Performance in Initiating and Delivering Clinical Research
|
|
|
- Eleanor Woods
- 6 years ago
- Views:
Transcription
1 Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get involved in Clinical Research. Moorfields delivers cutting edge treatments and has a diverse patient population that creates the opportunity to recruit patients into trials with wide ranging eye care needs. We have a dedicated research support team of nurses, trial co-ordinators and research managers that support this ambition. Delivering research across the Moorfields / UCL Institute of Ophthalmology partnership is a major priority, but we also strive to attract more and more global partnerships for delivering clinical trials as this both broadens the research portfolio and increases the interaction with industry to positively contribute to the UK Life Science economy. In order to speed up research translation we have a strong focus on research and clinical trial delivery performance and look continuously for ways to speed up set up times, improve our patient recruitment performance and time to target metrics. Currently we have: 322 research studies running and open 49 clinical trials in progress from Phase 1 to Phase 4 Contacts Julian.Hughes@moorfields.nhs.uk Geeta.Ghadiali@moorfields.nhs.uk Sabeena.Johal@moorfields.nhs.uk Tania.West@moorfields.nhs.uk 1
2 Contents Section/Chapter Page Table 1: Performance in Initiating Clinical Research 2 Table 2: Performance in Delivering Clinical Research 6 2
3 Table 1: Performance in Initiating Clinical Research 1 st April st March 2018 This table summarises Moorfields Eye Hospital s performance in initiating clinical trials by recruiting the first patient to the trial within 70 days of receiving a valid application by Selecting Study Site. The benchmark column indicates whether this target has been met. Where it was not, further information on the reason for any delays are provided in the final column. Name of Trial IRAS No. Research Ethics Committee Reference Date Site Invited Date Site Selected HRA Approval Date Date Site Confirmed by Date Site Confirmed Date Ready to Start First Recruit Date Reason if not hit benchmark Source of Delay An open label, multi-centre, Phase I/II dose escalation trial of a recombinant adeno-associated virus vector (AAV2/5- hrkp.rpgr) for gene therapy of adults and children with X-linked Retinitis Pigmentosa owing to defects in Retinitis Pigmentosa GTPase Regulator (RPGR) /LO/ /01/ /06/ /06/ /06/ /07/ /07/ /07/2017 3
4 Long-term followup study of participants following an open label, multicentre, Phase I/II dose escalation trial of a recombinant adeno-associated virus vector (AAV2/8- hcarp.hcngb3) for gene therapy of adults and children with achromatopsia owing to defects in CNGB /LO/ /03/ /04/ /04/ /05/ /05/ /06/ /06/2017 A Prospective, Randomized, Controlled, Multi- Center Clinical Study of the ACRYSOF IQ Extended Depth of Focus (EDF) IOL /EE/ /01/ /05/ /06/ /08/ /07/ /07/ /11/2017 Delays Delays 4
5 A prospective, double-masked, randomised, multicentre, active-controlled, parallel-group, 6- month study assessing the safety and ocular hypotensive efficacy of PG324 Opthalmic Solution compared to GANFORT in subjects with elevated intraocular pressure /LO/ /03/ /07/ /06/ /09/ /08/ /09/ /12/2017 Delays, Permissons Delays 5
6 Randomized, Assessor-Masked, Active-Controlled, Phase 3 Study to Evaluate Efficacy, Safety and Tolerability of 0.08% Polyhexamethylen e Biguanide (PHMB) Ophthalmic Solution in Comparison with 0.02% PHMB + 0.1% Propamidine Combination Therapy in Subjects Affected by Acanthamoeba keratitis /LO/ /02/ /06/ /06/ /07/ /07/ /08/ /08/2017 A Phase 2, Randomized, Placebo-Controlled Trial Evaluating the Efficacy and Safety of Filgotinib in Subjects with Active Non- Infectious Uveitis /LO/ /08/ /09/ /08/ /09/ /10/ /10/2017 No Patients Consented Neither 6
7 THE HYDRUS MICROSTENT FOR REFRACTORY OPEN?ANGLE GLAUCOMA: A PROSPECTIVE, MULTICENTER CLINICAL TRIAL (HYDRUS VII STUDY) /NS/ /10/ /12/ /12/ /02/ /02/ /02/2018 Randomized controlled trial comparing the use of Hilotherm cooling system with standard care in the postoperative treatment of bilateral Adnexal surgeries /LO/ /12/ /01/ /01/ /01/ /01/ /01/2018 Contracting Delays Staff availability and equipment delays Neither 7
8 Table 2: Performance in Delivering Clinical Research 1 st April st March 2018 This table summarises Moorfields Eye Hospital s performance in delivering clinical trials in line with the recruitment targets specified by the trials sponsor. The data illustrates whether the agreed number of patients were recruited by the site by the specified recruitment end date. Name of Trial Research Ethics Committee Reference IRAS No. Managing neovascular agerelated macular degeneration (namd) over 2 years with a Treat and Extend (T&E) regimen of 2 mg intravitreal aflibercept - a randomized, open-label, active-controlled, parallel-group phase IV/IIIb study (ARIES) 16/LO/ Target number of patients agreed Min Target no. of patients Max Target number of patients If Agreed, Date Agreed to recruit target number of patients of patients recruited by target date The total number of study particip ants recruite d Date trial closed Reason Trial Closed to to recruitment Recruitment Agreed /03/ /05/2017 Recruitment Finished 8
9 A PHASE II, MULTICENTER, RANDOMIZED, DOUBLE- MASKED, 4 PARALLEL ARMS, CONTROLLED 6-MONTH TRIAL DESIGNED TO EVALUATE THE SAFETY AND EFFICACY OF PAD CICLOSPORIN (CSA 0.06% AND 0.03%) OPHTHALMIC DISPERSION ADMINISTERED ONCE DAILY IN COMBINATION WITH LUBR 15/LO/ Agreed /02/ /04/2017 Recruitment Finished FAST: Fast Assessment of ocular Surface Trouble 16/YH/ Safety and Efficacy of Abicipar Pegol (AGN ) in Patients With Neovascular Age-related Macular Degeneration 15/SC/ Clinical performance of Compass in the diagnosis of glaucoma a comparison with HFA 15/LO/ A phase I study to evaluate the pharmacokinetics safety and tolerability of preservative free tafluprost ophthalmic solution in pediatric patients diagnosed with glaucoma or ocular hypertension 14/WM/ Agreed /06/ /04/2017 Recruitment Finished Agreed /06/ /04/2017 Recruitment Finished Agreed /07/ /08/2017 Recruitment Finished Agreed /06/ /07/2017 Recruitment Finished 9
