Genes Required for GLP-1 Asymmetry in the Early Caenorhabditis elegans Embryo
|
|
|
- Gyles Maxwell
- 6 years ago
- Views:
Transcription
1 DEVELOPMENTAL BIOLOGY 181, (1997) ARTICLE NO. DB Genes Required for GLP-1 Asymmetry in the Early Caenorhabditis elegans Embryo Sarah L. Crittenden,* David Rudel, Joe Binder,* Thomas C. Evans,,1 and Judith Kimble*,,,,2 *Howard Hughes Medical Institute, Departments of Biochemistry and Medical Genetics, and Laboratory of Molecular Biology, University of Wisconsin Madison, Madison, Wisconsin The translation of maternal glp-1 mrna is regulated both temporally and spatially in the early Caenorhabditis elegans embryo (T. C. Evans, S. L. Crittenden, V. Kodoyianni, and J. Kimble, Cell 77, , 1994). To investigate the control of embryonic glp-1 expression, we have examined the distribution of GLP-1 protein in selected maternal effect mutants that affect pattern or fate in the early embryo. We find that mutants that disrupt anterior posterior asymmetry in the early embryo (par-1 par-6, emb-8, Par(q537)) disrupt the spatial but not temporal control of GLP-1 expression: GLP-1 is observed at the normal stage of embryogenesis in par-like mutants; however, it is uniformly distributed. In contrast, mutants that alter blastomere identity (skn-1, pie-1, mex-1, apx-1) do not affect the normal GLP-1 pattern. We conclude that genes controlling the asymmetry of cellular components, including P granules, also control GLP-1 asymmetry in the early embryo. The finding that mutants that disrupt anterior posterior asymmetry translate GLP-1 in all blastomeres suggests that loss of embryonic asymmetry causes translational activation of GLP-1 in the posterior Academic Press INTRODUCTION rior P 1 blastomere; GLP-1 continues to be expressed in AB descendants through the 28-cell stage. Both temporal and spatial aspects of the maternal GLP-1 The specification of cell fates during metazoan developpattern rely on translational control (Evans et al., 1994). ment requires the precise temporal and spatial control of Reporter mrnas carrying the glp-1 3 untranslated region regulatory proteins. GLP-1, a transmembrane receptor re- (UTR) are expressed in a pattern similar to that of GLP-1. lated to Drosophila Notch, mediates several inductive in- Furthermore, a small deletion of only 61 nt from the middle teractions in the early Caenorhabditis elegans embryo of the glp-1 3 UTR eliminated spatial control but left tem- (Priess et al., 1987; Austin and Kimble, 1987; Yochem and poral regulation intact: the reporter was expressed at the Greenwald, 1989; Austin and Kimble, 1989; Roehl and Kim- correct stage but not in the right place (Evans et al., 1994). ble, 1993; Hutter and Schnabel, 1994, 1995; Mango et al., Because reporter RNA regulated by this mutant 3 UTR was 1994a; Mello et al., 1994; Moskowitz et al., 1994; Shelton expressed ectopically in posterior P 1 descendants, we preand Bowerman, 1996; see Fig. 1). The expression of GLP- dicted the existence of a trans-acting repressor localized in 1 protein from maternal glp-1 mrna is controlled both the P 1 blastomere (Evans et al., 1994). temporally and spatially (Evans et al., 1994; see Fig. 1). We have begun to investigate the genetic control of GLP- Whereas glp-1 mrna is uniformly distributed in both oo- 1 asymmetry in the early C. elegans embryo. Such genes cytes and early embryos (1 to 4 cells), GLP-1 protein is first might regulate glp-1 mrna specifically or they might be seen at the 2-cell stage, in the anterior AB but not the poste- required more generally for establishing embryonic polarity. In either case, genes regulating the early embryonic pattern of GLP-1 are predicted to act maternally because GLP-1 1 Present address: University of Colorado Health Sciences Center, asymmetry is visible early in embryogenesis, at the 2-cell Denver, CO stage. Furthermore, mutations in such genes are predicted 2 To whom reprint requests should be addressed. Fax: (608) 265- to cause lethality because release of glp-1 mrna from con jekimble@facstaff.wisc.edu. trol by its 3 UTR results in lethality (T.E., unpublished) /97 $25.00 Copyright 1997 by Academic Press All rights of reproduction in any form reserved.
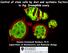
2 Control of GLP-1 Asymmetry 37 (AB) and a smaller posterior daughter (P 1 ) (Fig. 1). AB and P 1 differ in their molecular components as well as in their patterns of division and the types of tissues they generate (Laufer et al., 1980; Sulston et al., 1983; Cowan and McIntosh, 1985; Priess and Thomson, 1987; see Guo and Kemphues, 1996, for review). The maternal effect par genes (for partitioning defective) play a central role in controlling the earliest embryonic asymmetries (Kemphues et al., 1988; Kirby et al., 1990; Morton et al., 1992; Cheng et al., 1995; Guo and Kemphues, 1996). The phenotypes of par-1 4 include disruption of the asymmetry of the actin cytoskeleton, failure to localize P granules to the posterior, and loss of the normal asymmetric cleavage pattern. par-1 also disrupts the localization of a posterior determinant, SKN-1 (Bowerman et al., 1993). Three par genes have been characterized at the molecular level. PAR-1 is a serine/threonine kinase (Guo and Kemphues, 1995), PAR-2 protein contains a putative ATPbinding site and a zinc binding domain of the RING finger class (Levitan et al., 1994), and PAR-3 is a novel cytoplasmic protein (Etemad-Moghadam et al., 1995). PAR-1, PAR-2, and PAR-3 are localized in the 1-cell embryo soon after fertilization: both PAR-1 and PAR-2 localize to the posterior cortex (Guo and Kemphues, 1995; Boyd et al., 1996), while PAR-3 is found in the anterior cortex. PAR-3 is restricted FIG. 1. GLP-1 translation is regulated both temporally and spato the anterior by PAR-2 (Cheng et al., 1995; Etemadtially in the early C. elegans embryo. Thick lines represent GLP- Moghadam et al., 1995), and PAR-2 and PAR-3, in turn, are 1 in the membrane, shading represents GLP-1 in the cytoplasm. required for localization of PAR-1 to the posterior (Etemad- GLP-1 translation is repressed in oocytes and 1-cell embryos (temporal repression). GLP-1 translation is repressed in P 1 descendants Moghadam et al., 1995). The asymmetric distribution of from the 2-cell stage (spatial repression). At the 4-cell stage, GLPplay PAR proteins in the cell cortex of the zygote is thought to 1 in ABp is thought to interact with APX-1 in P 2 to promote ABp an important role in polarizing the embryonic cytoskel- fate (Mango et al., 1994a; Mello et al., 1994; Hutter and Schnabel, eton, which establishes multiple cellular and molecular 1994; Moskowitz et al., 1994; Mickey et al., 1996; Shelton and asymmetries (Guo and Kemphues, 1996). Bowerman, 1996). At the 12-cell stage, GLP-1 in ABal descendants While the par genes play a broad role in specifying anteand ABar descendants is thought to interact with an unknown rior posterior asymmetry, a separate set of maternal genes ligand in MS to promote development of the anterior pharynx (Genspecify the identities of individual blastomeres. SKN-1, a dreau et al., 1994; Hutter and Schnabel, 1994; Mango et al., 1994b; transcription factor found predominantly in the P 1 blasto- Moskowitz et al., 1994). Arrows indicate inductive interactions. mere at the 2-cell stage, is required for proper specification of one of the daughters of P 1, called EMS (Bowerman et al., 1992, 1993; Blackwell et al., 1994). Localization of SKN-1 In addition to GLP-1 asymmetry, several other asymmetries to P 1 depends on both par-1 and mex-1 activity: in both are established soon after fertilization in the early C. mutants, SKN-1 is found ectopically in AB descendants elegans embryo. The point of sperm entry determines the (Bowerman et al., 1993; Mello et al., 1992). Activity of the future posterior of the embryo (Goldstein and Hird, 1996). pie-1 gene is required for specification of the P 2 blastomere After fertilization, cytoplasmic rearrangements occur (Hird (Mello et al., 1992, 1996). In addition, GLP-1 signaling is and White, 1993; Hird, 1996) and P granules, putative germ- aberrant in both skn-1 and pie-1 mutants (Bowerman et al., line determinants, are localized to the posterior end of the 1992; Mango et al., 1994a; Shelton and Bowerman, 1996). zygote (Strome and Wood, 1982, 1983). Disruption of the Finally, APX-1, a transmembrane protein and potential ligand actin cytoskeleton at this point has profound effects on the for GLP-1, is required for signaling from P 2 to ABp distribution of P granules as well as on the ability of the (Mello et al., 1994; Mango et al., 1994a; Mickey et al., 1996; treated embryo to cleave properly and to produce specific Shelton and Bowerman, 1996). tissue types (Hill and Strome, 1988, 1990). This period of To identify genes involved in regulating GLP-1 asymmetry, cytoplasmic rearrangement preceding first cleavage is likely we analyzed GLP-1 in a broad spectrum of maternal effect lethal mutant embryos. GLP-1 is mislocalized in par mutants, but not in mutants with altered blastomere iden- tity or defective GLP-1 signaling. In addition, we identified to be crucial for the asymmetric distribution of many regulatory proteins, including GLP-1. The first cleavage division splits the fertilized zygote into a larger anterior daughter
3 38 Crittenden et al. new mutants with phenotypes similar to those of the par was scored as mislocalized. If the level of GLP-1 was lower in two genes. of the blastomeres, the embryo was scored as having a mislocalized but still asymmetric distribution of GLP-1. For wild-type 8- to 12- cell embryos we detect levels of GLP-1 in E and MS that are distinctly lower than in AB descendants. If an 8- to 15-cell mutant MATERIALS AND METHODS embryo had GLP-1 in all blastomeres at levels higher than those C. elegans Strains and Maintenance in wild-type embryos, then it was scored as mislocalized. Embryos were only included if the distribution of GLP-1 could unambiguously C. elegans strains were maintained as described by Brenner (1974). be scored as either mislocalized or wild-type. Mutants and strains used in this study were as follows: LGI, emb- 6(hc65), emb-12(g5), emb-20(g27), zyg-2(b10), par-6(zu170)unc- 13(e450)/hT2; LGII, emb-21(g31), emb-23(g39), zyg-9(b244), emb- RESULTS 27(g48), zyg-1(b1), rol-1(e91)mex-1(zu121)/mnc1, rol-1(e91)mex- 1(zu120)/mnC1; LGIII, emb-5(hc61), emb-8(hc69), emb-13(h6), emb- To identify genes required for proper GLP-1 expression 16(g19), emb-25(g45), emb-30(g53), emb-33(g60), emb-1(hc62), embin the early embryo, we examined a variety of maternal 7(hc66), par-2(lw32)unc-45(e286)/sc1, par-2(it5ts), lon-1(e185) pareffect lethal (Mel) mutant embryos. In the wild-type adult 3(it71)/qC1, pie-1(zu154)unc-25(e156)/qc1; LGIV, emb-3(hc59), embgerm line, GLP-1 is not detected in meiotic cells or matur- 11(g1), emb-26(g47), emb-31(g55), par-5(it121) unc-22(e66)/dnt1, skn-1(zu67)/dnt1; LGV, emb-4(hc60), emb-18(g21), rol-4(sc8)par- ing oocytes (Crittenden et al., 1994); in wild-type embryos, 1(b274)/DnT1, dpy-21(e428)par-4(it33)/ozdf2, par-4(it57ts), apx-1(or- GLP-1 is not observed at the 1-cell stage and is found in AB 3)dpy-11(e224)/DnT1. See text for references. Temperature sensitive descendants by the 4-cell stage (Evans et al., 