Exercise 2: Effects of Cold Temperature Aim: To record changes in heart rate after the heart is bathed in cold Ringer s solution.
|
|
|
- Ophelia Wilkerson
- 5 years ago
- Views:
Transcription
1 Experiment AM-3: Heart Muscle Warning: The heart preparation used in this experiment is functional for a limited period of time. If the muscle is bathed periodically in Ringer s solution, it will work for about four hours. To conserve time, complete all the exercises in the experiment before analyzing the data. Exercise 1: The Heart Rate Aim: To record the mechanical trace produced by the contraction of a resting heart, and to determine the resting heart rate. Procedure 1. Type Resting in the Mark box to the right of the Mark button. 2. Click the Record button and press the Enter key on the keyboard to attach the comment to the record. Click AutoScale to increase the size of the deflection on the Main window. 3. Record the heart contractions for fifteen seconds. 4. Click Stop to halt the recording. 5. Select Save As in the File menu, type a name for the file. Choose a destination on the computer in which to save the file, like your lab group folder). Designate the file type as *.iwxdata. Click on the Save button to save the data file. 6. Moisten the chest cavity with Ringer's solution. Exercise 2: Effects of Cold Temperature Aim: To record changes in heart rate after the heart is bathed in cold Ringer s solution. Procedure 1. Type Room Temp Ringer s in the Mark box to the right of the Mark button. 2. Click the Record button. Click AutoScale to increase the size of the deflection on the Main window. 3. Record the heart contractions for fifteen seconds. 4. Apply ten drops of Ringer's solution (at room temperature) to the heart. Press the Enter key on the keyboard when the Ringer s solution is dropped on the heart. 5. Place the beaker with chilled Ringer's solution near the preparation. 6. Type Cold Ringer's in the Mark box. 7. Twenty seconds after the addition of room temperature Ringer s to the heart, apply five drops of cold Ringer's solution to the heart. Press the Enter key on the keyboard when the cold Ringer s solution is dropped on the heart. 8. Record until the heart has recovered from the effects of cold Ringer s solution. AM-3-1
2 Note: Recovery is when the amplitude and rate of the heart contraction have returned to the resting values. 9. Click Stop to halt the recording. 10. Select Save in the File menu. 11. Moisten the chest cavity with room temperature Ringer's solution. Exercise 3: Effects of Drugs Aim: To monitor the effects of Epinephrine, Acetylcholine and Atropine on the amplitude and rate of heart contraction. Procedure - Epinephrine 1. Type Resting in the Mark box to the right of the Mark button. 2. Click the Record button. Press the Enter key on the keyboard to mark the recording. Click AutoScale to increase the size of the deflection on the Main window. 3. Record the heart contractions for thirty seconds. 4. Type Epinephrine in the Mark box to the right of the Mark button. 5. Apply two drops of Epinephrine solution (at room temperature) to the heart. Press the Enter key on the keyboard when the Epinephrine solution is dropped on the heart. Continue recording. 6. After recording the effects of Epinephrine for sixty seconds, rinse the heart with room temperature Ringer s solution until the heart rate returns to the resting rate. 7. Click Stop to halt the recording. 8. Select Save in the File menu. 9. Moisten the chest cavity with Ringer's solution. Acetylcholine 1. Type Acetylcholine in the Mark box to the right of the Mark button. 2. Click the Record button. Click AutoScale to increase the size of the deflection on the Main window. 3. Record the heart contractions for thirty seconds. 4. Apply one drop of Acetylcholine solution (at room temperature) to the heart. Press the Enter key on the keyboard when the Acetylcholine solution is dropped on the heart. Continue recording. Warning: If the heart goes into cardiac arrest, rinse the Acetylcholine solution off the heart with fresh, room temperature Ringer s solution. If the heart is still in cardiac arrest after 10 seconds, add two drops of Epinephrine solution to the heart. AM-3-2
3 5. After recording the effects of Acetylcholine for sixty seconds, rinse the heart with room temperature Ringer s solution until the heart rate returns to the resting rate. 6. Click Stop to halt the recording. 7. Select Save in the File menu. 8. Moisten the chest cavity with Ringer's solution. Atropine 1. Type Atropine in the Mark box to the right of the Mark button. 2. Click the Record button. Click AutoScale to increase the size of the deflection on the Main window. 3. Record the heart contractions for thirty seconds. 4. Apply two drops of Atropine solution (at room temperature) to the heart. Press the Enter key on the keyboard when the Atropine solution is dropped on the heart. Continue recording. 5. After recording the effects of Atropine for thirty seconds, type Acetylcholine in the Mark box. Apply one drop of room temperature Acetylcholine to the heart and press the Enter key on the keyboard to mark the recording. Continue recording. 6. After recording the effects of Acetylcholine that followed the Atropine for sixty seconds, rinse the heart with room temperature Ringer s solution until the heart rate returns to the resting rate. 7. Click Stop to halt the recording. 8. Select Save in the File menu. 9. Moisten the chest cavity with Ringer's solution. Exercise 4: The Refractory Period of the Heart Aim: To stimulate the ventricle to produce extra ventricular contractions (extra-systoles), and to determine when the heart is in an absolute refractory period and unable to create extra-systoles. Program the Stimulator 1. On the LabScribe Main window, open the Edit menu and select Preferences to open the Preferences Dialog window. Click on the tab at the top of this window that is labeled Stimulator. 2. On the Stimulator Preferences window, turn on the stimulator by selecting Pulse from the stimulus mode menu on the upper left side of this window. 3. Program the other settings for the stimulus pulses to be delivered to the heart according to values listed in Table AM-3-L1. Click OK to return to the Main window. AM-3-3
4 Table AM-3-13: Settings on the Stimulator Window of the Preferences Dialog that Configure the iworx System for Experiment AM-3. Parameter Units/Title Setting Stimulus Mode Stimulator Start Pulse With Recording Time Resolution msec 0.01 Toolbar Step Frequency Hz 0.1 Toolbar Step Amplitude Volts 0.01 Toolbar Step Time Sec Delay Sec 0.05 Amplitude (Amp) Volt 4 Pulses (#pulses) Number 1 Pulse Width (W) msec 10 Frequency (F) Hz 30 Time Off Amplitude Volts 0 Holding Potential (HP) Volts 0 4. Commonly used stimulus parameters can be controlled from the Main window using the stimulator control panel. Click the Stimulator Preferences icon on the LabScribe toolbar (Figure AM-3-L2) to open the stimulator control panel (Figure AM-3-L3) on the Main window. Figure AM-3-L2: The LabScribe toolbar. AM-3-4
5 Figure AM-3-L3: The stimulator control panel 5. Attach the BNC connector of the A-BST-100 bipolar stimulator cable to the adapter on the stimulator outputs. Slow the Heart Rate (if needed) 1. Click the Record button. Click AutoScale to increase the size of the deflection on the Main window. 2. Record heart contractions for 30 seconds. Click Stop to halt the recording. 3. Determine the resting heart rate. If the rate is greater than sixty beats per minute, slow the heart s rate of contraction by dripping cold Ringer s solution on it. 4. If cold Ringer s solution was dripped on the heart to slow it, record the heart contractions for a second time. Determine the rate of contraction of the cooled heart. Procedure 1. Adjust the bipolar stimulating electrodes on the ring stand so the tips are touching either side of the ventricle, and the ventricle is able to move up and down as it contracts. 2. Type Refractory in the Mark box to the right of the Mark button. 3. Click the Record button and then AutoScale. Press the Enter key on the keyboard. Continue to record for thirty seconds. 4. Click Stop to halt the recording 5. Examine the recording for extra ventricular beats (Figure AM-3-L4). The time in the cardiac cycle when the stimulus is delivered to the heart, its amplitude, and its frequency are critical to the development of extra-systoles. Try to evoke extra-systoles by decreasing the stimulus frequency and/or increasing the stimulus amplitude. Adjust these parameters from the stimulator control panel on the Main window. The value for a stimulus parameter can be changed by either of two methods: click on the arrow buttons to the right of the window that displays the value of the parameter to increase or decrease the value; or, type the value of the parameter in the window next to the label of the parameter. Click the Apply button to finalize the change in any stimulus parameter. 6. Repeat Steps 3, 4, and 5 until an extra-systole is evoked. AM-3-5
6 7. Select Save in the File menu. Figure AM-3-L4: Stimulation of the ventricle produced an extra contraction of the ventricle when delivered at the appropriate time during the cardiac cycle. Exercise 5: Effects of a Ligature on the Heart Aim: To monitor the effects of isolating the ventricle from the SA node by tying a ligature around the heart (in the AV groove) to interrupt communication between the atria and the ventricle: Procedure 1. Obtain a piece of thread about 12 inches long. Place the center of the thread around the AV groove that separates the ventricle from the atria. 2. Tie a single overhand knot in the thread to form a loop around the AV groove. Don t tighten the loop at this time! 3. Type Normal in the Mark box to the right of the Mark button. 4. Click the Record button, and then AutoScale. Press the Enter key on the keyboard. 5. Record the contractions of the heart for about 15 seconds. 6. Click Stop to halt the recording. 7. Type Ligature in the Mark box to the right of the Mark button. 8. Click the Record button, and then AutoScale. Press the Enter key on the keyboard. Slowly tighten the knot, making sure the thread stays in the AV groove. 9. Examine the recording. If the atria and ventricle still contract in a coordinated fashion, tighten the ligature again. Mark the recording accordingly. AM-3-6
