Effect of Volume Expansion on Renal Citrate
|
|
|
- Rosanna Cameron
- 5 years ago
- Views:
Transcription
1 Effect of Volume Expansion on Renal Citrate and Ammonia Metabolism in KCl-Deficient Rats SHELDON ADLER, BARBARA ZETr, BARBARA ANDERSON, and DONALD S. FRALEY From the Department of Medicine, University of Pittsburgh School of Medicine and Montefiore Hospital, Pittsburgh, Pennsylvania A B S T R A C T When rats with desoxycorticosterone acetate (DOCA)-induced potassium chloride deficiency are given sodium chloride there is simultaneously a partial correction of metabolic alkalosis and a marked reduction in urinary citrate excretion and renal citrate content. To examine DOCA's role in this phenomenon and to determine how sodium chloride alters renal metabolism, rats were made KC1 deficient using furosemide and a KCl-deficient diet. Renal citrate and ammonia metabolism were then studied after chronic oral sodium chloride administration or acute volume expansion with isotonic mannitol. Although both maneuvers partially corrected metabolic alkalosis, sodium chloride raised serum chloride concentration while mannitol significantly decreased it. Urinary citrate excretion decreased to 10% of control in rats given NaCl and to 50% of control in rats infused with mannitol. The filtered load of citrate was constant or increased indicating increased tubular citrate reabsorption. Renal cortical citrate content also decreased approximately 50%. Renal cortical slices from KCl-deficient rats incubated in low or normal chloride media produced equal amounts of "CO2 from [1,5-"4C]citrate. In addition, urinary ammonia excretion increased by over 300% in both groups. This occurred in the mannitol group despite increased urinary ph and flow rate indicating a rise in renal ammonia production. It seems that neither DOCA nor an increase in serum chloride concentration explains the experimental results. Rather, it appears that volume expansion is responsible for increased renal tubular citrate reabsorption and renal ammonia production. As these renal metabolic This work was previously reported in abstract form: Adler, S., B. Zett, B. Anderson, and D. Fraley Role of chloride and volume expansion in regulating renal citrate metabolism. Clin. Res. 22: 654a. Received for publication 31 December 1974 and in revised form 14 April responses ordinarily occur in response to acidosis, the data are consistent with the hypothesis that volume expansion reduces renal cell ph in KCl-deficient rats. INTRODUCTION Recently, Adler, Zett, and Anderson (1) demonstrated that when desoxycorticosterone acetate (DOCA)'-induced chronic potassium chloride-deficient rats are given sodium chloride, but no potassium salts, renal cortical citrate content and urinary citrate excretion profoundly decrease. Renal citrate metabolism was examined because it has been shown that urinary citrate excretion and renal citrate content decrease in acidosis and increase in alkalosis (2, 3) presumably secondary to increased renal citrate oxidation in acidosis and a decrease in alkalosis (4). However, Adler and his colleagues noted that despite the enormous reductions in urinary and renal citrate seen after sodium chloride administration, metabolic alkalosis was still present (1). Inasmuch as changes in citrate metabolism in potassium-deficient diaphragm muscle have been shown to be directly related to alterations in intracellular rather than extracellular ph (5), it was postulated that sodium chloride administration in the potassium chloridedeficient rats may have caused a renal tubular cell acidosis. Unfortunately, direct measurement of renal tubular cell ph is presently not possible in vivo (6), so this was not able to be confirmed directly. To test the hypothesis that renal tubular cell ph is lowered by sodium chloride ingestion in potassium chloride deficiency and to determine the mechanism through which sodium chloride induces changes in renal citrate metabolism, further experiments were performed. To eliminate the possible role of exogenously 'Abbreviation used in this paper: DOCA, desoxycorticosterone acetate. The Journal of Clinical Investigation Volume 56 August
2 administered DOCA, rats were made potassium chloride deficient by intraperitoneal furosemide administration. The roles played by extracellular chloride concentration and volume expansion in altering renal cell ph were studied by examining their effects on renal citrate and ammonia metabolism. The results indicate that the response of renal citrate metabolism to sodium chloride ingestion in the furosemide-induced potassium chloridedeficient rats was identical to the response of renal citrate metabolism to sodium chloride ingestion in rats made deficient with DOCA. In addition to the changes in renal citrate metabolism, sodium chloride or mannitol induced volume expansion increased ammonia excretion. These renal metabolic alterations appear to be due to an expansion of intravascular volume rather than a change in plasma chloride concentration. The data are consistent with the hypothesis that expansion of intravascular volume in potassium chloride-deficient rats leads to a decrease in renal tubular cell ph. METHODS Potassium chloride depletion protocol. Male Sprague- Dawley rats weighing g were used in all experiments. Each animal was placed on a ero potassium, ero chloride diet (obtained from Nutritional Biochemical Corporation, Cleveland, Ohio). Animals were allowed water containing 75 meq/liter of sodium bicarbonate ad libitum during the entire study. Rats were maintained on the potassium chloride-deficient diet for a 15-day period. On days 8-12 each rat received a daily intraperitoneal dose of 2 mg of furosemide. Repletion protocol. At the end of the 15-day period all food was removed, and the rats were divided into four equal groups: a KCI-repleted group tube fed 10 ml of a 20% dextrose solution containing 200 meq/liter KCI twice daily; a K-depleted group tube fed 10 ml of 20%o dextrose solution containing no electrolytes twice daily; an NaClrepleted group tube fed 10 ml of a 20%o dextrose solution containing 200 meq/liter sodium chloride twice daily; and a KHCOa-repleted group tube fed 10 ml of a 20%o dextrose solution containing 200 meq/liter KHCO. twice daily. Solutions were administered for 3 days during which the sodium bicarbonate drinking water was continued ad libitum. Rats were weighed each morning during this 3-day repletion. After 3 days of tube feeding rats were sacrificed the next day as outlined below. Citrate and ammonia excretion experiments in repleted rats. After receiving the sixth tube feeding each rat was placed in an individual metabolic cage and given only the sodium bicarbonate solution ad libitum. Urine was collected for the next 16 h. At the end of this time rats were removed from the cages which were then rinsed with distilled water to insure complete urine collection. Each urine collection was filtered and analyed for creatinine, chloride, citrate, and ammonia. Tissue electrolyte and citrate experiments in repleted rats. Animals were anesthetied by the intraperitoneal injection of inactin, 100 mg/kg of body weight. Each rat was anesthetied separately to avoid any delay before killing the rat by exsanguination from the abdominal aorta into a heparinied syringe. Portions of renal cortical tissue, liver, and gluteal muscle were removed and rapidly froen in liquid 392 S. Adler, B. Zett, A. Anderson, and D. S. Fraley nitrogen for citrate analysis. Other portions of these tissues were placed in tared tubes for later determination of water and potassium content. All the froen tissues were weighed and homogenied in the froen state. The homogenate was centrifuged and the supernate saved for citrate analysis. 14CO0 production from [1,5-14C] citrate experiments. As previously described (2) renal cortical slices were placed into specially constructed 25-ml Erlenmeyer flasks having a center well. Approximately 100 mg of renal cortex was used in each flask. Each flask contained 4 ml of a carbon dioxide-oxygen saturated Krebs-Ringer bicarbonate solution containing 1.0 meq/liter of potassium. The substrate was 1 mm acetate and 1 mm citrate. In addition, each flask contained 0.5,hCi of [1,5-14C]citrate (obtained from New England Nuclear, Boston, Mass.). The solution in one group of flasks contained 70 meq of sodium chloride, while the solution in the other group contained 110 meq of sodium chloride. Isoosmolarity was achieved by the addition of mannitol to the first flask. Flasks were incubated for 90 min in a metabolic shaker at 370C, and "CO2 was collected and counted as previously described (2). Results are expressed as disintegrations per minute per flask per 200 mg. Calculations of this value have also been previously described (2). Production of 'CO2 from labeled citrate in the remainder of the paper will be termed citrate decarboxylation. Mannitol expansion experiments. Potassium chloride-depleted rats were employed in all experiments. After the 15-day depletion protocol the potassium chloride-depleted rats were anesthetied by the intraperitoneal injection of inactin, 100 mg/kg of body weight. A tracheostomy was performed in each animal to allow for removal of retained secretions. An indwelling jugular catheter was inserted for infusion, and a femoral artery line was placed for blood sampling. The infusion line was connected to a Harvard constant infusion pump (Harvard Apparatus Co., Inc., Millis, Mass.). A bladder catheter was inserted suprapubically. After a 30-min stabiliation period, animals were divided into two groups. The dextrose-infused group received 8.3 ml/h of a 5% dextrose solution over a 210-min period. The mannitol-infused group was given 8.3 ml/h of the 5% dextrose solution for 90 min to act as a control period. These animals were then infused with a 280 mm mannitol solution given at 37 ml/min for 150 min. In the dextrose group blood was obtained after the stabiliation period and at the conclusion of the experiment. In the mannitol-infused group blood was obtained at the end of the stabiliation period, at the end of the control period, and at the conclusion of the experimental period. Urine collected under oil in the dextrose-infused group was obtained as a single sample over the entire 210 min. In the mannitolinfused rats a 90-min control period urine was obtained, and 30 min after mannitol infusion was started a 60-min and two 30-min urines were collected under oil. Urines in both groups were analyed for ph, creatinine, citrate, and ammonia. Each group was infused with radioisotopes immediately after the stabiliation period. Dextrose-infused rats received 2 1ACi each of Na "Cl and ["C] DMO and 10,uCi of tritiated water. Double this quantity was given to the mannitol-infused group to compensate for increased urinary losses. Isotopes were injected early to allow sufficient tissue equilibration time to take place. At the conclusion of the experiment animals in each group were killed by exsanguination from the abdominal aorta into a heparinied syringe. Portions of renal cortical tissue were removed and immediately froen in liquid nitrogen, weighed, homogenied in a froen state, and saved for citrate analysis as previously
