FEP Medical Policy Manual
|
|
|
- Ernest Flynn
- 6 years ago
- Views:
Transcription
1 FEP Medical Policy Manual Effective Date: April 15, 2017 Related Policies: Recombinant and Autologous Platelet-Derived Growth Factors as a Treatment of Wound Healing and Other Conditions Amniotic Membrane and Amniotic Fluid Bioengineered Skin and Soft Tissue Substitutes Description Bioengineered skin and soft tissue substitutes may be derived from human tissue (autologous or allogeneic), nonhuman tissue (xenographic), synthetic materials, or a composite of these materials. Bioengineered skin and soft tissue substitutes are being evaluated for a variety of conditions, including breast reconstruction and healing lower-extremity ulcers and severe burns. Acellular dermal matrix (ADM) products are also being evaluated for soft tissue repair. FDA REGULATORY STATUS A large number of artificial skin products are commercially available or in development. The following summary of commercially available skin substitutes describes those products that have substantial relevant evidence on efficacy. Acellular Dermal Matrix Products Allograft acellular dermal matrix (ADM) products derived from donated human skin tissue are supplied by tissue banks compliant with standards of the American Association of Tissue Banks (AATB) and U.S. Food and Drug Administration (FDA) guidelines. The processing removes the cellular components (ie, epidermis, all viable dermal cells) that can lead to rejection and infection. ADM products from human skin tissue are regarded as minimally processed and not significantly changed in structure from the natural material; FDA classifies ADM products as banked human tissue and therefore, not requiring FDA approval. AlloDerm (LifeCell Corp.) is a n ADM (a llograft) tissue-replacement product created from native human skin and processed so that the basement membrane and cellular matrix remain intact. Originally, AlloDerm required refrigeration and rehydration before use. It is currently available in a ready-to-use product stored at room temperature. An injectable micronized form of AlloDerm (Cymetra) is available. AlloMax Surgical Graft (Bard Davol) is an acellular non-cross-linked human dermis allograft. (AlloMax was previously marketed as NeoForm.) AlloPatch (Musculoskeletal Transplant Foundation) is an acellular human dermis allograft derived from the reticular layer of the dermis and marketed for wound care. This product is also marketed as FlexHD for postmastectomy breast reconstruction. FlexHD (Musculoskeletal Transplant Foundation) is an acellular hydrated reticular dermis allograft derived from donated human skin. Original Policy Date: September 2011 Page: 1
2 Effective Policy Date: April 15, 2017 Page: 2 of 13 DermACELL (LifeNet Health) is an allogeneic ADM processed with proprietary technologies MATRACELL and PRESERVON. DermaMatrix (Synthes) is a freeze-dried ADM derived from donated human skin tissue. DermaMatrix Acellular Dermis is processed by the Musculoskeletal Transplant Foundation. DermaPure (Tissue Regenix Wound Care) is a single-layer decellularized human dermal allograft for the treatment of acute and chronic wounds. Graftjacket Regenerative Tissue Matrix (also called Graftjacket Skin Substitute; KCI) is an acellular regenerative tissue matrix that has been processed from human skin supplied from U.S. tissue banks. The allograft is minimally processed to remove the epidermal and dermal cells, while preserving dermal structure. Graftjacket Xpress is an injectable product. FDA product codes: FTM, OXF. Xenogenic Products Keramatrix (Keraplast Research) is an open-cell foam comprised of freeze-dried keratin that is derived from acellular animal protein. In 2009, it was cleared for marketing by FDA through the 510(k) process under the name of Keratec. The wound dressings are indicated in the management of the following types of dry, light, and moderately exudating partial and full-thickness wounds: pressure (stage I-IV) and venous stasis ulcers, ulcers caused by mixed vascular etiologies, diabetic ulcers, donor sites, and grafts. Helicoll (Encol) is an acellular collagen matrix derived from bovine dermis. In 2004, it was cleared for marketing by FDA through the 510(k) process for topical wound management that includes partial and full-thickness wounds, pressure ulcers, venous ulcers, chronic vascular ulcers, diabetic ulcers, trauma wounds (eg, abrasions, lacerations, second-degree bums, skin tears), and surgical wounds including donor sites/grafts. Cytal (previously called MatriStem ) Wound Matrix, Multilayer Wound Matrix, Pelvic Floor Matrix, MicroMatrix, and Burn Matrix (all manufactured by ACell) are composed of porcine-derived urinary bladder matrix. Permacol (Covidien) is xenogeneic and composed of cross-linked porcine dermal collagen. Crosslinking improves the tensile strength and long-term durability, but decreases pliability. PriMatrix (TEI Biosciences) is a xenogeneic ADM processed from fetal bovine dermis. It was cleared for marketing by FDA through the 510(k) process for partial- and full-thickness wounds; diabetic, pressure, and venous stasis ulcers; surgical wounds; and tunneling, draining, and traumatic wounds. FDA product code: KGN. SurgiMend PRS (TEI Biosciences) is a xenogeneic ADM processed from fetal bovine dermis. Strattice Reconstructive Tissue Matrix (LifeCell Corp.) is a xenogenic non-cross-linked porcine-derived ADM. There are pliable and firm versions, which are stored at room temperature and come fully hydrated. Oasis Wound Matrix (Cook Biotech) is a xenogeneic collagen scaffold derived from porcine small intestinal mucosa. In 2000, it was cleared for marketing by FDA through the 510(k) process for the management of partial- and full-thickness wounds, including pressure ulcers, venous ulcers, diabetic ulcers, chronic vascular ulcers, tunneled undermined wounds, surgical wounds, trauma wounds, and draining wounds. FDA Product code: KGN. Living Cell Therapy Apligraf (Organogenesis) is a bilayered living cell therapy composed of an epidermal layer of living human keratinocytes and a dermal layer of living human fibroblasts. Apligraf is supplied as needed, in 1
3 Effective Policy Date: April 15, 2017 Page: 3 of 13 size, with a shelf-life of 10 days. In 1998, it was approved by FDA for use in conjunction with compression therapy for the treatment of noninfected, partial- and full-thickness skin ulcers due to venous insufficiency and in 2001 for full-thickness neuropathic diabetic lower-extremity ulcers nonresponsive to standard wound therapy. FDA product code: FTM. Dermagraft (Organogenesis) is composed of cryopreserved human-derived fibroblasts and collagen derived from newborn human foreskin and cultured on a bioabsorbable polyglactin mesh scaffold. Dermagraft has been approved by FDA for repair of diabetic foot ulcers. FDA product code: PFC. TheraSkin (Soluble Systems) is a cryopreserved split-thickness human skin allograft composed of living fibroblasts and keratinocytes and an extracellular matrix in epidermal and dermal layers. TheraSkin is derived from human skin allograft supplied by tissue banks compliant with the AATB and FDA guidelines. It is considered a minimally processed human cell, tissue, and cellular- and tissue-based product by FDA. Epicel (Genzyme Biosurgery) is a cultured epithelial autograft and is FDA-approved under a humanitarian device exemption (HDE) for the treatment of deep dermal or full-thickness burns comprising a total body surface area of 30% or more. It may be used in conjunction with split-thickness autografts or alone in patients for whom split-thickness autografts may not be an option due to the severity and extent of their burns. FDA product code: OCE. OrCel (Forticell Bioscience; formerly Composite Cultured Skin) is an absorbable allogeneic bilayered cellular matrix, made of bovine collagen, in which human dermal cells have been cultured. It was approved by FDA premarket approval for healing donor site wounds in burn victims and under an HDE for use in patients with recessive dystrophic epidermolysis bullosa undergoing hand reconstruction surgery to close and heal wounds created by the surgery, including those at donor sites. FDA product code: ODS. Biosynthetic Products Biobrane /Biobrane-L (Smith and Nephew) is a biosynthetic wound dressing constructed of a silicon film with a nylon fabric partially imbedded into the film. The fabric creates a complex 3-dimensional structure of trifilament thread, which chemically binds collagen. Blood/sera clot in the nylon matrix, adhering the dressing to the wound until epithelialization occurs. FDA product code: FRO. Integra Dermal Regeneration Template (marketed as Omnigraft Dermal Regeneration Matrix; Integra LifeSciences) is a bovine, collagen/glycosaminoglycan dermal replacement covered by a silicone temporary epidermal substitute. It was approved by FDA for use in postexcisional treatment of lifethreatening full-thickness or deep partial-thickness thermal injury where sufficient autograft is not available at the time of excision or not desirable because of the physiologic condition of the patient. Integra Matrix Wound Dressing and Integra meshed Bilayer Wound Matrix are substantially equivalent skin substitutes was cleared for marketing by FDA through the 510(k) process for other indications. Integra Bilayer Wound Matrix (Integra LifeSciences) is designed to be used in conjunction with negative pressure wound therapy. The meshed bilayer provides a flexible wound covering and allows drainage of wound exudate. FDA product code: MDD. TransCyte (Advanced Tissue Sciences) consists of human dermal fibroblasts grown on nylon mesh, combined with a synthetic epidermal layer and was approved by FDA in TransCyte is intended as a temporary covering over burns until autografting is possible. It can also be used as a temporary covering for some burn wounds that heal without autografting. Synthetic Products Suprathel (PolyMedics Innovations) is a synthetic copolymer membrane fabricated from a tripolymer of polylactide, trimethylene carbonate, and s-caprolactone. It is used to provide temporary coverage of superficial dermal burns and wounds. Suprathel is covered with gauze and a dressing that is left in place until the wound has healed.
