Atrial Reentry around an Anatomic Barrier with a Partially Refractory Excitable Gap
|
|
|
- Harvey Newton
- 5 years ago
- Views:
Transcription
1 495 Atrial Reentry around an Anatomic Barrier with a Partially Refractory Excitable Gap A Canine Model of Atrial Flutter Lawrence H. Frame, Richard L. Page, and Brian F. Hoffman From the College of Physicians & Surgeons, Columbia University, New York, New York Downloaded from by on October 19, 2018 SUMMARY. We have characterized, in dogs, a model of inducible regular atrial tachycardia that resembles atrial flutter. The model involves creating a Y-shaped lesion comprised of an intercaval incision and a connected incision across the right atrium. It is suitable for serial studies of the effects of pacing or antiarrhythmic drugs in chronically instrumented animals studied in the awake state for at least several months. The postoperative cycle length of the induced tachycardia varies from 143 to 188 msec, depending on the size of the dog. The tachycardia cycle length was consistent for each dog, and the rhythm once induced was very stable until terminated by pacing. The mechanism of the tachycardia was reentry due to circus movement based on the ability to induce and terminate it by premature impulses or overdrive, the ability to reset the tachycardia by single premature stimuli, the pattern of entrainment during overdrive stimulation, and the ability to terminate the tachycardia by interrupting the conduction pathway. The window of reset determined by the range of coupling intervals of premature stimuli that were able to enter and reset the tachycardia ranged from 56 to 82 msec. There appears to be incomplete recovery of excitability by the end of the excitable gap as evidenced by the fact that even late premature impulses that enter the reentrant circuit conduct more slowly than the tachycardia impulse, and because stimulation of muscarinic receptors that shortens the duration of the action potential and refractoriness also reduces the cycle length of the tachycardia. Epicardial and endocardial activation mapping during tachycardia showed the reentrant pathway does not merely encircle the lesion, particularly over the left atrial epicardium near the intercaval lesion. Rather, the impulse appears to travel around the atrial tissue just above the tricuspid ring, including a portion that travels through the right side of the lower intraatrial septum. Thus, the model involves circus movement around an anatomic barrier through normal tissue that contains no depressed segments. During the circus movement, there is a relatively long excitable gap during which there is incomplete recovery of excitability. This model should be useful for studies of the mechanism of antiarrhythmic drug action and the responses to premature stimulation in this particular subclass of reentrant rhythms, and for comparison with the behavior and responses of other forms of reentry. (Circ Res 58: , 1986) PROGRESS toward developing satisfactory criteria for identifying the mechanisms for arrhythmias and toward explaining the mechanisms of initiation and termination by electrical stimulation and prevention or termination by drugs depends in part on studies of animal models in which the mechanisms for specific arrhythmias are known. For example, even if one restricts consideration to reentrant tachycardia caused by circus movement, the behavior of the arrhythmia and the effects of electrical stimulation and drugs depend on the characteristics of the reentrant circuit. One factor of importance is the nature of the barrier around which the impulse circulates. Another is the duration of the excitable gap. In the leading circle form of reentry (Allessie, 1977), the excitable gap must be quite short, and full recovery of excitability and responsiveness cannot occur. With circus movement around an inexcitable barrier, the duration of the excitable gap will be a function of path length, conduction velocity, and the duration of the effective refractory period. During the gap, normal excitability and responsiveness may or may not be attained. The nature of, and responses by, the reentrant rhythm also will be influenced by the presence or absence of discrete areas of slower conduction and persistent unidirectional block in the circus path, and by the conditions causing these properties. The present study was designed to develop and characterize a model of reentrant tachycardia due to circus movement around an inexcitable barrier. We intended that the reentrant impulse propagate in healthy, electrophysiologically uniform tissues that developed fast responses and action potentials of comparable durations, and that the path and cycle lengths would provide an excitable gap. We planned to compare findings for this model with results we have obtained through studies on a canine model
2 4% (Boyden and Hoffman, 1981) in which an inducible atrial tachycardia resembing flutter results from reentry in the absence of an atrial lesion (Boyden et al., 1983). We chose to modify a procedure described by Rosenblueth and Garcia-Ramos (1947). They crushed the tissues of the intercaval bridge in dogs to create a barrier around which the impulse could circulate. They also showed that when they made an additional crush lesion extending toward the right atrial appendage, the cycle length of induced tachycardia was increased. They and others (Kimura, 1954; Lanari et al., 1956; Takayasu et al., 1958; Hayden et al., 1967) obtained evidence that the path traversed by the impulse during induced tachycardia seemed to course around the intercaval lesion and include both right and left atrial myocardium. We succeeded in developing in chronically instrumented dogs a model of atrial flutter based on the two-lesion method of Rosenblueth and Garcia-Ramos, in which a consistent and persistent reentrant atrial arrhythmia could be induced and terminated at will. We discovered that the path for the circus movement is provided, in large part if not in toto, by the atrial muscle just above the bicuspid ring. During the induced reentrant excitation, a partially excitable gap is present. The arrhythmia satisfies all usual tests for reentrant excitation caused by circus movement around an inexcitable barrier, and the model should be of value in studies on the mode of action of antiarrhythmic drugs. We have also shown how electrocardiographic criteria for diagnosing reentry based on showing fusion during entrainment (Waldo et al., 1983, 1984) can be modified to apply to local bipolar electrograms. LA Circulation Research/Vol. 58, No. 4, April 1986 FIGURE 1. This diagram of the heart viewed from the right posterior oblique projection shows the location of the Y-shaped lesion used to create the model of inducible atrial tachycardia, and the position of electrodes used in various chronically instrumented animals. The intercaval lesion extended from the superior vena cava to the inferior vena cava, and another connected lesion extended across the right atrium toward the right atrial appendage. Each number indicates the position of a bipolar pair of electrodes. The electrode positions surrounded by solid circles are on the exposed epicardial surface. Positions enclosed by dashed circles are on the opposite epicardial surface toward the aorta. Sites 1, 4, and 6 were used in each of the six experiments. In addition, an electrode was placed either at site 2 or 3 and either site 6 or 7 in each experiment. Two experiments had electrodes in 10 of the 13 positions shown. One of these experiments used all but sites 5, 9, and 32, and the other used all but sites 5, 11, and 13. Methods Experiments were performed on mongrel dogs of either sex weighing kg. Six dogs were instrumented for chronic studies, and four were used only for acute experiments. Each dog was anesthetized with pentobarbital, 30 mg/kg, iv, intubated, and ventilated with a Harvard respirator. The chest was opened in the 4th right intercostal space, and the pericardium was incised to provide free access to the venae cavae and right atrium. The tissue on a line extending from superior to inferior venae cavae was clamped, incised, and sewn together in two stages. Then another incision, contiguous with the intercaval lesion, was made in the right atrium. This incision ran parallel to the atrioventricular (AV) groove and extended to the base of the right atrial appendage. Sufficiently wide margins of tissue on each side of the incision were held by the clamp to make certain that no atrial muscle bundles bridged the incision. The Y-shaped lesion produced in this manner is shown in Figure 1. To prepare the dogs for chronic experiments, we attached close bipolar stimulating and recording electrodes at selected sites on the epicardium. In four dogs, we attached several electrodes before making the intercaval lesion, so that the arrhythmia induced after this incision could be compared with that induced after the Y-shaped lesion had been completed. In two early chronically instrumented dogs, we attached 10 pairs of electrodes on the right and left atrium to include most sites that earlier studies suggested would be part of the pathway for circus movement (see Fig. 1). Subsequently, when we had formed a reasonable estimate of the location of the reentrant circuit, we employed only five bipolar electrodes at sites chosen from the locations 1-6 shown in Figure 1. One additional pair of electrodes was implanted subcutaneously, one cephalad, and one caudad to the right thoractomy incision and about 6 inches apart, to record a body surface electrocardiogram. Leads from the electrodes were attached to one or two Teflon buttons, each with 12 contacts, that protruded through the skin between the scapulae. A standard lead II electrocardiogram was recorded intraoperatively. In four dogs, a Tygon catheter was introduced through the azygos vein. The tip was placed at the junction between the superior vena cava and the right atrium. The other end of the catheter was brought out through the skin between the scapulae. During subsequent experiments, the transcutaneous buttons were attached to an external cable connected to stimulation and recording devices, and the catheter was attached to intravenous infusion tubing. Electrograms were recorded on an Electronics for Medicine model VR-12 recorder and an eight-channel Gould- Brush recorder. Data also were recorded on a Hewlett-
3 Frame et al. /Reentry with a Partially Excitable Gap 497 Downloaded from by on October 19, 2018 Packard tape recorder. Electrograms were filtered at 10 or 30 Hz (high pass) and 200 Hz (low pass). Stimuli were rectangular pulses isolated from ground and controlled by digital rimers and counters. To measure the moment of activation as indicated by close bipolar electrograms, we used, for predominantly biphasic complexes, the moment when the trace crossed the line of zero potential and for triphasic complexes the peak of the major deflection. The reproducibility of the measurements was ±1.0 msec. In two dogs, maps of the sequence of activation of the accessible epicardial surfaces of the right and left atrium were made with a close bipolar electrode probe during the arrhythmias induced after only the intercaval incision had been made, and, also, after completion of the Y-shaped lesion. A Tektronix 502 oscilloscope was triggered by an electrogram recorded through a fixed reference electrode pair. A sweep speed of 20 msec/cm was used to measure the relative activation time at each recording site. All measurements used to construct a map of activation sequence were obtained during a single episode of arrhythmia, and we required the activation time at each site to be constant for at least four beats. Two dogs were anesthetized and prepared in the usual manner and used only for acute experiments. In addition, two dogs were studied during total cardiopulmonary bypass to construct endocardial activation maps. Venous return was collected by catheters placed in the superior and inferior venae cavae about 5 cm above and below their entry into the right atrium, respectively. The blood was oxygenated and heated and returned to an arterial infusion line placed in the right carotid artery. The same incisions were made in the right atrium, but the atrium was left open for access to the endocardial surface. Efflux from the coronary sinus was collected with a suction catheter. Activation maps were recorded by sequentially measuring activation times at different points during sustained tachycardia with respect to a fixed reference electrode. A hand-held roving probe with a bipolar electrode as its tip was used for these maps. Two patterns of stimulation were used to initiate the atrial arrhythmias, both intraoperatively and postoperatively. In one, the atria were paced through a selected pair of electrodes at cycle lengths between 110 and 200 msec. The duration of the rapid pacing at any cycle length varied from 2 to 20 seconds. If pacing at a slower rate failed to induce the arrhythmia, the rate was increased progressively until the arrhythmia supervened. Since we were not prepared to record and study the spread of excitation during the induction of arrhythmia, systematic evaluation of each rate was not attempted. Alternatively, the atria were paced at a cycle length of 200 msec, and single premature stimuli were introduced progressively earlier during diastole in every 8-12th cycle until the arrhythmia was initiated. Induction was more likely by stimuli applied near the end of the effective refractory period than by stimuli applied later in the cycle. We did not systematically compare induction by stimuli applied through different electrode pairs, and usually selected the stimulus site on the basis of ease of induction of the arrhythmia. Results Experiments were performed on a total of 10 dogs. Six dogs were chronically instrumented for serial studies of the tachycardia in the awake state. Four dogs used for activation sequence mapping were studied only acutely while anesthetized. Effects of the Extent of the Lesion on the Cycle Length and Persistence of the Tachycardia In four experiments (Table 1), after only the intercaval lesion had been completed, we used rapid pacing to induce a regular rapid atrial arrhythmia. Two of these four were used only for acute studies and two were instrumented for later serial studies in the awake state. In each case we obtained a tachycardia with a cycle length of msec. However, the arrhythmia was always transient and stopped in each trial after 3-12 minutes. In all animals, after the Y-shaped lesion had been completed, rapid pacing once again initiated a rapid regular tachycardia. Now, however, the cycle length was 6-31 msec longer than before, and the arrhythmia was much more persistent. Characteristics of the Tachycardia in Chronically Instrumented Dogs Five of the six dogs that were instrumented and had the Y-shaped lesion produced under sterile conditions survived the postoperative period. The other dog died on the 2nd postoperative day because of thrombosis of the superior vena cava. The five surviving dogs remained healthy and were studied over periods of time that ranged from 27 to 193 days for different dogs (Table 2). Programmed stimulation successfully induced a rapid regular sustained tachycardia on 35 of 37 different days in these five dogs. The cycle length of the induced tachycardia was constant from beat to beat. When a standard lead-2 electrocardiogram was recorded, atrial activity produced a number of different waveforms. During counterclockwise circulation of the impulse (see below), we often recorded a continuously varying sawtoothed pattern with no isoelectric segments that is characteristic of atrial flutter (Fig. 2). During clockwise circulation, this pattern was less clear. In some dogs, the ECG showed a pattern of P-waves unlike classical human flutter, as may be the case for the human ECG (Waldo and MacLean, 1980). For a given dog, when the tachycardia was induced multiple times on the same day, or on consecutive days, the cycle length for different runs of tachycardia did not vary by more than 5 msec. The cycle length of the induced tachycardia recorded intraoperatively TABLE 1 Cycle Lengths (in msec) of Induced Tachycardia before and after Addition of the Transverse Right Atrial Lesion Dog Acute/ Intercaval lesion Intercaval plus no. chronic alone transverse lesion 1 Acute 2 Acute 3 Acute 4 Chronic no
4 498 Circulation Research/Vol. 58, No. 4, April 1986 TABLE 2 Characteristics of Tachycardia in Chronically Instrumented Dogs Cycle length of tachycardia (msec) Chronic dog no Dog wt (kg) Intra operative Postoperative day Window of reset (msec) No. of days Period of studied study (days) (days) Downloaded from by on October 19, 2018 after the Y-shaped lesion had been created ranged from 143 to 152 msec in the chronically instrumented dogs (Table 2). The postoperative cycle length of induced tachycardia was approximately 20 msec greater than the intraoperarive value. Thereafter, the value decreased slightly with time or remained unchanged. A correlation between the weight of the dog and the cycle length of induced tachycardia is shown in Figure 3. We did not measure the circumference of the tricuspid ring in these experiments, but include the data to indicate that, as expected, there is a correlation between the size of the heart and the cycle length of the tachycardia. This relationship would not be expected of automatic or triggered rhythms, but seems appropriate for circus movement around an inexcitable obstacle the dimensions of which vary with heart size. In our experiments (Wu and Hoffman, unpublished observations), we have found that apparent conduction velocity during the tachycardia also varies with the circumference of the tricuspid ring. Thus, for three dogs in which the tricuspid ring was small (10.2, 10.2, and 12 cm), apparent conduction velocity was 0.68, 0.66, and 0.63 m/sec, whereas, in three dogs with quite large rings (16.5, 17.2, and 18.5 cm), apparent conduction velocity during the tachycardia was 1.18, 1.1, and 1.23 m/sec. These latter findings also suggest, as would be expected, that in quite large dogs excitability probably recovers fully before each new impulse is generated. In dog number 1, the 2 days on which programmed stimulation failed to result in sustained tachycardia were the 14th and 15th postoperative days. On these days, programmed stimulation resulted only in nonsustained runs of tachycardia lasting seconds with cycle lengths ranging from msec. A stable sustained atrial tachycardia was easily induced both before and after these days in this dog. Tachycardia could be induced either by rapid pacing or by single premature stimuli. Rapid pacing alone at cycle lengths between 100 msec and the cycle length of the induced tachycardia induced tachycardia in every animal. Occasionally, pacing at cycle lengths longer than that of the induced tachycardia, up to 180 msec, was also effective in inducing tachycardia. An example of the induction of the tachycardia by 5 beats of rapid pacing is shown in Figure 4A. Single premature stimuli delivered during FIGURE 2. An example of the electrogram sequence and ECG configuration during a sustained atrial tachycardia resembling atrial putter is shown. The top four tracings are recorded from epicardial bipolar electrodes and the bottom tracing records a standard lead-2 ECG. Flutter waves are visible on the ECG tracing. Time lines are at intervals of 100 msec DOG WEIGHT,kg FIGURE 3. The relationship between dog weight and the cycle length of induced atrial tachycardia approximately 2 weeks after creation of the Y-shaped lesion is shown for the five surviving chronically instrumented dogs.
