Lecture 1 6/23/10. Biochemistry. Welcome to Chem 353 Nutritional Biochemistry. Carbohydrates. Properties of Carbohydrates. Types of Carbohydrates
|
|
|
- Jerome King
- 5 years ago
- Views:
Transcription
1 Welcome to hem 353 Nutritional Biochemistry ourse Objective The objective of this course is to gain a basic understanding of biochemistry with a particular emphasis on nutrition and its effects on metabolism. oncepts covered in this course include identifying and characterizing nutrient molecules involved in metabolism, and ultimately understanding the degradative pathways of these molecules. In addition, an introduction to genetic material, DNA eplication, Transcription, and Translation will also be given. Biochemistry It is the study of chemical processes in living organisms Specifically, it aims to understand the structure and function of biomolecules In this class we are going to focus on the four major macromolecules found in living organisms! arbohydrates! Lipids! Proteins! Nucleic Acids arbohydrates Lecture 1 arbohydrates - Monosaccharides and Dissaccharides hapter 19 Section 1-5 Primary source of energy for the cell omposed of arbon, Hydrogen and Oxygen in a ratio roughly 1:2:1 (generic formula n H 2n O n ) Included in this group are! Starches and fibers (complex carbohydrates)! Sugars (sweet tasting compounds, simple carbs)! Structural material (such as cellulose) Properties of arbohydrates They are alcohols (contain -OH functional groups) and carbonyls (=O functional groups, in the form of an aldehyde or ketone group) Because of the -OH groups they form extensive hydrogen bonds! The ability to hydrogen bond allows them to dissolve well in water! Have high melting points! Mono- and disaccharides are solids at room temp. Types of arbohydrates Monosaccharides - one saccharide unit Disaccharides - two saccharide units Oligosaccharides - a few saccharide units (about 3 to 10) Polysaccharides - formed of many repeating units of mono or disaccharides, can range from more than 10 to several 100 1
2 Monosaccharides Are simple carbohydrates that can not be hydrolyzed further They are the building blocks of more complex carbohydrates an range in size from 3 to 7carbon units! A 3 saccharide is called a triose! A 4 saccharide is called a tetrose! A 5 saccharide is called a pentose! A 6 saccharide is called a hexose! A 7 saccharide is called a heptose General Nomenclature for Monosaccharides The name is composed of three parts! First part identifies the type of carbonyl Aldehyde - aldose (aldo) O Ketone - ketose (keto)! Second part identifies the number of carbons in the saccharide Mono, di, tri, tetra, penta, hexa, O hepta! The last part identifies the compound as a sugar ose Examples aldopentose ketoheptose aldohexose aldotriose ketotriose ketotetrose 4 ketohexose Drawing Saccharide Structures Fisher Projections! German chemist Emil Fischer initiated the convention of depicting the formulas as follows Aldehyde or ketone towards the top The -H or -OH are positioned to the right or left The formulas that followed glyceraldehyde (or had the -OH positioned to the right of the chiral carbon furthest from the =O) represents the dextrorotatory isomer (D) The levorotatory isomer (L) has the -OH positioned the left! The D and L do not explain how the saccharides rotate plane-polarized light, they only tell you the absolute configuration D-Glyceraldehyde D-Galactose ommon Names for Monosaccharides Two Biologically Important Trioses D-(+)- Glyceraldehyde Dihydroxyacetone D-(-)- ibsose D-(-)-2- Deoxyribsose All of the sugars used by humans are in the D configuration, because our enzymes do not recognize the L configuration Phosphorylated derivatives of Glyceraldehyde and Dihydroxyacetone are very important intermediates in glycolysis More complex sugars are thought to be derived from glyceraldehyde and dihydroxyacetone Note that dihydroxyacetone does not have a chiral carbon D-(+)- Glyceraldehyde Dihydroxyacetone 2
3 Most ommonly Found Hexoses in Nature Information on the Abundant Hexoses D-(+)-Galactose D-(+)-Glucose D-(+)-Mannose D-(-)-Fructose Glucose (also known as dextrose, grape sugar, corn sugar)! Most abundant sugar found in nature! ommonly found in fruits (especially ripe grapes)! Most carbohydrates we eat eventually get converted to glucose in a series of metabolic pathways that produce energy! Is the circulating carbohydrate of animals (blood sugar) Mannose! Is a component of the polysaccharide mannan, found in some berries and vegetable ivory (white fleshy part) of palm nuts These are 6 arbon Sugars (Hexoses) that you should be able to * identify Galactose (also known as brain sugar)! Is obtained from hydrolyzing lactose (a disaccharide of glucose and galactose)! Does not occur in nature uncombined! Is an important constituent of the glycolipids that occur in the brain and in the myelin sheath of nerve cells Fructose (also known as levulose)! Is the only naturally occurring ketohexose! Is found in honey and sweet fruits, along with glucose and sucrose! Is the sweetest sucrose! Is the only sugar found in the semen of bulls and men. It is formed in the prostate gland and is the major energy source for spermatozoa yclic Structures of Monosaccharides emembering a little organic chemistry we know that aldehydes and ketones can react with alcohols as follows: O --H = Aldehyde O = Ketone OH + -OH --H Alcohol O Hemiacetal OH + -OH Alcohol O Hemiketal The fact that the two functional groups are close together in monosaccharides facilitates their ability to react Monosaccharides longer than tetroses exist mainly as cyclic hemiacetals and hemiketals Generally the carbonyl will react with the OH group that will lead to the formation of a stable 5 or 6 member ring (rings with these sizes have the least amount of strain) Therefore in solution D-glucose exists as a mixture of: Looking at examples of monosaccharides that form five membered rings -These two isomers are referred to as anomers -They differ in structure around the anomeric carbon (the carbon that was the carbonyl carbon in the straight chain form) ule of thumb: 1. Aldoses tend to form rings that represent the number of carbons the sugar has, so if it is a 5 carbon sugar it will form a 5 member ring 2. Ketoses tend to form rings that are 1 member smaller than the number of carbons the sugar has, so if it is a 6 carbon sugar then it will form a 5 member ring *And remember with both types, one member of the ring will always be oxygen 3
4 In solution the!-, "- and open chain forms of the saccharide exist in equilibrium The molecules will interconvert between the various forms in a process referred to as mutarotation Questions:! What will happen if a pure anomer of D-glucose is dissolved in water?! What will happen if a chemical is added that reacts with the open chain of D-glucose? Are a simplified projection of the cyclic version of saccharides The saccharide is placed on a plane with the darkened edges toward the viewer and H directly attached to the ring are not shown (generally) Any group of atoms written on the right in a Fisher projection are drawn down on a Haworth projection and any on the left are drawn up Haworth Projections H O Properties of Monosaccharides Glucose, mannose, galactose and fructose are crystalline solids at room temp. These sugars are readily soluble in water because of the many hydroxyl groups present The hydroxyl groups can be chemically changed to esters and ethers, but most importantly the aldehyde group can be oxidized with a mild oxidizing agent! Tollens s eaction _ H O O O + Ag(NH 3 ) Ag(s) An aldose Tollens s eagent (lear solution) arboxylate anion! Any carbohydrate capable of this reduction without first undergoing hydrolysis is said to be a reducing sugar Silver mirror Another reagent also used to determine if a sugar is a reducing sugar is Benedict s reagent H O + u(citrate) -2 2 Benedict s eagent An aldose (blue solution) O O + u 2 O(s) arboxylate anion Brick-red precipitate Modified versions of this reaction are used as diagnostic tools to determine the presence of glucose in blood or urine The advantage of these reactions are that they are simple and quick Disaccharides Monosaccharides can also form chains, Two monosaccharides can join together to form a disaccharide, one water molecule is eliminated in the process and the bond formed between the sugars is called a glycosidic bond! Glycosidic bonds form when the anomeric carbon of one monosacccharide reacts with the hydroxyl group of a second monosaccharide! The carbon-oxygen-carbon linkage is called the glycosidic linkage! Anomeric carbons involved in a glycosidic bond are non reducing emembering a little organic chemistry H O OH + OH --O + OH Aldehyde Alcohol H Hemiacetal (unstable) Second alcohol Acid O --O + H 2 O H Acetal (Stable) When starting with a ketone instead of an aldehyde the first product is referred to as a hemiketal and the end product is a ketal Forming glycosidic linkages makes the sugars more stable Still reducible hemiacetal hemiketal acetal Not reducible ketal 4