7 10. Tighten the ligature until the atria and ventricle contract independently (Figure AM-3-L5). The ligature may need to be very tight. Mark the recording accordingly. 11. Select Save in the File menu. Figure AM-3-L5: A ligature causes the chambers to contract independently. Data Analysis Exercise 2: Temperature Effects 1. Scroll to the data recorded from the heart fifteen seconds before cold Ringer s solution was added to the heart. Click the AutoScale button to maximize the size of the heart contractions on the window. 2. Use the Display Time icons to adjust the Display Time of the Main window to show five contractions on the Main window. The contractions can be selected by: Placing a cursor before the first contraction, and a cursor after the fifth contraction; and Clicking the Zoom between Cursors button on the LabScribe toolbar to expand the five selected contractions to the width of the Main window. 3. Click on the Analysis window icon in the toolbar or select Analysis from the Windows menu to transfer the data displayed in the Main window to the Analysis window. 4. Look at the Function Table that is above the uppermost channel displayed in the Analysis window. The mathematical functions, V2-V1 and T2-T1 should appear in this table. Values for V2-V1 and T2-T1 on each channel are seen in the table across the top margin of each channel. AM-3-7
8 5. Maximize the height of the trace on the Heart Contraction Channel by clicking on the arrow to the left of the channel s title to open the channel menu. Select Scale from the menu and AutoScale from the Scale submenu to increase the height of the data on that channel. 6. Once the cursors are placed in the correct positions for determining the amplitude and period of each heart contraction, the values of the parameters in the Function Table can be recorded in the on-line notebook of LabScribe by typing their names and values directly into the Journal, or on a separate data table. 7. The functions in the channel pull-down menus of the Analysis window can also be used to enter the names and values of the parameters from the recording to the Journal. To use these functions: Place the cursors at the locations used to measure the amplitude and period of each heart contraction. Transfer the names of the mathematical functions used to determine the amplitude and times to the Journal using the Add Title to Journal function in the Heart Contraction Channel pull-down menu. Transfer the values for the amplitude and period to the Journal using the Add Ch. Data to Journal function in the Heart Contraction Channel pull-down menu. 8. On the Heart Contraction Channel, use the mouse to click on and drag the cursors to specific points on the recording to measure the following parameters: Contraction Amplitude, which is the difference between the baseline level of tension in the heart tissue and the tension at the peak of the contraction. To measure this parameter, place one cursor at the beginning of the contraction, and the second cursor on the peak of the contraction (Figure AM-3-L6). The value for the V2-V1 function on the Heart Contraction Channel is the contraction amplitude. Contraction Period, which is the time between the peaks of two adjacent contractions. To measure this parameter, place one cursor on the peak of a heart contraction, and the other cursor on the peak of an adjacent heart contraction. The value for the T2-T1 function on the Heart Contraction Channel is the contraction period. 9. Record the values in the Journal using the one of the techniques described in Steps 6 or 7, and on Table AM-3-L Scroll to the section of data recorded when cold Ringer s solution was added to the heart. Click AutoScale to maximize the size of the response on the window. 11. Repeat Steps 8, 9 and 10 to measure and record the contraction amplitude and period of the heart at the time the cold Ringer s solution was added to the heart and at 10 second intervals for the first minute after the addition of the cold Ringer s. 12. Repeat Steps 8, 9 and 10 to measure and record the contraction amplitude and period of the heart at the end of the recovery period from the effects of cold Ringer s. AM-3-8
9 Figure AM-3-L6: Measuring the amplitude of a contraction with two cursors. 13. Determine the heart rate at the times reported in the Journal and on Table AM-3-L2 by converting the contraction periods to heart rates using the following equation: 14. Select Save in the File menu. AM-3-9
10 Table AM-3-L2: Amplitudes, Periods, and Rate of Heart Contractions at Different Temperatures. Contraction Treatment Amplitude (V) Period (sec) Room Temp Ringer s Cold Ringer s 10 sec after Cold Ringer s 20 sec after Cold Ringer s 30 sec after Cold Ringer s 40 sec after Cold Ringer s 50 sec after Cold Ringer s 60 sec after Cold Ringer s Recovered from Cold Exercise 3: Drug Effects Frequency (BPM) 1. Scroll to the beginning of the data from Exercise 3 and find the normal heart contractions that occurred before the first drug treatment. 2. Use the same techniques used in Exercise 2 to measure the contraction amplitudes and periods for the heart during the rest, treatment, and recovery periods for the various drugs applied to the heart. Calculate the heart rate during each period. 3. Record the values for the amplitudes, periods, and heart rates from this exercise in the Journal and on Table AM-3-L3 for Epinephrine, Table AM-3-L4 for Acetylcholine, and Table AM-3-L5 for Atropine. AM-3-10
11 Table AM-3-L3: Amplitudes, Periods, and Rates of Heart Contraction with Epinephrine Treatment. Contraction Treatment Amplitude (V) Period (sec) Resting Epinephrine 10 sec after Epinephrine 20 sec after Epinephrine 30 sec after Epinephrine 40 sec after Epinephrine 50 sec after Epinephrine 60 sec after Epinephrine Recovered Frequency (BPM) AM-3-11
12 Table AM-3-L4: Amplitudes, Periods, and Rate of Heart Contraction with Acetylcholine Treatment. Contraction Treatment Amplitude (V) Period (sec) Resting Acetylcholine 10 sec after Acetylcholine 20 sec after Acetylcholine 30 sec after Acetylcholine 40 sec after Acetylcholine 50 sec after Acetylcholine 60 sec after Acetylcholine Recovered Frequency (BPM) AM-3-12
13 Table AM-3-L5: Amplitudes, Periods, and Rates of Heart Contraction with Atropine Treatment. Contraction Treatment Amplitude (V) Period (sec) Resting Atropine 10 sec after Atropine 20 sec after Atropine 30 sec after Atropine Acetylcholine Added 10 sec after Acetylcholine 20 sec after Acetylcholine 30 sec after Acetylcholine 40 sec after Acetylcholine 50 sec after Acetylcholine 60 sec after Acetylcholine Recovered Exercise 4: Refractory Period Frequency (BPM) 1. Scroll to the beginning of the data from Exercise 4 and find the normal heart contractions that occurred before the inducement of extra-systoles. 2. Use the same techniques used in Exercise 2 to measure the normal contraction amplitude, period, and rate. Record the values for these parameters in the Journal and on Table AM-3-L6. AM-3-13
14 3. Locate a section of the data from Exercise 4 with an extra ventricle contraction. Transfer the data to the Analysis window. 4. On the Analysis window, place one cursor on the peak of the heart contraction preceding the extra contraction, and the second cursor on the peak of the extra contraction. Record the time difference, T2-T1, between these peaks in the Journal and on Table AM-3-L6. Table AM-3-L6: Amplitudes, Periods, and Rates of Heart Contraction during Extra-Systoles. Contraction Condition Amplitude (V) Period (sec) Resting Extra-Systole 1 Extra-Systole 2 Extra-Systole 3 Fastest Extra-Systole 5. Examine the complete recording of Exercise 4: Frequency (BPM) If you find any additional extra-systoles, measure the period between the preceding contraction and the extra-contraction. Determine the shortest time between the peak of a normal contraction and the extracontraction; this is the refractory period of the ventricle. Determine the phases of the cardiac contraction cycle where extra-ventricular contractions were recorded. 6. Determine the phases of the cardiac contraction cycle where extra-ventricular contractions were not recorded. Exercise 5: Ligatures 1. Scroll to the beginning of the data from Exercise 5 and find the normal heart contractions that occurred before the ligature was applied. 2. Use the same techniques used in Exercise 2 to measure and record the normal contraction AM-3-14
15 amplitude, contraction period, and heart rate. 3. Locate the section of the data from Exercise 5 when the ligature was first applied. Transfer the data to the Analysis window. 4. On the Analysis window, place the cursors on the window to measure the following periods: Ventricular Contraction Period, which is time difference between the cursors placed on adjacent peaks of ventricular contractions. This period is the value for the parameter, T2- T1. Atrial Contraction Period, which is the time difference between the cursors placed on adjacent peaks of atrial contractions. This period is the value for the parameter, T2-T1. If two atrial contractions appear in each cardiac cycle, measure the period between the first atrial peaks in two adjacent cardiac cycles. Then, measure the period between the second atrial peaks in the same adjacent cycles. Table AM-3-L7: Amplitudes, Periods, and Rates of Heart Contraction during Ligation. Contraction Condition Amplitude (V) Period (sec) Resting Stage 1 Ligation-Ventricle Stage 2 Ligation-Ventricle Stage 3 Ligation-Ventricle Final Stage-Atria Final Stage-Ventricle Questions Frequency (BPM) 1. What is the effect of cold Ringer's solution on the rate and amplitude of the ventricular contraction? What mechanism is responsible for this effect? 2. What effect does Epinephrine have on heart rate and amplitude of the ventricular contraction? 3. How does Epinephrine produce its effects on the heart rate and the amplitude of the ventricular contraction? AM-3-15
16 4. What effect does Acetylcholine have on heart rate and amplitude of the ventricular contraction? 5. How does Acetylcholine produce its effects on the heart rate and the amplitude of the ventricular contraction? 6. What effect does Atropine have on the heart? 7. How does Atropine work? 8. Do the time courses for the effect of each drug on the amplitude and the rate of ventricular contraction differ? Why? 9. In the cardiac cycle, when does the refractory period of the ventricle occur? 10. What is the significance of the long refractory period to the function of the heart? 11. How does the ligature across the AV groove work to separate the atrial and ventricular contractions? 12. In the ligated heart, the atria and ventricle beat at their own rate. Which chamber has a heart rate closest to the heart rate seen before the ligature? 13. Where are the pacemakers for the atrial and ventricular rhythms located? AM-3-16
Warning: Avoid putting the tip of the syringe needle too deeply into the hole to avoid damage to the heart tissue.