4 Effective Policy Date: April 15, 2017 Page: 4 of 13 POLICY STATEMENT Breast reconstructive surgery using allogeneic acellular dermal matrix products* (including each of the following: AlloDerm, AlloMax, AlloMend, DermaMatrix, FlexHD, Graftjacket ; see Policy Guidelines) may be considered medically necessary, when there is insufficient tissue expander or implant coverage by the pectoralis major muscle and additional coverage is required, when there is viable but compromised or thin postmastectomy skin flaps that are at risk of dehiscence or necrosis, or the inframammary fold and lateral mammary folds have been undermined during mastectomy and reestablishment of these landmarks is needed. Treatment of chronic, noninfected, full-thickness diabetic lower-extremity ulcers using the following tissueengineered skin substitutes may be considered medically necessary: AlloPatch * Apligraf ** Dermagraft ** Integra Dermal Regeneration Template Treatment of chronic, noninfected, partial- or full-thickness lower-extremity skin ulcers due to venous insufficiency, which have not adequately responded following a 1-month period of conventional ulcer therapy, using the following tissue-engineered skin substitutes may be considered medically necessary: Apligraf ** Oasis Wound Matrix*** Treatment of dystrophic epidermolysis bullosa using the following tissue-engineered skin substitutes may be considered medically necessary: OrCel (for the treatment of mitten-hand deformity when standard wound therapy has failed and when provided in accordance with the humanitarian device exemption (HDE) specifications of the U.S. Food and Drug Administration [FDA])**** Treatment of second- and third-degree burns using the following tissue-engineered skin substitutes may be considered medically necessary: Epicel (for the treatment of deep dermal or full-thickness burns comprising a total body surface area 30% when provided in accordance with the HDE specifications of the FDA)**** Integra Dermal Regeneration Template ** * Banked human tissue. ** FDA premarket approval. *** FDA 510(k) cleared. **** FDA-approved under an HDE. All other uses of the bioengineered skin and soft tissue substitutes listed above are considered investigational. All other skin and soft tissue substitutes not listed above are considered investigational, including, but not limited to: ACell UBM Hydrated/Lyophilized Wound Dressing AlloSkin AlloSkin RT Aongen Collagen Matrix Architect ECM, PX, FX ArthroFlex (Flex Graft) Atlas Wound Matrix
5 Effective Policy Date: April 15, 2017 Page: 5 of 13 Avagen Wound Dressing AxoGuard Nerve Protector (AxoGen) CellerateRX (CRXa ) CollaCare CollaCare Dental Collagen Wound Dressing (Oasis Research) CollaGUARD CollaMend CollaWound Collexa Collieva Conexa Coreleader Colla-Pad CorMatrix Cymetra (Micronized AlloDerm Cytal (previously MatriStem ) Dermadapt Wound Dressing DermaPure DermaSpan DressSkin Durepair Regeneration Matrix Endoform Dermal Template ENDURAGen Excellagen ExpressGraft E-Z Derm FlexiGraft GammaGraft Graftjacket Xpress, injectable Hyalomatrix Hyalomatrix PA hmatrix Integra Flowable Wound Matrix Integra Bilayer Wound Matrix Integra Omnigraft MariGen /Kerecis Omega3 MatriDerm Matrix HD Mediskin MemoDerm Microderm biologic wound matrix NeoForm NuCel Oasis Burn Matrix Oasis Wound Matrix Oasis Ultra Pelvicol /PelviSoft Permacol
6 Effective Policy Date: April 15, 2017 Page: 6 of 13 PriMatrix PriMatrix Dermal Repair Scaffold PuraPly Wound Matrix (previously FortaDerm ) PuraPly AM (Antimicrobial Wound Matrix) Puros Dermis RegenePro Repliform Repriza StrataGraft Strattice (xenograft) Suprathel SurgiMend Talymed TenoGlide TenSIX Acellular Dermal Matrix TissueMend TheraForm Standard/Sheet TheraSkin TruSkin Veritas Collagen Matrix XCM Biologic Tissue Matrix XenMatrix AB. POLICY GUIDELINES Note: Amniotic membrane and amniotic fluid products are reviewed in evidence review Clinical input indicated that the various acellular dermal matrix (ADM) products used in breast reconstruction have similar efficacy. The products listed are those that have been identified for use in breast reconstruction. Additional ADM products may become available for this indication. BENEFIT APPLICATION Experimental or investigational procedures, treatments, drugs, or devices are not covered (See General Exclusion Section of brochure). RATIONALE Summary of Evidence Acellular dermal matrix (ADM) products are also being evaluated for soft tissue repair. Breast Reconstruction For individuals who are undergoing breast reconstruction who receive allogeneic ADM products, the evidence incudes a randomized controlled trial (RCT) and systematic reviews. Relevant outcomes are symptoms, morbid events, functional outcomes, quality of life, and treatment-related morbidity. A recent systematic review found no difference in overall complication rates with ADM allograft compared to standard procedures for breast reconstruction. Reconstructions with ADM have been reported to have higher seroma, infection, and necrosis rates than reconstructions without ADM. However, capsular
7 Effective Policy Date: April 15, 2017 Page: 7 of 13 contracture and malposition of implants may be reduced. Thus, in cases where there is limited tissue coverage, including but not limited to when the use of ADM allows a single-stage reconstruction, the available evidence may be considered sufficient to permit conclusions about health outcomes that may inform patient decision making about reconstruction options. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. Tendon Repair For individuals who are undergoing tendon repair who receive Graftjacket ADM, the evidence incudes 1 RCT. Relevant outcomes are symptoms, morbid events, functional outcomes, quality of life, and treatment-related morbidity. One RCT identified found improved outcomes with Graftjacket ADM allograft for rotator cuff repair. Although these results were positive, additional study with a larger number of patients is needed to evaluate consistency of the effect. The evidence is insufficient to determine the effects of the technology on health outcomes. Surgical Repair of Hernias or Parastomal Reinforcement For individuals who are undergoing surgical repair of hernias or parastomal reinforcement who receive acellular collagen-based scaffolds, the evidence incudes RCTs. Relevant outcomes are symptoms, morbid events, functional outcomes, quality of life, and treatment-related morbidity. Several comparative studies including RCTs have shown no difference in outcomes between tissue-engineered skin substitutes and either standard synthetic mesh or no reinforcement. The evidence is sufficient to determine that the technology is unlikely to improve the net health outcome. Diabetic Lower-Extremity Ulcers For individuals who have diabetic lower-extremity ulcers who receive AlloPatch, Apligraf, Dermagraft, or Integra Dermal Regeneration Template, the evidence includes RCTs. Relevant outcomes are diseasespecific survival, symptoms, change in disease status, morbid events, and quality of life. RCTs have demonstrated the efficacy of AlloPatch (reticular ADM), Apligraf and Dermagraft (living cell therapy), and Integra Dermal Regeneration Template (biosynthetic) over the standard of care. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who have diabetic lower-extremity ulcers who receive other ADM products, cryopreserved skin allograft, or xenogenic skin substitutes, the evidence includes RCTs. Relevant outcomes are disease-specific survival, symptoms, change in disease status, morbid events, and quality of life. Additional study with a larger number of subjects is needed to compare the effect of other human ADM products, cryopreserved skin allograft (TheraSkin) and xenogenic skin substitutes (eg, Oasis Wound Matrix, PriMatrix) to the standard of care. The evidence is insufficient to determine the effects of the technology on health outcomes. Lower-Extremity Ulcers due to Venous Insufficiency For individuals who have lower-extremity ulcers due to venous insufficiency who receive Apligraf or Oasis Wound Matrix, the evidence includes RCTs. Relevant outcomes are disease-specific survival, symptoms, change in disease status, morbid events, and quality of life. RCTs have demonstrated the efficacy of Apligraf living cell therapy and xenogenic Oasis Wound Matrix over the standard of care. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who have lower-extremity ulcers due to venous insufficiency who receive bioengineered skin substitutes other than Apligraf or Oasis Wound Matrix, the evidence includes RCTs. Relevant outcomes are disease-specific survival, symptoms, change in disease status, morbid events, and quality of life. In a moderately large RCT, Dermagraft was not shown to be more effective than controls for the
