Kothari,Professor UGC-CAS Department of Biosciences Saurashtra
Islet Oxygen Consumption Assay using the XF24 Islet Capture Microplate Shirihai Lab and Seahorse Bioscience Aug
 1 Islet Oxygen Consumption Assay using the XF24 Islet Capture Microplate Shirihai Lab and Seahorse Bioscience Aug 6 2009 XF Analyzers are most commonly used with an adherent monolayer of cells attached
1 Islet Oxygen Consumption Assay using the XF24 Islet Capture Microplate Shirihai Lab and Seahorse Bioscience Aug 6 2009 XF Analyzers are most commonly used with an adherent monolayer of cells attached
How Cells Harvest Energy. Chapter 7. Respiration
 How Cells Harvest Energy Chapter 7 Respiration Organisms classified on how they obtain energy: autotrophs: produce their own organic molecules through photosynthesis heterotrophs: live on organic compounds
How Cells Harvest Energy Chapter 7 Respiration Organisms classified on how they obtain energy: autotrophs: produce their own organic molecules through photosynthesis heterotrophs: live on organic compounds
7 Pathways That Harvest Chemical Energy
 7 Pathways That Harvest Chemical Energy Pathways That Harvest Chemical Energy How Does Glucose Oxidation Release Chemical Energy? What Are the Aerobic Pathways of Glucose Metabolism? How Is Energy Harvested
7 Pathways That Harvest Chemical Energy Pathways That Harvest Chemical Energy How Does Glucose Oxidation Release Chemical Energy? What Are the Aerobic Pathways of Glucose Metabolism? How Is Energy Harvested
Chapter 7 Cellular Respiration and Fermentation*
 Chapter 7 Cellular Respiration and Fermentation* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. Life Is Work
Chapter 7 Cellular Respiration and Fermentation* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. Life Is Work
Respiration. Respiration. Respiration. How Cells Harvest Energy. Chapter 7
 How Cells Harvest Energy Chapter 7 Organisms can be classified based on how they obtain energy: autotrophs: are able to produce their own organic molecules through photosynthesis heterotrophs: live on
How Cells Harvest Energy Chapter 7 Organisms can be classified based on how they obtain energy: autotrophs: are able to produce their own organic molecules through photosynthesis heterotrophs: live on
4. Which step shows a split of one molecule into two smaller molecules? a. 2. d. 5
 1. Which of the following statements about NAD + is false? a. NAD + is reduced to NADH during both glycolysis and the citric acid cycle. b. NAD + has more chemical energy than NADH. c. NAD + is reduced
1. Which of the following statements about NAD + is false? a. NAD + is reduced to NADH during both glycolysis and the citric acid cycle. b. NAD + has more chemical energy than NADH. c. NAD + is reduced
SOD1 inhibition reduces non-small cell lung cancer by inducing cell death
 SUPPLEMENTAL INFORMATION SOD1 inhibition reduces non-small cell lung cancer by inducing cell death Andrea Glasauer, Laura A. Sena, Lauren P. Diebold, Andrew P. Mazar & Navdeep S. Chandel Inventory of Supplemental
SUPPLEMENTAL INFORMATION SOD1 inhibition reduces non-small cell lung cancer by inducing cell death Andrea Glasauer, Laura A. Sena, Lauren P. Diebold, Andrew P. Mazar & Navdeep S. Chandel Inventory of Supplemental
A cell has enough ATP to last for about three seconds.