10 A Randomized, Double-Masked, Sham-Controlled, Pivotal Clinical Trial to Evaluate the Efficacy of a Single Intravitreal Injection of GS010 (raav2/2- ND4) in Subjects Affected for 6 Months or Less by Leber Hereditary Optic Neuropathy Due to the G11778A 15/LO/ Chart review of patients with confirmed geographic atrophy (GA) diagnosis secondary to age-related macular degeneration and non- GA controls 16/NI/ A randomised, sham-controlled, double-masked, multi-centre study to evaluate the effect of ocriplasmin 0.125mg in subjects with non-proliferative diabetic retinopathy (NPDR) 15/EM/ An 8-week phase I/II, multicenter, randomized, double-masked, vehicle controlled parallel group study with a 48 or 56 week follow-up period to evaluate the safety and efficacy of two doses (10 Ág/ml and 20 Ág/ml) of recombinant human nerve growth fac 13/LO/ Agreed /03/ /07/2017 Recruitment Finished Agreed /08/ /07/2017 Recruitment Finished Agreed /12/ /12/2017 Recruitment Finished Agreed /09/ /10/2017 Recruitment Finished 10
11 An open-label, randomized, active-controlled, parallel-group, Phase-3b study of the efficacy, safety, and tolerability of 2 mg aflibercept administered by intravitreal injections using two different treatment regimens to subjects with neovascular age 15/NE/ An open-label, randomized, active-controlled, parallel-group, Phase-3b study of the efficacy, safety, and tolerability of three different treatment regimens of 2 mg aflibercept administered by intravitreal injections to subjects with diabetic macular 16/LO/ Agreed /04/ /10/2017 Recruitment Finished Agreed /06/ /09/2017 Recruitment Finished PANORAMA_ VGFTe-OD /SC/ A Long Term Surveillance study of Latanoprost to monitor hyperpigmentation changes in the eye in pediatric 14/LO/ A Randomized Study Comparing the Safety and Efficacy of the InnFocus MicroShunt Glaucoma Drainage system to standard trabeculectomy in subjects with primary open angle glaucoma 16/LO/ Agreed /08/ /07/2017 Recruitment Finished Agreed /07/ /04/2017 Recruitment Finished Agreed /12/ /12/2017 Recruitment Finished 11
12 Randomized, double-blind, placebo-controlled exploratory study to evaluate pharmacodynamics, safety and tolerability of orally administered BI for 12 weeks in patients with visual impairment due to central-involved diabetic macular edema (DME 16/LO/ Agreed /06/ /06/2017 Recruitment Finished 12
Performance in Initiating and Delivering Clinical Research
 Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
Performance in Initiating and Delivering Clinical Research
 Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
Performance in Initiating and Delivering Clinical Research
 Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many as possible the opportunity to get involved
Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many as possible the opportunity to get involved
As a global leading. Moorfields delivers. Moorfields. economy. Version: Status: Approved: Ratified:
 Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
Performance in Initiating and Delivering Clinical Research As a global leading centre for research in Eyes and Vision, it is a key priority to offer as many patients as possible the opportunity to get
Performance in Delivering Q4. Target number of patients
 Performance in Delivering Q4 Research Ethics Committee Reference Number Name of Trial Target number of patients Date Agreed to recruit target number of patients Trial Status Target met within the agreed
Performance in Delivering Q4 Research Ethics Committee Reference Number Name of Trial Target number of patients Date Agreed to recruit target number of patients Trial Status Target met within the agreed
Glaucoma research at Moorfields
 Recruiting Research Studies Glaucoma research at Moorfields Moorfields Eye Hospital wants to improve access to clinical research studies for all patients within the NHS and provide the opportunities for
Recruiting Research Studies Glaucoma research at Moorfields Moorfields Eye Hospital wants to improve access to clinical research studies for all patients within the NHS and provide the opportunities for
Darren J. Bell, M.D. Texas A&M University College of Medicine College Station, Texas Doctor of Medicine
 Darren J. Bell, M.D. Education Texas A&M University College of Medicine College Station, Texas Doctor of Medicine 1997-2001 Texas A&M University College Station, Texas Bachelor of Science, Biomedical Engineering
Darren J. Bell, M.D. Education Texas A&M University College of Medicine College Station, Texas Doctor of Medicine 1997-2001 Texas A&M University College Station, Texas Bachelor of Science, Biomedical Engineering
Figure 1. Illustration of the progression of visual acuity in RESCUE
 Press Release GenSight Biologics reports topline results at Week 48 of the RESCUE Phase III clinical trial of GS010 in subjects within six months of visual loss onset due to Leber Hereditary Optic Neuropathy
Press Release GenSight Biologics reports topline results at Week 48 of the RESCUE Phase III clinical trial of GS010 in subjects within six months of visual loss onset due to Leber Hereditary Optic Neuropathy
Vanderbilt Eye Institute Clinical Trials
 April, 2010 Vanderbilt Eye Institute Clinical Trials Ophthalmology Actively Recruiting Studies For information on our clinical trials and other studies, please contact: Sandy Owings, COA, CCRP Clinic Director
April, 2010 Vanderbilt Eye Institute Clinical Trials Ophthalmology Actively Recruiting Studies For information on our clinical trials and other studies, please contact: Sandy Owings, COA, CCRP Clinic Director
Ophthalmic VEGF Inhibitors. Eylea (aflibercept), Macugen (pegaptanib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Ophthalmic VEGF Inhibitors Page: 1 of 5 Last Review Date: September 20, 2018 Ophthalmic VEGF Inhibitors
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Ophthalmic VEGF Inhibitors Page: 1 of 5 Last Review Date: September 20, 2018 Ophthalmic VEGF Inhibitors
Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial
 ASX and Media Release 3 January 2018 Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial Melbourne, Australia; January 3 2018 Opthea Limited (ASX:OPT), a late stage biopharmaceutical company
ASX and Media Release 3 January 2018 Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial Melbourne, Australia; January 3 2018 Opthea Limited (ASX:OPT), a late stage biopharmaceutical company
Table 1 Salford Royal NHS Foundation Trust: Performance in initiating clinical research (HRA Approval) Reporting period 1 April March 2017
 Table 1 Salford Royal NHS Foundation Trust: Performance in initiating clinical research (HRA Approval) Reporting period 1 April 2016 31 March 2017 REC reference IRAS number Study title 16/NW/0428 207062
Table 1 Salford Royal NHS Foundation Trust: Performance in initiating clinical research (HRA Approval) Reporting period 1 April 2016 31 March 2017 REC reference IRAS number Study title 16/NW/0428 207062
Common Causes of Vision Loss
