IHCP banner page. IHCP to cover CPT code IHCP to cover CPT code INDIANA HEALTH COVERAGE PROGRAMS BR JULY 3, 2018
|
|
|
- Annabella Jones
- 5 years ago
- Views:
Transcription
1 IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR JULY 3, 2018 IHCP to cover CPT Effective August 3, 2018, the Indiana Health Coverage Programs (IHCP) will cover Current Procedural Terminology (CPT 1 ) Arterial pressure waveform analysis for assessment of central arterial pressures, includes obtaining waveform(s), digitization and application of nonlinear mathematical transformations to determine central arterial pressures and augmentation index, with interpretation and report, upper extremity artery, non-invasive. Coverage applies to all IHCP programs, subject to limitations established for certain benefit plans. Coverage applies to dates of service (DOS) on or after August 3, The following reimbursement information applies: Pricing: Resource-based relative value scale (RBRVS) Prior authorization (PA): None required Billing guidance: Standard billing guidance applies This change will be reflected in the next regular updates to the Professional Fee Schedule and the Outpatient Fee Schedule at indianamedicaid.com. Reimbursement, PA, and billing information apply to services delivered under the fee-for-service (FFS) delivery system. Individual managed care entities (MCEs) establish and publish reimbursement, PA, and billing criteria within the managed care delivery system. Questions about managed care billing and PA should be directed to the MCE with which the member is enrolled. 1 CPT copyright 2018 American Medical Association. All rights reserved. CPT is a registered trademark of the American Medical Association. IHCP to cover CPT Effective August 3, 2018, the Indiana Health Coverage Programs (IHCP) will cover Current Procedural Terminology (CPT) Implantation of intrastromal corneal ring segments. Coverage applies to all IHCP programs, subject to limitations established for certain benefit plans. Coverage applies to dates of service (DOS) on or after August 3, The following reimbursement information applies: Pricing: Professional (CMS-1500) Resource-based relative value scale (RBRVS) Outpatient Ambulatory Surgical Center (ASC) pricing indicator G Prior authorization (PA): Required MORE IN THIS ISSUE New program integrity audit process training now available Billing guidance: Standard billing guidance applies IHCP links additional procedure s to specialty 140 Podiatrist 1 of 11
2 PA for the coverage of procedure requires the following criteria be met: The member is 21 years of age or older. is for the treatment of keratoconus. The member has experienced a progressive deterioration in vision, such that he or she can no longer achieve adequate functional vision with contact lenses or spectacles. Corneal transplantation is the only alternative to improve the patient s functional vision. The patient has a clear central cornea with a corneal thickness of 450 microns or greater at the proposed incision site. The following coverage limitations apply: is considered not medically necessary and therefore is not allowed as a treatment of myopia is considered investigational and therefore is not allowed for all other conditions. This coverage information will be reflected in the next regular updates to the Professional Fee Schedule and the Outpatient Fee Schedule at indianamedicaid.com. Reimbursement, PA, and billing information apply to services delivered under the fee-for-service (FFS) delivery system. Questions about FFS PA should be directed to Cooperative Managed Care Services (CMCS) at Individual managed care entities (MCEs) establish and publish reimbursement, PA, and billing criteria within the managed care delivery system. Questions about managed care PA should be directed to the MCE with which the member is enrolled. New program integrity audit process training now available The Indiana Health Coverage Programs (IHCP) is making web-based Program Integrity Provider Education Training available to all providers. These training presentations are intended to supplement the provider reference modules and other IHCP-published provider reference materials. The latest Program Integrity provider training titled Program Integrity Audit Process Overview is now available. This training is designed for any IHCP provider and affiliated personnel. The purpose of this training is to supply IHCP providers with the information necessary to understand program integrity s role to guard against fraud, abuse, and waste of Medicaid program benefits and resources, as well as to explain the program integrity audit process. By the end of the course, providers should be able to: Explain the purpose, role, and mission of the Program Integrity Unit Define Medicaid provider fraud and abuse and its consequences Understand the steps of the Program Integrity audit process To access the training, navigate to the Program Integrity Provider Education Training page at indianamedicaid.com. Other training topics posted there are listed below. Watch upcoming IHCP provider publications for announcements when other trainings under development become available. Non-Emergency Transportation Documentation Requirements and Billing Guidelines Ambulance Transportation Documentation Requirements and Billing Guidelines Dental Provider Documentation Requirements and Billing Guidelines 2 of 11
3 IHCP links additional procedure s to specialty 140 Podiatrist Effective August 3, 2018, the Indiana Health Coverage Programs (IHCP) will link the procedure s in Table 1 to specialty 140 Podiatrist. These procedure s will be covered when rendered by a podiatrist for dates of service (DOS) on or after August 3, These changes will be reflected in the Podiatry Services Codes table on the Code Sets page at indianamedicaid.com. Reimbursement and billing guidelines for the procedure s in the podiatrist set remain unchanged and are subject to current policies, edits, and audits. Claims submitted by podiatrists for procedure s not included in the set will be denied for explanation of benefits (EOB) 1012 Service and or modifier billed not payable for your provider type/specialty. Table 1 Additions to covered procedure s for podiatrists (specialty 140) Biopsy of single growth of skin and/or tissue Biopsy of each additional growth of skin and/or tissue Repair of finger or toe nail bed Repair of wound (2.5 centimeters or less) of the scalp, neck, underarms, trunk, arms and/or legs Repair of wound (2.6 to 7.5 centimeters) of the scalp, neck, underarms, genitals, trunk, arms and/or legs Repair of separation of wound closure Repair of wound (2.5 centimeters or less) of neck, hands, feet, and/or genitals Second repair of surgical wound Tissue transfer repair of wound (10 sq centimeters or less) of the forehead, cheeks, chin, mouth, neck, underarms, genitals, hands, and/or feet Tissue transfer repair of wound (10.1 to 30.0 sq centimeters) of the forehead, cheeks, chin, mouth, neck, underarms, genitals, hands, and/or feet Repair of tissue loss of finger or toe Preparation of graft site at trunk, arms, or legs (first 100 sq cm or 1% body area infants and children) Preparation of graft site of face, scalp, eyelids, mouth, neck, ears, eye region, genitals, hands, feet, and/or multiple fingers or toes (first 100 sq cm or 1% body area of infants and children) Relocation of skin (100 sq cm or less) for tissue cultured graft Skin graft (2 centimeters) to tip of finger or toe Skin graft at trunk, arms, or legs (first 100 sq cm or less, or 1% body are of infants and children) Skin graft at trunk, arms, or legs (first 100 sq cm or less, or 1% body area of infants and children) Skin graft of face, scalp, eyelids, mouth, neck, ears, eye region, genitals, hands, feet, and/or multiple fingers or toes (first 100 sq cm or less, or 1% body area of infants and children) 3 of 11