5 Frame et al. /Reentry with a Partially Excitable Gap SCEG Sites 200 «l«c 200 msec FIGURE 4. Panel A: induction of a rapid atrial tachycardia by 5 beats of burst pacing at a cycle length of 120 msec. The dots below the bottom electrogram indicate the timing of pacing stimuli. Panel B. termination of the same tachycardia by rapid pacing for 3 beats at a cycle length of 120 msec In each figure, the top trace (SCEG) is an electrogram recorded from two chronically implanted subcutaneous electrodes that shows ventricular activity The position of electrodes at sites 3, 4, 6, and 7 are indicated in Figure 1. atrial pacing at a cycle length of 200 msec were employed in three of the five chronically instrumented dogs and two of the acute experiments to induce the tachycardia. The technique was successful in each animal. We did not explore in detail the initiation of arrhythmia by premature impulses, but the following observations were consistent. Premature impulses timed to fall near the end of the effective refractory period were more likely to start flutter than impulses ocurring later in the cycle. Initiation of arrhythmia by premature impulses was successful more often if the paced cycle length was short rather than long. We were consistently able to induce the arrhythmia by premature impulses at cycle lengths of 300, 250, and 200 msec, and on some occasions at a cycle length of 400 msec. Longer cycle lengths were not attempted because variations in sinus rate frequently resulted in escape from pacing. The ease with which we could initiate an arrhythmia by premature impulses was not equal for all electrode sites: sites with the longest effective refractory period were least effective. We did not find a consistent relationship between the apparent proximity of the electrode site to the path for circus movement and the ease with which an arrhythmia might be initiated from that site. An example of 499 induction of arrhythmia by a premature impulse is shown in Figure 5. We did not examine in each dog the ease of induction of tachycardia by stimulation through each pair of electodes. When this was done, we found that either rapid pacing or premature impulses during pacing at a cycle length of 200 msec could induce the same tachycardia, regardless of the pacing site. Among dogs, there was considerable variability in susceptibility to tachycardia. For example, in some dogs, repeated trials of rapid pacing or premature stimulation were required, while, in others, the first burst of rapid pacing was successful in most instances. Bouts of tachycardia that lasted more than 30 seconds rarely terminated spontaneously, even when observed for several house. On one occasion, a dog was left in tachycardia overnight and the rhythm was still present the next morning. The tachycardia could be easily terminated by rapid pacing (see Fig. 4b) or properly timed extra stimuli. The Excitable Gap The degree of recovery of the reentrant pathway from refractoriness after each beat of the tachycardia was evaluated by noting the response to single premature stimuli delivered at various times during the tachycardia cycle and by observing changes in the cycle length of the tachycardia during vagal stimulation or infusion of methacholine. The duration of the period during which single premature stimuli advanced the next beat of tachycardia defined the window of reset. The duration of the window of reset was measured in four of the five chronically instrumented dogs, and ranged from 56 to 82 msec (see Table 2). Figure 6 shows the effect of single premature stimuli delivered at three different coupling intervals at site 7 (see Fig. 1) during a single episode of tachycardia. Note that the J J V'l * "i v " J f A, II I ( i r * 1 i FIGURE 5. Induction of arrhythmia by a single premature stimulus. Traces from the top down are bipolar electrvgrams from sites 1, 2, 3, 4, and 5, and an ECC recorded through two subcutaneous electrodes. Pacing and premature stimuli applied through an electrode pair located near junction of superior vena cava with right atrium. Pacing cycle length = 250 msec. Premature stimulus applied in every 10th cycle 90 msec after the pacing stimulus Time lines at intervals of 50 msec. Bottom trace shows pacing artifacts and then the artifact of the premature stimulus. Note that, after a period of somewhat disorganized activity, the regular clockwise circulation of the atrial impulse begins.
6 , ,1 65J65 Oq FIGURE 6. Resetting of the tachycardia by single premature stimuli delivered at three different coupling intervals through electrodes at site 7. The stimulus artifact is indicated by a solid arrow. The cycle length in msec at each site and for each cycle is indicated. Panel A shows a late premature stimulus that advances the electrogram at site 1 by only 10 msec (cycle length = 155 msec). Note that the interval between the premature impulse electrogram and the next is longer (170 msec) than the basic cycle length. Panels B and C show progressively earlier premature stimuli that shorten the cycle length at site I to 140 and 135 msec. In panel Q the electrogram following the premature stimulus is inverted (site 6) (open arrow) because this site is activated by an antidromic impulse moving in the opposite direction from the reentrant impulse. latest premature stimulus, shown in Figure 6A, advances the next electrogram at site 1 by only 10 msec. However, the next cycle length at that site is longer than the tachycardia cycle length. This suggests that the premature impulse propagated more slowly than the reentrant impulses of the tachycardia. Since the premature impulse elicited electrograms similar in shape and sequence to those of the tachycardia, propagation over a different path is a less likely cause of the increased cycle length. The slow propagation over the same path indicates that Circulation Research/Vol. 58, No. 4, April 1986 at the time of the premature impulse, the path had recovered less fully from prior activity than for impulses of the tachycardia. Since the premature impulse arrived at this site only 10 msec in advance of the expected reentrant impulse, it seems probable that recovery of excitability in the reentrant path was incomplete for impulses of the tachycardia. Impulses caused by earlier premature stimuli (Fig. 6, B and C) reset the tachycardia to a greater extent and also conducted even more slowly so that the next cycle length was increased progressively. This presumably occurred because the premature impulse entered the reentrant path earlier during the relative refractory period or period of reduced responsiveness. We noted, as expected, that the duration of the window of reset differed among stimulus sites in relation to the duration of the effective refractory period at each site and also, perhaps, the presumed distance of the test site from the path for circus movement. For example, in one dog we determined the duration of the window of reset during flutter at a cycle length of 156 msec. At sites 1, 2, 3, 4, and 6 (see Fig. 1), the earliest effective stimuli were those applied 75, 90, 77, 110, and 67 msec after prior activity at that site. We then terminated the flutter and measured the duration of the effective refractory period during pacing at a cycle length of 200 msec. For the five sites, the ERP measured 80, 110, 105, 125, and 115 msec. Measurements of this sort were not made routinely in all dogs, because the induction of premature impulses early in the window of reset typically terminated the arrhythmia. Also (see Table 2), the duration of the window was related to the cycle length of the arrhythmia. To evaluate the degree of refractoriness at the end of the excitable gap, we also used muscarinic activation either by vagal stimulation (in one acute experiment) or infusion of methacholine ( mg), and observed the effect on the tachycardia cycle length. We assumed that if the reentrant impulse was traveling in incompletely recovered tissue, a decrease in action potential duration should result in a decrease in cycle length (Allessie, 1977). Gradually increasing the intensity of vagal stimulation or increasing doses of methacholine resulted in progressive shortening of the cycle length up to a maximum of 8-15 msec. Activation Sequence Certain information about the activation sequence during the tachycardia was obtained from the relative activation times at the five or more bipolar electrodes sutured to the arrial epicardium at the sites shown in Figure 1. In each dog during an episode of tachycardia, we observed one or the other of two activation sequences. Both activation sequences were seen in each animal, and the cycle lengths of the tachycardias were the same for each sequence. In one sequence, activation at electrodes
7 Frame et al./reentry with a Partially Excitable Gap Comttirclocliolsi 200 > U i 501 sites 7, 9, and 10 suggested that this portion of the left atrial epicardium was not involved in the reentrant pathway. For instance, during the counterclockwise activation sequence in Figure 7, when activation proceeded from site 6 to 4 to 3, activation at site 7 occurs after site 6 already had been activated. To identify better the location of the reentrant pathway, we mapped epicardial activation sequences during the tachycardia in two anesthetized dogs with a roving probe electrode (see Fig. 8). In one dog, tachycardia was induced, and activation maps recorded intraoperatively, both after only the intercaval lesion and also after the full Y-shaped lesion. In this animal, extending the lesion increased the cycle length from 110 to msec. For each state, the cycle lengths of the clockwise and counterclockwise rhythms were the same. Figure 8A shows a counterclockwise tachycardia after only the intercaval lesion. The impulse spreads from the lower right atrium near the inferior vena cava both cephalad toward the sinus node and laterally around the tricuspid ring. Figure 8B shows the activation sequence of a clockwise tachycardia from the same dog but after the full Y-shaped lesion had been created. Note that, in both maps, activation times at widely separate points on the left atrium near the intercaval lesion are within an interval of 5 or 6 msec. These differences in activation time are too small to result Clocknisi FIGURE 7. Reversal of activation sequence by rapid pacing At the left side of the figure there is a counterclockrvise activation sequence dunng the tachycardia. Three premature stimuli at a cycle length of 125 msec are shown m the middle of the figure. The activation sequence is somewhat irregular for several beats and then converts to a clockwise activation sequence on the righthand side of the figure. at sites 1, 4, and 6 (which were present in all five chronically instrumented dogs) occurred in ascending numerical order (clockwise activation sequence), and in the other sequence activation occurred in descending (counterclockwise) order. In each animal, the interval between activation at each of these adjacent sites was similar for both sequences of activation. During sustained tachycardia, rapid pacing from the right atrium sometimes switched the activation sequence from one pattern to the other. An example of this is shown in Figure 7. The riming of activation at electrodes located at Downloaded from by on October 19, RA FIGURE 8. Maps of epicardial activation sequence during tachycardia. Panel A shows the activation times during a counterclockwise activation pattern following only the intercaval lesion Numbers not enclosed in the rectangles represent activation times on the exposed epicardial surface. Numbers surrounded by rectangles indicate epicardial activation times on the opposite epicardial surface of the atnum toward the aorta. Panel B shozvs a clockwise activation sequence during tachycardia m the same dog after a full Y-shaped incision had been created. This increased the cycle length of the tachycardia from 110 msec to msec. Also shown are the sites of the three ligatures identified by A, B, and C referred to m the text. Panel C shows a diagram of a transverse section through the right atnal free wall to illustrate the amount of tissue enclosed by each of three ligatures. RA = right atrial free wall; TV = tricuspid valve leaflet; VM = ventricular muscle; 1 = transverse incision. The coarsely stippled area is fat surrounding the right coronary artery in the AV nng.
8 502 from an impulse spreading along the epicardial surface parallel to the lesion. Furthermore, in Figure 8B, a portion of the right atrium near the aortic root was activated at the same time as the left atrial sites so the left atrial sites could not be part of the reentrant circuit. This map suggests that the reentrant impulse must travel through the intraatrial septum. That tissues in the intraatrial septum constituted part of the reentrant circuit was shown by mapping the endocardial activation sequence in two dogs during total cardiopulmonary bypass. The right atrium was opened through the standard Y-shaped incision, and the margins of the incision were retracted to expose the endocardium. A roving probe was used to time activation at each site relative to activation at a fixed reference electrode. Figure 9 shows activation times at multiple sites on the right atrial endocardium during tachycardias with each of the two activation sequences in one experiment. Each map shows activation times recorded during a single episode of tachycardia. Figure 9A shows an example of a clockwise activation sequence. Note that activation times in the atrial tissue around the tricuspid ring span the tachycardia cycle. It can also be seen that tissues along the posterior portion of the intraatrial septum between the inferior and superior vena cavae are activated by the spread of an impulse upward from the region of the coronary sinus through the limbus of the fossa ovalis. This impulse, after spreading superiorly and posteriorly toward the epicardial surface of the free wall, divides to spread upward toward the superior vena cava and downward toward the inferior vena cava. Figure 9B shows a counterclockwise activation sequence. Again activation times in the atrial tissue around the tricuspid ring appear to span the tachycardia cycle. Again in this activation sequence, the wavefront of activation speads upward and posteriorly in the intraatrial septum from the tricuspid B Circulation Research/Vol. 58, No. 4, April 1986 ring through the limbus of the fossa ovalis. It was difficult to complete activation maps during a single episode of tachycardia because even light contact with endocardium by the probe on right atrial tissue in the region of the ostium of coronary sinus frequently terminated the tachycardia. This did not result from induction of a premature impulse. This observation lends further support to the concept that the region forms a necessary portion of the reentrant path because it is the only path for impulse propagation in this part of the circus path. Both maps suggest that the reentrant impulse travels in atrial tissue just above the tricuspid ring, and that part of the pathway involves the base of the intraatrial septum. The maps suggest that the entire path of the leading edge of the reentrant impulse is around the tricuspid ring, but the evidence is not conclusive for the region at the base of the right atrial appendage. For instance, in Figure 9B, the impulse spreading around the lower edge of the right atrial free wall appears to divide and travel around both sides of the right atrial appendage. One impulse continues around the tricuspid ring at the base of the right atrial appendage, and the other impulse turns around the lateral edge of the right atrial incision and progresses superiorly toward the region of the sinoatrial node and then caudally down the aortic surface of the right atrium toward the root of the aorta. Since it is not clear which of these two impulses arrives at the region of the AV node first, one cannot be certain which of these two impulses represents the pathway of the leading edge of reentrant excitation. Termination of the Tachycardia by Interruption of the Pathway Confirmation that reentry due to circus movement is the mechanism of the tachycardia can be obtained by showing that interruption of the pathway termi- FIGURE 9. Endocardial activation times recorded in the right atrium during tachycardia on total cardiopulmonary bypass. Panel A shows a clockwise activation sequence. Panel B shows a counterclockwise activation sequence.