8 Effective Policy Date: April 15, 2017 Page: 8 of 13 primary or secondary end points in the entire population and was only slightly more effective than controls (an 8%-15% increase in healing) in subgroups of patients with ulcer durations of 12 months or less or size of 10 cm or less. Additional study with a larger number of subjects is needed to evaluate the effect of the xenogenic PriMatrix skin substitute versus the current standard of care. The evidence is insufficient to determine the effects of the technology on health outcomes. Dystrophic Epidermolysis Bullosa For individuals who have dystrophic epidermolysis bullosa who receive OrCel, the evidence includes case series. Relevant outcomes are disease-specific survival, symptoms, change in disease status, morbid events, and quality of life. OrCel was approved under a humanitarian drug exemption for use in patients with dystrophic epidermolysis bullosa undergoing hand reconstruction surgery, to close and heal wounds created by the surgery, including those at donor sites. Outcomes have been reported in small series (eg, 5 patients). The evidence is insufficient to determine the effects of the technology on health outcomes. Deep Dermal Burns For individuals who have deep dermal burns who receive bioengineered skin substitutes (ie, Epicel, Integra Dermal Regeneration Template), the evidence includes RCTs. Relevant outcomes are symptoms, change in disease status, morbid events, functional outcomes, quality of life, and treatment-related morbidity. Overall, there are few skin substitutes approved, and the evidence is limited for each product. Epicel (living cell therapy) has received Food and Drug Administration approval under a humanitarian device exemption for the treatment of deep dermal or full-thickness burns comprising a total body surface area of 30% or more. Comparative studies have demonstrated improved outcomes for biosynthetic skin substitute Integra Dermal Regeneration Template for the treatment of burns. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. SUPPLEMENTAL INFORMATION Practice Guidelines and Position Statements American Society of Plastic Surgeons and Wound Healing Society A literature review for the 2013 guidelines from the American Society of Plastic Surgeons (ASPS) found that use of ADM, although increasingly common in postmastectomy expander/implant breast reconstruction, can result in increased risk of complications in the presence of certain risk factors. ASPS notes that cellular dermal matrix is currently used to increase soft tissue coverage, support the implant pocket, improve contour, and reduce pain with expansion. However, evidence to support these improved surgical outcomes are limited. Some evidence suggested that use of ADM is associated with increased postoperative complications, specifically related to infection and seroma. Overall, ASPS found that evidence on ADM products in postmastectomy expander/implant breast reconstruction was varied and conflicting, and gave a grade C recommendation based on level III evidence that surgeons should evaluate each clinical case individually and objectively determine the use of ADM. In 2006, ASPS endorsed guidelines from the Wound Healing Society (WHS) on the treatment of arterial insufficiency ulcers.the guidelines stated that extracellular matrix replacement therapy appears to be promising for mixed ulcers and may have a role as an adjuvant agent in arterial ulcers, but further study is required (level IIIC): Despite the existence of animal studies, case series, and a small number of random control trials to support biomaterial use for pressure ulcers, diabetic ulcers, and venous ulcers; there are no studies specifically on arterial ulcers. Therefore, studies in arterial ulcers must be conducted before the recommendation can be made. ASPS also endorsed WHS guidelines on the treatment of venous ulcers in The guidelines stated that various skin substitutes or biologically active dressings are
9 Effective Policy Date: April 15, 2017 Page: 9 of 13 emerging that provide temporary wound closure and serve as a source of stimuli (eg, growth factors) for healing of venous ulcers. Guideline 7b.1 stated that there is evidence that a bilayered artificial skin (biologically active dressing), used in conjunction with compression bandaging, increases the chance of healing a venous ulcer compared with compression and a simple dressing (level I). ASPS also endorsed WHS guidelines on the treatment of diabetic ulcers in The guidelines stated that healthy living skin cells assist in healing diabetic foot ulcers by releasing therapeutic amounts of growth factors, cytokines, and other proteins that stimulate the wound bed. Guideline stated that living skin equivalents may be of benefit in healing diabetic foot ulcers (level I). The 2007 ASPS guidelines on chronic wounds of the lower extremity stated that maintaining a moist environment, while simultaneously removing soluble factors detrimental to wound healing, might logically provide optimal conditions for wound healing. Classic dressings include gauze, foam, hydrocolloid, and hydrogels. Fluid-handling mechanisms include absorption, gelling, retention, and vapor transmission. Bioactive dressings include topical antimicrobials, bioengineered composite skin equivalent, bilaminar dermal regeneration template, and recombinant human growth factor. Not applicable. U.S. Preventive Services Task Force Recommendations Medicare National Coverage Centers for Medicare and Medicaid Services (CMS) issued the following national coverage determination: Porcine (pig) skin dressings are covered, if reasonable and necessary for the individual patient as an occlusive dressing for burns, donor sites of a homograft, and decubiti and other ulcers. Since 2014, CMS has no longer distinguished between different skin substitutes and classifies them as either high cost or low cost.89 CMS packages skin substitutes of the same class into the associated surgical procedures for hospital outpatient departments and ambulatory surgical centers. A separate payment might be made if the item is furnished on a different date of service as the primary service. REFERENCES 1. U.S. Food and Drug Administration. Guidance for industry: Chronic cutaneous ulcer and burn wounds. 2006; Accessed December 22, European Wound Management Association. Outcomes in controlled and comparative studies on non-healing wounds: recommendations to improve the quality of evidence in wound management. J Wound Care 2010; 19(6): Accessed December 22, Sheehan P, Jones P, Caselli A, et al. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. Jun 2003;26(6): PMID Davila AA, Seth AK, Wang E, et al. Human acellular dermis versus submuscular tissue expander breast reconstruction: a multivariate analysis of short-term complications. Arch Plast Surg. Jan 2013;40(1): PMID Lee KT, Mun GH. Updated Evidence of Acellular Dermal Matrix Use for Implant-Based Breast Reconstruction: A Meta-analysis. Ann Surg Oncol. Feb 2016;23(2): PMID
10 Effective Policy Date: April 15, 2017 Page: 10 of McCarthy CM, Lee CN, Halvorson EG, et al. The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter, blinded, randomized controlled trial. Plast Reconstr Surg. Nov 2012;130(5 Suppl 2):57S-66S. PMID Liu DZ, Mathes DW, Neligan PC, et al. Comparison of outcomes using AlloDerm versus FlexHD for implant-based breast reconstruction. Ann Plast Surg. May 2014;72(5): PMID Brooke S, Mesa J, Uluer M, et al. Complications in tissue expander breast reconstruction: a comparison of AlloDerm, DermaMatrix, and FlexHD acellular inferior pole dermal slings. Ann Plast Surg. Oct 2012;69(4): PMID Barber FA, Burns JP, Deutsch A, et al. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. Jan 2012;28(1):8-15. PMID Bellows CF, Smith A, Malsbury J, et al. Repair of incisional hernias with biological prosthesis: a systematic review of current evidence. Am J Surg. Jan 2013;205(1): PMID Zhong T, Janis JE, Ahmad J, et al. Outcomes after abdominal wall reconstruction using acellular dermal matrix: a systematic review. J Plast Reconstr Aesthet Surg. Dec 2011;64(12): PMID Espinosa-de-los-Monteros A, de la Torre JI, Marrero I, et al. Utilization of human cadaveric acellular dermis for abdominal hernia reconstruction. Ann Plast Surg. Mar 2007;58(3): PMID Gupta A, Zahriya K, Mullens PL, et al. Ventral herniorrhaphy: experience with two different biosynthetic mesh materials, Surgisis and Alloderm. Hernia. Oct 2006;10(5): PMID Bochicchio GV, De Castro GP, Bochicchio KM, et al. Comparison study of acellular dermal matrices in complicated hernia surgery. J Am Coll Surg. Oct 2013;217(4): PMID Bellows CF, Shadduck P, Helton WS, et al. Early report of a randomized comparative clinical trial of Strattice reconstructive tissue matrix to lightweight synthetic mesh in the repair of inguinal hernias. Hernia. Apr 2014;18(2): PMID Fleshman JW, Beck DE, Hyman N, et al. A prospective, multicenter, randomized, controlled study of non-crosslinked porcine acellular dermal matrix fascial sublay for parastomal reinforcement in patients undergoing surgery for permanent abdominal wall ostomies. Dis Colon Rectum. May 2014;57(5): PMID Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2016;2:CD PMID Martinson M, Martinson N. A comparative analysis of skin substitutes used in the management of diabetic foot ulcers. J Wound Care. Oct 2016;25(Sup10):S8-S17. PMID Veves A, Falanga V, Armstrong DG, et al. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. Feb 2001;24(2): PMID Blue Cross and Blue Shield Technology Evaluation Center (TEC). Graftskin for the treatment of skin ulcers. TEC Assessments. 