What s New Medical Policy Updates April 2017
|
|
|
- Nathaniel Dorsey
- 5 years ago
- Views:
Transcription
1 What s New Medical Policy Updates April 2017 Listed below are the recent changes made to policies within the Geisinger Health Plan Medical Policy Portfolio during the month of March that will become effective May 15, 2017 (unless otherwise specified). The Plan uses medical policies as guidelines for coverage decisions made within the insured individuals written benefit documents. Coverage may vary by line of business and providers and members are encouraged to verify benefit questions regarding eligibility before applying the terms of the policy. MP66 ESWT - REVISED (Added Exclusion) INDICATIONS: Skeletally mature members insured individuals diagnosed with chronic plantar fasciitis of six (6) months duration or more that has been refractory to three (3) conservative treatment options such as rest, antiinflammatory medications, physical therapy, corticosteroid injections and/or heel orthotics. LIMITATIONS: There is inadequate evidence in the published, peer-reviewed medical literature to establish the safety and/or efficacy of Extracorporeal shock wave treatment in the following groups: Insured Individuals Members on anticoagulation therapy Rheumatoid arthritis There is insufficient evidence in the published peer- reviewed medical literature to support ESWT for indications such as, but not limited to, Peyronie's disease, erectile dysfunction, angina pectoris, breast cancer-related lymphedema, and wound healing. There is insufficient evidence in the peer-reviewed published medical literature to establish the effectiveness of this test on health outcomes when compared to established tests or technologies. Use of ESWT for the treatment of these conditions is considered experimental, investigational or unproven and is NOT COVERED. MP75 Tissue Engineered Skin Substitutes - REVISED (Edited Language, Added Exclusions) Integra Dermal Regeneration Template, Matrix Wound, Bilayer Matrix Wound, and Meshed Bilayer Wound Matrix an acellular, biodegradable copolymer matrix coated is considered medically necessary for post-excision treatment of full thickness of deep partial thickness burns when autografting is not feasible due to the lack of suitable healthy tissue or the Insured individuals member s weak physiological state. The use of tissue engineered skin equivalents in any application outside of the current FDA approvals and/or not listed in this policy is considered experimental, investigational or unproven and is NOT COVERED. Due to limited studies, small study populations, variable outcomes and/ or poor study designs, there is insufficient evidence in the current published peer-reviewed medical literature to fully evaluate the clinical utility of any of the following products. Their use is limited to the FDA approved indication. support the use of Celaderm, Cymetra, Alloskin, PriMatrix, Biodesign, Conexa, DermaMatrix, ArthroFlex, BioDfence, FlexHD, GOREBio A, SportMesh, SurgiMend,and TissueMend. These products are considered experimental, investigational or unproven and are NOT COVERED. AlloDerm RTM Ready Conexa Integra Matrix Wound Repriza to Use AlloMax CorMatrix InteguPly Restore Orthobiologic AlloMend CRXa Jaloskin Seamguard
2 Allopatch HD CryoSkin LiquidGen SportMesh Alloskin Cuffpatch MariGen Omega3 SS Matrix Alloskin RT Cymetra Matriderm Stimulen Collagen AlloWrap DeNovo NT Graft Matristem StrataGraft AmnioCare Dermacell Matrix HD Strattice AmnioExcel Dermadapt Wound MediHoney Suprathel AmnioFix DermaMatrix Medeor SurgiMend Amniomatrix DermaSpan Mediskin Surgisis (including Surgisis AFP Anal Fistula Plug, Surgisis Gold Hernia Repair Grafts, and Surgisis ) AmnioMTM DressSkin Memoderm Talymed AmnioShield Duraform Menaflex TenoGlide Aongen Collagen Duragen XS Meso BioMatrix TenSIX Matrix Architect Extracellular Matrix Duragen Plus Neoform Dermis TheraForm Standard/Sheet ArthroFlex DuraMatrix NEOX 100 Quick- TissueMend Peel Atlas Wound Matrix Durepair NEOX 1k Wound TranzGraft Regeneration Matrix Matrix Avance Nerve Graft Endobon Xenograft NEOX FLO NuCel Unite Granules Avaulta Plus Endoform Neuragen Veritas Collagen Matrix AxoGuard nerve ENDURAgen NeuraWrap X-Repair connector AxoGuard nerve Epicel Neuroflex XCM Biologic protector Biobrane EpiDex NeuroMatrix Xelma BioDDryFlex EpiFix NeuroMend XenMatrix Biodesign Excellagen NuCel Xwrap (Hydro, DRY, and ECM) BioDExCel EZ Derm NuShield BioDfactor FlexHD Oasis BioDfence FloGraft OrthADAPT BioDOptix FortaDerm Wound OsseoGuard BioFiber Gammagraft Ovation C-QUR GORE Bio A Pelvicol Celaderm Grafix CORE Pelvisoft CellerateRX Grafix PRIME Peri-Guard Repair Patch CelluTome GraftJacket Peri-Strips Dry CLARIX 100 Quick- Peel Graftjacket Xpress injectable Permacol
3 CLARIX 1k HA Absorbent Wound PriMatrix CLARIX FLO Helicoll Promogran CollaFix hmatrix PTFE felt Collamend Hyalomatrix Puracol CollaSorb Inforce Puros Dermis CollaWound Integra Dermal Regeneration Repliform MP217 Polysomnography and Sleep Studies - REVISED (Added Eclusion) INDICATIONS: I. Polysomnography testing may be an eligible benefit when provided in a facility based sleep study laboratory which meets ALL of the following criteria: a. The center is under the direction and control of physicians. Diagnostic testing routinely performed in sleep disorder laboratories may be covered even in the absence of direct supervision by a physician when data is interpreted by a board certified sleep specialist or physician who fulfills eligibility criteria for the sleep medicine certification exam; and b. The insured individual member is referred to the sleep center by a physician after a comprehensive sleep evaluation is completed, and the center maintains a record of the physician s orders and comprehensive sleep evaluation; and c. The need for diagnostic testing is confirmed by medical evidence e.g. medical histories, examinations and laboratory tests; and d. Scheduling of a follow-up visit with a physician to review results is standard. II. The Plan considers facility-based polysomnography medically necessary for ANY of the following: a. The diagnosis of sleep related breathing disorders b. To monitor the response to treatment or adjust treatment c. In combination with multiple sleep latency testing in the evaluation of suspected narcolepsy d. In the evaluation of sleep related behaviors that are violent or potentially injurious to the patient or others e. In certain atypical or unusual parasomnias III. The Plan considers facility-based polysomnography medically necessary in ANY of the following: a. in patients with neuromuscular disorders and sleep related symptoms b. to assist in the diagnosis of paroxysmal arousals or other sleep disruptions thought to be seizure related c. in a presumed parasomnia or sleep related seizure disorder that does not respond to conventional therapy d. when there is a strong indication of periodic limb disorder IV. Polysomnography for CPAP titration is medically necessary to evaluate the response to CPAP treatment in insured individual members who meet EITHER of the following criteria: I. Polysomnography, cardiorespiratory sleep studies, and MSLT are NOT COVERED in the following situations: a. For the diagnosis of chronic insomnia; b. Preoperative evaluation for laser-assisted uvulopalatopharyngoplasty without clinical evidence of obstructive sleep apnea; c. For the diagnosis of chronic lung disease;
