Carotid revascularization and medical management for asymptomatic carotid stenosis: Protocol of the CREST-2 clinical trials
|
|
|
- Frederica Poole
- 6 years ago
- Views:
Transcription
1 Protocol Carotid revascularization and medical management for asymptomatic carotid stenosis: Protocol of the CREST-2 clinical trials International Journal of Stroke 0(0) 1 9! 2017 World Stroke Organization Reprints and permissions: sagepub.co.uk/journalspermissions.nav DOI: / journals.sagepub.com/home/wso Virginia J Howard 2, James F Meschia 1, Brajesh K Lal 3, Tanya N Turan 4, Gary S Roubin 5, Robert D Brown Jr 6, Jenifer H Voeks 4, Kevin M Barrett 1, Bart M Demaerschalk 7, John Huston III 8, Ronald M Lazar 9, Wesley S Moore 10, Virginia G Wadley 11, Seemant Chaturvedi 12, Claudia S Moy 13, Marc Chimowitz 4, George Howard 14 and Thomas G Brott 1 ;on behalf of the CREST-2 study investigators Abstract Rationale: Trials conducted decades ago demonstrated that carotid endarterectomy by skilled surgeons reduced stroke risk in asymptomatic patients. Developments in carotid stenting and improvements in medical prevention of stroke caused by atherothrombotic disease challenge understanding of the benefits of revascularization. Aim: Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial (CREST-2) will test whether carotid endarterectomy or carotid stenting plus contemporary intensive medical therapy is superior to intensive medical therapy alone in the primary prevention of stroke in patients with high-grade asymptomatic carotid stenosis. Methods and design: CREST-2 is two multicenter randomized trials of revascularization plus intensive medical therapy versus intensive medical therapy alone. One trial randomizes patients to carotid endarterectomy plus intensive medical therapy versus intensive medical therapy alone; the other, to carotid stenting plus intensive medical therapy versus intensive medical therapy alone. The risk factor targets of centrally directed intensive medical therapy are LDL cholesterol <70 mg/dl and systolic blood pressure <140 mmhg. Study outcomes: The primary outcome is the composite of stroke and death within 44 days following randomization and stroke ipsilateral to the target vessel thereafter, up to four years. Change in cognition and differences in major and minor stroke are secondary outcomes. Sample size: Enrollment of 1240 patients in each trial provides 85% power to detect a treatment difference if the event rate in the intensive medical therapy alone arm is 4.8% higher or 2.8% lower than an anticipated 3.6% rate in the revascularization arm. 1 Department of Neurology (JFM, KMB, TGB), Mayo Clinic, Jacksonville, FL, USA 2 Department of Epidemiology (VJH), University of Alabama at Birmingham, Birmingham, AL, USA 3 Department of Surgery (BKL), University of Maryland, Baltimore, MD, USA 4 Department of Neurology (TNT, JHV, MIC), Medical University of South Carolina, Charleston, SC, USA 5 Department of Cardiology (GSR), Brookwood Medical Center, Birmingham, AL, USA 6 Department of Neurology (RDB), Mayo Clinic, Rochester, MN, USA 7 Department of Neurology (BMD), Mayo Clinic, Phoenix, AZ, USA 8 Department of Neuroradiology (JH), Mayo Clinic, Rochester, MN, USA 9 Department of Neurology (RML), Columbia University, New York, NY, USA 10 Department of Surgery (WSM), University of California Los Angeles, Los Angeles, CA, USA 11 Department of Medicine (VGW), University of Alabama at Birmingham, Birmingham, AL, USA 12 Department of Neurology & Stroke Program (SC), University of Miami Miller School of Medicine, Miami, FL, USA 13 Department of Health & Human Services (CSM), National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA 14 Department of Biostatistics (GH), University of Alabama at Birmingham, Birmingham, AL, USA Corresponding author: Thomas G Brott, Mayo Clinic, 4500 San Pablo Road,Jacksonville, Florida, FL 32224, USA. brott.thomas@mayo.edu
2 2 International Journal of Stroke 0(0) Discussion: Management of asymptomatic carotid stenosis requires contemporary randomized trials to address whether carotid endarterectomy or carotid stenting plus intensive medical therapy is superior in preventing stroke beyond intensive medical therapy alone. Whether carotid endarterectomy or carotid stenting has favorable effects on cognition will also be tested. Trial registration: United States National Institutes of Health Clinicaltrials.gov NCT Keywords Carotid endarterectomy, carotid stenting, asymptomatic carotid stenosis, medical treatment, randomized clinical trial, stroke prevention, cognitive functioning Received: 30 January 2017; accepted: 10 March 2017 Introduction Revascularization of the carotid artery by either endarterectomy or stenting is used as a means of preventing first-time and recurrent cerebral infarction. None of the pivotal trials utilized an intensive medical management (IMM) program. 1 4 The Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial (CREST-2) is two trials assessing: (1) treatment differences between IMM alone compared to carotid endarterectomy (CEA) plus IMM, and (2) treatment differences between IMM alone compared to carotid stenting (CAS) plus IMM. Herein, we describe key elements of the two trials and unique aspects, including centralized IMM and assessment of cognitive function. Selection of which trial for which patient The two parallel trials share general inclusion/exclusion criteria, and primary and secondary endpoints. Based on patient preference and characteristics, the local investigative team determines whether it would be best to revascularize by endarterectomy or stenting. Patient population Eligibility includes those 35 years old who have highgrade asymptomatic stenosis involving the carotid bifurcation with or without involvement of the adjacent internal carotid artery. A patient is considered asymptomatic in the absence of ipsilateral symptoms <180 days prior to randomization. Stenosis is defined as high-grade if catheter angiography documents 70% stenosis per criteria used in NASCET 5 or duplex ultrasonography (DU) documents peak systolic velocity (PSV) of 230 cm/s in combination with at least one of the following four criteria: end diastolic velocity of 100 cm/s, internal carotid arteryto-common carotid artery PSV ratio 4.0, CT angiogram showing 70% stenosis, or a magnetic resonance angiogram (MRA) showing 70% stenosis. General eligibility criteria are listed in Table 1, general exclusion criteria in Table 2, CEA-trial-specific exclusion criteria in Table 3, and CAS-trial-specific exclusion criteria in Table 4. Intensive medical management Sites implement IMM for all patients with guidance from the Medical Management Core. Patients take aspirin 325 mg/day for the entire follow-up period (CAS patients also take clopidogrel for days after procedure). The primary risk factors, such as systolic blood pressure and LDL cholesterol are managed according to protocols targeting a systolic blood pressure <140 mm Hg and LDL <70 mg/dl. 6,7 Medications are adjusted at each visit if the patient is not in target. Management of secondary risk factors (diabetes, non- HDL cholesterol, smoking, weight, and physical activity) is coordinated with the patient s primary physician or other consultant as needed. A lifestyle modification program, INTERVENT is provided to each patient. 7 Carotid endarterectomy protocol Guidelines are provided for conduct of CEA. The technique of plaque removal (regular endarterectomy or eversion endarterectomy) is not dictated. Patch angioplasty is recommended for conventional CEA but not for eversion CEA. Other techniques and methods used such as shunts, or intra-operative monitoring are variable and depend upon the individual surgeon s practice. Anticoagulation with either heparin or bivalirudin (Angiomax Õ ) is required. Carotid artery stenting protocol Guidelines for CAS include that arterial access be established by the femoral route using the Seldinger technique and that local anesthesia with sedation is preferred over general anesthesia. Embolic protection devices should be used. CREST-2 permits the use of the
