Chemical fingerprint of essential oil components from fresh leaves of Glycosmis pentaphylla (Retz.) Correa
|
|
|
- Virginia Hood
- 6 years ago
- Views:
Transcription
1 2015; 3(12): ISSN: TPI 2015; 3(12): TPI Received: Accepted: Pressy P. Prakasia Department of Botany, University of Kerala, Kariavattom campus, Thiruvananthapuram, Pin Code: , Kerala, India. Ashalatha S. Nair Department of Botany, University of Kerala, Kariavattom campus, Thiruvananthapuram, Pin Code: , Kerala, India. Chemical fingerprint of essential oil components from fresh leaves of Glycosmis pentaphylla (Retz.) Correa Pressy P. Prakasia, Ashalatha S. Nair Abstract The present research was undertaken to characterize the promising bioactive constituents of Glycosmis pentaphylla leaves. The volatile oil was obtained by hydrodistillation and the components present were analyzed by gas chromatography-mass spectrometry. Elucidation on mass spectrum GC-MS was conducted using the database of National Institute of Standard and technology (NIST). The GC-MS analysis of essential oil of Glycosmis pentaphylla leaves resolved into sixty seven compounds representing 99.71% of essential oil. The active principles with their retention time, peak area, molecular formula, molecular weight, structure and category of the compound were predicted. Phytol (28.03%) was the dominant compound in the oil. From the present study, it is revealed that the oil present in leaf is very rich in diverse phytochemicals. Most of the identified compounds are basically biological important. The results are in conformity with the tribal conviction for which they use as traditional medicine for diverse bioactivities and curing of ailments. Keywords: Glycosmis pentaphylla, essential oil, GC-MS, Phytol, sesquiterpenes. Correspondence: Pressy P. Prakasia Research Scholar, Department of Botany, University of Kerala, Kariavattom campus, Thiruvananthapuram, Pin Code: , Kerala, India. 1. Introduction The therapeutic use of plants against critical human illnesses predates recorded history and represents the most significant direct antecedent to modern medicine [1]. Scientific research has allowed discovering a wide range of active constituents, of which the most important as far as health is concerned are essential oils, alkaloids, glycosides or heterosides, mucilage and gums, and tannins. The active constituents specific to a particular species are characterized as chemical markers. Use of chemical markers is an effective tool to resolve problems in standardization of botanicals, using chemical fingerprinting and in chromatographic finger printing of botanicals to demarcate them on the basis of their chemotypes and geographical origin [2]. Glycosmis pentaphylla, belonging to the Rutaceae family, is a shrub or small (1.5 5 m) tree, widely distributed, spanning from India, Malaysia and Southern China to the Philippine Islands where it occurs in tropical forests at low altitudes. It has been used as folk medicine for the treatment of fever, liver complaints, jaundice, cough, eczema, anaemia, diarrhoea, and rheumatism [3, 4]. Phytochemically speaking, Glycosmis pentaphylla were investigated on a few occasions. Most of the phytochemical work realized in this field resulted in the isolation of hydrophobic alkaloids acridone, carbazoles, quinolones and quinazolones [5-8]. Studies showed the extracts of G. pentaphylla having potent anthelmintic, antipyretic, hepatoprotective, antibacterial, antioxidant, antidiabetic and antinociceptive properties [9, 10]. Regarding the phytochemistry of essential oil of G. pentaphylla, the composition of essential oil was shown, and aliphatic ketones 2- tridecanone 6,10,14- trimethyl-2-pentadecanone were the major components identified [11]. Review of literature divulges that the essential oil composition within the species may vary significantly and such differences in the composition of their essential oil could be due to natural chemical variation called chemotype, which occur in the secondary metabolism of plants and could possibly due to organ of the plant studied and also induced by environmental factors such as soil type, altitude, sun exposure, rain and seasonal variation besides the method of oil isolation [12]. As far the literature investigation could ascertain, the fresh leaves of Glycosmis pentaphylla were never examined regarding their volatile oil composition. Hence, the aim of the present study is to provide the first detail GC- MS analysis of the volatile components of the fresh leaves of Glycosmis pentaphylla. ~ 50 ~
2 2. Materials and Methods 2.1 Plant material Fresh leaves of Glycosmis pentaphylla were collected from its natural habitat from district of Thiruvananthapuram (Latitude- 8.54ºN and Longitude ºE), Kerala, India, in January The titled plant was botanically identified by Curator, department of Botany, University of Kerala and a voucher specimen (KUBH 5858) has been deposited at the herbarium of Botany department, University of Kerala, India. 2.2 Extraction of essential oil The leaves were slightly washed to remove dust and other physical contaminants. The leaves were then reduced to a suitable size using electric blender and loaded them in the extraction flask. The essential oil was extracted by hydrodistillation for 8 h (60 g of sample in 500 ml of distilled water) using Clevenger apparatus [13]. The essential oil obtained was separated from aqueous phase and stored in sealed glass vial protected from the light at 4 ºC until analysis. The oil sample was subsequently analysed by GC-MS. 2.3 GC-MS analysis The analysis of the oil was performed using GC-MS (Model: GCMS- QP 2010, Shimadzu, Tokyo, Japan) equipped with a VF 5 ms fused silica capillary column of 30 m length, 0.25 mm diameter and 0.25 mm film thickness. For GC-MS detection, electron ionization energy of 70 ev was used. The carrier gas was Helium (99.99%) used ata constant flow rate of 1.51 ml/min. Injector and mass transfer line temperature were set at 2000 C and 2400 C respectively. The oven temperature was set from 70 to 2200 C at 100 C/min. Two µl of sample was injected in a split mode with a scan range of m/z. The total running time of GC-MS was 31 min. The relative percentage of the extract was expressed as percentage with peak area normalization. 2.4 Identification of the compounds Elucidation on mass spectrum GC-MS was conducted using the database of National Institute of Standard and technology (NIST). The spectrum of the unknown components was compared with the spectrum of the known components stored in the NIST08 library source [14]. The name, molecular weight and molecular mass of the identified compounds were further confirmed by comparison of their retention indices with literature data. For quantitative analysis, compounds concentrations (as % content) were calculated by integrating their corresponding chromatographic peak areas. 3. Results The essential oil of fresh leaves of Glycosmis pentaphylla was obtained by conventional hydro- distillation method using a Clevenger apparatus. The hydrodistillation gave characteristic odour, golden yellow oil. In this study GC-MS fingerprinting of essential oil of fresh leaves of Glycosmis pentaphylla revealed several peaks. The gas chromatogram shows the relative concentrations of various compounds getting eluted as a function of retention time (Figure 1). Identification of the compounds was accomplished by comparing their mass spectra and retention indices with those given in the literature and those authentic samples. The active principles with their retention time (RT), molecular formula, molecular weight (MW) and concentration (%) are presented in Table 1. They are listed by their order of retention times. The heights of the peak indicate the relative concentrations of the compounds present in essential oil of Glycosmis pentaphylla leaves. Sixty seven compounds were identified representing about 99.71% of the total oil s compounds. The most prevailing major compounds were Phytol (28.03%), Bicyclo[5.2.0]nonane, 2-methylene-4,8,8-trimethyl-4-vinyl (10.93%), 1,19-Eicosadiene (9.84%), 1,6-Cyclodecadiene, 1- methyl-5-methylene-8-(1-methylethyl)-,[s(e,e)]- (4.63%), Caryophyllene oxide (4.32%), (-)-Spathulenol (3.92%) and Bicyclogermacrene (3.55%)., and the minor compounds were Bicyclo[4.4.0]dec-1-ene, 2-isopropyl-5-methyl-9-methylene- (2.11%), Epiglobulol (1.75%), (-)-Globulol (1.59%), Naphthalene,1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1- methylethyl)-(1s-cis)-(1.53%), 1H Indene, (1.33%), Ledol (1.33%), Cyclohexane,1-ethenyl -1-methyl -2,4-bis (1- methylethenyl)-, [1S-(1.alpha.,2.beta.,4.beta.)]- (1.23%), Santolina triene (1.23%), Toluene (1.20%), Humulene (1.20%), gamma.