3 TABLE I Arterial Acid-Base Status and Arterial and Tissue Potassium Levels in Potassium Chloride-Repleted, Potassium-Depleted, Sodium Chloride-Repleted, and Potassium Bicarbonate-Repleted Rats* Blood Tissue potassium ph HCOS PCo2 K Muscle Kidney meq/liter mm Hg meq/liter meq/kg dry weight KCl repleted, n = $ $ ±0.01 i1.7 ±-3 ±0.2 ±9 ±14 K depleted, n = ±0.02 ±1.8 ±1 ±0.2 ±9 319 ±+9 NaCi repleted, n = t 35$ ±1.2 ±2 ±0.1 ± ±5 KHCO3 repleted, n = ±0O.01 ± 1.0 ±t1 ±0.2 ±12 46 * Values represent the mean ±SE of the mean. $ Differ from K deplete P < 0.01, using Student t test. Differ from KC1 replete P < 0.01, using Student t test. described (2). A portion of renal cortex, plus liver, gluteal muscle, and cardiac muscle tissue were placed in tared tubes for later determination of water and potassium content. A portion of gluteal muscle, cardiac muscle, and liver tissue was placed in weighed homogeniing tubes for determination of intracellular ph, as previously described (7). Analytic methods. Blood ph and PCo2 were measured on a Radiometer BM3 MK2 blood micro system at 37'C. Urine ph was measured using a Radiometer PMS27 ph meter. Bicarbonate concentration in the blood was calculated from the Henderson-Hasselbalch equation employing a pk of 6.1. Sodium and potassium were measured on an IL flame photometer while chloride, creatinine, and blood urea nitrogen (BUN) were determined by autoanalyer. Tissue water and electrolytes were determined by drying tissue to constant weight in a 700C oven for 16 h, as previously described (2). Citrate was determined enymatically (8), and ammonia was determined by microdiffusion (9). Triple isotope determination of tissue ph employed the methods described by Schloerb and Grantham (10). Radioactivity was measured in a three channel Packard liquid scintillation counter (Packard Instrument Co., Inc., Downers Grove, Ill.) using a AES standardiation and standard methods for determining efficiencies. RESULTS Effect of sodium chloride repletion on renal citrate metabolism and urinary ammonia excretion in KCldepleted rats. Extracellular acid-base status and serum and tissue potassium levels in the four groups of rats studied are shown in Table I. With the exception of the potassium chloride-repleted rats, metabolic alkalosis was present in each group. Compared to potassiumdepleted and potassium bicarbonate-repleted animals, however, the severity of the metabolic alkalosis was less in the sodium chloride-repleted rats, P < Thus, sodium chloride partially corrected the alkalosis, a result previously shown by other investigators in DOCA-induced potassium-deficient animals (1, 11). The furosemide and dietary KC1 restriction regimen induced a state of severe potassium depletion. Muscle potassium concentration was reduced 25% in each of the last three groups compared to values found both in the potassium chloride-repleted animals and normal values obtained in this and other laboratories (P < 0.001). Renal potassium concentration was also reduced but the degree of reduction, as expected, was less than in the muscle (1). It is important to note that despite administration of potassium bicarbonate serum and muscle potassium depletion was as severe as that found in sodium chloride and potassium-depleted animals who received no potassium. Administration of potassium bicarbonate, unlike potassium chloride, was unable to restore extracellular acid-base conditions, serum potassium concentration, or tissue potassium concentration to normal. The efficacy of sodium chloride administration in repleting chloride stores is shown both by the rise in serum chloride concentration from 80 to 94 meq/liter and by the large amounts of chloride in the urine of the NaCl-repleted rats (Table II). In addition to partially correcting the extracellular metabolic alkalosis, administration of sodium chloride also altered renal metabolism. As shown in Fig. 1 and 2 urinary citrate excretion fell while urinary ammonia excretion rose in the sodium chloride-repleted rats. Citrate excretion in the sodium chloride-repleted group, compared to each of the three other groups, differed significantly (P < 0.001) as did ammonia excretion (P < vs. K depleted and KHCOs and P < 0.02 compared to KCl-repleted animals). The alteration in Expansion, Renal Citrate, and Ammonia Metabolism in KCI Deficiency 393
4 TABLE It Arterial Blood Concentrations of Urea, Creatinine and Citrate, Urinary Chloride Excretion, and Renal Cortical Citrate Content in the Four Groups of Rats* Blood Urinary Urea chloride Renal cortical nitrogen Creatinine Citrate excretion citrate content g/100 ml g/100 ml pmol/ml meq/liter pmol/g wet weight KCI replete, n = $ t = O :0.060 K deplete, n = ±2.4 ± ± NaCl replete, n = t t ±t1.2 ± ±28 ±0.025 KHCO3 replete, n = t 4.2t 0.819t ±2.0 ±0.02 ±0.008 ±0.8 ±0.075 * Values represent the mean ±SE of the mean. $ Differ from deplete P < 0.01, using Student t test. Differ from KCI replete P < 0.01, using Student t test. citrate excretion in NaCl-repleted rats apparently was not secondary to changes in the filtered load of citrate. Table II shows that serum creatinine levels were not significantly different between groups, and only in the potassium-depleted group was BUN elevated (P < 0.01), indicating equal glomerular filtration rates in the several groups. Blood citrate levels were elevated only in the potassium bicarbonate rats so filtered citrate in each of the first three groups studied probably was the same. Increased citrate excretion in the KHCOs w co Q 44 E E i 14_ I o 12 w C.> 10 - w 8_ 0: co- M 2- Wo I KCI K-DEPLETE NaCI KHCO3 FIGURE 1 Urinary citrate excretion in the four groups of repleted rats. The brackets represent ±1 SE of the mean. 394 S. Adler, B. Zett, A. Anderson, and D. S. Fraley group is easily accounted for by the elevated filtered load. Evidence that the observed change in renal citrate metabolism in the NaCl-repleted rats was not due to filtered load is presented in the last column of Table II. The data show that in addition to the reduced citrate excretion, renal cortical citrate content also decreased significantly in the sodium chloride-repleted animals (P < vs. each of the three other groups). Sodium chloride given to KCl-deficient rats, therefore, induces changes in both renal citrate and ammonia metabolism. L~J w C-) E UV :1. 0 I- C.) x w 0- cr > rof cr- 3.0 F F I KCI K-DEPLETE NaCI ii KHCO3 FIGURE 2 Urinary ammonia excretion in the four groups of replqtqd rats. The brackets represent ±1 SE of the mean.
5 TABLE III Arterial Blood Values in KCO-Depleted Rats Infused with either Dextrose or Mannitol Solutions* ph PCO2 HCO3 Na C1 K Creatinine Citrate mm Hg meq/lifer meq/liter g/100 ml Mmol/ml Dextrose infused, n = 12 Initial ±0.02 ±3 i±1.8 Final ±40.02 ±3 ±1.6 ±2 ±2 ±0.1 ±t0.06 i0.007 Mannitol infused, n = 13 Initial ± ±1.0 ±4 ±0.1 Final : T < ±0.01 ±2 ± 1.3 ±-3 ±0.2 ±0.06 ±0.004 * Values represent the mean ±SE of the mean. : Value differs significantly from initial value (P < 0.05). Difference from final dextrose infused value statistically significant (P < 0.01). In an attempt to delineate the mechanism involved, further experiments were performed. 14CO5 production from [1,5-14C]citrate in vitro. The results of the previous experiments might be due to alterations in the concentration of serum chloride bathing the kidney. It has been shown that urinary citrate excretion is dependent on the ability of renal tubules to reabsorb filtered citrate (12), and this resorptive capacity is probably a function of the renal tubular cells ability to oxidie citrate accumulated intracellularly (4, 13). Measurement of citrate decarboxylation by renal cortical tissue at different external chloride concentrations should, therefore, partially describe chloride's effect upon renal citrate metabolism. Renal cortical slices were obtained from potassium chloride-deficient rats whose extracellular acid-base status and serum and tissue potassium concentrations were statistically identical to those of the potassium-depleted rats shown in Table I. The slices were incubated in low (70 meq) and "normal" (110 meq) chloride solutions and 14CO2 production was measured. The results are shown in Fig. 3. As "CO2 production is equal in the two groups it appears that chloride concentration does not directly affect renal citrate metabolism. To further examine the effect of chloride concentration on renal metabolism and to determine whether changes in extracellular volume might account for the observed results additional experiments were performed. Expansion of intravascular volume by mannitol in potassium-depleted rats: effect on renal citrate and ammonia metabolism. Mannitol infusions were given to potassium chloride-depleted animals. The effect of this infusion was to expand intravascular volume and lower serum chloride concentration. Another group of animals was given low volume dextrose infusion over a similar time period to serve as a control. Serum and tissue potassiuni concentrations in the animals before and after infusion were similar to that of the animals shown in Table I. Muscle potassium concentration after infusion in the dextrose and mannitol groups was 304 ±30 and 312±36 meq/kg of dry weight, respectively, compared to a muscle potassium concentration in nonpotassium chloride-depleted animals of 465 meq/kg dry weight (P < 0.001). Renal potassium content in the dextrose and mannitol rats was 267±26 and 252±32, 500 E 0, C-) Cr 100 0_ C-) NORMAL CHLORIDE LOW CHLORIDE FIGURE 3 The effect of medium chloride concentration on citrate decarboxylation by renal cortical slices. The numbers within the bars represent medium chloride concentrations in each group while the brackets depict ±1 SE of the mean. Expansion, Renal Citrate, and Ammonia Metabolism in KCl Deficiency 395
