via efferent arteriole dilation and inhibition of tubular sodium reabsorption.
|
|
|
- Eunice Crawford
- 6 years ago
- Views:
Transcription
1 Am J Physiol Renal Physiol 297: F1265 F1272, First published September 9, 2009; doi: /ajprenal Proinsulin C-peptide reduces diabetes-induced glomerular hyperfiltration via efferent arteriole dilation and inhibition of tubular sodium reabsorption Lina Nordquist, 1 Russell Brown, 1,2 Angelica Fasching, 1 Patrik Persson, 1 and Fredrik Palm 1,3 1 Division of Integrative Physiology, Department of Medical Cell Biology, Uppsala University, Uppsala, Sweden; 2 Department of Physiology, Monash University, Melbourne, Australia; and 3 Division of Nephrology and Hypertension, Department of Medicine, Georgetown University, Washington, District of Columbia Submitted 27 April 2009; accepted in final form 8 September 2009 Nordquist L, Brown R, Fasching A, Persson P, Palm F. Proinsulin C-peptide reduces diabetes-induced glomerular hyperfiltration via efferent arteriole dilation and inhibition of tubular sodium reabsorption. Am J Physiol Renal Physiol 297: F1265 F1272, First published September 9, 2009; doi: /ajprenal Cpeptide reduces diabetes-induced glomerular hyperfiltration in diabetic patients and experimental animal models. However, the mechanisms mediating the beneficial effect of C-peptide remain unclear. We investigated whether altered renal afferent-efferent arteriole tonus or alterations in tubular Na transport (T Na ) in response to C-peptide administration mediate the reduction of diabetes-induced glomerular hyperfiltration. Glomerular filtration rate, filtration fraction, total and cortical renal blood flow, total kidney O 2 consumption (QO 2 ), T Na, fractional Na and Li excretions, and tubular free-flow and stopflow pressures were measured in anesthetized adult male normoglycemic and streptozotocin-diabetic Sprague-Dawley rats. The specific effect of C-peptide on transport-dependent QO 2 was investigated in vitro in freshly isolated proximal tubular cells. C-peptide reduced glomerular filtration rate ( 24%), stop-flow pressure ( 8%), and filtration fraction ( 17%) exclusively in diabetic rats without altering renal blood flow. Diabetic rats had higher baseline T Na ( 40%), which was reduced by C-peptide. Similarly, C-peptide increased fractional Na ( 80%) and Li ( 47%) excretions only in the diabetic rats. None of these parameters was affected by vehicle treatments in either group. Baseline QO 2 was 37% higher in proximal tubular cells from diabetic rats than controls and was normalized by C-peptide. C-peptide had no effect on ouabain-pretreated diabetic cells from diabetic rats. C-peptide reduced diabetes-induced hyperfiltration via a net dilation of the efferent arteriole and inhibition of tubular Na reabsorption, both potent regulators of the glomerular net filtration pressure. These findings provide new mechanistic insight into the beneficial effects of C-peptide on diabetic kidney function. diabetes mellitus; oxygen consumption; sodium excretion THERE ARE SEVERAL HYPOTHESES for the mechanisms leading to the onset and progression of diabetes-induced renal dysfunction. The Diabetes Control and Complication Trial concluded that the degree of hyperglycemia is an important predictor of long-term renal complications in diabetes (7). The early augmented glomerular filtration may play a crucial role in the development of diabetic nephropathy, and inhibition of the diabetes-induced hyperfiltration has been shown to have beneficial effects, delaying the progression of kidney damage (33). C-peptide, the 31-residue cleavage product of insulin synthesis, is secreted from the islets of Langerhans together with insulin. Since impaired insulin synthesis will affect C-peptide release to the same extent, C-peptide was initially used as a Address for reprint requests and other correspondence: F. Palm, Department of Medical Cell Biology, Biomedical Center, Uppsala Univ., Box 571, Uppsala, Sweden ( Fredrik.Palm@mcb.uu.se). biomarker for estimation of insulin production. However, in the early 1980s, it was suggested that C-peptide may influence biological functions (59). Several recent studies have reported renoprotective effects of C-peptide when it is administered to patients with type 1 diabetes (13, 19, 20) and animal models of experimental insulinopenic diabetes (16, 36, 47 49, 51). The studies show that administration of physiologically relevant doses of C-peptide reduces diabetes-induced glomerular hyperfiltration, decreases albuminuria, reduces renal hypertrophy, and normalizes glomerular volume. Therefore, the lack of C-peptide has emerged as a possible mechanism for the development of diabetes-induced complications in diabetic patients. Peptides with the same amino acid composition as C-peptide, but in randomized sequences, have no interfering specificity (2, 35, 51). The exact mechanism resulting in the diabetes-induced glomerular hyperfiltration is not fully understood (46). However, altered vascular tone of the afferent and efferent arterioles and increased proximal tubular Na reabsorption, all potential regulators of the glomerular filtration pressure and, thus, glomerular filtration rate (GFR), may contribute (46, 54). Nevertheless, glomerular hyperfiltration increases tubular electrolyte load, resulting in increased tubular Na reabsorption to maintain homeostasis. This will undoubtedly increase the O 2 demand and, thereby, O 2 consumption (QO 2 ) (27). A wide range of different techniques and animal models have been used to show that the increased O 2 utilization eventually results in decreased tissue O 2 availability in the diabetic kidney (9, 18, 23, 39, 40, 44, 45). Na -K -ATPase is located in the basolateral membrane of tubular cells and creates the main driving force for tubular electrolyte reabsorption (24). Approximately 80% of total kidney QO 2 is related to tubular electrolyte transport (4). Numerous studies have reported increased renal Na -K - ATPase activity and increased QO 2 during the early hyperfiltration phase in streptozotocin (STZ)-induced diabetes (5, 27, 28, 58) and in other models of experimental diabetes (50). It has even been suggested that alterations in renal proximal Na -K -ATPase activity may regulate GFR (30). Thus regulation of GFR, tubular electrolyte handling, and renal blood flow (RBF) are interconnected via several mechanisms that must be investigated separately for a full understanding of how C-peptide might influence kidney function. The present study aimed to provide mechanistic insights into the effect of C-peptide on GFR. We therefore tested the hypothesis that C-peptide reduces the diabetes-induced glomerular hyperfiltration via alteration of vascular tone of the afferent and/or efferent arterioles (measured as blood flow and tubular pressures) or proximal tubular Na reabsorption (mea /09 $8.00 Copyright 2009 the American Physiological Society F1265
2 F1266 sured as fractional urinary Na and Li excretions and inhibition of cellular QO 2 related to electrolyte transport), or a combination thereof. MATERIALS AND METHODS Animals and induction of diabetes. Age-matched male Sprague- Dawley rats ( g body wt) were purchased from M& B(Ry, Denmark). Animals had free access to water and standard rat chow (0.3% Na, 0.8% K, 21% protein; R3, Ewos, Södertälje, Sweden) throughout the study. All experiments were performed in accordance with the National Institutes of Health guidelines for use and care of laboratory animals and approved by the Animal Care and Use Committee of Uppsala University. Diabetes was induced by an injection of STZ (50 mg/kg body wt; Sigma-Aldrich, St. Louis, MO) into the tail vein. Animals were considered diabetic if blood glucose concentrations increased to 18 mm within 24 h after STZ injection and remained elevated. Test reagent strips (MediSense, Bedford, MA) were used to determine blood glucose concentrations from blood samples obtained from the cut tip of the tail in all animals. Surgical procedures. At 2 wk after allocation to the study, the animals were anesthetized with thiobutabarbital (120 mg/kg body wt ip; Inactin, Sigma-Aldrich), placed on a thermocontrolled operating table at 37 C, and tracheotomized. Polyethylene catheters were placed in the right femoral vein for infusion of Ringer solution (5 and 10 ml kg body wt 1 h 1 for normoglycemic controls and diabetic animals, respectively), the right femoral artery for blood pressure measurements (model P23dB, Statham Laboratories, Los Angeles, CA), and the left renal vein for blood sampling. The left ureter was catheterized for collection of urine for subsequent analysis, and the urinary bladder was catheterized to allow urinary drainage. The left kidney was exposed by a left subcostal flank incision, immobilized in a plastic cup, and embedded in pieces of saline-soaked cotton wool, and the surface was covered with paraffin oil (Apoteksbolaget, Gothenburg, Sweden). Simultaneous measurements of GFR, total and cortical RBF, and total Qo 2. GFR was estimated by measurements of [ 3 H]inulin clearance. 3 H (American Radiolabeled, St. Louis, MO) was initially administered as a 185-kBq bolus and then continuously infused in Ringer solution (185 kbq kg body wt 1 h 1 ). 