 Experiment AM-12: Crayfish Electrocardiogram (ECG) Exercise 1: The Crayfish ECG and Heart Rate Aim: To record the electrical trace (ECG) produced by the contraction of a resting heart, and to determine
Experiment AM-12: Crayfish Electrocardiogram (ECG) Exercise 1: The Crayfish ECG and Heart Rate Aim: To record the electrical trace (ECG) produced by the contraction of a resting heart, and to determine
Experiment HH-2: The Electrocardiogram and Heart Sounds
 Experiment HH-2: The Electrocardiogram and Heart Sounds Exercise 1: The ECG in a Resting Subject Aim: To measure the ECG in a resting individual. Procedure 1. Click on the Record button, located on the
Experiment HH-2: The Electrocardiogram and Heart Sounds Exercise 1: The ECG in a Resting Subject Aim: To measure the ECG in a resting individual. Procedure 1. Click on the Record button, located on the
Experiment HH-3: Exercise, the Electrocardiogram, and Peripheral Circulation
 Experiment HH-3: Exercise, the Electrocardiogram, and Peripheral Circulation Exercise 1: The ECG and the Pulse in a Resting Subject Aim: To measure and correlate the ECG and the pulse in a resting individual.
Experiment HH-3: Exercise, the Electrocardiogram, and Peripheral Circulation Exercise 1: The ECG and the Pulse in a Resting Subject Aim: To measure and correlate the ECG and the pulse in a resting individual.
iworx Sample Lab Experiment HC-3: Pulse Wave Velocity
 Experiment HC-3: Pulse Wave Velocity Exercise 1: The Pulse Wave Velocity at Rest Aim: To measure the pulse wave velocity in a resting individual. Procedure 1. Click on the Record button, located on the
Experiment HC-3: Pulse Wave Velocity Exercise 1: The Pulse Wave Velocity at Rest Aim: To measure the pulse wave velocity in a resting individual. Procedure 1. Click on the Record button, located on the
Experiment HH-3: Exercise, the Electrocardiogram, and Peripheral Circulation
 Experiment HH-3: Exercise, the Electrocardiogram, and Peripheral Circulation Background The arterial system functions as a pressure reservoir. Blood enters via the heart and exits through the capillaries.
Experiment HH-3: Exercise, the Electrocardiogram, and Peripheral Circulation Background The arterial system functions as a pressure reservoir. Blood enters via the heart and exits through the capillaries.
Exercise 1: Eye-Hand Reaction Times Aim: To measure the reaction time of a subject to a visual cue when responding with the hand.
 Experiment HN-4: Hand vs. Foot Reactions Exercise 1: Eye-Hand Reaction Times Aim: To measure the reaction time of a subject to a visual cue when responding with the hand. Procedure 1. Instruct the subject
Experiment HN-4: Hand vs. Foot Reactions Exercise 1: Eye-Hand Reaction Times Aim: To measure the reaction time of a subject to a visual cue when responding with the hand. Procedure 1. Instruct the subject
iworx Sample Lab Experiment HH-4: The Six-Lead Electrocardiogram
 Experiment HH-4: The Six-Lead Electrocardiogram Exercise 1: Six Lead ECG from Resting Subject Aim: To record a Six Lead ECG from a resting subject and determine the QRS axis of the subject s heart. Procedure
Experiment HH-4: The Six-Lead Electrocardiogram Exercise 1: Six Lead ECG from Resting Subject Aim: To record a Six Lead ECG from a resting subject and determine the QRS axis of the subject s heart. Procedure
Figure HN-5-L1: The Sequence menu containing the preprogramming light sequences used in this experiment.
 HN-5: Visual Reflexes and Color Stimulation Exercise 1: Reaction Time and Single Color Visual Cues Aim: To measure the reaction time of a subject to a visual cue. Procedure 1. Instruct the subject to:
HN-5: Visual Reflexes and Color Stimulation Exercise 1: Reaction Time and Single Color Visual Cues Aim: To measure the reaction time of a subject to a visual cue. Procedure 1. Instruct the subject to:
iworx Sample Lab Experiment AN-5: Cockroach Leg Mechanoreceptors
 Experiment AN-5: Cockroach Leg Mechanoreceptors Exercise 1: Chordotonal Organs Aim: To explore the basic characteristics of the chordotonal organs, their response to direction and intensity of leg movement,
Experiment AN-5: Cockroach Leg Mechanoreceptors Exercise 1: Chordotonal Organs Aim: To explore the basic characteristics of the chordotonal organs, their response to direction and intensity of leg movement,
iworx Sample Lab Experiment HC-5: Body Position, Exercise, and Cardiac Output
 Experiment HC-5: Body Position, Exercise, and Cardiac Output Exercise 1: Cardiac Output While Reclining Aim: To determine the cardiac output of a subject through the measurement of blood pressures and
Experiment HC-5: Body Position, Exercise, and Cardiac Output Exercise 1: Cardiac Output While Reclining Aim: To determine the cardiac output of a subject through the measurement of blood pressures and
iworx Sample Lab Experiment HH-4: The Six-Lead Electrocardiogram
 Experiment HH-4: The Six-Lead Electrocardiogram Background The cardiac cycle involves a sequential contraction of the atria and the ventricles. These contractions are triggered by the coordinated electrical
Experiment HH-4: The Six-Lead Electrocardiogram Background The cardiac cycle involves a sequential contraction of the atria and the ventricles. These contractions are triggered by the coordinated electrical
iworx Sample Lab Experiment AN-1: Membrane Potentials
 Experiment AN-1: Membrane Potentials Exercise 1: Impaling Muscle Fibers Aim: To measure the membrane potentials in different muscle fibers. Procedure 1. Look through the microscope; you should see the
Experiment AN-1: Membrane Potentials Exercise 1: Impaling Muscle Fibers Aim: To measure the membrane potentials in different muscle fibers. Procedure 1. Look through the microscope; you should see the
iworx Sample Lab Experiment HP-7: Hypothesis-driven Biofeedback Lab/Research Study
 Experiment HP-7: Hypothesis-driven Biofeedback Lab/Research Study What is Biofeedback? Biofeedback is a technique that people can use to learn to control their body's physiological functions. The definition
Experiment HP-7: Hypothesis-driven Biofeedback Lab/Research Study What is Biofeedback? Biofeedback is a technique that people can use to learn to control their body's physiological functions. The definition
Experiment HM-11: Electromyograms (EMG) for Paired Arm Wrestling
 Experiment HM-11: Electromyograms (EMG) for Paired Arm Wrestling Background The movement of parts of the body is accomplished through a system of levers composed of skeletal muscles and bones. In a lever,
Experiment HM-11: Electromyograms (EMG) for Paired Arm Wrestling Background The movement of parts of the body is accomplished through a system of levers composed of skeletal muscles and bones. In a lever,
Experiment HP-13: The Gaze Cue Paradigm
 Experiment HP-13: The Gaze Cue Paradigm Background During almost all social interactions, people s eyes convey information about their direction of attention as well as their emotional and mental status.