4 d. For the diagnosis of typical, uncomplicated and non-injurious parasomnia when the diagnosis is clearly delineated; e. Documented evidence of epilepsy with no specific complaints consistent with a sleep disorder; f. Documented evidence suggestive of periodic limb movement disorder or restless leg syndrome unless symptoms are suspected of being related to a covered indication; g. For the diagnosis of insomnia when related to depression; h. For the diagnosis of circadian rhythm disorders. II. The Plan considers the use of portable monitoring NOT medically necessary for patients who meet ANY of the following criteria: a. The diagnosis of OSA in patients with significant co-morbid medical conditions that may degrade the accuracy of PM, including but not limited to i. Severe Pulmonary Disease ii. Neuromuscular Disease iii. Congestive Heart Failure iv. Diagnostic evaluation of OSA in patient suspected of having other sleep disorders, including but not limited to: 1. Central Sleep Apnea 2. Periodic limb movement Apnea 3. Circadian rhythm disorders 4. Narcolepsy 5. General Screening of Asymptomatic patients b. Diagnostic evaluation of patients suspected of having co-morbid sleep disorders c. Diagnostic evaluation of patients with non-specific symptoms such as, but not limited to fatigue, malaise, etc., that may stem from other medical or psychological diagnoses. d. General screening of asymptomatic populations III. The Plan considers the use of Home MSLT not medically necessary and NOT COVERED. Home MSLT testing has not been proven to be equivalent to formal MSLT performed in a facility based sleep study laboratory. IV. The Plan considers diagnostic testing that is duplicative of previous sleep testing done by the attending physician NOT COVERED when there have been not significant clinical changes in the insured individual members documented medical history since the previous study. V. The Plan considers portable diagnostic sleep studies by home health agencies or durable medical equipment providers NOT COVERED. Devices /Procedures related to diagnosis sleep apnea The Plan does NOT provide coverage for the following devices/procedures for the diagnosis of OSA or other sleep disorders because they are considered experimental, investigational or unproven. The current body of evidence in the peerreviewed, published medical literature supporting the use of these devices/procedures for patients with sleep disorders is insufficient to allow adequate conclusions regarding their efficacy. (This list may not be all inclusive): SNAP Testing Watch PAT SleepStrip Actigraphy (Requires Program Exception for Medicaid business segment) The following policies have been reviewed with no change to the policy section. Additional references or background information was added to support the current policy. MP01 Neuromuscular Electrical Stim MP10 Blepharoplasty
5 MP25 Transcatheter Closure Devices MP34 Foot Orthotics MP68 Reduction Mammaplasty MP78 Sexual Dysfunction Therapies MP90 Inj. Bulking Agents/Incontinence MP92 Imp. Cardiac Loop Recorder MP94 Unilateral Pallidotomy MP106 Ultrasound/ Pregnancy MP112 Wireless Capsule Endoscopy MP113 Electrical Stim Wound Healing MP158 Continuous Passive Motion MP172 MicroVas Vascular Treatment System MP174 Exhaled Nitric Oxide for Asthma Management MP176 Meniett Device MP179 Photodynamic Therapy for Esophageal and Lung Cancer MP189 Computer Aided Detection Technology MP196 Convection-Enhanced Drug Delivery MP209 Medical Error Never Events MP261 Aqueous Drainage Shunt MP273 Prolaris Post-Prostatectomy MP280 Whole Exome Sequencing
Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds
 Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds WINDY COLE, DPM ADJUNCT PROFESSOR AND DIRECTOR OF WOUND CARE RESEARCH KSUCPM MEDICAL DIRECTOR, WOUND CENTER, UH AHUJA HOSPITAL Goals
Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds WINDY COLE, DPM ADJUNCT PROFESSOR AND DIRECTOR OF WOUND CARE RESEARCH KSUCPM MEDICAL DIRECTOR, WOUND CENTER, UH AHUJA HOSPITAL Goals
MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures
 POLICY: PG0203 ORIGINAL EFFECTIVE: 01/15/09 LAST REVIEW: 03/13/18 MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures GUIDELINES This policy does not certify benefits or authorization of benefits,
POLICY: PG0203 ORIGINAL EFFECTIVE: 01/15/09 LAST REVIEW: 03/13/18 MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures GUIDELINES This policy does not certify benefits or authorization of benefits,
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP075 Section: Medical Benefit Policy Subject: Tissue Engineered Skin Substitutes I. Policy: Tissue Engineered Skin Substitutes II. Purpose/Objective: To provide a
POLICIES AND PROCEDURE MANUAL Policy: MP075 Section: Medical Benefit Policy Subject: Tissue Engineered Skin Substitutes I. Policy: Tissue Engineered Skin Substitutes II. Purpose/Objective: To provide a
MEDICAL POLICY No R8 SKIN SUBSTITUTES & SOFT TISSUE GRAFTS
 Summary of Changes MEDICAL POLICY SKIN SUBSTITUTES & SOFT TISSUE GRAFTS Effective Date: August 25, 2017 Review Dates: 12/08, 8/09, 8/10, 8/11, 8/12, 4/13, 5/14, 5/15, 5/16, 5/17 Date Of Origin: December
Summary of Changes MEDICAL POLICY SKIN SUBSTITUTES & SOFT TISSUE GRAFTS Effective Date: August 25, 2017 Review Dates: 12/08, 8/09, 8/10, 8/11, 8/12, 4/13, 5/14, 5/15, 5/16, 5/17 Date Of Origin: December
See Policy CPT/HCPCS CODE section below for any prior authorization requirements
 Effective Date: 1/1/2019 Section: MED Policy No: 378 Medical Officer 1/1/2019 Date Technology Assessment Committee Approved Date: 3/09; 10/14; 1/16 Medical Policy Committee Approved Date: 4/02; 4/03; 9/04;
Effective Date: 1/1/2019 Section: MED Policy No: 378 Medical Officer 1/1/2019 Date Technology Assessment Committee Approved Date: 3/09; 10/14; 1/16 Medical Policy Committee Approved Date: 4/02; 4/03; 9/04;
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/28/2017 Section:
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/28/2017 Section:
Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association