5 Writing Names for Disaccharides The common names for disaccharides have an! or " placed before the name, this refers to the position of the OH group attached to the reducible anomeric carbon Example:!-Maltose "-Maltose But the linkage for both is labeled as an!-1,4-glycosidic linkage because the Oxygen connected to the anomeric carbon involved in the bond is pointing down and down is referred to as!, if the bond were pointing up as in lactose then the linkage would have been " ommon Disaccharides The three common disaccharides we are going to focus on are:! Maltose (malt sugar) is a disaccharide produced during the breakdown of starch It is important in the manufacture of beer and is produced when barley germinates and breaks down starch stores It is 30% as sweet as sucrose In humans it is broken down into its component, two D-glucose molecules by the action of the enzyme maltase In the laboratory it can be broken down with dilute acid acting as a catalyst Maltose is a reducing sugar, it exhibits mutarotation at the free anomeric carbon D-glucose!-1,4-glycosidic bond Maltose D-glucose! Lactose (milk sugar) is a disaccharide found in milk produced by humans (7.5%), cows (4.5%) and other mammals It is only synthesized by mammary tissue (most other carbohydrates are produced by plants) The alpha form is commercially important in baby formula and in the production of penicillin Drug dealers use it to cut heroin It is 1/6 th as sweet as sucrose In humans it is broken down into its component, D-galactose and D-glucose by the action of the enzyme lactase, people who are lactose intolerant have a deficiency in lactase In the laboratory it can be broken down with dilute acid acting as a catalyst It is a reducing sugar and exhibits mutarotation at the free anomeric carbon D-galactose "-1,4-glycosidic linkage Lactose D-glucose! Sucrose (beet sugar, cane sugar, table sugar, sugar) is obtained from sugar canes and sugar beets. Juice obtained from sugar canes and sugar beets contain 14-20% sucrose. The sucrose is recovered by evaporating the water and recrystallizing the sucrose. The glycosidic linkage in sucrose is unique among common disaccharides because it is a head-to-head linkage Because the two anomeric carbons are stable (both saccharides formed either an acetal or ketal) the sugar cannot be reduced or undergo mutarotation. It exists in one state in both solid and solution form and as a consequence can crystallize easily In humans it is broken down into its component, D-glucose and D-fructose by the action of the enzyme sucrase (invertase). The two sugars are produced in a 1:1 ratio and the mixture is referred to as invert sugar Glucose Fructose Sucrose!-1,"-2- glycosidic linkage Invert Sugar and hocolate- overed herries What is the secret to the extra sweet juice in chocolatecovered cherries! Before being dipped in chocolate the cherries are covered in a sugary paste that contains sucrase, then the cherries are dipped and stored for one to two weeks During storage a chemical process occurs, sucrase converts sucrose into invert sugar (a 1:1 mixture of glucose and fructose)! Invert sugar remains in solution better so they stay dissolved in the cherries juice! Fructose produced from the hydrolysis is sweeter than sucrose The chocolate has to be a of an optimum particle size onsequences of Too Much Sugar The average American consumes more than 100 lbs of sucrose a year and 2/3 of this comes from! Soft drinks! Sugary cereals! Other highly processed food Excess consumption of sugar has led to increased obesity, tooth decay and possibly other problems! Obesity can result from excess carbohydrates being converted and stored in the body as fat! Tooth decay is a result of sucrose becoming a part of plaque, the bacteria in plaque then uses the sucrose as an adhesive and a food source. The bacteria can also breakdown the sucrose to lactic acid, which corrodes teeth and leads to gum destruction 5
6 Artificial Sweeteners Synthetic organic compounds that are much sweeter then natural sugars (high intensity sweeteners) They are noncaloric or used in such a small amount that they do not add significantly to the caloric value of the food!sachcarin is noncaloric because it passes right through unchanged and it is x sweeter than sucrose, it needs to be combined with cyclamate to avoid a metallic aftertaste!yclamate is 30-50x sweeter than sucrose, when combined with saccharin in a ratio of 10 to 1 (cyclamate to saccharin) the aftertaste of both is masked, it is stable after heating! Aspartame is 160x sweeter than sucrose and has no aftertaste. In the body it is hydrolyzed to aspartic acid, phenylalanine, and methanol. The only major risk is to people with PKU disease. Lastly it is not heat stable and cannot be used in baked goods!sucralose is 600x sweeter than sugar, it is synthesized from sucrose but has 3-OH replaced by l, it passes right through and is heat stable!acesulfame K is 200x sweeter than sugar, is heat stable and has no aftertaste Lactose Intolerance and Galactosemia Lactose intolerance is caused by an inability to digest lactose! Lactose makes up 40% of an infant s diet during the first year, but infants and small children have a potent form of lactase in their small intestine that allows them to easily digest lactose! Adults have a less active form of the enzyme and about 70% of the world s population (20% of US population) have some lactase deficiency and for some people as they age they produce less and less enzyme. Symptoms of intolerance are caused by the unhydrolyzed lactose passing straight into the colon, there its presence causes fluid to be drawn in to the colon by osmosis and at the same time bacteria may act on the lactose to produce organic acids and gases which all results in:! Abdominal distention, cramps and diarrhea In most cases these symptoms are alleviated by:! Limiting or excluding milk from diet! Purchasing milk that has already been treated with lactase or treating food with lactase (lactaid)! onsuming milk that has been cook or fermented, because these process result in the partial hydrolysis of lactose A more severe enzyme deficiency associated with the inability to digest milk is galactosemia (ate in US is 1 in every 65,000) Galactosemia is a genetic disease, in which either of the two enzymes needed to convert galactose to glucose is lacking! As a result blood galactose is markedly elevated and galactose is found in the urine! An infant with the disease may exhibit lack of appetite, weight loss, diarrhea, and jaundice! The disease can result in impaired liver function, cataracts, mental retardation, and even death! If recognized early the effects of the disease can be prevented by eliminating all galactose from the diet! As the child ages, the child will develop an alternate pathway for metabolizing galactose Where Do arbohydrates ome From? People cannot synthesize carbohydrates from O 2 and H 2 O so we obtain them from plants, by eating! Seeds, roots, tubers, fruits, etc Plants synthesize sugar glucose as follows: 6O 2 + 6H 2 O kcal # 6 H 12 O 6 + 6O 2 Solar Energy The processes is known as photosynthesis The glucose can either be used by the plant for energy or it can be converted to a more complex carbohydrate that stores energy for later use or provides structure 6
7 arbohydrates Are Very Important Because they are the primary energy source for plants and animals AND Questions????? Because they provide the building blocks for the synthesis of proteins, lipids and nucleic acids Guess the Main Sugar 7
Carbohydrates. Chapter 12
 Carbohydrates Chapter 12 Educational Goals 1. Given a Fischer projection of a monosaccharide, classify it as either aldoses or ketoses. 2. Given a Fischer projection of a monosaccharide, classify it by
Carbohydrates Chapter 12 Educational Goals 1. Given a Fischer projection of a monosaccharide, classify it as either aldoses or ketoses. 2. Given a Fischer projection of a monosaccharide, classify it by