Experiment HP-13: The Gaze Cue Paradigm Background During almost all social interactions, people s eyes convey information about their direction of attention as well as their emotional and mental status.
1724 Lab: Frog Skeletal Muscle Physiology (Marieb Exercise 16A) Marieb/iWorx / Ziser, 2002
 1724 Lab: Frog Skeletal Muscle Physiology (Marieb Exercise 16A) Marieb/iWorx / Ziser, 2002 I. Introduction. Read the introductory material in your lab manual Marieb Ex 16A: Skeletal Muscle Physiology Frog
1724 Lab: Frog Skeletal Muscle Physiology (Marieb Exercise 16A) Marieb/iWorx / Ziser, 2002 I. Introduction. Read the introductory material in your lab manual Marieb Ex 16A: Skeletal Muscle Physiology Frog
Lab 5: Electromyograms (EMGs)
 Lab 5: Electromyograms (EMGs) Overview A motorneuron and all the muscle fibers that it innervates is known as a motor unit. Under normal circumstances, a neuronal action potential activates all of the
Lab 5: Electromyograms (EMGs) Overview A motorneuron and all the muscle fibers that it innervates is known as a motor unit. Under normal circumstances, a neuronal action potential activates all of the
iworx Sample Lab Experiment HP-6: Cynicism/Hostility and the Hot Reactor
 Experiment HP-6: Cynicism/Hostility and the Hot Reactor Exercise 1: Baseline Heart Rate and Blood Pressure Aim: To determine the resting heart rate and blood pressure of the subject. Procedure 1. Select
Experiment HP-6: Cynicism/Hostility and the Hot Reactor Exercise 1: Baseline Heart Rate and Blood Pressure Aim: To determine the resting heart rate and blood pressure of the subject. Procedure 1. Select
FT-302 Force Transducer
 Technical Note FT-302 LabScribe is a trademark of 2015 Overview The FT-302 is a high-sensitivity dual-range research grade force transducer designed to measure forces in the 0.005 to 10 gram and 0 to 100
Technical Note FT-302 LabScribe is a trademark of 2015 Overview The FT-302 is a high-sensitivity dual-range research grade force transducer designed to measure forces in the 0.005 to 10 gram and 0 to 100
Experiment HC-1: Blood Pressure, Peripheral Circulation, and Body Position
 Experiment HC-1: Blood Pressure, Peripheral Circulation, and Body Position Exercise 1: Blood Pressures from the Left Arm Aim: To determine the systolic and diastolic blood pressures in a reclining subject,
Experiment HC-1: Blood Pressure, Peripheral Circulation, and Body Position Exercise 1: Blood Pressures from the Left Arm Aim: To determine the systolic and diastolic blood pressures in a reclining subject,
Lab #4: Physiology of the in situ Amphibian Heart
 Lab #4: Physiology of the in situ Amphibian Heart This experiment explores the basic principles of cardiac muscle physiology, including contraction force, ECG, the effect of temperature, and the effect
Lab #4: Physiology of the in situ Amphibian Heart This experiment explores the basic principles of cardiac muscle physiology, including contraction force, ECG, the effect of temperature, and the effect
Metabolic and Thermal Response to Exercise
 iworx Physiology Lab Experiment Experiment HE-1 Metabolic and Thermal Response to Exercise Note: The lab presented here is intended for evaluation purposes only. iworx users should refer to the User Area
iworx Physiology Lab Experiment Experiment HE-1 Metabolic and Thermal Response to Exercise Note: The lab presented here is intended for evaluation purposes only. iworx users should refer to the User Area
Lab #3: Electrocardiogram (ECG / EKG)
 Lab #3: Electrocardiogram (ECG / EKG) An introduction to the recording and analysis of cardiac activity Introduction The beating of the heart is triggered by an electrical signal from the pacemaker. The
Lab #3: Electrocardiogram (ECG / EKG) An introduction to the recording and analysis of cardiac activity Introduction The beating of the heart is triggered by an electrical signal from the pacemaker. The
BP-600 Noninvasive Blood Pressure Sensor
 Technical Note BP-600 Overview A person's cardiac output, peripheral vascular resistance, blood pressure, and other cardiovascular parameters change in response to the activities and events taking place
Technical Note BP-600 Overview A person's cardiac output, peripheral vascular resistance, blood pressure, and other cardiovascular parameters change in response to the activities and events taking place
The Gaze Cueing Paradigm with Eye Tracking Background Set-up Lab
 iworx Physiology Lab Experiment Experiment HP-17 The Gaze Cueing Paradigm with Eye Tracking Background Set-up Lab Note: The lab presented here is intended for evaluation purposes only. iworx users should
iworx Physiology Lab Experiment Experiment HP-17 The Gaze Cueing Paradigm with Eye Tracking Background Set-up Lab Note: The lab presented here is intended for evaluation purposes only. iworx users should
LABORATORY INVESTIGATION
 LABORATORY INVESTIGATION Recording Electrocardiograms The taking of an electrocardiogram is an almost universal part of any complete physical examination. From the ECG record of the electrical activity
LABORATORY INVESTIGATION Recording Electrocardiograms The taking of an electrocardiogram is an almost universal part of any complete physical examination. From the ECG record of the electrical activity
Cardiac muscle is different from other types of muscle in that cardiac muscle
 6 E X E R C I S E Cardiovascular Physiology O B J E C T I V E S 1. To define autorhythmicity, sinoatrial node, pacemaker cells, and vagus nerves 2. To understand the effects of the sympathetic and parasympathetic
6 E X E R C I S E Cardiovascular Physiology O B J E C T I V E S 1. To define autorhythmicity, sinoatrial node, pacemaker cells, and vagus nerves 2. To understand the effects of the sympathetic and parasympathetic
Experiment HM-7: Electromyogram (EMG) Activity in Antagonistic Muscles and Range of Motion
 Experiment HM-7: Electromyogram (EMG) Activity in Antagonistic Muscles and Range of Motion Exercise 1: Antagonistic Muscles in Forearm Aim: To study the EMG activity in muscles that work in opposition
Experiment HM-7: Electromyogram (EMG) Activity in Antagonistic Muscles and Range of Motion Exercise 1: Antagonistic Muscles in Forearm Aim: To study the EMG activity in muscles that work in opposition
Biology 13A Lab #10: Cardiovascular System II ECG & Heart Disease
 Biology 13A Lab #10: Cardiovascular System II ECG & Heart Disease Lab #10 Table of Contents: Expected Learning Outcomes...... 83 Introduction....... 84 Activity 1: Collecting ECG Data..... 85 Activity
Biology 13A Lab #10: Cardiovascular System II ECG & Heart Disease Lab #10 Table of Contents: Expected Learning Outcomes...... 83 Introduction....... 84 Activity 1: Collecting ECG Data..... 85 Activity
Lab 7. Physiology of Electrocardiography
 7.1 Lab 7. Physiology of Electrocardiography The heart is a muscular pump that circulates blood throughout the body. To efficiently pump the blood, cardiac contractions must be coordinated and are regulated
7.1 Lab 7. Physiology of Electrocardiography The heart is a muscular pump that circulates blood throughout the body. To efficiently pump the blood, cardiac contractions must be coordinated and are regulated
Cardiovascular Physiology
 Cardiovascular Physiology The mammalian heart is a pump that pushes blood around the body and is made of four chambers: right and left atria and right and left ventricles. The two atria act as collecting
Cardiovascular Physiology The mammalian heart is a pump that pushes blood around the body and is made of four chambers: right and left atria and right and left ventricles. The two atria act as collecting
Lab #3: Electrocardiogram (ECG / EKG)
 Lab #3: Electrocardiogram (ECG / EKG) An introduction to the recording and analysis of cardiac activity Introduction The beating of the heart is triggered by an electrical signal from the pacemaker. The
Lab #3: Electrocardiogram (ECG / EKG) An introduction to the recording and analysis of cardiac activity Introduction The beating of the heart is triggered by an electrical signal from the pacemaker. The
Red Light - Green Light