9 Frame et al. /Reentry with a Partially Excitable Gap 503 nates the tachycardia. Tissue was squeezed by successively tightening each of the three ligatures that encircled parts of the lower right atrium as shown in Figure 8, B and C. Ligature A encircled right atrial free-wall tissue from the transverse incision caudally to the superior edge of the coronary fat pad in the AV groove. Tightening this ligature very firmly several times did not affect the tachycardia. Ligature B extended from the caudal end of ligature A through the tricuspid valve leaflet and out through the right ventricular free wall. It therefore included the atrial tissue just above the tricuspid ring. Tissue encompassed by ligatures A and B were mutually exclusive. Gentle tightening of ligature B initially increased the tachycardia cycle length by msec and then terminated the tachycardia; this occurred in the absence of any premature impulses. After this light tension was released, a stable tachycardia could be reinduced. Subsequent tightening of ligature C that encompassed all the tissue encircled by either ligature A or B also terminated the tachycardia. The position of these ligatures was confirmed after the animal had been killed. Thus, interruption of the circular sequence of activation around the tricuspid ring interrupted the tachycardia. Entrainment and Termination of Tachycardia by Overdrive Pacing Figures 10 and 11 illustrate features of overdrive pacing during the tachycardia that suggest that reentry is the mechanism for the arrhythmia (Waldo et al., 1983, 1984), and, more importantly, show how the paced impulses interact with the impulses of the arrhythmia. Figure 10 demonstrates examples of entrainment with resumption of tachycardia, and Figure 11 shows that loss of entrainment during pacing results in termination of the tachycardia. Entrainment implies that the mechanism of the tachycardia is not abolished by overdrive pacing / but that the rate of impulses in the circus path is B Site iy- i Silt I Downloaded from by on October 19, 2018 (stimulus) ECG LEAD! (stimulus) ECG LEAD II \ Silt I Silt 5 Silt 4 I Slim,I,i) Pacing CL 110 BIK 200 msic FIGURE 10. Entrainment by overdrive pacing followed by resumption of tachycardia. The left side of panel A shows the end of a period of overdrive pacing at a cycle length of 140 msec Seven pacing stimuli are shown. The left side of panel C shows the end of a period of overdrive pacing at a cycle length of 7 70 msec (nine pacing stimuli are shown). In each case, this stimulus was delivered at site 4 C). Panels B and D diagram the activation patterns and sites of collision of the orthodromic and antidromic impulses (open arrows) for these two pacing cycle lengths, respectively. In panels A and B, sites 2, 7, 6, and 5 were all activated by the orthodromic impulse before it collided with the antidromic impulse. In panels C and D at the faster pacing cycle length, site 5 was activated by the antidromic impulse. Sites Z 1, and 6 were still activated by the orthodromic impulse, but the next stimulus had been delivered before this impulse reached site 6. As a result, two electrograms recorded after the last stimulus were entrained to the paced cycle length of 110 msec. At this rapid cycle length, a collision between the antidromic impulse from the most recent stimulus and the orthodromic impulse from the preceding stimulus must occur between sites 5 and 6 during overdrive pacing. The right sides of panels A and C demonstrate resumption of the tachycardia after overdrive pacing. The unblocked orthodromic impulse continued to propagate around the reentrant circuit.
10 504 7 tshm.l.$i FIGURE 11. Interruption of entrainment and termination of tachycardia by rapid overdrive pacing. Panels A and B show two episodes of overdrive pacing at a cycle length of 110 msec. Electrograms recorded from sites 1, 4, and 6 are shown. The stimulus was delivered at site 5 (indicated by *). In panel A, overdrive pacing resulted in entrainment of tachycardia with resumption of the tachycardia sequence when the pacing was stopped. In panel B, entrainment was interrupted after stimulus 3 7 because the orthodromic impulse was blocked near its site of origin. This is diagrammed in panel C As a result, A^ did not collide with On- Instead, it continued to propagate until it collided with its own orthodromic impulse Oi». From this point on, the tachycardia could not have resumed after cessation of pacing because the antidromic and orthodromic impulses from each stimulus collided with each other so that the last orthodromic impulse could not reinitiate reentrant excitation. accelerated to the pacing rate. During entrainment of a reentrant rhythm, the impulse elicited by the stimulus enters and travels in both directions around the reentrant pathway. For this discussion, the N th stimulus will be labeled S N - This impulse originating from S N that enters the reentrant circuit and travels in the same direction as the reentrant impulse during the tachycardia is called the orthodromic impulse (ON), and the impulse from S N that enters the circuit and travels in the opposite direction is called the antidromic impulse (A N ). In terms of this model, since these impulses travel in opposite directions Circulation Research/Vol. 58, No. 4, April 1986 around the reentrant circuit, each antidromic impulse must collide with and block an orthodromic impulse. However, during entrainment of a reentrant rhythm, the antidromic impulse of the first paced beat that enters the reentrant circuit (A,) collides with and blocks the preexisting reentrant impulse, but Oi reinitiates the sequence of reentrant excitation. Resumption of the tachycardia following overdrive pacing requires preservation of this pattern of entrainment in which, after each stimulus, the antidromic impulse (A N ) collides with a preexisting orthodromic impulse (O N -i) while O N reinitiates the sequence of reentrant excitation. If this pattern continues throughout the period of pacing, then the last orthodromic impulse will not be blocked, and will be able to continue to circulate around the reentrant pathway. On the other hand, if this sequence is interrupted anytime during pacing so that the antidromic impulse (A N ) collides with the orthodromic impulse from the same stimulus (O N ), then the tachycardia will not resume after pacing because the last orthodromic impulse will be blocked. Figure 10 demonstrates three features that are important for confirming that the mechanism of the tachycardia is reentrant excitation due to circus movement. The first is that the sequence of activation by the orthodromic impulse during pacing is the same as by the reentrant impulse during spontaneous tachycardia. The right side of Figure 10, A and C, shows that the sequence of activation during the tachycardia after pacing follows descending numerical order from sites 6 to 5 to 2 to 1 and then again to 6. The cycle length, sequence of activation, and electrogram configuration during tachycardia before overdrive pacing (not shown) were the same in each case. The sequence and configuration of electrograms at sites 2, 1,6, and 5 are similar during overdrive pacing. The biggest difference is that the time between activation at sites 5 and 2 is reduced during pacing because another stimulus delivered between these sites initiates a new orthodromic impulse that advances the sequence of activation as recorded first at site 2. The second important feature of Figure 10 is that, during entrainment, the orthodromic impulse continued to propagate after the time of application of next stimulus. For instance, in Figure IOC, O N -2 activated tissue near site 6 after application of S N -i and at the same time that O N -i and A N _i were activating tissue at sites 2 and 5, respectively. The validity of this interpretation requires proof that the electrogram complex at site 6 labeled ON-2 in fact was produced by an impulse that originated from S N -2 rather than S N -i- Two tests confirm the origin of these electrograms. The first is to use the activation delay between the stimulus and local electrogram during pacing at long cycle lengths to determine which stimulus is related to the local electrogram at short cycle lengths. In Figure 10A,
11 Frame et al./reentry with a Partially Excitable Gap the S N -i to O N -i interval at site 6 is about 100 msec. In Figure IOC at site 6, there is a similar interval between S N _ 2 and the complex labeled O N -2, whereas the S N -i to O N -2 interval is less than 10 msec. Thus, O N -2 is related to S N -2 even though it occurs after SN-I- The second method for confirming the correct identification of electrograms is by examining their relationship to the last stimulus at the end of pacing. In Figure IOC at site 6, two electrogram complexes with coupling intervals that match the pacing cycle length are seen after the last stimulus. Since these two electrograms have this coupling interval only because they are entrained by the pacing stimulus, the second one, labeled Os, must have originated from the last stimulus S N. Therefore the electrogram labelled O N -i must be correctly attributed to S N -i even though it occurs after SN- An even more dramatic example of two electrograms entrained to the pacing cycle length after the last stimulus is seen in Figure 11A at site 6. The third important feature illustrated in Figure 10 is that the degree of penetration by the antidromic impulse increases and the site of collison between the antidromic and orthodromic impulses changes as the cycle length of overdrive pacing is decreased. At the longer pacing cycle length (Fig. 10A), each orthodromic impulse sequentially activates sites 2, 1, 6, and 5 before the next stimulus occurs. This is shown schematically in Figure 10B. At the shorter pacing cycle length (Fig. 10, C and D), the orthodromic impulse follows the same sequence except that tissue at site 5 is activated earlier than expected, suggesting that the antidromic impulse reached this site first. These observations indicate that the collision between antidromic and orthodromic impulses occurred between the stimulus and site 5 in Figure 10A and between sites 5 and 6 at the shorter pacing cycle length in Figure IOC. This pattern is more consistent with a collision between O N -i and A N than between ON and A N, because the relative timing of O N -i and A N should vary as the pacing cycle length is changed. These three features of figure 10 described above indicate that, during entrainment of the tachycardia, the orthodromic impulse causes the same sequence of activation over part of the pathway as the reentrant impulse during the tachycardia, and that this orthodromic impulse continues to propagate even after the next stimulus. Taken together, these features suggest first, that there is a pathway in which propagation is slow enough to allow reentrant excitation, and second, that the pattern of collision between A N and O N -i that is necessary for resumption of a reentrant tachycardia after the end of pacing is present. However, to prove conclusively from the response to overdrive pacing that reentry is the mechanism of the spontaneous tachyardia, it is also necessary to show that failure of the tachycardia to resume 505 after pacing is due to a disruption of the pattern of entrainment that prevents the last orthodromic impulse from reinitiating reentrant excitation, as shown in Figure 11. Figure 11, A and B, shows two examples of overdrive pacing at a cycle length of 100 msec during the same tachycardia recorded in Figure 10. However, in this case, the pacing stimulus is delivered at site 5, and only electrograms from sites 2, 4, and 6 are recorded. In Figure 11 A, a period of entrainment during overdrive pacing is followed by resumption of the tachycardia. In Figure 11B, the pattern of entrainment (ON-I colliding with AN) continues until the 17th stimulus. Then, O ]7 apparently is blocked close to the site of stimulation and does not reach either site 4 or site 2. Propagation of O17 presumbaly fails, not because of a collision with an antidromic impulse, but rather because it encounters residual refractoriness left by a previous orthodromic impulse Oj 6 as diagrammed in Figure 11C. Becuase of this event, A 18 does not collide with O17, but continues to propagate until it encounters Oie (see Fig. 11D). Site 6 is never activated by O ]7 but is activated by A 18 earlier than it would have been if Oi 8 had reached this site. Thus, for this beat and each subsequent beat, the orthodromic and antidromic impulses for the same stimulus (O N and A N ) collide with each other. When this pattern of collision becomes established during pacing, a tachycardia due to reentry cannot resume at the end of pacing because the last orthodromic impulse will be blocked by the last antidromic impulse. This strongly suggests that reentrant excitation by the last orthodromic impulse is the mechanism for resumption of the tachycardia after pacing and therefore that reentrant excitation over this pathway is the mechanism for this tachycardia. Discussion We have characterized and described a model of rapid regular, inducible atrial tachycardia resembling atrial flutter that should prove useful for study of both the mechanism of induction and termination of reentrant rhythms by pacing and the mechanisms of action of antiarrhythmic drugs. The model is easy to create as it requires only two simple incisions. The tachycardia is stable and highly reproducible in chronically instrumented dogs that can be studied serially under many conditions over a long period of time. This tachycardia is due to circus movement, and evidence discussed subsequently strongly supports the conclusion that the reentrant pathway is localized for the most part, if not in toto, to the atrial tissue around the bicuspid ring. During the tachyarrhythmia, cells in the reentrant path have an excitable gap that lasts more than 50 msec, but there is not complete recovery of excitability even at the end of the excitable gap. The conclusion that the tachycardia is due to reentry is based on a number of observations. The