 Energy Transformation: Cellular Respiration Outline 1. Energy and carbon sources in living cells 2. Sources of cellular ATP 3. Turning chemical energy of covalent bonds between C-C into energy for cellular
Energy Transformation: Cellular Respiration Outline 1. Energy and carbon sources in living cells 2. Sources of cellular ATP 3. Turning chemical energy of covalent bonds between C-C into energy for cellular
Cellular metabolism made easy: Microplate assays for mitochondrial function & glycolytic flux
 Cellular metabolism made easy: Microplate assays for mitochondrial function & glycolytic flux Convenient Microplate Based Analysis of Cell Metabolism Resuspend Add Reagent Measure A simple push-button
Cellular metabolism made easy: Microplate assays for mitochondrial function & glycolytic flux Convenient Microplate Based Analysis of Cell Metabolism Resuspend Add Reagent Measure A simple push-button
How Cells Harvest Chemical Energy
 How Cells Harvest Chemical Energy Chapter 6 Introduction: How Is a Marathoner Different from a Sprinter? Individuals inherit various percentages of the two main types of muscle fibers, slow and fast The
How Cells Harvest Chemical Energy Chapter 6 Introduction: How Is a Marathoner Different from a Sprinter? Individuals inherit various percentages of the two main types of muscle fibers, slow and fast The
Citric Acid Cycle and Oxidative Phosphorylation
 Citric Acid Cycle and Oxidative Phosphorylation Bởi: OpenStaxCollege The Citric Acid Cycle In eukaryotic cells, the pyruvate molecules produced at the end of glycolysis are transported into mitochondria,
Citric Acid Cycle and Oxidative Phosphorylation Bởi: OpenStaxCollege The Citric Acid Cycle In eukaryotic cells, the pyruvate molecules produced at the end of glycolysis are transported into mitochondria,
Collin County Community College BIOL Muscle Physiology. Muscle Length-Tension Relationship
 Collin County Community College BIOL 2401 Muscle Physiology 1 Muscle Length-Tension Relationship The Length-Tension Relationship Another way that muscle cells can alter their force capability, is determined
Collin County Community College BIOL 2401 Muscle Physiology 1 Muscle Length-Tension Relationship The Length-Tension Relationship Another way that muscle cells can alter their force capability, is determined
Chemical Energy. Valencia College
 9 Pathways that Harvest Chemical Energy Valencia College 9 Pathways that Harvest Chemical Energy Chapter objectives: How Does Glucose Oxidation Release Chemical Energy? What Are the Aerobic Pathways of
9 Pathways that Harvest Chemical Energy Valencia College 9 Pathways that Harvest Chemical Energy Chapter objectives: How Does Glucose Oxidation Release Chemical Energy? What Are the Aerobic Pathways of
Energy Transformation: Cellular Respiration Outline 1. Sources of cellular ATP 2. Turning chemical energy of covalent bonds between C-C into energy
 Energy Transformation: Cellular Respiration Outline 1. Sources of cellular ATP 2. Turning chemical energy of covalent bonds between C-C into energy for cellular work (ATP) 3. Importance of electrons and
Energy Transformation: Cellular Respiration Outline 1. Sources of cellular ATP 2. Turning chemical energy of covalent bonds between C-C into energy for cellular work (ATP) 3. Importance of electrons and
III. 6. Test. Respiració cel lular
 III. 6. Test. Respiració cel lular Chapter Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex molecules? A) anabolic pathways B) catabolic pathways
III. 6. Test. Respiració cel lular Chapter Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex molecules? A) anabolic pathways B) catabolic pathways
RESPIRATION Worksheet
 A.P. Bio L.C. RESPIRATION Worksheet 1. In the conversion of glucose and oxygen to carbon dioxide and water a) which molecule becomes reduced? b) which molecule becomes oxidized? c) what happens to the
A.P. Bio L.C. RESPIRATION Worksheet 1. In the conversion of glucose and oxygen to carbon dioxide and water a) which molecule becomes reduced? b) which molecule becomes oxidized? c) what happens to the
How Cells Harvest Chemical Energy
 Chapter 6 How Cells Harvest Chemical Energy INTRODUCTION TO CELLULAR RESIRATION hotosynthesis and cellular respiration provide energy for life Cellular respiration makes and consumes O During the oxidation
Chapter 6 How Cells Harvest Chemical Energy INTRODUCTION TO CELLULAR RESIRATION hotosynthesis and cellular respiration provide energy for life Cellular respiration makes and consumes O During the oxidation
CELLULAR BIOENERGETICS FOR THE 21st CENTURY
