SINGLE INTRAVENOUS ADMINISTRATION CARDIOVASCULAR SAFETY STUDY OF P1 IN MALE AND FEMALE BEAGLE DOGS
|
|
|
- Kelley Carter
- 5 years ago
- Views:
Transcription
1 Final Report April 12, 2007 SINGLE INTRAVENOUS ADMINISTRATION CARDIOVASCULAR SAFETY STUDY OF P1 IN MALE AND FEMALE BEAGLE DOGS Author: Linda Rausch, M. S., (Study Director) Testing Facility: SRI International Biosciences Division 333 Ravenswood Avenue Menlo Park, CA Principal Investigator: Hanna Ng, Ph.D., D.A.B.T. SRI Study No.: M SRI Project No.: P Study Initiation: November 28, 2006 Experimental Work Performed: Start: November 29, 2006 Finish: January 25, 2007 Study Completion: April 12, 2007 Sponsor: National Institute of Mental Health, NIH, DHHS NCS Building, Rm (MSC 9641) 6001 Executive Blvd. Bethesda, Maryland Sponsor's Representative: Jamie Driscoll, Program Analyst, NIMH Robert Innis, Ph.D., NIMH NIMH Contract No. N01-MH-32001, WA#26
2
3 TABLE OF CONTENTS APPROVAL SIGNATURES...2 SUMMARY...4 SRI QUALITY ASSURANCE STATEMENT...6 KEY PERSONNEL...7 I. PURPOSE AND OBJECTIVE OF STUDY...8 II. EXPERIMENTAL DESIGN...8 III. MATERIALS AND METHODS...9 A. Test and Control Articles...9 B. Test System...11 C. Experimental Procedure (In-Life Evaluations)...13 D. Evaluation of Data:...15 E. Control of Bias...15 F. Good Laboratory Practices Compliance...15 G. Retention of Records and Study Samples...16 IV. RESULTS AND DISCUSSION...16 A. Mortality/Morbidity and Clinical Observations...16 B. Body Weights...16 C. Cardiology...16 V. CONCLUSIONS...17 APPENDICES A. PROTOCOL AND AMENDMENT... A-1 B. ANALYTICAL CHEMISTRY...B-1 B-1. CERTIFICATES OF ANALYSIS...B-2 B-2. DOSE VERIFICATION, HOMOGENEITY AND STABILITY ANALYSES...B-9 C. CLINICAL OBSERVATIONS FREQUENCY BY ANIMAL...C-1 D. INDIVIDUAL ANIMAL BODY WEIGHTS... D-1 E. CARDIOLOGY...E-1 E-1. CARDIOLOGY NARRATIVE...E-2 E-2. BLOOD PRESSURE...E-6 E-3. INDIVIDUAL ANIMAL EKG DATA...E-11 3
4 SUMMARY The objective of this study was to determine any potential cardiovascular or toxicity effects of P1 after a single intravenous (IV) injection of 100 or 500 times the proposed human dose level, and to determine a no-observed-adverse-effect-level (NOAEL) in male and female Beagle dogs. This study involved three (Days 1, 8, and 15) dose administrations of P1 separated by one week intervals. Body weights were collected to determine dose calculations for each dose administration. Cardiovascular (CV) baseline data were collected prior to each dose administration including electrocardiogram (ECG), heart rate, respiratory rate, and blood pressure (indirect). Body temperature was also collected. Following administration of P1, each CV parameter was collected at 2, 30, 60, 120, 240 min, and 24 hr post dose administration. Clinical observations were made immediately post dose and daily thereafter for the duration of the study. The first IV administration of P1 was µg/kg (528.6 µg/m 2 ), 100 times the proposed human dose level. No adverse signs were observed for 7 days following administration. On Day 8, dose administration was escalated to 500 times the proposed human dose level ( µg/kg; 2643 µg/m 2 ). Again, no adverse clinical observations were observed for the week following administration. On Day 15, 1 naïve male and female dog were administered µg/kg P1 to confirm the NOAEL. Body weights and body temperatures were normal for all dogs for the duration of the study except for a minor body temperature decrease at the 24 hr time point for one dog administered µg/kg P1 on Day 1. The minor change of body temperature for this dog is considered to be a sporadic variation and not test article-related. There were no test article related CV effects, including ST segment abnormalities, pronounced U waves, nor T wave changes such as changes in polarity, increased amplitude, or flattening seen in any of the dogs after a single intravenous injection of 100x or 500x the human dose level of P1 for the duration of the study, except for dog #4, which was administered µg/kg P1, had mild ST segment elevation on Day 15 at time points of 2 min and 4 hr. Also in dog #4, there was a change in T wave polarity from pre-dose positive T wave to 2 min negative T wave, to time 4 hr positive T wave, returning to negative T waves at time 24 hour. Dog #3 had increased QRS amplitude at baseline, pre-dose and throughout the study; and this was not due to a treatment effect. The ST segment elevation changes seen in dog #4 administered µg/kg P1 on Day 15 were transient, which may represent regional myocardial hypoxia and may have normalized at 24 hr post-dose. However, dog #3, also administered µg/kg P1on Day 15, had no adverse test article related CV effects. In addition, Dogs #1 and #2 were administered µg/kg P1 on Day 8, with no test article related CV effects; therefore, the changes seen in dog #4 were not definitively due to a treatment related adverse effect. 4
5 Several dogs (#s 1, 2 and 4) had left and/or right mean electrical axis (MEA) deviations at the baseline, pre-dose and post dose administration. These changes can be affected by many extra-cardiac factors and are not considered to be test article-related. One dog (#2) had trivial hypotension at 4 hr post dose on Day 1. Animals with only reduced diastolic pressures were not considered to be hypotensive, as the diastolic pressure may be more likely to be erroneously low with oscillometric techniques; therefore, this effect is not considered to be test article related. In conclusion, the study results confirmed that there were no overt cardiovascular or toxicity effects of P1 after a single intravenous (IV) injection of 100 times the proposed human dose level of P1 in Beagle dogs. Effects at 500 times the proposed human dose ( µg/kg, 2643 µg/m 2 ) are inconclusive, with minor, transient effects on ST segment elevation seen in a single dog out of 4 evaluated at this dose level. The NOAEL for P1 is considered to be greater than µg/kg (528.6 µg/m 2 ) and possibly less than µg/kg (2643 µg/m 2 ) via a single intravenous administration to male and female Beagle dogs. The maximum tolerated dose (MTD) was not determined, but is considered to be greater than µg/kg (2643 µg/m 2 ). 5
6
7 Name KEY PERSONNEL Title Joseph Duran Jane Han, Ph.D. Ken López, D.V.M., M.P.H., D.A.C.L.A.M. Kristin MacDonald, D.V.M., D.A.C.V.I.M. Hanna Ng, Ph.D. Rhodora Ojerio Cecelia Paculba Sandra Phillips Linda Rausch, M. S. Elaine Tran, B.S. Study Monitor Manager, Analytical Chemistry Attending Veterinarian Veterinary Cardiologist Principal Investigator Veterinary Assistant Study Monitor Director, Toxicology Support Services Study Director Supervisor, Dose Preparation 7
8 I. PURPOSE AND OBJECTIVE OF STUDY The purpose of this study was to support an application for research or marketing purposes. This study, therefore, was performed in accordance with the U.S. FDA "Good Laboratory Practice for Nonclinical Laboratory Studies," as described in 21 CFR Part 58. The objective of this study was to determine any potential cardiovascular or toxicity effects of P1 after a single intravenous injection of 10, 100, and 500 times the human dose level, and to determine a No Adverse Effect Level (NOAEL) in male and female Beagle dogs. Information from this study may be used to determine the suitability of the proposed human dose. The protocol and amendment are presented in Appendix A. II. EXPERIMENTAL DESIGN Dose Level Target Dose A µg/m 2 /day µg/kg/day B Concentration # Dogs (µg/ml) Evaluated 10x Human Dose M/1F 100x Human Dose M/1F 500x Human Dose M/1F Confirm NOAEL M/1F A : Maximum human dose is 10 µg per 70 kg person. 10 µg/70 kg = µg/kg x 37 (human surface area conversion) = µg/m 2. Scaling for the dog gives µg/m 2 / 20 (dog surface area conversion) = µg/kg as an equivalent human dose. B : One male and one female dog were initially treated at a dose of 100x human dose. No effect was observed at 100x human dose, so the dose level was escalated to 500x the human dose in the same two animals (1/sex). No treatment at 10x human dose was performed. Two naive animals (1/sex) were used to confirm 500X human dose NOAEL. Species and strain: Route of administration and justification: Frequency: Beagle dog Intravenous (iv), slow bolus. This is the route intended for human clinical trials; therefore iv injection was selected to model the intended route of human administration. Single dose for each escalation. First day of dose administration was Study Day 1. 8
9 Data for each escalation was evaluated by the Study Director before determining the next dose to be administered. Dose Administration Volume: 2.5 ml/kg III. MATERIALS AND METHODS A. Test and Control Articles 1. Test Article: P1 (PBR28) Molecular Weight: Manufacturer: National Institute of Mental Health, NIH Supplier: National Institute of Mental Health, NIH Lot No.: Physical Description: Off white powder Storage Conditions: 18 to 23 C Characterization of Test Article: Characterization, identity, and purity of the test article was the responsibility of the Sponsor. A Certificate of Analysis (C of A), with information of identity and purity, has been included in the final report. The C of A does not include information on stability of the test article; however, SRI performed chromatographic purity of the test article before study initiation and after study termination to cover the duration of the study. These results have been used for evaluation of the test article stability. The chromatographic purity was calculated by normalized peak area percentage. The same HPLC method was used in concentration and purity analyses of the dose formulations. 2. Control Article/Vehicle: 5% ethanol (v/v) and 95% (v/v) sterile saline Ethanol: Ethyl Alcohol 200 Proof, USP Manufacturer: Pharmco (Brookfield, CT) Lot No.: AC2057 Physical Description: Clear, colorless liquid Storage Conditions: 18 to 28 C 9