6 TABLE IV Creatinine Clearance, Urinary ph, and Renal Citrate Content in KCL-Depleted Rats Infused with either Dextrose or Mannitol Solutions* Ccr Urinary ph Renal cortical citrate content ml/min pmol/g wet weight Dextrose infused, n = ± Mannitol infused, n = 13 Control Experiment t 7.064± t14:0.005 Experiment Experiment t * Each value represents the mean±sem. Statistically significant differences from dextrose-infused animals (P < 0.05). Statistically significant differences from control values in mannitolinfused rats (P < 0.05). mannitol-infused on extracellular conditions, therefore, was similar to that previously shown in the rats chronically given so- The effect of the dextrose and mantlitol infusions on dium chloride. Serum bicarbonate concentration and extracellular conditions in potassium chloride-depleted arterial PCo2 fell, metabolic alkalosis was maintained, rats is shown in Table III. Arterial ph before and and blood citrate levels were unaffected. A significant after infusion was unchanged in either group. Mannitol difference is that mannitol infusion decreased the infusion had no effect on the serum potassium level serum chloride concentration while sodium chloride ad- 0.6F 0.4F TIME (MINUTES) FIGURE 4 The effect of mannitol-induced on urinary citrate excretion. The closed circle is the value however, shows that changes in filtered load do not obtained after 210 min of dextrose iinfusion while the account for the observed decrease in urinary citrate opened circles are values obtained in ti he mannitol-infused excretion. Glomerular filtration rate, calculated from rats. The dashed horiontal line representts the value for the the endogenous creatinine clearance, was increased durdextrose-infused group. The brackets de!pict ±1 SE of the t ing mannitol infusion compared both to control period mean. meq/kg dry weight, respectively, comlpared to 312±18 meq/kg of dry weight in nondepleted control animals. Muscle and renal potassium content did not signifi- cantly differ between dextrose and rats (P > 0.4). I-- w C., E -C cr- 0 I-- Z- 0 ẕ co TIME (MINUTES) FIGURE 5 The effect of mannitol-induced volume expansion on urinary ammonia excretion. Conventions in this figure are the same as for Fig. 4. but did lower extracellular bicarbonaate concentration ministration raised serum chloride concentration to significantly (P < 0.01). Neither serum creatinine nor normal levels. blood citrate levels were significantly affected by man- As was seen with sodium chloride ingestion, manniannitol infusions tol infusion lowered urinary citrate excretion. This is nitol infusion. The result of acute m shown in Fig. 4. Citrate excretion in each of the experimental periods is significantly reduced compared to control period values or to excretion in the dextroseinfused animals (P < 0.01). Fig. 5 shows the effect on 1.2 F 0 ammonia excretion. In this case, however, urinary x ;_- L.J 1.0 ammonia was significantly elevated in each of the i I- DEXTRO)SE mannitol experimental periods compared to control cr. LicK0: I- I 396 S. Adler, B. Zett, A. Anderson, and D. S. Fraley period values or to results obtained in dextrose-infused animals (P < 0.001). Citrate and ammonia excretions during the control period were similar in the two groups (P > 0.5). The alteration in citrate excretion after mannitol infusion is probably secondary to changes in the filtered load of citrate as experiments employing radioactive labeled citrate indicate that the citrate in the urine comes from extrarenal citrate (13). Thus, urinary citrate essentially represents the net sum of I volume expansion filtered citrate less reabsorbed citrate (14). Table IV,
7 TABLE V Skeletal Muscle, Cardiac, and Liver Cell ph in KCI-Depleted1Rats Infused with either Dextrose or Mannitol Solutions* Skeletal muscle Cardiac muscle Liver K phdxo K phdmo K PHDmo meq/kg dry weight meq/kg dry weight meq/kg dry weight Dextrose infused, n = : Mannitol infused, n = : :19 :1:0.02 4:8 ±0.02 * Values represent the mean ±SE of the mean. t Differs significantly from dextrose-infused animals (P < 0.01). values and to the filtration rate of the dextrose-infused animals. This increase was statistically significant only in periods two and four (P <0.05). This change in filtration rate combined with the slight increase in blood citrate (Table III) shows that filtered citrate increased rather than decreased during mannitol infusion. Thus, decreased urinary citrate excretion is probably due to increased tubular reabsorption of filtered citrate. Further proof that the alterations in urinary citrate excretion reflect altered renal citrate metabolism is given in Table IV. The data show that renal cortical citrate content in the mannitol-infused rats was significantly reduced compared to that found in the control rats (P < 0.001). Increased urinary ammonia excretion similarly appears to be secondary to changes in renal metabolism rather than to a nonspecific effect of the mannitol infusion. Urinary ammonia excretion ordinarily rises when urinary ph falls (15). The data presented in Table IV show that urinary ph rose rather than fell during mannitol infusion. The rise was not significant, however, compared to control period values or to values obtained in the dextrose-infused rats (P < 0.1). Thus, chronic NaCl ingestion and acute mannitol infusion in KCldepleted rats reduce renal cortical citrate content, lower urinary citrate excretion, and increase urinary ammonia excretion. Effect of mannitol-induced volume expansion on muscle, liver, and cardiac ph. Although renal tubular cell ph cannot be directly determined in vivo, skeletal muscle, liver, and cardiac muscle cell ph may be calculated from distribution of the weak acid 5,5-dimethyl- 2,-4-oxaolidinedione (DMO) (16). Table V shows the effect of mannitol infusion on the intracellular ph of these three tissues. Extracellular potassium concentration was approximately 2.5 meq/liter (Table III) in these animals. The concentration of potassium in each of the three tissues (Table V) demonstrates that a higher intracellular to extracellular gradient existed at the conclusion of the infusion in each of the tissues studied. Membrane selectivity was thus maintained and the DMO distribution method for measuring cell ph may be employed (16). It is apparent that mannitol infusion did not lower intracellular ph in any of the three tissues. Indeed, there was a tendency in skeletal and cardiac muscle for intracellular ph to increase. This increase was not statistically significant (P > 0.05). As shown in Table III, the slight elevation of cell ph cannot be due to a change in extracellular ph for final arterial blood ph in the mannitol-infused rats was slightly lower than the final blood ph of the dextrose-infused animals. The rise in cell ph might be explained by the decreased C02 tension in the mannitol-infused rats since carbon dioxide readily penetrates cell membranes and might influence cell ph more than bicarbonate (17). Citrate content was determined in liver and gluteal muscle tissue. No differences were found between dextrose and mannitol-infused animals. This is consistent with the absence of significant intracellular ph differences since tissue citrate content apparently is a function of intracellular ph (5). DISCUSSION These experiments extend previous work which demonstrated that administration of sodium chloride to potassium chloride-deficient rats alters renal citrate metabolism (1). Whether sodium chloride altered renal citrate metabolism by changing extracellular chloride concentration or extracellular volume was not, however, examined in the earlier study. The present results show that the alteration in renal citrate metabolism is not due to changes in serum chloride concentration since volume expansion with mannitol had the same effect as sodium chloride on extracellular acid base status and renal citrate metabolism despite having an opposite effect on serum chloride concentration. Rather, it appears that increased circulating volume is responsible Expansion, Renal Citrate, and Ammonia Metabolism in KCI Deficiency 397
8 for the altered renal metabolism. Although circulating volume was not measured in the tube fed rats, weight loss in the NaCl-repleted animals over the 3 days of feeding was 10 g less than in K-depleted or KHCOa rats. There was sufficient scatter in the data to make this difference nonsignificant (P> 0.05, <0.1). Most studies, however, show only small increases or decreases in extracellular volume after chloride repletion or depletion (18), so the inability to obtain significant data under the conditions of the present experiments is not surprising. In addition, expansion of extracellular volume in rats decreases renal bicarbonate reabsorptive capacity (19). Thus, the increase in urinary ph during mannitol infusion and the fall in serum bicarbonate after NaCl mannitol administration is consistent with an increased circulating volume. In addition to its effect on renal citrate metabolism volume expansion increased ammonia excretion. The latter probably reflects increased renal ammonia production for two reasons. First, the increase was accompanied by a simultaneous rise in urine ph in the mannitol-infused rats. PNHI3 is in equilibrium between peritubular and luminal fluid (20), so the concentration of total urinary ammonia (NH8 plus NH4+) varies directly with the ph gradient between peritubular and luminal fluid; the greater the gradient the higher the urinary ammonia excretion. As blood ph was constant the gradient must have fallen. Less of the relatively nondiffusable NH4+ is then trapped in the tubular lumen, and urinary ammonia excretion should fall if renal ammonia production is constant (15, 21). Elevated urinary ammonia in these circumstances indicates increased renal ammonia production. This mechanism probably explains the decreased ammonia excretion seen in the rats given potassium bicarbonate. A second reason why increased production is probably responsible for the increased ammonia excretion is that renal blood flow and glomerular filtration rate ordinarily rise after volume expansion (22). Endogenous creatinine clearance increased in the rats infused with mannitol so renal blood flow probably also increased. As peritubular blood traps ammonia increased renal blood flow should increase ammonia transport into renal venous blood and decrease the amount of ammonia available for urinary excretion especially when the urine is only weakly acid and urine flow rate is high. Increased renal blood flow plus a rise in urinary ph indicate that elevated urinary ammonia excretion after NaCl or mannitol administration is due, therefore, to increased renal ammonia production. The volume expansion-induced