12 506 arrhythmia is consistently induced and terminated either by rapid pacing at a suitably short cycle length or by properly timed premature stimulation. When multiple electrograms were recorded simultaneously from sites judged to be in proximity to the path for circus movement, the sequences of activation at these sites spanned the tachycardia cycle, and the sequence could be reversed without significant changes in the cycle length of the arrhythmia. During the arrhythmia, properly timed premature impulses caused reset of the rhythm, and overdrive pacing over a range of cycle lengths resulted in entrainment. However, overdrive at a critically short cycle length caused disruption of the conduction pattern of entrainment and resulted in termination of the tachycardia. The cycle length of the arrhythmia was a function of the weight of the dog and, thus, of the circumference of the tricuspid ring. In other studies (Page et al., unpublished observations), we have shown that the atrial muscle fibers above the tricuspid ring are oriented in a circumferential direction and can sustain reentry due to circus movement in vitro. Finally, and most important, pressure on tissue thought to include part of the reentrant path resulted in slowing and termination of the arrhythmia. We used the window of reset by variably timed single extra stimuli delivered through electrodes near the reentrant path to define the duration of the excitable gap. The window of reset ranged from 56 to 82 msec and represented 35-45% of the tachycardia cycle length. Since the timed extra stimuli were delivered at a point outside the pathway of the leading edge of the reentrant wave, the window of reset almost certainly underestimates the true duration of the excitable gap by as much as twice the conduction time from the stimulus site to the closest point on the reentrant path. In spite of the long duration of the excitable gap, conduction of premature impulses occurring at the end of the window of reset was slowed. This suggests that there was incomplete recovery of excitability. That recovery of excitability is incomplete at the end of the excitable gap also is suggested by the finding that a muscarinic agonist or vagal stimulation shortens the cycle length of the tachycardia (Allessie et al., 1977). This effect is assumed to result from the ability of muscarinic agonists to shorten atrial action potentials and refractoriness, and thus speed conduction of the impulse as it travels through less refractory tissue. Interaction of acetylcholine with muscarinic receptors in atrium increases potassium conductance, and this has a number of effects important to impulse propagation: resting potential increases, membrane resistance decreases, and repolarization is accelerated. The direct effect of the increase in resting potential is to slow conduction as more current is needed to bring membrane potential to threshold. The decrease in membrane resistance also will slow conduction (Dominguez and Fozzard, Circulation Research/Vol. 58, No. 4, April ; Walton and Fozzard, 1983). The speeding of repolarization will speed conduction if the impulse is propagating in sufficiently refractory tissue, because in such tissue the depolarizing current is attenuated, outward current is enhanced, and the threshold potential is shifted positive. One change might be expected to speed conduction, i.e., the hyperpolarization might remove inactivation of some fast channels. Whether this would counterbalance the other effects of acetylcholine depends on quantitative considerations. If the tissues initially were depolarized, a significant increase in resting potential due to acetylcholine most likely would augment inward current and speed propagation. Indeed, this is the effect we favor, with the increase in resting potential resulting from more rapid repolarization of not yet fully repolarized tissues. In normally polarized tissues, acetylcholine does not necessarily speed conduction (Hoffman and Suckling, 1953), and in some parts of the atrium, it significantly decreases the amplitude of the action potential (Paes de Carvalho et al., 1969). The tissues of the tricuspid ring may be partially depolarized by K + accumulation in interfiber spaces, and because of the rapid rhythm, intracellular [Na + ] may be higher than normal. The effects of K + accumulation on conduction velocity cannot be estimated as an increase in [K + ]o can either speed or slow conduction (Dominguez and Fozzard, 1970). Regardless of the initial condition, acetylcholine will augment K + efflux and K + accumulation. If [Na + ], is elevated, it seems likely that any hyperpolarization may increase this elevation through a direct effect of the resting potential, and also, because of reduced inactivation of fast channels, by increasing Na + influx during each action potential upstroke. Incomplete recovery of excitability at the end of the excitable gap is an important characteristic of this tachycardia and implies that factors that influence the duration of refractoriness, such as antiarrhythmic drugs or changes in autonomic tone, can influence the conduction velocity of the reentrant impulse and, therefore, the cycle length of tachycardia. In contrast, the cycle length of a reentrant tachycardia around an anatomic barrier would not be changed by a decrease in duration of refractoriness if recovery of excitability were complete before the end of the gap. Our model also suggests that a long excitable gap does not preclude incomplete recovery of excitability. During the tachycardia, full recovery of excitability and conduction velocity required more than 50 msec. The time-dependent recovery of excitability would probably last even longer in tachycardias dependent on conduction of slow response action potentials. We were surprised by the pathway for circus movement of the reentrant impulse indicated by our epicardial and endocardial maps. Our initial hypothesis, based on our extrapolation from the conclusions of previous studies that will be discussed below, was
13 Frame et al./reentry with a Partially Excitable Gap that after creation of the Y-shaped lesion, the reentrant impulse would follow pathway 1 as diagrammed in Figure 12A. Instead, we found that part or all of the path for the reentrant impulse is provided by atrial tissue just above the tricuspid ring, as diagrammed in Figure 12B (pathways 2 and 3). The reentrant impulse travels around the tricuspid ring in part through the lower portion of the intraatrial septum. Sites near the left side of the intercaval lesion, such as those on the posterior wall of the left atrium, appear to be activated by posterior and caphalad spread of a secondary wave front moving away from the tricuspid ring through the region of the limbus of the fossa ovalis. We have shown from the progression of activation times over the posterior wall of the left atrium toward the superior vena cava that these tissues do not constitute part of the pathway of the reentrant impulse. Our endocardial activation maps suggest that the reentrant impulse divides to travel simultaneously around both sides of the right atrial appendage, as indicated by pathways 2 and 3 in Figure 12B. One impulse (pathway 2) travels around the rim of tissue just above the tricuspid ring. The other impulse travels over the superior aspect of the right atrium near the sinoatrial node and over the free wall of the right atrium near the right lateral end of the Y- shaped incision (pathway 3). If an impulse traveling along one of these two pathways arrives at the point where they reunite ahead of an impulse traveling along the other pathway, then the former pathway would represent the primary path of the reentrant impulse and would govern the cycle length of the FIGURE 12. Panels A and B illustrate possible pathways for reentrant excitation following the creation of the Y-shaped lesion. Panels C and D illustrate, possible pathways after the simple intercaval lesion. (See text for discussion.) AIP = anterior inteniodal pathway; TR = tricuspid ring; LAE = left atrial epicardium; CT = crista termmalis. 507 tachycardia. Our endocardial maps did not allow us to resolve whether one or the other of these pathways represents the primary reentrant circuit. The maps of activation sequence are important because they suggest that the circumference of a natural obstacle, the tricuspid orifice, is long enough to support sustained reentrant excitation if conduction in the remainder of the atrium permits it. Knowledge of the location of the reentrant pathway in the present model may have implications for the location of the reentrant circuit in inducible atrial flutter following only an intercaval crush lesion, and perhaps also for naturally occurring atrial flutter as well. Lewis first proposed the circus movement hypothesis for the mechanism of atrial flutter based on studies of the activation sequence of electrically induced atrial flutter in several dogs in which atrial flutter lasted long enough to map activation times at different sites (Lewis et al., 1920). They concluded that atrial flutter usually was due to a reentrant impulse traveling around an obstacle made up of one or both venae cavae, although one animal had an activation sequence during flutter that was more consistent with reentry around the mitral valve orifice. Based on these observations, Rosenblueth and Garcia-Ramos (1947) described a stable, inducible tachycardia in dogs following a crush lesion of the muscular bridge between the superior and inferior venae cavae. The cycle length resembled that of a brief episode of atrial flutter occasionally induced by stimulation in dogs without the crush lesion. This model has subsequently been employed by many investigators studying atrial flutter due to circus movement (Brown, 1952; Kimura et al., 1954; Lanari et al., 1956; Takayasu et al., 1958; Hayden et al., 1967). The location of the reentrant pathway assumed by each of these investigators is shown as pathway 4 in Figure 12C. The Rosenblueth and Garcia-Ramos study presented good evidence that a portion of the pathway extended along the sulcus terminalis at the caval junction with the right atrial free wall, by showing that extending the lesion across this region (producing a Y-shaped lesion similar to the one we used) increased the cycle length of the induced tachycardia. Furthermore, in other experiments, when they made an incision extending from the intercaval lesion across the sulcus terminalis all the way to the AV ring, the tachycardia stopped and could not be reinitiated. However, the portion of the presumed pathway over the posterior wall of the left atrium has not been proven by similar experiments. Kimura et al. (1954), Takaysau et al. (1958), and Hayden et al. (1967) demonstrated progressive delay of activation times over the left atrial epicardial surface, but that finding does not prove that this activation sequence represents impulse propagation in a portion of the reentrant circuit. Furthermore, these investigators all observed an apparent conduction velocity across the left atrial free wall nearly three times faster than
14 508 over the right atrial free wall. We know of no structural basis for rapid conduction in this region. Our epicardial activation maps during tachycardia initiated after only an intercaval lesion demonstrated at widely separated points along the left atrial epicardium near the lesion differences in activation time was too small to be due to sequential propagation over this surface (Fig. 8A). We therefore believe that, during tachycardia following the intercaval lesion alone, the posterior left atrial wall is not part of the reentrant pathway. If this is true, then the reentrant impulse must travel through the intraatrial septum. We believe that sites on the posterior wall of the left atrium are activated by secondary spread away from the tricuspid ring through the intraatrial septum, just as during the tachycardia following the full Y-shaped lesion. Although we did not map the activation sequence through the lower intraatrial septum following only the intercaval lesion, this portion of pathway 4 is identical to the similar portions of pathways 2 and 3 that we did verify after making the Y-shaped lesion. We did confirm that part of pathway 4 is located on the right atrial free wall, by showing that the tachycardia cycle length was consistently increased when the intercaval lesion was extended across the right atrial free wall into the full Y-shaped lesion. Role of the Y-Shaped Lesion It is important to consider how the lesion we have used makes the atria susceptible to sustained tachycardia due to circus movement around the tricuspid ring. It is generally true that when the path for circus movement contains an excitable gap, the circulation of the impulse can be interrupted by a suitably timed impulse originating outside the circus path and entering the path during the excitable gap. We have provided examples of this for our model. Since circus movement of the type we have induced is susceptible to termination by impulses originating outside the circus path, the rhythm is more likely to be sustained if the circus path is protected from such interruption by nonconducting barriers. We judge that the lesion we employ provides such protection. Thus, a wave of excitation spreading from the circus path into the atrium, perhaps over the anterior internodal path (James, 1963; Waldo et al., 1970,1971, 1975; Pastalin et al., 1978), cannot spread rapidly to the posterior path and then return to preexcite part of the tricuspid ring. Obviously, we do not know all possible paths for interrupting impulses that may be blocked by our lesion. Obviously, other lesions or combinations of lesions might have the same effect as the Y-shaped lesion we employ. However, these possibilities are not our concern. It also may be of some interest to consider the role of the lesion in relation to the initiation of the arrhythmia. The circus movement can be initiated by rapid pacing or premature stimulation through any electrode pair around the tricuspid ring or Circulation Research/Vol. 58, No. 4, April 1986 through electrodes at other sites on the right atrium. It thus is clear that there is no unique pathway involved in initiation of the arrhythmia. Also, when the circus movement is initiated by a premature impulse (Fig. 5) or when the direction of the circus movement is reversed by extrinsic impulses (Fig. 7), there is an initial irregularity of electrograms (in terms of both sequence and cycle length) before the regular rhythm supervenes. Brief irregular rhythms typically can be initiated in the normal canine atria (Boyden and Hoffman, 1981); the transition from these irregular rhythms to regular circus movement around the tricuspid ring obviously is made more likely by the Y-shaped lesion. We assume that this transition involves events like those usually thought to be causally related to initiation of reentry. In this case, the Y-shaped lesion may protect a long isthmus of tissue after block of an impulse at one end, so that an impulse entering the other end will reach the site of block with enough delay to allow recovery of excitability. After initiation of reentry, the lesion may block paths for the propagation of impulses that otherwise would block the circus path and prevent the establishment of sustained reentry. The observations that we have made on entrainment and termination of the tachycardia by overdrive pacing are derived from the criteria proposed by Waldo et al. (1983, 1984) for demonstrating a reentrant mechanism for tachycardia. He also demonstrated changes in electrogtam morphology and timing as evidence of fusion. One difference between our analysis and that of Waldo is that his criteria rely in part on the demonstration of fusion complexes that are most clearly recorded using distant recording electrodes such as standard electrocardiographic leads, whereas our analysis is based solely on local bipolar electrograms. His first criterion involves the demonstration of a constant degree of fusion during entrainment for a particular pacing cycle length, and progressive changes in the degree of fusion as the pacing cycle length is changed. When present, these observations suggest that fusion results from activation of the myocardium in part by a wavefront related to the most recent stimulus (the direct wavefront) and in part by a wavefront related to the preceding stimulus by a fixed delay (the delayed wavefront). Changes in the degree of fusion then result from activation of different amounts of myocardium by these two wavefronts as the time between successive stimuli is changed. For reentrant rhythms, the delayed component is easily explained by the return of the orthodromic impulse after it has traveled around the reentrant circuit. For other mechanisms of tachycardia, the source of delayed component is not easily explained. In lieu of this criterion, we showed (Fig. 10), with local electrograms, that the orthodromic impulse O N -i causes the same activation sequence as the reentrant impulse during tachycardia, and it continues to propagate, even after the next stimulus, until