10 Saline: Manufacturer: Supplier: Lot No.: 0.9% Sodium Chloride for Injection, USP Baxter Healthcare (Deerlfield, IL) VWR International, Inc. (Brisbane, CA) C Physical Description: Clear, colorless liquid Storage Conditions: 20 to 23 C Water: Sterile Water for Injection, USP Manufacturer: Baxter Healthcare (Deerlfield, IL) Lot No.: C Physical Description: Clear, colorless liquid Storage Conditions: 18 to 28 C Sodium Hydroxide: Manufacturer: Lot No.: Physical Description: Storage Conditions: Sodium Hydroxide DiLut-IT Analytical Concentrate, 0.1 N J.T. Baker (Phillipsburg, NJ) B03426 Clear, colorless liquid used immediately upon receipt Characterization of Control Article/Vehicle: Preparation of Dose Formulations: Information on the identity, purity, and stability was obtained by recording all of the pertinent information provided on the container labels or from the Certificate of Analysis (C of A). Dose solutions for all three escalation were prepared by dissolving the appropriate amount of test article in ethanol, mixed for 30 min, sonicated for 30 sec, diluted with saline to achieve the target concentration, and mixed 22 min (including monitoring of ph). The initial ph of the solution was The ph was adjusted using 0.1N NaOH DILUT_IT (Lot B03426; Phillipsburg, NJ). The final ph was The solution was mixed for another 15 minutes using a stir bar. Solutions were sterile filtered into sterile serum vials. Dose formulations were stored refrigerated at 3.0 to 4.1 C until use. 10
11 3. Characterization of Dose Formulations: Determination of formulation stability and homogeneity was conducted by SRI prior to, and concurrently with, the study (see Attachment A). Dose formulations were used within the window of stability determined in the stability study. Results of the dose verification have been included in Appendix B. Dose formulation concentrations were determined using an analytical method developed for these compounds and described in Attachment A. The analytical methods have been provided in Appendix B. 4. Test Article Handling: At the minimum, the test article and dose formulations were handled with the use of eye protection, latex gloves, and a protective smock or laboratory coat. An MSDS was provided which included recommended procedures for safe handling of the test article, for handling an accidental spill, and for disposal of the waste contaminated with the test articles. 5. Disposition: Unused bulk test article will be returned to the Supplier following the end of the study. Residual dose solutions will be discarded upon acceptance of the final report unless otherwise instructed by the Sponsor. 6. Method for Assuring The test article was weighed with calibrated Correct Dose: balances. The preparation and administration of each formulation was properly documented and records have been maintained showing the amount administered to each animal. B. Test System Species: Strain: Dog Beagle 11
12 Supplier: Gender: Age at Initiation: Weight at Initiation: Number of Animals: Animal Care: Quarantine: Housing: Light Cycle: Temperature: Humidity: Marshall Farms (North Rose, New York) Male and female 15 months 8.3 to 13 kg 4 assigned to test General procedures for animal care and housing was in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals (1996) and the Animal Welfare Standards incorporated in 9 CFR Part 3, These dogs were used previously for another study and went through at least 3 weeks of wash out period. The dogs appeared normal before the initiation of the study. Baseline and pre-dose information on cardiovascular evaluation parameters and blood pressure were obtained. Housed 1-2 dogs in a 4' x 8' wire mesh enclosure (run) with a concrete floor. 12 hr light/12 hr dark 60 to 75 F; protocol states 64 to 84 F. Unable to determine duration since digital hygrothermograph was used but minimum adverse affects to the study are expected due to this deviation. The temperature was not recorded on Day 5 due to a malfunction of the instrument. This is not expected to have adversely affected the study since the animals appeared healthy during this time period %, this deviated from the protocol-specified range of 30-70%; however, this is not expected to affect the study since the animals appeared healthy. The humidity was not recorded on Day 5 due to a malfunction of the instrument. This is not expected to have adversely affected the study since the animals appeared healthy during this time period. 12
13 Ventilation: Food: Water: Identification: Test System Justification: Welfare of the Animals: At least 10 room volumes per hour, with no recirculation of air Harlan Teklad 2025-C Certified Canine Chow (Hayward, CA). Dogs were given their daily ration of food. The quantity of the daily ration was sufficient to meet nutritional requirements. Feed is analyzed periodically to ensure that contaminants known to be capable of interfering with the study and reasonably expected to be present in such feed are not present at levels that would impact the study. Documentation of feed analyses is maintained in the study records. Public water supply was provided ad libitum. No contaminants that could interfere with and affect the results of the study are expected to have been present in the water, based on previous analysis reports. Copies of annual analysis reports will be maintained at SRI for reference. Individually identified by a unique ear tattoo. This is an accepted species to support studies of compounds used or intended for use in humans. Every effort was made to minimize, if not eliminate, pain and suffering in all animals in this study. Assignment of Animals to Study: Day: Group Assignment: Prior to initiation of treatment. Manually assigned to treatment groups. C. Experimental Procedure (In-Life Evaluations) Mortality/Morbidity: At least once daily. Clinical Observation: Body Weights: Observations were made immediately post dose and daily for the remainder of the study. Prior to dosing on each escalation 13
14 Cardiovascular Evaluation (Days 1 and 8). Parameters Evaluated: Frequency: Electrocardiograms (ECG), heart rate respiratory rate, and blood pressure (indirect) recordings from Prestudy, Day 8, and Day 15 were evaluated by Dr. Kristin MacDonald, D.V.M., D.A.C.V.I.M. The cardiologist s report is included in Appendix E-1. All cardiac measurements were performed pre-dose and initiated at 2, 30, 60, 120, and 240 min postdose. ECGs were recorded continuously between 2 and 15 min post dose. In addition, measurements were collected at 24 hr post-dose. Method: All ECGs were measured using a minimum of 4 leads and a lead II rhythm strip recorded at 25 mm/sec paper speed at standard sensitivity (1mm/mV). Blood pressure was measured using a SurgiVet V6004 oscillometric monitor. ECGs were evaluated for HR, rhythm, and mean electrical axis (MEA). HR and MEA were obtained from the automatic calculations generated by the electrocardiograph, when available. The rhythm was determined subjectively. P-wave duration, PR interval, QRS duration, and QT interval were calculated from manually recorded measurements. Each parameter was averaged from three measurements from three different beats. The corrected QT interval (QTc) was calculated using Fredericia s formula. RR was measured by counting the number of inhaled breaths in a 15-second interval and multiplying by 4 to determine the number of respirations per minute. BP was measured five times, when possible, at each time point using an oscillometric monitor, according to the manufacturer s instructions, with the cuff placed on the right or left forelimb. Average BPs were calculated from measurements recorded at each time point. 14
15 Body Temperature: Frequency: Method: Body temperature measurements were performed pre-dose and initiated at 2, 30, 60, 120, and 240 min post-dose. Body temperature was obtained by a digital rectal thermometer. D. Evaluation of Data: Statistical Test: No statistical evaluations were performed. E. Control of Bias When evaluating the animals, the technical staff was aware of the each animal s treatment history. However, based on the relatively objective endpoints to be examined, bias is not expected to have influenced the results of the study. F. Good Laboratory Practices Compliance This study is intended to be submitted to and reviewed by the U.S. Food and Drug Administration (FDA) or an equivalent regulatory agency and, therefore, was performed in accordance with the U.S. FDA "Good Laboratory Practice for Nonclinical Laboratory Studies," as described in 21 CFR Part 58, with the following exceptions. Animal water and food analysis were not performed under GLP compliance by the vendors. A digital temperature station, manufactured by La Crosse Technology, Inc, was used to measure the temperature and humidity of the animals housing conditions during the course of the study. This instrument measures minimum and maximum readings for the day and does not provide continuous measurement of the temperature and humidity. Characterization of the test article was the responsibility of the Sponsor. The Sponsor submitted a Certificate of Analysis (C of A) that included information on chemical identity and purity. The C of A of the test article was not conducted in accordance with the U.S. FDA GLP compliance. The C of A did not include information on stability of the test article; however, SRI performed chromatographic purity of the test article by measuring both before and after several days of test article in the formulation. Purity was calculated by normalized peak area percentage and purity results were used for evaluation of the test article stability. 15