alterations in renal citrate and ammonia metabolism cannot, obviously, be explained by changes in chloride concentration or by a nonspecific effect of mannitol. Three possibilities, not 398 S. Adler, B. Zett, A. Anderson, and D. S. Fraley mutually exclusive, may explain the results: the renal metabolic changes could be due to the observed isohydric reduction in extracellular bicarbonate; they could be secondary to a decrease in renal tubular cell potassium concentration; or the changes could represent a response to a reduction in renal tubular cell ph. Supporting the first possibility are the many studies showing that varying extracellular bicarbonate concentration at a constant extracellular ph alters intermediary metabolism in a variety of tissues (23, 24). Indeed, Kamm, Fruis, Goodman, and Cahill (25) have shown that gluconeogenesis in rat renal cortical slices varies inversely with the bicarbonate concentration of the medium at a constant extracellular ph of Since ammonia and glucose production by rat renal cortical slices obtained from metabolically acidotic animals is greater than that seen in slices obtained from alkalotic animals (26), it is possible the increased ammonia production in rats given NaCl or mannitol is due to the decreased bicarbonate concentration. Citrate metabolism also appears to be bicarbonate dependent. Adler (27) has demonstrated that the citrate content of rat diaphragm muscle is decreased in an iso-ph system when bicarbonate concentration is lowered. Furthermore, Simpson (4) has shown that under isohydric conditions in vitro citrate decarboxylation by renal mitochondria proceeds more rapidly as bicarbonate concentration is progressively lowered. This apparently is due to increased transport of citrate into the mitochondria (28). Increased citrate decarboxylation intramitochondrially would decrease renal citrate content and increase reabsorption of citrate, the results observed in the present study. Other work of Simpson (4), however, is not in accord with his observations in isolated mitochondria. Rabbit renal cortical slices incubated at a constant external ph of 7.39 and at bicarbonate concentrations varying from 10 to 50 meq/ liter showed identical citrate decarboxylation rates. These slices, however, were quite responsive to alterations in external ph as the citrate decarboxylation rate varied inversely with the ph of the bathing medium. If the whole kidney responds like the slice then a change in bicarbonate concentration occurring isohydrically would not be expected to alter renal citrate metabolism. The exact role of the decreased bicarbonate concentration, therefore, remains unclear. A second possibility is that the altered renal metabolism is a consequence of altered intracellular potassium concentration. When normal animals are made alkalotic, urinary citrate excretion (29) and renal citrate content (2) increase while renal ammonia production and excretion decrease (30). In potassium deficiency, however, urinary citrate excretion falls (12), and urinary ammonia excretion rises (30) despite the presence
9 of an extracellular alkalosis. This has been attributed to coexisting intracellular acidosis and extracellular alkalosis in potassium depletion. Because the changes in ph are accompanied by a fall in tissue potassium concentration, some investigators believe that the renal metabolic alterations are due to decreased intracellular potassium concentration rather than renal intracellular acidosis. In the present experiment, however, renal potassium content did not decrease further after sodium chloride ingestion or mannitol infusion. In addition, Adler, Anderson, and Zett (5) have shown that the citrate content of potassium-depleted rat diaphragm muscle in vitro is a function of cell ph (calculated from DMO distribution) and not the tissue potassium content. Thus, a fall in diaphragm muscle cell ph is accompanied by a decrease in tissue citrate content without any change in cellular potassium. It seems unlikely, therefore, that changes in intrarenal potassium concentration account for the renal metabolic alterations although changes in potassium concentration within specific areas of the cell cannot be ruled out. Finally, the alterations in renal citrate and ammonia metabolism may reflect reduced renal tubular cell ph. Acidosis apparently increases renal decarboxylation of exogenous citrate leading to a decrease in urinary citrate excretion and renal citrate content while the converse occurs in alkalosis (4). The observed changes in renal citrate content and excretion thus are compatible with renal cell acidosis. Indeed, Crawford, Milne, and Scribner (12), as well as other investigators (28), have postulated that renal citrate metabolism reflects intracellular ph, using as supporting evidence for this hypothesis the decreased citrate excretion and renal cortical citrate content found in potassium deficiency alkalosis (12) and the known decrease in muscle cell ph which occurs simultaneously (31, 32). In muscle there is direct evidence supporting the concept that citrate metabolism is a function of cell ph not of extracellular acidity. Rats acutely administered potassium chloride intraperitoneally develop extracellular metabolic acidosis, intracellular metabolic alkalosis, and elevated skeletal muscle citrate content (33). Ordinarily, tissue citrate decreases in acidosis (2), so the 'change in citrate content presumably reflects the intracellular not the extracellular ph state. Also, as men-,tioned previously, Adler and coworkers (5) have been able to relate citrate metabolism in diaphragm muscle to intracellular ph, not to cell potassium content or extracellular ph. Renal ammonia production is also felt to reflect intracellular rather than extracellular ph conditions. As is the case with renal citrate metabolism this hypothesis is based on indirect evidence obtained in abnormal metabolic states since renal cell ph has only been determined in an isolated renal tubular cell preparation (34). Most investigators agree, nevertheless, that both hydrogen ion secretion and renal ammonia production are regulated by intracellular ph. Both, for example, are increased in potassium deficiency despite a concomitant metabolic alkalosis (35). Also glutamine oxidation and presumably ammonia production in renal cortical slices and mitochondria is directly related to changes in ph (36). Finally, Lowance, Garfinkel, Mattern, and Schwart (37) demonstrated that hypotonic volume expansion in dogs with chronic metabolic acidosis increases net acid excretion and restores plasma bicarbonate concentration to normal. The increased net acid excretion probably was due in part to an increase in ammonia excretion. Although it is unclear how volume expansion might decrease renal cell ph, the data are consistent with the hypothesis that volume expansion lowers renal tubular cell ph. ACKNOWLEDGMENTS The authors wish to thank Mrs. Elaine R. New for her assistance in manuscript preparation. This work was supported in part by United States Public Health Service Grant HL REFERENCES 1. Adler, S., B. Zett, and B. Anderson Renal citrate in the potassium-deficient rat: role of potassium and chloride ions. J. Lab. Clin. Med. 84: Adler, S., B. Anderson, and L. Zemotel Metabolic acid-base effects on tissue citrate content and metabolism in the rat. Am. J. Physiol. 220: Ostberg, Studien uber die Zitronen saureausscherdung der Menschennisre in normalen and patholgischen Zustanden. Skand. Arch. Physiol. 62: Simpson, D. P Regulation of renal citrate metabolism by bicarbonate ion and ph. Observations in tissue slices and mitochondria. J. Clin. Invest. 46: Adler, S., B. Anderson, and B. Zett Regulation of citrate metabolism by cell ph in potassium-depleted rat diaphragm. Kidney Int. 6: Waddell, W. J., and R. G. Bates Intracellular ph. Physiol. Rev. 49: Adler, S., A. Roy, and A. S. Relman Intracellular acid-base regulation. I. The response of muscle cells to changes in CO2 tension or extracellular bicarbonate concentration. J. Clin. Invest. 44: Moellering, H., and W. Gruber Determination of citrate with citrate lyase. Anal. Biochem. 17: Conway, E. J., and E. O'Malley Microdiffusion methods. Ammonia and urea using buffered absorbants. Biochem. J. 36: Schloerb, P. R., and J. J. Grantham Intracellular ph measurement with tritiated water, carbon-14 labeled 5,5-dimethyl-2,4-oxaolidinedione, and chloride-36. J. Lab. Clin. Med. 65: Luke, R. G., and H. Levitin Impaired renal conservation of chloride and the acid-base changes associated with potassium depletion in the rat. Clin. Sci. (Of.). 32: Crawford, M. A., M. D. Milne, and B. H. Scribner The effects of changes in acid-base balance on Expansion, Renal Citrate, and Ammonia Metabolism in KCI Deficiency 399
10 urinary citrate in the rat. J. Physiol. (Lond.). 149: Gordon, E. E The metabolism of citrate-1'c in normal and in fluoro inhibitor-poisoned rats. J. Clin. Invest. 40: Grollman, A. P., H. C. Harrison, and H. E. Harrison The renal excretion of citrate. J. Clin. Invest. 40: Orloff, J., and R. W. Berliner The mechanism of the excretion of ammonia in the dog. J. Clin. Invest. 35: Waddell, W. J., and T. C. Butler Calculation of intracellular ph from the distribution of 5,5-dimethyl- 2,4-oxaolidinedione (DMO). Application to skeletal muscle of the dog. J. Clin. Invest. 38: Adler, S., A. Roy, and A. S. Relman Intracellular acid-base regulation. II. The interaction between CO2 tension and extracellular bicarbonate in the determination of muscle cell ph. J. Clin. Invest. 44: Atkins, E. L., and W. B. Schwart Factors governing correction of the alkalosis associated with potassium deficiency; the critical role of chloride in the recovery process. J. Clin. Invest. 41: Purkerson, M. L., H. Lubowit, R. W. White, and N. S. Bricker On the influence of extracellular fluid volume expansion on bicarbonate reabsorption in the rat. J. Clin. Invest. 48: Denis, G., H. Preuss, and R. Pitts The PNHS of renal tubular cells. J. Clin. Invest. 43: Milne, M. D., B. H. Schribner, and M. A, Crawford. 1958, Nonionic diffusion and the excretion of weak acids and bases. Am. J. Med. 24: Earley, L. E., and R. M. Friedler Changes in renal blood flow and possibly the intrarenal distribution of blood during natriuresis accompanying saline loading in the dog. J. Clin. Invest. 44: Katman, R., C. A. Villee, and H. K. Beecher Effect of increased carbon dioxide concentrations on fixed acid production in vitro. Am. J. Physiol. 