 Bio-Engineered Skin and Page 1 of 58 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See Also: Bio-Engineered Skin and Periodontal Soft Tissue Grafting dental
Bio-Engineered Skin and Page 1 of 58 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See Also: Bio-Engineered Skin and Periodontal Soft Tissue Grafting dental
Number: (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end
 1 of 278 Number: 0244 (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. I. Aetna considers the following products for wound care medically necessary according
1 of 278 Number: 0244 (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. I. Aetna considers the following products for wound care medically necessary according
Skin Substitutes for Wound Care AHM
 Skin Substitutes for Wound Care AHM Clinical Indications Any 1 or more of the following products for wound care are considered medically necessary if the individual criteria are met Apligraf (graftskin)
Skin Substitutes for Wound Care AHM Clinical Indications Any 1 or more of the following products for wound care are considered medically necessary if the individual criteria are met Apligraf (graftskin)
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2018 Origination: 4/2008 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2018 Origination: 4/2008 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy
 Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy File Name: Bioengineered Skin and Soft Tissue Substitutes File Code: UM.SURG.16 Origination: 09/2016 Last Review: 10/2018 Next Review:
Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy File Name: Bioengineered Skin and Soft Tissue Substitutes File Code: UM.SURG.16 Origination: 09/2016 Last Review: 10/2018 Next Review:
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
IHCP banner page. IHCP to cover CPT code IHCP to cover CPT code INDIANA HEALTH COVERAGE PROGRAMS BR JULY 3, 2018
 IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR201827 JULY 3, 2018 IHCP to cover CPT 93050 Effective August 3, 2018, the Indiana Health Coverage Programs (IHCP) will cover Current Procedural Terminology
IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR201827 JULY 3, 2018 IHCP to cover CPT 93050 Effective August 3, 2018, the Indiana Health Coverage Programs (IHCP) will cover Current Procedural Terminology
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Bio-Engineered Skin and Page 1 of 62 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Bio-Engineered Skin and Amniotic Membrane and Amniotic Fluid medical
Bio-Engineered Skin and Page 1 of 62 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Bio-Engineered Skin and Amniotic Membrane and Amniotic Fluid medical
Bioengineered Skin and Soft Tissue Substitutes
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Cigna Medical Coverage Policy
 Cigna Medical Coverage Policy Subject Tissue-Engineered Skin Substitutes Table of Contents Coverage Policy... 1 General Background... 15 Coding/Billing Information... 62 References... 66 Effective Date...
Cigna Medical Coverage Policy Subject Tissue-Engineered Skin Substitutes Table of Contents Coverage Policy... 1 General Background... 15 Coding/Billing Information... 62 References... 66 Effective Date...
Medical Policy. MP Bioengineered Skin and Soft Tissue Substitutes
 Medical Policy MP 7.01.113 BCBSA Ref. Policy: 7.01.113 Last Review: 02/26/2018 Effective Date: 05/30/2018 Section: Surgery Related Policies 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors
Medical Policy MP 7.01.113 BCBSA Ref. Policy: 7.01.113 Last Review: 02/26/2018 Effective Date: 05/30/2018 Section: Surgery Related Policies 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2014 Origination: 4/2008 Next Review: 3/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2014 Origination: 4/2008 Next Review: 3/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid
 02-10000-11 Original Effective Date: 01/01/01 Reviewed: 03/22/18 Revised: 01/01/19 Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid THIS MEDICAL COVERAGE GUIDELINE
02-10000-11 Original Effective Date: 01/01/01 Reviewed: 03/22/18 Revised: 01/01/19 Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid THIS MEDICAL COVERAGE GUIDELINE
Policy Specific Section: October 7, 2011 January 30, 2015
 Medical Policy Bio-Engineered Skin and Soft Tissue Substitutes Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Surgery Original Policy Date: Effective Date: October
Medical Policy Bio-Engineered Skin and Soft Tissue Substitutes Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Surgery Original Policy Date: Effective Date: October
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Acellular dermal allografts for arthroplasty, 633 642 for plantar soft tissue augmentation, 543 555 Achilles tendon repair extracellular matrix
Note: Page numbers of article titles are in boldface type. A Acellular dermal allografts for arthroplasty, 633 642 for plantar soft tissue augmentation, 543 555 Achilles tendon repair extracellular matrix
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL Policy Number: MP.083.MH Last Review Date: 11/03/2016 Effective Date: 01/01/2017
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: April 15, 2017 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors as a Treatment of Wound Healing and Other Conditions 7.01.149
FEP Medical Policy Manual Effective Date: April 15, 2017 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors as a Treatment of Wound Healing and Other Conditions 7.01.149
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Skin Replacement Therapy for Chronic Non healing Wounds in the Outpatient Setting MP-032-MD-DE Medical Management Provider
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Skin Replacement Therapy for Chronic Non healing Wounds in the Outpatient Setting MP-032-MD-DE Medical Management Provider
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: July 15, 2018 Related Policies: 7.01.149 Amniotic Membrane and Amniotic Fluid Bioengineered Skin and Soft Tissue Substitutes Description Bioengineered skin and
FEP Medical Policy Manual Effective Date: July 15, 2018 Related Policies: 7.01.149 Amniotic Membrane and Amniotic Fluid Bioengineered Skin and Soft Tissue Substitutes Description Bioengineered skin and
SKIN AND SOFT TISSUE SUBSTITUTES
 SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Community Plan Medical Policy Policy Number: CS153.B Effective Date: November 1, 2018 Instructions for Use Table of Contents Page COVERAGE RATIONALE...
SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Community Plan Medical Policy Policy Number: CS153.B Effective Date: November 1, 2018 Instructions for Use Table of Contents Page COVERAGE RATIONALE...