-elemene (1.18%), 2-Pyrrolidinone (1.10%) and Cyclohexane, 1-ethenyl-1-methyl-2-(1-methyethenyl)-4- (1-methylethylidene)- (1.05%). The remaining compounds were present in less than 1%. Chemical class of the detected volatile compounds are displayed in Table 2. The compounds were separated into monoterpenes, sesquiterpenes, diterpenes, alkanes and alkenes, fatty acids and others. The most representative compounds were sesquiterpenes (46.39%) followed by diterpenes (28.58%), fatty acids (10.61%), monoterpenes (5.81%) and alkane and alkene (3.83%). Major phytocompounds and its biological activities obtained through the GC-MS study of leaves of Glycosmis pentaphylla are presented in Table 3. The biological activities listed are based on Dr. Duke s phytochemical and ethnobotanical online databases by Dr. Jim Duke of Agricultural Research Service/ USDA. 4. Discussion The chemical and chromatographic fingerprints provide adequate information about the safety and credibility, with evidence for the product. A chemical profile of sixty seven compounds was identified representing about 99.71% of the total oil s compounds. Usually the major compounds mirror the biological activities of the essential oil from which it is extracted. Essential oil of G. pentaphylla composed mainly of terpenes (monoterpenes, sesquiterpene and diterpene) and its biological activity may be attributed to its high concentration. However, the activity of major components may be modulated by other minor components present in the oil. Among the identified compounds, the diterpene alcohol, Phytol, which is at hand in highest with 28.09%, is vital in the dispensation of glucose and can trigger enzymes within the body that have strong positive effects on insulin level. This means that Phytol in the human diet could perhaps help reinstate the metabolic activities of those with type-2 diabetes. Phytol confirmed a strong antioxidant effect in vitro in its capacity to remove hydroxyl radicals and nitric oxide as well as to prevent the formation of thiobarbituric acid reactive substances (TBARS) [15]. biosynthesis seems to be complex since the formation via either pathway (mevalonic or methylerythrytol) or a combination of both has been reported [16]. Nevertheless, these appear to be ubiquitous in plant taxa and some insects, and are associated to the cytosol mitochondria. s were reported to have antihyperlipidemic activity. ~ 51 ~
3 Fig 1: GC-MS Chromatogram of essential oil of Glycosmis pentaphylla leaves Table 1: Phytocompounds identified in the essential oil of Glycosmis pentaphylla leaf by GC-MS. Molecular Peak Molecular Area Name of the compound RT weight no formula % (g/mol) 1 Toluene 2.29 C7H Hexen-1-ol, (Z) 3.21 C6H12O beta. Pinene 5.39 C10H Pyrrolidinone, 1-methyl C4H7NO beta.-Ocimene 6.62 C10H ,6- Octadien-3-ol, 3,7-dimethyl C11H18O Terpinen-4-ol 9.11 C10H18O alpha.-Terpineol 9.36 C10H18O cis-3-hexenyl isovalerate 9.86 C9H16O ,5,5-Trimethyl-6-methylene-cyclohexene C10H Carene 11.6 C10H alpha.-Cubebene C15H alfa.-Copaene C15H Amino-1-phenylpyrazole C9H9N Cyclohexane, 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl)-,[1S-(1.alpha.,2.beta.,4.beta.)] C15H Caryophyllene C15H H-Cyclopro[e]azulene, 1a,2,3,4,4a,5,6,7b-octahydro- 1,1,4,7-tetramethyl-,[1arR C15H (1a.alpha.,4.alpha.,4a.beta.,7b.alpha.)]- 18 Bicyclo[5.2.0]nonane, 2-methylene-4,8,8-trimethyl-4-vinyl C15H gamma.-Elemene C15H beta.-copaene C15H Aromadendrene C15H Humulene C15H ~ 52 ~
4 23 Alloaromadendrene C15H gamma.-Muurolene C15H Naphthalene, 1,2,4a,5,6,8a-hexahydro-4,7-dimethyl-1-(-1- methylethyl) C15H ,6-Cyclodecadiene, 1-methyl-5-methylene-8-(1- methylethyl)-, [S(E,E)] C15H Naphthalene, 1,2,3,5,6,7,8,8a-octahydro-1,8a-dimethyl-7- (1-methylethylene)-,[1R-(1.alpha.,alpha.,7.beta.,8a.alpha.)] C15H Bicyclogermacrene C15H H-Cycloprop[e]azulene, decahydro 1,1,7-trimethyl-4- methylene C15H gamma.-Muurolene C15H Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1- methylethyl)-(1s-cis) C15H Hotrienol C10H16O Aromandendrene C15H Caryophyllene oxide C15H24O Cyclohexane, 1-ethenyl-1-methyl-2-(1-methyethenyl)-4- (1-methylethylidene) C6H Hexen-1-ol, benzoate, (Z) C13H16O ,7-Methanoazulene, 1,2,3,4,5,6,7,8-octahydro-1,4,9,9- tetramethyl-[1s-(1.alpha.,4.alpha.,7.alpha.)] C15H (-)-Spathulenol C15H24O Caryophyllene oxide C15H24O (-)-Globulol C15H26O H Indene, 1-ethylideneoctahydro-7a-methyl-(1E, 3a.alpha.,7a.beta.) C9H cis-thujopsene C15H Ledol C15H26O Santolina triene C10H Bicyclo[4.4.0]dec-1-ene, 2-isopropyl-5-methyl-9- methylene C15H Alloaromadendrene C15H Epiglobulol C15H26O beta.-Humulene C15H Isoaromadendrene epoxide 16.2 C15H24O trans-z-.alpha.-bisabolene epoxide C15H24O H-Cycloprop[e]azulene C15H Benzoic acid, heptadecyl ester C23H28N2O trans-.beta.-ionone C13H20O Isoshyobunone C15H24O Pentadecanone, 6,10,14-trimethyl C18H36O Isophytol C20H40O Phytol C20H40O alpha.-Pyrrolidone, 5-[3-hydroxybutyl] C8H15NO Phytol C20H40O Cyclopentane, 1,2,3,4,5-pentamethyl C10H Eicosene, (E) C20H trans-geranylgeraniol 21.6 C20H34O Fumaric acid, cis-hex-3-enyl tetra decyl ester C4H4O Cyclopentane,1,1,3-trimethyl C5H Undecen-2-one, 6,10-dimethyl C13H24O ,19- Eicosadiene C20H ,4-Bis(trimethylsilyl)benzene C12H22 Si Grouped components 1 Monoterpenes s Diterpenes Alkane and Alkene Fatty acids Others 4.49 Total identified components RT: Retention time Area %: relative percentage obtained from peak area ~ 53 ~
5 Table 2: Nature of the compound Peak no Name of the compound Nature of compound 1 Toluene Aromatic hydrocarbon 2 3- Hexen-1-ol, (Z) Alcohol 3.beta. Pinene Monoterpene 4 2-Pyrrolidinone,1-methyl- Pyrrolidines (aliphatic heteromonocyclic compounds ) 5.beta.-Ocimene Monoterpene 6 1,6- Octadien-3-ol, 3,7-dimethyl- Monoterpene 7 Terpinen-4-ol Monoterpene 8.alpha.-Terpineol Monoterpene alcohol 9 cis-3-hexenyl isovalerate Fatty acid 10 1,5,5-Trimethyl-6-methylene-cyclohexene Monoterpene 11 2-Carene Monoterpene 12.alpha.-Cubebene 13.alpha.-Copaene 14 5-Amino-1-phenylpyrazole Azoles 15 Cyclohexane, 1-ethenyl-1-methyl-2, 4-bis(1-methylethenyl)-,[1S- Cycloalkane (1.alpha.,2.beta.,4.beta.)]- 16 Caryophyllene Bicyclic sesquiterpene 17 1H-Cyclopro[e]azulene, 1a,2,3,4,4a,5,6,7b- octahydro-1,1,4,7-tetramethyl-,[1arr- Tricyclic sesquiterpene (1a.alpha.,4.alpha.,4a.beta.,7b.alpha.)]- 18 Bicyclo[5.2.0]nonane, 2-methylene-4,8,8- trimethyl-4-vinyl- 19.gamma.-Elemene Monocyclic sesquiterpene 20.beta.-copaene Tricyclic sesquiterpene 21 Aromandendrene Tricyclic sesquiterpene 22 Humulene Monocyclic sesquiterpene 23 Alloarmadendrene Tricyclic sesquiterpene 24.gamma.-Muurolene Oxygenated sesquiterpene 25 Naphthalene, 1,2,4a,5,6,8a-hexahydro-4,7- dimethyl-1-(-1-methylethyl)- 26 1,6-Cyclodecadiene, 1-methyl-5-methylene- 8-(1-methylethyl)-,[S(E,E)]- 27 Naphthalene, 1,2,3,5,6,7,8,8a-octahydro- 1,8a-dimethyl-7-(1-methylethylene)-,[1R- (1.alpha.,alpha.,7.beta.,8a.alpha.)]- 28 Bicyclogermacrene Monocyclic sesquiterpene 29 1H-Cycloprop[e]azulene, decahydro 1,1,7- trimethyl-4-methylene- Tricyclic sesquiterpene 30.gamma.-Muurolene Oxygenated sesquiterpene 31 Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7- dimethyl-1-(1-methylethyl)-(1s-cis)- 32 Hotrienol Monoterpene 33 Aromandendrene Tricyclic sesquiterpene 34 Caryophyllene oxide Bicyclic sesquiterpene 35 Cyclohexane, 1-ethenyl-1-methyl-2-(1- methyethenyl)-4-(1-methylethylidene)- Cycloalkane 36 3-Hexen-1-ol, benzoate, (Z) Monoterpene 37 4,7-Methanoazulene, 1,2,3,4,5,6,7,8- octahydro-1,4,9,9-tetramethyl-[1s- (1.alpha.,4.alpha.,7.alpha.)]- 38 (-)-Spathulenol Oxygenated sesquiterpene 39 Caryophyllene oxide Bicyclic sesquiterpene 40 (-)-Globulol Tricyclic hydroazulene sesquiterpene 41 1H Indene, 1-ethylideneoctahydro-7amethyl-, (1E,3a.alpha.,7a.beta.) Bicyclic aromatic compound 42 cis-thujopsene 43 Ledol Crystalline sesquiterpene 44 Santolina triene Oxygenated monoterpene 45 Bicyclo[4.4.0]dec-1-ene, 2-isopropyl-5- methyl-9-methylene- 46 Alloaromadendrene Tricyclic sesquiterpene 47 Epiglobulol Oxygenated sesquiterpene 48.beta.-Humulene Monocyclic sesquiterpene 49 Isoaromadendrene epoxide Tricyclic sesquiterpene ~ 54 ~
6 50 trans-z-.alpha.-bisabolene epoxide Oxygenated sesquiterpene 51 1H-Cycloprop[e]azulene Tricyclic sesquiterpene 52 Benzoic acid, heptadecyl ester Aromatic carboxylic acid 53 trans-.beta.-ionone Monocyclic monoterpene 54 Isoshyobunone Monocyclic monoterpene 55 2-Pentadecanone, 6,10,14-trimethyl Fatty acid ketone 56 Isophytol Acyclic diterpene 57 Phytol Acyclic diterpene alcohol 58.alpha.