1994). Some mutants were grown at 15 C to maintain stocks; other strains were 2-cell embryos possess weak GLP-1 staining that also is grown at 20 C unless noted otherwise. restricted to AB, suggesting that translation of maternal glp- 1 mrna first occurs at the 2-cell stage in AB blastomeres. After the 4-cell stage, strong GLP-1 staining persists in AB Screen for New Strict Maternal Effect Lethal descendants until the 28-cell stage (Evans et al., 1994), Mutants while weak GLP-1 is detected in membranes between cer- N2 hermaphrodites were mutagenized with ethylmethanesulfo- tain P 1 descendants beginning at the 8-cell stage (between nate as described (Brenner, 1974). From mutagenized L4 hermaphro- E and MS and between E and P 3, data not shown; R. Feichdites, F1 self-progeny were plated individually, and to identify ma- tinger and R. Schnabel, personal communication). ternal effect lethal mutants, F2 were then picked to individual To examine GLP-1 expression in Mel mutants, we stained plates and grown at 25 C. F2 that laid all or mostly dead eggs mutant embryos derived from homozygous mutant herwere crossed with wild-type males to identify strict maternal effect maphrodites with anti-glp-1 and anti-p granule antibodies. lethal (Mel) mutants (those not rescued by zygotic expression of the sperm s genome). From 4734 haploid genomes screened, we We refer to such embryos as x embryos where x represents obtained 182 independently isolated strict Mel mutants. Of these, the gene. For example, a par-1 embryo refers to a homozythree mislocalized GLP-1 (see Results). Two of these, q514 and gous par-1 mutant embryo derived from a homozygous mu- q516, mapped to linkage group V and failed to complement par- tant par-1 hermaphrodite. 4(it33). The third, q537, mapped to linkage group II and comple- To score spatial regulation of GLP-1, we examined GLPments mex-1(zu121). 1 in 4- to 15-cell embryos. Mutant embryos were scored as having a wild-type GLP-1 pattern if the levels of GLP-1 in Immunofluorescence AB and P 1 descendants were similar to those in wild-type. Embryos were scored as having mislocalized GLP-1 if GLP- To detect GLP-1, we used a mixture of anti-egfl, anti-lng, 1 was present in P 1 descendants at a level higher than that and anti-ank polyclonal antibodies (Crittenden et al., 1994); to in wild-type embryos (see Materials and Methods for more detect P granules, we used either OICID4 or K76 monoclonal anti- details). To score temporal regulation of GLP-1, we looked bodies (Strome and Wood, 1983). Embryos and germ lines were for the presence or absence of GLP-1 in oocytes and 1-cell stained as described (Evans et al., 1994). Secondary antibodies were labeled with FITC, LRSC, Cy3, or Cy5. Images were collected on embryos, when it is not normally detected. Bio-Rad MRC1000 and 1024 laser scanning confocal microscopes. To stain temperature-sensitive mutants, L4 hermaphrodites were Mutations in par-1 and par-4 Always Disrupt grown at 25 C for hr after which embryos were processed GLP-1 Asymmetry for staining. In par-1 embryos, the first cleavage division is symmetrical Scoring Embryos and P granules are not localized to P 1 but instead are found in both AB and P 1 (Kemphues et al., 1988; Guo and In wild-type 4-cell embryos we do not detect GLP-1 in P 1 descengenerates Kemphues, 1995). In par-4 embryos, the first cleavage often dants. If all blastomeres of a mutant 4-cell embryo had levels of large AB and small P 1 blastomeres as in wild-type, GLP-1 comparable to those found in wild-type AB blastomeres, it but P granules are distributed to both AB and P 1 (Kemphues
4 Control of GLP-1 Asymmetry 39 et al., 1988; Morton et al., 1992). We stained embryos homo- see also Kemphues et al., 1988), while 25% had P granules zygous for a strong par-1 allele, par-1(b274) (Kemphues et only in P 1 descendants. We also noticed two par-3 embryos al., 1988) or for a strong par-4 allele, par-4(it33). All par-1 with a very low level of GLP-1 in P 1 descendants, but with embryos (n Å 30) and par-4 embryos (n Å 30) mislocalized P granules distributed equally in all four blastomeres (Fig. GLP-1. 2G). Adult germ lines (n Å 14), oocytes (n Å 13), and 1-cell In 100% of both par-1 and par-4 mutant embryos we detected embryos (n Å 2) had normal distributions of GLP-1. GLP-1 in all blastomeres (Fig. 2I). For par-1, all em- Thus, in par-2 and par-3 mutant embryos, GLP-1 is mislo- bryos had approximately equal levels of GLP-1 expression calized in less than 100% of the embryos, indicating that in all blastomeres (Fig. 2B). In embryos where P granules the activities of PAR-2 and PAR-3 are not essential for GLPcould be scored, they were distributed equally among the 1 asymmetry. blastomeres (n Å 4; Fig. 2B; see also Kemphues et al., 1988; We also stained weak alleles of two par genes that are less Guo and Kemphues, 1995). Adult germ lines (n Å 9), oocytes well characterized. In par-5(it121) mutant embryos, which (n Å 9), and 1-cell embryos (n Å 8) had a normal GLP-1 have a phenotype similar to that of par-2 (K. Kemphues, pattern. For par-4, 90% (27/30) had similar levels of GLP-1 personal communication), 14% (1/7) had mislocalized GLP- in all blastomeres, while three 4-cell embryos had notice- 1. In par-6(zu170) mutant embryos, which have a phenotype ably lower levels of GLP-1 in two blastomeres. P granules similar to that of par-3 (Watts et al., 1996), 5% (1/20) had stained weakly and were present in all blastomeres (n Å 4; mislocalized GLP-1. Fig. 2C; see also Kemphues et al., 1988). Adult germ lines (n Å 20), oocytes (n Å 12), and 1-cell embryos (n Å 5) had emb-8 Is Required for both GLP-1 and P Granule a normal GLP-1 pattern. Asymmetry Thus, both par-1 and par-4 are required for spatial but not temporal regulation of GLP-1 asymmetry. The time of In an attempt to identify other Mel mutants affecting onset of GLP-1 translation appears to be normal in these GLP-1 asymmetry, we screened a collection of 24 previously mutant embryos, and the presence of GLP-1 in P 1 descen- isolated temperature-sensitive strict Mel mutants (Wood et dants is likely to reflect ectopic translation there. al., 1980; Miwa et al., 1980; Cassada et al., 1981) with anti- GLP-1 and anti-p granule antibodies. Of these only one, emb-8(hc69) mislocalized GLP-1 (see below). Four other Mutations in par-2 and par-3 Often Disrupt GLP-1 mutants arrested at the 1-cell stage with no detectable GLP- Asymmetry 1(emb-1(hc62), n Å 3; emb-7(hc66), n Å 3; emb-27(g48), n Many par-2 and par-3 embryos segregate P granules properly Å 75; zyg-1(b1), n Å 49), indicating that temporal control to P 1 at the first division; however, all of them exhibit of GLP-1 expression is normal. However, since embryos synchronous and symmetric first cleavage divisions. The arrested at the 1-cell stage, the asymmetric distribution of second cleavage planes differ between the two mutants GLP-1 could not be scored. Finally, 19 localized GLP-1 properly (Kemphues et al., 1988; Cheng et al., 1995). (emb-3(hc59), n Å 3; emb-4(hc60), n Å 1; emb-5(hc61), We stained embryos homozygous for the nonsense muta- n Å 16; emb-6(hc65), n Å 25; emb-11(g1), n Å 26; embtion par-2(lw32) (Levitan et al., 1994). Thirty-seven percent 12(g5), n Å 4; emb-13(g6), n Å 6; emb-16(g19), n Å 42; emb- (12/32) mislocalized GLP-1 (Fig. 2F). Among embryos mislo- 18(g21), n Å 4; emb-20(g27), n Å 9; emb-21(g31), n Å 9; calizing GLP-1, three 4-cell embryos had lower levels of emb-23(g39), n Å 8; emb-25(g45), n Å 22; emb-26(g47), n GLP-1 in P 1 descendants. In embryos where P granules could Å 3; emb-30(g53), n Å 2; emb-31(g55), n Å 2; emb-33(g60), be scored, 86% (12/14) had P granules localized to P 1 descendants n Å 10; zyg-2(b10), n Å 16; zyg-9(b244), n Å 13). (Fig. 2E), while 14% (2/14) had P granules in AB as For emb-8(hc69) embryos, 68% (13/19) had a normal dis- well as P 1 descendants (Fig. 2D; see also Kemphues et al., tribution of GLP-1 (Figs. 3A, 3B, and 3E). Thirty-two percent 1988). One embryo that had GLP-1 only in AB descendants (6/19) of the embryos stained for GLP-1 in both AB and P 1 also had a large number of P granules in both AB and P 1 descendants at the 4- to 12-cell stages (Figs. 3C and 3E). In descendants (Fig. 2D), raising the possibility that anterior all six of the 4-cell embryos that mislocalized GLP-1, the localization of GLP-1 may not depend on proper posterior level of GLP-1 was higher in two of the four blastomeres, localization of P granules. Adult germ lines (n Å 7), oocytes indicating that some asymmetry still exists (Fig. 3C). In (n Å 7), and 1-cell embryos (n Å 3) had normal distributions those emb-8(hc69) embryos scored for both GLP-1 and P of GLP-1. granules, 70% (7/10) had P granules only in P 1 descendants We stained embryos homozygous for the null mutant par- (Fig. 3A) and 30% (3/10) had P granules in both AB and P 1 3(it71) (Etemad-Moghadam et al., 1995). Seventy-seven per- descendants (Figs. 3B and 3E). Interestingly, in several 4- cent (23/30) mislocalized GLP-1 (Figs. 2G, 2H, and 2I). cell embryos, P granules were found either mostly in EMS Among embryos mislocalizing GLP-1, five 4-cell embryos (Fig. 3A) or in all blastomeres except EMS (Fig. 3B; also had lower levels of GLP-1 in P 1 descendants (Figs. 2G and observed by L. A. Khan and S. Siddiqui, personal communi- 2H). In embryos where P granules could be scored, 75% had cation). In two 4-cell embryos, GLP-1 was localized properly P granules in both AB and P 1 descendants (Figs. 2G and 2H; to AB descendants even though P granules were present in
5 40 Crittenden et al. 2
6 Control of GLP-1 Asymmetry