4 Table 1 Additions to Covered Codes for Podiatrists (Specialty 140) () Skin graft of face, scalp, eyelids, mouth, neck, ears, eye region, genitals, hands, feet, and/or multiple fingers or toes (first 100 sq cm or less, or 1% body area of infants and children) Skin graft at trunk, arms, or legs (first 100 sq cm or less, or 1% body area of infants and children) Skin graft of face, scalp, eyelids, mouth, neck, ears, eye region, genitals, hands, feet, and/or multiple fingers or toes (first 100 sq cm or less, or 1% body area of infants and children) Skin graft at trunk, arms, or legs (first 25 sq centimeters or less) Skin graft of face, scalp, eyelids, mouth, neck, ears, eye region, genitals, hands, feet, and/or multiple fingers or toes (first 25 sq centimeters or less) Relocation of patient skin (20 sq centimeters or less) to scalp, arms, and/or legs Relocation of patient skin to forehead, cheeks, chin, mouth, neck, underarms, genitals, hands, and/or feet (20 sq centimeters or less) Application of skin substitute (wound surface up to 100 sq cm) to trunk, arms, or legs (first 25 sq cm or less) Application of skin substitute (wound surface up to 100 sq cm) to trunk, arms, or legs Application of skin substitute (wound surface greater or equal to 100 sq cm) to trunk, arms, or legs (first 100 sq cm or 1% body area of infants and children) Application of skin substitute (wound surface greater or equal to 100 sq cm) to trunk, arms, or legs Application of skin substitute (wound surface up to 100 sq cm) to face, scalp, eyelids, mouth, neck, ears, eye region, genitals, hands, feet, and/or multiple fingers or toes (first 25 sq cm or less) Application of skin substitute (wound surface up to 100 sq cm) to face, scalp, eyelids, mouth, neck, ears, eye region, genitals, hands, feet, and/or multiple fingers or toes Application of skin substitute (wound surface great than or equal to 100 sq cm) to face, scalp, eyelids, mouth, neck, ears, eye region, genitals, hands, feet, and/or multiple fingers or toes (first 100 sq cm or 1% body area of infants and children) Application of skin substitute (wound surface great than or equal to 100 sq cm) to face, scalp, eyelids, mouth, neck, ears, eye region, genitals, hands, feet, and/or multiple fingers or toes Destruction of skin growth Destruction of 2-14 skin growths Destruction of 15 or more skin growths Application of chemical agent to excessive wound tissue Exploration of penetrating wound of arm or leg Biopsy of bone using needle or trocar Deep biopsy of bone using needle or trocar 4 of 11
5 Table 1 Additions to Covered Codes for Podiatrists (Specialty 140) () Biopsy of bone, open procedure Aspiration and/or injection of cysts Removal of bone implant Removal of deep bone implant Application of uniplane external bone fixation on one arm or leg Application of multiplane external bone fixation system on one arm or leg Removal of external bone fixation under anesthesia Application of multiplane external bone fixation system on one arm or leg Application of multiplane external bone fixation system Small bone graft harvest Incision of tissue of front and/or lateral muscle compartments of lower leg Incision of tissue of rear muscle compartments of lower leg Incision of tissue of front and/or lateral and rear muscle compartments of lower leg Drainage of infected fluid-filled sac (bursa) of leg or ankle Incision of Achilles tendon, accessed through the skin using local anesthetic Incision of Achilles tendon, accessed through the skin requiring general anesthesia Incision of bone of leg or ankle Exploration, drainage, or removal of foreign body of ankle Release of ankle joint capsule Exploration of ankle joint Removal of membrane covering of ankle joint Removal of membrane covering ankle joint and tendon Removal of growth of leg and/or ankle tendon lining or capsule Removal or scraping of cyst or growth of either bone of lower leg Removal or scraping of cyst or growth of either bone of lower leg with patient-derived bone graft Removal or scraping of cyst or growth of either bone of lower leg with donor bone graft Partial removal of shin bone Partial removal of leg bone Repair of ruptured Achilles tendon, open or through skin procedure 5 of 11
6 Table 1 Additions to Covered Codes for Podiatrists (Specialty 140) () Repair of ruptured Achilles tendon with graft, open or through skin procedure Repair of ruptured Achilles tendon Repair of leg tendon Repair of leg tendon Repair of leg tendon Repair of leg tendon Repair of dislocating lower leg tendons Repair of dislocating lower leg tendons Release of leg and/or ankle tendon Release of multiple tendons of leg and/or ankle Lengthening or shortening of tendon of leg or ankle Lengthening or shortening of multiple tendons of leg or ankle Lengthening of calf muscle Transplant of tendon and muscle rerouting at lower leg or ankle Transplant of deep tendon with muscle rerouting at lower leg or ankle Transplant of tendon and muscle rerouting at lower leg or ankle Repair of disrupted collateral ligament of ankle Repair of disruption of both collateral ligaments of ankle Repair of disrupted collateral ligament of ankle Repair of ankle joint Repair of ankle joint with prosthesis Repair of ankle joint with revision of prosthesis Removal of ankle implant Incision of shin bone Incision of leg bone Incision of shin and outer lower leg bones Closed treatment of broken ankle Closed treatment of broken ankle with manipulation Open treatment of broken ankle 6 of 11
7 Table 1 Additions to Covered Codes for Podiatrists (Specialty 140) () Closed treatment of broken ankle Closed treatment of broken ankle with manipulation Open treatment of broken ankle Closed treatment of broken ankle Closed treatment of broken ankle with manipulation Open treatment of broken ankle Closed treatment of broken ankle Closed treatment of broken ankle with manipulation Open treatment of broken ankle Closed treatment of broken ankle Closed treatment of broken ankle with manipulation Open treatment of broken ankle Open treatment of broken ankle Closed treatment of fracture of lower weight bearing joint of shin bone Closed treatment of fracture of lower weight bearing joint of shin bone with traction and/or manipulation Open treatment of ligament tear at ankle joint Closed treatment of knee joint dislocation Closed treatment of knee joint dislocation under anesthesia Closed treatment of ankle dislocation Closed treatment of ankle dislocation under anesthesia Open treatment of ankle dislocation Open treatment of ankle dislocation with repair or internal or external hardware Manipulation of ankle under general anesthesia Fusion of ankle joint, open procedure Repair of foot tendon Correction of rigid deformity of first joint of big toe using implant Correction of bunion Removal or bivalving of gauntlet, boot, or body cast Casting or strapping procedure 7 of 11