 Common Causes of Vision Loss Learning Objectives To identify the most common causes of vision loss in the United States To differentiate the most common forms of agerelated macular degeneration and diabetic
Common Causes of Vision Loss Learning Objectives To identify the most common causes of vision loss in the United States To differentiate the most common forms of agerelated macular degeneration and diabetic
FROM OUTDATED TO UPDATED Eminence-Based Medicine
 FROM OUTDATED TO UPDATED Eminence-Based Medicine Evidence-Based Medicine A REVIEW OF KEY CLINICAL TRIALS Anthony DeWilde, OD FAAO 1 EMINENCE BASED MEDICINE 2 EVIDENCE BASED MEDICINE 3 4 CLINICAL TRIALS
FROM OUTDATED TO UPDATED Eminence-Based Medicine Evidence-Based Medicine A REVIEW OF KEY CLINICAL TRIALS Anthony DeWilde, OD FAAO 1 EMINENCE BASED MEDICINE 2 EVIDENCE BASED MEDICINE 3 4 CLINICAL TRIALS
Target date to recruit patients agreed? Target range minimum. Target range maximum
 No REC Reference IRAS No Title Target number of patients agreed? Target range minimum Target range maximum Target date to recruit patients agreed? Planned Recruitment end date Total no of patients recruited
No REC Reference IRAS No Title Target number of patients agreed? Target range minimum Target range maximum Target date to recruit patients agreed? Planned Recruitment end date Total no of patients recruited
James K. Luu, M.D Present Retina Consultants of Southern Colorado, P.C. Colorado Springs, CO
 Retina Consultants of Southern Colorado, P.C. 2770 North Union Blvd., Suite 140 Colorado Springs, CO 80909 719-473-9595 CURRENT POSITION 2004 - Present Retina Consultants of Southern Colorado, P.C. Colorado
Retina Consultants of Southern Colorado, P.C. 2770 North Union Blvd., Suite 140 Colorado Springs, CO 80909 719-473-9595 CURRENT POSITION 2004 - Present Retina Consultants of Southern Colorado, P.C. Colorado
NEPTUNE RED BANK BRICK
 NEPTUNE RED BANK BRICK Diabetes & The Eye Diabetics are more likely to develop Cataracts at a younger age. Diabetics are twice as likely to develop Glaucoma when compared to non-diabetics. The primary
NEPTUNE RED BANK BRICK Diabetes & The Eye Diabetics are more likely to develop Cataracts at a younger age. Diabetics are twice as likely to develop Glaucoma when compared to non-diabetics. The primary
Rising to the Challenge of Satisfying Unmet Medical Needs. Back-of-the-eye. Main Back-of-the-Eye Diseases. Uveitis. Behcet s disease.
 Feature Rising to the Challenge of Satisfying Unmet Medical Needs Eyeing Further Specialization Back-of-the-eye Diseases Main Back-of-the-Eye Diseases Age-related macular degeneration Uveitis Behcet s
Feature Rising to the Challenge of Satisfying Unmet Medical Needs Eyeing Further Specialization Back-of-the-eye Diseases Main Back-of-the-Eye Diseases Age-related macular degeneration Uveitis Behcet s
Corporate Medical Policy
 Corporate Medical Policy Voretigene Neparvovec-rzyl (Luxturna) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: voretigene_neparvovec_rzyl_luxturna 1/2018 N/A 6/2018 2/2018 Description
Corporate Medical Policy Voretigene Neparvovec-rzyl (Luxturna) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: voretigene_neparvovec_rzyl_luxturna 1/2018 N/A 6/2018 2/2018 Description
2017 MIPS Quality Category Measures and Benchmarks for Ophthalmology
 2017 MIPS Quality Category Measures and Benchmarks for Ophthalmology Physicians must report on 50% of all patients, if reporting via registry or EHR, and 50% of all Medicare Part B patients if reporting
2017 MIPS Quality Category Measures and Benchmarks for Ophthalmology Physicians must report on 50% of all patients, if reporting via registry or EHR, and 50% of all Medicare Part B patients if reporting
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Performance in Initiating and Delivering Clinical Research Why are we doing this?
 Performance in Initiating and Delivering Clinical Research Why are we doing this? Through the NIHR (National Institute for Health Research) the Government wishes to see a dramatic and sustained improvement
Performance in Initiating and Delivering Clinical Research Why are we doing this? Through the NIHR (National Institute for Health Research) the Government wishes to see a dramatic and sustained improvement
EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO
 EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO Yehia Hashad, M.D. Vice President and Global Therapeutic Area Head
EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO Yehia Hashad, M.D. Vice President and Global Therapeutic Area Head
Cornea & External Disease research at Moorfields
 Recruiting Research Studies Cornea & External Disease research at Moorfields Moorfields Eye Hospital wants to improve access to clinical research studies for all patients within the NHS and provide the
Recruiting Research Studies Cornea & External Disease research at Moorfields Moorfields Eye Hospital wants to improve access to clinical research studies for all patients within the NHS and provide the
2019 COLLECTION TYPE: MIPS CLINICAL QUALITY MEASURES (CQMS) MEASURE TYPE: Outcome High Priority
 Quality ID #191 (NQF 0565): Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Management
Quality ID #191 (NQF 0565): Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Management
Measure #191: Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery
 Measure #191: Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery 2012 PHYSICIAN QUALITY REPORTING OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION: Percentage
Measure #191: Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery 2012 PHYSICIAN QUALITY REPORTING OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION: Percentage
SCIENTIFIC PROGRAM. Pediatric ophthalmology- Optometry challenges (max 20 attendees)
 FRIDAY, 22 SEPTEMBER 2017 WORKSHOPS (OPHTHALMICA Eye Institute) 08:30-09:00 Registration-Welcome 09:00-11:30 Workshops SCIENTIFIC PROGRAM I. Cornea & Refractive surgery (max 30 attendees) - Clinical examination
FRIDAY, 22 SEPTEMBER 2017 WORKSHOPS (OPHTHALMICA Eye Institute) 08:30-09:00 Registration-Welcome 09:00-11:30 Workshops SCIENTIFIC PROGRAM I. Cornea & Refractive surgery (max 30 attendees) - Clinical examination
nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd
 nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
The Pennine Acute Hospitals NHS Trust Performance in initiating clinical research Reporting period 1 October 2017 to 30 September 2018
 The Pennine Acute Hospitals NHS Trust Performance in initiating clinical research Reporting period 1 October 2017 to 30 September 2018 REC reference IRAS reference Name of trial First patient recruited?