3 Howard et al. 3 Table 1. Primary inclusion criteria for participant selection either trial 1. Patient age 35 years old (no upper age limit) 2. Carotid stenosis defined as: OR Stenosis 70% by catheter angiography (NASCET criteria); by Doppler ultrasonography with 70% stenosis defined by a peak systolic velocity of at least 230 cm/s plus at least one of the following: (a) an end diastolic velocity 100 cm/s, or (b) internal carotid/common carotid artery peak systolic velocity ratio 4.0, or (c) computed tomography angiography with 70% stenosis, or (d) magnetic resonance angiography with 70% stenosis. 3. No medical history of stroke or transient ischemic attack ipsilateral to the stenosis within 180 days of randomization. 4. Modified Rankin Scale score of 0 or 1 at the time of informed consent. 5. Woman has no childbearing potential or, if of childbearing potential, has a negative pregnancy test prior to randomization. 6. Patients must agree to comply with all protocol-specified follow-up appointments. 7. Patients must sign a consent form that has been approved by the local governing Institutional Review Board (IRB)/Medical Ethics Committee (MEC) of the respective clinical site. 8. Randomization to treatment group will apply to only one carotid artery for patients with bilateral carotid stenosis. 9. Carotid stenosis treatable with carotid endarterectomy (CEA), or carotid artery stenting (CAS) Table 2. Primary exclusion criteria for participant selection either trial 1. Intolerance or allergic reaction to a study medication without a suitable management alternative. 2. GI hemorrhage within one month prior to enrollment that would preclude antiplatelet therapy. 3. Prior major ipsilateral stroke in the past with substantial residual disability (mrs 2) that is likely to confound study outcomes. 4. Severe dementia. 5. History of major symptomatic intracranial hemorrhage within the past 12 months that was not related to anticoagulation. 6. Prior intracranial hemorrhage that the investigator believes represents a contraindication to the perioperative or periprocedural antithrombotic and antiplatelet treatments necessary to complete endarterectomy or stenting per protocol. 7. Current neurologic illness characterized by fleeting or fixed neurologic deficits that cannot be distinguished from TIA or stroke. 8. Patient objects to future blood transfusions. 9. Platelet count <100,000/ml or history of heparin-induced thrombocytopenia. 10. Anticoagulation with Phenprocoumon (Marcumar Õ ), warfarin, or a direct thrombin inhibitor, or anti-xa agents. 11. Chronic atrial fibrillation. 12. Any episode of atrial fibrillation within the past six months or history of paroxysmal atrial fibrillation that is deemed to require chronic anticoagulation. 13. Other high-risk cardiac sources of emboli, including left ventricular aneurysm, severe cardiomyopathy, aortic or mitral mechanical heart valve, severe calcific aortic stenosis (valve area <1.0 cm 2 ), endocarditis, moderate to severe mitral stenosis, left atrial thrombus, or any intracardiac mass, or known unrepaired PFO with prior paradoxical embolism. (continued)
4 4 International Journal of Stroke 0(0) Table 2. Continued 14. Unstable angina defined as rest angina with ECG changes that is not amenable to revascularization (patients should undergo planned coronary revascularization at least 30 days before randomization). 15. Left ventricular ejection fraction <30% or admission for heart failure in prior 6 months. 16. Respiratory insufficiency with life expectancy <4 years or FEV 1 <30% of predicted value. 17. Known malignancy other than basal cell non-melanoma skin cancer. There are two exceptions: patients with prior cancer treatment and no recurrence for >5 years are eligible, and cancer patients with life expectancy greater than five years are eligible for enrollment. 18. Any major surgery, major trauma, revascularization procedure, or acute coronary syndrome within the past 1 month. 19. Either the serum creatinine is 2.5 mg/dl or the estimated GFR is <30 cc/min. 20. Major (non-carotid) surgery procedure planned within three months after enrollment. 21. Currently listed or being evaluated for major organ transplantation (i.e. heart, lung, liver, kidney). 22. Actively participating in another drug or aortic arch or cerebrovascular device trial for which participation in CREST-2 would be compromised with regard to follow-up assessment of outcomes or continuation in CREST Inability to understand and cooperate with study procedures or provide informed consent. 24. Non-atherosclerotic carotid stenosis (dissection, fibromuscular dysplasia, or stenosis following radiation therapy). 25. Previous ipsilateral CEA or CAS. 26. Ipsilateral internal or common carotid artery occlusion. 27. Intra-carotid floating thrombus. 28. Ipsilateral intracranial aneurysm >5 mm. 29. Extreme morbid obesity that would compromise patient safety during the procedure or would compromise patient safety during the periprocedural period. 30. Coronary artery disease with two or more proximal or major diseased coronary arteries with 70% stenosis that have not, or cannot, be revascularized. GI: gastrointestinal; mrs: modified Rankin Scale; TIA: transient ischemic attack; PFO: patent foramen ovale; ECG: electrocardiogram; FEV: forced expiratory volume; GFR: glomerular filtration rate; CEA: carotid endarterectomy; CAS: carotid stenting. Table 3. Exclusion criteria specific to the carotid endarterectomy trial 1. Serious adverse reaction to anesthesia not able to be overcome by pre-medication. 2. Distal/intracranial stenosis greater than index lesion. 3. Any of the following anatomical: radical neck dissection; surgically inaccessible lesions (e.g. above cervical spine level 2 (C2)); adverse neck anatomy that limits surgical exposure (e.g. spinal immobility inability to flex neck beyond neutral or kyphotic deformity, or short obese neck); presence of tracheostomy stoma; laryngeal nerve palsy contralateral to target vessel; or previous extracranial-intracranial or subclavian bypass procedure ipsilateral to the target vessel. carotid stenting and embolic protection devices that have received U.S. FDA approval. The list of approved devices will evolve and CREST-2 will accommodate additional stents and/or embolic protection devices if shown to be safe and effective. Periprocedural drug recommendations for carotid stenting are summarized in Table 5. Baseline and follow-up data collection summary Information collected at baseline includes: demographics, vascular risk factors, height, weight, arterial blood pressure, cigarette smoking status, stroke symptoms using the
5 Howard et al. 5 Table 4. Exclusion criteria specific to the carotid artery stenting trial 1. Allergy to intravascular contrast dye not amenable to pre-medication. 2. Type III, aortic arch anatomy. 3. Angulation or tortuosity (90 degree) of the innominate and common carotid artery that precludes safe, expeditious sheath placement or that will transmit a severe loop to the internal carotid after sheath placement. 4. Severe angulation or tortuosity of the internal carotid artery (including calyceal origin from the carotid bifurcation) that precludes safe deployment of embolic protection device or stent. Severe tortuosity is defined as 2 or more 90 degree angles within 4 cm of the target stenosis. 5. Proximal/ostial CCA, innominate stenosis or distal/intracranial stenosis greater than index lesion. Excessive circumferential calcification of the stenotic lesion defined as >3 mm thickness of calcification seen in orthogonal views on fluoroscopy. (Note: Anatomic considerations such as tortuosity, arch anatomy, and calcification must be evaluated even more carefully in elderly subjects (70 years).) 6. Target ICA vessel reference diameter <4.0 mm or >9.0 mm. Target ICA measurements may be made from angiography of the contralateral artery. The reference diameter must be appropriate for the devices to be used. 7. Inability to deploy or utilize an FDA-approved Embolic Protection Device (EPD). 8. Non-contiguous lesions and long lesions (>3 cm). 9. Qualitative characteristics of stenosis and stenosis-length of the carotid bifurcation (common carotid) and/or ipsilateral external carotid artery, that preclude safe sheath placement. 10. Occlusive or critical ilio-femoral disease including severe tortuosity or stenosis that necessitates additional endovascular procedures to facilitate access to the aortic arch or that prevents safe and expeditious femoral access to the aortic arch. String sign of the ipsilateral common or internal carotid artery. 11. Angiographic, CT, MR or ultrasound evidence of severe atherosclerosis of the aortic arch or origin of the innominate or common carotid arteries that would preclude safe passage of sheath and other endovascular devices to the target artery as needed for carotid stenting. CCA: common carotid artery; ICA: internal carotid artery; FDA: Food and Drug Administration; CT: computed tomography; MR: magnetic resonance. Table 5. Periprocedural drug therapy for all carotid stent patients Medication Pre-procedure Intra-procedure Post-procedure Post-discharge Heparin a PRN Maintain ACT s a PRN b None Aspirin 325 mg p.o. b.i.d. (Begin 48 hours before) None 325 mg p.o. daily for 30 days c 325 mg c,d 1 tablet p.o. daily thereafter Clopidogrel 75 mg p.o. b.i.d. daily (begin 48 h before) None 75 mg 1 tablet p.o. daily for 30 days Ticlopidine (instead of clopidogrel) 250 mg p.o. b.i.d. (begin 48 h before) None 250 mg p.o. b.i.d. for 30 days Ticagrelor 180 mg p.o. once d None 90 mg p.o. b.i.d. for 4 weeks Atorvastatin (or dose equivalent of other statin) Total of 80 mg None Continue on the dose of statin started on the day of randomization Continue on the dose of statin started on the day of randomization a Bivalirudin may be substituted for heparin. Use in accordance with manufacturer s instructions. Activated clotting time are not collected when bivalirudin is used as the procedural anticoagulant. b Heparin may be given post-procedure as needed. c May be substituted with 81 mg tablet if patient cannot tolerate 325 mg dosage. d Dose is for those not currently taking ticagrelor.