7 42 Crittenden et al. both AB and P 1 descendants (Fig. 3B). All blastomeres of many 4-cell emb-8 embryos had similar sizes and nuclei appeared to be at the same stage of the cell cycle, suggesting that emb-8 embryos may have synchronous and symmetrical early divisions like par mutants. Screen for New Genes That Control GLP-1 Asymmetry To identify new genes that control GLP-1 asymmetry, we screened for strict Mel mutants and stained them to examine GLP-1 expression. To determine whether mutants af- fected other embryonic asymmetries, we also stained with anti-p granule antibodies and noted relative size and orientation of blastomeres in the stained embryos. We screened 4734 haploid genomes and isolated 182 strict maternal effect lethal mutants. GLP-1 was detectable in all 182 strict Mels. Therefore, we did not identify any candidate activators of GLP-1 translation. However, 3/182 mutants expressed GLP-1 in both AB and P 1 descendants at the 4- cell stage. All three new mutants also mislocalized P granules and appeared to have altered early cleavage patterns (Fig. 3D and data not shown). GLP-1 was not detected in oocytes or 1-cell embryos of any of these mutants. Two of the three new mutants, q514 and q516 are new alleles of par-4 since they map to the approximate position of par-4 on chromosome V and fail to complement par-4. The other mutant, q537, appears to be an allele of a new par gene. Par(q537) maps to chromosome II; none of the 6 known par genes is found on chromosome II. For Par(q537), 39% (23/38; Fig. 3E) had GLP-1 in both AB and P 1 descendants. Of these, 10 had less GLP-1 in P 1 descendants than in AB descendants (Fig. 3D). In those embryos in which P granule staining was scored, 37% (10/27) had P granules in both AB and P 1 descendants. P granules were often clustered in the middle of the embryo (Fig. 3D). The lack of mutants that specifically disrupt GLP-1 asymmetry can be interpreted in several ways. One possibility is that only one or a few genes can mutate to this specific phenotype and that our screen was not large enough to re- cover these mutants. Alternatively, such a regulator may have a zygotic phenotype if it is also required for the proper regulation of GLP-1 (or other regulatory proteins) at other times or in different tissues. Genes That Affect Blastomere Identity and/or GLP- 1-Mediated Inductions Do Not Affect GLP-1 Level or Distribution A number of maternal effect genes affect the identity of individual blastomeres at the 4-cell stage. In skn-1 and pie- FIG. 2. par gene activity is required for establishing GLP-1 asymmetry. Wild-type and par embryos were double stained with anti-glp- 1 and anti-p granule antibodies. GLP-1 is green; P granules are red. Embryos are approximately 40 mm long. (A) Wild-type 4-cell embryo. GLP-1 is detected in membranes and cytoplasms of ABa and ABp, but not in EMS or P 2. Levels of GLP-1 staining are variable in both wild-type and par mutants. (B) par-1(b274) 4-cell embryo. GLP-1 is present in membranes and cytoplasms of all four cells at levels comparable to that of wild-type AB blastomeres. There does appear to be slightly less GLP-1 in the membrane between EMS and P 2.P granules are present in the cytoplasm of all four blastomeres. (C) par-4(it33) 4-cell embryo. The pattern of GLP-1 is similar to that of par- 1. The membrane between EMS and P 2 is stained; however, it is not completely visible in this particular embryo. P granules are found in all blastomeres. (D F) Three different par-2(lw32) 4-cell embryos showing different phenotypes. The second cleavage plane in both AB and P 1 in par-2 embryos is transverse, thus their blastomere arrangement is somewhat different from that of wild-type. (D) GLP-1 is localized properly, but P granules occur in all blastomeres. (E) Both GLP-1 and P granules are asymmetric: P granules are present in both presumed P 1 descendants. Therefore, P granules were segregated correctly at the 2-cell stage, but not thereafter. (F) GLP-1 is mislocalized. P granules were not stained in this embryo. (G and H) Two different par-3(it71) embryos. Both embryos have GLP-1 in posterior blastomeres, but the levels are not as high as those of par-1 or par-4. P granules are found in all blastomeres in both embryos. While P granules appear to be symmetrically distributed, GLP-1 is still somewhat asymmetric. (I) Summary of staining results with par embryos. Percentages of 4- to 15-cell embryos mislocalizing GLP-1 and/or P granules are shown. FIG. 3. emb-8 and Par(q537) activities are required for both GLP-1 and P granule asymmetry. emb-8(hc69) and Par(q537) embryos were double stained with anti-glp-1 and anti-p granule antibodies. GLP-1 is green; P granules are red. emb-8 embryos are fragile making it difficult to preserve morphology; membranes are often less distinct than wild-type. Embryos are approximately 40 mm long. (A C) Three different emb-8(hc69) embryos are shown, each representing a distinct phenotype. (A) Both GLP-1 and P granules are asymmetrically distributed, though P granules are detected only in EMS. (B) GLP-1 is asymmetric, but P granules are found in both AB and P 1 descendants. In contrast to A, P granules are found in all blastomeres except EMS. (C) GLP-1 occurs in all membranes; P granules were not stained. (D) A Par(q537) embryo. GLP-1 is present in all membranes; P granules are present in three of four cells and are clustered together in one region. (E) Summary of results with emb-8 and Par(q537) embryos. Percentages of 4- to 12-cell embryos mislocalizing GLP-1 and/or P granules are shown. FIG. 4. GLP-1 asymmetry is independent of signaling and posterior blastomere identity. apx-1 (A), pie-1 (B), skn-1 (C), and mex-1 (D) mutant 4-cell embryos were double stained with anti-glp-1 and anti-p granule antibodies. GLP-1 is green; P granules are red. In all cases, GLP-1 is similar to wild-type; it is detected in the membranes and cytoplasm of AB descendants and is not detected in either the membranes of cytoplasms of P 1 descendants. Variability in brightness of the cytoplasm occurs in wild-type embryos and does not seem to be a feature of any particular mutant. P granules are distributed normally in all mutants except mex-1, where they are found in both P 1 descendants. Embryos are approximately 40 mm long.
8 Control of GLP-1 Asymmetry 43 1 mutants, fates of the two P 1 descendants, EMS and P 2, asymmetries. par-1 and par-4 which strongly affect GLP-1 are altered; in mex-1 mutants, AB fates are altered at least and P granule asymmetry only weakly affect cytoplasmic in part due to mislocalization of SKN-1. In addition, P gran- streaming, the position of pronuclear fusion, and the asymmetric ules are not segregated normally in mex-1 embryos (Mello distribution of actin in the 1-cell embryo (Kemphues et al., 1992; Schnabel et al., 1996). We stained 4- to 12-cell et al., 1988; Kirby et al., 1990). In addition, the first division skn-1(zu67) embryos (n Å 18; Fig. 4A), pie-1(zu154) embryos in par-4 mutants is asymmetric (Kemphues et al., 1988; (n Å 27; Fig. 4B), and embryos from two alleles of mex-1, Morton et al., 1992), indicating that proper placement of mex-1(zu121) (n Å 17; Fig. 4C), and mex-1(zu120) (n Å 18; the first cleavage plane is not sufficient for proper GLP-1 data not shown) and found that GLP-1 staining was similar asymmetry. In contrast, par-2 and par-3, which have a to wild-type. Thus, the correct fates of AB and P 1 descendants weaker effect on GLP-1 and P granule asymmetry, have are not essential for asymmetric GLP-1 expression. strong effects on the other early embryonic asymmetries The apx-1 gene encodes a signaling ligand for GLP-1 that that par-1 and par-4 only affect weakly (Kemphues et al., is used in P 2 to induce ABp at the 4-cell stage (Mello et al., 1988; Kirby et al., 1990). Thus, par-1 and par-4 play a central 1994; Mango et al., 1994a; Mickey et al., 1996; Shelton and role in controlling the asymmetric distribution of proteins, Bowerman, 1996). To ask whether the level of GLP-1 in AB including GLP-1, within the early embryo. In contrast, pardescendants is affected by the absence of its signaling ligand, 2 and par-3 appear to play a central role in cytoskeletal we scored apx-1(or3) (n Å 17; Fig. 4D) 4- to 12-cell asymmetry. embryos. GLP-1 staining was similar to wild-type at all A pathway for par gene activity has been proposed in stages. which PAR-2 and PAR-3 are required for the proper localization of PAR-1 (Etemad-Moghadam et al., 1995). However, in contrast to PAR-1, PAR-2 and PAR-3 are not absolutely DISCUSSION required for GLP-1 asymmetry. This dependence on PAR-1 suggests that sufficient PAR-1 activity remains in many Evans et al. (1994) showed that maternal glp-1 mrna is par-2 and par-3 mutant embryos to allow proper regulation translationally regulated by elements in its 3 UTR to of GLP-1 asymmetry. Consistent with this, it has been proposed achieve an asymmetric expression of GLP-1 protein in the that PAR-1 localization may not be absolutely reachieve early embryo. In this paper, we have begun to identify genes quired for its activity (Boyd et al., 1996). required for GLP-1 asymmetry. Specifically, we have identified eight genes, par-1 par-6, emb-8, and Par(q537), that Temporal Regulation Can Be Separated from influence GLP-1 asymmetry in the early embryo and have Spatial Regulation in the Early Embryo shown that many others do not affect GLP-1 expression. Our results have a number of implications for the control of GLP-1 asymmetry in the early embryo, which are discussed below. The Initial Establishment of Polarity Is Linked with the Establishment of GLP-1 Asymmetry We have found that the activity of genes regulating many aspects of anterior posterior asymmetry in the early embryo, par-1 6, emb-8, and Par(q537), are required for the proper spatial but not temporal regulation of maternal GLP- 1 expression. In par mutant embryos, we observe extra GLP- 1 expression in posterior blastomeres, indicating that GLP- 1 can be translated in all blastomeres in the absence of par activity. In contrast, we have found that GLP-1 distribution in the early embryo is independent of both GLP-1-mediated signaling and the proper specification of anterior and posterior blastomere identity. Several Individual Aspects of Embryonic Polarity Can Be Uncoupled from the Establishment of GLP-1 Asymmetry Phenotypic differences among the par mutants indicate that GLP-1 asymmetry is not coupled to all early embryonic The glp-1 3 UTR mediates both temporal and spatial regulation of GLP-1 translation in the early embryo (Evans et al., 1994). Deletion analysis of the 3 UTR raised the possibility that temporal and spatial regulation were mediated by different regions of the 3 UTR, possibly with separate regulators binding each element. Consistent with this idea, GLP-1 appears to be translated at the normal time in par mutants; however, its spatial asymmetry is lost. Alternatively, a single element in the 3 UTR may direct both temporal and spatial regulation, but temporal and spatial regula- tion may be controlled by distinct factors. Translational Regulation of glp-1 in the Early Embryo: Repression or Activation? Two simple ways to create GLP-1 asymmetry are (1) presence of a translational activator in the anterior of the em- bryo, or (2) presence of a translational repressor in the posterior of the embryo (see Fig. 5). In addition, temporal control of GLP-1 translation is necessary to ensure that GLP-1 protein is not synthesized in a 1-cell embryo and subsequently inherited by all embryonic blastomeres. The role of the par genes in GLP-1 translational control is not understood; however, because par gene activity is required for a wide range of embryonic asymmetries, it is likely that the main