16 G. Retention of Records and Study Samples The original protocol and amendments, original final report, and all raw data, supporting documents, and records specific to this study will be retained and stored in the Records Center at SRI International, 333 Ravenswood Avenue, Menlo Park, CA All records will be maintained for a period of at least 5 years. At the end of the retention period, the Sponsor will be contacted regarding further disposition of these records. IV. RESULTS AND DISCUSSION A. Mortality/Morbidity and Clinical Observations Individual animal clinical observations are presented in Appendix C. All animals survived the duration of the study. One female administered µg/kg P1 had slightly loose stool on Day 8. No adverse clinical observations were seen for any of the other dogs at any dose level for the duration of the study. B. Body Weights Individual animal body weights are presented in Appendix D. All animals consistently gained body weight for the duration of the study compared with their respective Day 1 body weights. C. Cardiology The cardiologist s report, and individual animal blood pressure and ECG data are presented in Appendix E. Electrocardiograms (ECG) There were no rhythm abnormalities at any time point in the study. Dog #3, administered µg/kg P1, had persistently increased QRS amplitude at baseline and at Day 15. Dog #4, administered µg/kg P1 had mild ST segment elevation on Day 15 at 2 min and 4 hr post dose administration. Also for dog #4 at the baseline pre-study ECG, the T waves were negative; however, there was a change in T wave polarity from positive at pre-dose, to negative T wave at 2 min post dose, positive T wave at 4 hr, returning to negative at 24 hr post dose administration. At baseline, dogs #1 and #2 had left axis deviations, and dog #4 had a significant left axis deviation. On Day 1, Dog #1, administered µg/kg P1, had right axis 16
17 deviation at pre-dose, 2 min, and 2 hr post dose administration, and left axis deviation at 30 min, 1, and 24 hr post dose administration. Dog #2, administered µg/kg P1, had left axis deviation at pre-dose, and significant left axis deviations at 30 min, 1, 2, 4, and 24 hr post dos administration. On Day 8, dog #1, administered µg/kg P1, had trivial left axis deviations at 30 min, 2, and 4 hr post dose administration. Dog #2, also administered µg/kg P1, had significant left axis deviations at pre-dose, 30 min, 2, and 24 hr post dose administration, and left axis deviations at 2 min, 1, and 4 hr post dose administration. On Day 15, dog #4 had significant left axis deviations at pre-dose, 30 min, 2 hr post dose, and significant right axis deviations at 2 min, 4, and 24 hr post dose, as well as a trivial right axis deviation at 1 hr post dose administration of µg/kg P1. In animals with mean electrical axis (MEA) deviations, there were normal QRS durations, indicating there were no partial bundle branch blocks. The axis deviations do not reflect persistent, significant conduction system disturbances and are likely affected by subtle changes in body position or electrode placement. MEA can be affected by many extra-cardiac factors including thoracic shape, body position, inappropriate or incorrect lead placement. MEA is a poor indication of left or right ventricular chamber size, and is mainly useful to determine presence of severe right ventricular hypertrophy or to evaluate for intraventricular conduction defects such as bundle branch block. Dog #4 had wide fluctuations of the MEA at Day 15, which may represent positional changes of the body or may be due to altered intraventricular conduction, since there was also ST segment and T wave changes at the same time points. Blood Pressure On Day 1, dog #2, administered µg/kg P1, had a trivial decrease in diastolic blood pressure (55 mmhg) 2 hr post dose administration, and a trivial hypotension with reduced diastolic, systolic, and mean arterial pressure (55 mmhg, 95 mmhg, and 73 mmhg respectively) 4 hr after dose administration. On Day 8, dog #2, administered µg/kg P1, had decreased diastolic pressure (48 mmhg) 24 hr post dose administration. On Day 15 no abnormalities in blood pressure were seen. Body Temperature Body temperatures were normal for all dogs for the duration of the study except for dog #2 administered µg/kg P1 on Day 1 which had a slightly decreased body temperature of 99.7 F at 24 hr post dose administration compared with pre-dose body temperature of F. The minor change of body temperature for this dog is considered to be a sporadic variation and not test article-related. V. CONCLUSIONS This study involved three (Days 1, 8, and 15) dose administrations of P1 separated by one week intervals. Body weights were collected to determine dose calculations for each dose administration. Cardiovascular (CV) baseline data was collected prior to each dose 17
18 administration including electrocardiogram (ECG), heart rate, respiratory rate, and blood pressure (indirect). Body temperature was also collected. Following administration of P1, each CV parameter was collected at 2, 30, 60, 120, 240 min, and 24 hr post dose administration. Clinical observations were made immediately post dose and daily thereafter for the duration of the study. The first IV administration of P1 was µg/kg (528.6 µg/m 2 ), 100 times the proposed human dose level. No adverse signs were observed for 7 days following administration. On Day 8, dose administration was escalated to 500 times the proposed human dose level ( µg/kg; 2643 µg/m 2 ). Again, no adverse clinical observations were observed for the week following administration. On Day 15, 1 naïve male and female dog were administered µg/kg P1 to confirm the NOAEL. Body weights and body temperatures were normal for all dogs for the duration of the study except for a minor body temperature decrease 24 hr post dose for one dog administered µg/kg P1 on Day 1. This minor change of body temperature is considered to be a sporadic variation and not test article related. There were no test article related CV effects, including ST segment abnormalities, pronounced U waves, nor T wave changes such as changes in polarity, increased amplitude, or flattening seen in any of the dogs after a single intravenous injection of 100x or 500x the human dose level of P1 for the duration of the study, except for dog #4, which was administered µg/kg P1, had mild ST segment elevation on Day 15 at time points of 2 min and 4 hr. Also in dog #4, there was a change in T wave polarity from pre-dose positive T wave to 2 min negative T wave, to 4 hr positive T wave, returning to negative T waves at 24 hr. The ST segment elevation changes seen in Dog #4 administered µg/kg P1 on Day 15 were transient, which may represent regional myocardial hypoxia and may have normalized at 24 hr post-dose. However, dog #3, also administered µg/kg P1on Day 15, had no adverse test article related CV effects. In addition, Dog 1 and 2 were administered µg/kg P1 on Day 8, with no test article related CV effects. Due to the small number of dogs tested (2 males and 2 females), it is not clear whether the ST segment and T wave changes seen in Dog #4 are conclusively linked to a test article related effect; however, 3 of the 4 dogs tested in this study at this dose level had no adverse signs related to ST segment and T wave changes. In addition, the CV changes were transient, only seen at 2 min and 4 hr post dose administration, and returned to normal by 24 hr. The significance of the ST segment elevation related to test article-treatment in dog #4 may be minimal, but not conclusive. Several dogs (#s 1, 2 and 4) had left and/or right mean electrical axis (MEA) deviations at the baseline, pre-dose and post dose administration. These changes can be affected by many extra-cardiac factors and are not considered to be test article-related. There was no treatment effect on blood pressure observed that was considered to be related to test article treatment. In conclusion, the study results confirm that there are no overt cardiovascular or toxicity effects of P1 after a single intravenous (IV) injection of 100 times the proposed human dose 18
19 level of P1 in Beagle dogs. Effects at 500 times the proposed human dose ( µg/kg, 2643 µg/m 2 ) are inconclusive, with minor, transient effects on ST segment elevation seen in a single dog out of 4 evaluated at this dose level. The NOAEL for P1 is considered to be greater than µg/kg (528.6 µg/m 2 ) and possibly less than µg/kg (2643 µg/m 2 ) via a single intravenous administration to male and female Beagle dogs. The maximum tolerated dose (MTD) was not determined, but is considered to be greater than µg/kg (2643 µg/m 2 ). 19
