MR-Guided Assessment and Management of Ventricular Tachycardia
|
|
|
- Darrell Fitzgerald
- 5 years ago
- Views:
Transcription
1 MR-Guided Assessment and Management of Ventricular Tachycardia by Samuel Oluwagbemiga Oduneye A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Graduate Department of Medical Biophysics University of Toronto Copyright by Samuel O. Oduneye 2013
2 ii Abstract MR-Guided Assessment and Management of Ventricular Tachycardia Samuel O. Oduneye Doctor of Philosophy Graduate Department of Medical Biophysics University of Toronto 2013 This thesis describes the electrical and physiological characterization of cardiac tissue with myocardial infarction (MI) responsible for abnormal cardiac rhythms such as ventricular tachycardia (VT), using a newly-developed magnetic resonance imaging (MRI) electrophysiology system. In electrophysiology (EP), radiofrequency (RF) catheter ablation combined with cardioverter-defibrillator implantation is a first-line action to manage ventricular VT. Unfortunately, this therapy is known to have sub-optimal success rates in a large number of patients because of difficulties to accurately identifying the arrhythmic target regions. Currently, characterization of post-mi scars is performed by using catheters to measure electrical signals of the endocardial tissue (electroanatomical mapping), under x-ray fluoroscopy guidance. Prolonged radiation exposure to both the cardiologist and the patient have made the use of MRI extremely attractive; further, unlike x-ray imaging, MRI provides post-mi scars with direct visualization, characterization in three dimensions and the ability to visualize ablation lesions. Although recent research has focused on registration between pre-acquired MR images and electroanatomical maps, a potentially more useful approach is to use real-time MRI to directly ii
3 locate and characterize potential arrhythmogenic regions during the EP procedure. A real-time MR-guided EP system was developed and validated to perform EP diagnostic procedures, such as mapping and pacing. In a series of animal studies, the system demonstrated the ability to use active catheter tracking and intra-procedural MR imaging to navigate to specific regions in the left ventricle and record intracardiac electrical signals. A study correlating myocardial fibrotic scar detected by multicontrast late enhancement (MCLE) MRI and electroanatomical voltage mapping demonstrated that MRI information (transmurality, tissue classification, and relaxation rate) can accurately predict areas of myocardial fibrosis identified with bipolar voltage mapping. Finally, MCLE-derived gray zone was shown to have a high correspondence to regions with a high proportion of abnormal intracardiac signals. The methods described in this thesis help advance the understanding of infarcted tissue responsible for ventricular tachycardia. Further studies are proposed to perform RF ablation lesions and correlate pre- and post-ablation tissue electrophysiological properties with MRI. iii iii
4 iv Acknowledgments The acknowledgement section of my Masters thesis read as I approach the end of this chapter of my life, I can only look back and appreciate the greatness of all of the events that led me to this moment and most important I thank the people that have crossed my path and made a contribution. As I am extremely excited for what lies ahead, I am forever grateful to all of you.... Those words couldn t resonate more powerful and true today. The path to this degree has been amazing and I have learned so much about myself. I would like to thank my supervisor, Graham Wright, for his guidance and for making this journey a much more enjoyable experience while still challenging me along the way. I also need to thank my committee, Don Plewes, and Rajiv Chopra for the insightful advice provided along the way. A special thanks goes to all the members for the Wright Group. I owe a huge debt of gratitude to Labonny Biswas, Jennifer Barry, Sudip Ghate, Venkat Ramanan, and Roey Flor for tirelessly supporting me during lengthy and demanding experiments: you are the best team that a doctorate student can have. I am particularly grateful for Mihaela Pop for showing me the ropes when I first joined the lab, and she has continued to be an invaluable resource over the years. I also would like to thank other Wright Group members Yingli Lu, Kevan Anderson, Haydar Celik, Garry Liu, and Nick Yak for useful conversations on both software and hardware aspects of my research. None of this work would have been possible without the support of Eugene Crystal and the Arrhythmia Services group at Sunnybrook Hospital. Thank you to Ilan lashevsky, Ehud Kadmon, Tawfiq Zeidan Shwiri, Avishag Laish FarKash and Mohammed Shurrab for providing your clinical expertise during the electrophysiology procedures and showing me how to use a catheter. I am grateful to Andriy Shmatukha for giving me my start in interventional MRI. A dearest thank you goes to my parents, family and friends for caring and been always there for me. Last but not least, I would like to thank Onye Nnorom, without her unconditional support and love I m not sure I would have been able to learn the many teachings outside the lab. I said it once and I ll say it again Change her and I am changed also. I look forward to many more exciting years together. iv
5 v Table of Contents Acknowledgments... iv Table of Contents... v List of Tables... ix List of Figures... x Statement of Contribution... xii Chapter Introduction Overview Cardiac System Post-infarction Ventricular Tachycardia and Clinical Motivation VT Mechanism Therapy And Management Options Diagnostic and Assessment Tools Electrophysiology study The Role of MRI Interventional Cardiac MRI MR Tracking Techniques Motivation Overview of Thesis References Chapter Electroanatomical Mapping With Real Time MR-Guided System Introduction Methods v
6 2.2.1 Electroanatomical Mapping with MR-guided Electrophysiology System EAM with CARTO Animal preparation Statistical and error analysis Results Electroanatomical mapping results EAM analysis Infarct EAM comparison Discussion Conclusion References Chapter Comparison Between Late Enhancement Magnetic Resonance Imaging And Voltage Mapping Introduction Material and Methods Animal preparation Magnetic resonance imaging and image processing MR-guided three-dimensional electroanatomical voltage mapping and analysis Correlation between multi-contrast late enhancement and voltage signal of electroanatomical map Statistical analysis Histopathology Results MR-guided three-dimensional electroanatomical voltage mapping and analysis vi vi
7 3.3.2 Correlation between multi-contrast late enhancement signal classification and voltage signal of electroanatomical map Discussion Conclusion References Chapter Distribution of Abnormal Intracardiac Signals In Infarct Regions Introduction Methods Animal preparation Imaging protocol and image processing Real-time MR-guided electrophysiology system Electrophysiology procedure Statistical analysis Results Electrophysiology mapping Distribution of Abnormal Potential Signals In Different Cardiac Rhythms Distribution of Abnormal Signal potentials Discussion Abnormal Potential Signals Distribution Limitations of electrophysiology procedure Conclusion References Chapter Summary and Future Directions Summary vii vii
8 5.2 Biophysics of RF Ablation and Lesion Characterization Future directions: MR-guided RF ablation Future directions: Lesion Characterization Alternative Modalities Final Words References viii viii
9 ix List of Tables Table 1.1: Average arrival time of action potential in ventricular regions of animal Table 3.1: Results of magnetic resonance imaging studies...53 Table 4.1: Distribution of abnormal potential EGMs in scar, gray zone and healthy regions...69 ix
10 x List of Figures Figure 1-1 Propagation of impulse in the myocardium... 3 Figure 1-2 Standard model of cardiac action potential... 4 Figure 1-3 Electrophysiology of the heart... 5 Figure 1-4 Schematic representation of the reentrant circuit... 6 Figure 1-5 Effect of scar on electrical propagation Figure 1-6 Example of typical electrophysiology study Figure 1-7 Influence of electrode spacing and size on bipolar voltage signal Figure 1-8 Local activation time map Figure 1-9 Voltage map of the left ventricle in a patient with ventricular tachycardia Figure 1-10 Contrast Enhanced Imaging. Early to late inversion time change shown. Various...19 Figure 1-11 In vivo active catheter guidance with image fusion Figure 1-12 In vivo active catheter guidance projection method Figure 2-1 MR-compatible catheter with three tracking coils Figure 2-2 MR-guided real-time electrophysiology system Figure 2-3 Local activation time map using CARTO vs MR-guided EP system Figure 2-4 Analysis LAT segments Figure 2-5 2D MCLE images of infarcted swine Figure 2-6 Isochronal LAT maps, comparison between a healthy heart and infarct heart Figure 2-7 Local activation map, voltage bipolar map and; MCLE MRI in-situ image x
11 Figure 3-1 Multi-contrast late enhancement MR images Figure 3-2 Description of sector division for short-axis slice tissue analysis Figure 3-3 Median bipolar voltage of points by percentage transmurality of scar Figure 3-4 Distribution of points for tissue classification Figure 3-5 Bipolar voltage vs. normalized R1* Figure 3-6 Approximate Masson Trichrome histology slice of corresponding region Figure 4-1 Examples of abnormal EGMs recordings Figure 4-2 Distribution of abnormal potential recordings in sinus and paced rhythm Figure 4-3 Activation map recorded during mapping procedure Figure 4-4 Distributions of abnormal potentials Figure dimensional voltage maps using the MRI-guided electrophysiology system Figure 5-2 SSFP image of left ventricle after ablation with corresponding voltage mapping Figure 5-3 Direct visual comparison of right ventricular radio frequency ablation lesion Figure 5-4 RFA lesion images xi xi
12 xii Statement of Contribution This thesis presents work done by myself in conjunction with other members of the Wright group lab and with electrophysiology fellows at Sunnybrook Health Science Centre. The ideas for the research presented were developed by me, but with significant feedback and guidance from my supervisor, Dr. Graham Wright. All MRI, electrophysiology, data, and statistical analysis was done solely by me. With the exception of the first few catheter procedures performed by Dr. Eugene Crystal and his electrophysiology fellows, the majority of the catheter manipulation procedures in the animal were done by me under the supervision of Jennifer Barry, veterinarian tech. The visualization software VURTIGO was developed by Roey Flor and the real-time MRtracking software by Bonny Biswas, however the electrophysiology analysis code used in Chapters 2 and 3, was written solely by me. I also wrote code to fuse MRI information with electrophysiology information, which was later adapted by Roey Flor to run natively on Vurtigo. The MRI data in Chapter 2 was acquired by Venkat Ramanan. The MRI data in Chapters 3 and 4 was acquired by me. All data analysis in chapters 3 and 4 was done by me with exception of segmentation process, which was developed in house by Dr. Yingli Lu. All studies described in chapter 5 were organized by me with the exception of the preliminary non-contrast imaging studies reported in the text, which are led by Dr. Kevan Anderson and Dr. Haydar Celik. xii
13 1 Chapter 1 1 Introduction 1.1 Overview Ischemia denotes the restriction of blood supply to tissues, where the oxygen demand is not met by the supply of oxygen due to reduced blood flow or complete obstruction of blood flow. In the heart, severe and prolonged ischemia can lead to permanent damage in the form of cell death, also known as myocardial infarction (MI). In a patient, following a MI episode, scar tissue may develop within the myocardium, creating the substrate for the generation of ventricular tachycardia (VT). VT is a rapid heart-rate condition that originates in the ventricles and can manifest with pulse rates of more than 100 beats per minute (BPM), with at least three irregular heartbeats in a row. VT can degenerate into ventricular fibrillation - an uncoordinated contraction of the cardiac muscle of the ventricles where blood is no longer pumped from the ventricles and quickly leads to sudden cardiac death (SCD) [1]. Patients who experience ventricular arrhythmic episodes and seek treatment depend on the accuracy of the diagnostic tools to identify the origin of the arrhythmogenicity, which affects the success of therapeutic electrophysiological (EP) procedures. This thesis deals with the assessment of myocardial tissue in post-infarct chronic settings. The ventricular myocardium containing scar tissue has been shown to support VT substrate. Accurate electrical and morphological characterization of the cardiac substrate is an essential component of an electrophysiology study. In recent years, magnetic resonance imaging (MRI) has played a major role in the identification of ventricular scar tissue, becoming the de-facto gold standard for the assessment and the detection of MI; therefore in this thesis we proposed research work that combines electrophysiology and MRI information. The aim is to improve the characterization of the cardiac tissue to better target the origin of the arrhythmia and eventually to improve the outcome of the intervention. Chapter 1 discusses the context in which an electrophysiology assessment of myocardial tissue is performed, the role of MRI myocardial imaging in the context of electrophysiology, and the application of these two modalities. The limitations of the current electrophysiology methods for tachycardia management and limitations of cardiac MRI are also described and provide the motivation for the bodies of work described in
14 Chapters 2, 3, and 4. This thesis concludes with future directions for MR-guided therapy in Chapter Cardiac System The normal cardiac heart rate (sinus rhythm) is beats/minute [2]. The sinus rhythm normally controls both atrial and ventricular rhythm. Typically, the heart rate is determined by a pacemaker site called the sinoatrial (SA) node located in the posterior wall of the right atrium near the superior vena cava (see Figure 1-1). The SA node consists of specialized cells (pacemaker cells) that undergo spontaneous generation of action potentials (electrical signal). Action potentials generated by the SA node spread throughout the atria, depolarizing this tissue and causing atrial contraction. The impulse then travels into the ventricles via the atrioventricular node (AV node). Then, within the ventricles, specialized branched conduction pathways (right and left bundle branches and the Purkinje fibers) rapidly conduct the wave of depolarization throughout the ventricles to initiate ventricular contraction. Normally, the only pathway available for action potentials to enter the ventricles is through the AV node located in the inferior-posterior region of the interatrial septum. As mentioned above, the AV node, a highly specialized conducting tissue, is also responsible for slowing down the electrical impulse conduction enough to allow sufficient time for complete atrial depolarization and contraction (systole) to occur prior to ventricular depolarization and contraction. Afterwards, the action potential enters the base of the ventricle at the Bundle of His, then continues to the left and right bundle branches. The bundle branches then divide into an extensive and complex system of Purkinje fibers that conduct the impulses throughout the ventricles. This results in rapid depolarization of ventricular myocytes throughout both ventricles. Overall, the sequence of cardiac electrical activation follows the following pattern: SA node -> AV node -> Bundle of His -> Left & Right bundle branches -> Purkinje Fibers (see Figure 1-1). The atria and ventricles are electrically separated by collagenous and electrically inert structures. The valve rings, central body and skeleton of the heart consisting of collagen are impermeable to electrical propagation, which results in a necessary slow-down of electrical propagation that allows sequential contraction of atria and ventricles. 2
15 3 Figure 1-1 As the SA node fires, each electrical impulse travels through the right and left atrium. This electrical activity causes the two upper chambers of the heart to contract then the two lower chambers to contract (modified from: Many excitable cells in the body have the ability to undergo a transient depolarization and repolarization that is either triggered by external mechanisms (e.g., motor nerve stimulation of skeletal muscle or cell-to-cell depolarization in the heart) or by intracellular, spontaneous mechanisms (e.g., cardiac pacemaker cells). There are two general types of cardiac action potentials: non-pacemaker action potentials, also called "fast response" action potentials because of their rapid depolarization, are found throughout the heart except for the pacemaker cells. The pacemaker cells generate spontaneous action potentials that are also termed "slow response" action potentials because of their slower rate of depolarization. These are found in the sinoatrial and atrioventricular nodes of the heart. In the heart muscle cell, or myocyte, electric activation takes place by inflow of sodium (Na + or Ca ++ ) ions across the cell membrane. This process is called depolarization and is shown in Figure 1-2. In the resting state the intracellular potential is approximate -90mV (also called the resting membrane potential), where the extra-cellular space is considered at 0mV or more positive. Calcium (Ca ++ ) influx prolongs the duration of the action potential and produces a characteristic plateau phase. Finally, the repolarization process is governed by the inflow of potassium (K + ). Pacemaker cells are able to initiate sodium transfer and are part of the conduction fibers.
16 4 Figure 1-2 The standard model used to describe the cardiac action potential is the action potential of the ventricular myocyte. a) Action potentials are generated by the movement of ions through the transmembrane ion channels in the cardiac cells. In b) the intracellular action potential phases are shown to describe the dynamics of the potential change. (modified from Electrical coupling is responsible for inter-cellular signaling and electrical signal transmission. Atrial myocytes, ventricular myocytes and Purkinje cells are examples of non-pacemaker cells in the heart. Unlike pacemaker cells found in nodal tissue within the heart, non-pacemaker cells have a true resting membrane potential (phase 4) that remains near the equilibrium potential for K + at about 90 mv (see Figure 1-2). When these cells are depolarized by an action potential in an adjacent cell, there is a rapid depolarization (phase 0) that is caused by a transient increase of Na + ions, through sodium channels. Phase 1 represents an initial repolarization that is caused by a short-lived opening of transient outward K + channel. Following phase 1, a large increase in slow inward Ca ++ begins, the repolarization is delayed and there is a plateau phase in the action potential (phase 2). Repolarization (phase 3) occurs when an outflow of K+ increases. The action potential represents the electrical activity in a single myocyte, the intracardial electrogram records the local extracellular electrical on the endocardial surface and the temporal summation of the action potentials from all the myocytes in the heart is recorded on the body using surface electrocardiograms (ECG), see Figure 1-3. The ECG recordings typically shows three main deflections described as the QRS complex, which corresponds to the depolarization of the right and left ventricles.
17 5 Figure 1-3 Electrophysiology of the heart. The different waveforms for each of the specialized cells found in the heart are shown: SN (Sino-atrial node), AVN (Atrio-ventricular node), HB (Bundle of HIS), LBB/RBB (Left Bundle Branch, Right Bundle Branch) and Purkinje Fibers. Intracardiac electrogram records the extracellular electrical activity detected with catheters placed on the endocardial surface. The spread of electrical activity, recorded on the chest of the patient produces the QRS complex on the ECG: typically an ECG has five deflections, arbitrarily named P, Q, R, S and T waves (modified: from presentation by F.M.Leonelli M.D). 1.3 Post-infarction Ventricular Tachycardia and Clinical Motivation The rate of sudden cardiac death (SCD) is 300,000 to 400,000 annually in the United States[3] and is approximately 20,000 annually in Canada [4]. The mechanism of death in the majority of patients dying of sudden cardiac death is ventricular fibrillation. In these cases ventricular fibrillation is often caused by the onset of ventricular tachycardia (VT). The most common finding at postmortem examination is chronic high-grade stenosis of at least one segment of a major coronary artery, or an identifiable thrombus (clot) in a major coronary artery, which causes transmural occlusion of that vessel. In other words, these results suggest that myocardial infarct (MI) and ischemia are the most common causes of the arrhythmia leading to SCD. Chronic (healed) infarctions are present in over half of all victims of SCD and survivors of sudden cardiac arrest [3].
18 The most common pathophysiologic mechanism for abnormal conduction results from localized or regional depolarization due to hypoxia caused by impaired coronary blood flow. Depolarization decreases the action potential amplitude and rate of depolarization (phase 0 slope is decreased), both of which decreases the velocity of action potential conduction or completely stop the conduction of action potentials (i.e., conduction block). When the conduction block does not form a complete AV block (e.g., left bundle branch block), electrical impulses can travel along alternate conduction pathways to depolarize the ventricles. When this occurs, it takes longer for the ventricles to depolarize. This is manifested as an increase in the duration of the QRS complex, and a change in its shape. Sometimes, the abnormal conduction pathways can cause a self-perpetuating, circular movement of electrical activation. This is termed reentry and is a major cause of ventricular and supraventricular tachycardias. 6 Figure 1-4 Schematic representation of the reentrant circuit; a wavefront is shown propagating through the tissue of the reentrant circuit (red arrow). a) and b) activation pathway of depolarization wave. c), d) and e) A stylized tachycardia circuit is shown in which propagation proceeds through a central pathway (red arrow) constrained by scars and then around the outside of these same barriers.
19 VT Mechanism As mentioned above, VT can be a fatal arrhythmia because it may degenerate into ventricular fibrillation[5]. In many patients, myocardial infarction scar tissue within the myocardium creates the condition for a reentrant circuit or pathway. In Figure 1-4 the reentry circuit is shown when formed between two scars (reentry circuit can also occur between an anatomical obstacle and a single scar [5]). The depolarization wave propagates through the myocardium, and when it encounters areas of scars, it may travel through a region of slow conduction and upon exiting the channel the now re-excitable surrounding tissue can be re-excited before the next sinus (normal) wave arrives (see Figure 1-4). The slow conduction central pathway in the figure above is the main factor creating a loop or circular reentry pathway between the two scars, and is referred to as the isthmus. Typically, when tissue pathology analysis is performed in the regions suspected to contain isthmuses, the result display tissues highly heterogeneous in nature [6], [7]: bundles of viable myocytes mixed with fibrotic, collagenous bundles. The mechanism for the slow action potential propagation has been explained in [8]: bundles of surviving fibers are connected heterogeneously, resulting in a zig-zag pattern of transverse conduction along a pathway lengthened by branching and merging bundles of surviving myocytes. Slow action potential propagation as previously mentioned can also result from hypoxia, which affects gap junctions and the cell-to-cell coupling. Also, the effect of scar on the electrical signal has been well characterized [9]: the normal electrogram is the result of normal cell to cell coupling and produces synchronous depolarization measurable with a distinct sharp deflection as shown in Figure 1-5; heterogeneous tissue that contains scar gives rise to a fractionated intracardiac electrogram due to poor cell to cell coupling of the surviving myocytes, which results in asynchronous depolarization as seen in Figure 1-5. Therefore, there is disruption of the smooth transverse pattern of conduction characteristic of uniform propagation, which results in a markedly irregular sequence or zig-zag conduction, producing the low-amplitude fractionated extracellular electrograms characteristic of nonuniform anisotropic conduction [8].
20 8 Figure 1-5 Effect of scar on electrical propagation. a) healthy myocardium conducts an electrical wavefront consistently, with synchronous activation of a large number of cells, leading to a sharp signal deflection whereas in b) healthy myocardium mixed with collagen fibers produces nonuniform propagation, resulting in a low-amplitude electrogram, with multiple fragmented peaks Therapy And Management Options To manage VT, drug treatment is the initial option.; anti-arrhythmic drugs interact with ion channels reducing or facilitating ion current flow. However, 40% of treated patients will still experience a VT episode within 2 years [10] Therefore, the American College of Cardiology guidelines recommend that any patient on anti-arrhythmic drugs for the management of VT be protected by an implantable cardioverter-defibrillator (ICD). With an ICD, termination of sustained VT is achieved by controlled pacing and/or cardioversion shock, which are used to reset the overall electrical activity of the heart. However, ICD therapy is painful and decreases the quality of life of the patient and is considered a palliative measure that doesn t prevent VT from occurring.