Ch13. Sugars. What biology does with monosaccharides disaccharides and polysaccharides. version 1.0
 Ch13 Sugars What biology does with monosaccharides disaccharides and polysaccharides. version 1.0 Nick DeMello, PhD. 2007-2015 Ch13 Sugars Haworth Structures Saccharides can form rings. That creates a
Ch13 Sugars What biology does with monosaccharides disaccharides and polysaccharides. version 1.0 Nick DeMello, PhD. 2007-2015 Ch13 Sugars Haworth Structures Saccharides can form rings. That creates a
!"#$%&'()*+(!,-./012-,345(
 (!"#$%&'()*+(!,-./012-,345( (!"#"$%&'()$*%#+,'(-(.+/&/*+,%&(01"2+34$5( 6%#+,"(!/$75#38+(92+41( CAPTER 20: Learning Objectives:! >
(!"#$%&'()*+(!,-./012-,345( (!"#"$%&'()$*%#+,'(-(.+/&/*+,%&(01"2+34$5( 6%#+,"(!/$75#38+(92+41( CAPTER 20: Learning Objectives:! >
Chemistry B11 Chapters 13 Esters, amides and carbohydrates
 Chapters 13 Esters, amides and carbohydrates Esters: esters are derived from carboxylic acids (the hydrogen atom in the carboxyl group of carboxylic acid is replaced by an alkyl group). The functional
Chapters 13 Esters, amides and carbohydrates Esters: esters are derived from carboxylic acids (the hydrogen atom in the carboxyl group of carboxylic acid is replaced by an alkyl group). The functional
Chem 263 Nov 22, Carbohydrates (also known as sugars or saccharides) See Handout
 hem 263 Nov 22, 2016 arbohydrates (also known as sugars or saccharides) See andout Approximately 0.02% of the sun s energy is used on this planet for photosynthesis in which organisms convert carbon dioxide
hem 263 Nov 22, 2016 arbohydrates (also known as sugars or saccharides) See andout Approximately 0.02% of the sun s energy is used on this planet for photosynthesis in which organisms convert carbon dioxide
CHAPTER 27 CARBOHYDRATES SOLUTIONS TO REVIEW QUESTIONS
 27 09/17/2013 11:12:35 Page 397 APTER 27 ARBYDRATES SLUTINS T REVIEW QUESTINS 1. In general, the carbohydrate carbon oxidation state determines the carbon s metabolic energy content. The more oxidized
27 09/17/2013 11:12:35 Page 397 APTER 27 ARBYDRATES SLUTINS T REVIEW QUESTINS 1. In general, the carbohydrate carbon oxidation state determines the carbon s metabolic energy content. The more oxidized
24.1 Introduction to Carbohydrates
 24.1 Introduction to Carbohydrates Carbohydrates (sugars) are abundant in nature: They are high energy biomolecules. They provide structural rigidity for organisms (plants, crustaceans, etc.). The polymer
24.1 Introduction to Carbohydrates Carbohydrates (sugars) are abundant in nature: They are high energy biomolecules. They provide structural rigidity for organisms (plants, crustaceans, etc.). The polymer
This is Carbohydrates, chapter 16 from the book Introduction to Chemistry: General, Organic, and Biological (index.html) (v. 1.0).
 This is Carbohydrates, chapter 16 from the book Introduction to Chemistry: General, Organic, and Biological (index.html) (v. 1.0). This book is licensed under a Creative Commons by-nc-sa 3.0 (http://creativecommons.org/licenses/by-nc-sa/
This is Carbohydrates, chapter 16 from the book Introduction to Chemistry: General, Organic, and Biological (index.html) (v. 1.0). This book is licensed under a Creative Commons by-nc-sa 3.0 (http://creativecommons.org/licenses/by-nc-sa/
MahaAbuAjamieh. BahaaNajjar. MamoonAhram
 7 MahaAbuAjamieh BahaaNajjar MamoonAhram Carbohydrates (saccharides) can be classified into these main categories: 1. Monosaccharides, they are simplesugars (the simplest units), such as glucose, galactose
7 MahaAbuAjamieh BahaaNajjar MamoonAhram Carbohydrates (saccharides) can be classified into these main categories: 1. Monosaccharides, they are simplesugars (the simplest units), such as glucose, galactose
Biomolecules are organic molecules produced by living organisms which consists mainly of the following elements:
 Biomolecules are organic molecules produced by living organisms which consists mainly of the following elements: These elements are non-metals which combine in various ways to form biomolecules through
Biomolecules are organic molecules produced by living organisms which consists mainly of the following elements: These elements are non-metals which combine in various ways to form biomolecules through
A Getting-It-On Review and Self-Test. . Carbohydrates are
 A Getting-It-n Review and Self-Test arbohydrates arbohydrates, one of the three principal classes of foods, contain only three elements: (1), (2), and (3). The name carbohydrate is derived from the French
A Getting-It-n Review and Self-Test arbohydrates arbohydrates, one of the three principal classes of foods, contain only three elements: (1), (2), and (3). The name carbohydrate is derived from the French
Carbohydrates. Green plants turn H 2 O, CO 2, and sunlight into carbohydrates.
 Chapter 27 Carbohydrates Green plants turn 2 O, CO 2, and sunlight into carbohydrates. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris ein, Scott Pattison, and Susan
Chapter 27 Carbohydrates Green plants turn 2 O, CO 2, and sunlight into carbohydrates. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris ein, Scott Pattison, and Susan
Fundamentals of Organic Chemistry. CHAPTER 6: Carbohydrates
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 6: Carbohydrates Carbohydrates
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 6: Carbohydrates Carbohydrates
Chapter 27 Carbohydrates
 Chapter 27 Carbohydrates Green plants turn 2 O, CO 2, and sunlight into carbohydrates. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris ein, Scott Pattison, and Susan
Chapter 27 Carbohydrates Green plants turn 2 O, CO 2, and sunlight into carbohydrates. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris ein, Scott Pattison, and Susan
Chapter 20 Carbohydrates Chapter 20
 Chapter 20 Carbohydrates Chapter 20 1 Carbohydrates Carbohydrate: A polyhydroxyaldehyde or polyhydroxyketone, or a substance that gives these compounds on hydrolysis. Monosaccharide: A carbohydrate that
Chapter 20 Carbohydrates Chapter 20 1 Carbohydrates Carbohydrate: A polyhydroxyaldehyde or polyhydroxyketone, or a substance that gives these compounds on hydrolysis. Monosaccharide: A carbohydrate that
Carbohydrates 1. Steven E. Massey, Ph.D. Assistant Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras
 Carbohydrates 1 Steven E. Massey, Ph.D. Assistant Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras Office & Lab: NCN#343B Tel: 787-764-0000 ext. 7798 E-mail: stevenemassey@gmail.com
Carbohydrates 1 Steven E. Massey, Ph.D. Assistant Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras Office & Lab: NCN#343B Tel: 787-764-0000 ext. 7798 E-mail: stevenemassey@gmail.com
Lecture 2 Carbohydrates
 Lecture 2 Carbohydrates Sources of CHOs Wholegrains major dietary intake Vegetables, legumes ad fruit contain dietary fibre Milk products provide lactose essential for infants Glycogen is a storage carbohydrate,
Lecture 2 Carbohydrates Sources of CHOs Wholegrains major dietary intake Vegetables, legumes ad fruit contain dietary fibre Milk products provide lactose essential for infants Glycogen is a storage carbohydrate,
Biochemistry: Macromolecules
 1 Biology: Macromolecules 2 Carbohydrates Carbohydrate organic compound containing carbon, hydrogen, & oxygen in a 1:2:1 ratio Meaning: hydrated carbon ratio of h:0 is 2:1 (same as in water) Source: plants
1 Biology: Macromolecules 2 Carbohydrates Carbohydrate organic compound containing carbon, hydrogen, & oxygen in a 1:2:1 ratio Meaning: hydrated carbon ratio of h:0 is 2:1 (same as in water) Source: plants
2/25/2015. Chapter 6. Carbohydrates. Outline. 6.1 Classes of Carbohydrates. 6.1 Classes of Carbohydrates. 6.1 Classes of Carbohydrates
 Lecture Presentation Chapter 6 Carbohydrates Julie Klare Fortis College Smyrna, GA Outline 6.7 Carbohydrates and Blood The simplest carbohydrates are monosaccharides (mono is Greek for one, sakkhari is
Lecture Presentation Chapter 6 Carbohydrates Julie Klare Fortis College Smyrna, GA Outline 6.7 Carbohydrates and Blood The simplest carbohydrates are monosaccharides (mono is Greek for one, sakkhari is
I. Carbohydrates Overview A. Carbohydrates are a class of biomolecules which have a variety of functions. 1. energy
 Chapter 22 Carbohydrates Chem 306 Roper I. Carbohydrates Overview A. Carbohydrates are a class of biomolecules which have a variety of functions. 1. energy 2. energy storage 3. structure 4. other functions!