15 Frame et al./reentry with a Partially Excitable Gap it collides with the next antidromic impulse, A N. We also showed that the distance traveled by A N and the site of collision between O N _i and A N vary as the overdrive pacing cycle length is decreased. Waldo's second criterion states that when the tachycardia resumes after cessation of overdrive pacing, the first beat of the tachycardia is entrained but there is no fusion. The fact that this beat is entrained to the pacing cycle length suggests that it is related to the last pacing stimulus. This observation suggests that the mechanism responsible for the delayed wavefront that contributed to fusion during pacing is the same as the mechanism of the tachycardia, or, at least, that this delayed wavefront when it occurs by itself after the last stimulus is able to reinitiate the tachycardia. In our study, we made the analogous observation that the configuration and sequence of electrograms at several recording sites following the last stimulus that lead to resumption of the tachycardia are the same as the activation sequence of the reentrant impulse during tachycardia. One caveat to this approach is that interference from stimulus artifacts and rate-related changes in the relative amplitude of multiple components of complex electrograms during overdrive pacing can make this analysis difficult. These problems can limit the certainty about the similarity of local activation sequence at particular sites during entrainment compared with that during the tachycardia (e.g. site 1 in Fig. 10). Waldo's third cirterion is that failure of the tachycardia to resume after overdrive pacing is attributable to block of an orthodromic impulse at a site proximal to the expected site of collision with an antidromic impulse. This is shown by activation of sites beyond the area of block (by continued propagation of the antidromic impulse) at times earlier than those that would have resulted from propagation of the next orthodromic impulse had entrainment continued. As discussed above, this event leads to a situation in which each orthodromic impulse (O N ) collides with the antidromic impulse from the same stimulus (A N ). As a result, the last orthodromic impulse is blocked and unable to continue reentrant excitation. Although Waldo's first two criteria are very suggestive of reentry, demonstration of the third criterion provides much stronger proof because, of the three, it is the only one that shows that a particular pattern of conduction of the orthodromic impulse around a protected circuit is necessary for the continuation of the tachycardia. We satisfied this criterion by observations like those made with Figure 11. We recognize that our records of bipolar electrograms do not provide definitive proof of collison of impulses in the reentrant path indeed cannot be expected to do so. What we interpret to be collision might in fact represent failure of propagation of both the antidromic and orthodromic impulses for some other but unknown reason as the impulses 509 approach each other. We assume that collision did occur, because this seems most likely in terms of all the findings. A striking feature of the model of atrial flutter we studied in chronically instrumented dogs was the stability and persistance of the tachycardia. Several factors may contribute to this stability. The impulse propagates through healthy tissue that generates fast response action potentials, and these typically have a large safety factor. We found no localized areas of depressed conduction or unidirectional block in the reentrant circuit. Finally, propagation of the impulse in a continuous circumferential band of atrial muscle just above the tricuspid ring may minimize structural complexities that favor block because of local discontinuities in effective axial resistance (Spach et al., 1982). However, it is noteworthy that the addition of the second lesion to form a connected Y-shaped incision made the tachycardia much more stable than with the intercaval lesion alone. Several factors may contribute to this increased stability. First, during part of the cycle, the reentrant impulse travels through a relatively long isthmus of tissue between the transverse lesion and the AV ring. During this interval, it may be protected from block by ectopic impulses or secondary wavefronts from other parts of the right or left atrium. The greater stability of the tachycardia after the Y-shaped lesion may also be related to the fact that the tachycardia cycle length is longer, and this results in a somewhat longer excitable gap. When the impulse follows a longer excitable gap, it will propagate through more completely repolarized tissue and therefore probably have a higher safety factor for propagation. In addition, slight variations in the duration of refractoriness in tissue around the reentrant pathway will be less likely to reduce the excitability of tissue encountered by the reentrant impulse to the point where it blocks. Finally, the rate of change of excitability at the end of the excitable gap may be an important determinant of stability. Simson et al. (1981) have developed this concept to explain differences in the degree of stability in a model of reentrant tachycardia utilizing an analogue atrioventricular bypass tract. The refractory curve relates the conduction time of a premature impulse to its coupling interval. Very early in the excitable gap, slight reductions in the coupling interval of a premature impulse are associated with large increases in conduction time. A tachycardia whose cycle length falls in the part of this curve where the slope is very steep is likely to be unstable. A slightly premature impulse that enters the tachycardia circuit is likely to conduct much more slowly than the normal tachycardia impulse. This slow conduction will make the cycle length of the next beat of tachycardia longer than normal. When the slope of the refractory curve is steep, perturbations of the cycle length tend to produce ever-increasing oscillations of the cycle length of tachycardia until a
16 510 cycle length is sufficiently short that the impulse is blocked. On the other hand, a tachycardia with a longer cycle length in which the impulse travels through more completely recovered tissue will show a damped oscillation of cycle lengths following a premature impulse because the slope of this portion of the refractory curve is not steep. Although there are several important differences between our model and that of Simson et al. (1981), it is possible that the slower tachycardia induced after creating the Y- shaped lesion is more stable because the impulse travels through more completely recovered tissue where changes in cycle length are associated with small changes in conduction velocity of the reentrant impulse. We have emphasized the usefulness of the tachycardia we studied as a model for a particular subcategory of reentrant rhythms. However, a comparison of our results with those of recent clinical studies suggest that this tachycardia may also be useful as a model of the common form of atrial flutter in man which has been called type I flutter (Waldo et al., 1979; Wells, 1979). Important points of similarity include the response to pacing, the duration of the excitable gap, and the stability of the rhythm. Type I human atrial flutter can be entrained or terminated by overdrive pacing (Waldo et al., 1977). The presence of an excitable gap in this type of atrial flutter was demonstrated using single premature extrastimuli by Inoue et al. (1981) and Disertori et al. (1983). In both studies, stimuli delivered to the low right atrium shortened the tachycardia cycle length that encompassed the stimulus, whereas stimuli delivered to the high right atrium shortened the cycle following the one that encompassed the stimulus. These findings suggested that this type of human atrial flutter is caused by macroreentry with an excitable gap, and that the region of the low right atrium is involved. Inoue et al. (1981) found that the duration of the excitable gap in human atrial flutter ranged from 30 to 60 msec. They found that the excitable gap comprised about 20% of the cycle length both in human flutter and in the classical canine model created by an intercaval lesion (Rosenblueth and Garcia-Ramos, 1947), although the absolute duration was shorter (25-35 msec) in the faster canine tachycardia. In contrast, the abolute duration of the excitable gap during flutter in the canine model we studied was similar to that measured during human atrial flutter (Inoue et al., 1981). Finally, we found that the tachycardia induced after the Y-shaped lesion is more stable than the one induced in the classic Rosenblueth and Garcia-Ramos model. It therefore may be a better model for the long-lasting form of type I atrial flutter seen in many patients. Of course, such similarities do not per se indicate that the location of the reentrant pathway is the same in the canine model and human atria flutter. On the other hand, the model we studied is less Circulation Research/Vol. 58, No. 4, April 1986 similar to type II atrial flutter in man (Waldo et al., 1978; Wells et al., 1979). The inability of overdrive pacing to entrain or terminate this type of flutter, which is faster than type I flutter, suggests that it does not have an excitable gap. Allessie et al. (1984) have suggested that type II flutter may be similar to the canine flutter he could induce in dogs by infusion of acetylcholine and rapid pacing. The reentrant impulse in that canine model does not appear to circulate around fixed obstacles, and therefore is similar to the "leading circle" form of reentry (Allessie et al., 1977) in which the pathway is determined by a functional central barrier. We believe this model will be useful for studies comparing the mechanism of antiarrhythmic drug action and the response to premature stimulation in various specific forms of reentry. The model involves reentry through normal tissue around an anatomic barrier with a relatively long excitable gap during which there is incomplete recovery of excitability. Supported in part by Program-Project Grant HL and Grants HL and HL from the National Heart, Lung, and Blood Institute, National Institutes of Health. Dr. Frame's present address is: Cardiovascular Section, Hospital of the University of Pennsylvania, 3400 Spruce Street, Philadelphia, Pennsylvania Dr. Page's present address is: Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts Address for reprints: Brian F. Hoffman, M.D., College of Physicians & Surgeons, 630 West 168th Street, New York, New York Received October 8, 1984; accepted for publication December 31, References Allessie MA, Bonke FIM, Schopman FJG (1977) Circus movement in rabbit atnal muscle as a mechanism of tachycardia. III. The "leading circle* concept: A new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res 41: 9-18 Allessie MA, Lammers WJEP, Bonke IM, Hollen J (1984) Intraatrial reentry as a mechanism for atrial flutter induced by acetylcholine and rapid pacing in the dog. Circulation 70: Boyden PA, Hoffman BF (1981) The effects on atrial electrophysiology and structure of surgically induced right atrial enlargement in dogs. Circ Res 49: Boyden PA, Tischler A, Wit AL (1983) Circus movement causes atnal flutter in the canine heart with enlarged right atrium (abstr). Circulation 68 (suppl III): 361 Brown BB (1952) A study of factors related to effects of quinidine in experimental auricular flutter. Circulation 5: Disertori M, Inama G, Vergara G, Guarnerio M, Favero AD, Furlanello F (1983) Evidence of a reentry circuit in the common type of atrial flutter in man. Circulation 67: Dominguez G, Fozzard HA (1970) Influence of extracellular K + concentration on cable properties and excitability of sheep cardiac Purkinje fibers. Circ Res 26: Hayden WG, Hurley EJ, Rytand DA (1967) The mechanism of canine atrial flutter. Circ Res 20: Hoffman BF, Suckling EE (1953) Cardiac cellular potentials: Effect of vagal stimulation and acetylcholine. Am ] Physiol 173: Inoue H, Matsuo H, Takayanagi K, Murao S (1981) Clinical and
17 Frame et al. /Reentry with a Partially Excitable Gap 511 experimental studies of the effects of atnal extrastimulation and rapid pacing on atrial flutter cycle. Am J Cardiol 48: James TN (1964) Anatomy of the A-V node of a dog. Anat Rec 148: Kimura R, Kato K, Murao S, Ajisaka H, Koyama S, Omiya 2 (1954) Experimental study on the mechanism of auricular flutter. Tohuku J Exp Med 60: Lanari A, Lambertini A, Ravin A (1956) Mechanisms of experimental atria flutter. Circ Res 4: Lewis T, Fell HS, Stroud WD (1920) Observations upon flutter and fibrillation: Part II: The nature of auricular flutter. Heart 7: 191 Paes de Carvalho A, Hoffman BF, De Paula Carvalho M (1969) Two components of the cardiac action potential. 1. Voltagetime course and the effect of acetylcholine on atrial and nodal cells of the rabbit heart. J Gen Physiol 54: Rosenblueth A, Garcia-Ramos J (1947) Studies on flutter and fibrillation. II. The influence of artificial obstacles on experimental auricular flutter. Am Heart J 33: Simson MB, Spear JF, Moore EN (1981) Stability of an experimental atrioventricular tachycardia in dogs. Am J Physiol 240: H947-H953 Spach MS, Miller WT, Dolber PC, Kootsey JM, Sommer JR, Masher CE (1982) The functional role of structural complexities in propagation of depolarization in the atrium of the dog. Circ Res 50: Takayasu M, Tateishi Y, Osawa M, Fu]iwara M, Ikuta S (1958) Experimental studies on the auricular flutter and fibrillation. JpnCircJ21:477 Waldo AL, MacLean WAH (1980) Diagnosis and Treatment of Cardiac Arrhythmias Following Open Heart Surgery. New York, Furura Waldo AL, Vihkainen KJ, Kaiser GA, Malm JR, Hoffman BF (1970) The P wave and P-R interval: Effects of the site of origin of atrial depolarization. Circulation 42: Waldo AL, Bush HL Jr, Gelband H, Zorn GL Jr, Vitikainen KJ, Hoffman BF (1971) Effects on the canine P waves of discrete lesions in the specialized atnal tracts. Circ Res 29: Waldo AL, Vitikainen KJ, Hoffman BF (1975) The sequence of retrograde atrial activation in the canine heart. Circ Res 37: Waldo AL, MacLean WAH, Karp RB, Kouchoukos NT, James TN (1977) Entrainment and interruption of atrial flutter with atrial pacing. Circulation 56: Waldo AL, Wells JL, Plumb VJ, Cooper IB, MacLean WAH (1979) Studies of atrial flutter following open heart surgery. Annu Rev Med 30: Waldo AL, Plumb VJ, Arciniegas JG, MacLean WAH, Cooper TB, Priest MF, James TN (1983) Transient entrainment and interruption of A-V bypass pathway type paroxysmal atrial tachycardia. A model for understanding and identifying reentrant arrhythmias in man. Circulation 67: Waldo AL, Henthom RW, Plumb VJ, MacLean WAH (1984) Demonstration of the mechanism of transient entrainment and interruption of ventricular tachycardia with rapid atrial pacing. J Am Coll Cardiol 3: Walton MK, Fozzard HA (1983) The conducted action potential. Model and comparison to experiments. Biophys J 44: 9-26 Wells JL, MacLean WAH, James TN, Waldo AL (1979) Characterization of atrial flutter. Studies in man after open heart surgery using fixed atnal electrodes. Circulation 60: INDEX TERMS: Reentry Circus movement Atrial tachycardia Excitable gap Downloaded from by on October 19, 2018
Ventricular Reentry Around a Fixed Barrier
 267 Ventricular Reentry Around a Fixed Barrier Resetting With Advancement in an In Vitro Model Robert C. Bernstein, MD, and Lawrence H. Frame, MD We studied an in vitro model of reentrant tachycardia in
267 Ventricular Reentry Around a Fixed Barrier Resetting With Advancement in an In Vitro Model Robert C. Bernstein, MD, and Lawrence H. Frame, MD We studied an in vitro model of reentrant tachycardia in
Circus movement in the canine atrium around the tricuspid ring during experimental atrial flutter and during reentry in vitro
 LABORATORY INVESTIGATION ELECTROPHYSIOLOGY Circus movement in the canine atrium around the tricuspid ring during experimental atrial flutter and during reentry in vitro LAWRENCE H. FRAME, M.D.,* RICHARD
LABORATORY INVESTIGATION ELECTROPHYSIOLOGY Circus movement in the canine atrium around the tricuspid ring during experimental atrial flutter and during reentry in vitro LAWRENCE H. FRAME, M.D.,* RICHARD
V. TACHYCARDIAS Rapid rhythm abnormalities