21 A potential curative treatment for VT is represented by radio frequency (RF) ablation. The cardiologist identifies the potential VT substrates and then uses RF ablation therapy to eliminate the reentrant circuit by producing a permanent but contained anatomical block through the creation of a lesion in the cardiac tissue. Generally, these lesions are created in areas with already compromised substrate such as isthmuses [1]. Before RF ablation is conducted, the target regions that are suspected of harboring the arrhythmia are identified during detailed electrophysiology studies. These studies involve several tools, techniques, and maneuvers that aim to characterize the morphology and structure of the electrical disruption. 1.4 Diagnostic and Assessment Tools Electrophysiology study Cardiac EP is the minimally invasive procedure used to assess the electrical activity and conduction pathways of the heart to identify arrhythmias such as VT. All techniques are based on the introduction of a catheter able to record the electrical status of the underlying myocardium into the cardiac chamber via a major artery or vein. EP studies are conducted during normal cardiac rhythm (sinus rhythm), during ventricular arrhythmia or during programmed electrical stimulation (pacing). EP catheters, in combination with conventional surface ECGs, are used as a diagnostic tool to record electrical signals propagating on endocardial or epicardial surfaces. Catheters are used to navigate to the various cardiac anatomical structures with the aid of x-ray fluoroscopy (see Figure 1-6). There are several techniques used during an EP study to investigate, diagnose and plan for the management of potential system abnormality of the heart. For instance, scar regions may be identified as low voltage regions on ventricular voltage maps or may be identified as regions with diminished excitability; a variety of techniques have been developed to map these regions. If a location is found that is the cause of the arrhythmia, a RF ablation (electrical block by heating RF waves) may be created, in order to decrease the episodes of arrhythmia. Catheter guidance is achieved using X-ray fluoroscopy, which provides high temporal resolution but does not give soft tissue information or three-dimensional information and makes it difficult it is difficult to guide a catheter repeatedly to the area of interest. Thus, recent 3D systems, which integrate electrophysiological signals with anatomy to provide a threedimensional geometry have been introduced and are discussed in the following section (see sec ). 9
22 10 Figure 1-6 Electrophysiology (EP) is a subspecialty of cardiology and is the science of elucidating, diagnosing, and treating the electrical activities of the heart. It involves measurements of voltage change or electric current within the patient's heart. An EP Study is an accurate method for assessing the heart's electrical system. EP catheters are introduced, under x- ray guidance in the cardiac chambers, monitoring of the electrical signals also provides guidance and anatomical references. (figure modified from: Recording Techniques and Electrical Measurements Basics During a typical EP study, electrode catheters are used for recording and pacing. These catheters consist of insulated wires; at the distal tip of the catheter, each wire is attached to an electrode, which is exposed to the endocardial or epicardial surface. At the proximal end of the catheter, each wire is attached to a plug which connects to an external monitoring and recording system. Electrode catheters come in different sizes (3 to 8 Fr). Recordings derived from electrodes can be unipolar (one electrode) or bipolar (two electrodes). The electrodes are typically 1 to 2 mm in width. The interelectrode distance can range from 1 to 10 mm or more. Catheters with a 2- or 5- mm interelectrode distance are most commonly used [9]. Many modern catheters also have a fixed or deflectable tip; deflectable catheters allow deflection of the tip in one or two directions in a single plane; some of these catheters have asymmetrical bidirectional deflectable curves. Catheters that are also used for ablation have tip electrodes that are conventionally 4 mm long and are available in sizes up to 10 mm in length. The larger tip electrodes on ablation catheters reduces the resolution of a map obtained using recordings from the distal pair of electrodes but increases the surface area of energy delivery. As mentioned in the previous section, while the
23 surface ECG records a summation of the electrical activity of the entire heart, intracardiac electrograms (EGMs) are most sensitive to local electrical activity of tissue directly underneath the electrodes, especially for bipolar recordings. Unipolar recordings are obtained by positioning the exploring electrode in the heart, subtracting this signal from the reference signal associated with a remote second electrode (referred to as the indifferent electrode), typically represented by the surface ECG base signal. The unipolar recordings can provide information about the direction of the action potential; positive deflections are generated by propagation toward the recording electrode, and negative deflections are generated by propagation away from the electrode. The major disadvantage of unipolar recordings is that they contain substantial far-field signals generated by depolarization of tissue remote from the recording electrode [9]; these farfield effects can inject noise or simply unrelated signals into the wanted recordings. Conversely, bipolar recordings are the result of potential differential of two electrodes near the catheter tip that are connected to a recording amplifier. At each point in time, the potential at the negative input is inverted and subtracted from the potential at the positive input so that the final bipolar recording is the difference between the two [9], [11]. Several elements can affect bipolar electrogram signal: the mass of the activated tissue, the distance between the electrode and the propagating wavefront, the direction of propagation relative to the bipoles, and the interelectrode distance. Unlike unipolar recordings, bipolar electrodes are relatively unaffected by far-field effects because the difference between the two unipolar electrograms recorded at the two local poles cancels out the virtually identical far-field signals during subtraction [11]. An important aspect of bipolar measures is shown in Figure 1-7: the tissue contribution to the final voltage signal is inversely proportional to distance, so that tissue furthest away from the electrodes has a lower contribution to the bipolar voltage, whereas tissue closest to the electrodes has the greatest contribution[12]. Although it was shown that other factors contribute to the final amplitude of the signal (e.g. electrode size, inter-electrode distance), it was confirmed that distance to the measured tissue was the most influential factor. Overall, recorded electrograms can provide various information: the tissue property of the tissue laying below the electrodes (e.g. healthy tissue has higher voltage than scar tissue), local activation time (e.g. the time of activation of myocardium immediately adjacent to the recording electrode relative to a reference), and the morphology of myocardial activation within the field of view of the recording electrode(s). 11
24 12 Figure 1-7 Depicted here is the influence of electrode as a function of myocardium fiber distance from the electrodes on the signal detected by bipolar electrodes when an action potential propagates in a muscle fiber below the endocardial surface. Although size of electrodes (D, L) and inter-electrode spacing (IS) is a factor that influences electric potential, the strongest factor is the distance (z) between tissue and recording electrodes Mapping techniques Cardiac mapping is defined as the process of identifying the temporal and spatial distributions of myocardial EGMs during a particular heart rhythm. Cardiac mapping also encompasses several modes and techniques of mapping such as body surface, endocardial, and epicardial mapping. Also, some of these techniques involve mapping while the patient is in sinus rhythm while others are performed during tachyarrhythmia. They may involve pacing (electrical stimulation) in a specific chamber at particular intervals to: 1) control the direction of the action potential propagation wave; 2) initiate the tachyarrhythmia and assess its response to pacing maneuvers; or 3) terminate the tachyarrhythmia. All of these maneuvers aim to elucidate the mechanism of the tachycardia, and identify critical sites of conduction to serve as a target for catheter ablation. The success of ablation is very much dependent on localization of ventricular tachycardia foci or reentrant circuits. Conventional fluoroscopy and the use of a single mapping catheter has limited success in ablation of complex arrhythmias that may originate from sites without characteristic fluoroscopic landmarks or having variable electrograms as recorded from the catheter tip. Advanced mapping techniques have been developed as adjuncts to conventional methods to improve the efficacy of catheter ablation for arrhythmias that are transient, focal, or
25 hemodynamically unstable and thus require rapid mapping. Advanced mapping systems have revolutionized the clinical EP laboratory in recent years and have filled the gaps inherent with conventional mapping, allowing new insights into arrhythmia mechanisms [13]-[15]. These new mapping systems are able to provide three-dimensional (3-D) spatial localization and decrease the time of acquisition of cardiac activation maps. The accurate 3-D location of the mapping catheter is determined using different principles depending on the system, while local electrograms are acquired using conventional, well-established methods. Then recorded data of the catheter location and intracardiac electrogram at that location are used to reconstruct in real time the 3-D geometry of the chamber, color-coded with relevant electrophysiological information. CARTO (Bionsense Webster, Diamond Bar California) is an electroanatomic mapping system that uses a magnetic field to localize the mapping catheter tip in three-dimensional space. Three coils in a locator pad positioned underneath the patient s back generate a static ultra-lowintensity magnetic field (5µT - 50µT). A sensor in the catheter tip measures the relative strength and hence the distance from each of the coils. This allows for the recording of the spatial and temporal location of the catheter. Electrodes at the catheter tip record local EGMs, and this information is displayed on screen as a three-dimensional map of the voltage amplitudes or of the local activation times relative to a reference catheter in a color-coded fashion (for detailed explanation of voltage and activation map, see sections and ). In order to characterize the full chamber the mapping catheter is moved around sequentially to individual locations, eventually covering the chamber. Data from multiple single mapping points can be acquired during tachycardia and used to show the reconstructed animated sequences of the action potential propagation. Voltage maps can be obtained to delineate regions of scar and diseased myocardium. A similar endocardial mapping system, EnSite NavX (St. Jude Medical, St. Paul, Minnesota), consists of a catheter with a woven braid. A low frequency 5.6KHz locator signal is generated between an array of skin patches placed orthogonally on the patient and a standard mapping catheter to permit nonfluoroscopic localization of the catheter to regions of interest. The recorded voltage and impedance at each catheter s electrode generated from this current allows their distance from each skin patch and location in space to be triangulated. Therefore, the chamber geometry can be determined by moving the mapping catheter along the endocardial surface. 13
26 With both the CARTO and the EnSite NavX systems, electroanatomical maps of various information can be produced (e.g. activation map, isochronal map or voltage map) Activation Mapping The activation maps display the local activation time color-coded and overlaid on the reconstructed 3-D geometry. Local activation time at a site is defined as the time from the onset of the surface QRS (or intracardiac signal from an alternative chamber) to the time at which the largest rapid deflection crossed the baseline measured in the mapping bipolar electrogram. An example of the measurement technique in several recording sites is shown in Figure 1-8a. A sensible number of points relatively homogeneously distributed (at the discretion of the cardiologist) in the target chamber have to be recorded. The points of local activation time can be color-coded red for the earliest electrical activation areas; orange, yellow, green, blue, and purple for progressively delayed activation areas (see Figure 1-8). The main goal of activation mapping of post-mi VT is to uncover sites with earlier or late activity than normal or expected. Early or presystolic activity occurs only in close proximity of zone of slow conduction. Endocardial activation time in the left ventricle is typically calculated relative to the earliest ventricular depolarization time (e.g. QRS peak signal R ) or from the time of the atrial depolarization signal recorded in the right ventricle (see Figure 1-8a). Wavefront direction is also an important characteristics derived during activation mapping. Then the activation map created is used to define the activation sequence of the wavefront propagation as show in Figure 1-8b. Electrogram amplitude is in part a reflection of the depolarizing muscle mass as well as wavefront direction. Furthermore, multipotential electrograms are sometimes detectable during normal sinus rhythm, but low-amplitude potentials can be obscured by the signal from the surrounding larger mass of myocardium. In target regions, changing the direction of depolarization can produce a separation between activation of adjacent bundles [17], [18], creating distinguishable signals. Regions containing isthmuses and conduction blocks can be detected by analyzing electrograms recorded during different ventricular activation sequences, such as atrial pacing or right ventricular pacing [19]. 14
27 Figure 1-8 a) Local activation time map of the left ventricle during VT. Colours represent activation time relative to the Reference signal. Recorded potentials in the slow conducting area with increasing R-P intervals are marked by pink dots (figure modified from [16]); b) local activation time map can be used to reconstruct the wavefront activation sequence as shown in this example. A 3D electroanatomical (CARTO) activation map of the right atrium is constructed during counterclockwise atrial arrhythmia. During tachycardia, the depolarization wavefront travels counterclockwise around the tricuspid annulus (TA), as indicated by a continuous progression of colors (from red to purple) with close proximity of earliest and latest local activation (red meeting purple). 1 to 7, Propagation map of the RA during counterclockwise atrial tachycardia (figure modified from [9]). 15
28 Theoretically, multiple potentials can become superimposed on each other, preventing their detection, especially if travelling in a parallel manner. Conversely, a wavefront traveling perpendicular to a fiber long axis that encounters bundles would be expected to result in greater temporal separation of potentials in these regions. Therefore, RV pacing using a catheter in a different location can be used to suppress the sinoatrial node pacemaker and change the normal direction of the electrical activation wavefront, which can unmask some areas of block and slow conduction. Abnormal electrograms with multiple deflections, highly predictive of a reentrant circuit [20], [21], are more frequently recorded during RV pacing. Theoretically, this can be related to the fact that during sinus rhythm several simultaneous activation wavefronts can coexist, making the probability of electrogram overlap higher than during RV pacing when only one activation wavefront is present Voltage mapping The voltage map displays the peak-to-peak amplitude of the electrogram recorded in various locations. These can be superimposed on an anatomical model as color-coded values; typically, red for the lowest amplitude and orange, yellow, green, blue, and purple indicating progressively higher amplitudes and purple for healthy myocardium (see Figure 1-9). Voltage mapping during sinus, paced, or any other rhythm can help define anatomically associated regions of no voltage (presumed scars or electrical scars), low voltage, and normal voltage, where peak-to-peak voltage is measured for the recorded intracardiac electrogram. Conventionally, myocardial scars are typically defined as low voltage (<1.5mV) with dense scar defined as <0.5mV. Therefore, voltage mapping refers to delineation of the infarcted myocardium based on the physiological correlation between amount of active myocytes and their contribution to voltage potential.
29 17 Figure 1-9 Voltage map of the left ventricle in a patient with ventricular tachycardia after anteroapical myocardial infarction. An adjusted voltage scale is shown; all sites with voltage less than 0.9 mv are colored red on the map, and those with voltage more than 0.9 mv are purple, with interpolation of color for intermediate amplitudes. The gray area denotes no detectable signal (scar). A large anteroapical infarction is clearly evident. Red circles denote ablation sites. (Right figure modified from [9]) Further, voltage mapping also targets the identification of abnormal local electrogram (isolated electrograms, fractionated electrograms, and/or electrograms with multiple delayed components [18], [22] - detailed description to follow in Chapter 4). Abnormal electrogram identification, is of great value when activation mapping cannot be performed during VT because the patient is unable to tolerate clinically induced VT (hemodynamically unstable patient) [23]. Recent studies have shown the value of abnormal potentials [17], [18], [23]-[25]. The reentry circuit isthmuses consist of a proximal part (entrance), a central part, and an exit from which the wavefront leaves the abnormal region and rapidly depolarizes the normal myocardium. Conduction through the isthmus or at its entrance and/or exit is often slow because of decreased cell-to-cell coupling. During VT, multiple potentials and isolated potentials are often recorded from these regions. The presence of multiple discrete potentials suggests that conduction block is present between adjacent myocyte bundles. A typical local electrogram in the border of an infarct has a
30 low voltage but is adjacent to more normal myocardium in the border, causing a fractionated electrogram that has markedly different amplitudes (see Figure 1-5), (reflecting a far-field signal from activation of the large mass of surrounding tissue) and a small sharp potential (reflecting local depolarization of a small mass of fibers in the infarct) The Role of MRI Late gadolinium enhanced MRI Cardiovascular magnetic resonance (CMR) imaging has emerged as a fundamental diagnostic technique for a wide range of pathologies. CMR is considered the gold standard for assessment of myocardial infarction, systolic function, cardiac viability, and congenital heart disease [26]. Myocardial infarction can be examined and quantified using late-gadolinium enhancement (LGE-MRI) via the injection of gadolinium contrast agent. The mechanism of gadolinium distribution in tissue with cellular necrosis [27] depends on whether the injury is acute or chronic: (1) in the acute case, contrast media accumulates in both the extra-cellular and intracellular spaces as a result of cell membrane disruption and increased permeability; (2) in the chronic case, interstitial space between collagen fibers is greater than the space between densely packed living myocytes; therefore the contrast agent accumulates within the collagen matrix to a greater extent than in healthy tissue. An LGE-MRI imaging sequence uses an inversion recovery followed by a gradient echo acquisition with optimization of the inversion time set to null the signal of normal myocardium. In this setting, images are acquired 10 to 15 minutes after intravenous administration of gadolinium; the result is hyper-enhanced regions due to increased distribution volume of the contrast agent in the injured myocardium, shortening of the longitudinal relaxation time (T 1 ) and increasing the contrast difference between regions. This delayed time interval from contrast delivery to image acquisition allows clearance of the contrast medium from the normal myocardium, whereas it accumulates in the nonviable myocardium; the result is increased signal in infarct on T1-weighted images in LGE-MRI due to the enhanced relaxation rate excited protons adjacent to retained gadolinium. Three factors affect the extent of infarct enhancement on LGE-MRI: (1) the dose of contrast; (2) the time from contrast injection to imaging; and (3) the MR pulse sequence inversion time used to null the signal of normal myocardium (see Figure 1-10).
31 19 Figure 1-10 Contrast Enhanced Imaging. Early to late inversion time change shown. Various contrast images with their corresponding inversion time (TI) of47-year old male patient. (Image edited from Detsky et. al., MRM 2007) LGE-MRI in the assessment of arrhythmias As mentioned in previous sections, arrhythmic risk assessment has traditionally relied on the clinical history, electrocardiographic features, morphological evaluation of ejection fraction, and electrophysiology study results. LGE-MRI is capable of imaging nonviable tissue and may identify potential substrates for VT reentry. Several studies have highlighted a role for LGE-MRI to complement traditional approaches for risk stratification of ventricular arrhythmia [28]. Currently, LV ejection fraction is the most used clinical parameter in post myocardial infarction risk stratification and in guidance of critical treatment decisions such as prophylactic implantation of cardioverter-defibrillators [29]; however, current risk assessment remains suboptimal, and the need for other accurate predictors of outcome has been evident. An enhancing infarct on LGE-MRI images can be separated into the dense infarct and the gray zone. It is now accepted that this gray zone represents heterogeneous infarct, an area with a mixture of viable and non-viable peri-infarct myocardium. Further, recent studies have
32 determined the existence of a stronger correlation between gray zone and VT morbidity. Specifically, the heterogeneous region likely involving a mixture of healthy tissue and infarct tissue has been shown to correlate with inducibility of VT [30], post-mi all-cause mortality[29] and VT occurrence followed by ICD shocks [31]. However, there is no consensus regarding the definition of what constitutes the gray zone. Similarly, the relationship that correlates gray zone to the border zone identified with electrophysiology tools is still under study. 20 Figure 1-11 In vivo catheter guidance inside an iliac artery into the right renal artery of a pig. The real-time MR images were acquired using the (a) active guidewire (red), (b) active catheter (green), and (c) surface phased array coils as RF receivers. d: The resulting real-time fusion image following individual image processing (image modified from [32]). 1.5 Interventional Cardiac MRI MR Tracking Techniques MR technology has also been used for catheter tracking and interventional guidance [33]. Several techniques have been developed to monitor the position of a small device inside the MR magnet bore. These devices are classified as active because they contain electronic components within the mechanical structures. Specifically, in active tracking the catheter incorporates microcoil receivers (preferably at the tip) that are used with imaging or non-imaging pulse sequences to determine their coordinates in space. Active catheter tracking techniques have been shown to be effective in identifying the location of catheters and guidewires [34]. One implementation of these active techniques consists of the overlay of an image obtained from receive tracking coils
33 located on the catheter onto an anatomical image that is obtained from a conventional RF imaging coil [35] (see Figure 1-11). This technique suffers from two significant limitations: first, the catheter may appear blurred when visualized as a result of the reception of MR signal from a significant volume around the coils, decreasing localization accuracy; second, the catheter needs to be contained in the imaging plane otherwise the tracked markers will not be visualized. Another common implementation of active-tracking in catheters consists of projecting MR signals obtained from solenoid receive coils located on the catheter onto three orthogonal dimensions [36]. Specifically, pulse sequences employing nonselective RF pulses are used to excite the entire volume; then readout magnetic field gradient pulses are applied along each of the primary axes of the imaging system. Then frequency encoding is used to determine the position of the receive coil along each axis; specifically, the data is Fourier transformed and the resulting signal peaks are used to determine the position of each receiver coil along the three orthogonal axes defined by the applied field gradients. Figure 1-12 shows an example of a tracking pulse sequence. Execution of this projection technique can be interleaved with a standard imaging sequence or can be performed in a continuous manner by superimposing the detected coil on a previously acquired image. Although there are some drawbacks to this technique (e.g. potential catheter heating), this method provides important advantages: (1) fast position detection; and (2) free choice of imaging plane with (3) additional automatic scan plane adjustment and catheter localization. 21
34 22 Figure 1-12 a) A simple pulse sequence for the determination of the location of an MR signal source. The sequence employs a non-selective RF pulse and a readout magnetic field gradient pulse. The sequence is repeated three times with gradients played on X, Y, and Z to obtain the location of the signal source in the X, Y and Z direction. The position of a signal source with respect to the applied magnetic field gradient is determined by Fourier transformation of the acquired signal and peak detection in the resulting 1D map; b) Schematic drawing of threeelement capacitively coupled tracking coil. Circuit is tuned to Larmor frequency of MR-scanner and matched to 50 ohm at the tip of catheter 1.6 Motivation As reported in the latest EHRA/HRS consensus publication [1] many unanswered questions remain in the management and treatment of ventricular tachycardia. One of the major issues of this arrhythmia is the high percentage of recurring VT episodes post RF ablation. In long-term follow-up studies, there is a VT recurrence up to 37% in successful ablation procedures, compared to 80% recurrence in non-successful ablation studies [1]. Several factors can hamper the success rate of this procedure. First, MRI images used for navigation but acquired several hours or days prior to the procedure cannot reflect changes in the patients physiology at the time of the procedure. Second, arbitrary single-voltage cut-off value for the presence of scar may not fully reflect the complex scar architecture and its use may not result in accurate delineation of scar especially in the case of non-transmural infarct. Third, for patients that are haemodynamically unstable and cannot tolerate VT induction or mapping, accurate identification
35 of target ablation region becomes problematic, resulting in an approximate delineation of the presumed reentrant circuit. Therefore, the goals of this thesis are to address these limitations by developing a real-time MR-guided electrophysiology system capable of integrating electrical information and MRI-derived structural information during the procedure; by correlating regions of VT substrate detected in EP and depicted by MRI imaging and further, by investigating potential surrogate MRI markers that can provide additional information of potential ablation target regions to the interventional cardiologists. 1.7 Overview of Thesis In Chapter 2, we describe a feasibility study where a newly constructed real-time MR-guided electrophysiology system was used to acquire data to construct local activation time maps and analyze propagation properties by performing MR-guided mapping of the porcine left ventricle while pacing from the right ventricle. Anatomical and myocardial late gadolinium enhancement images were used for catheter navigation and identification of scar regions. Our system was compared to the CARTO clinical eletroanatomical mapping system by performing the same mapping procedure in all the animals with the two systems. In Chapter 3 real-time MR-guided electroanatomical voltage mapping (EAVM) was performed in six chronically infarcted animals in a 1.5T MR system The purpose of this study was to characterize the relationship between chronic myocardial fibrotic scar detected by multi-contrast late enhancement (MCLE) MRI (a variant of LGE-MRI) and by EAVM obtained using the MRguided electrophysiology system, to better understand how these measures may improve identification of potentially arrhythmogenic substrates. Chapter 4 describes the relationship between MRI-based tissue classification (dense scar, gray zone and healthy myocardium) and abnormal potential electrograms. The purpose of this study was to identify regions of abnormal potentials in sinus rhythm and controlled-paced rhythm in post-infarct animals and to characterize the relative prevalence of these abnormal potentials in areas labeled as gray zone as quantified by LGE-MRI. Finally, chapter 5 discusses future directions and planned studies aimed at expanding and validating techniques developed in Chapters 2 to 4. The focus will be on using the real-time MRguided system to perform the therapeutic component of the electrophysiology study, the radio- 23
36 frequency ablation. One of the key components of RF ablation is lesion visualization. Some preliminary results are shown. 1.8 References 24 [1] E. M. Aliot, W. G. Stevenson, J. M. Almendral-Garrote, F. Bogun, C. H. Calkins, E. Delacretaz, P. Della Bella, G. Hindricks, P. Jaïs, M. E. Josephson, J. Kautzner, G. N. Kay, K.-H. Kuck, B. B. Lerman, F. Marchlinski, V. Reddy, M.-J. Schalij, R. Schilling, K. Soejima, and D. Wilber, EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias, Heart Rhythm, vol. 6, no. 6, pp , 01-Jun [2] F. Kusumoto, ECG Concepts Using Electrophysiologic Principles [3] D. P. Zipes and H. Wellens, Sudden cardiac death, Circulation, [4] A. S. Tang, H. Ross, C. S. Simpson, L. B. Mitchell, P. Dorian, R. Goeree, B. Hoffmaster, M. Arnold, M. Talajic, C. H. R. Society, and C. C. Society, Canadian Cardiovascular Society/Canadian Heart Rhythm Society position paper on implantable cardioverter defibrillator use in Canada., Canadian Journal of Cardiology, vol. 21. pp. 11A 18A, 01-May [5] W. G. Stevenson and K. Soejima, Catheter ablation for ventricular tachycardia, Circulation, vol. 115, no. 21, pp , May [6] H. L. Estner, M. M. Zviman, D. Herzka, F. Miller, V. Castro, S. Nazarian, H. Ashikaga, Y. Dori, R. D. Berger, H. Calkins, A. C. Lardo, and H. R. Halperin, The critical isthmus sites of ischemic ventricular tachycardia are in zones of tissue heterogeneity, visualized by magnetic resonance imaging., Heart rhythm : the official journal of the Heart Rhythm Society, vol. 8, no. 12, pp , Dec [7] M. Pop, M. Sermesant, T. Mansi, E. Crystal, J. Detsky, Y. Yang, P. Fefer, E. R. McVeigh, A. Dick, N. Ayache, and G. A. Wright, Characterization of Post-infarct Scars in a Porcine Model A Combined Experimental and Theoretical Study, Functional Imaging and Modeling of the Heart, pp. 1 10, [8] J. De Bakker, F. Van Capelle, M. Janse, S. Tasseron, J. Vermeulen, N. De Jonge, and J. Lahpor, Slow conduction in the infarcted human heart. Zigzag course of activation, Circulation, vol. 88, no. 3, p. 915, Sep [9] Z. Issa, J. M. Miller, and D. P. Zipes, Clinical Arrhythmology and Electrophysiology: A Companion to Braunwald's Heart Disease: Expert Consult: Online and Print, [10] K. Soejima and W. G. Stevenson, Ventricular tachycardia associated with myocardial infarct scar: a spectrum of therapies for a single patient, Circulation, vol. 106, no. 2, pp , Jul [11] W. G. Stevenson and K. Soejima, Recording techniques for clinical electrophysiology, J Cardiovasc Electrophysiol, vol. 16, no. 9, pp , Sep [12] J. M. Stinnett-Donnelly, N. Thompson, N. Habel, V. Petrov-Kondratov, D. D. Correa de Sa, J. H. T. Bates, and P. S. Spector, Effects of electrode size and spacing on the resolution of intracardiac electrograms, Coronary Artery Disease, vol. 23, no. 2, pp , Mar [13] N. D. Skadsberg, B. He, T. G. Laske, and P. A. Iaizzo, Cardiac Mapping Technology, in in Handbook of Cardiac, no. 29, Totowa, NJ: Humana Press, 2009, pp [14] J. Sra and J. M. Thomas, New techniques for mapping cardiac arrhythmias, Indian
37 Heart J, vol. 53, no. 4, pp , [15] R. Juneja, Radiofrequency ablation for cardiac tachyarrhythmias: principles and utility of 3D mapping systems, Current science, [16] M. M. Volkmer, F. F. Ouyang, F. F. Deger, S. S. Ernst, M. M. Goya, D. D. Bänsch, K. K. Berodt, K.-H. K. Kuck, and M. M. Antz, Substrate mapping vs. tachycardia mapping using CARTO in patients with coronary artery disease and ventricular tachycardia: impact on outcome of catheter ablation., Europace, vol. 8, no. 11, pp , Nov [17] A. Arenal, E. Glez-Torrecilla, M. Ortiz, J. Villacastín, J. Fdez-Portales, E. Sousa, S. del Castillo, L. Perez de Isla, J. Jimenez, and J. Almendral, Ablation of electrograms with an isolated, delayed component as treatment of unmappable monomorphic ventricular tachycardias in patients with structural heart disease., J Am Coll Cardiol, vol. 41, no. 1, pp , Jan [18] F. F. Bogun, B. B. Bender, Y.-G. Y. Li, G. G. Groenefeld, S. H. S. Hohnloser, F. F. Pelosi, B. B. Knight, S. A. S. Strickberger, and F. F. Morady, Analysis during sinus rhythm of critical sites in reentry circuits of postinfarction ventricular tachycardia., Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing, vol. 7, no. 1, pp , Aug [19] C. B. Brunckhorst, W. G. Stevenson, W. M. Jackman, K.-H. Kuck, K. Soejima, H. Nakagawa, R. Cappato, and S. A. Ben-Haim, Ventricular mapping during atrial and ventricular pacing. Relationship of multipotential electrograms to ventricular tachycardia reentry circuits after myocardial infarction, European Heart Journal, vol. 23, no. 14, pp. 8 8, Jul [20] S. Nakahara, M. Vaseghi, R. J. Ramirez, C. G. Fonseca, C. K. Lai, J. P. Finn, A. Mahajan, N. G. Boyle, and K. Shivkumar, Characterization of myocardial scars: electrophysiological imaging correlates in a porcine infarct model, Heart rhythm : the official journal of the Heart Rhythm Society, vol. 8, no. 7, pp , Jul [21] S. S. Nakahara, R. R. Tung, R. J. R. Ramirez, J. J. Gima, I. I. Wiener, A. A. Mahajan, N. G. N. Boyle, and K. K. Shivkumar, Distribution of late potentials within infarct scars assessed by ultra high-density mapping, Heart Rhythm, vol. 7, no. 12, pp. 8 8, Dec [22] F. Bogun, E. Good, S. Reich, D. Elmouchi, P. Igic, K. Lemola, D. Tschopp, K. Jongnarangsin, H. Oral, A. Chugh, F. Pelosi, and F. Morady, Isolated potentials during sinus rhythm and pace-mapping within scars as guides for ablation of post-infarction ventricular tachycardia., J Am Coll Cardiol, vol. 47, no. 10, pp , May [23] H. H. Hsia, D. D. Lin, W. H. Sauer, D. J. Callans, and F. E. Marchlinski, Relationship of late potentials to the ventricular tachycardia circuit defined by entrainment., Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing, vol. 26, no. 1, pp , Oct [24] J. M. T. de Bakker and F. H. M. Wittkampf, The pathophysiologic basis of fractionated and complex electrograms and the impact of recording techniques on their detection and interpretation., Circ Arrhythm Electrophysiol, vol. 3, no. 2, pp , Apr [25] T. Harada, W. G. Stevenson, D. Z. Kocovic, and P. L. Friedman, Catheter ablation of ventricular tachycardia after myocardial infarction: relation of endocardial sinus rhythm late potentials to the reentry circuit., J Am Coll Cardiol, vol. 30, no. 4, pp , Oct [26] H. W. Kim, A. L. Crowley, and R. J. Kim, A clinical cardiovascular magnetic 25
38 resonance service: operational considerations and the basic examination, Cardiology clinics, vol. 25, no. 1, pp. 1 13, v, Feb [27] J. W. Weinsaft, I. Klem, and R. M. Judd, MRI for the assessment of myocardial viability, Cardiology clinics, vol. 25, no. 1, pp , v, Feb [28] R. Macedo, A. Schmidt, C. E. Rochitte, J. A. C. Lima, and D. A. Bluemke, MRI to assess arrhythmia and cardiomyopathies, J. Magn. Reson. Imaging, vol. 24, no. 6, pp , Dec [29] A. T. Yan, A. J. Shayne, K. A. Brown, S. N. Gupta, C. W. Chan, T. M. Luu, M. F. Di Carli, H. G. Reynolds, W. G. Stevenson, and R. Y. Kwong, Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality, Circulation, vol. 114, no. 1, pp , Jul [30] A. Schmidt, C. F. Azevedo, A. Cheng, S. N. Gupta, D. A. Bluemke, T. K. Foo, G. Gerstenblith, R. G. Weiss, E. Marbán, G. F. Tomaselli, J. A. C. Lima, and K. C. Wu, Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction, Circulation, vol. 115, no. 15, pp , Apr [31] S. D. Roes, C. J. W. Borleffs, R. J. van der Geest, J. J. M. Westenberg, N. A. Marsan, T. A. M. Kaandorp, J. H. C. Reiber, K. Zeppenfeld, H. J. Lamb, A. de Roos, M. J. Schalij, and J. J. Bax, Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator., Circ Cardiovasc Imaging, vol. 2, no. 3, pp , May [32] H. H. Quick and M. E. Ladd, Interventional MRA: concepts for active visualization of catheters and stents, Zeitschrift für medizinische Physik, vol. 13, no. 3, pp , [33] M. Moche, R. Trampel, T. Kahn, and H. Busse, Navigation concepts for MR imageguided interventions, J. Magn. Reson. Imaging, vol. 27, no. 2, pp , Feb [34] K. K. Ratnayaka, A. Z. A. Faranesh, M. A. M. Guttman, O. O. Kocaturk, C. E. C. Saikus, and R. J. R. Lederman, Interventional cardiovascular magnetic resonance: still tantalizing., Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance, vol. 10, pp , Jan [35] J. Serfaty, X. Yang, P. Aksit, and H. Quick, Toward MRI-guided coronary catheterization: visualization of guiding catheters, guidewires,, J. Magn. Reson. Imaging, [36] C. L. Dumoulin, S. P. Souza, and R. D. Darrow, Real-time position monitoring of invasive devices using magnetic resonance, Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine, vol. 29, no. 3, pp , Mar
39 27 Chapter 2 2 Electroanatomical Mapping With Real Time MR- Guided System Introduction Radio frequency (RF) ablation has been shown to reduce recurrence rate of ventricular tachycardia (VT) in patients with structural heart disease [1]. For sustained monomorphic VTs, scar tissue within the myocardium creates the conditions for a reentrant VT; specifically, a reentrant pathway may arise between dense infarct scar zones or between a scar and an anatomical obstacle [2]-[4]. Such pathways have been shown to contain bundles of surviving myocytes intermingled with fibrosis, creating areas of slow conduction [5], [6]; the depolarization wave propagates in the narrow bundles of viable myocardium often following a circuitous route, effectively producing a slow conduction velocity channel needed for the reentrant tachycardia. To eliminate these arrhythmogenic slow conduction zones associated with present and potential VTs in patients with myocardial infarctions, these channels must be mapped and ablated. Recently, the understanding of VT mechanisms has greatly increased in part due to the introduction of electroanatomic mapping (EAM) systems. These systems, such as Ensite NavX and CARTO, are used to define the geometry of the cardiac chambers and to determine the regions responsible for the arrhythmia by creating voltage and/or activation time maps. In the case of VT, careful and detailed identification of reentry circuits is extremely important to increase success rates in ablation treatments. Previous studies have presented alternative methods to delineate scar regions in sinus rhythm and during right ventricular apex stimulation with voltage maps [7], [8]. However, in [2], it was also shown that conventionally defining all tissue with voltage potential below 0.5 mv as scar, might not be applicable to all cases and may lead to missed identification of slow conduction channels. 1 This chapter based on the original research article: The feasibility of endocardial propagation mapping using magnetic resonance guidance in a Swine model, and comparison with standard electroanatomic mapping. Oduneye SO, Biswas L, Ghate S, Ramanan V, Barry J, Laish-FarKash A, Kadmon E, Zeidan Shwiri T, Crystal E, Wright GA. IEEE Trans Med Imaging Apr;31(4): Nov
40 This case is especially true when the slow conduction channel (which has higher voltage potential) is completely surrounded by a low voltage potential area. Therefore, assessment of local activation times (LAT) may provide additional and complementary information, particularly a critical functional description of slow conduction channels, during cardiac mapping in patients affected by VT. Recently, there has been an effort to guide electrophysiology (EP) studies directly with magnetic resonance imaging in real-time (MRI) [9]-[11]. Three aspects make this approach extremely attractive: 1) MRI has a superior soft tissue contrast, which increases the ability to identify arrhythmogenic areas, 2) both the patient and the interventional cardiologist are not exposed to ionizing radiation and 3) three-dimensional navigation of catheters and visualization of anatomical structures. Despite the success of MR-guided EP procedures, studies in the ventricle have, to date, mainly focused on creating voltage maps, which may be of limited utility, as previously mentioned in identifying slow conduction channels often responsible for VT. The objectives of the current research are (1) to show the feasibility and performance of an electrophysiology (EP) study in the LV using an MR-guided EP system during RV pacing to verify the local activation times in different regions and (2) to show reliable imaging and EP correspondence of the endocardial substrate during MR-guided procedures in healthy and infarct models. 28 Figure 2-1 MR-compatible catheter (8.5 Fr.). Three tracking coils are embedded into this device and connected to the MR receivers (locations of first two tracking coils indicated as Coil #1 and #2; third coil is not shown). Bipolar intracardiac electrogram measurements where made between the first pair of electrodes (distal tip and adjacent ring electrode), filtered at Hz.