AN ACUTE ORAL TOXICITY STUDY IN RATS WITH ADVANTRA Z
 AN ACUTE ORAL TOXICITY STUDY IN RATS WITH ADVANTRA Z AMENDED FINAL REPORT Author Deborah A Douds, M.S. Original Study Completion Date July 8, 1997 Amended Study Completion Date November 19, 1997 Performing
AN ACUTE ORAL TOXICITY STUDY IN RATS WITH ADVANTRA Z AMENDED FINAL REPORT Author Deborah A Douds, M.S. Original Study Completion Date July 8, 1997 Amended Study Completion Date November 19, 1997 Performing
Acute Oral Toxicity Study in Rats with Argentyn 23 (EPA/OECD Guidelines) Study Title. Monica M. Vegarra, BS. Sponsor
 Note: This study was done using our Professional strength 23 ppm product (Argentyn 23), therefore making Sovereign Silver at 10 ppm, 2.3 times safer. Conclusion on page 6 shows the product to be non-toxic
Note: This study was done using our Professional strength 23 ppm product (Argentyn 23), therefore making Sovereign Silver at 10 ppm, 2.3 times safer. Conclusion on page 6 shows the product to be non-toxic
ACUTE INTRAVENOUS TOXICITY STUDY OF PBR-06 AND MNPA IN SPRAGUE DAWLEY RATS
 Unaudited Draft Report April 14, 2006 ACUTE INTRAVENOUS TOXICITY STUDY OF PBR-06 AND MNPA IN SPRAGUE DAWLEY RATS Author: Linda Rausch, M.S. Testing Facility SRI International Biosciences Division 333 Ravenswood
Unaudited Draft Report April 14, 2006 ACUTE INTRAVENOUS TOXICITY STUDY OF PBR-06 AND MNPA IN SPRAGUE DAWLEY RATS Author: Linda Rausch, M.S. Testing Facility SRI International Biosciences Division 333 Ravenswood
INTRAVENOUS TOXICITY STUDY OF P1 IN SPRAGUE-DAWLEY RATS
 Final Report May 16, 2007 INTRAVENOUS TOXICITY STUDY OF P1 IN SPRAGUE-DAWLEY RATS Author: Edward S. Riccio, B.S, (Study Director) Testing Facility: SRI International Biosciences Division 333 Ravenswood
Final Report May 16, 2007 INTRAVENOUS TOXICITY STUDY OF P1 IN SPRAGUE-DAWLEY RATS Author: Edward S. Riccio, B.S, (Study Director) Testing Facility: SRI International Biosciences Division 333 Ravenswood
SINGLE DOSE TOXICITY STUDY OF ER176 IN SPRAGUE DAWLEY RATS
 Final Report February 12, 2014 SINGLE DOSE TOXICITY STUDY OF ER176 IN SPRAGUE DAWLEY RATS Author: Howard Stock, PhD (Study Director) Testing Facility: SRI International Biosciences Division 333 Ravenswood
Final Report February 12, 2014 SINGLE DOSE TOXICITY STUDY OF ER176 IN SPRAGUE DAWLEY RATS Author: Howard Stock, PhD (Study Director) Testing Facility: SRI International Biosciences Division 333 Ravenswood
Final Report. Acute oral toxicity study in Wistar rats. Mr. S. Haribabu, B Tech (Biotech), MSc
 Study Title Test Item Study Director Sponsor Study Monitor Test Facility Acute oral toxicity study in Wistar rats Nualgi Nano Nutrients Mr. S. Haribabu, B Tech (Biotech), MSc Nualgi Nano Biotech Co 651,
Study Title Test Item Study Director Sponsor Study Monitor Test Facility Acute oral toxicity study in Wistar rats Nualgi Nano Nutrients Mr. S. Haribabu, B Tech (Biotech), MSc Nualgi Nano Biotech Co 651,
ACUTE INTRAVENOUS TOXICITY STUDY OF M1 IN SPRAGUE DAWLEY RATS
 Unaudited Draft Report October 30, 2006 ACUTE INTRAVENOUS TOXICITY STUDY OF M1 IN SPRAGUE DAWLEY RATS Author: Hanna Hongchin Ng, Ph.D., D.A.B.T (Study Director) Testing Facility: SRI International Toxicology
Unaudited Draft Report October 30, 2006 ACUTE INTRAVENOUS TOXICITY STUDY OF M1 IN SPRAGUE DAWLEY RATS Author: Hanna Hongchin Ng, Ph.D., D.A.B.T (Study Director) Testing Facility: SRI International Toxicology
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET SECTION 1 - CHEMICAL PRODUCT(S) AND COMPANY IDENTIFICATION Supplier Name and address: Genlantis, A division of Gene Therapy Systems, Inc. 10190 Telesis Court San Diego, CA 92121
MATERIAL SAFETY DATA SHEET SECTION 1 - CHEMICAL PRODUCT(S) AND COMPANY IDENTIFICATION Supplier Name and address: Genlantis, A division of Gene Therapy Systems, Inc. 10190 Telesis Court San Diego, CA 92121
PRODUCT STUDY TITLE DATA REQUIREMENT AUTHOR STUDY COMPLETED ON PERFORMING LABORATORY LABORATORY STUDY NUMBER
 Product Safety Laboratories 2394 Highway 130 Dayton, NJ 08810 Tel: 732.438.5100 Fax: 732.355.3275 e-mail: PSL@productsafetylabs.com PRODUCT Stalosan F STUDY TITLE Acute Dermal Toxicity Study in Rats -
Product Safety Laboratories 2394 Highway 130 Dayton, NJ 08810 Tel: 732.438.5100 Fax: 732.355.3275 e-mail: PSL@productsafetylabs.com PRODUCT Stalosan F STUDY TITLE Acute Dermal Toxicity Study in Rats -
Page 1132
 Page 1131 Page 1132 Page 1133 Page 1134 Page 1135 Huntingdon Life Sciences 03-6140 Page 6. Final Report TABLE OF CONTENTS Page 1136 COVER PAGE...1 GLP STATEMENT...2 SIGNATURE PAGE...3 QUALITY ASSURANCE
Page 1131 Page 1132 Page 1133 Page 1134 Page 1135 Huntingdon Life Sciences 03-6140 Page 6. Final Report TABLE OF CONTENTS Page 1136 COVER PAGE...1 GLP STATEMENT...2 SIGNATURE PAGE...3 QUALITY ASSURANCE
247 CMR BOARD OF REGISTRATION IN PHARMACY
 247 CMR 18.00: NON-STERILE COMPOUNDING Section 18.01: Authority and Purpose 18.02: Non-Sterile Compounding Process 18.03: Non-Sterile Compounding Facility 18.04: Non-Sterile Compounding Equipment 18.05:
247 CMR 18.00: NON-STERILE COMPOUNDING Section 18.01: Authority and Purpose 18.02: Non-Sterile Compounding Process 18.03: Non-Sterile Compounding Facility 18.04: Non-Sterile Compounding Equipment 18.05:
Final Report. Acute dermal toxicity study in Wistar rats. Mr. M. Vasanthan, M Tech (Biotech)
 Study Title Test Item Study Director Sponsor Study Monitor Test Facility Acute dermal toxicity study in Wistar rats Nualgi Nano Nutrients Mr. M. Vasanthan, M Tech (Biotech) Nualgi Nano Biotech Co 651,
Study Title Test Item Study Director Sponsor Study Monitor Test Facility Acute dermal toxicity study in Wistar rats Nualgi Nano Nutrients Mr. M. Vasanthan, M Tech (Biotech) Nualgi Nano Biotech Co 651,
ECG Interpretation Cat Williams, DVM DACVIM (Cardiology)
 ECG Interpretation Cat Williams, DVM DACVIM (Cardiology) Providing the best quality care and service for the patient, the client, and the referring veterinarian. GOAL: Reduce Anxiety about ECGs Back to
ECG Interpretation Cat Williams, DVM DACVIM (Cardiology) Providing the best quality care and service for the patient, the client, and the referring veterinarian. GOAL: Reduce Anxiety about ECGs Back to
MATERIAL SAFETY DATA SHEET FILE NO.: AB GoliBridge ELISA kit MSDS DATE: 24 Oct 2017
 SECTION 1: PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: SYNONYMS: PRODUCT CODES: MANUFACTURER: ADDRESS: GoliBridge ELISA kit AB000215 Array Bridge, Inc 4320 Forest Park Avenue, Saint Louis, MO 63108,
SECTION 1: PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: SYNONYMS: PRODUCT CODES: MANUFACTURER: ADDRESS: GoliBridge ELISA kit AB000215 Array Bridge, Inc 4320 Forest Park Avenue, Saint Louis, MO 63108,
3/26/15 HTEC 91. EKG Sign-in Book. The Cardiac Cycle. Parts of the ECG. Waves. Waves. Review of protocol Review of placement of chest leads (V1, V2)
 EKG Sign-in Book HTEC 91 Review of protocol Review of placement of chest leads (V1, V2) Medical Office Diagnostic Tests Week 2 http://www.cvphysiology.com/arrhythmias/a013c.htm The Cardiac Cycle Represents
EKG Sign-in Book HTEC 91 Review of protocol Review of placement of chest leads (V1, V2) Medical Office Diagnostic Tests Week 2 http://www.cvphysiology.com/arrhythmias/a013c.htm The Cardiac Cycle Represents
Residue Chemistry Studies for Veterinary Drugs. Human Food Safety Assessment. Presentation Topic. Improving residue chemistry submissions
 Residue Chemistry Studies for Veterinary Drugs Julia A. Oriani, Ph.D. Food and Drug Administration Center for Veterinary Medicine Division of Human Food Safety Human Food Safety Assessment Risk = Hazard
Residue Chemistry Studies for Veterinary Drugs Julia A. Oriani, Ph.D. Food and Drug Administration Center for Veterinary Medicine Division of Human Food Safety Human Food Safety Assessment Risk = Hazard
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress ECG INTERPRETATION Adrian Boswood MA VetMB DVC DECVIM-CA(Cardiology) MRCVS The Royal Veterinary College, Hawkshead
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress ECG INTERPRETATION Adrian Boswood MA VetMB DVC DECVIM-CA(Cardiology) MRCVS The Royal Veterinary College, Hawkshead
PacificBeam SHELLIII/PB-SHELL3: ACUTE DERMAL IRRITATION IN THE RABBIT PROJECT NUMBER: 2533/0014 AUTHOR:
 PAGE 1 OF 14 PAGES PacificBeam SHELLIII/PB-SHELL3: ACUTE DERMAL IRRITATION IN THE RABBIT PROJECT NUMBER: 2533/0014 AUTHOR: A Sanders STUDY SPONSOR: M.I.C.(Medical Intelligence Corporation) Co., Ltd. 2-2-15
PAGE 1 OF 14 PAGES PacificBeam SHELLIII/PB-SHELL3: ACUTE DERMAL IRRITATION IN THE RABBIT PROJECT NUMBER: 2533/0014 AUTHOR: A Sanders STUDY SPONSOR: M.I.C.(Medical Intelligence Corporation) Co., Ltd. 2-2-15
ECG interpretation basics
 ECG interpretation basics Michał Walczewski, MD Krzysztof Ozierański, MD 21.03.18 Electrical conduction system of the heart Limb leads Precordial leads 21.03.18 Precordial leads Precordial leads 21.03.18
ECG interpretation basics Michał Walczewski, MD Krzysztof Ozierański, MD 21.03.18 Electrical conduction system of the heart Limb leads Precordial leads 21.03.18 Precordial leads Precordial leads 21.03.18
Ingredient Listing Qty. Unit NDC # Supplier. q.s. to ml