-Pyrrolidone, 5-[3-hydroxybutyl]- Pyrrolidines 59 Phytol Acyclic diterpene alcohol 60 Cyclopentane, 1,2,3,4,5-pentamethyl- Cycloalkane 61 3-Eicosene, (E)- Acyclic alkene 62 trans-geranylgeraniol Diterpene alcohol 63 Fumaric acid, cis-hex-3-enyl tetra decyl ester Unsaturated fatty acid 64 Cyclopentane, 1,1,3-trimethyl- Cycloalkane 65 9-Undecen-2-one, 6,10-dimethyl Acyclic monoterpene 66 1,19- Eicosadiene Aliphatic fatty acid 67 1,4-Bis(trimethylsilyl)benzene Cycloalkane Name of the compound Table 3: Biological activities of major compounds Peak area % Bicyclogermacrene 3.55 (-)-Spathulenol 3.92 Caryophyllene oxide ,6-Cyclodecadiene, 1-methyl-5- methylene-8-(1-methylethyl)-, [S(E,E)] Biological activity* Antimicrobial, anti-inflammatory, anticancer, antiplasmodial, antifeedant, phytotoxic, inhibitor of tumour necrosis and interleukin-6 Immunomodulatory effects, mosquito repellant activity, antimicrobial, anti-inflammatory Trypanocidal activity,antiedemic, antifeedant, antiinflammatory, antitumor, calcium antagonist, fungicide, insecticide, pesticide Antimicrobial, antioxidant, deterrent effects against herbivores, insecticidal activity against mosquitoes, antibacterial Nature of compound Oxygenated Oxygenated 1,19- Eicosadiene 9.84 No activity reported Fatty acid Bicyclo[5.2.0]nonane, 2-methylene- Antimicrobial, anti-inflammatory, antihyperlipidemic, ,8,8-trimethyl-4-vinyl- antioxidant Cytotoxic, antinociceptive, antioxidant, antimicrobial, Phytol Diterpene anti-inflammatory, anticancer, diuretic *Biological Activity: Dr. Duke's Phytochemical and Ethnobotanical Database. Some sesquiterpene hydrocarbons present in this oil have been reported to exhibit antibacterial activity such as Bicyclo[5.2.0]nonane, 2-methylene-4,8,8-, (-)-Spathulenol, Bicyclogermacrene and 1,6-Cyclodecadiene, 1-methyl-5- methylene-8-(1-methylethyl)-, [S(E,E)]-. The compound Caryophyllene oxide is an oxygenated sesquiterpene, and it has been suggested to function as trypanocidal acivity [17]. It also has biological properties of antiedemic, antifeedant, antiinflammatory, antitumor etc. Immunomodulatory effects of Spathulenol have been reported. Bicyclo [5.2.0]nonane, 2- methylene-4,8,8-trimethyl-4-vinyl- is a sesquiterpene, is known to possess anti-inflammatory, antihyperlipidemic properties. Monoterpenes, have shown sound effects on mevalonate metabolism, linked to the maintenance of cell membrane, which could add to terpene tumor suppressive action. Thus, the presence of monoterpenes in the selected active fractions explains their antiproliferative actions against some tumor cell lines. Studies indicated that the activity of the essential oil may be due to the synergistic effects of the active compounds. The reports on the chemical composition of the essential oils of G. pentaphylla leaves are few in the literature. An assessment with literature data showed that the compounds identified in the present study showed variation in the chemical composition pattern to those reported for the same species growing in another geographical region [18]. It is noteworthy to point out that the constituents of the plants essential oils are normally influenced by several factors such as geographical, climatic, seasonal and experimental conditions. 5. Conclusion This is the first report on the chemical composition of essential oil of fresh leaves of Glycosmis pentaphylla. The result reveals the existence of various bioactive compounds and validates the earlier reports of therapeutic importance of the plant. It is strongly recommended that this medicinal plant needs further research in many-sided field of natural products to isolate, typify and explicate the structure of bioactive molecules to endure the clinical trials to develop a safety and effectual plant-based natural drug for various ailments in the point of health security. ~ 55 ~
7 6. Acknowledgement The authors are thankful to Dr. P.M Rhadhamany, Associate Professor and Head, Department of Botany, University of Kerala, Kariyavattom, for providing the required facilities for the conduct of this research work. 7. References 1. Dutt R, Garg V, Madan AK. Can plants growing in diverse hostile environments provide a vital source of anticancer drugs? Journal of Cancer Therapy 2014; 10: Soumya PR, Choudary KA, Kar DM, Lopanudra Das, Jain A. Plants in traditional medicinal system- Future source of new drugs. International Journal of Pharmacy and Pharmaceutical Science. 2009; 1: Kirtikar KR, Basu BD. Indian Medicinal Plants. Dehradun, International Book Publisher. 1993, 16: Ghani A. Medicinal plants of Bangladesh. 2 nd ed. Bangladesh, Asiatic society of Bangladesh, 2003, Bhattacharyya P, Chowdhury BK. Glycolone, a quinolone alkaloid from Glycosmis pentaphylla. Phytochemistry 1985; 24: Chowdhury BK, Mustapha A, Garba M, Bhattacharya P. Carbazole and 3- methylcarbazole from Glycosmis pentaphylla. Phytochemistry 1987; 26: Roy KS, Chakraborty DP. Mupamine from Glycosmis pentaphylla. Phytochemistry1989; 28: Sarkar M, Chakraborty DP. Some minor constituents from Glycosmis pentaphylla. Phytochemistry 1977; 16: Jaya RN, Rao BG. Evaluation of hepatoprotective activity of Glycosmis pentaphylla roots against CCl 4 induced acute liver injury in rats. International Journal of Pharmacy Science Review and Research 2010; 4(2): Gupta N, Agarwal M, Bhatia V, Jha SK, Dinesh J. In vitro antioxidant activity of crude extracts of the plant Glycosmis pentaphylla Correa. International Journal of Pharmacy Science Review and Research 2011; 6(2): Nayive PB, Erika MML, Elena ES. Essential Oil Composition from Two Species of Piperaceae Family Grown in Colombia. Journal of Chromatographic Science 2009; 47: Singariya P, Kumar P, Mourya KK. Identification of New Bioactive Compounds by GC-MS and Estimation of Physiological and Biological Activity of Kala Dhaman (Cenchrus setigerus). International Journal of Pharmaceutical and Biological Archives 2012; 3(3): Clevenger JF. Apparatus for determination of volatile oil. Journal of American Pharmacists Association 1928; 17: Stein SE. National Institute of Standards and Technology (NIST), Mass Spectral Database and Software. Version Gaithersburg, USA, Camila Carolina de Mnezes Patricio Santos, Mirian Stiebbe Salvadori, Vanine Gomes Mota, Luciana Muratori Costa, Antonia Amanda Cardoso de Almeida, Guilherme Antonio Lopes de Oliveira, et al. Antinociceptive and antioxidant activities of Phytol In vivo and In vitro Models. Journal of Neuroscience 2013, Umlauf D, Zapp J, Becker H, Adam KP. Biosynthesis of the irregular monoterpene artemisia ketone, the sesquiterpene germacrene D and other isoprenoids in ~ 56 ~ Tanacetum vulgare L. (Asteraceae). Phytochemistry 2004; 65(17): Polanco-Hernández G, Escalante-Erosa F, García-Sosa K, María ER, Eugenia GM, Karla Y, Acosta-Viana et al. Synergistic Effect of Lupenone and Caryophyllene Oxide against Trypanosoma cruzi. Evidance Based Complement and Alternative Medicine 2013, Riyazzudin A, Sadananda C, Ingrid V, Piet AL. Essential Oils of Glycosmis pentaphylla (Cor.). A New Report from Assam, India. Journal of Essential Oil Research 2000; 12(4):
GC/MS BATCH NUMBER: N10101
 GC/MS BATCH NUMBER: N10101 ESSENTIAL OIL: NEROLI BOTANICAL NAME: CITRUS AURANTIUM ORIGIN: EGYPT KEY CONSTITUENTS PRESENT IN THIS BATCH OF NEROLI OIL % LINALOOL 36.1 LIMONENE 14.0 LINALYL ACETATE 10.9 -TERPINEOL
GC/MS BATCH NUMBER: N10101 ESSENTIAL OIL: NEROLI BOTANICAL NAME: CITRUS AURANTIUM ORIGIN: EGYPT KEY CONSTITUENTS PRESENT IN THIS BATCH OF NEROLI OIL % LINALOOL 36.1 LIMONENE 14.0 LINALYL ACETATE 10.9 -TERPINEOL
GC/MS BATCH NUMBER: P50102
 GC/MS BATCH NUMBER: P50102 ESSENTIAL OIL: PEPPERMINT BOTANICAL NAME: MENTHA PIPERITA ORIGIN: INDIA KEY CONSTITUENTS PRESENT IN THIS BATCH OF PEPPERMINT OIL % MENTHOL 39.5 MENTHONE 23.2 1,8-CINEOLE 5.5
GC/MS BATCH NUMBER: P50102 ESSENTIAL OIL: PEPPERMINT BOTANICAL NAME: MENTHA PIPERITA ORIGIN: INDIA KEY CONSTITUENTS PRESENT IN THIS BATCH OF PEPPERMINT OIL % MENTHOL 39.5 MENTHONE 23.2 1,8-CINEOLE 5.5
GC/MS BATCH NUMBER: F50101
 GC/MS BATCH NUMBER: F50101 ESSENTIAL OIL: ORGANIC FRANKINCENSE SERRATA BOTANICAL NAME: BOSWELLIA SERRATA ORGANIC ORIGIN: INDIA KEY CONSTITUENTS IN THIS BATCH OF ORGANIC FRANKINCENSE SERRATA OIL % -THUJENE
GC/MS BATCH NUMBER: F50101 ESSENTIAL OIL: ORGANIC FRANKINCENSE SERRATA BOTANICAL NAME: BOSWELLIA SERRATA ORGANIC ORIGIN: INDIA KEY CONSTITUENTS IN THIS BATCH OF ORGANIC FRANKINCENSE SERRATA OIL % -THUJENE
GC/MS BATCH NUMBER: C80101
 GC/MS BATCH NUMBER: C80101 ESSENTIAL OIL: GERMAN CHAMOMILE BOTANICAL NAME: MATRICARIA RECUTITA ORIGIN: EGYPT KEY CONSTITUENTS PRESENT IN THIS BATCH OF GERMAN CHAMOMILE OIL % α-bisabolol OXIDE A 44.6 trans-β-farnesene
GC/MS BATCH NUMBER: C80101 ESSENTIAL OIL: GERMAN CHAMOMILE BOTANICAL NAME: MATRICARIA RECUTITA ORIGIN: EGYPT KEY CONSTITUENTS PRESENT IN THIS BATCH OF GERMAN CHAMOMILE OIL % α-bisabolol OXIDE A 44.6 trans-β-farnesene
GC/MS BATCH NUMBER: F90100
 GC/MS BATCH NUMBER: F90100 ESSENTIAL OIL: FRANKINCENSE SERRATA CO2 BOTANICAL NAME: BOSWELLIA SERRATA ORIGIN: INDIA KEY CONSTITUENTS PRESENT IN THIS BATCH OF FRANKINCENSE SERRATA OIL % α-thujene 54.9 INCENSYL
GC/MS BATCH NUMBER: F90100 ESSENTIAL OIL: FRANKINCENSE SERRATA CO2 BOTANICAL NAME: BOSWELLIA SERRATA ORIGIN: INDIA KEY CONSTITUENTS PRESENT IN THIS BATCH OF FRANKINCENSE SERRATA OIL % α-thujene 54.9 INCENSYL
GC/MS BATCH NUMBER: J10105
 GC/MS BATCH NUMBER: J10105 ESSENTIAL OIL: JASMINE ABSOLUTE BOTANICAL NAME: JASMINUM SAMBAC ORIGIN: INDIA KEY CONSTITUENTS PRESENT IN THIS BATCH OF JASMINE ABSOLUTE OIL % (3E,6E)-α-FARNESENE 12.0 BENZYL
GC/MS BATCH NUMBER: J10105 ESSENTIAL OIL: JASMINE ABSOLUTE BOTANICAL NAME: JASMINUM SAMBAC ORIGIN: INDIA KEY CONSTITUENTS PRESENT IN THIS BATCH OF JASMINE ABSOLUTE OIL % (3E,6E)-α-FARNESENE 12.0 BENZYL
Presence of Compounds in Ubos (Spondias mombin)
 Presence of Compounds in Ubos (Spondias mombin) Compound Chemical Type Plant Part Plant Origin Quantity Ref # Ascorbic acid Vitamin 165.8 mg M09539 Benzaldehyde Benzenoid Benzene, 4-allyl-1-2-3-trimethoxy:
Presence of Compounds in Ubos (Spondias mombin) Compound Chemical Type Plant Part Plant Origin Quantity Ref # Ascorbic acid Vitamin 165.8 mg M09539 Benzaldehyde Benzenoid Benzene, 4-allyl-1-2-3-trimethoxy:
Customer : Comments and Conclusions : Daniel Dantin - Laboratory director
 - Page 1/5 - Customer : ANN HARMAN Circle H Institute 5951 RIVERBEND BAY FRUITLAND, WA 99129 - USA Sample Nature : ESSENTIAL OIL Botanical name : THYMUS CITRIODORA Common name : THYMUS CITRODORUS Batch
- Page 1/5 - Customer : ANN HARMAN Circle H Institute 5951 RIVERBEND BAY FRUITLAND, WA 99129 - USA Sample Nature : ESSENTIAL OIL Botanical name : THYMUS CITRIODORA Common name : THYMUS CITRODORUS Batch
GC/MS BATCH NUMBER: G30103
 GC/MS BATCH NUMBER: G30103 ESSENTIAL OIL: GERANIUM EGYPTIAN BOTANICAL NAME: PELARGONIUM X ASPERUM ORIGIN: EGYPT KEY CONSTITUENTS PRESENT IN THIS BATCH OF GERANIUM EGYPTIAN OIL % CITRONELLOL 31.6 GERANIOL
GC/MS BATCH NUMBER: G30103 ESSENTIAL OIL: GERANIUM EGYPTIAN BOTANICAL NAME: PELARGONIUM X ASPERUM ORIGIN: EGYPT KEY CONSTITUENTS PRESENT IN THIS BATCH OF GERANIUM EGYPTIAN OIL % CITRONELLOL 31.6 GERANIOL
GC/MS BATCH NUMBER: G50106
 GC/MS BATCH NUMBER: G50106 ESSENTIAL OIL: GRAPEFRUIT PINK BOTANICAL NAME: CITRUS X PARADISI ORIGIN: SOUTH AFRICA KEY CONSTITUENTS PRESENT IN THIS BATCH OF GRAPEFRUIT PINK OIL % LIMONENE + Β-PHELLANDRENE
GC/MS BATCH NUMBER: G50106 ESSENTIAL OIL: GRAPEFRUIT PINK BOTANICAL NAME: CITRUS X PARADISI ORIGIN: SOUTH AFRICA KEY CONSTITUENTS PRESENT IN THIS BATCH OF GRAPEFRUIT PINK OIL % LIMONENE + Β-PHELLANDRENE
GC/MS BATCH NUMBER: J10101
 GC/MS BATCH NUMBER: J10101 ESSENTIAL OIL: JASMINE ABSOLUTE BOTANICAL NAME: JASMINUM SAMBAC ORIGIN: EGYPT KEY CONSTITUENTS PRESENT IN THIS BATCH OF JASMINE ABSOLUTE OIL (E,E)-α-FARNESENE 13.9 LINALOOL 9.5
GC/MS BATCH NUMBER: J10101 ESSENTIAL OIL: JASMINE ABSOLUTE BOTANICAL NAME: JASMINUM SAMBAC ORIGIN: EGYPT KEY CONSTITUENTS PRESENT IN THIS BATCH OF JASMINE ABSOLUTE OIL (E,E)-α-FARNESENE 13.9 LINALOOL 9.5
Phytochemical and Biosynthetic Studies of Lignans, with a Focus on Indonesian Medicinal Plants Elfahmi, [No Value]
![Phytochemical and Biosynthetic Studies of Lignans, with a Focus on Indonesian Medicinal Plants Elfahmi, [No Value] Phytochemical and Biosynthetic Studies of Lignans, with a Focus on Indonesian Medicinal Plants Elfahmi, [No Value]](/thumbs/94/119407236.jpg) University of Groningen Phytochemical and Biosynthetic Studies of Lignans, with a Focus on Indonesian Medicinal Plants Elfahmi, [No Value] IMPORTANT NOTE: You are advised to consult the publisher's version
University of Groningen Phytochemical and Biosynthetic Studies of Lignans, with a Focus on Indonesian Medicinal Plants Elfahmi, [No Value] IMPORTANT NOTE: You are advised to consult the publisher's version
Figure 1 Separation of Ginger oil by (a) Cold Maceration (b) Hot Extraction (c) Liquid- Liquid Extraction -
 Effect of Methods of Separation on Ginger Oil Method of extractions affects how many components of a sample is successfully extracted from the sample. Figure one shows the chromatogram obtained for ginger
Effect of Methods of Separation on Ginger Oil Method of extractions affects how many components of a sample is successfully extracted from the sample. Figure one shows the chromatogram obtained for ginger
GC/MS BATCH NUMBER: L70106
 GC/MS BATCH NUMBER: L70106 ESSENTIAL OIL: LEMON ORGANIC BOTANICAL NAME: CITRUS X LIMON ORIGIN: USA KEY CONSTITUENTS PRESENT IN THIS BATCH OF LEMON ORGANIC OIL % LIMONENE 68.9 β-pinene 9.6 γ-terpinene 8.0
GC/MS BATCH NUMBER: L70106 ESSENTIAL OIL: LEMON ORGANIC BOTANICAL NAME: CITRUS X LIMON ORIGIN: USA KEY CONSTITUENTS PRESENT IN THIS BATCH OF LEMON ORGANIC OIL % LIMONENE 68.9 β-pinene 9.6 γ-terpinene 8.0
ICC Iranian Chemical Communication
 ICC Iranian Chemical Communication Payame Noor University http://icc.journals.pnu.ac.ir Separation identification and antioxidant evaluation of zingiber officinale essential oil Ali Saberi*, Mehri Alimohammadi
ICC Iranian Chemical Communication Payame Noor University http://icc.journals.pnu.ac.ir Separation identification and antioxidant evaluation of zingiber officinale essential oil Ali Saberi*, Mehri Alimohammadi
ENREGISTREMENT DES BULLETINS ANALYTIQUES : CHROMATOGRAPHIE ESSENTIAL OIL CHROMATOGRAPHY SHEET RECORDS
 FORM-LAB005-B Page 1 sur 6 Date : 01/10/2013 Référence produit / Product reference : FLE015 Huile essentielle de / Essential oil of : Camomille Matricaire / German chamomile Numéro de lot / Lot Number
FORM-LAB005-B Page 1 sur 6 Date : 01/10/2013 Référence produit / Product reference : FLE015 Huile essentielle de / Essential oil of : Camomille Matricaire / German chamomile Numéro de lot / Lot Number
SAMPLE IDENTIFICATION ANALYSIS. Date : January 19, 2017
 Date : January 19, 2017 SAMPLE IDENTIFICATION Internal code : 17A03-TOB24-1-DM Customer identification : Geranium - 07-2530-BP Type : Essential Oil Source : Pelargonium graveolens Customer : Serene Living
Date : January 19, 2017 SAMPLE IDENTIFICATION Internal code : 17A03-TOB24-1-DM Customer identification : Geranium - 07-2530-BP Type : Essential Oil Source : Pelargonium graveolens Customer : Serene Living
Study on New Extraction Technology and Chemical Composition of Litsea Cubeba Essential Oil
 The Open Materials Science Journal, 2011, 5, 93-99 93 Open Access Study on New Extraction Technology and Chemical Composition of Litsea Cubeba Essential Oil Guoen Yang *, Guiwu Wang, Xiangzhou Li and Min
The Open Materials Science Journal, 2011, 5, 93-99 93 Open Access Study on New Extraction Technology and Chemical Composition of Litsea Cubeba Essential Oil Guoen Yang *, Guiwu Wang, Xiangzhou Li and Min
GC/MS BATCH NUMBER: J10102
 GC/MS BATCH NUMBER: J10102 ESSENTIAL OIL: JASMINE ABSOLUTE BOTANICAL NAME: JASMINUM SAMBAC ORIGIN: INDIA KEY CONSTITUENTS PRESENT IN THIS BATCH OF JASMINE ABSOLUTE OIL % (E,E)-α-FARNESENE 8.3 δ-cadinene
GC/MS BATCH NUMBER: J10102 ESSENTIAL OIL: JASMINE ABSOLUTE BOTANICAL NAME: JASMINUM SAMBAC ORIGIN: INDIA KEY CONSTITUENTS PRESENT IN THIS BATCH OF JASMINE ABSOLUTE OIL % (E,E)-α-FARNESENE 8.3 δ-cadinene
Chemical Composition and Larvicidal Activities of Zanthoxylum armatum (Rutaceae) against Diamondback Moth, Plutella xylostella
 Chemical Composition and Larvicidal Activities of Zanthoxylum armatum (Rutaceae) against Diamondback Moth, Plutella xylostella Dr. S.G. Eswara Reddy Scientist (Agril. Entomology) CSIR - Institute of Himalayan
Chemical Composition and Larvicidal Activities of Zanthoxylum armatum (Rutaceae) against Diamondback Moth, Plutella xylostella Dr. S.G. Eswara Reddy Scientist (Agril. Entomology) CSIR - Institute of Himalayan
GC/MS BATCH NUMBER: BG0100
 GC/MS BATCH NUMBER: BG0100 ESSENTIAL OIL: BLUE CYPRESS BOTANICAL NAME: CALLITRIS INTRATROPICA ORIGIN: AUSTRALIA KEY CONSTITUENTS PRESENT IN THIS BATCH OF BLUE CYPRESS OIL % GUAIOL 17.1 BULNESOL 10.4 DIHYDROCOLUMELLARIN
GC/MS BATCH NUMBER: BG0100 ESSENTIAL OIL: BLUE CYPRESS BOTANICAL NAME: CALLITRIS INTRATROPICA ORIGIN: AUSTRALIA KEY CONSTITUENTS PRESENT IN THIS BATCH OF BLUE CYPRESS OIL % GUAIOL 17.1 BULNESOL 10.4 DIHYDROCOLUMELLARIN
FT-IR Analysis of column extract (F4) of B.zeylanica. FT-IR Analysis of column extract (F5) of B.zeylanica
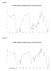 Fig. 39 FT-IR Analysis of column extract (F4) of B.zeylanica Fig. 40 FT-IR Analysis of column extract (F5) of B.zeylanica Fig. 41 FT-IR Analysis of column extract (F1) of P.persica Fig. 42 FT-IR Analysis
Fig. 39 FT-IR Analysis of column extract (F4) of B.zeylanica Fig. 40 FT-IR Analysis of column extract (F5) of B.zeylanica Fig. 41 FT-IR Analysis of column extract (F1) of P.persica Fig. 42 FT-IR Analysis
GC/MS BATCH NUMBER: G40106
 GC/MS BATCH NUMBER: G40106 ESSENTIAL OIL: GINGER ROOT C02 BOTANICAL NAME: ZINGIBER OFFICINALE ORIGIN: INDONESIA KEY CONSTITUENTS PRESENT IN THIS BATCH OF GINGER ROOT C02 OIL % ar-curcumene 13.7 [6]-GINGEROL
GC/MS BATCH NUMBER: G40106 ESSENTIAL OIL: GINGER ROOT C02 BOTANICAL NAME: ZINGIBER OFFICINALE ORIGIN: INDONESIA KEY CONSTITUENTS PRESENT IN THIS BATCH OF GINGER ROOT C02 OIL % ar-curcumene 13.7 [6]-GINGEROL
ENREGISTREMENT DES BULLETINS ANALYTIQUES : CHROMATOGRAPHIE ESSENTIAL OIL CHROMATOGRAPHY SHEET RECORDS
 FORM-LAB005-B Page 1 sur 10 Date : 05/07/2011 Huile essentielle de / Essential oil of : Verveine odorante / Lemon Verbena Numéro de lot / Lot Number : FLE094H110411F Densité à 20 C (g/cm 3 ) / Density
FORM-LAB005-B Page 1 sur 10 Date : 05/07/2011 Huile essentielle de / Essential oil of : Verveine odorante / Lemon Verbena Numéro de lot / Lot Number : FLE094H110411F Densité à 20 C (g/cm 3 ) / Density
GC-MS analysis of rhizome of Gloriosa superba
 RUT Printer and Publisher Print & Online, Open Access, Research Journal Available on http://jbsd.in ISSN: 2229-3469 (Print); ISSN: 2231-024X (Online) Research Article GC-MS analysis of rhizome of Gloriosa
RUT Printer and Publisher Print & Online, Open Access, Research Journal Available on http://jbsd.in ISSN: 2229-3469 (Print); ISSN: 2231-024X (Online) Research Article GC-MS analysis of rhizome of Gloriosa
Analysis of the fatty acids from Periploca sepium by GC-MS and GC-FID
 Analysis of the fatty acids from Periploca sepium by GC-MS and GC-FID Ling Tong, Lei Zhang, Shuanghui Yu, Xiaohui Chen, Kaishun Bi * Department of Pharmacy, Shenyang Pharmaceutical University, Wenhua Road