9 44 Crittenden et al. ules. We have also observed several embryos in which GLP- 1 is properly localized, but P granules are found in both AB and P 1 descendants. These results raise the possibility that proper P granule localization may not be absolutely required for proper regulation of GLP-1 asymmetry. Signaling Is Not Required for GLP-1 Expression in the Early Embryo Positive feedback does not appear to play a role in maintaining GLP-1 expression levels in the early embryo: neither active ligand nor active GLP-1 itself (S.C., unpublished) is required for proper expression. In contrast to the embryo, glp-1 activity is required for continued GLP-1 expression in the germ line (S.C., unpublished), and positive autoregulation has been suggested (Kodoyianni et al., 1992; Christensen et al., 1996; Berry and Schedl, in press). Positive FIG. 5. Two models for how par genes could affect GLP-1 asym- feedback has also been proposed to maintain expression of metry. Shading indicates presence of GLP-1; / indicates presence a C. elegans GLP-1 homolog, LIN-12, in the larval somatic of an activator, 0 indicates presence of repressor. 0 with an x gonad (Seydoux and Greenwald, 1989; Wilkinson et al., through it indicates an inactive repressor. See discussion for more 1994). While positive feedback does not appear to control details. (A) In wild-type embryos, an activator of GLP-1 translation levels of GLP-1 in 2- to 28-cell embryos, activity of maternal may be restricted to the anterior blastomere, AB. In par mutants GLP-1 appears to activate LIN-12 expression in AB descenthis activator would be found in both AB and P 1. (B) In wild-type dants later in embryonic development (Moskowitz and embryos a repressor of GLP-1 translation may be restricted to the posterior blastomere, P 1.Inpar mutants this repressor would be found in both AB and P 1 ; however, it must not be active since GLP- 1 is present in all blastomeres. GLP-1 asymmetry is established early and requires the activity of the par genes. However, genes that establish blas- tomere identity do not affect GLP-1 asymmetry. For exam- ple, the presence of GLP-1 in mex-1(zu120) AB blastomeres indicates that even though these blastomeres contain SKN- 1 (Bowerman et al., 1993) and produce tissue types typical of MS (Mello et al., 1992), they still regulate GLP-1 in an AB-specific manner. In addition, SKN-1 is neither necessary nor sufficient to repress GLP-1 expression. Therefore, SKN- 1 specifies EMS identity after GLP-1 asymmetry is estab- lished and establishment of blastomere identity is not closely linked to regulation of GLP-1 asymmetry in the early embryo. requirement for par activity is to properly localize a regulator of GLP-1 translation (Fig. 5). They might localize a translational activator (or its RNA) to the anterior (Fig. 5A) or a translational repressor (or its RNA) to the posterior (Fig. 5B). In the latter case, the repressor would have to be inactive when not localized properly since GLP-1 is present in all blastomeres in par mutant embryos. This situation occurs in Drosophila where Nanos, a translational repressor of hunchback mrna, is not translated unless its mrna is localized properly (Gavis and Lehmann, 1994). Is a Repressor of glp-1 Translation Associated with P Granules? In Drosophila, the proper formation and posterior localization of polar granules are required for the posterior repression of hunchback translation and the determination of abdominal and germ cell fates (see Lehmann, 1995, for review). By analogy, it is possible that factors required for the asymmetric expression of embryonic proteins are associated with P granules in C. elegans. We have found that in many par mutant embryos there is a correlation between P granule mislocalization and GLP-1 mislocalization. The presence of P granules in the same blastomeres as GLP-1 suggests that, at least in par mutant embryos, an active repressor of GLP-1 translation is not associated with P gran- Rothman, 1996). Blastomere Identity Is Established after GLP-1 Asymmetry Is There a Separate Pathway for Regulation of GLP-1 Asymmetry? The mechanisms for generating the asymmetric localization of certain regulators in the early embryo can be sepa- rated genetically. Thus, mex-1(zu120) affects SKN-1 but not GLP-1 asymmetry (Bowerman et al., 1993; this paper), and mex-3 affects PAL-1 asymmetry but not GLP-1, SKN-1, or APX-1 asymmetries (B. Draper and J. Priess, 1996; C. Hunter and C. Kenyon, 1996). Furthermore, par-2 6 may not affect GLP-1 and SKN-1 asymmetry to the same extent (B. Bow- erman, personal communication; this paper). Thus, there are both global regulators of embryonic asymmetry, such
10 Control of GLP-1 Asymmetry 45 as par-1, and regulators affecting a more restricted group of ing in Caenorhabditis elegans, is homologous to human CBF1 asymmetries, such as mex-3. Specific regulators of GLP-1 and Drosophila Su(H). Development 122, asymmetry have not yet been identified, and their existence Cowan, A. E., and McIntosh, J. R. (1985). Mapping the distribution of differentiation potential for intestine, muscle, and hypodermis remains an open question for future investigation. during early development in Caenorhabditis elegans. Cell 41, ACKNOWLEDGMENTS Crittenden, S. L., Troemel, E. R., Evans, T. C., and Kimble, J. (1994). GLP-1 is localized to the mitotic region of the C. elegans germ line. Development 120, We thank Deena Schuster, Phil Balandyk, Kim Oas, and Eileen Draper, B. W., Mello, C. M., Bowerman, B., Hardin, J., and Priess, Durkin for technical assistance, Ken Kemphues, Bruce Bowerman, J. R. (1996). MEX-3 is a KH domain protein that regulates blasto- Susana Guedes, Craig Mello, and past and present members of the mere identity in early C. elegans embryos. Cell, in press. Kimble lab for helpful discussions, Lisa Kadyk, Maria Gallegos, Etemad-Moghadam, B., Guo, S., and Kemphues, K. J. (1995). Asymand the reviewers for comments on the manuscript, and Bruce metrically distributed PAR-3 protein contributes to cell polarity Bowerman, Laura Berry, Tim Schedl, Richard Feichtinger, Ralf and spindle alignment in early C. elegans embryos. Cell 83, 743 Schnabel, L. A. Khan, and S. Siddiqui for sharing unpublished data We also thank Ken Kemphues, Lynn Boyd, Bruce Bowerman, and Evans, T. C., Crittenden, S. L., Kodoyianni, V., and Kimble, J. especially the Caenorhabditis Genetics Center for sending strains. (1994). Translational control of maternal glp-1 mrna establishes Anti-P granule monoclonal antibodies K76 and OICID4 were pro- an asymmetry in the C. elegans embryo. Cell 77, vided by the Developmental Studies Hybridoma Bank maintained Gavis, E. R., and Lehmann, R. (1994). Localization of nanos RNA by a contract from NICHD (N01-HD ). This work was sup- controls embryonic polarity. Nature 369, ported by grants from NIH and NSF to J.K. and Wisconsin Alumni Gendreau, S. B., Moskowitz, I. P. G., Terns, R. M., and Rothman, Research Foundation and NIH Molecular Biosciences Training J. H. (1994). The potential to differentiate epidermis is unequally grant fellowships for D.R. J.K. is an investigator with the Howard distributed in the AB lineage during early embryonic develop- Hughes Medical Institute. ment in C. elegans. Dev. Biol. 166, Goldstein, B., and Hird, S. N. (1996). Specification of the anteroposterior axis in Caenorhabditis elegans. Development 122, REFERENCES Guo, S., and Kemphues, K. J. (1995). par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/ Austin, J., and Kimble, J. (1987). glp-1 is required in the germ line Thr kinase that is asymmetrically distributed. Cell 81, for regulation of the decision between mitosis and meiosis in C. Guo, S., and Kemphues, K. J. (1996). Molecular genetics of asymelegans. Cell 51, metric cleavage in the early Caenorhabditis elegans embryo. Austin, J., and Kimble, J. (1989). Transcript analysis of glp-1 and Curr. Opin. Genet. Dev. 6, lin-12 homologous genes required for cell interactions during Hill, D. P., and Strome, S. (1988). An analysis of the role of microdevelopment of C. elegans. Cell 58, Berry, L. W., Westlund, B., and Schedl, T. (1996). Germline tumor filaments in the establishment and maintenance of asymmetry formation caused by activation of glp-1, a member of the Notch in Caenorhabditis elegans zygotes. Dev. Biol. 125, family of receptors. Development, in press. Hill, D. P., and Strome, S. (1990). Brief cytochalasin-induced disrup- Blackwell, T. K., Bowerman, B., Priess, J. R., and Weintraub, H. tion of microfilaments during a critical interval in 1-cell C. eleg- (1994). Formation of a monomeric DNA binding domain by Skntions to the 2-cell embryo. Development 108, ans embryos alters the partitioning of developmental instruc- 1 bzip and homeodomain elements. Science 266, Bowerman, B., Draper, B. W., Mello, C. C., and Priess, J. F. (1993). Hird, S. (1996). Cortical actin movements during the first cell cycle The maternal gene skn-1 encodes a protein that is distributed of the Caenorhabditis elegans embryo. J. Cell Sci. 109, unequally in early C. elegans embryos. Cell 74, Hird, S. N., and White, J. G. (1993). Cortical and cytoplasmic flow Bowerman, B., Eaton, B. A., and Priess, J. R. (1992). skn-1, a mater- polarity in early embryonic cells of Caenorhabditis elegans. J. nally expressed gene required to specify the fate of ventral blasto- Cell Biol. 121, meres in the early C. elegans embryo. Cell 68, Hunter, C. P., and Kenyon, C. (1996). Spatial and temporal controls Boyd, L., Guo, S., Levitan, D., Stinchcomb, D. T., and Kemphues, target pal-i blastomere specification activity to a single blasto- K. J. (1996). PAR-2 is asymmetrically distributed and promotes mere lineage in C. elegans embryos. Cell 87, association of P granules and PAR-1 with the cortex in C. elegans Hutter, H., and Schnabel, R. (1994). glp-1 and inductions establish- embryos. Development 122, ing embryonic axes in C. elegans. Development 120, Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetasymmetry Hutter, H., and Schnabel, R. (1995). Establishment of left-right ics 77, in the Caenorhabditis elegans embryo: A multistep Cassada, R., Isnenghi, E., Culotti, M., and von Ehrenstein, G. (1981). process involving a series of inductive events. Development 121, Genetic analysis of temperature-sensitive embryogenesis mu tants in Caenorhabditis elegans. Dev. Biol. 84, Kemphues, K. J., Priess, J. R., Morton, D. G., and Cheng, N. (1988). Cheng, N. N., Kirby, C. M., and Kemphues, K. J. (1995). Control of Identification of genes required for cytoplasmic localization of cleavage spindle orientation in Caenorhabditis elegans: The role early C. elegans embryos. Cell 52, of the genes par-2 and par-3. Genetics 139, Kirby, C., Kusch, M., and Kemphues, K. (1990). Mutations in the Christensen, S., Kodoyianni, V., Bosenberg, M., Friedman, L., and par genes of Caenorhabditis elegans affect cytoplasmic reorgani- Kimble, J. (1996). lag-1, a gene required for lin-12 and glp-1 signal- zation during the first cell cycle. Dev. Biol. 142,