 V. TACHYCARDIAS Rapid rhythm abnormalities Tachyarrhythmias currently account for up to 350,000 deaths annually in the US. In addition to these clearly dangerous rhythm disturbances, other forms of more
V. TACHYCARDIAS Rapid rhythm abnormalities Tachyarrhythmias currently account for up to 350,000 deaths annually in the US. In addition to these clearly dangerous rhythm disturbances, other forms of more
EHRA Accreditation Exam - Sample MCQs Invasive cardiac electrophysiology
 EHRA Accreditation Exam - Sample MCQs Invasive cardiac electrophysiology Dear EHRA Member, Dear Colleague, As you know, the EHRA Accreditation Process is becoming increasingly recognised as an important
EHRA Accreditation Exam - Sample MCQs Invasive cardiac electrophysiology Dear EHRA Member, Dear Colleague, As you know, the EHRA Accreditation Process is becoming increasingly recognised as an important
How to ablate typical atrial flutter
 Europace (1999) 1, 151 155 HOW TO... SERIES How to ablate typical atrial flutter A. Takahashi, D. C. Shah, P. Jaïs and M. Haïssaguerre Electrophysiologie Cardiaque, Hopital Cardiologique du Haut-Lévêque,
Europace (1999) 1, 151 155 HOW TO... SERIES How to ablate typical atrial flutter A. Takahashi, D. C. Shah, P. Jaïs and M. Haïssaguerre Electrophysiologie Cardiaque, Hopital Cardiologique du Haut-Lévêque,
Reentry in a Pulmonary Vein as a Possible Mechanism of Focal Atrial Fibrillation
 824 Reentry in a Pulmonary Vein as a Possible Mechanism of Focal Atrial Fibrillation BERNARD BELHASSEN, M.D., AHARON GLICK, M.D., and SAMI VISKIN, M.D. From the Department of Cardiology, Tel-Aviv Sourasky
824 Reentry in a Pulmonary Vein as a Possible Mechanism of Focal Atrial Fibrillation BERNARD BELHASSEN, M.D., AHARON GLICK, M.D., and SAMI VISKIN, M.D. From the Department of Cardiology, Tel-Aviv Sourasky
Basic Electrophysiology Protocols
 Indian Journal of Cardiology ISSN-0972-1622 2012 by the Indian Society of Cardiology Vol. 15, (3-4), 27-37 [ 27 Review Article Shomu Bohora Assistant Professor, Deptt. of Cardiology, U.N. Mehta Institute
Indian Journal of Cardiology ISSN-0972-1622 2012 by the Indian Society of Cardiology Vol. 15, (3-4), 27-37 [ 27 Review Article Shomu Bohora Assistant Professor, Deptt. of Cardiology, U.N. Mehta Institute
A atrial rate of 250 to 350 beats per minute that usually
 Use of Intraoperative Mapping to Optimize Surgical Ablation of Atrial Flutter Shigeo Yamauchi, MD, Richard B. Schuessler, PhD, Tomohide Kawamoto, MD, Todd A. Shuman, MD, John P. Boineau, MD, and James
Use of Intraoperative Mapping to Optimize Surgical Ablation of Atrial Flutter Shigeo Yamauchi, MD, Richard B. Schuessler, PhD, Tomohide Kawamoto, MD, Todd A. Shuman, MD, John P. Boineau, MD, and James
PART I. Disorders of the Heart Rhythm: Basic Principles
 PART I Disorders of the Heart Rhythm: Basic Principles FET01.indd 1 1/11/06 9:53:05 AM FET01.indd 2 1/11/06 9:53:06 AM CHAPTER 1 The Cardiac Electrical System The heart spontaneously generates electrical
PART I Disorders of the Heart Rhythm: Basic Principles FET01.indd 1 1/11/06 9:53:05 AM FET01.indd 2 1/11/06 9:53:06 AM CHAPTER 1 The Cardiac Electrical System The heart spontaneously generates electrical
Cardiac Arrhythmias Simulated by Concealed Bundle of His Extrasystoles in the Dog
 Cardiac Arrhythmias Simulated by Concealed Bundle of His Extrasystoles in the Dog By Anthony N. Da ma to, Sun H. Lau, and Gustavus Bobb ABSTRACT In 0 open-chest intact dog hearts, multiple close bipolar
Cardiac Arrhythmias Simulated by Concealed Bundle of His Extrasystoles in the Dog By Anthony N. Da ma to, Sun H. Lau, and Gustavus Bobb ABSTRACT In 0 open-chest intact dog hearts, multiple close bipolar
Influences of Anisotropic Tissue Structure on Reentrant Circuits in the Epicardial Border Zone of Subacute Canine Infarcts
 182 Influences of Anisotropic Tissue Structure on Reentrant Circuits in the Epicardial Border Zone of Subacute Canine Infarcts Stephen M. Dillon, Maurits A. Allessie, Philip C. Ursell, and Andrew L. Wit
182 Influences of Anisotropic Tissue Structure on Reentrant Circuits in the Epicardial Border Zone of Subacute Canine Infarcts Stephen M. Dillon, Maurits A. Allessie, Philip C. Ursell, and Andrew L. Wit
The radial procedure was developed as an outgrowth
 The Radial Procedure for Atrial Fibrillation Takashi Nitta, MD The radial procedure was developed as an outgrowth of an alternative to the maze procedure. The atrial incisions are designed to radiate from
The Radial Procedure for Atrial Fibrillation Takashi Nitta, MD The radial procedure was developed as an outgrowth of an alternative to the maze procedure. The atrial incisions are designed to radiate from
THE HEART. A. The Pericardium - a double sac of serous membrane surrounding the heart
 THE HEART I. Size and Location: A. Fist-size weighing less than a pound (250 to 350 grams). B. Located in the mediastinum between the 2 nd rib and the 5 th intercostal space. 1. Tipped to the left, resting
THE HEART I. Size and Location: A. Fist-size weighing less than a pound (250 to 350 grams). B. Located in the mediastinum between the 2 nd rib and the 5 th intercostal space. 1. Tipped to the left, resting
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists
 PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
Conventional Mapping. Introduction
 Conventional Mapping Haitham Badran Ain Shams University it Introduction The mapping approach used to guide ablation depends on the type of arrhythmia being assessed. Simple fluoroscopic anatomy is essential
Conventional Mapping Haitham Badran Ain Shams University it Introduction The mapping approach used to guide ablation depends on the type of arrhythmia being assessed. Simple fluoroscopic anatomy is essential
Factors Determining Vulnerability to Ventricular Fibrillation Induced by 60-CPS Alternating Current
 Factors Determining Vulnerability to Ventricular Fibrillation Induced by 60-CPS Alternating Current By Tsuneoki Sugimoto, M.D., Stephen F. School, M.D., and Andrew G. Wallace, M.D. ABSTRACT Very weak,
Factors Determining Vulnerability to Ventricular Fibrillation Induced by 60-CPS Alternating Current By Tsuneoki Sugimoto, M.D., Stephen F. School, M.D., and Andrew G. Wallace, M.D. ABSTRACT Very weak,
the Cardiovascular System I
 the Cardiovascular System I By: Dr. Nabil A Khouri MD, MsC, Ph.D MEDIASTINUM 1. Superior Mediastinum 2. inferior Mediastinum Anterior mediastinum. Middle mediastinum. Posterior mediastinum Anatomy of
the Cardiovascular System I By: Dr. Nabil A Khouri MD, MsC, Ph.D MEDIASTINUM 1. Superior Mediastinum 2. inferior Mediastinum Anterior mediastinum. Middle mediastinum. Posterior mediastinum Anatomy of
Chapter 16: Arrhythmias and Conduction Disturbances
 Complete the following. Chapter 16: Arrhythmias and Conduction Disturbances 1. Cardiac arrhythmias result from abnormal impulse, abnormal impulse, or both mechanisms together. 2. is the ability of certain
Complete the following. Chapter 16: Arrhythmias and Conduction Disturbances 1. Cardiac arrhythmias result from abnormal impulse, abnormal impulse, or both mechanisms together. 2. is the ability of certain
Case 1 Left Atrial Tachycardia
 Case 1 Left Atrial Tachycardia A 16 years old woman was referred to our institution because of recurrent episodes of palpitations and dizziness despite previous ablation procedure( 13 years ago) of postero-septal
Case 1 Left Atrial Tachycardia A 16 years old woman was referred to our institution because of recurrent episodes of palpitations and dizziness despite previous ablation procedure( 13 years ago) of postero-septal
Mapping the Conversion of Atrial Flutter to
 882 Mapping the Conversion of Atrial Flutter to Atrial Fibrillation and Atrial Fibrillation to Atrial Flutter Insights Into Mechanisms Jose Ortiz, Shinichi Niwano, Haruhiko Abe, Yoram Rudy, Nancy J. Johnson,
882 Mapping the Conversion of Atrial Flutter to Atrial Fibrillation and Atrial Fibrillation to Atrial Flutter Insights Into Mechanisms Jose Ortiz, Shinichi Niwano, Haruhiko Abe, Yoram Rudy, Nancy J. Johnson,
Introduction. Circulation
 Introduction Circulation 1- Systemic (general) circulation 2- Pulmonary circulation carries oxygenated blood to all parts of the body carries deoxygenated blood to the lungs From Lt. ventricle aorta From
Introduction Circulation 1- Systemic (general) circulation 2- Pulmonary circulation carries oxygenated blood to all parts of the body carries deoxygenated blood to the lungs From Lt. ventricle aorta From
Electrocardiogram and Heart Sounds
 Electrocardiogram and Heart Sounds Five physiologic properties of cardiac muscle Automaticity: SA node is the primary pacemaker of the heart, but any cells in the conduction system can initiate their
Electrocardiogram and Heart Sounds Five physiologic properties of cardiac muscle Automaticity: SA node is the primary pacemaker of the heart, but any cells in the conduction system can initiate their
human anatomy 2016 lecture thirteen Dr meethak ali ahmed neurosurgeon
 Heart The heart is a hollow muscular organ that is somewhat pyramid shaped and lies within the pericardium in the mediastinum. It is connected at its base to the great blood vessels but otherwise lies
Heart The heart is a hollow muscular organ that is somewhat pyramid shaped and lies within the pericardium in the mediastinum. It is connected at its base to the great blood vessels but otherwise lies
ECG. Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13
 ECG Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13 The Concept When the cardiac impulse passes through the heart, electrical current
ECG Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13 The Concept When the cardiac impulse passes through the heart, electrical current
How to Ablate Atrial Tachycardia Mechanisms and Approach. DrJo Jo Hai
 How to Ablate Atrial Tachycardia Mechanisms and Approach DrJo Jo Hai Contents Mechanisms of focal atrial tachycardia Various mapping techniques Detailed discussion on activation sequence mapping and entrainment
How to Ablate Atrial Tachycardia Mechanisms and Approach DrJo Jo Hai Contents Mechanisms of focal atrial tachycardia Various mapping techniques Detailed discussion on activation sequence mapping and entrainment
Case Report Electroanatomical Mapping and Ablation of Upper Loop Reentry Atrial Flutter
 Hellenic J Cardiol 46: 74-78, 2005 Case Report Electroanatomical Mapping and blation of Upper Loop Reentry trial Flutter POSTOLOS KTSIVS, PNGIOTIS IONNIDIS, CHRLMOS VSSILOPOULOS, THIN GIOTOPOULOU, THNSIOS
Hellenic J Cardiol 46: 74-78, 2005 Case Report Electroanatomical Mapping and blation of Upper Loop Reentry trial Flutter POSTOLOS KTSIVS, PNGIOTIS IONNIDIS, CHRLMOS VSSILOPOULOS, THIN GIOTOPOULOU, THNSIOS
Atrioventricular (AV) Nodal Reentry Associated with 2:1 Infra-His Conduction Block during Tachycardia in a Patient with AV Nodal Triple Pathways
 Atrioventricular (AV) Nodal Reentry Associated with 2:1 Infra-His Conduction Block during Tachycardia in a Patient with AV Nodal Triple Pathways Haruhiko ABE, M.D., Takashi OHKITA, M.D., Masasuke FUJITA,
Atrioventricular (AV) Nodal Reentry Associated with 2:1 Infra-His Conduction Block during Tachycardia in a Patient with AV Nodal Triple Pathways Haruhiko ABE, M.D., Takashi OHKITA, M.D., Masasuke FUJITA,
Paroxysmal Supraventricular Tachycardia PSVT.
 Atrial Tachycardia; is the name for an arrhythmia caused by a disorder of the impulse generation in the atrium or the AV node. An area in the atrium sends out rapid signals, which are faster than those
Atrial Tachycardia; is the name for an arrhythmia caused by a disorder of the impulse generation in the atrium or the AV node. An area in the atrium sends out rapid signals, which are faster than those
and fibrillation in pre-excitation (Wolff-Parkinson-
 British Heart Journal, 973, 35, 8ii-8i6. Factors regulating ventricular rates during atrial flutter and fibrillation in pre-excitation (Wolff-Parkinson- White) syndrome Agustin Castellanos, Jr., Robert
British Heart Journal, 973, 35, 8ii-8i6. Factors regulating ventricular rates during atrial flutter and fibrillation in pre-excitation (Wolff-Parkinson- White) syndrome Agustin Castellanos, Jr., Robert
CARDIOVASCULAR SYSTEM
 CARDIOVASCULAR SYSTEM Overview Heart and Vessels 2 Major Divisions Pulmonary Circuit Systemic Circuit Closed and Continuous Loop Location Aorta Superior vena cava Right lung Pulmonary trunk Base of heart
CARDIOVASCULAR SYSTEM Overview Heart and Vessels 2 Major Divisions Pulmonary Circuit Systemic Circuit Closed and Continuous Loop Location Aorta Superior vena cava Right lung Pulmonary trunk Base of heart
Lab #3: Electrocardiogram (ECG / EKG)
 Lab #3: Electrocardiogram (ECG / EKG) An introduction to the recording and analysis of cardiac activity Introduction The beating of the heart is triggered by an electrical signal from the pacemaker. The
Lab #3: Electrocardiogram (ECG / EKG) An introduction to the recording and analysis of cardiac activity Introduction The beating of the heart is triggered by an electrical signal from the pacemaker. The
Lab Activity 23. Cardiac Anatomy. Portland Community College BI 232
 Lab Activity 23 Cardiac Anatomy Portland Community College BI 232 Cardiac Muscle Histology Branching cells Intercalated disc: contains many gap junctions connecting the adjacent cell cytoplasm, creates
Lab Activity 23 Cardiac Anatomy Portland Community College BI 232 Cardiac Muscle Histology Branching cells Intercalated disc: contains many gap junctions connecting the adjacent cell cytoplasm, creates
37 1 The Circulatory System
 H T H E E A R T 37 1 The Circulatory System The circulatory system and respiratory system work together to supply cells with the nutrients and oxygen they need to stay alive. a) The respiratory system:
H T H E E A R T 37 1 The Circulatory System The circulatory system and respiratory system work together to supply cells with the nutrients and oxygen they need to stay alive. a) The respiratory system:
A Narrow QRS Complex Tachycardia With An Apparently Concentric Retrograde Atrial Activation Sequence
 www.ipej.org 125 Case Report A Narrow QRS Complex Tachycardia With An Apparently Concentric Retrograde Atrial Activation Sequence Miguel A. Arias MD, PhD; Eduardo Castellanos MD, PhD; Alberto Puchol MD;
www.ipej.org 125 Case Report A Narrow QRS Complex Tachycardia With An Apparently Concentric Retrograde Atrial Activation Sequence Miguel A. Arias MD, PhD; Eduardo Castellanos MD, PhD; Alberto Puchol MD;
Cardiovascular System
 Cardiovascular System The Heart Cardiovascular System The Heart Overview What does the heart do? By timed muscular contractions creates pressure gradients blood moves then from high pressure to low pressure
Cardiovascular System The Heart Cardiovascular System The Heart Overview What does the heart do? By timed muscular contractions creates pressure gradients blood moves then from high pressure to low pressure
BIPN100 F15 Human Physiology I (Kristan) Problem set #5 p. 1
 BIPN100 F15 Human Physiology I (Kristan) Problem set #5 p. 1 1. Dantrolene has the same effect on smooth muscles as it has on skeletal muscle: it relaxes them by blocking the release of Ca ++ from the
BIPN100 F15 Human Physiology I (Kristan) Problem set #5 p. 1 1. Dantrolene has the same effect on smooth muscles as it has on skeletal muscle: it relaxes them by blocking the release of Ca ++ from the
Anatomical Problems with Identification and Interruption of Posterior Septa1 Kent Bundles
 Anatomical Problems with Identification and Interruption of Posterior Septa1 Kent Bundles Will C. Sealy, M.D., and Eileen M. Mikat, Ph.D. ABSTRACT To gain insight into the cause of the complex anatomical
Anatomical Problems with Identification and Interruption of Posterior Septa1 Kent Bundles Will C. Sealy, M.D., and Eileen M. Mikat, Ph.D. ABSTRACT To gain insight into the cause of the complex anatomical
Kent Bundles in the Anterior Septal Space Will C. Sealy, M.D.