 iworx Physiology Lab Experiment Experiment HN-9 Red Light - Green Light Background Setup Lab Note: The lab presented here is intended for evaluation purposes only. iworx users should refer to the User
iworx Physiology Lab Experiment Experiment HN-9 Red Light - Green Light Background Setup Lab Note: The lab presented here is intended for evaluation purposes only. iworx users should refer to the User
Labs #7 and #8: Vertebrate Skeletal Muscle
 Labs #7 and #8: Vertebrate Skeletal Muscle In this experiment, you will investigate the physiological properties of skeletal muscle from the isolated toad gastrocnemius. Concepts to understand include
Labs #7 and #8: Vertebrate Skeletal Muscle In this experiment, you will investigate the physiological properties of skeletal muscle from the isolated toad gastrocnemius. Concepts to understand include
Cardiovascular Effects of Exercise. Background Cardiac function
 Cardiovascular Effects of Exercise In this experiment, you will record an electrocardiogram, or ECG, and finger pulse from a healthy volunteer. You will then compare the ECG and pulse recordings when the
Cardiovascular Effects of Exercise In this experiment, you will record an electrocardiogram, or ECG, and finger pulse from a healthy volunteer. You will then compare the ECG and pulse recordings when the
Kidney Dialysis and Conductivity Background Set-up Lab Appendix
 iworx Physiology Lab Experiment Experiment HK-2 Kidney Dialysis and Conductivity Background Set-up Lab Appendix Note: The lab presented here is intended for evaluation purposes only. iworx users should
iworx Physiology Lab Experiment Experiment HK-2 Kidney Dialysis and Conductivity Background Set-up Lab Appendix Note: The lab presented here is intended for evaluation purposes only. iworx users should
Lesson 2 EMG 2 Electromyography: Mechanical Work
 Physiology Lessons for use with the Biopac Science Lab MP40 Lesson 2 EMG 2 Electromyography: Mechanical Work PC running Windows XP or Mac OS X 10.3-10.4 Lesson Revision 5.23.2006 BIOPAC Systems, Inc. 42
Physiology Lessons for use with the Biopac Science Lab MP40 Lesson 2 EMG 2 Electromyography: Mechanical Work PC running Windows XP or Mac OS X 10.3-10.4 Lesson Revision 5.23.2006 BIOPAC Systems, Inc. 42
Lab 4: Introduction to Physiological Measurements - Cardiovascular
 Lab 4: Introduction to Physiological Measurements - Cardiovascular INTRODUCTION: This lab will demonstrate cardiovascular measurements by creating an ECG with instruments used in previous labs. Students
Lab 4: Introduction to Physiological Measurements - Cardiovascular INTRODUCTION: This lab will demonstrate cardiovascular measurements by creating an ECG with instruments used in previous labs. Students
iworx Sample Lab Experiment HP-1: The Galvanic Skin Response (GSR) and Emotion
 Experiment HP-1: The Galvanic Skin Response (GSR) and Emotion What is a GSR? The Galvanic Skin Response (GSR) is one of several electrodermal responses (EDRs). EDRs are changes in the electrical properties
Experiment HP-1: The Galvanic Skin Response (GSR) and Emotion What is a GSR? The Galvanic Skin Response (GSR) is one of several electrodermal responses (EDRs). EDRs are changes in the electrical properties
Investigation of human cardiovascular physiology is very interesting, but many
 6 E X E R C I S E Frog Cardiovascular Physiology O B J E C T I V E S 1. To list the properties of cardiac muscle as automaticity and rhythmicity, and to define each. 2. To explain the statement, Cardiac
6 E X E R C I S E Frog Cardiovascular Physiology O B J E C T I V E S 1. To list the properties of cardiac muscle as automaticity and rhythmicity, and to define each. 2. To explain the statement, Cardiac
Experiment HE-12: Targeted Exercise with Wireless Electrocardiogram (ECG)
 Experiment HE-12: Targeted Exercise with Wireless Electrocardiogram (ECG) Get Your Cardio On Preparations for this experiment need to be reviewed with the students in advance. There will be exercise routines
Experiment HE-12: Targeted Exercise with Wireless Electrocardiogram (ECG) Get Your Cardio On Preparations for this experiment need to be reviewed with the students in advance. There will be exercise routines
Biopac Student Lab Lesson 6 ELECTROCARDIOGRAPHY (ECG) II Analysis Procedure. Rev
 42 Aero Camino, Goleta, CA 93117 www.biopac.com Biopac Student Lab Lesson 6 ELECTROCARDIOGRAPHY (ECG) II Analysis Procedure Rev. 12292017 Richard Pflanzer, Ph.D. Associate Professor Emeritus Indiana University
42 Aero Camino, Goleta, CA 93117 www.biopac.com Biopac Student Lab Lesson 6 ELECTROCARDIOGRAPHY (ECG) II Analysis Procedure Rev. 12292017 Richard Pflanzer, Ph.D. Associate Professor Emeritus Indiana University
Biopac Student Lab Lesson 3 ELECTROENCEPHALOGRAPHY (EEG) I Analysis Procedure. Rev
 42 Aero Camino, Goleta, CA 93117 www.biopac.com Biopac Student Lab Lesson 3 ELECTROENCEPHALOGRAPHY (EEG) I Analysis Procedure Rev. 12112014 Richard Pflanzer, Ph.D. Associate Professor Emeritus Indiana
42 Aero Camino, Goleta, CA 93117 www.biopac.com Biopac Student Lab Lesson 3 ELECTROENCEPHALOGRAPHY (EEG) I Analysis Procedure Rev. 12112014 Richard Pflanzer, Ph.D. Associate Professor Emeritus Indiana
ECE ECE PRINCIPLES OF BIOMEDICAL SYSTEMS & DEVICES LAB 1 - ELECTROCARDIOGRAM
 ECE0909.404.01 ECE 0909.504.03 PRINCIPLES OF BIOMEDICAL SYSTEMS & DEVICES LAB 1 - ELECTROCARDIOGRAM The purpose of this laboratory is to introduce you to electrocardiogram, its acquisition and interpretation.
ECE0909.404.01 ECE 0909.504.03 PRINCIPLES OF BIOMEDICAL SYSTEMS & DEVICES LAB 1 - ELECTROCARDIOGRAM The purpose of this laboratory is to introduce you to electrocardiogram, its acquisition and interpretation.
Exercise 6: Muscle Physiology II Twitch & Summation
 Exercise 6: Muscle Physiology II Twitch & Summation Text Reading: Silverthorn, 5 th ed. 412 419, 425 427; 6 th ed. pg. 410 420 In this exercise, we will investigate the physiology of contraction in the
Exercise 6: Muscle Physiology II Twitch & Summation Text Reading: Silverthorn, 5 th ed. 412 419, 425 427; 6 th ed. pg. 410 420 In this exercise, we will investigate the physiology of contraction in the
Biopac Student Lab Lesson 8 RESPIRATORY CYCLE I Analysis Procedure. Rev
 42 Aero Camino, Goleta, CA 93117 www.biopac.com Biopac Student Lab Lesson 8 RESPIRATORY CYCLE I Analysis Procedure Rev. 12292017 Richard Pflanzer, Ph.D. Associate Professor Emeritus Indiana University
42 Aero Camino, Goleta, CA 93117 www.biopac.com Biopac Student Lab Lesson 8 RESPIRATORY CYCLE I Analysis Procedure Rev. 12292017 Richard Pflanzer, Ph.D. Associate Professor Emeritus Indiana University
Biopac Student Lab Lesson 5 ELECTROCARDIOGRAPHY (ECG) I Procedure. Rev
 42 Aero Camino, Goleta, CA 93117 www.biopac.com Biopac Student Lab Lesson 5 ELECTROCARDIOGRAPHY (ECG) I Procedure Rev. 07112013 Richard Pflanzer, Ph.D. Associate Professor Emeritus Indiana University School
42 Aero Camino, Goleta, CA 93117 www.biopac.com Biopac Student Lab Lesson 5 ELECTROCARDIOGRAPHY (ECG) I Procedure Rev. 07112013 Richard Pflanzer, Ph.D. Associate Professor Emeritus Indiana University School
HUMAN ANATOMY AND PHYSIOLOGY
 HUMAN ANATOMY AND PHYSIOLOGY NAME Detection of heart sounds. Clean the ear pieces of the stethoscope before using. The ear pieces should be pointing slightly forward when inserted into the ears because
HUMAN ANATOMY AND PHYSIOLOGY NAME Detection of heart sounds. Clean the ear pieces of the stethoscope before using. The ear pieces should be pointing slightly forward when inserted into the ears because
Biopac Student Lab Lesson 20 SPINAL CORD REFLEXES Analysis Procedure. Rev
 42 Aero Camino, Goleta, CA 93117 www.biopac.com Biopac Student Lab Lesson 20 SPINAL CORD REFLEXES Analysis Procedure Rev. 12292017 Richard Pflanzer, Ph.D. Associate Professor Emeritus Indiana University
42 Aero Camino, Goleta, CA 93117 www.biopac.com Biopac Student Lab Lesson 20 SPINAL CORD REFLEXES Analysis Procedure Rev. 12292017 Richard Pflanzer, Ph.D. Associate Professor Emeritus Indiana University
CORONARY ARTERIES. LAD Anterior wall of the left vent Lateral wall of left vent Anterior 2/3 of interventricluar septum R & L bundle branches