41 Methods Electroanatomical Mapping with MR-guided Electrophysiology System In this chapter ten animals were used in the experiments (eight healthy, two infarct); the healthy animals were divided into two groups: the first group underwent LV mapping with MR-guided EP first, before using the CARTO system, and the second group underwent CARTO mapping procedure, before the MR-guided procedure. For the infarct model, only paced LV substrate mapping with our MR-guided EP system was performed. 8.5Fr MR-compatible catheters (Imricor Medical Systems, Burnsville, USA) were navigated to and around the RV and LV either completely under MR guidance or after initial placement under x-ray fluoroscopy for the MRguided studies. EP measurements were performed using a prototype Bridge EP Recording System (Imricor Medical Systems, Burnsville, MN): the system has an integrated stimulator for pacing and each catheter had one or three MR-tracking coils, with four total electrodes, 4-mm catheter tip with mm inter-electrode spacing for intracardiac electrogram measurements (see Figure 2-1). Pacing was conducted with the following parameters: 400-ms cycle length (or 150 BPM), 2mA to 3mA amplitude and 5-ms pulse duration. To define the activation time sequence map on the endocardial surface, the local activation time (LAT) at each site was calculated as the time difference between the pacing intracardiac electrogram signal from the catheter tip in the RV apical area (reference), and the intracardiac bipolar recording of the activation time on the second catheter in the LV (Figure 2-2a); LAT Maps were constructed with an average of 300 points per LV. Time reference was taken consistently at the maximum (positive peak) deflection. For each location the average of four to 10 LAT values was used. Intracardiac electrograms from sites showing poor contact and electrodes floating in blood, displayed very distinct EGMs and were rejected by an observer. The remaining recordings at each time point were associated with the spatial coordinates, which were determined using an MR-tracking coil at the tip of the catheter (see Figure 2-1). Temporal and spatial synchronization between EP signals and recorded points was performed using Matlab. Tracking information packets were also tagged with corresponding respiration and time reference information relative to the R-peak of the surface electrocardiogram (ECG). Finally, after labeling each recording point with EP data (LAT or voltage), the data is mapped to a LV endocardial surface shell (Figure 2-2)
42 Figure 2-2 (a) Bipolar pacing stimulation from the right ventricle (RV) from catheter electrodes 1-2 pair and recording of the arrival time in the left ventricle (LV) with the mapping catheter LV 1-2 pair. The signals from the second pair of electrodes on both catheters, RV 3-4 and LV 3-4, was not used (b) Short-axis and long-axis views were displayed in Vurtigo to help navigation in the LV. A stack of images were acquired using a breath-held 2D SSFP-CINE pulse sequence, once imported in the visualization software, the endocardial surface was created using an automatic segmentation algorithm. Finally, under electrical stimulation several spatial and intracardiac signals were simultaneously recorded and used to create an EAM based on the catheter tip touching the endocardium (red dots). Though the points were recorded and displayed in real-time, the final map (as displayed in Figure 2-3) was generated after reviewing the point in post-processing. The RV tip is shown as the green dot. 30
43 31 LV Segmentation LV endocardial surfaces resolved over the cardiac cycle were generated using a fully automatic segmentation algorithm from cine short-axis images, as previously described by Lu et al. [12], [13]. The diastolic shell was then imported into a visualization tool, Vurtigo [14], developed inhouse (Figure 2-2b). Both the endocardial shell and the contact points recorded were acquired in the same frame of reference. Consequently, the arrival time of the action potential at specific position was matched to a location on the endocardial surface computed for the same cardiac phase to minimize any potential discrepancy between the shell and the mapped points. MR Acquisition and Tracking Anatomical images were obtained at the beginning of each study on a 1.5T GE Signa system (GE Healthcare, Milwaukee, USA) using a 5-inch surface coil for signal reception. A stack of 2D MRI slices were acquired covering the ventricular chambers in their entirety using breath-held short-axis and long-axis 2D SSFP-CINE sequences with the following parameters: TE/TR = 1.1/3.6 ms, FA = 45º, BW = 62.5 khz, FOV = 210 mm, slice thickness = 6 mm, Nx = 224, Ny = 160 (resolution of 0.9x1.3 mm), NEX = 1, Phase FOV = 0.8 and views per segment (VPS) = 12. The cine SSFP sequence produced 20 phases over the heart cycle, acquired on average over second breath-holds. Finally, the catheters were tracked using the MR projection method [15] at a temporal resolution of 27 frames-per-second; 2D and 3D multicontrast late enhancement (MCLE) imaging sequences were also acquired to visualize the infarct in the LV. The sequence, described in [16], uses a retrospective gating and applies an inversion pulse once every R-R interval, then a segmented SSFP acquisition using the same retrospective gating as the CINE acquisition to produce a cardiac-phase resolved set of images with varying inversion times and hence T1 contrasts across the cardiac cycle. The parameters used for MCLE were: TE/TR = 1.4/3.3 ms, FA = 45º, BW = 100 khz, FOV = 210 mm, slice thickness = 5 mm, Nx = 224, Ny = 160 (resolution of 0.9x1.3 mm), NEX = 1 Phase FOV = 0.8 and VPS = EAM with CARTO CARTO was used as the gold standard for the LV mapping studies in healthy animals. Catheter placement was performed under x-ray fluoroscopy, (OEC 9800, GE Healthcare, Salt Lake City, USA), using two 4-mm 7F catheters (Navistar, Biosense Webster, Diamond Bar, CA). The first
44 catheter was placed in the RV apex or near the septum for pacing, while the second catheter was placed in the LV for mapping. Pacing resulting in myocardial capture was achieved, performed at a 400-ms cycle length or 150 BPM at double the capturing threshold (EP-4, St. Jude Medical, Minnetonka, USA), usually between 2mA to 3mA amplitude and 5-ms pulse width. Signals were filtered from 10 to 400 Hz and the peak-to-peak bipolar amplitude was recorded by CARTO. The LAT maps of the LV were generated at each site by taking the difference between the intracardiac electrogram signal from the RV catheter and the activation time recorded in the LV. Timing references were taken at the maximum peak of the positive deflection. Then the local activation time color-coded maps were created along with the reconstructed 3D geometry. To avoid recording potential cross-talk between the two catheters, a 10-ms window after the pacing initiation was ignored for the LV signal using CARTO windowing feature. The timing window refers to the range of activation times surrounding reference electrogram activation Animal preparation A total of ten young swine kg were used for all the experiments (eight healthy models, two infarct models). The animal care committee at Sunnybrook Health Sciences Centre approved this protocol and all the procedures were conducted following institutional guidelines. Each porcine subject was placed under general anesthesia induced with an intramuscular injection of ketamine (30 mg/kg) and atropine (0.05 mg/kg), and maintained by inhalation of 1-5% isoflurane. Also lidocaine (30 ml of 2% lidocaine in 250 ml of saline) and amiodarone is 50mg/ml, were administered during the procedure. Finally, anticoagulation with intravenous heparin (70 units/kg) was also administered. Then, each animal was intubated and mechanically ventilated (20-25 breaths/minute). Incisions of femoral vessels, carotid artery or jugular vein were performed and secured with 9F introducer sheaths, for the purpose of administering medications and insertion of catheters. Finally, ECG leads I, II, and III were monitored throughout the experiment. The procedural plan for the eight healthy swine consisted of undergoing LV substrate mapping with both our MR-guided EP system and CARTO XP (Biosense Webster, Diamond Bar, CA). For the infarct subjects, myocardial infarction was induced by complete coronary occlusion distal to the second diagonal branch of the left anterior descending artery for 60 minutes, via the inflation of a percutaneous balloon dilation catheter 32
45 under x-ray guidance, followed by reperfusion. These animals were then left to recover, with MR and EP studies performed 4 weeks post-infarction Statistical and error analysis Local activation times are presented as mean ± SD. Arrival times measures were compared using the one-way ANOVA technique. Also, the unpaired t-test and one-way analysis of variance were used to determine the statistical significance of the difference in means between variables (pvalue < 0.05). Also we calculated spatial error associated with fusion of EAM surface and EP points by measuring the average perpendicular distance between EP points and mesh surface. Table Results Of the 10 swine that began the study, three (two healthy and one infarcted) died during catheter placement or initial navigation. In those that died early, there was no evidence of perforation from gross histology; however all three animals went into ventricular tachycardia and then ventricular fibrillation. Two healthy animals were excluded from our analysis because the LV map was acquired only in sinus rhythm; out of the remaining 5 pigs, we were able to obtain intracardiac electrograms recordings under programmed stimulation with the MR-EP or CARTO
46 system or both, the results are summarized in table 2.1. Of the two infarcted swine, the MRguided EP procedure was performed in the one that survived beyond the point of imaging; however CARTO mapping was not available in this animal due to premature death. Under MRI guidance, using intra-procedural images as navigational roadmaps, we were able to simultaneously track the catheters in both ventricular chambers, and record electrophysiology signals. Image signal-to-noise ratio, contrast-to-noise ratio and resolution were sufficient to construct 3D endocardial shells for navigation within the LV. Image quality analysis with and without the system was not performed; however, a qualitative assessment determined that introduction of the system did notimpact image quality dramatically. In two cases, catheters had to be repositioned back in the LV and RV using solely MR-guidance after they were erroneously pulled out of the chambers. Re-inserting the catheters took only a few minutes. Endocardial mapping procedural time progressively decreased as we became more familiar with the tools and the setup process. Finally, the average location and tracking error (point to surface distance) of the acquired mapping points was calculated to be 2.1±1.1 mm. Our spatial error seems to be significantly lower than the CartoMerge point to surface registration errors in the ventricle reported in the literature: 3.8±0.6 mm [17], 4.8±2.0 mm [18], 4.6±3.1 mm [19], 4.3±3.2 mm [20] Electroanatomical mapping results MR-guided EAM of the LV was successfully performed during pacing; we were able to navigate the catheters in several endocardial locations of the LV for direct electrical characterization of the different activation times. We determined adequate contact between catheter tip and the endocardium via direct MR image and via quality of the intracardiac electrogram signal assessment. Effective pacing with myocardium capture was established by confirming change in heart rate via ECG. All results presented here were achieved while pacing in the RV with the MR-guided system with confirmed myocardial capture. For example, for the pig shown in Figure 2-2, myocardial contraction was performed at a 400-ms cycle length or 150 BPM and achieved using a range between 2mA and 3mA pacing amplitude and 5-ms pulse duration. Qualitatively, the MR-guided LAT map agreed well with the CARTO LAT map, in terms of the range of activation times observed, spatial position and anatomical areas corresponding to activation times. In the example in Figure 2-3, the CARTO map shows the earliest activation point 34
47 originating in the septal and mid-inferoseptal regions. The depolarization wave traversed the septal wall, after pacing from the RV, which resulted in the earliest activation being recorded after a time delay from the reference stimulation signal. The latest point was recorded in the basal-lateral region of the LV. Similarly, in the MR-guided map, earliest activation times also originate in the septal region and the latest in the same region as the CARTO (see Figure 2-3 second row). During the MR-EP procedure, the catheters in the RV were placed approximately in the same position as in the CARTO procedure and overall, when the hemodynamic condition of the animal remained stable, the propagation patterns had high correspondence. Using standardized myocardial segmentation and nomenclature as reported in [21], we calculated local activation times in four distinct regions as shown in the following section 35 Figure 2-3 (First row) Local activation time map using CARTO in RAO (Right Anterior Oblique) AP (Anterior Posterior) LAO (Left Anterior Oblique) views. (Second row) MR-guided EP system activation maps in the same healthy animal. Earliest activation regions in both CARTO and MR-guided EP system occurred in the apical septal region, whereas the latest point we recorded was in the basal anterolateral region. Points and anatomical images were all acquired in the same frame of reference. The LV endocardial surface was segmented from a stack of 2D SSFP-CINE MR images.
48 EAM analysis Results of the average arrival times in the LV in the healthy group are summarized in Table 2.1. The standardized segments as described in [21], were grouped into major ventricular areas. The earliest activation times were recorded at the apex (segment-17) or at the apical septal segment (segment-14). As expected, there was variability in average times across animals, however there were common characteristics. Average time of action potential propagation increased with procedural time resulting in higher arrival times during the second mapping procedure; the second study of the session, usually occurring several hours after, with the animal remaining under anesthesia. Within each animal, arrival times show that propagation occurs almost symmetrically in lateral and septal wall; comparably when the earliest activation is recorded in segment-14, the depolarization wave seem to travel earlier in the septum. The graph in Figure 2-4 provides a more detailed analysis of the LATs, taking as an example pig #2. Specifically, the earliest activation points in this example were recorded in the apical septal region (segment-14) for CARTO and the apical region (segment-17) for MR-EP. The segments taken in consideration were 9, 7, 12 and 6. The first region of interest (segment-9) measured as 12.8 ± 1.96 ms for CARTO and 16.7 ± 1.15 ms for the MR-guided EP system (P<0.01). Segment-7 apical anterior activation times measured 17.7 ± ms and 19.5 ± 3.67 ms, for the CARTO and MR-EP system respectively (P<0.05). In the mid anterolateral region, the arrival time was recorded and found to be 25.4 ± 1.42 ms and 21.3 ± 2.31 ms respectively (P<0.03). Finally, the basal anterolateral region (segment-6), measured 34.5 ± 5.90 ms for the CARTO 26.0 ± 1.73 ms and for the MR-guided EP system (P<0.05). These results are summarized in the graph of Figure 2-4; overall, the propagation times increase comparably as the region of interest gets further away from the point of earliest activation. The points recorded in segment-6 are the furthest away from the pacing location and in the largest variation.
49 37 Figure 2-4 Analysis for pig #2 with pacing at 150 BPM of CARTO and MR-guided EP system LAT for the areas of interest: LAT are expressed as mean and standard deviation displayed as error bars; The standard classification is shown (right), where the segments of interest are highlighted and the earliest arrival time (at 0ms) for the two systems is marked as MR-EP and CARTO Infarct EAM comparison MCLE images were loaded into the visualization system Vurtigo and were used to identify the location of the infarct scars in our porcine models Figure 2-5. The images were acquired at the beginning of the study session, prior to the endocardial mapping and were used to successfully target the scar situated in the apical septal regions. Then, the catheter was navigated to the infarct area and as well as the healthy tissue. The resulting MR-guided EP maps revealed a distinct slower propagation area on the apical septal wall (see Figure 2-6b) when compared to a healthy animal (see Figure 2-6a). Additionally, in the apical region in the proximity of the infarct we recorded intracardiac fractionated delayed electrograms (Figure 2-6d), which are typical of scar tissue and very distinct from normal activation electrograms (Figure 2-6c) in healthy tissue. Finally, the in-situ 3D MCLE MRI also identified the apical septal scar. This pulse sequence with higher spatial resolution and SNR confirmed the location of the sub-endocardial scars with higher level of accuracy. The scar location also matched well with the locations of low-voltage areas in the voltage electroanatomical map (see Figure 2-7).
50 Figure 2-5 2D MCLE images (4 of 12 acquired) of infarcted swine; the first two images (a, b) are acquired at a different inversion time than the second two (c, d). The top two images were used to visualize the infero-apical infarct (inversion time = 350ms), while the bottom two images show anatomical structure (inversion time = 900ms). Enhancement from catheter in RV can be also seen where the coil is located, due to lack of decoupling between tracking and imaging coils. 38
51 39 Figure 2-6 Isochronal LAT maps - comparison between a healthy heart (a) and an infarct heart (b). In both cases the stimulation is applied from the RV and the activation times have been normalized with respect to the earliest activation. The resulting LAT map shows in the healthy subject an extended area of early activation times (red and orange surface). However, in the presence of infarct in the apical septal region, the area of early activation is reduced considerably. Intracardiac electrograms in the healthy area (c) and infarct areas (d) are also shown. In (d) late fractionated signals with reduced amplitude are shown. Figure 2-7 In infarcted swine (Left) Local activation map; (middle) voltage bipolar map; (right) MCLE MRI in-situ images, combination of short-axis and long-axis. The presence of infarct in the apical-septal region, shown in the voltage map and MRI in-situ images, is responsible for longer activation times in LAT map. Also, the voltage map shows higher voltage potential than 0mv, because the scar is only < 25% transmural.
52 Discussion In this chapter, we demonstrated the feasibility of MR-guided EP mapping of the LV during RV pacing, and we also characterized different activation times within the ventricular chamber. First, we showed the performance of the MR-guided EP system compared to a clinical standard system, CARTO. EAMs were created in healthy swine to verify that the two systems qualitatively yielded similar propagation patterns. Second, we compared the local activation times in healthy models, identifying the different ventricle segments and comparing the arrival times of the depolarization wave in those segments for CARTO and MR-EP. Finally, we observed the effects of infarcted tissue on the local activation time maps. The acquisition of LAT maps with an MR-guided mapping system has several potential benefits in an EP study. LAT maps, have the potential of identifying a reentry circuit responsible for VT, in cases when a voltage map does not detect slow conducting channels associated with the presence heterogeneous tissue with mixed electrical properties. The conventional classification of dense infarct scar as voltage potential below 0.5 mv has significant limitations including the fact that it may miss bundles of surviving myocytes surrounded by low voltage regions which are still able to depolarize, creating the substrate for VT. Therefore, MR-guided LAT maps may complement voltage maps. Our MR-guided EP system also reduces registration processing steps when compared to CARTO, potentially reducing the sources of registration errors. Both the endocardial shell and the touch-point geometry were acquired in the same frame of reference; no manual image segmentation and landmark selection process was required to align the anatomy to the EP information, reducing procedural steps. This study presented several challenges. For example, the missing data in Table 2.1 was the result of some animals undergoing sudden cardiac events. The majority of them had episodes of ventricular tachycardia/fibrillation leading to deaths hours into the procedure. In all those cases, cardiac defibrillation and mechanical compression were performed by our veterinary staff. In two cases we were able to revive the animals, but the ensuing cardiac function was extremely compromised, resulting in early termination of the study. In one case the pig died following a sudden depression of cardiac function and heart rate, which could not be reversed with heart rate increasing medications (i.e. epinephrine).