Analysis of the fatty acids from Periploca sepium by GC-MS and GC-FID Ling Tong, Lei Zhang, Shuanghui Yu, Xiaohui Chen, Kaishun Bi * Department of Pharmacy, Shenyang Pharmaceutical University, Wenhua Road
GC-MS ANALYSIS OF BIOACTIVE CONSTITUENTS OF HYPERICUM MYSORENSE (HYPERICACEAE)
 J. Curr. Chem. Pharm. Sc.: 3(1), 213, 6-15 ISSN 2277-2871 GC-MS ANALYSIS F BIACTIVE CNSTITUENTS F HYPERICUM MYSRENSE (HYPERICACEAE) S. GPINATH, G. SAKTHIDEVI, S. MUTHUKUMARASWAMY a and V. R. MHAN * Ethnopharmacology
J. Curr. Chem. Pharm. Sc.: 3(1), 213, 6-15 ISSN 2277-2871 GC-MS ANALYSIS F BIACTIVE CNSTITUENTS F HYPERICUM MYSRENSE (HYPERICACEAE) S. GPINATH, G. SAKTHIDEVI, S. MUTHUKUMARASWAMY a and V. R. MHAN * Ethnopharmacology
GC/MS BATCH NUMBER: O10106
 GC/MS BATCH NUMBER: O10106 ESSENTIAL OIL: ORANGE BLOOD BOTANICAL NAME: CITRUS SINENSIS ORIGIN: ITALY KEY CONSTITUENTS PRESENT IN THIS BATCH OF ORANGE BLOOD OIL % LIMONENE 93.2 MYRCENE 1.8 Comments from
GC/MS BATCH NUMBER: O10106 ESSENTIAL OIL: ORANGE BLOOD BOTANICAL NAME: CITRUS SINENSIS ORIGIN: ITALY KEY CONSTITUENTS PRESENT IN THIS BATCH OF ORANGE BLOOD OIL % LIMONENE 93.2 MYRCENE 1.8 Comments from
Jan Karlsen and A. Baerheim Svendsen Department of Pharmaceutics, Faculty of Science University of Oslo, P.O.Box 1068, 0316 Oslo, Norway
 Scientia Pharmaceutica (Sci. Pharm.) 70, 87-92 (2002) 0 Osterreichische Apotheker-Verlagsgesellschaft m. b.h., Wien, Printed in Austria The influence of the isolation method on the composition of the essential
Scientia Pharmaceutica (Sci. Pharm.) 70, 87-92 (2002) 0 Osterreichische Apotheker-Verlagsgesellschaft m. b.h., Wien, Printed in Austria The influence of the isolation method on the composition of the essential
CERTIFICATE OF ANALYSIS - GC PROFILING
 Date : May 22, 2018 CERTIFICATE OF ANALYSIS - GC PROFILING SAMPLE IDENTIFICATION Internal code : 18E08-NAD3-1-CC Customer identification : Grapefruit Oil Pink - R122257-04 Type : Essential oil Source :
Date : May 22, 2018 CERTIFICATE OF ANALYSIS - GC PROFILING SAMPLE IDENTIFICATION Internal code : 18E08-NAD3-1-CC Customer identification : Grapefruit Oil Pink - R122257-04 Type : Essential oil Source :
Chemical investigation of semi purified shorea robusta gum resin by using GCMS
 IJPAR Vol.4 Issue 1 Jan-Mar-2015 Journal Home page: www.ijpar.com ISSN: 2320-2831 Research article Open Access Chemical investigation of semi purified shorea robusta gum resin by using GCMS D.Vishakante
IJPAR Vol.4 Issue 1 Jan-Mar-2015 Journal Home page: www.ijpar.com ISSN: 2320-2831 Research article Open Access Chemical investigation of semi purified shorea robusta gum resin by using GCMS D.Vishakante
Determining Terpene Profiles of Cannabis Strains Using GC and GCxGC with High Performance TOFMS
 Determining Terpene Profiles of Cannabis Strains Using GC and GCxGC with High Performance TOFMS LECO Corporation; Saint Joseph, Michigan USA Key Words: GC-MS, GC GC-MS, GC GC-TOFMS, Pegasus BT 4D, cannabis,
Determining Terpene Profiles of Cannabis Strains Using GC and GCxGC with High Performance TOFMS LECO Corporation; Saint Joseph, Michigan USA Key Words: GC-MS, GC GC-MS, GC GC-TOFMS, Pegasus BT 4D, cannabis,
GC/MS BATCH NUMBER: J10104
 GC/MS BATCH NUMBER: J10104 ESSENTIAL OIL: JASMINE ABSOLUTE BOTANICAL NAME: JASMINUM SAMBAC ORIGIN: INDIA KEY CONSTITUENTS PRESENT IN THIS BATCH OF JASMINE OIL (E,E)-α-FARNESENE 9.4 BENZYL ACETATE 6.3 %
GC/MS BATCH NUMBER: J10104 ESSENTIAL OIL: JASMINE ABSOLUTE BOTANICAL NAME: JASMINUM SAMBAC ORIGIN: INDIA KEY CONSTITUENTS PRESENT IN THIS BATCH OF JASMINE OIL (E,E)-α-FARNESENE 9.4 BENZYL ACETATE 6.3 %
GC/MS BATCH NUMBER: H20106
 GC/MS BATCH NUMBER: H20106 ESSENTIAL OIL: HELICHRYSUM ITALICUM BOTANICAL NAME: HELICHRYSUM ITALICUM ORIGIN: BOSNIA AND HERZEGOVINA KEY CONSTITUENTS PRESENT IN THIS BATCH OF HELICHRYSUM ITALICUM OIL % Α-PINENE
GC/MS BATCH NUMBER: H20106 ESSENTIAL OIL: HELICHRYSUM ITALICUM BOTANICAL NAME: HELICHRYSUM ITALICUM ORIGIN: BOSNIA AND HERZEGOVINA KEY CONSTITUENTS PRESENT IN THIS BATCH OF HELICHRYSUM ITALICUM OIL % Α-PINENE
Scholars Research Library. Annals of Biological Research, 2012, 3 (2): (http://scholarsresearchlibrary.com/archive.html)
 Available online at www.scholarsresearchlibrary.com Annals of Biological Research, 2012, 3 (2):1117-1124 (http://scholarsresearchlibrary.com/archive.html) ISSN 0976-1233 CODEN (USA): ABRNBW The Quantitative
Available online at www.scholarsresearchlibrary.com Annals of Biological Research, 2012, 3 (2):1117-1124 (http://scholarsresearchlibrary.com/archive.html) ISSN 0976-1233 CODEN (USA): ABRNBW The Quantitative
GC/MS BATCH NUMBER: F00100
 GC/MS BATCH NUMBER: F00100 ESSENTIAL OIL: FRANKINCENSE ORGANIC BOTANICAL NAME: BOSWELLIA CARTERI ORIGIN: SOMALIA KEY CONSTITUENTS IN THIS BATCH OF ORGANIC FRANKINCENSE CARTERI OIL % 1-METHOXY DECANE 22.5
GC/MS BATCH NUMBER: F00100 ESSENTIAL OIL: FRANKINCENSE ORGANIC BOTANICAL NAME: BOSWELLIA CARTERI ORIGIN: SOMALIA KEY CONSTITUENTS IN THIS BATCH OF ORGANIC FRANKINCENSE CARTERI OIL % 1-METHOXY DECANE 22.5
GC/MS BATCH NUMBER: C50101
 GC/MS BATCH NUMBER: C50101 ESSENTIAL OIL: CEDARWOOD HIMALAYAN BOTANICAL NAME: CEDRUS DEODARA ORIGIN: NEPAL KEY CONSTITUENTS PRESENT IN THIS BATCH OF CEDARWOOD DEODORA OIL % -HIMACHALENE 37.0 -HIMACHALENE
GC/MS BATCH NUMBER: C50101 ESSENTIAL OIL: CEDARWOOD HIMALAYAN BOTANICAL NAME: CEDRUS DEODARA ORIGIN: NEPAL KEY CONSTITUENTS PRESENT IN THIS BATCH OF CEDARWOOD DEODORA OIL % -HIMACHALENE 37.0 -HIMACHALENE
GC/MS BATCH NUMBER: R30102
 GC/MS BATCH NUMBER: R30102 ESSENTIAL OIL: ROSE ABSOLUTE BOTANICAL NAME: ROSA X DAMASCENA ORIGIN: MOROCCO KEY CONSTITUENTS PRESENT IN THIS BATCH OF ROSE ABSOLUTE OIL % PHENYLETHYL ALCOHOL 24.6 CITRONELLOL
GC/MS BATCH NUMBER: R30102 ESSENTIAL OIL: ROSE ABSOLUTE BOTANICAL NAME: ROSA X DAMASCENA ORIGIN: MOROCCO KEY CONSTITUENTS PRESENT IN THIS BATCH OF ROSE ABSOLUTE OIL % PHENYLETHYL ALCOHOL 24.6 CITRONELLOL
CERTIFICATE OF ANALYSIS - GC PROFILING
 Date : May 22, 2018 CERTIFICATE OF ANALYSIS - GC PROFILING SAMPLE IDENTIFICATION Internal code : 18E08-NAD2-1-CC Customer identification : Lemon Oil - Argentina - R122257-02 Type : Essential oil Source
Date : May 22, 2018 CERTIFICATE OF ANALYSIS - GC PROFILING SAMPLE IDENTIFICATION Internal code : 18E08-NAD2-1-CC Customer identification : Lemon Oil - Argentina - R122257-02 Type : Essential oil Source
Journal of Global Trends in Pharmaceutical Sciences
 An Elsevier Indexed Journal ISSN-2230-7346 Journal of Global Trends in Pharmaceutical Sciences Research Article STIGMASTEROL, CAMPESTEROL AND BRASSICOSTEROL FROM HIBISCUS SABDARIFFA SEED Devarakonda Ramadevi*
An Elsevier Indexed Journal ISSN-2230-7346 Journal of Global Trends in Pharmaceutical Sciences Research Article STIGMASTEROL, CAMPESTEROL AND BRASSICOSTEROL FROM HIBISCUS SABDARIFFA SEED Devarakonda Ramadevi*
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Chemical composition and biological activities of the essential oil from Rubus pungens var. oldhamii Yaojie Zhang, Jiajing Chen, Lizhi Wang, Jingjing Cao,and Lishan Xu* College of
SUPPLEMENTARY MATERIAL Chemical composition and biological activities of the essential oil from Rubus pungens var. oldhamii Yaojie Zhang, Jiajing Chen, Lizhi Wang, Jingjing Cao,and Lishan Xu* College of
GC/MS BATCH NUMBER: C90110
 GC/MS BATCH NUMBER: C90110 ESSENTIAL OIL: CHAMOMILE ROMAN BOTANICAL NAME: CHAMAEMELUM NOBILE ORIGIN: UNITED KINGDOM KEY CONSTITUENTS PRESENT IN THIS BATCH OF CHAMOMILE ROMAN OIL % 3-METHYLAMYL ANGELATE
GC/MS BATCH NUMBER: C90110 ESSENTIAL OIL: CHAMOMILE ROMAN BOTANICAL NAME: CHAMAEMELUM NOBILE ORIGIN: UNITED KINGDOM KEY CONSTITUENTS PRESENT IN THIS BATCH OF CHAMOMILE ROMAN OIL % 3-METHYLAMYL ANGELATE
Received: ; Revised; Accepted:
 International Journal of Institutional Pharmacy and Life Sciences 2(5): September-October 2012 INTERNATIONAL JOURNAL OF INSTITUTIONAL PHARMACY AND LIFE SCIENCES Life Sciences Original Article!!! Received:
International Journal of Institutional Pharmacy and Life Sciences 2(5): September-October 2012 INTERNATIONAL JOURNAL OF INSTITUTIONAL PHARMACY AND LIFE SCIENCES Life Sciences Original Article!!! Received:
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Chemical composition and antimicrobial activities of essential oil from Wedelia urticifolia growing wild in Hunan Province, China Junpeng Hu a,mengmeng Jia a,liang Zhu a * a School
SUPPLEMENTARY MATERIAL Chemical composition and antimicrobial activities of essential oil from Wedelia urticifolia growing wild in Hunan Province, China Junpeng Hu a,mengmeng Jia a,liang Zhu a * a School
Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry Journal home page:
 Banu Rekha J and Jaykar. / Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry. 3(2), 2015, 76-81. Research Article CODEN: AJPAD7 ISSN: 2321-0923 Asian Journal of Pharmaceutical Analysis and
Banu Rekha J and Jaykar. / Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry. 3(2), 2015, 76-81. Research Article CODEN: AJPAD7 ISSN: 2321-0923 Asian Journal of Pharmaceutical Analysis and
Screening of Phytochemistry and Secondary Metabolites: A Case Study on Nyctanthes arboritis