6 6 International Journal of Stroke 0(0) Table 6. Baseline and follow-up data collection schedule in CREST-2 Evaluation Visit number Time Month Baseline a Postprocedure b 44 days Informed consent X Demographics Medical history X X Interval medical history X X X X X X X X X X Stroke questionnaire (QVSS) X X X X X X X X X X X Modified Rankin X X X X X X X X X X X National Institutes of Health Stroke Scale (NIHSS) X X X X X X X X X X X X Cognitive testing X X X X X X Ultrasound X X X X X CTA/MRA/CBA c X Blood pressure X X X X X X X X X X X Laboratory d X X X X X Note: For patients who undergo CAS and prescribed ticlopidine, a complete blood count will be required at 2 weeks and 30 days per standard medical practice. a Must be collected prior to procedure or initiation of medical management therapy. b NIHSS to be collected h post procedure. c CTA indicates computed tomography angiography; MRA indicates magnetic resonance angiography; CBA indicates catheter-based angiogram. d Baseline blood tests include lipids, hemoglobin A1c, creatinine, potassium, alanine and aspartate transaminases, and creatine kinase. QVSS: questionnaire for verifying stroke-free status. Questionnaire for Verifying Stroke-free Status (QVSS), 8 the modified Rankin Scale (mrs), 9 the National Institutes of Health Stroke Scale (NIHSS), 10 cognitive testing and selected blood tests (Table 6). The neurocognitive battery, measuring vascular cognitive status, is administered centrally via telephone at baseline, 44 days, 12 months and annually thereafter to 48 months. This assessment includes administration of five tests comprising four domains of cognitive function: Learning (CERAD Word List Learning Test), memory (CERAD Word List Delayed Recall), 11 executive function/processing speed (animal naming and letter fluency), and attention/working memory (digit span). The follow-up schedule is provided in Table 6. An NIHSS is performed 12 to 36 h post-cea or CAS. For all patients, NIHSS, medical history, QVSS, mrs, and blood pressure measurements are collected at each follow-up clinic visit. For patients unable to return for a clinic visit, telephone follow-up will include an abbreviated medical history, mrs, QVSS, and cognitive assessment (if applicable.) Primary and secondary endpoints In each trial, the primary outcome is any stroke or death from randomization to 44 days or ipsilateral ischemic stroke from 45 days up to four years of follow-up. Stroke will be defined according to the World Health Organization as rapidly developing clinical signs of focal disturbance of cerebral function, lasting more than 24 h, with no apparent cause other than that of vascular origin. 12 Outcome is determined by a masked Adjudication Committee. Major and minor stroke Stroke is defined as major when the NIHSS is 6at least 30 days after date of stroke onset, and minor
7 Howard et al. 7 otherwise, or as determined by the Stroke Adjudication Committee based on clinical data. Disabling and non-disabling stroke A stroke is classified as disabling if the mrs is 3 or more at least 30 days after onset of stroke, and non-disabling if the mrs is 2 or less. If the mrs was not performed, the score is estimated based on available clinical records. Statistical consideration The sample size in each trial is Power calculations assumed a 5% cross-over rate, a 2.5% annual dropout rate, and a 0.05 level to declare a significant difference. The anticipated 4-year event rate in the revascularization arms (CEA or CAS) is 3.6%, comprising 2.0% during the 44-day periprocedural period plus 1.6% (0.4% per year) over the four-year post-procedural period. Under these assumptions, there is approximately 85% power to detect a difference if the event rate in the IMM arm is 4.8% greater (a rate of 8.4%) or 2.8% less (a rate of 0.8%) than the anticipated 3.6% rate in the revascularization arm. Analysis will use superiority testing in an intention-to-treat approach. Within each trial, the four-year event rate in both arms will be estimated using Kaplan-Meier methods, and the difference between arms will be assessed with a re-randomization test. The analysis of cognitive function will also be estimated at the four-year follow-up point using a randomeffects regression approach that includes assessments performed at each time point. Additional secondary analyses also include: (1) treatment differences in the area under the Kaplan-Meier event curves, (2) potential effect modification of the difference in event rates by age, sex, severity of stenosis, risk factor profile, and remote ipsilateral symptoms and contralateral symptoms, (3) treatment differences in major stroke and minor stroke, (4) the effect of stenosis progression or restenosis on stroke risk, and (5) effect modifiers and predictors of the pattern of cognitive change. Sensitivity analyses will assess the impact of participant withdrawal from the study and the level of risk factor control. Imaging Core The Imaging Core oversees examination and interpretation for DU, CT, MRA and catheter-based angiography, and brain CT and MR imaging. The Core has a standardized carotid DU protocol and certifies each ultrasound laboratory s equipment, personnel, and testing technique. The Core reviews and re-reads all imaging tests performed on CREST-2 patients. Accordingly, exclusion of patients without high grade stenosis will be maximized and accurate imaging characterization of brain lesions associated with clinical strokes during the trial will be accomplished. Important secondary endpoints such as restenosis/occlusion after revascularization and stenosis progression/occlusion during medical therapy will be detected. Site selection and ongoing site management Careful selection and timely activation of clinical sites in multicenter clinical trials is critical for successful enrollment, subject safety, and generalizability of results. The first potential clinical sites were identified from the sites in CREST that provided the highest number of randomizations as well as high-quality data. 13 Additional sites were invited to apply through submission of a data form containing extensive information on experience with the trial procedures, and clinical trials (see Table 7). Sites from StrokeNet, a centrally coordinated US network of 25 regional centers, were especially invited to apply. 14 Information was reviewed and sites were scored as high, medium or low. The initial target was 120 sites in North America. By the end of January 2017, 153 sites had been approved by the Site Selection and Management Committee. Of these, 104 had completed all the training and regulatory requirements and been approved to randomize. Monitoring assures adequate protection of the rights and safety of human subjects. The trial monitor visits every site, verifies source and study documents, proper informed consent, and patient eligibility. Data quality reports are produced by the Statistical and Data Coordinating Center and protocol deviations and incompleteness of data are addressed for corrective and/or preventive actions by study leadership. An independent, NINDS-appointed Data Safety and Monitoring Board monitors safety and integrity of the trials and advises the funding agency. Training and credentialing Protocol and medical management training is conducted prior to study start-up and annually for site coordinators and Investigators via in-person, teleconferences, webinars, and e-learning platforms. Multi-disciplinary Surgical and Interventional Management Committees (IMC) credentialed operators. Surgeon candidates not previously credentialed in CREST submitted procedural reports and discharge information on at least 50 consecutive cases.
8 8 International Journal of Stroke 0(0) Table 7. Data submitted for site selection consideration 1. Number of carotid endarterectomy and carotid stenting procedures performed at participating hospital for the past two years (including minority and sex distribution) 2. Qualifications and experience of principal investigator 3. Presence of an experienced clinical investigative team (e.g. neurologists, vascular surgeons, neurosurgeons, interventional cardiologists, interventional radiologists, interventional neuroradiologists, medical management physicians, research coordinators, etc.) 4. Presence of affiliate sites 5. Status of vascular ultrasonography laboratory (i.e. whether approved by the Intersocietal Commission on Accreditation of Vascular Laboratories or American College of Radiology Certification) 6. Availability of central institutional ethics review board 7. Receipt of any FDA warning letters 8. Experience in other stroke trials 9. Scientific/publication experience of the investigators 10. StrokeNet site Interventionist candidates submitted 25 consecutive cases completed within five years as primary operator out of a required total experience of 50 cases (20 for operators completing training). Credentialing requires appropriate case selection, technique, and a combined stroke and death rate of <3% for asymptomatic patients. Companion registry A CREST-2 Companion Registry (C2R) (clinicaltrials.gov NCT ) was developed to promote rapid enrollment into the CREST-2 CAS trial because many stent operator applicants did not have sufficient experience to apply for credentialing. Once reviewed by the IMC, operators receive conditional approval allowing them to perform CAS procedures within C2R. The registry ensures that procedures are performed by skilled operators at well-resourced sites. Summary and conclusions Building on the recent successes of the CREST trial, 13,15 CREST-2 is uniquely positioned to test the merits of CEA and CAS in the context of IMM. CREST-2 is needed to compare CEA and CAS to IMM in asymptomatic patients primarily because of substantial changes in medical management in the past two decades that may have substantially reduced the risk of asymptomatic carotid stenosis. By using real-world applicable best practices from SAMMPRIS, 7 CREST-2 will achieve substantially better rates of risk factor control than all prior cervical carotid atherosclerosis trials through more active centralized intervention. Acknowledgements The authors thank the other investigators, the staff, and the participants of the CREST-2 trials for their valuable contributions. A full list of participating CREST-2 investigators and institutions can be found at Authors contributions All authors were involved in the study design, protocol preparation, and acquisition of funding. VJH was responsible for first draft and final revision. All authors provided critical revision of the manuscript for important intellectual content. All authors have reviewed and approved the final manuscript. Declaration of conflicting interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The CREST-2 trials and the CREST-2 Registry are supported by cooperative agreements U01 NS080168, and U01 NS from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. Additional support for CREST-2 comes from StrokeNet U01 NS Additional support for the CREST-2 Registry comes from Industry (Abbott Vascular, Boston Scientific, Cardinal Health, Covidien, Gore Medical and Silk Road Medical).