53 In our animal studies, we observed measurement variability that can be classified into two types: variability across the animals and variability within each animal. The discrepancies observed across the animals have already been explained in the literature [22]. During intracardiac procedures, prophylactic measures are used to prevent arrhythmias and vasospasm because swine are very prone to induction of ventricular fibrillation when the myocardium is stimulated or irritated. Specifically, anti-arrhythmic drugs are given along with anesthetic drugs. As a consequence, there is a challenge in reproducing the exact electrophysiological conditions across animals, since both anesthetic and anti-arrhythmic drugs have a varied degree of effect in each animal. Despite our efforts to mitigate these effects we still observed variability across animals. The author in [22] suggests that analysis results would benefit from using each animal as its own control, comparing each animal s experimental measurements to its own baseline measurements. The intra-animal variability, which is responsible for decreasing MRI-CARTO agreement observed, is a complex issue and it depends on several factors. Placing two pacing catheters in the same identical positions between the CARTO and MR-EP procedures was a challenge and may have also contributed to LAT difference in segments closer to pacing origin observed across measurements within the same animal. Attaching (via screw) a pacing lead onto the myocardium may improve results due to location consistency. An additional contributing factor to the deviation between measurements (accentuated in segments further away from pacing origin) is the mode of action of anesthetic gases on ion currents and ion channels in cardiomyocytes; this topic is still the subject of investigation [23]. In the case of isoflurane, it is believed that it interferes with the action potential duration by changing the kinetics of Na +, decisive for the upstroke component of the action potential. Most notably, this anesthetic extends the recovery time of the channels required to restore the resting state from which a renewed depolarization is capable of evoking a full activation, resulting in slowing down conduction [24]. Effects of isoflurane in swine models and other volatile gasses have been inconsistent with some studies showing increase action potential duration while others showing shortening. However, the overall consensus seems to be that these gases are certainly contributing to changes in cardiomyocytes behavior. In our studies we observed a relative slow conduction effect with time, which seems to be in agreement with Suzuki et al. observations [24], which found isoflurane to increase the effective refractory period. Most notably, in our studies propagation delays were typically longer in the second mapping session compared to the first in each study (with either CARTO or MR-EP system); this is most likely due to isoflurane accumulation effects on 41
54 conduction velocity in the ventricular myocardium after several hours of anesthetic [25]. Therefore, the combination of isoflurane effect on ionic currents and concentration of isoflurane, is the most likely cause of intra-animal variability. In our infarct studies, 2D and 3D MRI results provided critical information including wall thickness, scar presence, extent of scars, and local structure. In clinical settings, this information could affect procedure management. The MCLE images also provided different contrasts across different cardiac phases, which made the MR-guided EP procedure easier: the visualization of the scar facilitated navigation of the catheter tip directly to the specific location. In the resulting EAM, we observed voltage higher than 0mV and slower conduction time on the endocardial surface immediately adjacent to the infarct, which may suggest that the depolarization wave still traveled around or through the infarct region, albeit at a reduced velocity. The result was not totally unexpected since the infarct was < 25% transmural and also bundles of viable tissue may have been still present in the region. Our system has also some known limitations. Although acquired points are displayed in real time onto the LV shell, the raw EP signals are recorded by the Bridge TM system, whereas imaging and tracking information are displayed on Vurtigo, which runs on a separate system; after the completion of the study, processing the data consist of synchronizing timing and spatial information and the elimination of rejected points. These results are then imported into Vurtigo for creation of the EAM volumes. Mapped points in the LV depicting poor contact according to the electrogram signal were rejected. Additional filtering was performed by using only points from the same cardiac phase cycle as the surface shell (generally diastole). Ideally, a real-time data processing approach would facilitate immediate data re-acquisition if poor data were acquired, more closely resembling the conventional clinical systems. Furthermore, there is a fusion error between prior MRI volumes and EP points. The catheter touch-points were filtered to match the cardiac phase (diastole); since each point was tagged with time since R-trigger field, the time window was chosen to be around the third quarter of the cardiac cycle. However, a limiting factor is the temporal resolution (50 ms) of the prior MRI volume (for faster heart rate, number of points acquirable during diastole is reduced). We measured the latency of communication between the scanner acquisition board to display in Vurtigo and found it to be 64 ms ± 14 ms. Therefore, the spatial error would correspond to the displacement of the cardiac wall over this time interval due to a small diastolic motion and a small respiratory motion 42
55 (approximately 1-2 mm). We calculated this error by measuring the average perpendicular distance from point to surface (in diastolic phase). Additional spatial registration errors that require estimation and/or compensation are the cycle-to-cycle variation due to cardiac and respiratory motion, although the latter is small, that is in the order of ~1 mm. Furthermore, tracking accuracy is limited by the size of the microcoil. As explained in the previous chapter, the tracking depends on the projection profile of the signal from the coil, which is related to the physical size of the coil. Finally, the tip of the catheter, is interpolated from the two tracking coils, since the actual tip is 1mm further along the distal length than the actual microcoil. In our case the size of the coil is around 1 mm. Validation and improvement of tracking, spatial and temporal fusion is a work in progress Conclusion In this chapter, we performed EP studies in the porcine LV using an MR-guided EP system while pacing from the RV; we found qualitative agreement between the activation times and a clinical gold standard. We showed that imaging and MR guided electrophysiological measurements were possible in a few animals with myocardial infarction. 2.6 References [1] W. G. Stevenson and E. Delacretaz, Radiofrequency catheter ablation of ventricular tachycardia, Heart, vol. 84, no. 5, pp , Nov [2] A. Arenal, S. del Castillo, E. Gonzalez-Torrecilla, F. Atienza, M. Ortiz, J. Jimenez, A. Puchol, J. García, and J. Almendral, Tachycardia-related channel in the scar tissue in patients with sustained monomorphic ventricular tachycardias: influence of the voltage scar definition, Circulation, vol. 110, no. 17, pp , Oct [3] C. de Chillou, D. Lacroix, D. Klug, I. Magnin-Poull, C. Marquié, M. Messier, M. Andronache, C. Kouakam, N. Sadoul, J. Chen, E. Aliot, and S. Kacet, Isthmus characteristics of reentrant ventricular tachycardia after myocardial infarction, Circulation, vol. 105, no. 6, pp , Feb [4] C. Knackstedt, P. Schauerte, and P. Kirchhof, Electro-anatomic mapping systems in arrhythmias, Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology, vol. 10, pp. iii28 34, Nov [5] J. De Bakker, F. Van Capelle, M. Janse, S. Tasseron, J. Vermeulen, N. De Jonge,
56 and J. Lahpor, Slow conduction in the infarcted human heart. Zigzag course of activation, Circulation, vol. 88, no. 3, p. 915, Sep [6] J. M. de Bakker, F. J. van Capelle, M. J. Janse, A. A. Wilde, R. Coronel, A. E. Becker, K. P. Dingemans, N. M. van Hemel, and R. N. Hauer, Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation, Circulation, vol. 77, no. 3, pp , Mar [7] C. B. Brunckhorst, W. G. Stevenson, W. M. Jackman, K.-H. Kuck, K. Soejima, H. Nakagawa, R. Cappato, and S. A. Ben-Haim, Ventricular mapping during atrial and ventricular pacing. Relationship of multipotential electrograms to ventricular tachycardia reentry circuits after myocardial infarction, European Heart Journal, vol. 23, no. 14, pp. 8 8, Jul [8] K. Soejima, W. G. Stevenson, W. H. Maisel, J. L. Sapp, and L. M. Epstein, Electrically unexcitable scar mapping based on pacing threshold for identification of the reentry circuit isthmus: feasibility for guiding ventricular tachycardia ablation, Circulation, vol. 106, no. 13, pp , Sep [9] S. R. Dukkipati, R. Mallozzi, E. J. Schmidt, G. Holmvang, A. d'avila, R. Guhde, R. D. Darrow, G. Slavin, M. Fung, Z. Malchano, G. Kampa, J. D. Dando, C. McPherson, T. K. Foo, J. N. Ruskin, C. L. Dumoulin, and V. Y. Reddy, Electroanatomic mapping of the left ventricle in a porcine model of chronic myocardial infarction with magnetic resonance-based catheter tracking, Circulation, vol. 118, no. 8, pp , Aug [10] S. Nazarian, A. Kolandaivelu, M. Zviman, G. Meininger, R. Kato, R. Susil, A. Roguin, T. Dickfeld, H. Ashikaga, H. Calkins, R. Berger, D. Bluemke, A. Lardo, and H. Halperin, Feasibility of Real-Time Magnetic Resonance Imaging for Catheter Guidance in Electrophysiology Studies, Circulation, vol. 118, no. 3, p. 223, Jul [11] E. Schmidt, R. Mallozzi, A. Thiagalingam, G. Holmvang, A. d'avila, R. Guhde, R. Darrow, G. Slavin, M. Fung, J. Dando, L. Foley, C. Dumoulin, and V. Reddy, Electro-Anatomic Mapping and Radio-Frequency Ablation of Porcine Left Atria and Atrio-Ventricular Nodes using Magnetic Resonance Catheter Tracking, Circ Arrhythm Electrophysiol, pp , Oct [12] Y. Lu, P. Radau, K. Connelly, A. Dick, and G. A. Wright, Pattern Recognition of Abnormal Left Ventricle Wall Motion in Cardiac MR, Med Image Comput Comput Assist Interv. 2009;12(Pt 2): [13] Y. Lu, P. Radau, K. Connelly, A. Dick, and G. A. Wright, Segmentation of Left Ventricle in Cardiac Cine MRI: An Automatic Image-Driven Method, Functional Im and Mod of the Hear t, Vol. 5528, pp , [14] S. Pintilie, L. Biswas, K. Anderson, S. Dick, G. Wright, and P. Radau, VURTIGO: Visualization Software for Real-time, Image-guided Interventions, Medical image computing and computer-assisted intervention (MICCAI): Cardiac Interventional Imaging and Biophysical Modeling Workshop, London, England, Sept.20,, [15] C. L. Dumoulin, S. P. Souza, and R. D. Darrow, Real-time position monitoring of invasive devices using magnetic resonance, Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine, vol. 29, no. 3, pp , Mar [16] J. S. Detsky, J. A. Stainsby, R. Vijayaraghavan, J. J. Graham, A. J. Dick, and G. A. 44
57 Wright, Inversion-recovery-prepared SSFP for cardiac-phase-resolved delayedenhancement MRI., Magn. Reson. Med., vol. 58, no. 2, pp , Aug [17] A. P. Wijnmaalen, R. J. Van Der Geest, C. F. B. Van Huls Van Taxis, H.-M. J. Siebelink, L. J. M. Kroft, J. J. Bax, J. H. C. Reiber, M. J. Schalij, and K. Zeppenfeld, Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: real-time image integration and reversed registration, European Heart Journal, vol. 32, no. 1, pp , [18] Y. Okumura, B. D. Henz, S. B. Johnson, T. J. Bunch, C. J. O'Brien, D. O. Hodge, A. Altman, A. Govari, and D. L. Packer, Three-dimensional ultrasound for imageguided mapping and intervention: methods, quantitative validation, and clinical feasibility of a novel multimodality image mapping system, vol. 1, no. 2, pp , Jun [19] T. Dickfeld, J. Tian, G. Ahmad, A. Jimenez, A. Turgeman, R. Kuk, M. Peters, A. Saliaris, M. Saba, S. Shorofsky, and J. Jeudy, MRI-Guided Ventricular Tachycardia Ablation: Integration of Late Gadolinium-Enhanced 3D Scar in Patients With Implantable Cardioverter-Defibrillators, Circ Arrhythm Electrophysiol, vol. 4, no. 2, pp , Apr [20] B. Desjardins, T. Crawford, E. Good, H. Oral, A. Chugh, F. Pelosi, F. Morady, and F. Bogun, Infarct architecture and characteristics on delayed enhanced magnetic resonance imaging and electroanatomic mapping in patients with postinfarction ventricular arrhythmia, Heart rhythm : the official journal of the Heart Rhythm Society, vol. 6, no. 5, pp , May [21] M. D. Cerqueira, N. J. Weissman, V. Dilsizian, A. K. Jacobs, S. Kaul, W. K. Laskey, D. J. Pennell, J. A. Rumberger, T. Ryan, M. S. Verani, and American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging, Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association, presented at the Circulation, 2002, vol. 105, no. 4, pp [22] M. Michael Swindle, Swine in the laboratory: surgery, anesthesia, imaging, and experimental..., p. 471, [23] R. Hüneke, J. Fassl, R. Rossaint, and A. Lückhoff, Effects of volatile anesthetics on cardiac ion channels, Acta Anaesthesiol Scand, vol. 48, no. 5, pp , May [24] A. Suzuki, K. Aizawa, S. Gassmayr, Z. J. Bosnjak, and W.-M. Kwok, Biphasic effects of isoflurane on the cardiac action potential: an ionic basis for anestheticinduced changes in cardiac electrophysiology, Anesthesiology, vol. 97, no. 5, pp , Nov [25] S. Ozaki, H. Nakaya, Y. Gotoh, M. Azuma, O. Kemmotsu, and M. Kanno, Effects of isoflurane on conduction velocity and maximum rate of rise of action potential upstroke in guinea pig papillary muscles, Anesth Analg, vol. 70, no. 6, pp , Jun
58 46 Chapter 3 3 Comparison Between Late Enhancement Magnetic Resonance Imaging And Voltage Mapping Introduction Characterization of the myocardial substrate of ventricular tachycardia (VT) is a critical component for therapy planning prior to catheter ablation. The substrate supporting scar-related VT is characterized by regions of low voltage, slow conduction and fractionated electrograms [1]. The associated tissue is composed of a heterogeneous mix of surviving healthy myocyte bundles and dense fibrotic tissue through which a re-entry wave is likely to propagate. These arrhythmogenic areas are the ablative targets, which can be identified via careful substrate mapping, where the endocardial surface is examined through point-by-point direct catheter contact with the endocardial surface and electrical data are collected as a function of position; the acquired data are processed to create an electroanatomical voltage map (EAVM) or an activation time map, displaying both anatomical and electrophysiological information. In EAVMs, it is generally accepted that normal left ventricle (LV) endocardial bipolar electrograms have peakto-peak amplitudes of >1.5 mv and abnormal tissue or scar is identified by low voltage regions < 1.5 mv [1]-[3]; also, it has been shown that the border zone or peri-infarct zone (between 1.5mV and 0.5mV) is of particular importance given its correlation with the location of radiofrequency linear endocardial lesions that control VT [4]. To complement the conventional electrophysiology (EP) study, magnetic resonance imaging (MRI) has been used during the planning and procedural phases of a ventricular ablation procedure [5]. Cardiac MRI is a valuable tool to assess chronic myocardial infarction scar, due to its high spatial resolution and soft tissue discrimination. Specifically, contrast-enhanced MRI or late-enhancement MRI (LE-MRI) has been established as an accurate method for tissue characterization [6], [7]. 1 This chapter based on the original research article: Post-Infarction Ventricular Tachycardia Substrate Characterization: A Comparison Between Late Enhancement Magnetic Resonance Imaging And Voltage Mapping Using a MR-Guided Electrophysiology System. Oduneye S, Pop M, Biswas L, Flor R, Ghate S, Ramanan V, Barry J, Celik H, Crystal E, Wright G. IEEE Trans Biomed Eng Apr 12
59 Infarcted myocardium has a higher concentration of a gadolinium-based extracellular contrast agent, exhibiting a higher signal due to its reduced spin-lattice relaxation time (T1) (which results in an increased contrast between infarcted and healthy areas on the resulting images). Therefore, myocardial T1 characterization has been used for infarct extent quantification as T1 values are sensitive to the amount of damaged tissue and gadolinium contained in those regions in patients with acute and chronic myocardial infarction [8], [9]. Thus far, most studies characterizing arrhythmogenic substrates have not considered the spatial resolution or sensitive recording region of bipolar electrodes. Past studies have shown that bipolar measurements are highly influenced by electrode size and electrode spacing of the recording catheters [10], [11]. By modelling myocardial electrical activity as a dipole current source, the electrical potential field generated by this source was simulated in relation to bipolar electrodes located at an arbitrary distance. Specifically, the bipolar electrograms were affected by the electrode size, spacing between electrodes and the contribution to the amplitude signal decreases gradually as the distance from the bipolar electrodes to the tissue was increased. In recent years, in an effort to take advantage of the tissue characterization capabilities of MRI, clinical electrophysiology systems have incorporated the capacity to import and fuse preprocedural MR images [12], and MR systems have been designed to integrate electrophysiology measurement capabilities [13]-[15]. Although interpretation of the relationship between scar extent identified by bipolar or unipolar EAVM and by MRI has been described in various papers [16]-[18], it remains unclear how localized regions of VT substrate depicted in electrophysiology and detected by voltage measurement correlate with MRI. The relationship between T1 in the myocardium and bipolar signal has not been explored in depth; T1 quantification is important for myocardial injury assessment, because its measurements are directly linked to the property of the tissue and less sensitive to the influences of variations in signal enhancement [8]. Further, we believe that there is a relationship between the local voltage detected by bipolar measurements and the endocardial infarct detected by late-enhancement MRI in the sensitive region of the bipolar electrode measurement. Thus, the aims of this chapter are to: (1) characterize the relationship between chronic myocardial scar detectable by LE-MRI and intracardiac voltage recording using our MR-guided electrophysiology system [19], and (2) to introduce a new approach for myocardial infarct assessment relating bipolar voltage to a continuous parameter reflecting the relaxation rate R1 (1/T1), which is proportional to the amount of scar in the 47
60 corresponding tissue volume. In the previous chapter, we showed the advantages of our MRguided EP system, especially its capacity to minimize registration errors (2.1±1.1 mm [15]) typically seen when importing pre-acquired MR images into clinical electroanatomical mapping systems. Furthermore, LE-MRI was performed using a recent sequence and image processing technique called multi-contrast late enhancement (MCLE) that has shown improved reliability and reproducibility in tissue measurements of core infarct and gray zone, when compared to other conventional techniques [20], [21]. As described in [21], these conventional methods include the SD method (standard deviation) and FWHM method (full width half max) used IR- GRE images. The first method defines the infarct core and gray zone voxels as SI core > Mean remote + 3*SD remote and Mean remote + 2*SD remote < SI gray_zone < Mean remote +3*SD remote where SI core is the signal intensity of a voxel classified as the infarct core, and SI gray_zone is the signal intensity of a voxel classified as the gray zone. The second method defines the infarct core and gray zone voxels: SI core > 0.5 * Peak infarct and Peak remote < SI gray_zone < 0.5 * Peak infarct where Peak infarct is the peak signal intensity of all infarcted voxels. 3.2 Material and Methods Animal preparation Six pigs weighing approximately 30 to 40 kg were used for this set of experiments. Each animal underwent two procedures: infarct induction and, after 3 to 5 weeks the MR-guided EP procedure. Before each procedure, animals were placed under general anaesthesia induced with an intramuscular injection of ketamine (30 mg/kg) and atropine (0.05 mg/kg), and maintained by inhalation of 1-5% isoflurane. Also lidocaine (30 ml of 2% lidocaine in 250 ml of saline) and 50mg/ml amiodarone, were administered during the procedure; anticoagulation with intravenous heparin (70 units/kg) was also administered. Then, the animals were intubated and mechanically ventilated (20-25 breaths/minute). For infarct induction, complete coronary occlusion was achieved distal to the second diagonal branch of the left anterior descending artery (LAD) for 90 minutes by inflation of a percutaneous balloon dilation catheter (Sprinter Legend Balloon Catheter, Medtronic, Minneapolis, MN), and was followed by reperfusion. For the EP procedure, incisions of femoral vessels, carotid artery and/or jugular vein were performed and secured with 9F introducer sheaths for the purpose of administering medications and insertion of mapping catheters. Finally, ECG leads I, II, and III were monitored throughout the infarct induction 48
61 procedure as well as the electrophysiology procedure to track animal health. The animal care committee at Sunnybrook Research Institute approved this protocol and all the procedures were conducted following institutional guidelines Magnetic resonance imaging and image processing Several anatomical images were obtained at the beginning of each study on a 1.5T GE Signa system (GE Healthcare, Milwaukee, USA) using a 5-inch surface coil for signal reception. A stack of 2D MRI slices was acquired covering the ventricular chambers in their entirety using breath-held short-axis and long-axis sections. The typical parameters for the SSFP-CINE were: TE/TR = 1.1/3.6 ms, FA = 45, BW = 62.5 khz, FOV = 210 mm (phase FOV 0.8/0.9), slice thickness = 5 mm, Nx = 224, Ny = 160 (resolution of 1.1x1.2 mm), NEX = 1, and views per segment (vps) = 12. The cine SSFP sequence produced 20 phases over the heart cycle, acquired on average over second breath-holds. Late-enhancement imaging was started 5 minutes after the injection of 0.44 mmol/kg of Gd-DTPA (Magnevist, Berlex Inc., Wayne, NJ). The parameters for the MCLE sequence were: TR/TE = 2.7/1.3 ms, readout FA = 45, BW = ±125 khz, VPS = 16, FOV = 210 mm, slice thickness = 5 mm, 192 x 160 (phase FOV 0.8 or 0.9) imaging matrix and NEX = 1. The inversion pulse was placed such that the infarct-enhanced images are acquired during diastole. MCLE required a breath-hold of approximately seconds for each imaging slice. The MCLE sequence produces images with varying contrast where infarct can be visualized as an area of fast T1* recovery (T1* is the apparent T1 relaxation, shorter than the true T1 due to the continuous SSFP readout). Between six and eight images acquired during diastole to minimize motion between images (at the earliest inversion times) were used to extract the signal intensity and the steady-state plateau of the recovery curve signal for each pixel within the LV. After obtaining T1* and steady-state values for each pixel, we used a fuzzy C-means algorithm to automatically classify each pixel as infarct, healthy myocardium, gray zone, or blood, as previously described.[21]. Image noise plays a much smaller role in determining the categorization of each voxel using the MCLE analysis when compared to other inversion recovery approaches. The resulting classification for each voxel is therefore less sensitive to noise in any one image[21]. The change in R1* (1/T1*) relaxation rate of tissue is directly
62 proportional to the concentration of the contrast agent, which is increased in the extracellular space in chronic infarct, due to the presence of collagen fibers in the scar tissue and loss of intracellular volume. In our analyses, we normalized R1* on a per slice basis to minimize the effect of the contrast agent clearing the tissue across imaging slice acquisitions [22] (were entire imaging procedure lasted about 30~45 minutes. Thus, the pixel value was normalized with respect to the maximum R1* value of that particular slice and values between 0 and 1 represented the variation between no infarct (healthy myocardium) and core infarct respectively MR-guided three-dimensional electroanatomical voltage mapping and analysis Using our MR-guided EP system, we conducted substrate voltage mapping during sinus rhythm for each animal. The EP apparatus consisted of 8.5Fr MR-compatible Vision TM 50 catheters, (Imricor Medical Systems, Burnsville, USA) and a prototype Bridge EP Recording System (Imricor Medical Systems, Burnsville, MN); the catheters had four electrodes with 2mm spacing between the first two distal electrodes, 3 mm spacing between second and third electrode and 1 mm spacing between third and fourth electrode; bipolar recordings were made with the distal electrode pair (1 and 2). The catheters were placed in the LV using active catheter tracking with MR guidance as reported previously[19]. The catheters were positioned with the aid of a 3D LV shell created from segmentation of SSFP-cine images acquired minutes prior to the mapping procedure, with the animal resting in the same position; this eliminated the need for additional registration or alignment. The MCLE images were loaded into the visualization software Vurtigo (vurtigo.ca) to directly identify the navigation targets, (i.e., the infarcted areas). In these regions, an effort was made to acquire higher density voltage maps. At each point of contact the intracardiac signals and the three-dimensional spatial coordinates were recorded. This spatial and electrical information was used to create the EAVM (see Figure 3-1b) during off-line analysis. In the EP studies, tissue with bipolar voltages < 1.5 mv was defined as abnormal and tissue > 1.5mV was defined as healthy myocardium. It s important to note that high density map was acquired in the region of scar, whereas in healthy myocardium a more sparse approach was used to acquire points. Further points acquire in healthy myocardium were acquired around structures that are electrically inert.
63 51 Figure 3-1 (a) Multi-contrast late enhancement (MCLE) MR images were processed using a fuzzy clustering algorithm to classify each pixel as infarct (red), healthy myocardium (purple), or gray zone (yellow). (b) SSFP-derived meshes were overlaid with voltage amplitude information obtained from the MR-Guided endocardial mapping procedure, resulting in an EAVM (color coded as per color bar). (c) After the procedure, EAVM meshes were fused with the MCLEderived images. Figure 3-2 Description of sector division for short-axis slice tissue analysis. (a) Short-axis MCLE image with superimposed mapping points (points are colour coded as per bipolar voltage amplitude colorbar). (b) Stylized example of tissue classification (red = infarct, yellow = gray zone) (c) Each slice was subdivided into 40 radial sectors and coded based on the most common pixel class in that segment from the endocardial border to different depths, 2mm, 4mm and 6mm. Bipolar signals were then compared to the respective nearest segment Correlation between multi-contrast late enhancement and voltage signal of electroanatomical map As previously described, using the technique and image analysis tools, we performed further analysis off-line; we segmented the infarct core, gray zone and healthy myocardium in each of our MCLE short-axis images (see Figure 3-1a). The classification results were processed and
64 imported into Vurtigo as an endocardial mesh (Figure 3-1c). Correlation analysis was performed between the bipolar voltage at a given endocardial location and three main MR parameters associated with that location: 1) percentage of infarct across wall thickness; 2) tissue classification of sectors with varying depth from the endocardial surface; and 3) normalized R1* (1/T1*) within the same segments. First, we compared bipolar voltages to the percentage of infarct across the wall thickness at the corresponding location; each short-axis LV slice was evenly divided into 40 sectors by radial lines originating from the centroid of the LV (see Figure 3-2b). Then, infarct transmurality was calculated as the ratio of amount of over full wall thickness for each sector. Second, we examined voltages vs. sector tissue classification for each slice. Each of the 40 sectors was subdivided and reclassified as 1 of 9 categories based on tissue class (healthy myocardium, gray zone, infarct) and extent of tissue (2mm, 4mm and 6mm) see Figure 3-2c. Specifically, we determined the percentage of each tissue type (in pixels) in each sub-segment and re-categorized each sub-segment. 52 Figure 3-3 Median bipolar voltage of points located on the endocardial surface divided into sectors where percentage transmurality of scar is determined from the MCLE short-axis image. Median and mean voltage between each group differs significantly (P<0.01). The dotted line represents 1.5mV mark.
65 53 Starting at the endocardial surface, the first 2-mm sub-segment class is determined based on the highest percentage of pixels in one class. If the class changes when considering the 4-mm subsegment, the sector is classified as a sector 2-mm wide with class as determined. If the class remains the same over 4-mm, it s re-categorized as a 4-mm segment instead and similarly for 6- mm sub-segments. The layer increment of 2mm was chosen to eliminate any potential single pixel bias (~1 mm) in MCLE classification results, due to noise. Then, a bipolar voltage at the corresponding location from the maps was assigned to the segment for analysis similar to the 'points by segment' method described in this study[17]. Finally, we examined voltages vs. average normalized R1* calculated over the 6-mm sub-segment for each sector. As mentioned above, in all the sectors R1* values were normalized with respect to the minimum and maximum R1* value of each slice. Therefore, the resulting normalized R1* for each pixel is between 0 and 1, where 0 was the normalized R1* equivalent to healthy myocardium and 1 to core infarct Statistical analysis Bipolar voltages appearing in transmurality and classification results were expressed as median and 25th and 75th percentile (inter-quartile range); the whiskers extend to the most extreme data points not considered outliers. The rest of the measurements or graphs are expressed (or displayed) as mean ± SD. As appropriate, the Kruskal-Wallis test, modified from Mann Whitney U test (MATLAB, The Mathworks, Natick, MA) and one-way ANOVA test ( were used to evaluate median or mean values and were considered statistically significant at P-value of Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cutpoints for R1* that delineated healthy tissue and abnormal tissue. Optimal cost effective cutpoint was defined as the point on the curve closest to (0, 1), maximum efficiency cutpoint was defined as the point with the highest fraction of subjects that are correctly classified and finally the area under the ROC curve measured the test accuracy. Statistical analysis was performed with MATLAB (
66 Histopathology After completion of the electrophysiology study and mapping, the animals were sacrificed, and the heart was explanted immediately. The gross specimens were photographed, then placed in 10% neutral-buffered formalin for a minimum of 72 hours, then sectioned, and embedded in paraffin. Slices containing infarct lesions were cut to match short-axis MR views in reference to the geometry of the MR images (based on the distance from the apex of the heart). Thin tissue sections (4μm thickness) were prepared for whole-mount fixation on glass slides and underwent routine Trichrome Masson staining. 3.3 Results MR-guided three-dimensional electroanatomical voltage mapping and analysis In our infarct model, obtained by 90 minutes ligation of the LAD, the lesion is produced in the anterior and antero-septal aspect of the LV. At the time of the MR-EP study (3-5 weeks postinfarct), the mean LV ejection fraction for these animals was 30.4 ± 7.7 %. In our sample group, the mean mass of the infarct core was 4.69 ± 1.55 g and the mean gray zone mass was 2.34 ± 0.63 g. The results of the imaging studies are summarized in Table 3.1. In all our electrophysiology studies a catheter was placed inside the LV under MR guidance via carotid artery access with the animal placed feet-first in the scanner. No complications were experienced during visualization and guidance. The tracking FOV was adjusted in real time to include the aortic arch, allowing visualization of the catheter tip until it reached the aortic valve; once the catheter tip reached the valve the FOV was re-adjusted to enclose just the heart (improving tracking resolution). Navigation within the LV was facilitated by the mesh created from the SSFP short-axis images, which was displayed during the procedure in Vurtigo. In four animals the apical-most slices, were not included in the meshed volume because the blood pool was not distinguishable from myocardium. Overall, voltage maps of the LV were created with an average of 231 ± 35 points per LV. MCLE images were displayed along with the mesh, to highlight the location of the infarct (Figure 3-1c).