Chapter 22 Carbohydrates Chem 306 Roper I. Carbohydrates Overview A. Carbohydrates are a class of biomolecules which have a variety of functions. 1. energy 2. energy storage 3. structure 4. other functions!
Chapter 18. Carbohydrates with an Introduction to Biochemistry. Carbohydrates with an Introduction to Biochemistry page 1
 Chapter 18 Carbohydrates with an Introduction to Biochemistry Carbohydrates with an Introduction to Biochemistry page 1 Introduction to Proteins, Carbohydrates, Lipids, and Bioenergetics Metabolism and
Chapter 18 Carbohydrates with an Introduction to Biochemistry Carbohydrates with an Introduction to Biochemistry page 1 Introduction to Proteins, Carbohydrates, Lipids, and Bioenergetics Metabolism and
Chemistry 1120 Exam 2 Study Guide
 Chemistry 1120 Exam 2 Study Guide Chapter 6 6.1 Know amines are derivatives of ammonia, which is not an amine. Classify amines as primary, secondary or tertiary. Master Tutor Section 6.1 Review Section
Chemistry 1120 Exam 2 Study Guide Chapter 6 6.1 Know amines are derivatives of ammonia, which is not an amine. Classify amines as primary, secondary or tertiary. Master Tutor Section 6.1 Review Section
Chapter 23 Carbohydrates and Nucleic Acids. Carbohydrates
 Chapter 23 Carbohydrates and Nucleic Acids Carbohydrates Synthesized by plants using sunlight to convert CO 2 and H 2 O to glucose and O 2. Polymers include starch and cellulose. Starch is storage unit
Chapter 23 Carbohydrates and Nucleic Acids Carbohydrates Synthesized by plants using sunlight to convert CO 2 and H 2 O to glucose and O 2. Polymers include starch and cellulose. Starch is storage unit
Dr. Nafith Abu Tarboush. Rana N. Talj
 2 Dr. Nafith Abu Tarboush June 19 th 2013 Rana N. Talj Review: Fischer suggested a projection in which the horizontal bonds are projecting towards the viewer and the vertical ones project away from the
2 Dr. Nafith Abu Tarboush June 19 th 2013 Rana N. Talj Review: Fischer suggested a projection in which the horizontal bonds are projecting towards the viewer and the vertical ones project away from the
Number of Carbohydrate Units
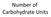 Number of Carbohydrate Units Monosaccharides = single unit Disaccharides = two units Oligiosaccharide = 3 10 units Polysaccharide = 11+ units Bonus: Can you name the most common Mono (4), Di(3), and Poly(4)
Number of Carbohydrate Units Monosaccharides = single unit Disaccharides = two units Oligiosaccharide = 3 10 units Polysaccharide = 11+ units Bonus: Can you name the most common Mono (4), Di(3), and Poly(4)
Carbohydrates CHAPTER SUMMARY
 14 2 cellulose 2 2 arbohydrates 2 amylose APTER SUMMARY 14.1 hemical Nature of arbohydrates - Polyhydroxy Aldehydes and Ketones arbohydrates are a class of organic biopolymers which consist of polyhydroxy
14 2 cellulose 2 2 arbohydrates 2 amylose APTER SUMMARY 14.1 hemical Nature of arbohydrates - Polyhydroxy Aldehydes and Ketones arbohydrates are a class of organic biopolymers which consist of polyhydroxy
CLASS 12th. Biomolecules
 CLASS 12th Biomolecules 01. Introduction Biomolecules may be defined as complex lifeless chemical substances which form the basis of life. i.e. they not only build up living system (creatures) but are
CLASS 12th Biomolecules 01. Introduction Biomolecules may be defined as complex lifeless chemical substances which form the basis of life. i.e. they not only build up living system (creatures) but are
Chemistry 1050 Exam 3 Study Guide
 Chapter 12 Chemistry 1050 Exam 3 Study Guide 12.1 a) Identify alkenes, alkynes and aromatics as unsaturated hydrocarbons. Determine the number of hydrogen atoms needed to complete an alkene structure.
Chapter 12 Chemistry 1050 Exam 3 Study Guide 12.1 a) Identify alkenes, alkynes and aromatics as unsaturated hydrocarbons. Determine the number of hydrogen atoms needed to complete an alkene structure.
Chemistry 107 Exam 3 Study Guide
 Chapter 7 Chemistry 107 Exam 3 Study Guide 7.1 Recognize the aldehyde, ketone and hydroxyl (-OH) functional groups found in carbohydrates. Differentiate between mono-, di-, and polysaccharides. Master
Chapter 7 Chemistry 107 Exam 3 Study Guide 7.1 Recognize the aldehyde, ketone and hydroxyl (-OH) functional groups found in carbohydrates. Differentiate between mono-, di-, and polysaccharides. Master
CARBOHYDRATES (SUGARS)
 ARBYDRATES (SUGARS) ARBYDRATES: 1. Most Abundant Molecules on Earth: (100 MILLIN METRI TNS f 2 And 2 0 onverted To ellulose and ther Plant Products/Year) 2. FUNTINS: Diet, Energy, Structural, Signalling
ARBYDRATES (SUGARS) ARBYDRATES: 1. Most Abundant Molecules on Earth: (100 MILLIN METRI TNS f 2 And 2 0 onverted To ellulose and ther Plant Products/Year) 2. FUNTINS: Diet, Energy, Structural, Signalling
Carbohydrates. Chapter 18
 Carbohydrates Chapter 18 Biochemistry an overview Biochemistry is the study of chemical substances in living organisms and the chemical interactions of these substances with each other. Biochemical substances
Carbohydrates Chapter 18 Biochemistry an overview Biochemistry is the study of chemical substances in living organisms and the chemical interactions of these substances with each other. Biochemical substances
Carbohydrates are a large group of organic compounds occurring in and including,, and. They contain hydrogen and oxygen in the same ratio as (2:1).
 Carbohydrates are a large group of organic compounds occurring in and and including,, and. They contain hydrogen and oxygen in the same ratio as (2:1). Why we study carbohydrates 1) carbohydrates are the
Carbohydrates are a large group of organic compounds occurring in and and including,, and. They contain hydrogen and oxygen in the same ratio as (2:1). Why we study carbohydrates 1) carbohydrates are the
Review from last lecture
 eview from last lecture D-glucose has the structure shown below (you are responsible for its structure on the exam). It is an aldohexose ( aldo since it contains aldehyde functionality and hexose since
eview from last lecture D-glucose has the structure shown below (you are responsible for its structure on the exam). It is an aldohexose ( aldo since it contains aldehyde functionality and hexose since
Carbohydrates - Chemical Structure
 Carbohydrates - Chemical Structure Carbohydrates consist of the elements carbon (C), hydrogen (H) and oxygen (O) with a ratio of hydrogen twice that of carbon and oxygen. Carbohydrates include sugars,
Carbohydrates - Chemical Structure Carbohydrates consist of the elements carbon (C), hydrogen (H) and oxygen (O) with a ratio of hydrogen twice that of carbon and oxygen. Carbohydrates include sugars,
Carbohydrates. Learning Objective
 , one of the four major classes of biomolecules, are aldehyde or ketone compounds with multiple hydroxyl groups. They function as energy stores, metabolic intermediates and important fuels for the body.
, one of the four major classes of biomolecules, are aldehyde or ketone compounds with multiple hydroxyl groups. They function as energy stores, metabolic intermediates and important fuels for the body.
BIOMOLECULES & SPECTROSCOPY TABLE OF CONTENTS S.NO. TOPIC PAGE NO. i) Carbohydrates B3. ii) Proteins & Nucleic Acids.