 Research Article Screening of Phytochemistry and Secondary Metabolites: A Case Study on Nyctanthes arboritis *B. Ramachandran, M. Kamaraj, V. Subramani, J. Jerome Jeyakumar PG and Research Department of
Research Article Screening of Phytochemistry and Secondary Metabolites: A Case Study on Nyctanthes arboritis *B. Ramachandran, M. Kamaraj, V. Subramani, J. Jerome Jeyakumar PG and Research Department of
Simplified Cannabis Terpene Profiling by GCMS
 Gas Chromatograph Mass Spectrometer Simplified Cannabis Terpene Profiling by GCMS No. GCMS-1604 Introduction Terpene and terpenoid compounds are naturally occurring aromatic compounds that give cannabis
Gas Chromatograph Mass Spectrometer Simplified Cannabis Terpene Profiling by GCMS No. GCMS-1604 Introduction Terpene and terpenoid compounds are naturally occurring aromatic compounds that give cannabis
DETECTION OF BEESWAX ADULTERATION WITH HYDROCARBONS USING GAS CHROMATOGRAPHY WITH MASS DETECTOR (GC-MS)
 DETECTION OF BEESWAX ADULTERATION WITH HYDROCARBONS USING GAS CHROMATOGRAPHY WITH MASS DETECTOR (GC-MS) Ewa Waś,, Helena Rybak-Chmielewska, Teresa Szczęsna e-mail: ewa.was@man.pulawy.pl Research Institute
DETECTION OF BEESWAX ADULTERATION WITH HYDROCARBONS USING GAS CHROMATOGRAPHY WITH MASS DETECTOR (GC-MS) Ewa Waś,, Helena Rybak-Chmielewska, Teresa Szczęsna e-mail: ewa.was@man.pulawy.pl Research Institute
Isolation and characterization of stigmast- 5-en-3β-ol (β-sitosterol) from the leaves of Hygrophila spinosa T. Anders
 Isolation and characterization of stigmast- 5-en-3β-ol (β-sitosterol) from the leaves of Hygrophila spinosa T. Anders Arjun Patra 1*, S. Jha 2, P.N. Murthy 3, Manik 2, A. Sharone 2 1 College of Pharmacy,
Isolation and characterization of stigmast- 5-en-3β-ol (β-sitosterol) from the leaves of Hygrophila spinosa T. Anders Arjun Patra 1*, S. Jha 2, P.N. Murthy 3, Manik 2, A. Sharone 2 1 College of Pharmacy,
Received; accepted CHEMICAL COMPOSITION AND ANTIMICROBIAL PROPERTIES OF ESSENTIAL OIL OF AGONIS FLEXUOSA
 International Journal of Institutional Pharmacy and Life Sciences 1(2): September-October 2011 INTERNATIONAL JOURNAL OF INSTITUTIONAL PHARMACY AND LIFE SCIENCES Life Sciences Short Communication!!! Received;
International Journal of Institutional Pharmacy and Life Sciences 1(2): September-October 2011 INTERNATIONAL JOURNAL OF INSTITUTIONAL PHARMACY AND LIFE SCIENCES Life Sciences Short Communication!!! Received;
Oxidation of essential oil of Chloroxylon swietenia (Roxb. corom)
 Abstract Research Journal of Recent Sciences ISSN 2277-2502 Oxidation of essential oil of Chloroxylon swietenia (Roxb. corom) Telang T. Department of Chemistry and Applied Sciences, Career Point University,
Abstract Research Journal of Recent Sciences ISSN 2277-2502 Oxidation of essential oil of Chloroxylon swietenia (Roxb. corom) Telang T. Department of Chemistry and Applied Sciences, Career Point University,
Essential Oil Composition from Juniperus communis Originated from Albania
 Essential Oil Composition from Juniperus communis Originated from Albania A. Buci 1, E. Hodaj-Çeliku 2,3,, H. Manaj 1, S. Abazi 4, S. Drushku 1, D. Lazari 3. 1 Industrial Chemistry Department, Natural
Essential Oil Composition from Juniperus communis Originated from Albania A. Buci 1, E. Hodaj-Çeliku 2,3,, H. Manaj 1, S. Abazi 4, S. Drushku 1, D. Lazari 3. 1 Industrial Chemistry Department, Natural
Keywords: fragrance, callus culture, Michelia alba, essential oil, linalool, hydrodistillation
 The Fragrance Production from Callus Cultures of Gynoecium and Stamen of Michelia alba Flower Nor aishah Abu Shah 1,, Radzali Muse 2 and Mohd Aspollah Sukari 3, 1 Faculty of Applied Science, UiTM Negeri
The Fragrance Production from Callus Cultures of Gynoecium and Stamen of Michelia alba Flower Nor aishah Abu Shah 1,, Radzali Muse 2 and Mohd Aspollah Sukari 3, 1 Faculty of Applied Science, UiTM Negeri
Analysis of FAMEs Using Cold EI GC/MS for Enhanced Molecular Ion Selectivity
 APPLICATION NOTE Gas Chromatography/ Mass Spectrometry Author: Adam Patkin PerkinElmer, Inc. Shelton, CT Analysis of FAMEs Using GC/MS for Enhanced Molecular Ion Selectivity Introduction Characterization
APPLICATION NOTE Gas Chromatography/ Mass Spectrometry Author: Adam Patkin PerkinElmer, Inc. Shelton, CT Analysis of FAMEs Using GC/MS for Enhanced Molecular Ion Selectivity Introduction Characterization
International Journal of Ayurveda and Pharma Research
 Int. J. Ayur. Pharma Research, 2014; 2(2): 47-52 ISSN: 2322-0910 International Journal of Ayurveda and Pharma Research Research Article ANALYSIS OF THE ESSENTIAL OIL FROM THE LEAVES OF WRIGHTIA TINCTORIA
Int. J. Ayur. Pharma Research, 2014; 2(2): 47-52 ISSN: 2322-0910 International Journal of Ayurveda and Pharma Research Research Article ANALYSIS OF THE ESSENTIAL OIL FROM THE LEAVES OF WRIGHTIA TINCTORIA
GC-MS analysis of bioactive constituents of Hibiscus flower
 AUSTRALIAN JOURNAL OF BASIC AND APPLIED SCIENCES ISSN:1991-8178 EISSN: 2309-8414 Journal home page: www.ajbasweb.com GC-MS analysis of bioactive constituents of Hibiscus flower Hesham H. A. Rassem, Abdurahman
AUSTRALIAN JOURNAL OF BASIC AND APPLIED SCIENCES ISSN:1991-8178 EISSN: 2309-8414 Journal home page: www.ajbasweb.com GC-MS analysis of bioactive constituents of Hibiscus flower Hesham H. A. Rassem, Abdurahman
Received: ; Revised; Accepted: PHYTOCHEMICAL AND GC-MS ANALYSIS OF EUPHORBIA HIRTA LINN.LEAF
 International Standard Serial Number (ISSN): 2249-6807 International Journal of Institutional Pharmacy and Life Sciences 2(3): May-June 2012 INTERNATIONAL JOURNAL OF INSTITUTIONAL PHARMACY AND LIFE SCIENCES
International Standard Serial Number (ISSN): 2249-6807 International Journal of Institutional Pharmacy and Life Sciences 2(3): May-June 2012 INTERNATIONAL JOURNAL OF INSTITUTIONAL PHARMACY AND LIFE SCIENCES
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 9841 Third edition 13-10-15 Essential oil of hyssop (Hyssopus officinalis L. ssp. officinalis) Huile essentielle d hysope (Hyssopus officinalis L. ssp. officinalis) Reference
INTERNATIONAL STANDARD ISO 9841 Third edition 13-10-15 Essential oil of hyssop (Hyssopus officinalis L. ssp. officinalis) Huile essentielle d hysope (Hyssopus officinalis L. ssp. officinalis) Reference
GC-MS Analysis of Bioactive Components of Aerial parts of Fluggea leucopyrus Willd. (Euphorbiaceae)
 . Journal of Applied Pharmaceutical Science Vol. 3 (5), pp. 126-13, May, 213 Available online at http://www.japsonline.com DOI: 1.7324/JAPS.213.3524 ISSN 2231-3354 Short Communication GC-MS Analysis of
. Journal of Applied Pharmaceutical Science Vol. 3 (5), pp. 126-13, May, 213 Available online at http://www.japsonline.com DOI: 1.7324/JAPS.213.3524 ISSN 2231-3354 Short Communication GC-MS Analysis of
Unit title: Aromatherapy Chemistry (SCQF level 8)
 Higher National unit specification General information Unit code: HL91 35 Superclass: HK Publication date: May 2017 Source: Scottish Qualifications Authority Version: 01 Unit purpose This unit is designed
Higher National unit specification General information Unit code: HL91 35 Superclass: HK Publication date: May 2017 Source: Scottish Qualifications Authority Version: 01 Unit purpose This unit is designed
Isolation and identification of six terpenes extracted from leaves of Inula viscosa (L.) grown in Syria
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2016, 8(1):27-31 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Isolation and identification of six terpenes extracted
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2016, 8(1):27-31 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Isolation and identification of six terpenes extracted
Compounds Identification from Hypersaline Oscillatoria Salina Using GC-MS Analysis
 International Journal of Research Studies in Biosciences (IJRSB) Volume 3, Issue 9, September 2015, PP 6-10 ISSN 2349-0357 (Print) & ISSN 2349-0365 (Online) www.arcjournals.org Compounds Identification
International Journal of Research Studies in Biosciences (IJRSB) Volume 3, Issue 9, September 2015, PP 6-10 ISSN 2349-0357 (Print) & ISSN 2349-0365 (Online) www.arcjournals.org Compounds Identification
Chromatography Vacuum Ultraviolet Spectroscopy
 Application Note Differentiation and Determination Differentiation and Determination of Fatty Acid Methyl of Fatty Esters Acid by Gas Methyl Chromatography Esters by Vacuum Gas Ultraviolet Spectroscopy
Application Note Differentiation and Determination Differentiation and Determination of Fatty Acid Methyl of Fatty Esters Acid by Gas Methyl Chromatography Esters by Vacuum Gas Ultraviolet Spectroscopy
terpenoids terpenoids isoprenoids terpenes isoprene units terpenoids isoprene units
 terpenoids In general, terpenoids, may be defined as natural products whose structures are considered to be divided into several isoprene units; therefore, these compounds are invariably termed as isoprenoids.
terpenoids In general, terpenoids, may be defined as natural products whose structures are considered to be divided into several isoprene units; therefore, these compounds are invariably termed as isoprenoids.