8 Table 1 Additions to Covered Codes for Podiatrists (Specialty 140) () Destruction of peripheral nerve or branch Removal of growth of skin nerve Removal of growth of finger or toe nerve Removal of growth of finger or toe nerve Removal of growth of hand or foot nerve Removal of growth of hand or foot nerve Implantation of nerve end into bone or muscle X-ray of lower leg, 2 views Q4100 Skin substitute, not otherwise specified Q4101 Apligraf, per square centimeter Q4102 Oasis wound matrix, per square centimeter Q4103 Q4104 Q4105 Q4106 Q4107 Q4108 Q4110 Q4111 Q4112 Q4113 Q4114 Q4115 Q4116 Q4117 Q4118 Q4121 Q4122 Oasis burn matrix, per square centimeter Integra bilayer matrix wound dressing (BMWD), per square centimeter Integra dermal regeneration template (DRT) or integra omnigraft dermal regeneration matrix, per square centimeter Dermagraft, per square centimeter Graftjacket, per square centimeter Integra matrix, per square centimeter Primatrix, per square centimeter Gammagraft, per square centimeter Cymetra, injectable, 1 cc Graftjacket xpress, injectable, 1 cc Integra flowable wound matrix, injectable, 1 cc Alloskin, per square centimeter Alloderm, per square centimeter Hyalomatrix, per square centimeter Matristem micromatrix, 1 mg Theraskin, per square centimeter Dermacell, per square centimeter 8 of 11
9 Table 1 Additions to Covered Codes for Podiatrists (Specialty 140) () Q4123 Q4124 Q4125 Q4126 Q4127 Q4128 Q4130 Q4131 Q4132 Q4133 Q4134 Q4135 Q4136 Q4137 Q4138 Q4139 Q4140 Q4141 Q4142 Q4143 Q4145 Q4146 Q4147 Q4148 Q4149 Q4150 Q4151 Q4152 Alloskin RT, per square centimeter Oasis ultra tri-layer wound matrix, per square centimeter Arthroflex, per square centimeter Memoderm, dermaspan, tranzgraft or integuply, per square centimeter Talymed, per square centimeter FlexHD, allopatch hd, or matrix hd, per square centimeter Strattice TM, per square centimeter EpiFix or EpiCrd, per square centimeter Grafix core and grafixpl core, per square centimeter Grafix prime and grafixpl prime, per square centimeter hmatrix, per square centimeter Mediskin, per square centimeter E-Z Derm, per square centimeter Amnioexcel or biodexcel, per square centimeter Biodfence dryflex, per square centimeter Amniomatrix or biodmatrix, injectable, 1 cc Biodfence, per square centimeter Alloskin ac, per square centimeter XCM BIOLOGIC tissue matrix, per square centimeter Repriza, per square centimeter EpiFix, injectable, 1 mg Tensix, per square centimeter Architect, architect px, or architect fx, extracellular matrix, per square centimeter Neox cord 1k, NEOX CORD RT, or clarix cord 1k, per square centimeter Excellagen, 0.1 cc Allowrap DS or dry, per square centimeter Amnioband or guardian, per square centimeter Dermapure, per square centimeter 9 of 11
10 Table 1 Additions to Covered Codes for Podiatrists (Specialty 140) () Q4153 Q4154 Q4155 Q4156 Q4157 Q4158 Q4159 Q4160 Q4161 Q4162 Q4163 Q4164 Q4165 Q4166 Q4167 Q4168 Q4169 Q4170 Q4171 Q4172 Q4173 Q4174 Q4175 Q4176 Q4177 Q4178 Q4179 Q4180 Dermavest and plurivest, per square centimeter Biovance, per square centimeter Neoxflo or clarixflo, 1 mg Neox 100 or clarix 100, per square centimeter Revitalon, per square centimeter Kerecis omega3, per square centimeter Affinity, per square centimeter Nushield, per square centimeter bio-connekt wound matrix, per square centimeter Woundex flow, bioskin flow, 0.5 cc Woundex, bioskin, per square centimeter Helicoll, per square centimeter Keramatrix, per square centimeter Cytal, per square centimeter Truskin, per square centimeter Amnioband, 1 mg Artacent wound, per square centimeter Cygnus, per square centimeter Interfyl, 1 mg Puraply or puraply am, per square centimeter Palingen or palingen xplus, per square centimeter Palingen or promatrx, 0.36 mg per 0.25 cc Miroderm, per square centimeter Neopatch, per square centimeter Floweramnioflo, 0.1 cc Floweramniopatch, per square centimeter Flowerderm, per square centimeter Revita, per square centimeter 10 of 11
11 Table 1 Additions to Covered Codes for Podiatrists (Specialty 140) () Q4181 Q4182 Amnio wound, per square centimeter Transcyte, per square centimeter QUESTIONS? If you have questions about this publication, please contact Customer Assistance at SIGN UP FOR IHCP NOTIFICATIONS To receive notices of IHCP publications, subscribe by clicking the blue subscription envelope here or on the pages of indianamedicaid.com. COPIES OF THIS PUBLICATION If you need additional copies of this publication, please download them from indianamedicaid.com. TO PRINT A printer-friendly version of this publication, in black and white and without graphics, is available for your convenience. 11 of 11
INDIANA HEALTH COVERAGE PROGRAMS
 INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
See Policy CPT/HCPCS CODE section below for any prior authorization requirements
 Effective Date: 1/1/2019 Section: MED Policy No: 378 Medical Officer 1/1/2019 Date Technology Assessment Committee Approved Date: 3/09; 10/14; 1/16 Medical Policy Committee Approved Date: 4/02; 4/03; 9/04;
Effective Date: 1/1/2019 Section: MED Policy No: 378 Medical Officer 1/1/2019 Date Technology Assessment Committee Approved Date: 3/09; 10/14; 1/16 Medical Policy Committee Approved Date: 4/02; 4/03; 9/04;
MEDICAL POLICY No R8 SKIN SUBSTITUTES & SOFT TISSUE GRAFTS
 Summary of Changes MEDICAL POLICY SKIN SUBSTITUTES & SOFT TISSUE GRAFTS Effective Date: August 25, 2017 Review Dates: 12/08, 8/09, 8/10, 8/11, 8/12, 4/13, 5/14, 5/15, 5/16, 5/17 Date Of Origin: December
Summary of Changes MEDICAL POLICY SKIN SUBSTITUTES & SOFT TISSUE GRAFTS Effective Date: August 25, 2017 Review Dates: 12/08, 8/09, 8/10, 8/11, 8/12, 4/13, 5/14, 5/15, 5/16, 5/17 Date Of Origin: December
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Skin Replacement Therapy for Chronic Non healing Wounds in the Outpatient Setting MP-032-MD-DE Medical Management Provider
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Skin Replacement Therapy for Chronic Non healing Wounds in the Outpatient Setting MP-032-MD-DE Medical Management Provider
SKIN AND SOFT TISSUE SUBSTITUTES
 SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Community Plan Medical Policy Policy Number: CS153.B Effective Date: November 1, 2018 Instructions for Use Table of Contents Page COVERAGE RATIONALE...
SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Community Plan Medical Policy Policy Number: CS153.B Effective Date: November 1, 2018 Instructions for Use Table of Contents Page COVERAGE RATIONALE...