3 H activities in urine and plasma were measured using a standard liquid scintillation technique, and GFR was calculated as follows: GFR U V /P, where U and P denote the activity of [ 3 H]inulin in the urine and plasma, respectively, and V denotes the urine flow rate (in ml/min). After a 45-min recovery period, baseline measurements were obtained for 30 min. A single bolus dose of rat C-peptide (5 nmol/kg body wt; Sigma-Genosys, Cambridge, UK; 95% purity as measured by HPLC) was followed by a 40-min continuous infusion of C-peptide (50 pmol kg body wt 1 min 1 ), which should result in plasma C-peptide concentrations within the physiological range (51). Thereafter, all parameters were measured during a second 30-min experimental period. Additional control and diabetic animals were given only saline vehicle and served as time controls, since the scrambled C-peptide has previously been shown to have no physiological effects (2, 35, 51). Total RBF was measured using an ultrasound probe (Transonic Systems, Ithaca, NY) placed around the left renal artery, and cortical RBF was measured with laser-doppler flowmetry (model PF , Perimed, Stockholm, Sweden) (10). All measured parameters were continuously recorded with a MacLab instrument (AD Instruments, Hastings, UK) connected to a Macintosh Power-PC Blood gas parameters were analyzed on samples drawn from the left renal vein and femoral artery during the first and second measurement periods. Kidney weights were determined at the end of the experiment. Urine volumes were measured gravimetrically, and urinary Na concentrations were determined by flame spectrophotometry (model IL543, Instrumentation Lab, Milan, Italy). Estimations of proximal tubular reabsorption using Li clearance. In a separate set of control and diabetic rats (n 7 8/group), Li clearance was measured according to a previously published procedure (32). Briefly, the animals were subjected to the surgical protocol described above (see Surgical procedures), except only the bladder was catheterized for urine collection, and the subcostal flank incision and the immobilization of the kidney with the plastic cup were not performed. Li was administered as a 4-mg ip bolus of LiCl at the onset of anesthesia followed by a continuous intravenous infusion (2.1 mg h 1 rat 1 ), resulting in plasma concentration of mm. After surgery, the experimental protocol described above was performed for measurements of Li and inulin clearance in these animals. Since C-peptide reduces GFR and, therefore, tubular Li load via the same mechanism, estimations of proximal tubular reabsorption were calculated as fractional urinary Li excretion. Calculations. The filtration fraction (FF) was estimated as follows: FF GFR/RBF (1 Hct). Renal vascular resistance (RVR) was calculated as mean arterial pressure (MAP) divided by RBF. In vivo renal QO 2 ( mol/min) was estimated from the arteriovenous difference in O 2 content (O 2ct [Hb] oxygen saturation 1.34 PO ) total RBF. Tubular Na transport (T Na, mol/min) was calculated as follows: T Na [P Na] GFR, where [P Na] is plasma Na concentration. T Na per QO 2 was calculated as T Na/QO 2. Fractional Na excretion was estimated from [U Na] [P inulin]/[p Na] [U inulin], and fractional Li excretion was calculated according to [U Li] [P inulin]/[p Li] [U inulin], where [U Na] is urinary Na concentration, [P inulin] and [U inulin] represent plasma and urinary inulin concentration, and [P Li] is plasma Li concentration. Measurements of tubular pressures. The net glomerular filtration pressure (P net) was estimated by stop-flow technique at baseline and after 40 min of infusion of rat C-peptide (5 nmol/kg body wt bolus continuous infusion of 50 pmol kg body wt 1 min 1 in 5 control and 6 diabetic animals). Early proximal tubular segments ( 5/rat) were randomly chosen on the kidney surface before and after C-peptide administration. A pipette, filled with 1 M NaCl and Lissamine green and connected to a servo-null pressure system (World Precision Instruments, New Haven, CT), was used to determine proximal tubular free-flow pressure (P ff) and stop-flow pressure (P sf), the latter after tubular flow was interrupted with a wax blockade distal to the pressure pipette. P net was calculated as P sf P ff. Measurements of QO 2 in vitro. Proximal tubular cells were isolated from normoglycemic controls (n 9) and STZ-diabetic rats (n 12) as previously described (27, 39, 40). Briefly, the renal cortical tissue Fig. 1. Glomerular filtration rate in normoglycemic control and hyperglycemic diabetic rats before and after infusion of vehicle or C-peptide. Values are means SE (n 8 10/group). NS, not significant.
3 F1267 Fig. 2. Filtration fraction in normoglycemic control and hyperglycemic diabetic rats before and after infusion of vehicle or C-peptide. Values are means SE (n 8 10/group). was minced through a metallic mesh strainer and immediately placed in an ice-cooled buffer solution and incubated in collagenase (0.05% wt/vol; Sigma-Aldrich) at 37 C for 60 min while the buffer was equilibrated with 95% O 2-5% CO 2. Thereafter, the cell suspension was cooled to 4 C and filtered through graded filters with pore sizes of 180, 75, 53, and 38 m, respectively. The cells were pelleted by a slow centrifugation (100 g, 4 min) and resuspended in a collagenasefree buffer, a procedure that was repeated three times. The suspension was kept on ice until QO 2 was measured according to a previously described procedure (19). Briefly, a custom-made thermostatically controlled (37 C) gas-tight Plexiglas chamber with a total volume of 1.10 ml was used. The chamber was filled with buffer solution (113.0 mm NaCl, 4.0 mm KCl, 27.2 mm NaHCO 3, 1.0 mm KH 2PO 4, 1.2 mm MgCl 2, 1.0 mm CaCl 2, 10.0 mm HEPES, 0.5 mm Ca lactate, 2.0 mm glutamine, and 50 U/ml streptomycin, mosm, ph 7.40) and continuously stirred with an air-driven magnetic stirrer. Glucose concentration in the medium was 5.8 mm for cells from normoglycemic controls and 23.2 mm for cells from diabetic animals. O 2 content was measured by a modified O 2 sensor (model 500, Unisense, Aarhus, Denmark) calibrated with the air-equilibrated buffer solution as 228 M O 2 and Na 2S 2O 5-saturated buffer as zero. Cell suspension (100 l) was then injected into the chamber, and the rate of O 2 disappearance was measured. At the end of each experiment, a 100- l sample was taken for determination of the protein concentration using DC protein assay (Bio-Rad Laboratories, Hercules, CA). QO 2 was calculated as the rate of O 2 disappearance adjusted for protein concentration. QO 2 was measured during baseline and after incubation with rat C-peptide (1 nm), ouabain (1 mm), N -nitro-l-arginine methyl ester (L-NAME, 37 M), or a combination of C-peptide and either ouabain or L-NAME. Statistical evaluation. All statistical analyses were performed using GraphPad Prism software (San Diego, CA). Multiple comparisons between different groups were performed using ANOVA followed by Fisher s protected least significant difference test. Multiple comparisons within the same group were performed using repeated-measures ANOVA followed by Dunnett s post hoc test for paired comparisons. For comparison before and after a treatment within the same animals, paired Student s t-test was used. Descriptive statistics are presented as means SE. For all comparisons, P 0.05 was considered statistically significant. RESULTS All diabetic animals were hyperglycemic compared with normoglycemic controls ( vs mm). Diabetic animals gained less weight than the age-matched normoglycemic controls (281 5 vs g). Left kidney weight increased in diabetic animals compared with normoglycemic controls ( vs g). Diabetic rats displayed increased GFR (Fig. 1), which was reduced by C-peptide. FF was elevated in diabetic rats (Fig. 2) compared with controls. C-peptide selectively reduced FF by 17% in diabetic animals. MAP was similar at baseline in all groups (Table 1) and decreased in vehicle- and C-peptidetreated controls. However, MAP only decreased after C-peptide administration in the diabetic animals. Baseline T Na was 70% higher in diabetic animals than in controls (Fig. 3) and was only reduced by C-peptide in the diabetic group. Baseline fractional Na excretion was similar in all groups (Fig. 4), but C-peptide increased fractional Na excretion by 77% selectively in the diabetic group. Furthermore, fractional Li excretion increased only in the diabetic animals treated with C- peptide (Fig. 5). There was no difference in total or cortical RBF in the diabetic animals compared with the normoglycemic controls (Table 1). C-peptide did not alter RBF in any of the groups. Total in vivo QO 2 was 50% higher in the diabetic animals (Table 1), although QO 2 did not differ between diabetic and Table 1. MAP, total RBF, RVR, cortical RBF, in vivo renal O 2 consumption, and T Na per O 2 consumed in normoglycemic control and hyperglycemic diabetic rats acutely treated with vehicle or C-peptide n MAP, mmhg RBF, ml/min RVR, mmhg ml 1 min Cortical RBF, LU O2 Consumption, mol/min TNa/O2 Consumed Normoglycemic 10 Baseline Vehicle 106 4* * * Normoglycemic 8 Baseline C-peptide 108 2* * Diabetic 9 Baseline Vehicle * Diabetic 9 Baseline C-peptide 113 3* * Values are means SE. LU, laser units; MAP, mean arterial blood pressure; RBF, renal blood flow; RVR, renal vascular resistance; T Na,Na transport. *P 0.05 vs. baseline in the same group. P 0.05 vs. normoglycemic at baseline.