2001;Volume 16:Tab Steinberg JS, Edmonds M, Hurley DP, Jr., et al. Confirmatory data from EU study supports Apligraf for the treatment of neuropathic diabetic foot ulcers. J Am Podiatr Med Assoc. Jan-Feb 2010;100(1): PMID Kirsner RS, Warriner R, Michela M, et al. Advanced biological therapies for diabetic foot ulcers. Arch Dermatol. Aug 2010;146(8): PMID Marston WA, Hanft J, Norwood P, et al. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. Jun 2003;26(6): PMID Frykberg RG, Marston WA, Cardinal M. The incidence of lower-extremity amputation and bone resection in diabetic foot ulcer patients treated with a human fibroblast-derived dermal substitute. Adv Skin Wound Care. Jan 2015;28(1): PMID Frykberg RG, Cazzell SM, Arroyo-Rivera J, et al. Evaluation of tissue engineering products for the management of neuropathic diabetic foot ulcers: an interim analysis. J Wound Care. Jul 2016;25 Suppl 7:S PMID Sanders L, Landsman AS, Landsman A, et al. A prospective, multicenter, randomized, controlled clinical trial comparing a bioengineered skin substitute to a human skin allograft. Ostomy Wound Manage. Sep 2014;60(9): PMID DiDomenico L, Landsman AR, Emch KJ, et al. A prospective comparison of diabetic foot ulcers treated with either a cryopreserved skin allograft or a bioengineered skin substitute. Wounds. Jul 2011;23(7): PMID Zelen CM, Orgill DP, Serena T, et al. A prospective, randomised, controlled, multicentre clinical trial examining healing rates, safety and cost to closure of an acellular reticular allogenic human dermis versus standard of care in the treatment of chronic diabetic foot ulcers. Int Wound J. Apr PMID
11 Effective Policy Date: April 15, 2017 Page: 11 of Brigido SA, Boc SF, Lopez RC. Effective management of major lower extremity wounds using an acellular regenerative tissue matrix: a pilot study. Orthopedics. Jan 2004;27(1 Suppl):s PMID Reyzelman A, Crews RT, Moore JC, et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. Int Wound J. Jun 2009;6(3): PMID Reyzelman AM, Bazarov I. Human acellular dermal wound matrix for treatment of DFU: literature review and analysis. J Wound Care. Mar 2015;24(3):128; PMID Brigido SA. The use of an acellular dermal regenerative tissue matrix in the treatment of lower extremity wounds: a prospective 16-week pilot study. Int Wound J. Sep 2006;3(3): PMID Walters J, Cazzell S, Pham H, et al. Healing rates in a multicenter assessment of a sterile, room temperature, acellular dermal matrix versus conventional care wound management and an active comparator in the treatment of full-thickness diabetic foot ulcers. Eplasty. 2016;16:e10. PMID Driver VR, Lavery LA, Reyzelman AM, et al. A clinical trial of Integra Template for diabetic foot ulcer treatment. Wound Repair Regen. Nov ;23(6): PMID Niezgoda JA, Van Gils CC, Frykberg RG, et al. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care. Jun 2005;18(5 Pt 1): PMID Kavros SJ, Dutra T, Gonzalez-Cruz R, et al. The use of PriMatrix, a fetal bovine acellular dermal matrix, in healing chronic diabetic foot ulcers: a prospective multicenter study. Adv Skin Wound Care. Aug 2014;27(8): PMID Karr JC. Retrospective comparison of diabetic foot ulcer and venous stasis ulcer healing outcome between a dermal repair scaffold (PriMatrix) and a bilayered living cell therapy (Apligraf). Adv Skin Wound Care. Mar 2011;24(3): PMID Falanga V, Margolis D, Alvarez O, et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Human Skin Equivalent Investigators Group. Arch Dermatol. Mar 1998;134(3): PMID Harding K, Sumner M, Cardinal M. A prospective, multicentre, randomised controlled study of human fibroblastderived dermal substitute (Dermagraft) in patients with venous leg ulcers. Int Wound J. Apr 2013;10(2): PMID Mostow EN, Haraway GD, Dalsing M, et al. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg. May 2005;41(5): PMID Romanelli M, Dini V, Bertone M, et al. OASIS wound matrix versus Hyaloskin in the treatment of difficult-to-heal wounds of mixed arterial/venous aetiology. Int Wound J. Mar 2007;4(1):3-7. PMID Romanelli M, Dini V, Bertone MS. Randomized comparison of OASIS wound matrix versus moist wound dressing in the treatment of difficult-to-heal wounds of mixed arterial/venous etiology. Adv Skin Wound Care. Jan 2010;23(1): PMID Fivenson DP, Scherschun L, Cohen LV. Apligraf in the treatment of severe mitten deformity associated with recessive dystrophic epidermolysis bullosa. Plast Reconstr Surg. Aug 2003;112(2): PMID Carsin H, Ainaud P, Le Bever H, et al. Cultured epithelial autografts in extensive burn coverage of severely traumatized patients: a five year single-center experience with 30 patients. Burns. Jun 2000;26(4): PMID Lagus H, Sarlomo-Rikala M, Bohling T, et al. Prospective study on burns treated with Integra(R), a cellulose sponge and split thickness skin graft: comparative clinical and histological study--randomized controlled trial. Burns. Dec 2013;39(8): PMID Branski LK, Herndon DN, Pereira C, et al. Longitudinal assessment of Integra in primary burn management: a randomized pediatric clinical trial. Crit Care Med. Nov 2007;35(11): PMID Heimbach DM, Warden GD, Luterman A, et al. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil. Jan-Feb 2003;24(1): PMID Still J, Glat P, Silverstein P, et al. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns. Dec 2003;29(8): PMID Lukish JR, Eichelberger MR, Newman KD, et al. The use of a bioactive skin substitute decreases length of stay for pediatric burn patients. J Pediatr Surg. Aug 2001;36(8): PMID Amani H, Dougherty WR, Blome-Eberwein S. Use of Transcyte and dermabrasion to treat burns reduces length of stay in burns of all size and etiology. Burns. Nov 2006;32(7): PMID Lazic T, Falanga V. Bioengineered skin constructs and their use in wound healing. Plast Reconstr Surg. Jan 2011;127 Suppl 1:75S-90S. PMID
12 Effective Policy Date: April 15, 2017 Page: 12 of Saffle JR. Closure of the excised burn wound: temporary skin substitutes. Clin Plast Surg. Oct 2009;36(4): PMID American Society of Plastic Surgeons. Evidence-Based Clinical Practice Guideline: Breast Reconstruction with Expanders and Implants. 2013; Accessed December 22, Hopf HW, Ueno C, Aslam R, et al. Guidelines for the treatment of arterial insufficiency ulcers. Wound Repair Regen. Nov-Dec 2006;14(6): PMID Robson MC, Cooper DM, Aslam R, et al. Guidelines for the treatment of venous ulcers. Wound Repair Regen. Nov-Dec 2006;14(6): PMID Steed DL, Attinger C, Colaizzi T, et al. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen. Nov- Dec 2006;14(6): PMID American Society of Plastic Surgeons (ASPS). Evidence-based Clinical Practice Guideline: Chronic Wounds of the Lower Extremity. 2007; Accessed December 22, National Institute for Health and Care Excellence (NICE). Diabetic Foot Problems: Prevention and Management [NG19]. 2015; Accessed December 22, Frykberg RG, Zgonis T, Armstrong DG, et al. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg. Sep-Oct 2006;45(5 Suppl):S1-66. PMID Lipsky BA, Berendt AR, Cornia PB, et al Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. Jun 2012;54(12):e PMID Centers for Medicare and Medicaid Services (CMS). Porcine skin and gradient pressure dressing Accessed December 22, Centers for Medicare and Medicaid Services (CMS). CMS issues hospital outpatient department and ambulatory surgical center policy and payment changes for ; Accessed December 22, POLICY HISTORY Date Action Description September 2011 New Policy March 2013 Update Policy Policy updated and scope expanded; policy statements added for other indications; title changed to Bio- Engineered Skin and Soft Tissue Substitutes March 2014 Update Policy Policy updated with literature search adding references 1, 13-15, 24, 36, 40, 48, 59, 66, and 68. First policy statement expanded to include other acellular dermal matrix products. March 2015 Update Policy Policy updated with literature review through December 3, 2014; references 2, 17, 27, 37, 41, 42, and 44 added; EpiFix considered medically necessary for treatment of diabetic foot ulcers was added to the policy statement. December 2016 Update Policy Policy updated with literature review through October 30, 2015; references added and renumbered. Clinical input reviewed. Integra Dermal Regeneration Template, Biovance and Grafix were added as medically necessary for the treatment of diabetic foot ulcers. TransCyte removed from the medically necessary statement; it is no longer commercially available. HCPCS codes updated. Acellular dermal matrix products used in breast reconstruction clarified; investigational list
13 Effective Policy Date: April 15, 2017 Page: 13 of 13 updated with new products and name changes; wound dressing products removed from list March 2017 Update Policy Policy updated with literature review through November 7, 2016; references 6, 19, 26, and added. Review of amniotic membrane products moved to evidence review ; section on laryngoplasty removed. Products with new HCPCS codes (Microderm, TruSkin) added to investigational statement; Unite Biomatrix no longer available; MatriStem renamed Cytal; FortaDerm renamed PuraPly. Rationale revised to focus on randomized controlled trials and some references removed. AlloMend added to medically necessary statement for breast reconstructive surgery. AlloPatch added to medically necessary statement for diabetic lower-extremity ulcers.