The Pennine Acute Hospitals NHS Trust Performance in initiating clinical research Reporting period 1 October 2017 to 30 September 2018 REC reference IRAS reference Name of trial First patient recruited?
New STAIRWAY study data shows potential for extended durability with Roche s faricimab in neovascular age-related macular degeneration (namd)
 Media Release New STAIRWAY study data shows potential for extended durability with Roche s faricimab in neovascular age-related macular degeneration (namd) Faricimab the first bispecific antibody designed
Media Release New STAIRWAY study data shows potential for extended durability with Roche s faricimab in neovascular age-related macular degeneration (namd) Faricimab the first bispecific antibody designed
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Outcome
 Quality ID #191 (NQF 0565): Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES:
Quality ID #191 (NQF 0565): Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES:
From Outdated to Updated: A Review of Important Clinical Trials in Ocular Disease from 2014
 From Outdated to Updated: A Review of Important Clinical Trials in Ocular Disease from 2014 1. This course is designed to review the important ophthalmic literature that was released between October 2013
From Outdated to Updated: A Review of Important Clinical Trials in Ocular Disease from 2014 1. This course is designed to review the important ophthalmic literature that was released between October 2013
EyeGene Inc. Contact Information Korea Health Industry Development Institute
 EG-Mirotin: First-in-Class recombinant polypeptide novel subcutaneous drug containing RGD-motif targeted to suppress edema and vascular leakage for Nonproliferative Diabetic Retinopathy (NPDR) EyeGene
EG-Mirotin: First-in-Class recombinant polypeptide novel subcutaneous drug containing RGD-motif targeted to suppress edema and vascular leakage for Nonproliferative Diabetic Retinopathy (NPDR) EyeGene
Note: This is an outcome measure and can be calculated solely using registry data.
 Measure #191 (NQF 0565): Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery -- National Quality Strategy Domain: Effective Clinical Care DESCRIPTION: Percentage of patients
Measure #191 (NQF 0565): Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery -- National Quality Strategy Domain: Effective Clinical Care DESCRIPTION: Percentage of patients
Micro-Invasive Glaucoma Surgery (Aqueous Stents)
 Micro-Invasive Glaucoma Surgery (Aqueous Stents) Policy Number: Original Effective Date: MM.06.029 02/01/2019 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 02/01/2019 Section:
Micro-Invasive Glaucoma Surgery (Aqueous Stents) Policy Number: Original Effective Date: MM.06.029 02/01/2019 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 02/01/2019 Section:
Perfomance in Delivering (Commercial Trials)
 Perfomance in Delivering (Commercial Trials) REC Ref IRAS ID Name of Trial Target Of? Minimum Of Maximum Of Target Date To Recruit? Total Date Of to recruit target number Recruited At of patients The Target
Perfomance in Delivering (Commercial Trials) REC Ref IRAS ID Name of Trial Target Of? Minimum Of Maximum Of Target Date To Recruit? Total Date Of to recruit target number Recruited At of patients The Target
Sponsor. Generic Drug Name. Trial Indications. Protocol Number. Protocol Title. Clinical Trial Phase. Study Start/End Dates. Reason for Termination
 Sponsor Alcon Research, Ltd. Generic Drug Name Travoprost/timolol maleate Trial Indications Open-angle glaucoma or ocular hypertension Protocol Number C-09-007 Protocol Title An Evaluation of Patient Reported
Sponsor Alcon Research, Ltd. Generic Drug Name Travoprost/timolol maleate Trial Indications Open-angle glaucoma or ocular hypertension Protocol Number C-09-007 Protocol Title An Evaluation of Patient Reported
Clinically Significant Macular Edema (CSME)
 Clinically Significant Macular Edema (CSME) 1 Clinically Significant Macular Edema (CSME) Sadrina T. Shaw OMT I Student July 26, 2014 Advisor: Dr. Uwaydat Clinically Significant Macular Edema (CSME) 2
Clinically Significant Macular Edema (CSME) 1 Clinically Significant Macular Edema (CSME) Sadrina T. Shaw OMT I Student July 26, 2014 Advisor: Dr. Uwaydat Clinically Significant Macular Edema (CSME) 2
MEASURING THE SIZE OF A TREATMENT EFFECT: RELATIVE RISK REDUCTION, ABSOLUTE RISK REDUCTION, AND NUMBER NEEDED TO TREAT
 MEASURING THE SIZE OF A TREATMENT EFFECT: RELATIVE RISK REDUCTION, ABSOLUTE RISK REDUCTION, AND NUMBER NEEDED TO TREAT Hussein Hollands, MD, MS (epid) Peter J. Kertes, MD, CM, FRCS(C) 72 The purpose of
MEASURING THE SIZE OF A TREATMENT EFFECT: RELATIVE RISK REDUCTION, ABSOLUTE RISK REDUCTION, AND NUMBER NEEDED TO TREAT Hussein Hollands, MD, MS (epid) Peter J. Kertes, MD, CM, FRCS(C) 72 The purpose of
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Lucentis) Reference Number: CP.PHAR.186 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at
Clinical Policy: (Lucentis) Reference Number: CP.PHAR.186 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at
Oxurion NV Business Update Q End Q cash position 95.1 million
 Oxurion NV Business Update Q3 2018 Shareholders Approval and Launch of Oxurion NV as new Company Name First patient enrolled in Phase 1 study evaluating THR-687, a Novel Pan-RGD Integrin Antagonist, for
Oxurion NV Business Update Q3 2018 Shareholders Approval and Launch of Oxurion NV as new Company Name First patient enrolled in Phase 1 study evaluating THR-687, a Novel Pan-RGD Integrin Antagonist, for
Appendix Table 1. Ophthalmic drugs approved by the US Food and Drug Administration,
 SUPPLEMENTARY DATA Appendix Table 1. Ophthalmic drugs approved by the US Food and Drug Administration, 2002-2012 Approval Year Name Indication Pivotal Trial Design Randomized Comparator Masked Post-Approval
SUPPLEMENTARY DATA Appendix Table 1. Ophthalmic drugs approved by the US Food and Drug Administration, 2002-2012 Approval Year Name Indication Pivotal Trial Design Randomized Comparator Masked Post-Approval
Aging & Ophthalmology
 Aging & Ophthalmology Pr Jean-Marie Rakic Dr Denis Malaise January 2018 Major ocular diseases 1. Cataract 2. Age-related macular degeneration 3. Ischemic optic neuropathy 4. Horton arteritis 5. Glaucoma
Aging & Ophthalmology Pr Jean-Marie Rakic Dr Denis Malaise January 2018 Major ocular diseases 1. Cataract 2. Age-related macular degeneration 3. Ischemic optic neuropathy 4. Horton arteritis 5. Glaucoma
Dan Roberts. International Low Vision Support Group NEWSLETTER. This Month. In This Issue. 1: This Month 2-5: News
 In This Issue 1: This Month 2-5: News Eye & Body Complete from Biosyntrx Extra Strength AREDS-2 Multisupplement Call for information 800-688-6815 The ILVSG is sponsored by MD Foundation www.eyesight.org
In This Issue 1: This Month 2-5: News Eye & Body Complete from Biosyntrx Extra Strength AREDS-2 Multisupplement Call for information 800-688-6815 The ILVSG is sponsored by MD Foundation www.eyesight.org
The Pennine Acute Hospitals NHS Trust Performance in delivering clinical research Reporting period 1 October 2017 to 30 September 2018
 The Pennine Acute Hospitals NHS Trust Performance in delivering clinical research Reporting period 1 October 2017 to 30 September 2018 REC reference IRAS reference Name of trial Target number of? Minimum
The Pennine Acute Hospitals NHS Trust Performance in delivering clinical research Reporting period 1 October 2017 to 30 September 2018 REC reference IRAS reference Name of trial Target number of? Minimum
Completed Clinical Research Trials Monte Dirks, M.D.