SKIN AND SOFT TISSUE SUBSTITUTES
 SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Commercial Medical Policy Policy Number: 2018T0592A Effective Date: October 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Commercial Medical Policy Policy Number: 2018T0592A Effective Date: October 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
MP.083.MH Skin Substitutes- Human Skin Equivalents
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 10/01/2013 Section: Surgery Place(s)
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 10/01/2013 Section: Surgery Place(s)
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP217 Section: Medical Benefit Policy Subject: Polysomnography and Sleep Studies I. Policy: Polysomnography and Sleep Studies II. Purpose/Objective: To provide a policy
POLICIES AND PROCEDURE MANUAL Policy: MP217 Section: Medical Benefit Policy Subject: Polysomnography and Sleep Studies I. Policy: Polysomnography and Sleep Studies II. Purpose/Objective: To provide a policy
INDIANA HEALTH COVERAGE PROGRAMS
 INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 03/12
 Protocol Bio-Engineered Skin and Soft Tissue Substitutes (701113) Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 03/12 The following Protocol contains
Protocol Bio-Engineered Skin and Soft Tissue Substitutes (701113) Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 03/12 The following Protocol contains
Coding for Sleep Disorders Jennifer Rose V. Molano, MD
 Practice Coding for Sleep Disorders Jennifer Rose V. Molano, MD Accurate coding is an important function of neurologic practice. This section of is part of an ongoing series that presents helpful coding
Practice Coding for Sleep Disorders Jennifer Rose V. Molano, MD Accurate coding is an important function of neurologic practice. This section of is part of an ongoing series that presents helpful coding
Review Services Update September 2015
 Review Services Update September 2015 Unless otherwise indicated, prior authorization guidelines are applicable to members with active Group Health coverage. Prior Authorization Updates Procedure Notifications
Review Services Update September 2015 Unless otherwise indicated, prior authorization guidelines are applicable to members with active Group Health coverage. Prior Authorization Updates Procedure Notifications
POLICY The proposal is to add words in red text and to delete words or statements with a strikethrough:
 Bi-Engineered Skin and Sft Tissue Substitutes DESCRIPTION Bi-engineered skin and sft tissue substitutes may be derived frm human tissue (autlgus r allgeneic), nnhuman tissue (xengraphic), synthetic materials,
Bi-Engineered Skin and Sft Tissue Substitutes DESCRIPTION Bi-engineered skin and sft tissue substitutes may be derived frm human tissue (autlgus r allgeneic), nnhuman tissue (xengraphic), synthetic materials,
PORTABLE OR HOME SLEEP STUDIES FOR ADULT PATIENTS:
 Sleep Studies: Attended Polysomnography and Portable Polysomnography Tests, Multiple Sleep Latency Testing and Maintenance of Wakefulness Testing MP9132 Covered Service: Prior Authorization Required: Additional
Sleep Studies: Attended Polysomnography and Portable Polysomnography Tests, Multiple Sleep Latency Testing and Maintenance of Wakefulness Testing MP9132 Covered Service: Prior Authorization Required: Additional
Sleep Medicine. Maintenance of Certification Examination Blueprint. Purpose of the exam
 Sleep Medicine Maintenance of Certification Examination Blueprint Purpose of the exam The exam is designed to evaluate the knowledge, diagnostic reasoning, and clinical judgment skills expected of the
Sleep Medicine Maintenance of Certification Examination Blueprint Purpose of the exam The exam is designed to evaluate the knowledge, diagnostic reasoning, and clinical judgment skills expected of the
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: January 15, 2018 Related Policies: 2.01.18 Diagnosis and Medical Management of Obstructive Sleep Apnea Syndrome Diagnosis and Medical Management of Obstructive
FEP Medical Policy Manual Effective Date: January 15, 2018 Related Policies: 2.01.18 Diagnosis and Medical Management of Obstructive Sleep Apnea Syndrome Diagnosis and Medical Management of Obstructive
What s New Medical Policy Updates April 2018
 What s New Medical Policy Updates April 2018 Listed below are the recent changes made to policies within the Geisinger Health Plan Medical Policy Portfolio during the month of March that will become effective
What s New Medical Policy Updates April 2018 Listed below are the recent changes made to policies within the Geisinger Health Plan Medical Policy Portfolio during the month of March that will become effective
Tissue-Engineered Skin Substitutes
 Tissue-Engineered Skin Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO 06/01/2012 Section: Surgery Place(s) of Service: Outpatient/Office
Tissue-Engineered Skin Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO 06/01/2012 Section: Surgery Place(s) of Service: Outpatient/Office
Sleep Studies: Attended Polysomnography and Portable Polysomnography Tests, Multiple Sleep Latency Testing and Maintenance of Wakefulness Testing
 Portable Polysomnography Tests, Multiple Sleep Latency Testing and Maintenance of Wakefulness Testing MP9132 Covered Service: Yes when meets criteria below Prior Authorization Required: Yes as indicated
Portable Polysomnography Tests, Multiple Sleep Latency Testing and Maintenance of Wakefulness Testing MP9132 Covered Service: Yes when meets criteria below Prior Authorization Required: Yes as indicated
BREAST RECONSTRUCTION/REMOVAL AND REPLACEMENT OF IMPLANTS
 Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: October 15, 2018 Related Policies: 2.01.18 Diagnosis and Medical Management of Obstructive Sleep Apnea Syndrome Polysomnography for Non-Respiratory Sleep Disorders
FEP Medical Policy Manual Effective Date: October 15, 2018 Related Policies: 2.01.18 Diagnosis and Medical Management of Obstructive Sleep Apnea Syndrome Polysomnography for Non-Respiratory Sleep Disorders
medical policy update bulletin
 January 2019 medical policy update bulletin Medical Policy, Medical Benefit Drug Policy & Coverage Determination Guideline Updates UnitedHealthcare respects the expertise of the physicians, health care
January 2019 medical policy update bulletin Medical Policy, Medical Benefit Drug Policy & Coverage Determination Guideline Updates UnitedHealthcare respects the expertise of the physicians, health care
Medical Services Protocol Updates
 Protocol Medical Services Protocol Updates Distribution Date: September 1, 2016 The following Medical Protocol update includes information on protocols that have undergone a review over the last several
Protocol Medical Services Protocol Updates Distribution Date: September 1, 2016 The following Medical Protocol update includes information on protocols that have undergone a review over the last several
Index. sleep.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Actigraphy, 475, 485, 496 Adolescents, sleep disorders in, 576 578 Adults, sleep disorders in, 578 580 Advanced sleep phase disorder, 482 Age,
Note: Page numbers of article titles are in boldface type. A Actigraphy, 475, 485, 496 Adolescents, sleep disorders in, 576 578 Adults, sleep disorders in, 578 580 Advanced sleep phase disorder, 482 Age,
Is there an icd 10 code for smoking vapor
 Is there an icd 10 code for smoking vapor Thanks for your response, I'm just thinking that 305.1 is tobacco use and there is no tobacco in the e-cig. Just the nicotine and other chemicals. I have been
Is there an icd 10 code for smoking vapor Thanks for your response, I'm just thinking that 305.1 is tobacco use and there is no tobacco in the e-cig. Just the nicotine and other chemicals. I have been
Polysomnography (PSG) (Sleep Studies), Sleep Center
 Policy Number: 1036 Policy History Approve Date: 07/09/2015 Effective Date: 07/09/2015 Preauthorization All Plans Benefit plans vary in coverage and some plans may not provide coverage for certain service(s)
Policy Number: 1036 Policy History Approve Date: 07/09/2015 Effective Date: 07/09/2015 Preauthorization All Plans Benefit plans vary in coverage and some plans may not provide coverage for certain service(s)
Sleep 101. Kathleen Feeney RPSGT, RST, CSE Business Development Specialist
 Sleep 101 Kathleen Feeney RPSGT, RST, CSE Business Development Specialist 2016 Why is Sleep Important More than one-third of the population has trouble sleeping (Gallup) Obstructive Sleep Apnea Untreated
Sleep 101 Kathleen Feeney RPSGT, RST, CSE Business Development Specialist 2016 Why is Sleep Important More than one-third of the population has trouble sleeping (Gallup) Obstructive Sleep Apnea Untreated
MOVE BEYOND. to new hope for enterocutaneous fistulas. Enterocutaneous Fistula Repair
 MOVE BEYOND to new hope for enterocutaneous fistulas. Enterocutaneous Fistula Repair to an alternative treatment option Enterocutaneous fistulas can significantly affect patient health and quality of life.