 CELLULAR BIOENERGETICS FOR THE 21st CENTURY Seahorse XF Extracellular Flux Analyzers XF Extracellular Flux Analyzer PROVIDING A NEW WINDOW INTO CELLULAR BIOENERGETICS Before Seahorse developed With an
CELLULAR BIOENERGETICS FOR THE 21st CENTURY Seahorse XF Extracellular Flux Analyzers XF Extracellular Flux Analyzer PROVIDING A NEW WINDOW INTO CELLULAR BIOENERGETICS Before Seahorse developed With an
Name Class Date. 1. Cellular respiration is the process by which the of "food"
 Name Class Date Cell Respiration Introduction Cellular respiration is the process by which the chemical energy of "food" molecules is released and partially captured in the form of ATP. Carbohydrates,
Name Class Date Cell Respiration Introduction Cellular respiration is the process by which the chemical energy of "food" molecules is released and partially captured in the form of ATP. Carbohydrates,
7 Cellular Respiration and Fermentation
 CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 7 Cellular Respiration and Fermentation Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 7 Cellular Respiration and Fermentation Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
Cellular Pathways That Harvest Chemical Energy. Cellular Pathways That Harvest Chemical Energy. Cellular Pathways In General
 Cellular Pathways That Harvest Chemical Energy A. Obtaining Energy and Electrons from Glucose Lecture Series 12 Cellular Pathways That Harvest Chemical Energy B. An Overview: Releasing Energy from Glucose
Cellular Pathways That Harvest Chemical Energy A. Obtaining Energy and Electrons from Glucose Lecture Series 12 Cellular Pathways That Harvest Chemical Energy B. An Overview: Releasing Energy from Glucose
Harvesting energy: photosynthesis & cellular respiration
 Harvesting energy: photosynthesis & cellular respiration Learning Objectives Know the relationship between photosynthesis & cellular respiration Know the formulae of the chemical reactions for photosynthesis
Harvesting energy: photosynthesis & cellular respiration Learning Objectives Know the relationship between photosynthesis & cellular respiration Know the formulae of the chemical reactions for photosynthesis
CHY2026: General Biochemistry UNIT 7& 8: CARBOHYDRATE METABOLISM
 CHY2026: General Biochemistry UNIT 7& 8: CARBOHYDRATE METABOLISM Metabolism Bioenergetics is the transfer and utilization of energy in biological systems The direction and extent to which a chemical reaction
CHY2026: General Biochemistry UNIT 7& 8: CARBOHYDRATE METABOLISM Metabolism Bioenergetics is the transfer and utilization of energy in biological systems The direction and extent to which a chemical reaction
g) Cellular Respiration Higher Human Biology
 g) Cellular Respiration Higher Human Biology What can you remember about respiration? 1. What is respiration? 2. What are the raw materials? 3. What are the products? 4. Where does it occur? 5. Why does
g) Cellular Respiration Higher Human Biology What can you remember about respiration? 1. What is respiration? 2. What are the raw materials? 3. What are the products? 4. Where does it occur? 5. Why does
Fatty Acid Oxidation Assay on the XF24 Analyzer
 Fatty Acid Oxidation Assay on the XF24 Analyzer Mitochondria oxidize a variety of fuels to generate ATP through oxidative phosphorylation. Cells can utilize fatty acid, glucose and amino acids as their
Fatty Acid Oxidation Assay on the XF24 Analyzer Mitochondria oxidize a variety of fuels to generate ATP through oxidative phosphorylation. Cells can utilize fatty acid, glucose and amino acids as their
Chapter 9: Cellular Respiration: Harvesting Chemical Energy
 AP Biology Reading Guide Name: Date: Period Chapter 9: Cellular Respiration: Harvesting Chemical Energy Overview: Before getting involved with the details of cellular respiration and photosynthesis, take
AP Biology Reading Guide Name: Date: Period Chapter 9: Cellular Respiration: Harvesting Chemical Energy Overview: Before getting involved with the details of cellular respiration and photosynthesis, take
Cellular Respiration
 Cellular Respiration C 6 H 12 O 6 + 6O 2 -----> 6CO 2 + 6H 2 0 + energy (heat and ATP) 1. Energy Capacity to move or change matter Forms of energy are important to life include Chemical, radiant (heat
Cellular Respiration C 6 H 12 O 6 + 6O 2 -----> 6CO 2 + 6H 2 0 + energy (heat and ATP) 1. Energy Capacity to move or change matter Forms of energy are important to life include Chemical, radiant (heat
Class XI Chapter 14 Respiration in Plants Biology. 1. It is a biochemical process. 1. It is a physiochemical process.
 Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Supplementary Figure 1
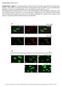 Supplementary Figure 1. (A) Representative confocal analysis of cardiac mesenchymal cells from nondiabetic (ND-CMSC) and diabetic (D-CMSC) immunostained with anti-h3k9ac and anti-h3k27me3 antibodies. Analysis
Supplementary Figure 1. (A) Representative confocal analysis of cardiac mesenchymal cells from nondiabetic (ND-CMSC) and diabetic (D-CMSC) immunostained with anti-h3k9ac and anti-h3k27me3 antibodies. Analysis
In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic
 Glycolysis 1 In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic glycolysis. If this pyruvate is converted instead
Glycolysis 1 In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic glycolysis. If this pyruvate is converted instead
Cellular Respiration
 Cellular Respiration 1. To perform cell work, cells require energy. a. A cell does three main kinds of work: i. Mechanical work, such as the beating of cilia, contraction of muscle cells, and movement
Cellular Respiration 1. To perform cell work, cells require energy. a. A cell does three main kinds of work: i. Mechanical work, such as the beating of cilia, contraction of muscle cells, and movement
Reading Assignments. A. Energy and Energy Conversions. Lecture Series 9 Cellular Pathways That Harvest Chemical Energy. gasoline) or elevated mass.
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Aerobic vs Anaerobic Respiration. 1. Glycolysis 2. Oxidation of Pyruvate and Krebs Cycle
 CELLULAR RESPIRATION Student Packet SUMMARY ALL LIVING SYSTEMS REQUIRE CONSTANT INPUT OF FREE ENERGY Cellular respiration is a catabolic pathway in which glucose and other organic fuels (such as starch,
CELLULAR RESPIRATION Student Packet SUMMARY ALL LIVING SYSTEMS REQUIRE CONSTANT INPUT OF FREE ENERGY Cellular respiration is a catabolic pathway in which glucose and other organic fuels (such as starch,
2. What is molecular oxygen directly converted into? a. Carbon Dioxide b. Water c. Glucose d. None of the Above
 Biochem 1 Mock Exam 3 Chapter 11: 1. What is glucose completely oxidized into? a. Carbon Dioxide and Water b. Carbon Dioxide and Oxygen c. Oxygen and Water d. Water and Glycogen 2. What is molecular oxygen
Biochem 1 Mock Exam 3 Chapter 11: 1. What is glucose completely oxidized into? a. Carbon Dioxide and Water b. Carbon Dioxide and Oxygen c. Oxygen and Water d. Water and Glycogen 2. What is molecular oxygen
10/25/2010 CHAPTER 9 CELLULAR RESPIRATION. Life is Work. Types of cellular respiration. Catabolic pathways = oxidizing fuels
 CHAPTER 9 CELLULAR RESPIRATION Life is Work Living cells require transfusions of energy from outside sources to perform their many tasks: Chemical work Transport work Mechanical work Energy stored in the
CHAPTER 9 CELLULAR RESPIRATION Life is Work Living cells require transfusions of energy from outside sources to perform their many tasks: Chemical work Transport work Mechanical work Energy stored in the
 Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Plant Respiration. Exchange of Gases in Plants:
 Plant Respiration Exchange of Gases in Plants: Plants do not have great demands for gaseous exchange. The rate of respiration in plants is much lower than in animals. Large amounts of gases are exchanged
Plant Respiration Exchange of Gases in Plants: Plants do not have great demands for gaseous exchange. The rate of respiration in plants is much lower than in animals. Large amounts of gases are exchanged
Electron Transport and oxidative phosphorylation (ATP Synthesis) Dr. Howaida Nounou Biochemistry department Sciences college