 Completed Clinical Research Trials Monte Dirks, M.D. Monte Dirks, M.D. - Primary Investigator Norfloxacin -Merck (1986) Safety and efficacy of norfloxacin vs. tobramycin in the treatment of external ocular
Completed Clinical Research Trials Monte Dirks, M.D. Monte Dirks, M.D. - Primary Investigator Norfloxacin -Merck (1986) Safety and efficacy of norfloxacin vs. tobramycin in the treatment of external ocular
HTA Systematic review of treatment of dry age-related macular degeneration and Stargardt disease.
 HTA 16.09.10 Systematic review of treatment of dry age-related macular degeneration and Stargardt disease. Supplementary file 3. Cell therapies Schwartz et al See Appendi 2 (Stargardt s disease) Song et
HTA 16.09.10 Systematic review of treatment of dry age-related macular degeneration and Stargardt disease. Supplementary file 3. Cell therapies Schwartz et al See Appendi 2 (Stargardt s disease) Song et
Coding Implications Revision Log. See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Eylea) Reference Number: CP.PHAR.184 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at the
Clinical Policy: (Eylea) Reference Number: CP.PHAR.184 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at the
AMD research at Moorfields
 Recruiting Research Studies AMD research at Moorfields Moorfields Eye Hospital wants to improve access to clinical research studies for all patients within the NHS and provide the opportunities for patients
Recruiting Research Studies AMD research at Moorfields Moorfields Eye Hospital wants to improve access to clinical research studies for all patients within the NHS and provide the opportunities for patients
Micro-Invasive Glaucoma Surgery (Aqueous Stents)
 Micro-Invasive Glaucoma Surgery (Aqueous Stents) Policy Number: Original Effective Date: MM.06.029 02/01/2019 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 02/01/2019 Section:
Micro-Invasive Glaucoma Surgery (Aqueous Stents) Policy Number: Original Effective Date: MM.06.029 02/01/2019 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 02/01/2019 Section:
Ocular Pathology. I. Congenital and/or developmental. A. Trisomy 21. Hypertelorism (widely spaced eyes) Keratoconus (cone shaped cornea)
 I. Congenital and/or developmental Robbins Pathologic Basis of Disease, 6 th Ed. A. Trisomy 21 Hypertelorism (widely spaced eyes) Keratoconus (cone shaped cornea) Focal hypoplasia of iris Cataracts frequently
I. Congenital and/or developmental Robbins Pathologic Basis of Disease, 6 th Ed. A. Trisomy 21 Hypertelorism (widely spaced eyes) Keratoconus (cone shaped cornea) Focal hypoplasia of iris Cataracts frequently
Diagnosis and treatment of diabetic retinopathy. Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City
 Diagnosis and treatment of diabetic retinopathy Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City Disclosures Consulted for Novo Nordisk 2017,2018. Will be discussing
Diagnosis and treatment of diabetic retinopathy Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City Disclosures Consulted for Novo Nordisk 2017,2018. Will be discussing
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company AAO October 25, 2018 NASDAQ: EYPT
 psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ AAO October 25, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ AAO October 25, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
International Journal of Health Sciences and Research ISSN:
 International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article A Multivariate Analysis of Intravitreal Injection of Anti-VEGF Bevacizumab in the Treatment
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article A Multivariate Analysis of Intravitreal Injection of Anti-VEGF Bevacizumab in the Treatment
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #14 (NQF 0087): Age-Related Macular Degeneration (AMD): Dilated Macular Examination National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Quality ID #14 (NQF 0087): Age-Related Macular Degeneration (AMD): Dilated Macular Examination National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
 measure of your overall performance. An isolated glucose test is helpful to let you know what your sugar level is at one moment, but it doesn t tell you whether or not your diabetes is under adequate control
measure of your overall performance. An isolated glucose test is helpful to let you know what your sugar level is at one moment, but it doesn t tell you whether or not your diabetes is under adequate control
CENTENE PHARMACY AND THERAPEUTICS NEW DRUG REVIEW 2Q17 April May
 BRAND NAME Lucentis GENERIC NAME ranibizumab MANUFACTURER Genentech, Inc. DATE OF APPROVAL June 30, 2006 PRODUCT LAUNCH DATE July 13, 2006 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of
BRAND NAME Lucentis GENERIC NAME ranibizumab MANUFACTURER Genentech, Inc. DATE OF APPROVAL June 30, 2006 PRODUCT LAUNCH DATE July 13, 2006 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of
2018 OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS ONLY. MEASURE TYPE: Process
 Quality ID #14 (NQF 0087): Age-Related Macular Degeneration (AMD): Dilated Macular Examination National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS ONLY
Quality ID #14 (NQF 0087): Age-Related Macular Degeneration (AMD): Dilated Macular Examination National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS ONLY
These issues are covered in more detail below.