67 55 TABLE RESULTS OF MAGNETIC RESONANCE IMAGING STUDIES MRI Results (n = 6) Cardiac output (l/min) 1.50 ± 0.58 LV ejection fraction (%) 30.4 ± 7.7 LV volume ED (ml) ± 8.01 LV volume ES (ml) ± 9.17 Core infarct (g) 4.69 ± 1.55 Gray zone (g) 2.34 ± Correlation between multi-contrast late enhancement signal classification and voltage signal of electroanatomical map The bipolar voltage for infarct regions decreased significantly with increasing scar transmurality percentage. These results are in agreement with results previously reported in the literature [16], [17]. Bipolar voltages are reported as the median values, with 25 th percentile-75 th percentile marks and whiskers extending to most extreme data points. The median values were 3.45 mv ( mv), 1.65 mv ( mv), 1.37 mv ( mv), 1.23 ( mv) and 1.18 mv ( mv) for infarct transmurality ranges of 0-12%, 13-37%, 38-62%, 63-87%, and % respectively (Figure 3-3). Bipolar voltage amplitudes were compared to classification results, and the results were combined to the tissue classification coded segments to determine how tissue classification with varying extent or depth affects the overall voltage (see Figure 3-4). For 2 mm segments myocardial sectors of the endocardial surface, the median values for myocardium, gray zone and infarct were calculated and found to be 1.08 mv ( mv), 1.54 mv ( mv) and 3.34 mv; for 4mm segments 0.65 mv ( mv), 1.59 mv ( mv) and 1.92 mv ( mv) for myocardium, gray zone and infarct respectively; for 6mm segments 3.63 mv ( mv), 1.62 mv ( mv) and 1.34 mv ( mv). Beyond 6mm at full width the results remained unchanged. At 6mm healthy tissue has a median bipolar voltage value
68 > 1.5 mv, gray zone around 1.5mV and infarct < 1.5 mv, consistent with the literature. Therefore, these results suggest that the sensitivity of the bipolar electrode is the most accurate at 6mm tissue depth. Bipolar voltage as a function of normalized R1* was calculated and plotted (see Figure 3-5a). Only 6mm depth analysis is shown, as a result of previous finding that suggests that 6mm represents the most accurate sensitivity of the bipolar electrode measurements of our catheters. To determine the accuracy of R1* to distinguish normal tissue (>1.5mV) from abnormal tissue (<1.5mV), ROC curve analysis was performed. The optimal cost effective cutpoint of R1* = resulted in sensitivity of and specificity of with an efficiency of Maximum efficiency was obtained at cutpoint of R1* = 0.642, which resulted in sensitivity of and specificity of and efficiency of The ROC curve analysis and diagnostic results to separate abnormal tissue (voltage < 1.5mV) are shown in Figure 3-5, and the area under the ROC curve was found to be ± Figure 3-4 The distribution of points for each classification category according to MCLE classification results plotted against bipolar voltages as recorded during voltage mapping (P<0.001 between each group). Dotted line represents delineation of abnormal tissue at 1.5mV. The p-value differences between mean bipolar voltages of each category are shown for 2mm, 4mm and 6mm subsectors.
69 57 Figure 3-5 a) Bipolar voltage vs. normalized R1* is plotted and color-coded according to the tissue classification (red = infarct, yellow = gray zone, purple = myocardium); dashed line is 1.5 mv line. 6mm depth shown only. b) ROC analysis for R1* for 6mm depth applied to separate healthy tissue and abnormal tissue. Corresponding ROC curve area and confidence intervals are shown. (AUC = Area Under Curve, CI = confidence interval). 3.4 Discussion This chapter presents data indicating good correspondence between electrophysiological characterization and MR characterization of endocardial scar with an MR-guided electrophysiology system. MR-derived parameters such as transmurality, tissue classification and normalized R1* have the potential to be used routinely in clinical electrophysiology studies to contribute to differentiation between healthy and non-healthy tissue and identification of arrhythmogenic areas. In this chapter we observed that bipolar voltages decreased with increasing scar transmurality, which is in accordance with the findings in animals models[16]. However, in humans it has been reported that sectors with bipolar voltage < 1.5mV were associated with 75% transmural infarct [17], whereas in our animal studies the presence of infarct of at least 50% transmurality resulted in median voltages of 1.5mV or less; this could be the result of our infarct model or the catheter configuration. Nonetheless, infarct description using transmurality percentage is an important indicator in identifying arrhythmogenic regions. High transmurality and infarct heterogeneity is believed to be responsible for facilitating electrical instability and tachycardic events due to the presence of reentrant pathways between the islands of necrotic tissue[17], [23].
70 Further, recent studies have demonstrated the presence of arrhythmogenic sources within the scars visualized by MRI[24], [25] ; therefore we believe that accurate MRI information well complements the EP study. Specifically, R1*-type of quantification may be especially important, as it more directly reflects the actual degree of fibrosis and is less sensitive to windowing and variations in signal enhancement [26]. While we derived R1* with MCLE, a more conventional approach would be to map R1 (1/T1). A widely used cardiac T1 mapping method is MOLLI [26]. MOLLI is acquired in a breath hold which limits spatial resolution and restricts coverage to a single slice. The MOLLI sequence has the advantage of T1 determination at the same phase of the cardiac cycle, which reduces heart rate dependency and increases T1 measurement accuracy. The current study suggests that combining information about 1) scar detected by low voltage and 2) abnormal tissue identified by MRI-driven tissue classification and by normalized R1*, may have synergistic benefit. We believe the criteria under the two modalities used to identify scar regions, low bipolar voltages in EAVMs and hyper-enhanced regions in MRI, can be used in a complementary manner to identify VT substrate regions; accurate localization of VT substrate is an important factor for planning RF ablation procedures. Specifically, the high discriminatory power of R1* in detecting normal from abnormal tissue, implies that, when R1* maps are integrated into the clinical mapping system, identification of ablation sites is possible without the performance of a full and lengthy LV voltage map. Further, low-voltage regions in sinus rhythm EAVMs are a sensitive indicator of damage but are not always sensitive to thin infarcts as suggested by our results and are highly specific in the detection of VT substrate. This is due to the fact that bipolar voltage is directly influenced by catheter contact, which if inconsistent may give rise to mis-classifications; the presence of bundles of viable myocytes contributing to higher voltage within scar area may also contribute to mis-classifications. Similarly, late-enhancement MRI represents an accurate method to depict the three-dimensional nature of a scar, specifically if the scar extends past the region of sensitivity of a catheter. However, in-vivo imaging has some challenges due to limited spatial resolution of the images as it may miss sectors with small regions of surviving tissue near the endocardium. It is also only an indirect indicator of regions with altered electrical properties, which determine the nature of re-entry circuits. Our data analysis presented two types of outliers in the bipolar voltage measurements. The first were low voltage amplitude points in regions classified as healthy myocardium and second were high voltage amplitude signals in regions classified as scar. The former were caused by sporadic poor 58
71 catheter-tissue contact. Conversely, the latter group of outliers required a more in-depth investigation. The histology images revealed the presence of an endocardial healthy tissue layer (~1mm thickness) adjacent to the subendocardial scar; this tissue morphology resulted in higher than expected voltage recordings due to signal contribution from these bundles of healthy myocardium (see Figure 3-6). While this abnormal layer was the result of this particular infarct model, we believe it reinforces the idea that additional signatures to establish arrhythmogenic regions are needed. We envision discordance between EP and MR results triggering follow-up measurements in the associated region to more accurately determine arrhythmogenic risk. Effectively, the outliers represent a limitation of the current measurements. Furthermore, and related to the outlier issue, the large variance in healthy myocardium and small variance in gray zone of voltages shown in Figure 3-4 at 6mm may be due to the mapping methodology explained in section 3.2.3: more points were acquired in infarct regions by executing small movements of the catheter tip, in comparison to less spatially concentrated recording in healthy tissue. Furthermore, some healthy tissue points were acquired adjacent to electrically inert regions so that partial voluming of sensitive region together with small errors in misregistration could result in lower voltages. Because of data processing, gray zone regions were at least several pixels in extent and usually adjacent to core infarct (with even lower voltage but in a small range). These facts could lead to the lower voltage variance obtained in gray zone voltage potentials in Fig. 3.4 at 6mm points. 59 Figure 3-6 Example of high voltage outliers. A) Bipolar Voltage vs. normalized R1*, normalized values. The points in the box are above 1.5 mv. Dashed black line is 1.5 mv threshold. B) Localization of corresponding bipolar voltage points acquired on endocardial surface, shown on 3D visualization system (color bar is the same as Figure 3-2a). C) Approximate Masson Trichrome histology slice of corresponding region.
72 In this chapter we presented an analysis of electroanatomic voltage information with MR information. Both methodologies provide a structural depiction of the arrhythmogenic region; however an increased level of accuracy in the identification of VT substrate can be obtained with the addition of functional information in the suspected region generating the arrhythmia. This information can be extracted from activation time maps and the morphology of the intracardiac signals; Future studies are planned so that we can better correlate intracardiac recording morphology with MR information. Adding scar substrate functional information to MR information represents an ideal way to improve VT substrate identification. The advantage of our integrated MR-system is in the ability to potentially provide iterative acquisition of MR images along with structural and functional EP information in a single environment that does not require time-consuming switches from one modality to another. An interventional physician would have the capability to easily use the most convenient modality to fill in the gaps of knowledge in the diagnostic phase and verify suspected arrhythmogenic regions via MRI or EP, prior to performing ablation. For example, bipolar signals could be better interpreted and related to the distance between measuring electrodes as a result of myocardium information obtained via the extended MRI field of view. Also, MRI-based 3-D computer modeling can also provide a valuable tool to understand the propagation of abnormal activation patterns and provide targets for ablation[27]. Overall we believe that a dynamic exchange of information between the imaging / mapping modalities (MRI and electrocardiographic) could improve the specificity of VT substrate identification and represents an attainable solution. 3.5 Conclusion At the time of the study, it was the first systematic assessment of the myocardial substrate as defined by integrated normalized R1* in vivo imaging and high-resolution electroanatomic voltage mapping in an animal infarct model. Our results demonstrate that MRI information (transmurality, tissue classification and relaxation rate) can accurately predict areas that represent regions scar identified with bipolar voltage mapping, as demonstrated by ROC analysis. MCLE can help overcome limitations of bipolar voltage mapping including long durations and lower spatial discrimination and may help identify the sites within scars, which are commonly believed to trigger arrhythmic events in post-infarction patients. 60
73 3.6 References [1] E. M. Aliot, W. G. Stevenson, J. M. Almendral-Garrote, F. Bogun, C. H. Calkins, E. Delacretaz, P. Della Bella, G. Hindricks, P. Jaïs, M. E. Josephson, J. Kautzner, G. N. Kay, K.-H. Kuck, B. B. Lerman, F. Marchlinski, V. Reddy, M.-J. Schalij, R. Schilling, K. Soejima, and D. Wilber, EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias, Heart Rhythm, vol. 6, no. 6, pp , 01-Jun [2] K. Soejima, W. G. Stevenson, W. H. Maisel, J. L. Sapp, and L. M. Epstein, Electrically unexcitable scar mapping based on pacing threshold for identification of the reentry circuit isthmus: feasibility for guiding ventricular tachycardia ablation, Circulation, vol. 106, no. 13, pp , Sep [3] A. Arenal, S. del Castillo, E. Gonzalez-Torrecilla, F. Atienza, M. Ortiz, J. Jimenez, A. Puchol, J. García, and J. Almendral, Tachycardia-related channel in the scar tissue in patients with sustained monomorphic ventricular tachycardias: influence of the voltage scar definition, Circulation, vol. 110, no. 17, pp , Oct [4] Marchlinski, D. Callans, C. Gottlieb, and E. Zado, Linear ablation lesions for control of unmappable ventricular tachycardia in patients with Ischemic and Nonischemic Cardiomyopathy, Circulation, [5] W. G. Stevenson and K. Soejima, Catheter ablation for ventricular tachycardia, Circulation, vol. 115, no. 21, pp , May [6] J. W. Weinsaft, I. Klem, and R. M. Judd, MRI for the assessment of myocardial viability, Cardiology clinics, vol. 25, no. 1, pp , v, Feb [7] R. Macedo, A. Schmidt, C. E. Rochitte, J. A. C. Lima, and D. A. Bluemke, MRI to assess arrhythmia and cardiomyopathies, J. Magn. Reson. Imaging, vol. 24, no. 6, pp , Dec [8] D. R. Messroghli, K. Walters, S. Plein, P. Sparrow, M. G. Friedrich, J. P. Ridgway, and M. U. Sivananthan, Myocardial T1 mapping: Application to patients with acute and chronic myocardial infarction, Magn. Reson. Med., vol. 58, no. 1, pp , Jul [9] D. R. Messroghli, T. Niendorf, J. Schulz-Menger, R. Dietz, and M. G. Friedrich, T1 mapping in patients with acute myocardial infarction., Journal of cardiovascular magnetic resonance, vol. 5, no. 2, pp , [10] S. Kun and R. A. Peura, Influence of the electrode configuration on the results of electrophysiological studies, presented at the Engineering in Medicine and Biology Society, 1995., IEEE 17th Annual Conference, 1995, vol. 2, pp [11] J. M. Stinnett-Donnelly, N. Thompson, N. Habel, V. Petrov-Kondratov, D. D. Correa de Sa, J. H. T. Bates, and P. S. Spector, Effects of electrode size and spacing on the resolution of intracardiac electrograms, Coronary Artery Disease, vol. 23, no. 2, pp , Mar [12] S. Nazarian, D. A. Bluemke, and H. R. Halperin, Applications of cardiac magnetic resonance in electrophysiology, Circ Arrhythm Electrophysiol, vol. 2, no. 1, pp , Feb [13] E. Schmidt, R. Mallozzi, A. Thiagalingam, G. Holmvang, A. d'avila, R. Guhde, R. Darrow, G. Slavin, M. Fung, J. Dando, L. Foley, C. Dumoulin, and V. Reddy, Electro- Anatomic Mapping and Radio-Frequency Ablation of Porcine Left Atria and Atrio- Ventricular Nodes using Magnetic Resonance Catheter Tracking, Circ Arrhythm Electrophysiol, pp , Oct [14] S. R. Dukkipati, R. Mallozzi, E. J. Schmidt, G. Holmvang, A. d'avila, R. Guhde, R. D. Darrow, G. Slavin, M. Fung, Z. Malchano, G. Kampa, J. D. Dando, C. McPherson, T. K. 61
74 Foo, J. N. Ruskin, C. L. Dumoulin, and V. Y. Reddy, Electroanatomic mapping of the left ventricle in a porcine model of chronic myocardial infarction with magnetic resonance-based catheter tracking, Circulation, vol. 118, no. 8, pp , Aug [15] S. Nazarian, A. Kolandaivelu, M. Zviman, G. Meininger, R. Kato, R. Susil, A. Roguin, T. Dickfeld, H. Ashikaga, H. Calkins, R. Berger, D. Bluemke, A. Lardo, and H. Halperin, Feasibility of Real-Time Magnetic Resonance Imaging for Catheter Guidance in Electrophysiology Studies, Circulation, vol. 118, no. 3, p. 223, Jul [16] T. Wolf, L. Gepstein, U. Dror, and G. Hayam, Detailed endocardial mapping accurately predicts the transmural extent of myocardial infarction, J Am Coll Cardiol, [17] A. P. Wijnmaalen, R. J. Van Der Geest, C. F. B. Van Huls Van Taxis, H.-M. J. Siebelink, L. J. M. Kroft, J. J. Bax, J. H. C. Reiber, M. J. Schalij, and K. Zeppenfeld, Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: real-time image integration and reversed registration, European Heart Journal, vol. 32, no. 1, pp , [18] S. Nakahara, M. Vaseghi, R. J. Ramirez, C. G. Fonseca, C. K. Lai, J. P. Finn, A. Mahajan, N. G. Boyle, and K. Shivkumar, Characterization of myocardial scars: electrophysiological imaging correlates in a porcine infarct model, Heart rhythm : the official journal of the Heart Rhythm Society, vol. 8, no. 7, pp , Jul [19] S. Oduneye, L. Biswas, S. Ghate, V. Ramanan, J. Barry, A. Laish-Farkash, E. Kadmon, T. Zeidan-Shwiri, E. Crystal, and G. A. Wright, The Feasibility of Endocardial Propagation Mapping Using Magnetic Resonance Guidance in a Swine Model, and Comparison with Standard Electroanatomic Mapping, Medical Imaging, IEEE Transactions on, vol. 31, no. 4, pp , [20] K. A. Connelly, J. S. Detsky, J. J. Graham, G. Paul, R. Vijayaragavan, A. J. Dick, and G. A. Wright, Multicontrast late gadolinium enhancement imaging enables viability and wall motion assessment in a single acquisition with reduced scan times., J. Magn. Reson. Imaging, vol. 30, no. 4, pp , Oct [21] J. S. Detsky, G. Paul, A. J. Dick, and G. A. Wright, Reproducible classification of infarct heterogeneity using fuzzy clustering on multicontrast delayed enhancement magnetic resonance images., IEEE Trans Med Imaging, vol. 28, no. 10, pp , Oct [22] R. E. Hendrick and E. M. Haacke, Basic physics of MR contrast agents and maximization of image contrast., J. Magn. Reson. Im, vol. 3, no. 1, pp , [23] B. Desjardins, T. Crawford, E. Good, H. Oral, A. Chugh, F. Pelosi, F. Morady, and F. Bogun, Infarct architecture and characteristics on delayed enhanced magnetic resonance imaging and electroanatomic mapping in patients with postinfarction ventricular arrhythmia, Heart rhythm, vol. 6, no. 5, pp , May [24] H. Ashikaga, T. Sasano, J. Dong, M. M. Zviman, R. Evers, B. Hopenfeld, V. Castro, R. H. Helm, T. Dickfeld, S. Nazarian, J. K. Donahue, R. D. Berger, H. Calkins, M. R. Abraham, E. Marbán, A. C. Lardo, E. R. McVeigh, and H. R. Halperin, Magnetic resonance-based anatomical analysis of scar-related ventricular tachycardia: implications for catheter ablation., Circ Res, vol. 101, no. 9, pp , Oct [25] H. L. Estner, M. M. Zviman, D. Herzka, S. Nazarian, H. Ashikaga, Y. Dori, R. D. Berger, H. Calkins, A. C. Lardo, and H. R. Halperin, The critical isthmus sites of ischemic ventricular tachycardia are in zones of tissue heterogeneity, visualized by 62
75 magnetic resonance imaging., Heart rhythm, vol. 8, no. 12, pp , Dec [26] M. S. Nacif, E. B. Turkbey, N. Gai, S. Nazarian, R. J. van der Geest, R. A. Noureldin, C. T. Sibley, M. Ugander, S. Liu, A. E. Arai, J. A. C. Lima, and D. A. Bluemke, Myocardial T1 mapping with MRI: Comparison of look-locker and MOLLI sequences, J. Magn. Reson. Imaging, vol. 34, no. 6, pp , Sep [27] M. Pop, M. Sermesant, T. Mansi, E. Crystal, S. Ghate, J.-M. Peyrat, I. Lashevsky, B. Qiang, E. McVeigh, N. Ayache, and G. A. Wright, Correspondence Between Simple 3- D MRI-Based Computer Models and In-Vivo EP Measurements in Swine With Chronic Infarctions., IEEE Trans Biomed Eng, vol. 58, no. 12, pp , Dec
76 64 Chapter 4 4 Distribution of Abnormal Intracardiac Signals In Infarct Regions 4.1 Introduction One of the most important determinants to achieving successful ablation is the accurate delineation of the reentry pathway. The conventional substrate mapping method delineates the infarct area using voltage mapping (1.5-mV cut-off value), then uses pace-mapping to define the VT exit as well as EGM characteristics to identify areas with slow conduction [1]. In the majority of ventricular tachycardia cases arising after the patient has suffered a myocardial infarction, slow conduction regions are part of the re-entry circuit in the arrhythmogenic substrate [2], often detected with the voltage maps as well. These regions of the re-entrant path have been found to contain presystolic abnormal electrograms (EGMs) [3]. These abnormal EGMs originate from the action potential propagating across surviving viable myocardium separated by fibrosis in a zig-zag activation pattern[4], [5]. The combined local conduction delays produce abnormal signals with multiple signal components, each representing the nonsynchronous depolarization of the different bundles of the surviving myocardial fibers. These abnormal potentials, recorded during electroanatomical mapping, are classified as fractionated potentials or as isolated/late potentials. Identifying these abnormal potentials becomes important in cases where the patient has hemodynamically unstable VT, or non-inducible VT; otherwise, in these patients, electrophysiologists only have the information from the voltage maps, which has low specificity for VT substrate identification [6]. Abnormal potentials have been analyzed in several studies. Hsia et al. [7] compared the correlation between late potentials and arrhythmogenic substrate of reentrant VT circuits and found that, out of the 133 sites recorded, a higher prevalence of late potentials were recorded near the isthmus (89%) compared to entrance (57%) or exit (20%). Similarly, comparable studies have identified higher prevalence of late potentials at sites classified as central or proximal in the reentry circuit as compared to other sites (exit or outer loop sites) [8]. Abnormal potentials were shown not only to correlate with the reentry pathway but also to have direct correspondence to successful ablation targets. When ablation strategies have focused on targeting isolated potentials
77 the results have proven to be effective [9]. Furthermore, other studies have also indicated that abnormal potentials can be used to identify critical isthmuses with signals recorded during sinus rhythm (SR) [6], [10], [11], paced-controlled rhythm [6] or both [9], [12]. It is believed that overlapping activation fronts may limit the identification of multiple components during sinus rhythm. This limitation can be mitigated by simplifying the evaluation to one propagation front; specifically, right ventricular apex (RVA) pacing from a known location creates a dominant action potential wavefront that can identify abnormal potentials otherwise not detected [6]. Point-by-point mapping of the left ventricle (LV) is time consuming and leads to prolonged procedure/anesthesia time, and the low specificity of this technique requires additional diagnostic procedures [6]. In the previous chapter [13] we showed that MRI information used in electrophysiology studies can accurately predict areas of myocardial scar identified with bipolar voltage mapping. We believe that a strategy for identification of sites for mapping and ablation is achievable when using MRI combined with abnormal electrical signal information; this added information is desirable to due to its greater specificity compared to peak voltage mapping alone. More specifically, gray zone volume on late enhancement-mri (LE-MRI) images has been shown to correlate with all-cause post-mi mortality and with inducibility for ventricular tachycardia (VT) [14], [15]. Therefore, we hypothesized that gray zone quantified by LE-MRI have high correspondence to the spatial locations with high likelihood of abnormal potentials. To test this hypothesis we aimed to: 1) identify regions of isolated/late and fractionated potentials in sinus rhythm and controlled-paced rhythm in post-infarct animals; and 2) to characterize the relative prevalence of these abnormal potentials in critical sites and areas labeled as gray zone as quantified by LE MRI Methods Animal preparation Six pigs weighing an average of 35 kg were used for the study. Four of these animals were used in the work of the previous chapter; overall four had 90-minute infarcts, whereas two had 60- minute infarcts. Each animal underwent infarct induction and, after approximately 4 weeks, an MR-guided EP procedure. Before each procedure, animals were placed under general anesthesia
78 induced with an intramuscular injection of ketamine (30 mg/kg) and atropine (0.05 mg/kg), and maintained by inhalation of 1-5% isoflurane. Also lidocaine (30 ml of 2% lidocaine in 250 ml of saline) and 50mg/ml amiodarone, were administered during the procedure; anticoagulation with intravenous heparin (70 units/kg) was also administered. Then, the animals were intubated and mechanically ventilated (20-25 breaths/minute). For infarct induction, complete coronary occlusion was achieved distal to the second diagonal branch of the left anterior descending artery (LAD) for 90 minutes or 60 minutes by inflation of a percutaneous balloon dilation catheter (Sprinter Legend Balloon Catheter, Medtronic, Minneapolis, MN), and was followed by reperfusion. The different occlusion times change the extent and heterogeneity of the infarct. For the EP procedure, incisions of femoral vessels, carotid artery and/or jugular vein were performed and access secured with 9F introducer sheaths for the purpose of administering medications and inserting mapping catheters. Finally, ECG leads I, II, and III were monitored throughout the infarct induction procedure as well as the electrophysiology procedure to track animal health. The animal care committee at Sunnybrook Research Institute approved this protocol and all the procedures were conducted following institutional guidelines Imaging protocol and image processing A 1.5T GE Signa (GE Healthcare, Milwaukee, USA) with a 5-inch surface coil was used to acquire anatomical scans. The left and right ventricle were fully imaged in long axis and short axis directions yielding multiple 2D MR images using a respiratory gated sequence. The principal anatomical scans were performed with SSFP-CINE with the following parameters: TE/TR = 1.1/3.6 ms, FA = 45, BW = 62.5 khz, NEX = 1, and views per segment (vps) = 12, slice thickness = 5 mm, FOV = 210 mm (phase FOV 0.8 or 0.9), Nx = 224, Ny = 160. This resulted in an approximate in-plane resolution of 0.9x1.2 mm. The cine SSFP sequence produced 20 phases over the heart cycle, acquired on average over second breath-holds. Following the anatomical scans, we performed the late-enhancement imaging using a multi contrast late enhancement (MCLE) sequence recently developed in our lab [16]. MCLE was shown to yield more reliable and reproducible measures of the infarct core and gray zones than conventional techniques. This sequence produces images with multiple contrasts where infarct
79 can be visualized as an area of fast T1* recovery (T1* is the apparent T1 relaxation, shorter than the true T1 due to the continuous SSFP readout). The MR scans started 5 minutes after injection of 0.44 mmol/kg of Gd-DTPA (Magnevist, Berlex Inc., Wayne, NJ). The parameters for the MCLE sequence were: TR/TE = 2.7/1.3 ms, readout FA = 45, BW = ±125 khz, VPS = 16, FOV = 210 mm, slice thickness = 5 mm, 192 x 160 (phase FOV 0.8 or 0.9) imaging matrix and NEX = 1. An inversion pulse was placed such that the infarct-enhanced images from the continuous SSFP acquisition are acquired during diastole. The resulting images from the MCLE sequence were used to classify the different myocardial tissues states. Six to eight images were utilized from the diastolic cardiac phase to extract the signal recovery rate (1/T1*) and the steady-state plateau of the recovery curve signal for each pixel within the LV. After obtaining T1* and steady-state values for each pixel, we used a fuzzy C-means algorithm to automatically classify each pixel as infarct, healthy myocardium, gray zone, or blood, as previously described in [16] Real-time MR-guided electrophysiology system The MR-guided EP apparatus was comprised of 8.5Fr MR-compatible Vision TM catheters, (Imricor Medical Systems, Burnsville, USA) and a prototype Bridge TM EP Recording System (Imricor Medical Systems, Burnsville, MN); we used a 4-electrode catheter with 2mm spacing between the first two distal electrodes, 3 mm spacing between second and third electrode and 1 mm spacing between third and fourth electrode. Bipolar recordings were made with the distal electrode pair (1 and 2). The catheters were placed in the (right ventricle) RV or LV using active catheter tracking with MR-guidance as reported previously in chapter 3. The catheters were advanced under full MR guidance with a 3D LV shell created from segmentation of SSFP-cine images acquired in the same session, with the animal resting in the same position; this eliminated the need for additional registration or alignment. The MCLE images were loaded into the visualization software Vurtigo (vurtigo.ca) to directly identify the navigation targets, (i.e., the infarcted areas). In these regions, an effort was made to acquire higher density voltage maps. At each point of contact, the intracardiac signals and the three-dimensional spatial coordinates were recorded.