172: Longmore, W. J., A. B. Hastings, and T. A. Mahowald Effect of environmental C02 and ph on glycerol metabolism by rat liver in vitro. J. Biol. Chem. 239: Kamm, D. E., R. E. Fuis, A. D. Goodman, and G. F. Cahill, Jr Acid-base alterations and renal gluconeogenesis: effect of ph, bicarbonate concentration, and Pco2. J. Clin. Invest. 46: Goodman, A. D., R. E. Fuis, and G. F. Cahill, Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production J. Clin. Invest. 45: Adler, S The role of ph, Pco2 and bicarbonate in regulating rat diaphragm citrate content. J. Clin. Invest. 49: Simpson, D. P., and S. Angielski Regulation by bicarbonate ion of intramitochondrial citrate concentration in kidney mitochondria. Biochim. Biophys. Acta. 298: Simpson, D. P Influence of plasma bicarbonate concentration and ph on citrate excretion. Am. J. Physiol. 206: Balagura-Baruch, S Renal metabolism and transfer of ammonia. In The Kidney. C. Rouiller and A. F. Muller, editors. Academic Press, Inc, New York. 3: Cooke, R. E., W. E. Segar, D. B. Cheek, F. E. Colville, and D. C. Darrow The extrarenal correction of alkalosis associated with potassium deficiency. J. Clin. Invest. 31: Adler, S., B. Zett, and B. Anderson The effect of acute potassium depletion on muscle cell ph in vitro. Kidney Int. 2: Hudson, J. B., and A. S. Relman Effects of potassium and rubidium on muscle cell bicarbonate. Am. J. Physiol. 203: Struyvenberg, A., R. B. Morrison, and A. S. Relman Acid-base behaviour of separated canine renal tubular cells. Am. J. Physiol. 214: Huth, E. J., R. D. Squires, and J. R. Elkinton Experimental potassium depletion in normal human subjects. II. Renal and hormonal factors in the development of extracellular alkalosis during depletion. J. Clin. Invest. 38: Simpson, D. P., and J. D. Sherrard Regulation of glutamine metabolism in vitro by bicarbonate ion and ph. J. Clin. Invest. 48: Lowance, D. C., H. B. Garfinkel, W. D. Mattern, and W. B. Schwart The effect of chronic hypotonic volume expansion on the renal regulation of acid-base equilibrium. J. Clin. Invest. 51: S. Adler, B. Zett, A. Anderson, and D. S. Fraley
Acid-Base Alterations and Renal Gluconeogenesis: Effect
 Journal of Clinical Investigation Vol. 46, No. 7, 1967 Acid-Base Alterations and Renal Gluconeogenesis: Effect of ph, Bicarbonate Concentration, and Pco2 * DONALD E. KAMM,t ROBERT E. Fuisz,4 A. DAVID GOODMAN,
Journal of Clinical Investigation Vol. 46, No. 7, 1967 Acid-Base Alterations and Renal Gluconeogenesis: Effect of ph, Bicarbonate Concentration, and Pco2 * DONALD E. KAMM,t ROBERT E. Fuisz,4 A. DAVID GOODMAN,
The kidney. (Pseudo) Practical questions. The kidneys are all about keeping the body s homeostasis. for questions Ella
 The kidney (Pseudo) Practical questions for questions Ella (striemit@gmail.com) The kidneys are all about keeping the body s homeostasis Ingestion Product of metabolism H 2 O Ca ++ Cl - K + Na + H 2 O
The kidney (Pseudo) Practical questions for questions Ella (striemit@gmail.com) The kidneys are all about keeping the body s homeostasis Ingestion Product of metabolism H 2 O Ca ++ Cl - K + Na + H 2 O
Acid Base Balance. Professor Dr. Raid M. H. Al-Salih. Clinical Chemistry Professor Dr. Raid M. H. Al-Salih
 Acid Base Balance 1 HYDROGEN ION CONCENTRATION and CONCEPT OF ph Blood hydrogen ion concentration (abbreviated [H + ]) is maintained within tight limits in health, with the normal concentration being between
Acid Base Balance 1 HYDROGEN ION CONCENTRATION and CONCEPT OF ph Blood hydrogen ion concentration (abbreviated [H + ]) is maintained within tight limits in health, with the normal concentration being between
The Induction of Metabolic Alkalosis by Correction of Potassium Deficiency *
 lournal of Clinical Investigation Vol. 45, No. 4, 1966 The Induction of Metabolic Alkalosis by Correction of Potassium Deficiency * HOWARD L. BLEICH,t RICHARD L. TANNEN,t AND WILLIAM B. SCHWARTZ t (From
lournal of Clinical Investigation Vol. 45, No. 4, 1966 The Induction of Metabolic Alkalosis by Correction of Potassium Deficiency * HOWARD L. BLEICH,t RICHARD L. TANNEN,t AND WILLIAM B. SCHWARTZ t (From
rapidity. It has also been reported that addition
 THE ACIDIFYING EFFECT OF RUBIDIUM IN NORMAL AND POTASSIUM-DEFICIENT ALKALOTIC RATS 1 By ARNOLD S. RELMAN, ARLENE M. ROY, AND WILLIAM B. SCHWARTZ (From the Evans Memorial, Massachusetts Memorial Hospitals,
THE ACIDIFYING EFFECT OF RUBIDIUM IN NORMAL AND POTASSIUM-DEFICIENT ALKALOTIC RATS 1 By ARNOLD S. RELMAN, ARLENE M. ROY, AND WILLIAM B. SCHWARTZ (From the Evans Memorial, Massachusetts Memorial Hospitals,
adam.com (http://www.adam.com/) Benjamin/Cummings Publishing Co (http://www.awl.com/bc) -42-
 Graphics are used with permission of : adam.com (http://www.adam.com/) Benjamin/Cummings Publishing Co (http://www.awl.com/bc) -42-74. (1) Carbon dioxide arrives at the kidney tubule cell in the proximal
Graphics are used with permission of : adam.com (http://www.adam.com/) Benjamin/Cummings Publishing Co (http://www.awl.com/bc) -42-74. (1) Carbon dioxide arrives at the kidney tubule cell in the proximal
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
CASE 27. What is the response of the kidney to metabolic acidosis? What is the response of the kidney to a respiratory alkalosis?
 CASE 27 A 21-year-old man with insulin-dependent diabetes presents to the emergency center with mental status changes, nausea, vomiting, abdominal pain, and rapid respirations. On examination, the patient
CASE 27 A 21-year-old man with insulin-dependent diabetes presents to the emergency center with mental status changes, nausea, vomiting, abdominal pain, and rapid respirations. On examination, the patient
STUDY ON MECHANISM OF ACID AND AMMONIA EXCRETION BY KIDNEY AFTER ACID LOAD. Hisato YOSHIMURA, Mamoru FUJIMOTO AND Junichi SUGIMOTO
 STUDY ON MECHANISM OF ACID AND AMMONIA EXCRETION BY KIDNEY AFTER ACID LOAD Hisato YOSHIMURA, Mamoru FUJIMOTO AND Junichi SUGIMOTO Department of Physiology, Kyoto Prefectural University of Medicine, Kyoto
STUDY ON MECHANISM OF ACID AND AMMONIA EXCRETION BY KIDNEY AFTER ACID LOAD Hisato YOSHIMURA, Mamoru FUJIMOTO AND Junichi SUGIMOTO Department of Physiology, Kyoto Prefectural University of Medicine, Kyoto
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
only. Analysis of human muscle obtained by biopsy suggests that changes
 A PROPOSED MECHANISM OF EXTRACELLLAR REGLATION OF MSCLE COMPOSITION* ROBERT E. COOKE** AND WILLIAM E. SEGAR The changes in muscle composition which occur in disturbances of extracellular electrolyte composition
A PROPOSED MECHANISM OF EXTRACELLLAR REGLATION OF MSCLE COMPOSITION* ROBERT E. COOKE** AND WILLIAM E. SEGAR The changes in muscle composition which occur in disturbances of extracellular electrolyte composition
to assess the renal reabsorptive capacity after restoration of normal carbon dioxide tension in
 Journal of Clinical nvestigation Vol. 41, No. 12, 1962 EFFECTS OF CHRONC HYPERCAPNA ON ELECTROLYTE AND ACD-BASE EQULBRUM.. CHARACTERSTCS OF THE ADAPTVE AND RECOVERY PROCESS AS EVALUATED BY PROVSON OF ALKAL
Journal of Clinical nvestigation Vol. 41, No. 12, 1962 EFFECTS OF CHRONC HYPERCAPNA ON ELECTROLYTE AND ACD-BASE EQULBRUM.. CHARACTERSTCS OF THE ADAPTVE AND RECOVERY PROCESS AS EVALUATED BY PROVSON OF ALKAL
Diffusion Equilibrium for Ammonia in the Kidney of the Acidotic Dog *
 The Journal of Clinical Investigation Vol. 46, No. 10, 1967 Diffusion Equilibrium for Ammonia in the Kidney of the Acidotic Dog * WILLIAM J. STONE,4 SULAMITA BALAGURA, AND ROBERT F. PITTS WITH THE TECHNICAL
The Journal of Clinical Investigation Vol. 46, No. 10, 1967 Diffusion Equilibrium for Ammonia in the Kidney of the Acidotic Dog * WILLIAM J. STONE,4 SULAMITA BALAGURA, AND ROBERT F. PITTS WITH THE TECHNICAL
Acids and Bases their definitions and meanings
 Acids and Bases their definitions and meanings Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. (HCl H + Cl ) (H 2 CO 3 H + HCO 3 ) A base is an
Acids and Bases their definitions and meanings Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. (HCl H + Cl ) (H 2 CO 3 H + HCO 3 ) A base is an
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
Other Factors Affecting GFR. Chapter 25. After Filtration. Reabsorption and Secretion. 5 Functions of the PCT
 Other Factors Affecting GFR Chapter 25 Part 2. Renal Physiology Nitric oxide vasodilator produced by the vascular endothelium Adenosine vasoconstrictor of renal vasculature Endothelin a powerful vasoconstrictor
Other Factors Affecting GFR Chapter 25 Part 2. Renal Physiology Nitric oxide vasodilator produced by the vascular endothelium Adenosine vasoconstrictor of renal vasculature Endothelin a powerful vasoconstrictor
Answers and Explanations
 Answers and Explanations 1. The answer is D [V B 4 b]. Distal K + secretion is decreased by factors that decrease the driving force for passive diffusion of K + across the luminal membrane. Because spironolactone
Answers and Explanations 1. The answer is D [V B 4 b]. Distal K + secretion is decreased by factors that decrease the driving force for passive diffusion of K + across the luminal membrane. Because spironolactone
Objectives. Blood Buffers. Definitions. Strong/Weak Acids. Fixed (Non-Volatile) Acids. Module H Malley pages
 Blood Buffers Module H Malley pages 120-126 Objectives Define a buffer system and differentiate between the buffering systems present in the body. Given an arterial blood-gas result, determine the degree
Blood Buffers Module H Malley pages 120-126 Objectives Define a buffer system and differentiate between the buffering systems present in the body. Given an arterial blood-gas result, determine the degree
meq of sodium, less than 1 meq of chloride, and 0.1 meq
 Journal of Clinical Investigation Vol. 4, No., 96 ON THE MECHANISM OF NITRATE-INDUCED ALKALOSIS. THE POSSIBLE ROLE OF SELECTIVE CHLORIDE DEPLETION IN ACID-BASE REGULATION * By PAUL F. GULYASSY,t CHARLES
Journal of Clinical Investigation Vol. 4, No., 96 ON THE MECHANISM OF NITRATE-INDUCED ALKALOSIS. THE POSSIBLE ROLE OF SELECTIVE CHLORIDE DEPLETION IN ACID-BASE REGULATION * By PAUL F. GULYASSY,t CHARLES
(5), the presumed site of potassium secretion. RENAL EXCRETION OF POTASSIUM IN NORMAL AND SODIUM
 RENAL EXCRETION OF POTASSIUM IN NORMAL AND SODIUM DEPLETED DOGS' By HELEN M. ANDERSON AND JOHN H. LARAGH WITH THE TECHNICAL ASSISTANCE OF CLARA W. HALL, SALLY MOORE, AND JOSEPH HARTOG (From the Department
RENAL EXCRETION OF POTASSIUM IN NORMAL AND SODIUM DEPLETED DOGS' By HELEN M. ANDERSON AND JOHN H. LARAGH WITH THE TECHNICAL ASSISTANCE OF CLARA W. HALL, SALLY MOORE, AND JOSEPH HARTOG (From the Department
EXCRETION QUESTIONS. Use the following information to answer the next two questions.
 EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
Nephron Structure inside Kidney:
 In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
Chapter 26 Fluid, Electrolyte, and Acid- Base Balance
 Chapter 26 Fluid, Electrolyte, and Acid- Base Balance 1 Body Water Content Infants: 73% or more water (low body fat, low bone mass) Adult males: ~60% water Adult females: ~50% water (higher fat content,
Chapter 26 Fluid, Electrolyte, and Acid- Base Balance 1 Body Water Content Infants: 73% or more water (low body fat, low bone mass) Adult males: ~60% water Adult females: ~50% water (higher fat content,
simultaneously excreted. They also brought forward some evidence to
 THE EXCRETION OF CHLORIDES AND BICARBON- ATES BY THE HUMAN KIDNEY. BY H. W. DAVIES, M.B., B.S., J. B. S. HALDANE, M.A. AND G. L. PESKETT, B.A. (From the Laboratory, Cherwell, Oxford.) AM BARD and PAPI
THE EXCRETION OF CHLORIDES AND BICARBON- ATES BY THE HUMAN KIDNEY. BY H. W. DAVIES, M.B., B.S., J. B. S. HALDANE, M.A. AND G. L. PESKETT, B.A. (From the Laboratory, Cherwell, Oxford.) AM BARD and PAPI
HUMAN SUBJECT 1. Syracuse, N. Y.) the urine of increasing quantities of these buffers, it has been found in man as in the dog that (1)
 THE RENAL REGULATION OF ACID-BASE BALANCE IN MAN. II. FACTORS AFFECTING THE EXCRETION OF TITRATABLE ACID BY THE NORMAL HUMAN SUBJECT 1 By W. A. SCHIESS, J. L. AYER, W. D. LOTSPEICH AND R. F. PITTS WITH
THE RENAL REGULATION OF ACID-BASE BALANCE IN MAN. II. FACTORS AFFECTING THE EXCRETION OF TITRATABLE ACID BY THE NORMAL HUMAN SUBJECT 1 By W. A. SCHIESS, J. L. AYER, W. D. LOTSPEICH AND R. F. PITTS WITH
The Excretory System. Biology 20
 The Excretory System Biology 20 Introduction Follow along on page 376 What dangers exist if your body is unable to regulate the fluid balance of your tissues? What challenged would the body have to respond
The Excretory System Biology 20 Introduction Follow along on page 376 What dangers exist if your body is unable to regulate the fluid balance of your tissues? What challenged would the body have to respond
Chapter 15 Fluid and Acid-Base Balance
 Chapter 15 Fluid and Acid-Base Balance by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Fluid Balance Water constitutes ~60% of body weight. All cells and tissues are surrounded by an aqueous environment.
Chapter 15 Fluid and Acid-Base Balance by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Fluid Balance Water constitutes ~60% of body weight. All cells and tissues are surrounded by an aqueous environment.
estimates were made of the normal rate of increase in plasma urea over periods in skin and in plasma, hypertonic sodium chloride solution was
 482 J. Physiol. (I95I) II5, 482-487 THE STTE OF BODY WTER IN THE CT BY M. GRCE EGGLETON From the Department of Physiology, University College, London (Received 5 July 1951) In the course of an investigation
482 J. Physiol. (I95I) II5, 482-487 THE STTE OF BODY WTER IN THE CT BY M. GRCE EGGLETON From the Department of Physiology, University College, London (Received 5 July 1951) In the course of an investigation
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + )
 Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration in body fluids Precise regulation of ph at
Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration in body fluids Precise regulation of ph at
Excretory System 1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
D fini n tion: p = = -log [H+] ph=7 me m an s 10-7 Mol M H+ + (100 nmol m /l); ) p ; H=8 me m an s 10-8 Mol M H+ + (10 (10 n nmol m /l) Nor
![D fini n tion: p = = -log [H+] ph=7 me m an s 10-7 Mol M H+ + (100 nmol m /l); ) p ; H=8 me m an s 10-8 Mol M H+ + (10 (10 n nmol m /l) Nor D fini n tion: p = = -log [H+] ph=7 me m an s 10-7 Mol M H+ + (100 nmol m /l); ) p ; H=8 me m an s 10-8 Mol M H+ + (10 (10 n nmol m /l) Nor](/thumbs/75/72756397.jpg) Definition: ph regulation ph = -log [H + ] ph=7 means 10-7 Mol H + (100 nmol/l); ph=8 means 10 Normal plasma value: 7.35-7.45; 7.45; (H Acidosis: ph7.45 Intracellular ph = 7.1-7.3
Definition: ph regulation ph = -log [H + ] ph=7 means 10-7 Mol H + (100 nmol/l); ph=8 means 10 Normal plasma value: 7.35-7.45; 7.45; (H Acidosis: ph7.45 Intracellular ph = 7.1-7.3
ELECTROLYTE DISTURBANCES IN CONGESTIVE HEART FAILURE*
 ELECTROLYTE DISTURBANCES IN CONGESTIVE HEART FAILURE* DAVID P. B.AUMANN, M.D. Department of Medicine, University of Arkansas School of Medicine, Little Rock, Arkansas The retention of salt and water secondary
ELECTROLYTE DISTURBANCES IN CONGESTIVE HEART FAILURE* DAVID P. B.AUMANN, M.D. Department of Medicine, University of Arkansas School of Medicine, Little Rock, Arkansas The retention of salt and water secondary
Acid - base equilibrium
 Acid base equilibrium ph concept ph = log [H + ] ph [H+] 1 100 mmol/l D = 90 mmol/l 2 10 mmol/l D = 9 mmol/l 3 1 mmol/l 2 ph = log [H + ] 3 ph ph = log [H + ] ph of capillary blood norm: 7,35 7,45 Sorensen
Acid base equilibrium ph concept ph = log [H + ] ph [H+] 1 100 mmol/l D = 90 mmol/l 2 10 mmol/l D = 9 mmol/l 3 1 mmol/l 2 ph = log [H + ] 3 ph ph = log [H + ] ph of capillary blood norm: 7,35 7,45 Sorensen
RENAL FUNCTION An Overview
 RENAL FUNCTION An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ. Temple 1 Kidneys
RENAL FUNCTION An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ. Temple 1 Kidneys
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
BIOL 2402 Fluid/Electrolyte Regulation
 Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Acid-Base Balance Dr. Gary Mumaugh
 Acid-Base Balance Dr. Gary Mumaugh Introduction Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration
Acid-Base Balance Dr. Gary Mumaugh Introduction Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration
Introduction. Acids, Bases and ph; a review
 0 P a g e Introduction In this sheet, we discuss acidbase balance in our body and the role of kidneys in its establishment. Arrangement of topics is different from that of the lecture, to assure consistency
0 P a g e Introduction In this sheet, we discuss acidbase balance in our body and the role of kidneys in its establishment. Arrangement of topics is different from that of the lecture, to assure consistency
Acid-Base Balance 11/18/2011. Regulation of Potassium Balance. Regulation of Potassium Balance. Regulatory Site: Cortical Collecting Ducts.
 Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
Nephron Function and Urine Formation. Ms. Kula December 1, 2014 Biology 30S
 Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
12/7/10. Excretory System. The basic function of the excretory system is to regulate the volume and composition of body fluids by:
 Excretory System The basic function of the excretory system is to regulate the volume and composition of body fluids by: o o removing wastes returning needed substances to the body for reuse Body systems
Excretory System The basic function of the excretory system is to regulate the volume and composition of body fluids by: o o removing wastes returning needed substances to the body for reuse Body systems
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Hyperaldosteronism: Conn's Syndrome
 RENAL AND ACID-BASE PHYSIOLOGY 177 Case 31 Hyperaldosteronism: Conn's Syndrome Seymour Simon is a 54-year-old college physics professor who maintains a healthy lifestyle. He exercises regularly, doesn't
RENAL AND ACID-BASE PHYSIOLOGY 177 Case 31 Hyperaldosteronism: Conn's Syndrome Seymour Simon is a 54-year-old college physics professor who maintains a healthy lifestyle. He exercises regularly, doesn't
Use the following diagram to answer the next question. 1. In the diagram above, pressure filtration occurs in a. W b. X c. Y d. Z
 Part A: Multiple Choice Questions Value: 32 Marks Suggested time: 40 minutes Instructions: For each question select the best answer and record your choice on the Scantron card provided. Using an HB pencil,
Part A: Multiple Choice Questions Value: 32 Marks Suggested time: 40 minutes Instructions: For each question select the best answer and record your choice on the Scantron card provided. Using an HB pencil,
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 27 Fluid, Electrolyte, and Acid Base Fluid Compartments and Fluid In adults, body fluids make up between 55% and 65% of total body mass. Body
Principles of Anatomy and Physiology 14 th Edition CHAPTER 27 Fluid, Electrolyte, and Acid Base Fluid Compartments and Fluid In adults, body fluids make up between 55% and 65% of total body mass. Body
RENAL TUBULAR ACIDOSIS An Overview
 RENAL TUBULAR ACIDOSIS An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY CLINICAL BIOCHEMISTRY PBL MBBS IV VJ. Temple 1 What is Renal Tubular
RENAL TUBULAR ACIDOSIS An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY CLINICAL BIOCHEMISTRY PBL MBBS IV VJ. Temple 1 What is Renal Tubular
A&P 2 CANALE T H E U R I N A R Y S Y S T E M
 A&P 2 CANALE T H E U R I N A R Y S Y S T E M URINARY SYSTEM CONTRIBUTION TO HOMEOSTASIS Regulates body water levels Excess water taken in is excreted Output varies from 2-1/2 liter/day to 1 liter/hour
A&P 2 CANALE T H E U R I N A R Y S Y S T E M URINARY SYSTEM CONTRIBUTION TO HOMEOSTASIS Regulates body water levels Excess water taken in is excreted Output varies from 2-1/2 liter/day to 1 liter/hour
had no effect on the production of aldosterone, corticosterone, or cortisol after
 INHIBITION OF THE EFFECTS OF ANGIOTENSIN II ON ADRENAL STEROID PRODUCTION BY DIETARY SODIUM BY WARREN W. DAVIS,* LAWRENCE R. BURWELL,t AND FREDERIC C. BARTTERt ENDOCRINOLOGY BRANCH, NATIONAL HEART INSTITUTE,
INHIBITION OF THE EFFECTS OF ANGIOTENSIN II ON ADRENAL STEROID PRODUCTION BY DIETARY SODIUM BY WARREN W. DAVIS,* LAWRENCE R. BURWELL,t AND FREDERIC C. BARTTERt ENDOCRINOLOGY BRANCH, NATIONAL HEART INSTITUTE,
Excretion Chapter 29. The Mammalian Excretory System consists of. The Kidney. The Nephron: the basic unit of the kidney.
 Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
3/19/2009. The task of the kidney in acid-base balance Excretion of the daily acid load. Buffering of an acid load. A o B - + H + B - A o +OH - C +
 The task of the kidney in acid-base balance Excretion of the daily acid load Buffering of an acid load Oxidation of amino acids, fats and carbohydrates often lead to acid production. On an average American
The task of the kidney in acid-base balance Excretion of the daily acid load Buffering of an acid load Oxidation of amino acids, fats and carbohydrates often lead to acid production. On an average American
Acid-Base Tutorial 2/10/2014. Overview. Physiology (2) Physiology (1)
 Overview Acid-Base Tutorial Nicola Barlow Physiology Buffering systems Control mechanisms Laboratory assessment of acid-base Disorders of H + ion homeostasis Respiratory acidosis Metabolic acidosis Respiratory
Overview Acid-Base Tutorial Nicola Barlow Physiology Buffering systems Control mechanisms Laboratory assessment of acid-base Disorders of H + ion homeostasis Respiratory acidosis Metabolic acidosis Respiratory
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
2.5 per cent solution). A Magill endotracheal tube fitted. with an inflatable balloon was introduced into the trachea
 THE REGULATION OF RENAL BICARBONATE REABSORPTION BY PLASMA CARBON DIOXIDE TENSION ' By ARNOLD S. RELMAN, BENJAMIN ETSTEN, AND WILLIAM B. SCHWARTZ (From the Evans Memorial, Massachusetts Memorial Hospitals
THE REGULATION OF RENAL BICARBONATE REABSORPTION BY PLASMA CARBON DIOXIDE TENSION ' By ARNOLD S. RELMAN, BENJAMIN ETSTEN, AND WILLIAM B. SCHWARTZ (From the Evans Memorial, Massachusetts Memorial Hospitals
After studying this lecture, you should be able to...
 Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
MIGUEL CHIAPPORI 4. Renal function. Twelve healthy Peruvian males between the ages of 20 and 28 years were studied. None
 ORAL SODIUM LOADING IN NORMAL INDIVIDUALS By KEHL MARKLEY,1 MANUEL BOCANEGRA,2 GUILLERMO MORALES,3 AND MIGUEL CHIAPPORI 4 (From the U. S. Public Health Service, U. S. Department of Health, Education, and
ORAL SODIUM LOADING IN NORMAL INDIVIDUALS By KEHL MARKLEY,1 MANUEL BOCANEGRA,2 GUILLERMO MORALES,3 AND MIGUEL CHIAPPORI 4 (From the U. S. Public Health Service, U. S. Department of Health, Education, and
Ch 17 Physiology of the Kidneys
 Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
2) This is a Point and Click question. You must click on the required structure.
 Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Carbon Dioxide Transport. Carbon Dioxide. Carbon Dioxide Transport. Carbon Dioxide Transport - Plasma. Hydrolysis of Water
 Module H: Carbon Dioxide Transport Beachey Ch 9 & 10 Egan pp. 244-246, 281-284 Carbon Dioxide Transport At the end of today s session you will be able to : Describe the relationship free hydrogen ions
Module H: Carbon Dioxide Transport Beachey Ch 9 & 10 Egan pp. 244-246, 281-284 Carbon Dioxide Transport At the end of today s session you will be able to : Describe the relationship free hydrogen ions
Potassium regulation. -Kidney is a major regulator for potassium Homeostasis.
 Potassium regulation. -Kidney is a major regulator for potassium Homeostasis. Normal potassium intake, distribution, and output from the body. Effects of severe hyperkalemia Partial depolarization of cell
Potassium regulation. -Kidney is a major regulator for potassium Homeostasis. Normal potassium intake, distribution, and output from the body. Effects of severe hyperkalemia Partial depolarization of cell
Physiology questions review
 Physiology questions review 1- The consumption of O2 by the kidney: a- decrease as blood flow increases b- regulated by erythropoiten c- remains constant as blood flow increase d- direct reflects the level
Physiology questions review 1- The consumption of O2 by the kidney: a- decrease as blood flow increases b- regulated by erythropoiten c- remains constant as blood flow increase d- direct reflects the level
mid ihsan (Physiology ) GFR is increased when A -Renal blood flow is increased B -Sym. Ganglion activity is reduced C-A and B **
 (Physiology ) mid ihsan GFR is increased when A -Renal blood flow is increased B -Sym. Ganglion activity is reduced C-A and B ** Colloid pressure in the efferent arteriole is: A- More than that leaving
(Physiology ) mid ihsan GFR is increased when A -Renal blood flow is increased B -Sym. Ganglion activity is reduced C-A and B ** Colloid pressure in the efferent arteriole is: A- More than that leaving
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University
 Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
BIOLOGY - CLUTCH CH.44 - OSMOREGULATION AND EXCRETION.
 !! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
!! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
CONTROLLING THE INTERNAL ENVIRONMENT
 AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
UNIT VI: ACID BASE IMBALANCE
 UNIT VI: ACID BASE IMBALANCE 1 Objectives: Review the physiological mechanism responsible to regulate acid base balance in the body i.e.: Buffers (phosphate, hemoglobin, carbonate) Renal mechanism Respiratory
UNIT VI: ACID BASE IMBALANCE 1 Objectives: Review the physiological mechanism responsible to regulate acid base balance in the body i.e.: Buffers (phosphate, hemoglobin, carbonate) Renal mechanism Respiratory
The average potassium content during the last 5. solids. This average decrease of 2.2 meq. per 100. initial potassium content of the arteries.
 THE EFFECT OF NOR-EPINEPHRINE ON THE ELECTROLYTE COMPOSITION OF ARTERIAL SMOOTH MUSCLE' By LOUIS TOBIAN 2 AND ADACIE FOX (From the Departments of Pharmacology and Internal Medicine, Southwesters Medical
THE EFFECT OF NOR-EPINEPHRINE ON THE ELECTROLYTE COMPOSITION OF ARTERIAL SMOOTH MUSCLE' By LOUIS TOBIAN 2 AND ADACIE FOX (From the Departments of Pharmacology and Internal Medicine, Southwesters Medical
20 Barton et al. Fourteen female mongrel dogs, weighing between 10 to 20 kg, were studied. Light pentobarbital anesthesia
 20 Barton et al. critical determinant of the occurrence and magnitude of natriuresis. It is possible that volume expansion suppresses sodium reabsorption in the loop, and that in the absence of this inhibitory
20 Barton et al. critical determinant of the occurrence and magnitude of natriuresis. It is possible that volume expansion suppresses sodium reabsorption in the loop, and that in the absence of this inhibitory
Renal physiology V. Regulation of acid-base balance. Dr Alida Koorts BMS
 Renal physiology V Regulation of acidbase balance Dr Alida Koorts BMS 712 012 319 2921 akoorts@medic.up.ac.za Hydrogen ions (H + ): Concentration and origin Concentration in arterial blood, resting: [H
Renal physiology V Regulation of acidbase balance Dr Alida Koorts BMS 712 012 319 2921 akoorts@medic.up.ac.za Hydrogen ions (H + ): Concentration and origin Concentration in arterial blood, resting: [H
Kidney Physiology. Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed
 Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
ACID-BASE BALANCE URINE BLOOD AIR
 ACIDBASE BALANCE URINE BLOOD AIR H 2 PO 4 NH 4 HCO 3 KIDNEY H H HCO 3 CELLS Hb H LUNG H 2 CO 3 HHb CO 2 H 2 O ph = 7.4 [HCO 3 ] = 24 meq/l PCO 2 = 40 mm Hg CO 2 PRIMARY RENAL MECHANISMS INVOLVED IN ACIDBASE
ACIDBASE BALANCE URINE BLOOD AIR H 2 PO 4 NH 4 HCO 3 KIDNEY H H HCO 3 CELLS Hb H LUNG H 2 CO 3 HHb CO 2 H 2 O ph = 7.4 [HCO 3 ] = 24 meq/l PCO 2 = 40 mm Hg CO 2 PRIMARY RENAL MECHANISMS INVOLVED IN ACIDBASE
1. remove: waste products: urea, creatinine, and uric acid foreign chemicals: drugs, water soluble vitamins, and food additives, etc.
 Making Water! OR is it really Just Water Just Ask the Nephron!! Author: Patricia L. Ostlund ostlundp@faytechcc.edu (910) 678-9892 Fayetteville Technical Community College Fayetteville, NC 28303 Its just
Making Water! OR is it really Just Water Just Ask the Nephron!! Author: Patricia L. Ostlund ostlundp@faytechcc.edu (910) 678-9892 Fayetteville Technical Community College Fayetteville, NC 28303 Its just
NORMOSOL -R MULTIPLE ELECTROLYTES INJECTION TYPE 1, USP For Replacing Acute Losses of Extracellular Fluid Flexible Plastic Container
 NORMOSOL -R MULTIPLE ELECTROLYTES INJECTION TYPE 1, USP For Replacing Acute Losses of Extracellular Fluid Flexible Plastic Container R x only DESCRIPTION Normosol-R is a sterile, nonpyrogenic isotonic
NORMOSOL -R MULTIPLE ELECTROLYTES INJECTION TYPE 1, USP For Replacing Acute Losses of Extracellular Fluid Flexible Plastic Container R x only DESCRIPTION Normosol-R is a sterile, nonpyrogenic isotonic
Renal Physiology. April, J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine.
 Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Inorganic pharmaceutical chemistry. Replacement Therapy Lec 2
 Inorganic pharmaceutical chemistry Replacement Therapy Lec 2 Replacement Therapy The basic objective of replacement therapy is to restore the volume and composition of the body fluids to normal one. Volume
Inorganic pharmaceutical chemistry Replacement Therapy Lec 2 Replacement Therapy The basic objective of replacement therapy is to restore the volume and composition of the body fluids to normal one. Volume
CONCERNING THE EFFECTS OF MAGNESIUM SULFATE ON RENAL FUNCTION, ELECTROLYTE EXCRETION, AND CLEARANCE OF MAGNESIUM
 CONCERNING THE EFFECTS OF MAGNESIUM SULFATE ON RENAL FUNCTION, ELECTROLYTE EXCRETION, AND CLEARANCE OF MAGNESIUM B. I. Heller,, J. F. Hammarsten, F. L. Stutzman J Clin Invest. 1953;32(9):858-861. https://doi.org/10.1172/jci102803.