MOVE BEYOND to new hope for enterocutaneous fistulas. Enterocutaneous Fistula Repair to an alternative treatment option Enterocutaneous fistulas can significantly affect patient health and quality of life.
TOP 10 LIST OF SLEEP QUESTIONS. Kenneth C. Sassower, MD Sleep Disorders Unit Massachusetts General Hospital for Children
 TOP 10 LIST OF SLEEP QUESTIONS Kenneth C. Sassower, MD Sleep Disorders Unit Massachusetts General Hospital for Children QUESTION #1: ARE SLEEP ISSUES IN CHILDREN THE SAME AS IN ADULTS? Distinctive Features
TOP 10 LIST OF SLEEP QUESTIONS Kenneth C. Sassower, MD Sleep Disorders Unit Massachusetts General Hospital for Children QUESTION #1: ARE SLEEP ISSUES IN CHILDREN THE SAME AS IN ADULTS? Distinctive Features
SLEEP DISORDERS. Kenneth C. Sassower, MD Division of Sleep Medicine; Department of Neurology Massachusetts General Hospital for Children
 SLEEP DISORDERS Kenneth C. Sassower, MD Division of Sleep Medicine; Department of Neurology Massachusetts General Hospital for Children Distinctive Features of Pediatric Sleep Daytime sleepiness uncommon
SLEEP DISORDERS Kenneth C. Sassower, MD Division of Sleep Medicine; Department of Neurology Massachusetts General Hospital for Children Distinctive Features of Pediatric Sleep Daytime sleepiness uncommon
Sleep Medicine Maintenance of Certification Examination Blueprint
 Sleep Medicine Maintenance of Certification Examination Blueprint Purpose of the exam The exam is designed to evaluate the knowledge, diagnostic reasoning, and clinical judgment skills expected of the
Sleep Medicine Maintenance of Certification Examination Blueprint Purpose of the exam The exam is designed to evaluate the knowledge, diagnostic reasoning, and clinical judgment skills expected of the
Basic Standards for Fellowship Training in Sleep Medicine
 Basic Standards for Fellowship Training in Sleep Medicine American Osteopathic Association and American College of Osteopathic Neurologists and Psychiatrists and American College of Osteopathic Internists
Basic Standards for Fellowship Training in Sleep Medicine American Osteopathic Association and American College of Osteopathic Neurologists and Psychiatrists and American College of Osteopathic Internists
Rocky Mountain Health Plans
 Rocky Mountain Health Plans Procedures that Require Prior Authorization Revised 12/29/16 The procedures for which Rocky Mountain Health Plans requires prior authorization include the list below. If you
Rocky Mountain Health Plans Procedures that Require Prior Authorization Revised 12/29/16 The procedures for which Rocky Mountain Health Plans requires prior authorization include the list below. If you
THIS WEBSITE IS FOR INFORMATIONAL PURPOSE ONLY. MEDICAID FEE FOR SERVICES: UPDATE JANUARY 1, 2018 Codes for Out-Patient Hospital Billing ONLY
 A4641 A9505 A9512 A9516 A9521 A9528 A9537 A9540 A9541 A9555 A9556 A9558 A9560 A9561 A9562 A9567 A9587 A9588 C1300 C1713 C1714 C1715 C1716 C1717 C1719 C1721 C1722 C1724 C1725 C1726 RADIOPHARMACEUTICAL,
A4641 A9505 A9512 A9516 A9521 A9528 A9537 A9540 A9541 A9555 A9556 A9558 A9560 A9561 A9562 A9567 A9587 A9588 C1300 C1713 C1714 C1715 C1716 C1717 C1719 C1721 C1722 C1724 C1725 C1726 RADIOPHARMACEUTICAL,
Coding for Wound Care
 Coding for Wound Care ****IMPORTANT*** Disclaimer ***Information provided is to the best of our knowledge and as current as possible. ***Please verify all policy and reimbursement information with your
Coding for Wound Care ****IMPORTANT*** Disclaimer ***Information provided is to the best of our knowledge and as current as possible. ***Please verify all policy and reimbursement information with your
Medical Policy Original Effective Date:01/23/2019
 Page 1 of 17 Disclaimer Description Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans or the plan may have broader or more
Page 1 of 17 Disclaimer Description Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans or the plan may have broader or more
Biodesign E NTEROCUTANEOUS FISTULA PLUG
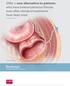 Offer a new alternative to patients who have enterocutaneous fistulas, even after standard treatments have been tried. Illustration by Lisa Clark Biodesign E NTEROCUTANEOUS FISTULA PLUG MEDICAL How is
Offer a new alternative to patients who have enterocutaneous fistulas, even after standard treatments have been tried. Illustration by Lisa Clark Biodesign E NTEROCUTANEOUS FISTULA PLUG MEDICAL How is
MLA HASS LUNG. SLEEP CENTER, Leominster Campus 100 Erdman Way, Leomjnster, MA Phone: Fax:
 MLA HASS LUNG SLEEP CENTER, Leominster Campus 100 Erdman Way, Leomjnster, MA 01453 Phone: 978-728-4641 Fax: 978-978-1382 MEICAL IRECTOR: Payam Aghassi, M, FCCP Thank you for your sleep study order! Attached
MLA HASS LUNG SLEEP CENTER, Leominster Campus 100 Erdman Way, Leomjnster, MA 01453 Phone: 978-728-4641 Fax: 978-978-1382 MEICAL IRECTOR: Payam Aghassi, M, FCCP Thank you for your sleep study order! Attached
Practice Parameters for the Indications for Polysomnography and Related Procedures
 Sleep. 20(6):406-422 1997 American Sleep Disorders Association and Sleep Research Society An American Sleep Disorders Association Report " Practice Parameters for the Indications for Polysomnography and
Sleep. 20(6):406-422 1997 American Sleep Disorders Association and Sleep Research Society An American Sleep Disorders Association Report " Practice Parameters for the Indications for Polysomnography and