9 Howard et al. 9 References 1. Howard VJ, Grizzle J, Diener HC, Hobson RW 2nd, Mayberg MR and Toole JF. Comparison of multicenter study designs for investigation of carotid endarterectomy efficacy. Stroke 1992; 23: Benavente O, Moher D and Pham B. Carotid endarterectomy for asymptomatic carotid stenosis: a meta-analysis. BMJ 1998; 317: Rothwell PM, Eliasziw M, Gutnikov SA, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003; 361: Howard G, Roubin GS, Jansen O, et al. Association between age and risk of stroke or death from carotid endarterectomy and carotid stenting: a meta-analysis of pooled patient data from four randomised trials. Lancet 2016; 387: Eliasziw M, Smith RF, Singh N, Holdsworth DW, Fox AJ and Barnett HJ. Further comments on the measurement of carotid stenosis from angiograms. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Stroke 1994; 25: Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011; 365: Meschia JF, Lojacono MA, Miller MJ, Brott TG, Atkinson EJ and O Brien PC. Reliability of the questionnaire for verifying stroke-free status. Cerebrovasc Dis 2004; 17: Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol 2006; 5: Lyden P, Lu M, Jackson C, et al. Underlying structure of the National Institutes of Health Stroke Scale: results of a factor analysis. NINDS tpa Stroke Trial Investigators. Stroke 1999; 30: Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer s disease. Neurology 1989; 39: Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE and Strasser T. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Org 1980; 58: Brott TG, Hobson RW 2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. New Engl J Med 2010; 363: Mott M, Janis S and Koroshetz WJ. StrokeNet takes off: National Institute of Neurological Disorders and Stroke Organizational Update. Stroke 2016; 47: e Brott TG, Howard G, Roubin GS, et al. Long-term results of stenting versus endarterectomy for carotidartery stenosis. N Engl J Med 2016; 374:
Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis
 Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis William A. Gray MD System Chief of Cardiovascular Services, President, Wynnewood, PA USA Two parallel multi-center randomized,
Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis William A. Gray MD System Chief of Cardiovascular Services, President, Wynnewood, PA USA Two parallel multi-center randomized,
New Trials in Progress: ACT 1. Jon Matsumura, MD Cannes, France June 28, 2008
 New Trials in Progress: ACT 1 Jon Matsumura, MD Cannes, France June 28, 2008 Faculty Disclosure I disclose the following financial relationships: Consultant, CAS training director, and/or research grants
New Trials in Progress: ACT 1 Jon Matsumura, MD Cannes, France June 28, 2008 Faculty Disclosure I disclose the following financial relationships: Consultant, CAS training director, and/or research grants
Carotid Artery Disease and What s Pertinent JOSEPH A PAULISIN DO
 Carotid Artery Disease and What s Pertinent JOSEPH A PAULISIN DO Goal of treatment of carotid disease Identify those at risk of developing symptoms Prevent patients at risk from developing symptoms Prevent
Carotid Artery Disease and What s Pertinent JOSEPH A PAULISIN DO Goal of treatment of carotid disease Identify those at risk of developing symptoms Prevent patients at risk from developing symptoms Prevent
Disclosures. State of the Art Management of Carotid Stenosis. NIH funding for clinical trials Consultant for Scientia Vascular and Medtronic
 State of the Art Management of Carotid Stenosis Mark R. Harrigan, MD UAB Stroke Center Professor of Neurosurgery, Neurology, and Radiology University of Alabama, Birmingham Disclosures NIH funding for
State of the Art Management of Carotid Stenosis Mark R. Harrigan, MD UAB Stroke Center Professor of Neurosurgery, Neurology, and Radiology University of Alabama, Birmingham Disclosures NIH funding for
03/30/2016 DISCLOSURES TO OPERATE OR NOT THAT IS THE QUESTION CAROTID INTERVENTION IS INDICATED FOR ASYMPTOMATIC CAROTID OCCLUSIVE DISEASE
 CAROTID INTERVENTION IS INDICATED FOR ASYMPTOMATIC CAROTID OCCLUSIVE DISEASE Elizabeth L. Detschelt, M.D. Allegheny Health Network Vascular and Endovascular Symposium April 2, 2016 DISCLOSURES I have no
CAROTID INTERVENTION IS INDICATED FOR ASYMPTOMATIC CAROTID OCCLUSIVE DISEASE Elizabeth L. Detschelt, M.D. Allegheny Health Network Vascular and Endovascular Symposium April 2, 2016 DISCLOSURES I have no
Slide 1. Slide 2 Conflict of Interest Disclosure. Slide 3 Stroke Facts. The Treatment of Intracranial Stenosis. Disclosure
 Slide 1 The Treatment of Intracranial Stenosis Helmi Lutsep, MD Vice Chair and Dixon Term Professor, Department of Neurology, Oregon Health & Science University Chief of Neurology, VA Portland Health Care
Slide 1 The Treatment of Intracranial Stenosis Helmi Lutsep, MD Vice Chair and Dixon Term Professor, Department of Neurology, Oregon Health & Science University Chief of Neurology, VA Portland Health Care
Contemporary Management of Carotid Disease What We Know So Far
 Contemporary Management of Carotid Disease What We Know So Far Ammar Safar, MD, FSCAI, FACC, FACP, RPVI Interventional Cardiology & Endovascular Medicine Disclosers NONE Epidemiology 80 % of stroke are
Contemporary Management of Carotid Disease What We Know So Far Ammar Safar, MD, FSCAI, FACC, FACP, RPVI Interventional Cardiology & Endovascular Medicine Disclosers NONE Epidemiology 80 % of stroke are
Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease: Executive summary
 SOCIETY FOR VASCULAR SURGERY DOCUMENT Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease: Executive summary John J. Ricotta, MD, a Ali AbuRahma, MD, FACS, b
SOCIETY FOR VASCULAR SURGERY DOCUMENT Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease: Executive summary John J. Ricotta, MD, a Ali AbuRahma, MD, FACS, b
Carotid Artery Stenting
 Carotid Artery Stenting JESSICA MITCHELL, ACNP CENTRAL ILLINOIS RADIOLOGICAL ASSOCIATES External Carotid Artery (ECA) can easily be identified from Internal Carotid Artery (ICA) by noticing the branches.
Carotid Artery Stenting JESSICA MITCHELL, ACNP CENTRAL ILLINOIS RADIOLOGICAL ASSOCIATES External Carotid Artery (ECA) can easily be identified from Internal Carotid Artery (ICA) by noticing the branches.
Treatment Considerations for Carotid Artery Stenosis. Danielle Zielinski, RN, MSN, ACNP Rush University Neurosurgery
 Treatment Considerations for Carotid Artery Stenosis Danielle Zielinski, RN, MSN, ACNP Rush University Neurosurgery 4.29.2016 There is no actual or potential conflict of interest in regards to this presentation
Treatment Considerations for Carotid Artery Stenosis Danielle Zielinski, RN, MSN, ACNP Rush University Neurosurgery 4.29.2016 There is no actual or potential conflict of interest in regards to this presentation
Vivek R. Deshmukh, MD Director, Cerebrovascular and Endovascular Neurosurgery Chairman, Department of Neurosurgery Providence Brain and Spine
 Vivek R. Deshmukh, MD Director, Cerebrovascular and Endovascular Neurosurgery Chairman, Department of Neurosurgery Providence Brain and Spine Institute The Oregon Clinic Disclosure I declare that neither
Vivek R. Deshmukh, MD Director, Cerebrovascular and Endovascular Neurosurgery Chairman, Department of Neurosurgery Providence Brain and Spine Institute The Oregon Clinic Disclosure I declare that neither
CAROTID ARTERY ANGIOPLASTY
 CAROTID ARTERY ANGIOPLASTY Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
CAROTID ARTERY ANGIOPLASTY Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
Quality ID #195 (NQF 0507): Radiology: Stenosis Measurement in Carotid Imaging Reports National Quality Strategy Domain: Effective Clinical Care
 Quality ID #195 (NQF 0507): Radiology: Stenosis Measurement in Carotid Imaging Reports National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Quality ID #195 (NQF 0507): Radiology: Stenosis Measurement in Carotid Imaging Reports National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Peter A. Soukas, M.D., FACC, FSVM, FSCAI, RPVI
 Peter A. Soukas, M.D., FACC, FSVM, FSCAI, RPVI Director, Peripheral Vascular Interventional Laboratory Director, Vascular & Endovascular Medicine Fellowship Program Assistant Professor of Medicine The
Peter A. Soukas, M.D., FACC, FSVM, FSCAI, RPVI Director, Peripheral Vascular Interventional Laboratory Director, Vascular & Endovascular Medicine Fellowship Program Assistant Professor of Medicine The
Carotid Artery Revascularization: Current Strategies. Shonda Banegas, D.O. Vascular Surgery Carondelet Heart and Vascular Institute September 6, 2014
 Carotid Artery Revascularization: Current Strategies Shonda Banegas, D.O. Vascular Surgery Carondelet Heart and Vascular Institute September 6, 2014 Disclosures None 1 Stroke in 2014 Stroke kills almost
Carotid Artery Revascularization: Current Strategies Shonda Banegas, D.O. Vascular Surgery Carondelet Heart and Vascular Institute September 6, 2014 Disclosures None 1 Stroke in 2014 Stroke kills almost
MORTALITY AND MORBIDITY RISK FROM CAROTID ARTERY ATHEROSCLEROSIS. 73 year old NS right-handed male applicant for $1 Million Life Insurance
 MORTALITY AND MORBIDITY RISK FROM CAROTID ARTERY ATHEROSCLEROSIS October 17, 2012 AAIM Triennial Conference, San Diego Robert Lund, MD What Is The Risk? 73 year old NS right-handed male applicant for $1
MORTALITY AND MORBIDITY RISK FROM CAROTID ARTERY ATHEROSCLEROSIS October 17, 2012 AAIM Triennial Conference, San Diego Robert Lund, MD What Is The Risk? 73 year old NS right-handed male applicant for $1
ROADSTER 2. SPONSOR: Silk Road Medical
 ROADSTER 2 CONDITION: Carotid artery disease PI: Jessica Titus, MD CONTACT INFO: Jo Anne Goldman JoAnne.Goldman@allina.com 612-863-3793 DESCRIPTION: The study is intended to evaluate real world usage of
ROADSTER 2 CONDITION: Carotid artery disease PI: Jessica Titus, MD CONTACT INFO: Jo Anne Goldman JoAnne.Goldman@allina.com 612-863-3793 DESCRIPTION: The study is intended to evaluate real world usage of
MORTALITY AND MORBIDITY RISK FROM CAROTID ARTERY ATHEROSCLEROSIS. 73 year old NS right-handed male applicant for $1 Million life insurance
 MORTALITY AND MORBIDITY RISK FROM CAROTID ARTERY ATHEROSCLEROSIS October 17, 2012 AAIM Triennial Conference, San Diego Robert Lund, MD What Is The Risk? 73 year old NS right-handed male applicant for $1
MORTALITY AND MORBIDITY RISK FROM CAROTID ARTERY ATHEROSCLEROSIS October 17, 2012 AAIM Triennial Conference, San Diego Robert Lund, MD What Is The Risk? 73 year old NS right-handed male applicant for $1
Measure #195 (NQF 0507): Radiology: Stenosis Measurement in Carotid Imaging Reports National Quality Strategy Domain: Effective Clinical Care
 Measure #195 (NQF 0507): Radiology: Stenosis Measurement in Carotid Imaging Reports National Quality Strategy Domain: Effective Clinical Care 2017 OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS ONLY MEASURE TYPE:
Measure #195 (NQF 0507): Radiology: Stenosis Measurement in Carotid Imaging Reports National Quality Strategy Domain: Effective Clinical Care 2017 OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS ONLY MEASURE TYPE:
Carotid Artery Stenting (CAS) Pathophysiology. Technical Considerations. Plaque characteristics: relevant concepts. CAS and CEA
 Carotid Artery Stenting (CAS) Carotid Artery Stenting for Stroke Risk Reduction Matthew A. Corriere MD, MS, RPVI Assistant Professor of Surgery Department of Vascular and Endovascular Surgery Rationale:
Carotid Artery Stenting (CAS) Carotid Artery Stenting for Stroke Risk Reduction Matthew A. Corriere MD, MS, RPVI Assistant Professor of Surgery Department of Vascular and Endovascular Surgery Rationale:
Carotid Artery Stenting
 Carotid Artery Stenting Woong Chol Kang M.D. Gil Medical Center, Gachon University of Medicine and Science, Incheon, Korea Carotid Stenosis and Stroke ~25% of stroke is due to carotid disease, the reminder
Carotid Artery Stenting Woong Chol Kang M.D. Gil Medical Center, Gachon University of Medicine and Science, Incheon, Korea Carotid Stenosis and Stroke ~25% of stroke is due to carotid disease, the reminder
Pre-and Post Procedure Non-Invasive Evaluation of the Patient with Carotid Disease
 Pre-and Post Procedure Non-Invasive Evaluation of the Patient with Carotid Disease Michael R. Jaff, D.O., F.A.C.P., F.A.C.C. Assistant Professor of Medicine Harvard Medical School Director, Vascular Medicine
Pre-and Post Procedure Non-Invasive Evaluation of the Patient with Carotid Disease Michael R. Jaff, D.O., F.A.C.P., F.A.C.C. Assistant Professor of Medicine Harvard Medical School Director, Vascular Medicine
Carotid Artery Stent: Is it ready for prime time?