SKIN AND SOFT TISSUE SUBSTITUTES
 SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Commercial Medical Policy Policy Number: 2018T0592A Effective Date: October 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Commercial Medical Policy Policy Number: 2018T0592A Effective Date: October 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL Policy Number: MP.083.MH Last Review Date: 11/03/2016 Effective Date: 01/01/2017
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures
 POLICY: PG0203 ORIGINAL EFFECTIVE: 01/15/09 LAST REVIEW: 03/13/18 MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures GUIDELINES This policy does not certify benefits or authorization of benefits,
POLICY: PG0203 ORIGINAL EFFECTIVE: 01/15/09 LAST REVIEW: 03/13/18 MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures GUIDELINES This policy does not certify benefits or authorization of benefits,
Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy
 Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy File Name: Bioengineered Skin and Soft Tissue Substitutes File Code: UM.SURG.16 Origination: 09/2016 Last Review: 10/2018 Next Review:
Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy File Name: Bioengineered Skin and Soft Tissue Substitutes File Code: UM.SURG.16 Origination: 09/2016 Last Review: 10/2018 Next Review:
Understanding Your Costs and Coverage
 Understanding Your Costs and Coverage Thank you for choosing UW. We know that understanding your healthcare costs can be a challenge we re here to help. Your healthcare costs depend on many factors such
Understanding Your Costs and Coverage Thank you for choosing UW. We know that understanding your healthcare costs can be a challenge we re here to help. Your healthcare costs depend on many factors such
Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid
 02-10000-11 Original Effective Date: 01/01/01 Reviewed: 03/22/18 Revised: 01/01/19 Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid THIS MEDICAL COVERAGE GUIDELINE
02-10000-11 Original Effective Date: 01/01/01 Reviewed: 03/22/18 Revised: 01/01/19 Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid THIS MEDICAL COVERAGE GUIDELINE
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/28/2017 Section:
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/28/2017 Section:
WPS Medicare Policy Primer
 WPS Medicare Policy Primer Medicare Jurisdiction (J5 & J8) NE, KS, IA, MO, IN, & MI Retired skin substitute LCD 3/2016 Indications Apligraf Non-infected partial and full-thickness skin ulcers due to VSU
WPS Medicare Policy Primer Medicare Jurisdiction (J5 & J8) NE, KS, IA, MO, IN, & MI Retired skin substitute LCD 3/2016 Indications Apligraf Non-infected partial and full-thickness skin ulcers due to VSU
MP.083.MH Skin Substitutes- Human Skin Equivalents
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
INDIANA HEALTH COVERAGE PROGRAMS
 INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP075 Section: Medical Benefit Policy Subject: Tissue Engineered Skin Substitutes I. Policy: Tissue Engineered Skin Substitutes II. Purpose/Objective: To provide a
POLICIES AND PROCEDURE MANUAL Policy: MP075 Section: Medical Benefit Policy Subject: Tissue Engineered Skin Substitutes I. Policy: Tissue Engineered Skin Substitutes II. Purpose/Objective: To provide a
Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds
 Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds WINDY COLE, DPM ADJUNCT PROFESSOR AND DIRECTOR OF WOUND CARE RESEARCH KSUCPM MEDICAL DIRECTOR, WOUND CENTER, UH AHUJA HOSPITAL Goals
Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds WINDY COLE, DPM ADJUNCT PROFESSOR AND DIRECTOR OF WOUND CARE RESEARCH KSUCPM MEDICAL DIRECTOR, WOUND CENTER, UH AHUJA HOSPITAL Goals
IHCP banner page. Transportation providers are reminded of IHCP guidance for billing ALS/BLS oxygen services
 IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR201725 JUNE 20, 2017 Transportation providers are reminded of IHCP guidance for billing ALS/BLS oxygen services The Indiana Health Coverage Programs
IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR201725 JUNE 20, 2017 Transportation providers are reminded of IHCP guidance for billing ALS/BLS oxygen services The Indiana Health Coverage Programs
Palmetto Medicare Policy Primer
 Palmetto Medicare Policy Primer Medicare Jurisdiction (JM) NC, SC, WV & VA Application of Skin Substitutes LCD #L36466 Indications Presence of neuropathic diabetic foot ulcer(s) having failed to respond
Palmetto Medicare Policy Primer Medicare Jurisdiction (JM) NC, SC, WV & VA Application of Skin Substitutes LCD #L36466 Indications Presence of neuropathic diabetic foot ulcer(s) having failed to respond
medical policy update bulletin
 January 2019 medical policy update bulletin Medical Policy, Medical Benefit Drug Policy & Coverage Determination Guideline Updates UnitedHealthcare respects the expertise of the physicians, health care
January 2019 medical policy update bulletin Medical Policy, Medical Benefit Drug Policy & Coverage Determination Guideline Updates UnitedHealthcare respects the expertise of the physicians, health care
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 05 01 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 05 01 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
medically necessary as the evidence is insufficient to determine the effects of the technology on health outcomes.
 Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 10 02 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 10 02 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Surgical Preparation Codes for Skin Replacement Surgery** Hospital Outpatient/Ambulatory Surgical Center Setting
 2018 National Medicare Reimbursement Rate Summary* for Integra Dermal Regeneration Template, & Office Settings Integra LifeSciences Corporation compiles this summary of Medicare payment rates to provide
2018 National Medicare Reimbursement Rate Summary* for Integra Dermal Regeneration Template, & Office Settings Integra LifeSciences Corporation compiles this summary of Medicare payment rates to provide
Wound & Burn. Reimbursement & Coding Guide
 Wound & Burn Reimbursement & Coding Guide Wound & Burn Reimbursement and Coding Guide MicroMatrix and Cytal devices facilitate the remodeling of functional, site-appropriate tissue. Comprised of ACell
Wound & Burn Reimbursement & Coding Guide Wound & Burn Reimbursement and Coding Guide MicroMatrix and Cytal devices facilitate the remodeling of functional, site-appropriate tissue. Comprised of ACell
What s New Medical Policy Updates April 2017
 What s New Medical Policy Updates April 2017 Listed below are the recent changes made to policies within the Geisinger Health Plan Medical Policy Portfolio during the month of March that will become effective
What s New Medical Policy Updates April 2017 Listed below are the recent changes made to policies within the Geisinger Health Plan Medical Policy Portfolio during the month of March that will become effective
Summary of Package Insert 1 for PuraPly Wound Matrix
 Summary of Package Insert 1 for PuraPly Wound Matrix For NGS Indications Indicated for the management of wounds including: Partial and full-thickness wounds Venous ulcers Diabetic ulcers Drainage wounds
Summary of Package Insert 1 for PuraPly Wound Matrix For NGS Indications Indicated for the management of wounds including: Partial and full-thickness wounds Venous ulcers Diabetic ulcers Drainage wounds
Summary of Package Insert 1 for PuraPly Antimicrobial Wound Matrix
 Summary of Package Insert 1 for PuraPly Antimicrobial Wound Matrix For States with Non-Published Policies-Novitas Indications Indicated for the management of wounds as an effective barrier to resist microbial
Summary of Package Insert 1 for PuraPly Antimicrobial Wound Matrix For States with Non-Published Policies-Novitas Indications Indicated for the management of wounds as an effective barrier to resist microbial
Novitas Medicare Policy Primer
 Novitas Medicare Policy Primer Medicare Jurisdiction (JL and JH) AR, CO, LA, MS, NM, OK, TX, DC, DE, MD, NJ, & PA Counties of Arlington and Fairfax in Virginia and the city of Alexandria in Virginia Application
Novitas Medicare Policy Primer Medicare Jurisdiction (JL and JH) AR, CO, LA, MS, NM, OK, TX, DC, DE, MD, NJ, & PA Counties of Arlington and Fairfax in Virginia and the city of Alexandria in Virginia Application
Cigna Medical Coverage Policy
 Cigna Medical Coverage Policy Subject Tissue-Engineered Skin Substitutes Table of Contents Coverage Policy... 1 General Background... 15 Coding/Billing Information... 62 References... 66 Effective Date...
Cigna Medical Coverage Policy Subject Tissue-Engineered Skin Substitutes Table of Contents Coverage Policy... 1 General Background... 15 Coding/Billing Information... 62 References... 66 Effective Date...