 Kent Bundles in the Anterior Septal Space Will C. Sealy, M.D. ABSTRACT Kent bundles in the anterior septal area of the heart occupy a region of complex morphology. In this study, the anatomical characteristics
Kent Bundles in the Anterior Septal Space Will C. Sealy, M.D. ABSTRACT Kent bundles in the anterior septal area of the heart occupy a region of complex morphology. In this study, the anatomical characteristics
ELECTROCARDIOGRAPHY (ECG)
 ELECTROCARDIOGRAPHY (ECG) The heart is a muscular organ, which pumps blood through the blood vessels of the circulatory system. Blood provides the body with oxygen and nutrients, as well as assists in
ELECTROCARDIOGRAPHY (ECG) The heart is a muscular organ, which pumps blood through the blood vessels of the circulatory system. Blood provides the body with oxygen and nutrients, as well as assists in
EKG Abnormalities. Adapted from:
 EKG Abnormalities Adapted from: http://www.bem.fi/book/19/19.htm Some key terms: Arrhythmia-an abnormal rhythm or sequence of events in the EKG Flutter-rapid depolarizations (and therefore contractions)
EKG Abnormalities Adapted from: http://www.bem.fi/book/19/19.htm Some key terms: Arrhythmia-an abnormal rhythm or sequence of events in the EKG Flutter-rapid depolarizations (and therefore contractions)
LAB 12-1 HEART DISSECTION GROSS ANATOMY OF THE HEART
 LAB 12-1 HEART DISSECTION GROSS ANATOMY OF THE HEART Because mammals are warm-blooded and generally very active animals, they require high metabolic rates. One major requirement of a high metabolism is
LAB 12-1 HEART DISSECTION GROSS ANATOMY OF THE HEART Because mammals are warm-blooded and generally very active animals, they require high metabolic rates. One major requirement of a high metabolism is
Cardiovascular System Notes: Heart Disease & Disorders
 Cardiovascular System Notes: Heart Disease & Disorders Interesting Heart Facts The Electrocardiograph (ECG) was invented in 1902 by Willem Einthoven Dutch Physiologist. This test is still used to evaluate
Cardiovascular System Notes: Heart Disease & Disorders Interesting Heart Facts The Electrocardiograph (ECG) was invented in 1902 by Willem Einthoven Dutch Physiologist. This test is still used to evaluate
11/10/2014. Muscular pump Two atria Two ventricles. In mediastinum of thoracic cavity 2/3 of heart's mass lies left of midline of sternum
 It beats over 100,000 times a day to pump over 1,800 gallons of blood per day through over 60,000 miles of blood vessels. During the average lifetime, the heart pumps nearly 3 billion times, delivering
It beats over 100,000 times a day to pump over 1,800 gallons of blood per day through over 60,000 miles of blood vessels. During the average lifetime, the heart pumps nearly 3 billion times, delivering
Cardiovascular System Notes: Physiology of the Heart
 Cardiovascular System Notes: Physiology of the Heart Interesting Heart Fact Capillaries are so small it takes ten of them to equal the thickness of a human hair. Review What are the 3 parts of the cardiovascular
Cardiovascular System Notes: Physiology of the Heart Interesting Heart Fact Capillaries are so small it takes ten of them to equal the thickness of a human hair. Review What are the 3 parts of the cardiovascular
4. The two inferior chambers of the heart are known as the atria. the superior and inferior vena cava, which empty into the left atrium.
 Answer each statement true or false. If the statement is false, change the underlined word to make it true. 1. The heart is located approximately between the second and fifth ribs and posterior to the
Answer each statement true or false. If the statement is false, change the underlined word to make it true. 1. The heart is located approximately between the second and fifth ribs and posterior to the
Step by step approach to EKG rhythm interpretation:
 Sinus Rhythms Normal sinus arrhythmia Small, slow variation of the R-R interval i.e. variation of the normal sinus heart rate with respiration, etc. Sinus Tachycardia Defined as sinus rhythm with a rate
Sinus Rhythms Normal sinus arrhythmia Small, slow variation of the R-R interval i.e. variation of the normal sinus heart rate with respiration, etc. Sinus Tachycardia Defined as sinus rhythm with a rate
UNDERSTANDING YOUR ECG: A REVIEW
 UNDERSTANDING YOUR ECG: A REVIEW Health professionals use the electrocardiograph (ECG) rhythm strip to systematically analyse the cardiac rhythm. Before the systematic process of ECG analysis is described
UNDERSTANDING YOUR ECG: A REVIEW Health professionals use the electrocardiograph (ECG) rhythm strip to systematically analyse the cardiac rhythm. Before the systematic process of ECG analysis is described
CASE 10. What would the ST segment of this ECG look like? On which leads would you see this ST segment change? What does the T wave represent?
 CASE 10 A 57-year-old man presents to the emergency center with complaints of chest pain with radiation to the left arm and jaw. He reports feeling anxious, diaphoretic, and short of breath. His past history
CASE 10 A 57-year-old man presents to the emergency center with complaints of chest pain with radiation to the left arm and jaw. He reports feeling anxious, diaphoretic, and short of breath. His past history
Cardiovascular Physiology
 Cardiovascular Physiology The mammalian heart is a pump that pushes blood around the body and is made of four chambers: right and left atria and right and left ventricles. The two atria act as collecting
Cardiovascular Physiology The mammalian heart is a pump that pushes blood around the body and is made of four chambers: right and left atria and right and left ventricles. The two atria act as collecting
Chapter 18 - Heart. I. Heart Anatomy: size of your fist; located in mediastinum (medial cavity)
 Chapter 18 - Heart I. Heart Anatomy: size of your fist; located in mediastinum (medial cavity) A. Coverings: heart enclosed in double walled sac called the pericardium 1. Fibrous pericardium: dense connective
Chapter 18 - Heart I. Heart Anatomy: size of your fist; located in mediastinum (medial cavity) A. Coverings: heart enclosed in double walled sac called the pericardium 1. Fibrous pericardium: dense connective
Cardiac Cycle. Each heartbeat is called a cardiac cycle. First the two atria contract at the same time.
 The Heartbeat Cardiac Cycle Each heartbeat is called a cardiac cycle. First the two atria contract at the same time. Next the two ventricles contract at the same time. Then all the chambers relax. http://www.youtube.com/watch?v=frd3k6lkhws
The Heartbeat Cardiac Cycle Each heartbeat is called a cardiac cycle. First the two atria contract at the same time. Next the two ventricles contract at the same time. Then all the chambers relax. http://www.youtube.com/watch?v=frd3k6lkhws
myocardial infarction period: prevention of reentry by dual stimulation during basic rhythm
 LABORATORY INVESTIGATION ARRHYTMA Reentrant ventricular rhythms in the late myocardial infarction period: prevention of reentry by dual stimulation during basic rhythm MARK RESTIVO, PH.D., WILLIAM B. GOUGH,
LABORATORY INVESTIGATION ARRHYTMA Reentrant ventricular rhythms in the late myocardial infarction period: prevention of reentry by dual stimulation during basic rhythm MARK RESTIVO, PH.D., WILLIAM B. GOUGH,
The Mammalian Circulatory System
 The Mammalian Heart The Mammalian Circulatory System Recall: What are the 3 cycles of the mammalian circulatory system? What are their functions? What are the three main vessel types in the mammalian circulatory
The Mammalian Heart The Mammalian Circulatory System Recall: What are the 3 cycles of the mammalian circulatory system? What are their functions? What are the three main vessel types in the mammalian circulatory
Unit 6: Circulatory System. 6.2 Heart
 Unit 6: Circulatory System 6.2 Heart Functions of Circulatory System 1. The heart is the pump necessary to circulate blood to all parts of the body 2. Arteries, veins and capillaries are the structures
Unit 6: Circulatory System 6.2 Heart Functions of Circulatory System 1. The heart is the pump necessary to circulate blood to all parts of the body 2. Arteries, veins and capillaries are the structures
Circulation. Circulation = is a process used for the transport of oxygen, carbon! dioxide, nutrients and wastes through-out the body
 Circulation Circulation = is a process used for the transport of oxygen, carbon! dioxide, nutrients and wastes through-out the body Heart = muscular organ about the size of your fist which pumps blood.
Circulation Circulation = is a process used for the transport of oxygen, carbon! dioxide, nutrients and wastes through-out the body Heart = muscular organ about the size of your fist which pumps blood.
LONG RP TACHYCARDIA MAPPING AND RF ABLATION
 LONG RP TACHYCARDIA MAPPING AND RF ABLATION Dr. Hayam Eldamanhoury Ain shams univeristy Arrhythmia is a too broad topic SVT is broadly defined as narrow complex ( unless aberrant conduction ) Requires
LONG RP TACHYCARDIA MAPPING AND RF ABLATION Dr. Hayam Eldamanhoury Ain shams univeristy Arrhythmia is a too broad topic SVT is broadly defined as narrow complex ( unless aberrant conduction ) Requires
Intraoperative and Postoperative Arrhythmias: Diagnosis and Treatment
 Intraoperative and Postoperative Arrhythmias: Diagnosis and Treatment Karen L. Booth, MD, Lucile Packard Children s Hospital Arrhythmias are common after congenital heart surgery [1]. Postoperative electrolyte
Intraoperative and Postoperative Arrhythmias: Diagnosis and Treatment Karen L. Booth, MD, Lucile Packard Children s Hospital Arrhythmias are common after congenital heart surgery [1]. Postoperative electrolyte
Arrival of excitation at right ventricular apical endocardium in Wolff-Parkinson-White syndrome type A, with and without right bundle-branch block'
 British Heart Journal, 973, 35, 594-600. Arrival of excitation at right ventricular apical endocardium in Wolff-Parkinson-White syndrome type A, with and without right bundle-branch block' Cesar A. Castillo,
British Heart Journal, 973, 35, 594-600. Arrival of excitation at right ventricular apical endocardium in Wolff-Parkinson-White syndrome type A, with and without right bundle-branch block' Cesar A. Castillo,
Outline. Electrical Activity of the Human Heart. What is the Heart? The Heart as a Pump. Anatomy of the Heart. The Hard Work
 Electrical Activity of the Human Heart Oguz Poroy, PhD Assistant Professor Department of Biomedical Engineering The University of Iowa Outline Basic Facts about the Heart Heart Chambers and Heart s The
Electrical Activity of the Human Heart Oguz Poroy, PhD Assistant Professor Department of Biomedical Engineering The University of Iowa Outline Basic Facts about the Heart Heart Chambers and Heart s The
All About the Heart. Structures of the heart. Layers. Chambers
 All About the Heart Your heart is a muscle. It is slightly larger than your fist and weighs less than a pound. It is located to the left of the middle of your chest. Your heart pumps blood to the lungs
All About the Heart Your heart is a muscle. It is slightly larger than your fist and weighs less than a pound. It is located to the left of the middle of your chest. Your heart pumps blood to the lungs
Chapter 20 (1) The Heart
 Chapter 20 (1) The Heart Learning Objectives Describe the location and structure of the heart Describe the path of a drop of blood from the superior vena cava or inferior vena cava through the heart out
Chapter 20 (1) The Heart Learning Objectives Describe the location and structure of the heart Describe the path of a drop of blood from the superior vena cava or inferior vena cava through the heart out
Electrophyslology, Pacing, and Mythmla
 Clin. Cardiol. 15, 667-673 (1992) Electrophyslology, Pacing, and Mythmla This section edited by A. John Camm, MD., FR.C.P., FA. C.C. Electrophysiologic Studies in Atrial flutter FRANCISCO G. COSIO, M.D.,
Clin. Cardiol. 15, 667-673 (1992) Electrophyslology, Pacing, and Mythmla This section edited by A. John Camm, MD., FR.C.P., FA. C.C. Electrophysiologic Studies in Atrial flutter FRANCISCO G. COSIO, M.D.,
THE CARDIOVASCULAR SYSTEM. Part 1
 THE CARDIOVASCULAR SYSTEM Part 1 CARDIOVASCULAR SYSTEM Blood Heart Blood vessels What is the function of this system? What other systems does it affect? CARDIOVASCULAR SYSTEM Functions Transport gases,
THE CARDIOVASCULAR SYSTEM Part 1 CARDIOVASCULAR SYSTEM Blood Heart Blood vessels What is the function of this system? What other systems does it affect? CARDIOVASCULAR SYSTEM Functions Transport gases,
Mapping Cardiac Pacemaker Circuits: Methodological puzzles of SAN optical mapping
 Mapping Cardiac Pacemaker Circuits: Methodological puzzles of SAN optical mapping Igor R. Efimov, Vadim V. Fedorov, Boyoung Joung, and Shien-Fong Lin Journal of American College of Cardiology, 66(18):1976-86,
Mapping Cardiac Pacemaker Circuits: Methodological puzzles of SAN optical mapping Igor R. Efimov, Vadim V. Fedorov, Boyoung Joung, and Shien-Fong Lin Journal of American College of Cardiology, 66(18):1976-86,
CORONARY ARTERIES. LAD Anterior wall of the left vent Lateral wall of left vent Anterior 2/3 of interventricluar septum R & L bundle branches
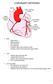 CORONARY ARTERIES RCA Right atrium Right ventricle SA node 55% AV node 90% Posterior wall of left ventricle in 90% Posterior third of interventricular septum 90% LAD Anterior wall of the left vent Lateral
CORONARY ARTERIES RCA Right atrium Right ventricle SA node 55% AV node 90% Posterior wall of left ventricle in 90% Posterior third of interventricular septum 90% LAD Anterior wall of the left vent Lateral
The Heart. Happy Friday! #takeoutyournotes #testnotgradedyet
 The Heart Happy Friday! #takeoutyournotes #testnotgradedyet Introduction Cardiovascular system distributes blood Pump (heart) Distribution areas (capillaries) Heart has 4 compartments 2 receive blood (atria)
The Heart Happy Friday! #takeoutyournotes #testnotgradedyet Introduction Cardiovascular system distributes blood Pump (heart) Distribution areas (capillaries) Heart has 4 compartments 2 receive blood (atria)
This presentation will deal with the basics of ECG description as well as the physiological basics of
 Snímka 1 Electrocardiography basics This presentation will deal with the basics of ECG description as well as the physiological basics of Snímka 2 Lecture overview 1. Cardiac conduction system functional
Snímka 1 Electrocardiography basics This presentation will deal with the basics of ECG description as well as the physiological basics of Snímka 2 Lecture overview 1. Cardiac conduction system functional
Atrial fibrillation (AF) is associated with increased morbidity
 Ablation of Atrial Fibrillation with Concomitant Surgery Edward G. Soltesz, MD, MPH, and A. Marc Gillinov, MD Atrial fibrillation (AF) is associated with increased morbidity and mortality in coronary artery
Ablation of Atrial Fibrillation with Concomitant Surgery Edward G. Soltesz, MD, MPH, and A. Marc Gillinov, MD Atrial fibrillation (AF) is associated with increased morbidity and mortality in coronary artery
This study was supported by the National Institutes of Health, Grant HE Received for publication December 6,
 Mechanisms of Ventricular Fibrillation Yoshio WATANABE, M.D. and Leonard S. DREIFUS, M.D. T was pointed out more than two decades ago, by Wegria and Wiggers, that any satisfactory theory of fibrillation
Mechanisms of Ventricular Fibrillation Yoshio WATANABE, M.D. and Leonard S. DREIFUS, M.D. T was pointed out more than two decades ago, by Wegria and Wiggers, that any satisfactory theory of fibrillation
PARA-HISSIAN CONCEALED ACCESSORY PATHWAY
 PARA-HISSIAN CONCEALED ACCESSORY PATHWAY Anamnestic Findings 41 y.o. man with normal cardiac findings on echocardiography, suffering for paroxysmal supra-ventricular tachycardia since 1982 with rapid onset
PARA-HISSIAN CONCEALED ACCESSORY PATHWAY Anamnestic Findings 41 y.o. man with normal cardiac findings on echocardiography, suffering for paroxysmal supra-ventricular tachycardia since 1982 with rapid onset
The Circulatory System. The Heart, Blood Vessels, Blood Types
 The Circulatory System The Heart, Blood Vessels, Blood Types The Closed Circulatory System Humans have a closed circulatory system, typical of all vertebrates, in which blood is confined to vessels and
The Circulatory System The Heart, Blood Vessels, Blood Types The Closed Circulatory System Humans have a closed circulatory system, typical of all vertebrates, in which blood is confined to vessels and
Goals 2/10/2016. Voltage Gradient Mapping: A Novel Approach for Successful Ablation of AV Nodal Reentry Tachycardia
 Voltage Gradient Mapping: A Novel Approach for Successful Ablation of AV Nodal Reentry Tachycardia Steven J. Bailin*, MD ; FACC, FHRS Iowa Heart Center, Des Moines, IA University of Iowa Hospital and Clinics,
Voltage Gradient Mapping: A Novel Approach for Successful Ablation of AV Nodal Reentry Tachycardia Steven J. Bailin*, MD ; FACC, FHRS Iowa Heart Center, Des Moines, IA University of Iowa Hospital and Clinics,
Emergency Medical Training Services Emergency Medical Technician Paramedic Program Outlines Outline Topic: WPW Revised: 11/2013
 Emergency Medical Training Services Emergency Medical Technician Paramedic Program Outlines Outline Topic: WPW Revised: 11/2013 Wolff-Parkinson-White syndrome (WPW) is a syndrome of pre-excitation of the
Emergency Medical Training Services Emergency Medical Technician Paramedic Program Outlines Outline Topic: WPW Revised: 11/2013 Wolff-Parkinson-White syndrome (WPW) is a syndrome of pre-excitation of the
Chapter 12: Cardiovascular Physiology System Overview
 Chapter 12: Cardiovascular Physiology System Overview Components of the cardiovascular system: Heart Vascular system Blood Figure 12-1 Plasma includes water, ions, proteins, nutrients, hormones, wastes,
Chapter 12: Cardiovascular Physiology System Overview Components of the cardiovascular system: Heart Vascular system Blood Figure 12-1 Plasma includes water, ions, proteins, nutrients, hormones, wastes,
Multiple Recording During Electrically Induced Atrial Fibrillation
 Multiple Recording During Electrically Induced Atrial Fibrillation By Toyomi Sano, M.D., and Allen M. Scher, Ph.D. With the technical assistance of O. F. Brown The mechanisms of atrial fibrillation and
Multiple Recording During Electrically Induced Atrial Fibrillation By Toyomi Sano, M.D., and Allen M. Scher, Ph.D. With the technical assistance of O. F. Brown The mechanisms of atrial fibrillation and
The HEART. What is it???? Pericardium. Heart Facts. This muscle never stops working It works when you are asleep
 This muscle never stops working It works when you are asleep The HEART It works when you eat It really works when you exercise. What is it???? Located between the lungs in the mid thoracic region Apex
This muscle never stops working It works when you are asleep The HEART It works when you eat It really works when you exercise. What is it???? Located between the lungs in the mid thoracic region Apex
Cardiovascular system
 BIO 301 Human Physiology Cardiovascular system The Cardiovascular System: consists of the heart plus all the blood vessels transports blood to all parts of the body in two 'circulations': pulmonary (lungs)
BIO 301 Human Physiology Cardiovascular system The Cardiovascular System: consists of the heart plus all the blood vessels transports blood to all parts of the body in two 'circulations': pulmonary (lungs)
Circulatory System Review
 Circulatory System Review 1. Know the diagrams of the heart, internal and external. a) What is the pericardium? What is myocardium? What is the septum? b) Explain the 4 valves of the heart. What is their
Circulatory System Review 1. Know the diagrams of the heart, internal and external. a) What is the pericardium? What is myocardium? What is the septum? b) Explain the 4 valves of the heart. What is their
Figure 2. Normal ECG tracing. Table 1.