 BIOMOLECULES & SPECTROSCOPY TABLE OF CONTENTS S.NO. TOPIC PAGE NO. 1. Biomolecules i) Carbohydrates B3 ii) Proteins & Nucleic Acids iii) Steroids iv) Terpenes & Cartenoids B27 B61 B65 2. Spectroscopy v)
BIOMOLECULES & SPECTROSCOPY TABLE OF CONTENTS S.NO. TOPIC PAGE NO. 1. Biomolecules i) Carbohydrates B3 ii) Proteins & Nucleic Acids iii) Steroids iv) Terpenes & Cartenoids B27 B61 B65 2. Spectroscopy v)
BCH 4053 Spring 2001 Chapter 7 Lecture Notes
 BC 4053 Spring 2001 Chapter 7 Lecture Notes 1 Chapter 7 Carbohydrates 2 Carbohydrates: Nomenclature ydrates of carbon General formula (C 2 ) n (simple sugars) or C x ( 2 0) y Monosaccharides (simple sugars)
BC 4053 Spring 2001 Chapter 7 Lecture Notes 1 Chapter 7 Carbohydrates 2 Carbohydrates: Nomenclature ydrates of carbon General formula (C 2 ) n (simple sugars) or C x ( 2 0) y Monosaccharides (simple sugars)
Carbohydrates - General Description
 arbohydrates - General Description A. Polyhydroxy Aldehydes or Ketones ARBN AIN B. Serve a variety of functions ARBN AIN ARBN AIN 1. Energy storage (Glucose, Glycogen, Starch) 2. Structural Support (ellulose,
arbohydrates - General Description A. Polyhydroxy Aldehydes or Ketones ARBN AIN B. Serve a variety of functions ARBN AIN ARBN AIN 1. Energy storage (Glucose, Glycogen, Starch) 2. Structural Support (ellulose,
Farah Al-Khaled. Razi Kittaneh. Mohammad Omari
 7 Farah Al-Khaled Razi Kittaneh Mohammad Omari Dr. Mamoun Ahram In this lecture we are going to talk about modified sugars. Remember: The Fischer projection can be turned into a ring structure (which is
7 Farah Al-Khaled Razi Kittaneh Mohammad Omari Dr. Mamoun Ahram In this lecture we are going to talk about modified sugars. Remember: The Fischer projection can be turned into a ring structure (which is
Carbohydrates- Disaccharides. By Dr. Bhushan R. Kavimandan
 Carbohydrates- Disaccharides By Dr. Bhushan R. Kavimandan Disaccharides ofbiological importance: Disaccharides consist of two monosaccharides joined by glycosidic linkages. They are crystalline, water-soluble
Carbohydrates- Disaccharides By Dr. Bhushan R. Kavimandan Disaccharides ofbiological importance: Disaccharides consist of two monosaccharides joined by glycosidic linkages. They are crystalline, water-soluble
Carbohydrates. Lecture2
 Carbohydrates Lecture2 Disaccharides Consist of two monosaccharides covalently bound to each other. All of which are isomers with the molecular formula C 12 22 O 11. The differences in these disaccharides
Carbohydrates Lecture2 Disaccharides Consist of two monosaccharides covalently bound to each other. All of which are isomers with the molecular formula C 12 22 O 11. The differences in these disaccharides
Chapter 22 Carbohydrates
 Chapter 22 Carbohydrates Introduction Classification of Carbohydrates Carbohydrates have the general formula C x (H 2 O) y Carbohydrates are defined as polyhydroxy aldehydes or ketones or substances that
Chapter 22 Carbohydrates Introduction Classification of Carbohydrates Carbohydrates have the general formula C x (H 2 O) y Carbohydrates are defined as polyhydroxy aldehydes or ketones or substances that
A. Incorrect! No, this is not the description of this type of molecule. B. Incorrect! No, this is not the description of this type of molecule.
 Biochemistry - Problem Drill 08: Carbohydrates No. 1 of 10 1. have one aldehyde (-CHO) or one keto (-C=O) group and many hydroxyl (-OH) groups. (A) Amino acids (B) Proteins (C) Nucleic Acids (D) Carbohydrates
Biochemistry - Problem Drill 08: Carbohydrates No. 1 of 10 1. have one aldehyde (-CHO) or one keto (-C=O) group and many hydroxyl (-OH) groups. (A) Amino acids (B) Proteins (C) Nucleic Acids (D) Carbohydrates
Polymers: large molecules made up of repeating smaller units (monomer) peptides and proteins (Chapter 25) nucleic acids (Chapter 26)
 Chapter 23: Carbohydrates hydrates of carbon: general formula C n (H 2 O) n Plants: photosynthesis 6 CO 2 + 6 H 2 O hν C 6 H 12 O 6 + 6 O 2 Polymers: large molecules made up of repeating smaller units
Chapter 23: Carbohydrates hydrates of carbon: general formula C n (H 2 O) n Plants: photosynthesis 6 CO 2 + 6 H 2 O hν C 6 H 12 O 6 + 6 O 2 Polymers: large molecules made up of repeating smaller units
Chemistry 110. Bettelheim, Brown, Campbell & Farrell. Ninth Edition. Introduction to General, Organic and Biochemistry Chapter 20 Carbohydrates
 hemistry 110 Bettelheim, Brown, ampbell & Farrell Ninth Edition Introduction to General, rganic and Biochemistry hapter 20 arbohydrates Polyhydroxy Aldehydes & Ketones arbohydrates A A arbohydrate is a
hemistry 110 Bettelheim, Brown, ampbell & Farrell Ninth Edition Introduction to General, rganic and Biochemistry hapter 20 arbohydrates Polyhydroxy Aldehydes & Ketones arbohydrates A A arbohydrate is a
Long time ago, people who sacrifice their sleep, family, food, laughter, and other joys of life were called SAINTS. But now, they are called STUDENTS!
 Long time ago, people who sacrifice their sleep, family, food, laughter, and other joys of life were called SAINTS. But now, they are called STUDENTS! Monosaccharaides Q. Can hydrolysis occur at anytime
Long time ago, people who sacrifice their sleep, family, food, laughter, and other joys of life were called SAINTS. But now, they are called STUDENTS! Monosaccharaides Q. Can hydrolysis occur at anytime
Chem 263 Apr 11, 2017
 hem 263 Apr 11, 2017 arbohydrates- emiacetal Formation You know from previous lectures that carbonyl compounds react with all kinds of nucleophiles. ydration and hemiacetal formation are typical examples.
hem 263 Apr 11, 2017 arbohydrates- emiacetal Formation You know from previous lectures that carbonyl compounds react with all kinds of nucleophiles. ydration and hemiacetal formation are typical examples.
Chapter-8 Saccharide Chemistry
 Chapter-8 Saccharide Chemistry Page 217-228 Carbohydrates (Saccharides) are most abundant biological molecule, riginally produced through C 2 fixation during photosynthesis I (C 2 ) n or - C - I where
Chapter-8 Saccharide Chemistry Page 217-228 Carbohydrates (Saccharides) are most abundant biological molecule, riginally produced through C 2 fixation during photosynthesis I (C 2 ) n or - C - I where
You know from previous lectures that carbonyl react with all kinds of nucleophiles. Hydration and hemiacetal formation are typical examples.
 hem 263 Nov 17, 2009 D,L onfiguration of Sugars Glyceraldehyde has only one stereogenic center and therefore has two enantiomers (mirror image) forms. A D-sugar is defined as one that has configuration
hem 263 Nov 17, 2009 D,L onfiguration of Sugars Glyceraldehyde has only one stereogenic center and therefore has two enantiomers (mirror image) forms. A D-sugar is defined as one that has configuration
Learning Target: Describe characteristics and functions of carbohydrates, lipids, and proteins. Compare and contrast the classes of organic
 Learning Target: Describe characteristics and functions of carbohydrates, lipids, and proteins. Compare and contrast the classes of organic compounds. What are inorganic molecules? Molecules that CANNOT
Learning Target: Describe characteristics and functions of carbohydrates, lipids, and proteins. Compare and contrast the classes of organic compounds. What are inorganic molecules? Molecules that CANNOT
Structural Polysaccharides
 Carbohydrates & ATP Carbohydrates include both sugars and polymers of sugars. The simplest carbohydrates are the monosaccharides, or simple sugars; these are the monomers from which more complex carbohydrates
Carbohydrates & ATP Carbohydrates include both sugars and polymers of sugars. The simplest carbohydrates are the monosaccharides, or simple sugars; these are the monomers from which more complex carbohydrates
Sheet #8 Dr. Nafeth Abu-Tarboush
 1 arbohydrates There are two topic goals in our study of carbohydrates: Monosaccharides: to recognize their structure, properties, & their stereochemistry. The nature of di-, oligo- & polysaccharides.