 26.3.07 Comments from Novartis on the Assessment Report for the Health Technology Appraisal of Pegaptinib and Ranibizumab for the treatment of age-related macular degeneration In general we feel that the
26.3.07 Comments from Novartis on the Assessment Report for the Health Technology Appraisal of Pegaptinib and Ranibizumab for the treatment of age-related macular degeneration In general we feel that the
Eylea (aflibercept) Document Number: IC-0026
 Eylea (aflibercept) Document Number: IC-0026 Last Review Date: 3/1/2018 Date of Origin: 02/07/2013 Dates Reviewed: 03/07/2013, 06/2013, 09/2013, 12/2013, 03/2014, 06/2014, 09/2014, 12/2014, 03/2015, 04/2015,
Eylea (aflibercept) Document Number: IC-0026 Last Review Date: 3/1/2018 Date of Origin: 02/07/2013 Dates Reviewed: 03/07/2013, 06/2013, 09/2013, 12/2013, 03/2014, 06/2014, 09/2014, 12/2014, 03/2015, 04/2015,
South London and Maudsley NHS Foundation Trust: FY1819 Q2 Performance in Initiating
 of First 17/YH/0195 228739 17/YH/0196 228435 17/LO/0987 215947 17/LO/1314 224111 17/WM/0441 224759 17/LO/1867 229005 A Randomized, Double-Blind, Placebo Controlled, Two-Period Cross-Over, Proof of Activity
of First 17/YH/0195 228739 17/YH/0196 228435 17/LO/0987 215947 17/LO/1314 224111 17/WM/0441 224759 17/LO/1867 229005 A Randomized, Double-Blind, Placebo Controlled, Two-Period Cross-Over, Proof of Activity
Drug Class Update: Vascular Endothelial Growth Factors
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Performance in delivering Clinical Research at PHT Q3 14/15 study met the 70 day target study within 70 day target study not met 70 day target
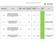 Performance in delivering Clinical Research at PHT Q3 14/15 study met the 70 day target study within 70 day target study not met 70 day target Research Ethics Committee 14/ES/0004 ALA-BCC-CT008 - A randomized,
Performance in delivering Clinical Research at PHT Q3 14/15 study met the 70 day target study within 70 day target study not met 70 day target Research Ethics Committee 14/ES/0004 ALA-BCC-CT008 - A randomized,
Management of Neovascular AMD
 Kapusta AMD Part 1 Management of Neovascular AMD Dr. Michael A. Kapusta, MD, FRCSC Ophthalmologist in Chief Jewish General Hospital Vitreoretinal Surgeon 1 FINANCIAL DISCLOSURES Consulting honoraria Bayer,
Kapusta AMD Part 1 Management of Neovascular AMD Dr. Michael A. Kapusta, MD, FRCSC Ophthalmologist in Chief Jewish General Hospital Vitreoretinal Surgeon 1 FINANCIAL DISCLOSURES Consulting honoraria Bayer,
aflibercept 40mg/mL solution for injection (Eylea ) SMC No. (1074/15) Bayer
 aflibercept 40mg/mL solution for injection (Eylea ) SMC No. (1074/15) Bayer 07 August 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS Boards
aflibercept 40mg/mL solution for injection (Eylea ) SMC No. (1074/15) Bayer 07 August 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS Boards
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME
 Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
Sudden Vision Loss. Brendan Girschek, MD, FRCSC, FACS Vitreoretinal Surgery Cedar Valley Medical Specialists
 Sudden Vision Loss Brendan Girschek, MD, FRCSC, FACS Vitreoretinal Surgery Cedar Valley Medical Specialists My Credentials -Residency in Ophthalmology at the LSU Eye Center in New Orleans, LA -Fellowship
Sudden Vision Loss Brendan Girschek, MD, FRCSC, FACS Vitreoretinal Surgery Cedar Valley Medical Specialists My Credentials -Residency in Ophthalmology at the LSU Eye Center in New Orleans, LA -Fellowship
11 Eye. regarding this product in this setting. As a result we cannot recommend its use within NHSScotland.
 Recommendations from the Lothian Formulary Committee (FC) following Scottish Medicines Consortium (SMC) advice, NICE MTA advice, (FAF3) unlicensed and off-label medicines and (FAF2) medicines not considered
Recommendations from the Lothian Formulary Committee (FC) following Scottish Medicines Consortium (SMC) advice, NICE MTA advice, (FAF3) unlicensed and off-label medicines and (FAF2) medicines not considered
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME
 Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
The Common Clinical Competency Framework for Non-medical Ophthalmic Healthcare Professionals in Secondary Care
 The Common Clinical Competency Framework for Non-medical Ophthalmic Healthcare Professionals in Secondary Care Medical Retina November 2016 Association of Health Professions in Ophthalmology General basic
The Common Clinical Competency Framework for Non-medical Ophthalmic Healthcare Professionals in Secondary Care Medical Retina November 2016 Association of Health Professions in Ophthalmology General basic
Clinical Trials in Diabetic Retinopathy. Harry W. Flynn Jr., M.D. Nidhi Relhan Batra, M.D.
 1 Clinical Trials in Diabetic Retinopathy 2018 Harry W. Flynn Jr., M.D. Nidhi Relhan Batra, M.D. Bascom Palmer Eye Institute 900 N.W. 17th Street Miami, FL 33136 Phone: (305) 326-6118 Fax: (305) 326-6417
1 Clinical Trials in Diabetic Retinopathy 2018 Harry W. Flynn Jr., M.D. Nidhi Relhan Batra, M.D. Bascom Palmer Eye Institute 900 N.W. 17th Street Miami, FL 33136 Phone: (305) 326-6118 Fax: (305) 326-6417
Target date to recruit patients agreed? Target range. maximum. minimum. Date. 5 Agreed 26/01/ /11/2015 Recruitment Finished.