11 46 Crittenden et al. Kodoyianni, V., Maine, E. M., and Kimble, J. (1992). Molecular basis required zygotically for early embryonic cellular interactions and of loss-of-function mutations in the glp-1 gene of Caenorhabditis are regulated by maternal GLP-1 signalling in Caenorhabditis elegans. Mol. Biol. Cell 3, elegans. Development 122, Laufer, J. S., Bazzicalupo, P., and Wood, W. B. (1980). Segregation Priess, J. R., Schnabel, H., and Schnabel, R. (1987). The glp-1 locus of developmental potential in early embryos of Caenorhabditis and cellular interactions in early C. elegans embryos. Cell 51, elegans. Cell 19, Lehmann, R. (1995). Establishment of embryonic polarity during Priess, J. R., and Thomson, J. N. (1987). Cellular interactions in Drosophila oogenesis. Semin. Dev. Biol. 6, early C. elegans embryos. Cell 48, Levitan, D. J., Boyd, L., Mello, C. C., Kemphues, K. J., and Stinchby Roehl, H., and Kimble, J. (1993). Control of cell fate in C. elegans comb, D. T. (1994). par-2, a gene required for blastomere asymture a GLP-1 peptide consisting primarily of ankyrin repeats. Na- metry in Caenorhabditis elegans, encodes zinc-finger and ATPbinding 364, motifs. Proc. Natl. Acad. Sci. USA 91, Schnabel, R., Weigner, C., Hutter, H., Feichtinger, R., and Schnabel, Mango, S. E., Thorpe, C. J., Martin, P. R., Chamberlain, S. H., and H. (1996). mex-1 and the general partitioning of cell fate in the Bowerman, B. (1994a). Two maternal genes, apx-1 and pie-1, are early C. elegans embryo. Mech. Dev. 54, required to distinguish the fates of equivalent blastomeres in the Seydoux, G., and Greenwald, I. (1989). Cell autonomy of lin-12 early Caenorhabditis elegans embryo. Development 120, 2305 function in a cell fate decision in C. elegans. Cell 57, Shelton, C. A., and Bowerman, B. (1996). Time-dependent re- Mango, S. E., Lambie, E. J., and Kimble, J. (1994b). The pha-4 gene sponses to glp-1 mediated inductions in early C. elegans embryos. is required to generate the pharyngeal primordium of Caenorhab- Development 122, ditis elegans. Development 120, Strome, S., and Wood, W. B. (1982). Immunofluorescence visualiza- Mello, C. C., Draper, B. W., Krause, M., Weintraub, H., and Preiss, tion of germ-line-specific cytoplasmic granules in embryos, lar- J. R. (1992). The pie-1 and mex-1 genes and maternal control of vae, and adults of Caenorhabditis elegans. Proc. Natl. Acad. Sci. blastomere identity in early C.elegans embryos. Cell 70, 163 USA 79, Strome, S., and Wood, W. B. (1983). Generation of asymmetry and Mello, C. C., Draper, B. W., and Priess, J. R. (1994). The maternal segregation of germ-line granules in early C. elegans embryos. genes apx-1 and glp-1 and establishment of dorsal ventral polar- Cell 35, ity in the early C. elegans embryo. Cell 77, Sulston, J. E., Schierenberg, E., White, J. G., and Thomson, J. N. Mello, C. C., Schubert, C., Draper, B., Zhang, W., Lobel, R., and (1983). The embryonic lineage of the nematode Caenorhabditis Priess, J. R. (1996). The PIE-1 protein and germline specification elegans. Dev. Biol. 100, in C. elegans embryos. Nature 382, Watts, J. L., Etemad-Moghadam, B., Guo, S., Boyd, L., Draper, B. W., Mickey, K. M., Mello, C. C., Montgomery, M. K., Fire, A., and Mello, C. C., Priess, J. R., and Kemphues, K. K. (1996). par-6, a Priess, J. R. (1996). An inductive interaction in 4-cell stage C. gene involved in the establishment of asymmetry in early C. elegans embryos involves APX-1 expression in the signaling cell. elegans embryos, mediates the asymmetric localization of PAR- Development 122, Development 122, Miwa, J., Schierenberg, E., Miwa, S., and von Ehrenstein, G. (1980). Wilkinson, H. A., Fitzgerald, K., and Greenwald, I. (1994). Recipro- Genetics and mode of expression of temperature-sensitive muta- cal changes in expression of the receptor LIN-12 and its ligand tions arresting embryonic development in Caenorhabditis eleg- LAG-2 prior to commitment in a C. elegans cell fate decision. ans. Dev. Biol. 76, Cell 79, Morton, D. G., Roos, J. M., and Kemphues, K. J. (1992). par-4, a Wood, W. B., Hecht, R., Carr, S., Vanderslice, R., Wolf, N., and gene required for cytoplasmic localization and determination of Hirsh, D. (1980). Parental effects and phenotypic characterization specific cell types in Caenorhabditis elegans embryogenesis. Geelegans. of mutations that affect early development in Caenorhabditis netics 130, Dev. Biol. 74, Moskowitz, I. P. G., Gendreau, S. B., and Rothman, J. H. (1994). Yochem, J., and Greenwald, I. (1989). glp-1 and lin-12, genes impli- Combinatorial specification of blastomere identity by glp-1 delar transmembrane proteins. Cell 58, cated in distinct cell cell interactions in C. elegans, encode simipendent cellular interactions in the nematode Caenorhabditis elegans. Development 120, Received for publication August 23, 1996 Moskowitz, I. P. G., and Rothman, J. (1996). LIN-12 and GLP-1 are Accepted September 23, 1996
glp-1 and inductions establishing embryonic axes in C. elegans
 Development 120, 2051-2064 (1994) Printed in Great Britain The Company of Biologists Limited 1994 2051 glp-1 and inductions establishing embryonic axes in C. elegans Harald Hutter and Ralf Schnabel Max-Planck-Institut
Development 120, 2051-2064 (1994) Printed in Great Britain The Company of Biologists Limited 1994 2051 glp-1 and inductions establishing embryonic axes in C. elegans Harald Hutter and Ralf Schnabel Max-Planck-Institut
C. elegans Embryonic Development
 Autonomous Specification in Tunicate development Autonomous & Conditional Specification in C. elegans Embryonic Development Figure 8.36 Bilateral Symmetry in the Egg of the Tunicate Styela partita Fig.
Autonomous Specification in Tunicate development Autonomous & Conditional Specification in C. elegans Embryonic Development Figure 8.36 Bilateral Symmetry in the Egg of the Tunicate Styela partita Fig.
MEX-5 and MEX-6 Function to Establish Soma/Germline Asymmetry in Early C. elegans Embryos
 Molecular Cell, Vol. 5, 671 682, April, 2000, Copyright 2000 by Cell Press MEX-5 and MEX-6 Function to Establish Soma/Germline Asymmetry in Early C. elegans Embryos Charlotte M. Schubert,* Rueyling Lin,*
Molecular Cell, Vol. 5, 671 682, April, 2000, Copyright 2000 by Cell Press MEX-5 and MEX-6 Function to Establish Soma/Germline Asymmetry in Early C. elegans Embryos Charlotte M. Schubert,* Rueyling Lin,*
JCB. Myosin and the PAR proteins polarize microfilament-dependent forces that shape and position mitotic spindles in Caenorhabditis elegans
 JCB Published Online: 14 April, 2003 Supp Info: http://doi.org/10.1083/jcb.200210171 Downloaded from jcb.rupress.org on January 15, 2019 Report Myosin and the PAR proteins polarize microfilament-dependent
JCB Published Online: 14 April, 2003 Supp Info: http://doi.org/10.1083/jcb.200210171 Downloaded from jcb.rupress.org on January 15, 2019 Report Myosin and the PAR proteins polarize microfilament-dependent
pod-2, along with pod-1, Defines a New Class of Genes Required for Polarity in the Early Caenorhabditis elegans Embryo
 Developmental Biology 233, 412 424 (2001) doi:10.1006/dbio.2001.0234, available online at http://www.idealibrary.com on pod-2, along with pod-1, Defines a New Class of Genes Required for Polarity in the
Developmental Biology 233, 412 424 (2001) doi:10.1006/dbio.2001.0234, available online at http://www.idealibrary.com on pod-2, along with pod-1, Defines a New Class of Genes Required for Polarity in the
GLP-1 is localized to the mitotic region of the C. elegans germ line
 Development 120, 2901-2911 (1994) Printed in Great Britain The Company of Biologists Limited 1994 2901 GLP-1 is localized to the mitotic region of the C. elegans germ line Sarah L. Crittenden, Emily R.
Development 120, 2901-2911 (1994) Printed in Great Britain The Company of Biologists Limited 1994 2901 GLP-1 is localized to the mitotic region of the C. elegans germ line Sarah L. Crittenden, Emily R.
Problem Set 5 KEY
 2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
Translational control of maternal glp-1 mrna by POS-1 and its interacting protein SPN-4 in Caenorhabditis elegans
 Development 130, 2495-2503 2003 The Company of Biologists Ltd doi:10.1242/dev.00469 2495 Translational control of maternal glp-1 mrna by POS-1 and its interacting protein SPN-4 in Caenorhabditis elegans
Development 130, 2495-2503 2003 The Company of Biologists Ltd doi:10.1242/dev.00469 2495 Translational control of maternal glp-1 mrna by POS-1 and its interacting protein SPN-4 in Caenorhabditis elegans
A Genetic Program for Embryonic Development
 Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Programmed Cell Death (apoptosis)
 Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach
![klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach](/thumbs/91/104639484.jpg) DOI: 10.1038/ncb1891 A. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach embryos let hatch overnight transfer to RNAi plates; incubate 5 days at 15 C RNAi food L1 worms adult worms
DOI: 10.1038/ncb1891 A. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach embryos let hatch overnight transfer to RNAi plates; incubate 5 days at 15 C RNAi food L1 worms adult worms
Biology Developmental Biology Spring Quarter Midterm 1 Version A
 Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Identification of Grandchildless Loci Whose Products Are Required for Normal Germ-Line Development in the Nematode Caenorhabditis elegans
 Copyright 0 1991 by the Genetics Society of America Identification of Grandchildless Loci Whose Products Are Required for Normal Germ-Line Development in the Nematode Caenorhabditis elegans Elizabeth E.
Copyright 0 1991 by the Genetics Society of America Identification of Grandchildless Loci Whose Products Are Required for Normal Germ-Line Development in the Nematode Caenorhabditis elegans Elizabeth E.
Biology 4361 Developmental Biology Exam 1 ID#: October 11, 2005
 Biology 4361 Developmental Biology Name: Key Exam 1 ID#: October 11, 2005 Multiple choice (one point each) 1. Primordial germ cells a. are immortal b. produce polar bodies c. are haploid d. are somatic
Biology 4361 Developmental Biology Name: Key Exam 1 ID#: October 11, 2005 Multiple choice (one point each) 1. Primordial germ cells a. are immortal b. produce polar bodies c. are haploid d. are somatic
Carol Garvin, Richard Holdeman and Susan Strome
 Copyright 1998 by the Genetics Society of America The Phenotype of mes-2, mes-3, mes-4 and mes-6, Maternal-Effect Genes Required for Survival of the Germline in Caenorhabditis elegans, Is Sensitive to
Copyright 1998 by the Genetics Society of America The Phenotype of mes-2, mes-3, mes-4 and mes-6, Maternal-Effect Genes Required for Survival of the Germline in Caenorhabditis elegans, Is Sensitive to
Chapter 11. Robert C. Johnsen and Denise V. Clark. Department of Biological Sciences, Simon Fraser University, Burnaby, B.C.
 Chapter 11 Mapping Genes in C. elegans Robert C. Johnsen and Denise V. Clark Department of Biological Sciences, Simon Fraser University, Burnaby, B.C., Canada, V5A 1S6 Robert C. Johnsen received his B.Sc.
Chapter 11 Mapping Genes in C. elegans Robert C. Johnsen and Denise V. Clark Department of Biological Sciences, Simon Fraser University, Burnaby, B.C., Canada, V5A 1S6 Robert C. Johnsen received his B.Sc.
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment
 Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
meiosis asexual reproduction CHAPTER 9 & 10 The Cell Cycle, Meiosis & Sexual Life Cycles Sexual reproduction mitosis
 meiosis asexual reproduction CHAPTER 9 & 10 The Cell Cycle, Meiosis & Sexual Sexual reproduction Life Cycles mitosis Chromosomes Consists of a long DNA molecule (represents thousands of genes) Also consists
meiosis asexual reproduction CHAPTER 9 & 10 The Cell Cycle, Meiosis & Sexual Sexual reproduction Life Cycles mitosis Chromosomes Consists of a long DNA molecule (represents thousands of genes) Also consists
ALTHOUGH the nematode Caenorhabditis elegans spermatids undergo spermiogenesis after ejaculation
 Copyright 1999 by the Genetics Society of America Sperm Competition in the Absence of Fertilization in Caenorhabditis elegans Andrew Singson, Katherine L. Hill and Steven W. L Hernault Department of Biology,
Copyright 1999 by the Genetics Society of America Sperm Competition in the Absence of Fertilization in Caenorhabditis elegans Andrew Singson, Katherine L. Hill and Steven W. L Hernault Department of Biology,
Linkage Mapping in Drosophila Melanogaster
 Linkage Mapping in Drosophila Melanogaster Genetics: Fall 2012 Joshua Hanau Introduction: An experiment was performed in order to determine the presence and degree of gene linkage in Drosophila Melanogaster.