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 10/01/2013 Section: Surgery Place(s)
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 10/01/2013 Section: Surgery Place(s)
2012 Head and Neck Reconstruction/ENT Repair Coding Observations
 Health Policy, Economics & Reimbursement Reimbursement Hotline Tel: 888.543.3656 Fax: 866.262.6977 reimbursement@lifecell.com www.lifecell.com 2012 Head and Neck Reconstruction/ENT Repair Coding Observations
Health Policy, Economics & Reimbursement Reimbursement Hotline Tel: 888.543.3656 Fax: 866.262.6977 reimbursement@lifecell.com www.lifecell.com 2012 Head and Neck Reconstruction/ENT Repair Coding Observations
CGS Medicare Policy Primer
 CGS Medicare Policy Primer Medicare Jurisdiction (J15) OH & KY Wound Application of Cellular and/or Tissue Based Products (CTPs) Lower Extremities #L36690 Indications Application of a CTP graft for lower
CGS Medicare Policy Primer Medicare Jurisdiction (J15) OH & KY Wound Application of Cellular and/or Tissue Based Products (CTPs) Lower Extremities #L36690 Indications Application of a CTP graft for lower
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Chapter 11 Worksheet Code It
 Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
Alberta Health Care Insurance Plan. Schedule Of Anaesthetic Rates Applicable To Podiatric Surgery. Procedure List. As Of.
 Alberta Health Care Insurance Plan Procedure List As Of 01 April 2016 Alberta Health Care Insurance Plan Page i Generated 2016/03/22 TABLE OF CONTENTS As of 2016/04/01 07 PHYSICAL MEDICINE, REHABILITATION,
Alberta Health Care Insurance Plan Procedure List As Of 01 April 2016 Alberta Health Care Insurance Plan Page i Generated 2016/03/22 TABLE OF CONTENTS As of 2016/04/01 07 PHYSICAL MEDICINE, REHABILITATION,
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2018 Origination: 4/2008 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2018 Origination: 4/2008 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
First Coast Service Options (FCSO) Medicare Policy Primer
 First Coast Service Options (FCSO) Medicare Policy Primer Medicare Jurisdiction (JN) Florida, Puerto Rico and U.S. Virgin Islands Application of Skin Substitute Grafts for the treatment of DFU and VLU
First Coast Service Options (FCSO) Medicare Policy Primer Medicare Jurisdiction (JN) Florida, Puerto Rico and U.S. Virgin Islands Application of Skin Substitute Grafts for the treatment of DFU and VLU
INVESTIGATIONAL NOT COVERED
 Acoustic Heart Sound Recorder (93799) Actigraphy for Sleep Disturbances (95803) Anoscopy with Thermal Energy (46999) Aqueous Drainage Devices for Glaucoma (0253T, 0449T, 0450T, and 0474T ) Effective 10/01/17
Acoustic Heart Sound Recorder (93799) Actigraphy for Sleep Disturbances (95803) Anoscopy with Thermal Energy (46999) Aqueous Drainage Devices for Glaucoma (0253T, 0449T, 0450T, and 0474T ) Effective 10/01/17
Suture of Tendon Sheath of Hand , , Delayed suture of other tendon of hand , Other Suture of Flexor Tendon of Hand
 Coding and Reimbursement Guide for Integra BioFix Amniotic Membrane Allograft, Integra BioFix Plus Amniotic Membrane Allograft & Integra BioFix Flow Placental Tissue Matrix Allograft For Use In Repair
Coding and Reimbursement Guide for Integra BioFix Amniotic Membrane Allograft, Integra BioFix Plus Amniotic Membrane Allograft & Integra BioFix Flow Placental Tissue Matrix Allograft For Use In Repair
Appendix C Podiatriac Services
 Appendix C Podiatriac Services CPT/ HCPCS Codes Description Auth required Y or N Mod Service Limits Age Limits Notes 10021-10022 Fine Needle Aspiration w/ or w/o imaging guidance 10060-10061 10120-10121
Appendix C Podiatriac Services CPT/ HCPCS Codes Description Auth required Y or N Mod Service Limits Age Limits Notes 10021-10022 Fine Needle Aspiration w/ or w/o imaging guidance 10060-10061 10120-10121
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Bio-Engineered Skin and Page 1 of 62 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Bio-Engineered Skin and Amniotic Membrane and Amniotic Fluid medical
Bio-Engineered Skin and Page 1 of 62 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Bio-Engineered Skin and Amniotic Membrane and Amniotic Fluid medical
SCHEDULE OF BENEFITS GAI
 SCHEDULE OF BENEFITS The Schedule of Benefits provides a brief outline of the coverage and benefits including the maximum benefit amount, benefit periods, and any limitations applicable to benefits provided
SCHEDULE OF BENEFITS The Schedule of Benefits provides a brief outline of the coverage and benefits including the maximum benefit amount, benefit periods, and any limitations applicable to benefits provided
Alberta Health Care Insurance Plan. Schedule Of Anaesthetic Rates Applicable To Podiatry. Procedure List. As Of. 01 April Government of Alberta
 Alberta Health Care Insurance Plan Procedure List As Of 01 April 2017 Alberta Health Care Insurance Plan Page i Generated 2017/03/14 TABLE OF CONTENTS As of 2017/04/01 II. OPERATIONS ON THE NERVOUS SYSTEM.......................
Alberta Health Care Insurance Plan Procedure List As Of 01 April 2017 Alberta Health Care Insurance Plan Page i Generated 2017/03/14 TABLE OF CONTENTS As of 2017/04/01 II. OPERATIONS ON THE NERVOUS SYSTEM.......................
DermACELL AWM Comprehensive Reimbursement Resource Guide. Prepared by Musculoskeletal Clinical Regulatory Advisers, LLC. Version 01/2018.
 2018 Comprehensive Reimbursement Resource Guide Prepared by Musculoskeletal Clinical Regulatory Advisers, LLC. Version 01/2018. DermACELL AWM Disclaimer: This information is for educational/informational
2018 Comprehensive Reimbursement Resource Guide Prepared by Musculoskeletal Clinical Regulatory Advisers, LLC. Version 01/2018. DermACELL AWM Disclaimer: This information is for educational/informational
code it ACTISHIELD HCPCS Device Codes Amniotic Barrier Membrane HCPCS Code C9399 Description Q4100 C5271
 code it HCPCS Device Codes 2015 Reimbursement Codes The following codes contained within this document are representative of possible services or diagnoses that may be associated with use of Wright products.
code it HCPCS Device Codes 2015 Reimbursement Codes The following codes contained within this document are representative of possible services or diagnoses that may be associated with use of Wright products.
IHCP banner page. IHCP will remove age restrictions on certain diagnosis codes and mass reprocess claims that denied inappropriately
 IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR201745 NOVEMBER 7, 2017 IHCP will remove age restrictions on certain diagnosis codes and mass reprocess claims that denied inappropriately The Indiana
IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR201745 NOVEMBER 7, 2017 IHCP will remove age restrictions on certain diagnosis codes and mass reprocess claims that denied inappropriately The Indiana
Skin Deep. Agenda. Burns Wounds Debridement Evaluation and Management Services. Presented by: Mike Strong, SFM The Work Comp Experts.