 Electron Transport and oxidative phosphorylation (ATP Synthesis) Dr. Howaida Nounou Biochemistry department Sciences college The Metabolic Pathway of Cellular Respiration All of the reactions involved
Electron Transport and oxidative phosphorylation (ATP Synthesis) Dr. Howaida Nounou Biochemistry department Sciences college The Metabolic Pathway of Cellular Respiration All of the reactions involved
3.2 Aerobic Respiration
 3.2 Aerobic Respiration Aerobic Cellular Respiration Catabolic pathways Breaks down energy-rich compounds to make ATP Requires oxygen Occurs in different parts of the cell C 6 H 12 O 6 (s) + 6O 2 (g) 6CO
3.2 Aerobic Respiration Aerobic Cellular Respiration Catabolic pathways Breaks down energy-rich compounds to make ATP Requires oxygen Occurs in different parts of the cell C 6 H 12 O 6 (s) + 6O 2 (g) 6CO
Background knowledge
 Background knowledge This is the required background knowledge: State three uses of energy in living things Give an example of an energy conversion in a living organism State that fats and oils contain
Background knowledge This is the required background knowledge: State three uses of energy in living things Give an example of an energy conversion in a living organism State that fats and oils contain
3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP]
![3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP] 3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP]](/thumbs/72/66350985.jpg) 3.7 Cell respiration ( Chapter 9 in Campbell's book) 3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP] Organic compounds store
3.7 Cell respiration ( Chapter 9 in Campbell's book) 3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP] Organic compounds store
Metabolism of Herpes Simplex Virus 1 infected RAW macrophages
 Metabolism of Herpes Simplex Virus 1 infected RAW 264.7 macrophages A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science By Mary Kathryn Jenkins B.S., Bowling
Metabolism of Herpes Simplex Virus 1 infected RAW 264.7 macrophages A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science By Mary Kathryn Jenkins B.S., Bowling
Cellular Respiration
 Cellular I can describe cellular respiration Cellular respiration is a series of metabolic pathways releasing energy from a foodstuff e.g. glucose. This yields energy in the form of ATP adenosine P i P
Cellular I can describe cellular respiration Cellular respiration is a series of metabolic pathways releasing energy from a foodstuff e.g. glucose. This yields energy in the form of ATP adenosine P i P
AP BIOLOGY Chapter 7 Cellular Respiration =
 1 AP BIOLOGY Chapter 7 Cellular Respiration = Day 1 p. I. Overview A. Cellular Respiration 1. Respiration breathing, exchange of O 2 for CO 2 2. Cellular respiration aerobic harvesting of energy from food
1 AP BIOLOGY Chapter 7 Cellular Respiration = Day 1 p. I. Overview A. Cellular Respiration 1. Respiration breathing, exchange of O 2 for CO 2 2. Cellular respiration aerobic harvesting of energy from food
Chapter 6 : How Cells Harvest Energy (B) Dr. Chris Doumen 10/28/14 CITRIC ACID CYCLE. Acetyl CoA CoA CoA CO 2 NAD + FADH 2 NADH FAD + 3 H + ADP + ATP
 Chapter 6 : How Cells Harvest Energy (B) Dr. Chris Doumen Acetyl CoA CoA CoA Oxaloacetate Citrate CITRIC ACID CYCLE CO FADH 3 NAD + FAD 3 NADH ADP + P + 3 1 Pyruvate oxida.on and Citric Acid Cycle Thus
Chapter 6 : How Cells Harvest Energy (B) Dr. Chris Doumen Acetyl CoA CoA CoA Oxaloacetate Citrate CITRIC ACID CYCLE CO FADH 3 NAD + FAD 3 NADH ADP + P + 3 1 Pyruvate oxida.on and Citric Acid Cycle Thus
Chapter 5. Microbial Metabolism
 Chapter 5 Microbial Metabolism Metabolism Collection of controlled biochemical reactions that take place within a microbe Ultimate function of metabolism is to reproduce the organism Metabolic Processes