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2018 Origination: 4/2008 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2018 Origination: 4/2008 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Bioengineered Skin and Soft Tissue Substitutes
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: July 15, 2018 Related Policies: 7.01.149 Amniotic Membrane and Amniotic Fluid Bioengineered Skin and Soft Tissue Substitutes Description Bioengineered skin and
FEP Medical Policy Manual Effective Date: July 15, 2018 Related Policies: 7.01.149 Amniotic Membrane and Amniotic Fluid Bioengineered Skin and Soft Tissue Substitutes Description Bioengineered skin and
Medical Policy. MP Bioengineered Skin and Soft Tissue Substitutes
 Medical Policy MP 7.01.113 BCBSA Ref. Policy: 7.01.113 Last Review: 02/26/2018 Effective Date: 05/30/2018 Section: Surgery Related Policies 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors
Medical Policy MP 7.01.113 BCBSA Ref. Policy: 7.01.113 Last Review: 02/26/2018 Effective Date: 05/30/2018 Section: Surgery Related Policies 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Bio-Engineered Skin and Page 1 of 62 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Bio-Engineered Skin and Amniotic Membrane and Amniotic Fluid medical
Bio-Engineered Skin and Page 1 of 62 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Bio-Engineered Skin and Amniotic Membrane and Amniotic Fluid medical
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/28/2017 Section:
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/28/2017 Section:
Tissue-Engineered Skin Substitutes
 Tissue-Engineered Skin Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO 06/01/2012 Section: Surgery Place(s) of Service: Outpatient/Office
Tissue-Engineered Skin Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO 06/01/2012 Section: Surgery Place(s) of Service: Outpatient/Office
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2014 Origination: 4/2008 Next Review: 3/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2014 Origination: 4/2008 Next Review: 3/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy
 Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy File Name: Bioengineered Skin and Soft Tissue Substitutes File Code: UM.SURG.16 Origination: 09/2016 Last Review: 10/2018 Next Review:
Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy File Name: Bioengineered Skin and Soft Tissue Substitutes File Code: UM.SURG.16 Origination: 09/2016 Last Review: 10/2018 Next Review:
Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 03/12
 Protocol Bio-Engineered Skin and Soft Tissue Substitutes (701113) Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 03/12 The following Protocol contains
Protocol Bio-Engineered Skin and Soft Tissue Substitutes (701113) Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 03/12 The following Protocol contains
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 10/01/2013 Section: Surgery Place(s)
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 10/01/2013 Section: Surgery Place(s)
Policy Specific Section: October 7, 2011 January 30, 2015
 Medical Policy Bio-Engineered Skin and Soft Tissue Substitutes Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Surgery Original Policy Date: Effective Date: October
Medical Policy Bio-Engineered Skin and Soft Tissue Substitutes Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Surgery Original Policy Date: Effective Date: October
MEDICAL POLICY No R8 SKIN SUBSTITUTES & SOFT TISSUE GRAFTS
 Summary of Changes MEDICAL POLICY SKIN SUBSTITUTES & SOFT TISSUE GRAFTS Effective Date: August 25, 2017 Review Dates: 12/08, 8/09, 8/10, 8/11, 8/12, 4/13, 5/14, 5/15, 5/16, 5/17 Date Of Origin: December
Summary of Changes MEDICAL POLICY SKIN SUBSTITUTES & SOFT TISSUE GRAFTS Effective Date: August 25, 2017 Review Dates: 12/08, 8/09, 8/10, 8/11, 8/12, 4/13, 5/14, 5/15, 5/16, 5/17 Date Of Origin: December
Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association
 Bio-Engineered Skin and Page 1 of 58 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See Also: Bio-Engineered Skin and Periodontal Soft Tissue Grafting dental
Bio-Engineered Skin and Page 1 of 58 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See Also: Bio-Engineered Skin and Periodontal Soft Tissue Grafting dental
See Policy CPT/HCPCS CODE section below for any prior authorization requirements
 Effective Date: 1/1/2019 Section: MED Policy No: 378 Medical Officer 1/1/2019 Date Technology Assessment Committee Approved Date: 3/09; 10/14; 1/16 Medical Policy Committee Approved Date: 4/02; 4/03; 9/04;
Effective Date: 1/1/2019 Section: MED Policy No: 378 Medical Officer 1/1/2019 Date Technology Assessment Committee Approved Date: 3/09; 10/14; 1/16 Medical Policy Committee Approved Date: 4/02; 4/03; 9/04;
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Acellular dermal allografts for arthroplasty, 633 642 for plantar soft tissue augmentation, 543 555 Achilles tendon repair extracellular matrix
Note: Page numbers of article titles are in boldface type. A Acellular dermal allografts for arthroplasty, 633 642 for plantar soft tissue augmentation, 543 555 Achilles tendon repair extracellular matrix
What s New Medical Policy Updates April 2017
 What s New Medical Policy Updates April 2017 Listed below are the recent changes made to policies within the Geisinger Health Plan Medical Policy Portfolio during the month of March that will become effective
What s New Medical Policy Updates April 2017 Listed below are the recent changes made to policies within the Geisinger Health Plan Medical Policy Portfolio during the month of March that will become effective
Clinical Policy: EpiFix Wound Treatment
 Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
The Georgetown Team Approach to Diabetic Limb Salvage: 2013
 The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
MOVE BEYOND. to new hope for enterocutaneous fistulas. Enterocutaneous Fistula Repair
 MOVE BEYOND to new hope for enterocutaneous fistulas. Enterocutaneous Fistula Repair to an alternative treatment option Enterocutaneous fistulas can significantly affect patient health and quality of life.
MOVE BEYOND to new hope for enterocutaneous fistulas. Enterocutaneous Fistula Repair to an alternative treatment option Enterocutaneous fistulas can significantly affect patient health and quality of life.
Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds
 Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds WINDY COLE, DPM ADJUNCT PROFESSOR AND DIRECTOR OF WOUND CARE RESEARCH KSUCPM MEDICAL DIRECTOR, WOUND CENTER, UH AHUJA HOSPITAL Goals
Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds WINDY COLE, DPM ADJUNCT PROFESSOR AND DIRECTOR OF WOUND CARE RESEARCH KSUCPM MEDICAL DIRECTOR, WOUND CENTER, UH AHUJA HOSPITAL Goals
Skin Substitutes for Wound Care AHM
 Skin Substitutes for Wound Care AHM Clinical Indications Any 1 or more of the following products for wound care are considered medically necessary if the individual criteria are met Apligraf (graftskin)
Skin Substitutes for Wound Care AHM Clinical Indications Any 1 or more of the following products for wound care are considered medically necessary if the individual criteria are met Apligraf (graftskin)
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL Policy Number: MP.083.MH Last Review Date: 11/03/2016 Effective Date: 01/01/2017
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Skin Replacement Therapy for Chronic Non healing Wounds in the Outpatient Setting MP-032-MD-DE Medical Management Provider
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Skin Replacement Therapy for Chronic Non healing Wounds in the Outpatient Setting MP-032-MD-DE Medical Management Provider
Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds
 Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds D. Heath Stacey, MD Northwest Arkansas Center for Plastic Surgery, Fayetteville, Ark Correspondence: dheathstacey@gmail.com Keywords:
Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds D. Heath Stacey, MD Northwest Arkansas Center for Plastic Surgery, Fayetteville, Ark Correspondence: dheathstacey@gmail.com Keywords:
Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid
 02-10000-11 Original Effective Date: 01/01/01 Reviewed: 03/22/18 Revised: 01/01/19 Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid THIS MEDICAL COVERAGE GUIDELINE
02-10000-11 Original Effective Date: 01/01/01 Reviewed: 03/22/18 Revised: 01/01/19 Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid THIS MEDICAL COVERAGE GUIDELINE
MP.083.MH Skin Substitutes- Human Skin Equivalents
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures
 POLICY: PG0203 ORIGINAL EFFECTIVE: 01/15/09 LAST REVIEW: 03/13/18 MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures GUIDELINES This policy does not certify benefits or authorization of benefits,
POLICY: PG0203 ORIGINAL EFFECTIVE: 01/15/09 LAST REVIEW: 03/13/18 MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures GUIDELINES This policy does not certify benefits or authorization of benefits,
IHCP banner page. IHCP to cover CPT code IHCP to cover CPT code INDIANA HEALTH COVERAGE PROGRAMS BR JULY 3, 2018
 IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR201827 JULY 3, 2018 IHCP to cover CPT 93050 Effective August 3, 2018, the Indiana Health Coverage Programs (IHCP) will cover Current Procedural Terminology
IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR201827 JULY 3, 2018 IHCP to cover CPT 93050 Effective August 3, 2018, the Indiana Health Coverage Programs (IHCP) will cover Current Procedural Terminology
Equine Pericardium Collagen Wound Dressing in the Treatment of the Neuropathic Diabetic Foot Wound
 ORIGINAL ARTICLES Equine Pericardium Collagen Wound Dressing in the Treatment of the Neuropathic Diabetic Foot Wound A Pilot Study John G. Fleischli, DPM* Terese J. Laughlin, DPM* Jeffery W. Fleischli,
ORIGINAL ARTICLES Equine Pericardium Collagen Wound Dressing in the Treatment of the Neuropathic Diabetic Foot Wound A Pilot Study John G. Fleischli, DPM* Terese J. Laughlin, DPM* Jeffery W. Fleischli,
Number: (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end
 1 of 278 Number: 0244 (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. I. Aetna considers the following products for wound care medically necessary according
1 of 278 Number: 0244 (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. I. Aetna considers the following products for wound care medically necessary according
WOUND CARE UPDATE. -Commonly Used Skin Substitute Products For Wound. -Total Contact Casting. Jack W. Hutter DPM, FACFAS, C. ped.
 WOUND CARE UPDATE -Commonly Used Skin Substitute Products For Wound Closure -Total Contact Casting Jack W. Hutter DPM, FACFAS, C. ped. Commonly Used Skin Substitute Products for Wound Closure why are they
WOUND CARE UPDATE -Commonly Used Skin Substitute Products For Wound Closure -Total Contact Casting Jack W. Hutter DPM, FACFAS, C. ped. Commonly Used Skin Substitute Products for Wound Closure why are they
Smart Solutions for Serious Wounds. An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers.