 Presented by: Mike Strong, SFM The Work Comp Experts Agenda Wounds Debridement Evaluation and Management Services 2 1 Types of First Degree Second Degree Third Degree Rule of 9 Adults Infants Burn Coding
Presented by: Mike Strong, SFM The Work Comp Experts Agenda Wounds Debridement Evaluation and Management Services 2 1 Types of First Degree Second Degree Third Degree Rule of 9 Adults Infants Burn Coding
Cahaba Medicare Policy Primer 1,2 for Apligraf
 Cahaba Medicare Policy Primer 1,2 for Apligraf MAC A: AL, GA & TN MAC B: AL, GA, & TN LCD# 31428 Indications Applied to partial- or full-thickness ulcers of the lower extremities (see individual product
Cahaba Medicare Policy Primer 1,2 for Apligraf MAC A: AL, GA & TN MAC B: AL, GA, & TN LCD# 31428 Indications Applied to partial- or full-thickness ulcers of the lower extremities (see individual product
Coding and Reimbursement Guide for TenoGlide Tendon Protector Sheet 2018
 Coding and Reimbursement Guide for TenoGlide Protector Sheet 2018 Effective October 1, 2015, the Centers for & Medicaid Services (CMS) is implementing International Classification of Diseases, 10 th Revision
Coding and Reimbursement Guide for TenoGlide Protector Sheet 2018 Effective October 1, 2015, the Centers for & Medicaid Services (CMS) is implementing International Classification of Diseases, 10 th Revision
Arkansas Medicaid Health Care Providers Podiatrist Provider Manual Update Transmittal #99 Page 2
 Division of Medical Services Program Planning & Development P.O. Box 1437, Slot S-295 Little Rock, AR 72203-1437 501-682-8368 Fax: 501-682-2480 TDD: 501-682-6789 TO: Arkansas Medicaid Health Care Providers
Division of Medical Services Program Planning & Development P.O. Box 1437, Slot S-295 Little Rock, AR 72203-1437 501-682-8368 Fax: 501-682-2480 TDD: 501-682-6789 TO: Arkansas Medicaid Health Care Providers
Coding for Wound Care
 Coding for Wound Care ****IMPORTANT*** Disclaimer ***Information provided is to the best of our knowledge and as current as possible. ***Please verify all policy and reimbursement information with your
Coding for Wound Care ****IMPORTANT*** Disclaimer ***Information provided is to the best of our knowledge and as current as possible. ***Please verify all policy and reimbursement information with your
Clinical Policy: EpiFix Wound Treatment
 Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
Coding and Reimbursement Guide for Integra Reinforcement Matrix 2018
 Coding and Reimbursement Guide for Integra Reinforcement Matrix 2018 Effective October 1, 2015, the Centers for & Medicaid Services (CMS) is implementing International Classification of Diseases, 10 th
Coding and Reimbursement Guide for Integra Reinforcement Matrix 2018 Effective October 1, 2015, the Centers for & Medicaid Services (CMS) is implementing International Classification of Diseases, 10 th
ASPEN MEDICAL SURGERY REGINA
 It is hereby certified that ASPEN MEDICAL SURGERY REGINA Has successfully completed an inspection as is required under the College s Bylaw 26.1 and is therefore approved as a Non Hospital Treatment Facility
It is hereby certified that ASPEN MEDICAL SURGERY REGINA Has successfully completed an inspection as is required under the College s Bylaw 26.1 and is therefore approved as a Non Hospital Treatment Facility
Medical Policy. MP Bioengineered Skin and Soft Tissue Substitutes
 Medical Policy MP 7.01.113 BCBSA Ref. Policy: 7.01.113 Last Review: 02/26/2018 Effective Date: 05/30/2018 Section: Surgery Related Policies 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors
Medical Policy MP 7.01.113 BCBSA Ref. Policy: 7.01.113 Last Review: 02/26/2018 Effective Date: 05/30/2018 Section: Surgery Related Policies 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors
Bioengineered Skin and Soft Tissue Substitutes
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
2013 MCT CPC-H Quiz #8 Chapters 13 and 14
 2013 MCT CPC-H Quiz #8 Chapters 13 and 14 Name: Date: Instructor: Score: 1. A female patient presents to the outpatient clinic for excision of a 4.8 cm malignant melanoma of the left inner thigh. A 6 cm
2013 MCT CPC-H Quiz #8 Chapters 13 and 14 Name: Date: Instructor: Score: 1. A female patient presents to the outpatient clinic for excision of a 4.8 cm malignant melanoma of the left inner thigh. A 6 cm
Is there an icd 10 code for smoking vapor
 Is there an icd 10 code for smoking vapor Thanks for your response, I'm just thinking that 305.1 is tobacco use and there is no tobacco in the e-cig. Just the nicotine and other chemicals. I have been
Is there an icd 10 code for smoking vapor Thanks for your response, I'm just thinking that 305.1 is tobacco use and there is no tobacco in the e-cig. Just the nicotine and other chemicals. I have been
CODING COMPANION. Podiatry A comprehensive illustrated guide to coding and reimbursement. Sample page. Power up your coding optum360coding.
 Podiatry A comprehensive illustrated guide to coding and reimbursement 2020 CODING COANION Power up your coding optum360coding.com Contents Getting Started with Coding Companion...i Resequencing of CPT
Podiatry A comprehensive illustrated guide to coding and reimbursement 2020 CODING COANION Power up your coding optum360coding.com Contents Getting Started with Coding Companion...i Resequencing of CPT
IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR MARCH 1, 2016
 IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR201609 MARCH 1, 2016 IHCP creates separate Hearing Aid Dealer and Audiologist code sets Effective April 1, 2016, the Indiana Health Coverage Programs
IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR201609 MARCH 1, 2016 IHCP creates separate Hearing Aid Dealer and Audiologist code sets Effective April 1, 2016, the Indiana Health Coverage Programs
Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of PriMatrix AG Antimicrobial Dermal Repair Scaffold
 Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of PriMatrix AG Antimicrobial Dermal Repair Scaffold Effective October 1, 2015, the Centers for & Medicaid Services (CMS) is
Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of PriMatrix AG Antimicrobial Dermal Repair Scaffold Effective October 1, 2015, the Centers for & Medicaid Services (CMS) is