 Figure 2. Normal ECG tracing that navigates through the left ventricle. Following these bundle branches the impulse finally passes to the terminal points called Purkinje fibers. These Purkinje fibers are
Figure 2. Normal ECG tracing that navigates through the left ventricle. Following these bundle branches the impulse finally passes to the terminal points called Purkinje fibers. These Purkinje fibers are
TEST BANK FOR ECGS MADE EASY 5TH EDITION BY AEHLERT
 Link download full: http://testbankair.com/download/test-bank-for-ecgs-made-easy-5thedition-by-aehlert/ TEST BANK FOR ECGS MADE EASY 5TH EDITION BY AEHLERT Chapter 5 TRUE/FALSE 1. The AV junction consists
Link download full: http://testbankair.com/download/test-bank-for-ecgs-made-easy-5thedition-by-aehlert/ TEST BANK FOR ECGS MADE EASY 5TH EDITION BY AEHLERT Chapter 5 TRUE/FALSE 1. The AV junction consists
Case Report Simultaneous Accessory Pathway and AV Node Mechanical Block
 185 Case Report Simultaneous Accessory Pathway and AV Node Mechanical Block Daniel Garofalo, MD, FRACP, Alfonso Gomez Gallanti, MD, David Filgueiras Rama, MD, Rafael Peinado Peinado, PhD, FESC Unidad de
185 Case Report Simultaneous Accessory Pathway and AV Node Mechanical Block Daniel Garofalo, MD, FRACP, Alfonso Gomez Gallanti, MD, David Filgueiras Rama, MD, Rafael Peinado Peinado, PhD, FESC Unidad de
2. right heart = pulmonary pump takes blood to lungs to pick up oxygen and get rid of carbon dioxide
 A. location in thorax, in inferior mediastinum posterior to sternum medial to lungs superior to diaphragm anterior to vertebrae orientation - oblique apex points down and to the left 2/3 of mass on left
A. location in thorax, in inferior mediastinum posterior to sternum medial to lungs superior to diaphragm anterior to vertebrae orientation - oblique apex points down and to the left 2/3 of mass on left
Case Report Wide-QRS Tachycardia Inducible by Both Atrial and Ventricular Pacing
 Hellenic J Cardiol 2008; 49: 446-450 Case Report Wide-QRS Tachycardia Inducible by Both Atrial and Ventricular Pacing ELEFTHERIOS GIAZITZOGLOU, DEMOSTHENES G. KATRITSIS Department of Cardiology, Athens
Hellenic J Cardiol 2008; 49: 446-450 Case Report Wide-QRS Tachycardia Inducible by Both Atrial and Ventricular Pacing ELEFTHERIOS GIAZITZOGLOU, DEMOSTHENES G. KATRITSIS Department of Cardiology, Athens
MR Advance Techniques. Cardiac Imaging. Class IV
 MR Advance Techniques Cardiac Imaging Class IV Heart The heart is a muscular organ responsible for pumping blood through the blood vessels by repeated, rhythmic contractions. Layers of the heart Endocardium
MR Advance Techniques Cardiac Imaging Class IV Heart The heart is a muscular organ responsible for pumping blood through the blood vessels by repeated, rhythmic contractions. Layers of the heart Endocardium
ECG ABNORMALITIES D R. T AM A R A AL Q U D AH
 ECG ABNORMALITIES D R. T AM A R A AL Q U D AH When we interpret an ECG we compare it instantaneously with the normal ECG and normal variants stored in our memory; these memories are stored visually in
ECG ABNORMALITIES D R. T AM A R A AL Q U D AH When we interpret an ECG we compare it instantaneously with the normal ECG and normal variants stored in our memory; these memories are stored visually in
The Cardiovascular System
 The Cardiovascular System The Manila Times College of Subic Prepared by: Stevens B. Badar, RN, MANc THE HEART Anatomy of the Heart Location and Size approx. the size of a person s fist, hollow and cone-shaped,
The Cardiovascular System The Manila Times College of Subic Prepared by: Stevens B. Badar, RN, MANc THE HEART Anatomy of the Heart Location and Size approx. the size of a person s fist, hollow and cone-shaped,
The cardiovascular system is composed of the heart and blood vessels that carry blood to and from the body s organs. There are 2 major circuits:
 1 The cardiovascular system is composed of the heart and blood vessels that carry blood to and from the body s organs. There are 2 major circuits: pulmonary and systemic. The pulmonary goes out to the
1 The cardiovascular system is composed of the heart and blood vessels that carry blood to and from the body s organs. There are 2 major circuits: pulmonary and systemic. The pulmonary goes out to the
By the end of this lecture, you will be able to: Understand the 12 lead ECG in relation to the coronary circulation and myocardium Perform an ECG
 By the end of this lecture, you will be able to: Understand the 12 lead ECG in relation to the coronary circulation and myocardium Perform an ECG recording Identify the ECG changes that occur in the presence
By the end of this lecture, you will be able to: Understand the 12 lead ECG in relation to the coronary circulation and myocardium Perform an ECG recording Identify the ECG changes that occur in the presence
How to ablate typical slow/fast AV nodal reentry tachycardia
 Europace (2000) 2, 15 19 Article No. eupc.1999.0070, available online at http://www.idealibrary.com on OW TO... SERIES ow to ablate typical slow/fast AV nodal reentry tachycardia. eidbüchel Department
Europace (2000) 2, 15 19 Article No. eupc.1999.0070, available online at http://www.idealibrary.com on OW TO... SERIES ow to ablate typical slow/fast AV nodal reentry tachycardia. eidbüchel Department
Characteristics of systolic and diastolic potentials recorded in the left interventricular septum in verapamil-sensitive left ventricular tachycardia
 CASE REPORT Cardiology Journal 2012, Vol. 19, No. 4, pp. 418 423 10.5603/CJ.2012.0075 Copyright 2012 Via Medica ISSN 1897 5593 Characteristics of systolic and diastolic potentials recorded in the left
CASE REPORT Cardiology Journal 2012, Vol. 19, No. 4, pp. 418 423 10.5603/CJ.2012.0075 Copyright 2012 Via Medica ISSN 1897 5593 Characteristics of systolic and diastolic potentials recorded in the left
Repetitive narrow QRS tachycardia in a 61-year-old female patient with recent palpitations
 Journal of Geriatric Cardiology (2018) 15: 193 198 2018 JGC All rights reserved; www.jgc301.com Case Report Open Access Repetitive narrow QRS tachycardia in a 61-year-old female patient with recent palpitations
Journal of Geriatric Cardiology (2018) 15: 193 198 2018 JGC All rights reserved; www.jgc301.com Case Report Open Access Repetitive narrow QRS tachycardia in a 61-year-old female patient with recent palpitations
The Heart. The Heart A muscular double pump. The Pulmonary and Systemic Circuits
 C H A P T E R 19 The Heart The Heart A muscular double pump circuit takes blood to and from the lungs Systemic circuit vessels transport blood to and from body tissues Atria receive blood from the pulmonary
C H A P T E R 19 The Heart The Heart A muscular double pump circuit takes blood to and from the lungs Systemic circuit vessels transport blood to and from body tissues Atria receive blood from the pulmonary
The Electrocardiogram
 The Electrocardiogram Chapters 11 and 13 AUTUMN WEDAN AND NATASHA MCDOUGAL The Normal Electrocardiogram P-wave Generated when the atria depolarizes QRS-Complex Ventricles depolarizing before a contraction
The Electrocardiogram Chapters 11 and 13 AUTUMN WEDAN AND NATASHA MCDOUGAL The Normal Electrocardiogram P-wave Generated when the atria depolarizes QRS-Complex Ventricles depolarizing before a contraction
Cardiac Conduction System
 Cardiac Conduction System What causes the Heart to Beat? Heart contracts by electrical signals! Cardiac muscle tissue contracts on its own an electrical signal is sent out by the heart so that all cells
Cardiac Conduction System What causes the Heart to Beat? Heart contracts by electrical signals! Cardiac muscle tissue contracts on its own an electrical signal is sent out by the heart so that all cells
Sinus rhythm with premature atrial beats 2 and 6 (see Lead II).
 Cardiac Pacemaker Premature Beats When one of ectopic foci becomes irritable, it may spontaneously fire, leading to one or more premature beats. Atrial and junctional foci may become irritable from excess
Cardiac Pacemaker Premature Beats When one of ectopic foci becomes irritable, it may spontaneously fire, leading to one or more premature beats. Atrial and junctional foci may become irritable from excess
Collin County Community College. ! BIOL Anatomy & Physiology! WEEK 5. The Heart
 Collin County Community College! BIOL. 2402 Anatomy & Physiology! WEEK 5 The Heart 1 (1578-1657) A groundbreaking work in the history of medicine, English physician William Harvey s Anatomical Essay on
Collin County Community College! BIOL. 2402 Anatomy & Physiology! WEEK 5 The Heart 1 (1578-1657) A groundbreaking work in the history of medicine, English physician William Harvey s Anatomical Essay on
LECTURE 5. Anatomy of the heart
 LECTURE 5. Anatomy of the heart Main components of the CVS: Heart Blood circulatory system arterial compartment haemomicrocirculatory (=microvascular) compartment venous compartment Lymphatic circulatory
LECTURE 5. Anatomy of the heart Main components of the CVS: Heart Blood circulatory system arterial compartment haemomicrocirculatory (=microvascular) compartment venous compartment Lymphatic circulatory
Pearson's Comprehensive Medical Assisting Administrative and Clinical Competencies
 Pearson's Comprehensive Medical Assisting Administrative and Clinical Competencies THIRD EDITION CHAPTER 27 The Cardiovascular System Lesson 1: Overview of the Cardiovascular System Lesson Objectives Upon
Pearson's Comprehensive Medical Assisting Administrative and Clinical Competencies THIRD EDITION CHAPTER 27 The Cardiovascular System Lesson 1: Overview of the Cardiovascular System Lesson Objectives Upon
Spontaneous clockwise (CW) and counterclockwise
 Atypical Right Atrial Flutter Patterns Yanfei Yang, MD; Jie Cheng, MD, PhD; Andy Bochoeyer, MD; Mohamed H. Hamdan, MD; Robert C. Kowal, MD, PhD; Richard Page, MD; Randall J. Lee, MD, PhD; Paul R. Steiner,
Atypical Right Atrial Flutter Patterns Yanfei Yang, MD; Jie Cheng, MD, PhD; Andy Bochoeyer, MD; Mohamed H. Hamdan, MD; Robert C. Kowal, MD, PhD; Richard Page, MD; Randall J. Lee, MD, PhD; Paul R. Steiner,
Exercise 2: Effects of Cold Temperature Aim: To record changes in heart rate after the heart is bathed in cold Ringer s solution.
 Experiment AM-3: Heart Muscle Warning: The heart preparation used in this experiment is functional for a limited period of time. If the muscle is bathed periodically in Ringer s solution, it will work
Experiment AM-3: Heart Muscle Warning: The heart preparation used in this experiment is functional for a limited period of time. If the muscle is bathed periodically in Ringer s solution, it will work