80 Electrophysiology procedure Using the MR-guided EP system, we conducted substrate voltage mapping during sinus rhythm for each animal. The EP procedure was executed and the mapping data was collected in the following sequence: 1) a voltage map was constructed for the endocardial surface of the left ventricle under real-time MR guidance; 2) RVA programmed stimulation was then performed, with a second catheter advanced into the right ventricle to reduce overlap of action potential signals commonly seen in the porcine model. For the latter, performing pacing at a rate faster than the animal s normal sinus rhythm (on average 150 BPM) and increasing the current until muscle capture was assured, the natural pacemaker was overridden. Abnormal potentials were defined in the following manner: 1) distinct bipolar electrogram recordings inscribed after the end of the RVA reference signal (R-peak), separated from the major initial component of the local ventricular electrogram with an isoelectric interval greater than 20 ms in both sinus and paced cases; and 2) fractionated potentials as multicomponent signals of long duration >100ms and greater than underlying signal noise (0.1mV) in both sinus and paced rhythms. The remaining EGMs displaying both above-mentioned characteristics were also defined as abnormal. Abnormal signals were distinct and distinguishable from repolarization signals, because of the sharp high-frequency delayed components (see Figure 4-1). This process was performed by an observer. Only endocardial sites with bipolar electrogram recordings acquired during sinus or right ventricular pacing were analyzed for abnormal EGMs. All EGMs recordings within the LV EP map were analyzed for the presence or absence of abnormal potentials. EGMs with high electrical noise, or poor signal quality reducing accurate assessment of abnormal EGMs were excluded from the analysis. To estimate the degree of local activation time delay, an isoelectric interval was measured as the timing interval from the onset of the second upstroke in the ventricular electrogram to the earliest component of the abnormal EGM. The incidence of abnormal EGMs in different infarct regions as defined by voltage amplitudes and by LE-MRI was determined. Regions of low voltage potentials were classified as those with voltage amplitude < 1.5 mv, specifically < 0.5 mv for dense scar and 0.5mV-1.5mV for gray zone [1]. Critical central pathways were defined as regions of late arrival time in infarct or gray zone tissue and it was hypothesized to support VT, in a similar manner as the isthmus of the reentry circuit.
81 Figure 4-1 Examples of abnormal EGMs recordings from animals with myocardial infarction. Shown are the EGM recordings in RV and LV in the sinus (a, c, e, g) and paced (b, d, f, h) condition. Tracings (a) and (b) show RVA signals, c and d represent normal healthy myocardium recordings. The following recordings are all acquired in non-healthy tissue. In (e) and (f) the local EGM recorded by the mapping catheter displays an isolated potential (oblique arrows) that is separated from the ventricular EGM by an isoelectric line of at least 20 ms. Finally, (g) and (h) are the recorded EGM fractionated and the width is > 102 ms; (RVA = Right ventricular apex, LV = left ventricle) 69
82 Statistical analysis Categorical variables were compared by a chi-squared test. Continuous variables were compared using one-way ANOVA test ( To determine the statistical significance of the frequency of potentials in the different presumed regions of the reentry (entry, exit and critical central pathway), we compared observed and expected proportion with a chisquare test; this is applicable when you wish to compare two or more groups, and the outcome variable is categorical rather than a continuous variable ( A value of p <.05 was considered statistically significant. MATLAB (The Mathworks, Natick, MA) was used for statistical analyses. 4.3 Results Electrophysiology mapping In our studies during the electrophysiology mapping, we recorded a total of 3945 EGMs from 6 animals. EGMs were recorded in both SR (2469 points) and paced rhythm (1476 points). During SR the signals recorded resulted in 609 low voltage EGMs and 558 abnormal EGMs; for the paced rhythm, 308 and 360 EGMs were founds to be low voltage and abnormal respectively. Also, our analysis focused on the relationship between abnormal potentials and tissue properties as classified by MCLE. Tissue classification results were processed and imported into Vurtigo as an endocardial mesh. Abnormal potentials recorded during the mapping procedure were assigned to locations on the tissue classification mesh using a closest point algorithm, this process resulted in EGM characteristic to tissue property pairings for various regions. We recorded 758, 147 and 1544 EGMs from MR-defined regions of dense infarct, gray zone and healthy myocardium respectively in the SR case. For the paced case we recorded 378, 55 and 1043 EGMs for MR-defined dense infarct, gray zone and healthy myocardium respectively. Results are summarized in Figure 4-2. Abnormal potentials contained isolated/late potentials as well as fractionated potentials.
83 71 Figure 4-2 Distribution of abnormal potential recordings in sinus and paced rhythm for low voltage potentials and abnormal potentials (left); and within scar, gray zone and healthy myocardium regions (right) of the endocardium. Low Volt = low voltage, DS = dense scar, GZ = gray zone, HM = healthy myocardium. TABLE 4.1 DISTRIBUTION OF ABNORMAL POTENTIAL EGMS RECORDED IN SCAR, GRAY ZONE AND HEALTHY REGIONS Animals N = 6 SINUS RHYTHM Dense Scar Gray zone Healthy myo p-value PACED RHYTHM Dense Scar Gray zone Healthy myo p-value Total EGMs recorded Low voltage potentials 214 (28%) 38 (26%) 337 (22%) p< (26%) 2 (4%) 208 (20%) p<0.01 Abnormal potentials 183 (24%) 39 (27%) 336 (22%) p< (30%) 23 (42%) 224 (21%) p<0.001 Values in parentheses indicate relative percentage of low voltage or abnormal potentials over total EGMs in either scar or gray zone or myocardium. EGMs are shown in rhythm and vs. paced rhythm. p-values are calculated as the difference between the tissue groups Distribution of Abnormal Potential Signals In Different Cardiac Rhythms Sites classified as dense scar, gray zone, and healthy myocardium by MRI within the low voltage and abnormal potentials groups were further examined. Relative prevalence of abnormal EGMs in a given region was calculated as the ratio of EGMs classified as low voltage or abnormal over the total number of EGMs recorded in scar, gray zone and healthy myocardium, respectively. In
84 general, our analysis determined that low-voltage potentials had a higher relative prevalence in dense scar, whereas abnormal EGMs had a higher relative percentage in gray zone regions in paced rhythm. Specifically, for low-voltage in sinus rhythm, relative prevalence was 28%, 26% and 22% in dense scar, gray zone and healthy myocardium respectively (p=0.01 among the three groups). For low-voltage during pacing the relative prevalence of low-voltage potentials was 26%, 4% and 20% for dense scar, gray zone and healthy myocardium respectively (p<0.001). Conversely, for abnormal potentials in sinus rhythm, relative prevalence was equivalent for all EGM measurements in those regions, compared to 24%, 27% and 22% (p = 0.3) in dense scar, gray zone and healthy tissue respectively; in the paced rhythm case the relative prevalence of abnormal EGMs was 30%, 42% and 21% (p = between each group) in dense scar, gray zone and healthy myocardium respectively. These results are summarized in Table 1. When performing pair-wise statistical comparison instead of across all three groups, in the paced rhythm case of abnormal EGMs we obtain gray zone vs. healthy myocardium (p<0.01), dense scar vs. healthy myocardium (p<0.01), and dense scar vs. gray zone (p = 0.1). These results are summarized in Table 4.1. These results suggest that pacing increased the difference in abnormal EGM prevalence between the classification groups. 72 Figure 4-3 Activation map recorded during mapping procedure. The color index reveals the continuity between the earliest and latest activated areas typical of later arrival time capable of supporting VT. The presumed depolarization wavefront is drawn to indicate potential pathway. a) classification of tissue b) presumed depolarization wavefront examples; c) presumed regions of hypothetical arrhythmic pathway Distribution of Abnormal Signal potentials Distributions of abnormal potentials across locations in assumed target regions as a function of rhythm were also analyzed. The target regions (entry, critical central pathway and exit, see
85 Figure 4-3) were inferred from depolarization times of local activation/arrival signals relative to all arrival times. Local activation times for recorded points classified as dense infarct and gray zone were subdivided into quartiles for each animal: 1) the first quartile of the earliest arrival times within MR-determined regions of dense infarct or gray zone were classified as being associated with the entry; 2) 2 nd and 3 rd quartile were classified as within the critical central pathway; and 3) latest arrival times (4 th quartile) are considered to be within the exit region. Therefore, the distribution of all measured potentials across entry, critical central pathway and exit will be, by definition, 25%-50%-25% respectively. Considering only the abnormal potentials observed in these regions we asked if their spatial distribution was different than that for all potentials. Our results show that, in the sinus case, there is an unexpectedly high relative fraction at the exit, specifically 21.2%, 42.8%, and 36.0% of the abnormal potentials in the regions were seen in the entry, central pathway, and exit regions respectively; in the paced case, the abnormal EGMs were more likely to be seen in the central pathway region, with a distribution of 19.9%, 65.4% and 14.7% in the entry, critical central pathway and exit regions respectively (as shown in Figure 4-4). Using a comparison of proportion test, we determined that each of these distributions differed from the expected distribution of 25:50:25 in a statistically significant way (p<0.05). 73 Figure 4-4 Distributions of abnormal potentials. Entry, critical central pathway and exit are shown in the figure. The distribution of abnormal potentials is based on Figure 4-2. The critical central pathway location is associated with the presence of abnormal potentials. Using a comparison of proportion test, we determined that the frequencies between each distribution were statistically significant p<0.05.
86 Discussion Abnormal Potential Signals Distribution For patients who are hemodynamically unstable or have unmappable VTs, the identification of regions of abnormal potentials is extremely important, because abnormal potentials are more associated with the location of the VT isthmus than low amplitude potentials [6], [7], [10], [17]. Conversely, locations of VT-related conducting channels have been shown to be only grossly estimated by low peak bipolar voltages (<1.5mV) measured during SR without tachycardia induction; notably, single threshold cannot be applied universally to all patients [18]. Furthermore, straight-line radiofrequency ablation lesions designed to transect these channels of slow conduction have been shown to be on average longer in length by several cms when the isthmus has not been effectively identified and only voltage maps are known (e.g. in hemodynamically unstable patients) [19]. However, abnormal EGMs reflecting anisotropic late arrival times can identify VT-related slow conduction regions. Specifically, sites with isolated/late potentials and fractionated potentials during sinus/paced rhythm reflect late local activation of bundles of healthy myocytes that enable conduction and become critical components of a reentry circuit. Abnormal potentials acquired during endocardial mapping have been shown to correspond to appropriate ablation targets with greater specificity than simple peak voltage mapping [9]. Also, as previously stated, MRI-determined gray zone has been shown to correlate with all-cause post-mi mortality and with inducibility for ventricular tachycardia for patients with myocardial infarct scars; therefore it would be important to characterize the prevalence of abnormal potentials in areas labeled as gray zone as quantified by LE MRI. Our data suggest that abnormal potentials are more prevalent in the MR-defined gray zone, particularly during pacing. This is a novel finding, and provides additional insights into the analysis of RVA stimulation; these results are consistent with the hypothesis that RVA pacing suppresses overlapping signals and reduces the influence of adrenergic and cholinergic nerve fibers present on the swine endocardium. The heterogeneous nature of gray zone with bundles of surviving myocytes responsible for the irregular propagation of the action potential may explain these results. Also, these results are in line with previous studies that hypothesized that overlapping of EGMs or a particular orientation of a line of block with respect to the activation front may preclude the identification of multiple components during SR [6]. This limitation can be overcome by changing the activation front, such as during RVA pacing, to identify otherwise
87 undetected abnormal EGMs. This is important because the authors of the previous study also claim that these EGMs could better identify VT-related slow conduction areas relative to lowamplitude electrograms [6]. We also found that, in the paced case, a higher relative percentage of abnormal signal potentials was associated with sites classified as critical central pathway compared to entry or exit, based on activation arrival times. These findings are in line with findings of Nakahara et al [12], where a higher concentration of abnormal EGMs (late potentials) was found in the putative isthmus and also with Hsia et al. [7], where the majority of these delayed and fractionated endocardial electrogram recordings were found in critical central pathway sites. Our LGE-MRI spatial resolution is approximately ~1.67mm in-plane and 5 mm through plane, however gray-zone analysis would benefit from increased resolution in the order of 1mm isotropic. This high resolution would facilitate the reduction of partial volume effects. Currently, in our tissue analysis single pixels of gray zone found in healthy myocardium are eliminated to minimize partial volume concerns, while limiting our analysis to larger gray zone regions. Improved resolution will solve this limitation as well Limitations of electrophysiology procedure Our underlying assumptions are that abnormal EGMs correlate with reentry VT pathway as shown in the literature[11]; however, VT was not induced in our animals. Also, abnormal morphology signals could be recorded on endocardial regions arising from bystander sites adjacent to the reentry pathways that may not participate in a VT circuit. Ideally, in future studies, specificity and sensitivity of recorded abnormal signal potentials may be assessed if one could induce VT in the swine model and characterize multiple morphologies of VT in detail. After VT induction, linear ablation could be performed to confirm accuracy of target sites identified via increased distribution of abnormal potentials in the gray zone of our analysis. 4.5 Conclusion Our data suggests that gray zone quantified by LE-MRI has high correspondence to regions with a high proportion of abnormal potentials. This correspondence is higher when right ventricular apex stimulation is employed. Abnormal electrograms are commonly observed in sinus rhythm and paced rhythm from and in close proximity to critical central pathways in other studies. 75
88 Future investigation into the clinical outcome associated with ablation of VT using gray zone information and signal morphology is warranted. 4.6 References [1] E. M. Aliot, W. G. Stevenson, J. M. Almendral-Garrote, F. Bogun, C. H. Calkins, E. Delacretaz, P. Della Bella, G. Hindricks, P. Jaïs, M. E. Josephson, J. Kautzner, G. N. Kay, K.-H. Kuck, B. B. Lerman, F. Marchlinski, V. Reddy, M.-J. Schalij, R. Schilling, K. Soejima, and D. Wilber, EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias, Heart Rhythm, vol. 6, no. 6, pp , 01-Jun [2] W. G. Stevenson and K. Soejima, Catheter ablation for ventricular tachycardia, Circulation, vol. 115, no. 21, pp , May [3] F. Bogun, S. Krishnan, M. Siddiqui, E. Good, J. E. Marine, C. Schuger, H. Oral, A. Chugh, F. Pelosi, and F. Morady, Electrogram characteristics in postinfarction ventricular tachycardia: effect of infarct age., J Am Coll Cardiol, vol. 46, no. 4, pp , Aug [4] J. De Bakker, F. Van Capelle, M. Janse, S. Tasseron, J. Vermeulen, N. De Jonge, and J. Lahpor, Slow conduction in the infarcted human heart. Zigzag course of activation, Circulation, vol. 88, no. 3, p. 915, Sep [5] J. M. T. de Bakker and F. H. M. Wittkampf, The pathophysiologic basis of fractionated and complex electrograms and the impact of recording techniques on their detection and interpretation., Circ Arrhythm Electrophysiol, vol. 3, no. 2, pp , Apr [6] A. Arenal, E. Glez-Torrecilla, M. Ortiz, J. Villacastín, J. Fdez-Portales, E. Sousa, S. del Castillo, L. Perez de Isla, J. Jimenez, and J. Almendral, Ablation of electrograms with an isolated, delayed component as treatment of unmappable monomorphic ventricular tachycardias in patients with structural heart disease., J Am Coll Cardiol, vol. 41, no. 1, pp , Jan [7] H. H. Hsia, D. D. Lin, W. H. Sauer, D. J. Callans, and F. E. Marchlinski, Relationship of late potentials to the ventricular tachycardia circuit defined by entrainment., Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing, vol. 26, no. 1, pp , Oct [8] T. Harada, W. G. Stevenson, D. Z. Kocovic, and P. L. Friedman, Catheter ablation of ventricular tachycardia after myocardial infarction: relation of endocardial sinus rhythm late potentials to the reentry circuit., J Am Coll Cardiol, vol. 30, no. 4, pp , Oct [9] F. F. Bogun, B. B. Bender, Y.-G. Y. Li, G. G. Groenefeld, S. H. S. Hohnloser, F. F. Pelosi, B. B. Knight, S. A. S. Strickberger, and F. F. Morady, Analysis during sinus rhythm of critical sites in reentry circuits of postinfarction ventricular tachycardia., Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing, vol. 7, no. 1, pp , Aug
89 [10] F. Bogun, E. Good, S. Reich, D. Elmouchi, P. Igic, K. Lemola, D. Tschopp, K. Jongnarangsin, H. Oral, A. Chugh, F. Pelosi, and F. Morady, Isolated potentials during sinus rhythm and pace-mapping within scars as guides for ablation of post-infarction ventricular tachycardia., J Am Coll Cardiol, vol. 47, no. 10, pp , May [11] C. B. Brunckhorst, W. G. Stevenson, W. M. Jackman, K.-H. Kuck, K. Soejima, H. Nakagawa, R. Cappato, and S. A. Ben-Haim, Ventricular mapping during atrial and ventricular pacing. Relationship of multipotential electrograms to ventricular tachycardia reentry circuits after myocardial infarction, European Heart Journal, vol. 23, no. 14, pp. 8 8, Jul [12] S. S. Nakahara, R. R. Tung, R. J. R. Ramirez, J. J. Gima, I. I. Wiener, A. A. Mahajan, N. G. N. Boyle, and K. K. Shivkumar, Distribution of late potentials within infarct scars assessed by ultra high-density mapping, Heart Rhythm, vol. 7, no. 12, pp. 8 8, Dec [13] S. O. Oduneye, M. Pop, L. Biswas, R. Flor, S. Ghate, V. Ramanan, J. Barry, H. Celik, E. Crystal, and G. A. Wright, Post-Infarction Ventricular Tachycardia Substrate Characterization: A Comparison Between Late Enhancement Magnetic Resonance Imaging And Voltage Mapping Using a MR-Guided Electrophysiology System, Biomedical Engineering, IEEE Transactions on, no. 99, p. 1, [14] A. T. Yan, A. J. Shayne, K. A. Brown, S. N. Gupta, C. W. Chan, T. M. Luu, M. F. Di Carli, H. G. Reynolds, W. G. Stevenson, and R. Y. Kwong, Characterization of the periinfarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality, Circulation, vol. 114, no. 1, pp , Jul [15] A. Schmidt, C. F. Azevedo, A. Cheng, S. N. Gupta, D. A. Bluemke, T. K. Foo, G. Gerstenblith, R. G. Weiss, E. Marbán, G. F. Tomaselli, J. A. C. Lima, and K. C. Wu, Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction, Circulation, vol. 115, no. 15, pp , Apr [16] J. S. Detsky, G. Paul, A. J. Dick, and G. A. Wright, Reproducible classification of infarct heterogeneity using fuzzy clustering on multicontrast delayed enhancement magnetic resonance images., IEEE Trans Med Imaging, vol. 28, no. 10, pp , Oct [17] S. Nakahara, M. Vaseghi, R. J. Ramirez, C. G. Fonseca, C. K. Lai, J. P. Finn, A. Mahajan, N. G. Boyle, and K. Shivkumar, Characterization of myocardial scars: electrophysiological imaging correlates in a porcine infarct model, Heart rhythm : the official journal of the Heart Rhythm Society, vol. 8, no. 7, pp , Jul [18] A. Arenal, S. del Castillo, E. Gonzalez-Torrecilla, F. Atienza, M. Ortiz, J. Jimenez, A. Puchol, J. García, and J. Almendral, Tachycardia-related channel in the scar tissue in patients with sustained monomorphic ventricular tachycardias: influence of the voltage scar definition, Circulation, vol. 110, no. 17, pp , Oct
90 [19] K. Soejima, M. Suzuki, W. H. Maisel, C. B. Brunckhorst, E. Delacretaz, L. Blier, S. Tung, H. Khan, and W. G. Stevenson, Catheter ablation in patients with multiple and unstable ventricular tachycardias after myocardial infarction: short ablation lines guided by reentry circuit isthmuses and sinus rhythm mapping., Circulation, vol. 104, no. 6, pp , Aug
91 79 Chapter 5 5 Summary and Future Directions 5.1 Summary In Chapter 2, we described a feasibility study that tested the performance of a newly designed and built MR-guided electrophysiology (EP) system. We used the system to conduct some basic EP maneuvers to demonstrate reliable imaging and EP signal fidelity during MR-guided procedures in healthy and infarct models. The overall performance of the system was validated against the current clinical gold standard for electroanatomical mapping, a system called CARTO. Each procedure was conducted in healthy animals; one catheter was inserted in the left ventricle (LV) and one in the right ventricle (RV). The latter was used to pace the heart at higher rate than normal sinus rhythm. This electrical stimulation was used to create activation time maps on the LV endocardial surface. The relative arrival time of the action potential wavefront from the fixed RV pacing location to each location in the LV was recorded and calculated; the measurements at multiple positions in the LV were used to reconstruct an activation time map. Each animal underwent mapping with the MR-guided EP system inside the MR scanner and mapping with the CARTO system outside the scanner. The comparison of the two systems suggested that there was significant correspondence between the MR-EP and CARTO-derived maps; furthermore, our system resulted in lower spatial error when compared to the CARTOMerge software registration error reported in the literature (2.1± 1.1mm against 4.3 ± 3.2mm See Chapter 2). CARTOMerge registers preacquired MR images with electroanatomical maps. Our MR-guided system does not require registration; however we currently do not compensate for respiration motion, hence the spatial discrepancy. In Chapter 3, we characterized the relationship between chronic myocardial fibrotic scar detected by multi-contrast late enhancement (MCLE) MRI and detected by electroanatomical voltage mapping (EAVM) obtained using our real-time MR-Guided electrophysiology system. We also explored how these measures might improve identification of suspected arrhythmogenic VT substrates. The MCLE images were analyzed to identify the location and extent of fibrotic infarct. Correlation analysis was conducted between bipolar voltages and three MR parameters (infarct transmurality, tissue classification, and normalized relaxation rate R1 ) at corresponding
92 locations. In general, tissue regions classified as scar by normalized R1 values were well correlated with locations of low bipolar voltage amplitude. Moreover, our results demonstrate that MRI information (transmurality, tissue classification, and relaxation rate) can accurately predict areas of myocardial fibrosis identified with bipolar voltage mapping, as demonstrated by the ROC analysis. In Chapter 4, we analyzed abnormal electrograms as a marker of VT, since abnormal electrograms have more discriminatory power than voltage maps. Our study in six infarcted animals identified regions of abnormal potentials (isolated and fractionated potentials) during sinus rhythm and paced rhythm to characterize the relative prevalence of these potentials in areas labeled as gray zone identified by MCLE. Using our real-time MR-Guided EP system, highdensity electrogram recordings were acquired and used to generate endocardial electroanatomical maps. We conducted substrate voltage mapping, created voltage and activation time maps, and fused the corresponding MRI information for each data set. Then, tissue classification (dense infarct, gray zone and healthy myocardium) was correlated with locations of abnormal potentials. Our results suggested that gray zone has high correspondence to regions with a high relative proportion of abnormal potentials. This correspondence was higher when right ventricular apex stimulation was used. Furthermore, the abnormal potential prevalence was higher in central critical ablation regions. The work conducted in chapter 3 and chapter 4, created the platform to tackle two critical questions that remain unanswered: 1) Will targeting therapy to gray zone identified regions during EP procedures, improve the outcome of radiofrequency ablations? 2) Can MR-guided radiofrequency ablation procedures, augmented with direct RF lesion characterization by MRI provide a better solution to the high rate of post-treatment arrhythmia recurrence? Biophysics of RF Ablation and Lesion Characterization In patients suffering from VT, the basic idea for radiofrequency ablation (RFA) is to position a catheter on a critical area of the arrhythmogenic substrate within the ventricle and to apply RF power to create a lesion via thermal energy. The presence of scars in the myocardial tissue disrupts the pathways responsible for arrhythmia genesis. Specifically, in the case of reentrant
93 VT, the goal of RFA is to create a non-conducting linear transmural lesion pattern across the isthmus connecting areas of non-conducting scar. Although procedural success has improved in the past two decades, VT recurrence afflicts at least 30% percent of patients treated with RFA [1]; thus protective implantable cardioverter defibrillators (ICDs) are currently the preferred treatment option (though palliative in nature). Currently used guiding tools in RFA therapy, notably X-ray fluoroscopy and three-dimensional mapping devices, do not provide sufficiently accurate information on the exact target location, and cannot directly depict the presence and extent of ablation lesions. All these are factors that impact procedural outcomes. MRI has been identified as a possible solution to these challenges. Previous studies have demonstrated the potential to map RF lesions using T2-weighted or T1-weighted contrast-enhanced sequences [2], [3]; however it remains a challenge to quickly distinguish edema (which is a transient lesion) from permanently damaged tissue. Therefore, our goal moving forward is to: 1) validate the findings in Chapter 3, Chapter 4 with respect to VT substrate markers represented by gray zone and (R1* and abnormal potentials); 2) to establish the effectiveness of our system immediately post-ablation for predicting successful elimination of VT in the porcine model using our novel real-time imaging and EP system; and 3) to monitor the RFA lesion formation progression. The goal of RF ablation is to transform electromagnetic energy into thermal energy and to destroy the target tissue through thermal coagulation. The frequencies used for RF ablation are generally in the range of KHz [4]. This mode is considered resistive heating (or electrical heating) because as the electrical current flows in a closed loop from the tip of the catheter to the indifferent electrode (an electrode used as ground) placed on the skin, it encounters resistive material producing heat. Applying lower frequency energy (<100Hz) to the myocardium can potentially interact with cardiac muscle and nerves, creating muscle contraction, pain sensation and arrhythmia generation. In the MHz range, resistive heating becomes dielectric heating (as seen in microwave energy) and becomes less effective at transferring heat to the tissue; it also requires more expensive hardware. Resistive heating is proportional to the RF power density, which is in turn proportional to current density [5]. The current density decreases as a function of radial distance from the electrode source. Therefore, resistive heating of the tissue decreases proportionally with the fourth power of the distance from the electrode. Practically, this translates to a radial rim of 2 to 3 mm around the tip that is generally directly heated by catheter tip. During RF catheter ablation, direct heating is 81
94 principally responsible for lesion formation. In the case of a catheter, although heat is generated in deeper tissue through thermal conduction, a lot of heat generated by the catheter tip is carried away by the surrounding flowing blood, convective cooling [6]. As a result, transfer of thermal energy to deeper tissue and efficiency of energy coupling to the tissue can be as low as 10% [7]. This fact coupled with the hemorrhagic reaction of injured tissue may be the difference between a linear lesion without gaps and one that exhibits a transient electrical block despite containing gaps. Arguably, one of the most important features of MR-guided electrophysiology procedures is the ability to visualize RFA lesions with sufficient temporal and spatial resolution. RFA lesions are typically imaged with a T2-weighted Fast Spin Echo (FSE) sequence or a gadolinium-enhanced T1- weighted Fast Gradient Recalled Echo (FGRE) sequence [3]. RFA lesions in images obtained using T2-weighted FSE sequences appear as hyperintense regions; the zones of reversible and irreversible damage are not discernable [3]. The acute interstitial edema associated with the ablation is the primary cause for the hyperintense regions representing the area of damage observed by T2-weighted FSE imaging. The release of vasoactive polypeptides from local inflammatory cells immediately following the injury during edema formation causes water and proteins to escape through gaps in the endothelial cells lining the vessel and enter the interstitial space. This rapid local increase in the number of unbound protons increases the T2 relaxation constant of the tissue and gives rise to the hyperintense regions that appear to represent the spatial extent of the anatomic lesion. The delayed lesion response after ablation, over ~10 minutes, is consistent with the temporal physiology of local acute interstitial edema and probably represents the time required for hydrostatic and osmotic capillary pressures to equilibrate. The underlying mechanism for RFA lesion visualization using T1-weigthed imaging is distinctly different. After administration of Gadolinium-DTPA, the agent exerts its signal-enhancing effect by interacting with water protons resulting in shorter T1 relaxation times. In healthy tissue, this large molecule cannot penetrate cell membranes and is therefore restricted to the extracellular space. After endocardial ablation, however, damaged/ruptured cell membranes allow diffusion and penetration of the contrast agent into the intracellular space, increasing volume distribution of the contrast agent and resulting in hyperintense voxels. 82
95 83 Figure 5-1 Three-dimensional bipolar voltage maps using the MRI-guided electrophysiology system in an animal with prior myocardial infarction. The map demonstrates an apical-septal wall myocardial scar. The scale bar units are in mv. Figure 5-2 SSFP image of left ventricle after ablation with corresponding intracardiac voltage mapping points. A lesion directly adjacent to the catheter tip could be visualized after RF delivery. Intracardiac electrogram amplitude decreased 50% after ablation.