Chapter 5 Microbial Metabolism Metabolism Collection of controlled biochemical reactions that take place within a microbe Ultimate function of metabolism is to reproduce the organism Metabolic Processes
Ch 9: Cellular Respiration
 Ch 9: Cellular Respiration Cellular Respiration An overview Exergonic reactions and catabolic pathway Energy stored in bonds of food molecules is transferred to ATP Cellular respiration provides the energy
Ch 9: Cellular Respiration Cellular Respiration An overview Exergonic reactions and catabolic pathway Energy stored in bonds of food molecules is transferred to ATP Cellular respiration provides the energy
How Cells Harvest Chemical Energy
 How Cells Harvest Chemical Energy Global Athlete Outreach Program US CytoThesis Systems Medicine Center www.cytothesis.us US OncoTherapy Systems BioMedicine Group CytoThesis Bioengineering Research Group
How Cells Harvest Chemical Energy Global Athlete Outreach Program US CytoThesis Systems Medicine Center www.cytothesis.us US OncoTherapy Systems BioMedicine Group CytoThesis Bioengineering Research Group
Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 6 How Cells Harvest Chemical Energy. 6.1 Multiple-Choice Questions
 Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 6 How Cells Harvest Chemical Energy 6.1 Multiple-Choice Questions 1) Which of the following statements regarding photosynthesis and
Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 6 How Cells Harvest Chemical Energy 6.1 Multiple-Choice Questions 1) Which of the following statements regarding photosynthesis and
PHM142 Energy Production + The Mitochondria
 PHM142 Energy Production + The Mitochondria 1 The Endosymbiont Theory of Mitochondiral Evolution 1970: Lynn Margulis Origin of Eukaryotic Cells Endosymbiant Theory: the mitochondria evolved from free-living
PHM142 Energy Production + The Mitochondria 1 The Endosymbiont Theory of Mitochondiral Evolution 1970: Lynn Margulis Origin of Eukaryotic Cells Endosymbiant Theory: the mitochondria evolved from free-living
Cellular Respiration. 3. In the figure, which step of the citric acid cycle requires both NAD+ and ADP as reactants? a. Step 1. c. Step 3 b.
 Cellular Respiration 1. Enzymes are organic catalysts. How do they increase the rate of chemical reactions? a. By decreasing the free-energy change of the reaction b. By increasing the free-energy change
Cellular Respiration 1. Enzymes are organic catalysts. How do they increase the rate of chemical reactions? a. By decreasing the free-energy change of the reaction b. By increasing the free-energy change
Objective: You will be able to construct an explanation for how each phase of respiration captures and stores free energy.
 Objective: You will be able to construct an explanation for how each phase of respiration captures and stores free energy. Do Now: Compare and contrast the three black equations below ADP + P + Energy
Objective: You will be able to construct an explanation for how each phase of respiration captures and stores free energy. Do Now: Compare and contrast the three black equations below ADP + P + Energy
Cellular Respiration Harvesting Chemical Energy ATP
 Cellular Respiration Harvesting Chemical Energy ATP 2009-2010 Ch.8.3 Section Objectives: Compare and contrast cellular respiration and fermentation. Explain how cells obtain energy from cellular respiration.
Cellular Respiration Harvesting Chemical Energy ATP 2009-2010 Ch.8.3 Section Objectives: Compare and contrast cellular respiration and fermentation. Explain how cells obtain energy from cellular respiration.
Cellular Respiration. Cellular Respiration. C 6 H 12 O 6 + 6O > 6CO 2 + 6H energy. Heat + ATP. You need to know this!