 Smart Solutions for Serious Wounds An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers. A New Approach to Diabetic Foot Ulcer Care Supported by Over Two
Smart Solutions for Serious Wounds An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers. A New Approach to Diabetic Foot Ulcer Care Supported by Over Two
Regenerative Tissue Matrix in Treatment of Wounds
 Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
WPS Medicare Policy Primer
 WPS Medicare Policy Primer Medicare Jurisdiction (J5 & J8) NE, KS, IA, MO, IN, & MI Retired skin substitute LCD 3/2016 Indications Apligraf Non-infected partial and full-thickness skin ulcers due to VSU
WPS Medicare Policy Primer Medicare Jurisdiction (J5 & J8) NE, KS, IA, MO, IN, & MI Retired skin substitute LCD 3/2016 Indications Apligraf Non-infected partial and full-thickness skin ulcers due to VSU
Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU)
 Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU) Sami Khan, MD FACS Associate Professor of Surgery Division of Plastic and Reconstructive Surgery SUNY-Stony
Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU) Sami Khan, MD FACS Associate Professor of Surgery Division of Plastic and Reconstructive Surgery SUNY-Stony
Integra. PriMatrix Dermal Repair Scaffold PATIENT INFORMATION. Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1
 Integra PriMatrix Dermal Repair Scaffold PATIENT INFORMATION Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Your Path to Recovery Your health care provider has chosen to use
Integra PriMatrix Dermal Repair Scaffold PATIENT INFORMATION Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Your Path to Recovery Your health care provider has chosen to use
Is there an icd 10 code for smoking vapor
 Is there an icd 10 code for smoking vapor Thanks for your response, I'm just thinking that 305.1 is tobacco use and there is no tobacco in the e-cig. Just the nicotine and other chemicals. I have been
Is there an icd 10 code for smoking vapor Thanks for your response, I'm just thinking that 305.1 is tobacco use and there is no tobacco in the e-cig. Just the nicotine and other chemicals. I have been
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP075 Section: Medical Benefit Policy Subject: Tissue Engineered Skin Substitutes I. Policy: Tissue Engineered Skin Substitutes II. Purpose/Objective: To provide a
POLICIES AND PROCEDURE MANUAL Policy: MP075 Section: Medical Benefit Policy Subject: Tissue Engineered Skin Substitutes I. Policy: Tissue Engineered Skin Substitutes II. Purpose/Objective: To provide a
SKIN AND SOFT TISSUE SUBSTITUTES
 SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Community Plan Medical Policy Policy Number: CS153.B Effective Date: November 1, 2018 Instructions for Use Table of Contents Page COVERAGE RATIONALE...
SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Community Plan Medical Policy Policy Number: CS153.B Effective Date: November 1, 2018 Instructions for Use Table of Contents Page COVERAGE RATIONALE...
Cigna Medical Coverage Policy
 Cigna Medical Coverage Policy Subject Tissue-Engineered Skin Substitutes Table of Contents Coverage Policy... 1 General Background... 15 Coding/Billing Information... 62 References... 66 Effective Date...
Cigna Medical Coverage Policy Subject Tissue-Engineered Skin Substitutes Table of Contents Coverage Policy... 1 General Background... 15 Coding/Billing Information... 62 References... 66 Effective Date...
Dressings do not heal wounds properly selected dressings enhance the body s ability to heal the wound. Progression Towards Healing
 Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
SKIN AND SOFT TISSUE SUBSTITUTES
 SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Commercial Medical Policy Policy Number: 2018T0592A Effective Date: October 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Commercial Medical Policy Policy Number: 2018T0592A Effective Date: October 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
Wound & Burn. Reimbursement & Coding Guide
 Wound & Burn Reimbursement & Coding Guide Wound & Burn Reimbursement and Coding Guide MicroMatrix and Cytal devices facilitate the remodeling of functional, site-appropriate tissue. Comprised of ACell
Wound & Burn Reimbursement & Coding Guide Wound & Burn Reimbursement and Coding Guide MicroMatrix and Cytal devices facilitate the remodeling of functional, site-appropriate tissue. Comprised of ACell
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Case Report Treatment of severe burn with DermACELL, an acellular dermal matrix
 Int J Burn Trauma 2012;2(2):105-109 www.ijbt.org /ISSN: 2160-2126/IJBT1208002 Case Report Treatment of severe burn with DermACELL, an acellular dermal matrix Shyi-Gen Chen, Yuan-Sheng Tzeng, Chih-Hsin
Int J Burn Trauma 2012;2(2):105-109 www.ijbt.org /ISSN: 2160-2126/IJBT1208002 Case Report Treatment of severe burn with DermACELL, an acellular dermal matrix Shyi-Gen Chen, Yuan-Sheng Tzeng, Chih-Hsin
Diabetic Foot Ulcer Treatment and Prevention
 Diabetic Foot Ulcer Treatment and Prevention Alexander Reyzelman DPM, FACFAS Associate Professor California School of Podiatric Medicine at Samuel Merritt University Diabetic Foot Ulcers One of the most
Diabetic Foot Ulcer Treatment and Prevention Alexander Reyzelman DPM, FACFAS Associate Professor California School of Podiatric Medicine at Samuel Merritt University Diabetic Foot Ulcers One of the most
Integra. Tissue Technologies. Limit uncertainty with a leader in collagen technology
 Limit uncertainty with a leader in collagen technology Integra Dermal Regeneration Template PriMatrix Dermal Repair Scaffold A Pioneer in Regenerative Medicine Integra LifeSciences, a wordwide leader in
Limit uncertainty with a leader in collagen technology Integra Dermal Regeneration Template PriMatrix Dermal Repair Scaffold A Pioneer in Regenerative Medicine Integra LifeSciences, a wordwide leader in
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: July15, 2017 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors for Healing and Other Non Orthopedic Conditions 7.01.113 Bioengineered
FEP Medical Policy Manual Effective Date: July15, 2017 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors for Healing and Other Non Orthopedic Conditions 7.01.113 Bioengineered
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: April 15, 2018 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors for Healing and Other Non Orthopedic Conditions 7.01.113 Bioengineered
FEP Medical Policy Manual Effective Date: April 15, 2018 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors for Healing and Other Non Orthopedic Conditions 7.01.113 Bioengineered
REPRESENTATIVE REPORTS USING DERMACELL AWM
 REPRESENTATIVE REPORTS USING DERMACELL AWM Documents available upon request. Clinical Reports using DermACELL AWM in Advanced Wound Management Published & Peer-Reviewed Articles, Posters, & Abstracts from
REPRESENTATIVE REPORTS USING DERMACELL AWM Documents available upon request. Clinical Reports using DermACELL AWM in Advanced Wound Management Published & Peer-Reviewed Articles, Posters, & Abstracts from
Jeffrey A. Ross, DPM, MD, FACFAS Associate Professor Baylor College of Medicine Houston, Texas DFCon 2018 Houston, Texas
 Jeffrey A. Ross, DPM, MD, FACFAS Associate Professor Baylor College of Medicine Houston, Texas DFCon 2018 Houston, Texas A Bridge to Healing S.T.E.P Save The Extremity Program Brooklyn Bridge Abnormal
Jeffrey A. Ross, DPM, MD, FACFAS Associate Professor Baylor College of Medicine Houston, Texas DFCon 2018 Houston, Texas A Bridge to Healing S.T.E.P Save The Extremity Program Brooklyn Bridge Abnormal
Application Guide for Full-Thickness Wounds
 Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
Use of AlloPatch Pliable, a Human Acellular Dermal Matrix, as an Adjunctive Therapy for Chronic Non-Healing Diabetic Foot Ulcers
 Use of AlloPatch Pliable, a Human Acellular Dermal Matrix, as an Adjunctive Therapy for Chronic Non-Healing Diabetic Foot Ulcers Case Studies and Clinical Review Charles M Zelen, DPM FACFAS 1, Jarrod Kaufman,
Use of AlloPatch Pliable, a Human Acellular Dermal Matrix, as an Adjunctive Therapy for Chronic Non-Healing Diabetic Foot Ulcers Case Studies and Clinical Review Charles M Zelen, DPM FACFAS 1, Jarrod Kaufman,
Treating Massive Rotator Cuff Tears and Revisions
 Treating Massive Rotator Cuff Tears and Revisions The solutions leading surgeons are using to help achieve higher success rates and better patient outcomes. Shoulder Restoration System To learn more about
Treating Massive Rotator Cuff Tears and Revisions The solutions leading surgeons are using to help achieve higher success rates and better patient outcomes. Shoulder Restoration System To learn more about
THE WOUND SOLUTION. (Freeze dried Acellular Dermal Matrix) (Cryopreserved Acellular Dermal Matrix)
 (Freeze dried Acellular Dermal Matrix) (Cryopreserved Acellular Dermal Matrix) What is / CGCryoDerm?? /CGCryoDerm are processed by the CGBio co. and is available through Daewoong Bio. /CGCryoDerm are an
(Freeze dried Acellular Dermal Matrix) (Cryopreserved Acellular Dermal Matrix) What is / CGCryoDerm?? /CGCryoDerm are processed by the CGBio co. and is available through Daewoong Bio. /CGCryoDerm are an
A Comparative Study of CG CryoDerm and AlloDerm in Direct-to-Implant Immediate Breast Reconstruction
 A Comparative Study of CG CryoDerm and AlloDerm in Direct-to-Implant Immediate Breast Reconstruction Original Article Jun Ho Lee 1, Ki Rin Park 1, Tae Gon Kim 1, Ju-Ho Ha 1, Kyu-Jin Chung 1, Yong-Ha Kim
A Comparative Study of CG CryoDerm and AlloDerm in Direct-to-Implant Immediate Breast Reconstruction Original Article Jun Ho Lee 1, Ki Rin Park 1, Tae Gon Kim 1, Ju-Ho Ha 1, Kyu-Jin Chung 1, Yong-Ha Kim
Equine Pericardium as a Biological Covering for the Treatment of Diabetic Foot Wounds
 ORIGINAL ARTICLES Equine Pericardium as a Biological Covering for the Treatment of Diabetic Foot Wounds A Prospective Study Jeffery H. Alexander, DPM* David A. Yeager, DPM Dean S. Stern, DPM* Carlo A.