4 F1268 Fig. 3. Transported Na in normoglycemic control and hyperglycemic diabetic rats before and after infusion of vehicle or C-peptide. Values are means SE (n 8 10/group). normoglycemic control rats during baseline conditions (Table 1). C-peptide did not affect total QO 2 or T Na /QO 2 (Table 1). Infusion of vehicle did not alter any of the parameters in controls or diabetic animals, except for RVR, which decreased in all groups as a function of time, and T Na /QO 2, which decreased in the normoglycemic controls (Table 1). C-peptide reduced P ff by 5.2% ( vs mmhg), P sf by 8.4% ( vs mmhg), and calculated P net by 9.6% ( vs mmhg) in the diabetic animals, whereas it had no effect in controls (Fig. 6). Baseline QO 2 was increased in isolated proximal tubular cells from diabetic animals compared with controls (39 3 vs nmol mg protein 1 min 1 ; Fig. 7). C-peptide reduced QO 2 in diabetic cells (28 3 nmol mg protein 1 min 1 ) but had no effect on cells from normoglycemic controls (26 5 nmol mg protein 1 min 1 ). C-peptide had no effect on ouabain-pretreated cells (17 6 vs nmol mg protein 1 min 1 ; Fig. 7). Pretreatment with L-NAME had no Fig. 4. Fractional Na excretion in normoglycemic control and hyperglycemic diabetic rats before and after infusion of vehicle or C-peptide. Values are means SE (n 8 10/group). Fig. 5. Fractional Li excretion in normoglycemic control and hyperglycemic diabetic rats before and after infusion of vehicle or C-peptide. Values are means SE (n 7 8/group). effect in any of the groups and did not alter the effect of C-peptide in the diabetic cells (Fig. 7). DISCUSSION The main new finding from the present study was that acute administration of C-peptide reduced GFR in diabetic rats via two seemingly different mechanisms. 1) C-peptide caused a net dilation of the efferent arteriole in the diabetic rats, as evident from decreased FF and P sf in the absence of altered RBF. 2) C-peptide inhibited tubular Na reabsorption in diabetic rats, as evident from increased fractional Na and Li excretions and reduced transport-dependent QO 2 in isolated proximal tubule cells from diabetic rats. Acute alterations in GFR are determined by the glomerular capillary hydrostatic pressure (P cap ), the hydrostatic pressure in Bowman s space, and the oncotic pressure ( onc ). Changes in P cap are a result of alterations in the interplay of afferent and efferent arteriolar diameter, which alter the pressure transmitted to the glomerular capillaries (41, 43). Constriction of the afferent arteriole or dilation of the efferent arteriole will reduce P cap and lower P net, which reduces GFR. Intratubular hydrostatic pressure is mainly determined by early proximal tubular reabsorption and, under certain conditions, by the hydraulic resistance in the distal nephron segments, e.g., obstruction of the tubules (22). onc is determined by differences in the protein concentration between the blood in the glomerular capillaries and the fluid in Bowman s space. The main intrarenal factor influencing acute changes in onc is the FF (3, 38), which reflects the balance between the afferent and efferent arterioles during normal conditions. Reduced FF will decrease the concentration of proteins in the blood that leaves the glomerulus through the afferent arteriole and, thus, reduce the driving force for reabsorption along the proximal part of the nephron into the peritubular capillaries, commonly referred to as the glomerulotubular balance. Diabetes-induced glomerular hyperfiltration is reduced by C-peptide in patients with type 1 diabetes (19, 20) and in animal models of insulinopenic diabetes (16, 36, 48, 49, 51), with concomitant reduction of diabetes-induced renal damage (14, 16, 48). The glomerular arterioles exert critical actions to
5 F1269 Downloaded from Fig. 6. Tubular free-flow pressure (top), stop-flow pressure (middle), and calculated glomerular net filtration pressure (bottom) in control (n 5) and diabetic (n 6) rats before and after C-peptide. Values are means SE. P 0.05 vs. control at baseline. control P cap, thereby influencing the GFR. We previously reported that C-peptide constricts isolated afferent glomerular arterioles from diabetic mice in vitro (35). A similarly pronounced constriction of the afferent arterioles in vivo with no effect on efferent arterioles would undoubtedly reduce GFR and RBF and increase RVR. However, C-peptide reduced the elevated GFR and FF in the diabetic rats in the present study, but did not affect RBF, which is consistent with previous reports (20, 49). None of these parameters were altered in the animals treated with vehicle only. Furthermore, RVR was not increased in the diabetic animals treated with C-peptide but, rather, decreased as a function of time in all groups. Taken together with our finding of reduced P sf after C-peptide administration, this can only be attributed to a balancing dilation of the efferent arteriole. Similar dual actions have been reported for nitric oxide (NO) synthase inhibitors, Ca 2 channel blockers, atrial natriuretic peptide, and adenosine (1, 11, 15, 31). However, given the lowering of P net, this mechanism could have a significant effect on the outcome for diabetic patients, since it lowers GFR and, thus, the tubular Na load while maintaining RBF. C-peptide may also decrease diabetic hyperfiltration through inhibition of Na -K -ATPase activity, as evident from the increase in fractional urinary Na and Li excretions and decreased transport-independent QO 2 in isolated proximal tubular cells from diabetic rats. It has previously been postulated that diabetic hyperfiltration occurs as a result of primary hyperreabsorption upstream of the macula densa and the normal physiology of tubuloglomerular feedback (53, 54). However, it has been suggested that diabetes-induced glomerular hyperfiltration is caused by TGF-independent mechanisms (10, 46), although conflicting results have been reported (55). These by on November 22, 2017
6 F1270 Fig. 7. O 2 consumption in isolated proximal tubular cells from normoglycemic control (n 9) and hyperglycemic diabetic (n 12) rats at baseline and after incubation with C-peptide, ouabain, N -nitro-l-arginine methyl ester (L-NAME), or a combination of C-peptide and ouabain or L-NAME. Values are means SE. *P 0.05 vs. untreated control at baseline. studies utilize adenosine A 1 receptor-deficient mice, which have been shown to lack the TGF mechanism, but additional TGF-independent effects of adenosine A 1 receptors on afferent arteriolar tone complicate the interpretation of these findings. Also, the degree of hyperglycemia must be considered in a discussion of the potential role of TGF in diabetes-induced glomerular hyperfiltration (55). The diabetic rats displayed reduced proximal tubular Na reabsorption after C-peptide administration, and it is possible that the reduced GFR in these animals is caused by a TGF activation. Furthermore, earlier work demonstrated that tubular reabsorption directly influences hydraulic resistance in the proximal tubule and, therefore, affects GFR (30). It has been shown that sustained hyperglycemia in patients (56), as well as experimental animal models (27, 54), results in increased proximal tubular Na reabsorption secondary to increased Na -linked glucose reabsorption. It has also been shown that early distal tubular Na concentrations are decreased during hyperglycemia, together with increased cortical Na -K -ATPase activity (27, 50). Conversely, decreased Na reabsorption in the early proximal tubule will increase hydraulic resistance, which in turn lowers P net and, therefore, reduces GFR. This hypothesis is consistent with reports of a linear correlation between GFR and Na -K - ATPase activity in the kidney cortex during experimental diabetes (27, 50). Therefore, a reduced or normalized Na reabsorption in the early proximal tubule is likely to reduce GFR in the diabetic kidney. The differences in the reduction of P sf ( 8.4%) and P ff ( 5.2%) in this study are not definite proof but indicate that proximal tubular reabsorption is involved in the decrease in GFR. No reduction was seen in total Na excretion after C-peptide, a finding that is consistent with previous studies (42). However, the reduction in tubular Na load, due to reduced GFR after C-peptide, will conceal the inhibitory effect on total urinary Na excretion. The calculated fractional Na and Li excretions increased in the diabetic animals after C-peptide administration, which shows that C- peptide directly inhibits Na reabsorption exclusively in the diabetic rats. Electrolyte transport via Na -K -ATPase activity accounts for 80% of total QO 2 in the kidney (52). Therefore, diabetesinduced increase in Na -K -ATPase activity is likely to influence total QO 2 and, therefore, possibly limit renal O 2 availability. The finding of increased cellular QO 2 in diabetic rats in the present study is consistent with previous reports (27, 39). However, the present study shows that C-peptide directly inhibits QO 2 in isolated proximal tubular cells but that the in vivo total kidney QO 2 was not altered by C-peptide administration, even though T Na decreased by 17%. A possible explanation can be a shift of the Na reabsorption site along the nephron after inhibition of proximal tubular Na -K - ATPase activity. QO 2 /T Na is significantly less in the proximal tubule than in more distal parts of the nephron, since a notable part of the Na reabsorption in the proximal tubule is achieved via paracellular transport (29). This explanation would account for lack of reduced QO 2 in the diabetic rats in vivo after C-peptide administration.