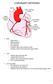 CORONARY ARTERIES RCA Right atrium Right ventricle SA node 55% AV node 90% Posterior wall of left ventricle in 90% Posterior third of interventricular septum 90% LAD Anterior wall of the left vent Lateral
CORONARY ARTERIES RCA Right atrium Right ventricle SA node 55% AV node 90% Posterior wall of left ventricle in 90% Posterior third of interventricular septum 90% LAD Anterior wall of the left vent Lateral
Experiment HM-5: Kinesiology and Electromyogram (EMG) Activity in Targeted Muscles
 Experiment HM-5: Kinesiology and Electromyogram (EMG) Activity in Targeted Muscles Lab written in conjunction with: Kelly Helm PhD, Director Exercise Science, Department of Kinesiology; Amanda Ulin & Kelsey
Experiment HM-5: Kinesiology and Electromyogram (EMG) Activity in Targeted Muscles Lab written in conjunction with: Kelly Helm PhD, Director Exercise Science, Department of Kinesiology; Amanda Ulin & Kelsey
Science in Sport. 204a ECG demonstration (Graph) Read. The Electrocardiogram. ECG Any 12 bit EASYSENSE. Sensors: Loggers: Logging time: 10 seconds
 Sensors: Loggers: ECG Any 12 bit EASYSENSE Science in Sport Logging time: 10 seconds 204a ECG demonstration (Graph) Read Regular medical check ups are essential part of the life of a professional sports
Sensors: Loggers: ECG Any 12 bit EASYSENSE Science in Sport Logging time: 10 seconds 204a ECG demonstration (Graph) Read Regular medical check ups are essential part of the life of a professional sports
Experiment HP-4: The Galvanic Skin Response (GSR) and Investigation into Cheating
 Experiment HP-4: The Galvanic Skin Response (GSR) and Investigation into Cheating This iworx lab experiment was graciously provided by Dr. Paul Wagner and Dr. Tracy Wagner, Asst. Professors, Washburn University,
Experiment HP-4: The Galvanic Skin Response (GSR) and Investigation into Cheating This iworx lab experiment was graciously provided by Dr. Paul Wagner and Dr. Tracy Wagner, Asst. Professors, Washburn University,
Experiment HP-28: Control of EEG and Biofeedback Lab/Research Study
 Experiment HP-28: Control of EEG and Biofeedback Lab/Research Study Equipment Required PC or Mac Computer IXTA, USB cable and power supply iwire-b3g cable and EEG leads Reusable Gold-Cup EEG Electrodes
Experiment HP-28: Control of EEG and Biofeedback Lab/Research Study Equipment Required PC or Mac Computer IXTA, USB cable and power supply iwire-b3g cable and EEG leads Reusable Gold-Cup EEG Electrodes
Physiology Lessons for use with the Biopac Student Lab. Lesson 1 ELECTROMYOGRAPHY I Standard and Integrated EMG
 Physiology Lessons for use with the Biopac Student Lab Lesson 1 ELECTROMYOGRAPHY I Standard and Integrated EMG Richard Pflanzer, Ph.D. Associate Professor Emeritus Indiana University School of Medicine
Physiology Lessons for use with the Biopac Student Lab Lesson 1 ELECTROMYOGRAPHY I Standard and Integrated EMG Richard Pflanzer, Ph.D. Associate Professor Emeritus Indiana University School of Medicine
iworx Sample Lab Experiment AN-10: Crustacean Stretch Receptor
 Experiment AN-10: Crustacean Stretch Receptor Background Animals use their senses to gather information about their environment. Most senses use specialized sensory organs (eyes, ears, nares) to receive
Experiment AN-10: Crustacean Stretch Receptor Background Animals use their senses to gather information about their environment. Most senses use specialized sensory organs (eyes, ears, nares) to receive
Exercise 5: Muscle Physiology I - Electromyography
 Exercise 5: Muscle Physiology I - Electromyography Readings: Silverthorn, 6 th ed. pg. 410 420 Your brain communicates with your muscles through action potentials on the motor neurons, which are then transmitted
Exercise 5: Muscle Physiology I - Electromyography Readings: Silverthorn, 6 th ed. pg. 410 420 Your brain communicates with your muscles through action potentials on the motor neurons, which are then transmitted
Lesson 5 BIOPAC Systems, Inc. Manual Revision PL3.7.5
 Physiology Lessons for use with the Biopac Student Lab Lesson 5 ELECTROCARDIOGRAPHY I Components of the ECG Richard Pflanzer, Ph.D. Associate Professor Emeritus Indiana University School of Medicine Purdue
Physiology Lessons for use with the Biopac Student Lab Lesson 5 ELECTROCARDIOGRAPHY I Components of the ECG Richard Pflanzer, Ph.D. Associate Professor Emeritus Indiana University School of Medicine Purdue
BSL PRO Lesson H03: Nerve Conduction Velocity: Along the Ulnar Nerve of a Human Subject
 Updated 12-22-03 BSL PRO Lesson H03: Nerve Conduction Velocity: Along the Ulnar Nerve of a Human Subject This PRO lesson describes hardware and software setup of the BSL PRO System to record and measure
Updated 12-22-03 BSL PRO Lesson H03: Nerve Conduction Velocity: Along the Ulnar Nerve of a Human Subject This PRO lesson describes hardware and software setup of the BSL PRO System to record and measure
Figure 1 The respiratory cycle
 RESPIRATORY CYCLE Introduction Three primary functions of the respiratory system are to provide oxygen for the body's energy needs, provide an outlet for C0 2 and help maintain the ph of the blood plasma.
RESPIRATORY CYCLE Introduction Three primary functions of the respiratory system are to provide oxygen for the body's energy needs, provide an outlet for C0 2 and help maintain the ph of the blood plasma.
Heart Rate and Blood Pressure as Vital Signs
 Heart Rate and Blood Pressure as Vital Signs Computer 10 Since the earliest days of medicine heart rate has been recognized as a vital sign an indicator of health, disease, excitement, and stress. Medical
Heart Rate and Blood Pressure as Vital Signs Computer 10 Since the earliest days of medicine heart rate has been recognized as a vital sign an indicator of health, disease, excitement, and stress. Medical
Experiment HP-3: The Galvanic Skin Response, Deception, Cognitive Complexity, and Vigilance
 Experiment HP-3: The Galvanic Skin Response, Deception, Cognitive Complexity, and Vigilance Exercise 4: GSR and Deliberate Deception Aim: To test an experimental hypothesis about deliberate deception and
Experiment HP-3: The Galvanic Skin Response, Deception, Cognitive Complexity, and Vigilance Exercise 4: GSR and Deliberate Deception Aim: To test an experimental hypothesis about deliberate deception and
The heart's "natural" pacemaker is called the sinoatrial (SA) node or sinus node.
 PACEMAKER Natural pacemaker: The heart's "natural" pacemaker is called the sinoatrial (SA) node or sinus node. Artificial pacemaker: It is a small, battery-operated device that helps the heart beat in
PACEMAKER Natural pacemaker: The heart's "natural" pacemaker is called the sinoatrial (SA) node or sinus node. Artificial pacemaker: It is a small, battery-operated device that helps the heart beat in
Posner s Attention Test
 iworx Physiology Lab Experiment Experiment HP-18 Posner s Attention Test Background Setup Lab Note: The lab presented here is intended for evaluation purposes only. iworx users should refer to the User
iworx Physiology Lab Experiment Experiment HP-18 Posner s Attention Test Background Setup Lab Note: The lab presented here is intended for evaluation purposes only. iworx users should refer to the User
Our Heart Rate. Measuring our heart rate at rest and after physical exercise
 Dimension 2 Cross Cutting Concepts Dimension 1 Science and Engineering Practices Our Heart Rate USA Standards Correlation FRAMEWORK FOR K-12 SCIENCE EDUCATION 2012 The Dimension I practices listed below
Dimension 2 Cross Cutting Concepts Dimension 1 Science and Engineering Practices Our Heart Rate USA Standards Correlation FRAMEWORK FOR K-12 SCIENCE EDUCATION 2012 The Dimension I practices listed below
Biology 325 Fall 2003 Human anatomy and physiology
 SKELETAL MUSCLE PROPERTIES Background : In this experiment, you will investigate the physiological properties of skeletal muscle from the isolated amphibian gastrocnemius. You will examine skeletal muscle
SKELETAL MUSCLE PROPERTIES Background : In this experiment, you will investigate the physiological properties of skeletal muscle from the isolated amphibian gastrocnemius. You will examine skeletal muscle
Charts Worksheet using Excel Obesity Can a New Drug Help?