ORIGINAL ARTICLES Equine Pericardium as a Biological Covering for the Treatment of Diabetic Foot Wounds A Prospective Study Jeffery H. Alexander, DPM* David A. Yeager, DPM Dean S. Stern, DPM* Carlo A.
Care of Wound Reconstruction Patients, 2018 ed.
 Care of Wound Reconstruction Patients, 2018 ed. STEVE GROSSO, MD, FACS BILLINGS PLASTIC SURGERY A Smorgasbord of Plastic Surgery Stuff Disclaimer/Conflicts of Interest It is not possible to discuss wound
Care of Wound Reconstruction Patients, 2018 ed. STEVE GROSSO, MD, FACS BILLINGS PLASTIC SURGERY A Smorgasbord of Plastic Surgery Stuff Disclaimer/Conflicts of Interest It is not possible to discuss wound
BREAST RECONSTRUCTION/REMOVAL AND REPLACEMENT OF IMPLANTS
 Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Wright Medical Technology, Inc Airline Road Arlington, TN phone toll-free
 References 1 Brigido SA, Boc SF, Lopez RC. Effective Management of Major Lower Extremity Wounds Using an Acellular Regenerative Tissue Matrix: A Pilot Study. Orthopedics 2004; 27(1S): pp145-149. 2 Brigido
References 1 Brigido SA, Boc SF, Lopez RC. Effective Management of Major Lower Extremity Wounds Using an Acellular Regenerative Tissue Matrix: A Pilot Study. Orthopedics 2004; 27(1S): pp145-149. 2 Brigido
Single-Stage Full-Thickness Scalp Reconstruction Using Acellular Dermal Matrix and Skin Graft
 Single-Stage Full-Thickness Scalp Reconstruction Using Acellular Dermal Matrix and Skin Graft Yoon S. Chun, MD, a and Kapil Verma, BA b a Division of Plastic and Reconstructive Surgery, Department of Surgery,
Single-Stage Full-Thickness Scalp Reconstruction Using Acellular Dermal Matrix and Skin Graft Yoon S. Chun, MD, a and Kapil Verma, BA b a Division of Plastic and Reconstructive Surgery, Department of Surgery,
AlloMend AlloSkin TM AlloWrap DS. AlloSkin TM AC AlloSkin TM RT AlloSkin TM REGENERATIVE WOUND CARE JOINT OPERATIONS LLC
 Allosource-Woundcare-no prices_allosource Price List 15/12/2015 17:54 Page 1 AlloMend AlloSkin TM AlloWrap DS AlloSkin TM AC AlloSkin TM RT AlloSkin TM JOINT OPERATIONS LLC Suite 20, Anchor Business Centre,
Allosource-Woundcare-no prices_allosource Price List 15/12/2015 17:54 Page 1 AlloMend AlloSkin TM AlloWrap DS AlloSkin TM AC AlloSkin TM RT AlloSkin TM JOINT OPERATIONS LLC Suite 20, Anchor Business Centre,
Lower Extremity Wound Evaluation and Treatment
 Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18
 Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18 See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18 See Important Reminder at the end of this policy for important regulatory and
The choice for aesthetic and functional results in loss of substances 1 SKIN EXPERTISE WITH A NEW COLLAGEN TECHNOLOGY
 The choice for aesthetic and functional results in loss of substances 1 SKIN EXPERTISE WITH A NEW COLLAGEN TECHNOLOGY SYMATESE: OUR EXPERTISE IN THE FIELD OF COLLAGEN AND SKIN SYMATESE GROUP is recognized
The choice for aesthetic and functional results in loss of substances 1 SKIN EXPERTISE WITH A NEW COLLAGEN TECHNOLOGY SYMATESE: OUR EXPERTISE IN THE FIELD OF COLLAGEN AND SKIN SYMATESE GROUP is recognized
QUICK GUIDE SNAP THERAPY SYSTEM
 QUICK GUIDE SNAP THERAPY SYSTEM Clinical Pathway to SNAP System Full holistic assessment of patient and wound Is the wound type indicated for NPWT use without contraindications 1? SNAP System is indicated
QUICK GUIDE SNAP THERAPY SYSTEM Clinical Pathway to SNAP System Full holistic assessment of patient and wound Is the wound type indicated for NPWT use without contraindications 1? SNAP System is indicated
NovoSorb BTM. A unique synthetic biodegradable wound scaffold. Regenerating tissue. Changing lives.
 NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
Patient Care Information
 Patient Care Information A Guide to Healing Diabetic Foot Ulcers Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Dermal Regeneration Matrix Overview Diabetic foot ulcers are
Patient Care Information A Guide to Healing Diabetic Foot Ulcers Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Dermal Regeneration Matrix Overview Diabetic foot ulcers are
FUTURE Local Coverage Determination (LCD): Application of Bioengineered Skin Substitutes to Lower Extremity Chronic Non-Healing Wounds (L35041)
 FUTURE Local Coverage Determination (LCD): Application of Bioengineered Skin Substitutes to Lower Extremity Chronic Non-Healing Wounds (L35041) Links in PDF documents are not guaranteed to work. To follow
FUTURE Local Coverage Determination (LCD): Application of Bioengineered Skin Substitutes to Lower Extremity Chronic Non-Healing Wounds (L35041) Links in PDF documents are not guaranteed to work. To follow
Allograft Based Breast Reconstruction: Opportunity for a Second Look
 Allograft Based Breast Reconstruction: Opportunity for a Second Look Martin I. Newman, MD, FACS Director of Resident Education and Associate Program Director Department of Plastic and Reconstructive Surgery
Allograft Based Breast Reconstruction: Opportunity for a Second Look Martin I. Newman, MD, FACS Director of Resident Education and Associate Program Director Department of Plastic and Reconstructive Surgery
ALLIANCE OF WOUND CARE STAKEHOLDERS. Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015
 ALLIANCE OF WOUND CARE STAKEHOLDERS Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015 1 ALLIANCE OF WOUND CARE Who is the Alliance? STAKEHOLDERS A non-profit
ALLIANCE OF WOUND CARE STAKEHOLDERS Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015 1 ALLIANCE OF WOUND CARE Who is the Alliance? STAKEHOLDERS A non-profit
Original Article Tissue engineered skin for diabetic foot ulcers: a meta-analysis
 Int J Clin Exp Med 2015;8(10):18191-18196 www.ijcem.com /ISSN:1940-5901/IJCEM0014309 Original Article Tissue engineered skin for diabetic foot ulcers: a meta-analysis Xuedong Li 1, Geliang Xu 2, Jianqiu
Int J Clin Exp Med 2015;8(10):18191-18196 www.ijcem.com /ISSN:1940-5901/IJCEM0014309 Original Article Tissue engineered skin for diabetic foot ulcers: a meta-analysis Xuedong Li 1, Geliang Xu 2, Jianqiu
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons
 Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander.