Linkage Mapping in Drosophila Melanogaster Genetics: Fall 2012 Joshua Hanau Introduction: An experiment was performed in order to determine the presence and degree of gene linkage in Drosophila Melanogaster.
Sexual Reproduction. For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species.
 Sex Determination Sexual Reproduction For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species. Meiosis in the sexual organs of parents produces haploid
Sex Determination Sexual Reproduction For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species. Meiosis in the sexual organs of parents produces haploid
Biology 4361 Developmental Biology. October 11, Multiple choice (one point each)
 Biology 4361 Developmental Biology Exam 1 October 11, 2005 Name: ID#: Multiple choice (one point each) 1. Sertoli cells a. surround spermatocytes b. are the structural components of the seminiferous tubules
Biology 4361 Developmental Biology Exam 1 October 11, 2005 Name: ID#: Multiple choice (one point each) 1. Sertoli cells a. surround spermatocytes b. are the structural components of the seminiferous tubules
Cellular innovations at the origin of new reproductive modes: the case of pseudogamy in nematodes
 Cellular innovations at the origin of new reproductive modes: the case of pseudogamy in nematodes Marie Delattre Plasticité et Evolution de la Division Cellulaire ENS Lyon Marie-Anne Félix Evolution des
Cellular innovations at the origin of new reproductive modes: the case of pseudogamy in nematodes Marie Delattre Plasticité et Evolution de la Division Cellulaire ENS Lyon Marie-Anne Félix Evolution des
TO divide, a cell must be able to replicate its DNA, to exploring the mechanisms of cell division. In addition
 Copyright 1998 by the Genetics Society of America A Genetic Screen for Temperature-Sensitive Cell-Division Mutants of Caenorhabditis elegans Kevin F. O Connell,* Charles M. Leys* and John G. White*, *Laboratory
Copyright 1998 by the Genetics Society of America A Genetic Screen for Temperature-Sensitive Cell-Division Mutants of Caenorhabditis elegans Kevin F. O Connell,* Charles M. Leys* and John G. White*, *Laboratory
Cell cycle and apoptosis
 Cell cycle and apoptosis Cell cycle Definition Stages and steps Cell cycle Interphase (G1/G0, S, and G2) Mitosis (prophase, metaphase, anaphase, telophase, karyokinesis, cytokinesis) Control checkpoints
Cell cycle and apoptosis Cell cycle Definition Stages and steps Cell cycle Interphase (G1/G0, S, and G2) Mitosis (prophase, metaphase, anaphase, telophase, karyokinesis, cytokinesis) Control checkpoints
Transgenerational epigenetics in the germline cycle of Caenorhabditis elegans
 Transgenerational epigenetics in the germline cycle of Caenorhabditis elegans William G Kelly, Emory University Journal Title: Epigenetics and Chromatin Volume: Volume 7, Number 6 Publisher: BioMed Central
Transgenerational epigenetics in the germline cycle of Caenorhabditis elegans William G Kelly, Emory University Journal Title: Epigenetics and Chromatin Volume: Volume 7, Number 6 Publisher: BioMed Central
CAENORHABDITIS ELEGANS
 NONDISJUNCTION MUTANTS OF THE NEMATODE CAENORHABDITIS ELEGANS JONATHAN HODGKIN, H. ROBERT HORVITL* AND SYDNEY BRENNER M.R.C. Lrrboratory of Molecular Biology, Hills Road, Cambridge, CB2 ZQH, England Manuscript
NONDISJUNCTION MUTANTS OF THE NEMATODE CAENORHABDITIS ELEGANS JONATHAN HODGKIN, H. ROBERT HORVITL* AND SYDNEY BRENNER M.R.C. Lrrboratory of Molecular Biology, Hills Road, Cambridge, CB2 ZQH, England Manuscript
MCB140: Second Midterm Spring 2010
 MCB140: Second Midterm Spring 2010 Before you start, print your name and student identification number (S.I.D) at the top of each page. There are 11 pages including this page. You will have 150 minutes
MCB140: Second Midterm Spring 2010 Before you start, print your name and student identification number (S.I.D) at the top of each page. There are 11 pages including this page. You will have 150 minutes
Why do cells reproduce?
 Outline Cell Reproduction 1. Overview of Cell Reproduction 2. Cell Reproduction in Prokaryotes 3. Cell Reproduction in Eukaryotes 1. Chromosomes 2. Cell Cycle 3. Mitosis and Cytokinesis Examples of Cell
Outline Cell Reproduction 1. Overview of Cell Reproduction 2. Cell Reproduction in Prokaryotes 3. Cell Reproduction in Eukaryotes 1. Chromosomes 2. Cell Cycle 3. Mitosis and Cytokinesis Examples of Cell
Early Embryonic Development
 Early Embryonic Development Maternal effect gene products set the stage by controlling the expression of the first embryonic genes. 1. Transcription factors 2. Receptors 3. Regulatory proteins Maternal
Early Embryonic Development Maternal effect gene products set the stage by controlling the expression of the first embryonic genes. 1. Transcription factors 2. Receptors 3. Regulatory proteins Maternal
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis
 Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Chromosomes Chromosomes were first observed by the German embryologist Walther Fleming in 1882. Chromosome number varies among organisms most
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Chromosomes Chromosomes were first observed by the German embryologist Walther Fleming in 1882. Chromosome number varies among organisms most
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis
 Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Prokaryotes Have a Simpler Cell Cycle Cell division in prokaryotes takes place in two stages, which together make up a simple cell cycle 1. Copy
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Prokaryotes Have a Simpler Cell Cycle Cell division in prokaryotes takes place in two stages, which together make up a simple cell cycle 1. Copy
Axis Formation and Mesoderm Induction
 Developmental Biology Biology 4361 Axis Formation and Mesoderm Induction October 27, 2005 Amphibian anteroposterior specification polarized eggs animal/vegetal pigment yolk v. clear cytoplasm mitochondrial
Developmental Biology Biology 4361 Axis Formation and Mesoderm Induction October 27, 2005 Amphibian anteroposterior specification polarized eggs animal/vegetal pigment yolk v. clear cytoplasm mitochondrial
Suppressors of glp-1, a Gene Required for Cell Communication During Development in Caenorhabditis elegans, Define a Set of Interacting Genes
 Syracuse University SURFACE Biology College of Arts and Sciences 1993 Suppressors of glp-1, a Gene Required for Cell Communication During Development in Caenorhabditis elegans, Define a Set of Interacting
Syracuse University SURFACE Biology College of Arts and Sciences 1993 Suppressors of glp-1, a Gene Required for Cell Communication During Development in Caenorhabditis elegans, Define a Set of Interacting
MSP and GLP-1/Notch signaling coordinately regulate actomyosin-dependent cytoplasmic streaming and oocyte growth in C. elegans
 RESEARCH ARTICLE 2223 Development 136, 2223-2234 (2009) doi:10.1242/dev.034603 MSP and GLP-1/Notch signaling coordinately regulate actomyosin-dependent cytoplasmic streaming and oocyte growth in C. elegans
RESEARCH ARTICLE 2223 Development 136, 2223-2234 (2009) doi:10.1242/dev.034603 MSP and GLP-1/Notch signaling coordinately regulate actomyosin-dependent cytoplasmic streaming and oocyte growth in C. elegans
Midterm 1. Number of students Score. Mean: 73 Median: 75 Top Score: 98
 Midterm 1 14 12 Number of students 10 8 6 4 2 0 35-40 41-45 Mean: 73 Median: 75 Top Score: 98 46-50 51-55 56-60 61-65 66-70 71-75 Score 76-80 81-85 86-90 91-95 96-100 Write your name and student ID# on
Midterm 1 14 12 Number of students 10 8 6 4 2 0 35-40 41-45 Mean: 73 Median: 75 Top Score: 98 46-50 51-55 56-60 61-65 66-70 71-75 Score 76-80 81-85 86-90 91-95 96-100 Write your name and student ID# on
Chapter 4 The Chromosome Theory of Inheritance
 Chapter 4 The Chromosome Theory of Inheritance 4-1 Sections to study 4.1 Chromosomes: The carriers of genes 4.2 Mitosis: Cell division that preserves chromosome number 4.3 Meiosis: Cell division that halve
Chapter 4 The Chromosome Theory of Inheritance 4-1 Sections to study 4.1 Chromosomes: The carriers of genes 4.2 Mitosis: Cell division that preserves chromosome number 4.3 Meiosis: Cell division that halve
General Embryology. School of Medicine Department of Anatomy and Histology School of medicine The University of Jordan
 General Embryology 2019 School of Medicine Department of Anatomy and Histology School of medicine The University of Jordan https://www.facebook.com/dramjad-shatarat What is embryology? Is the science that
General Embryology 2019 School of Medicine Department of Anatomy and Histology School of medicine The University of Jordan https://www.facebook.com/dramjad-shatarat What is embryology? Is the science that
Chapter 2. Mitosis and Meiosis
 Chapter 2. Mitosis and Meiosis Chromosome Theory of Heredity What structures within cells correspond to genes? The development of genetics took a major step forward by accepting the notion that the genes
Chapter 2. Mitosis and Meiosis Chromosome Theory of Heredity What structures within cells correspond to genes? The development of genetics took a major step forward by accepting the notion that the genes
Regulation of Gene Expression in Eukaryotes
 Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
7.013 Problem Set 5 FRIDAY April 2nd, 2004
 MIT Department of Biology 7.013: Introductory Biology - Spring 2004 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Problem Set 5 FRIDAY April 2nd, 2004 Problem sets
MIT Department of Biology 7.013: Introductory Biology - Spring 2004 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Problem Set 5 FRIDAY April 2nd, 2004 Problem sets
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10643 Supplementary Table 1. Identification of hecw-1 coding polymorphisms at amino acid positions 322 and 325 in 162 strains of C. elegans. WWW.NATURE.COM/NATURE 1 Supplementary Figure
doi:10.1038/nature10643 Supplementary Table 1. Identification of hecw-1 coding polymorphisms at amino acid positions 322 and 325 in 162 strains of C. elegans. WWW.NATURE.COM/NATURE 1 Supplementary Figure
The subcortical maternal complex controls symmetric division of mouse zygotes by
 The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics Xing-Jiang Yu 1,2, Zhaohong Yi 1, Zheng Gao 1,2, Dan-dan Qin 1,2, Yanhua Zhai 1, Xue Chen 1,
The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics Xing-Jiang Yu 1,2, Zhaohong Yi 1, Zheng Gao 1,2, Dan-dan Qin 1,2, Yanhua Zhai 1, Xue Chen 1,
life Lab 7 Centromere region One (replicated) chromosome Sister Figure I. The Cell Cycle. Figure 2. A Replicated Chromosome.