 No REC Reference IRAS No Title Target number of agreed? Target range minimum Target range maximum Target date to recruit agreed? Planned Recruitment end date Total no of recruited at the agreed target
No REC Reference IRAS No Title Target number of agreed? Target range minimum Target range maximum Target date to recruit agreed? Planned Recruitment end date Total no of recruited at the agreed target
Building a Major Ophthalmic Pharmaceutical Company. Aerie Pharmaceuticals, Inc. ASRS August 8, 2016
 Building a Major Ophthalmic Pharmaceutical Company Aerie Pharmaceuticals, Inc. OIS @ ASRS August 8, 2016 1 Important Information Any discussion of the potential use or expected success of our product candidates
Building a Major Ophthalmic Pharmaceutical Company Aerie Pharmaceuticals, Inc. OIS @ ASRS August 8, 2016 1 Important Information Any discussion of the potential use or expected success of our product candidates
Jay M. Haynie, O.D.; F.A.A.O. Olympia Tacoma Renton Kennewick Washington
 Jay M. Haynie, O.D.; F.A.A.O. Olympia Tacoma Renton Kennewick Washington I Jay M. Haynie, OD, FAAO have received honoraria from the following companies: Reichert Technologies Notal Vision Carl Zeiss Meditec
Jay M. Haynie, O.D.; F.A.A.O. Olympia Tacoma Renton Kennewick Washington I Jay M. Haynie, OD, FAAO have received honoraria from the following companies: Reichert Technologies Notal Vision Carl Zeiss Meditec
CDEC FINAL RECOMMENDATION
 CDEC FINAL RECOMMENDATION AFLIBERCEPT (Eylea Bayer Inc.) Indication: Wet Age-related Macular Degeneration Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that aflibercept be listed
CDEC FINAL RECOMMENDATION AFLIBERCEPT (Eylea Bayer Inc.) Indication: Wet Age-related Macular Degeneration Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that aflibercept be listed
There are no published randomized, double-blind trials comparing aflibercept to other therapies in neovascular AMD.
 Subject: Eylea (aflibercept) Original Effective Date: 7/11/2014 Policy Number: MCP-191 Revision Date(s): 10/11/2016 Review Date(s): 12/16/2015; 10/11/2016, 6/22/2017, 7/10/2018 DISCLAIMER This Medical
Subject: Eylea (aflibercept) Original Effective Date: 7/11/2014 Policy Number: MCP-191 Revision Date(s): 10/11/2016 Review Date(s): 12/16/2015; 10/11/2016, 6/22/2017, 7/10/2018 DISCLAIMER This Medical
FEP Medical Policy Manual
 FEP Medical Policy Manual Last Review: September 20 Next Review: September 2017 Related Policies 9.03.21 Aqueous Shunts for Glaucoma Intravitreal Corticosteroid Implants Summary An intravitreal implant
FEP Medical Policy Manual Last Review: September 20 Next Review: September 2017 Related Policies 9.03.21 Aqueous Shunts for Glaucoma Intravitreal Corticosteroid Implants Summary An intravitreal implant
What's hot and current in ophthalmology. ... and what is missing?
 What's hot and current in ophthalmology... and what is missing? 30 April 2014 Cross-sectional review of ophthalmology clinical trials 2 Sources Online searches, BioPharmClinica, clinicaltrials.gov, EU
What's hot and current in ophthalmology... and what is missing? 30 April 2014 Cross-sectional review of ophthalmology clinical trials 2 Sources Online searches, BioPharmClinica, clinicaltrials.gov, EU
CURRICULUM VITAE. SCOTT G. FOXMAN, M.D. Retinal and Ophthalmic Consultants, P.C Tilton Road, Northfield, NJ 08225
 CURRICULUM VITAE SCOTT G. FOXMAN, M.D. Retinal and Ophthalmic Consultants, P.C. OFFICE LOCATIONS: PROFESSIONAL EXPERIENCE: 1500 Tilton Road, Northfield, NJ 08225 2466 East Chestnut Avenue, Vineland, NJ
CURRICULUM VITAE SCOTT G. FOXMAN, M.D. Retinal and Ophthalmic Consultants, P.C. OFFICE LOCATIONS: PROFESSIONAL EXPERIENCE: 1500 Tilton Road, Northfield, NJ 08225 2466 East Chestnut Avenue, Vineland, NJ
Integrated Research Application System Number. Total Number Of Study Participants Recruited. Research Ethics Committee Reference Number
 Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial Minimum Number Of Patients Agreed Maximum Number Of Patients Agreed Date That The Trial Closed To
Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial Minimum Number Of Patients Agreed Maximum Number Of Patients Agreed Date That The Trial Closed To
Clinical Research Institute HUCH Sponsored, fully signed Clinical Trial Agreements (except grants)
 1. 2. The is Sponsor Full name of the Hospital clinic where the is details of the January January NightstaR Ltd A Randomised, Open Label, Outcomes-Assessor Masked, Prospective, Parallel Controlled Group,
1. 2. The is Sponsor Full name of the Hospital clinic where the is details of the January January NightstaR Ltd A Randomised, Open Label, Outcomes-Assessor Masked, Prospective, Parallel Controlled Group,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Contents METHODS... 2 Inclusion and exclusion criteria... 2 Supplementary table S1... 2 Assessment of abnormal ocular signs and symptoms... 3 Supplementary table S2... 3 Ocular
SUPPLEMENTARY INFORMATION Contents METHODS... 2 Inclusion and exclusion criteria... 2 Supplementary table S1... 2 Assessment of abnormal ocular signs and symptoms... 3 Supplementary table S2... 3 Ocular
Preventing Avoidable Vision loss from Diabetic Retinopathy in Indian Country
 Diabetes in Indian Country- 2017 Preventing Avoidable Vision loss from Diabetic Retinopathy in Indian Country Albuquerque, NM 20 September2017 Mark B. Horton, OD, MD Director, IHS/JVN Teleophthalmology
Diabetes in Indian Country- 2017 Preventing Avoidable Vision loss from Diabetic Retinopathy in Indian Country Albuquerque, NM 20 September2017 Mark B. Horton, OD, MD Director, IHS/JVN Teleophthalmology
Advanced Vitreoretinal Techniques & Technology Symposium
 14th ANNUAL Advanced Vitreoretinal Techniques & Technology Symposium JULY 25-27, 2014 SWISSÔTEL CHICAGO CHICAGO, IL CME ACCREDITATION AND CREDIT STATEMENT This activity has been planned and implemented
14th ANNUAL Advanced Vitreoretinal Techniques & Technology Symposium JULY 25-27, 2014 SWISSÔTEL CHICAGO CHICAGO, IL CME ACCREDITATION AND CREDIT STATEMENT This activity has been planned and implemented
Intraocular Radiation Therapy for Age-Related Macular Degeneration
 Medical Policy Manual Medicine, Policy No. 134 Intraocular Radiation Therapy for Age-Related Macular Degeneration Next Review: April 2019 Last Review: June 2018 Effective: August 1, 2018 IMPORTANT REMINDER