Analysis of non-polar chemical profile of Melia azedarach L.
 Analysis of non-polar chemical profile of Melia azedarach L. Habib R. * 1, A. Mohyuddin 1, Z. Khan 2, T. Mahmood 3 1 Department of Chemistry, University of Management and Technology, C-II, Johar Town,
Analysis of non-polar chemical profile of Melia azedarach L. Habib R. * 1, A. Mohyuddin 1, Z. Khan 2, T. Mahmood 3 1 Department of Chemistry, University of Management and Technology, C-II, Johar Town,
Scholars Research Library. Phytochemical studies and GC/MS analysis on the isolated essential oil from the leaves of Citrus Aurantium Linn
 Available online at www.scholarsresearchlibrary.com J. Nat. Prod. Plant Resour., 2013, 3 (6):19-23 (http://scholarsresearchlibrary.com/archive.html) ISSN : 2231 3184 CODEN (USA): JNPPB7 Phytochemical studies
Available online at www.scholarsresearchlibrary.com J. Nat. Prod. Plant Resour., 2013, 3 (6):19-23 (http://scholarsresearchlibrary.com/archive.html) ISSN : 2231 3184 CODEN (USA): JNPPB7 Phytochemical studies
Chemical Composition of the Essential Oil from Aerial Parts of Astragalus schahrudensis Bge. from Northeast of Iran
 Jeobp 12 (1) 2009 pp 59-63 59 ISSN 0972-060X Chemical Composition of the Essential Oil from Aerial Parts of Astragalus schahrudensis Bge. from Northeast of Iran Hashem Akhlaghi Department of Basic Sciences,
Jeobp 12 (1) 2009 pp 59-63 59 ISSN 0972-060X Chemical Composition of the Essential Oil from Aerial Parts of Astragalus schahrudensis Bge. from Northeast of Iran Hashem Akhlaghi Department of Basic Sciences,
Key Words:- Crude oil, Egyptian central Gulf of Suez, Chemical fingerprint, Capillary gas chromatography, Carbon number distribution.
 Chemical Fingerprinting of some Egyptian crude oils by gas chromatography Ashraf Yehia El-Naggar Chemistry department, Faculty of Science, Taif University, Kingdom of Saudi Arabia Egyptian Petroleum Research
Chemical Fingerprinting of some Egyptian crude oils by gas chromatography Ashraf Yehia El-Naggar Chemistry department, Faculty of Science, Taif University, Kingdom of Saudi Arabia Egyptian Petroleum Research
Rapid Analysis of 37 FAMEs with the Agilent 8860 Gas Chromatograph
 Application Note Food Rapid Analysis of 37 FAMEs with the Agilent 88 Gas Chromatograph Author Youjuan Zhang Agilent Technologies (Shanghai) Co. Ltd., Shanghai 131 P. R. China Abstract An Agilent 88 GC
Application Note Food Rapid Analysis of 37 FAMEs with the Agilent 88 Gas Chromatograph Author Youjuan Zhang Agilent Technologies (Shanghai) Co. Ltd., Shanghai 131 P. R. China Abstract An Agilent 88 GC
Chemical compositions and mosquito repellency of essential oils from Artabotrys hexapetalus and Artabotrys rupestris
 Available online at http://ajol.info/index.php/ijbcs Int. J. Biol. Chem. Sci. 8(6): 2804-2812, December 2014 ISSN 1997-342X (Online), ISSN 1991-8631 (Print) Chemical compositions and mosquito repellency
Available online at http://ajol.info/index.php/ijbcs Int. J. Biol. Chem. Sci. 8(6): 2804-2812, December 2014 ISSN 1997-342X (Online), ISSN 1991-8631 (Print) Chemical compositions and mosquito repellency
DET REPORT NO. 69 JUNE 2015
 1.) THINKING BEYOND THE NPD BOX - INEXPENSIVE CONVERSION OF NPD EQUIPMENT TO MULTIPLE MODES OF SELECTIVE GC DETECTION. 2.) GC-CCID DIFFERENTIATION BETWEEN SATURATE VS. UNSATURATE AND MONO-UNSATURATE VS.
1.) THINKING BEYOND THE NPD BOX - INEXPENSIVE CONVERSION OF NPD EQUIPMENT TO MULTIPLE MODES OF SELECTIVE GC DETECTION. 2.) GC-CCID DIFFERENTIATION BETWEEN SATURATE VS. UNSATURATE AND MONO-UNSATURATE VS.
creening of Natural/Synthetic Compounds for Antimalarial Activity
 PARASITE BILGY S creening of atural/synthetic Compounds for Antimalarial Activity Plants have been used as a traditional medicine for the treatment of malaria. Plants may provide drugs directly such as
PARASITE BILGY S creening of atural/synthetic Compounds for Antimalarial Activity Plants have been used as a traditional medicine for the treatment of malaria. Plants may provide drugs directly such as
Determination of Bioactive Components of Cynodon dactylon by GC-MS Analysis. R.K. Jananie, V. Priya, K. Vijaya Lakshmi a*
 Determination of Bioactive Components of Cynodon dactylon by GC-MS Analysis R.K. Jananie, V. Priya, K. Vijaya Lakshmi a* * Department of Biochemistry, Barathi Women s College, Chennai-600108, Tamil Nadu,
Determination of Bioactive Components of Cynodon dactylon by GC-MS Analysis R.K. Jananie, V. Priya, K. Vijaya Lakshmi a* * Department of Biochemistry, Barathi Women s College, Chennai-600108, Tamil Nadu,
ENREGISTREMENT DES BULLETINS ANALYTIQUES : CHROMATOGRAPHIE ESSENTIAL OIL CHROMATOGRAPHY SHEET RECORDS
 FORM-LAB005-B Page 1 sur 6 Date : 18/11/2013 Référence produit / Product reference : FLE043 Huile essentielle de / Essential oil of : Helichryse Italienne / Helichrysum Italian Numéro de lot / Lot Number
FORM-LAB005-B Page 1 sur 6 Date : 18/11/2013 Référence produit / Product reference : FLE043 Huile essentielle de / Essential oil of : Helichryse Italienne / Helichrysum Italian Numéro de lot / Lot Number
Chemistry 324W Fall 2010 Experiment 3 GC-MS Analysis of Commercial Product Fragrances E. S. (student)
 Chemistry 324W Fall 2010 Experiment 3 GC-MS Analysis of Commercial Product Fragrances E. S. (student) Introduction In this work we analyzed the common fragrance compounds in shampoo.it is assumed that
Chemistry 324W Fall 2010 Experiment 3 GC-MS Analysis of Commercial Product Fragrances E. S. (student) Introduction In this work we analyzed the common fragrance compounds in shampoo.it is assumed that
COMPARATIVE STUDIES ON EXTRACTION OF OIL FROM LEMON GRASS
 NATIONAL JOURNAL OF ARTS, COMMERCE & SCIENTIFIC RESEARCH REVIEW A REFEREED PEER REVIEW BI-ANNUAL ONLINE JOURNAL PAPER ID:- NJACSRR/JAN-JUN 2018/VOL-5/ ISS-1/A2 PAGE NO.10-14 IMPACT FACTOR (2017): 5.9781
NATIONAL JOURNAL OF ARTS, COMMERCE & SCIENTIFIC RESEARCH REVIEW A REFEREED PEER REVIEW BI-ANNUAL ONLINE JOURNAL PAPER ID:- NJACSRR/JAN-JUN 2018/VOL-5/ ISS-1/A2 PAGE NO.10-14 IMPACT FACTOR (2017): 5.9781
ABTRACT. Key words: solvent extraction, XAD-2 resin, free and glycosidically bound volatile compound, kaffir lime leaves
 Kasetsart J. (Nat. Sci.) 41 : 300-306 (2007) Comparative Study on Extraction Methods of Free and Glycosidically Bound Volatile Compounds from Kaffir Lime Leaves by Solvent Extraction and Solid Phase Extraction
Kasetsart J. (Nat. Sci.) 41 : 300-306 (2007) Comparative Study on Extraction Methods of Free and Glycosidically Bound Volatile Compounds from Kaffir Lime Leaves by Solvent Extraction and Solid Phase Extraction
Catalysts of Lipid Oxidation
 Catalysts of Lipid Oxidation Iron The most important nonenzymic catalyst for initiation of lipid peroxidation The most abundant transitional metal in biological systems Possibility of various oxidation
Catalysts of Lipid Oxidation Iron The most important nonenzymic catalyst for initiation of lipid peroxidation The most abundant transitional metal in biological systems Possibility of various oxidation
TABLE - 1. Physicochemical analysis and extractive values of (Percentage) leaf powder of Aegle marmelos Var. I and Var. III
 TABLE - 1 Physicochemical analysis and extractive values of (Percentage) leaf powder of Aegle marmelos Var. I and Var. III Sl.No Parameters Variant-I (%) 1 Organoleptic characteristics a. Appearance b.
TABLE - 1 Physicochemical analysis and extractive values of (Percentage) leaf powder of Aegle marmelos Var. I and Var. III Sl.No Parameters Variant-I (%) 1 Organoleptic characteristics a. Appearance b.
Research Article. GC-MS studies and phytochemical screening of Sesbania grandiflora L.