 Cellular Respiration LISA Biology Cellular Respiration C 6 H 12 O 6 + 6O 2 - - - - - > 6CO 2 + 6H 2 0 + energy You need to know this! Heat + ATP 1 Did that equation look familiar? * The equation for cellular
Cellular Respiration LISA Biology Cellular Respiration C 6 H 12 O 6 + 6O 2 - - - - - > 6CO 2 + 6H 2 0 + energy You need to know this! Heat + ATP 1 Did that equation look familiar? * The equation for cellular
How Cells Harvest Chemical Energy. Chapter 9
 How Cells Harvest Chemical Energy Chapter 9 Cellular Respiration Releasing energy (ATP) from glucose (chemical energy) in the presence of O 2 Energy flows Matter cycles True or False Plants only perform
How Cells Harvest Chemical Energy Chapter 9 Cellular Respiration Releasing energy (ATP) from glucose (chemical energy) in the presence of O 2 Energy flows Matter cycles True or False Plants only perform
McWilliams et al., http :// /cgi /content /full /jcb /DC1
 Supplemental material JCB McWilliams et al., http ://www.jcb.org /cgi /content /full /jcb.201603039 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. In vitro characterization of mito-qc. (A and B) Analysis
Supplemental material JCB McWilliams et al., http ://www.jcb.org /cgi /content /full /jcb.201603039 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. In vitro characterization of mito-qc. (A and B) Analysis
Higher Biology. Unit 2: Metabolism and Survival Topic 2: Respiration. Page 1 of 25
 Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
Section B: The Process of Cellular Respiration
 CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL ENERGY Section B: The Process of Cellular Respiration 1. Respiration involves glycolysis, the Krebs cycle, and electron transport: an overview 2. Glycolysis
CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL ENERGY Section B: The Process of Cellular Respiration 1. Respiration involves glycolysis, the Krebs cycle, and electron transport: an overview 2. Glycolysis
Cell Injury MECHANISMS OF CELL INJURY
 Cell Injury MECHANISMS OF CELL INJURY The cellular response to injurious stimuli depends on the following factors: Type of injury, Its duration, and Its severity. Thus, low doses of toxins or a brief duration
Cell Injury MECHANISMS OF CELL INJURY The cellular response to injurious stimuli depends on the following factors: Type of injury, Its duration, and Its severity. Thus, low doses of toxins or a brief duration
How Cells Release Chemical Energy. Chapter 7
 How Cells Release Chemical Energy Chapter 7 7.1 Overview of Carbohydrate Breakdown Pathways All organisms (including photoautotrophs) convert chemical energy of organic compounds to chemical energy of
How Cells Release Chemical Energy Chapter 7 7.1 Overview of Carbohydrate Breakdown Pathways All organisms (including photoautotrophs) convert chemical energy of organic compounds to chemical energy of
Supporting Information
 Supporting Information van der Windt et al. 10.1073/pnas.1221740110 SI Materials and Methods Mice and Reagents. C57BL/6 and major histocompatibility complex class I-restricted OVA-specific T-cell receptor
Supporting Information van der Windt et al. 10.1073/pnas.1221740110 SI Materials and Methods Mice and Reagents. C57BL/6 and major histocompatibility complex class I-restricted OVA-specific T-cell receptor
AWARD NUMBER: W81XWH TITLE: BIOENERGETICS OF STROMAL CELLS AS A PREDICTOR OF AGGRESSIVE PROSTATE CANCER
 AWARD NUMBER: W81XWH-14-1-0255 TITLE: BIOENERGETICS OF STROMAL CELLS AS A PREDICTOR OF AGGRESSIVE PROSTATE CANCER PRINCIPAL INVESTIGATOR: Praveen K. Vayalil RECIPIENT: University of Alabama at Birmingham
AWARD NUMBER: W81XWH-14-1-0255 TITLE: BIOENERGETICS OF STROMAL CELLS AS A PREDICTOR OF AGGRESSIVE PROSTATE CANCER PRINCIPAL INVESTIGATOR: Praveen K. Vayalil RECIPIENT: University of Alabama at Birmingham
Human induced pluripotent stem cell derived cardiomyocytes are a more relevant model for assessing drug-induced effects on mitochondrial function
 Human induced pluripotent stem cell derived cardiomyocytes are a more relevant model for assessing drug-induced effects on mitochondrial function than H9C2 cells 16 January 2013 SLAS2013 Presentation Outline
Human induced pluripotent stem cell derived cardiomyocytes are a more relevant model for assessing drug-induced effects on mitochondrial function than H9C2 cells 16 January 2013 SLAS2013 Presentation Outline