CONCERNING THE EFFECTS OF MAGNESIUM SULFATE ON RENAL FUNCTION, ELECTROLYTE EXCRETION, AND CLEARANCE OF MAGNESIUM B. I. Heller,, J. F. Hammarsten, F. L. Stutzman J Clin Invest. 1953;32(9):858-861. https://doi.org/10.1172/jci102803.
organs of the urinary system
 organs of the urinary system Kidneys (2) bean-shaped, fist-sized organ where urine is formed. Lie on either sides of the vertebral column, in a depression beneath peritoneum and protected by lower ribs
organs of the urinary system Kidneys (2) bean-shaped, fist-sized organ where urine is formed. Lie on either sides of the vertebral column, in a depression beneath peritoneum and protected by lower ribs
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
THE RENAL EXCRETION OF CITRATE
 THE RENAL EXCRETION OF CITRATE Arthur P. Grollman,, Helen C. Harrison, Harold E. Harrison J Clin Invest. 1961;4(7):129-1296. https://doi.org/1.1172/jci14358. Research Article Find the latest version: http://jci.me/14358-pdf
THE RENAL EXCRETION OF CITRATE Arthur P. Grollman,, Helen C. Harrison, Harold E. Harrison J Clin Invest. 1961;4(7):129-1296. https://doi.org/1.1172/jci14358. Research Article Find the latest version: http://jci.me/14358-pdf
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (2) Dr. Attila Nagy 2017 TUBULAR FUNCTIONS (Learning objectives 54-57) 1 Tubular Transport About 99% of filtrated water and more than 90% of the filtrated
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (2) Dr. Attila Nagy 2017 TUBULAR FUNCTIONS (Learning objectives 54-57) 1 Tubular Transport About 99% of filtrated water and more than 90% of the filtrated
What is excretion? Excretion is the removal of metabolic waste from the body.
 Excretion What is excretion? Excretion is the removal of metabolic waste from the body. Excretion in Plants Plants produce very little waste products. Plants lose oxygen and water vapour through the stomata.
Excretion What is excretion? Excretion is the removal of metabolic waste from the body. Excretion in Plants Plants produce very little waste products. Plants lose oxygen and water vapour through the stomata.
BIOL2030 Human A & P II -- Exam 6
 BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
Non-protein nitrogenous substances (NPN)
 Non-protein nitrogenous substances (NPN) A simple, inexpensive screening test a routine urinalysis is often the first test conducted if kidney problems are suspected. A small, randomly collected urine
Non-protein nitrogenous substances (NPN) A simple, inexpensive screening test a routine urinalysis is often the first test conducted if kidney problems are suspected. A small, randomly collected urine
Deficiency: Its Possible Role in Regulation of Renal
 Journal of Clinical Investigation Vol. 45, No. 4, 1966 Renal Gluconeogenesis in Acidosis, Alkalosis, and Potassium Deficiency: Its Possible Role in Regulation of Renal Ammonia Production * A. DAVID GOODMAN,t
Journal of Clinical Investigation Vol. 45, No. 4, 1966 Renal Gluconeogenesis in Acidosis, Alkalosis, and Potassium Deficiency: Its Possible Role in Regulation of Renal Ammonia Production * A. DAVID GOODMAN,t
1. Urinary System, General
 S T U D Y G U I D E 16 1. Urinary System, General a. Label the figure by placing the numbers of the structures in the spaces by the correct labels. 7 Aorta 6 Kidney 8 Ureter 2 Inferior vena cava 4 Renal
S T U D Y G U I D E 16 1. Urinary System, General a. Label the figure by placing the numbers of the structures in the spaces by the correct labels. 7 Aorta 6 Kidney 8 Ureter 2 Inferior vena cava 4 Renal
Mannitol-induced Metabolic Alkalosis
 Electrolyte & Blood Pressure :, 00 ) Mannitolinduced Metabolic Alkalosis Kyung Pyo Kang, M.D., Sik Lee, M.D., Kyung Hoon Lee, M.D., and Sung Kyew Kang, M.D. Department of Internal Medicine, Research Institute
Electrolyte & Blood Pressure :, 00 ) Mannitolinduced Metabolic Alkalosis Kyung Pyo Kang, M.D., Sik Lee, M.D., Kyung Hoon Lee, M.D., and Sung Kyew Kang, M.D. Department of Internal Medicine, Research Institute
Acid and Base Balance
 Acid and Base Balance 1 2 The Body and ph Homeostasis of ph is tightly controlled Extracellular fluid = 7.4 Blood = 7.35 7.45 < 7.35: Acidosis (acidemia) > 7.45: Alkalosis (alkalemia) < 6.8 or > 8.0: death
Acid and Base Balance 1 2 The Body and ph Homeostasis of ph is tightly controlled Extracellular fluid = 7.4 Blood = 7.35 7.45 < 7.35: Acidosis (acidemia) > 7.45: Alkalosis (alkalemia) < 6.8 or > 8.0: death
Salt and Water Balance and Nitrogen Excretion
 Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
Normal Renal Function
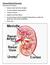 Normal Renal Function Functions of the Kidney: balances solute and water transport excretes metabolic waste products conserves nutrient regulates acid-base balance secretes hormones that help regulate
Normal Renal Function Functions of the Kidney: balances solute and water transport excretes metabolic waste products conserves nutrient regulates acid-base balance secretes hormones that help regulate
ADRENALECTOMIZED rats drink less than normal rats when 2 per cent saline. daily by stomach tube and water to drink freely, died quickly but such
 THE EFFECT OF PROLONGED INTRAGASTRIC INFUSIONS OF ISOTONIC AND HYPERTONIC SALINE ON WATER AND SODIUM EXCRETION AND ON EXCHANGEABLE BODY SODIUM IN NORMAL AND ADRENALECTOMIZED RATS. By C. J. EDMONDS. From
THE EFFECT OF PROLONGED INTRAGASTRIC INFUSIONS OF ISOTONIC AND HYPERTONIC SALINE ON WATER AND SODIUM EXCRETION AND ON EXCHANGEABLE BODY SODIUM IN NORMAL AND ADRENALECTOMIZED RATS. By C. J. EDMONDS. From
Pharmacology I [PHL 313] Diuretics. Dr. Mohammad Nazam Ansari
![Pharmacology I [PHL 313] Diuretics. Dr. Mohammad Nazam Ansari Pharmacology I [PHL 313] Diuretics. Dr. Mohammad Nazam Ansari](/thumbs/90/104323364.jpg) Pharmacology I [PHL 313] Diuretics Dr. Mohammad Nazam Ansari Renal Pharmacology Kidneys: Each adult kidney weighs 125-170g in males and 115-155g in females, represent 0.5% of total body weight, but receive
Pharmacology I [PHL 313] Diuretics Dr. Mohammad Nazam Ansari Renal Pharmacology Kidneys: Each adult kidney weighs 125-170g in males and 115-155g in females, represent 0.5% of total body weight, but receive
ACTIVE TRANSPORT OF SODIUM BY THE ISOLATED MIDGUT OF HYALOPHORA CECROPIA
 J. Exp. Biol. (1971). 54. 269-374 269 With 1 text-figure Printed in Great Britain ACTIVE TRANSPORT OF SODIUM BY THE ISOLATED MIDGUT OF HYALOPHORA CECROPIA BY W. R. HARVEY AND K. ZERAHN* Department of Biology,
J. Exp. Biol. (1971). 54. 269-374 269 With 1 text-figure Printed in Great Britain ACTIVE TRANSPORT OF SODIUM BY THE ISOLATED MIDGUT OF HYALOPHORA CECROPIA BY W. R. HARVEY AND K. ZERAHN* Department of Biology,
RENAL. 6. Renal acid secretion is affected by all of the following except a. pco 2 b. K c. Carbonic anhydrase d. Aldosterone e. Ca
 RENAL 1. The reabsorption of Na in the proximal tubules a. Reabsorbs 80% of the filtered sodium load b. Causes increased hypertonicity c. Is powered by N/H ATPase d. Shares a common carrier with glucose
RENAL 1. The reabsorption of Na in the proximal tubules a. Reabsorbs 80% of the filtered sodium load b. Causes increased hypertonicity c. Is powered by N/H ATPase d. Shares a common carrier with glucose
NOTES: CH 44 Regulating the Internal Environment (Homeostasis & The Urinary System)
 NOTES: CH 44 Regulating the Internal Environment (Homeostasis & The Urinary System) HOMEOSTASIS **Recall HOMEOSTASIS is the steady-state physiological condition of the body. It includes: 1) Thermoregulation:
NOTES: CH 44 Regulating the Internal Environment (Homeostasis & The Urinary System) HOMEOSTASIS **Recall HOMEOSTASIS is the steady-state physiological condition of the body. It includes: 1) Thermoregulation:
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal Physiology Part II. Bio 219 Napa Valley College Dr. Adam Ross
 Renal Physiology Part II Bio 219 Napa Valley College Dr. Adam Ross Fluid and Electrolyte balance As we know from our previous studies: Water and ions need to be balanced in order to maintain proper homeostatic
Renal Physiology Part II Bio 219 Napa Valley College Dr. Adam Ross Fluid and Electrolyte balance As we know from our previous studies: Water and ions need to be balanced in order to maintain proper homeostatic
clamped. At 30- or 60-minute intervals urine specimens were collected and the bladder washed out with saline
 Downloaded from http://www.jci.org on January 11, 218. https://doi.org/1.1172/jci11171 THE MECHANISM OF THE EXCRETION OF VITAMIN C BY THE HUMAN KIDNEY AT LOW AND NORMAL PLASMA LEVELS OF ASCORBIC ACID 1
Downloaded from http://www.jci.org on January 11, 218. https://doi.org/1.1172/jci11171 THE MECHANISM OF THE EXCRETION OF VITAMIN C BY THE HUMAN KIDNEY AT LOW AND NORMAL PLASMA LEVELS OF ASCORBIC ACID 1