7 Na -K -ATPase is inhibited by ouabain, although complete inhibition cannot be achieved in rats (12, 34). Ouabain treatment reduced cellular QO 2 in both groups. C-peptide had no further effect on ouabain-treated cells, which indicates that C-peptide reduces Na -K -ATPase activity. C-peptide reduced blood pressure in the present study, similar to previously reported findings (36), although most studies show no such effect. However, it is predicted that increased NaCl load to the macula densa will decrease renin release and, therefore, angiotensin II production (25), and it is possible that reduced tubular Na -K -ATPase activity may reduce long-term arterial blood pressure, which should further add to the renoprotective effects of C-peptide. However, the effect of long-term C-peptide administration on arterial blood pressure control in diabetic patients is beyond the scope of this study. The beneficial effects of C-peptide on diabetes mellitus have been observed in various tissues (14, 17). However, on the molecular level, the data may seem contradictory. In the present study, C-peptide decreased the ouabain-sensitive QO 2 of cells from diabetic rats but did not elicit an effect in cells isolated from normoglycemic rats. The inhibitory effect of C-peptide on isolated diabetic proximal tubular cells persisted also when the cells where pretreated with a nonselective NO synthase blocker, indicating that the mechanism does not involve a stimulated NO release with concomitant inhibition of mitochondrial respiration. Several previous studies report stimulatory effects of C-peptide on Na -K -ATPase, but these studies were performed under normoglycemic conditions (37, 60). Furthermore, Na -K -ATPase activities in neurons and erythrocytes are reduced during diabetes, and C-peptide normalizes these impairments (8, 13, 17). However, Na -K - ATPase activity in the kidney is elevated during the early onset of diabetes, probably due to the increased tubular electrolyte load resulting from the elevated GFR (6, 27, 57, 58). We report increased fractional urinary Na excretion and decreased transport-related cellular QO 2 after C-peptide administration, which demonstrates an inhibitory effect of C-peptide on Na - K -ATPase in the kidney. Indeed, the effects of C-peptide on endothelial NO synthase under normoglycemic conditions are different from those under hyperglycemic conditions (21, 26). In conclusion, these results indicate that C-peptide reduces the diabetes-induced hyperfiltration via a net dilation of the efferent arteriole and inhibition of tubular Na reabsorption, both potent regulators of P net. These findings provide new mechanistic insight into the beneficial effects of C-peptide on diabetic kidney function. GRANTS This study was supported by the Swedish Medical Research Council, the Swedish Society for Medical Research, the Fredrik and Ingrid Thuring Foundation, the Marcus and Amalia Wallenberg Foundation, the Magnus Bergvall Foundation, and National Institute of Diabetes and Digestive and Kidney Diseases Grant K99/R00 DK REFERENCES 1. Al-Mashhadi RH, Skott O, Vanhoutte PM, Hansen PB. Activation of A 2 adenosine receptors dilates cortical efferent arterioles in mouse. Kidney Int 75: , Al-Rasheed NM, Meakin F, Royal EL, Lewington AJ, Brown J, Willars GB, Brunskill NJ. Potent activation of multiple signalling pathways by C-peptide in opossum kidney proximal tubular cells. Diabetologia 47: , F Brenner BM, Troy JL. Postglomerular vascular protein concentration: evidence for a causal role in governing fluid reabsorption and glomerulotublar balance by the renal proximal tubule. J Clin Invest 50: , Brodwall EK, Laake H. The relation between oxygen consumption and transport of sodium in the human kidney. Scand J Clin Lab Invest 16: , Chen CC. Differentiation of renal Na -K -ATPase in control and streptozotocin-induced diabetic mice by G-protein acting toxins and phorbol esters. Eur J Pharmacol 225: , Cohen RA, MacGregor LC, Spokes KC, Silva P, Epstein FH. Effect of myo-inositol on renal Na-K-ATPase in experimental diabetes. Metabolism 39: , Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: , Djemli-Shipkolye A, Gallice P, Coste T, Jannot MF, Tsimaratos M, Raccah D, Vague P. The effects ex vivo and in vitro of insulin and C-peptide on Na/K adenosine triphosphatase activity in red blood cell membranes of type 1 diabetic patients. Metabolism 49: , dos Santos EA, Li LP, Ji L, Prasad PV. Early changes with diabetes in renal medullary hemodynamics as evaluated by fiberoptic probes and BOLD magnetic resonance imaging. Invest Radiol 42: , Faulhaber-Walter R, Chen L, Oppermann M, Kim SM, Huang Y, Hiramatsu N, Mizel D, Kajiyama H, Zerfas P, Briggs JP, Kopp JB, Schnermann J. Lack of A 1 adenosine receptors augments diabetic hyperfiltration and glomerular injury. J Am Soc Nephrol 19: , Feng MG, Navar LG. Nitric oxide synthase inhibition activates L- and T-type Ca 2 channels in afferent and efferent arterioles. Am J Physiol Renal Physiol 290: F873 F879, Feraille E, Barlet-Bas C, Cheval L, Rousselot M, Carranza ML, Dreher D, Arystarkhova E, Doucet A, Favre H. Presence of two isoforms of Na,K-ATPase with different pharmacological and immunological properties in the rat kidney. Pflügers Arch 430: , Forst T, De La Tour DD, Kunt T, Pfutzner A, Goitom K, Pohlmann T, Schneider S, Johansson BL, Wahren J, Lobig M, Engelbach M, Beyer J, Vague P. Effects of proinsulin C-peptide on nitric oxide, microvascular blood flow and erythrocyte Na,K -ATPase activity in diabetes mellitus type I. Clin Sci (Lond) 98: , Forst T, Kunt T, Pfutzner A, Beyer J, Wahren J. New aspects on biological activity of C-peptide in IDDM patients. Exp Clin Endocrinol Diabetes 106: , Hayashi K, Wakino S, Sugano N, Ozawa Y, Homma K, Saruta T. Ca 2 channel subtypes and pharmacology in the kidney. Circ Res 100: , Huang DY, Richter K, Breidenbach A, Vallon V. Human C-peptide acutely lowers glomerular hyperfiltration and proteinuria in diabetic rats: a dose-response study. Naunyn Schmiedebergs Arch Pharmacol 365: 67 73, Ido Y, Vindigni A, Chang K, Stramm L, Chance R, Heath WF, DiMarchi RD, Di Cera E, Williamson JR. Prevention of vascular and neural dysfunction in diabetic rats by C-peptide. Science 277: , Izuhara Y, Nangaku M, Inagi R, Tominaga N, Aizawa T, Kurokawa K, van Ypersele de Strihou C, Miyata T. Renoprotective properties of angiotensin receptor blockers beyond blood pressure lowering. JAmSoc Nephrol 16: , Johansson BL, Borg K, Fernqvist-Forbes E, Kernell A, Odergren T, Wahren J. Beneficial effects of C-peptide on incipient nephropathy and neuropathy in patients with type 1 diabetes mellitus. Diabet Med 17: , Johansson BL, Sjoberg S, Wahren J. The influence of human C-peptide on renal function and glucose utilization in type 1 (insulin-dependent) diabetic patients. Diabetologia 35: , Kamikawa A, Ishii T, Shimada K, Makondo K, Inanami O, Sakane N, Yoshida T, Saito M, Kimura K. Proinsulin C-peptide abrogates type-1 diabetes-induced increase of renal endothelial nitric oxide synthase in rats. Diabetes Metab Res Rev 24: , Karlsen FM, Holstein-Rathlou NH, Leyssac PP. A re-evaluation of the determinants of glomerular filtration rate. Acta Physiol Scand 155: , Katavetin P, Miyata T, Inagi R, Tanaka T, Sassa R, Ingelfinger JR, Fujita T, Nangaku M. High glucose blunts vascular endothelial growth