Basic Standards for Osteopathic Fellowship Training in Sleep Medicine
 Basic Standards for Osteopathic Fellowship Training in Sleep Medicine American Osteopathic Association and the American College of Osteopathic Neurologists and Psychiatrists and the American College of
Basic Standards for Osteopathic Fellowship Training in Sleep Medicine American Osteopathic Association and the American College of Osteopathic Neurologists and Psychiatrists and the American College of
Medical Policy New Technology Assessment and Non-Covered Services
 Medical Policy New Technology Assessment and Non-Covered Services Subject: New Technology Assessment and Non-Covered Services Background: Harvard Pilgrim Health Care (HPHC) does not cover services or technology
Medical Policy New Technology Assessment and Non-Covered Services Subject: New Technology Assessment and Non-Covered Services Background: Harvard Pilgrim Health Care (HPHC) does not cover services or technology
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Abscesses, 27 30 Acellular products, for bone healing, 55 56 Acetaminophen, 79 80 Achilles tendon lengthening, for pediatric flatfoot,
Index Note: Page numbers of article titles are in boldface type. A Abscesses, 27 30 Acellular products, for bone healing, 55 56 Acetaminophen, 79 80 Achilles tendon lengthening, for pediatric flatfoot,
Medical Affairs Policy
 Medical Affairs Policy Service: Sleep Disorder Testing (Polysomnogram, Split Night Polysomnogram, Sleep Study, Multiple Sleep Latency Test (MSLT), Maintenance of Wakefulness Testing (MWT), Home Sleep Apnea
Medical Affairs Policy Service: Sleep Disorder Testing (Polysomnogram, Split Night Polysomnogram, Sleep Study, Multiple Sleep Latency Test (MSLT), Maintenance of Wakefulness Testing (MWT), Home Sleep Apnea
Checklist for Completion of Training Requirements in Sleep Medicine Pathway 2
 Checklist for Completion of Training Requirements in Sleep Medicine Pathway 2 This checklist follows the mandatory components of training in sleep medicine for physicians who have followed Pathway 2. Please
Checklist for Completion of Training Requirements in Sleep Medicine Pathway 2 This checklist follows the mandatory components of training in sleep medicine for physicians who have followed Pathway 2. Please
Care of Wound Reconstruction Patients, 2018 ed.
 Care of Wound Reconstruction Patients, 2018 ed. STEVE GROSSO, MD, FACS BILLINGS PLASTIC SURGERY A Smorgasbord of Plastic Surgery Stuff Disclaimer/Conflicts of Interest It is not possible to discuss wound
Care of Wound Reconstruction Patients, 2018 ed. STEVE GROSSO, MD, FACS BILLINGS PLASTIC SURGERY A Smorgasbord of Plastic Surgery Stuff Disclaimer/Conflicts of Interest It is not possible to discuss wound
What s New Medical Policy Updates September 2017
 What s New Medical Policy Updates September 2017 Listed below are the recent changes made to policies within the Geisinger Health Plan Medical Policy Portfolio during the month of August that will become
What s New Medical Policy Updates September 2017 Listed below are the recent changes made to policies within the Geisinger Health Plan Medical Policy Portfolio during the month of August that will become
FUTURE Local Coverage Determination (LCD): Application of Bioengineered Skin Substitutes to Lower Extremity Chronic Non-Healing Wounds (L35041)
 FUTURE Local Coverage Determination (LCD): Application of Bioengineered Skin Substitutes to Lower Extremity Chronic Non-Healing Wounds (L35041) Links in PDF documents are not guaranteed to work. To follow
FUTURE Local Coverage Determination (LCD): Application of Bioengineered Skin Substitutes to Lower Extremity Chronic Non-Healing Wounds (L35041) Links in PDF documents are not guaranteed to work. To follow
Introduction to Physical Agents Part II: Principles of Heat for Thermotherapy
 Introduction to Physical Agents Part II: Principles of Heat for Thermotherapy Mohammed TA, Omar momarar@ksu.edu.sa Dr.taher_m@yahoo.com Mobile : 542115404 Office number: 2074 Objectives After studying
Introduction to Physical Agents Part II: Principles of Heat for Thermotherapy Mohammed TA, Omar momarar@ksu.edu.sa Dr.taher_m@yahoo.com Mobile : 542115404 Office number: 2074 Objectives After studying
WOUND CARE UPDATE. -Commonly Used Skin Substitute Products For Wound. -Total Contact Casting. Jack W. Hutter DPM, FACFAS, C. ped.
 WOUND CARE UPDATE -Commonly Used Skin Substitute Products For Wound Closure -Total Contact Casting Jack W. Hutter DPM, FACFAS, C. ped. Commonly Used Skin Substitute Products for Wound Closure why are they
WOUND CARE UPDATE -Commonly Used Skin Substitute Products For Wound Closure -Total Contact Casting Jack W. Hutter DPM, FACFAS, C. ped. Commonly Used Skin Substitute Products for Wound Closure why are they
SLEEP MEDICINE CLINICAL PRIVILEGES
 Name: Page 1 Initial Appointment Reappointment All new applicants must meet the following requirements as approved by the governing body effective: 4/3/2013. Applicant: Check off the Requested box for
Name: Page 1 Initial Appointment Reappointment All new applicants must meet the following requirements as approved by the governing body effective: 4/3/2013. Applicant: Check off the Requested box for
Index SLEEP MEDICINE CLINICS. Note: Page numbers of article titles are in boldface type.