 12/20/2018; Page 1 SUGGESTED FORMULATION Ingredient Listing Qty. Unit NDC # Supplier, USP 10 MU Sucrose, NF 23.00 g Propylene Glycol, USP 7.0 ml Medisca Oral Mix (Flavored Suspending Vehicle) 50.0 ml Medisca
12/20/2018; Page 1 SUGGESTED FORMULATION Ingredient Listing Qty. Unit NDC # Supplier, USP 10 MU Sucrose, NF 23.00 g Propylene Glycol, USP 7.0 ml Medisca Oral Mix (Flavored Suspending Vehicle) 50.0 ml Medisca
*Sections or subsections omitted from the full prescribing information are not listed.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GIAPREZA TM safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA (angiotensin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GIAPREZA TM safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA (angiotensin
GLP VIRUCIDAL TESTING OF ZEROMOLD PLUS FOR HIV-1
 Southwest Research Institute Page 1 of 8 Microencapsulation and Nanomaterials Department Amended Final Report: GLP Virucidal Testing of ZeroMold Plus for HIV-1 Protocol No.: GLP-SP-239 FINAL REPORT STUDY
Southwest Research Institute Page 1 of 8 Microencapsulation and Nanomaterials Department Amended Final Report: GLP Virucidal Testing of ZeroMold Plus for HIV-1 Protocol No.: GLP-SP-239 FINAL REPORT STUDY
SHRIRAM INSTITUTE FOR INDUSTRIAL RESEARCH. ACUTE ORAL TOXICITY STUDY IN RATS WITH Cry 1 C a 1 PROTEIN
 ACUTE ORAL TOXICITY STUDY IN RATS WITH Cry 1 C a 1 PROTEIN REPORT FOR METAHELIX LIFE SCIENCES PVT. LTD., PLOT NO.-3, KIADB 4 TH PHASE, BOMMASANDRA, BANGALORE-500 099, INDIA. GUIDELINES DBT Guidelines TEST
ACUTE ORAL TOXICITY STUDY IN RATS WITH Cry 1 C a 1 PROTEIN REPORT FOR METAHELIX LIFE SCIENCES PVT. LTD., PLOT NO.-3, KIADB 4 TH PHASE, BOMMASANDRA, BANGALORE-500 099, INDIA. GUIDELINES DBT Guidelines TEST
LEAD SAFETY PROGRAM. Purpose. Scope. Responsibilities. Southern Heat Exchanger Services Safety Program
 Page: Page 1 of 5 Purpose The purpose of this procedure is to identify the controls and actions necessary to prevent adverse health effects to employees from occupational exposure to lead, and to ensure
Page: Page 1 of 5 Purpose The purpose of this procedure is to identify the controls and actions necessary to prevent adverse health effects to employees from occupational exposure to lead, and to ensure
Sandoz Inc. 12-Aug-08
 Sandoz Inc. 12-Aug-08 Department of Health and Human Services Public Health Service Food and Drug Administration Atlanta District Office 60 8th Street, N.E. Atlanta, Georgia 30309 August 12, 2008 VIA FEDERAL
Sandoz Inc. 12-Aug-08 Department of Health and Human Services Public Health Service Food and Drug Administration Atlanta District Office 60 8th Street, N.E. Atlanta, Georgia 30309 August 12, 2008 VIA FEDERAL
Electrical Conduction
 Sinoatrial (SA) node Electrical Conduction Sets the pace of the heartbeat at 70 bpm AV node (50 bpm) and Purkinje fibers (25 40 bpm) can act as pacemakers under some conditions Internodal pathway from
Sinoatrial (SA) node Electrical Conduction Sets the pace of the heartbeat at 70 bpm AV node (50 bpm) and Purkinje fibers (25 40 bpm) can act as pacemakers under some conditions Internodal pathway from
ECG. Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13
 ECG Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13 The Concept When the cardiac impulse passes through the heart, electrical current
ECG Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13 The Concept When the cardiac impulse passes through the heart, electrical current
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Diploma in Electrocardiography
 The Society for Cardiological Science and Technology Diploma in Electrocardiography The Society makes this award to candidates who can demonstrate the ability to accurately record a resting 12-lead electrocardiogram
The Society for Cardiological Science and Technology Diploma in Electrocardiography The Society makes this award to candidates who can demonstrate the ability to accurately record a resting 12-lead electrocardiogram
Cardiac Cycle. Each heartbeat is called a cardiac cycle. First the two atria contract at the same time.
 The Heartbeat Cardiac Cycle Each heartbeat is called a cardiac cycle. First the two atria contract at the same time. Next the two ventricles contract at the same time. Then all the chambers relax. http://www.youtube.com/watch?v=frd3k6lkhws
The Heartbeat Cardiac Cycle Each heartbeat is called a cardiac cycle. First the two atria contract at the same time. Next the two ventricles contract at the same time. Then all the chambers relax. http://www.youtube.com/watch?v=frd3k6lkhws
Corporate Medical Policy Electrocardiographic Body Surface Mapping
 Corporate Medical Policy Electrocardiographic Body Surface Mapping File Name: Origination: Last CAP Review: Next CAP Review: Last Review: eletrocardiographic_body_surface_mapping 6/2009 10/2016 10/2017
Corporate Medical Policy Electrocardiographic Body Surface Mapping File Name: Origination: Last CAP Review: Next CAP Review: Last Review: eletrocardiographic_body_surface_mapping 6/2009 10/2016 10/2017
(angiotensin II) injection for intravenous infusion
 ADMINISTERING GIAPREZA TM (angiotensin II) injection for intravenous infusion Visit www.giapreza.com INITIATE Recommended starting dose of GIAPREZA is 20 ng/kg/min, which is equivalent to 0.02 mcg/kg/min
ADMINISTERING GIAPREZA TM (angiotensin II) injection for intravenous infusion Visit www.giapreza.com INITIATE Recommended starting dose of GIAPREZA is 20 ng/kg/min, which is equivalent to 0.02 mcg/kg/min
SAFETY DATA SHEET. Emergency Telephone Number: (24/7/365) English Only
 PRODUCT NAME: SAFETY DATA SHEET PNEUMOVAX 23 Page: 1/6 1. Product and Company Identification Manufactured/Supplied by Label Name Chemical Name Synonyms Material Product Number Intended Use Merck Sharp
PRODUCT NAME: SAFETY DATA SHEET PNEUMOVAX 23 Page: 1/6 1. Product and Company Identification Manufactured/Supplied by Label Name Chemical Name Synonyms Material Product Number Intended Use Merck Sharp
Safety Data Sheet Product Name: Heparin / PF4 Serum Panel - For In Vitro Diagnostic Use Only
 Section 1 Identification Product Name Catalog Numbers Heparin / PF4 Assayed Antibody Serum Panel 4036027, 2 Member QC Panel 4036028, Multi-Member Qualification Panel (6 member) Company Identification AKERS
Section 1 Identification Product Name Catalog Numbers Heparin / PF4 Assayed Antibody Serum Panel 4036027, 2 Member QC Panel 4036028, Multi-Member Qualification Panel (6 member) Company Identification AKERS
AKA Good Manufacturing Practice (GMP) Certification Program
 AKA Good Manufacturing Practice (GMP) Certification Program Preamble The American Kratom Association (AKA) is establishing this program to assure the safety and integrity of kratom dietary supplements
AKA Good Manufacturing Practice (GMP) Certification Program Preamble The American Kratom Association (AKA) is establishing this program to assure the safety and integrity of kratom dietary supplements
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Chapter 08. Health Screening and Risk Classification
 Chapter 08 Health Screening and Risk Classification Preliminary Health Screening and Risk Classification Protocol: 1) Conduct a Preliminary Health Evaluation 2) Determine Health /Disease Risks 3) Determine
Chapter 08 Health Screening and Risk Classification Preliminary Health Screening and Risk Classification Protocol: 1) Conduct a Preliminary Health Evaluation 2) Determine Health /Disease Risks 3) Determine
SHRIRAM INSTITUTE FOR INDUSTRIAL RESEARCH
 IN RATS SUB CHRONIC ORAL TOXICITY WITH NHH 44 Bt-COTTON SEEDS Report for: UNIVERSITY OF AGRICULTURAL SCIENCES AGRICULTURAL RESEARCH STATION DHARWAD-580007 KARNATAKA Guidelines: DBT, Guidelines for Toxicity
IN RATS SUB CHRONIC ORAL TOXICITY WITH NHH 44 Bt-COTTON SEEDS Report for: UNIVERSITY OF AGRICULTURAL SCIENCES AGRICULTURAL RESEARCH STATION DHARWAD-580007 KARNATAKA Guidelines: DBT, Guidelines for Toxicity
ECG Basics Sonia Samtani 7/2017 UCI Resident Lecture Series
 ECG Basics Sonia Samtani 7/2017 UCI Resident Lecture Series Agenda I. Introduction II.The Conduction System III.ECG Basics IV.Cardiac Emergencies V.Summary The Conduction System Lead Placement avf Precordial
ECG Basics Sonia Samtani 7/2017 UCI Resident Lecture Series Agenda I. Introduction II.The Conduction System III.ECG Basics IV.Cardiac Emergencies V.Summary The Conduction System Lead Placement avf Precordial
Model Standards for Pharmacy Compounding of Non-Sterile Preparations
 Model Standards for Pharmacy Compounding of Non-Sterile Preparations Published with the Guidance Document For Pharmacy Compounding of Non-Sterile Preparations National Association of Pharmacy Regulatory
Model Standards for Pharmacy Compounding of Non-Sterile Preparations Published with the Guidance Document For Pharmacy Compounding of Non-Sterile Preparations National Association of Pharmacy Regulatory
DR QAZI IMTIAZ RASOOL OBJECTIVES
 PRACTICAL ELECTROCARDIOGRAPHY DR QAZI IMTIAZ RASOOL OBJECTIVES Recording of electrical events in heart Established electrode pattern results in specific tracing pattern Health of heart i. e. Anatomical
PRACTICAL ELECTROCARDIOGRAPHY DR QAZI IMTIAZ RASOOL OBJECTIVES Recording of electrical events in heart Established electrode pattern results in specific tracing pattern Health of heart i. e. Anatomical
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Medical Electronics Dr. Neil Townsend Michaelmas Term 2001 ( The story so far.