1 arbohydrates There are two topic goals in our study of carbohydrates: Monosaccharides: to recognize their structure, properties, & their stereochemistry. The nature of di-, oligo- & polysaccharides.
Chapter 7 Carbohydrates
 Chapter 7 Carbohydrates Definition of Carbohydrates carbohydrate: hydrate of carbon ; C n ( 2 ) m Examples: glucose (C 6 12 6 or C 6 ( 2 ) 6 ), sucrose (C 12 22 11 or C 12 ( 2 ) 11 ) saccharide: simple
Chapter 7 Carbohydrates Definition of Carbohydrates carbohydrate: hydrate of carbon ; C n ( 2 ) m Examples: glucose (C 6 12 6 or C 6 ( 2 ) 6 ), sucrose (C 12 22 11 or C 12 ( 2 ) 11 ) saccharide: simple
Chapter 11. Learning objectives: Structure and function of monosaccharides, polysaccharide, glycoproteins lectins.
 Chapter 11 Learning objectives: Structure and function of monosaccharides, polysaccharide, glycoproteins lectins. Carbohydrates Fuels Structural components Coating of cells Part of extracellular matrix
Chapter 11 Learning objectives: Structure and function of monosaccharides, polysaccharide, glycoproteins lectins. Carbohydrates Fuels Structural components Coating of cells Part of extracellular matrix
Definition of a Carbohydrate
 * Atoms held together by covalent bonds Definition of a Carbohydrate * Organic macromolecules * Consist of C, H, & O atoms * Usually in a 1:2:1 ratio of C:H : O Functions Performed by Carbohydrates Used
* Atoms held together by covalent bonds Definition of a Carbohydrate * Organic macromolecules * Consist of C, H, & O atoms * Usually in a 1:2:1 ratio of C:H : O Functions Performed by Carbohydrates Used
Dr. Mahendra P. Bhatt (BMLT, MS-Ph.D., Post-doctorate) Associate Professor Clinical Biochemistry
 Dr. Mahendra P. Bhatt (BMLT, MS-Ph.D., Post-doctorate) Associate Professor Clinical Biochemistry mahendramlt@gmail.com Students will be able to describe: Biochemical organization of the cell Transport
Dr. Mahendra P. Bhatt (BMLT, MS-Ph.D., Post-doctorate) Associate Professor Clinical Biochemistry mahendramlt@gmail.com Students will be able to describe: Biochemical organization of the cell Transport
Carbohydrates. Organic compounds which comprise of only C, H and O. C x (H 2 O) y
 Carbohydrates Organic compounds which comprise of only C, H and O C x (H 2 O) y Carbohydrates Monosaccharides Simple sugar Soluble in water Precursors in synthesis triose sugars of other (C3) molecules
Carbohydrates Organic compounds which comprise of only C, H and O C x (H 2 O) y Carbohydrates Monosaccharides Simple sugar Soluble in water Precursors in synthesis triose sugars of other (C3) molecules
Questions- Carbohydrates. A. The following structure is D-sorbose. (Questions 1 7) CH 2 OH C = O H C OH HO C H H C OH
 Questions- Carbohydrates A. The following structure is D-sorbose. (Questions 1 7) CH 2 C = O H C HO C H H C CH 2 1. 2. 3. 4. 5. Which characteristic is different when comparing the open-chain forms of
Questions- Carbohydrates A. The following structure is D-sorbose. (Questions 1 7) CH 2 C = O H C HO C H H C CH 2 1. 2. 3. 4. 5. Which characteristic is different when comparing the open-chain forms of
Module-04: Food carbohydrates: Monosaccharides and Oligosaccharides
 Paper No. 01 Paper Title: Food Chemistry Module-04: Food carbohydrates: Monosaccharides and Oligosaccharides Monosaccharides The simplest form of carbohydrates is the monosaccharide. Monosaccharides are
Paper No. 01 Paper Title: Food Chemistry Module-04: Food carbohydrates: Monosaccharides and Oligosaccharides Monosaccharides The simplest form of carbohydrates is the monosaccharide. Monosaccharides are
B.sc. III Chemistry Paper b. Submited by :- Dr. Sangeeta Mehtani Associate Professor Deptt. Of Chemistry PGGCG, sec11 Chd
 B.sc. III Chemistry Paper b Submited by :- Dr. Sangeeta Mehtani Associate Professor Deptt. Of Chemistry PGGCG, sec11 Chd CARBOYDRATES Carbohydrates polyhydroxyaldehydes or polyhydroxyketones of formula
B.sc. III Chemistry Paper b Submited by :- Dr. Sangeeta Mehtani Associate Professor Deptt. Of Chemistry PGGCG, sec11 Chd CARBOYDRATES Carbohydrates polyhydroxyaldehydes or polyhydroxyketones of formula
Topic 4 - #2 Carbohydrates Topic 2
 Topic 4 - #2 Carbohydrates Topic 2 Biologically Important Monosaccharide Derivatives There are a large number of monosaccharide derivatives. A variety of chemical and enzymatic reactions produce these
Topic 4 - #2 Carbohydrates Topic 2 Biologically Important Monosaccharide Derivatives There are a large number of monosaccharide derivatives. A variety of chemical and enzymatic reactions produce these
CLASS 11th. Biomolecules
 CLASS 11th 01. Carbohydrates These are the compound of carbon, hydrogen and oxygen having hydrogen and oxygen in the same ratio as that of water, i.e. 2 : 1. They are among the most widely distributed
CLASS 11th 01. Carbohydrates These are the compound of carbon, hydrogen and oxygen having hydrogen and oxygen in the same ratio as that of water, i.e. 2 : 1. They are among the most widely distributed
CHAPTER 23. Carbohydrates
 CAPTER 23 Carbohydrates 1 Introduction Carbohydrates are naturally occurring compounds of carbon, hydrogen, and oxygen. Carbohydrates have the empirical formula C 2. Carbohydrates have the general formula
CAPTER 23 Carbohydrates 1 Introduction Carbohydrates are naturally occurring compounds of carbon, hydrogen, and oxygen. Carbohydrates have the empirical formula C 2. Carbohydrates have the general formula
Carbohydrates hydrates of carbon: general formula C n (H 2 O) n. Polymers: large molecules made up of repeating smaller units (monomer)
 Carbohydrates hydrates of carbon: general formula C n ( ) n Plants: photosynthesis hν 6 C + 6 C 6 6 + 6 Polymers: large molecules made up of repeating smaller units (monomer) Biopolymers: carbohydrates
Carbohydrates hydrates of carbon: general formula C n ( ) n Plants: photosynthesis hν 6 C + 6 C 6 6 + 6 Polymers: large molecules made up of repeating smaller units (monomer) Biopolymers: carbohydrates
Carbohydrates. Monosaccharides
 Carbohydrates Carbohydrates (also called saccharides) are molecular compounds made from just three elements: carbon, hydrogen and oxygen. Monosaccharides (e.g. glucose) and disaccharides (e.g. sucrose)
Carbohydrates Carbohydrates (also called saccharides) are molecular compounds made from just three elements: carbon, hydrogen and oxygen. Monosaccharides (e.g. glucose) and disaccharides (e.g. sucrose)
Chapter 24: Carbohydrates
 Chapter 24: Carbohydrates [Sections: 24.1 24.10] 1. Carbohydrates definition naturally occuring compounds derived from carbon, oxygen and hydrogen the net molecular formula comes from each carbon having
Chapter 24: Carbohydrates [Sections: 24.1 24.10] 1. Carbohydrates definition naturally occuring compounds derived from carbon, oxygen and hydrogen the net molecular formula comes from each carbon having
Chapter 1. Chemistry of Life - Advanced TABLE 1.2: title
 Condensation and Hydrolysis Condensation reactions are the chemical processes by which large organic compounds are synthesized from their monomeric units. Hydrolysis reactions are the reverse process.