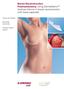 Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
The Use of the. in Clinical Practice
 The Use of the SNAP Therapy System in Clinical Practice It s an ultraportable, mechanically-powered disposable NPWT. By Animesh Bhatia, DPM, CWS This article is written exclusively for PM and appears courtesy
The Use of the SNAP Therapy System in Clinical Practice It s an ultraportable, mechanically-powered disposable NPWT. By Animesh Bhatia, DPM, CWS This article is written exclusively for PM and appears courtesy
NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION
 NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION 42 yo female healthy athlete Right breast mass. Past medical history: none Family history: aunt with Breast cancer Candidates for nipple-sparing mastectomy
NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION 42 yo female healthy athlete Right breast mass. Past medical history: none Family history: aunt with Breast cancer Candidates for nipple-sparing mastectomy
Clinical Summary. Introduction. What are Scars? There are three main types of scars:
 Introduction Clinical Summary Since the introduction of silicone in the early 1980s for the prevention and treatment of hypertrophic & keloid scars, it s therapeutic effects have been well documented in
Introduction Clinical Summary Since the introduction of silicone in the early 1980s for the prevention and treatment of hypertrophic & keloid scars, it s therapeutic effects have been well documented in
HUMAN AMNIOTIC MEMBRANE GRAFTS TO ENHANCE HEALING. Frank Burrows, MBA, EMT David Mason, MD
 HUMAN AMNIOTIC MEMBRANE GRAFTS TO ENHANCE HEALING Frank Burrows, MBA, EMT David Mason, MD Good Wound Care Treat the whole patient, not just the hole in the patient Ensure adequate perfusion to wound site
HUMAN AMNIOTIC MEMBRANE GRAFTS TO ENHANCE HEALING Frank Burrows, MBA, EMT David Mason, MD Good Wound Care Treat the whole patient, not just the hole in the patient Ensure adequate perfusion to wound site
D-WOUND SOLUTION. From the start to completion of wound healing
 From the start to completion of wound healing From the start to completion of wound healing Treatment for ALL type of wound Burn, Pressure ulcer Diabetic Foot ulcer Scar prevention 244, Galmachi-ro, Jungwon-gu,
From the start to completion of wound healing From the start to completion of wound healing Treatment for ALL type of wound Burn, Pressure ulcer Diabetic Foot ulcer Scar prevention 244, Galmachi-ro, Jungwon-gu,
MY STRATEGY FOR TREATING BURN INJURIES. Warren Garner MD FACS Keck School of Medicine at USC Los Angeles, CA
 MY STRATEGY FOR TREATING BURN INJURIES Warren Garner MD FACS Keck School of Medicine at USC Los Angeles, CA ASSUMPTIONS: Burns which heal to normal have best outcome. Medical risk, functional recovery,
MY STRATEGY FOR TREATING BURN INJURIES Warren Garner MD FACS Keck School of Medicine at USC Los Angeles, CA ASSUMPTIONS: Burns which heal to normal have best outcome. Medical risk, functional recovery,
Infectious Complications Leading to Explantation in Implant-Based Breast Reconstruction With AlloDerm
 Infectious Complications Leading to Explantation in Implant-Based Breast Reconstruction With AlloDerm Minh-Doan Nguyen, MD, PhD, a Chen Chen, MS, b Salih Colakoğlu, MD, b Donald J. Morris, MD, b Adam M.
Infectious Complications Leading to Explantation in Implant-Based Breast Reconstruction With AlloDerm Minh-Doan Nguyen, MD, PhD, a Chen Chen, MS, b Salih Colakoğlu, MD, b Donald J. Morris, MD, b Adam M.
Fish Skin Grafts Promote Superior Cell Ingrowth Compared to Amnion Allografts, Human Cadaver Skin and Mammalian Extracellular Matrix (ECM)
 Fish Skin Grafts Promote Superior Cell Ingrowth Compared to Amnion Allografts, Human Cadaver Skin and Mammalian Extracellular Matrix (ECM) Christopher L. Winters, DPM American Health Network Indianapolis,
Fish Skin Grafts Promote Superior Cell Ingrowth Compared to Amnion Allografts, Human Cadaver Skin and Mammalian Extracellular Matrix (ECM) Christopher L. Winters, DPM American Health Network Indianapolis,
The management of chronic
 Amniotic Membranes for Diabetic Foot Ulcerations Here s an update on their use. By Alison Migonis, DPM and John M. Giurini, DPM The management of chronic diabetic foot wounds poses a significant economic
Amniotic Membranes for Diabetic Foot Ulcerations Here s an update on their use. By Alison Migonis, DPM and John M. Giurini, DPM The management of chronic diabetic foot wounds poses a significant economic
Coding for Wound Care
 Coding for Wound Care ****IMPORTANT*** Disclaimer ***Information provided is to the best of our knowledge and as current as possible. ***Please verify all policy and reimbursement information with your
Coding for Wound Care ****IMPORTANT*** Disclaimer ***Information provided is to the best of our knowledge and as current as possible. ***Please verify all policy and reimbursement information with your
The Strong New Choice MILLION. From A Provider. You Already Know
 The Strong New Choice MILLION From A Provider You Already Know MILLION With more than three million implants distributed with zero incidence of implantassociated infection, the Tutoplast Tissue Sterilization
The Strong New Choice MILLION From A Provider You Already Know MILLION With more than three million implants distributed with zero incidence of implantassociated infection, the Tutoplast Tissue Sterilization
BASICS OF BURN MANAGEMENT
 BASICS OF BURN MANAGEMENT Dr S M Keswani Cosmetic Surgeon National Burns Centre, Airoli,Navi-Mumbai Breach Candy Hospital Wockhardt Hospital National Burns Centre, Airoli, Navi-Mumbai. CLASSIFICATION 1.
BASICS OF BURN MANAGEMENT Dr S M Keswani Cosmetic Surgeon National Burns Centre, Airoli,Navi-Mumbai Breach Candy Hospital Wockhardt Hospital National Burns Centre, Airoli, Navi-Mumbai. CLASSIFICATION 1.
Hernia & Surgical. Reimbursement & Coding Guide
 Hernia & Surgical Reimbursement & Coding Guide Hernia and General Surgery Reimbursement and Coding Guide Gentrix devices facilitate the remodeling of functional, site-appropriate tissue. Compromised of
Hernia & Surgical Reimbursement & Coding Guide Hernia and General Surgery Reimbursement and Coding Guide Gentrix devices facilitate the remodeling of functional, site-appropriate tissue. Compromised of
Clinical Policy Title: Vacuum assisted closure in surgical wounds
 Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference
 2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
BONE AUGMENTATION AND GRAFTING
 1 A Computer-Guided Bone Block Harvesting Procedure: A Proof-of-Principle Case Report and Technical Notes Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review
1 A Computer-Guided Bone Block Harvesting Procedure: A Proof-of-Principle Case Report and Technical Notes Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review
Technology Report. Artificial Skin Grafts in Chronic Wound Care: A Meta-analysis of Clinical Efficacy and a Review of Cost-effectiveness
 Technology Report Issue 52 February 2005 Artificial Skin Grafts in Chronic Wound Care: A Meta-analysis of Clinical Efficacy and a Review of Cost-effectiveness Publications can be requested from: CCOHTA
Technology Report Issue 52 February 2005 Artificial Skin Grafts in Chronic Wound Care: A Meta-analysis of Clinical Efficacy and a Review of Cost-effectiveness Publications can be requested from: CCOHTA
2008 American Medical Association and National Committee for Quality Assurance. All Rights Reserved. CPT Copyright 2007 American Medical Association
 Chronic Wound Care ASPS #1: Use of wound surface culture technique in patients with chronic skin ulcers (overuse measure) This measure may be used as an Accountability measure Clinical Performance Measure
Chronic Wound Care ASPS #1: Use of wound surface culture technique in patients with chronic skin ulcers (overuse measure) This measure may be used as an Accountability measure Clinical Performance Measure
UvA-DARE (Digital Academic Repository) Ischemic and diabetic wounds of the lower extremity Santema, T.B. Link to publication
 UvA-DARE (Digital Academic Repository) Ischemic and diabetic wounds of the lower extremity Santema, T.B. Link to publication Citation for published version (APA): Santema, T. B. (2017). Ischemic and diabetic
UvA-DARE (Digital Academic Repository) Ischemic and diabetic wounds of the lower extremity Santema, T.B. Link to publication Citation for published version (APA): Santema, T. B. (2017). Ischemic and diabetic
A wide variety of membrane options
 A wide variety of membrane options BOVINE Bovine collagen membrane Neomem is a resorbable collagen membrane derived from highly purified type I collagen fibres from bovine Achilles tendon. The macromolecular
A wide variety of membrane options BOVINE Bovine collagen membrane Neomem is a resorbable collagen membrane derived from highly purified type I collagen fibres from bovine Achilles tendon. The macromolecular
LARS (Ligament Augmentation & Reconstruction System) Literature
 LARS-Related Studies and Papers ACL: 1. Level of Evidence: IV Li, H. et al (2011). Enhancement of the osseointegration of a polyethylene Terephthalate artificial ligament graft in a bone tunnel using 58S
LARS-Related Studies and Papers ACL: 1. Level of Evidence: IV Li, H. et al (2011). Enhancement of the osseointegration of a polyethylene Terephthalate artificial ligament graft in a bone tunnel using 58S
- Plans Commercial Launch of three dosing options for Excellagen in pre-filled syringes
 Generex Biotechnology Subsidiary Olaregen Therapeutix Inc. Plans Launch of FDA Cleared Excellagen Wound Conforming Gel Matrix with Three Dosage Options - Plans Commercial Launch of three dosing options
Generex Biotechnology Subsidiary Olaregen Therapeutix Inc. Plans Launch of FDA Cleared Excellagen Wound Conforming Gel Matrix with Three Dosage Options - Plans Commercial Launch of three dosing options
Negative Pressure Wound Therapy in the Outpatient Setting
 Negative Pressure Wound Therapy in the Outpatient Setting Policy Number: 1.01.16 Last Review: 9/2017 Origination: 7/2001 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Negative Pressure Wound Therapy in the Outpatient Setting Policy Number: 1.01.16 Last Review: 9/2017 Origination: 7/2001 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