 549 SLEEP MEDICINE CLINICS Sleep Med Clin 1 (2007) 549 553 Note: Page numbers of article titles are in boldface type. A Abdominal motion, in assessment of sleep-related breathing disorders, 452 454 Adherence,
549 SLEEP MEDICINE CLINICS Sleep Med Clin 1 (2007) 549 553 Note: Page numbers of article titles are in boldface type. A Abdominal motion, in assessment of sleep-related breathing disorders, 452 454 Adherence,
Medical Services Protocol Updates
 Protocol Medical Services Protocol Updates Distribution Date: September 2, 2014 The following Medical Protocol update includes information on protocols that have undergone a review over the last several
Protocol Medical Services Protocol Updates Distribution Date: September 2, 2014 The following Medical Protocol update includes information on protocols that have undergone a review over the last several
Sweet Dreams: The Relationship between Sleep Health and Your Weight
 Sweet Dreams: The Relationship between Sleep Health and Your Weight Jason C. Ong, PhD Associate Professor Department of Neurology Center for Circadian and Sleep Medicine Northwestern University Feinberg
Sweet Dreams: The Relationship between Sleep Health and Your Weight Jason C. Ong, PhD Associate Professor Department of Neurology Center for Circadian and Sleep Medicine Northwestern University Feinberg
Index. sleep.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Accidents, risk of, with insufficient sleep, 318 Acquired immunodeficiency syndrome (AIDS), comorbid with narcolepsy, 298 299 Actigraphy, in
Note: Page numbers of article titles are in boldface type. A Accidents, risk of, with insufficient sleep, 318 Acquired immunodeficiency syndrome (AIDS), comorbid with narcolepsy, 298 299 Actigraphy, in
PNEUMATIC COMPRESSION DEVICES IN THE HOME SETTING
 Status Active Medical and Behavioral Health Policy Section: Medicine Policy Number: II-60 Effective Date: 05/19/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Status Active Medical and Behavioral Health Policy Section: Medicine Policy Number: II-60 Effective Date: 05/19/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
SLEEP DISORDERED BREATHING The Clinical Conditions
 SLEEP DISORDERED BREATHING The Clinical Conditions Robert G. Hooper, M.D. In the previous portion of this paper, the definitions of the respiratory events that are the hallmarks of problems with breathing
SLEEP DISORDERED BREATHING The Clinical Conditions Robert G. Hooper, M.D. In the previous portion of this paper, the definitions of the respiratory events that are the hallmarks of problems with breathing
KAPC Practice Guideline Title: Presurgical Testing Guidelines. Date Approved: 2/13/2017
 KAPC Practice Guideline Title: Presurgical ing Guidelines Date Approved: 2/13/2017 Policy Overview: Several specialty societies, most notably the American Society of Anesthesiologists and the American
KAPC Practice Guideline Title: Presurgical ing Guidelines Date Approved: 2/13/2017 Policy Overview: Several specialty societies, most notably the American Society of Anesthesiologists and the American
Today there are several working hypothesis for the physical effects of acoustic pressure waves:
 EPAT Shockwave Therapy This scientifically proven procedure represents a breakthrough in regenerative medicine treatment options for a broad range of musculoskeletal disorders/conditions utilizing a proprietary
EPAT Shockwave Therapy This scientifically proven procedure represents a breakthrough in regenerative medicine treatment options for a broad range of musculoskeletal disorders/conditions utilizing a proprietary
Integra. PriMatrix Dermal Repair Scaffold PATIENT INFORMATION. Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1
 Integra PriMatrix Dermal Repair Scaffold PATIENT INFORMATION Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Your Path to Recovery Your health care provider has chosen to use
Integra PriMatrix Dermal Repair Scaffold PATIENT INFORMATION Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Your Path to Recovery Your health care provider has chosen to use
DOWNLOAD OR READ : WHAT ARE SLEEP DISORDERS PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : WHAT ARE SLEEP DISORDERS PDF EBOOK EPUB MOBI Page 1 Page 2 what are sleep disorders what are sleep disorders pdf what are sleep disorders PAMF's Sleep Medicine Center doctors can help
DOWNLOAD OR READ : WHAT ARE SLEEP DISORDERS PDF EBOOK EPUB MOBI Page 1 Page 2 what are sleep disorders what are sleep disorders pdf what are sleep disorders PAMF's Sleep Medicine Center doctors can help
Title: Polysomnography and Sleep Studies Division: Medical Management Department: Utilization Management
 Retired Date: Page 1 of 14 1. POLICY DESCRIPTION: Polysomnography and Sleep Studies 2. RESPONSIBLE PARTIES: Medical Management Administration, Utilization Management, Integrated Care Management, Pharmacy,
Retired Date: Page 1 of 14 1. POLICY DESCRIPTION: Polysomnography and Sleep Studies 2. RESPONSIBLE PARTIES: Medical Management Administration, Utilization Management, Integrated Care Management, Pharmacy,
Index SLEEP MEDICINE CLINICS. Note: Page numbers of article titles are in boldface type. Cerebrospinal fluid analysis, for Kleine-Levin syndrome,
 165 SLEEP MEDICINE CLINICS Index Sleep Med Clin 1 (2006) 165 170 Note: Page numbers of article titles are in boldface type. A Academic performance, effects of sleepiness in children on, 112 Accidents,
165 SLEEP MEDICINE CLINICS Index Sleep Med Clin 1 (2006) 165 170 Note: Page numbers of article titles are in boldface type. A Academic performance, effects of sleepiness in children on, 112 Accidents,
RUN DESCRIPTION. NZRSI Sleep and Physiology Advanced Trainee. NZ Respiratory & Sleep Institute Service
 RUN DESCRIPTION POSITION: NZRSI Sleep and Physiology Advanced Trainee DEPARTMENT: NZ Respiratory & Sleep Institute Service PLACE OF WORK: Primary- New Zealand Respiratory & Sleep Institute(NZRSI), Greenlane
RUN DESCRIPTION POSITION: NZRSI Sleep and Physiology Advanced Trainee DEPARTMENT: NZ Respiratory & Sleep Institute Service PLACE OF WORK: Primary- New Zealand Respiratory & Sleep Institute(NZRSI), Greenlane