Bioenergetics. Chapter 3. Objectives. Objectives. Introduction. Photosynthesis. Energy Forms
 Objectives Chapter 3 Bioenergetics Discuss the function of cell membrane, nucleus, & mitochondria Define: endergonic, exergonic, coupled reactions & bioenergetics Describe how enzymes work Discuss nutrients
Objectives Chapter 3 Bioenergetics Discuss the function of cell membrane, nucleus, & mitochondria Define: endergonic, exergonic, coupled reactions & bioenergetics Describe how enzymes work Discuss nutrients
THE GLUCOSE-FATTY ACID-KETONE BODY CYCLE Role of ketone bodies as respiratory substrates and metabolic signals
 Br. J. Anaesth. (1981), 53, 131 THE GLUCOSE-FATTY ACID-KETONE BODY CYCLE Role of ketone bodies as respiratory substrates and metabolic signals J. C. STANLEY In this paper, the glucose-fatty acid cycle
Br. J. Anaesth. (1981), 53, 131 THE GLUCOSE-FATTY ACID-KETONE BODY CYCLE Role of ketone bodies as respiratory substrates and metabolic signals J. C. STANLEY In this paper, the glucose-fatty acid cycle
Metabolism Energy Pathways Biosynthesis. Catabolism Anabolism Enzymes
 Topics Microbial Metabolism Metabolism Energy Pathways Biosynthesis 2 Metabolism Catabolism Catabolism Anabolism Enzymes Breakdown of complex organic molecules in order to extract energy and dform simpler
Topics Microbial Metabolism Metabolism Energy Pathways Biosynthesis 2 Metabolism Catabolism Catabolism Anabolism Enzymes Breakdown of complex organic molecules in order to extract energy and dform simpler
Metabolism. Metabolism. Energy. Metabolism. Energy. Energy 5/22/2016
 5//016 Metabolism Metabolism All the biochemical reactions occurring in the body Generating, storing and expending energy ATP Supports body activities Assists in constructing new tissue Metabolism Two
5//016 Metabolism Metabolism All the biochemical reactions occurring in the body Generating, storing and expending energy ATP Supports body activities Assists in constructing new tissue Metabolism Two
Cellular Respiration Checkup Quiz. 1. Of the following products, which is produced by both anaerobic respiration and aerobic respiration in humans?
 1. Of the following products, which is produced by both anaerobic respiration and aerobic respiration in humans? I. Pyruvate II. III. ATP Lactate A. I only B. I and II only C. I, II and III D. II and III
1. Of the following products, which is produced by both anaerobic respiration and aerobic respiration in humans? I. Pyruvate II. III. ATP Lactate A. I only B. I and II only C. I, II and III D. II and III
MITOCHONDRIA LECTURES OVERVIEW
 1 MITOCHONDRIA LECTURES OVERVIEW A. MITOCHONDRIA LECTURES OVERVIEW Mitochondrial Structure The arrangement of membranes: distinct inner and outer membranes, The location of ATPase, DNA and ribosomes The
1 MITOCHONDRIA LECTURES OVERVIEW A. MITOCHONDRIA LECTURES OVERVIEW Mitochondrial Structure The arrangement of membranes: distinct inner and outer membranes, The location of ATPase, DNA and ribosomes The
Concept 9.1: Catabolic pathways yield energy by oxidizing organic fuels Several processes are central to cellular respiration and related pathways
 Overview: Life Is Work Living cells require energy from outside sources Some animals, such as the chimpanzee, obtain energy by eating plants, and some animals feed on other organisms that eat plants Energy
Overview: Life Is Work Living cells require energy from outside sources Some animals, such as the chimpanzee, obtain energy by eating plants, and some animals feed on other organisms that eat plants Energy
Enzymes and Metabolism
 PowerPoint Lecture Slides prepared by Vince Austin, University of Kentucky Enzymes and Metabolism Human Anatomy & Physiology, Sixth Edition Elaine N. Marieb 1 Protein Macromolecules composed of combinations
PowerPoint Lecture Slides prepared by Vince Austin, University of Kentucky Enzymes and Metabolism Human Anatomy & Physiology, Sixth Edition Elaine N. Marieb 1 Protein Macromolecules composed of combinations
Electron Transport Chain and Oxidative phosphorylation
 Electron Transport Chain and Oxidative phosphorylation So far we have discussed the catabolism involving oxidation of 6 carbons of glucose to CO 2 via glycolysis and CAC without any oxygen molecule directly
Electron Transport Chain and Oxidative phosphorylation So far we have discussed the catabolism involving oxidation of 6 carbons of glucose to CO 2 via glycolysis and CAC without any oxygen molecule directly