 Worksheet using Excel 2000 Obesity Can a New Drug Help? Introduction Obesity is known to be a major health risk. The data here arise from a study which aimed to investigate whether or not a new drug, used
Worksheet using Excel 2000 Obesity Can a New Drug Help? Introduction Obesity is known to be a major health risk. The data here arise from a study which aimed to investigate whether or not a new drug, used
BIO 360: Vertebrate Physiology Performing and analyzing an EKG Lab 11: Performing and analyzing an EKG Lab report due April 17 th
 BIO 60: Vertebrate Physiology Lab : Lab report due April 7 th All muscles produce an electrical current when they contract. The heart is no exception. An electrocardiogram (ECG or EKG) is a graphical recording
BIO 60: Vertebrate Physiology Lab : Lab report due April 7 th All muscles produce an electrical current when they contract. The heart is no exception. An electrocardiogram (ECG or EKG) is a graphical recording
Allergy Basics. This handout describes the process for adding and removing allergies from a patient s chart.
 Allergy Basics This handout describes the process for adding and removing allergies from a patient s chart. Accessing Allergy Information Page 1 Recording No Known Medication Allergies Page 2 Recording
Allergy Basics This handout describes the process for adding and removing allergies from a patient s chart. Accessing Allergy Information Page 1 Recording No Known Medication Allergies Page 2 Recording
Kinesiology and Electromyogram (EMG) Activity in Targeted Muscles
 iworx Physiology Lab Experiment Experiment HM-9 Kinesiology and Electromyogram (EMG) Activity in Targeted Muscles Note: The lab presented here is intended for evaluation purposes only. iworx users should
iworx Physiology Lab Experiment Experiment HM-9 Kinesiology and Electromyogram (EMG) Activity in Targeted Muscles Note: The lab presented here is intended for evaluation purposes only. iworx users should
Lesson 4 ECG 2 Electrocardiography
 Physiology Lessons for use with the Biopac Science Lab MP40 Lesson 4 ECG 2 Electrocardiography PC running Windows XP or Mac OS X 10.3-10.4 Lesson Revision 1.20.2006 BIOPAC Systems, Inc. 42 Aero Camino,
Physiology Lessons for use with the Biopac Science Lab MP40 Lesson 4 ECG 2 Electrocardiography PC running Windows XP or Mac OS X 10.3-10.4 Lesson Revision 1.20.2006 BIOPAC Systems, Inc. 42 Aero Camino,
Heart Rate, Blood Pressure, and Exercise. Evaluation copy
 Heart Rate, Blood Pressure, and Exercise Computer 11 The adaptability of the heart can be observed during exercise, when the metabolic activity of skeletal muscles increases. The cardiovascular system,
Heart Rate, Blood Pressure, and Exercise Computer 11 The adaptability of the heart can be observed during exercise, when the metabolic activity of skeletal muscles increases. The cardiovascular system,
Lesson 10 Aerobic Exercise Physiology
 Physiology Lessons for use with the Biopac Science Lab MP40 Lesson 10 Aerobic Exercise Physiology PC running Windows XP or Mac OS X 10.3-10.4 Lesson Revision 12.19.2005 BIOPAC Systems, Inc. 42 Aero Camino,
Physiology Lessons for use with the Biopac Science Lab MP40 Lesson 10 Aerobic Exercise Physiology PC running Windows XP or Mac OS X 10.3-10.4 Lesson Revision 12.19.2005 BIOPAC Systems, Inc. 42 Aero Camino,
Each student should record the ECG of one of the members of the lab group and have their own ECG recorded.
 EXPERIMENT 1 ELECTROCARDIOGRAPHY The purpose of this experiment is to introduce you to the techniques of electrocardiography and the interpretation of electrocardiograms. In Part A of the experiment, you
EXPERIMENT 1 ELECTROCARDIOGRAPHY The purpose of this experiment is to introduce you to the techniques of electrocardiography and the interpretation of electrocardiograms. In Part A of the experiment, you
Arrhythmias. Pulmonary Artery
 Arrhythmias Introduction Cardiac arrhythmia is an irregularity of the heart beat that causes the heart to beat too slowly, too fast, or irregularly. There are different types of arrhythmias. Most arrhythmias
Arrhythmias Introduction Cardiac arrhythmia is an irregularity of the heart beat that causes the heart to beat too slowly, too fast, or irregularly. There are different types of arrhythmias. Most arrhythmias
Figure 1 muscle tissue to its resting state. By looking at several beats you can also calculate the rate for each component.
 ANALYZING THE HEART WITH EKG WITH LABQUEST LAB From Human Physiology with Vernier Westminster College INTRODUCTION An electrocardiogram (ECG or EKG) is a graphical recording of the electrical events occurring
ANALYZING THE HEART WITH EKG WITH LABQUEST LAB From Human Physiology with Vernier Westminster College INTRODUCTION An electrocardiogram (ECG or EKG) is a graphical recording of the electrical events occurring
Lab #9: Muscle Physiology
 Background Overview of Skeletal Muscle Contraction Sarcomere Thick Filaments Skeletal muscle fibers are very large, elongated cells (Fig 9.1). Roughly 80% of the content of each muscle fiber consists of
Background Overview of Skeletal Muscle Contraction Sarcomere Thick Filaments Skeletal muscle fibers are very large, elongated cells (Fig 9.1). Roughly 80% of the content of each muscle fiber consists of
Lesson 1 ELECTROMYOGRAPHY I Standard and Integrated EMG
 Physiology Lessons for use with the PC running Windows 98SE, Me, 2000 Pro, XP Pro/Home/Media Lesson 1 ELECTROMYOGRAPHY I Standard and Integrated EMG Manual Revision PL3.7.0 080706 Richard Pflanzer, Ph.D.
Physiology Lessons for use with the PC running Windows 98SE, Me, 2000 Pro, XP Pro/Home/Media Lesson 1 ELECTROMYOGRAPHY I Standard and Integrated EMG Manual Revision PL3.7.0 080706 Richard Pflanzer, Ph.D.
Sample. Analyzing the Heart with EKG. Computer
 Analyzing the Heart with EKG Computer An electrocardiogram (ECG or EKG) is a graphical recording of the electrical events occurring within the heart. In a healthy heart there is a natural pacemaker in
Analyzing the Heart with EKG Computer An electrocardiogram (ECG or EKG) is a graphical recording of the electrical events occurring within the heart. In a healthy heart there is a natural pacemaker in
SANAKO Lab 100 STS USER GUIDE
 SANAKO Lab 100 STS USER GUIDE Copyright 2008 SANAKO Corporation. All rights reserved. Microsoft is a registered trademark. Microsoft Windows 2000 and Windows XP are trademarks of Microsoft Corporation.
SANAKO Lab 100 STS USER GUIDE Copyright 2008 SANAKO Corporation. All rights reserved. Microsoft is a registered trademark. Microsoft Windows 2000 and Windows XP are trademarks of Microsoft Corporation.