Condensation and Hydrolysis Condensation reactions are the chemical processes by which large organic compounds are synthesized from their monomeric units. Hydrolysis reactions are the reverse process.
I (CH 2 O) n or H - C - OH I
 V. ARBYDRATE arbohydrates (glycans) have the following basic composition: I ( ) n or - - I Many carbohydrates are soluble in water. The usual chemical test for the simpler carbohydrates is heating with
V. ARBYDRATE arbohydrates (glycans) have the following basic composition: I ( ) n or - - I Many carbohydrates are soluble in water. The usual chemical test for the simpler carbohydrates is heating with
Disaccharides. Three Important Disaccharides Maltose, Lactose, and Sucrose. The formation of these three common disaccharides are:
 DISACCHARIDES Disaccharides Three Important Disaccharides Maltose, Lactose, and Sucrose The formation of these three common disaccharides are: 2 Disaccharides Maltose (Malt Sugar) Maltose is known as malt
DISACCHARIDES Disaccharides Three Important Disaccharides Maltose, Lactose, and Sucrose The formation of these three common disaccharides are: 2 Disaccharides Maltose (Malt Sugar) Maltose is known as malt
Carbohydrates. What are they? What do cells do with carbs? Where do carbs come from? O) n. Formula = (CH 2
 Carbohydrates What are they? Formula = (C 2 O) n where n > 3 Also called sugar Major biomolecule in body What do cells do with carbs? Oxidize them for energy Store them to oxidize later for energy Use
Carbohydrates What are they? Formula = (C 2 O) n where n > 3 Also called sugar Major biomolecule in body What do cells do with carbs? Oxidize them for energy Store them to oxidize later for energy Use
Carbohydrates. Dr. Mamoun Ahram Summer,
 Carbohydrates Dr. Mamoun Ahram Summer, 2017-2018 Resource This lecture Campbell and Farrell s Biochemistry, Chapter 16 What are they? Carbohydrates are polyhydroxy aldehydes or ketones Saccharide is another
Carbohydrates Dr. Mamoun Ahram Summer, 2017-2018 Resource This lecture Campbell and Farrell s Biochemistry, Chapter 16 What are they? Carbohydrates are polyhydroxy aldehydes or ketones Saccharide is another
BCH302 [Practical] 1
![BCH302 [Practical] 1 BCH302 [Practical] 1](/thumbs/81/83984357.jpg) BCH302 [Practical] 1 Carbohydrates are defined as the polyhydroxy aldehydes or polyhydroxy ketones. Most, but not all carbohydrate have a formula (CH 2 O)n (hence the name hydrate of carbon). Sugars ends
BCH302 [Practical] 1 Carbohydrates are defined as the polyhydroxy aldehydes or polyhydroxy ketones. Most, but not all carbohydrate have a formula (CH 2 O)n (hence the name hydrate of carbon). Sugars ends
BCH 445 Biochemistry of nutrition Dr. Mohamed Saad Daoud
 BCH 445 Biochemistry of nutrition Dr. Mohamed Saad Daoud 1 Carbohydrates Carbohydrates: Compounds composed of carbon, oxygen, and hydrogen arranged as monosaccharides or multiples of monosaccharides. Most,
BCH 445 Biochemistry of nutrition Dr. Mohamed Saad Daoud 1 Carbohydrates Carbohydrates: Compounds composed of carbon, oxygen, and hydrogen arranged as monosaccharides or multiples of monosaccharides. Most,
Biochemistry lecturer Bio- chemical Eng. Zahraa Abdulhussein Mousa. Bio.Eng Zahraa A.A. Mousa
 Biochemistry lecturer Bio- chemical Eng. Zahraa Abdulhussein Mousa Overview Carbohydrates are the most abundant organic molecules in nature Wide range of functions e.g., a significant fraction of the energy
Biochemistry lecturer Bio- chemical Eng. Zahraa Abdulhussein Mousa Overview Carbohydrates are the most abundant organic molecules in nature Wide range of functions e.g., a significant fraction of the energy
among the most important organic compounds in the living organisms;
 CARBOHYDRATES Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine and Pharmacy "Nicolae Testemitanu" CARBOHYDRATESare among the most
CARBOHYDRATES Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine and Pharmacy "Nicolae Testemitanu" CARBOHYDRATESare among the most
Carbohydrate Chemistry
 Carbohydrate Chemistry The term carbohydrate is derived from the Cn(2O)n general chemical formula Carbohydrates are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis
Carbohydrate Chemistry The term carbohydrate is derived from the Cn(2O)n general chemical formula Carbohydrates are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis
A BEGINNER S GUIDE TO BIOCHEMISTRY
 A BEGINNER S GUIDE TO BIOCHEMISTRY Life is basically a chemical process Organic substances: contain carbon atoms bonded to other carbon atom 4 classes: carbohydrates, lipids, proteins, nucleic acids Chemical
A BEGINNER S GUIDE TO BIOCHEMISTRY Life is basically a chemical process Organic substances: contain carbon atoms bonded to other carbon atom 4 classes: carbohydrates, lipids, proteins, nucleic acids Chemical
Waseem Abu Obeida. Salsabeel Fleifal. Mamoon Ahram
 8 Waseem Abu Obeida Salsabeel Fleifal Mamoon Ahram Anomers Anomers cyclic monosaccharides or glycosides that are epimers, they differ from each other in the configuration of C-1 if they are aldoses or
8 Waseem Abu Obeida Salsabeel Fleifal Mamoon Ahram Anomers Anomers cyclic monosaccharides or glycosides that are epimers, they differ from each other in the configuration of C-1 if they are aldoses or
Dehydration Synthesis and Hydrolysis Reactions. ne_content/animations/reaction_types.ht ml
 Glucose Molecule Macromolecules Carbohydrates, proteins, and nucleic acids are polymers Polymers long molecules made from building blocks linked by covalent bonds Monomers the building blocks to polymers
Glucose Molecule Macromolecules Carbohydrates, proteins, and nucleic acids are polymers Polymers long molecules made from building blocks linked by covalent bonds Monomers the building blocks to polymers
Organic Chemistry III
 rganic Chemistry III (Yuki Goto, Bioorganic Chemistry Lab.) rganic chemistry of biomolecules rganic chemistry of radicals 6/6 (Wed) 6/13 (Wed) 6/20 (Wed) 6/27 (Wed) 7/4 (Wed) Examples of biomolecules?
rganic Chemistry III (Yuki Goto, Bioorganic Chemistry Lab.) rganic chemistry of biomolecules rganic chemistry of radicals 6/6 (Wed) 6/13 (Wed) 6/20 (Wed) 6/27 (Wed) 7/4 (Wed) Examples of biomolecules?
QUALITATIVE TESTS OF CARBOHYDRATE
 QUALITATIVE TESTS OF CARBOHYDRATE MACROMOLECULE CARBOHYDRATES Are the key source of energy used by living things. Also serve as extracellular structural elements as in cell wall of bacteria and plant.
QUALITATIVE TESTS OF CARBOHYDRATE MACROMOLECULE CARBOHYDRATES Are the key source of energy used by living things. Also serve as extracellular structural elements as in cell wall of bacteria and plant.
Biochemistry. Definition-
 Biochemistry Notes Biochemistry Definition- the scientific study of the chemical composition of living matter AND of the chemical processes that go on in living organisms. Biochemistry Facts 1. The human
Biochemistry Notes Biochemistry Definition- the scientific study of the chemical composition of living matter AND of the chemical processes that go on in living organisms. Biochemistry Facts 1. The human
2.2: Sugars and Polysaccharides François Baneyx Department of Chemical Engineering, University of Washington
 2.2: Sugars and Polysaccharides François Baneyx Department of hemical Engineering, University of Washington baneyx@u.washington.edu arbohydrates or saccharides are abundant compounds that play regulatory
2.2: Sugars and Polysaccharides François Baneyx Department of hemical Engineering, University of Washington baneyx@u.washington.edu arbohydrates or saccharides are abundant compounds that play regulatory
Biological Molecules
 SIM Tuition Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t
SIM Tuition Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t
Name: Class: Honors Biology Period: Question: What is the molecular formula of this molecule?