96 Future directions: MR-guided RF ablation The MRI-EP system developed for these studies has been described in Chapter 3 and 4. Initial assessment studies were conducted in anticipation of future RFA studies; for the latter, the MRI- EP system was combined with an additional integrated RFA system. The RFA generator (Stockert EP Shuttle) was operated in manual mode to bypass the impedance control/monitor. The generator was located outside the scanner room. Temperature-monitored RFA delivery, without noise introduction to real-time tracking, was achieved by adding RF filters designed by Imricor Medical Systems. The EP catheter tip temperature was monitored by a thermocouple, which was connected via fiber-optic cable to a temperature monitoring system also outside the scanner. We conducted tests to ensure that the RFA system didn t impact tracking and imaging: with the system ON tracking and imaging were possible; with the system delivering power tracking was functional, however imaging displayed a high level of noise. In one of our preliminary animal studies, we first recorded an electroanatomical voltage map as shown in Figure 5-1. With MR-tracking active, we then proceeded to deliver several 30-watt 30-seconds RF transmissions on the healthy lateral wall of the animal. Immediately after the power delivery (RF energy OFF), we acquired short-axis and long-axis SSFP-CINE images, and a few illustrative post-ablation voltage mapping points. SSFP-CINE pulse sequence had the following parameters: TE/TR = 1.1/3.6 ms, FA = 45, BW = 62.5 khz, FOV = 210 mm (phase FOV 0.8/0.9), slice thickness = 5 mm, Nx = 224, Ny = 160, NEX = 1. The resulting image is shown in Figure 5-2. A large lesion was visualized directly adjacent to the ablation catheter tip minutes after the injury was induced. In the same figure we showed an example of the change in voltage amplitude for an intracardiac electrogram recording: pre-ablation voltage amplitude decreased by 50% compared to post-ablation amplitude, from 3.7mV to 1.5mV. In Figure 5-3, direct visual comparison of the lesions between the MR-derived image (few minutes post-ablation) and gross pathology examination is shown. Lesion width measured at gross examination matched well with MR-derived measurements. This study was a feasibility study to validate the hardware and software functionality of our system for RFA purposes. Our primary concern was the potential of the RF generator injecting noise into the MR scanner rendering tracking or imaging unusable; we did not experience any of these issues.
97 Figure 5-3 Direct visual comparison of right ventricular apex lesion appearance by MRI and by gross examination. Ablation lesion appears only a few minutes RFA in MRI slices. Both images also show manually estimated lesion size. 85
98 Future directions: Lesion Characterization A large effort is under way in our lab towards a comprehensive characterization of RFA lesions in the left ventricle of a swine model. One of the goals of this work is to further our understanding of the evolution of lesion formation, and link imaging characteristics to the underlying pathophysiology. This work being conducted at Sunnybrook Research Institute by Dr. Celik and others [8] examines the intrinsic tissue contrast mechanisms for characterization of acute RFA lesions in the heart; the study is based on the notion that the RF-induced chemical and structural alterations change both the T1 and T2 characteristics of the tissue, which may yield a more repeatable and reliable method to delineate lesion extent than the contrast-enhanced methods. The motivation of this study is to be found in some of the limitations of current contrast-based imaging methods: late gadolinium enhanced imaging is less favorable because of contrast variability of lesions due to wash-in/wash-out kinetics. Preliminary results of this experiment are shown in Figure 5-4. IR-SSFP with contrast agent displays very promising lesion characterization. Prior to ablation, a baseline MRI of the heart was acquired. Active ablation catheters were advanced into the LV of swine and several localized lesions were created through the application of RF energy. Several lesions were created per animal at various locations in the myocardium using different degrees of RF power and/or duration of power deposition to create lesions of varying severity. Preliminary results indicate that an IR-SSFP sequence provides good depiction of both the lesions and the edema in various images with different TIs [8]; imaging thermal injury in the myocardium using the IR-SSFP approach, during or immediately after ablation, has the potential to provide a reliable estimation of the myocardium viability and reversibility of the original thermal damage in the myocardium. The majority of the advantages of an IR-SSFP based procedure over the conventional late-gadolinium enhancement come from the independence from contrast agent wash-in/wash-out kinetics: the procedure can be repeated within short period of times (without waiting for full contrast agent washout), and the full extent of the lesion can be characterized earlier as contrast is not required to fully enter the lesion space. Thus, this study will focus on IR-SSFP reliability and consistency for RFA lesion characterization compared to late-gadolinium enhanced methods.
99 Combining the results from the lesion characterization studies in this chapter and the results from the studies in Chapter 4 will provide the necessary tools to validate the performance of MR- Guided RFA to eliminate VT. After identifying the critical ablation region via MCLE, abnormal potentials and other key markers, RFA will be conducted and its effectiveness in terminating the VT will be evaluated for different markers. For example, VT termination outcomes could be compared for procedures guided by maps of various parameters: low-voltage amplitude; normalized R1*; tissue classification (gray zone); abnormal electrograms; and location of abnormal electrograms. By using each map or parameter as an ablation target, the effective capacity to terminate the VT could be used to rank and validate each marker and parameter. 87 Figure 5-4 Lesion images: a) InSitu, 3D IR-GRE: TI=600ms, b) IR-SSFP, t=5min after ablation c) IR-SSFP, t=14min after ablation d) IR-SSFP, t=48min after ablation e) late gadolinium enhancement TI=600ms, 7min after Gad injection f) MCLE 3min after Gad injection. The RFA lesions (b, c, d) were delineated using the multi-contrast IR-SSFP imaging sequence but without injecting Gd-DTPA. This sequence is based on a segmented and cardiac-gated SSFP continuous data acquisition following an inversion pulse, which generates multiple images with varying contrast weighted by intrinsic tissue T1 and T2
PART I. Disorders of the Heart Rhythm: Basic Principles
 PART I Disorders of the Heart Rhythm: Basic Principles FET01.indd 1 1/11/06 9:53:05 AM FET01.indd 2 1/11/06 9:53:06 AM CHAPTER 1 The Cardiac Electrical System The heart spontaneously generates electrical
PART I Disorders of the Heart Rhythm: Basic Principles FET01.indd 1 1/11/06 9:53:05 AM FET01.indd 2 1/11/06 9:53:06 AM CHAPTER 1 The Cardiac Electrical System The heart spontaneously generates electrical
CARDIOVASCULAR SYSTEM
 CARDIOVASCULAR SYSTEM Overview Heart and Vessels 2 Major Divisions Pulmonary Circuit Systemic Circuit Closed and Continuous Loop Location Aorta Superior vena cava Right lung Pulmonary trunk Base of heart
CARDIOVASCULAR SYSTEM Overview Heart and Vessels 2 Major Divisions Pulmonary Circuit Systemic Circuit Closed and Continuous Loop Location Aorta Superior vena cava Right lung Pulmonary trunk Base of heart
Where are the normal pacemaker and the backup pacemakers of the heart located?
 CASE 9 A 68-year-old woman presents to the emergency center with shortness of breath, light-headedness, and chest pain described as being like an elephant sitting on her chest. She is diagnosed with a
CASE 9 A 68-year-old woman presents to the emergency center with shortness of breath, light-headedness, and chest pain described as being like an elephant sitting on her chest. She is diagnosed with a
ELECTROCARDIOGRAPHY (ECG)
 ELECTROCARDIOGRAPHY (ECG) The heart is a muscular organ, which pumps blood through the blood vessels of the circulatory system. Blood provides the body with oxygen and nutrients, as well as assists in
ELECTROCARDIOGRAPHY (ECG) The heart is a muscular organ, which pumps blood through the blood vessels of the circulatory system. Blood provides the body with oxygen and nutrients, as well as assists in
CASE 10. What would the ST segment of this ECG look like? On which leads would you see this ST segment change? What does the T wave represent?
 CASE 10 A 57-year-old man presents to the emergency center with complaints of chest pain with radiation to the left arm and jaw. He reports feeling anxious, diaphoretic, and short of breath. His past history
CASE 10 A 57-year-old man presents to the emergency center with complaints of chest pain with radiation to the left arm and jaw. He reports feeling anxious, diaphoretic, and short of breath. His past history
How to Ablate Atrial Tachycardia Mechanisms and Approach. DrJo Jo Hai
 How to Ablate Atrial Tachycardia Mechanisms and Approach DrJo Jo Hai Contents Mechanisms of focal atrial tachycardia Various mapping techniques Detailed discussion on activation sequence mapping and entrainment
How to Ablate Atrial Tachycardia Mechanisms and Approach DrJo Jo Hai Contents Mechanisms of focal atrial tachycardia Various mapping techniques Detailed discussion on activation sequence mapping and entrainment
Figure 2. Normal ECG tracing. Table 1.
 Figure 2. Normal ECG tracing that navigates through the left ventricle. Following these bundle branches the impulse finally passes to the terminal points called Purkinje fibers. These Purkinje fibers are
Figure 2. Normal ECG tracing that navigates through the left ventricle. Following these bundle branches the impulse finally passes to the terminal points called Purkinje fibers. These Purkinje fibers are
Ablation Lesion Assessment
 HRC 2016 Ablation Lesion Assessment The creation of effective and permanent lesions Ian Wright Imperial College Healthcare Wed 09:00-09:30 Hall 11 Objective Examine the role of existing strategies and
HRC 2016 Ablation Lesion Assessment The creation of effective and permanent lesions Ian Wright Imperial College Healthcare Wed 09:00-09:30 Hall 11 Objective Examine the role of existing strategies and
Chapter 16: Arrhythmias and Conduction Disturbances
 Complete the following. Chapter 16: Arrhythmias and Conduction Disturbances 1. Cardiac arrhythmias result from abnormal impulse, abnormal impulse, or both mechanisms together. 2. is the ability of certain
Complete the following. Chapter 16: Arrhythmias and Conduction Disturbances 1. Cardiac arrhythmias result from abnormal impulse, abnormal impulse, or both mechanisms together. 2. is the ability of certain
Chapter 20 (2) The Heart
 Chapter 20 (2) The Heart ----------------------------------------------------------------------------------------------------------------------------------------- Describe the component and function of
Chapter 20 (2) The Heart ----------------------------------------------------------------------------------------------------------------------------------------- Describe the component and function of
Chapter 12: Cardiovascular Physiology System Overview
 Chapter 12: Cardiovascular Physiology System Overview Components of the cardiovascular system: Heart Vascular system Blood Figure 12-1 Plasma includes water, ions, proteins, nutrients, hormones, wastes,
Chapter 12: Cardiovascular Physiology System Overview Components of the cardiovascular system: Heart Vascular system Blood Figure 12-1 Plasma includes water, ions, proteins, nutrients, hormones, wastes,
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
Outline. Electrical Activity of the Human Heart. What is the Heart? The Heart as a Pump. Anatomy of the Heart. The Hard Work
 Electrical Activity of the Human Heart Oguz Poroy, PhD Assistant Professor Department of Biomedical Engineering The University of Iowa Outline Basic Facts about the Heart Heart Chambers and Heart s The
Electrical Activity of the Human Heart Oguz Poroy, PhD Assistant Professor Department of Biomedical Engineering The University of Iowa Outline Basic Facts about the Heart Heart Chambers and Heart s The
Cardiac Arrhythmias. Cathy Percival, RN, FALU, FLMI VP, Medical Director AIG Life and Retirement Company
 Cardiac Arrhythmias Cathy Percival, RN, FALU, FLMI VP, Medical Director AIG Life and Retirement Company The Cardiovascular System Three primary functions Transport of oxygen, nutrients, and hormones to
Cardiac Arrhythmias Cathy Percival, RN, FALU, FLMI VP, Medical Director AIG Life and Retirement Company The Cardiovascular System Three primary functions Transport of oxygen, nutrients, and hormones to
Step by step approach to EKG rhythm interpretation:
 Sinus Rhythms Normal sinus arrhythmia Small, slow variation of the R-R interval i.e. variation of the normal sinus heart rate with respiration, etc. Sinus Tachycardia Defined as sinus rhythm with a rate
Sinus Rhythms Normal sinus arrhythmia Small, slow variation of the R-R interval i.e. variation of the normal sinus heart rate with respiration, etc. Sinus Tachycardia Defined as sinus rhythm with a rate
Cardiovascular system
 BIO 301 Human Physiology Cardiovascular system The Cardiovascular System: consists of the heart plus all the blood vessels transports blood to all parts of the body in two 'circulations': pulmonary (lungs)
BIO 301 Human Physiology Cardiovascular system The Cardiovascular System: consists of the heart plus all the blood vessels transports blood to all parts of the body in two 'circulations': pulmonary (lungs)
Chapter 13 The Cardiovascular System: Cardiac Function
 Chapter 13 The Cardiovascular System: Cardiac Function Overview of the Cardiovascular System The Path of Blood Flow through the Heart and Vasculature Anatomy of the Heart Electrical Activity of the Heart
Chapter 13 The Cardiovascular System: Cardiac Function Overview of the Cardiovascular System The Path of Blood Flow through the Heart and Vasculature Anatomy of the Heart Electrical Activity of the Heart
ECG. Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13
 ECG Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13 The Concept When the cardiac impulse passes through the heart, electrical current
ECG Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13 The Concept When the cardiac impulse passes through the heart, electrical current
4. The two inferior chambers of the heart are known as the atria. the superior and inferior vena cava, which empty into the left atrium.
 Answer each statement true or false. If the statement is false, change the underlined word to make it true. 1. The heart is located approximately between the second and fifth ribs and posterior to the
Answer each statement true or false. If the statement is false, change the underlined word to make it true. 1. The heart is located approximately between the second and fifth ribs and posterior to the
V. TACHYCARDIAS Rapid rhythm abnormalities
 V. TACHYCARDIAS Rapid rhythm abnormalities Tachyarrhythmias currently account for up to 350,000 deaths annually in the US. In addition to these clearly dangerous rhythm disturbances, other forms of more
V. TACHYCARDIAS Rapid rhythm abnormalities Tachyarrhythmias currently account for up to 350,000 deaths annually in the US. In addition to these clearly dangerous rhythm disturbances, other forms of more
Shock-induced termination of cardiac arrhythmias
 Shock-induced termination of cardiac arrhythmias Group members: Baltazar Chavez-Diaz, Chen Jiang, Sarah Schwenck, Weide Wang, and Jinglei Zhang Cardiac arrhythmias, also known as irregular heartbeat, occur
Shock-induced termination of cardiac arrhythmias Group members: Baltazar Chavez-Diaz, Chen Jiang, Sarah Schwenck, Weide Wang, and Jinglei Zhang Cardiac arrhythmias, also known as irregular heartbeat, occur
Basic Electrophysiology Protocols
 Indian Journal of Cardiology ISSN-0972-1622 2012 by the Indian Society of Cardiology Vol. 15, (3-4), 27-37 [ 27 Review Article Shomu Bohora Assistant Professor, Deptt. of Cardiology, U.N. Mehta Institute
Indian Journal of Cardiology ISSN-0972-1622 2012 by the Indian Society of Cardiology Vol. 15, (3-4), 27-37 [ 27 Review Article Shomu Bohora Assistant Professor, Deptt. of Cardiology, U.N. Mehta Institute
Lab #3: Electrocardiogram (ECG / EKG)
 Lab #3: Electrocardiogram (ECG / EKG) An introduction to the recording and analysis of cardiac activity Introduction The beating of the heart is triggered by an electrical signal from the pacemaker. The
Lab #3: Electrocardiogram (ECG / EKG) An introduction to the recording and analysis of cardiac activity Introduction The beating of the heart is triggered by an electrical signal from the pacemaker. The
Conventional Mapping. Introduction
 Conventional Mapping Haitham Badran Ain Shams University it Introduction The mapping approach used to guide ablation depends on the type of arrhythmia being assessed. Simple fluoroscopic anatomy is essential
Conventional Mapping Haitham Badran Ain Shams University it Introduction The mapping approach used to guide ablation depends on the type of arrhythmia being assessed. Simple fluoroscopic anatomy is essential
Cardiovascular System
 Cardiovascular System The Heart Cardiovascular System The Heart Overview What does the heart do? By timed muscular contractions creates pressure gradients blood moves then from high pressure to low pressure
Cardiovascular System The Heart Cardiovascular System The Heart Overview What does the heart do? By timed muscular contractions creates pressure gradients blood moves then from high pressure to low pressure
Cardiac Cycle. Each heartbeat is called a cardiac cycle. First the two atria contract at the same time.
 The Heartbeat Cardiac Cycle Each heartbeat is called a cardiac cycle. First the two atria contract at the same time. Next the two ventricles contract at the same time. Then all the chambers relax. http://www.youtube.com/watch?v=frd3k6lkhws
The Heartbeat Cardiac Cycle Each heartbeat is called a cardiac cycle. First the two atria contract at the same time. Next the two ventricles contract at the same time. Then all the chambers relax. http://www.youtube.com/watch?v=frd3k6lkhws
Emergency Medical Training Services Emergency Medical Technician Paramedic Program Outlines Outline Topic: WPW Revised: 11/2013
 Emergency Medical Training Services Emergency Medical Technician Paramedic Program Outlines Outline Topic: WPW Revised: 11/2013 Wolff-Parkinson-White syndrome (WPW) is a syndrome of pre-excitation of the
Emergency Medical Training Services Emergency Medical Technician Paramedic Program Outlines Outline Topic: WPW Revised: 11/2013 Wolff-Parkinson-White syndrome (WPW) is a syndrome of pre-excitation of the
Lab Activity 24 EKG. Portland Community College BI 232
 Lab Activity 24 EKG Reference: Dubin, Dale. Rapid Interpretation of EKG s. 6 th edition. Tampa: Cover Publishing Company, 2000. Portland Community College BI 232 Graph Paper 1 second equals 25 little boxes
Lab Activity 24 EKG Reference: Dubin, Dale. Rapid Interpretation of EKG s. 6 th edition. Tampa: Cover Publishing Company, 2000. Portland Community College BI 232 Graph Paper 1 second equals 25 little boxes
EHRA Accreditation Exam - Sample MCQs Invasive cardiac electrophysiology
 EHRA Accreditation Exam - Sample MCQs Invasive cardiac electrophysiology Dear EHRA Member, Dear Colleague, As you know, the EHRA Accreditation Process is becoming increasingly recognised as an important
EHRA Accreditation Exam - Sample MCQs Invasive cardiac electrophysiology Dear EHRA Member, Dear Colleague, As you know, the EHRA Accreditation Process is becoming increasingly recognised as an important
Shock-induced termination of cardiac arrhythmias
 Shock-induced termination of cardiac arrhythmias Group members: Baltazar Chavez-Diaz, Chen Jiang, Sarah Schwenck, Weide Wang, and Jinglei Zhang Abstract: Cardiac arrhythmias occur when blood flow to the
Shock-induced termination of cardiac arrhythmias Group members: Baltazar Chavez-Diaz, Chen Jiang, Sarah Schwenck, Weide Wang, and Jinglei Zhang Abstract: Cardiac arrhythmias occur when blood flow to the
MR Advance Techniques. Cardiac Imaging. Class IV
 MR Advance Techniques Cardiac Imaging Class IV Heart The heart is a muscular organ responsible for pumping blood through the blood vessels by repeated, rhythmic contractions. Layers of the heart Endocardium
MR Advance Techniques Cardiac Imaging Class IV Heart The heart is a muscular organ responsible for pumping blood through the blood vessels by repeated, rhythmic contractions. Layers of the heart Endocardium
UNDERSTANDING YOUR ECG: A REVIEW
 UNDERSTANDING YOUR ECG: A REVIEW Health professionals use the electrocardiograph (ECG) rhythm strip to systematically analyse the cardiac rhythm. Before the systematic process of ECG analysis is described
UNDERSTANDING YOUR ECG: A REVIEW Health professionals use the electrocardiograph (ECG) rhythm strip to systematically analyse the cardiac rhythm. Before the systematic process of ECG analysis is described
Principles and Applications of Electrical Circuits and Signal Theories in EP
 Principles and Applications of Electrical Circuits and Signal Theories in EP Graydon Beatty, VP Therapy Development Atrial Fibrillation Division, St. Jude Medical, Inc. CardioRhythm 2009 Background Biophysics
Principles and Applications of Electrical Circuits and Signal Theories in EP Graydon Beatty, VP Therapy Development Atrial Fibrillation Division, St. Jude Medical, Inc. CardioRhythm 2009 Background Biophysics
EKG Abnormalities. Adapted from:
 EKG Abnormalities Adapted from: http://www.bem.fi/book/19/19.htm Some key terms: Arrhythmia-an abnormal rhythm or sequence of events in the EKG Flutter-rapid depolarizations (and therefore contractions)
EKG Abnormalities Adapted from: http://www.bem.fi/book/19/19.htm Some key terms: Arrhythmia-an abnormal rhythm or sequence of events in the EKG Flutter-rapid depolarizations (and therefore contractions)
The Electrocardiogram
 The Electrocardiogram Chapters 11 and 13 AUTUMN WEDAN AND NATASHA MCDOUGAL The Normal Electrocardiogram P-wave Generated when the atria depolarizes QRS-Complex Ventricles depolarizing before a contraction
The Electrocardiogram Chapters 11 and 13 AUTUMN WEDAN AND NATASHA MCDOUGAL The Normal Electrocardiogram P-wave Generated when the atria depolarizes QRS-Complex Ventricles depolarizing before a contraction
This presentation will deal with the basics of ECG description as well as the physiological basics of
 Snímka 1 Electrocardiography basics This presentation will deal with the basics of ECG description as well as the physiological basics of Snímka 2 Lecture overview 1. Cardiac conduction system functional
Snímka 1 Electrocardiography basics This presentation will deal with the basics of ECG description as well as the physiological basics of Snímka 2 Lecture overview 1. Cardiac conduction system functional
NEIL CISPER TECHNICAL FIELD ENGINEER ICD/CRTD BASICS
 NEIL CISPER TECHNICAL FIELD ENGINEER ICD/CRTD BASICS OBJECTIVES Discuss history of ICDs Review the indications for ICD and CRT therapy Describe basic lead and device technology Discuss different therapies
NEIL CISPER TECHNICAL FIELD ENGINEER ICD/CRTD BASICS OBJECTIVES Discuss history of ICDs Review the indications for ICD and CRT therapy Describe basic lead and device technology Discuss different therapies
37 1 The Circulatory System
 H T H E E A R T 37 1 The Circulatory System The circulatory system and respiratory system work together to supply cells with the nutrients and oxygen they need to stay alive. a) The respiratory system:
H T H E E A R T 37 1 The Circulatory System The circulatory system and respiratory system work together to supply cells with the nutrients and oxygen they need to stay alive. a) The respiratory system:
Introduction. Circulation
 Introduction Circulation 1- Systemic (general) circulation 2- Pulmonary circulation carries oxygenated blood to all parts of the body carries deoxygenated blood to the lungs From Lt. ventricle aorta From
Introduction Circulation 1- Systemic (general) circulation 2- Pulmonary circulation carries oxygenated blood to all parts of the body carries deoxygenated blood to the lungs From Lt. ventricle aorta From
Cardiovascular System Notes: Physiology of the Heart
 Cardiovascular System Notes: Physiology of the Heart Interesting Heart Fact Capillaries are so small it takes ten of them to equal the thickness of a human hair. Review What are the 3 parts of the cardiovascular
Cardiovascular System Notes: Physiology of the Heart Interesting Heart Fact Capillaries are so small it takes ten of them to equal the thickness of a human hair. Review What are the 3 parts of the cardiovascular
The cardiovascular system is composed of the heart and blood vessels that carry blood to and from the body s organs. There are 2 major circuits:
 1 The cardiovascular system is composed of the heart and blood vessels that carry blood to and from the body s organs. There are 2 major circuits: pulmonary and systemic. The pulmonary goes out to the
1 The cardiovascular system is composed of the heart and blood vessels that carry blood to and from the body s organs. There are 2 major circuits: pulmonary and systemic. The pulmonary goes out to the
Health Science 20 Circulatory System Notes
 Health Science 20 Circulatory System Notes Functions of the Circulatory System The circulatory system functions mainly as the body s transport system. It transports: o Oxygen o Nutrients o Cell waste o
Health Science 20 Circulatory System Notes Functions of the Circulatory System The circulatory system functions mainly as the body s transport system. It transports: o Oxygen o Nutrients o Cell waste o
The Heart. Happy Friday! #takeoutyournotes #testnotgradedyet
 The Heart Happy Friday! #takeoutyournotes #testnotgradedyet Introduction Cardiovascular system distributes blood Pump (heart) Distribution areas (capillaries) Heart has 4 compartments 2 receive blood (atria)
The Heart Happy Friday! #takeoutyournotes #testnotgradedyet Introduction Cardiovascular system distributes blood Pump (heart) Distribution areas (capillaries) Heart has 4 compartments 2 receive blood (atria)
Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition
 Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition Table of Contents Volume 1 Chapter 1: Cardiovascular Anatomy and Physiology Basic Cardiac
Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition Table of Contents Volume 1 Chapter 1: Cardiovascular Anatomy and Physiology Basic Cardiac
BIPN100 F15 Human Physiology I (Kristan) Problem set #5 p. 1
 BIPN100 F15 Human Physiology I (Kristan) Problem set #5 p. 1 1. Dantrolene has the same effect on smooth muscles as it has on skeletal muscle: it relaxes them by blocking the release of Ca ++ from the
BIPN100 F15 Human Physiology I (Kristan) Problem set #5 p. 1 1. Dantrolene has the same effect on smooth muscles as it has on skeletal muscle: it relaxes them by blocking the release of Ca ++ from the
Cardiac physiology. b. myocardium -- cardiac muscle and fibrous skeleton of heart
 I. Heart anatomy -- general gross. A. Size/orientation - base/apex B. Coverings D. Chambers 1. parietal pericardium 2. visceral pericardium 3. Layers of heart wall a. epicardium Cardiac physiology b. myocardium
I. Heart anatomy -- general gross. A. Size/orientation - base/apex B. Coverings D. Chambers 1. parietal pericardium 2. visceral pericardium 3. Layers of heart wall a. epicardium Cardiac physiology b. myocardium
Ablation of Post-Infarct VTs Unmasking VT Isthmuses Using Pace-Mapping
 Ablation of Post-Infarct VTs Unmasking VT Isthmuses Using Pace-Mapping Christian de Chillou, MD, PhD Department of Cardiology University Hospital Nancy, France 29/05/2015 Post-infarct mappable VT Isthmus
Ablation of Post-Infarct VTs Unmasking VT Isthmuses Using Pace-Mapping Christian de Chillou, MD, PhD Department of Cardiology University Hospital Nancy, France 29/05/2015 Post-infarct mappable VT Isthmus
Paramedic Rounds. Tachyarrhythmia's. Sean Sutton Dallas Wood
 Paramedic Rounds Tachyarrhythmia's Sean Sutton Dallas Wood Objectives At the end of this session, the paramedic will be able to: State the key components of the cardiac conduction pathway, along with the
Paramedic Rounds Tachyarrhythmia's Sean Sutton Dallas Wood Objectives At the end of this session, the paramedic will be able to: State the key components of the cardiac conduction pathway, along with the
Cardiovascular Physiology
 Cardiovascular Physiology The mammalian heart is a pump that pushes blood around the body and is made of four chambers: right and left atria and right and left ventricles. The two atria act as collecting
Cardiovascular Physiology The mammalian heart is a pump that pushes blood around the body and is made of four chambers: right and left atria and right and left ventricles. The two atria act as collecting
Cardiovascular System Notes: Heart Disease & Disorders
 Cardiovascular System Notes: Heart Disease & Disorders Interesting Heart Facts The Electrocardiograph (ECG) was invented in 1902 by Willem Einthoven Dutch Physiologist. This test is still used to evaluate
Cardiovascular System Notes: Heart Disease & Disorders Interesting Heart Facts The Electrocardiograph (ECG) was invented in 1902 by Willem Einthoven Dutch Physiologist. This test is still used to evaluate
Collin County Community College. ! BIOL Anatomy & Physiology! WEEK 5. The Heart
 Collin County Community College! BIOL. 2402 Anatomy & Physiology! WEEK 5 The Heart 1 (1578-1657) A groundbreaking work in the history of medicine, English physician William Harvey s Anatomical Essay on
Collin County Community College! BIOL. 2402 Anatomy & Physiology! WEEK 5 The Heart 1 (1578-1657) A groundbreaking work in the history of medicine, English physician William Harvey s Anatomical Essay on
Biology 212: Anatomy and Physiology II Lab #4: CARDIOVASCULAR PHYSIOLOGY AND THE ELECTROCARDIOGRAM
 Biology 212: Anatomy and Physiology II Lab #4: CARDIOVASCULAR PHYSIOLOGY AND THE ELECTROCARDIOGRAM References: Saladin, KS: Anatomy and Physiology, The Unity of Form and Function 7 th (2015). Be sure you
Biology 212: Anatomy and Physiology II Lab #4: CARDIOVASCULAR PHYSIOLOGY AND THE ELECTROCARDIOGRAM References: Saladin, KS: Anatomy and Physiology, The Unity of Form and Function 7 th (2015). Be sure you
Case Report Catheter ablation of ventricular tachycardia related to a septo-apical left ventricular aneurysm
 Int J Clin Exp Med 2015;8(10):19576-19580 www.ijcem.com /ISSN:1940-5901/IJCEM0014701 Case Report Catheter ablation of ventricular tachycardia related to a septo-apical left ventricular aneurysm Radu Rosu
Int J Clin Exp Med 2015;8(10):19576-19580 www.ijcem.com /ISSN:1940-5901/IJCEM0014701 Case Report Catheter ablation of ventricular tachycardia related to a septo-apical left ventricular aneurysm Radu Rosu
DR QAZI IMTIAZ RASOOL OBJECTIVES
 PRACTICAL ELECTROCARDIOGRAPHY DR QAZI IMTIAZ RASOOL OBJECTIVES Recording of electrical events in heart Established electrode pattern results in specific tracing pattern Health of heart i. e. Anatomical
PRACTICAL ELECTROCARDIOGRAPHY DR QAZI IMTIAZ RASOOL OBJECTIVES Recording of electrical events in heart Established electrode pattern results in specific tracing pattern Health of heart i. e. Anatomical
WHAT S THAT RHYTHM I AM HEARING? GUIDE TO AUSCULTATION OF ARRHYTHMIAS IN HORSES
 WHAT S THAT RHYTHM I AM HEARING? GUIDE TO AUSCULTATION OF ARRHYTHMIAS IN HORSES Michelle Henry Barton DVM, PhD, DACVIM University of Georgia, Athens, GA INTRODUCTION The purpose of this talk is to review
WHAT S THAT RHYTHM I AM HEARING? GUIDE TO AUSCULTATION OF ARRHYTHMIAS IN HORSES Michelle Henry Barton DVM, PhD, DACVIM University of Georgia, Athens, GA INTRODUCTION The purpose of this talk is to review
Chapter 20: Cardiovascular System: The Heart
 Chapter 20: Cardiovascular System: The Heart I. Functions of the Heart A. List and describe the four functions of the heart: 1. 2. 3. 4. II. Size, Shape, and Location of the Heart A. Size and Shape 1.