3. The prescribed fee shall be accepted as payment in full for the podiatry services.
 WorkSafeBC Schedule for Podiatry Services 1. The Payment Schedule includes the services of podiatrists who are registered members in good standing of the College of Podiatric Surgeons of British Columbia,
WorkSafeBC Schedule for Podiatry Services 1. The Payment Schedule includes the services of podiatrists who are registered members in good standing of the College of Podiatric Surgeons of British Columbia,
Number: (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end
 1 of 278 Number: 0244 (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. I. Aetna considers the following products for wound care medically necessary according
1 of 278 Number: 0244 (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. I. Aetna considers the following products for wound care medically necessary according
Chiropractic Services
 INDIANA HEALTH COVERAGE PROGRAMS PROVIDER REFERENCE M ODULE Chiropractic Services L I B R A R Y R E F E R E N C E N U M B E R : P R O M O D 0 0 0 2 1 P U B L I S H E D : A U G U S T 1, 2 0 1 7 P O L I
INDIANA HEALTH COVERAGE PROGRAMS PROVIDER REFERENCE M ODULE Chiropractic Services L I B R A R Y R E F E R E N C E N U M B E R : P R O M O D 0 0 0 2 1 P U B L I S H E D : A U G U S T 1, 2 0 1 7 P O L I
Final Rule CMS-1676-F was released on November 2, 2017 and finalized policies first proposed
 November 8, 2017 Subject: (CMS 1676 F) Summary of the Centers for Medicare and Medicaid Services Revisions to Payment Policies under the Physician Fee Schedule and Other Revisions to Part B for CY 2018
November 8, 2017 Subject: (CMS 1676 F) Summary of the Centers for Medicare and Medicaid Services Revisions to Payment Policies under the Physician Fee Schedule and Other Revisions to Part B for CY 2018
Orthopedic Coding Changes for 2012
 Orthopedic Coding Changes for Lynn M. Anderanin, CPC,CPC-I, COSC Vertebroplasty 22520- Percutaneous vertebroplasty, 1 vertebral body, unilateral or bilateral injection; thoracic 22520- Percutaneous vertebroplasty,
Orthopedic Coding Changes for Lynn M. Anderanin, CPC,CPC-I, COSC Vertebroplasty 22520- Percutaneous vertebroplasty, 1 vertebral body, unilateral or bilateral injection; thoracic 22520- Percutaneous vertebroplasty,
Rocky Mountain Health Plans
 Rocky Mountain Health Plans Procedures that Require Prior Authorization Revised 12/29/16 The procedures for which Rocky Mountain Health Plans requires prior authorization include the list below. If you
Rocky Mountain Health Plans Procedures that Require Prior Authorization Revised 12/29/16 The procedures for which Rocky Mountain Health Plans requires prior authorization include the list below. If you
Foot and Ankle Systems Coding Reference Guide
 Foot and Ankle Systems Coding Reference Guide Physician Arthrodesis 27870 Arthrodesis, ankle, open 27871 Arthrodesis, tibiofibular joint, proximal or distal 28705 Arthrodesis; pantalar 28715 Arthrodesis;
Foot and Ankle Systems Coding Reference Guide Physician Arthrodesis 27870 Arthrodesis, ankle, open 27871 Arthrodesis, tibiofibular joint, proximal or distal 28705 Arthrodesis; pantalar 28715 Arthrodesis;
SAMPLE. Relative Values for Dentists Relative values based on survey data from Relative Value Studies, Inc. ICD-10
 www.optumcoding.com Relative Values for Dentists Relative values based on survey data from Relative Value Studies, Inc. 2017 a ICD-10 A full suite of resources including the latest code set, mapping products,
www.optumcoding.com Relative Values for Dentists Relative values based on survey data from Relative Value Studies, Inc. 2017 a ICD-10 A full suite of resources including the latest code set, mapping products,
THIS WEBSITE IS FOR INFORMATIONAL PURPOSE ONLY. MEDICAID FEE FOR SERVICES: UPDATE JANUARY 1, 2018 Codes for Out-Patient Hospital Billing ONLY
 A4641 A9505 A9512 A9516 A9521 A9528 A9537 A9540 A9541 A9555 A9556 A9558 A9560 A9561 A9562 A9567 A9587 A9588 C1300 C1713 C1714 C1715 C1716 C1717 C1719 C1721 C1722 C1724 C1725 C1726 RADIOPHARMACEUTICAL,
A4641 A9505 A9512 A9516 A9521 A9528 A9537 A9540 A9541 A9555 A9556 A9558 A9560 A9561 A9562 A9567 A9587 A9588 C1300 C1713 C1714 C1715 C1716 C1717 C1719 C1721 C1722 C1724 C1725 C1726 RADIOPHARMACEUTICAL,
Musculoskeletal System
 Musculoskeletal System CPT CPT copyright 2011 American Medical Association. All rights reserved. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the
Musculoskeletal System CPT CPT copyright 2011 American Medical Association. All rights reserved. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the
OMNIBUS CODES. Policy Number: 2018T0535TT Effective Date: January 1, 2018
 OMNIBUS CODES UnitedHealthcare Commercial Medical Policy Policy Number: 2018T0535TT Effective Date: January 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 COVERAGE SUMMARY... 1 COVERAGE RATIONALE/CLINICAL
OMNIBUS CODES UnitedHealthcare Commercial Medical Policy Policy Number: 2018T0535TT Effective Date: January 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 COVERAGE SUMMARY... 1 COVERAGE RATIONALE/CLINICAL
CYGNUS REIMBURSEMENT GUIDE
 CYGNUS REIMBURSEMENT GUIDE The CYGNUS amnion patch is an immune-privileged tissue containing the natural regenerative factors responsible for creating a wound healing environment conducive to tissue regeneration.
CYGNUS REIMBURSEMENT GUIDE The CYGNUS amnion patch is an immune-privileged tissue containing the natural regenerative factors responsible for creating a wound healing environment conducive to tissue regeneration.
The Orthopaedic Coding Coach 2010 Orthopaedic Coding Tips By Karen Zupko & Associates
 The Orthopaedic Coding Coach 2010 Orthopaedic Coding Tips By Karen Zupko & Associates Use of Modifiers October 14, 2010 I was recently told that when applying more than one modifier, they should be listed
The Orthopaedic Coding Coach 2010 Orthopaedic Coding Tips By Karen Zupko & Associates Use of Modifiers October 14, 2010 I was recently told that when applying more than one modifier, they should be listed
Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of Integra Bilayer Wound Matrix
 Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of Integra Bilayer Wound Matrix Effective October 1, 2015, the Centers for & Medicaid Services (CMS) is implementing International
Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of Integra Bilayer Wound Matrix Effective October 1, 2015, the Centers for & Medicaid Services (CMS) is implementing International
Chapter 12 Worksheet Code It
 Class: Date: Chapter 12 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. The type of fracture corresponds to the type of treatment. 2. An open fracture is always treated
Class: Date: Chapter 12 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. The type of fracture corresponds to the type of treatment. 2. An open fracture is always treated
2016 MEDICARE PHYSICIAN & FACILITY REIMBURSEMENT INFORMATION*
 2016 MEDICARE & FACILITY REIMBURSEMENT INFORMATION* CODING FOR CLARIX PRODUCTS UTILIZED AS SURGICAL COVERINGS, WRAPS OR BARRIERS CLARIX CORD 1K, CLARIX 100 and CLARIX FLO are cryopreserved human umbilical
2016 MEDICARE & FACILITY REIMBURSEMENT INFORMATION* CODING FOR CLARIX PRODUCTS UTILIZED AS SURGICAL COVERINGS, WRAPS OR BARRIERS CLARIX CORD 1K, CLARIX 100 and CLARIX FLO are cryopreserved human umbilical
Transplantable Organs
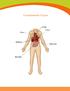 Transplantable Organs Liver Kidneys Pancreas Intestine Organ Information Heart The body s hardest working muscle, the heart beats 70 times each minute as it pumps blood throughout the body. Some conditions
Transplantable Organs Liver Kidneys Pancreas Intestine Organ Information Heart The body s hardest working muscle, the heart beats 70 times each minute as it pumps blood throughout the body. Some conditions
code it PRO-TOE C2 HCPCS Device Codes CPT Codes Physician Coding Hammertoe Implant HCPCS Code Description C1713 CPT CODE Description RVUs
 code it HCPCS Device Codes 2015 Reimbursement Codes The following codes contained within this document are representative of possible services or diagnoses that may be associated with use of Wright products.
code it HCPCS Device Codes 2015 Reimbursement Codes The following codes contained within this document are representative of possible services or diagnoses that may be associated with use of Wright products.