8 F1272 factor response to hypoxia via the oxidative stress-regulated hypoxiainducible factor/hypoxia-responsible element pathway. J Am Soc Nephrol 17: , Katz AI, Epstein FH. The role of sodium-potassium-activated adenosine triphosphatase in the reabsorption of sodium by the kidney. J Clin Invest 46: , Kim SM, Mizel D, Huang YG, Briggs JP, Schnermann J. Adenosine as a mediator of macula densa-dependent inhibition of renin secretion. Am J Physiol Renal Physiol 290: F1016 F1023, Kitamura T, Kimura K, Makondo K, Furuya DT, Suzuki M, Yoshida T, Saito M. Proinsulin C-peptide increases nitric oxide production by enhancing mitogen-activated protein-kinase-dependent transcription of endothelial nitric oxide synthase in aortic endothelial cells of Wistar rats. Diabetologia 46: , Korner A, Eklof AC, Celsi G, Aperia A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes 43: , Ku DD, Sellers BM, Meezan E. Development of renal hypertrophy and increased renal Na,K-ATPase in streptozotocin-diabetic rats. Endocrinology 119: , Larsen EH, Mobjerg N, Sorensen JN. Fluid transport and ion fluxes in mammalian kidney proximal tubule: a model analysis of isotonic transport. Acta Physiol (Oxf) 187: , Leyssac PP. Dependence of glomerular filtration rate on proximal tubular reabsorption of salt. Acta Physiol Scand 58: , Marin-Grez M, Fleming JT, Steinhausen M. Atrial natriuretic peptide causes pre-glomerular vasodilatation and post-glomerular vasoconstriction in rat kidney. Nature 324: , Miracle CM, Rieg T, Blantz RC, Vallon V, Thomson SC. Combined effects of carbonic anhydrase inhibitor and adenosine A 1 receptor antagonist on hemodynamic and tubular function in the kidney. Kidney Blood Press Res 30: , Nath KA, Kren SM, Hostetter TH. Dietary protein restriction in established renal injury in the rat. Selective role of glomerular capillary pressure in progressive glomerular dysfunction. J Clin Invest 78: , Noel F, Fagoo M, Godfraind T. A comparison of the affinities of rat (Na K )-ATPase isozymes for cardioactive steroids, role of lactone ring, sugar moiety and KCl concentration. Biochem Pharmacol 40: , Nordquist L, Lai EY, Sjoquist M, Patzak A, Persson AE. Proinsulin C-peptide constricts glomerular afferent arterioles in diabetic mice. A potential renoprotective mechanism. Am J Physiol Regul Integr Comp Physiol 294: R836 R841, Nordquist L, Moe E, Sjoquist M. The C-peptide fragment EVARQ reduces glomerular hyperfiltration in streptozotocin-induced diabetic rats. Diabetes Metab Res Rev 23: , Ohtomo Y, Aperia A, Sahlgren B, Johansson BL, Wahren J. C-peptide stimulates rat renal tubular Na,K -ATPase activity in synergism with neuropeptide Y. Diabetologia 39: , Ott CE, Marchand GR, Diaz-Buxo JA, Knox FG. Determinants of glomerular filtration rate in the dog. Am J Physiol 231: , Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia 46: , Palm F, Hansell P, Ronquist G, Waldenstrom A, Liss P, Carlsson PO. Polyol-pathway-dependent disturbances in renal medullary metabolism in experimental insulin-deficient diabetes mellitus in rats. Diabetologia 47: , Persson AE, Gushwa LC, Blantz RC. Feedback pressure-flow responses in normal and angiotensin-prostaglandin-blocked rats. Am J Physiol Renal Fluid Electrolyte Physiol 247: F925 F931, Rebsomen L, Pitel S, Boubred F, Buffat C, Feuerstein JM, Raccah D, Vague P, Tsimaratos M. C-peptide replacement improves weight gain and renal function in diabetic rats. Diabetes Metab 32: , Ren YL, Garvin JL, Ito S, Carretero OA. Role of neuronal nitric oxide synthase in the macula densa. Kidney Int 60: , Ries M, Basseau F, Tyndal B, Jones R, Deminiere C, Catargi B, Combe C, Moonen CW, Grenier N. Renal diffusion and BOLD MRI in experimental diabetic nephropathy. Blood oxygen level-dependent. J Magn Reson Imaging 17: , Rosenberger C, Khamaisi M, Abassi Z, Shilo V, Weksler-Zangen S, Goldfarb M, Shina A, Zibertrest F, Eckardt KU, Rosen S, Heyman SN. Adaptation to hypoxia in the diabetic rat kidney. Kidney Int 73: 34 42, Sallstrom J, Carlsson PO, Fredholm BB, Larsson E, Persson AE, Palm F. Diabetes-induced hyperfiltration in adenosine A 1-receptor deficient mice lacking the tubuloglomerular feedback mechanism. Acta Physiol (Oxf) 190: , Samnegard B, Jacobson SH, Jaremko G, Johansson BL, Ekberg K, Isaksson B, Eriksson L, Wahren J, Sjoquist M. C-peptide prevents glomerular hypertrophy and mesangial matrix expansion in diabetic rats. Nephrol Dial Transplant 20: , Samnegard B, Jacobson SH, Jaremko G, Johansson BL, Sjoquist M. Effects of C-peptide on glomerular and renal size and renal function in diabetic rats. Kidney Int 60: , Samnegard B, Jacobson SH, Johansson BL, Ekberg K, Isaksson B, Wahren J, Sjoquist M. C-peptide and captopril are equally effective in lowering glomerular hyperfiltration in diabetic rats. Nephrol Dial Transplant 19: , Scherzer P, Nachliel I, Bar-On H, Popovtzer MM, Ziv E. Renal Na-K-ATPase hyperactivity in diabetic Psammomys obesus is related to glomerular hyperfiltration but is insulin-independent. J Endocrinol 167: , Sjoquist M, Huang W, Johansson BL. Effects of C-peptide on renal function at the early stage of experimental diabetes. Kidney Int 54: , Thaysen JH, Lassen NA, Munck O. Sodium transport and oxygen consumption in the mammalian kidney. Nature 190: , Thomson SC, Vallon V, Blantz RC. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol 286: F8 F15, Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol 10: , Vallon V, Schroth J, Satriano J, Blantz RC, Thomson SC, Rieg T. Adenosine A 1 receptors determine glomerular hyperfiltration and the salt paradox in early streptozotocin diabetes mellitus. Nephron Physiol 111: p30 p38, Vervoort G, Veldman B, Berden JH, Smits P, Wetzels JF. Glomerular hyperfiltration in type 1 diabetes mellitus results from primary changes in proximal tubular sodium handling without changes in volume expansion. Eur J Clin Invest 35: , Wald H, Scherzer P, Popovtzer MM. Enhanced renal tubular ouabainsensitive ATPase in streptozotocin diabetes mellitus. Am J Physiol Renal Fluid Electrolyte Physiol 251: F164 F170, Wald H, Scherzer P, Rasch R, Popovtzer MM. Renal tubular Na -K - ATPase in diabetes mellitus: relationship to metabolic abnormality. Am J Physiol Endocrinol Metab 265: E96 E101, Wojcikowski C, Maier V, Dominiak K, Fussganger R, Pfeiffer EF. Effects of synthetic rat C-peptide in normal and diabetic rats. Diabetologia 25: , Zhong Z, Kotova O, Davidescu A, Ehren I, Ekberg K, Jornvall H, Wahren J, Chibalin AV. C-peptide stimulates Na,K -ATPase via activation of ERK1/2 MAP kinases in human renal tubular cells. Cell Mol Life Sci 61: , 2004.
Malou Friederich-Persson, Patrik Persson, Angelica Fasching, Peter Hansell, Lina Nordquist, and Fredrik Palm
 Chapter 2 Increased Kidney Metabolism as a Pathway to Kidney Tissue Hypoxia and Damage: Effects of Triiodothyronine and Dinitrophenol in Normoglycemic Rats Malou Friederich-Persson, Patrik Persson, Angelica
Chapter 2 Increased Kidney Metabolism as a Pathway to Kidney Tissue Hypoxia and Damage: Effects of Triiodothyronine and Dinitrophenol in Normoglycemic Rats Malou Friederich-Persson, Patrik Persson, Angelica
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Review Article C-Peptide Effects on Renal Physiology and Diabetes
 Hindawi Publishing Corporation Experimental Diabetes Research Volume 2008, Article ID 281536, 5 pages doi:10.1155/2008/281536 Review Article C-Peptide Effects on Renal Physiology and Diabetes L. Rebsomen,
Hindawi Publishing Corporation Experimental Diabetes Research Volume 2008, Article ID 281536, 5 pages doi:10.1155/2008/281536 Review Article C-Peptide Effects on Renal Physiology and Diabetes L. Rebsomen,
MAJOR FUNCTIONS OF THE KIDNEY
 MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION
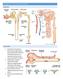 LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM
 RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
Copyright 2009 Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. Figure 19-1c. Efferent arteriole. Juxtaglomerular apparatus
 /6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
/6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Reduced adenosine A 2a receptor mediated efferent arteriolar vasodilation contributes to diabetes-induced glomerular hyperfiltration
 http://www.kidney-international.org & 214 International Society of Nephrology Reduced adenosine A 2a receptor mediated efferent arteriolar vasodilation contributes to diabetes-induced glomerular hyperfiltration
http://www.kidney-international.org & 214 International Society of Nephrology Reduced adenosine A 2a receptor mediated efferent arteriolar vasodilation contributes to diabetes-induced glomerular hyperfiltration
Glomerular Capillary Blood Pressure
 Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Introduction to the kidney: regulation of sodium & glucose. Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health
 Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Physiology Lecture 2. What controls GFR?
 Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences. Body fluids and.
 By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences Body fluids and Renal physiology 25 Volume and Osmolality of Extracellular and Intracellular Fluids
By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences Body fluids and Renal physiology 25 Volume and Osmolality of Extracellular and Intracellular Fluids
RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY
 RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY GROSS ANATOMY Location *Bean-shaped *Retroperitoneal *At level of T12 L1 vertebrae. *The right kidney lies slightly inferior to left
RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY GROSS ANATOMY Location *Bean-shaped *Retroperitoneal *At level of T12 L1 vertebrae. *The right kidney lies slightly inferior to left
KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin
![KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin](/thumbs/87/96174332.jpg) Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
RENAL PHYSIOLOGY. Physiology Unit 4
 RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
C-peptide prevents glomerular hypertrophy and mesangial matrix expansion in diabetic rats
 NDT Advance Access published January 21, 25 Nephrol Dial Transplant (25) 1 of 7 doi:1.193/ndt/gfh683 Original Article C-peptide prevents glomerular hypertrophy and mesangial matrix expansion in diabetic
NDT Advance Access published January 21, 25 Nephrol Dial Transplant (25) 1 of 7 doi:1.193/ndt/gfh683 Original Article C-peptide prevents glomerular hypertrophy and mesangial matrix expansion in diabetic
Renal Physiology Part II. Bio 219 Napa Valley College Dr. Adam Ross
 Renal Physiology Part II Bio 219 Napa Valley College Dr. Adam Ross Fluid and Electrolyte balance As we know from our previous studies: Water and ions need to be balanced in order to maintain proper homeostatic
Renal Physiology Part II Bio 219 Napa Valley College Dr. Adam Ross Fluid and Electrolyte balance As we know from our previous studies: Water and ions need to be balanced in order to maintain proper homeostatic
Renal Physiology - Lectures
 Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
The functional unit of the kidney, the single nephron, shows
 Tubuloglomerular Feedback and the Control of Glomerular Filtration Rate Volker Vallon Institute of Pharmacology and Toxicology, University of Tübingen, D-72074 Tübingen, Germany In every nephron the glomerular
Tubuloglomerular Feedback and the Control of Glomerular Filtration Rate Volker Vallon Institute of Pharmacology and Toxicology, University of Tübingen, D-72074 Tübingen, Germany In every nephron the glomerular
Fal Fal P h y s i o l o g y 6 1 1, S a n F r a n c i s c o S t a t e U n i v e r s i t y
 Fall 12 OSMOTIC REGULATION OF THE RENAL SYSTEM: Effects of fasting and ingestion of water, coke, or Gatorade on urine flow rate and specific gravity Dorette Franks The purpose of the physiology experiment
Fall 12 OSMOTIC REGULATION OF THE RENAL SYSTEM: Effects of fasting and ingestion of water, coke, or Gatorade on urine flow rate and specific gravity Dorette Franks The purpose of the physiology experiment
Basic Functions of the Kidneys
 Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Nephron Anatomy Nephron Anatomy
 Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Renal physiology II. Basic renal processes. Dr Alida Koorts BMS
 Renal physiology II Basic renal processes Dr Alida Koorts BMS 7-12 012 319 2921 akoorts@medic.up.ac.za Basic renal processes 1. filtration 2. reabsorption 3. secretion Glomerular filtration The filtration
Renal physiology II Basic renal processes Dr Alida Koorts BMS 7-12 012 319 2921 akoorts@medic.up.ac.za Basic renal processes 1. filtration 2. reabsorption 3. secretion Glomerular filtration The filtration
Ch 19: The Kidneys. Functional unit of kidneys:?? Developed by John Gallagher, MS, DVM
 Ch 19: The Kidneys Homeostatic regulation of ECF volume and BP Osmolarity 290 mosm Ion balance Na+ and K+, etc. ph (acid-base balance Excretion of wastes & foreign substances Hormone production EPO Renin
Ch 19: The Kidneys Homeostatic regulation of ECF volume and BP Osmolarity 290 mosm Ion balance Na+ and K+, etc. ph (acid-base balance Excretion of wastes & foreign substances Hormone production EPO Renin
modulating the tubuloglomerular feed-back mechanism in the canine kidney; Sciences Center, 4200 East Ninth Avenue, Denver, CO 80262, U.S.A.
 J. Physiol. (1986), 380, pp. 35-43 35 With 3 text-figures Printed in Great Britain RENAL VASOCONSTRICTOR RESPONSE TO HYPERTONIC SALINE IN THE DOG: EFFECTS OF PROSTAGLANDINS, INDOMETHACIN AND THEOPHYLLINE
J. Physiol. (1986), 380, pp. 35-43 35 With 3 text-figures Printed in Great Britain RENAL VASOCONSTRICTOR RESPONSE TO HYPERTONIC SALINE IN THE DOG: EFFECTS OF PROSTAGLANDINS, INDOMETHACIN AND THEOPHYLLINE
Renal Regulation of Sodium and Volume. Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM
 Renal Regulation of Sodium and Volume Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM Maintaining Volume Plasma water and sodium (Na + ) are regulated independently - you are already familiar
Renal Regulation of Sodium and Volume Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM Maintaining Volume Plasma water and sodium (Na + ) are regulated independently - you are already familiar
Renal System Physiology
 M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
The kidneys are excretory and regulatory organs. By
 exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
COMPLETE INHIBITION OF RENAL RESERVE IN CHRONIC RENAL FAILURE BY A COMBINATION OF ACEI AND ARB
 COMPLETE INHIBITION OF RENAL RESERVE IN CHRONIC RENAL FAILURE BY A COMBINATION OF ACEI AND ARB CG Musso ¹, J Reynaldi¹, N Imperiali ¹, L Algranati ¹, DG Oreopoulos ² Nephrology Department Hospital Italiano
COMPLETE INHIBITION OF RENAL RESERVE IN CHRONIC RENAL FAILURE BY A COMBINATION OF ACEI AND ARB CG Musso ¹, J Reynaldi¹, N Imperiali ¹, L Algranati ¹, DG Oreopoulos ² Nephrology Department Hospital Italiano
Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION
 3 Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION David Shirley, Giovambattista Capasso and Robert Unwin The kidney has three homeostatic functions that can broadly be described as excretory, regulatory
3 Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION David Shirley, Giovambattista Capasso and Robert Unwin The kidney has three homeostatic functions that can broadly be described as excretory, regulatory