 71 life.. -' - \ Lab 7 Cell Division Cellular reproduction in the cells is accomplished by mitosis or meiosis. The chromosomes of the cell have to repli cate themselves in both processes and then move
71 life.. -' - \ Lab 7 Cell Division Cellular reproduction in the cells is accomplished by mitosis or meiosis. The chromosomes of the cell have to repli cate themselves in both processes and then move
Foundational questions Oocyte-derived functional mediators of early embryonic development (EST and candidate gene) JY-1 Nobox Importin 8 Oocyte and cu
 Models for study of oocyte competence: George W. Smith (Smithge7@msu.edu) Foundational questions Oocyte-derived functional mediators of early embryonic development (EST and candidate gene) JY-1 Nobox Importin
Models for study of oocyte competence: George W. Smith (Smithge7@msu.edu) Foundational questions Oocyte-derived functional mediators of early embryonic development (EST and candidate gene) JY-1 Nobox Importin
Patterns of Single-Gene Inheritance Cont.
 Genetic Basis of Disease Patterns of Single-Gene Inheritance Cont. Traditional Mechanisms Chromosomal disorders Single-gene gene disorders Polygenic/multifactorial disorders Novel mechanisms Imprinting
Genetic Basis of Disease Patterns of Single-Gene Inheritance Cont. Traditional Mechanisms Chromosomal disorders Single-gene gene disorders Polygenic/multifactorial disorders Novel mechanisms Imprinting
GENETICS - NOTES-
 GENETICS - NOTES- Warm Up Exercise Using your previous knowledge of genetics, determine what maternal genotype would most likely yield offspring with such characteristics. Use the genotype that you came
GENETICS - NOTES- Warm Up Exercise Using your previous knowledge of genetics, determine what maternal genotype would most likely yield offspring with such characteristics. Use the genotype that you came
Structure of Ovaries
 Structure of Ovaries What Are Oocyte Accessory Cells? Follicle cells somatic connected by desmosomes, gap junctions, interdigitating microvilli for anchorage and exchange function in yolk transport, egg
Structure of Ovaries What Are Oocyte Accessory Cells? Follicle cells somatic connected by desmosomes, gap junctions, interdigitating microvilli for anchorage and exchange function in yolk transport, egg
Article. Regulation of Sperm Activation by SWM-1 Is Required for Reproductive Success of C. elegans Males
 Current Biology 16, 252 263, February 7, 2006 ª2006 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2005.12.041 Regulation of Sperm Activation by SWM-1 Is Required for Reproductive Success of C. elegans
Current Biology 16, 252 263, February 7, 2006 ª2006 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2005.12.041 Regulation of Sperm Activation by SWM-1 Is Required for Reproductive Success of C. elegans
Cancer. October is National Breast Cancer Awareness Month
 Cancer October is National Breast Cancer Awareness Month Objectives 1: Gene regulation Explain how cells in all the different parts of your body develop such different characteristics and functions. Contrast
Cancer October is National Breast Cancer Awareness Month Objectives 1: Gene regulation Explain how cells in all the different parts of your body develop such different characteristics and functions. Contrast
Nature Genetics: doi: /ng Supplementary Figure 1. Immunofluorescence (IF) confirms absence of H3K9me in met-2 set-25 worms.
 Supplementary Figure 1 Immunofluorescence (IF) confirms absence of H3K9me in met-2 set-25 worms. IF images of wild-type (wt) and met-2 set-25 worms showing the loss of H3K9me2/me3 at the indicated developmental
Supplementary Figure 1 Immunofluorescence (IF) confirms absence of H3K9me in met-2 set-25 worms. IF images of wild-type (wt) and met-2 set-25 worms showing the loss of H3K9me2/me3 at the indicated developmental
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division The Cell Cycle: Cell Growth, Cell Division 2007-2008 2007-2008 Getting from there to here Going from egg to baby. the original
Biology is the only subject in which multiplication is the same thing as division The Cell Cycle: Cell Growth, Cell Division 2007-2008 2007-2008 Getting from there to here Going from egg to baby. the original
Human Molecular Genetics Prof. S. Ganesh Department of Biological Sciences and Bioengineering Indian Institute of Technology, Kanpur
 Human Molecular Genetics Prof. S. Ganesh Department of Biological Sciences and Bioengineering Indian Institute of Technology, Kanpur Module - 02 Lecture - 06 Let us test your understanding of Pedigree
Human Molecular Genetics Prof. S. Ganesh Department of Biological Sciences and Bioengineering Indian Institute of Technology, Kanpur Module - 02 Lecture - 06 Let us test your understanding of Pedigree
SEX DETERMINATION AND SEX CHROMOSOMES
 Klug et al. 2006, 2009 Concepts of Genetics Chapter 7 STUDY UNIT 5 SEX DETERMINATION AND SEX CHROMOSOMES Some species reproduce asexually Most diploid eukaryotes reproduce sexually Parent (2n) Parent (2n)
Klug et al. 2006, 2009 Concepts of Genetics Chapter 7 STUDY UNIT 5 SEX DETERMINATION AND SEX CHROMOSOMES Some species reproduce asexually Most diploid eukaryotes reproduce sexually Parent (2n) Parent (2n)
Identification of genes that interact with glp-1, a gene required for inductive cell interactions in Caenorhabditis elegans
 Development 15, 133-143 (1989) Printed in Great Britain The Company of Biologists Limited 1989 133 Identification of genes that interact with glp-1, a gene required for iuctive cell interactions in Caenorhabditis
Development 15, 133-143 (1989) Printed in Great Britain The Company of Biologists Limited 1989 133 Identification of genes that interact with glp-1, a gene required for iuctive cell interactions in Caenorhabditis
Daniela Drummond-Barbosa, Ph.D. Department of Biochemistry and Molecular Biology
 Control of stem cells by diet and systemic factors in the Drosophila ovary Daniela Drummond-Barbosa, Ph.D. Department of Biochemistry and Molecular Biology Multiple levels of stem cell regulation local
Control of stem cells by diet and systemic factors in the Drosophila ovary Daniela Drummond-Barbosa, Ph.D. Department of Biochemistry and Molecular Biology Multiple levels of stem cell regulation local
Karen L.P. McNally, Amy S. Fabritius, Marina L. Ellefson, Jonathan R. Flynn, Jennifer A. Milan, and Francis J. McNally
 Developmental Cell, Volume 22 Supplemental Information Kinesin-1 Prevents Capture of the Oocyte Meiotic Spindle by the Sperm Aster Karen L.P. McNally, Amy S. Fabritius, Marina L. Ellefson, Jonathan R.
Developmental Cell, Volume 22 Supplemental Information Kinesin-1 Prevents Capture of the Oocyte Meiotic Spindle by the Sperm Aster Karen L.P. McNally, Amy S. Fabritius, Marina L. Ellefson, Jonathan R.
Unit 4: Cell Division Guided Notes
 Unit 4: Cell Division Guided Notes 1 Chromosomes are structures that contain material When Eukaryotes are not dividing, DNA and Proteins are in a mass called: When the cell divides, it condenses and becomes
Unit 4: Cell Division Guided Notes 1 Chromosomes are structures that contain material When Eukaryotes are not dividing, DNA and Proteins are in a mass called: When the cell divides, it condenses and becomes
The Cell Cycle and How Cells Divide
 The Cell Cycle and How Cells Divide 1 Phases of the Cell Cycle The cell cycle consists of Interphase normal cell activity The mitotic phase cell divsion INTERPHASE Growth G 1 (DNA synthesis) Growth G 2
The Cell Cycle and How Cells Divide 1 Phases of the Cell Cycle The cell cycle consists of Interphase normal cell activity The mitotic phase cell divsion INTERPHASE Growth G 1 (DNA synthesis) Growth G 2
Inheritance of Aldehyde Oxidase in Drosophila melanogaster
 Inheritance of Aldehyde Oxidase in Drosophila melanogaster (adapted from Morgan, J. G. and V. Finnerty. 1991. Inheritance of aldehyde oxidase in Drosophilia melanogaster. Pages 33-47, in Tested studies
Inheritance of Aldehyde Oxidase in Drosophila melanogaster (adapted from Morgan, J. G. and V. Finnerty. 1991. Inheritance of aldehyde oxidase in Drosophilia melanogaster. Pages 33-47, in Tested studies
Gene Regulation. Bacteria. Chapter 18: Regulation of Gene Expression
 Chapter 18: Regulation of Gene Expression A Biology 2013 1 Gene Regulation rokaryotes and eukaryotes alter their gene expression in response to their changing environment In multicellular eukaryotes, gene
Chapter 18: Regulation of Gene Expression A Biology 2013 1 Gene Regulation rokaryotes and eukaryotes alter their gene expression in response to their changing environment In multicellular eukaryotes, gene
-19. -Mousa Salah. -Shahd Alqudah. -Dr Belal
 التزام -19 -Mousa Salah -Shahd Alqudah -Dr Belal 1 P a g e In the previous lecture we talked about the numerical chromosomal abnormalities, they are either autosomal or sex, and we said that the chromosomal
التزام -19 -Mousa Salah -Shahd Alqudah -Dr Belal 1 P a g e In the previous lecture we talked about the numerical chromosomal abnormalities, they are either autosomal or sex, and we said that the chromosomal
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Getting from there to here Going from egg to baby. the original
Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Getting from there to here Going from egg to baby. the original
BIOLOGY - CLUTCH CH.15 - CHROMOSOMAL THEORY OF INHERITANCE
 !! www.clutchprep.com Chromosomal theory of inheritance: chromosomes are the carriers of genetic material. Independent Assortment alleles for different characters sort independently of each other during
!! www.clutchprep.com Chromosomal theory of inheritance: chromosomes are the carriers of genetic material. Independent Assortment alleles for different characters sort independently of each other during
IN THE NEMATODE CAENORHABDZTZS ELEGANS
 MUTATIONS CAUSING TRANSFORMATION OF SEXUAL PHENOTYPE IN THE NEMATODE CAENORHABDZTZS ELEGANS JONATHAN A. HODGKIN AND SYDNEY BRENNER MCR Laboratory of Molecular Biology, Hills Road, Cambridge CB2 ZQH, England
MUTATIONS CAUSING TRANSFORMATION OF SEXUAL PHENOTYPE IN THE NEMATODE CAENORHABDZTZS ELEGANS JONATHAN A. HODGKIN AND SYDNEY BRENNER MCR Laboratory of Molecular Biology, Hills Road, Cambridge CB2 ZQH, England
Lecture 17: Human Genetics. I. Types of Genetic Disorders. A. Single gene disorders
 Lecture 17: Human Genetics I. Types of Genetic Disorders A. Single gene disorders B. Multifactorial traits 1. Mutant alleles at several loci acting in concert C. Chromosomal abnormalities 1. Physical changes
Lecture 17: Human Genetics I. Types of Genetic Disorders A. Single gene disorders B. Multifactorial traits 1. Mutant alleles at several loci acting in concert C. Chromosomal abnormalities 1. Physical changes
Meiosis. 4. There are multiple alleles for the ABO blood group. Why are there only two of these alleles normally present in any one individual?