Effects of Temperature, Stretch, and Various Drug Treatments on the
 Nicole Rodi Bio 235: Animal Physiology Heart Muscle Lab Report 10/24/2014 Effects of Temperature, Stretch, and Various Drug Treatments on the Cardiac Muscle Activity of Rana pipiens Abstract Mechanical
Nicole Rodi Bio 235: Animal Physiology Heart Muscle Lab Report 10/24/2014 Effects of Temperature, Stretch, and Various Drug Treatments on the Cardiac Muscle Activity of Rana pipiens Abstract Mechanical
12.2 Monitoring the Human Circulatory System
 12.2 Monitoring the Human Circulatory System Video 1: 3D Animation of Heart Pumping Blood blood flow through the heart... Video 2: Hank Reviews Everything on the Heart https://www.youtube.com/watch?v=x9zz6tcxari
12.2 Monitoring the Human Circulatory System Video 1: 3D Animation of Heart Pumping Blood blood flow through the heart... Video 2: Hank Reviews Everything on the Heart https://www.youtube.com/watch?v=x9zz6tcxari
Lesson 2 ELECTROMYOGRAPHY II Motor unit recruitment Fatigue
 Physiology Lessons for use with the PC running Windows 98SE, Me, 2000 Pro, XP Pro/Home/Media Lesson 2 ELECTROMYOGRAPHY II Motor unit recruitment Fatigue Manual Revision PL3.7.0 M3.0.6 080706 Richard Pflanzer,
Physiology Lessons for use with the PC running Windows 98SE, Me, 2000 Pro, XP Pro/Home/Media Lesson 2 ELECTROMYOGRAPHY II Motor unit recruitment Fatigue Manual Revision PL3.7.0 M3.0.6 080706 Richard Pflanzer,
CAMOSUN COLLEGE BIOLOGY 144 (2010) LABS
 LAB 8: CARDIOVASCULAR PHYSIOLOGY PART 1. HEART SOUNDS AND PULSE DETERMINATIONS Introduction Two distinct sounds can be heard during each cardiac cycle. These sounds are commonly described as lub and dup
LAB 8: CARDIOVASCULAR PHYSIOLOGY PART 1. HEART SOUNDS AND PULSE DETERMINATIONS Introduction Two distinct sounds can be heard during each cardiac cycle. These sounds are commonly described as lub and dup
Chapter 1: Introduction to iworx and LabScribe
 Chapter 1: Introduction to iworx and LabScribe Overview The iworx/204 data acquisition unit is a four-channel recording instrument (Figure 1-1 on page 1). Channels 1 and 2 receive electrical inputs through
Chapter 1: Introduction to iworx and LabScribe Overview The iworx/204 data acquisition unit is a four-channel recording instrument (Figure 1-1 on page 1). Channels 1 and 2 receive electrical inputs through
X-Plain Atrial Fibrillation Reference Summary
 X-Plain Atrial Fibrillation Reference Summary Introduction Atrial fibrillation is a common heart condition that affects approximately 2.5 million Americans every year. Atrial fibrillation requires immediate
X-Plain Atrial Fibrillation Reference Summary Introduction Atrial fibrillation is a common heart condition that affects approximately 2.5 million Americans every year. Atrial fibrillation requires immediate
ELECTROCARDIOGRAPHY (ECG)
 ELECTROCARDIOGRAPHY (ECG) The heart is a muscular organ, which pumps blood through the blood vessels of the circulatory system. Blood provides the body with oxygen and nutrients, as well as assists in
ELECTROCARDIOGRAPHY (ECG) The heart is a muscular organ, which pumps blood through the blood vessels of the circulatory system. Blood provides the body with oxygen and nutrients, as well as assists in
Electrocardiography I Laboratory
 Introduction The body relies on the heart to circulate blood throughout the body. The heart is responsible for pumping oxygenated blood from the lungs out to the body through the arteries and also circulating
Introduction The body relies on the heart to circulate blood throughout the body. The heart is responsible for pumping oxygenated blood from the lungs out to the body through the arteries and also circulating
A. User s Guide. CareCenter MD Stress and Resting ECG
 70-00533-02 A User s Guide CareCenter MD Stress and Resting ECG CARECENTER MD STRESS AND RESTING USER S GUIDE 70-00533-02 A Information in this document is subject to change without notice. Names and data
70-00533-02 A User s Guide CareCenter MD Stress and Resting ECG CARECENTER MD STRESS AND RESTING USER S GUIDE 70-00533-02 A Information in this document is subject to change without notice. Names and data
Using Freezing-Point Depression to Find Molecular Weight. Evaluation copy
 Using Freezing-Point Depression to Find Molecular Weight Computer 4 When a solute is dissolved in a solvent, the freezing temperature is lowered in proportion to the number of moles of solute added. This
Using Freezing-Point Depression to Find Molecular Weight Computer 4 When a solute is dissolved in a solvent, the freezing temperature is lowered in proportion to the number of moles of solute added. This
Analyzing the Heart with EKG
 Analyzing the Heart with EKG LabQuest An electrocardiogram (ECG or EKG) is a graphical recording of the electrical events occurring within the heart. In a healthy heart there is a natural pacemaker in
Analyzing the Heart with EKG LabQuest An electrocardiogram (ECG or EKG) is a graphical recording of the electrical events occurring within the heart. In a healthy heart there is a natural pacemaker in
Heart Rate and Exercise. Evaluation copy. Figure 1
 Heart Rate and Exercise Computer 4 The adaptability of the heart can be observed during exercise, when the metabolic activity of muscle tissue increases. The cardiovascular system, consisting of the heart
Heart Rate and Exercise Computer 4 The adaptability of the heart can be observed during exercise, when the metabolic activity of muscle tissue increases. The cardiovascular system, consisting of the heart
Cloud Condensation Nuclei Counter (CCN) Module
 Particle Analysis and Display System (PADS): Cloud Condensation Nuclei Counter (CCN) Module Operator Manual DOC-0190 A-1 PADS 2.5.6, CCN Module 2.5.1 5710 Flatiron Parkway, Unit B Boulder, CO 80301 USA
Particle Analysis and Display System (PADS): Cloud Condensation Nuclei Counter (CCN) Module Operator Manual DOC-0190 A-1 PADS 2.5.6, CCN Module 2.5.1 5710 Flatiron Parkway, Unit B Boulder, CO 80301 USA
SARASOTA MEMORIAL HOSPITAL
 SARASOTA MEMORIAL HOSPITAL TITLE: NURSING PROCEDURE PACEMAKERS AND DETERMINING PACEMAKER DATE: REVIEWED: PAGES: 10/99 11/18 1 of 7 PS1094 ISSUED FOR: Nursing RESPONSIBILITY: *Qualified RN PURPOSE: To establish
SARASOTA MEMORIAL HOSPITAL TITLE: NURSING PROCEDURE PACEMAKERS AND DETERMINING PACEMAKER DATE: REVIEWED: PAGES: 10/99 11/18 1 of 7 PS1094 ISSUED FOR: Nursing RESPONSIBILITY: *Qualified RN PURPOSE: To establish
UNDERSTANDING YOUR ECG: A REVIEW
 UNDERSTANDING YOUR ECG: A REVIEW Health professionals use the electrocardiograph (ECG) rhythm strip to systematically analyse the cardiac rhythm. Before the systematic process of ECG analysis is described
UNDERSTANDING YOUR ECG: A REVIEW Health professionals use the electrocardiograph (ECG) rhythm strip to systematically analyse the cardiac rhythm. Before the systematic process of ECG analysis is described
Electrical Conduction
 Sinoatrial (SA) node Electrical Conduction Sets the pace of the heartbeat at 70 bpm AV node (50 bpm) and Purkinje fibers (25 40 bpm) can act as pacemakers under some conditions Internodal pathway from
Sinoatrial (SA) node Electrical Conduction Sets the pace of the heartbeat at 70 bpm AV node (50 bpm) and Purkinje fibers (25 40 bpm) can act as pacemakers under some conditions Internodal pathway from
THE INFLUENCE OF IONS ON THE CONTRACTION OF ISOLATED EARTHWORM SMOOTH MUSCLE
 THE INFLUENCE OF IONS ON THE CONTRACTION OF ISOLATED EARTHWORM SMOOTH MUSCLE BACKGROUND READING Animal Physiology by Hill, Wyse & Anderson, 2004: pp. 484 486. ANIMALS & EQUIPMENT Living material Large
THE INFLUENCE OF IONS ON THE CONTRACTION OF ISOLATED EARTHWORM SMOOTH MUSCLE BACKGROUND READING Animal Physiology by Hill, Wyse & Anderson, 2004: pp. 484 486. ANIMALS & EQUIPMENT Living material Large
Cardiac Output. Graphics are used with permission of: adam.com ( Benjamin Cummings Publishing Co (
 Interactive Physiology Cardiac Output Graphics are used with permission of: adam.com (http://www.adam.com/) Benjamin Cummings Publishing Co (http://www.aw.com/bc) Page 1. Introduction Cardiac output is
Interactive Physiology Cardiac Output Graphics are used with permission of: adam.com (http://www.adam.com/) Benjamin Cummings Publishing Co (http://www.aw.com/bc) Page 1. Introduction Cardiac output is
ELECTROMYOGRAM ANALYSIS OF MUSCLE FUNCTION INTRODUCTION
 ELECTROMYOGRAM ANALYSIS OF MUSCLE FUNCTION STANDARDS: 3.3.10.B - Explain cell functions and processes in terms of chemical reactions and energy changes. 3.3.12.B - Evaluate relationships between structure
ELECTROMYOGRAM ANALYSIS OF MUSCLE FUNCTION STANDARDS: 3.3.10.B - Explain cell functions and processes in terms of chemical reactions and energy changes. 3.3.12.B - Evaluate relationships between structure
Sanako Lab 100 STS USER GUIDE
 Sanako Lab 100 STS USER GUIDE Copyright 2002-2015 SANAKO Corporation. All rights reserved. Microsoft is a registered trademark. Microsoft Windows XP, Windows Vista and Windows 7 are trademarks of Microsoft
Sanako Lab 100 STS USER GUIDE Copyright 2002-2015 SANAKO Corporation. All rights reserved. Microsoft is a registered trademark. Microsoft Windows XP, Windows Vista and Windows 7 are trademarks of Microsoft