Chapter 20: Cardiovascular System: The Heart I. Functions of the Heart A. List and describe the four functions of the heart: 1. 2. 3. 4. II. Size, Shape, and Location of the Heart A. Size and Shape 1.
A request for a log book extension must be put in writing and sent to BHRS, Unit 6B, Essex House, Cromwell Business Park, Chipping Norton,
 7 7. A request for a log book extension must be put in writing and sent to BHRS, Unit 6B, Essex House, Cromwell Business Park, Chipping Norton, Oxfordshire OX7 5SR. E-mail: admin@bhrs.com. Tel: 01789 867
7 7. A request for a log book extension must be put in writing and sent to BHRS, Unit 6B, Essex House, Cromwell Business Park, Chipping Norton, Oxfordshire OX7 5SR. E-mail: admin@bhrs.com. Tel: 01789 867
Sample. Analyzing the Heart with EKG. Computer
 Analyzing the Heart with EKG Computer An electrocardiogram (ECG or EKG) is a graphical recording of the electrical events occurring within the heart. In a healthy heart there is a natural pacemaker in
Analyzing the Heart with EKG Computer An electrocardiogram (ECG or EKG) is a graphical recording of the electrical events occurring within the heart. In a healthy heart there is a natural pacemaker in
UNDERSTANDING ELECTROPHYSIOLOGY STUDIES
 UNDERSTANDING ELECTROPHYSIOLOGY STUDIES Testing and Treating Your Heart s Electrical System A Problem with Your Heart Rhythm The speed and pattern of a heartbeat is called the heart rhythm. The rhythm
UNDERSTANDING ELECTROPHYSIOLOGY STUDIES Testing and Treating Your Heart s Electrical System A Problem with Your Heart Rhythm The speed and pattern of a heartbeat is called the heart rhythm. The rhythm
Electrocardiogram ECG. Hilal Al Saffar FRCP FACC College of medicine,baghdad University
 Electrocardiogram ECG Hilal Al Saffar FRCP FACC College of medicine,baghdad University Tuesday 29 October 2013 ECG introduction Wednesday 30 October 2013 Abnormal ECG ( ischemia, chamber hypertrophy, heart
Electrocardiogram ECG Hilal Al Saffar FRCP FACC College of medicine,baghdad University Tuesday 29 October 2013 ECG introduction Wednesday 30 October 2013 Abnormal ECG ( ischemia, chamber hypertrophy, heart
Cardiac Telemetry Self Study: Part One Cardiovascular Review 2017 THINGS TO REMEMBER
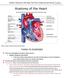 Please review the above anatomy of the heart. THINGS TO REMEMBER There are 3 electrolytes that affect cardiac function o Sodium, Potassium, and Calcium When any of these electrolytes are out of the normal
Please review the above anatomy of the heart. THINGS TO REMEMBER There are 3 electrolytes that affect cardiac function o Sodium, Potassium, and Calcium When any of these electrolytes are out of the normal
The Normal Electrocardiogram
 C H A P T E R 1 1 The Normal Electrocardiogram When the cardiac impulse passes through the heart, electrical current also spreads from the heart into the adjacent tissues surrounding the heart. A small
C H A P T E R 1 1 The Normal Electrocardiogram When the cardiac impulse passes through the heart, electrical current also spreads from the heart into the adjacent tissues surrounding the heart. A small
Biology 212: Anatomy and Physiology II. Lab #5: Physiology of the Cardiovascular System For Labs Associated With Dr. Thompson s Lectures
 Biology 212: Anatomy and Physiology II Lab #5: Physiology of the Cardiovascular System For Labs Associated With Dr. Thompson s Lectures References: Saladin, KS: Anatomy and Physiology, The Unity of Form
Biology 212: Anatomy and Physiology II Lab #5: Physiology of the Cardiovascular System For Labs Associated With Dr. Thompson s Lectures References: Saladin, KS: Anatomy and Physiology, The Unity of Form
ECG Interpretation Made Easy
 ECG Interpretation Made Easy Dr. A Tageldien Abdellah, MSc MD EBSC Lecturer of Cardiology- Hull University Hull York Medical School 2007-2008 ECG Interpretation Made Easy Synopsis Benefits Objectives Process
ECG Interpretation Made Easy Dr. A Tageldien Abdellah, MSc MD EBSC Lecturer of Cardiology- Hull University Hull York Medical School 2007-2008 ECG Interpretation Made Easy Synopsis Benefits Objectives Process
Systems Biology Across Scales: A Personal View XXVII. Waves in Biology: Cardiac Arrhythmia. Sitabhra Sinha IMSc Chennai
 Systems Biology Across Scales: A Personal View XXVII. Waves in Biology: Cardiac Arrhythmia Sitabhra Sinha IMSc Chennai The functional importance of biological waves Spiral Waves Cardiac Arrhythmias Arrhythmias:
Systems Biology Across Scales: A Personal View XXVII. Waves in Biology: Cardiac Arrhythmia Sitabhra Sinha IMSc Chennai The functional importance of biological waves Spiral Waves Cardiac Arrhythmias Arrhythmias:
Image-guided Minimally-invasive Cardiac Interventions
 Image-guided Minimally-invasive Cardiac Interventions Terry Peters PhD Biomedical Imaging Research Centre University of Western Ontario Imaging Research Labs - Robarts Research Institute Surgical Cardiac
Image-guided Minimally-invasive Cardiac Interventions Terry Peters PhD Biomedical Imaging Research Centre University of Western Ontario Imaging Research Labs - Robarts Research Institute Surgical Cardiac
Full file at
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What electrical event must occur for atrial kick to occur? 1) A) Atrial repolarization B) Ventricular
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What electrical event must occur for atrial kick to occur? 1) A) Atrial repolarization B) Ventricular
Lab 2. The Intrinsic Cardiac Conduction System. 1/23/2016 MDufilho 1
 Lab 2 he Intrinsic Cardiac Conduction System 1/23/2016 MDufilho 1 Figure 18.13 Intrinsic cardiac conduction system and action potential succession during one heartbeat. Superior vena cava ight atrium 1
Lab 2 he Intrinsic Cardiac Conduction System 1/23/2016 MDufilho 1 Figure 18.13 Intrinsic cardiac conduction system and action potential succession during one heartbeat. Superior vena cava ight atrium 1
Pearson's Comprehensive Medical Assisting Administrative and Clinical Competencies
 Pearson's Comprehensive Medical Assisting Administrative and Clinical Competencies THIRD EDITION CHAPTER 27 The Cardiovascular System Lesson 1: Overview of the Cardiovascular System Lesson Objectives Upon
Pearson's Comprehensive Medical Assisting Administrative and Clinical Competencies THIRD EDITION CHAPTER 27 The Cardiovascular System Lesson 1: Overview of the Cardiovascular System Lesson Objectives Upon
THE CARDIOVASCULAR SYSTEM. Heart 2
 THE CARDIOVASCULAR SYSTEM Heart 2 PROPERTIES OF CARDIAC MUSCLE Cardiac muscle Striated Short Wide Branched Interconnected Skeletal muscle Striated Long Narrow Cylindrical PROPERTIES OF CARDIAC MUSCLE Intercalated
THE CARDIOVASCULAR SYSTEM Heart 2 PROPERTIES OF CARDIAC MUSCLE Cardiac muscle Striated Short Wide Branched Interconnected Skeletal muscle Striated Long Narrow Cylindrical PROPERTIES OF CARDIAC MUSCLE Intercalated
Electrocardiography Abnormalities (Arrhythmias) 7. Faisal I. Mohammed, MD, PhD
 Electrocardiography Abnormalities (Arrhythmias) 7 Faisal I. Mohammed, MD, PhD 1 Causes of Cardiac Arrythmias Abnormal rhythmicity of the pacemaker Shift of pacemaker from sinus node Blocks at different
Electrocardiography Abnormalities (Arrhythmias) 7 Faisal I. Mohammed, MD, PhD 1 Causes of Cardiac Arrythmias Abnormal rhythmicity of the pacemaker Shift of pacemaker from sinus node Blocks at different
ECG ABNORMALITIES D R. T AM A R A AL Q U D AH
 ECG ABNORMALITIES D R. T AM A R A AL Q U D AH When we interpret an ECG we compare it instantaneously with the normal ECG and normal variants stored in our memory; these memories are stored visually in
ECG ABNORMALITIES D R. T AM A R A AL Q U D AH When we interpret an ECG we compare it instantaneously with the normal ECG and normal variants stored in our memory; these memories are stored visually in
Catheter Ablation of a Complex Atrial Tachycardia after Surgical Repair of Tetralogy of Fallot Guided by Combined Noncontact and Contact Mapping
 J Arrhythmia Vol 26 No 1 2010 Case Report Catheter Ablation of a Complex Atrial Tachycardia after Surgical Repair of Tetralogy of Fallot Guided by Combined Noncontact and Contact Mapping Eitaro Fujii MD,
J Arrhythmia Vol 26 No 1 2010 Case Report Catheter Ablation of a Complex Atrial Tachycardia after Surgical Repair of Tetralogy of Fallot Guided by Combined Noncontact and Contact Mapping Eitaro Fujii MD,
11/18/13 ECG SIGNAL ACQUISITION HARDWARE DESIGN. Origin of Bioelectric Signals
 ECG SIGNAL ACQUISITION HARDWARE DESIGN Origin of Bioelectric Signals 1 Cell membrane, channel proteins Electrical and chemical gradients at the semi-permeable cell membrane As a result, we get a membrane
ECG SIGNAL ACQUISITION HARDWARE DESIGN Origin of Bioelectric Signals 1 Cell membrane, channel proteins Electrical and chemical gradients at the semi-permeable cell membrane As a result, we get a membrane
Paroxysmal Supraventricular Tachycardia PSVT.
 Atrial Tachycardia; is the name for an arrhythmia caused by a disorder of the impulse generation in the atrium or the AV node. An area in the atrium sends out rapid signals, which are faster than those
Atrial Tachycardia; is the name for an arrhythmia caused by a disorder of the impulse generation in the atrium or the AV node. An area in the atrium sends out rapid signals, which are faster than those
A. Incorrect! The left ventricle receives oxygenated blood from the lungs via the left atrium.
 Anatomy and Physiology - Problem Drill 16: The Cardiovascular System No. 1 of 10 Instruction: (1) Read the problem statement and answer choices carefully (2) Work the problems on paper as needed (3) Pick
Anatomy and Physiology - Problem Drill 16: The Cardiovascular System No. 1 of 10 Instruction: (1) Read the problem statement and answer choices carefully (2) Work the problems on paper as needed (3) Pick
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists
 PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
Dr David Begley Papworth Hospital, Cambridge HRUK Certificate of Accreditation Course: Core Heart Rhythm Congress 2011
 Dr David Begley Papworth Hospital, Cambridge HRUK Certificate of Accreditation Course: Core Heart Rhythm Congress 2011 The AV node is the soul of the heart, and whoever understands its anatomy and electrophysiology
Dr David Begley Papworth Hospital, Cambridge HRUK Certificate of Accreditation Course: Core Heart Rhythm Congress 2011 The AV node is the soul of the heart, and whoever understands its anatomy and electrophysiology
Πρώτης γραμμή θεραπεία η κατάλυση κοιλιακής ταχυκαρδίας στην ισχαιμική μυοκαρδιοπάθεια
 Πρώτης γραμμή θεραπεία η κατάλυση κοιλιακής ταχυκαρδίας στην ισχαιμική μυοκαρδιοπάθεια Δ. Τσιαχρής Διευθυντής Εργαστηρίου Ηλεκτροφυσιολογίας - Βηματοδότησης, Ιατρικό Κέντρο Αθηνών, Αθήνα Ventricular tachycardia
Πρώτης γραμμή θεραπεία η κατάλυση κοιλιακής ταχυκαρδίας στην ισχαιμική μυοκαρδιοπάθεια Δ. Τσιαχρής Διευθυντής Εργαστηρίου Ηλεκτροφυσιολογίας - Βηματοδότησης, Ιατρικό Κέντρο Αθηνών, Αθήνα Ventricular tachycardia
Different indications for pacemaker implantation are the following:
 Patient Resources: ICD/Pacemaker Overview ICD/Pacemaker Overview What is a pacemaker? A pacemaker is a device that uses low energy electrical pulses to prompt the heart to beat whenever a pause in the
Patient Resources: ICD/Pacemaker Overview ICD/Pacemaker Overview What is a pacemaker? A pacemaker is a device that uses low energy electrical pulses to prompt the heart to beat whenever a pause in the
Collin County Community College
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 5 The Heart 1 The Heart Beat and the EKG 2 1 The Heart Beat and the EKG P-wave = Atrial depolarization QRS-wave = Ventricular depolarization
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 5 The Heart 1 The Heart Beat and the EKG 2 1 The Heart Beat and the EKG P-wave = Atrial depolarization QRS-wave = Ventricular depolarization
Ventricular Parasystole
 Ventricular Parasystole 1 WHAT IS IT? In addition to the sinus node, there are many accessory pacemakers throughout the conducting system of the atria, junction and ventricles that are ready to assume
Ventricular Parasystole 1 WHAT IS IT? In addition to the sinus node, there are many accessory pacemakers throughout the conducting system of the atria, junction and ventricles that are ready to assume
A Dynamic model of Pulmonary Vein Electrophysiology. Harry Green 2 nd year Ph.D. student University of Exeter
 A Dynamic model of Pulmonary Vein Electrophysiology Harry Green 2 nd year Ph.D. student University of Exeter Background to the Project Cardiac disease is the leading cause of death in most developed countries
A Dynamic model of Pulmonary Vein Electrophysiology Harry Green 2 nd year Ph.D. student University of Exeter Background to the Project Cardiac disease is the leading cause of death in most developed countries
Kadlec Regional Medical Center Cardiac Electrophysiology
 Definition of electrophysiology study and ablation Kadlec Regional Medical Center Cardiac Electrophysiology Electrophysiology Study and Ablation An electrophysiology, or EP, study is a test of the heart
Definition of electrophysiology study and ablation Kadlec Regional Medical Center Cardiac Electrophysiology Electrophysiology Study and Ablation An electrophysiology, or EP, study is a test of the heart
Lab 7. Physiology of Electrocardiography
 7.1 Lab 7. Physiology of Electrocardiography The heart is a muscular pump that circulates blood throughout the body. To efficiently pump the blood, cardiac contractions must be coordinated and are regulated
7.1 Lab 7. Physiology of Electrocardiography The heart is a muscular pump that circulates blood throughout the body. To efficiently pump the blood, cardiac contractions must be coordinated and are regulated
BME 5742 Bio-Systems Modeling and Control. Lecture 41 Heart & Blood Circulation Heart Function Basics
 BME 5742 Bio-Systems Modeling and Control Lecture 41 Heart & Blood Circulation Heart Function Basics Dr. Zvi Roth (FAU) 1 Pumps A pump is a device that accepts fluid at a low pressure P 1 and outputs the
BME 5742 Bio-Systems Modeling and Control Lecture 41 Heart & Blood Circulation Heart Function Basics Dr. Zvi Roth (FAU) 1 Pumps A pump is a device that accepts fluid at a low pressure P 1 and outputs the
CORONARY ARTERIES. LAD Anterior wall of the left vent Lateral wall of left vent Anterior 2/3 of interventricluar septum R & L bundle branches
 CORONARY ARTERIES RCA Right atrium Right ventricle SA node 55% AV node 90% Posterior wall of left ventricle in 90% Posterior third of interventricular septum 90% LAD Anterior wall of the left vent Lateral
CORONARY ARTERIES RCA Right atrium Right ventricle SA node 55% AV node 90% Posterior wall of left ventricle in 90% Posterior third of interventricular septum 90% LAD Anterior wall of the left vent Lateral
ICD: Basics, Programming and Trouble-shooting
 ICD: Basics, Programming and Trouble-shooting Amir AbdelWahab, MD Electrophysiology and Pacing Service Cardiology Department Cairo University Feb 2013 Evolution of ICD Technology ICD Evolution Indications
ICD: Basics, Programming and Trouble-shooting Amir AbdelWahab, MD Electrophysiology and Pacing Service Cardiology Department Cairo University Feb 2013 Evolution of ICD Technology ICD Evolution Indications
QUIZ/TEST REVIEW NOTES SECTION 1 CARDIAC MYOCYTE PHYSIOLOGY [CARDIOLOGY]
![QUIZ/TEST REVIEW NOTES SECTION 1 CARDIAC MYOCYTE PHYSIOLOGY [CARDIOLOGY] QUIZ/TEST REVIEW NOTES SECTION 1 CARDIAC MYOCYTE PHYSIOLOGY [CARDIOLOGY]](/thumbs/96/126998162.jpg) QUIZ/TEST REVIEW NOTES SECTION 1 CARDIAC MYOCYTE PHYSIOLOGY [CARDIOLOGY] Learning Objectives: Describe the ionic basis of action potentials in cardiac contractile and autorhythmic cells Explain the relationship
QUIZ/TEST REVIEW NOTES SECTION 1 CARDIAC MYOCYTE PHYSIOLOGY [CARDIOLOGY] Learning Objectives: Describe the ionic basis of action potentials in cardiac contractile and autorhythmic cells Explain the relationship
Objectives of the Heart
 Objectives of the Heart Electrical activity of the heart Action potential EKG Cardiac cycle Heart sounds Heart Rate The heart s beat separated into 2 phases Relaxed phase diastole (filling of the chambers)
Objectives of the Heart Electrical activity of the heart Action potential EKG Cardiac cycle Heart sounds Heart Rate The heart s beat separated into 2 phases Relaxed phase diastole (filling of the chambers)
Electrical Conduction
 Sinoatrial (SA) node Electrical Conduction Sets the pace of the heartbeat at 70 bpm AV node (50 bpm) and Purkinje fibers (25 40 bpm) can act as pacemakers under some conditions Internodal pathway from
Sinoatrial (SA) node Electrical Conduction Sets the pace of the heartbeat at 70 bpm AV node (50 bpm) and Purkinje fibers (25 40 bpm) can act as pacemakers under some conditions Internodal pathway from
Comparison of Different ECG Signals on MATLAB
 International Journal of Electronics and Computer Science Engineering 733 Available Online at www.ijecse.org ISSN- 2277-1956 Comparison of Different Signals on MATLAB Rajan Chaudhary 1, Anand Prakash 2,
International Journal of Electronics and Computer Science Engineering 733 Available Online at www.ijecse.org ISSN- 2277-1956 Comparison of Different Signals on MATLAB Rajan Chaudhary 1, Anand Prakash 2,
TEST BANK FOR ECGS MADE EASY 5TH EDITION BY AEHLERT
 Link download full: http://testbankair.com/download/test-bank-for-ecgs-made-easy-5thedition-by-aehlert/ TEST BANK FOR ECGS MADE EASY 5TH EDITION BY AEHLERT Chapter 5 TRUE/FALSE 1. The AV junction consists
Link download full: http://testbankair.com/download/test-bank-for-ecgs-made-easy-5thedition-by-aehlert/ TEST BANK FOR ECGS MADE EASY 5TH EDITION BY AEHLERT Chapter 5 TRUE/FALSE 1. The AV junction consists
Arrhythmias. 1. beat too slowly (sinus bradycardia). Like in heart block
 Arrhythmias It is a simple-dysfunction caused by abnormalities in impulse formation and conduction in the myocardium. The heart is designed in such a way that allows it to generate from the SA node electrical
Arrhythmias It is a simple-dysfunction caused by abnormalities in impulse formation and conduction in the myocardium. The heart is designed in such a way that allows it to generate from the SA node electrical
Use of Catheter Ablation in the Treatment of Ventricular Tachycardia Triggered by Premature Ventricular Contraction
 J Arrhythmia Vol 22 No 3 2006 Case Report Use of Catheter Ablation in the Treatment of Ventricular Tachycardia Triggered by Premature Ventricular Contraction sao Kato MD, Toru wa MD, Yasushi Suzuki MD,
J Arrhythmia Vol 22 No 3 2006 Case Report Use of Catheter Ablation in the Treatment of Ventricular Tachycardia Triggered by Premature Ventricular Contraction sao Kato MD, Toru wa MD, Yasushi Suzuki MD,
SAMPLE CHAPTER CHAPTER
 2.1 Introduction CHAPTER 2 Cardiovascular Structure and Function One may question why it is important for a biomedical engineer to study physiology. To answer this question, we can begin by recognizing
2.1 Introduction CHAPTER 2 Cardiovascular Structure and Function One may question why it is important for a biomedical engineer to study physiology. To answer this question, we can begin by recognizing
ECG Interpretation Cat Williams, DVM DACVIM (Cardiology)
 ECG Interpretation Cat Williams, DVM DACVIM (Cardiology) Providing the best quality care and service for the patient, the client, and the referring veterinarian. GOAL: Reduce Anxiety about ECGs Back to
ECG Interpretation Cat Williams, DVM DACVIM (Cardiology) Providing the best quality care and service for the patient, the client, and the referring veterinarian. GOAL: Reduce Anxiety about ECGs Back to
Heart. Heart 2-Tunica media: middle layer (media ='middle') muscle fibers (smooth or cardiac).
 t. innermost lumenal General Circulatory system heart and blood vessels walls have 3 layers (inside to outside) 1-Tunica interna: aka tunica intima layer--lumenal layer epithelium--endothelium simple squamous
t. innermost lumenal General Circulatory system heart and blood vessels walls have 3 layers (inside to outside) 1-Tunica interna: aka tunica intima layer--lumenal layer epithelium--endothelium simple squamous
Case Report Substrate Based Ablation of Ventricular Tachycardia Through An Epicardial Approach
 www.ipej.org 364 Case Report Substrate Based Ablation of Ventricular Tachycardia Through An Epicardial Approach Aman Makhija DM 1, Ajit Thachil DM 1, C Sridevi, DNB 2, B Hygriv Rao, DM 2, S Jaishankar
www.ipej.org 364 Case Report Substrate Based Ablation of Ventricular Tachycardia Through An Epicardial Approach Aman Makhija DM 1, Ajit Thachil DM 1, C Sridevi, DNB 2, B Hygriv Rao, DM 2, S Jaishankar
Circulation: Arrhythmia and Electrophysiology CHALLENGE OF THE WEEK
 A 14-year-old girl with Wolff-Parkinson-White syndrome and recurrent paroxysmal palpitations due to atrioventricular reentry tachycardia had undergone two prior failed left lateral accessory pathway ablations
A 14-year-old girl with Wolff-Parkinson-White syndrome and recurrent paroxysmal palpitations due to atrioventricular reentry tachycardia had undergone two prior failed left lateral accessory pathway ablations