 Name: ate: 1. The diagram shown represents a cell that will undergo mitosis. Which diagrams below best illustrate the nuclei of the daughter cells that result from a normal mitotic cell division of the
Name: ate: 1. The diagram shown represents a cell that will undergo mitosis. Which diagrams below best illustrate the nuclei of the daughter cells that result from a normal mitotic cell division of the
Cell Division Questions. Mitosis and Meiosis
 Cell Division Questions Mitosis and Meiosis 1 10 Do not write outside the box 5 Figure 3 shows a pair of chromosomes at the start of meiosis. The letters represent alleles. Figure 3 E E e e F F f f 5 (a)
Cell Division Questions Mitosis and Meiosis 1 10 Do not write outside the box 5 Figure 3 shows a pair of chromosomes at the start of meiosis. The letters represent alleles. Figure 3 E E e e F F f f 5 (a)
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
Animal Development. Lecture 3. Germ Cells and Sex
 Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
Regulation of Gene Expression
 Chapter 18 Regulation of Gene Expression PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
Chapter 18 Regulation of Gene Expression PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
By Mir Mohammed Abbas II PCMB 'A' CHAPTER CONCEPT NOTES
 Chapter Notes- Genetics By Mir Mohammed Abbas II PCMB 'A' 1 CHAPTER CONCEPT NOTES Relationship between genes and chromosome of diploid organism and the terms used to describe them Know the terms Terms
Chapter Notes- Genetics By Mir Mohammed Abbas II PCMB 'A' 1 CHAPTER CONCEPT NOTES Relationship between genes and chromosome of diploid organism and the terms used to describe them Know the terms Terms
Cellular Reproduction, Part 2: Meiosis Lecture 10 Fall 2008
 Mitosis & 1 Cellular Reproduction, Part 2: Lecture 10 Fall 2008 Mitosis Form of cell division that leads to identical daughter cells with the full complement of DNA Occurs in somatic cells Cells of body
Mitosis & 1 Cellular Reproduction, Part 2: Lecture 10 Fall 2008 Mitosis Form of cell division that leads to identical daughter cells with the full complement of DNA Occurs in somatic cells Cells of body
On the Control of Oocyte Meiotic Maturation and Ovulation in Caenorhabditis elegans
 Developmental Biology 205, 111 128 (1999) Article ID dbio.1998.9109, available online at http://www.idealibrary.com on On the Control of Oocyte Meiotic Maturation and Ovulation in Caenorhabditis elegans
Developmental Biology 205, 111 128 (1999) Article ID dbio.1998.9109, available online at http://www.idealibrary.com on On the Control of Oocyte Meiotic Maturation and Ovulation in Caenorhabditis elegans
A gene is a sequence of DNA that resides at a particular site on a chromosome the locus (plural loci). Genetic linkage of genes on a single
 8.3 A gene is a sequence of DNA that resides at a particular site on a chromosome the locus (plural loci). Genetic linkage of genes on a single chromosome can alter their pattern of inheritance from those
8.3 A gene is a sequence of DNA that resides at a particular site on a chromosome the locus (plural loci). Genetic linkage of genes on a single chromosome can alter their pattern of inheritance from those
Exam #2 BSC Fall. NAME_Key correct answers in BOLD FORM A
 Exam #2 BSC 2011 2004 Fall NAME_Key correct answers in BOLD FORM A Before you begin, please write your name and social security number on the computerized score sheet. Mark in the corresponding bubbles
Exam #2 BSC 2011 2004 Fall NAME_Key correct answers in BOLD FORM A Before you begin, please write your name and social security number on the computerized score sheet. Mark in the corresponding bubbles
GENES AND CHROMOSOMES CHAPTER 5
 CHAPTER 5 GENES AND CHROMOSOMES For many years the standard authority on the chromosomes was Wilson s The Cell in Development and Inheritance. The second edition of this work was published in 1900; it
CHAPTER 5 GENES AND CHROMOSOMES For many years the standard authority on the chromosomes was Wilson s The Cell in Development and Inheritance. The second edition of this work was published in 1900; it
Specification of the anteroposterior axis in Caenorhabditis elegans
 Development 122, 1467-1474 (1996) rinted in Great Britain The Company of Biologists Limited 1996 DEV8317 1467 Specification of the anteroposterior axis in Caenorhabditis elegans Bob Goldstein and Steven
Development 122, 1467-1474 (1996) rinted in Great Britain The Company of Biologists Limited 1996 DEV8317 1467 Specification of the anteroposterior axis in Caenorhabditis elegans Bob Goldstein and Steven
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division Ch. 10 Where it all began You started as a cell smaller than a period
Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division Ch. 10 Where it all began You started as a cell smaller than a period
The vagaries of non-traditional mendelian recessive inheritance in uniparental disomy: AA x Aa = aa!
 Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL AT INIST-CNRS Deep Insight Section The vagaries of non-traditional mendelian recessive inheritance in uniparental disomy:
Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL AT INIST-CNRS Deep Insight Section The vagaries of non-traditional mendelian recessive inheritance in uniparental disomy:
Genetics Review. Alleles. The Punnett Square. Genotype and Phenotype. Codominance. Incomplete Dominance
 Genetics Review Alleles These two different versions of gene A create a condition known as heterozygous. Only the dominant allele (A) will be expressed. When both chromosomes have identical copies of the
Genetics Review Alleles These two different versions of gene A create a condition known as heterozygous. Only the dominant allele (A) will be expressed. When both chromosomes have identical copies of the
5/25/2015. Replication fork. Replication fork. Replication fork. Replication fork
 Mutations Chapter 5 Cellular Functions Lecture 3: and Cell Division Most DNA mutations alter the protein product May Make it function better (rarely) Change its function Reduce its function Make it non-functional
Mutations Chapter 5 Cellular Functions Lecture 3: and Cell Division Most DNA mutations alter the protein product May Make it function better (rarely) Change its function Reduce its function Make it non-functional
Cell Division. The Process of Cell Division Section Section 10.2: The Process of Cell Division 12/8/2010
 The Process of Cell Division Section 10.2 Biology B Section 10.2: The Process of Cell Division The student will investigate and understand common mechanisms of inheritance and protein synthesis. Key concepts
The Process of Cell Division Section 10.2 Biology B Section 10.2: The Process of Cell Division The student will investigate and understand common mechanisms of inheritance and protein synthesis. Key concepts
BSC 2010C SI EXAM 3 REVIEW REVIEW SESSION AT: Wednesday, 12 2 PM In CB2 Room 105
 BSC 2010C SI EXAM 3 REVIEW REVIEW SESSION AT: Wednesday, 7/26 @ 12 2 PM In CB2 Room 105 Ch. 10 1) Where does the light cycle happen? Thylakoid membrane 2) Where does the calvin cycle happen? Stroma Ch.
BSC 2010C SI EXAM 3 REVIEW REVIEW SESSION AT: Wednesday, 7/26 @ 12 2 PM In CB2 Room 105 Ch. 10 1) Where does the light cycle happen? Thylakoid membrane 2) Where does the calvin cycle happen? Stroma Ch.
Cell Division and Inheritance
 Cell Division and Inheritance Continuing life relies on reproduction Individual organism replacing dead or damaged cells Species making more of same species Reproduction Cells divide, grow, divide again
Cell Division and Inheritance Continuing life relies on reproduction Individual organism replacing dead or damaged cells Species making more of same species Reproduction Cells divide, grow, divide again
Excretion. 2- The kidney s filtrate consists of a) solutes b) urea c) water d) vitamins e) all of the above choices are correct
 Excretion 1- Homeostasis is the : a) ability to regulate internal environment. b) maintenance of steady internal conditions despite fluctuations in the external environment. c) maintenance of internal
Excretion 1- Homeostasis is the : a) ability to regulate internal environment. b) maintenance of steady internal conditions despite fluctuations in the external environment. c) maintenance of internal
Cell Division. Chromosome structure. Made of chromatin (mix of DNA and protein) Only visible during cell division
 Chromosome structure Made of chromatin (mix of DNA and protein) Only visible during cell division Chromosome structure The DNA in a cell is packed into an elaborate, multilevel system of coiling and folding.
Chromosome structure Made of chromatin (mix of DNA and protein) Only visible during cell division Chromosome structure The DNA in a cell is packed into an elaborate, multilevel system of coiling and folding.
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
APGRU4L1 Chap 12 Extra Reading Cell Cycle and Mitosis
 APGRU4L1 Chap 12 Extra Reading Cell Cycle and Mitosis Dr. Ramesh Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008
APGRU4L1 Chap 12 Extra Reading Cell Cycle and Mitosis Dr. Ramesh Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008
Genetics and Cellular Function
 Genetics and Cellular Function DNA replication and the cell cycle Mitosis Mitosis Mitosis: division of cells that results in daughter cells with the same the genetic information that the original cell
Genetics and Cellular Function DNA replication and the cell cycle Mitosis Mitosis Mitosis: division of cells that results in daughter cells with the same the genetic information that the original cell
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
A Role for Set1/MLL-Related Components in Epigenetic Regulation of the Caenorhabditis elegans Germ Line
 A Role for Set1/MLL-Related Components in Epigenetic Regulation of the Caenorhabditis elegans Germ Line Tengguo Li, Emory University William G Kelly, Emory University Journal Title: PLoS Genetics Volume:
A Role for Set1/MLL-Related Components in Epigenetic Regulation of the Caenorhabditis elegans Germ Line Tengguo Li, Emory University William G Kelly, Emory University Journal Title: PLoS Genetics Volume:
Biology Unit III Exam» Form C
 Directions: For each of the following questions, decide which of the choices is best and fill in the corresponding space on the answer document. 1. Which of these sets of chromosomes is found in a single
Directions: For each of the following questions, decide which of the choices is best and fill in the corresponding space on the answer document. 1. Which of these sets of chromosomes is found in a single
1042SCG Genetics & Evolutionary Biology Semester Summary
 1042SCG Genetics & Evolutionary Biology Semester Summary Griffith University, Nathan Campus Semester 1, 2014 Topics include: - Mendelian Genetics - Eukaryotic & Prokaryotic Genes - Sex Chromosomes - Variations
1042SCG Genetics & Evolutionary Biology Semester Summary Griffith University, Nathan Campus Semester 1, 2014 Topics include: - Mendelian Genetics - Eukaryotic & Prokaryotic Genes - Sex Chromosomes - Variations
To General Embryology Dr: Azza Zaki
 Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Molecular Determination of Gender in Drosophila
 Molecular Determination of Gender in Drosophila 5th edition 20.1.3 Alternate Splicing of RNA p. 595 20.2.2 Proteins Involved in Control of Transcription: Transcription Factors p. 602 20.6.2 Hyperactivation
Molecular Determination of Gender in Drosophila 5th edition 20.1.3 Alternate Splicing of RNA p. 595 20.2.2 Proteins Involved in Control of Transcription: Transcription Factors p. 602 20.6.2 Hyperactivation
DRB666 Applied Developmental and Reproductive Biology (Spring 2013)
 DRB666 Applied Developmental and Reproductive Biology (Spring 2013) Director: 651 Ilalo Street, BSB163-3 e-mail: yyamazak@hawaii.edu Phone: (808) 692-1416 Instructors (e-mail): Steve Ward Yusuke Marikawa
DRB666 Applied Developmental and Reproductive Biology (Spring 2013) Director: 651 Ilalo Street, BSB163-3 e-mail: yyamazak@hawaii.edu Phone: (808) 692-1416 Instructors (e-mail): Steve Ward Yusuke Marikawa
Gametogenesis. To complete this worksheet, select: Module: Continuity Activity: Animations Title: Gametogenesis. Introduction
 Gametogenesis To complete this worksheet, select: Module: Continuity Activity: Animations Title: Gametogenesis Introduction 1. a. Define gametogenesis. b. What cells are gametes? c. What are the two cell
Gametogenesis To complete this worksheet, select: Module: Continuity Activity: Animations Title: Gametogenesis Introduction 1. a. Define gametogenesis. b. What cells are gametes? c. What are the two cell
Chromosomes and Cell Cycle
 Chromosomes and Cell Cycle Cell Basics There are trillions of cells in your body Cells are microscopic Cells have DNA inside a structure called the nucleus The nucleus is enclosed by a structure called
Chromosomes and Cell Cycle Cell Basics There are trillions of cells in your body Cells are microscopic Cells have DNA inside a structure called the nucleus The nucleus is enclosed by a structure called