 Medical Electronics Dr Neil Townsend Michaelmas Term 2001 (wwwrobotsoxacuk/~neil/teaching/lectures/med_elec) Blood Pressure has been measured (in some way) for centuries However, clinical interpretation
Medical Electronics Dr Neil Townsend Michaelmas Term 2001 (wwwrobotsoxacuk/~neil/teaching/lectures/med_elec) Blood Pressure has been measured (in some way) for centuries However, clinical interpretation
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Animal Health Pfizer Inc 235 East 42nd Street New York, NY 10017 Poison Control Center Phone: 1-866-531-8896
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Animal Health Pfizer Inc 235 East 42nd Street New York, NY 10017 Poison Control Center Phone: 1-866-531-8896
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Santa Clarita Community College District HEARING CONSERVATION PROGRAM. Revised
 Santa Clarita Community College District HEARING CONSERVATION PROGRAM Revised March 2018 TABLE OF CONTENTS INTRODUCTION...1 HEARING CONSERVATION PROGRAM...2 1.1 District Policy...2 1.2 Plan Review...2
Santa Clarita Community College District HEARING CONSERVATION PROGRAM Revised March 2018 TABLE OF CONTENTS INTRODUCTION...1 HEARING CONSERVATION PROGRAM...2 1.1 District Policy...2 1.2 Plan Review...2
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
PROFICIENCY TESTING POLICY
 Supersedes Prepared by: APPROVALS in this section Approved by: Date: Laboratory Director RECORD OF REVIEWS Date Signature Title Procedural Changes/Review VERSION HISTORY Revision # 0 Section #/Changes
Supersedes Prepared by: APPROVALS in this section Approved by: Date: Laboratory Director RECORD OF REVIEWS Date Signature Title Procedural Changes/Review VERSION HISTORY Revision # 0 Section #/Changes
Ingredient Listing Qty. Unit NDC # Supplier g. Sterile Preparation
 Note: Glucosamine Sulfate Potassium Chloride 1267 mg is equivalent to Glucosamine 750 mg. 11/8/2016; Page 1 SUGGESTED FORMULATION Ingredient Listing Qty. Unit NDC # Supplier Chondroitin Sulfate Sodium,
Note: Glucosamine Sulfate Potassium Chloride 1267 mg is equivalent to Glucosamine 750 mg. 11/8/2016; Page 1 SUGGESTED FORMULATION Ingredient Listing Qty. Unit NDC # Supplier Chondroitin Sulfate Sodium,
Subject experienced tachycardia and atrial fibrillation while engaged in a study in the Human Studies Facility
 IRB Number: 04-1677 Legacy ID: GCRC-2067 PI: Robert Devlin IRB: Biomedical Sponsor: Study Title: US EPA Physiological Changes in Adults with Metabolic Syndrome Exposed to Concentrated Ultrafine Chapel
IRB Number: 04-1677 Legacy ID: GCRC-2067 PI: Robert Devlin IRB: Biomedical Sponsor: Study Title: US EPA Physiological Changes in Adults with Metabolic Syndrome Exposed to Concentrated Ultrafine Chapel
Material Safety Data Sheet
 MSDS Date: 7/20/07 Product Name: Hand Sanitizers Importer Name: Delta Brands, Inc. 1. Product and Company Description Delta Brands Inc. 1890 Palmer Avenue Larchmont, NY 10538 For Product Information/Emergency:
MSDS Date: 7/20/07 Product Name: Hand Sanitizers Importer Name: Delta Brands, Inc. 1. Product and Company Description Delta Brands Inc. 1890 Palmer Avenue Larchmont, NY 10538 For Product Information/Emergency:
Environmental Health & Safety Policy Manual
 Environmental Health & Safety Policy Manual Issue Date: 5/31/2017 Policy # EHS-400.17 Isoflurane Use and Exposure Control Procedures 1.0 PURPOSE: LSUHSC is committed to keeping all exposures to hazardous
Environmental Health & Safety Policy Manual Issue Date: 5/31/2017 Policy # EHS-400.17 Isoflurane Use and Exposure Control Procedures 1.0 PURPOSE: LSUHSC is committed to keeping all exposures to hazardous
Cardiac Telemetry Self Study: Part One Cardiovascular Review 2017 THINGS TO REMEMBER
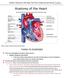 Please review the above anatomy of the heart. THINGS TO REMEMBER There are 3 electrolytes that affect cardiac function o Sodium, Potassium, and Calcium When any of these electrolytes are out of the normal
Please review the above anatomy of the heart. THINGS TO REMEMBER There are 3 electrolytes that affect cardiac function o Sodium, Potassium, and Calcium When any of these electrolytes are out of the normal
Module 1: Introduction to ECG & Normal ECG
 Module 1: Introduction to ECG & Normal ECG Importance of Correct anatomical positions Measurements & Morphologies ONLY accurate if Precise anatomical positions adhered to Standardised techniques are used
Module 1: Introduction to ECG & Normal ECG Importance of Correct anatomical positions Measurements & Morphologies ONLY accurate if Precise anatomical positions adhered to Standardised techniques are used
Safety Data Sheet. Product Name: DetectX Palladium Screening Fluorescent Detection Kit. Section 1: Identification. Section 2: Hazard(s) Identification
 Safety Data Sheet Revision Date: 17 October 2016 Product Name: DetectX Palladium Screening Fluorescent Detection Kit Section 1: Identification Product Name: DetectX Palladium Screening Fluorescent Detection
Safety Data Sheet Revision Date: 17 October 2016 Product Name: DetectX Palladium Screening Fluorescent Detection Kit Section 1: Identification Product Name: DetectX Palladium Screening Fluorescent Detection
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Testing the Accuracy of ECG Captured by Cronovo through Comparison of ECG Recording to a Standard 12-Lead ECG Recording Device
 Testing the Accuracy of ECG Captured by through Comparison of ECG Recording to a Standard 12-Lead ECG Recording Device Data Analysis a) R-wave Comparison: The mean and standard deviation of R-wave amplitudes
Testing the Accuracy of ECG Captured by through Comparison of ECG Recording to a Standard 12-Lead ECG Recording Device Data Analysis a) R-wave Comparison: The mean and standard deviation of R-wave amplitudes
INTRODUCTION TO ECG. Dr. Tamara Alqudah
 INTRODUCTION TO ECG Dr. Tamara Alqudah Excitatory & conductive system of the heart + - The ECG The electrocardiogram, or ECG, is a simple & noninvasive diagnostic test which records the electrical
INTRODUCTION TO ECG Dr. Tamara Alqudah Excitatory & conductive system of the heart + - The ECG The electrocardiogram, or ECG, is a simple & noninvasive diagnostic test which records the electrical
Final Rule for Preventive Controls for Animal Food
 Final Rule for Preventive Controls for Animal Food http://www.fda.gov/fsma THE FUTURE IS NOW 1 Background Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Food
Final Rule for Preventive Controls for Animal Food http://www.fda.gov/fsma THE FUTURE IS NOW 1 Background Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Food
Multi Analyte Custom Grade Solution