Medical Policy Manual Medicine, Policy No. 134 Intraocular Radiation Therapy for Age-Related Macular Degeneration Next Review: April 2019 Last Review: June 2018 Effective: August 1, 2018 IMPORTANT REMINDER
INTRAVITREAL IMPLANTS
 INTRAVITREAL IMPLANTS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
INTRAVITREAL IMPLANTS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Abbreviated Drug Evaluation: Fluocinolone acetonide intravitreal implant (Retisert )
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Grand Rounds: Interesting and Exemplary Cases From Guanajuato and Djibouti
 Learning Community: January 25, 2015 Grand Rounds: Interesting and Exemplary Cases From Guanajuato and Djibouti JORGE CUADROS, OD, PHD EyePACS In Guanajuato Program started in 2007 Cameras go from clinic
Learning Community: January 25, 2015 Grand Rounds: Interesting and Exemplary Cases From Guanajuato and Djibouti JORGE CUADROS, OD, PHD EyePACS In Guanajuato Program started in 2007 Cameras go from clinic
Coding Implications Revision Log. See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Verteporfin (Visudyne) Reference Number: CP.PHAR.187 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important
Clinical Policy: Verteporfin (Visudyne) Reference Number: CP.PHAR.187 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important
Wayne A. Solley, M.D. Curriculum Vitae
 Wayne A. Solley, M.D. Curriculum Vitae Work Texas Retina Associates 801 W. Randol Mill Road, Suite 101 Arlington, Texas 76012 Telephone 817-261-9625 Fax 817-261-9586 BIOGRAPHICAL DATA Date of Birth 03-18-1968
Wayne A. Solley, M.D. Curriculum Vitae Work Texas Retina Associates 801 W. Randol Mill Road, Suite 101 Arlington, Texas 76012 Telephone 817-261-9625 Fax 817-261-9586 BIOGRAPHICAL DATA Date of Birth 03-18-1968
Clinical Policy: Aflibercept (Eylea) Reference Number: CP.PHAR.184
 Clinical Policy: (Eylea) Reference Number: CP.PHAR.184 Effective Date: 03/16 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Eylea) Reference Number: CP.PHAR.184 Effective Date: 03/16 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
The Common Clinical Competency Framework for Non-medical Ophthalmic Healthcare Professionals in Secondary Care
 The Common Clinical Competency Framework for Non-medical Ophthalmic Healthcare Professionals in Secondary Care Cataract November 2016 Association of Health Professions in Ophthalmology General basic competences
The Common Clinical Competency Framework for Non-medical Ophthalmic Healthcare Professionals in Secondary Care Cataract November 2016 Association of Health Professions in Ophthalmology General basic competences
Vision loss in elderly. Erica Weir, April 2015
 Vision loss in elderly Erica Weir, April 2015 1 Burden Enter nursing homes 3 years earlier Twice the risk of falling 4x the risk of hip fracture Independent risk factor for delirium What are the leading
Vision loss in elderly Erica Weir, April 2015 1 Burden Enter nursing homes 3 years earlier Twice the risk of falling 4x the risk of hip fracture Independent risk factor for delirium What are the leading
Vision loss in elderly
 Vision loss in elderly Erica Weir, February 2016 1 Burden Enter nursing homes 3 years earlier Twice the risk of falling 4x the risk of hip fracture Independent risk factor for delirium What are the leading
Vision loss in elderly Erica Weir, February 2016 1 Burden Enter nursing homes 3 years earlier Twice the risk of falling 4x the risk of hip fracture Independent risk factor for delirium What are the leading
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
ThromboGenics Business Update Q1 2018
 Press release 9 May 2018 Regulated Information ThromboGenics Business Update Q1 2018 Advancing Diabetic Eye Disease Portfolio Positive Initial Topline Data from Phase 1/ 2a Clinical Study evaluating THR-
Press release 9 May 2018 Regulated Information ThromboGenics Business Update Q1 2018 Advancing Diabetic Eye Disease Portfolio Positive Initial Topline Data from Phase 1/ 2a Clinical Study evaluating THR-
11 Eye. systemic disease or both eyes are affected (or 1 eye is affected if the second eye has poor visual acuity) and
 Recommendations from the Lothian Formulary Committee (FC) following Scottish Medicines Consortium (SMC) advice, NICE MTA advice, (FAF3) unlicensed and off-label medicines and (FAF2) medicines not considered
Recommendations from the Lothian Formulary Committee (FC) following Scottish Medicines Consortium (SMC) advice, NICE MTA advice, (FAF3) unlicensed and off-label medicines and (FAF2) medicines not considered
and at the same patient encounter. Code has been deleted. For scanning computerized ophthalmic diagnostic imaging of optic nerve and retin
 92227: Remote imaging for detection of retinal disease (eg, retinopathy in a patient with diabetes) with analysis and report under physician supervision, unilateral or bilateral. For Medicare, bill only
92227: Remote imaging for detection of retinal disease (eg, retinopathy in a patient with diabetes) with analysis and report under physician supervision, unilateral or bilateral. For Medicare, bill only
TREATMENT STRATEGIES FOR CHORIORETINAL VASCULAR DISEASES: ADVANTAGES AND DISADVANTAGES OF INDIVIDUALISED THERAPY
 TREATMENT STRATEGIES FOR CHORIORETINAL VASCULAR DISEASES: ADVANTAGES AND DISADVANTAGES OF INDIVIDUALISED THERAPY *Michael W. Stewart Professor and Chairman, Mayo School of Medicine, Department of Ophthalmology,
TREATMENT STRATEGIES FOR CHORIORETINAL VASCULAR DISEASES: ADVANTAGES AND DISADVANTAGES OF INDIVIDUALISED THERAPY *Michael W. Stewart Professor and Chairman, Mayo School of Medicine, Department of Ophthalmology,
The Wilmer Eye Institute s 34 th Annual Current Concepts in Ophthalmology March 13-17, 2017 Vail Marriott * Vail, Colorado
 The Wilmer Eye Institute s 34 th Annual Current Concepts in Ophthalmology March 13-17, 2017 Vail Marriott * Vail, Colorado Tentative 1/23/17 PROGRAM MONDAY, MARCH 13, 2017 Morning Session 6:00-7:00 Registration
The Wilmer Eye Institute s 34 th Annual Current Concepts in Ophthalmology March 13-17, 2017 Vail Marriott * Vail, Colorado Tentative 1/23/17 PROGRAM MONDAY, MARCH 13, 2017 Morning Session 6:00-7:00 Registration