 2010 CATH LAB SYMPOSIUM Carotid Artery Stent: Is it ready for prime time? Luis F. Tami, MD, FACC, FSCAI Interventional Cardiology and Vascular Medicine Memorial Regional Hospital August 2010 CAE and CAS
2010 CATH LAB SYMPOSIUM Carotid Artery Stent: Is it ready for prime time? Luis F. Tami, MD, FACC, FSCAI Interventional Cardiology and Vascular Medicine Memorial Regional Hospital August 2010 CAE and CAS
2015 Update in Diagnosis and Management of Stroke
 2015 Update in Diagnosis and Management of Stroke S. Andrew Josephson MD Carmen Castro Franceschi and Gladyne K. Mitchell Neurohospitalist Distinguished Professor Senior Executive Vice Chair, Department
2015 Update in Diagnosis and Management of Stroke S. Andrew Josephson MD Carmen Castro Franceschi and Gladyne K. Mitchell Neurohospitalist Distinguished Professor Senior Executive Vice Chair, Department
Management of intracranial atherosclerotic stenosis (ICAS)/intracranial atherosclerosis
 Management of intracranial atherosclerotic stenosis (ICAS)/intracranial atherosclerosis Tim Mikesell, D.O. Oct 22, 2016 Stroke facts Despite progress in decreasing stroke incidence and mortality, stroke
Management of intracranial atherosclerotic stenosis (ICAS)/intracranial atherosclerosis Tim Mikesell, D.O. Oct 22, 2016 Stroke facts Despite progress in decreasing stroke incidence and mortality, stroke
CAROTID DEBATE High-Grade Asymptomatic Disease Should Be Repaired Selectively; Medical Management is NOT Enough
 Todd W GenslerMD April 28, 2018 CAROTID DEBATE High-Grade Asymptomatic Disease Should Be Repaired Selectively; Medical Management is NOT Enough DISCLOSURES I have no financial disclosures Presenter name
Todd W GenslerMD April 28, 2018 CAROTID DEBATE High-Grade Asymptomatic Disease Should Be Repaired Selectively; Medical Management is NOT Enough DISCLOSURES I have no financial disclosures Presenter name
Assessment of the procedural etiology of stroke resulting from carotid artery stenting
 Assessment of the procedural etiology of stroke resulting from carotid artery stenting 1. Study Purpose and Rationale: A. Background Stroke is the 3 rd leading cause of death in the United States and carries
Assessment of the procedural etiology of stroke resulting from carotid artery stenting 1. Study Purpose and Rationale: A. Background Stroke is the 3 rd leading cause of death in the United States and carries
APPENDIX A NORTH AMERICAN SYMPTOMATIC CAROTID ENDARTERECTOMY TRIAL
 APPENDIX A Primary Findings From Selected Recent National Institute of Neurological Disorders and Stroke-Sponsored Clinical Trials That Have shaped Modern Stroke Prevention Philip B. Gorelick 178 NORTH
APPENDIX A Primary Findings From Selected Recent National Institute of Neurological Disorders and Stroke-Sponsored Clinical Trials That Have shaped Modern Stroke Prevention Philip B. Gorelick 178 NORTH
Carotid. The. Issue. Now approved by the FDA, carotid stenting moves into the spotlight in endovascular care.
 The Carotid Issue 33 Carotid Revascularization in 2004 By Thomas G. Brott, MD; Jamie Roberts, LPN, CRC; Robert W. Hobson II, MD; and Susan Hughes, BSN Now approved by the FDA, carotid stenting moves into
The Carotid Issue 33 Carotid Revascularization in 2004 By Thomas G. Brott, MD; Jamie Roberts, LPN, CRC; Robert W. Hobson II, MD; and Susan Hughes, BSN Now approved by the FDA, carotid stenting moves into
Index. interventional.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A ACAS (Asymptomatic Carotid Atherosclerosis Study), 65 66 ACST (Asymptomatic Carotid Surgery Trial), 6 7, 65, 75 Age factors, in carotid
Index Note: Page numbers of article titles are in boldface type. A ACAS (Asymptomatic Carotid Atherosclerosis Study), 65 66 ACST (Asymptomatic Carotid Surgery Trial), 6 7, 65, 75 Age factors, in carotid
NCDR CARE Registry Carotid Artery Revascularization and Endarterectomy Registry Carotid Artery Stenting Form v1.09
 NCDR CARE Registry Carotid Artery Revascularization and Endarterectomy Registry Carotid Artery Stenting Fm v1.09 A. PARTICIPANT ADMINISTRATION Participant ID 1000 : Participant Name 1010 : Medicare Provider
NCDR CARE Registry Carotid Artery Revascularization and Endarterectomy Registry Carotid Artery Stenting Fm v1.09 A. PARTICIPANT ADMINISTRATION Participant ID 1000 : Participant Name 1010 : Medicare Provider
Carotid Artery Stenting Today: A Few Updating Remarks
 Carotid Artery Stenting Today: A Few Updating Remarks Camilo R. Gomez, MD, MBA Director, Alabama Neurological Institute Birmingham, Alabama Disclaimer & Warning Company Pharmaceutical BMS-Sanofi-Aventis
Carotid Artery Stenting Today: A Few Updating Remarks Camilo R. Gomez, MD, MBA Director, Alabama Neurological Institute Birmingham, Alabama Disclaimer & Warning Company Pharmaceutical BMS-Sanofi-Aventis
Corporate Medical Policy
 Corporate Medical Policy Endovascular Therapies for Extracranial Vertebral Artery Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: endovascular_therapies_for_extracranial_vertebral_artery_disease
Corporate Medical Policy Endovascular Therapies for Extracranial Vertebral Artery Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: endovascular_therapies_for_extracranial_vertebral_artery_disease
Canadian Best Practice Recommendations for Stroke Care. (Updated 2008) Section # 3 Section # 3 Hyperacute Stroke Management
 Canadian Best Practice Recommendations for Stroke Care (Updated 2008) Section # 3 Section # 3 Hyperacute Stroke Management Reorganization of Recommendations 2008 2006 RECOMMENDATIONS: 2008 RECOMMENDATIONS:
Canadian Best Practice Recommendations for Stroke Care (Updated 2008) Section # 3 Section # 3 Hyperacute Stroke Management Reorganization of Recommendations 2008 2006 RECOMMENDATIONS: 2008 RECOMMENDATIONS:
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service
 M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
Cryptogenic Stroke: What Don t We Know. Siddharth Sehgal, MD Medical Director, TMH Stroke Center Tallahassee Memorial Healthcare
 Cryptogenic Stroke: What Don t We Know Siddharth Sehgal, MD Medical Director, TMH Stroke Center Tallahassee Memorial Healthcare Financial Disclosures None Objectives Principles of diagnostic evaluation
Cryptogenic Stroke: What Don t We Know Siddharth Sehgal, MD Medical Director, TMH Stroke Center Tallahassee Memorial Healthcare Financial Disclosures None Objectives Principles of diagnostic evaluation
Carotid Stenosis (carotid artery disease)
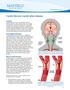 1 Carotid Stenosis (carotid artery disease) Overview Carotid stenosis is a narrowing of the carotid arteries, the two major arteries that carry oxygenrich blood from the heart to the brain. Also called
1 Carotid Stenosis (carotid artery disease) Overview Carotid stenosis is a narrowing of the carotid arteries, the two major arteries that carry oxygenrich blood from the heart to the brain. Also called
Carotid Revascularization
 Options for Carotid Disease Carotid Revascularization Wayne Causey, MD 2 nd Year Vascular Surgery Fellow Best medical therapy, Carotid Endarterectomy, and Carotid Stenting Who benefits from best medical
Options for Carotid Disease Carotid Revascularization Wayne Causey, MD 2 nd Year Vascular Surgery Fellow Best medical therapy, Carotid Endarterectomy, and Carotid Stenting Who benefits from best medical
a physician-initiated study investigating the RoadSaver stent in carotid lesions Dr. Michel Bosiers
 The study a physician-initiated study investigating the RoadSaver stent in carotid lesions Dr. Michel Bosiers Conflict of interest have the following potential conflicts of interest to report: Consulting
The study a physician-initiated study investigating the RoadSaver stent in carotid lesions Dr. Michel Bosiers Conflict of interest have the following potential conflicts of interest to report: Consulting
Lecture Outline: 1/5/14
 John P. Karis, MD Lecture Outline: Provide a clinical overview of stroke: Risk Prevention Diagnosis Intervention Illustrate how MRI is used in the diagnosis and management of stroke. Illustrate how competing
John P. Karis, MD Lecture Outline: Provide a clinical overview of stroke: Risk Prevention Diagnosis Intervention Illustrate how MRI is used in the diagnosis and management of stroke. Illustrate how competing
AN ASSESSMENT OF INTER-RATER RELIABILITY IN THE TREATMENT OF CAROTID ARTERY STENOSIS
 Pak Heart J ORIGINAL ARTICLE AN ASSESSMENT OF INTER-RATER RELIABILITY IN THE TREATMENT OF CAROTID ARTERY STENOSIS 1 2 3 4 5 Abhishek Nemani, Arshad Ali, Arshad Rehan, Ali Aboufaris, Jabar Ali 1-4 Guthrie
Pak Heart J ORIGINAL ARTICLE AN ASSESSMENT OF INTER-RATER RELIABILITY IN THE TREATMENT OF CAROTID ARTERY STENOSIS 1 2 3 4 5 Abhishek Nemani, Arshad Ali, Arshad Rehan, Ali Aboufaris, Jabar Ali 1-4 Guthrie
Trans-Carotid Artery Revascularization
 Trans-Carotid Artery Revascularization How I do it John Rollo Clinical Assistant Professor University of Washington Vascular Surgery 6/15/2018 DISCLOSURE John Rollo, MD, RPVI Consultant / Advisory Board:
Trans-Carotid Artery Revascularization How I do it John Rollo Clinical Assistant Professor University of Washington Vascular Surgery 6/15/2018 DISCLOSURE John Rollo, MD, RPVI Consultant / Advisory Board:
UPMC HAMOT CAROTID ARTERY DISEASE WHERE DO WE GO FROM HERE?