 1.0 ACCREDITATION / REGISTRATION INORGANIC VENTURES is accredited to ISO Guide 34, "General Requirements for the Competence of Reference Material Producers" and ISO/IEC 17025, "General Requirements for
1.0 ACCREDITATION / REGISTRATION INORGANIC VENTURES is accredited to ISO Guide 34, "General Requirements for the Competence of Reference Material Producers" and ISO/IEC 17025, "General Requirements for
THE PERMANENT PACEMAKER SYSTEM FOR THE TREATMENT OF HEART BLOCK IN THE DOG. Lanqford House, Lanqford, Bristol
 - 30 - THE PERMANENT PACEMAKER SYSTEM FOR THE TREATMENT OF HEART BLOCK IN THE DOG J. N. Lucke - Department of Veterinary Surqery, University of Bristol, Lanqford House, Lanqford, Bristol -- I IGTRODUCT
- 30 - THE PERMANENT PACEMAKER SYSTEM FOR THE TREATMENT OF HEART BLOCK IN THE DOG J. N. Lucke - Department of Veterinary Surqery, University of Bristol, Lanqford House, Lanqford, Bristol -- I IGTRODUCT
Procine sphingomyelin ELISA Kit
 Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Lecture outline. Electrical properties of the heart. Automaticity. Excitability. Refractoriness. The ABCs of ECGs Back to Basics Part I
 Lecture outline The ABCs of ECGs Back to Basics Part I Meg Sleeper VMD, DACVIM (cardiology) University of Florida Veterinary School Electrical properties of the heart Action potentials Normal intracardiac
Lecture outline The ABCs of ECGs Back to Basics Part I Meg Sleeper VMD, DACVIM (cardiology) University of Florida Veterinary School Electrical properties of the heart Action potentials Normal intracardiac
Analytical method validation. Presented by Debbie Parker 4 July, 2016
 Analytical method validation Presented by Debbie Parker 4 July, 2016 Introduction This session will cover: Guidance and references The types of test methods Validation requirements Summary Slide 2 PharmOut
Analytical method validation Presented by Debbie Parker 4 July, 2016 Introduction This session will cover: Guidance and references The types of test methods Validation requirements Summary Slide 2 PharmOut
General Concepts in the European Pharmacopoeia. Anne-Sophie Bouin European Pharmacopoeia Department, EDQM, Council of Europe
 General Concepts in the European Pharmacopoeia Anne-Sophie Bouin European Pharmacopoeia Department, EDQM, Council of Europe General notices Anne-Sophie Bouin, 28/10/09 2009 EDQM, Council of Europe, All
General Concepts in the European Pharmacopoeia Anne-Sophie Bouin European Pharmacopoeia Department, EDQM, Council of Europe General notices Anne-Sophie Bouin, 28/10/09 2009 EDQM, Council of Europe, All
NHA Certified EKG Technician (CET) Test Plan for the CET Exam
 NHA Certified EKG Technician (CET) Test Plan for the CET Exam 100 scored items Exam Time: 2 hours *Based on the results of a job analysis completed in 2017 This document provides both a summary and detailed
NHA Certified EKG Technician (CET) Test Plan for the CET Exam 100 scored items Exam Time: 2 hours *Based on the results of a job analysis completed in 2017 This document provides both a summary and detailed
12-Lead ECG Interpretation. Kathy Kuznar, RN, ANP
 12-Lead ECG Interpretation Kathy Kuznar, RN, ANP The 12-Lead ECG Objectives Identify the normal morphology and features of the 12- lead ECG. Perform systematic analysis of the 12-lead ECG. Recognize abnormalities
12-Lead ECG Interpretation Kathy Kuznar, RN, ANP The 12-Lead ECG Objectives Identify the normal morphology and features of the 12- lead ECG. Perform systematic analysis of the 12-lead ECG. Recognize abnormalities
Human CD4+T Cell Care Manual
 Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.04 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.04 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
510(k) SUMMARY. Bristol, PA President. PhysioFlow Q-Link. Classification Name: Impedance plethysmograph (21 CER 870.
 KC140102 Page 1 of 3 510(k) SUMMARY FB121 This 5 10(k) summary is submitted in accordance with the requirements of SMDA 1990 and 21 CER 807.87 Establishment Relistration Number: 3005467960 Address of Manufacturer:
KC140102 Page 1 of 3 510(k) SUMMARY FB121 This 5 10(k) summary is submitted in accordance with the requirements of SMDA 1990 and 21 CER 807.87 Establishment Relistration Number: 3005467960 Address of Manufacturer:
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. COMPANY AND PRODUCT IDENTIFICATION DUNCAN ENTERPRISES EMERGENCY TELEPHONE NUMBERS 5673 East Shields Avenue Health Emergency: 559-291-4444 7:00 am 3:30 pm Fresno, CA 93727
MATERIAL SAFETY DATA SHEET 1. COMPANY AND PRODUCT IDENTIFICATION DUNCAN ENTERPRISES EMERGENCY TELEPHONE NUMBERS 5673 East Shields Avenue Health Emergency: 559-291-4444 7:00 am 3:30 pm Fresno, CA 93727
Disclosures. STEMI:To Call or Not to Call. Disclosures 9/18/2017. Alternate Title: Hey Doc, If you re not doing anything Saturday Night
 STEMI:To Call or Not to Call Disclosures No financial disclosures September, 2017 Frederick James Trip Meine III MD, FACC, FSCAI Cape Fear Heart Associates, Wilmington, NC Disclosures Alternate Title:
STEMI:To Call or Not to Call Disclosures No financial disclosures September, 2017 Frederick James Trip Meine III MD, FACC, FSCAI Cape Fear Heart Associates, Wilmington, NC Disclosures Alternate Title:
MR Advance Techniques. Cardiac Imaging. Class IV
 MR Advance Techniques Cardiac Imaging Class IV Heart The heart is a muscular organ responsible for pumping blood through the blood vessels by repeated, rhythmic contractions. Layers of the heart Endocardium
MR Advance Techniques Cardiac Imaging Class IV Heart The heart is a muscular organ responsible for pumping blood through the blood vessels by repeated, rhythmic contractions. Layers of the heart Endocardium
Biosafety Level 3 (BL3)
 Biosafety Level 3 (BL3) NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (Appendix G-II-C) - April 2016 Biosafety Level 3 (BL3) Biosafety Level 3 is applicable to clinical,
Biosafety Level 3 (BL3) NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (Appendix G-II-C) - April 2016 Biosafety Level 3 (BL3) Biosafety Level 3 is applicable to clinical,
Collin County Community College
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 5 The Heart 1 The Heart Beat and the EKG 2 1 The Heart Beat and the EKG P-wave = Atrial depolarization QRS-wave = Ventricular depolarization
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 5 The Heart 1 The Heart Beat and the EKG 2 1 The Heart Beat and the EKG P-wave = Atrial depolarization QRS-wave = Ventricular depolarization
II. PROCEDURE DESCRIPTION A. Normal Waveform from an Electrocardiogram Figure 1 shows two cycles of a normal ECG waveform.