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(9):4347 Research Article ISSN : 09757384 CODEN(USA) : JCPRC5 GCMS studies and phytochemical screening of Sesbania
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(9):4347 Research Article ISSN : 09757384 CODEN(USA) : JCPRC5 GCMS studies and phytochemical screening of Sesbania
Reduction of Volatiles from Odorous Personal Care Ingredients with Honeywell Asensa DS 912 Odor Absorbing Zeolite
 SYNOPSIS Test results show that by adding Honeywell Asensa DS 912 odor absorbing zeolite at a 5% level to personal care ingredients which have malodors can substantially reduce the total volatiles measured
SYNOPSIS Test results show that by adding Honeywell Asensa DS 912 odor absorbing zeolite at a 5% level to personal care ingredients which have malodors can substantially reduce the total volatiles measured
Quantitative Analysis of -Hydroxybutyrate in Hair Using Target Analyte Finding Processing of Comprehensive GC-HRT Data
 Quantitative Analysis of -Hydroxybutyrate in Hair Using Target Analyte Finding Processing of Comprehensive GC-HRT Data LECO Corporation; Saint Joseph, Michigan USA Key Words: Pegasus GC-HRT, GHB, Hair,
Quantitative Analysis of -Hydroxybutyrate in Hair Using Target Analyte Finding Processing of Comprehensive GC-HRT Data LECO Corporation; Saint Joseph, Michigan USA Key Words: Pegasus GC-HRT, GHB, Hair,
Chemical Composition of the Essential Oil of Lavandula angustifolia from Syria
 Chemical Composition of the Essential Oil of Lavandula angustifolia from Syria Gassan al-wassouf Ph.D. Department of Organic Chemistry, Al-Baath University Homs, Syria Abstract Chemical composition of
Chemical Composition of the Essential Oil of Lavandula angustifolia from Syria Gassan al-wassouf Ph.D. Department of Organic Chemistry, Al-Baath University Homs, Syria Abstract Chemical composition of
Characterization of sixty alkenes in a cat-cracked gasoline naphtha by gas chromatography
 REFINERY OLEFINES HEXENES ISOMERS ANALYSIS PETROL PROPORTIONS HEPTENES SEPARATION GC-MS Open access accepted manuscript version of Chromatographia 38 (1994) 222-226 Link to publisher Characterization of
REFINERY OLEFINES HEXENES ISOMERS ANALYSIS PETROL PROPORTIONS HEPTENES SEPARATION GC-MS Open access accepted manuscript version of Chromatographia 38 (1994) 222-226 Link to publisher Characterization of
CHEMICAL INVESTIGATION OF THE BARK OF ADENANTHERA PAVONINA LINN
 Int. J. Chem. Sci.: 10(1), 2012, 98-103 ISSN 0972-768X www.sadgurupublications.com CHEMICAL INVESTIGATION OF THE BARK OF ADENANTHERA PAVONINA LINN ARZUMAND ARA, MD. ABUL HASHEM * and TANVIR MUSLIM a Department
Int. J. Chem. Sci.: 10(1), 2012, 98-103 ISSN 0972-768X www.sadgurupublications.com CHEMICAL INVESTIGATION OF THE BARK OF ADENANTHERA PAVONINA LINN ARZUMAND ARA, MD. ABUL HASHEM * and TANVIR MUSLIM a Department
Mass Spectral Fragmentation Studies of Coumarin-Type Compounds Using GC High-Resolution MS
 The Open Analytical Chemistry Journal, 2011, 5, 27-36 27 Open Access Mass Spectral Fragmentation Studies of Coumarin-Type Compounds Using GC High-Resolution MS Viorica Lopez-Avila * and George Yefchak
The Open Analytical Chemistry Journal, 2011, 5, 27-36 27 Open Access Mass Spectral Fragmentation Studies of Coumarin-Type Compounds Using GC High-Resolution MS Viorica Lopez-Avila * and George Yefchak
Determination of New Isomer of Palmitoleic Acid in Mucuna Pruriens Seed Oil
 Determination of New Isomer of Palmitoleic Acid in Mucuna Pruriens Seed Oil A.Rastogi (Corresponding author) Department of Chemistry, National Institute of Technology, Raipur-492001 INDIA arti.rastogi20@gmail.com
Determination of New Isomer of Palmitoleic Acid in Mucuna Pruriens Seed Oil A.Rastogi (Corresponding author) Department of Chemistry, National Institute of Technology, Raipur-492001 INDIA arti.rastogi20@gmail.com
Type Analysis of Citrus Essential Oils by Multidimensional Supercritical Fluid Chromatography/ Gas Chromatography
 25 Type Analysis of Citrus Essential Oils by Multidimensional Supercritical Fluid Chromatography/ Gas Chromatography Takashi YARITA, Akira NoMURA and Yoshiyuki HoRIMOTo Department of Analytical Chemistry,
25 Type Analysis of Citrus Essential Oils by Multidimensional Supercritical Fluid Chromatography/ Gas Chromatography Takashi YARITA, Akira NoMURA and Yoshiyuki HoRIMOTo Department of Analytical Chemistry,
Amudha S et al., Asian Journal of Pharmthiaceutical Technology & Innovation, 04 (21); 2016; Research Article
 Asian Journal of Pharmaceutical Technology & Innovation ISSN: 2347-8810 Research Article Received on: 09-11-2016 Accepted on: 20-11-2016 Published on: 15-12-2016 Corresponding Author: * Amudha S, Dept.
Asian Journal of Pharmaceutical Technology & Innovation ISSN: 2347-8810 Research Article Received on: 09-11-2016 Accepted on: 20-11-2016 Published on: 15-12-2016 Corresponding Author: * Amudha S, Dept.
Analysis of Light Crude Oil Using Gas Chromatography High Resolution Time-of-Flight Mass Spectrometry
 nalysis of Light Crude Oil Using Gas Chromatography High Resolution Time-of-Flight ass pectrometry LECO Corporation; aint Joseph, ichigan U Keywords: GC, High Resolution, Time-of-Flight, Light Crude Oil
nalysis of Light Crude Oil Using Gas Chromatography High Resolution Time-of-Flight ass pectrometry LECO Corporation; aint Joseph, ichigan U Keywords: GC, High Resolution, Time-of-Flight, Light Crude Oil
CHEMICAL COMPOSITION OF VOLATILE OIL OF NIGELLA SATIVA SEEDS
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES SJIF Impact Factor 2.786 Volume 3, Issue 10, 1588-1594. Research Article ISSN 2278 4357 CHEMICAL COMPOSITION OF VOLATILE OIL OF NIGELLA SATIVA SEEDS
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES SJIF Impact Factor 2.786 Volume 3, Issue 10, 1588-1594. Research Article ISSN 2278 4357 CHEMICAL COMPOSITION OF VOLATILE OIL OF NIGELLA SATIVA SEEDS
PROFILING OF CENTELLA ASIATICA (L.) URBAN EXTRACT
 The Malaysian Journal of Analytical Sciences, Vol 12, No 2 (2008): 322-327 PROFILING OF CENTELLA ASIATICA (L.) URBAN EXTRACT N.A. Zainol, S.C. Voo, M.R. Sarmidi, R.A. Aziz Faculty Of Chemical and Natural
The Malaysian Journal of Analytical Sciences, Vol 12, No 2 (2008): 322-327 PROFILING OF CENTELLA ASIATICA (L.) URBAN EXTRACT N.A. Zainol, S.C. Voo, M.R. Sarmidi, R.A. Aziz Faculty Of Chemical and Natural
Determination of Allergens in Fragrance Products Using Agilent Deconvolution Reporting Software Application Brief
 Determination of Allergens in Fragrance Products Using Agilent Deconvolution Reporting Software Application Brief Wei Luan, Chris Sandy, and Mike Szelewski Flavor and Fragrance The 7th amendment of the
Determination of Allergens in Fragrance Products Using Agilent Deconvolution Reporting Software Application Brief Wei Luan, Chris Sandy, and Mike Szelewski Flavor and Fragrance The 7th amendment of the
Extraction and Qualitative Analysis of Piper Betle Leaves for Antimicrobial Activities
 Extraction and Qualitative Analysis of Piper Betle Leaves for Antimicrobial Activities Lee Weng Foo, Eraricar Salleh and Siti Nur Hana Mamat Bioprocess Engineering Department, Faculty of Chemical Engineering,Universiti
Extraction and Qualitative Analysis of Piper Betle Leaves for Antimicrobial Activities Lee Weng Foo, Eraricar Salleh and Siti Nur Hana Mamat Bioprocess Engineering Department, Faculty of Chemical Engineering,Universiti
Supplement of Evaluation of NO + reagent ion chemistry for online measurements of atmospheric volatile organic compounds
 Supplement of Atmos. Meas. Tech., 9, 2909 2925, 2016 http://www.atmos-meas-tech.net/9/2909/2016/ doi:10.5194/amt-9-2909-2016-supplement Author(s) 2016. CC Attribution 3.0 License. Supplement of Evaluation
Supplement of Atmos. Meas. Tech., 9, 2909 2925, 2016 http://www.atmos-meas-tech.net/9/2909/2016/ doi:10.5194/amt-9-2909-2016-supplement Author(s) 2016. CC Attribution 3.0 License. Supplement of Evaluation
PHARMACOGNOSTIC, PHYTOCHEMICAL STUDIES ON THE ROOT OF CLERODENDRON INFORTUNATUM LINN ROOT
 INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article PHARMACOGNOSTIC, PHYTOCHEMICAL STUDIES ON THE ROOT OF CLERODENDRON INFORTUNATUM LINN ROOT
INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article PHARMACOGNOSTIC, PHYTOCHEMICAL STUDIES ON THE ROOT OF CLERODENDRON INFORTUNATUM LINN ROOT
FATTY ACID PROFILING BY GAS CHROMATOGRAPHY FOR THE SHERLOCK MIS
 FATTY ACID PROFILING BY GAS CHROMATOGRAPHY FOR THE SHERLOCK MIS Traditional gas chromatography of complex mixtures of compounds requires precision on the part of the chromatography equipment and considerable
FATTY ACID PROFILING BY GAS CHROMATOGRAPHY FOR THE SHERLOCK MIS Traditional gas chromatography of complex mixtures of compounds requires precision on the part of the chromatography equipment and considerable
Analysis of Omega 3 and Omega 6 FAMEs in Fish Oil and Animal Fat Using an Agilent J&W DB-FATWAX Ultra Inert GC Column
 Application Note Food Analysis of Omega 3 and Omega 6 FAMEs in Fish Oil and Animal Fat Using an Agilent J&W DB-FATWAX Ultra Inert GC Column Authors Ingrid Van Der Meer, Yun Zou, and Gustavo Serrano Agilent
Application Note Food Analysis of Omega 3 and Omega 6 FAMEs in Fish Oil and Animal Fat Using an Agilent J&W DB-FATWAX Ultra Inert GC Column Authors Ingrid Van Der Meer, Yun Zou, and Gustavo Serrano Agilent
DiscovIR-LC. Application Note 026 May 2008 READING TEA LEAVES SUMMARY INTRODUCTION
 TM DiscovIR-LC Deposition and Detection System Application Note 026 May 2008 READING TEA LEAVES The DiscovIR-LC is a powerful new tool for materials analysis. When connected to the outlet of an LC column,
TM DiscovIR-LC Deposition and Detection System Application Note 026 May 2008 READING TEA LEAVES The DiscovIR-LC is a powerful new tool for materials analysis. When connected to the outlet of an LC column,