 UPMC HAMOT CAROTID ARTERY DISEASE WHERE DO WE GO FROM HERE? Richard W. Petrella M.D. FACP,FACC,FASCI DEPARTMENT CHAIRMAN CVM&S UPMC HAMOT MEDICAL CENTER 1 LEARNING OBJECTIVES REVIEW THE RISK FACTORS FOR
UPMC HAMOT CAROTID ARTERY DISEASE WHERE DO WE GO FROM HERE? Richard W. Petrella M.D. FACP,FACC,FASCI DEPARTMENT CHAIRMAN CVM&S UPMC HAMOT MEDICAL CENTER 1 LEARNING OBJECTIVES REVIEW THE RISK FACTORS FOR
Section Editor Scott E Kasner, MD
 1 of 6 9/29/2013 6:55 PM Official reprint from UpToDate www.uptodate.com 2013 UpToDate The content on the UpToDate website is not intended nor recommended as a substitute for medical advice, diagnosis,
1 of 6 9/29/2013 6:55 PM Official reprint from UpToDate www.uptodate.com 2013 UpToDate The content on the UpToDate website is not intended nor recommended as a substitute for medical advice, diagnosis,
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
Disclosures. CREST Trial: Summary. Lecture Outline 4/16/2015. Cervical Atherosclerotic Disease
 Disclosures Your Patient Has Carotid Bulb Stenosis and a Tandem Intracranial Stenosis: How Do SAMMPRIS and Other Evidence Inform Your Treatment? UCSF Vascular Symposium 2015 Steven W. Hetts, MD Associate
Disclosures Your Patient Has Carotid Bulb Stenosis and a Tandem Intracranial Stenosis: How Do SAMMPRIS and Other Evidence Inform Your Treatment? UCSF Vascular Symposium 2015 Steven W. Hetts, MD Associate
Recommendations for Follow-up After Vascular Surgery Arterial Procedures SVS Practice Guidelines
 Recommendations for Follow-up After Vascular Surgery Arterial Procedures 2018 SVS Practice Guidelines vsweb.org/svsguidelines About the guidelines Published in the July 2018 issue of Journal of Vascular
Recommendations for Follow-up After Vascular Surgery Arterial Procedures 2018 SVS Practice Guidelines vsweb.org/svsguidelines About the guidelines Published in the July 2018 issue of Journal of Vascular
Carotid Artery Stenting
 Carotid Artery Stenting Natural history of the carotid stenosis Asymptomatic 80% carotid stenosis - 6% risk of stroke / year Symptomatic carotid stenosis have 10% risk of CVA at one year and 40% at 5 years
Carotid Artery Stenting Natural history of the carotid stenosis Asymptomatic 80% carotid stenosis - 6% risk of stroke / year Symptomatic carotid stenosis have 10% risk of CVA at one year and 40% at 5 years
Carotid Endarterectomy vs. Carotid artery Stenting (Surgeon Perspective)
 Carotid Endarterectomy vs. Carotid artery Stenting (Surgeon Perspective) T-Woei Tan, MD, FACS, RPVI Assistant Professor of Surgery Vascular and Endovascular Surgery Louisiana State University Health -
Carotid Endarterectomy vs. Carotid artery Stenting (Surgeon Perspective) T-Woei Tan, MD, FACS, RPVI Assistant Professor of Surgery Vascular and Endovascular Surgery Louisiana State University Health -
Long-Term Care Updates
 Long-Term Care Updates October/November 2015 By Daniel Kerner, PharmD A stroke occurs when blood flow to the brain is stopped or slowed, resulting in death or damage to brain cells. There are three main
Long-Term Care Updates October/November 2015 By Daniel Kerner, PharmD A stroke occurs when blood flow to the brain is stopped or slowed, resulting in death or damage to brain cells. There are three main
Carotid stenosis management: CAS or CEA? Yaoguo Yang, Chen Zhong Beijing Anzhen Hospital,China
 Carotid stenosis management: CAS or CEA? Yaoguo Yang, Chen Zhong Beijing Anzhen Hospital,China Disclosure Speaker name:... I have the following potential conflicts of interest to report: Consulting Employment
Carotid stenosis management: CAS or CEA? Yaoguo Yang, Chen Zhong Beijing Anzhen Hospital,China Disclosure Speaker name:... I have the following potential conflicts of interest to report: Consulting Employment
Cryptogenic Strokes: Evaluation and Management
 Cryptogenic Strokes: Evaluation and Management 77 yo man with hypertension and hyperlipidemia developed onset of left hemiparesis and right gaze preference, last seen normal at 10:00 AM Brought to ZSFG
Cryptogenic Strokes: Evaluation and Management 77 yo man with hypertension and hyperlipidemia developed onset of left hemiparesis and right gaze preference, last seen normal at 10:00 AM Brought to ZSFG
SCAI Fall Fellows Course Subclavian/Innominate Case Presentation
 SCAI Fall Fellows Course 2012 Subclavian/Innominate Case Presentation Daniel J. McCormick DO, FACC, FSCAI Director, Cardiovascular Interventional Therapy Pennsylvania Hospital University of Pennsylvania
SCAI Fall Fellows Course 2012 Subclavian/Innominate Case Presentation Daniel J. McCormick DO, FACC, FSCAI Director, Cardiovascular Interventional Therapy Pennsylvania Hospital University of Pennsylvania
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33200-37186, 37195-37785, 92950-93272, 93303-93581,
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33200-37186, 37195-37785, 92950-93272, 93303-93581,
2018 Update in Diagnosis and Management of Stroke
 2018 Update in Diagnosis and Management of Stroke S. Andrew Josephson MD Carmen Castro Franceschi and Gladyne K. Mitchell Neurohospitalist Distinguished Professor Chair, Department of Neurology Director,
2018 Update in Diagnosis and Management of Stroke S. Andrew Josephson MD Carmen Castro Franceschi and Gladyne K. Mitchell Neurohospitalist Distinguished Professor Chair, Department of Neurology Director,
Asymptomatic Carotid Stenosis To Do or Not To Do
 Asymptomatic Carotid Stenosis To Do or Not To Do October 22, 2016 Neurosciences: Updates and Controversies Andrew C. MacDougall, MD Advocate Medical Group Advocate Lutheran General Hospital Principle
Asymptomatic Carotid Stenosis To Do or Not To Do October 22, 2016 Neurosciences: Updates and Controversies Andrew C. MacDougall, MD Advocate Medical Group Advocate Lutheran General Hospital Principle
Special Topic Section
 Special Topic Section Cerebrovasc Dis 2004;18:69 74 DOI: 10.1159/000078753 Received: March 8, 2004 Accepted: March 8, 2004 Published online: June 1, 2004 International Carotid Stenting Study: Protocol
Special Topic Section Cerebrovasc Dis 2004;18:69 74 DOI: 10.1159/000078753 Received: March 8, 2004 Accepted: March 8, 2004 Published online: June 1, 2004 International Carotid Stenting Study: Protocol
Carotid Imaging. Dr Andrew Farrall. Consultant Neuroradiologist
 20121123 SSCA http://www.neuroimage.co.uk/network Andrew Farrall Carotid Imaging Dr Andrew Farrall Consultant Neuroradiologist SFC Brain Imaging Research Centre (www.sbirc.ed.ac.uk), SINAPSE Collaboration
20121123 SSCA http://www.neuroimage.co.uk/network Andrew Farrall Carotid Imaging Dr Andrew Farrall Consultant Neuroradiologist SFC Brain Imaging Research Centre (www.sbirc.ed.ac.uk), SINAPSE Collaboration
TCT mdbuyline.com Clinical Trial Results Summary
 TCT 2012 Clinical Trial Results Summary FAME2 Trial: FFR (fractional flow reserve) guided PCI in all target lesions Patients with significant ischemia, randomized 1:1 Control arm: not hemodynamically significant
TCT 2012 Clinical Trial Results Summary FAME2 Trial: FFR (fractional flow reserve) guided PCI in all target lesions Patients with significant ischemia, randomized 1:1 Control arm: not hemodynamically significant
5/2/2016. Outpatient Stroke Management Sheila Smith MD May 5, 2016
 Outpatient Stroke Management Sheila Smith MD May 5, 2016 1 Management of Outpatient Stroke Objectives Review blood pressure management post stroke Review antithrombotic therapy Review statin therapy Discuss
Outpatient Stroke Management Sheila Smith MD May 5, 2016 1 Management of Outpatient Stroke Objectives Review blood pressure management post stroke Review antithrombotic therapy Review statin therapy Discuss
ESC Congress 2011 SIMULTANEOUS HYBRID REVASCULARIZATION OF CAROTID AND CORONARY DISEASE INITIAL RESULTS OF A NEW THERAPEUTIC APPROACH
 ESC Congress 2011 SIMULTANEOUS HYBRID REVASCULARIZATION OF CAROTID AND CORONARY DISEASE IN PATIENTS WITH ACUTE CORONARY SYNDROME: INITIAL RESULTS OF A NEW THERAPEUTIC APPROACH AUTHORS: Marta Ponte 1, RICARDO
ESC Congress 2011 SIMULTANEOUS HYBRID REVASCULARIZATION OF CAROTID AND CORONARY DISEASE IN PATIENTS WITH ACUTE CORONARY SYNDROME: INITIAL RESULTS OF A NEW THERAPEUTIC APPROACH AUTHORS: Marta Ponte 1, RICARDO
WHI Form Report of Cardiovascular Outcome Ver (For items 1-11, each question specifies mark one or mark all that apply.