 Cardiac Monitor with Mobile Application and Alert System Miguel A. Goenaga-Jimenez, Ph.D. 1, Abigail C. Teron, BS. 1, Pedro A. Rivera 1 1 Universidad del Turabo, Puerto Rico, mgoenaga1@suagm.edu, abigailteron@gmail.com,
Cardiac Monitor with Mobile Application and Alert System Miguel A. Goenaga-Jimenez, Ph.D. 1, Abigail C. Teron, BS. 1, Pedro A. Rivera 1 1 Universidad del Turabo, Puerto Rico, mgoenaga1@suagm.edu, abigailteron@gmail.com,
International Journal of Pharma and Bio Sciences DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR THE ESTIMATION OF STRONTIUM RANELATE IN SACHET
 International Journal of Pharma and Bio Sciences RESEARCH ARTICLE ANALYTICAL CHEMISTRY DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR THE ESTIMATION OF STRONTIUM RANELATE IN SACHET K.MYTHILI *, S.GAYATRI,
International Journal of Pharma and Bio Sciences RESEARCH ARTICLE ANALYTICAL CHEMISTRY DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR THE ESTIMATION OF STRONTIUM RANELATE IN SACHET K.MYTHILI *, S.GAYATRI,
1. IDENTIFICATION OF SUBSTANCE OXYMETAZOLINE & TETRACAINE TOPICAL Name: SOLUTION
 Page 1 of 5 1. IDENTIFICATION OF SUBSTANCE OXYMETAZOLINE & TETRACAINE TOPICAL Name: SOLUTION Manufacturer: Information Department: Department of Pharmacy Duke University Medical Center Box 3089 Durham,
Page 1 of 5 1. IDENTIFICATION OF SUBSTANCE OXYMETAZOLINE & TETRACAINE TOPICAL Name: SOLUTION Manufacturer: Information Department: Department of Pharmacy Duke University Medical Center Box 3089 Durham,
Appendix D Output Code and Interpretation of Analysis
 Appendix D Output Code and Interpretation of Analysis 8 Arrhythmia Code No. Description 8002 Marked rhythm irregularity 8110 Sinus rhythm 8102 Sinus arrhythmia 8108 Marked sinus arrhythmia 8120 Sinus tachycardia
Appendix D Output Code and Interpretation of Analysis 8 Arrhythmia Code No. Description 8002 Marked rhythm irregularity 8110 Sinus rhythm 8102 Sinus arrhythmia 8108 Marked sinus arrhythmia 8120 Sinus tachycardia
Raritan Pharmaceuticals, Inc. 6/20/17
 Raritan Pharmaceuticals, Inc. 6/20/17 U.S. Food & Drug Administration Division of Pharmaceutical Quality Operations I New Jersey District 10 Waterview Boulevard, 3rd Floor Parsippany, NJ 07054 June 20,
Raritan Pharmaceuticals, Inc. 6/20/17 U.S. Food & Drug Administration Division of Pharmaceutical Quality Operations I New Jersey District 10 Waterview Boulevard, 3rd Floor Parsippany, NJ 07054 June 20,
HUMAN LIVER SLICE EXPERIMENT 1 Effects of Propylene Glycol on Ethylene Glycol Metabolism
 HUMAN LIVER SLICE EXPERIMENT 1 Effects of Propylene Glycol on Ethylene Glycol Metabolism Determination of Ethylene Glycol, Propylene Glycol, Oxalic Acid and Glycolic Acid BioReliance Study Number Testing
HUMAN LIVER SLICE EXPERIMENT 1 Effects of Propylene Glycol on Ethylene Glycol Metabolism Determination of Ethylene Glycol, Propylene Glycol, Oxalic Acid and Glycolic Acid BioReliance Study Number Testing
Sodium Bromide, Liquid Sodium Bromide
 MATERIAL SAFETY DATA SHEET SODIUM BROMIDE SOLUTION January 2017 A. GENERAL INFORMATION Trade Name (Common Name or Synonym) - Sodium Bromide, Liquid Sodium Bromide B. COMPOSITION INFORMATION ON INGREDIENTS
MATERIAL SAFETY DATA SHEET SODIUM BROMIDE SOLUTION January 2017 A. GENERAL INFORMATION Trade Name (Common Name or Synonym) - Sodium Bromide, Liquid Sodium Bromide B. COMPOSITION INFORMATION ON INGREDIENTS
SAFETY DATA SHEETS. Date: 1 July 2014 Update: - SECTION 2 HAZARDS IDENTIFICATION EMERGENCY OVERVIEW:
 SAFETY DATA SHEETS SECTION 1 CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Quality Shampoo Company Identification: RDI USA INC Company Address: 2999 N. Blackstock Rd. Spartanburg,
SAFETY DATA SHEETS SECTION 1 CHEMICAL SUBSTANCE/PRODUCT AND COMPANY IDENTIFICATION Finished Product Name: Quality Shampoo Company Identification: RDI USA INC Company Address: 2999 N. Blackstock Rd. Spartanburg,
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
1. COMPANY AND PRODUCT IDENTIFICATION 2. HAZARDS IDENTIFICATION
 Safety Data Sheet 1. COMPANY AND PRODUCT IDENTIFICATION ilovetocreate A Duncan Enterprises Company 5673 East Shields Avenue Fresno, CA 93727 559-291-4444 559-291-9444 (Fax) EMERGENCY TELEPHONE NUMBERS
Safety Data Sheet 1. COMPANY AND PRODUCT IDENTIFICATION ilovetocreate A Duncan Enterprises Company 5673 East Shields Avenue Fresno, CA 93727 559-291-4444 559-291-9444 (Fax) EMERGENCY TELEPHONE NUMBERS
1. IDENTIFICATION OF SUBSTANCE Name: ALUMINUM CHLORIDE SOLUTION 30% Manufacturer:
 Page 1 of 5 1. IDENTIFICATION OF SUBSTANCE Name: ALUMINUM CHLORIDE SOLUTION 30% Manufacturer: Department of Pharmacy Duke University Medical Center Box 3089 Durham, NC 27710 919-684-5125 Information Department:
Page 1 of 5 1. IDENTIFICATION OF SUBSTANCE Name: ALUMINUM CHLORIDE SOLUTION 30% Manufacturer: Department of Pharmacy Duke University Medical Center Box 3089 Durham, NC 27710 919-684-5125 Information Department:
LABORATORY INVESTIGATION
 LABORATORY INVESTIGATION Recording Electrocardiograms The taking of an electrocardiogram is an almost universal part of any complete physical examination. From the ECG record of the electrical activity
LABORATORY INVESTIGATION Recording Electrocardiograms The taking of an electrocardiogram is an almost universal part of any complete physical examination. From the ECG record of the electrical activity
Safety Data Sheet. Oligonucleotide Resolution Standard, Part Number x 2 ml vial, 4 nmol full-length product, 2 nmole per additional oligo
 Safety Data Sheet Oligonucleotide Resolution Standard, Part Number 5190-9028 Section 1. Product and company identification 1.1 Product identifier Product name : Oligonucleotide Resolution Standard Part
Safety Data Sheet Oligonucleotide Resolution Standard, Part Number 5190-9028 Section 1. Product and company identification 1.1 Product identifier Product name : Oligonucleotide Resolution Standard Part
IMPORTANT DRUG INFORMATION
 IMPORTANT DRUG INFORMATION 2 nd January 2019 Subject: ERWINAZE Batch 191K Notice of *New* Special Handling Instructions Use a 0.2-micron, low protein binding, in-line filter for IV administration of ERWINAZE
IMPORTANT DRUG INFORMATION 2 nd January 2019 Subject: ERWINAZE Batch 191K Notice of *New* Special Handling Instructions Use a 0.2-micron, low protein binding, in-line filter for IV administration of ERWINAZE
Sheet 5 physiology Electrocardiography-
 *questions asked by some students Sheet 5 physiology Electrocardiography- -why the ventricles lacking parasympathetic supply? if you cut both sympathetic and parasympathetic supply of the heart the heart
*questions asked by some students Sheet 5 physiology Electrocardiography- -why the ventricles lacking parasympathetic supply? if you cut both sympathetic and parasympathetic supply of the heart the heart
Assessment of pro-arrhythmic effects using Pluricyte Cardiomyocytes. on the ACEA xcelligence RTCA CardioECR
 Assessment of pro-arrhythmic effects using Pluricyte Cardiomyocytes on the ACEA xcelligence RTCA CardioECR User Guide Version 3.0 / March 2018 Contents 1. Introduction 2 2. Workflow 3 3. Important recommendations
Assessment of pro-arrhythmic effects using Pluricyte Cardiomyocytes on the ACEA xcelligence RTCA CardioECR User Guide Version 3.0 / March 2018 Contents 1. Introduction 2 2. Workflow 3 3. Important recommendations
Study Day 1 Study Days 2 to 9 Sequence 1 Placebo for moxifloxacin Study Days 2 to 8: placebo for pazopanib (placebopaz) 800 mg;
 The study listed may include approved non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Package leaflet: Information for the patient. NEGABAN 1g, powder for solution for injection or infusion Temocillin
 Package leaflet: Information for the patient NEGABAN 1g, powder for solution for injection or infusion Temocillin Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the patient NEGABAN 1g, powder for solution for injection or infusion Temocillin Read all of this leaflet carefully before you start using this medicine because it contains
CERTIFICATE OF ANALYSIS. tel: fax:
 CERTIFICATE OF ANALYSIS 300 Technology Drive Christiansburg, VA 24073. USA inorganicventures.com tel: 800.669.6799. 540.585.3030 fax: 540.585.3012 info@inorganicventures.com 1.0 ACCREDITATION / REGISTRATION
CERTIFICATE OF ANALYSIS 300 Technology Drive Christiansburg, VA 24073. USA inorganicventures.com tel: 800.669.6799. 540.585.3030 fax: 540.585.3012 info@inorganicventures.com 1.0 ACCREDITATION / REGISTRATION
Paediatric ECG Interpretation
 Paediatric ECG Interpretation Dr Sanj Fernando (thanks to http://lifeinthefastlane.com/ecg-library/paediatric-ecginterpretation/) 3 yo boy complaining of abdominal pain and chest pain Child ECG vs Adult
Paediatric ECG Interpretation Dr Sanj Fernando (thanks to http://lifeinthefastlane.com/ecg-library/paediatric-ecginterpretation/) 3 yo boy complaining of abdominal pain and chest pain Child ECG vs Adult
ALLERGEN STATEMENT. Ultra Fresh Supreme 400. Product Allergens and Sensitizers Wheat
 ALLERGEN STATEMENT Ultra Fresh Supreme 400 Updated On: 7/11/2013 K Page 1 of 1 Product Allergens and Sensitizers Wheat Facility Allergens and Sensitizers Egg Gluten, Barley Gluten, Oats Gluten, Rye Milk
ALLERGEN STATEMENT Ultra Fresh Supreme 400 Updated On: 7/11/2013 K Page 1 of 1 Product Allergens and Sensitizers Wheat Facility Allergens and Sensitizers Egg Gluten, Barley Gluten, Oats Gluten, Rye Milk
Full file at
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What electrical event must occur for atrial kick to occur? 1) A) Atrial repolarization B) Ventricular
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What electrical event must occur for atrial kick to occur? 1) A) Atrial repolarization B) Ventricular
HUMAN ANATOMY AND PHYSIOLOGY
 HUMAN ANATOMY AND PHYSIOLOGY NAME Detection of heart sounds. Clean the ear pieces of the stethoscope before using. The ear pieces should be pointing slightly forward when inserted into the ears because
HUMAN ANATOMY AND PHYSIOLOGY NAME Detection of heart sounds. Clean the ear pieces of the stethoscope before using. The ear pieces should be pointing slightly forward when inserted into the ears because