 Chapter 3: The Chemistry of Organic Molecules Exercise 1 Diversity of Carbon-Based Molecules (3.1) The great variety of organic compounds results from the ability of carbon atoms to bond with four other
Chapter 3: The Chemistry of Organic Molecules Exercise 1 Diversity of Carbon-Based Molecules (3.1) The great variety of organic compounds results from the ability of carbon atoms to bond with four other
Chemistry 106 Lecture Notes Examination 5 Materials. *Hydrated Carbons.
 hemistry 106 Lecture Notes Examination 5 Materials hapter 23: arbohydrates & Nucleic Acids arbohydrates Definition: *ompounds made of,, &. Example: *ydrated arbons. Glucose: 6 12 6 an be written as 6(
hemistry 106 Lecture Notes Examination 5 Materials hapter 23: arbohydrates & Nucleic Acids arbohydrates Definition: *ompounds made of,, &. Example: *ydrated arbons. Glucose: 6 12 6 an be written as 6(
Dr. Entedhar Carbohydrates Carbohydrates are carbon compounds that have aldehyde (C-H=0) or ketone (C=O) moiety and comprises polyhyroxyl alcohol
 Dr. Entedhar Carbohydrates Carbohydrates are carbon compounds that have aldehyde (C-H=0) or ketone (C=O) moiety and comprises polyhyroxyl alcohol (polyhydroxyaldehyde or polyhyroxyketone); their polymers,which
Dr. Entedhar Carbohydrates Carbohydrates are carbon compounds that have aldehyde (C-H=0) or ketone (C=O) moiety and comprises polyhyroxyl alcohol (polyhydroxyaldehyde or polyhyroxyketone); their polymers,which
Chem 263 Nov 21, 2013
 hem 263 Nov 21, 2013 arbohydrates- emiacetal Formation You know from previous lectures that carbonyl compounds react with all kinds of nucleophiles. ydration and hemiacetal formation are typical examples.
hem 263 Nov 21, 2013 arbohydrates- emiacetal Formation You know from previous lectures that carbonyl compounds react with all kinds of nucleophiles. ydration and hemiacetal formation are typical examples.
HW #9: 21.36, 21.52, 21.54, 21.56, 21.62, 21.68, 21.70, 21.76, 21.82, 21.88, 21.94, Carbohydrates
 Chemistry 131 Lectures 16 & 17: Carbohydrates Chapter 21 in McMurry, Ballantine, et. al. 7 th edition 05/24/18, 05/25/18 W #9: 21.36, 21.52, 21.54, 21.56, 21.62, 21.68, 21.70, 21.76, 21.82, 21.88, 21.94,
Chemistry 131 Lectures 16 & 17: Carbohydrates Chapter 21 in McMurry, Ballantine, et. al. 7 th edition 05/24/18, 05/25/18 W #9: 21.36, 21.52, 21.54, 21.56, 21.62, 21.68, 21.70, 21.76, 21.82, 21.88, 21.94,
IB Biology BIOCHEMISTRY. Biological Macromolecules SBI3U7. Topic 3. Thursday, October 4, 2012
 + IB Biology SBI3U7 BIOCHEMISTRY Topic 3 Biological Macromolecules Essential Questions: 1.What are the 4 main types of biological macromolecules and what is their function within cells? 2.How does the
+ IB Biology SBI3U7 BIOCHEMISTRY Topic 3 Biological Macromolecules Essential Questions: 1.What are the 4 main types of biological macromolecules and what is their function within cells? 2.How does the
IntroducKon to Carbohydrates
 Carbohidratos IntroducKon to Carbohydrates Carbohydrates (sugars) are abundant in nature: They are high energy biomolecules. They provide structural rigidity for organisms (plants, crustaceans, etc.).
Carbohidratos IntroducKon to Carbohydrates Carbohydrates (sugars) are abundant in nature: They are high energy biomolecules. They provide structural rigidity for organisms (plants, crustaceans, etc.).
Lecture Notes Chem 51C S. King. Chapter 28 Carbohydrates. Starch, Glycogen and cellulose are all polymers of glucose.
 Lecture otes hem 51 S. King hapter 28 arbohydrates arbohydrates are the most abundant class of organic compounds in the plant world. They are synthesized by nearly all plants and animals, which use them
Lecture otes hem 51 S. King hapter 28 arbohydrates arbohydrates are the most abundant class of organic compounds in the plant world. They are synthesized by nearly all plants and animals, which use them
Sheet #9 Dr. Mamoun Ahram
 1 Carbohydrates Life is defined by its chemistry and every single class of molecules contributes to the defining features of it.today we are going to talk about carbohydrates a class of macromolecules-
1 Carbohydrates Life is defined by its chemistry and every single class of molecules contributes to the defining features of it.today we are going to talk about carbohydrates a class of macromolecules-
Name LastName Student ID
 Name LastName Student ID 1) (12 points) Imidazopyridine derivatives such as 1-deaza-9H-purines (like 1) and 3- deaza-9h-purines (like 2) represent privileged structures in medicinal chemistry and they
Name LastName Student ID 1) (12 points) Imidazopyridine derivatives such as 1-deaza-9H-purines (like 1) and 3- deaza-9h-purines (like 2) represent privileged structures in medicinal chemistry and they
Chem 60 Takehome Test 2 Student Section
 Multiple choice: 1 point each. Mark only one answer for each question. 1. are composed primarily of carbon and hydrogen, but may also include oxygen, nitrogen, sulfur, phosphorus, and a few other elements.
Multiple choice: 1 point each. Mark only one answer for each question. 1. are composed primarily of carbon and hydrogen, but may also include oxygen, nitrogen, sulfur, phosphorus, and a few other elements.
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
BIOCHEMISTRY UNIT 2 Part 4 ACTIVITY #4 (Chapter 5) CARBOHYDRATES
 AP BIOLOGY BIOCHEMISTRY UNIT 2 Part 4 ACTIVITY #4 (Chapter 5) NAME DATE PERIOD CARBOHYDRATES GENERAL CHARACTERISTICS: Polymers of simple sugars Classified according to number of simple sugars Sugars 3
AP BIOLOGY BIOCHEMISTRY UNIT 2 Part 4 ACTIVITY #4 (Chapter 5) NAME DATE PERIOD CARBOHYDRATES GENERAL CHARACTERISTICS: Polymers of simple sugars Classified according to number of simple sugars Sugars 3
Biochemistry: A Short Course
 Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 10 Carbohydrates 2013 W. H. Freeman and Company Chapter 10 Outline Monosaccharides are aldehydes or ketones that contain two or
Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 10 Carbohydrates 2013 W. H. Freeman and Company Chapter 10 Outline Monosaccharides are aldehydes or ketones that contain two or
Chapter 23: Carbohydrates hydrates of carbon: general formula C n (H 2 O) n. Polymers: large molecules made up of repeating smaller units (monomer)
 Chapter : Carbohydrates hydrates of carbon: general formula C n ( ) n Plants: photosynthesis hν C + C + Polymers: large molecules made up of repeating smaller units (monomer) Biopolymers: Monomer units:
Chapter : Carbohydrates hydrates of carbon: general formula C n ( ) n Plants: photosynthesis hν C + C + Polymers: large molecules made up of repeating smaller units (monomer) Biopolymers: Monomer units:
Carbohydrates: structure and Function. Important. 436 Notes Original slides. 438 notes Extra information
 Carbohydrates: structure and Function Important. 436 Notes Original slides. 438 notes Extra information Objectives: To understand: 1- The structure of carbohydrates of physiological significance. 2- The
Carbohydrates: structure and Function Important. 436 Notes Original slides. 438 notes Extra information Objectives: To understand: 1- The structure of carbohydrates of physiological significance. 2- The