 WHI Form - Report of Cardiovascular Outcome Ver. 6. COMMENTS To be completed by Physician Adjudicator Date Completed: - - (M/D/Y) Adjudicator Code: OMB# 095-044 Exp: 4/06 -Affix label here- Clinical Center/ID:
WHI Form - Report of Cardiovascular Outcome Ver. 6. COMMENTS To be completed by Physician Adjudicator Date Completed: - - (M/D/Y) Adjudicator Code: OMB# 095-044 Exp: 4/06 -Affix label here- Clinical Center/ID:
2/7/
 Disclosure Intracranial Atherosclerosis an update None Mai N. Nguyen-Huynh, MD, MAS Assistant Professor of Neurology UCSF Neurovascular Service February 7, 2009 Case #1 60 y.o. Chinese-speaking speaking
Disclosure Intracranial Atherosclerosis an update None Mai N. Nguyen-Huynh, MD, MAS Assistant Professor of Neurology UCSF Neurovascular Service February 7, 2009 Case #1 60 y.o. Chinese-speaking speaking
TCAR: TransCarotid Artery Revascularization Angela A. Kokkosis, MD, RPVI, FACS
 TCAR: TransCarotid Artery Revascularization Angela A. Kokkosis, MD, RPVI, FACS Assistant Professor of Surgery Director of Carotid Interventions Division of Vascular & Endovascular Surgery Stony Brook University
TCAR: TransCarotid Artery Revascularization Angela A. Kokkosis, MD, RPVI, FACS Assistant Professor of Surgery Director of Carotid Interventions Division of Vascular & Endovascular Surgery Stony Brook University
Michael Horowitz, MD Pittsburgh, PA
 Michael Horowitz, MD Pittsburgh, PA Introduction Cervical Artery Dissection occurs by a rupture within the arterial wall leading to an intra-mural Hematoma. A possible consequence is an acute occlusion
Michael Horowitz, MD Pittsburgh, PA Introduction Cervical Artery Dissection occurs by a rupture within the arterial wall leading to an intra-mural Hematoma. A possible consequence is an acute occlusion
ORIGINAL CONTRIBUTION
 ORIGINAL CONTRIBUTION Safety of Latest-Generation Self-expanding Stents in Patients With NASCET-Ineligible Severe Symptomatic Extracranial Internal Carotid Artery Stenosis Italo Linfante, MD; Joshua A.
ORIGINAL CONTRIBUTION Safety of Latest-Generation Self-expanding Stents in Patients With NASCET-Ineligible Severe Symptomatic Extracranial Internal Carotid Artery Stenosis Italo Linfante, MD; Joshua A.
Comparison of Five Major Recent Endovascular Treatment Trials
 Comparison of Five Major Recent Endovascular Treatment Trials Sample size 500 # sites 70 (100 planned) 316 (500 planned) 196 (833 estimated) 206 (690 planned) 16 10 22 39 4 Treatment contrasts Baseline
Comparison of Five Major Recent Endovascular Treatment Trials Sample size 500 # sites 70 (100 planned) 316 (500 planned) 196 (833 estimated) 206 (690 planned) 16 10 22 39 4 Treatment contrasts Baseline
2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Outcome
 Measure #344: Rate of Carotid Artery Stenting (CAS) for Asymptomatic Patients, Without Major Complications (Discharged to Home by Post-Operative Day #2) National Quality Strategy Domain: Effective Clinical
Measure #344: Rate of Carotid Artery Stenting (CAS) for Asymptomatic Patients, Without Major Complications (Discharged to Home by Post-Operative Day #2) National Quality Strategy Domain: Effective Clinical
2014 Update in Diagnosis and Management of Stroke
 2014 Update in Diagnosis and Management of Stroke S. Andrew Josephson MD Carmen Castro Franceschi and Gladyne K. Mitchell Neurohospitalist Distinguished Professor Acting Chairman, Department of Neurology
2014 Update in Diagnosis and Management of Stroke S. Andrew Josephson MD Carmen Castro Franceschi and Gladyne K. Mitchell Neurohospitalist Distinguished Professor Acting Chairman, Department of Neurology
Advances in Prevention and Treatment of Stroke: What Every Primary Care Physician Needs to Know. Case 1 4/5/11. What treatment should you initiate?
 Advances in Prevention and Treatment of Stroke: What Every Primary Care Physician Needs to Know S. Andrew Josephson, MD Director, Neurohospitalist Program Medical Director, Inpatient Neurology University
Advances in Prevention and Treatment of Stroke: What Every Primary Care Physician Needs to Know S. Andrew Josephson, MD Director, Neurohospitalist Program Medical Director, Inpatient Neurology University
Subclavian artery Stenting
 Subclavian artery Stenting Etiology Atherosclerosis Takayasu s arteritis Fibromuscular dysplasia Giant Cell Arteritis Radiation-induced Vascular Injury Thoracic Outlet Syndrome Neurofibromatosis Incidence
Subclavian artery Stenting Etiology Atherosclerosis Takayasu s arteritis Fibromuscular dysplasia Giant Cell Arteritis Radiation-induced Vascular Injury Thoracic Outlet Syndrome Neurofibromatosis Incidence
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
The CARENET all-comer trial using the CGuard micronet covered carotid embolic prevention stent
 The CARENET all-comer trial using the CGuard micronet covered carotid embolic prevention stent 6 month data Piotr Musialek, MD DPhil FESC Jagiellonian University Dept. of Cardiac & Vascular Diseases John
The CARENET all-comer trial using the CGuard micronet covered carotid embolic prevention stent 6 month data Piotr Musialek, MD DPhil FESC Jagiellonian University Dept. of Cardiac & Vascular Diseases John
Beyond Stenosis Severity: Top 5 Important Duplex Characteristics to Identify in a Patient with Carotid Disease
 Beyond Stenosis Severity: Top 5 Important Duplex Characteristics to Identify in a Patient with Carotid Disease Jan M. Sloves RVT, RCS, FASE Technical Director New York Cardiovascular Associates Disclosures
Beyond Stenosis Severity: Top 5 Important Duplex Characteristics to Identify in a Patient with Carotid Disease Jan M. Sloves RVT, RCS, FASE Technical Director New York Cardiovascular Associates Disclosures
Emergency Department Stroke Registry Process of Care Indicator Specifications (July 1, 2011 June 30, 2012 Dates of Service)
 Specifications Description Methodology NIH Stroke Scale (NIHSS) Performed in Initial Evaluation used to assess the percentage of adult stroke patients who had the NIHSS performed during their initial evaluation
Specifications Description Methodology NIH Stroke Scale (NIHSS) Performed in Initial Evaluation used to assess the percentage of adult stroke patients who had the NIHSS performed during their initial evaluation
Carotid Artery Stenosis
 Evidence-Based Approach to Carotid Artery Stenosis Seong-Wook Park, MD Division of Cardiology, Asan Medical Center University of Ulsan College of Medicine, Seoul, Korea Carotid Artery Stenosis Carotid
Evidence-Based Approach to Carotid Artery Stenosis Seong-Wook Park, MD Division of Cardiology, Asan Medical Center University of Ulsan College of Medicine, Seoul, Korea Carotid Artery Stenosis Carotid
Introducing the COAPT Trial
 physician INFORMATION Eligible patients Symptomatic functional mitral regurgitation 3+ Not suitable candidates for open mitral valve surgery NYHA functional class II, III, or ambulatory IV Introducing
physician INFORMATION Eligible patients Symptomatic functional mitral regurgitation 3+ Not suitable candidates for open mitral valve surgery NYHA functional class II, III, or ambulatory IV Introducing
CardioLucca2014. Fare luce sulla scelta ottimale del trattamento nella rivascolarizzazione delle stenosi carotidee. Fabrizio Tomai
 CardioLucca2014 Fare luce sulla scelta ottimale del trattamento nella rivascolarizzazione delle stenosi carotidee Fabrizio Tomai European Hospital e Aurelia Hospital Roma Treatment of Carotid Artery Disease
CardioLucca2014 Fare luce sulla scelta ottimale del trattamento nella rivascolarizzazione delle stenosi carotidee Fabrizio Tomai European Hospital e Aurelia Hospital Roma Treatment of Carotid Artery Disease
Surgical Treatment of Carotid Disease
 Department of Cardiothoracic & Vascular Surgery McGovern Medical School / The University of Texas Health Science Center at Houston Surgical Treatment of Carotid Disease The Old, the New, and the Future
Department of Cardiothoracic & Vascular Surgery McGovern Medical School / The University of Texas Health Science Center at Houston Surgical Treatment of Carotid Disease The Old, the New, and the Future
Non-commercial use only