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
** Accordingly GFR can be estimated by using one urine sample and do creatinine testing.
 This sheet includes the lecture and last year s exam. When a patient goes to a clinic, we order 2 tests: 1) kidney function test: in which we measure UREA and CREATININE levels, and electrolytes (Na+,
This sheet includes the lecture and last year s exam. When a patient goes to a clinic, we order 2 tests: 1) kidney function test: in which we measure UREA and CREATININE levels, and electrolytes (Na+,
Renal Functions: Renal Functions: Renal Function: Produce Urine
 Renal Functions: Excrete metabolic waste products Reabsorb vital nutrients Regulate osmolarity: Maintain ion balance Regulate extracellular fluid volume (and thus blood pressure) Renal Functions: Regulate
Renal Functions: Excrete metabolic waste products Reabsorb vital nutrients Regulate osmolarity: Maintain ion balance Regulate extracellular fluid volume (and thus blood pressure) Renal Functions: Regulate
Salt Sensitivity: Mechanisms, Diagnosis, and Clinical Relevance
 Salt Sensitivity: Mechanisms, Diagnosis, and Clinical Relevance Matthew R. Weir, MD Professor and Director Division of Nephrology University of Maryland School of Medicine Overview Introduction Mechanisms
Salt Sensitivity: Mechanisms, Diagnosis, and Clinical Relevance Matthew R. Weir, MD Professor and Director Division of Nephrology University of Maryland School of Medicine Overview Introduction Mechanisms
renoprotection therapy goals 208, 209
 Subject Index Aldosterone, plasminogen activator inhibitor-1 induction 163, 164, 168 Aminopeptidases angiotensin II processing 64 66, 214 diabetic expression 214, 215 Angiotensin I intrarenal compartmentalization
Subject Index Aldosterone, plasminogen activator inhibitor-1 induction 163, 164, 168 Aminopeptidases angiotensin II processing 64 66, 214 diabetic expression 214, 215 Angiotensin I intrarenal compartmentalization
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1
 BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
Renal Blood flow; Renal Clearance. Dr Sitelbanat
 Renal Blood flow; Renal Clearance Dr Sitelbanat Objectives At the end of this lecture student should be able to describe: Renal blood flow Autoregulation of GFR and RBF Regulation of GFR The Calcuation
Renal Blood flow; Renal Clearance Dr Sitelbanat Objectives At the end of this lecture student should be able to describe: Renal blood flow Autoregulation of GFR and RBF Regulation of GFR The Calcuation
Filtration and Reabsorption Amount Filter/d
 Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD Contact me at lharris@lsuhsc.edu Renal Physiology Lecture 3 Renal Clearance and Glomerular Filtration Filtration and Reabsorption Amount Filter/d Amount
Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD Contact me at lharris@lsuhsc.edu Renal Physiology Lecture 3 Renal Clearance and Glomerular Filtration Filtration and Reabsorption Amount Filter/d Amount
Functional morphology of kidneys Clearance
 Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Renal physiology D.HAMMOUDI.MD
 Renal physiology D.HAMMOUDI.MD Functions Regulating blood ionic composition Regulating blood ph Regulating blood volume Regulating blood pressure Produce calcitrol and erythropoietin Regulating blood glucose
Renal physiology D.HAMMOUDI.MD Functions Regulating blood ionic composition Regulating blood ph Regulating blood volume Regulating blood pressure Produce calcitrol and erythropoietin Regulating blood glucose
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
Functions of the kidney
 Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
Regulation of fluid and electrolytes balance
 Regulation of fluid and electrolytes balance Three Compartment Fluid Compartments Intracellular = Cytoplasmic (inside cells) Extracellular compartment is subdivided into Interstitial = Intercellular +
Regulation of fluid and electrolytes balance Three Compartment Fluid Compartments Intracellular = Cytoplasmic (inside cells) Extracellular compartment is subdivided into Interstitial = Intercellular +
Ch 17 Physiology of the Kidneys
 Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Nephron Function and Urine Formation. Ms. Kula December 1, 2014 Biology 30S
 Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
2) This is a Point and Click question. You must click on the required structure.
 Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
MATHEMATICAL MODELING OF THE TUBULOGLOMERULAR FEEDBACK MECHANISM IN THE KIDNEY
 MATHEMATICAL MODELING OF THE TUBULOGLOMERULAR FEEDBACK MECHANISM IN THE KIDNEY Susanne Ditlevsen Department of Mathematical Sciences, University of Copenhagen Donald Marsh Department of Molecular Pharmacology,
MATHEMATICAL MODELING OF THE TUBULOGLOMERULAR FEEDBACK MECHANISM IN THE KIDNEY Susanne Ditlevsen Department of Mathematical Sciences, University of Copenhagen Donald Marsh Department of Molecular Pharmacology,
Managing patients with renal disease
 Managing patients with renal disease Hiddo Lambers Heerspink, MD University Medical Centre Groningen, The Netherlands Asian Cardio Diabetes Forum April 23 24, 216 Kuala Lumpur, Malaysia Prevalent cases,
Managing patients with renal disease Hiddo Lambers Heerspink, MD University Medical Centre Groningen, The Netherlands Asian Cardio Diabetes Forum April 23 24, 216 Kuala Lumpur, Malaysia Prevalent cases,
Septic Acute Kidney Injury (AKI) Rinaldo Bellomo Australian and New Zealand Intensive Care Research Centre (ANZIC-RC) Melbourne Australia
 Septic Acute Kidney Injury (AKI) Rinaldo Bellomo Australian and New Zealand Intensive Care Research Centre (ANZIC-RC) Melbourne Australia Things we really, honestly know about septic AKI AKI is common
Septic Acute Kidney Injury (AKI) Rinaldo Bellomo Australian and New Zealand Intensive Care Research Centre (ANZIC-RC) Melbourne Australia Things we really, honestly know about septic AKI AKI is common
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Glomerular filtration rate (GFR)
 LECTURE NO (2) Renal Physiology Glomerular filtration rate (GFR) Faculty Of Medicine Dept.Of Physiology The glomerulus Is a tuft of capillaries enclosed within a Bowman capsule. It is supplied by an afferent
LECTURE NO (2) Renal Physiology Glomerular filtration rate (GFR) Faculty Of Medicine Dept.Of Physiology The glomerulus Is a tuft of capillaries enclosed within a Bowman capsule. It is supplied by an afferent
RENAL PHYSIOLOGY. Zekeriyya ALANOGLU, MD, DESA Ahmet Onat Bermede, MD, Ankara University School of Medicine Dept. Anesthesiology and ICM
 RENAL PHYSIOLOGY Zekeriyya ALANOGLU, MD, DESA Ahmet Onat Bermede, MD, Ankara University School of Medicine Dept. Anesthesiology and ICM Kidneys Stabilize the composition of the ECF (electrolyte, H
RENAL PHYSIOLOGY Zekeriyya ALANOGLU, MD, DESA Ahmet Onat Bermede, MD, Ankara University School of Medicine Dept. Anesthesiology and ICM Kidneys Stabilize the composition of the ECF (electrolyte, H
Urinary System Organization. Urinary System Organization. The Kidneys. The Components of the Urinary System
 Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Chapter 25 The Urinary System
 Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Osmoregulation and Renal Function
 1 Bio 236 Lab: Osmoregulation and Renal Function Fig. 1: Kidney Anatomy Fig. 2: Renal Nephron The kidneys are paired structures that lie within the posterior abdominal cavity close to the spine. Each kidney
1 Bio 236 Lab: Osmoregulation and Renal Function Fig. 1: Kidney Anatomy Fig. 2: Renal Nephron The kidneys are paired structures that lie within the posterior abdominal cavity close to the spine. Each kidney
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Use the following diagram to answer the next question. 1. In the diagram above, pressure filtration occurs in a. W b. X c. Y d. Z
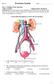 Part A: Multiple Choice Questions Value: 32 Marks Suggested time: 40 minutes Instructions: For each question select the best answer and record your choice on the Scantron card provided. Using an HB pencil,
Part A: Multiple Choice Questions Value: 32 Marks Suggested time: 40 minutes Instructions: For each question select the best answer and record your choice on the Scantron card provided. Using an HB pencil,
Nephron Structure inside Kidney:
 In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
Urinary System. consists of the kidneys, ureters, urinary bladder and urethra
 Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Physiology (4) 2/4/2018. Wael abu-anzeh
 Physiology (4) 2/4/2018 Wael abu-anzeh In the previous lectures we have discussed the filtration and the reabsorption processes but in this lecture we will talk about the factor that will regulate or control
Physiology (4) 2/4/2018 Wael abu-anzeh In the previous lectures we have discussed the filtration and the reabsorption processes but in this lecture we will talk about the factor that will regulate or control
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
RENAL PHYSIOLOGY. Zekeriyya ALANOGLU, MD, DESA. Ahmet Onat Bermede, MD. Ankara University School of Medicine Dept. Anesthesiology and ICM
 RENAL PHYSIOLOGY Zekeriyya ALANOGLU, MD, DESA. Ahmet Onat Bermede, MD. Ankara University School of Medicine Dept. Anesthesiology and ICM Kidneys Stabilize the composition of the ECF (electrolyte,
RENAL PHYSIOLOGY Zekeriyya ALANOGLU, MD, DESA. Ahmet Onat Bermede, MD. Ankara University School of Medicine Dept. Anesthesiology and ICM Kidneys Stabilize the composition of the ECF (electrolyte,
Excretion Chapter 29. The Mammalian Excretory System consists of. The Kidney. The Nephron: the basic unit of the kidney.
 Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
Pressure Diuresis 9 Sample Student Essays
 Pressure Diuresis 9 Sample Student Essays Below please find assembled consecutively in one document the brief analyses submitted by nine students in Mammalian Physiology 08 to the Teach Yourself Pressure
Pressure Diuresis 9 Sample Student Essays Below please find assembled consecutively in one document the brief analyses submitted by nine students in Mammalian Physiology 08 to the Teach Yourself Pressure
Lecture 16: The Nephron
 Lecture 16: The Nephron Reading: OpenStax A&P Text Chapter 25 Primary functions of the kidneys 1. Regulating osmolarity (blood concentration!) A. Regulating blood pressure B. Maintaining ion balance C.