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference
 2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
Modifier 62 - Co-surgery (Two Surgeons)
 Manual: Policy Title: Reimbursement Policy Modifier 62 - Co-surgery (Two Surgeons) Section: Modifiers Subsection: None Date of Origin: 1/1/2000 Policy Number: RPM035 Last Updated: 7/5/2017 Last Reviewed:
Manual: Policy Title: Reimbursement Policy Modifier 62 - Co-surgery (Two Surgeons) Section: Modifiers Subsection: None Date of Origin: 1/1/2000 Policy Number: RPM035 Last Updated: 7/5/2017 Last Reviewed:
NEW YORK STATE MEDICAID PROGRAM PHYSICIAN PROCEDURE CODES SECTION 5 - SURGERY
 NEW YORK STATE MEDICAID PROGRAM PHYSICIAN PROCEDURE CODES SECTION 5 - SURGERY Table of Contents ANESTHESIA SECTION------------------------------------------------------------------------2 GENERAL INFORMATION
NEW YORK STATE MEDICAID PROGRAM PHYSICIAN PROCEDURE CODES SECTION 5 - SURGERY Table of Contents ANESTHESIA SECTION------------------------------------------------------------------------2 GENERAL INFORMATION
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: July 15, 2018 Related Policies: 7.01.149 Amniotic Membrane and Amniotic Fluid Bioengineered Skin and Soft Tissue Substitutes Description Bioengineered skin and
FEP Medical Policy Manual Effective Date: July 15, 2018 Related Policies: 7.01.149 Amniotic Membrane and Amniotic Fluid Bioengineered Skin and Soft Tissue Substitutes Description Bioengineered skin and
Hip $5,200. Wrist or Elbow $1,430 $715. Toe or Finger $390 $195. (except toes/heel), Wrist,
 High 24 Hour Module 1 Accident Emergency Treatment Accident Emergency Treatment Benefit For physician treatment and X-rays in a hospital or doctor's office within 96 hours of the accident. Major Diagnostic
High 24 Hour Module 1 Accident Emergency Treatment Accident Emergency Treatment Benefit For physician treatment and X-rays in a hospital or doctor's office within 96 hours of the accident. Major Diagnostic
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: April 15, 2017 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors as a Treatment of Wound Healing and Other Conditions 7.01.149
FEP Medical Policy Manual Effective Date: April 15, 2017 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors as a Treatment of Wound Healing and Other Conditions 7.01.149
Schedule of Benefits for General Practitioners
 Schedule of Benefits for General Practitioners Aviva Health Insurance Ireland Limited SCHEDULE OF BENEFITS FOR GENERAL PRACTITIONERS FROM AVIVA HEALTH INSURANCE IRELAND LIMITED Welcome to Aviva s schedule
Schedule of Benefits for General Practitioners Aviva Health Insurance Ireland Limited SCHEDULE OF BENEFITS FOR GENERAL PRACTITIONERS FROM AVIVA HEALTH INSURANCE IRELAND LIMITED Welcome to Aviva s schedule
Anesthesia Cross Coder. Essential links from CPT codes to ICD-9-CM and HCPCS codes
 Anesthesia Cross Coder Essential links from CPT codes to ICD-9-CM and HCPCS codes 2015 Contents Introduction... i CPT Anesthesia to Procedure Crosswalk...i Format...i Icon Key...ii CPT Codes...ii Resequenced
Anesthesia Cross Coder Essential links from CPT codes to ICD-9-CM and HCPCS codes 2015 Contents Introduction... i CPT Anesthesia to Procedure Crosswalk...i Format...i Icon Key...ii CPT Codes...ii Resequenced
For the Physical Therapist
 Coding and Payment Guide www.optumcoding.com For the Physical Therapist n essential coding, billing, and reimbursement resource for the physical therapist 216 a ICD-1 full suite of resources including
Coding and Payment Guide www.optumcoding.com For the Physical Therapist n essential coding, billing, and reimbursement resource for the physical therapist 216 a ICD-1 full suite of resources including
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Amniotic Membrane and Amniotic Fluid Page 1 of 26 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Amniotic Membrane and Amniotic Fluid Bio-Engineered Skin
Amniotic Membrane and Amniotic Fluid Page 1 of 26 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Amniotic Membrane and Amniotic Fluid Bio-Engineered Skin
Release Notes and Installation Instructions. Medtech32. ACC Subsidy Updates
 Release Notes and Installation Instructions Medtech32 ACC Subsidy Updates General Practitioners, Medical Specialists, Nurses, Podiatrists and Specified Treatment Providers (April 2014) These Release Notes
Release Notes and Installation Instructions Medtech32 ACC Subsidy Updates General Practitioners, Medical Specialists, Nurses, Podiatrists and Specified Treatment Providers (April 2014) These Release Notes
Reimbursement Information for Diagnostic Musculoskeletal Ultrasound and Ultrasound-guided Procedures 1
 GE Healthcare Reimbursement Information for Diagnostic Musculoskeletal Ultrasound and Ultrasound-guided Procedures 1 January, 2013 www.gehealthcare.com/reimbursement This overview addresses coding, coverage,
GE Healthcare Reimbursement Information for Diagnostic Musculoskeletal Ultrasound and Ultrasound-guided Procedures 1 January, 2013 www.gehealthcare.com/reimbursement This overview addresses coding, coverage,
Subcutaneous Tissues
 Podiatry Fee Schedule Effective January 1, 2013 The Base Fee listed below for each code is reimbursement for services rendered to adult recipients age 21 and over. To calculate the fee for children under
Podiatry Fee Schedule Effective January 1, 2013 The Base Fee listed below for each code is reimbursement for services rendered to adult recipients age 21 and over. To calculate the fee for children under
SCOPE OF PRACTICE PGY-6 PGY-7 PGY-8
 PGY-6 Round on all plastic surgery inpatients every day. Assess progress of patients and identify real or potential problems. Review patients progress with attending physicians daily and participate in
PGY-6 Round on all plastic surgery inpatients every day. Assess progress of patients and identify real or potential problems. Review patients progress with attending physicians daily and participate in
All Providers. All Hospice Providers. All Ambulance Providers
 INDIANA HEALTH COVERAGE PROGRAMS B A N N E R P A G E All Providers B R 2 0 0 8 0 5 J A N U A R Y 2 9, 2 0 0 8 CPT 90660 Influenza Virus Vaccine, Live for Intranasal Use The age restriction for Current
INDIANA HEALTH COVERAGE PROGRAMS B A N N E R P A G E All Providers B R 2 0 0 8 0 5 J A N U A R Y 2 9, 2 0 0 8 CPT 90660 Influenza Virus Vaccine, Live for Intranasal Use The age restriction for Current
2017 Coding and Reimbursement Survival Guide
 2017 Coding and Reimbursement Survival Guide Chapter 8: General Surgery Integumentary Procedures: 4 Questions Focus Your Skin Substitute Graft Coding Hint: Graft size doesn t matter. If your surgeon treats
2017 Coding and Reimbursement Survival Guide Chapter 8: General Surgery Integumentary Procedures: 4 Questions Focus Your Skin Substitute Graft Coding Hint: Graft size doesn t matter. If your surgeon treats
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Acellular dermal allografts for arthroplasty, 633 642 for plantar soft tissue augmentation, 543 555 Achilles tendon repair extracellular matrix
Note: Page numbers of article titles are in boldface type. A Acellular dermal allografts for arthroplasty, 633 642 for plantar soft tissue augmentation, 543 555 Achilles tendon repair extracellular matrix
Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association
 Bio-Engineered Skin and Page 1 of 58 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See Also: Bio-Engineered Skin and Periodontal Soft Tissue Grafting dental
Bio-Engineered Skin and Page 1 of 58 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See Also: Bio-Engineered Skin and Periodontal Soft Tissue Grafting dental