 Sleep Studies Sleep studies are tests that measure how well you sleep and how your body responds to sleep problems. These tests can help your doctor find out whether you have a sleep disorder and how severe
Sleep Studies Sleep studies are tests that measure how well you sleep and how your body responds to sleep problems. These tests can help your doctor find out whether you have a sleep disorder and how severe
Sleep Disorders The Effect on our Lives Every Day
 Sleep Disorders The Effect on our Lives Every Day Julie Hatleli, RN Sheri Krenz, RN Regional Sleep Disorders Center-Mankato, MN 2016 MFMER slide-1 Agenda Sleep Stages Sleep Apnea Sleep Disorders DOT requirements
Sleep Disorders The Effect on our Lives Every Day Julie Hatleli, RN Sheri Krenz, RN Regional Sleep Disorders Center-Mankato, MN 2016 MFMER slide-1 Agenda Sleep Stages Sleep Apnea Sleep Disorders DOT requirements
Integra. Tissue Technologies. Limit uncertainty with a leader in collagen technology
 Limit uncertainty with a leader in collagen technology Integra Dermal Regeneration Template PriMatrix Dermal Repair Scaffold A Pioneer in Regenerative Medicine Integra LifeSciences, a wordwide leader in
Limit uncertainty with a leader in collagen technology Integra Dermal Regeneration Template PriMatrix Dermal Repair Scaffold A Pioneer in Regenerative Medicine Integra LifeSciences, a wordwide leader in
Negative Pressure Wound Therapy
 Origination: 6/29/04 Revised: 8/24/16 Annual Review: 11/10/16 Purpose: To provide Negative Pressure Wound Therapy (wound care treatment) guidelines for the Medical Department staff to reference when making
Origination: 6/29/04 Revised: 8/24/16 Annual Review: 11/10/16 Purpose: To provide Negative Pressure Wound Therapy (wound care treatment) guidelines for the Medical Department staff to reference when making
MEDICAID FEE FOR SERVICES: UPDATE NOVEMBER 1, 2015 Codes for Out-Patient Hospital Billing ONLY
 90785 PSYTX_COMPLEX_INTERACTIVE 90837 PSYTX_PT&/FAMILY_60_MINUTES 90838 PSYTX_PT&/FAM_W/E&M_60_MIN 90839 PSYTX_CRISIS_INITIAL_60_MIN 90840 PSYTX_CRISIS_EA_ADDL_30_MIN 90846 FAMILY MEDICAL PSYCHOTH..1 HR.
90785 PSYTX_COMPLEX_INTERACTIVE 90837 PSYTX_PT&/FAMILY_60_MINUTES 90838 PSYTX_PT&/FAM_W/E&M_60_MIN 90839 PSYTX_CRISIS_INITIAL_60_MIN 90840 PSYTX_CRISIS_EA_ADDL_30_MIN 90846 FAMILY MEDICAL PSYCHOTH..1 HR.
Patient Care Information
 Patient Care Information A Guide to Healing Diabetic Foot Ulcers Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Dermal Regeneration Matrix Overview Diabetic foot ulcers are
Patient Care Information A Guide to Healing Diabetic Foot Ulcers Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Dermal Regeneration Matrix Overview Diabetic foot ulcers are
Chapter 11 Worksheet Code It
 Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
Polysomnography Course Session: Sept 2017
 Polysomnography Course Session: Sept 2017 General Information Polysomnography course will be held at SLEEP AND ALERTNESS CLINIC Med-West Medical centre 750 Dundas St. W., Suite 2-259 (Conference Room)
Polysomnography Course Session: Sept 2017 General Information Polysomnography course will be held at SLEEP AND ALERTNESS CLINIC Med-West Medical centre 750 Dundas St. W., Suite 2-259 (Conference Room)
Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of Integra Bilayer Wound Matrix
 Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of Integra Bilayer Wound Matrix Effective October 1, 2015, the Centers for & Medicaid Services (CMS) is implementing International
Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of Integra Bilayer Wound Matrix Effective October 1, 2015, the Centers for & Medicaid Services (CMS) is implementing International
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL Policy Number: PA.010.MH Last Review Date: 05/11/2017 Effective Date: 07/01/2017
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL Last Review Date: 05/11/2017 Effective Date: 07/01/2017 PA.010.MH Durable Medical Equipment, Corrective Appliances and This policy applies to the following
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL Last Review Date: 05/11/2017 Effective Date: 07/01/2017 PA.010.MH Durable Medical Equipment, Corrective Appliances and This policy applies to the following
What is a sleep center? Mercy Sleep Centers Staff Mercy Sleep Center Clive What is a sleep evaluation? Mercy Sleep Center Ames
 Mercy Sleep Center What is a sleep center? A sleep center is a medical facility dedicated to diagnosing and treating patients with sleep-related problems. Mercy Sleep Center is staffed by board certified/eligible
Mercy Sleep Center What is a sleep center? A sleep center is a medical facility dedicated to diagnosing and treating patients with sleep-related problems. Mercy Sleep Center is staffed by board certified/eligible
CPAP. The CPAP will be covered
 CPAP CPAP Did your patient have a face to face visit with the physician prior to having a sleep study that documented (1) Sleep History and symptoms and/or (2) Epworth Scale and/or (3) Physical Examination?
CPAP CPAP Did your patient have a face to face visit with the physician prior to having a sleep study that documented (1) Sleep History and symptoms and/or (2) Epworth Scale and/or (3) Physical Examination?
Smart Solutions for Serious Wounds. An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers.
 Smart Solutions for Serious Wounds An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers. A New Approach to Diabetic Foot Ulcer Care Supported by Over Two
Smart Solutions for Serious Wounds An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers. A New Approach to Diabetic Foot Ulcer Care Supported by Over Two
11/9/2015. Lehigh Valley Health Network Allentown, PA Lisa LePage, OTR/L
 Lehigh Valley Health Network Allentown, PA Lisa LePage, OTR/L Regional Burn Center 18 Bed Unit located in Kasych Building Critical and non-critical beds on the unit 3 Burn Surgeons, 6 Physician Assistants
Lehigh Valley Health Network Allentown, PA Lisa LePage, OTR/L Regional Burn Center 18 Bed Unit located in Kasych Building Critical and non-critical beds on the unit 3 Burn Surgeons, 6 Physician Assistants