 Italian Journal of Medicine 2016; volume 10:202-206 Embolic stroke of undetermined source: a retrospective analysis from an Italian Stroke Unit Marco Masina, 1 Annalena Cicognani, 1 Carla Lofiego, 2 Simona
Italian Journal of Medicine 2016; volume 10:202-206 Embolic stroke of undetermined source: a retrospective analysis from an Italian Stroke Unit Marco Masina, 1 Annalena Cicognani, 1 Carla Lofiego, 2 Simona
Stroke Topics. Advances in the Prevention and Treatment of Stroke. Non-Contrast Head CT. Patient 1-68 yo man
 Stroke Topics Advances in the Prevention and Treatment of Stroke August 10, 2009 John W. Engstrom, M.D. Professor of Neurology Acute treatment options for ischemic stroke tpa, clot retraction, future directions
Stroke Topics Advances in the Prevention and Treatment of Stroke August 10, 2009 John W. Engstrom, M.D. Professor of Neurology Acute treatment options for ischemic stroke tpa, clot retraction, future directions
ORIGINAL CONTRIBUTION. Early Stroke Risk After Transient Ischemic Attack Among Individuals With Symptomatic Intracranial Artery Stenosis
 ORIGINAL CONTRIBUTION Early Stroke Risk After Transient Ischemic Attack Among Individuals With Symptomatic Intracranial Artery Stenosis Bruce Ovbiagele, MD; Salvador Cruz-Flores, MD; Michael J. Lynn, MS;
ORIGINAL CONTRIBUTION Early Stroke Risk After Transient Ischemic Attack Among Individuals With Symptomatic Intracranial Artery Stenosis Bruce Ovbiagele, MD; Salvador Cruz-Flores, MD; Michael J. Lynn, MS;
CAROTID STENTING A 2009 UPDATE. Hoang Duong, MD Director of Interventional Neuroradiology Memorial Regional Hospital
 CAROTID STENTING A 2009 UPDATE Hoang Duong, MD Director of Interventional Neuroradiology Memorial Regional Hospital TREATMENT FOR CAROTID STENOSIS Best medical management Antiplatelet therapy Antihypertensive
CAROTID STENTING A 2009 UPDATE Hoang Duong, MD Director of Interventional Neuroradiology Memorial Regional Hospital TREATMENT FOR CAROTID STENOSIS Best medical management Antiplatelet therapy Antihypertensive
Supplementary Online Content
 Supplementary Online Content Inohara T, Manandhar P, Kosinski A, et al. Association of renin-angiotensin inhibitor treatment with mortality and heart failure readmission in patients with transcatheter
Supplementary Online Content Inohara T, Manandhar P, Kosinski A, et al. Association of renin-angiotensin inhibitor treatment with mortality and heart failure readmission in patients with transcatheter
Dabigatran following acute transient ischemic attack and minor stroke II (DATAS II)
 Protocol Dabigatran following acute transient ischemic attack and minor stroke II (DATAS II) International Journal of Stroke 2017, Vol. 12(8) 910 914! 2017 World Stroke Organization Reprints and permissions:
Protocol Dabigatran following acute transient ischemic attack and minor stroke II (DATAS II) International Journal of Stroke 2017, Vol. 12(8) 910 914! 2017 World Stroke Organization Reprints and permissions:
2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
 Measure #260: Rate of Carotid Endarterectomy (CEA) for Asymptomatic Patients, without Major Complications (Discharged to Home by Post-Operative Day #2) National Quality Strategy Domain: Patient Safety
Measure #260: Rate of Carotid Endarterectomy (CEA) for Asymptomatic Patients, without Major Complications (Discharged to Home by Post-Operative Day #2) National Quality Strategy Domain: Patient Safety
Protocol. This trial protocol has been provided by the authors to give readers additional information about their work.
 Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive
Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive
PFO Management update
 PFO Management update May 12, 2017 Peter Casterella, MD Swedish Heart and Vascular 1 PFO Update 2017: Objectives Review recently released late outcomes of RESPECT trial and subsequent FDA approval of PFO
PFO Management update May 12, 2017 Peter Casterella, MD Swedish Heart and Vascular 1 PFO Update 2017: Objectives Review recently released late outcomes of RESPECT trial and subsequent FDA approval of PFO
CAROTID ARTERY ANGIOPLASTY
 CAROTID ARTERY ANGIOPLASTY Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and
CAROTID ARTERY ANGIOPLASTY Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and
Carotid Stenosis 1/24/2019. Review of Primary Studies. NASCET- Moderate stenosis. ACAS (Asymptomatic Carotid Atherosclerosis Study) NASCET
 Review of Primary Studies Carotid Stenosis NINDS National Institute of Neurological Disorders and Stroke 2 large studies to determine who would benefit from surgery NASCET North American Symptomatic Carotid
Review of Primary Studies Carotid Stenosis NINDS National Institute of Neurological Disorders and Stroke 2 large studies to determine who would benefit from surgery NASCET North American Symptomatic Carotid
Update on Carotid Disease
 Update on Carotid Disease L. Nelson Hopkins, MD Elad Levy, MD Adnan Siddiqui, MD,PhD Ken Snyder, MD,PhD Gates Vascular Institute LN Hopkins, MD I disclose the following financial relationship(s): President,
Update on Carotid Disease L. Nelson Hopkins, MD Elad Levy, MD Adnan Siddiqui, MD,PhD Ken Snyder, MD,PhD Gates Vascular Institute LN Hopkins, MD I disclose the following financial relationship(s): President,
CANADIAN STROKE BEST PRACTICE RECOMMENDATIONS
 CANADIAN STROKE BEST PRACTICE RECOMMENDATIONS Management of Extracranial Carotid Disease and Intracranial Atherosclerosis Wein T, Gladstone D (Writing Group Chairs) on Behalf of the PREVENTION of STROKE
CANADIAN STROKE BEST PRACTICE RECOMMENDATIONS Management of Extracranial Carotid Disease and Intracranial Atherosclerosis Wein T, Gladstone D (Writing Group Chairs) on Behalf of the PREVENTION of STROKE
Endovascular treatment for pseudoocclusion of the internal carotid artery
 Endovascular treatment for pseudoocclusion of the internal carotid artery Daqiao Guo, Xiao Tang, Weiguo Fu Institute of Vascular Surgery, Fudan University, Department of Vascular Surgery, Zhongshan Hospital
Endovascular treatment for pseudoocclusion of the internal carotid artery Daqiao Guo, Xiao Tang, Weiguo Fu Institute of Vascular Surgery, Fudan University, Department of Vascular Surgery, Zhongshan Hospital
CEREBRO VASCULAR ACCIDENTS
 CEREBRO VASCULAR S MICHAEL OPONG-KUSI, DO MBA MORTON CLINIC, TULSA, OK, USA 8/9/2012 1 Cerebrovascular Accident Third Leading cause of deaths (USA) 750,000 strokes in USA per year. 150,000 deaths in USA
CEREBRO VASCULAR S MICHAEL OPONG-KUSI, DO MBA MORTON CLINIC, TULSA, OK, USA 8/9/2012 1 Cerebrovascular Accident Third Leading cause of deaths (USA) 750,000 strokes in USA per year. 150,000 deaths in USA
CLINICAL TIMELINE EVA-3S CREST ICSS SPACE SAPPHIRE
 Normal Risk Symptomatic Patients: Ongoing Debate CAS vs CEA John R. Laird, MD Professor of Medicine Medical Director of the Vascular Center University of California, Davis CLINICAL TIMELINE Randomized
Normal Risk Symptomatic Patients: Ongoing Debate CAS vs CEA John R. Laird, MD Professor of Medicine Medical Director of the Vascular Center University of California, Davis CLINICAL TIMELINE Randomized
More than strokes occur
 Surgery vs Stent: Treatment for Carotid Artery Disease Imad A. Alhaddad, MD ABSTRACT PURPOSE: This article summarizes and compares the roles of surgery and stent in the management of carotid artery disease.
Surgery vs Stent: Treatment for Carotid Artery Disease Imad A. Alhaddad, MD ABSTRACT PURPOSE: This article summarizes and compares the roles of surgery and stent in the management of carotid artery disease.
I, (Issam Moussa) DO NOT have a financial interest/arrangement t/ t or affiliation with one or more organizations that could be perceived as a real
 PFO Closure: Where We Are Going to after CLOSURE I Study? Issam D. Moussa, MD Professor of Medicine Chair, Division of Cardiovascular Diseases Mayo Clinic Jacksonville, Florida Disclosure Statement of
PFO Closure: Where We Are Going to after CLOSURE I Study? Issam D. Moussa, MD Professor of Medicine Chair, Division of Cardiovascular Diseases Mayo Clinic Jacksonville, Florida Disclosure Statement of