Lecture 16: The Nephron Reading: OpenStax A&P Text Chapter 25 Primary functions of the kidneys 1. Regulating osmolarity (blood concentration!) A. Regulating blood pressure B. Maintaining ion balance C.
NOTES: CH 44 Regulating the Internal Environment (Homeostasis & The Urinary System)
 NOTES: CH 44 Regulating the Internal Environment (Homeostasis & The Urinary System) HOMEOSTASIS **Recall HOMEOSTASIS is the steady-state physiological condition of the body. It includes: 1) Thermoregulation:
NOTES: CH 44 Regulating the Internal Environment (Homeostasis & The Urinary System) HOMEOSTASIS **Recall HOMEOSTASIS is the steady-state physiological condition of the body. It includes: 1) Thermoregulation:
osmoregulation mechanisms in gills, salt glands, and kidneys
 Ionic & Osmotic Homeostasis osmoregulation mechanisms in gills, salt glands, and kidneys extracellular intracellular 22 23 Salt Secretion: recycle Figure in Box 26.2 Hill et al. 2004 active Down electrochemical
Ionic & Osmotic Homeostasis osmoregulation mechanisms in gills, salt glands, and kidneys extracellular intracellular 22 23 Salt Secretion: recycle Figure in Box 26.2 Hill et al. 2004 active Down electrochemical
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1)
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional role of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional role of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
Renal Physiology - Lectures
 Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD lharris@lsuhsc.edu Renal Physiology - Lectures Physiology of Body Fluids 2. Structure & Function of the Kidneys 3. Renal Clearance & Glomerular Filtration
Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD lharris@lsuhsc.edu Renal Physiology - Lectures Physiology of Body Fluids 2. Structure & Function of the Kidneys 3. Renal Clearance & Glomerular Filtration
Physiology questions review
 Physiology questions review 1- The consumption of O2 by the kidney: a- decrease as blood flow increases b- regulated by erythropoiten c- remains constant as blood flow increase d- direct reflects the level
Physiology questions review 1- The consumption of O2 by the kidney: a- decrease as blood flow increases b- regulated by erythropoiten c- remains constant as blood flow increase d- direct reflects the level
Acute Kidney Injury. APSN JSN CME for Nephrology Trainees May Professor Robert Walker
 Acute Kidney Injury APSN JSN CME for Nephrology Trainees May 2017 Professor Robert Walker Kidney International (2017) 91, 1033 1046; http://dx.doi.org/10.1016/ j.kint.2016.09.051 Case for discussion 55year
Acute Kidney Injury APSN JSN CME for Nephrology Trainees May 2017 Professor Robert Walker Kidney International (2017) 91, 1033 1046; http://dx.doi.org/10.1016/ j.kint.2016.09.051 Case for discussion 55year
Neuronal nitric oxide synthase inhibition sensitizes the tubuloglomerular feedback mechanism after volume expansion
 Kidney International, Vol. 65 (04), pp. 1349 16 Neuronal nitric oxide synthase inhibition sensitizes the tubuloglomerular feedback mechanism after volume expansion RUSSELL BROWN, 1 ANNA OLLERSTAM, 1 and
Kidney International, Vol. 65 (04), pp. 1349 16 Neuronal nitric oxide synthase inhibition sensitizes the tubuloglomerular feedback mechanism after volume expansion RUSSELL BROWN, 1 ANNA OLLERSTAM, 1 and
PHGY210 Renal Physiology
 PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
Excretory Lecture Test Questions Set 1
 Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
** TMP mean page 340 in 12 th edition. Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2:
 QUESTION Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2: Urine flow rate = 1 ml/min Urine inulin concentration = 100 mg/ml Plasma inulin concentration = 2 mg/ml
QUESTION Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2: Urine flow rate = 1 ml/min Urine inulin concentration = 100 mg/ml Plasma inulin concentration = 2 mg/ml
Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note
![Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note](/thumbs/77/75431210.jpg) Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
Collin County Community College RENAL PHYSIOLOGY
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Kidney and urine formation
 Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
2- Maintain the proper balance between water and. 3- Maintain the proper acid base balance. glucose by gluconeogenesis. pressure
 Filter ~180 liters of blood plasma daily, allowing toxins, metabolic wastes, and excess ions to leave the body in urine 1 Regulate volume and chemical composition of the blood 2 Maintain the proper balance
Filter ~180 liters of blood plasma daily, allowing toxins, metabolic wastes, and excess ions to leave the body in urine 1 Regulate volume and chemical composition of the blood 2 Maintain the proper balance
Principles of Renal Physiology. 4th Edition
 Principles of Renal Physiology 4th Edition Principles of Renal Physiology 4th Edition Chris Lote Professor of Experimental Nephrology, University of Birmingham, UK SPRINGER SCIENCE+BUSINESS MEDIA, B.V.
Principles of Renal Physiology 4th Edition Principles of Renal Physiology 4th Edition Chris Lote Professor of Experimental Nephrology, University of Birmingham, UK SPRINGER SCIENCE+BUSINESS MEDIA, B.V.
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015
 RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology
 Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper
Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper
Key words: Macula densa Afferent and efferent arterioles Sodium balance Angiotensin II Autoregulation Nitric oxide Adenosine
 Special Article Tubuloglomerular Feedback Sadayoshi ITO, MD, and Keishi ABE, MD SUMMARY The macula densa, a plaque of specialized tubular epithelial cells located in the distal tubule, monitors the NaCl
Special Article Tubuloglomerular Feedback Sadayoshi ITO, MD, and Keishi ABE, MD SUMMARY The macula densa, a plaque of specialized tubular epithelial cells located in the distal tubule, monitors the NaCl
Urinary System. BSC 2086 A & P 2 Professor Tcherina Duncombe Palm Beach State College
 Urinary System BSC 2086 A & P 2 Professor Tcherina Duncombe Palm Beach State College Filter plasma, separate and eliminate wastes Functions Regulate blood volume and pressure Regulate osmolarity of body
Urinary System BSC 2086 A & P 2 Professor Tcherina Duncombe Palm Beach State College Filter plasma, separate and eliminate wastes Functions Regulate blood volume and pressure Regulate osmolarity of body
SGLT2 inhibition in diabetes: extending from glycaemic control to renal and cardiovascular protection
 SGLT2 inhibition in diabetes: extending from glycaemic control to renal and cardiovascular protection Hiddo Lambers Heerspink Department of Clinical Pharmacy and Pharmacology University Medical Center
SGLT2 inhibition in diabetes: extending from glycaemic control to renal and cardiovascular protection Hiddo Lambers Heerspink Department of Clinical Pharmacy and Pharmacology University Medical Center
BIOL2030 Human A & P II -- Exam 6
 BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Original Contribution. R. Mileva 1, A. Tolekova 2, G. Ilieva 2, D. Popov 1, P. Markova 1* 50 Trakia Journal of Sciences, Vol.
 Trakia Journal of Sciences, No 1, pp 50-54, 2013 Copyright 2013 Trakia University Available online at: http://www.uni-sz.bg ISSN 1313-7050 (print) ISSN 1313-3551 (online) Original Contribution PLASMA RENIN
Trakia Journal of Sciences, No 1, pp 50-54, 2013 Copyright 2013 Trakia University Available online at: http://www.uni-sz.bg ISSN 1313-7050 (print) ISSN 1313-3551 (online) Original Contribution PLASMA RENIN
Autoregulation of Single Nephron Filtration Rate in the Presence and the Absence of Flow to the Macula Densa
 Autoregulation of Single Nephron Filtration Rate in the Presence and the Absence of Flow to the Macula Densa By Franklyn G. Knox, Cobern Ott, Jean-Louis Cuche, Josianne Gasser, and John Haas ABSTRACT Flow
Autoregulation of Single Nephron Filtration Rate in the Presence and the Absence of Flow to the Macula Densa By Franklyn G. Knox, Cobern Ott, Jean-Louis Cuche, Josianne Gasser, and John Haas ABSTRACT Flow
The Excretory System. Biology 20
 The Excretory System Biology 20 Introduction Follow along on page 376 What dangers exist if your body is unable to regulate the fluid balance of your tissues? What challenged would the body have to respond
The Excretory System Biology 20 Introduction Follow along on page 376 What dangers exist if your body is unable to regulate the fluid balance of your tissues? What challenged would the body have to respond
Outline the functional anatomy, and the physiological factors, that determine oxygen delivery to the renal medulla.
 2011-2-21 Outline the functional anatomy, and the physiological factors, that determine oxygen delivery to the renal medulla. Oxygen delivery = Blood flow CaO 2 Where Blood flow determined by (arterial
2011-2-21 Outline the functional anatomy, and the physiological factors, that determine oxygen delivery to the renal medulla. Oxygen delivery = Blood flow CaO 2 Where Blood flow determined by (arterial
